User login
HIV testing still suboptimal
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
from the Centers for Disease Control and Prevention. The reasons are complex and could jeopardize goals of ending the AIDS epidemic by 2030.
Patients and doctors alike face system challenges, including stigma, confidentiality concerns, racism, and inequitable access. Yet doctors, public health authorities, and even some patients agree that testing does work: In 2022, 81% of people diagnosed with HIV were linked to care within 30 days. Moreover, many patients are aware of where and how they wish to be tested. So, what would it take to achieve what ostensibly should be the lowest hanging fruit in the HIV care continuum?
“We didn’t look at the reasons for not testing,” Marc Pitasi, MPH, CDC epidemiologist and coauthor of the CDC study said in an interview. But “we found that the majority of people prefer the test in a clinical setting, so that’s a huge important piece of the puzzle,” he said.
The “never-tested” populations (4,334 of 6,072) in the study were predominantly aged 18-29 years (79.7%) and 50 years plus (78.1%). A total of 48% of never-tested adults also indicated that they had engaged in past-year risky behaviors (that is, injection drug use, treated for a sexually transmitted disease, exchanged sex/drugs for money, engaged in condomless anal sex, or had more than four sex partners). However, the difference between never-tested adults who live in EHE (Ending the HIV Epidemic in the U.S.)–designated jurisdictions (comprising 50 areas and 7 U.S. states responsible for more than 50% of new HIV infections) and those residing in non-EHE areas was only about 5 percentage points (69.1% vs. 74.5%, respectively), underscoring the need for broader engagement.
“There’s definitely a lack of testing across the board,” explained Lina Rosengren-Hovee, MD, MPH, MS, an infectious disease epidemiologist at the University of North Carolina at Chapel Hill. “There are all sorts of biases on how we make decisions and how we stratify … and these heuristics that we have in our minds to identify who is at risk and who needs testing,” she said.
“If we just look at the need for HIV testing based on who is at risk, I think that we are always going to fall short.”
Conflicting priorities
Seventeen years have passed since the CDC recommended that HIV testing and screening be offered at least once to all people aged 13-64 years in a routine clinical setting, with an opt-out option and without a separate written consent. People at higher risk (sexually active gay, bisexual, and other men who have sex with men) should be rescreened at least annually.
These recommendations were subsequently reinforced by numerous organizations, including the U.S. Preventive Services Task Force in 2013 and again in 2019, and the American Academy of Pediatrics in 2021.
But Dr. Rosengren-Hovee said that some clinicians remain unaware of the guidelines; for others, they’re usually not top-of-mind because of conflicting priorities.
This is especially true of pediatricians, who, despite data demonstrating that adolescents account for roughly 21% of new HIV diagnoses, rarely recognize or take advantage of HIV-testing opportunities during routine clinical visits.
“Pediatricians want to do the right thing for their patients but at the same time, they want to do the right thing on so many different fronts,” said Sarah Wood, MD, of the University of Pennsylvania, Philadelphia, and attending physician of adolescent medicine at Children’s Hospital of Philadelphia.
Dr. Wood is coauthor of a study published in Implementation Science Communicationsexamining pediatrician perspectives on implementing HIV testing and prevention. Participants identified confidentiality and time constraints as the most important challenges across every step of their workflow, which in turn, influenced perceptions about patients’ perceived risks for acquiring HIV – perceptions that Dr. Wood believes can be overcome.
“We need to really push pediatricians (through guideline-making societies like AAP and USPSTF) that screening should be universal and not linked to sexual activity or pinned to behavior, so the offer of testing is a universal opt-out,” she said. Additionally, “we need to make it easier for pediatricians to order the test,” for example, “through an office rapid test … and a redesigned workflow that moves the conversation away from physicians and nurse practitioners to medical assistants.”
Dr. Wood also pointed out that any effort would require pediatricians and other types of providers to overcome discomfort around sexual health conversations, noting that, while pediatricians are ideally positioned to work with parents to do education around sexual health, training and impetus are needed.
A fractured system
A fractured, often ill-funded U.S. health care system might also be at play according to Scott Harris, MD, MPH, state health officer of the Alabama Department of Public Health in Montgomery, and Association of State and Territorial Health Officials’ Infectious Disease Policy Committee chair.
“There’s a general consensus among everyone in public health that [HIV testing] is an important issue that we’re not addressing as well as we’d like to,” he said.
Dr. Harris acknowledged that, while COVID diverted attention away from HIV, some states have prioritized HIV more than others.
“We don’t have a national public health program; we have a nationwide public health program,” he said. “Everyone’s different and has different responsibilities and authorities ... depending on where their funding streams come from.”
The White House recently announced that it proposed a measure in its Fiscal Year 2023 budget to increase funding for HIV a further $313 million to accelerate efforts to end HIV by 2030, also adding a mandatory program to increase preexposure prophylaxis (PrEP) access. Without congressional approval, the measures are doomed to fail, leaving many states without the proper tools to enhance existing programs, and further painting overworked clinicians into a corner.
For patients, the ramifications are even greater.
“The majority of folks [in the CDC study] that were not tested said that if they were to get tested, they’d prefer to do that within the context of their primary care setting,” said Justin C. Smith, MS, MPH, director of the Campaign to End AIDS, Positive Impact Health Centers; a behavioral scientist at Emory University’s Rollins School of Public Health in Atlanta; and a member of the Presidential Advisory Council on HIV/AIDS.
“When you create a more responsive system that really speaks to the needs that people are expressing, that can provide better outcomes,” Dr. Smith said.
“It’s vital that we create health care and public health interventions that change the dynamics ... and make sure that we’re designing systems with the people that we’re trying to serve at the center.”
