User login
Evidence supports accuracy of COPD diagnosis tool
The ratio of the forced expiratory volume in 1 second to the forced vital capacity (FEV1:FVC) at the recommended threshold of 0.70 effectively diagnosed individuals at risk for clinically significant COPD, a longitudinal study of more than 24,000 individuals has found.
Guidelines from respiratory societies have long recommended a diagnosis of airflow obstruction when the FEV1:FVC is less than 0.70, but no rigorous, population-based studies have been conducted to support this recommendation, wrote Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and colleagues.
“The selection of a threshold for defining airflow obstruction has major implications for patient care and public health as the prevalence of airflow obstruction can vary by as much as 33% depending on which threshold is selected,” they said.
In a study published in JAMA, the researchers reviewed data from 24,207 participants in the National Heart, Lung, and Blood Institute Pooled Cohorts Study to assess the accuracy of different thresholds in predicting COPD events in a large, multiethnic, U.S. population. All participants underwent spirometry; the average age at spirometry was 63 years, and 54% of the patients were women. Patients were enrolled during 1987-2000 and received follow-up longitudinally through 2016.
Overall, 3,925 participants experienced COPD-related events during an average of 15 years of follow-up (more than 340,757 person-years). These events included 3,563 hospitalizations and 447 deaths related to COPD.
The researchers compared three thresholds for FEV1:FVC ratios: a fixed optimal threshold of 0.71, a lower limit of normal (LLN) defined as 0.034, and the currently recommended 0.70.
The optimal 0.71 was not significantly different from the recommended 0.70 but was significantly more accurate than the LLN of 0.034. In addition, the 0.70 value was the optimal predictor in a subgroup analysis of ever-smokers and in multivariate analysis.
The findings were limited by several factors including the use of prebronchodilator spirometry, lack of adjustment for medication use, and limitation of outcomes to COPD mortality or clinical events mainly caused by COPD, which might exclude patients with mild to moderate disease, the researchers noted.
However, ” to help clinicians identify patients at increased risk for significant COPD, they said.
Lead author Dr. Bhatt disclosed a National Institutes of Health grant, consulting fees from Sunovion and research funds from Proterix Bio. The study was supported by grants from multiple agencies of the National Institutes of Health, including the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging.
The ratio of the forced expiratory volume in 1 second to the forced vital capacity (FEV1:FVC) at the recommended threshold of 0.70 effectively diagnosed individuals at risk for clinically significant COPD, a longitudinal study of more than 24,000 individuals has found.
Guidelines from respiratory societies have long recommended a diagnosis of airflow obstruction when the FEV1:FVC is less than 0.70, but no rigorous, population-based studies have been conducted to support this recommendation, wrote Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and colleagues.
“The selection of a threshold for defining airflow obstruction has major implications for patient care and public health as the prevalence of airflow obstruction can vary by as much as 33% depending on which threshold is selected,” they said.
In a study published in JAMA, the researchers reviewed data from 24,207 participants in the National Heart, Lung, and Blood Institute Pooled Cohorts Study to assess the accuracy of different thresholds in predicting COPD events in a large, multiethnic, U.S. population. All participants underwent spirometry; the average age at spirometry was 63 years, and 54% of the patients were women. Patients were enrolled during 1987-2000 and received follow-up longitudinally through 2016.
Overall, 3,925 participants experienced COPD-related events during an average of 15 years of follow-up (more than 340,757 person-years). These events included 3,563 hospitalizations and 447 deaths related to COPD.
The researchers compared three thresholds for FEV1:FVC ratios: a fixed optimal threshold of 0.71, a lower limit of normal (LLN) defined as 0.034, and the currently recommended 0.70.
The optimal 0.71 was not significantly different from the recommended 0.70 but was significantly more accurate than the LLN of 0.034. In addition, the 0.70 value was the optimal predictor in a subgroup analysis of ever-smokers and in multivariate analysis.
The findings were limited by several factors including the use of prebronchodilator spirometry, lack of adjustment for medication use, and limitation of outcomes to COPD mortality or clinical events mainly caused by COPD, which might exclude patients with mild to moderate disease, the researchers noted.
