User login
Endometriosis and Pain: Expert Answers to 6 Questions Targeting Your Management Options
IN THIS ARTICLE
• When is laparoscopy indicated?
• Excision versus ablation
• How to reduce the risk for postoperative recurrence
Endometriosis has always posed a treatment challenge. In the early 19th century, before the widespread advent of surgery, the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
In the 21st century, the picture is clearer, though still not crystal clear. The optimal approach to endometriosis depends on many factors, foremost the patient’s chief complaint: pain or infertility (or both).
This article focuses on medical and surgical management of pain. Six experts address such questions as: When is laparoscopy indicated? Is excision or ablation of lesions preferred? What is the role of hysterectomy in eliminating pain? And what can be done about the problem of recurrence?
1. WHAT ARE THE OPTIONS FOR EMPIRIC THERAPY?
One reason for the diagnostic delay with endometriosis, which still averages about six years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. (Editor’s Note: See below for biographical information on each clinician interviewed in this article.)
Medical and hormonal options include
• NSAIDs, often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
• Cyclic combined OCs “are recommended as firstline therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of three to six months of cyclic OCs, consider switching to continuous low-dose combined OCs for an additional six months,” Dr. Lue adds. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P < .001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within six months and by 75% at two years” (P < .001).2
• Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
• Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
• Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
• Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
• Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches, such as physical therapy, may be beneficial to treat myofascial dysfunction and sensitization. “Chronic pain conditions that overlap with endometriosis-associated pain—such as migraines, irritable bowel syndrome, or painful bladder syndrome—should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Continue on to find out when laparoscopy is indicated >>
2. WHEN IS LAPAROSCOPY INDICATED?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.”
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy: “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone adds. “Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting one year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes sought.”
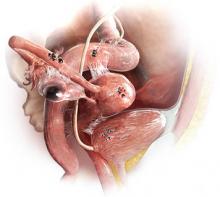
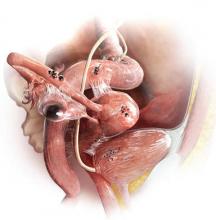
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
Continue to find out how disease should be staged >>
3. HOW SHOULD DISEASE BE STAGED?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton. The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into four stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, visit www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
Continue to learn whether excision or ablation is preferable >>
4. WHICH IS PREFERABLE: EXCISION OR ABLATION?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at three, six, nine, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at five years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at five years.12
In an accompanying editorial, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13 “When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation. In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease, you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone adds. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk for recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well, and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatologic skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way—and regardless of the primary indication for surgery (pain versus infertility)—a minimally invasive gynecologic surgeon is expected to have the ability to perform both techniques, Dr. Nezhat says.
Continue to find out if hysterectomy is definitive treatment >>
5. IS HYSTERECTOMY DEFINITIVE TREATMENT?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a seven-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if not. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at two, five, and seven years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women younger than 40, we recommend keeping normal ovaries if all disease is removed.”17
Continue on to find out if the risk for postoperative recurrence can be reduced >>
6. CAN THE RISK FOR POSTOPERATIVE RECURRENCE BE REDUCED?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at seven years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way, and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Adenomyosis is another reason pain may persist or recur after surgery.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2-weighted MRI,” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI.”
The frequent recurrence of pain after surgery makes the disease a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO), typically results in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
• No hormonal therapy
• Estrogen-progestin contraceptives, either cyclic or continuous
• The LNG-IUS
• Norethindrone acetate (5 mg/d)
• DMPA (150 mg every three months)
• Leuprolide acetate depot (3.75 mg IM monthly)
• Nafarelin nasal spray (200 µg bid)
• Danazol (200 mg bid).
“I explain the common adverse effects with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20-22
In addition, he notes, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk for recurrent pain and repetitive surgical procedures in the future.”23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are two major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
REFERENCES
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1-S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389-2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370-1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, et al. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373-393, 396-406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268-279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
9. Hsu AL, Sinaii N, Segars J, et al. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223-230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536-2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double blinded trial after 5-year follow-up. JMIG. 2014;21(6):999-1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212-2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396-1400.
17. Shakiba K, Bena JF, McGill KM, et al. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1-3):255-264.
19. Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711-715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729-2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860-864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927-935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
IN THIS ARTICLE
• When is laparoscopy indicated?
• Excision versus ablation
• How to reduce the risk for postoperative recurrence
Endometriosis has always posed a treatment challenge. In the early 19th century, before the widespread advent of surgery, the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
In the 21st century, the picture is clearer, though still not crystal clear. The optimal approach to endometriosis depends on many factors, foremost the patient’s chief complaint: pain or infertility (or both).
This article focuses on medical and surgical management of pain. Six experts address such questions as: When is laparoscopy indicated? Is excision or ablation of lesions preferred? What is the role of hysterectomy in eliminating pain? And what can be done about the problem of recurrence?
1. WHAT ARE THE OPTIONS FOR EMPIRIC THERAPY?
One reason for the diagnostic delay with endometriosis, which still averages about six years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. (Editor’s Note: See below for biographical information on each clinician interviewed in this article.)
Medical and hormonal options include
• NSAIDs, often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
• Cyclic combined OCs “are recommended as firstline therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of three to six months of cyclic OCs, consider switching to continuous low-dose combined OCs for an additional six months,” Dr. Lue adds. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P < .001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within six months and by 75% at two years” (P < .001).2
• Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
• Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
• Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
• Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
• Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches, such as physical therapy, may be beneficial to treat myofascial dysfunction and sensitization. “Chronic pain conditions that overlap with endometriosis-associated pain—such as migraines, irritable bowel syndrome, or painful bladder syndrome—should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Continue on to find out when laparoscopy is indicated >>
2. WHEN IS LAPAROSCOPY INDICATED?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.”
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy: “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone adds. “Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting one year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes sought.”
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
Continue to find out how disease should be staged >>
3. HOW SHOULD DISEASE BE STAGED?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton. The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into four stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, visit www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
Continue to learn whether excision or ablation is preferable >>
4. WHICH IS PREFERABLE: EXCISION OR ABLATION?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at three, six, nine, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at five years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at five years.12
In an accompanying editorial, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13 “When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation. In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease, you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone adds. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk for recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well, and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatologic skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way—and regardless of the primary indication for surgery (pain versus infertility)—a minimally invasive gynecologic surgeon is expected to have the ability to perform both techniques, Dr. Nezhat says.
Continue to find out if hysterectomy is definitive treatment >>
5. IS HYSTERECTOMY DEFINITIVE TREATMENT?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a seven-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if not. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at two, five, and seven years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women younger than 40, we recommend keeping normal ovaries if all disease is removed.”17
Continue on to find out if the risk for postoperative recurrence can be reduced >>
6. CAN THE RISK FOR POSTOPERATIVE RECURRENCE BE REDUCED?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at seven years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way, and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Adenomyosis is another reason pain may persist or recur after surgery.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2-weighted MRI,” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI.”
The frequent recurrence of pain after surgery makes the disease a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO), typically results in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
• No hormonal therapy
• Estrogen-progestin contraceptives, either cyclic or continuous
• The LNG-IUS
• Norethindrone acetate (5 mg/d)
• DMPA (150 mg every three months)
• Leuprolide acetate depot (3.75 mg IM monthly)
• Nafarelin nasal spray (200 µg bid)
• Danazol (200 mg bid).
“I explain the common adverse effects with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20-22
In addition, he notes, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk for recurrent pain and repetitive surgical procedures in the future.”23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are two major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
REFERENCES
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1-S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389-2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370-1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, et al. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373-393, 396-406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268-279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
9. Hsu AL, Sinaii N, Segars J, et al. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223-230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536-2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double blinded trial after 5-year follow-up. JMIG. 2014;21(6):999-1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212-2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396-1400.
17. Shakiba K, Bena JF, McGill KM, et al. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1-3):255-264.
19. Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711-715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729-2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860-864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927-935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
IN THIS ARTICLE
• When is laparoscopy indicated?
• Excision versus ablation
• How to reduce the risk for postoperative recurrence
Endometriosis has always posed a treatment challenge. In the early 19th century, before the widespread advent of surgery, the disease was managed by applying leeches to the cervix. In fact, as Nezhat and colleagues note in their comprehensive survey of the 4,000-year history of endometriosis, “leeches were considered a mainstay in treating any condition associated with menstruation.”1
In the 21st century, the picture is clearer, though still not crystal clear. The optimal approach to endometriosis depends on many factors, foremost the patient’s chief complaint: pain or infertility (or both).
This article focuses on medical and surgical management of pain. Six experts address such questions as: When is laparoscopy indicated? Is excision or ablation of lesions preferred? What is the role of hysterectomy in eliminating pain? And what can be done about the problem of recurrence?
1. WHAT ARE THE OPTIONS FOR EMPIRIC THERAPY?
One reason for the diagnostic delay with endometriosis, which still averages about six years, is that definitive diagnosis is achieved only through laparoscopic investigation and histologic confirmation. For many women who experience pain thought to be associated with endometriosis, however, clinicians begin empiric treatment with medical agents as a way to avert the need for surgery, if at all possible.
“There is no cure for endometriosis,” says John R. Lue, MD, MPH, “but there are many ways that endometriosis can be treated” and the impact of the disease reduced in a patient’s life. (Editor’s Note: See below for biographical information on each clinician interviewed in this article.)
Medical and hormonal options include
• NSAIDs, often used with combined oral contraceptives (OCs). NSAIDs are not a long-term treatment option because of their effect on cyclo-oxygenase (COX) 1 and 2 enzymes, says Dr. Lue. COX-1 protects the gastrointestinal (GI) system, and prolonged use of NSAIDs can cause adverse GI effects.
• Cyclic combined OCs “are recommended as firstline therapy in the absence of contraindications,” says Dr. Lue, and are often used in combination with NSAIDs. However, the failure rate may be as high as 20% to 25%.2 “If pain persists after a trial of three to six months of cyclic OCs, consider switching to continuous low-dose combined OCs for an additional six months,” Dr. Lue adds. When combined OCs were compared with placebo in the treatment of dysmenorrhea, they reduced baseline pain scores by 45% to 52%, compared with 14% to 17% for placebo (P < .001).2 They also reduced the volume of endometriomas by 48%, compared with 32% for placebo (P = .04). According to Linda C. Giudice, MD, PhD, “In women with severe dysmenorrhea who have been treated with cyclic combined OCs, a switch to continuous combined OCs reduced pain scores by 58% within six months and by 75% at two years” (P < .001).2
• Depot medroxyprogesterone acetate (DMPA) or the levonorgestrel-releasing intrauterine system (LNG-IUS). These agents suppress the hypothalamic-pituitary-ovarian (HPO) axis to different degrees. DMPA suppresses the HPO completely, preventing ovulation. The LNG-IUS does not fully suppress the HPO but acts directly on endometrial tissue, with antiproliferative effects on eutopic and endometriotic implants, says Dr. Lue. The LNG-IUS also is effective at suppressing disease after surgical treatment, says Dr. Giudice.2
• Gonadotropin-releasing hormone (GnRH) agonist therapy, with estrogen and/or progestin add-back therapy to temper the associated loss in bone mineral density, “may be effective—if only temporarily—as it inhibits the HPO axis and blocks ovarian function, thereby greatly reducing systemic estrogen levels and inducing artificial menopause,” says Dr. Lue.
• Norethindrone acetate, a synthetic progestational agent, is occasionally used as empiric therapy for endometriosis because of its ability to inhibit ovulation. It has antiandrogenic and antiestrogenic effects.
• Aromatase inhibitors. Dr. Lue points to considerable evidence that endometriotic implants are an autocrine source of estrogen.3 “This locally produced estrogen results from overexpression of the enzyme P450 aromatase by endometriotic tissue,” he says. Consequently, in postmenopausal women, “aromatase inhibitors may be used orally in a daily pill form to curtail endometriotic implant production of estrogen and subsequent implant growth.”4 In women of reproductive age, aromatase inhibitors are combined with an HPO-suppressive therapy, such as norethindrone acetate. These strategies represent off-label use of aromatase inhibitors.
• Danazol, a synthetic androgen, has been used in the past to treat dysmenorrhea and dyspareunia. Because of its severe androgenic effects, however, it is not widely used today.
“For those using medical approaches, endometriosis-related pain may be reduced by using hormonal treatments to modify reproductive tract events, thereby decreasing local peritoneal inflammation and cytokine production,” says Pamela Stratton, MD. Because endometriosis is a “central sensitivity syndrome,” multidisciplinary approaches, such as physical therapy, may be beneficial to treat myofascial dysfunction and sensitization. “Chronic pain conditions that overlap with endometriosis-associated pain—such as migraines, irritable bowel syndrome, or painful bladder syndrome—should be identified and treated. Mood changes of depression and anxiety common to women with endometriosis-associated pain also warrant treatment,” she says.
Continue on to find out when laparoscopy is indicated >>
2. WHEN IS LAPAROSCOPY INDICATED?
When medical and hormonal treatments fail to control a patient’s pain, laparoscopy is indicated to confirm the diagnosis of endometriosis. During that procedure, it is also advisable to treat any endometriosis that is present, provided the surgeon is highly experienced in such treatment.
Proper treatment is preferable—even if it requires expert consultation. “No treatment and referral to a more experienced surgeon are better than incomplete treatment by an inexperienced surgeon,” says Ceana Nezhat, MD. “Not all GYN surgeons have the expertise to treat advanced endometriosis.”
Dr. Stratton agrees about the importance of thorough treatment of endometriosis at the time of diagnostic laparoscopy: “At the laparoscopy, the patient benefits if all potential sources of pain are investigated and addressed.” At surgery, the surgeon should look for and treat any lesions suspicious for endometriosis, as well as any other finding that might contribute to pain, she says. “For example, routinely inspecting the appendix for endometriosis or other lesions, and removing affected appendices is reasonable; also, lysis and, where possible, excision of adhesions is an important strategy.”
If a medical approach fails for a patient, “then surgery is indicated to confirm the diagnosis and treat the disease,” agrees Tommaso Falcone, MD.
“Surgery is very effective in treating the pain associated with endometriosis,” Dr. Falcone adds. “Randomized clinical trials have shown that up to 90% of patients who obtain pain relief from surgery will have an effect lasting one year.6 If patients do not get relief, then the association of the pain with endometriosis should be questioned and other causes sought.”
The most common anatomic sites of implants
“The most common accepted theory for pathogenesis of endometriosis suggests that implants develop when debris from retrograde menstruation attaches to the pelvic peritoneum,” says Dr. Stratton.7 “Thus, the vast majority of lesions occur in the dependent portions of the pelvis, which include the ovarian fossae (posterior broad ligament under the ovaries), cul de sac, and the uterosacral ligaments.8 The bladder peritoneum, ovarian surface, uterine peritoneal surface, fallopian tube, and pelvic sidewall are also frequent sites. The colon and appendix are less common sites, and small bowel lesions are rare.”
“However, pain location does not correlate with lesion location,” Dr. Stratton notes. “For this reason, the goal at surgery is to treat all lesions, even ones that are not in sites of pain.”
Continue to find out how disease should be staged >>
3. HOW SHOULD DISEASE BE STAGED?
Most surgeons with expertise in treating endometriosis attempt to stage the disease at the time of initial laparoscopy, even though a patient’s pain does not always correlate with the stage of disease.
“The staging system for endometriosis is a means to systematically catalogue where lesions are located,” says Dr. Stratton. The most commonly used classification system was developed by the American Society for Reproductive Medicine (ASRM). It takes into account such characteristics as how deep an implant lies, the extent to which it obliterates the posterior cul de sac, and the presence and extent of adhesions. Although the classification system is broken down into four stages ranging from minimal to severe disease, it is fairly complex. For example, it assigns a score for each lesion as well as the size and location of that lesion, notes Dr. Stratton. The presence of an endometrioma automatically renders the disease as stage III or IV, and an obliterated cul de sac means the endometriosis is graded as stage IV.
“This system enables us to communicate with each other about patients and may guide future surgeries for assessment of lesion recurrence or the planning of treatment for lesions the surgeon was unable to treat at an initial surgery,” says Dr. Stratton.
“Women with uterosacral nodularity, fixed pelvic organs, or severe pain with endometriomas may have deep infiltrating lesions. These lesions, in particular, are not captured well with the current staging system,” says Dr. Stratton. Because they appear to be innervated, “the greatest benefit to the patient is achieved by completely excising these lesions.” Preoperative imaging may help confirm the existence, location, and extent of these deep lesions and help the surgeon plan her approach “based on clinical and imaging findings.”
“Severity of pain or duration of surgical effect does not correlate with stage or extent of disease,” Dr. Stratton says.9 “In fact, patients with the least amount of disease noted at surgery experience pain sooner, suggesting that the central nervous system may have been remodeled prior to surgery or that the pain is in part due to some other cause.10 This observation underscores the principle that, while endometriosis may initiate pain, the pain experience is determined by engagement of the central nervous system.”
For more information on the ASRM revised classification of endometriosis, visit www.fertstert.org/article/S0015-0282(97)81391-X/pdf.
Continue to learn whether excision or ablation is preferable >>
4. WHICH IS PREFERABLE: EXCISION OR ABLATION?
In a prospective, randomized, double-blind study, Healey and colleagues compared pain levels following laparoscopic treatment of endometriosis with either excision or ablation. Preoperatively, women in the study completed a questionnaire rating various types of pain using visual analogue scales. They then were randomly assigned to treatment of endometriosis via excision or ablation. Postoperatively, they again completed a questionnaire about pain levels at three, six, nine, and 12 months. Investigators found no significant difference in pain scores at 12 months.11
Five-year follow-up of the same population yielded slightly different findings, however. Although there was a reduction in all pain scores at five years in both the excision and ablation groups, a significantly greater reduction in dyspareunia was observed in the excision group at five years.12
In an accompanying editorial, Dr. Falcone and a coauthor called excision versus ablation of ovarian, bowel, and peritoneal endometriosis one of the “great debates” in the surgical management of endometriosis.13 “When there is deep involvement of adjacent organs, there is general consensus that excision is best for optimal surgical outcome,” they write. “However, for disease involving the peritoneum alone, there are proponents for either option.”13
“This is a very controversial issue,” says Dr. Falcone, “and the debate can sometimes be somewhat inflammatory…. It is hard to understand how a comparative trial could even be accomplished between excision and ablation. In my experience, deep disease typically occurs on the pelvic sidewall over the ureter or in the cul de sac on the bowel or infiltrating the bladder peritoneum. Therefore, ablation would increase the risk of damaging any of these structures. With superficial disease away from critical structures, it should be fine to ablate. Everywhere else and with deep disease, you need to excise or leave disease behind.”
“Endometriomas are a special situation,” Dr. Falcone adds. “Excision of the cyst has been shown in randomized controlled trials (RCTs) to be associated with less risk for recurrence.14 Therefore, it should be the treatment of choice. However, in patients interested in future fertility, we must take into consideration the potential damage to ovarian reserve associated with excision.”
Endometriosis of the ovaries has unique manifestations. “My approach to ovarian cysts depends on their classification,” says Dr. Nezhat.15 In general, primary endometriomas (type 1) are small, superficial cysts that contain dark “chocolate” fluid. They tend to be firmly adherent to the ovarian tissue and difficult to remove surgically.
Secondary endometriomas (type 2) are follicular or luteal cysts that have been involved or invaded by cortical endometriotic implants or by primary endometrioma. Secondary endometriomas are further classified by the relationship between cortical endometriosis and the cyst wall. Type 2A endometriomas are usually large, with a capsule that is easily separated from ovarian tissue. Type 2B endometriomas have some features of functional cysts but show deep involvement with surface endometriosis. Type 2C endometriomas are similar, showing extensive surface endometrial implants but with deep penetration of the endometriosis into the cyst wall.15
“For type 1 endometriomas, I biopsy the cyst to ensure the lesion is benign, then vaporize the endometrioma,” Dr. Nezhat says. “In cases of type 2A and 2B endometriomas, the cyst capsule is easily enucleated and removed. Type 2C endometriomas are biopsied as well, and then I proceed with vaporizing the fibrotic area with a low-power energy source, such as neutral argon plasma, avoiding excessive coagulation and thermal injury.” Recent literature supports the idea of evaluation and biopsy of fibrotic endometriomas to confirm benign conditions, followed by ablation without compromising ovarian function.16
“Excision and ablation both have indications,” Dr. Nezhat asserts. “It depends on the location and depth of penetration of implants, as well as the patient’s ultimate goal. For example, if the patient desires future fertility and has endometriosis on the ovary, removal by excision could damage ovarian function. The same holds true for endometriosis on the fallopian tubes. It’s better in such cases to ablate.”
“Ablation is different from coagulation, which is not recommended,” Dr. Nezhat explains. “Ablation vaporizes the diseased area layer by layer, like peeling an onion, until the disease is eradicated. It is similar to dermatologic skin resurfacing. Vaporization is preferable for endometriosis on the tubes and ovaries in patients who desire pregnancy. The choice between excision and ablation depends on the location, depth of penetration, and the patient’s desire for fertility.”
Either way—and regardless of the primary indication for surgery (pain versus infertility)—a minimally invasive gynecologic surgeon is expected to have the ability to perform both techniques, Dr. Nezhat says.
Continue to find out if hysterectomy is definitive treatment >>
5. IS HYSTERECTOMY DEFINITIVE TREATMENT?
“Not necessarily,” says Dr. Nezhat. “Hysterectomy by itself doesn’t take care of endometriosis unless the patient has adenomyosis. If a patient has endometriosis, the first step is complete treatment of the disease to restore the anatomy. Then the next step might be hysterectomy to give a better long-term result, especially in cases of adenomyosis. Removal of the ovaries at the time of hysterectomy has to be individualized.”
“The implication that hysterectomy ‘cures’ endometriosis is false yet is stated in some textbooks,” says Dr. Nezhat. “Even at the time of hysterectomy, the first step should be complete treatment of endometriosis and restoration of anatomy, followed by the hysterectomy. Leaving endometriosis behind, believing it will go away by itself or not cause future issues, is a gross misperception.”
Removal of the ovaries at hysterectomy?
“There are few comparative studies on the long-term follow-up of patients who have undergone hysterectomy with or without removal of both ovaries,” says Dr. Falcone. “The conventional dogma has been that, in women undergoing definitive surgery for endometriosis, both ovaries should be removed, even if they are normal. I personally believe that this was because hysterectomy was often performed without excision of the endometriosis. So the uterus was removed and disease was left behind. In these cases, recurrent symptoms were due to persistent disease.”
