User login
“Thank You for Not Letting Me Crash and Burn”: The Imperative of Quality Physician Onboarding to Foster Job Satisfaction, Strengthen Workplace Culture, and Advance the Quadruple Aim
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).
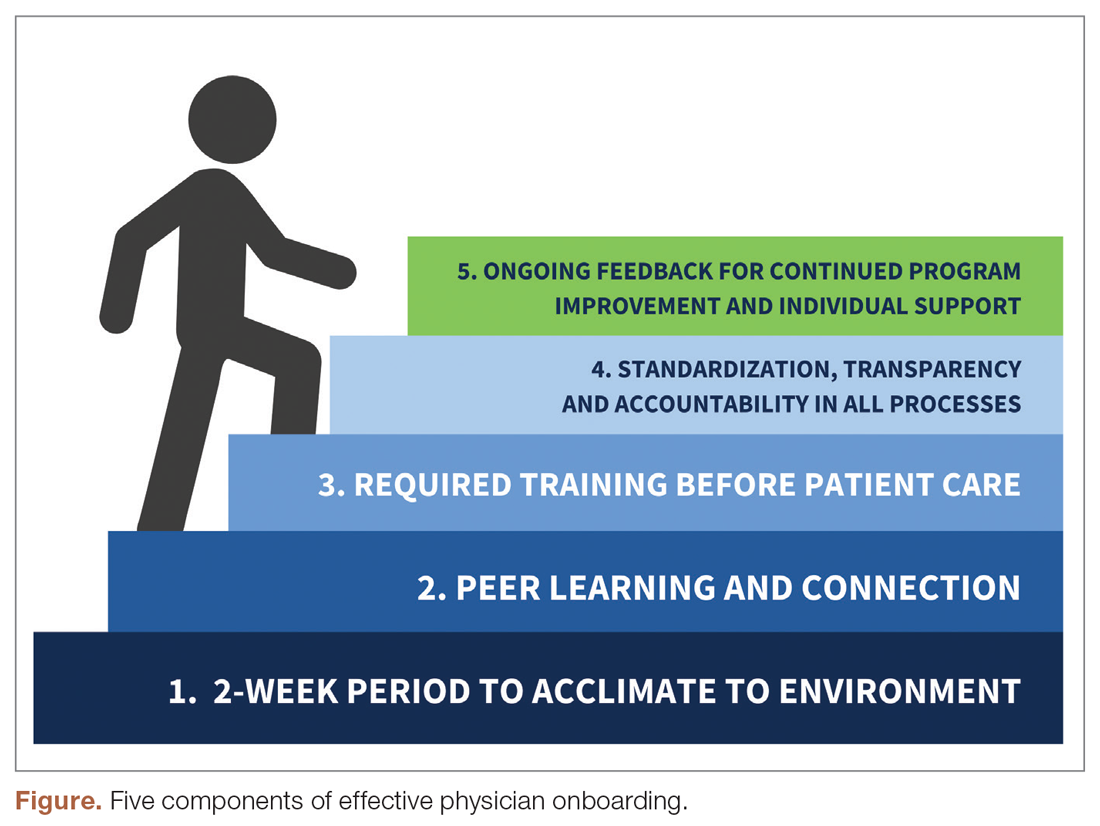
The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
Change is hard: Lessons from an EHR conversion
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Artifactual hypoglycemia: When there’s a problem in the tube
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
If you are looking for zebras you might consider adrenal insufficiency, which could cause both hyperkalemia and hypoglycemia, but this would make no sense in someone asymptomatic.
This pattern is one I have seen commonly when I am on call, and I am contacted about abnormal labs. The lab reported no hemolysis seen, but this is the typical pattern seen with hemolytic specimens and/or specimens that have been held a long time before they are analyzed.
Lippi and colleagues reported on the clinically significant increase in potassium in samples that visually appeared not to be hemolyzed.1 Hemolyzed specimens can also drop glucose values, but not as profoundly as raising potassium values. When left unprocessed, glycolysis occurs in the white blood cells of a blood sample and may consume 5%-7% of the sample’s glucose content per hour.2
Khaled and colleagues looked at the drop in glucose levels in samples over time based on what anticoagulants were used.3 They found that, at 3 hours, glucose measurements were decreased by 28.4 mg/dL when sodium citrate is used, 58 mg/dL when EDTA was used, 15.4 mg/dL when fluoride oxalate was used, and 60.2 mg/dL when no anticoagulant is used.
