User login
Patient‐Centered Blood Management
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
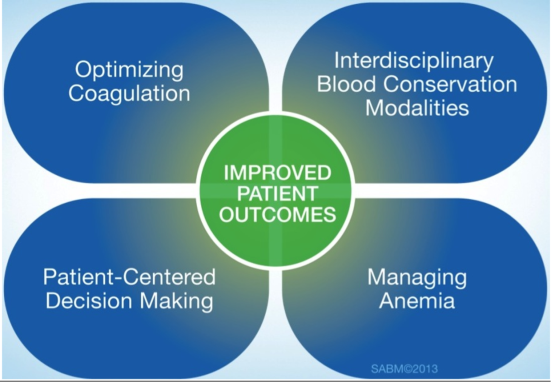
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).
| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin 13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.
Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).

| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin 13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
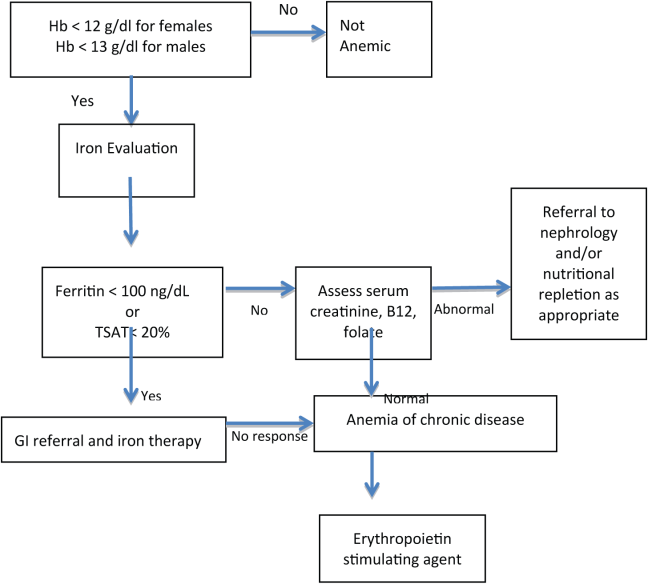
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.

Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
The transfusion of blood is the most frequently performed procedure in US hospitals.[1] Every year, approximately 14 million units of packed red blood cells are used,[2] and 1 in 10 hospitalized patients is transfused.[3] In a recent large retrospective analysis, the prevalence of anemia at hospital discharge was 12.8%.[4] In some patients hospitalized with heart failure or pneumonia, the prevalence of anemia may exceed 50%.[5, 6] Randomized controlled trials in multiple patient populations show that a restrictive transfusion strategy (using lower hemoglobin thresholds for transfusion) is safe and may be associated with less morbidity and mortality compared to a liberal transfusion strategy.[7, 8, 9] In a recent randomized clinical trial of patients with acute upper gastrointestinal bleeding, Villanueva and colleagues found a 4% increased risk of mortality when a liberal rather than a restrictive transfusion strategy was used.[10]
Transfusions are considered to be a high risk procedure, with morbidity and mortality increasing with each unit of blood received.[11] The costs associated with transfusion are substantial, with a median cost of $761 per unit (in 2010 dollars), which translates into >$11 billion annually for red cells alone.[12] Despite this, health outcomes research shows that more than half of red cell transfusions may be inappropriate.[13] Furthermore, there is wide variation in practice that is unexplained by patient characteristics.[14, 15] Given the financial and human costs, the status quo of overuse and practice variation is no longer acceptable.
The traditional focus of transfusion medicine, blood banks, and blood utilization committees has been on ensuring that we have an adequate supply of product (ie, blood components) and safe and reliable methods of administering the product. The work that has been done to secure the supply of blood components and the safety of transfusion is both necessary and laudable. More recently, attention has focused on the promise of a restrictive approach to transfusion.[7, 8, 9] This approach, in addition to easing the supply side, has the potential to improve patient care by avoiding transfusions in situations where the probability of harm may exceed the probability of benefit. The American Medical Association and the Joint Commission have recently identified blood transfusions as 1 of 5 overused medical procedures that pose a quality and safety concern.[16] The Society for Hospital Medicine recognizes blood overutilization as a high priority issue by urging avoidance of red blood cell transfusions for arbitrary hemoglobin levels in their Choosing Wisely campaign.[17]
A transformational next step is to move beyond the decision of whether and when to transfuse blood components and to focus on patient blood management.
