User login
Eosinophil-guided therapy reduces corticosteroid use in COPD
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
in terms of the number of days out of hospital and alive, new research has found.
Writing in the Lancet Respiratory Medicine, researchers reported the outcomes of a multicenter, controlled, open-label trial comparing eosinophil-guided and standard therapy with systemic corticosteroids in 318 patients with COPD.
Pradeesh Sivapalan, MD, of the respiratory medicine section of Herlev and Gentofte Hospital at the University of Copenhagen, and coauthors wrote that eosinophilic inflammation had been seen in 20%-40% of patients with acute exacerbations of COPD. Patients with higher eosinophilic blood counts were at increased risk of acute exacerbations but were also more likely to benefit from corticosteroid treatment.
In the eosinophil-guided therapy arm of the study, 159 patients received 80 mg of intravenous methylprednisolone on day 1, then from the second day were treated with 37.5 mg of prednisolone oral tablet daily – up to 4 days – only on days when their blood eosinophil count was at least 0.3 x 10⁹ cells/L. In the control arm, 159 patients also received 80 mg of intravenous methylprednisolone on day 1, followed by 37.5 mg of prednisolone tablets daily for 4 days.
After 14 days, there were no significant differences between the two groups for mean days alive and out of hospital.
There were 12 more cases of readmission with COPD, including three fatalities, in the eosinophil-guided group within the first month. However the authors said these differences were not statistically significant, but “because the study was not powered to detect differences in this absolute risk range, we cannot rule out that this was an actual harm effect from the interventional strategy.”
The eosinophil-guided therapy group did show more than a 50% reduction in the median duration of systemic corticosteroid therapy, which was 2 days in the eosinophil-guided group, compared with 5 days in the control group (P less than .0001), and the differences between the two groups remained significant at days 30 and 90.
“The tested strategy was successful in reducing the exposure to systemic corticosteroids, but we cannot exclude the possibility that a more aggressive algorithm, such as a single dose of systemic corticosteroid, might have been more effective,” the authors wrote.
At the 90-day follow-up, there were no differences in the number of infections requiring antibiotic treatment, nor in dyspepsia, ulcer complications, or initiation of new proton-pump inhibitor treatment.
The study was supported by the Danish Regions Medical Fund and the Danish Council for Independent Research. Two authors declared personal fees from pharmaceutical companies outside the submitted work. No other conflicts were declared.
SOURCE: Sivapalan P et al. Lancet Respir Med. 2019, May 20. doi: 10.1016/S2213-2600(19)30176-6.
FROM LANCET RESPIRATORY MEDICINE
FDA invites sample submission for FDA-ARGOS database
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
which seeks to support research and regulatory decisions regarding DNA testing for pathogens with quality-controlled and curated genomic sequence data. Such testing and devices could be used as medical countermeasures against biothreats such as Ebola and Zika.
Infectious disease next-generation sequencing could use DNA analysis to help identify pathogens – from viruses to parasites – faster and more efficiently by, in theory, accomplishing with one test what was only possible before with many, according to the FDA. In order to not only further development of such tests and devices but also aid regulatory and scientific review of them, the FDA has collaborated with the Department of Defense, the National Center for Biotechnology Information, and Institute for Genome Sciences at the University of Maryland, Baltimore, to create FDA-ARGOS.
However, the FDA and its collaborators need samples of pathogens to continue developing the database, so they’ve invited health care professionals to submit samples for that purpose. More information, including preferred organism list and submission guidelines, can be found on the FDA-ARGOS website.
Reducing adverse drug reactions
Easing the inpatient/outpatient transition
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Easing the inpatient/outpatient transition
Easing the inpatient/outpatient transition
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Adverse drug reactions are a problem hospitalists encounter often. An estimated 9% of hospital admissions in older adults are the result of adverse drug reactions, and up to one in five adults experience an adverse drug reaction during hospitalization.
“Many interventions have been tried to solve this problem, and certain of them have worked, but to date we don’t have any great solutions that meaningfully impact the rate of these events in a way that’s feasible in most health care environments, so any efforts to reduce the burden of these problems in older adults could be hugely beneficial,” said Michael Steinman, MD, author of an editorial highlighting a new approach.
