User login
Lefamulin found noninferior to moxifloxacin for bacterial pneumonia
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
“The development and approval of a new antibiotic is a rare occurrence and a reason to celebrate” given the scientific, regulatory, and economic challenges to antibiotic development, wrote Preeti N. Malani, MD, in an accompanying editorial. Lefamulin in both oral and intravenous forms was approved by the Food and Drug Administration in August 2019 for the treatment of community-acquired bacterial pneumonia, Dr. Malani said.
Lefamulin will likely be an expensive option. According to a manufacturer press release, lefamulin may cost $205/day for intravenous treatment and $275/day for oral treatment. “This is severalfold more than moxifloxacin or levofloxacin, which are the most commonly prescribed fluoroquinolones for CABP [community-acquired bacterial pneumonia],” said Dr. Malani. However, the addition of lefamulin to the array of antibiotics is important because of the persistent burden of bacterial pneumonia as an indication for antibiotic use, Dr. Malani emphasized.
Dr. Malani is affiliated with the University of Michigan, Ann Arbor, and serves as an associate editor of JAMA, but had no financial conflicts to disclose. These remarks were taken from an accompanying editorial (JAMA. 2019 Sep 27. doi:10.1001/jama.2019.16215).
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
Persistent high rates of bacterial resistance to current treatments have created the need for more options, especially for the treatment of community-acquired bacterial pneumonia (CABP), which remains a leading cause of hospitalization and death in the United States, wrote Elizabeth Alexander, MD, of Nabriva Therapeutics in King of Prussia, Penn., and colleagues. Lefamulin, “the first pleuromutilin antibiotic approved for intravenous and oral use in humans,” has demonstrated activity against many CABP-causing pathogens, including some not susceptible to other classes of antimicrobials, they noted.
Findings of Lefamulin Evaluation Against Pneumonia 2 (LEAP2) were published in JAMA. In this study, the researchers randomized 370 patients to 600 mg of oral lefamulin every 12 hours for 5 days and 368 patients to 400 mg of oral moxifloxacin every 24 hours for 7 days.
Early clinical response rates at 96 hours were 90.8% for both medications (difference of 0.1%). In addition, the rates of clinical response success were similar between the groups in both the modified intent-to-treat population (87.5% with lefamulin and 89.1% with moxifloxacin) and the clinically evaluable population (89.7% with lefamulin and 93.6% with moxifloxacin).
Gastrointestinal issues of diarrhea and nausea were the two most frequently reported treatment-emergent adverse events in both groups. Both conditions occurred more often in the lefamulin group, compared with the moxifloxacin group, but the differences were not significant (12.2% vs. 1.1% and 5.2% vs. 1.9%, respectively).
The study findings were limited by several factors including strict exclusion criteria that may limit the generalizability of the results, as well as a lack of testing for viral copathogens, low recovery of resistant pathogens, and possible misclassification of patient ethnicity, the researchers noted.
However, the results were strengthened by the randomized design, inclusion of patients with more severe CABP, and low rate of discontinuation, they said. The data support previous studies of lefamulin. Its lack of cross-resistance to other drug classes, coverage of typical and atypical CABP pathogens, and options for both oral and intravenous use suggest that it “may provide an alternative approach for the treatment of vulnerable patients,” the researchers said.
The study was supported by Nabriva Therapeutics. Dr. Alexander and several coauthors are employees of Nabriva Therapeutics and own stock in the company.
SOURCE: Alexander E et al. JAMA. 2019 Sep 27. doi:10.1001/jama.2019.15468.
FROM JAMA
Oral anticoagulant and PPI cotherapy cuts upper GI bleed risk
Background: PPIs reduce gastric acid production, promote ulcer healing, and prevent ulcer recurrence; however, limited evidence is available describing the incidence of anticoagulant-related serious upper GI tract bleeding from the newer non–vitamin K anticoagulants and PPI cotherapy.

Study design: Retrospective cohort.
Setting: Medicare enrollees.
