User login
Sessile serrated polyps: Cancer risk and appropriate surveillance
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
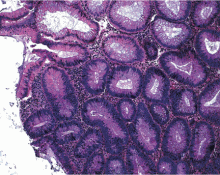
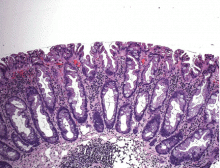
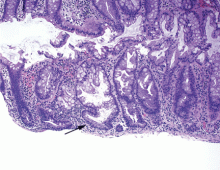
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
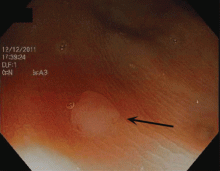
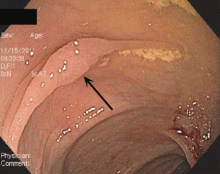
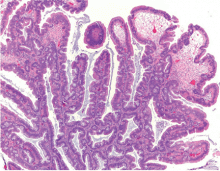
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
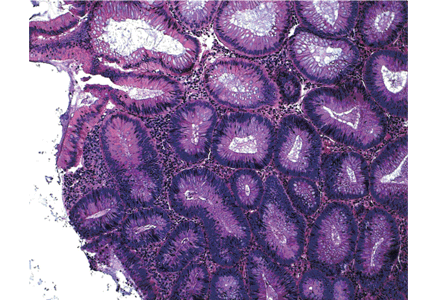
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
Sessile serrated polyps are a type of polyp recently recognized to be a precursor of colorectal cancer. They arise from a pathway of genetic alterations different from the pathway that causes the more common and well-understood conventional adenomas (also called tubular adenomas, tubulovillous adenomas, and villous adenomas).
We do not yet know enough about the lifetime colorectal cancer risk for individuals with sessile serrated polyps, nor do we know the optimal surveillance interval for patients who have these polyps on colonoscopy. It is believed that sessile serrated polyps may be the cause of a substantial number of “interval” colorectal cancers—ie, cancers that occur after colonoscopy but before the next scheduled examination.
Serrated polyps get their name from their jagged appearance on microscopy. In the past, all serrated colorectal lesions were called hyperplastic polyps. But with the advent of molecular and genetic diagnostics and with the ability to recognize the subtle morphologic differences of serrated lesions, they have been reclassified into those without malignant potential (hyperplastic polyps) and those that are neoplastic (sessile serrated polyps and traditional serrated adenomas) (Table 1).
In this article, we discuss the evolving understanding of the different types of serrated polyps, and we offer our thoughts on a reasonable postpolypectomy surveillance plan in patients with these lesions. We focus on sessile serrated polyps, the most common form of serrated polyp with cancerous potential, since it may be one of our greatest challenges in optimal colorectal cancer prevention.
CLINICAL SCENARIO
A 65-year-old woman with no family history of colorectal cancer undergoes screening colonoscopy, during which three polyps are found and removed—a 3-mm tubular adenoma in the sigmoid colon, an 8-mm sessile serrated polyp at the hepatic flexure, and a 2-mm hyperplastic polyp in the rectum. When should she undergo follow-up colonoscopy?
Based on the number, size, and pathologic makeup of the polyps in this patient, we would recommend follow-up surveillance colonoscopy in 5 years.
THE SERRATED POLYP PATHWAY: A DIFFERENT PATH TO COLORECTAL CANCER
Colorectal cancer is the third most common cancer in the United States.1 From 70% to 80% of these cancers arise from adenomatous polyps via the adenoma-carcinoma pathway. This molecular pathway develops through chromosomal instability (CIN) and involves the loss of heterozygosity (the loss of function of one allele). This leads to the progressive accumulation of mutations in tumor-suppressor genes such as adenomatous polyposis coli (APC) and p53, and oncogenes such as KRAS. The result of these mutations is the development of adenomatous polyps that lead to microsatellite-stable colorectal cancers (Figure 1).2
More recently, studies have shown that the other 20% to 30% of colorectal cancers likely arise through a separate pathway, called the serrated polyp pathway or serrated neoplasia pathway. In contrast to CIN, this pathway is characterized by methylation of CpG islands (CIMP–CpG island methylation phenotype, CIMP) in the promoter regions of specific genes.3 Central to the serrated polyp pathway is progressive methylation in colonic mucosa; mutation in the BRAF oncogene, activating cell proliferation leading to a sessile serrated polyp; and epigenetic silencing of the DNA mismatch repair gene hMLH1, which is a key step in the progression to a sessile serrated polyp with dysplasia, which may rapidly become a microsatellite-unstable colorectal cancer.4
Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium, and they include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (sessile serrated adenomas).
Sessile serrated polyps and traditional serrated adenomas (which are rare) are thought to be precancerous, whereas hyperplastic polyps do not have malignant potential.
COMMON, BUT PREVALENCE IS NOT CLEARLY ESTABLISHED
The histologic criteria for sessile serrated polyps and traditional serrated adenomas have been elucidated,4–7 but the epidemiology of these serrated polyps is not clear. Small studies have shown that sessile serrated polyps account for 2% to 9% of all polyps removed at colonoscopy8–10; however, larger studies are needed to determine the prevalence because detection by an endoscopist and pathologic diagnosis of these polyps are both operator-dependent.
Traditional serrated adenomas are the least common type of serrated polyp, with a reported prevalence of 0.3%.7 Hyperplastic polyps are by far the most common, accounting for 20% to 30% of all polyps removed at colonoscopy.9,11 Sessile serrated polyps have a predilection for the proximal colon and are associated with female sex and with smoking, 12,13 but no consistent effect of other factors on their formation has been reported. In contrast, Wallace et al13 found that obesity, cigarette smoking, dietary fat intake, total caloric intake, and the consumption of red meat were associated with an increased risk of distal (but not proximal) serrated polyps, including hyperplastic polyps, sessile serrated polyps, and traditional serrated adenomas.
HYPERPLASTIC POLYPS
Hyperplastic polyps usually occur in the rectosigmoid colon. They appear as slightly elevated, whitish lesions with a diameter less than 5 mm (Figure 2). Microscopically, the serrated architecture is present in the upper half of their crypts (Figure 3). The proliferative zone is more or less normally located in the basal half of the crypt (the nonserrated portion), with nuclei that are small, uniform, and basally located.14 The bases of the crypts have a rounded contour and do not grow laterally along the muscularis mucosae.
SESSILE SERRATED POLYPS
Endoscopically, sessile serrated polyps are often subtle, appear flat or slightly elevated, and can be covered by yellow mucus (Figure 4). They are typically found in the proximal colon and are usually larger than typical adenomas, with 50% being larger than 10 mm.10
Histologically, the serrations are more prominent than those of hyperplastic polyps and involve the entire length of the crypt (Figure 5). The crypt bases are often dilated and display lateral growth along the lamina muscularis mucosae, resembling a letter t or l. The lamina muscularis mucosae is often thinner than normal. Crypts from sessile serrated polyps are occasionally found beneath the muscularis mucosae, a condition called pseudoinvasion.7
TRADITIONAL SERRATED ADENOMAS
Traditional serrated adenomas are usually left-sided. In contrast to the other types of serrated polyps, they are histologically often villiform and are lined by cells with elongated nuclei and abundant eosinophilic cytoplasm (Figure 6). Unlike those in sessile serrated polyps, the crypt bases do not display an abnormal architecture; rather, traditional serrated adenomas have abundant ectopic crypts (“budding crypts”) in the long, slender villi.7
Traditional serrated adenomas also appear to be genetically distinct from sessile serrated polyps. They are most often characterized by a KRAS (or less commonly, BRAF) mutation and commonly have methylation of the DNA repair gene MGMT (O-6-methylguanine-DNA methyltransferase) rather than hMLH1.
CHALLENGES TO EFFECTIVE COLONOSCOPY
Colonoscopic polypectomy of adenomatous polyps reduces the incidence of colorectal cancer and the rate of death from it.15,16 However, recent data show that colonoscopy may not be as effective as once thought. As many as 9% of patients with colorectal cancer have had a “normal” colonoscopic examination in the preceding 3 years.17,18 In addition, the reduction in incidence and mortality rates was less for cancers in the proximal colon than for cancers in the distal colon.19,20
Possible explanations for this discrepancy include the skill of the endoscopist, technical limitations of the examination, incomplete removal of polyps, and inadequate bowel preparation. Several studies have shown that interval colorectal cancers are more likely to be found in the proximal colon and to have the same molecular characteristics as sessile serrated polyps and the serrated colorectal cancer pathway (CIMP-high and MSI-H).21,22 Therefore, it is now thought that sessile serrated polyps may account for a substantial portion of “postcolonoscopy cancers” (ie, interval cancers) that arise in the proximal colon.
Two large studies of screening colonoscopy confirmed that the ability to detect sessile serrated polyps depends greatly on the skill of the endoscopist. Hetzel et al9 studied the differences in the rates of polyp detection among endoscopists performing more than 7,000 colonoscopies. Detection rates varied significantly for adenomas, hyperplastic polyps, and sessile serrated polyps, with the greatest variability noted in the detection of sessile serrated polyps. Significant variability was also noted in the ability of the pathologist to diagnose sessile serrated polyps.9
In the other study, a strong correlation was found between physicians who are “high detectors” of adenomas and their detection rates for proximal serrated polyps.23 There is widespread acceptance that screening colonoscopy in average-risk patients age 50 and older should detect adenomas in more than 25% of men and more than 15% of women. There is no current minimum recommended detection rate for sessile serrated polyps, but some have suggested 1.5%.8
POLYPS AS PREDICTORS OF CANCER RISK
Certain polyp characteristics predict the risk of metachronous, advanced neoplasia. Advanced neoplasms are defined as invasive carcinomas, adenomas 10 mm or larger, or adenomas with any villous histology or high-grade dysplasia. Patients with one or two small tubular adenomas have a much lower risk of metachronous advanced neoplasia than do patients with more than two adenomas or advanced neoplasms.24 Current recommended surveillance intervals vary on that basis (Table 2).25
People who harbor serrated neoplasms are at high risk of synchronous serrated polyps and advanced adenomatous neoplasia. Pai et al26 found that patients with one sessile serrated polyp were four times more likely to have additional serrated polyps at the same time than an unselected population. The authors suggested that this indicates a strong colonic mucosal-field defect in patients with sessile serrated polyps, thereby predisposing them to the development of synchronous serrated polyps.
Li et al27 found that large serrated polyps (ie, > 10 mm) are associated with a risk of synchronous advanced neoplasia that is three times higher than in patients without adenomas. Schreiner et al28 determined that patients with either a proximal or a large serrated polyp were at higher risk of synchronous advanced neoplasia compared with patients who did not have those lesions. Vu et al29 found that patients who have both sessile serrated polyps and conventional adenomas have significantly larger and more numerous lesions of both types.29 In addition, these lesions are more likely to be pathologically advanced when compared with people with only one or the other type.
In the only study of the risk of advanced neoplasia on follow-up colonoscopy,28 patients with advanced neoplasia and proximal serrated polyps at baseline examination were twice as likely to have advanced neoplasia during subsequent surveillance than those with only advanced neoplasia at baseline examination.28
Therefore, it seems clear that the presence of large or proximal serrated polyps or serrated neoplasms predicts the presence of synchronous and likely metachronous advanced neoplasms.
Guidelines for postpolypectomy surveillance for individuals with serrated lesions of the colon have recently been published.25 Patients with large serrated lesions (≥ 10 mm) or an advanced serrated lesion (a sessile serrated polyp with or without cytologic dysplasia or a traditional serrated adenoma) should be followed closely. Patients with small (< 10-mm) rectosigmoid hyperplastic polyps should be followed as average-risk patients. If a patient with a sessile serrated polyp also has adenomas, the surveillance interval should be the shortest interval recommended for either lesion.29
SURVEILLANCE FOR OUR PATIENT
In our patient, given the number, size, and histologic features of the polyps found, surveillance colonoscopy should be considered in 5 years. Although the clinical significance of the serrated pathway to colorectal cancer cannot be argued, further study is required to understand the lifetime risk to patients with serrated neoplasms and the optimal surveillance interval.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010; 138;2059–2072.
- Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010; 138:2088–2100.
- Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011; 42:1–10.
- O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006; 30:1491–1501.
- Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003; 27:65–81.
- Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs traditional serrated adenoma (TSA). Am J Surg Pathol 2008; 32:21–29.
- Sanaka MR, Gohel T, Podugu A, et al. Quality indicators to enhance adenoma detection rate: should there be reconsideration of the current standard? Gastrointest Endosc 2011; 73:AB138.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010; 105:2656–2664.
- Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006; 131:1400–1407.
- Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005; 47:32–40.
- Lieberman DA, Prindiville S, Weiss DG, Willett W; VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 2003; 290:2959–2967.
- Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009; 18:2310–2317.
- Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012; 107:1315–1329.
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329:1977–1981.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696.
- Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology 2006; 131:1700–1705.
- Baxter NN, Sutradhar R, Forbes SS, Paszat lF, Saskin R, Rabeneck l. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology 2011; 140:65–72.
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 2010; 139:1128–1137.
- Baxter NN, Goldwasser MA, Paszat lF, Saskin R, Urbach DR, Rabeneck l. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009; 150:1–8.
- Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol 2010; 105:1189–1195.
- Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol 2006; 4:1259–1264.
- Kahi CJ, Hewett DG, Norton Dl, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 2011; 9:42–46.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009; 136:832–841.
- Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143:844–857.
- Pai RK, Hart J, Noffsinger AE. Sessile serrated adenomas strongly predispose to synchronous serrated polyps in nonsyndromic patients. Histopathology 2010; 56:581–588.
- Li D, Jin C, McCulloch C, et al. Association of large serrated polyps with synchronous advanced colorectal neoplasia. Am J Gastroenterol 2009; 104:695–702.
- Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology 2010; 139:1497–1502.
- Vu HT, Lopez R, Bennett A, Burke CA. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum 2011; 54:1216–1223.
KEY POINTS
- From 20% to 30% of colorectal cancers arise through the serrated polyp pathway (the serrated neoplasia pathway.)
- Histologically, serrated polyps have a serrated or sawtooth appearance from the folding in of the crypt epithelium. Types of serrated polyps include hyperplastic polyps, traditional serrated adenomas, and sessile serrated polyps (also known as sessile serrated adenomas).
- Guidelines for surveillance after polypectomy of serrated lesions recommend that patients with a large (≥ 10-mm) or a sessile serrated polyp with cytologic dysplasia or a traditional serrated adenoma be followed more closely than patients with a sessile serrated polyp smaller than 10 mm. Patients with small rectosigmoid hyperplastic polyps should be followed the same as people at average risk.
Mild cognitive impairment: Hope for stability, plan for progression
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
As our population ages, people are thinking more about preserving their quality of life, especially with regard to maintaining their cognitive and functional abilities. Older patients and caregivers often raise concerns about cognitive issues to their primary care providers: many patients have memory complaints, are worried about whether these are merely part of normal aging or symptoms of early dementia, and want strategies to forestall the progression of cognitive impairment.
Mild cognitive impairment (MCI) is a heterogeneous syndrome that in some cases represents a transition between normal aging and dementia. However, this condition is not yet well understood. Although some patients progress to dementia, others remain stable, or even improve. This article will review the current definitions and the underlying physiology of MCI, as well as diagnostic and management strategies.
COGNITIVE CHANGES OCCUR WITH NORMAL AGING
Cognition is defined as a means of acquiring and processing information about ourselves and our world. It includes memory as well as other domains such as attention, visuospatial skills, mental processing speed, language, and executive function. Cognitive abilities typically peak between ages 30 and 40, plateau in our 50s and 60s, and decline in our late 70s.
With age come detectable changes in the brain: brain weight declines by 10% by age 80, blood flow diminishes, neurons are lost throughout life, and nerve conduction slows. Despite these changes, the brain has a great deal of functional reserve capacity.
Table 1 compares the signs of normal aging, MCI, and dementia. Normally, cognitive abilities decline gradually with age without affecting overall function or activities of daily living. Even in normal aging, the processing of new information (new learning) is reduced. Mental processing becomes less efficient and slower. Visuospatial skills gradually decline, recall slows, and ultimately, the speed of performance slows as well. Additionally, distractibility increases. On the other hand, normal aging does not affect recognition, intelligence, or long-term memory.1
The line between the normal effects of aging on cognition and true pathologic cognitive decline is blurry. In a busy clinical practice, it is often difficult to determine whether problems with memory and cognition that elderly patients and their family members describe represent true pathologic decline. In general, the clinical presentation of MCI is more profound than that of age-associated cognitive impairment: whereas normal aging may involve forgetting names and words and misplacing things, MCI frequently involves forgetting conversations, information that one would ordinarily remember, appointments, and planned events.
