User login
Aerosolization of COVID-19 and Contamination Risks During Respiratory Treatments
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
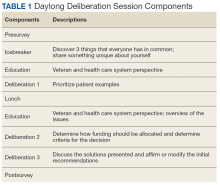
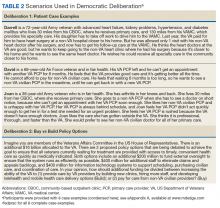
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
FDA provides flexibility to improve COVID-19 test availability
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
To Prevent Pernicious Political Activities: The Hatch Act and Government Ethics
The impeachment trial has concluded. By the time you read this editorial, Super Tuesday will be over. Then there will be the political party conventions, and finally the general election. Politics is everywhere and will be for the rest of 2020. As a preventive ethics measure, the legal arms of almost every federal agency will be sending cautionary e-mails to employees to remind us that any political activity undertaken must comply with the Hatch Act. Many of you who have worked in federal health care for some years may have heard a fellow employee say, “be careful you don’t violate the Hatch Act.”
Most readers probably had not heard of the statute before entering federal service. And you may have had an experience similar to mine in my early federal career when through osmosis I absorbed my peers fear and trembling when the Hatch Act was mentioned. This was the situation even though you were not at all sure you understood what the lawyers were warning you not to do. In my decades in federal service, I have heard that the Hatch Act dictates everything from you cannot vote to you can run for political office.
All this makes the timing right to review a piece of legislation that governs the political actions of every federal health and administrative professional. The Hatch Act sets apart federal employees from many, if not most, of our civilian counterparts, who, depending on your perspective, have more freedom to express their political views or are not held to such a high standard of ethical conduct.
In legalese, the Hatch Act is Political Activity Authorized; Prohibitions, 5 USC §7323 (1939). The title of this editorial, “To Prevent Pernicious Political Activities” is the formal title of the Hatch Act enacted at a time when government legislation was written in more ornamental rhetoric than the staid language of the current bureaucratic style. The alliterative title phrase of the act is an apt, if dated, encapsulation of the legislative intention of the act, which in modern parlance:
The law’s purpose is to ensure that federal programs are administered in a nonpartisan fashion, to protect federal employees from political coercion in the workplace, and to ensure that federal employees are advanced based on merit and not based on political affiliation. 2
For all its poetic turn of phrase, the title is historically accurate. The Hatch Act was passed in response to rampant partisan activity in public office. It was a key part of an effort to professionalize civil service, and as an essential aspect of that process, to protect federal employees from widespread political influence. The ethical principle behind the legislation is the one that still stands as the ideal for federal practitioners: to serve the people and act for the good of the public and republic.
The Hatch Act was intended to prevent unscrupulous politicians from intimidating federal employees and usurping the machinery of major government agencies to achieve their political ambitions. Imagine if your supervisor was running for office or supporting a particular candidate and ordered you to put a campaign sign in your yard, attend a political rally, and wear a campaign button on your lapel or you would be fired. All that and far worse happened in the good old USA before the Hatch Act.3
The Office of Special Counsel (OSC) is the authoritative guardian of the Hatch Act providing opinions on whether an activity is permitted under the act; investigating compliance with the provisions of the act; taking disciplinary action against the employee for serious violations; and prosecuting those violations before the Merit Systems Protection Board. Now I understand why the incantation “Hatch Act” casts a chill on our civil service souls. While there have been recent allegations against a high-profile political appointee, federal practitioners are not immune to prosecution.4 In 2017, Federal Times reported that the OSC sought disciplinary action against a VA physician for 15 violations of the Hatch Act after he ran for a state Senate seat in 2014.5
Fortunately, the OSC has produced a handy list of “Though Shalt Nots” and “You Cans” as a guide to the Hatch Act.6 Only the highpoints are mentioned here:
- Thou shalt not be a candidate for nomination or election to a partisan public office;
- Thou shalt not use a position of official public authority to influence or interfere with the result of an election;
- Thou shalt not solicit or host, accept, or receive a donation or contribution to a partisan political party, candidate, or group; and
- Thou shalt not engage in political activity on behalf of a partisan political party, candidate, or group while on duty, in a federal space, wearing a federal uniform, or driving a federal vehicle.
Covered under these daunting prohibitions is ordinary American politicking like hosting fundraisers or inviting your coworkers to a political rally, distributing campaign materials, and wearing a T-shirt with your favorite candidates smiling face at work. The new hotbed of concern for the Hatch Act is, you guessed it, social media—you cannot use your blog, Facebook, Instagram, or e-mail account to comment pro or con for a partisan candidate, party, office, or group.6
You may be asking at this point whether you can even watch the political debates? Yes, that is allowed under the Hatch Act along with running for nonpartisan election and participating in nonpartisan campaigns; voting, and registering others to vote; you can contribute money to political campaigns, parties, or partisan groups; attend political rallies, meetings and fundraisers; and even join a political party. Of course these activities must be on your own time and dime, not that of your federal employer. All of these “You Cans” enable a federal employee to engage in the bare minimum of democracy: voting in elections, but opponents argue they bar the civil servant from fully participating in the complex richness of the American political process.7
Nonetheless, since its inception the Hatch Act has been a matter of fierce debate among federal employees and other advocates of civil liberties. Those who feel it should be relaxed contend that the modern merit-based system of government service has rendered the provisions of the Hatch Act unnecessary, even obsolete. In addition, unlike in 1939, critics of the act claim there are now formidable whistleblower protections for employees who experience political coercion. Over the years there have been several efforts to weaken the conflict of interest safeguards that the act contains, leading many commentators to think that some of the amendments and reforms have blurred the tight boundaries between the professional and the political. Others such as the government unions and the American Civil Liberties Union (ACLU) believe that the tight line drawn between public and private binds the liberty of civil servants.8 Those who defend the Hatch Act believe that the wall it erects between professional and personal in the realm of political activities for federal employees must remain high and strong to protect the integrity of the administrative branch and the public trust.9
So, as political advertisements dominate television programming and the texts never stop asking for campaign donations, you can cast your own vote for or against the Hatch Act. As for me and my house, we will follow President Jefferson in preferring to be the property of the people rather than be indebted to the powerful. You need never encounter a true conflict of interest if you have no false conflict of obligation: history teaches us that serving 2 masters usually turns out badly for the slave. Many of you will completely disagree with my stance, holding that your constitutional rights as a citizen are being curtailed, if not outright denied, simply because you choose to serve your country. Our ability to freely hold and express our differences of opinions about the Hatch Act and so much else is what keeps democracy alive.
1. Rayner BL. Life of Thomas Jefferson With Selections From the Most Valuable Portions of his Voluminous and Unrivalled Private Correspondence. Boston, MA: Lilly, Wait, Colman, and Holden; 1834:356.
2. US Office of Special Counsel. Hatch Act overview. https://osc.gov/Services/Pages/HatchAct.aspx. Accessed February 24, 2020.
3. Brown AJ. Public employee participation: Hatch Acts in the federal and state governments. Public Integrity. 2000;2(2):105-120.
4. Phillips A. What is the Hatch Act, and why did Kellyanne Conway get accused of violating it so egregiously? Washington Post. June 13, 2019. https://www.washingtonpost.com/politics/2019/06/13/what-is-hatch-act-why-did-kellyanne-conway-get-accused-violating-it-so-egregiously. Accessed February 24, 2020.
5. Bur J. Special counsel: VA doctor violated Hatch Act while campaigning. https://www.federaltimes.com/federal-oversight/watchdogs/2017/11/22/special-counsel-va-doctor-violated-hatch-act-while-campaigning. Published November 22, 2017. Accessed February 24, 2020.
6. US Office of Special Counsel. A guide to the Hatch Act for the federal employee. https://osc.gov/Documents/Outreach%20and%20Training/Handouts/A%20Guide%20to%20the%20Hatch%20Act%20for%20Federal%20Employees.pdf. Published September 2014. Accessed February 24, 2020.
7. Brown C, Maskell J. Hatch Act restrictions on federal employee’s political activities in the digital age. https://fas.org/sgp/crs/misc/R44469.pdf. Published April 13, 2016. Accessed February 24, 2020.
8. Thurber KT Jr. Revising the Hatch Act: a practitioner’s perspective. Public Manag. 1993;22(1):43.
9. Pearson WM, Castle DS. Expanding the opportunity for partisan activity among government employees: potential effects of federal executive’s political involvement. Int J Public Adm. 2007;16(4):511-525.
The impeachment trial has concluded. By the time you read this editorial, Super Tuesday will be over. Then there will be the political party conventions, and finally the general election. Politics is everywhere and will be for the rest of 2020. As a preventive ethics measure, the legal arms of almost every federal agency will be sending cautionary e-mails to employees to remind us that any political activity undertaken must comply with the Hatch Act. Many of you who have worked in federal health care for some years may have heard a fellow employee say, “be careful you don’t violate the Hatch Act.”
Most readers probably had not heard of the statute before entering federal service. And you may have had an experience similar to mine in my early federal career when through osmosis I absorbed my peers fear and trembling when the Hatch Act was mentioned. This was the situation even though you were not at all sure you understood what the lawyers were warning you not to do. In my decades in federal service, I have heard that the Hatch Act dictates everything from you cannot vote to you can run for political office.
All this makes the timing right to review a piece of legislation that governs the political actions of every federal health and administrative professional. The Hatch Act sets apart federal employees from many, if not most, of our civilian counterparts, who, depending on your perspective, have more freedom to express their political views or are not held to such a high standard of ethical conduct.
