User login
What’s new in transcranial magnetic stimulation
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
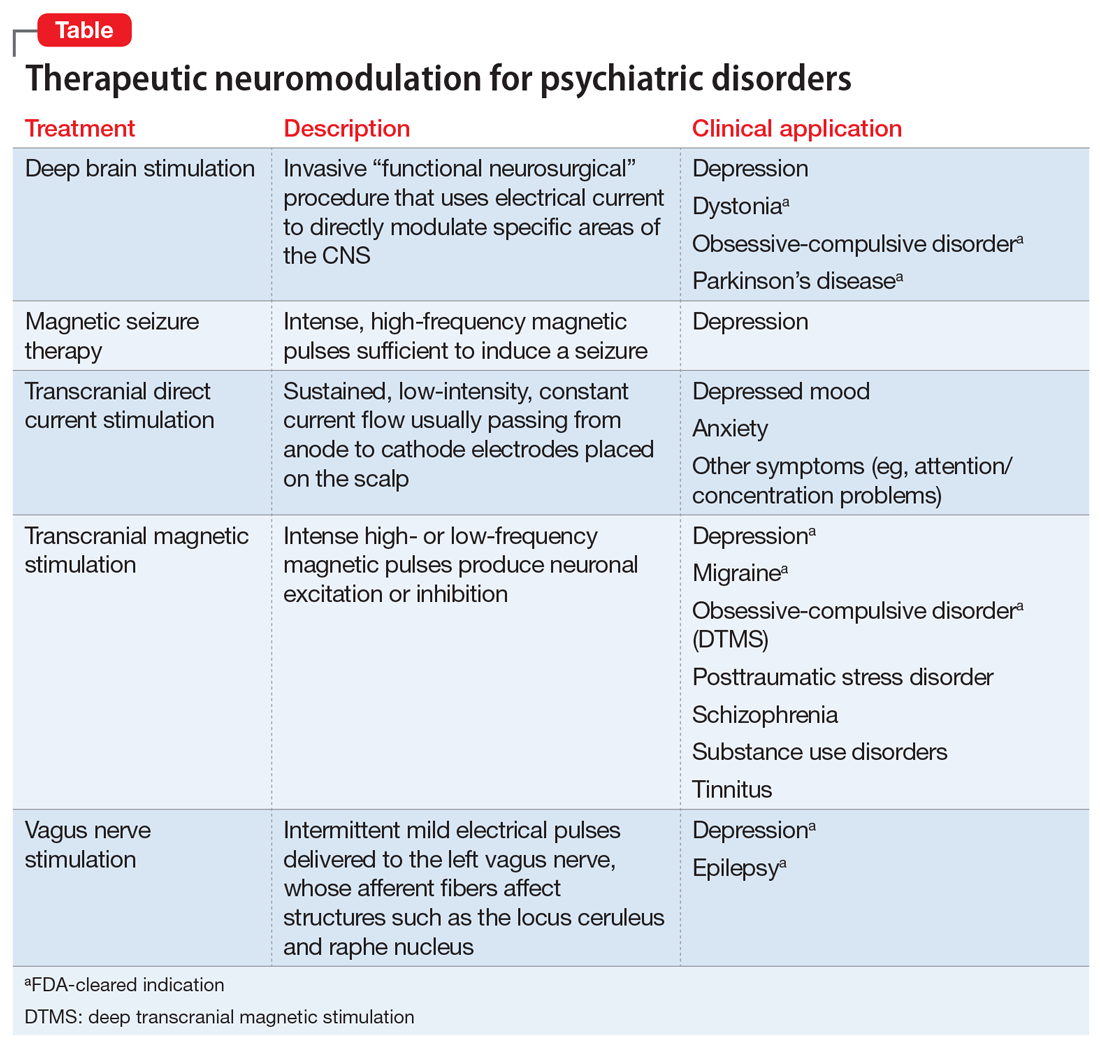
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
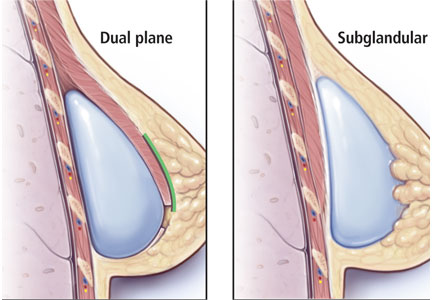
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
Therapeutic neuromodulation takes advantage of the brain’s electrochemical makeup. This allows for treatment devices that modulate neurocircuits relevant to behaviors disrupted in disorders such as major depressive disorder (MDD) (eg, sleep quality, appetite, cognitive, and executive functions). The default mode network (comprised of structures such as the medial prefrontal cortex [MPFC], the posterior cingulate cortex, the hippocampus, and their functional connectivity) serves as a prime example of circuitry that can be targeted by this approach.1
For 80 years, electroconvulsive therapy (ECT) has been an important neuromodulation option for patients with more severe illness. Recently, additional neuromodulatory approaches have been FDA-cleared, including transcranial magnetic stimulation (TMS), vagus nerve stimulation (VNS), and deep brain stimulation (DBS). Another approach, transcranial direct current stimulation (tDCS), has been extensively studied for its potential clinical utility but is not FDA-cleared. The Table provides descriptions of these therapies.
Since being cleared by the FDA in 2008, TMS has arguably made the greatest strides in providing an alternate neuromodulation treatment option for patients with MDD, with >1,000 centers nationally and 7 TMS devices FDA-cleared for treatment of depression. In this article, we review recent developments in TMS.
An evolving therapeutic option
While primarily studied as a monotherapy for MDD, in clinical practice TMS (Box) is typically used as an adjunct to medication and psychotherapy.2,3 In this context, it has demonstrated efficacy for more difficult-to-treat mood disorders with an excellent safety and tolerability profile whether used with or without medication.4-6
To further improve the efficiency and efficacy of TMS while maintaining its safety and tolerability, researchers and clinicians have been exploring a few initiatives.
Box
- Transcranial magnetic stimulation (TMS) utilizes intense, localized magnetic fields to alter activity in neural circuits implicated in the pathophysiology of depression
- Randomized, sham-controlled acute trials have demonstrated the efficacy of TMS for treatment-resistant depression
- Clinical availability of TMS has grown steadily over the past 10 years as >1,000 centers have been opened and additional devices have been FDA-cleared
- TMS has the potential to avoid safety and tolerability concerns associated with antidepressant pharmacotherapy (eg, weight gain, sexual dysfunction) and electroconvulsive therapy (eg, cognitive deficits)
- Greater sophistication in the choice of stimulation parameters, as well as other ongoing efforts to optimize the benefits of TMS, are yielding better clinical outcomes
Altered treatment parameters
One initiative is assessing the feasibility of altering various treatment parameters, such as the total number of treatment sessions (30 to 60 sessions); the frequency of sessions (eg, more than once daily); the total number of magnetic pulses per session (eg, >3,000); the stimulation coil localization (eg, left vs right dorsal lateral prefrontal cortex [DLPFC]; MPFC; and various methods to determine optimal coil placement (eg, EEG F3 coordinate or MRI-guided neuro-navigational methods). Such refinements offer the potential for enhanced efficacy, shorter treatment sessions, and/or improved tolerability. For example, lower frequency right DLPFC stimulations (eg, 1 Hz) can decrease the risk of seizures and improve overall tolerability. While this has not been studied as extensively as higher frequency left DLPFC stimulations (eg, 5 to 20 Hz), existing evidence supports similar efficacy between these 2 approaches.7
Theta burst stimulation. Some TMS devices can be adapted to deliver theta burst stimulation (TBS). This produces trains of triple, 50 Hz, pulsed bursts (usually with 200 ms inter-burst intervals occurring at a rate of 5 Hz; at 80% MT) to model naturally occurring theta rhythms. These bursts can be administered in stimulation protocols using intermittent TBS (iTBS) (eg, 10 bursts of triplets over 2 seconds every 10 seconds; 30 pulses per burst; for approximately 3 minutes; totaling 600 pulses) or continuous TBS (cTBS) bursts given in an uninterrupted train (eg, 40 seconds, 600 pulses). Evidence indicates these protocols facilitate long-term potentiation (ie, iTBS) and long-term depression (ie, cTBS), which in turn can modulate synaptic plasticity.
Continue to: While some clinicians are using...
While some clinicians are using TBS off-label, a recent non-inferiority trial (N = 395) reported similar efficacy and safety comparing standard 10 Hz TMS to an iTBS protocol at 120% of resting motor threshold (both over the left DLPFC).8 This has led to FDA clearance of the TMS device adapted to provide iTBS in this trial.8
From a more practical perspective, TBS has the potential to reduce the number of pulses (eg, 600 vs 3,000) and the total number of sessions required, as well as the duration of treatment sessions (eg, 37.5 minutes to <5 minutes). This can accelerate the time to response and decrease patient and staff commitment, with resulting cost savings.9 Despite this recent progress, ongoing research still needs to clarify issues such as the risk/benefit profile, particularly in younger and older populations, as well as assessment of duration of initial benefit and appropriate maintenance strategies.
New devices
Another initiative is the development of alternative TMS equipment. For example, newer coil designs with enhanced cooling ability allow for a substantial decrease in the required inter-train interval duration between stimulation trains, thus shortening the total session duration by approximately 50% (eg, from 37.5 to 19 minutes). The use of different coil arrays (eg, the H-coil capable of deeper vs surface stimulation) may allow for more direct stimulation of relevant neurocircuitry (eg, cingulate cortex), possibly improving efficacy and shortening time to onset of benefit. However, in head-to-head comparisons with single-coil devices, enhanced efficacy for depression has not been clearly demonstrated. One caveat is that the increase in depth of magnetic field penetration results in a loss of focality, resulting in the stimulation of larger brain areas. This might increase the risk of adverse effects such as seizures.
Increasing durability of effect
Because high relapse and recurrence rates compromise the initial benefit of any antidepressant therapy, appropriate maintenance strategies are essential. Several studies have evaluated strategies to maintain the acute benefit of TMS for treatment-resistant depression.
One was a 6-month, open-label TMS durability of effect trial for acute responders (n = 99) in the pivotal registration study.5 During this study, all participants were given antidepressant medication monotherapy. In addition, with early indication of relapse, patients received a reintroduction of TMS sessions (32/99 patients; mean number of sessions = 14.3). With this protocol, approximately 84% re-achieved their response status. The overall relapse rate was approximately 13%.5
Continue to: In a 1-year naturalistic study...
In a 1-year naturalistic study, 63% of patients (75/120) who met response or remission criteria after an acute course of TMS still met response criteria after 12 months. These patients received clinician-determined maintenance treatment that included reintroduction of TMS when indicated.3
In a prospective, 12-month, multisite, randomized pilot study, 67 patients with treatment-resistant MDD underwent an antidepressant medication washout and then received 30 sessions of TMS monotherapy.10 Those who met criteria for improvement (n = 49) were then randomized to once-monthly TMS or observation only. All patients remained medication-free but could receive TMS re-introduction if they deteriorated. At the end of the study, both groups demonstrated comparable outcomes, with a trend to a longer time before relapse among participants who received once-monthly TMS. Although these results are preliminary, they suggest that some patients could be treated both acutely and then maintained with TMS alone.
Re-introducing TMS in patients who show early signs of relapse after having an initial response achieves rates of sustained improvement that compare favorably with those of other strategies used to manage patients with treatment-resistant depression.
TMS vs ECT
The question often arises as to whether TMS is a viable alternate treatment to ECT. I believe the answer is unequivocally yes and no. By this, I mean some patients who in the past only had ECT as their next option when medications and psychotherapy were insufficient may now consider TMS. In support, there is evidence of comparable efficacy between TMS and ECT in a subgroup of patients who were considered clinically appropriate for ECT.11-13
How to best identify this group remains unclear, but investigators are exploring predictive biomarkers. For example, a large study (N = 1,188), with functional magnetic resonance imaging (fMRI) reported that depressed patients could be divided into 4 neurophysiological “biotypes” based on different patterns of aberrant connectivity in limbic and fronto-striatal networks.14 The authors further noted that such distinctions were helpful in predicting response in a subgroup of patients (n = 154) who received TMS.
Continue to: For now...
For now, experience indicates certain clinical factors may provide some guidance. Patients are usually better served by ECT if they:
- have depressive episodes of longer duration (eg, >3 years)
- have a high risk of suicide
- have psychotic or catatonic features associated with their depression
- have difficulty maintaining their physical well-being
- have bipolar depression.
Although existing evidence supports a possible benefit with TMS for bipolar depression (used in combination with a mood stabilizer), the lack of a definitive trial (precluding FDA clearance for this indication) and the lack of insurance coverage both limit the routine use of TMS for this indication.15
One potential advantage of TMS over ECT is a lower cost.13 Transcranial magnetic stimulation also may make it possible to achieve similar efficacy as ECT with fewer cognitive adverse effects when used in combination with ECT to reduce the number of acute ECT treatments required or as part of a maintenance strategy after a patient experiences an acute response to ECT.13
Magnetic seizure therapy (MST) vs ECT. An experimental treatment, MST uses a TMS device capable of producing more intense magnetic fields sufficient to induce a seizure.16 The advantage of MST over ECT-induced seizures is better control of intra-cerebral current path and density, thus avoiding deeper cortical areas associated with memory (eg, hippocampus) and minimizing cognitive adverse effects. As with ECT, however, anesthesia and muscle relaxation are required. Presently, MST remains investigational.
Other potential indications
In addition to MDD, TMS is also being studied as a potential treatment for other neuropsychiatric disorders.
Continue to: Obsessive-compulsive disorder
Obsessive-compulsive disorder (OCD). A recent double-blind study that evaluated a deep TMS (DTMS) device reported a significantly better outcome based on the Yale-Brown Obsessive-Compulsive Scale score with active high-frequency (20 Hz) DTMS (n = 18) vs a sham control (n = 15).17 The initial benefit persisted up to 1 month after the end of treatment. The authors speculated that this benefit may be due to direct modulation of the anterior cingulate cortex. These results led to the first FDA clearance of a deep TMS device for treating OCD.
Cognition. Because TMS does not require a seizure to produce its antidepressant effect and does not require anesthesia, the risk of neurocognitive disruption is low. In fact, evidence suggests TMS may have beneficial cognitive effects.18
In an effort to take advantage of this benefit, researchers have explored providing psychoeducation and psychotherapy sessions (eg, behavioral activation) during TMS treatments (“online”).19,20 The rationale is that neurocircuitry subserving various cognitive functions may be in a heightened state of receptivity during a TMS treatment, which would allow patients to assimilate and better utilize the therapeutic information provided.19,20
Researchers are also looking at the use of TMS to treat patients with mild cognitive impairment or early dementia. These patients often experience comorbid depression, and TMS could potentially improve memory via both its pro-cognitive and antidepressant effects.1 The lack of effective treatments for dementia supports pursuing TMS as a therapeutic option for these patients.
Other neuropsychiatric disorders. In addition to early-onset cognitive problems, other neurologic indications with promising data for TMS include chronic pain syndromes, Parkinson’s disease, tinnitus, and migraine headaches (a hand-held FDA-cleared device is now available for treating migraines). In addition to OCD and bipolar depression, other psychiatric indications with promising data include schizophrenia (eg, refractory auditory hallucinations, negative symptoms), posttraumatic stress disorder, and various addictive disorders.21 Because results have been mixed for most of these disorders, definitive trials are needed to clearly characterize the potential role of TMS.
Continue to: An ongoing evolution
An ongoing evolution
Neuromodulation is undergoing a renaissance spurred on by the need for more effective treatments to manage some of our most challenging illnesses. Transcranial magnetic stimulation and other forms of therapeutic neuromodulation are welcome additions for managing treatment-resistant depression, OCD, and possibly other disorders. But perhaps their greatest value is as a bellwether for what’s to come. In addition to the ongoing refinements to existing neuromodulation devices, newer modulation approaches (eg, temporal interference stimulation) and the search for reliable biomarkers may dramatically expand and enhance our clinical options.14,22
Bottom Line
Transcranial magnetic stimulation (TMS) continues to evolve as a nonpharmacologic treatment for mood disorders, obsessive-compulsive disorder, and potentially for other indications. Recent developments, including altered treatment parameters, new devices, and strategies for increasing the durability of antidepressant effects, have enhanced the benefits of TMS.
Related Resources
- Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973-984.
- Janicak PG, Sackett V, Kudrna K, et al. Transcranial magnetic stimulation for the treatment of major depression: an update on recent advances. Current Psychiatry. 2016:15(6):49-56.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
1. Koch G, Bonnì S, Pellicciari MC, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2018;169: 302-310.
2. O’Reardon JP, Solvason B, Janicak PG, et al. Efficacy and safety of repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of major depression: results of a multicenter randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
3. Dunner DL, Aaronson ST, Sackheim HA, et al. A multisite, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a one-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
4. Janicak PG, O’Reardon JP, Sampson SM, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008;69:222-232.
5. Janicak PG, Nahas Z, Lisanby SH, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3(4):187-199.
6. Janicak PG. Risk management issues in transcranial magnetic stimulation for treatment of major depression. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
7. Chen J, Zhou C, Wu B, et al. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 2013;210(3):1260-1264.
8. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391(10131):1683-1692.
9. Chung SW, Hoy KE, Fitzgerald PB. Theta-burst stimulation: a new form of TMS treatment for depression? Depress Anxiety. 2015;32(3):182-192.
10. Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul. 2016;9(2):251-257.
11. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prop Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
12. Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depressive: preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659-667.
13. Lanocha K, Janicak PG. TMS for depression: relationship to ECT and other therapeutic neuromodulation approaches. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
14. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38.
15. Aaronson ST, Croarkin PE. Transcranial magnetic stimulation for the treatment of other mood disorders. In: Bermudes R, Lanocha K, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
16. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi: 10.1155/2015/521398.
17. Carmi L, Alyagon U, Barnea-Ygael N, et al. Clinical and electrophysiological outcomes of deep TMS over the medial prefrontal and anterior cingulate cortices in OCD patients. Brain Stimul. 2018;11(1):158-165.
18. Martis B, Alam D, Dowd SM, et al. Neurocognitive effects of repetitive transcranial magnetic stimulation in severe major depression. Clin Neurophysiol. 2003;114:1125-1132.
19. Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. 2018;11(2):337-345.
20. Russo GB, Tirrell E, Busch A, et al. Behavioral activation therapy during transcranial magnetic stimulation for major depressive disorder. J Affect Disord. 2018;236:101-104.
21. Pannu J, DE Souza DD, Samara Z, et al. Transcranial magnetic stimulation for disorders other than depression. In: Bermudes RA, Lanocha KI, Janicak PG (eds). Transcranial magnetic stimulation: clinical applications for psychiatric practice. Washington, DC: American Psychiatric Association Publishing; 2018.
22. Grossman N. Modulation without surgical intervention. Science. 2018;361:461-462.
Limitation of Life-Sustaining Care in the Critically Ill: A Systematic Review of the Literature
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
Access to life-sustaining treatment (LST) became a mainstay in hospitals across the United States in the 1970s. This has raised complex ethical questions surrounding the use of these therapies, particularly in the face of a poor prognosis or significant morbidity. The Society for Critical Care Medicine formed a consensus panel in 1989 to construct ethical guidelines regarding the initiation, continuation, and withdrawal of intensive care.1 These guidelines emphasized that withdrawing and withholding are not only permissible but may be necessary to preserve the balance between quantity and quality of life. Nevertheless, an increasing number of Americans are dying after aggressive LST in the hospital, and greater than one in five deaths occur after admission to the ICU.2 Understanding the factors associated with decisions to withhold or withdraw LST are important to policy makers, ethicists, and healthcare leaders because they affect resources used at the end of life and the need for palliative care and hospice in the ICU setting.
Several studies have characterized the patient characteristics, incidence, and variability associated with limitation of LST in various populations of critically ill patients in the US. We are unaware of another systematic review of the literature that has examined data from these studies in order to understand the process and outcomes of LST limitations. We defined limitations of LST as decisions to withdraw or withhold cardiopulmonary resuscitation through Do Not Resuscitate (DNR) orders, mechanical ventilation, renal replacement therapy, intravenous blood pressure support, or artificial nutrition (enteric or intravenous).
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for reporting. A comprehensive literature search was performed by a medical librarian (TWE) in Ovid MEDLINE, PubMed, Embase, the full Cochrane Library, CINAHL, PsycINFO, the Philosopher’s Index, Scopus, Web of Science, and Google Scholar. PubMed was limited to non-MEDLINE records in order to complement the Ovid results. The Georgetown Bioethics Research Library at the Kennedy Institute (https://bioethics.georgetown.edu/) was also searched for any unpublished literature. Initial searches were conducted in December 2014, and an update was performed in April 2017. All databases were searched from inception, and bibliographies of relevant studies were reviewed for additional references (Appendix 1).
Database-specific subject headings and keyword variants for each of the five main concepts—intensive care, end-of-life, decision making, limitation of treatment, and death—were identified and combined. Results were limited to the adult population and to the English language.
Two authors independently reviewed article titles and abstracts (KM, AMT). The full text of potentially eligible studies was then reviewed for inclusion. All disputes were discussed and resolved by consensus. The criteria for inclusion were reporting of patient-level data, critical care patients only (or reported separately from other unit types), US setting, and reporting of data on limitations of LST. The exclusion criteria were studies published only as research abstracts, surveys of physicians or families, organ donors, studies of brain death, surveys, patients less than 18 years old, and long-term intensive care settings (ie, long-term acute care hospitals, long-term respiratory units). Also excluded were studies in which an intervention was performed; as a result, all included studies were observational. Research abstracts were excluded because they lacked sufficient detail from which to abstract study quality or results. Studies of organ donation, brain death, and pediatrics were excluded due to differences in the decision-making context that would make it difficult to draw conclusions about adult ICU care. Studies which included an intervention were excluded to avoid affecting the rate of limitation of LST as a result of the intervention, since our goal was to quantify the number of limitations of LST in usual medical practice.
For each article, we abstracted the number of patients who experienced a limitation of LST out of the total population and factors associated with the limitation. If a multivariable analysis was performed, we reported only variables that remained significant in this analysis. We also reported the number of patients who died, and of those, the number of decedents who underwent a limitation of LST before death. In some cases, this proportion was not reported in the manuscript but could be calculated based on the data presented. This number was calculated based on the number of deaths that were preceded by a limitation in life-sustaining care divided by the total number of deaths. Patients with brain death were not counted as having had a “limitation” if support was withdrawn after the declaration of brain death. We were unable to conduct a meta-analysis of the findings because of the wide variation in study populations and criteria used to define limitations of care.
To assess risk of bias in individual studies, the two raters independently made a yes/no determination regarding several quality metrics established at the outset of the review: clarity of the eligibility criteria for participant inclusion, whether a power or sample size calculation was done, adequacy of the description of the sampling approach and recruitment, and generalizability. Disagreements were resolved by consensus.
RESULTS
Study Selection
A total of 2,460 references were identified, and after removal of 578 duplicates, 1,882 unique titles and abstracts were reviewed. One hundred thirteen titles met the inclusion criteria. After review of complete texts, 83 were excluded based on the above criteria (Appendix). This led to a final number of 36 studies included for analysis.
Fifteen articles were prospective, observational studies. The rest were retrospective analyses of patient-level data. Seven were large, multicenter studies with greater than 20 centers involved (including Project IMPACT); six such studies included medical and surgical patients. The remaining large, multicenter study examined a surgical trauma cohort.
Fifteen of the studies addressed DNR as a limitation and 25 addressed other limitations such as withdrawing or withholding LST (several addressed both DNR and another limitation). Nine studies enrolled only patients who had died and the remaining 27 enrolled all ICU admissions.
Historical Trends
Examination of the three studies that looked at >20 regionally diverse ICUs revealed a trend over time toward increased limitation prior to death (Figure). Jayes looked at the number of DNR orders preceding death from 1979 to 1980 then compared that to a cohort from 1988 to 1990; Prendergast included withholding/withdrawing of LST prior to death from 1994 to 1995;and Quill used the IMPACT database to examine limitations prior to death from 2001 to 2009.3-5
Effect of Unit Specialty
Twelve studies were mixed (surgical/medical or medical/neuro) ICUs, 11 were medical/cardiac units, five were neurologic units, and six were surgical/trauma units only. Two studies did not report unit specialty. Four studies that compared surgical and medical ICUs found that surgical patients were more likely to die with full intervention.4-7 In all of these studies, medical patients were more likely to have limitations of LST preceding death. Quill, et al. further detailed that emergency surgery was more likely to be associated with limitation than elective surgery.5
Patient Factors
In 15 studies, older age was associated with an increased likelihood of limitations on LST.3,5-18 In one study, advanced age was associated with early versus late withdrawal.19 Poor performance status and multiple medical comorbidities were also associated with limitations of LST. The largest population-based study by Quill et al. found that being fully dependent on others upon admission to the ICU was associated with an increased likelihood of limiting LST.5 Sise et al. found, in an analysis performed over 10 years in one trauma center, that increased age, comorbidities, and a fall as the reason for trauma admission were associated with limitation of LST.9 Salottolo et al. found that if the reason for trauma admission was a fall, there was an increased odds ratio of DNR status.18 Many studies found that having medical comorbidities prior to admission was associated with increased likelihood of limiting LST in both medical and surgical patients.3,7,9,13,15,18
Five studies found a statistically significant difference between women and men in the likelihood of limitation of LST,3,5,9,14,16 and another study reported that women who were trauma patients had an increased odds ratio of changing to DNR code status.18 Only one study found that males were associated with an increased likelihood of limiting aggressive treatment.20
White race was associated with increased limitation of LST in nine studies.4,5,10,11,14-16,21,22 One study in neurocritical care patients found that both white and Hispanic races were correlated with a higher likelihood of limitations.23 Muni et al. found that nonwhite patients had a statistically significantly lower likelihood of having comfort measures and DNR orders written prior to death, but discussion of prognosis was more likely to be documented in nonwhite patients.21
In summary, white race, female gender, and older age were the most frequent factors associated with a higher likelihood of limiting LST.
Factors Related to Critical Illness
There were several illness severity indicators that were associated with limitations. The Acute Physiology and Chronic Health Evaluation (APACHE) scores were the most common for medical patients and Glasgow Coma Scale (GCS) was the most common for patients with neurologic injury. Eight studies reported that a higher APACHE score was associated with an increased likelihood of limitations.3,7,10,15,17,20,22,24 Similar associations were found based on the Sepsis Related Organ Failure Assessment score in one study and a scoring system developed by the author in a second study.25,26
Seven studies, consisting of three neurologic, two medical-surgical, and two trauma cohorts, reported that a lower GCS score increased the likelihood that the patient would have limited LST.5,10,11,13,14,18,22 Additionally, Geocadin and colleagues discussed the difficulty with neurological prognostication in clinical practice; they reported that the cortical evoked potential (CEP) was correlated with the time to withdrawLST if the CEP was malignant, and the time to withdraw LST was less in malignant than in benign CEP.27
Mortality and End Effects of Limiting LST
Chen and colleagues used propensity scores to control for mortality differences between patients who had full interventions versus those with limitations and found that higher mortality correlated with the decision to withhold or withdraw LST.10 Weimer and colleagues used modeling to predict the probable outcome of patients who experienced an intracranial hemorrhage who had limitation of LST. Based on this model, nearly all the patients in their study would have died or had severe disability at 12 months despite having maximal therapy; they concluded that withdrawal of LST may not have been a self-fulfilling prophecy as others have proposed.28 Mulder and colleagues reported that in a small cohort of out-of-hospital cardiac arrest survivors admitted to the hospital, over one-third had good neurological outcomes after coding after 72 hours.29 The study highlighted the importance of timing in neurological prognostication.