His editorial in BMJ Quality & Safety cites research on the Pharm2Pharm program, implemented in six Hawaiian hospitals, in which hospital-based pharmacists identified inpatients at high risk of medication misadventures with criteria such as use of multiple medications, presence of high-risk medications such as warfarin or glucose-lowering drugs, and a history of previous acute care use resulting from medication-related problems. The hospital pharmacist would then meet with the patient to reconcile medications and facilitate a coordinated hand-off to a community pharmacist, who would meet with the patient after discharge.
In addition to a 36% reduction in the rate of medication-related hospitalizations, the intervention generated an estimated savings of $6.6 million per year in avoided hospitalizations.
There are two major takeaways, said Dr. Steinman, who is based in the division of geriatrics at the University of California, San Francisco: It’s critical to focus on transitions and coordination between inpatient and outpatient care to address medication-related problems, and pharmacists can be extremely helpful in that.
“Decisions about drug therapy in the hospital may seem reasonable in the short term but often won’t stick in the long term unless there is a coordinated care that can help ensure appropriate follow-through once patients return home,” Dr. Steinman said. “The study that the editorial references is a systems intervention that hospitalists can advocate for in their own institutions, but in the immediate day-to-day, trying to ensure solid coordination of medication management from the inpatient to outpatient setting is likely to be very helpful for their patients.”
The long-term outcomes of hospitalized patients are largely influenced by getting them set up with appropriate community resources and supports once they leave the hospital, he added, and the hospital can play a critical role in putting these pieces into place.
Reference
1. Steinman MA. Reducing hospital admissions for adverse drug events through coordinated pharmacist care: learning from Hawai’i without a field trip. BMJ Qual Saf. Epub 2018 Nov 24. doi: 10.1136/bmjqs-2018-008815. Accessed Dec. 11, 2018.
Reducing pediatric RSV burden is top priority
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.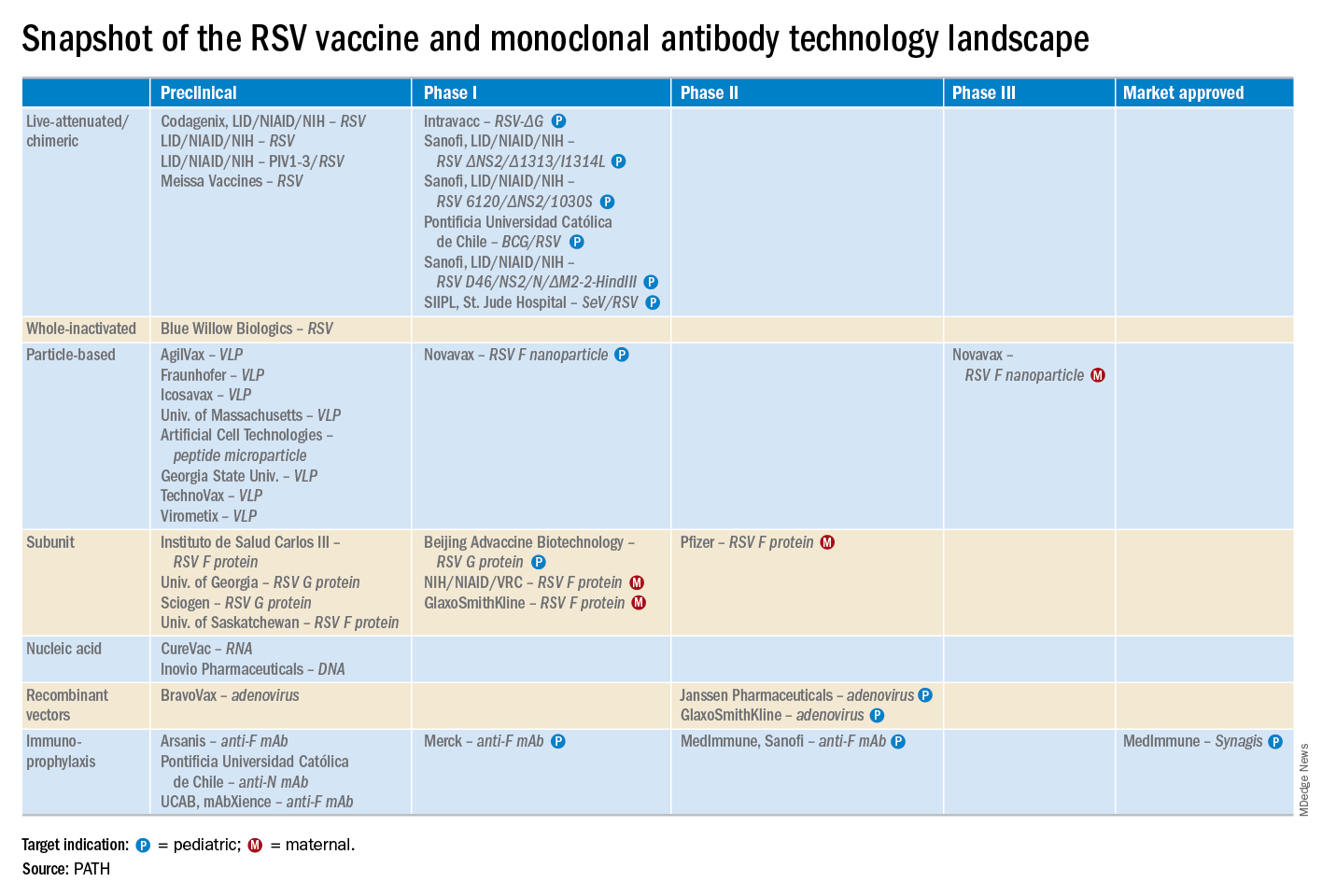
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