Mr. Pitasi, Dr. Rosengren-Hovee, Dr. Wood, Dr. Harris, and Dr. Smith have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Liquid albuterol shortage effects reduced by alternative drugs, similar shortages may be increasingly common
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
New hope for MDS, with AML treatments
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
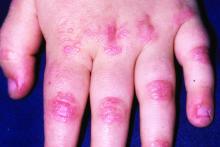
Novel therapy shows promise for treating skin-predominant dermatomyositis
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – in a double-blind, placebo-controlled phase 2 trial, according to results presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
“These findings support the inhibition of IFN-beta as a promising therapeutic strategy in skin-predominant disease,” said principal investigator Aaron Mangold, MD, associate professor of dermatology, Mayo Clinic, Scottsdale, Ariz.
Dermatomyositis, a rare autoimmune inflammatory condition that typically involves both skeletal muscles and skin, is a challenging disease with a diverse set of potential complications.
Immunosuppressive and immunomodulatory agents are used with mixed success for myositis, but skin manifestations, which include papular eruptions, heliotrope rash, photoerythema, burning, and pruritus, are often the most troublesome and the most difficult to control. Treatment options other than immunomodulators that target cutaneous involvement – which include steroids, emollients, and photoprotection – are generally modestly effective, according to Dr. Mangold.
Targeting an elevated cytokine
Interest in IFN-beta, which is elevated in the blood of individuals with dermatomyositis, was triggered by evidence that this cytokine plays an important role in driving the skin inflammation, Dr. Mangold explained.
“The blood concentrations of IFN-beta are positively correlated with cutaneous disease activity and severity,” he said.
The study drug, currently known as PF-06823859 (Dazukibart), “is a potent, selective humanized IgG1-neutralizing antibody directed at IFN-beta,” Dr. Mangold said. A dose-ranging phase 1 study published 2 years ago provided evidence of acceptable pharmacokinetics and safety in healthy individuals to support treatment studies for disorders associated with elevated IFN-beta levels. In addition to dermatomyositis, this includes systemic lupus erythematosus.
In this phase 2 trial, patients whose condition was not improved by at least one standard-care therapy for skin manifestations of dermatomyositis were eligible if they had moderate to severe disease as measured with the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), according to Dr. Mangold. During the study, patients were allowed to remain on a disease modifying antirheumatic drug and/or prednisone if they had been on stable doses and did not change the dose.
After a screening run-in, the trial had two blinded stages. In stage 1, 30 patients were randomly assigned either to 600 mg of PF-06823859 or to placebo, both administered intravenously every 4 weeks. A second cohort of 25 patients was randomly assigned in stage 2 to placebo, 150 mg of PF-06823859, or 600 mg of PF-06823859. The primary endpoint assessed at 12 weeks was a greater than 5-point reduction in CDASI score or greater than 40% CDASI improvement from baseline.
Both endpoints are associated with a clinically meaningful response in regard to an improved quality of life, Dr. Mangold noted.
Both doses better than placebo
In results from the stage 1 portion, the mean reduction in CDASI at 12 weeks after three doses of the assigned therapy was 18.8 points in the active-treatment group versus 3.9 points in the placebo group. In pooled data from stage 1 and 2, the reductions were 16.6 points, 19.2 points, and 2.9 points for the 150-mg, 600-mg, and placebo arms, respectively. Both doses achieved a highly significant advantage over placebo.
For both stages and doses, the response curves of the active-treatment groups and the placebo group diverged almost immediately. By 4 weeks, both measures of CDASI reductions on active therapy were significantly improved relative to placebo, and the response curves had a consistent downward slope through the end of the 12-week study, Dr. Mangold reported.
The majority of patients responded by either of the primary endpoint criteria. For a CDASI reduction of greater than 5 points, the response rates were 100% and 96% for the 150-mg and 600-mg doses of PF-06823859, respectively. The placebo response was 35.7%. For the CDASI reduction of greater than 40%, the rates were 80%, 82.1%, and 7.1% for the 150-mg, 600-mg, and placebo arms, respectively.
“There were no major safety concerns. Most of the treatment-emergent adverse events were mild, and adverse events did not have a relationship to dose,” Dr. Mangold said. Notably, there were no cases of herpes zoster, and infections of any kind were low in all study groups.
A phase 3 study is being planned with the 600-mg dose, according to Dr. Mangold, but he acknowledged that regulatory authorities have generally required endpoints for both cutaneous and muscle manifestations in previous trials of therapies for dermatomyositis.
It is not yet certain that “there will be a carve-out for skin,” he said in answer to a question about investigations moving forward. So far, studies have been focused on skin response. However, a meaningful degree of benefit against muscle involvement, which has not yet been well studied, has not been ruled out.
Even though this is a phase 2 trial with small numbers, it was controlled and blinded, and the potential of an inhibitor of IFN-beta to control the skin manifestations of dermatomyositis “is kind of a big deal,” said Paul Nghiem, MD, PhD, professor of dermatology, University of Washington, Seattle.
“There is definitely an unmet need for better therapies to control the skin involvement,” Dr. Nghiem said.
Hensin Tsao, MD, PhD, clinical director of the Melanoma and Pigmented Lesion Center at Massachusetts General Hospital, Boston, agreed. Like Dr. Nghiem, Dr. Tsao was a panelist during the late-breaker session where the study was presented, and he was impressed by the data.
“This is something that is definitely newsworthy,” Dr. Tsao said.
Dr. Mangold reports financial relationships with Actelion, Amgen, Corbus, Eli Lilly, Incyte, miRagen, Novartis, Regeneron, Solagenix, Sun Pharmaceuticals, Teva, and Pfizer, which provided funding for this trial. Both Dr. Nghiem and Dr. Tsao reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2023
‘Harm avoidance’ temperament predicts depression in adults
Temperament has been defined as “an individual’s propensity to react emotionally, learn behavior and to form attachments without conscious effort by associative conditioning,” wrote Aleksi Ahola, PhD, of the University of Oulu, Finland, and colleagues. “Temperament is a potential endophenotype for depression, as it is inheritable and genetically linked with depression,” they said. The Temperament and Character Inventory (TCI) includes four temperament traits: harm avoidance (HA), novelty seeking (NS), reward dependence (RD), and persistence (P); previous studies have shown associations between higher HA and depression, but long-term data are limited, they wrote.