Variation in Limitation Rates among Centers
In the 36 studies, we found an overall range of DNR orders from 5.4%7 to 82.0%.30 For other limitations, the rates ranged from 6.3%13 to 80.4%.31 Hart reported a low rate of limitations (4.8%) at the time of ICU admission.16 Four large, multicenter studies drew attention to the large variability between critical care centers and the limitation of end-of-life care.3-5,14 Jayes first described this phenomenon when examining the frequency of DNR orders from 1979 to 1980 and 1988 to 1990.3 This study found a range from 1.5% to 22%. Later, in another large, multicenter study, Prendergast et al. looked at 131 ICUs at 110 different institutions in 38 states that participated in postgraduate training and found variability in CPR attempts prior to death between 4% and 79%.4 In 2008, Nathens et al. reported significant variation in DNR rates across trauma centers; they found a higher incidence of DNR orders when there was an open ICU structure.14
Overall, there was wide variation in the proportion of deaths preceded by limitation of LST, ranging from 29.5% in one study of trauma patients8 to 92% in another study of trauma patients whose death occurred after 24 hours of care.9 In the largest study to date by Quill and colleagues utilizing the IMPACT database, they found large variability in the number of deaths preceded by full intervention based on differences in practice patterns of critical care centers.5
Bias
All studies indicated clear eligibility criteria for inclusion and described their sampling approach in adequate detail. All but one stated their method of participant recruitment, and the one remaining study was a secondary analysis and referenced the earlier manuscript.30 No study provided a power or sample size calculation, and sample sizes varied widely. Generalizability was most affected by the fact that many studies were conducted in a single ICU.
DISCUSSION
This systematic review of LST in US ICUs found several patient and illness factors that were associated with limitation of LST. The association of preadmission functional status and comorbidities with limitation of LST suggest that prior health is a factor in decision making. Further, ICU severity of illness, as measured by several commonly used indices, was associated with limitations.
Although variations in study design precluded meta-analysis, examination of the largest studies suggests that limitations are becoming more frequent over time. Also, early studies generally addressed DNR status, while later studies included withdrawal or withholding of LST, most commonly artificial ventilation. These findings reflect the current consensus in US medicine that it is ethically acceptable to limit LSTs in cases when they no longer benefit the patient or the patient would no longer want them.32,33
Some studies found variability by unit type, suggesting that decision making may differ among surgical, medical, and neurologic illness. Mayerand Kossoff concluded, in study of a cohort of neurocritical care ICU patients, that medical patients often have issues of physiologic futility and imminent death, whereas neurologic patients more often confront issues of quality of life. They also note that there is a difference in how patients with differing illnesses die; medical patients will have limitation of hemodialysis or vasopressors, whereas neurologic surrogate decision makers often confront decisions around terminal extubation.23
Some patient-level factors, such as race or ethnicity, may point to cultural differences in preferences for LST at the end of life. Other authors have documented that African American patients are more likely to choose end-of-life care for themselves or their family members, which may be due to cultural or religious factors as well as to a history of unequal access to medical care.34 Reasons for the finding that women are more likely to have limitations has not been as well described. Further research could explore whether this is due to differences in patient preferences by gender or to other factors.
Even when examining patient-level factors, illness severity and type of ICU, the wide variability in end-of-life care in critical care units across the country is still large. A worldwide review also found a high degree of variability, even within geographical regions.35 More research is needed to understand the factors associated with this wide variability, as this seems to indicate that approaches to end-of-life care may vary based on the ICU as much as individual patient preferences or clinical factors.
These findings can inform clinicians about variables that are important in the decision-making process. Patient age and race are factors to consider in the likelihood of reaching a decision to set limitations. Information about patients’ health status prior to critical illness, as well as ICU illness severity, are also important considerations.
The limitations of this review include the wide variety of LSTs assessed, including code status change, ventilator withdrawal, removal of pressors, and cessation of renal replacement therapy. Also, there was variation in sample size and the number of included units. There was also significant heterogeneity in the outcomes addressed and the variety of methods used in the included studies. We attempted to address this with an analysis of the quality of the studies, but given the wide variability, we were unable to account for all of the differences; unfortunately, this is a standard issue within studies that utilize systematic reviews, as well as similar concepts such as meta-analyses.
In conclusion, the increase in the frequency of limitations of LST in critically ill patients and a change in the nature of limitations from DNR order to withdrawal or withholding of LST suggests a trend toward growing acceptance of limiting treatments in critical illness. The wide variation in withdrawal of care in US ICUs does not seem fully explained by patient variables including preferences, illness type, or changes over time. Factors such as poor prefunctional status, a higher number of comorbid conditions prior to critical illness, and the severity of critical illness are likely important for surrogates and clinicians to consider during goals of care discussions. Further research is needed to explore why patients may receive very different types of care at the end of life depending the institution and ICU in which they receive their care.
Disclosures
The authors have no conflicts of interest to disclose. This work was performed at the Indiana University School of Medicine.
Funding
Financial support for Dr. Torke was provided by a Midcareer Investigator Award in Patient Oriented Research from the National Institute on Aging (K24AG053794). Dr. McPherson was supported by the Indiana University Department of Medicine.
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
1. Sprung CL, Raphaely RC, Hynninen M, et al. Consensus report on the ethics of foregoing life-sustaining treatments in the critically ill. Task Force on Ethics of the Society of Critical Care Medicine. Crit Care Med. 1990;18(12):1435-1439. PubMed
2. Angus DC, Barnato AE, Linde-Zwirble WT, et al. Use of intensive care at the end of life in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638-643. PubMed
3. Jayes RL, Zimmerman JE, Wagner DP, Draper EA, Knaus WA. Do-not-resuscitate orders in intensive care units. Current practices and recent changes. JAMA. 1993;270(18):2213-2217. doi: 10.1001/jama.1993.03510180083039. PubMed
4. Prendergast TJ, Claessens MT, Luce JM. A national survey of end-of-life care for critically ill patients. Am J Respir Crit Care Med. 1998;158(4):1163-1167. doi: 10.1164/ajrccm.158.4.9801108. PubMed
5. Quill CM, Ratcliffe SJ, Harhay MO, Halpern SD. Variation in decisions to forgo life-sustaining therapies in US ICUs. Chest. 2014;146(3):573-582. doi: 10.1378/chest.13-2529. PubMed
6. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med. 2014;42(2):296-302. doi: 10.1097/CCM.0b013e3182a272db. PubMed
7. Zimmerman JE, Knaus WA, Sharpe SM, Anderson AS, Draper EA, Wagner DP. The use and implications of do not resuscitate orders in intensive care units. JAMA. 1986;255(3):351-356. doi: 10.1001/jama.1986.03370030071030. PubMed
8. Weireter LJ, Jr., Collins JN, Britt RC, Novosel TJ, Britt LD. Withdrawal of care in a trauma intensive care unit: the impact on mortality rate. Am Surg. 2014;80(8):764-767. PubMed
9. Sise MJ, Sise CB, Thorndike JF, Kahl JE, Calvo RY, Shackford SR. Withdrawal of care: A 10-year perspective at a Level I trauma center. J Trauma Acute Care Surg. 2012;72(5):1186-1191. doi: 10.1097/TA.0b013e31824d0e57. PubMed
10. Chen Y-Y, Connors AF, Jr., Garland A. Effect of decisions to withhold life support on prolonged survival. Chest. 2008;133(6):1312-1318. doi: 10.1378/chest.07-1500. PubMed
11. Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29(9):1792-1797. PubMed
12. Huynh TN, Walling AM, Le TX, Kleerup EC, Liu H, Wenger NS. Factors associated with palliative withdrawal of mechanical ventilation and time to death after withdrawal. J Palliat Med. 2013;16(11):1368-1374. doi: 10.1089/jpm.2013.0142. PubMed
13. Kowalski RG, Chang TR, Carhuapoma JR, Tamargo RJ, Naval NS. Withdrawal of technological life support following subarachnoid hemorrhage. Neurocrit Care. 2013;19:269-275. doi: 10.1007/s12028-013-9929-8. PubMed
14. Nathens AB, Rivara FP, Wang J, Mackenzie EJ, Jurkovich GJ. Variation in the rates of do not resuscitate orders after major trauma and the impact of intensive care unit environment. J Trauma. 2008;64(1):81-88;discussion 8-91. doi: 10.1097/TA.0b013e31815dd4d7. PubMed
15. Youngner SJ, Lewandowski W, McClish DK, Juknialis BW, Coulton C, Bartlett ET. ‘Do not resuscitate’ orders. Incidence and implications in a medical-intensive care unit. JAMA. 1985;253(1):54-57. doi: 10.1001/jama.1985.03350250062023. PubMed
16. Hart JL, Harhay MO, Gabler NB, Ratcliffe SJ, Quill CM, Halpern SD. Variability among US intensive care units in managing the care of patients admitted with preexisting limits on life-sustaining therapies. JAMA Intern Med. 2015;175(6):1019-1026. doi: 10.1001/jamainternmed.2015.0372. PubMed
17. Mehter HM, Wiener RS, Walkey AJ. “Do not resuscitate” decisions in acute respiratory distress syndrome: a secondary analysis of clinical trial data. Ann Am Thorac Soc. 2014;11(10):1592-1596. doi: 10.1513/AnnalsATS.201406-244BC. PubMed
18. Salottolo K, Offner PJ, Orlando A, et al. The epidemiology of do-not-resuscitate orders in patients with trauma: a community level one trauma center observational experience. Scand J Trauma Resusc Emerg Med. 2015;23(1):9. doi: 10.1186/s13049-015-0094-2. PubMed
19. Albaeni A, Chandra-Strobos N, Vaidya D, Eid SM. Predictors of early care withdrawal following out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1455-1461. doi: 10.1016/j.resuscitation.2014.08.030. PubMed
20. Lissauer ME, Naranjo LS, Kirchoffner J, Scalea TM, Johnson SB. Patient characteristics associated with end-of-life decision making in critically ill surgical patients. J Am Coll Surg. 2011;213(6):766-770. doi: 10.1016/j.jamcollsurg.2011.09.003. PubMed
21. Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139(5):1025-1033. doi: 10.1378/chest.10-3011. PubMed
22. Rubin MA, Dhar R, Diringer MN. Racial differences in withdrawal of mechanical ventilation do not alter mortality in neurologically injured patients. J Crit Care. 2014;29(1):49-53. doi: 10.1016/j.jcrc.2013.08.023. PubMed
23. Mayer SA, Kossoff SB. Withdrawal of life support in the neurological intensive care unit. Neurology. 1999;52(8):1602-1609. doi: 10.1212/WNL.52.8.1602. PubMed
24. 2nd National Congress on Medicinal Plants. Iranian J Pharm Res. 2013;12:43.
25. Hamel MB, Phillips R, Teno J, et al. Cost effectiveness of aggressive care for patients with nontraumatic coma. Crit Care Med. 2002;30(6):1191-1196. PubMed
26. Reichner CA, Thompson JA, O’Brien S, Kuru T, Anderson ED. Outcome and code status of lung cancer patients admitted to the medical ICU. Chest. 2006;130(3):719-723. doi: 10.1378/chest.130.3.719. PubMed
27. Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67(1):105-108. doi: 10.1212/01.wnl.0000223335.86166.b4. PubMed
28. Weimer JM, Nowacki AS, Frontera JA. Withdrawal of life-sustaining therapy in patients with intracranial hemorrhage: self-fulfilling prophecy or accurate prediction of outcome? Crit Care Med. 2016;44(5):1161-1172. doi: 10.1097/CCM.0000000000001570. PubMed
29. Mulder M, Gibbs HG, Smith SW, et al. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42(12):2493-2499. doi: 10.1097/CCM.0000000000000540. PubMed
30. Brown CE, Engelberg RA, Nielsen EL, Curtis JR. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684-689. doi: 10.1513/AnnalsATS.201510-667OC. PubMed
31. Plaisier BR, Blostein PA, Hurt KJ, Malangoni MA. Withholding/withdrawal of life support in trauma patients: is there an age bias? Am Surg. 2002;68(2):159-162. PubMed
32. Beauchamp, Childress JF. Principles of Biomedical Ethics. 13th ed. Oxford: Oxford University Press; 2013.
33. Jonson AR, Siegler M, Winslade WJ. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York: McGraw Hill; 2015.
34. Johnson KS, Elbert Avila KI, Tulsky JA. The influence of spiritual beliefs and practices on the treatment preferences of African Americans: a review of the literature. J Am Geriatr Soc. 2005;53(4):711-719. doi: 10.1111/j.1532-5415.2005.53224.x. PubMed
35. Mark NM, Rayner SG, Lee NJ, Curtis JR. Global variability in withholding and withdrawal of life-sustaining treatment in the intensive care unit: a systematic review. Intensive Care Med. 2015;41(9):1572-1585. doi: 10.1007/s00134-015-3810-5. PubMed
36. Creutzfeldt CJ, Wunsch H, Curtis JR, Hua M. Prevalence and Outcomes of Patients Meeting Palliative Care Consultation Triggers in Neurological Intensive Care Units. Neurocrit Care. 2015;23:14-21. PubMed
37. Mulder M, Smith SW, Dhaliwal RS, Goodwin HE, Scott NL, Geocadin RG. Comatose survivors of cardiac arrest and therapeutic hypothermia: Time of awakening and withdrawal of life sustaining therapies. Neurocrit Care. 2013;19:S281. PubMed
38. Naib T, Lahewala S, Arora S, Gidwani U. Palliative care in the cardiac intensive care unit. Am J Cardiol. 2015;115:687-90. PubMed
39. Prendergast TJ, Luce JM. Increasing incidence of withholding and withdrawal of life support from the critically ill. Am J Respir Crit Care Med. 1997;155:15-20. PubMed
40. Smedira NG, Evans BH, Grais LS, et al. Withholding and withdrawal of life support from the critically ill. N Engl J Med. 1990;322:309-15. PubMed
41. Van Scoy LJ, Sherman M. Factors Affecting Code Status in a University Hospital Intensive Care Unit. Death Stud. 2013;37:768-81. PubMed
42. White DB, Curtis JR, Lo B, Luce JM. Decisions to limit life-sustaining treatment for critically ill patients who lack both decision-making capacity and surrogate decision-makers. Crit Care Med. 2006;34:2053-9. PubMed
43. Kerlin MP, Harhay MO, Kahn JM, Halpern SD. Nighttime intensivist staffing, mortality, and limits on life support; a retrospective cohort study. Chest. 2015;147(4):951-958. PubMed
44. Kish Wallace S, Martin CG, Shaw AD, Price KJ. Influence of an advance directive on the initiation of life support technology in critically ill cancer patients. Crit Care Med. 2001;29(12):2294-2298. PubMed
© 2019 Society of Hospital Medicine
Serious Choices: A Systematic Environmental Scan of Decision Aids and Their Use for Seriously Ill People Near Death
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
People often do not receive the kind of care they want at the end of their lives.1,2 Although most people say they do not wish to have aggressive interventions if they are dying,3-5 nearly one in five dies in the hospital and one in seven dies in the intensive care unit (ICU), where aggressive care is usually provided.6 Coming demographic shifts will put this phenomenon in relief. The US Census Bureau estimates the number of people over age 85 will balloon to 20 million by 2050.7
A proposed strategy for reducing this mismatch is to expand shared decision making for people facing life-sustaining treatment decisions.8-10 Patient decision aids are tools that help people make informed healthcare decisions in light of their values and preferences, facilitating shared decision making.8,11 Decision aids can take many forms: paper-based, audio/video-based, or online. They can be intended for the clinical encounter (used in partnership with a physician, nurse, or other clinician), independent patient use, or peer-to-peer use.8 In a 2017 review, Stacey and colleagues found that patient decision aids improve knowledge, clarify values, encourage more active decision making, and improve risk perception, across a variety of treatment and screening decisions.12 They also concluded that decision aids might help people make decisions that are more aligned with their values, without affecting health outcomes negatively. 12
The number of available patient decision aids for people making life-sustaining treatment choices during serious illness near death is currently unknown. A 2014 review of all advanced care planning decision aids, including those for people who are healthy and people who are seriously ill, found 16 published studies in the peer-reviewed literature that tested patient decision aids for advanced care planning, but they did not systematically search the Internet and query key informants.13
Given the frequency of serious illness and death in hospital settings, awareness of potentially useful tools, their quality, and their use may be of interest to practicing hospitalists. This awareness may inform their decision making around whether or not to use decision aids in their own practice.
METHODS
Study Aims and Design
With our systematic environmental scan, we aimed to identify all decision aids available to seriously ill people near death facing choices about life-sustaining treatments, developed by both academic researchers and private organizations. We set out to articulate their quality and the degree to which they are used.
Protocol
We developed four research questions to address our study objectives. Our questions were as follows: (1) What English-language patient decision aids are available? (2) What are the characteristics of these patient decision aids? (3) What is the quality of these patient decision aids, including readability? (4) What organizations use these patient decision aids in routine care (exploratory)? 14-16 See protocol: doi: 10.1007/s40271-017-0268-2.17
Decision Aid Search Strategy
We searched for patient decision aids among published systematic reviews, Internet search results (Google.com), and app stores (Google Play and Apple App Store). To identify previously published systematic reviews, we searched MEDLINE via PubMed, with the date range from inception to 2017. We chose not to include other academic databases because the unit of observation for this environmental scan was the decision aids themselves, not the published articles. Additionally, we were aware of systematic reviews concerning this issue and felt that adding additional databases would not appreciably improve our likelihood of identifying eligible decision aids. We conducted searches using Google.com on November 30, 2016, and January 26, 2017, and included the first 100 search results. We also contacted shared decision-making and palliative care experts using a previously established list, via an online survey and one-on-one interviews between April 17, 2017, and August 30, 2017.
Published Reviews
Using a search strategy developed with a librarian, we identified reviews of decision aids that met our inclusion criteria using the MEDLINE database.17 The primary reviewer (CHS) examined the results of the search, identifying reviews appropriate for further investigation and the secondary reviewer (KP) extracted patient decision aids potentially eligible for our study. See Appendix Table 1 and our published protocol.17 Notably, given that the decision aids themselves, not published articles, were the unit of observation for our environmental scan, we did not perform dual coding on the MEDLINE extraction.
Google and App Stores
Two reviewers (CHS and MAD) performed the Google and application screening, including both the Apple App Store and Google Play.17 Using Google Advanced Search, we ran the queries detailed in Appendix Table 2. We disabled cookies and limited our search to English.
The primary reviewer ran each Google search and app store search, archiving the first 100 results of Google searches and first 50 results of app store searches.18 Then, the primary reviewer opened each page and scanned for patient decision aids or references to patient decision aids, marking those that met our inclusion criteria, those that might meet our inclusion criteria with further research, and those that were not appropriate. We documented specific reasons for exclusion. The secondary reviewer assessed a randomly-selected, 10% subsample. We calculated interrater reliability using a Cohen’s Kappa statistic.
Key Informants
To identify decision aids that did not appear in our online search, we surveyed 187 key informants who work in or study issues related to aging, death and dying and shared decision making.19 We developed a questionnaire for these informants and deployed it using the online survey software Qualtrics (see Appendix 1. Key Informant Survey). We used a snowball approach, asking participants for other individuals they thought we should speak with about other relevant decision aids. We corresponded with individuals who suggested decision aids that were not already in our decision aid database.
Decision Aid Selection Criteria
We included patient decision aids designed to help seriously ill people near death or their caregivers make decisions about life-sustaining treatments. See Appendix Table 1 for an explanation of terms. We saved decision aids that met our inclusion criteria in an online database, organizing them by target user or index decision(s). When identified decision aids were unavailable online, we e-mailed developers three times to ask for access to the decision aid. If after three queries, we did not receive access to the decision aid, we excluded the tool from our review. Similarly, if developers explicitly refused to participate in the study, we excluded them.
Once we banked and organized the decision aids, one reviewer (KP) systematically collected information about decision aid characteristics using a data collection form (see Appendix 2. Table 3). The data we collected for decision aids from all sources included (1) the index decision, (2) secondary decision(s), (3) the disease/condition, (4) availability (whether the decision aids are available publicly or proprietorially), and (5) use, ie, whether we learned anything about routine use in clinical environments.
Decision Aid Quality Grading Methods
At least two or three reviewers (C.H.S., K.P., M.A.D.), independently assessed the quality of each included patient decision aid, using the NQF standards. Before assessing the quality of each decision aid, we tested an NQF quality assessment form on five decision aids. We subsequently added specificity to the NQF quality criteria for this review. At least two of three reviewers (CHS, KP, MAD) assessed the quality of all included patient decision aids. We calculated interrater reliability using both Cohen’s Kappa statistic for individual quality categories and Spearman’s correlations for overall scores.
Notably, one of the NQF items concerns plain language. We assessed plain language using average readability scores, generated via Readable.io. If readability scores were below seventh-grade level, we considered them plain language. When we could not assess readability using an average score, ie, in the case of video decision aids, the researchers made a qualitative judgment about the plain language criteria.
Statistical Analysis
Our primary outcome was the number and variety of decision aids available for seriously ill individuals near death facing choices about life-sustaining treatments. Secondary outcomes included the quality, actual availability, and use of the available decision aids. We used Stata 13 to synthesize our results. We also reported overall quality and use. We conducted subgroup analyses, including quality, availability, and use of decision aids by category.
RESULTS
Decision Aid Selection Process
We identified 608 links with information about potential decision aids from our Google search. The two raters had substantial interrater reliability according to Cohen’s Kappa statistic (K = 0.64).20 We did not detect any possible decision aids with our app store searches. We identified 31 studies from our MEDLINE search with information about potential decision aids eligible for inclusion. We received 60 responses to our expert survey from the 187 administered (a 32% response rate).
Altogether, we identified 105 potential decision aids from these sources. We excluded 22/105 potential decision aids from our analysis because they were not publicly accessible, and we could not successfully obtain them from the developers. It remains unknown whether these tools would have qualified for inclusion in our review. We excluded 55/105 tools for not meeting one of the following criteria: 1) not being decision aids according to the NQF criteria 2) not concerning life-sustaining treatments 3) not being targeted at people with serious illness near death. A majority of decision aids for life-sustaining treatment decisions are intended for people who do not yet have an advanced serious illness or are not near death. There were 27 decision aids in our final review (Figure 1).
Characteristics of Included Decision Aids
Of the 27 decision aids we included in our review, 14 (52%) were tailored to seriously ill individuals with specific conditions. Eleven decision aids (41%) concerned specific life-sustaining treatments. Two decision aids concerned general treatment approaches, such as life-sustaining care versus palliative care (Table 1).
The decision aids were of variable length and approach. Some were text only, while others were image heavy. The mean length of decision aids was 19 pages, while the median length was 10 pages. Included decision aids offered interventions meant to return patients to health, as well as palliative interventions and comfort care.
Notably, most of the decision aids we included in our review (25 decision aids; 93%) were freely available online. Three (11%) were not. Seventeen (63%) decision aids were developed in the U.S., eight (30%) in Canada, two (7%) in Australia, and one (4%) in the Netherlands (in Dutch, translated using Google Translate). Additionally, there were 22 potentially eligible decision aids that we could not access to review and therefore could not include.
Quality of Included Decision Aids
The overall correlation of scores between the two reviewers was high (0.85). Agreement was high for both reviewers for all categories (balanced 90%, K = 0.0; outcome probabilities 86%, K = 0.7; publication date 93%, K = 0.8; update policy 93%, K = 0.7; funding sources 96%, K = 0.8), except the category concerning the rigor of the decision aid development process (66%, K = 0.2) and the evidence sources used (79%, K = 0.6) categories.
The quality of the decision aids was high in some categories. Of 27 decision aids, most presented options in a balanced way (24, 89%) and identified funding sources (23, 85%). They also reported publication dates most of the time (19, 70%). Readability of the included decision aids was mixed. The average readability grade level was 7.5, with a low score of 4.1 and a high score of 10.7. Eleven decision aids (41%) had readability levels less than seventh grade (Table 2). Thirteen had plain language, including video decision aids that we agreed used plain language.
The decision aids also had consistently low scores in some categories. Of 27, only 11 listed their evidence sources (41%), 11 reported a rigorous evidence-synthesis method (41%), six stated their competing interests (22%), and three offered an update policy (11%). There were no notable differences in the quality of the decision aids in each of the three category types (condition-specific, treatment-specific, general).
Use of Included and Excluded Decision Aids (exploratory)
We received 60 of 187 responses to our key informant survey. We asked every respondent if they were aware of any relevant decision aids. Of the 60 respondents, 45 (75%) said they were aware of decision aids, but only 38 (63%) offered the names of potential tools. Twenty-six respondents (43%) said they were aware of institutions that used the decision aids in routine and sustained care. Twenty-four respondents (40%) offered names of organizations, but most of the suggestions concerned decision aids that did not qualify for inclusion in our review or care that was not routine or sustained. In this preliminary use estimation, we found evidence for the use of only three decision aids or similar tools in routine care, two of which we included in our review.
DISCUSSION
We found many decision aids of varying quality for people with serious illnesses facing decisions about life-sustaining treatments. Most available decision aids are customized for people with particular diseases or conditions, like cancer or heart failure, with few generalized tools. This may make it difficult for practicing clinicians to find tools that are appropriate for their patients. It could also contribute to the gap between their availability and use in routine care, which is an essential but exploratory finding of this systematic environmental scan. Even if seriously ill people or those who cared for them wanted to obtain and use a decision aid independently, a large proportion of them are not publicly accessible.
Concerning the quality of decision aids, they were usually balanced and listed their funding sources, but other quality areas we often missing concerning their development, content, and disclosures. These deficiencies may affect the trustworthiness of decision aids, which may make practicing clinicians less likely to use them in hospital settings. Reporting of outcome probabilities was particularly weak. Reporting outcome probabilities in ways that people who are ill and their relatives can understand, especially during times of heightened emotion, is critically important. Therefore, it is a cause for concern that the available decision aids often neglect to use evidence-based techniques for conveying outcome information.
Our work built on Butler and colleagues’ “state of the science” review in 2014.13 Focusing specifically on proximal life-sustaining treatment decisions, we found many more decision aids by expanding our search beyond the peer-reviewed literature to include the Internet and experts.13 We also identified an important gap worthy of further exploration between the decision aids available and their usage in real-world clinical environments.