In legalese, the Hatch Act is Political Activity Authorized; Prohibitions, 5 USC §7323 (1939). The title of this editorial, “To Prevent Pernicious Political Activities” is the formal title of the Hatch Act enacted at a time when government legislation was written in more ornamental rhetoric than the staid language of the current bureaucratic style. The alliterative title phrase of the act is an apt, if dated, encapsulation of the legislative intention of the act, which in modern parlance:
The law’s purpose is to ensure that federal programs are administered in a nonpartisan fashion, to protect federal employees from political coercion in the workplace, and to ensure that federal employees are advanced based on merit and not based on political affiliation. 2
For all its poetic turn of phrase, the title is historically accurate. The Hatch Act was passed in response to rampant partisan activity in public office. It was a key part of an effort to professionalize civil service, and as an essential aspect of that process, to protect federal employees from widespread political influence. The ethical principle behind the legislation is the one that still stands as the ideal for federal practitioners: to serve the people and act for the good of the public and republic.
The Hatch Act was intended to prevent unscrupulous politicians from intimidating federal employees and usurping the machinery of major government agencies to achieve their political ambitions. Imagine if your supervisor was running for office or supporting a particular candidate and ordered you to put a campaign sign in your yard, attend a political rally, and wear a campaign button on your lapel or you would be fired. All that and far worse happened in the good old USA before the Hatch Act.3
The Office of Special Counsel (OSC) is the authoritative guardian of the Hatch Act providing opinions on whether an activity is permitted under the act; investigating compliance with the provisions of the act; taking disciplinary action against the employee for serious violations; and prosecuting those violations before the Merit Systems Protection Board. Now I understand why the incantation “Hatch Act” casts a chill on our civil service souls. While there have been recent allegations against a high-profile political appointee, federal practitioners are not immune to prosecution.4 In 2017, Federal Times reported that the OSC sought disciplinary action against a VA physician for 15 violations of the Hatch Act after he ran for a state Senate seat in 2014.5
Fortunately, the OSC has produced a handy list of “Though Shalt Nots” and “You Cans” as a guide to the Hatch Act.6 Only the highpoints are mentioned here:
- Thou shalt not be a candidate for nomination or election to a partisan public office;
- Thou shalt not use a position of official public authority to influence or interfere with the result of an election;
- Thou shalt not solicit or host, accept, or receive a donation or contribution to a partisan political party, candidate, or group; and
- Thou shalt not engage in political activity on behalf of a partisan political party, candidate, or group while on duty, in a federal space, wearing a federal uniform, or driving a federal vehicle.
Covered under these daunting prohibitions is ordinary American politicking like hosting fundraisers or inviting your coworkers to a political rally, distributing campaign materials, and wearing a T-shirt with your favorite candidates smiling face at work. The new hotbed of concern for the Hatch Act is, you guessed it, social media—you cannot use your blog, Facebook, Instagram, or e-mail account to comment pro or con for a partisan candidate, party, office, or group.6
You may be asking at this point whether you can even watch the political debates? Yes, that is allowed under the Hatch Act along with running for nonpartisan election and participating in nonpartisan campaigns; voting, and registering others to vote; you can contribute money to political campaigns, parties, or partisan groups; attend political rallies, meetings and fundraisers; and even join a political party. Of course these activities must be on your own time and dime, not that of your federal employer. All of these “You Cans” enable a federal employee to engage in the bare minimum of democracy: voting in elections, but opponents argue they bar the civil servant from fully participating in the complex richness of the American political process.7
Nonetheless, since its inception the Hatch Act has been a matter of fierce debate among federal employees and other advocates of civil liberties. Those who feel it should be relaxed contend that the modern merit-based system of government service has rendered the provisions of the Hatch Act unnecessary, even obsolete. In addition, unlike in 1939, critics of the act claim there are now formidable whistleblower protections for employees who experience political coercion. Over the years there have been several efforts to weaken the conflict of interest safeguards that the act contains, leading many commentators to think that some of the amendments and reforms have blurred the tight boundaries between the professional and the political. Others such as the government unions and the American Civil Liberties Union (ACLU) believe that the tight line drawn between public and private binds the liberty of civil servants.8 Those who defend the Hatch Act believe that the wall it erects between professional and personal in the realm of political activities for federal employees must remain high and strong to protect the integrity of the administrative branch and the public trust.9
So, as political advertisements dominate television programming and the texts never stop asking for campaign donations, you can cast your own vote for or against the Hatch Act. As for me and my house, we will follow President Jefferson in preferring to be the property of the people rather than be indebted to the powerful. You need never encounter a true conflict of interest if you have no false conflict of obligation: history teaches us that serving 2 masters usually turns out badly for the slave. Many of you will completely disagree with my stance, holding that your constitutional rights as a citizen are being curtailed, if not outright denied, simply because you choose to serve your country. Our ability to freely hold and express our differences of opinions about the Hatch Act and so much else is what keeps democracy alive.
The impeachment trial has concluded. By the time you read this editorial, Super Tuesday will be over. Then there will be the political party conventions, and finally the general election. Politics is everywhere and will be for the rest of 2020. As a preventive ethics measure, the legal arms of almost every federal agency will be sending cautionary e-mails to employees to remind us that any political activity undertaken must comply with the Hatch Act. Many of you who have worked in federal health care for some years may have heard a fellow employee say, “be careful you don’t violate the Hatch Act.”
Most readers probably had not heard of the statute before entering federal service. And you may have had an experience similar to mine in my early federal career when through osmosis I absorbed my peers fear and trembling when the Hatch Act was mentioned. This was the situation even though you were not at all sure you understood what the lawyers were warning you not to do. In my decades in federal service, I have heard that the Hatch Act dictates everything from you cannot vote to you can run for political office.
All this makes the timing right to review a piece of legislation that governs the political actions of every federal health and administrative professional. The Hatch Act sets apart federal employees from many, if not most, of our civilian counterparts, who, depending on your perspective, have more freedom to express their political views or are not held to such a high standard of ethical conduct.
In legalese, the Hatch Act is Political Activity Authorized; Prohibitions, 5 USC §7323 (1939). The title of this editorial, “To Prevent Pernicious Political Activities” is the formal title of the Hatch Act enacted at a time when government legislation was written in more ornamental rhetoric than the staid language of the current bureaucratic style. The alliterative title phrase of the act is an apt, if dated, encapsulation of the legislative intention of the act, which in modern parlance:
The law’s purpose is to ensure that federal programs are administered in a nonpartisan fashion, to protect federal employees from political coercion in the workplace, and to ensure that federal employees are advanced based on merit and not based on political affiliation. 2
For all its poetic turn of phrase, the title is historically accurate. The Hatch Act was passed in response to rampant partisan activity in public office. It was a key part of an effort to professionalize civil service, and as an essential aspect of that process, to protect federal employees from widespread political influence. The ethical principle behind the legislation is the one that still stands as the ideal for federal practitioners: to serve the people and act for the good of the public and republic.
The Hatch Act was intended to prevent unscrupulous politicians from intimidating federal employees and usurping the machinery of major government agencies to achieve their political ambitions. Imagine if your supervisor was running for office or supporting a particular candidate and ordered you to put a campaign sign in your yard, attend a political rally, and wear a campaign button on your lapel or you would be fired. All that and far worse happened in the good old USA before the Hatch Act.3
The Office of Special Counsel (OSC) is the authoritative guardian of the Hatch Act providing opinions on whether an activity is permitted under the act; investigating compliance with the provisions of the act; taking disciplinary action against the employee for serious violations; and prosecuting those violations before the Merit Systems Protection Board. Now I understand why the incantation “Hatch Act” casts a chill on our civil service souls. While there have been recent allegations against a high-profile political appointee, federal practitioners are not immune to prosecution.4 In 2017, Federal Times reported that the OSC sought disciplinary action against a VA physician for 15 violations of the Hatch Act after he ran for a state Senate seat in 2014.5
Fortunately, the OSC has produced a handy list of “Though Shalt Nots” and “You Cans” as a guide to the Hatch Act.6 Only the highpoints are mentioned here:
- Thou shalt not be a candidate for nomination or election to a partisan public office;
- Thou shalt not use a position of official public authority to influence or interfere with the result of an election;
- Thou shalt not solicit or host, accept, or receive a donation or contribution to a partisan political party, candidate, or group; and
- Thou shalt not engage in political activity on behalf of a partisan political party, candidate, or group while on duty, in a federal space, wearing a federal uniform, or driving a federal vehicle.