In a population-based study published in Comprehensive Psychiatry, the researchers followed 3,999 adults from age 31 to 54 years. The participants were part of the Northern Finland Birth Cohort 1966 Study.
The primary outcome was the onset of depression in a previously mentally healthy adult population. Temperament was assessed using the TCI, and depression was based on the Hopkins system checklist-25 (SCL-25). Individuals with previous psychiatric disorders related to depression, bipolar disorder, or psychosis were excluded. Effect size was measured using the Cohen’s d test.
Overall, 240 individuals were diagnosed with depression over the follow-up period. Women later diagnosed with depression had higher baseline TCI scores for HA, compared with those without depression. After controlling for multiple variables, higher TCI scores for HA, NS, and P were significantly associated with increased risk of any depression.
Among men, the TCI HA score was associated with significantly increased risk of any depression after adjustments, but no association appeared for other TCI scores. However, higher RD was associated with a reduced risk of psychotic depression in men (odds ratio, 0.79), although the study was not designed to assess psychotic depression, the researchers noted.
In an additional analysis of temperament cluster groups, shy and pessimistic traits were associated with depression in men (OR, 1.89), but not in women. In women, the cluster group with no specific extreme personality traits (cluster III) appeared to show an association with depression, which may be related to the association of NS and P with depression, the researchers wrote in their discussion.
The study is the first known to show differences between genders in the prediction of depression based on temperament traits, notably the link between high persistence and the onset of any depression in women, they said.
The study findings were limited by several factors including the potential for missed cases of less severe depression not reported in a national register, and by the relatively small number of men in the study, the researchers noted. In addition, the TCI’s three character traits of self-directedness, cooperativeness, and self-transcendence were not part of the current study, they said.
However, the results were strengthened by the large sample size, premorbid temperament assessment, and long follow-up period, although more research is needed in larger populations using real-world personalities to confirm the findings, they said.
“Research regarding temperament is important as it may have clinical significance as predictor of psychiatric morbidity and even suicide risk,” they said.
“Understanding those potentially at risk of depression could help in preventing the onset of the disease, and creating cluster profiles to match real-world personas could offer a clinical tool for this kind of prevention,” they concluded.
The study was supported by the University of Oulu, Oulu University Hospital, the Ministry of Health and Social Affairs, the National Institute for Health and Welfare, and the Regional Institute of Occupational Health. The researchers had no financial conflicts to disclose.
Temperament has been defined as “an individual’s propensity to react emotionally, learn behavior and to form attachments without conscious effort by associative conditioning,” wrote Aleksi Ahola, PhD, of the University of Oulu, Finland, and colleagues. “Temperament is a potential endophenotype for depression, as it is inheritable and genetically linked with depression,” they said. The Temperament and Character Inventory (TCI) includes four temperament traits: harm avoidance (HA), novelty seeking (NS), reward dependence (RD), and persistence (P); previous studies have shown associations between higher HA and depression, but long-term data are limited, they wrote.
In a population-based study published in Comprehensive Psychiatry, the researchers followed 3,999 adults from age 31 to 54 years. The participants were part of the Northern Finland Birth Cohort 1966 Study.
The primary outcome was the onset of depression in a previously mentally healthy adult population. Temperament was assessed using the TCI, and depression was based on the Hopkins system checklist-25 (SCL-25). Individuals with previous psychiatric disorders related to depression, bipolar disorder, or psychosis were excluded. Effect size was measured using the Cohen’s d test.
Overall, 240 individuals were diagnosed with depression over the follow-up period. Women later diagnosed with depression had higher baseline TCI scores for HA, compared with those without depression. After controlling for multiple variables, higher TCI scores for HA, NS, and P were significantly associated with increased risk of any depression.
Among men, the TCI HA score was associated with significantly increased risk of any depression after adjustments, but no association appeared for other TCI scores. However, higher RD was associated with a reduced risk of psychotic depression in men (odds ratio, 0.79), although the study was not designed to assess psychotic depression, the researchers noted.
In an additional analysis of temperament cluster groups, shy and pessimistic traits were associated with depression in men (OR, 1.89), but not in women. In women, the cluster group with no specific extreme personality traits (cluster III) appeared to show an association with depression, which may be related to the association of NS and P with depression, the researchers wrote in their discussion.
The study is the first known to show differences between genders in the prediction of depression based on temperament traits, notably the link between high persistence and the onset of any depression in women, they said.
The study findings were limited by several factors including the potential for missed cases of less severe depression not reported in a national register, and by the relatively small number of men in the study, the researchers noted. In addition, the TCI’s three character traits of self-directedness, cooperativeness, and self-transcendence were not part of the current study, they said.
However, the results were strengthened by the large sample size, premorbid temperament assessment, and long follow-up period, although more research is needed in larger populations using real-world personalities to confirm the findings, they said.
“Research regarding temperament is important as it may have clinical significance as predictor of psychiatric morbidity and even suicide risk,” they said.
“Understanding those potentially at risk of depression could help in preventing the onset of the disease, and creating cluster profiles to match real-world personas could offer a clinical tool for this kind of prevention,” they concluded.
The study was supported by the University of Oulu, Oulu University Hospital, the Ministry of Health and Social Affairs, the National Institute for Health and Welfare, and the Regional Institute of Occupational Health. The researchers had no financial conflicts to disclose.
