User login
Seasonality of birth and psychiatric illness
“To every thing there is a season, and a time to every purpose under the heaven.”
— Ecclesiastes
The month of birth is not just relevant to one’s astrological sign. It may have medical consequences. An impressive number of published studies have found that the month and season of birth may be related to a higher risk of various medical and psychiatric disorders.
For decades, it has been reported in more than 250 studies1 that a disproportionate number of individuals with schizophrenia are born during the winter months (January/February/March in the Northern Hemisphere and July/August/September in the Southern Hemisphere). This seasonal pattern was eventually linked to the lack of sunlight during winter months and a deficiency of vitamin D, a hormone that is critical for normal brain development. Recent studies have reported that very low serum levels of vitamin D during pregnancy significantly increase the risk of schizophrenia in offspring.2
But the plot thickens. Numerous studies over the past 20 to 30 years have reported an association between month or season of birth with sundry general medical and psychiatric conditions. Even longevity has been reported to vary with season of birth, with a longer life span for people born in autumn (October to December), compared with those born in spring (April to June).3 Of note, a longer life span for an individual born in autumn has been attributed to a higher birth weight during that season compared with those born in other seasons. In addition, the shorter life span of those with spring births has been attributed to factors during fetal life that increase the susceptibility to disease later in life (after age 50).
The following studies have reported an association between month/season of birth and general medical disorders:
- Higher rate of myopia for summer births4
- Tenfold higher risk of respiratory syntactical virus in babies born in January compared with October, and a 2 to 3 times higher risk of hospitalization5
- Higher rates of asthma during childhood for March and April births6
- Lower rate of lung cancer for winter births compared with all other seasons7
- An excess of colon and rectal cancer for people born in September, and the lowest rate for spring births8
- Lowest diabetes risk for summer births9
- For males: Cardiac mortality is 11% less likely for 4th-quarter births compared with 1st-quarter births. For females: Cancer mortality is lowest in 3rd-quarter vs 1st-quarter births10
- The peak risk for both Hodgkin and non-Hodgkin lymphoma is for April births compared with other months11
- A strong trend for malignant neoplasm in males was reported for births during the 1st trimester of the year (January through April) compared with the rest of the year12
- Higher rate of spring births among patients who have insulin-dependent diabetes13
- Breast cancer is 5% higher for June births compared with December births14
- Higher risk of developing an allergy later in life for those born approximately 3 months before the main allergy season.15
The above studies may imply that birth seasonality is medical destiny. However, most such reports need further replication, or may be due to chance findings in various databases. However, they are worth considering as hypothesis-generating signals.
Continue to: And now for the risk of psychiatric disorders...
And now for the risk of psychiatric disorders and month or season of birth. Here, too, there are multiple published reports:
- Higher social anhedonia and schizoid features among persons born in June and July16
- Higher autism rates for children conceived in December to March compared with those conceived during summer months17
- In contrast to the above report, the risk of autism spectrum disorders in the United Kingdom was higher for those born in summer18
- Another study labeled seasonality of birth in autism as “fiction”!19
- Significant spring births for persons with anxiety20
- Highest occurrence of postpartum depression in December21
- High prepartum depression in winter and postpartum depression in fall22
- Lower performance IQ among spring births23
- Disproportionate excess of births in April, May, and June for those who die by suicide24
- Suicide by burning oneself is higher among individuals born in January compared with any other month25
- Relative increase in March and August births among patients with anorexia26
- Season of birth is a predictor of emotional and behavioral regulation27
- Serotonin metabolites show a peak in spring and a trough in fall28
- Increase of spring births in individuals with Down syndrome29
- Excess of spring births among patients with Alzheimer’s disease.30
As with the seasonality of medical illness risk, the association of the month or season of birth with psychiatric disorders may be based on skewed samples or simply a chance finding. However, there may be some seasonal environmental factors that could increase the risk for disorders of the body or the brain/mind. The most plausible factors may be season-related fetal developmental disruptions caused by maternal infection, diet, lack of sunlight, temperature, substance use, or immune dysregulation from comorbid medical conditions during pregnancy. Some researchers have speculated that fluctuations in the availability of various fresh fruits and vegetables during certain seasons of the year may influence fetal development or increase the susceptibility to some medical disorders. This may be at the time of conception or during the 2nd trimester of pregnancy, when the brain develops.
On the other hand, those studies, published in peer-reviewed journals, may constitute a sophisticated form of “psychiatric astrology” whose credibility could be as suspect as the imaginative predictions of one’s horoscope in the daily newspaper…
To comment on this editorial or other topics of interest: [email protected].
1. Torrey EF, Miller J, Rawlings R, et al. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1-38.
2. McGrath J, Welham J, Pemberton M. Month of birth, hemisphere of birth and schizophrenia. Br J Psychiatry. 1995;167(6):783-785.
3. Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci U S A. 2001;98(5):2934-2939.
4. Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology. 2008;115(4):686-692.
5. Lloyd PC, May L, Hoffman D, et al. The effect of birth month on the risk of respiratory syncytial virus hospitalization in the first year of life in the United States. Pediatr Infect Dis J. 2014;33(6):e135-e140.
6. Gazala E, Ron-Feldman V, Alterman M, et al. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41(12):1125-1128.
7. Hao Y, Yan L, Ke E, et al. Birth in winter can reduce the risk of lung cancer: A retrospective study of the birth season of patients with lung cancer in Beijing area, China. Chronobiol Int. 2017;34(4):511-518.
8. Francis NK, Curtis NJ, Noble E, et al. Is month of birth a risk factor for colorectal cancer? Gastroenterol Res Pract. 2017;2017:5423765. doi: 10.1155/2017/5423765.
9. Si J, Yu C, Guo Y, et al; China Kadoorie Biobank Collaborative Group. Season of birth and the risk of type 2 diabetes in adulthood: a prospective cohort study of 0.5 million Chinese adults. Diabetologia. 2017;60(5):836-842.
10. Sohn K. The influence of birth season on mortality in the United States. Am J Hum Biol. 2016;28(5):662-670.
11. Crump C, Sundquist J, Sieh W, et al. Season of birth and risk of Hodgkin and non-Hodgkin lymphoma. Int J Cancer. 2014;135(11):2735-2739.
12. Stoupel E, Abramson E, Fenig E. Birth month of patients with malignant neoplasms: links to longevity? J Basic Clin Physiol Pharmacol. 2012;23(2):57-60.
13. Rothwell PM, Gutnikov SA, McKinney PA, et al. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319(7214):887-888.
14. Yuen J, Ekbom A, Trichopoulos D, et al. Season of birth and breast cancer risk in Sweden. Br J Cancer. 1994;70(3):564-568.
15. Aalberse RC, Nieuwenhuys EJ, Hey M, et al. ‘Horoscope effect’ not only for seasonal but also for non-seasonal allergens. Clin Exp Allergy. 1992;22(11):1003-1006.
16. Kirkpatrick B, Messias E, LaPorte D. Schizoid-like features and season of birth in a nonpatient sample. Schizophr Res. 2008;103:151-155.
17. Zerbo O, Iosif AM, Delwiche L, et al. Month of conception and risk of autism. Epidemiology. 2011;22(4):469-475.
18. Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res. 2010;3(4):185-190.
19. Landau EC, Cicchetti DV, Klin A, et al. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29(5):385-393.
20. Parker G, Neilson M. Mental disorder and season of birth--a southern hemisphere study. Br J Psychiatry. 1976;129:355-361.
21. Sit D, Seltman H, Wisner KL. Seasonal effects on depression risk and suicidal symptoms in postpartum women. Depress Anxiety. 2011;28(5):400-405.
22. Chan JE, Samaranayaka A, Paterson H. Seasonal and gestational variation in perinatal depression in a prospective cohort in New Zealand. Aust N Z J Obstet Gynaecol. 2018. [Epub ahead of print]. doi: 10.1111/ajo.12912.
23. Grootendorst-van Mil NH, Steegers-Theunissen RP, Hofman A, et al. Brighter children? The association between seasonality of birth and child IQ in a population-based birth cohort. BMJ Open. 2017;7(2):e012406. doi: 10.1136/bmjopen-2016-012406.
24. Salib E, Cortina-Borja M. Effect of month of birth on the risk of suicide. Br J Psychiatry. 2006;188:416-422.
25. Salib E, Cortina-Borja M. An association between month of birth and method of suicide. Int J Psychiatry Clin Pract. 2010;14(1):8-17.
26. Brewerton TD, Dansky BS, O’Neil PM, et al. Seasonal patterns of birth for subjects with bulimia nervosa, binge eating, and purging: results from the National Women’s Study. Int J Eat Disord. 2012;45(1):131-134.
27. Asano R, Tsuchiya KJ, Harada T, et al; for Hamamatsu Birth Cohort (HBC) Study Team. Season of birth predicts emotional and behavioral regulation in 18-month-old infants: Hamamatsu birth cohort for mothers and children (HBC Study). Front Public Health. 2016;4:152.
28. Luykx JJ, Bakker SC, Lentjes E, et al. Season of sampling and season of birth influence serotonin metabolite levels in human cerebrospinal fluid. PLoS One. 2012;7(2):e30497. doi: 10.1371/journal.pone.0030497.
29. Videbech P, Nielsen J. Chromosome abnormalities and season of birth. Hum Genet. 1984;65(3):221-231.
30. Vézina H, Houde L, Charbonneau H, et al. Season of birth and Alzheimer’s disease: a population-based study in Saguenay-Lac-St-Jean/Québec (IMAGE Project). Psychol Med. 1996;26(1):143-149.
“To every thing there is a season, and a time to every purpose under the heaven.”
— Ecclesiastes
The month of birth is not just relevant to one’s astrological sign. It may have medical consequences. An impressive number of published studies have found that the month and season of birth may be related to a higher risk of various medical and psychiatric disorders.
For decades, it has been reported in more than 250 studies1 that a disproportionate number of individuals with schizophrenia are born during the winter months (January/February/March in the Northern Hemisphere and July/August/September in the Southern Hemisphere). This seasonal pattern was eventually linked to the lack of sunlight during winter months and a deficiency of vitamin D, a hormone that is critical for normal brain development. Recent studies have reported that very low serum levels of vitamin D during pregnancy significantly increase the risk of schizophrenia in offspring.2
But the plot thickens. Numerous studies over the past 20 to 30 years have reported an association between month or season of birth with sundry general medical and psychiatric conditions. Even longevity has been reported to vary with season of birth, with a longer life span for people born in autumn (October to December), compared with those born in spring (April to June).3 Of note, a longer life span for an individual born in autumn has been attributed to a higher birth weight during that season compared with those born in other seasons. In addition, the shorter life span of those with spring births has been attributed to factors during fetal life that increase the susceptibility to disease later in life (after age 50).