Synopsis: With use of computerized Medicare beneficiaries files, researchers identified 1,643,123 patients with 1,713,183 new episodes of oral anticoagulant treatment between Jan. 1, 2011, and Sept. 30, 2015. This analysis showed that cotherapy with PPIs was associated with a lower incidence of upper GI bleed, with the largest difference associated with dabigatran with an incidence rate ratio of 0.49 (95% CI, 0.52-0.85), followed by warfarin (IRR, 0.65; 95%CI, 0.62-0.69), apixaban (IRR, 0.66; 95% CI, 0.52-0.85), and rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84).
Generalizability was limited by population (Medicare enrollees) and the study excluded prior hospitalizations for GI bleed, as well as switches in anticoagulant therapy during the study period.
Bottom line: PPI cotherapy with oral anticoagulation reduces risk of hospitalization for upper GI bleed.
Citation: Ray WA et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018 Dec 4;320(21):2221-30.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: PPIs reduce gastric acid production, promote ulcer healing, and prevent ulcer recurrence; however, limited evidence is available describing the incidence of anticoagulant-related serious upper GI tract bleeding from the newer non–vitamin K anticoagulants and PPI cotherapy.

Study design: Retrospective cohort.
Setting: Medicare enrollees.
Synopsis: With use of computerized Medicare beneficiaries files, researchers identified 1,643,123 patients with 1,713,183 new episodes of oral anticoagulant treatment between Jan. 1, 2011, and Sept. 30, 2015. This analysis showed that cotherapy with PPIs was associated with a lower incidence of upper GI bleed, with the largest difference associated with dabigatran with an incidence rate ratio of 0.49 (95% CI, 0.52-0.85), followed by warfarin (IRR, 0.65; 95%CI, 0.62-0.69), apixaban (IRR, 0.66; 95% CI, 0.52-0.85), and rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84).
Generalizability was limited by population (Medicare enrollees) and the study excluded prior hospitalizations for GI bleed, as well as switches in anticoagulant therapy during the study period.
Bottom line: PPI cotherapy with oral anticoagulation reduces risk of hospitalization for upper GI bleed.
Citation: Ray WA et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018 Dec 4;320(21):2221-30.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: PPIs reduce gastric acid production, promote ulcer healing, and prevent ulcer recurrence; however, limited evidence is available describing the incidence of anticoagulant-related serious upper GI tract bleeding from the newer non–vitamin K anticoagulants and PPI cotherapy.

Study design: Retrospective cohort.
Setting: Medicare enrollees.
Synopsis: With use of computerized Medicare beneficiaries files, researchers identified 1,643,123 patients with 1,713,183 new episodes of oral anticoagulant treatment between Jan. 1, 2011, and Sept. 30, 2015. This analysis showed that cotherapy with PPIs was associated with a lower incidence of upper GI bleed, with the largest difference associated with dabigatran with an incidence rate ratio of 0.49 (95% CI, 0.52-0.85), followed by warfarin (IRR, 0.65; 95%CI, 0.62-0.69), apixaban (IRR, 0.66; 95% CI, 0.52-0.85), and rivaroxaban (IRR, 0.75; 95% CI, 0.68-0.84).
Generalizability was limited by population (Medicare enrollees) and the study excluded prior hospitalizations for GI bleed, as well as switches in anticoagulant therapy during the study period.
Bottom line: PPI cotherapy with oral anticoagulation reduces risk of hospitalization for upper GI bleed.
Citation: Ray WA et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018 Dec 4;320(21):2221-30.
Dr. Ho is an assistant professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Vitamin C infusion falls short for sepsis and ARDS patients
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Although none of the primary outcomes was significant, “the difference in mortality is tantalizing and likely to spur much debate,” wrote Emily B. Brant, MD, and Derek C. Angus, MD, in an accompanying editorial.
“However, this outcome was one of many secondary outcomes, and although reported as statistically significant, that finding was without adjustment for multiple comparisons,” they said.