BETWEEN NORMAL AGING AND DEMENTIA
MCI is a transitional state between normal cognition and dementia. But the course is not inevitably downward: on follow-up, patients with MCI may be better, stable, or worse (see PROGNOSIS VARIES, below).
On autopsy studies, the brains of people with MCI appear intermediate between normal brains and brains of people with Alzheimer-type dementia, which have neurofibrillary tangles, amyloid senile plaques, and neuronal degeneration.
Definitions of MCI vary
True cognitive decline that is more profound than normal aging was named and defined differently in different studies, making comparisons difficult. The concept of MCI arose from the term “benign senescent forgetfulness,” used by Kral in 1962.2 Other early terms include “cognitive impairment no dementia,” “memory impairment,” “mild cognitive disorder,” and “mild neurocognitive disorder.”3,4
MCI was first defined as a precursor to Alzheimer dementia. The term later described a sometimes reversible but abnormal state. It is a heterogeneous syndrome in terms of etiology, incidence, prevalence, presentation, and overall prognosis.
Most recently, MCI has been defined as5,6:
- Subjective memory complaints, preferably qualified by another person
- Memory impairment, with consideration for age and education
- Preserved general cognitive function
- Intact activities of daily living
- Absence of overt dementia.
MCI may arise from vascular, neurodegenerative, traumatic, metabolic, psychiatric, and other underlying medical disorders.7–9
The prevalence of MCI is difficult to determine because of the various definitions, populations studied (eg, clinic-based vs community-dwelling), and evaluation techniques. Published rates vary from 2% to 4% in all patients to 10% to 20% in the elderly. Incidence rates in the elderly vary from 14 to 75 per 1,000 patient-years.10–14
EARLY RECOGNITION ALLOWS PROMPT EVALUATION AND PLANNING
Pathologic cognitive decline is best detected early, for many reasons. Early recognition and intervention may help delay further decline. Establishing a diagnosis can also lessen family and caregiver stress and misunderstanding. Education of caregivers is important so that they can prepare for likely behavioral changes and plan for future care. Advance care planning, including advance directives, power of attorney, and designation of proxy for decision-making, is extremely important and is best considered before cognitive impairment becomes severe.
The diagnosis of MCI also provides the opportunity to assess safety concerns related to driving, working, medication compliance, the home environment, and firearms. Because patients with MCI are still highly functional, these issues need not be fully evaluated and should be handled on a case-by-case basis, depending on concerns raised. For example, if depression is an active concern, firearms safety should be addressed.
MEMORY LOSS MAY NOT BE THE PRIMARY CONCERN
MCI is categorized into two types based on whether memory loss is the primary cognitive deficit.
The amnestic type predominantly involves memory problems and is more common. Generally, several years elapse between initial memory concerns and a clinical diagnosis of MCI. Patients with amnestic MCI that progresses to dementia are more likely to develop Alzheimer disease.2,15
Nonamnestic types involve domains of cognition other than memory, such as executive function, attention, visuospatial ability, and language. Nonamnestic MCI can be subcategorized through extensive neuropsychological evaluation as involving single or multiple impaired domains.16,17 Such categorization is particularly important in determining prognosis, as patients with involvement of multiple domains are at higher risk of progressing to dementia.
Patients with nonamnestic MCI who progress to dementia are more likely to have non-Alzheimer types of dementia, such as Lewy body dementia and frontotemporal dementias.10
HISTORY SHOULD FOCUS ON FUNCTION, MEDICATIONS, AND DEPRESSION
Cognitive impairment should be clinically evaluated within the context of cognition, function, and behavior. Clinicians should focus on the time course of cognitive concerns, the specifics of the concerns, and their impact on day-to-day living and functioning. In assessing functional capacity, it is important to determine the level of assistance the patient needs to perform specific activities of daily living and instrumental activities of daily living (ie, the more advanced skills needed to live independently) (Table 2).
A thorough history includes consideration of baseline education, intellect, and previous learning disabilities; sensory impairments with emphasis on sight and hearing impairments; uncontrolled pain; head trauma; sleep disorders; concurrent medical and psychosocial illnesses such as depression and anxiety; substance abuse; and polypharmacy.
Depression, delirium, and the use of anticholinergic drugs are particularly important to evaluate, as these can result in cognitive deficits associated with MCI. The cognitive deficits may resolve with treatment or with stopping the drug.
Behavioral concerns such as wandering, agitation, and anger and sleep concerns, eating habits, and social etiquette are also important to evaluate.
PHYSICAL EVALUATION: RULE OUT REVERSIBLE CONDITIONS
The differential diagnosis of MCI includes delirium, depression, dementia, possibly reversible conditions affecting cognition (vitamin B12 deficiency, hypothyroidism, effects of anticholinergic drugs), and uncommonly, central nervous system conditions (normal pressure hydrocephalus, subdural hematoma, tumor, stroke), and others (Table 3).18
A thorough physical examination should include neurologic, cardiovascular, hearing, and vision examinations, as well as an evaluation of functional status.
Laboratory studies. Although evidence is lacking to support a laboratory diagnostic workup for MCI, a selective evaluation including a comprehensive metabolic profile, complete blood count, thyroid studies, and a vitamin B12 level can be useful. Occasionally, a treatable cause of impaired cognition such as vitamin B12 deficiency or thyroid disease can be identified and resolved. A further comprehensive laboratory evaluation should be obtained if a patient progresses to dementia.
Imaging can be used in conjunction with other supportive evidence but should not be used solely to establish a diagnosis of MCI. Magnetic resonance imaging (MRI) can detect metastatic disease, normal pressure hydrocephalus, and subdural hematoma, in addition to traumatic, inflammatory, infectious, and vascular causes of cognitive impairment. MRI can also determine focal areas of atrophy; temporal lobe atrophy is a risk factor for progression to dementia.
Other studies. Structural MRI using techniques to evaluate the hippocampus, functional imaging, genetic testing for ApoE4 alleles, and biomarkers in cerebrospinal fluid are currently under evaluation to identify those at risk of progression to dementia. Recently published guidelines by the Alzheimer’s Association and the National Institute on Aging indicate that pathophysiologic findings in MCI that may predict future Alzheimer disease are meant to guide research and are not part of clinical practice at this time.19
COGNITIVE AND NEUROLOGIC TESTING IDENTIFIES DEFICITS
A number of global measures of cognition can be used in the office in clinical practice to help in evaluating significant cognitive concerns and to determine areas and severity of deficits at presentation. These include the Mini-Mental State Examination, the Montreal Cognitive Assessment, the Saint Louis University Mental Status, and many others (Table 4).20
Caveats about interpreting the results: each of these tests has different sensitivities and specificities for detecting MCI. Also, we need to take into account the patient’s level of education, as highly educated people tend to do better on these tests.21–23 It is important to note that some patients with MCI have normal results or only minimally abnormal results on these tests.
Neuropsychological testing is reserved for patients needing further evaluation, eg, those with atypical or complex cases, and those in whom the specific domains of cognition involved need to be identified. It can also provide additional insight into the contribution of depression to cognitive deficits. Neuropsychological testing is usually very time-intensive and requires patients to be able to perform complicated cognitive tasks. Not all patients are good candidates for this testing; sensory and motor impairments must be considered to determine if patients can adequately participate in testing. The cost of neuropsychological testing for MCI may not be covered by insurance and should be discussed with patients before referral. Specific concerns about cognitive problems that need further evaluation should be stated in the referral.
No one test should be used to make a diagnosis of MCI or dementia; clinical judgment is also necessary. The need for referral to a neurologist, geriatrician, or psychiatrist depends on the nature of the cognitive and behavioral concerns, the complexity of making a diagnosis, the need for further assessment of functional ability, and the need for evaluation of risk of progression to dementia.
MEDICATIONS HAVE LITTLE ROLE IN MANAGEMENT
No drug has yet been approved by the US Food and Drug Administration for treating MCI.
The acetylcholinesterase inhibitors donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon) have undergone clinical trials for treatment of MCI but have not been definitely shown to significantly reduce the risk of progression to dementia.24
On the other hand, Diniz et al25 performed a meta-analysis of the use of cholinesterase inhibitors in patients with MCI as a means of delaying the progression to Alzheimer disease.25 They calculated that 15.4% of patients who received these drugs progressed to dementia, compared with 20.4% of those who received placebo, for a relative risk of 0.75 (95% confidence interval 0.66–0.87, P < .001). They concluded that the use of these drugs in patients with MCI “may attenuate the risk of progression” to Alzheimer disease and dementia.
In addition to not being approved for this indication and showing mixed evidence of efficacy, these drugs have well-known side effects such as diarrhea, nausea, vomiting, anorexia, and rhinitis, as well as significant but lesser-known side effects such as syncope, bradycardia, gastrointestinal bleeding, and vivid dreams.26
Nevertheless, some patients with MCI, particularly those at high risk with amnestic MCI, may still want to try these medications. In these cases, the risks and possible benefits (or lack of them) should be reviewed thoroughly with the patient and family, and the discussion should be documented before starting therapy. The lowest starting dose of acetylcholinesterase inhibitor should be used to determine tolerability; generally, the dose is increased after 4 weeks to a maintenance dosage, with particular consideration of side effects.
Other agents have also been evaluated for MCI but have shown no evidence of benefit. Nonsteroidal anti-inflammatory drugs have not been found to either improve symptoms or delay progression to dementia. Ginkgo biloba has shown unclear benefit in achieving important treatment goals for MCI,27 and it increases the risk of bleeding in the elderly. Vitamin E was evaluated in one study and did not slow progression to dementia.28
STAYING HEALTHY AND ACTIVE MAY HELP
We recommend optimizing vascular risk factors such as diabetes, blood pressure, smoking, and lipid levels in managing MCI, given that uncontrolled vascular risk factors may lead to progression to dementia. However, we can point to no research to support this recommendation.
Cognitive rehabilitation involves training in deficient domains and developing strategies to compensate for deficits. Different interventions are used, including computerized simulation exercises, memory aids, organizational techniques, personal digital assistants, crossword puzzles, mind games, and other mentally engaging activities.29
Increasing physical activity is another aspect of treatment. Some studies have shown that it improves cognitive performance in MCI, at least in the short term.30,31
Optimizing mood and emotions is also important. If present, depression should be identified and optimally treated. Social activity can be useful and leads to less emotional stress and to better coping mechanisms.
A multidisciplinary approach may help patients and may also help relieve the burden on the caregiver. Periodic reassessment of cognitive and functional symptoms may be warranted.
Maintaining disease-specific registries of patients who have MCI may be useful to longitudinally follow patients and ensure that they get the care they need.
PROGNOSIS VARIES
MCI is a heterogeneous condition that often does not predictably progress to dementia. Patients and families should be told that having MCI does not mean that the patient will necessarily get dementia.
Several studies have shown that the annual risk of progression to dementia for patients with MCI is 5% to 10% in community-dwelling populations and up to 15% in specialty-clinic patients.24,32 In comparison, the incidence of dementia in the general elderly population is 1% to 3% per year.
On the other hand, a number of studies show that MCI improves significantly in up to 15% to 40% of patients and sometimes reverts to a normal cognitive state.33,34 But prospective studies of patients with clinically diagnosed MCI usually find a low rate of reversion to a normal state.35,36 Many are short-term follow-up studies of different populations, making generalizations difficult.14
Patients with impairment in instrumental activities of daily living may be more likely to have nonreversible MCI and may be at higher risk of progressing to dementia.37
PATIENT AND FAMILY EDUCATION AND FOLLOW-UP CONSIDERATIONS
Caregiver education and stress management are important components of managing patients with MCI. Formally assessing caregiver stress is useful. Steps to prevent caregiver burnout include making use of respite care, counseling, education, and community resources such as adult day care and those offered by the Alzheimer’s Association.
Clinicians should follow patients with MCI closely to evaluate progression, address specific concerns, minimize risks, emphasize healthy habits, manage concurrent illnesses, and evaluate management.
Functional status, as demonstrated by activities of daily living, is the most important determinant of progression of MCI to dementia and should be evaluated at each visit. Repeat cognitive testing should be done on patients who have significant loss of functional status. Changes in work habits also warrant further attention.
Patients diagnosed with MCI or those who have persistent cognitive concerns should be considered for neuropsychological evaluation after 1 year to assess specific deficits and progression of cognitive impairment.
Finally, consideration should be given to current clinical research, and referrals should be made to research centers that focus on MCI management and treatment.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
- Keefover RW. Aging and cognition. Neurol Clin 1998; 16:635–648.
- Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J 1962; 86:257–260.
- Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment—a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand 2002; 106:403–414.
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1133–1142.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308.
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009; 66:1447–1455.
- Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology 2005; 64:834–841.
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 2006; 63:665–672.
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol 2003; 60:729–736.
- Molano J, Boeve B, Ferman T, et al. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 2010; 133:540–556.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology 2010; 75:889–897.
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63:494–506.
- Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58:1903–1910.
- Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30:58–69.
- Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 2001; 58:411–416.
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2003; 2:15–21.
- Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet 1997; 349:1793–1796.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:270–279.
- Tariq SH, Tumosa N, Chibnall JT, Perry MH, Morley JE. Comparison of the Saint Louis University mental status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006; 14:900–910.
- Tang-Wai DF, Knopman DS, Geda YE, et al. Comparison of the short test of mental status and the Mini-Mental State Examination in mild cognitive impairment. Arch Neurol 2003; 60:1777–1781.
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699.
- Banks WA, Morley JE. Memories are made of this: recent advances in understanding cognitive impairments and dementia. J Gerontol A Biol Sci Med Sci 2003; 58:314–321.
- Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med 2011; 364:2227–2234.
- Diniz BS, Pinto JA, Gonzaga MLC, Guimaraes FM, Gattaz WF, Forlenza OV. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Neurosci 2009; 259:248–256.
- Patel BB, Holland NW. Adverse effects of acetylcholinesterase inhibitors. Clin Geriatr 2011; 19:27–30.
- Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 1:CD003120.
- Petersen RC, Thomas RG, Grundman M, et al; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005; 352:2379–2388.
- Jean L, Bergeron ME, Thivierge S, Simard M. Cognitive intervention programs for individuals with mild cognitive impairment: systematic review of the literature. Am J Geriatr Psychiatry 2010; 18:281–296.
- Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300:1027–1037.
- van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med 2008; 42:344–351.
- Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009; 66:1151–1157.
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 2001; 56:37–42.
- Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59:1594–1599.
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 2006; 67:2176–2185.
- Fischer P, Jungwirth S, Zehetmayer S, et al. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology 2007; 68:288–291.
- Pérès K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006; 67:461–466.
KEY POINTS
- MCI that primarily involves memory or multiple domains has a higher risk of progressing to dementia.
- Depression and the effects of anticholinergic medication can mimic MCI, and these should be looked for in patients presenting with cognitive loss.
- Impaired functional status as reflected in activities of daily living is an important sign of progression from MCI to dementia.
- Acetylcholinesterase inhibitors are not approved for treating MCI, have shown little efficacy in altering progression to dementia, and have multiple side effects.
- Enhancing physical and mental health and developing strategies to compensate for deficits are key management approaches.
Is an adult with Asperger syndrome sitting in your waiting room?
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
In 1944, Hans Asperger described a subset of children who exhibited “a lack of empathy, little ability to form friendships, one-sided conversation, intense absorption in a special interest, and clumsy movements.”1
In recent years, Asperger syndrome has become increasingly recognized in the medical community and by the general public. It has been popularized in the media in John Elder Robison’s bestselling book, Look Me in the Eye; with the television character Sheldon Cooper in The Big Bang Theory; and in the 2009 film, Adam, a romantic comedy with the title character accurately portraying a young man with Asperger syndrome.
In this article, we discuss the causes and characteristics of Asperger syndrome, with special focus on adults: how it presents, how to treat it, and how to enhance the delivery of care.
PREVALENCE SEEMS TO BE INCREASING
One in 88 children is diagnosed with an autism spectrum disorder, and the rates of Asperger syndrome and other autism spectrum disorders appear to be increasing.2 Whether this increase is the result of more thorough assessment and identification or of environmental changes is hotly debated.3 The rise began before the proposed changes to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) to combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise specified to simplify diagnosis.4 Asperger syndrome affects males three to four times more often than females.5 For most patients, the effects persist throughout life.
BEHAVIORAL IMPAIRMENTS CHARACTERIZE THE SYNDROME
Poor social skills are a hallmark
People with Asperger syndrome struggle with social interaction and face challenges in forming and maintaining relationships. They tend to have less eye contact (often the first indicator), smiling, animated speech, and physical communication such as hand gestures. They tend not to solicit another’s attention to something they themselves find interesting. They often lack social and emotional reciprocity and have difficulty understanding another person’s thoughts or feelings,6 and they have marked difficulty reading social cues. Some adults may appear rigid, selfish, or narrow-minded.
