User login
Computerized Physician Handoff Tools
Physician handoff is a common and essential component of daily patient care that includes transfer of important clinical patient information and accountability of patient care. Thus, high‐quality physician handoffs are crucial to ensure patient safety and continuity of patient care, especially with the new resident work hour restriction in North America.[1, 2] As such, healthcare organizations including the World Health Organization[3] have issued specific goals and organizational challenges to improve the effectiveness and coordination of communication among the care/service providers and with the recipients of care/service across the continuum in healthcare.[4, 5]
It has been well‐documented that physician handoffs in hospital settings are often unstructured and not standardized, which leads to medical errors and jeopardizes patient safety.[2, 6, 7, 8, 9, 10, 11, 12] This lack of standardization of physician handoff for hospitalized patients occurs in every major in‐hospital service and affects trainees and staff.[2, 6, 7, 9, 10, 12, 13] It has been demonstrated in healthcare and in other domains that a standardized handoff protocol that involves both verbal communication and written handoff documents is likely to be an effective method of handoff to decrease miscommunication and associated errors.[14, 15, 16, 17] Computerized physician handoff tools (CHTs) have been increasingly deployed to address these challenges and have quickly gained popularity among physicians for documenting patient information during physician handoff for hospitalized patients.[18] CHTs can be an complementary part of electronic medical record (EMR) systems, but not a substitute since their focus is to deliver concise and essential information vital for patient care during interfaces of patient care.
Two recent systematic reviews have examined information technology (IT) systems to promote the handoff process in healthcare.[17, 19] However, to our knowledge, there has not been a systematic review of the potential role of CHT in physician handoff and quality of patient care for hospitalized patients. We therefore conducted a systematic review to examine the current evidence for CHTs in physician handoff for hospitalized patients, focusing specifically on potential effects on continuity of patient care, physician work efficiency, quality of handoffs, and patient outcomes.
METHODS
Criteria for Considering Eligible Studies
We included randomized controlled trials, controlled clinical trial, quasi‐experimental studies, and controlled beforeafter studies that evaluated CHTs during physician handoff of hospitalized patients. Studies needed to report patient outcomes (adverse events, missing patients at rounds, or in‐hospital mortality), physician work efficiency, quality of handoff (accuracy, consistency, or completeness), continuity of care, or physician satisfaction. Articles that met all these inclusion criteria were considered to be eligible for the review. We excluded review articles, commentaries, case reports, and retrospective studies.
Search Strategy
CHTs were defined as computer‐based platforms, designed specifically for the purpose of physician handoff, to allow distributed access and synchronous archiving of patient information via Internet protocols (ie, electronic tool to allow physician data access and data entry for handoff from different computers at multiple locations within the authorized hospitals or clinics). A search strategy was developed based on a MEDLINE search format combined with our inclusion criteria and with this definition of CHTs. We used search terms related to physician communication and information technology, and relevant Medical Subjects Headings, which include handover, handoff, signoff, sign‐over, off‐duty, post‐call, computerized, Web‐based, communication tool. The databases, including MEDLINE, PUBMED, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane database for systematic reviews, and the Cochrane CENTRAL register of controlled trials, were initially searched from 1985 to December 2011 in all languages. The Cochrane Collaboration filter for controlled interventional studies was used to select the above‐mentioned interventional trial designs. In addition, the first 2 authors hand searched the references of included articles and relevant systematic reviews.
Screening for Eligible Studies
All articles identified in the database searches described above were included for screening in 2 stages. First, 2 reviewers (P.L., S.A.) independently reviewed the title and abstracts of the identified articles for eligibility. The articles selected in the first stage of screening were then further assessed by a full‐text review independently by the 2 reviewers. Any discrepancy was resolved by consensus or by involvement of a third reviewer (C.T.).
Data Abstraction and Analysis
Data abstraction from selected studies was conducted independently by 3 authors based on a predefined template. All discrepancies in this stage were resolved by consensus among the 3 authors. For each study, we analyzed study design, data collection, intervention, main outcomes, and components of physician handoffs in the study. Due to heterogeneity of study outcomes, measures used, and results, a meta‐analysis was not performed. Study outcomes, which included adverse events, missing patients at rounds, time spent on rounding patient, accuracy, consistency or completeness of handoff information, and continuity of care, were summarized.
RESULTS
Study Selection
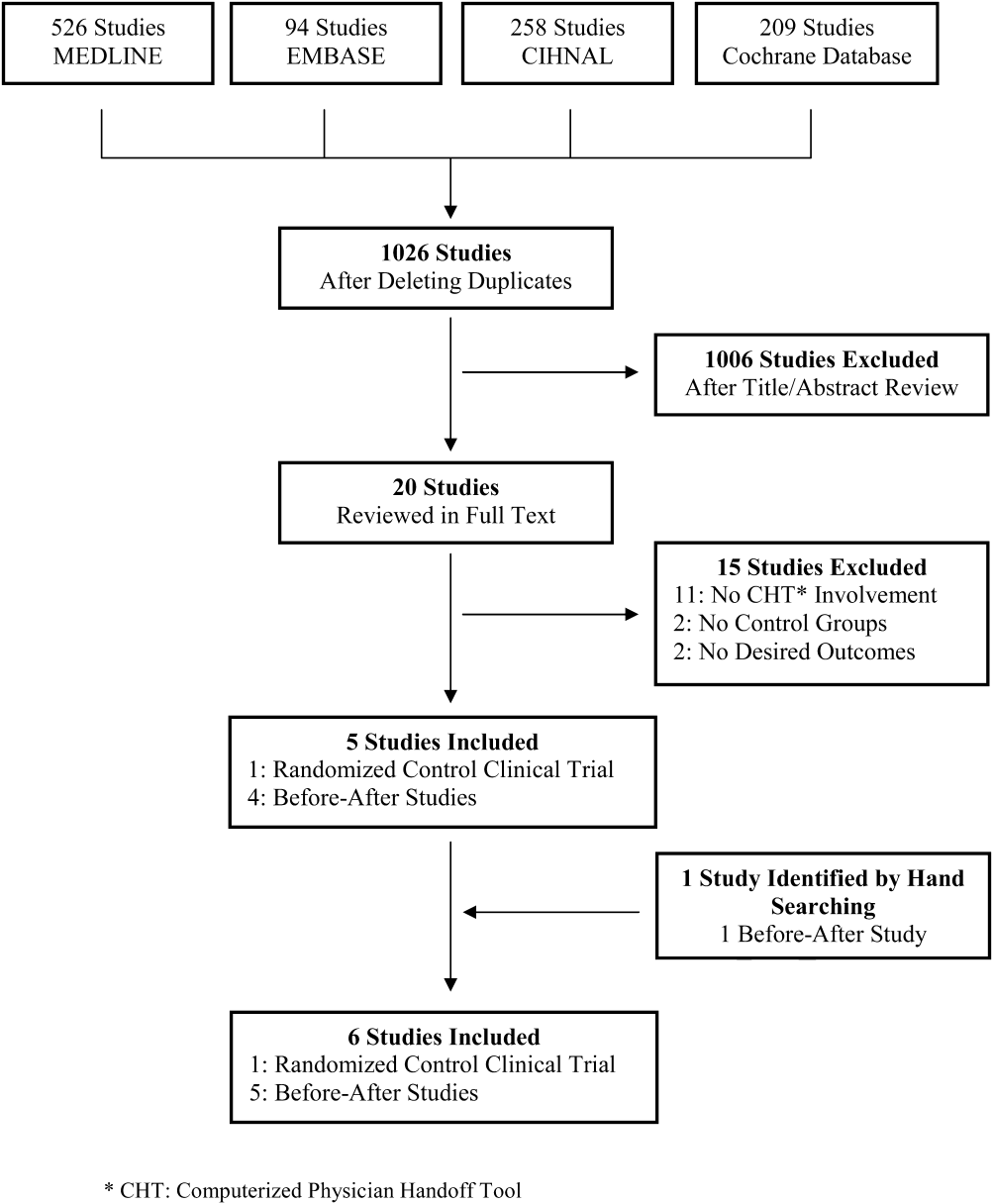
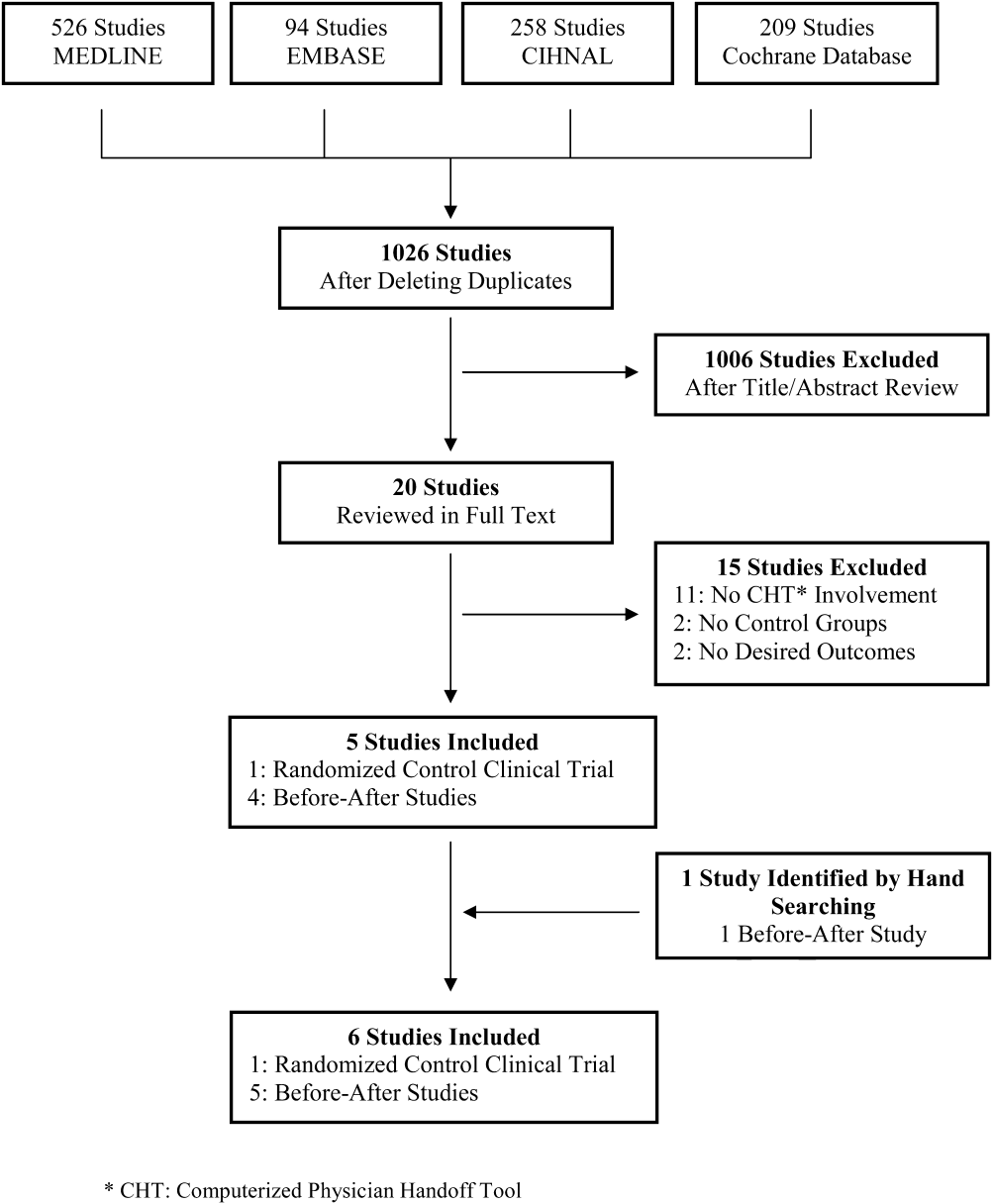
A total of 1026 citations were identified in the initial search, of which 1006 studies did not evaluate CHT and were excluded by title and abstract screening. Of the 20 studies evaluated further by full‐text review, 5 were selected for the final analysis. One additional study was identified by hand searching references. The kappa score of inter‐reviewer agreement on article selection in the first stage of screening was 0.7, and for the second stage of article selection, kappa was 1.0. The reasons for exclusion in the second selection step are presented in Figure 1.
Study Characteristics
Of the 6 studies identified, 1 study was a randomized controlled trial[20] and the other 5 were controlled beforeafter studies.[21, 22, 23, 24, 25] All studies were conducted in teaching hospitals in English‐speaking high‐income countries. All were single‐center studies, except the study by Van Eaton et[20] that involved 2 centers. All the studies investigated physician handoffs conducted by trainees. Two studies included staff physicians.[22, 24] Van Eaton et al's study included general medical, general surgical, and subspecialty surgical services.[20] The other 5 studies assessed physician handoffs in family medicine,[25] internal medicine services,[21, 23] a surgical service,[22] and a neonatal intensive care unit.[24] The study by Van Eaton et al[20] enrolled the largest study population. The intervention or observation phase ranged from 1 month[20] to 6 months[24] (Table 1).
| Study | Design | Setting | Target Services | Intervention Group | Control Group | Data Collection and Validation |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Beforeafter study | 150‐bed urban hospital in USA | Family Medicine Residents (N = 7) | Patient no. not reported 1 mo of intervention No CHT training prior to the intervention reported | Patient no. not reported Traditional handoff note (on index card or previous list) Components of handoff note not reported | Questionnaire No data validation |
| Peterson et al[21] (1998) | Beforeafter study | 720‐bed tertiary care hospital in USA | All Internal Medicine Services Residents (N = 99) | 3747 patients 4 mo of intervention 8 wk of run‐in period | 1874 patients Handwritten handoff Components of handoff note not reported | Self‐report using e‐mail, report card, in person chart review for unreported adverse events in 250 samples |
| Van Eaton et al[20] (2005) | Randomized cross‐over trial | 450‐bed tertiary care hospital and a 368‐bed trauma center in USA | General Medicine, General Surgery, and Subspecialties Trauma Residents (N = 7 teams) | 8018 patients 14 wk of randomized crossover period 6 wk of run‐in period | 7569 patients Individual written lists, cards, a team‐developed computer‐generated spreadsheet Components of handoff note not reported | Telephone interview and anonymous online survey No validation of data |
| Cheah et al[22] (2005) | Beforesfter atudy | A 400‐bed regional teaching hospital in Australia | General Surgery Registrars and Residents (N = 714) | Patient no. not reported 3 mo of observation period (for weekend coverage only) No CHT training prior to the intervention reported | Patient no. not reported No description of pre‐intervention handoff method reported | In‐person interview and survey No validation of data |
| Flanagan et al[23] (2009) | Beforesfter atudy | Tertiary care hospital in USA | Internal Medicine, Medical Intensive Care Unit First‐year Residents (N = 35) | 1264 patient handoff forms 1 mo of observation Orientation session and 1 cross‐over shift of run‐in period | Patient no. not reported No description of pre‐CHT implementation handoff method reported | In‐person interview and survey No validation of data |
| Palma et al[24] (2011) | Beforeafter study | 304‐bed quaternary care women and children hospital in USA | NICU Attendings, Residents, Nursing staffs (N = 4652) | Patient no. not reported 6 mo of intervention of NICU handoff tool Instruction document by e‐mail and informal instructional session | Patient no. not reported A Microsoft‐based standalone handoff tool or EMR integrated Medical/Surgical handoff tool Components of handoff note not reported | Online survey No validation of data |
CHT Characteristics
Three CHTs were standalone applications designed specifically for physician handoffs.[20, 22, 25] The other 3 CHTs were add‐on functions to existing hospital Electric Medical Record (EMR) systems.[21, 23, 24] All CHTs except one[25] interfaced with existing EMR systems, allowing for variable degrees of data transfer depending on CHT design and the functionalities of the EMR systems. CHT users were actively involved in designing and modifying the CHTs in most of the studies.[20, 21, 23, 25] The characteristics of the CHTs were summarized in Table 2.