Temperament has been defined as “an individual’s propensity to react emotionally, learn behavior and to form attachments without conscious effort by associative conditioning,” wrote Aleksi Ahola, PhD, of the University of Oulu, Finland, and colleagues. “Temperament is a potential endophenotype for depression, as it is inheritable and genetically linked with depression,” they said. The Temperament and Character Inventory (TCI) includes four temperament traits: harm avoidance (HA), novelty seeking (NS), reward dependence (RD), and persistence (P); previous studies have shown associations between higher HA and depression, but long-term data are limited, they wrote.
In a population-based study published in Comprehensive Psychiatry, the researchers followed 3,999 adults from age 31 to 54 years. The participants were part of the Northern Finland Birth Cohort 1966 Study.
The primary outcome was the onset of depression in a previously mentally healthy adult population. Temperament was assessed using the TCI, and depression was based on the Hopkins system checklist-25 (SCL-25). Individuals with previous psychiatric disorders related to depression, bipolar disorder, or psychosis were excluded. Effect size was measured using the Cohen’s d test.
Overall, 240 individuals were diagnosed with depression over the follow-up period. Women later diagnosed with depression had higher baseline TCI scores for HA, compared with those without depression. After controlling for multiple variables, higher TCI scores for HA, NS, and P were significantly associated with increased risk of any depression.
Among men, the TCI HA score was associated with significantly increased risk of any depression after adjustments, but no association appeared for other TCI scores. However, higher RD was associated with a reduced risk of psychotic depression in men (odds ratio, 0.79), although the study was not designed to assess psychotic depression, the researchers noted.
In an additional analysis of temperament cluster groups, shy and pessimistic traits were associated with depression in men (OR, 1.89), but not in women. In women, the cluster group with no specific extreme personality traits (cluster III) appeared to show an association with depression, which may be related to the association of NS and P with depression, the researchers wrote in their discussion.
The study is the first known to show differences between genders in the prediction of depression based on temperament traits, notably the link between high persistence and the onset of any depression in women, they said.
The study findings were limited by several factors including the potential for missed cases of less severe depression not reported in a national register, and by the relatively small number of men in the study, the researchers noted. In addition, the TCI’s three character traits of self-directedness, cooperativeness, and self-transcendence were not part of the current study, they said.
However, the results were strengthened by the large sample size, premorbid temperament assessment, and long follow-up period, although more research is needed in larger populations using real-world personalities to confirm the findings, they said.
“Research regarding temperament is important as it may have clinical significance as predictor of psychiatric morbidity and even suicide risk,” they said.
“Understanding those potentially at risk of depression could help in preventing the onset of the disease, and creating cluster profiles to match real-world personas could offer a clinical tool for this kind of prevention,” they concluded.
The study was supported by the University of Oulu, Oulu University Hospital, the Ministry of Health and Social Affairs, the National Institute for Health and Welfare, and the Regional Institute of Occupational Health. The researchers had no financial conflicts to disclose.
FROM COMPREHENSIVE PSYCHIATRY
Logistical hassles hinder lifesaving lung cancer screenings
Screening high-risk populations for lung cancer saves lives. The National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual screening over 3 years with low-dose CT as compared with x-rays. The NELSON trial found a higher benefit: Men at high risk for lung cancer had a 26% reduced risk of dying from lung cancer and women had a 61% reduced risk over 10 years. However,
There are many reasons, but I submit that at least one hurdle is related to the difficulties associated with ordering the low-dose CT in the electronic medical record (EMR) and following the results. The rules and regulations around lung cancer screening are complex. First, the ordering provider must be able to determine if the patient is eligible for screening and has insurance coverage – a complicated procedure, which is constantly in flux, and is based on age, smoking history, smoke-free interval, and type of insurance coverage. Most EMRs do not have a way of flagging high-risk individuals, and clinic coordinators (for those practices that have one) are often put in charge of determining eligibility.
Secondly, the health care provider must order the scan. Unlike mammography, people must have a prescreening visit with a physician or other health care provider – a visit which is poorly compensated, and often must be supported by the institution. Many EMRs also do not have a smooth mechanism to make sure all the “boxes have been checked” before the scan can be ordered. Is there a complete smoking history? Has the patient had their prescreening visit? Has the patient been counseled regarding tobacco use? Has eligibility for insurance payment been confirmed?
Coordinating follow-up is cumbersome. Abnormal findings are common and usually nonmalignant, but must be followed up, and the follow-up recommendations are complicated and are based upon the appearance of the finding. This may be difficult for a general practitioner, so referrals to pulmonologists are often scheduled. Best practices state the patient be followed in a multidisciplinary pulmonary module clinic, but again, most multidisciplinary pulmonary module clinics are found in the academic setting.
All this involves a lot of back-and-forth for the patient: First to see their primary care physician, then to see a pulmonologist or other health care provider for the counseling regarding risks and benefits of screening and the importance of smoking cessation, and then a visit to a radiologist as well as a visit to a smoking cessation clinic, then a return follow-up visit. Academic medical centers and NCI-approved cancer centers often have these procedures worked out, but many private or smaller practices do not. Yes, the local IT folks can modify an EMR, but in a small practice, there are other “more important” problems that take precedence.
Would coupling lung cancer screening with breast cancer scanning help? One study followed 874 women who attended mammographic screening and found that over 11% were at high risk for lung cancer. This would appear to be an ideal “teaching moment” to educate the importance of lung cancer screening to women. It could also cut down on some of the logistical issues associated with lung cancer screening, particularly if a health care provider and coordinator were immediately available for counseling and eligibility determination at the time of the mammography visit. The radiology clinic staff could schedule a scan and return visit while the patient was still in the mammography suite.