The study was well-designed, and resulted in the collection of considerable patient data, they said. Previous studies have suggested that approximately 40% of sepsis patients are vitamin C deficient, and vitamin C is considered safe and inexpensive, which may be reason to pursue research in this area, they added.
Study design for addition research should keep in mind the timing and dosage that were limitations in the current study; the lack of effect on organ dysfunction may have occurred because vitamin C was given too late, they said.
Researchers planning further evaluation might “reconsider optimal dosing and timing, as well as the likelihood that any potential benefits may only accrue to subsets of patients, given the underlying heterogeneity of sepsis,” they concluded (JAMA. 2019 Oct 1; 322:1257-8).
Dr. Brant and Dr. Angus are affiliated with the department of critical care medicine, University of Pittsburgh. Dr. Angus serves as a associate editor for JAMA and disclosed receiving consulting fees from Ferring, Bristol-Myers Squibb, and Beckman Coulter; holding stock in Alung Technologies; and holding pending patents for selepressin and for proteomic biomarkers of sepsis in elderly patients. Dr. Brant had no financial conflicts to disclose.
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
Vitamin C infusion did not improve outcomes related to organ failure, inflammation, or vascular injury for patients with sepsis and acute respiratory distress syndrome, based on data from 167 adults.
“Previous research found that vitamin C attenuates systemic inflammation, corrects sepsis-induced coagulopathy, and attenuates vascular injury,” wrote Alpha A. Fowler III, MD, of Virginia Commonwealth University, Richmond, and colleagues.
To examine the impact of vitamin C infusion on patients with sepsis and acute respiratory distress syndrome (ARDS), the researchers designed the CITRIS-ALI trial, a randomized, double-blind, placebo-controlled study conducted at 7 medical intensive care units in the United States.
In the study, published in JAMA, the researchers randomized 167 adults with sepsis and ARDS to receive high-dose intravenous vitamin C (50 mg/kg in 5% dextrose in water) or placebo (5% dextrose in water only) every 6 hours for 96 hours. The primary outcomes were measures of organ failure based on changes in the modified Sequential Organ Failure Assessment score (mSOFA), inflammation (based on changes in C-reactive protein), and vascular injury based on thrombomodulin.
Overall, no significant differences appeared between the vitamin C and placebo groups, respectively in the three primary outcome measures: change in average SOFA score (3-point change vs. a 3.5-point change) at 96 hours; change in C-reactive protein levels (change of 54.1 mcg/mL vs. 46.1 mcg/mL) at 168 hours; and change in thrombomodulin levels (14.5 ng/mL vs. 13.8 ng/mL) at 168 hours.
The average age of the patients was 55 years, and 54% were men.
The researchers also assessed 46 secondary outcomes. Most of these showed no significant differences between the groups, but 28-day all-cause mortality was significantly lower in the vitamin C group, compared with the placebo group (46.3% vs. 29.8%), the researchers said. Vitamin C also was significantly associated with increased ICU-free days to day 28 and hospital-free days to day 60, compared with placebo.
No significant differences were seen between the groups on 43 other secondary outcomes including ventilator-free days and vasopressor use. However, “these findings were based on analyses that did not account for multiple comparisons and therefore must be considered exploratory,” they said.
“The inability of vitamin C to affect C-reactive protein and thrombomodulin levels in this trial possibly resulted from the advanced stages of sepsis that were present before the development of ARDS,” the researchers noted.
The findings were limited by several factors including the variability in the timing of vitamin C administration and the use of a single high dose of vitamin C, they emphasized. However, the results suggest that further research may be needed to determine the potential of vitamin C for improving outcomes in patients with sepsis and ARDS, they said.
The study was supported by the National Heart, Lung, and Blood Institute, National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Polytechnic Institute and State University, Richmond; the NHLBI; and study materials from McGuff Pharmaceuticals.
SOURCE: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi:10.1001/jama.2019.11825.
FROM JAMA
Key clinical point: Vitamin C infusion failed to improve outcomes for patients with ARDS and sepsis.