Covered under these daunting prohibitions is ordinary American politicking like hosting fundraisers or inviting your coworkers to a political rally, distributing campaign materials, and wearing a T-shirt with your favorite candidates smiling face at work. The new hotbed of concern for the Hatch Act is, you guessed it, social media—you cannot use your blog, Facebook, Instagram, or e-mail account to comment pro or con for a partisan candidate, party, office, or group.6
You may be asking at this point whether you can even watch the political debates? Yes, that is allowed under the Hatch Act along with running for nonpartisan election and participating in nonpartisan campaigns; voting, and registering others to vote; you can contribute money to political campaigns, parties, or partisan groups; attend political rallies, meetings and fundraisers; and even join a political party. Of course these activities must be on your own time and dime, not that of your federal employer. All of these “You Cans” enable a federal employee to engage in the bare minimum of democracy: voting in elections, but opponents argue they bar the civil servant from fully participating in the complex richness of the American political process.7
Nonetheless, since its inception the Hatch Act has been a matter of fierce debate among federal employees and other advocates of civil liberties. Those who feel it should be relaxed contend that the modern merit-based system of government service has rendered the provisions of the Hatch Act unnecessary, even obsolete. In addition, unlike in 1939, critics of the act claim there are now formidable whistleblower protections for employees who experience political coercion. Over the years there have been several efforts to weaken the conflict of interest safeguards that the act contains, leading many commentators to think that some of the amendments and reforms have blurred the tight boundaries between the professional and the political. Others such as the government unions and the American Civil Liberties Union (ACLU) believe that the tight line drawn between public and private binds the liberty of civil servants.8 Those who defend the Hatch Act believe that the wall it erects between professional and personal in the realm of political activities for federal employees must remain high and strong to protect the integrity of the administrative branch and the public trust.9
So, as political advertisements dominate television programming and the texts never stop asking for campaign donations, you can cast your own vote for or against the Hatch Act. As for me and my house, we will follow President Jefferson in preferring to be the property of the people rather than be indebted to the powerful. You need never encounter a true conflict of interest if you have no false conflict of obligation: history teaches us that serving 2 masters usually turns out badly for the slave. Many of you will completely disagree with my stance, holding that your constitutional rights as a citizen are being curtailed, if not outright denied, simply because you choose to serve your country. Our ability to freely hold and express our differences of opinions about the Hatch Act and so much else is what keeps democracy alive.
1. Rayner BL. Life of Thomas Jefferson With Selections From the Most Valuable Portions of his Voluminous and Unrivalled Private Correspondence. Boston, MA: Lilly, Wait, Colman, and Holden; 1834:356.
2. US Office of Special Counsel. Hatch Act overview. https://osc.gov/Services/Pages/HatchAct.aspx. Accessed February 24, 2020.
3. Brown AJ. Public employee participation: Hatch Acts in the federal and state governments. Public Integrity. 2000;2(2):105-120.
4. Phillips A. What is the Hatch Act, and why did Kellyanne Conway get accused of violating it so egregiously? Washington Post. June 13, 2019. https://www.washingtonpost.com/politics/2019/06/13/what-is-hatch-act-why-did-kellyanne-conway-get-accused-violating-it-so-egregiously. Accessed February 24, 2020.
5. Bur J. Special counsel: VA doctor violated Hatch Act while campaigning. https://www.federaltimes.com/federal-oversight/watchdogs/2017/11/22/special-counsel-va-doctor-violated-hatch-act-while-campaigning. Published November 22, 2017. Accessed February 24, 2020.
6. US Office of Special Counsel. A guide to the Hatch Act for the federal employee. https://osc.gov/Documents/Outreach%20and%20Training/Handouts/A%20Guide%20to%20the%20Hatch%20Act%20for%20Federal%20Employees.pdf. Published September 2014. Accessed February 24, 2020.
7. Brown C, Maskell J. Hatch Act restrictions on federal employee’s political activities in the digital age. https://fas.org/sgp/crs/misc/R44469.pdf. Published April 13, 2016. Accessed February 24, 2020.
8. Thurber KT Jr. Revising the Hatch Act: a practitioner’s perspective. Public Manag. 1993;22(1):43.
9. Pearson WM, Castle DS. Expanding the opportunity for partisan activity among government employees: potential effects of federal executive’s political involvement. Int J Public Adm. 2007;16(4):511-525.
1. Rayner BL. Life of Thomas Jefferson With Selections From the Most Valuable Portions of his Voluminous and Unrivalled Private Correspondence. Boston, MA: Lilly, Wait, Colman, and Holden; 1834:356.
2. US Office of Special Counsel. Hatch Act overview. https://osc.gov/Services/Pages/HatchAct.aspx. Accessed February 24, 2020.
3. Brown AJ. Public employee participation: Hatch Acts in the federal and state governments. Public Integrity. 2000;2(2):105-120.
4. Phillips A. What is the Hatch Act, and why did Kellyanne Conway get accused of violating it so egregiously? Washington Post. June 13, 2019. https://www.washingtonpost.com/politics/2019/06/13/what-is-hatch-act-why-did-kellyanne-conway-get-accused-violating-it-so-egregiously. Accessed February 24, 2020.
5. Bur J. Special counsel: VA doctor violated Hatch Act while campaigning. https://www.federaltimes.com/federal-oversight/watchdogs/2017/11/22/special-counsel-va-doctor-violated-hatch-act-while-campaigning. Published November 22, 2017. Accessed February 24, 2020.
6. US Office of Special Counsel. A guide to the Hatch Act for the federal employee. https://osc.gov/Documents/Outreach%20and%20Training/Handouts/A%20Guide%20to%20the%20Hatch%20Act%20for%20Federal%20Employees.pdf. Published September 2014. Accessed February 24, 2020.
7. Brown C, Maskell J. Hatch Act restrictions on federal employee’s political activities in the digital age. https://fas.org/sgp/crs/misc/R44469.pdf. Published April 13, 2016. Accessed February 24, 2020.
8. Thurber KT Jr. Revising the Hatch Act: a practitioner’s perspective. Public Manag. 1993;22(1):43.
9. Pearson WM, Castle DS. Expanding the opportunity for partisan activity among government employees: potential effects of federal executive’s political involvement. Int J Public Adm. 2007;16(4):511-525.
Breach of migrant youths’ confidentiality is unethical, unacceptable
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
We are in the healing profession. We practice a trade. We are doctors, therapists, counselors. We work with children, adults, and couples. We document the physical form of our patient after examination, setting the stage for interventions that heal and alleviate suffering. With those who we do not touch physically, we hold out our psychological arms to embrace them in a therapeutic relationship.
We are privileged to appreciate their deeper selves through voice, unsaid words, and body language. A trust evolves (or might not); deeper exploration where our intuition and technical skill discover what troubles the soul. Healing begins as a delicate dance: As trust is earned, our patients risk vulnerability by revealing their weakest selves.
As healers, we often find ourselves adrift with our own insecurities, our own histories that make us human; our styles may differ but training and the tenets and guidelines set by our professional societies keep us in safe waters. These guidelines are informed by the science of health care research and vetted through centuries of observation and experience of process. “Do no harm” is perhaps one of the major rules of engaging with patients. The scaffolding that our code of ethics provides healing professions trumps external pressures to deviate. If you violate these codes, the consequences are borne by the patient and the potential loss of your license.
Some of you may have read about Kevin Euceda, an adolescent who reportedly was waiting for his immigration interview and ordered to undergo mandatory therapy as part of the immigration protocol. Kevin revealed to his therapist the history of violence he experienced as a child growing up in Honduras. His subsequent initiation into a gang was the only option he had to escape a violent death. Those of us who work with youth from gang cultures know fully that allegiance to a gang is a means to find an identity and brotherhood with the payment by a lifestyle of violence. A therapist faced with this information does not judge but helps the person deal with PTSD, nightmares, and guilt that become part of an identity just as the memories of mines blowing up in the face of combat affect veterans.
But the therapist, who reportedly holds a master’s in rehabilitation counseling and was “a year away from passing her licensing exam,” according to an article published in the Washington Post, followed policy of the Office of Refugee Resettlement. The therapist betrayed Kevin by reporting the information he shared with her confidentially to Immigration and Customs Enforcement. The reason the therapist gave for the breach was that she was compelled do so because Kevin reported participating in gang activity in Honduras. Subsequently, Kevin was sent to a high-security detention center – and is now facing deportation.
Betraying a patient, profession
Therapy begins as a contract between patient and therapist. The contract stipulates that all that transpires in the process of therapy (usually a 50-minute block of time, usually weekly) is information held by the therapist and patient – and is not to be shared with anyone, including parents, guardians, legal entities, and health care agencies. This allows the gradual sharing of events, emotions, behaviors, and reactions akin to peeling an onion. Memories, reactions, and feelings assist the therapist as they start their quest of discovery of the conflict and how to resolve it. Trust is the central tenet of this journey. The patient thinks: “You will hear me; you will see me you will understand me and help me understand myself.” The doctor responds: “Even I don’t yet know fully what ails you; we will discover that together. … I will not fail your trust.”
So how does this interface with external pressures? The constitution of a free country provides some inviolable protections that prevent derailment of the codes of ethics based on science. The fine line between what are considered sacrosanct ethics of a field – be it health care, climatology, or architecture – and what could be sacrificed in the name of prevailing forces (political or otherwise) has to be under constant scrutiny by the members of the guild. In health care, when patients cannot trust the science, its implementation, or is let down by the clinician, they are unlikely to benefit from treatment. A foundation of distrust paves the way for future therapeutic relationships that are stained with distrust and noncompliance.
The ethics guidelines of the American Academy of Psychiatry and the Law specify that psychiatrists in forensic roles “should be clear about limitations on confidentiality in the treatment relationship and ensure that these limitations are communicated to the patient.” Again, the therapist in this case is not a psychiatrist, but I would argue that the same rules would apply.
It is reassuring to know that several key groups, including the American Psychiatric Association, American Academy of Child and Adolescent Psychiatry, and the American Psychological Association, have all condemned the therapist’s actions. Psychiatrists and other mental health professionals must do no harm. We must not stand idly by and allow the kind of professional breach that happened to Kevin continue. Patients who confide in mental health professionals with the promise of confidentiality must be able to do so without fear. Only with confidentiality can the therapeutic relationship thrive.
Dr. Sood is professor of psychiatry and pediatrics, and senior professor of child mental health policy, at Virginia Commonwealth University, Richmond.
