User login
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
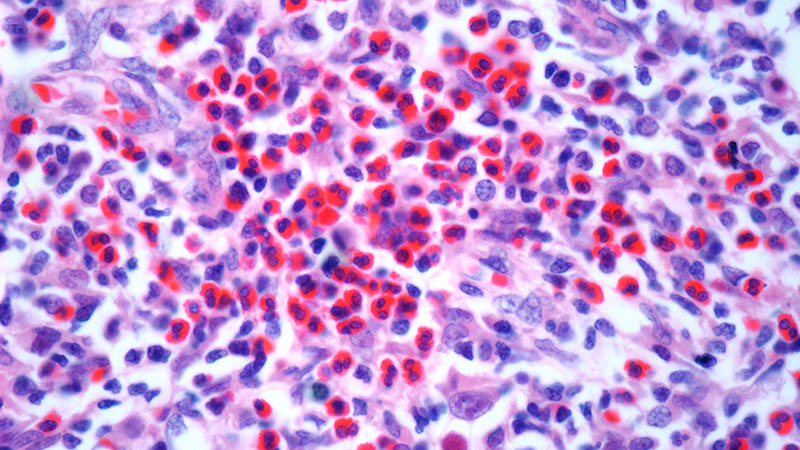
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Case Presentation: First Ever VA "Bloodless" Autologous Stem Cell Transplant Was a Success
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Background
Autologous stem cell transplant (ASCT) is an important part of the treatment paradigm for patients with multiple myeloma (MM) and remains the standard of care for newly diagnosed patients. Blood product transfusion support in the form of platelets and packed red blood cells (pRBCs) is part of the standard of practice as supportive measures during the severely pancytopenic period. Some MM patients, such as those of Jehovah’s Witness (JW) faith, may have religious beliefs or preferences that preclude acceptance of such blood products. Some transplant centers have developed protocols to allow safe “bloodless” ASCT that allows these patients to receive this important treatment while adhering to their beliefs or preferences.
Case Presentation
A 61-year-old veteran of JW faith with newly diagnosed IgG Kappa Multiple Myeloma was referred to the Tennessee Valley Healthcare System (TVHS) Stem Cell Transplant program for consideration of “bloodless” ASCT. With the assistance and expertise of the academic affiliate, Vanderbilt University Medical Center’s established bloodless ASCT protocol, this same protocol was established at TVHS to optimize the patient’s care pretransplant (use of erythropoiesis stimulating agents, intravenous iron, B12 supplementation) as well as post-transplant (use of antifibrinolytics, close inpatient monitoring). Both Ethics and Legal consultation was obtained, and guidance was provided to create a life sustaining treatment (LST) note in the veteran’s electronic health record that captured the veteran’s blood product preference. Once all protocols and guidance were in place, the TVHS SCT/CT program proceeded to treat the veteran with a myeloablative melphalan ASCT. The patient tolerated the procedure exceptionally well with minimal complications. He achieved full engraftment on day +14 after ASCT as expected and was discharged from the inpatient setting. He was monitored in the outpatient setting until day +30 without further complications.
Conclusions
The TVHS SCT/CT performed the first ever bloodless autologous stem cell transplant within the VA. This pioneering effort to establish such protocols to provide care to all veterans whatever their personal or religious preferences is a testament to commitment of VA to provide care for all veterans and the willingness to innovate to do so.
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
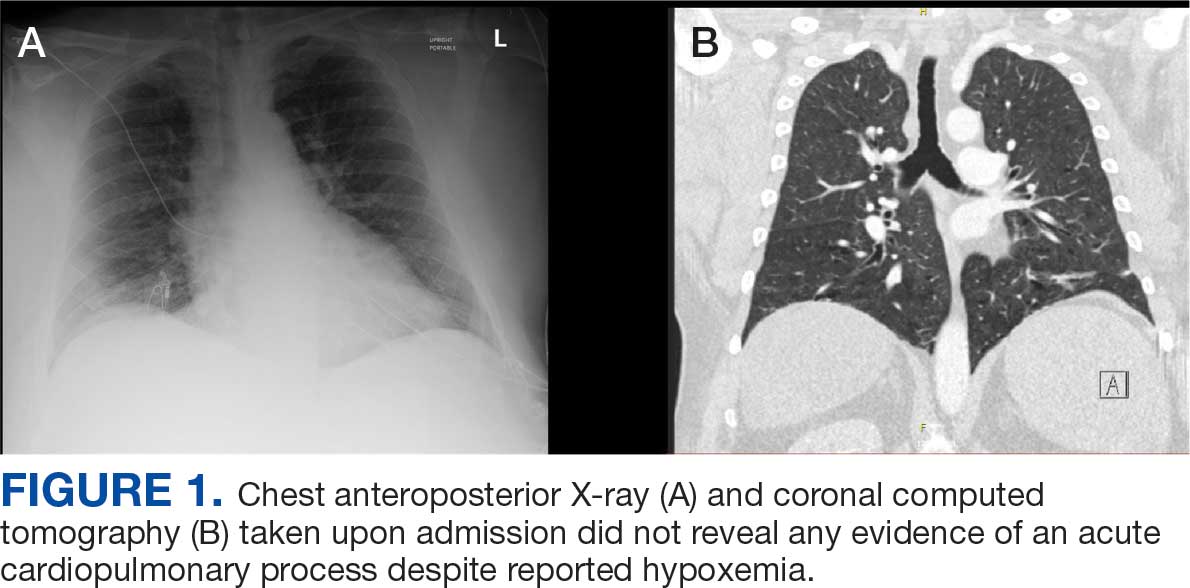
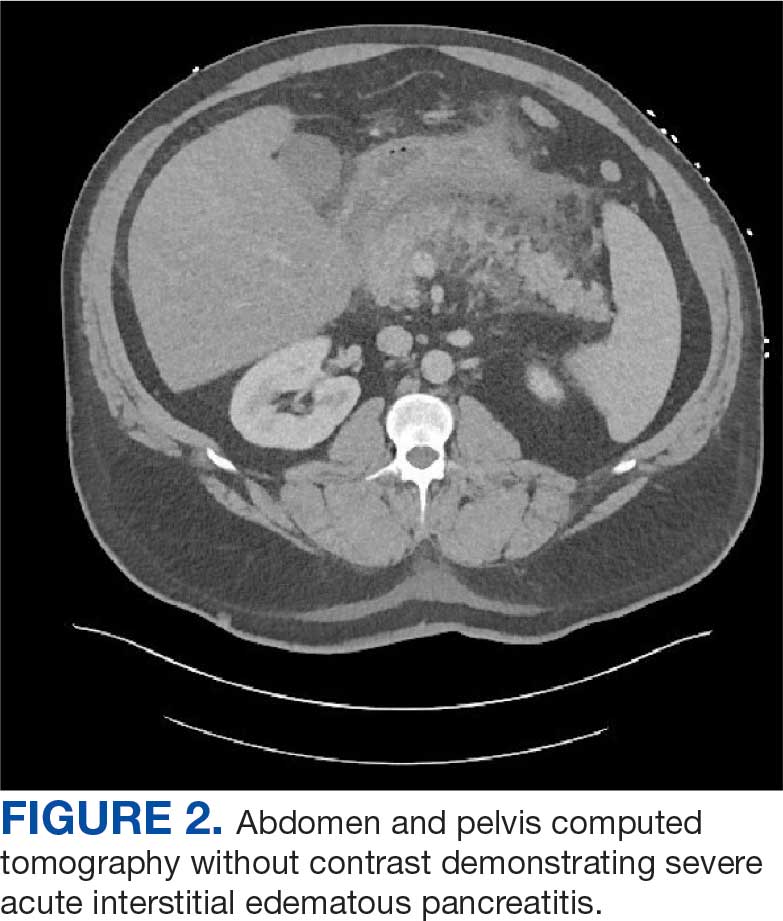
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.
The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
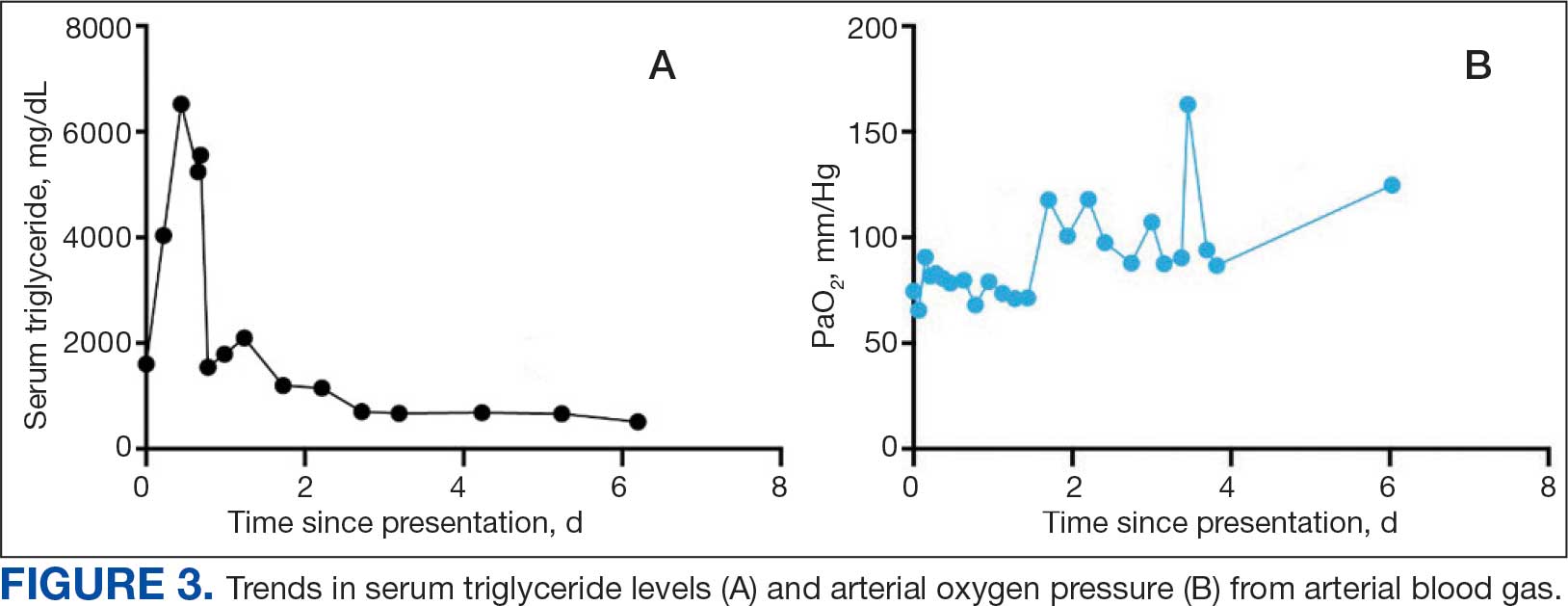
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).
Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1
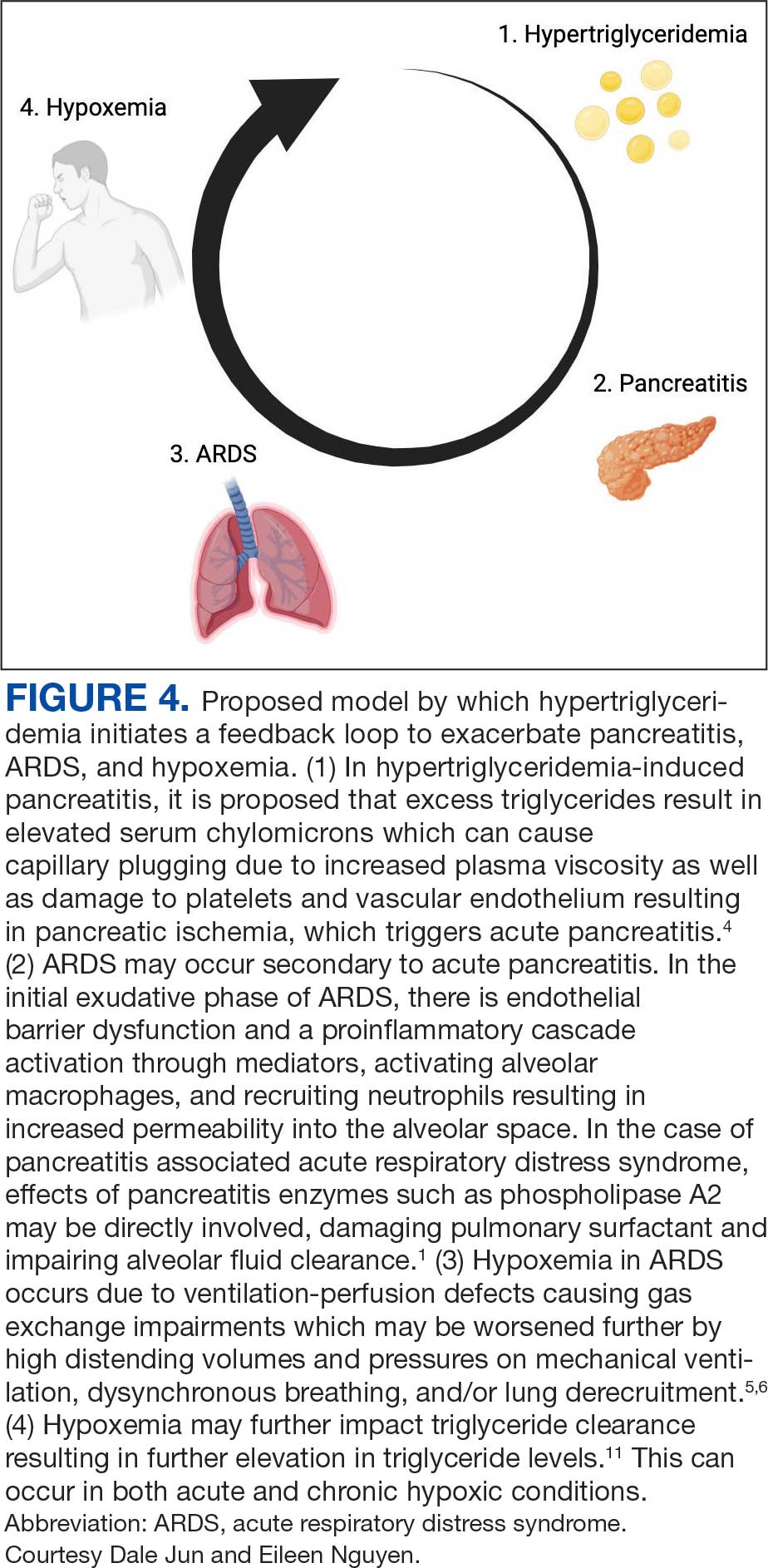
Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
Acute pancreatitis can be associated with multiorgan system failure, including respiratory failure, which has a high mortality rate. Acute respiratory distress syndrome (ARDS) is a known complication of severe, acute pancreatitis, and is fatal in up to 40% of cases. Mortality rates exceed 80% in patients with PaO2/FiO2 < 100 mm Hg.2 Although ARDS is typically associated with bilateral pulmonary infiltrates, severe hypoxemia in pancreatitis may not be visible in radiography in up to 50% of cases.1
Hypertriglyceridemia is the third-most common cause of acute pancreatitis, with an incidence of 2% to 10% among patients diagnosed with acute pancreatitis.3.4 Elevated serum triglycerides have been proposed to trigger acute pancreatitis by increasing plasma viscosity, which leads to ischemia and inflammation of the pancreas.4 In severe cases of hypertriglyceridemia-induced acute pancreatitis, plasmapheresis is used to rapidly reduce serum chylomicron and triglyceride levels.3
This case report discusses a patient with acute pancreatitis whose hypoxemia coincided with the severity of hypertriglyceridemia, but without radiographic evidence of pulmonary infiltrates or other known pulmonary causes.