Major finding: The average SOFA score to measure organ failure changed by 3 points in the vitamin C group vs. 3.5 points in the placebo group.
Study details: The data come from a randomized trial of 167 adults with ARDS and sepsis.
Disclosures: The study was supported by the National Heart, Lung, and Blood Institute, the National Center for Advancing Translational Sciences, VCU Wright Center for Translational Science Award, VCU Investigational Drug Services, and McGuff Pharmaceuticals, who supplied the vitamin C free of charge. Dr. Fowler disclosed funding from Virginia Tech School of Medicine, the NHLBI, and study materials from McGuff Pharmaceuticals.
Source: Fowler AA et al. JAMA. 2019 Oct 1;322:1261-70. doi: 10.1001/jama.2019.11825.
Caution with IVC filters in elderly
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
FDA adds diabetic kidney disease, heart failure indications to canagliflozin
The Food and Drug Administration has approved canagliflozin (Invokana) for the treatment of diabetic kidney disease and for reduction of the risk of hospitalization for heart failure in patients with type 2 diabetes and diabetic kidney disease, which makes it the first drug indicated for diabetic kidney disease treatment in 20 years.
FDA approval, which was announced in a press release by Janssen, the drug’s manufacturer, is based on results from the phase 3 CREDENCE trial. In that study patients with type 2 diabetes and chronic diabetic kidney disease received either 100 mg canagliflozin or placebo. Patients who received canagliflozin experienced a 30% reduction in the risk of the primary composite endpoint, which included end-stage kidney disease, doubling of serum creatinine, and renal or cardiovascular death. The risk of secondary outcomes were also reduced in patients receiving canagliflozin, including a 39% reduction in the risk of hospitalization for heart failure.
The most common adverse events associated with canagliflozin, according to the label, are female genital mycotic infections, urinary tract infection, and increased urination. Serious adverse events associated with canagliflozin include ketoacidosis, kidney problems, serious urinary tract infections, hypoglycemia, necrotizing fasciitis, serious allergic reaction, and bone fractures.
“The real battle to turn the tide on kidney disease is in early detection and slowing its progression so that patients stay healthier and fewer patients reach kidney failure,” LaVerne A. Burton, president and CEO of the American Kidney Fund, said in the press release. “We are so grateful that advances in kidney disease research are producing treatment options that help to slow the progression of diabetic kidney disease and reduce the risk of hospitalization for heart failure.”
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) for the treatment of diabetic kidney disease and for reduction of the risk of hospitalization for heart failure in patients with type 2 diabetes and diabetic kidney disease, which makes it the first drug indicated for diabetic kidney disease treatment in 20 years.
FDA approval, which was announced in a press release by Janssen, the drug’s manufacturer, is based on results from the phase 3 CREDENCE trial. In that study patients with type 2 diabetes and chronic diabetic kidney disease received either 100 mg canagliflozin or placebo. Patients who received canagliflozin experienced a 30% reduction in the risk of the primary composite endpoint, which included end-stage kidney disease, doubling of serum creatinine, and renal or cardiovascular death. The risk of secondary outcomes were also reduced in patients receiving canagliflozin, including a 39% reduction in the risk of hospitalization for heart failure.
The most common adverse events associated with canagliflozin, according to the label, are female genital mycotic infections, urinary tract infection, and increased urination. Serious adverse events associated with canagliflozin include ketoacidosis, kidney problems, serious urinary tract infections, hypoglycemia, necrotizing fasciitis, serious allergic reaction, and bone fractures.
“The real battle to turn the tide on kidney disease is in early detection and slowing its progression so that patients stay healthier and fewer patients reach kidney failure,” LaVerne A. Burton, president and CEO of the American Kidney Fund, said in the press release. “We are so grateful that advances in kidney disease research are producing treatment options that help to slow the progression of diabetic kidney disease and reduce the risk of hospitalization for heart failure.”
Find the full press release on the Janssen website.