“We reported our experience at the Cleveland Clinic with a seven-year follow-up,” Dr. Falcone continues. “Hysterectomy was performed with excision of all visible disease. Ovaries were conserved if normal and removed if not. We looked at the reoperation-free frequency over time. In women undergoing hysterectomy with excision of visible disease but ovarian preservation, the reoperation-free percentages at two, five, and seven years were 95%, 86%, and 77%, respectively, versus 96%, 91%, and 91% in those without ovarian preservation. So, overall, there was an advantage over time for removal of the ovaries. However, in the subset of women between ages 30 and 39, there was no difference in the long-term recurrence rate if the ovaries were left in. For this reason, in women younger than 40, we recommend keeping normal ovaries if all disease is removed.”17
Continue on to find out if the risk for postoperative recurrence can be reduced >>
6. CAN THE RISK FOR POSTOPERATIVE RECURRENCE BE REDUCED?
“The main problem with surgery is the recurrence rate,” Dr. Falcone says. “Studies have shown that the recurrence rate of pain at seven years may be as high as 50%.”17 Furthermore, “the recurrence of pain may not be associated with visualized endometriosis at laparoscopy.”
“Incomplete removal of lesions may be associated with an increase in pain after surgery,” says Dr. Stratton.18 “Incomplete removal of lesions may occur because of varying technical skill or specific lesion characteristics. The lesions may be difficult to remove because of their location. Lesions may not be recognized because their appearance can vary from subtle (red or clear or white) to classic (blue-black). The depth of the lesion may not be appreciated until surgery is under way, and a surgeon may not be adequately prepared to treat deep lesions when they are identified.”
Adenomyosis is another reason pain may persist or recur after surgery.19 “Adenomyosis appears as either diffuse or focal thickening of the junctional zone between the endometrium and myometrium of the uterus on T2-weighted MRI,” says Dr. Stratton. “After excision of endometriosis, chronic pelvic pain is significantly more likely to persist in women who have a junctional zone thickness of more than 11 mm on MRI.”
The frequent recurrence of pain after surgery makes the disease a long-term challenge.
“Pelvic pain caused by endometriosis is a chronic problem that requires a multiyear management plan, involving both surgery and hormonal therapy,” says Robert L. Barbieri, MD. “To reduce the number of surgical procedures in the lifetime of a woman with endometriosis and pain, I suggest hormonal medical therapy following conservative surgery for endometriosis.”
“Definitive surgery, such as hysterectomy or hysterectomy plus bilateral salpingo-oophorectomy (BSO), typically results in prolonged symptom relief,” Dr. Barbieri says. “Following hysterectomy, hormonal therapy may not be needed. Following BSO, low-dose hormonal therapy is often needed to reduce the severity of menopausal symptoms.”
After surgical treatment of endometriosis associated with pain, Dr. Barbieri presents the patient with the following menu of hormonal options:
• No hormonal therapy
• Estrogen-progestin contraceptives, either cyclic or continuous
• The LNG-IUS
• Norethindrone acetate (5 mg/d)
• DMPA (150 mg every three months)
• Leuprolide acetate depot (3.75 mg IM monthly)
• Nafarelin nasal spray (200 µg bid)
• Danazol (200 mg bid).
“I explain the common adverse effects with each approach and have the patient select what she determines to be her best option,” says Dr. Barbieri. “In my experience, conservative surgery followed by hormonal therapy is effective in more than 75% of women.”
“The evidence to support postoperative hormonal therapy is modest,” Dr. Barbieri notes. “The best evidence is available for use of the LNG-IUS, estrogen-progestin contraceptives, and GnRH agonists.”20-22
In addition, he notes, “major professional societies have highlighted the option of postoperative hormonal therapy to reduce the risk for recurrent pain and repetitive surgical procedures in the future.”23,24
When pain recurs after surgery for endometriosis, it pays to consider what type of pain it is, says Dr. Barbieri.
“There are two major types of pain—nociceptive and neuropathic,” he says. “Nociceptive pain is caused by an injury, acute or chronic. Neuropathic pain is caused by ‘activation’ of neural circuits, sometimes in the absence of an ongoing injury. Many women with endometriosis and chronic pain have both nociceptive and neuropathic pain. Consequently, it is important to consider the use of a multidisciplinary pain practice in the management of chronic pain syndromes. Multidisciplinary pain practices have special expertise in the management of neuropathic pain. Standard conservative surgical intervention is unlikely to improve pain caused by neuropathic mechanisms. Likewise, opioid analgesics are not recommended for the treatment of neuropathic pain.”
REFERENCES
1. Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6S):S1-S62.
2. Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389-2398.
3. Pavone ME, Bulun SE. Aromatase inhibitors for the treatment of endometriosis: a review. Fertil Steril. 2012;98(6):1370-1379.
4. Nothnick WB. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod Biol Endocrinol. 2011;9:87.
5. Chwalisz K, Garg R, Brenner RM, et al. Selective progesterone receptor modulators (SPRMs): a novel therapeutic concept in endometriosis. Ann N Y Acad Sci. 2002;955:373-393, 396-406.
6. Duffy JM, Arambage K, Correa FJ, et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2014;(4):CD011031.
7. Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268-279.
8. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
9. Hsu AL, Sinaii N, Segars J, et al. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 pt 1):223-230.
10. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
11. Healey M, Ang WC, Cheng C. Surgical treatment of endometriosis: a prospective randomized double-blinded trial comparing excision and ablation. Fertil Steril. 2010;94(7):2536-2540.
12. Healey M, Chang C, Kaur H. To excise or ablate endometriosis? A prospective randomized double blinded trial after 5-year follow-up. JMIG. 2014;21(6):999-1004.
13. Falcone T, Wilson JR. Surgical management of endometriosis: excision or ablation. JMIG. 2014;21(6):969.
14. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
15. Nezhat C, Nezhat F, Nezhat CH, Seidman D. Classification of endometriosis: improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212-2216.
16. Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril. 2011;96(6):1396-1400.
17. Shakiba K, Bena JF, McGill KM, et al. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
18. McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147(1-3):255-264.
19. Parker JD, Leondires M, Sinaii N, et al. Persistence of dysmenorrhea and nonmenstrual pain after optimal endometriosis surgery may indicate adenomyosis. Fertil Steril. 2006;86(3):711-715.
20. Abou-Setta AM, Al-Inany HG, Farquar CM. Levonorgestrel-releasing intrauterine device for symptomatic endometriosis following surgery. Cochrane Database Syst Rev. 2006;(1):CD005072.
21. Seracchioli R, Mabrouk M, Manuzzi L, et al. Postoperative use of oral contraceptive pills for prevention of anatomic relapse or symptom recurrence following surgery. Hum Reprod. 2009;24(11):2729-2735.
22. Hornstein MD, Hemmings R, Yuzpe AA, Heinrichs WL. Use of nafarelin versus placebo after reductive laparoscopic surgery for endometriosis. Fertil Steril. 1997;68(5):860-864.
23. Practice Committee of the American Society for Reproductive Medicine. Treatment of pain associated with endometriosis: a committee opinion. Fertil Steril. 2014;101(4):927-935.
24. Dunselman GA, Vermeulen N, Becker C, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
Randomized trial: When a vaginal approach is feasible, the robot offers no advantages for benign hysterectomy
When investigators compared the cost of vaginal hysterectomy with robot-assisted laparoscopic hysterectomy head to head, they found hospital costs of $4,579 and $7,059, respectively, with no other significant differences between the approaches. Accordingly, they concluded that vaginal hysterectomy should be the “first-choice” approach when it is feasible.
The randomized controlled trial by Lönnerfors and colleagues also compared “traditional” minimally invasive hysterectomy (vaginal or laparoscopic approach) with robot-assisted hysterectomy in 122 women undergoing hysterectomy for benign conditions. Women with a uterine size of 16 gestational weeks or smaller were randomly allocated to:
- traditional minimally invasive hysterectomy (n = 61) or
- robotic assisted hysterectomy (n = 61).
In the traditional group, vaginal hysterectomy was the first-choice approach when it was feasible; otherwise, laparoscopic hysterectomy was performed. Vaginal hysterectomy was possible in 41% of cases in this group.
When costs for vaginal and laparoscopic approaches were consolidated and compared with the cost of the robot-assisted approach, the differential was $993 for the robotic approach when the robot was considered a preexisting investment. The hospital cost increased by $1,607 for the robotic approach when investment costs and maintenance expenses were included.
When laparoscopic hysterectomy was compared directly with robot-assisted hysterectomy, costs were similar ($7,016 vs $7,059, respectively) when the robot was considered a preexisting investment, and the robotic approach was associated with less blood loss and fewer postoperative complications.
Investigators noted that: “per-protocol analysis indicates that laparoscopic and robotic-assisted hysterectomy can be performed at similar hospital cost because of higher robot capacity that entails excluding the cost of investment and maintenance, i.e., the basic cost of the robot. This cost differs among institutions, depending on the number of procedures performed; however, the difference becomes less pronounced when 300 to 400 procedures or more are performed annually and the cost for instruments and disposables accounts for most of the cost of the procedure.”
This randomized controlled trial was awarded the Robert B. Hunt Award at the 2015 AAGL Global Congress in Las Vegas as the best paper published over the past year in the Journal of Minimally Invasive Gynecology.
Reference
Lönnerfors C, Reynisson P, Persson J. A randomized trial comparing vaginal and laparoscopic hysterectomy vs robot-assisted hysterectomy. J Minim Invasive Gynecol. 2015;22(1):78–86.
When investigators compared the cost of vaginal hysterectomy with robot-assisted laparoscopic hysterectomy head to head, they found hospital costs of $4,579 and $7,059, respectively, with no other significant differences between the approaches. Accordingly, they concluded that vaginal hysterectomy should be the “first-choice” approach when it is feasible.
The randomized controlled trial by Lönnerfors and colleagues also compared “traditional” minimally invasive hysterectomy (vaginal or laparoscopic approach) with robot-assisted hysterectomy in 122 women undergoing hysterectomy for benign conditions. Women with a uterine size of 16 gestational weeks or smaller were randomly allocated to:
- traditional minimally invasive hysterectomy (n = 61) or
- robotic assisted hysterectomy (n = 61).
In the traditional group, vaginal hysterectomy was the first-choice approach when it was feasible; otherwise, laparoscopic hysterectomy was performed. Vaginal hysterectomy was possible in 41% of cases in this group.
When costs for vaginal and laparoscopic approaches were consolidated and compared with the cost of the robot-assisted approach, the differential was $993 for the robotic approach when the robot was considered a preexisting investment. The hospital cost increased by $1,607 for the robotic approach when investment costs and maintenance expenses were included.
When laparoscopic hysterectomy was compared directly with robot-assisted hysterectomy, costs were similar ($7,016 vs $7,059, respectively) when the robot was considered a preexisting investment, and the robotic approach was associated with less blood loss and fewer postoperative complications.
Investigators noted that: “per-protocol analysis indicates that laparoscopic and robotic-assisted hysterectomy can be performed at similar hospital cost because of higher robot capacity that entails excluding the cost of investment and maintenance, i.e., the basic cost of the robot. This cost differs among institutions, depending on the number of procedures performed; however, the difference becomes less pronounced when 300 to 400 procedures or more are performed annually and the cost for instruments and disposables accounts for most of the cost of the procedure.”
This randomized controlled trial was awarded the Robert B. Hunt Award at the 2015 AAGL Global Congress in Las Vegas as the best paper published over the past year in the Journal of Minimally Invasive Gynecology.
When investigators compared the cost of vaginal hysterectomy with robot-assisted laparoscopic hysterectomy head to head, they found hospital costs of $4,579 and $7,059, respectively, with no other significant differences between the approaches. Accordingly, they concluded that vaginal hysterectomy should be the “first-choice” approach when it is feasible.
The randomized controlled trial by Lönnerfors and colleagues also compared “traditional” minimally invasive hysterectomy (vaginal or laparoscopic approach) with robot-assisted hysterectomy in 122 women undergoing hysterectomy for benign conditions. Women with a uterine size of 16 gestational weeks or smaller were randomly allocated to:
- traditional minimally invasive hysterectomy (n = 61) or
- robotic assisted hysterectomy (n = 61).
In the traditional group, vaginal hysterectomy was the first-choice approach when it was feasible; otherwise, laparoscopic hysterectomy was performed. Vaginal hysterectomy was possible in 41% of cases in this group.
When costs for vaginal and laparoscopic approaches were consolidated and compared with the cost of the robot-assisted approach, the differential was $993 for the robotic approach when the robot was considered a preexisting investment. The hospital cost increased by $1,607 for the robotic approach when investment costs and maintenance expenses were included.
When laparoscopic hysterectomy was compared directly with robot-assisted hysterectomy, costs were similar ($7,016 vs $7,059, respectively) when the robot was considered a preexisting investment, and the robotic approach was associated with less blood loss and fewer postoperative complications.
Investigators noted that: “per-protocol analysis indicates that laparoscopic and robotic-assisted hysterectomy can be performed at similar hospital cost because of higher robot capacity that entails excluding the cost of investment and maintenance, i.e., the basic cost of the robot. This cost differs among institutions, depending on the number of procedures performed; however, the difference becomes less pronounced when 300 to 400 procedures or more are performed annually and the cost for instruments and disposables accounts for most of the cost of the procedure.”
This randomized controlled trial was awarded the Robert B. Hunt Award at the 2015 AAGL Global Congress in Las Vegas as the best paper published over the past year in the Journal of Minimally Invasive Gynecology.
Reference
Lönnerfors C, Reynisson P, Persson J. A randomized trial comparing vaginal and laparoscopic hysterectomy vs robot-assisted hysterectomy. J Minim Invasive Gynecol. 2015;22(1):78–86.
Reference
Lönnerfors C, Reynisson P, Persson J. A randomized trial comparing vaginal and laparoscopic hysterectomy vs robot-assisted hysterectomy. J Minim Invasive Gynecol. 2015;22(1):78–86.
A surge in congenital syphilis reveals gaps in obstetric practice
The rate of congenital syphilis increased to 11.6 cases per 100,000 live births in 2014 in the United States—the highest rate documented since 2001, according to a new report from the Centers for Disease Control and Prevention (CDC).1 The increase in the rate of congenital syphilis reflects a rise in the rate of primary and secondary syphilis among US women, from 0.9 to 1.1 cases per 100,000 women, during the same period.1
The rate of congenital syphilis had declined between 2008 and 2012, from 10.5 cases to 8.4 cases per 100,000 live births.
Congenital syphilis occurs when an infected mother transmits the disease to her fetus during pregnancy. Among the adverse effects of congenital syphilis are deformities, stillbirth, and early infant death. “However, among mothers identified with syphilis who deliver past 20 weeks’ gestation, treatment with penicillin at least 30 days before delivery is 98% effective at preventing [congenital syphilis],” the CDC report notes.1
For the purposes of the CDC report, congenital syphilis includes “both infants and stillbirths with clinical evidence” of the disease, “as well as those infants and stillbirths born to mothers with untreated or inadequately treated syphilis, regardless of the infant’s manifestation of clinical disease.”1
CDC recommendations
The CDC notes that most of the increases in the rates of maternal and congenital syphilis likely stem from inadequate prenatal care.
“A large percentage of [congenital syphilis] cases continue to be attributable to a lack of prenatal care; even among those receiving some prenatal care, the detection and treatment of maternal syphilis is often not early enough….At particular risk are those who are uninsured or underinsured and those with substance use issues.”1
Among the recommendations of the CDC:
- Screen all pregnant women for syphilis at their first prenatal visit.
- Screen women at elevated risk for syphilis, as well as those who live in “high-morbidity geographic areas” at the beginning of the third trimester and again at delivery.
- In cases where prenatal care has been lacking, screen the woman for syphilis using a rapid plasma reagin (RPR) card and treat the patient who tests positive at the time the pregnancy is confirmed.
- Do not discharge an infant from the hospital unless the syphilis serologic status of the mother has been tested at least once during pregnancy and, preferably, again at delivery (in high-risk cases).
- Test any woman who delivers a stillborn infant for syphilis.
Reference
1. Bowen V, Su J, Torrone E, Kidd S, Weinstock H. Increase in incidence of congenital syphilis—United States, 2008–2014. MMWR. 2015;64(44):1241–1245.
The rate of congenital syphilis increased to 11.6 cases per 100,000 live births in 2014 in the United States—the highest rate documented since 2001, according to a new report from the Centers for Disease Control and Prevention (CDC).1 The increase in the rate of congenital syphilis reflects a rise in the rate of primary and secondary syphilis among US women, from 0.9 to 1.1 cases per 100,000 women, during the same period.1
The rate of congenital syphilis had declined between 2008 and 2012, from 10.5 cases to 8.4 cases per 100,000 live births.
Congenital syphilis occurs when an infected mother transmits the disease to her fetus during pregnancy. Among the adverse effects of congenital syphilis are deformities, stillbirth, and early infant death. “However, among mothers identified with syphilis who deliver past 20 weeks’ gestation, treatment with penicillin at least 30 days before delivery is 98% effective at preventing [congenital syphilis],” the CDC report notes.1
For the purposes of the CDC report, congenital syphilis includes “both infants and stillbirths with clinical evidence” of the disease, “as well as those infants and stillbirths born to mothers with untreated or inadequately treated syphilis, regardless of the infant’s manifestation of clinical disease.”1
CDC recommendations
The CDC notes that most of the increases in the rates of maternal and congenital syphilis likely stem from inadequate prenatal care.
“A large percentage of [congenital syphilis] cases continue to be attributable to a lack of prenatal care; even among those receiving some prenatal care, the detection and treatment of maternal syphilis is often not early enough….At particular risk are those who are uninsured or underinsured and those with substance use issues.”1
Among the recommendations of the CDC:
- Screen all pregnant women for syphilis at their first prenatal visit.
- Screen women at elevated risk for syphilis, as well as those who live in “high-morbidity geographic areas” at the beginning of the third trimester and again at delivery.
- In cases where prenatal care has been lacking, screen the woman for syphilis using a rapid plasma reagin (RPR) card and treat the patient who tests positive at the time the pregnancy is confirmed.
- Do not discharge an infant from the hospital unless the syphilis serologic status of the mother has been tested at least once during pregnancy and, preferably, again at delivery (in high-risk cases).
- Test any woman who delivers a stillborn infant for syphilis.
The rate of congenital syphilis increased to 11.6 cases per 100,000 live births in 2014 in the United States—the highest rate documented since 2001, according to a new report from the Centers for Disease Control and Prevention (CDC).1 The increase in the rate of congenital syphilis reflects a rise in the rate of primary and secondary syphilis among US women, from 0.9 to 1.1 cases per 100,000 women, during the same period.1
The rate of congenital syphilis had declined between 2008 and 2012, from 10.5 cases to 8.4 cases per 100,000 live births.
Congenital syphilis occurs when an infected mother transmits the disease to her fetus during pregnancy. Among the adverse effects of congenital syphilis are deformities, stillbirth, and early infant death. “However, among mothers identified with syphilis who deliver past 20 weeks’ gestation, treatment with penicillin at least 30 days before delivery is 98% effective at preventing [congenital syphilis],” the CDC report notes.1
For the purposes of the CDC report, congenital syphilis includes “both infants and stillbirths with clinical evidence” of the disease, “as well as those infants and stillbirths born to mothers with untreated or inadequately treated syphilis, regardless of the infant’s manifestation of clinical disease.”1
CDC recommendations
The CDC notes that most of the increases in the rates of maternal and congenital syphilis likely stem from inadequate prenatal care.
“A large percentage of [congenital syphilis] cases continue to be attributable to a lack of prenatal care; even among those receiving some prenatal care, the detection and treatment of maternal syphilis is often not early enough….At particular risk are those who are uninsured or underinsured and those with substance use issues.”1
Among the recommendations of the CDC:
- Screen all pregnant women for syphilis at their first prenatal visit.
- Screen women at elevated risk for syphilis, as well as those who live in “high-morbidity geographic areas” at the beginning of the third trimester and again at delivery.
- In cases where prenatal care has been lacking, screen the woman for syphilis using a rapid plasma reagin (RPR) card and treat the patient who tests positive at the time the pregnancy is confirmed.
- Do not discharge an infant from the hospital unless the syphilis serologic status of the mother has been tested at least once during pregnancy and, preferably, again at delivery (in high-risk cases).
- Test any woman who delivers a stillborn infant for syphilis.
Reference
1. Bowen V, Su J, Torrone E, Kidd S, Weinstock H. Increase in incidence of congenital syphilis—United States, 2008–2014. MMWR. 2015;64(44):1241–1245.
Reference
1. Bowen V, Su J, Torrone E, Kidd S, Weinstock H. Increase in incidence of congenital syphilis—United States, 2008–2014. MMWR. 2015;64(44):1241–1245.
Endometriosis: Expert Answers to 7 Crucial Questions on Diagnosis
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
IN THIS ARTICLE
• The “why” of endometriosis
• Environmental factors, estrogen, and endometriosis
• Is imaging useful?
• When is diagnostic laparoscopy clearly indicated?
CASE M.L. is a 32-year-old nulliparous woman who is referred to your office by her primary care provider for chronic pelvic pain. She reports severe dysmenorrhea as her main symptom, but she also mentions dyspareunia. She says these symptoms have been present for several years but have increased in intensity gradually. She asks what you consider to be the most likely diagnosis.
What potential diagnoses do you mention to her? And how do you identify the cause of her pain?
Although endometriosis—the presence of endometrial tissue outside the uterus—affects at least 5 million women of reproductive age in the United States alone, it can be a challenging diagnosis for several reasons.
“Endometriosis is a great masquerader,” says Linda Giudice, MD, PhD. “It presents with a variety of pain patterns, intensities, and triggers. It can also involve symptoms that overlap those of other disorders, including disorders of the gastrointestinal and urinary tracts.”
Although endometriosis falls within the differential diagnosis of chronic pelvic pain, “it is usually not high on the list in the primary care setting (adult and adolescent),” adds Dr. Giudice.
John R. Lue, MD, MPH, an author of the most recent practice bulletin on endometriosis from the American College of Obstetricians and Gynecologists,1 sees the situation similarly.
“The main challenge in the diagnosis of endometriosis is that its presentation mimics other causes of chronic pelvic pain,” he says. “Pelvic pain due to endometriosis is usually chronic (lasting ≥ 6 months). It is associated with dysmenorrhea in 50% to 90% of cases, as well as with dyspareunia, deep pelvic pain, and lower abdominal pain with or without back and loin pain. The pain can occur unpredictably and intermittently throughout the menstrual cycle or it can be continuous. In addition, it can be dull, throbbing, or sharp and may be exacerbated by physical activity.2,3 Up to 20% of women with endometriosis have concurrent pain conditions.”4
Among other diseases of the female pelvis that have a relatively similar presentation, Dr. Lue adds, are pathologies of the
• Uterus (adenomyosis, fibroids)
• Fallopian tube (hydrosalpinx)
• Ovaries (ovarian cysts)
• Bladder (interstitial cystitis)
• Bowel (irritable bowel syndrome)
• Musculoskeletal system (piriformis syndrome).