Case Presentation
A 60-year-old male presented to the emergency department with several hours of diffuse abdominal pain, nausea, and vomiting. The patient reported that his symptoms began after eating fried chicken. He reported no dyspnea, fever, chills, or other symptoms. His medical history included type 2 diabetes (hemoglobin A1c, 11.1%), Hashimoto hypothyroidism, severe obstructive sleep apnea not on continuous positive airway pressure (apnea-hypoxia index, 59/h), and obesity (body mass index, 52). Initial vital signs were afebrile, heart rate of 90 beats/min, and oxygen saturation (SpO2) of 85% on 6L oxygen via nasal cannula. He was admitted to the intensive care unit and quickly maximized on high flow nasal cannula, ultimately requiring endotracheal intubation and mechanical ventilation.
Initial laboratory studies were remarkable for serum sodium of 120 mmol/L (reference range, 136-146 mmol/L), creatinine of 1.65 mg/dL (reference range, 0.52-1.28 mg/dL), anion gap of 18 mEq/L (reference range, 3-11 mEq/L), lipase level of 1115 U/L (reference range, 11-82 U/L), glucose level of 334 mg/dL (reference range, 70-110 mg/dL), white blood count of 13.1 K/uL (reference range, 4.5-11.0 K/uL), lactate level of 3.8 mmol/L (reference range, 0.5-2.2 mmol/L), triglyceride level of 1605 mg/dL (reference range, 40-160 mg/dL), cholesterol level of 565 mg/dL (reference range, < 200 mg/dL), aminotransferase of 21 U/L (reference range, 13-36 U/L), alanine aminotransferase of < 3 U/L (reference range, 7-45 U/L), and total bilirubin level of 1.6 mg/dL (reference range, 0.2-1 mg/dL).
The patient had an initial arterial blood gas pH of 7.26, partial pressure of CO2 and O2 of 64.1 mm Hg and 74.1 mm Hg, respectively, on volume control with a tidal volume of 500 mL, positive end-expiratory pressure of 10 cm H2O, respiratory rate of 26 breaths/min, and FiO2 was 100%, which yielded a PaO2/FiO2 of 74 mm Hg. The patient was maintained in steep reverse-Trendelenburg position with moderate improvement in his SpO2.
Chest X-ray and computed tomography angiogram did not reveal pleural effusions, pulmonary infiltrates, or pulmonary embolism (Figure 1). Computed tomography of the abdomen and pelvis demonstrated severe acute interstitial edematous pancreatitis with no evidence of pancreatic necrosis or evidence of gallstones (Figure 2). A transthoracic echocardiogram with bubble was negative for intracardiac right to left shunting.


The leading diagnosis was ARDS secondary to acute pancreatitis with hypoxemia exacerbated by morbid obesity and untreated obstructive sleep apnea leading to hypoventilation.
Treatment
The patient was intubated and restricted to nothing by mouth and provided fluid resuscitation with crystalloids. On hospital day 1, he remained intubated and on mechanical ventilation, started on plasmapheresis and continued insulin infusion for severe hypertriglyceridemia. The patient’s PaO2/FiO2 ratio remained persistently < 100 mm Hg despite maximal ventilatory support. After 3 sessions of plasmapheresis, the serum triglyceride levels and oxygen requirements improved (Figure 3).

Due to prolonged intubation, the patient ultimately required a tracheostomy. By hospital day 48, the patient was successfully weaned off mechanical ventilation. His tracheostomy was decannulated uneventfully on hospital day 55 and the stoma was closed. The patient was discharged to a skilled nursing home for rehabilitation and received intensive physical therapy for deconditioning from prolonged hospitalization.
Discussion
Respiratory insufficiency is a common and potentially lethal complication observed in one-third of patients with acute pancreatitis.1 Radiographic evidence of pleural effusions, atelectasis and pulmonary infiltrates are often present. Acute lung injury (ALI) and ARDS are the most severe pulmonary complications of acute pancreatitis.5 It has been proposed that ALI and ARDS are driven by a hyperinflammatory state, which has multiple downstream effects. Pulmonary parenchymal and vascular damage has been associated with activated proinflammatory cytokines, trypsin, phospholipase A, and free fatty acids (Figure 4).1

Hypoxemia secondary to acute pancreatitis may occur without initial radiographic findings and has been observed in up to half of patients.1 Hypoxemia in ARDS occurs due to ventilation-perfusion defects causing gas exchange impairments which may be worsened further by high distending volumes and pressures on mechanical ventilation, dyssynchronous breathing, and/or lung derecruitment.6 Patients who require intubation for pancreatitis-associated ALI or ARDS eventually exhibit imaging findings consistent with their disease.1 The patient in this case exhibited severe hypoxemia for several days despite persistently negative radiographic studies. His history of obstructive sleep apnea and a body mass index of 52 may have contributed to respiratory failure; however, assessment of other contributors to the acute and profound hypoxemia yielded largely unremarkable results. The patient did not have a history or evidence of heart failure and his hypoxemia did not improve with diuresis. He tested positive for COVID-19 on admission and was briefly treated with remdesivir and dexamethasone, but it was determined that the test was likely a false positive due to negative subsequent tests and elevated cycle thresholds (> 40). A concomitant COVID-19 infection likely did not contribute to his symptoms.
