User login
As the Affordable Care Act comes of age, a look behind the headlines
The Affordable Care Act (ACA) faced—and failed—an important test on October 1, 2013, when open enrollment began in the new health-care marketplaces. Plenty has been written about Web site crashes, technical glitches, and what seems to be general mismanagement of this crucial aspect of implementation.
Let’s look behind the headlines to see which aspects of the ACA are working, and which aren’t, and why.
KNOWING THE FACTS CAN HELP YOU HELP YOUR PATIENTS
ObGyns are scientists. As a scientist, you know the importance of facts. In your research and clinical care, you seek out and rely on scientific facts and evidence. You leave aside unsubstantiated thinking.
It’s imperative that we take the same approach with this subject. Far too many misleading and unsubstantiated claims and headlines are crowding out reliable factual information, seriously hindering physicians’ ability to understand this important health-care system change and respond to it appropriately on behalf of patients. As much as we all love Facebook, for example, it may not be the most accurate source of information on the ACA.
Plenty of reliable, factual, unbiased sources of information about the ACA exist, such as “Understanding Obamacare, Politico’s Guide to the Affordable Care Act” (http://www.politico.com/obamacare-guide/). Other helpful sources of ACA outreach and enrollment information:
HealthCare.gov is the federal government’s main portal for information on the Affordable Care Act. A Spanish version of this site can be accessed at www.CuidadoDeSalud.gov.
“FAQ: What you need to know about the new online marketplaces” features questions and answers from Kaiser Health News at http://www.kaiserhealthnews.org/stories/2013/september/17/marketplace-faq-insurance-exchange-obamacare-aca.aspx.
“Fact sheets: Why the Affordable Care Act matters for women” offers links to summaries of ACA provisions; information on health care for pregnant, low-income and older women; preventive care; and more from the National Partnership for Women and Families at http://go.nationalpartnership.org/site/PageServer?pagename=issues_health_reform_anniversary.
Webinars, speakers, FAQs, and more from Doctors for America at http://www.drsforamerica.org/take-action/get-people-covered.
Reports, blog posts, and links to information on enrollment from Enroll America at www.enrollamerica.org.
An informative video on coverage decisions from the Kaiser Family Foundation at http://kff.org/health-reform/video/youtoons-obamacare-video/.
A REVIEW OF THE CHANGES UNDER ACA
Let’s start with one key fact: The ACA offers a lot of good for women’s health care. Many of these improvements hinge on individuals’ ability to enroll in private health insurance policies sold in the marketplaces.
Each state’s marketplace is similar to the system used by the Federal Employees Health Benefits Program (FEHBP), the insurance marketplace used nationwide by federal employees, including members of Congress. Private plans, such as Blue Cross Blue Shield, Aetna, and United Healthcare, offer health insurance on the FEHBP marketplace to the millions of federal employees each year.
In state marketplaces, private health insurers will offer plans to potentially millions of previously under- or uninsured individuals and families. In exchange for access to this huge new group of consumers, private insurers must abide by a number of important consumer protections in order to be eligible to sell their policies in a state marketplace:
Insurers must agree to abide by the 80/20 rule. Under this game-changer, insurers agree to return the actuarial value of 80% of an enrollee’s premium to health care, keeping only a maximum of 20% for profits and other non-health-care categories.
Insurers must agree to cover 10 essential benefits, including maternity care.
Insurers must agree to cover key preventive services, without copays or deductibles, helping our patients stay healthy.
Insurers must abide by significant insurance protections. They can’t, for example, deny a woman coverage because she has a preexisting condition, was once the victim of domestic violence, or once had a cesarean delivery.
Essential benefits and preventive services
All private health insurance plans sold in the state marketplaces must cover the 10 essential health benefits:
ambulatory patient services
emergency services
hospitalization
maternity and newborn care
mental health and substance use disorder services, including behavioral health treatment
prescription drugs
rehabilitative and habilitative services and devices
laboratory services
preventive and wellness services and chronic disease management
pediatric services, including oral and vision care.
These insurers also must cover—with no charge to the patient—preventive services:
well-woman visits (one or more)
all FDA-approved contraceptive methods and contraception counseling
gestational diabetes screening
mammograms
Pap tests
HIV and other sexually transmitted infection screening and counseling
breastfeeding support, supplies, and counseling
domestic violence screening and counseling.
Related Article: Your age-based guide to comprehensive well-woman care Robert L. Barbieri, MD (October 2012)
In addition, private insurers must offer additional preventive services, although they can charge copays for them:
anemia screening on a routine basis for pregnant women
screening for urinary tract or other infection for pregnant women
counseling about genetic testing for a BRCA mutation for women at higher risk
counseling about chemoprevention of breast cancer for women at higher risk
cervical cancer screening for sexually active women
folic acid supplementation for women who may become pregnant
osteoporosis screening for women over age 60, depending on risk factors
screening for Rh incompatibility for all pregnant women and follow-up testing for women at higher risk
tobacco use screening and interventions for all women, and expanded counseling for pregnant users of tobacco.
An end to preexisting-condition exclusions and other harmful practices
Insurers offering plans in the state marketplaces also must abide by important insurance reforms:
They must eliminate exclusions for preexisting conditions. Insurers cannot deny individuals coverage because they already have a condition that requires medical care, including pregnancy. Before this ACA rule, private insurers often rejected applicants who needed care, as well as those who accessed health care in the past. Insurers regularly denied coverage to women who had had a cesarean delivery or had once been a victim of domestic violence.
They cannot charge women more than men for the same coverage. Before the ACA, women seeking health-care coverage often faced higher premiums than men for identical coverage. This made private coverage less affordable for our patients.
They cannot impose a 9-month waiting period. (Need I say more?)
They must eliminate any annual lifetime limits on coverage. Insurers selling policies in the marketplaces cannot end coverage after a certain dollar amount has been reached, a common practice before the ACA. This change is good news for you and your patients. A patient needing long or expensive care won’t lose coverage when the cost of her care hits an arbitrary ceiling.
They cannot rescind coverage unless fraud is proven. Before the ACA, private health insurers would often drop an individual if he or she started racking up high health-care costs. Patients in the middle of expensive cancer treatments, for example, would find themselves suddenly without health insurance. This won’t happen for policies sold in marketplaces unless the patient lied on her enrollment forms or failed to keep up her premium payments.
What these changes mean, in real numbers
These protections are critically important to your ability to care for your patients. Here’s what they mean in real life:
Health-care coverage for about 10,000 insured women is no longer subject to an annual lifetime coverage limit.
Private insurers can’t drop coverage, a change that will affect about 5.5 million insured women.
Insurance companies cannot deny coverage for preexisting conditions, which will help insure about 100,000 women.
Each state marketplace offers four types of plans, the idea being to help people compare policies side by side. All plans sold in the marketplaces must abide by the consumer protections I just reviewed. Each tier is differentiated by the average percentage of an enrollee’s health-care expenses paid by the insurer. The more an enrollee agrees to pay out of pocket, the lower his or her premium.
The tiers are:
Bronze – The insurer covers 60% of health-care costs, and the insured covers 40%. This tier offers the cheapest premiums.
Silver – The insurer covers 70% of costs.
Gold – The insurer covers 80% of costs.
Platinum – The insurer covers 90% of costs.
WHAT WENT WRONG
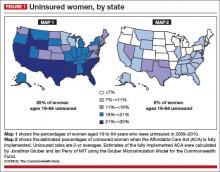
If everything had gone according to plan, women’s access to health insurance would have increased dramatically nationwide, including in the dark blue states in FIGURE 1 (Map 1)—states that rank lowest in access to care. You’ll notice that many of the states that are dark blue in Map 1 shift to light blue in Map 2. Take a careful look at those dark blue states and see what colors they are in the next two maps (FIGURES 1 and 2). Hint: There’s a pattern.
Problem 1 – Strains on Healthcare.gov
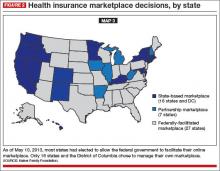
When the ACA was signed into law, most states were expected to build and run their own online marketplaces. The federal government offered to run a state exchange if a state didn’t. Few ACA engineers anticipated that the federal government would have to run the marketplaces in more than half the states—politics-fueled decisions in many states. Now you can see that many of the states that are dark blue in Map 1 are gray in Map 3 (FIGURE 2). Many states whose populations have the greatest need left it to the federal government to run the marketplaces.
Data indicate that state-run marketplaces are doing pretty well—a fact not often caught in frenzied headlines. If we look at the percentage of the target population actually enrolled in marketplace insurance plans during the first month, we see that the lowest state marketplace enrollment was in Washington State, with 30% of its target population enrolled. The highest target-population enrollment was achieved in Connecticut, with 191%.
Compare these numbers to the rates of target-population enrollment in states with marketplaces run by the federal government, which range from 3% to 20% (FIGURE 2). During the first month, federal and state-run marketplaces together enrolled 106,000 individuals into new coverage, 21% of the national target.
The news media have focused on the federal online enrollment debacle. Easier enrollment options are available to people who live in federally run marketplace states, including direct enrollment. Strongly supported by America’s health insurance industry, direct enrollment lets potential enrollees purchase coverage directly from insurance companies participating in the marketplaces.
Problem 2: People lost their current coverage
This is a problem worth exploring—one that affects people who previously bought insurance on the individual insurance market. This is the market that offers people comparatively limited coverage, usually with no maternity care coverage, for comparatively high premiums.
So why are these individuals losing coverage?
They are losing coverage because, as of January 2014, new individual plans must abide by the 80/20 rule, abide by insurance protections, and cover 10 essential services with no cost sharing.
You may recall that, in August 2009, Americans were demanding that they be able to keep the health-care coverage they currently had, and President Barack Obama promised that they would be able to. Consequently, health insurance policies in effect before March 2010, when the ACA was signed into law, were exempted—“grandfathered”—from most ACA requirements. If people liked their old policies, they could keep them.
Grandfathered plans are exempt from:
the requirement to cover the 10 essential health benefits
the requirement that plans must provide preventive services with no patient cost sharing
state or federal review of insurance premium increases of 10% or more for non-group and small business plans
a rule allowing consumers to appeal denials of claims to a third-party reviewer.
Most ACA requirements apply to new policies—those offered after March 2010 and those that have been changed significantly by the insurance companies. Some examples of changes in coverage that would cause a plan to lose grandfathering include:
the elimination of benefits to diagnose or treat a particular condition
an increase in the up-front deductible patients must pay before coverage kicks in by more than the cumulative growth in medical inflation since March 2010 plus 15%
a reduction in the share of the premium that the employer pays by more than 5% since March 2010.
How many people are we talking about? Not the 40% of Americans who have employer-based coverage or the 20% of Americans on Medicare, Medicaid, or Tricare. This provision affects about 5% of the insured, as many as 15 million people—many with plans that offer little coverage for high premiums.
The ACA intention was that many people previously covered in the individual insurance market would find better and cheaper coverage in their state marketplaces. That may be a good option for people in states that have chosen to run their own marketplaces, and a good option for people in other states, too, as federal online enrollment issues get fixed.
Problem 3: Medicaid expansion became a state option
When the US Supreme Court upheld the constitutionality of the ACA’s individual mandate, it also effectively turned the ACA Medicaid expansion into a state option.