Before pelvic pain is attributed to endometriosis, he says, the provider should rule out bowel, bladder, musculoskeletal, and psychiatric causes.
This article focuses on seven questions, the answers to which are critical to narrowing in on the diagnosis of endometriosis, including essential factors to consider in the patient history, imaging and other diagnostic tools, and considerations in surgical exploration.
1. WHY SUCH A LONG DELAY IN DIAGNOSIS?
Investigators exploring the length of time from a patient’s presentation with symptoms to diagnosis have found it to be particularly long for endometriosis, ranging from six to 11 years.
Because endometriosis is usually not high on the list of differential diagnoses for chronic pelvic pain in the primary care setting, a patient may not be referred to a gynecologist unless those symptoms include severe dysmenorrhea, dyspareunia, or similar findings. Once the referral is made, the gynecologist “will usually try contraceptive steroids, NSAIDs, or second-line progestins before a diagnosis is made,” says Dr. Giudice.5
The delay in diagnosis “is astounding,” she adds, “and has its roots in empiric medical therapies and a combination of patients fearing a diagnosis of cancer and reluctance of gynecologists to perform laparoscopy on adolescents.”6 Another possible cause of diagnostic delay: Some adolescent girls may not realize when their pain is severe. Because they may have always experienced a high degree of pain since menarche, they may assume it to be a normal aspect of womanhood and delay seeking help, says Pamela Stratton, MD.
Continue to learn any biomarkers proved to be useful diagnostic tools >>
2. HAVE ANY BIOMARKERS PROVED TO BE USEFUL DIAGNOSTIC TOOLS?
Any biomarker proven to reliably identify endometriosis would be a boon to medicine, as it would provide a noninvasive or minimally invasive alternative to diagnostic laparoscopy, the current gold standard. Regrettably, the search for such a biomarker has produced “disappointing results,” says Dr. Giudice.
“Recent systematic reviews of all proposed endometriosis-related biomarkers over the past 25 years in serum, plasma, urine, and endometrium could not identify an unequivocally clinically useful biomarker or panel of biomarkers,” she notes.7,8 “This is due mainly to low numbers of subjects, small populations for validations, cycle/hormonal- and disease stage–dependence, poorly defined controls, and low sensitivity and specificity.”
One hopeful development: “Whole genome transcriptomics of archived endometrial tissue and machine learning found several classifiers to diagnose and stage endometriosis with high accuracy that were validated on an independent sample set,” says Dr. Giudice.9 “However, these data now warrant a prospective, multisite study for further validation.”
Continue for key aspects of patient history >>
3. WHAT ASPECTS OF THE PATIENT HISTORY ARE KEY?
Dr. Stratton recommends that clinicians begin their evaluation of the patient with pain by asking her to describe that pain: how long she has had it, when it occurs, and which areas are affected.
“Most women with endometriosis-associated pain have chronic pelvic pain,” Dr. Stratton continues.5 “Up to 90% of those have dysmenorrhea or cyclic pain with menses.”10 In addition, women with endometriosis “commonly report having pain with any bleeding or spotting. About 30% of women diagnosed with endometriosis initially present to their gynecologist with dyspareunia.”11
“Episodic pain with menses may become more constant, lasting for many days of the month,” says Dr. Stratton. “Women with dyschezia or dysuria may have endometriosis lesions associated with the bowel or bladder, respectively.12 When women with these symptoms do not have lesions on the bowel or bladder, these pain symptoms may occur because of higher peritoneal hormone and inflammatory factor levels or because adjacent organs share the neural networks.”
Dr. Giudice views the history similarly: “I believe listening to the patient is essential in evaluating the possibility of her having endometriosis. This involves asking her to describe where her pain is, grading it on a scale of 1 to 10, identifying when in her cycle it occurs, and learning what makes it better or worse.”
“It also is important to assess the quality of the pain,” she adds. “Does it radiate, does it limit her daily activities, does it interfere with her relationships, intercourse, work, school? Is it associated with bowel movements, urination, other pain syndromes?”
“Having a pain questionnaire is a great help so that patients have a chance to reflect on these and other questions that help to frame the pain associated with endometriosis when they come for consultation,” she adds.
By determining if pain is associated with menstruation or spotting, the clinician is better informed about the value of menstrual suppression, says Dr. Stratton. “Determining what makes the pain better or worse can help define triggers which, if treated, can decrease the likelihood of episodes of pain.”
“A detailed history of any medical or surgical treatments and their outcome is helpful in guiding future treatment,” she adds. “While hormonal therapy has been a mainstay of treatment, in some women, some hormonal treatments may worsen pain or have unacceptable adverse effects, such as worsening depression or anxiety. In addition, some pain—especially that associated with deep lesions—may be relieved by surgical treatment;13,14 pain that worsened after surgery may suggest neural damage.”
“As there is an engagement of the central nervous system, endometriosis is considered a central sensitivity syndrome in which women may also have other sites of pain,” Dr. Stratton says. “Thus, obtaining a history about current symptoms or prior diagnosis of irritable bowel syndrome, interstitial cystitis/painful bladder, migraines, fibromyalgia, or chronic fatigue syndrome is beneficial.10,15-17 Facilitating treatment for these comorbidities is a key principle in helping women with endometriosis-associated pain, as any condition that triggers or perpetuates pain warrants treatment.”
Continue for what the physical exam entails >>
4. WHAT SHOULD THE PHYSICAL EXAM ENTAIL?
“An abdominal exam and a pelvic exam are essential in evaluating pain in a woman when endometriosis is suspected,” says Dr. Giudice. “Sometimes the latter is challenging in young teens and can be deferred.” Overall, however, “the pelvic exam can give insight into pain triggers, adnexal masses (possible endometriomas), and mobility of pelvic organs. A rectovaginal exam is important in evaluating deep infiltrating disease and to gauge the pelvic pain landscape overall. In addition, palpating the pelvic floor musculature is important to distinguish pelvic floor muscle spasm from endometriosis pain.”
“The challenge for clinicians is to think beyond the endometrial implants, taking into account multiple factors that influence pain perception,” says Dr. Stratton. During the examination, the clinician should begin by mapping the regions of pain in the abdomen and back, “distinguishing musculoskeletal pain from deep pain. Determining whether pains are focused or diffuse is also important.”
Dr. Stratton recommends that the routine pelvic exam be modified because a standard bimanual exam “confuses pain signals from the pelvic floor, abdominal wall, bladder, and other viscera. For this reason, a pain-oriented assessment is mandatory.”
Begin with a single digital examination to map tender areas, Dr. Stratton advises. Then consider the size, shape, and mobility of reproductive and pelvic organs. “A bimanual exam will help identify adnexal masses like endometriomas,” she says.
Endometriomas usually are not associated with pain, she adds, but “they are associated with deep infiltrating lesions. Nodularity along the uterosacral ligaments, limited reproductive organ mobility, and thickening of the rectovaginal septum also suggest deep infiltrating lesions. Importantly, deep infiltrating lesions are the lesion type most associated with pain.”18,19
Continue to learn if imaging is useful in the diagnosis of endometriosis >>
5. IS IMAGING USEFUL IN THE DIAGNOSIS OF ENDOMETRIOSIS?
Laparoscopy remains the gold standard for diagnosis of endometriosis, observes Steven R. Goldstein, MD. Visualization of endometriotic implants at the time of surgery—with histologic assessment—offers definitive confirmation of the diagnosis. The physical examination, too, can offer a strong suggestion of endometriosis, he says.
“In the past, the pelvic examination and history often were the sine qua non for patients with pain,” Dr. Goldstein says. “Extreme dysmenorrhea and pain between periods, especially with intercourse, defecation, and exercise, all increased the suspicion of endometriosis. People used to talk about feeling nodularity in the uterosacral ligaments and finding decreased mobility of pelvic structures—but I don’t have any question that the skill of today’s gynecologists in doing a bimanual pelvic exam is a fraction of what it was in years gone by, because they haven’t had the necessity of experience. The first thing they do if there’s any question is send the patient for an ultrasound.”
Of course, ultrasound can be especially helpful in identifying endometriomas—sometimes called chocolate cysts—in the ovary. Endometriomas can have a solid appearance on ultrasound, says Dr. Goldstein, because the fluid they contain (dried blood) is sonolucent or pure black on ultrasound, similar to amniotic fluid or the fluid seen in the bladder. “This ‘chocolate’ fluid contained in endometriomas is homogeneous, particulate, and very monotonous in its appearance, in contrast to the internal echoes observed in hemorrhagic corpus lutea, which are very cobweb-like and can sometimes mimic papillary projections,” he adds.
“What’s absolutely essential when imaging a suspected endometrioma by ultrasound is that there be no evidence of any blood flow contained within that structure. Because it’s dried blood, it shouldn’t have any vascularity. If you see blood flow inside what you would call an endometrioma, you need to rethink your diagnosis,” he says.
In some cases, a supposed endometrioma lacks a black, sonolucent appearance, but “the clinician often can tell that it’s a cystic structure by the very bright posterior wall—what we call posterior wall acoustic enhancement—even though the interior of the structure may appear sort of grayish or whitish rather than the pure black of a simple cyst. It’s still fluid-filled,” Dr. Goldstein says.
In some instances, even endometriotic nodules can be imaged by ultrasound, he adds. “There’s an increasing body of literature that suggests that, if you look carefully in people with deep infiltrating endometriosis, you can often see solid-appearing nodules in the rectovaginal septum or between the uterus and bladder. With the kind of resolution that we now have with the vaginal probe, some of these nodules can be seen. That’s somewhat new, and it’s a function of two things—people looking for endometriosis and the better resolution of more modern equipment.”
Dr. Goldstein believes that MRI is “almost never” indicated in the diagnosis of endometriosis. A more helpful approach would be a consultative ultrasound with someone with more experience. However, when that is not available, or “in areas where you have excellent backup in terms of pelvic MRI, that may be the way to go. I don’t think so,” he demurs, “and some of my colleagues would be very upset at the thought of needing to use MRI to diagnose endometriosis. But in the occasional confusing or difficult case, depending on the quality of the referral pattern you have, it might make sense."
Continue to learn when diagnostic laparoscopy is clearly indicated >>
6. WHEN IS DIAGNOSTIC LAPAROSCOPY CLEARLY INDICATED?
Dr. Giudice believes that laparoscopy—with the intention to treat endometriosis, if present—“is essential when firstline medical therapy fails or when pain is acute and severe.”5
Dr. Stratton concurs. “Any woman with chronic pain wants to know what is causing the pain,” she says. Therefore, “women report a benefit from knowing that their pain is associated with endometriosis.6 However, diagnostic laparoscopy alone, with the sole purpose of determining the presence of endometriosis but not treating the lesions, is no longer performed, as it poses little benefit to the patient other than peace of mind.”
“The general trend in the US has been to first use hormonal treatments when the diagnosis of endometriosis is suspected, prior to performing surgery,” Dr. Stratton says.1 In many cases, by using cyclic combined hormonal contraceptives to reduce menstrual flow or “suppressing menstruation with continuous combined hormonal contraceptives,” gonadotropin-releasing hormone analogues (combined with progestin to prevent bone loss) “or continuous progestin alone may be effective in decreasing pain. Not surprisingly, these hormonal approaches are effective for any chronic pelvic pain, even for women who do not have the surgical diagnosis of endometriosis.”20
“When the firstline approach to chronic pelvic pain is hormonal treatment, laparoscopy is considered when these medical treatments have failed to control the pain or are poorly tolerated, or when the diagnosis of endometriosis is in question,” Dr. Stratton says.
“Laparoscopy to treat endometriomas is indicated if an endometrioma is enlarging or measures more than 4 cm in diameter, or if the diagnosis of an ovarian mass is in question,” she explains. “While surgeons have previously been aggressive in removing endometriomas, this practice may have negative consequences on ovarian function. Because endometriomas are pseudocysts, removing them completely leads to the removal of viable ovarian tissue and may diminish ovarian reserve.”21,22
Continue for the surgical appearance of endometriosis >>
7. WHAT IS THE SURGICAL APPEARANCE OF ENDOMETRIOSIS?
Dr. Giudice returns to the enigmatic nature of endometriosis in addressing this question, mentioning its “many faces” at the time of surgery. “It is imperative that the surgeon recognize the disease in its many forms,” she says. “Also, it is especially helpful at the time of surgery if suspected lesions are biopsied and sent to pathology to have the diagnosis made unequivocally.”5
As for the surgical appearance of endometriosis, Dr. Stratton notes that there are three types of lesions—“superficial lesions, deep infiltrating lesions, and endometriomas. Endometriomas occur almost exclusively in the ovary and are pseudocysts without an identifiable cystic lining. They vary in dimension from a few millimeters to several centimeters.”
“Superficial peritoneal endometriosis lesions have a variable appearance, with some lesions being clear or red; some brown, blue or black; and some having a white appearance, like a scar,” says Dr. Stratton. “Endometriosis can be diagnosed on histologic examination of any of these lesion types."
“Overall, single-color lesions have similar frequencies of biopsy-confirmed endometriosis (59% to 62%),” she says.23 “These lesion appearances likely represent different stages of development of endometriosis, with red or clear lesions occurring first, soon after endometrial tissue implantation; black, blue, or brown lesions occurring later, in response to the hormones varying in the menstrual cycle; and white lesions occurring as the lesions age. Deep infiltrating lesions generally have blue/black or white features.”
“Wide, deep, multiple-color lesions in the cul-de-sac, ovarian fossa, or uterosacral ligaments are most likely endometriosis,” Dr. Stratton adds.23 Only lesions with multiple colors have a significantly higher percentage of positive biopsies (76%). Importantly, more than half of women with only subtle lesions (small red or white lesions) have endometriosis.
You tell the patient that endometriosis is one of the possible diagnoses for her chronic pelvic pain, and you take a focused history. During a pelvic examination, you observe that her right ovary lacks mobility, and you map a number of trigger points for her pain. Transvaginal ultrasound results suggest the presence of nodules in the rectovaginal septum. You begin empiric treatment with continuous combined hormonal contraceptives to suppress menstruation. On her next visit, M.L. reports reduced but still bothersome pain. Laparoscopy reveals a 2-cm endometrioma in the right ovary and deep infiltrating lesions in the cul-de-sac. The endometrioma is resected. Histology confirms the diagnosis of endometriosis.
REFERENCES
1. American College of Obstetricians and Gynecologists. Practice Bulletin #114: Management of endometriosis. Obstet Gynecol. 2010; 116(1):223-236.
2. Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986; 154(1):39-43.
3. Taylor HS, Bagot C, Kardana A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999; 14(5):1328-1331.
4. Berkley KJ, Stratton P. Mechanisms: lessons from translational studies of endometriosis. In: Giamberardino MA, ed. Visceral Pain: Clinical, Pathophysiological and Therapeutic Aspects. Oxford, UK: Oxford University Press; 2009:39-50.
5. Giudice LC. Clinical practice: endometriosis. N Engl J Med. 2010;362(25): 2389-2398.
6. Ballard K, Lowton K, Wright J. What’s the delay: a qualitative study of women’s experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296-1301.
7. May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update. 2010; 16(6):651-674.
8. May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011; 17(5):637-653.
9. Tamaresis JS, Irwin JC, Goldfien GA, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986-4999.
10. Sinaii N, Cleary SD, Ballweg ML, et al. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17(10):2715-2724.
11. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28(10):2677-2685.
12. Lafay Pillet MC, Huchon C, Santulli P, et al. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum Reprod. 2014;29(8):1666-1676.
13. Healey M, Cheng C, Kaur H. To excise or ablate endometriosis? A prospective randomized double-blinded trial after 5-year follow-up. J Minim Invasive Gynecol. 2014;21(6):999-1004.
14. Anaf V, El Nakadi I, De Moor V, et al. Increased nerve density in deep infiltrating endometriotic nodules. Gynecol Obstet Invest. 2011;71(2):112-117.
15. Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327-346.
16. Karp BI, Sinaii N, Nieman LK, et al. Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril. 2011;95(3):895-899.
17. Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86(3):272-280.
18. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
19. Vercellini P, Fedele L, Aimi G, et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266-271.
20. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93(1):51-58.
21. Muzii L, Di Tucci C, Di Feliciantonio M, et al. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod. 2014;29(10):2190-2198.
22. Muzii L, Luciano AA, Zupi E, Panici PB. Effect of surgery for endometrioma on ovarian function: a different point of view. J Minim Invasive Gynecol. 2014;21(4):531-533.
23. Stegmann BJ, Sinaii N, Liu S, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertil Steril. 2008;89(6):1632-1636.
What you need to know (and do) to prescribe the new drug flibanserin
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
It was a long road to approval by the US Food and Drug Administration (FDA), but flibanserin (Addyi) got the nod on August 18, 2015. Its New Drug Application (NDA) originally was filed October 27, 2009. The drug launched October 17, 2015.
Although there has been a lot of fanfare about approval of this drug, most of the coverage has focused on its status as the “first female Viagra”—a less than accurate depiction. For a more realistic and practical assessment of the drug, OBG Management turned to Michael Krychman, MD, executive director of the Southern California Center for Sexual Health and Survivorship Medicine in Newport Beach, to determine the types of information clinicians need to know to begin prescribing flibanserin. This article highlights 11 questions (and answers) to help you get started.
1. How did the FDA arrive
at its approval?
In 2012, the agency determined that female sexual dysfunction was one of 20 disease areas that warranted focused attention. In October 2014, as part of its intensified look at female sexual dysfunction, the FDA convened a 2-day meeting “to advance our understanding,” reports Andrea Fischer, FDA press officer.
“During the first day of the meeting, the FDA solicited patients’ perspectives on their condition and its impact on daily life. While this meeting did not focus on flibanserin, it provided an opportunity for the FDA to hear directly from patients about the impact of their condition,” Ms. Fischer says. During the second day of the meeting, the FDA “discussed scientific issues and challenges with experts in sexual medicine.”
As a result, by the time of the FDA’s June 4, 2015 Advisory Committee meeting on the flibanserin NDA, FDA physician-scientists were well versed in many nuances of female sexual function. That meeting included an open public hearing “that provided an opportunity for members of the public, including patients, to provide input specifically on the flibanserin application,” Ms. Fischer notes.
Nuances of the deliberations
“The FDA’s regulatory decision making on any drug product is a science-based process that carefully weighs each drug in terms of its risks and benefits to the patient population for which the drug would be indicated,” says Ms. Fischer.
The challenge in the case of flibanserin was determining whether the drug provides “clinically meaningful” improvements in sexual activity and desire.
“For many conditions and diseases, what constitutes ‘clinically meaningful’ is well known and accepted,” Ms. Fischer notes, “such as when something is cured or a severe symptom that is life-altering resolves completely. For others, this is not the case. For example, a condition that has a wide range of degree of severity can offer challenges in assessing what constitutes a clinically meaningful treatment effect. Ascertaining this requires a comprehensive knowledge of the disease, affected patient population, management strategies and the drug in question, as well as an ability to look at the clinical trial data taking this all into account.”
“In clinical trials, an important method for assessing the impact of a treatment on a patient’s symptoms, mental state, or functional status is through direct self-report using well developed and thoughtfully integrated patient-reported outcome (PRO) assessments,” Ms. Fischer says. “PROs can provide valuable information on the patient perspective when determining whether benefits outweigh risks, and they also are used to support medical product labeling claims, which are a key source of information for both health care providers and patients. PROs have been and continue to be a high priority as part of FDA’s commitment to advance patient-focused drug development, and we fully expect this to continue. The clinical trials in the flibanserin NDA all utilized PRO assessments.”
Those assessments found that patients taking flibanserin had a significant increase in “sexually satisfying events.” Three 24-week randomized controlled trials explored this endpoint for flibanserin (studies 1–3).
As for improvements in desire, the first 2 trials utilized an e-diary to assess this aspect of sexual function, while the 3rd trial utilized the Female Sexual Function Index (FSFI).
Although the e-diary reflected no statistically significant improvement in desire in the first 2 trials, the FSFI did find significant improvement in the 3rd trial. In addition, when the FSFI was considered across all 3 trials, results in the desire domain were consistent. (The FSFI was used as a secondary tool in the first 2 trials.)
In addition, sexual distress, as measured by the Female Sexual Distress Scale (FSDS), was decreased in the trials with use of flibanserin, notes Dr. Krychman. The Advisory Committee determined that these findings were sufficient to demonstrate clinically meaningful improvements with use of the drug.
Although the drug was approved by the FDA, the agency was sufficiently concerned about some of its potential risks (see questions 4 and 5) that it implemented rigorous mitigation strategies (see question 7). Additional investigations were requested by the agency, including drug-drug interaction, alcohol challenge, and driving studies.
2. What are the indications?
Flibanserin is intended for use in premenopausal women who have acquired, generalized hypoactive sexual desire disorder (HSDD). That diagnosis no longer is included in the 5th edition of the Diagnostic and
Statistical Manual of Mental Disorders but is described in drug package labeling as “low sexual desire that causes marked distress or interpersonal difficulty and is not due to:
- a coexisting medical or psychiatric condition,
- problems within the relationship, or
- the effects of a medication or other drug substance.”1
- the effects of a medication or other drug substance.”
Although the drug has been tested in both premenopausal and postmenopausal women, it was approved for use only in premenopausal women. Also note inclusion of the term “acquired” before the diagnosis of HSDD, indicating that the drug is inappropriate for women who have never experienced a period of normal sexual desire.
3. How is HSDD diagnosed?
One of the best screening tools is the
Decreased Sexual Desire Screener, says
Dr. Krychman. It is available at http://obgynalliance.com/files/fsd/DSDS_Pocketcard.pdf. This tool is a validated instrument to help clinicians identify what HSDD is and is not.
4. Does the drug carry
any warnings?
Yes, it carries a black box warning about the risks of hypotension and syncope:
- when alcohol is consumed by users of the drug. (Alcohol use is contraindicated.)
- when the drug is taken in conjunction with moderate or strong CYP3A4 inhibitors or by patients with hepatic impairment. (The drug is contraindicated in both circumstances.) See question 9 for a list of drugs that are CYP3A4 inhibitors.
5. Are there any other risks worth noting?
The medication can increase the risks of hypotension and syncope even without concomitant use of alcohol. For example, in clinical trials, hypotension was reported in 0.2% of flibanserin-treated women versus less than 0.1% of placebo users. And syncope was reported in 0.4% of flibanserin users versus 0.2% of placebo-treated patients. Flibanserin is prescribed as a once-daily medication that is to be taken at bedtime; the risks of hypotension and syncope are increased if flibanserin is taken during waking hours.