Ventilation-perfusion mismatch is a well-recognized complication of pancreatitis, which results in right-to-left shunting.5 While we considered whether an intracardiac shunt may have contributed to the patient’s hypoxemia, a transthoracic echocardiogram with bubble contrast was negative.
The patient had a peak serum triglyceride of > 6000 mg/dl, which meets the criteria for very severe hypertriglyceridemia.7 As observed in prior reports, the extent of the hypertriglyceridemia in this patient resulted in pronounced lipemic blood, which was appreciable by the eye and necessitated several rounds of centrifugation to analyze the laboratory studies.8 In this case, plasmapheresis was used to rapidly treat the hypertriglyceridemia, thereby reducing inflammation and further damage to the pancreas.9
It is possible the patient’s hypertriglyceridemia may have been associated with his hypoxemia. His hypoxemia was most pronounced approximately 24 hours postadmission, which coincided with the peak of the hypertriglyceridemia. It remains unclear whether the severity of triglyceride elevation could accurately predict the severity of respiratory insufficiency. Hypoxemia is thought to modulate triglyceride metabolism through stimulation of intracellular lipolysis, upregulation of very low-density lipoproteins production in the liver, and inhibition of triglyceride-rich lipoprotein metabolism.10 Evidence from rodent studies supports the idea that acute hypoxemia increases triglycerides, and the degree of hypoxemia correlates with the elevated triglyceride levels.11 However, this has not been consistently observed in humans and may vary by prandial state.12,13 Thus, dysfunction of lipid metabolism may be a relevant clinical indicator of hypoxemia; further work is needed to elucidate this association.
Patient Perspective
The patient continues to undergo extensive rehabilitation following his prolonged illness and hospitalization. He expressed gratitude for the care received. However, he has limited and distorted recollection of the events during his hospitalization and stated that it felt “like an extraterrestrial state.”
Conclusions
This report describes a case of marked hypoxemia in the setting of acute pancreatitis. Pulmonary insufficiency in acute pancreatitis is commonly associated with imaging findings such as atelectasis, pleural effusions, and pulmonary infiltrates; however, up to half of cases initially lack any radiographic findings. Plasmapheresis is an effective treatment for hypertriglyceridemia-induced pancreatitis to both directly reduce circulating triglycerides and inflammation. Plasmapheresis also represents a promising therapy for the prevention of further episodes of pancreatitis in patients with recurrent pancreatitis. We propose a feedback mechanism through which pancreatitis induces severe hypoxemia, which may modulate lipid metabolism and severe hypertriglyceridemia correlates with respiratory failure.
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
- Zhou M-T, Chen C-S, Chen B-C, Zhang Q-Y, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094-2099. doi:10.3748/wjg.v16.i17.2094
- Peek GJ, White S, Scott AD, et al. Severe acute respiratory distress syndrome secondary to acute pancreatitis successfully treated with extracorporeal membrane oxygenation in three patients. Ann Surg. 1998;227(4):572-574. doi:10.1097/00000658-199804000-00020
- Searles GE, Ooi TC. Underrecognition of chylomicronemia as a cause of acute pancreatitis. Can Med Assoc J. 1992;147(12):1806-1808.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649-655. doi:10.1177/2050640618755002
- Ranson JH, Turner JW, Roses DF, et al. Respiratory compli cations in acute pancreatitis. Ann Surg. 1974;179(5):557-566. doi:10.1097/00000658-197405000-00006 6. Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Swenson KE, Swenson ER. Pathophysiology of acute respiratory distress syndrome and COVID- 19 lung injury. Crit Care Clin. 2021;37(4):749-776. doi:10.1016/j.ccc.2021.05.003
- Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(9):2969-2989. doi:10.1210/jc.2011-3213
- Ahern BJ, Yi HJ, Somma CL. Hypertriglyceridemia-induced pancreatitis and a lipemic blood sample: a case report and brief clinical review. J Emerg Nurs. 2022;48(4):455-459. doi:10.1016/j.jen.2022.02.001
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:4721357. doi:10.1155/2018/4721357
- Morin R, Goulet N, Mauger J-F, Imbeault P. Physiological responses to hypoxia on triglyceride levels. Front Physiol. 2021;12:730935. doi:10.3389/fphys.2021.730935
- Jun JC, Shin M-K, Yao Q, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab. 2012;303(3):E377-88. doi:10.1152/ajpendo.00641.2011
- Mahat B, Chassé É, Lindon C, Mauger J-F, Imbeault P. No effect of acute normobaric hypoxia on plasma triglyceride levels in fasting healthy men. Appl Physiol Nutr Metab. 2018;43(7):727-732. doi:10.1139/apnm-2017-0505
- Mauger J-F, Chassé É, Mahat B, Lindon C, Bordenave N, Imbeault P. The effect of acute continuous hypoxia on triglyceride levels in constantly fed healthy men. Front Physiol. 2019;10:752. doi:10.3389/fphys.2019.00752
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Profound Hypoxemia in a Patient With Hypertriglyceridemia-Induced Pancreatitis
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.