The following studies have reported an association between month/season of birth and general medical disorders:
- Higher rate of myopia for summer births4
- Tenfold higher risk of respiratory syntactical virus in babies born in January compared with October, and a 2 to 3 times higher risk of hospitalization5
- Higher rates of asthma during childhood for March and April births6
- Lower rate of lung cancer for winter births compared with all other seasons7
- An excess of colon and rectal cancer for people born in September, and the lowest rate for spring births8
- Lowest diabetes risk for summer births9
- For males: Cardiac mortality is 11% less likely for 4th-quarter births compared with 1st-quarter births. For females: Cancer mortality is lowest in 3rd-quarter vs 1st-quarter births10
- The peak risk for both Hodgkin and non-Hodgkin lymphoma is for April births compared with other months11
- A strong trend for malignant neoplasm in males was reported for births during the 1st trimester of the year (January through April) compared with the rest of the year12
- Higher rate of spring births among patients who have insulin-dependent diabetes13
- Breast cancer is 5% higher for June births compared with December births14
- Higher risk of developing an allergy later in life for those born approximately 3 months before the main allergy season.15
The above studies may imply that birth seasonality is medical destiny. However, most such reports need further replication, or may be due to chance findings in various databases. However, they are worth considering as hypothesis-generating signals.
Continue to: And now for the risk of psychiatric disorders...
And now for the risk of psychiatric disorders and month or season of birth. Here, too, there are multiple published reports:
- Higher social anhedonia and schizoid features among persons born in June and July16
- Higher autism rates for children conceived in December to March compared with those conceived during summer months17
- In contrast to the above report, the risk of autism spectrum disorders in the United Kingdom was higher for those born in summer18
- Another study labeled seasonality of birth in autism as “fiction”!19
- Significant spring births for persons with anxiety20
- Highest occurrence of postpartum depression in December21
- High prepartum depression in winter and postpartum depression in fall22
- Lower performance IQ among spring births23
- Disproportionate excess of births in April, May, and June for those who die by suicide24
- Suicide by burning oneself is higher among individuals born in January compared with any other month25
- Relative increase in March and August births among patients with anorexia26
- Season of birth is a predictor of emotional and behavioral regulation27
- Serotonin metabolites show a peak in spring and a trough in fall28
- Increase of spring births in individuals with Down syndrome29
- Excess of spring births among patients with Alzheimer’s disease.30
As with the seasonality of medical illness risk, the association of the month or season of birth with psychiatric disorders may be based on skewed samples or simply a chance finding. However, there may be some seasonal environmental factors that could increase the risk for disorders of the body or the brain/mind. The most plausible factors may be season-related fetal developmental disruptions caused by maternal infection, diet, lack of sunlight, temperature, substance use, or immune dysregulation from comorbid medical conditions during pregnancy. Some researchers have speculated that fluctuations in the availability of various fresh fruits and vegetables during certain seasons of the year may influence fetal development or increase the susceptibility to some medical disorders. This may be at the time of conception or during the 2nd trimester of pregnancy, when the brain develops.
On the other hand, those studies, published in peer-reviewed journals, may constitute a sophisticated form of “psychiatric astrology” whose credibility could be as suspect as the imaginative predictions of one’s horoscope in the daily newspaper…
To comment on this editorial or other topics of interest: [email protected].
“To every thing there is a season, and a time to every purpose under the heaven.”
— Ecclesiastes
The month of birth is not just relevant to one’s astrological sign. It may have medical consequences. An impressive number of published studies have found that the month and season of birth may be related to a higher risk of various medical and psychiatric disorders.
For decades, it has been reported in more than 250 studies1 that a disproportionate number of individuals with schizophrenia are born during the winter months (January/February/March in the Northern Hemisphere and July/August/September in the Southern Hemisphere). This seasonal pattern was eventually linked to the lack of sunlight during winter months and a deficiency of vitamin D, a hormone that is critical for normal brain development. Recent studies have reported that very low serum levels of vitamin D during pregnancy significantly increase the risk of schizophrenia in offspring.2
But the plot thickens. Numerous studies over the past 20 to 30 years have reported an association between month or season of birth with sundry general medical and psychiatric conditions. Even longevity has been reported to vary with season of birth, with a longer life span for people born in autumn (October to December), compared with those born in spring (April to June).3 Of note, a longer life span for an individual born in autumn has been attributed to a higher birth weight during that season compared with those born in other seasons. In addition, the shorter life span of those with spring births has been attributed to factors during fetal life that increase the susceptibility to disease later in life (after age 50).
The following studies have reported an association between month/season of birth and general medical disorders:
- Higher rate of myopia for summer births4
- Tenfold higher risk of respiratory syntactical virus in babies born in January compared with October, and a 2 to 3 times higher risk of hospitalization5
- Higher rates of asthma during childhood for March and April births6
- Lower rate of lung cancer for winter births compared with all other seasons7
- An excess of colon and rectal cancer for people born in September, and the lowest rate for spring births8
- Lowest diabetes risk for summer births9
- For males: Cardiac mortality is 11% less likely for 4th-quarter births compared with 1st-quarter births. For females: Cancer mortality is lowest in 3rd-quarter vs 1st-quarter births10
- The peak risk for both Hodgkin and non-Hodgkin lymphoma is for April births compared with other months11
- A strong trend for malignant neoplasm in males was reported for births during the 1st trimester of the year (January through April) compared with the rest of the year12
- Higher rate of spring births among patients who have insulin-dependent diabetes13
- Breast cancer is 5% higher for June births compared with December births14
- Higher risk of developing an allergy later in life for those born approximately 3 months before the main allergy season.15
The above studies may imply that birth seasonality is medical destiny. However, most such reports need further replication, or may be due to chance findings in various databases. However, they are worth considering as hypothesis-generating signals.
Continue to: And now for the risk of psychiatric disorders...
And now for the risk of psychiatric disorders and month or season of birth. Here, too, there are multiple published reports:
- Higher social anhedonia and schizoid features among persons born in June and July16
- Higher autism rates for children conceived in December to March compared with those conceived during summer months17
- In contrast to the above report, the risk of autism spectrum disorders in the United Kingdom was higher for those born in summer18
- Another study labeled seasonality of birth in autism as “fiction”!19
- Significant spring births for persons with anxiety20
- Highest occurrence of postpartum depression in December21
- High prepartum depression in winter and postpartum depression in fall22
- Lower performance IQ among spring births23
- Disproportionate excess of births in April, May, and June for those who die by suicide24
- Suicide by burning oneself is higher among individuals born in January compared with any other month25
- Relative increase in March and August births among patients with anorexia26
- Season of birth is a predictor of emotional and behavioral regulation27
- Serotonin metabolites show a peak in spring and a trough in fall28
- Increase of spring births in individuals with Down syndrome29
- Excess of spring births among patients with Alzheimer’s disease.30
As with the seasonality of medical illness risk, the association of the month or season of birth with psychiatric disorders may be based on skewed samples or simply a chance finding. However, there may be some seasonal environmental factors that could increase the risk for disorders of the body or the brain/mind. The most plausible factors may be season-related fetal developmental disruptions caused by maternal infection, diet, lack of sunlight, temperature, substance use, or immune dysregulation from comorbid medical conditions during pregnancy. Some researchers have speculated that fluctuations in the availability of various fresh fruits and vegetables during certain seasons of the year may influence fetal development or increase the susceptibility to some medical disorders. This may be at the time of conception or during the 2nd trimester of pregnancy, when the brain develops.
On the other hand, those studies, published in peer-reviewed journals, may constitute a sophisticated form of “psychiatric astrology” whose credibility could be as suspect as the imaginative predictions of one’s horoscope in the daily newspaper…
To comment on this editorial or other topics of interest: [email protected].
1. Torrey EF, Miller J, Rawlings R, et al. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1-38.
2. McGrath J, Welham J, Pemberton M. Month of birth, hemisphere of birth and schizophrenia. Br J Psychiatry. 1995;167(6):783-785.
3. Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci U S A. 2001;98(5):2934-2939.
4. Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology. 2008;115(4):686-692.
5. Lloyd PC, May L, Hoffman D, et al. The effect of birth month on the risk of respiratory syncytial virus hospitalization in the first year of life in the United States. Pediatr Infect Dis J. 2014;33(6):e135-e140.
6. Gazala E, Ron-Feldman V, Alterman M, et al. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41(12):1125-1128.
7. Hao Y, Yan L, Ke E, et al. Birth in winter can reduce the risk of lung cancer: A retrospective study of the birth season of patients with lung cancer in Beijing area, China. Chronobiol Int. 2017;34(4):511-518.
8. Francis NK, Curtis NJ, Noble E, et al. Is month of birth a risk factor for colorectal cancer? Gastroenterol Res Pract. 2017;2017:5423765. doi: 10.1155/2017/5423765.
9. Si J, Yu C, Guo Y, et al; China Kadoorie Biobank Collaborative Group. Season of birth and the risk of type 2 diabetes in adulthood: a prospective cohort study of 0.5 million Chinese adults. Diabetologia. 2017;60(5):836-842.
10. Sohn K. The influence of birth season on mortality in the United States. Am J Hum Biol. 2016;28(5):662-670.
11. Crump C, Sundquist J, Sieh W, et al. Season of birth and risk of Hodgkin and non-Hodgkin lymphoma. Int J Cancer. 2014;135(11):2735-2739.
12. Stoupel E, Abramson E, Fenig E. Birth month of patients with malignant neoplasms: links to longevity? J Basic Clin Physiol Pharmacol. 2012;23(2):57-60.
13. Rothwell PM, Gutnikov SA, McKinney PA, et al. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319(7214):887-888.
14. Yuen J, Ekbom A, Trichopoulos D, et al. Season of birth and breast cancer risk in Sweden. Br J Cancer. 1994;70(3):564-568.
15. Aalberse RC, Nieuwenhuys EJ, Hey M, et al. ‘Horoscope effect’ not only for seasonal but also for non-seasonal allergens. Clin Exp Allergy. 1992;22(11):1003-1006.
16. Kirkpatrick B, Messias E, LaPorte D. Schizoid-like features and season of birth in a nonpatient sample. Schizophr Res. 2008;103:151-155.
17. Zerbo O, Iosif AM, Delwiche L, et al. Month of conception and risk of autism. Epidemiology. 2011;22(4):469-475.
18. Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res. 2010;3(4):185-190.
19. Landau EC, Cicchetti DV, Klin A, et al. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29(5):385-393.
20. Parker G, Neilson M. Mental disorder and season of birth--a southern hemisphere study. Br J Psychiatry. 1976;129:355-361.
21. Sit D, Seltman H, Wisner KL. Seasonal effects on depression risk and suicidal symptoms in postpartum women. Depress Anxiety. 2011;28(5):400-405.
22. Chan JE, Samaranayaka A, Paterson H. Seasonal and gestational variation in perinatal depression in a prospective cohort in New Zealand. Aust N Z J Obstet Gynaecol. 2018. [Epub ahead of print]. doi: 10.1111/ajo.12912.
23. Grootendorst-van Mil NH, Steegers-Theunissen RP, Hofman A, et al. Brighter children? The association between seasonality of birth and child IQ in a population-based birth cohort. BMJ Open. 2017;7(2):e012406. doi: 10.1136/bmjopen-2016-012406.
24. Salib E, Cortina-Borja M. Effect of month of birth on the risk of suicide. Br J Psychiatry. 2006;188:416-422.
25. Salib E, Cortina-Borja M. An association between month of birth and method of suicide. Int J Psychiatry Clin Pract. 2010;14(1):8-17.
26. Brewerton TD, Dansky BS, O’Neil PM, et al. Seasonal patterns of birth for subjects with bulimia nervosa, binge eating, and purging: results from the National Women’s Study. Int J Eat Disord. 2012;45(1):131-134.
27. Asano R, Tsuchiya KJ, Harada T, et al; for Hamamatsu Birth Cohort (HBC) Study Team. Season of birth predicts emotional and behavioral regulation in 18-month-old infants: Hamamatsu birth cohort for mothers and children (HBC Study). Front Public Health. 2016;4:152.
28. Luykx JJ, Bakker SC, Lentjes E, et al. Season of sampling and season of birth influence serotonin metabolite levels in human cerebrospinal fluid. PLoS One. 2012;7(2):e30497. doi: 10.1371/journal.pone.0030497.
29. Videbech P, Nielsen J. Chromosome abnormalities and season of birth. Hum Genet. 1984;65(3):221-231.
30. Vézina H, Houde L, Charbonneau H, et al. Season of birth and Alzheimer’s disease: a population-based study in Saguenay-Lac-St-Jean/Québec (IMAGE Project). Psychol Med. 1996;26(1):143-149.
1. Torrey EF, Miller J, Rawlings R, et al. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1-38.
2. McGrath J, Welham J, Pemberton M. Month of birth, hemisphere of birth and schizophrenia. Br J Psychiatry. 1995;167(6):783-785.
3. Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci U S A. 2001;98(5):2934-2939.
4. Mandel Y, Grotto I, El-Yaniv R, et al. Season of birth, natural light, and myopia. Ophthalmology. 2008;115(4):686-692.
5. Lloyd PC, May L, Hoffman D, et al. The effect of birth month on the risk of respiratory syncytial virus hospitalization in the first year of life in the United States. Pediatr Infect Dis J. 2014;33(6):e135-e140.
6. Gazala E, Ron-Feldman V, Alterman M, et al. The association between birth season and future development of childhood asthma. Pediatr Pulmonol. 2006;41(12):1125-1128.
7. Hao Y, Yan L, Ke E, et al. Birth in winter can reduce the risk of lung cancer: A retrospective study of the birth season of patients with lung cancer in Beijing area, China. Chronobiol Int. 2017;34(4):511-518.
8. Francis NK, Curtis NJ, Noble E, et al. Is month of birth a risk factor for colorectal cancer? Gastroenterol Res Pract. 2017;2017:5423765. doi: 10.1155/2017/5423765.
9. Si J, Yu C, Guo Y, et al; China Kadoorie Biobank Collaborative Group. Season of birth and the risk of type 2 diabetes in adulthood: a prospective cohort study of 0.5 million Chinese adults. Diabetologia. 2017;60(5):836-842.
10. Sohn K. The influence of birth season on mortality in the United States. Am J Hum Biol. 2016;28(5):662-670.
11. Crump C, Sundquist J, Sieh W, et al. Season of birth and risk of Hodgkin and non-Hodgkin lymphoma. Int J Cancer. 2014;135(11):2735-2739.
12. Stoupel E, Abramson E, Fenig E. Birth month of patients with malignant neoplasms: links to longevity? J Basic Clin Physiol Pharmacol. 2012;23(2):57-60.
13. Rothwell PM, Gutnikov SA, McKinney PA, et al. Seasonality of birth in children with diabetes in Europe: multicentre cohort study. European Diabetes Study Group. BMJ. 1999;319(7214):887-888.
14. Yuen J, Ekbom A, Trichopoulos D, et al. Season of birth and breast cancer risk in Sweden. Br J Cancer. 1994;70(3):564-568.
15. Aalberse RC, Nieuwenhuys EJ, Hey M, et al. ‘Horoscope effect’ not only for seasonal but also for non-seasonal allergens. Clin Exp Allergy. 1992;22(11):1003-1006.
16. Kirkpatrick B, Messias E, LaPorte D. Schizoid-like features and season of birth in a nonpatient sample. Schizophr Res. 2008;103:151-155.
17. Zerbo O, Iosif AM, Delwiche L, et al. Month of conception and risk of autism. Epidemiology. 2011;22(4):469-475.
18. Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res. 2010;3(4):185-190.
19. Landau EC, Cicchetti DV, Klin A, et al. Season of birth in autism: a fiction revisited. J Autism Dev Disord. 1999;29(5):385-393.
20. Parker G, Neilson M. Mental disorder and season of birth--a southern hemisphere study. Br J Psychiatry. 1976;129:355-361.
21. Sit D, Seltman H, Wisner KL. Seasonal effects on depression risk and suicidal symptoms in postpartum women. Depress Anxiety. 2011;28(5):400-405.
22. Chan JE, Samaranayaka A, Paterson H. Seasonal and gestational variation in perinatal depression in a prospective cohort in New Zealand. Aust N Z J Obstet Gynaecol. 2018. [Epub ahead of print]. doi: 10.1111/ajo.12912.
23. Grootendorst-van Mil NH, Steegers-Theunissen RP, Hofman A, et al. Brighter children? The association between seasonality of birth and child IQ in a population-based birth cohort. BMJ Open. 2017;7(2):e012406. doi: 10.1136/bmjopen-2016-012406.
24. Salib E, Cortina-Borja M. Effect of month of birth on the risk of suicide. Br J Psychiatry. 2006;188:416-422.
25. Salib E, Cortina-Borja M. An association between month of birth and method of suicide. Int J Psychiatry Clin Pract. 2010;14(1):8-17.
26. Brewerton TD, Dansky BS, O’Neil PM, et al. Seasonal patterns of birth for subjects with bulimia nervosa, binge eating, and purging: results from the National Women’s Study. Int J Eat Disord. 2012;45(1):131-134.
27. Asano R, Tsuchiya KJ, Harada T, et al; for Hamamatsu Birth Cohort (HBC) Study Team. Season of birth predicts emotional and behavioral regulation in 18-month-old infants: Hamamatsu birth cohort for mothers and children (HBC Study). Front Public Health. 2016;4:152.
28. Luykx JJ, Bakker SC, Lentjes E, et al. Season of sampling and season of birth influence serotonin metabolite levels in human cerebrospinal fluid. PLoS One. 2012;7(2):e30497. doi: 10.1371/journal.pone.0030497.
29. Videbech P, Nielsen J. Chromosome abnormalities and season of birth. Hum Genet. 1984;65(3):221-231.
30. Vézina H, Houde L, Charbonneau H, et al. Season of birth and Alzheimer’s disease: a population-based study in Saguenay-Lac-St-Jean/Québec (IMAGE Project). Psychol Med. 1996;26(1):143-149.
Fulfillment within success: A physician’s dilemma
They say success without fulfillment is of little value in life. Whether this concept is actually driving the spate of depression and substance abuse currently experienced by youth and middle-aged adults in developed countries is rarely discussed and needs to be explored.
We have all reflected on the tragic ends of Anthony Bourdain, Kate Spade, and Robin Williams. Much has been said about the accolades they achieved and the heights they scaled, and just as much about their struggles with substance abuse over the years. Sensational portrayals by the media also encouraged youth to spend time dissecting the details of these high-profile deaths, lending popularity to the notion of suicide contagion. But somewhere in the myriad theories and conclusions, we still seem baffled by the questions of why these suicides occurred, and why no one had seen them coming.
As humans, we are designed to build. For many people, including physicians, the final product is a rewarding career built on years of hard work, or a flourishing family to look back on be proud of. Sometimes, however, these larger ideas barely intersect with our pictures of success.
As physicians and high achievers, we dream of goals and ambitions and set stringent deadlines for achieving them. Falling short sometimes finds us grappling with self-punishment and doubt. When one goal is achieved, another one is automatically created, or the goal post is pushed further. And the cycle continues.
Having said this, I will ask: What are you looking for? What is it that will give you a sense of purpose?
This is not a redundant question, nor is it an easy one. So are you really taking the time to think about it? Does any of this border on self-reflection and self-awareness for you? If it does, then developing that insight into yourself is perhaps a better way of serving your patients.
Peace and gratification often lie in the little things; not everything you do has to be acknowledged with an award. There is a sense of fulfillment that comes from developing others. The key is to realize that there is never a moment to start doing that—it is an ongoing journey. Therefore, give generously, of your time, of your skills, of your knowledge, but above all, of your kindness. Do it because in the end, you will have something to look back on and be proud of. Do it because maybe somewhere you will find meaning in it. And your success may not be bereft of fulfillment.
They say success without fulfillment is of little value in life. Whether this concept is actually driving the spate of depression and substance abuse currently experienced by youth and middle-aged adults in developed countries is rarely discussed and needs to be explored.
We have all reflected on the tragic ends of Anthony Bourdain, Kate Spade, and Robin Williams. Much has been said about the accolades they achieved and the heights they scaled, and just as much about their struggles with substance abuse over the years. Sensational portrayals by the media also encouraged youth to spend time dissecting the details of these high-profile deaths, lending popularity to the notion of suicide contagion. But somewhere in the myriad theories and conclusions, we still seem baffled by the questions of why these suicides occurred, and why no one had seen them coming.
As humans, we are designed to build. For many people, including physicians, the final product is a rewarding career built on years of hard work, or a flourishing family to look back on be proud of. Sometimes, however, these larger ideas barely intersect with our pictures of success.
As physicians and high achievers, we dream of goals and ambitions and set stringent deadlines for achieving them. Falling short sometimes finds us grappling with self-punishment and doubt. When one goal is achieved, another one is automatically created, or the goal post is pushed further. And the cycle continues.
Having said this, I will ask: What are you looking for? What is it that will give you a sense of purpose?
This is not a redundant question, nor is it an easy one. So are you really taking the time to think about it? Does any of this border on self-reflection and self-awareness for you? If it does, then developing that insight into yourself is perhaps a better way of serving your patients.
Peace and gratification often lie in the little things; not everything you do has to be acknowledged with an award. There is a sense of fulfillment that comes from developing others. The key is to realize that there is never a moment to start doing that—it is an ongoing journey. Therefore, give generously, of your time, of your skills, of your knowledge, but above all, of your kindness. Do it because in the end, you will have something to look back on and be proud of. Do it because maybe somewhere you will find meaning in it. And your success may not be bereft of fulfillment.
They say success without fulfillment is of little value in life. Whether this concept is actually driving the spate of depression and substance abuse currently experienced by youth and middle-aged adults in developed countries is rarely discussed and needs to be explored.
We have all reflected on the tragic ends of Anthony Bourdain, Kate Spade, and Robin Williams. Much has been said about the accolades they achieved and the heights they scaled, and just as much about their struggles with substance abuse over the years. Sensational portrayals by the media also encouraged youth to spend time dissecting the details of these high-profile deaths, lending popularity to the notion of suicide contagion. But somewhere in the myriad theories and conclusions, we still seem baffled by the questions of why these suicides occurred, and why no one had seen them coming.
As humans, we are designed to build. For many people, including physicians, the final product is a rewarding career built on years of hard work, or a flourishing family to look back on be proud of. Sometimes, however, these larger ideas barely intersect with our pictures of success.
As physicians and high achievers, we dream of goals and ambitions and set stringent deadlines for achieving them. Falling short sometimes finds us grappling with self-punishment and doubt. When one goal is achieved, another one is automatically created, or the goal post is pushed further. And the cycle continues.
Having said this, I will ask: What are you looking for? What is it that will give you a sense of purpose?
This is not a redundant question, nor is it an easy one. So are you really taking the time to think about it? Does any of this border on self-reflection and self-awareness for you? If it does, then developing that insight into yourself is perhaps a better way of serving your patients.
Peace and gratification often lie in the little things; not everything you do has to be acknowledged with an award. There is a sense of fulfillment that comes from developing others. The key is to realize that there is never a moment to start doing that—it is an ongoing journey. Therefore, give generously, of your time, of your skills, of your knowledge, but above all, of your kindness. Do it because in the end, you will have something to look back on and be proud of. Do it because maybe somewhere you will find meaning in it. And your success may not be bereft of fulfillment.
Debunking Psoriasis Myths: Psoriasis Is More Than Skin Deep
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
Myth: Psoriasis Is Only a Skin Problem
Psoriasis is predominantly regarded as a skin disease because of the outward clinical presentation of the condition. However, psoriasis is a disorder of the immune system and its damage may be more than skin deep.
Psoriasis commonly presents on the skin and nails, but a growing body of evidence has suggested that psoriasis is associated with systemic comorbidities. Up to 25% of psoriasis patients develop joint inflammation, and psoriatic arthritis (PsA) may precede skin involvement. There also is a risk for cardiovascular complications. Because of the emotional distress caused by psoriasis, patients may develop psychosocial disorders. Other conditions in patients with psoriasis include diabetes mellitus, high blood pressure, Crohn disease, and the metabolic syndrome.
Results from surveys conducted by the National Psoriasis Foundation from 2003 to 2011 found that the diagnosis of psoriasis preceded PsA in the majority of patients by a mean period of 14.6 years. Patients with moderate to severe psoriasis were more likely to develop PsA than patients with mild psoriasis. Furthermore, patients with severe psoriasis were more likely to develop diabetes mellitus and cardiovascular disease.
In a Cutis editorial, Dr. Jeffrey Weinberg emphasizes that the role of the dermatologist “is to identify and educate patients with psoriasis who are at risk of systemic complications and ensure appropriate follow-up for their treatment and overall health.” An infographic created by the American Academy of Dermatology illustrates areas of the body that may be impacted by psoriasis beyond the skin; for example, patients may develop eye problems, weight gain, or mood changes. Consider distributing this infographic to patients to show how psoriasis can affect more than their skin.
More Cutis content is available on psoriasis comorbidities:
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
- Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation surveys 2003 to 2011. Dermatology. 2012;225:121-126.
- Can psoriasis affect more than my skin? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/psoriasis-signs-and-symptoms/can-psoriasis-affect-more-than-my-skin. Accessed December 10, 2018.
- Psoriasis: more than skin deep. Harv Mens Health Watch. 2010;14:4-5. https://www.health.harvard.edu/newsletter_article/psoriasis-more-than-skin-deep. Accessed December 10, 2018.
- Weinberg JM. More than skin deep. Cutis. 2008;82:175.
Gatekeeper
One evening as the oncology fellow on call, I received a phone call from the ICU fellow.
“Can you meet me in the emergency room?” he asked. “I want to make sure we’re on the same page.”
A patient we had discharged from the hospital 2 days before was back. He had metastatic stomach cancer that had spread into his lungs and the lymph nodes in his chest. While he was in the hospital, he had required several liters of oxygen to maintain a normal work of breathing.
But now, he was in the emergency room, he was requiring a full face mask to help him breathe – and his oxygen levels were still dropping.
The ICU had been called. The next step along the algorithm of worsening breathing would be intubation. They would have to sedate him, put a breathing tube down his throat, and connect him to a ventilator to keep him alive.
But they didn’t want to do that if he was dying from his cancer.
Hence the call to me. My job, as the oncologist on call, was to answer the question: Is he dying?
Specifically, that meant weigh in on his cancer prognosis. Put his disease into context. Does he have any more options, chemotherapy or otherwise?
As an oncology fellow, I’ve found this to be one of the most common calls I get. Someone is critically ill and they need something to survive – maybe it’s intubation; maybe it’s surgery. The patient also happens to have metastatic cancer. The question posed to me is: Should we proceed?
It’s also one of the most difficult calls. Because doctors are historically bad at prognosticating. Because often I’m meeting the patient for the first time. Because the decision is huge and often final, and because both options are bad.
Suppose I say he has a good year or 2 ahead of him, and we intubate him – and then he never comes off the ventilator. We are eventually forced to withdraw care, and to the family it’s as though they are killing their father. It’s traumatic; it’s painful; and it deprives someone of a comfortable passing. Suppose I say he is dying from his cancer and we decide against a breathing tube. If I am wrong in that direction, a person’s life is cut short. It’s a perfect storm of high risk and low certainty.
Many people with metastatic cancer say they wouldn’t want invasive treatment near the end of life. But how do we know when it’s the end? There is still a moment when you must determine: Is this it? The truth is it’s not always clear.
Whenever I can, I reach out to the primary oncologist who knows the patient best. Then, I do a quick search for something reversible. Did the patient take too much morphine at home, and should we trial a dose of Narcan? Does he have a pneumonia that could be cured with antibiotics, a blood clot that could improve with blood thinners, or some extra fluid that can be diuresed? But usually it’s a mix, and even if there is a reversible injury, it can tip the very ill person over to the irreversible. This is how passing away from an aggressive cancer plays out.
Down in the emergency room, my patient’s breathing is rapid. His chest is heaving. The nurse shows me his blood gas with a carbon dioxide level more than twice the upper limit of normal. Now fading in and out of consciousness, he is a different man from the one who had walked out of the hospital 2 days earlier.
His daughter stands next to him. “He always said he wanted to do everything. I think we should give the breathing tube a try,” she says.
I tell her my concerns. I am afraid if we do it the likelihood of ever coming off is slim. And if we place a breathing tube he would have to be sedated so as not to be uncomfortable, and you won’t be able to communicate with him. You can’t say good bye, or I love you. If we keep the mask, he may wake up enough to interact.
The daughter – whom I knew well from prior visits, who was always articulate and poised and the spokesperson for the family – had held it together this entire time. Now, she breaks down. We all wait as I hand her a box of tissues. I look down, channeling all of my energy into not crying in front of her.
He’s waking up, one of us notes.
She goes over. “I need to ask him,” she says.
“Papa.”
At first he doesn’t answer.
“Papa, do you want the breathing tube?”
“No,” he says.
“Without it you can die. You know that, Papa?”
“No breathing tube,” he says.
“OK,” she turns to us, with tears of sadness but also what seems like relief.
Forty-eight hours later, he passed away. His family had time to come in, and he had periods of alertness where he could speak with them. They were able to say good-bye. He was able to say I love you.
Another patient’s wife once told me he had given her the “gift of clarity” when he plainly stated before he passed that he didn’t want to be saved. She didn’t have to make the decision for him, and neither did the doctors. I liked that term, and I thought about it then.
I am grateful my patient’s wishes were clear. But we aren’t always so lucky. It’s a chilling part of the job description, being a gatekeeper to the question: Is this the end?
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
One evening as the oncology fellow on call, I received a phone call from the ICU fellow.
“Can you meet me in the emergency room?” he asked. “I want to make sure we’re on the same page.”
A patient we had discharged from the hospital 2 days before was back. He had metastatic stomach cancer that had spread into his lungs and the lymph nodes in his chest. While he was in the hospital, he had required several liters of oxygen to maintain a normal work of breathing.
But now, he was in the emergency room, he was requiring a full face mask to help him breathe – and his oxygen levels were still dropping.
The ICU had been called. The next step along the algorithm of worsening breathing would be intubation. They would have to sedate him, put a breathing tube down his throat, and connect him to a ventilator to keep him alive.
But they didn’t want to do that if he was dying from his cancer.
Hence the call to me. My job, as the oncologist on call, was to answer the question: Is he dying?
Specifically, that meant weigh in on his cancer prognosis. Put his disease into context. Does he have any more options, chemotherapy or otherwise?
As an oncology fellow, I’ve found this to be one of the most common calls I get. Someone is critically ill and they need something to survive – maybe it’s intubation; maybe it’s surgery. The patient also happens to have metastatic cancer. The question posed to me is: Should we proceed?
It’s also one of the most difficult calls. Because doctors are historically bad at prognosticating. Because often I’m meeting the patient for the first time. Because the decision is huge and often final, and because both options are bad.
Suppose I say he has a good year or 2 ahead of him, and we intubate him – and then he never comes off the ventilator. We are eventually forced to withdraw care, and to the family it’s as though they are killing their father. It’s traumatic; it’s painful; and it deprives someone of a comfortable passing. Suppose I say he is dying from his cancer and we decide against a breathing tube. If I am wrong in that direction, a person’s life is cut short. It’s a perfect storm of high risk and low certainty.
Many people with metastatic cancer say they wouldn’t want invasive treatment near the end of life. But how do we know when it’s the end? There is still a moment when you must determine: Is this it? The truth is it’s not always clear.
Whenever I can, I reach out to the primary oncologist who knows the patient best. Then, I do a quick search for something reversible. Did the patient take too much morphine at home, and should we trial a dose of Narcan? Does he have a pneumonia that could be cured with antibiotics, a blood clot that could improve with blood thinners, or some extra fluid that can be diuresed? But usually it’s a mix, and even if there is a reversible injury, it can tip the very ill person over to the irreversible. This is how passing away from an aggressive cancer plays out.
Down in the emergency room, my patient’s breathing is rapid. His chest is heaving. The nurse shows me his blood gas with a carbon dioxide level more than twice the upper limit of normal. Now fading in and out of consciousness, he is a different man from the one who had walked out of the hospital 2 days earlier.
His daughter stands next to him. “He always said he wanted to do everything. I think we should give the breathing tube a try,” she says.
I tell her my concerns. I am afraid if we do it the likelihood of ever coming off is slim. And if we place a breathing tube he would have to be sedated so as not to be uncomfortable, and you won’t be able to communicate with him. You can’t say good bye, or I love you. If we keep the mask, he may wake up enough to interact.
The daughter – whom I knew well from prior visits, who was always articulate and poised and the spokesperson for the family – had held it together this entire time. Now, she breaks down. We all wait as I hand her a box of tissues. I look down, channeling all of my energy into not crying in front of her.
He’s waking up, one of us notes.
She goes over. “I need to ask him,” she says.
“Papa.”
At first he doesn’t answer.
“Papa, do you want the breathing tube?”
“No,” he says.
“Without it you can die. You know that, Papa?”
“No breathing tube,” he says.
“OK,” she turns to us, with tears of sadness but also what seems like relief.
Forty-eight hours later, he passed away. His family had time to come in, and he had periods of alertness where he could speak with them. They were able to say good-bye. He was able to say I love you.
Another patient’s wife once told me he had given her the “gift of clarity” when he plainly stated before he passed that he didn’t want to be saved. She didn’t have to make the decision for him, and neither did the doctors. I liked that term, and I thought about it then.
I am grateful my patient’s wishes were clear. But we aren’t always so lucky. It’s a chilling part of the job description, being a gatekeeper to the question: Is this the end?
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
One evening as the oncology fellow on call, I received a phone call from the ICU fellow.
“Can you meet me in the emergency room?” he asked. “I want to make sure we’re on the same page.”
A patient we had discharged from the hospital 2 days before was back. He had metastatic stomach cancer that had spread into his lungs and the lymph nodes in his chest. While he was in the hospital, he had required several liters of oxygen to maintain a normal work of breathing.
But now, he was in the emergency room, he was requiring a full face mask to help him breathe – and his oxygen levels were still dropping.
The ICU had been called. The next step along the algorithm of worsening breathing would be intubation. They would have to sedate him, put a breathing tube down his throat, and connect him to a ventilator to keep him alive.
But they didn’t want to do that if he was dying from his cancer.
Hence the call to me. My job, as the oncologist on call, was to answer the question: Is he dying?
Specifically, that meant weigh in on his cancer prognosis. Put his disease into context. Does he have any more options, chemotherapy or otherwise?
As an oncology fellow, I’ve found this to be one of the most common calls I get. Someone is critically ill and they need something to survive – maybe it’s intubation; maybe it’s surgery. The patient also happens to have metastatic cancer. The question posed to me is: Should we proceed?
It’s also one of the most difficult calls. Because doctors are historically bad at prognosticating. Because often I’m meeting the patient for the first time. Because the decision is huge and often final, and because both options are bad.
Suppose I say he has a good year or 2 ahead of him, and we intubate him – and then he never comes off the ventilator. We are eventually forced to withdraw care, and to the family it’s as though they are killing their father. It’s traumatic; it’s painful; and it deprives someone of a comfortable passing. Suppose I say he is dying from his cancer and we decide against a breathing tube. If I am wrong in that direction, a person’s life is cut short. It’s a perfect storm of high risk and low certainty.
Many people with metastatic cancer say they wouldn’t want invasive treatment near the end of life. But how do we know when it’s the end? There is still a moment when you must determine: Is this it? The truth is it’s not always clear.
Whenever I can, I reach out to the primary oncologist who knows the patient best. Then, I do a quick search for something reversible. Did the patient take too much morphine at home, and should we trial a dose of Narcan? Does he have a pneumonia that could be cured with antibiotics, a blood clot that could improve with blood thinners, or some extra fluid that can be diuresed? But usually it’s a mix, and even if there is a reversible injury, it can tip the very ill person over to the irreversible. This is how passing away from an aggressive cancer plays out.
Down in the emergency room, my patient’s breathing is rapid. His chest is heaving. The nurse shows me his blood gas with a carbon dioxide level more than twice the upper limit of normal. Now fading in and out of consciousness, he is a different man from the one who had walked out of the hospital 2 days earlier.
His daughter stands next to him. “He always said he wanted to do everything. I think we should give the breathing tube a try,” she says.
I tell her my concerns. I am afraid if we do it the likelihood of ever coming off is slim. And if we place a breathing tube he would have to be sedated so as not to be uncomfortable, and you won’t be able to communicate with him. You can’t say good bye, or I love you. If we keep the mask, he may wake up enough to interact.
The daughter – whom I knew well from prior visits, who was always articulate and poised and the spokesperson for the family – had held it together this entire time. Now, she breaks down. We all wait as I hand her a box of tissues. I look down, channeling all of my energy into not crying in front of her.
He’s waking up, one of us notes.
She goes over. “I need to ask him,” she says.
“Papa.”
At first he doesn’t answer.
“Papa, do you want the breathing tube?”
“No,” he says.
“Without it you can die. You know that, Papa?”
“No breathing tube,” he says.
“OK,” she turns to us, with tears of sadness but also what seems like relief.
Forty-eight hours later, he passed away. His family had time to come in, and he had periods of alertness where he could speak with them. They were able to say good-bye. He was able to say I love you.
Another patient’s wife once told me he had given her the “gift of clarity” when he plainly stated before he passed that he didn’t want to be saved. She didn’t have to make the decision for him, and neither did the doctors. I liked that term, and I thought about it then.
I am grateful my patient’s wishes were clear. But we aren’t always so lucky. It’s a chilling part of the job description, being a gatekeeper to the question: Is this the end?
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
The daunting challenge of schizophrenia: Hundreds of biotypes and dozens of theories
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.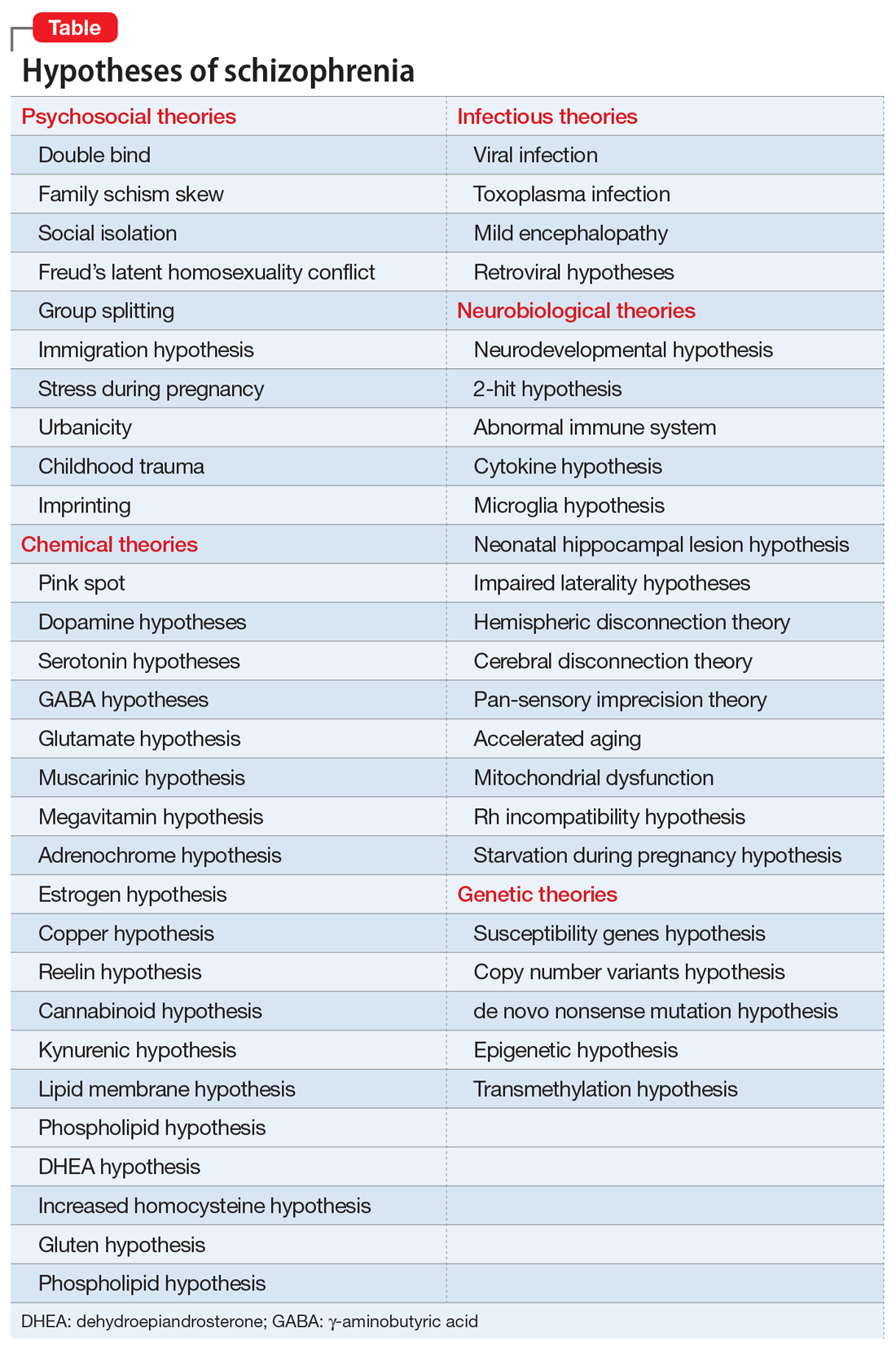
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
A transgender adolescent with chronic pain, depression, and PTSD
X, a 17-year-old Mexican-American transgender male (assigned female at birth) experienced a traumatic brain injury (TBI) 4 years ago and subsequently developed posttraumatic stress disorder (PTSD). I came to treat X at a pediatric outpatient psychiatric clinic after he developed physiologic dysregulation of his nervous system and began to experience panic attacks, major depressive disorder, and auditory hallucinations. X also developed chronic widespread pain during the next few years, including migraines, abdominal pain, and back pain, which significantly impaired his ability to function socially and academically. X was treated by a child and adolescent psychiatrist who used an integrative approach of traditional and complementary medical practices in a pediatric chronic pain clinic.
X’s treatment course at the pediatric psychiatric clinic included 2 years of field capable mental health services. During this time, fluoxetine was started and titrated up to 40 mg/d to target anxiety and depressive symptoms such as pervasive sadness, poor self-esteem, poor concentration, physiologic arousal, and sleep disruption. Risperidone, 2 mg/d, was temporarily added to address residual mood symptoms and the auditory hallucinations X experienced at school. Neuropsychological testing did not indicate that X had cognitive impairments from the TBI. In the pain clinic, X was encouraged to continue with psychotherapy and the selective serotonin reuptake inhibitor. Another recommendation was to seek out acupuncture and yoga. Over the course of 1 year, X’s pain symptoms began to resolve, and his functioning improved significantly. It was during this year that X came out as transgender, first to his friends, and then to his family and his physicians.
The link between PTSD and chronic pain
X’s PTSD presented as nightmares, hypervigilance, and anxiety, especially when he was in school. He would often describe how his chronic pain symptoms prevented him from functioning academically and socially. I wondered if X’s presentation of PTSD indicated a predisposition for chronic widespread pain symptoms or pain syndromes. This theory could be approximated by an association, but research suggests there is a significant temporal relationship between PTSD and widespread pain symptoms, such as in fibromyalgia.
One multicenter study of patients with fibromyalgia found that the prevalence of comorbid PTSD was 45%.1 In two-thirds of patients with fibromyalgia, traumatic life events and PTSD symptoms preceded the onset of chronic widespread pain, while in roughly one-third, traumatic life events and PTSD symptoms followed the onset of chronic widespread pain.1 This study suggests that PTSD could be viewed as a marker of stress vulnerability in which individuals susceptible to stress are more likely to develop chronic widespread pain and other health problems, including fibromyalgia, when a traumatic event occurs.
Benefits of transgender-specific care
During the course of X’s psychiatric treatment, he eventually revealed that he had been experiencing gender dysphoria for many years. His gender transition was occurring during adolescence; during this time, identity formation is a central developmental task.2 X was not comfortable asking others to use his preferred pronouns until he had physiologically transitioned. Any further delay to accessing transgender-specific services would increase the likelihood of a poor prognosis, both behaviorally and medically, because sexual minority adolescents are 3 to 4 times more likely to meet criteria for an internalizing disorder and 2 to 5 times more likely to meet criteria for externalizing disorders.3 My understanding of the minority stress model raised concerns that if X did not get appropriate treatment, the interdependence of stressors of being a sexual minority as well as an ethnic minority would further burden his mental health.
Now that X had access to transgender-specific care, how would management affect his pain symptoms or response to treatment? While some of his pain symptoms began to remit before he came out as transgender, I considered whether hormone therapy might improve his subjective pain. Little research has been conducted in transgender patients to determine whether sex-steroid administration might alter nociception. One study that examined daily fluctuations of sex hormones in 8 women with fibromyalgia found trends suggesting progesterone and testosterone are inversely associated with pain, with peaks of those hormones occurring on days with lower reported pain.4 A small study of female-to-male transgender patients found that administration of sex steroids was associated with relief from chronic painful conditions (headaches, musculoskeletal pain) in 6 of 16 patients who received testosterone injections.5 What little evidence I found in regards to an association between gender-affirming hormone therapy and chronic pain left me feeling optimistic that hormone therapy would not negatively affect the prognosis of X’s chronic pain.
Another consideration in treating X was the practice of chest binding, the compression of chest tissue for masculine gender expression among people who were assigned female sex at birth. One study found that chest binding can improve mood; decrease suicidality, anxiety, and dysphoria; and increase self-esteem.6 However, 97.2% of participants reported at least one negative outcome they attributed to binding. The most common was back pain (53.8%), which X had been experiencing before he began chest binding. I found it notable that X’s primary doctors in the transgender clinic kept this adverse effect in mind when they recommended that he take breaks and limit daily hours of chest binding to minimize the risk of increased chronic back pain.
This particular case spanned several specialized services and required coordination and careful consideration to address X’s developmental and gender-related needs. X experienced significant symptoms incited by a TBI; however, the manifestation of his chronic pain symptoms were more than likely influenced by several overlapping stressors, including belonging to an ethnic minority, transitioning into adulthood, transitioning publicly as a male, and mood symptoms. While it pleased me to see how X responded positively to the integrative and holistic treatment he received, I remain concerned that simply not enough research exists that addresses how transgender individuals are affected, physically and affectively, by chronic levels of stress attributable to their minority status.
1. Häuser W, Galek A, Erbslöh-Möller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain. 2013;154(8):1216-1223.
2. Erikson EH. Identity: Youth and crisis. New York, NY: W.W. Norton & Company; 1968.
3. Fergusson DM, Horwood LJ, Beautrais AL. Is sexual orientation related to mental health problems and suicidality in young people? Arch Gen Psychiatry. 1999;56(10):876-880.
4. Schertzinger M, Wesson-Sides K, Parkitny L, et al. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain. 2018;19(4):410-417.
5. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(suppl 1):S60-S67.
6. Peitzmeier S, Gardner I, Weinand J et al. Health impact of chest binding among transgender adults: a community-engaged, cross-sectional study. Cult Health Sex. 2017;19(1):64-75.
X, a 17-year-old Mexican-American transgender male (assigned female at birth) experienced a traumatic brain injury (TBI) 4 years ago and subsequently developed posttraumatic stress disorder (PTSD). I came to treat X at a pediatric outpatient psychiatric clinic after he developed physiologic dysregulation of his nervous system and began to experience panic attacks, major depressive disorder, and auditory hallucinations. X also developed chronic widespread pain during the next few years, including migraines, abdominal pain, and back pain, which significantly impaired his ability to function socially and academically. X was treated by a child and adolescent psychiatrist who used an integrative approach of traditional and complementary medical practices in a pediatric chronic pain clinic.
X’s treatment course at the pediatric psychiatric clinic included 2 years of field capable mental health services. During this time, fluoxetine was started and titrated up to 40 mg/d to target anxiety and depressive symptoms such as pervasive sadness, poor self-esteem, poor concentration, physiologic arousal, and sleep disruption. Risperidone, 2 mg/d, was temporarily added to address residual mood symptoms and the auditory hallucinations X experienced at school. Neuropsychological testing did not indicate that X had cognitive impairments from the TBI. In the pain clinic, X was encouraged to continue with psychotherapy and the selective serotonin reuptake inhibitor. Another recommendation was to seek out acupuncture and yoga. Over the course of 1 year, X’s pain symptoms began to resolve, and his functioning improved significantly. It was during this year that X came out as transgender, first to his friends, and then to his family and his physicians.
The link between PTSD and chronic pain
X’s PTSD presented as nightmares, hypervigilance, and anxiety, especially when he was in school. He would often describe how his chronic pain symptoms prevented him from functioning academically and socially. I wondered if X’s presentation of PTSD indicated a predisposition for chronic widespread pain symptoms or pain syndromes. This theory could be approximated by an association, but research suggests there is a significant temporal relationship between PTSD and widespread pain symptoms, such as in fibromyalgia.
One multicenter study of patients with fibromyalgia found that the prevalence of comorbid PTSD was 45%.1 In two-thirds of patients with fibromyalgia, traumatic life events and PTSD symptoms preceded the onset of chronic widespread pain, while in roughly one-third, traumatic life events and PTSD symptoms followed the onset of chronic widespread pain.1 This study suggests that PTSD could be viewed as a marker of stress vulnerability in which individuals susceptible to stress are more likely to develop chronic widespread pain and other health problems, including fibromyalgia, when a traumatic event occurs.
Benefits of transgender-specific care
During the course of X’s psychiatric treatment, he eventually revealed that he had been experiencing gender dysphoria for many years. His gender transition was occurring during adolescence; during this time, identity formation is a central developmental task.2 X was not comfortable asking others to use his preferred pronouns until he had physiologically transitioned. Any further delay to accessing transgender-specific services would increase the likelihood of a poor prognosis, both behaviorally and medically, because sexual minority adolescents are 3 to 4 times more likely to meet criteria for an internalizing disorder and 2 to 5 times more likely to meet criteria for externalizing disorders.3 My understanding of the minority stress model raised concerns that if X did not get appropriate treatment, the interdependence of stressors of being a sexual minority as well as an ethnic minority would further burden his mental health.
Now that X had access to transgender-specific care, how would management affect his pain symptoms or response to treatment? While some of his pain symptoms began to remit before he came out as transgender, I considered whether hormone therapy might improve his subjective pain. Little research has been conducted in transgender patients to determine whether sex-steroid administration might alter nociception. One study that examined daily fluctuations of sex hormones in 8 women with fibromyalgia found trends suggesting progesterone and testosterone are inversely associated with pain, with peaks of those hormones occurring on days with lower reported pain.4 A small study of female-to-male transgender patients found that administration of sex steroids was associated with relief from chronic painful conditions (headaches, musculoskeletal pain) in 6 of 16 patients who received testosterone injections.5 What little evidence I found in regards to an association between gender-affirming hormone therapy and chronic pain left me feeling optimistic that hormone therapy would not negatively affect the prognosis of X’s chronic pain.
Another consideration in treating X was the practice of chest binding, the compression of chest tissue for masculine gender expression among people who were assigned female sex at birth. One study found that chest binding can improve mood; decrease suicidality, anxiety, and dysphoria; and increase self-esteem.6 However, 97.2% of participants reported at least one negative outcome they attributed to binding. The most common was back pain (53.8%), which X had been experiencing before he began chest binding. I found it notable that X’s primary doctors in the transgender clinic kept this adverse effect in mind when they recommended that he take breaks and limit daily hours of chest binding to minimize the risk of increased chronic back pain.
This particular case spanned several specialized services and required coordination and careful consideration to address X’s developmental and gender-related needs. X experienced significant symptoms incited by a TBI; however, the manifestation of his chronic pain symptoms were more than likely influenced by several overlapping stressors, including belonging to an ethnic minority, transitioning into adulthood, transitioning publicly as a male, and mood symptoms. While it pleased me to see how X responded positively to the integrative and holistic treatment he received, I remain concerned that simply not enough research exists that addresses how transgender individuals are affected, physically and affectively, by chronic levels of stress attributable to their minority status.
X, a 17-year-old Mexican-American transgender male (assigned female at birth) experienced a traumatic brain injury (TBI) 4 years ago and subsequently developed posttraumatic stress disorder (PTSD). I came to treat X at a pediatric outpatient psychiatric clinic after he developed physiologic dysregulation of his nervous system and began to experience panic attacks, major depressive disorder, and auditory hallucinations. X also developed chronic widespread pain during the next few years, including migraines, abdominal pain, and back pain, which significantly impaired his ability to function socially and academically. X was treated by a child and adolescent psychiatrist who used an integrative approach of traditional and complementary medical practices in a pediatric chronic pain clinic.
X’s treatment course at the pediatric psychiatric clinic included 2 years of field capable mental health services. During this time, fluoxetine was started and titrated up to 40 mg/d to target anxiety and depressive symptoms such as pervasive sadness, poor self-esteem, poor concentration, physiologic arousal, and sleep disruption. Risperidone, 2 mg/d, was temporarily added to address residual mood symptoms and the auditory hallucinations X experienced at school. Neuropsychological testing did not indicate that X had cognitive impairments from the TBI. In the pain clinic, X was encouraged to continue with psychotherapy and the selective serotonin reuptake inhibitor. Another recommendation was to seek out acupuncture and yoga. Over the course of 1 year, X’s pain symptoms began to resolve, and his functioning improved significantly. It was during this year that X came out as transgender, first to his friends, and then to his family and his physicians.
The link between PTSD and chronic pain
X’s PTSD presented as nightmares, hypervigilance, and anxiety, especially when he was in school. He would often describe how his chronic pain symptoms prevented him from functioning academically and socially. I wondered if X’s presentation of PTSD indicated a predisposition for chronic widespread pain symptoms or pain syndromes. This theory could be approximated by an association, but research suggests there is a significant temporal relationship between PTSD and widespread pain symptoms, such as in fibromyalgia.
One multicenter study of patients with fibromyalgia found that the prevalence of comorbid PTSD was 45%.1 In two-thirds of patients with fibromyalgia, traumatic life events and PTSD symptoms preceded the onset of chronic widespread pain, while in roughly one-third, traumatic life events and PTSD symptoms followed the onset of chronic widespread pain.1 This study suggests that PTSD could be viewed as a marker of stress vulnerability in which individuals susceptible to stress are more likely to develop chronic widespread pain and other health problems, including fibromyalgia, when a traumatic event occurs.
Benefits of transgender-specific care
During the course of X’s psychiatric treatment, he eventually revealed that he had been experiencing gender dysphoria for many years. His gender transition was occurring during adolescence; during this time, identity formation is a central developmental task.2 X was not comfortable asking others to use his preferred pronouns until he had physiologically transitioned. Any further delay to accessing transgender-specific services would increase the likelihood of a poor prognosis, both behaviorally and medically, because sexual minority adolescents are 3 to 4 times more likely to meet criteria for an internalizing disorder and 2 to 5 times more likely to meet criteria for externalizing disorders.3 My understanding of the minority stress model raised concerns that if X did not get appropriate treatment, the interdependence of stressors of being a sexual minority as well as an ethnic minority would further burden his mental health.
Now that X had access to transgender-specific care, how would management affect his pain symptoms or response to treatment? While some of his pain symptoms began to remit before he came out as transgender, I considered whether hormone therapy might improve his subjective pain. Little research has been conducted in transgender patients to determine whether sex-steroid administration might alter nociception. One study that examined daily fluctuations of sex hormones in 8 women with fibromyalgia found trends suggesting progesterone and testosterone are inversely associated with pain, with peaks of those hormones occurring on days with lower reported pain.4 A small study of female-to-male transgender patients found that administration of sex steroids was associated with relief from chronic painful conditions (headaches, musculoskeletal pain) in 6 of 16 patients who received testosterone injections.5 What little evidence I found in regards to an association between gender-affirming hormone therapy and chronic pain left me feeling optimistic that hormone therapy would not negatively affect the prognosis of X’s chronic pain.
Another consideration in treating X was the practice of chest binding, the compression of chest tissue for masculine gender expression among people who were assigned female sex at birth. One study found that chest binding can improve mood; decrease suicidality, anxiety, and dysphoria; and increase self-esteem.6 However, 97.2% of participants reported at least one negative outcome they attributed to binding. The most common was back pain (53.8%), which X had been experiencing before he began chest binding. I found it notable that X’s primary doctors in the transgender clinic kept this adverse effect in mind when they recommended that he take breaks and limit daily hours of chest binding to minimize the risk of increased chronic back pain.
This particular case spanned several specialized services and required coordination and careful consideration to address X’s developmental and gender-related needs. X experienced significant symptoms incited by a TBI; however, the manifestation of his chronic pain symptoms were more than likely influenced by several overlapping stressors, including belonging to an ethnic minority, transitioning into adulthood, transitioning publicly as a male, and mood symptoms. While it pleased me to see how X responded positively to the integrative and holistic treatment he received, I remain concerned that simply not enough research exists that addresses how transgender individuals are affected, physically and affectively, by chronic levels of stress attributable to their minority status.
1. Häuser W, Galek A, Erbslöh-Möller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain. 2013;154(8):1216-1223.
2. Erikson EH. Identity: Youth and crisis. New York, NY: W.W. Norton & Company; 1968.
3. Fergusson DM, Horwood LJ, Beautrais AL. Is sexual orientation related to mental health problems and suicidality in young people? Arch Gen Psychiatry. 1999;56(10):876-880.
4. Schertzinger M, Wesson-Sides K, Parkitny L, et al. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain. 2018;19(4):410-417.
5. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(suppl 1):S60-S67.
6. Peitzmeier S, Gardner I, Weinand J et al. Health impact of chest binding among transgender adults: a community-engaged, cross-sectional study. Cult Health Sex. 2017;19(1):64-75.
1. Häuser W, Galek A, Erbslöh-Möller B, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain. 2013;154(8):1216-1223.
2. Erikson EH. Identity: Youth and crisis. New York, NY: W.W. Norton & Company; 1968.
3. Fergusson DM, Horwood LJ, Beautrais AL. Is sexual orientation related to mental health problems and suicidality in young people? Arch Gen Psychiatry. 1999;56(10):876-880.
4. Schertzinger M, Wesson-Sides K, Parkitny L, et al. Daily fluctuations of progesterone and testosterone are associated with fibromyalgia pain severity. J Pain. 2018;19(4):410-417.
5. Aloisi AM, Bachiocco V, Costantino A, et al. Cross-sex hormone administration changes pain in transsexual women and men. Pain. 2007;132(suppl 1):S60-S67.
6. Peitzmeier S, Gardner I, Weinand J et al. Health impact of chest binding among transgender adults: a community-engaged, cross-sectional study. Cult Health Sex. 2017;19(1):64-75.
Resilience: Our only remedy?
Resilience is like patience; we all wish we had more of it, but we hope to avoid getting it the hard way. This wasn’t really an area of interest for me, until it needed to be. When one academic year brings the suicide of one colleague and the murder of another, resilience becomes the only alternative to despair.
I realize that even though the particular pain or trauma we endured may be unique, it’s becoming increasingly common. The alarming studies of resident depression and suicide are too difficult for us to ignore. Now we must look in that evidence-based mirror and decide where we will go from here, as a profession and as trainees. The 2018 American Psychiatric Association annual meeting gave us a rude awakening that we may not have it figured out. Even during a year-long theme on wellness, and several sessions at the meeting focusing on the same, we all found ourselves mourning the loss of 2 colleagues to suicide that very weekend only a few miles away from the gathering of the world’s experts.
It brought an eerie element to the conversation.
The wellness “window dressing” will not get the job done. I recently had a candid discussion with a mentor in administrative leadership, and his words surprised as well as challenged me. He told me that the “system” will not save you. You must save yourself. I have decided to respectfully reject that. I think everyone should be involved, including the “system” that is entrusted with my training, and the least that it ought to ensure is that I get out alive.
Has that really become too much to ask of our profession?
We must hold our system to a higher standard. More mindfulness and better breathing will surely be helpful—but I hope we can begin to admit that this is not the answer. Unfortunately, the culture of “pay your dues” and “you know how much harder it was when I was a resident?” is still the norm. We now receive our training in an environment where the pressure is extraordinarily high, the margin for error very low, and the possibility of support is almost a fantasy. “Sure, you can get the help you need ... but don’t take time off or you will be off cycle and create extra work for all your colleagues, who are also equally stressed and will hate you. In the meantime … enjoy this free ice cream and breathing exercise to mindfully cope with the madness around you.”
The perfectly resilient resident may very well be a mythical figure, a clinical unicorn, that we continue chasing. This is the resident who remarkably discovers posttraumatic growth in every stressor. The vicarious trauma they experience from their patients only bolsters their deep compassion, and they thrive under pressure, so we can continue to pile it on. In our search for this “super resident,” we seem to continue to lose a few ordinary residents along the way.
Are we brave enough as a health care culture to take a closer look at the way we are training the next generation of healers? As I get to the end of this article, I wish I had more answers. I’m just a trainee. What do I know? My fear is that we’ve been avoiding this question altogether and have had our eyes closed to the real problem while pacifying ourselves with one “wellness” activity after another. My sincere hope is that this article will make you angry enough to be driven by a conviction that this is not
Resilience is like patience; we all wish we had more of it, but we hope to avoid getting it the hard way. This wasn’t really an area of interest for me, until it needed to be. When one academic year brings the suicide of one colleague and the murder of another, resilience becomes the only alternative to despair.
I realize that even though the particular pain or trauma we endured may be unique, it’s becoming increasingly common. The alarming studies of resident depression and suicide are too difficult for us to ignore. Now we must look in that evidence-based mirror and decide where we will go from here, as a profession and as trainees. The 2018 American Psychiatric Association annual meeting gave us a rude awakening that we may not have it figured out. Even during a year-long theme on wellness, and several sessions at the meeting focusing on the same, we all found ourselves mourning the loss of 2 colleagues to suicide that very weekend only a few miles away from the gathering of the world’s experts.
It brought an eerie element to the conversation.
The wellness “window dressing” will not get the job done. I recently had a candid discussion with a mentor in administrative leadership, and his words surprised as well as challenged me. He told me that the “system” will not save you. You must save yourself. I have decided to respectfully reject that. I think everyone should be involved, including the “system” that is entrusted with my training, and the least that it ought to ensure is that I get out alive.
Has that really become too much to ask of our profession?
We must hold our system to a higher standard. More mindfulness and better breathing will surely be helpful—but I hope we can begin to admit that this is not the answer. Unfortunately, the culture of “pay your dues” and “you know how much harder it was when I was a resident?” is still the norm. We now receive our training in an environment where the pressure is extraordinarily high, the margin for error very low, and the possibility of support is almost a fantasy. “Sure, you can get the help you need ... but don’t take time off or you will be off cycle and create extra work for all your colleagues, who are also equally stressed and will hate you. In the meantime … enjoy this free ice cream and breathing exercise to mindfully cope with the madness around you.”
The perfectly resilient resident may very well be a mythical figure, a clinical unicorn, that we continue chasing. This is the resident who remarkably discovers posttraumatic growth in every stressor. The vicarious trauma they experience from their patients only bolsters their deep compassion, and they thrive under pressure, so we can continue to pile it on. In our search for this “super resident,” we seem to continue to lose a few ordinary residents along the way.
Are we brave enough as a health care culture to take a closer look at the way we are training the next generation of healers? As I get to the end of this article, I wish I had more answers. I’m just a trainee. What do I know? My fear is that we’ve been avoiding this question altogether and have had our eyes closed to the real problem while pacifying ourselves with one “wellness” activity after another. My sincere hope is that this article will make you angry enough to be driven by a conviction that this is not
Resilience is like patience; we all wish we had more of it, but we hope to avoid getting it the hard way. This wasn’t really an area of interest for me, until it needed to be. When one academic year brings the suicide of one colleague and the murder of another, resilience becomes the only alternative to despair.
I realize that even though the particular pain or trauma we endured may be unique, it’s becoming increasingly common. The alarming studies of resident depression and suicide are too difficult for us to ignore. Now we must look in that evidence-based mirror and decide where we will go from here, as a profession and as trainees. The 2018 American Psychiatric Association annual meeting gave us a rude awakening that we may not have it figured out. Even during a year-long theme on wellness, and several sessions at the meeting focusing on the same, we all found ourselves mourning the loss of 2 colleagues to suicide that very weekend only a few miles away from the gathering of the world’s experts.
It brought an eerie element to the conversation.
The wellness “window dressing” will not get the job done. I recently had a candid discussion with a mentor in administrative leadership, and his words surprised as well as challenged me. He told me that the “system” will not save you. You must save yourself. I have decided to respectfully reject that. I think everyone should be involved, including the “system” that is entrusted with my training, and the least that it ought to ensure is that I get out alive.
Has that really become too much to ask of our profession?
We must hold our system to a higher standard. More mindfulness and better breathing will surely be helpful—but I hope we can begin to admit that this is not the answer. Unfortunately, the culture of “pay your dues” and “you know how much harder it was when I was a resident?” is still the norm. We now receive our training in an environment where the pressure is extraordinarily high, the margin for error very low, and the possibility of support is almost a fantasy. “Sure, you can get the help you need ... but don’t take time off or you will be off cycle and create extra work for all your colleagues, who are also equally stressed and will hate you. In the meantime … enjoy this free ice cream and breathing exercise to mindfully cope with the madness around you.”
The perfectly resilient resident may very well be a mythical figure, a clinical unicorn, that we continue chasing. This is the resident who remarkably discovers posttraumatic growth in every stressor. The vicarious trauma they experience from their patients only bolsters their deep compassion, and they thrive under pressure, so we can continue to pile it on. In our search for this “super resident,” we seem to continue to lose a few ordinary residents along the way.
Are we brave enough as a health care culture to take a closer look at the way we are training the next generation of healers? As I get to the end of this article, I wish I had more answers. I’m just a trainee. What do I know? My fear is that we’ve been avoiding this question altogether and have had our eyes closed to the real problem while pacifying ourselves with one “wellness” activity after another. My sincere hope is that this article will make you angry enough to be driven by a conviction that this is not
Pharmacologic performance enhancement: What to consider before prescribing
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
Performance enhancement in sports (“doping”) dates back to Ancient Greece. This was an era when Olympic athletes would attempt to improve their physical performance by consuming magic potions, herbal medications, and even exotic meats such as sheep testicles—a delicacy high in testosterone. Advances in medical and pharmaceutical technologies have increased both the range of enhancement agents available and their efficacy, leading to the development of anti-doping agencies and routine screening for doping in athletics. This has led to the renouncement of titles, medals, and financial sponsorship of athletes found to have been using prohibited substances during competition.
While doping in elite athletes often forms the nidus of media attention, the pressure to compete and perform at, or even beyond, one’s potential extends into many facets of today’s achievementfocused society. In the face of these pressures, individuals are increasingly seeking medications to enhance their performance across numerous domains, including cognitive, athletic, and artistic endeavors. Medication classes used to enhance performance include stimulants, which increase attention, executive function, and energy; cholinesterase inhibitors, which may ameliorate age-related memory decline; and beta-blockers, which decrease physiologic symptoms of anxiety and have been demonstrated to be beneficial for musical performance.1 Fifty-three percent of college athletes report using prescription medications to enhance athletic performance,2 and most college students who take stimulants without a prescription use them to study (84%) or stay awake (51%).3
Pharmacologic performance enhancement is the use of medications by healthy individuals to improve function in the absence of mental illness. Psychiatrists are increasingly finding themselves in the controversial position of “gatekeeper” of these medications for enhancement purposes. In this article we:
- outline arguments that support the use of psychopharmacology for performance enhancement, as well as some serious concerns with this practice
- discuss special considerations for pediatric populations and the risk of malpractice when prescribing for performance enhancement
- offer practice guidelines for approaching requests for psychopharmacologic performance enhancement.
Performance enhancement: The wave of the future?
The ethical principle that supports providing medication for performance enhancement is beneficence, the promotion of the patient’s well-being. In other words, it is a physician’s duty to help his or her patient in need. Individuals seeking performance enhancement typically present with suffering, and the principle of beneficence would call upon the psychiatrist to help ameliorate that suffering. Furthermore, patients who seek performance enhancement may present with impairing “subsyndromal” psychiatric symptoms (for example, low-grade attentional difficulty that occurs only in one setting), which, even if they do not rise to the threshold of a DSM diagnosis, may improve with psychiatric medications.
Using medical knowledge and skills beyond the traditional physician duty to diagnose and treat medical conditions is not unprecedented (eg, when surgeons perform cosmetic enhancement). Might elective enhancement of cognition and psychological performance through the judicious use of medication be part of the future of psychiatry? If cognitive and emotional enhancement becomes a more widely accepted standard of care, might this increase both individual and societal innovation and productivity?
Dilemma: Cautions against performance enhancement
One of the major cautions against prescribing psychotropics for the purpose of performance enhancement is the lack of clearly supported efficacy. Psychiatric medications generally are studied in individuals who meet criteria for mental illness, and they are FDA-approved for use in ill persons. It may be erroneous to extrapolate that a medication that improves symptoms in a patient with an illness would achieve the same target effect in a healthy individual. For example, data on whether stimulants provide neurocognitive enhancement in healthy individuals without attention-deficit/hyperactivity disorder is mixed, and these agents may even promote risky behavior in healthy controls.4 Furthermore, dopamine agonism may compress cognitive performance in both directions,5 as it has been observed that methylphenidate improves executive function in healthy controls, but is less beneficial for those with strong executive function at baseline.6
In the face of unclear benefit, it is particularly important to consider the risk of medications used for performance enhancement. Pharmacologic performance enhancement in individuals without psychopathology can be considered an “elective” intervention, for which individuals typically tolerate less risk. Physical risks, including medication-related adverse effects, must be considered, particularly in settings where there may be temptation to use more than prescribed, or to divert medication to others who may use it without medical monitoring. In addition to physical harm, there may be psychological harm associated with prescribing performance enhancers, such as pathologizing variants of “normal,” diminishing one’s sense of self-efficacy, or decreasing one’s ability to bear failure.
Continue to: Finally, there are ethical quandaries
Finally, there are ethical quandaries regarding using medications for performance enhancement. Widespread adoption of pharmacologic performance enhancement may lead to implicit coercion for all individuals to enhance their abilities. As a greater proportion of society receives pharmacologic enhancement, society as a whole faces stronger pressures to seek pharmacologic enhancement, ultimately constricting an individual’s freedom of choice to enhance.6 In this setting, distributive justice would become a consideration, because insurance companies are unlikely to reimburse for medications used for enhancement,7 which would give an advantage to individuals with higher socioeconomic status. Research shows that children from higher socioeconomic communities and from states with higher academic standards are more likely to use stimulants.8
Areas of controversy
Pediatric populations. There are special considerations when prescribing performance-enhancing medications for children and adolescents. First, such prescribing may inhibit normal child development, shifting the focus away from the normative tasks of social and emotional development that occur through leisure and creativity, experimentation, and play to an emphasis on performance and outcomes-based achievement.9 Second, during childhood and adolescence, one develops a sense of his or her identity, morals, and values. Taking a medication during childhood to enhance performance may inhibit the process of learning to tolerate failure, become aware of one’s weaknesses, and value effort in addition to outcome.
Malpractice risk. Practicing medicine beyond the scope of one’s expertise is unethical and unlawful. In the past 30 years, medical malpractice has become one of the most difficult health care issues in the U.S.10 In addition to billions of dollars in legal fees and court costs, medical malpractice premiums in the U.S. total more than $5 billion annually,11 and “defensive medicine”— procedures performed to protect against litigation—is estimated to cost more than $14 billion a year.12
When considering performance-enhancing treatment, it is the physician’s duty to conduct a diagnostic assessment, including noting target symptoms that are interfering with the patient’s function, and to tailor such treatment toward measurable goals and outcomes. Aside from medication, this could include a therapeutic approach to improving performance that might include cognitive-behavioral therapy and promotion of a healthy diet and exercise.
Treatment rises to the level of malpractice when there is a dereliction of duty that directly leads to damages.13 Part of a physician’s duty is to educate patients about the pros and cons of different treatment options. For performance-enhancing medications, the risks of addiction and dependence are adverse effects that require discussion. And for a pediatric patient, this would require the guardian’s engagement and understanding.
Continue to: What to do if you decide to prescribe
What to do if you decide to prescribe
Inevitably, the decision to prescribe psychotropic medications for performance enhancement is a physician-specific one. Certainly, psychiatrists should not feel obligated to prescribe performance enhancers. Given our current state of pharmacology, it is unclear whether medications would be helpful in the absence of psychopathology. When deciding whether to prescribe for performance enhancement in the absence of psychopathology, we suggest first carefully considering how to maintain the ethical value of nonmaleficence by weighing both the potential physical and psychologic harms of prescribing as well as the legal risks and rules of applicable sport governing bodies.
For a psychiatrist who chooses to prescribe for performance enhancement, we recommend conducting a thorough psychiatric assessment to determine whether the patient has a treatable mental illness. If so, then effective treatment of that illness should take priority. Before prescribing, the psychiatrist and patient should discuss the patient’s specific performance goals and how to measure them.
Any prescription for a performance-enhancing medication should be given in conjunction with nonpharmacologic approaches, including optimizing diet, exercise, and sleep. Therapy to address problem-solving techniques and skills to cope with stress may also be appropriate. The patient and psychiatrist should engage in regular follow-up to assess the efficacy of the medication, as well as its safety and tolerability. Finally, if a medication is not efficacious as a performance enhancer, then both the patient and psychiatrist should be open to re-evaluating the treatment plan, and when appropriate, stopping the medication.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
1. Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72(1):88-94.
2. Hoyte CO, Albert D, Heard KJ. The use of energy drinks, dietary supplements, and prescription medications by United States college students to enhance athletic performance. J Community Health. 2013;38(3):575-850.
3. Advokat CD, Guidry D, Martino L. Licit and illicit use of medications for attention-deficit hyperactivity disorder in undergraduate college students. J Am Coll Health. 2008;56(6):601-606.
4. Advokat C, Scheithauer M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front Neurosci. 2013;7:82.
5. Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8(16):3581-3585.
6. Farah MJ, Illes J, Cook-Deegan R, et al. Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci. 2004;5(5):421-425.
7. Larriviere D, Williams MA, Rizzo M, et al; AAN Ethics, Law and Humanities Committee. Responding to requests from adult patients for neuroenhancements: guidance of the Ethics, Law and Humanities Committee. Neurology. 2009;73(17):1406-1412.
8. Colaneri N, Sheldon M, Adesman A. Pharmacological cognitive enhancement in pediatrics. Curr Opin Pediatr. 2018;30(3):430-437.
9. Gaucher N, Payot A, Racine E. Cognitive enhancement in children and adolescents: Is it in their best interests? Acta Paediatr. 2013;102(12):1118-1124.
10. Moore PJ, Adler, NE, Robertson, PA. Medical malpractice; the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244-250.
11. Hiatt H. Medical malpractice. Bull N Y Acad Med. 1992;68(2):254-260.
12. Rubin RJ, Mendelson DN. How much does defensive medicine cost? J Am Health Policy. 1994;4(4):7-15.
13. Kloss D. The duty of care: medical negligence. Br Med J (Clin Res Ed). 1984;289(6436):66-68.
The powerful virus inflammatory response
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Veterans are not ‘ticking time bombs’
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.
Like all of us, I was very troubled by the recent mass shooting in Thousand Oaks, Calif. This shooting was on top of the massacre at Pittsburgh’s Tree of Life synagogue, the shootings in a yoga studio ... the sickening list goes on and on.
As both a veteran and a psychiatrist with expertise in posttraumatic stress disorder, I was especially dismayed by the assumption that the Thousand Oaks shooter, who had served in the Marine Corps, had PTSD, and that the PTSD had led to the shooting.
The overall effect of these assumptions is to reinforce the stigma against veterans as “ticking time bombs.”
No question, there are plenty of other stereotypes to go around, especially those of Muslims as terrorists. In reality, as reports from the GAO and independent news sources show, most “terrorist” attacks in the United States have been carried out by right-wing extremists, mainly white, and born in this country.
Back to veterans. It is true that there have been several mass shootings by service members and veterans, including the massacre at Fort Hood, Tex., in 2009 by an Army major, the 2017 shooting up of a church in Texas by someone who had served in the Air Force, and this most recent one by a former Marine.
But there have been many other shootings and acts of political violence by numerous others, including those for whom “life is going down the toilet.” When you look at these situations, the driving factors are usually anger, irritability, and a sense of being wronged. Often, delusions and paranoia emerge.
It is true that there are many barriers to treatment for both veterans and nonveterans, including stigma, lack of insurance, and the dearth of mental health providers.
Those factors have nothing to do with being a veteran, who are normally very proud of both their country and their military service.
Let us celebrate those who have given so much to this country, America’s sons and daughters.
Dr. Ritchie is chief of psychiatry at MedStar Washington Hospital Center.