Low blood sugars caused by elevated WBCs in blood samples has been well described.4 It has been described with moderate and very high WBC counts, as well as with the leukocytosis seen with polycythemia vera.5 The term “leukocyte larceny” has been used to describe high WBC counts that can not only utilize glucose, but also oxygen.
Saccheti and colleagues described a patient with a WBC greater than 500,000 who had repeatedly low oxygen levels on blood gases, that did not correlate with the normal oxygen saturations measured by pulse oximetry.6 This same issue has been seen in patients with extreme thrombocytosis.7Pearl: When labs don’t make sense clinically, always look at the possibility that there may be a problem in the tube and not in the person. Especially think of this when blood samples may have been held for a long time before they are run, such as with visiting nurse visits and blood draws at shelters and nursing homes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Lippi G et al. Clin Chem Lab Med. 2006;44(3):311-6.
2. Mikesh LM and Bruns DE. Clin Chem. 2008 May;54(5):930-2.
3. Khaled S et al. Al-Mukhtar Journal of Sciences. 2018;33(2):100-6.
4. Goodenow TJ and Malarkey WB. JAMA. 1977;237(18):1961-2.
5. R Arem et al. Arch Intern Med. 1982 Nov;142(12):2199-201.
6. Sacchetti A et al. J Emerg Med. 1990;8:567–569.
7. A Mehta et al. Eur Respir J. 2008 Feb;31(2):469-72.
Recurrent miscarriage: What’s the evidence-based evaluation and management?
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.
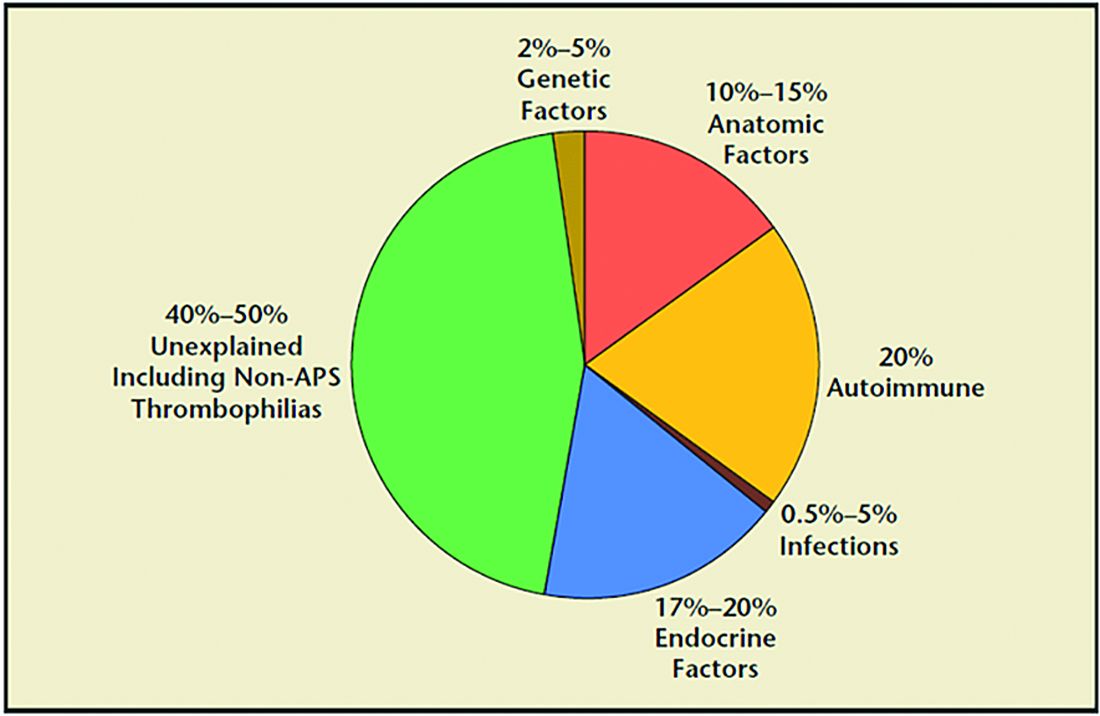
Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Reproductive safety of treatments for women with bipolar disorder
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since March 2020, my colleagues and I have conducted Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital. It has been an opportunity to review the basic tenets of care for reproductive age women before, during, and after pregnancy, and also to learn of extraordinary cases being managed both in the outpatient setting and in the context of the COVID-19 pandemic.