However, ” to help clinicians identify patients at increased risk for significant COPD, they said.
Lead author Dr. Bhatt disclosed a National Institutes of Health grant, consulting fees from Sunovion and research funds from Proterix Bio. The study was supported by grants from multiple agencies of the National Institutes of Health, including the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging.
The ratio of the forced expiratory volume in 1 second to the forced vital capacity (FEV1:FVC) at the recommended threshold of 0.70 effectively diagnosed individuals at risk for clinically significant COPD, a longitudinal study of more than 24,000 individuals has found.
Guidelines from respiratory societies have long recommended a diagnosis of airflow obstruction when the FEV1:FVC is less than 0.70, but no rigorous, population-based studies have been conducted to support this recommendation, wrote Surya P. Bhatt, MD, of the University of Alabama at Birmingham, and colleagues.
“The selection of a threshold for defining airflow obstruction has major implications for patient care and public health as the prevalence of airflow obstruction can vary by as much as 33% depending on which threshold is selected,” they said.
In a study published in JAMA, the researchers reviewed data from 24,207 participants in the National Heart, Lung, and Blood Institute Pooled Cohorts Study to assess the accuracy of different thresholds in predicting COPD events in a large, multiethnic, U.S. population. All participants underwent spirometry; the average age at spirometry was 63 years, and 54% of the patients were women. Patients were enrolled during 1987-2000 and received follow-up longitudinally through 2016.
Overall, 3,925 participants experienced COPD-related events during an average of 15 years of follow-up (more than 340,757 person-years). These events included 3,563 hospitalizations and 447 deaths related to COPD.
The researchers compared three thresholds for FEV1:FVC ratios: a fixed optimal threshold of 0.71, a lower limit of normal (LLN) defined as 0.034, and the currently recommended 0.70.
The optimal 0.71 was not significantly different from the recommended 0.70 but was significantly more accurate than the LLN of 0.034. In addition, the 0.70 value was the optimal predictor in a subgroup analysis of ever-smokers and in multivariate analysis.
The findings were limited by several factors including the use of prebronchodilator spirometry, lack of adjustment for medication use, and limitation of outcomes to COPD mortality or clinical events mainly caused by COPD, which might exclude patients with mild to moderate disease, the researchers noted.
However, ” to help clinicians identify patients at increased risk for significant COPD, they said.
Lead author Dr. Bhatt disclosed a National Institutes of Health grant, consulting fees from Sunovion and research funds from Proterix Bio. The study was supported by grants from multiple agencies of the National Institutes of Health, including the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, and the National Institute on Aging.
FROM JAMA
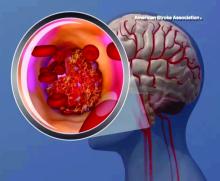
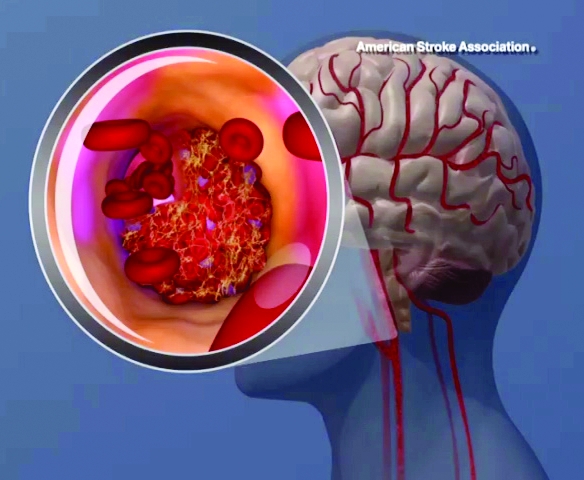
Lipoprotein(a) levels tied to higher ischemic stroke risk
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: Stroke risk was 1.6X higher with high Lp(a) levels.
Study details: Analysis of 49,699 individuals in the Copenhagen General Population Study, and 10,813 individuals in the Copenhagen City Heart Study.
Disclosures: Both studies were supported by the sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures.
Source: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
Mitral valve repair improves prognosis in heart failure patients with secondary MR
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: In patients with primary degenerative MR, MVR is curative, with the transcatheter approach being safer than surgical repair. However, it is unknown whether patients with secondary MR from left ventricular dilatation would confer the same benefit of MVR.