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
“We appreciate and applaud the FDA and FTC in recognizing that biosimilar development and approval has not been as robust as many stakeholders had hoped,” said Colin Edgerton, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “We continue to see anticompetitive activities that prevent manufacturers from developing biosimilar products. We hope that a greater focus on these practices will pave the way for more biosimilars to be developed.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also will be sponsoring a public workshop on March 9 to discuss competition for biologics.
“This workshop is the first step,” Dr. Edgerton said. “ACR will continue to work with other organizations and patient groups to help educate providers and patients on the scientific rigor that is required in developing and approving biosimilars. Additionally, we look forward to working with the FDA and FTC to continue this conversation on ways to encourage more development of biosimilar products and greater education for the providers and patients.”
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Dr. Edgerton highlighted why this agreement between the two agencies is so important.
“Biologics are life-changing treatments for many of our patients,” he said. “Due to the high cost of discovery and development, the cost of biologics has resulted in delayed access and financial hardships for so many. It has always been our hope that biosimilars would offer the same life-changing treatment for patients at a lower price point. A robust biosimilars market is imperative to allow greater access to these treatments that can help patients to have a better quality of life.”
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
Trump seeks to cut NIH, CDC budgets, some Medicare spending
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
The Trump administration on Feb. 10 argued for cutting spending for a federal agency at the forefront of the efforts to combat the coronavirus, while also seeking to slow spending in certain parts of the Medicare and Medicaid programs.
President Donald Trump presented his fiscal 2021 request to Congress for refilling the coffers of federal agencies. In any administration, an annual budget serves only as a political blueprint, as the White House document itself makes no changes in federal spending.
In Mr. Trump’s case, several of his requests for agencies within the Department of Health & Human Services run counter to recent budget trends. Republicans and Democrats in Congress have worked together in recent years to increase budgets for major federal health agencies.
But Mr. Trump asked Congress to cut annual budget authority for the National Institute of Allergy and Infectious Diseases by $430 million to $5.446 billion for fiscal 2021. In contrast, Congress has raised the annual budget for NIAID, a key agency in combating the coronavirus, from $5.545 billion in fiscal 2019 to $5.876 billion in fiscal 2020, which began in October, according to an HHS summary of Mr. Trump’s request.
For the Centers for Disease Control and Prevention, which is central to the battle against the coronavirus, Mr. Trump proposed a drop in discretionary funding to $5.627 billion. In contrast, Congress raised the CDC budget from $6.544 billion in fiscal 2019 to $6.917 in fiscal 2020.
Mr. Trump also wants to cut $559 million from the budget of the National Cancer Institute, dropping it to $5.881 billion in fiscal 2021. In contrast, Congress raised NCI’s budget from $6.121 billion in fiscal 2019 to $6.440 billion in fiscal 2020.
Mr. Trump requested a $2.6 billion reduction in the National Institutes of Health’s total discretionary budget, seeking to drop it to $37.70 billion. In contrast, Congress raised NIH’s budget from $37.887 in fiscal 2019 to $40.304 billion in fiscal 2020.
Mr. Trump’s budget proposal also includes an estimate of $152 billion in savings over a decade for Medicaid through the implementation of what the administration calls “community engagement” requirements.
The Trump administration has been at odds with Democrats for years about whether work requirements should be attached to Medicaid. “Well-designed community engagement incentives have great potential to improve health and well-being while empowering beneficiaries to rise out of poverty,” HHS said in a budget document.
Yet researchers last year reported that Arkansas’ attempt to attach work requirements to Medicaid caused almost 17,000 adults to lose this health care coverage within the first 6 months, and there was no significant difference in employment.
The researchers say this loss of coverage was partly a result of bureaucratic obstacles and confusion about the new rules. In June 2018, Arkansas became the first state to implement work requirements for Medicaid, Benjamin D. Sommers, MD, PhD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues wrote in the New England Journal of Medicine (2019 Sep 12;381[11]:1073-82).
Budget ‘would thwart’ progress
A few medical groups on Monday quickly criticized Mr. Trump’s proposals.
“In a time where our nation continues to face significant public health challenges — including 2019 novel coronavirus, climate change, gun violence, and costly chronic diseases such as heart disease and cancer – the administration should be investing more resources in better health, not cutting federal health budgets,” said Georges C. Benjamin, MD, executive director of the American Public Health Association, in a statement.
David J. Skorton, MD, chief executive and president of the Association of American Medical Colleges (AAMC) also urged increased investment in fighting disease.
“We must continue the bipartisan budget trajectory set forth by Congress over the last several years, not reverse course,” Dr. Skorton said in a statement.
Mr. Trump’s proposed cuts in medical research “would thwart scientific progress on strategies to prevent, diagnose, treat, and cure medical conditions that affect countless patients nationwide,” he said.
In total, the new 2021 appropriations for HHS would fall by $9.46 billion to $85.667 billion under Mr. Trump’s proposal. Appropriations, also called discretionary budget authority, represents the operating budgets for federal agencies. These are decided through annual spending bills.
Congress has separate sets of laws for handling payments the federal government makes through Medicare and Medicaid. These are known as mandatory spending.
‘Untenable cuts’
AAMC’s Dr. Skorton also objected to what he termed Mr. Trump’s bid “to reduce and consolidate Medicare, Medicaid, and children’s hospital graduate medical education into a single grant program.”
This would force teaching hospitals to absorb $52 billion in “untenable cuts,” he said.
“The proposal ignores the intent of the Medicare GME program, which is to ensure an adequate physician workforce to care for Medicare beneficiaries and support the critical patient care missions of America’s teaching hospitals,” Dr. Skorton said.
The budget also seeks cuts to Medicaid, which come in addition to the administration’s “recent proposals to scale back Medicaid coverage,” Dr. Skorton said.
“More than 26% of all Medicaid hospitalizations occur at AAMC-member teaching hospitals, even though these institutions represent only 5% of all hospitals,” Dr. Skorton said. “Each of the administration’s proposals on their own would be devastating for patients – and combined, they would be disastrous.”
Rick Pollack, the chief executive and president of the American Hospital Association, described Mr. Trump’s fiscal 2021 proposal as another bid to undermine medical care in the United States.
“Every year, we adapt to a constantly changing environment, but every year, the administration aims to gut our nation’s health care infrastructure,” Mr. Pollack said in a statement.
In it, he noted that about one in five people in America depend on Medicaid, with children accounting for a large proportion of those covered by the state-federal program.
“The budget’s proposal on Medicaid financing and service delivery would cut hundreds of billions of dollars from the Medicaid program annually,” Mr. Pollack said.
He also objected to “hundreds of billions of proposed reductions to Medicare” endorsed by Mr. Trump.
Medical malpractice overhaul
The Trump administration also offered many suggestions for changing federal laws to reduce health care spending. Among these was a proposed overhaul of the approach to medical malpractice cases.
The president’s budget proposal estimates $40 billion in savings over a decade from steps to limit medical liability, according to a report from the Office of Management and Budget (OMB).
“The current medical liability system does not work for patients or providers, nor does it promote high-quality, evidence-based care,” OMB said. “Providers practice with a threat of potentially frivolous lawsuits, and injured patients often do not receive just compensation for their injuries.”
Mr. Trump’s fiscal 2021 budget calls for a cap on noneconomic damage awards of $250,000, which would increase with inflation over time, and a 3-year statute of limitations. Under this plan, courts could also modify attorney’s fee arrangements. HHS could provide guidance to states on how to create expert panels and administrative health care tribunals to review medical liability.
These steps would lead to lower health care spending, with clinicians dropping “defensive medicine practices,” OMB said. That would benefit the Medicare and Medicaid programs as well as lowering costs of health insurance in general.
Mr. Trump’s fiscal 2021 budget also includes a series of proposals for Medicare that it estimates would, in aggregate, save $755.5 billion over a decade.
Site-neutral policy
A large chunk of the estimated Medicare savings in Mr. Trump’s fiscal 2021 health budget would come from lowering payments to hospitals for services provided in their outpatient and physician offices.
In the fiscal 2021 proposal, HHS noted that “Medicare generally pays on-campus hospital outpatient departments substantially more than physician offices for the same services.”
Mr. Trump’s budget proposal seeks a more expansive shift to what’s called a “site-neutral” payment for services delivered in hospital outpatient programs or physician offices. This would bring these payments more in line with those made to independent physician practices.
“This proposal would eliminate the often significant disparity between what Medicare pays in these different settings for the same services,” HHS said in the budget summary.
HHS estimated this change in policy would generate $117.2 billion in savings over a decade. Combined with saving from medical malpractice reforms, the Trump administration estimates these two moves combined could save about $164 billion over a decade.
The site-neutral policy has been a legal battleground, with hospital and physician groups winning a round last year.
Another Medicare proposal included in Mr. Trump’s fiscal 2021 budget homes in on this issue for cases where a hospital owns a physician office. Medicare now pays most off-campus hospital outpatient departments higher rates than the program’s physician fee schedule dictates for the same services.
Switching to a site-neutral policy for these hospital-owned physician offices would result in $47.2 billion in savings over a decade, HHS said in the budget document.
This article first appeared on Medscape.com.
Surgeon General scolds docs for failing to help patients quit smoking
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
The U.S. Surgeon General is calling on all physicians to help patients stop smoking, noting that two-thirds of adult smokers say they want to quit, but only 40% report that their doctor has advised them to stop.
“I’ve got to own this as the nation’s doctor, and our health providers in this room and in this country need to own this stat,” said Surgeon General Jerome Adams, MD, at a press briefing releasing a new report on smoking cessation.