As I’ve noted in previous columns, we have seen a heightening of symptoms of anxiety and insomnia during the pandemic in women who visit our center, and at the centers of the more than 100 clinicians who join Virtual Rounds each week. These colleagues represent people in rural areas, urban environments, and underserved communities across America that have been severely affected by the pandemic. It is clear that the stress of the pandemic is undeniable for patients both with and without psychiatric or mental health issues. We have also seen clinical roughening in women who have been well for a long period of time. In particular, we have noticed that postpartum women are struggling with the stressors of the postpartum period, such as figuring out the logistics of support with respect to childcare, managing maternity leave, and adapting to shifting of anticipated support systems.
Hundreds of women with bipolar disorder come to see us each year about the reproductive safety of the medicines on which they are maintained. Those patients are typically well, and we collaborate with them and their doctors about the safest treatment recommendations. With that said, women with bipolar disorder are at particular risk for postpartum worsening of their mood. The management of their medications during pregnancy requires extremely careful attention because relapse of psychiatric disorder during pregnancy is the strongest predictor of postpartum worsening of underlying psychiatric illness.
This is an opportunity to briefly review the reproductive safety of treatments for these women. We know through initiatives such as the Massachusetts General Hospital National Pregnancy Registry for Psychiatric Medications that the most widely used medicines for bipolar women during pregnancy include lamotrigine, atypical antipsychotics, and lithium carbonate.
Lamotrigine
The last 15 years have generated the most consistent data on the reproductive safety of lamotrigine. One of the issues, however, with respect to lamotrigine is that its use requires very careful and slow titration and it is also more effective in patients who are well and in the maintenance phase of the illness versus those who are more acutely manic or who are suffering from frank bipolar depression.
Critically, the literature does not support the use of lamotrigine for patients with bipolar I or with more manic symptoms. That being said, it remains a mainstay of treatment for many patients with bipolar disorder, is easy to use across pregnancy, and has an attractive side-effect profile and a very strong reproductive safety profile, suggesting the absence of an increased risk for major malformations.
Atypical antipsychotics
We have less information but have a growing body of evidence about atypical antipsychotics. Both data from administrative databases as well a growing literature from pregnancy registries, such as the National Pregnancy Registry for Atypical Antipsychotics, fail to show a signal for teratogenicity with respect to use of the medicines as a class, and also with specific reference to some of the most widely used atypical antipsychotics, particularly quetiapine and aripiprazole. Our comfort level, compared with a decade ago, with using the second-generation antipsychotics is much greater. That’s a good thing considering the extent to which patients presenting on a combination of, for example, lamotrigine and atypical antipsychotics.
Lithium carbonate
Another mainstay of treatment for women with bipolar I disorder and prominent symptoms of mania is lithium carbonate. The data for efficacy of lithium carbonate used both acutely and for maintenance treatment of bipolar disorder has been unequivocal. Concerns about the teratogenicity of lithium go back to the 1970s and indicate a small increased absolute and relative risk for cardiovascular malformations. More recently, a meta-analysis of lithium exposure during pregnancy and the postpartum period supports this older data, which suggests this increased risk, and examines other outcomes concerning to women with bipolar disorder who use lithium, such as preterm labor, low birth weight, miscarriage, and other adverse neonatal outcomes.
In 2021, with the backdrop of the pandemic, what we actually see is that, for our pregnant and postpartum patients with bipolar disorder, the imperative to keep them well, keep them out of the hospital, and keep them safe has often required careful coadministration of drugs like lamotrigine, lithium, and atypical antipsychotics (and even benzodiazepines). Keeping this population well during the perinatal period is so critical. We were all trained to use the least number of medications when possible across psychiatric illnesses. But the years, data, and clinical experience have shown that polypharmacy may be required to sustain euthymia in many patients with bipolar disorder. The reflex historically has been to stop medications during pregnancy. We take pause, particularly during the pandemic, before reverting back to the practice of 25 years ago of abruptly stopping medicines such as lithium or atypical antipsychotics in patients with bipolar disorder because we know that the risk for relapse is very high following a shift from the regimen that got the patient well.
