User login
Endometriosis and infertility – Combining a chronic physical and emotional pain
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Pain is classified as chronic when it lasts or recurs for more than 3-6 months (“Classification of chronic pain” 2nd ed. Seattle: IASP Press, 1994). This universally accepted definition does not distinguish between physical and emotional pain. Categorically, pain is pain. Two prevalent chronic gynecologic diseases are closely related medically and emotionally. Forty percent to 50% of women with endometriosis have infertility; 30%-50% of women with infertility are found to have coexisting endometriosis. The approach to both is, typically, symptomatic treatment. In this month’s column, I examine the relationship between these ailments and how we can advise women on management.
Endometriosis is simply defined as the displacement of normal endometrial glands and stroma from their natural anatomical location to elsewhere in the body. With the recent identification of the disease in the spleen, endometriosis has been found in every organ system. Endometriosis is identified in 6%-10% of the general female population. The prevalence ranges from 2% to 11% among asymptomatic women and from 5% to 21% in women hospitalized for pelvic pain (Best Pract Res Clin Obstet Gynaecol. 2018;51:1-15). Compared with fertile women, infertile women are six to eight times more likely to have endometriosis (Fertil Steril. 2012;98:591-8).
Retrograde menstruation is the presumed theory for the origins of endometriosis, that is, the reflux of menstrual debris containing active endometrial cells through the fallopian tubes into the peritoneal cavity (Am J Obstet Gynecol. 1927;14:422-69). Because of the varied etiologies of the most common symptoms of endometriosis, dysmenorrhea, dyspareunia, dyschezia, and infertility, women visit, on average, seven physicians before being diagnosed (Fertil Steril. 2011;96:366). The delay in promptly identifying endometriosis is further impaired by the lack of specific biomarkers, awareness, and inadequate evaluation (N Engl J Med. 2020;382:1244-56).
The 2008 U.S. health care costs for endometriosis were approximately $4,000 per affected woman, analogous to the costs for other chronic conditions such as type 2 diabetes, Crohn’s disease, and rheumatoid arthritis (Hum Reprod. 2012;27:1292-9). The management of symptoms further increases the financial burden because of the effect of the disease on physical, mental, sexual, and social well-being, as well as productivity (Health Qual Life Outcomes. 2019;17:123).
We have known the paradoxical relationship between the stage of endometriosis and symptoms: Women with low-stage disease may present with severe pain and/or infertility but those with advanced-stage disease may be asymptomatic. Endometriotic cells and tissue elicit a localized immune and inflammatory response with the production of cytokines, chemokines, and prostaglandins. Given the usual intra-abdominal location and the small size of implants, endometriosis requires a surgical diagnosis, ideally with histopathology for confirmation. However, imaging – transvaginal ultrasound or MRI – has more than 90% sensitivity and specificity for identifying endometriomas (Cochrane Database Syst Rev. 2016;2[2]:CD009591).
The effect of endometriosis on fertility, particularly in women with minimal to mild stages, is not clear, and many studies have been retrospective. Tubal factor infertility can be a result of endometriosis. Per the 2020 Cochrane Database Systemic Reviews (2020 Oct;2020[10]:CD011031), “Compared to diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis; no data were reported on live birth. There is moderate-quality evidence that laparoscopic surgery increases viable intrauterine pregnancy rates confirmed by ultrasound compared to diagnostic laparoscopy only.” In women undergoing IVF, more advanced stages of endometriosis have reduced pregnancy outcomes as shown in recent meta-analyses (Obstet Gynecol. 2015;125:79-88).
The revised ASRM (rASRM) surgical staging classification of endometriosis has been widely used to describe the degree, although it poorly correlates with fertility potential (Fertil Steril. 2012;98:591-8). Women diagnosed with endometriosis may benefit from the Endometriosis Fertility Index (EFI), published in 2010 as a useful scoring system to predict postoperative non-IVF pregnancy rates (both by natural means and intrauterine insemination) based on patient characteristics, rASRM staging and “least function” score of the adnexa (Fertil Steril. 2010;94:1609-15).
Compared with diagnostic laparoscopy only, it is uncertain whether laparoscopic surgery reduces overall pain associated with minimal to severe endometriosis. “Further research is needed considering the management of different subtypes of endometriosis and comparing laparoscopic interventions with lifestyle and medical interventions (Cochrane Database Syst Rev. 2020 Oct;2020[10]:CD011031).”
The treatment of endometriosis is directly related to the desire for and timing of fertility since therapy is often contraceptive, as opposed to surgery. Because endometriosis is exacerbated by estradiol, the mainstay of medical therapy is initially combined hormonal or progestin-only contraception as a means of reducing pelvic pain by reducing estradiol production and action, respectively. GnRH-agonist suppression of follicle stimulation hormone and luteinizing hormone remains the standard for inactivating endogenous estradiol. In 2018, the U.S. Food and Drug Administration approved elagolix for the treatment of pain associated with endometriosis – the first pill specifically approved for endometriosis pain relief. An off-label approach for women is letrozole, the aromatase inhibitor, to reduce circulating estradiol levels. Unfortunately, estradiol suppression cannot be used solely long term without add-back therapy, because of the risk of bone loss and vasomotor symptoms.
Excision of endometriomas adversely affects ovarian follicular reserve (as indicated by lower levels of anti-müllerian hormone and reduced ovarian antral follicle counts on ultrasound). For women who want to preserve their fertility, the potential benefits of surgery should be weighed against these negative effects. Surgical treatment of endometriosis in women without other identifiable infertility factors may improve rates of spontaneous pregnancy. In women with moderate to severe endometriosis, intrauterine insemination with ovarian stimulation may be of value, particularly with preceding GnRH-agonist therapy (J Endometr Pelvic Pain Disord. 2018;10[3]:158-73).
Despite the reduction in IVF outcomes in women with moderate to severe endometriosis, it remains unclear whether surgery improves the likelihood of pregnancy with IVF as does the concurrent use of prolonged GnRH agonist during IVF stimulation. (Fertil Steril. 2012;98:591-8).
Summary
- Medical therapy alone does not appear to improve fertility in endometriosis.
- Surgical treatment of endometriosis improves natural fertility, particularly in lower-stage endometriosis.
- EFI is a useful tool to predict postoperative natural fertility and assess the need for IVF.
- Despite advanced endometriosis reducing IVF outcomes, surgery or medical pretreatment to increase IVF success remains unproven.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Reassessing benzodiazepines: What role should this medication class play in psychiatry?
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Many psychiatrists have had the grim experience of a newly referred patient explaining that her (and it is most often “her”) primary care doctor has been prescribing lorazepam 8 mg per day or alprazolam 6 mg per day and is sending her to you for help with ongoing anxiety. For conscientious psychiatrists, this means the beginning of a long tapering process along with a great deal of reassuring of a patient who is terrified of feeling overwhelmed with anxiety. The same problem occurs with patients taking large doses of sedatives who are still unable to sleep.
Mark Olfson and coauthors quantified benzodiazepine use in the United States in 2008 using a large prescription database, and found that 5.2% of adults between 18 and 80 years old were taking these drugs.1 The percentage increased with age, to 8.7% of those 65-80 years, in whom 31% received long-term prescriptions from a psychiatrist. Benzodiazepine use was twice as prevalent in women, compared with men. This occurs despite peer-reviewed publications and articles in the popular press regarding the risks of long-term benzodiazepine use in the elderly. Fang-Yu Lin and coauthors documented a 2.23-fold higher risk of hip fracture in zolpidem users that increased with age; elderly users had a 21-fold higher incidence of fracture, compared with younger users, and were twice as likely to sustain a fracture than elderly nonusers.2
Rashona Thomas and Edid Ramos-Rivas reviewed the risks of benzodiazepines in older patients with insomnia and document the increase in serious adverse events such as falls, fractures, and cognitive and behavioral changes.3 Many patients have ongoing prescriptions that make discontinuation difficult, given the potential for withdrawal agitation, seizures, insomnia, nightmares and even psychosis.
Greta Bushnell and coauthors pointed to the problem of simultaneous prescribing of a new antidepressant with a benzodiazepine by 10% of doctors initiating antidepressants.4 Over 12% of this group of patients continued benzodiazepines long term, even though there was no difference in the response to antidepressant treatment at 6 months. Those with long-term benzodiazepine use were also more likely to have recent prescriptions for opiates.