Think of the Medicaid expansion as Medicaid Part 2. Regular Medicaid remains largely unchanged, with the same eligibility rules and coverage requirements.
The ACA included a provision under which every state would add a new part to its Medicaid program. Beginning in 2014, this part—the expansion—would cover individuals in each state with incomes under 138% of the federal poverty line—about $32,000 for a family of four in 2014. Medicaid expansion coverage is based only on income eligibility, a major change for women, many of whom currently qualify for Medicaid only if they’re pregnant.
Who would pay for the new coverage?
In 2014, 2015, and 2016, the federal government pays 100% of the cost of care for Medicaid expansion. From 2017 to 2020, the federal share gradually drops to 90%.
Medicaid expansion is an integral part of reducing the number of uninsured under the ACA and is expected to reduce the uninsured rate by almost 30% if adopted by every state. Medicaid expansion plus the ACA marketplaces were expected to cut our uninsured rate almost in half.
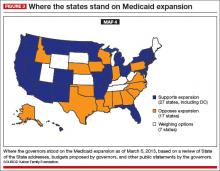
FIGURE 3 shows how states responded when the Supreme Court effectively converted the Medicaid expansion into an option, leaving us, again, with coverage gaps. Many of the states that have opted not to expand Medicaid are the same states that declined to operate their own state marketplaces, the same states with highest percentages of the uninsured.
The ACA has many interdependent parts. Make the Medicaid expansion a state option, and you end up with higher than expected rates of uninsured. Trigger big changes in the individual market when there are still bugs in the system, and people are left in the lurch.
Related Article: ACOG to legislators: Partnership, not interference Lucia DiVenere, MA (April 2013)
What’s happening now
As this article is going to press, enrollment in the marketplaces is getting easier, with Web site fixes and useful alternatives to Web-based enrollment. Small changes are being made to some deadlines to help people who have gotten stuck in the process. We’ll likely continue to see steps forward and back over the next many months.
Two things remain important:
We need to stick with the facts. If you see something in the news that seems too crazy to be real, your hunch may be right.
Your patients can benefit significantly from the ACA. An ACOG Fellow recently told me about one of his patients who has a severe health condition, no insurance, and needs expensive treatment. The ACA, with its marketplace rules outlawing exclusions for preexisting conditions and offering premium assistance, may be a lifesaver to her. But first she needs to enroll.
As your patients’ trusted physician, you can help point them in the right direction, possibly toward coverage that they never had before.
That’s good for all of us.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
The Affordable Care Act (ACA) faced—and failed—an important test on October 1, 2013, when open enrollment began in the new health-care marketplaces. Plenty has been written about Web site crashes, technical glitches, and what seems to be general mismanagement of this crucial aspect of implementation.
Let’s look behind the headlines to see which aspects of the ACA are working, and which aren’t, and why.
KNOWING THE FACTS CAN HELP YOU HELP YOUR PATIENTS
ObGyns are scientists. As a scientist, you know the importance of facts. In your research and clinical care, you seek out and rely on scientific facts and evidence. You leave aside unsubstantiated thinking.
It’s imperative that we take the same approach with this subject. Far too many misleading and unsubstantiated claims and headlines are crowding out reliable factual information, seriously hindering physicians’ ability to understand this important health-care system change and respond to it appropriately on behalf of patients. As much as we all love Facebook, for example, it may not be the most accurate source of information on the ACA.
Plenty of reliable, factual, unbiased sources of information about the ACA exist, such as “Understanding Obamacare, Politico’s Guide to the Affordable Care Act” (http://www.politico.com/obamacare-guide/). Other helpful sources of ACA outreach and enrollment information:
HealthCare.gov is the federal government’s main portal for information on the Affordable Care Act. A Spanish version of this site can be accessed at www.CuidadoDeSalud.gov.
“FAQ: What you need to know about the new online marketplaces” features questions and answers from Kaiser Health News at http://www.kaiserhealthnews.org/stories/2013/september/17/marketplace-faq-insurance-exchange-obamacare-aca.aspx.
“Fact sheets: Why the Affordable Care Act matters for women” offers links to summaries of ACA provisions; information on health care for pregnant, low-income and older women; preventive care; and more from the National Partnership for Women and Families at http://go.nationalpartnership.org/site/PageServer?pagename=issues_health_reform_anniversary.
Webinars, speakers, FAQs, and more from Doctors for America at http://www.drsforamerica.org/take-action/get-people-covered.
Reports, blog posts, and links to information on enrollment from Enroll America at www.enrollamerica.org.
An informative video on coverage decisions from the Kaiser Family Foundation at http://kff.org/health-reform/video/youtoons-obamacare-video/.
A REVIEW OF THE CHANGES UNDER ACA
Let’s start with one key fact: The ACA offers a lot of good for women’s health care. Many of these improvements hinge on individuals’ ability to enroll in private health insurance policies sold in the marketplaces.
Each state’s marketplace is similar to the system used by the Federal Employees Health Benefits Program (FEHBP), the insurance marketplace used nationwide by federal employees, including members of Congress. Private plans, such as Blue Cross Blue Shield, Aetna, and United Healthcare, offer health insurance on the FEHBP marketplace to the millions of federal employees each year.
In state marketplaces, private health insurers will offer plans to potentially millions of previously under- or uninsured individuals and families. In exchange for access to this huge new group of consumers, private insurers must abide by a number of important consumer protections in order to be eligible to sell their policies in a state marketplace:
Insurers must agree to abide by the 80/20 rule. Under this game-changer, insurers agree to return the actuarial value of 80% of an enrollee’s premium to health care, keeping only a maximum of 20% for profits and other non-health-care categories.
Insurers must agree to cover 10 essential benefits, including maternity care.
Insurers must agree to cover key preventive services, without copays or deductibles, helping our patients stay healthy.
Insurers must abide by significant insurance protections. They can’t, for example, deny a woman coverage because she has a preexisting condition, was once the victim of domestic violence, or once had a cesarean delivery.
Essential benefits and preventive services
All private health insurance plans sold in the state marketplaces must cover the 10 essential health benefits:
ambulatory patient services
emergency services
hospitalization
maternity and newborn care
mental health and substance use disorder services, including behavioral health treatment
prescription drugs
rehabilitative and habilitative services and devices
laboratory services
preventive and wellness services and chronic disease management
pediatric services, including oral and vision care.
These insurers also must cover—with no charge to the patient—preventive services:
well-woman visits (one or more)
all FDA-approved contraceptive methods and contraception counseling
gestational diabetes screening
mammograms
Pap tests
HIV and other sexually transmitted infection screening and counseling
breastfeeding support, supplies, and counseling
domestic violence screening and counseling.
Related Article: Your age-based guide to comprehensive well-woman care Robert L. Barbieri, MD (October 2012)
In addition, private insurers must offer additional preventive services, although they can charge copays for them:
anemia screening on a routine basis for pregnant women
screening for urinary tract or other infection for pregnant women
counseling about genetic testing for a BRCA mutation for women at higher risk
counseling about chemoprevention of breast cancer for women at higher risk
cervical cancer screening for sexually active women
folic acid supplementation for women who may become pregnant
osteoporosis screening for women over age 60, depending on risk factors
screening for Rh incompatibility for all pregnant women and follow-up testing for women at higher risk
tobacco use screening and interventions for all women, and expanded counseling for pregnant users of tobacco.
An end to preexisting-condition exclusions and other harmful practices
Insurers offering plans in the state marketplaces also must abide by important insurance reforms:
They must eliminate exclusions for preexisting conditions. Insurers cannot deny individuals coverage because they already have a condition that requires medical care, including pregnancy. Before this ACA rule, private insurers often rejected applicants who needed care, as well as those who accessed health care in the past. Insurers regularly denied coverage to women who had had a cesarean delivery or had once been a victim of domestic violence.
They cannot charge women more than men for the same coverage. Before the ACA, women seeking health-care coverage often faced higher premiums than men for identical coverage. This made private coverage less affordable for our patients.
They cannot impose a 9-month waiting period. (Need I say more?)
They must eliminate any annual lifetime limits on coverage. Insurers selling policies in the marketplaces cannot end coverage after a certain dollar amount has been reached, a common practice before the ACA. This change is good news for you and your patients. A patient needing long or expensive care won’t lose coverage when the cost of her care hits an arbitrary ceiling.
They cannot rescind coverage unless fraud is proven. Before the ACA, private health insurers would often drop an individual if he or she started racking up high health-care costs. Patients in the middle of expensive cancer treatments, for example, would find themselves suddenly without health insurance. This won’t happen for policies sold in marketplaces unless the patient lied on her enrollment forms or failed to keep up her premium payments.
What these changes mean, in real numbers
These protections are critically important to your ability to care for your patients. Here’s what they mean in real life:
Health-care coverage for about 10,000 insured women is no longer subject to an annual lifetime coverage limit.
Private insurers can’t drop coverage, a change that will affect about 5.5 million insured women.
Insurance companies cannot deny coverage for preexisting conditions, which will help insure about 100,000 women.
Each state marketplace offers four types of plans, the idea being to help people compare policies side by side. All plans sold in the marketplaces must abide by the consumer protections I just reviewed. Each tier is differentiated by the average percentage of an enrollee’s health-care expenses paid by the insurer. The more an enrollee agrees to pay out of pocket, the lower his or her premium.
The tiers are:
Bronze – The insurer covers 60% of health-care costs, and the insured covers 40%. This tier offers the cheapest premiums.
Silver – The insurer covers 70% of costs.
Gold – The insurer covers 80% of costs.
Platinum – The insurer covers 90% of costs.
WHAT WENT WRONG
If everything had gone according to plan, women’s access to health insurance would have increased dramatically nationwide, including in the dark blue states in FIGURE 1 (Map 1)—states that rank lowest in access to care. You’ll notice that many of the states that are dark blue in Map 1 shift to light blue in Map 2. Take a careful look at those dark blue states and see what colors they are in the next two maps (FIGURES 1 and 2). Hint: There’s a pattern.
Problem 1 – Strains on Healthcare.gov
When the ACA was signed into law, most states were expected to build and run their own online marketplaces. The federal government offered to run a state exchange if a state didn’t. Few ACA engineers anticipated that the federal government would have to run the marketplaces in more than half the states—politics-fueled decisions in many states. Now you can see that many of the states that are dark blue in Map 1 are gray in Map 3 (FIGURE 2). Many states whose populations have the greatest need left it to the federal government to run the marketplaces.
Data indicate that state-run marketplaces are doing pretty well—a fact not often caught in frenzied headlines. If we look at the percentage of the target population actually enrolled in marketplace insurance plans during the first month, we see that the lowest state marketplace enrollment was in Washington State, with 30% of its target population enrolled. The highest target-population enrollment was achieved in Connecticut, with 191%.
Compare these numbers to the rates of target-population enrollment in states with marketplaces run by the federal government, which range from 3% to 20% (FIGURE 2). During the first month, federal and state-run marketplaces together enrolled 106,000 individuals into new coverage, 21% of the national target.
The news media have focused on the federal online enrollment debacle. Easier enrollment options are available to people who live in federally run marketplace states, including direct enrollment. Strongly supported by America’s health insurance industry, direct enrollment lets potential enrollees purchase coverage directly from insurance companies participating in the marketplaces.