The COVID-19 pandemic in many respects has highlighted a need to clinically thread the needle with respect to developing a regimen that minimizes risk of reproductive safety concerns but maximizes the likelihood that we can sustain the emotional well-being of these women across pregnancy and into the postpartum period.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The revenge of the ‘late COVID adopters’
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has stressed all aspects of the world’s health care systems. The sheer volume of pandemic-related research produced over the past year has been challenging to process. This is as it should be, given its unprecedented spread and related morbidity and mortality. However, such rapid production and application leaves little time for proper vetting. Large numbers of providers adopted suggested, but largely unproven, practices that deviated from pre–COVID-19 guidelines. These “early adopters” theorized that COVID-19–related disease processes were different, necessitating a modification to existing practices.
Other equally prominent researchers countered this argument. Martin Tobin drew on physiology, while Arthur Slutsky and Niall Ferguson used emerging data to make their case. Tobin and colleagues cautioned against early intubation for anyone who could be maintained using noninvasive support. In August 2020 (well into the pandemic and after more data were available), Slutsky and colleagues argued that ARDS caused by COVID-19 wasn’t much different from lung injury due to other causes.
Two more recent studies published online recently are relevant to the debate over COVID-19 ARDS. One was a prospective study and the other a retrospective study; both had comparison groups, and both came to the same conclusions. Overall, COVID-19 ARDS isn’t much different from ARDS due to other causes. These studies were comprehensive in their comparisons and measures of outcomes, but they were both rather small and included patients from one and two hospitals, respectively. The discussions of both provide a nice review of the existing literature on COVID-19 ARDS.
A second controversial, but unproven, COVID-19 practice is aggressive anticoagulation. Early reports of a high prevalence of venous thromboembolism (VTE) in patients with COVID-19 pushed many to recommend empirically increasing prophylaxis. Most of the data guiding this approach were from retrospective, observational studies that suffered from selection bias. Early on, many of the studies were from China, where baseline VTE prophylaxis rates were low. Despite these limitations, many physicians acted on the basis of these data. An arbitrarily defined “intermediate” or treatment dose for prophylaxis was used, with some measuring D-dimer to guide their approach. An evidence-based argument against this practice, published in the New England Journal of Medicine, failed to sway readers. (Look at the poll at the end of the article and you’ll see how readers answered.)
Two articles recently published online in CHEST attempted to bring clarity to the debate over COVID-19 and VTE prophylaxis. The first study evaluated critically ill patients in France, and researchers found that higher doses of anticoagulation reduced thrombotic complications without an associated increase in bleeding events. The study is well done but certainly has its flaws. It is observational and retrospective, and it essentially uses a before-after comparison technique. Such an approach is particularly prone to bias during COVID-19, given that practice patterns change quickly.
The second paper is a systematic review looking at VTE and bleeding rates among patients hospitalized with COVID-19. The authors found high rates of VTE (17.0% overall), with screening, admission to the ICU, and the prospective study design all being associated with increased rates. Of importance, unlike the retrospective trial cited in the previous paragraph, the authors of the systematic review found treatment-dose anticoagulation was associated with higher bleeding rates.
I admit, the title of this piece is a bit of a misnomer. The “late adopters” would truly have their revenge if deviation from guidelines for COVID-19–related ARDS and VTE prophylaxis proves to be harmful. It’s not clear that’s the case, and at least for VTE prophylaxis, results from several randomized, controlled trials (REMAP-CAP, ATTACC, and ACTIV-4a) will be released soon. These are sure to provide more definitive answers. If nothing else, the COVID-19–related ARDS and VTE data reinforce how difficult it is to obtain high-quality data that yield clear results. Until something more definitive is published and released, I will remain a “late adopter.” Standard non–COVID-19 guidelines for ARDS and VTE prophylaxis are good enough for me.
Dr. Holley is program director of the Pulmonary and Critical Care Medical Fellowship at Walter Reed National Military Medical Center, Bethesda, Md.
A version of this article first appeared on Medscape.com.
A young girl presents with ‘itchy, rashy’ hands
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Examination findings of the bilateral hands and wrists demonstrate plaques of erythema, lichenification, and scale of the dorsal surfaces of the hands and digits. Closer inspection reveals fissuring and erythematous crust of the affected skin but normal nails. The rest of the skin exam is unremarkable.
How family medicine has changed over the past half century
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
Dialing back pandemic screen time
The light at the end of the pandemic tunnel seems even brighter than it did just a month ago and in its glow it’s tempting to look back on the adjustments we have made in our lives and consider how many of those adjustments will solidify into new standards. Certainly, near the top of the changes wrought by SARS-CoV-2 is an explosive use of the Internet as a vehicle for group interaction and communication. Did you even know what Zoom was a year ago?