A Finnish research team found that 34% of middle-aged and 55% of elderly people developed long-term use of benzodiazepines after an initial prescription.5 Those who became long-term users were more often older male receivers of social benefits, with psychiatric comorbidities and substance abuse histories.
Kevin Xu and coauthors reviewed a National Health and Nutrition Examination Survey dataset from 1999 to 2015 with follow-up on over 5,000 individuals in that period.6 They found doubling of all-cause mortality in users of benzodiazepines with or without accompanying use of opiates, a statistically significant increase.
Perhaps most alarming is the increased risk for Alzheimer’s dementia diagnosis in users of benzodiazepines. Two separate studies (Billoti de Gage and colleagues and Ettcheto and colleagues7,8) provided reviews of evidence for the relationship between use of benzodiazepines and development of dementia, and repeated warnings about close monitoring of patients and the need for alternative treatments for anxiety and insomnia in the elderly.
Be alert to underlying issues
Overburdened primary practitioners faced with complaints about sleep and anxiety understandably turn to medication rather than taking time to discuss the reasons for these problems or to describe nonmedication approaches to relief of symptoms. Even insured patients may have very limited options for “covered” psychiatric consultation, as many competent psychiatrists have moved to a cash-only system. It is easier to renew prescriptions than to counsel patients or refer them, and many primary care practitioners have limited experience with diagnosing causes of anxiety and insomnia, much less alternative medication approaches.
Psychiatrists should be aware of the frequency of underlying mood disorders that include sleep and anxiety as prominent symptoms; in fact, these symptoms are often what motivates patients to pursue treatment. It is critical to obtain not only a personal history of symptoms beginning in childhood up to the present, but also a family history of mood and anxiety problems. Mood dysregulation disorders are highly hereditary and a family history of mania or psychosis should raise concern about the cause of symptoms in one’s patient. A strong personal and/or family history of alcohol abuse and dependence may cover underlying undiagnosed mood dysregulation. Primary care physicians may not recognize mood dysregulation unless a patient is clearly manic or psychotic.
There is a cohort of patients who do well on antidepressant medication, but anorgasmia, fatigue, and emotional blunting are common side effects that affect compliance. When patients have unexpected responses to SSRI medications such as euphoria, agitation, anxiety, insomnia, and more prominent mood swings, primary care physicians may add a benzodiazepine, expecting the problem to abate with time. Unfortunately, this often leads to ongoing use of benzodiazepines, since attempts to stop them causes withdrawal effects that are indistinguishable from the original anxiety symptoms.
Most psychiatrists are aware that some patients need mood stabilization rather than mood elevation to maintain an adequate baseline mood. Lithium, anticonvulsants, and second-generation antipsychotics may be effective without adding antidepressant medication. Managing dosing and side effects requires time for follow-up visits with patients after initiating treatment but leads to more stability and better outcomes.
Benzodiazepines are appropriate and helpful in situations that cause transient anxiety and with patients who have done poorly with other options. Intermittent use is key to avoiding tolerance and inevitable dose increases. Some individuals can take low daily doses that are harmless, though these likely only prevent withdrawal rather than preventing anxiety. The placebo effect of taking a pill is powerful. And some patients take more doses than they admit to. Most practitioners have heard stories about the alprazolam that was accidentally spilled into the sink or the prescription bottle of diazepam that was lost or the lorazepam supply that was stolen by the babysitter.
These concepts are illustrated in case examples below.
Case one
Ms. A, a 55-year-old married female business administrator, admitted to using zolpidem at 40 mg per night for the past several months. She began with the typical dose of 10 mg at bedtime prescribed by her internist, but after several weeks, needed an additional 10 mg at 2 a.m. to stay asleep. As weeks passed, she found that she needed an additional 20 mg when she awoke at 2 a.m. Within months, she needed 20 mg to initiate sleep and 20 mg to maintain sleep. She obtained extra zolpidem from her gynecologist and came for consultation when refill requests were refused.
Ms. A had a family history of high anxiety in her mother and depressed mood in multiple paternal relatives, including her father. She had trouble sleeping beginning in adolescence, significant premenstrual dysphoria, and postpartum depression that led to a prescription for sertraline. Instead of feeling better, Ms. A remembers being agitated and unable to sleep, so she stopped it. Ms. A was now perimenopausal, and insomnia was worse. She had gradually increased wine consumption to a bottle of wine each night after work to “settle down.” This allowed her to fall asleep, but she inevitably awoke within 4 hours. Her internist noted an elevation in ALT and asked Ms. A about alcohol consumption. She was alarmed and cut back to one glass of wine per night but again couldn’t sleep. Her internist started zolpidem at that point.
The psychiatrist explained the concepts of tolerance and addiction and a plan to slowly taper off zolpidem while using quetiapine for sleep. She decreased to 20 mg of zolpidem at bedtime with quetiapine 50 mg and was able to stay asleep. After 3 weeks, Ms. A took zolpidem 10 mg at bedtime with quetiapine 75 mg and again, was able to fall asleep and stay asleep. After another 3 weeks, she increased quetiapine to 100 mg and stopped zolpidem without difficulty. This dose of quetiapine has continued to work well without significant side effects.
Case two
Ms. B, a 70-year-old married housewife, was referred for help with longstanding anxiety when her primary care doctor recognized that lorazepam, initially helpful at 1 mg twice daily, had required titration to 2 mg three times daily. Ms. B was preoccupied with having lorazepam on hand and never missed a dose. She had little interest in activities beyond her home, rarely socialized, and had fallen twice. She napped for 2 hours each afternoon, and sometimes had trouble staying asleep through the night.
Ms. B was reluctant to talk about her childhood history of hostility and undermining by her mother, who clearly preferred her older brother and was competitive with Ms. B. Her father traveled for work during the week and had little time for her. Ms. B had always seen herself as stupid and unlovable, which interfered with making friends. She attended college for 1 year but dropped out to marry her husband. He was also anxious and had difficulty socializing, but they found reassurance in each other. Their only child, a son in his 40s, was estranged from them, having married a woman who disliked Ms. B. Ms. B felt hopeless about developing a relationship with her grandchildren who were rarely allowed to visit. Despite her initial shame in talking about these painful problems, Ms. B realized that she felt better and scheduled monthly visits to check in.
Ms. B understood the risks of using lorazepam and wanted to stop it but was terrified of becoming anxious again. We set up a very slow tapering schedule that lowered her total dose by 0.5 mg every 2 weeks. At the same time, she began escitalopram which was effective at 20 mg. Ms. B noted that she no longer felt anxious upon awakening but was still afraid to miss a dose of lorazepam. As she felt more confident and alert, Ms. B joined a painting class at a local community center and was gratified to find that she was good at working with watercolors. She invited her neighbors to come for dinner and was surprised at how friendly and open they were. Once she had tapered to 1 mg twice daily, Ms. B began walking for exercise as she now had enough energy that it felt good to move around. After 6 months, she was completely off lorazepam, and very grateful to have discovered her capacity to improve her pleasure in life.
Case three
Ms. C, a 48-year-old attorney was referred for help with anxiety and distress in the face of separation from her husband who had admitted to an affair after she heard him talking to his girlfriend from their basement. She was unsure whether she wanted to save the marriage or end it and was horrified at the thought of dating. She had never felt especially anxious or depressed and had a supportive circle of close friends. She was uncharacteristically unable to concentrate long enough to consider her options because of anxiety.
A dose of clonazepam 0.5 mg allowed her to stay alert but calm enough to reflect on her feelings. She used it intermittently over several months and maintained regular individual psychotherapy sessions that allowed her to review the situation thoroughly. On her psychiatrist’s recommendation, she contacted a colleague to represent her if she decided to initiate divorce proceedings. She attempted to engage her husband in marital therapy, and his reluctance made it clear to her that she could no longer trust him. Ms. C offered him the option of a dissolution if he was willing to cooperate, or to sue for divorce if not. Once Ms. C regained her confidence and recognized that she would survive this emotionally fraught situation, she no longer needed clonazepam.
Summary
The risks, which include cognitive slowing, falls and fractures, and withdrawal phenomena when abruptly stopped, make this class dangerous for all patients but particularly the elderly. Benzodiazepines are nonetheless useful medications for patients able to use them intermittently, whether on an alternating basis with other medications (for example, quetiapine alternating with clonazepam for chronic insomnia) or because symptoms of anxiety are intermittent. Psychiatrists treating tolerant patients should be familiar with the approach of tapering slowly while introducing more appropriate medications at adequate doses to manage symptoms.