| Study | CHT Design | EMR Interface | Physician Daily Progress Note | Participants' Role in CHT Design | Components of CHT | Components That Require Manual Input |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Standalone application | No interface | Paper‐based | Designing | Patient demographics Medications Diagnosis Problem lists Comment line | All the information |
| Peterson et al[21] (1998) | A part of existing EMR | Bi‐directional interface | Paper‐based | Designing | Patient demographics Current medication Allergy Code status Recent lab value A problem list A to do list | A problem list A to do list |
| Van Eaton et al[20] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Designing and modifying | Patient demographics Diagnosis Medication Allergy Vital signs Lab and investigation A problem list A to do list | Diagnosis Medication A problem list A to do list |
| Cheah et al[22] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | No | Patient demographics Diagnosis Length of stay Recent investigations Free‐text note (Not standardized) | Free‐text note |
| Flanagan et al[23] (2009) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Evaluating and modifying | Patient demographics Medication Allergy Lab and investigation Physician daily note Free‐text note (not standardized) | Free‐text note (may contain assessment, a problem list, venous access, short‐term concerns and long‐term plan, and follow‐up tasks) |
| Palma et al[24] (2011) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Paper‐based | No | Patient demographics Lab and measurement Free‐text note (not standardized) | Free‐text note (including patient description, active medical issues, ongoing care and a to do list) |
CHT's Impact on Adverse Events
The impact of CHTs on preventable adverse events was evaluated in a single study by Peterson et al.[21] The authors defined an adverse event as an injury due to medical treatment which prolonged hospital stay or produced disability at discharge in the study. Preventability was determined by using a 6‐point scale and assessed independently by 3 reviewers. Fewer adverse events were found after implementation of CHTs (2.38% vs 3.94%, P 0.001). They also reported nonsignificant reductions in preventable adverse events (1.23% vs 1.72%, P 0.1) with implementation of the CHT, and preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10). The odds ratio for a patient experiencing a preventable adverse event during cross‐coverage compared to noncross‐coverage time was reduced from 5.2 (95% confidence interval [CI], 1.518.2) to 1.5 (95% CI, 0.29.0) following implementation of the CHT (Table 3).
| Study | Outcomes of Interest | Results | Implication for CHT Design and Use |
|---|---|---|---|
| |||
| Ram and Block[25] (1992) | Physician satisfaction Importance and accessibility of clinical information | Improved physician satisfaction Handoff documentation more legible, more consistent, and more comprehensive Information required to be typed in by residents and not up‐to‐date | The most important data for handoff: a to do list and code status A CHT interfaced with hospital IT system, and in a format that can focus on physician needs |
| Petersen et al[21] (1998) | Adverse event rate Preventable adverse events rate | Fewer adverse events (2.38% vs 3.94%, P 0.001) Fewer preventable adverse events (1.23% vs 1.72%, P 0.1) Few preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10) Lower OR of preventable adverse events during cross‐coverage (1.5; 95% CI 0.29.0 vs 5.2; 95% CI 1.58.2) | Active involvement in the design of CHT by house staff likely contributes to high participation and CHT use rate in the study |
| Van Eaton et al[20] (2005) | No. of patients missed on rounds Perception on continuity of care quality and workflow efficiency Daily self‐reported pre‐rounding and rounding times and tasks | Reduced the no. of patients missed on rounds (2.5 patients/team/mo) (P = 0.0001) Spent 40% more time with patients at pre‐rounds Reduced time on team rounds by 1.5 min per patient Reduced time on manual copying at pre‐rounding by 50% Improved handoff quality Improved continuity of care No reduction of overall pre‐rounding time | The largest benefit from CHTs varies between clinical services, from more time assessing patients before rounds in Internal Medicine to reduced backtracking and locating patients in Surgery |
| Cheah et al[22] (2005) | Completeness and usefulness of handoff information Desirability of electronic handoff system | Identified information set for handoff Free text entry in CHT often deficient in particular patient information Concerns of the completeness and consistency of information delivered in CHT | CHT needs to be linked to hospital information system |
| Flanagan et al[23] (2009) | Common data elements of interest extracted during physician handoff Missing data required during handoff Physicians' perception of CHT | Additional important information needed that not included during handoff in 25% cases | Code status, relevant lab data, short‐term concerns, a problem list, and a if‐then list should be included in CHT template A standard form reduces variability of handoff information |
| Palma et al[24] (2011) | Accuracy of handoff information Healthcare provider satisfaction | Improved perceived accuracy of handoff information (91% vs 78%, P 0.01) Improved satisfaction with handoff process (71% vs 35%, P 0.01) Improved satisfaction with handoff documents (98% vs 91%, P 0.01) More time spent on updating handoff information (1620 min vs 1115 min, P = 0.03) | A discipline‐specific handoff tool results in perceived handoff accuracy and satisfaction A more efficient handoff tool can be achieved by more extensive data transfer from hospital IT system |
CHT's Impact on Physician Work Efficiency
Van Eaton et al's study examined the effect of CHTs on physician work efficiency.[20] Improved physician work efficiency was found following implementation of CHT. Self‐reported time spent on hand‐copying patient information was reduced by 50%, while the portion of time spent on seeing patients during pre‐rounding increased. Similarly, self‐reported time spent on each patient during rounding (routine patient assessment by the primary team) was decreased by 1.5 minutes. Overall, resident physicians subjectively reported an average time saving of 45 minutes daily for junior residents and 30 minutes for senior residents, and 81% of residents reported finishing their work sooner when using CHTs. Although no data were reported in the pre‐CHT period described in the study by Cheah et al, they indicated that work efficiency was felt to be improved because all physicians could locate their patients quickly and were pleased to be able to check patients' lab results in the CHT.[22] Conversely, Palma et al and Ram and Block reported perceived increased work load with CHTs by users due to time spent updating handoff information.[24, 25]
CHT's Impact on Quality of Physician Handoff
Overall quality of physician handoff and completeness of the handoff document was improved in 3 studies.[20, 24, 25] Flanagan et al reported that patient identifiers and medications were extracted most of the time.[23] However, there were concerns regarding consistency,[22] completeness[22, 23] of information provided during physician handoff using CHTs. Palma et al's and Ram and Block's studies[24, 25] commented on the accuracy of patient information communicated during physician handoff. While Ram and Block's study suggested that it may be poorer during the intervention period,[25] Palma et al's study found improved perceived accuracy of handoff information postimplementation of a CHT (98% vs 91%, P 0.01).[24]
CHT's Impact on Continuity of Patient Care
Using CHTs was associated with a decreased number of patients missed on rounds after handoff (new admitted patients who were not assessed by the primary team in the morning rounds because cross‐covering physicians did not inform the primary team) in Van Eaton et al's study.[20] On the other hand, Cheah et al[22] reported that documented handoffs after physicians returned to duty occurred on 50% of patients who had experienced important clinical events on weekends.
DISCUSSION
Our systematic review identified 6 controlled studies of CHT. Outcome parameters reported in these studies included quality of the handoff (including completeness, accuracy, and consistency), physician time management, continuity of care, adverse events, and missed patients. Our results suggest that while CHT are a promising tool, further evaluation using rigorous study methodologies is needed. These findings are somewhat surprising given increasing popularity of CHTs in daily patient care.[19, 24, 26, 27, 28] This might be due to the fact that IT adoption and use in healthcare is still in a phase of relative infancy,[29] and that the success of adopting IT systems in healthcare depends on various factors.[30]
Roles of CHT in Physician Handoff for Hospitalized Patients
Our study indicates that CHT can potentially improve continuity of patient care by reducing the number of missing patients during rounds following handoff,[20] and similarly improve patient safety by decreasing adverse events and preventable adverse events.[21] Of note, users reported that they were able to spend more time with patients during pre‐rounding[20] which will likely enhance quality and continuity of patient care. However, it is unclear whether these improvements translate into better patient outcomes. Although Peterson et al attempted to minimize the risk of bias by using anonymous reporting and blinding participants to the timing of data collection,[21] adverse events during the intervention period could have been underestimated due to surveillance bias or decreased self‐reporting. Nevertheless, the results suggest that CHTs may have affected quality of patient care in a positive manner from included studies.
The findings from our review also point to a positive impact of CHT on physician work efficiency. Specifically, residents spent less time rounding on patients after handoff and finished their work sooner after introduction of the intervention.[20] Several other published studies on CHT also indicated potential benefits on work efficiency and/or patient safety,[31, 33, 34, 35] although they did not meet the inclusion criteria for our study (prespecified outcomes not reported,[31, 35] or study design[33, 34, 35]). In the studies in which the majority of handoff information was manually typed in the CHT, the work load was perceived to be increased with CHT implementation.[24, 25] On the other hand, the study conducted by Van Eaton et al demonstrated that a CHT that had broad integration with the hospital main IT system, and could automatically transfer important patient information such as medication, medical problems, recent investigation, and vital signs into CHT, quickly gained popularity among residents and staff due to its user‐friendly features.[20] This integration can also potentially reduce miscommunication and associated medical errors during physician handoff. Palma et al's study reported higher perceived workload due to manual entry of patient data.[24] Although the CHT used in their study was developed within their existing EMR system, large amounts of information needed to be manually imputed, and thus increased time spent on updating handoff information. This information included patient demographics, active medical issues, a to do list, and on‐going issues,[24] some of which could be imputed automatically with better CHT design. It is also possible that users spent more time in updating the handoff because they were able to deliver more information using a CHT.[24] However, this may allow cross‐covering physicians to spend less time on looking for patient information from other sources and thus actually decrease workload during cross‐coverage. Although there are numerous factors that could affect physician work efficiency when using a new IT system,[30] it was felt that a well‐designed and easy‐to‐use CHT that is integrated with the hospital information system can improve physician productivity.
The role of CHT in improving quality of handoff is less clear. Three studies[20, 24, 25] found an overall improvement in the quality of handoff after implementation of CHT, such that the handoff information was more complete and more consistent. On the other hand, physicians were concerned about the comprehensiveness of physician handoff after implementation of CHT in 2 studies.[22, 23] In Ram and Block's study,[25] physicians relied heavily on an unstructured free‐text entry system to deliver the majority of patient information that physicians thought to be important. In Flanagan et al's study,[23] resident physicians had to search for alternative sources, such as patient charts and electronic order systems, to obtain vital information in many cases in spite of a structured CHT. As a result, the information available was often not sufficient to help on‐call physicians make patient care decisions.[23]
Implication of CHT Design and Use
It has been demonstrated in many non‐healthcare domains,[15, 36, 37] as well as nursing care,[38] that a standardized handoff protocol is vital to decrease medical errors and improve patient safety. In our review, we found that physicians generally reported being satisfied with the accuracy of handoff information and the overall handoff when using standardized CHTs interfaced with hospital IT systems. This suggests, as recommended by Flanagan et al,[23] Palma et al,[24] and Ram and Block[25] that CHTs be developed with a standardized protocol and wide integration into hospital IT systems.
In order to achieve this goal, key patient information necessary for patient care need to be communicated during physician handoff. As hospitals consist of a wide range of disciplines and specialties with varying cultures and focuses of patient information, it is likely difficult to develop a single panacea CHT template for all the in‐hospital services.[1] This may be even particularly relevant when developing CHTs for different hospital services. However, some patient information appears to be universally important for physician handoff for inpatient care. Key elements, such as patient demographics, diagnosis, outstanding investigation results, code status, a problem list, and a to do list, were noted to be consistently present in the CHTs that were evaluated in our review (Table 2). Other studies have also demonstrated that information items such as a to do list, outstanding investigation results, and patients' code status were regarded as the most important information during physician handoff.[1, 2, 17, 23, 39, 40] Based on these findings, a potential solution for CHT standardization would be to develop a core CHT which includes the universally important components of physician handoff identified in this review, and provides options for adding well‐categorized service‐specific information as needed (eg, type and date of surgical procedures for surgical patients). It also appears that active involvement of physicians in CHT design and modification facilitates successful implementation of CHT, as demonstrated in Van Eaton et al's and Peterson et al's studies.[20, 21]
It is difficult to recommend metrics for CHT evaluation based on the limited literature identified in our review. However, it appears to be reasonable to consider integration into existing IT system, user friendly features, impact on quality of handoff documents, work efficiency, and processes and outcomes of patient care when assessing CHTs.
Limitations
There are several limitations in the studies included in our review. None of the studies were multi‐centered. The majority of the included studies had a beforeafter design.[21, 22, 23, 24, 25] Some studies did not have user training or a run in period to ensure familiarity of CHTs by users.[22, 24, 25] None of the studies described the key components of handoff in the control groups, or used quality control measurements for user familiarity with the CHTs. Furthermore, outcomes reported by the studies were heterogeneous, subjective, based on participant self‐report, and not independently validated.
Our review also has also several limitations. First, in spite of a comprehensive search effort, it is possible that we failed to identify all relevant articles. However, this is unlikely, given that we searched multiple databases and performed hand searches of all references identified from the included articles, as well as content‐related previously published systematic reviews. Second, we were not able to perform a meta‐analysis, given the heterogeneity seen in outcomes assessed across studies, measures applied, and results presented.
CONCLUSIONS AND IMPLICATIONS FOR PRACTICE
Although the current literature suggests that implementation of CHTs is likely to improve physician work efficiency, satisfaction, and quality of patient care during physician handoff for hospitalized patients, the evidence supporting these potential benefits is limited. Furthermore, it is unknown what impacts CHTs may have on clinical outcomes, such as hospital length of stay and mortality. Further studies with larger sample size, multiple center involvement, and more objective patient outcome measurements are therefore needed to evaluate the roles of CHTs in physician handoff and improving the quality of patient care.
In the absence of larger studies evaluating major clinical outcomes, such as length of stay and mortality, hospitals considering innovations in the domain of computerized platforms for physician handoffs will need to consider the pros and cons of immediate system implementation on the basis of the evidence presented here versus waiting until there is more evidence from more definitive studies. In addition, our study suggests that organizations engage physicians during CHT design and develop a standardized CHT protocol that is interfaced with hospital IT systems and includes key components of handoff information, but provides flexibility to meet service‐specific needs. The evidence summarized here, while far from definitive for major outcomes, is nonetheless rather positive for the general benefits of CHTan impetus for careful design, implementation, and modification, whenever and wherever possible. Any such system implementations should, however, incorporate an evaluative component so that the evidence‐base surrounding CHT can be enhanced.
Acknowledgments
Disclosure: Nothing to report.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- World Health Organization. Patient safety solution: communication during patient handovers. Available at: http://www.who.int/patientsafety/solutions/patientsafety/PS‐Solution3.pdf Accessed January 20, 2011.
- Accreditation Canada. Required Organizational Practices: Communication. Available at: http://wwwaccreditationca/uploadedFiles/information%20transferpdf?n=1212. Accessed January 20, 2010.
- Joint Commission on Accreditation of Healthcare Organizations National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.html. Accessed January 20, 2010.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- . Fumbled handoffs: one dropped ball after another. Ann Intern Med. 2005;142(5):352–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , et al. Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167(19):2030–2036.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , . Utility of a standardized sign‐out card for new medical interns. J Gen Intern Med. 1996;11(12):753–755.
- , , , et al. Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , . Enhancing patient safety: improving the patient handoff process through appreciative inquiry. J Nurs Adm. 2007;37(2):95–104.
- , , , et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , et al. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , et al. A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , , et al. Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improve. 1998;24(2):77–87.
- , , , . Electronic medical handover: towards safer medical care. Med J Aust. 2005;183(7):369–372.
- , , , . Evaluation of a physician informatics tool to improve patient handoffs. J Am Med Inform Assoc. 2009;16(4):509–515.
- , , . Impact of electronic medical record integration of a handoff tool on sign‐out in a newborn intensive care unit. J Perinatol. 2011;31(5):311–317.
- , . Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods. Proceedings—The Annual Symposium on Computer Applications in Medical Care. 1992;114–118.
- , . MediSign: using a Web‐based SignOut system to improve provider identification. Proc AMIA Symp. 1999:550–554.
- , , , et al. Implementation of electronic medical records in hospitals: two case studies. Health Policy. 2007;84(2–3):181–190.
- , . Signing on to sign out, part 2: describing the success of a Web‐based patient sign‐out application and how it will serve as a platform for an electronic discharge summary program. Healthc Q. 2007;10(1):120–124.
- , , , et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Affairs. 2005;24(5):1103–1117.
- , , , et al. Interventions for promoting information and communication technologies adoption in healthcare professionals. Cochrane Database Syst Rev. 2009;Jan21(1):CD006093.
- , , . Improving physician communication through an automated, integrated sign‐out system. J Healthc Inf Manag. 2005;19(4):68–74.
- , , , et al. SynopSIS: integrating physician sign‐out with the electronic medical record. J Hosp Med. 2007;2(5):336–342.
- , , , . Improved physician work flow after integrating sign‐out notes into the electronic medical record. Jt Comm J Qual Patient Saf. 2010;36(2):72–78.