From remote education to international business meetings our screen time has increased dramatically. In homes across the country families have relaxed any restrictions they might have had on video exposure as they struggled to amuse and entertain children who have been shut off from their playmates. As reported in the Washington Post, the monitoring company Bark found that children sent and received 144% more Internet messages in 2020 than they had the year before..
Even families that I know who have been incredibly creative in finding physical activities, both indoor and outdoor, for their children have scaled back their restrictions on screen time. While the term “survival mode” is a bit too strong to describe this phenomenon, it was simply a matter of finding solutions given a limited supply of options.
The increase in screen time has prompted many parents to worry about its effect on their children. The American Academy of Pediatrics has already expressed concern about the cumulative effects of screen exposure on visual acuity. And it seems reasonable to expect that the obesity epidemic will accelerate as more children become more sedentary watching video screens. Whether the dire predictions of educators about lost learning will come true remains to be seen.
We can hope that this relaxation of screen time limits will be temporary. But that hope has a slim chance of becoming a reality as we have realized how powerful the Internet can be as an imperfect but effective educational tool. We have seen that apps such as Zoom, GoToMeeting, and FaceTime can allow families to connect on holidays when to face-to-face meetings are impractical. How should parents, and those of us who advise them, begin to restructure sensible and enforceable guidelines for screen time given the sea change we have just experienced?
There will certainly be significant resistance on the part of children to unlearn screen habits developed during the darkest hours of the pandemic: Texting a friend whom you will now be able to see in school, playing a video game instead of biking around the neighborhood with on a sunny afternoon, or, binging on sitcoms in the evening with your parents when they knew you didn’t have to get up early to catch the school bus.
It could be a herculean task to nudge the screen time pendulum back toward the prepandemic “norm.” In the past we haven’t done a very good job of promoting a healthy screen time diet for children. When the only screen in town was television the American Academy of Pediatrics’ focus was more on content than quantity. Quality is often difficult to assess and parents, like most everyone, seem more comfortable with guidelines that include a time metric – even if they don’t seem to be very good at enforcing it.
Maybe screen time is too big a boulder to roll up the hill. The good news is that during the pandemic, activity – particularly outdoor activity – has increased dramatically. Bicycles went off the shelves like toilet paper. National and state parks have been overflowing with families. While we must not ignore the downside of excess screen time, we should put more effort into promoting the healthy alternative of outdoor recreation. Let’s not allow a positive trend slip into becoming a short-lived fad.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The light at the end of the pandemic tunnel seems even brighter than it did just a month ago and in its glow it’s tempting to look back on the adjustments we have made in our lives and consider how many of those adjustments will solidify into new standards. Certainly, near the top of the changes wrought by SARS-CoV-2 is an explosive use of the Internet as a vehicle for group interaction and communication. Did you even know what Zoom was a year ago?
From remote education to international business meetings our screen time has increased dramatically. In homes across the country families have relaxed any restrictions they might have had on video exposure as they struggled to amuse and entertain children who have been shut off from their playmates. As reported in the Washington Post, the monitoring company Bark found that children sent and received 144% more Internet messages in 2020 than they had the year before..
Even families that I know who have been incredibly creative in finding physical activities, both indoor and outdoor, for their children have scaled back their restrictions on screen time. While the term “survival mode” is a bit too strong to describe this phenomenon, it was simply a matter of finding solutions given a limited supply of options.
The increase in screen time has prompted many parents to worry about its effect on their children. The American Academy of Pediatrics has already expressed concern about the cumulative effects of screen exposure on visual acuity. And it seems reasonable to expect that the obesity epidemic will accelerate as more children become more sedentary watching video screens. Whether the dire predictions of educators about lost learning will come true remains to be seen.
We can hope that this relaxation of screen time limits will be temporary. But that hope has a slim chance of becoming a reality as we have realized how powerful the Internet can be as an imperfect but effective educational tool. We have seen that apps such as Zoom, GoToMeeting, and FaceTime can allow families to connect on holidays when to face-to-face meetings are impractical. How should parents, and those of us who advise them, begin to restructure sensible and enforceable guidelines for screen time given the sea change we have just experienced?
There will certainly be significant resistance on the part of children to unlearn screen habits developed during the darkest hours of the pandemic: Texting a friend whom you will now be able to see in school, playing a video game instead of biking around the neighborhood with on a sunny afternoon, or, binging on sitcoms in the evening with your parents when they knew you didn’t have to get up early to catch the school bus.