WHAT IS PATIENT BLOOD MANAGEMENT?
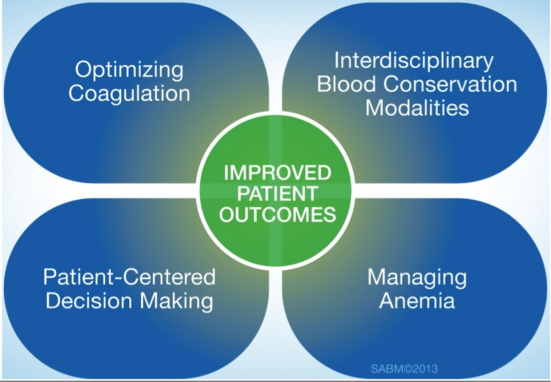
Patient‐centered blood management (PBM) aspires to improve patient outcomes by actively managing the patient's own blood and hematopoietic system recognizing that the transfusion of blood components is but 1 of many therapeutic options. PBM is a multimodal, multidisciplinary effort and is defined as the timely application of evidence‐based medical and surgical concepts designed to maintain hemoglobin concentration, optimize hemostasis, and minimize blood loss in an effort to improve patient outcomes.[18] These principles are shown in Figure 1. PBM strategies should be implemented in surgical and nonsurgical settings in virtually all stages of patient care.[18, 19] These strategies fall into 4 general categories: anemia management, coagulation optimization, blood conservation, and patient‐centered decision making (Table 1).

| Patient Blood Management Strategies | |
|---|---|
| |
| Managing anemia | Optimizing coagulation |
| Create methods for early and ongoing detection of anemia | Evaluate both quantitative and qualitative measures to assess true coagulation status |
| Employ timely evidence‐based pharmaceutical and nutritional intervention to support erythropoiesis | Employ goal‐directed therapy to correct coagulation abnormalities |
| Determine causes and contributing factors of anemia | Accurately assess true cause of bleeding dysfunction |
| Apply evidence‐based rationale for use of red cells | Apply evidence‐based rationale for use of plasma |
| Enhance physiologic tolerance of anemia by minimizing oxygen consumption | |
| Interdisciplinary blood conservation modalities | Patient‐centered decision making |
| Adopt precise and meticulous surgical technique using all available methods of hemostasis | Listen to patient needs, desires, and concerns |
| Rapidly diagnose and promptly arrest blood loss in all situations | Explore treatment possibilities, provide patient with current information about all PBM interventions |
| Employ appropriate intraoperative blood conservation modalities in an evidence‐based fashion | Inform patients of risks, benefits, and alternatives of treatment choices |
| Use available intra‐ and postoperative autologous blood conservation modalities | Integrate patient values and autonomy in decision making, decide together on a course of action, and tailor a plan of care that incorporates patient choice |
| Use methods to measure and assess hemoglobin loss | Document and communicate patient preferences |
| Control diagnostic blood loss | |
The appropriate use of these tools as part of an evidence‐based, multidisciplinary, patient‐focused program has the potential to reduce transfusions and improve patient outcomes.[18, 19, 20] The value of PBM programs as tools for improved outcomes has been endorsed by many regulatory and professional organizations including the American Association of Blood Banks,[21] the Joint Commission,[22] and the US Department of Health and Human Services.[23] There are currently 92 self‐identified PBM Programs in the United States.[18] Internationally, initiatives are underway to bring about change and implement PBM. In 2008, the Western Australia Department of Health implemented a comprehensive health‐systemwide PBM program. As a result of this program, despite increasing activity, red blood cell utilization to the entire state progressively decreased from 70,103 units in 2008 to 65,742 units in 2011.[24]
Although transfusion rates are an easy end point to measure, PBM's ultimate aim is to improve patient outcomes, not simply lower transfusion rates. To date, randomized clinical trials have not been performed comparing patient populations managed with PBM principles to a control arm. However, in large joint arthroplasty, a PBM approach was associated with decreased length of stay, decreased readmission, in addition to a decreased transfusion rate compared to historical controls.[25] Similar findings have been described in other patient populations when comparing Jehovah's Witnesses patients to non‐Jehovah's Witnesses patients.[26]