Problem 2: People lost their current coverage
This is a problem worth exploring—one that affects people who previously bought insurance on the individual insurance market. This is the market that offers people comparatively limited coverage, usually with no maternity care coverage, for comparatively high premiums.
So why are these individuals losing coverage?
They are losing coverage because, as of January 2014, new individual plans must abide by the 80/20 rule, abide by insurance protections, and cover 10 essential services with no cost sharing.
You may recall that, in August 2009, Americans were demanding that they be able to keep the health-care coverage they currently had, and President Barack Obama promised that they would be able to. Consequently, health insurance policies in effect before March 2010, when the ACA was signed into law, were exempted—“grandfathered”—from most ACA requirements. If people liked their old policies, they could keep them.
Grandfathered plans are exempt from:
the requirement to cover the 10 essential health benefits
the requirement that plans must provide preventive services with no patient cost sharing
state or federal review of insurance premium increases of 10% or more for non-group and small business plans
a rule allowing consumers to appeal denials of claims to a third-party reviewer.
Most ACA requirements apply to new policies—those offered after March 2010 and those that have been changed significantly by the insurance companies. Some examples of changes in coverage that would cause a plan to lose grandfathering include:
the elimination of benefits to diagnose or treat a particular condition
an increase in the up-front deductible patients must pay before coverage kicks in by more than the cumulative growth in medical inflation since March 2010 plus 15%
a reduction in the share of the premium that the employer pays by more than 5% since March 2010.
How many people are we talking about? Not the 40% of Americans who have employer-based coverage or the 20% of Americans on Medicare, Medicaid, or Tricare. This provision affects about 5% of the insured, as many as 15 million people—many with plans that offer little coverage for high premiums.
The ACA intention was that many people previously covered in the individual insurance market would find better and cheaper coverage in their state marketplaces. That may be a good option for people in states that have chosen to run their own marketplaces, and a good option for people in other states, too, as federal online enrollment issues get fixed.
Problem 3: Medicaid expansion became a state option
When the US Supreme Court upheld the constitutionality of the ACA’s individual mandate, it also effectively turned the ACA Medicaid expansion into a state option.
Think of the Medicaid expansion as Medicaid Part 2. Regular Medicaid remains largely unchanged, with the same eligibility rules and coverage requirements.
The ACA included a provision under which every state would add a new part to its Medicaid program. Beginning in 2014, this part—the expansion—would cover individuals in each state with incomes under 138% of the federal poverty line—about $32,000 for a family of four in 2014. Medicaid expansion coverage is based only on income eligibility, a major change for women, many of whom currently qualify for Medicaid only if they’re pregnant.
Who would pay for the new coverage?
In 2014, 2015, and 2016, the federal government pays 100% of the cost of care for Medicaid expansion. From 2017 to 2020, the federal share gradually drops to 90%.
Medicaid expansion is an integral part of reducing the number of uninsured under the ACA and is expected to reduce the uninsured rate by almost 30% if adopted by every state. Medicaid expansion plus the ACA marketplaces were expected to cut our uninsured rate almost in half.
FIGURE 3 shows how states responded when the Supreme Court effectively converted the Medicaid expansion into an option, leaving us, again, with coverage gaps. Many of the states that have opted not to expand Medicaid are the same states that declined to operate their own state marketplaces, the same states with highest percentages of the uninsured.
The ACA has many interdependent parts. Make the Medicaid expansion a state option, and you end up with higher than expected rates of uninsured. Trigger big changes in the individual market when there are still bugs in the system, and people are left in the lurch.
Related Article: ACOG to legislators: Partnership, not interference Lucia DiVenere, MA (April 2013)
What’s happening now
As this article is going to press, enrollment in the marketplaces is getting easier, with Web site fixes and useful alternatives to Web-based enrollment. Small changes are being made to some deadlines to help people who have gotten stuck in the process. We’ll likely continue to see steps forward and back over the next many months.
Two things remain important:
We need to stick with the facts. If you see something in the news that seems too crazy to be real, your hunch may be right.
Your patients can benefit significantly from the ACA. An ACOG Fellow recently told me about one of his patients who has a severe health condition, no insurance, and needs expensive treatment. The ACA, with its marketplace rules outlawing exclusions for preexisting conditions and offering premium assistance, may be a lifesaver to her. But first she needs to enroll.
As your patients’ trusted physician, you can help point them in the right direction, possibly toward coverage that they never had before.
That’s good for all of us.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
The Affordable Care Act (ACA) faced—and failed—an important test on October 1, 2013, when open enrollment began in the new health-care marketplaces. Plenty has been written about Web site crashes, technical glitches, and what seems to be general mismanagement of this crucial aspect of implementation.
Let’s look behind the headlines to see which aspects of the ACA are working, and which aren’t, and why.
KNOWING THE FACTS CAN HELP YOU HELP YOUR PATIENTS
ObGyns are scientists. As a scientist, you know the importance of facts. In your research and clinical care, you seek out and rely on scientific facts and evidence. You leave aside unsubstantiated thinking.
It’s imperative that we take the same approach with this subject. Far too many misleading and unsubstantiated claims and headlines are crowding out reliable factual information, seriously hindering physicians’ ability to understand this important health-care system change and respond to it appropriately on behalf of patients. As much as we all love Facebook, for example, it may not be the most accurate source of information on the ACA.
Plenty of reliable, factual, unbiased sources of information about the ACA exist, such as “Understanding Obamacare, Politico’s Guide to the Affordable Care Act” (http://www.politico.com/obamacare-guide/). Other helpful sources of ACA outreach and enrollment information:
HealthCare.gov is the federal government’s main portal for information on the Affordable Care Act. A Spanish version of this site can be accessed at www.CuidadoDeSalud.gov.
“FAQ: What you need to know about the new online marketplaces” features questions and answers from Kaiser Health News at http://www.kaiserhealthnews.org/stories/2013/september/17/marketplace-faq-insurance-exchange-obamacare-aca.aspx.
“Fact sheets: Why the Affordable Care Act matters for women” offers links to summaries of ACA provisions; information on health care for pregnant, low-income and older women; preventive care; and more from the National Partnership for Women and Families at http://go.nationalpartnership.org/site/PageServer?pagename=issues_health_reform_anniversary.
Webinars, speakers, FAQs, and more from Doctors for America at http://www.drsforamerica.org/take-action/get-people-covered.
Reports, blog posts, and links to information on enrollment from Enroll America at www.enrollamerica.org.
An informative video on coverage decisions from the Kaiser Family Foundation at http://kff.org/health-reform/video/youtoons-obamacare-video/.
A REVIEW OF THE CHANGES UNDER ACA
Let’s start with one key fact: The ACA offers a lot of good for women’s health care. Many of these improvements hinge on individuals’ ability to enroll in private health insurance policies sold in the marketplaces.
Each state’s marketplace is similar to the system used by the Federal Employees Health Benefits Program (FEHBP), the insurance marketplace used nationwide by federal employees, including members of Congress. Private plans, such as Blue Cross Blue Shield, Aetna, and United Healthcare, offer health insurance on the FEHBP marketplace to the millions of federal employees each year.
In state marketplaces, private health insurers will offer plans to potentially millions of previously under- or uninsured individuals and families. In exchange for access to this huge new group of consumers, private insurers must abide by a number of important consumer protections in order to be eligible to sell their policies in a state marketplace:
Insurers must agree to abide by the 80/20 rule. Under this game-changer, insurers agree to return the actuarial value of 80% of an enrollee’s premium to health care, keeping only a maximum of 20% for profits and other non-health-care categories.
Insurers must agree to cover 10 essential benefits, including maternity care.
Insurers must agree to cover key preventive services, without copays or deductibles, helping our patients stay healthy.
Insurers must abide by significant insurance protections. They can’t, for example, deny a woman coverage because she has a preexisting condition, was once the victim of domestic violence, or once had a cesarean delivery.
Essential benefits and preventive services
All private health insurance plans sold in the state marketplaces must cover the 10 essential health benefits:
ambulatory patient services
emergency services
hospitalization
maternity and newborn care
mental health and substance use disorder services, including behavioral health treatment
prescription drugs
rehabilitative and habilitative services and devices
laboratory services
preventive and wellness services and chronic disease management
pediatric services, including oral and vision care.
These insurers also must cover—with no charge to the patient—preventive services:
well-woman visits (one or more)
all FDA-approved contraceptive methods and contraception counseling
gestational diabetes screening
mammograms
Pap tests
HIV and other sexually transmitted infection screening and counseling
breastfeeding support, supplies, and counseling
domestic violence screening and counseling.
Related Article: Your age-based guide to comprehensive well-woman care Robert L. Barbieri, MD (October 2012)
In addition, private insurers must offer additional preventive services, although they can charge copays for them:
anemia screening on a routine basis for pregnant women
screening for urinary tract or other infection for pregnant women
counseling about genetic testing for a BRCA mutation for women at higher risk
counseling about chemoprevention of breast cancer for women at higher risk
cervical cancer screening for sexually active women
folic acid supplementation for women who may become pregnant
osteoporosis screening for women over age 60, depending on risk factors
screening for Rh incompatibility for all pregnant women and follow-up testing for women at higher risk
tobacco use screening and interventions for all women, and expanded counseling for pregnant users of tobacco.
An end to preexisting-condition exclusions and other harmful practices
Insurers offering plans in the state marketplaces also must abide by important insurance reforms:
They must eliminate exclusions for preexisting conditions. Insurers cannot deny individuals coverage because they already have a condition that requires medical care, including pregnancy. Before this ACA rule, private insurers often rejected applicants who needed care, as well as those who accessed health care in the past. Insurers regularly denied coverage to women who had had a cesarean delivery or had once been a victim of domestic violence.
They cannot charge women more than men for the same coverage. Before the ACA, women seeking health-care coverage often faced higher premiums than men for identical coverage. This made private coverage less affordable for our patients.
They cannot impose a 9-month waiting period. (Need I say more?)
They must eliminate any annual lifetime limits on coverage. Insurers selling policies in the marketplaces cannot end coverage after a certain dollar amount has been reached, a common practice before the ACA. This change is good news for you and your patients. A patient needing long or expensive care won’t lose coverage when the cost of her care hits an arbitrary ceiling.
They cannot rescind coverage unless fraud is proven. Before the ACA, private health insurers would often drop an individual if he or she started racking up high health-care costs. Patients in the middle of expensive cancer treatments, for example, would find themselves suddenly without health insurance. This won’t happen for policies sold in marketplaces unless the patient lied on her enrollment forms or failed to keep up her premium payments.
What these changes mean, in real numbers
These protections are critically important to your ability to care for your patients. Here’s what they mean in real life:
Health-care coverage for about 10,000 insured women is no longer subject to an annual lifetime coverage limit.
Private insurers can’t drop coverage, a change that will affect about 5.5 million insured women.
Insurance companies cannot deny coverage for preexisting conditions, which will help insure about 100,000 women.
Each state marketplace offers four types of plans, the idea being to help people compare policies side by side. All plans sold in the marketplaces must abide by the consumer protections I just reviewed. Each tier is differentiated by the average percentage of an enrollee’s health-care expenses paid by the insurer. The more an enrollee agrees to pay out of pocket, the lower his or her premium.
