User login
Liraglutide for obesity: New indication
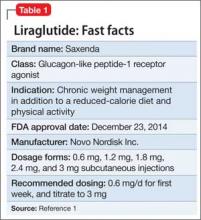
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
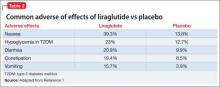
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
Reference
1. Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk A/S; 2015.
Managing first-episode psychosis: An early stage of schizophrenia with distinct treatment needs
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
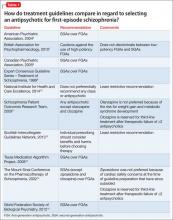
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
The less time that passes between the onset of psychosis and initiation of appropriate treatment, the greater the patient’s odds of recovery.1 However, relapse prevention is a major clinical challenge because >80% of patients will relapse within 5 years, and, on average, 40% to 50% of patients with a first-episode schizophrenia will relapse within 2 years depending on the definition used and patient characteristics.2 Although there are several explanations and contributing factors to relapses, nonadherence—partial or complete discontinuation of antipsychotics—is a primary risk factor, contributing to a 5-fold increase in relapse risk.3
As such, optimal antipsychotic selection, dosing, and monitoring play an important role in managing this illness. Patients with first-episode psychosis (FEP) are unusual in some ways, compared with patients with multiple episodes of psychosis and represent a different stage of schizophrenia.
In this 2-part series, we will discuss pharmacotherapy for FEP. This article focuses on antipsychotic selection, dosage, and duration of treatment among these patients. The second article, in the July 2015 issue, reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Defining FEP
FEP refers to a patient who has presented, been evaluated, and received treatment for the first psychotic episode associated with a schizophrenia spectrum diagnosis.4 FEP is part of a trajectory marked by tran sitional periods. The patient transitions from being “healthy” to a prodromal state characterized by: (1) nonpsychotic behavioral disturbances such as depression or obsessive-compulsive disorder, (2) attenuated psychotic symptoms not requiring treatment, then converting to (3) psychotic symptoms prompting initial presentation for antipsychotic pharmacotherapy, leading to (4) a formal diagnosis of schizophreniform disorder and, subsequently, schizophrenia, requiring treatment to stabilize symptoms.
There are 2 critical periods along this continuum: prodromal stage and the duration of untreated psychosis (DUP). The prodromal period is a retrospectively identified time where the patient shows initial nonpsychotic disturbances (eg, cognitive and behavioral symptoms) before exhibiting clinical diagnostic criteria for a schizophrenia spectrum disorder. Approximately one-third of patients exhibiting these symptoms convert to psychosis within 1 year, and early treatment engagement at this stage has been shown to improve outcomes.5 The DUP is the time from when a patient has noticeable psychotic symptoms to initiation of drug treatment. The DUP is a consistent predictor of clinical outcome in schizophrenia, including negative symptoms, quality of life, and functional capacity.1
Antipsychotic selection
Treatment goals for FEP patients include:
• minimizing the DUP
• rapidly stabilizing psychosis
• achieving full symptomatic remission
• preventing relapse.
Several treatment guidelines for managing schizophrenia offer variable recommendations for initial antipsychotic treatment in patients with first-episode schizophrenia (Table 1).6-15 Most recommend second-generation antipsychotics (SGAs) over first-generation antipsychotics (FGAs)6,8,9,13,15 with specific recommendations on minimizing neurologic and metabolic adverse effects—to which FEP patients are susceptible—by avoiding high-potency and neurotoxic FGAs (eg, haloperidol and fluphenazine),7 clozapine,11,14 olanzapine,11 or ziprasidone.14 Two guidelines—the National Institute for Health and Care Excellence and the Scottish Intercollegiate Guidelines Network—do not state a preference for antipsychotic selection.10,12
The rationale for these recommendations is based on efficacy data, tolerability differences, FDA-approved indications, and recent FDA approvals with sparse post-marketing data. Of note, there are a lack of robust data for newer antipsychotics (eg, aripiprazole, paliperidone, iloperidone, asenapine, and lurasidone) in effectively and safely treating FEP; however, given the results of other antipsychotics studies, it is likely the efficacy and tolerability of these drugs can be extrapolated from experience with multi-episode patients.
Study design and demographics. Research studies of FEP share some similarities in study design; however, there is enough variability to make it difficult to compare studies and generalize findings (Table 2).16 The variability of DUP is a limitation when comparing studies because it is a significant predictor of clinical outcome. Patients who abuse substances—and often are more challenging to treat17—typically are excluded from these trials, which could explain the high response rate documented in studies of first-episode schizophrenia.
In addition, some FEP patients included in clinical trials might not be truly antipsychotic naïve; an estimated 25% to 75% of patients in these studies are antipsychotic naïve. This is an important consideration when comparing data on adverse effects that occur early in treatment. Additionally, acknowledging the advantages and disadvantages of how to handle missing data is critical because of the high dropout rate observed in these studies.18
Efficacy. There is a high response rate to antipsychotic therapy—ranging from 46% to 96%, depending on the study—in patients with first-episode schizophrenia.3 The response mainly is seen in reduction of positive symptoms because typically negative and cognitive symptoms do not respond to antipsychotics. One study reported only 29% of patients achieved both positive and negative symptom remission.19 It is likely that secondary negative symptoms caused by social withdrawal, reduced speech, and avoidance improve when positive symptoms subside, but primary negative symptoms endure.In general, there is a lack of evidence suggesting that 1 antipsychotic class or agent is more effective than another. Studies mainly assess effectiveness using the primary outcome measure of all-cause discontinuation, such as the Clinical Antipsychotic Trials of Intervention Effectiveness study.20 This outcome measure is a mixture of patient preference, tolerability, and efficacy that provides a more generalizable gauge on how well the treatment works in the clinic rather than tightly regulated settings such as clinical trials. A recent meta-analysis supports no differences in efficacy among antipsychotics in early-episode psychosis.21
Tolerability. Because there are no significant differences among antipsychotic classes or agents in terms of efficacy in first-episode schizophrenia, drug selection is guided mainly by (1) the adverse effect profile and (2) what should be avoided depending on patient-specific variables. Evidence suggests first-episode patients are more sensitive to adverse effects of antipsychotics, particularly neurologic side effects (see this article at CurrentPsychiatry.com for a table comparing adverse effects of antipsychotics in first-episode psychosis).18,22-29 Overall adverse effect profiles remain similar across FEP or multi-episode patients, but tend to be more exaggerated in drug-naïve patients with FEP.
Regarding FGA side effects, McEvoy et al18 demonstrated the neuroleptic threshold occurs at 50% lower haloperidol dosages in patients with first-episode schizophrenia (2.1 mg/d) compared with multi-episode schizophrenia (4.3 mg/d). Other trials suggest SGAs are associated with a lower risk of extrapyramidal side effects (EPS) or use of adjunctive therapies such as anticholinergic drugs or benzodiazepines.23-27 An exception to this statement is that higher risperidone dosages (≥4 to 6 mg/d) have been found to have higher rates of EPS and use of adjunctive medications to treat these symptoms in FEP.26 This is important because studies report higher discontinuation rates with more severe adverse effects of antipsychotics.
Cardiometabolic effects are of particular concern in first-episode patients because most weight gain happens in the first 3 to 4 months of treatment and remains throughout the first year.18,24,29,30 Studies have shown that olanzapine, quetiapine, and risperidone are associated with more clinically significant weight gain compared with haloperidol and ziprasidone.23-25 Olanzapine-associated weight gain has been reported to be twice that of quetiapine and risperidone.18 Regardless, the EUFEST trial did not find a difference in clinically significant weight gain after 12 months among the antipsychotics studied, including haloperidol and ziprasidone.25
Weight gain associated with these antipsychotics is accompanied by changes in fasting triglycerides, glucose, total cholesterol,23 and high-density lipoprotein cholesterol as well as an increase in body mass index (BMI) categorization29 (eg, shift from normal to overweight).18,25 Patients with lower baseline BMI and in racial minority groups might experience more rapid weight gain regardless of antipsychotic selection.29,30
Hyperprolactinemia could be under-recognized and could contribute to early treatment discontinuation.31 Evidence in patients with first-episode schizophrenia suggests similar outcomes as those seen in multi-episode patients, in whom risperidone is associated with higher prolactin elevations and clinically significant hyperprolactinemia (eg, galactorrhea and gynecomastia) compared with olanzapine, quetiapine, and low-dose haloperidol.18,23,24 However, there is a lack of studies that assess whether long-term therapy with strong D2 receptor antagonists increases the risk of bone demineralization or pathological fractures when started before patients’ bones reach maximum density in their mid-20s.31
Antipsychotic dosing
Given the high rate of treatment response in FEP and patients’ higher sensitivity to antipsychotic adverse effects, particularly EPS, guidelines recommend antipsychotic dosages lower than those used for multi-episode schizophrenia,11 especially FGAs. Based on trial data, commonly used dosages include:
• haloperidol, ≤5 mg/d23-25,29
• olanzapine, 10 mg/d18,23,25,29
• risperidone, ≤4 to 6 mg/d.18,24,29,32
In general, haloperidol and risperidone, 2 to 3 mg/d, were well tolerated and effective in trials. Higher quetiapine dosages of 500 to 600 mg/d could be required.11,18,25
According to a survey on prescribing practices of antipsychotic selection and dosing in first-episode schizophrenia,4 clinical prescribing practices tend to use unnecessarily high initial antipsychotic dosing compared with trial data. There also is variability in the usual target antipsychotic dosage ranging from 50% lower dosages to normal dosages in chronic schizophrenia to above FDA-approved maximum dosages for olanzapine (which may be necessary to counteract tobacco-induced cytochrome P450 1A2 enzyme induction).
In addition, these clinicians reported prescribing aripiprazole, an antipsychotic with weaker evidence (eg, case reports, case series, open-label studies) supporting its efficacy and tolerability in FEP. These prescribing practices could reflect attempts to reduce the DUP and achieve symptom remission, so long as tolerability is not a concern.
Essentially, prescribed dosages should be based on symptom improvement and tolerability. This ideal dosage will vary as illustrated by Kapur et al,33 who reported that FEP patients (N = 20) given haloperidol, 1 mg or 2.5 mg/d, had D2 receptor occupancy rates of 38% to 87%, which was significantly dose-related (1 mg/d mean = 59%, 2.5 mg/d mean = 75%). Clinical response and EPS significantly increased as D2 receptor occupancy exceeded 65% and 78%, respectively.
Antipsychotic response
When should you expect to see symptom improvement in patients with first-episode schizophrenia?
Emsley et al34 reported a 77.6% response rate among first-episode patients (N = 522) treated with low dosages of risperidone (mean modal dosage [MMD] = 3.3 mg/d) and haloperidol (MMD = 2.9 mg/d). They found variable response times that were evenly dispersed over a 10-week period. Nearly one-quarter (22.5%) did not respond until after week 4 and 11.2% did not respond until after week 8. In a study of FEP patients (N = 112) treated with olanzapine (MMD = 11.8 mg/d) or risperidone (MMD = 3.9 mg/d), Gallego et al35 reported a cumulative response of 39.6% at week 8 and 65.1% at week 16.
Although there is evidence that, among multi-episode patients, early nonresponse to antipsychotic therapy could predict subsequent nonresponse,36 the evidence is mixed for first-episode schizophrenia. Studies by Emsley et al34 and Gallego et al35 did not find that early nonresponse at weeks 1 or 2 predicted subsequent nonresponse at week 4 or later. However, other studies support the idea that early nonresponse predicts subsequent nonresponse and early antipsychotic response predicts future response in first-episode patients, with good specificity and sensitivity.37,38
Overall, treatment response in first-episode schizophrenia is variable. An adequate antipsychotic trial may be longer, 8 to 16 weeks, compared with 4 to 8 weeks in multi-episode patients. Because research suggests that failure to respond to treatment may lead to medication nonadherence,39 it is reasonable to consider switching antipsychotics when a patient experiences minimal or no response to antipsychotic therapy at week 2; however, this should be a patient-specific decision.
How long should you continue therapy after symptom remission?
There is a lack of consensus on the duration of therapy for a patient treated for first-episode schizophrenia because a small percentage (10% to 20%) do not relapse after the first psychotic episode.3 In general, treatment guidelines and expert consensus statements recommend at least 1 to 2 years of treatment before considering a discontinuation trial.7,10-11 Discuss the benefits and risks of maintenance treatment with your patient and obtain informed consent. With patients with minimal insight, obtaining proper consent is not possible and the physician must exercise judgment unilaterally, if necessary, after educating the family.
After at least 12 months of treatment, antipsychotic therapy could continue indefinitely, depending on patient-specific factors. There are no predictors for identifying patients who do not require maintenance therapy beyond the first psychotic episode. The absence of negative and cognitive deficits could provide clues that a patient might be a candidate for antipsychotic tapering.
Predicting the treatment course
Research investigating clinical predictors or biomarkers that forecast whether a patient will respond to treatment is preliminary. Many characteristics have been identified (Table 31,3,4,23,25,40) and include shorter DUP,1 poorer premorbid function,3 antipsychotic discontinuation,3 a trusting patient-doctor relationship,41 and antipsychotic-related adverse effects,23,25 which are predictive of response, nonresponse, relapse, adherence, and nonadherence, respectively.
Bottom Line
The goals of pharmacological treatment of first-episode schizophrenia are to minimize the duration of untreated psychosis and target full remission of positive symptoms using the lowest possible antipsychotic dosages. Pharmacotherapy should continued for 1 to 2 years, with longer duration considered if it is discussed with the patient and with vigilant monitoring for adverse effects and suboptimal medication nonadherence to prevent relapse.
Editor’s note: The second article in this series in the July 2015 issue reviews the rationale and evidence for non-standard, first-line therapies, including long-acting injectable antipsychotics and clozapine.
Related Resources
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
• Martens L, Baker S. Promoting recovery from first episode psychosis: a guide for families. Centre for Addiction and Mental Health. http://www.camh.ca/en/hospital/ Documents/www.camh.net/AboutCAMH/Guideto CAMH/MentalHealthPrograms/SchizophreniaProgram/ 3936PromotingRecoveryFirstEpisodePsychosisfinal.pdf.
Drug Brand Names
Aripiprazole • Abilify Lurasidone • Latuda
Asenapine • Saphris Olanzapine • Zyprexa
Clozapine • Clozaril Paliperidone • Invega
Fluphenazine • Prolixin Quetiapine • Seroquel
Iloperidone • Fanapt Risperidone • Risperdal
Haloperidol • Haldol Ziprasidone • Geodon
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following a response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
4. Weiden PJ, Buckley PF, Grody M. Understanding and treating “first-episode” schizophrenia. Psychiatr Clin North Am. 2007;30(3):481-510.
5. Madaan V, Bestha DP, Kolli V. Schizophrenia prodrome: an optimal approach. Current Psychiatry. 2014;13(3):16-20, 29-30.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
8. Canadian Psychiatric Association. Clinical practice guideline. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
9. McEvoy JP, Scheifler PL, Frances A. Treatment of schizophrenia 1999. Expert consensus guideline series. J Clin Psychiatry. 1999;60(suppl 11):4-80.
10. National Institute for Health and Care Excellence (NICE). Clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2014.
11. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
12. Scottish Intercollegiate Guidelines Network (SIGN). Management of schizophrenia. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2013. SIGN publication no. 131.
13. Argo TR, Crismon ML, Miller AL, et al. Texas Medication Algorithm Project procedural manual. Schizophrenia treatment algorithms. Austin, Texas: Texas Department of State Health Services; 2008.
14. Marder SR, Essock SM, Miller Al, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
15. Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry. 2008;9(4):248-312.
16. Robinson DG, Woerner MG, Alvir JMJ, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psych. 1999;56(3):241-247.
17. Green AI, Tohen MF, Hamer RM, et al. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res. 2004;66(2-3):125-135.
18. McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1050-1060.
19. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71(6):716-728.
20. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New Engl J Med. 2005; 353(12):1209-1223.
21. Crossley NA, Constante M, McGuire P, et al. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196(6):434-439.
22. McEvoy JP, Hogarty GE, Steingard S. Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psych. 1991;48(8):739-745.
23. Lieberman JA, Tollefson G, Tohen M, et al; HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160(8):1396-1404.
24. Schooler N, Rabinowitz J, Davidson M, et al; Early Psychosis Global Working Group. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947-953.
25. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
26. Emsley RA; Risperidone Working Group. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull. 1999;25(4):721-729.
27. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naïve first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
28. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
29. Robinson DG, Woerner MG, Napolitano B, et al. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry. 2006;163(12):2096-2102.
30. Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537-543.
31. Taylor M, Waight A, Leonard B. Advances in the understanding and challenges facing the management of first-episode schizophrenia. J Psychopharmacol. 2012; 26(suppl 5):3-5.
32. Merlo MC, Hofer H, Gekle W, et al. Risperidone, 2mg/day vs. 4mg/day, in first-episode, acutely psychotic patients: treatment efficacy and effects on fine motor functioning. J Clin Psychiatry. 2002;63(10):885-891.
33. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
34. Emsley R, Rabinowitz J, Medori R. Time course for antipsychotic treatment response in first-episode schizophrenia. Am J Psychiatry. 2006;163(4):743-745.
35. Gallego JA, Robinson DG, Sevy SM, et al. Time to treatment response in first-episode schizophrenia: should acute treatment trials last several months? J Clin Psychiatry. 2011;72(12):1691-1696.
36. Gardner KN, Bostwick JR. Antipsychotic treatment response in schizophrenia. Am J Health Sys Pharm. 2012;69(21):1872-1879.