“Smoking is the No. 1 preventable cause of death, disease, and disability in the United States,” he said. “So why are 40% of our health providers out there not advising smokers to quit when they come in?”
In the first U.S. Surgeon General report on smoking cessation in 30 years, the 700-page report suggests smoking cessation-related quality measures that include physician reimbursement would increase treatment.
The evidence also suggests that using electronic health records to prompt clinicians to inquire about smoking would increase cessation treatment.
EHRs could be used to “empower and enable” physicians to advise people to quit, said Dr. Adams. Physicians also need “the education and the confidence to be able to have that conversation, because too many of them look at someone and say: ‘Nope, too hard, too much effort, no, that’s not what they’re here for today,’ ” he said.
However, “simply asking, advising, and referring can be enough to get someone on the pathway to quitting,” Dr. Adams said.
34 million still smoke
The new report is the first on the topic released since 1990, and the 34th on tobacco control since the first one was issued in 1964, said Dr. Adams. Since that first report, adult smoking has declined 70%, but some 34 million Americans (14%) still smoke, he said.
In addition, Dr. Adams said that many subpopulations have been left behind, noting: “Cigarette smoking remains highest among LGBTQ adults, people with disabilities or limitations, American Indians and Alaska Natives, and people with mental health conditions or substance use disorders.”
He also noted that 40% of cigarettes are consumed by those with a mental illness or a substance use disorder.
Quitting is beneficial at any age and can add as much as a decade to life expectancy, the report notes. Quitting also reduces the risk of 12 cancers, cuts the risk of chronic obstructive pulmonary disease, and reduces cardiovascular and stroke morbidity and mortality.
Pregnant women who quit also reduce their own morbidity and mortality risk and that of unborn children and infants, the report says.
“We know more about the science of quitting than ever before. We can, and must, do more to ensure that evidence-based cessation treatments are reaching the people that need them,” said Dr. Adams.
Less than one-third of those who have quit have used Food and Drug Administration–approved cessation medications or behavioral counseling, Dr. Adams said.
Barriers to care
Despite the existence of five nicotine replacement therapies and two nonnicotine oral medications, and more widespread availability of proven counseling methods – including web- or text-based programs – barriers to access remain.
These include a lack of insurance coverage for comprehensive, evidence-based smoking cessation treatment, which, when offered, increases availability and use.
“These are cost-effective interventions,” said Dr. Adams. “It’s penny wise and pound foolish to not give someone access to what we know works,” he said.
Because of the diversity of e-cigarette products and the variety of ways they are used, coupled with little research, it’s not currently possible to determine whether they are, or are not, useful smoking cessation tools, the report notes.
However, experts who compiled the report found some evidence to suggest that e-cigarettes containing nicotine may be “associated with increased smoking cessation compared with the use of e-cigarettes not containing nicotine.”
Asked whether the report’s conclusions might be interpreted as supportive of e-cigarettes, Dr. Adams said the report focused on smoking cessation, not initiation.
“I’m terribly concerned about the clear data that shows youth are initiating tobacco product use with e-cigarettes,” he said.
The Trump administration’s current proposal to partially restrict sales of some flavored e-cigarettes “reflects the science,” and “a balance between a desire to really make sure that people aren’t initiating with these products, but also a desire to again try to maintain a pathway for adults who want to use these products to quit to use them,” Dr. Adams said.
The focus, said Dr. Adams, should not be on e-cigarettes and whether they do, or do not, work.
“People want to quit,” he said. “We know what works. Not enough of them are getting it, and there are terrible disparities in who is and who is not getting access to effective and evidence-based treatment – that’s the story here.”
This article first appeared on Medscape.com.
Using Democratic Deliberation to Engage Veterans in Complex Policy Making for the Veterans Health Administration
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
Providing high-quality, patient-centered health care is a top priority for the US Department of Veterans Affairs (VA) Veteran Health Administration (VHA), whose core mission is to improve the health and well-being of US veterans. Thus, news of long wait times for medical appointments in the VHA sparked intense national attention and debate and led to changes in senior management and legislative action. 1 On August 8, 2014, President Bara c k Obama signed the Veterans Access, Choice, and Accountability Act of 2014, also known as the Choice Act, which provided an additional $16 billion in emergency spending over 3 years to improve veterans’ access to timely health care. 2 The Choice Act sought to develop an integrated health care network that allowed qualified VHA patients to receive specific health care services in their communities delivered by non-VHA health care providers (HCPs) but paid for by the VHA. The Choice Act also laid out explicit criteria for how to prioritize who would be eligible for VHA-purchased civilian care: (1) veterans who could not get timely appointments at a VHA medical facility within 30 days of referral; or (2) veterans who lived > 40 miles from the closest VHA medical facility.
VHA decision makers seeking to improve care delivery also need to weigh trade-offs between alternative approaches to providing rapid access. For instance, increasing access to non-VHA HCPs may not always decrease wait times and could result in loss of continuity, limited care coordination, limited ability to ensure and enforce high-quality standards at the VHA, and other challenges.3-6 Although the concerns and views of elected representatives, advocacy groups, and health system leaders are important, it is unknown whether these views and preferences align with those of veterans. Arguably, the range of views and concerns of informed veterans whose health is at stake should be particularly prominent in such policy decision making.
To identify the considerations that were most important to veterans regarding VHA policy around decreasing wait times, a study was designed to engage a group of veterans who were eligible for civilian care under the Choice Act. The study took place 1 year after the Choice Act was passed. Veterans were asked to focus on 2 related questions: First, how should funding be used for building VHA capacity (build) vs purchasing civilian care (buy)? Second, under what circumstances should civilian care be prioritized?
The aim of this paper is to describe democratic deliberation (DD), a specific method that engaged veteran patients in complex policy decisions around access to care. DD methods have been used increasingly in health care for developing policy guidance, setting priorities, providing advice on ethical dilemmas, weighing risk-benefit trade-offs, and determining decision-making authority.7-12 For example, DD helped guide national policy for mammography screening for breast cancer in New Zealand.13 The Agency for Healthcare Research and Quality has completed a systematic review and a large, randomized experiment on best practices for carrying out public deliberation.8,13,14 However, despite the potential value of this approach, there has been little use of deliberative methods within the VHA for the explicit purpose of informing veteran health care delivery.
This paper describes the experience engaging veterans by using DD methodology and informing VHA leadership about the results of those deliberations. The specific aims were to understand whether DD is an acceptable approach to use to engage patients in the medical services policy-making process within VHA and whether veterans are able to come to an informed consensus.
Methods
Engaging patients and incorporating their needs and concerns within the policy-making process may improve health system policies and make those policies more patient centered. Such engagement also could be a way to generate creative solutions. However, because health-system decisions often involve making difficult trade-offs, effectively obtaining patient population input on complex care delivery issues can be challenging.
Although surveys can provide intuitive, top-of-mind input from respondents, these opinions are generally not sufficient for resolving complex problems.15 Focus groups and interviews may produce results that are more in-depth than surveys, but these methods tend to elicit settled private preferences rather than opinions about what the community should do.16 DD, on the other hand, is designed to elicit deeply informed public opinions on complex, value-laden topics to develop recommendations and policies for a larger community.17 The goal is to find collective solutions to challenging social problems. DD achieves this by giving participants an opportunity to explore a topic in-depth, question experts, and engage peers in reason-based discussions.18,19 This method has its roots in political science and has been used over several decades to successfully inform policy making on a broad array of topics nationally and internationally—from health research ethics in the US to nuclear and energy policy in Japan.7,16,20,21 DD has been found to promote ownership of public programs and lend legitimacy to policy decisions, political institutions, and democracy itself.18
A single day (8 hours) DD session was convened, following a Citizens Jury model of deliberation, which brings veteran patients together to learn about a topic, ask questions of experts, deliberate with peers, and generate a “citizen’s report” that contains a set of recommendations (Table 1). An overview of the different models of DD and rationale for each can be found elsewhere.8,15
Recruitment Considerations
A purposively selected sample of civilian care-eligible veterans from a midwestern VHA health care system (1 medical center and 3 community-based outpatient clinics [CBOCs]) were invited to the DD session. The targeted number of participants was 30. Female veterans, who comprise only 7% of the local veteran population, were oversampled to account for their potentially different health care needs and to create balance between males and females in the session. Oversampling for other characteristics was not possible due to the relatively small sample size. Based on prior experience,7 it was assumed that 70% of willing participants would attend the session; therefore 34 veterans were invited and 24 attended. Each participant received a $200 incentive in appreciation for their substantial time commitment and to offset transportation costs.
Background Materials
A packet with educational materials (Flesch-Kincaid Grade Level of 10.5) was mailed to participants about 2 weeks before the DD session. Participants were asked to review prior to attending the session. These materials described the session (eg, purpose, organizers, importance) and provided factual information about the Choice Act (eg, eligibility, out-of-pocket costs, travel pay, prescription drug policies).
Session Overview
The session was structured to accomplish the following goals: (1) Elicit participants’ opinions about access to health care and reasons for those opinions; (2) Provide in-depth education about the Choice Act through presentations and discussions with topical experts; and (3) Elicit reasoning and recommendations on both the criteria by which participants prioritize candidates for civilian care and how participants would allocate additional funding to improve access (ie, by building VHA capacity to deliver more timely health care vs purchasing health care from civilian HCPs).