Dr. Kaplan is training and supervising psychoanalyst at the Cincinnati Psychoanalytic Institute and volunteer professor of clinical psychiatry at the University of Cincinnati. The author reported no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Olfson M et al. JAMA Psychiatry. 2015 Feb;72(2):136-42. doi: 10.1001/jamapsychiatry.2014.1763.
2. Lin FY et al. Sleep. 2014 Apr 1;37(4):673-9. doi: 10.5665/sleep.3566.
3. Thomas R and Ramos-Rivas E. Psychiatr Ann. 2018;48(6):266-70. doi: 10.3928/00485713-20180513-01.
4. Bushnell GA et al. JAMA Psychiatry. 2017 Jul 1;74(7):747-55. doi: 10.1001/jamapsychiatry.2017.1273.
5. Taipale H et al. JAMA Netw Open. 2020;3(10):e2019029. doi: 10.1001/jamanetworkopen.2020.19029.
6. Xu KY et al. JAMA Netw Open. 2020;3(12):e2028557. doi: 10.1001/jamanetworkopen.2020.28557.
7. Billioti de Gage S et al. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205.
8. Ettcheto M et al. Front Aging Neurosci. 2020 Jan 8;11:344. doi: 10.3389/fnagi.2019.00344.
Burnout and stress of today: How do we cope?
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Interestingly, the group that seems to be least impacted by this was health care administrators (with 12% of them planning on leaving their jobs).
I couldn’t stop thinking about these percentages.
I am reminded every day of the commitment and excellence of my colleagues in the health care field, and I do not want to lose them. I am hoping the following information and my thoughts on this topic will be helpful for those thinking about leaving health care.
Surgeon general’s burnout report
The surgeon general recently released a report on addressing health care worker burnout.2 It includes several very interesting and appropriate observations. I will summarize the most important ones here:
1. Our health depends on the well-being of our health workforce.
2. Direct harm to health care workers can lead to anxiety, depression, insomnia, and interpersonal and relationship struggles.
3. Health care workers experience exhaustion from providing overwhelming care and empathy.
4. Health care workers spend less time with patients and too much time with EHRs.
5. There are health workforce shortages.
The report is comprehensive, and everything in it is correct. The real issue is how does it go from being a report to true actionable items that we as health care professionals benefit from? I think in regards to exhaustion from overwhelming care responsibilities, and empathy fatigue, we need better boundaries.
Those who go into medicine, and especially those who go into primary care, always put the patients’ needs first. When operating in a broken system, it stays broken when individuals cover for the deficiencies in the system. Adding four extra patients every day because there is no one to refer them to with availability is injurious to the health care provider, and those providers who accept these additional patients will eventually be part of the 23% who want to leave their jobs. It feels awful to say no, but until the system stops accommodating there will not be substantial change.
The empathy drain
One of the unreported stresses of open access for patients through EHR communications is the empathy drain on physicians. When I see a patient in clinic with chronic symptoms or issues, I spend important time making sure we have a plan and an agreed upon time frame.
With the EHR, patients frequently send multiple messages for the same symptoms between visits. It is okay to redirect the patient and share that these issues will be discussed at length at appointments. My reasoning on this is that I think it is better for me to better care for myself and stay as the doctor for my patients, than always say yes to limitless needs and soon be looking for the off ramp.
The following statistic in the surgeon general’s report really hit home. For every hour of direct patient care, physicians currently spend 2 hours on the EHR system. Most practices allow 10%-20% of time for catch up, where with statistics like this it should be 50%. This concept is fully lost on administrators, or ignored.
It is only when we refuse to continue to accept and follow a broken system that it will change. A minority of internal medicine and family doctors (4.5% in 2018) practice in direct primary care models, where these issues are addressed. Unfortunately, this model as it is currently available is not an option for lower income patients.
A major theme in the surgeon general’s report was that administrative burdens need to be reduced by 75% by 2025. When I look at the report, I see the suggestions, I just don’t see how it will be achieved. Despite almost all clinics moving to the EHR, paperwork in the form of faxes and forms has increased.
A sweeping reform would be needed to eliminate daily faxes from PT offices, visiting nurse services, prior authorization, patients reminders from insurance companies, and disability forms from patients. I am glad that there is acknowledgment of the problem, but this change will take more than 3 years.
Takeaways
So what do we do?
Be good to yourself, and your colleagues. The pandemic has isolated us, which accelerates burnout.
Reach out to people you care about.
We are all feeling this. Set boundaries that allow you to care for yourself, and accept that you are doing your best, even if you can’t meet the needs of all your patients all the time.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Sinsky CA et al. Covid-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021 Dec;5(6):1165-73.
2. Addressing health worker burnout. The U.S. Surgeon General’s advisory on building a thriving health workforce.
Author Q&A: Intravenous Immunoglobulin for Treatment of COVID-19 in Select Patients
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
Dr. George Sakoulas is an infectious diseases clinician at Sharp Memorial Hospital in San Diego and professor of pediatrics at the University of California, San Diego School of Medicine. He was the lead investigator in a study published in the May/June 2022 issue of JCOM that found that, when allocated to the appropriate patient type, intravenous immunoglobulin can reduce hospital costs for COVID-19 care. 1 He joined JCOM’s Editor-in-Chief, Dr. Ebrahim Barkoudah, to discuss the study’s background and highlight its main findings.
The following has been edited for length and clarity.
Dr. Barkoudah Dr. Sakoulas is an investigator and a clinician, bridging both worlds to bring the best evidence to our patients. We’re discussing his new article regarding intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia. Dr. Sakoulas, could you please share with our readers the clinical question your study addressed and what your work around COVID-19 management means for clinical practice?
Dr. Sakoulas Thank you. I’m an infectious disease physician. I’ve been treating patients with viral acute respiratory distress syndrome for almost 20 years as an ID doctor. Most of these cases are due to influenza or other viruses. And from time to time, anecdotally and supported by some literature, we’ve been using IVIG, or intravenous immunoglobulin, in some of these cases. And again, I can report anecdotal success with that over the years.
So when COVID emerged in March of 2020, we deployed IVIG in a couple of patients early who were heading downhill. Remember, in March of 2020, we didn’t have the knowledge of steroids helping, patients being ventilated very promptly, and we saw some patients who made a turnaround after treatment with IVIG. We were able to get some support from an industry sponsor and perform and publish a pilot study, enrolling patients early in the pandemic. That study actually showed benefits, which then led the sponsor to fund a phase 3 multicenter clinical trial. Unfortunately, a couple of things happened. First, the trial was designed with the knowledge we had in April of 2020, and again, this is before steroids, before we incorporated proning patients in the ICU, or started ventilating people early. So there were some management changes and evolutions and improvements that happened. And second, the trial was enrolling a very broad repertoire of patients. There were no age limitations, and the trial, ultimately a phase 3 multicenter trial, failed to meet its endpoint.
There were some trends for benefit in younger patients, and as the trial was ongoing, we continued to evolve our knowledge, and we really honed it down to seeing a benefit of using IVIG in patients with COVID with specific criteria in mind. They had to be relatively younger patients, under 65, and not have any major comorbidities. In other words, they weren’t dialysis patients or end-stage disease patients, heart failure patients, cancer or malignancy patients. So, you know, we’re looking at the patients under 65 with obesity, diabetes, and hypertension, who are rapidly declining, going from room air to BiPAP or high-flow oxygen in a short amount of time. And we learned that when using IVIG early, we actually saw patients improve and turn around.
What this article in JCOM highlighted was, number one, incorporating that outcome or that patient type and then looking at the cost of hospitalization of patients who received IVIG versus those that did not. There were 2 groups that were studied. One was the group of patients in that original pilot trial that I discussed who were randomized to receive 1 or the other prospectively; it was an unblinded randomized study. And the second group was a matched case-control study where we had patients treated with IVIG matched by age and comorbidity status and level of hypoxia to patients that did not receive IVIG. We saw a financial benefit in shortening or reducing hospitalizations, really coming down to getting rid of that 20% tail of patients that wound up going to the ICU, getting intubated, and using a high amount of hospital resources that would ramp up the cost of hospitalization. We saw great mitigation of that with IVIG, and even with a small subset of patients, we were able to show a benefit.