It could be a herculean task to nudge the screen time pendulum back toward the prepandemic “norm.” In the past we haven’t done a very good job of promoting a healthy screen time diet for children. When the only screen in town was television the American Academy of Pediatrics’ focus was more on content than quantity. Quality is often difficult to assess and parents, like most everyone, seem more comfortable with guidelines that include a time metric – even if they don’t seem to be very good at enforcing it.
Maybe screen time is too big a boulder to roll up the hill. The good news is that during the pandemic, activity – particularly outdoor activity – has increased dramatically. Bicycles went off the shelves like toilet paper. National and state parks have been overflowing with families. While we must not ignore the downside of excess screen time, we should put more effort into promoting the healthy alternative of outdoor recreation. Let’s not allow a positive trend slip into becoming a short-lived fad.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The light at the end of the pandemic tunnel seems even brighter than it did just a month ago and in its glow it’s tempting to look back on the adjustments we have made in our lives and consider how many of those adjustments will solidify into new standards. Certainly, near the top of the changes wrought by SARS-CoV-2 is an explosive use of the Internet as a vehicle for group interaction and communication. Did you even know what Zoom was a year ago?
From remote education to international business meetings our screen time has increased dramatically. In homes across the country families have relaxed any restrictions they might have had on video exposure as they struggled to amuse and entertain children who have been shut off from their playmates. As reported in the Washington Post, the monitoring company Bark found that children sent and received 144% more Internet messages in 2020 than they had the year before..
Even families that I know who have been incredibly creative in finding physical activities, both indoor and outdoor, for their children have scaled back their restrictions on screen time. While the term “survival mode” is a bit too strong to describe this phenomenon, it was simply a matter of finding solutions given a limited supply of options.
The increase in screen time has prompted many parents to worry about its effect on their children. The American Academy of Pediatrics has already expressed concern about the cumulative effects of screen exposure on visual acuity. And it seems reasonable to expect that the obesity epidemic will accelerate as more children become more sedentary watching video screens. Whether the dire predictions of educators about lost learning will come true remains to be seen.
We can hope that this relaxation of screen time limits will be temporary. But that hope has a slim chance of becoming a reality as we have realized how powerful the Internet can be as an imperfect but effective educational tool. We have seen that apps such as Zoom, GoToMeeting, and FaceTime can allow families to connect on holidays when to face-to-face meetings are impractical. How should parents, and those of us who advise them, begin to restructure sensible and enforceable guidelines for screen time given the sea change we have just experienced?
There will certainly be significant resistance on the part of children to unlearn screen habits developed during the darkest hours of the pandemic: Texting a friend whom you will now be able to see in school, playing a video game instead of biking around the neighborhood with on a sunny afternoon, or, binging on sitcoms in the evening with your parents when they knew you didn’t have to get up early to catch the school bus.
It could be a herculean task to nudge the screen time pendulum back toward the prepandemic “norm.” In the past we haven’t done a very good job of promoting a healthy screen time diet for children. When the only screen in town was television the American Academy of Pediatrics’ focus was more on content than quantity. Quality is often difficult to assess and parents, like most everyone, seem more comfortable with guidelines that include a time metric – even if they don’t seem to be very good at enforcing it.
Maybe screen time is too big a boulder to roll up the hill. The good news is that during the pandemic, activity – particularly outdoor activity – has increased dramatically. Bicycles went off the shelves like toilet paper. National and state parks have been overflowing with families. While we must not ignore the downside of excess screen time, we should put more effort into promoting the healthy alternative of outdoor recreation. Let’s not allow a positive trend slip into becoming a short-lived fad.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Addressing mental health for transgender patients during the pandemic
Obstetrician/gynecologists are often first-line providers in addressing mental health concerns for our patients. Routine screening for intimate partner violence, obtaining a history of sexual assault, and assessing patients for postpartum depression are among the many tools that we use to ascertain the psychological well-being of cisgender women. As transgender patients continue to seek care from ob.gyns., it is vital that we not only screen transgender patients for depression and intimate partner violence, but also assess factors relating to social support.