Participants were asked to fill out a survey on arrival in the morning and were assigned to 1 of 3 tables or small groups. Each table had a facilitator who had extensive experience in qualitative data collection methods and guided the dialogue using a scripted protocol that they helped develop and refine. The facilitation materials drew from and used previously published studies.22,23 Each facilitator audio recorded the sessions and took notes. Three experts presented during plenary education sessions. Presentations were designed to provide balanced factual information and included a veteran’s perspective. One presenter was a clinician on the project team, another was a local clinical leader responsible for making decisions about what services to provide via civilian care (buy) vs enhancing the local VHA health system’s ability to provide those services (build), and the third presenter was a veteran who was on the project team.
Education Session 1
The first plenary education session with expert presentations was conducted after each table completed an icebreaker exercise. The project team physician provided a brief history and description of the Choice Act to reinforce educational materials sent to participants prior to the session. The health system clinical leader described his decision process and principles and highlighted constraints placed on him by the Choice Act that were in place at the time of the DD session. He also described existing local and national programs to provide civilian care (eg, local fee-basis non-VHA care programs) and how these programs sought to achieve goals similar to the Choice Act. The veteran presenter focused on the importance of session participants providing candid insight and observations and emphasized that this session was a significant opportunity to “have their voices heard.”
Deliberation 1: What criteria should be used to prioritize patients for receiving civilian care paid for by the VHA? To elicit preferences on the central question of this deliberation, participants were presented with 8 real-world cases that were based on interviews conducted with Choice Act-eligible veterans (Table 2 and eAppendices A
Education Session 2
In the second plenary session, the project team physician provided information about health care access issues, both inside and outside of the VHA, particularly between urban and rural areas. He also discussed factors related to the insufficient capacity to meet growing demand that contributed to the VHA wait-time crisis. The veteran presenter shared reflections on health care access from a veteran’s perspective.
Deliberation 2: How should additional funding be divided between increasing the ability of the VHA to (1) provide care by VHA HCPs; and (2) pay for care from non-VHA civilian HCPs? Participants were presented the patient examples and Choice Act funding scenarios (the buy policy option) and contrasted that with a build policy option. Participants were explicitly encouraged to shift their perspectives from thinking about individual cases to considering policy-level decisions and the broader social good (Table 2).
Ensuring Robust Deliberations
If participants do not adequately grasp the complexities of the topic, a deliberation can fail. To facilitate nuanced reasoning, real-world concrete examples were developed as the starting point of each deliberation based on interviews with actual patients (deliberation 1) and actual policy proposals relevant to the funding allocation decisions within the Choice Act (deliberation 2).
A deliberation also can fail with self-silencing, where participants withhold opinions that differ from those articulated first or by more vocal members of the group.24 To combat self-silencing, highly experienced facilitators were used to ensure sharing from all participants and to support an open-minded, courteous, and reason-based environment for discourse. It was specified that the best solutions are achieved through reason-based and cordial disagreement and that success can be undermined when participants simply agree because it is easier or more comfortable.
A third way a deliberation can fail is if individuals do not adopt a group or system-level perspective. To counter this, facilitators reinforced at multiple points the importance of taking a broader social perspective rather than sharing only one’s personal preferences.
Finally, it is important to assess the quality of the deliberative process itself, to ensure that results are trustworthy.25 To assess the quality of the deliberative process, participants knowledge about key issues pre- and postdeliberation were assessed. Participants also were asked to rate the quality of the facilitators and how well they felt their voice was heard and respected, and facilitators made qualitative assessments about the extent to which participants were engaged in reason-based and collaborative discussion.
Data
Quantitative data were collected via pre- and postsession surveys. The surveys contained items related to knowledge about the Choice Act, expectations for the DD session, beliefs and opinions about the provision of health care for veterans, recommended funding allocations between build vs buy policy options, and general demographics. Qualitative data were collected through detailed notes taken by the 3 facilitators. Each table’s deliberations were audio recorded so that gaps in the notes could be filled.
The 3 facilitators, who were all experienced qualitative researchers, typed their written notes into a template immediately after the session. Two of the 3 facilitators led the analysis of the session notes. Findings within and across the 3 deliberation tables were developed using content and matrix analysis methods.26 Descriptive statistics were generated from survey responses and compared survey items pre- and postsession using paired t tests or χ2 tests for categorical responses.
Results
Thirty-three percent of individuals invited (n = 127) agreed to participate. Those who declined cited conflicts related to distance, transportation, work/school, medical appointments, family commitments, or were not interested. In all, 24 (69%) of the 35 veterans who accepted the invitation attended the deliberation session. Of the 11 who accepted but did not attend, 5 cancelled ahead of time because of conflicts (Figure). Most participants were male (70%), 48% were aged 61 to 75 years, 65% were white, 43% had some college education, 43% reported an annual income of between $25,000 and $40,000, and only 35% reported very good health (eAppendix D).
Deliberation 1
During the deliberation on the prioritization criteria, the concept of “condition severity” emerged as an important criterion for veterans. This criterion captured simultaneous consideration of both clinical necessity and burden on the veteran to obtain care. For example, participants felt that patients with a life-threatening illness should be prioritized for civilian care over patients who need preventative or primary care (clinical necessity) and that elderly patients with substantial difficulty traveling to VHA appointments should be prioritized over patients who can travel more easily (burden). The Choice Act regulations at the time of the DD session did not reflect this nuanced perspective, stipulating only that veterans must live > 40 miles from the nearest VHA medical facility.
One of the 3 groups did not prioritize the patient cases because some members felt that no veteran should be constrained from receiving civilian care if they desired it. Nonetheless, this group did agree with prioritizing the first 2 cases in Table 3. The other groups prioritized all 8 cases in generally similar ways.
Deliberation 2
No clear consensus emerged on the buy vs build question. A representative from each table presented their group’s positions, rationale, and recommendations after deliberations were completed. After hearing the range of positions, the groups then had another opportunity to deliberate based on what they heard from the other tables; no new recommendations or consensus emerged.
Participants who were in favor of allocating more funds toward the build policy offered a range of rationales, saying that it would (1) increase access for rural veterans by building CBOCs and deploying more mobile units that could bring outlets for health care closer to their home communities; (2) provide critical and unique medical expertise to address veteran-specific issues such as prosthetics, traumatic brain injury, posttraumatic stress disorder, spinal cord injury, and shrapnel wounds that are typically not available through civilian providers; (3) give VHA more oversight over the quality and cost of care, which is more challenging to do with civilian providers; and (4) Improve VHA infrastructure by, for example, upgrading technology and attracting the best clinicians and staff to support “our VHA.”
Participants who were in favor of allocating more funds toward the buy policy also offered a range of rationales, saying that it would (1) decrease patient burden by increasing access through community providers, decreasing wait time, and lessening personal cost and travel time; (2) allow more patients to receive civilian care, which was generally seen as beneficial by a few participants because of perceptions that the VHA provides lower quality care due to a shortage of VHA providers, run-down/older facilities, lack of technology, and poorer-quality VHA providers; and (3) provide an opportunity to divest of costly facilities and invest in other innovative approaches. Regarding this last reason, a few participants felt that the VHA is “gouged” when building medical centers that overrun budgets. They also were concerned that investing in facilities tied VHA to specific locations when current locations of veterans may change “25 years from now.”
Survey Results
Twenty-three of the 24 participants completed both pre- and postsession surveys. The majority of participants in the session felt people in the group respected their opinion (96%); felt that the facilitator did not try to influence the group with her own opinions (96%); indicated they understood the information enough to participate as much as they wanted (100%); and were hopeful that their reasoning and recommendations would help inform VHA policy makers (82%).
The surveys also provided an opportunity to examine the extent to which knowledge, attitudes, and opinions changed from before to after the deliberation. Even with the small sample, responses revealed a trend toward improved knowledge about key elements of the Choice Act and its goals. Further, there was a shift in some participants’ opinions about how patients should be prioritized to receive civilian care. For example, before the deliberation participants generally felt that all veterans should be able to receive civilian care, whereas postdeliberation this was not the case. Postdeliberation, most participants felt that primary care should not be a high priority for civilian care but continued to endorse prioritizing civilian care for specialty services like orthopedic or cardiology-related care. Finally, participants moved from more diverse recommendations regarding additional funds allocations, toward consensus after the deliberation around allocating funds to the build policy. Eight participants supported a build policy beforehand, whereas 16 supported this policy afterward.
Discussion
This study explored DD as a method for deeply engaging veterans in complex policy making to guide funding allocation and prioritization decisions related to the Choice Act, decisions that are still very relevant today within the context of the Mission Act and have substantial implications for how health care is delivered in the VHA. The Mission Act passed on June 6, 2018, with the goal of improving access to and the reliability of civilian or community care for eligible veterans.27 Decisions related to appropriating scarce funding to improve access to care is an emotional and value-laden topic that elicited strong and divergent opinions among the participants. Veterans were eager to have their voices heard and had strong expectations that VHA leadership would be briefed about their recommendations. The majority of participants were satisfied with the deliberation process, felt they understood the issues, and felt their opinions were respected. They expressed feelings of comradery and community throughout the process.
In this single deliberation session, the groups did not achieve a single, final consensus regarding how VHA funding should ultimately be allocated between buy and build policy options. Nonetheless, participants provided a rich array of recommendations and rationale for them. Session moderators observed rich, sophisticated, fair, and reason-based discussions on this complex topic. Participants left with a deeper knowledge and appreciation for the complex trade-offs and expressed strong rationales for both sides of the policy debate on build vs buy. In addition, the project yielded results of high interest to VHA policy makers.