Of course, the logistical hassle is just one of many associated with lung cancer screening. The internal stigma the patient may experience about their smoking history, the unconscious bias on the part of many health professionals, the many other screening and prevention regulations providers are now required to follow, the institution’s reluctance to support a screening program, the rushed pace in many clinics: These all certainly contribute to the problem. However, we have overcome all of these issues when it comes to mammography, such as work flow, stigma, logistical issues, seamless incorporation of ordering scans and referrals to specialists in the EMR, etc. We can and must do the same for our patients at high risk for lung cancer, and routine scheduling of mammograms and low-dose CTs may help.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
Screening high-risk populations for lung cancer saves lives. The National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual screening over 3 years with low-dose CT as compared with x-rays. The NELSON trial found a higher benefit: Men at high risk for lung cancer had a 26% reduced risk of dying from lung cancer and women had a 61% reduced risk over 10 years. However,
There are many reasons, but I submit that at least one hurdle is related to the difficulties associated with ordering the low-dose CT in the electronic medical record (EMR) and following the results. The rules and regulations around lung cancer screening are complex. First, the ordering provider must be able to determine if the patient is eligible for screening and has insurance coverage – a complicated procedure, which is constantly in flux, and is based on age, smoking history, smoke-free interval, and type of insurance coverage. Most EMRs do not have a way of flagging high-risk individuals, and clinic coordinators (for those practices that have one) are often put in charge of determining eligibility.
Secondly, the health care provider must order the scan. Unlike mammography, people must have a prescreening visit with a physician or other health care provider – a visit which is poorly compensated, and often must be supported by the institution. Many EMRs also do not have a smooth mechanism to make sure all the “boxes have been checked” before the scan can be ordered. Is there a complete smoking history? Has the patient had their prescreening visit? Has the patient been counseled regarding tobacco use? Has eligibility for insurance payment been confirmed?
Coordinating follow-up is cumbersome. Abnormal findings are common and usually nonmalignant, but must be followed up, and the follow-up recommendations are complicated and are based upon the appearance of the finding. This may be difficult for a general practitioner, so referrals to pulmonologists are often scheduled. Best practices state the patient be followed in a multidisciplinary pulmonary module clinic, but again, most multidisciplinary pulmonary module clinics are found in the academic setting.
All this involves a lot of back-and-forth for the patient: First to see their primary care physician, then to see a pulmonologist or other health care provider for the counseling regarding risks and benefits of screening and the importance of smoking cessation, and then a visit to a radiologist as well as a visit to a smoking cessation clinic, then a return follow-up visit. Academic medical centers and NCI-approved cancer centers often have these procedures worked out, but many private or smaller practices do not. Yes, the local IT folks can modify an EMR, but in a small practice, there are other “more important” problems that take precedence.
Would coupling lung cancer screening with breast cancer scanning help? One study followed 874 women who attended mammographic screening and found that over 11% were at high risk for lung cancer. This would appear to be an ideal “teaching moment” to educate the importance of lung cancer screening to women. It could also cut down on some of the logistical issues associated with lung cancer screening, particularly if a health care provider and coordinator were immediately available for counseling and eligibility determination at the time of the mammography visit. The radiology clinic staff could schedule a scan and return visit while the patient was still in the mammography suite.
Of course, the logistical hassle is just one of many associated with lung cancer screening. The internal stigma the patient may experience about their smoking history, the unconscious bias on the part of many health professionals, the many other screening and prevention regulations providers are now required to follow, the institution’s reluctance to support a screening program, the rushed pace in many clinics: These all certainly contribute to the problem. However, we have overcome all of these issues when it comes to mammography, such as work flow, stigma, logistical issues, seamless incorporation of ordering scans and referrals to specialists in the EMR, etc. We can and must do the same for our patients at high risk for lung cancer, and routine scheduling of mammograms and low-dose CTs may help.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
Screening high-risk populations for lung cancer saves lives. The National Lung Screening Trial (NLST) demonstrated a 20% relative reduction in lung cancer mortality with annual screening over 3 years with low-dose CT as compared with x-rays. The NELSON trial found a higher benefit: Men at high risk for lung cancer had a 26% reduced risk of dying from lung cancer and women had a 61% reduced risk over 10 years. However,
There are many reasons, but I submit that at least one hurdle is related to the difficulties associated with ordering the low-dose CT in the electronic medical record (EMR) and following the results. The rules and regulations around lung cancer screening are complex. First, the ordering provider must be able to determine if the patient is eligible for screening and has insurance coverage – a complicated procedure, which is constantly in flux, and is based on age, smoking history, smoke-free interval, and type of insurance coverage. Most EMRs do not have a way of flagging high-risk individuals, and clinic coordinators (for those practices that have one) are often put in charge of determining eligibility.
Secondly, the health care provider must order the scan. Unlike mammography, people must have a prescreening visit with a physician or other health care provider – a visit which is poorly compensated, and often must be supported by the institution. Many EMRs also do not have a smooth mechanism to make sure all the “boxes have been checked” before the scan can be ordered. Is there a complete smoking history? Has the patient had their prescreening visit? Has the patient been counseled regarding tobacco use? Has eligibility for insurance payment been confirmed?
Coordinating follow-up is cumbersome. Abnormal findings are common and usually nonmalignant, but must be followed up, and the follow-up recommendations are complicated and are based upon the appearance of the finding. This may be difficult for a general practitioner, so referrals to pulmonologists are often scheduled. Best practices state the patient be followed in a multidisciplinary pulmonary module clinic, but again, most multidisciplinary pulmonary module clinics are found in the academic setting.
All this involves a lot of back-and-forth for the patient: First to see their primary care physician, then to see a pulmonologist or other health care provider for the counseling regarding risks and benefits of screening and the importance of smoking cessation, and then a visit to a radiologist as well as a visit to a smoking cessation clinic, then a return follow-up visit. Academic medical centers and NCI-approved cancer centers often have these procedures worked out, but many private or smaller practices do not. Yes, the local IT folks can modify an EMR, but in a small practice, there are other “more important” problems that take precedence.