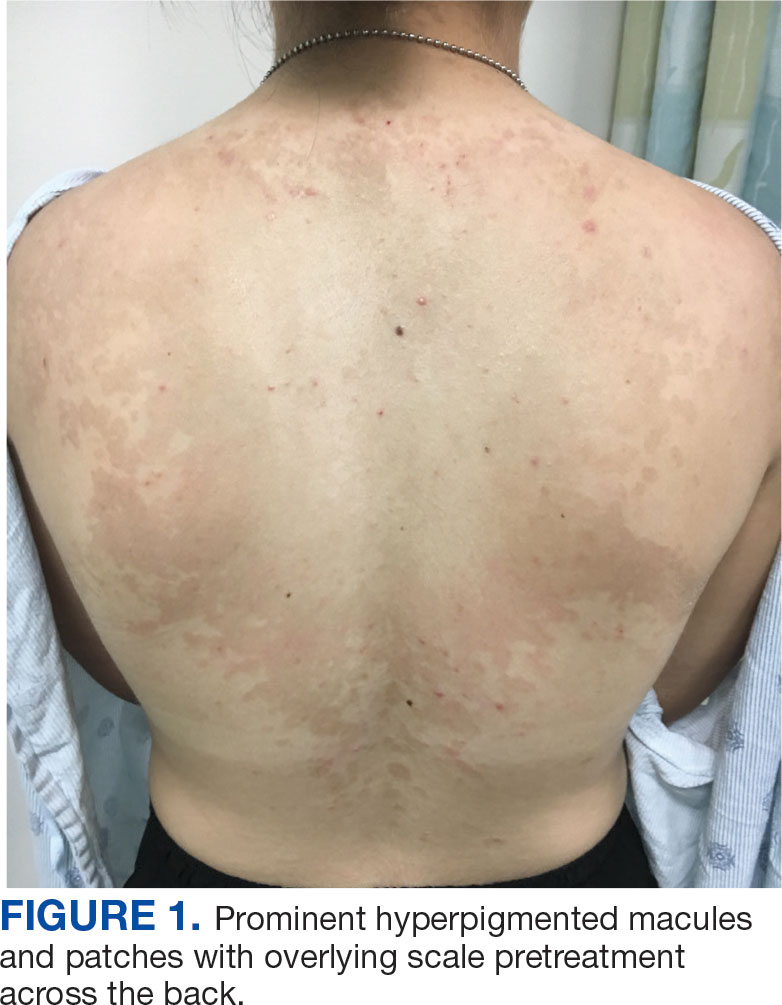
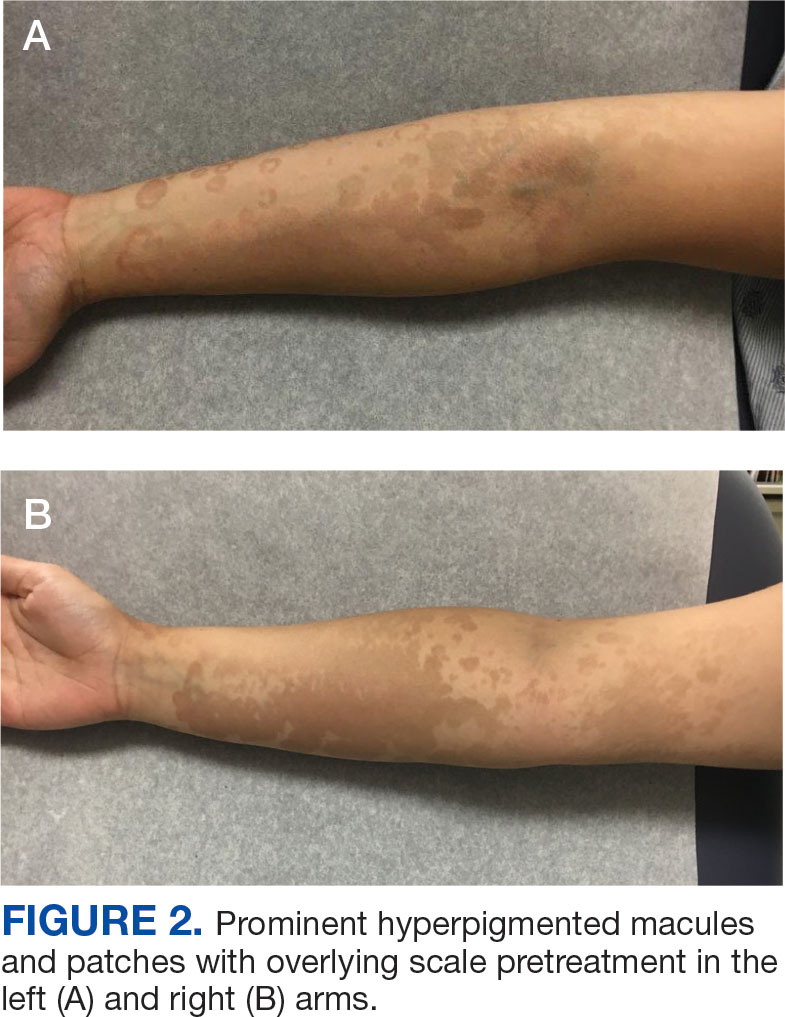
The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.