This work was presented in multiple venues between 2015 to 2016, and to both local and national VHA leadership, including the local Executive Quality Leadership Boards, the VHA Central Office Committee on the Future State of VA Community Care, the VA Office of Patient Centered Care, and the National Veteran Experience Committee. Through these discussions and others, we saw great interest within the VHA system and high-level leaders to explore ways to include veterans’ voices in the policy-making process. This work was invaluable to our research team (eAppendix E
Many health system decisions regarding what care should be delivered (and how) involve making difficult, value-laden choices in the context of limited resources. DD methods can be used to target and obtain specific viewpoints from diverse populations, such as the informed perspectives of minority and underrepresented populations within the VHA.19 For example, female veterans were oversampled to ensure that the informed preferences of this population was obtained. Thus, DD methods could provide a valuable tool for health systems to elicit in-depth diverse patient input on high-profile policies that will have a substantial impact on the system’s patient population.
Limitations
One potential downside of DD is that, because of the resource-intensive nature of deliberation sessions, they are often conducted with relatively small groups.9 Viewpoints of those within these small samples who are willing to spend an entire day discussing a complex topic may not be representative of the larger patient community. However, the core goal of DD is diversity of opinions rather than representativeness.
A stratified random sampling strategy that oversampled for underrepresented and minority populations was used to help select a diverse group that represents the population on key characteristics and partially addresses concern about representativeness. Efforts to optimize participation rates, including providing monetary incentives, also are helpful and have led to high participation rates in past deliberations.7
Health system communication strategies that promote the importance of becoming involved in DD sessions also may be helpful in improving rates of recruitment. On particularly important topics where health system leaders feel a larger resource investment is justified, conducting larger scale deliberations with many small groups may obtain more generalizable evidence about what individual patients and groups of patients recommend.7 However, due to the inherent limitations of surveys and focus group approaches for obtaining informed views on complex topics, there are no clear systematic alternatives to the DD approach.
Conclusion
DD is an effective method to meaningfully engage patients in deep deliberations to guide complex policy making. Although design of deliberative sessions is resource-intensive, patient engagement efforts, such as those described in this paper, could be an important aspect of a well-functioning learning health system. Further research into alternative, streamlined methods that can also engage veterans more deeply is needed. DD also can be combined with other approaches to broaden and confirm findings, including focus groups, town hall meetings, or surveys.
Although this study did not provide consensus on how the VHA should allocate funds with respect to the Choice Act, it did provide insight into the importance and feasibility of engaging veterans in the policy-making process. As more policies aimed at improving veterans’ access to civilian care are created, such as the most recent Mission Act, policy makers should strongly consider using the DD method of obtaining informed veteran input into future policy decisions.
Acknowledgments
Funding was provided by the US Department of Veterans Affairs Office of Analytics and Business Intelligence (OABI) and the VA Quality Enhancement Research Initiative (QUERI). Dr. Caverly was supported in part by a VA Career Development Award (CDA 16-151). Dr. Krein is supported by a VA Health Services Research and Development Research Career Scientist Award (RCS 11-222). The authors thank the veterans who participated in this work. They also thank Caitlin Reardon and Natalya Wawrin for their assistance in organizing the deliberation session.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
1. VA Office of the Inspector General. Veterans Health Administration. Interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix Health Care System. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf. Published May 28, 2014. Accessed December 9, 2019.
2. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
3. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
4. Thorpe JM, Thorpe CT, Schleiden L, et al. Association between dual use of Department of Veterans Affairs and Medicare Part D drug benefits and potentially unsafe prescribing. JAMA Intern Med. 2019; July 22. [Epub ahead of print.]
5. Moyo P, Zhao X, Thorpe CT, et al. Dual receipt of prescription opioids from the Department of Veterans Affairs and Medicare Part D and prescription opioid overdose death among veterans: a nested case-control study. Ann Intern Med. 2019;170(7):433-442.
6. Meyer LJ, Clancy CM. Care fragmentation and prescription opioids. Ann Intern Med. 2019;170(7):497-498.
7. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
8. Street J, Duszynski K, Krawczyk S, Braunack-Mayer A. The use of citizens’ juries in health policy decision-making: a systematic review. Soc Sci Med. 2014;109:1-9.
9. Paul C, Nicholls R, Priest P, McGee R. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85(3):314-320.
10. Martin D, Abelson J, Singer P. Participation in health care priority-setting through the eyes of the participants. J Health Serv Res Pol. 2002;7(4):222-229.
11. Mort M, Finch T. Principles for telemedicine and telecare: the perspective of a citizens’ panel. J Telemed Telecare. 2005;11(suppl 1):66-68.
12. Kass N, Faden R, Fabi RE, et al. Alternative consent models for comparative effectiveness studies: views of patients from two institutions. AJOB Empir Bioeth. 2016;7(2):92-105.
13. Carman KL, Mallery C, Maurer M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Soc Sci Med. 2015;133:11-20.
14. Carman KL, Maurer M, Mangrum R, et al. Understanding an informed public’s views on the role of evidence in making health care decisions. Health Aff (Millwood). 2016;35(4):566-574.
15. Kim SYH, Wall IF, Stanczyk A, De Vries R. Assessing the public’s views in research ethics controversies: deliberative democracy and bioethics as natural allies, J Empir Res Hum Res Ethics. 2009;4(4):3-16.
16. Gastil J, Levine P, eds. The Deliberative Democracy Handbook: Strategies for Effective Civic Engagement in the Twenty-First Century. San Francisco, CA: Jossey-Bass; 2005.
17. Dryzek JS, Bächtiger A, Chambers S, et al. The crisis of democracy and the science of deliberation. Science. 2019;363(6432):1144-1146.
18. Blacksher E, Diebel A, Forest PG, Goold SD, Abelson J. What is public deliberation? Hastings Cent Rep. 2012;4(2):14-17.
19. Wang G, Gold M, Siegel J, et al. Deliberation: obtaining informed input from a diverse public. J Health Care Poor Underserved. 2015;26(1):223-242.
20. Simon RL, ed. The Blackwell Guide to Social and Political Philosophy. Malden, MA: Wiley-Blackwell; 2002.
21. Stanford University, Center for Deliberative Democracy. Deliberative polling on energy and environmental policy options in Japan. https://cdd.stanford.edu/2012/deliberative-polling-on-energy-and-environmental-policy-options-in-japan. Published August 12, 2012. Accessed December 9, 2019.
22. Damschroder LJ, Pritts JL, Neblo MA, Kalarickal RJ, Creswell JW, Hayward RA. Patients, privacy and trust: patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64(1):223-235.
23. Carman KL, Maurer M, Mallery C, et al. Community forum deliberative methods demonstration: evaluating effectiveness and eliciting public views on use of evidence. Final report. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/deliberative-methods_research-2013-1.pdf. Published November 2014. Accessed December 9, 2019.
24. Sunstein CR, Hastie R. Wiser: Getting Beyond Groupthink to Make Groups Smarter. Boston, MA: Harvard Business Review Press; 2014.
25. Damschroder LJ, Kim SY. Assessing the quality of democratic deliberation: a case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70(12):1896-1903.
26. Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd ed. Thousand Oaks: SAGE Publications, Inc; 1994.
27. US Department of Veterans Affairs. Veteran community care – general information. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/VHA-FS_MISSION-Act.pdf. Published September 9 2019. Accessed December 9, 2019.
Military Health Care at a Crossroads
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
The certainty that federal health care will be different, and the equal uncertainty about when and how the systems will evolve, were major topics at the recent AMSUS annual meeting. The Veterans Health Administration (VHA) and Military Health System (MHS) are in the midst of major transformations, although they are at very different points in the process and the final outcomes are yet unknown. This editorial, written at the end of 2019, will review some of the highlights of a discussion that is sure to continue in 2020 and beyond.
Almost everyone in the VA and many of the public can pinpoint the exact place (and time) the VHA’s upheaval began: Phoenix, Arizona, in 2014. “The attack on our system,” as VHA Executive in Charge Richard A. Stone, MD, described it at AMSUS, happened because “we were just too slow a bureaucracy,” he explained.1 “We can debate how many veterans died while waiting for care, but the answer is that 1 was too many and it had to be fixed. We had to become a more agile organization.”
The US Department of Veterans Affairs (VA) response to the media firestorm and congressional outrage was uncharacteristically swift and sweeping. Both the VA Secretary and Deputy Under Secretary of Health were removed, as were many others in leadership at Phoenix and elsewhere. The VA faced an existential crisis as many loud voices called for dismantling the entire system in the wake of its perceived inability or unwillingness to care for those it was legally mandated to serve.2 The Veterans’ Access to Care through Choice, Accountability, and Transparency Act of 2014 and its successor the VA Mission Act of 2018 dramatically expanded veterans’ access to covered health care from non-VA health care providers (HCPs).
Debate continues in the veteran community and the wider society about whether this expansion constitutes an abandonment of a health care system dedicated to veterans and their unique health problems or a commitment to deliver the most efficient and high-quality care to veterans that can be obtained.3-5 Many see this as a crossroads for the VA. Still, even if the VA will continue to exist, the question remains: in what form?
The increased use of private sector HCPs has wrought significant and long-lasting modifications to the traditional VA organization. In fiscal year (FY) 2017, the VA paid for care that non-VA HCPs provided for 24% of patients.6 Veterans with higher service-connected disability ratings and aged > 65 years were more likely to rely on the VA for care than were less disabled and younger patients.6 The Mission Act is expected to increase the VA expenditures by nearly $19 billion between FY 2019 and FY 2023, with the bulk of the patients still going to the VHA for their care.6 Stakeholders from unions to politicians are concerned that every dollar spent on community care is one less they can spend in VA institutions. It is unclear to what degree this concern will be actualized, as smaller hospitals and those in rural areas have always had contact with the private sector to obtain the specialty care veterans needed that the VA could not provide.