The tiers are:
Bronze – The insurer covers 60% of health-care costs, and the insured covers 40%. This tier offers the cheapest premiums.
Silver – The insurer covers 70% of costs.
Gold – The insurer covers 80% of costs.
Platinum – The insurer covers 90% of costs.
WHAT WENT WRONG
If everything had gone according to plan, women’s access to health insurance would have increased dramatically nationwide, including in the dark blue states in FIGURE 1 (Map 1)—states that rank lowest in access to care. You’ll notice that many of the states that are dark blue in Map 1 shift to light blue in Map 2. Take a careful look at those dark blue states and see what colors they are in the next two maps (FIGURES 1 and 2). Hint: There’s a pattern.
Problem 1 – Strains on Healthcare.gov
When the ACA was signed into law, most states were expected to build and run their own online marketplaces. The federal government offered to run a state exchange if a state didn’t. Few ACA engineers anticipated that the federal government would have to run the marketplaces in more than half the states—politics-fueled decisions in many states. Now you can see that many of the states that are dark blue in Map 1 are gray in Map 3 (FIGURE 2). Many states whose populations have the greatest need left it to the federal government to run the marketplaces.
Data indicate that state-run marketplaces are doing pretty well—a fact not often caught in frenzied headlines. If we look at the percentage of the target population actually enrolled in marketplace insurance plans during the first month, we see that the lowest state marketplace enrollment was in Washington State, with 30% of its target population enrolled. The highest target-population enrollment was achieved in Connecticut, with 191%.
Compare these numbers to the rates of target-population enrollment in states with marketplaces run by the federal government, which range from 3% to 20% (FIGURE 2). During the first month, federal and state-run marketplaces together enrolled 106,000 individuals into new coverage, 21% of the national target.
The news media have focused on the federal online enrollment debacle. Easier enrollment options are available to people who live in federally run marketplace states, including direct enrollment. Strongly supported by America’s health insurance industry, direct enrollment lets potential enrollees purchase coverage directly from insurance companies participating in the marketplaces.
Problem 2: People lost their current coverage
This is a problem worth exploring—one that affects people who previously bought insurance on the individual insurance market. This is the market that offers people comparatively limited coverage, usually with no maternity care coverage, for comparatively high premiums.
So why are these individuals losing coverage?
They are losing coverage because, as of January 2014, new individual plans must abide by the 80/20 rule, abide by insurance protections, and cover 10 essential services with no cost sharing.
You may recall that, in August 2009, Americans were demanding that they be able to keep the health-care coverage they currently had, and President Barack Obama promised that they would be able to. Consequently, health insurance policies in effect before March 2010, when the ACA was signed into law, were exempted—“grandfathered”—from most ACA requirements. If people liked their old policies, they could keep them.
Grandfathered plans are exempt from:
the requirement to cover the 10 essential health benefits
the requirement that plans must provide preventive services with no patient cost sharing
state or federal review of insurance premium increases of 10% or more for non-group and small business plans
a rule allowing consumers to appeal denials of claims to a third-party reviewer.
Most ACA requirements apply to new policies—those offered after March 2010 and those that have been changed significantly by the insurance companies. Some examples of changes in coverage that would cause a plan to lose grandfathering include:
the elimination of benefits to diagnose or treat a particular condition
an increase in the up-front deductible patients must pay before coverage kicks in by more than the cumulative growth in medical inflation since March 2010 plus 15%
a reduction in the share of the premium that the employer pays by more than 5% since March 2010.
How many people are we talking about? Not the 40% of Americans who have employer-based coverage or the 20% of Americans on Medicare, Medicaid, or Tricare. This provision affects about 5% of the insured, as many as 15 million people—many with plans that offer little coverage for high premiums.
The ACA intention was that many people previously covered in the individual insurance market would find better and cheaper coverage in their state marketplaces. That may be a good option for people in states that have chosen to run their own marketplaces, and a good option for people in other states, too, as federal online enrollment issues get fixed.
Problem 3: Medicaid expansion became a state option
When the US Supreme Court upheld the constitutionality of the ACA’s individual mandate, it also effectively turned the ACA Medicaid expansion into a state option.
Think of the Medicaid expansion as Medicaid Part 2. Regular Medicaid remains largely unchanged, with the same eligibility rules and coverage requirements.
The ACA included a provision under which every state would add a new part to its Medicaid program. Beginning in 2014, this part—the expansion—would cover individuals in each state with incomes under 138% of the federal poverty line—about $32,000 for a family of four in 2014. Medicaid expansion coverage is based only on income eligibility, a major change for women, many of whom currently qualify for Medicaid only if they’re pregnant.
Who would pay for the new coverage?
In 2014, 2015, and 2016, the federal government pays 100% of the cost of care for Medicaid expansion. From 2017 to 2020, the federal share gradually drops to 90%.
Medicaid expansion is an integral part of reducing the number of uninsured under the ACA and is expected to reduce the uninsured rate by almost 30% if adopted by every state. Medicaid expansion plus the ACA marketplaces were expected to cut our uninsured rate almost in half.
FIGURE 3 shows how states responded when the Supreme Court effectively converted the Medicaid expansion into an option, leaving us, again, with coverage gaps. Many of the states that have opted not to expand Medicaid are the same states that declined to operate their own state marketplaces, the same states with highest percentages of the uninsured.
The ACA has many interdependent parts. Make the Medicaid expansion a state option, and you end up with higher than expected rates of uninsured. Trigger big changes in the individual market when there are still bugs in the system, and people are left in the lurch.
Related Article: ACOG to legislators: Partnership, not interference Lucia DiVenere, MA (April 2013)
What’s happening now