Study design: Multicenter, randomized, controlled, parallel-group, open-label trial.
Setting: 78 sites in the United States and Canada.
Synopsis: From December 2012 to June 2017, 614 patients from 78 centers in the United States and Canada with symptomatic MR were enrolled with 302 patients assigned to the device group (transcatheter MVR and medical treatment) and 312 to the control group (medical therapy). Over 2 years, the device group’s annual rate for heart failure hospitalizations was significantly lower (35.8%/patient-year versus 67.9%/patient-year in the control group), as was all-cause mortality (29.1% for the device group versus 46.1%). The rate of freedom from device-related complications was 96.6%, better than the goal of 88%. There was improvement in quality of life, functional capacity, severity of MR, and left ventricular remodeling.
Limitations include that investigators were not blinded because the device was visible on imaging. Longer follow-up in the device group may have contributed to the observed decreased mortality. It is unknown whether less-symptomatic patients would attain the same benefit.
Bottom line: In patients with symptomatic, moderate to severe, and severe secondary MR, MVR lowers rates of hospitalization, decreases mortality, and improves quality of life.
Citation: Stone GW et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018 Sep 23. doi: 10.1056/NEJMoa1806640.
Dr. Kochar is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Daily aspirin use may not improve CV outcomes in healthy elderly
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: What are the benefits and risks of daily aspirin use for primary prevention in healthy elderly adults?
Background: Prior studies have shown the efficacy of aspirin for secondary prevention of cardiovascular disease and stroke, but the evidence supporting the use of aspirin for primary prevention is less certain.
Study design: Randomized, double-blind, placebo-controlled prospective study with a 5-year study period.
Setting: Australia and the United States.
Synopsis: The Aspirin in Reducing Events in the Elderly (ASPREE) trial included 19,114 community-dwelling healthy people (aged 70 years and older overall and aged 65 years and older if black or Hispanic), without cardiovascular disease, dementia or disability. The goal was to investigate the effect of daily low-dose aspirin (100 mg, enteric coated) on healthy life span (without dementia or disability), with prespecified secondary outcomes (cardiovascular events and major hemorrhage).
Analysis was by intention-to-treat. Participants were predominantly Caucasian, approximately 10% of patients had diabetes, 74% had hypertension, and 65% had dyslipidemia. There was high adherence to the intervention. There was no significant difference in the primary outcome (disability-free survival) or in the secondary outcome of cardiovascular event (fatal or nonfatal MI or stroke, or hospitalization for heart failure.) The rate of major hemorrhage (hemorrhagic stroke, symptomatic intracranial bleeding, clinically significant extracranial bleeding) was higher in the aspirin group (P less than .001). In contrast to prior studies, subgroup analysis showed higher mortality in the aspirin group (attributed to an increase in the risk of cancer-related death.) The authors warn that this finding should be interpreted with caution.
Bottom line: Aspirin use for primary prevention in healthy elderly persons over a 5-year period did not change disability-free survival, did not decrease cardiovascular risk, and increased the rate of major hemorrhage.
Citation: McNeil JJ et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519-28.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
FDA issues warning on insulin pump cybersecurity weakness
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
The Food and Drug Administration has issued a warning to patients and health care providers that a pair of Medtronic insulin pumps are being recalled because of potential cybersecurity risks, according to a press release.
The affected devices are the MiniMed 508 and MiniMed Paradigm series insulin pumps, which wirelessly connect to both the patient’s blood glucose meter and continuous glucose monitoring system. A remote controller and CareLink USB – a thumb-sized wireless device that plugs into a computer – are used to operate the devices; the remote controller sends insulin dosing commands to the pump and the CareLink USB can be used to download and share data with the patient’s health care provider.
The potential risk involves the wireless communication between the pumps and related devices such as the blood glucose meter and remote controller. The FDA has identified a cybersecurity vulnerability within the insulin pumps, and is concerned that a third party could connect to the device and change the pump’s settings. Insulin could be given in excess, causing hypoglycemia, or stopped, causing hyperglycemia or diabetic ketoacidosis.