Our review confirms that implementation of decision aids in routine care is a continued challenge, especially for seriously ill people facing life-sustaining treatment decisions.53 Why tools that are efficacious in controlled trial environments have failed to gain acceptance in real-world settings remains unanswered for this population.54 For decision aids in general, researchers have reported barriers concerning clinician awareness, perception, and comfort, as well as usability issues.55,56 Additionally, systems-level barriers exist, like culture and priorities, difficulty incorporating decision aids into the workflow, resistance from parties who favor other interventions, and the costs associated with implementation.56 There may also be particular barriers related to the topics of death and dying.A strength of this work is thatwe applied the rigor of the systematic review method to the environmental scan, a newer method that answers different questions, such as “How many?”, “How much?”, and “How often?” We hope our use of the word systematic will reinforce perception among the scientific community that the environmental scan method is thorough, valid and worthwhile. We believe this method unearthed more decision aids than a traditional systematic review limited to the academic literature would have revealed. Another strength of our review was the rigor of screening and assessment.
A limitation of our work is the challenge of defining serious illness. We worked with palliative care physicians to make these judgments as grounded in clinical practice as possible. The preliminary nature and selection of experts for our sustained—use survey are limitations as well. Despite our efforts to conduct a comprehensive review of a vast environment of tools, we may have missed some decision aids that met our inclusion criteria. An additional limitation of our work is that due to the exploratory nature of our sustained-use survey, we cannot determine with accuracy how often these tools are used, although we have provided the first preliminary assessment of use, to our knowledge.
The gap between prolific patient decision aid development and real-world usage is puzzling. It is possible that using a tool at all is inappropriate for the complex, emotionally-laden decision-making process associated with death and dying. Alternatively, the tools may be inappropriate for serious illness, due to their design, their content, or some other characteristics. Perhaps the existing tools are too tailored for specific conditions and interventions―less appropriate for generalized use. Indeed, only two decision aids included in our final review addressed general care pathways, like life-sustaining care, palliative care, and hospice care. The others were highly specific, concerning particular diseases like kidney disease and particular interventions, like CPR. We know that most people die with comultimorbidities, meaning such specificity may paradoxically make it more difficult for individuals and their families to identify with the content in the materials.57,58 Without having data from real-world use, we cannot know whether any particular tool is suited or helpful for hospital practice.
It is essential for practicing hospitalists to know whether patient decision aids are appropriate for use in routine care. We hope that our review will help clinicians and health systems find appropriate tools to use with their patients. We also believe there should be mechanisms for providing feedback on whether decision aids are feasible and acceptable to hospitalized people and their caregivers and to practicing hospitalists and what leads to their sustained implementation.55,56 This can be explored with on-the-ground observational research or through health system quality improvement efforts.
Acknowledgments
Pamela J. Bagley provided search strategy support. Meredith MacMartin provided clinical counsel. Amber Barnato provided comments and insight as an advisor and a new member of Catherine’s Ph.D. committee.
Author contributions
Catherine H. Saunders designed the study, with support from Marie-Anne Durand, Glyn Elwyn, and Kathryn Kirkland. Catherine H. Saunders conducted all screening, with support from Marie-Anne Durand. Khusbu Patel managed the inventory of decision aids. Catherine H. Saunders designed and distributed the key informant survey, with support from Marie-Anne Durand. Hyunkyung Kang and Catherine H. Saunders managed follow-up with key informants. Khusbu Patel and Catherine H. Saunders conducted the decision aid quality review. Catherine H. Saunders, Marie-Anne Durand, and Kathryn Kirkland screened decision aids to determine appropriateness for people with serious illness. Catherine H. Saunders drafted the manuscript, and all authors reviewed and approved it.
Ethical approval
The Dartmouth College Committee for the Protection of Human Subjects designated this project as exempt from further review. All survey participants confirmed their consent via an online form.
Disclosures
Ms. Saunders, Ms. Patel, Ms. Kang, and Dr. Kirkland have nothing to disclose. Dr. Elwyn reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, personal fees from Chicago (Federally Qualified Medical Centers), outside the submitted work, and as Director of &think LLC, which owns the registered trademark for OptionGrids(TM) patient decision aids. He owns copyright in measures of shared decision making and care integration, namely collaboRATE, integRATE, Observer OPTION-5, and Observer OPTION-12, which are freely available for use. He is codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. He has received reimbursement for travel, accommodations, and expenses from EBSCO Health, ACCESS Community Health Network, and Chicago (Federally Qualified Medical Centers). Dr. Durand reports personal fees from ACCESS Community Health Network, personal fees from EBSCO Health, outside the submitted work, and as codeveloper of the OptionGrid patient decision aids, which are licensed to EBSCO Health. She has received reimbursement for travel, accommodations, and expenses from EBSCO Health and ACCESS Community Health Network.
Financial conflicts of interest
Glyn Elwyn (GE) and Marie-Anne Durand (M-A D) have developed the Option Grid patient decision aids, which are licensed to EBSCO Health. They receive consulting income from EBSCO Health and may receive royalties in the future. M-A D is a consultant for ACCESS Community Health Network. No other competing interests declared.
Funding
The authors did not receive funding for this research.
Published protocol linked here: https://www.ncbi.nlm.nih.gov/pubmed/28825182
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
1. Getting Ready to Go. AARP Bull Poll. 2008;(January):Executive summary.
2. Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470-477. doi:10.1001/jama.2012.207624. PubMed
3. Nelson JE, Danis M. End-of-life care in the intensive care unit: where are we now? Crit Care Med. 2001;29(2):N2-N9. PubMed
4. Steinhauser KE, Christakis NA, Clipp EC, et al. Preparing for the end of life: preferences of patients, families, physicians, and other care providers. J Pain Symptom Manage. 2001;22(3):727-737. doi:10.1016/S0885-3924(01)00334-7. PubMed
5. Gross MD. What do patients express as their preferences in advance directives? Arch Intern Med. 1998;158(4):363. doi:10.1001/archinte.158.4.363. PubMed
6. Goodman D, Fisher E. The Dartmouth Atlas of Health Care. 2013. http://www.dartmouthatlas.org/.
7. Bureau USC. American FactFinder.
8. Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med Decis Mak. 2010;30(6):701-711. doi:10.1177/0272989X10386231. PubMed
9. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345(3):e6572. doi:10.1136/bmj.e6572. PubMed
10. Warren C, McGraw AP, Van Boven L. Values and preferences: defining preference construction. Wiley Interdiscip Rev Cogn Sci. 2011;2(2):193-205. doi:10.1002/wcs.98. PubMed
11. Drug and Therapeutics Bulletin Editorial Office. An introduction to patient decision aids. BMJ. 2013;347:f4147. doi:10.1136/BMJ.F4147.
12. Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4: CD001431. doi:10.1002/14651858.CD001431.pub5. PubMed
13. Butler M, Ratner E, McCreedy E, Shippee N, Kane RL. Decision aids for advance care planning: an overview of the state of the science. Ann Intern Med. 2014;161(6):408-418. doi:10.7326/M14-0644. PubMed
14. Aslakson RA, Schuster ALR, Miller J, Weiss M, Volandes AE, Bridges JFP. An environmental scan of advance care planning decision AIDS for patients undergoing major surgery: a study protocol. Patient. 2014;7(2):207-217. doi:10.1007/s40271-014-0046-3. PubMed
15. Legare F, Politi MC, Drolet R, Desroches S, Stacey D, Bekker H. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159-169. doi:10.1016/j.pec.2012.01.002. PubMed
16. Donnelly KZ, Thompson R. Medical versus surgical methods of early abortion: protocol for a systematic review and environmental scan of patient decision aids. BMJ Open. 2015;5(7):e007966. doi:10.1136/bmjopen-2015-007966. PubMed
17. Saunders CH, Elwyn G, Kirkland K, Durand M-A. Serious choices: a protocol for an environmental scan of patient decision aids for seriously ill people at risk of death facing choices about life-sustaining treatments. Patient. 2018;11(1):97-106. doi:10.1007/s40271-017-0268-2. PubMed
18. Tsulukidze M, Grande SW, Thompson R, Rudd K, Elwyn G. Patients covertly recording clinical encounters: threat or opportunity? A qualitative analysis of online texts. PLoS One. 2015;10(5):e0125824. doi:10.1371/journal.pone.0125824. PubMed
19. Elwyn G, Dannenberg M, Blaine A, Poddar U, Durand M-A. Trustworthy patient decision aids: a qualitative analysis addressing the risk of competing interests. BMJ Open. 2016;6(9):e012562. doi:10.1136/bmjopen-2016-012562. PubMed
20. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159. doi:10.2307/2529310. PubMed
21. Tummers M, Oostendorp L, Stalmeier P O. Gedeelde besluitvorming - keuzehulpen voor de palliatieve zorg. http://gedeeldebesluitvorming.nl/. Accessed November 15, 2018.
22. Coping with Advanced Cancer - National Cancer Institute. https://www.cancer.gov/publications/patient-education/advanced-cancer. Accessed December 5, 2018.
23. PDQ Supportive and Palliative Care Editorial Board. Planning the Transition to End-of-Life Care in Advanced Cancer (PDQ®): Patient Version.; 2002. http://www.ncbi.nlm.nih.gov/pubmed/26389514. Accessed December 5, 2018. PubMed
24. National Cancer Institute. Choices for Care When Treatment May Not Be an Option. https://www.cancer.gov/about-cancer/advanced-cancer/care-choices. Accessed November 16, 2018.
25. Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29(15):2077-2084. doi:10.1200/JCO.2010.32.0754. PubMed
26. Choice Map. choicemap.com.
27. Left Ventricular Assist Device – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/lvad/. Accessed November 16, 2018.
28. Thompson JS, Matlock DD, Morris MA, McIlvennan CK, Allen LA. Organic Dissemination and Real-World Implementation of Patient Decision Aids for Left Ventricular Assist Device. MDM Policy Pract. 2018;3(1):238146831876765. doi:10.1177/2381468318767658. PubMed
29. Thompson JS, Matlock DD, McIlvennan CK, Jenkins AR, Allen LA. Development of a Decision Aid for Patients With Advanced Heart Failure Considering a Destination Therapy Left Ventricular Assist Device. JACC Hear Fail. 2015;3(12):965-976. doi:10.1016/j.jchf.2015.09.007. PubMed
30. Implantable Cardioverter Defibrillator – Colorado Program for Patient Centered Decisions. https://patientdecisionaid.org/icd/. Accessed November 16, 2018.
31. healthwise. Heart Failure: Should I Get a Pacemaker (Cardiac Resynchronization Therapy)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9843. Published October . Accessed November 16, 2018.
32. Healthwise. Heart Failure: Should I Get an Implantable Cardioverter-Defibrillator (ICD)? https://www.healthwise.net/ohridecisionaid/Content/StdDocument.aspx?DOCHWID=uf9848.
33. DECIDING TOGETHER. https://docs.wixstatic.com/ugd/56c3c3_57e7a9edbcda46c595c96eb4b360f400.pdf. Accessed November 16, 2018.
34. A Decision Aid for the Treatment of Kidney Disease A Guide for Health Professionals about This Tool. https://www.kidneys.co.nz/resources/file/decision_aid.pdf. Accessed November 16, 2018.
35. Making Choices Feeding Options for Patients with Dementia. 2011. https://decisionaid.ohri.ca/docs/das/feeding_options.pdf. Accessed December 5, 2018.
36. End-of-life decisions honoring the wishes of a person with alzheimer’s disease preparing for the end of life. https://www.alz.org/national/documents/brochure_endoflifedecisions.pdf. Accessed December 5, 2018.
37. What Is Artificial Hydration? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Artificial-Hydration.pdf. Accessed November 16, 2018.
38. What Is Tube Feeding? https://www.talkaboutwhatmatters.org/documents/Tools/Decision-Guide-Tube-Feeding.pdf. Accessed November 16, 2018.
39. Deciding About Tube Feeding Providing Patient and Family Centred Care. www.stjoes.ca. Accessed November 16, 2018.
40. Patient and Family Guidelines: Making Decisions about Long-Term Tube Feeding Deciding about Long-Term Tube Feeding. https://cloudfront.ualberta.ca/-/media/dossetor/publications/patientandfamilyguidelines.pdf. Accessed November 16, 2018.
41. Mitchell SL, Tetroe J, O’Connor AM. A Decision Aid for Long-Term Tube Feeding in Cognitively Impaired Older Persons. J Am Geriatr Soc. 2001;49(3):313-316. doi:10.1046/j.1532-5415.2001.4930313.x. PubMed
42. Health O. Long Term Feeding Tube Placement in Elderly Patients. https://decisionaid.ohri.ca/docs/Tube_Feeding_DA/PDF/TubeFeeding.pdf. Accessed November 16, 2018.
43. CPR Decision Aids - Speak Up | Parlons en. http://www.advancecareplanning.ca/resource/cpr-decision-aids/. Accessed November 16, 2018.
44. Frank C, Pichora D, Suurdt J, Heyland D. Development and use of a decision aid for communication with hospitalized patients about cardiopulmonary resuscitation preference. Patient Educ Couns. 2010;79(1):130-133. doi:10.1016/J.PEC.2009.08.002. PubMed
45. A Decision Aid to Prepare Patients And Their Families For Shared Decision-Making About Cardio-Pulmonary Resuscitation (CPR) on Vimeo. https://vimeo.com/48147363. Accessed November 16, 2018.
46. Plaisance A, Witteman HO, LeBlanc A, et al. Development of a decision aid for cardiopulmonary resuscitation and invasive mechanical ventilation in the intensive care unit employing user-centered design and a wiki platform for rapid prototyping. Hart J, ed. PLoS One. 2018;13(2):e0191844. doi:10.1371/journal.pone.0191844. PubMed
47. Patient Decision Aid: Sharing Goals for ICU Care. https://www.wikidecision.org/_media/english:final_da_english.pdf. Accessed November 16, 2018.
48. What Is CPR? https://coalitionccc.org/wp-content/uploads/2014/06/cccc_cpr_web_SAMPLE.pdf. Accessed December 5, 2018.
49. Cox CE, Lewis CL, Hanson LC, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Crit Care Med. 2012;40(8):2327-2334. doi:10.1097/CCM.0b013e3182536a63. PubMed
50. What Is a Ventilator? https://coalitionccc.org/wp-content/uploads/2019/01/Ventilator_2018_web_SAMPLE.pdf. Accessed January 3, 2019.
51. Kryworuchko BScN CNCC JR. An Intervention to Involve Family in Decisions about Life Support. https://ruor.uottawa.ca/bitstream/10393/20448/1/Kryworuchko_Jennifer_2011_thesis.pdf. Accessed November 16, 2018.
52. Looking Ahead: Choices for medical care when you’re seriously ill. https://med.dartmouth-hitchcock.org/documents/8L_looking_ahead.pdf. Accessed November 16, 2018.
53. Elwyn G, Scholl I, Tietbohl C, et al. “Many miles to go …”: a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S14. doi:10.1186/1472-6947-13-S2-S14. PubMed
54. Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213-1221. doi:10.1001/jamainternmed.2015.1679. PubMed
55. O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice*. J Eval Clin Pract. 2006;12(2):174-181. doi:10.1111/j.1365-2753.2006.00613.x. PubMed
56. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One. 2015;10(2):e0116629. doi:10.1371/journal.pone.0116629. PubMed
57. van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54(7):675-679. doi: 10.1016/S0895-4356(00)00358-9. PubMed
58. Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223-228. doi:10.1370/afm.272. PubMed
© 2019 Society of Hospital Medicine
Aspiration Pneumonia in Older Adults
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
Aspiration pneumonia refers to an infection of the lung parenchyma in an individual who has inhaled a bolus of endogenous flora that overwhelms the natural defenses of the respiratory system. It primarily affects older adults with almost 80% of cases occurring in those 65 years and older.1 Compared with nonaspiration pneumonia, aspiration pneumonia (whether community acquired or healthcare associated) results in more ICU stays, mechanical ventilation, increased length of hospital stay, and higher mortality.2
The etiology of aspiration pneumonia comes from aspirated bacteria from the oropharynx or stomach.3 However, aspiration alone is a common occurrence and does not always lead to clinical pneumonia. Indeed, one study demonstrated that 45% of “normal subjects” aspirate in their sleep,4 illustrating that our bodies have evolved defense mechanisms to protect us from aspirated bacteria. Thus, it is only when these systems are overwhelmed, after compromise of both glottic closure and the cough reflex in addition to dysphagia,3 that an infection manifests.
ASPIRATION PNEUMONITIS
Aspiration pneumonitis refers to a significant inflammation of the lung parenchyma that results from inhalation of regurgitated gastric contents.5 It can produce fever, cough, wheezing, shortness of breath, hypoxemia, leukocytosis, and a pulmonary infiltrate as well as lead to severe acute respiratory distress syndrome and even death. In the past, the use of antibiotics shortly after aspiration in patients who develop a fever, leukocytosis, or a pulmonary infiltrate was discouraged.5 Empiric antibiotics were recommended only for patients who aspirate gastric contents and who have conditions associated with colonization of gastric contents, such as small-bowel obstruction.5 Yet, it is difficult to distinguish aspiration pneumonitis from pneumonia6 and there are no randomized trials in older adults to help guide their management.
PRESENTATION OF ASPIRATION PNEUMONIA
Pneumonia in older adults can present in an atypical fashion. In one study of community-acquired pneumonia (CAP), the combination of cough, fever, and dyspnea is present in only 31% of patients, although separately, they are present in 67%, 64%, and 71% of patients, respectively. The same study also showed that delirium was present in 45% of patients with CAP.7 Nonrespiratory symptoms were present during the initial presentation of CAP in 55% of patients, with confusion in 42%, and falls in 16% of cases.8 The same is true of aspiration pneumonia where altered mental status is seen in approximately 30% of community-acquired aspiration pneumonia (CAAP) patients and in 19% of continuing care facility patients with aspiration pneumonia.2 Another study that compared CAP, CAAP, and healthcare-associated aspiration pneumonia (HCAAP) showed that confusion is present in 5.1%, 12.7%, and 18.6%, respectively.9 The absence of fever in older adults is shown in studies where fever, defined as greater than or equal to 37.5°C, is absent in 32% of the very old10and in 40% of patients 65 years or older when it was defined as greater than 37°C.8 The inconsistencies regarding typical symptoms of pneumonia in the older adult population are also confirmed in nursing home residents.11 Ultimately, it is important to remember that any infection in older adults, especially in those residing in long-term care facilities, may present with subtle findings such as an acute change in cognitive and functional status.12
Risk Factors for Aspiration Pneumonia
Risk factors for aspiration pneumonia, while not universally agreed upon, are important to recognize as they increase the probability of the diagnosis when present. A 2011 systematic review identified age, male gender, lung disease, dysphagia, and diabetes mellitus (level 2a), as well as severe dementia, angiotensin I-converting enzyme deletion/deletion genotype, and poor oral health (level 2b) as risk factors.13 In 2016, a panel of experts reached a consensus (modified Delphi Method) on the following risk factors for the diagnosis of aspiration pneumonia in nursing home residents: history of dysphagia, choking incident, tube feeding, neurologic disease, and cognitive impairment. The presence of one or more of these risk factors in the appropriate clinical setting may suggest a diagnosis of aspiration pneumonia.14
Radiographic/Ultrasonographic Imaging
In the appropriate scenario, the diagnosis of aspiration pneumonia is supported with an image representative of pneumonia. The pulmonary segment involved in aspiration pneumonia depends on the position of the patient during the aspiration event. If the aspiration event occurs while the patient is in the recumbent position, development of pneumonia is more common in the posterior segments of the upper lobes and the apical segments of the lower lobes; whereas if it occurs while the patient is in an upright position, the location changes to the basal segments of the lower lobes.3
Overall, the sensitivity of a chest X-ray to diagnose pneumonia ranges between 32%-77.7%,15-17 suggesting that a significant proportion of patients suspected of having pneumonia in past research studies, may have been misdiagnosed. Studies using lung ultrasound to identify pneumonia demonstrate a higher sensitivity, but additional research is needed to validate these findings.17-19 Noncontrast CT scans of the chest remain the reference standard for diagnosing pneumonia and currently tend to have the largest impact on diagnosis and subsequent treatment decisions.15,16,20,21 As a result, if radiation exposure risks are not a concern for the patient, we recommend utilizing noncontrast CT imaging whenever the diagnosis is in doubt until future research elucidates the most appropriate approach to imaging.
Diagnosis
Diagnosing aspiration pneumonia is difficult, in part because there is no universal definition or set of diagnostic criteria. The diagnosis of aspiration pneumonia is supported by the fulfillment of three criteria. First, appropriate risk factors for aspiration, as documented above, should be present. Second, there should be evidence of clinical signs and symptoms of pneumonia (typical or atypical). Third, radiographic representation of pneumonia in a dependent pulmonary segment confirms the diagnosis. Once these criteria are met, it is important to distinguish between CAAP and HCAAP with particular attention to risk factors for multidrug-resistant (MDR) organisms and Pseudomonas aeruginosa (PA).
MICROBIOLOGY
Many studies have tried to determine the exact bacterial etiology of aspiration pneumonia as documented in the Table.
Even when an ideal method is used to obtain a good sample, however, the results are limited by other variables in the study. For example, in studies that use protected brush specimens and protected bronchoalveolar lavage to acquire samples for culture, many patients received antibiotics prior to sampling, and the studies are small (Table). Although anaerobes have traditionally been implicated in aspiration pneumonia, only El-Solh et al.22 were able to culture a significant proportion of anaerobes. The study, however, was limited to institutionalized older adults requiring mechanical ventilation and it did not require the typical radiographic location for aspiration pneumonia. Even under the best circumstances, it is difficult to determine causality because the antibiotics used to treat these cases of aspiration pneumonia cover a broad range of organisms. Based on the studies in the Table, causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods in addition to traditional organisms classically thought to cause aspiration pneumonia-anaerobes. Microbiologic etiology, however, may also be insinuated from the studies discussed in the therapeutic strategies section below as some include antibiotics with limited antimicrobial activity.
Therapeutic Strategies
The management of aspiration pneumonia has evolved significantly since it was first studied in the 1970s because of the development of antibiotic resistance patterns, newer antibiotics, and increasing information on the diversity of pathogens involved in each subset of aspiration syndromes. The antimicrobial treatment of aspiration pneumonia was classically directed against anaerobic pathogens; treatment of these infections, however, was extrapolated from studies of pulmonary abscesses and other anaerobic pulmonary infections.
A randomized controlled trial in the mid-1980s comparing penicillin and clindamycin demonstrated a significantly improved cure rate in the clindamycin group.23 A follow-up study in 1990 implicated a significant number of penicillin-resistant Bacteroides infections—the majority of these infections were subsequently reclassified as Prevotella melaninogenica—as the cause for high rates of penicillin resistance in lung abscesses and necrotizing pneumonias, further supporting clindamycin as the treatment of choice for these infections.24 Amoxicillin-clavulanic acid (IV and PO regimens), studied in the treatment of community-acquired necrotizing pneumonia/lung abscess, shows good efficacy as well.25 This study also attempted to elucidate the underlying causative organisms in these patients. Organisms associated with CAP as well as anaerobic organisms were isolated, giving more credence to the idea of broader coverage for aspiration pneumonia.
Community-Acquired Aspiration Pneumonia/Healthcare-Associated Aspiration Pneumonia
The importance of making a diagnostic distinction between CAAP versus HCAAP is critical for management strategies. A prospective population-based study demonstrated that ICU length of stay and 30-day mortality is highest for HCAAP, followed by CAAP, and lastly for those with CAP.9 Although some studies use different nomenclature for identifying aspiration pneumonia patients at risk for a wider array of microorganisms, we attempt to standardize the language by using HCAAP. The literature on nonaspiration pneumonia is changing from terms such as CAP and healthcare-associated pneumonia (HCAP) to pneumonia with the risk of MDR organisms. One study proposed a new treatment algorithm for CAP based on the presence or absence of the following six risk factors: prior hospitalization of greater than or equal to two days in the preceding 90 days, immunosuppression, previous antibiotic use within the preceding 90 days, use of gastric acid-suppressive agents, tube feeding, and nonambulatory status.26 A similar approach proposed years earlier for HCAP patients found the following to be risk factors for MDR organisms: hospitalization in the past 90 days, antibiotic therapy in the past six months, poor functional status as defined by activities of daily living score, and immune suppression.27 Other factors, such as structural lung disease, that increase the risk of organisms resistant to standard antibiotic treatment regimens28-31 should be considered in aspiration pneumonia as well. Aspiration pneumonia is following a similar trajectory where the risk of MDR organisms is taking precedence over the environment of acquisition. The final nomenclature will allow the healthcare provider to understand the organisms that need to be targeted when choosing an appropriate antibiotic treatment regimen.
There is evidence supporting the premise that CAAP and nursing home patients with no risk factors for MDR organisms can be treated with standard regimens used for patients with CAP. A prospective cohort study in 2014 did not show any statistically significant differences in clinical outcomes in nursing and healthcare-associated aspiration pneumonia patients (with no risks of MDR organisms) treated with azithromycin versus ampicillin/sulbactam. However, only 36 patients were included in the azithromycin arm, and the therapeutic choices were made by the treating physician.32
A prospective study of 95 long-term care residents reported that of those patients admitted to the ICU with severe aspiration pneumonia, the causative organisms are gram-negative enteric bacilli in 49% of isolates, anaerobes in 16%, and Staphylococcus aureus in 12%.22 This study mentioned that six of seven anaerobic pneumonia cases had inadequate anaerobic coverage yet were effectively treated; based on the organisms represented, however, the antibiotics administered did provide some coverage.22 Prevotella was one of the common anaerobic organisms that could be treated by levofloxacin or ceftriaxone/azithromycin, possibly explaining the success of azithromycin in the study quoted previously.22,32 Therefore, although anaerobic organisms still need to be considered, some may be treated by traditional CAP coverage.22
In a 2005 randomized prospective study of 100 patients aged 71 to 94 years, clindamycin was found to have clinical efficacy equivalent to ampicillin-sulbactam and panipenem in the treatment of mild-to-moderate aspiration pneumonia.33 Most patients in this study are nursing home residents, and 53% of sputum cultures in the clindamycin arm grew gram-negative rods. In contrast to the previous study, the significance of gram-negative rod infections in this population of patients, with less severe infections, is called into question, as clindamycin has no coverage against these organisms. This premise is supported by a more recent study using azithromycin in nursing and healthcare-associated aspiration pneumonia patients, mentioned previously.32 Taken together, these three studies suggest that the severity of aspiration pneumonia may be a risk factor that needs to be taken into account when considering broad-spectrum antimicrobial coverage.
While further research is needed to validate treatment approaches, based on the current literature we propose the following:
CAAP requiring hospitalization but without any of the following-risk for PA or MDR organisms, septic shock, the need for ICU admission, or mechanical ventilation-can be treated with standard CAP therapy that covers anaerobes.26,32-34 Patients with CAAP and either of the following—risk factors for MDR organisms, septic shock, need for ICU admission, or mechanical ventilation—should be considered for broader coverage with vancomycin or linezolid, antipseudomonal antibiotics, and anaerobic coverage. CAAP with specific risk for a PA infection should be considered for two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic, and one has anaerobic coverage).