- , , , et al. Electronic inpatient whiteboards: improving multidisciplinary communication and coordination of care. Int J Med Inform. 2009;78(4):239–247.
- , , , et al. Systematically improving physician assignment during in‐hospital transitions of care by enhancing a preexisting hospital electronic health record. J Hosp Med. 2009;4(5):308–312.
- . On error management: lessons from aviation. BMJ. 2000;320(7237):781–785.
- , , , . There is more to monitoring a nuclear power plant than meets the eye. Hum Factors 2000;42(1):36–55.
- , , . Handoffs in care—can we make them safer?Pediatr Clin North Am. 2006;53(6):1185–1195.
- , , , et al. The top 10 list for a safe and effective sign‐out. Arch Surg. 2008;143(10):1008–1010.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
Physician handoff is a common and essential component of daily patient care that includes transfer of important clinical patient information and accountability of patient care. Thus, high‐quality physician handoffs are crucial to ensure patient safety and continuity of patient care, especially with the new resident work hour restriction in North America.[1, 2] As such, healthcare organizations including the World Health Organization[3] have issued specific goals and organizational challenges to improve the effectiveness and coordination of communication among the care/service providers and with the recipients of care/service across the continuum in healthcare.[4, 5]
It has been well‐documented that physician handoffs in hospital settings are often unstructured and not standardized, which leads to medical errors and jeopardizes patient safety.[2, 6, 7, 8, 9, 10, 11, 12] This lack of standardization of physician handoff for hospitalized patients occurs in every major in‐hospital service and affects trainees and staff.[2, 6, 7, 9, 10, 12, 13] It has been demonstrated in healthcare and in other domains that a standardized handoff protocol that involves both verbal communication and written handoff documents is likely to be an effective method of handoff to decrease miscommunication and associated errors.[14, 15, 16, 17] Computerized physician handoff tools (CHTs) have been increasingly deployed to address these challenges and have quickly gained popularity among physicians for documenting patient information during physician handoff for hospitalized patients.[18] CHTs can be an complementary part of electronic medical record (EMR) systems, but not a substitute since their focus is to deliver concise and essential information vital for patient care during interfaces of patient care.
Two recent systematic reviews have examined information technology (IT) systems to promote the handoff process in healthcare.[17, 19] However, to our knowledge, there has not been a systematic review of the potential role of CHT in physician handoff and quality of patient care for hospitalized patients. We therefore conducted a systematic review to examine the current evidence for CHTs in physician handoff for hospitalized patients, focusing specifically on potential effects on continuity of patient care, physician work efficiency, quality of handoffs, and patient outcomes.
METHODS
Criteria for Considering Eligible Studies
We included randomized controlled trials, controlled clinical trial, quasi‐experimental studies, and controlled beforeafter studies that evaluated CHTs during physician handoff of hospitalized patients. Studies needed to report patient outcomes (adverse events, missing patients at rounds, or in‐hospital mortality), physician work efficiency, quality of handoff (accuracy, consistency, or completeness), continuity of care, or physician satisfaction. Articles that met all these inclusion criteria were considered to be eligible for the review. We excluded review articles, commentaries, case reports, and retrospective studies.
Search Strategy
CHTs were defined as computer‐based platforms, designed specifically for the purpose of physician handoff, to allow distributed access and synchronous archiving of patient information via Internet protocols (ie, electronic tool to allow physician data access and data entry for handoff from different computers at multiple locations within the authorized hospitals or clinics). A search strategy was developed based on a MEDLINE search format combined with our inclusion criteria and with this definition of CHTs. We used search terms related to physician communication and information technology, and relevant Medical Subjects Headings, which include handover, handoff, signoff, sign‐over, off‐duty, post‐call, computerized, Web‐based, communication tool. The databases, including MEDLINE, PUBMED, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane database for systematic reviews, and the Cochrane CENTRAL register of controlled trials, were initially searched from 1985 to December 2011 in all languages. The Cochrane Collaboration filter for controlled interventional studies was used to select the above‐mentioned interventional trial designs. In addition, the first 2 authors hand searched the references of included articles and relevant systematic reviews.
Screening for Eligible Studies
All articles identified in the database searches described above were included for screening in 2 stages. First, 2 reviewers (P.L., S.A.) independently reviewed the title and abstracts of the identified articles for eligibility. The articles selected in the first stage of screening were then further assessed by a full‐text review independently by the 2 reviewers. Any discrepancy was resolved by consensus or by involvement of a third reviewer (C.T.).
Data Abstraction and Analysis
Data abstraction from selected studies was conducted independently by 3 authors based on a predefined template. All discrepancies in this stage were resolved by consensus among the 3 authors. For each study, we analyzed study design, data collection, intervention, main outcomes, and components of physician handoffs in the study. Due to heterogeneity of study outcomes, measures used, and results, a meta‐analysis was not performed. Study outcomes, which included adverse events, missing patients at rounds, time spent on rounding patient, accuracy, consistency or completeness of handoff information, and continuity of care, were summarized.
RESULTS
Study Selection
A total of 1026 citations were identified in the initial search, of which 1006 studies did not evaluate CHT and were excluded by title and abstract screening. Of the 20 studies evaluated further by full‐text review, 5 were selected for the final analysis. One additional study was identified by hand searching references. The kappa score of inter‐reviewer agreement on article selection in the first stage of screening was 0.7, and for the second stage of article selection, kappa was 1.0. The reasons for exclusion in the second selection step are presented in Figure 1.

Study Characteristics
Of the 6 studies identified, 1 study was a randomized controlled trial[20] and the other 5 were controlled beforeafter studies.[21, 22, 23, 24, 25] All studies were conducted in teaching hospitals in English‐speaking high‐income countries. All were single‐center studies, except the study by Van Eaton et[20] that involved 2 centers. All the studies investigated physician handoffs conducted by trainees. Two studies included staff physicians.[22, 24] Van Eaton et al's study included general medical, general surgical, and subspecialty surgical services.[20] The other 5 studies assessed physician handoffs in family medicine,[25] internal medicine services,[21, 23] a surgical service,[22] and a neonatal intensive care unit.[24] The study by Van Eaton et al[20] enrolled the largest study population. The intervention or observation phase ranged from 1 month[20] to 6 months[24] (Table 1).
| Study | Design | Setting | Target Services | Intervention Group | Control Group | Data Collection and Validation |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Beforeafter study | 150‐bed urban hospital in USA | Family Medicine Residents (N = 7) | Patient no. not reported 1 mo of intervention No CHT training prior to the intervention reported | Patient no. not reported Traditional handoff note (on index card or previous list) Components of handoff note not reported | Questionnaire No data validation |
| Peterson et al[21] (1998) | Beforeafter study | 720‐bed tertiary care hospital in USA | All Internal Medicine Services Residents (N = 99) | 3747 patients 4 mo of intervention 8 wk of run‐in period | 1874 patients Handwritten handoff Components of handoff note not reported | Self‐report using e‐mail, report card, in person chart review for unreported adverse events in 250 samples |
| Van Eaton et al[20] (2005) | Randomized cross‐over trial | 450‐bed tertiary care hospital and a 368‐bed trauma center in USA | General Medicine, General Surgery, and Subspecialties Trauma Residents (N = 7 teams) | 8018 patients 14 wk of randomized crossover period 6 wk of run‐in period | 7569 patients Individual written lists, cards, a team‐developed computer‐generated spreadsheet Components of handoff note not reported | Telephone interview and anonymous online survey No validation of data |
| Cheah et al[22] (2005) | Beforesfter atudy | A 400‐bed regional teaching hospital in Australia | General Surgery Registrars and Residents (N = 714) | Patient no. not reported 3 mo of observation period (for weekend coverage only) No CHT training prior to the intervention reported | Patient no. not reported No description of pre‐intervention handoff method reported | In‐person interview and survey No validation of data |
| Flanagan et al[23] (2009) | Beforesfter atudy | Tertiary care hospital in USA | Internal Medicine, Medical Intensive Care Unit First‐year Residents (N = 35) | 1264 patient handoff forms 1 mo of observation Orientation session and 1 cross‐over shift of run‐in period | Patient no. not reported No description of pre‐CHT implementation handoff method reported | In‐person interview and survey No validation of data |
| Palma et al[24] (2011) | Beforeafter study | 304‐bed quaternary care women and children hospital in USA | NICU Attendings, Residents, Nursing staffs (N = 4652) | Patient no. not reported 6 mo of intervention of NICU handoff tool Instruction document by e‐mail and informal instructional session | Patient no. not reported A Microsoft‐based standalone handoff tool or EMR integrated Medical/Surgical handoff tool Components of handoff note not reported | Online survey No validation of data |
CHT Characteristics
Three CHTs were standalone applications designed specifically for physician handoffs.[20, 22, 25] The other 3 CHTs were add‐on functions to existing hospital Electric Medical Record (EMR) systems.[21, 23, 24] All CHTs except one[25] interfaced with existing EMR systems, allowing for variable degrees of data transfer depending on CHT design and the functionalities of the EMR systems. CHT users were actively involved in designing and modifying the CHTs in most of the studies.[20, 21, 23, 25] The characteristics of the CHTs were summarized in Table 2.
| Study | CHT Design | EMR Interface | Physician Daily Progress Note | Participants' Role in CHT Design | Components of CHT | Components That Require Manual Input |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Standalone application | No interface | Paper‐based | Designing | Patient demographics Medications Diagnosis Problem lists Comment line | All the information |
| Peterson et al[21] (1998) | A part of existing EMR | Bi‐directional interface | Paper‐based | Designing | Patient demographics Current medication Allergy Code status Recent lab value A problem list A to do list | A problem list A to do list |
| Van Eaton et al[20] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Designing and modifying | Patient demographics Diagnosis Medication Allergy Vital signs Lab and investigation A problem list A to do list | Diagnosis Medication A problem list A to do list |
| Cheah et al[22] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | No | Patient demographics Diagnosis Length of stay Recent investigations Free‐text note (Not standardized) | Free‐text note |
| Flanagan et al[23] (2009) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Evaluating and modifying | Patient demographics Medication Allergy Lab and investigation Physician daily note Free‐text note (not standardized) | Free‐text note (may contain assessment, a problem list, venous access, short‐term concerns and long‐term plan, and follow‐up tasks) |
| Palma et al[24] (2011) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Paper‐based | No | Patient demographics Lab and measurement Free‐text note (not standardized) | Free‐text note (including patient description, active medical issues, ongoing care and a to do list) |
CHT's Impact on Adverse Events
The impact of CHTs on preventable adverse events was evaluated in a single study by Peterson et al.[21] The authors defined an adverse event as an injury due to medical treatment which prolonged hospital stay or produced disability at discharge in the study. Preventability was determined by using a 6‐point scale and assessed independently by 3 reviewers. Fewer adverse events were found after implementation of CHTs (2.38% vs 3.94%, P 0.001). They also reported nonsignificant reductions in preventable adverse events (1.23% vs 1.72%, P 0.1) with implementation of the CHT, and preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10). The odds ratio for a patient experiencing a preventable adverse event during cross‐coverage compared to noncross‐coverage time was reduced from 5.2 (95% confidence interval [CI], 1.518.2) to 1.5 (95% CI, 0.29.0) following implementation of the CHT (Table 3).
| Study | Outcomes of Interest | Results | Implication for CHT Design and Use |
|---|---|---|---|
| |||
| Ram and Block[25] (1992) | Physician satisfaction Importance and accessibility of clinical information | Improved physician satisfaction Handoff documentation more legible, more consistent, and more comprehensive Information required to be typed in by residents and not up‐to‐date | The most important data for handoff: a to do list and code status A CHT interfaced with hospital IT system, and in a format that can focus on physician needs |
| Petersen et al[21] (1998) | Adverse event rate Preventable adverse events rate | Fewer adverse events (2.38% vs 3.94%, P 0.001) Fewer preventable adverse events (1.23% vs 1.72%, P 0.1) Few preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10) Lower OR of preventable adverse events during cross‐coverage (1.5; 95% CI 0.29.0 vs 5.2; 95% CI 1.58.2) | Active involvement in the design of CHT by house staff likely contributes to high participation and CHT use rate in the study |
| Van Eaton et al[20] (2005) | No. of patients missed on rounds Perception on continuity of care quality and workflow efficiency Daily self‐reported pre‐rounding and rounding times and tasks | Reduced the no. of patients missed on rounds (2.5 patients/team/mo) (P = 0.0001) Spent 40% more time with patients at pre‐rounds Reduced time on team rounds by 1.5 min per patient Reduced time on manual copying at pre‐rounding by 50% Improved handoff quality Improved continuity of care No reduction of overall pre‐rounding time | The largest benefit from CHTs varies between clinical services, from more time assessing patients before rounds in Internal Medicine to reduced backtracking and locating patients in Surgery |
| Cheah et al[22] (2005) | Completeness and usefulness of handoff information Desirability of electronic handoff system | Identified information set for handoff Free text entry in CHT often deficient in particular patient information Concerns of the completeness and consistency of information delivered in CHT | CHT needs to be linked to hospital information system |
| Flanagan et al[23] (2009) | Common data elements of interest extracted during physician handoff Missing data required during handoff Physicians' perception of CHT | Additional important information needed that not included during handoff in 25% cases | Code status, relevant lab data, short‐term concerns, a problem list, and a if‐then list should be included in CHT template A standard form reduces variability of handoff information |
| Palma et al[24] (2011) | Accuracy of handoff information Healthcare provider satisfaction | Improved perceived accuracy of handoff information (91% vs 78%, P 0.01) Improved satisfaction with handoff process (71% vs 35%, P 0.01) Improved satisfaction with handoff documents (98% vs 91%, P 0.01) More time spent on updating handoff information (1620 min vs 1115 min, P = 0.03) | A discipline‐specific handoff tool results in perceived handoff accuracy and satisfaction A more efficient handoff tool can be achieved by more extensive data transfer from hospital IT system |
CHT's Impact on Physician Work Efficiency
Van Eaton et al's study examined the effect of CHTs on physician work efficiency.[20] Improved physician work efficiency was found following implementation of CHT. Self‐reported time spent on hand‐copying patient information was reduced by 50%, while the portion of time spent on seeing patients during pre‐rounding increased. Similarly, self‐reported time spent on each patient during rounding (routine patient assessment by the primary team) was decreased by 1.5 minutes. Overall, resident physicians subjectively reported an average time saving of 45 minutes daily for junior residents and 30 minutes for senior residents, and 81% of residents reported finishing their work sooner when using CHTs. Although no data were reported in the pre‐CHT period described in the study by Cheah et al, they indicated that work efficiency was felt to be improved because all physicians could locate their patients quickly and were pleased to be able to check patients' lab results in the CHT.[22] Conversely, Palma et al and Ram and Block reported perceived increased work load with CHTs by users due to time spent updating handoff information.[24, 25]
CHT's Impact on Quality of Physician Handoff
Overall quality of physician handoff and completeness of the handoff document was improved in 3 studies.[20, 24, 25] Flanagan et al reported that patient identifiers and medications were extracted most of the time.[23] However, there were concerns regarding consistency,[22] completeness[22, 23] of information provided during physician handoff using CHTs. Palma et al's and Ram and Block's studies[24, 25] commented on the accuracy of patient information communicated during physician handoff. While Ram and Block's study suggested that it may be poorer during the intervention period,[25] Palma et al's study found improved perceived accuracy of handoff information postimplementation of a CHT (98% vs 91%, P 0.01).[24]
CHT's Impact on Continuity of Patient Care
Using CHTs was associated with a decreased number of patients missed on rounds after handoff (new admitted patients who were not assessed by the primary team in the morning rounds because cross‐covering physicians did not inform the primary team) in Van Eaton et al's study.[20] On the other hand, Cheah et al[22] reported that documented handoffs after physicians returned to duty occurred on 50% of patients who had experienced important clinical events on weekends.