The Food and Drug Administration has approved canagliflozin (Invokana) for the treatment of diabetic kidney disease and for reduction of the risk of hospitalization for heart failure in patients with type 2 diabetes and diabetic kidney disease, which makes it the first drug indicated for diabetic kidney disease treatment in 20 years.
FDA approval, which was announced in a press release by Janssen, the drug’s manufacturer, is based on results from the phase 3 CREDENCE trial. In that study patients with type 2 diabetes and chronic diabetic kidney disease received either 100 mg canagliflozin or placebo. Patients who received canagliflozin experienced a 30% reduction in the risk of the primary composite endpoint, which included end-stage kidney disease, doubling of serum creatinine, and renal or cardiovascular death. The risk of secondary outcomes were also reduced in patients receiving canagliflozin, including a 39% reduction in the risk of hospitalization for heart failure.
The most common adverse events associated with canagliflozin, according to the label, are female genital mycotic infections, urinary tract infection, and increased urination. Serious adverse events associated with canagliflozin include ketoacidosis, kidney problems, serious urinary tract infections, hypoglycemia, necrotizing fasciitis, serious allergic reaction, and bone fractures.
“The real battle to turn the tide on kidney disease is in early detection and slowing its progression so that patients stay healthier and fewer patients reach kidney failure,” LaVerne A. Burton, president and CEO of the American Kidney Fund, said in the press release. “We are so grateful that advances in kidney disease research are producing treatment options that help to slow the progression of diabetic kidney disease and reduce the risk of hospitalization for heart failure.”
Find the full press release on the Janssen website.
Ticagrelor monotherapy tops DAPT for high-risk PCI patients
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
REPORTING FROM TCT 2019
CDC reports most vaping lung disease linked to THC-containing cartridges
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
and most products used were prepackaged, prefilled cartridges, according to new data released by the Centers for Disease Control and Prevention.
The majority of these products (66%) were THC-containing cartridges marketed under the brand name Dank. Dank cartridges are available at legal dispensaries and online in areas where they are legal. The Dank company posted a statement on its website warning buyers about fake cartridges and showing images of genuine cartridges. However, 89% of the cartridges were obtained on the street, from dealers, online, or from friends or social contacts, Jennifer Layden, MD, of the Illinois Department of Public Health said during a CDC telebriefing.
The illness was first recognized in Wisconsin and Illinois. Marijuana is illegal in Wisconsin; Illinois licensed recreational marijuana in 2009.
Other commonalties among cases have also emerged, Anne Schuchat, MD, deputy director of CDC, said during the call. More than two-thirds of the 805 confirmed or probable cases were male, and the median age was 23 years. The illness crosses age barriers, she said. About 62% were 18-24 years of age, and 54% under age 25. However, among the 12 deaths so far reported, the median age was 50 years. The age range was wide, from 27 to 71 years. Dr. Schuchat said data about medical comorbidities potentially linking the deaths is not yet available, although it is part of the ongoing investigation.
Other clinical commonalities included intensive use of THC-containing products and, in a small number of cases, concomitant use of benzodiazepenes, opioids, and narcotics.
Cases have now emerged in 46 states and in the U.S. Virgin Islands, although the number reported each week is dropping. However, this decrease may not represent a drop in newly occurring cases, but instead reflect delays in clinical recognition or reporting to local health departments, Dr. Schuchat said.
Regardless of the recent decline in reported cases, she said, the epidemic is serious, far reaching, and ongoing.
“I want to stress that this is a serious, life-threatening disease occurring mostly in otherwise healthy young people. These illnesses and deaths are occurring in the context of a dynamic marketplace with mix of products with mixes of ingredients, including potentially illicit substances. Users don’t know what’s in them and cannot tell from the ingredients listed on the packaging.”
Dr. Schuchat drew her data from two reports issued in the Morbidity and Mortality Weekly Report: a national case update by Peter A. Briss, MD, chair of CDC’s Lung Injury Response Epidemiology/Surveillance Group, and colleagues, and a regional report coauthored by Dr. Layden of cases in Illinois and Wisconsin.