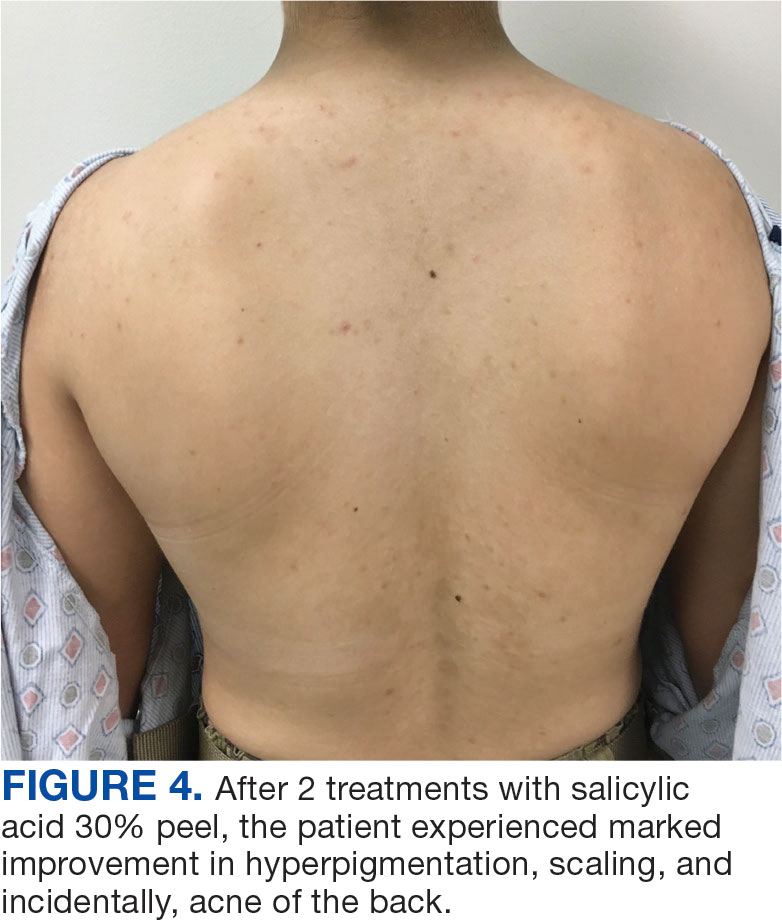
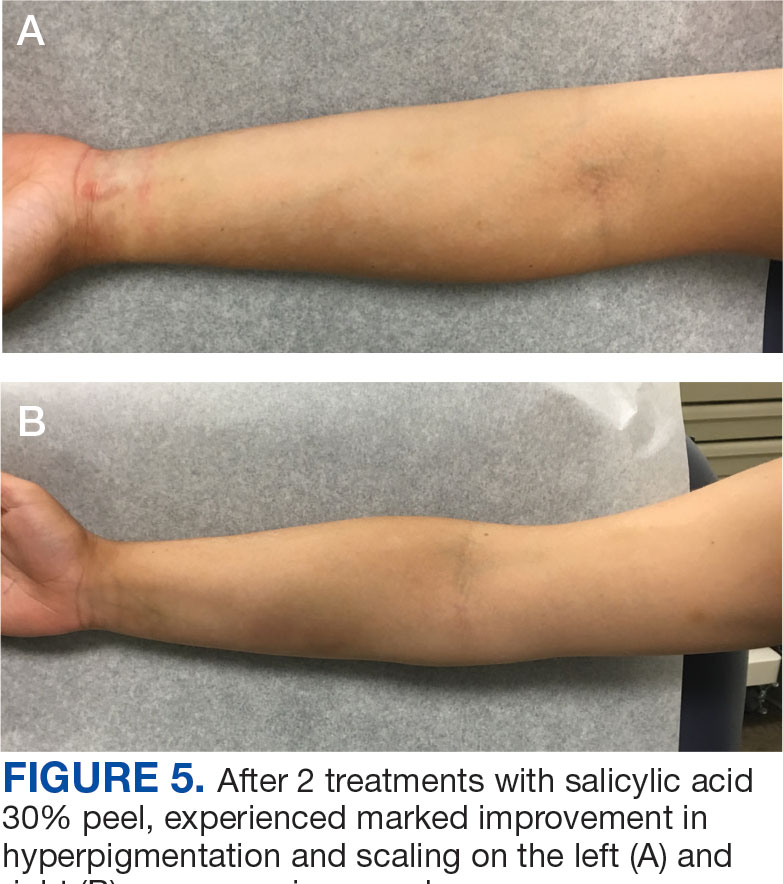
Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
Tinea versicolor (TV) is a common, chronic, and recurrent superficial fungal infection caused by Malassezia species, most commonly Malassezia furfur (M. furfur)—a dimorphic fungus that is a part of the normal skin flora and resides in the stratum corneum.1 TV manifests as hypopigmented, hyperpigmented, or erythematous macules and patches with scaling, typically found on the trunk and proximal upper extremities. The condition is most common among young to middle-aged individuals exposed to high temperatures and humidity.1
While many cases respond to topical antifungal treatment, application can be cumbersome, particularly in large areas that are difficult to reach. An efficient and cost effective in-office treatment option could alleviate patient burden and improve satisfaction. This article presents a case of TV successfully treated with an in-office salicylic acid (SA) 30% peel, an uncommon application of this medication.