ANEMIA MANAGEMENT
Anemia has been identified as an independent predictor of morbidity, including increased postoperative infection, length of stay, and mortality.[27] The presence of anemia is also a risk factor for blood transfusion.[22] However, transfusion has not been proven to decrease the morbidity and mortality associated with anemia. Anemia is a highly prevalent finding in both medical and surgical patients.[3] Its prevalence increases after the age 50 years, to over 20% in the elderly (85 years).[28] Patients should be screened and evaluated for anemia throughout their course of care.[20] An audit of more than 9000 patients undergoing elective orthopedic surgery found that more than one‐third of patients were considered to be anemic (hemoglobin 13 g/dL) during preadmission testing.[29] Despite the association with negative outcomes, preexisting anemia is often ignored and remains untreated.[30]
PBM includes the identification of patients at risk of anemia and development of a treatment plan. The detection, evaluation, and correction of preoperative anemia should be undertaken 3 to 4 weeks before elective surgery, so treatment can be initiated prior to surgery with appropriate therapy.[31] Management of anemia consists of treating the underlying cause and use of hematinic agents to rapidly restore hemoglobin levels to normal.[20] Anemia therapy, which often includes iron supplementation and erythropoietic‐stimulating drugs, increases red blood cell mass, thus reducing or eliminating the need for allogenic blood.[32] An overview of the management of preoperative anemia can be found in Figure 2.

Available evidence suggests that in many clinical situations, transfusion of red blood cells for modest anemia does not improve outcomes and may cause harm.[7, 8, 9, 10] Although using transfusion trigger hemoglobin levels of 7 to 8 g/dL appears to be preferable to using triggers of 9 to 10 g/dL, we have no high‐quality evidence to suggest what, if any, the optimal trigger should be. Furthermore, although the traditional rationale for red cell transfusion is to improve tissue oxygen delivery, some evidence suggests that tissue oxygen delivery is maintained even at hemoglobin levels as low as 5 g/dL.[33] Available evidence suggests that for nonhemorrhaging patients, routinely transfusing at a hemoglobin level of greater than 7 to 8 g/dL should be avoided. Whether a hemoglobin of 8, 7, 6, or 5 g/dL should serve as a trigger for transfusion is unclear. Our recommendation is to focus less on the number and more on the patient with regard to assessing symptoms and treatment preferences.
OPTIMIZING COAGULATION
Prior to surgery, patients should be screened for bleeding disorders by taking a structured bleeding history and performing coagulation testing if areas of concern arise. The first‐line coagulation tests commonly used are activated partial thromboplastin time and prothrombin time.[34] Testing may also be considered in patients with conditions potentially associated with hemorrhage such as liver disease, sepsis, diffuse intravascular coagulation, preeclampsia, cholestasis, and poor nutritional states.[35]
Point‐of‐care (POC) testing for rapid testing of hemostatic function can provide fast and accurate identification of coagulation abnormalities. Platelet function has been assessed using impedance or turbidimetric aggregometry testing of whole‐blood samples. Viscoelastic tests using thromboelastometry and thromboelastography measure time and dynamics of clot formation and stability of clots over time.[36] POC coagulation testing has shown positive outcomes in surgery, critical care, organ transplantation, and trauma patients.[36] In surgical and organ transplant patients, POC testing has been shown to lower perioperative blood losses and decrease the use of allogenic transfusions.