In the national report, 514 patients self-reported their history of e-cigarette and vaping use. Among those, 395 (76.9%) reported using THC-containing products, and 292 (56.8%) reported using nicotine-containing products in the 30 days preceding symptom onset. Almost half (210; 40.9%) reported using both THC- and nicotine-containing products.
But there appeared to be no clear pattern of use, said Dr. Briss, who also participated in the briefing. More than a third (185; 36.0%) reported exclusive use of THC-containing products, and 82 (16.0%) reported exclusive use of nicotine-containing products.
The regional report added additional details.
Among the 86 patients who self-reported details, there were 234 unique cases of e-cigarette or THC vaping in 87 brands.
“Patients reported using numerous products and brands,” Dr. Layden noted. “Those who reported using THC products used an average of 2.1 different products and those who reported using nicotine products used about 1.3 different ones. Some patients reported using up to seven different brands, and these were used at least daily and sometimes numerous times in the day.”
According to the MMWR regional report, among the urinary THC screens obtained for 32 patients, “29 (91%) were positive for THC. One of these patients reported smoking combustible marijuana. Urinary THC levels for four patients who reported using THC-containing products exceeded 400 ng/ml, indicating intensive use of THC or THC-containing products.”
About 40% of THC users and 65% of nicotine-product users reported using the product at least five times a day; 52% said they used combustible marijuana in addition to the vapes, and 24% reported also smoking combustible tobacco.
There was a very low level of concomitant drug use. Two patients reported using LSD; one reported misusing dextroamphetamine-amphetamine (Adderall), and one reported misusing oxycodone. Two tested positive for benzodiazepines and opioids, and one each for only benzodiazepines, only opioids, only amphetamines. One patient screened positive for unidentified narcotics.
Palliative care programs continue growth in U.S. hospitals
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.
“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
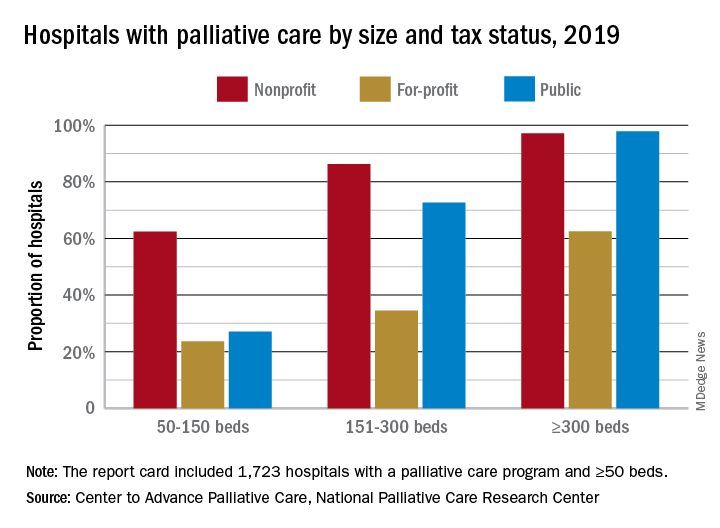
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Apixaban prevents clots with cancer
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Dual therapy best for AFib with ACS no matter the treatment strategy
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
SAN FRANCISCO – Anticoagulation with apixaban and a P2Y12 inhibitor without aspirin provides superior safety and similar efficacy in patients with atrial fibrillation who have an acute coronary syndrome, compared with regimens that include vitamin K antagonists, aspirin, or both.
The findings come from a prespecified analysis of data from the AUGUSTUS trial presented by Stephan Windecker, MD, at the Transcatheter Cardiovascular Therapeutics annual meeting.
“This study adds very important information [to the notion] that triple therapy in the setting of atrial fibrillation and PCI [percutaneous coronary intervention] is really not the way to go,” Ori Ben-Yehuda, MD, FACC, executive director of the Cardiovascular Research Foundation’s Clinical Trials Center, said during a media briefing.