LJUBLJANA, SLOVENIA – Prevention or early effective treatment of respiratory syncytial virus (RSV) infection in infants and small children holds the promise of sharply reduced burdens of both acute otitis media (AOM) and pneumonia, Terho Heikkinen, MD, PhD, predicted in the Bill Marshall Award Lecture presented at the annual meeting of the European Society for Paediatric Infectious Diseases (ESPID).
RSV is by far the hottest virus in the world,” declared Dr. Heikkinen, professor of pediatrics at the University of Turku (Finland).
“A lot of progress is being made with respect to RSV. This increased understanding holds great promise for new interventions,” he explained. “Lots of different types of vaccines are being developed, monoclonal antibodies, antivirals. So
Today influenza is the only respiratory viral infection that’s preventable via vaccine or effectively treatable using antiviral drugs. That situation has to change, as Dr. Heikkinen demonstrated early in his career; RSV is the respiratory virus that’s most likely to invade the middle ear during AOM. It’s much more ototropic than influenza, parainfluenza, enteroviruses, or adenoviruses (N Engl J Med. 1999 Jan 28;340[4]:260-4), he noted.
The Bill Marshall Award and Lecture, ESPID’s most prestigious award, is given annually to an individual recognized as having significantly advanced the field of pediatric infectious diseases. Dr. Heikkinen was singled out for his decades of work establishing that viruses, including RSV, play a key role in AOM, which had traditionally been regarded as a bacterial infection. He and his coinvestigators demonstrated that in about two-thirds of cases, AOM is actually caused by a combination of bacteria and viruses, which explains why patients’ clinical response to antibiotic therapy for AOM often is poor. They also described the chain of events whereby viral infection of the upper airway epithelium triggers an inflammatory response in the nasopharynx, with resultant Eustachian tube dysfunction and negative middle ear pressure, which in turn encourages microbial invasion of the middle ear. Moreover, they showed that the peak incidence of AOM isn’t on day 1 after onset of upper respiratory infection symptoms, but on day 3 or 4.
“What this tells us is that, once a child has a viral respiratory infection, there is a certain window of opportunity to try to prevent the development of the complication if we have the right tools in place,” Dr. Heikkinen said.