DISCUSSION
Our systematic review identified 6 controlled studies of CHT. Outcome parameters reported in these studies included quality of the handoff (including completeness, accuracy, and consistency), physician time management, continuity of care, adverse events, and missed patients. Our results suggest that while CHT are a promising tool, further evaluation using rigorous study methodologies is needed. These findings are somewhat surprising given increasing popularity of CHTs in daily patient care.[19, 24, 26, 27, 28] This might be due to the fact that IT adoption and use in healthcare is still in a phase of relative infancy,[29] and that the success of adopting IT systems in healthcare depends on various factors.[30]
Roles of CHT in Physician Handoff for Hospitalized Patients
Our study indicates that CHT can potentially improve continuity of patient care by reducing the number of missing patients during rounds following handoff,[20] and similarly improve patient safety by decreasing adverse events and preventable adverse events.[21] Of note, users reported that they were able to spend more time with patients during pre‐rounding[20] which will likely enhance quality and continuity of patient care. However, it is unclear whether these improvements translate into better patient outcomes. Although Peterson et al attempted to minimize the risk of bias by using anonymous reporting and blinding participants to the timing of data collection,[21] adverse events during the intervention period could have been underestimated due to surveillance bias or decreased self‐reporting. Nevertheless, the results suggest that CHTs may have affected quality of patient care in a positive manner from included studies.
The findings from our review also point to a positive impact of CHT on physician work efficiency. Specifically, residents spent less time rounding on patients after handoff and finished their work sooner after introduction of the intervention.[20] Several other published studies on CHT also indicated potential benefits on work efficiency and/or patient safety,[31, 33, 34, 35] although they did not meet the inclusion criteria for our study (prespecified outcomes not reported,[31, 35] or study design[33, 34, 35]). In the studies in which the majority of handoff information was manually typed in the CHT, the work load was perceived to be increased with CHT implementation.[24, 25] On the other hand, the study conducted by Van Eaton et al demonstrated that a CHT that had broad integration with the hospital main IT system, and could automatically transfer important patient information such as medication, medical problems, recent investigation, and vital signs into CHT, quickly gained popularity among residents and staff due to its user‐friendly features.[20] This integration can also potentially reduce miscommunication and associated medical errors during physician handoff. Palma et al's study reported higher perceived workload due to manual entry of patient data.[24] Although the CHT used in their study was developed within their existing EMR system, large amounts of information needed to be manually imputed, and thus increased time spent on updating handoff information. This information included patient demographics, active medical issues, a to do list, and on‐going issues,[24] some of which could be imputed automatically with better CHT design. It is also possible that users spent more time in updating the handoff because they were able to deliver more information using a CHT.[24] However, this may allow cross‐covering physicians to spend less time on looking for patient information from other sources and thus actually decrease workload during cross‐coverage. Although there are numerous factors that could affect physician work efficiency when using a new IT system,[30] it was felt that a well‐designed and easy‐to‐use CHT that is integrated with the hospital information system can improve physician productivity.
The role of CHT in improving quality of handoff is less clear. Three studies[20, 24, 25] found an overall improvement in the quality of handoff after implementation of CHT, such that the handoff information was more complete and more consistent. On the other hand, physicians were concerned about the comprehensiveness of physician handoff after implementation of CHT in 2 studies.[22, 23] In Ram and Block's study,[25] physicians relied heavily on an unstructured free‐text entry system to deliver the majority of patient information that physicians thought to be important. In Flanagan et al's study,[23] resident physicians had to search for alternative sources, such as patient charts and electronic order systems, to obtain vital information in many cases in spite of a structured CHT. As a result, the information available was often not sufficient to help on‐call physicians make patient care decisions.[23]
Implication of CHT Design and Use
It has been demonstrated in many non‐healthcare domains,[15, 36, 37] as well as nursing care,[38] that a standardized handoff protocol is vital to decrease medical errors and improve patient safety. In our review, we found that physicians generally reported being satisfied with the accuracy of handoff information and the overall handoff when using standardized CHTs interfaced with hospital IT systems. This suggests, as recommended by Flanagan et al,[23] Palma et al,[24] and Ram and Block[25] that CHTs be developed with a standardized protocol and wide integration into hospital IT systems.
In order to achieve this goal, key patient information necessary for patient care need to be communicated during physician handoff. As hospitals consist of a wide range of disciplines and specialties with varying cultures and focuses of patient information, it is likely difficult to develop a single panacea CHT template for all the in‐hospital services.[1] This may be even particularly relevant when developing CHTs for different hospital services. However, some patient information appears to be universally important for physician handoff for inpatient care. Key elements, such as patient demographics, diagnosis, outstanding investigation results, code status, a problem list, and a to do list, were noted to be consistently present in the CHTs that were evaluated in our review (Table 2). Other studies have also demonstrated that information items such as a to do list, outstanding investigation results, and patients' code status were regarded as the most important information during physician handoff.[1, 2, 17, 23, 39, 40] Based on these findings, a potential solution for CHT standardization would be to develop a core CHT which includes the universally important components of physician handoff identified in this review, and provides options for adding well‐categorized service‐specific information as needed (eg, type and date of surgical procedures for surgical patients). It also appears that active involvement of physicians in CHT design and modification facilitates successful implementation of CHT, as demonstrated in Van Eaton et al's and Peterson et al's studies.[20, 21]
It is difficult to recommend metrics for CHT evaluation based on the limited literature identified in our review. However, it appears to be reasonable to consider integration into existing IT system, user friendly features, impact on quality of handoff documents, work efficiency, and processes and outcomes of patient care when assessing CHTs.
Limitations
There are several limitations in the studies included in our review. None of the studies were multi‐centered. The majority of the included studies had a beforeafter design.[21, 22, 23, 24, 25] Some studies did not have user training or a run in period to ensure familiarity of CHTs by users.[22, 24, 25] None of the studies described the key components of handoff in the control groups, or used quality control measurements for user familiarity with the CHTs. Furthermore, outcomes reported by the studies were heterogeneous, subjective, based on participant self‐report, and not independently validated.
Our review also has also several limitations. First, in spite of a comprehensive search effort, it is possible that we failed to identify all relevant articles. However, this is unlikely, given that we searched multiple databases and performed hand searches of all references identified from the included articles, as well as content‐related previously published systematic reviews. Second, we were not able to perform a meta‐analysis, given the heterogeneity seen in outcomes assessed across studies, measures applied, and results presented.
CONCLUSIONS AND IMPLICATIONS FOR PRACTICE
Although the current literature suggests that implementation of CHTs is likely to improve physician work efficiency, satisfaction, and quality of patient care during physician handoff for hospitalized patients, the evidence supporting these potential benefits is limited. Furthermore, it is unknown what impacts CHTs may have on clinical outcomes, such as hospital length of stay and mortality. Further studies with larger sample size, multiple center involvement, and more objective patient outcome measurements are therefore needed to evaluate the roles of CHTs in physician handoff and improving the quality of patient care.
In the absence of larger studies evaluating major clinical outcomes, such as length of stay and mortality, hospitals considering innovations in the domain of computerized platforms for physician handoffs will need to consider the pros and cons of immediate system implementation on the basis of the evidence presented here versus waiting until there is more evidence from more definitive studies. In addition, our study suggests that organizations engage physicians during CHT design and develop a standardized CHT protocol that is interfaced with hospital IT systems and includes key components of handoff information, but provides flexibility to meet service‐specific needs. The evidence summarized here, while far from definitive for major outcomes, is nonetheless rather positive for the general benefits of CHTan impetus for careful design, implementation, and modification, whenever and wherever possible. Any such system implementations should, however, incorporate an evaluative component so that the evidence‐base surrounding CHT can be enhanced.
Acknowledgments
Disclosure: Nothing to report.
Physician handoff is a common and essential component of daily patient care that includes transfer of important clinical patient information and accountability of patient care. Thus, high‐quality physician handoffs are crucial to ensure patient safety and continuity of patient care, especially with the new resident work hour restriction in North America.[1, 2] As such, healthcare organizations including the World Health Organization[3] have issued specific goals and organizational challenges to improve the effectiveness and coordination of communication among the care/service providers and with the recipients of care/service across the continuum in healthcare.[4, 5]
It has been well‐documented that physician handoffs in hospital settings are often unstructured and not standardized, which leads to medical errors and jeopardizes patient safety.[2, 6, 7, 8, 9, 10, 11, 12] This lack of standardization of physician handoff for hospitalized patients occurs in every major in‐hospital service and affects trainees and staff.[2, 6, 7, 9, 10, 12, 13] It has been demonstrated in healthcare and in other domains that a standardized handoff protocol that involves both verbal communication and written handoff documents is likely to be an effective method of handoff to decrease miscommunication and associated errors.[14, 15, 16, 17] Computerized physician handoff tools (CHTs) have been increasingly deployed to address these challenges and have quickly gained popularity among physicians for documenting patient information during physician handoff for hospitalized patients.[18] CHTs can be an complementary part of electronic medical record (EMR) systems, but not a substitute since their focus is to deliver concise and essential information vital for patient care during interfaces of patient care.
Two recent systematic reviews have examined information technology (IT) systems to promote the handoff process in healthcare.[17, 19] However, to our knowledge, there has not been a systematic review of the potential role of CHT in physician handoff and quality of patient care for hospitalized patients. We therefore conducted a systematic review to examine the current evidence for CHTs in physician handoff for hospitalized patients, focusing specifically on potential effects on continuity of patient care, physician work efficiency, quality of handoffs, and patient outcomes.
METHODS
Criteria for Considering Eligible Studies
We included randomized controlled trials, controlled clinical trial, quasi‐experimental studies, and controlled beforeafter studies that evaluated CHTs during physician handoff of hospitalized patients. Studies needed to report patient outcomes (adverse events, missing patients at rounds, or in‐hospital mortality), physician work efficiency, quality of handoff (accuracy, consistency, or completeness), continuity of care, or physician satisfaction. Articles that met all these inclusion criteria were considered to be eligible for the review. We excluded review articles, commentaries, case reports, and retrospective studies.
Search Strategy
CHTs were defined as computer‐based platforms, designed specifically for the purpose of physician handoff, to allow distributed access and synchronous archiving of patient information via Internet protocols (ie, electronic tool to allow physician data access and data entry for handoff from different computers at multiple locations within the authorized hospitals or clinics). A search strategy was developed based on a MEDLINE search format combined with our inclusion criteria and with this definition of CHTs. We used search terms related to physician communication and information technology, and relevant Medical Subjects Headings, which include handover, handoff, signoff, sign‐over, off‐duty, post‐call, computerized, Web‐based, communication tool. The databases, including MEDLINE, PUBMED, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Cochrane database for systematic reviews, and the Cochrane CENTRAL register of controlled trials, were initially searched from 1985 to December 2011 in all languages. The Cochrane Collaboration filter for controlled interventional studies was used to select the above‐mentioned interventional trial designs. In addition, the first 2 authors hand searched the references of included articles and relevant systematic reviews.
Screening for Eligible Studies
All articles identified in the database searches described above were included for screening in 2 stages. First, 2 reviewers (P.L., S.A.) independently reviewed the title and abstracts of the identified articles for eligibility. The articles selected in the first stage of screening were then further assessed by a full‐text review independently by the 2 reviewers. Any discrepancy was resolved by consensus or by involvement of a third reviewer (C.T.).
Data Abstraction and Analysis
Data abstraction from selected studies was conducted independently by 3 authors based on a predefined template. All discrepancies in this stage were resolved by consensus among the 3 authors. For each study, we analyzed study design, data collection, intervention, main outcomes, and components of physician handoffs in the study. Due to heterogeneity of study outcomes, measures used, and results, a meta‐analysis was not performed. Study outcomes, which included adverse events, missing patients at rounds, time spent on rounding patient, accuracy, consistency or completeness of handoff information, and continuity of care, were summarized.
RESULTS
Study Selection
A total of 1026 citations were identified in the initial search, of which 1006 studies did not evaluate CHT and were excluded by title and abstract screening. Of the 20 studies evaluated further by full‐text review, 5 were selected for the final analysis. One additional study was identified by hand searching references. The kappa score of inter‐reviewer agreement on article selection in the first stage of screening was 0.7, and for the second stage of article selection, kappa was 1.0. The reasons for exclusion in the second selection step are presented in Figure 1.

Study Characteristics
Of the 6 studies identified, 1 study was a randomized controlled trial[20] and the other 5 were controlled beforeafter studies.[21, 22, 23, 24, 25] All studies were conducted in teaching hospitals in English‐speaking high‐income countries. All were single‐center studies, except the study by Van Eaton et[20] that involved 2 centers. All the studies investigated physician handoffs conducted by trainees. Two studies included staff physicians.[22, 24] Van Eaton et al's study included general medical, general surgical, and subspecialty surgical services.[20] The other 5 studies assessed physician handoffs in family medicine,[25] internal medicine services,[21, 23] a surgical service,[22] and a neonatal intensive care unit.[24] The study by Van Eaton et al[20] enrolled the largest study population. The intervention or observation phase ranged from 1 month[20] to 6 months[24] (Table 1).
| Study | Design | Setting | Target Services | Intervention Group | Control Group | Data Collection and Validation |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Beforeafter study | 150‐bed urban hospital in USA | Family Medicine Residents (N = 7) | Patient no. not reported 1 mo of intervention No CHT training prior to the intervention reported | Patient no. not reported Traditional handoff note (on index card or previous list) Components of handoff note not reported | Questionnaire No data validation |
| Peterson et al[21] (1998) | Beforeafter study | 720‐bed tertiary care hospital in USA | All Internal Medicine Services Residents (N = 99) | 3747 patients 4 mo of intervention 8 wk of run‐in period | 1874 patients Handwritten handoff Components of handoff note not reported | Self‐report using e‐mail, report card, in person chart review for unreported adverse events in 250 samples |
| Van Eaton et al[20] (2005) | Randomized cross‐over trial | 450‐bed tertiary care hospital and a 368‐bed trauma center in USA | General Medicine, General Surgery, and Subspecialties Trauma Residents (N = 7 teams) | 8018 patients 14 wk of randomized crossover period 6 wk of run‐in period | 7569 patients Individual written lists, cards, a team‐developed computer‐generated spreadsheet Components of handoff note not reported | Telephone interview and anonymous online survey No validation of data |
| Cheah et al[22] (2005) | Beforesfter atudy | A 400‐bed regional teaching hospital in Australia | General Surgery Registrars and Residents (N = 714) | Patient no. not reported 3 mo of observation period (for weekend coverage only) No CHT training prior to the intervention reported | Patient no. not reported No description of pre‐intervention handoff method reported | In‐person interview and survey No validation of data |
| Flanagan et al[23] (2009) | Beforesfter atudy | Tertiary care hospital in USA | Internal Medicine, Medical Intensive Care Unit First‐year Residents (N = 35) | 1264 patient handoff forms 1 mo of observation Orientation session and 1 cross‐over shift of run‐in period | Patient no. not reported No description of pre‐CHT implementation handoff method reported | In‐person interview and survey No validation of data |
| Palma et al[24] (2011) | Beforeafter study | 304‐bed quaternary care women and children hospital in USA | NICU Attendings, Residents, Nursing staffs (N = 4652) | Patient no. not reported 6 mo of intervention of NICU handoff tool Instruction document by e‐mail and informal instructional session | Patient no. not reported A Microsoft‐based standalone handoff tool or EMR integrated Medical/Surgical handoff tool Components of handoff note not reported | Online survey No validation of data |
CHT Characteristics
Three CHTs were standalone applications designed specifically for physician handoffs.[20, 22, 25] The other 3 CHTs were add‐on functions to existing hospital Electric Medical Record (EMR) systems.[21, 23, 24] All CHTs except one[25] interfaced with existing EMR systems, allowing for variable degrees of data transfer depending on CHT design and the functionalities of the EMR systems. CHT users were actively involved in designing and modifying the CHTs in most of the studies.[20, 21, 23, 25] The characteristics of the CHTs were summarized in Table 2.