As this article is going to press, enrollment in the marketplaces is getting easier, with Web site fixes and useful alternatives to Web-based enrollment. Small changes are being made to some deadlines to help people who have gotten stuck in the process. We’ll likely continue to see steps forward and back over the next many months.
Two things remain important:
We need to stick with the facts. If you see something in the news that seems too crazy to be real, your hunch may be right.
Your patients can benefit significantly from the ACA. An ACOG Fellow recently told me about one of his patients who has a severe health condition, no insurance, and needs expensive treatment. The ACA, with its marketplace rules outlawing exclusions for preexisting conditions and offering premium assistance, may be a lifesaver to her. But first she needs to enroll.
As your patients’ trusted physician, you can help point them in the right direction, possibly toward coverage that they never had before.
That’s good for all of us.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
Pediatric Discharge Systematic Review
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
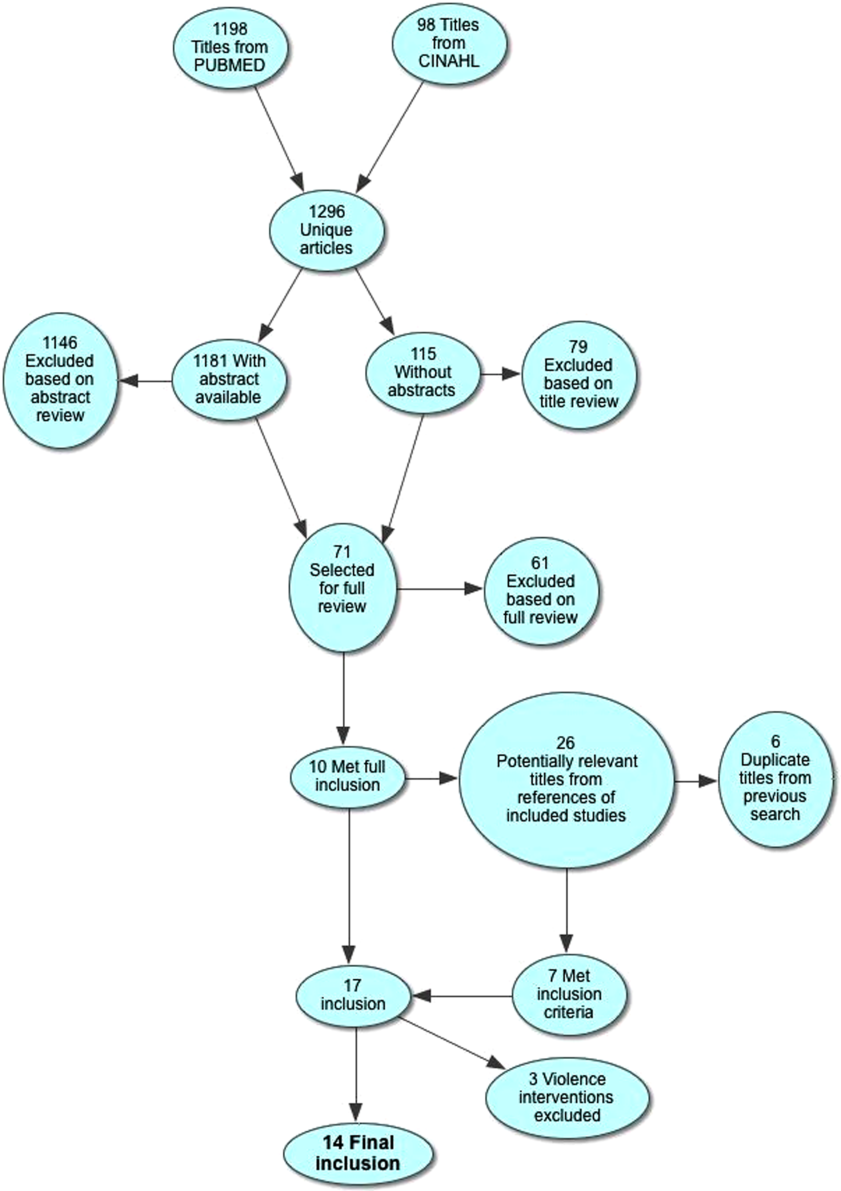
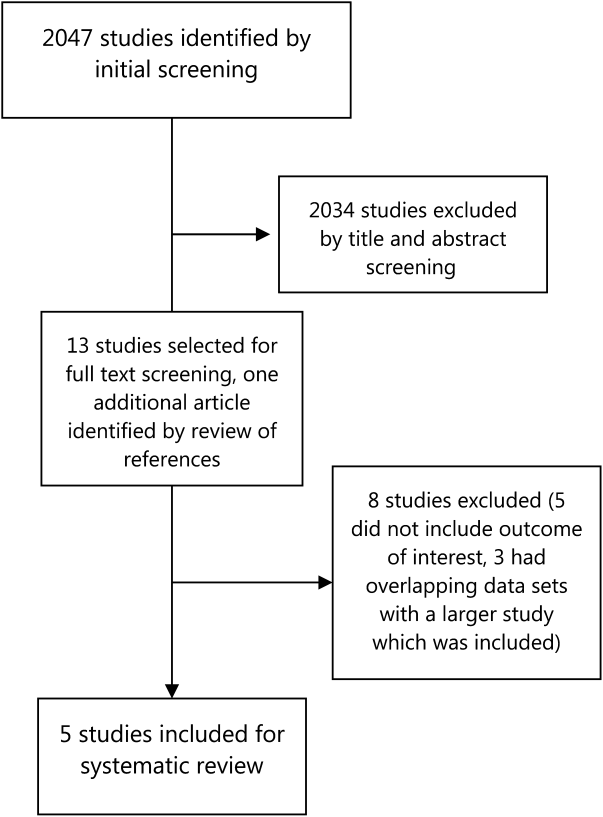
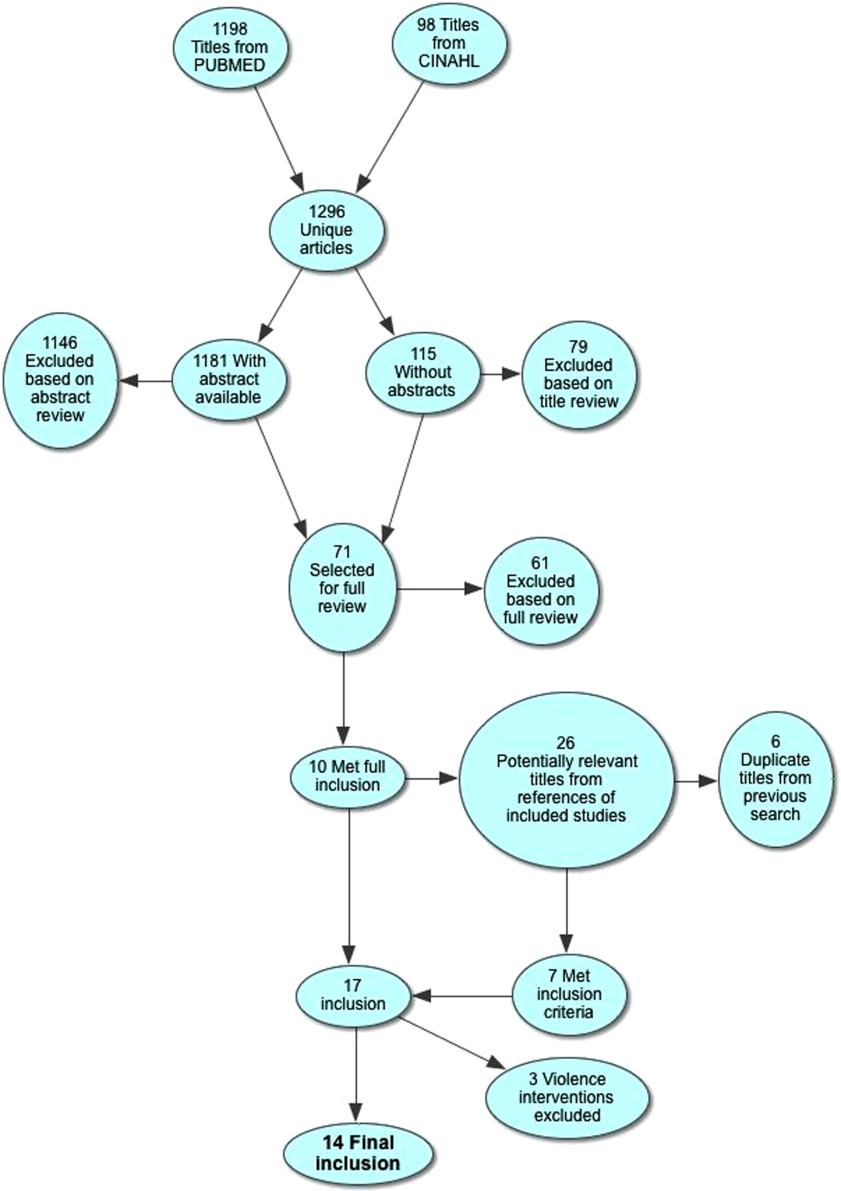
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).
Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
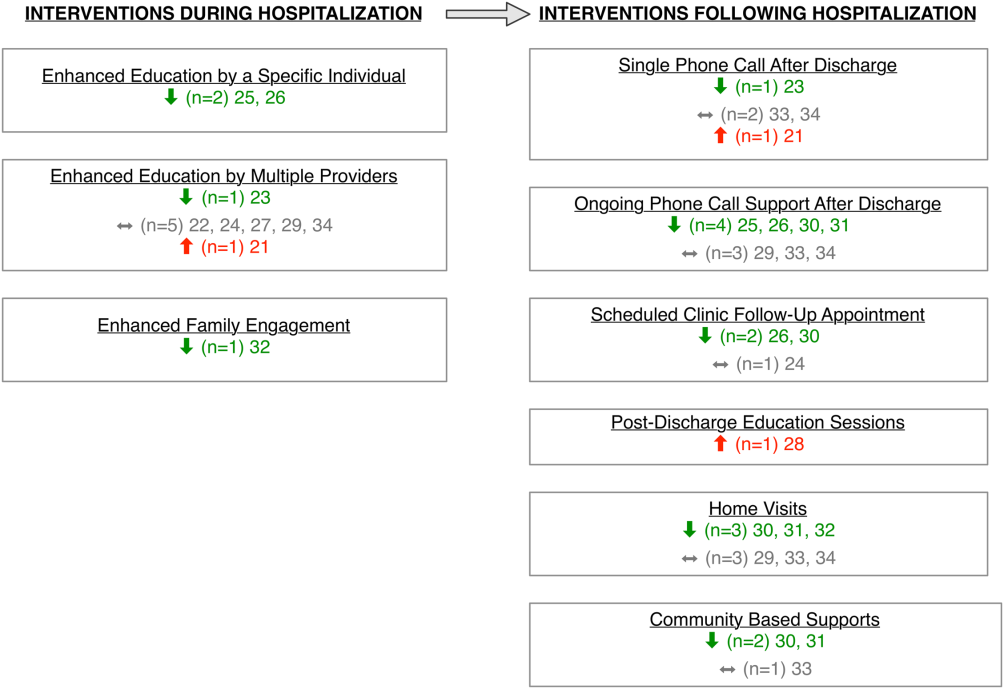
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]
Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).

Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]

Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).

Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]

Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.
Key issues in the management of gastrointestinal immune-related adverse events associated with ipilimumab administration
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
Ipilimumab is an anticytotoxic T lymphocyte antigen-4 (CTLA-4) monoclonal antibody that attenuates negative signaling from CTLA-4 and potentiates T-cell activation and proliferation. Two phase 3 randomized trials in advanced melanoma demonstrated a significant improvement in overall survival, the first of which led to regulatory approval in the United States and Europe for treatment of unresectable or metastatic melanoma. Ipilimumab administration is associated with immune-related adverse events (irAEs). Gastrointestinal (GI) irAEs are among the most common and although they are typically mild to moderate in severity, if they are left unrecognized or untreated, they can become life-threatening. These toxicities can be managed effectively in almost all patients by using established guidelines that stress vigilance and the use of corticosteroids and other immunosuppressive agents when necessary. The goal of this review is to educate physicians on the recognition and challenges associated with management of GI irAEs.
*Click on the link to the left for a PDF of the full article.
A planning and evaluation program for assessing telecommunications applications in community radiation oncology programs
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Quick Diagnosis Units
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.
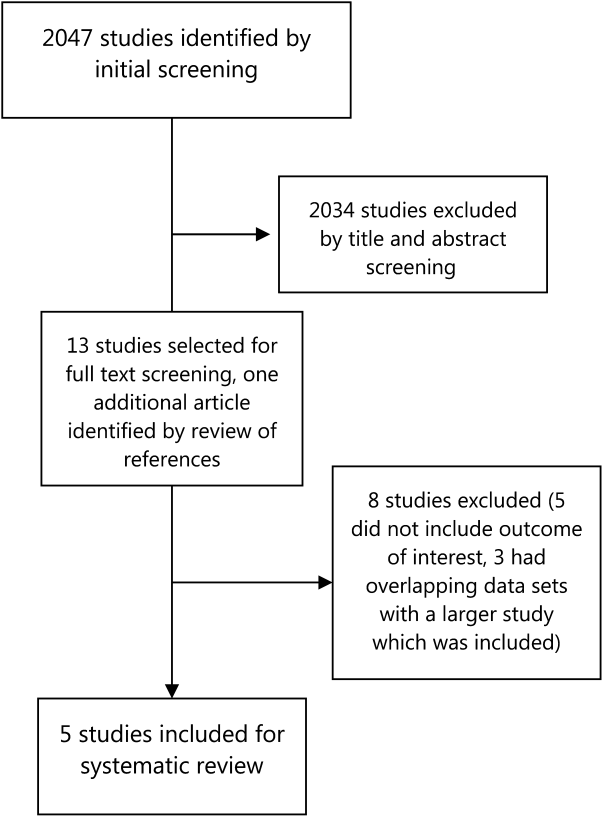
Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.

Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.

Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
Is your patient’s poor recall more than just a ‘senior moment’?
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Memory and other cognitive complaints are common among the general population and become more prevalent with age.1 People who have significant emotional investment in their cognitive competence, mood disturbance, somatic symptoms, and anxiety or related disorders are likely to worry more about their cognitive functioning as they age.
Common complaints
Age-related complaints, typically beginning by age 50, often include problems retaining or retrieving names, difficulty recalling details of conversations and written materials, and hazy recollection of remote events and the time frame of recent life events. Common complaints involve difficulties with mental calculations, multi-tasking (including vulnerability to distraction), and problems keeping track of and organizing information. The most common complaint is difficulty with remembering the reason for entering a room.
More concerning are complaints involving recurrent lapses in judgment or forgetfulness with significant implications for everyday living (eg, physical safety, job performance, travel, and finances), especially when validated by friends or family members and coupled with decline in at least 1 activity of daily living, and poor insight.
Helping your forgetful patient
Office evaluation with brief cognitive screening instruments—namely, the Montreal Cognitive Assessment and the recent revision of the Mini-Mental State Examination—might help clarify the clinical presentation. Proceed with caution: Screening tests tap a limited number of neurocognitive functions and can generate a false-negative result among brighter and better educated patients and a false-positive result among the less intelligent and less educated.2 Applying age- and education-corrected norms can reduce misclassification but does not eliminate it.
Screening measures can facilitate decision-making regarding the need for more comprehensive psychometric assessment. Such evaluations sample a broader range of neurobehavioral domains, in greater depth, and provide a more nuanced picture of a patient’s neurocognition.
Findings on a battery of psychological and neuropsychological tests that might evoke concern include problems with incidental, anterograde, and recent memory that are not satisfactorily explained by: age and education or vocational training; estimated premorbid intelligence; residual neurodevelopmental disorders (attention, learning, and autistic-spectrum disorders); situational, sociocultural, and psychiatric factors; and motivational influences—notably, malingering.
Some difficulties with memory are highly associated with mild cognitive impairment or early dementia:
• anterograde memory (involving a reduced rate of verbal and nonverbal learning over repeated trials)
• poor retention
• accelerated forgetting of newly learned information
• failure to benefit from recognition and other mnemonic cues
• so-called source error confusion—a misattribution that involves difficulty differentiating target information from competing information, as reflected in confabulation errors and an elevated rate of intrusion errors.
Disclosure
Dr. Pollak reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
1. Weiner MF, Garrett R, Bret ME. Neuropsychiatric assessment and diagnosis. In: Weiner MF, Lipton AM, eds. Clinical manual of Alzheimer disease and other dementias. Arlington, VA: American Psychiatric Publishing, Inc.; 2012: 3-46.
2. Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms and commentary: third edition. New York, NY: Oxford University Press; 2006.
Discharging your patients who display contingency-based suicidality: 6 steps
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Discharging patients from a hospital or emergency department despite his (her) ongoing suicidal ideation is a clinical dilemma. Typically, these patients do not respond to hospital care and do not follow up after discharge. They often have a poorly treated illness and many unmet psychosocial and interpersonal needs.1 These patients may communicate their suicidality as conditional, aimed at satisfying unmet needs; secondary gain; dependency needs; or remaining in the sick role. Faced with impending discharge, such a patient might increase the intensity of his suicidal statements or engage in behaviors that subvert discharge. Some go as far as to engage in behaviors with apparent suicidal intent soon after discharge.
A complicated decision
Such patients often are at a chronically elevated risk for suicide because of mood disorders, personality pathology, substance use disorder, or a history of serious suicide attempt.2 Do not dismiss a patient’s suicidal statements; he is ill and may end his own life.
Managing these situations can put you under a variety of pressures: your own negative emotional and psychological reactions to the patient; pressure from staff to avoid admission or expedite discharge of the patient; and administrative pressure to efficiently manage resources.3 You’re faced with a difficult decision: Discharge a patient who might self-harm or commit suicide, or continue care that may be counterproductive.
We propose 6 steps that have helped us promote good clinical care while documenting the necessary information to manage risk in these complex situations.
1. Define and document the clinical situation. Summarize the clinical dilemma.
2. Assess and document current suicide risk.4 Conduct a formal suicide risk assessment; if necessary, reassess throughout care. Focus on dynamic risk factors; protective risk factors (static and dynamic); acute stressors (or lack thereof) that would increase their risk of suicide above their chronically elevated baseline; and access to lethal means—firearms, stockpiled medication, etc.
3. Document modified dynamic or protective factors. Review the dynamic risk and protective factors you have identified and how they have been modified by treatment to date. If dynamic factors have not been modified, indicate why and document the recommended plan to address these matters. You might not be able to provide relief, but you should be able to outline a plan for eventual relief.
4. Document the reasons continued care in the acute setting is not indicated. Reasons might include: the patient isn’t participating in recommended care or treatment; the patient isn’t improving, or is becoming worse, in the care environment; continued care is preventing or interfering with access to more effective care options; is counterproductive to the patient’s stated goals; or compromising the safety benefit of the structured care environment because the patient is not collaborating with his care team.
5. Document your discussion of discharge with the patient. Highlight attempts to engage the patient in adaptive problem solving. Work out a crisis or suicide safety plan and give the patient a copy and keep a copy in his (her) chart.
If the patient refuses to engage in safety planning, document it in the chart. Note the absence of any conditions that might impair the patient’s volitional capacity to not end their life—intoxication, delirium, acute psychosis, etc. Explicitly frame the patient’s responsibility for his life. Discuss and document a follow-up plan and make direct contact with providers and social supports, documenting whether contacting these providers was successful.
6. Consult with a colleague. An informal non-visit consultation with a colleague demonstrates your recognition of the complexity of the situation and your due diligence in arriving at a discharge decision. Consultation often will result in useful additional strategies for managing or engaging the patient. A colleague’s agreement helps demonstrate that “average practitioner” and “prudent practitioner” standards of care have been met with respect to clinical decision-making.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
1. Lambert MT, Bonner J. Characteristics and six-month outcome of patients who use suicide threats to seek hospital admission. Psychiatr Serv. 1996;47(8):871-873.
2. Zaheer J, Links PS, Liu E. Assessment and emergency management of suicidality in personality disorders. Psychiatr Clin North Am. 2008;31(3):527-543, viii-ix.
3. Gutheil TG, Schetky D. A date with death: management of time-based and contingent suicidal intent. Am J Psychiatry. 1998;155(11):1502-1507.
4. Haney EM, O’Neil ME, Carson S, et al. Suicide risk factors and risk assessment tools: a systematic review. VA Evidence-based Synthesis Program Reports. Washington, DC: Department of Veterans Affairs; 2012.
Medication for alcohol use disorder: Which agents work best?
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Historically, alcohol use disorder (AUD; classified as alcohol abuse or dependence in DSM-IV-TR) has been treated with psychosocial therapies, but many patients treated this way relapse into heavy drinking patterns and are unable to sustain sobriety (Box 11). Although vital for treating AUD, psychosocial methods have, to date, a modest success rate. Research has demonstrated that combining pharmacotherapy with psychosocial programs is effective for treating AUD.2
Patients and clinicians might associate AUD medications with so-called aversion therapy because, for many years, the only treatment was disulfiram, which causes unpleasant physical effects when consumed with alcohol. However, newer medications help patients maintain abstinence by targeting brain neurotransmitters relevant to addiction neurocircuitry, such as dopamine, serotonin, ϒ-aminobutyric acid (GABA), glutamate, and opioid.3 These medications may help patients with AUD achieve sobriety, avoid relapse, decrease heavy drinking days, and delay time to recurrent drinking.
In this article, we review FDA-approved medications (Table 1)4-6 and off-label agents (Table 2)3,7-18 and provide recommendations for treating patients with AUD (Box 2).19-21
FDA-approved treatments
Naltrexone is an opiate antagonist that blocks the mu receptor and is believed to interrupt the dopamine reward pathway in the brain for alcohol. A meta-analysis of 2,861 patients in 24 combined randomized controlled trials (RCTs) demonstrated naltrexone to be an effective short-term (12 weeks) treatment for alcoholism, significantly decreasing relapses.22 The large multisite COMBINE study (N = 1,383) showed that naltrexone, 100 mg/d, and medical treatment without behavioral treatment over 16 weeks was more effective than placebo in increasing percentage of days abstinent (80.6% vs 75.1%, respectively) and reducing the percentage of patients experiencing heavy drinking days (66.2% vs 73.1%, respectively).2 Patients who have a family history of AUD or strong cravings, or both, may benefit most from naltrexone.3,7,23 Despite evidence of the effectiveness of naltrexone for AUD, not all studies have yielded positive results.24
Common side effects of naltrexone, if present, appear early in treatment and include GI upset (eg, nausea, vomiting, abdominal pain), headache, and fatigue.2,3,22,23 Hepatotoxicity has been reported with dosages of 100 to 300 mg/d, but lab values typically normalize when naltrexone is discontinued.2,3,23 Monitor markers of liver function including ϒ-glutamyltransferase, aspartate aminotransferase, alanine aminotransferase, and bilirubin before and during naltrexone treatment (we check patients 1 to 3 months after starting treatment and yearly thereafter). Obtain a negative urine drug screen for opioids before administering naltrexone, because if opioids were consumed recently naltrexone could precipitate withdrawal. Because of the unknown teratogenicity of naltrexone, women of childbearing age should undergo pregnancy testing before and periodically during naltrexone therapy.
Naltrexone also is available in a once-monthly, 380-mg injectable formulation. Studies show that, similar to its oral counterpart, injectable naltrexone effectively reduces heavy drinking days and number of drinks a day compared with placebo.25,26 Advantages of injectable naltrexone are its extended steady release of medication and its efficacy for patients who do not adhere to oral dosing.7,27 Side effects are similar to oral naltrexone, except for injection site reactions and pain.
Contraindications to naltrexone include current opioid use because its antagonistic effects on opioid receptors render opioid analgesia ineffective. Patients who have used opioids within 7 to 10 days or who may be surreptitiously using opioids should not take naltrexone because it may cause opioid withdrawal. Some patients may try to override the opioid receptor blockade of naltrexone with higher opioid doses, which could result in overdose.
Naltrexone is approved for treating opioid use disorder and may be useful for persons with comorbid opioid use disorder and AUD, if the patient has been adequately detoxified from opioids and intends to abstain from these drugs. Patients who have extensive liver damage secondary to acute hepatitis or uncompensated cirrhosis would not be good candidates for naltrexone because of a risk of hepatotoxicity.2,3,22
Because of ease of dosing, we recommend naltrexone as a first-line treatment for AUD, unless the patient requires opioids or has severe liver disease. We recommend increasing naltrexone from 50 mg to 100 mg before switching to acamprosate, based on European studies.
Acamprosate is a glutamate antagonist that is thought to modulate overactive glutamatergic brain activity that occurs after stopping chronic heavy alcohol use. In a meta-analysis of 17 studies (N = 4,087), continuous abstinence rates at 6 months were significantly higher in acamprosate-treated patients (36.1%) than in patients receiving placebo (23.4%).28 In a review of European studies, acamprosate benefited patients who have increased anxiety, physiological dependence, negative family history of AUD, and late age of onset (age >25) of alcohol dependence.7 However, in the COMBINE trial acamprosate was no more effective than placebo.2
We consider acamprosate an effective option for patients who do not respond to naltrexone or have a contraindication. Dosages of 333 mg to 666 mg, 3 times a day, are recommended, although dosages up to 3 g/d have been studied; titration is not required.2,7,28 We recommend advising patients to continue treatment even if they relapse, because these medications may mitigate relapse severity. Adherence to multiple daily doses can be problematic for some patients, but pairing medications with meals or bedtime may improve adherence.
Diarrhea is the most common side effect of acamprosate; nervousness, fatigue, insomnia, and depression have been reported with high dosages.2,3,7,28 Acamprosate is excreted through the kidney and is safe for patients with liver disorders such as acute hepatitis or cirrhosis. The drug is contraindicated in patients with acute or chronic renal failure with creatinine clearance <30 mL/min; those with less severe renal insufficiency might need a lower dosage. Obtain baseline renal function before starting acamprosate; women of childbearing age should undergo a pregnancy test.
Disulfiram inhibits alcohol metabolism, resulting in acetaldehyde accumulation, which causes unpleasant physical effects such as nausea, vomiting, and hypotension. This creates a negative rather than a positive experience with drinking. A US Veterans Administration Cooperative Study randomized 605 participants to riboflavin, disulfiram, 1 mg/d (an inactive dose), or disulfiram, 250 mg/d (standard dose). There was no difference in percentage of patients remaining abstinent or time to first drink.8 Participants receiving disulfiram, 250 mg/d, had fewer drinking days after relapse compared with the other groups.8
Adverse physical effects produced when disulfiram and alcohol interact include tremor, diaphoresis, unstable blood pressure, and severe diarrhea and vomiting. Disulfiram can cause medically serious reactions in a small percentage of patients, especially those with significant medical comorbidity or advanced age. Patients with severe hypertension, diabetes mellitus, heart disease, a history of stroke, peripheral neuropathy, epilepsy, or renal or hepatic insufficiency should not use disulfiram.3 Patients taking disulfiram should avoid casual exposures to food, aftershave, mouthwash, and hand sanitizer that might contain alcohol. Disulfiram has no significant effect on alcohol craving. Social support to help oversee dosing may enhance adherence.7
Off-label medications
Topiramate is FDA-approved to treat migraine headaches and some seizure disorders. Topiramate facilitates GABA-mediated neuronal inhibition and antagonizes certain glutamate receptor subtypes. In an RCT (N = 150), topiramate, up to 300 mg/d, was more effective than placebo at reducing heavy drinking days and number of drinks per day, increasing days abstinent, and alleviating cravings.9 In a 12-week, double-blind RCT (N = 150), topiramate increased “safe drinking”—defined as ≤1 standard drink per day for women and ≤2 per day for men—vs placebo.10 Dosages were 75 mg to 300 mg/d in twice daily divided doses. Dosing starts at 25 mg/d and increases by 25 to 50 mg a day at weekly intervals. We recommend reserving topiramate for persons who do not respond to or cannot tolerate naltrexone and acamprosate because of the slow titration needed to prevent side effects. Although not studied, it may seem that topiramate’s antiepileptic actions could prevent seizures during alcohol withdrawal, but the protracted titration would limit its utility.