Mental health disorders disproportionately affect the transgender population. A large online survey showed that 41% of transgender patients had experienced suicidality, with rates among transgender youth even higher.1 While the rates of sexual violence are higher among LGBTQ patients compared with cisgender counterparts, the rate of sexual assault is as high as 47% in the transgender population.2,3 Additional surveys and studies have demonstrated that more than 70% of transgender individuals report discrimination in school (K-12), 27% have lost their jobs because of their gender identity; and 30% have experienced homelessness at some point.3 Tragically, these rates are further affected by race and ethnicity with American Indian (65%), multiracial (59%), Middle Eastern (58%), and Black (53%) respondents in the survey stating they were assaulted at some point.3
In a prepandemic world, mental health for transgender patients was influenced by several factors, such as stigmatization, health care disparities, limited access to health care, prolonged exposure to discrimination, lack of a supportive environment, and history of trauma or violence. During the pandemic, these factors have been magnified. Furthermore, many of the supportive services such as group meetings at LGBTQ centers, networking events/conferences, LGBTQ pride events, and social gatherings at bars or restaurants have been postponed, reduced to accommodate social distancing measures, or moved to virtual platforms.
While the pandemic has led to increased unemployment rates, concerns over housing and rent payments, and limiting one’s social circle in the general population, these rates are increased among LGBTQ adults. Data are limited on how significantly the pandemic has affected LGBTQ adults, but an analysis conducted by the Kaiser Family Foundation found that 56% of LGBTQ adults reported that they or someone they know lost a job, compared with 44% of non-LGBTQ adults.4 In addition, 75% of LGBTQ adults report that the pandemic has negatively affected their mental health, compared with 49% of the non-LGBTQ population.4 To my dismay, I’ve seen these statistics reflected in my own patient population.
Given this knowledge, it is even more crucial that obstetrician/gynecologists screen for depression, substance use, and intimate partner violence, in addition to assessing the patient’s social determinants for overall well-being. These often include determining a patient’s living situation, employment status, familial support, and social support. In my practice, if concerns are raised in any of these areas, we have a streamlined referral system connecting patients to a variety of therapists, psychologists, and/or social workers, with close follow-up on either a weekly or monthly basis depending on the particular issue the patient is facing. While many patients may be hesitant to go to in-office appointments or where transportation poses a barrier, telemedicine visits are useful adjuncts to assess patient’s overall well-being.
While the pandemic has been extraordinarily difficult for us all, it is important for us to be even stronger advocates for communities that have experienced further challenges as a result of this global tragedy.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier; 2020:8-11.
2. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
3. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
4. Dawson L et al. The impact of the COVID-19 pandemic on LGBT people. KFF COVID-19 Vaccine Monitor. Kaiser Family Foundation. March 11, 2021.
Obstetrician/gynecologists are often first-line providers in addressing mental health concerns for our patients. Routine screening for intimate partner violence, obtaining a history of sexual assault, and assessing patients for postpartum depression are among the many tools that we use to ascertain the psychological well-being of cisgender women. As transgender patients continue to seek care from ob.gyns., it is vital that we not only screen transgender patients for depression and intimate partner violence, but also assess factors relating to social support.
Mental health disorders disproportionately affect the transgender population. A large online survey showed that 41% of transgender patients had experienced suicidality, with rates among transgender youth even higher.1 While the rates of sexual violence are higher among LGBTQ patients compared with cisgender counterparts, the rate of sexual assault is as high as 47% in the transgender population.2,3 Additional surveys and studies have demonstrated that more than 70% of transgender individuals report discrimination in school (K-12), 27% have lost their jobs because of their gender identity; and 30% have experienced homelessness at some point.3 Tragically, these rates are further affected by race and ethnicity with American Indian (65%), multiracial (59%), Middle Eastern (58%), and Black (53%) respondents in the survey stating they were assaulted at some point.3
In a prepandemic world, mental health for transgender patients was influenced by several factors, such as stigmatization, health care disparities, limited access to health care, prolonged exposure to discrimination, lack of a supportive environment, and history of trauma or violence. During the pandemic, these factors have been magnified. Furthermore, many of the supportive services such as group meetings at LGBTQ centers, networking events/conferences, LGBTQ pride events, and social gatherings at bars or restaurants have been postponed, reduced to accommodate social distancing measures, or moved to virtual platforms.
While the pandemic has led to increased unemployment rates, concerns over housing and rent payments, and limiting one’s social circle in the general population, these rates are increased among LGBTQ adults. Data are limited on how significantly the pandemic has affected LGBTQ adults, but an analysis conducted by the Kaiser Family Foundation found that 56% of LGBTQ adults reported that they or someone they know lost a job, compared with 44% of non-LGBTQ adults.4 In addition, 75% of LGBTQ adults report that the pandemic has negatively affected their mental health, compared with 49% of the non-LGBTQ population.4 To my dismay, I’ve seen these statistics reflected in my own patient population.