Sometimes behavior is in the normal range but is out of context for a particular situation.7 For example, a preprofessional student with Asperger syndrome might walk into a psychiatric evaluation to assess fitness for duty and take a seat cross-legged on the floor and have a snack. Poor grooming inappropriate for the occasion may also be observed, such as showing up for a formal photo with unkempt hair and in a stained shirt that is half tucked in.
Many adults with autism spectrum disorders are oblivious to their social reputation.8 They are often unaware that their behavior is out of place and only learn that it is not normal when they are told. Others recognize that they have trouble empathizing with or understanding the perspectives of others, but they are at a loss as to how to improve. The syndrome has a tremendous impact on broader aspects of life, such as employment, functional independence, relationships, and social networks.
Other odd behaviors are common
Repetitive behaviors. Many patients with Asperger syndrome have repetitive behaviors, which can manifest as repeating phrases or expressions, attempting to imitate others, and rocking. They tend to follow routines, do not enjoy spontaneity, and are more inflexible and uncomfortable when their planned regimen is altered.
Gait or balance issues may be observed on physical examination.9 Uncoordinated motion and clumsiness are common,10 and some patients may have a bouncy, stilted gait or may walk on their toes, although the latter is more common in children than adults. Many patients have illegible handwriting.11
Fixations. Many Asperger patients have unusual and intense obsessions with subjects like numbers, dates, or aerodynamics of planes. Children with such fascinations are described as “little professors” or as having “geek syndrome.”12 Certain obsessions often continue into adulthood, although one area of interest may fade and another may take over. Such “expertise” in adults may gain them respect, even though they may seem very odd in other ways.
Lack of boundaries. Patients with Asperger syndrome tend to have poor spatial awareness and to be unaware of physical boundaries, standing too close for others’ comfort or unusually far away. Lack of boundaries may extend beyond the physical, as patients may inappropriately help themselves to food or use an item belonging to another without invitation, being unaware that the behavior may be intrusive or inappropriate.
BEHAVIORAL ASSESSMENTS HELP MAKE DIAGNOSIS
Asperger syndrome is most often diagnosed in early childhood, although it may remain undiagnosed into adulthood. Coexisting depression, attention deficit hyperactivity disorder, or anxiety disorders are also often present.
Establishing the diagnosis is aided by information from family members and others who interact with the patient, from the observations of trained professionals, and from self-reported data. However, self-reported assessments are not always reliable, because the syndrome can affect insight.
The most common assessment tool for autism spectrum disorders is the Autism Diagnostic Interview-Revised (ADI-R),13 a battery of tests given in a structured interview to identify and quantify symptoms, determine where a patient falls on the autism spectrum,14 and point toward interventions. The ADI-R also organizes critical developmental history to evaluate if something else is present, such as prodromal schizophrenia. Although the ADI-R can be very useful in the diagnostic process, it is based on parental reporting, which is neither always available nor fully reliable.
A specific diagnostic tool for adults is the Adult Asperger Assessment.15 Patients are asked to complete two screening questionnaires that gauge cognitive function and gather information about thinking, processing, and behavior.
Table 1 lists the criteria for Asperger syndrome from the DSM Fourth Edition, Text Revision (DSM-IV-TR).16 Asperger syndrome differs from general autism in that it is not associated with language delay. In addition, patients with Asperger syndrome usually have average or above-average IQ scores.17 Still, determining whether a patient has Asperger syndrome or high-functioning autism is sometimes challenging.6
In DSM-V, Asperger syndrome will be subsumed under autism spectrum disorder
In 2013, the DSM-V will replace the DSMIV-TR and will combine autism, Asperger syndrome, and pervasive developmental disorder not otherwise classified into a single diagnosis: autism spectrum disorder. The new system uses two instead of the previous three clusters of core symptoms, centered on “social reciprocity and communication” in one arm and “restricted interests and repetitive behavior” in the other.18 There will be less emphasis on play and imagination than in the past. Some authors suggest adding sensory criteria, particularly reduced pain and increased hearing sensitivity.19
The proposed system is sensitive and specific for autism spectrum disorders, allows early diagnosis, and indicates degree of severity.20 It is hoped that the new system, which accounts for the range and severity of symptoms, should help physicians refer patients to the correct level of treatment.
On the other hand, it may be difficult to think of the three disorders as a single diagnosis. Asperger syndrome manifests in distinct ways, and clear behavioral criteria for diagnosis can be invaluable in helping people with the syndrome. Also, the public may continue to refer to it as Asperger syndrome, and parents and patients may feel uncomfortable having it considered to be the same diagnosis as autism.
BEHAVIORAL THERAPY CAN HELP ACHIEVE INDEPENDENCE
Although there is no cure for Asperger syndrome, various interventions can dramatically improve quality of life and independence. The health care team may include a primary care physician, psychologist, psychiatrist, neurologist, and speech therapist.
Behavioral therapies can help patients with Asperger syndrome learn skills to reduce their symptoms. Occupational and physical therapy can improve dexterity, fluidity, and coordination. Desensitization training may help patients adapt to uncomfortable sights, sounds, or smells that may arise. This can be critical in a job situation. For example, while an average person exposed to a foul odor in public is likely to react tactfully, a person with Asperger syndrome may scream loudly, make inappropriate comments, or run from the room. Social training, especially targeted to the workplace, can provide strategies for promoting typical behaviors and be key to maximizing functional independence.
Speech therapists can teach patients how to sound more relaxed and help them master the natural give-and-take of conversational exchange. Psychotherapy can provide a safe place to work on anxiety, express emotions, and manage restricted interests or repeated behaviors. Group therapy or social training can be a venue for learning improved interactions.
Living independently can be very challenging, and patients with Asperger syndrome may need functional independence training to help with a variety of skills, from handling finances to organizing the home.
Improving quality of life includes determining the best learning environments from childhood into college years and beyond.21–23 Socialization can be enhanced with additional social support at home or on campus, through family interactions and collaborative learning, and by teaching empathy.24 Vocational training can be extremely useful.
DRUG THERAPY MAY HAVE A ROLE
Medications are not usually prescribed unless depression or anxiety is also present, but they may also help manage irritability, anger, stereotypical mannerisms, and disturbing movements. Fluoxetine (Prozac) helps reduce repetitive behaviors in adults with Asperger syndrome. Propranolol (Inderal), a well-known antihypertensive, is also used for performance anxiety and improves word fluency, understanding of verbal communication, and verbal problem-solving in patients with an autism spectrum disorder.25
Giving oxytocin (Pitocin) intranasally in a spray formulation is currently being tested to enhance social skills. Patients with an autism spectrum disorder were more able to perceive emotions of others and to respond more appropriately.26 Oxytocin has long been associated with bonding and is believed to enhance mothering skills. It is naturally present in both sexes, but levels are higher in women, which may in part explain the lower rate of autism spectrum disorders in females.27
HEALTH CARE REQUIRES SPECIAL CONSIDERATIONS
Medical care for patients with Asperger syndrome is enhanced by understanding the patient’s experience. Adults, in particular, may have learned to suppress symptoms of Asperger syndrome to better function in society but still experience stress in situations in which others would not. Patients with Asperger syndrome may struggle with social interactions during medical examinations or procedures, and clinicians may find interaction with the patient challenging.
It is important for health care providers to be calm and patient and to understand that anxiety may prevent people with Asperger syndrome from making eye contact. The clinician should confirm that a patient is engaged but should avoid seeming pushy or invasive.
When anxious, patients may employ strange gestures that they find soothing, such as flapping the hands, rocking, or cracking the knuckles. It is usually easier to allow them to continue unless the activity hinders the examination or treatment.
Patients are likely to respond better to direct requests than to subtle questions: eg, “Open your mouth, please” instead of “Could you open your mouth?” Using clear, specific language and avoiding metaphors, irony, and nonverbal communication are best. It is important to explicitly ask for everything needed, as patients may not volunteer information and may have trouble articulating what they are thinking or feeling. While educating patients about their health needs, physicians may need to reiterate guidance several times or approach the same topic from different angles in order for the patient to accept a concern.
All actions, especially touching the patient, should be explained clearly beforehand. If possible, the doctor should demonstrate using visuals or on his or her own body if appropriate. For invasive procedures, anesthetizing the local area is recommended.
People with Asperger syndrome often rely heavily on a regular routine to maintain a sense of organization. By interrupting this routine, a doctor’s visit can induce anxiety. Waiting also increases anxiety, so scheduling patients with Asperger syndrome either first or last in the day may help.
Hypersensitivity poses challenges
Many people with Asperger syndrome have abnormal sensitivity to stimuli, with differences in pain sensation and hearing perhaps most prominent. Loud noises, such as beeping equipment, whirring fans, or buzzing lights may be distressful and should be reduced if possible. Patients may also be strongly affected by bright lights or scents such as perfumes.
Patients may also have an altered sense of taste, with consequences that go beyond simple “picky eating.” Patients should be asked about unusual eating patterns, diets, or food aversions. People with autism spectrum disorders often do not consume adequate vitamin C because of an aversion to fruits and vegetables. Vitamin deficiency may have originated in infancy but may not be identified or treated until adulthood.28
The sense of touch may be intensified, causing patients to be extremely ticklish; they may actually prefer to be touched more firmly. When it is necessary to make physical contact with patients, it will make the process easier if the physician determines their comfort level and finds ways to help them endure the experience with the least amount of discomfort.
Some patients with impaired sensory expression may have a high tolerance for extreme temperatures and pain, leading to delay in seeking aid.29 Patients may downgrade pain levels, masking the severity of an illness or injury.
Transition from pediatric to adult care
Pediatrics is often a warm environment in which children develop a trusting relationship with their care providers. The transition to adult care can be daunting for patients with Asperger syndrome and their families, and many postpone the change for as long as possible.
Although time-consuming, a collaborative effort between the pediatric and adult care teams can dramatically smooth the transition. It can help to have a familiar person from the pediatric team, such as a nurse, be present at the initial interaction with the new adult care team. Both teams should be familiar with the other’s clinical practices and be aware of the patient’s stressors and ways to ameliorate them.30
THE SEARCH FOR A CAUSE CONTINUES
Numerous studies are attempting to understand the anatomic and physiologic causes of autism spectrum disorders, and to find effective treatments and improve the quality of life.
Prenatal factors implicated
Several recent studies have focused on environmental factors during pregnancy as risk factors for autism spectrum disorder. Selective serotonin reuptake inhibitors were found to increase the risk,31 but the severity of the mother’s depressive illness must be considered before counseling against using these drugs. Older maternal or paternal age was also found to increase the risk of an autism spectrum disorder.32 Recent research indicates that older fathers are in particular more likely to have children with disorders such as autism because of an increase in random mutations associated with advanced age.33
Maternal illness during pregnancy is also associated. Preliminary studies found an increased risk of autism if the mother had had a prenatal viral infection.34 A more recent study found that untreated fever during pregnancy rather than a specific viral infection is more strongly linked.35
Maternal antibodies have been implicated as well. One review found that psoriasis is the only maternal autoimmune condition significantly associated with the development of an autism spectrum disorder.36 Elevated levels of antibodies against the fetal brain have been found in mothers with autistic children.37 One study found that autistic children and their siblings have elevated antibrain antibodies in distinct brain regions, including the caudate nucleus, putamen, prefrontal cortex, cerebellum, and cingulate gyrus (why the siblings are spared from having the disorder is unclear).38 Some have questioned whether a child’s own immune system might even be involved.39
Functional magnetic resonance imaging reveals multiple differences
Functional magnetic resonance imaging (fMRI) has been used to investigate impaired social interaction, specific deficits of facial perception and recognition, sensory processing, working memory, and “theory of mind.” Hypoactivation, hyperactivation, and decreased functional connectivity have been observed depending on the mental processes evaluated.40
When undergoing facial perception tasks, subjects with autism spectrum disorders exhibit hypoactivation in the lateral aspect of the middle region of the fusiform gyrus, responsible for face identification. But they have significant activation of the limbic system, specifically the amygdala, during facial recognition. Hypoactivity in the fusiform gyrus is observed when trying to identify faces or read facial expressions.41,42 This cluster of findings helps explain misinterpretations, misidentification, and heightened affect.
A hallmark characteristic of autism is the difficulty patients have in determining intentions and interpreting others’ behavior, thoughts, or emotions. Studies of people with autism spectrum disorders show that areas often responsible for “sensitivity to others” are hypoactive.43 There is also diminished activation in the medial cingulate cortex, normally activated when these people are asked to think about themselves and who they are.44
The resting state in the brains of people with autism spectrum disorders is abnormally activated.45 They are often particularly good at attention to detail but challenged in integrating information needed for general executive functioning. Impaired sensory processing makes it difficult for them to simultaneously interpret multiple sources of sensory input.46
Perhaps some of the most exciting fMRI news comes from infant studies. Radical and axial diffusivity and fractional anisotropy techniques demonstrate differences in the brains of infants 6 to 24 months old, before symptoms of autism spectrum disorders are observed. It is hoped that early intervention could come into play before the syndrome develops fully.47
The synthesis of input of social and emotional cues is sometimes referred to in the literature as “theory of mind.” It is impaired in Asperger syndrome,48 as manifested by a lack of empathy and by challenges in perceiving others’ thoughts and feelings. The basis of impairment may be related to abnormalities in the amygdala.49 Normal awareness involves the integration of multiple neural networks in the anterior paracingulate cortex, the superior temporal sulci, and the temporal poles bilaterally, but different regions appear to be used in patients with Asperger syndrome.50 A small series of five case studies using positron emission tomography indicated that the left prefrontal cortex was the primary location for theory of mind in Asperger syndrome.51
Epilepsy, gastrointestinal problems, and sleep disturbances are associated
About 25% of people with autism spectrum disorders have epilepsy vs 2% to 3% in the general population. Asperger syndrome is associated with a much lower but still elevated risk of 4% to 6%.47,52
Gastrointestinal complaints, most often constipation or chronic diarrhea, are much more common in children with autism spectrum disorders than in the general population. Preliminary data showed that children with an autism spectrum disorder have a 42% rate of gastrointestinal problems vs 12% in unaffected siblings. There is also a correlation between the severity of gastrointestinal problems and severity of autistic symptoms.53
Research is ongoing to determine the prevalence of insomnia or interrupted sleep in those with autism spectrum disorders.54–56 Changes in sleep architecture can explain nighttime activity.
NONTRADITIONAL CONSIDERATIONS
Dietary treatment: Mixed findings
A popular hypothesis is that adherence to a gluten-free or casein-free diet can reduce symptoms of autism spectrum disorders. Preliminary reports identified several cases of children showing improvement.57 However, this has not been replicated, and more studies refute benefits of these diets.58
Essential nutritional needs should be met with any diet, whether it is designed to reduce symptoms or not. Patients with autism spectrum disorders may have strong food aversions, and dietary supplements of vitamins and minerals may be required.
Vaccines do not cause autism
Despite popular concern, recent research indicates that vaccines do not cause autism. Thimerosal, a mercury-based preservative used in childhood vaccines, was at one time implicated as a risk factor for autism spectrum disorders. The US Centers for Disease Control and Prevention (CDC) issued a precaution against using thimerosal-containing vaccines while testing was done to determine the effects on neuropsychological development.59 The CDC study as well as newer studies did not demonstrate that exposure to mercury causes these neuropsychological concerns, but researchers have continued to study the subject.60–62 The original study implicating thimerosal was disproven as scientifically unsound and fraught with conflict of interest and legal concerns. It has since been retracted, and its findings have been completely discredited.63
Other areas of research
Current research is exploring the higher prevalence of autism spectrum disorders in particular families.64–66 Autism and autism spectrum disorders may be caused by hundreds of simultaneous gene alterations or may develop as a result of reduced gene expression in two areas of the cerebral cortex where higher-order processing occurs, in the frontal and temporal lobes.67
Although genetic theories of autism predominate, a 2011 project suggests that environment is also important. A study of twins found that genetics accounted for 40% or less of cases of autism spectrum disorder, with at least 55% of cases being attributable to environmental factors.68
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
- Frith U, editor. Autism and Asperger Syndrome. New York: Cambridge University Press, 1991:37–92.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 2012; 61( 3):1–19.
- Rutter M. Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 2005; 94:2–15.
- Happé F. Criteria, categories, and continua: autism and related disorders in DSM-5. J Am Acad Child Adolesc Psychiatry 2011; 50:540–542.
- National Institute of Neurological Disorders and Stroke. Asperger syndrome fact sheet. http://www.ninds.nih.gov/disorders/asperger/detail_asperger.htm. Accessed October 11, 2012.
- Toth K, King BH. Asperger’s syndrome: diagnosis and treatment. Am J Psychiatry 2008; 165:958–963.