The risk of adverse effects when flibanserin is taken with alcohol is highlighted by one case reported in package labeling: A 54-year-old postmenopausal woman died after taking flibanserin (100 mg daily at bedtime) for 14 days. This patient had a history of hypertension and hypercholesterolemia and consumed a baseline amount of 1 to 3 alcoholic beverages daily. She died of acute alcohol intoxication, with a blood alcohol concentration of 0.289 g/dL.1 Whether this patient’s death was related to flibanserin use is unknown.1
It is interesting to note that, in the studies of flibanserin leading up to the drug’s approval, alcohol use was not an exclusion, says Dr. Krychman. “Approximately 58% of women were self-described as mild to moderate drinkers. The clinical program was extremely large—more than 11,000 women were studied.”
Flibanserin is currently not approved for use in postmenopausal women, and concomitant alcohol consumption is contraindicated.
6. What is the dose?
The recommended dose is one tablet of
100 mg daily. The drug is to be taken at
bedtime to reduce the risks of hypotension, syncope, accidental injury, and central nervous system (CNS) depression, which can occur even in the absence of alcohol.
7. Are there any requirements for clinicians who want to prescribe the drug?
Yes. Because of the risks of hypotension, syncope, and CNS depression, the drug is subject to Risk Evaluation and Mitigation Strategies (REMS), as determined by the FDA. To prescribe the drug, providers must:
- review its prescribing information
- review the Provider and Pharmacy Training Program
- complete and submit the Knowledge Assessment Form
- enroll in REMS by completing and submitting the Prescriber Enrollment Form.
Before giving a patient her initial prescription, the provider must counsel her about the risks of hypotension and syncope and the interaction with alcohol using the Patient-Provider Agreement Form. The provider must then complete that form, provide a designated portion of it to the patient, and retain the remainder for the patient’s file.
For more information and to download the relevant forms, visit https://www.addyirems.com.
8. What are the most common
adverse reactions to the drug?
According to package labeling, the most common adverse reactions, with an incidence greater than 2%, are dizziness, somnolence, nausea, fatigue, insomnia, and dry mouth.
Less common reactions include anxiety, constipation, abdominal pain, rash, sedation, and vertigo.
In studies of the drug, appendicitis was reported among 0.2% of flibanserin-treated patients, compared with no reports of appendicitis among placebo-treated patients. The FDA has requested additional investigation of the association, if any, between flibanserin and appendicitis.
9. What drug interactions are notable?
As stated earlier, the concomitant use of flibanserin with alcohol or a moderate or strong CYP3A4 inhibitor can result in severe hypotension and syncope. Flibanserin also should not be prescribed for patients who use other CNS depressants such as diphenhydramine, opioids, benzodiazepines, and hypnotic agents.
Some examples of strong CYP3A4 inhibitors are ketoconazole, itraconazole, posaconazole, clarithromycin, nefazodone, ritonavir, saquinavir, nelfinavir, indinavir, boceprevir, telaprevir, telithromycin, and conivaptan.
Moderate CYP3A4 inhibitors include amprenavir, atazanavir, ciprofloxacin, diltiazem, erythromycin, fluconazole, fosamprenavir, verapamil, and grapefruit juice.
In addition, the concomitant use of flibanserin with multiple weak CYP3A4 inhibitors—which include herbal supplements such as ginkgo and resveratrol and nonprescription drugs such as cimetidine—also may increase the risks of hypotension and syncope.
The concomitant use of flibanserin with digoxin increases the digoxin concentration and may lead to toxicity.
10. Is the drug safe in pregnancy
and lactation?
There are currently no data on the use of flibanserin in human pregnancy. In animals, fetal toxicity occurred only in the presence of significant maternal toxicity. Adverse effects included decreased fetal weight, structural anomalies, and increases in fetal loss when exposure exceeded 15 times the recommended human dosage.
As for the advisability of using flibanserin during lactation, it is unknown whether the drug is excreted in human milk, whether it might have adverse effects in the breastfed infant, or whether it affects milk production. Package labeling states: “Because of the potential for serious adverse reactions, including sedation in a breastfed infant, breastfeeding is not recommended during treatment with [flibanserin].”1
11. When should the drug
be discontinued?
If there is no improvement in sexual desire after an 8-week trial of flibanserin, the drug should be
discontinued.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
Reference
- Addyi [package insert]. Raleigh, NC: Sprout Pharmaceuticals; 2015.
In this Article
- How is HSDD diagnosed?
- What are clinicians required to do?
- Is the drug safe in pregnancy?
What you should know about the latest change in mammography screening guidelines
When the American Cancer Society (ACS) updated its guidelines for screening mammography earlier this week,1 the effect was that of a stone being tossed into a tranquil pond, generating ripples in all directions.
The new guidelines focus on women at average risk for breast cancer (TABLE 1) and were updated for the first time since 2003, based on new evidence, a new emphasis on eliminating as many screening harms as possible, and a goal of “supporting the interplay among values, preferences, informed decision making, and recommendations.”1 Earlier ACS guidelines recommended annual screening starting at age 40.
TABLE 1 What constitutes “average risk” of breast cancer?
|
The new guidelines are graded according to the strength of the rec ommendation as being either “strong” or “qualified.” The ACS defines a “strong” recommendation as one that most individuals should follow. “Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator,” the guidelines note.1
A “qualified” recommendation indicates that “Clinicians should acknowledge that different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her or his values and preferences.”1
The recommendations are:
- Regular screening mammography should start at age 45 years (strong recommendation)
- Screening should be annual in women aged 45 to 54 years (qualified recommendation)
- Screening should shift to biennial intervals at age 55, unless the patient prefers to continue screening annually (qualified recommendation)
- Women who desire to initiate annual screening between the ages of 40 and 44 years should be accommodated (qualified recommendation)
- Screening mammography should continue as long as the woman is in good health and has a life expectancy of at least 10 years (qualified recommendation)
- Clinical breast examination (CBE) is not recommended at any age (qualified recommendation).1
ACOG weighs in
Shortly after publication of the new ACS guidelines, the American College of Obstetricians and Gynecologists (ACOG) issued a formal statement in response2:
Response of the USPSTF
The US Preventive Services Task Force (USPSTF) also issued a statement in response to the new ACS guidelines:
The USPSTF currently recommends biennial screening beginning at age 50.
A leader in breast health cites pros and cons of ACS recommendations
Mark Pearlman, MD, professor of obstetrics and gynecology at the University of Michigan health system, is a nationally recognized expert on breast cancer screening. He sits on the National Comprehensive Cancer Network (NCCN) breast cancer screening and diagnosis group, helped author ACOG guidelines on mammography screening, and serves as a Contributing Editor to OBG Management.
“I believe the overall ACS mammography benefit evidence synthesis is reasonable and is in keeping with both NCCN and ACOG’s current recommendations. NCCN and ACOG mammography screening recommendations have both valued lives saved more highly than the ‘harms’ such as recalls and needle biopsies,” Dr. Pearlman says.
“If one combines ACS ‘strong’ and ‘qualified’ recommendations, ACS recommendations are similar to current ACOG and NCCN recommendations for mammography,” he adds.
Dr. Pearlman finds 7 areas of agreement between NCCN/ACOG and ACS recommendations, using both strong and qualified recommendations:
- “They reaffirm that screening from age 40 to 69 years is associated with a reduction in breast cancer deaths.
- They support annual screening for women in their 40s [although the ACS’ ‘strong’ recommendation is that regular screening begin at age 45 instead of 40].
- They support screening for women 70 and older who are in good health (10-year life expectancy).
- They support the finding that annual screening yields a larger mortality reduction than biennial screening.
- They confirm much uncertainty about the “over-diagnosis/overtreatment” issue.
- They endorse insurance coverage at all ages and intervals of screening (not just USPSTF ‘A’ or ‘B’ recommendations).
- They involve the patient in informed decision making.”
Where the ACS and ACOG/NCCN disagree is over the issue of the physical exam (abandoning CBE in average-risk women).
In regard to this last item, Dr. Pearlman says, “The ACS made a qualified recommendation against clinical breast exam. There is no high-level data to support such a marked change in practice. For example, when recommendations against breast self-examinations (BSE) were made, there were randomized controlled trials (RCTs) showing a lack of benefit and significant harms with BSE. With RCT-level data, it made sense to make a recommendation against the long-taught practice of SBE in average-risk women. That was not the case here. In fact, there are small amounts of data showing benefits of clinical breast exam.”
“One of my biggest concerns is not just the recommendation against CBE,” says Dr. Pearlman, “but that this may lead many women to interpret [this statement] as if they do not need to see their health care provider anymore. As you may recall, the American College of Physicians (ACP) recommended against annual pelvic examinations in asymptomatic patients. The ACS recommendation statement—taken together with the ACP statement—basically suggests that average-risk women don’t ever need to see a provider for a pelvic or breast examination except every 5 years for a Pap smear. That thinking does not recognize the importance of the clinical encounter (not just the CBE or pelvic exam), which is the opportunity to perform risk assessment and provide risk-reduction recommendations and healthy lifestyle recommendations.”
Radiologists resist new recommendations
Although the American College of Radiology (ACR) and the Society of Breast Imaging (SBI) agree with the ACS that mammography screening saves lives and should be available to women aged 40 and older, the 2 imaging organizations continue to recommend that annual screening begin at age 40. Their rationale: The latest ACS breast cancer screening guidelines, and earlier data used by the USPSTF to create its recommendations, both note that starting annual mammography at age 40 “saves the most lives.”
Where the organizations differ from the ACR is summed up by a formal statement on the ACR Web site: “The ACR and SBI strongly encourage women to obtain the maximum lifesaving benefits from mammography by continuing to get annual screening.”4
When OBG Management touched base with radiologist Barbara Monsees, MD, professor of radiology and Evens Professor of Women’s Health at Washington University Medical Center in St. Louis, Missouri, she expressed dismay at early news reports on the ACS guidelines.
“I’m dismayed that the headlines don’t seem to correlate with what the ACS actually recommended. The ACS did not state that women should wait until age 45 to begin screening. I believe the ACS was going for a more nuanced approach, but since that’s a bit complicated, I think that reporters have misconstrued what was intended,” Dr. Monsees says.
“The ACS guideline says that women between 40 and 44 years should have the opportunity to begin annual screening,” she says, noting that this recommendation was graded as “qualified.”
“The ACS states that a qualified recommendation indicates that ‘there is clear evidence of benefit of screening, but less certainty about the balance of benefits and harms, or about patients’ values and preferences, which could lead to different decisions about screening.’” The guideline also articulates the view “that the meaning of a qualified recommendation for patients is that the ‘majority of individuals in this situation would want the suggested course of action, but many would not.’ Therefore, I find it mind-boggling that this has been interpreted to mean that women should not begin screening until age 45.”1
“It is my opinion that it is clear that if women want to achieve the most lifesaving benefit from screening, they should adhere to a schedule of yearly mammograms beginning at age 40,” says Dr. Monsees. However, she also agrees with the ACS notation that clinicians should acknowledge that “different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her values and preferences.”1
The word from an expert ObGyn
“By changing its guidance to begin screening at age 45 instead of 40, and in recommending biennial rather than annual screens in women 55 years of age and older, the updated ACS guidance will reduce harms (overdiagnosis and unnecessary additional imaging and biopsies) and moves closer to USPSTF guidance,” says Andrew M. Kaunitz, MD. He is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
“As one editorialist points out, the ACS recommendation that women begin screening at age 45 years is based on observational comparisons of screened and unscreened cohorts—a type of analysis which the USPSTF does not consider due to concerns regarding bias,” notes Dr. Kaunitz.5
“The ACS recommendation for annual screening in women aged 45 to 54 is largely based on the findings of a report showing that, for premenopausal (but not postmenopausal) women, tumor stage was higher and size larger for screen-detected lesions among women undergoing biennial screens."6
As for the recommendation against screening CBE, Dr. Kaunitz considers that “a dramatic change from prior guidance. It is based on the absence of data finding benefits with CBE (alone or with screening mammography). Furthermore, the updated ACS guidance does not change its 2003 guidance, which does not support routine performance of or instruction regarding SBE.”
“These updated ACS guidelines should result in more women starting screening mammograms later in life, and they endorse biennial screening for many women, meaning that patients following ACS guidance will have fewer lifetime screens than with earlier recommendations,” says Dr. Kaunitz.
“Another plus is that performing fewer breast examinations during well-woman visits will allow us more time to assess family history and other risk factors for breast cancer, and to discuss screening recommendations.”
The bottom line
What is one to make of the many viewpoints on screening? For now, it probably is best to adhere to either the new ACS guidelines or current ACOG guidelines (TABLE 2), says OBG Management Editor in Chief Robert L. Barbieri, MD. He is chief of the Department of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, and Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School.
TABLE 2 What are ACOG’s current recommendations?
|
ACOG recommends screening mammography every year for women starting at age 40. ACOG also states that “breast self-awareness has the potential to detect palpable breast cancer and can be recommended”; it also recommends CBE every year for women aged 19 or older.
These recommendations may change early next year, after ACOG convenes a consensus conference on the subject. The aim: “To develop a consistent set of uniform guidelines for breast cancer screening that can be implemented nationwide. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail.”2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk. 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists. ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 20, 2015.
- US Preventive Services Task Force. Email communication, USPSTF Newsroom, October 20, 2015.
- American College of Radiology. News Release: ACR and SBI Continue to Recommend Regular Mammography Starting at Age 40. http://www.acr.org/About-Us/Media-Center/Press-Releases/2015-Press-Releases/20151020-ACR-SBI-Recommend-Mammography-at-Age-40. Published October 20, 2015. Accessed October 21, 2015.
- Kerlikowske K. Progress toward consensus on breast cancer screening guidelines and reducing screening harms [published online ahead of print October 20, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6466.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status [published online ahead of print October 20, 2015]. JAMA. doi:10.1001/jamaoncol.2015.3084.
When the American Cancer Society (ACS) updated its guidelines for screening mammography earlier this week,1 the effect was that of a stone being tossed into a tranquil pond, generating ripples in all directions.
The new guidelines focus on women at average risk for breast cancer (TABLE 1) and were updated for the first time since 2003, based on new evidence, a new emphasis on eliminating as many screening harms as possible, and a goal of “supporting the interplay among values, preferences, informed decision making, and recommendations.”1 Earlier ACS guidelines recommended annual screening starting at age 40.
TABLE 1 What constitutes “average risk” of breast cancer?
|
The new guidelines are graded according to the strength of the rec ommendation as being either “strong” or “qualified.” The ACS defines a “strong” recommendation as one that most individuals should follow. “Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator,” the guidelines note.1
A “qualified” recommendation indicates that “Clinicians should acknowledge that different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her or his values and preferences.”1
The recommendations are:
- Regular screening mammography should start at age 45 years (strong recommendation)
- Screening should be annual in women aged 45 to 54 years (qualified recommendation)
- Screening should shift to biennial intervals at age 55, unless the patient prefers to continue screening annually (qualified recommendation)
- Women who desire to initiate annual screening between the ages of 40 and 44 years should be accommodated (qualified recommendation)
- Screening mammography should continue as long as the woman is in good health and has a life expectancy of at least 10 years (qualified recommendation)
- Clinical breast examination (CBE) is not recommended at any age (qualified recommendation).1
ACOG weighs in
Shortly after publication of the new ACS guidelines, the American College of Obstetricians and Gynecologists (ACOG) issued a formal statement in response2:
Response of the USPSTF
The US Preventive Services Task Force (USPSTF) also issued a statement in response to the new ACS guidelines:
The USPSTF currently recommends biennial screening beginning at age 50.
A leader in breast health cites pros and cons of ACS recommendations
Mark Pearlman, MD, professor of obstetrics and gynecology at the University of Michigan health system, is a nationally recognized expert on breast cancer screening. He sits on the National Comprehensive Cancer Network (NCCN) breast cancer screening and diagnosis group, helped author ACOG guidelines on mammography screening, and serves as a Contributing Editor to OBG Management.
“I believe the overall ACS mammography benefit evidence synthesis is reasonable and is in keeping with both NCCN and ACOG’s current recommendations. NCCN and ACOG mammography screening recommendations have both valued lives saved more highly than the ‘harms’ such as recalls and needle biopsies,” Dr. Pearlman says.
“If one combines ACS ‘strong’ and ‘qualified’ recommendations, ACS recommendations are similar to current ACOG and NCCN recommendations for mammography,” he adds.
Dr. Pearlman finds 7 areas of agreement between NCCN/ACOG and ACS recommendations, using both strong and qualified recommendations:
- “They reaffirm that screening from age 40 to 69 years is associated with a reduction in breast cancer deaths.
- They support annual screening for women in their 40s [although the ACS’ ‘strong’ recommendation is that regular screening begin at age 45 instead of 40].
- They support screening for women 70 and older who are in good health (10-year life expectancy).
- They support the finding that annual screening yields a larger mortality reduction than biennial screening.
- They confirm much uncertainty about the “over-diagnosis/overtreatment” issue.
- They endorse insurance coverage at all ages and intervals of screening (not just USPSTF ‘A’ or ‘B’ recommendations).
- They involve the patient in informed decision making.”
Where the ACS and ACOG/NCCN disagree is over the issue of the physical exam (abandoning CBE in average-risk women).
In regard to this last item, Dr. Pearlman says, “The ACS made a qualified recommendation against clinical breast exam. There is no high-level data to support such a marked change in practice. For example, when recommendations against breast self-examinations (BSE) were made, there were randomized controlled trials (RCTs) showing a lack of benefit and significant harms with BSE. With RCT-level data, it made sense to make a recommendation against the long-taught practice of SBE in average-risk women. That was not the case here. In fact, there are small amounts of data showing benefits of clinical breast exam.”
“One of my biggest concerns is not just the recommendation against CBE,” says Dr. Pearlman, “but that this may lead many women to interpret [this statement] as if they do not need to see their health care provider anymore. As you may recall, the American College of Physicians (ACP) recommended against annual pelvic examinations in asymptomatic patients. The ACS recommendation statement—taken together with the ACP statement—basically suggests that average-risk women don’t ever need to see a provider for a pelvic or breast examination except every 5 years for a Pap smear. That thinking does not recognize the importance of the clinical encounter (not just the CBE or pelvic exam), which is the opportunity to perform risk assessment and provide risk-reduction recommendations and healthy lifestyle recommendations.”
Radiologists resist new recommendations
Although the American College of Radiology (ACR) and the Society of Breast Imaging (SBI) agree with the ACS that mammography screening saves lives and should be available to women aged 40 and older, the 2 imaging organizations continue to recommend that annual screening begin at age 40. Their rationale: The latest ACS breast cancer screening guidelines, and earlier data used by the USPSTF to create its recommendations, both note that starting annual mammography at age 40 “saves the most lives.”
Where the organizations differ from the ACR is summed up by a formal statement on the ACR Web site: “The ACR and SBI strongly encourage women to obtain the maximum lifesaving benefits from mammography by continuing to get annual screening.”4
When OBG Management touched base with radiologist Barbara Monsees, MD, professor of radiology and Evens Professor of Women’s Health at Washington University Medical Center in St. Louis, Missouri, she expressed dismay at early news reports on the ACS guidelines.
“I’m dismayed that the headlines don’t seem to correlate with what the ACS actually recommended. The ACS did not state that women should wait until age 45 to begin screening. I believe the ACS was going for a more nuanced approach, but since that’s a bit complicated, I think that reporters have misconstrued what was intended,” Dr. Monsees says.
“The ACS guideline says that women between 40 and 44 years should have the opportunity to begin annual screening,” she says, noting that this recommendation was graded as “qualified.”
“The ACS states that a qualified recommendation indicates that ‘there is clear evidence of benefit of screening, but less certainty about the balance of benefits and harms, or about patients’ values and preferences, which could lead to different decisions about screening.’” The guideline also articulates the view “that the meaning of a qualified recommendation for patients is that the ‘majority of individuals in this situation would want the suggested course of action, but many would not.’ Therefore, I find it mind-boggling that this has been interpreted to mean that women should not begin screening until age 45.”1
“It is my opinion that it is clear that if women want to achieve the most lifesaving benefit from screening, they should adhere to a schedule of yearly mammograms beginning at age 40,” says Dr. Monsees. However, she also agrees with the ACS notation that clinicians should acknowledge that “different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her values and preferences.”1
The word from an expert ObGyn
“By changing its guidance to begin screening at age 45 instead of 40, and in recommending biennial rather than annual screens in women 55 years of age and older, the updated ACS guidance will reduce harms (overdiagnosis and unnecessary additional imaging and biopsies) and moves closer to USPSTF guidance,” says Andrew M. Kaunitz, MD. He is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
“As one editorialist points out, the ACS recommendation that women begin screening at age 45 years is based on observational comparisons of screened and unscreened cohorts—a type of analysis which the USPSTF does not consider due to concerns regarding bias,” notes Dr. Kaunitz.5
“The ACS recommendation for annual screening in women aged 45 to 54 is largely based on the findings of a report showing that, for premenopausal (but not postmenopausal) women, tumor stage was higher and size larger for screen-detected lesions among women undergoing biennial screens."6
As for the recommendation against screening CBE, Dr. Kaunitz considers that “a dramatic change from prior guidance. It is based on the absence of data finding benefits with CBE (alone or with screening mammography). Furthermore, the updated ACS guidance does not change its 2003 guidance, which does not support routine performance of or instruction regarding SBE.”
“These updated ACS guidelines should result in more women starting screening mammograms later in life, and they endorse biennial screening for many women, meaning that patients following ACS guidance will have fewer lifetime screens than with earlier recommendations,” says Dr. Kaunitz.
“Another plus is that performing fewer breast examinations during well-woman visits will allow us more time to assess family history and other risk factors for breast cancer, and to discuss screening recommendations.”
The bottom line
What is one to make of the many viewpoints on screening? For now, it probably is best to adhere to either the new ACS guidelines or current ACOG guidelines (TABLE 2), says OBG Management Editor in Chief Robert L. Barbieri, MD. He is chief of the Department of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, and Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School.
TABLE 2 What are ACOG’s current recommendations?
|
ACOG recommends screening mammography every year for women starting at age 40. ACOG also states that “breast self-awareness has the potential to detect palpable breast cancer and can be recommended”; it also recommends CBE every year for women aged 19 or older.
These recommendations may change early next year, after ACOG convenes a consensus conference on the subject. The aim: “To develop a consistent set of uniform guidelines for breast cancer screening that can be implemented nationwide. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail.”2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
When the American Cancer Society (ACS) updated its guidelines for screening mammography earlier this week,1 the effect was that of a stone being tossed into a tranquil pond, generating ripples in all directions.