37. Stauffer VL, Case M, Kinon BJ, et al. Early response to antipsychotic therapy as a clinical marker of subsequent response in the treatment of patients with first-episode psychosis. Psychiatry Res. 2011;187(1-2):42-48.
38. Schennach-Wolff R, Seemüller FH, Mayr A, et al. An early improvement threshold to predict response and remission in first-episode schizophrenia. Br J Psychiatry. 2010;196(6):460-466.
39. Perkins DO, Gu H, Weiden PJ, et al; Comparison of Atypicals in First Episode study group. Predictors of treatment discontinuation and medication nonadherence in patients recovering from a first episode of schizophrenia, schizophreniform disorder, or schizoaffective disorder: a randomized, double-blind, flexible-dose, multicenter study. J Clin Psychiatry. 2008;69(1):106-113.
40. Garner B, Berger GE, Nicolo JP, et al. Pituitary volume and early treatment response in drug-naïve first-episode psychosis patients. Schizophr Res. 2009;113(1):65-71.
41. Sapra M, Weiden PJ, Schooler NR, et al. Reasons for adherence and nonadherence: a pilot study comparing first-and multi-episode schizophrenia patients. Clin Schizophr Relat Psychoses. 2014;7(4):199-206.
When it’s time for ‘the talk’: Sexuality and your geriatric patient
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Recent studies suggest that most older adults maintain sexual interest well into late life; many, however, experience sexual dysfunction. This article provides psychiatric practitioners with current information regarding sexuality and aging, as well as psychiatric and systemic medical comorbidities and sexual side effects of medications. Practice guidelines for assessing and managing sexual dysfunction have been developed for use in many medical specialties, and such guidance would be welcome in psychiatric practice.
This article addresses the myth of “geriatric asexuality” and its potential impact on clinical practice, the effects of age-related physiological changes on sexual activity, the importance of sexuality in the lives of older adults, and sensitive questions clinicians can pose about geriatric sexuality. We also will discuss:
• the importance of including a sexual assessment in the comprehensive psychiatric evaluation
• recognizing sexual dysfunction
• providing appropriate management within a multi-disciplinary, collaborative approach.
Sexuality after 65
Regardless of age, sexual activity can provide a sense of comfort and elicit a positive emotional and physical response.1 Hillman2 defined human sexuality as any combination of sexual behavior, emotional intimacy, and sense of sexual identity.
Sexuality in the aging population generally is an understudied area, obscured by the myth of “geriatric asexuality” and subject to numerous psychosocial variables.1 Previous research, focused on a biological perspective of sexuality, has largely overlooked psychological and social influences.3 It has been assumed that, with age, physical and hormonal changes or chronic illness ordinarily reduce or eliminate sexual desire and sexual behavior.3 However, the majority of older adults (defined as age ≥65) report a moderate-to-high level of sexual interest well into late life.1,3
Sexual function remains a subject often neglected in psychiatry. Sexual dysfunctions, as described in the DSM-5,4 do not include age-related changes in sexual function. In addition to physiological changes, sexual difficulties can result from relationship strain, systemic medical or psychiatric disorders, and sexual side effects of medications.
CASE REPORT
Mr. C, age 71 and married, is being treated for a major depressive episode that followed a course of shingles and persistent postherpetic neuralgia. Medications are: escitalopram, 20 mg/d; pregabalin, 150 mg/d; and ramipril, 5 mg/d. Mr. C is physically active and involved in social activities; he has no substance use history. He attends clinic visits with his wife.
Mr. C reports that despite significant improvement of his depressive and pain symptoms, he now experiences sexual difficulties, which he seems hesitant to discuss in detail. According to his wife, Mr. C appears to lack sexual desire and has difficulty initiating and maintaining an erection. She asks Mr. C’s psychiatrist whether she should stop her estrogen treatment, intended to enhance her sexual function, given that the couple is no longer engaging in sexual intercourse.
Mr. C admits to missing physical intimacy; however, he states, “If I have to make a choice between having sex with my wife and getting this depression out of my head, I’m going to pick getting rid of the depression.” Mrs. C says she is becoming dissatisfied with their marriage and the limited time she and her husband now spend together. Mr. C’s psychiatrist suggests that Mr. C and his wife undergo couples counseling.
Physiological changes with aging
In both women and men, the reproductive system undergoes age-related physiological changes.
Women. In women, the phase of decline in ovarian function and resulting decline in sex steroid production (estradiol and progesterone) is referred to as the climacteric, with menopause being determined retrospectively by the cessation of a menstrual period for 1 year.5
Menopausal symptoms typically occur between age 40 and 58; the average age of menopause is 51.6,7 Both estradiol and progesterone levels decline with menopause, and anovulation and ovarian failure ensue. A more gradual decline of female testosterone levels also occurs with aging, starting in the fourth decade of life.8
Clinical manifestations of menopause include vasomotor symptoms (ie, “hot flushes”), sleep disturbances, anxiety and depressive symptoms, decreased bone mineral density, and increased risk of cardiovascular disease.6,7 Loss of estrogen as well as continued loss of testosterone can result in dyspareunia because of atrophy and decreased vulvar and vaginal lubrication, with sexual excitement achieved less quickly, and a decreased intensity of orgasm.7
Men. Research has shown that testosterone levels are highest in men in the second and third decades, with a subsequent gradual decline.9 Older men with a low testosterone level are described as experiencing “late-onset hypogonadism,” also known by the popularized term “andropause.”10 This is attributed to decreased activity at the testicular and hypothalamic levels.10
Nonetheless, only a small fraction of older men with confirmed androgen deficiency are clinically symptomatic.11,12 Low testosterone is associated with decreased libido; it can hinder morning erections, contribute to erectile dysfunction, and result in erections that require physical stimulation.13
Notably, erectile dysfunction involves several other etiologic factors: psychiatric (eg, relationship difficulties, depression), neurogenic (eg, spinal cord injury), endocrine (eg, hyperprolactinemia), arteriogenic (eg, hypertension, type 2 diabetes mellitus), and drug-induced (eg, antidepressants, antihypertensives).14 A low testosterone level also has been associated with potential cognitive changes, decreased bone mineral density, metabolic syndrome (eg, increased risk of type 2 diabetes mellitus), and cardiovascular mortality.10
Effects on sexual activity. How much age-related physiological changes impact sexual practices in the geriatric population is uncertain. A study following 3,302 women through menopause over 6 years found some decline in sexual activity; however, this decline was not associated with increased sexual pain, decreased desire, or lack of arousal.15 Men continue to find ways to remain sexually active despite physiological changes (eg, erectile difficulties), but the etiology of sexual dysfunction in later life remains multi-modal, involving physical, psychological, and relational factors.16,17
Sexual practices in older adults
Researchers for the National Social Life, Health, and Aging Project (NSHAP) have examined sexual activities, behaviors, and problems in >3,000 older community-dwelling men and women across the United States, using information collected from in-home interviews.18 This study found that sexual activity, defined as any mutually voluntary sexual contact with another person, declines with age; however, even in the oldest age group (age 75 to 85), 39% of men and 17% of women reported being sexually active in the last 12 months. Among these persons, 54% reported sexual activity at least 2 times per month; 23% reported having sex at least once a week; and 32% reported engaging in oral sex. Partner availability predicted sexual activity.
Respondents with self-reported poor physical health were more likely to experience sexual problems (eg, difficulty with erection or lubrication, dyspareunia, and lack of pleasure). The most commonly reported reason for sexual inactivity in those with a spouse or other intimate partner was the male partner’s poor physical health.18
A longitudinal study, part of the Women’s Healthy Ageing Project, examined changes in sexual function at late menopause compared with early menopause. Although the researchers also found an age-related decrease in sexual activity, 50% of their late-menopause respondents (mean age, 70; range, 64 to 77) still reported sexual activity in the previous month, with 35% of this subgroup reporting sexual activity at least once a week; 83% reported sexual thoughts or fantasies.19 Availability of a partner, absence of a history of depression, moderate (compared with no) alcohol consumption, and better cognitive function were significantly associated with a higher level of sexual activity.19
In the Successful Aging Evaluation study, conducted in San Diego County, California, community-dwelling older partnered adults age 50 to 99 (mean age, 75) were surveyed about their sexual health after a cognitive screen by telephone20; rating scales for depression, anxiety, and physical function also were included. Results included 41% of men and 35% of women reporting sexual activity at least once a week, and only 21% of men and 24% of women reporting no sexual activity in the previous year. Depressive symptoms were most highly correlated with lack of sexual activity.20
Overall, these studies reveal that positive physical and mental health, access to a healthy partner, and a positive attitude toward sex are correlated with sexual activity in later life, whereas barriers to sexual activity include lack of a healthy sexual partner, depression, and chronic systemic medical illnesses, such as coronary artery disease or type 2 diabetes mellitus.13,17,21-24 Sexual activity and satisfaction have been positively associated with perceived general well-being and self-esteem.25,26 Conversely, sexual difficulties secondary to disease can be a source of distress for couples.27
Possibly overlooked? It is important to note that sexuality itself is a subjective area. Psychological intimacy is a universal phenomenon, and its physical expression may evolve as couples accommodate to age-related bodily changes. Means of achieving physical closeness, other than intercourse (eg, intimate touching, hand holding, kissing, or even acts of caretaking), may not be adequately captured in studies that look specifically at sexual activity.
Taking a sexual history in a geriatric patient
Because sexuality can be an uncomfortable topic for geriatric patients to discuss, sexual problems in this population often go unrecognized. It has been suggested that psychiatrists are more likely to inquire about sexual activity in middle-aged patients than geriatric patients with the same psychiatric presentation—perhaps illustrating a bias against taking a sexual history from a geriatric patient.28 However, because many older patients can experience depression or anxiety disorders in relation to normal sexual changes or sexual dysfunction within the context of their intimate relationships, it is essential to bring these issues to light.
Although a sexual history may not be the focus of a first clinical encounter, consider making such an assessment at a relatively early stage of patient care. The importance of such dialogue is 2-fold:
• It demonstrates to the patient that talking about sexuality in a respectful and empathic manner is appropriate and can encourage patients to communicate more effectively about sexuality with clinicians and with sexual partners.
• It helps elicit medical information needed to make an accurate diagnosis and provide adequate management.
How to begin. As a starting point to taking a sexual history, an open-ended invitation for the geriatric patient to share information may be best, such as “What would you like to tell me about your sexual life?” See further suggestions (Table 1) and examples of more detailed questions to ask once a dialogue has been initiated (Table 2). Additional factors that may contribute to sexual dysfunction are presented in Table 3.1,27,29,30
CASE CONTINUED
In Mr. C’s case, an assessment of his sexual history, including risk factors for sexual dysfunction, is completed. Results from laboratory investigations, including a total testosterone level, are within normal limits.
Mr. C asks about using medications with fewer sexual side effects (he has been taking 3 medications that can contribute to sexual dysfunction). A gradual cross-taper of escitalopram, 20 mg/d, to mirtazapine, 45 mg/d, is implemented, along with tapering pregabalin to 50 mg/d.
Mr. C’s psychiatric and pain symptom improvement is maintained. He notices a boost in his sexual desire but has minimal improvement in erectile dysfunction. He is encouraged to speak with his primary care physician about an antihypertensive agent with less impact on sexual function, as well as therapeutic agents for erectile dysfunction; these, he declines.
At a subsequent visit, Mr. C reports feeling less apprehension about sexual performance. He is now willing to consider further medication options with his primary care physician, and agrees to a recommendation for couples psychotherapy.
As illustrated in Mr. C’s case, the recommended sexual assessment and management strategies to consider at a minimum in psychiatric practice are listed in Table 4.
STI risk in geriatric patients
The risk of sexually transmitted infections (STIs), including human immunodeficiency virus (HIV), often is overlooked in sexually active older adults. Although STIs are more common among younger adults, there is recent evidence of increased incidence in the geriatric population31 (with the highest risk of incident HIV and some STIs in older men who have sex with men32). These increased rates can be explained, at least in part, by:
• older men being less likely to use a condom during sexual activity
• promotion of viral entry in older women through a drier, thinner vaginal wall
• increased longevity of HIV-positive persons.31
Routine STI screening is not warranted in all older adults, but education and prevention strategies in sexually active seniors who are at greater risk of STIs are recommended. Particularly, clinicians should seek opportunities to discuss risk factors and safe sex practices (eg, using condoms, limiting number of sexual partners, practicing good hygiene, engaging in preventive care), and provide behavioral counseling where appropriate.31,33
Additional considerations in geriatric sexuality
Because psychiatric and systemic medical conditions can hinder sexual function, it is essential to identify and manage these conditions. Several neuropsychiatric disorders, including mood and neurocognitive disorders, can not only cause sexual dysfunction, but also can raise ethical dilemmas for clinicians, such as reduced decisional capacity in cognitively impaired patients to consent to sexual activity.1,34
In some patients, psychological, environmental, and pharmacological treatment options may help. A phosphodiesterase type 5 inhibitor for erectile dysfunction can be prescribed by the primary care physician, a psychiatrist, or another specialist, depending on the physician’s expertise and comfort level.
Sequencing of sexual dysfunction. Notably, there is a common paradox in mood disorders. Decreased sexual interest or performance may represent an aspect of anhedonia associated with depression, whereas sexual dysfunction could also result from medication use (particularly that of serotonergic antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine inhibitors), even as other depressive symptoms improve. Therefore, it is critical to analyze sequencing of sexual dysfunction—as part of the presenting mood symptoms or dysfunction induced by antidepressant treatment.
Geriatric sexuality in the digital age. Because older adults represent a rapidly growing segment of digital device users,35 Internet use is likely to play a role in the future of sexuality and “digital intimacy,” in that older adults can engage in online sexual activities. The Internet also can be a tool to access medical education.
Related Resources
• Burghardt KJ, Gardner KN. Sildenafil for SSRI-induced sexual dysfunction. Current Psychiatry. 2013;12(4):29-32,A.
• Maciel M, Laganà L. Older women’s sexual desire problems: biopsychosocial factors impacting them and barriers to their clinical assessment [published online January 5, 2014]. Biomed Res Int. 2014;2014:107217. doi: 10.1155/2014/107217.
Drug Brand Names
Bupropion • Wellbutrin, Zyban Mirtazapine • Remeron
Carbamazepine • Tegretol Oxcarbazepine • Trileptal
Clonidine • Catapres Phenobarbital • Luminal
Donepezil • Aricept Phenytoin • Dilantin
Escitalopram • Lexapro Pregabalin • Lyrica
Gabapentin • Neurontin Ramipril • Altace
Lamotrigine • Lamictal Rivastigmine • Exelon
Lithium • Eskalith, Lithobid Trazodone • Desyrel
Memantine • Namenda Valproic acid • Depakote
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
1. Jagus CE, Benbow SM. Sexuality in older men with mental health problems. Sex Relation Ther. 2002;17(3):271-279.
2. Hillman JL. Clinical perspectives on elderly sexuality. New York, NY: Springer; 2000.
3. DeLamater JD, Sill M. Sexual desire in later life. J Sex Res. 2005;42(2):138-149.
4. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
5. Laufer LR, Gambone JC. Climacteric: menopause and peri-and postmenopause. In: Hacker NF, Gambone JC, Hobel CJ. Hacker and Moore’s essentials of obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders/Elsevier; 2010:379-385.
6. Wilson MM. Menopause. Clin Geriatr Med. 2003;19(3): 483-506.
7. Reid R, Abramson BL, Blake J, et al. Managing menopause. J Obstet Gynaecol Can. 2014;36(9):830-838.
8. Horstman AM, Dillon EL, Urban RJ, et al. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67(11):1140-1152.
9. Wu FC, Tajar A, Pye SR, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
10. Basaria S. Reproductive aging in men. Endocrinol Metab Clin North Am. 2013;42(2):255-270.
11. Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123-135.
12. Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241-4247.
13. Lochlainn MN, Kenny RA. Sexual activity and aging. J Am Med Dir Assoc. 2013;14(8):565-572.
14. McMahon CG. Erectile dysfunction. Intern Med J. 2014;44(1):18-26.
15. Avis NE, Brockwell S, Randolph JF Jr, et al. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women’s Health Across the Nation. Menopause. 2009;16(3):442-452.
16. Perelman M, Shabsigh R, Seftel A, et al. Attitudes of men with erectile dysfunction: a cross-national survey. J Sex Med. 2005;2(3):397-406.
17. Corona G, Rastrelli G, Maseroli E, et al. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab. 2013;27(4):581-601.
18. Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762-774.
19. Lonnèe-Hoffmann RA, Dennerstein L, Lehert P, et al. Sexual function in the late postmenopause: a decade of follow-up in a population-based cohort of Australian women. J Sex Med. 2014;11(8):2029-2038.
20. Wang V, Depp CA, Ceglowski J, et al. Sexual health and function in later life: a population-based study of 606 older adults with a partner. Am J Geriatr Psychiatry. 2015;23(3):227-233.
21. Garrett D. Psychosocial barriers to sexual intimacy for older people. Br J Nurs. 2014;23(6):327-331.
22. DeLamater J, Karraker A. Sexual functioning in older adults. Curr Psychiatry Rep. 2009;11(1):6-11.
23. DeLamater J. Sexual expression in later life: a review and synthesis. J Sex Res. 2012;49(2-3):125-141.
24. Inelmen EM, Sergi G, Girardi A, et al. The importance of sexual health in the elderly: breaking down barriers and taboos. Aging Clin Exp Res. 2012;24(suppl 3):31-34.
25. Choi KB, Jang SH, Lee MY, et al. Sexual life and self-esteem in married elderly. Arch Gerontol Geriatr. 2011;53(1):e17-e20.