Protocols are needed for discontinuing drugs that may affect coagulation or increase bleeding such as warfarin, aspirin, clopidogrel, herbal supplements,[32] low molecular weight heparins, selective factor Xa inhibitors, and direct thrombin inhibitors.[36] Interruption of oral anticoagulant therapy provides gradual reduction of the coagulation effects of warfarin but provides more rapid reduction from agents such as dabigatran.[37] Warfarin‐treated patients in emergency situations, such as excessive bleeding, emergent surgery, or international normalized ratio (INR) >10 require rapid anticoagulation reversal that cannot be achieved by drug discontinuation alone. Vitamin K (phytonadione) therapy can be used in these situations and may be given intravenously or orally; however, the intramuscular and subcutaneous routes are not recommended.[37]
Fresh frozen plasma (FFP) provides fast, partial reversal of coagulopathy by replacement of factors II, VII, IX, and X; however, volume overload may make it difficult to administer an adequate FFP dose. In patients with very high INRs, replacement of hemostatic levels of these factors cannot be achieved with tolerable doses of FFP.[37] Prothrombin complex concentrates (PCC) are an alternative to FFP for reversal of warfarin and other oral anticoagulants.[37] Both 3‐factor PCC and 4‐factor PCC products are available, all containing factors II, IX, and X with variable amounts of FVII.[37] The 4 factor products provide larger amounts of factor VII compared to the 3 factor products.[37] In studies comparing PCCs to FFP, PCCs showed superior efficacy in decreasing time to INR correction, with a lower risk of thrombotic adverse events.[37]
Although some aspects of optimizing coagulation are well within the domain of hospital medicine, others require collaboration with hematology. As with all aspects of patient blood management, the optimal approach is often multidisciplinary and multimodal.
INTERDISCIPLINARY BLOOD CONSERVATION MODALITIES
The minimization of intraoperative bleeding is one of the cornerstones of effective PBM. Perioperative blood loss is an important factor in increasing postoperative morbidity and mortality.[19] Blood loss during surgery increases patient exposure to blood transfusions and their associated risks.[27] In postoperative patients, blood transfusion has been shown to be an independent risk factor for respiratory complications, infection, and intensive care unit (ICU) admissions. Patients receiving more than 2 U of blood had twice the risk of complications and ICU admissions.[39]
The management of surgical bleeding requires multiple techniques, including excellent surgical technique, the use of minimally invasive surgery, reinfusion of shed blood, and the use of topical hemostatic agents. Meticulous surgical technique is the cornerstone of intraoperative blood conservation.[32] During surgery, various techniques can be used to help decrease allogeneic blood exposure. These include techniques such as intraoperative blood recovery and acute normovolemic hemodilution.[40] Energy‐based technologies, such as electrosurgery, harmonic scalpels, argon beam coagulation, and radiofrequency technology have also been used to aid in hemostasis.[41] Interventions such as pharmacologic agents and topical hemostatic/sealant agents can also be utilized to minimize intraoperative blood loss. Not surprisingly, operative blood loss has been associated with an increased risk of death.[42] Blood loss and allogeneic blood transfusion can be greatly reduced with the utilization of an appropriate combination of therapies.
Hospital‐acquired anemia is a common complication affecting almost two‐thirds of patients admitted to the hospital. Although anemia of chronic disease is the leading cause of hospital‐acquired anemia, phlebotomy‐induced blood loss is an important contributing factor.[43] In critical care patients, phlebotomy volume is an independent predictor of transfusion requirements. On average, these patients undergo 4 to 5 blood draws per day.[44] Healthcare professionals can help decrease the development of hospital‐acquired anemia by employing strategies aimed at decreasing phlebotomy blood loss.[32] Losses in the range of 41 to 65 mL of blood per day have been reported in the medical literature and are associated with development of anemia.[45] Phlebotomy blood loss can be reduced by strategies that include eliminating arterial line blood discard, using small volume (ie, pediatric size) blood collection tubes, and ordering laboratory tests only when clinically justified.[45]
PATIENT‐CENTERED DECISION MAKING
Patient‐centered medicine is the practice of taking into account patients' individual preferences, objectives, and values.[46] Physicians are responsible for providing patients with complete and understandable information regarding treatment, and potential benefits and risks of available treatment options. Patients, in turn, must communicate their preferences and feelings with regard to their treatment.[47] A recent observational study by Weiner confirmed that employing theses practices is associated with improved health outcomes.[48]
An individualized approach to PBM helps ensure the right fit for each individual patient by informing them of risks, benefits, and alternatives of treatment choices and listening to their needs, desires, and concerns. Patients may have specific religious or cultural factors that may need to be considered. Some patients, such as Jehovah's Witnesses, decline blood products and may refuse agents derived from human or animal plasma. Some patients from other cultural or religious backgrounds may refuse agents that have factors derived from a specific animal.