Would coupling lung cancer screening with breast cancer scanning help? One study followed 874 women who attended mammographic screening and found that over 11% were at high risk for lung cancer. This would appear to be an ideal “teaching moment” to educate the importance of lung cancer screening to women. It could also cut down on some of the logistical issues associated with lung cancer screening, particularly if a health care provider and coordinator were immediately available for counseling and eligibility determination at the time of the mammography visit. The radiology clinic staff could schedule a scan and return visit while the patient was still in the mammography suite.
Of course, the logistical hassle is just one of many associated with lung cancer screening. The internal stigma the patient may experience about their smoking history, the unconscious bias on the part of many health professionals, the many other screening and prevention regulations providers are now required to follow, the institution’s reluctance to support a screening program, the rushed pace in many clinics: These all certainly contribute to the problem. However, we have overcome all of these issues when it comes to mammography, such as work flow, stigma, logistical issues, seamless incorporation of ordering scans and referrals to specialists in the EMR, etc. We can and must do the same for our patients at high risk for lung cancer, and routine scheduling of mammograms and low-dose CTs may help.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation. Ivy Elkins, cofounder of EGFR Resisters, a patient, survivor, and caregiver advocacy group, contributed to this article.
Brain imaging markers of breathlessness-expectation predict COPD rehabilitation success
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
In an experimental medicine study of D-cycloserine given during chronic obstructive pulmonary disease (COPD) rehabilitation, only models including brain imaging markers of breathlessness-expectation successfully predicted Dyspnea-12 score improvement. D-cycloserine was independently associated with breathlessness improvement, according to original research published in Thorax.
Chronic breathlessness persisting despite maximal medical therapy is a key feature of COPD. While pulmonary rehabilitation is the best treatment for chronic breathlessness in COPD, responses to treatment are variable, with 30% deriving no clinical benefit, Sarah L. Finnegan, PhD, with the Nuffield Department of Clinical Neurosciences, University of Oxford (England), and colleagues wrote.
While recent research has shown fear and anxiety to be key components of the expectation that plays an important role in the mechanisms and maintenance of breathlessness, expectation-related effects have not previously been considered in prediction studies of pulmonary rehabilitation outcomes. The authors’ prior research showed a clear correlation between improvements in breathlessness through pulmonary rehabilitation and expectation-related brain activity in areas that include the anterior insula, anterior cingulate cortex, and prefrontal cortex. That research methodology, however, did not attempt to predict individual responses.
The current study focused on brain activity changes within preselected regions associated with breathlessness-expectation and body and symptom perception. Its purpose was to predict improvements in breathlessness during pulmonary rehabilitation by analyzing baseline data from a longitudinal experimental medicine study of D-cycloserine on breathlessness during pulmonary rehabilitation. D-cycloserine, a partial agonist of brain N-methyl-D-aspartate receptors, was chosen because of its effects on neural plasticity and influence on brain expectation mechanisms associated with cognitive behavioral therapies. The authors hypothesized that baseline brain activity in response to breathlessness-related expectation would predict improvement in breathlessness through pulmonary rehabilitation, with D-cycloserine emerging as a significant factor in the prediction model.
The researchers recruited 71 participants (18 women, median age 71 years [46-85 years]) with mild to moderate COPD immediately prior to enrollment in a National Health Service–prescribed course of pulmonary rehabilitation. They were randomized double-blind to receive either 250 mg oral D-cycloserine or a matched placebo. Participants received a single dose on four occasions 30 minutes prior to the onset of the first four pulmonary rehabilitation sessions.
Baseline variables, including brain-activity, self-report questionnaires responses, clinical measures of respiratory function, and drug allocation were used to train three machine-learning models to predict the outcome, a minimally clinically relevant change in the Dyspnea-12 score.
Improvements in Dyspnea-12 score occurred only in the two models including brain imaging markers of breathlessness-expectation (sensitivity 0.88, specificity 0.77). The model that combined brain and behavior metrics produced the best classification performance (accuracy, 0.83 [95% confidence interval, 0.75-0.90]; sensitivity, 0.88; specificity, 0.77; P < 0.001). While the brain-only model was able to correctly categorize participants with statistically significant likelihood (accuracy, 0.70 [95% CI, 0.58-0.81]), it demonstrated poor goodness of fit, a measure of how well sample data fit a distribution from a population with a normal distribution. “By enriching the brain-only models with questionnaires and physiology measures improved performance considerably,” the researchers stated.
“Our findings demonstrate the first predictive model of change in breathlessness across pulmonary rehabilitation and, for the first time, the clinical relevance of expectation-related brain activity as a therapeutic target in the treatment of breathlessness. ... This was achieved using sensitive brain imaging techniques in order to capture personalized responses to breathlessness-expectation which has, until recently remained relatively unexplored.”
“This study raises interesting questions about breathlessness-expectations,” commented assistant professor of medicine Mary Jo S. Farmer, MD, PhD, director pulmonary hypertension service, University of Massachusetts, Worcester, in an interview. “There is much more to be understood about expectations pathways as to how these pathways are built upon prior experience and pave the way for reaction to future experiences. There is need for a similar study with larger sample size and clarification of the role of the effect of the agent D-cycloserine on breathlessness-expectation.”
The researchers noted their study’s limitations, pointing out that the small sample size precluded holding out a proportion of the original data to create an external validation dataset.
Dr. Finnegan and Dr. Farmer declared no disclosures relevant to this study. This work was supported by the JABBS Foundation and Dunhill Medical Trust. This research was funded in whole, or in part, by the Wellcome Trust.
FROM THORAX
California picks generic drug company Civica to produce low-cost insulin
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Mediterranean diet linked to 24% reduction in CVD risk in women
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Mediterranean diet appears to be associated with a lower incidence of cardiovascular disease (CVD) and mortality in women, new observational data suggest.
Those who had a higher adherence to a Mediterranean diet had a 24% lower risk for cardiovascular disease and 23% lower risk for death.
“A healthy diet is a huge factor in preventing heart disease. However, current guidelines on preventing heart disease lack sex-specific recommendations,” said senior author Sarah Zaman, MBBS, PhD, an associate professor of medicine and principal research fellow at the University of Sydney’s Westmead Applied Research Centre.