Dr. Barkoudah Any thoughts on where we can implement the new findings from your article in our practice at the moment, knowing we now have practice guidelines and protocols to treat COVID-19? There was a tangible benefit in treating the patients the way you approached it in your important work. Could you share with us what would be implementable at the moment?
Dr. Sakoulas I think, fortunately, with the increasing host immunity in the population and decreased virulence of the virus, perhaps we won’t see as many patients of the type that were in these trials going forward, but I suspect we will perhaps in the unvaccinated patients that remain. I believe one-third of the United States is not vaccinated. So there is certainly a vulnerable group of people out there. Potentially, an unvaccinated patient who winds up getting very sick, the patient who is relatively young—what I’m looking at is the 30- to 65-year-old obese, hypertensive, or diabetic patient who comes in and, despite the steroids and the antivirals, rapidly deteriorates into requiring high-flow oxygen. I think implementing IVIG in that patient type would be helpful. I don’t think it’s going to be as helpful in patients who are very elderly, because I think the mechanism of the disease is different in an 80-year-old versus a 50-year-old patient. So again, hopefully, it will not amount to a lot of patients, but I still suspect hospitals are going to see, perhaps in the fall, when they’re expecting a greater number of cases, a trickling of patients that do meet the criteria that I described.
Dr. Barkoudah JCOM’s audience are the QI implementers and hospital leadership. And what caught my eye in your article is your perspective on the pharmacoeconomics of treating COVID-19, and I really appreciate your looking at the cost aspect. Would you talk about the economics of inpatient care, the total care that we provide now that we’re in the age of tocilizumab, and the current state of multiple layers of therapy?
Dr. Sakoulas The reason to look at the economics of it is because IVIG—which is actually not a drug, it’s a blood product—is very expensive. So, we received a considerable amount of administrative pushback implementing this treatment at the beginning outside of the clinical trial setting because it hadn’t been studied on a large scale and because the cost was so high, even though, as a clinician at the bedside, I was seeing a benefit in patients. This study came out of my trying to demonstrate to the folks that are keeping the economics of medicine in mind that, in fact, investing several thousand dollars of treatment in IVIG will save you cost of care, the cost of an ICU bed, the cost of a ventilator, and the cost even of ECMO, which is hugely expensive.
If you look at the numbers in the study, for two-thirds or three-quarters of the patients, your cost of care is actually greater than the controls because you’re giving them IVIG, and it’s increasing the cost of their care, even though three-quarters of the patients are going to do just as well without it. It’s that 20% to 25% of patients that really are going to benefit from it, where you’re reducing your cost of care so much, and you’re getting rid of that very, very expensive 20%, that there’s a cost savings across the board per patient. So, it’s hard to understand when you say you’re losing money on three-quarters of the patients, you’re only saving money on a quarter of the patients, but that cost of saving on that small subset is so substantial it’s really impacting all numbers.
Also, abandoning the outlier principle is sort of an underlying theme in how we think of things. We tend to ignore outliers, not consider them, but I think we really have to pay attention to the more extreme cases because those patients are the ones that drive not just the financial cost of care. Remember, if you’re down to 1 ventilator and you can cut down the use of scarce ICU resources, the cost is sort of even beyond the cost of money. It’s the cost of resources that may become scarce in some settings. So, I think it speaks to that as well.
A lot of the drugs that we use, for example, tocilizumab, were able to be studied in thousands of patients. If you look at the absolute numbers, the benefit of tocilizumab from a magnitude standpoint—low to mid twenties to high twenties—you know, reducing mortality from 29% to 24%. I mean, just take a step back and think about that. Even though it’s statistically significant, try telling a patient, “Well, I’m going to give you this treatment that’s going to reduce mortality from 29% to 24%.” You know, that doesn’t really change anything from a clinical significance standpoint. But they have a P value less than .05, which is our standard, and they were able to do a study with thousands of patients. We didn’t have that luxury with IVIG. No one studied thousands of patients, only retrospectively, and those retrospective studies don’t get the attention because they’re considered biased with all their limitations. But I think one of the difficulties we have here is the balance between statistical and clinical significance. For example, in our pilot study, our ventilation rate was 58% with the non-IVIG patients versus 14% for IVIG patients. So you might say, magnitude-wise, that’s a big number, but the statistical significance of it is borderline because of small numbers.
Anyway, that’s a challenge that we have as clinicians trying to incorporate what’s published—the balancing of statistics, absolute numbers, and practicalities of delivering care. And I think this study highlights some of the nuances that go into that incorporation and those clinical decisions.
Dr. Barkoudah Would you mind sharing with our audience how we can make the connection between the medical outcomes and pharmacoeconomics findings from your article and link it to the bedside and treatment of our patients?
Dr. Sakoulas One of the points this article brings out is the importance of bringing together not just level 1A data, but also small studies with data such as this, where the magnitude of the effect is pretty big but you lose the statistics because of the small numbers. And then also the patients’ aspects of things. I think, as a bedside clinician, you appreciate things, the nuances, much sooner than what percolates out from a level 1A study. Case in point, in the sponsored phase 3 study that we did, and in some other studies that were prospectively done as well, these studies of IVIG simply had an enrollment of patients that was very broad, and not every patient benefits from the same therapy. A great example of this is the sepsis trials with Xigris and those types of agents that failed. You know, there are clinicians to this day who believe that there is a subset of patients that benefit from agents like this. The IVIG story falls a little bit into that category. It comes down to trying to identify the subset of patients that might benefit. And I think we’ve outlined this subset pretty well in our study: the younger, obese diabetic or hypertensive patient who’s rapidly declining.
It really brings together the need to not necessarily toss out these smaller studies, but kind of summarize everything together, and clinicians who are bedside, who are more in tune with the nuances of individual decisions at the individual patient level, might better appreciate these kinds of data. But I think we all have to put it together. IVIG does not make treatment guidelines at national levels and so forth. It’s not even listed in many of them. But there are patients out there who, if you ask them specifically how they felt, including a friend of mine who received the medication, there’s no question from their end, how they felt about this treatment option. Now, some people will get it and will not benefit. We just have to be really tuned into the fact that the same drug does not have the same result for every patient. And just to consider this in the high-risk patients that we talked about in our study.
Dr. Barkoudah While we were prepping for this interview, you made an analogy regarding clinical evidence along the lines of, “Do we need randomized clinical trials to do a parachute-type of experiment,” and we chatted about clinical wisdom. Would you mind sharing with our readers your thoughts on that?
Dr. Sakoulas Sometimes, we try a treatment and it’s very obvious for that particular patient that it helped them. Then you study the treatment in a large trial setting and it doesn’t work. For us bedside clinicians, there are some interventions sometimes that do appear as beneficial as a parachute would be, but yet, there has never been a randomized clinical trial proving that parachutes work. Again, a part of the challenge we have is patients are so different, their immunology is different, the pathogen infecting them is different, the time they present is different. Some present early, some present late. There are just so many moving parts to treating an infection that only a subset of people are going to benefit. And sometimes as clinicians, we’re so nuanced, that we identify a specific subset of patients where we know we can help them. And it’s so obvious for us, like a parachute would be, but to people who are looking at the world from 30,000 feet, they don’t necessarily grasp that because, when you look at all comers, it doesn’t show a benefit.
So the problem is that now those treatments that might help a subset of patients are being denied, and the subset of patients that are going to benefit never get the treatment. Now we have to balance that with a lot of stuff that went on during the pandemic with, you know, ivermectin, hydroxychloroquine, and people pushing those things. Someone asked me once what I thought about hydroxychloroquine, and I said, “Well, somebody in the lab probably showed that it was beneficial, analogous to lighting tissue paper on fire on a plate and taking a cup of water and putting the fire out. Well, now, if you take that cup of water to the Caldor fire that’s burning in California on thousands of acres, you’re not going to be able to put the fire out with that cup of water.” So while it might work in the lab, it’s truly not going to work in a clinical setting. We have to balance individualizing care for patients with some information people are pushing out there that may not be necessarily translatable to the clinical setting.
I think there’s nothing better than being at the bedside, though, and being able to implement something and seeing what works. And really, experience goes a long way in being able to individually treat a patient optimally.
Dr. Barkoudah Thank you for everything you do at the bedside and your work on improving the treatment we have and how we can leverage knowledge to treat our patients. Thank you very much for your time and your scholarly contribution. We appreciate it and I hope the work will continue. We will keep working on treating COVID-19 patients with the best knowledge we have.