He and his colleagues put this lesson to good use nearly a decade ago in a randomized, double-blind trial in which they showed that giving oseltamivir (Tamiflu) within 12 hours after onset of influenza symptoms in children aged 1-3 years reduced the subsequent incidence of AOM by 85%, compared with placebo (Clin Infect Dis. 2010 Oct 15;51[8]:887-94).
These observations paved the way for the ongoing intensive research effort exploring ways of preventing AOM through interventions at two different levels: by developing viral vaccines to prevent a healthy child from contracting the viral upper respiratory infection that precedes AOM and by coming up with antiviral drugs or bacterial vaccines to prevent a upper respiratory infection from evolving into AOM.
The same applies to pneumonia. Other investigators showed years ago that both respiratory viruses and bacteria were present in two-thirds of sputum samples obtained from children with community-acquired pneumonia (Clin Microbiol Infect. 2012 Mar;18[3]:300-7).
RSV is the top cause of hospitalization for acute respiratory infection – pneumonia and bronchiolitis – in infants. Worldwide, it’s estimated that RSV accounts for more than 33 million episodes of pneumonia annually, with 3.2 million hospitalizations and 118,200 deaths.
Beyond the hospital, however, Dr. Heikkinen and colleagues conducted a prospective cohort study in Turku over the course of two consecutive respiratory infection seasons in which they captured the huge burden of RSV as an outpatient illness. It hit hardest in children younger than 3 years, in whom the average annual incidence of RSV infection was 275 cases per 1,000 children. In that youngest age population, RSV upper respiratory infection was followed by AOM 58% of the time, with antibiotics prescribed in 66% of the cases of this complication of RSV illness. The mean duration of RSV illness was greatest in this young age group, at 13 days, and it was associated with parental absenteeism from work at a rate of 136 days per 100 children with RSV illness.
Moreover, while AOM occurred less frequently in children aged 3-6 years, 46% of the cases were attributed to a preceding RSV infection, which led to antibiotic treatment nearly half of the time (J Infect Dis. 2017 Jan 1;215[1]:17-23). This documentation has spurred further efforts to develop RSV vaccines and antivirals.
He reported serving as a consultant to a half-dozen pharmaceutical companies, as well as having received research funding from Janssen, GlaxoSmithKline, and Novavax.
EXPERT ANALYSIS FROM ESPID 2019
Fewer antibiotics prescribed with PCR than conventional stool testing
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
SAN DIEGO – However, antibiotics were still prescribed for more than one in three patients tested by any method.
“A positive test by any modality did result in decreased utilization of endoscopy, radiology, and antibiotic prescribing, but this effect appeared to be much greater for the GI PCR assay,” said Jordan Axelrad, MD, speaking at the annual Digestive Disease Week.
“Overall, patients who received GI PCR were 12% less likely to undergo endoscopy, 7% less likely to undergo abdominal radiography, and 11% less likely to be prescribed any antibiotic,” compared with patients who were tested by conventional stool culture, said Dr. Axelrad, a gastroenterologist at New York University.
In a cross-sectional study, Dr. Axelrad and his coauthors looked at patients who underwent stool testing for the 26 months before (n = 5,986) and after (n = 9,402) March 2015, when Dr. Axelrad’s home institution switched from conventional stool culture to the GI PCR panel. For the earlier time period, the investigators included patients who received stool culture both with and without an ova and parasites exam, as well as those who underwent enzyme-linked immunosorbent assay viral testing for rotavirus and adenovirus.
Patient demographic data were included as study variables; additionally, the study tracked utilization of endoscopy, abdominal, or other radiology studies, and ED visits for 30 days after testing. They also included any antibiotic prescribing within the 14 days post testing.
Roughly one-third of patients were tested as outpatients, 1 in 10 in the ED, and the remainder as inpatients. Patient age was a mean 46.7 years for the culture group, and 45.5 years for the GI PCR group.
The multiplex PCR test used in the study tested for 12 gastrointestinal pathogenic bacteria, 4 parasites, and 5 viruses.