| Study | CHT Design | EMR Interface | Physician Daily Progress Note | Participants' Role in CHT Design | Components of CHT | Components That Require Manual Input |
|---|---|---|---|---|---|---|
| ||||||
| Ram and Block[25] (1992) | Standalone application | No interface | Paper‐based | Designing | Patient demographics Medications Diagnosis Problem lists Comment line | All the information |
| Peterson et al[21] (1998) | A part of existing EMR | Bi‐directional interface | Paper‐based | Designing | Patient demographics Current medication Allergy Code status Recent lab value A problem list A to do list | A problem list A to do list |
| Van Eaton et al[20] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Designing and modifying | Patient demographics Diagnosis Medication Allergy Vital signs Lab and investigation A problem list A to do list | Diagnosis Medication A problem list A to do list |
| Cheah et al[22] (2005) | Standalone application | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | No | Patient demographics Diagnosis Length of stay Recent investigations Free‐text note (Not standardized) | Free‐text note |
| Flanagan et al[23] (2009) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Electronic‐based | Evaluating and modifying | Patient demographics Medication Allergy Lab and investigation Physician daily note Free‐text note (not standardized) | Free‐text note (may contain assessment, a problem list, venous access, short‐term concerns and long‐term plan, and follow‐up tasks) |
| Palma et al[24] (2011) | A part of existing EMR | Uni‐directional interface (data input from hospital IT system) | Paper‐based | No | Patient demographics Lab and measurement Free‐text note (not standardized) | Free‐text note (including patient description, active medical issues, ongoing care and a to do list) |
CHT's Impact on Adverse Events
The impact of CHTs on preventable adverse events was evaluated in a single study by Peterson et al.[21] The authors defined an adverse event as an injury due to medical treatment which prolonged hospital stay or produced disability at discharge in the study. Preventability was determined by using a 6‐point scale and assessed independently by 3 reviewers. Fewer adverse events were found after implementation of CHTs (2.38% vs 3.94%, P 0.001). They also reported nonsignificant reductions in preventable adverse events (1.23% vs 1.72%, P 0.1) with implementation of the CHT, and preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10). The odds ratio for a patient experiencing a preventable adverse event during cross‐coverage compared to noncross‐coverage time was reduced from 5.2 (95% confidence interval [CI], 1.518.2) to 1.5 (95% CI, 0.29.0) following implementation of the CHT (Table 3).
| Study | Outcomes of Interest | Results | Implication for CHT Design and Use |
|---|---|---|---|
| |||
| Ram and Block[25] (1992) | Physician satisfaction Importance and accessibility of clinical information | Improved physician satisfaction Handoff documentation more legible, more consistent, and more comprehensive Information required to be typed in by residents and not up‐to‐date | The most important data for handoff: a to do list and code status A CHT interfaced with hospital IT system, and in a format that can focus on physician needs |
| Petersen et al[21] (1998) | Adverse event rate Preventable adverse events rate | Fewer adverse events (2.38% vs 3.94%, P 0.001) Fewer preventable adverse events (1.23% vs 1.72%, P 0.1) Few preventable adverse events during cross‐coverage (0.24% vs 0.38%, P > 0.10) Lower OR of preventable adverse events during cross‐coverage (1.5; 95% CI 0.29.0 vs 5.2; 95% CI 1.58.2) | Active involvement in the design of CHT by house staff likely contributes to high participation and CHT use rate in the study |
| Van Eaton et al[20] (2005) | No. of patients missed on rounds Perception on continuity of care quality and workflow efficiency Daily self‐reported pre‐rounding and rounding times and tasks | Reduced the no. of patients missed on rounds (2.5 patients/team/mo) (P = 0.0001) Spent 40% more time with patients at pre‐rounds Reduced time on team rounds by 1.5 min per patient Reduced time on manual copying at pre‐rounding by 50% Improved handoff quality Improved continuity of care No reduction of overall pre‐rounding time | The largest benefit from CHTs varies between clinical services, from more time assessing patients before rounds in Internal Medicine to reduced backtracking and locating patients in Surgery |
| Cheah et al[22] (2005) | Completeness and usefulness of handoff information Desirability of electronic handoff system | Identified information set for handoff Free text entry in CHT often deficient in particular patient information Concerns of the completeness and consistency of information delivered in CHT | CHT needs to be linked to hospital information system |
| Flanagan et al[23] (2009) | Common data elements of interest extracted during physician handoff Missing data required during handoff Physicians' perception of CHT | Additional important information needed that not included during handoff in 25% cases | Code status, relevant lab data, short‐term concerns, a problem list, and a if‐then list should be included in CHT template A standard form reduces variability of handoff information |
| Palma et al[24] (2011) | Accuracy of handoff information Healthcare provider satisfaction | Improved perceived accuracy of handoff information (91% vs 78%, P 0.01) Improved satisfaction with handoff process (71% vs 35%, P 0.01) Improved satisfaction with handoff documents (98% vs 91%, P 0.01) More time spent on updating handoff information (1620 min vs 1115 min, P = 0.03) | A discipline‐specific handoff tool results in perceived handoff accuracy and satisfaction A more efficient handoff tool can be achieved by more extensive data transfer from hospital IT system |
CHT's Impact on Physician Work Efficiency
Van Eaton et al's study examined the effect of CHTs on physician work efficiency.[20] Improved physician work efficiency was found following implementation of CHT. Self‐reported time spent on hand‐copying patient information was reduced by 50%, while the portion of time spent on seeing patients during pre‐rounding increased. Similarly, self‐reported time spent on each patient during rounding (routine patient assessment by the primary team) was decreased by 1.5 minutes. Overall, resident physicians subjectively reported an average time saving of 45 minutes daily for junior residents and 30 minutes for senior residents, and 81% of residents reported finishing their work sooner when using CHTs. Although no data were reported in the pre‐CHT period described in the study by Cheah et al, they indicated that work efficiency was felt to be improved because all physicians could locate their patients quickly and were pleased to be able to check patients' lab results in the CHT.[22] Conversely, Palma et al and Ram and Block reported perceived increased work load with CHTs by users due to time spent updating handoff information.[24, 25]
CHT's Impact on Quality of Physician Handoff
Overall quality of physician handoff and completeness of the handoff document was improved in 3 studies.[20, 24, 25] Flanagan et al reported that patient identifiers and medications were extracted most of the time.[23] However, there were concerns regarding consistency,[22] completeness[22, 23] of information provided during physician handoff using CHTs. Palma et al's and Ram and Block's studies[24, 25] commented on the accuracy of patient information communicated during physician handoff. While Ram and Block's study suggested that it may be poorer during the intervention period,[25] Palma et al's study found improved perceived accuracy of handoff information postimplementation of a CHT (98% vs 91%, P 0.01).[24]
CHT's Impact on Continuity of Patient Care
Using CHTs was associated with a decreased number of patients missed on rounds after handoff (new admitted patients who were not assessed by the primary team in the morning rounds because cross‐covering physicians did not inform the primary team) in Van Eaton et al's study.[20] On the other hand, Cheah et al[22] reported that documented handoffs after physicians returned to duty occurred on 50% of patients who had experienced important clinical events on weekends.
DISCUSSION
Our systematic review identified 6 controlled studies of CHT. Outcome parameters reported in these studies included quality of the handoff (including completeness, accuracy, and consistency), physician time management, continuity of care, adverse events, and missed patients. Our results suggest that while CHT are a promising tool, further evaluation using rigorous study methodologies is needed. These findings are somewhat surprising given increasing popularity of CHTs in daily patient care.[19, 24, 26, 27, 28] This might be due to the fact that IT adoption and use in healthcare is still in a phase of relative infancy,[29] and that the success of adopting IT systems in healthcare depends on various factors.[30]
Roles of CHT in Physician Handoff for Hospitalized Patients
Our study indicates that CHT can potentially improve continuity of patient care by reducing the number of missing patients during rounds following handoff,[20] and similarly improve patient safety by decreasing adverse events and preventable adverse events.[21] Of note, users reported that they were able to spend more time with patients during pre‐rounding[20] which will likely enhance quality and continuity of patient care. However, it is unclear whether these improvements translate into better patient outcomes. Although Peterson et al attempted to minimize the risk of bias by using anonymous reporting and blinding participants to the timing of data collection,[21] adverse events during the intervention period could have been underestimated due to surveillance bias or decreased self‐reporting. Nevertheless, the results suggest that CHTs may have affected quality of patient care in a positive manner from included studies.
The findings from our review also point to a positive impact of CHT on physician work efficiency. Specifically, residents spent less time rounding on patients after handoff and finished their work sooner after introduction of the intervention.[20] Several other published studies on CHT also indicated potential benefits on work efficiency and/or patient safety,[31, 33, 34, 35] although they did not meet the inclusion criteria for our study (prespecified outcomes not reported,[31, 35] or study design[33, 34, 35]). In the studies in which the majority of handoff information was manually typed in the CHT, the work load was perceived to be increased with CHT implementation.[24, 25] On the other hand, the study conducted by Van Eaton et al demonstrated that a CHT that had broad integration with the hospital main IT system, and could automatically transfer important patient information such as medication, medical problems, recent investigation, and vital signs into CHT, quickly gained popularity among residents and staff due to its user‐friendly features.[20] This integration can also potentially reduce miscommunication and associated medical errors during physician handoff. Palma et al's study reported higher perceived workload due to manual entry of patient data.[24] Although the CHT used in their study was developed within their existing EMR system, large amounts of information needed to be manually imputed, and thus increased time spent on updating handoff information. This information included patient demographics, active medical issues, a to do list, and on‐going issues,[24] some of which could be imputed automatically with better CHT design. It is also possible that users spent more time in updating the handoff because they were able to deliver more information using a CHT.[24] However, this may allow cross‐covering physicians to spend less time on looking for patient information from other sources and thus actually decrease workload during cross‐coverage. Although there are numerous factors that could affect physician work efficiency when using a new IT system,[30] it was felt that a well‐designed and easy‐to‐use CHT that is integrated with the hospital information system can improve physician productivity.
The role of CHT in improving quality of handoff is less clear. Three studies[20, 24, 25] found an overall improvement in the quality of handoff after implementation of CHT, such that the handoff information was more complete and more consistent. On the other hand, physicians were concerned about the comprehensiveness of physician handoff after implementation of CHT in 2 studies.[22, 23] In Ram and Block's study,[25] physicians relied heavily on an unstructured free‐text entry system to deliver the majority of patient information that physicians thought to be important. In Flanagan et al's study,[23] resident physicians had to search for alternative sources, such as patient charts and electronic order systems, to obtain vital information in many cases in spite of a structured CHT. As a result, the information available was often not sufficient to help on‐call physicians make patient care decisions.[23]
Implication of CHT Design and Use
It has been demonstrated in many non‐healthcare domains,[15, 36, 37] as well as nursing care,[38] that a standardized handoff protocol is vital to decrease medical errors and improve patient safety. In our review, we found that physicians generally reported being satisfied with the accuracy of handoff information and the overall handoff when using standardized CHTs interfaced with hospital IT systems. This suggests, as recommended by Flanagan et al,[23] Palma et al,[24] and Ram and Block[25] that CHTs be developed with a standardized protocol and wide integration into hospital IT systems.
In order to achieve this goal, key patient information necessary for patient care need to be communicated during physician handoff. As hospitals consist of a wide range of disciplines and specialties with varying cultures and focuses of patient information, it is likely difficult to develop a single panacea CHT template for all the in‐hospital services.[1] This may be even particularly relevant when developing CHTs for different hospital services. However, some patient information appears to be universally important for physician handoff for inpatient care. Key elements, such as patient demographics, diagnosis, outstanding investigation results, code status, a problem list, and a to do list, were noted to be consistently present in the CHTs that were evaluated in our review (Table 2). Other studies have also demonstrated that information items such as a to do list, outstanding investigation results, and patients' code status were regarded as the most important information during physician handoff.[1, 2, 17, 23, 39, 40] Based on these findings, a potential solution for CHT standardization would be to develop a core CHT which includes the universally important components of physician handoff identified in this review, and provides options for adding well‐categorized service‐specific information as needed (eg, type and date of surgical procedures for surgical patients). It also appears that active involvement of physicians in CHT design and modification facilitates successful implementation of CHT, as demonstrated in Van Eaton et al's and Peterson et al's studies.[20, 21]
It is difficult to recommend metrics for CHT evaluation based on the limited literature identified in our review. However, it appears to be reasonable to consider integration into existing IT system, user friendly features, impact on quality of handoff documents, work efficiency, and processes and outcomes of patient care when assessing CHTs.
Limitations
There are several limitations in the studies included in our review. None of the studies were multi‐centered. The majority of the included studies had a beforeafter design.[21, 22, 23, 24, 25] Some studies did not have user training or a run in period to ensure familiarity of CHTs by users.[22, 24, 25] None of the studies described the key components of handoff in the control groups, or used quality control measurements for user familiarity with the CHTs. Furthermore, outcomes reported by the studies were heterogeneous, subjective, based on participant self‐report, and not independently validated.
Our review also has also several limitations. First, in spite of a comprehensive search effort, it is possible that we failed to identify all relevant articles. However, this is unlikely, given that we searched multiple databases and performed hand searches of all references identified from the included articles, as well as content‐related previously published systematic reviews. Second, we were not able to perform a meta‐analysis, given the heterogeneity seen in outcomes assessed across studies, measures applied, and results presented.
CONCLUSIONS AND IMPLICATIONS FOR PRACTICE
Although the current literature suggests that implementation of CHTs is likely to improve physician work efficiency, satisfaction, and quality of patient care during physician handoff for hospitalized patients, the evidence supporting these potential benefits is limited. Furthermore, it is unknown what impacts CHTs may have on clinical outcomes, such as hospital length of stay and mortality. Further studies with larger sample size, multiple center involvement, and more objective patient outcome measurements are therefore needed to evaluate the roles of CHTs in physician handoff and improving the quality of patient care.
In the absence of larger studies evaluating major clinical outcomes, such as length of stay and mortality, hospitals considering innovations in the domain of computerized platforms for physician handoffs will need to consider the pros and cons of immediate system implementation on the basis of the evidence presented here versus waiting until there is more evidence from more definitive studies. In addition, our study suggests that organizations engage physicians during CHT design and develop a standardized CHT protocol that is interfaced with hospital IT systems and includes key components of handoff information, but provides flexibility to meet service‐specific needs. The evidence summarized here, while far from definitive for major outcomes, is nonetheless rather positive for the general benefits of CHTan impetus for careful design, implementation, and modification, whenever and wherever possible. Any such system implementations should, however, incorporate an evaluative component so that the evidence‐base surrounding CHT can be enhanced.
Acknowledgments
Disclosure: Nothing to report.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- World Health Organization. Patient safety solution: communication during patient handovers. Available at: http://www.who.int/patientsafety/solutions/patientsafety/PS‐Solution3.pdf Accessed January 20, 2011.
- Accreditation Canada. Required Organizational Practices: Communication. Available at: http://wwwaccreditationca/uploadedFiles/information%20transferpdf?n=1212. Accessed January 20, 2010.
- Joint Commission on Accreditation of Healthcare Organizations National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.html. Accessed January 20, 2010.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- . Fumbled handoffs: one dropped ball after another. Ann Intern Med. 2005;142(5):352–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , et al. Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167(19):2030–2036.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , . Utility of a standardized sign‐out card for new medical interns. J Gen Intern Med. 1996;11(12):753–755.
- , , , et al. Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , . Enhancing patient safety: improving the patient handoff process through appreciative inquiry. J Nurs Adm. 2007;37(2):95–104.
- , , , et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , et al. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , et al. A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , , et al. Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improve. 1998;24(2):77–87.
- , , , . Electronic medical handover: towards safer medical care. Med J Aust. 2005;183(7):369–372.
- , , , . Evaluation of a physician informatics tool to improve patient handoffs. J Am Med Inform Assoc. 2009;16(4):509–515.
- , , . Impact of electronic medical record integration of a handoff tool on sign‐out in a newborn intensive care unit. J Perinatol. 2011;31(5):311–317.
- , . Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods. Proceedings—The Annual Symposium on Computer Applications in Medical Care. 1992;114–118.
- , . MediSign: using a Web‐based SignOut system to improve provider identification. Proc AMIA Symp. 1999:550–554.
- , , , et al. Implementation of electronic medical records in hospitals: two case studies. Health Policy. 2007;84(2–3):181–190.
- , . Signing on to sign out, part 2: describing the success of a Web‐based patient sign‐out application and how it will serve as a platform for an electronic discharge summary program. Healthc Q. 2007;10(1):120–124.
- , , , et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Affairs. 2005;24(5):1103–1117.
- , , , et al. Interventions for promoting information and communication technologies adoption in healthcare professionals. Cochrane Database Syst Rev. 2009;Jan21(1):CD006093.
- , , . Improving physician communication through an automated, integrated sign‐out system. J Healthc Inf Manag. 2005;19(4):68–74.
- , , , et al. SynopSIS: integrating physician sign‐out with the electronic medical record. J Hosp Med. 2007;2(5):336–342.
- , , , . Improved physician work flow after integrating sign‐out notes into the electronic medical record. Jt Comm J Qual Patient Saf. 2010;36(2):72–78.
- , , , et al. Electronic inpatient whiteboards: improving multidisciplinary communication and coordination of care. Int J Med Inform. 2009;78(4):239–247.