Q&A participants: George Sakoulas, MD, Sharp Rees-Stealy Medical Group, La Jolla, CA, and University of California San Diego School of Medicine, San Diego, CA; and Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Disclosures: None reported.
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
1. Poremba M, Dehner M, Perreiter A, et al. Intravenous immunoglobulin in treating nonventilated COVID-19 patients with moderate-to-severe hypoxia: a pharmacoeconomic analysis. J Clin Outcomes Manage. 2022;29(3):123-129. doi:10.12788/jcom.0094
Comorbidity Coding and Its Impact on Hospital Complexity: Reply
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).
We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).

We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Authors' Response
We agree with the valid comments made by Dr. Kerguelen and will respond to each set of questions in order.
Regarding the first set of questions on how we knew that our CMI was low and our patient acuity was under- represented, the University of Miami Health System is a designated cancer center with a Prospective Payment System exempt model (PPS exempt), and is one of 11 hospitals in the United States excluded for payment under the Inpatient Prospective Payment System. We know, therefore, that we care for a very complex patient population. Additionally, we benchmark ourselves against other academic medical centers (AMCs) with similarly complex patients and had noted that our patients appeared “less complex.” Specifically, our baseline CMI was 1.77 in early 2018 compared with an overall higher CMI for the AMC cohort; also, the total number of diagnoses we captured was lower than that in other AMCs. These 2 facts together alerted us that we likely had coding and clinical documentation improvement (CDI) opportunities. We recognized that our complexity was not being captured both because the clinical information was not documented in a manner readily translatable to ICD-10 codes and codes were missed when the documentation did exist. To remedy these problems, we implemented multiple immediate “fixes,” which included revamping our CDI efforts, re-education, and enhancements to our electronic health record for providers, CDIs, and coders. Since publication of our article, our CMI has continued to increase month over month, up to 2.57 most recently in May 2022, as we have continued to focus on several additional initiatives to impact both better documentation and coding.
The second set of questions asked whether the perceived low CMI was causing problems with payers and about the risk of artificially increasing the CMI through overdiagnosis as well as audit mechanisms to avoid this, and changes in expected mortality and observed mortality. To our knowledge, the lower CMI did not cause any problems with payers, but this is something we are currently tracking. Coding and documentation are constantly audited both internally (by our quality department) and externally (using Inter-Rater Reliability audits and validation), with no noted trend or targeted opportunities. We only include comorbidities that are current, actively monitored/managed, and pertinent to the care of our patients. We have not noted a change in denials, which gives us confidence we are not now overdiagnosing.
Our observed mortality has also increased. We, like all institutions, experienced the confounding factor of the COVID-19 pandemic, which coincided with the higher observed mortality over the course of the past 2 years. While the observed mortality (indicating sicker patients assuming no worsening of care processes) may partly explain our increased coding complexity, our decreasing mortality index (observed:expected mortality) suggests that our efforts to improve documentation and coding likely reflect improved capture of missed complexity (Figure).

We understand the concerns raised by Dr. Kerguelen about potential mis(over)coding. As part of this quality initiative, therefore, we plan long-term evaluations of our processes and metrics to better determine and guide our understanding of the impact of what we have already implemented and future interventions. In fact, we are in the process of analyzing additional interventions and hope to share results from these evaluations soon.
Marie Anne Sosa, MD
Tanira Ferreira, MD
Hayley Gershengorn, MD
Melissa Soto
Estin Kelly
Ameena Shrestha
Julianne Burgos
Sandeep Devabhaktuni
Dipen Parekh, MD
Maritza Suarez, MD
University of Miami Hospital and Clinics, Miami, FL
[email protected]
Disclosures: None reported.
Comorbidity Coding and Its Impact on Hospital Complexity
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
To the Editor:
I read with interest the article by Sosa and colleagues1 in which they present some stimulating analyses pertaining to a topic that we have been discussing at my institution for several years. Part of this discussion deals with the complexity of our hospital and how complexity is affected by comorbidity coding.
In 2013, we implemented the International Refined-DRGs (IR-DRGs) system to measure complexity at our hospital in Bogotá, Colombia. Our perception at that time was that the case mix index (CMI) was very low (0.7566), even for a general hospital with a high volume of pathologies with low relative weight (RW). Two medical auditors were assigned to review the medical records in order to improve the quality, quantity, and order of diagnoses. Emphasis was placed on patients with stays longer than 5 days and with only 1 diagnosis coded at admission. Additionally, International Classification of Diseases 10th Revision (World Health Organization version) diagnoses from chapters R (Symptoms and Signs Not Elsewhere Classified) and V through Y (External Causes) were blocked in the electronic health record. With these measures, our CMI increased 74%, reaching 1.3151 by the end of 2021, with a maximum peak of 1.6743 in May 2021, which coincided with the third peak of COVID-19 in Colombia.
However, the article by Sosa and colleagues draws my attention to the following: why do the authors state that their CMI is low and the patient acuity was under-represented? Is this due to a comparison with similar hospitals, or to a recommendation from a regulatory agency? We have found our CMI remains low because of a high volume of nonsurgical care (60%), deliveries, and digestive, respiratory, and urinary pathologies of low RW.
Also, was the perceived low CMI causing problems with payers? And further, how did the authors avoid the risk of artificially increasing the CMI through overdiagnosis of patients, and were there audit mechanisms to avoid this? While there was a clear change in expected mortality, did the observed mortality also change with the strategies implemented? This last question is relevant because, if the observed mortality were maintained, this would provide evidence that a coding problem was the cause of their hospital’s low CMI.
I reiterate my congratulations to the authors for presenting analyses that are very useful to other providers and researchers worldwide interested in addressing management issues related to the correct identification and classification of patients.
Carlos Kerguelen, MD, MA
Fundacion Santa Fe de Bogotá, Bogotá, Colombia
[email protected]
Disclosures: None reported.
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
1. Sosa M, Ferreira T, Gershengorn H, et al. Improving hospital metrics through the implementation of a comorbidity capture tool and other quality initiatives. J Clin Outcomes Manage. 2022;29(2):80-87. doi:10.12788/jcom.0088
Supporting Patients on Complex Care Journeys: How Technology Can Bridge the Gaps
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
From Memora Health (Dr. Flyckt and Dr. Colbert), San Francisco, CA; and Harvard Medical School (Dr. Colbert), Boston, MA.
A close relative was recently diagnosed with follicular lymphoma. He was cared for at a high-ranked cancer center by physicians with demonstrated expertise, and even had the support of a care navigator. Still, he was often left feeling overwhelmed and confused, holding an inch-thick stack of papers, instructions, and pamphlets. As he left his treatment planning visit, reeling from the emotional burden of his diagnosis and all the unfamiliar terminology, he didn’t know what to do or what to expect. Later, when he experienced early signs of tumor lysis syndrome, he struggled to reach his care team for triage and guidance. When he went to the emergency room, his oncologist was never informed.
This scenario is unfortunately common, and versions of this scenario play out thousands of times each day across the US health system. Within the clinic and hospital setting, patients receive excellent care from their providers, but a disconnect emerges once the patient leaves these medical settings: patients at home struggle to find guidance and support, while care teams lack the tools to engage patients between visits or monitor their health across care settings, providers, or episodes of care.
Leveraging Technology to Move From Episodes of Care to Complex Care Journeys
The use of automated messaging, artificial intelligence and natural language processing–driven chat experiences, and text-based support is becoming more common. However, health care lags behind other industries in the adoption of these technologies.1,2 The slow pace can be warranted, given that health care is more complicated and higher risk than inquiring about a lost package, ordering groceries, or applying for a mortgage. At the same time, many of the consumer engagement tools used to guide an applicant through the multiple steps and complexities of their home loan process or to prompt viewers to select new shows to binge have applications in health care.
Over the past few years, technologies have emerged that guide patients through complex care journeys and allow care teams to monitor and engage patients between visits. These solutions come in different formats, but generally patients can receive messages on their phones that contain disease-specific educational content, prompts to fill prescriptions and take medications, and reminders and guidance on how to prepare for appointments and procedures. These programs also collect relevant data from patients through survey and electronic patient-reported outcomes instruments, as well as connected patient monitoring devices, that help track patient progress and identify issues as they arise. Many programs also incorporate symptom triage pathways and use natural language processing to respond automatically to patient questions and concerns.3,4
These technology solutions can automate many tasks that in the past required a care team member to spend hours on the phone. Newly freed from such repetitive tasks, care teams can now focus on more in-depth interactions with those patients who are most in need—the types of interactions that are more satisfying and rewarding. Such assistance is particularly needed today with the staffing shortages faced by most health systems.5
In addition, technology allows teams to see the panel of patients they are caring for and to quickly identify and take action on any specific needs or issues. Care teams can focus on any patient and see where they are in their journey. When appropriate, some solutions also allow care teams to engage directly with patients through text-messaging, creating a seamless experience and unified communication channel. Ideally, these solutions should be linked or embedded within the electronic health record or other primary system of record, so that teams can easily access these tools through their existing workflows and avoid creating yet another interface to navigate.