The new guidelines focus on women at average risk for breast cancer (TABLE 1) and were updated for the first time since 2003, based on new evidence, a new emphasis on eliminating as many screening harms as possible, and a goal of “supporting the interplay among values, preferences, informed decision making, and recommendations.”1 Earlier ACS guidelines recommended annual screening starting at age 40.
TABLE 1 What constitutes “average risk” of breast cancer?
|
The new guidelines are graded according to the strength of the rec ommendation as being either “strong” or “qualified.” The ACS defines a “strong” recommendation as one that most individuals should follow. “Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator,” the guidelines note.1
A “qualified” recommendation indicates that “Clinicians should acknowledge that different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her or his values and preferences.”1
The recommendations are:
- Regular screening mammography should start at age 45 years (strong recommendation)
- Screening should be annual in women aged 45 to 54 years (qualified recommendation)
- Screening should shift to biennial intervals at age 55, unless the patient prefers to continue screening annually (qualified recommendation)
- Women who desire to initiate annual screening between the ages of 40 and 44 years should be accommodated (qualified recommendation)
- Screening mammography should continue as long as the woman is in good health and has a life expectancy of at least 10 years (qualified recommendation)
- Clinical breast examination (CBE) is not recommended at any age (qualified recommendation).1
ACOG weighs in
Shortly after publication of the new ACS guidelines, the American College of Obstetricians and Gynecologists (ACOG) issued a formal statement in response2:
Response of the USPSTF
The US Preventive Services Task Force (USPSTF) also issued a statement in response to the new ACS guidelines:
The USPSTF currently recommends biennial screening beginning at age 50.
A leader in breast health cites pros and cons of ACS recommendations
Mark Pearlman, MD, professor of obstetrics and gynecology at the University of Michigan health system, is a nationally recognized expert on breast cancer screening. He sits on the National Comprehensive Cancer Network (NCCN) breast cancer screening and diagnosis group, helped author ACOG guidelines on mammography screening, and serves as a Contributing Editor to OBG Management.
“I believe the overall ACS mammography benefit evidence synthesis is reasonable and is in keeping with both NCCN and ACOG’s current recommendations. NCCN and ACOG mammography screening recommendations have both valued lives saved more highly than the ‘harms’ such as recalls and needle biopsies,” Dr. Pearlman says.
“If one combines ACS ‘strong’ and ‘qualified’ recommendations, ACS recommendations are similar to current ACOG and NCCN recommendations for mammography,” he adds.
Dr. Pearlman finds 7 areas of agreement between NCCN/ACOG and ACS recommendations, using both strong and qualified recommendations:
- “They reaffirm that screening from age 40 to 69 years is associated with a reduction in breast cancer deaths.
- They support annual screening for women in their 40s [although the ACS’ ‘strong’ recommendation is that regular screening begin at age 45 instead of 40].
- They support screening for women 70 and older who are in good health (10-year life expectancy).
- They support the finding that annual screening yields a larger mortality reduction than biennial screening.
- They confirm much uncertainty about the “over-diagnosis/overtreatment” issue.
- They endorse insurance coverage at all ages and intervals of screening (not just USPSTF ‘A’ or ‘B’ recommendations).
- They involve the patient in informed decision making.”
Where the ACS and ACOG/NCCN disagree is over the issue of the physical exam (abandoning CBE in average-risk women).
In regard to this last item, Dr. Pearlman says, “The ACS made a qualified recommendation against clinical breast exam. There is no high-level data to support such a marked change in practice. For example, when recommendations against breast self-examinations (BSE) were made, there were randomized controlled trials (RCTs) showing a lack of benefit and significant harms with BSE. With RCT-level data, it made sense to make a recommendation against the long-taught practice of SBE in average-risk women. That was not the case here. In fact, there are small amounts of data showing benefits of clinical breast exam.”
“One of my biggest concerns is not just the recommendation against CBE,” says Dr. Pearlman, “but that this may lead many women to interpret [this statement] as if they do not need to see their health care provider anymore. As you may recall, the American College of Physicians (ACP) recommended against annual pelvic examinations in asymptomatic patients. The ACS recommendation statement—taken together with the ACP statement—basically suggests that average-risk women don’t ever need to see a provider for a pelvic or breast examination except every 5 years for a Pap smear. That thinking does not recognize the importance of the clinical encounter (not just the CBE or pelvic exam), which is the opportunity to perform risk assessment and provide risk-reduction recommendations and healthy lifestyle recommendations.”
Radiologists resist new recommendations
Although the American College of Radiology (ACR) and the Society of Breast Imaging (SBI) agree with the ACS that mammography screening saves lives and should be available to women aged 40 and older, the 2 imaging organizations continue to recommend that annual screening begin at age 40. Their rationale: The latest ACS breast cancer screening guidelines, and earlier data used by the USPSTF to create its recommendations, both note that starting annual mammography at age 40 “saves the most lives.”
Where the organizations differ from the ACR is summed up by a formal statement on the ACR Web site: “The ACR and SBI strongly encourage women to obtain the maximum lifesaving benefits from mammography by continuing to get annual screening.”4
When OBG Management touched base with radiologist Barbara Monsees, MD, professor of radiology and Evens Professor of Women’s Health at Washington University Medical Center in St. Louis, Missouri, she expressed dismay at early news reports on the ACS guidelines.
“I’m dismayed that the headlines don’t seem to correlate with what the ACS actually recommended. The ACS did not state that women should wait until age 45 to begin screening. I believe the ACS was going for a more nuanced approach, but since that’s a bit complicated, I think that reporters have misconstrued what was intended,” Dr. Monsees says.
“The ACS guideline says that women between 40 and 44 years should have the opportunity to begin annual screening,” she says, noting that this recommendation was graded as “qualified.”
“The ACS states that a qualified recommendation indicates that ‘there is clear evidence of benefit of screening, but less certainty about the balance of benefits and harms, or about patients’ values and preferences, which could lead to different decisions about screening.’” The guideline also articulates the view “that the meaning of a qualified recommendation for patients is that the ‘majority of individuals in this situation would want the suggested course of action, but many would not.’ Therefore, I find it mind-boggling that this has been interpreted to mean that women should not begin screening until age 45.”1
“It is my opinion that it is clear that if women want to achieve the most lifesaving benefit from screening, they should adhere to a schedule of yearly mammograms beginning at age 40,” says Dr. Monsees. However, she also agrees with the ACS notation that clinicians should acknowledge that “different choices will be appropriate for different patients and that clinicians must help each patient arrive at a management decision consistent with her values and preferences.”1
The word from an expert ObGyn
“By changing its guidance to begin screening at age 45 instead of 40, and in recommending biennial rather than annual screens in women 55 years of age and older, the updated ACS guidance will reduce harms (overdiagnosis and unnecessary additional imaging and biopsies) and moves closer to USPSTF guidance,” says Andrew M. Kaunitz, MD. He is University of Florida Research Foundation Professor and Associate Chairman, Department of Obstetrics and Gynecology, at the University of Florida College of Medicine–Jacksonville. He also serves on the OBG Management Board of Editors.
“As one editorialist points out, the ACS recommendation that women begin screening at age 45 years is based on observational comparisons of screened and unscreened cohorts—a type of analysis which the USPSTF does not consider due to concerns regarding bias,” notes Dr. Kaunitz.5
“The ACS recommendation for annual screening in women aged 45 to 54 is largely based on the findings of a report showing that, for premenopausal (but not postmenopausal) women, tumor stage was higher and size larger for screen-detected lesions among women undergoing biennial screens."6
As for the recommendation against screening CBE, Dr. Kaunitz considers that “a dramatic change from prior guidance. It is based on the absence of data finding benefits with CBE (alone or with screening mammography). Furthermore, the updated ACS guidance does not change its 2003 guidance, which does not support routine performance of or instruction regarding SBE.”
“These updated ACS guidelines should result in more women starting screening mammograms later in life, and they endorse biennial screening for many women, meaning that patients following ACS guidance will have fewer lifetime screens than with earlier recommendations,” says Dr. Kaunitz.
“Another plus is that performing fewer breast examinations during well-woman visits will allow us more time to assess family history and other risk factors for breast cancer, and to discuss screening recommendations.”
The bottom line
What is one to make of the many viewpoints on screening? For now, it probably is best to adhere to either the new ACS guidelines or current ACOG guidelines (TABLE 2), says OBG Management Editor in Chief Robert L. Barbieri, MD. He is chief of the Department of Obstetrics and Gynecology at Brigham and Women’s Hospital in Boston, and Kate Macy Ladd Professor of Obstetrics, Gynecology, and Reproductive Biology at Harvard Medical School.
TABLE 2 What are ACOG’s current recommendations?
|
ACOG recommends screening mammography every year for women starting at age 40. ACOG also states that “breast self-awareness has the potential to detect palpable breast cancer and can be recommended”; it also recommends CBE every year for women aged 19 or older.
These recommendations may change early next year, after ACOG convenes a consensus conference on the subject. The aim: “To develop a consistent set of uniform guidelines for breast cancer screening that can be implemented nationwide. Major organizations and providers of women’s health care, including ACS, will gather to evaluate and interpret the data in greater detail.”2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk. 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists. ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 20, 2015.
- US Preventive Services Task Force. Email communication, USPSTF Newsroom, October 20, 2015.
- American College of Radiology. News Release: ACR and SBI Continue to Recommend Regular Mammography Starting at Age 40. http://www.acr.org/About-Us/Media-Center/Press-Releases/2015-Press-Releases/20151020-ACR-SBI-Recommend-Mammography-at-Age-40. Published October 20, 2015. Accessed October 21, 2015.
- Kerlikowske K. Progress toward consensus on breast cancer screening guidelines and reducing screening harms [published online ahead of print October 20, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6466.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status [published online ahead of print October 20, 2015]. JAMA. doi:10.1001/jamaoncol.2015.3084.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk. 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists. ACOG Statement on Revised American Cancer Society Recommendations on Breast Cancer Screening. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Recommendations-on-Breast-Cancer-Screening. Published October 20, 2015. Accessed October 20, 2015.
- US Preventive Services Task Force. Email communication, USPSTF Newsroom, October 20, 2015.
- American College of Radiology. News Release: ACR and SBI Continue to Recommend Regular Mammography Starting at Age 40. http://www.acr.org/About-Us/Media-Center/Press-Releases/2015-Press-Releases/20151020-ACR-SBI-Recommend-Mammography-at-Age-40. Published October 20, 2015. Accessed October 21, 2015.
- Kerlikowske K. Progress toward consensus on breast cancer screening guidelines and reducing screening harms [published online ahead of print October 20, 2015]. JAMA Intern Med. doi:10.1001/jamainternmed.2015.6466.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status [published online ahead of print October 20, 2015]. JAMA. doi:10.1001/jamaoncol.2015.3084.
A survey of liability claims against obstetric providers highlights major areas of contention
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
An analysis of 882 obstetric claims closed between 2007 and 2014 highlighted 3 common allegationsby patients1:
- a delay in the treatment of fetal distress (22%). The term “fetal distress” remains a common allegation in malpractice claims. Cases in this category most often reflected a delay or failure to act in the face of Category II or III fetal heart-rate tracings.
- improper performance of vaginal delivery (20%). Almost half of the cases in this category involved brachial plexus injuries linked to shoulder dystocia. Patients alleged that improper maneuvers were used to resolve the dystocia. The remainder of cases in this category involved forceps and vacuum extraction deliveries.
- improper management of pregnancy (17%). Among the allegations were a failure to test for fetal abnormalities, failure to recognize complications of pregnancy, and failure to address abnormal findings.
Together, these 3 allegations accounted for 59% of claims. Other allegations included diagnosis-related claims, delay in delivery, improper performance of operative delivery, retained foreign bodies, and improper choice of delivery method.1
Where are the really big malpractice awards?
Everything may be bigger in Texas, but New York is the biggest in at least 1 area: large medical malpractice payments. New York had more than 3 times as many $1 million-plus malpractice awards as any other state in 2014, according to data from the National Practitioner Data Bank (NPDB).1
New York physicians had 210 malpractice payments of $1 million or more reported to the NPDB last year, compared with 61 for Illinois, the next-highest state. Rounding out the top 5 were Massachusetts with 49, followed by California with 43, and New Jersey with 41, the NPDB data show.
After taking population into account, New York was still the leader with 10.66 large awards per million residents. Next in this category was the New England trio of Rhode Island, which had 9.42 such payments per 1 million population; Massachusetts (7.26); and Connecticut (6.39).
In 2014, there were 4 states that had no malpractice payments of at least $1 million reported to the NPDB: Alaska, Kansas, North Dakota, and Nebraska, with Kansas having the largest population. In states with at least one $1 million-plus malpractice payment, Texas physicians had the lowest rate per million population, 0.22—just 6 awards from a population of 27 million.
Reference
1. NPDB Research Statistics. National Practitioner Data Bank. http://www.npdb.hrsa.gov/resources/npdbstats/npdbStatistics.jsp. Accessed
July 17, 2015.
Copyright © 2015 Ob.Gyn. News Digital Network, Frontline Medical Communications. Available at: http://www.obgynews.com/?id=11146&tx_ttnews[tt_news]=417377&cHash=5cc8cd69fa7c8a1186aaeec0e814e4e4
The Obstetrics Closed Claims Study findings were released earlier this spring by the Napa, California−based Doctors Company, the nation’s largest physician-owned medical malpractice insurer.1 Susan Mann, MD,a spokesperson for the company, provided expert commentary on the study at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco (see “Frequent sources of malpractice claims” below).
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
Frequent sources of malpractice claims
Communication breakdowns and treatment delays are frequent sources of malpractice claims. Susan Mann, MD, spokesperson for The Doctors Company, the nation’s largest physician-owned medical malpractice insurer, discusses the underlying practice vulnerabilities revealed by the Obstetrics Closed Claims Study.
Dr. Mann practices obstetrics and gynecology in Brookline, Massachusetts, and at Beth Israel Deaconess Medical Center in Boston. She is president of the QualBridge Institute, a consulting firm focused on issues of quality and safety.
Top 7 factors contributing to patient injury
The Doctors Company identified specific factors that contributed to patient injury in the closed claims1:
- Selection and management of therapy(34%). Among the issues here were decisions involving augmentation of labor, route of delivery, and the timing of interventions. This factor also related to medications—for example, a failure to order antibiotics for Group A and Group B strep, a failure to order Rho(D) immune globulin for Rh-negative mothers, and a failure to provide magnesium sulfate for women with eclampsia.
- Patient-assessment issues (32%). The Doctors Company reviewers found that physicians frequently failed to consider information that was available, or overlooked abnormal findings.
- Technical performance (18%). This factor involved problems associated with known risks of various procedures, such as postpartum hemorrhage and brachial plexus injuries. It also included poor technique.
- Communication problems among providers (17%).
- Patient factors (16%). These factors included a failure to comply with therapy or to show up for appointments.
- Insufficient notes or a lack of documentation (14%).
- Communication problems between patient/family and provider (14%).
“Studying obstetrical medical malpractice claims sheds light on the wide array of problems that may arise during pregnancy and in labor and delivery,” the study authors conclude. “Many of these cases reflect unusual maternal or neonatal conditions that can be diagnosed only with vigilance. Examples include protein deficiencies, clotting abnormalities, placental abruptions, infections, and genetic abnormalities. More common conditions should be identified with close attention to vital signs, laboratory studies, changes to maternal and neonatal conditions, and patient complaints.”1 See “Tips for reducing malpractice claims in obstetrics” below.
Tips for reducing malpractice claims in obstetrics1
The Obstetrics Closed Claim Study identified a number of “underlying vulnerabilities” that place patients at risk and increase liability for clinicians. The Doctors Company offers the following tips to help reduce these claims:
Require periodic training and certification for physicians and nurses to maintain competency and facilitate conversations about fetal heart-rate (FHR) tracing interpretation. Both parties should use the same terminology when discussing the strips.
Use technology that allows physicians to review FHR patterns from remote locations so that physicians and nurses are able to see the same information when discussing next steps.
When operative vaginal delivery is attempted in the face of a Category III FHR tracing, a contingency team should be available for possible emergent cesarean delivery.
Foster a culture in which caregivers feel comfortable speaking up if they have a concern. Ensure that the organization has a well-defined escalation guideline.
“Obstetric departments must plan for clinical emergencies by developing and maintaining physician and staff competencies through mock drills and simulations that reduce the likelihood of injuries to mothers and their infants,” the study authors conclude.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
Reference
1. The Doctors Company. Obstetrics Closed Claim Study. http://www.thedoctors.com/KnowledgeCenter/Pa tient Safety/articles/CON_ID_011803. Published April 2015. Accessed May 6, 2015.
In this Article
- Tips for reducing malpractice claims in obstetrics
- Where are the really big malpractice awards?
- Top 7 factors contributing topatient injury
ObGyn salaries continue gradual improvement
The mean income for ObGyns rose by 2% in 2014 over 2013 to $249,000, according to the 2015 Medscape Compensation Report.1 This slight rise continues a gradual increase over the past few years ($242,000 in 2012; $220,000 in 2011).1–4 The 2015 report took into account survey responses from 19,657 physicians across 26 specialties, 5% (982) of whom were ObGyns.
The highest earners among all physician specialties were orthopedists ($421,000), cardiologists, and gastroenterologists. The lowest earners were pediatricians, family physicians, endocrinologists, and internists ($196,000). The highest ObGyn earners lived in the Northwest ($289,000) and Great Lakes ($268,000) regions; the lowest earners lived in the Mid-Atlantic ($230,000) and Northeast ($235,000) areas.1
Survey findings
Career satisfaction for ObGyns is dipping
In 2011, 69%, 53%, and 48% of ObGyns indicated they would choose a career in medicine again, select the same specialty, and pick the same practice setting, respectively.4 In the 2015 survey, 67% of ObGyns reported that they would still choose medicine; however, only 40% would pick obstetrics and gynecology as their specialty, and only 22% would select the same practice setting.1
Employment over private practice: Who feels best compensated?
Overall, 63% of all physicians are now employed, with only 23% reporting to be in private practice. Employment appears to be more popular for women: 59% of men and 72% of women responded that they work for a salary. Slightly more than a third (36%) of men and about a quarter (23%) of women are self-employed.5
The gender picture. Half of all ObGyns are women, and almost half of medical school graduates are women, yet male ObGyns continue to make more money than their female counterparts.1,5,6 The 9% difference between compensation rates for self-employed male and female ObGyns ($265,000 vs $242,000, respectively) is less than the 14% difference between their employed colleagues ($266,000 vs $229,000, respectively).1 Women tend to work shorter hours, fewer weeks, and see fewer patients than men, which could account for the lower compensation rate for female ObGyns. Studies suggest that greater schedule flexibility and fewer hours are key factors that improve satisfaction rates for female physicians.5
Male and female ObGyns tend to agree on their income satisfaction: less than half are satisfied (male, 44%; female, 46%). Many more employed ObGyns (55%) than self-employed ObGyns (31%) believe that they are fairly compensated.1
Which practice settings pay better?
Compensation rates for ObGyns in 2015 are greatest for those in office-based multispecialty group practice ($280,000), followed by those who work in1:
- health care organizations ($269,000)
- office-based single-specialty group practices ($266,000)
- outpatient clinics ($223,000)
- academic settings (nonhospital), research, military, and government ($219,000).
The lowest paid practice settings are office-based solo practices ($218,000) and hospital-employed ObGyns ($209,000).
In 2013, ObGyns who earned the most worked for health care organizations ($273,000); those who earned the least worked for outpatient clinics ($207,000).1
Do you take insurance, Medicare, Medicaid?
More employed (82%) than self-employed (53%) ObGyns will continue to take new and current Medicare or Medicaid patients, which is a rise from data published in the 2014 report (employed, 72%; self-employed, 46%).1
More than half (58%) of all physicians received less than $100 from private insurers for a new-patient office visit in 2014. Among ObGyns, 26% said they would drop insurers that pay poorly; 29% replied that they would not drop an insurer because they need all payers.1
The rate of participation in Accountable Care Organizations (ACOs) has increased from 25% in 2013 to 35% in 2014, with 8% more expecting to join an ACO in 2015. Concierge practice (2%) and cash-only practice (5%) were reportedly not significant payment models for ObGyns in 2014.1
Only 26% of ObGyns are planning to participate in health insurance exchanges; 23% said they are not participating, and 51% are not sure whether they will participate. Close to half (41%) of ObGyns believe their income will decrease because of health insurance exchanges, whereas 54% do not anticipate a change in income.1
Do you offer ancillary services?
When asked, 11% of employed ObGyns and 28% of self-employed ObGyns revealed that they have offered new ancillary services within the past 3 years. These ancillary services can include mammography, bone density testing, ultrasound, in-house laboratory services, bioidentical hormone replacement therapy, and weight management.1
How much time do you spend with patients?
In 2014, 62% of ObGyns reported spending 9 to 16 minutes with a patient during a visit. This is compared to 56% of family physicians and 44% of internists (TABLE).1,5
More than one-half (52%) of ObGynsspend 30 to 45 hours per week seeing patients. Fewer (38%) spend more than 45 hours per week, and 9% spend less than 30 hours per week with patients. This decline may be due to the increasing proportion of women and older physicians who tend to work shorter hours and fewer weeks.1
In the general physician population, 24% of women and 13% of men work part time, whereas 16% of both male and female ObGyns work part time. ObGyns aged 65 years or older constitute 35% of part-timers; 9% of those aged 35 to 49 years, and 11% of those aged 50 to 64 years, work part time. Only 2% of those younger than age 35 work part time.1
Would you select a career in obstetrics and gynecology all over again?
If given a second chance, would you rather choose orthopedic surgery as your specialty, or even choose medicine as a career again? OBG Management recently asked readers to weigh in, through its Quick Poll posted at obgmanagement.com, on whether or not they would choose ObGyn all over again. Ninety-one readers answered “yes” and 70 answered “no,” for a total of 161 respondents.
When this same question was posed to OBG Management’s Virtual Board of Editors (VBE), the perspectives were as split as the Quick Poll results:
- “No, no, no, I would not choose ObGyn all over again.”
- “Yes, I still love what I do.”
- “Yes, it is still the most unique specialty in medicine because it involves both surgery and primary care.”
- “Yes, for all the reasons I first loved the specialty: every week’s schedule, and every day is different. There is a mix of office care, surgery, and call.”
- “No! There is constant concern of litigation for complications, poor reimbursement, and compromised lifestyle.”
“There are much easier ways to make a living,” said one respondent, and another replied, “Work is very tough right now and the payment is too low.”