- , , , et al. Systematically improving physician assignment during in‐hospital transitions of care by enhancing a preexisting hospital electronic health record. J Hosp Med. 2009;4(5):308–312.
- . On error management: lessons from aviation. BMJ. 2000;320(7237):781–785.
- , , , . There is more to monitoring a nuclear power plant than meets the eye. Hum Factors 2000;42(1):36–55.
- , , . Handoffs in care—can we make them safer?Pediatr Clin North Am. 2006;53(6):1185–1195.
- , , , et al. The top 10 list for a safe and effective sign‐out. Arch Surg. 2008;143(10):1008–1010.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , . Lost in translation: challenges and opportunities in physician‐to‐physician communication during patient handoffs. Acad Med. 2005;80(12):1094–1099.
- World Health Organization. Patient safety solution: communication during patient handovers. Available at: http://www.who.int/patientsafety/solutions/patientsafety/PS‐Solution3.pdf Accessed January 20, 2011.
- Accreditation Canada. Required Organizational Practices: Communication. Available at: http://wwwaccreditationca/uploadedFiles/information%20transferpdf?n=1212. Accessed January 20, 2010.
- Joint Commission on Accreditation of Healthcare Organizations National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.html. Accessed January 20, 2010.
- , , . Communicating in the “gray zone”: perceptions about emergency physician hospitalist handoffs and patient safety. Acad Emerg Med. 2007;14(10):884–894.
- . Fumbled handoffs: one dropped ball after another. Ann Intern Med. 2005;142(5):352–358.
- , , , . Transfers of patient care between house staff on internal medicine wards: a national survey. Arch Intern Med. 2006;166(11):1173–1177.
- , , , et al. Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , et al. What are covering doctors told about their patients? Analysis of sign‐out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248–255.
- , , , et al. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701–710.
- , , , . Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167(19):2030–2036.
- , , , et al. Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , . Utility of a standardized sign‐out card for new medical interns. J Gen Intern Med. 1996;11(12):753–755.
- , , , et al. Handoff strategies in settings with high consequences for failure: lessons for health care operations. Int J Qual Health Care. 2004;16(2):125–132.
- , , . Enhancing patient safety: improving the patient handoff process through appreciative inquiry. J Nurs Adm. 2007;37(2):95–104.
- , , , et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , , , . Organizing the transfer of patient care information: the development of a computerized resident sign‐out system. Surgery. 2004;136(1):5–13.
- , , , et al. Hospitalist handoffs: a systematic review and task force recommendations. J Hosp Med. 2009;4(7):433–440.
- , , , et al. A randomized, controlled trial evaluating the impact of a computerized rounding and sign‐out system on continuity of care and resident work hours. J Am Coll Surg. 2005;200(4):538–545.
- , , , et al. Using a computerized sign‐out program to improve continuity of inpatient care and prevent adverse events. Jt Comm J Qual Improve. 1998;24(2):77–87.
- , , , . Electronic medical handover: towards safer medical care. Med J Aust. 2005;183(7):369–372.
- , , , . Evaluation of a physician informatics tool to improve patient handoffs. J Am Med Inform Assoc. 2009;16(4):509–515.
- , , . Impact of electronic medical record integration of a handoff tool on sign‐out in a newborn intensive care unit. J Perinatol. 2011;31(5):311–317.
- , . Signing out patients for off‐hours coverage: comparison of manual and computer‐aided methods. Proceedings—The Annual Symposium on Computer Applications in Medical Care. 1992;114–118.
- , . MediSign: using a Web‐based SignOut system to improve provider identification. Proc AMIA Symp. 1999:550–554.
- , , , et al. Implementation of electronic medical records in hospitals: two case studies. Health Policy. 2007;84(2–3):181–190.
- , . Signing on to sign out, part 2: describing the success of a Web‐based patient sign‐out application and how it will serve as a platform for an electronic discharge summary program. Healthc Q. 2007;10(1):120–124.
- , , , et al. Can electronic medical record systems transform health care? Potential health benefits, savings, and costs. Health Affairs. 2005;24(5):1103–1117.
- , , , et al. Interventions for promoting information and communication technologies adoption in healthcare professionals. Cochrane Database Syst Rev. 2009;Jan21(1):CD006093.
- , , . Improving physician communication through an automated, integrated sign‐out system. J Healthc Inf Manag. 2005;19(4):68–74.
- , , , et al. SynopSIS: integrating physician sign‐out with the electronic medical record. J Hosp Med. 2007;2(5):336–342.
- , , , . Improved physician work flow after integrating sign‐out notes into the electronic medical record. Jt Comm J Qual Patient Saf. 2010;36(2):72–78.
- , , , et al. Electronic inpatient whiteboards: improving multidisciplinary communication and coordination of care. Int J Med Inform. 2009;78(4):239–247.
- , , , et al. Systematically improving physician assignment during in‐hospital transitions of care by enhancing a preexisting hospital electronic health record. J Hosp Med. 2009;4(5):308–312.
- . On error management: lessons from aviation. BMJ. 2000;320(7237):781–785.
- , , , . There is more to monitoring a nuclear power plant than meets the eye. Hum Factors 2000;42(1):36–55.
- , , . Handoffs in care—can we make them safer?Pediatr Clin North Am. 2006;53(6):1185–1195.
- , , , et al. The top 10 list for a safe and effective sign‐out. Arch Surg. 2008;143(10):1008–1010.
- , , . Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196–204.
Twelve Reasons for Considering Buprenorphine as a Frontline Analgesic in the Management of Pain
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Mellar P. Davis, MD, FCCP, FAAHPM
ABSTRACT: Buprenorphine is an opioid that has a complex and unique pharmacology which provides some advantages over other potent mu agonists. We review 12 reasons for considering buprenorphine as a frontline analgesic for moderate to severe pain: (1) Buprenorphine is effective in cancer pain; (2) buprenorphine is effective in treating neuropathic pain; (3) buprenorphine treats a broader array of pain phenotypes than do certain potent mu agonists, is associated with less analgesic tolerance, and can be combined with other mu agonists; (4) buprenorphine produces less constipation than do certain other potent mu agonists, and does not adversely affect the sphincter of Oddi; (5) buprenorphine has a ceiling effect on respiratory depression but not analgesia; (6) buprenorphine causes less cognitive impairment than do certain other opioids; (7) buprenorphine is not immunosuppressive like morphine and fentanyl; (8) buprenorphine does not adversely affect the hypothalamic-pituitary-adrenal axis or cause hypogonadism; (9) buprenorphine does not significantly prolong the QTc interval, and is associated with less sudden death than is methadone; (10) buprenorphine is a safe and effective analgesic for the elderly; (11) buprenorphine is one of the safest opioids to use in patients in renal failure and those on dialysis; and (12) withdrawal symptoms are milder and drug dependence is less with buprenorphine. In light of evidence for efficacy, safety, versatility, and cost, buprenorphine should be considered as a first-line analgesic.
*For a PDF of the full article and a Commentary by Paul Sloan, MD, click on the links to the left of this introduction.
Androgen deficiency in older men: Indications, advantages, and pitfalls of testosterone replacement therapy
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
KEY POINTS
- General health benefits and safety of TRT in asymptomatic patients are not clearly defined by current data.
- Treatment of low testosterone is discouraged in the absence of clinical symptoms.
- A morning serum testosterone should be obtained after ruling out other causes of symptoms. It should also be repeated to confirm androgen deficiency in older men.
- Androgen deficiency in older men is associated with metabolic syndrome, type 2 diabetes mellitus, obesity, osteoporosis, renal failure, anemia, and previous treatment with steroids or opiates.
- TRT in men with a history of prostate cancer remains controversial. The existing limited data suggest that TRT is safe after curative therapy for prostate cancer. Patients treated should be monitored closely and informed of the risks of cancer progression and recurrence while they are on TRT.
Emergency contraception: Separating fact from fiction
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
KEY POINTS
- Levonorgestrel-based emergency contraceptives such as Plan B One-Step, Next Choice, and generics are now available over the counter, which has the advantage of avoiding the delays and hassles of calling the doctor’s office and waiting for prescriptions. But patients still need our guidance on how and when to use emergency contraception.
- Even if patients now have easy access to over-the-counter emergency contraceptives, we physicians should take every opportunity to discuss effective contraceptive options with our patients.
- Ulipristal and copper intrauterine devices (ParaGard) are likely to be more effective than levonorgestrel and should be considered in women at highest risk of pregnancy, such as those who are obese.
- Prescribers should feel comfortable addressing tough questions about mechanisms of action, as controversies and myths about emergency contraception are regularly discussed in the media and on the Internet.
Tattooing: Medical uses and problems
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
People have been marking the skin with pigments for at least 4,000 years.1 Tattoos have been found on Egyptian mummies, and Roman gladiators are known to have used tattoos for identification.2 Tattooing was considered fashionable among royalty in the first half of the 20th century.3 And today it is perhaps more popular than ever.
But tattooing is not confined to popular culture and decoration. It has established uses in medicine, as well as other medically related uses that represent more recent trends. In this review, we explore the range of medical tattooing.
MEDICAL ALERT TATTOOING
Medical alert tattooing is a form of medical identification similar to medical alert jewelry, ie, bracelets and necklaces, to alert first-responders to a medical condition or to specific desires for care, such as do-not-resuscitate (DNR) directives.
Some people choose to have their medical condition tattooed rather than wear medical alert jewelry, which can break or be misplaced. 4–6
This practice is currently unregulated by the medical community, and the few reports of its use published to date include two people with diabetes who had the word “diabetic” tattooed on their bodies,4,5 and a woman with a tattoo warning of a past severe reaction to succinylcholine during anesthesia.6 She had been advised to wear medical alert jewelry, but she instead chose a tattoo.
Blood-type tattooing was briefly used in a few communities in the United States in the early 1950s as part of a program to provide a “walking blood bank.”7 However, the practice fell out of favor as physicians questioned the reliability of tattoos for medical information.7
This type of tattooing could also benefit patients with adrenal insufficiency, O-negative blood type, and allergies, and patients taking an anticoagulant drug (after discussing the risks of bleeding with their primary physician).
Emergency medical technicians are trained to search unresponsive patients for health-related items, including medical alert necklaces and bracelets. Since tattooing for disease identification purposes is not an officially recognized procedure, these personnel need to be aware that this practice is increasing among the general public. Identifying medical alert tattoos in emergency situations is much more difficult in people with extensive decorative tattooing.
Tattoos indicating health directives
Reports of people with tattoos indicating health directives (DNR, do-not-defibrillate) have prompted debate over the validity of tattoos as a type of advance directive.8–13 These types of tattoos pose practical and ethical problems: they may not reflect a person’s current wishes, and they may have even been applied as a joke.13 Furthermore, they are not recognized as meeting any of the legal requirements for advance directives, so they cannot be considered as valid health directives, but only as a way to guide treatment decisions.14
The same is true for the other ways of notifying first-responders to one’s treatment wishes, ie, wallet cards and medical alert bracelets and necklaces. One manufacturer of medical alert bracelets and necklaces offers to engrave that the wearer has a living will and to keep on file a copy of the document, which they can fax or read out loud to paramedics if they are contacted.11
Organ donor tattoo
In the case of a man who had his consent to be an organ donor tattooed on his chest,15 the tattoo was viewed as not equivalent to signed documentation; however, such tattoos can be used to help guide management.15
DIABETIC PATIENTS AND MEDICAL ALERT TATTOOS
Medical alert tattooing is increasingly common in people with diabetes. Discussions on social-networking sites on the Internet indicate that diabetic patients often do this on their own without consulting their physician.
In our clinic, we have encountered patients with tattoos on the wrist (Figure 1), similar to those seen on the Internet, typically displaying a six-pointed star of life, a caduceus (physician’s staff), and the word “diabetic.” Patients we have encountered in the past 3 to 4 years have cited the same rationale for resorting to medical tattooing—ie, the cost of repeatedly replacing broken and lost medical alert jewelry.
We believe there is a convincing rationale for diabetic patients to undergo medical tattooing, and we believe that diabetes organizations need to evaluate this and provide education to patients and clinicians about it, so that patients can discuss it with their care providers before taking action on their own.
Risks of tattooing in diabetic patients
Diabetic patients who ask their physician about getting a diabetes-alert tattoo should be informed about the dangers of tattooing in diabetes. The diabetes should be optimally controlled, as gauged by both hemoglobin A1c and mean blood glucose profile at the time of tattooing, in order to promote healing of the tattooed area and to prevent wound infection.
Also helpful is to advise diabetic patients to avoid tattooing of the feet or lower legs in view of the risk of diabetes-related neurovascular disease that may impair healing or incite infection.
RECONSTRUCTIVE AND COSMETIC TATTOOING
Areolar reconstruction
Breast reconstruction after mastectomy is fundamental to the psychosocial health of the patient and helps her regain a positive body image.16,17 Tattooing of the nipple-areola complex16 is usually the final step of the breast reconstruction process.
Complications of areolar tattooing are rare but can include local erythema and infection. 18 And patients should be informed that the tattoos will likely fade over time and require re-tattooing.18
Tattooing as camouflage
Tattooing is used to repigment the skin in conditions that cause hypopigmentation or hyperpigmentation, 2 including burns.19 It is also used as an alternative to laser treatment in port-wine stain and in cosmetic surgery of the scalp.20
Tattooing is used for micropigmentation of the lips and fingertips in patients who have vitiligo. However, this should be reserved for those with stable vitiligo, since tattooing may trigger another patch of vitiligo at tattoo sites.21
Although medical management exists for vitiligo, it is often ineffective for lip vitiligo since the success of medical therapy depends on the pigment-cell reservoir at the site of depigmentation. The lips lack such a reservoir of melanocytes, so tattooing may be an option.22
Corneal scarring
Perforating injury, measles keratitis, and other conditions can result in cosmetically disfiguring discoloration of the cornea. When microsurgical reconstruction is ineffective or is not an option, corneal tattooing has been reported to provide satisfactory results at up to 4 years.23 Reopacification, increased opacity, fading of the tattoo pigment, and epithelial growth have been reported, and in one series, most patients required reoperation.24
Tattooing to hide surgical scars
Spyropoulou and Fatah25 reported three patients in a plastic surgery practice who underwent decorative tattooing to camouflage cosmetically undesirable scars. The authors suggested this as a valid option, especially in younger patients, among whom tattooing is common and acceptable.25
‘Permanent makeup’
Tattooing is also used to simulate makeup (“permanent makeup”) and may be beneficial to people allergic to conventional makeup or people with disabilities that make applying makeup difficult.26 Complications of this procedure include bleeding, crusting, swelling, infection, allergic reactions, hypertrophic scars, keloid, loss of eyelashes, eyelid necrosis, and ectropion, as well as complications related to magnetic resonance imaging (described further below).
Most pigments used for this purpose do not have an established history of safe use, and patients may experience severe allergic reactions. A recent report described severe allergic reactions resistant to topical or systemic therapy with steroids in combination with topical tacrolimus (Prograf), especially after exposure to red dye 181.27 Researchers have recommended the regulation and control of colorants in permanent makeup.27
RADIATION ONCOLOGY
Tattooing is used in radiation oncology to ensure accurate targeting of radiation therapy. Typically, several small, black marks 1 to 2 mm in size are applied by a medical professional using an 18- or 19-gauge hypodermic needle and india ink.2 The marks are permanent.
Although these markings are clearly helpful during radiation treatment, they can be psychologically upsetting to patients, as they are a constant reminder of the disease and the treatment, both during the treatment course and long after it is finished.
An alternative is to use temporary marks for the 6 to 7 weeks that patients typically need them. However, temporary tattooing is prone to fading, and this is a key limitation.