- Vermeulen P. Autism: from mind blindness to context blindness. Asperger’s Digest November/December 2011. http://autismdigest.com/autism-from-mind-blindness-to-context-blindness/. Accessed October 11, 2012.
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proc Natl Acad Sci U S A. 2011; 108:17302–17307.
- Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger’s syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr 2001; 22:92–101.
- Siaperas P, Ring HA, McAllister CJ, et al. Atypical movement performance and sensory integration in Asperger’s syndrome. J Autism Dev Disord 2012; 42:718–725.
- Kushki A, Chau T, Anagnostou E. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J Autism Dev Disord 2011; 41:1706–1716.
- Nash JM, Bonesteel A. The geek syndrome. Time Magazine U.S. 2002. http://www.time.com/time/magazine/article/0,9171,1002365-1,00.html. Accessed October 11, 2012.
- Le Couteur A, Rutter M, Lord C, et al. Autism diagnostic interview: a standardized investigatorbased instrument. J Autism Dev Disord 1989; 19:363–387.
- Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised WPS Edition Manual. Los Angeles, CA. Western Psychological Services; 2003.
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger’s Assessment (AAA): a diagnostic method. J Autism Developmental Disord 2005; 35:807–819.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Text Revision DSM IV–TR 4th Ed. 2000; Washington, DC: American Psychiatric Association; 2000:80–84.
- Centers for Disease Control. Asperger syndrome fact sheet. http://www.cdc.gov/ncbddd/actearly/pdf/parents_pdfs/Asperger_Syndrome.pdf. Accessed October 11, 2012.
- Peckham C. The current state in autism—still tough to treat but encouraging progress. An expert interview with Fred R. Volkmar, MD. Medscape Pediatrics 2010. http://www.medscape.com/viewarticle/720802?src=mp&spon=17. Accessed October 31, 2012.
- Muscari ME. How should I evaluate an adult for possible Asperger’s syndrome? Medscape News Today 2006.
- Hollander E. Can we treat core symptoms of autism spectrum disorders in adults? December 21, 2011; 1( 18). http://www.medscape.com/viewarticle/531750. Accessed October 1, 2012.
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger’s syndrome and other autism spectrum disabilities. Autism 2008; 12:173–190.
- Helman T, Berger O. Parents of children with Asperger’s syndrome or with learning disabilities: family environment and social support. Res Dev Disabil 2008; 29;289–300.
- Taylor CM. Campus commons. When pigs fly: a new perspective on learning. About Campus 2011; 16:30–32.
- Cheng Y, Chiang H, Ye J, Cheng L. Enhancing empathy instruction using a collaborative virtual learning environment for children with autistic spectrum conditions. Comput Edu 2010; 55:1449–1458.
- Beversdorf DQ, Saklayen S, Higgins KF, Bodner KE, Kanne SM, Christ SE. Effect of propranolol on word fluency in autism. Cogn Behav Neurol 2011; 24:11–17.
- Kuehn BM. Scientists probe oxytocin therapy for social deficits in autism, schizophrenia. JAMA 2011; 305:659–661.
- Pfaff DW, Rapin I, Goldman S. Male dominance in autism: neuroendocrine influences on arousal and social anxiety. Autism Res 2011; 4:163–176.
- Brauser D. Children with autism routinely exhibit feeding difficulties in infancy. Medscape Medical News 2010. http://www.medscape.org/viewarticle/726060. Accessed October 31, 2012.
- Baron MG, Groden J, Groden G, Lipsitt L. Stress and coping in autism. New York: Oxford University Press; 2006:355.
- Camfield P, Camfield C. Transition to adult care for children with chronic neurological disorders. Ann Neurol 2001; 69:437–444.
- Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011; 68:1104–1112.
- Croen LA, Najjar DV, Fireman B, Grether JK. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007; 161:334–340.
- Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 2012; 488:471–475.
- Atlandóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010; 40:1423–1430.
- Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2012; 10.1007/s10803-012-1540-x.
- Crown LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med 2005; 159:151–157.
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neruoimmunol 2008; 194:165–172.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol 2006; 178:149–155.
- Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity Rev 2004; 3:557–562.
- South M, Diehl JJ. Functional magnetic resonance imaging. In:Hollander E, Kolevzon A, Coyle J, editors. Textbook of Autism Spectrum Disorders. Washington, DC: American Psychiatric Publishing; 2011:409–414.
- Shultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry 2000; 57:331–340.
- Wang AT, Dapretto M, Hariri AR, et al. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:481–490.
- Mason RA, Williams DL, Kana RK, et al. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia 2008; 46:269–280.
- Chiu PH, Kayali MA, Kishida KT, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 2008; 57:463–437.
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A 2006; 103:8275–8280.
- Bölte S, Hubl D, Dierks T, et al. An fMRI-study of locally oriented perception in autism: altered early visual processing of the block design test. J Neural Transm 2008; 115:545–552.
- Maski KP, Jeste SS, Spencer SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011; 23:609–615.
- Kleinman J, Marciano P, Ault RL. Advanced theory of mind in high functioning adults with autism. J Autism Dev Disord 2011; 31:29–36.
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind and executive functions in a patient with early left amygdala damage. Brain 2001; 124:287–298.
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind.’ Trends Cogn Sci 2003; 7:77–83.
- Happé F, Ehlers S, Pletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger’s syndrome. Neuroreport 1996; 8:197–207.
- Kugimiya S. Clinical features and possible correlations between autism and epilepsy. Neurology Asia 2010; 15(suppl 1):44–45.
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32:351–360.
- Bruni O, Ferri R, Vittori E, et al. Sleep architecture and NREM alterations in children and adolescents with Asperger syndrome. Sleep 2007; 30:1577–1585.
- Richdale AL, Schreck KA. Sleep problems in autism spectrum disorders: prevalence, nature, & possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411.
- Paavonen EJ, Vehkalahti K, Vanhala R, von Wendt L, Nieminen-von Wendt T, Aronen ET. Sleep in children with Asperger’s syndrome. J Autism Dev Disord 2007; 38:41–51.
- Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord 2006; 36:413–420.
- Keller DM. Diet free of gluten and casein has no effect on autism symptoms. Medscape News May 24, 2010. http://www.medscape.com/viewarticle/722283.
- Centers for Disease Control and Prevention (CDC). Recommendations regarding the use of vaccines that contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep 1999; 48:996–998.
- Thompson WW, Price C, Goodson B, et al; Vaccine Safety Datalink Team. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med 2007; 357:1281–1292.
- Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics 2010; 126:656–664.
- Centers for Disease Control and Prevention (CDC). CDC study on “Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism.” www.cdc.gov/vaccinesafety/Concerns/Thimerosal/QA_Pediatrics-thimerosal-autism.html. Accessed November 5, 2012.
- Deer B. How the case against the MMR vaccine was fixed. BMJ 2011; 342:c5347.
- Losh M, Sullivan PF, Trembath D, Piven J. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol 2008; 67:829–837.
- Curran S, Bolton P. Genetics of autism. In:Kim Y-K, editor. Handbook of Behavior Genetics, Part IV. New York, NY: Springer; 2009:397–410.
- State MW. The genetics of child psychiatric disorders: focus on autism and Tourette syndrome. Neuron 2010; 68:254–269.
- Voineagu I, Wang X, Johnston P, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474:380–384.
- Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095–1102.
KEY POINTS
- Indicators of Asperger syndrome include lack of eye contact, inappropriate comments, odd posture, high anxiety, and intensely focused interests.
- Asperger syndrome is evident in childhood, but it also presents undiagnosed in adulthood.
- Physicians should be aware of patients’ social differences and increased sensitivities in order to improve health care delivery.
- Episodic cognitive behavioral therapy addressing interpersonal skills can dramatically improve quality of life and independence.
- Proposed diagnostic changes scheduled to take effect in 2013 involve including Asperger syndrome as an autism spectrum disorder.
Why (and how) we must repeal the sustainable growth rate
Imagine this: Your 20-year-old daughter tells you she wants to attend an expensive school for 5 years of intensive postgraduate training, amassing tens of thousands of dollars of debt, to provide expert services to the US population. There is no good substitute for the services she hopes to provide, and they are vitally needed. The services also carry risk. Despite this, she tells you that her salary will not increase every year in tandem with the cost of living; in fact, she expects her salary to be cut by nearly one-third each year. Compensation in her chosen field hasn’t increased in real dollars for many years.
Sound like a good plan?
By now you have recognized this as your own story, at least if you’re among the 92% of ObGyns who participate in Medicare.
ObGyn participation in the Medicare program reflects ObGyn training and commitment to serve as lifelong principal care physicians for women of all ages, including women with disabilities. Fifty-six percent of all Medicare beneficiaries are women. With continuing shortages of primary care physicians and the transitioning of the Baby Boomer generation to Medicare, it is likely that ObGyns will become more involved in delivering health care to this population.
Medicare physician payments matter to ObGyns in other ways, too, because TRICARE and private payers often follow Medicare payment and coverage policies. Clearly, the Medicare program is a pretty big gorilla in every exam room. We all have much at stake in ensuring a stable Medicare system for years to come, starting with an improved physician payment system.
In 2011, Medicare paid $68 billion for physician care provided to nearly 50 million elderly and disabled individuals—about 12% of total Medicare spending—covering just over 1 billion distinct physician services. Physicians received a 10-month reprieve from a 27% cut in Medicare payments that had been scheduled for March 1, 2011, extending current payment rates through the end of this year. The agreement is part of a deal to extend a payroll tax cut and unemployment benefits. It is the 14th short-term patch to the sustainable growth rate (SGR) in the past 10 years. On January 1, 2013, we now face a 26.5% cut that Congress will have to find $245 billion to eliminate altogether.
How did we get here?
In 1997, Congress passed the Balanced Budget Act (BBA), at a time when many members of Congress were frustrated by continued increases in Medicare costs, fueled on the physician side, in part, by increases in the number of visits, tests, and procedures. To control these costs, Congress included in the BBA a complicated formula to peg Medicare physician payments to an economic growth target—the SGR. For the first few years, Medicare expenditures stayed within the target, and doctors received modest pay increases. But in 2002, expenditures rose faster than the SGR, and doctors were slated for a 4.8% pay cut.
Every year since, the SGR has signaled physician pay cuts, and every year, Congress has stopped the cuts from taking effect. But each deferral just made the next cut bigger and increased the price tag of stopping each pay cut. Today, the price of eliminating the SGR is $245 billion over the next 10 years. In these days of sequestration and deficit reduction, $245 billion is hard to find.

What now?
The good news is that support for eliminating the SGR is bicameral and bipartisan, rare in these hyperpartisan political days. Both Republicans and Democrats in the US House and Senate agree: The SGR has got to go. It’s a topic of conversation that wore out its welcome long ago.
The bad news? The $245 billion price tag. Remember, the SGR is in statute, so it requires an Act of Congress, signed by the President, to repeal it—and every Act is scored by the Congressional Budget Office.
The likeliest scenario is one we’ve seen many times before: Congress returns from a difficult election for a short, lame-duck session, during which it will have to address the cut before January 1. A real solution won’t be within reach, so Congress will likely kick that well-dented can a few more yards down the road, delaying the cut for yet another legislative cliffhanger.
In October 2012, the American Congress of Obstetricians and Gynecologists (ACOG) joined the American Medical Association (AMA) and 110 state and national medical societies in providing the US Congress with a clear and definitive document—Driving Principles and Core Elements—that describes a way to transition to a Medicare payment system that will endure and ensure high-quality care for the individuals who rely on that program, and for many millions more whose care is linked to Medicare payment policies.
This document is unique in many ways, perhaps especially in the unity it demonstrates among all physician organizations. It echoes ACOG’s earlier guidance to the US Congress on essential elements for a Medicare payment system that benefits women’s health. Among ACOG’s recommendations:
Make the new system simple, coordinated, and transparent. A new Medicare physician payment system should coordinate closely with other health-care programs; ensure that information technology is interoperable; and guarantee that quality-measurement programs are the same across all payers and rely on high-quality, risk-adjusted data.
Maintain the global obstetric care package. Medicare currently uses this package to reimburse for pregnancy. It works well and may be a model for global payment options for care provided by other physician types. The global obstetric care payment covers 10 months of care, from the first antepartum visit through the final postdelivery office visit.
Global payments allow a physician to manage costs and care for a patient’s course of treatment, rather than for a patient’s individual medical encounters.
Maintain fee for service for women’s health physicians who have small Medicare populations. Depending on the practice mix, type, and area, ObGyns and ObGyn subspecialists could see relatively few Medicare patients; unique Medicare requirements can pose significant administrative challenges and create inefficiencies with participation. Physicians who have small numbers of Medicare patients must be accommodated—and not penalized—in a new payment system.
Ensure that payment fairly and accurately reflects the cost of care. Medicare payments to obstetricians are already well below the cost of maternity care; no further cuts should be allowed for this care.
Support innovative care models, including a women’s medical home. These models should recognize the dual role that ObGyns may play as primary care and specialty care physicians.
Repeal the Independent Medicare Payment Advisory Board. Leaving Medicare payment decisions in the hands of an unelected, unaccountable body with minimal Congressional oversight is just a bad idea.
Pass medical liability reform. Congress must enact meaningful medical liability reform, which the Congressional Budget Office says could save $40 billion—enough for a small downpayment on SGR repeal.
A continuing promise
Rest assured that ACOG’s work to ensure appropriate Medicare payments to physicians, and to ensure that your patients have access to needed care, won’t stop until the job is done.
ACOG, AMA, and 110 state and national medical societies think so, and prescribe driving principles and core elements for the transition
In their letter to Congressional leaders, ACOG, AMA, and other societies acknowledged the “profound change” sweeping through the US health-care system, noting that it offers a “unique opportunity to improve and restructure how we deliver and pay for care.” When it comes to the SGR, however, these organizations conclude that it is “an enormous impediment to successful health-care delivery and payment reforms that can improve the quality of patient care while lowering growth in costs. Physicians facing the constant specter of severe cuts under the SGR cannot invest their time, energy, and resources in care redesign. The first step in moving to a higher-performing Medicare program must be the elimination of the SGR formula,” they write, based on the following principles, values, and key reforms.
Driving principles
- Successful delivery reform is an essential foundation for transitioning to a high-performing Medicare program that provides patient choice and meets the health-care needs of a diverse patient population.
- The Medicare program must invest in and support physician infrastructure that provides the platform for delivery and payment reform.
- Medicare payment updates should reflect the cost of providing services as well as efforts and progress on quality improvements and managing costs.
Core elements of reform
- Reflect the diversity of physician practices and provide opportunities for physicians to choose payment models that work for their patients, practice, specialty, and region.
- Encourage incremental changes with positive incentives and rewards during a defined timetable instead of using penalties to order abrupt changes in the delivery of care.
- Provide a way to measure progress and show policymakers that physicians are taking accountability for quality and costs.
Recommended structural improvements
- Reward physicians for savings achieved across the health-care spectrum.
- Enhance prospects for physicians adopting new models to achieve positive updates.
- Tie incentives to physicians’ own actions, rather than the actions of others or variables beyond their influence.
- Enhance prospects to harmonize measures and alter incentives in current law.
- Encourage systems of care, regional collaborative efforts, and primary care and specialist cooperation while preserving patient choice.
- Allow specialty and state society initiatives to be credited as delivering improvements (deeming authority) and recognize the central role of the profession in determining and measuring quality.
- Provide exemptions and alternative pathways for physicians in practice situations in which making or recovering the investments that may be needed to improve care delivery would constitute a hardship.
We want to hear from you! Tell us what you think.
Women’s health under the Affordable Care Act: What is covered?
(September 2012)
Lay midwives and the ObGyn: Is collaboration risky?
(May 2012)
How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012)
Is private ObGyn practice on its way out?
(October 2011)
14 questions (and answers) about health reform and you
with Janelle Yates, Senior Editor (August 2010)
Imagine this: Your 20-year-old daughter tells you she wants to attend an expensive school for 5 years of intensive postgraduate training, amassing tens of thousands of dollars of debt, to provide expert services to the US population. There is no good substitute for the services she hopes to provide, and they are vitally needed. The services also carry risk. Despite this, she tells you that her salary will not increase every year in tandem with the cost of living; in fact, she expects her salary to be cut by nearly one-third each year. Compensation in her chosen field hasn’t increased in real dollars for many years.
Sound like a good plan?
By now you have recognized this as your own story, at least if you’re among the 92% of ObGyns who participate in Medicare.
ObGyn participation in the Medicare program reflects ObGyn training and commitment to serve as lifelong principal care physicians for women of all ages, including women with disabilities. Fifty-six percent of all Medicare beneficiaries are women. With continuing shortages of primary care physicians and the transitioning of the Baby Boomer generation to Medicare, it is likely that ObGyns will become more involved in delivering health care to this population.