“The specialty has changed,” said Mary Vanko, MD, who practices in the suburbs of “blue collar Indiana.” “The public has very little idea of the breadth of our knowledge. The ObGyn generalist has the ability to serve as a woman’s doctor throughout her lifetime, not just perform the deliveries and surgeries. All of a sudden we are excluded from primary care status and people have to fight to see us. The newbies will never experience what it used to be as an ObGyn, the woman’s primary. Now we are the doctors to see when someone wants an IUD or is bleeding or pregnant. Big difference.”
Wesley Hambright, MD, practices in a small community hospital, but feels that “a larger hospital with more specialties may offer more flexibility and support in dealing with external pressures.” Tameka O’Neal, MD, is currently hospital employed but feels “as though I have little say in my practice.” Shaukat Ashai, MD, who is retired after 35 years in practice, says he would have preferred an academic setting on a full-time basis, citing long hours and poor compensation.
Robert del Rosario, MD, is in a large single-specialty suburban practice and would choose this practice setting again, although he would not choose a career as an ObGyn again. “The work demands have taken away too much from family,” he says. In addition, “as a male ObGyn, I am regularly faced with patients who choose their doctors based on gender rather than on skill. Our colleagues are no better. Early in my career and until the present, I hear people say, ‘Oh, I can’t hire Dr. X because we’re looking to hire a female.’”
Joe Walsh, MD, of Philadelphia, Pennsylvania, expresses similar discontent as a male ObGyn practicing in today’s female-populated specialty. In a letter to the editor in response to Editor in Chief Robert L. Barbieri, MD’s Editorial in the May 2015 issue, “Why is obstetrics and gynecology a popular choice for medical students?” Dr. Walsh states: “The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the field today. Perhaps job advertisements touting physician opportunities in ‘all female groups’ discourage men. Perhaps hospitals’ ‘Women’s Health Centers’ with such slogans as ‘Women taking care of women’ discourage men. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns.”
Many VBE members express some frustrations—with their practice setting, compensation, and longer work hours—but say that the patient relationships are the most rewarding aspect of their jobs. After 29 years in practice, Patrick Pevoto, MD, says the most rewarding aspect of his job is “being part of the legacy in people’s lives.”
Others say what keeps them engaged is:
- Enjoying “good outcomes.”
- “The patient contact. It’s fun having someone come up to me in the grocery store and introduce me to a teenager that I delivered 15 years ago.”
- “Surgery.”
- “Helping patients and teaching fellows.”
- “Knowing that I am making a difference in people’s lives.”
What is most rewarding?
When given several choices to select as the most rewarding aspect of their jobs, more female ObGyns (47%) than males (41%) reported that their physician-patient relationships are the major source of satisfaction. More men (10%) than women (7%) cite that making good money at a job they like is most gratifying. Only 3% of men and 2% of women reported no reward to being an ObGyn.1
Survey methodology
Medscape reports that the recruitment period for the 2015 Physician Compensation Report was from December 30, 2014, through March 11, 2015. Data were collected via a third-party online survey collection site. The margin of error for the survey was ±0.69%.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Peckham C. Medscape OB/GYN Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/womenshealth. Published April 21, 2015. Accessed May 13, 2015.
2. Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
3. Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
4. Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
5. Peckham C. Medscape Physician Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/public/overview. Published April 21, 2015. Accessed May 13, 2015.
6. Distribution of medical school graduates by gender. Henry Kaiser Family Foundation Web site. http://kff.org/other/state-indicator/medical-school-graduates-by-gender/. Accessed May 13, 2015.
The mean income for ObGyns rose by 2% in 2014 over 2013 to $249,000, according to the 2015 Medscape Compensation Report.1 This slight rise continues a gradual increase over the past few years ($242,000 in 2012; $220,000 in 2011).1–4 The 2015 report took into account survey responses from 19,657 physicians across 26 specialties, 5% (982) of whom were ObGyns.
The highest earners among all physician specialties were orthopedists ($421,000), cardiologists, and gastroenterologists. The lowest earners were pediatricians, family physicians, endocrinologists, and internists ($196,000). The highest ObGyn earners lived in the Northwest ($289,000) and Great Lakes ($268,000) regions; the lowest earners lived in the Mid-Atlantic ($230,000) and Northeast ($235,000) areas.1
Survey findings
Career satisfaction for ObGyns is dipping
In 2011, 69%, 53%, and 48% of ObGyns indicated they would choose a career in medicine again, select the same specialty, and pick the same practice setting, respectively.4 In the 2015 survey, 67% of ObGyns reported that they would still choose medicine; however, only 40% would pick obstetrics and gynecology as their specialty, and only 22% would select the same practice setting.1
Employment over private practice: Who feels best compensated?
Overall, 63% of all physicians are now employed, with only 23% reporting to be in private practice. Employment appears to be more popular for women: 59% of men and 72% of women responded that they work for a salary. Slightly more than a third (36%) of men and about a quarter (23%) of women are self-employed.5
The gender picture. Half of all ObGyns are women, and almost half of medical school graduates are women, yet male ObGyns continue to make more money than their female counterparts.1,5,6 The 9% difference between compensation rates for self-employed male and female ObGyns ($265,000 vs $242,000, respectively) is less than the 14% difference between their employed colleagues ($266,000 vs $229,000, respectively).1 Women tend to work shorter hours, fewer weeks, and see fewer patients than men, which could account for the lower compensation rate for female ObGyns. Studies suggest that greater schedule flexibility and fewer hours are key factors that improve satisfaction rates for female physicians.5
Male and female ObGyns tend to agree on their income satisfaction: less than half are satisfied (male, 44%; female, 46%). Many more employed ObGyns (55%) than self-employed ObGyns (31%) believe that they are fairly compensated.1
Which practice settings pay better?
Compensation rates for ObGyns in 2015 are greatest for those in office-based multispecialty group practice ($280,000), followed by those who work in1:
- health care organizations ($269,000)
- office-based single-specialty group practices ($266,000)
- outpatient clinics ($223,000)
- academic settings (nonhospital), research, military, and government ($219,000).
The lowest paid practice settings are office-based solo practices ($218,000) and hospital-employed ObGyns ($209,000).
In 2013, ObGyns who earned the most worked for health care organizations ($273,000); those who earned the least worked for outpatient clinics ($207,000).1
Do you take insurance, Medicare, Medicaid?
More employed (82%) than self-employed (53%) ObGyns will continue to take new and current Medicare or Medicaid patients, which is a rise from data published in the 2014 report (employed, 72%; self-employed, 46%).1
More than half (58%) of all physicians received less than $100 from private insurers for a new-patient office visit in 2014. Among ObGyns, 26% said they would drop insurers that pay poorly; 29% replied that they would not drop an insurer because they need all payers.1
The rate of participation in Accountable Care Organizations (ACOs) has increased from 25% in 2013 to 35% in 2014, with 8% more expecting to join an ACO in 2015. Concierge practice (2%) and cash-only practice (5%) were reportedly not significant payment models for ObGyns in 2014.1
Only 26% of ObGyns are planning to participate in health insurance exchanges; 23% said they are not participating, and 51% are not sure whether they will participate. Close to half (41%) of ObGyns believe their income will decrease because of health insurance exchanges, whereas 54% do not anticipate a change in income.1
Do you offer ancillary services?
When asked, 11% of employed ObGyns and 28% of self-employed ObGyns revealed that they have offered new ancillary services within the past 3 years. These ancillary services can include mammography, bone density testing, ultrasound, in-house laboratory services, bioidentical hormone replacement therapy, and weight management.1
How much time do you spend with patients?
In 2014, 62% of ObGyns reported spending 9 to 16 minutes with a patient during a visit. This is compared to 56% of family physicians and 44% of internists (TABLE).1,5
More than one-half (52%) of ObGynsspend 30 to 45 hours per week seeing patients. Fewer (38%) spend more than 45 hours per week, and 9% spend less than 30 hours per week with patients. This decline may be due to the increasing proportion of women and older physicians who tend to work shorter hours and fewer weeks.1
In the general physician population, 24% of women and 13% of men work part time, whereas 16% of both male and female ObGyns work part time. ObGyns aged 65 years or older constitute 35% of part-timers; 9% of those aged 35 to 49 years, and 11% of those aged 50 to 64 years, work part time. Only 2% of those younger than age 35 work part time.1
Would you select a career in obstetrics and gynecology all over again?
If given a second chance, would you rather choose orthopedic surgery as your specialty, or even choose medicine as a career again? OBG Management recently asked readers to weigh in, through its Quick Poll posted at obgmanagement.com, on whether or not they would choose ObGyn all over again. Ninety-one readers answered “yes” and 70 answered “no,” for a total of 161 respondents.
When this same question was posed to OBG Management’s Virtual Board of Editors (VBE), the perspectives were as split as the Quick Poll results:
- “No, no, no, I would not choose ObGyn all over again.”
- “Yes, I still love what I do.”
- “Yes, it is still the most unique specialty in medicine because it involves both surgery and primary care.”
- “Yes, for all the reasons I first loved the specialty: every week’s schedule, and every day is different. There is a mix of office care, surgery, and call.”
- “No! There is constant concern of litigation for complications, poor reimbursement, and compromised lifestyle.”
“There are much easier ways to make a living,” said one respondent, and another replied, “Work is very tough right now and the payment is too low.”
“The specialty has changed,” said Mary Vanko, MD, who practices in the suburbs of “blue collar Indiana.” “The public has very little idea of the breadth of our knowledge. The ObGyn generalist has the ability to serve as a woman’s doctor throughout her lifetime, not just perform the deliveries and surgeries. All of a sudden we are excluded from primary care status and people have to fight to see us. The newbies will never experience what it used to be as an ObGyn, the woman’s primary. Now we are the doctors to see when someone wants an IUD or is bleeding or pregnant. Big difference.”
Wesley Hambright, MD, practices in a small community hospital, but feels that “a larger hospital with more specialties may offer more flexibility and support in dealing with external pressures.” Tameka O’Neal, MD, is currently hospital employed but feels “as though I have little say in my practice.” Shaukat Ashai, MD, who is retired after 35 years in practice, says he would have preferred an academic setting on a full-time basis, citing long hours and poor compensation.
Robert del Rosario, MD, is in a large single-specialty suburban practice and would choose this practice setting again, although he would not choose a career as an ObGyn again. “The work demands have taken away too much from family,” he says. In addition, “as a male ObGyn, I am regularly faced with patients who choose their doctors based on gender rather than on skill. Our colleagues are no better. Early in my career and until the present, I hear people say, ‘Oh, I can’t hire Dr. X because we’re looking to hire a female.’”
Joe Walsh, MD, of Philadelphia, Pennsylvania, expresses similar discontent as a male ObGyn practicing in today’s female-populated specialty. In a letter to the editor in response to Editor in Chief Robert L. Barbieri, MD’s Editorial in the May 2015 issue, “Why is obstetrics and gynecology a popular choice for medical students?” Dr. Walsh states: “The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the field today. Perhaps job advertisements touting physician opportunities in ‘all female groups’ discourage men. Perhaps hospitals’ ‘Women’s Health Centers’ with such slogans as ‘Women taking care of women’ discourage men. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns.”
Many VBE members express some frustrations—with their practice setting, compensation, and longer work hours—but say that the patient relationships are the most rewarding aspect of their jobs. After 29 years in practice, Patrick Pevoto, MD, says the most rewarding aspect of his job is “being part of the legacy in people’s lives.”
Others say what keeps them engaged is:
- Enjoying “good outcomes.”
- “The patient contact. It’s fun having someone come up to me in the grocery store and introduce me to a teenager that I delivered 15 years ago.”
- “Surgery.”
- “Helping patients and teaching fellows.”
- “Knowing that I am making a difference in people’s lives.”
What is most rewarding?
When given several choices to select as the most rewarding aspect of their jobs, more female ObGyns (47%) than males (41%) reported that their physician-patient relationships are the major source of satisfaction. More men (10%) than women (7%) cite that making good money at a job they like is most gratifying. Only 3% of men and 2% of women reported no reward to being an ObGyn.1
Survey methodology
Medscape reports that the recruitment period for the 2015 Physician Compensation Report was from December 30, 2014, through March 11, 2015. Data were collected via a third-party online survey collection site. The margin of error for the survey was ±0.69%.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The mean income for ObGyns rose by 2% in 2014 over 2013 to $249,000, according to the 2015 Medscape Compensation Report.1 This slight rise continues a gradual increase over the past few years ($242,000 in 2012; $220,000 in 2011).1–4 The 2015 report took into account survey responses from 19,657 physicians across 26 specialties, 5% (982) of whom were ObGyns.
The highest earners among all physician specialties were orthopedists ($421,000), cardiologists, and gastroenterologists. The lowest earners were pediatricians, family physicians, endocrinologists, and internists ($196,000). The highest ObGyn earners lived in the Northwest ($289,000) and Great Lakes ($268,000) regions; the lowest earners lived in the Mid-Atlantic ($230,000) and Northeast ($235,000) areas.1
Survey findings
Career satisfaction for ObGyns is dipping
In 2011, 69%, 53%, and 48% of ObGyns indicated they would choose a career in medicine again, select the same specialty, and pick the same practice setting, respectively.4 In the 2015 survey, 67% of ObGyns reported that they would still choose medicine; however, only 40% would pick obstetrics and gynecology as their specialty, and only 22% would select the same practice setting.1
Employment over private practice: Who feels best compensated?
Overall, 63% of all physicians are now employed, with only 23% reporting to be in private practice. Employment appears to be more popular for women: 59% of men and 72% of women responded that they work for a salary. Slightly more than a third (36%) of men and about a quarter (23%) of women are self-employed.5
The gender picture. Half of all ObGyns are women, and almost half of medical school graduates are women, yet male ObGyns continue to make more money than their female counterparts.1,5,6 The 9% difference between compensation rates for self-employed male and female ObGyns ($265,000 vs $242,000, respectively) is less than the 14% difference between their employed colleagues ($266,000 vs $229,000, respectively).1 Women tend to work shorter hours, fewer weeks, and see fewer patients than men, which could account for the lower compensation rate for female ObGyns. Studies suggest that greater schedule flexibility and fewer hours are key factors that improve satisfaction rates for female physicians.5
Male and female ObGyns tend to agree on their income satisfaction: less than half are satisfied (male, 44%; female, 46%). Many more employed ObGyns (55%) than self-employed ObGyns (31%) believe that they are fairly compensated.1
Which practice settings pay better?
Compensation rates for ObGyns in 2015 are greatest for those in office-based multispecialty group practice ($280,000), followed by those who work in1:
- health care organizations ($269,000)
- office-based single-specialty group practices ($266,000)
- outpatient clinics ($223,000)
- academic settings (nonhospital), research, military, and government ($219,000).
The lowest paid practice settings are office-based solo practices ($218,000) and hospital-employed ObGyns ($209,000).
In 2013, ObGyns who earned the most worked for health care organizations ($273,000); those who earned the least worked for outpatient clinics ($207,000).1
Do you take insurance, Medicare, Medicaid?
More employed (82%) than self-employed (53%) ObGyns will continue to take new and current Medicare or Medicaid patients, which is a rise from data published in the 2014 report (employed, 72%; self-employed, 46%).1
More than half (58%) of all physicians received less than $100 from private insurers for a new-patient office visit in 2014. Among ObGyns, 26% said they would drop insurers that pay poorly; 29% replied that they would not drop an insurer because they need all payers.1
The rate of participation in Accountable Care Organizations (ACOs) has increased from 25% in 2013 to 35% in 2014, with 8% more expecting to join an ACO in 2015. Concierge practice (2%) and cash-only practice (5%) were reportedly not significant payment models for ObGyns in 2014.1
Only 26% of ObGyns are planning to participate in health insurance exchanges; 23% said they are not participating, and 51% are not sure whether they will participate. Close to half (41%) of ObGyns believe their income will decrease because of health insurance exchanges, whereas 54% do not anticipate a change in income.1
Do you offer ancillary services?
When asked, 11% of employed ObGyns and 28% of self-employed ObGyns revealed that they have offered new ancillary services within the past 3 years. These ancillary services can include mammography, bone density testing, ultrasound, in-house laboratory services, bioidentical hormone replacement therapy, and weight management.1
How much time do you spend with patients?
In 2014, 62% of ObGyns reported spending 9 to 16 minutes with a patient during a visit. This is compared to 56% of family physicians and 44% of internists (TABLE).1,5
More than one-half (52%) of ObGynsspend 30 to 45 hours per week seeing patients. Fewer (38%) spend more than 45 hours per week, and 9% spend less than 30 hours per week with patients. This decline may be due to the increasing proportion of women and older physicians who tend to work shorter hours and fewer weeks.1
In the general physician population, 24% of women and 13% of men work part time, whereas 16% of both male and female ObGyns work part time. ObGyns aged 65 years or older constitute 35% of part-timers; 9% of those aged 35 to 49 years, and 11% of those aged 50 to 64 years, work part time. Only 2% of those younger than age 35 work part time.1
Would you select a career in obstetrics and gynecology all over again?
If given a second chance, would you rather choose orthopedic surgery as your specialty, or even choose medicine as a career again? OBG Management recently asked readers to weigh in, through its Quick Poll posted at obgmanagement.com, on whether or not they would choose ObGyn all over again. Ninety-one readers answered “yes” and 70 answered “no,” for a total of 161 respondents.
When this same question was posed to OBG Management’s Virtual Board of Editors (VBE), the perspectives were as split as the Quick Poll results:
- “No, no, no, I would not choose ObGyn all over again.”
- “Yes, I still love what I do.”
- “Yes, it is still the most unique specialty in medicine because it involves both surgery and primary care.”
- “Yes, for all the reasons I first loved the specialty: every week’s schedule, and every day is different. There is a mix of office care, surgery, and call.”
- “No! There is constant concern of litigation for complications, poor reimbursement, and compromised lifestyle.”
“There are much easier ways to make a living,” said one respondent, and another replied, “Work is very tough right now and the payment is too low.”
“The specialty has changed,” said Mary Vanko, MD, who practices in the suburbs of “blue collar Indiana.” “The public has very little idea of the breadth of our knowledge. The ObGyn generalist has the ability to serve as a woman’s doctor throughout her lifetime, not just perform the deliveries and surgeries. All of a sudden we are excluded from primary care status and people have to fight to see us. The newbies will never experience what it used to be as an ObGyn, the woman’s primary. Now we are the doctors to see when someone wants an IUD or is bleeding or pregnant. Big difference.”
Wesley Hambright, MD, practices in a small community hospital, but feels that “a larger hospital with more specialties may offer more flexibility and support in dealing with external pressures.” Tameka O’Neal, MD, is currently hospital employed but feels “as though I have little say in my practice.” Shaukat Ashai, MD, who is retired after 35 years in practice, says he would have preferred an academic setting on a full-time basis, citing long hours and poor compensation.
Robert del Rosario, MD, is in a large single-specialty suburban practice and would choose this practice setting again, although he would not choose a career as an ObGyn again. “The work demands have taken away too much from family,” he says. In addition, “as a male ObGyn, I am regularly faced with patients who choose their doctors based on gender rather than on skill. Our colleagues are no better. Early in my career and until the present, I hear people say, ‘Oh, I can’t hire Dr. X because we’re looking to hire a female.’”
Joe Walsh, MD, of Philadelphia, Pennsylvania, expresses similar discontent as a male ObGyn practicing in today’s female-populated specialty. In a letter to the editor in response to Editor in Chief Robert L. Barbieri, MD’s Editorial in the May 2015 issue, “Why is obstetrics and gynecology a popular choice for medical students?” Dr. Walsh states: “The unaddressed question is why is it unpopular for half of medical students? Ninety-three percent of resident graduates in the field are women, while women account for half of medical student graduates. Men rarely go into the field today. Perhaps job advertisements touting physician opportunities in ‘all female groups’ discourage men. Perhaps hospitals’ ‘Women’s Health Centers’ with such slogans as ‘Women taking care of women’ discourage men. Perhaps receptionists’ asking patients whether they prefer a male or female physician discourages male ObGyns.”
Many VBE members express some frustrations—with their practice setting, compensation, and longer work hours—but say that the patient relationships are the most rewarding aspect of their jobs. After 29 years in practice, Patrick Pevoto, MD, says the most rewarding aspect of his job is “being part of the legacy in people’s lives.”
Others say what keeps them engaged is:
- Enjoying “good outcomes.”
- “The patient contact. It’s fun having someone come up to me in the grocery store and introduce me to a teenager that I delivered 15 years ago.”
- “Surgery.”
- “Helping patients and teaching fellows.”
- “Knowing that I am making a difference in people’s lives.”
What is most rewarding?
When given several choices to select as the most rewarding aspect of their jobs, more female ObGyns (47%) than males (41%) reported that their physician-patient relationships are the major source of satisfaction. More men (10%) than women (7%) cite that making good money at a job they like is most gratifying. Only 3% of men and 2% of women reported no reward to being an ObGyn.1
Survey methodology
Medscape reports that the recruitment period for the 2015 Physician Compensation Report was from December 30, 2014, through March 11, 2015. Data were collected via a third-party online survey collection site. The margin of error for the survey was ±0.69%.1
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Peckham C. Medscape OB/GYN Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/womenshealth. Published April 21, 2015. Accessed May 13, 2015.
2. Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
3. Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
4. Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
5. Peckham C. Medscape Physician Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/public/overview. Published April 21, 2015. Accessed May 13, 2015.
6. Distribution of medical school graduates by gender. Henry Kaiser Family Foundation Web site. http://kff.org/other/state-indicator/medical-school-graduates-by-gender/. Accessed May 13, 2015.
1. Peckham C. Medscape OB/GYN Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/womenshealth. Published April 21, 2015. Accessed May 13, 2015.
2. Peckham C. Medscape OB/GYN Compensation Report 2014. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2014/womenshealth. Published April 15, 2014. Accessed June 2, 2014.
3. Medscape News. Ob/Gyn Compensation Report 2013. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2013/womenshealth. Accessed June 30, 2013.
4. Reale D. Mean income for ObGyns increased in 2012. OBG Manag. 2013;25(8):34–36.
5. Peckham C. Medscape Physician Compensation Report 2015. Medscape Web site. http://www.medscape.com/features/slideshow/compensation/2015/public/overview. Published April 21, 2015. Accessed May 13, 2015.
6. Distribution of medical school graduates by gender. Henry Kaiser Family Foundation Web site. http://kff.org/other/state-indicator/medical-school-graduates-by-gender/. Accessed May 13, 2015.
In this article
- Which practice settings pay better?
- Would you select a career in ObGyn again?