“Historically, research trials and studies have had predominantly male participants or lacked sex-specific analysis,” she said. “Our results will pave the way to bridge this gap and also highlight the need for more research to ensure health guidelines and policies include diverse perspectives.”
The study was published online in the journal Heart.
Analyzing cardiovascular outcomes
Dr. Zaman and colleagues conducted a systematic review and meta-analysis of 16 studies published between 2006 and 2021 that reported a Mediterranean diet score and included either all women or had stratified outcomes by sex. They excluded studies that referred to only certain components of the Mediterranean diet or combined it with other lifestyle-related factors.
The studies, which were mainly conducted in the United States and Europe, included 722,495 adult women without previous clinical or subclinical CVD, with a median follow-up of 12.5 years.
Higher Mediterranean diet adherence was defined as the highest category reporting the highest range of Mediterranean diet scores, and lower adherence was defined as the lowest category reporting lowest scores. Incident CVD included coronary heart disease, myocardial infarction, stroke, heart failure, cardiovascular death, major adverse cardiovascular events, major adverse cardiac cerebrovascular events, and patient-reported CVD.
Overall, higher adherence to a Mediterranean diet was associated with lower CVD incidence (hazard ratio, 0.76; 95% confidence interval, 0.72-0.81), total mortality (HR, 0.77; 95% CI, 0.74-0.80), and coronary heart disease (HR, 0.75; 95% CI, 0.65-0.87).
Stroke incidence was also lower among women who adhered to the Mediterranean diet, although it wasn’t considered statistically significant (HR, 0.87; 95% CI, 0.76-1.01).
Additional analyses found similar reductions in risk across women of different ethnicities. Higher Mediterranean diet adherence was associated with lower CVD incidence for both women of European descent (HR, 0.76; 95% CI, 0.59-0.98) and women of non-European descent – Asian, Native Hawaiian, and African American – (HR, 0.79; 95% CI, 0.72-0.87).
The results didn’t materially change in sensitivity analyses, the authors note. Excluding one study at a time, the pooled HRs for the highest versus the lowest Mediterranean diet adherence ranged from 0.76 (95% CI, 0.72-0.80) to 0.83 (95% CI, 0.70-0.98) for incident CVD and from 0.77 (95% CI, 0.75-0.80) to 0.77 (95% CI, 0.74-0.81) for total mortality among women.
At the same time, the authors pointed to several limitations, including the observational nature of all of the studies, the reliance on self-reported food frequency questionnaires, and heterogeneity in the adjustments for influential factors across the studies.
Additional considerations
Dr. Zaman and colleagues called for more sex-specific research in cardiology, including risk factors related to premature menopause, preeclampsia, gestational diabetes, and autoimmune diseases such as systemic lupus.
Future studies should also explore the underlying mechanisms that may explain the links between the Mediterranean diet, cardiovascular disease, and death, the authors write. For instance, the diet may reduce inflammation and cardiovascular risk factors through antioxidant and beneficial gut microbiome pathways. Other components of the diet – such as polyphenols, nitrates, omega-3 fatty acids, higher fiber intake, and reduced glycemic load – may also play a role.
“It was striking to see how strong the long-term cardioprotective properties of a Mediterranean-type dietary pattern were,” said Samia Mora, MD, MHS, a professor of medicine at Harvard Medical School and director of the Center for Lipid Metabolomics at Brigham and Women’s Hospital.
Dr. Mora, who wasn’t involved with this study, has researched potential mechanisms related to the Mediterranean diet, cardiovascular events, and diabetes in women. She and colleagues have found that women with high adherence to the diet are more likely to have lower inflammation, insulin resistance, body mass index, and blood pressure, as well as improved lipid and metabolic profiles.
“This could represent an opportunity to intervene earlier and more intensively on improving inflammation, insulin resistance, and cardiometabolic health through evidence-based dietary approaches such as the Mediterranean diet,” she said. “As health care providers, we should promote the healthy dietary attributes of the Mediterranean diet, especially as many of our patients in the U.S. are less familiar with the Mediterranean diet and how to incorporate its components into daily food intake.”
The study did not receive any funding. Dr. Zaman was supported by a Heart Foundation Future Leader Fellowship. The authors declared no conflicts of interest. Dr. Mora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AI-assisted colonoscopy doesn’t always improve adenoma detection: Study
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized clinical trial using EndoVigilant, there wasn’t a significant difference in adenomas per colonoscopy (APC) in procedures with the CADe tool versus those without it. In addition, the adenoma detection rate (ADR) and serrated polyp detection rate were similar in the CADe and non-CADe groups.
“Although we were disappointed that AI [artificial intelligence] did not improve detection of adenomas or serrated polyps in our study, we are still optimistic that this exciting technology will eventually impact endoscopy in a very positive way,” senior author Shai Friedland, MD, a professor of medicine at Stanford (Calif.) University and gastroenterologist with the Veterans Affairs Palo Alto Health Care System, said in an interview.
“The ultimate goal should be to improve the ability of colonoscopy to prevent morbidity and mortality from colon cancer, especially for endoscopists who may not be performing as well as they could be,” he said. “AI can potentially help prevent missed lesions due to fatigue or distraction, much like a warning system that averts car accidents. It can also potentially help endoscopists recognize dangerous – but rare – subtle lesions such as small, flat, and depressed cancers.”
The study was published online in the American Journal of Gastroenterology.
Analyzing detection rates
Several studies have evaluated the use of different CADe devices to reduce adenoma miss rates during colonoscopy, and some have found that the technology contributed to significantly higher ADR and APC, the study authors write. However, most of these studies have been performed in academic settings.