As expected, PCR testing yielded a higher positive test rate than conventional stool testing, even when EIA tests were included (29.2% vs. 4.1%). In the 2,746 patients with a positive GI PCR test, a total of 3,804 pathogens were identified. Adenovirus accounted for 39% of these positive results. Positive bacterial results were seen in about 65.0% of the positive subgroup, with Escherichia coli subtypes seen in 51.7% of the positive tests.
Overall, positive results for viruses, bacteria, and multiple pathogens were more likely with GI PCR testing, compared with conventional testing (P = .001 for all). Parasites accounted for only 8.2% of the positive PCR test results, but this was significantly more than the 3.7% seen with conventional testing (P = .011).
At the 14-day mark post testing, “Patients who underwent a GI panel were less likely to be prescribed any antibiotic. But overall, antibiotics were fairly common in both groups,” said Dr. Axelrad, noting that 41% of patients who underwent stool culture received an antibiotic by 14 days, compared with 36% for patients who underwent a GI PCR panel (P = .001).
By the end of 30 days, most patients in each group had not received an endoscopic procedure, with significantly more procedure-free patients in the PCR group (91.6% vs. 90.4%; P = .008).
Against a backdrop of slightly higher overall radiology utilization in the PCR group – potentially attributable to practice trends over time – abdominal radiology was less likely for these patients than for the culture group (11.4% vs. 12.8%; P = .011).
The 30-day ED visit rate was low and similar between groups (11.4% for PCR vs. 12.8% for culture; P = .116).
The much quicker turnaround for the GI PCR panel didn’t translate into a shorter length of stay, though: Inpatient length of stay was a median 5 days in both groups.
“We feel that the outcomes that we noted were likely due to the increased sensitivity and specificity” of the PCR-based testing, said Dr. Axelrad. “Obviously, if you have more pathogen-positive findings, you may be less likely to order extensive testing. And if you’ve identified something like norovirus, you may feel reassured, and not order further testing.”
Dr. Axelrad pointed out that his institution’s overall PCR positivity rates were lower than the 70% rates some other studies have reported. “We feel that, given our large sample size, our results may more accurately reflect clinical practice, and perhaps that lower positivity rate may reflect increased use of this test in an inpatient setting,” he said. “We’re looking at that.”
Study limitations included the retrospective nature of the study. “Also, as we all know, PCR testing fails to discriminate between active infection and asymptomatic colonization,” raising questions about whether a positive PCR test really indicates true infection, noted Dr. Axelrad.
“Coupled with a high-sensitivity rapid turnaround, there’s the potential to reduce costs, but the cost-effectiveness of these assays has not been fully determined. There are several studies looking at this,” with results still to come, he said.
The notable reduction in antibiotic prescribing for those patients who received PCR-based testing means that GI PCR panels could be a useful tool to promote antibiotic stewardship, though Dr. Axelrad also noted that “antibiotics were still used in about a third of all patients.”
Dr. Axelrad reported no outside sources of funding. He has performed consulting services for and received research funding from BioFire, which manufactured the GI PCR assay used in the study, but BioFire did not fund this research.
SOURCE: Axelrad J et al. DDW 2019, Presentation 978.
REPORTING FROM DDW 2019
Opioid prescriptions declined 33% over 5 years
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
Fewer opioid retail prescriptions are being filled, according to a new report issued by the American Medical Association Opioid Task Force.
Opioid prescribing declined by 33% over a 5-year period based on the total number of opioid retail prescriptions filled. Total prescriptions declined from 251.8 million in 2013 to 168.8 million in 2018, according to the report.
The numbers come as the most recent data from the Centers for Disease Control and Prevention show a leveling of deaths involving prescription opioids. The CDC data were most recently updated in January 2019 and cover the period 1999-2017.
A closer look shows that deaths involving prescription opioids, but not other synthetic narcotics, peaked in 2011 and have generally declined since then. Deaths involving other synthetic narcotics, however, have been rising, offsetting the reduction and keeping the total number of deaths involving opioids relatively stable between 2016 and 2017.