- Comparing time spent with patients
ACOG, SMFM, and others address safety concerns in labor and delivery
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.3
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.3
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. Patients should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.3
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinicianto lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.3
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.3
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.3
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. Patients should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.3
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinicianto lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.3
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.3
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.3
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. Patients should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.3
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinicianto lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.3
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
In this article
- Why handoffs should be structured
- Goals for teamleaders
Endometriosis and infertility: Expert answers to 6 questions to help pinpoint the best route to pregnancy
Although endometriosis and infertility are clearly linked—in life as well as the medical literature—no causal relationship has been established. Nevertheless, data suggest that 25% to 50% of infertile women have endometriosis, and that as many as 30% to 50% of women who have endometriosis are infertile.1
Among the mechanisms that have been proposed to explain this link are:
- distorted pelvic anatomy
- endocrine and ovulatory abnormalities
- impaired implantation
- impaired quality of the oocyte and embryo
- altered peritoneal function
- altered hormonal and cell-mediated function
- abnormal uterotubal transport.2
Recent studies by Kao and colleagues and Giudice and colleagues have led to new findings in regard to endometriosis and infertility, says Ceana Nezhat, MD.3,4 Dr. Nezhat is Director of the Nezhat Medical Center in Atlanta, Georgia, and Medical Director of Training and Education at Northside Hospital in Atlanta. “These researchers have discovered that endometriosis causes changes to the endometrium that contribute to infertility.”
“There are no studies that have specifically assessed whether one anatomic site is associated with increased infertility over another,” says Tommaso Falcone, MD. “However, it is assumed that disease that involves the tubes and ovaries would impede fertility the most. Adhesive disease and endometriomas around the tubes and ovaries are associated with a worsening prognosis. Although peritoneal disease probably influences fertility solely on the basis of inflammation, disease around the tubes and ovaries is thought to have a mechanical effect as well.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
“Endometriosis is a chronic and heterogeneous disease process,” says Stephanie J. Estes, MD, Director of Robotic Surgical Services and Associate Professor, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, at Penn State Hershey Medical Center in Hershey, Pennsylvania.
“It is likely that no single site is the causative factor,” Dr. Estes says. “Endometriosis alters prostaglandins, cytokines, and proteases that may adversely affect eggs, sperm, or embryo development. In addition, altered endometrial receptivity may play a role. It has been shown that, when donor oocytes from women with endometriosis are transferred to women without endometriosis, there are lower implantation rates and poorer embryo quality.”5
Does it follow, then, that eradication of endometriosis improves fertility?
The answer is not clear. Rather, it depends on a number of variables, including the stage of the disease, its location, the presence of symptoms, and more.
“The approach for endometiosis-associated infertility is completely different from the approach for pain,” says Dr. Nezhat. “In a patient with pain, complete eradication of endometriosis is necessary. However, when addressing infertility, a surgeon must be cautious in the vicinity of the reproductive organs, even if a multistage approach is required. Fertility preservation is the goal.6,7 However, thorough treatment of endometriosis improves fertility rates even in cases of failed in vitro fertilization” (IVF).8
In this article, the focus is on 6 critical questions concerning endometriosis and infertility, including the role of medical therapy, when surgery is indicated, and whether an endometrioma warrants removal or referral for IVF.
In Part 1 of this 3-part series, which appeared in the April 2015 issue of OBG Management, the subject was diagnosis of endometriosis. In Part 2, which appeared in May 2015, the focus was endometriosis and pain.
1. Is there a role for medical therapy?
In women who have endometriosis-related infertility, medical therapy does not appear to produce any benefit. In its committee opinion on the subject, the American Society for Reproductive Medicine (ASRM) states as much: “There is no evidence that medical treatment of endometriosis improves fertility.”2
In fact, observes Dr. Estes, trials of medical treatment, involving such medications as combined estrogen-progestin therapy, danazol, progestins, or gonadotropin-releasing hormone (GnRH) agonists, may cause an unnecessary “delay in the use of more effective treatments that could result in pregnancy.”
Dr. Nezhat believes that medical therapy is effective in patients who have adenomyosis in addition to endometriosis. “Three months of treatment with a GnRH agonist will improve fertility rates in these patients,” he says.
“Medical treatments inhibit ovulation,” notes Dr. Estes. “These therapies have no role in pretreatment of patients with endometriosis prior to infertility treatment. On the other hand, medical therapy with GnRH agonists for 3 to 6 months prior to an IVF cycle does result in increased pregnancy rates.”9
2. What is the role of clomiphene and intrauterine insemination?
Should women who have endometriosis, open fallopian tubes, and infertility be treated with clomiphene and intrauterine insemination (IUI) prior to a cycle of IVF?
“This is a complex question,” says Dr. Estes, “as clinical parameters such as age, symptoms, duration of infertility, and the ability to proceed with IVF are important, as well as stage of disease.”
“Overall, in patients younger than 35 years, clomiphene-IUI is an option and has an increased pregnancy rate over timed intercourse” (9% vs 3%), she says.10 “However, clearly IVF is much more successful and is the most effective treatment, with pregnancy rates of approximately 46% for women younger than 35 years (3% of whom have a ‘diagnosis’ of endometriosis, and 13% of whom have an ‘unknown’ infertility factor).11 Women who are older than 35 or who have additional infertility factors, such as male factor, should consider IVF first.”
3. When is surgery indicated?
“Patients who have significant pain associated with their infertility will benefit from surgery, which offers both pain relief and an improvement in the spontaneous pregnancy rate,” says Dr. Falcone. “Patients who are infertile and desire spontaneous pregnancy without pharmacologic intervention or assisted reproductive technology (ART) also benefit from surgery.”
The benefit of surgery in asymptomatic women with minimal to mild endometriosis is unclear. In a randomized controlled trial of 341 women (aged 20–39 years) with infertility and minimal to mild endometriosis, laparoscopic resection or ablation of visible lesions resulted in pregnancy in 30.7% of women, compared with 17.7% of women who underwent diagnostic laparoscopy only.12
Another randomized study of 96 women with minimal to mild endometriosis who underwent resection/ablation or diagnostic laparoscopy found no difference in the birth rate at 1 year.13
When the results of these 2 studies are combined, the number needed to treat is 12 laparoscopies in women with endometriosis, says Dr. Falcone. “So 1 additional pregnancy would be gained from performing endometriosis surgery in 12 patients. If we assume a prevalence of 30% in asymptomatic women with infertility, then you need to perform 40 diagnostic laparoscopies to achieve an extra pregnancy.”
For women with infertility and severe endometriosis, a nonrandomized study found cumulative pregnancy rates of 45% and 63% after laparoscopy and laparotomy, respectively, in 216 women followed for up to 2 years.14 The difference in rates was not statistically significant.
“Although surgery is indicated to diagnose endometriosis, multiple repeat procedures are not an effective treatment for infertility,” says Dr. Estes. “Plus, especially for stage I and II endometriosis [according to the ASRM classification system], approximately 12 patients will need surgery for 1 additional pregnancy—and many more will have needed the procedure to even get to those who have endometriosis. While patients often want us to ‘do something’—often a covered service such as surgery—we need to consider that surgery for early disease does not provide a significant or long-lasting enhancement to women’s ability to conceive.”
In the absence of male-factor infertility, surgical diagnosis with conservative and precise treatment of endometriosis at the same time is better than going directly to IVF, says Dr. Nezhat. Younger patients who undergo surgical treatment have a better chance of achieving more than 1 spontaneous pregnancy than they do with IVF. And older patients also will have an improved conception rate with ART when they are treated surgically, he says.8
Coding and reimbursement
For endometriosis, the correct diagnostic code helps establish the medical necessity of later treatments
Several diagnostic codes are available to describe the location of endometriotic implants, but the fact remains that these codes can be reported only after a definitive diagnosis is made, which generally comes after confirmatory surgery has been performed. When the patient is in the diagnostic phase, she may present with complaints of pelvic pain, dyspareunia, dysmenorrhea, or infertility. Knowing which codes to use at the time of evaluation goes a long way toward establishing medical necessity for any later surgery or medical treatment.
In the TABLE, you will find common diagnostic codes that can be reported during evaluation of endometriosis, including both International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM equivalents. Note that, with ICD-10-CM, payers are looking for a more specific type of dysmenorrhea in the documentation. In the case of painful menstruation suspected to arise from endometriosis, the code for secondary dysmenorrhea should be reported.
Diagnostic coding in cases in which the patient first presents with a complaint of infertility in the absence of other symptoms can be problematic, as many insurers still do not cover the treatment of infertility. However, in the diagnostic stage, codes for fertility testing can be reported instead of codes that confirm infertility when no other symptoms are present. Once the clinician has verified that endometriosis is the source of the infertility and begins treatment, the primary code for the type of endometriosis would be reported, not a diagnosis of infertility.
The procedure performed to diagnose endometriosis would be diagnostic laparoscopy (Current Procedure Terminology [CPT] code 49320, Laparoscopy, abdomen, peritoneum, and omentum, diagnostic, with or without collection of specimen[s] by brushing or washing [separate procedure]) if medical treatment is being pursued. If the plan is to confirm and then immediately remove the implants, the CPT codes would be either 58662, Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method, or 49203–49205, Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors, based on the largest tumor diameter, if they are removed via an abdominal incision.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
4. Surgery or IVF for endometriomas?
When an endometrioma is present, should it be surgically treated or should the patient go straight to IVF? And does the optimal approach depend on the size of the endometrioma?
“The answer to this question ultimately depends on the skill and experience of the surgeon and the type of endometrioma,” says Dr. Nezhat, “because improper removal of an endometrioma may compromise the function of the ovary.”
“I recommend removal of the endometrioma by a skilled surgeon experienced in the management of ovarian cysts and preservation of ovarian function, especially in patients who have failed IVF,” he says.6,15
According to the ASRM committee opinion on endometriosis and infertility, laparoscopic cystectomy for endometriomas larger than 4 cm improves fertility, compared with cyst drainage and coagulation, which is associated with a high risk of cyst recurrence.2 However, no randomized trials have compared laparoscopic excision of an endometrioma to expectant management prior to IVF/intracytoplasmic sperm injection (ICSI). One case-control study found that laparoscopic cystectomy prior to IVF had no effect on fertility outcomes.16 At the same time, however, “conservative surgical treatment in symptomatic patients did not impair the success rates of IVF or ICSI,” notes the ASRM.2 Therefore, “evidence suggests that surgery does not benefit asymptomatic women with an endometrioma prior to scheduled IVF/ICSI,” the ASRM concludes.2
Dr. Nezhat cautions against the practice of indiscriminate IVF in the setting of ovarian endometriomas because he has seen patients who developed tubo-ovarian abscess and infected endometriomas after transvaginal egg retrieval. He notes that there are additional case reports of similar findings from other surgeons.
As far as excision versus ablation is concerned, all studies have shown some effect on ovarian function after excision, says Dr. Falcone. “The studies assessed this parameter using several endpoints, such as the number of oocytes retrieved at IVF. Recently, anti-Müllerian hormone has been used and has confirmed this observation. The decreased ovarian reserve is especially problematic with bilateral ovarian disease. The extent of the decreased ovarian function is dependent on several parameters, especially how much energy is used to achieve hemostasis. Several techniques have been proposed to reduce damage, including less traumatic ways of achieving hemostasis in the ovarian hilus.”
“The dilemma is that, if we excise the disease, the pregnancy rates are better than with ablation, but if we excise, there is more damage to the ovary. In my practice, I excise if there is minimal associated disease, such as adhesions, because the pregnancy rate after surgery is good. If, however, there are extensive adhesions with a low chance of spontaneous pregnancy, I minimize excision,” Dr. Falcone says.
As for going straight to IVF, “many patients cannot afford IVF; therefore, surgery is their only option. Furthermore, many patients have severe associated pain; therefore, surgery is required to improve quality of life. However, if the decision is made to proceed to IVF, there is no evidence that cystectomy prior to treatment with ART improves the pregnancy rate,” Dr. Falcone says.
“Basically, if a woman has no pain and no endometrioma, and infertility alone is the issue, I treat her in the same manner as I would a patient with idiopathic infertility—no surgery,” Dr. Falcone says. “If a patient has severe pain and infertility, I treat her surgically but conservatively, especially when an endometrioma is present. The patient will get pain relief but also derive fertility benefit. If a patient has had previous surgery for endometriosis-associated infertility, additional surgery is not the next step—as our study has shown that pregnancy rates are better with IVF than repeat surgery.”17
5. Is surgery ever effective after failed IVF?
To answer this question, Dr. Nezhat points to a retrospective case series by Littman and colleagues in which 29 women with a history of failed IVF underwent laparoscopic evaluation and treatment of endometriosis (by the same surgeon).8 Of 29 patients, 22 conceived after laparoscopic treatment of endometriosis, including 15 “non-IVF” pregnancies and 7 IVF pregnancies, the study results show.8
“It is not unusual for patients and health care providers to perceive IVF as the final treatment for infertility,” Littman and colleagues write. “When this definitive therapy fails repeatedly, clinicians and patients may be inclined to pursue oocyte donation or elect to forego further treatment altogether. This is especially true in women of advanced age and in patients with borderline embryo quality. Presently, as a result of our clinical observation in patients with failed IVF, before egg donation or adoption, we offer the option to have meticulous laparoscopic evaluation and treatment by a skilled surgeon. Furthermore, we would not classify an infertility condition as unexplained without confirming the absence of endometriosis by a thorough laparoscopy. In our experience, patients under 35 years old with unexplained infertility who are found to have endometriosis at the time of laparoscopy have an excellent chance of pregnancy following surgical treatment without ART.”8
6. Is repeat surgery ever helpful?
In the treatment of endometriosis-associated infertility, repeat surgery should be avoided if no symptoms are present, says Dr. Estes. “In women with continued symptoms, the benefits of repeat surgery are small, with known risks, including the potential for adhesive disease and the iatrogenic detrimental effect on ovarian function—often in a setting of already-present decreased ovarian reserve.”
Dr. Nezhat agrees that multiple surgeries carry the potential for harm. “However, there is one caveat,” he says. “What was done at the previous surgery and how? The skill and experience of the surgeon and proper technique are of paramount importance to the surgical outcome.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796.
2. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
3. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881.
4. Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–264; discussion 293–295, 396–406.
5. Garrido N, Navarro J, Garcia-Velasco J, et al. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2012;8(1):95-103.
6. Lewis M, Baker V, Nezhat C. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(6):e81–83.
7. Tsolakidis D, Pados G, Vavilis D, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(1):71–77.
8. Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
9. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;(1):CD004635.
10. Deaton JL, Gibson M, Blackmer KM, et al. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990; 54:1083–1088.
11. Society for Assisted Reproductive Technology. Clinic Summary Report [all member clinics]: 2013. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0. Accessed May 13, 2015.
12. Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–222.
13. Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum Reprod. 1999;14(5):1332–1334.
14. Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66(5):706–711.
15. Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
16. Garcia-Velasco JA, Mahutte NG, Corona J, et al. Removal of endometriomas before in vitro fertilization does not improve fertility outcomes: a matched, case-control study. Fertil Steril. 2004;81(5):1194–1197.
17. Pagidas K, Falcone T, Hemmings R, Miron R. Comparison of surgical treatment of moderate (stage III) and severe (stage IV) endometriosis-related infertility with IVF–embryo transfer. Fertil Steril. 1996;65(4):791–795.
Although endometriosis and infertility are clearly linked—in life as well as the medical literature—no causal relationship has been established. Nevertheless, data suggest that 25% to 50% of infertile women have endometriosis, and that as many as 30% to 50% of women who have endometriosis are infertile.1
Among the mechanisms that have been proposed to explain this link are:
- distorted pelvic anatomy
- endocrine and ovulatory abnormalities
- impaired implantation
- impaired quality of the oocyte and embryo
- altered peritoneal function
- altered hormonal and cell-mediated function
- abnormal uterotubal transport.2
Recent studies by Kao and colleagues and Giudice and colleagues have led to new findings in regard to endometriosis and infertility, says Ceana Nezhat, MD.3,4 Dr. Nezhat is Director of the Nezhat Medical Center in Atlanta, Georgia, and Medical Director of Training and Education at Northside Hospital in Atlanta. “These researchers have discovered that endometriosis causes changes to the endometrium that contribute to infertility.”
“There are no studies that have specifically assessed whether one anatomic site is associated with increased infertility over another,” says Tommaso Falcone, MD. “However, it is assumed that disease that involves the tubes and ovaries would impede fertility the most. Adhesive disease and endometriomas around the tubes and ovaries are associated with a worsening prognosis. Although peritoneal disease probably influences fertility solely on the basis of inflammation, disease around the tubes and ovaries is thought to have a mechanical effect as well.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
“Endometriosis is a chronic and heterogeneous disease process,” says Stephanie J. Estes, MD, Director of Robotic Surgical Services and Associate Professor, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, at Penn State Hershey Medical Center in Hershey, Pennsylvania.
“It is likely that no single site is the causative factor,” Dr. Estes says. “Endometriosis alters prostaglandins, cytokines, and proteases that may adversely affect eggs, sperm, or embryo development. In addition, altered endometrial receptivity may play a role. It has been shown that, when donor oocytes from women with endometriosis are transferred to women without endometriosis, there are lower implantation rates and poorer embryo quality.”5
Does it follow, then, that eradication of endometriosis improves fertility?
The answer is not clear. Rather, it depends on a number of variables, including the stage of the disease, its location, the presence of symptoms, and more.
“The approach for endometiosis-associated infertility is completely different from the approach for pain,” says Dr. Nezhat. “In a patient with pain, complete eradication of endometriosis is necessary. However, when addressing infertility, a surgeon must be cautious in the vicinity of the reproductive organs, even if a multistage approach is required. Fertility preservation is the goal.6,7 However, thorough treatment of endometriosis improves fertility rates even in cases of failed in vitro fertilization” (IVF).8
In this article, the focus is on 6 critical questions concerning endometriosis and infertility, including the role of medical therapy, when surgery is indicated, and whether an endometrioma warrants removal or referral for IVF.
In Part 1 of this 3-part series, which appeared in the April 2015 issue of OBG Management, the subject was diagnosis of endometriosis. In Part 2, which appeared in May 2015, the focus was endometriosis and pain.
1. Is there a role for medical therapy?
In women who have endometriosis-related infertility, medical therapy does not appear to produce any benefit. In its committee opinion on the subject, the American Society for Reproductive Medicine (ASRM) states as much: “There is no evidence that medical treatment of endometriosis improves fertility.”2
In fact, observes Dr. Estes, trials of medical treatment, involving such medications as combined estrogen-progestin therapy, danazol, progestins, or gonadotropin-releasing hormone (GnRH) agonists, may cause an unnecessary “delay in the use of more effective treatments that could result in pregnancy.”
Dr. Nezhat believes that medical therapy is effective in patients who have adenomyosis in addition to endometriosis. “Three months of treatment with a GnRH agonist will improve fertility rates in these patients,” he says.
“Medical treatments inhibit ovulation,” notes Dr. Estes. “These therapies have no role in pretreatment of patients with endometriosis prior to infertility treatment. On the other hand, medical therapy with GnRH agonists for 3 to 6 months prior to an IVF cycle does result in increased pregnancy rates.”9
2. What is the role of clomiphene and intrauterine insemination?
Should women who have endometriosis, open fallopian tubes, and infertility be treated with clomiphene and intrauterine insemination (IUI) prior to a cycle of IVF?
“This is a complex question,” says Dr. Estes, “as clinical parameters such as age, symptoms, duration of infertility, and the ability to proceed with IVF are important, as well as stage of disease.”
“Overall, in patients younger than 35 years, clomiphene-IUI is an option and has an increased pregnancy rate over timed intercourse” (9% vs 3%), she says.10 “However, clearly IVF is much more successful and is the most effective treatment, with pregnancy rates of approximately 46% for women younger than 35 years (3% of whom have a ‘diagnosis’ of endometriosis, and 13% of whom have an ‘unknown’ infertility factor).11 Women who are older than 35 or who have additional infertility factors, such as male factor, should consider IVF first.”
3. When is surgery indicated?
“Patients who have significant pain associated with their infertility will benefit from surgery, which offers both pain relief and an improvement in the spontaneous pregnancy rate,” says Dr. Falcone. “Patients who are infertile and desire spontaneous pregnancy without pharmacologic intervention or assisted reproductive technology (ART) also benefit from surgery.”
The benefit of surgery in asymptomatic women with minimal to mild endometriosis is unclear. In a randomized controlled trial of 341 women (aged 20–39 years) with infertility and minimal to mild endometriosis, laparoscopic resection or ablation of visible lesions resulted in pregnancy in 30.7% of women, compared with 17.7% of women who underwent diagnostic laparoscopy only.12
Another randomized study of 96 women with minimal to mild endometriosis who underwent resection/ablation or diagnostic laparoscopy found no difference in the birth rate at 1 year.13
When the results of these 2 studies are combined, the number needed to treat is 12 laparoscopies in women with endometriosis, says Dr. Falcone. “So 1 additional pregnancy would be gained from performing endometriosis surgery in 12 patients. If we assume a prevalence of 30% in asymptomatic women with infertility, then you need to perform 40 diagnostic laparoscopies to achieve an extra pregnancy.”
For women with infertility and severe endometriosis, a nonrandomized study found cumulative pregnancy rates of 45% and 63% after laparoscopy and laparotomy, respectively, in 216 women followed for up to 2 years.14 The difference in rates was not statistically significant.
“Although surgery is indicated to diagnose endometriosis, multiple repeat procedures are not an effective treatment for infertility,” says Dr. Estes. “Plus, especially for stage I and II endometriosis [according to the ASRM classification system], approximately 12 patients will need surgery for 1 additional pregnancy—and many more will have needed the procedure to even get to those who have endometriosis. While patients often want us to ‘do something’—often a covered service such as surgery—we need to consider that surgery for early disease does not provide a significant or long-lasting enhancement to women’s ability to conceive.”
In the absence of male-factor infertility, surgical diagnosis with conservative and precise treatment of endometriosis at the same time is better than going directly to IVF, says Dr. Nezhat. Younger patients who undergo surgical treatment have a better chance of achieving more than 1 spontaneous pregnancy than they do with IVF. And older patients also will have an improved conception rate with ART when they are treated surgically, he says.8
Coding and reimbursement
For endometriosis, the correct diagnostic code helps establish the medical necessity of later treatments
Several diagnostic codes are available to describe the location of endometriotic implants, but the fact remains that these codes can be reported only after a definitive diagnosis is made, which generally comes after confirmatory surgery has been performed. When the patient is in the diagnostic phase, she may present with complaints of pelvic pain, dyspareunia, dysmenorrhea, or infertility. Knowing which codes to use at the time of evaluation goes a long way toward establishing medical necessity for any later surgery or medical treatment.
In the TABLE, you will find common diagnostic codes that can be reported during evaluation of endometriosis, including both International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM equivalents. Note that, with ICD-10-CM, payers are looking for a more specific type of dysmenorrhea in the documentation. In the case of painful menstruation suspected to arise from endometriosis, the code for secondary dysmenorrhea should be reported.