Medtronic has identified 4,000 patients in the United States who are affected by the security weakness. Because the company is unable to adequately update or patch the device to remove the weakness, the FDA is working to ensure that Medtronic addresses the issue in any way possible, including helping patients with affected pumps switch to newer models.
“While we are not aware of patients who may have been harmed by this particular cybersecurity vulnerability, the risk of patient harm if such a vulnerability were left unaddressed is significant. The safety communication issued today contains recommendations for what actions patients and health care providers should take to avoid the risk this vulnerability could pose,” said Suzanne Schwartz, MD, MBA, deputy director of the Office of Strategic Partnerships and Technology Innovation.
Find the full press release on the FDA website.
Short Takes
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Restrictive transfusion strategy for cardiac surgery patients remains noninferior at 6 months post op
The authors previously reported that, in moderate- to high-risk cardiac surgery patients, a restrictive transfusion strategy was noninferior to a liberal strategy based on the clinical outcomes of all-cause mortality, MI, stroke, or new renal failure with dialysis. The groups continued to show no significant difference in outcomes at 6 months post op.
Citation: Mazer CD et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N Engl J Med. 2018;379:1224-33.
Subset of patients benefits from in-hospital sleep apnea screening
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
EXPERT ANALYSIS FROM SLEEP 2019
What’s new in pediatric sepsis
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
LJUBLJANA, SLOVENIA – The dogma of the “Golden Hour” for the immediate management of pediatric sepsis has been oversold and actually is based upon weak evidence, Luregn J. Schlapbach, MD, asserted at the annual meeting of the European Society for Paediatric Infectious Diseases.
The true Golden Hour – that is, the time frame within which it’s imperative to administer the sepsis bundle comprised of appropriate antibiotics, fluids, and inotropes – is probably more like 3 hours.
“The evidence suggests that up to 3 hours you don’t really have a big difference in outcomes for sepsis. If you recognize shock there’s no question: You should not even wait 1 hour. But if you’re not certain, it may be better to give up to 3 hours to work up the child and get the senior clinician involved before you make decisions about treatment. So I’m not advocating to delay anything, said Dr. Schlapbach, a pediatric intensivist at the Child Health Research Center at the University of Queensland in South Brisbane, Australia.
The problem with a 1-hour mandate for delivery of the sepsis bundle, as recommended in guidelines by the Surviving Sepsis Campaign and the American College of Critical Care Medicine, and endorsed in quality improvement initiatives, is that the time pressure pushes physicians to overprescribe antibiotics to children who don’t actually have a serious bacterial infection. And that, he noted, contributes to the growing problem of antimicrobial resistance.
“You may have a child where you’re not too sure. Usually you would have done a urine culture because UTI [urinary tract infection] is quite a common cause of these infections, and many of these kids aren’t necessarily septic. But if people tell you that within 1 hour you need to treat, are you going to take the time to do the urine culture, or are you just going to decide to treat?” he asked rhetorically.
Dr. Schlapbach is a world-renowned pediatric sepsis researcher. He is far from alone in his reservations about the Golden Hour mandate.
“This is one of the reasons why IDSA [the Infectious Diseases Society of America] has not endorsed the Surviving Sepsis Campaign,” according to the physician, who noted that, in a position statement, IDSA officials have declared that discrimination of sepsis from noninfectious conditions remains a challenge, and that a 60-minute time to antibiotics may jeopardize patient reassessment (Clin Infect Dis. 2018 May 15;66[10]:1631-5).
Dr. Schlapbach highlighted other recent developments in pediatric sepsis.
The definition of adult sepsis has changed, and the pediatric version needs to as well
The revised definition of sepsis, known as Sepsis-3, issued by the International Sepsis Definition Task Force in 2016 notably dropped systemic inflammatory response syndrome (SIRS), as a requirement for sepsis (JAMA. 2016;315[8]:801-10). The revised definition characterizes sepsis as a dysregulated host response to infection resulting in life-threatening organ dysfunction. But Sepsis-3 is based entirely on adult data and is not considered applicable to children.
The current Pediatric Sepsis Consensus Conference definition dates back to 2005. A comprehensive revision is getting underway. It, too, is likely to drop SIRS into the wastebasket, Dr. Schlapbach said.