Given this knowledge, it is even more crucial that obstetrician/gynecologists screen for depression, substance use, and intimate partner violence, in addition to assessing the patient’s social determinants for overall well-being. These often include determining a patient’s living situation, employment status, familial support, and social support. In my practice, if concerns are raised in any of these areas, we have a streamlined referral system connecting patients to a variety of therapists, psychologists, and/or social workers, with close follow-up on either a weekly or monthly basis depending on the particular issue the patient is facing. While many patients may be hesitant to go to in-office appointments or where transportation poses a barrier, telemedicine visits are useful adjuncts to assess patient’s overall well-being.
While the pandemic has been extraordinarily difficult for us all, it is important for us to be even stronger advocates for communities that have experienced further challenges as a result of this global tragedy.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier; 2020:8-11.
2. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
3. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
4. Dawson L et al. The impact of the COVID-19 pandemic on LGBT people. KFF COVID-19 Vaccine Monitor. Kaiser Family Foundation. March 11, 2021.
Obstetrician/gynecologists are often first-line providers in addressing mental health concerns for our patients. Routine screening for intimate partner violence, obtaining a history of sexual assault, and assessing patients for postpartum depression are among the many tools that we use to ascertain the psychological well-being of cisgender women. As transgender patients continue to seek care from ob.gyns., it is vital that we not only screen transgender patients for depression and intimate partner violence, but also assess factors relating to social support.
Mental health disorders disproportionately affect the transgender population. A large online survey showed that 41% of transgender patients had experienced suicidality, with rates among transgender youth even higher.1 While the rates of sexual violence are higher among LGBTQ patients compared with cisgender counterparts, the rate of sexual assault is as high as 47% in the transgender population.2,3 Additional surveys and studies have demonstrated that more than 70% of transgender individuals report discrimination in school (K-12), 27% have lost their jobs because of their gender identity; and 30% have experienced homelessness at some point.3 Tragically, these rates are further affected by race and ethnicity with American Indian (65%), multiracial (59%), Middle Eastern (58%), and Black (53%) respondents in the survey stating they were assaulted at some point.3
In a prepandemic world, mental health for transgender patients was influenced by several factors, such as stigmatization, health care disparities, limited access to health care, prolonged exposure to discrimination, lack of a supportive environment, and history of trauma or violence. During the pandemic, these factors have been magnified. Furthermore, many of the supportive services such as group meetings at LGBTQ centers, networking events/conferences, LGBTQ pride events, and social gatherings at bars or restaurants have been postponed, reduced to accommodate social distancing measures, or moved to virtual platforms.
While the pandemic has led to increased unemployment rates, concerns over housing and rent payments, and limiting one’s social circle in the general population, these rates are increased among LGBTQ adults. Data are limited on how significantly the pandemic has affected LGBTQ adults, but an analysis conducted by the Kaiser Family Foundation found that 56% of LGBTQ adults reported that they or someone they know lost a job, compared with 44% of non-LGBTQ adults.4 In addition, 75% of LGBTQ adults report that the pandemic has negatively affected their mental health, compared with 49% of the non-LGBTQ population.4 To my dismay, I’ve seen these statistics reflected in my own patient population.
Given this knowledge, it is even more crucial that obstetrician/gynecologists screen for depression, substance use, and intimate partner violence, in addition to assessing the patient’s social determinants for overall well-being. These often include determining a patient’s living situation, employment status, familial support, and social support. In my practice, if concerns are raised in any of these areas, we have a streamlined referral system connecting patients to a variety of therapists, psychologists, and/or social workers, with close follow-up on either a weekly or monthly basis depending on the particular issue the patient is facing. While many patients may be hesitant to go to in-office appointments or where transportation poses a barrier, telemedicine visits are useful adjuncts to assess patient’s overall well-being.
While the pandemic has been extraordinarily difficult for us all, it is important for us to be even stronger advocates for communities that have experienced further challenges as a result of this global tragedy.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Karasic D. Mental health care for the adult transgender patient. In: Ferrando CA, ed. Comprehensive Care of the Transgender Patient. Philadelphia, PA: Elsevier; 2020:8-11.
2. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
3. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
4. Dawson L et al. The impact of the COVID-19 pandemic on LGBT people. KFF COVID-19 Vaccine Monitor. Kaiser Family Foundation. March 11, 2021.