Diagnostic coding in cases in which the patient first presents with a complaint of infertility in the absence of other symptoms can be problematic, as many insurers still do not cover the treatment of infertility. However, in the diagnostic stage, codes for fertility testing can be reported instead of codes that confirm infertility when no other symptoms are present. Once the clinician has verified that endometriosis is the source of the infertility and begins treatment, the primary code for the type of endometriosis would be reported, not a diagnosis of infertility.
The procedure performed to diagnose endometriosis would be diagnostic laparoscopy (Current Procedure Terminology [CPT] code 49320, Laparoscopy, abdomen, peritoneum, and omentum, diagnostic, with or without collection of specimen[s] by brushing or washing [separate procedure]) if medical treatment is being pursued. If the plan is to confirm and then immediately remove the implants, the CPT codes would be either 58662, Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method, or 49203–49205, Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors, based on the largest tumor diameter, if they are removed via an abdominal incision.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
4. Surgery or IVF for endometriomas?
When an endometrioma is present, should it be surgically treated or should the patient go straight to IVF? And does the optimal approach depend on the size of the endometrioma?
“The answer to this question ultimately depends on the skill and experience of the surgeon and the type of endometrioma,” says Dr. Nezhat, “because improper removal of an endometrioma may compromise the function of the ovary.”
“I recommend removal of the endometrioma by a skilled surgeon experienced in the management of ovarian cysts and preservation of ovarian function, especially in patients who have failed IVF,” he says.6,15
According to the ASRM committee opinion on endometriosis and infertility, laparoscopic cystectomy for endometriomas larger than 4 cm improves fertility, compared with cyst drainage and coagulation, which is associated with a high risk of cyst recurrence.2 However, no randomized trials have compared laparoscopic excision of an endometrioma to expectant management prior to IVF/intracytoplasmic sperm injection (ICSI). One case-control study found that laparoscopic cystectomy prior to IVF had no effect on fertility outcomes.16 At the same time, however, “conservative surgical treatment in symptomatic patients did not impair the success rates of IVF or ICSI,” notes the ASRM.2 Therefore, “evidence suggests that surgery does not benefit asymptomatic women with an endometrioma prior to scheduled IVF/ICSI,” the ASRM concludes.2
Dr. Nezhat cautions against the practice of indiscriminate IVF in the setting of ovarian endometriomas because he has seen patients who developed tubo-ovarian abscess and infected endometriomas after transvaginal egg retrieval. He notes that there are additional case reports of similar findings from other surgeons.
As far as excision versus ablation is concerned, all studies have shown some effect on ovarian function after excision, says Dr. Falcone. “The studies assessed this parameter using several endpoints, such as the number of oocytes retrieved at IVF. Recently, anti-Müllerian hormone has been used and has confirmed this observation. The decreased ovarian reserve is especially problematic with bilateral ovarian disease. The extent of the decreased ovarian function is dependent on several parameters, especially how much energy is used to achieve hemostasis. Several techniques have been proposed to reduce damage, including less traumatic ways of achieving hemostasis in the ovarian hilus.”
“The dilemma is that, if we excise the disease, the pregnancy rates are better than with ablation, but if we excise, there is more damage to the ovary. In my practice, I excise if there is minimal associated disease, such as adhesions, because the pregnancy rate after surgery is good. If, however, there are extensive adhesions with a low chance of spontaneous pregnancy, I minimize excision,” Dr. Falcone says.
As for going straight to IVF, “many patients cannot afford IVF; therefore, surgery is their only option. Furthermore, many patients have severe associated pain; therefore, surgery is required to improve quality of life. However, if the decision is made to proceed to IVF, there is no evidence that cystectomy prior to treatment with ART improves the pregnancy rate,” Dr. Falcone says.
“Basically, if a woman has no pain and no endometrioma, and infertility alone is the issue, I treat her in the same manner as I would a patient with idiopathic infertility—no surgery,” Dr. Falcone says. “If a patient has severe pain and infertility, I treat her surgically but conservatively, especially when an endometrioma is present. The patient will get pain relief but also derive fertility benefit. If a patient has had previous surgery for endometriosis-associated infertility, additional surgery is not the next step—as our study has shown that pregnancy rates are better with IVF than repeat surgery.”17
5. Is surgery ever effective after failed IVF?
To answer this question, Dr. Nezhat points to a retrospective case series by Littman and colleagues in which 29 women with a history of failed IVF underwent laparoscopic evaluation and treatment of endometriosis (by the same surgeon).8 Of 29 patients, 22 conceived after laparoscopic treatment of endometriosis, including 15 “non-IVF” pregnancies and 7 IVF pregnancies, the study results show.8
“It is not unusual for patients and health care providers to perceive IVF as the final treatment for infertility,” Littman and colleagues write. “When this definitive therapy fails repeatedly, clinicians and patients may be inclined to pursue oocyte donation or elect to forego further treatment altogether. This is especially true in women of advanced age and in patients with borderline embryo quality. Presently, as a result of our clinical observation in patients with failed IVF, before egg donation or adoption, we offer the option to have meticulous laparoscopic evaluation and treatment by a skilled surgeon. Furthermore, we would not classify an infertility condition as unexplained without confirming the absence of endometriosis by a thorough laparoscopy. In our experience, patients under 35 years old with unexplained infertility who are found to have endometriosis at the time of laparoscopy have an excellent chance of pregnancy following surgical treatment without ART.”8
6. Is repeat surgery ever helpful?
In the treatment of endometriosis-associated infertility, repeat surgery should be avoided if no symptoms are present, says Dr. Estes. “In women with continued symptoms, the benefits of repeat surgery are small, with known risks, including the potential for adhesive disease and the iatrogenic detrimental effect on ovarian function—often in a setting of already-present decreased ovarian reserve.”
Dr. Nezhat agrees that multiple surgeries carry the potential for harm. “However, there is one caveat,” he says. “What was done at the previous surgery and how? The skill and experience of the surgeon and proper technique are of paramount importance to the surgical outcome.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Although endometriosis and infertility are clearly linked—in life as well as the medical literature—no causal relationship has been established. Nevertheless, data suggest that 25% to 50% of infertile women have endometriosis, and that as many as 30% to 50% of women who have endometriosis are infertile.1
Among the mechanisms that have been proposed to explain this link are:
- distorted pelvic anatomy
- endocrine and ovulatory abnormalities
- impaired implantation
- impaired quality of the oocyte and embryo
- altered peritoneal function
- altered hormonal and cell-mediated function
- abnormal uterotubal transport.2
Recent studies by Kao and colleagues and Giudice and colleagues have led to new findings in regard to endometriosis and infertility, says Ceana Nezhat, MD.3,4 Dr. Nezhat is Director of the Nezhat Medical Center in Atlanta, Georgia, and Medical Director of Training and Education at Northside Hospital in Atlanta. “These researchers have discovered that endometriosis causes changes to the endometrium that contribute to infertility.”
“There are no studies that have specifically assessed whether one anatomic site is associated with increased infertility over another,” says Tommaso Falcone, MD. “However, it is assumed that disease that involves the tubes and ovaries would impede fertility the most. Adhesive disease and endometriomas around the tubes and ovaries are associated with a worsening prognosis. Although peritoneal disease probably influences fertility solely on the basis of inflammation, disease around the tubes and ovaries is thought to have a mechanical effect as well.” Dr. Falcone is Professor and Chair of Obstetrics and Gynecology at the Cleveland Clinic in Cleveland, Ohio.
“Endometriosis is a chronic and heterogeneous disease process,” says Stephanie J. Estes, MD, Director of Robotic Surgical Services and Associate Professor, Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, at Penn State Hershey Medical Center in Hershey, Pennsylvania.
“It is likely that no single site is the causative factor,” Dr. Estes says. “Endometriosis alters prostaglandins, cytokines, and proteases that may adversely affect eggs, sperm, or embryo development. In addition, altered endometrial receptivity may play a role. It has been shown that, when donor oocytes from women with endometriosis are transferred to women without endometriosis, there are lower implantation rates and poorer embryo quality.”5
Does it follow, then, that eradication of endometriosis improves fertility?
The answer is not clear. Rather, it depends on a number of variables, including the stage of the disease, its location, the presence of symptoms, and more.
“The approach for endometiosis-associated infertility is completely different from the approach for pain,” says Dr. Nezhat. “In a patient with pain, complete eradication of endometriosis is necessary. However, when addressing infertility, a surgeon must be cautious in the vicinity of the reproductive organs, even if a multistage approach is required. Fertility preservation is the goal.6,7 However, thorough treatment of endometriosis improves fertility rates even in cases of failed in vitro fertilization” (IVF).8
In this article, the focus is on 6 critical questions concerning endometriosis and infertility, including the role of medical therapy, when surgery is indicated, and whether an endometrioma warrants removal or referral for IVF.
In Part 1 of this 3-part series, which appeared in the April 2015 issue of OBG Management, the subject was diagnosis of endometriosis. In Part 2, which appeared in May 2015, the focus was endometriosis and pain.
1. Is there a role for medical therapy?
In women who have endometriosis-related infertility, medical therapy does not appear to produce any benefit. In its committee opinion on the subject, the American Society for Reproductive Medicine (ASRM) states as much: “There is no evidence that medical treatment of endometriosis improves fertility.”2
In fact, observes Dr. Estes, trials of medical treatment, involving such medications as combined estrogen-progestin therapy, danazol, progestins, or gonadotropin-releasing hormone (GnRH) agonists, may cause an unnecessary “delay in the use of more effective treatments that could result in pregnancy.”
Dr. Nezhat believes that medical therapy is effective in patients who have adenomyosis in addition to endometriosis. “Three months of treatment with a GnRH agonist will improve fertility rates in these patients,” he says.
“Medical treatments inhibit ovulation,” notes Dr. Estes. “These therapies have no role in pretreatment of patients with endometriosis prior to infertility treatment. On the other hand, medical therapy with GnRH agonists for 3 to 6 months prior to an IVF cycle does result in increased pregnancy rates.”9
2. What is the role of clomiphene and intrauterine insemination?
Should women who have endometriosis, open fallopian tubes, and infertility be treated with clomiphene and intrauterine insemination (IUI) prior to a cycle of IVF?
“This is a complex question,” says Dr. Estes, “as clinical parameters such as age, symptoms, duration of infertility, and the ability to proceed with IVF are important, as well as stage of disease.”
“Overall, in patients younger than 35 years, clomiphene-IUI is an option and has an increased pregnancy rate over timed intercourse” (9% vs 3%), she says.10 “However, clearly IVF is much more successful and is the most effective treatment, with pregnancy rates of approximately 46% for women younger than 35 years (3% of whom have a ‘diagnosis’ of endometriosis, and 13% of whom have an ‘unknown’ infertility factor).11 Women who are older than 35 or who have additional infertility factors, such as male factor, should consider IVF first.”
3. When is surgery indicated?
“Patients who have significant pain associated with their infertility will benefit from surgery, which offers both pain relief and an improvement in the spontaneous pregnancy rate,” says Dr. Falcone. “Patients who are infertile and desire spontaneous pregnancy without pharmacologic intervention or assisted reproductive technology (ART) also benefit from surgery.”
The benefit of surgery in asymptomatic women with minimal to mild endometriosis is unclear. In a randomized controlled trial of 341 women (aged 20–39 years) with infertility and minimal to mild endometriosis, laparoscopic resection or ablation of visible lesions resulted in pregnancy in 30.7% of women, compared with 17.7% of women who underwent diagnostic laparoscopy only.12
Another randomized study of 96 women with minimal to mild endometriosis who underwent resection/ablation or diagnostic laparoscopy found no difference in the birth rate at 1 year.13
When the results of these 2 studies are combined, the number needed to treat is 12 laparoscopies in women with endometriosis, says Dr. Falcone. “So 1 additional pregnancy would be gained from performing endometriosis surgery in 12 patients. If we assume a prevalence of 30% in asymptomatic women with infertility, then you need to perform 40 diagnostic laparoscopies to achieve an extra pregnancy.”
For women with infertility and severe endometriosis, a nonrandomized study found cumulative pregnancy rates of 45% and 63% after laparoscopy and laparotomy, respectively, in 216 women followed for up to 2 years.14 The difference in rates was not statistically significant.
“Although surgery is indicated to diagnose endometriosis, multiple repeat procedures are not an effective treatment for infertility,” says Dr. Estes. “Plus, especially for stage I and II endometriosis [according to the ASRM classification system], approximately 12 patients will need surgery for 1 additional pregnancy—and many more will have needed the procedure to even get to those who have endometriosis. While patients often want us to ‘do something’—often a covered service such as surgery—we need to consider that surgery for early disease does not provide a significant or long-lasting enhancement to women’s ability to conceive.”
In the absence of male-factor infertility, surgical diagnosis with conservative and precise treatment of endometriosis at the same time is better than going directly to IVF, says Dr. Nezhat. Younger patients who undergo surgical treatment have a better chance of achieving more than 1 spontaneous pregnancy than they do with IVF. And older patients also will have an improved conception rate with ART when they are treated surgically, he says.8
Coding and reimbursement
For endometriosis, the correct diagnostic code helps establish the medical necessity of later treatments
Several diagnostic codes are available to describe the location of endometriotic implants, but the fact remains that these codes can be reported only after a definitive diagnosis is made, which generally comes after confirmatory surgery has been performed. When the patient is in the diagnostic phase, she may present with complaints of pelvic pain, dyspareunia, dysmenorrhea, or infertility. Knowing which codes to use at the time of evaluation goes a long way toward establishing medical necessity for any later surgery or medical treatment.
In the TABLE, you will find common diagnostic codes that can be reported during evaluation of endometriosis, including both International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM equivalents. Note that, with ICD-10-CM, payers are looking for a more specific type of dysmenorrhea in the documentation. In the case of painful menstruation suspected to arise from endometriosis, the code for secondary dysmenorrhea should be reported.
Diagnostic coding in cases in which the patient first presents with a complaint of infertility in the absence of other symptoms can be problematic, as many insurers still do not cover the treatment of infertility. However, in the diagnostic stage, codes for fertility testing can be reported instead of codes that confirm infertility when no other symptoms are present. Once the clinician has verified that endometriosis is the source of the infertility and begins treatment, the primary code for the type of endometriosis would be reported, not a diagnosis of infertility.
The procedure performed to diagnose endometriosis would be diagnostic laparoscopy (Current Procedure Terminology [CPT] code 49320, Laparoscopy, abdomen, peritoneum, and omentum, diagnostic, with or without collection of specimen[s] by brushing or washing [separate procedure]) if medical treatment is being pursued. If the plan is to confirm and then immediately remove the implants, the CPT codes would be either 58662, Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method, or 49203–49205, Excision or destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary tumors, based on the largest tumor diameter, if they are removed via an abdominal incision.
—Melanie Witt, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
4. Surgery or IVF for endometriomas?
When an endometrioma is present, should it be surgically treated or should the patient go straight to IVF? And does the optimal approach depend on the size of the endometrioma?
“The answer to this question ultimately depends on the skill and experience of the surgeon and the type of endometrioma,” says Dr. Nezhat, “because improper removal of an endometrioma may compromise the function of the ovary.”
“I recommend removal of the endometrioma by a skilled surgeon experienced in the management of ovarian cysts and preservation of ovarian function, especially in patients who have failed IVF,” he says.6,15
According to the ASRM committee opinion on endometriosis and infertility, laparoscopic cystectomy for endometriomas larger than 4 cm improves fertility, compared with cyst drainage and coagulation, which is associated with a high risk of cyst recurrence.2 However, no randomized trials have compared laparoscopic excision of an endometrioma to expectant management prior to IVF/intracytoplasmic sperm injection (ICSI). One case-control study found that laparoscopic cystectomy prior to IVF had no effect on fertility outcomes.16 At the same time, however, “conservative surgical treatment in symptomatic patients did not impair the success rates of IVF or ICSI,” notes the ASRM.2 Therefore, “evidence suggests that surgery does not benefit asymptomatic women with an endometrioma prior to scheduled IVF/ICSI,” the ASRM concludes.2
Dr. Nezhat cautions against the practice of indiscriminate IVF in the setting of ovarian endometriomas because he has seen patients who developed tubo-ovarian abscess and infected endometriomas after transvaginal egg retrieval. He notes that there are additional case reports of similar findings from other surgeons.
As far as excision versus ablation is concerned, all studies have shown some effect on ovarian function after excision, says Dr. Falcone. “The studies assessed this parameter using several endpoints, such as the number of oocytes retrieved at IVF. Recently, anti-Müllerian hormone has been used and has confirmed this observation. The decreased ovarian reserve is especially problematic with bilateral ovarian disease. The extent of the decreased ovarian function is dependent on several parameters, especially how much energy is used to achieve hemostasis. Several techniques have been proposed to reduce damage, including less traumatic ways of achieving hemostasis in the ovarian hilus.”
“The dilemma is that, if we excise the disease, the pregnancy rates are better than with ablation, but if we excise, there is more damage to the ovary. In my practice, I excise if there is minimal associated disease, such as adhesions, because the pregnancy rate after surgery is good. If, however, there are extensive adhesions with a low chance of spontaneous pregnancy, I minimize excision,” Dr. Falcone says.
As for going straight to IVF, “many patients cannot afford IVF; therefore, surgery is their only option. Furthermore, many patients have severe associated pain; therefore, surgery is required to improve quality of life. However, if the decision is made to proceed to IVF, there is no evidence that cystectomy prior to treatment with ART improves the pregnancy rate,” Dr. Falcone says.
“Basically, if a woman has no pain and no endometrioma, and infertility alone is the issue, I treat her in the same manner as I would a patient with idiopathic infertility—no surgery,” Dr. Falcone says. “If a patient has severe pain and infertility, I treat her surgically but conservatively, especially when an endometrioma is present. The patient will get pain relief but also derive fertility benefit. If a patient has had previous surgery for endometriosis-associated infertility, additional surgery is not the next step—as our study has shown that pregnancy rates are better with IVF than repeat surgery.”17
5. Is surgery ever effective after failed IVF?
To answer this question, Dr. Nezhat points to a retrospective case series by Littman and colleagues in which 29 women with a history of failed IVF underwent laparoscopic evaluation and treatment of endometriosis (by the same surgeon).8 Of 29 patients, 22 conceived after laparoscopic treatment of endometriosis, including 15 “non-IVF” pregnancies and 7 IVF pregnancies, the study results show.8
“It is not unusual for patients and health care providers to perceive IVF as the final treatment for infertility,” Littman and colleagues write. “When this definitive therapy fails repeatedly, clinicians and patients may be inclined to pursue oocyte donation or elect to forego further treatment altogether. This is especially true in women of advanced age and in patients with borderline embryo quality. Presently, as a result of our clinical observation in patients with failed IVF, before egg donation or adoption, we offer the option to have meticulous laparoscopic evaluation and treatment by a skilled surgeon. Furthermore, we would not classify an infertility condition as unexplained without confirming the absence of endometriosis by a thorough laparoscopy. In our experience, patients under 35 years old with unexplained infertility who are found to have endometriosis at the time of laparoscopy have an excellent chance of pregnancy following surgical treatment without ART.”8
6. Is repeat surgery ever helpful?
In the treatment of endometriosis-associated infertility, repeat surgery should be avoided if no symptoms are present, says Dr. Estes. “In women with continued symptoms, the benefits of repeat surgery are small, with known risks, including the potential for adhesive disease and the iatrogenic detrimental effect on ovarian function—often in a setting of already-present decreased ovarian reserve.”
Dr. Nezhat agrees that multiple surgeries carry the potential for harm. “However, there is one caveat,” he says. “What was done at the previous surgery and how? The skill and experience of the surgeon and proper technique are of paramount importance to the surgical outcome.”
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796.
2. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
3. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881.
4. Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–264; discussion 293–295, 396–406.
5. Garrido N, Navarro J, Garcia-Velasco J, et al. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2012;8(1):95-103.
6. Lewis M, Baker V, Nezhat C. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(6):e81–83.
7. Tsolakidis D, Pados G, Vavilis D, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(1):71–77.
8. Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
9. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;(1):CD004635.
10. Deaton JL, Gibson M, Blackmer KM, et al. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990; 54:1083–1088.
11. Society for Assisted Reproductive Technology. Clinic Summary Report [all member clinics]: 2013. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0. Accessed May 13, 2015.
12. Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–222.
13. Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum Reprod. 1999;14(5):1332–1334.
14. Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66(5):706–711.
15. Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
16. Garcia-Velasco JA, Mahutte NG, Corona J, et al. Removal of endometriomas before in vitro fertilization does not improve fertility outcomes: a matched, case-control study. Fertil Steril. 2004;81(5):1194–1197.
17. Pagidas K, Falcone T, Hemmings R, Miron R. Comparison of surgical treatment of moderate (stage III) and severe (stage IV) endometriosis-related infertility with IVF–embryo transfer. Fertil Steril. 1996;65(4):791–795.
1. Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784–796.
2. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591–598.
3. Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881.
4. Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002;955:252–264; discussion 293–295, 396–406.
5. Garrido N, Navarro J, Garcia-Velasco J, et al. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2012;8(1):95-103.
6. Lewis M, Baker V, Nezhat C. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(6):e81–83.
7. Tsolakidis D, Pados G, Vavilis D, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: a prospective randomized study. Fertil Steril. 2010;94(1):71–77.
8. Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
9. Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006;(1):CD004635.
10. Deaton JL, Gibson M, Blackmer KM, et al. A randomized, controlled trial of clomiphene citrate and intrauterine insemination in couples with unexplained infertility or surgically corrected endometriosis. Fertil Steril. 1990; 54:1083–1088.
11. Society for Assisted Reproductive Technology. Clinic Summary Report [all member clinics]: 2013. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=0. Accessed May 13, 2015.
12. Marcoux S, Maheux R, Bérubé S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N Engl J Med. 1997;337(4):217–222.
13. Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: a randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum Reprod. 1999;14(5):1332–1334.
14. Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil Steril. 1996;66(5):706–711.
15. Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
16. Garcia-Velasco JA, Mahutte NG, Corona J, et al. Removal of endometriomas before in vitro fertilization does not improve fertility outcomes: a matched, case-control study. Fertil Steril. 2004;81(5):1194–1197.
17. Pagidas K, Falcone T, Hemmings R, Miron R. Comparison of surgical treatment of moderate (stage III) and severe (stage IV) endometriosis-related infertility with IVF–embryo transfer. Fertil Steril. 1996;65(4):791–795.
In This Article
- The role of medical therapy
- When is surgery indicated?
- Coding and reimbursement
- Is surgery ever effective after failed IVF?