Severe HCAAP without risk for MDR organisms or PA but with any of the following-septic shock, ICU admission, or mechanical ventilation-can be treated based on the 2016 Infectious Diseases Society of America guideline recommendation for hospital-acquired pneumonia, with a regimen that also provides adequate anaerobic coverage.35 If patients have HCAAP with one or more risk factors for MDR organisms, no septic shock, and no need for ICU admission or mechanical ventilation, provide coverage with a similar regimen. In contrast, HCAAP with risk factors for PA or severe HCAAP causing septic shock, requiring ICU admission, or needing mechanical ventilation, which occurs in the setting of one or more risk factors for MDR organisms, or structural lung disease, should receive two antipseudomonal antibiotics (where only one can be a beta-lactam antibiotic and one has anaerobic coverage) in addition to vancomycin or linezolid.
A recent systematic review demonstrates the paucity of studies of ideal methodologic design which complicates the ability to recommend, with confidence, one guideline-based antimicrobial regimen over another.36 Future studies may determine that despite the severity of the infection, if patients do not carry any risk for MDR pathogens or PA, they may only require CAAP coverage. When a patient presents with an acute infection, it is prudent to review previous cultures, and although it may be necessary to treat with broad-spectrum antibiotics initially, it is always important to narrow the spectrum based on reliable culture results. If future studies support the results of many studies cited in this article, we may be using fewer antibiotics with narrower spectrums in the near future.
Prevention
Although the healthcare system has practices in place to prevent aspiration pneumonia, the evidence supporting them are either inconclusive or not of ideal methodological design. Two systematic reviews failed to show statistically significant decreases in rates of aspiration pneumonia or mortality using the standard of care positioning strategies or thickened fluids in patients with chronic dysphagia.37,38 One study showed a decreased incidence of all pneumonia in dysphasic patients with dementia or Parkinson disease when a chin-down posture (with thin liquids) or thickened fluids in a head-neutral position was used. The study, however, has significant limitations, including a lack of a “no treatment” group for comparison, which did not allow investigators to conclude that the decreased incidence was from their interventions.39
There are preventive strategies that show a decreased risk of aspiration pneumonia. Poor oral hygiene seems to be a modifiable risk factor to establish better control of oral flora and decrease aspiration pneumonia. A systematic review of five studies, evaluating the effects of oral healthcare on the incidence of aspiration pneumonia in frail older people, found that tooth brushing after each meal along with cleaning dentures once a day and professional oral healthcare once a week decreases febrile days, pneumonia, and dying from pneumonia.40A two-year historical cohort study using aromatherapy with black pepper oil, followed by application of capsaicin troches, and finally menthol gel, as the first meal, leads to a decreased incidence of pneumonia and febrile days in older adults with dysphagia.41 Well-designed validation studies may establish these practices as the new standard of care for preventing pneumonia in patients with dysphagia.
Feeding Tubes
Multiple studies show that in older adults with advanced dementia there is no survival benefit from percutaneous endoscopic gastrostomy (PEG) tube placement42-44 and more recent systematic reviews also conclude that there is currently no evidence to support the use of PEG tubes in this specific population.45,46 In February 2013, as part of the American Board of Internal Medicine Foundation Choosing Wisely® campaign, the American Geriatrics Society advised providers not to recommend percutaneous feeding tubes in patients with advanced dementia, rather, “offer assisted oral feeding.”47 It is worth noting, however, that none of the studies reviewed were of ideal methodological design, so opinions may change with future studies.
A more recent study compared liquid feeds versus semisolid feeds in patients with PEG tubes. The study shows a 22.2% incidence of aspiration pneumonia in the liquid feed group, which is comparable to prior studies, but the incidence of aspiration pneumonia is only 2.2% in the semisolid feed group (P < .005).48 A benefit of this size warrants future studies for validation.
CONCLUSION
Aspiration pneumonia leads to increased mortality when compared with CAP and HCAP.2 Until future studies validate or refute the current understanding surrounding its management, the following should provide some guidance: aspiration pneumonia should be suspected in any individual with risk factors of aspiration who presents with typical or atypical symptoms of pneumonia. Confirmation of the diagnosis requires an image representative of pneumonia in the typical dependent lung segment on chest X-ray, lung ultrasound, or noncontrast CT scan of the chest. Treatment of aspiration pneumonia should take into account the site of acquisition, severity of illness, and risk for MDR organisms as the causative organisms may include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and gram-negative rods, in addition to the traditional organisms classically thought to cause aspiration pneumonia-anaerobes.
Disclosures
The authors have nothing to disclose.
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
1. Wu CP, Chen YW, Wang MJ, Pinelis E. National trends in admission for aspiration pneumonia in the United States, 2002-2012. Ann Am Thorac Soc. 2017;14(6):874-879. doi: 10.1513/AnnalsATS.201611-867OC. PubMed
2. Reza Shariatzadeh M, Huang JQ, Marrie TJ. Differences in the features of aspiration pneumonia according to site of acquisition: community or continuing care facility. J Am Geriatr Soc. 2006;54(2):296-302. doi: 10.1111/j.1532-5415.2005.00608.x. PubMed
3. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. doi: 10.1378/chest.68.4.560. PubMed
4. Huxley EJ, Viroslav J, Gray WR, Pierce AK. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564-568. doi: 10.1016/0002-9343(78)90574-0. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39(4):818-826. doi: 10.1097/CCM.0b013e31820a856b. PubMed
7. Riquelme R, Torres A, el-Ebiary M, et al. Community-acquired pneumonia in the elderly. Clinical and nutritional aspects. Am J Respir Crit Care Med. 1997;156(6):1908-1914. doi: 10.1164/ajrccm.156.6.9702005. PubMed
8. Venkatesan P, Gladman J, Macfarlane JT, et al. A hospital study of community acquired pneumonia in the elderly. Thorax. 1990;45(4):254-258. doi: 10.1136/thx.45.4.254. PubMed
9. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
10. Fernández-Sabé N, Carratalà J, Rosón B, et al. Community-acquired pneumonia in very elderly patients: causative organisms, clinical characteristics, and outcomes. Medicine (Baltimore). 2003;82(3):159-169. doi: 10.1097/01.md.0000076005.64510.87. PubMed
11. Mehr DR, Binder EF, Kruse RL, et al. Clinical findings associated with radiographic pneumonia in nursing home residents. J Fam Pract. 2001;50(11):931-937. PubMed
12. Bentley DW, Bradley S, High K, et al. Practice guideline for evaluation of fever and infection in long-term care facilities. Clin Infect Dis. 2000;31(3):640-653. doi: 10.1086/314013. PubMed
13. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344-354. doi: 10.1016/j.jamda.2010.12.099. PubMed
14. Hollaar V, van der Maarel-Wierink C, van der Putten GJ, et al. Defining characteristics and risk indicators for diagnosing nursing home-acquired pneumonia and aspiration pneumonia in nursing home residents, using the electronically-modified Delphi Method. BMC Geriatr. 2016;16:60. doi: 10.1186/s12877-016-0231-4. PubMed
15. Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88.e1-88.e5. doi: 10.1016/j.amjmed.2009.09.012. PubMed
16. Claessens YE, Debray MP, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974-982. doi: 10.1164/rccm.201501-0017OC. PubMed
17. Liu XL, Lian R, Tao YK, Gu CD, Zhang GQ. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433-438. doi: 10.1136/emermed-2013-203039. PubMed
18. Bourcier JE, Paquet J, Seinger M, et al. Performance comparison of lung ultrasound and chest x-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115-118. doi: 10.1016/j.ajem.2013.10.003. PubMed
19. Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15:50. doi: 10.1186/1465-9921-15-50. PubMed
20. Syrjälä H, Broas M, Suramo I, Ojala A, Lähde S. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358-363. doi: 10.1086/514675. PubMed
21. Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266-270. doi: 10.1016/j.jemermed.2007.11.042. PubMed
22. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. doi: 10.1164/rccm.200212-1543OC. PubMed
23. Levison ME, Mangura CT, Lorber B, et al. Clindamycin compared with penicillin for the treatment of anaerobic lung abscess. Ann Intern Med. 1983;98(4):466-471. doi: 10.7326/0003-4819-98-4-466. PubMed
24. Gudiol F, Manresa F, Pallares R, et al. Clindamycin vs penicillin for anaerobic lung infections. High rate of penicillin failures associated with penicillin-resistant Bacteroides melaninogenicus. Arch Intern Med. 1990;150(12):2525-2529. doi: 10.1001/archinte.150.12.2525. PubMed
25. Germaud P, Poirier J, Jacqueme P, et al. Monotherapy using amoxicillin/clavulanic acid as treatment of first choice in community-acquired lung abscess. Apropos of 57 cases. Rev Pneumol Clin. 1993;49(3):137-141. PubMed
26. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. doi: 10.1164/rccm.201301-0079OC. PubMed
27. Brito V, Niederman MS. Healthcare-associated pneumonia is a heterogeneous disease, and all patients do not need the same broad-spectrum antibiotic therapy as complex nosocomial pneumonia. Curr Opin Infect Dis. 2009;22(3):316-325. doi: 10.1097/QCO.0b013e328329fa4e. PubMed
28. Restrepo MI, Babu BL, Reyes LF, et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: a multinational point prevalence study of hospitalised patients. Eur Respir J. 2018;52(2). doi: 10.1183/13993003.01190-2017. PubMed
29. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Supplement 2:S27-S72. doi: 10.1086/511159. PubMed
30. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415-425. doi: 10.1016/j.chest.2016.03.042. PubMed
31. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc. 2015;12(2):153-160. doi: 10.1513/AnnalsATS.201407-305OC. PubMed
32. Marumo S, Teranishi T, Higami Y, et al. Effectiveness of azithromycin in aspiration pneumonia: a prospective observational study. BMC Infect Dis. 2014;14:685. doi: 10.1186/s12879-014-0685-y. PubMed
33. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. doi: 10.1378/chest.127.4.1276. PubMed
34. Maruyama T, Fujisawa T, Okuno M, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57(10):1373-1383. doi: 10.1093/cid/cit571. PubMed
35. Kalil AC, Metersky ML, Klompas M, et al. Executive Summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. doi: 10.1093/cid/ciw504. PubMed
36. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. doi: 10.2147/CIA.S183344. PubMed
37. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
38. Andersen UT, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (≥18 years) with oropharyngeal dysphagia. Clin Nutr ESPEN. 2013;8(4):e127-e134.
39. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
40. van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology. 2013;30(1):3-9. doi: 10.1111/j.1741-2358.2012.00637.x. PubMed
41. Ebihara T, Ebihara S, Yamazaki M, et al. Intensive stepwise method for oral intake using a combination of transient receptor potential stimulation and olfactory stimulation inhibits the incidence of pneumonia in dysphagic older adults. J Am Geriatr Soc. 2010;58(1):196-198. doi: 10.1111/j.1532-5415.2009.02648.x. PubMed
42. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95(6):1472-1475. doi: 10.1111/j.1572-0241.2000.02079.x. PubMed
43. Murphy LM, Lipman TO. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch Intern Med. 2003;163(11):1351-1353. doi: 10.1001/archinte.163.11.1351. PubMed
44. Rimon E, Kagansky N, Levy S. Percutaneous endoscopic gastrostomy; evidence of different prognosis in various patient subgroups. Age Ageing. 2005;34(4):353-357. doi: 10.1093/ageing/afi085. PubMed
45. Candy B, Sampson EL, Jones L. Enteral tube feeding in older people with advanced dementia: findings from a Cochrane systematic review. Int J Palliat Nurs. 2009;15(8):396-404. doi: 10.12968/ijpn.2009.15.8.43799. PubMed
46. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733-1739. doi: 10.2147/CIA.S53153. PubMed
47. Workgroup AGSCW. American Geriatrics Society identifies five things that healthcare providers and patients should question. J Am Geriatr Soc. 2013;61(4):622-631. doi: 10.1111/jgs.12226. PubMed
48. Toh Yoon EW, Yoneda K, Nishihara K. Semi-solid feeds may reduce the risk of aspiration pneumonia and shorten postoperative length of stay after percutaneous endoscopic gastrostomy (PEG). Endosc Int Open. 2016;4(12):E1247-E1251. doi: 10.1055/s-0042-117218. PubMed
49. Mier L, Dreyfuss D, Darchy B, et al. Is penicillin-G an adequate initial treatment for aspiration pneumonia? A prospective evaluation using a protected specimen brush and quantitative cultures. Intens Care Med. 1993;19(5):279-284. doi: 10.1007/BF01690548. PubMed
50. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. doi: 10.1378/chest.115.1.178. PubMed
© 2019 Society of Hospital Medicine
Subclinical hypothyroidism: When to treat
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
Whether subclinical hypothyroidism is clinically important and should be treated remains controversial. Studies have differed in their findings, and although most have found this condition to be associated with a variety of adverse outcomes, large randomized controlled trials are needed to clearly demonstrate its clinical impact in various age groups and the benefit of levothyroxine therapy.
Currently, the best practical approach is to base treatment decisions on the magnitude of elevation of thyroid-stimulating hormone (TSH) and whether the patient has thyroid autoantibodies and associated comorbid conditions.
HIGH TSH, NORMAL FREE T4 LEVELS
Subclinical hypothyroidism is defined by elevated TSH along with a normal free thyroxine (T4).1
The hypothalamic-pituitary-thyroid axis is a balanced homeostatic system, and TSH and thyroid hormone levels have an inverse log-linear relation: if free T4 and triiodothyronine (T3) levels go down even a little, TSH levels go up a lot.2
TSH secretion is pulsatile and has a circadian rhythm: serum TSH levels are 50% higher at night and early in the morning than during the rest of the day. Thus, repeated measurements in the same patient can vary by as much as half of the reference range.3
WHAT IS THE UPPER LIMIT OF NORMAL FOR TSH?
The upper limit of normal for TSH, defined as the 97.5th percentile, is approximately 4 or 5 mIU/L depending on the laboratory and the population, but some experts believe it should be lower.3
In favor of a lower upper limit: the distribution of serum TSH levels in the healthy general population does not seem to be a typical bell-shaped Gaussian curve, but rather has a tail at the high end. Some argue that some of the individuals with values in the upper end of the normal range may actually have undiagnosed hypothyroidism and that the upper 97.5th percentile cutoff would be 2.5 mIU/L if these people were excluded.4 Also, TSH levels higher than 2.5 mIU/L have been associated with a higher prevalence of antithyroid antibodies and a higher risk of clinical hypothyroidism.5
On the other hand, lowering the upper limit of normal to 2.5 mIU/L would result in 4 times as many people receiving a diagnosis of subclinical hypothyroidism, or 22 to 28 million people in the United States.4,6 Thus, lowering the cutoff may lead to unnecessary therapy and could even harm from overtreatment.
Another argument against lowering the upper limit of normal for TSH is that, with age, serum TSH levels shift higher.7 The third National Health and Nutrition Education Survey (NHANES III) found that the 97.5th percentile for serum TSH was 3.56 mIU/L for age group 20 to 29 but 7.49 mIU/L for octogenarians.7,8
It has been suggested that the upper limit of normal for TSH be adjusted for age, race, sex, and iodine intake.3 Currently available TSH reference ranges are not adjusted for these variables, and there is not enough evidence to suggest age-appropriate ranges,9 although higher TSH cutoffs for treatment are advised in elderly patients.10 Interestingly, higher TSH in older people has been linked to lower mortality rates in some studies.11
Authors of the NHANES III8 and Hanford Thyroid Disease study12 have proposed a cutoff of 4.1 mIU/L for the upper limit of normal for serum TSH in patients with negative antithyroid antibodies and normal findings on thyroid ultrasonography.
SUBCLINICAL HYPOTHYROIDISM IS COMMON
In different studies, the prevalence of subclinical hypothyroidism has been as low as 4% and as high as 20%.1,8,13 The prevalence is higher in women and increases with age.8 It is higher in iodine-sufficient areas, and it increases in iodine-deficient areas with iodine supplementation.14 Genetics also plays a role, as subclinical hypothyroidism is more common in white people than in African Americans.8
A difficulty in estimating the prevalence is the disagreement about the cutoff for TSH, which may differ from that in the general population in certain subgroups such as adolescents, the elderly, and pregnant women.10,15
A VARIETY OF CAUSES
The most common cause of subclinical hypothyroidism, accounting for 60% to 80% of cases, is Hashimoto (autoimmune) thyroiditis,2 in which thyroid peroxidase antibodies are usually present.2,16
Also important to rule out are false-positive elevations due to substances that interfere with TSH assays (eg, heterophile antibodies, rheumatoid factor, biotin, macro-TSH); reversible causes such as the recovery phase of euthyroid sick syndrome; subacute, painless, or postpartum thyroiditis; central hypo- or hyperthyroidism; and thyroid hormone resistance.
SUBCLINICAL HYPOTHYROIDISM CAN RESOLVE OR PROGRESS
“Subclinical” suggests that the disease is in its early stage, with changes in TSH already apparent but decreases in thyroid hormone levels yet to come.17 And indeed, subclinical hypothyroidism can progress to overt hypothyroidism,18 although it has been reported to resolve spontaneously in half of cases within 2 years,19 typically in patients with TSH values of 4 to 6 mIU/L.20 The rate of progression to overt hypothyroidism is estimated to be 33% to 55% over 10 to 20 years of follow-up.18
Figure 1 shows the natural history of subclinical hypothyroidism.1
GUIDELINES FOR SCREENING DIFFER
Guidelines differ on screening for thyroid disease in the general population, owing to lack of large-scale randomized controlled trials showing treatment benefit in otherwise-healthy people with mildly elevated TSH values.
Various professional societies have adopted different criteria for aggressive case-finding in patients at risk of thyroid disease. Risk factors include family history of thyroid disease, neck irradiation, partial thyroidectomy, dyslipidemia, atrial fibrillation, unexplained weight loss, hyperprolactinemia, autoimmune disorders, and use of medications affecting thyroid function.23
The US Preventive Services Task Force in 2014 found insufficient evidence on the benefits and harms of screening.24
The American Thyroid Association (ATA) recommends screening adults starting at age 35, with repeat testing every 5 years in patients who have no signs or symptoms of hypothyroidism, and more frequently in those who do.25
The American Association of Clinical Endocrinologists recommends screening in women and older patients. Their guidelines and those of the ATA also suggest screening people at high risk of thyroid disease due to risk factors such as history of autoimmune diseases, neck irradiation, or medications affecting thyroid function.26
The American Academy of Family Physicians recommends screening after age 60.18
The American College of Physicians recommends screening patients over age 50 who have symptoms.18
Our approach. Although evidence is lacking to recommend routine screening in adults, aggressive case-finding and treatment in patients at risk of thyroid disease can, we believe, offset the risks associated with subclinical hypothyroidism.24
CLINICAL PRESENTATION
About 70% of patients with subclinical hypothyroidism have no symptoms.13
Tiredness was more common in subclinical hypothyroid patients with TSH levels lower than 10 mIU/L compared with euthyroid controls in 1 study, but other studies have been unable to replicate this finding.27,28
Other frequently reported symptoms include dry skin, cognitive slowing, poor memory, muscle weakness, cold intolerance, constipation, puffy eyes, and hoarseness.13
The evidence in favor of levothyroxine therapy to improve symptoms in subclinical hypothyroidism has varied, with some studies showing an improvement in symptom scores compared with placebo, while others have not shown any benefit.29–31
In one study, the average TSH value for patients whose symptoms did not improve with therapy was 4.6 mIU/L.31 An explanation for the lack of effect in this group may be that the TSH values for these patients were in the high-normal range. Also, because most subclinical hypothyroid patients have no symptoms, it is difficult to ascertain symptomatic improvement. Though it is possible to conclude that levothyroxine therapy has a limited role in this group, it is important to also consider the suggestive evidence that untreated subclinical hypothyroidism may lead to increased morbidity and mortality.
ADVERSE EFFECTS OF SUBCLINICAL HYPOTHYROIDISM, EFFECTS OF THERAPY
INDIVIDUALIZED MANAGEMENT AND SHARED DECISION-MAKING
The management of subclinical hypothyroidism should be individualized on the basis of extent of thyroid dysfunction, comorbid conditions, risk factors, and patient preference.118 Shared decision-making is key, weighing the risks and benefits of levothyroxine treatment and the patient’s goals.
The risks of treatment should be kept in mind and explained to the patient. Levothyroxine has a narrow therapeutic range, causing a possibility of overreplacement, and a half-life of 7 days that can cause dosing errors to have longer effect.118,119
Adherence can be a challenge. The drug needs to be taken on an empty stomach because foods and supplements interfere with its absorption.118,120 In addition, the cost of medication, frequent biochemical monitoring, and possible need for titration can add to financial burden.
When choosing the dose, one should consider the degree of hypothyroidism or TSH elevation and the patient’s weight, and adjust the dose gently.
If the TSH is high-normal
It is proposed that a TSH range of 3 to 5 mIU/L overlaps with normal thyroid function in a great segment of the population, and at this level it is probably not associated with clinically significant consequences. For these reasons, levothyroxine therapy is not thought to be beneficial for those with TSH in this range.
Pollock et al121 found that, in patients with symptoms suggesting hypothyroidism and TSH values in the upper end of the normal range, there was no improvement in cognitive function or psychological well-being after 12 weeks of levothyroxine therapy.
However, due to the concern for possible adverse maternal and fetal outcomes and low IQ in children of pregnant patients with subclinical hypothyroidism, levothyroxine therapy is advised in those who are pregnant or planning pregnancy who have TSH levels higher than 2.5 mIU/L, especially if they have thyroid peroxidase antibody. Levothyroxine therapy is not recommended for pregnant patients with negative thyroid peroxidase antibody and TSH within the pregnancy-specific range or less than 4 mIU/L if the reference ranges are unavailable.
Keep in mind that, even at these TSH values, there is risk of progression to overt hypothyroidism, especially in the presence of thyroid peroxidase antibody, so patients in this group should be monitored closely.
If TSH is mildly elevated
The evidence to support levothyroxine therapy in patients with subclinical hypothyroidism with TSH levels less than 10 mIU/L remains inconclusive, and the decision to treat should be based on clinical judgment.2 The studies that have looked at the benefit of treating subclinical hypothyroidism in terms of cardiac, neuromuscular, cognitive, and neuropsychiatric outcomes have included patients with a wide range of TSH levels, and some of these studies were not stratified on the basis of degree of TSH elevation.
The risk that subclinical hypothyroidism will progress to overt hypothyroidism in patients with TSH higher than 8 mIU/L is high, and in 70% of these patients, the TSH level rises to more than 10 mIU/L within 4 years. Early treatment should be considered if the TSH is higher than 7 or 8 mIU/L.
If TSH is higher than 10 mIU/L
Figure 2 outlines an algorithmic approach to subclinical hypothyroidism in nonpregnant patients as suggested by Peeters.122
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012; 379(9821):1142–1154. doi:10.1016/S0140-6736(11)60276-6
- Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009; 84(1):65–71. doi:10.4065/84.1.65
- Laurberg P, Andersen S, Carle A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol 2011; 7(4):232–239. doi:10.1038/nrendo.2011.13
- Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005; 90(9):5483–5488. doi:10.1210/jc.2005-0455
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 2007; 92(11):4236–4240. doi:10.1210/jc.2007-0287
- Fatourechi V, Klee GG, Grebe SK, et al. Effects of reducing the upper limit of normal TSH values. JAMA 2003; 290(24):3195–3196. doi:10.1001/jama.290.24.3195-a
- Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 2007; 92(12):4575–4582. doi:10.1210/jc.2007-1499
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87(2):489–499. doi:10.1210/jcem.87.2.8182
- Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 2014; 24(12):1670–1751. doi:10.1089/thy.2014.0028
- Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc 2015; 63(8):1663–1673. doi:10.1111/jgs.13532
- Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SH. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: a metaanalysis. J Clin Endocrinol Metab 2008; 93(8):2998–3007. doi:10.1210/jc.2008-0167
- Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab 2008; 93(4):1224–1230. doi:10.1210/jc.2006-2300
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160(4):526–534. pmid:10695693
- Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006; 354(26):2783–2793. doi:10.1056/NEJMoa054022
- Negro R, Stagnaro-Green A. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 2014; 349:g4929. doi:10.1136/bmj.g4929
- Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly 2014; 144:w14058. doi:10.4414/smw.2014.14058
- Stedman TL. Stedman’s Medical Dictionary. 28th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006.
- Raza SA, Mahmood N. Subclinical hypothyroidism: controversies to consensus. Indian J Endocrinol Metab 2013; 17(suppl 3):S636–S642. doi:10.4103/2230-8210.123555
- Huber G, Staub JJ, Meier C, et al. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 2002; 87(7):3221–3226. doi:10.1210/jcem.87.7.8678
- Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 2005; 90(7):4124–4127. doi:10.1210/jc.2005-0375
- Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995; 43(1):55–68. pmid:7641412
- Li Y, Teng D, Shan Z, et al. Antithyroperoxidase and antithyroglobulin antibodies in a five-year follow-up survey of populations with different iodine intakes. J Clin Endocrinol Metab 2008; 93(5):1751–1757. doi:10.1210/jc.2007-2368
- Hennessey JV, Klein I, Woeber KA, Cobin R, Garber JR. Aggressive case finding: a clinical strategy for the documentation of thyroid dysfunction. Ann Intern Med 2015; 163(4):311–312. doi:10.7326/M15-0762
- Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the US.Preventive Services Task Force. Ann Intern Med 2015; 162(1):35–45. doi:10.7326/M14-1456
- Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 2000; 160(11):1573–1575. pmid:10847249
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012; 18(6):988–1028. doi:10.4158/EP12280.GL
- Jorde R, Waterloo K, Storhaug H, Nyrnes A, Sundsfjord J, Jenssen TG. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab 2006; 91(1):145–153. doi:10.1210/jc.2005-1775
- Joffe RT, Pearce EN, Hennessey JV, Ryan JJ, Stern RA. Subclinical hypothyroidism, mood, and cognition in older adults: a review. Int J Geriatr Psychiatry 2013; 28(2):111–118. doi:10.1002/gps.3796
- Cooper DS, Halpern R, Wood LC, Levin AA, Ridgway EC. L-thyroxine therapy in subclinical hypothyroidism. A double-blind, placebo-controlled trial. Ann Intern Med 1984; 101(1):18–24. pmid:6428290
- Nystrom E, Caidahl K, Fager G, Wikkelso C, Lundberg PA, Lindstedt G. A double-blind cross-over 12-month study of L-thyroxine treatment of women with ‘subclinical’ hypothyroidism. Clin Endocrinol (Oxf) 1988; 29(1):63–75. pmid:3073880
- Monzani F, Del Guerra P, Caraccio N, et al. Subclinical hypothyroidism: neurobehavioral features and beneficial effect of L-thyroxine treatment. Clin Investig 1993; 71(5):367–371. pmid:8508006
- Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab 2010; 95(8):3614–3617. doi:10.1210/jc.2010-1245
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest 2011; 34(7):488–492. doi:10.3275/7202
- Javed Z, Sathyapalan T. Levothyroxine treatment of mild subclinical hypothyroidism: a review of potential risks and benefits. Ther Adv Endocrinol Metab 2016; 7(1):12–23. doi:10.1177/2042018815616543
- Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2(4):215–228. doi:10.1159/000356507
- Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013; 2013:390534. doi:10.1155/2013/390534
- Razvi S, Weaver JU, Vanderpump MP, Pearce SH. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham survey cohort. J Clin Endocrinol Metab 2010; 95(4):1734–1740. doi:10.1210/jc.2009-1749
- Bindels AJ, Westendorp RG, Frolich M, Seidell JC, Blokstra A, Smelt AH. The prevalence of subclinical hypothyroidism at different total plasma cholesterol levels in middle aged men and women: a need for case-finding? Clin Endocrinol (Oxf) 1999; 50(2):217–220. pmid:10396365
- Pearce EN. Hypothyroidism and dyslipidemia: modern concepts and approaches. Curr Cardiol Rep 2004; 6(6):451–456. pmid:15485607
- Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab 2012; 97(2):326–333. doi:10.1210/jc.2011-2532
- Rizos CV, Elisaf MS, Liberopoulos EN. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J 2011; 5:76–84. doi:10.2174/1874192401105010076
- Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011:575840. doi:10.1155/2011/575840
- Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. the HUNT study. Eur J Endocrinol 2007;1 56(2):181–186. doi:10.1530/eje.1.02333
- Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab 2000; 85(9):2993–3001. doi:10.1210/jcem.85.9.6841
- Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92(5):1715–1723. doi:10.1210/jc.2006-1869
- Abreu IM, Lau E, de Sousa Pinto B, Carvalho D. Subclinical hypothyroidism: to treat or not to treat, that is the question! A systematic review with meta-analysis on lipid profile. Endocr Connect 2017; 6(3):188–199. doi:10.1530/EC-17-0028
- Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8(4):401–407. doi:10.1016/j.jacl.2014.05.005
- Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam study. Ann Intern Med 2000; 132(4):270–278. pmid:10681281
- Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 2010; 72(3):404–410. doi:10.1111/j.1365-2265.2009.03640.x
- Andersen MN, Olsen AM, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One 2015; 10(6):e0129793. doi:10.1371/journal.pone.0129793
- Biondi B. Cardiovascular effects of mild hypothyroidism. Thyroid 2007; 17(7):625–630. doi:10.1089/thy.2007.0158
- Brenta G, Mutti LA, Schnitman M, Fretes O, Perrone A, Matute ML. Assessment of left ventricular diastolic function by radionuclide ventriculography at rest and exercise in subclinical hypothyroidism, and its response to L-thyroxine therapy. Am J Cardiol 2003; 91(11):1327–1330. pmid:12767425
- Taddei S, Caraccio N, Virdis A, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab 2003; 88(8):3731–3737. doi:10.1210/jc.2003-030039
- Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis 2013; 227(1):18–25. doi:10.1016/j.atherosclerosis.2012.10.070
- Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29(1):76–131. doi:10.1210/er.2006-0043
- Chaker L, Baumgartner C, den Elzen WP, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab 2015; 100(6):2181–2191. doi:10.1210/jc.2015-1438
- Monzani F, Di Bello V, Caraccio N, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86(3):1110–1115. doi:10.1210/jcem.86.3.7291
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001; 358(9285):861-865. doi:10.1016/S0140-6736(01)06067-6
- Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172(10):811–817. doi:10.1001/archinternmed.2012.1159
- Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab 2013; 98(6):2256–2266. doi:10.1210/jc.2012-3818
- Mooijaart SP, IEMO 80-plus Thyroid Trial Collaboration. Subclinical thyroid disorders. Lancet 2012; 380(9839):335. doi:10.1016/S0140-6736(12)61241-0
- Rodondi N, Bauer DC. Subclinical hypothyroidism and cardiovascular risk: how to end the controversy. J Clin Endocrinol Metab 2013; 98(6):2267–2269. doi:10.1210/jc.2013-1875
- Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165(21):2460–2466. doi:10.1001/archinte.165.21.2460
- Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. The Cardiovascular Health study. J Am Coll Cardiol 2008; 52(14):1152–1159. doi:10.1016/j.jacc.2008.07.009
- Haggerty JJ Jr, Garbutt JC, Evans DL, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med 1990; 20(2):193–208. doi:10.2190/ADLY-1UU0-1A8L-HPXY
- Baldini IM, Vita A, Mauri MC, et al. Psychopathological and cognitive features in subclinical hypothyroidism. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21(6):925–935. pmid:9380789
- del Ser Quijano T, Delgado C, Martinez Espinosa S, Vazquez C. Cognitive deficiency in mild hypothyroidism. Neurologia 2000; 15(5):193–198. Spanish. pmid:10850118
- Correia N, Mullally S, Cooke G, et al. Evidence for a specific defect in hippocampal memory in overt and subclinical hypothyroidism. J Clin Endocrinol Metab 2009; 94(10):3789–3797. doi:10.1210/jc.2008-2702
- Aghili R, Khamseh ME, Malek M, et al. Changes of subtests of Wechsler memory scale and cognitive function in subjects with subclinical hypothyroidism following treatment with levothyroxine. Arch Med Sci 2012; 8(6):1096–1101. doi:10.5114/aoms.2012.32423
- Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab 2015; 100(11):4240–4248. doi:10.1210/jc.2015-2046
- Christ-Crain M, Meier C, Huber PR, Staub J, Muller B. Effect of L-thyroxine replacement therapy on surrogate markers of skeletal and cardiac function in subclinical hypothyroidism. Endocrinologist 2004; 14(3):161–166. doi:10.1097/01.ten.0000127932.31710.4f
- Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid 2006; 16(4):375–380. doi:10.1089/thy.2006.16.375
- Reuters VS, Teixeira Pde F, Vigario PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci 2009; 338(4):259–263. doi:10.1097/MAJ.0b013e3181af7c7c
- Mainenti MR, Vigario PS, Teixeira PF, Maia MD, Oliveira FP, Vaisman M. Effect of levothyroxine replacement on exercise performance in subclinical hypothyroidism. J Endocrinol Invest 2009; 32(5):470–473. doi:10.3275/6106
- Lankhaar JA, de Vries WR, Jansen JA, Zelissen PM, Backx FJ. Impact of overt and subclinical hypothyroidism on exercise tolerance: a systematic review. Res Q Exerc Sport 2014; 85(3):365–389. doi:10.1080/02701367.2014.930405
- Lee JS, Buzkova P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010; 170(21):1876–1883. doi:10.1001/archinternmed.2010.424
- Svare A, Nilsen TI, Asvold BO, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol 2013; 169(6):845–852. doi:10.1530/EJE-13-0546
- Di Mase R, Cerbone M, Improda N, et al. Bone health in children with long-term idiopathic subclinical hypothyroidism. Ital J Pediatr 2012; 38:56. doi:10.1186/1824-7288-38-56
- Boelaert K. The association between serum TSH concentration and thyroid cancer. Endocr Relat Cancer 2009; 16(4):1065–1072. doi:10.1677/ERC-09-0150
- Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H. Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 2009; 71(3):434–439. doi:10.1111/j.1365-2265.2008.03489.x
- Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 2012; 97(4):1134–1145. doi:10.1210/jc.2011-2735
- Fiore E, Rago T, Provenzale MA, et al. L-thyroxine-treated patients with nodular goiter have lower serum TSH and lower frequency of papillary thyroid cancer: results of a cross-sectional study on 27,914 patients. Endocr Relat Cancer 2010; 17(1):231–239. doi:10.1677/ERC-09-0251
- Hercbergs AH, Ashur-Fabian O, Garfield D. Thyroid hormones and cancer: clinical studies of hypothyroidism in oncology. Curr Opin Endocrinol Diabetes Obes 2010; 17(5):432–436. doi:10.1097/MED.0b013e32833d9710
- Thvilum M, Brandt F, Brix TH, Hegedus L. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 2012; 8(7):417–424. doi:10.1038/nrendo.2012.29
- Stott DJ, Rodondi N, Kearney PM, et al; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376(26):2534–2544. doi:10.1056/NEJMoa1603825
- Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril 2015; 104(3):545–753. doi:10.1016/j.fertnstert.2015.05.028
- Stagnaro-Green A, Abalovich M, Alexander E, et al; American Thyroid Association Taskforce on Thyroid Disease During Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21(10):1081–1125. doi:10.1089/thy.2011.0087
- Goldsmith RE, Sturgis SH, Lerman J, Stanbury JB. The menstrual pattern in thyroid disease. J Clin Endocrinol Metab. 1952; 12(7):846-855. doi:10.1210/jcem-12-7-846
- Plowden TC, Schisterman EF, Sjaarda LA, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab 2016; 101(6):2358–2365. doi:10.1210/jc.2016-1049
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27(3):315–389. doi:10.1089/thy.2016.0457
- Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006; 91(7):2587–2591. doi:10.1210/jc.2005-1603
- Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem 2001; 38(pt 4):329–332. doi:10.1258/0004563011900830
- Lepoutre T, Debieve F, Gruson D, Daumerie C. Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases. Gynecol Obstet Invest 2012; 74(4):265–273. doi:10.1159/000343759
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(8):2543–2565. doi:10.1210/jc.2011-2803
- Crawford NM, Steiner AZ. Thyroid autoimmunity and reproductive function. Semin Reprod Med 2016; 34(6):343–350. doi:10.1055/s-0036-1593485
- Maraka S, Ospina NM, O’Keeffe DT, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid 2016; 26(4):580–590. doi:10.1089/thy.2015.0418
- Wiles KS, Jarvis S, Nelson-Piercy C. Are we overtreating subclinical hypothyroidism in pregnancy? BMJ 2015; 351:h4726. doi:10.1136/bmj.h4726
- Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol 2012; 119(5):983–988. doi:10.1097/AOG.0b013e318250aeeb
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J 2014; 3(2):76–94. doi:10.1159/000362597
- Karakosta P, Alegakis D, Georgiou V, et al. Thyroid dysfunction and autoantibodies in early pregnancy are associated with increased risk of gestational diabetes and adverse birth outcomes. J Clin Endocrinol Metab 2012; 97(12):4464–4472. doi:10.1210/jc.2012-2540
- Toulis KA, Stagnaro-Green A, Negro R. Maternal subclinical hypothyroidsm and gestational diabetes mellitus: a meta-analysis. Endocr Pract 2014; 20(7):703–714. doi:10.4158/EP13440.RA
- van den Boogaard E, Vissenberg R, Land JA, et al. Significance of subclinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update 2011; 17(5):605–619. doi:10.1093/humupd/dmr024
- Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol 2012; 119(2 Pt 1):315–320. doi:10.1097/AOG.0b013e318240de6a
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH. Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid 2010; 20(9):989–993. doi:10.1089/thy.2010.0058
- Casey BM, Dashe JS, Wells CE, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol 2005; 105(2):239–245. doi:10.1097/01.AOG.0000152345.99421.22
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab 2010; 95(9):E44–E48. doi:10.1210/jc.2010-0340
- Su PY, Huang K, Hao JH, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab 2011; 96(10):3234–3241. doi:10.1210/jc.2011-0274
- Allan WC, Haddow JE, Palomaki GE, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen 2000; 7(3):127–130. doi:10.1136/jms.7.3.127
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol 2009; 160(6):985–991. doi:10.1530/EJE-08-0953
- Korevaar TI, Medici M, de Rijke YB, et al. Ethnic differences in maternal thyroid parameters during pregnancy: the generation R study. J Clin Endocrinol Metab 2013; 98(9):3678–3686. doi:10.1210/jc.2013-2005
- Cleary-Goldman J, Malone FD, Lambert-Messerlian G, et al. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol 2008; 112(1):85–92. doi:10.1097/AOG.0b013e3181788dd7
- Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010; 72(6):825–829. doi:10.1111/j.1365-2265.2009.03743.x
- Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 1999; 341(8):549–555. doi:10.1056/NEJM199908193410801
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab 2010; 95(9):4227–4234. doi:10.1210/jc.2010-0415
- Behrooz HG, Tohidi M, Mehrabi Y, Behrooz EG, Tehranidoost M, Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid 2011; 21(10):1143–1147. doi:10.1089/thy.2011.0053
- Julvez J, Alvarez-Pedrerol M, Rebagliato M, et al. Thyroxine levels during pregnancy in healthy women and early child neurodevelopment. Epidemiology 2013; 24(1):150–157. doi:10.1097/EDE.0b013e318276ccd3
- Casey BM, Thom EA, Peaceman AM, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017; 376(9):815–825. doi:10.1056/NEJMoa1606205
- Burns RB, Bates CK, Hartzband P, Smetana GW. Should we treat for subclinical hypothyroidism?: Grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med 2016; 164(11):764–770. doi:10.7326/M16-0857
- Kucukler FK, Akbaba G, Arduc A, Simsek Y, Guler S. Evaluation of the common mistakes made by patients in the use of levothyroxine. Eur J Intern Med 2014; 25(9):e107–e108. doi:10.1016/j.ejim.2014.09.002
- McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D 2016; 16(1):53–68. doi:10.1007/s40268-015-0116-6
- Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: Randomised double blind placebo controlled crossover trial. BMJ 2001; 323(7318):891–895. pmid:11668132
- Peeters RP. Subclinical hypothyroidism. N Engl J Med 2017; 376(26):2556–2565. doi:10.1056/NEJMcp1611144
KEY POINTS
- From 4% to 20% of adults have subclinical hypothyroidism, with a higher prevalence in women, older people, and those with thyroid autoimmunity.
- Subclinical hypothyroidism can progress to overt hypothyroidism, especially if antithyroid antibodies are present, and has been associated with adverse metabolic, cardiovascular, reproductive, maternal-fetal, neuromuscular, and cognitive abnormalities and lower quality of life.
- Some studies have suggested that levothyroxine therapy is beneficial, but others have not, possibly owing to variability in study designs, sample sizes, and patient populations.
- Further trials are needed to clearly demonstrate the clinical impact of subclinical hypothyroidism and the effect of levothyroxine therapy.
Managing malignant pleural effusion
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
Managing patients with malignant pleural effusion can be challenging. Symptoms are often distressing, and its presence signifies advanced disease. Median survival after diagnosis is 4 to 9 months,1–3 although prognosis varies considerably depending on the type and stage of the malignancy.
How patients are best managed depends on clinical circumstances. Physicians should consider the risks and benefits of each option while keeping in mind realistic goals of care.
This article uses brief case presentations to review management strategies for malignant pleural effusion.
CANCER IS A COMMON CAUSE OF PLEURAL EFFUSION
Physicians and surgeons, especially in tertiary care hospitals, must often manage malignant pleural effusion.4 Malignancy is the third leading cause of pleural effusion after heart failure and pneumonia, accounting for 44% to 77% of exudates.5 Although pleural effusion can arise secondary to many different malignancies, the most common causes are lung cancer in men and breast cancer in women; these cancers account for about 75% of all cases of malignant pleural effusion.6,7
A WOMAN ON CHEMOTHERAPY WITH ASYMPTOMATIC PLEURAL EFFUSION
An 18-year-old woman with non-Hodgkin lymphoma has received her first cycle of chemotherapy and is now admitted to the hospital for diarrhea. A routine chest radiograph reveals a left-sided pleural effusion covering one-third of the thoracic cavity. She is asymptomatic and reports no shortness of breath at rest or with exertion. Her oxygen saturation level is above 92% on room air without supplemental oxygen.
Thoracentesis reveals an exudative effusion, and cytologic study shows malignant lymphoid cells, consistent with a malignant pleural effusion. Cultures are negative.
What is the appropriate next step to manage this patient’s effusion?
Observation is reasonable
This patient is experiencing no symptoms and has just begun chemotherapy for her lymphoma. Malignant pleural effusion associated with lymphoma, small-cell lung cancer, and breast cancer is most sensitive to chemotherapy.5 For patients who do not have symptoms from the pleural effusion and who are scheduled to receive further chemotherapy, a watch-and-wait approach is reasonable.
It is important to follow the patient for developing symptoms and obtain serial imaging to evaluate for an increase in the effusion size. We recommend repeat imaging at 2- to 4-week intervals, and sooner if symptoms develop.
If progression is evident or if the patient’s oncologist indicates that the cancer is unresponsive to systemic therapy, further intervention may be necessary with one of the options discussed below.
A MAN WITH LUNG CANCER WITH PLEURAL EFFUSION, LUNG COLLAPSE
A 42-year-old man with a history of lung cancer is admitted for worsening shortness of breath. Chest radiography reveals a large left-sided pleural effusion with complete collapse of the left lung and contralateral shift of midline structures (Figure 1). Large-volume thoracentesis improves his symptoms. Pleural fluid cytology is positive for malignant cells. A repeat chest radiograph shows incomplete expansion of the left lung, thick pleura, and pneumothorax, indicating a trapped lung (ie, one unable to expand fully). Two weeks later, his symptoms recur, and chest radiography reveals a recurrent effusion.
How should this effusion be managed?
Indwelling pleural catheter placement
In a retrospective cohort study,8 malignant pleural effusion recurred in 97% of patients within 1 month (mean, 4.2 days) of therapeutic aspiration, highlighting the need for definitive treatment.
In the absence of lung expansion, pleurodesis is rarely successful, and placing an indwelling pleural catheter in symptomatic patients is the preferred strategy. The US Food and Drug Administration approved this use in 1997.9
Indwelling pleural catheters are narrow (15.5 French, or about 5 mm in diameter) and soft (made of silicone), with distal fenestrations. The distal end remains positioned in the pleural cavity to enable drainage of pleural fluid. The middle portion passes through subcutaneous tissue, where a polyester cuff prevents dislodgement and infection. The proximal end of the catheter remains outside the patient’s skin and is connected to a 1-way valve that prevents air or fluid flow into the pleural cavity.
Pleural fluid is typically drained every 2 or 3 days for palliation. Patients must be educated about home drainage and proper catheter care.
Indwelling pleural catheters are now initial therapy for many
Although indwelling pleural catheters were first used for patients who were not candidates for pleurodesis, they are now increasingly used as first-line therapy.
Since these devices were introduced, several clinical series including more than 800 patients have found that their use for malignant pleural infusion led to symptomatic improvement in 89% to 100% of cases, with 90% of patients needing no subsequent pleural procedures after catheter insertion.10–13
Davies et al14 randomized 106 patients with malignant pleural effusion to either receive an indwelling pleural catheter or undergo pleurodesis. In the first 6 weeks, the 2 groups had about the same incidence of dyspnea, but the catheter group had less dyspnea at 6 months, shorter index hospitalization (0 vs 4 days), fewer hospital days in the first year for treatment-related complications (1 vs 4.5 days), and fewer patients needing follow-up pleural procedures (6% vs 22%). On the other hand, adverse events were more frequent in the indwelling pleural catheter group (40% vs 13%). The most frequent events were pleural infection, cellulitis, and catheter blockage.
Fysh et al15 also compared indwelling pleural catheter insertion and pleurodesis (based on patient choice) in patients with malignant pleural effusion. As in the previous trial, those who received a catheter required significantly fewer days in the hospital and fewer additional pleural procedures than those who received pleurodesis. Safety profiles and symptom control were comparable.
Indwelling pleural catheters have several other advantages. They have been found to be more cost-effective than talc pleurodesis in patients not expected to live long (survival < 14 weeks).16 Patients with an indwelling pleural catheter can receive chemotherapy, and concurrent treatment does not increase risk of infection.17 And a systematic review18 found a 46% rate of autopleurodesis at a median of 52 days after insertion of an indwelling pleural catheter.
Drainage rate may need to be moderated
Chest pain has been reported with the use of indwelling pleural catheters, related to rapid drainage of the effusion in the setting of failed reexpansion of the trapped lung due to thickened pleura. Drainage schedules may need to be adjusted, with more frequent draining of smaller volumes, to control dyspnea without causing significant pain.
A WOMAN WITH RECURRENT PLEURAL EFFUSION, GOOD PROGNOSIS
A 55-year-old woman with a history of breast cancer presents with shortness of breath. Chest radiography reveals a right-sided effusion, which on thoracentesis is found to be malignant. After fluid removal, repeat chest radiography shows complete lung expansion.
One month later, she returns with symptoms and recurrence of the effusion. Ultrasonography does not reveal any adhesions in the pleural space. Her oncologist informs you that her expected survival is in years.
What is the next step?
Chemical pleurodesis
Chemical pleurodesis involves introducing a sclerosant into the pleural space to provoke an intense inflammatory response, creating adhesions and fibrosis that will obliterate the space. The sclerosing agent (typically talc) can be delivered by tube thoracostomy, video-assisted thoracic surgery (VATS), or medical pleuroscopy. Although the latter 2 methods allow direct visualization of the pleural space and, in theory, a more even distribution of the sclerosing agent, current evidence does not favor 1 option over the other,19 and practice patterns vary between institutions.
Tube thoracostomy. Typically, the sclerosing agent is administered once a chest radiograph shows lung reexpansion, and tube output of pleural fluid is less than 150 mL/day.19 However, some studies indicate that if pleural apposition can be confirmed using ultrasonography, then sclerosant administration at that time leads to optimal pleurodesis efficacy and shorter hospitalization.20,21
VATS is usually done in the operating room with the patient under general anesthesia. A double-lumen endotracheal tube allows for single-lung ventilation; a camera is then inserted into the pleural space of the collapsed lung. Multiple ports of entry are usually employed, and the entire pleural space can be visualized and the sclerosing agent instilled uniformly. The surgeon may alternatively choose to perform mechanical pleurodesis, which entails abrading the visceral and parietal pleura with dry gauze to provoke diffuse petechial hemorrhage and an inflammatory reaction. VATS can also be used to perform biopsy, lobectomy, and pneumonectomy.
Medical pleuroscopy. Medical pleuroscopy is usually done using local anesthesia with the patient awake, moderately sedated, and not intubated. Because no double-lumen endotracheal tube is used, lung collapse may not be complete, making it difficult to completely visualize the entire pleural surfaces.
Although no randomized study of VATS vs medical pleuroscopy exists, a retrospective case-matched study22 comparing VATS (under general anesthesia) to single-port VATS (under local anesthesia) noted equivalent rates of pleurodesis. However, the local anesthesia group had a lower perioperative mortality rate (0% vs 2.3%), a lower postoperative major morbidity rate (5.2% vs 9%), earlier improvement in quality of life, and shorter hospitalization (3 vs 5 days).22 In general, the diagnostic sensitivity of pleuroscopy for pleural malignancy is similar to that of VATS (93% vs 97%).23,24
A MAN WITH PLEURAL EFFUSION AND A POOR PROGNOSIS
A 60-year-old man with metastatic pancreatic cancer is brought to the clinic for worsening shortness of breath over the past 2 months. During that time, he has lost 6 kg and has become bedridden.
On examination, he has severe cachexia and is significantly short of breath at rest with associated hypoxia. His oncologist expects him to survive less than 3 months.
His laboratory investigations reveal hypoalbuminemia and leukocytosis. A chest radiograph shows a large left-sided pleural effusion that was not present 2 months ago.
What should be done for him?
Thoracentesis, repeat as needed
Malignant pleural effusion causing dyspnea is not uncommon in certain advanced malignancies and may contribute to significant suffering at the end of life. A study of 298 patients with malignant pleural effusion noted that the presence of leukocytosis, hypoalbuminemia, and hypoxemia was associated with a poorer prognosis. Patients having all 3 factors had a median survival of 42 days.25
Thoracentesis, the least invasive option that may improve dyspnea, can be done in the clinic setting and is a reasonable strategy for patients with advanced cancer and an expected survival of less than 3 months.26 Although recurrence is expected, it may take up to a few weeks, and repeat thoracentesis can be performed as needed.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40. doi:10.1136/thx.2010.136994
- Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22(2 suppl 3):58–63. pmid:7740322
- Bielsa S, Martín-Juan J, Porcel JM, Rodríguez-Panadero F. Diagnostic and prognostic implications of pleural adhesions in malignant effusions. J Thorac Oncol 2008; 3(11):1251–1256. doi:10.1097/JTO.0b013e318189f53d
- 35th Annual meeting of the European Association for the Study of Diabetes. Brussels, Belgium, 28 September–2 October, 1999. Abstracts. Diabetologia 1999;42(suppl 1):A1–A354. pmid:10505080
- Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001; 18(2):402–419. pmid:11529302
- Sahn SA. Malignancy metastatic to the pleura. Clin Chest Med 1998; 19(2):351–361. pmid:9646986
- Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10(8):1907–1913. pmid:9272937
- Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33(4):916–922. pmid:4362107
- Uzbeck MH, Almeida FA, Sarkiss MG, et al. Management of malignant pleural effusions. Adv Ther 2010; 27(6):334–347. doi:10.1007/S12325-010-0031-8
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011; 6(4):762–767. doi:10.1097/JTO.0b013e31820d614f
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129(2):362–368. doi:10.1378/chest.129.2.362
- Warren WH, Kalimi R, Khodadadian LM, Kim AW. Management of malignant pleural effusions using the Pleur(x) catheter. Ann Thorac Surg 2008; 85(3):1049–1055 doi:10.1016/j.athoracsur.2007.11.039
- Murthy SC, Okereke I, Mason DP, Rice TW. A simple solution for complicated pleural effusions. J Thorac Oncol 2006; 1(7):697–700. pmid:17409939
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307(22):2383–2389. doi:10.1001/jama.2012.5535
- Fysh ETH, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142(2):394–400. doi:10.1378/chest.11-2657
- Olfert JA, Penz ED, Manns BJ, et al. Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22(4):764–770. doi:10.1111/resp.12962
- Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax 2011; 66(5):448–449. doi:10.1136/thx.2009.133504
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26(1):70–76. doi:10.1007/s11606-010-1472-0
- Lee YCG, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries. Chest 2003; 124(6):2229–2238. pmid:14665505
- Villanueva AG, Gray AW Jr, Shahian DM, Williamson WA, Beamis JF Jr. Efficacy of short term versus long term tube thoracostomy drainage before tetracycline pleurodesis in the treatment of malignant pleural effusions. Thorax 1994; 49(1):23–25. pmid:7512285
- Sartori S, Tombesi P, Tassinari D, et al. Sonographically guided small-bore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med 2004; 23(9):1171–1176. pmid:15328431
- Mineo TC, Sellitri F, Tacconi F, Ambrogi V. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014; 17(7):761–768. doi:10.1089/jpm.2013.0617
- Michaud G, Berkowitz DM, Ernst A. Pleuroscopy for diagnosis and therapy for pleural effusions. Chest 2010; 138(5):1242–1246. doi:10.1378/chest.10-1259
- Bhatnagar R, Maskell NA. Medical pleuroscopy. Clin Chest Med 2013; 34(3):487–500. doi:10.1016/j.ccm.2013.04.001
- Pilling JE, Dusmet ME, Ladas G, Goldstraw P. Prognostic factors for survival after surgical palliation of malignant pleural effusion. J Thorac Oncol 2010; 5(10):1544–1550. doi:10.1097/JTO.0b013e3181e95cb8
- Beyea A, Winzelberg G, Stafford RE. To drain or not to drain: an evidence-based approach to palliative procedures for the management of malignant pleural effusions. J Pain Symptom Manage 2012; 44(2):301–306. doi:10.1016/j.jpainsymman.2012.05.002
KEY POINTS
- Asymptomatic pleural effusion in patients currently on chemotherapy does not require treatment but should be monitored for progression.
- Indwelling pleural catheters are best used to treat effusion with lung collapse and are increasingly used as first-line therapy in other settings.
- Chemical or mechanical pleurodesis results in filling the pleural space to prevent further fluid accumulation and can be accomplished by one of several methods.
- For patients near the end of life, simple thoracentesis, repeated as needed, is a reasonable strategy.
Breast augmentation surgery: Clinical considerations
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
At present, 300,000 US women undergo breast augmentation surgery each year,1 making this the second most common aesthetic procedure in women (after liposuction),2–4 and making it extremely likely that clinicians will encounter women who have breast implants. In addition, approximately 110,000 women undergo breast reconstructive surgery after mastectomy, of whom more than 88,000 (81%) receive implants (2016 data).5
This review discusses the evolution of breast implants, their complications, and key considerations with regard to aesthetic and reconstructive breast surgery, as the principles are similar.
EVOLUTION OF IMPLANTS
Reports of breast augmentation surgery, also known as augmentation mammoplasty, date back to 1895, when a fatty tumor (lipoma) was successfully transplanted from a patient’s back to a breast defect in a mastectomy patient.2,3,6,7 In the 1930s, implantation of a glass ball into a patient’s breast marked the first implant-based breast augmentation.6 By 1954, attempts at breast augmentation using local dermal-fat flaps, adipose tissue, and even omentum were described.
Alloplastic materials gained popularity throughout the 1950s and 1960s and included polyurethane, polytetrafluoroethylene (Teflon), and other synthetics. Adverse reactions associated with alloplastic materials were plentiful: local tissue reactions, distortion of the breast mound, increased firmness, and discomfort all contributed to the eventual discontinuation of their use. The history of alloplastic breast augmentation also included epoxy resin, shellac, beeswax, paraffin, rubber, petroleum jelly, and liquefied silicone. Outcomes were not good, and many patients ultimately needed mastectomy.7
The first modern breast prosthesis was developed in 1961, and since then, implant composition and design have evolved significantly.8
From silicone to saline, and back again
The first silicone gel implants, introduced in the early 1960s,8–19 had high complication rates—some centers reported an incidence of capsular contracture of up to 70%.8,11 This is a foreign body reaction in which pathologic scar tissue encases the implant, causing it to distort, appear misshapen, harden, and even become painful.11 Attempts to minimize this reaction led to later generations of silicone implants with polyurethane shells.12
Inflatable implants filled with sterile saline solution were originally developed in France in 1965. Unlike silicone implants, saline implants have undergone minimal changes since their inception, and grew in popularity during the 1970s in view of the high rates of capsular contracture with silicone implants.8 However, saline implants have their own problems, and as they became increasingly popular, deflation and the unnatural feel of saline sparked a renewed interest in silicone gel.
By the late 1980s, the thinner-shelled generation of silicone implants displayed its own frustrating complications including implant rupture, capsular contracture, infection, and possible systemic and disseminated granulomatous disease. From 1992 to 2006, the US Food and Drug Administration (FDA) placed a moratorium on silicone implants due to concerns about a possible link with autoimmune and connective tissue diseases and the possible carcinogenic nature of silicone.
While silicone implants were prohibited in the United States, development continued abroad, and eventually the moratorium was lifted after several meta-analyses failed to reveal any link regarding the aforementioned concerns.13
Today, silicone gel implants dominate the world market.14 In the United States, approximately 60% of implants contain silicone gel filler, and trends are similar in Europe.7
Table 1 summarizes the evolution of silicone breast implants over the last 50 years.2,6,11,12Table 2 lists the advantages and disadvantages of silicone and saline breast implants.2,6,8,15
CURRENT IMPLANT OPTIONS
Currently, 3 companies (Allergan, Mentor, Sientra) manufacture and distribute breast implants and implant-associated products such as tissue expanders and sizers in the US market.6
Another company, Motiva, makes an implant that is available in Europe, Asia, and Australia, and the device is currently undergoing a 10-year clinical trial in the United States that began recruiting patients in 16 centers in April 2018.16 Pending final approval, the Cleveland Clinic Department of Plastic Surgery may be among the centers involved in the clinical trial of the Motiva implant. Innovations in the Motiva implant include a high-performance shell that maintains consistent strength and includes a proprietary barrier layer, improved silicone gel filler, 3-D imprinted surface texturing, and an implant shape that adapts with vertical and horizontal movement. It also contains radio-frequency identification transponders that can transmit data about the implant wirelessly.17–19
Surface (textured vs smooth)
Developed in the 1980s, texturing of the implant surface disrupts capsule formation around the prosthesis. Additionally, texturing stabilizes an anatomically shaped (teardrop) implant within the breast pocket, reducing malrotation.20,21
The first textured implants were covered with polyurethane foam, but they were ultimately withdrawn from the US market because of concern for in vivo degradation to carcinogenic compounds. The focus subsequently turned to texturing implant shells by mechanically creating pores of different sizes. Smooth implants, by contrast, are manufactured by repeatedly dipping the implant shell into liquid silicone.2
The capsular contraction rate has been shown to be lower with textured silicone than with smooth silicone (number needed to treat = 7–9), and evidence suggests a lower risk of needing a secondary procedure.21
Form-stable vs fluid-form
Silicone is a polymer. The physical properties of polymers vary greatly and depend on the length of the individual chains and the degree to which those chains are cross-linked. Liquid silicone contains short chains and sparse cross-linking, resulting in an oily compound well suited for lubrication. Silicone gel contains longer chains and more cross-linking and is therefore more viscous.
In “form-stable” implants, the silicone interior has sufficient chain length and cross-linking to retain the designed shape even at rest,2 but they require slightly larger incisions.7 “Fluid-form” refers to an implant with silicone filler with shorter chain length, less cross-linking, and more fluidity.6
Shell
As with silicone fillers, the properties of silicone implant shells also depend on chain length and cross-linking within the polymer. Silicone elastomer shells (Table 1) contain extensively cross-linked chains that impart a flexible yet rubbery character. Silicone elastomers can also be found in facial implants and tissue expanders.2
Implant shape (round vs anatomic)
The shape of an implant is determined by the gel distribution inside of it. To understand gel distribution and implant shape, one must understand the gel-shell ratio. This ratio increases as cohesivity of the filler increases, and it represents increased bonding of the gel filler to the shell and a preserved implant shape at rest.
The gel-shell ratio varies among manufacturers, and a less-viscous filler may be more prone to rippling or loss of upper pole fullness in some patients. For this reason, careful analysis, patient and implant selection, and discussion of complications remain paramount.2
No anatomically shaped implant is manufactured with a smooth shell, but rather with a textured shell that resists malrotation.6,15 However, in the United States, 95% of patients receive round implants.16
PATIENT ASSESSMENT
Before breast augmentation surgery, the surgeon assesses a number of factors—physical and psychosocial—and helps the patient choose a type and size of implant. The surgeon and patient also plan where the implants will be placed—ie, above or beneath the chest wall muscle—and where the incisions will be made. Every decision is made in close consultation with the patient, taking into account the patient’s desires and expectations, as well as what the patient’s anatomy allows. An integral component of this shared decision-making process is a discussion of the possible complications, and often photographs to better illustrate what to expect postoperatively.
Psychosocial factors
One must consider the patient’s psychology, motivations for surgery, and emotional stability. Here, we look for underlying body dysmorphic disorder; excessive or unusual encouragement to undergo the procedure by a spouse, friends, or others; a history of other aesthetic procedures; unrealistic expectations; and other factors influencing the desire to undergo this surgery.
Choosing an implant
Implant selection must take into account the patient’s height, weight,7 and overall body morphology: taller patients and those with wider hips or shoulders usually require larger implants. A reliable method for determining the appropriate implant must include the current breast shape, dimensions, volume, skin elasticity, soft-tissue thickness, and overall body habitus. Ultimately, the most important considerations include breast base diameter, implant volume,20 and soft-tissue envelope.
Preoperative sizing can involve placing sample implants within a brassiere so that the patient can preview possible outcomes. This method is particularly effective in minimizing dissatisfaction because it shares ownership of the decision-making process.15
A computerized implant selection program available in Europe suggests a “best-fit” implant based on a clinician’s measurements.7
Anatomic placement
Traditionally, plastic surgeons place breast implants either beneath the pectoralis major muscle (submuscular placement) or over the pectoralis8 but beneath the glandular breast parenchyma (subglandular placement) (Figure 2).7
Advantages of submuscular placement are a smoother transition of the upper breast pole from the chest wall and less rippling visible through the skin, due to the additional muscular coverage of the implant. Another advantage is that capsular contraction rates are lower with submuscular placement, likely due to possible contamination of implants by lactiferous ductal microbes when accessing the subglandular plane.14,20 Disadvantages are pronounced discomfort after surgery and animation deformities with muscle contraction, particularly in young, highly active patients.
A popular modification of submuscular placement involves creating a surgical dissection plane between the subglandular tissue and the pectoralis major fascia. This “dualplane” approach allows the parenchyma to retract superiorly and reduce breast ptosis.7
Incisions
Table 3 highlights important considerations with regard to incision location.15,20,21
ANTIBIOTICS
Many surgeons give a single prophylactic dose of antibiotic before surgery, a practice that some studies have shown to be effective in reducing the risk of infection.15 However, the benefit of routine postoperative use of antibiotics remains unsubstantiated15: postoperative antibiotic use does not appear to protect against infection, capsular contracture, or overall complications in primary or secondary breast augmentation surgery.20
PERIOPERATIVE PERIOD
At our institution, breast augmentation surgery is an ambulatory procedure—the patient goes home the same day unless circumstances such as pain control warrant admission. This is, however, according to surgeon preference, and differs on a case-by-case basis. General anesthesia is the standard of care.15
POSTOPERATIVE PERIOD
In the immediate postoperative period, patients are instructed to wear a surgical bra for up to 6 weeks to allow stable scarring. Early mobilization is encouraged.7,15 Depending on the patient’s situation, recovery, and healing, she may be out of work for about 1 week, sometimes more, sometimes less.
Additional instructions are surgeon-specific. However, the patient is instructed to avoid bathing, swimming, immersion in water, and wearing underwire brassieres that could impair healing of an inferior incision; instead, patients are often instructed to wear a surgical bra provided on the day of surgery until cleared in the clinic.
Showering is allowed the next day or the second day after surgery, and of course there is no driving while on narcotics. Additionally, patients are counseled extensively regarding hematoma formation and the signs and symptoms of infection.
Patients are typically seen in clinic 1 week after surgery.
The cost of surgery may be $5,000 to $6,000 but can vary significantly from center to center depending on who the patient sees and where, and whether the patient presents for breast reconstruction after cancer or repair of congenital anomalies, or in certain cases of transgender surgery. The patient is typically responsible for the fee, but again this depends on the patient, indications, and particular insurance concerns.
IMPLANT LONGEVITY AND RUPTURE
In the United States, implant rupture rates range from 1.1% to 17.7% at 6 to 10 years after primary augmentation, 2.9% to 14.7% after revision augmentation, 1.5% to 35.4% after primary breast reconstruction, and 0% to 19.6% after revision reconstruction.11
Unfortunately, the existence of multiple implant manufacturers, numerous implant generations, and poorly standardized screening protocols and reporting systems make the true rate of implant rupture difficult to assess without definitive imaging or implant retrieval.11
Damage from surgical instrumentation during implantation is the most common cause of silicone breast implant rupture (50% to 64% of cases).22 Other causes include underfilling and fold flaw from capsular contracture.
Leakage of silicone gel filler may be confined to the periprosthetic capsule (intracapsular rupture) or extend beyond and into the breast parenchyma (extracapsular rupture). One study reported that only 10% of intracapsular ruptures progressed extracapsularly, while 84% of patients with extracapsular involvement remained stable for up to 2 years,23 indicating that intracapsular rupture may not portend worsening disease.11
Implant rupture occurs silently in most cases, with no clinically detectable signs or symptoms. In other cases, patients may present with alterations in breast shape and size, sudden asymmetry, firmness, pronounced capsular contracture, contour irregularity, or pain.
Aside from physical examination, comprehensive diagnostic testing includes imaging—ultrasonography, mammography, computed tomography, and magnetic resonance imaging (MRI). Of these, MRI is the method of choice, with sensitivity and specificity exceeding 90% for detecting implant rupture.11 Classic findings on MRI include the “linguine” sign from a deflating implant shell, or the teardrop sign from implant sagging. Classic findings on ultrasonography include the “snowstorm” sign of extracapsular rupture and the “stepladder” sign of intracapsular rupture.
Mammography effectively detects free silicone in breast tissue with extracapsular rupture (25% of ruptures according to some studies)23; however, it cannot detect rupture within the implant capsule. As an aside, submuscular implant placement may interfere less with screening mammography than subglandular implants do.14,24
Current FDA recommendations to detect implant rupture encourage women with silicone breast implants to undergo screening 3 years after implantation and then every 2 years thereafter; no long-term monitoring is suggested for saline implants.15 Many plastic surgeons evaluate silicone breast implant patients every 1 to 2 years for contracture and rupture.8 Of note, capsular contracture impairs the effectiveness of ultrasonography and may require MRI confirmation.11
If implant rupture is confirmed, the current recommendation is to remove the implant and the capsule. Another implant may be placed depending on the patient’s preference. Rigorous washout remains a key feature of any surgical intervention for ruptured breast implants; however, in the event of extracapsular rupture, resection of silicone granulomas may also be required.11
Reoperation rates for primary breast augmentation surgery approach 20% and are even higher for secondary augmentation over a patient’s lifetime—the highest rate of all aesthetic procedures.7,14
CAPSULAR CONTRACTURE
Capsular contracture is the most common complication of breast augmentation,25 typically presenting within the first postoperative year,26,27 and the risk increases over time.28 It occurs with both silicone and saline breast implants.
In some studies, the incidence exceeded 4% in the first 2 years after surgery,29 and nearly 50% by 10 years.30 Other studies found rates of 0% to 20% over 13 years.20
The etiology is not well understood and is presumed to be multifactorial, with proposed mechanisms and factors that include bacterial contamination, surface texturing, the implant pocket selected, the incision type, drain placement, antibiotic use, and smoking.25
A meta-analysis from 17,000 implants found that the risk of capsular contracture was significantly higher when an implant was placed in a subglandular pocket than in a submuscular pocket,22,26 and that although texturing decreased capsular contracture compared with smooth implants, the effect was modest when a textured or smooth implant was placed in a submuscular location.28 With regard to incision location, studies have reported that the incidence of capsular contracture is highest with transaxillary and periareolar incisions, and lowest with inframammary incisions.20,21
The leading theory is that contamination of the implant (primarily from the mammary ducts) results in biofilm formation. Subclinical hematoma surrounding the implant may also provide key bacterial nutrients.20
Textured implants induce a greater inflammatory response in the capsular tissue, resulting in a thicker capsule; however, contracture rates remain lower with textured than with smooth implants.14,31 Interestingly, lower rates of capsular contracture have been observed with later-generation, cohesive-gel, form-stable implants than with those of earlier generations.12
Although more research is needed, silicone implants appear to confer a higher risk of capsular contracture than saline implants.14,20
Irrigating the breast pocket intraoperatively with triple antibiotic solution (bacitracin, cefazolin, and gentamicin) before placing the implant may decrease the capsular contracture rate.15,20
Treatments for capsular contracture include pocket modifications such as capsulotomy (making releasing, relaxing incisions in the scar capsule encasing the implant), capsulectomy (removing portions of or the entire capsule), and replacing the implant in the other pocket (ie, if the original implant was subglandular, the replacement is placed in the submuscular pocket). Patients who have contractures that fail to respond to these treatments may ultimately benefit from implant removal and autologous reconstruction (autoaugmentation) rather than implant replacement.32,33
ADDITIONAL COMPLICATIONS
Other complications include infection, malposition, rippling, seroma, hematoma, and sensory alterations.
Irrigation during the implantation procedure with a triple antibiotic solution consisting of bacitracin, gentamycin, and cephalexin in normal saline decreases infection and seroma rates.15,20,34
Some surgeons also choose to irrigate the pocket with a betadine solution, or to cleanse the skin with betadine and place sterile towels and redrape before inserting the implant. Additionally, many prefer using a sterile device much like a pastry funnel called a Keller funnel to insert the implant into the breast pocket.35
Infection is less common with cosmetic augmentations than with implant-based breast reconstruction, likely because of healthier, well-vascularized tissue in patients undergoing cosmetic surgery than in those undergoing mastectomy.14
Seroma is thought to be a consequence of texturing, and more so with macro- vs microtexturing. Though poorly understood, an association between texturing and double capsules has also been reported.12,20
After primary breast augmentation, 10-year follow-up rates of capsular contracture, seroma, rippling, and malposition vary across the 3 major silicone implant manufacturers.12 Hematoma and infection occur in less than 1% of primary augmentation patients.15
Malposition of the implant over time is less frequent with textured implants because of the higher coefficient of friction compared with smooth implants.6,8,15
Visible skin rippling may be a consequence of texturing and also of thin body habitus, eg, in patients with a body mass index less than 18.5 kg/m2. If the soft-tissue layer of the breast is thin, the natural rippling of smooth saline implant shells are more likely to show when placed in the subglandular pocket. Form-stable implants, by contrast, resist rippling.12,15
Large implants and extensive lateral dissection can cause alterations in nipple sensation and sensory loss within lower breast pole skin. Axillary incisions may traumatize or damage the intercostobrachial nerve, resulting in upper inner arm sensory aberrations.
Ultimately, the 10-year incidence of secondary surgery ranges from 0% to 36% and the 10-year incidence of capsular contracture ranges from 11% to 19%.15 Additional cosmetic complaints after augmentation with implants include enlargement of the areola and engorgement of breast veins.14
BREAST CANCER AND DETECTION
Patients with or without implants do not seem to differ with regard to breast cancer stage upon detection, tumor burden, recurrence, or survival. However, more patients with implants may present with palpable masses, invasive tumors, axillary metastasis, and falsely negative mammograms.
Breast implants may actually facilitate cancer detection on physical examination by providing a more dense or stable surface upon which to palpate the breast tissue. Although they do not necessarily impair mastectomy or breast reconstruction, they may result in an increased rate of revision surgery after breast conservation therapy.24,36 Mammography remains the standard of care for radiologic diagnosis but can be further supported by MRI and ultrasonography if necessary in patients with implants.
AUTOIMMUNE DISEASES
Although concerns persist, multiple studies have demonstrated the safety of fourth- and fifth-generation silicone breast implants with regard to autoimmune disease.7
In various clinical studies in mastectomy patients who underwent breast reconstruction with either silicone implants or autologous tissue, no difference was found with regard to the incidence of autoimmune diseases.2 Additionally, in meta-analyses of data from more than 87,000 women, no association was found between connective tissue disease and silicone breast implants.2,11 One study11,23 noted no increase in autoantibodies in patients with undamaged silicone implants vs patients who experienced rupture.
Studies have also demonstrated that in children born to mothers with breast implants, the risk of rheumatic disease, esophageal disorders, congenital malformations, and death during the perinatal period is comparable with that in controls.37 Another study, examining breastfeeding in women with silicone breast implants, showed no significant difference in silicon levels (used as a proxy for silicone) in breast milk compared with controls without implants; silicon levels were found to be significantly higher in cow’s milk and store-bought formulas.38
BREAST IMPLANT-ASSOCIATED ANAPLASTIC LARGE-CELL LYMPHOMA
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a subtype of T-cell lymphoma that develops in tissue adjacent to breast implants. It typically presents as breast swelling 2 to 38 years (mean of 8 years) after implant insertion.39,40 The swelling may be secondary to periprosthetic seroma formation or, more rarely, palpable disease in the axilla. Patients occasionally complain of pain and, rarely, constitutional symptoms.20 BIA-ALCL is not a disease of the surrounding breast tissue, but rather of the fibrous periprosthetic capsule.21
Of note, there is no documented case involving smooth implants,41–43 but it may be related to fifth-generation textured implants.6 At present, it is not possible to definitively state which implant is associated with this condition; hence, more data are needed, and this association is currently under study.
The absolute risk of BIA-ALCL was reported in a Dutch study39 as 1 in 35,000 by age 50, 1 in 12,000 by age 70, and 1 in 7,000 by age 75, with a number needed to harm of 6,920. Overall lifetime risk was estimated at 1 in 30,000 for women with textured implants in a 2015 US study.40 In comparison, breast cancer risk is about 1 in 8 women. There is no apparent predilection for patients who underwent cosmetic augmentation vs reconstruction, or who received silicone vs saline implants.
The diagnosis is confirmed by ultrasonographically guided fine-needle aspiration of seroma fluid and subsequent immunohistochemical testing for CD30-positive and ALK-negative T lymphocytes. Other than positron-emission tomography for staging after diagnosis confirmation, imaging is ineffective. Expert opinion does not recommend routine screening unless the aforementioned symptoms arise.
Treatment involves implant removal and total capsulectomy, with samples sent for pathology study with cytokeratin staining.12 Of note, in all cases of BIA-ALCL in which the disease was limited to the circumscribed scar tissue of the breast capsule, complete surgical excision has proved curative, whereas incomplete capsulectomy portends a greater risk of recurrence and decreased survival.44
In cases of advanced or recurrent ALCL, diagnosed late or inappropriately, the National Comprehensive Cancer Network recommends a multidisciplinary approach involving adjuvant chemotherapy and radiation.44 Anecdotally, at our institution, we have recently treated several cases of advanced ALCL presenting with invasive chest wall masses with extirpative surgery and subsequent reconstruction with the assistance of our thoracic surgery colleagues, as well as the aforementioned multidisciplinary approach using adjuvant therapy.
The mechanism of this malignancy is currently under investigation, but the current theory implicates an exaggerated lymphoproliferative response to bacterial contamination of the capsule superimposed upon genetic factors in susceptible patients.42,43
National societies advise plastic surgeons to discuss the risk of BIA-ALCL with all patients at the time of breast augmentation consultation and to report all confirmed cases to the PROFILE registry (Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology).45
ARE PATIENTS HAPPIER AFTERWARD?
Studies have shown that after undergoing breast augmentation surgery, patients note improvement in body image, and satisfaction rates range from 85% to 95% with respect to self-confidence and body image.46 An evaluation of patient responses on the validated BREAST-Q Augmentation Questionnaire showed the following satisfaction rates: breasts 83%, psychosocial well-being 88%, and sexual functioning 81%.15
Although epidemiologic studies have reported higher suicide rates in women with cosmetic breast implants, this likely stems from preoperative psychological factors and underscores the role of psychiatric referral in patients with a mental health history or in those whom the surgeon deems it necessary.46
Several high-quality studies have demonstrated that quality of life and psychosocial functioning (including depression) markedly improve after breast augmentation surgery.47 Among a cohort of Norwegian patients, breast implant surgery resulted in improved motivation to perform daily activities, as well as improved quality of life from both a psychosocial and aesthetic perspective.48 Interestingly, a recent study reported that patients who underwent breast implant surgery alone reported greater satisfaction and psychosocial quality of life than patients who underwent combination breast augmentation and mastopexy (breast-lifting) surgery.49
Additional data are needed to refine our understanding of the complex interplay between psychosocial factors before and after surgery in patients seeking and undergoing breast augmentation procedures.
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Maxwell GP, Gabriel A. Breast implant design. Gland Surg 2017; 6(2):148–153. doi:10.21037/gs.2016.11.09
- Gabriel A, Maxwell GP. The evolution of breast implants. Clin Plast Surg 2015; 42(4):399–404. doi:10.1016/j.cps.2015.06.015
- American Society of Plastic Surgeons. Procedural statistics trends 1992–2012. www.plasticsurgery.org/documents/News/Statistics/2012/plastic-surgery-statistics-full-report-2012.pdf. Accessed January 17, 2019.
- American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 17, 2019.
- Henderson PW, Nash D, Laskowski M, Grant RT. Objective comparison of commercially available breast implant devices. Aesthetic Plast Surg 2015; 39(5):724–732. doi:10.1007/s00266-015-0537-1
- Adams WP Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg 2012; 130(4):597e–611e. doi:10.1097/PRS.0b013e318262f607
- Spear SL, Jespersen MR. Breast implants: saline or silicone? Aesthet Surg J 2010; 30(4):557–570. doi:10.1177/1090820X10380401
- Cronin TD, Gerow FJ. Augmentation mammaplasty: a new “natural feel” prosthesis. In: Transactions of the Third International Conference of Plastic Surgery: October 13–18, 1963, Washington, DC.
- Maxwell GP, Gabriel A. The evolution of breast implants. Plast Reconstr Surg 2014; 134(suppl 1):12S–17S. doi:10.1097/PRS.0000000000000348
- Hillard C, Fowler JD, Barta R, Cunningham B. Silicone breast implant rupture: a review. Gland Surg 2017; 6(2):163–168. doi:10.21037/gs.2016.09.12
- Derby BM, Codner MA. Textured silicone breast implant use in primary augmentation: core data update and review. Plast Reconstr Surg 2015; 135(1):113–124. doi:10.1097/PRS.0000000000000832
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum 2001; 44(11):2477–2484. pmid:11710703
- Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg 2008; 121(suppl 4):1–7. doi:10.1097/01.prs.0000305933.31540.5d
- Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg 2014; 133(4):567e–583e. doi:10.1097/PRS.0000000000000033
- ClinicalTrials.gov. Study of the safety and effectiveness of Motiva Implants®. https://clinicaltrials.gov/ct2/show/NCT03579901. Accessed January 17, 2019.
- Establishment Labs. Motiva Implants. https://motivaimplants.com/why-motiva/innovation-for-enhanced-safety/. Accessed January 17, 2019.
- Sforza M, Zaccheddu R, Alleruzzo A, et al. Preliminary 3-year evaluation of experience with silksurface and velvetsurface Motiva silicone breast implants: a single-center experience with 5813 consecutive breast augmentation cases. Aesthet Surg J 2018; 38(suppl 2):S62–S73. doi:10.1093/asj/sjx150
- Huemer GM, Wenny R, Aitzetmüller MM, Duscher D. Motiva ergonomix round silksurface silicone breast implants: outcome analysis of 100 primary breast augmentations over 3 years and technical considerations. Plast Reconstr Surg 2018; 141(6):831e–842e. doi:10.1097/PRS.0000000000004367
- Lista F, Ahmad J. Evidence-based medicine: augmentation mammaplasty. Plast Reconstr Surg 2013; 132(6):1684–1696. doi:10.1097/PRS.0b013e3182a80880
- Namnoum JD, Largent J, Kaplan HM, Oefelein MG, Brown MH. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg 2013; 66(9):1165–1172. doi:10.1016/j.bjps.2013.04.046
- Handel N, Garcia ME, Wixtrom R. Breast implant rupture: causes, incidence, clinical impact, and management. Plast Reconstr Surg 2013; 132(5):1128–1137. doi:10.1097/PRS.0b013e3182a4c243
- Hölmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg 2003; 138(7):801–806. doi:10.1001/archsurg.138.7.801
- Mccarthy CM, Pusic AL, Disa JJ, Cordeiro PG, Cody HS 3rd, Mehrara B. Breast cancer in the previously augmented breast. Plast Reconstr Surg 2007; 119(1):49–58. doi:10.1097/01.prs.0000244748.38742.1f
- Egeberg A, Sørensen JA. The impact of breast implant location on the risk of capsular contraction. Ann Plast Surg 2016; 77(2):255–259. doi:10.1097/SAP.0000000000000227
- Wickman M. Rapid versus slow tissue expansion for breast reconstruction: a three-year follow-up. Plast Reconstr Surg 1995; 95(4):712–718. pmid:7892316
- Kjøller K, Hölmich LR, Jacobsen PH, et al. Epidemiological investigation of local complications after cosmetic breast implant surgery in Denmark. Ann Plast Surg 2002; 48(3):229–237. pmid:11862025
- Handel N, Jensen JA, Black Q, Waisman JR, Silverstein MJ. The fate of breast implants: a critical analysis of complications and outcomes. Plast Reconstr Surg 1995; 96(7):1521–1533. pmid:7480271
- Henriksen TF, Hölmich LR, Fryzek JP, et al. Incidence and severity of short-term complications after breast augmentation: results from a nationwide breast implant registry. Ann Plast Surg 2003; 51(6):531–539. doi:10.1097/01.sap.0000096446.44082.60
- Fernandes JR, Salinas HM, Broelsch GF, et al. Prevention of capsular contracture with photochemical tissue passivation. Plast Reconstr Surg 2014; 133(3):571–577. doi:10.1097/01.prs.0000438063.31043.79
- Wong CH, Samuel M, Tan BK, Song C. Capsular contracture in subglandular breast augmentation with textured versus smooth breast implants: a systematic review. Plast Reconstr Surg 2006; 118(5):1224–1236. doi:10.1097/01.prs.0000237013.50283.d2
- Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg 2013; 132(2):304–315. doi:10.1097/PRS.0b013e31829e7d9e
- Gurunluoglu R, Shafighi M, Schwabegger A, Ninkovic M. Secondary breast reconstruction with deepithelialized free flaps from the lower abdomen for intractable capsular contracture and maintenance of breast volume. J Reconstr Microsurg 2005; 21(1):35–41. doi:10.1055/s-2005-862779
- Adams WP Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstru Surg 2006; 118(7 suppl):46S–52S. doi:10.1097/01.prs.0000185671.51993.7e
- Moyer HR, Ghazi B, Saunders N, Losken A. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J 2012; 32(2):194–199. doi:10.1177/1090820X11434505
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg 2007; 120(7 suppl 1):81S–93S. doi:10.1097/01.prs.0000286578.94102.2b
- Kjøller K, Friis S, Lipworth L, Mclaughlin JK, Olsen JH. Adverse health outcomes in offspring of mothers with cosmetic breast implants: a review. Plast Reconstr Surg 2007; 120(7 suppl 1):129S–134S. doi:10.1097/01.prs.0000286571.93392.00
- Semple JL. Breast-feeding and silicone implants. Plast Reconstr Surg 2007; 120(7 suppl 1):123S–128S. doi:10.1097/01.prs.0000286579.27852.ed
- de Boer M, van leeuwen FE, Hauptmann M, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol 2018; 4(3):335–341. doi:10.1001/jamaoncol.2017.4510
- McCarthy CM, Horwitz SM. Association of breast implants with anaplastic large-cell lymphoma. JAMA Oncol 2018; 4(3):341–342. doi:10.1001/jamaoncol.2017.4467
- American Society of Plastic Surgeons. BIA-ALCL physician resources. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-physician-resources. Accessed December 17, 2018.
- The American Society for Aesthetic Plastic Surgery, Inc. Member FAQs: latest information on ALCL. www.surgery.org/sites/default/files/Member-FAQs_1.pdf. Accessed January 17, 2019.
- The American Society of Plastic Surgeons. BIA-ALCL resources: summary and quick facts. www.plasticsurgery.org/for-medical-professionals/health-policy/bia-alcl-summary-and-quick-facts. Accessed January 17, 2019.
- National Comprehensive Cancer Network. T-cell lymphomas. www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf.
- The Plastic Surgery Foundation PROFILE Registry. www.thepsf.org/research/registries/profile. Accessed January 17, 2019.
- Sarwer DB. The psychological aspects of cosmetic breast augmentation. Plast Reconstr Surg 2007; 120(7 suppl 1):110S–117S. doi:10.1097/01.prs.0000286591.05612.72
- Rohrich RJ, Adams WP, Potter JK. A review of psychological outcomes and suicide in aesthetic breast augmentation. Plast Reconstr Surg 2007; 119(1):401–408. doi:10.1097/01.prs.0000245342.06662.00
- Kalaaji A, Bjertness CB, Nordahl C, Olafsen K. Survey of breast implant patients: characteristics, depression rate, and quality of life. Aesthet Surg J 2013; 33(2):252–257. doi:10.1177/1090820X12473106
- Kalaaji A, Dreyer S, Brinkmann J, Maric I, Nordahl C, Olafsen K. Quality of life after breast enlargement with implants versus augmentation mastopexy: a comparative study. Aesthet Surg J 2018; 38(12):1304–1315. doi:10.1093/asj/sjy047
KEY POINTS
- Nearly 300,000 breast augmentation surgeries are performed annually, making this the second most common aesthetic procedure in US women (after liposuction).
- Today, silicone gel implants dominate the world market, and in the United States, approximately 60% of implants contain silicone gel filler.
- Capsular contracture is the most common complication of breast augmentation, typically presenting within the first postoperative year and with increasing risk over time. It occurs with both silicone and saline breast implants.
- Numerous studies have demonstrated the safety of silicone breast implants with regard to autoimmune disease incidence. However, the risk of associated anaplastic large-cell lymphoma must be discussed at every consultation, and confirmed cases should be reported to a national registry.
Heart failure guidelines: What you need to know about the 2017 focused update
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and Heart Failure Society of America (HFSA) jointly released a focused update1 of the 2013 ACC/AHA guideline for managing heart failure.2 This is the second focused update of the 2013 guidelines; the first update,3 in 2016, covered 2 new drugs (sacubitril-valsartan and ivabradine) for chronic stage C heart failure with reduced ejection fraction (HFrEF).
Rather than focus on new medication classes, this second update provides recommendations regarding:
- Preventing the progression to left ventricular dysfunction or heart failure in patients at high risk (stage A) through screening with B-type natriuretic peptide (BNP) and aiming for more aggressive blood pressure control
- Inpatient biomarker use
- Medications in heart failure with preserved ejection fraction (HFpEF, or diastolic heart failure)
- Blood pressure targets in stage C heart failure
- Managing important comorbidities such as iron deficiency and sleep-disordered breathing to decrease morbidity, improve functional capacity, and enhance quality of life.
These guidelines and the data that underlie them are explored below. We also discuss potential applications to the management of hospitalization for acute decompensated heart failure (ADHF).
COMMON, COSTLY, AND DEBILITATING
Heart failure—defined by the ACC/AHA as the complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood—remains one of the most common, costly, and debilitating diseases in the United States.2 Based on National Health and Nutrition Examination Survey data from 2011 to 2014, an estimated 6.5 million US adults have it, with projections of more than 8 million by 2030.4,5 More than 960,000 new cases are thought to occur annually, with a lifetime risk of developing it of roughly 20% to 45%.6
Despite ever-growing familiarity and some significant strides in management, the death rate in this syndrome is substantial. After admissions for heart failure (which number 1 million per year), the mortality rate is roughly 10% at 1 year and 40% at 5 years.6 Also staggering are the associated costs, with $30.7 billion attributed to heart failure in 2012 and a projected $69.7 billion annually by 2030.5 Thus, we must direct efforts not only to treatment, but also to prevention.
Preventive efforts would target patients with ACC/AHA stage A heart failure—those at high risk for developing but currently without evidence of structural heart disease or heart failure symptoms (Table 1).7 This group may represent up to one-third of the US adult population, or 75 million people, when including the well-recognized risk factors of coronary artery disease, hypertension, diabetes mellitus, and chronic kidney disease in those without left ventricular dysfunction or heart failure.8
BIOMARKERS FOR PREVENTION
Past ACC/AHA heart failure guidelines2 have included recommendations on the use of biomarkers to aid in diagnosis and prognosis and, to a lesser degree, to guide treatment of heart failure. Largely based on 2 trials (see below), the 2017 guidelines go further, issuing a recommendation on the use of natriuretic peptide biomarkers in a screening strategy to prompt early intervention and prevent the progression to clinical heart failure in high-risk patients (stage A heart failure).
The PONTIAC trial
The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) trial9 randomized 300 outpatients with type 2 diabetes mellitus and an elevated N-terminal proBNP (NT-proBNP) level (> 125 pg/mL) to standard medical care vs standard care plus intensive up-titration of renin-angiotensin system antagonists and beta-blockers in a cardiac clinic over 2 years.
Earlier studies10 had shown NT-proBNP levels to have predictive value for cardiac events in diabetic patients, while the neurohormonal treatments were thought to have an established record of preventing primary and secondary cardiovascular events. In PONTIAC, a significant reduction was seen in the primary end point of hospitalization or death due to cardiac disease (hazard ratio [HR] 0.351, P = .044), as well as in the secondary end point of hospitalization due to heart failure (P < .05), in the aggressive-intervention group. These results laid the foundation for the larger St. Vincent’s Screening to Prevent Heart Failure (STOP-HF) trial.11
The STOP-HF trial
The STOP-HF trial randomized 1,235 outpatients who were at high risk but without left ventricular dysfunction or heart failure symptoms (stage A) to annual screening alone vs annual screening plus BNP testing, in which a BNP level higher than 50 pg/mL triggered echocardiography and evaluation by a cardiologist who would then assist with medications.11
Eligible patients were over age 40 and had 1 or more of the following risk factors:
- Diabetes mellitus
- Hypertension
- Hypercholesterolemia
- Obesity (body mass index > 30 kg/m2)
- Vascular disease (coronary, cerebral, or peripheral arterial disease)
- Arrhythmia requiring treatment
- Moderate to severe valvular disease.
After a mean follow-up of 4.3 years, the primary end point, ie, asymptomatic left ventricular dysfunction with or without newly diagnosed heart failure, was found in 9.7% of the control group and in only 5.9% of the intervention group with BNP screening, a 42% relative risk reduction (P = .013).
Similarly, the incidence of secondary end points of emergency hospitalization for a cardiovascular event (arrhythmia, transient ischemic attack, stroke, myocardial infarction, peripheral or pulmonary thrombosis or embolization, or heart failure) was also lower at 45.2 vs 24.4 per 1,000 patient-years, a 46% relative risk reduction.
An important difference in medications between the 2 groups was an increase in subsequently prescribed renin-angiotensin-aldosterone system therapy, mainly consisting of angiotensin II receptor blockers (ARBs), in those with elevated BNP in the intervention group. Notably, blood pressure was about the same in the 2 groups.11
Although these findings are encouraging, larger studies are needed, as the lack of blinding, low event rates, and small absolute risk reduction make the results difficult to generalize.
New or modified recommendations for screening
Employing this novel prevention strategy in the extremely large number of patients with stage A heart failure, thought to be up to one-third of the US adult population, may serve as a way to best direct and utilize limited medical resources.8
BIOMARKERS FOR PROGNOSIS OR ADDED RISK STRATIFICATION
The 2013 guidelines2 recognized that a significant body of work had accumulated showing that natriuretic peptide levels can predict outcomes in both chronic and acute heart failure. Thus, in both conditions, the guidelines contained separate class Ia recommendations to obtain a natriuretic peptide level, troponin level, or both to establish prognosis or disease severity.
The 2017 update1 underscores the importance of timing in measuring natriuretic peptide levels during admission for ADHF, with emphasis on obtaining them at admission and at discharge for acute and postdischarge prognosis. The completely new class IIa recommendation to obtain a predischarge natriuretic peptide level for postdischarge prognosis was based on a number of observational studies, some of which we explore below.
The ELAN-HF meta-analysis
The European Collaboration on Acute Decompensated Heart Failure (ELAN-HF)12 performed a meta-analysis to develop a discharge prognostication score for ADHF that included both absolute level and percent change in natriuretic peptide levels at the time of discharge.
Using data from 7 prospective cohorts totaling 1,301 patients, the authors found that incorporation of these values into a subsequently validated risk model led to significant improvements in the ability to predict the end points of all-cause mortality and the combined end point of all-cause mortality or first readmission for a cardiovascular reason within 180 days.
The OPTIMIZE-HF retrospective analysis
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) were retrospectively analyzed13 to determine whether postdischarge outcomes were best predicted by natriuretic peptide levels at admission or discharge or by the relative change in natriuretic peptide level. More than 7,000 patients age 65 or older, in 220 hospitals, were included, and Cox prediction models were compared using clinical variables alone or in combination with the natriuretic peptide levels.
The model that included the discharge natriuretic peptide level was found to be the most predictive, with a c-index of 0.693 for predicting mortality and a c-index of 0.606 for mortality or rehospitalization at 1 year.
New or modified recommendations on biomarkers for prognosis
The 2017 update1 modified the earlier recommendation to obtain a natriuretic peptide or troponin level or both at admission for ADHF to establish prognosis. This now has a class Ia recommendation, emphasizing that such levels be obtained on admission. In addition, a new class IIa recommendation is made to obtain a predischarge natriuretic peptide level for postdischarge prognosis. The former class Ia recommendation to obtain a natriuretic peptide level in chronic heart failure to establish prognosis or disease severity remains unchanged.
Also worth noting is what the 2017 update does not recommend in regard to obtaining biomarker levels. It emphasizes that many patients, particularly those with advanced (stage D) heart failure, have a poor prognosis that is well established with or without biomarker levels. Additionally, there are many cardiac and noncardiac causes of natriuretic peptide elevation; thus, clinical judgment remains paramount.
The 2017 update1 also cautions against setting targets of percent change in or absolute levels of natriuretic peptide at discharge despite observational and retrospective studies demonstrating better outcomes when levels are reduced, as treating for any specific target has never been studied in a large prospective study. Thus, doing so may result in unintended harm. Rather, clinical judgment and optimization of guideline-directed management and therapy are encouraged (Table 2).
PHARMACOLOGIC TREATMENT FOR STAGE C HFpEF
Although the 2013 guidelines2 contain many class I recommendations for various medications in chronic HFrEF, not a single such recommendation is found for chronic HFpEF. A review by Okwuosa et al7 covered HFrEF, including the most recent additions on which the 2016 update was based, sacubitril-valsartan and ivabradine. The 2016 update was similarly devoid of recommendations regarding specific medications in HFpEF, leaving only the 2013 class IIb recommendation to consider using an ARB to decrease hospitalizations in HFpEF.
Evidence behind this recommendation came from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity program’s randomized controlled trial in 3,025 patients with New York Heart Association (NYHA) class II to IV heart failure and left ventricular ejection fraction over 40%, who were treated with candesartan or placebo.14 Over a median follow-up of 36.6 months, there was no significant difference in the primary composite outcome of cardiovascular death or admission for heart failure, but significantly fewer patients in the candesartan arm were admitted (230 vs 270, P = .017). Thus the recommendation.
Although this finding was encouraging, it was clear that no blockbuster drug for HFpEF had been identified. Considering that roughly half of all heart failure patients have preserved ejection fraction, the discovery of such a drug for HFpEF would be met with much excitement.15 Subsequently, other medication classes have been evaluated in the hope of benefit, allowing the 2017 update to provide specific recommendations for aldosterone antagonists, nitrates, and phosphodiesterase-5 inhibitors in HFpEF.
ALDOSTERONE ANTAGONISTS FOR HFpEF
Mineralocorticoid receptor antagonists had previously been shown to significantly reduce morbidity and mortality rates in patients with HFrEF.16 In addition to aldosterone’s effects on sodium retention and many other pathophysiologic mechanisms relating to heart failure, this hormone is also known to play a role in promoting myocardial fibrosis.17 Accordingly, some have wondered whether aldosterone antagonists could improve diastolic dysfunction, and perhaps outcomes, in HFpEF.
The Aldo-DHF trial
The Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF) trial investigated whether the aldosterone antagonist spironolactone would improve diastolic function or maximal exercise capacity in chronic HFpEF.18 It randomized 422 ambulatory patients with NYHA stage II or III heart failure, preserved left ventricular ejection fraction (≥ 50%), and echocardiographic evidence of diastolic dysfunction to receive spironolactone 25 mg daily or placebo.
Although no significant difference was seen in maximal exercise capacity, follow-up over 1 year nevertheless showed significant improvement in echocardiographic diastolic dysfunction (E/e') and perhaps reverse remodeling (decreased left ventricular mass index). These improvements spurred larger trials powered to detect whether clinical outcomes could also be improved.
The TOPCAT trial
The Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial19 was a large, multicenter, international, double-blind, placebo-controlled trial that investigated whether spironolactone could improve clinical outcomes in HFpEF. It randomized 3,445 patients with symptomatic heart failure and left ventricular ejection fraction of 45% or more to spironolactone 15 to 45 mg daily or placebo.
The effect on a composite primary outcome of death from cardiovascular cause, aborted cardiac arrest, or hospitalization for heart failure was evaluated over a mean follow-up of 3.3 years, with only a small (HR 0.89), nonclinically significant reduction evident. Those in the spironolactone group did have a significantly lower incidence of hospitalization for heart failure (12.0% vs 14.2%, P = .04).
Although the results were disappointing in this essentially negative trial, significant regional variations evident on post hoc analysis prompted further investigation and much controversy since the trial’s publication in 2014.
Participants came in roughly equal proportions from the Americas (United States, Canada, Brazil, and Argentina—51%) and from Russia and Georgia (49%), but outcomes between the two groups were markedly different. Concern was first raised when immediate review discovered a 4-fold lower rate of the primary outcome in the placebo groups from Russia and Georgia (8.4%), a rate in fact similar to that in patients without heart failure.19 This led to further exploration that identified other red flags that called into question the data integrity from the non-American sites.20
Not only did patients receiving spironolactone in Russia and Georgia not experience the reduction in clinical outcomes seen in their American counterparts, they also did not manifest the expected elevations in potassium and creatinine, and spironolactone metabolites were undetectable in almost one-third of patients.21
These findings prompted a post hoc analysis that included only the 51% (1,767 patients) of the study population coming from the Americas; in this subgroup, treatment with spironolactone was associated with a statistically significant 18% relative risk reduction in the primary composite outcome, a 26% reduction in cardiovascular mortality, and an 18% reduction in hospitalization for heart failure.20
New or modified recommendations on aldosterone receptor antagonists
Nitrates and phosphodiesterase-5 inhibitors
Earlier studies indicated that long-acting nitrates are prescribed in 15% to 50% of patients with HFpEF, perhaps based on extrapolation from studies in HFrEF suggesting that they might improve exercise intolerance.22 Some have speculated that the hemodynamic effects of nitrates, such as decreasing pulmonary congestion, might improve exercise intolerance in those with the stiff ventricles of HFpEF as well, prompting further study.
The NEAT-HFpEF trial
The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEAT-HFpEF) trial22 investigated whether extended-release isosorbide mononitrate would increase daily activity levels in patients with HFpEF. This double-blind, crossover study randomized 110 patients with HFpEF (ejection fraction ≥ 50%) and persistent dyspnea to escalating doses of isosorbide mononitrate or placebo over 6 weeks, then to the other arm for another 6 weeks. Daily activity levels during the 120-mg phase were measured with a continuously worn accelerometer.
No beneficial effect of nitrates was evident, with a nonsignificant trend towards decreased activity levels, a significant decrease in hours of activity per day (–0.30 hours, P = .02), and no change in the other secondary end points such as quality-of-life score, 6-minute walk distance, or natriuretic peptide level.
Suggested explanations for these negative findings include the possibility of rapid dose escalation leading to increased subtle side effects (headache, dizziness, fatigue) that, in turn, decreased activity. Additionally, given the imprecise diagnostic criteria for HFpEF, difficulties with patient selection may have led to inclusion of a large number of patients without elevated left-sided filling pressures.23
The RELAX trial
The Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure With Preserved Ejection Fraction (RELAX) trial24 investigated whether the phosphodiesterase-5 inhibitor sildenafil would improve exercise capacity in HFpEF. Improvements in both exercise capacity and clinical outcomes had already been seen in earlier trials in patients with pulmonary hypertension, as well as in those with HFrEF.25 A smaller study in HFpEF patients with pulmonary hypertension was also encouraging.26
Thus, it was disappointing that, after randomizing 216 outpatients with HFpEF to sildenafil or placebo for 24 weeks, no benefit was seen in the primary end point of change in peak oxygen consumption or in secondary end points of change in 6-minute walk distance or composite clinical score. Unlike in NEAT-HFpEF, patients here were required to have elevated natriuretic peptide levels or elevated invasively measured filling pressures.
The study authors speculated that pulmonary arterial hypertension and right ventricular systolic failure might need to be significant for patients with HFpEF to benefit from phosphodiesterase-5 inhibitors, with their known effects of dilation of pulmonary vasculature and increasing contractility of the right ventricle.24
New or modified recommendations on nitrates or phosphodiesterase-5 drugs
Given these disappointing results, the 2017 update provides a class III (no benefit) recommendation against the routine use of nitrates or phosphodiesterase-5 inhibitors to improve exercise tolerance or quality of life in HFpEF, citing them as ineffective (Table 3).1
IRON DEFICIENCY IN HEART FAILURE
Not only is iron deficiency present in roughly 50% of patients with symptomatic heart failure (stage C and D HFrEF),27 it is also associated with increased heart failure symptoms such as fatigue and exercise intolerance,28 reduced functional capacity, decreased quality of life, and increased mortality.
Notably, this association exists regardless of the hemoglobin level.29 In fact, even in those without heart failure or anemia, iron deficiency alone results in worsened aerobic performance, exercise intolerance, and increased fatigue.30 Conversely, improvement in symptoms, exercise tolerance, and cognition have been shown with repletion of iron stores in such patients.31
At the time of the 2013 guidelines, only a single large trial of intravenous iron in HFrEF and iron deficiency had been carried out (see below), and although the results were promising, it was felt that the evidence base on which to make recommendations was inadequate. Thus, recommendations were deferred until more data could be obtained.
Of note, in all the trials discussed below, iron deficiency was diagnosed in the setting of heart failure as ferritin less than 100 mg/mL (absolute iron deficiency) or as ferritin 100 to 300 mg/mL with transferrin saturation less than 20% (relative deficiency).32
The CONFIRM-HF trial
As in the Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR-HF) trial,33 the subsequent Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) trial34 involved the intravenous infusion of iron (ferric carboxymaltose) in outpatients with symptomatic HFrEF and iron deficiency. It showed that benefits remained evident with a more objective primary end point (change in 6-minute walk test distance at 24 weeks), and that such benefits were sustained, as seen in numerous secondary end points related to functional capacity at 52 weeks. Benefits in CONFIRM-HF were evident independently from anemia, specifically whether hemoglobin was under or over 12 g/dL.
Although these results were promising, it remained unclear whether such improvements could be obtained with a much easier to administer, more readily available, and less expensive oral iron formulation.
The IRONOUT-HF trial
The Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT-HF) trial35 investigated whether oral, rather than intravenous, iron supplementation could improve peak exercise capacity in patients with HFrEF and iron deficiency. This double-blind, placebo-controlled trial randomized 225 patients with NYHA class II to IV HFrEF and iron deficiency to treatment with oral iron polysaccharide (150 mg twice daily) or placebo for 16 weeks.
Contrary to the supportive findings above, no significant change was seen in the primary end point of change in peak oxygen uptake or in any of the secondary end points (change in 6-minute walk, quality of life). Also, despite a 15-fold increase in the amount of iron administered in oral form compared with intravenously, little change was evident in the indices of iron stores over the course of the study, with only a 3% increase in transferrin saturation and an 11 ng/mL increase in ferritin. The intravenous trials resulted in a 4-fold greater increase in transferrin saturation and a 20-fold greater increase in ferritin.36
What keeps heart failure patients from absorbing oral iron? It is unclear why oral iron administration in HFrEF, such as in IRONOUT-HF, seems to be so ineffective, but hepcidin—a protein hormone made by the liver that shuts down intestinal iron absorption and iron release from macrophages—may play a central role.37 When iron stores are adequate, hepcidin is upregulated to prevent iron overload. However, hepcidin is also increased in inflammatory states, and chronic heart failure is often associated with inflammation.
With this in mind, the IRONOUT-HF investigators measured baseline hepcidin levels at the beginning and at the end of the 16 weeks and found that high baseline hepcidin levels predicted poorer response to oral iron. Other inflammatory mediators, such as interleukin 6, may also play a role.38,39 Unlike oral iron formulations such as iron polysaccharide, intravenous iron (ferric carboxymaltose) bypasses these regulatory mechanisms, which may partly explain its much more significant effect on the indices of iron stores and outcomes.
New or modified recommendations on iron
The 2017 update1 makes recommendations regarding iron deficiency and anemia in heart failure for the first time.
A class IIb recommendation states that it might be reasonable to treat NYHA class II and III heart failure patients with iron deficiency with intravenous iron to improve functional status and quality of life. A strong recommendation has been deferred until more is known about morbidity and mortality effects from adequately powered trials, some of which are under way and explored further below.
The 2017 update also withholds any recommendations regarding oral iron supplementation in heart failure, citing an uncertain evidence base. Certainly, the subsequent IRONOUT-HF trial does not lend enthusiasm for this approach.
Lastly, given the lack of benefit coupled with the increased risk of thromboembolic events evident in a trial of darbepoetin alfa vs placebo in non-iron deficiency-related anemia in HFrEF,40,41 the 2017 update provides a class III (no benefit) recommendation against using erythropoietin-stimulating agents in heart failure and anemia.
HYPERTENSION IN HEART FAILURE
The 2013 guidelines for the management of heart failure simply provided a class I recommendation to control hypertension and lipid disorders in accordance with contemporary guidelines to lower the risk of heart failure.1
SPRINT
The Systolic Blood Pressure Intervention Trial (SPRINT)42 sought to determine whether a lower systolic blood pressure target (120 vs 140 mm Hg) would reduce clinical events in patients at high risk for cardiovascular events but without diabetes mellitus. Patients at high risk were defined as over age 75, or with known vascular disease, chronic kidney disease, or a Framingham Risk Score higher than 15%. This multicenter, open-label controlled trial randomized 9,361 patients to intensive treatment (goal systolic blood pressure < 120 mm Hg) or standard treatment (goal systolic blood pressure < 140 mm Hg).
SPRINT was stopped early at a median follow-up of 3.26 years when a 25% relative risk reduction in the primary composite outcome of myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes became evident in the intensive-treatment group (1.65% vs 2.19% per year, HR 0.75, P < .0001).
All-cause mortality was also lower in the intensive-treatment group (HR 0.73, P = .003), while the incidence of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury, and noninjurious falls) was only slightly higher (38.3% vs 37.1%, P = .25). Most pertinent, a significant 38% relative risk reduction in heart failure and a 43% relative risk reduction in cardiovascular events were also evident.
Of note, blood pressure measurements were taken as the average of 3 measurements obtained by an automated cuff taken after the patient had been sitting quietly alone in a room for 5 minutes.