Other data released by the AMA Opioid Task Force show that physicians are increasing their use of state-level prescription drug monitoring programs (PDMPs).
In 2017, there were 1.5 million physicians registered to use state PDMPs. That number rose to 1.97 million in 2019. And the physicians are using PDMPs. In 2018, physicians made 460 million PDMP queries, up 56% from 2017 and up 651% from 2014.
More education about opioid prescribing is being sought, with 700,000 physicians completing CME training and accessing other training related to opioid prescribing, pain management, screening for substance use disorders, and other related topics.
While the report does show positive trends, the task force is calling for more action, including more access to naloxone and better access to mental health treatment.
The report notes that more than 66,000 physicians and other health professionals have a federal waiver to prescribe buprenorphine, up more than 28,000 since 2016.
A number of policy recommendations are made in the report, including removing inappropriate administrative burdens or barriers that delay access to medications used in medication-assisted treatment (MAT); removing barriers to comprehensive pain care and rehabilitation programs, and reforming the civil and criminal justice system to help ensure access to high-quality, evidence-based care for opioid use disorder.
“We are at a crossroads in our nation’s efforts to end the opioid epidemic,” AMA Opioid Task Force Chair Patrice A. Harris, MD, stated in the report. “It is time to end delays and barriers to medication-assisted treatment – evidence based care proven to save lives; time for payers, [pharmacy benefit managers] and pharmacy chains to reevaluate and revise policies that restrict opioid therapy to patients based on arbitrary thresholds; and time to commit to helping all patients access evidence-based care for pain and substance use disorders.”
Dr. Harris continued: “Physicians must continue to demonstrate leadership, but unless these actions occur, the progress we are making will not stop patients from dying.”
New help for peanut allergies
Breakthrough therapy holds potential
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.
Breakthrough therapy holds potential
Breakthrough therapy holds potential
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.
When it comes to anaphylaxis episodes leading to pediatric intensive care–unit stays, peanuts are the most common culprit. Now the results of a recent clinical trial may lead to approval of the first oral medication to ameliorate reactions in children with severe peanut allergies.
After 6 months of treatment and 6 months of maintenance therapy, two-thirds of the 372 children who received this treatment could ingest the equivalent of two peanuts without allergic symptoms. Just 4% of the 124 children given a placebo powder were able to consume that amount of peanut without reacting. The treatment was not effective for the small number of adults in the study.
This trial of the drug, called AR101 and developed by Aimmune Therapeutics, was published in Nov. 2018 in the New England Journal of Medicine. The company has submitted a biologics license application to the U.S. Food and Drug Administration, and because the drug has been designated a breakthrough therapy, it will go through an accelerated approval process. It could be on the market by the end of 2019.
Reference
1. Rabin RC. New Peanut Allergy Drug Shows ‘Lifesaving’ Potential. New York Times. Nov. 18, 2018. https://www.nytimes.com/2018/11/18/well/live/new-peanut-allergy-drug-shows-lifesaving-potential.html. Accessed Nov. 26, 2018.
Lipoprotein(a) levels can guide CV risk assessment and treatment
Lipoprotein(a) is an independent risk factor for atherosclerotic cardiovascular disease–related events, and plasma levels of Lp(a) could help refine risk assessment and influence treatment decisions, say the authors of a scientific statement from the National Lipid Association.
Don P. Wilson, MD, of Cook Children’s Medical Center, Fort Worth, Tex., and coauthors reviewed the evidence around testing of Lp(a) in clinical practice and its use in guiding treatment for both primary and secondary prevention. Their report is in the Journal of Clinical Lipidology.
Prospective, population-based studies point to a clear link between high Lp(a) levels and high risk of myocardial infarction, coronary heart disease, coronary artery stenosis, carotid stenosis, valvular aortic stenosis, ischemic stroke, cardiovascular mortality, and all-cause mortality, the authors wrote. This association was independent of the effect of other risk factors, including LDL cholesterol.
However, existing Lp(a) assays have not been globally standardized, and there is only incomplete evidence for age, sex, or ethnicity-specific cutoff points for high risk.