Compounding these trends is the VA’s ongoing staffing challenges. To meet the demand and eliminate wait times between September 2014 and September 2018, the VHA grew its workforce by > 40,000 individuals, a 13% growth rate. In FY 2019 alone, the VHA hired 28,000 new employees. And yet despite the rapid growth, a lower than average turnover rate, and relatively high employee satisfaction measures (at least when compared with those of other federal employees), the VHA still has 43,000 vacancies.7,8
Which brings us to the very different set of challenges facing the Defense Health Agency (DHA). In an era of ballooning military budgets the DHA is being asked to “transform the MHS into an integrated readiness and health system, eliminate redundancies, and create a common high-quality experience for our beneficiaries.”9 The seeds of change were tucked into the National Defense Authorization Act (NDAA) of 2017, and their ramifications are only now becoming apparent. Among the most consequential of these changes are transfer of the management of hundreds of MHS hospitals and clinics from the medical services of the Army, Navy, and Air Force to the DHA.
“If we don’t shape our future, others will step in and do it for us,” Tom McCaffrey, Assistant Secretary of Defense for Health Affairs explained at AMSUS.10 In October 2019, DoD transitioned the first group of facilities to the DHA, and the remainder will change management by the end of 2022. In the next step of the process, facilities will be combined—along with TRICARE providers—in 21 geographically based “markets” to streamline management and avoid “redundancies.”
Lost in the bland language, though, is the scale of the contemplated changes. Although the exact shape of the changes have not been finalized, up to 18,000 MHS health care providers—civilian or uniformed—may be eliminated as DHA relies more heavily on TRICARE providers.11 Not even the future of the Uniformed Service University for the Health Sciences and its leadership training and health care research are guaranteed.12 The ominous possibility that the nation could lose its only military medical school has raised alarm among medical educators. They fear that the country may sacrifice its ability to train physicians with the highly skilled specialities needed on the battlefield and the familiarity with military culture that enables doctors in uniform to relate to the problems of active-duty families and retired service members.12VHA and MHS colleagues are undergoing a similar organizational transition with all the trepidation and expectation that accompanies the turning of an enormous ship in stormy seas. In the midst of these major institutional transformations, VHA and MHS need to band together if the unique specialty of military and VA medicine is to survive. Unless these unprecedented changes can establish a new spirit of solidarity to 2 often separate partners in one mission to care for those who serve, we may well be asking in the next few years, “Where have all the federal practitioners gone?”
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
1. Stone R. Plenary session. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
2. Lane C. Why don’t we just abolish the VA? Washington Post. April 22, 2015. https://www.washingtonpost.com/opinions/caring-for-veterans-is-our-national-responsibility/2015/04/22/ae61eb88-e929-11e4-aae1-d642717d8afa_story.html. Accessed December 18, 2019.
3. Lemle RB. Choice Program expansion jeopardizes high-quality VHA mental health services. Fed Pract. 2018;35(3):18-24.
4. Shulkin D. Implications for Veterans’ health care: the danger becomes clearer. JAMA Intern Med. 2019;10.1001/jamainternmed.2019.2996. [Published online ahead of print, 2019 Jul 22.]
5. Kullgren JT, Fagerlin A, Kerr EA. Completing the MISSION: a blueprint for helping veterans make the most of new choices. J Gen Intern Med. 2019;10.1007/s11606-019-05404-w. [Published online ahead of print, 2019 Oct 24.]
6. Statement of Merideth Randles, FSA, MAAA Principal and Consulting Actuary, Milliman, Inc. For Presentation Before the Senate Committee on Veterans’ Affairs. VA Mission Act: Implementing the Veterans Community Care Program. https://www.veterans.senate.gov/imo/media/doc/04.10.19%20Milliman%20Testimony.pdf. Submitted April 10, 2019. Accessed December 18, 2019.
7. Sitterly DR. Statement of Daniel R. Sitterly, Assistant Secretary, Office of Human Resources and Administration/Operations Security, and Preparedness, on behalf of U.S. Department of Veterans Affairs Before the House Committee on Veterans Affairs, September 18, 2019. https://docs.house.gov/meetings/VR/VR00/20190918/109925/HHRG-116-VR00-Wstate-SitterlyD-20190918.pdf. Published September 18, 2019. Accessed December 22, 2019.
8. US Office of Personnel Management, FedScope. Federal workforce data. https://www.fedscope.opm.gov. Accessed December 22, 2019.
9. US Department of Defense. Defense Health Program Fiscal Year (FY) 2020 President’s Budget Operation and Maintenance Introductory Statement. https://comptroller.defense.gov/Portals/45/Documents/defbudget/fy2020/budget_justification/pdfs/09_Defense_Health_Program/Vol_I_Sec_1_PBA-19_Introductory_Statement_DHP_PB20.pdf. Accessed December 23, 2019.
10. McCaffery T. MHS vision. Presented at: AMSUS Annual Meeting; December 2019; National Harbor, MD.
11. Sternberg S. Military Health System in the crosshairs. https://www.usnews.com/news/health-news/articles/2019-12-11/military-health-system-in-the-crosshairs. Published December 11, 2019. Accessed December 23, 2019.
12. Novak D. Officials warn Pentagon cuts could force closing of Bethesda military medical university. https://cnsmaryland.org/2019/11/20/officials-warn-pentagon-cuts-could-force-closing-of-bethesda-military-medical-university. Published November 20, 2019. Accessed December 23, 2019.
HHS drug importation proposals aim to address high costs
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.
The Department of Health & Human Services is taking the first steps in allowing drugs to be imported into the United States.
HHS proposes to offer two different pathways for importation: One allowing states to design programs to import certain drugs directly from Canada and another allowing manufacturers to obtain a new National Drug Code (NDC) number to import their own Food and Drug Administration–approved products manufactured outside of the United States.
“The importation proposals we are rolling out ... are a historic step forward in efforts to bring down drug prices and out-of-pocket costs,” HHS Secretary Alex Azar said during a Dec. 17, 2019, press conference. “New pathways for importation can move us toward a more open and competitive marketplace that supplies American patients with safe, effective, affordable prescription drugs.”
The proposals were made public on Dec. 18, the day the House Rules committee was scheduled to vote on impeaching President Trump.
He emphasized that these proposals “are both important steps in advancing the FDA’s safe-importation action plan, [which] aims to insure that importation is done in a way that prioritizes safety and includes elements to help insure importation does not put patients or the U.S. drug supply chain at risk.”
The pathway for states to import drugs from Canada will be proposed through the federal regulatory process. The notice of proposed rulemaking, which implements authority for FDA regulation of importation granted in the Medicare Modernization Act of 2003, will outline a process by which states, potentially working with wholesalers and/or pharmacies, will submit proposals for FDA review and approval on how they would implement an importation program.
Only certain drugs would be eligible for importation from Canada under this proposal. The drugs would need to be approved in Canada and, except for Canadian labeling, need to meet the conditions of an FDA-approved new drug application or abbreviated new drug application.
Controlled substances, biologics, intravenously injected drugs, drugs with a risk evaluation and management strategy, and drugs injected into the spinal column or eye would be excluded from importation.
Drugs coming in from Canada would be relabeled with U.S.-approved labels and would be subject to testing to ensure they are authentic, not degraded, and compliant with U.S. standards.
States would be required to show that importing drugs poses no additional risk in public health and safety and it would result in the reduction of costs, according to information provided by HHS.
Many of the most expensive drugs, as well as insulins, would not be eligible for importation under this pathway, Mr. Azar acknowledged, adding that “I would envision that as we demonstrate the safety as well as the cost savings from this pathway, [this could serve as] a pilot and a proof of concept that Congress could then look to and potentially take up for more complex molecules that involve cold-chain storage and more complex distribution channels.”
The proposed regulations do not offer any estimates on how much savings could be achieved. He said that there is no way to estimate which states might develop importation plans and how those plans might work.
The second proposed pathway would involve FDA guidance to manufacturers allowing them to import their own FDA-approved products manufactured abroad. Under this proposal, there would be no restriction on which type or kind of FDA-approved product to be imported.
“The FDA has become aware that manufacturers of some brand-name drugs want to offer their drugs at lower costs in the U.S. market but, due to certain challenges in the private market, are not readily [able] to do so without obtaining a different national drug code for their drugs,” Adm. Brett Giroir, MD, HHS assistant secretary for health, said during the press conference.
Obtaining a separate NDC for imported drugs could address the challenges, particularly those posed by the incentives to raise list prices and offer higher rebates to pharmacy benefit managers, Mr. Azar said.
The draft guidance outlines procedures manufacturers could follow to get that NDC for those products and how manufacturers can demonstrate that these products meet U.S. regulatory standards. Products imported in this pathway could be made available to patients in hospitals, physician offices, and pharmacies. Generic drugs are not part of this guidance, but the proposed guidance asked for feedback on whether a similar approach is needed for generic products.
“This would potentially allow for the sale of these drugs at lower prices than currently offered to American consumers, giving drugmakers new flexibility to reduce list prices,” Mr. Azar said.
The proposed regulation on state-level importation will have a 75-day comment period from the day it is published in the Federal Register, and Mr. Azar said that the FDA is committing resources to getting the comments analyzed and reflected in the final rule.
“We will be moving as quickly as we possibly can,” Mr. Azar said, adding that the FDA guidance to manufacturers may move more quickly through its approval process because it is not a formal rule.