ENDOSCOPIC TATTOOING
In laparoscopic gastrointestinal surgery, lesions are often difficult to visualize and localize since the surgeon is unable to palpate the bowel directly to identify the diseased segment; this increases the risk of resecting the wrong segment of bowel.28 Endoscopic tattooing of the segment to be resected greatly improves the accuracy of laparoscopic procedures. Endoscopic tattooing is also used to facilitate identification of subtle mucosal lesions or endoscopic resection sites at the time of subsequent endoscopy.29,30
India ink or a similar presterilized commercial preparation is commonly used.31 Complications are rare but include mild chronic inflammation, hyperplastic changes, inflammatory bowel disease, abdominal abscess, inflammatory pseudotumor, focal peritonitis, peritoneal staining, and, very rarely, seeding of tumor via the tattooing needle.30
FORENSIC MEDICINE
Specialists in forensic medicine use primary markers such as fingerprints and dental records and secondary markers such as birthmarks, scarring, and tattoos to identify victims.32 Tattoos are useful for identification when finger-prints or dental records are unavailable,33 as in the tsunami of December 2004 in Southeast Asia34 and the London Paddington train crash of October 1999.35 However, as the body decomposes, tattoos can discolor and fade, making them hard to identify. Application of 3% hydrogen peroxide to the tattoo site has been reported to aid in identification, and infrared imaging has shown promise.32
GENERAL RISKS AND COMPLICATIONS OF TATTOOING
Improper sterilization of tattooing needles and tattoo ink in public tattoo parlors can cause a wide range of diseases and skin reactions.36–44
Infection
Pyodermal infections can include temporary inflammation at the sites of needle punctures, superficial infections such as impetigo and ecthyma, and deeper infections such as cellulitis, erysipelas, and furunculosis.
Other transmissible infections include hepatitis, syphilis, leprosy, tuberculosis cutis, rubella, chancroid, tetanus, and molluscum contagiosum. An outbreak of infection with Mycobacterium chelonae from premixed tattoo ink has also been reported.44
Hepatitis C has been shown in epidemiologic studies to be transmissible via nonsterile needles. Human immunodeficiency virus is also theoretically transmissible this way, but this is difficult to confirm because the virus has a long incubation period.36
Cutaneous reactions
Skin reactions to tattooing include aseptic inflammation and acquired sensitivity to tattoo dyes, especially red dyes, but also to chromium in green dyes, cadmium in yellow dyes, and cobalt in blue dyes.38 The reaction can manifest as either allergic contact dermatitis or photoallergic dermatitis.
Cutaneous conditions that localize in tattooed areas include vaccinia, verruca vulgaris, herpes simplex, herpes zoster, psoriasis, lichen planus, keratosis follicularis (Darier disease), chronic discoid lupus erythematosus, and keratoacanthoma.
Other possible conditions include keloid, sarcoidal granuloma, erythema multiforme, localized scleroderma, and lymphadenopathy.36,37
Malignancy
Malignancies reported to arise within tattoos include squamous cell carcinoma, basal cell carcinoma, malignant melanoma, leiomyosarcoma, primary non-Hodgkin lymphoma, and dermatofibrosarcoma protuberans.39 These malignancies may be considered coincidental, but carcinogenicity of the tattooing colorants is a concern to be addressed. Nevertheless, a malignancy within a tattoo is more difficult to identify on skin examination.
Burns during magnetic resonance imaging
The metallic ferric acid pigments used in tattoos can conduct heat on the skin during magnetic resonance imaging,40 resulting in traumatic burns. This has also been reported to occur with tattoos with nonferrous pigments. 41 Patients should be asked before this procedure if they have tattooing so that this complication can be avoided.
Two other complications
Two interesting complications of tattooing have been described. First, tattoo pigments have been noted within lymph nodes in patients with melanoma.42 This finding during surgery could cause the surgeon to mistake tattoo pigment for disease and to complete a regional lymph node dissection if biopsy of the sentinel node is not performed.
The other involved disseminated hyperalgesia after volar wrist tattooing. The authors speculated that the pain associated with volar tattooing may have been related to the proximity of the tattoo to the palmar cutaneous branch of the median nerve.43
Acknowledgment: The authors would like to acknowledge the patients in Figure 1 for their permission to use their photos and Nicolas Kluger, MD, Departments of Dermatology, Allergology, and Venereology, University of Helsinki, Finland, for his input into an early draft of this manuscript.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
- Grumet GW. Psychodynamic implications of tattoos. Am J Orthopsychiatry 1983; 53:482–492.
- Vassileva S, Hristakieva E. Medical applications of tattooing. Clin Dermatol 2007; 25:367–374.
- van der Velden EM, de Jong BD, van der Walle HB, Stolz E, Naafs B. Tattooing and its medical aspects. Int J Dermatol 1993; 32:381–384.
- Nag S, McCulloch A. An informative tattoo. Postgrad Med J 2003; 79:402.
- Aldasouqi S. A medical alert tattoo. Am Fam Physician 2011; 83:796.
- Barclay P, King H. Tattoo medi-alert. Anaesthesia 2002; 57:625.
- Wolf EK, Laumann AE. The use of blood-type tattoos during the Cold War. J Am Acad Dermatol 2008; 58:472–476.
- Lawn A, Bassi D. An unusual resuscitation request. Resuscitation 2008; 78:5–6.
- Gupta D. Tattoo flash: consider “do not resuscitate.” J Palliat Med 2010; 13:1155–1156.
- Sullivan W. The “emergency” DNR order. ED Legal Letter 2005; 16:133–144.
- Polack C. Is a tattoo the answer? BMJ 2001; 323:1063.
- Sokol DK, McFadzean WA, Dickson WA, Whitaker IS. Ethical dilemmas in the acute setting: a framework for clinicians. BMJ 2011; 343:d5528.
- Cooper L, Aronowitz P. DNR tattoos: a cautionary tale. J Gen Intern Med 2012; E-pub ahead of print.
- Iserson KV. The ‘no code’ tattoo—an ethical dilemma. West J Med 1992; 156:309–312.
- Kämäräinen A, Länkimäki S. A tattooed consent for organ donation. Resuscitation 2009; 80:284–285.
- Chen SG, Chiu TF, Su WF, Chou TD, Chen TM, Wang HJ. Nipple-areola complex reconstruction using badge flap and intradermal tattooing. Br J Surg 2005; 92:435–437.
- Hoffman S, Mikell A. Nipple-areola tattooing as part of breast reconstruction. Plast Surg Nurs 2004; 24:155–157.
- Goh SC, Martin NA, Pandya AN, Cutress RI. Patient satisfaction following nipple-areolar complex reconstruction and tattooing. J Plast Reconstr Aesthet Surg 2011; 64:360–363.
- van der Velden EM, Baruchin AM, Jairath D, Oostrom CA, Ijsselmuiden OE. Dermatography: a method for permanent repigmentation of achromic burn scars. Burns 1995; 21:304–307.
- Traquina AC. Micropigmentation as an adjuvant in cosmetic surgery of the scalp. Dermatol Surg 2001; 27:123–128.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010; 1:CD003263.
- Singh AK, Karki D. Micropigmentation: tattooing for the treatment of lip vitiligo. J Plast Reconstr Aesthet Surg 2010; 63:988–991.
- Pitz S, Jahn R, Frisch L, Duis A, Pfeiffer N. Corneal tattooing: an alternative treatment for disfiguring corneal scars. Br J Ophthalmol 2002; 86:397–399.
- Kim C, Kim KH, Han YK, Wee WR, Lee JH, Kwon JW. Five-year results of corneal tattooing for cosmetic repair in disfigured eyes. Cornea 2011; 30:1135–1139.
- Spyropoulou GA, Fatah F. Decorative tattooing for scar camouflage: patient innovation. J Plast Reconstr Aesthet Surg 2009; 62:e353–e355.
- De Cuyper C. Permanent makeup: indications and complications. Clin Dermatol 2008; 26:30–34.
- Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis 2010; 63:223–227.
- Wexner SD, Cohen SM, Ulrich A, Reissman P. Laparoscopic colorectal surgery—are we being honest with our patients? Dis Colon Rectum 1995; 38:723–727.
- ASGE Technology Committee; Kethu SR, Banerjee S, Desilets D, et al. Endoscopic tattooing. Gastrointest Endosc 2010; 72:681–685.
- Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery—making the mark? Colorectal Dis 2009; 11:527–530.
- Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005; 365:305–311.
- Starkie A, Birch W, Ferllini R, Thompson TJ. Investigation into the merits of infrared imaging in the investigation of tattoos postmortem. J Forensic Sci 2011; 56:1569–1573.
- Mallon WK, Russell MA. Clinical and forensic significance of tattoos. Top Emerg Med 1999; 21:21–29.
- Lessig R, Grundmann C, Dahlmann F, Rçtzcher K, Edelmann J, Schneider PM. Review article: Tsunami 2004—a review of one year of continuous forensic medical work for victim identification. EXCLI 2006; 5:128–139.
- Sutherland C, Groombridge L. The Paddington rail crash: identification of the deceased following mass disaster. Sci Justice 2001; 41:179–184.
- Sperry K. Tattoos and tattooing. Part II: gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 1992; 13:7–17.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg 2002; 28:962–965.
- Kaur RR, Kirby W, Maibach H. Cutaneous allergic reactions to tattoo ink. J Cosmet Dermatol 2009; 8:295–300.
- Reddy KK, Hanke CW, Tierney EP. Malignancy arising within cutaneous tattoos: case of dermatofibrosarcoma protuberans and review of literature. J Drugs Dermatol 2011; 10:837–842.
- Price RR. The AAPM/RSNA physics tutorial for residents. MR imaging safety considerations. Radiological Society of North America. Radiographics 1999; 19:1641–1651.
- Franiel T, Schmidt S, Klingebiel R. First-degree burns on MRI due to nonferrous tattoos. AJR Am J Roentgenol 2006; 187:W556.
- Chikkamuniyappa S, Sjuve-Scott R, Lancaster-Weiss K, Miller A, Yeh IT. Tattoo pigment in sentinel lymph nodes: a mimicker of metastatic malignant melanoma. Dermatol Online J 2005; 11:14.
- Morte PD, Magee LM. Hyperalgesia after volar wrist tattoo: a case of complex regional pain syndrome? J Clin Neuromuscul Dis 2011; 12:118–121.
- Kennedy BS, Bedard B, Younge M, et al. Outbreak of Mycobacterium chelonae infection associated with tattoo ink. http://www.nejm.org/doi/full/10.1056/NEJMoa1205114?query=TOC#t=article. Accessed August 28, 2012.
KEY POINTS
- Tattoos that state an advance directive for health care are not recognized as meeting the legal requirements for advance directives. They should only be considered as a guide to treatment decisions.
- Tattooing for medical-alert purposes is part of current culture. People with diabetes should avoid tattooing of feet or lower legs in view of impaired healing.
- Endoscopic tattooing is commonly used to aid visualization of diseased bowel segments during laparoscopic surgical procedures. Complications are rare but include mild chronic inflammation, abscesses, inflammatory pseudotumors, focal peritonitis, and peritoneal staining.
- Improper sterilization of tattooing needles can cause a wide range of infectious diseases and skin reactions.
2012–2013 Influenza update: Hitting a rapidly moving target
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
KEY POINTS
- A recent outbreak of swine flu in children exposed to pigs at agricultural fairs is unprecedented. Seasonal influenza vaccine does not protect against this strain, designated H3N2v. The neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza) are the drugs of choice for treatment.
- A highly lethal bird flu, designated H5N1, is still a pandemic threat. In the event of an outbreak, an inactivated whole-virus vaccine is available.
- A community outbreak of oseltamivir-resistant H1N1 in Australia sounded an alarm for a potential drug-resistant flu epidemic. Inhaled zanamivir would be the only effective therapy available in the event of such an epidemic.
- An emerging new antiviral drug is effective against oseltamivir-resistant influenza.
Infant’s brain damage blamed on delayed delivery … and more
DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.
DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.

DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.

DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.

DURING DELIVERY, THE MOTHER’S PERINATOLOGIST recognized a severe shoulder dystocia. The perinatologist abandoned vaginal delivery and ordered an emergency cesarean delivery. The mother was transferred to an operating room (OR) with the baby’s head out between her legs. In the OR, the perinatologist pushed the baby’s head back into the uterus and performed a cesarean extraction. Nineteen minutes elapsed from when the vaginal delivery was abandoned and the baby was delivered.
The child was unresponsive at birth with no spontaneous movement or respiration. She was intubated and transferred to the NICU, where she was resuscitated. MRI confirmed that the child had hypoxic ischemia and severe, permanent brain damage from acute birth asphyxia. The child is blind, deaf, hypertensive, and has diffuse spasticity. She has a tracheostomy, a gastrostomy tube, and requires 24-hour care.
PARENTS’ CLAIM The perinatologist was negligent for abandoning vaginal delivery when delivery was progressing appropriately and there was no fetal distress. If the perinatologist had rotated the baby’s shoulder to the oblique position and/or used suprapubic pressure, the shoulder would have become disimpacted and the baby would have been safely delivered within seconds. Delay in delivery allowed for 19 minutes of umbilical cord compression, resulting in brain damage.
PHYSICIAN’S DEFENSE Cesarean delivery was appropriate; the baby did not suffer cord compression. Injury to the brain occurred days before delivery, based on prenatal ultrasonography.
VERDICT A $5.5 million California settlement was reached.
Failure to diagnose breast cancer: death
A 38-YEAR-OLD WOMAN went to her primary care physician (PCP) 3 years after giving birth. She reported breast pain, nipple discharge, and a dime-sized lump. The woman was still breastfeeding. An exam by the nurse practitioner (NP) was limited because the patient had breast implants. The NP suspected a galactocele and advised the patient to stop breastfeeding and apply ice packs. When the patient returned in 2 weeks, only the lump remained. The PCP determined that she had mastitis.
Five months later, she returned with additional lumps in both breasts, and was referred to a gynecologist. Ultrasonography (US) was ordered, but the patient never followed up. A year later, the patient was found to have metastatic breast cancer and died after 3 years of treatment.
ESTATE’S CLAIM The PCP and NP were negligent for not referring her for a breast biopsy when a lump was first detected.
DEFENDANTS’ DEFENSE Proper care was given. An earlier diagnosis would not have changed the outcome.
VERDICT A $750,000 Massachusetts settlement was reached.
What caused this child’s autism?
AFTER 33 HOURS OF LABOR, a baby was delivered vaginally by an ObGyn, nurse, and midwife. The child was diagnosed with autism several years later. His development is delayed, and he suffers cognitive impairment.
PARENTS’ CLAIM The child’s autism is due to a prolonged hypoxic event during labor. Fetal heart-rate monitoring demonstrated fetal distress, with a bradycardia. A cesarean delivery should have been performed.
PHYSICIAN’S DEFENSE The child has genetic autism unrelated to the birth process.
VERDICT A $1.35 million New York settlement was reached.
Was oxytocin the culprit?
DURING AN EXTENDED LABOR, the ObGyn continued to give the mother oxytocin, although there were signs of fetal distress. The child was born with brain damage, cannot walk, talk, or see, and requires 24-hour care.
PATIENT’S CLAIM The use of oxytocin was inappropriate given the signs of fetal distress. Oxytocin caused a lack of oxygen to the child, resulting in brain damage. A cesarean delivery should have been performed when fetal distress was identified.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $12 million Illinois settlement was reached: $11 million from the hospital and $1 million from the ObGyn.

DURING LEFT OOPHORECTOMY, the ObGyn encountered adhesions. Five days later, the 41-year-old patient reported severe pain. A second procedure revealed sepsis and perforation of the large bowel. A colostomy was performed. The patient underwent additional corrective operations.
PATIENT’S CLAIM The ObGyn was negligent for causing tissue damage to the colon that perforated and escalated into sepsis. A surgeon should have been consulted when the ObGyn found the adhesions, so the bowel could be properly inspected before the abdomen was closed. The physician was also negligent for not recognizing symptoms of sepsis earlier.
PHYSICIAN’S DEFENSE Bowel injury is a known complication of oophorectomy. The patient appeared to be making a fairly good recovery until infection became evident; she was immediately treated.
VERDICT A $6.3 million New Jersey verdict was returned, including $300,000 for the husband’s loss of consortium.
Traumatic delivery causes seizures
DURING CESAREAN DELIVERY, the ObGyn rotated the baby from a transverse to a cephalic lie, and used a vacuum extractor to deliver the head through the hysterotomy incision.
When the child was 25 hours old, he suffered a seizure that lasted 6 minutes. Focal seizure activity involving the left side of his body and a skull fracture were identified. He was transferred to another hospital, where radiologic studies indicated a middle right cerebral artery infarct. The child developed an ongoing seizure disorder, speech and language delays, and mild, left-sided weakness.
PARENTS’ CLAIM The baby’s head was not properly delivered through the cesarean incision nor should the ObGyn have used vacuum extraction. The combination of the rotation and use of vacuum caused trauma to the infant’s head. In addition, the baby was placed in the well-baby nursery, which was inappropriate because he was born through thick meconium, resuscitated by a neonatal nurse, and had a depressed skull fracture.
PHYSICIAN’S DEFENSE Delivery was not traumatic; all treatment was appropriate.
VERDICT A $4.6 million New York settlement was reached with the hospital and ObGyn’s insurer.
Mother gets severe headache during birth
A 35-YEAR-OLD WOMAN began having a severe headache during delivery that continued after birth. She was discharged from the hospital and collapsed at home a day later. She was returned to the ED, where she was left in a hallway for 6 hours. She lost consciousness while in the hallway. Imaging and neurologic evaluation determined that she suffered a hypoxic brain injury from intracranial bleeding. She has slow response time, difficulty with all aspects of everyday life, and requires full-time attendant care.
PATIENT’S CLAIM Although she complained of a headache, no testing was done prior to her hospital discharge. Treatment was extremely delayed in the ED; an earlier diagnosis could have prevented brain damage.
PHYSICIAN’S DEFENSE Nothing could have prevented the brain damage.
VERDICT A $3.5 million California settlement was mediated.
Shoulder dystocia; brachial plexus injury
WHEN SHOULDER DYSTOCIA was encountered during delivery, the ObGyn applied gentle pressure to deliver the head. He was assisted by an ObGyn resident. The child was born with a brachial plexus injury, causing left-arm paralysis. She underwent surgery that increased her range of motion, but she will need years of physical therapy.
PATIENT’S CLAIM The ObGyn applied excessive traction and the resident improperly applied fundal pressure.
DEFENDANTS’ DEFENSE Only gentle traction was used. The resident did not apply fundal pressure.
VERDICT A New York jury found the ObGyn at fault and awarded the patient $3.5 million. The resident was vindicated.

AN EXPECTANT MOTHER MISCARRIED AT HOME at 6 months’ gestation, and an ambulance was called. After the EMTs helped the mother to the ambulance, they retrieved the fetus. When the baby was seen moving its head, the EMTs requested assistance from the advanced life support (ALS) team. ALS personnel visually assessed the fetus, determined it was nonviable, and placed the baby in a small container. The mother and baby arrived at the hospital 17 minutes after the ambulance was called.
At the hospital, a nurse noticed that the fetus was warm and had a heartbeat. The baby was taken to a special-care nursery for resuscitation and then transferred to another hospital’s NICU. The baby died after 46 days from severe brain damage due to lack of oxygen.
PARENTS’ CLAIM The EMTs and ALS team should have provided better evaluation and treatment for the infant; they were not trained to determine an infant’s viability. Placing the infant inside a plastic bag inside a box with a lid further deprived the baby of oxygen.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $1 million Massachusetts settlement was reached.
Were records altered because of a delayed diagnosis?
A WOMAN FOUND A LUMP in her left breast. A gynecologist ordered mammography. In January 2006, the radiologist requested ultrasonography (US), and reported that it conclusively indicated that the mass was a cyst. The gynecologist told the patient the tests were normal; further action was unnecessary. The patient saw the gynecologist four more times before being referred to a breast surgeon. In June 2006, she underwent surgical resection and chemotherapy for a malignant breast tumor.
PATIENT’S CLAIM The gynecologist was negligent for not referring the patient to a surgeon earlier. The gynecologist altered records: excerpts from the mammogram and US reports had been scanned in with a notation that the gynecologist had told the patient to follow up with a surgeon. When the gynecologist faxed the same reports to the surgeon, the annotations were absent. The gynecologist also changed the December 2005 chart, which referred to an US she never ordered.
PHYSICIAN’S DEFENSE The gynecologist stated that she regularly “merged” two reports into one document in her practice.
VERDICT A $700,000 Pennsylvania verdict was returned.
Excessive force or standard of care?
SHOULDER DYSTOCIA occurred during labor. The child sustained left brachial plexus palsy. At age 6, his left arm is paralyzed and smaller than the right arm. He has trouble performing normal daily tasks.
PATIENT’S CLAIM The ObGyn used excessive force by pulling on the baby’s head to complete the delivery. Standard of care required the ObGyn to take a more gentle approach to achieve delivery.
PHYSICIAN’S DEFENSE Delivery was performed appropriately, and did not deviate from standard of care.
VERDICT A $20.881 million Maryland verdict was returned, including $20 million for pain and suffering. The total award was reduced to $1,531,082 when the pain and suffering award was cut to $650,000 under the state’s statutory cap.
Preterm birth from an asymptomatic UTI?
A BABY WAS BORN AT 31 WEEKS’ gestation. The child has cerebral palsy, spastic quadriplegia, and requires assistance in all aspects of life.
PARENTS’ CLAIM Chorioamnionitis from a urinary tract infection (UTI) caused preterm birth. Urinalysis performed 7 weeks earlier indicated an infection, but the second-year resident caring for the mother failed to treat the UTI. The resident should have obtained a confirming urine culture, prescribed antibiotics, and monitored the mother more closely. The resident was poorly supervised.
DEFENDANTS’ DEFENSE Chorioamnionitis developed just before birth and could not be detected or prevented. A UTI cannot remain asymptomatic for 7 weeks and still cause premature birth. The mother was at increased risk of premature delivery because she had given birth to an anencephalic infant a year earlier. She began prenatal care in the middle of her pregnancy and ignored a referral to a high-risk maternal fetal specialist.
VERDICT A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Taking an extended leave: What to do before you go
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Arranging coverage and adjusting workload duties before taking an extended leave of absence from clinical practice—eg, for vacation, family leave, medical illness—can be challenging. During extended absences, clinicians depend on colleagues for assistance. In clinical settings such as residency training programs, arranging coverage for a maternity leave could be complicated by differences in attitudes toward pregnancy.1 However, an anticipated leave allows for advanced planning that can help ease transfer of care.
A smooth transition
Begin planning far in advance of your leave date because complications may necessitate a sudden, early departure. All clinical documentation, such as progress notes, should be completed so that a covering colleague can seamlessly assume patient care. It may be helpful to create a spreadsheet of all patients’ information, including name, contact number, diagnoses, medications, and a risk category (eg, low to high), along with notes—eg, lab results that need to be followed up on or labs to be ordered. This spreadsheet can be updated weekly and kept in a secure location so colleagues can access it in case your leave begins earlier than anticipated. To reduce workload burden on covering colleagues, it may be helpful to see as many stable, medication-only patients as possible before you leave to ensure that you have provided enough refills to cover the duration of your leave, assuming these patients typically are seen every other month or less.
It may be helpful to arrange for colleagues to take on a greater proportion of new consultations within the practice as the leave draws closer, because usually this is not a good time to begin treating new patients. However, it may be desirable for you to see a greater proportion of 1-time consultations, such as pre-surgical evaluations and second-opinion consultations. If time allows, arrange meetings among yourself, the colleague who will be covering for you, and high-risk patients before your leave. This can help promote familiarity and comfort between patients and the covering physician and increase the likelihood that patients in crisis will reach out to the covering physician. In some cases it may be advisable to consider a patient’s diagnosis, treatment history, and past experiences when selecting which colleague will provide care, assuming a choice is available—ie, female patients with a history of sexual trauma may feel more comfortable with a female physician.
Although taking an extended leave of absence from clinical practice can present many practical challenges, working with colleagues in advance can help promote a smoother transition of care and decrease workload burden.
Disclosure
Dr. Troy reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Reference
1. Tamburrino MB, Evans CL, Campbell NB, et al. Physician pregnancy: male and female colleagues’ attitudes. J Am Med Womens Assoc. 1992;47(3):82-84.
Vitamin D deficiency in older adults
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
Low vitamin D levels can impact cognitive functioning in older adults.1 As vitamin D levels decrease, cognitive impairment increases.
Vitamin D deficiency can occur because few foods contain this nutrient2 and patients have limited exposure to sunlight—vitamin D is produced when sunlight strikes the skin.2 In addition to rickets, low levels of vitamin D have been linked to slower information processing in middle age and older men, cognitive decline, mood disorders, and altered brain development and function resulting in neurodegenerative diseases and other medical disorders.3
One study suggested that one-half of adults age >60 do not get sufficient vitamin D, with an even higher rate among women with Alzheimer’s disease.4 Patients in dementia units typically are not tested for vitamin D levels. These patients rarely leave the unit, which leaves them deprived of the vitamin D provided by sunlight. Even patients exposed to sunlight may receive minimal vitamin D because they use sunscreen.
The following protocol can help patients who may benefit from vitamin D supplementation and increased sun exposure.
Obtain and assess vitamin D levels. Evaluate your patient’s level in the context of physical or cognitive symptoms and other lab values:
- deficient: <12 ng/mL
- inadequate: 12 to 20 ng/mL
- adequate: ≥20 ng/mL.2
Order dietary assessment to identify foods that may increase vitamin D levels. The best sources are fish—salmon, tuna, and mackerel—fish oils, beef, liver, cheese, and egg yolks.2 Several food products, including milk and orange juice, are fortified with vitamin D.
Suggest a daily vitamin D supplement ranging from 400 IU/d to 1,000 IU/d. The Institute of Medicine suggests 600 IU/d for patients age 60 to 70 and 800 IU/d for those age ≥71. For vitamin D deficient patients, recommend >1,000 IU/d.1
Recommend 15 minutes per day in the sun without sunscreen from spring to autumn; late summer to fall is ideal because vitamin D’s half-life is 30 days. Midday is the best time to produce vitamin D.5
Recheck the patient’s Mini-Mental State Examination score every 4 months. Vitamin D supplementation is correlated with cognitive functioning.6
Disclosure
Dr. LaFerney reports no financial, relationship with any company whose, products are mentioned in this article, or with manufacturers of competing, products.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
1. Mayo Clinic. Vitamin D. http://www.mayoclinic.com/health/vitamin-d/NS_patient-vitamind/DSECTION=dosing. Updated October 1 2011. Accessed September 26, 2012.
2. National Institutes of Health. Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional. Accessed September 26, 2012.
3. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
4. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
5. Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82(6):1697-1703.
6. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
An open-label trial of escitalopram for PPD: Considerations for research
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
Challenges in recruiting women to postpartum depression (PPD) antidepressant treatment trials, which we encountered when conducting a trial of escitalopram, contribute to the limited body of knowledge about PPD treatment. Here we discuss results from a preliminary trial of escitalopram for PPD, and challenges of research in this area.
Escitalopram, the S-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with high selectivity and potency that is FDA-approved for treating major depressive disorder (MDD) and generalized anxiety disorder. An agent with antidepressant and anxiolytic effects is particularly desirable for PPD because anxiety is more common in postpartum major depressive episodes than non-postpartum MDD.1 Anxiety and depressive disorders commonly are comorbid in postpartum women.2
We conducted an open-label trial of escitalopram for women with PPD and anxiety. We initially attempted to recruit 20 women.
Methods
Patients received 8 weeks of treatment with escitalopram, 10 to 20 mg/d (flexible dose). After completing the initial phone screen, patients had 5 follow-up visits, once every 2 weeks for 8 weeks. The institutional review board at Massachusetts General Hospital approved this study and we obtained written informed consent from all patients at the first visit. Twelve patients completed the phone screen and 7 eligible patients were enrolled in the study over 32 months. Reasons for ineligibility included having a history of psychosis, onset of symptoms >3 months postpartum, or presenting >6 months after onset. Others declined to participate because of concern about the time commitment or because they pursued nonpharmacologic treatments after the evaluation visit. One patient was lost to follow-up. Three patients completed the study. The study was halted because of the slow pace of recruitment.
Patient selection. Patients were screened for a major depressive episode with postpartum onset within 3 months of childbirth; depressive symptoms may have developed during pregnancy and worsened postpartum to meet criteria for MDD. Women were eligible for the study if they:
- were age 18 to 45
- experienced a major depressive episode with symptoms developing within 3 months of childbirth
- presented within 6 months of childbirth
- had a Montgomery-Åsberg Depression Rating Scale (MADRS) score >15
- had a Beck Anxiety Inventory (BAI) score >10.
Patients who were pregnant or breast-feeding were excluded from the study per an agreement with the sponsor. In addition, women were excluded if they had taken any psychotropic medication within 2 weeks of enrollment; had active suicidal ideation, homicidal ideation, or presence of psychotic symptoms; had chronic depression or dysthymia; had chronic or treatment-resistant anxiety disorders; had a history of mania or hypomania; or had active alcohol or substance abuse within the past year.
Treatment. Patients received escitalopram, 10 mg/d, after the baseline visit. At the investigator’s discretion, the dose could be increased to 20 mg/d or lowered to 5 mg/d if side effects occurred.
Measures. At the first visit, patients were assessed with the Mini-International Neuropsychiatric Interview to verify MDD and exclude diagnoses that would determine ineligibility. MADRS and Edinburgh Postnatal Depression Scale (EPDS) were used at each visit to measure depressive symptoms.3,4 The BAI was completed at each visit to measure anxiety symptoms. Obsessions and compulsions were measured with the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)5 at baseline, and at all following visits if the patient scored >8 at baseline. The Clinical Global Impression Scales for severity and improvement were completed at each visit.6
Results
Of 7 patients enrolled, 3 completed the study, 2 were ineligible after the baseline visit, and 2 did not participate after the baseline visit (1 selected to pursue psychotherapy, and 1 was lost to follow-up).
Two of 3 patients responded to escitalopram (≥50% decrease on MADRS), and both were remitters (MADRS score <7). All 3 patients were responders on EPDS and BAI. One patient had Y-BOCS >8 at baseline (Total Y-BOCS score of 9, and final Y-BOCS score of 8) (Table).
Table
Symptom rating scale scores at baseline and study end
| Baseline (Visit 1) | Final (Visit 5) | |||||
|---|---|---|---|---|---|---|
| Patient | MADRS | BAI | EPDS | MADRS | BAI | EPDS |
| Ms. A | 21 | 18 | 22 | 12 | 0 | 0 |
| Ms. B | 28 | 28 | 19 | 4 | 5 | 2 |
| Ms. C | 37 | 6 | 19 | 6 | 2 | 0 |
| BAI: Beck Anxiety Inventory; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery-Åsberg Depression Rating Scale | ||||||
Discussion
Patients who stayed in treatment improved during the course of this study. Recruitment was difficult; we were able to recruit only 7 patients out of a projected 20 for the screening visit. We solicited feedback from local obstetrics health care providers and social workers on recruitment and attractiveness of the study as part of our routine collaboration with obstetrical services that screen for PPD. Primary reasons patients were not referred were that they were breast-feeding or they stated they would prefer to receive treatment from their primary care doctor. Recruitment difficulty in this study was in stark contrast to other recent studies completed at our center. For example, we have successfully recruited for menopausal depression and premenstrual dysphoric disorder treatment studies, and have completed large naturalistic studies of women with unipolar depression and bipolar disorder across pregnancy and postpartum. We suspect that many patients who were eligible for the study preferred to seek care from an obstetrician or primary care doctor with whom they already had a therapeutic alliance, and we also suspect that many women with PPD do not seek treatment at all, which is consistent with findings from other research groups.
Lessons learned from PPD research include:
- Including women who are breast-feeding is important because many women choose to breast-feed and suffer from PPD. Because antidepressant use during breast-feeding has been closely studied, it is appropriate to include potential research participants who are breast-feeding as long as they receive adequate information and are able to provide informed consent.
- Participants in PPD studies may require accommodations that take into account their role as a new mother, such as on-site childcare, home visits, or other strategies.
- Because of recruitment challenges in postpartum patients, multisite trials may be required to include adequate numbers of participants.
Related Resource
- Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-21.
Drug Brand Names
- Citalopram • Celexa
- Escitalopram • Lexapro
Disclosures
Dr. Freeman has received grant or research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, is on the advisory boards of Otsuka and Takeda/Lundbeck, and is a consultant for PamLab LLC.
Dr. Joffe has received grant or research support from Cephalon/Teva, and is a consultant to Noven and Sunovion.
Dr. Cohen has received research support from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, National Institutes of Health, Ortho-McNeil Janssen, and Pfizer and has served on an advisory board for PamLab LLC.
This study was funded as an investigator-initiated trial by Forest Pharmaceuticals.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.
1. Bernstein IH, Rush AJ, Yonkers K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20-26.
2. Wenzel A, Haugen EN, Jackson LC, et al. Anxiety symptoms and disorders at eight weeks postpartum. J Anxiety Disord. 2005;19(3):295-311.
3. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
4. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389.
5. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011.
6. Guy W. ECDEU assessment manual for psychopharmacology. Rockville MD: US Department of Health and Human Services; 1976. Department of Health, Education, and Welfare Publication (ADM) 76–338.