New or modified recommendations on hypertension in heart failure
Given the impressive 25% relative risk reduction in myocardial infarction, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes in SPRINT,42 the 2017 update1 incorporated the intensive targets of SPRINT into its recommendations. However, to compensate for what are expected to be higher blood pressures obtained in real-world clinical practice as opposed to the near-perfect conditions used in SPRINT, a slightly higher blood pressure goal of less than 130/80 mm Hg was set.
Although not specifically included in SPRINT, given the lack of trial data on specific blood pressure targets in HFrEF and the decreased cardiovascular events noted above, a class I (level of evidence C, expert opinion) recommendation to target a goal systolic blood pressure less than 130 mm Hg in stage C HFrEF with hypertension is also given. Standard guideline-directed medications in the treatment of HFrEF are to be used (Table 4).
Similarly, a new class I (level of evidence C, expert opinion) recommendation is given for hypertension in HFpEF to target a systolic blood pressure of less than 130 mm Hg, with special mention to first manage any element of volume overload with diuretics. Other than avoiding nitrates (unless used for angina) and phosphodiesterase inhibitors, it is noted that few data exist to guide the choice of antihypertensive further, although perhaps renin-angiotensin-aldosterone system inhibition, especially aldosterone antagonists, may be considered. These recommendations are fully in line with the 2017 ACC/AHA high blood pressure clinical practice guidelines,43 ie, that renin-angiotensin-aldosterone system inhibition with an angiotensin-converting enzyme (ACE) inhibitor or ARB and especially mineralocorticoid receptor antagonists would be the preferred choice (Table 4).
SLEEP-DISORDERED BREATHING IN HEART FAILURE
Sleep-disordered breathing, either obstructive sleep apnea (OSA) or central sleep apnea, is quite commonly associated with symptomatic HFrEF.44 Whereas OSA is found in roughly 18% and central sleep apnea in 1% of the general population, sleep-disordered breathing is found in nearly 60% of patients with HFrEF, with some studies showing a nearly equal proportion of OSA and central sleep apnea.45 A similar prevalence is seen in HFpEF, although with a much higher proportion of OSA.46 Central sleep apnea tends to be a marker of more severe heart failure, as it is strongly associated with severe cardiac systolic dysfunction and worse functional capacity.47
Not surprisingly, the underlying mechanism of central sleep apnea is quite different from that of OSA. Whereas OSA predominantly occurs because of repeated obstruction of the pharynx due to nocturnal pharyngeal muscle relaxation, no such airway patency issues or strained breathing patterns exist in central sleep apnea. Central sleep apnea, which can manifest as Cheyne-Stokes respirations, is thought to occur due to an abnormal ventilatory control system with complex pathophysiology such as altered sensitivity of central chemoreceptors to carbon dioxide, interplay of pulmonary congestion, subsequent hyperventilation, and prolonged circulation times due to reduced cardiac output.48
What the two types of sleep-disordered breathing have in common is an association with negative health outcomes. Both appear to induce inflammation and sympathetic nervous system activity via oxidative stress from intermittent nocturnal hypoxemia and hypercapnea.49 OSA was already known to be associated with significant morbidity and mortality rates in the general population,50 and central sleep apnea had been identified as an independent predictor of mortality in HFrEF.51
At the time of the 2013 guidelines, only small or observational studies with limited results had been done evaluating treatment effects of continuous positive airway pressure therapy (CPAP) on OSA and central sleep apnea. Given the relative paucity of data, only a single class IIa recommendation stating that CPAP could be beneficial to increase left ventricular ejection fraction and functional status in concomitant sleep apnea and heart failure was given in 2013. However, many larger trials were under way,52–59 some with surprising results such as a significant increase in cardiovascular and all-cause mortality (Table 5).54
New or modified recommendations on sleep-disordered breathing
Given the common association with heart failure (60%)45 and the marked variation in response to treatment, including potential for harm with adaptive servo-ventilation and central sleep apnea, a class IIa recommendation is made stating that it is reasonable to obtain a formal sleep study in any patient with symptomatic (NYHA class II–IV) heart failure.1
Due to the potential for harm with adaptive servo-ventilation in patients with central sleep apnea and NYHA class II to IV HFrEF, a class III (harm) recommendation is made against its use.
Largely based on the results of the Sleep Apnea Cardiovascular Endpoints (SAVE) trial,56 a class IIb, level of evidence B-R (moderate, based on randomized trials) recommendation is given, stating that the use of CPAP in those with OSA and known cardiovascular disease may be reasonable to improve sleep quality and reduce daytime sleepiness.
POTENTIAL APPLICATIONS IN ACUTE DECOMPENSATED HEART FAILURE
Although the 2017 update1 is directed mostly toward managing chronic heart failure, it is worth considering how it might apply to the management of ADHF.
SHOULD WE USE BIOMARFER TARGETS TO GUIDE THERAPY IN ADHF?
The 2017 update1 does offer direct recommendations regarding the use of biomarker levels during admissions for ADHF. Mainly, they emphasize that the admission biomarker levels provide valuable information regarding acute prognosis and risk stratification (class I recommendation), while natriuretic peptide levels just before discharge provide the same for the postdischarge timeframe (class IIa recommendation).
The update also explicitly cautions against using a natriuretic peptide level-guided treatment strategy, such as setting targets for predischarge absolute level or percent change in level of natriuretic peptides during admissions for ADHF. Although observational and retrospective studies have shown better outcomes when levels are reduced at discharge, treating for any specific inpatient target has never been tested in any large, prospective study; thus, doing so could result in unintended harm.
So what do we know?
McQuade et al systematic review
McQuade et al57 performed a systematic review of more than 40 ADHF trials, which showed that, indeed, patients who achieved a target absolute natriuretic peptide level (BNP ≤ 250 pg/mL) or percent reduction (≥ 30%) at time of discharge had significantly improved outcomes such as reduced postdischarge all-cause mortality and rehospitalization rates. However, these were mostly prospective cohort studies that did not use any type of natriuretic peptide level-guided treatment protocol, leaving it unclear whether such a strategy could positively influence outcomes.
For this reason, both McQuade et al57 and, in an accompanying editorial, Felker et al58 called for properly designed, randomized controlled trials to investigate such a strategy. Felker noted that only 2 such phase II trials in ADHF have been completed,59,60 with unconvincing results.
PRIMA II
The Multicenter, Randomized Clinical Trial to Study the Impact of In-hospital Guidance for Acute Decompensated Heart Failure Treatment by a Predefined NT-ProBNP Target on the Reduction of Readmission and Mortality Rates (PRIMA II)60 randomized patients to natriuretic peptide level-guided treatment or standard care during admission for ADHF.
Many participants (60%) reached the predetermined target of 30% reduction in natriuretic peptide levels at the time of clinical stabilization and randomization; 405 patients were randomized. Patients in the natriuretic peptide level-guided treatment group underwent a prespecified treatment algorithm, with repeat natriuretic peptide levels measured again after the protocol.
Natriuretic peptide-guided therapy failed to show any significant benefit in any clinical outcomes, including the primary composite end point of mortality or heart failure readmissions at 180 days (36% vs 38%, HR 0.99, 95% confidence interval 0.72–1.36). Consistent with the review by McQuade et al,57 achieving the 30% reduction in natriuretic peptide at discharge, in either arm, was associated with a better prognosis, with significantly lower mortality and readmission rates at 180 days (HR 0.39 for rehospitalization or death, 95% confidence interval 0.27–0.55).
As in the observational studies, those who achieved the target natriuretic peptide level at the time of discharge had a better prognosis than those who did not, but neither study showed an improvement in clinical outcomes using a natriuretic peptide level-targeting treatment strategy.
No larger randomized controlled trial results are available for guided therapy in ADHF. However, additional insight may be gained from a subsequent trial61 that evaluated biomarker-guided titration of guideline-directed medical therapy in outpatients with chronic HFrEF.
The GUIDE-IT trial
That trial, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT)61 trial, was a large multicenter attempt to determine whether a natriuretic peptide-guided treatment strategy was more effective than standard care in the management of 894 high-risk outpatients with chronic HFrEF. Earlier, promising results had been obtained in a meta-analysis62 of more than 11 similar trials in 2,000 outpatients, with a decreased mortality rate (HR 0.62) seen in the biomarker-guided arm. However, the results had not been definitive due to being underpowered.62
Unfortunately, the results of GUIDE-IT were disappointing, with no significant difference in either the combined primary end point of mortality or hospitalization for heart failure, or the secondary end points evident at 15 months, prompting early termination for futility.61 Among other factors, the study authors postulated that this may have partly resulted from a patient population with more severe heart failure and resultant azotemia, limiting the ability to titrate neurohormonal medications to the desired dosage.
The question of whether patients who cannot achieve such biomarker targets need more intensive therapy or whether their heart failure is too severe to respond adequately echoes the question often raised in discussions of inpatient biomarker-guided therapy.58 Thus, only limited insight is gained, and it remains unclear whether a natriuretic peptide-guided treatment strategy can improve outpatient or inpatient outcomes. Until this is clarified, clinical judgment and optimization of guideline-directed management and therapy should remain the bedrock of treatment.
SHOULD ALDOSTERONE ANTAGONISTS BE USED IN ACUTE HFpEF?
Given the encouraging results in chronic HFpEF from post hoc analyses of TOPCAT, are there any additional recent data suggesting a role for aldosterone antagonists such as spironolactone in acute HFpEF?
The ATHENA-HF trial
The Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure (ATHENA-HF) trial63 compared treatment with high-dose spironolactone (100 mg) for 96 hours vs usual care in 360 patients with ADHF. The patient population included those with HFrEF and HFpEF, and usual care included low-dose spironolactone (12.5–25 mg) in roughly 15% of patients. High-dose mineralocorticoid receptor antagonists have been shown to overcome diuretic resistance, improve pulmonary vascular congestion, and partially combat the adverse neurohormonal activation seen in ADHF.
Unfortunately, the trial was completely neutral in regard to the primary end point of reduction in natriuretic peptide levels as well as to the secondary end points of 30-day mortality rate, heart failure readmission, clinical congestion scores, urine output, and change in weight. No suggestion of additional benefit was seen in subgroup analysis of patients with acute HFpEF (ejection fraction > 45%), which yielded similar results.63
Given these lackluster findings, routine use of high-dose spironolactone in ADHF is not recommended.64 However, the treatment was well tolerated, without significant adverse effects of hyperkalemia or kidney injury, leaving the door open as to whether it may have utility in selected patients with diuretic resistance.
Should ARNIs and ivabradine be started during ADHF admissions?
The first half of the focused update3 of the 2013 guidelines,2 reviewed by Okwuosa et al,7 provided recommendations for the use of sacubitril-valsartan, an angiotensin-neprilysin inhibitor (ARNI), and ivabradine, a selective sinoatrial node If channel inhibitor, in chronic HFrEF.
Sacubitril-valsartan was given a class I recommendation for use in patients with NYHA class II or III chronic HFrEF who tolerate an ACE inhibitor or an ARB. This recommendation was given largely based on the benefits in mortality and heart failure hospitalizations seen in PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure)65 compared with enalapril (HR 0.80, 95% CI 0.73–0.87, P < .001).
There is currently no recommendation on initiation or use of ARNIs during admissions for ADHF, but a recent trial may lend some insight.66
THE PIONEER-HF trial
The Comparison of Sacubitril/Valsartan vs Enalapril on Effect on NT-proBNP in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial66 randomized patients admitted for acute HFrEF, once stabilized, to sacubitril-valsartan or enalapril. Encouragingly, the percentage change of natriuretic peptide levels from the time of inpatient initiation to 4 and 8 weeks thereafter, the primary efficacy end point, was 46.7% with sacubitril-valsartan versus 25.3% with enalapril alone (ratio of change 0.71, 95% CI 0.63–0.81, P < .001). Although not powered for such, a prespecified analysis of a composite of clinical outcomes was also favorable for sacubitril-valsartan, largely driven by a 44% decreased rate of rehospitalization. More definitive, and quite reassuring, was that no significant difference was seen in the key safety outcomes of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema. These results were also applicable to the one-third of study participants who had no former diagnosis of heart failure, the one-third identifying as African American, and the one-third who had not been taking an ACE inhibitor or ARB. These results, taken together with the notion that at study completion the patients become similar to those included in PARADIGM-HF, have led some to assert that PIONEER-HF has the potential to change clinical practice.
Ivabradine was given a class IIa recommendation for use in patients with NYHA class II or III chronic HFrEF with a resting heart rate of at least 70 bpm, in sinus rhythm, despite being on optimal medical therapy including a beta-blocker at a maximum tolerated dose.
This recommendation was largely based on SHIFT (Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial), which randomized patients to ivabradine or placebo to evaluate the effects of isolated lowering of the heart rate on the composite primary outcome of cardiovascular death or hospitalization. A significant reduction was seen in the ivabradine arm (HR 0.82, 95% CI 0.75–0.90, P < .0001), mainly driven by decreased hospitalizations.67
Subsequently, a small unblinded single-center study was undertaken to evaluate the efficacy and safety of initiating ivabradine during admissions for ADHF.68
THE ETHIC-AHF trial
The Effect of Early Treatment With Ivabradine Combined With Beta-Blockers vs Beta-Blockers Alone in Patients Hospitalized With Heart Failure and Reduced Left Ventricular Ejection Fraction (ETHIC-AHF) trial68 sought to determine the safety and effectiveness of early coadministration of ivabradine with beta-blockers in patients with acute HFrEF.
This single-center, unblinded study randomized 71 patients to ivabradine and beta-blockade or beta-blockade alone upon clinical stabilization (24–48 hours) after admission for acute decompensated HFrEF.
The primary end point was heart rate at 28 days, with the ivabradine group showing a statistically significant decrease (64 vs 70 bpm, P = .01), which persisted at 4 months. There was no significant difference in the secondary end points of adverse drug effects or the composite of clinical event outcomes (all-cause mortality, admission for heart failure or cardiovascular cause), but a number of surrogate end points including left ventricular ejection fraction, BNP level, and NYHA functional class at 4 months showed mild improvement.
Although this study provided evidence that the coadministration of ivabradine and a beta-blocker is safe and was positive in regard to clinical outcomes, the significant limitations due to its size and study design (single-center, unblinded, 4-month follow-up) simply serve to support the pursuit of larger studies with more stringent design and longer follow-up in order to determine the clinical efficacy.
The PRIME-HF trial
The Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF) trial69 is a randomized, open-label, multicenter trial comparing standard care vs the initiation of ivabradine before discharge, but after clinical stabilization, during admissions for ADHF in patients with chronic HFrEF (left ventricular ejection fraction ≤ 35%). At subsequent outpatient visits, the dosage can be modified in the ivabradine group, or ivabradine can be initiated at the provider’s discretion in the usual-care group.
PRIME-HF is attempting to determine whether initiating ivabradine before discharge will result in more patients taking ivabradine at 180 days, its primary end point, as well as in changes in secondary end points including heart rate and patient-centered outcomes. The study is active, with reporting expected in 2019.
As these trials all come to completion, it will not be long before we have further guidance regarding the inpatient initiation of these new and exciting therapeutic agents.
SHOULD INTRAVENOUS IRON BE GIVEN DURING ADHF ADMISSIONS?
Given the high prevalence of iron deficiency in symptomatic HFrEF, its independent association with mortality, improvements in quality of life and functional capacity suggested by repleting with intravenous iron (in FAIR-HF and CONFIRM-HF), the seeming inefficacy of oral iron in IRONOUT, and the logistical challenges of intravenous administration during standard clinic visits, could giving intravenous iron soon be incorporated into admissions for ADHF?
Caution has been advised for several reasons. As discussed above, larger randomized controlled trials powered to detect more definitive clinical end points such as death and the rate of hospitalization are still needed before a stronger recommendation can be made for intravenous iron in HFrEF. Also, without such data, it seems unwise to add the considerable economic burden of routinely assessing for iron deficiency and providing intravenous iron during ADHF admissions to the already staggering costs of heart failure.
The effects seen on morbidity and mortality that become evident in these trials over the next 5 years will help determine future guidelines and whether intravenous iron is routinely administered in bridge clinics, during inpatient admissions for ADHF, or not at all in patients with HFrEF and iron deficiency.
INTERNISTS ARE KEY
Heart failure remains one of the most common, morbid, complex, and costly diseases in the United States, and its prevalence is expected only to increase.4,5 The 2017 update1 of the 2013 guideline2 for the management of heart failure provides recommendations aimed not only at management of heart failure, but also at its comorbidities and, for the first time ever, at its prevention.
Internists provide care for the majority of heart failure patients, as well as for their comorbidities, and are most often the first to come into contact with patients at high risk of developing heart failure. Thus, a thorough understanding of these guidelines and how to apply them to the management of acute decompensated heart failure is of critical importance.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70(6):776–803. doi:10.1016/j.jacc.2017.04.025
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128(16):e240–e327. doi:10.1161/CIR.0b013e31829e8776
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2016; 134(13):e282–e293. doi:10.1161/CIR.0000000000000435
- Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017; 135(10):e146–e603. doi:10.1161/CIR.0000000000000485
- Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013; 6(3):606–619. doi:10.1161/HHF.0b013e318291329a
- Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol 2013; 61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
- Okwuosa IS, Princewill O, Nwabueze C, et al. The ABCs of managing systolic heart failure: past, present, and future. Cleve Clin J Med 2016; 83(10):753–765. doi:10.3949/ccjm.83a.16006
- Kovell LC, Juraschek SP, Russell SD. Stage A heart failure is not adequately recognized in US adults: analysis of the National Health and Nutrition Examination Surveys, 2007–2010. PLoS One 2015; 10(7):e0132228. doi:10.1371/journal.pone.0132228
- Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62(15):1365–1372. doi:10.1016/j.jacc.2013.05.069
- Clodi M, Resl M, Neuhold S, et al. A comparison of NT-proBNP and albuminuria for predicting cardiac events in patients with diabetes mellitus. Eur J Prev Cardiol 2012; 19(5):944–951. doi:10.1177/1741826711420015
- Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013; 310(1):66–74. doi:10.1001/jama.2013.7588
- Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompensated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on Acute decompeNsated Heart Failure: ELAN-HF Score. Heart 2014; 100(2):115–125. doi:10.1136/heartjnl-2013-303632
- Kociol RD, Horton JR, Fonarow GC, et al. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail 2011; 4(5):628–636. doi:10.1161/CIRCHEARTFAILURE.111.962290
- Yusuf S, Pfeffer MA, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003; 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355(3):251–259. doi:10.1056/NEJMoa052256
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341(10):709–717. doi:10.1056/NEJM199909023411001
- MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35(1):30–34. pmid:9302344
- Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 2013; 309(8):781–791. doi:10.1001/jama.2013.905
- Pitt B, Pfeffer MA, Assmann SF, et al; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370(15):1383–1392. doi:10.1056/NEJMoa1313731
- Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015; 31(1):34–42. doi:10.1161/CIRCULATIONAHA.114.013255
- de Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376(17):1690–1692. doi:10.1056/NEJMc1612601
- Redfield MM, Anstrom KJ, Levine JA, et al; NHLBI Heart Failure Clinical Research Network. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373(24):2314–2324. doi:10.1056/NEJMoa1510774
- Walton-Shirley M. Succinct thoughts on NEAT-HFpEF: true, true, and unrelated? Medscape 2015. https://www.medscape.com/viewarticle/854116. Accessed January 17, 2019.
- Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013; 309(12):1268–1277. doi:10.1001/jama.2013.2024
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo controlled study. Circ Heart Fail 2011; 4(1):8–17. doi:10.1161/CIRCHEARTFAILURE.110.944694
- Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011; 124(2):164–174. doi:10.1161/CIRCULATIONAHA.110.983866
- Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165(4):575–582.e3. doi:10.1016/j.ahj.2013.01.017
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013; 34(11):816–829. doi:10.1093/eurheartj/ehs224
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011; 17(11):899–906. doi:10.1016/j.cardfail.2011.08.003
- Haas JD, Brownlie T 4th. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001; 131(2S–2):676S-690S. doi:10.1093/jn/131.2.676S
- Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982; 242(6):E418–E427. doi:10.1152/ajpendo.1982.242.6.E418
- Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs 2017; 17(3):183–201. doi:10.1007/s40256-016-0211-2
- Anker SD, Comin Colet J, Filippatos G, et al; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361(25):2436–2448. doi:10.1056/NEJMoa0908355
- Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36(11):657–668. doi:10.1093/eurheartj/ehu385
- Lewis GD, Malhotra R, Hernandez AF, et al; NHLBI Heart Failure Clinical Research Network. Effect of Oral Iron Repletion on Exercise Capacity in Patients With Heart Failure With Reduced Ejection Fraction and Iron Deficiency: The IRONOUT HF randomized clinical trial. JAMA 2017; 317(19):1958–1966. doi:10.1001/jama.2017.5427
- Wendling P. Iron supplementation in HF: trials support IV but not oral. Medscape 2016. https://www.medscape.com/viewarticle/872088. Accessed January 17, 2019.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood 2011; 117(17):4425–4433. doi:10.1182/blood-2011-01-258467
- Jankowska EA, Kasztura M, Sokolski M, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35(36):2468–2476. doi:10.1093/eurheartj/ehu235
- Jankowska EA, Malyszko J, Ardehali H, et al. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34(11):827–834. doi:10.1093/eurheartj/ehs377
- Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013; 368(13):1210–1219. doi:10.1056/NEJMoa1214865
- Ghali JK, Anand IS, Abraham WT, et al; Study of Anemia in Heart Failure Trial (STAMINA-HeFT) Group. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008; 117(4):526–535. doi:10.1161/CIRCULATIONAHA.107.698514
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Whelton PK, Carey RM, Arnow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Young T, Shahar E, Nieto FJ, et al; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002; 162(8):893–900. pmid:11966340
- MacDonald M, Fang J, Pittman SD, White DP, Malhotra A.The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008; 4(1):38-42. pmid:18350960
- Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11(6):602–608. doi:10.1093/eurjhf/hfp057
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med 1999; 160(4):1101–1106. doi:10.1164/ajrccm.160.4.9903020
- Ng AC, Freedman SB. Sleep disordered breathing in chronic heart failure. Heart Fail Rev 2009; 14(2):89–99. doi:10.1007/s10741-008-9096-8
- Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol 2011; 57(2):119–127. doi:10.1016/j.jacc.2010.08.627
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
- Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49(20):2028–2034. doi:10.1016/j.jacc.2007.01.084
- Bradley TD, Logan AG, Kimoff RJ, et al; CANPAP Investigators. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005; 353(19):2025–2033. doi:10.1056/NEJMoa051001
- Arzt M, Floras JS, Logan AG, et al; CANPAP Investigators. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115(25):3173–3180. doi:10.1161/CIRCULATIONAHA.106.683482
- Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373(12):1095–1105. doi:10.1056/NEJMoa1506459
- O’Connor CM, Whellan DJ, Fiuzat M, et al. Cardiovascular outcomes with minute ventilation-targeted adaptive servo-ventilation therapy in heart failure: the CAT-HF Trial. J Am Coll Cardiol 2017; 69(12):1577–1587. doi:10.1016/j.jacc.2017.01.041
- McEvoy RD, Antic NA, Heeley E, et al; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375(10):919–931. doi:10.1056/NEJMoa1606599
- McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med 2017; 166(3):180–190. doi:10.7326/M16-1468
- Felker GM, Whellan DJ. Inpatient management of heart failure: are we shooting at the right target? Ann Intern Med 2017; 166(3):223–224. doi:10.7326/M16-2667
- Carubelli V, Lombardi C, Lazzarini V, et al. N-terminal pro-B-type natriuretic peptide-guided therapy in patients hospitalized for acute heart failure. J Cardiovasc Med (Hagerstown) 2016; 17(11):828–839. doi:10.2459/JCM.0000000000000419
- Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and mortality rates. Am Heart J 2014; 168(1):30–36. doi:10.1016/j.ahj.2014.04.008
- Felker GM, Anstrom KJ, Adams KF, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2017; 318(8):713–720. doi:10.1001/jama.2017.10565
- Troughton RW, Frampton CM, Brunner-La Rocca HP, et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J 2014; 35(23):1559–1567. doi:10.1093/eurheartj/ehu090
- van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993; 71(3):21A–28A. pmid:8422000
- Butler J, Anstrom KJ, Felker GM, et al; National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and safety of spironolactone in acute heart failure. The ATHENA-HF randomized clinical trial. JAMA Cardiol 2017; 2(9):950–958. doi:10.1001/jamacardio.2017.2198
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371(11):993–1004. doi:10.1056/NEJMoa1409077
- ClinicalTrials.gov. ComParIson Of Sacubitril/valsartaN Versus Enalapril on Effect on NTpRo-BNP in patients stabilized from an acute Heart Failure episode (PIONEER-HF). https://clinicaltrials.gov/ct2/show/NCT02554890. Accessed January 17, 2019.
- Swedberg K, Komajda M, Böhm M, et al; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010; 376(9744):875–885. doi:10.1016/S0140-6736(10)61198-1
- Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016; 217:7–11. doi:10.1016/j.ijcard.2016.04.136
- ClinicalTrials.gov. Predischarge Initiation of Ivabradine in the Management of Heart Failure (PRIME-HF). https://clinicaltrials.gov/ct2/show/NCT02827500. Accessed January 17, 2019.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018; 20(1):125–133. doi:10.1002/ejhf.823
- ClinicalTrials.gov. Intravenous Iron in Patients With Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality (FAIR-HF2). https://clinicaltrials.gov/ct2/show/NCT03036462. Accessed January 17, 2019.
- ClinicalTrials.gov. Study to Compare Ferric Carboxymaltose With Placebo in Patients With Acute Heart Failure and Iron Deficiency (AFFIRM-AHF). https://clinicaltrials.gov/ct2/show/record/NCT02937454. Accessed January 17, 2019.
- ClinicalTrials.gov. Randomized Placebo-controlled Trial of Ferric Carboxymaltose as Treatment for Heart Failure With Iron Deficiency (HEART-FID). https://clinicaltrials.gov/ct2/show/NCT03037931. Accessed January 17, 2019.
- ClinicalTrials.gov. Intravenous Iron Treatment in Patients With Heart Failure and Iron Deficiency (IRONMAN). https://clinicaltrials.gov/ct2/show/NCT02642562. Accessed January 17, 2019.
KEY POINTS
- Despite advances in treatment, heart failure remains highly morbid, common, and costly. Prevention is key.
- Strategies to prevent progression to clinical heart failure in high-risk patients include new blood pressure targets (< 130/80 mm Hg) and B-type natriuretic peptide screening to prompt referral to a cardiovascular specialist.
- An aldosterone receptor antagonist might be considered to decrease hospitalizations in appropriately selected stage C HFpEF patients. Routine use of nitrates or phosphodiesterase-5 inhibitors in such patients is not recommended.
- Outpatient intravenous iron infusions are reasonable in persistently symptomatic New York Heart Association stage II to III heart failure with reduced ejection fraction (HFrEF) to improve functional capacity and quality of life.
- The new systolic blood pressure target is less than 130 mm Hg for stage A heart failure, stage C HFrEF, and stage C HFpEF.