Informed consent for transfusions is often obtained via a printed form offered without discussion with the patient by clerical or nursing staff. Obtaining a patient's signature to comply with Joint Commission and CMS mandates is too often the goal of this process. True informed consent requires that patients understand treatments and are informed of both the possible benefits and risks of the proposed treatment. Patients should also be informed of available treatment alternatives.[27] The benefit of transfusions are sometimes overstated, whereas the risks, such as transfusion‐related acute lung injury and transfusion‐associated circulatory overload, are often overlooked.[49] A comprehensive informed consent process, including a frank and open discussion between physician and patient, is a vital component of patient‐centered decision making.
THE HOSPITALIST'S ROLE IN PBM
Hospitalists often have the responsibility for prescribing and obtaining consent for the administration of blood components. Therefore, understanding the complexities that surround PBM and the transfusion process, including the potential for harm vs the potential for benefit, as well as the economic impact of transfusions, are essential for providing effective patient care.
Although hospitalists are not primarily based in the operating room, they are uniquely positioned to champion the value of PBM throughout their institution. Many hospitalists play a vital role in preoperative anemia detection and management via clinical and administrative roles in preadmission testing. In addition, hospitalists can serve as the connectors that bring anesthesiologists, surgeons, and others to the table to explore ways to decrease the widespread incidence of hospital‐acquired anemia. Improving perioperative blood conservation, optimizing coagulation, and managing anemia all require a multidisciplinary approach.
Hospitalists can play a major role in affecting gradual changes in organizational culture. Whether it is helping a subspecialist become comfortable with not reflexively transfusing at a threshold hemoglobin, or working with pharmacists and nurses to increase their comfort level with intravenous iron and vitamin K, a sustained effort with ongoing communication and education is required to change practice. Recognizing and engaging existing institutional stakeholders and existent efforts related to blood management (eg, transfusion committees, blood banks, blood utilization committees) is also essential to successful implementation of patient blood‐management principles. Hospitalists are often the ones who combine the credibility and the connections to the disparate stakeholders to drive the necessary culture change forward.
It is the dual role as both front‐line care provider and champion for quality improvement that uniquely positions hospitalists to lead implementation of PBM strategies. Improving quality and safety while decreasing costs, and centering decision making on the patient, are goals of effective PBM that are intimately aligned with the goals of hospital medicine. By developing, implementing, and practicing PBM, hospitalists have the opportunity to yet again lead the way in improving patient care within their organizations.
Disclosures
Disclosures: Maria Ashton received payments from the Society for the Advancement of Blood Management to assist in writing and reviewing this article and for travel to meetings. The authors report no other conflicts of interest.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.
- Agency for Healthcare Research and Quality. Healthcare Cost Utilization Project Statistical Brief #149. Most frequent procedures performed in U.S. hospitals 2010. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb149.pdf. Accessed July 18, 2013.
- Department of Health and Human Services. The 2011 national blood collection and utilization survey report. Washington, DC: DHHS, 2013.
- Agency for Healthcare Research and Quality. HCUP facts and figures: statistics on hospital‐based care in the United States, 2007. Available at: http://www.hcup‐us.ahrq.gov/reports/factsandfigures/2007/pdfs/FF_report_2007.pdf. Accessed June 16, 2013.
- , , , , . Prevalence and impact of anemia in hospitalized patients. South Med J. 2013;106(3):202–206.
- , , , et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol. 2004;96(1):79–87.
- , , , et al. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15.
- , , . Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4):CD002042.
- , , , et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417.
- , , , et al.; FOCUS Investigators. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21.