Medicare physician payments matter to ObGyns in other ways, too, because TRICARE and private payers often follow Medicare payment and coverage policies. Clearly, the Medicare program is a pretty big gorilla in every exam room. We all have much at stake in ensuring a stable Medicare system for years to come, starting with an improved physician payment system.
In 2011, Medicare paid $68 billion for physician care provided to nearly 50 million elderly and disabled individuals—about 12% of total Medicare spending—covering just over 1 billion distinct physician services. Physicians received a 10-month reprieve from a 27% cut in Medicare payments that had been scheduled for March 1, 2011, extending current payment rates through the end of this year. The agreement is part of a deal to extend a payroll tax cut and unemployment benefits. It is the 14th short-term patch to the sustainable growth rate (SGR) in the past 10 years. On January 1, 2013, we now face a 26.5% cut that Congress will have to find $245 billion to eliminate altogether.
How did we get here?
In 1997, Congress passed the Balanced Budget Act (BBA), at a time when many members of Congress were frustrated by continued increases in Medicare costs, fueled on the physician side, in part, by increases in the number of visits, tests, and procedures. To control these costs, Congress included in the BBA a complicated formula to peg Medicare physician payments to an economic growth target—the SGR. For the first few years, Medicare expenditures stayed within the target, and doctors received modest pay increases. But in 2002, expenditures rose faster than the SGR, and doctors were slated for a 4.8% pay cut.
Every year since, the SGR has signaled physician pay cuts, and every year, Congress has stopped the cuts from taking effect. But each deferral just made the next cut bigger and increased the price tag of stopping each pay cut. Today, the price of eliminating the SGR is $245 billion over the next 10 years. In these days of sequestration and deficit reduction, $245 billion is hard to find.

What now?
The good news is that support for eliminating the SGR is bicameral and bipartisan, rare in these hyperpartisan political days. Both Republicans and Democrats in the US House and Senate agree: The SGR has got to go. It’s a topic of conversation that wore out its welcome long ago.
The bad news? The $245 billion price tag. Remember, the SGR is in statute, so it requires an Act of Congress, signed by the President, to repeal it—and every Act is scored by the Congressional Budget Office.
The likeliest scenario is one we’ve seen many times before: Congress returns from a difficult election for a short, lame-duck session, during which it will have to address the cut before January 1. A real solution won’t be within reach, so Congress will likely kick that well-dented can a few more yards down the road, delaying the cut for yet another legislative cliffhanger.
In October 2012, the American Congress of Obstetricians and Gynecologists (ACOG) joined the American Medical Association (AMA) and 110 state and national medical societies in providing the US Congress with a clear and definitive document—Driving Principles and Core Elements—that describes a way to transition to a Medicare payment system that will endure and ensure high-quality care for the individuals who rely on that program, and for many millions more whose care is linked to Medicare payment policies.
This document is unique in many ways, perhaps especially in the unity it demonstrates among all physician organizations. It echoes ACOG’s earlier guidance to the US Congress on essential elements for a Medicare payment system that benefits women’s health. Among ACOG’s recommendations:
Make the new system simple, coordinated, and transparent. A new Medicare physician payment system should coordinate closely with other health-care programs; ensure that information technology is interoperable; and guarantee that quality-measurement programs are the same across all payers and rely on high-quality, risk-adjusted data.
Maintain the global obstetric care package. Medicare currently uses this package to reimburse for pregnancy. It works well and may be a model for global payment options for care provided by other physician types. The global obstetric care payment covers 10 months of care, from the first antepartum visit through the final postdelivery office visit.
Global payments allow a physician to manage costs and care for a patient’s course of treatment, rather than for a patient’s individual medical encounters.
Maintain fee for service for women’s health physicians who have small Medicare populations. Depending on the practice mix, type, and area, ObGyns and ObGyn subspecialists could see relatively few Medicare patients; unique Medicare requirements can pose significant administrative challenges and create inefficiencies with participation. Physicians who have small numbers of Medicare patients must be accommodated—and not penalized—in a new payment system.
Ensure that payment fairly and accurately reflects the cost of care. Medicare payments to obstetricians are already well below the cost of maternity care; no further cuts should be allowed for this care.
Support innovative care models, including a women’s medical home. These models should recognize the dual role that ObGyns may play as primary care and specialty care physicians.
Repeal the Independent Medicare Payment Advisory Board. Leaving Medicare payment decisions in the hands of an unelected, unaccountable body with minimal Congressional oversight is just a bad idea.
Pass medical liability reform. Congress must enact meaningful medical liability reform, which the Congressional Budget Office says could save $40 billion—enough for a small downpayment on SGR repeal.
A continuing promise
Rest assured that ACOG’s work to ensure appropriate Medicare payments to physicians, and to ensure that your patients have access to needed care, won’t stop until the job is done.
ACOG, AMA, and 110 state and national medical societies think so, and prescribe driving principles and core elements for the transition
In their letter to Congressional leaders, ACOG, AMA, and other societies acknowledged the “profound change” sweeping through the US health-care system, noting that it offers a “unique opportunity to improve and restructure how we deliver and pay for care.” When it comes to the SGR, however, these organizations conclude that it is “an enormous impediment to successful health-care delivery and payment reforms that can improve the quality of patient care while lowering growth in costs. Physicians facing the constant specter of severe cuts under the SGR cannot invest their time, energy, and resources in care redesign. The first step in moving to a higher-performing Medicare program must be the elimination of the SGR formula,” they write, based on the following principles, values, and key reforms.
Driving principles
- Successful delivery reform is an essential foundation for transitioning to a high-performing Medicare program that provides patient choice and meets the health-care needs of a diverse patient population.
- The Medicare program must invest in and support physician infrastructure that provides the platform for delivery and payment reform.
- Medicare payment updates should reflect the cost of providing services as well as efforts and progress on quality improvements and managing costs.
Core elements of reform
- Reflect the diversity of physician practices and provide opportunities for physicians to choose payment models that work for their patients, practice, specialty, and region.
- Encourage incremental changes with positive incentives and rewards during a defined timetable instead of using penalties to order abrupt changes in the delivery of care.
- Provide a way to measure progress and show policymakers that physicians are taking accountability for quality and costs.
Recommended structural improvements
- Reward physicians for savings achieved across the health-care spectrum.
- Enhance prospects for physicians adopting new models to achieve positive updates.
- Tie incentives to physicians’ own actions, rather than the actions of others or variables beyond their influence.
- Enhance prospects to harmonize measures and alter incentives in current law.
- Encourage systems of care, regional collaborative efforts, and primary care and specialist cooperation while preserving patient choice.
- Allow specialty and state society initiatives to be credited as delivering improvements (deeming authority) and recognize the central role of the profession in determining and measuring quality.
- Provide exemptions and alternative pathways for physicians in practice situations in which making or recovering the investments that may be needed to improve care delivery would constitute a hardship.
We want to hear from you! Tell us what you think.
Women’s health under the Affordable Care Act: What is covered?
(September 2012)
Lay midwives and the ObGyn: Is collaboration risky?
(May 2012)
How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012)
Is private ObGyn practice on its way out?
(October 2011)
14 questions (and answers) about health reform and you
with Janelle Yates, Senior Editor (August 2010)
Imagine this: Your 20-year-old daughter tells you she wants to attend an expensive school for 5 years of intensive postgraduate training, amassing tens of thousands of dollars of debt, to provide expert services to the US population. There is no good substitute for the services she hopes to provide, and they are vitally needed. The services also carry risk. Despite this, she tells you that her salary will not increase every year in tandem with the cost of living; in fact, she expects her salary to be cut by nearly one-third each year. Compensation in her chosen field hasn’t increased in real dollars for many years.
Sound like a good plan?
By now you have recognized this as your own story, at least if you’re among the 92% of ObGyns who participate in Medicare.
ObGyn participation in the Medicare program reflects ObGyn training and commitment to serve as lifelong principal care physicians for women of all ages, including women with disabilities. Fifty-six percent of all Medicare beneficiaries are women. With continuing shortages of primary care physicians and the transitioning of the Baby Boomer generation to Medicare, it is likely that ObGyns will become more involved in delivering health care to this population.
Medicare physician payments matter to ObGyns in other ways, too, because TRICARE and private payers often follow Medicare payment and coverage policies. Clearly, the Medicare program is a pretty big gorilla in every exam room. We all have much at stake in ensuring a stable Medicare system for years to come, starting with an improved physician payment system.
In 2011, Medicare paid $68 billion for physician care provided to nearly 50 million elderly and disabled individuals—about 12% of total Medicare spending—covering just over 1 billion distinct physician services. Physicians received a 10-month reprieve from a 27% cut in Medicare payments that had been scheduled for March 1, 2011, extending current payment rates through the end of this year. The agreement is part of a deal to extend a payroll tax cut and unemployment benefits. It is the 14th short-term patch to the sustainable growth rate (SGR) in the past 10 years. On January 1, 2013, we now face a 26.5% cut that Congress will have to find $245 billion to eliminate altogether.
How did we get here?
In 1997, Congress passed the Balanced Budget Act (BBA), at a time when many members of Congress were frustrated by continued increases in Medicare costs, fueled on the physician side, in part, by increases in the number of visits, tests, and procedures. To control these costs, Congress included in the BBA a complicated formula to peg Medicare physician payments to an economic growth target—the SGR. For the first few years, Medicare expenditures stayed within the target, and doctors received modest pay increases. But in 2002, expenditures rose faster than the SGR, and doctors were slated for a 4.8% pay cut.
Every year since, the SGR has signaled physician pay cuts, and every year, Congress has stopped the cuts from taking effect. But each deferral just made the next cut bigger and increased the price tag of stopping each pay cut. Today, the price of eliminating the SGR is $245 billion over the next 10 years. In these days of sequestration and deficit reduction, $245 billion is hard to find.

What now?
The good news is that support for eliminating the SGR is bicameral and bipartisan, rare in these hyperpartisan political days. Both Republicans and Democrats in the US House and Senate agree: The SGR has got to go. It’s a topic of conversation that wore out its welcome long ago.
The bad news? The $245 billion price tag. Remember, the SGR is in statute, so it requires an Act of Congress, signed by the President, to repeal it—and every Act is scored by the Congressional Budget Office.
The likeliest scenario is one we’ve seen many times before: Congress returns from a difficult election for a short, lame-duck session, during which it will have to address the cut before January 1. A real solution won’t be within reach, so Congress will likely kick that well-dented can a few more yards down the road, delaying the cut for yet another legislative cliffhanger.
In October 2012, the American Congress of Obstetricians and Gynecologists (ACOG) joined the American Medical Association (AMA) and 110 state and national medical societies in providing the US Congress with a clear and definitive document—Driving Principles and Core Elements—that describes a way to transition to a Medicare payment system that will endure and ensure high-quality care for the individuals who rely on that program, and for many millions more whose care is linked to Medicare payment policies.
This document is unique in many ways, perhaps especially in the unity it demonstrates among all physician organizations. It echoes ACOG’s earlier guidance to the US Congress on essential elements for a Medicare payment system that benefits women’s health. Among ACOG’s recommendations:
Make the new system simple, coordinated, and transparent. A new Medicare physician payment system should coordinate closely with other health-care programs; ensure that information technology is interoperable; and guarantee that quality-measurement programs are the same across all payers and rely on high-quality, risk-adjusted data.
Maintain the global obstetric care package. Medicare currently uses this package to reimburse for pregnancy. It works well and may be a model for global payment options for care provided by other physician types. The global obstetric care payment covers 10 months of care, from the first antepartum visit through the final postdelivery office visit.
Global payments allow a physician to manage costs and care for a patient’s course of treatment, rather than for a patient’s individual medical encounters.
Maintain fee for service for women’s health physicians who have small Medicare populations. Depending on the practice mix, type, and area, ObGyns and ObGyn subspecialists could see relatively few Medicare patients; unique Medicare requirements can pose significant administrative challenges and create inefficiencies with participation. Physicians who have small numbers of Medicare patients must be accommodated—and not penalized—in a new payment system.
Ensure that payment fairly and accurately reflects the cost of care. Medicare payments to obstetricians are already well below the cost of maternity care; no further cuts should be allowed for this care.
Support innovative care models, including a women’s medical home. These models should recognize the dual role that ObGyns may play as primary care and specialty care physicians.
Repeal the Independent Medicare Payment Advisory Board. Leaving Medicare payment decisions in the hands of an unelected, unaccountable body with minimal Congressional oversight is just a bad idea.
Pass medical liability reform. Congress must enact meaningful medical liability reform, which the Congressional Budget Office says could save $40 billion—enough for a small downpayment on SGR repeal.
A continuing promise
Rest assured that ACOG’s work to ensure appropriate Medicare payments to physicians, and to ensure that your patients have access to needed care, won’t stop until the job is done.
ACOG, AMA, and 110 state and national medical societies think so, and prescribe driving principles and core elements for the transition
In their letter to Congressional leaders, ACOG, AMA, and other societies acknowledged the “profound change” sweeping through the US health-care system, noting that it offers a “unique opportunity to improve and restructure how we deliver and pay for care.” When it comes to the SGR, however, these organizations conclude that it is “an enormous impediment to successful health-care delivery and payment reforms that can improve the quality of patient care while lowering growth in costs. Physicians facing the constant specter of severe cuts under the SGR cannot invest their time, energy, and resources in care redesign. The first step in moving to a higher-performing Medicare program must be the elimination of the SGR formula,” they write, based on the following principles, values, and key reforms.
Driving principles
- Successful delivery reform is an essential foundation for transitioning to a high-performing Medicare program that provides patient choice and meets the health-care needs of a diverse patient population.
- The Medicare program must invest in and support physician infrastructure that provides the platform for delivery and payment reform.
- Medicare payment updates should reflect the cost of providing services as well as efforts and progress on quality improvements and managing costs.
Core elements of reform
- Reflect the diversity of physician practices and provide opportunities for physicians to choose payment models that work for their patients, practice, specialty, and region.
- Encourage incremental changes with positive incentives and rewards during a defined timetable instead of using penalties to order abrupt changes in the delivery of care.
- Provide a way to measure progress and show policymakers that physicians are taking accountability for quality and costs.
Recommended structural improvements
- Reward physicians for savings achieved across the health-care spectrum.
- Enhance prospects for physicians adopting new models to achieve positive updates.
- Tie incentives to physicians’ own actions, rather than the actions of others or variables beyond their influence.
- Enhance prospects to harmonize measures and alter incentives in current law.
- Encourage systems of care, regional collaborative efforts, and primary care and specialist cooperation while preserving patient choice.
- Allow specialty and state society initiatives to be credited as delivering improvements (deeming authority) and recognize the central role of the profession in determining and measuring quality.
- Provide exemptions and alternative pathways for physicians in practice situations in which making or recovering the investments that may be needed to improve care delivery would constitute a hardship.
We want to hear from you! Tell us what you think.
Women’s health under the Affordable Care Act: What is covered?
(September 2012)
Lay midwives and the ObGyn: Is collaboration risky?
(May 2012)
How state budget crises are putting the squeeze on Medicaid (and you)
(February 2012)
Is private ObGyn practice on its way out?
(October 2011)
14 questions (and answers) about health reform and you
with Janelle Yates, Senior Editor (August 2010)
Telepsychiatry: Overcoming barriers to implementation
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Although many states have substantial health services in urban areas, these services—particularly mental health care—are relatively scarce in rural areas.1 Telepsychiatry, in which clinicians provide mental health care from a distance in real time by using interactive, 2-way, audio-video communication (videoconferencing), could mitigate workforce shortages that affect remote and underserved areas.2 Psychiatry is one of the biggest users of telemedicine, which refers to any combination of communication technology and medicine.3-5 This article discusses telepsychiatry’s effectiveness in providing psychiatric diagnosis and treatment, and the clinical implications of this technology, including improving access, cost, and quality of mental health services.
Outcomes comparable to face-to-face care
Telepsychiatry is used primarily in rural areas or correctional institutions or with underserved populations such as veterans with posttraumatic stress disorder or children. Although the literature generally is weak, there has been more research on psychiatry than other medical specialties because psychiatric clinicians rely on mental status examinations and verbal communications, not physical exams. Telepsychiatry can be considered a part of an evolving “connected health” system that offers many benefits to patients and clinicians (Table).
Table
Benefits of telepsychiatry as part of a ‘connected health’ system
| Available to everyone |
| Health care is provided at the point of convenience |
| Patients are informed and empowered |
| Facilitates patient compliance, continuing education, ease of access into the health care system, and healthy behaviors |
| Clinical data are integrated with longitudinal electronic health records |
| Data are available to patients via his or her personal electronic medical record and authorized clinical providers |
| Data and transactions are secure to greatest practical extent |
| Other telehealth applications with demonstrated efficacy—eg, telephone, internet, e-mail, and text messaging interventions—can be used as well |
Barriers to implementation
Although telepsychiatry offers tremendous promise, implementation has not been widespread or easy. Potential barriers to implementation, such as cost and resistance to change, are associated with acceptance of new technology or practice in health care. In addition, there are several legal, regulatory, and technical barriers.
Institutional barriers. Physicians and other providers may not have access to timely, evidence-based information and may face challenges, such as time constraints, access to technical support, and complexity of large health care institutions, when integrating this information into clinical practice.16 Two studies17 found that after controlling for other barriers—eg, reimbursement and regulatory issues—providers are the most significant initial gatekeepers that affect telemedicine use. When designing a telemedicine system, project managers should prioritize providers’ needs, such as ease of use and incentives.18
States do not cover services provided by other mental health providers, except for Utah’s coverage for social workers. The American Psychiatric Association has 2 suggestions regarding this issue3:
- reimbursement for telepsychiatry services should follow customary charges for delivering the appropriate current procedural terminology code(s)
- a structure for reimbursement of collateral charges, such as technician and line time, should be identified.
Licensure. A physician conducting a telemedicine session with a patient in another state must be licensed in both his or her state and the patient’s state. Nurses and other allied health professionals have similar state licensing constraints. Sanders22 suggests 3 potential solutions:
- establishing a national licensing system
- assigning the responsibility of care to the referring physician, with the consulting physician’s opinion serving as “recommendation only”
- determining that the patient is being “electronically transmitted” to the consultant’s state.
Although telemedicine has embraced many communication technologies, live, interactive, 2-way, audio-video communication—called videoconferencing—is broadly synonymous with telemedicine and, more specifically, telepsychiatry.
Telepsychiatry primarily uses interactive audiovisual conferencing systems over high-bandwidth networks. The central component of interactive telepsychiatry is the codec (coder/decoder), which provides compression, decompression, and synchronization of audio and video signals; both patients and clinicians need a codec. A codec can be a separate device, but personal computer-based codecs are being used more frequently. A typical setup also includes a video camera, microphone, speakers or headset, and 1 or 2 display monitors at both the clinician’s and patient’s end of the system. Often, separate displays or a picture-in-picture display are used to see both outgoing and incoming video. Another consideration is pan-zoom-tilt control of video cameras. This allows clinicians to remotely control his or her view of the patient’s site or control the view being transmitted to the patient.
Historically, interactive telepsychiatry applications have used point-to-point network connections, usually as full or fractional T-1 or integrated services digital network circuits. However, the rapid diffusion of internet and ethernet networks has led to the development of videoconferencing systems that can work over internet protocol (IP) networks. If using an IP network, ensure security by using encrypted codecs or by setting up a virtual private network and/or a virtual local area network (LAN). The principal advantage of IP networks is that by implementing proper security solutions, they can be shared by several applications—eg, internet, e-mail, LAN, etc. This means that the telecommunications network costs can be shared or considered a sunk cost (ie, not an additional cost of the telepsychiatry application).
The U.S. Federal Communications Commission’s (FCC) Universal Service Fund (USF) subsidies can reduce the cost of telepsychiatry network connections. The FCC implemented the USF to bring high bandwidth telecommunications to rural schools, libraries, and health care providers. Funding for the USF is generated from fees paid by telecommunications providers. However, the USF subsidies are not being widely used for several reasons, including a cumbersome application process, limitations on eligible facilities and locations, and questions regarding costs to the health care provider.19
Individual states also have developed funding streams to support telemedicine. The Centers for Medicare and Medicaid Services will pay a facility site fee to the host site (where the patient is located), but only if the site is in a rural area. Providers can charge patients a fee to support telepsychiatry infrastructure and maintenance, but typically this arrangement is not affordable and is not standard practice.
The future
Telepsychiatry’s ability to improve access to mental health care to underserved populations is becoming more evident. Technology is adequate for most uses and is constantly advancing. Numerous applications already have been defined, and more are ripe for exploration. Barriers to implementation are primarily of the human variety and will require a combination of consumer, provider, and governmental advocacy to overcome.
- American Telemedicine Association. www.americantelemed.org.
- International Society for Telemedicine & eHealth. www.isfteh.org.
- American Psychiatric Association. Telepsychiatry internet resources. www.psychiatry.org/practice/professional-interests/underserved-communities/telepsychiatry-internet-resources.
- Mossman D. Practicing psychiatry via Skype: Medicolegal considerations. Current Psychiatry. 2011;10(12):30-32,39.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
1. President’s New Freedom Commission on Mental Health. Subcommittee on rural issues: background paper. Rockville MD: Substance Abuse and Mental Health Administration; 2004.
2. Antonacci DJ, Bloch RM, Saeed SA, et al. Empirical evidence on the use and effectiveness of telepsychiatry via videoconferencing: implications for forensic and correctional psychiatry. Behav Sci Law. 2008;26(3):253-269.
3. American Psychiatric Association. Resource document on telepsychiatry via videoconferencing. http://www.psychiatry.med.uwo.ca/ecp/info/toronto/telepsych/Appendix%20II.htm. Accessed November 5 2012.
4. Grigsby J, Rigby M, Hiemstra A, et al. Telemedicine/telehealth: an international perspective. The diffusion of telemedicine. Telemed J E Health. 2002;8(1):79-94.
5. Krupinski E, Nypaver M, Poropatich R, et al. Telemedicine/telehealth: an international perspective. Clinical applications in telemedicine/telehealth. Telemed J E Health. 2002;8(1):13-34.
6. Diamond JM, Bloch RM. Telepsychiatry assessments of child or adolescent behavior disorders: a review of evidence and issues. Telemed J E Health. 2010;16(6):712-716.
7. Buono S, Città S. Tele-assistance in intellectual disability. J Telemed Telecare. 2007;13(5):241-245.
8. Manguno-Mire GM, Thompson JW, Jr, Shore JH, et al. The use of telemedicine to evaluate competency to stand trial: a preliminary randomized controlled study. J Am Acad Psychiatry Law. 2007;35(4):481-489.
9. Fortney JC, Maciejewski ML, Tripathi SP, et al. A budget impact analysis of telemedicine-based collaborative care for depression. Med Care. 2011;49(9):872-880.
10. Pyne JM, Fortney JC, Tripathi SP, et al. Cost-effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Arch Gen Psychiatry. 2010;67(8):812-821.
11. Morland LA, Greene CJ, Rosen CS, et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J Clin Psychiatry. 2010;71(7):855-863.
12. Mitchell JE, Crosby RD, Wonderlich SA, et al. A randomized trial comparing the efficacy of cognitive-behavioral therapy for bulimia nervosa delivered via telemedicine versus face-to-face. Behav Res Ther. 2008;46(5):581-592.
13. Steel K, Cox D, Garry H. Therapeutic videoconferencing interventions for the treatment of long-term conditions. J Telemed Telecare. 2011;17(3):109-117.
14. Greene CJ, Morland LA, Macdonald A, et al. How does tele-mental health affect group therapy process? Secondary analysis of a noninferiority trial. J Consult Clin Psychol. 2010;78(5):746-750.
15. Morland LA, Greene CJ, Grubbs K, et al. Therapist adherence to manualized cognitive-behavioral therapy for anger management delivered to veterans with PTSD via videoconferencing. J Clin Psychol. 2011;67(6):629-638.
16. Saeed SA, Diamond J, Bloch RM. Use of telepsychiatry to improve care for people with mental illness in rural North Carolina. N C Med J. 2011;72(3):219-222.
17. Whitten PS, Mackert MS. Addressing telehealth’s foremost barrier: provider as initial gatekeeper. Int J Technol Assess Health Care. 2005;21(4):517-521.
18. Coleman JR. HMOs and the future of telemedicine and telehealth: part 2. Case Manager. 2002;13(4):38-43.
19. Puskin DS. Telemedicine: follow the money modalities. Online J Issues Nurs. 2001;6(3):2.-
20. American Telemedicine Association. Medicare payment of telemedicine and telehealth services. http://www.americantelemed.org/files/public/membergroups/businessfinance/reimbursement/
BF_MedicarePaymentofTelemedicine.pdf. Published May 15 2006. Accessed November 5, 2012.
21. Fishbein M. Developing effective behavior change interventions: some lessons learned from behavioral research. NIDA Res Monogr. 1995;155:246-261.
22. Sanders JH. Telemedicine: challenges to implementation. Paper presented at: Rural Telemedicine Workshop; November 4 1993; Washington, DC.
23. Kumekawa JK. Health information privacy protection: crisis or common sense? Online J Issues Nurs. 2001;6(3):3.-
Monoamine oxidase inhibitors: Forgotten treatment for depression
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
Discuss this article at www.facebook.com/CurrentPsychiatry
For patients with major depressive disorder (MDD), monoamine oxidase inhibitors (MAOIs) have efficacy comparable to that of other antidepressants. However, concerns about side effects—particularly hypertensive crisis—and drug-drug interactions have led clinicians to prescribe MAOIs less often than newer antidepressants. A 1999 survey of 573 Michigan psychiatrists found that 30% had not prescribed an MAOI within the past 3 years, and 12% had never prescribed an MAOI.1 Although there are challenges to using these agents, we prefer prescribing MAOIs to depressed patients who have not responded to previous antidepressant trials over trying untested antidepressant combinations.
Currently, MAOIs are used primarily for patients who have not responded to other antidepressant trials and are considered treatment resistant. Treatment-resistant depression (TRD) typically is defined as nonresponse to ≥3 adequate antidepressant trials. TRD is a major cause of disability and loss of productivity. These patients tend to do poorly over the long term, with high rates of hospitalization and suicide attempts. Several controlled trials have shown that patients who fail other antidepressants may respond to MAOIs.2-4
Our knowledge regarding MAOIs has grown considerably. We have learned more about depression subtypes that MAOIs may help. As we learned more about dietary restrictions for patients taking MAOIs, the list of “forbidden foods” has decreased. Advances in treating a hypertensive crisis have decreased the need for hospitalization. By educating ourselves and our patients about MAOIs, we can provide another option for treating MDD.
An older antidepressant class
MAOIs were introduced approximately 60 years ago. Their potential for treating depression was discovered when a tuberculosis treatment—iproniazid—was found to reduce depressive symptoms. Researchers determined iproniazid’s antidepressant effects were the result of blocking removal of the amine group by monoamine oxidase (MAO) from dopamine, norepinephrine, and serotonin.5 A second MAOI, tranylcypromine, was discovered when it was found to be ineffective for treating decongestion.6
MAOI use in psychiatric practice has undergone significant changes since these medications were introduced. The discovery of hypertensive crises related to tyramine consumption led to decreased MAOI use, as did the rise of tricyclic antidepressants (TCAs) shortly thereafter. In the 1960s, research compared the relative efficacy of MAOIs to TCAs, and they became second-line antidepressants after the TCAs. In the late 1980s, the introduction of fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) resulted in a significant drop-off in MAOI use.
Pharmacologic effects
MAO is a class of enzymes that initiate oxidation of extracellular neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs can be classified based on their relative affinity to MAO as well as their enzyme selectivity. The first distinguishing characteristic is whether the drug binds to MAO in a reversible or irreversible manner. Currently, all MAOIs that are FDA-approved for treating depression bind irreversibly to MAO. As a result, the body must renew its MAO levels before a patient is no longer at risk for a hypertensive crisis, a process that may take up to 2 weeks. Clinicians must take care to ensure their patients avoid foods that contain tyramine and medications contraindicated with MAOIs during this period.
MAOIs also differ from each other in enzyme selectivity. There are 2 subtypes of MAO enzymes—MAOA and MAOB. Generally, the antidepressant activity of MAOIs appears to be directed toward MAOA inhibition. MAOA has been found to be more specific for binding to serotonin and norepinephrine and MAOB to be more specific for phenylethylamine. Dopamine is equally deaminated by both MAOA and MAOB.
Reversible MAOA inhibitors require fewer restrictions on diet or concurrent medications, but efficacy data of reversible MAOA inhibitors is mixed.
Clinical use of MAOIs
Four MAOIs are available in the United States: tranylcypromine, phenelzine, isocarboxazid, and selegiline. Selegiline is the only MAOI available as a transdermal patch. Transdermal administration results in fewer effects on MAO in the gastrointestinal tract, which means no dietary restrictions at the 6 mg/d starting dose, although the manufacturer recommends patients follow the MAO diet at 9 mg/d and 12 mg/d doses.7 Although selegiline is selective for MAOB at low doses, it becomes nonselective at therapeutic doses for depression. Recommended dosages for MAOIs can be found in Table 1.8
Table 1
Recommended dosages of monoamine oxidase inhibitors
| Medication | Starting dosages | Usual therapeutic dosage |
|---|---|---|
| Isocarboxazid | 10 mg twice a day | 30 to 60 mg/d |
| Phenelzine | 15 mg twice a day | 45 to 90 mg/d |
| Selegiline transdermal | 6 mg patch/d | 6 to 12 mg patch/d |
| Tranylcypromine | 10 mg, 2 or 3 times a day | 30 to 60 mg/d |
| Source: Adapted from reference 8 | ||
10,11
Several controlled trials have found a better response rate to MAOI therapy in outpatients with MDD who have not responded to other antidepressants.2,12 In a 6-week, double-blind trial, Vallejo et al10 reported that the TCA imipramine and high-dose phenelzine were equally efficacious in 32 patients with major depression with melancholia. In 32 dysthymic patients, high-dose phenelzine was superior to imipramine. Himmelhoch et al13 compared efficacy of tranylcypromine with that of imipramine in treating anergic bipolar depressive illness. Patients receiving tranylcypromine experienced significantly greater symptomatic improvement and higher global response without increased risk of treatment-emergent hypomania or mania.
Serum monitoring of MAOIs is not clinically indicated and there are no correlations between drug levels and effectiveness.14 In a study that examined the correlation of inhibiting platelet MAO and MAOIs’ antidepressant effects, researchers found that a higher dose of phenelzine (60 mg/d) was significantly better in treating depression and anxiety than a lower dose (30 mg/d), and only the higher dose achieved 80% of platelet MAO inhibition.15 Further studies with other MAOIs did not reproduce this effect and platelet MAO inhibition is not regularly used to assess adequate dosing.
A refined view of side effects
In a hypertensive crisis, patients experience significant hypertension, headaches, tachycardia, diaphoresis, and vomiting. Intravenous phentolamine—an α-adrenergic receptor blocker—can be used as an antidote; often a single dose is effective.16 Alternatively, calcium channel blockers such as nifedipine can be prescribed. A patient can take 10 mg/hour and be observed in the emergency room until symptoms are relieved (usually only 1 or 2 doses are needed) without being admitted to the hospital.
Table 2
Food restrictions with MAOIs
| Severe |
| Aged cheeses Aged meats (pepperoni, sausage, salami) Sauerkraut Soy sauce Fava or broad bean pods Banana peels All beers on tap |
| Use in moderation (≤2 servings/d) |
| Red wine (4 oz) White wine (4 oz) Bottled or canned beers (12 oz) |
| Mild to none |
| Avocados Banana pulp Bouillon Chocolate Fresh cheeses (cottage cheese, cream cheese, processed cheese slices) Fresh or processed meat |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from references 4,17,18 |
Orthostatic hypotension is a common cardiovascular side effect of MAOIs that may lead to dizziness or syncope. Typically this is seen 2 to 3 weeks after initiating MAOI treatment. If hypotension remains a problem, mineralocorticoids can be prescribed with monitoring of serum potassium for hypokalemia. Increasing doses of tranylcypromine can increase supine—but not standing—diastolic blood pressure.19 Distinguish this type of blood pressure elevation from a hypertensive crisis by monitoring blood pressure with the patient sitting and standing and before and after he or she walks for 60 seconds.
Insomnia and day-night shifting—sleeping during the day and staying awake at night—are common and patients often cite these as reasons for discontinuing MAOIs. Many patients who respond to MAOIs report periods of substantial sleepiness in the mid to late afternoon. Table 320 provides a more complete list of reported side effects and their frequencies.
Table 3
MAOIs: Stay vigilant for side effects
| Medication | Common side effects |
|---|---|
| Isocarboxazid | Anxiety, blurred vision, constipation, dizziness, headache, insomnia, mania, somnolence, weight gain, xerostomia |
| Phenelzine | Constipation, disorder of ejaculation and/or orgasm, dizziness, edema, fatigue, headache, hyperreflexia, impotence, elevated values on liver function tests, orthostatic hypotension, sleep disorders, somnolence, tremor, weight gain, xerostomia |
| Selegiline transdermal | Application site reaction, decreased systolic blood pressure, diarrhea, headache, indigestion, insomnia, orthostatic hypotension, weight loss, xerostomia |
| Tranylcypromine | Agitation, anxiety, constipation, diarrhea, dizziness, headache, impotence, insomnia, loss of appetite, mania, nausea, orthostatic hypotension, somnolence, weight gain, xerostomia |
| MAOIs: monoamine oxidase inhibitors Source: Adapted from reference 20 | |
Practice guidelines
The American Psychiatric Association’s practice guidelines for treating major depression state that MAOIs are effective in treating subgroups of patients with MDD with atypical features who have failed TCA trials.21 These guidelines also state that MAOIs have been shown to be effective treatment for some patients who have failed other antidepressants. However, for TRD patients who have not responded to SSRIs or SNRIs, the effectiveness of MAOIs compared with other strategies is unclear.22
MAOIs have been used for >6 decades, and published studies continue to document their efficacy and safety when patients are monitored appropriately.11,12,14,15,25 However, based on our observations we suspect MAOIs are underutilized in clinical practice today. We are concerned that such practices can trickle down into residency training programs. Psychiatric residents typically do not receive adequate training in prescribing MAOIs, largely because many training faculty are not prescribing MAOIs themselves. Despite MAOIs’ limitations, concerns about an increased risk of suicide in patients with TRD26 and the high likelihood of a poor outcome associated with persistent nonresponse to prior treatments must be weighed against the relatively low risk of a hypertensive event with MAOIs.6
- McCabe-Sellers BJ, Staggs CG, Bogle ML. Tyramine in foods and monoamine oxidase inhibitor drugs: a crossroad where medicine, nutrition, pharmacy, and food industry converge. Journal of Food Composition and Analysis. 2006;19(suppl):S58-S65.
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248.
- Clomipramine • Anafranil
- Epinephrine • Adrenalin, EpiPen
- Fluoxetine • Prozac
- Imipramine • Tofranil
- Isocarboxazid • Marplan
- Lithium • Eskalith, Lithobid
- Nifedipine • Adalat, Afeditab
- Phenelzine • Nardil
- Phentolamine • OraVerse, Regitine
- Selegiline • EMSAM
- Tranylcypromine • Parnate
Dr. Kosinski reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rothschild receives grant or research support from Cyberonics, the National Institute of Mental Health, St. Jude Medical, and Takeda, and is a consultant to Allergan, Eli Lilly and Company, GlaxoSmithKline, Noven Pharmaceuticals, Pfizer Inc., Shire Pharmaceuticals, and Sunovion.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
1. Balon R, Mufti R, Arfken CL. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatr Serv. 1999;50(7):945-947.
2. Nolen WA, van de Putte JJ, Dijken WA, et al. Treatment strategy in depression. II. MAO inhibitors in depression resistant to cyclic antidepressants: two controlled crossover studies with tranylcypromine versus L-5-hydroxytryptophan and nomifensine. Acta Psychiatr Scand. 1988;78(6):676-683.
3. McGrath PJ, Stewart JW, Harrison W, et al. Treatment of tricyclic refractory depression with a monoamine oxidase inhibitor antidepressant. Psychopharmacol Bull. 1987;23(1):169-172.
4. Amsterdam JD. Monoamine oxidase inhibitor therapy in severe and resistant depression. Psychiatr Ann. 2006;36(9):607-613.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122(5):509-522.
6. Kennedy SH, Holt A, Baker GB. Monoamine oxidase inhibitors. In: Sadock BJ Sadock VA, eds. Kaplan and Sadock’s comprehensive textbook of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 1076–1080.
7. EMSAM [package insert]. Napa CA: Dey Pharm LP; 2011.
8. Amsterdam JD, Chopra M. Monoamine oxidase inhibitors revisited. Psychiatric Ann. 2001;31(6):361-370.
9. Quitkin FM, Stewart JW, McGrath PJ, et al. Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry. 1988;145(3):306-311.
10. Vallejo J, Gasto C, Catalan R, et al. Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry. 1987;151:639-642.
11. White K, Razani J, Cadow B, et al. Trancylpromine vs. nortriptyline vs. placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl). 1984;82(3):258-262.
12. Thase ME, Frank E, Mallinger AG, et al. Treatment of imipramine-resistant recurrent depression, III: efficacy of monoamine oxidase inhibitors. J Clin Psychiatry. 1992;53(1):5-11.
13. Himmelhoch JM, Thase ME, Mallinger AG, et al. Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry. 1991;148(7):910-916.
14. Rothschild AJ. ed. The evidence-based guide to antidepressant medications. Arlington, VA: American Psychiatric Publishing, Inc.; 2012:15–20.
15. Ravaris CL, Nies A, Robinson DS, et al. A multiple-dose, controlled study of phenelzine in depression-anxiety states. Arch Gen Psychiatry. 1976;33(3):347-350.
16. Cockhill LA, Remick RA. Blood pressure effects of monoamine oxidase inhibitors—the highs and lows. Can J Psychiatry. 1987;32(9):803-808.
17. Shulman KI, Walker SE. A reevaluation of dietary restrictions for irreversible monoamine oxidase inhibitors. Psychiatr Ann. 2001;31(6):378-384.
18. Gardner DM, Shulman KI, Walker SE, et al. The making of a user friendly MAOI diet. J Clin Psychiatry. 1996;57(3):99-104.
19. Keck PE, Jr, Carter WP, Nierenberg AA, et al. Acute cardiovascular effects of tranylcypromine: correlation with plasma drug, metabolite, norepinephrine, and MHPG levels. J Clin Psychiatry. 1991;52(6):250-254.
20. Micromedex Healthcare Series [UMass Memorial Healthcare Intranet System]. Version 5.1. Greenwood Village CO: Thomson Reuters (Healthcare) Inc.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third edition. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1667485. Published October 2010. Accessed October 26, 2012.
22. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541; quiz 1666.
23. Nelson JC, Byck R. Rapid response to lithium in phenelzine non-responders. Br J Psychiatry. 1982;141:85-86.
24. Guze BH, Baxter LR, Jr, Rego J. Refractory depression treated with high doses of monoamine oxidase inhibitor. J Clin Psychiatry. 1987;48(1):31-32.
25. Robinson DS, Gilmor ML, Yang Y, et al. Treatment effects of selegiline transdermal system on symptoms of major depressive disorder: a meta analysis of short term, placebo controlled, efficacy trials. Psychopharmacol Bull. 2007;40(3):15-28.
26. Keller MB, Lavori PW, Rice J, et al. The persistent risk of chronicity in recurrent episodes of nonbipolar major depressive disorder: a prospective follow-up. Am J Psychiatry. 1986;143(1):24-28.
QUIT: A mnemonic to help patients stop smoking
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Research indicates that even brief physician advice on a regular basis can increase quit rates for patients who smoke.1 This is particularly important in mental health settings, where there are more smokers than in the general population (50% to 90% vs 25% to 27%, respectively) but quit rates are lower.2
There is no “one size fits all” solution to quitting smoking; there are many individual factors to take into account for each patient. In addition to environmental factors that can make quitting smoking more challenging—eg, the patient’s partner also smokes—a patient’s genetic makeup can make it easier or harder to become addicted or to quit smoking, and can make pharmacologic approaches to cessation more or less successful.3,4 A patient’s failed attempt to quit in the past does not indicate that quitting is impossible.
Although we encourage the use of traditional mnemonics such as the “5 A’s”5 and the “5 R’s,”5 we introduce QUIT as an easy-to-remember, compassionate, realistic way of discussing smoking cessation with patients.
Question each patient to understand the pros and cons of quitting. Ask your patients about the “benefits” of smoking and understand what role cigarettes serve in their lives. Remind patients of immediate benefits that would make quitting smoking a “trade” rather than a loss—eg, how would they use the extra $200 a month they would save by giving up cigarettes?
If patients say they are not interested in quitting, find out why they are not motivated to quit and collaborate with them to try to address their concerns. Additionally, ask if they would be comfortable discussing smoking cessation at each visit, even if they are not expressing interest.
Understand the nature of addiction. The trajectory of tobacco dependence—similar to other addictions—involves a chronic and relapsing course. Most patients require multiple quit attempts using several strategies before they succeed. Find out what they have tried in the past and build on previous successes. Be persistent in offering evidence-based treatments to help patients quit, even when motivation is low and patients have multiple failed attempts.
Keep in mind that only 4% to 7% of unaided quit attempts are successful.6 Most patients require counseling and/or medication, as well as help from a caring physician. By understanding the nature of addiction, you can be optimistic and supportive of your patients as they face the often disheartening process of quitting.
Identify risk factors and triggers. Studies have demonstrated that stimuli related to smoking increase a patient’s craving to smoke; this response is stronger than triggers encountered by patients addicted to alcohol or opiates.7 A plan for handling cravings and avoiding triggers can empower your patients and help them stay on track.
Talk with—not to—your patient. Discussing smoking can help clarify your patient’s feelings rather than avoiding them. Although patients may aspire to eventually quit smoking, the unspoken concerns they harbor combined with the “benefits” of smoking may lead to a failure to act.
Talk is powerful and with training, physicians can move patients toward change. Motivational interviewing is evidence-based and offers techniques that enable physicians to use conversation with their patients as a way of overcoming ambivalence about unhealthy behaviors and eliciting talk about changing these behaviors, and eventually help them to change.
You can make an impact
Physicians need to recognize their potential impact on this life-threatening behavior. Through an active, conversational style, develop a big-picture understanding of your patient’s pros and cons of quitting smoking; strengths and weaknesses; past failures and successes; barriers to success; available supports; etc. This information, combined with encouragement, support, and knowledge of evidence-based practices, can yield a thorough plan for quitting.
Although quitting smoking can be extremely challenging for clinicians and patients, expanding your knowledge in this area will allow you to help your patients make life-saving changes. The best care comes from direct communication and unconditional support.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
1. Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ. 2000;321(7257):355-358.
2. Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction. 2009;104(5):719-733.
3. Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368.
4. Tillie-Louise H. Genetic determinants of smoking cessation. European Respiratory Disease. 2009;5(1):37-40.
5. U.S. Department of Health and Human Services. Treating tobacco use and dependence. Quick reference guide for clinicians. 2008 update. http://www.ahrq.gov/clinic/tobacco/tobaqrg.pdf. Accessed November 15, 2012.
6. Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297-314.
7. Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36(3):235-243.
Teens, social media, and ‘sexting’: What to tell parents
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Children and adolescents who have unrestricted use of the internet and cell phones are at increased risk for being exposed to sexually explicit material. One study found almost 1 in 5 high school students have “sexted”—sending a text message with sexually explicit pictures—and almost twice as many reported that they had received a sexually explicit picture via cell phone.1 More than 25% of students acknowledged forwarding a sexually explicit picture to others; >33% did so despite knowing the legal consequences, including being arrested and facing pornography charges.1
Concerned parents may seek advice on how to prevent their child from receiving or sending sexually inappropriate material on the internet or on their cell phones. You can help parents keep their children safe by sharing the following tips from The American Academy of Pediatrics (AAP)2:
Keep up with technology. Advise parents to become familiar with popular social networking websites such as Facebook. Creating their own Facebook page and “friending” their child may help them facilitate a conversation about their individual online experiences.
Enable privacy features. Instruct parents to install parental controls on their child’s computer. Explain to parents that these monitoring systems can help them check their child’s e-mail, chat records, and instant messages. Many social networking sites have privacy features that can help block unwanted users from contacting a child.
Check up on your children. Parents should let children know they are aware of their online presence and will be keeping an eye on them. They should periodically check a child’s chat logs, messages, e-mails, and social networking profiles for inappropriate content, friends, messages, and images. Instruct parents to teach their children that nothing is private once it’s posted on the internet. Suggest keeping the child’s computer in a public location such as the family room or kitchen.
Limit time spent online. Explain to parents that they should limit their child’s internet and cell phone access.
Combating ‘sexting’
Suggest to parents that they explain to their child in an age-appropriate manner what sexting is before giving their child a cell phone. The AAP2 recommends that parents make sure their children understand the legal ramifications of sexting. A child who is caught sexting could be arrested, which may hurt his or her chances of being accepted into college or getting a job. A simple way to reduce a child’s opportunities for sexting is to restrict his or her access to a cell phone during social situations where peer pressure could influence behavior.
Disclosure
Dr. Jain reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
1. Strassberg DS, McKinnon RK, Sustaíta MA. Sexting by high school students: an exploratory and descriptive study [published online June 7, 2012]. Arch Sex Behav. doi: 10.1007/s10508-012-9969-8.
2. American Academy of Pediatrics. Talking to kids and teens about social media and sexting. http://www.aap.org/en-us/about-the-aap/aap-press-room/news-features-and-safety-tips/pages/Talking-to-Kids-and-Teens-About-Social-Media-and-Sexting.aspx?. Published June 2009. Updated March 2, 2011. Accessed August 14, 2012.
How to provide culturally sensitive care to Arab American patients
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
Since September 11, 2001, many Arab Americans have faced increased discrimination, which puts them at greater risk for depression and low self-esteem.1 Children and adolescents in particular have been the victims of teasing and taunts. Many Muslim Arab Americans turned to their imams—a mosque’s spiritual leader—rather than a mental health clinician to help them deal with the national tragedy and the fallout that followed.2
Arab Americans may struggle to bridge their personal identity with their cultural one. Traditional Arab values stress the importance of family—both immediate and extended—loyalty to parents, religious adherence, and respect for elders and authority. Adapting those values to typical American values can cause dissonance as Arab Americans grapple to find a balance between renouncing their Arab culture in hopes of fitting in and feeling like outcasts in the country they call home.
Understanding cultural nuances
Be aware of the stigma of mental illness within Arab American communities. Unlike diabetes or heart disease, psychiatric disorders can carry a negative connotation for many Arab Americans.3 They may view mental illness as a personal shortcoming or ascribe their symptoms to supernatural spirits. The fear of being discriminated against for being culturally different and mentally ill may delay or prevent individuals from seeking care.
Understanding these dynamics, as well as Arab American culture, is the first step to evaluating these patients. Being aware of cultural nuances also is important. Patients may say they don’t smoke, but some prodding may reveal that they use a tobacco water pipe, or hookah.
Be cognizant of any preconceived notions that can seep into an assessment. It’s easy to assume that Arab American patients fall into stereotypical gender roles or are unhappy with what may be perceived as inadequate assimilation. Conversely, a patient’s appearance, devotion to cultural and religious values, and family support may lead to an assumption that the patient does not abuse substances or engage in high-risk behavior.
In addition, note that Arab Americans tend to present their mental illness as somatic complaints, which may make them more comfortable seeing a primary care physician than a psychiatrist.
Adjusting treatment
Many Arab Americans’ first choice is to seek support from family, friends, and religious leaders.4 A patient may need to be convinced to take psychotropics the same as they would other medications. Therefore, it may be necessary to involve family members to ensure treatment compliance. Clinicians may need to spend more time with Arab American patients, which can help the clinician grasp the complexity of their issues and allow patients to feel that they’re being cared for by a clinician who respects their cultural and religious beliefs. In conjunction, these steps will help you provide culturally sensitive care that best addresses Arab Americans’ mental health needs.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
1. Amer MM, Hovey JD. Socio-demographic differences in acculturation and mental health for a sample of 2nd generation/early immigrant Arab Americans. J Immigr Minor Health. 2007;9(4):335-347.
2. Abu-Ras W, Gheith A, Cournos F. The imam’s role in mental health promotion: a study at 22 mosques in New York City’s Muslim community. J Muslim Ment Health. 2008;3(2):155-176.
3. Carolan MT, Bagherinia G, Juhari R, et al. Contemporary Muslim families: research and practice. Contemp Fam Ther. 2000;22(1):67-79.
4. Moradi B, Hasan NT. Arab American persons’ reported experiences of discrimination and mental health: the mediating role of personal control. J Couns Psychol. 2004;51(4):418-428.
How to stabilize an acutely psychotic patient
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).
Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21
Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).

Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21

Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acute psychosis is a symptom that can be caused by many psychiatric and medical conditions. Psychotic patients might be unable to provide a history or participate in treatment if they are agitated, hostile, or violent. An appropriate workup may reveal the etiology of the psychosis; secondary causes, such as medical illness and substance use, are prevalent in the emergency room (ER) setting. If the patient has an underlying primary psychotic disorder, such as schizophrenia or mania, illness-specific intervention will help acutely and long-term. With agitated and uncooperative psychotic patients, clinicians often have to intervene quickly to ensure the safety of the patient and those nearby.
This article focuses on the initial evaluation and treatment of psychotic patients in the ER, either by a psychiatric emergency service or a psychiatric consultant. This process can be broken down into:
- triage or initial clinical assessment
- initial psychiatric stabilization, including pharmacologic interventions and agitation management
- diagnostic workup to evaluate medical and psychiatric conditions
- further psychiatric evaluation
- determining safe disposition.1
Triage determines the next step
Initial clinical assessment and triage are necessary to select the appropriate immediate intervention. When a patient arrives in the ER, determine if he or she requires urgent medical attention. Basic initial screening should include:
- vital signs
- finger stick blood glucose
- medical history
- signs or symptoms of intoxication or withdrawal
- signs of trauma (eg, neck ligature marks, gunshot wounds, lacerations)
- asking the patient to give a brief history leading up to the current presentation.
A review of medical records may reveal patients’ medical and psychiatric history and allergies. Collateral documentation—such as ambulance run sheets or police reports—may provide additional information. If no immediate medical intervention is warranted, determine if the patient can wait in an open, unlocked waiting area or if he or she needs to be in an unlocked area with a sitter, a locked open area, or a secluded room with access to restraints. In general, psychotic patients who pose a threat of harm to themselves or others or cannot care for themselves because of their psychosis need locked areas or observation.
Initial psychiatric stabilization
Agitation is diagnostically unspecific but can occur in patients with psychosis. Psychotic patients can become unpredictably and impulsively aggressive and assaultive. Rapid intervention is necessary to minimize risk of bodily harm to the patient and those around the patient. Physicians often must make quick interventions based on limited clinical information. It is important to recognize early signs and symptoms of agitation, including:
- restlessness (pacing, fidgeting, hand wringing, fist clenching, posturing)
- irritability
- decreased attention
- inappropriate or hostile behaviors.2
Pharmacologic interventions. The initial goals of pharmacologic treatment are to calm the patient without oversedation, thereby allowing the patient to take part in his or her care and begin treatment for the primary psychotic illness.3,4 Offering oral medications first and a choice of medications may help a patient feel more in control of the situation. If a patient has to be physically restrained, pharmacotherapy may limit the amount of time spent in restraints.
Medication choice depends on several factors, including onset of action, available formulation (eg, IM, liquid, rapidly dissolving), the patient’s previous medication response, side effect profile, allergies or adverse reactions to medications, and medical comorbidities.3 If a patient has a known psychotic illness, it may be helpful to administer the patient’s regular antipsychotic or anxiolytic medication. Some medications, such as lithium, are not effective in the acute setting and should be avoided. Additionally, benzodiazepines other than lorazepam or midazolam should not be administered IM because of erratic absorption.
Antipsychotics can be used for psychotic patients with or without agitation. Benzodiazepines may treat agitation, but are not specific for psychosis. Haloperidol can be used to treat acute psychosis and has proven efficacy for agitation. Benzodiazepines can decrease acute agitation and have efficacy similar to haloperidol, but with more sedation.5 A combination of lorazepam and haloperidol is thought to be superior to either medication alone.6 Lorazepam helps maintain sedation and decreases potential side effects caused by haloperidol. Consensus guidelines from 2001 and 2005 recommend combined haloperidol and lorazepam for first-line treatment of acute agitation.3,7 High-potency antipsychotics such as haloperidol have an increased risk for extrapyramidal symptoms (EPS), particularly acute dystonic reactions—involuntary, sustained muscle contractions—in susceptible patients (eg, antipsychotic-naïve patients); consider starting diphenhydramine, 25 to 50 mg, or benztropine, 0.5 to 2 mg, to prevent EPS from high-potency antipsychotics (Algorithm 1).

Algorithm 1: Treating acute psychosis: Choosing pharmacologic agents
EPS: extrapyramidal symptoms; PO: by mouth; SL: sublingual
Second-generation antipsychotics (SGAs) increasingly have been used for managing acute agitation in patients with an underlying psychotic disorder. Guidelines from a 2012 American Association for Emergency Psychiatry workgroup recommend using an SGA as monotherapy or in combination with another medication instead of haloperidol to treat agitated patients with a known psychotic disorder.8 Clinical policy guidelines from the American College of Emergency Physicians recommend antipsychotic monotherapy for agitation and initial treatment in patients with a known psychiatric illness for which antipsychotic treatment is indicated (eg, schizophrenia).9 For patients with known psychotic illness, expert opinion recommends oral risperidone or olanzapine.3,8 The combination of oral risperidone plus lorazepam may be as effective as the IM haloperidol and IM lorazepam combination.10 Patients who are too agitated to take oral doses may require parenteral medications. Ziprasidone, olanzapine, and aripiprazole are available in IM formulations. Ziprasidone, 20 mg IM, is well tolerated and has been shown to be effective in decreasing acute agitation symptoms in patients with psychotic disorders.11 Olanzapine is as effective as haloperidol in decreasing agitation in patients with schizophrenia, with lower rates of EPS.12 In a double-blind, placebo-controlled trial, psychotic symptoms in patients with schizophrenia or schizoaffective disorder decreased within 2 hours of IM olanzapine administration.13 Both IM ziprasidone and olanzapine have a relatively rapid onset of action (within 30 minutes), which makes them reasonable choices in the acute setting. Olanzapine has a long half-life (21 to 50 hours); therefore, patients’ comorbid medical conditions, such as cardiac abnormalities or hypotension, must be considered. If parenteral medication is required, IM olanzapine or IM ziprasidone is recommended.8 IM haloperidol with a benzodiazepine also can be considered.3
Coadministration of parenteral olanzapine and a benzodiazepine can lead to severe orthostatic hypotension and cardiac or respiratory depression and should be avoided in geriatric patients.14 Finally, it is important to rule out presentations that may worsen with antipsychotic treatment, including phencyclidine (PCP) toxicity (could worsen dystonic reactions), anticholinergic delirium, neuroleptic malignant syndrome (NMS), or catatonia.
If a patient does not respond to the initial dose of a medication, the dose may be repeated. However, doses should not be repeated until a patient is so sedated that he or she cannot take part in his or her care, or until he or she has developed significant EPS.
In addition to antipsychotics, consider loading with oral divalproex for patients who are acutely psychotic in the context of a manic episode (Table).15,16 Higher serum divalproex levels—target serum levels >94 μg/mL—are associated with greater efficacy as measured by change from baseline in Mania Rating Scale or Young Mania Rating Scale scores compared with placebo.15 For acutely psychotic schizophrenia patients, there is evidence of benefit with initial treatment with divalproex combined with an SGA. In a randomized, double-blind study, patients treated with divalproex plus olanzapine or risperidone showed quicker initial resolution of psychotic symptoms compared with olanzapine or risperidone monotherapy, but no better long-term benefit.16 Clinicians may consider this well-tolerated combination after an appropriate medical workup. This finding of early benefit was not replicated with divalproex extended-release.17
Table
Divalproex dosing for patients with acute psychosis and mania
| Initial dose | Titration | |
|---|---|---|
| Acute mania15 | Divalproex delayed-release: 750 mg/d Divalproex extended-release: 20 mg/kg/d | Increase to clinical effectiveness or maximum serum level of 125 μg/mL |
| Exacerbation of psychosis16 | Divalproex: 15 mg/kg/d (in 2 doses) | Increase to clinical effectiveness over 12 days or maximum dosage of 30 mg/kg/d |
Side effects and adverse reactions. Treatment with antipsychotics may cause QTc interval prolongation, which can lead to increased risk for torsades de pointes and sudden death due to ventricular fibrillation. However, there have been few cases of torsades de pointes after oral haloperidol and none with IM haloperidol compared with at least 30 cases of torsades de pointes after IV haloperidol treatment. Torsades de pointes after risperidone, olanzapine, or ziprasidone treatment has not been reported.18
Hypotension and bradycardia may occur in patients treated with olanzapine; however, these signs occur less frequently in agitated patients.18 Antipsychotic treatment increases risk for EPS, including acute dystonia, akathisia (subjective restlessness with desire to move), and parkinsonism (shuffling gait, resting tremor, rigidity and bradykinesia), as well as NMS.
Nonpharmacologic interventions. Verbal intervention to try to de-escalate an agitated, psychotic patient should be attempted first; however, this is not always possible. Other behavioral interventions include offering a meal, blanket, or pillow, or other comforting options to decrease the patient’s anxiety associated with psychosis.2 However, if agitated psychotic patients continue to display aggressive behaviors and pose a risk of harm to themselves or those around them, physical restraints should be considered because the clinician must balance protecting the patient’s rights with others’ safety. If physical restraints are used, medication also should be administered. Remove physical restraints as soon as safely possible; the Joint Commission has established standards for minimizing harm when using physical restraints.19
Diagnostic workup
Once a patient is medically stable in the ER, begin further workup of the etiology of the psychosis (Algorithm 2). All patients should have a physical exam, provided they are calm and in behavioral control. Monitor vital signs; patients at risk of withdrawal from substances should be monitored more frequently. Although there is no established standard for “medical clearance” of a psychiatric patient,20 all patients should undergo basic laboratory tests, including basic metabolic panel, complete blood count, and urine toxicology. The extent of the workup is determined by the clinical situation and suspected cause of psychosis.21

Algorithm 2: Diagnostic workup of an acutely psychotic patient
ER: emergency room; EEG: electroencephalography; LP: lumbar puncture; TSH: thyroid-stimulating hormone
If you suspect delirium, the underlying medical etiology must be identified and treated. Up to 40% of hospitalized patients with delirium may have psychosis.22 Psychosis in a delirious patient may be characterized by poorly formed delusions and visual hallucinations. Delirious patients often are inattentive, easily distracted, and disoriented, with a fluctuating clinical course. Patients with psychosis generally do not have impaired attention and are alert with intact memory. However, acutely psychotic patients may be quite disorganized and uncooperative, which makes it difficult to distinguish between these 2 diagnoses. Serial exams may help clarify the clinical picture. It is important to remember that patients with a history of a psychotic disorder may have a superimposed delirium.
In young patients (age 18 to 30) with new-onset psychosis, consider drug-induced psychosis; PCP, lysergic acid diethylamide, and methamphetamine intoxication and withdrawal can lead to psychotic presentations. Additionally, comorbid substance use is common among patients with primary psychotic disorders. One study found 37% of first-episode psychotic patients misused drugs or alcohol, similar to the lifetime rate of patients with chronic psychotic disorders.23,24 Check urine and serum toxicology screens and obtain relevant substance use history. Brain MRI may be considered for patients with first presentation of psychosis; however, there is little evidence to support head CT imaging unless there is known head trauma.25 Electroencephalography and lumbar puncture can be considered if clinically indicated.
Further psychiatric evaluation
Obtaining a psychiatric history is necessary to determine the etiology of the acute psychotic presentation. The timing and duration of psychotic symptoms are key. Acute symptom onset with fluctuating course and impaired attention suggests a delirious process. A gradual decline in functioning over several months to years in a young person suggests a first episode of a psychotic disorder (eg, schizophrenia). Drug abuse is common among young persons with a psychotic disorder and a positive drug screen for a psychogenic substance does not exclude a primary psychotic disorder.
If a patient has a history of schizophrenia, bipolar disorder, or psychotic depression, acutely worsening psychosis may be considered an acute or chronic presentation. Even in patients diagnosed with a psychotic illness, it is necessary to determine the cause of symptom exacerbation. Medication nonadherence (which can be partial), substance use, psychosocial stressors, or underlying medical illness should be considered. Collateral information from family or friends may be crucial to understanding a patient’s presentation.
Safe disposition
Patients who pose a risk of harm to themselves or others or who are so impaired by their psychosis that they cannot care for themselves generally should be admitted to an inpatient psychiatric facility. For some psychotic patients who are agreeable to treatment and not prone to violence, less restrictive settings—such as a crisis intervention unit or respite facility—may be appropriate. A patient with first-episode psychosis could be admitted for further diagnostic clarification and treatment initiation. Manic patients often have no insight into their illness and may need hospitalization for containment and assurance of medication adherence. Goals of inpatient care include initiating or resuming pharmacologic treatment to reduce psychotic symptoms and beginning the recovery process. Response rates—defined as ≥20% improvement in total score on a psychopathology scale such as the Positive and Negative Syndrome Scale—will vary, but can take ≥4 weeks in some patients with first-episode schizophrenia.26 However, most patients will be stabilized and ready for discharge before 4 weeks. Family education and alliance building with the patient and family are important during hospitalization.
Related Resources
- Schwartz S, Weathers, M. The psychotic patient. In: Riba MB, Ravindranath D, eds. Clinical manual of emergency psychiatry. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:115-140.
- American Association for Emergency Psychiatry. http://emergencypsychiatry.org.
Drug Brand Names
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Diphenhydramine • Benadryl
- Divalproex • Depakote
- Haloperidol • Haldol
- Lithium • Eskalith, Lithobid
- Lorazepam • Ativan
- Midazolam • Versed
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr. Freudenreich receives grant or research support from Beacon Health Strategies, Global Medical Education, MGH Psychiatry Academy, Optimal Medicine, Pfizer Inc., and PsychoGenics.
Drs. Brown and Stoklosa report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
1. Marco CA, Vaughan J. Emergency management of agitation in schizophrenia. Am J Emerg Med. 2005;23(6):767-776.
2. Freudenreich O. Emergency management of acute psychosis. In: Freudenreich O ed. Psychotic disorders: a practical guide. New York, NY: Wolter Kluwer/Lippincott Williams & Wilkins; 2008:72–78.
3. Allen MH, Currier GW, Carpenter D, et al. Expert Consensus Panel for Behavioral Emergencies 2005. The expert consensus guideline series. Treatment of behavioral emergencies 2005. J Psychiatr Pract. 2005;11(1 suppl):S5-S108.
4. National Institute for Health and Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in primary and secondary care. London United Kingdom: National Institute for Clinical Excellence; 2002.
5. Allen MH. Managing the agitated psychotic patient: a reappraisal of the evidence. J Clin Psychiatry. 2000;61 (14 suppl):S11-S20.
6. Battaglia J, Moss S, Rush J, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997;15(4):335-340.
7. Allen MH, Currier GW, Hughes DH, et al. Expert Consensus Panel for Behavioral Emergencies. The expert consensus guideline series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec no):1-88.
8. Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of American Association for Emergency Psychiatry project BETA psychopharmacology workgroup. West J Emerg Med. 2012;13(1):26-34.
9. Lukens TW, Wolf SJ, Edlow JA, et al. American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Diagnosis and Management of the Adult Psychiatric Patient in the Emergency Department. Clinical policy: critical issues in the diagnosis and management of the adult psychiatric patient in the emergency department. Ann Emerg Med. 2006;47(1):79-99.
10. Currier GW, Chou JC, Feifel D, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J Clin Psychiatry. 2004;65(3):386-394.
11. Daniel DG, Potkin SG, Reeves KR, et al. Intramuscular (IM) ziprasidone 20 mg is effective in reducing agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl). 2001;155(2):128-134.
12. Wright P, Birkett M, David SR, et al. Double-blind, placebo-controlled comparison of intramuscular olanzapine and intramuscular haloperidol in the treatment of acute agitation in schizophrenia. Am J Psychiatry. 2001;158(7):1149-1151.
13. Kapur S, Arenovic T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry. 2005;162(5):939-946.
14. Marder SR, Sorasburu S, Dunayevic E, et al. Case reports of postmarketing adverse event experiences with olanzapine intramuscular treatment in patients with agitation. J Clin Psychiatry. 2010;71(4):433-441.
15. Allen MH, Hirschfeld RM, Wozniak PJ, et al. Linear relationship of valproate serum concentration to response and optimal serum levels for acute mania. Am J Psychiatry. 2006;163(2):272-275.
16. Casey DE, Daniel DG, Wassef AA, et al. Effect of divalproex combined with olanzapine or risperidone in patients with an acute exacerbation of schizophrenia. Neuropsychopharmacology. 2003;28(1):182-192.
17. Casey DE, Daniel DG, Tamminga C, et al. Divalproex ER combined with olanzapine or risperidone for treatment of acute exacerbations of schizophrenia. Neuropsychopharmacology. 2009;34(5):1330-1338.
18. Currier GW, Allen MH, Bunney EB, et al. Safety of medications used to treat acute agitation. J Emerg Med. 2004;27(4 suppl):S19-S24.
19. The Joint Commission. Sentinel event alert. Preventing restraint deaths. Published November 18 1998. http://www.jointcommission.org/assets/1/18/SEA_8.pdf. Accessed October 26, 2012
20. Janiak BD, Atteberry S. Medical clearance of the psychiatric patient in the emergency department. J Emerg Med. 2012;43(5):866-870.
21. Freudenreich O, Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3(1):10-18.
22. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
23. Cantwell R, Brewin J, Glazebrook C, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150-153.
24. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
25. Goulet K, Deschamps B, Evoy F, et al. Use of brain imaging (computed tomography and magnetic resonance imaging) in first-episode psychosis: review and retrospective study. Can J Psychiatry. 2009;54(7):493-501.
26. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.