In the recent multicenter AUGUSTUS trial, Dr. Windecker, of the department of cardiology at Bern University Hospital, Switzerland, and colleagues found that among 4,614 patients with atrial fibrillation and a recent acute coronary syndrome or PCI treated with a P2Y12 inhibitor, apixaban without aspirin resulted in less bleeding, fewer hospitalizations, and no significant differences in ischemic events compared with regimens that included a vitamin K antagonist (VKA), aspirin, or both (N Engl J Med. 2019;380:1509-24). For this prespecified analysis, the researchers used a 2×2 factorial design to compare apixaban with VKA and aspirin with placebo in the AUGUSTUS trial participants with ACS treated medically (group 1; 1,097 patients, or 24%), those with ACS treated with PCI (group 2; 1,714 patients, or 37%), and those undergoing elective PCI (group 3; 1,784 patients, or 39%). The outcomes of interest were bleeding, death, and hospitalization as well as death and ischemic events by antithrombotic strategy in the study participants. This marks the only trial in the field that included patients with ACS managed medically, Dr. Windecker said.
At baseline, the median age of patients was 71 years, 30% were female, 36% had diabetes, and 45% had heart failure. Patients managed medically were younger (a median age of 70) and more frequently female; 57% presented with heart failure. The groups had identical CHA2DS2VASc scores (4), and very similar HAS-BLED scores (2 in groups 1 and 2, and 3 in group 3).
Apixaban compared with VKA showed lower International Society on Thrombosis and Haemostasis–defined major or clinically relevant nonmajor bleeding among patients in group 1 (HR, 0.44), group 2 (HR, 0.68), and group 3 (HR, 0.82) (P for interaction = .052). Apixaban compared with VKA reduced death or hospitalization among patients in group 1 (HR, 0.71), group 2 (HR 0.88), and group 3 (HR, 0.87) (P for interaction = .345). Compared with VKA, apixaban resulted in a similar effect on death and ischemic events among patients in all three treatment groups (P for interaction = .356).
Compared with placebo, aspirin had a higher rate of bleeding among patients in group 1 (HR, 1.49), group 2 (HR, 2.02) and group 3 (HR, 1.91) (P for interaction = .479). For the same comparison, there was no difference in outcomes among the three groups for the composite of death or hospitalization and death and ischemic events.
“The overall results of the AUGUSTUS trial are consistent across the three clinically important subgroups,” Dr. Windecker said. The reasons why patients received medical therapy remain unclear, “because it was at the physician’s discretion as to whether they were treated medically or underwent PCI,” he said. “The proportion very much reflects our clinical practice, where 20%-25% of patients are treated medically. What was surprising for me is that I would have anticipated there would be more elderly patients with comorbidities, but I did anticipate that there would be more female patients (in this subgroup).”
Robert A. Harrington, MD, an interventional cardiologist at Stanford (Calif.) University who served on the Data Safety and Monitoring Board for the trial, noted that the patients with atrial fibrillation represent 7%-10% of all ACS patients, “so it’s a big population,” he said. “What’s been disappointing is that none of the trials have been big enough to uncouple the bleeding vs. ischemic issue. We don’t know the answer for how long do you need the triple therapy versus when you can switch to the double therapy.”
Dr. Windecker said that the optimal duration of short-term aspirin remains unclear in this patient population. “Whether there is a benefit of giving aspirin for 2 weeks or 4 weeks remains unanswered,” he said. “Triple therapy is not the way to go, but we need to fine-tune, and probably individualize, which patients may benefit from a certain duration of aspirin.”
The study results were published online at the time of presentation (Circulation 2019 Sep 26. doi: 10.1161/CIRCULATIONAHA.119.043308.
AUGUSTUS was funded by Bristol-Myers Squibb and Pfizer Inc. Dr. Windecker reported having received institutional research and educational grants to Bern University Hospital from Abbott, Amgen, Bayer, BMS, CSL Behring, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Polares, and Sinomed. His coauthors reported having numerous financial ties to the pharmaceutical and device industries.
SOURCE: Windecker S. TCT 2019, Late-Breaking Trials 1 session.
AT TCT 2019