“It is probably time to abandon the old view of sepsis disease progression, which proposes a progression from infection to SIRS to severe sepsis with organ dysfunction to septic shock, because most children with infection do manifest signs of SIRS, such as tachycardia, tachypnea, and fever, and these probably should be considered as more of an adaptive rather than a maladaptive response,” he explained.
The goal of the pediatric sepsis redefinition project is to come up with something more useful for clinicians than the Sepsis-3 definition. While the Sepsis-3 concept of a dysregulated host response to infection sounds nice, he explained, “we don’t actually know what it is.
“One of the challenges that you all know as pediatricians is that children who develop sepsis get sick very, very quickly. We all have memories of children who we saw and may have discharged, and they were dead 12 hours later,” he noted.
Indeed, he and others have shown in multiple studies that up to 50% of pediatric deaths caused by sepsis happen within 24 hours of presentation.
“So whatever happens, it happens very quickly. The true question for us is actually how and why do children progress from no organ dysfunction, where the mortality is close to zero, to organ dysfunction, where all of a sudden mortality jumps up dramatically. It’s this progression that we don’t understand at all,” according to Dr. Schlapbach.
The genetic contribution to fulminant sepsis in children may be substantial
One-third of pediatric sepsis deaths in high-income countries happen in previously healthy children. In a proof-of-concept study, Dr. Schlapbach and coinvestigators in the Swiss Pediatric Sepsis Study Group conducted exome-sequencing genetic studies in eight previously healthy children with no family history of immunodeficiency who died of severe sepsis because of community-acquired Pseudomonas aeruginosa infection. Two of the eight had rare loss-of-function mutations in genes known to cause primary immunodeficiencies. The investigators proposed that unusually severe sepsis in previously healthy children warrants exome sequencing to look for underlying previously undetected primary immunodeficiencies. That’s important information for survivors and/or affected families to have, they argued (Front Immunol. 2016 Sep 20;7:357. eCollection 2016).
“There are some indications that the genetic contribution in children with sepsis may be larger than previously assumed,” he said.
The longstanding practice of fluid bolus therapy for resuscitation in pediatric sepsis is being reexamined
The FEAST (Fluid Expansion As Supportive Therapy) study, a randomized trial of more than 3,000 children with severe febrile illness and impaired perfusion in sub-Saharan Africa, turned heads with its finding that fluid boluses significantly increased 48-hour mortality (BMC Med. 2013 Mar 14;11:67).
Indeed, the FEAST findings, supported by mechanistic animal studies, were sufficiently compelling that the use of fluid boluses in both pediatric and adult septic shock is now under scrutiny in two major randomized trials: RIFTS (the Restrictive IV Fluid Trial in Severe Sepsis and Septic Shock), and CLOVERS (Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis). Stay tuned.
Dr. Schlapbach reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM ESPID 2019
Repeated qSOFA measurements better predict in-hospital mortality from sepsis
Clinical question: Do repeated quick Sepsis-Related Organ Failure Assessment (qSOFA) measurements improve predictive validity for sepsis using in-hospital mortality, compared with a single qSOFA measurement at the time a clinician first suspects infection?
Background: Sepsis in hospitalized patients is associated with poor outcomes, but it is not clear how to best identify patients at risk. For non-ICU patients, the qSOFA score (made up of three simple clinical variables: respiratory rate greater than or equal to 22 breaths/minute, systolic blood pressure less than or equal to 100 mm Hg, and Glasgow Coma Scale score less than 15) has predictive validity for important outcomes including in-hospital mortality. qSOFA is relatively new in clinical practice, and the optimal utilization of the score has not yet been defined.
Study design: Retrospective Cohort Study.
Setting: All adult medical and surgical encounters in the ED, hospital ward, postanesthesia care unit (PACU), and ICU at 12 hospitals in Pennsylvania in 2012.