- , , , et al. Morbidity and mortality risk associated with red blood cell and blood‐component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616.
- , , , , , . Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765.
- , , , et al.; International Consensus Conference on Transfusion Outcomes Group. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25(3):232–246.
- , , , . Utilization of blood transfusion among older adults in the United States. Transfusion. 2011;51(4):710–718.
- , , , et al. Variation of blood transfusion in patients undergoing major noncardiac surgery. Ann Surg. 2013;257(2):266–278.
- The Joint Commission continues to study overuse issues. Jt Comm Perspect. 2012;32(5):4, 8.
- ABIM Foundation. Choosing Wisely. Available at: http://www.choosingwisely.org/. Accessed July 18, 2013.
- Society for the Advancement of Blood Management. Administrative and clinical standards for patient blood management programs. Englewood, New Jersey; 2012. Available at: http://www.sabm.org/publications. Accessed June 16, 2013.
- . Patient blood management: a patient‐oriented approach to blood replacement with the goal of reducing anemia, blood loss and the need for blood transfusion in elective surgery. Transfus Med Hemother. 2012;39:67–72.
- , , , et al. From bloodless surgery to patient blood management. Mt Sinai J Med. 2012;79(1):56–65.
- , , , et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58.
- The Joint Commission implementation guide for the joint commission patient blood management performance measures 2011. Available at: http://www.jointcommission.org/assets/1/6/PBM_Implementation_Guide_20110624.pdf. Accessed June, 16, 2013.
- U.S. Department of Health and Human Services. Advisory Committee on Blood Safety and Availability. Recommendations, November 2010. Available at: http://www.hhs.gov/ash/bloodsafety/advisorycommittee/recommendations/recommendations201011.pdf. Accessed June 16, 2013.
- , , , . Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013;27(1):43–58.
- , , . Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952.
- , , , et al. How good patient blood management leads to excellent outcomes in Jehovah's witness patients undergoing cardiac surgery. Interact Cardiovasc Thorac Surg. 2011;12(2):183–188.
- . Anemia and patient blood management in hip and knee surgery. Anesthesiology. 2010;113:482–495.
- , , , . Hematology Am Soc Hematol Educ Program 2005;528–532.
- , , . Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106(1):13–22.
- , , , . Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47(8):1468–1480.
- , , , et al. Detection, evaluation, and management of anemia in the elective surgery patient. Anest Analg. 2005;101:1858–1861.
- , . Blood management: a primer for clinicians. Pharmacotherapy. 2007;27(10):1394–1411.
- , , , et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279(3):217–221.
- , , , et al. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504.
- , , . Routine preoperative coagulation tests: an outdated practice? Br J Anaesth. 2011;106(1):1–3.
- , , . Point‐of‐care coagulation management in intensive care medicine. Crit Care. 2013;17:218.
- . Pharmacologic interventions for reversing the effects of oral anticoagulants. Am J Health Syst Pharm. 2013;70(10 supp 1):S12–S21.
- , , , . The combination of platelet‐enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma. 2004;56(1):76–79.
- . Indications and contraindications of cell salvage. Transfusion. 2004;44(12 suppl):40S–44S.
- , , , et al. Application of energy‐based technologies and topical hemostatic agents in the management of surgical hemostasis. Vascular. 2010;18(4):197–204.
- , , , et al. Effect of anemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060.
- , . Hospital‐acquired anemia. J Med Assoc Thai. 2006;89(1):63–67.
- , , . Anemia, transfusion, and phlebotomy practices in critically ill patients with prolonged ICU length of stay: a cohort study. Crit Care. 2006;10(5):R140.
- . Blood conservation in the critically ill patient. Anesthesiol Clin North Am. 2005;23(2):363–372.
- . Patient‐centered medicine and patient‐oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6.
- , , Supporting patients to make the best decisions. BMJ. 2011;342:775–777.
- . Patient‐centered decision making and health care outcomes: an observational study. Ann Intern Med. 2013;158(8):573–579.
- , , , et al. Informed consent for blood transfusion: what do medicine residents tell? What do patients understand? Am J Clin Pathol. 2012;138(4):559–565.