The Impact of Low-Tech Solutions to Deliver High-Touch Support
There is evidence showing that digital patient navigation tools impact patient care. In the oncology setting, patients with a digital navigator have achieved over 95% adherence rates with complex oral chemotherapy regimens (Memora Health Unpublished Data. 2022.). In the postpartum setting, a text message–based program improved screening rates for postpartum depression and did so with very high patient satisfaction ratings.6 Particularly notable is the fact that this depression screening program achieved these results in a population that was predominantly low income, with more than half belonging to underrepresented minority populations.6
We believe these digital patient navigation technologies, specifically low-tech solutions that don’t require app downloads, portal log-ins, or high-speed internet, will transform care delivery over the next 5 to 10 years. Successful management of complex conditions like diabetes or cancer requires more than 3 hours of care each day,7 yet most patients spend only 1 or 2 hours per month directly interacting with their health care providers. However, most patients carry their phones with them at all times, and artificial intelligence–enabled text support is “always on” to provide support, monitoring, and guidance, wherever a patient happens to be when assistance is needed.
Shifting the Model to Support a Lifetime of Care
While still in the early stages of development, these tools have the potential to radically alter the practice of medicine, shifting the focus from episodic interactions to continuous journey-based care delivery. Outside of an acute event bringing a patient into the clinic or emergency room, many patients go a year or more without seeing their primary care providers.8 During that time, an immense amount of information is underreported or completely lost. Capturing this information in real-time and more holistically over a person’s lifetime of care could provide physicians better insight to both better manage and more fully evaluate the success of treatment plans by tracking patient symptoms, pain, and functional status over time. With this more longitudinal view of the patient, we see a pathway towards achieving the Quadruple Aim: patients who are more supported will achieve better outcomes at lower cost, they will have a better experience, and care teams will be empowered to focus their time on more satisfying activities rather than repetitive administrative tasks.
Corresponding author: James A. Colbert, MD, MBA; [email protected]
Disclosures: Dr. Flyckt and Dr. Colbert are employed by Memora Health, an organization that helps health care systems digitize and automate care journeys.
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
1. Hermes S, Riasanow T, Clemons EK, et al. The digital transformation of the healthcare industry: exploring the rise of emerging platform ecosystems and their influence on the role of patients. Bus Res. 2020;13:1033-1069. doi:10.1007/s40685-020-00125-x
2. Van Velthoven MH, Cordon C. Sustainable adoption of digital health innovations: perspectives from a stakeholder workshop. J Med Internet Res. 2019;21(3):e11922. doi:10.2196/11922
3. Campbell K, Louie P, Levine B, Gililland J. Using patient engagement platforms in the postoperative management of patients. Curr Rev Musculoskelet Med. 2020;13(4):479-484. doi:10.1007/s12178-020-09638-8
4. Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi:10.2196/27850
5. Data brief: health care workforce challenges threaten hospitals’ ability to care for patients. American Hospital Association. Accessed July 24, 2022. www.aha.org/fact-sheets/2021-11-01-data-brief-health-care-workforce-challenges-threaten-hospitals-ability-care
6. Gaulton JS, Leitner K, Hahn L, et al. Healing at home: applying innovation principles to redesign and optimise postpartum care. BMJ Innovations. 2022;8:37-41.
7. Østbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. doi:10.1370/afm.310
8. Ganguli I, Shi Z, E. Orav J, et al. Declining use of primary care among commercially insured adults in the united states, 2008–2016. Ann Intern Med. 2020;172:240-247. doi:10.7326/M19-1834
Reversing depression: A plethora of therapeutic strategies and mechanisms
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7
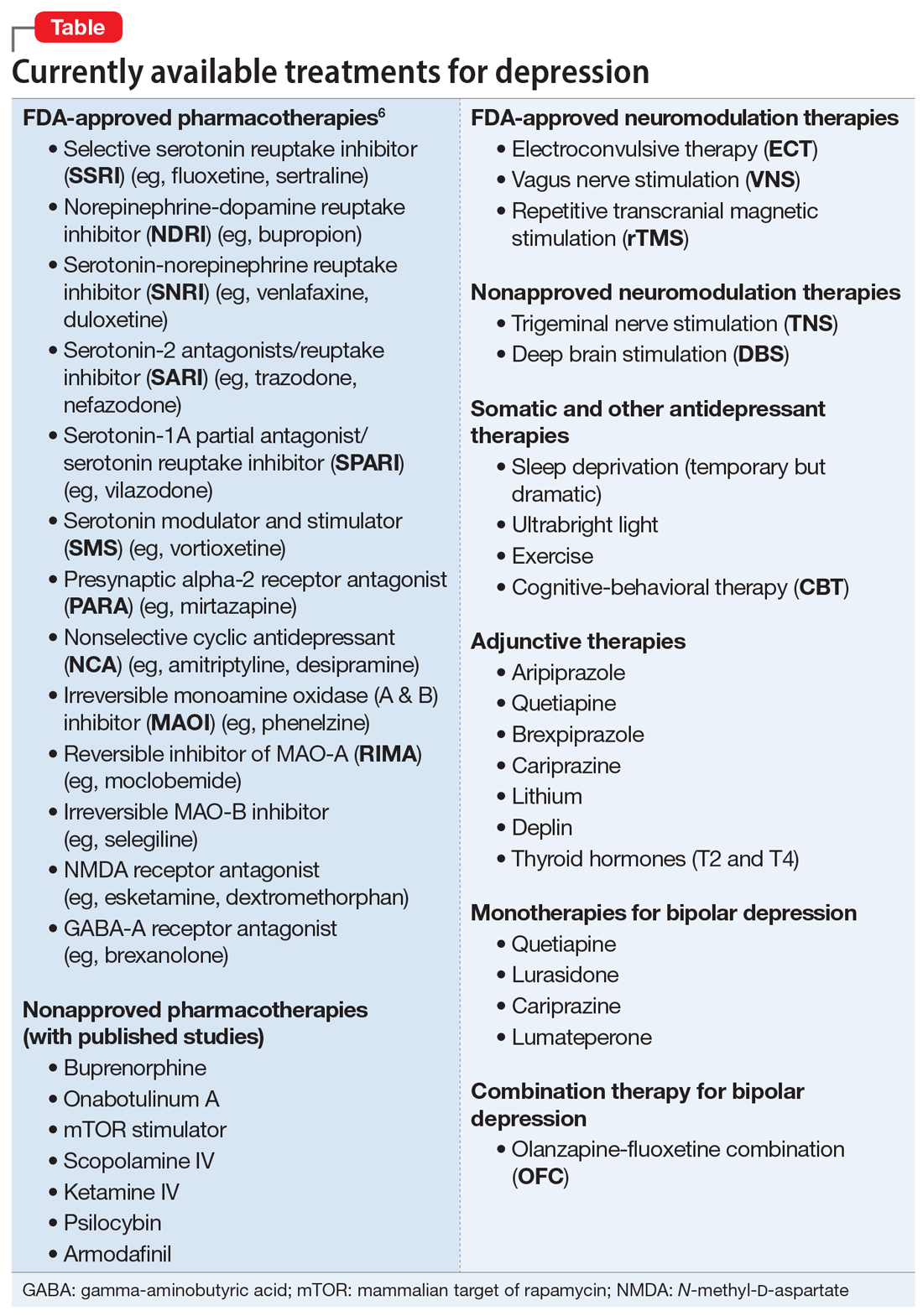
These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
Despite much progress, major depressive disorder (MDD) continues to be a challenging and life-threatening neuropsychiatric disorder. It is highly prevalent and afflicts tens of millions of Americans.
It is also ranked as the No. 1 disabling medical (not just psychiatric) condition by the World Health Organization.1 A significant proportion of patients with MDD do not respond adequately to several rounds of antidepressant medications,2 and many are labeled as having “treatment-resistant depression” (TRD).
In a previous article, I provocatively proposed that TRD is a myth.3 What I meant is that in a heterogeneous syndrome such as depression, failure to respond to 1, 2, or even 3 antidepressants should not imply TRD, because there is a “right treatment” that has not yet been identified for a given depressed patient. Most of those labeled as TRD have simply not yet received the pharmacotherapy or somatic therapy with the requisite mechanism of action for their variant of depression within a heterogeneous syndrome. IV ketamine, which, astonishingly, often reverses severe TRD of chronic duration within a few hours, is a prime example of why the term TRD is often used prematurely. Ketamine’s mechanism of action (immediate neuroplasticity via glutamate N-methyl-
Some clinicians may not be aware of the abundance of mechanisms of action currently available for the treatment of MDD as well as bipolar depression. Many practitioners, in both psychiatry and primary care, usually start the treatment of depression with a selective serotonin reuptake inhibitor, and if that does not produce a response or remission, they might switch to a serotonin-norepinephrine reuptake inhibitor. If that does not control the patient’s depressive symptoms, they start entertaining the notion that the patient may have TRD, not realizing that they have barely scratched the surface of the many therapeutic options and mechanisms of action, one of which could be the “best match” for a given patient.4
There will come a day when “precision psychiatry” finally arrives, and specific biomarkers will be developed to identify the “right” treatment for each patient within the heterogenous syndrome of depression.5 Until that day arrives, the treatment of depression will continue to be a process of trial and error, and hit or miss. But research will eventually discover genetic, neurochemical, neurophysiological, neuroimaging, or neuroimmune biomarkers that will rapidly guide clinicians to the correct treatment. This is critical to avoid inordinate delays in achieving remission and avert the ever-present risk of suicidal behavior.
The Table6 provides an overview of the numerous treatments currently available to manage depression. All increase brain-derived neurotrophic factor and restore healthy neuroplasticity and neurogenesis, which are impaired in MDD and currently believed to be a final common pathway for all depression treatments.7

These 41 therapeutic approaches to treating MDD or bipolar depression reflect the heterogeneity of mechanisms of action to address an equally heterogeneous syndrome. This implies that clinicians have a wide array of on-label options to manage patients with depression, aiming for remission, not just a good response, which typically is defined as a ≥50% reduction in total score on one of the validated rating scales used to quantify depression severity, such as the Montgomery-Åsberg Depression Rating Scale, Hamilton Depression Rating Scale, or Calgary Depression Scale for Schizophrenia.
Continue to: When several FDA-approved pharmacotherapies...
When several FDA-approved pharmacotherapies fall short and produce a suboptimal response, clinicians can resort to other treatment options known to have a higher efficacy than oral antidepressants. These include electroconvulsive therapy, repetitive transcranial magnetic stimulation, and vagus nerve stimulation. Other on-label options include adjunctive therapy with one of the approved second-generation antipsychotic agents or with adjunctive esketamine.
But if the patient still does not improve, one of many emerging off-label treatment options may work. One of the exciting new discoveries is the hallucinogen psilocybin, whose mechanism of action is truly unique. Unlike standard antidepressant medications, which modulate neurotransmitters, psilocybin increases the brain’s network flexibility, decreases the modularity of several key brain networks (especially the default-brain network, or DMN), and alters the dark and distorted mental perspective of depression to a much healthier and optimistic outlook about the self and the world.8 Such novel breakthroughs in the treatment of severe depression will shed some unprecedented insights into the core neurobiology of depression, and may lead to early intervention and prevention.
As the saying goes, all roads lead to Rome. Psychiatric clinicians should rejoice that there are abundant approaches and therapeutic mechanisms to relieve their severely melancholic (and often suicidal) patients from the grips of this disabling and life-altering brain syndrome.
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
1. World Health Organization. Depression: let’s talk says WHO, as depression tops list of causes of ill health. March 30, 2017. Accessed July 5, 2022. www.who.int/news/item/30-03-2017--depression-let-s-talk-says-who-as-depression-tops-list-of-causes-of-ill-health
2. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12)1243-1252.
3. Nasrallah HA. Treatment resistance is a myth! Current Psychiatry. 2021;20(3):14-16,28.
4. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
5. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi:10.1016/j.bionps.2019.100001
6. Procyshyn RM, Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs. 23rd ed. Hogrefe; 2019.
7. Tartt AN, Mariani, MB, Hen R, et al. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol Psychiatry. 2022;27(6):2689-2699.
8. Lowe H, Toyang N, Steele B, et al. The therapeutic potential of psilocybin. Molecules. 2021;26(10):2948. doi: 10.3390/molecules26102948
More on stigma
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
I just finished reading your editorial “A PSYCHIATRIC MANIFESTO: Stigma is hate speech and a hate crime” (
Our son went from an honor roll student before the pandemic to a child I barely recognized. Approximately 6 months into the pandemic, he was using drugs, vaping nicotine, destroying our property, and eloping at night. The journey of watching his decline and getting him help was agonizing. But the stigma around what was happening to him was an entirely separate animal.
Our society vilifies, ridicules, dismisses, and often makes fun of those with mental health issues. I experience it daily with my son and am on constant guard to shoot down any comments and to calmly teach those who say such cruel things. But the shame my son feels is the most devastating part. Although we keep reminding him that his condition is a medical condition like diabetes or heart disease, for a teenage boy, that makes no sense. He just wants to be “normal.” And living in a world that rarely represents mental illness this way, it’s almost a lost cause to get him to let go of this shame. All we can do is love him, be there for him, support him, and do what we can to educate those around us about the stigma of mental illness.
What a powerful and accurate article. Thank you for putting into words what I have been thinking and feeling, and for being as outraged as we are at how this vulnerable population is treated. My husband is a psychiatrist and we live in an affluent urban area, so we are not in the middle of nowhere with no knowledge of what is happening to our son. And despite that, we still suffer from the stigma.
Thank you, Dr. Nasrallah.
Name withheld
I need to take a moment to thank you for your editorial about stigma being hate speech and a hate crime. I really agree with you, and I think the way you formulated and articulated this message is very compelling.
I have focused on normalizing mental health differences among entrepreneurs as a destigmatization strategy (see https://www.sciencedirect.com/science/article/pii/S0883902622000027 and https://link.springer.com/article/10.1007/s11187-018-0059-8). Entrepreneurs clearly illustrate the fallacy of stigma. As a simple example, Elon Musk—the wealthiest person in the world—talks openly about being autistic, and possibly bipolar. These mental health differences help him create jobs and contribute to our shared prosperity. Nothing to be ashamed of there.
Thanks again for being such an effective advocate.
Michael A. Freeman, MD
Kentfield, California
Continue to: Thank you...
Thank you so much for your “Psychiatric Manifesto.” I will do my best to disseminate it amongst colleagues, patients, friends, family, and as many others as possible.
Daniel N. Pistone, MD
San Francisco, California
Once again, your words hit the pin on the head.
Robert W. Pollack, MD, ABPN, DLFAPA
Fort Myers, Florida
Legal abortion is a matter of public health
On June 24, the U.S. Supreme Court overturned Roe v. Wade, a decision that was issued in 1973. From now on, each state will be able to choose the laws that it wants to put in place regarding abortion. Several states have already decided to ban abortion altogether. As a physician, but also as a woman, I am stunned to see this opposition to a right that, in my opinion, is also a matter of public health.
International data
In Belgium, voluntary termination of pregnancy (VTP) has been allowed since 1990. Except in the case of a serious medical problem, the abortion must take place before the end of the 12th week after conception. So, 14 weeks from the last menstrual period (LMP).
Beyond that time frame, a VTP can be performed only when the continuation of the pregnancy endangers the health of the woman or when it is certain that the unborn child will be affected by a condition of particular gravity and recognized as incurable at the time of diagnosis. This is referred to as termination for medical reasons (TFMR).
First observation
The annual number of VTPs did not climb following legalization. For the past 20 years in Belgium, that number has remained stable, hovering around 19,000. Abortion continues to be an action – neither trivialized nor minimized – that is difficult for any woman to take, no matter what her reason.
Second observation
Over 60% of women who had an abortion were using a form of contraception. So, while the burden of contraception still rests almost exclusively on the woman, it cannot be said that those who had a VTP did not use some method of birth control.
Even more important, legal abortions have very few complications, either physical or psychological. Studies show that pregnancy itself carries a higher risk for psychopathological manifestations than a VTP. These VTPs are safe, and women quickly recover from them. The most sensitive time seems to be the period before the abortion, and it’s at this stage that most of the psychological and psychopathological manifestations accumulate. The majority of women facing a VTP experience feelings of relief, and only a minority develop psychological problems, usually when there is already a history of mental disorder. The literature shows that the levels of anxiety and depression decrease in the month following the abortion. Being denied a VTP, on the other hand, significantly increases the woman’s risk of developing a mental disorder.
Should a VTP be denied, a woman, if she determines that she doesn’t have any other choice, may then end up turning to a back-alley abortion. The methods used for this are medieval, dangerous, and may not prove successful – things like using chemicals, piercing the amniotic sac with a needle or sharp object (the famous coat hanger), eating or drinking abortifacient herbs, taking large quantities of medication, punching the stomach, falling down stairs, and engaging in intense physical exercise.
From there, these risky methods inevitably lead to numerous complications: Incomplete abortions, infections, septicemia, breakthrough bleeding, subsequent sterility, laceration of the uterine wall, or death.
Around one-third of women who undergo risky abortions develop complications, while less than half receive care.
The World Health Organization estimates that back-alley abortions represent 49% of abortions worldwide. It puts the number of illegal abortions performed each year at 20 million.
Each year, around 60,000 women worldwide die as a result of an unsafe VTP. That’s one woman every 9 minutes. And odds are that these figures are underestimated.
Making the decision to resort to a VTP is always difficult. Ideally, you should be able to discuss it with your partner, when there is one, and with your close friends and family, to have someone go with you as support, to weigh the pros and cons, and to make a choice in line with your convictions and your conscience. But first and foremost, the law must guarantee the right to be able to ask oneself this question, because guaranteeing this right is also guaranteeing the health and safety of women, and that is why this remains a public health imperative.
A version of this article first appeared on Medscape.com. This article was translated from MediQuality.
On June 24, the U.S. Supreme Court overturned Roe v. Wade, a decision that was issued in 1973. From now on, each state will be able to choose the laws that it wants to put in place regarding abortion. Several states have already decided to ban abortion altogether. As a physician, but also as a woman, I am stunned to see this opposition to a right that, in my opinion, is also a matter of public health.
International data
In Belgium, voluntary termination of pregnancy (VTP) has been allowed since 1990. Except in the case of a serious medical problem, the abortion must take place before the end of the 12th week after conception. So, 14 weeks from the last menstrual period (LMP).
Beyond that time frame, a VTP can be performed only when the continuation of the pregnancy endangers the health of the woman or when it is certain that the unborn child will be affected by a condition of particular gravity and recognized as incurable at the time of diagnosis. This is referred to as termination for medical reasons (TFMR).
First observation
The annual number of VTPs did not climb following legalization. For the past 20 years in Belgium, that number has remained stable, hovering around 19,000. Abortion continues to be an action – neither trivialized nor minimized – that is difficult for any woman to take, no matter what her reason.
Second observation
Over 60% of women who had an abortion were using a form of contraception. So, while the burden of contraception still rests almost exclusively on the woman, it cannot be said that those who had a VTP did not use some method of birth control.
Even more important, legal abortions have very few complications, either physical or psychological. Studies show that pregnancy itself carries a higher risk for psychopathological manifestations than a VTP. These VTPs are safe, and women quickly recover from them. The most sensitive time seems to be the period before the abortion, and it’s at this stage that most of the psychological and psychopathological manifestations accumulate. The majority of women facing a VTP experience feelings of relief, and only a minority develop psychological problems, usually when there is already a history of mental disorder. The literature shows that the levels of anxiety and depression decrease in the month following the abortion. Being denied a VTP, on the other hand, significantly increases the woman’s risk of developing a mental disorder.
Should a VTP be denied, a woman, if she determines that she doesn’t have any other choice, may then end up turning to a back-alley abortion. The methods used for this are medieval, dangerous, and may not prove successful – things like using chemicals, piercing the amniotic sac with a needle or sharp object (the famous coat hanger), eating or drinking abortifacient herbs, taking large quantities of medication, punching the stomach, falling down stairs, and engaging in intense physical exercise.
From there, these risky methods inevitably lead to numerous complications: Incomplete abortions, infections, septicemia, breakthrough bleeding, subsequent sterility, laceration of the uterine wall, or death.
Around one-third of women who undergo risky abortions develop complications, while less than half receive care.
The World Health Organization estimates that back-alley abortions represent 49% of abortions worldwide. It puts the number of illegal abortions performed each year at 20 million.
Each year, around 60,000 women worldwide die as a result of an unsafe VTP. That’s one woman every 9 minutes. And odds are that these figures are underestimated.
Making the decision to resort to a VTP is always difficult. Ideally, you should be able to discuss it with your partner, when there is one, and with your close friends and family, to have someone go with you as support, to weigh the pros and cons, and to make a choice in line with your convictions and your conscience. But first and foremost, the law must guarantee the right to be able to ask oneself this question, because guaranteeing this right is also guaranteeing the health and safety of women, and that is why this remains a public health imperative.
A version of this article first appeared on Medscape.com. This article was translated from MediQuality.
On June 24, the U.S. Supreme Court overturned Roe v. Wade, a decision that was issued in 1973. From now on, each state will be able to choose the laws that it wants to put in place regarding abortion. Several states have already decided to ban abortion altogether. As a physician, but also as a woman, I am stunned to see this opposition to a right that, in my opinion, is also a matter of public health.
International data
In Belgium, voluntary termination of pregnancy (VTP) has been allowed since 1990. Except in the case of a serious medical problem, the abortion must take place before the end of the 12th week after conception. So, 14 weeks from the last menstrual period (LMP).
Beyond that time frame, a VTP can be performed only when the continuation of the pregnancy endangers the health of the woman or when it is certain that the unborn child will be affected by a condition of particular gravity and recognized as incurable at the time of diagnosis. This is referred to as termination for medical reasons (TFMR).
First observation
The annual number of VTPs did not climb following legalization. For the past 20 years in Belgium, that number has remained stable, hovering around 19,000. Abortion continues to be an action – neither trivialized nor minimized – that is difficult for any woman to take, no matter what her reason.
Second observation
Over 60% of women who had an abortion were using a form of contraception. So, while the burden of contraception still rests almost exclusively on the woman, it cannot be said that those who had a VTP did not use some method of birth control.
Even more important, legal abortions have very few complications, either physical or psychological. Studies show that pregnancy itself carries a higher risk for psychopathological manifestations than a VTP. These VTPs are safe, and women quickly recover from them. The most sensitive time seems to be the period before the abortion, and it’s at this stage that most of the psychological and psychopathological manifestations accumulate. The majority of women facing a VTP experience feelings of relief, and only a minority develop psychological problems, usually when there is already a history of mental disorder. The literature shows that the levels of anxiety and depression decrease in the month following the abortion. Being denied a VTP, on the other hand, significantly increases the woman’s risk of developing a mental disorder.
Should a VTP be denied, a woman, if she determines that she doesn’t have any other choice, may then end up turning to a back-alley abortion. The methods used for this are medieval, dangerous, and may not prove successful – things like using chemicals, piercing the amniotic sac with a needle or sharp object (the famous coat hanger), eating or drinking abortifacient herbs, taking large quantities of medication, punching the stomach, falling down stairs, and engaging in intense physical exercise.
From there, these risky methods inevitably lead to numerous complications: Incomplete abortions, infections, septicemia, breakthrough bleeding, subsequent sterility, laceration of the uterine wall, or death.
Around one-third of women who undergo risky abortions develop complications, while less than half receive care.
The World Health Organization estimates that back-alley abortions represent 49% of abortions worldwide. It puts the number of illegal abortions performed each year at 20 million.
Each year, around 60,000 women worldwide die as a result of an unsafe VTP. That’s one woman every 9 minutes. And odds are that these figures are underestimated.
Making the decision to resort to a VTP is always difficult. Ideally, you should be able to discuss it with your partner, when there is one, and with your close friends and family, to have someone go with you as support, to weigh the pros and cons, and to make a choice in line with your convictions and your conscience. But first and foremost, the law must guarantee the right to be able to ask oneself this question, because guaranteeing this right is also guaranteeing the health and safety of women, and that is why this remains a public health imperative.
A version of this article first appeared on Medscape.com. This article was translated from MediQuality.