Synopsis: Kievlan et al. studied whether repeated qSOFA scores improved prediction of in-hospital mortality and allowed identification of specific clinical trajectories. The study included approximately 37,600 encounters. Authors abstracted demographic data, vital signs, laboratory results, and antibiotic/culture orders. An infection cohort was identified by a combination of orders for body fluid culture and antibiotics. The qSOFA scores were gathered at 6-hour intervals from the culture/antibiotic event (suspected sepsis). Scores were low (0), moderate (1), or high (greater than or equal to 2). Mean initial qSOFA scores were greater for patients who died, and remained higher during the 48-hours after suspected infection. Mortality was less than 2% in patients with an initial low qSOFA. 25% of patients with an initial moderate qSOFA had subsequent higher qSOFAs, and they had higher mortality, compared with patients with subsequent low qSOFA scores (16% vs. 4%).
Only those patients with initial qSOFA scores at the time of suspected infection were included, and missing data was common. The results may not be applicable to hospitals with a different sepsis case mix from the those of study institutions.
Bottom line: Repeated qSOFA measurements improve predictive validity for in-hospital mortality for patients with sepsis. Patients with low initial qSOFA scores have a low chance (less than 2%) of in-hospital mortality. Further studies are needed to determine how repeat qSOFA measurements can be used to improve management of patients with sepsis.
Citation: Kievlan DR et al. Evaluation of repeated quick sepsis-related organ failure assessment measurements among patients with suspected infection. Crit Care Med. 2018. doi: 10.1097/CCM.0000000000003360.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Do repeated quick Sepsis-Related Organ Failure Assessment (qSOFA) measurements improve predictive validity for sepsis using in-hospital mortality, compared with a single qSOFA measurement at the time a clinician first suspects infection?
Background: Sepsis in hospitalized patients is associated with poor outcomes, but it is not clear how to best identify patients at risk. For non-ICU patients, the qSOFA score (made up of three simple clinical variables: respiratory rate greater than or equal to 22 breaths/minute, systolic blood pressure less than or equal to 100 mm Hg, and Glasgow Coma Scale score less than 15) has predictive validity for important outcomes including in-hospital mortality. qSOFA is relatively new in clinical practice, and the optimal utilization of the score has not yet been defined.
Study design: Retrospective Cohort Study.
Setting: All adult medical and surgical encounters in the ED, hospital ward, postanesthesia care unit (PACU), and ICU at 12 hospitals in Pennsylvania in 2012.
Synopsis: Kievlan et al. studied whether repeated qSOFA scores improved prediction of in-hospital mortality and allowed identification of specific clinical trajectories. The study included approximately 37,600 encounters. Authors abstracted demographic data, vital signs, laboratory results, and antibiotic/culture orders. An infection cohort was identified by a combination of orders for body fluid culture and antibiotics. The qSOFA scores were gathered at 6-hour intervals from the culture/antibiotic event (suspected sepsis). Scores were low (0), moderate (1), or high (greater than or equal to 2). Mean initial qSOFA scores were greater for patients who died, and remained higher during the 48-hours after suspected infection. Mortality was less than 2% in patients with an initial low qSOFA. 25% of patients with an initial moderate qSOFA had subsequent higher qSOFAs, and they had higher mortality, compared with patients with subsequent low qSOFA scores (16% vs. 4%).
Only those patients with initial qSOFA scores at the time of suspected infection were included, and missing data was common. The results may not be applicable to hospitals with a different sepsis case mix from the those of study institutions.
Bottom line: Repeated qSOFA measurements improve predictive validity for in-hospital mortality for patients with sepsis. Patients with low initial qSOFA scores have a low chance (less than 2%) of in-hospital mortality. Further studies are needed to determine how repeat qSOFA measurements can be used to improve management of patients with sepsis.
Citation: Kievlan DR et al. Evaluation of repeated quick sepsis-related organ failure assessment measurements among patients with suspected infection. Crit Care Med. 2018. doi: 10.1097/CCM.0000000000003360.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Clinical question: Do repeated quick Sepsis-Related Organ Failure Assessment (qSOFA) measurements improve predictive validity for sepsis using in-hospital mortality, compared with a single qSOFA measurement at the time a clinician first suspects infection?