26. Davison SL, Bell RJ, LaChina M, et al. The relationship between self-reported sexual satisfaction and general well-being in women. J Sex Med. 2009;6(10):2690-2697.
27. Morley JE, Tariq SH. Sexuality and disease. Clin Geriatr Med. 2003;19(3):563-573.
28. Bouman WP, Arcelus J. Are psychiatrists guilty of “ageism” when it comes to taking a sexual history? Int J Geriatr Psychiatry. 2001;16(1):27-31.
29. La Torre A, Giupponi G, Duffy DM, et al. Sexual dysfunction related to psychotropic drugs: a critical review. Part III: mood stabilizers and anxiolytic drugs. Pharmacopsychiatry. 2014;47(1):1-6.
30. Tucker I. Management of inappropriate sexual behaviors in dementia: a literature review. Int Psychogeriatr. 2010; 22(5):683-692.
31. Imparato T, Sanders D. STD prevalence demands clinical awareness. Aging Well. 2012;5(1):14.
32. Poynten IM, Grulich AE, Templeton DJ. Sexually transmitted infections in older populations. Curr Opin Infect Dis. 2013;26(1):80-85.
33. Talashek ML, Tichy AM, Epping H. Sexually transmitted diseases in the elderly—issues and recommendations. J Gerontol Nurs. 1990;16(4):33-40.
34. Benbow SM, Jagus CE. Sexuality in older women with mental health problems. Sex Relation Ther. 2002;17(3):261-270.
35. Veenhof B, Timusk P. Online activities of Canadian boomers and seniors. http://www.statcan.gc.ca/pub/ 11-008-x/2009002/article/10910-eng.htm#tphp. Accessed March 26, 2015.
Use PRESS to craft a concise psychodynamic formulation
More and more time is being allocated to training psychiatric residents in cognitive-behavioral therapy and crafting a cognitive-behavioral formulation. However, the psychodynamic formulation, once considered the backbone of psychiatry, should not be forgotten. The psychodynamic formulation is a cohesive portrait of an individual’s inner world based on the biopsychosocial approach.1 The purpose in crafting a psychodynamic formulation is to create a succinct and focused case conceptualization that can guide treatment and anticipate possible outcomes.2,3 To teach this in a simple, practical, and relatable way, we propose an approach that can be summarized with the acronym PRESS.
Psychologically minded
Can the patient be introspective and contemplate his (her) thoughts and feelings before acting? Without the capacity to look within—distinct from intelligence—a patient could struggle with the psychodynamic approach and could benefit from a more supportive form of psychotherapy.4
Relationships
Examine the patient’s relationships with others:
• Who are the prominent people in his (her) life?
• What are his interpersonal relations like?
• How does he (she) recall important relationships from the past?
• Do these relationships appear to be recurring?4
Just as themes and patterns recur, so do relationships. Predict how the patient’s relationship pattern could be recreated in the therapeutic dynamic and how this could influence treatment. Then, by examining this transference and countertransference data, you can illustrate a pattern from past relationships that is being recreated in the doctor-patient relationship.3,5
Ego strength
Determining how the patient expresses or inhibits wishes and exhibits impulse control can shed light onto how he operates on a daily basis:
• Does he have the ability to regulate his impulses?
• Is he capable of anticipating the consequences of inappropriate action?
• Does he show a lack of insight and judgment by exhibiting too many repetitive maladaptive behaviors?
Additionally, how does the patient keep unwanted fantasies, wishes, and memories out of conscious awareness?
Identifying which constellation of defense mechanisms the patient is using can help categorize his level of functioning and personality type, and identify anxiety-provoking thoughts and events.1,6 Often, one of these situations has consciously or subconsciously triggered the need for psychotherapy.
Stimulus
The hallmark of any psychodynamic formulation starts with a concise summarizing statement that describes the fundamental details about the patient and his motivation for treatment.2 Determining the patient’s impetus for treatment is 2-fold: Why does the patient want to receive treatment? Why now?
Superego
Review the patient’s ego ideal—what one should not do—and the moral conscience— what one should do.1 Do there seem to be any deficits (recurrent shoplifting, criminality, etc.)? Who contributed to his sense of right and wrong, and how harsh or lax is it? Is the patient self-defeating or self-punishing? Contrarily, does the patient seem to have little conscience?
Acknowledgment
Franklin Maleson, MD, provided advice and input to the authors.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Gabbard GO. Long-term psychodynamic psychotherapy: a basic text. Arlington, VA: American Psychiatric Publishing; 2010.
2. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psychiatry. 1987;144(5):543-550.
3. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry. 2002;159(5):721-726.
4. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington, VA: American Psychiatric Publishing; 2004.
5. Faden J, McFadden RF. The avoidant psychotherapy patient. Current Psychiatry. 2012;11(8):44-47,A.
6. Blackman JS. 101 Defenses: how the mind shields itself. New York, NY: Brunner-Routledge; 2004.
More and more time is being allocated to training psychiatric residents in cognitive-behavioral therapy and crafting a cognitive-behavioral formulation. However, the psychodynamic formulation, once considered the backbone of psychiatry, should not be forgotten. The psychodynamic formulation is a cohesive portrait of an individual’s inner world based on the biopsychosocial approach.1 The purpose in crafting a psychodynamic formulation is to create a succinct and focused case conceptualization that can guide treatment and anticipate possible outcomes.2,3 To teach this in a simple, practical, and relatable way, we propose an approach that can be summarized with the acronym PRESS.
Psychologically minded
Can the patient be introspective and contemplate his (her) thoughts and feelings before acting? Without the capacity to look within—distinct from intelligence—a patient could struggle with the psychodynamic approach and could benefit from a more supportive form of psychotherapy.4
Relationships
Examine the patient’s relationships with others:
• Who are the prominent people in his (her) life?
• What are his interpersonal relations like?
• How does he (she) recall important relationships from the past?
• Do these relationships appear to be recurring?4
Just as themes and patterns recur, so do relationships. Predict how the patient’s relationship pattern could be recreated in the therapeutic dynamic and how this could influence treatment. Then, by examining this transference and countertransference data, you can illustrate a pattern from past relationships that is being recreated in the doctor-patient relationship.3,5
Ego strength
Determining how the patient expresses or inhibits wishes and exhibits impulse control can shed light onto how he operates on a daily basis:
• Does he have the ability to regulate his impulses?
• Is he capable of anticipating the consequences of inappropriate action?
• Does he show a lack of insight and judgment by exhibiting too many repetitive maladaptive behaviors?
Additionally, how does the patient keep unwanted fantasies, wishes, and memories out of conscious awareness?
Identifying which constellation of defense mechanisms the patient is using can help categorize his level of functioning and personality type, and identify anxiety-provoking thoughts and events.1,6 Often, one of these situations has consciously or subconsciously triggered the need for psychotherapy.
Stimulus
The hallmark of any psychodynamic formulation starts with a concise summarizing statement that describes the fundamental details about the patient and his motivation for treatment.2 Determining the patient’s impetus for treatment is 2-fold: Why does the patient want to receive treatment? Why now?
Superego
Review the patient’s ego ideal—what one should not do—and the moral conscience— what one should do.1 Do there seem to be any deficits (recurrent shoplifting, criminality, etc.)? Who contributed to his sense of right and wrong, and how harsh or lax is it? Is the patient self-defeating or self-punishing? Contrarily, does the patient seem to have little conscience?
Acknowledgment
Franklin Maleson, MD, provided advice and input to the authors.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
More and more time is being allocated to training psychiatric residents in cognitive-behavioral therapy and crafting a cognitive-behavioral formulation. However, the psychodynamic formulation, once considered the backbone of psychiatry, should not be forgotten. The psychodynamic formulation is a cohesive portrait of an individual’s inner world based on the biopsychosocial approach.1 The purpose in crafting a psychodynamic formulation is to create a succinct and focused case conceptualization that can guide treatment and anticipate possible outcomes.2,3 To teach this in a simple, practical, and relatable way, we propose an approach that can be summarized with the acronym PRESS.
Psychologically minded
Can the patient be introspective and contemplate his (her) thoughts and feelings before acting? Without the capacity to look within—distinct from intelligence—a patient could struggle with the psychodynamic approach and could benefit from a more supportive form of psychotherapy.4
Relationships
Examine the patient’s relationships with others:
• Who are the prominent people in his (her) life?
• What are his interpersonal relations like?
• How does he (she) recall important relationships from the past?
• Do these relationships appear to be recurring?4
Just as themes and patterns recur, so do relationships. Predict how the patient’s relationship pattern could be recreated in the therapeutic dynamic and how this could influence treatment. Then, by examining this transference and countertransference data, you can illustrate a pattern from past relationships that is being recreated in the doctor-patient relationship.3,5
Ego strength
Determining how the patient expresses or inhibits wishes and exhibits impulse control can shed light onto how he operates on a daily basis:
• Does he have the ability to regulate his impulses?
• Is he capable of anticipating the consequences of inappropriate action?
• Does he show a lack of insight and judgment by exhibiting too many repetitive maladaptive behaviors?
Additionally, how does the patient keep unwanted fantasies, wishes, and memories out of conscious awareness?
Identifying which constellation of defense mechanisms the patient is using can help categorize his level of functioning and personality type, and identify anxiety-provoking thoughts and events.1,6 Often, one of these situations has consciously or subconsciously triggered the need for psychotherapy.
Stimulus
The hallmark of any psychodynamic formulation starts with a concise summarizing statement that describes the fundamental details about the patient and his motivation for treatment.2 Determining the patient’s impetus for treatment is 2-fold: Why does the patient want to receive treatment? Why now?
Superego
Review the patient’s ego ideal—what one should not do—and the moral conscience— what one should do.1 Do there seem to be any deficits (recurrent shoplifting, criminality, etc.)? Who contributed to his sense of right and wrong, and how harsh or lax is it? Is the patient self-defeating or self-punishing? Contrarily, does the patient seem to have little conscience?
Acknowledgment
Franklin Maleson, MD, provided advice and input to the authors.
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. Gabbard GO. Long-term psychodynamic psychotherapy: a basic text. Arlington, VA: American Psychiatric Publishing; 2010.
2. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psychiatry. 1987;144(5):543-550.
3. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry. 2002;159(5):721-726.
4. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington, VA: American Psychiatric Publishing; 2004.
5. Faden J, McFadden RF. The avoidant psychotherapy patient. Current Psychiatry. 2012;11(8):44-47,A.
6. Blackman JS. 101 Defenses: how the mind shields itself. New York, NY: Brunner-Routledge; 2004.
1. Gabbard GO. Long-term psychodynamic psychotherapy: a basic text. Arlington, VA: American Psychiatric Publishing; 2010.
2. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psychiatry. 1987;144(5):543-550.
3. Kassaw K, Gabbard GO. Creating a psychodynamic formulation from a clinical evaluation. Am J Psychiatry. 2002;159(5):721-726.
4. Ursano RJ, Sonnenberg SM, Lazar SG. Concise guide to psychodynamic psychotherapy. Arlington, VA: American Psychiatric Publishing; 2004.
5. Faden J, McFadden RF. The avoidant psychotherapy patient. Current Psychiatry. 2012;11(8):44-47,A.
6. Blackman JS. 101 Defenses: how the mind shields itself. New York, NY: Brunner-Routledge; 2004.
Sleep disorders in patients with cancer
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
Sleep disturbances are common among patients with cancer for many reasons. Sleep problems can be present at any stage during treatment for cancer and in some patients, sleep disturbance may be the presenting symptoms that lead to the diagnosis of some types of cancer. Poor sleep impairs quality of life In people with cancer, but most do not specifically complain of sleep problems unless they are explicitly asked. Insomnia and fatigue are most common sleep disorders in this cohort, although primary sleep disorders, including obstructive sleep apnea and restless legs syndrome, which are common in the general population, have not been carefully studied in the oncology setting despite significant their impairment of quality of life.
Click on the PDF icon at the top of this introduction to read the full article.
disorder
disorder
Research Agenda for Older Patient Care
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.
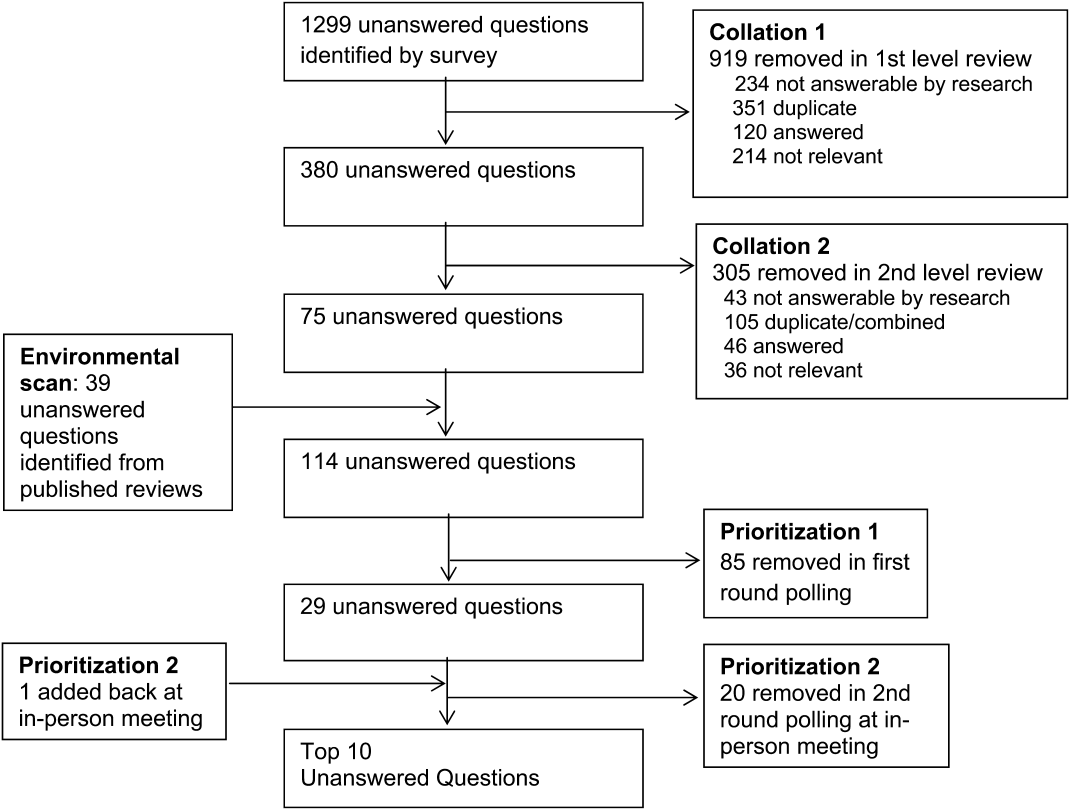
In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Older adults with high levels of medical complexity occupy an increasing fraction of beds in acute‐care hospitals in the United States.[1, 2] By 2007, patients age 65 years and older accounted for nearly half of adult inpatient days of care.[1] These patients are commonly cared for by hospitalists who number more than 40,000.[3] Although hospitalists are most often trained in internal medicine, they have typically received limited formal geriatrics training. Increasingly, access to experts in geriatric medicine is limited.[4] Further, hospitalists and others who practice in acute care are limited by the lack of research to address the needs of the older adult population, specifically in the diagnosis and management of conditions encountered during acute illness.
To better support hospitalists in providing acute inpatient geriatric care, the Society of Hospital Medicine (SHM) partnered with the Association of Specialty Professors to develop a research agenda to bridge this gap. Using methodology from the James Lind Alliance (JLA) and the Patient Centered Outcomes Research Institute (PCORI), the SHM joined with older adult advocacy groups, professional societies of providers, and funders to create a geriatric‐focused acute‐care research agenda, highlighting 10 key research questions.[5, 6, 7] The goal of this approach was to produce and promote high integrity, evidence‐based information that comes from research guided by patients, caregivers, and the broader healthcare community.[8] In this article, we describe the methodology and results of this agenda‐setting process, referred to as the Acute Care of Older Patients (ACOP) Priority Setting Partnership.
METHODS
Overview
This project focused on topic generation, the first step in the PCORI framework for identification and prioritization of research areas.[5] We employed a specific and defined methodology to elicit and prioritize potential research topics incorporating input from representatives of older patients, family caregivers, and healthcare providers.[6]
To elicit this input, we chose a collaborative and consultative approach to stakeholder engagement, drawing heavily from the published work of the JLA, an initiative promoting patient‐clinician partnerships in health research developed in the United Kingdom.[6] We previously described the approach elsewhere.[7]
The ACOP process for determining the research agenda consisted of 4 steps: (1) convene, (2) consult, (3) collate, and (4) prioritize.[6] Through these steps, detailed below, we were able to obtain input from a broad group of stakeholders and engage the stakeholders in a process of reducing and refining our research questions.
Convene
The steering committee (the article's authors) convened a stakeholder partnership group that included stakeholders representing patients and caregivers, advocacy organizations for the elderly, organizations that address diseases and conditions common among hospitalized older patients, provider professional societies (eg, hospitalists, subspecialists, and nurses and social workers), payers, and funders. Patient, caregiver, and advocacy organizations were identified based on their engagement in aging and health policy advocacy by SHM staff and 1 author who had completed a Health and Aging Policy Fellowship (H.L.W.).
The steering committee issued e‐mail invitations to stakeholder organizations, making initial inquiries through professional staff and relevant committee chairs. Second inquiries were made via e‐mail to each organization's volunteer leadership. We developed a webinar that outlined the overall research agenda setting process and distributed the webinar to all stakeholders. The stakeholder organizations were asked to commit to (1) surveying their memberships and (2) participating actively in prioritization by e‐mail and at a 1‐day meeting in Washington DC.
Consult
Each stakeholder organization conducted a survey of its membership via an Internet‐based survey in the summer of 2013 (see Supporting Information, Appendix A, in the online version of this article). Stakeholder organizations were asked to provide up to 75 survey responses each. Though a standard survey was used, the steering committee was not prescriptive in the methodology of survey distribution to accommodate the structure and communication methods of the individual stakeholder organizations. Survey respondents were asked to identify up to 5 unanswered questions relevant to the acute care of older persons and also provide demographic information.
Collate
In the collating process, we clarified and categorized the unanswered questions submitted in the individual surveys. Each question was initially reviewed by a member of the steering committee, using explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Questions that did not meet all 4 criteria were removed. For questions that met all criteria, we clarified language, combined similar questions, and categorized each question. Categories were created in a grounded process, in which individual reviewers assigned categories based on the content of the questions. Each question could be assigned to up to 2 categories. Each question was then reviewed by a second member of the steering committee using the same 4 criteria. As part of this review, similar questions were consolidated, and when possible, questions were rewritten in a standard format.[6]
Finally, the steering committee reviewed previously published research agendas looking for additional relevant unanswered questions, specifically the New Frontiers Research Agenda created by the American Geriatrics Society in conjunction with participating subspecialty societies,[9] the Cochrane Library, and other systematic reviews identified in the literature via PubMed search.[10, 11, 12, 13, 14, 15]
Prioritize
The resulting list of unanswered questions was prioritized in 2 phases. First, the list was e‐mailed to all stakeholder organizations. The organizations were asked to vote on their top 10 priorities from this list using an online ballot, assigning 10 points to their highest priority down to 1 point for their lowest priority. In so doing, they were asked to consider explicit criteria (see Supporting Information, Appendix B, in the online version of this article). Each organization had only 1 ballot and could arrive at their top 10 list in any manner they wished. The balloting from this phase was used to develop a list of unanswered questions for the second round of in‐person prioritization. Each priority's scores were totaled across all voting organizations. The 29 priorities with the highest point totals were brought to the final prioritization round because of a natural cut point at priority number 29, rather than number 30.
For the final prioritization round, the steering committee facilitated an in‐person meeting in Washington, DC in October 2013 using nominal group technique (NGT) methodologies to arrive at consensus.[16] During this process stakeholders were asked to consider additional criteria (see Supporting Information, Appendix B, in the online version of this article).
RESULTS
Table 1 lists the organizations who engaged in 1 or more parts of the topic generation process. Eighteen stakeholder organizations agreed to participate in the convening process. Ten organizations did not respond to our solicitation and 1 declined to participate.
| Organization (N=18) | Consultation % of Survey Responses (N=580) | Prioritization Round 1 | Prioritization Round 2 |
|---|---|---|---|
| Alzheimer's Association | 7.0% | Yes | Yes |
| American Academy of Neurology | 3.4% | Yes | Yes |
| American Association of Retired Persons | 0.8% | No | No |
| American College of Cardiology | 11.4% | Yes | Yes |
| American College of Emergency Physicians | 1.3% | No | No |
| American College of Surgeons | 1.0% | Yes | Yes |
| American Geriatrics Society | 7.6% | Yes | Yes |
| American Hospital Association | 1.7% | Yes | No |
| Centers for Medicare & Medicaid Services | 0.8% | Yes | Yes |
| Gerontological Society of America | 18.9% | Yes | Yes |
| National Alliance for Caregiving | 1.0% | Yes | Yes |
| National Association of Social Workers | 5.9% | Yes | Yes |
| National Coalition for Healthcare | 0.6% | No | No |
| National Institute on Aging | 2.1% | Yes | Yes |
| National Partnership for Women and Families | 0.0% | Yes | Yes |
| Nursing Improving Care for Healthsystem Elders | 28.6% | Yes | No |
| Society of Critical Care Medicine | 12.0% | Yes | Yes |
| Society of Hospital Medicine | 4.6% | Yes | Yes |
Seventeen stakeholder organizations obtained survey responses from a total of 580 individuals (range, 3150 per organization), who were asked to identify important unanswered questions in the acute care of older persons. Survey respondents were typically female (77%), white (85%), aged 45 to 65 years (65%), and identified themselves as health professionals (90%). Twenty‐six percent of respondents also identified as patients or family caregivers. Their surveys included 1299 individual questions.
Figure 1 summarizes our collation and prioritization process and reports the numbers of questions resulting at each stage. Nine hundred nineteen questions were removed during the first review conducted by steering committee members, and 31 question categories were identified. An additional 305 questions were removed in the second review, with 75 questions remaining. As the final step of the collating process, literature review identified 39 relevant questions not already suggested or moved forward through our consultation and collation process. These questions were added to the list of unanswered questions.

In the first round of prioritization, this list of 114 questions was emailed to each stakeholder organization (Table 1). After the stakeholder voting process was completed, 29 unanswered questions remained (see Supporting Information, Appendix C, in the online version of this article). These questions were refined and prioritized in the in‐person meeting to create the final list of 10 questions. The stakeholders present in the meeting represented 13 organizations (Table 1). Using the NGT with several rounds of small group breakouts and large group deliberation, 9 of the top 10 questions were selected from the list of 29. One additional highly relevant question that had been removed earlier in the collation process regarding workforce was added back by the stakeholder group.
This prioritized research agenda appears in Table 2 and below, organized alphabetically by topic.
- Advanced care planning: What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients?
- Care transitions: What is the comparative effectiveness of transitional care models on patient‐centered outcomes for hospitalized older adults?
- Delirium: What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes among hospitalized older adults?
- Dementia: Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient‐centered outcomes?
- Depression: Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes?
- Medications: What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in the hospital and postacute care?
- Models of care: For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes?
- Physical function: What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients?
- Surgery: What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients?
- Training: What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies?
| Topic | Scope of Problem | What Is known | Unanswered Question | Proposed Dimensions |
|---|---|---|---|---|
| ||||
| Advanced‐care planning | Older persons who lack decision‐making capacity often do not have surrogates or clear goals of care documented.[19] Advanced‐care directives are associated with an increase in patient autonomy and empowerment, and although 15% to 25% of adults completed the documentation in 2004,[20] a recent study found completion rates have increased to 72%.[21] | Nursing home residents with advanced directives are less likely to be hospitalized.[22, 23] Advanced directive tools, such as POLST, work to translate patient preferences to medical order.[24] standardized patient transfer tools may help to improve transitions between nursing homes and hospitals.[25] However, advanced care planning fails to integrate into courses of care if providers are unwilling or unskilled in using advanced care documentation.[26] | What approaches for determining and communicating goals of care across and within healthcare settings are most effective in promoting goal‐concordant care for hospitalized older patients? | Potential interventions: |
| Decision aids | ||||
| Standard interdisciplinary advanced care planning approach | ||||
| Patient advocates | ||||
| Potential outcomes might include: | ||||
| Completion of advanced directives and healthcare power of attorney | ||||
| Patient‐centered outcomesa | ||||
| Care transitions | Hospital readmission from home and skilled nursing facilities occurs within 30 days in up to a quarter of patients.[27, 28] The discharge of complex older hospitalized patients is fraught with challenges. The quality of the hospital discharge process can influence outcomes for vulnerable older patients.[29, 30, 31, 32] Studies measuring the quality of hospital discharge frequently find deficits in documentation of assessment of geriatric syndromes,[33] poor patient/caregiver understanding,[34, 35] and poor communication and follow‐up with postacute providers.[35, 36, 37, 38] | As many as 10 separate domains may influence the success of a discharge.[39] There is limited evidence, regarding quality‐of‐care transitions for hospitalized older patients. The Coordinated‐Transitional Care Program found that follow‐up with telecommunication decreased readmission rates and improved transitional care for a high‐risk condition veteran population.[40] There is modest evidence for single interventions,[41] whereas the most effective hospital‐to‐community care interventions address multiple processes in nongeriatric populations.[39, 42, 43] | What is the comparative effectiveness of the transitional care models on patient‐centered outcomes for hospitalized older adults? | Possible models: |
| Established vs novel care‐transition models | ||||
| Disease‐specific vs general approaches | ||||
| Accountable care models | ||||
| Caregiver and family engagement | ||||
| Community engagement | ||||
| Populations of interest: | ||||
| Patients with dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Patients with psychiatric disease | ||||
| Racially and ethnically diverse patients | ||||
| Outcomes: | ||||
| Readmission | ||||
| Other adverse events | ||||
| Cost and healthcare utilization | ||||
| Patient‐centered outcomesa | ||||
| Delirium | Among older inpatients, the prevalence of delirium varies with severity of illness. Among general medical patients, in‐hospital prevalence ranges from 10% to 25 %.[44, 45] In the ICU, prevalence estimates are higher, ranging from 25% to as high as 80%.[46, 47] Delirium independently predicts increased length of stay,[48, 49] long‐term cognitive impairment,[50, 51] functional decline,[51] institutionalization,[52] and short‐ and long‐term mortality.[52, 53, 54] | Multicomponent strategies have been shown to be effective in preventing delirium. A systematic review of 19 such interventions identified the most commonly included such as[55]: early mobilization, nutrition supplements, medication review, pain management, sleep enhancement, vision/hearing protocols, and specialized geriatric care. Studies have included general medical patients, postoperative patients, and patients in the ICU. The majority of these studies found reductions in either delirium incidence (including postoperative), delirium prevalence, or delirium duration. Although medications have not been effective in treating delirium in general medical patients,[48] the choice and dose of sedative agents has been shown to impact delirium in the ICU.[56, 57, 58] | What practices are most effective for consistent recognition, prevention, and treatment of delirium subtypes (hypoactive, hyperactive, and mixed) among hospitalized older adults? | Outcomes to examine: |
| Delirium incidence (including postoperative) | ||||
| Delirium duration | ||||
| Delirium‐/coma‐free days | ||||
| Delirium prevalence at discharge | ||||
| Subsyndromal delirium | ||||
| Potential prevention and treatment modalities: | ||||
| Family education or psychosocial interventions | ||||
| Pharmacologic interventions | ||||
| Environmental modifications | ||||
| Possible areas of focus: | ||||
| Special populations | ||||
| Patients with varying stages of dementia | ||||
| Patients with multimorbidity | ||||
| Patients with geriatric syndromes | ||||
| Observation patients | ||||
| Diverse settings | ||||
| Emergency department | ||||
| Perioperative | ||||
| Skilled nursing/rehab/long‐term acute‐care facilities | ||||
| Dementia | 13% to 63% of older persons in the hospital have dementia.[59] Dementia is often unrecognized among hospitalized patients.[60] The presence of dementia is associated with a more rapid functional decline during admission and delayed hospital discharge.[59] Patients with dementia require more nursing hours, and are more likely to have complications[61] or die in care homes rather than in their preferred site.[59] | Several tools have been validated to screen for dementia in the hospital setting.[62] Studies have assessed approaches to diagnosing delirium in hospitalized patients with dementia.[63] Cognitive and functional stimulation interventions may have a positive impact on reducing behavioral issues.[64, 65] | Does universal assessment of hospitalized older adults for cognitive impairment (eg, at presentation and/or discharge) lead to more appropriate application of geriatric care principles and improve patient centered outcomes? | Potential interventions: |
| Dementia or delirium care | ||||
| Patient/family communication and engagement strategies | ||||
| Maintenance/recovery of independent functional status | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Length of stay, cost, and healthcare utilization (including palliative care) | ||||
| Immediate invasive vs early conservative treatments pursued | ||||
| Depression | Depression is a common geriatric syndrome among acutely ill older patients, occurring in up to 45% of patients.[66, 67] Rates of depression are similar among patients discharged following a critical illness, with somatic, rather than cognitive‐affective complaints being the most prevalent.[68] Depression among inpatients or immediately following hospitalization independently predicts worse functional outcomes,[69] cognitive decline,[70] hospital readmission,[71, 72] and long‐term mortality.[69, 73] Finally, geriatric patients are known to respond differently to medical treatment.[74, 75] | Although highly prevalent, depression is poorly recognized and managed in the inpatient setting. Depression is recognized in only 50% of patients, with previously undiagnosed or untreated depression being at highest risk for being missed.[76] The role of treatment of depression in the inpatient setting is poorly understood, particularly for those with newly recognized depression or depressive symptoms. Some novel collaborative care and telephone outreach programs have led to increases in depression treatment in patients with specific medical and surgical conditions, resulting in early promising mental health and comorbid outcomes.[77, 78] The efficacy of such programs for older patients is unknown. | Does identifying depressive symptoms during a hospital stay and initiating a therapeutic plan prior to discharge improve patient‐centered and/or disease‐specific outcomes? | Possible areas of focus: |
| Comprehensive geriatric and psychosocial assessment; | ||||
| Inpatient vs outpatient initiation of pharmacological therapy | ||||
| Integration of confusion assessment method into therapeutic approaches | ||||
| Linkages with outpatient mental health resources | ||||
| Medications | Medication exposure, particularly potentially inappropriate medications, is common in hospitalized elders.[79] Medication errorsof dosage, type, and discrepancy between what a patient takes at home and what is known to his/her prescribing physicianare common and adversely affects patient safety.[80] Geriatric populations are disproportionately affected, especially those taking more than 5 prescription medications per day.[81] | Numerous strategies including electronic alerts, screening protocols, and potentially inappropriate medication lists (Beers list, STOPP) exist, though the optimal strategies to limit the use of potentially inappropriate medications is not yet known.[82, 83, 84] | What systems interventions improve medication management for older adults (ie, appropriateness of medication choices and dosing, compliance, cost) in hospital and post‐acute care? | Possible areas of focus: |
| Use of healthcare information technology | ||||
| Communication across sites of care | ||||
| Reducing medication‐related adverse events | ||||
| Engagement of family caregivers | ||||
| Patient‐centered strategies to simplify regimens | ||||
| Models of care | Hospitalization marks a time of high risk for older patients. Up to half die during hospitalization or within the year following the hospitalization. There is high risk of nosocomial events, and more than a third experience a decline in health resulting in longer hospitalizations and/or placement in extended‐care facilities.[73, 85, 86] | Comprehensive inpatient care for older adults (acute care for elders units, geriatric evaluation and management units, geriatric consultation services) were studied in 2 meta‐analyses, 5 RCTs, and 1 quasiexperimental study and summarized in a systematic review.[87] The studies reported improved quality of care (1 of 1 article), quality of life (3 of 4), functional autonomy (5 of 6), survival (3 of 6), and equal or lower healthcare utilization (7 of 8). | For which populations of hospitalized older adults does systematic implementation of geriatric care principles/processes improve patient‐centered outcomes? | Potential populations: |
| Patients of the emergency department, critical care, perioperative, and targeted medical/surgical units | ||||
| Examples of care principles: | ||||
| Geriatric assessment, early mobility, medication management, delirium prevention, advanced‐care planning, risk‐factor modification, caregiver engagement | ||||
| Potential outcomes: | ||||
| Patient‐centered outcomesa | ||||
| Cost | ||||
| Physical function | Half of older patients will lose functional capacity during hospitalization.[88] Loss of physical function, particularly of lower extremities, is a risk factor for nursing home placement.[89, 90] Older hospitalized patients spend the majority (up to 80%) of their time lying in bed, even when they are capable of walking independently.[91] | Loss of independences with ADL capabilities is associated with longer hospital stays, higher readmission rates, and higher mortality risk.[92] Excessive time in bed during a hospital stay is also associated with falls.[93] Often, hospital nursing protocols and physician orders increase in‐hospital immobility in patients.[91, 94] However, nursing‐driven mobility protocols can improve functional outcomes of older hospitalized patients.[95, 96] | What is the comparative effectiveness of interventions that promote in‐hospital mobility, improve and preserve physical function, and reduce falls among older hospitalized patients? | Potential interventions: |
| Intensive physical therapy | ||||
| Incidental functional training | ||||
| Restraint reduction | ||||
| Medication management | ||||
| Potential outcomes: | ||||
| Discharge location | ||||
| Delirium, pressure ulcers, and falls | ||||
| Surgery | An increasing number of persons over age 65 years are undergoing surgical procedures.[97] These persons are at increased risk for developing delirium/cogitative dysfunction,[98] loss of functional status,[99] and exacerbations of chronic illness.[97] Additionally, pain management may be harder to address in this population.[100] Current outcomes may not reflect the clinical needs of elder surgical patients.[101] | Tailored drug selection and nursing protocols may prevent delirium.[98] Postoperative cognitive dysfunction may require weeks for resolution. Identifying frail patients preoperatively may lead to more appropriate risk stratification and improved surgical outcomes.[99] Pain management strategies focused on mitigating cognitive impact and other effects may also be beneficial.[100] Development of risk‐adjustment tools specific to older populations, as well as measures of frailty and patient‐centered care, have been proposed.[101] | What perioperative strategies can be used to optimize care processes and improve outcomes in older surgical patients? | Potential strategies: |
| Preoperative risk assessment and optimization for frail or multimorbid older patients | ||||
| Perioperative management protocols for frail or multimorbid older patients | ||||
| Potential outcomes: | ||||
| Postoperative patient centered outcomesa | ||||
| Perioperative cost, healthcare utilization | ||||
| Training | Adults over age 65 years comprise 13.2 % of the US population, but account for >30% of hospital discharges and 50% of hospital days.[86, 102, 103] By 2030, there will only be 1 geriatrician for every 3798 Americans >75 years.[4] Between 1997 and 2006, the odds that a hospitalist would treat a hospitalized Medicare patient rose 29% per year.[3] | Train the trainer programs for physicians include the CHAMP, the AGESP, and the PAGE. Education for nurses include the NICHE. Outcomes include improved self‐confidence, attitudes, teaching skills, and geriatric care environment.[104, 105, 106] | What is the most effective approach to training hospital‐based providers in geriatric and palliative care competencies? | Potential interventions: |
| Mentored implementation | ||||
| Train the trainer | ||||
| Technical support | ||||
Table 2 also contains a capsule summary of the scope of the problem addressed by each research priority, a capsule summary of related work in the content area (what is known) not intended as a systematic review, and proposed dimensions or subquestions suggested by the stakeholders at the final prioritization meeting
DISCUSSION
Older hospitalized patients account for an increasing number and proportion of hospitalized patients,[1, 2] and hospitalists increasingly are responsible for inpatient care for this population.[3] The knowledge required for hospitalists to deliver optimal care and improve outcomes has not kept pace with the rapid growth of either hospitalists or hospitalized elders. Through a rigorous prioritization process, we identified 10 areas that deserve the highest priority in directing future research efforts to improve care for the older hospitalized patient. Assessment, prevention, and treatment of geriatric syndromes in the hospital account for almost half of the priority areas. Additional research is needed to improve advanced care planning, develop new care models, and develop training models for future hospitalists competent in geriatric and palliative care competencies.
A decade ago, the American Geriatric Society and the John A. Hartford Foundation embarked upon a research agenda aimed at improving the care of hospitalized elders cared for by specialists (ie, New Frontiers in Geriatrics Research: An Agenda for Surgical and Related Medical Specialties).[9] This effort differed in many important ways from the current priortization process. First, the New Frontiers agenda focused upon specific diseases, whereas the ACOP agenda addresses geriatric syndromes that cut across multiple diseases. Second, the New Frontiers agenda was made by researchers and based upon published literature, whereas the ACOP agenda involved the input of multiple stakeholders. Finally, the New Frontiers prioritized a research agenda across a number of surgical specialties, emergency medicine, and geriatric rehabilitation. Hospital medicine, however, was still early in its development and was not considered a unique specialty. Since that time, hospital medicine has matured into a unique specialty, with increased numbers of hospitalists,[3] increased research in hospital medicine,[17] and a separate recertification pathway for internal medicine licensure.[18] To date, there has not been a similar effort performed to direct geriatric research efforts for hospital medicine.
For researchers working in the field of hospital medicine, this list of topics has several implications. First, as hospitalists are commonly generalists, hospitalist researchers may be particularly well‐suited to study syndromes that cut across specialties. However, this does raise concerns about funding sources, as most National Institutes of Health institutes are disease‐focused. Funders that are not disease‐focused such as PCORI, National Institute on Aging, National Institute of Nursing Research, and Agency for Healthcare Research and Quality, and private foundations (Hartford, Robert Wood Johnson, and Commonwealth) may be more fruitful sources of funding for this work, but funding may be challenging. Nonetheless, the increased focus on patient‐centered work may increase funders' interest in such work. Second, the topics on this list would suggest that interventions will not be pharmacologic, but will focus on nonpharmacologic, behavioral, and social interventions. Similarly, outcomes of interest must expand beyond utilization metrics such as length of stay and mortality, to include functional status and symptom management, and goal‐concordant care. Therefore, research in geriatric acute care will necessarily be multidisciplinary.
Although these 10 high‐priority areas have been selected, this prioritized list is inherently limited by our methodology. First, our survey question was not focused on a disease state, and this wording may have resulted in the list favoring geriatric syndromes rather than common disease processes. Additionally, the resulting questions encompass large research areas and not specific questions about discrete interventions. Our results may also have been skewed by the types of engaged respondents who participated in the consultation, collating, and prioritization phases. In particular, we had a large response from geriatric medicine nurses, whereas some stakeholder groups provided no survey responses. Thus, these respondents were not representative of all possible stakeholders, nor were the survey respondents necessarily representative of each of their organizations. Nonetheless, the participants self‐identified as representative of diverse viewpoints that included patients, caregivers, and advocacy groups, with the majority of stakeholder organizations remaining engaged through the completion of the process. Thus, the general nature of this agenda helps us focus upon larger areas of importance, leaving researchers the flexibility to choose to narrow the focus on a specific research question that may include potential interventions and unique outcomes. Finally, our methodology may have inadvertently limited the number of patient and family caregiver voices in the process given our approach to large advocacy groups, our desire to be inclusive of healthcare professional organizations, and our survey methodology. Other methodologies may have reached more patients and caregivers, yet many healthcare professionals have served as family caregivers to frail elders requiring hospitalization and may have been in an ideal position to answer the survey.
In conclusion, several forces are shaping the future of acute inpatient care. These include the changing demographics of the hospitalized patient population, a rapid increase in the proportion of multimorbid hospitalized older adults, an inpatient workforce (hospitalists, generalists, and subspecialists) with potentially limited geriatrics training, and gaps in evidence‐based guidance to inform diagnostic and therapeutic decision making for acutely ill older patients. Training programs in hospital medicine should be aware of and could benefit from the resulting list of unanswered questions. Our findings also have implications for training to enrich education in geriatrics. Moreover, there is growing recognition that patients and other stakeholders deserve a greater voice in determining the direction of research. In addition to efforts to improve patient‐centeredness of research, these areas have been uniquely identified by stakeholders as important, and therefore are in line with newer priorities of PCORI. This project followed a road map resulting in a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine.[7] In creating this agenda, we relied heavily on the framework proposed by PCORI. We propose to pursue a dissemination and evaluation strategy for this research agenda as well as additional prioritization steps. We believe the adoption of this methodology will create a knowledge base that is rigorously derived and most relevant to the care of hospitalized older adults and their families. Its application will ultimately result in improved outcomes for hospitalized older adults.
Acknowledgements
The authors acknowledge Claudia Stahl, Society of Hospital Medicine; Cynthia Drake, University of Colorado; and the ACOP stakeholder organizations.
Disclosures: This work was supported by the Association of Specialty Professors/American Society of Internal Medicine and the John A. Hartford Foundation. Dr. Vasilevskis was supported by the National Institute on Aging of the National Institutes of Health under award number K23AG040157 and the Veterans Affairs Clinical Research Center of Excellence, and the Geriatric Research, Education and Clinical Center (GRECC). Dr. Vasilevskis' institution receives grant funding for an aspect of submitted work. Dr. Meltzer is a PCORI Methodology Committee member. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
- , , , , . National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010(29):1–20, 24.
- Centers for Medicare 2012. Available at: http://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Chronic‐Conditions/Downloads/2012Chartbook.pdf. Accessed December 12, 2014.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . A revelation of numbers: will America's eldercare workforce be ready to care for an aging America? Generations. 2010;34(4):11–19.
- Patient‐Centered Outcomes Research Institute Methodology Committee. The PCORI methodology report. Available at: http://www.pcori.org/assets/2013/11/PCORI‐Methodology‐Report.pdf. Published November 2013. Accessed December 19, 2013.
- The James Lind Alliance. JLA method. Available at: http://www.lindalliance.org/JLA_Method.asp. Accessed December 19, 2013.
- , , , , . Road map to a patient‐centered research agenda at the intersection of hospital medicine and geriatric medicine. J Gen Intern Med. 2014;29(6):926–931.
- Patient‐Centered Outcomes Research Institute. About us. Available at: http://www.pcori.org/about‐us. Accessed February 23, 2015.
- , , . New Frontiers of Geriatrics Research: An Agenda for Surgical and Related Medical Specialties. New York, NY: American Geriatrics Society; 2004.
- , , , . Linking the NIH strategic plan to the research agenda for social workers in health and aging. J Gerontol Soc Work. 2010;53(1):77–93.
- , . Assessing the capacity to make everyday decisions: a guide for clinicians and an agenda for future research. Am J Geriatr Psychiatry. 2007;15(2):101–111.
- , , , et al. Practitioners' views on elder mistreatment research priorities: recommendations from a Research‐to‐Practice Consensus conference. J Elder Abuse Negl. 2011;23(2):115–126.
- , . The intersection between geriatrics and palliative care: a call for a new research agenda. J Am Geriatr Soc. 2005;53(9):1593–1598.
- . The cancer aging interface: a research agenda. J Clin Oncol. 2007;25(14):1945–1948.
- , , , . Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc. 2012;60(9):1742–1748.
- , . A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–492.
- , , , , . Research and publication trends in hospital medicine. J Hosp Med. 2014;9(3):148–154.
- . ABFM and ABIM to jointly participate in recognition of focused practice (rfp) in hospital medicine pilot approved by abms. Ann Fam Med. 2010;8(1):87.
- , , , . Medical decision‐making for older adults without family. J Am Geriatr Soc. 2012;60(11):2144–2150.
- , , , , , . Promoting advance directives among elderly primary care patients. J Gen Intern Med. 2004;19(9):944–951.
- , , . Advance directive completion by elderly Americans: a decade of change. J Am Geriatr Soc. 2014;62(4):706–710.
- , . Care transitions by older adults from nursing homes to hospitals: implications for long‐term care practice, geriatrics education, and research. J Am Med Dir Assoc. 2010;11(4):231–238.
- , , , . Decisions to hospitalize nursing home residents dying with advanced dementia. J Am Geriatr Soc. 2005;53(8):1396–1401.
- , , , , , . A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life‐sustaining treatment program. J Am Geriatr Soc. 2010;58(7):1241–1248.
- , , , , . Interventions to improve transitional care between nursing homes and hospitals: a systematic review. J Am Geriatr Soc. 2010;58(4):777–782.
- , , . Opening end‐of‐life discussions: how to introduce Voicing My CHOiCES, an advance care planning guide for adolescents and young adults [published online ahead of print March 13, 2014]. Palliat Support Care. doi: 10.1017/S1478951514000054.
- , , , . The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood). 2010;29(1):57–64.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Predictors of rehospitalization among elderly patients admitted to a rehabilitation hospital: the role of polypharmacy, functional status, and length of stay. J Am Med Dir Assoc. 2013;14(10):761–767.
- , , , et al. Mobility after hospital discharge as a marker for 30‐day readmission. J Gerontol A Biol Sci Med Sci. 2013;68(7):805–810.
- , , , , . The association between the quality of inpatient care and early readmission: a meta‐analysis of the evidence. Med Care. 1997;35(10):1044–1059.
- , , , , , . Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. May 2014;9(5):277–282.
- , , , , , . A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59(11):2001–2008.
- , , , et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498.
- , , , et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385–391.
- , , , et al. Communication and information deficits in patients discharged to rehabilitation facilities: an evaluation of five acute care hospitals. J Hosp Med. 2009;4(8):E28–E33.
- , , , , , . The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;61(7):1095–1102.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , et al. Low‐cost transitional care with nurse managers making mostly phone contact with patients cut rehospitalization at a VA hospital. Health Aff (Millwood). 2012;31(12):2659–2668.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- , , , , , . Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):433–440.
- , , , . Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13(4):234–242.
- , , , . Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73.
- , , , et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM‐ICU). JAMA. 2001;286(21):2703–2710.
- , , , et al. Impact and recognition of cognitive impairment among hospitalized elders. J Hosp Med. 2010;5(2):69–75.
- , , , et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900.
- , , . Long‐term cognitive impairment after critical illness. N Engl J Med. 2014;370(2):185–186.
- , , , et al. Delirium in the ICU and subsequent long‐term disability among survivors of mechanical ventilation. Crit Care Med. 2014;42(2):369–377.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762.
- , , , , , . Days of delirium are associated with 1‐year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097.
- , . In‐facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):375–380.
- , , , et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499.
- , , , et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653.
- , , , , , . Protocolized intensive care unit management of analgesia, sedation, and delirium improves analgesia and subsyndromal delirium rates. Anesth Analg. 2010;111(2):451–463.
- , . A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23(3):344–355.
- , , , . Cognitive impairment is undetected in medical inpatients: a study of mortality and recognition amongst healthcare professionals. BMC Geriatr. 2012;12:47.
- , , . How can we keep patients with dementia safe in our acute hospitals? A review of challenges and solutions. J R Soc Med. 2013;106(9):355–361.
- , , . Screening for dementia in general hospital inpatients: a systematic review and meta‐analysis of available instruments. Age Ageing. 2013;42(6):689–695.
- , , , et al. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60(11):2005–2013.
- , , , , , . Functional analysis‐based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2:CD006929.
- , , , . Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
- , , , et al. The prevalence and correlates of major and minor depression in older medical inpatients. J Am Geriatr Soc. 2005;53(8):1344–1353.
- , , , , , . Major depressive disorder in hospitalized medically ill patients: an examination of young and elderly male veterans. J Am Geriatr Soc. 1991;39(9):881–890.
- , , , et al. Depression, post‐traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN‐ICU study: a longitudinal cohort study. Lancet Respir Med. 2014;2(5):369–379.
- , , , et al. Depressive symptoms after hospitalization in older adults: function and mortality outcomes. J Am Geriatr Soc. 2012;60(12):2254–2262.
- , , , , . 12‐month cognitive outcomes of major and minor depression in older medical patients. Am J Geriatr Psychiatry. 2008;16(9):742–751.
- , , , et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256–262.
- , , , , , . Dose‐response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358–364.
- , , , , , . Depressive symptoms and 3‐year mortality in older hospitalized medical patients. Ann Intern Med. 1999;130(7):563–569.
- , , , et al. Support for the vascular depression hypothesis in late‐life depression: results of a 2‐site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285.
- , , , , , . Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210.
- , , , , . Recognition of depression in older medical inpatients. J Gen Intern Med. 2007;22(5):559–564.
- , , , , , . A collaborative care depression management program for cardiac inpatients: depression characteristics and in‐hospital outcomes. Psychosomatics. 2011;52(1):26–33.
- , , , , , . Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;4(2):198–205.
- , , , et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3(2):91–102.
- , , , , , . Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32(5):379–389.
- , . Minimizing adverse drug events in older patients. Am Fam Physician. 2007;76(12):1837–1844.
- , , , , . STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72–83.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616–631.
- , , , . Preventing potentially inappropriate medication use in hospitalized older patients with a computerized provider order entry warning system. Arch Intern Med. 2010;170(15):1331–1336.
- . Hazards of Hospitalization of the Elderly. Ann Intern Med. 1993;118(3):219–223.
- . Improving health care for older persons. Ann Intern Med. 2003;139(5 part 2):421–424.
- , , , , , . Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine's "retooling for an aging America" report. J Am Geriatr Soc. 2009;57(12):2328–2337.
- , , , et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179.
- , , , . Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993;48(3):S94–S101.
- , , . Risk factors for nursing home placement in a population‐based dementia cohort. J Am Geriatr Soc. 2000;48(5):519–525.
- , , , . The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665.
- , , , , . A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57.
- . Immobility and falls. Clin Geriatr Med. 1998;14(4):699–726.
- , , . Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52(8):1263–1270.
- , , . Impact of a nurse‐driven mobility protocol on functional decline in hospitalized older adults. J Nurs Care Qual. 2009;24(4):325–331.
- , . Impact of early mobilization protocol on the medical‐surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87–94.
- , , , . The aging population and its impact on the surgery workforce. Ann Surg. 2003;238(2):170–177.
- . Perioperative care of the elderly patient: an update. Cleve Clin J Med. 2009;76(suppl 4):S16–S21.
- , , . Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142–147.
- . The assessment and management of peri‐operative pain in older adults. Anaesthesia. 2014;69(suppl 1):54–60.
- , . National Research Strategies: what outcomes are important in peri‐operative elderly care? Anaesthesia. 2014;69(suppl 1):61–69.
- , . 2002 National Hospital Discharge Survey. Adv Data. 2004;342:1–30.
- , . Hospitalization in the United States, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- , , , et al. The Curriculum for the Hospitalized Aging Medical Patient program: a collaborative faculty development program for hospitalists, general internists, and geriatricians. J Hosp Med. 2008;3(5):384–393.
- , . Advancement of geriatrics education. J Hosp Med. 2011;6(6):370.
- , , , , , . Advancing geriatrics education: an efficient faculty development program for academic hospitalists increases geriatric teaching. J Hosp Med. 2010;5(9):541–546.
Alcoholic hepatitis: Challenges in diagnosis and management
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
Alcoholic hepatitis, a severe manifestation of alcoholic liver disease, is rising in incidence. Complete abstinence from alcohol remains the cornerstone of treatment, while other specific interventions aim to decrease short-term mortality rates.
Despite current treatments, about 25% of patients with severe alcoholic hepatitis eventually die of it. For those who survive hospitalization, measures need to be taken to prevent recidivism. Although liver transplantation seems to hold promise, early transplantation is still largely experimental in alcoholic hepatitis and will likely be available to only a small subset of patients, especially in view of ethical issues and the possible wider implications for transplant centers.
New treatments will largely depend on a better understanding of the disease’s pathophysiology, and future clinical trials should evaluate therapies that improve short-term as well as long-term outcomes.
ACUTE HEPATIC DECOMPENSATION IN A HEAVY DRINKER
Excessive alcohol consumption is very common worldwide, is a major risk factor for liver disease, and is a leading cause of preventable death. Alcoholic cirrhosis is the eighth most common cause of death in the United States and in 2010 was responsible for nearly half of cirrhosis-related deaths worldwide.1
Alcoholic liver disease is a spectrum. Nearly all heavy drinkers (ie, those consuming 40 g or more of alcohol per day, Table 1) have fatty liver changes, 20% to 40% develop fibrosis, 10% to 20% progress to cirrhosis, and of those with cirrhosis, 1% to 2% are diagnosed with hepatocellular carcinoma every year.2
Within this spectrum, alcoholic hepatitis is a well-defined clinical syndrome characterized by acute hepatic decompensation that typically results from long-standing alcohol abuse. Binge drinkers may also be at risk for alcoholic hepatitis, but good data on the association between drinking patterns and the risk of alcoholic hepatitis are limited.
Alcoholic hepatitis varies in severity from mild to life-threatening.3 Although its exact incidence is unknown, its prevalence in alcoholics has been estimated at 20%.4 Nearly half of patients with alcoholic hepatitis have cirrhosis at the time of their acute presentation, and these patients generally have a poor prognosis, with a 28-day death rate as high as 50% in severe cases.5,6 Moreover, although alcoholic hepatitis develops in only a subset of patients with alcoholic liver disease, hospitalizations for it are increasing in the United States.7
Women are at higher risk of developing alcoholic hepatitis, an observation attributed to the effect of estrogens on oxidative stress and inflammation, lower gastric alcohol dehydrogenase levels resulting in slower first-pass metabolism of alcohol, and higher body fat content causing a lower volume of distribution for alcohol than in men.8 The incidence of alcoholic hepatitis is also influenced by a number of demographic and genetic factors as well as nutritional status and coexistence of other liver diseases.9 Most patients diagnosed with alcoholic hepatitis are active drinkers, but it can develop even after significantly reducing or stopping alcohol consumption.
FATTY ACIDS, ENZYMES, CYTOKINES, INFLAMMATION
Alcohol consumption induces fatty acid synthesis and inhibits fatty acid oxidation, thereby promoting fat deposition in the liver.
The major enzymes involved in alcohol metabolism are cytochrome P450 2E1 (CYP2E1) and alcohol dehydrogenase. CYP2E1 is inducible and is up-regulated when excess alcohol is ingested, while alcohol dehydrogen-
ase function is relatively stable. Oxidative degradation of alcohol by these enzymes generates reactive oxygen species and acetaldehyde, inducing liver injury.10 Interestingly, it has been proposed that variations in the genes for these enzymes influence alcohol consumption and dependency as well as alcohol-driven tissue damage.
In addition, alcohol disrupts the intestinal mucosal barrier, allowing lipopolysaccharides from gram-negative bacteria to travel to the liver via the portal vein. These lipopolysaccharides then bind to and activate sinusoidal Kupffer cells, leading to production of several cytokines such as tumor necrosis factor alpha, interleukin 1, and transforming growth factor beta. These cytokines promote hepatocyte inflammation, apoptosis, and necrosis (Figure 1).11
Besides activating the innate immune system, the reactive oxygen species resulting from alcohol metabolism interact with cellular components, leading to production of protein adducts. These act as antigens that activate the adaptive immune response, followed by B- and T-lymphocyte infiltration, which in turn contribute to liver injury and inflammation.12
THE DIAGNOSIS IS MAINLY CLINICAL
The diagnosis of alcoholic hepatitis is mainly clinical. In its usual presentation, jaundice develops rapidly in a person with a known history of heavy alcohol use. Other symptoms and signs may include ascites, encephalopathy, and fever. On examination, the liver may be enlarged and tender, and a hepatic bruit has been reported.13
Other classic signs of liver disease such as parotid enlargement, Dupuytren contracture, dilated abdominal wall veins, and spider nevi can be present, but none is highly specific or sensitive for alcoholic hepatitis.
Elevated liver enzymes and other clues
Laboratory tests are important in evaluating potential alcoholic hepatitis, although no single laboratory marker can definitively establish alcohol as the cause of liver disease. To detect alcohol consumption, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), mean corpuscular volume, carbohydrate-deficient transferrin, and, more commonly, gamma-glutamyl transpeptidase are used.
In the acute setting, typical biochemical derangements in alcoholic hepatitis include elevated AST (up to 2 to 6 times the upper limit of normal; usually less than 300 IU/L) and elevated ALT to a lesser extent,14 with an AST-to-ALT ratio greater than 2. Neutrophilia, anemia, hyperbilirubinemia, and coagulopathy with an elevated international normalized ratio are common.
Patients with alcoholic hepatitis are also prone to develop bacterial infections, and about 7% develop hepatorenal syndrome, itself an ominous sign.15
Imaging studies are valuable in excluding other causes of abnormal liver test results in patients who abuse alcohol, such as biliary obstruction, infiltrative liver diseases, and hepatocellular carcinoma.
Screen for alcohol intake
During the initial evaluation of suspected alcoholic hepatitis, one should screen for excessive drinking. In a US Centers for Disease Control and Prevention study, only one of six US adults, including binge drinkers, said they had ever discussed alcohol consumption with a health professional.16 Many patients with alcoholic liver disease in general and alcoholic hepatitis in particular deny alcohol abuse or underreport their intake.17
Screening tests such as the CAGE questionnaire and the Alcohol Use Disorders Identification Test can be used to assess alcohol dependence or abuse.18,19 The CAGE questionnaire consists of four questions:
- Have you ever felt you should cut down on your drinking?
- Have people annoyed you by criticizing your drinking?
- Have you ever felt guilty about your drinking?
- Have you ever had a drink first thing in the morning (an eye-opener) to steady your nerves or to get rid of a hangover?
A yes answer to two or more questions is considered clinically significant.
Is liver biopsy always needed?
Although alcoholic hepatitis can be suspected on the basis of clinical and biochemical clues, liver biopsy remains the gold standard diagnostic tool. It confirms the clinical diagnosis of alcoholic hepatitis in about 85% of all patients and in up to 95% when significant hyperbilirubinemia is present.20
However, whether a particular patient needs a biopsy is not always clear. The American Association for the Study of Liver Diseases (AASLD) recommends biopsy in patients who have a clinical diagnosis of severe alcoholic hepatitis for whom medical treatment is being considered and in those with an uncertain underlying diagnosis.
Findings on liver biopsy in alcoholic hepatitis include steatosis, hepatocyte ballooning, neutrophilic infiltration, Mallory bodies (which represent aggregated cytokeratin intermediate filaments and other proteins), and scarring with a typical perivenular distribution as opposed to the periportal fibrosis seen in chronic viral hepatitis. Some histologic findings, such as centrilobular necrosis, may overlap alcoholic hepatitis and nonalcoholic steatohepatitis.
In addition to confirming the diagnosis and staging the disease, liver biopsy has prognostic value. The severity of inflammation and cholestatic changes correlates with poor prognosis and may also predict response to corticosteroid treatment in severe cases of alcoholic hepatitis.21
However, the utility of liver biopsy in confirming the diagnosis and assessing the prognosis of alcoholic hepatitis is controversial for several reasons. Coagulopathy, thrombocytopenia, and ascites are all common in patients with alcoholic hepatitis, often making percutaneous liver biopsy contraindicated. Trans-
jugular liver biopsy is not universally available outside tertiary care centers.
Needed is a minimally invasive test for assessing this disease. Breath analysis might be such a test, offering a noninvasive means to study the composition of volatile organic compounds and elemental gases and an attractive method to evaluate health and disease in a patient-friendly manner. Our group devised a model based on breath levels of trimethylamine and pentane. When we tested it, we found that it distinguishes patients with alcoholic hepatitis from those with acute liver decompensation from causes other than alcohol and controls without liver disease with up to 90% sensitivity and 80% specificity.22
ASSESSING THE SEVERITY OF ALCOHOLIC HEPATITIS
Several models have been developed to assess the severity of alcoholic hepatitis and guide treatment decisions (Table 2).
The MDF (Maddrey Discriminant Function)6 system was the first scoring system developed and is still the most widely used. A score of 32 or higher indicates severe alcoholic hepatitis and has been used as the threshold for starting treatment with corticosteroids.6
The MDF has limitations. Patients with a score lower than 32 are considered not to have severe alcoholic hepatitis, but up to 17% of them still die. Also, since it uses the prothrombin time, its results can vary considerably among laboratories, depending on the sensitivity of the thromboplastin reagent used.
The MELD (Model for End-stage Liver Disease) score. Sheth et al23 compared the MELD and the MDF scores in assessing the severity of alcoholic hepatitis. They found that the MELD performed as well as the MDF in predicting 30-day mortality. A MELD score of greater than 11 had a sensitivity in predicting 30-day mortality of 86% and a specificity of 81%, compared with 86% and 48%, respectively, for MDF scores greater than 32.
Another study found a MELD score of 21 to have the highest sensitivity and specificity in predicting mortality (an estimated 90-day death rate of 20%). Thus, a MELD score of 21 is an appropriate threshold for prompt consideration of specific therapies such as corticosteroids.24
The MELD score has become increasingly important in patients with alcoholic hepatitis, as some of them may become candidates for liver transplantation (see below). Also, serial MELD scores in hospitalized patients have prognostic implications, since an increase of 2 or more points in the first week has been shown to predict in-hospital mortality.25
The GAHS (Glasgow Alcoholic Hepatitis Score)26 was shown to identify patients with alcoholic hepatitis who have an especially poor prognosis and need corticosteroid therapy. In those with a GAHS of 9 or higher, the 28-day survival rate was 78% with corticosteroid treatment and 52% without corticosteroid treatment; survival rates at 84 days were 59% and 38%, respectively.26
The ABIC scoring system (Age, Serum Bilirubin, INR, and Serum Creatinine) stratifies patients by risk of death at 90 days27:
- Score less than 6.71: low risk (100% survival)
- A score 6.71–8.99: intermediate risk (70% survival)
- A score 9.0 or higher: high risk (25% survival).
Both the GAHS and ABIC score are limited by lack of external validation.
The Lille score.28 While the above scores are used to identify patients at risk of death from alcoholic hepatitis and to decide on starting corticosteroids, the Lille score is designed to assess response to corticosteroids after 1 week of treatment. It is calculated based on five pretreatment variables and the change in serum bilirubin level at day 7 of corticosteroid therapy. Lille scores range from 0 to 1; a score higher than 0.45 is associated with a 75% mortality rate at 6 months and indicates a lack of response to corticosteroids and that these drugs should be discontinued.28
MANAGEMENT
Supportive treatment
Abstinence from alcohol is the cornerstone of treatment of alcoholic hepatitis. Early management of alcohol abuse or dependence is, therefore, warranted in all patients with alcoholic hepatitis. Referral to addiction specialists, motivational therapies, and anticraving drugs such as baclofen can be utilized.
Treat alcohol withdrawal. Alcoholics who suddenly decrease or discontinue their alcohol use are at high risk of alcohol withdrawal syndrome. Within 24 hours after the last drink, patients can experience increases in their heart rate and blood pressure, along with irritability and hyperreflexia. Within the next few days, more dangerous complications including seizures and delirium tremens can arise.
Alcohol withdrawal symptoms should be treated with short-acting benzodiazepines or clomethiazole, keeping the risk of worsening encephalopathy in mind.29 If present, complications of cirrhosis such as encephalopathy, ascites, and variceal bleeding should be managed.
Nutritional support is important. Protein-calorie malnutrition is common in alcoholics, as are deficiencies of vitamin A, vitamin D, thiamine, folate, pyridoxine, and zinc.30 Although a randomized controlled trial comparing enteral nutrition (2,000 kcal/day) vs corticosteroids (prednisolone 40 mg/day) in patients with alcoholic hepatitis did not show any difference in the 28-day mortality rate, those who received nutritional support and survived the first month had a lower mortality rate than those treated with corticosteroids (8% vs 37%).31 A daily protein intake of 1.5 g per kilogram of body weight is therefore recommended, even in patients with hepatic encephalopathy.15
Combining enteral nutrition and corticosteroid treatment may have a synergistic effect but is yet to be investigated.
Screen for infection. Patients with alcoholic hepatitis should be screened for infection, as about 25% of those with severe alcoholic hepatitis have an infection at admission.32 Since many of these patients meet the criteria for systemic inflammatory response syndrome, infections can be particularly difficult to diagnose. Patients require close clinical monitoring as well as regular pancultures for early detection. Antibiotics are frequently started empirically even though we lack specific evidence-based guidelines on this practice.33
Corticosteroids
Various studies have evaluated the role of corticosteroids in treating alcoholic hepatitis, differing considerably in sample populations, methods, and end points. Although the results of individual trials differ, meta-analyses indicate that corticosteroids have a moderate beneficial effect in patients with severe alcoholic hepatitis.
For example, Rambaldi et al34 performed a meta-analysis that concluded the mortality rate was lower in alcoholic hepatitis patients with MDF scores of at least 32 or hepatic encephalopathy who were treated with corticosteroids than in controls (relative risk 0.37, 95% confidence interval 0.16–0.86).
Therefore, in the absence of contraindications, the AASLD recommends starting corticosteroids in patients with severe alcoholic hepatitis, defined as an MDF score of 32 or higher.21 The preferred agent is oral prednisolone 40 mg daily or parenteral methylprednisolone 32 mg daily for 4 weeks and then tapered over the next 2 to 4 weeks or abruptly discontinued. Because activation of prednisone is decreased in patients with liver disease, prednisolone (the active form) is preferred over prednisone (the inactive precursor).35 In alcoholic hepatitis, the number needed to treat with corticosteroids to prevent one death has been calculated36 at 5.
As mentioned, response to corticosteroids is commonly assessed at 1 week of treatment using the Lille score. A score higher than 0.45 predicts a poor response and should trigger discontinuation of corticosteroids, particularly in those classified as null responders (Lille score > 0.56).
Adverse effects of steroids include sepsis, gastrointestinal bleeding, and steroid psychosis. Of note, patients who have evidence of hepatorenal syndrome or gastrointestinal bleeding tend to have a less favorable response to corticosteroids. Also, while infections were once considered a contraindication to steroid therapy, recent evidence suggests that steroid use might not be precluded in infected patients after appropriate antibiotic therapy. Infections occur in about a quarter of all alcoholic hepatitis patients treated with steroids, more frequently in null responders (42.5%) than in responders (11.1%), which supports corticosteroid discontinuance at 1 week in null responders.32
Pentoxifylline
An oral phosphodiesterase inhibitor, pentoxifylline, also inhibits production of several cytokines, including tumor necrosis factor alpha. At a dose of 400 mg orally three times daily for 4 weeks, pentoxifylline has been used in treating severe alcoholic hepatitis (MDF score ≥ 32) and is recommended especially if corticosteroids are contraindicated, as with sepsis.21
An early double-blind clinical trial randomized patients with severe alcoholic hepatitis to receive either pentoxifylline 400 mg orally three times daily or placebo. Of the patients who received pentoxifylline, 24.5% died during the index hospitalization, compared with 46.1% of patients who received placebo. This survival benefit was mainly related to a markedly lower incidence of hepatorenal syndrome as the cause of death in the pentoxifylline group than in the placebo group (50% vs 91.7% of deaths).37
In a small clinical trial in patients with severe alcoholic hepatitis, pentoxifylline recipients had a higher 3-month survival rate than prednisolone recipients (35.29% vs 14.71%, P = .04).38 However, a larger trial showed no improvement in 6-month survival with the combination of prednisolone and pentoxifylline compared with prednisolone alone (69.9% vs 69.2%, P = .91).39 Also, a meta-analysis of five randomized clinical trials found no survival benefit with pentoxifylline therapy.40
Of note, in the unfortunate subgroup of patients who have a poor response to corticosteroids, no alternative treatment, including pentoxifylline, has been shown to be effective.41
Prednisone or pentoxifylline? Very recently, results of the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial have been released.42 This is a large, multicenter, double-blinded clinical trial that aimed to provide a definitive answer to whether corticosteroids or pentoxifylline (or both) are beneficial in patients with alcoholic hepatitis. The study included 1,103 adult patients with severe alcoholic hepatitis (MDF score ≥ 32) who were randomized to monotherapy with prednisolone or pentoxifylline, combination therapy, or placebo. The primary end point was mortality at 28 days, and secondary end points included mortality at 90 days and at 1 year. Prednisolone reduced 28-day mortality by about 39%. In contrast, the 28-day mortality rate was similar in patients who received pentoxifylline and those who did not. Also, neither drug was significantly associated with a survival benefit beyond 28 days. The investigators concluded that pentoxifylline has no impact on disease progression and should not be used for the treatment of severe alcoholic hepatitis.42
Other tumor necrosis factor alpha inhibitors not recommended
Two other tumor necrosis factor alpha inhibitors, infliximab and etanercept, have been tested in clinical trials in alcoholic hepatitis. Unfortunately, the results were not encouraging, with no major reduction in mortality.43–45 In fact, these trials demonstrated a significantly increased risk of infections in the treatment groups. Therefore, these drugs are not recommended for treating alcoholic hepatitis.
A possible explanation is that tumor necrosis factor alpha plays an important role in liver regeneration, aiding in recovery from alcohol-induced liver injury, and inhibiting it can have deleterious consequences.
Other agents
A number of other agents have undergone clinical trials in alcoholic hepatitis.
N-acetylcysteine, an antioxidant that replenishes glutathione stores in hepatocytes, was evaluated in a randomized clinical trial in combination with prednisolone.46 Although the 1-month mortality rate was significantly lower in the combination group than in the prednisolone-only group (8% vs 24%, P = .006), 3-month and 6-month mortality rates were not. Nonetheless, the rates of infection and hepatorenal syndrome were lower in the combination group. Therefore, corticosteroids and N-acetylcysteine may have synergistic effects, but the optimum duration of N-acetylcysteine therapy needs to be determined in further studies.
Vitamin E, silymarin, propylthiouracil, colchicine, and oxandrolone (an anabolic steroid) have also been studied, but with no convincing benefit.21
Role of liver transplantation
Liver transplantation for alcoholic liver disease has been a topic of great medical and social controversy. The view that alcoholic patients are responsible for their own illness led to caution when contemplating liver transplantation. Many countries require 6 months of abstinence from alcohol before placing a patient on the liver transplant list, posing a major obstacle to patients with alcoholic hepatitis, as almost all are active drinkers at the time of presentation and many will die within 6 months. Reasons for this 6-month rule include donor shortage and risk of recidivism.47
With regard to survival following alcoholic hepatitis, a study utilizing the United Network for Organ Sharing database matched patients with alcoholic hepatitis and alcoholic cirrhosis who underwent liver transplantation. Rates of 5-year graft survival were 75% in those with alcoholic hepatitis and 73% in those with alcoholic cirrhosis (P = .97), and rates of patient survival were 80% and 78% (P = .90), respectively. Proportional regression analysis adjusting for other variables showed no impact of the etiology of liver disease on graft or patient survival. The investigators concluded that liver transplantation could be considered in a select group of patients with alcoholic hepatitis who do not improve with medical therapy.48
In a pivotal case-control prospective study,49 26 patients with Lille scores greater than 0.45 were listed for liver transplantation within a median of 13 days after nonresponse to medical therapy. The cumulative 6-month survival rate was higher in patients who received a liver transplant early than in those who did not (77% vs 23%, P < .001). This benefit was maintained through 2 years of follow-up (hazard ratio 6.08, P = .004). Of note, all these patients had supportive family members, no severe coexisting conditions, and a commitment to alcohol abstinence (although 3 patients resumed drinking after liver transplantation).49
Although these studies support early liver transplantation in carefully selected patients with severe alcoholic hepatitis, the criteria for transplantation in this group need to be refined. Views on alcoholism also need to be reconciled, as strong evidence is emerging that implicates genetic and environmental influences on alcohol dependence.
Management algorithm
Figure 2 shows a suggested management algorithm for alcoholic hepatitis, adapted from the guidelines of the AASLD and European Association for the Study of the Liver.
NEW THERAPIES NEEDED
Novel therapies for severe alcoholic hepatitis are urgently needed to help combat this devastating condition. Advances in understanding its pathophysiology have uncovered several new therapeutic targets, and new agents are already being evaluated in clinical trials.
IMM 124-E, a hyperimmune bovine colostrum enriched with immunoglobulin G anti-lipopolysaccharide, is going to be evaluated in combination with prednisolone in patients with severe alcoholic hepatitis.
Anakinra, an interleukin 1 receptor antagonist, has significant anti-inflammatory activity and is used to treat rheumatoid arthritis. A clinical trial to evaluate its role in alcoholic hepatitis has been designed in which patients with severe alcoholic hepatitis (defined as a MELD score ≥ 21) will be randomized to receive either methylprednisolone or a combination of anakinra, pentoxifylline, and zinc (a mineral that improves gut integrity).
Emricasan, an orally active caspase protease inhibitor, is another agent currently being tested in a phase 2 clinical trial in patients with severe alcoholic hepatitis. Since caspases induce apoptosis, inhibiting them should theoretically dampen alcohol-induced hepatocyte injury.
Interleukin 22, a hepatoprotective cytokine, shows promise as a treatment and will soon be evaluated in alcoholic hepatitis.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
- Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol 2013; 59:160–168.
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 1995; 346:987–990.
- Alcoholic liver disease: morphological manifestations. Review by an international group. Lancet 1981; 1:707–711.
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997; 25:108–111.
- Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 2011; 3:108–113.
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978; 75:193–199.
- Jinjuvadia R, Liangpunsakul S, for the Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol 2014 Jun 25 (Epub ahead of print).
- Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res 2001; 25(suppl s1):40S–45S.
- Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol 2014; 12:555–564.
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem 2006; 387:349–360.
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol 1998; 275:G605–G611.
- Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis 2012; 16:687–698.
- Lischner MW, Alexander JF, Galambos JT. Natural history of alcoholic hepatitis. I. The acute disease. Am J Dig Dis 1971; 16:481–494.
- Cohen JA, Kaplan MM. The SGOT/SGPT ratio—an indicator of alcoholic liver disease. Dig Dis Sci 1979; 24:835–838.
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009; 360:2758–2769.
- McKnight-Eily LR, Liu Y, Brewer RD, et al; Centers for Disease Control and Prevention (CDC). Vital signs: communication between health professionals and their patients about alcohol use—44 states and the District of Columbia, 2011. MMWR Morb Mortal Wkly Rep 2014; 63:16–22.
- Grant BF. Barriers to alcoholism treatment: reasons for not seeking treatment in a general population sample. J Stud Alcohol 1997; 58:365–371.
- Aertgeerts B, Buntinx F, Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol 2004; 57:30–39.
- The Alcohol Use Disorders Identification Test Guidelines for Use in Primary Care. Second Edition. World Health Organization. Department of Mental Health and Substance Dependence. http://whqlibdoc.who.int/hq/2001/who_msd_msb_01.6a.pdf. Accessed February 3, 2015.
- Hamid R, Forrest EH. Is histology required for the diagnosis of alcoholic hepatitis? A review of published randomised controlled trials. Gut 2011; 60(suppl 1):A233.
- O’Shea RS, Dasarathy S, McCullough AJ; Practice Guideline Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51:307–328.
- Hanouneh IA, Zein NN, Cikach F, et al. The breathprints in patients with liver disease identify novel breath biomarkers in alcoholic hepatitis. Clin Gastroenterol Hepatol 2014; 12:516–523.
- Sheth M, Riggs M, Patel T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol 2002; 2:2.
- Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005; 41:353–358.
- Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol 2005; 42:700–706.
- Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007; 56:1743–1746.
- Dominguez M, Rincón D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008; 103:2747–2756.
- Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354.
- Mayo-Smith MF, Beecher LH, Fischer TL, et al; Working Group on the Management of Alcohol Withdrawal Delirium, Practice Guidelines Committee, American Society of Addiction Medicine. Management of alcohol withdrawal delirium. An evidence-based practice guideline. Arch Intern Med 2004; 164:1405–1412.
- Mezey E. Interaction between alcohol and nutrition in the pathogenesis of alcoholic liver disease. Semin Liver Dis 1991; 11:340–348.
- Cabré E, Rodríguez-Iglesias P, Caballería J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology 2000; 32:36–42.
- Louvet A, Wartel F, Castel H, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009; 137:541–548.
- European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012; 57:399–420.
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis—a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther 2008; 27:1167–1178.
- Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut 1972; 13:690–696.
- Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011; 60:255–260.
- Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:1637–1648.
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J Gastroenterol 2009; 15:1613–1619.
- Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the CORPENTOX trial (abstract). Hepatology 2011; 54(suppl 1):81A.
- Whitfield K, Rambaldi A, Wetterslev J, Gluud C. Pentoxifylline for alcoholic hepatitis. Cochrane Database Syst Rev 2009; CD007339.
- Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol 2008; 48:465–470.
- Thursz MR, Richardson P, Allison ME, et al. Steroids or pentoxifylline for alcoholic hepatitis: results of the STOPAH trial [abstract LB-1]. 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, MA.
- Naveau S, Chollet-Martin S, Dharancy S, et al; Foie-Alcool group of the Association Française pour l’Etude du Foie. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology 2004; 39:1390–1397.
- Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol 2004; 99:255–260.
- Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology 2008; 135:1953–1960.
- Nguyen-Khac E, Thevenot T, Piquet MA, et al; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med 2011; 365:1781–1789.
- Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: current status and future development. World J Hepatol 2011; 3:215–218.
- Singal AK, Bashar H, Anand BS, Jampana SC, Singal V, Kuo YF. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: exploratory analysis from the UNOS database. Hepatology 2012; 55:1398–1405.
- Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800.
KEY POINTS
- One should assess the severity of alcoholic hepatitis, using defined scoring systems, to allocate resources and initiate appropriate therapy.
- Supportive care should focus on alcohol withdrawal and enteral nutrition while managing the complications of liver failure.
- Corticosteroids or pentoxifylline are commonly used, but increase the survival rate only by about 50%.
- Opinion is shifting toward allowing some patients with alcoholic hepatitis to receive liver transplants early in the course of their disease.
- Many new therapies are undergoing clinical trials.
Resuming anticoagulation after hemorrhage: A practical approach
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
KEY POINTS
- Not all patients on anticoagulation at the time of a bleeding event have a strong indication to continue anticoagulation afterward.
- Important considerations when deciding whether to resume anticoagulation after hemorrhage are whether the source of bleeding has been found and controlled and, if the patient is receiving warfarin, whether he or she can be expected to maintain the target international normalized ratio.
- The newer oral anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, lack antidotes or reversal agents, and their risk of causing bleeding compared with warfarin varies by site of bleeding.
5 ways to wake up your Web site
Web sites are not like wine and cheese—they don’t necessarily get better with age. You may have started your Web page 20 years ago by moving your 3-color trifold brochure onto the Internet. It may have worked then, but to compete today you must have a robust, interactive, attractive Web site that is continuously being updated with new content. What prospective patients are looking for in a Web site has evolved rapidly. How to get these patients to take action and call for an appointment requires a process or a system.
Trying to keep your Web site current can be daunting for most medical practices. If you find that your Web site is not generating new patients and that your existing patients are not using the site in an interactive fashion, then it is time to upgrade. In this article we suggest 5 practical ways to make your Web site a useful adjunct to your medical practice—an automatic patient conversion system.
1. Go mobile
Make your Web site “thumb friendly.” Mobile technology has taken over the desktop and laptop worlds. Now nearly everyone is using a hand-held smartphone or tablet for their Internet needs.
To attract patients your Web page must be responsive to the screen size of a smartphone or tablet—very different from your Web site, which is accessed from a desktop or a laptop computer. The majority of users navigate not with a mouse but with their fingers and thumbs. To ensure they can find their way on your Web page on a mobile device, the screen view should adjust automatically to the mobile device being used. Whether that is accomplished through a mobile responsive design or an entirely different mobile Web site, you do not want the user to have to resize, zoom, or pinch their way through the page in order to read the content. All the buttons must be large enough to be easily pressed without having to zoom in, and the font should be easy-to-read in style and size.
Having your current Web site programmed to be responsive to these devices will increase the time a mobile user spends on your site and make it easier for her to make an appointment.
2. Add patient reviews
What others say about you is far more important than anything you can say about yourself. Almost half of prospective patients will check out your online reviews before calling you to schedule an appointment.1 Therefore, it is very important that you ask for positive feedback from your patients and post it to your Web site. We recommend that you capture compliments from your existing patients when they are in the office. Have a computer or iPad handy for them to create a positive review; patients who “promise” to do it when they get back to the office or home rarely follow through. Testimonials should be visible on your homepage and can link to another testimonial page or review site.
“as many as 8 out of 10 people will look online for information about individual doctors. And all of that happens long before they make an appointment … and what they find—positive, negative, neutral or nothing at all—influences their decision to call or not to call.”2
Always invite your patients to evaluate you, your practice partners, and the practice online. There are numerous patient review Web sites, including: Google Plus, http://www.RateMDs.com, http://www.Vitals.com, and http://www.HealthGrades.com. And check out what your patients are saying about you on a regular basis. Just type “Reviews for Dr. <your name>” into your search bar to find the results.
Although we hope they will, happy patients rarely fill out these online reviews. However, it takes just 2 or 3 unhappy patients to ruin your online reputation. That could be costing you tens of thousands of dollars in lost billing.
3. Share your videos
What’s hot and what’s not? To answer that, just take a look at how many people watch videos on YouTube every day! People don’t want to read anymore; they want to be entertained and spoon-fed information.
Take advantage of this trend by placing videos on your homepage. Post a video that introduces your practice, provides testimonials of satisfied patients, explains some of the procedures you perform, or shows you describing the latest breakthrough in medical technology.
Your videos don’t have to be long. One to 2 minutes is plenty. They don’t have to feature you talking about medical symptoms or procedures (what’s called a talking head video). Use a PowerPoint presentation with voice overlay—and you don’t have to be the one talking.
Your Web site isn’t the only place you’ll want to post your videos. YouTube is second only to Google as the most popular search engine.3 Just about everyone goes to YouTube to view videos on whatever interests them. See our April 2014 article, titled “Using the Internet in your practice. Part 2: Generating new patients using social media,” to learn more on getting started with YouTube.
Videos will improve your Web site rankings and will increase the time visitors spend on the site. When done properly—labeling the videos with relevant keywords, making the videos short, and presenting information in layman’s language with reasons why it is important to seek a professional if the viewer is experiencing these types of symptoms—they are a great way to convert visitors to patients.
4. Hook‘em on the homepage
If you want your Web site to create a favorable first impression, your homepage should reflect that positive impression. Remember, the homepage, as the face of your practice, is the first thing that a patient will see long before she picks up the phone or comes to the office.
A potential patient visiting your site will make a snap judgment within a few seconds. Think of your homepage as a highway billboard. There are about 3 seconds to make an impression and for a driver to decide whether or not she will exit the highway to buy gas or eat at a restaurant or even contact a business in the future by telephone or, most likely, online. A visit to your Web site has the same attraction timing.
Your homepage must be attractive; provide useful, current information; and have pleasing graphics—all without requiring the visitor to scroll down too far. Your Web site is your opportunity to create a good first impression—an opportunity that won’t happen again.
Use compelling headlines with keyword-related content. You want to make sure you use keywords that a prospective patient might search for in a main headline and in the main body of your homepage. But patients are not the only ones who spot those key terms. Search engines also crawl your Web site for keywords that prospective patients may type into the Google search bar—words like gynecologist, ObGyn, urinary leakage, breast lump, pelvic pain, menopause, etc. Using those keywords helps your site to be found more often by patients and helps those prospective patients find information relevant to their medical needs.
5. Place calls to action on every page
Contact us! This is so rudimentary, yet many Web sites do not have easy-to-find contact information on their homepages. Be sure to include your phone number (which could be different than your regular phone office number so you can track how many calls you get from your Web site).
Add a “schedule an appointment” icon in a prominent position on the homepage so the visitor does not have to scroll down to search for it. But don’t just stop at the homepage. Your contact information should be on every page so that, when the visitor is on a page reading about a condition or procedure, the “schedule an appointment” button is right there for her to click.
Be sure to evaluate your contact page. Make sure it’s easy for patients to find multiple ways to connect with you and your office: phone, fax, email, and snail mail.
Interactivity is important. Why not have an “Ask the doctor your question” field? It makes the site interactive and gives you the opportunity to communicate and develop a relationship with your patients.
Additional interactivity
Social media is the new buzz word-of-mouth. Your patients use Facebook, YouTube, blogging, and Twitter every day. It is the easiest way to stay connected and make your practice and your brand part of their daily lives. Social media builds loyalty. Integrating social media into your Web site provides new opportunities to engage your existing patients and to attract new ones to your practice.
Connect to medical records. Your Web site should have an easy portal for patients to connect to their medical records and laboratory results in a secure, encrypted fashion to comply with HIPAA regulations.
You can do this yourself!
You and your staff should be able to make changes on your Web site without having to contact your Web developer, even if you do not have full-time IT assistance. For example, in Dr. Baum’s practice, his support staff can add testimonials, content, and pictures without contacting the Web developer or knowing code.
Make sure that function is designed into your site and that your Web developer teaches you and your staff how to keep your site updated.
The bottom line
Web sites are like a farmer’s fence, they are always under construction. Merely having a Web site, regardless of the size, specialty, or location of your practice, is not enough. Be sure your site attracts, holds, and converts viewers into paying patients. We hope you will consider these 5 suggestions as a roadmap to develop a robust site, so that when you ask a patient who referred her to your practice, her answer will be “your Web site” or “the Internet.” This will bring cockles to your heart and bucks in your bank account.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Online reputation management for doctors. Vanguard Communications Web site. http://vanguardcommuni cations.net/medical-marketing-portfolio/reputation-management. Accessed March 17, 2015.
2. Gandolf S. Ten commandments of online reputation management for physicians [Part one]. Healthcare Success Web site. http://www.healthcaresuccess.com/blog/internet-marketing-advertising/10-commandments-online-reputation-management-physicians-2.html. Published May 12, 2014. Accessed March 9, 2015.
3. YouTube—The 2nd Largest Search Engine. Mushroom Networks Web site. http://www.mushroomnetworks.com/infographics/youtube---the-2nd-largest-search-engine-infographic. Accessed March 17, 2015.
Web sites are not like wine and cheese—they don’t necessarily get better with age. You may have started your Web page 20 years ago by moving your 3-color trifold brochure onto the Internet. It may have worked then, but to compete today you must have a robust, interactive, attractive Web site that is continuously being updated with new content. What prospective patients are looking for in a Web site has evolved rapidly. How to get these patients to take action and call for an appointment requires a process or a system.
Trying to keep your Web site current can be daunting for most medical practices. If you find that your Web site is not generating new patients and that your existing patients are not using the site in an interactive fashion, then it is time to upgrade. In this article we suggest 5 practical ways to make your Web site a useful adjunct to your medical practice—an automatic patient conversion system.
1. Go mobile
Make your Web site “thumb friendly.” Mobile technology has taken over the desktop and laptop worlds. Now nearly everyone is using a hand-held smartphone or tablet for their Internet needs.
To attract patients your Web page must be responsive to the screen size of a smartphone or tablet—very different from your Web site, which is accessed from a desktop or a laptop computer. The majority of users navigate not with a mouse but with their fingers and thumbs. To ensure they can find their way on your Web page on a mobile device, the screen view should adjust automatically to the mobile device being used. Whether that is accomplished through a mobile responsive design or an entirely different mobile Web site, you do not want the user to have to resize, zoom, or pinch their way through the page in order to read the content. All the buttons must be large enough to be easily pressed without having to zoom in, and the font should be easy-to-read in style and size.
Having your current Web site programmed to be responsive to these devices will increase the time a mobile user spends on your site and make it easier for her to make an appointment.
2. Add patient reviews
What others say about you is far more important than anything you can say about yourself. Almost half of prospective patients will check out your online reviews before calling you to schedule an appointment.1 Therefore, it is very important that you ask for positive feedback from your patients and post it to your Web site. We recommend that you capture compliments from your existing patients when they are in the office. Have a computer or iPad handy for them to create a positive review; patients who “promise” to do it when they get back to the office or home rarely follow through. Testimonials should be visible on your homepage and can link to another testimonial page or review site.
“as many as 8 out of 10 people will look online for information about individual doctors. And all of that happens long before they make an appointment … and what they find—positive, negative, neutral or nothing at all—influences their decision to call or not to call.”2
Always invite your patients to evaluate you, your practice partners, and the practice online. There are numerous patient review Web sites, including: Google Plus, http://www.RateMDs.com, http://www.Vitals.com, and http://www.HealthGrades.com. And check out what your patients are saying about you on a regular basis. Just type “Reviews for Dr. <your name>” into your search bar to find the results.
Although we hope they will, happy patients rarely fill out these online reviews. However, it takes just 2 or 3 unhappy patients to ruin your online reputation. That could be costing you tens of thousands of dollars in lost billing.
3. Share your videos
What’s hot and what’s not? To answer that, just take a look at how many people watch videos on YouTube every day! People don’t want to read anymore; they want to be entertained and spoon-fed information.
Take advantage of this trend by placing videos on your homepage. Post a video that introduces your practice, provides testimonials of satisfied patients, explains some of the procedures you perform, or shows you describing the latest breakthrough in medical technology.
Your videos don’t have to be long. One to 2 minutes is plenty. They don’t have to feature you talking about medical symptoms or procedures (what’s called a talking head video). Use a PowerPoint presentation with voice overlay—and you don’t have to be the one talking.
Your Web site isn’t the only place you’ll want to post your videos. YouTube is second only to Google as the most popular search engine.3 Just about everyone goes to YouTube to view videos on whatever interests them. See our April 2014 article, titled “Using the Internet in your practice. Part 2: Generating new patients using social media,” to learn more on getting started with YouTube.
Videos will improve your Web site rankings and will increase the time visitors spend on the site. When done properly—labeling the videos with relevant keywords, making the videos short, and presenting information in layman’s language with reasons why it is important to seek a professional if the viewer is experiencing these types of symptoms—they are a great way to convert visitors to patients.
4. Hook‘em on the homepage
If you want your Web site to create a favorable first impression, your homepage should reflect that positive impression. Remember, the homepage, as the face of your practice, is the first thing that a patient will see long before she picks up the phone or comes to the office.
A potential patient visiting your site will make a snap judgment within a few seconds. Think of your homepage as a highway billboard. There are about 3 seconds to make an impression and for a driver to decide whether or not she will exit the highway to buy gas or eat at a restaurant or even contact a business in the future by telephone or, most likely, online. A visit to your Web site has the same attraction timing.
Your homepage must be attractive; provide useful, current information; and have pleasing graphics—all without requiring the visitor to scroll down too far. Your Web site is your opportunity to create a good first impression—an opportunity that won’t happen again.
Use compelling headlines with keyword-related content. You want to make sure you use keywords that a prospective patient might search for in a main headline and in the main body of your homepage. But patients are not the only ones who spot those key terms. Search engines also crawl your Web site for keywords that prospective patients may type into the Google search bar—words like gynecologist, ObGyn, urinary leakage, breast lump, pelvic pain, menopause, etc. Using those keywords helps your site to be found more often by patients and helps those prospective patients find information relevant to their medical needs.
5. Place calls to action on every page
Contact us! This is so rudimentary, yet many Web sites do not have easy-to-find contact information on their homepages. Be sure to include your phone number (which could be different than your regular phone office number so you can track how many calls you get from your Web site).
Add a “schedule an appointment” icon in a prominent position on the homepage so the visitor does not have to scroll down to search for it. But don’t just stop at the homepage. Your contact information should be on every page so that, when the visitor is on a page reading about a condition or procedure, the “schedule an appointment” button is right there for her to click.
Be sure to evaluate your contact page. Make sure it’s easy for patients to find multiple ways to connect with you and your office: phone, fax, email, and snail mail.
Interactivity is important. Why not have an “Ask the doctor your question” field? It makes the site interactive and gives you the opportunity to communicate and develop a relationship with your patients.
Additional interactivity
Social media is the new buzz word-of-mouth. Your patients use Facebook, YouTube, blogging, and Twitter every day. It is the easiest way to stay connected and make your practice and your brand part of their daily lives. Social media builds loyalty. Integrating social media into your Web site provides new opportunities to engage your existing patients and to attract new ones to your practice.
Connect to medical records. Your Web site should have an easy portal for patients to connect to their medical records and laboratory results in a secure, encrypted fashion to comply with HIPAA regulations.
You can do this yourself!
You and your staff should be able to make changes on your Web site without having to contact your Web developer, even if you do not have full-time IT assistance. For example, in Dr. Baum’s practice, his support staff can add testimonials, content, and pictures without contacting the Web developer or knowing code.
Make sure that function is designed into your site and that your Web developer teaches you and your staff how to keep your site updated.
The bottom line
Web sites are like a farmer’s fence, they are always under construction. Merely having a Web site, regardless of the size, specialty, or location of your practice, is not enough. Be sure your site attracts, holds, and converts viewers into paying patients. We hope you will consider these 5 suggestions as a roadmap to develop a robust site, so that when you ask a patient who referred her to your practice, her answer will be “your Web site” or “the Internet.” This will bring cockles to your heart and bucks in your bank account.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Web sites are not like wine and cheese—they don’t necessarily get better with age. You may have started your Web page 20 years ago by moving your 3-color trifold brochure onto the Internet. It may have worked then, but to compete today you must have a robust, interactive, attractive Web site that is continuously being updated with new content. What prospective patients are looking for in a Web site has evolved rapidly. How to get these patients to take action and call for an appointment requires a process or a system.
Trying to keep your Web site current can be daunting for most medical practices. If you find that your Web site is not generating new patients and that your existing patients are not using the site in an interactive fashion, then it is time to upgrade. In this article we suggest 5 practical ways to make your Web site a useful adjunct to your medical practice—an automatic patient conversion system.
1. Go mobile
Make your Web site “thumb friendly.” Mobile technology has taken over the desktop and laptop worlds. Now nearly everyone is using a hand-held smartphone or tablet for their Internet needs.
To attract patients your Web page must be responsive to the screen size of a smartphone or tablet—very different from your Web site, which is accessed from a desktop or a laptop computer. The majority of users navigate not with a mouse but with their fingers and thumbs. To ensure they can find their way on your Web page on a mobile device, the screen view should adjust automatically to the mobile device being used. Whether that is accomplished through a mobile responsive design or an entirely different mobile Web site, you do not want the user to have to resize, zoom, or pinch their way through the page in order to read the content. All the buttons must be large enough to be easily pressed without having to zoom in, and the font should be easy-to-read in style and size.
Having your current Web site programmed to be responsive to these devices will increase the time a mobile user spends on your site and make it easier for her to make an appointment.
2. Add patient reviews
What others say about you is far more important than anything you can say about yourself. Almost half of prospective patients will check out your online reviews before calling you to schedule an appointment.1 Therefore, it is very important that you ask for positive feedback from your patients and post it to your Web site. We recommend that you capture compliments from your existing patients when they are in the office. Have a computer or iPad handy for them to create a positive review; patients who “promise” to do it when they get back to the office or home rarely follow through. Testimonials should be visible on your homepage and can link to another testimonial page or review site.
“as many as 8 out of 10 people will look online for information about individual doctors. And all of that happens long before they make an appointment … and what they find—positive, negative, neutral or nothing at all—influences their decision to call or not to call.”2
Always invite your patients to evaluate you, your practice partners, and the practice online. There are numerous patient review Web sites, including: Google Plus, http://www.RateMDs.com, http://www.Vitals.com, and http://www.HealthGrades.com. And check out what your patients are saying about you on a regular basis. Just type “Reviews for Dr. <your name>” into your search bar to find the results.
Although we hope they will, happy patients rarely fill out these online reviews. However, it takes just 2 or 3 unhappy patients to ruin your online reputation. That could be costing you tens of thousands of dollars in lost billing.
3. Share your videos
What’s hot and what’s not? To answer that, just take a look at how many people watch videos on YouTube every day! People don’t want to read anymore; they want to be entertained and spoon-fed information.
Take advantage of this trend by placing videos on your homepage. Post a video that introduces your practice, provides testimonials of satisfied patients, explains some of the procedures you perform, or shows you describing the latest breakthrough in medical technology.
Your videos don’t have to be long. One to 2 minutes is plenty. They don’t have to feature you talking about medical symptoms or procedures (what’s called a talking head video). Use a PowerPoint presentation with voice overlay—and you don’t have to be the one talking.
Your Web site isn’t the only place you’ll want to post your videos. YouTube is second only to Google as the most popular search engine.3 Just about everyone goes to YouTube to view videos on whatever interests them. See our April 2014 article, titled “Using the Internet in your practice. Part 2: Generating new patients using social media,” to learn more on getting started with YouTube.
Videos will improve your Web site rankings and will increase the time visitors spend on the site. When done properly—labeling the videos with relevant keywords, making the videos short, and presenting information in layman’s language with reasons why it is important to seek a professional if the viewer is experiencing these types of symptoms—they are a great way to convert visitors to patients.
4. Hook‘em on the homepage
If you want your Web site to create a favorable first impression, your homepage should reflect that positive impression. Remember, the homepage, as the face of your practice, is the first thing that a patient will see long before she picks up the phone or comes to the office.
A potential patient visiting your site will make a snap judgment within a few seconds. Think of your homepage as a highway billboard. There are about 3 seconds to make an impression and for a driver to decide whether or not she will exit the highway to buy gas or eat at a restaurant or even contact a business in the future by telephone or, most likely, online. A visit to your Web site has the same attraction timing.
Your homepage must be attractive; provide useful, current information; and have pleasing graphics—all without requiring the visitor to scroll down too far. Your Web site is your opportunity to create a good first impression—an opportunity that won’t happen again.
Use compelling headlines with keyword-related content. You want to make sure you use keywords that a prospective patient might search for in a main headline and in the main body of your homepage. But patients are not the only ones who spot those key terms. Search engines also crawl your Web site for keywords that prospective patients may type into the Google search bar—words like gynecologist, ObGyn, urinary leakage, breast lump, pelvic pain, menopause, etc. Using those keywords helps your site to be found more often by patients and helps those prospective patients find information relevant to their medical needs.
5. Place calls to action on every page
Contact us! This is so rudimentary, yet many Web sites do not have easy-to-find contact information on their homepages. Be sure to include your phone number (which could be different than your regular phone office number so you can track how many calls you get from your Web site).
Add a “schedule an appointment” icon in a prominent position on the homepage so the visitor does not have to scroll down to search for it. But don’t just stop at the homepage. Your contact information should be on every page so that, when the visitor is on a page reading about a condition or procedure, the “schedule an appointment” button is right there for her to click.
Be sure to evaluate your contact page. Make sure it’s easy for patients to find multiple ways to connect with you and your office: phone, fax, email, and snail mail.
Interactivity is important. Why not have an “Ask the doctor your question” field? It makes the site interactive and gives you the opportunity to communicate and develop a relationship with your patients.
Additional interactivity
Social media is the new buzz word-of-mouth. Your patients use Facebook, YouTube, blogging, and Twitter every day. It is the easiest way to stay connected and make your practice and your brand part of their daily lives. Social media builds loyalty. Integrating social media into your Web site provides new opportunities to engage your existing patients and to attract new ones to your practice.
Connect to medical records. Your Web site should have an easy portal for patients to connect to their medical records and laboratory results in a secure, encrypted fashion to comply with HIPAA regulations.
You can do this yourself!
You and your staff should be able to make changes on your Web site without having to contact your Web developer, even if you do not have full-time IT assistance. For example, in Dr. Baum’s practice, his support staff can add testimonials, content, and pictures without contacting the Web developer or knowing code.
Make sure that function is designed into your site and that your Web developer teaches you and your staff how to keep your site updated.
The bottom line
Web sites are like a farmer’s fence, they are always under construction. Merely having a Web site, regardless of the size, specialty, or location of your practice, is not enough. Be sure your site attracts, holds, and converts viewers into paying patients. We hope you will consider these 5 suggestions as a roadmap to develop a robust site, so that when you ask a patient who referred her to your practice, her answer will be “your Web site” or “the Internet.” This will bring cockles to your heart and bucks in your bank account.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Online reputation management for doctors. Vanguard Communications Web site. http://vanguardcommuni cations.net/medical-marketing-portfolio/reputation-management. Accessed March 17, 2015.
2. Gandolf S. Ten commandments of online reputation management for physicians [Part one]. Healthcare Success Web site. http://www.healthcaresuccess.com/blog/internet-marketing-advertising/10-commandments-online-reputation-management-physicians-2.html. Published May 12, 2014. Accessed March 9, 2015.
3. YouTube—The 2nd Largest Search Engine. Mushroom Networks Web site. http://www.mushroomnetworks.com/infographics/youtube---the-2nd-largest-search-engine-infographic. Accessed March 17, 2015.
1. Online reputation management for doctors. Vanguard Communications Web site. http://vanguardcommuni cations.net/medical-marketing-portfolio/reputation-management. Accessed March 17, 2015.
2. Gandolf S. Ten commandments of online reputation management for physicians [Part one]. Healthcare Success Web site. http://www.healthcaresuccess.com/blog/internet-marketing-advertising/10-commandments-online-reputation-management-physicians-2.html. Published May 12, 2014. Accessed March 9, 2015.
3. YouTube—The 2nd Largest Search Engine. Mushroom Networks Web site. http://www.mushroomnetworks.com/infographics/youtube---the-2nd-largest-search-engine-infographic. Accessed March 17, 2015.
Hospital Management of AECOPD
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]
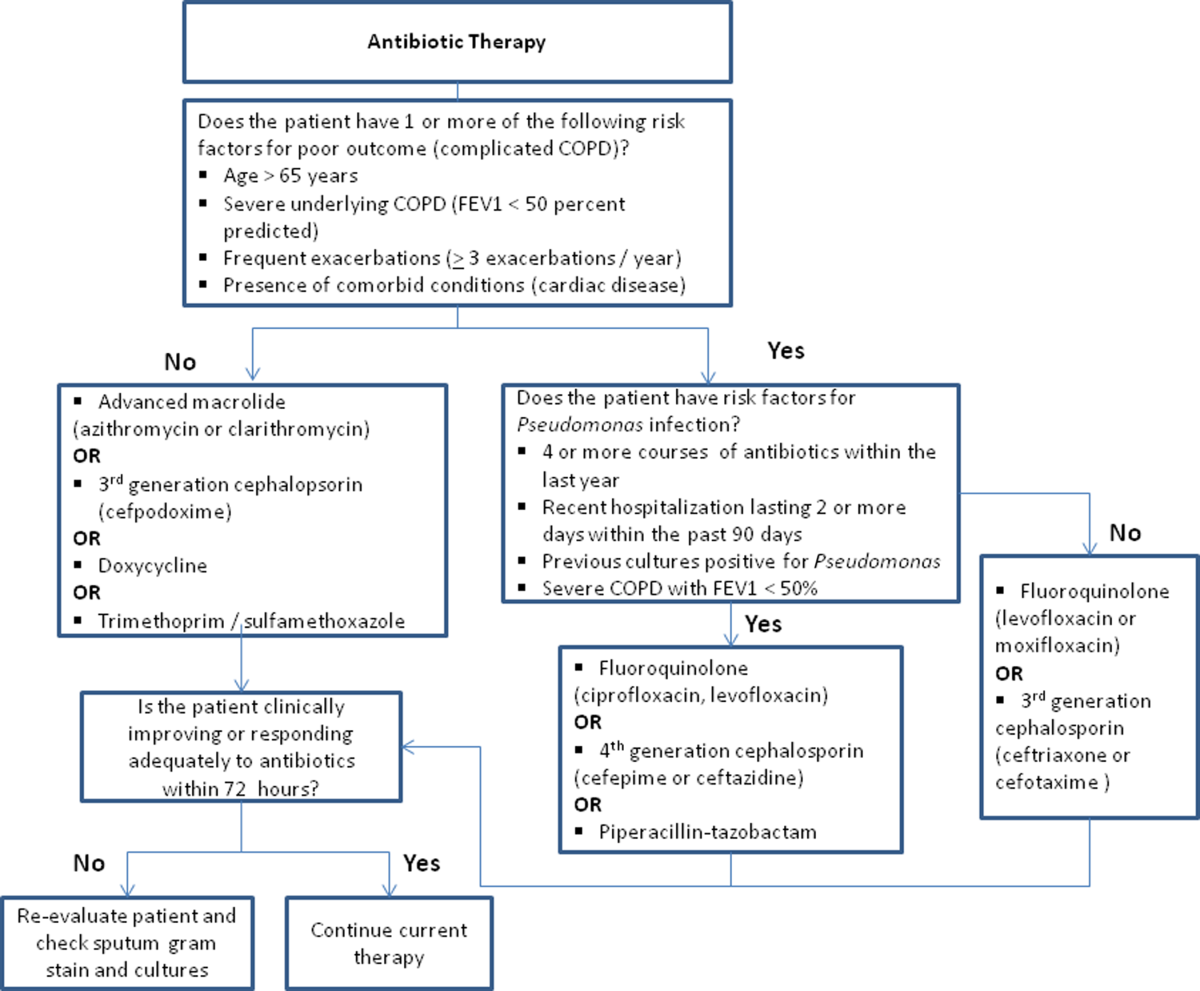
Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.
Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.