The authors suggested Lp(a) levels greater than 50 mg/dL (100 nmol/L) could be considered a risk factor that justifies the initiation of statin therapy. However ,they pointed out this level corresponded to the 80th population percentile in predominantly white populations, while in African American populations the equivalent cutoff was around 150 nmol/L.
On the issue of whom to test for Lp(a) serum levels, the authors said testing could reasonably be used to refine risk assessment for atherosclerotic cardiovascular disease in adults with first-degree relatives who experienced premature atherosclerotic cardiovascular disease, those with a personal history of the disease, or in those with severe hypercholesterolemia or suspected familial hypercholesterolemia.
However, statin therapy does not decrease Lp(a) levels, and there is also evidence that patients with high Lp(a) levels may not show as much LDL-C lowering in response to statin therapy.
“There is a lack of current evidence demonstrating that lowering Lp(a), independently of LDL-C, reduces ASCVD events in individuals with established ASCVD,” the authors wrote. “It appears that large absolute reductions in Lp(a) may be needed to demonstrate a significant clinical benefit.”
Despite this, the authors argued that in primary prevention, it was reasonable to use a Lp(a) level greater than 50 mg/dL (100 nmol/L) as a “risk-enhancing factor,” and in high-risk or very-high-risk patients with elevated LDL-C, it could prompt use of more intensive therapies.
Five authors disclosed honorarium or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Clin Lipidol. 2019 May 17. doi: 10.1016/j.jacl.2019.04.010.
Lipoprotein(a) is an independent risk factor for atherosclerotic cardiovascular disease–related events, and plasma levels of Lp(a) could help refine risk assessment and influence treatment decisions, say the authors of a scientific statement from the National Lipid Association.
Don P. Wilson, MD, of Cook Children’s Medical Center, Fort Worth, Tex., and coauthors reviewed the evidence around testing of Lp(a) in clinical practice and its use in guiding treatment for both primary and secondary prevention. Their report is in the Journal of Clinical Lipidology.
Prospective, population-based studies point to a clear link between high Lp(a) levels and high risk of myocardial infarction, coronary heart disease, coronary artery stenosis, carotid stenosis, valvular aortic stenosis, ischemic stroke, cardiovascular mortality, and all-cause mortality, the authors wrote. This association was independent of the effect of other risk factors, including LDL cholesterol.
However, existing Lp(a) assays have not been globally standardized, and there is only incomplete evidence for age, sex, or ethnicity-specific cutoff points for high risk.
The authors suggested Lp(a) levels greater than 50 mg/dL (100 nmol/L) could be considered a risk factor that justifies the initiation of statin therapy. However ,they pointed out this level corresponded to the 80th population percentile in predominantly white populations, while in African American populations the equivalent cutoff was around 150 nmol/L.
On the issue of whom to test for Lp(a) serum levels, the authors said testing could reasonably be used to refine risk assessment for atherosclerotic cardiovascular disease in adults with first-degree relatives who experienced premature atherosclerotic cardiovascular disease, those with a personal history of the disease, or in those with severe hypercholesterolemia or suspected familial hypercholesterolemia.
However, statin therapy does not decrease Lp(a) levels, and there is also evidence that patients with high Lp(a) levels may not show as much LDL-C lowering in response to statin therapy.
“There is a lack of current evidence demonstrating that lowering Lp(a), independently of LDL-C, reduces ASCVD events in individuals with established ASCVD,” the authors wrote. “It appears that large absolute reductions in Lp(a) may be needed to demonstrate a significant clinical benefit.”
Despite this, the authors argued that in primary prevention, it was reasonable to use a Lp(a) level greater than 50 mg/dL (100 nmol/L) as a “risk-enhancing factor,” and in high-risk or very-high-risk patients with elevated LDL-C, it could prompt use of more intensive therapies.
Five authors disclosed honorarium or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Clin Lipidol. 2019 May 17. doi: 10.1016/j.jacl.2019.04.010.
