User login
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.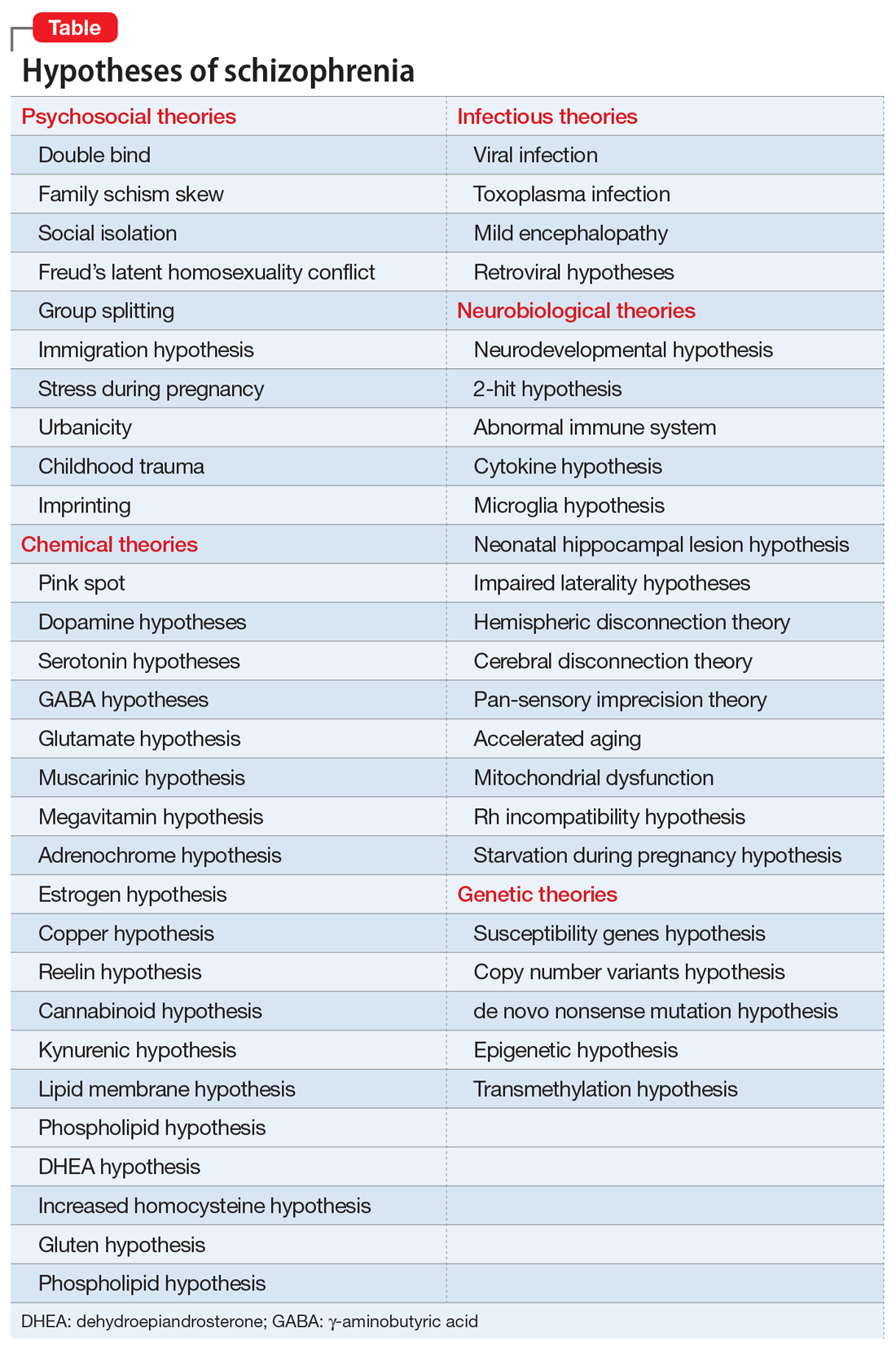
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
Islands of knowledge in an ocean of ignorance. That summarizes the advances in unraveling the enigma of schizophrenia, arguably the most complex psychiatric brain disorder. The more breakthroughs are made, the more questions emerge.
Progress is definitely being made and the published literature, replete with new findings, is growing logarithmically. Particularly exciting are the recent advances in the etiology of schizophrenia, both genetic and environmental. Collaboration among geneticists around the world has enabled genome-wide association studies on almost 50,000 DNA samples and has revealed 3 genetic pathways to disrupted brain development, which lead to schizophrenia in early adulthood. Those genetic pathways include:
1. Susceptibility genes—more than 340 of them—are found significantly more often in patients with schizophrenia compared with the general population. These risk genes are scattered across all 23 pairs of chromosomes. They influence neurotransmitter functions, neuroplasticity, and immune regulation. The huge task that lies ahead is identifying what each of the risk genes disrupts in brain structure and/or function.
2. Copy number variants (CNVs), such as deletions (1 allele instead of the normal 2) or duplications (3 alleles), are much more frequent in patients with schizophrenia compared with the general population. That means too little or too much protein is made, which can disrupt the 4 stages of brain development (proliferation, migration, differentiation, and elimination).
3. de novo nonsense mutations, leading to complete absence of protein coding by the affected genes, with adverse ripple effects on brain development.
Approximately 10,000 genes (close to 50% of all 22,000 coding genes in the human genome) are involved in constructing the human brain. The latest estimate is that 79% of the hundreds of biotypes of schizophrenia are genetic in etiology.
In addition, multiple environmental factors can disrupt brain development and lead to schizophrenia. These include older paternal age (>45 years) at the time of conception, pregnancy complications (infections, gestational diabetes, vitamin D deficiency, hypoxia during delivery), childhood maltreatment (sexual or physical abuse or neglect) in the first 5 to 6 years of life, as well as migration and urbanicity (being born and raised in a large metropolitan area).
The bottom line: Schizophrenia is not only very complex, but also an extremely heterogeneous brain syndrome, both biologically and clinically. Psychiatric practitioners are fully cognizant of the extensive clinical variability in patients with schizophrenia, including the presence, absence, or severity of various signs and symptoms, such as insight, delusions, hallucinations, conceptual disorganization, bizarre behaviors, emotional withdrawal, agitation, depression, suicidality, anxiety, substance use, somatic concerns, hostility, idiosyncratic mannerisms, blunted affect, apathy, avolition, self-neglect, poor attention, memory impairment, and problems with decision-making, planning ahead, or organizing one’s life.
In addition, heterogeneity is encountered in such variables as age of onset, minor physical anomalies, soft neurologic signs, naturally occurring movement disorders, premorbid functioning, family history, general medical comorbidities, psychiatry comorbidities, structural brain abnormalities on neuroimaging, neurophysiological deviations (pre-pulse inhibition, p50, p300, N100, mismatch negativity, smooth pursuit eye movements), pituitary volume, rapidity and extent of response to antipsychotics, type and frequency of adverse effects, and functional disability or restoration of vocational functioning.
No wonder, then, given the daunting biologic and clinical heterogeneity of this complex brain syndrome, that myriad hypotheses have been proposed over the past century. The Table lists approximately 50 hypotheses, some discredited but others plausible and still viable. The most absurd hypotheses are the double bind theory of schizophrenia in the 1950s by Gregory Bateson et al, or the latent homosexuality theory of Freud. Some hypotheses may be related to a specific biotype within the schizophrenia syndrome (such as the megavitamin theory) that do not apply to other biotypes. Some of the hypotheses seem to be the product of the rich imagination of an enthusiastic researcher based on limited data.
Another consequence of the extensive heterogeneity of schizophrenia is the large number of “lab tests” that have been reported over the past few decades.1 Those hundreds of biomarkers probably mirror the biologies of the numerous disease subtypes within the schizophrenia syndrome. Some are blood tests, others neurophysiological or neuroimaging, others molecular or genetic, along with many postmortem tissue markers. Obviously, none of these “lab tests” can be used clinically because there would be an unacceptably large number of false positives and false negatives when applied to a heterogeneous sample of patients with schizophrenia.
Heterogeneity also represents a formidable challenge for researchers. Replication of a research finding by investigators across the world can be quite challenging because of the variable composition of biotypes in different countries. This heterogeneity also complicates FDA clinical trials by pharmaceutical companies seeking approval for a new drug to treat schizophrenia. The FDA requires use of DSM diagnostic criteria, which would include patients with similar clinical symptoms, but who can vary widely at the biological level. This results in failed clinical trials where only 20% or 30% of patients with schizophrenia show significant improvement compared with placebo. Given the advances in schizophrenia, a better strategy is to recruit participants who share a specific biomarker to assemble a biologically more homogeneous sample of schizophrenia. If the clinical trial is successful, the same biomarker can then be used by practitioners to predict response to the new drug. That would fulfill the aspirations of applying precision medicine in psychiatric practice.
The famous Eugen Bleuler (whose sister suffered from schizophrenia) was prescient when a century ago he published his classic book titled Dementia Praecox or the Group of Schizophrenias.2 His astute clinical observations led him to recognize the heterogeneity of the syndrome whose name he coined (schizophrenia, or disconnected thoughts). His conceptualization of schizophrenia as a spectrum of disorders of variable outcomes contrasted with that of Emil Kraepelin’s model,3 which regarded dementia praecox as a single, homogeneous, deteriorating disease. But neither Bleuler nor Kraepelin, both of whom relied on clinical observations without any biologic studies, could even imagine the spectacular complexity of the neurobiology of the schizophrenia syndrome and how difficult it is to identify its many biotypes. The monumental advances in neuroscience and neurogenetics, with their sophisticated methodologies, will eventually decipher the mysteries of this neuropsychiatric syndrome, which generates so many aberrations in thought, affect, mood, cognition, and behavior, often leading to severe functional disability among young adults, and untold anguish for their families.
To comment on this editorial or other topics of interest: [email protected].
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
1. Nasrallah HA. Lab tests for psychiatric disorders: Few clinicians are aware of them. Current Psychiatry. 2013;12(2):5-7.
2. Bleuler E. Dementia praecox or the group of schizophrenias. New York, NY: International University Press; 1950.
3. Hippius H, Muller N. The work of Emil Kraepelin and his research group in Munich. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):3-11.