Background: Sepsis in hospitalized patients is associated with poor outcomes, but it is not clear how to best identify patients at risk. For non-ICU patients, the qSOFA score (made up of three simple clinical variables: respiratory rate greater than or equal to 22 breaths/minute, systolic blood pressure less than or equal to 100 mm Hg, and Glasgow Coma Scale score less than 15) has predictive validity for important outcomes including in-hospital mortality. qSOFA is relatively new in clinical practice, and the optimal utilization of the score has not yet been defined.
Study design: Retrospective Cohort Study.
Setting: All adult medical and surgical encounters in the ED, hospital ward, postanesthesia care unit (PACU), and ICU at 12 hospitals in Pennsylvania in 2012.
Synopsis: Kievlan et al. studied whether repeated qSOFA scores improved prediction of in-hospital mortality and allowed identification of specific clinical trajectories. The study included approximately 37,600 encounters. Authors abstracted demographic data, vital signs, laboratory results, and antibiotic/culture orders. An infection cohort was identified by a combination of orders for body fluid culture and antibiotics. The qSOFA scores were gathered at 6-hour intervals from the culture/antibiotic event (suspected sepsis). Scores were low (0), moderate (1), or high (greater than or equal to 2). Mean initial qSOFA scores were greater for patients who died, and remained higher during the 48-hours after suspected infection. Mortality was less than 2% in patients with an initial low qSOFA. 25% of patients with an initial moderate qSOFA had subsequent higher qSOFAs, and they had higher mortality, compared with patients with subsequent low qSOFA scores (16% vs. 4%).
Only those patients with initial qSOFA scores at the time of suspected infection were included, and missing data was common. The results may not be applicable to hospitals with a different sepsis case mix from the those of study institutions.
Bottom line: Repeated qSOFA measurements improve predictive validity for in-hospital mortality for patients with sepsis. Patients with low initial qSOFA scores have a low chance (less than 2%) of in-hospital mortality. Further studies are needed to determine how repeat qSOFA measurements can be used to improve management of patients with sepsis.
Citation: Kievlan DR et al. Evaluation of repeated quick sepsis-related organ failure assessment measurements among patients with suspected infection. Crit Care Med. 2018. doi: 10.1097/CCM.0000000000003360.
Dr. Linker is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
What drives intensification of antihypertensive therapy at discharge?
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.
Background: Transient elevations in blood pressure are common among adult patients, yet there are no data or guidelines that support long-term medication changes based on these readings. Tight control of blood pressure is likely to improve outcomes among patients with heart failure), myocardial infarction, and stroke. Patients with reduced life expectancy, dementia, or metastatic cancer are less likely to benefit from tight control.
Study design: Retrospective cohort study.
Setting: U.S. Veterans Administration (VA) Health System.
Synopsis: The investigators reviewed data from 14,915 adults over 65 (median age, 76 years) admitted to the VA with a diagnosis of pneumonia, urinary tract infection, or venous thromboembolism. Most patients (65%) had well-controlled blood pressure prior to admission.
A total of 2,074 (14%) patients were discharged with an intensified hypertension regimen (additional medication or higher dose). While both elevated inpatient and outpatient blood pressures were predictive of intensification, the association with elevated inpatient blood pressure was much stronger (odds ratio, 3.66; 95% confidence interval, 3.29-4.08) than it was with elevated outpatient blood pressure (OR, 1.75; 95% CI, 1.58-1.93).
In a multivariate regression analysis, the investigators found no significant differences in intensification by life expectancy (P = .07), diagnosis of dementia (P = .95), or metastatic malignancy (P = .13). There was a small increased probability of intensification among patients with heart failure, but no such difference for patients with history of MI (P = .53), stroke (P = .37), or renal disease (P = .73).
The generalizability of this trial may be limited given the cohort was predominantly male (97%), white (77%), and 53% had at least four major comorbidities.
Bottom line: Intensification of antihypertensive therapy at discharge is often driven by inpatient blood pressure readings rather than the broader context of their disease, such as prior long-term outpatient blood pressure control or major comorbidities.
Citation: Anderson TS et al. Intensification of older adults’ outpatient blood pressure treatment at hospital discharge: A national retrospective cohort study. BMJ. 2018:362:k3503.
Dr. Holzer is an assistant professor of medicine in the division of hospital medicine at Mount Sinai Hospital, New York.