Side effects of topiramate include impaired memory and concentration, paresthesia, and anorexia and are more likely to present during rapid titration or with a high dosage.7,8 Rare reports of spontaneous myopia, angle-closure glaucoma, increased intraocular pressure, ocular pain, and blurry vision have been reported, but these complications often resolve with discontinuation of topiramate.7,8
Topiramate primarily is excreted through the kidney, and its action in the renal tubules can lead to metabolic acidosis or nephrolithiasis.8 Relative contraindications include acute or chronic kidney disease, including kidney stones. Consider slower titration and a 50% reduction in dosing if creatinine clearance is <70 mL/min. Obtain renal function tests before starting topiramate and consider monitoring serum bicarbonate for metabolic acidosis (we test at 3 and 6 months, then every 6 months). Because of teratogenic effects of topiramate (eg, cleft lip and palate), rule out pregnancy in all women of childbearing age.
Baclofen is a GABAb receptor agonist that is FDA approved for treating spasticity. Because GABA transmission is down-regulated in chronic AUD, it is a commonly targeted neurotransmitter when developing medications for AUD. GABAa receptors are fast-acting inhibitory ion channels, and its agonists (eg, benzodiazepines) have a significant abuse and cross-addiction liability. GABAb receptors, however, are slow-acting through a complex cascade of intracellular signals, and therefore GABAb agonists such as baclofen have been studied for treating addiction.
In a randomized double-blind, placebo-controlled trial (N = 39), baclofen was superior to placebo in suppressing obsessive aspects of cravings and decreasing state anxiety.11 Baclofen, 10 mg 3 times daily, in another randomized double-blind, placebo-controlled trial (n = 42) reduced the number of drinks per day by 53% vs placebo; 20 mg 3 times a day resulted in a 68% reduction in drinks per day vs placebo.12 However, a placebo-controlled RCT (n = 80) reported that baclofen, 10 mg 3 times daily, was not superior to placebo for primary outcomes related to alcohol consumption, although it did significantly decrease cravings and anxiety among persons with AUD.13 Evidence suggests that baclofen might be effective for promoting abstinence, reducing the risk of relapse, and alleviating cravings and anxiety in persons with AUD, although further investigation is needed.
In studies for AUD, the side-effect profile for baclofen was relatively benign.11-13 Nausea, fatigue, sleepiness, vertigo, and abdominal pain were reported; overall, baclofen was found to be safe and to have no abuse liability.7,10,12 The addictive potential of other muscle relaxers may have dissuaded providers from using baclofen for AUD, but we consider it a reasonable alternative when FDA-approved treatments fail.
Because baclofen is primarily eliminated by the kidneys, it may be safe for people with cirrhosis or severe liver disease.12 Baseline renal labs should be performed before administering baclofen and a negative pregnancy test obtained for women of childbearing age.
Ondansetron is a serotonin receptor type 3 (5-HT3) antagonist that has shown promising results for AUD.3,7 Research suggests that 5-HT3 receptors are an action site for alcohol in the brain and are thought to play a role in its rewarding effects.7 Ondansetron may be more effective for early-onset alcoholism (EOA) than late-onset alcoholism (LOA).14,15 EOA (age ≤25) is characterized by strong family history of AUD and prominent antisocial traits. A randomized double-blind, placebo-controlled trial (n = 271) reported that ondansetron, 4 mcg/kg twice daily, was superior to placebo in reducing number of drinks per day, increasing days abstinent, and reducing cravings in patients with EOA.14 Among persons with EOA, ondansetron, 16 mcg/kg twice daily, significantly reduced the severity of symptoms of fatigue, confusion, and overall mood disturbance such as depression, anxiety, and hostility compared with placebo in an RCT (n = 321).15 The lowest available oral dosage of ondansetron is 4 mg tablets or 4 mg/5 mL solution. We have used 4 mg twice daily for patients who have failed naltrexone and acamprosate or when these agents are contraindicated.
Common side effects of ondansetron include constipation, diarrhea, elevated liver enzymes, tachycardia, headache, and fatigue. Contraindications include congenital long QT syndrome, QTc prolongation risk, or significant hepatic impairment. We suggest evaluating baseline electrocardiogram and liver function tests. Women should undergo a pregnancy test before receiving medications.
Gabapentin is an anticonvulsant that is FDA-approved for treating epilepsy and postherpetic neuralgia. Gabapentin is related structurally to GABA and may potentiate central nervous system GABA activity, inhibit glutamate activity, and reduce norepinephrine and dopamine release.16 Gabapentin is thought to balance the GABA/glutamate dysregulation found in early alcohol abstinence and reduce risk for alcohol relapse.16A randomized, double-blind, placebo-controlled trial (N = 60) demonstrated that gabapentin, 600 mg/d, significantly reduced number of drinks per day and heavy drinking days and increased days of abstinence compared with placebo over 28 days.17 Another double-blind, randomized, placebo-controlled trial of 150 people used gabapentin, 900 or 1,800 mg/d; there was a linear dose response for increased days abstinent and no heavy drinking days in favor of gabapentin.18
An RCT (N = 150) evaluated adding gabapentin, up to 1200 mg/d, to naltrexone, 50 mg/d, vs naltrexone with placebo or double placebo over 6 weeks of treatment. The combined gabapentin-naltrexone group outperformed the other 2 groups on time to heavy drinking, number of heavy drinking days, and number of drinks per day. Gabapentin’s positive effects on sleep may have mediated some of its beneficial effects.29 In an open-label pilot study, gabapentin was more effective than trazodone for insomnia during early alcohol abstinence.30 Of note, gabapentin is a safe alternative to benzodiazepines for alcohol detoxification in patients with severe hepatic disease or those at risk of interacting with alcohol (eg, outpatients at high risk to drink during detoxification).31 Gabapentin, 400 mg/d to 1,600 mg/d, generally is safe and well tolerated and has some support for improving cravings, reducing alcohol consumption, delaying relapse, and improving sleep in patients with AUD.
Side effects of gabapentin include daytime sedation, dizziness, ataxia, fatigue, and dyspepsia. Using 3 divided doses might enhance tolerability. To reduce daytime sedation, we recommend administering most of the dose at night, which also may relieve insomnia. Gabapentin is excreted through the kidney; baseline renal function tests should be performed before initiating treatment, because the dosage might need to be adjusted in people with renal insufficiency.
Bottom Line
FDA-approved (acamprosate, naltrexone, and disulfiram) and off-label (baclofen, gabapentin, ondansetron, and topiramate) agents can help patients with alcohol use disorder achieve abstinence, reduce heavy drinking days, prevent relapse, and maintain sobriety. Research supports the use of pharmacotherapy combined with psychosocial modalities, such as 12-step programs, motivational interviewing, and cognitive-behavioral therapy.
Related Resources
- National Institute on Drug Abuse. Principles of drug addiction treatment: A research-based guide (third edition). www.drugabuse.gov/publications/principles-drug-addiction-treatment-research-based-guide-third-edition/evidence-based-approaches-to-drug-addiction-treatment/pharmacotherapi-1.
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. http://162.99.3.213/products/manuals/tips/pdf/TIP49.pdf.
- Pettinati HM, Mattson ME. Medical management treatment manual: A clinical guide for researchers and clinicians providing pharmacotherapy for alcohol dependence. http://pubs.niaaa.nih.gov/publications/MedicalManual/MMManual.pdf.
Drug Brand Names
Acamprosate • Campral Naltrexone • Vivitrol, ReVia
Baclofen • Lioresal Ondansetron • Zofran
Disulfiram • Antabuse Topiramate • Topamax
Gabapentin • Neurontin Trazodone • Desyrel, Oleptro
Metronidazole • Flagyl
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
1. World Health Organization. Alcohol fact sheet. http://www.who.int/mediacentre/factsheets/fs349/en/index.html. Published February 2011. Accessed April 30, 2013.
2. Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence. The COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
3. Mann K. Pharmacotherapy of alcohol dependence a review of the clinical data. CNS Drugs. 2004;18(8):485-504.
4. ReVia [package insert]. Pomona, NY: Barr Pharmaceuticals; 2009.
5. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals; 2004.
6. Antabuse [package insert]. Pomona, NY: Barr Pharmaceuticals; 2010.
7. Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75(1):34-56.
8. Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism: a Veterans Administration cooperative study. JAMA. 1986;256(11):1449-1454.
9. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):
1677-1685.
10. Ma JZ, Ait-Daoud N, Johnson BA. Topiramate reduces the harm of excessive drinking: implications for public health and primary care. Addiction. 2006;101:1561-1568.
11. Addolorato G, Caputo F, Capristo E, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37(5):504-508.
12. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
13. Garbutt JC, Kampov-Polevoy AB, Gallop R, et al. Efficacy and safety of baclofen for alcohol dependence: a randomized, double-blind placebo-controlled trial. Alcohol Clin Exp Res. 2010;34(11):1849-1857.
14. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971.
15. Johnson BA, Ait-Daoud N, Ma JZ, et al. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003;27(11):1773-1779.
16. Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227.
17. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691-1700.
18. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial [published online November 4, 2013]. JAMA Intern Med. doi: 10.1001/jamainternmed.2013.11950.
19. The Management of Substance Use Disorders Working Group. VA/DoD clinical practice guideline for management of substance use disorders (SUD). http://www.healthquality.va.gov/sud/sud_full_601f.pdf. Published August 2009. Accessed November 22, 2013.
20. Mark TL, Kranzler HR, Song X, et al. Physicians’ opinions about medication to treat alcoholism. Addiction. 2003;98(5):617-626.
21. Thomas CP, Wallack SS, Lee S, et al. Research to practice: adoption of naltrexone in alcoholism treatment. J Subst Abuse Treat. 2003;24(1):1-11.
22. Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267-280.
23. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715-721.
24. Gueorguieva R, Wu R, Pittman B, et al. New insights into the efficacy of naltrexone based on trajectory-based reanalysis of two negative clinical trials. Biol Psychiatry. 2007;61(11): 1290-1295.
25. Lapham S, Forman R, Alexander M, et al. The effects of extended-release naltrexone on holiday drinking in alcohol-dependent patients. J Subst Abuse. 2009;36(1):1-6.
26. Ciraulo DA, Dong Q, Silverman BL, et al. Early treatment response in alcohol dependence with extended-release naltrexone. J Clin Psychiatry. 2008;69(2):190-195.
27. Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Managed Care. 2010;16(12): 879-888.
28. Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28(1):51-63.
29. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
30. Karam-Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542-544.
31. Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588.