Lipoprotein(a) is an independent risk factor for atherosclerotic cardiovascular disease–related events, and plasma levels of Lp(a) could help refine risk assessment and influence treatment decisions, say the authors of a scientific statement from the National Lipid Association.
Don P. Wilson, MD, of Cook Children’s Medical Center, Fort Worth, Tex., and coauthors reviewed the evidence around testing of Lp(a) in clinical practice and its use in guiding treatment for both primary and secondary prevention. Their report is in the Journal of Clinical Lipidology.
Prospective, population-based studies point to a clear link between high Lp(a) levels and high risk of myocardial infarction, coronary heart disease, coronary artery stenosis, carotid stenosis, valvular aortic stenosis, ischemic stroke, cardiovascular mortality, and all-cause mortality, the authors wrote. This association was independent of the effect of other risk factors, including LDL cholesterol.
However, existing Lp(a) assays have not been globally standardized, and there is only incomplete evidence for age, sex, or ethnicity-specific cutoff points for high risk.
The authors suggested Lp(a) levels greater than 50 mg/dL (100 nmol/L) could be considered a risk factor that justifies the initiation of statin therapy. However ,they pointed out this level corresponded to the 80th population percentile in predominantly white populations, while in African American populations the equivalent cutoff was around 150 nmol/L.
On the issue of whom to test for Lp(a) serum levels, the authors said testing could reasonably be used to refine risk assessment for atherosclerotic cardiovascular disease in adults with first-degree relatives who experienced premature atherosclerotic cardiovascular disease, those with a personal history of the disease, or in those with severe hypercholesterolemia or suspected familial hypercholesterolemia.
However, statin therapy does not decrease Lp(a) levels, and there is also evidence that patients with high Lp(a) levels may not show as much LDL-C lowering in response to statin therapy.
“There is a lack of current evidence demonstrating that lowering Lp(a), independently of LDL-C, reduces ASCVD events in individuals with established ASCVD,” the authors wrote. “It appears that large absolute reductions in Lp(a) may be needed to demonstrate a significant clinical benefit.”
Despite this, the authors argued that in primary prevention, it was reasonable to use a Lp(a) level greater than 50 mg/dL (100 nmol/L) as a “risk-enhancing factor,” and in high-risk or very-high-risk patients with elevated LDL-C, it could prompt use of more intensive therapies.
Five authors disclosed honorarium or advisory board positions with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Clin Lipidol. 2019 May 17. doi: 10.1016/j.jacl.2019.04.010.
FROM THE JOURNAL OF CLINICAL LIPIDOLOGY
DOACs surpass warfarin in low-weight AFib patients
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
REPORTING FROM HEART RHYTHM 2019
Tick-borne disease has become a national issue
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.
There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”
Pennsylvania had more reported cases of tick-borne disease from 2004 to 2016 than any other state, but these diseases are becoming a national threat, according to the Centers for Disease Control and Prevention.

There were 73,000 cases reported in Pennsylvania over that period, and tick-borne diseases, including Lyme disease, anaplasmosis/ehrlichiosis, spotted fever rickettsiosis, babesiosis, tularemia, and Powassan virus, among others, affected almost 492,000 people nationwide, with Lyme disease representing the majority of cases, the CDC said in a Vital Signs report.
Although it’s no surprise that Pennsylvania, New York, and Connecticut were tick-borne disease hot spots, non-Northeastern states like Virginia, Wisconsin, and Minnesota also were among the top 10 in cases. States even further away from the Northeast can be found in the next 10: Arkansas had more than 7,000 cases in 13 years, and Oklahoma had over 4,600 cases, data from the National Notifiable Diseases Surveillance System show.
Nationally, the number of cases more than doubled from 23,000 in 2004 to 49,000 in 2016, and tick-borne disease hit every state except Hawaii. Over that same time, seven new tick-borne pathogens were discovered or introduced into the United States, the CDC reported.
“Local and state health departments and vector control organizations face increasing demands to respond to these threats,” the CDC said, but “more than 80% of vector control organizations report needing improvement in one or more of five core competencies, such as testing for pesticide resistance [and using] data to drive local decisions about vector control.”