Dr. Friedland and colleagues conducted a randomized controlled trial, called AI-SEE, to evaluate the use of CADe during colonoscopy in four community-based endoscopy centers located in California, Connecticut, Maryland, and New Jersey between September 2020 and September 2021. The trial included seven board-certified clinicians, who had ADR of 25%-37% before the study. The participants were randomly assigned to colonoscopies with or without CADe in blocks of 16 patients to ensure masking. Both groups had similar patient demographics.
The research team enrolled patients aged 45 years or older who presented for screening or low-risk surveillance colonoscopy, which was defined as a patient qualifying for a surveillance interval of 3 years or greater based on the U.S. Multi-Society Task Force 2020 Guidelines. Patients were excluded if they had a history of inflammatory bowel disease, known or suspected polyposis or hereditary colon cancer syndrome, history of colon resection, or a referral for a diagnostic colonoscopy.
Among 769 enrolled patients, 387 were randomly assigned to undergo colonoscopy with EndoVigilant, an AI-enabled CADe software for colonoscopy. It augments existing white-light colonoscopy in real time by highlighting colon polyps and displaying a graphic box around the lesion on the monitor. It can be deployed as a single- or dual-monitor device. Although the study was originally designed to use two monitors, three investigators expressed strong preference for the single-monitor mode, so the protocol allowed endoscopists to choose.
Primary outcomes included APC and adenoma per extraction (APE), which is the percentage of polyps removed that are adenomas. Secondary endpoints included procedural time, ADR, serrated polyp detection rate, serrated polyps per colonoscopy, and nonadenomatous, nonserrated polyps per colonoscopy.
Overall, the use of CADe didn’t show a significant difference in APC, at 0.73, compared with 0.67 for non-CADe.
Although the use of CADe didn’t lead to increased identification of serrated polyps per colonoscopy – both at 0.08 – CADe led to increased identification of nonadenomatous, nonserrated polyps per colonoscopy, at 0.90 versus 0.51.
There also wasn’t a significant difference in distribution regarding adenomatous polyp location, size, or morphology. However, there was a trend toward greater identification of 6-9 mm APC using CADe, at 0.13 versus 0.08.
Mean withdrawal time was longer in the CADe group, at 11.7 minutes versus 10.7 minutes. However, when no polyps were identified, the withdrawal times were similar, at 9.1 minutes versus 8.8 minutes.
In addition, there was no difference in ADR for screening colonoscopies between the non-CADe and CADe groups, at 34.6% versus 34.3%, or for surveillance procedures, at 43.9% versus 40%. CADe also didn’t improve serrated polyp detection rates for screening or surveillance.
CADe was also associated with decreased APE in all colonoscopies (44.8 vs. 56.8) as well as in screening colonoscopies (43 vs. 57.8).
A comparison of single-monitor CADe with dual-monitor CADe found no significant difference in the average number of adenomas or serrated polyps identified per colonoscopy. However, dual-monitor CADe identified significantly more non-adenomatous, nonserrated polyps per colonoscopy (1.18 vs. 0.42), more adenomas sized at least 10 mm (0.19 vs. 0.05), and more flat polyps (0.18 vs. 0).
The study was terminated early after the interim analysis point, marked by 769 valid subjects. At this point, the comparison of APC between the two groups resulted in a new sample size estimate required for final analysis of 6,557 per group. This revised large study size estimate made it impractical to continue, the study authors wrote. No adverse events were observed during the study.
“What our study shows is that current systems – and the one we used in this study performs very well when tested on a database of images or videos – don’t make a major impact on very crude outcome measures, such as the total number of adenomas detected by a group of endoscopists at typical private endoscopy centers,” Dr. Friedland said. “I’m not convinced that we have a good answer yet for where to go from here, but we need to keep working with our AI colleagues to figure out how to use this exciting technology to improve outcomes in colon cancer.”
Additional considerations
In a separate evaluation of EndoVigilant, the frame level sensitivity was 0.9 and the frame level specificity was 0.97. These calculations were conducted on a dataset not used in training or validation of this model, the authors noted.
In this study, it’s possible that experienced community-based endoscopists are proficient at detecting the adenomas highlighted by the CADe system, so the technology may not detect a significant number of additional adenomas, the authors wrote. It’s also possible that some endoscopists ignore lesions highlighted by CADe, including small lesions that might be difficult to identify as adenomas or are seen as clinically unimportant, which could reduce the potential benefit of CADe.
“It’s important to remember that these tools are meant to be endoscopist assistance devices, not endoscopist replacements. They provide added benefit by pointing out polyps while we do the best exam we can,” Aasma Shaukat, MD, a professor of medicine and gastroenterologist at NYU Langone Health, New York, said in an interview.
Dr. Shaukat, who wasn’t involved with this study, has researched CADe for screening and surveillance colonoscopies. She and colleagues found that CADe use improved APC without an increase in resection of nonneoplastic lesions.
“Different trials have reported different results, and at the end of the day, it’s an endoscopist assistance tool, like spellcheck in a document,” she said. “It’s nice if spellcheck points to an incorrect spelling, but you don’t have to use it. Similarly, we often don’t know in these studies what an endoscopist felt or believed about the tool when using it.”
The benefits of CADe could vary based on its software, setting, number of patients, patient characteristics, number of clinicians, provider experience and training, dual- versus single-monitor setup, and even time of day, she noted. Future studies could clarify these factors, as well as improve the technology.
“This is just the beginning of AI in this field, and while bounding boxes to indicate potential polyps is a good start, it’s not the be-all, end-all,” Dr. Shaukat said.
“We want AI software to be able to tell us more about the size of the polyp, histology, prep quality, landmarks in the colon, adequacy of resection, and more. There’s some work being geared toward developing the algorithms to do these additional aspects,” she added.
The study was sponsored by EndoVigilant. Some of the authors reported consultant roles with Neptune Medical, AgilTx, Intuitive Surgical, Capsovision, and EndoVigilant. Dr. Shaukat reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY