User login
Venous Thromboembolism
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 develop a VTE in the Western countries. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of patients diagnosed with thrombotic events will die within the first month after diagnosis. PE is a common consequence of DVT; 40% of patients who are diagnosed with a DVT will be subsequently found to have a PE upon further imaging. The high rate of association is also seen in those who present with a PE, 70% of whom will also be found to have a concomitant DVT.
To read the full article in PDF:
Antiplatelet therapy to prevent recurrent stroke: Three good options
After a stroke, an important goal is to prevent another one.1,2 And for patients who have had an ischemic stroke or transient ischemic attack (TIA) due to atherosclerosis, an important part of secondary preventive therapy is a drug that inhibits platelets—ie, aspirin, extended-release dipyridamole, or clopidogrel. This has taken years to establish.
In the following pages, we discuss the antiplatelet agents that have been shown to be beneficial after stroke of atherosclerotic origin, and we briefly review the indications for surgery and stenting for the subset of patients whose strokes are caused by symptomatic carotid disease.
(Although managing modifiable risk factors such as smoking, hypertension, diabetes, and dyslipidemia is also important, we will not cover this topic here, nor will we talk about hemorrhagic stroke or stroke due to atrial fibrillation. Also not discussed here is cilostazol, which, although shown to be effective in preventing recurrent stroke when compared with placebo and aspirin,3,4 has not been approved for this use by the US Food and Drug Administration, as of this writing.)
HOW WE REVIEWED THE LITERATURE
We searched PubMed using the terms aspirin, acetylsalicylic acid, clopidogrel, and/or dipyridamole, in combination with stroke, cerebral ische(ae)mia, transient ische(ae)mic attacks, or retinal artery occlusion. We reviewed only clinical trials or meta-analyses of these drugs for either primary or secondary prevention of cerebrovascular disease.
As our aim was to review the topic and not to perform a meta-analysis, no cutoffs were used to exclude trials. The references in the selected papers were also reviewed to expand the articles. Finally, the references in the current American Heart Association and American Stroke Association secondary stroke prevention guideline were also reviewed.
For a summary of the trials included in our review, see the Data Supplement as an appendix to the online version of this article.
ASPIRIN: THE GOLD STANDARD
Prescribed by Hippocrates in the form of willow bark extract, aspirin has long been known for its antipyretic and anti-inflammatory properties. Its antiplatelet and antithrombotic properties, first described in 1967 by Weiss and Aledort,5 are mediated by irreversible inhibition of cyclooxygenase, leading to decreased thromboxane A2, a platelet-aggregation activator.
Fields et al,6,7 in 1977 and 1978, reported that in a controlled trial in patients with TIA or monocular blindness, fewer subsequent TIAs occurred in patients who received aspirin, although the difference was not statistically significant, with lower rates of events only in nonsurgical patients. Over the next 20 years, the results remained mixed.
The Danish Cooperative study8 (1983) found no significant difference in the rate of recurrent stroke with aspirin vs placebo.
AICLA.9 The Accidents Ischémiques Cérébraux Liés à l’Athérosclérose study of 1983 did find a difference. However, both the Danish Cooperative study and the AICLA were limited by lacking standardized computed tomographic imaging to rule out hemorrhagic stroke and by being relatively small.
The Swedish Cooperative Study10 (1987) found no statistical difference between high-dose aspirin and placebo in preventing recurrent vascular events (stroke, TIA, or myocardial infarction [MI]) 1 to 3 weeks after a stroke. However, it had several limitations: the aspirin group contained more patients with ischemic heart disease (who are more likely to die of cardiac causes), there were significantly more men in the aspirin group, and nearly one-fourth of the deaths were a result of the initial stroke, potentially masking the effect of aspirin in secondary prevention.
Later studies began to show a consistently favorable effect of aspirin.
Boysen et al11 in 1988 reported a nonsignificant trend toward fewer adverse events with aspirin.
UK-TIA.12 The United Kingdom Transient Ischaemic Attack trial in 1991 found a similar trend.
SALT.13 The Swedish Aspirin Low-dose Trial, also in 1991, showed a significant 18% lower rate of stroke or death in patients with recent TIA, minor stroke, or retinal occlusion treated with low-dose aspirin. The inclusion of patients with TIA helped broaden the population that might benefit. However, the study may have favored the aspirin group by having a run-in period in which patients were nonrandomly treated either with aspirin or with anticoagulation at the discretion of the patient’s physician and, if they suffered “several” TIAs, a stroke, retinal artery occlusion, or MI, were removed from the study.
ESPS-2.14 The second European Stroke Prevention Study in 1996 added to the evidence that aspirin prevents recurrent stroke. Patients with a history of TIA or stroke were randomized in double-blind fashion to four treatment groups: placebo, low-dose aspirin, dipyridamole, or aspirin plus dipyridamole. At 2 years, strokes had occurred in 18% fewer patients in the aspirin group than in the placebo group, and TIAs had occurred in 21.9% fewer. However, aspirin was associated with an absolute 0.5% increase in severe and fatal bleeding. The power of the study was limited because patients from one center were excluded because of “serious inconsistencies in patient case record forms and compliance assay determinations.” 14
Comment. The mixed results with aspirin in studies predating ESPS-2 were partly because the study populations were too small to show benefit.
ATT.15 The Antithrombotic Trialists’ Collaboration performed a meta-analysis that conclusively confirmed the benefit of aspirin after stroke or TIA. The investigators analyzed individual patient data pooled from randomized controlled trials published before 1997 that compared antiplatelet regimens (mostly aspirin) against placebo and against each other. The rates of vascular events were 10.7% with treatment vs 13.2% with placebo (P < .0001). Antiplatelet therapy was particularly effective in preventing ischemic stroke, with a 25% reduction in the rate of nonfatal stroke, and with an overall absolute benefit in stroke prevention across all high-risk patient groups. This translated to 25 fewer nonfatal strokes per 1,000 patients treated with antiplatelet therapy.
What is the optimal aspirin dose?
Studies of aspirin have used different daily doses—the earliest studies used large doses of 1,000 to 1,500 mg.6–10
Boysen et al11 in 1988 found a trend toward benefit (not statistically significant) with doses ranging from 50 mg to 100 mg.
In 1991, three separate studies found that higher doses of aspirin were no more effective than lower doses.
The UK-TIA trial12 compared aspirin 300 mg vs 1,200 mg and found a higher risk of gastrointestinal bleeding with the higher dose.
The SALT Collaborative Group13 found 75 mg to be effective.
The Dutch TIA trial16 compared 30 mg vs 283 mg; end point outcomes were similar but the rate of adverse events was higher with 283 mg.
ESPS-2 was able to show efficacy at a dose of only 50 mg.14
Taylor et al17 compared lower doses (81 or 325 mg) vs higher doses (650 or 1,300 mg) for patients undergoing carotid endarterectomy and found that the risk of adverse events was twice as high with the higher doses.
The ATT Collaboration15 found that efficacy was 40% lower with the highest dose of aspirin than with the lowest doses.
Algra and van Gijn18 performed a meta-analysis of all these studies and found no difference in risk reduction between low-dose and high-dose aspirin, with an overall relative risk reduction of 13% at any dose above 30 mg.
Campbell et al,19 in a 2007 review, found that doses greater than 300 mg conferred no benefit, and that rapid and maximum suppression of thromboxane A2 can be achieved by chewing or ingesting dissolved forms of aspirin 162 mg.
Conclusion. Aspirin doses higher than 81 mg (the US standard) confer no greater benefit and may even decrease the efficacy of aspirin. In an emergency, rapid suppression of thromboxane A2 can be achieved by chewing a minimum dose of 162 mg.
DIPYRIDAMOLE CAN BE ADDED TO ASPIRIN
In 1967, Weiss and Aledort5 found that aspirin’s antiplatelet effect could be blocked by adenosine diphosphate, which is released by activated platelet cells and is an essential part of thrombus formation. Adjacent platelets are then activated, leading to up-regulation of thromboxane A2 and glycoprotein IIb/IIIa receptors and resulting in a cascade of platelet activation and clot formation.20 Dipyridamole inhibits aggregation of platelets by inhibiting their ability to take up adenosine diphosphate.
Studies of dipyridamole
AICLA.9 Bousser et al9 randomized patients who suffered one or more cerebral or retinal infarctions to receive placebo, aspirin 1 g, or aspirin 1 g plus dipyridamole 225 mg. Aspirin was significantly better than placebo in preventing a recurrence of stroke. The event rate with aspirin plus dipyridamole was similar to the rate with aspirin alone, although on 2-by-2 analysis, the difference between placebo and aspirin plus dipyridamole did not reach statistical significance. However, the rate of carotid-origin stroke was 17% with aspirin alone and 6% with aspirin plus dipyridamole, a statistically significant difference.
Thus, this study confirmed the benefit of aspirin in preventing ischemic events but did not fully support the addition of dipyridamole, except in preventing stroke of carotid origin. The study had a number of limitations: the sample size was small, TIA was not included as an end point, computed tomography was not required for entry, and many patients were lost to follow-up, decreasing the statistical power of the trial.
The ESPS study21 was also a randomized controlled trial of aspirin plus dipyridamole vs placebo. But unlike AICLA, ESPS included patients with TIA.
ESPS found a 38.1% relative risk reduction in stroke with aspirin plus dipyridamole compared with placebo, and a 30.6% reduction in death from all causes. Interestingly, patients who had a TIA as the qualifying event had a lower end-point incidence and larger end-point reduction than those who had a stroke as the qualifying event. However, ESPS did not resolve the question of whether adding dipyridamole to aspirin affords any benefit over aspirin alone.
ESPS-214 hoped to answer this question. Patients were randomized to placebo, aspirin, dipyridamole, or aspirin plus dipyridamole. On 2 × 2 analysis, the dipyridamole group had a 16% lower rate of recurrent stroke than the placebo group, and patients on aspirin plus dipyridamole had a 37% lower rate. Aspirin plus dipyridamole yielded a 23.1% reduction compared with aspirin alone, and a 24.7% reduction compared with dipyridamole alone. Similar benefit was reported for the end point of TIA with combination therapy compared with either agent alone.
However, nearly 25% of patients had to withdraw because of side effects, particularly in the dipyridamole-alone and aspirin-dipyridamole groups, and, as mentioned above, verification of compliance in the aspirin group was an issue.14,22 Nevertheless, ESPS-2 clearly showed that aspirin plus dipyridamole was better than either drug alone in preventing recurrent stroke. It also showed the effectiveness of dipyridamole, which AICLA and ESPS could not do, because it had a larger study population, used a lower dose of aspirin, and perhaps because it used an extended-release form of dipyridamole.23
The ATT meta-analysis15 showed a clear benefit of antiplatelet therapy. However, much of this benefit was derived from aspirin therapy, with the addition of dipyridamole resulting in a nonsignificant 6% reduction of vascular events. Most of the patients on dipyridamole were from the ESPS-2 study. In effect, the ATT was a meta-analysis of aspirin, as aspirin studies dominated at that time.
A Cochrane review24 publsihed in 2003 attempted to rectify this by analyzing randomized controlled trials of dipyridamole vs placebo.24 Like the ATT meta-analysis, it did not bear out the benefits of dipyridamole: compared with placebo, there was no effect on the rate of vascular death, and only a minimal benefit in reduction of vascular events—and this latter point is only because of the inclusion of ESPS-2.
Directly comparing aspirin plus dipyridamole vs aspirin alone, the reviewers found no effect on the rate of vascular death, and with the exclusion of ESPS-2, no effect on vascular events.
The Cochrane review had the same limitation as the ATT meta-analysis, ie, dependence on a single trial (ESPS-2) to show benefit, and perhaps the fact that ESPS-2 was the only study that used an extended-release form of dipyridamole.
Leonardi-Bee et al25 performed a meta-analysis that overcame the limitation of ESPS-2 being the only study at the time with positive findings: they used pooled individual patient data from randomized trials and analyzed them en masse. Patients on aspirin plus dipyridamole had a 39% lower risk than with placebo and a 22% lower risk than with aspirin alone. Unlike the ATT and the Cochrane review, excluding ESPS-2 did not alter the statistically significant lower stroke rate with aspirin plus dipyridamole compared with controls. This meta-analysis helped to confirm ESPS-2’s finding of the additive effect of aspirin plus dipyridamole compared with aspirin and placebo control.
ESPRIT.26,27 The European/Australasian Stroke Prevention in Reversible Ischaemia Trial confirmed these findings. This randomized controlled trial compared aspirin plus dipyridamole against aspirin alone in patients with a TIA or minor ischemic stroke of arterial origin within the past 6 months. For the primary end point (death from all vascular causes, nonfatal stroke, nonfatal MI, nonfatal major bleeding complication), the hazard ratio was 0.80 favoring aspirin plus dipyridamole, with a number needed to treat of 104 over a mean of 3.5 years (absolute risk reduction of 1% per year). Importantly, twice as many patients taking aspirin plus dipyridamole discontinued the medication.
Caveats to interpreting this study are that it was not blinded, the aspirin doses varied (although the median aspirin dose—75 mg—was the same between the two groups), and not all patients received the extended-release form of dipyridamole.
Conclusions about dipyridamole
ESPS-2, ESPRIT, and the meta-analysis by Leonardi-Bee et al showed that aspirin plus dipyridamole is more effective than placebo or aspirin alone in secondary prevention of vascular events, including stroke. Also, extended-release dipyridamole appears to be more effective.
Unfortunately, many patients stop taking dipyridamole because of side effects (primarily headache).
Based on the results of ESPRIT, the absolute benefit of dipyridamole used alone may be small.
CLOPIDOGREL: SIMILAR TO ASPIRIN IN EFFICACY?
Like dipyridamole, clopidogrel targets adenosine diphosphate to prevent clot formation, blocking its ability to bind to its receptor on platelets. It is a thienopyridine and, unlike its sister drug ticlopidine, does not seem to be associated with the potentially serious side effects of neutropenia. However, a few cases of thrombotic thrombocytopenic purpura have been reported.28 The other drugs in this class have not been evaluated in clinical trials for secondary stroke prophylaxis.
Trials of clopidogrel
CAPRIE.29 The Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events trial, in 1996, was one of the first to compare the clinical use of clopidogrel against aspirin. It was a randomized controlled noninferiority trial in patients over age 21 (inclusion criteria: ischemic stroke, MI, or peripheral arterial disease) randomized to aspirin 325 mg once daily or clopidogrel 75 mg once daily. Patients were followed for 1 to 3 years.
Patients on clopidogrel had a relative risk reduction of 8.7% in primary events (ischemic stroke, MI, or vascular death); patients on aspirin were at significantly higher risk of gastrointestinal hemorrhage. Patients with peripheral arterial disease as the qualifying event did particularly well on clopidogrel, with a significant relative risk reduction of 23.8%.
Limitations of the CAPRIE trial included its inability to measure the effect of treatment on individual outcomes, particularly stroke, and the fact that the relative risk reduction for patients with stroke as the qualifying event was not significant (P = .66). Another limitation was that it did not use TIA as an entry criterion or as part of the composite outcome. Also, the relative risk reduction had a wide confidence interval, and a large number of patients discontinued therapy for reasons other than the defined outcomes.
Nevertheless, the CAPRIE trial showed clopidogrel to be an effective antiplatelet prophylactic, particularly in patients with peripheral artery disease, but with no discernible difference from aspirin for those patients with MI or stroke as a qualifying event.
MATCH.30 The Management of Atherothrombosis With Clopidogrel in High-risk Patients trial hoped to better assess clopidogrel’s efficacy, particularly in patients with ischemic cerebral events. Cardiac studies leading up to MATCH suggested that adding a thienopyridine to aspirin might offer additive benefit in reducing the rate of vascular outcomes.15,31 MATCH randomized high-risk patients (inclusion criteria were ischemic stroke or TIA and a history of vascular disease) to clopidogrel or to aspirin plus clopidogrel.
There was a nonsignificant 6.4% relative risk reduction in the combined primary outcome of MI, ischemic stroke, vascular death, other vascular death, and re-hospitalization for acute ischemic events in the aspirin-plus-clopidogrel group compared with clopidogrel alone. However, this came at the cost of double the number of bleeding events in the combination group, mitigating most of the benefit of combination therapy.
An important caveat in interpreting the results of MATCH, as compared with the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study, is that aspirin was being added to clopidogrel, not vice versa. CURE, which looked at the addition of clopidogrel to aspirin vs aspirin alone in cardiac patients, found a significant reduction of ischemic events taken as a group (relative risk 0.8), and a trend toward a lower rate of stroke (relative risk 0.86, but 95% confidence interval encompassing 1) for aspirin plus clopidogrel vs aspirin alone.31 However, patients in the CURE trial did not have high-risk vasculopathy per se but rather non-ST-elevation MI, perhaps skewing the benefit of combination therapy and lessening the risk of intracranial bleeding.
CHARISMA.32 The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance trial, like the CURE trial, compared aspirin plus clopidogrel vs aspirin in patients with established cardiovascular, cerebrovascular, or peripheral arterial disease, or who were at high risk of events. As in the MATCH study, the findings for combination therapy were a nonsignificant relative risk of 0.93 for primary events (MI, stroke, or death from cardiovascular causes), and a significant reduction of secondary end points (primary end point event plus TIA or hospitalization for unstable angina) (relative risk 0.92, P = .04).
Importantly, combination therapy significantly increased the rate of bleeding events. In asymptomatic patients (those without documented vascular disease but with multiple atherothrombotic risk factors), there was actually harm with combined treatment. Conversely, for symptomatic patients (those with documented vascular disease), there was a negligible, but significant reduction in primary end points.
The result was that in patients with documented vascular disease, aspirin plus clopidogrel combination therapy provided little or no benefit over aspirin alone. For patients with elevated risk factors but no documented vascular burden, there may actually be harm from combination therapy.
PRoFESS.33 Logically following is the question of whether aspirin plus dipyridamole offers any benefit over clopidogrel as a stroke prophylactic. The Prevention Regimen for Effectively Avoiding Second Strokes trial hoped to answer this by comparing clopidogrel against aspirin plus dipyridamole, both with and without telmisartan, in patients with recent stroke.
The rate of recurrent stroke was similar in the two groups, but there were 25 fewer ischemic strokes in patients on aspirin plus dipyridamole, offset by an increase in hemorrhagic strokes. Rates of secondary outcomes of stroke, death, or MI were nearly identical between the groups. Early discontinuation of treatment was significantly more frequent in those patients taking aspirin plus dipyridamole, meaning better compliance for those taking clopidogrel.
Initially, patients were to be randomized to either aspirin plus dipyridamole or aspirin plus clopidogrel. However, after MATCH30 demonstrated a significantly higher bleeding risk with aspirin plus clopidogrel, patients were changed to clopidogrel alone. But despite this, the bleeding risk was still higher with aspirin plus dipyridamole.
During the trial, the entry criteria were expanded, allowing for the inclusion of younger patients and those with less recent strokes; but despite this change, the study remained underpowered to demonstrate its goal of noninferiority. Thus, it showed only a trend of noninferiority of clopidogrel vs aspirin plus dipyridamole.
What the clopidogrel trials tell us
Clopidogrel confers a benefit similar to that of aspirin (as shown in the CAPRIE study).29 Although aspirin plus dipyridamole confers greater benefit than aspirin alone (as shown in the ESPS-2,14 Leonardi-Bee,25 and ESPRIT26 studies), aspirin plus dipyridamole is not superior to clopidogrel, and may even be inferior.34
WARFARIN FOR ATRIAL FIBRILLATION ONLY
Warfarin acts by disrupting the coagulation cascade rather than acting at the site of platelet plug formation. In theory, warfarin should be as effective as the antiplatelet drugs in preventing clot formation, and so it was thought to possibly be effective in preventing stroke of arterial origin.
However, in at least three studies, warfarin increased the risk of death, MI, and hemorrhage, with perhaps a slight decrease in the risk of recurrent stroke in patients with suspected stroke or TIA.35–37 This should be differentiated from stroke originating from cardiac dysrhythmias, for which warfarin has clearly been shown to be beneficial.28
THREE GOOD MEDICAL OPTIONS FOR PREVENTING STROKE RECURRENCE
Antiplatelet therapy offers benefit in the primary and secondary prevention of stroke, with a 25% reduction in the rate of nonfatal stroke and a 17% reduction in the rate of death due to vascular causes.15
Aspirin is the best established
Aspirin is the best established, best tolerated, and least expensive of the three contemporary agents. Further, it is also the agent of choice for acute stroke care, to be given within 48 hours of a stroke to mitigate the risk of death and morbidity. The data for other agents in acute stroke management remain limited.38
Aspirin plus dipyridamole
Aspirin plus dipyridamole is slightly more efficacious than aspirin alone, and it is an alternative when aspirin is ineffective and when the patient can afford the additional cost. Aspirin plus dipyridamole offers up to a 22% relative risk reduction (but a small reduction in absolute risk) of stroke compared with aspirin alone, as demonstrated by ESPS-2,14 Leonardi-Bee et al,25 and ESPRIT.26
When is clopidogrel appropriate?
Up to one-third of patients may not tolerate aspirin plus dipyridamole because of side effects. Clopidogrel is an option for these patients. The CAPRIE study29 showed clopidogrel similar in efficacy to aspirin.
In contrast to aspirin plus dipyridamole, there is clearly no benefit to combining aspirin and clopidogrel for ischemic stroke prophylaxis. And data from PRoFESS33 suggested the combination was qualitatively inferior to aspirin plus dipyridamole. However, the PRoFESS trial was underpowered to fully bear this out.
Therefore, current guidelines consider all three agents as appropriate for secondary prevention of stroke. One is not preferred over another, and the selection should be based on individual patient characteristics and affordability.28
CAROTID SURGERY OR STENTING: BENEFITS AND LIMITATIONS
Atherosclerosis is the most common cause of stroke, and atherosclerosis of the common carotid bifurcation accounts for a small but significant percentage of all strokes.39–41
The degree of carotid stenosis and whether it is producing symptoms influence how it should be managed. For patients with symptomatic carotid stenosis of more than 70%, multicenter randomized trials have shown that surgery (ie, carotid endarterectomy) added to medical therapy decreases the rate of recurrent stroke by up to 17% and the rate of combined stroke and death by 10% to 12% over a 2- to 3-year follow-up period (level of evidence A).42–44 No study has proven the efficacy of surgery in patients with symptomatic stenosis of less than 50%.43,44
Similarly, in asymptomatic carotid disease, preventive surgery is a beneficial adjunct to medical therapy in certain patients. An approximate 6% reduction in the rate of stroke or death over 5 years has been shown in patients with moderate stenosis (> 60%), with men younger than age 75 and with greater than 70% stenosis deriving the most benefit.45–47
However, these robust, positive results with surgical intervention should not overshadow the importance of intensive and guided medical therapy, which has been shown to mitigate the risk of stroke.48,49
Is stenting as good as surgery? In the multicenter randomized Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), stenting resulted in similar rates of stroke and MI in patients with symptomatic and asymptomatic disease.50 However, stenting carried a greater risk of perioperative stroke, and endarterectomy carried a greater risk of MI. Those under age 70 benefited more from stenting, and those over age 70 benefited more from endarterectomy.
But another fact to keep in mind is that the relationship between carotid narrowing and an ipsilateral stroke is not necessarily direct. Two follow-up studies in patients from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that up to 45% of strokes that occurred after intervention in the distribution of the asymptomatic stenosed carotid artery were unrelated to the stenosis.51,52 Moreover, up to 20% of subsequent strokes in the distribution of the symptomatic artery were not of large-artery origin, increasing up to 35% for those with stenosis of less than 70%.51 Clearly, thorough screening of those with presumed symptomatic stenosis is needed to eliminate other possible causes.
- Zivin JA. Approach to cerebrovascular diseases. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier, 2012:2304–2309.
- Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999; 30:338–349.
- Gotoh F, Tohgi H, Hirai S, et al. Cilostazol Stroke Prevention Study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000; 9:147–157.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967; 2:495–497.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Stroke 1977; 8:301–314.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Part II: surgical group. Stroke 1978; 9:309–319.
- Sorensen PS, Pedersen H, Marquardsen J, et al. Acetylsalicylic acid in the prevention of stroke in patients with reversible cerebral ischemic attacks. A Danish cooperative study. Stroke 1983; 14:15–22.
- Bousser MG, Eschwege E, Haguenau M, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983; 14:5–14.
- High-dose acetylsalicylic acid after cerebral infarction. A Swedish Cooperative Study. Stroke 1987; 18:325–334.
- Boysen G, Sørensen PS, Juhler M, et al. Danish very-low-dose aspirin after carotid endarterectomy trial. Stroke 1988; 19:1211–1215.
- Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54:1044–1054.
- Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet 1991; 338:1345–1349.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, MI, and stroke in high risk patients. BMJ 2002; 324:71–86.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med 1991; 325:1261–1266.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Algra A, van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry 1996; 60:197–199.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47:2169–2180.
- European Stroke Prevention Study. ESPS Group. Stroke 1990; 21:1122–1130.
- Davis SM, Donnan GA. Secondary prevention for stroke after CAPRIE and ESPS-2. Opinion 1. Cerebrovasc Dis 1998; 8:73–77.
- Diener HC. Dipyridamole trials in stroke prevention. Neurology 1998; 51(suppl 3):S17–S19.
- De Schryver EL, Algra A, van Gijn J. Cochrane review: dipyridamole for preventing major vascular events in patients with vascular disease. Stroke 2003; 34:2072–2080.
- Leonardi-Bee J, Bath PM, Bousser MG, et al; Dipyridamole in Stroke Collaboration (DISC). Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005; 36:162–168.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006; 367:1665–1673.
- Tirschwell D. Aspirin plus dipyridamole was more effective than aspirin alone for preventing vascular events after minor cerebral ischemia. ACP J Club 2006; 145:57.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364:331–337.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Kent DM, Thaler DE. Stroke prevention—insights from incoherence. N Engl J Med 2008; 359:1287–1289.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352:1305–1316.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol 2007; 6:115–124.
- Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345:1444–1451.
- Jauch EC, Saver JL, Adams HP, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32:2735–2740.
- Zivin JA. Ischemic cerebrovascular disease. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine 24th ed. Philadelphia, PA: Elsevier; 2012: chap 414. www.mdconsult.com. Accessed November 7, 2013.
- Smith WS, Johnston C, Easton D. Cerebrovascular diseases. In:Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw Hill; 2005: chap 349. www.accessmedicine.com.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991; 325:445–453.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Hobson RW, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363:1491–1502.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Barnett HJ, Gunton RW, Eliasziw M, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA 2000; 283:1429–1436.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
After a stroke, an important goal is to prevent another one.1,2 And for patients who have had an ischemic stroke or transient ischemic attack (TIA) due to atherosclerosis, an important part of secondary preventive therapy is a drug that inhibits platelets—ie, aspirin, extended-release dipyridamole, or clopidogrel. This has taken years to establish.
In the following pages, we discuss the antiplatelet agents that have been shown to be beneficial after stroke of atherosclerotic origin, and we briefly review the indications for surgery and stenting for the subset of patients whose strokes are caused by symptomatic carotid disease.
(Although managing modifiable risk factors such as smoking, hypertension, diabetes, and dyslipidemia is also important, we will not cover this topic here, nor will we talk about hemorrhagic stroke or stroke due to atrial fibrillation. Also not discussed here is cilostazol, which, although shown to be effective in preventing recurrent stroke when compared with placebo and aspirin,3,4 has not been approved for this use by the US Food and Drug Administration, as of this writing.)
HOW WE REVIEWED THE LITERATURE
We searched PubMed using the terms aspirin, acetylsalicylic acid, clopidogrel, and/or dipyridamole, in combination with stroke, cerebral ische(ae)mia, transient ische(ae)mic attacks, or retinal artery occlusion. We reviewed only clinical trials or meta-analyses of these drugs for either primary or secondary prevention of cerebrovascular disease.
As our aim was to review the topic and not to perform a meta-analysis, no cutoffs were used to exclude trials. The references in the selected papers were also reviewed to expand the articles. Finally, the references in the current American Heart Association and American Stroke Association secondary stroke prevention guideline were also reviewed.
For a summary of the trials included in our review, see the Data Supplement as an appendix to the online version of this article.
ASPIRIN: THE GOLD STANDARD
Prescribed by Hippocrates in the form of willow bark extract, aspirin has long been known for its antipyretic and anti-inflammatory properties. Its antiplatelet and antithrombotic properties, first described in 1967 by Weiss and Aledort,5 are mediated by irreversible inhibition of cyclooxygenase, leading to decreased thromboxane A2, a platelet-aggregation activator.
Fields et al,6,7 in 1977 and 1978, reported that in a controlled trial in patients with TIA or monocular blindness, fewer subsequent TIAs occurred in patients who received aspirin, although the difference was not statistically significant, with lower rates of events only in nonsurgical patients. Over the next 20 years, the results remained mixed.
The Danish Cooperative study8 (1983) found no significant difference in the rate of recurrent stroke with aspirin vs placebo.
AICLA.9 The Accidents Ischémiques Cérébraux Liés à l’Athérosclérose study of 1983 did find a difference. However, both the Danish Cooperative study and the AICLA were limited by lacking standardized computed tomographic imaging to rule out hemorrhagic stroke and by being relatively small.
The Swedish Cooperative Study10 (1987) found no statistical difference between high-dose aspirin and placebo in preventing recurrent vascular events (stroke, TIA, or myocardial infarction [MI]) 1 to 3 weeks after a stroke. However, it had several limitations: the aspirin group contained more patients with ischemic heart disease (who are more likely to die of cardiac causes), there were significantly more men in the aspirin group, and nearly one-fourth of the deaths were a result of the initial stroke, potentially masking the effect of aspirin in secondary prevention.
Later studies began to show a consistently favorable effect of aspirin.
Boysen et al11 in 1988 reported a nonsignificant trend toward fewer adverse events with aspirin.
UK-TIA.12 The United Kingdom Transient Ischaemic Attack trial in 1991 found a similar trend.
SALT.13 The Swedish Aspirin Low-dose Trial, also in 1991, showed a significant 18% lower rate of stroke or death in patients with recent TIA, minor stroke, or retinal occlusion treated with low-dose aspirin. The inclusion of patients with TIA helped broaden the population that might benefit. However, the study may have favored the aspirin group by having a run-in period in which patients were nonrandomly treated either with aspirin or with anticoagulation at the discretion of the patient’s physician and, if they suffered “several” TIAs, a stroke, retinal artery occlusion, or MI, were removed from the study.
ESPS-2.14 The second European Stroke Prevention Study in 1996 added to the evidence that aspirin prevents recurrent stroke. Patients with a history of TIA or stroke were randomized in double-blind fashion to four treatment groups: placebo, low-dose aspirin, dipyridamole, or aspirin plus dipyridamole. At 2 years, strokes had occurred in 18% fewer patients in the aspirin group than in the placebo group, and TIAs had occurred in 21.9% fewer. However, aspirin was associated with an absolute 0.5% increase in severe and fatal bleeding. The power of the study was limited because patients from one center were excluded because of “serious inconsistencies in patient case record forms and compliance assay determinations.” 14
Comment. The mixed results with aspirin in studies predating ESPS-2 were partly because the study populations were too small to show benefit.
ATT.15 The Antithrombotic Trialists’ Collaboration performed a meta-analysis that conclusively confirmed the benefit of aspirin after stroke or TIA. The investigators analyzed individual patient data pooled from randomized controlled trials published before 1997 that compared antiplatelet regimens (mostly aspirin) against placebo and against each other. The rates of vascular events were 10.7% with treatment vs 13.2% with placebo (P < .0001). Antiplatelet therapy was particularly effective in preventing ischemic stroke, with a 25% reduction in the rate of nonfatal stroke, and with an overall absolute benefit in stroke prevention across all high-risk patient groups. This translated to 25 fewer nonfatal strokes per 1,000 patients treated with antiplatelet therapy.
What is the optimal aspirin dose?
Studies of aspirin have used different daily doses—the earliest studies used large doses of 1,000 to 1,500 mg.6–10
Boysen et al11 in 1988 found a trend toward benefit (not statistically significant) with doses ranging from 50 mg to 100 mg.
In 1991, three separate studies found that higher doses of aspirin were no more effective than lower doses.
The UK-TIA trial12 compared aspirin 300 mg vs 1,200 mg and found a higher risk of gastrointestinal bleeding with the higher dose.
The SALT Collaborative Group13 found 75 mg to be effective.
The Dutch TIA trial16 compared 30 mg vs 283 mg; end point outcomes were similar but the rate of adverse events was higher with 283 mg.
ESPS-2 was able to show efficacy at a dose of only 50 mg.14
Taylor et al17 compared lower doses (81 or 325 mg) vs higher doses (650 or 1,300 mg) for patients undergoing carotid endarterectomy and found that the risk of adverse events was twice as high with the higher doses.
The ATT Collaboration15 found that efficacy was 40% lower with the highest dose of aspirin than with the lowest doses.
Algra and van Gijn18 performed a meta-analysis of all these studies and found no difference in risk reduction between low-dose and high-dose aspirin, with an overall relative risk reduction of 13% at any dose above 30 mg.
Campbell et al,19 in a 2007 review, found that doses greater than 300 mg conferred no benefit, and that rapid and maximum suppression of thromboxane A2 can be achieved by chewing or ingesting dissolved forms of aspirin 162 mg.
Conclusion. Aspirin doses higher than 81 mg (the US standard) confer no greater benefit and may even decrease the efficacy of aspirin. In an emergency, rapid suppression of thromboxane A2 can be achieved by chewing a minimum dose of 162 mg.
DIPYRIDAMOLE CAN BE ADDED TO ASPIRIN
In 1967, Weiss and Aledort5 found that aspirin’s antiplatelet effect could be blocked by adenosine diphosphate, which is released by activated platelet cells and is an essential part of thrombus formation. Adjacent platelets are then activated, leading to up-regulation of thromboxane A2 and glycoprotein IIb/IIIa receptors and resulting in a cascade of platelet activation and clot formation.20 Dipyridamole inhibits aggregation of platelets by inhibiting their ability to take up adenosine diphosphate.
Studies of dipyridamole
AICLA.9 Bousser et al9 randomized patients who suffered one or more cerebral or retinal infarctions to receive placebo, aspirin 1 g, or aspirin 1 g plus dipyridamole 225 mg. Aspirin was significantly better than placebo in preventing a recurrence of stroke. The event rate with aspirin plus dipyridamole was similar to the rate with aspirin alone, although on 2-by-2 analysis, the difference between placebo and aspirin plus dipyridamole did not reach statistical significance. However, the rate of carotid-origin stroke was 17% with aspirin alone and 6% with aspirin plus dipyridamole, a statistically significant difference.
Thus, this study confirmed the benefit of aspirin in preventing ischemic events but did not fully support the addition of dipyridamole, except in preventing stroke of carotid origin. The study had a number of limitations: the sample size was small, TIA was not included as an end point, computed tomography was not required for entry, and many patients were lost to follow-up, decreasing the statistical power of the trial.
The ESPS study21 was also a randomized controlled trial of aspirin plus dipyridamole vs placebo. But unlike AICLA, ESPS included patients with TIA.
ESPS found a 38.1% relative risk reduction in stroke with aspirin plus dipyridamole compared with placebo, and a 30.6% reduction in death from all causes. Interestingly, patients who had a TIA as the qualifying event had a lower end-point incidence and larger end-point reduction than those who had a stroke as the qualifying event. However, ESPS did not resolve the question of whether adding dipyridamole to aspirin affords any benefit over aspirin alone.
ESPS-214 hoped to answer this question. Patients were randomized to placebo, aspirin, dipyridamole, or aspirin plus dipyridamole. On 2 × 2 analysis, the dipyridamole group had a 16% lower rate of recurrent stroke than the placebo group, and patients on aspirin plus dipyridamole had a 37% lower rate. Aspirin plus dipyridamole yielded a 23.1% reduction compared with aspirin alone, and a 24.7% reduction compared with dipyridamole alone. Similar benefit was reported for the end point of TIA with combination therapy compared with either agent alone.
However, nearly 25% of patients had to withdraw because of side effects, particularly in the dipyridamole-alone and aspirin-dipyridamole groups, and, as mentioned above, verification of compliance in the aspirin group was an issue.14,22 Nevertheless, ESPS-2 clearly showed that aspirin plus dipyridamole was better than either drug alone in preventing recurrent stroke. It also showed the effectiveness of dipyridamole, which AICLA and ESPS could not do, because it had a larger study population, used a lower dose of aspirin, and perhaps because it used an extended-release form of dipyridamole.23
The ATT meta-analysis15 showed a clear benefit of antiplatelet therapy. However, much of this benefit was derived from aspirin therapy, with the addition of dipyridamole resulting in a nonsignificant 6% reduction of vascular events. Most of the patients on dipyridamole were from the ESPS-2 study. In effect, the ATT was a meta-analysis of aspirin, as aspirin studies dominated at that time.
A Cochrane review24 publsihed in 2003 attempted to rectify this by analyzing randomized controlled trials of dipyridamole vs placebo.24 Like the ATT meta-analysis, it did not bear out the benefits of dipyridamole: compared with placebo, there was no effect on the rate of vascular death, and only a minimal benefit in reduction of vascular events—and this latter point is only because of the inclusion of ESPS-2.
Directly comparing aspirin plus dipyridamole vs aspirin alone, the reviewers found no effect on the rate of vascular death, and with the exclusion of ESPS-2, no effect on vascular events.
The Cochrane review had the same limitation as the ATT meta-analysis, ie, dependence on a single trial (ESPS-2) to show benefit, and perhaps the fact that ESPS-2 was the only study that used an extended-release form of dipyridamole.
Leonardi-Bee et al25 performed a meta-analysis that overcame the limitation of ESPS-2 being the only study at the time with positive findings: they used pooled individual patient data from randomized trials and analyzed them en masse. Patients on aspirin plus dipyridamole had a 39% lower risk than with placebo and a 22% lower risk than with aspirin alone. Unlike the ATT and the Cochrane review, excluding ESPS-2 did not alter the statistically significant lower stroke rate with aspirin plus dipyridamole compared with controls. This meta-analysis helped to confirm ESPS-2’s finding of the additive effect of aspirin plus dipyridamole compared with aspirin and placebo control.
ESPRIT.26,27 The European/Australasian Stroke Prevention in Reversible Ischaemia Trial confirmed these findings. This randomized controlled trial compared aspirin plus dipyridamole against aspirin alone in patients with a TIA or minor ischemic stroke of arterial origin within the past 6 months. For the primary end point (death from all vascular causes, nonfatal stroke, nonfatal MI, nonfatal major bleeding complication), the hazard ratio was 0.80 favoring aspirin plus dipyridamole, with a number needed to treat of 104 over a mean of 3.5 years (absolute risk reduction of 1% per year). Importantly, twice as many patients taking aspirin plus dipyridamole discontinued the medication.
Caveats to interpreting this study are that it was not blinded, the aspirin doses varied (although the median aspirin dose—75 mg—was the same between the two groups), and not all patients received the extended-release form of dipyridamole.
Conclusions about dipyridamole
ESPS-2, ESPRIT, and the meta-analysis by Leonardi-Bee et al showed that aspirin plus dipyridamole is more effective than placebo or aspirin alone in secondary prevention of vascular events, including stroke. Also, extended-release dipyridamole appears to be more effective.
Unfortunately, many patients stop taking dipyridamole because of side effects (primarily headache).
Based on the results of ESPRIT, the absolute benefit of dipyridamole used alone may be small.
CLOPIDOGREL: SIMILAR TO ASPIRIN IN EFFICACY?
Like dipyridamole, clopidogrel targets adenosine diphosphate to prevent clot formation, blocking its ability to bind to its receptor on platelets. It is a thienopyridine and, unlike its sister drug ticlopidine, does not seem to be associated with the potentially serious side effects of neutropenia. However, a few cases of thrombotic thrombocytopenic purpura have been reported.28 The other drugs in this class have not been evaluated in clinical trials for secondary stroke prophylaxis.
Trials of clopidogrel
CAPRIE.29 The Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events trial, in 1996, was one of the first to compare the clinical use of clopidogrel against aspirin. It was a randomized controlled noninferiority trial in patients over age 21 (inclusion criteria: ischemic stroke, MI, or peripheral arterial disease) randomized to aspirin 325 mg once daily or clopidogrel 75 mg once daily. Patients were followed for 1 to 3 years.
Patients on clopidogrel had a relative risk reduction of 8.7% in primary events (ischemic stroke, MI, or vascular death); patients on aspirin were at significantly higher risk of gastrointestinal hemorrhage. Patients with peripheral arterial disease as the qualifying event did particularly well on clopidogrel, with a significant relative risk reduction of 23.8%.
Limitations of the CAPRIE trial included its inability to measure the effect of treatment on individual outcomes, particularly stroke, and the fact that the relative risk reduction for patients with stroke as the qualifying event was not significant (P = .66). Another limitation was that it did not use TIA as an entry criterion or as part of the composite outcome. Also, the relative risk reduction had a wide confidence interval, and a large number of patients discontinued therapy for reasons other than the defined outcomes.
Nevertheless, the CAPRIE trial showed clopidogrel to be an effective antiplatelet prophylactic, particularly in patients with peripheral artery disease, but with no discernible difference from aspirin for those patients with MI or stroke as a qualifying event.
MATCH.30 The Management of Atherothrombosis With Clopidogrel in High-risk Patients trial hoped to better assess clopidogrel’s efficacy, particularly in patients with ischemic cerebral events. Cardiac studies leading up to MATCH suggested that adding a thienopyridine to aspirin might offer additive benefit in reducing the rate of vascular outcomes.15,31 MATCH randomized high-risk patients (inclusion criteria were ischemic stroke or TIA and a history of vascular disease) to clopidogrel or to aspirin plus clopidogrel.
There was a nonsignificant 6.4% relative risk reduction in the combined primary outcome of MI, ischemic stroke, vascular death, other vascular death, and re-hospitalization for acute ischemic events in the aspirin-plus-clopidogrel group compared with clopidogrel alone. However, this came at the cost of double the number of bleeding events in the combination group, mitigating most of the benefit of combination therapy.
An important caveat in interpreting the results of MATCH, as compared with the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study, is that aspirin was being added to clopidogrel, not vice versa. CURE, which looked at the addition of clopidogrel to aspirin vs aspirin alone in cardiac patients, found a significant reduction of ischemic events taken as a group (relative risk 0.8), and a trend toward a lower rate of stroke (relative risk 0.86, but 95% confidence interval encompassing 1) for aspirin plus clopidogrel vs aspirin alone.31 However, patients in the CURE trial did not have high-risk vasculopathy per se but rather non-ST-elevation MI, perhaps skewing the benefit of combination therapy and lessening the risk of intracranial bleeding.
CHARISMA.32 The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance trial, like the CURE trial, compared aspirin plus clopidogrel vs aspirin in patients with established cardiovascular, cerebrovascular, or peripheral arterial disease, or who were at high risk of events. As in the MATCH study, the findings for combination therapy were a nonsignificant relative risk of 0.93 for primary events (MI, stroke, or death from cardiovascular causes), and a significant reduction of secondary end points (primary end point event plus TIA or hospitalization for unstable angina) (relative risk 0.92, P = .04).
Importantly, combination therapy significantly increased the rate of bleeding events. In asymptomatic patients (those without documented vascular disease but with multiple atherothrombotic risk factors), there was actually harm with combined treatment. Conversely, for symptomatic patients (those with documented vascular disease), there was a negligible, but significant reduction in primary end points.
The result was that in patients with documented vascular disease, aspirin plus clopidogrel combination therapy provided little or no benefit over aspirin alone. For patients with elevated risk factors but no documented vascular burden, there may actually be harm from combination therapy.
PRoFESS.33 Logically following is the question of whether aspirin plus dipyridamole offers any benefit over clopidogrel as a stroke prophylactic. The Prevention Regimen for Effectively Avoiding Second Strokes trial hoped to answer this by comparing clopidogrel against aspirin plus dipyridamole, both with and without telmisartan, in patients with recent stroke.
The rate of recurrent stroke was similar in the two groups, but there were 25 fewer ischemic strokes in patients on aspirin plus dipyridamole, offset by an increase in hemorrhagic strokes. Rates of secondary outcomes of stroke, death, or MI were nearly identical between the groups. Early discontinuation of treatment was significantly more frequent in those patients taking aspirin plus dipyridamole, meaning better compliance for those taking clopidogrel.
Initially, patients were to be randomized to either aspirin plus dipyridamole or aspirin plus clopidogrel. However, after MATCH30 demonstrated a significantly higher bleeding risk with aspirin plus clopidogrel, patients were changed to clopidogrel alone. But despite this, the bleeding risk was still higher with aspirin plus dipyridamole.
During the trial, the entry criteria were expanded, allowing for the inclusion of younger patients and those with less recent strokes; but despite this change, the study remained underpowered to demonstrate its goal of noninferiority. Thus, it showed only a trend of noninferiority of clopidogrel vs aspirin plus dipyridamole.
What the clopidogrel trials tell us
Clopidogrel confers a benefit similar to that of aspirin (as shown in the CAPRIE study).29 Although aspirin plus dipyridamole confers greater benefit than aspirin alone (as shown in the ESPS-2,14 Leonardi-Bee,25 and ESPRIT26 studies), aspirin plus dipyridamole is not superior to clopidogrel, and may even be inferior.34
WARFARIN FOR ATRIAL FIBRILLATION ONLY
Warfarin acts by disrupting the coagulation cascade rather than acting at the site of platelet plug formation. In theory, warfarin should be as effective as the antiplatelet drugs in preventing clot formation, and so it was thought to possibly be effective in preventing stroke of arterial origin.
However, in at least three studies, warfarin increased the risk of death, MI, and hemorrhage, with perhaps a slight decrease in the risk of recurrent stroke in patients with suspected stroke or TIA.35–37 This should be differentiated from stroke originating from cardiac dysrhythmias, for which warfarin has clearly been shown to be beneficial.28
THREE GOOD MEDICAL OPTIONS FOR PREVENTING STROKE RECURRENCE
Antiplatelet therapy offers benefit in the primary and secondary prevention of stroke, with a 25% reduction in the rate of nonfatal stroke and a 17% reduction in the rate of death due to vascular causes.15
Aspirin is the best established
Aspirin is the best established, best tolerated, and least expensive of the three contemporary agents. Further, it is also the agent of choice for acute stroke care, to be given within 48 hours of a stroke to mitigate the risk of death and morbidity. The data for other agents in acute stroke management remain limited.38
Aspirin plus dipyridamole
Aspirin plus dipyridamole is slightly more efficacious than aspirin alone, and it is an alternative when aspirin is ineffective and when the patient can afford the additional cost. Aspirin plus dipyridamole offers up to a 22% relative risk reduction (but a small reduction in absolute risk) of stroke compared with aspirin alone, as demonstrated by ESPS-2,14 Leonardi-Bee et al,25 and ESPRIT.26
When is clopidogrel appropriate?
Up to one-third of patients may not tolerate aspirin plus dipyridamole because of side effects. Clopidogrel is an option for these patients. The CAPRIE study29 showed clopidogrel similar in efficacy to aspirin.
In contrast to aspirin plus dipyridamole, there is clearly no benefit to combining aspirin and clopidogrel for ischemic stroke prophylaxis. And data from PRoFESS33 suggested the combination was qualitatively inferior to aspirin plus dipyridamole. However, the PRoFESS trial was underpowered to fully bear this out.
Therefore, current guidelines consider all three agents as appropriate for secondary prevention of stroke. One is not preferred over another, and the selection should be based on individual patient characteristics and affordability.28
CAROTID SURGERY OR STENTING: BENEFITS AND LIMITATIONS
Atherosclerosis is the most common cause of stroke, and atherosclerosis of the common carotid bifurcation accounts for a small but significant percentage of all strokes.39–41
The degree of carotid stenosis and whether it is producing symptoms influence how it should be managed. For patients with symptomatic carotid stenosis of more than 70%, multicenter randomized trials have shown that surgery (ie, carotid endarterectomy) added to medical therapy decreases the rate of recurrent stroke by up to 17% and the rate of combined stroke and death by 10% to 12% over a 2- to 3-year follow-up period (level of evidence A).42–44 No study has proven the efficacy of surgery in patients with symptomatic stenosis of less than 50%.43,44
Similarly, in asymptomatic carotid disease, preventive surgery is a beneficial adjunct to medical therapy in certain patients. An approximate 6% reduction in the rate of stroke or death over 5 years has been shown in patients with moderate stenosis (> 60%), with men younger than age 75 and with greater than 70% stenosis deriving the most benefit.45–47
However, these robust, positive results with surgical intervention should not overshadow the importance of intensive and guided medical therapy, which has been shown to mitigate the risk of stroke.48,49
Is stenting as good as surgery? In the multicenter randomized Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), stenting resulted in similar rates of stroke and MI in patients with symptomatic and asymptomatic disease.50 However, stenting carried a greater risk of perioperative stroke, and endarterectomy carried a greater risk of MI. Those under age 70 benefited more from stenting, and those over age 70 benefited more from endarterectomy.
But another fact to keep in mind is that the relationship between carotid narrowing and an ipsilateral stroke is not necessarily direct. Two follow-up studies in patients from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that up to 45% of strokes that occurred after intervention in the distribution of the asymptomatic stenosed carotid artery were unrelated to the stenosis.51,52 Moreover, up to 20% of subsequent strokes in the distribution of the symptomatic artery were not of large-artery origin, increasing up to 35% for those with stenosis of less than 70%.51 Clearly, thorough screening of those with presumed symptomatic stenosis is needed to eliminate other possible causes.
After a stroke, an important goal is to prevent another one.1,2 And for patients who have had an ischemic stroke or transient ischemic attack (TIA) due to atherosclerosis, an important part of secondary preventive therapy is a drug that inhibits platelets—ie, aspirin, extended-release dipyridamole, or clopidogrel. This has taken years to establish.
In the following pages, we discuss the antiplatelet agents that have been shown to be beneficial after stroke of atherosclerotic origin, and we briefly review the indications for surgery and stenting for the subset of patients whose strokes are caused by symptomatic carotid disease.
(Although managing modifiable risk factors such as smoking, hypertension, diabetes, and dyslipidemia is also important, we will not cover this topic here, nor will we talk about hemorrhagic stroke or stroke due to atrial fibrillation. Also not discussed here is cilostazol, which, although shown to be effective in preventing recurrent stroke when compared with placebo and aspirin,3,4 has not been approved for this use by the US Food and Drug Administration, as of this writing.)
HOW WE REVIEWED THE LITERATURE
We searched PubMed using the terms aspirin, acetylsalicylic acid, clopidogrel, and/or dipyridamole, in combination with stroke, cerebral ische(ae)mia, transient ische(ae)mic attacks, or retinal artery occlusion. We reviewed only clinical trials or meta-analyses of these drugs for either primary or secondary prevention of cerebrovascular disease.
As our aim was to review the topic and not to perform a meta-analysis, no cutoffs were used to exclude trials. The references in the selected papers were also reviewed to expand the articles. Finally, the references in the current American Heart Association and American Stroke Association secondary stroke prevention guideline were also reviewed.
For a summary of the trials included in our review, see the Data Supplement as an appendix to the online version of this article.
ASPIRIN: THE GOLD STANDARD
Prescribed by Hippocrates in the form of willow bark extract, aspirin has long been known for its antipyretic and anti-inflammatory properties. Its antiplatelet and antithrombotic properties, first described in 1967 by Weiss and Aledort,5 are mediated by irreversible inhibition of cyclooxygenase, leading to decreased thromboxane A2, a platelet-aggregation activator.
Fields et al,6,7 in 1977 and 1978, reported that in a controlled trial in patients with TIA or monocular blindness, fewer subsequent TIAs occurred in patients who received aspirin, although the difference was not statistically significant, with lower rates of events only in nonsurgical patients. Over the next 20 years, the results remained mixed.
The Danish Cooperative study8 (1983) found no significant difference in the rate of recurrent stroke with aspirin vs placebo.
AICLA.9 The Accidents Ischémiques Cérébraux Liés à l’Athérosclérose study of 1983 did find a difference. However, both the Danish Cooperative study and the AICLA were limited by lacking standardized computed tomographic imaging to rule out hemorrhagic stroke and by being relatively small.
The Swedish Cooperative Study10 (1987) found no statistical difference between high-dose aspirin and placebo in preventing recurrent vascular events (stroke, TIA, or myocardial infarction [MI]) 1 to 3 weeks after a stroke. However, it had several limitations: the aspirin group contained more patients with ischemic heart disease (who are more likely to die of cardiac causes), there were significantly more men in the aspirin group, and nearly one-fourth of the deaths were a result of the initial stroke, potentially masking the effect of aspirin in secondary prevention.
Later studies began to show a consistently favorable effect of aspirin.
Boysen et al11 in 1988 reported a nonsignificant trend toward fewer adverse events with aspirin.
UK-TIA.12 The United Kingdom Transient Ischaemic Attack trial in 1991 found a similar trend.
SALT.13 The Swedish Aspirin Low-dose Trial, also in 1991, showed a significant 18% lower rate of stroke or death in patients with recent TIA, minor stroke, or retinal occlusion treated with low-dose aspirin. The inclusion of patients with TIA helped broaden the population that might benefit. However, the study may have favored the aspirin group by having a run-in period in which patients were nonrandomly treated either with aspirin or with anticoagulation at the discretion of the patient’s physician and, if they suffered “several” TIAs, a stroke, retinal artery occlusion, or MI, were removed from the study.
ESPS-2.14 The second European Stroke Prevention Study in 1996 added to the evidence that aspirin prevents recurrent stroke. Patients with a history of TIA or stroke were randomized in double-blind fashion to four treatment groups: placebo, low-dose aspirin, dipyridamole, or aspirin plus dipyridamole. At 2 years, strokes had occurred in 18% fewer patients in the aspirin group than in the placebo group, and TIAs had occurred in 21.9% fewer. However, aspirin was associated with an absolute 0.5% increase in severe and fatal bleeding. The power of the study was limited because patients from one center were excluded because of “serious inconsistencies in patient case record forms and compliance assay determinations.” 14
Comment. The mixed results with aspirin in studies predating ESPS-2 were partly because the study populations were too small to show benefit.
ATT.15 The Antithrombotic Trialists’ Collaboration performed a meta-analysis that conclusively confirmed the benefit of aspirin after stroke or TIA. The investigators analyzed individual patient data pooled from randomized controlled trials published before 1997 that compared antiplatelet regimens (mostly aspirin) against placebo and against each other. The rates of vascular events were 10.7% with treatment vs 13.2% with placebo (P < .0001). Antiplatelet therapy was particularly effective in preventing ischemic stroke, with a 25% reduction in the rate of nonfatal stroke, and with an overall absolute benefit in stroke prevention across all high-risk patient groups. This translated to 25 fewer nonfatal strokes per 1,000 patients treated with antiplatelet therapy.
What is the optimal aspirin dose?
Studies of aspirin have used different daily doses—the earliest studies used large doses of 1,000 to 1,500 mg.6–10
Boysen et al11 in 1988 found a trend toward benefit (not statistically significant) with doses ranging from 50 mg to 100 mg.
In 1991, three separate studies found that higher doses of aspirin were no more effective than lower doses.
The UK-TIA trial12 compared aspirin 300 mg vs 1,200 mg and found a higher risk of gastrointestinal bleeding with the higher dose.
The SALT Collaborative Group13 found 75 mg to be effective.
The Dutch TIA trial16 compared 30 mg vs 283 mg; end point outcomes were similar but the rate of adverse events was higher with 283 mg.
ESPS-2 was able to show efficacy at a dose of only 50 mg.14
Taylor et al17 compared lower doses (81 or 325 mg) vs higher doses (650 or 1,300 mg) for patients undergoing carotid endarterectomy and found that the risk of adverse events was twice as high with the higher doses.
The ATT Collaboration15 found that efficacy was 40% lower with the highest dose of aspirin than with the lowest doses.
Algra and van Gijn18 performed a meta-analysis of all these studies and found no difference in risk reduction between low-dose and high-dose aspirin, with an overall relative risk reduction of 13% at any dose above 30 mg.
Campbell et al,19 in a 2007 review, found that doses greater than 300 mg conferred no benefit, and that rapid and maximum suppression of thromboxane A2 can be achieved by chewing or ingesting dissolved forms of aspirin 162 mg.
Conclusion. Aspirin doses higher than 81 mg (the US standard) confer no greater benefit and may even decrease the efficacy of aspirin. In an emergency, rapid suppression of thromboxane A2 can be achieved by chewing a minimum dose of 162 mg.
DIPYRIDAMOLE CAN BE ADDED TO ASPIRIN
In 1967, Weiss and Aledort5 found that aspirin’s antiplatelet effect could be blocked by adenosine diphosphate, which is released by activated platelet cells and is an essential part of thrombus formation. Adjacent platelets are then activated, leading to up-regulation of thromboxane A2 and glycoprotein IIb/IIIa receptors and resulting in a cascade of platelet activation and clot formation.20 Dipyridamole inhibits aggregation of platelets by inhibiting their ability to take up adenosine diphosphate.
Studies of dipyridamole
AICLA.9 Bousser et al9 randomized patients who suffered one or more cerebral or retinal infarctions to receive placebo, aspirin 1 g, or aspirin 1 g plus dipyridamole 225 mg. Aspirin was significantly better than placebo in preventing a recurrence of stroke. The event rate with aspirin plus dipyridamole was similar to the rate with aspirin alone, although on 2-by-2 analysis, the difference between placebo and aspirin plus dipyridamole did not reach statistical significance. However, the rate of carotid-origin stroke was 17% with aspirin alone and 6% with aspirin plus dipyridamole, a statistically significant difference.
Thus, this study confirmed the benefit of aspirin in preventing ischemic events but did not fully support the addition of dipyridamole, except in preventing stroke of carotid origin. The study had a number of limitations: the sample size was small, TIA was not included as an end point, computed tomography was not required for entry, and many patients were lost to follow-up, decreasing the statistical power of the trial.
The ESPS study21 was also a randomized controlled trial of aspirin plus dipyridamole vs placebo. But unlike AICLA, ESPS included patients with TIA.
ESPS found a 38.1% relative risk reduction in stroke with aspirin plus dipyridamole compared with placebo, and a 30.6% reduction in death from all causes. Interestingly, patients who had a TIA as the qualifying event had a lower end-point incidence and larger end-point reduction than those who had a stroke as the qualifying event. However, ESPS did not resolve the question of whether adding dipyridamole to aspirin affords any benefit over aspirin alone.
ESPS-214 hoped to answer this question. Patients were randomized to placebo, aspirin, dipyridamole, or aspirin plus dipyridamole. On 2 × 2 analysis, the dipyridamole group had a 16% lower rate of recurrent stroke than the placebo group, and patients on aspirin plus dipyridamole had a 37% lower rate. Aspirin plus dipyridamole yielded a 23.1% reduction compared with aspirin alone, and a 24.7% reduction compared with dipyridamole alone. Similar benefit was reported for the end point of TIA with combination therapy compared with either agent alone.
However, nearly 25% of patients had to withdraw because of side effects, particularly in the dipyridamole-alone and aspirin-dipyridamole groups, and, as mentioned above, verification of compliance in the aspirin group was an issue.14,22 Nevertheless, ESPS-2 clearly showed that aspirin plus dipyridamole was better than either drug alone in preventing recurrent stroke. It also showed the effectiveness of dipyridamole, which AICLA and ESPS could not do, because it had a larger study population, used a lower dose of aspirin, and perhaps because it used an extended-release form of dipyridamole.23
The ATT meta-analysis15 showed a clear benefit of antiplatelet therapy. However, much of this benefit was derived from aspirin therapy, with the addition of dipyridamole resulting in a nonsignificant 6% reduction of vascular events. Most of the patients on dipyridamole were from the ESPS-2 study. In effect, the ATT was a meta-analysis of aspirin, as aspirin studies dominated at that time.
A Cochrane review24 publsihed in 2003 attempted to rectify this by analyzing randomized controlled trials of dipyridamole vs placebo.24 Like the ATT meta-analysis, it did not bear out the benefits of dipyridamole: compared with placebo, there was no effect on the rate of vascular death, and only a minimal benefit in reduction of vascular events—and this latter point is only because of the inclusion of ESPS-2.
Directly comparing aspirin plus dipyridamole vs aspirin alone, the reviewers found no effect on the rate of vascular death, and with the exclusion of ESPS-2, no effect on vascular events.
The Cochrane review had the same limitation as the ATT meta-analysis, ie, dependence on a single trial (ESPS-2) to show benefit, and perhaps the fact that ESPS-2 was the only study that used an extended-release form of dipyridamole.
Leonardi-Bee et al25 performed a meta-analysis that overcame the limitation of ESPS-2 being the only study at the time with positive findings: they used pooled individual patient data from randomized trials and analyzed them en masse. Patients on aspirin plus dipyridamole had a 39% lower risk than with placebo and a 22% lower risk than with aspirin alone. Unlike the ATT and the Cochrane review, excluding ESPS-2 did not alter the statistically significant lower stroke rate with aspirin plus dipyridamole compared with controls. This meta-analysis helped to confirm ESPS-2’s finding of the additive effect of aspirin plus dipyridamole compared with aspirin and placebo control.
ESPRIT.26,27 The European/Australasian Stroke Prevention in Reversible Ischaemia Trial confirmed these findings. This randomized controlled trial compared aspirin plus dipyridamole against aspirin alone in patients with a TIA or minor ischemic stroke of arterial origin within the past 6 months. For the primary end point (death from all vascular causes, nonfatal stroke, nonfatal MI, nonfatal major bleeding complication), the hazard ratio was 0.80 favoring aspirin plus dipyridamole, with a number needed to treat of 104 over a mean of 3.5 years (absolute risk reduction of 1% per year). Importantly, twice as many patients taking aspirin plus dipyridamole discontinued the medication.
Caveats to interpreting this study are that it was not blinded, the aspirin doses varied (although the median aspirin dose—75 mg—was the same between the two groups), and not all patients received the extended-release form of dipyridamole.
Conclusions about dipyridamole
ESPS-2, ESPRIT, and the meta-analysis by Leonardi-Bee et al showed that aspirin plus dipyridamole is more effective than placebo or aspirin alone in secondary prevention of vascular events, including stroke. Also, extended-release dipyridamole appears to be more effective.
Unfortunately, many patients stop taking dipyridamole because of side effects (primarily headache).
Based on the results of ESPRIT, the absolute benefit of dipyridamole used alone may be small.
CLOPIDOGREL: SIMILAR TO ASPIRIN IN EFFICACY?
Like dipyridamole, clopidogrel targets adenosine diphosphate to prevent clot formation, blocking its ability to bind to its receptor on platelets. It is a thienopyridine and, unlike its sister drug ticlopidine, does not seem to be associated with the potentially serious side effects of neutropenia. However, a few cases of thrombotic thrombocytopenic purpura have been reported.28 The other drugs in this class have not been evaluated in clinical trials for secondary stroke prophylaxis.
Trials of clopidogrel
CAPRIE.29 The Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events trial, in 1996, was one of the first to compare the clinical use of clopidogrel against aspirin. It was a randomized controlled noninferiority trial in patients over age 21 (inclusion criteria: ischemic stroke, MI, or peripheral arterial disease) randomized to aspirin 325 mg once daily or clopidogrel 75 mg once daily. Patients were followed for 1 to 3 years.
Patients on clopidogrel had a relative risk reduction of 8.7% in primary events (ischemic stroke, MI, or vascular death); patients on aspirin were at significantly higher risk of gastrointestinal hemorrhage. Patients with peripheral arterial disease as the qualifying event did particularly well on clopidogrel, with a significant relative risk reduction of 23.8%.
Limitations of the CAPRIE trial included its inability to measure the effect of treatment on individual outcomes, particularly stroke, and the fact that the relative risk reduction for patients with stroke as the qualifying event was not significant (P = .66). Another limitation was that it did not use TIA as an entry criterion or as part of the composite outcome. Also, the relative risk reduction had a wide confidence interval, and a large number of patients discontinued therapy for reasons other than the defined outcomes.
Nevertheless, the CAPRIE trial showed clopidogrel to be an effective antiplatelet prophylactic, particularly in patients with peripheral artery disease, but with no discernible difference from aspirin for those patients with MI or stroke as a qualifying event.
MATCH.30 The Management of Atherothrombosis With Clopidogrel in High-risk Patients trial hoped to better assess clopidogrel’s efficacy, particularly in patients with ischemic cerebral events. Cardiac studies leading up to MATCH suggested that adding a thienopyridine to aspirin might offer additive benefit in reducing the rate of vascular outcomes.15,31 MATCH randomized high-risk patients (inclusion criteria were ischemic stroke or TIA and a history of vascular disease) to clopidogrel or to aspirin plus clopidogrel.
There was a nonsignificant 6.4% relative risk reduction in the combined primary outcome of MI, ischemic stroke, vascular death, other vascular death, and re-hospitalization for acute ischemic events in the aspirin-plus-clopidogrel group compared with clopidogrel alone. However, this came at the cost of double the number of bleeding events in the combination group, mitigating most of the benefit of combination therapy.
An important caveat in interpreting the results of MATCH, as compared with the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study, is that aspirin was being added to clopidogrel, not vice versa. CURE, which looked at the addition of clopidogrel to aspirin vs aspirin alone in cardiac patients, found a significant reduction of ischemic events taken as a group (relative risk 0.8), and a trend toward a lower rate of stroke (relative risk 0.86, but 95% confidence interval encompassing 1) for aspirin plus clopidogrel vs aspirin alone.31 However, patients in the CURE trial did not have high-risk vasculopathy per se but rather non-ST-elevation MI, perhaps skewing the benefit of combination therapy and lessening the risk of intracranial bleeding.
CHARISMA.32 The Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance trial, like the CURE trial, compared aspirin plus clopidogrel vs aspirin in patients with established cardiovascular, cerebrovascular, or peripheral arterial disease, or who were at high risk of events. As in the MATCH study, the findings for combination therapy were a nonsignificant relative risk of 0.93 for primary events (MI, stroke, or death from cardiovascular causes), and a significant reduction of secondary end points (primary end point event plus TIA or hospitalization for unstable angina) (relative risk 0.92, P = .04).
Importantly, combination therapy significantly increased the rate of bleeding events. In asymptomatic patients (those without documented vascular disease but with multiple atherothrombotic risk factors), there was actually harm with combined treatment. Conversely, for symptomatic patients (those with documented vascular disease), there was a negligible, but significant reduction in primary end points.
The result was that in patients with documented vascular disease, aspirin plus clopidogrel combination therapy provided little or no benefit over aspirin alone. For patients with elevated risk factors but no documented vascular burden, there may actually be harm from combination therapy.
PRoFESS.33 Logically following is the question of whether aspirin plus dipyridamole offers any benefit over clopidogrel as a stroke prophylactic. The Prevention Regimen for Effectively Avoiding Second Strokes trial hoped to answer this by comparing clopidogrel against aspirin plus dipyridamole, both with and without telmisartan, in patients with recent stroke.
The rate of recurrent stroke was similar in the two groups, but there were 25 fewer ischemic strokes in patients on aspirin plus dipyridamole, offset by an increase in hemorrhagic strokes. Rates of secondary outcomes of stroke, death, or MI were nearly identical between the groups. Early discontinuation of treatment was significantly more frequent in those patients taking aspirin plus dipyridamole, meaning better compliance for those taking clopidogrel.
Initially, patients were to be randomized to either aspirin plus dipyridamole or aspirin plus clopidogrel. However, after MATCH30 demonstrated a significantly higher bleeding risk with aspirin plus clopidogrel, patients were changed to clopidogrel alone. But despite this, the bleeding risk was still higher with aspirin plus dipyridamole.
During the trial, the entry criteria were expanded, allowing for the inclusion of younger patients and those with less recent strokes; but despite this change, the study remained underpowered to demonstrate its goal of noninferiority. Thus, it showed only a trend of noninferiority of clopidogrel vs aspirin plus dipyridamole.
What the clopidogrel trials tell us
Clopidogrel confers a benefit similar to that of aspirin (as shown in the CAPRIE study).29 Although aspirin plus dipyridamole confers greater benefit than aspirin alone (as shown in the ESPS-2,14 Leonardi-Bee,25 and ESPRIT26 studies), aspirin plus dipyridamole is not superior to clopidogrel, and may even be inferior.34
WARFARIN FOR ATRIAL FIBRILLATION ONLY
Warfarin acts by disrupting the coagulation cascade rather than acting at the site of platelet plug formation. In theory, warfarin should be as effective as the antiplatelet drugs in preventing clot formation, and so it was thought to possibly be effective in preventing stroke of arterial origin.
However, in at least three studies, warfarin increased the risk of death, MI, and hemorrhage, with perhaps a slight decrease in the risk of recurrent stroke in patients with suspected stroke or TIA.35–37 This should be differentiated from stroke originating from cardiac dysrhythmias, for which warfarin has clearly been shown to be beneficial.28
THREE GOOD MEDICAL OPTIONS FOR PREVENTING STROKE RECURRENCE
Antiplatelet therapy offers benefit in the primary and secondary prevention of stroke, with a 25% reduction in the rate of nonfatal stroke and a 17% reduction in the rate of death due to vascular causes.15
Aspirin is the best established
Aspirin is the best established, best tolerated, and least expensive of the three contemporary agents. Further, it is also the agent of choice for acute stroke care, to be given within 48 hours of a stroke to mitigate the risk of death and morbidity. The data for other agents in acute stroke management remain limited.38
Aspirin plus dipyridamole
Aspirin plus dipyridamole is slightly more efficacious than aspirin alone, and it is an alternative when aspirin is ineffective and when the patient can afford the additional cost. Aspirin plus dipyridamole offers up to a 22% relative risk reduction (but a small reduction in absolute risk) of stroke compared with aspirin alone, as demonstrated by ESPS-2,14 Leonardi-Bee et al,25 and ESPRIT.26
When is clopidogrel appropriate?
Up to one-third of patients may not tolerate aspirin plus dipyridamole because of side effects. Clopidogrel is an option for these patients. The CAPRIE study29 showed clopidogrel similar in efficacy to aspirin.
In contrast to aspirin plus dipyridamole, there is clearly no benefit to combining aspirin and clopidogrel for ischemic stroke prophylaxis. And data from PRoFESS33 suggested the combination was qualitatively inferior to aspirin plus dipyridamole. However, the PRoFESS trial was underpowered to fully bear this out.
Therefore, current guidelines consider all three agents as appropriate for secondary prevention of stroke. One is not preferred over another, and the selection should be based on individual patient characteristics and affordability.28
CAROTID SURGERY OR STENTING: BENEFITS AND LIMITATIONS
Atherosclerosis is the most common cause of stroke, and atherosclerosis of the common carotid bifurcation accounts for a small but significant percentage of all strokes.39–41
The degree of carotid stenosis and whether it is producing symptoms influence how it should be managed. For patients with symptomatic carotid stenosis of more than 70%, multicenter randomized trials have shown that surgery (ie, carotid endarterectomy) added to medical therapy decreases the rate of recurrent stroke by up to 17% and the rate of combined stroke and death by 10% to 12% over a 2- to 3-year follow-up period (level of evidence A).42–44 No study has proven the efficacy of surgery in patients with symptomatic stenosis of less than 50%.43,44
Similarly, in asymptomatic carotid disease, preventive surgery is a beneficial adjunct to medical therapy in certain patients. An approximate 6% reduction in the rate of stroke or death over 5 years has been shown in patients with moderate stenosis (> 60%), with men younger than age 75 and with greater than 70% stenosis deriving the most benefit.45–47
However, these robust, positive results with surgical intervention should not overshadow the importance of intensive and guided medical therapy, which has been shown to mitigate the risk of stroke.48,49
Is stenting as good as surgery? In the multicenter randomized Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), stenting resulted in similar rates of stroke and MI in patients with symptomatic and asymptomatic disease.50 However, stenting carried a greater risk of perioperative stroke, and endarterectomy carried a greater risk of MI. Those under age 70 benefited more from stenting, and those over age 70 benefited more from endarterectomy.
But another fact to keep in mind is that the relationship between carotid narrowing and an ipsilateral stroke is not necessarily direct. Two follow-up studies in patients from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that up to 45% of strokes that occurred after intervention in the distribution of the asymptomatic stenosed carotid artery were unrelated to the stenosis.51,52 Moreover, up to 20% of subsequent strokes in the distribution of the symptomatic artery were not of large-artery origin, increasing up to 35% for those with stenosis of less than 70%.51 Clearly, thorough screening of those with presumed symptomatic stenosis is needed to eliminate other possible causes.
- Zivin JA. Approach to cerebrovascular diseases. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier, 2012:2304–2309.
- Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999; 30:338–349.
- Gotoh F, Tohgi H, Hirai S, et al. Cilostazol Stroke Prevention Study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000; 9:147–157.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967; 2:495–497.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Stroke 1977; 8:301–314.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Part II: surgical group. Stroke 1978; 9:309–319.
- Sorensen PS, Pedersen H, Marquardsen J, et al. Acetylsalicylic acid in the prevention of stroke in patients with reversible cerebral ischemic attacks. A Danish cooperative study. Stroke 1983; 14:15–22.
- Bousser MG, Eschwege E, Haguenau M, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983; 14:5–14.
- High-dose acetylsalicylic acid after cerebral infarction. A Swedish Cooperative Study. Stroke 1987; 18:325–334.
- Boysen G, Sørensen PS, Juhler M, et al. Danish very-low-dose aspirin after carotid endarterectomy trial. Stroke 1988; 19:1211–1215.
- Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54:1044–1054.
- Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet 1991; 338:1345–1349.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, MI, and stroke in high risk patients. BMJ 2002; 324:71–86.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med 1991; 325:1261–1266.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Algra A, van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry 1996; 60:197–199.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47:2169–2180.
- European Stroke Prevention Study. ESPS Group. Stroke 1990; 21:1122–1130.
- Davis SM, Donnan GA. Secondary prevention for stroke after CAPRIE and ESPS-2. Opinion 1. Cerebrovasc Dis 1998; 8:73–77.
- Diener HC. Dipyridamole trials in stroke prevention. Neurology 1998; 51(suppl 3):S17–S19.
- De Schryver EL, Algra A, van Gijn J. Cochrane review: dipyridamole for preventing major vascular events in patients with vascular disease. Stroke 2003; 34:2072–2080.
- Leonardi-Bee J, Bath PM, Bousser MG, et al; Dipyridamole in Stroke Collaboration (DISC). Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005; 36:162–168.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006; 367:1665–1673.
- Tirschwell D. Aspirin plus dipyridamole was more effective than aspirin alone for preventing vascular events after minor cerebral ischemia. ACP J Club 2006; 145:57.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364:331–337.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Kent DM, Thaler DE. Stroke prevention—insights from incoherence. N Engl J Med 2008; 359:1287–1289.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352:1305–1316.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol 2007; 6:115–124.
- Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345:1444–1451.
- Jauch EC, Saver JL, Adams HP, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32:2735–2740.
- Zivin JA. Ischemic cerebrovascular disease. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine 24th ed. Philadelphia, PA: Elsevier; 2012: chap 414. www.mdconsult.com. Accessed November 7, 2013.
- Smith WS, Johnston C, Easton D. Cerebrovascular diseases. In:Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw Hill; 2005: chap 349. www.accessmedicine.com.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991; 325:445–453.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Hobson RW, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363:1491–1502.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Barnett HJ, Gunton RW, Eliasziw M, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA 2000; 283:1429–1436.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
- Zivin JA. Approach to cerebrovascular diseases. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier, 2012:2304–2309.
- Samsa GP, Bian J, Lipscomb J, Matchar DB. Epidemiology of recurrent cerebral infarction: a Medicare claims-based comparison of first and recurrent strokes on 2-year survival and cost. Stroke 1999; 30:338–349.
- Gotoh F, Tohgi H, Hirai S, et al. Cilostazol Stroke Prevention Study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000; 9:147–157.
- Shinohara Y, Katayama Y, Uchiyama S, et al; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010; 9:959–968.
- Weiss HJ, Aledort LM. Impaired platelet-connective-tissue reaction in man after aspirin ingestion. Lancet 1967; 2:495–497.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Stroke 1977; 8:301–314.
- Fields WS, Lemak NA, Frankowski RF, Hardy RJ. Controlled trial of aspirin in cerebral ischemia. Part II: surgical group. Stroke 1978; 9:309–319.
- Sorensen PS, Pedersen H, Marquardsen J, et al. Acetylsalicylic acid in the prevention of stroke in patients with reversible cerebral ischemic attacks. A Danish cooperative study. Stroke 1983; 14:15–22.
- Bousser MG, Eschwege E, Haguenau M, et al. “AICLA” controlled trial of aspirin and dipyridamole in the secondary prevention of athero-thrombotic cerebral ischemia. Stroke 1983; 14:5–14.
- High-dose acetylsalicylic acid after cerebral infarction. A Swedish Cooperative Study. Stroke 1987; 18:325–334.
- Boysen G, Sørensen PS, Juhler M, et al. Danish very-low-dose aspirin after carotid endarterectomy trial. Stroke 1988; 19:1211–1215.
- Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 1991; 54:1044–1054.
- Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet 1991; 338:1345–1349.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996; 143:1–13.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, MI, and stroke in high risk patients. BMJ 2002; 324:71–86.
- A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med 1991; 325:1261–1266.
- Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet 1999; 353:2179–2184.
- Algra A, van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry 1996; 60:197–199.
- Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 2007; 297:2018–2024.
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47:2169–2180.
- European Stroke Prevention Study. ESPS Group. Stroke 1990; 21:1122–1130.
- Davis SM, Donnan GA. Secondary prevention for stroke after CAPRIE and ESPS-2. Opinion 1. Cerebrovasc Dis 1998; 8:73–77.
- Diener HC. Dipyridamole trials in stroke prevention. Neurology 1998; 51(suppl 3):S17–S19.
- De Schryver EL, Algra A, van Gijn J. Cochrane review: dipyridamole for preventing major vascular events in patients with vascular disease. Stroke 2003; 34:2072–2080.
- Leonardi-Bee J, Bath PM, Bousser MG, et al; Dipyridamole in Stroke Collaboration (DISC). Dipyridamole for preventing recurrent ischemic stroke and other vascular events: a meta-analysis of individual patient data from randomized controlled trials. Stroke 2005; 36:162–168.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006; 367:1665–1673.
- Tirschwell D. Aspirin plus dipyridamole was more effective than aspirin alone for preventing vascular events after minor cerebral ischemia. ACP J Club 2006; 145:57.
- Furie KL, Kasner SE, Adams RJ, et al; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996; 348:1329–1339.
- Diener HC, Bogousslavsky J, Brass LM, et al; MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004; 364:331–337.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Bhatt DL, Fox KA, Hacke W, et al; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Sacco RL, Diener HC, Yusuf S, et al; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359:1238–1251.
- Kent DM, Thaler DE. Stroke prevention—insights from incoherence. N Engl J Med 2008; 359:1287–1289.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352:1305–1316.
- ESPRIT Study Group; Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol 2007; 6:115–124.
- Mohr JP, Thompson JL, Lazar RM, et al; Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001; 345:1444–1451.
- Jauch EC, Saver JL, Adams HP, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44:870–947.
- Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001; 32:2735–2740.
- Zivin JA. Ischemic cerebrovascular disease. In:Goldman L, Schafer AI, editors. Goldman’s Cecil Medicine 24th ed. Philadelphia, PA: Elsevier; 2012: chap 414. www.mdconsult.com. Accessed November 7, 2013.
- Smith WS, Johnston C, Easton D. Cerebrovascular diseases. In:Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw Hill; 2005: chap 349. www.accessmedicine.com.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991; 325:445–453.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998; 351:1379–1387.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998; 339:1415–1425.
- Hobson RW, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med 1993; 328:221–227.
- Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995; 273:1421–1428.
- Halliday A, Mansfield A, Marro J, et al; MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004; 363:1491–1502.
- Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41:e11–e17.
- Spence JD, Coates V, Li H, et al. Effects of intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol 2010; 67:180–186.
- Brott TG, Hobson RW, Howard G, et al; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010; 363:11–23.
- Barnett HJ, Gunton RW, Eliasziw M, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA 2000; 283:1429–1436.
- Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342:1693–1700.
KEY POINTS
- After a stroke, antiplatelet therapy lowers the rate of recurrent nonfatal stroke by about 25%.
- Aspirin is the most established, best tolerated, and least expensive of the three approved drugs.
- Adding dipyridamole to aspirin increases the efficacy, with a 22% reduction in relative risk, but only a 1% reduction in absolute risk.
- Clopidogrel is similar in efficacy to aspirin and to dipyridamole.
- All three agents are regarded as equal and appropriate for secondary prevention of stroke; the choice is based on individual patient characteristics.
- A small number of strokes result from atherosclerotic disease of the common carotid bifurcation, and patients with symptomatic carotid disease can be treated with the combination of surgery or stenting and drug therapy, or with drug therapy alone.
Third universal definition of myocardial infarction: Update, caveats, differential diagnoses
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
In 2012, a task force of the European Society of Cardiology, the American College of Cardiology Foundation, the American Heart Association, and the World Heart Federation released its “third universal definition” of myocardial infarction (MI),1 replacing the previous (2007) definition. The new consensus definition reflects the increasing sensitivity of available troponin assays, which are commonly elevated in other conditions and after uncomplicated percutaneous coronary intervention or cardiac surgery. With a more appropriate definition of the troponin threshold after these procedures, benign myocardial injury can be differentiated from pathologic MI.
TROPONINS: THE PREFERRED MARKERS
Symptoms of MI such as nausea, chest pain, epigastric discomfort, syncope, and diaphoresis may be nonspecific, and findings on electrocardiography or imaging studies may be nondiagnostic. We thus rely on biomarker elevations to identify patients who need treatment.
Cardiac troponin I and cardiac troponin T have become the preferred markers for detecting MI, as they are more sensitive and tissue-specific than their main competitor, the MB fraction of creatine kinase (CK-MB).2 But the newer troponin assays, which are even more sensitive than earlier ones, have raised concerns about their ability to differentiate patients who truly have acute coronary syndromes from those with other causes of troponin elevation. This can have major effects on treatment, patient psyche, and hospital costs.
Troponin elevations can occur in patients with heart failure, end-stage renal disease, sepsis, acute pulmonary embolism, myopericarditis, arrhythmias, and many other conditions. As noted by the task force, these cases of elevated troponin in the absence of clinical supportive evidence should not be labeled as an MI but rather as myocardial injury.
Troponins bind actin and myosin filaments in a trimeric complex composed of troponins I, C, and T. Troponins are present in all muscle cells, but the cardiac isoforms are specific to myocardial tissue.
As a result, both cardiac troponin I and cardiac troponin T, as measured by fourth-generation assays, are highly sensitive (75.2%, 95% confidence interval [CI] 66.8%–83.4%) and specific (94.6%, 95% CI 93.4%–96.3%) for detecting pathologic processes involving the heart.3,4 Nonetheless, increases in cardiac troponin T (but not I) have been documented in patients with disease of skeletal muscles, likely secondary to re-expressed isoforms of the troponin C gene present in both cardiac and skeletal myocytes.3 There has been no evidence to suggest that either cardiac troponin I nor cardiac troponin T is superior to the other as a marker of MI.
Serum troponin levels detectably rise by 2 to 3 hours after myocardial injury. This temporal pattern is similar to that of CK-MB, which rises at about 2 hours and reaches a peak in 4 to 6 hours. However, troponins are more sensitive than CK-MB during this early time period, since a greater proportion is released from the heart during times of cardiac injury.
The definition of an abnormal troponin value is set by the precision of each individual assay. The task force has designated the optimal precision for troponin assays to be at a coefficient of variation of less than 10% when describing a value exceeding the 99th percentile in a reference population. The 99th percentile, which is the upper reference limit, corresponds to a value near 0.035 μg/L for fourth-generation troponin I and troponin T assays.5 Most assays have been adapted to ensure that they meet such criteria.
High-sensitivity assays
Over the past few years, “high-sensitivity” assays have been developed that can detect nanogram levels of troponin.
In one study, an algorithm that incorporated high-sensitivity cardiac troponin T levels was able to rule in or rule out acute MI in 77% of patients with chest pain within 1 hour.6 The algorithm had a sensitivity and negative predictive value of 100%.
Other studies have shown a sensitivity of 100.0%, a specificity of 34.0%, and a negative predictive value of 100.0% when using a cardiac troponin T cutoff of 3 ng/L, while a cutoff of 14 ng/L yielded a sensitivity of 85.4%, a specificity of 82.4%, and a negative predictive value of 96.1%.4 With cutoffs as low as 3 ng/L, some assays detect elevated troponin in up to 90% of people in normal reference populations without MI.7
Physicians thus need to be aware that high-sensitivity troponin assays should mainly be used to rule out acute coronary syndrome, as their high sensitivity substantially compromises their specificity. The appropriate thresholds for various patient populations, the appropriate testing procedures with high-sensitivity assays as compared with the fourth-generation troponin assays (ie, frequency of testing, change in level, and rise), and the cost and clinical outcomes of care based on algorithms that use these values remain unclear and will require further study.8,9
TYPES OF MYOCARDIAL INFARCTION
The task force defines the following categories of MI (Table 1):
Type 1: Spontaneous myocardial infarction
Type 1, or “spontaneous” MI, is an acute coronary syndrome, colloquially called a “heart attack.” It is primarily the result of rupture, fissuring, erosion, or dissection of atherosclerotic plaque. Most are the result of underlying atherosclerotic coronary artery disease, although some (ie, those caused by coronary dissection) are not.
To diagnose type 1 MI, a blood sample must detect a rise or fall (or both) of cardiac biomarker values (preferably cardiac troponin), with at least one value above the 99th percentile. However, an elevated troponin level is not sufficient. At least one of the following criteria must also be met:
- Symptoms of ischemia
- New ST-segment or T-wave changes or new left bundle branch block
- Development of pathologic Q waves
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality
- Finding of an intracoronary thrombus by angiography or autopsy.
Type 1 MI therapy requires antithrombotic drugs and, with the additional findings, revascularization.
Type 2: Due to ischemic imbalance
Type 2 MI is caused by a supply-demand imbalance in myocardial perfusion, resulting in ischemic damage. This specifically excludes acute coronary thrombosis, but can result from marked changes in demand or supply (eg, sepsis) or from a combination of acute changes and chronic conditions (eg, tachycardia with baseline coronary artery disease). Baseline stable coronary artery disease, left ventricular hypertrophy, endothelial dysfunction, coronary artery spasm, coronary embolism, arrhythmias, anemia, respiratory failure, hypotension, and hypertension can all contribute to a supply-demand mismatch sufficient to cause permanent myocardial damage.
The criteria for diagnosing type 2 MI are the same as for type 1: both elevated troponin levels and one of the clinical criteria (symptoms of ischemia, electrocardiographic changes, new wall-motion abnormality, or intracoronary thrombus) must be present.
Of importance, unlike those with type 1 MI, most patients with type 2 MI are unlikely to immediately benefit from antithrombotic therapy, as they typically have no acute thrombosis (except in cases of coronary embolism). Therapy should instead be directed at the underlying supply-demand imbalance and may include volume resuscitation, blood pressure support or control, or control of tachyarrhythmias.
In the long term, treatment to resolve or prevent supply-demand imbalances may also include revascularization or antithrombotic drugs, but these may be contraindicated in the acute setting.
Type 3: Sudden cardiac death from MI
The third type of MI occurs when myocardial ischemia results in sudden cardiac death before blood samples can be obtained. Before dying, the patient should have had symptoms suggesting myocardial ischemia and should have had presumed new ischemic electrocardiographic changes or new left bundle branch block.
This definition of MI is not very useful clinically but is important for population-based research studies.
Type 4a: Due to percutaneous coronary intervention
A rise in CK-MB levels after percutaneous coronary intervention has been associated with a higher rate of death or recurrent MI.10 Previously, type 4 MI was defined as an elevation of cardiac biomarker values (> 3 times the 99th percentile) after percutaneous coronary intervention in a patient who had a normal baseline value (< 99th percentile).11
Unfortunately, using troponin at this threshold, the number of cases is five times higher than when CK-MB is used, without a consistent correlation with the outcomes of death or complications.12 Currently, the increase in cardiac troponin after percutaneous coronary intervention is best interpreted as a marker of the patient’s atherothrombotic burden more than as a predictor of adverse outcomes.13
The updated definition of MI associated with percutaneous coronary intervention now requires an elevation of cardiac troponin values greater than 5 times the 99th percentile in a patient who had normal baseline values or an increase of more than 20% from baseline within 48 hours of the procedure. As this value has been arbitrarily assigned rather than based on an established threshold with clinical outcomes, a true MI must further meet one of the following criteria:
- Symptoms suggesting myocardial ischemia
- New ischemic electrocardiographic changes or new left bundle branch block
- Angiographic loss of patency of a major coronary artery or a side branch or persistent slow-flow or no-flow or embolization
- Imaging evidence of a new loss of viable myocardium or a new wall-motion abnormality.
Given that troponin levels may be elevated in up to 65% of patients after uncomplicated percutaneous coronary intervention and this elevation may be unavoidable,14 a higher troponin threshold to diagnose MI and the clear requirement of clinical correlates may resonate with physicians as a more appropriate definition. In turn, such guidelines may better identify those with an adverse event, while partly reducing unnecessary hospitalization and observation time in those for whom it is not necessary.
Type 4b: Due to stent thrombosis
Type 4b MI is MI caused by stent thrombosis. The thrombosis must be detected by coronary angiography or autopsy in the setting of myocardial ischemia and a rise or fall of cardiac biomarker values, with at least one value above the 99th percentile.
Type 4c: Due to restenosis
Proposed is the addition of type 4c MI, ie, MI resulting from restenosis of more than 50%, because restenosis after percutaneous coronary intervention can lead to MI without thrombosis.15
Type 5: After coronary artery bypass grafting
Similar to the situation after percutaneous coronary intervention, increased CK-MB levels after coronary artery bypass graft surgery are associated with poor outcomes.16 Although some studies have indicated that increased troponin levels within 24 hours of this surgery are associated with higher death rates, no study has established a troponin threshold that correlates with outcomes.17
The task force acknowledged this lack of prognostic value but arbitrarily defined type 5 MI as requiring biomarker elevations greater than 10 times the 99th percentile during the first 48 hours after surgery, with a normal baseline value. One of the following additional criteria must also be met:
- New pathologic Q waves or new left bundle branch block
- Angiographically documented new occlusion in the graft or native coronary artery
- Imaging evidence of new loss of viable myocardium or new wall-motion abnormality.
CHANGES FROM THE 2007 DEFINITIONS
Updates to the definitions of the MI types since the 2007 task force definition can be found in Table 1.
In type 1 and 2 MI, the finding of an intracoronary thrombus by angiography or autopsy was added as one of the possible criteria for evidence of myocardial ischemia.
In type 3 MI, the definition was simplified by deleting the former criterion of finding a fresh thrombus by angiography or autopsy.
In type 4a MI, by requiring clinical correlates, the updated definition in particular moves away from relying solely on troponin levels to diagnose an infarction after percutaneous coronary intervention, as was the case in 2007. Other changes from the 2007 definition: the troponin MI threshold was previously 3 times the 99th percentile, now it is 5 times. Also, if the patient had an elevated baseline value, he or she can now still qualify as having an MI if the level increases by more than 20%.
In type 5 MI, changes to the definition similarly reflect the need to address overly sensitive troponin values when diagnosing an MI after coronary artery bypass grafting. To address such concerns, the required cardiac biomarker values were increased from more than 5 to more than 10 times the 99th percentile.
The task force raised the troponin thresholds for type 4 and type 5 MI in response to evidence showing that troponins are excessively sensitive to minimal myocardial damage during revascularization, and the lack of a troponin threshold that correlates with clinical outcomes.12 Although higher, these values remain arbitrary, so physicians will need to exercise clinical judgment when deciding whether patients are experiencing benign myocardial injury or rather a true MI after revascularization procedures.
OTHER CONDITIONS THAT RAISE TROPONIN LEVELS
As troponin is a marker not only for MI but also for any form of cardiac injury, its levels are elevated in numerous conditions, such as heart failure, renal failure, and left ventricular hypertrophy. The task force identifies distinct troponin elevations above basal levels as the best indication of new pathology, yet several conditions other than acute coronary syndromes can also cause dynamic changes in troponin levels.
Troponin is a sensitive marker for ruling out MI and has tissue specificity for cardiac injury, but it is not specific for acute coronary syndrome as the cause of such injury. Troponin assays were tested and validated in patients in whom there was a high clinical suspicion of acute coronary syndrome, but when ordered indiscriminately, they have a poor positive predictive value (53%) for this disorder.18
Physicians must distinguish between acute coronary syndrome and other causes when deciding to give antithrombotics. Table 2 lists common causes of increased troponin other than acute coronary syndrome.
Heart failure
Some patients with acute congestive heart failure have elevated troponin levels. In one study, 6.2% of such patients had troponin I levels of 1 μg/L or higher or troponin T levels of 0.1 μg/L or higher, and these patients had poorer outcomes and more severe symptoms.19 Levels can also be elevated in patients with chronic heart failure, in whom they correlate with impaired hemodynamics, progressive ventricular dysfunction, and death.20 In an overview of two large trials of patients with chronic congestive heart failure, 86% and 98% tested positive for cardiac troponin using high-sensitivity assays.21
Troponin levels can rise from baseline and subsequently fall in congestive heart failure due to small amounts of myocardial injury, which may be very difficult to distinguish from MI based on the similar presenting symptoms of dyspnea and chest pressure.1,22 The increased troponin levels in chronic congestive heart failure may reflect apoptosis secondary to wall stretch or direct cell toxicity by neurohormones, alcohol, chemotherapy agents, or infiltrative disorders.23–26
End-stage renal disease
Troponin levels are increased in end-stage renal disease, with 25% to 75% of patients having elevated levels using currently available assays.27–29 With the advent of high-sensitivity assays, however, cardiac troponin T levels higher than the 99th percentile are found in 100% of patients who have end-stage renal disease without cardiac symptoms.30
Troponin values above the 99th percentile are therefore not diagnostic of MI in this population. Rather, a diagnosis of MI in patients with end-stage renal disease requires clinical signs and symptoms and serial changes in troponin levels from baseline levels. The task force and the National Academy of Clinical Biochemistry recommend requiring an elevation of more than 20% from baseline, representing a change in troponin of more than 3 standard deviations.31
Increases in troponin in renal failure are thought to be the result of chronic cardiac structural changes such as coronary artery disease, left ventricular hypertrophy, and elevated left ventricular end-diastolic pressure, rather than decreased clearance.32,33
In stable patients with end-stage renal disease, those who have high levels of cardiac troponin T have a higher mortality rate.34 Although the mechanism is not completely clear, decreased clearance of uremic toxins may contribute to myocardial damage beyond that of the cardiac structural changes.34
Sepsis
Approximately 50% of patients admitted to an intensive care unit with sepsis without acute coronary syndrome have elevated troponin levels.35
Elevated troponin in sepsis patients has been associated with left ventricular dysfunction, most likely from hemodynamic stress, direct cytotoxicity of bacterial endotoxins, and reperfusion injury.35,36 Critical illness places high demands on the myocardium, while oxygen supply may be diminished by hypotension, pulmonary edema, and intravascular volume depletion. This supply-demand mismatch is similar to the physiology of type 2 MI, with clinical signs and symptoms of MI potentially being the only differentiating factor.
Elevated troponin levels may represent either reversible or irreversible myocardial injury in patients with sepsis and are a predictor of severe illness and death.37 However, what to do about elevated troponin in patients with sepsis is not clear. When patients are in the intensive care unit with single-organ or multi-organ failure, the diagnosis and treatment of troponin elevations may not take priority.1 Diagnosing MI is further complicated by the inability of critically ill patients to communicate signs and symptoms. Physicians should also remember that diagnostic testing (electrocardiography, echocardiography) is often necessary to meet the clinical criteria for a type 1 or 2 MI in critically ill patients, and that treatment options may be limited.
Pulmonary embolism
Pulmonary embolism is a leading noncardiac cause of troponin elevation in patients in whom the clinical suspicion of acute coronary syndrome is initially high.38 It is thought that increased troponin levels in patients with pulmonary embolism are caused by increased right ventricular strain secondary to increased pulmonary artery resistance.
The signs and symptoms of MI and of pulmonary embolism overlap, and troponin can be elevated in both conditions, making the initial diagnosis difficult. Electrocardiography and early bedside echocardiography can identify the predominant right-sided dilatation and strain in the heart secondary to pulmonary embolism. Computed tomography should be performed if there is even a moderate clinical suspicion of pulmonary embolism.
The appropriate use of thrombolytics in a normotensive patient with pulmonary embolism remains controversial. The significant risks of hemorrhage need to be balanced with the risk of hemodynamic deterioration. For these patients, the combination of cardiac troponin I measurement and echocardiography provides more prognostic information than each does individually.39 Troponin elevation may therefore be a marker for poor outcomes without aggressive treatment with thrombolytics.
However, single troponin measurements in patients hospitalized early with pulmonary embolism can lead to substantial risk of misdiagnosing them with MI. Although the intensity of the peak is not particularly useful in the setting of pulmonary embolism, two consecutive troponin values 8 hours apart will allow for more appropriate risk stratification for pulmonary embolism patients, who may have a delay between right heart injury and troponin release.40
‘Myopericarditis’
It is reasonable to expect that myocarditis—inflammation of the myocardium—would cause release of troponin from myocytes.41 Interestingly, however, troponin levels can also be elevated in pericarditis.42 The reasons are not clear but have been hypothesized as being caused by nonspecific inflammation during pericarditis that also includes the superficial myocardium—hence, “myopericarditis.”
We have only limited data on the outcomes of patients who have pericarditis with troponin elevation, but troponin levels did correlate with an adverse prognosis in one study.43
Arrhythmias
A number of arrhythmias have been associated with elevated troponin levels. Some studies have shown arrhythmias to be the most common cause of high troponin levels in patients who are not experiencing an acute coronary syndrome.44,45
The reasons proposed for increased troponins in tachyarrhythmia are similar to those in other conditions of oxygen supply-demand mismatch.46 Tachycardia alone may lead to troponin release in the absence of myodepressive factors, inflammatory mediators, or coronary artery disease.46
Studies have provided only mixed data as to whether troponin levels predict newonset arrhythmias or recurrence of arrhythmias.47,48 Nonetheless, elevated troponin (≥ 0.040 μg/L) in patients with atrial fibrillation has independently correlated with increased risk of stroke or systemic embolism, death, and other cardiovascular events. This is clinically important, as troponin elevations higher than these levels adds prognostic information to that given by the CHADS2 stroke score (congestive heart failure, hypertension, age ≥ 75 years diabetes mellitus, and prior stroke or transient ischemic attack) and thus can inform appropriate anticoagulation therapy.49
USE OF TROPONIN VALUES
Troponins are highly sensitive assays with high tissue specificity for myocardial injury, but levels can be elevated in non-MI conditions and in MIs other than type 1. As with any diagnostic test applied to a population with a low prevalence of the disease, troponin elevation has a low positive predictive value—53% for acute coronary syndrome.18
Unfortunately, in clinical practice, troponins are measured in up to 50% of admitted patients, a small proportion of whom have clinical signs or symptoms of MI.50 Often, clinicians are left with a positive troponin of unknown significance, potentially leading to unnecessary diagnostic testing that detracts from the primary diagnosis.
Dynamic changes in troponin values (eg, a change of more than 20% in a patient with end-stage renal disease) are helpful in distinguishing acute from chronic causes of troponin elevation. However, such changes can also occur with acute or chronic congestive heart failure, tachycardia, hypotension, or other conditions other than acute coronary syndrome.
The absolute numerical value of troponin can help assess the significance of troponin elevation. In most non-MI and non-acute coronary syndrome causes of troponin elevation, the troponin level tends to be lower than 1 μg/mL (Figure 1). Occasional exceptions occur, especially when multiple conditions coexist (end-stage renal disease and congestive heart failure, for example). In contrast, most patients with acute coronary syndromes have either clear symptoms or electrocardiographic changes consistent with MI and a troponin that rises above 0.5 μg/mL.
The task force discourages the use of secondary thresholds for MI, as there is no level of troponin that is considered benign. While any troponin elevation carries a negative prognosis, such prognostic knowledge may not be particularly helpful in deciding whether to anticoagulate patients or attempt revascularization procedures.
We thus recommend using a threshold higher than the 99th percentile to distinguish acute coronary syndromes from other causes of troponin elevations. The particular threshold for decision-making should vary, depending on how strongly one clinically suspects an acute coronary syndrome. For instance, a cardiac troponin I level of 0.2 μg/mL in an otherwise healthy patient with chest pain and ST-segment depression is more than sufficient to diagnose acute coronary syndrome. In contrast, an end-stage renal disease patient with hypertensive cardiomyopathy who presents only with nausea should have a level markedly higher than his or her baseline value (and likely > 0.8 μg/mL) before acute coronary syndrome should be diagnosed.
CK-MB’S ROLE IN THE TROPONIN ERA
Some proponents of troponin assays, including those on the task force, have suggested that CK-MB may no longer be necessary in the evaluation of acute MI.51 In the past, CK-MB had more research supporting its use in quantifying myocardial damage and in diagnosing reinfarction, but some data suggest that troponin may be equally useful for these applications.52,53
These comments aside, CK-MB measurements are still widely ordered with troponin, a probable response to the clinical difficulty of determining the cause and significance of troponin elevations. Although likely less common with recent assays, a small subgroup of patients with acute coronary syndrome will be CK-MB–positive and troponin-negative and at higher risk of morbidity and death than those who are troponin- and CK-MB–negative.54,55
Troponin levels are elevated in many chronic conditions, whereas CK-MB levels may be unaffected or less affected. In some cases, such as congestive heart failure or renal failure, troponins may be both chronically elevated and more than 20% higher than at baseline. In a clinical context in which a false-positive troponin assay is likely, the addition of a CK-MB assay may help determine if a rise (and possibly a subsequent fall) in the troponin level represents true MI. More importantly, deciding on antithrombotic therapy or revascularization is often based on whether a patient has acute coronary syndrome, rather than a small MI from demand ischemia. CK-MB may thus serve as a less sensitive but more specific marker for the larger amount of myocardial damage that one might expect from an acute coronary syndrome.
CK-MB testing also may help determine the acuity of an acute coronary syndrome for patients with known causes of increased troponin. A negative CK-MB value in the presence of a troponin value elevated above baseline could indicate an event a few days prior.
Finally, the approach of ordering both troponin and CK-MB may be particularly helpful in diagnosing type 4 and 5 MIs, as current guidelines suggest that more research is needed to determine whether current troponin thresholds lead to clinical outcomes.
CLINICAL JUDGMENT IS NECESSARY
The updated definition raises the biomarker threshold required to diagnose MI after revascularization procedures and reemphasizes the need to look for other signs of infarction. This change reflects the sometimes excessive sensitivity of troponin assays for minimal and often unavoidable myocardial damage that occurs in numerous conditions.
With sensitive troponin assays, clinical judgment is essential for separating true MI from myocardial injury, and acute coronary syndrome from demand ischemia. Clinicians will now be forced to be cognizant of their suspicion for acute coronary syndrome in the presence of multiple noncoronary causes of increased troponin with little practical guideline guidance. In settings in which troponin elevation is expected (eg, congestive heart failure, end-stage renal failure, shock), a higher cardiac troponin threshold or CK-MB may be useful as a less sensitive but more specific marker of significant myocardial damage requiring aggressive treatment.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598.
- Perry SV. Troponin T: genetics, properties and function. J Muscle Res Cell Motil 1998; 19:575–602.
- Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol 2011; 58:1819–1824.
- Body R, Carley S, McDowell G, et al. Rapid exclusion of acute myocardial infarction in patients with undetectable troponin using a high-sensitivity assay. J Am Coll Cardiol 2011; 58:1332–1339.
- Jaffe AS, Apple FS, Morrow DA, Lindahl B, Katus HA. Being rational about (im)precision: a statement from the Biochemistry Subcommittee of the Joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the definition of myocardial infarction. Clin Chem 2010; 56:941–943.
- Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172:1211–1218.
- Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361:858–867.
- Kavsak PA, Worster A. Dichotomizing high-sensitivity cardiac troponin T results and important analytical considerations [letter]. J Am Coll Cardiol 2012; 59:1570; author reply 1571–1572.
- Newby LK. Myocardial infarction rule-out in the emergency department: are high-sensitivity troponins the answer? Comment on “one-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T.” Arch Intern Med 2012; 172:1218–1219.
- Califf RM, Abdelmeguid AE, Kuntz RE, et al. Myonecrosis after revascularization procedures. J Am Coll Cardiol 1998; 31:241–251.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol 2007; 50:2173–2195.
- Cockburn J, Behan M, de Belder A, et al. Use of troponin to diagnose periprocedural myocardial infarction: effect on composite endpoints in the British Bifurcation Coronary Study (BBC ONE). Heart 2012; 98:1431–1435.
- Zimarino M, Cicchitti V, Genovesi E, Rotondo D, De Caterina R. Isolated troponin increase after percutaneous coronary interventions: does it have prognostic relevance? Atherosclerosis 2012; 221:297–302.
- Loeb HS, Liu JC. Frequency, risk factors, and effect on long-term survival of increased troponin I following uncomplicated elective percutaneous coronary intervention. Clin Cardiol 2010; 33:E40–E44.
- Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol 2008; 20:401–403.
- Klatte K, Chaitman BR, Theroux P, et al; GUARDIAN Investigators (The GUARD during Ischemia Against Necrosis). Increased mortality after coronary artery bypass graft surgery is associated with increased levels of postoperative creatine kinase-myocardial band isoenzyme release: results from the GUARDIAN trial. J Am Coll Cardiol 2001; 38:1070–1077.
- Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011; 305:585–591.
- Alcalai R, Planer D, Culhaoglu A, Osman A, Pollak A, Lotan C. Acute coronary syndrome vs nonspecific troponin elevation: clinical predictors and survival analysis. Arch Intern Med 2007; 167:276–281.
- Peacock WF, De Marco T, Fonarow GC, et al; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008; 358:2117–2126.
- Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure. Circulation 2003; 108:833–838.
- Masson S, Anand I, Favero C, et al; Valsartan Heart Failure Trial (Val-HeFT) and Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca—Heart Failure (GISSI-HF) Investigators. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012; 125:280–288.
- Januzzi JL, Filippatos G, Nieminen M, Gheorghiade M. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J 2012; 33:2265–2271.
- Shih H, Lee B, Lee RJ, Boyle AJ. The aging heart and post-infarction left ventricular remodeling. J Am Coll Cardiol 2011; 57:9–17.
- Latini R, Masson S, Anand IS, et al; Val-HeFT Investigators. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116:1242–1249.
- Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003; 361:1787–1789.
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011; 107:1375–1380.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 2002; 106:2941–2945.
- Mallamaci F, Zoccali C, Parlongo S, et al. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2002; 40:68–75.
- Roppolo LP, Fitzgerald R, Dillow J, Ziegler T, Rice M, Maisel A. A comparison of troponin T and troponin I as predictors of cardiac events in patients undergoing chronic dialysis at a Veteran’s Hospital: a pilot study. J Am Coll Cardiol 1999; 34:448–454.
- Jacobs LH, van de Kerkhof J, Mingels AM, et al. Haemodialysis patients longitudinally assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and cardiac troponin I assays. Ann Clin Biochem 2009; 46:283–290.
- NACB Writing Group; Wu AH, Jaffe AS, Apple FS, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines: use of cardiac troponin and B-type natriuretic peptide or N-terminal proB-type natriuretic peptide for etiologies other than acute coronary syndromes and heart failure. Clin Chem 2007; 53:2086–2096.
- Schulz O, Kirpal K, Stein J, et al. Importance of low concentrations of cardiac troponins. Clin Chem 2006; 52:1614–1615.
- Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006; 48:1–11.
- deFilippi C, Wasserman S, Rosanio S, et al. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA 2003; 290:353–359.
- ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657.
- Fromm RE. Cardiac troponins in the intensive care unit: common causes of increased levels and interpretation. Crit Care Med 2007; 35:584–588.
- Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17.
- Ilva TJ, Eskola MJ, Nikus KC, et al. The etiology and prognostic significance of cardiac troponin I elevation in unselected emergency department patients. J Emerg Med 2010; 38:1–5.
- Kucher N, Wallmann D, Carone A, Windecker S, Meier B, Hess OM. Incremental prognostic value of troponin I and echocardiography in patients with acute pulmonary embolism. Eur Heart J 2003; 24:1651–1656.
- Ferrari E, Moceri P, Crouzet C, Doyen D, Cerboni P. Timing of troponin I measurement in pulmonary embolism. Heart 2012; 98:732–735.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Brandt RR, Filzmaier K, Hanrath P. Circulating cardiac troponin I in acute pericarditis. Am J Cardiol 2001; 87:1326–1328.
- Imazio M, Cecchi E, Demichelis B, et al. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008; 94:498–501.
- Bakshi TK, Choo MK, Edwards CC, Scott AG, Hart HH, Armstrong GP. Causes of elevated troponin I with a normal coronary angiogram. Intern Med J 2002; 32:520–525.
- Bukkapatnam RN, Robinson M, Turnipseed S, Tancredi D, Amsterdam E, Srivatsa UN. Relationship of myocardial ischemia and injury to coronary artery disease in patients with supraventricular tachycardia. Am J Cardiol 2010; 106:374–377.
- Jeremias A, Gibson CM. Narrative review: alternative causes for elevated cardiac troponin levels when acute coronary syndromes are excluded. Ann Intern Med 2005; 142:786–791.
- Beaulieu-Boire I, Leblanc N, Berger L, Boulanger JM. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis 2012; Epub ahead of print.
- Latini R, Masson S, Pirelli S, et al; GISSI-AF Investigators. Circulating cardiovascular biomarkers in recurrent atrial fibrillation: data from the GISSI-atrial fibrillation trial. J Intern Med 2011; 269:160–171.
- Hijazi Z, Oldgren J, Andersson U, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy. Circulation 2012; 125:1605–1616.
- Waxman DA, Hecht S, Schappert J, Husk G. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol 2006; 48:1755–1762.
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinase-MB. Circulation 2008; 118:2200–2206.
- Younger JF, Plein S, Barth J, Ridgway JP, Ball SG, Greenwood JP. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007; 93:1547–1551.
- Morrow DA, Cannon CP, Jesse RL, et al; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 2007; 115:e356–e375.
- Yee KC, Mukherjee D, Smith DE, et al. Prognostic significance of an elevated creatine kinase in the absence of an elevated troponin I during an acute coronary syndrome. Am J Cardiol 2003; 92:1442–1444.
- Newby LK, Roe MT, Chen AY, et al; CRUSADE Investigators. Frequency and clinical implications of discordant creatine kinase-MB and troponin measurements in acute coronary syndromes. J Am Coll Cardiol 2006; 47:312–318.
KEY POINTS
- Because newer assays for troponin can detect this biomarker at lower concentrations than earlier ones could, they are more sensitive but less specific.
- The high sensitivity of troponin assays makes them valuable for ruling out MI, but less so for ruling it in. Therefore, additional signs are required for the diagnosis.
- MI is categorized into several types, depending on whether it is spontaneous (acute coronary syndromes), caused by supply-demand mismatch, associated with sudden cardiac death, or a complication of percutaneous coronary intervention or of coronary artery bypass grafting.
- In settings in which nonspecific troponin elevations are frequently seen, a less sensitive but more specific test such as creatine kinase MB or troponin using a higher threshold value may be useful.
Breast biopsy delayed. $1.5M verdict
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
During a routine mammogram, an enlarged lymph node was found in the patient’s armpit. The patient’s primary care physician (PCP) ordered follow-up imaging and referred the patient to a surgeon for possible excisional biopsy. The surgeon suggested that the biopsy could be delayed until additional imaging studies were completed.
The patient transferred her care to another surgeon, who immediately performed the biopsy and found stage IV inoperable breast cancer. The patient underwent aggressive chemotherapy for 3 years, but died 39 months after diagnosis.
ESTATE’S CLAIM The first surgeon was negligent for not immediately performing the biopsy.
DEFENDANTS’ DEFENSE There was no negligence. An earlier biopsy would not have changed the outcome.
VERDICT A $1.5 million Massachusetts verdict was returned.
Treating bowel injury after uterine ablation
Following uterine ablation performed by a gynecologist, a 35-year-old woman suffered severe abdominal pain. Six days later, the gynecologist and a surgeon performed a hysterectomy.
Three days after discharge, the patient returned to the hospital with an abdominal infection and sepsis. During a third operation, a burn hole was found; the injured portion of bowel was resected. The patient has chronic abdominal pain.
PATIENT’S CLAIM Sepsis and infection could have been avoided if either physician had identified the injury during the second hospitalization and surgery. The patient developed psychological issues as a result of chronic pain.
DEFENDANTS’ DEFENSE A settlement was reached with the gynecologist during the trial. The surgeon denied negligence. During the second surgery, he examined her bowel for a possible injury but found none.
VERDICT A $3.5 million Illinois verdict was returned. It included
$1.5 million for past pain and suffering that was reduced by $100,000 due to the patient’s failure to report for psychological counseling. The jury found the gynecologist 65% at fault and the surgeon 35% at fault.
Mother in permanent vegetative state
When a 30-year-old woman went to a hospital in labor, she had gestational hypertension. The next morning, she suffered cardiopulmonary arrest. A healthy baby was born by emergency cesarean delivery, but the mother was left in a permanent vegetative state.
PATIENT’S CLAIM The nurses failed to ensure that the ObGyn came to the hospital and did not report blood pressure data to the ObGyn. Gestational hypertension progressed to preeclampsia. Early delivery should have been induced or magnesium sulfate should have been administered.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial.
The nurses were right to rely on the ObGyn to make decisions regarding the patient’s care. They provided appropriate treatment.
VERDICT A New Jersey defense verdict was returned for the hospital.
What caused the child’s brain injuries?
After vaginal delivery, the baby was not breathing and required intubation. He had a seizure and displayed signs of oxygen deprivation, hypoxic ischemic injury, and brain damage. The child uses a special walker and can only communicate using a computer that speaks for him.
PARENTS’ CLAIM The nurses and ObGyn failed to properly assess the baby. The fetal heart-rate monitor electrode should have been placed on the fetal scalp. A cesarean delivery should have been performed.
DEFENDANTS’ DEFENSE The fetal monitor was properly placed. The child’s injury occurred 24 to 72 hours prior to birth due to an umbilical cord accident. A cesarean delivery would have not changed the outcome.
VERDICT A Georgia defense verdict was returned.
Did a woman’s vaginal infection cause her baby’s death?
At 22 weeks’ gestation, a 26-year-old woman began to leak amniotic fluid and went to the hospital. She was in premature labor. The newborn died 19 minutes after birth.
PARENTS’ CLAIM The ObGyn and nurse midwife who provided prenatal care failed to diagnose and treat a vaginal infection. The infection resulted in premature rupture of membranes, leading to premature birth and the baby’s death.
DEFENDANTS’ DEFENSE A confidential settlement was reached with the ObGyn before trial. The nurse midwife claimed the patient did not have a vaginal infection; she never reported symptoms of a foul-smelling vaginal odor or discharge. Premature rupture of membranes was not caused by a vaginal infection. The newborn’s death was related to an umbilical cord defect, the patient’s delay in coming to the hospital, and the multiple obstetric procedures the mother had undergone before this pregnancy.
VERDICT A $456,024 New Jersey verdict was returned.
Inadvertent ligation, ureteral obstruction
A 41-year-old woman suffered pelvic pain and had a history of endometriosis. In January 2007, a CT scan revealed a ruptured ovarian cyst; her ObGyn performed laparotomy for a hysterectomy and oophorectomy.
During surgery, a resident working under the supervision of the ObGyn inadvertently ligated the left ureter. The injury was close to the bladder near the ureteral vesicle junction. A few days later, cystoscopy showed ureteral obstruction. The patient underwent operative repair with nephrostomy tube placement. In May 2007, the patient had a third operation to reimplant the ureter. She has chronic flank pain.
PATIENT’S CLAIM The resident and, therefore, the ObGyn, were negligent in the performance of the procedure. Proper bladder dissection would have moved the ureter to a position where it could not have been ligated.
DEFENDANTS’ DEFENSE Ureter injury is a known risk of the procedure.
VERDICT An Illinois defense verdict was returned.
Foot drop after tubal ligatioN?
During tubal ligation, a woman in her 30s was restrained by a belt. Venodyne boots were applied to promote blood circulation.
PATIENT’S CLAIM The belt and/or boot damaged the perineal and tibial nerves in her left leg, causing foot drop. When asked to definitely identify what caused the nerve damage, the patient invoked the doctrine of res ipsa loquitur (presumed negligence during surgery).
DEFENDANTS’ DEFENSE A $400,000 settlement was reached with the hospital before the trial.
The gynecologist and anesthesiologist denied negligence. The Venodyne boots could not have caused the injury, nor could the belt, which was applied in an area that did not involve the perineal or tibial nerves. The patient did not complain of pain after surgery.
VERDICT A New York defense verdict was returned for the physicians.
Avoid surgical menopause?
After a 10-year history of endometriosis and chronic pelvic pain, a 38-year-old woman underwent bilateral salpingo-oophorectomy. Postoperatively, she suffered surgical menopause that exacerbated pre-existing anxiety and depression.
PATIENT’S CLAIM It was unnecessary to remove the healthy right ovary; having it remain would have avoided early menopause. She would not have consented to the removal of both ovaries had she been properly advised. Alternative treatment was not offered. Her marriage dissolved, her children went to live with their grandparents, and she was unable to work because of complications.
PHYSICIAN’S DEFENSE Proper consent was obtained, including alternatives to surgery. Evidence of ovarian cancer or other medical necessity was not required because full consent was obtained. Removal of the ovaries was proper due to dense pelvic and bowel adhesions, cystic adnexal masses with questionable pathology, and her chronic pelvic pain. The patient’s appendix was adhesed, causing an unreasonable risk of ovarian torsion.
VERDICT A Michigan defense verdict was returned.
Do you enjoy reading Medical Verdicts?
Find more in the PROFESSIONAL LIABILITY Topic Collection.
Persistent voiding problems
A 52-year-old woman was given a diagnosis of stage II anterior pelvic organ prolapse, a high transverse fascial defect, stress urinary incontinence, and distal rectocele.
A gynecologist performed robotic supracervical hysterectomy and colposacropexy, with tension-free vaginal tape and perineal repair.
While in the hospital, she required a catheter to void, and was still unable to void 5 days after discharge. The gynecologist identified persistent urinary retention, released the tension-free vaginal tape, and performed a midurethral sling procedure, but the patient continued to have voiding problems.
The gynecologist suspected a neurogenic problem and referred the patient to a neuro-urologist. Continued intermittent catheterization was recommended by the neuro-urologist, but the patient had continued voiding problems and developed a urinary tract infection.
She went to her ObGyn, who performed a sling revision and cystoscopy and removed all the mesh that could be found. The patient underwent additional treatment, with some improvement.
PATIENT’S CLAIM The gynecologist was negligent for failing to offer further surgery to improve the patient’s condition.
PHYSICIAN’S DEFENSE There was no negligence. Further dissection in the presence of a neurogenic bladder carried a high risk of incontinence. The patient was told of the risk of urinary retention prior to the first procedure and signed an informed consent.
VERDICT A Virginia defense verdict was returned.
Did pathologists fail to diagnose early breast cancer?
After A 45-year-old woman underwent mammography in May 2008 at a local hospital, an oncologist noted a suspicious finding in the right breast. The patient had an incisional biopsy interpreted by Dr. A, a pathologist, and a core biopsy interpreted by Dr. B, another pathologist from the same diagnostic medical group. Both pathologists interpreted the mass as atypia, a benign abnormality.
In 2010, the patient went to a university medical center, where the mass was biopsied and the patient was found to have cancer. She underwent a right mastectomy.
PATIENT’S CLAIM The pathologists failed to diagnose her breast cancer at an early stage. Dr. A should have interpreted the 2008 incisional biopsy as malignant. A diagnosis in 2008 would have avoided the need for a mastectomy, allowing her to have a lumpectomy with chemotherapy.
DEFENDANTS’ DEFENSE The 2010 review of the 2008 data was an over-interpretation with hindsight bias; the diagnosis in 2008 was correct.
VERDICT The case against the local hospital and Dr. B were dismissed. The matter continued against Dr. A and the diagnostic medical group. A California defense verdict was returned.
Brachial plexus injury occurs after admitting physician leaves
A woman sought prenatal care from her family practitioner (FP). The FP admitted the mother to a hospital for induction of labor at 38 weeks’ gestation with concerns of increased uric acid, possible gestational hypertension, and leaking amniotic fluid. Labor progressed and the mother began pushing about 4 pm. After 30 minutes, the FP attempted vacuum extraction three times; the device popped off during one of the attempts.
The FP then left for a planned trip, and an ObGyn assumed her care. The ObGyn chose to allow the mother to rest. At 6 pm, the mother began to feel the urge to push. The ObGyn attempted vacuum extraction. Shoulder dystocia was encountered, and McRoberts and corkscrew maneuvers were used to deliver the fetus.
The child has C5–C6 brachial plexus injury with scapular winging and internal shoulder rotation.
PARENTS’ CLAIM A cesarean delivery should have been performed. The ObGyn applied excessive lateral traction, leading to the injury.
DEFENDANTS’ DEFENSE The FP and ObGyn argued that a cesarean delivery was not indicated because the fetus was not in distress. Fetal heart-rate monitoring strips were reassuring. The ObGyn denied using excessive lateral traction when freeing the shoulder dystocia.
VERDICT The hospital settled before trial for $300,000. An Illinois defense verdict was returned for the FP. The jury deadlocked as to the ObGyn’s negligence.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Patient‐Centered Blood Management
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).
| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin <13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.
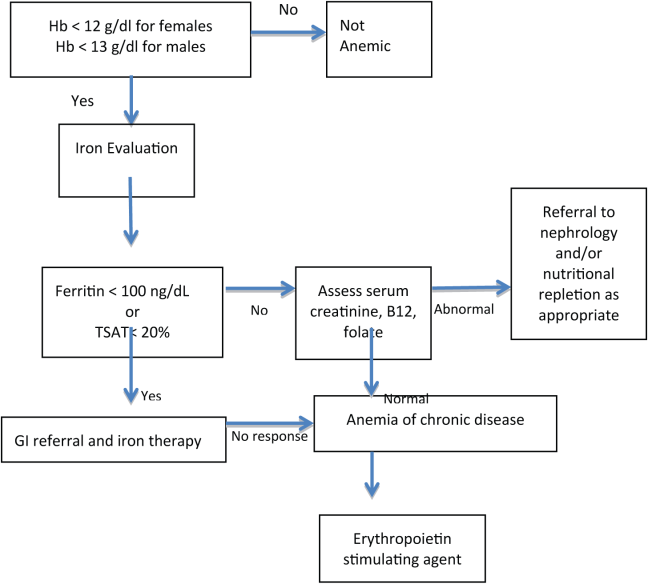
Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).

| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin <13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.

Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).

| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin <13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.

Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.
Update in Hospital Palliative Care
Seriously ill patients frequently receive care in hospitals,[1, 2, 3] and palliative care is a core competency for hospitalists.[4, 5] The goal of this update was to summarize and critique recently published research that has the highest potential to impact the clinical practice of palliative care in the hospital. We reviewed articles published between January 2012 and May 2013. To identify articles, we hand‐searched 22 leading journals (see Appendix) and the Cochrane Database of Systematic Reviews, and performed a PubMed keyword search using the terms hospice and palliative care. We evaluated identified articles based on scientific rigor and relevance to hospital practice. In this review, we summarize 9 articles that were collectively selected as having the highest impact on the clinical practice of hospital palliative care. We summarize each article and its findings and note cautions and implications for practice.
SYMPTOM MANAGEMENT
Indwelling Pleural Catheters and Talc Pleurodesis Provide Similar Dyspnea Relief in Patients With Malignant Pleural Effusions
Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion. JAMA. 2012;307:23832389.
Background
Expert guidelines recommend chest‐tube insertion and talc pleurodesis as a first‐line therapy for symptomatic malignant pleural effusions, but indwelling pleural catheters are gaining in popularity.[6] The optimal management is unknown.
Findings
A total of 106 patients with newly diagnosed symptomatic malignant pleural effusion were randomized to undergo talc pleurodesis or placement of an indwelling pleural catheter. Most patients had metastatic breast or lung cancer. Overall, there were no differences in relief of dyspnea at 42 days between patients who received indwelling catheters and pleurodesis; importantly, more than 75% of patients in both groups reported improved shortness of breath. The initial hospitalization was much shorter in the indwelling catheter group (0 days vs 4 days). There was no difference in quality of life, but in surviving patients, dyspnea at 6 months was better with the indwelling catheter. In the talc group, 22% of patients required further pleural procedures compared with 6% in the indwelling catheter group. Patients in the talc group had a higher frequency of adverse events than in the catheter group (40% vs 13%). In the catheter group, the most common adverse events were pleural infection, cellulitis, and catheter obstruction.
Cautions
The study was small and unblinded, and the primary outcome was subjective dyspnea. The study occurred at 7 hospitals, and the impact of institutional or provider experience was not taken into account. Last, overall costs of care, which could impact the choice of intervention, were not calculated.
Implications
This was a small but well‐done study showing that indwelling catheters and talc pleurodesis provide similar relief of dyspnea 42 days postintervention. Given these results, both interventions seem to be acceptable options. Clinicians and patients could select the best option based on local procedural expertise and patient factors such as preference, ability to manage a catheter, and life expectancy.
Most Dying Patients Do Not Experience Increased Respiratory Distress When Oxygen is Withdrawn
Campbell ML, Yarandi H, Dove‐Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517523.
Background
Oxygen is frequently administered to patients at the end of life, yet there is limited evidence evaluating whether oxygen reduces respiratory distress in dying patients.
Findings
In this double‐blind, repeated‐measure study, patients served as their own controls as the investigators evaluated respiratory distress with and without oxygen therapy. The study included 32 patients who were enrolled in hospice or seen in palliative care consultation and had a diagnosis such as lung cancer or heart failure that might cause dyspnea. Medical air (nasal cannula with air flow), supplemental oxygen, and no flow were randomly alternated every 10 minutes for 1 hour. Blinded research assistants used a validated observation scale to compare respiratory distress under each condition. At baseline, 27 of 32 (84%) patients were on oxygen. Three patients, all of whom were conscious and on oxygen at baseline, experienced increased respiratory distress without oxygen; reapplication of supplemental oxygen relieved their distress. The other 29 patients had no change in respiratory distress under the oxygen, medical air, and no flow conditions.
Cautions
All patients in this study were near death as measured by the Palliative Performance Scale, which assesses prognosis based on functional status and level of consciousness. Patients were excluded if they were receiving high‐flow oxygen by face mask or were experiencing respiratory distress at the time of initial evaluation. Some patients experienced increased discomfort after withdrawal of oxygen. Close observation is needed to determine which patients will experience distress.
Implications
The majority of patients who were receiving oxygen at baseline experienced no change in respiratory comfort when oxygen was withdrawn, supporting previous evidence that oxygen provides little benefit in nonhypoxemic patients. Oxygen may be an unnecessary intervention near death and has the potential to add to discomfort through nasal dryness and decreased mobility.
Sennosides Performed Similarly to Docusate Plus Sennosides in Managing Opioid‐Induced Constipation in Seriously Ill Patients
Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double‐blind, placebo‐controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45:213.
Background
Seriously ill patients frequently suffer from constipation, often as a result of opioid analgesics. Hospital clinicians should seek to optimize bowel regimens to prevent opioid‐induced constipation. A combination of the stimulant laxative sennoside and the stool softener docusate is often recommended to treat and prevent constipation. Docusate may not have additional benefit to sennoside, and may have significant burdens, including disturbing the absorption of other medications, adding to patients' pill burden and increasing nurse workload.[7]
Findings
In this double‐blinded trial, 74 patients in 3 inpatient hospices in Canada were randomized to receive sennoside plus either docusate 100 mg, or placebo tablets twice daily, or sennoside plus placebo for 10 days. Most patients had cancer as a life‐limiting diagnosis and received opioids during the study period. All were able to tolerate pills and food or sips of fluid. There was no significant difference between the 2 groups in stool frequency, volume, consistency, or patients' perceptions of difficulty with defecation. The percentage of patients who had a bowel movement at least every 3 days was 71% in the docusate plus sennoside group and 81% in the sennoside only group (P=0.45). There was also no significant difference between the groups in sennoside dose (which ranged between 13, 8.6 mg tablets daily), mean morphine equivalent daily dosage, or other bowel interventions.
Cautions
The trial was small, though it was adequately powered to detect a clinically meaningful difference between the 2 groups of 0.5 in the average number of bowel movements per day. The consent rate was low (26%); the authors do not detail reasons patients were not randomized. Patients who did not participate might have had different responses.
Implications
Consistent with previous work,[7] these results indicate that docusate is probably not needed for routine management of opioid‐induced constipation in seriously ill patients.
Sublingual Atropine Performed Similarly to Placebo in Reducing Noise Associated With Respiratory Rattle Near Death
Heisler M, Hamilton G, Abbott A, et al. Randomized double‐blind trial of sublingual atropine vs. placebo for the management of death rattle. J Pain Symptom Manage. 2012;45(1):1422.
Background
Increased respiratory tract secretions in patients near death can cause noisy breathing, often referred to as a death rattle. Antimuscarinic medications, such as atropine, are frequently used to decrease audible respirations and family distress, though little evidence exists to support this practice.
Findings
In this double‐blind, placebo‐controlled, parallel group trial at 3 inpatient hospices, 177 terminally ill patients with audible respiratory secretions were randomized to 2 drops of sublingual atropine 1% solution or placebo drops. Bedside nurses rated patients' respiratory secretions at enrollment, and 2 and 4 hours after receiving atropine or placebo. There were no differences in noise score between subjects treated with atropine and placebo at 2 hours (37.8% vs. 41.3%, P=0.24) or at 4 hours (39.7% and 51.7%, P=0.21). There were no differences in the safety end point of change in heart rate (P=0.47).
Cautions
Previous studies comparing different anticholinergic medications and routes of administration to manage audible respiratory secretions had variable response rates but suggested a benefit to antimuscarinic medications. However, these trials had significant methodological limitations including lack of randomization and blinding. The improvement in death rattle over time in other studies may suggest a favorable natural course for respiratory secretions rather than a treatment effect.
Implications
Although generalizability to other antimuscarinic medications and routes of administration is limited, in a randomized, double‐blind, placebo‐controlled trial, sublingual atropine did not reduce the noise from respiratory secretions when compared to placebo.
PATIENT AND FAMILY OUTCOMES AFTER CARDIOPULMONARY RESUSCITATION
Over Half of Older Adult Survivors of In‐Hospital Cardiopulmonary Resuscitation Were Alive At 1 Year
Chan PS, Krumholz HM, Spertus JA, et al. Long‐term outcomes in elderly survivors of in‐hospital cardiac arrest. N Engl J Med. 2013;368:10191026.
Background
Studies of cardiopulmonary resuscitation (CPR) outcomes have focused on survival to hospital discharge. Little is known about long‐term outcomes following in‐hospital cardiac arrest in older adults.
Findings
The authors analyzed data from the Get With the GuidelinesResuscitation registry from 2000 to 2008 and Medicare inpatient files from 2000 to 2010. The cohort included 6972 patients at 401 hospitals who were discharged after surviving in‐hospital arrest. Outcomes were survival and freedom from hospital readmission at 1 year after discharge. At discharge, 48% of patients had either no or mild neurologic disability at discharge; the remainder had moderate to severe neurologic disability. Overall, 58% of patients who were discharged were still alive at 1 year. Survival rates were lowest for patients who were discharged in coma or vegetative state (8% at 1 year), and highest for those discharged with mild or no disability (73% at 1 year). Older patients had lower survival rates than younger patients, as did men compared with women and blacks compared with whites. At 1 year, 34.4% of the patients had not been readmitted. Predictors of readmission were similar to those for lower survival rates.
Cautions
This study only analyzed survival data from patients who survived to hospital discharge after receiving in‐hospital CPR, not all patients who had a cardiac arrest. Thus, the survival rates reported here do not include patients who died during the original arrest, or who survived the arrest but died during their hospitalization. The 1‐year survival rate for people aged 65 years and above following a cardiac arrest is not reported but is likely to be about 10%, based on data from this registry.[8] Data were not available for health status, neurologic status, or quality of life of the survivors at 1 year.
Implications
Older patients who receive in‐hospital CPR and have a good neurologic status at hospital discharge have good long‐term outcomes. In counseling patients about CPR, it is important to note that most patients who receive CPR do not survive to hospital discharge.
Families Who Were Present During CPR Had Decreased Post‐traumatic Stress Symptoms
Jabre P, Belpomme V, Azoulay E, et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368:10081018.
Background
Family members who watch their loved ones undergo (CPR) might have increased emotional distress. Alternatively, observing CPR may allow for appreciation of the efforts taken for their loved one and provide comfort at a challenging time. The right balance of benefit and harm is unclear.
Findings
Between 2009 and 2011, 15 prehospital emergency medical service units in France were randomized to offer adult family members the opportunity to observe CPR or follow their usual practice. A total of 570 relatives were enrolled. In the intervention group, 79% of relatives observed CPR, compared to 43% in the control group. There was no difference in the effectiveness of CPR between the 2 groups. At 90 days, post‐traumatic stress symptoms were more common in the control group (adjusted odds ratio [OR]: 1.7; 95% confidence interval [CI]: 1.2‐2.5). At 90 days, those who were present for the resuscitation also had fewer symptoms of anxiety and fewer symptoms of depression (P<0.009 for both). Stress of the medical teams involved in the CPR was not different between the 2 groups. No malpractice claims were filed in either group.
Cautions
The study was conducted only in France, so the results may not be generalizable outside of France. In addition, the observed resuscitation was for patients who suffered a cardiac arrest in the home; it is unclear if the same results would be found in the emergency department or hospital.
Implications
This is the highest quality study to date in this area that argues for actively inviting family members to be present for resuscitation efforts in the home. Further studies are needed to determine if hospitals should implement standard protocols. In the meantime, providers who perform CPR should consider inviting families to observe, as it may result in less emotional distress for family members.
COMMUNICATION AND DECISION MAKING
Surrogate Decision Makers Interpreted Prognostic Information Optimistically
Zier LS, Sottile PD, Hong SY, et al. Surrogate decision makers' interpretation of prognostic information: a mixed‐methods study. Ann Intern Med. 2012;156:360366.
Background
Surrogates of critically ill patients often have beliefs about prognosis that are discordant from what is told to them by providers. Little is known about why this is the case.
Findings
Eighty surrogates of patients in intensive care units (ICUs) were given questionnaires with hypothetical prognostic statements and asked to identify a survival probability associated with each statement on a 0% to 100% scale. Interviewers examined the questionnaires to identify responses that were not concordant with the given prognostic statements. They then interviewed participants to determine why the answers were discordant. The researchers found that surrogates were more likely to offer an overoptimistic interpretation of statements communicating a high risk of death, compared to statements communicating a low risk of death. The qualitative interviews revealed that surrogates felt they needed to express ongoing optimism and that patient factors not known to the medical team would lead to better outcomes.
Cautions
The participants were surrogates who were present in the ICU at the time when study investigators were there, and thus the results may not be generalizable to all surrogates. Only a subset of participants completed qualitative interviews. Prognostic statements were hypothetical. Written prognostic statements may be interpreted differently than spoken statements.
Implications
Surrogate decision makers may interpret prognostic statements optimistically, especially when a high risk of death is estimated. Inaccurate interpretation may be related to personal beliefs about the patients' strengths and a need to hold onto hope for a positive outcome. When communicating with surrogates of critically ill patients, providers should be aware that, beyond the actual information shared, many other factors influence surrogates' beliefs about prognosis.
A Majority of Patients With Metastatic Cancer Felt That Chemotherapy Might Cure Their Disease
Weeks JC, Catalano PJ, Chronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:16161625.
Background
Chemotherapy for advanced cancer is not curative, and many cancer patients overestimate their prognosis. Little is known about patients' understanding of the goals of chemotherapy when cancer is advanced.
Findings
Participants were part of the Cancer Care Outcomes Research and Surveillance study. Patients with stage IV lung or colon cancer who opted to receive chemotherapy (n=1193) were asked how likely they thought it was that the chemotherapy would cure their cancer. A majority (69% of lung cancer patients and 81% of colon cancer patients) felt that chemotherapy might cure their disease. Those who rated their physicians very favorably in satisfaction surveys were more likely to feel that that chemotherapy might be curative, compared to those who rated their physician less favorably (OR: 1.90; 95% CI: 1.33‐2.72).
Cautions
The study did not include patients who died soon after diagnosis and thus does not provide information about those who opted for chemotherapy but did not survive to the interview. It is possible that responses were influenced by participants' need to express optimism (social desirability bias). It is not clear how or whether prognostic disclosure by physicians caused the lower satisfaction ratings.
Implications
Despite the fact that stage IV lung and colon cancer are not curable with chemotherapy, a majority of patients reported believing that chemotherapy might cure their disease. Hospital clinicians should be aware that many patients who they view as terminally ill believe their illness may be cured.
Older Patients Who Viewed a Goals‐of‐Care Video at Admission to a Skilled Nursing Facility Were More Likely to Prefer Comfort Care
Volandes AE, Brandeis GH, Davis AD, et al. A randomized controlled trial of a goals‐of‐care video for elderly patients admitted to skilled nursing facilities. J Palliat Med. 2012;15:805811.
Background
Seriously ill older patients are frequently discharged from hospitals to skilled nursing facilities (SNFs). It is important to clarify and document patients' goals for care at the time of admission to SNFs, to ensure that care provided there is consistent with patients' preferences. Previous work has shown promise using videos to assist patients in advance‐care planning, providing realistic and standardized portrayals of different treatment options.[9, 10]
Findings
English‐speaking patients at least 65 years of age who did not have altered mental status were randomized to hear a verbal description (n=51) or view a 6‐minute video (n=50) that presented the same information accompanied by pictures of patients of 3 possible goals of medical care: life‐prolonging care, limited medical care, and comfort care. After the video or narrative, patients were asked what their care preference would be if they became more ill while at the SNF. Patients who viewed the video were more likely to report a preference for comfort care, compared to patients who received the narrative, 80% vs 57%, P=0.02. In a review of medical records, only 31% of patients who reported a preference for comfort care had a do not resuscitate order at the SNF.
Cautions
The study was conducted at 2 nursing homes located in the Boston, Massachusetts area, which may limit generalizability. Assessors were not blinded to whether the patient saw the video or received the narrative, which may have introduced bias. The authors note that the video aimed to present the different care options without valuing one over the other, though it may have inadvertently presented one option in a more favorable light.
Implications
Videos may be powerful tools for helping nursing home patients to clarify goals of care, and might be applied in the hospital setting prior to transferring patients to nursing homes. There is a significant opportunity to improve concordance of care with preferences through better documentation and implementation of code status orders when transferring patients to SNFs.
Acknowledgments
Disclosures: Drs. Anderson and Johnson and Mr. Horton received an honorarium and support for travel to present findings resulting from the literature review at the Annual Assembly of the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association on March 16, 2013 in New Orleans, Louisiana. Dr. Anderson was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI grant number KL2TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
APPENDIX
Journals That Were Hand Searched to Identify Articles, By Topic Area
General:
- British Medical Journal
- Journal of the American Medical Association
- Lancet
- New England Journal of Medicine
Internal medicine:
- Annals Internal Medicine
- Archives Internal Medicine
- Journal of General Internal Medicine
- Journal of Hospital Medicine
Palliative care and symptom management:
- Journal Pain and Symptom Management
- Journal of Palliative Care
- Journal of Palliative Medicine
- Palliative Medicine
- Pain
Oncology:
- Journal of Clinical Oncology
- Supportive Care in Cancer
Critical care:
- American Journal of Respiratory and Critical Care Medicine
- Critical Care Medicine
Pediatrics:
- Pediatrics
Geriatrics:
- Journal of the American Geriatrics Society
Education:
- Academic Medicine
Nursing:
- Journal of Hospice and Palliative Nursing
- Oncology Nursing Forum
- The Dartmouth Atlas of Health Care. Percent of Medicare decedents hospitalized at least once during the last six months of life 2007. Available at: http://www.dartmouthatlas.org/data/table.aspx?ind=133. Accessed October 30, 2013.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. End‐of‐life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst. 2011;103(11):853–862.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- Society of Hospital Medicine; 2008.The core competencies in hospital medicine. http://www.hospitalmedicine.org/Content/NavigationMenu/Education/CoreCurriculum/Core_Competencies.htm. Accessed October 30, 2013.
- , , , , . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline. Thorax. 2010;65:ii32–ii40.
- , . A comparison of sennosides‐based bowel protocols with and without docusate in hospitalized patients with cancer. J Palliat Med. 2008;11(4):575–581.
- , , , , , . Trends in survival after In‐hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention‐postintervention study. Cancer. 2012;118(17):4331–4338.
Seriously ill patients frequently receive care in hospitals,[1, 2, 3] and palliative care is a core competency for hospitalists.[4, 5] The goal of this update was to summarize and critique recently published research that has the highest potential to impact the clinical practice of palliative care in the hospital. We reviewed articles published between January 2012 and May 2013. To identify articles, we hand‐searched 22 leading journals (see Appendix) and the Cochrane Database of Systematic Reviews, and performed a PubMed keyword search using the terms hospice and palliative care. We evaluated identified articles based on scientific rigor and relevance to hospital practice. In this review, we summarize 9 articles that were collectively selected as having the highest impact on the clinical practice of hospital palliative care. We summarize each article and its findings and note cautions and implications for practice.
SYMPTOM MANAGEMENT
Indwelling Pleural Catheters and Talc Pleurodesis Provide Similar Dyspnea Relief in Patients With Malignant Pleural Effusions
Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion. JAMA. 2012;307:23832389.
Background
Expert guidelines recommend chest‐tube insertion and talc pleurodesis as a first‐line therapy for symptomatic malignant pleural effusions, but indwelling pleural catheters are gaining in popularity.[6] The optimal management is unknown.
Findings
A total of 106 patients with newly diagnosed symptomatic malignant pleural effusion were randomized to undergo talc pleurodesis or placement of an indwelling pleural catheter. Most patients had metastatic breast or lung cancer. Overall, there were no differences in relief of dyspnea at 42 days between patients who received indwelling catheters and pleurodesis; importantly, more than 75% of patients in both groups reported improved shortness of breath. The initial hospitalization was much shorter in the indwelling catheter group (0 days vs 4 days). There was no difference in quality of life, but in surviving patients, dyspnea at 6 months was better with the indwelling catheter. In the talc group, 22% of patients required further pleural procedures compared with 6% in the indwelling catheter group. Patients in the talc group had a higher frequency of adverse events than in the catheter group (40% vs 13%). In the catheter group, the most common adverse events were pleural infection, cellulitis, and catheter obstruction.
Cautions
The study was small and unblinded, and the primary outcome was subjective dyspnea. The study occurred at 7 hospitals, and the impact of institutional or provider experience was not taken into account. Last, overall costs of care, which could impact the choice of intervention, were not calculated.
Implications
This was a small but well‐done study showing that indwelling catheters and talc pleurodesis provide similar relief of dyspnea 42 days postintervention. Given these results, both interventions seem to be acceptable options. Clinicians and patients could select the best option based on local procedural expertise and patient factors such as preference, ability to manage a catheter, and life expectancy.
Most Dying Patients Do Not Experience Increased Respiratory Distress When Oxygen is Withdrawn
Campbell ML, Yarandi H, Dove‐Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517523.
Background
Oxygen is frequently administered to patients at the end of life, yet there is limited evidence evaluating whether oxygen reduces respiratory distress in dying patients.
Findings
In this double‐blind, repeated‐measure study, patients served as their own controls as the investigators evaluated respiratory distress with and without oxygen therapy. The study included 32 patients who were enrolled in hospice or seen in palliative care consultation and had a diagnosis such as lung cancer or heart failure that might cause dyspnea. Medical air (nasal cannula with air flow), supplemental oxygen, and no flow were randomly alternated every 10 minutes for 1 hour. Blinded research assistants used a validated observation scale to compare respiratory distress under each condition. At baseline, 27 of 32 (84%) patients were on oxygen. Three patients, all of whom were conscious and on oxygen at baseline, experienced increased respiratory distress without oxygen; reapplication of supplemental oxygen relieved their distress. The other 29 patients had no change in respiratory distress under the oxygen, medical air, and no flow conditions.
Cautions
All patients in this study were near death as measured by the Palliative Performance Scale, which assesses prognosis based on functional status and level of consciousness. Patients were excluded if they were receiving high‐flow oxygen by face mask or were experiencing respiratory distress at the time of initial evaluation. Some patients experienced increased discomfort after withdrawal of oxygen. Close observation is needed to determine which patients will experience distress.
Implications
The majority of patients who were receiving oxygen at baseline experienced no change in respiratory comfort when oxygen was withdrawn, supporting previous evidence that oxygen provides little benefit in nonhypoxemic patients. Oxygen may be an unnecessary intervention near death and has the potential to add to discomfort through nasal dryness and decreased mobility.
Sennosides Performed Similarly to Docusate Plus Sennosides in Managing Opioid‐Induced Constipation in Seriously Ill Patients
Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double‐blind, placebo‐controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45:213.
Background
Seriously ill patients frequently suffer from constipation, often as a result of opioid analgesics. Hospital clinicians should seek to optimize bowel regimens to prevent opioid‐induced constipation. A combination of the stimulant laxative sennoside and the stool softener docusate is often recommended to treat and prevent constipation. Docusate may not have additional benefit to sennoside, and may have significant burdens, including disturbing the absorption of other medications, adding to patients' pill burden and increasing nurse workload.[7]
Findings
In this double‐blinded trial, 74 patients in 3 inpatient hospices in Canada were randomized to receive sennoside plus either docusate 100 mg, or placebo tablets twice daily, or sennoside plus placebo for 10 days. Most patients had cancer as a life‐limiting diagnosis and received opioids during the study period. All were able to tolerate pills and food or sips of fluid. There was no significant difference between the 2 groups in stool frequency, volume, consistency, or patients' perceptions of difficulty with defecation. The percentage of patients who had a bowel movement at least every 3 days was 71% in the docusate plus sennoside group and 81% in the sennoside only group (P=0.45). There was also no significant difference between the groups in sennoside dose (which ranged between 13, 8.6 mg tablets daily), mean morphine equivalent daily dosage, or other bowel interventions.
Cautions
The trial was small, though it was adequately powered to detect a clinically meaningful difference between the 2 groups of 0.5 in the average number of bowel movements per day. The consent rate was low (26%); the authors do not detail reasons patients were not randomized. Patients who did not participate might have had different responses.
Implications
Consistent with previous work,[7] these results indicate that docusate is probably not needed for routine management of opioid‐induced constipation in seriously ill patients.
Sublingual Atropine Performed Similarly to Placebo in Reducing Noise Associated With Respiratory Rattle Near Death
Heisler M, Hamilton G, Abbott A, et al. Randomized double‐blind trial of sublingual atropine vs. placebo for the management of death rattle. J Pain Symptom Manage. 2012;45(1):1422.
Background
Increased respiratory tract secretions in patients near death can cause noisy breathing, often referred to as a death rattle. Antimuscarinic medications, such as atropine, are frequently used to decrease audible respirations and family distress, though little evidence exists to support this practice.
Findings
In this double‐blind, placebo‐controlled, parallel group trial at 3 inpatient hospices, 177 terminally ill patients with audible respiratory secretions were randomized to 2 drops of sublingual atropine 1% solution or placebo drops. Bedside nurses rated patients' respiratory secretions at enrollment, and 2 and 4 hours after receiving atropine or placebo. There were no differences in noise score between subjects treated with atropine and placebo at 2 hours (37.8% vs. 41.3%, P=0.24) or at 4 hours (39.7% and 51.7%, P=0.21). There were no differences in the safety end point of change in heart rate (P=0.47).
Cautions
Previous studies comparing different anticholinergic medications and routes of administration to manage audible respiratory secretions had variable response rates but suggested a benefit to antimuscarinic medications. However, these trials had significant methodological limitations including lack of randomization and blinding. The improvement in death rattle over time in other studies may suggest a favorable natural course for respiratory secretions rather than a treatment effect.
Implications
Although generalizability to other antimuscarinic medications and routes of administration is limited, in a randomized, double‐blind, placebo‐controlled trial, sublingual atropine did not reduce the noise from respiratory secretions when compared to placebo.
PATIENT AND FAMILY OUTCOMES AFTER CARDIOPULMONARY RESUSCITATION
Over Half of Older Adult Survivors of In‐Hospital Cardiopulmonary Resuscitation Were Alive At 1 Year
Chan PS, Krumholz HM, Spertus JA, et al. Long‐term outcomes in elderly survivors of in‐hospital cardiac arrest. N Engl J Med. 2013;368:10191026.
Background
Studies of cardiopulmonary resuscitation (CPR) outcomes have focused on survival to hospital discharge. Little is known about long‐term outcomes following in‐hospital cardiac arrest in older adults.
Findings
The authors analyzed data from the Get With the GuidelinesResuscitation registry from 2000 to 2008 and Medicare inpatient files from 2000 to 2010. The cohort included 6972 patients at 401 hospitals who were discharged after surviving in‐hospital arrest. Outcomes were survival and freedom from hospital readmission at 1 year after discharge. At discharge, 48% of patients had either no or mild neurologic disability at discharge; the remainder had moderate to severe neurologic disability. Overall, 58% of patients who were discharged were still alive at 1 year. Survival rates were lowest for patients who were discharged in coma or vegetative state (8% at 1 year), and highest for those discharged with mild or no disability (73% at 1 year). Older patients had lower survival rates than younger patients, as did men compared with women and blacks compared with whites. At 1 year, 34.4% of the patients had not been readmitted. Predictors of readmission were similar to those for lower survival rates.
Cautions
This study only analyzed survival data from patients who survived to hospital discharge after receiving in‐hospital CPR, not all patients who had a cardiac arrest. Thus, the survival rates reported here do not include patients who died during the original arrest, or who survived the arrest but died during their hospitalization. The 1‐year survival rate for people aged 65 years and above following a cardiac arrest is not reported but is likely to be about 10%, based on data from this registry.[8] Data were not available for health status, neurologic status, or quality of life of the survivors at 1 year.
Implications
Older patients who receive in‐hospital CPR and have a good neurologic status at hospital discharge have good long‐term outcomes. In counseling patients about CPR, it is important to note that most patients who receive CPR do not survive to hospital discharge.
Families Who Were Present During CPR Had Decreased Post‐traumatic Stress Symptoms
Jabre P, Belpomme V, Azoulay E, et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368:10081018.
Background
Family members who watch their loved ones undergo (CPR) might have increased emotional distress. Alternatively, observing CPR may allow for appreciation of the efforts taken for their loved one and provide comfort at a challenging time. The right balance of benefit and harm is unclear.
Findings
Between 2009 and 2011, 15 prehospital emergency medical service units in France were randomized to offer adult family members the opportunity to observe CPR or follow their usual practice. A total of 570 relatives were enrolled. In the intervention group, 79% of relatives observed CPR, compared to 43% in the control group. There was no difference in the effectiveness of CPR between the 2 groups. At 90 days, post‐traumatic stress symptoms were more common in the control group (adjusted odds ratio [OR]: 1.7; 95% confidence interval [CI]: 1.2‐2.5). At 90 days, those who were present for the resuscitation also had fewer symptoms of anxiety and fewer symptoms of depression (P<0.009 for both). Stress of the medical teams involved in the CPR was not different between the 2 groups. No malpractice claims were filed in either group.
Cautions
The study was conducted only in France, so the results may not be generalizable outside of France. In addition, the observed resuscitation was for patients who suffered a cardiac arrest in the home; it is unclear if the same results would be found in the emergency department or hospital.
Implications
This is the highest quality study to date in this area that argues for actively inviting family members to be present for resuscitation efforts in the home. Further studies are needed to determine if hospitals should implement standard protocols. In the meantime, providers who perform CPR should consider inviting families to observe, as it may result in less emotional distress for family members.
COMMUNICATION AND DECISION MAKING
Surrogate Decision Makers Interpreted Prognostic Information Optimistically
Zier LS, Sottile PD, Hong SY, et al. Surrogate decision makers' interpretation of prognostic information: a mixed‐methods study. Ann Intern Med. 2012;156:360366.
Background
Surrogates of critically ill patients often have beliefs about prognosis that are discordant from what is told to them by providers. Little is known about why this is the case.
Findings
Eighty surrogates of patients in intensive care units (ICUs) were given questionnaires with hypothetical prognostic statements and asked to identify a survival probability associated with each statement on a 0% to 100% scale. Interviewers examined the questionnaires to identify responses that were not concordant with the given prognostic statements. They then interviewed participants to determine why the answers were discordant. The researchers found that surrogates were more likely to offer an overoptimistic interpretation of statements communicating a high risk of death, compared to statements communicating a low risk of death. The qualitative interviews revealed that surrogates felt they needed to express ongoing optimism and that patient factors not known to the medical team would lead to better outcomes.
Cautions
The participants were surrogates who were present in the ICU at the time when study investigators were there, and thus the results may not be generalizable to all surrogates. Only a subset of participants completed qualitative interviews. Prognostic statements were hypothetical. Written prognostic statements may be interpreted differently than spoken statements.
Implications
Surrogate decision makers may interpret prognostic statements optimistically, especially when a high risk of death is estimated. Inaccurate interpretation may be related to personal beliefs about the patients' strengths and a need to hold onto hope for a positive outcome. When communicating with surrogates of critically ill patients, providers should be aware that, beyond the actual information shared, many other factors influence surrogates' beliefs about prognosis.
A Majority of Patients With Metastatic Cancer Felt That Chemotherapy Might Cure Their Disease
Weeks JC, Catalano PJ, Chronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:16161625.
Background
Chemotherapy for advanced cancer is not curative, and many cancer patients overestimate their prognosis. Little is known about patients' understanding of the goals of chemotherapy when cancer is advanced.
Findings
Participants were part of the Cancer Care Outcomes Research and Surveillance study. Patients with stage IV lung or colon cancer who opted to receive chemotherapy (n=1193) were asked how likely they thought it was that the chemotherapy would cure their cancer. A majority (69% of lung cancer patients and 81% of colon cancer patients) felt that chemotherapy might cure their disease. Those who rated their physicians very favorably in satisfaction surveys were more likely to feel that that chemotherapy might be curative, compared to those who rated their physician less favorably (OR: 1.90; 95% CI: 1.33‐2.72).
Cautions
The study did not include patients who died soon after diagnosis and thus does not provide information about those who opted for chemotherapy but did not survive to the interview. It is possible that responses were influenced by participants' need to express optimism (social desirability bias). It is not clear how or whether prognostic disclosure by physicians caused the lower satisfaction ratings.
Implications
Despite the fact that stage IV lung and colon cancer are not curable with chemotherapy, a majority of patients reported believing that chemotherapy might cure their disease. Hospital clinicians should be aware that many patients who they view as terminally ill believe their illness may be cured.
Older Patients Who Viewed a Goals‐of‐Care Video at Admission to a Skilled Nursing Facility Were More Likely to Prefer Comfort Care
Volandes AE, Brandeis GH, Davis AD, et al. A randomized controlled trial of a goals‐of‐care video for elderly patients admitted to skilled nursing facilities. J Palliat Med. 2012;15:805811.
Background
Seriously ill older patients are frequently discharged from hospitals to skilled nursing facilities (SNFs). It is important to clarify and document patients' goals for care at the time of admission to SNFs, to ensure that care provided there is consistent with patients' preferences. Previous work has shown promise using videos to assist patients in advance‐care planning, providing realistic and standardized portrayals of different treatment options.[9, 10]
Findings
English‐speaking patients at least 65 years of age who did not have altered mental status were randomized to hear a verbal description (n=51) or view a 6‐minute video (n=50) that presented the same information accompanied by pictures of patients of 3 possible goals of medical care: life‐prolonging care, limited medical care, and comfort care. After the video or narrative, patients were asked what their care preference would be if they became more ill while at the SNF. Patients who viewed the video were more likely to report a preference for comfort care, compared to patients who received the narrative, 80% vs 57%, P=0.02. In a review of medical records, only 31% of patients who reported a preference for comfort care had a do not resuscitate order at the SNF.
Cautions
The study was conducted at 2 nursing homes located in the Boston, Massachusetts area, which may limit generalizability. Assessors were not blinded to whether the patient saw the video or received the narrative, which may have introduced bias. The authors note that the video aimed to present the different care options without valuing one over the other, though it may have inadvertently presented one option in a more favorable light.
Implications
Videos may be powerful tools for helping nursing home patients to clarify goals of care, and might be applied in the hospital setting prior to transferring patients to nursing homes. There is a significant opportunity to improve concordance of care with preferences through better documentation and implementation of code status orders when transferring patients to SNFs.
Acknowledgments
Disclosures: Drs. Anderson and Johnson and Mr. Horton received an honorarium and support for travel to present findings resulting from the literature review at the Annual Assembly of the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association on March 16, 2013 in New Orleans, Louisiana. Dr. Anderson was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI grant number KL2TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
APPENDIX
Journals That Were Hand Searched to Identify Articles, By Topic Area
General:
- British Medical Journal
- Journal of the American Medical Association
- Lancet
- New England Journal of Medicine
Internal medicine:
- Annals Internal Medicine
- Archives Internal Medicine
- Journal of General Internal Medicine
- Journal of Hospital Medicine
Palliative care and symptom management:
- Journal Pain and Symptom Management
- Journal of Palliative Care
- Journal of Palliative Medicine
- Palliative Medicine
- Pain
Oncology:
- Journal of Clinical Oncology
- Supportive Care in Cancer
Critical care:
- American Journal of Respiratory and Critical Care Medicine
- Critical Care Medicine
Pediatrics:
- Pediatrics
Geriatrics:
- Journal of the American Geriatrics Society
Education:
- Academic Medicine
Nursing:
- Journal of Hospice and Palliative Nursing
- Oncology Nursing Forum
Seriously ill patients frequently receive care in hospitals,[1, 2, 3] and palliative care is a core competency for hospitalists.[4, 5] The goal of this update was to summarize and critique recently published research that has the highest potential to impact the clinical practice of palliative care in the hospital. We reviewed articles published between January 2012 and May 2013. To identify articles, we hand‐searched 22 leading journals (see Appendix) and the Cochrane Database of Systematic Reviews, and performed a PubMed keyword search using the terms hospice and palliative care. We evaluated identified articles based on scientific rigor and relevance to hospital practice. In this review, we summarize 9 articles that were collectively selected as having the highest impact on the clinical practice of hospital palliative care. We summarize each article and its findings and note cautions and implications for practice.
SYMPTOM MANAGEMENT
Indwelling Pleural Catheters and Talc Pleurodesis Provide Similar Dyspnea Relief in Patients With Malignant Pleural Effusions
Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion. JAMA. 2012;307:23832389.
Background
Expert guidelines recommend chest‐tube insertion and talc pleurodesis as a first‐line therapy for symptomatic malignant pleural effusions, but indwelling pleural catheters are gaining in popularity.[6] The optimal management is unknown.
Findings
A total of 106 patients with newly diagnosed symptomatic malignant pleural effusion were randomized to undergo talc pleurodesis or placement of an indwelling pleural catheter. Most patients had metastatic breast or lung cancer. Overall, there were no differences in relief of dyspnea at 42 days between patients who received indwelling catheters and pleurodesis; importantly, more than 75% of patients in both groups reported improved shortness of breath. The initial hospitalization was much shorter in the indwelling catheter group (0 days vs 4 days). There was no difference in quality of life, but in surviving patients, dyspnea at 6 months was better with the indwelling catheter. In the talc group, 22% of patients required further pleural procedures compared with 6% in the indwelling catheter group. Patients in the talc group had a higher frequency of adverse events than in the catheter group (40% vs 13%). In the catheter group, the most common adverse events were pleural infection, cellulitis, and catheter obstruction.
Cautions
The study was small and unblinded, and the primary outcome was subjective dyspnea. The study occurred at 7 hospitals, and the impact of institutional or provider experience was not taken into account. Last, overall costs of care, which could impact the choice of intervention, were not calculated.
Implications
This was a small but well‐done study showing that indwelling catheters and talc pleurodesis provide similar relief of dyspnea 42 days postintervention. Given these results, both interventions seem to be acceptable options. Clinicians and patients could select the best option based on local procedural expertise and patient factors such as preference, ability to manage a catheter, and life expectancy.
Most Dying Patients Do Not Experience Increased Respiratory Distress When Oxygen is Withdrawn
Campbell ML, Yarandi H, Dove‐Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage. 2013;45(3):517523.
Background
Oxygen is frequently administered to patients at the end of life, yet there is limited evidence evaluating whether oxygen reduces respiratory distress in dying patients.
Findings
In this double‐blind, repeated‐measure study, patients served as their own controls as the investigators evaluated respiratory distress with and without oxygen therapy. The study included 32 patients who were enrolled in hospice or seen in palliative care consultation and had a diagnosis such as lung cancer or heart failure that might cause dyspnea. Medical air (nasal cannula with air flow), supplemental oxygen, and no flow were randomly alternated every 10 minutes for 1 hour. Blinded research assistants used a validated observation scale to compare respiratory distress under each condition. At baseline, 27 of 32 (84%) patients were on oxygen. Three patients, all of whom were conscious and on oxygen at baseline, experienced increased respiratory distress without oxygen; reapplication of supplemental oxygen relieved their distress. The other 29 patients had no change in respiratory distress under the oxygen, medical air, and no flow conditions.
Cautions
All patients in this study were near death as measured by the Palliative Performance Scale, which assesses prognosis based on functional status and level of consciousness. Patients were excluded if they were receiving high‐flow oxygen by face mask or were experiencing respiratory distress at the time of initial evaluation. Some patients experienced increased discomfort after withdrawal of oxygen. Close observation is needed to determine which patients will experience distress.
Implications
The majority of patients who were receiving oxygen at baseline experienced no change in respiratory comfort when oxygen was withdrawn, supporting previous evidence that oxygen provides little benefit in nonhypoxemic patients. Oxygen may be an unnecessary intervention near death and has the potential to add to discomfort through nasal dryness and decreased mobility.
Sennosides Performed Similarly to Docusate Plus Sennosides in Managing Opioid‐Induced Constipation in Seriously Ill Patients
Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double‐blind, placebo‐controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45:213.
Background
Seriously ill patients frequently suffer from constipation, often as a result of opioid analgesics. Hospital clinicians should seek to optimize bowel regimens to prevent opioid‐induced constipation. A combination of the stimulant laxative sennoside and the stool softener docusate is often recommended to treat and prevent constipation. Docusate may not have additional benefit to sennoside, and may have significant burdens, including disturbing the absorption of other medications, adding to patients' pill burden and increasing nurse workload.[7]
Findings
In this double‐blinded trial, 74 patients in 3 inpatient hospices in Canada were randomized to receive sennoside plus either docusate 100 mg, or placebo tablets twice daily, or sennoside plus placebo for 10 days. Most patients had cancer as a life‐limiting diagnosis and received opioids during the study period. All were able to tolerate pills and food or sips of fluid. There was no significant difference between the 2 groups in stool frequency, volume, consistency, or patients' perceptions of difficulty with defecation. The percentage of patients who had a bowel movement at least every 3 days was 71% in the docusate plus sennoside group and 81% in the sennoside only group (P=0.45). There was also no significant difference between the groups in sennoside dose (which ranged between 13, 8.6 mg tablets daily), mean morphine equivalent daily dosage, or other bowel interventions.
Cautions
The trial was small, though it was adequately powered to detect a clinically meaningful difference between the 2 groups of 0.5 in the average number of bowel movements per day. The consent rate was low (26%); the authors do not detail reasons patients were not randomized. Patients who did not participate might have had different responses.
Implications
Consistent with previous work,[7] these results indicate that docusate is probably not needed for routine management of opioid‐induced constipation in seriously ill patients.
Sublingual Atropine Performed Similarly to Placebo in Reducing Noise Associated With Respiratory Rattle Near Death
Heisler M, Hamilton G, Abbott A, et al. Randomized double‐blind trial of sublingual atropine vs. placebo for the management of death rattle. J Pain Symptom Manage. 2012;45(1):1422.
Background
Increased respiratory tract secretions in patients near death can cause noisy breathing, often referred to as a death rattle. Antimuscarinic medications, such as atropine, are frequently used to decrease audible respirations and family distress, though little evidence exists to support this practice.
Findings
In this double‐blind, placebo‐controlled, parallel group trial at 3 inpatient hospices, 177 terminally ill patients with audible respiratory secretions were randomized to 2 drops of sublingual atropine 1% solution or placebo drops. Bedside nurses rated patients' respiratory secretions at enrollment, and 2 and 4 hours after receiving atropine or placebo. There were no differences in noise score between subjects treated with atropine and placebo at 2 hours (37.8% vs. 41.3%, P=0.24) or at 4 hours (39.7% and 51.7%, P=0.21). There were no differences in the safety end point of change in heart rate (P=0.47).
Cautions
Previous studies comparing different anticholinergic medications and routes of administration to manage audible respiratory secretions had variable response rates but suggested a benefit to antimuscarinic medications. However, these trials had significant methodological limitations including lack of randomization and blinding. The improvement in death rattle over time in other studies may suggest a favorable natural course for respiratory secretions rather than a treatment effect.
Implications
Although generalizability to other antimuscarinic medications and routes of administration is limited, in a randomized, double‐blind, placebo‐controlled trial, sublingual atropine did not reduce the noise from respiratory secretions when compared to placebo.
PATIENT AND FAMILY OUTCOMES AFTER CARDIOPULMONARY RESUSCITATION
Over Half of Older Adult Survivors of In‐Hospital Cardiopulmonary Resuscitation Were Alive At 1 Year
Chan PS, Krumholz HM, Spertus JA, et al. Long‐term outcomes in elderly survivors of in‐hospital cardiac arrest. N Engl J Med. 2013;368:10191026.
Background
Studies of cardiopulmonary resuscitation (CPR) outcomes have focused on survival to hospital discharge. Little is known about long‐term outcomes following in‐hospital cardiac arrest in older adults.
Findings
The authors analyzed data from the Get With the GuidelinesResuscitation registry from 2000 to 2008 and Medicare inpatient files from 2000 to 2010. The cohort included 6972 patients at 401 hospitals who were discharged after surviving in‐hospital arrest. Outcomes were survival and freedom from hospital readmission at 1 year after discharge. At discharge, 48% of patients had either no or mild neurologic disability at discharge; the remainder had moderate to severe neurologic disability. Overall, 58% of patients who were discharged were still alive at 1 year. Survival rates were lowest for patients who were discharged in coma or vegetative state (8% at 1 year), and highest for those discharged with mild or no disability (73% at 1 year). Older patients had lower survival rates than younger patients, as did men compared with women and blacks compared with whites. At 1 year, 34.4% of the patients had not been readmitted. Predictors of readmission were similar to those for lower survival rates.
Cautions
This study only analyzed survival data from patients who survived to hospital discharge after receiving in‐hospital CPR, not all patients who had a cardiac arrest. Thus, the survival rates reported here do not include patients who died during the original arrest, or who survived the arrest but died during their hospitalization. The 1‐year survival rate for people aged 65 years and above following a cardiac arrest is not reported but is likely to be about 10%, based on data from this registry.[8] Data were not available for health status, neurologic status, or quality of life of the survivors at 1 year.
Implications
Older patients who receive in‐hospital CPR and have a good neurologic status at hospital discharge have good long‐term outcomes. In counseling patients about CPR, it is important to note that most patients who receive CPR do not survive to hospital discharge.
Families Who Were Present During CPR Had Decreased Post‐traumatic Stress Symptoms
Jabre P, Belpomme V, Azoulay E, et al. Family presence during cardiopulmonary resuscitation. N Engl J Med. 2013;368:10081018.
Background
Family members who watch their loved ones undergo (CPR) might have increased emotional distress. Alternatively, observing CPR may allow for appreciation of the efforts taken for their loved one and provide comfort at a challenging time. The right balance of benefit and harm is unclear.
Findings
Between 2009 and 2011, 15 prehospital emergency medical service units in France were randomized to offer adult family members the opportunity to observe CPR or follow their usual practice. A total of 570 relatives were enrolled. In the intervention group, 79% of relatives observed CPR, compared to 43% in the control group. There was no difference in the effectiveness of CPR between the 2 groups. At 90 days, post‐traumatic stress symptoms were more common in the control group (adjusted odds ratio [OR]: 1.7; 95% confidence interval [CI]: 1.2‐2.5). At 90 days, those who were present for the resuscitation also had fewer symptoms of anxiety and fewer symptoms of depression (P<0.009 for both). Stress of the medical teams involved in the CPR was not different between the 2 groups. No malpractice claims were filed in either group.
Cautions
The study was conducted only in France, so the results may not be generalizable outside of France. In addition, the observed resuscitation was for patients who suffered a cardiac arrest in the home; it is unclear if the same results would be found in the emergency department or hospital.
Implications
This is the highest quality study to date in this area that argues for actively inviting family members to be present for resuscitation efforts in the home. Further studies are needed to determine if hospitals should implement standard protocols. In the meantime, providers who perform CPR should consider inviting families to observe, as it may result in less emotional distress for family members.
COMMUNICATION AND DECISION MAKING
Surrogate Decision Makers Interpreted Prognostic Information Optimistically
Zier LS, Sottile PD, Hong SY, et al. Surrogate decision makers' interpretation of prognostic information: a mixed‐methods study. Ann Intern Med. 2012;156:360366.
Background
Surrogates of critically ill patients often have beliefs about prognosis that are discordant from what is told to them by providers. Little is known about why this is the case.
Findings
Eighty surrogates of patients in intensive care units (ICUs) were given questionnaires with hypothetical prognostic statements and asked to identify a survival probability associated with each statement on a 0% to 100% scale. Interviewers examined the questionnaires to identify responses that were not concordant with the given prognostic statements. They then interviewed participants to determine why the answers were discordant. The researchers found that surrogates were more likely to offer an overoptimistic interpretation of statements communicating a high risk of death, compared to statements communicating a low risk of death. The qualitative interviews revealed that surrogates felt they needed to express ongoing optimism and that patient factors not known to the medical team would lead to better outcomes.
Cautions
The participants were surrogates who were present in the ICU at the time when study investigators were there, and thus the results may not be generalizable to all surrogates. Only a subset of participants completed qualitative interviews. Prognostic statements were hypothetical. Written prognostic statements may be interpreted differently than spoken statements.
Implications
Surrogate decision makers may interpret prognostic statements optimistically, especially when a high risk of death is estimated. Inaccurate interpretation may be related to personal beliefs about the patients' strengths and a need to hold onto hope for a positive outcome. When communicating with surrogates of critically ill patients, providers should be aware that, beyond the actual information shared, many other factors influence surrogates' beliefs about prognosis.
A Majority of Patients With Metastatic Cancer Felt That Chemotherapy Might Cure Their Disease
Weeks JC, Catalano PJ, Chronin A, et al. Patients' expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:16161625.
Background
Chemotherapy for advanced cancer is not curative, and many cancer patients overestimate their prognosis. Little is known about patients' understanding of the goals of chemotherapy when cancer is advanced.
Findings
Participants were part of the Cancer Care Outcomes Research and Surveillance study. Patients with stage IV lung or colon cancer who opted to receive chemotherapy (n=1193) were asked how likely they thought it was that the chemotherapy would cure their cancer. A majority (69% of lung cancer patients and 81% of colon cancer patients) felt that chemotherapy might cure their disease. Those who rated their physicians very favorably in satisfaction surveys were more likely to feel that that chemotherapy might be curative, compared to those who rated their physician less favorably (OR: 1.90; 95% CI: 1.33‐2.72).
Cautions
The study did not include patients who died soon after diagnosis and thus does not provide information about those who opted for chemotherapy but did not survive to the interview. It is possible that responses were influenced by participants' need to express optimism (social desirability bias). It is not clear how or whether prognostic disclosure by physicians caused the lower satisfaction ratings.
Implications
Despite the fact that stage IV lung and colon cancer are not curable with chemotherapy, a majority of patients reported believing that chemotherapy might cure their disease. Hospital clinicians should be aware that many patients who they view as terminally ill believe their illness may be cured.
Older Patients Who Viewed a Goals‐of‐Care Video at Admission to a Skilled Nursing Facility Were More Likely to Prefer Comfort Care
Volandes AE, Brandeis GH, Davis AD, et al. A randomized controlled trial of a goals‐of‐care video for elderly patients admitted to skilled nursing facilities. J Palliat Med. 2012;15:805811.
Background
Seriously ill older patients are frequently discharged from hospitals to skilled nursing facilities (SNFs). It is important to clarify and document patients' goals for care at the time of admission to SNFs, to ensure that care provided there is consistent with patients' preferences. Previous work has shown promise using videos to assist patients in advance‐care planning, providing realistic and standardized portrayals of different treatment options.[9, 10]
Findings
English‐speaking patients at least 65 years of age who did not have altered mental status were randomized to hear a verbal description (n=51) or view a 6‐minute video (n=50) that presented the same information accompanied by pictures of patients of 3 possible goals of medical care: life‐prolonging care, limited medical care, and comfort care. After the video or narrative, patients were asked what their care preference would be if they became more ill while at the SNF. Patients who viewed the video were more likely to report a preference for comfort care, compared to patients who received the narrative, 80% vs 57%, P=0.02. In a review of medical records, only 31% of patients who reported a preference for comfort care had a do not resuscitate order at the SNF.
Cautions
The study was conducted at 2 nursing homes located in the Boston, Massachusetts area, which may limit generalizability. Assessors were not blinded to whether the patient saw the video or received the narrative, which may have introduced bias. The authors note that the video aimed to present the different care options without valuing one over the other, though it may have inadvertently presented one option in a more favorable light.
Implications
Videos may be powerful tools for helping nursing home patients to clarify goals of care, and might be applied in the hospital setting prior to transferring patients to nursing homes. There is a significant opportunity to improve concordance of care with preferences through better documentation and implementation of code status orders when transferring patients to SNFs.
Acknowledgments
Disclosures: Drs. Anderson and Johnson and Mr. Horton received an honorarium and support for travel to present findings resulting from the literature review at the Annual Assembly of the American Academy of Hospice and Palliative Medicine and Hospice and Palliative Nurses Association on March 16, 2013 in New Orleans, Louisiana. Dr. Anderson was funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI grant number KL2TR000143. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
APPENDIX
Journals That Were Hand Searched to Identify Articles, By Topic Area
General:
- British Medical Journal
- Journal of the American Medical Association
- Lancet
- New England Journal of Medicine
Internal medicine:
- Annals Internal Medicine
- Archives Internal Medicine
- Journal of General Internal Medicine
- Journal of Hospital Medicine
Palliative care and symptom management:
- Journal Pain and Symptom Management
- Journal of Palliative Care
- Journal of Palliative Medicine
- Palliative Medicine
- Pain
Oncology:
- Journal of Clinical Oncology
- Supportive Care in Cancer
Critical care:
- American Journal of Respiratory and Critical Care Medicine
- Critical Care Medicine
Pediatrics:
- Pediatrics
Geriatrics:
- Journal of the American Geriatrics Society
Education:
- Academic Medicine
Nursing:
- Journal of Hospice and Palliative Nursing
- Oncology Nursing Forum
- The Dartmouth Atlas of Health Care. Percent of Medicare decedents hospitalized at least once during the last six months of life 2007. Available at: http://www.dartmouthatlas.org/data/table.aspx?ind=133. Accessed October 30, 2013.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. End‐of‐life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst. 2011;103(11):853–862.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- Society of Hospital Medicine; 2008.The core competencies in hospital medicine. http://www.hospitalmedicine.org/Content/NavigationMenu/Education/CoreCurriculum/Core_Competencies.htm. Accessed October 30, 2013.
- , , , , . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline. Thorax. 2010;65:ii32–ii40.
- , . A comparison of sennosides‐based bowel protocols with and without docusate in hospitalized patients with cancer. J Palliat Med. 2008;11(4):575–581.
- , , , , , . Trends in survival after In‐hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention‐postintervention study. Cancer. 2012;118(17):4331–4338.
- The Dartmouth Atlas of Health Care. Percent of Medicare decedents hospitalized at least once during the last six months of life 2007. Available at: http://www.dartmouthatlas.org/data/table.aspx?ind=133. Accessed October 30, 2013.
- , , , et al. Change in end‐of‐life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477.
- , , , et al. End‐of‐life care for lung cancer patients in the United States and Ontario. J Natl Cancer Inst. 2011;103(11):853–862.
- , , , , . Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1(suppl 1):48–56.
- Society of Hospital Medicine; 2008.The core competencies in hospital medicine. http://www.hospitalmedicine.org/Content/NavigationMenu/Education/CoreCurriculum/Core_Competencies.htm. Accessed October 30, 2013.
- , , , , . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline. Thorax. 2010;65:ii32–ii40.
- , . A comparison of sennosides‐based bowel protocols with and without docusate in hospitalized patients with cancer. J Palliat Med. 2008;11(4):575–581.
- , , , , , . Trends in survival after In‐hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , et al. Augmenting advance care planning in poor prognosis cancer with a video decision aid: a preintervention‐postintervention study. Cancer. 2012;118(17):4331–4338.
Private practice remains strong despite an increase in hospital employment
Although more physicians today are employed by hospitals than in the past, the overwhelming majority of doctors still work in private practices, according to 2012 data from the Physician Practice Benchmark Study (PPBS) conducted by the American Medical Association (AMA).1
The survey shows that 53.2% of physicians were self-employed in 2012, and 60% were operating in practices wholly owned by physicians. Only 23% of physicians worked in practices that were partially or fully owned by a hospital, and only 5.6% were directly employed by a hospital.1
The AMA estimates that 18.4% of physicians worked in solo practices in 2012, a decline of about 6% from the previous AMA survey in 2207/2008.1 In 1983, 40.5% of physicians were in solo practice.1
“To paraphrase Mark Twain, the reports of the death of private practice medicine have been greatly exaggerated,” said AMA President Ardis Dee Hoven, MD, in presenting the figures.1
And AMA investigators Carol C. Kane, PhD, and David W. Emmons, PhD, who authored the report, noted: “After a 5-year gap in physician-level data, the 2012 PPBS offers an update on the status of physician practice arrangements, and allows for a nationally representative response to the numerous articles of the past several years that have highlighted a surge in the employment of physicians by hospitals and the ‘death’ of private practice.”1
Details of the survey
Like earlier AMA surveys, the PPBS involved a nationally representative random sample of physicians who had completed residency, practiced at least 20 hours per week, and were not employed by the federal government.
Unlike earlier AMA surveys, which targeted AMA members, the 2012 PPBS utilized the Epocrates Honors market research panel rather than the AMA Masterfile. The reason for this switch: declining participation rates for surveys utilizing the Masterfile.
Another distinction: Earlier surveys failed to ask specifically whether the respondent’s practice was owned by its physician members or by a larger entity, such as a hospital. They also overlooked the organizational structure of practices. The 2012 survey addressed both issues.
The PPBS went to 14,750 physicians. Of these, 3,466 physicians responded, a response rate of 28%.1
FINDINGS ON THE STRUCTURE OF PRACTICE
Ownership status
In 2012, 53.2% of physicians fully or partly owned their practice (a decline of 8.0% since 2007/2008), 41.8% were employed, and 5.0% were independent contractors.1
Younger physicians were less likely to own their practice than older physicians were. Among physicians under age 40, the ownership rate was 43.3%, compared with 60.0% among doctors aged 55 years or older.1
Women, too, were less likely to own their practice (38.7% vs 59.6% for men).1
Type of practice
The most common type of practice setting was the single-specialty practice, reported by 45.5% of physicians. Women were less likely to report single-specialty practice than men (39.7% vs 48.0%).1
Among ObGyns, single-specialty practice was reported by 52.7% of respondents.1
Multispecialty practice was reported by 22.1% of respondents. Among ObGyns, that figure was 17.9%.1
Solo practice was reported by 18.4% of respondents but varied significantly by age. Among physicians under age 40, only 10% reported solo practice, compared with 25.3% of physicians aged 55 or older. Among women, solo practice was reported by 21.0%, compared with 17.3% among men. Among all ObGyns (men and women), 20.6% reported solo practice.1
Only 5.6% of physicians reported direct hospital employment. Among ObGyns, the figure was 2.3%.1
Size of the practice
Sixty percent of respondents (in all practice settings) reported working in a practice with fewer than 10 physicians. Sixteen percent reported working in a practice with 10 to 24 physicians, 7.1% in practices with 25 to 49 physicians, and 12.2% in practices with more than 50 physicians. Hospital employees were not asked about the number of physicians in their practice setting.
Among physicians in single-specialty practices, 39.0% reported that their practice included no more than four physicians, compared with 5.3% who reported a practice of at least 50 physicians.1
Among physicians in multispecialty practice, only 9.9% reported having no more than four physicians, compared with 35.5% reporting at least 50 physicians.1
Hospital ownership
Twenty-three percent of all respondents reported working in a practice that was at least partially owned by a hospital. Of these physicians, 14.7% worked in practices fully owned by a hospital.
Physicians who worked in a single-specialty practice were more likely to report physician ownership of that practice (71.8%) than were doctors in multi-specialty practice (36.9%). And physicians in small practices (single- or multispecialty) were more likely to report physician ownership than physicians in large practices: 72% of physicians in groups of two to four reported physician ownership, compared with 45.6% of physicians in groups of 50 or more. Physicians in large practices (≥50 members) also were more likely to report ownership by a not-for-profit foundation.1
After exploring the issue of hospital ownership from several different angles, Kane and Emmons found that the association between increasing practice size and hospital ownership did not persist. Rather, they found that the “wider scope of practice in multispecialty groups, not practice size, drives hospital ownership.” They theorized that hospitals are more likely to buy primary care practices to gain a strong referral base, and this theory was borne out by the data, which showed that primary care physicians are more likely to report hospital ownership.1
RELATED ARTICLE: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Two final comments
Kane and Emmons point out that their analysis doesn’t “capture relationships that are short of full employment” and, therefore, may underestimate “the degree of integration between physicians and hospitals.”1
Although the decline in solo practice may have been accelerated by reform measures in recent years, the shift was “already well underway in the early 1990s,” Kane and Emmons observed.1
Reference
- Kane CK, Emmons DW. Policy Research Perspectives: New Data on Physician Practice Arrangements: Private Practice Remains Strong Despite Shifts Toward Hospital Employment. American Medical Association. September 2013. http://www.ama-assn.org/resources/doc/health-policy/prp-physician-practice-arrangements.pdf. Accessed October 25, 2013.
Although more physicians today are employed by hospitals than in the past, the overwhelming majority of doctors still work in private practices, according to 2012 data from the Physician Practice Benchmark Study (PPBS) conducted by the American Medical Association (AMA).1
The survey shows that 53.2% of physicians were self-employed in 2012, and 60% were operating in practices wholly owned by physicians. Only 23% of physicians worked in practices that were partially or fully owned by a hospital, and only 5.6% were directly employed by a hospital.1
The AMA estimates that 18.4% of physicians worked in solo practices in 2012, a decline of about 6% from the previous AMA survey in 2207/2008.1 In 1983, 40.5% of physicians were in solo practice.1
“To paraphrase Mark Twain, the reports of the death of private practice medicine have been greatly exaggerated,” said AMA President Ardis Dee Hoven, MD, in presenting the figures.1
And AMA investigators Carol C. Kane, PhD, and David W. Emmons, PhD, who authored the report, noted: “After a 5-year gap in physician-level data, the 2012 PPBS offers an update on the status of physician practice arrangements, and allows for a nationally representative response to the numerous articles of the past several years that have highlighted a surge in the employment of physicians by hospitals and the ‘death’ of private practice.”1
Details of the survey
Like earlier AMA surveys, the PPBS involved a nationally representative random sample of physicians who had completed residency, practiced at least 20 hours per week, and were not employed by the federal government.
Unlike earlier AMA surveys, which targeted AMA members, the 2012 PPBS utilized the Epocrates Honors market research panel rather than the AMA Masterfile. The reason for this switch: declining participation rates for surveys utilizing the Masterfile.
Another distinction: Earlier surveys failed to ask specifically whether the respondent’s practice was owned by its physician members or by a larger entity, such as a hospital. They also overlooked the organizational structure of practices. The 2012 survey addressed both issues.
The PPBS went to 14,750 physicians. Of these, 3,466 physicians responded, a response rate of 28%.1
FINDINGS ON THE STRUCTURE OF PRACTICE
Ownership status
In 2012, 53.2% of physicians fully or partly owned their practice (a decline of 8.0% since 2007/2008), 41.8% were employed, and 5.0% were independent contractors.1
Younger physicians were less likely to own their practice than older physicians were. Among physicians under age 40, the ownership rate was 43.3%, compared with 60.0% among doctors aged 55 years or older.1
Women, too, were less likely to own their practice (38.7% vs 59.6% for men).1
Type of practice
The most common type of practice setting was the single-specialty practice, reported by 45.5% of physicians. Women were less likely to report single-specialty practice than men (39.7% vs 48.0%).1
Among ObGyns, single-specialty practice was reported by 52.7% of respondents.1
Multispecialty practice was reported by 22.1% of respondents. Among ObGyns, that figure was 17.9%.1
Solo practice was reported by 18.4% of respondents but varied significantly by age. Among physicians under age 40, only 10% reported solo practice, compared with 25.3% of physicians aged 55 or older. Among women, solo practice was reported by 21.0%, compared with 17.3% among men. Among all ObGyns (men and women), 20.6% reported solo practice.1
Only 5.6% of physicians reported direct hospital employment. Among ObGyns, the figure was 2.3%.1
Size of the practice
Sixty percent of respondents (in all practice settings) reported working in a practice with fewer than 10 physicians. Sixteen percent reported working in a practice with 10 to 24 physicians, 7.1% in practices with 25 to 49 physicians, and 12.2% in practices with more than 50 physicians. Hospital employees were not asked about the number of physicians in their practice setting.
Among physicians in single-specialty practices, 39.0% reported that their practice included no more than four physicians, compared with 5.3% who reported a practice of at least 50 physicians.1
Among physicians in multispecialty practice, only 9.9% reported having no more than four physicians, compared with 35.5% reporting at least 50 physicians.1
Hospital ownership
Twenty-three percent of all respondents reported working in a practice that was at least partially owned by a hospital. Of these physicians, 14.7% worked in practices fully owned by a hospital.
Physicians who worked in a single-specialty practice were more likely to report physician ownership of that practice (71.8%) than were doctors in multi-specialty practice (36.9%). And physicians in small practices (single- or multispecialty) were more likely to report physician ownership than physicians in large practices: 72% of physicians in groups of two to four reported physician ownership, compared with 45.6% of physicians in groups of 50 or more. Physicians in large practices (≥50 members) also were more likely to report ownership by a not-for-profit foundation.1
After exploring the issue of hospital ownership from several different angles, Kane and Emmons found that the association between increasing practice size and hospital ownership did not persist. Rather, they found that the “wider scope of practice in multispecialty groups, not practice size, drives hospital ownership.” They theorized that hospitals are more likely to buy primary care practices to gain a strong referral base, and this theory was borne out by the data, which showed that primary care physicians are more likely to report hospital ownership.1
RELATED ARTICLE: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Two final comments
Kane and Emmons point out that their analysis doesn’t “capture relationships that are short of full employment” and, therefore, may underestimate “the degree of integration between physicians and hospitals.”1
Although the decline in solo practice may have been accelerated by reform measures in recent years, the shift was “already well underway in the early 1990s,” Kane and Emmons observed.1
Although more physicians today are employed by hospitals than in the past, the overwhelming majority of doctors still work in private practices, according to 2012 data from the Physician Practice Benchmark Study (PPBS) conducted by the American Medical Association (AMA).1
The survey shows that 53.2% of physicians were self-employed in 2012, and 60% were operating in practices wholly owned by physicians. Only 23% of physicians worked in practices that were partially or fully owned by a hospital, and only 5.6% were directly employed by a hospital.1
The AMA estimates that 18.4% of physicians worked in solo practices in 2012, a decline of about 6% from the previous AMA survey in 2207/2008.1 In 1983, 40.5% of physicians were in solo practice.1
“To paraphrase Mark Twain, the reports of the death of private practice medicine have been greatly exaggerated,” said AMA President Ardis Dee Hoven, MD, in presenting the figures.1
And AMA investigators Carol C. Kane, PhD, and David W. Emmons, PhD, who authored the report, noted: “After a 5-year gap in physician-level data, the 2012 PPBS offers an update on the status of physician practice arrangements, and allows for a nationally representative response to the numerous articles of the past several years that have highlighted a surge in the employment of physicians by hospitals and the ‘death’ of private practice.”1
Details of the survey
Like earlier AMA surveys, the PPBS involved a nationally representative random sample of physicians who had completed residency, practiced at least 20 hours per week, and were not employed by the federal government.
Unlike earlier AMA surveys, which targeted AMA members, the 2012 PPBS utilized the Epocrates Honors market research panel rather than the AMA Masterfile. The reason for this switch: declining participation rates for surveys utilizing the Masterfile.
Another distinction: Earlier surveys failed to ask specifically whether the respondent’s practice was owned by its physician members or by a larger entity, such as a hospital. They also overlooked the organizational structure of practices. The 2012 survey addressed both issues.
The PPBS went to 14,750 physicians. Of these, 3,466 physicians responded, a response rate of 28%.1
FINDINGS ON THE STRUCTURE OF PRACTICE
Ownership status
In 2012, 53.2% of physicians fully or partly owned their practice (a decline of 8.0% since 2007/2008), 41.8% were employed, and 5.0% were independent contractors.1
Younger physicians were less likely to own their practice than older physicians were. Among physicians under age 40, the ownership rate was 43.3%, compared with 60.0% among doctors aged 55 years or older.1
Women, too, were less likely to own their practice (38.7% vs 59.6% for men).1
Type of practice
The most common type of practice setting was the single-specialty practice, reported by 45.5% of physicians. Women were less likely to report single-specialty practice than men (39.7% vs 48.0%).1
Among ObGyns, single-specialty practice was reported by 52.7% of respondents.1
Multispecialty practice was reported by 22.1% of respondents. Among ObGyns, that figure was 17.9%.1
Solo practice was reported by 18.4% of respondents but varied significantly by age. Among physicians under age 40, only 10% reported solo practice, compared with 25.3% of physicians aged 55 or older. Among women, solo practice was reported by 21.0%, compared with 17.3% among men. Among all ObGyns (men and women), 20.6% reported solo practice.1
Only 5.6% of physicians reported direct hospital employment. Among ObGyns, the figure was 2.3%.1
Size of the practice
Sixty percent of respondents (in all practice settings) reported working in a practice with fewer than 10 physicians. Sixteen percent reported working in a practice with 10 to 24 physicians, 7.1% in practices with 25 to 49 physicians, and 12.2% in practices with more than 50 physicians. Hospital employees were not asked about the number of physicians in their practice setting.
Among physicians in single-specialty practices, 39.0% reported that their practice included no more than four physicians, compared with 5.3% who reported a practice of at least 50 physicians.1
Among physicians in multispecialty practice, only 9.9% reported having no more than four physicians, compared with 35.5% reporting at least 50 physicians.1
Hospital ownership
Twenty-three percent of all respondents reported working in a practice that was at least partially owned by a hospital. Of these physicians, 14.7% worked in practices fully owned by a hospital.
Physicians who worked in a single-specialty practice were more likely to report physician ownership of that practice (71.8%) than were doctors in multi-specialty practice (36.9%). And physicians in small practices (single- or multispecialty) were more likely to report physician ownership than physicians in large practices: 72% of physicians in groups of two to four reported physician ownership, compared with 45.6% of physicians in groups of 50 or more. Physicians in large practices (≥50 members) also were more likely to report ownership by a not-for-profit foundation.1
After exploring the issue of hospital ownership from several different angles, Kane and Emmons found that the association between increasing practice size and hospital ownership did not persist. Rather, they found that the “wider scope of practice in multispecialty groups, not practice size, drives hospital ownership.” They theorized that hospitals are more likely to buy primary care practices to gain a strong referral base, and this theory was borne out by the data, which showed that primary care physicians are more likely to report hospital ownership.1
RELATED ARTICLE: Is private ObGyn practice on its way out? Lucia DiVenere, MA (October 2011)
Two final comments
Kane and Emmons point out that their analysis doesn’t “capture relationships that are short of full employment” and, therefore, may underestimate “the degree of integration between physicians and hospitals.”1
Although the decline in solo practice may have been accelerated by reform measures in recent years, the shift was “already well underway in the early 1990s,” Kane and Emmons observed.1
Reference
- Kane CK, Emmons DW. Policy Research Perspectives: New Data on Physician Practice Arrangements: Private Practice Remains Strong Despite Shifts Toward Hospital Employment. American Medical Association. September 2013. http://www.ama-assn.org/resources/doc/health-policy/prp-physician-practice-arrangements.pdf. Accessed October 25, 2013.
Reference
- Kane CK, Emmons DW. Policy Research Perspectives: New Data on Physician Practice Arrangements: Private Practice Remains Strong Despite Shifts Toward Hospital Employment. American Medical Association. September 2013. http://www.ama-assn.org/resources/doc/health-policy/prp-physician-practice-arrangements.pdf. Accessed October 25, 2013.
Expanding medication options for pediatric ADHD
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Molly, age 9, is diagnosed with attention-deficit/hyperactivity disorder (ADHD) by her psychiatrist, who prescribes a long-acting methylphenidate formulation at 1 mg/kg. She tolerates the medication without side effects and shows significant improvement in her academic performance and on-task behavior in school. Molly takes methylphenidate before school at 7:00 am; this dose usually wears off at approximately 3:30 pm.
Molly and her parents are pleased with her response to methylphenidate, but report that she has difficulty getting ready for school because of distractibility. In the evenings Molly has trouble staying seated to do homework and often interrupts and argues with family members, but cannot tolerate afternoon dosing of immediate-release methylphenidate because of insomnia.
ADHD, the most common childhood neurobehavioral disorder, is characterized by difficulties with attention, impulse control, and modulating activity level. The pathophysiology of ADHD is thought to involve dysregulation of brain dopamine and norepinephrine systems.1 Managing ADHD includes pharmacotherapeutic and nonpharmacotherapeutic—ie, behavioral and psychoeducational—interventions.2,3
In this article, we provide an overview of the efficacy, side effects, and dosing for the 3 classes of ADHD medication—psychostimulants, atomoxetine, and α2 adrenergic agonists—including guidance on medication choice and combination treatment. We also discuss the effects of psychostimulants on tics, cardiovascular concerns, and substance abuse potential.
Psychostimulants
Methylphenidates and amphetamines are first-line agents for ADHD. Their primary mechanism of action involves blocking dopamine transporters, with additional effects including blockade of norepinephrine transporters, dampening action of monoamine oxidase (which slows dopamine and norepinephrine degradation), and enhanced release of dopamine into the synaptic space.1
Efficacy and response rates are similar for methylphenidate and amphetamine medications, although as many as 25% of patients may respond to only 1 agent.1 More than 90% of patients will have a positive response to one of the psychostimulants.1 The beneficial effects of psychostimulants on inattention, hyperactivity, and impulsivity are well documented.2Improvements in noncompliance, aggression, social interactions, and academic productivity also have been observed.4,5
Because of increased recognition of pervasive ADHD-related impairments, which can affect functioning in social, family, and extracurricular settings, practitioners have shifted to long-acting psychostimulants to reduce the need for in-school dosing, improve compliance, and obtain more after-school treatment effects. Long-acting formulations produce a slower rise and fall of psychostimulant levels in the brain, which may decrease side effects and potential for later drug abuse.6 See Table 12,7-9 and Table 22,7,9 for titration, dosing, and duration of action of psychostimulants.
The most common side effects of psychostimulants are appetite loss, abdominal pain, headaches, and sleep disturbances.2 Emotional symptoms—irritability and nervousness—may be observed with psychostimulant use, but these behaviors may improve, rather than become worse, with treatment.5 Methylphenidates and amphetamines share many of the same side effects,2 with many studies indicating no differences between their side-effect profiles.1 Other studies indicate that sleep and emotional side effects may be more prominent with amphetamines than methylphenidates,10 although response varies by individual.
There is little evidence that methylphenidate, low-dose amphetamine, or low-dose dextroamphetamine makes tics worse in most children who have them, although significant tic exacerbation has been observed with higher-dose dextroamphetamine.11,12 In patients with comorbid ADHD and tic disorders, a trial of psychostimulants with monitoring for worsening tics is appropriate.
Changes in heart rate and blood pressure generally are not clinically significant in patients taking psychostimulants (average increases: 1 or 2 beats per minute and 1 to 4 mm Hg for systolic and diastolic blood pressures).12 However, psychostimulants may be associated with more substantial increases in heart rate and blood pressure in a subset of individuals (5% to 15%).12 Large studies of children and adults in the general population have not found an association between psychostimulant use and severe cardiovascular events (sudden cardiac death, myocardial infarction, stroke).12-14 Because of reports of sudden cardiac death in children with underlying heart disease who take a psychostimulant,15 clinicians are advised to screen patients and consider an electrocardiogram or evaluation by a cardiologist before starting a psychostimulant in a patient who has a personal or family history of specific cardiovascular risk factors (see Perrin et al16 and Cortese et al12 for screening questions and conditions).
Modest reductions in height (1 or 2 cm after 3 years of psychostimulant treatment) appear to be dose-dependent, and are similar across the methylphenidate and amphetamine classes. Some studies have shown reversal of growth deficits after treatment is stopped treatment and no adverse effects on final adult height.12,17 More study is needed to clarify the effects of continuous psychostimulant treatment from childhood to adulthood on growth.
Studies have failed to show an increased risk of substance abuse in persons with ADHD who were treated with psychostimulants during childhood. Some studies document a lower rate of later substance abuse in youths who received ADHD medications, although other reports show no effect of psychostimulant treatment on subsequent substance use disorder risk.12 Be aware that psychostimulants can be misused (eg, to get “high,” for performance enhancement, to suppress appetite, etc.). Misuse of psychostimulants is most common with short-acting preparations, and generally more difficult with long-acting preparations because extracting the active ingredients for snorting is difficult.2,12 Monitor refill requests and patient behavior for signs of misuse, and be alert for signs of illegal drug use in the patient’s family.
Psychotic symptoms—including hallucinations, delusions, mania, and extreme agitation—with psychostimulant treatment are rare, occurring at a rate of 1.5%.12
Atomoxetine
Approved by the FDA in 2002 for ADHD, atomoxetine is effective and generally well tolerated, although it is not as effective as psychostimulants.2 Atomoxetine is a potent norepinephrine reuptake inhibitor18 that does not produce euphoria, does not have potential for abuse, and has not been linked to increased tic onset or severity.19 Atomoxetine treatment is associated with a lower rate of sleep initiation difficulty compared with psychostimulants.18 Some studies suggest that atomoxetine may have mild beneficial effects on anxiety disorders,18 making it a reasonable choice for patients with significant anxiety or insomnia during psychostimulant treatment. Table 12,7-9 and Table 32,7,9 include information on dosing and duration of action for atomoxetine.
Common side effects of atomoxetine include sedation and fatigue, upset stomach, nausea and vomiting, reduced appetite, headache, and irritability.18 Inform patients that atomoxetine carries an FDA black-box warning for suicide risk; a review of 14 studies showed suicidal ideation was more common with atomoxetine than placebo, although no suicides occurred in any trials.20
Hepatotoxicity is rare with atomoxetine.21 Although routine liver enzyme testing is not required, discontinue atomoxetine if jaundice develops or elevated levels of liver enzymes are noted. Other rare but potentially serious side effects include changes in heart rate (≥20 beats per min) or blood pressure that occur in 5% to 10% of patients taking atomoxetine.22 The risk of serious cardiovascular events and sudden cardiac death with atomoxetine is extremely low, but patients should be screened for a personal and family history of cardiovascular risk factors and, if any of these are present, evaluated further before starting atomoxetine. Routine heart rate and blood pressure monitoring is recommended for all patients.12-14,16
Last, atomoxetine has been linked to growth delays in the first 1 or 2 years of treatment, with a return to expected measurements after an average 2 or 3 years of treatment; persistent decreases in growth rate were observed in patients who were taller or heavier than average before treatment.23
α2 Adrenergic agonists
Guanfacine ER and clonidine ER, the extended release (ER) formulations of α2 adrenergic agonists, were FDA-approved for treating ADHD in 2009 and 2010, respectively. Short-acting guanfacine and clonidine also are used for treating ADHD.24 Their mechanism of action involves stimulation of the pre-synaptic and post-synapic α2 adrenergic receptors, which control the release of norepinephrine and the rate of cell firing.25 The α2 agonists are considered a second-line treatment for ADHD because their efficacy and response rate for core ADHD symptoms lags behind those of psychostimulants.25 In addition to treating core ADHD symptoms, guanfacine and clonidine are used to treat tics and oppositional/aggressive behavior comorbid with ADHD.24,26 Clonidine, which is more sedating than guanfacine, can be used to treat comorbid ADHD and sleep disorders.24 The α2 agonists do not produce euphoria and do not have drug abuse potential.2Table 12,7-9 and Table 32,7,9 provide guidelines for prescribing guanfacine ER and clonidine ER.
The most common adverse effect is drowsiness; other common side effects include dizziness, irritability, headache, and abdominal pain.24 Short-term studies of α2 agonist treatment of ADHD have shown small, non-clinically significant reductions in heart rate and blood pressure; α2 agonist-associated bradycardia, increased QT interval, and cardiac arrhythmias have been reported,7,24,27 as well as rebound hypertension with abrupt discontinuation.24 Screen patients for a personal and family history of cardiovascular risk factors and, if present, evaluate further before initiating α2 agonists.
Combining ADHD medication classes
Combination therapy with >1 ADHD medications is employed when 1 class does not provide adequate symptom coverage or produces problematic side effects.8,24 Psychostimulants can be combined with low-dose atomoxetine (0.5 to 1.0 mg/kg/d) when atomoxetine does not adequately cover ADHD symptoms in school, or when psychostimulants do not adequately cover evening symptoms or patients experience problems with evening psychostimulant rebound.8 To date, prospective data on the safety and efficacy of combining atomoxetine and psychostimulants are limited, but what evidence is available suggests improved symptom control for some, but not all, patients, and a lack of serious adverse events.28
Psychostimulants have been combined with α2agonists when children have an inadequate response to psychostimulants alone, or in cases of ADHD comorbid with aggression or tics.24 Although early case reports raised concern about the safety of combining psychostimulants and α2 agonists, subsequent studies suggest that clonidine and guanfacine generally are well-tolerated when co-administered with psychostimulants.24,27,29
Case continued
Molly has derived substantial benefit from long-acting methylphenidate during the school day, but continues to have significant ADHD-related impairment in the mornings and evenings. Her physician tried afternoon dosing of immediate-release methylphenidate to address evening difficulties, but Molly experienced insomnia. It would be reasonable to consider adjunctive therapy with a non-stimulant medication. A medication that can provide round-the-clock ADHD symptom coverage—such as atomoxetine, guanfacine ER, or clonidine ER—could be added to her current day-time psychostimulant treatment, potentially improving her functioning at home before school and in the evenings.
Additional considerations
Combining medication and behavior therapy offers greater improvements on academic, conduct, and family satisfaction measures than either treatment alone.2 Clinicians can choose to employ behavior therapy alone, particularly if parents feel uncomfortable with—or children have not tolerated—medication.2,3 Evidence-based behavioral parent training and classroom management strategies (implemented by teachers) have shown the strongest and most consistent effects among nonpharmacotherapeutic interventions for ADHD.2 Most studies comparing behavior therapy to psychostimulants have found a stronger effect on core ADHD symptoms from psychostimulants than from behavior therapy.
When a patient does not respond adequately to FDA-approved ADHD medications alone or in combination, consider bupropion, an antidepressant with indirect dopamine and noradrenergic effects. Off-label bupropion has been shown to be effective for ADHD in controlled trials of both children and adults.30
Clinicians often encounter children who meet criteria for ADHD and an anxiety or mood disorder. Table 48,31 summarizes treatment recommendations for these patients.
Clinical considerations
- Begin treatment with a psychostimulant at a low dosage, and titrate gradually until symptoms are controlled or side effects develop.
- Keep in mind that an effective dosage of a psychostimulant is not closely correlated with age, weight, or severity of symptoms.
- Monitor refill requests and patient behavior for signs of psychostimulant misuse. Be alert for signs of illegal drug use in patient family members.
- Lisdexamfetamine, dermal methylphenidate, and osmotic release oral system methylphenidate are the formulations least likely to be misused because their delivery systems make it difficult to extract the active ingredient for snorting or intravenous injection.
- Psychostimulants have not been shown to exacerbate tics in most children who have comorbid ADHD and a tic disorder. When a stimulant is associated with an exacerbation of tics, switching treatment to atomoxetine or α2 agonists is reasonable.
- For patients whose use of a stimulant is limited by an adverse effect on sleep, consider atomoxetine and α2 adrenergic agonists as alternative or adjunctive treatments.
- All 3 classes of FDA-approved ADHD medications (psychostimulants, atomoxetine, and adrenergic agonists) have been associated with adverse cardiac events in children who have underlying cardiovascular conditions. Before initiating treatment, screen patients for a personal or family history of cardiovascular risk factors, and undertake further evaluation as indicated.
Bottom Line
In general, the evidence supports psychostimulants as initial pharmacotherapy for ADHD, with additional options including atomoxetine and α2 agonists. When one medication class does not provide adequate coverage for ADHD symptoms, combining medication classes can be beneficial.
Related Resources
- National Institute of Mental Health. What is attention deficit hyperactivity disorder (ADHD, ADD)?” www.nimh.nih.gov/health/topics/attention-deficit-hyperactivity-disorder-adhd/index.shtml.
- National Resource Center on AD/HD. Managing medication for children and adolescents with ADHD. www.help4adhd.org/en/treatment/medication/WWK3.
Drug Brand Names
Atomoxetine • Strattera
Lisdexamfetamine • Vyvanse
Bupropion • Wellbutrin, Zyban
Clonidine extended release • Kapvay
Guanfacine extended release • Intuniv
Dexmethylphenidate • Focalin, Focalin XR
Mixed amphetamine salts • Adderall, Adderall XR
Dextroamphetamine • Dexedrine, Dexedrine SR, DextroStat, ProCentra
Methylphenidate • Ritalin, Methylin, Metadate CD, Metadate ER, Methylin ER, Ritalin LA, Ritalin SR, Concerta, Quillivant XR, Daytrana
Disclosures
Dr. Froehlich receives support from the National Institute of Mental Health Grant K23 MH083881. Dr. Delgado has received research support from Pfizer, Inc. Dr. Anixt reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
1. Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998; 94(1):127-152.
2. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management; Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
3. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
4. Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340(1):40-46.
5. Goldman LS, Genel M, Bezman RJ, et al. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279(14):1100-1107.
6. Swanson J, Gupta S, Lam A, et al. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry. 2003;60(2):204-211.
7. Vaughan B, Kratochvil CJ. Pharmacotherapy of pediatric attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2012;21(4):941-955.
8. Pliszka SR, Crismon ML, Hughes CW, et al; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(6):642-657.
9. Antshel KM, Hargrave TM, Simonescu M, et al. Advances in understanding and treating ADHD. BMC Med. 2011;9:72.
10. Efron D, Jarman F, Barker M. Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: a double-blind, crossover trial. Pediatrics. 1997;100(4):662-666.
11. Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. 2011(4):CD007990.
12. Cortese S, Holtmann M, Banaschewski T, et al. Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry. 2013; 54(3):227-246.
13. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
14. Martinez-Raga J, Knecht C, Szerman N, et al. Risk of serious cardiovascular problems with medications for attention-deficit hyperactivity disorder. CNS Drugs. 2013;27(1):15-30.
15. Vetter VL, Elia J, Erickson C, et al; American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee; American Heart Association Council on Cardiovascular Nursing. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
16. Perrin JM, Friedman RA, Knilans TK; Black Box Working Group; Section on Cardiology and Cardiac Surgery. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122(2):451-453.
17. Faraone SV, Biederman J, Morley CP, et al. Effect of stimulants on height and weight: a review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(9):994-1009.
18. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
19. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699-711.
20. Bangs ME, Tauscher-Wisniewski S, Polzer J, et al. Meta-analysis of suicide-related behavior events in patients treated with atomoxetine. J Am Acad Child Adolesc Psychiatry. 2008;47(2):209-218.
21. Bangs ME, Jin L, Zhang S, et al. Hepatic events associated with atomoxetine treatment for attention-deficit hyperactivity disorder. Drug Saf. 2008;31(4):345-354.
22. U.S. Food and Drug Administration. Strattera (atomoxetine hydrochloride) capsule. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm223889.htm. Published August 2013. Accessed October 31, 2013.
23. Spencer TJ, Kratochvil CJ, Sangal RB, et al. Effects of atomoxetine on growth in children with attention-deficit/hyperactivity disorder following up to five years of treatment. J Child Adolesc Psychopharmacol. 2007;17(5):689-700.
24. Connor DF. Other medications. In: Barkley RA, ed. Attention-deficit/hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:658-677.
25. May DE, Kratochvil CJ. Attention-deficit hyperactivity disorder: recent advances in paediatric pharmacotherapy. Drugs. 2010;70(1):15-40.
26. Connor DF, Findling RL, Kollins SH, et al. Effects of guanfacine extended release on oppositional symptoms in children aged 6-12 years with attention-deficit hyperactivity disorder and oppositional symptoms: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010; 24(9):755-768.
27. Croxtall JD. Clonidine extended-release: in attention-deficit hyperactivity disorder. Paediatr Drugs. 2011;13(5):329-336.
28. Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179-193.
29. Sallee FR. The role of alpha2-adrenergic agonists in attention-deficit/hyperactivity disorder. Postgrad Med. 2010;122(5):78-87.
30. Spencer TJ. Antidepressant and specific norepinephrine reuptake inhibitor treatments. In: Barkley RA, ed. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York, NY: The Guilford Press; 2006:648-657.
31. Singh MK, DelBello MP, Kowatch RA, et al. Co-occurrence of bipolar and attention-deficit hyperactivity disorders in children. Bipolar Disord. 2006;8(6):710-720.
Levomilnacipran for the treatment of major depressive disorder
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In July 2013, the FDA approved levomilnacipran for the treatment of major depressive disorder (MDD) in adults.1 It is available in a once-daily, extended-release formulation (Table 1).1 The drug is the fifth serotonin-norepinephrine reuptake inhibitor (SNRI) to be sold in the United States and the fourth to receive FDA approval for treating MDD.
Levomilnacipran is believed to be the more active enantiomer of milnacipran, which has been available in Europe for years and was approved by the FDA in 2009 for treating fibromyalgia. Efficacy of levomilnacipran for treating patients with MDD was established in three 8-week randomized controlled trials (RCTs).1
Clinical implications
Levomilnacipran is indicated for treating MDD in adults and is unique compared with other SNRIs because it is relatively more selective for norepinephrine reuptake inhibition (NRI) compared with serotonin reuptake inhibition (SRI).1 In vitro studies demonstrate that the drug has >10-fold greater selectivity for norepinephrine reuptake inhibition than it does for serotonin reuptake inhibition, compared with duloxetine or venlafaxine.2
This difference in selectivity could lend itself to treating symptoms of MDD that might be related to norepinephrine deficiency; these include decreased concentration, lassitude, mental and physical slowing, and decreased self-care.3,4 Some authors claim that individual patients could experience improvement in their social and occupational functioning in addition to improvement in the core symptoms of depression.5
Levomilnacipran is the more active enantiomer of milnacipran, an SNRI that is approved for treating fibromyalgia in the United States and approved for treating depression in many other countries.6 In general, enantiomeric formulations are believed to have advantages over racemic formulations because they are less complex and have a more selective pharmacodynamic profile, better therapeutic index, lower liability for drug-drug interactions (DDIs), and a less complicated relationship between plasma concentration and pharmacodynamic effect.6 In addition, regulatory guidelines in the United States recommend development of enantiomers over racemates where appropriate.7
How it works
Levomilnacipran’s exact mechanism of action is unknown. Similar to other SNRIs, it binds with high affinity to the serotonin (5-HT) and norepinephrine (NE) transporters and potently inhibits 5-HT and NE reuptake. Levomilnacipran lacks significant affinity for any other receptors, ion channels, or transporters tested in vitro.2 It differs from other SNRIs such as venlafaxine and duloxetine in having higher selectivity for norepinephrine vs serotonin reuptake inhibition. In vitro studies demonstrated a 2-fold preference for NE over 5-HT reuptake inhibition.2
Pharmacokinetics
Levomilnacipran reaches maximum plasma concentration within 6 to 8 hours of oral administration and has a half-life of approximately 12 hours, which makes it suitable for once-daily dosing. The concentration of levomilnacipran at steady state is proportional to the dosage of the drug when administered within the range of 25 to 300 mg once daily.1
The drug’s mean apparent total clearance is 21 to 29 liters/hour and its bioavailability is not significantly affected when taken with food. The drug is widely distributed in the body and is converted primarily to 2 metabolites: desethy levomilnacipran and p-hydroxy-levomilnacipran. Both metabolites are inactive and undergo further conjugation with glucuronide. The drug is eliminated primarily via renal excretion.1
The major enzyme that catalyzes metabolism of levomilnacipram is cytochrome P 450 (CYP) 3A4, which makes it susceptible to DDIs with drugs that inhibit or induce this enzyme. For example, a person taking levomilnacipran with a potent CYP3A4 inhibitor, such as ketoconazole, may require a dosage adjustment. No dosage adjustment is needed when given with a CYP3A4 inducer or substrate. Drinking alcohol with levomilnacipran may cause more rapid release of the drug into the blood stream.1
Efficacy
Levomilnacipran decreased core symptoms of MDD and showed a statistically significant separation from placebo in 2 phase III RCTs (Table 2).3,5 The first study was a 10-week flexible dose (75 or 100 mg) trial in 563 outpatients age 18 to 70 who met DSM-IV-TR criteria of MDD for >1 month and had a 17-item Hamilton Depression Rating Scale (HDRS-17) score >22 and a Sheehan Disability Scale (SDS) score >10.3 The primary efficacy measure was change in Montgomery-Åsberg Depression Rating Scale (MADRS) score from baseline to week 10. Secondary efficacy measures included the HDRS-17, SDS, and Clinical Global Impressions-improvement scale. Efficacy analyses included 276 subjects treated with levomilnacipran and 277 treated with placebo.3
Levomilnacipran was significantly superior to placebo on the MADRS and HDRS-17 from baseline to week 10. Response and remission rates were
significantly greater for the levomilnacipran group compared with placebo. Response exceeded the 10% average advantage for drug vs placebo and 46% of levomilnacipran-treated patients achieved remission.3
The number needed to treat (NNT), based on the MADRS scores for the levomilnacipran group compared with the placebo group, was 6 for response and 5 for remission.3 By comparison, most studies of venlafaxine demonstrate a NNT of 8.3
Levomilnacipran generally was reported to be safe and well tolerated. The most common adverse events leading to discontinuation in the levomilnacipran group were nausea, vomiting, change in systolic and diastolic blood pressure, and increase in heart rate. The favorable tolerability profile of levomilnacipran may relate to the 2-fold greater potency for NE reuptake inhibition compared with 5-HT reuptake inhibition.3
The second study was an 11-week, fixed-dose trial of levomilnacipran using 40, 80, or 120 mg. A total of 724 outpatients age 18 to 65 who met DSM-IV-TR criteria for MDD and who had an ongoing episode of depression lasting >8 weeks were randomly assigned to receive placebo (n = 179) or levomilnacipran at 40 mg (n = 181), 80 mg (n = 181), or 120 mg (n = 183) once daily for 8 weeks of double-blind treatment followed by a 2-week, double-blind taper of the drug.5 The primary efficacy parameter was change from baseline on the MADRS and the secondary efficacy parameter was change from baseline in SDS total score. HDRS-17, CGI-I, and CGI-S were included as secondary outcome measures.5
Significant difference in MADRS total score were seen in the levomilnacipran group compared with the placebo group (least mean squared difference: 40 mg/d, −3.23; 80 mg/d, −3.99; and 120 mg/d, −4.86). Higher dosages produced a numerically greater change and significant separation from placebo occurred sooner in the 80-mg and 120-mg groups compared with the 40-mg group.5
Significant differences vs placebo were consistently observed across secondary outcome measures for the higher dosages of levomilnacipran, and improvement in SDS total score was noted in all levomilnacipran groups compared with the placebo group. When dosed at 120 mg/d, levomilnacipran produced significant improvement vs placebo on all SDS subscales and was as well tolerated as the 80 mg dosage.5
No new safety concerns were observed in the study. A dose-response relationship in tolerability was not demonstrated and the number of patients reporting adverse events and who discontinued participation because of adverse events was higher in the 80-mg group than in the 40-mg and 120-mg groups.5
Tolerability
Overall, levomilnacipran was well tolerated in clinical trials, during which 2,673 subjects were exposed to the drug—translating to 942 patient-years of exposure. These patients ranged in age from 18 to 78; 825 of these subjects were enrolled in long-term studies for 1 year. Dosing of levomilnacipran during these studies ranged from 40 to 120 mg once daily, without regard to food.1
Nine percent of patients who received levomilnacipran in short-term studies discontinued because of adverse events, compared with 3% of patients who received placebo. The most common adverse event reported was nausea; other common adverse events reported included constipation, hyperhidrosis, elevated heart rate, erectile dysfunction, tachycardia, palpitations, and vomiting. Of these events, only erectile dysfunction and urinary hesitation were dose-related.1 Among levomilnacipran-treated female patients, <2% reported adverse events related to sexual dysfunction.
All SNRIs have well established associations with elevation in blood pressure and heart rate. Levomilnacipran resulted in a mean increase of 3 mm HG in systolic and 3.2 mm Hg in diastolic blood pressure in short-term, placebo-controlled trials.1
Orthostatic hypotension was observed in 11.6% of patients in the levomilnacipran groups, compared with 9.7% in placebo groups in all short-term studies. Orthostatic reductions of blood pressure occurred in 5.8%, 6.1%, and 9.8% of levomilnacipran-treated patients with dosages of 40, 80, and 120 mg/d respectively, indicating a dose-dependent adverse event. A mean increase in heart rate of 7.2 beats per minute (bpm) also was seen in short-term studies in the levomilnacipran-treated group compared with 0.3 bpm in the placebo-treated group.1 Clinicians should monitor blood pressure and heart rate routinely because of potential increases seen in some subjects in these studies, which excluded those who had significant cardiovascular disease.
Unique clinical issues
Both in-vitro and in-vivo studies found that levomilnacipran exhibited more potency for NE reuptake inhibition than for 5-HT reuptake inhibition at the lowest effective dosage (10 mg/kg). However as the dosage was increased (20 mg/kg and 40 mg/kg), it was equally potent at NE and 5-HT reuptake inhibition. This is in contrast to venlafaxine, which demonstrates a similar, but opposite, effect in terms of potentiation at the 5-HT and NE reuptake pumps.2
The greater noradrenergic effect of levomilnacipran could lend itself to treating certain subgroups of patients whose symptoms are believed to be related to deficiencies in NE (eg, lassitude).4 This concept is theoretical, and was not explicitly studied in clinical trials and the drug is not labeled in this way.
Contraindications
Contraindications to levomilnacipran are similar to those seen with SSRIs and SNRIs, including concomitant use of a monoamine oxidase inhibitor (MAOI) and the use of the levomilnacipran within 14 days of stopping an MAOI. Contraindications unique to levomilnacipran include hypersensitivity to levomilnacipran, milnacipran, or any component specific to the formulation; and uncontrolled narrow-angle glaucoma.1
Dosing
The recommended dosage range of levomilnacipran is 40 to 120 mg once
daily with or without food. The capsules should be swallowed whole and should not be opened or crushed. As with most psychotropics, levomilnacipran should be taken at approximately the same time each day.1
The manufacturer recommends an initial dose of levomilnacipran of 20 mg once daily for 2 days, increased to 40 mg once daily. Based on efficacy and tolerably, levomilnacipran can be increased in increments of 40 mg every 2 days.
Dosage adjustment is recommended for patients with moderate or severe renal impairment; and the maintenance dosage should not exceed 80 mg and 40 mg respectively in these populations. As with many antidepressants, gradual dosage reduction is recommended to avoid discontinuation symptoms.
Bottom Line
Levomilnacipran is FDA-approved for treating major depressive disorder in adults. In 2 randomized controlled trials, the drug showed a significant separation from placebo. Levomilnacipran generally was reported to be safe and well tolerated; common adverse events were nausea, vomiting, changes in blood pressure, and an increase in heart rate.
Related Resources
- Citrome L. Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? [published online September 8, 2013]. Int J Clin Pract. doi: 10.1111/ijcp.12298.
- Mago R, Forero G, Greenberg WM, et al. Safety and tolerability of levomilnacipran ER in major depressive disorder: results from an open-label, 48-week extension study. Clin Drug Investig. 2013;33(10):761-771.
Drug Brand Names
Duloxetine • Cymbalta Milnacipran • Savella
Ketoconazole • Nizoral Venlafaxine • Effexor Levomilnacipran • Fetzima
Disclosures
Dr. Macaluso has been the principal investigator for clinical trials for AbbVie, Eisai, Envivo, Janssen, Naurex, and Pfizer. All clinical trial and study contracts and payments were made through the Kansas University Medical Center Research Institute. Drs. Kazanchi and Malhotra report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
1. Fetzima [package insert]. St. Louis, MO: Forest Laboratories; 2013.
2. Auclair AL, Martel JC, Assié MB, et al. Levomilnacipran (F2695), a norepinephrine-preferring SNRI: profile in vitro and in models of depression and anxiety. Neuropharmacology. 2013;70:338-347.
3. Montgomery SA, Mansuy L, Ruth A, et al. Efficacy and Safety of levomilnacipran sustained release in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2013;74(4):363-369.
4. Kasper S, Meshkat D, Kutzelnigg A. Improvement of the noradrenergic symptom cluster following treatment with milnacipran. Neuropsychiatric Dis Treat. 2011; 7(suppl 1):21-27.
5. Asnis GM, Bose A, Gommoll CP, et al. Efficacy and safety of levomilnacipran sustained release 40 mg, 80 mg, or 120 mg in major depressive disorder: a phase 3, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(3):242-248.
6. Hutt AJ, Vanetová J. The chiral switch: the development of single enantiomer drugs from racemates. Acta Facultatis Pharmaceuticae Universitatis Comenianae. 2003; 50(7):23.
7. U.S. Food and Drug Administration. Development of new stereoisomeric drugs. Published May 1, 1992. http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm#.UKHEWm4ZyYE.email. Accessed October 8, 2013.
Out of the cupboard and into the clinic: Nutmeg-induced mood disorder
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Clinicians often are unaware of a patient’s misuse or abuse of easily accessible substances such as spices, herbs, and natural supplements. This can lead to misdiagnosed severe psychiatric disorders and, more alarmingly, unnecessary use of long-term psychotropics and psychiatric services.
Excessive ingestion of nutmeg (Myristica fragrans) can produce psychiatric symptoms because it contains myristicin, a psychoactive substance, in its aromatic oil.1 It is structurally similar to other hallucinogenic compounds such as mescaline. The effects of nutmeg could be attributed to metabolic formation of amphetamine derivatives from its core ingredients: elemicin, myristicin, and safrole.1-3 However, neither amphetamine derivatives nor core ingredients are detected in the urine of patients suspected of abusing nutmeg, which makes diagnosis challenging.
We present a case of nutmeg abuse leading to psychotic depression.
Nutmeg and depression
Mr. D, age 50, is admitted to our inpatient psychiatric unit with severe dysphoria, hopelessness, persecutory delusions, suicidal ideation, and a sense of impending doom for the third time in 2 years. At previous admissions, he was diagnosed with bipolar disorder.
During his first admission, Mr. D reported an intentional overdose by water intoxication; laboratory studies revealed hyponatremia, liver dysfunction, abnormal cardiac markers, increased creatine kinase, and leukocytosis with neutrophilia. With supportive treatment, all parameters returned to the normal range within 7 days.
At his third admission, Mr. D describes an extensive history of nutmeg abuse. He reports achieving desirable psychoactive effects such as excitement, euphoria, enhanced sensory perceptions, and racing thoughts within a half hour of ingesting 1 teaspoon (5 g) of nutmeg; effects lasted for 6 hours. He reports that consuming 2 teaspoons (10 g) produced a “stronger” effect, and that 1 tablespoon (15 g) was associated with severe dysphoria, fear, psychosis, suicidal ideation, and behavior, which led to his psychiatric admissions. He denies any other substance use.
Urine drug screen and other routine laboratory investigations are negative. Symptoms resolve spontaneously within 3 days and he is managed without pharmacotherapy. The diagnosis is revised to substance-induced mood disorder with psychotic features. We provide psychoeducation about nutmeg’s psychoactive effects, and Mr. D is motivated to stop abusing nutmeg. Three years later he remains in good health.
Effects of nutmeg
Acute nutmeg intoxication produces anxiety, fear, and hallucinations, and generally is self-limited, with most symptoms resolving within 24 hours.2 Chronic effects of nutmeg abuse resemble those of marijuana abuse (Table 1). Acute and long-term physical effects are listed in Table 2.
Be alert for presentations of a ‘natural high’
Nutmeg, other spices, and herbs can be used by persons looking for a ”natural high”; as we saw with Mr. D, nutmeg abuse can present as a mood disorder resembling bipolar disorder. An acute, atypical presentation of mood changes or suicidal ideation should prompt you to investigate causes other than primary mood or psychotic disorders, and should include consideration of the effects of atypical drugs—and spices—of abuse.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.
1. Weiss G. Hallucinogenic and narcotic-like effects of powdered Myristica (nutmeg). Psychiatr Q. 1960;34:346-356.
2. McKenna A, Nordt SP, Ryan J. Acute nutmeg poisoning. Eur J Emerg Med. 2004;11(4):240-241.
3. Brenner N, Frank OS, Knight E. Chronic nutmeg psychosis. J R Soc Med. 1993;86(3):179-180.