Performing capacity evaluations: What’s expected from your consult
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
One of the most common reasons medical colleagues seek consultation with a psychiatrist is to address the question of capacity. Indeed, this referral question often is posed as, “Is the patient competent?”
This referral question is incomplete and incorrectly phrased. The question should include the domain in which capacity is being questioned—for example, “Is the patient competent to refuse surgery?” Specifically identifying the area in which competency is questioned is necessary because a person might be competent in one area and incompetent in another (Box 1).
The question of competency should be modified as follows: “Does the patient have capacity to refuse surgery?” Competency is the degree of mental soundness necessary to make decisions about a specific issue or to carry out a specific act. Capacity is a person’s ability to make an informed decision. A determination of competency is a judicial finding made by the court. A physician can opine about a patient’s capacity, but cannot determine competency.
Adults are presumed to have capacity unless determined otherwise by the court. A person who lacks capacity to make an informed decision or give consent might need to be referred for a competency hearing or have a guardian appointed. Psychiatrists often are called on to provide an opinion to the court regarding a person’s capacity. Psychiatrists are particularly skilled at accessing a person’s mental status and gauging its potential for interfering with specific areas of functioning, but, in fact, any physician can make a determination of capacity.1
In this article, I:
- outline the components of a capacity evaluation
- describe the tools used in the determination of capacity
- review the typical features of patients and psychiatrists who perform capacity evaluations.
What constitutes a capacity evaluation?
The components of a capacity evaluation are comprehension, free choice, and reliability.
Comprehension refers to a patient’s factual understanding of his (her) medical condition—for example, including the risks and benefits of treatment and reasonable alternatives. The patient should show an understanding of 1) the situation as it relates to his condition, and 2) the consequences of his decisions. He also should demonstrate a rational manipulation of the information presented, applying a coherent and logical thought process to analyze possible courses of action.2
To determine if the patient has the requisite knowledge regarding his condition, the physician must be familiar with the patient’s clinical status. This might require consultation with the treating physician. Communication is a key component of capacity evaluations. Barriers to good communication can lead to the evaluating physician’s perception that the patient lacks capacity. If a patient does not understand his condition or the proposed treatments, the psychiatrist should educate him. It might be useful to arrange a meeting with the treating physician to facilitate communication.
Free choice. The patient’s decision to accept or reject a proposed treatment should be voluntary and free of coercion. In assessing a patient’s capacity, the psychiatrist should determine whether choices have been rendered impossible because of unrealistic fears or expectations about treatment, or because of impaired mental processes.
Reliability refers to a patient’s ability to provide a consistent choice over time. A patient who vacillates or is inconsistent does not have capacity to make decisions.
Features of patients referred for evaluation, and their evaluators
The most common reason for a capacity evaluation is a patient’s refusal of medical treatment. Between 3% and 25% of requests for psychiatric consultation in hospital settings involve questions about patients’ competence to make a treatment-related decision.3 Approximately 25% of adult medicine inpatients lack capacity for medical decision-making.4
Decision-making capacity is a functional evaluation. Decision-making capacity does not relate specifically to a person’s psychiatric diagnosis. In other words, the presence of a mental disorder does not render a person incapable of making decisions. However, people with Alzheimer’s disease or dementia have a high rate of impaired capacity for making treatment decisions.
Schizophrenia has been found to have the highest rate of impaired decision-making among psychiatric disorders; depression is second and bipolar disorder, third. The strongest predictor of incapacity in psychiatric patients is lack of insight.5 Positive symptoms, negative symptoms, severity of symptoms, involuntary admission, lack of insight, and treatment refusal were strong predictors of incapacity in a sample of psychiatric patients.6
The neuronal basis of decision-making is unknown. Studies have implicated functioning of the medial and lateral prefrontal cortex as an important correlate of decision-making capacity.7 As a result of these findings, a brain-based criterion could be added to the conceptual criteria of capacity. The specific neuropsychological components necessary for decision-making capacity are unknown. Some studies suggest that poor executive functioning and limited learning ability correlate with impaired decision-making capacity.8 Little is known about the relationship between emotion and capacity. Supady et al demonstrated that higher cognitive empathy and good emotion recognition were associated with increased decision-making capacity and higher rates of refusal to give informed consent.9
Physician bias has been identified in capacity evaluations. See Box 2.4,10-12
Tools used in capacity evaluations
Most capacity evaluations are conducted by clinical interview (Box 3). The reliability of physicians’ unstructured judgments of capacity has been poor.13 In a study of 5 physicians who made a determination of capacity after watching a videotape of capacity assessments, the rate of agreement among the subjects was no better than that of chance.14
There is no specific, simple, quick test to assess capacity.
Folstein Mini-Mental State Examination. The MMSE has not been found to be predictive of decision-making capacity. It has been found to correlate with clinical judgments of incapacity, and may be used to identify patients at the high and low ends of the range of capacity, especially among older persons who exhibit cognitive impairment.15 Patients who have severe dementia (MMSE score <16) have a high likelihood of being unable to consent to treatment.16
MacArthur Competence Assessment Tool-Treatment. The MacCAT-T is a structured interviewing tool used to evaluate a patient’s decision-making ability. It is the most commonly used screening tool to evaluate decision-making capacity. Advantages of the MacCAT-T include a higher inter-rater agreement and—unlike other assessment instruments—its ability to incorporate information specific to a patient’s decision-making situation.17 The MacCAT-T requires training and experience to administer.
Bottom Line
Physicians make decisions about a patient’s decision-making capacity. Courts determine competence by a formal judicial proceeding. The psychiatric consultant’s role in capacity evaluations is to determine if the patient 1) possesses the requisite knowledge about the specific referral issue and 2) demonstrates a voluntary and reliable decision.
Related Resources
- The MacArthur Treatment Competence Study. www.macarthur.virginia.edu/treatment.html.
- Resnick P, Sorrentino R: Forensic considerations (chapter. 8). In: Psychosomatic medicine. Blumenfield M, Strain JJ, eds. Baltimore, MD: Lippincott Williams & Wilkins; 2006:91-106.
- Appelbaum PS. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834-1840.
Disclosure
Dr. Sorrentino reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
1. Grisson T, Appelbaum PS. Assessing competence to consent to treatment—a guide for physicians and other health professionals. New York, NY: Oxford University Press; 1998.
2. Cohen LM, McCue JD, Green GM. Do clinical and formal assessments of capacity of patients in the intensive care unit to make decisions agree? Arch Intern Med. 1993;153(21): 2841-2845.
3. Farnsworth MG. Competency evaluations in a general hospital. Psychosomatics. 1990;31(1):60-66.
4. Sessums LL, Zembrzuska H, Jackson JL. Does this patient have medical decision-making capacity? JAMA. 2011; 306(4):420-427.
5. Cairns R, Maddock C, Buchanan A, et al. Prevalence and predictors of mental incapacity in psychiatric in-patients. Br J Psychiatry. 2005;187:379-385.
6. Candia PC, Barba AC. Mental capacity and consent to treatment in psychiatric patients: the state of the research. Curr Opin Psychiatry. 2011;24(5):442-446.
7. Duncan J. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475-483.
8. Mandarelli G, Parmigiani G, Tarsitani L, et al. The relationship between executive functions and capacity to consent to treatment in acute psychiatric hospitalization. J Empir Res Hum Res Ethics. 2012;7(5):63-70.
9. Supady A, Voelkel A, Witzel J, et al. How is informed consent related to emotions and empathy? An exploratory neuroethical investigation. J Med Ethics. 2011;37(5):311-317.
10. Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2006;32(1):121-128.
11. Feldman-Stewart D, Brundage MD. Challenges for designing and implementing decision aids. Patient Educ Couns. 2004;54(3):265-273.
12. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681-692.
13. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007; 357(18):1834-1840.
14. Marson DC, McIntruff B, Hawkins L, et al. Consistency of physician judgments of capacity to consent to mild Alzheimer’s disease. J Am Geriatr Soc. 1997;45(4):453-457.
15. Kim SY, Caine ED. Utility and limits of the mini mental status examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322-1324.
16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
17. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resource Press; 1998.
Is he DISTRACTED? Considerations when diagnosing ADHD in an adult
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Adult attention-deficit/hyperactivity disorder (ADHD) can be challenging to assess accurately. Adult ADHD differs significantly from childhood ADHD, in that hyperactivity often is absent or greatly diminished, comorbid disorders (depression or substance use) are common, and previously compensated attention deficits in school can manifest in the patient’s personal and professional life.1
The mnemonic DISTRACTED can help when recalling key components in assessing adult ADHD.2 Because ADHD is a developmental disorder—there are signs of onset in childhood—it is important to maintain a longitudinal view when asking about patterns of behavior or thinking.
Distractibility. Is there a pattern of getting “off track” in conversations or in school or work situations because of straying thoughts or daydreams? Is there a tendency to over-respond to extraneous stimuli (eg, cell phones, computers, television) that impedes the patient’s ability to converse, receive information, or follow directions?
Impulsivity. Does the patient have a history of saying things “off the cuff,” interrupting others, or “walking on” someone else’s words in a conversation? Is impulsivity evident in the person’s substance use or spending patterns?
School history. This domain is important in diagnosing ADHD in adults because there needs to be evidence that the disorder was present from an early age. How did the patient perform in school (ie, grades, organization, completion of homework assignments)? Was there a behavioral pattern that reflected hyperactivity (could not stay seated) or emotional dysregulation (frequent outbursts)?
Task completion. Does the patient have trouble finishing assignments at work, staying focused on a project that is considered boring, or completing a home project (eg, fixing a leaky faucet) in a timely fashion?
Rating scales. Rating scales should be used to help support the diagnosis, based on the patient’s history and life story. There are >12 scales that can be utilized in a
clinical setting3; the ADHD/Hyperactivity Disorder Self-Report Scale is a brief and easy measure of core ADHD symptoms.
Accidents. Adults with ADHD often are accident-prone because of inattention, hyperactivity, or impulsivity. Does the patient have a history of unintentionally hurting himself because he “wasn’t paying attention” (falls, burns), or was too impatient (traffic accidents or citations)?
Commitments. Does the patient fail to fulfill verbal obligations (by arriving late, forgetting to run errands)? Has this difficulty to commit created problems in relationships over time?
Time management. How difficult is it for the patient to stay organized while balancing work expectations, social obligations, and family needs? Is there a pattern of chaotic scheduling with regard to meals, work, or sleeping?
Employment. Has the patient changed jobs because the work becomes “too boring” or “uninteresting”? Is there a pattern of being terminated because of poor work quality based on time management or job performance?
Decisions. Adults with ADHD often make hasty, ill-informed choices or procrastinate so that they do not have to make a decision. Does the patient’s decision-making reveal a pattern of being too distracted to hear the information needed, or too impatient to consider all the details?
Remember: No single component of this mnemonic alone suffices to make a diagnosis of adult ADHD. However, these considerations will help clarify what lies behind your DISTRACTED patient’s search for self-understanding and appropriate medical care.
Disclosure
Dr. Christensen reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.
1. Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008;13(11):977-984.
2. Barkley R. Taking charge of adult ADHD. New York, NY: Guilford Press; 2010.
3. Attwell C. ADHD, rating scales, and your practice today. The Carlat Psychiatry Report. 2012;10(12):1,3,5-8.