Case Presentation
An 18-year-old female active-duty US Army service member with a history of acne vulgaris presented to a dermatology clinic with a mildly pruritic rash that had been present for several weeks. An examination revealed hyperpigmented macules and patches with overlying fine scales across the patient’s back and bilateral arms (Figures 1 and 2). She reported no history of similar lesions. The patient had recently completed a military basic training course during which she wore a uniform jacket and trousers daily in hot and humid conditions. A skin scraping was obtained. Microscopic examination with potassium hydroxide preparation revealed hyphae and spores, consistent with TV.


The diagnosis of TV and treatment options (topical ketoconazole 2% shampoo, topical terbinafine, or oral fluconazole) were discussed with the patient. Due to military training-related constraints, residence in the barracks, and personal preference, the patient felt unable to regularly apply topical medications to the entirety of the affected area and preferred to avoid oral medication. The decision was made to pursue in-clinic treatment with a SA 30% peel. The affected areas (back and bilateral arms) were thoroughly cleansed and prepped with alcohol. SA 30% in hydroethanolic solution was applied evenly to the affected area. The patient was observed for pseudofrosting, a precipitation of SA crystals that indicates peel completion (Figure 3). The peel was left in place, as it is self-neutralizing, and the patient was instructed to shower that same day with a gentle cleanser. This procedure was repeated 10 days later. Both treatments were well tolerated, with only a transient burning sensation reported during the application. At 3-week follow-up, the patient presented with complete resolution of her arm lesions and significant improvement of the back lesions (Figures 4 and 5). She also reported improvement in the acne vulgaris on her back.



Discussion
SA 30% is a lipid-soluble hydroxybenzoic acid with comedolytic and desmolytic qualities. This results in the disruption of epidermal cell cohesion and promotes exfoliation.2 Lipophilic properties allow SA to penetrate sebaceous glands and disrupt sebum production, making it particularly effective in seborrheic conditions such as acne. This mechanism may have increased therapeutic effect in this case.3 Additionally, as a salicylate, SA possesses anti-inflammatory properties, though this effect is most pronounced at lower concentrations. SA 30% is considered a superficial peel, as the depth of chemexfoliation is limited to the epidermis.3 A modified SA preparation is a safe and effective treatment for moderate-to-severe acne vulgaris. The apparent pseudofrost during application is due to precipitated SA, rather than the precipitation of dermal proteins seen in deeper peels, such as trichloroacetic acid.2 Unlike glycolic or pyruvic acid peels, SA does not require neutralization.
SA is cost-effective and has been used safely in all skin types to treat various epidermal conditions, including acne vulgaris, melasma, photodamage, freckles, lentigines and postinflammatory hyperpigmentation (PIH).2 Mild adverse effects occur in about 15% to 30% of patients and include prolonged erythema, intense exfoliation, dryness, crusting, and pigmentary dyschromias. Rare adverse effects include systemic toxicity (salicylism) and hypoglycemia. Contraindications to SA 30% peels include history of allergy to salicylates, active bacterial or viral infection, dermatitis in the treatment area, pregnancy, and skin malignancy.2
Chemical peels are typically used with caution in patients with skin of color due to a higher risk of PIH. However, SA 30% has been shown to be safe and effective in these populations.4 A study by Grimes found that 88% of patients with Fitzpatrick skin types V and VI experienced significant improvement in PIH, melasma, or enlarged pores with minimal to no adverse effects.4 Subsequent larger studies have reinforced these findings. In a study involving 250 patients with Fitzpatrick skin types IV and V, no patients experienced PIH, confirming the safety of SA in darker skin tones. This is likely due to the superficial nature of the peel, which does not affect the basal layer of the epidermis where melanocytes reside, reducing the risk of pigmentary complications. Additionally, SA peels are self-neutralizing, unlike glycolic or trichloroacetic acid peels, which require manual neutralization and carry a higher risk of PIH if not neutralized properly.5
SA has been as shown to be a moderately successful treatment for PIH. The Grimes study found that 4 of 5 patients with Fitzpatrick skin types IV and V saw a 75% improvement in PIH after SA peels.4 Davis et al found a nonsignificant trend toward skin lightening in Korean adults treated for acne and PIH, with significant decreases in erythema and improvements in greasiness, dryness, and scaliness.6 Importantly, the risk of PIH following TV is higher in patients with skin of color.7 SA may be effective in treating TV and PIH, offering a multifactorial approach by addressing both conditions while posing a low risk for causing PIH.8
TV and other Malassezia spp infections are common concerns in dermatology and primary care, with Malassezia-associated superficial mycoses (eg, dandruff, pityriasis versicolor, and folliculitis) affecting up to 50% of the population worldwide.9 Despite this, there has been little recent advancement in antifungal treatments. Ketoconazole, terbinafine, and fluconazole have been in use since the 1980s and 1990s.8 Most antifungal drugs target ergosterol, a component of the fungal cell wall.10 Additionally, Malassezia spp have been increasingly reported to cause invasive infections in immunocompromised patients.11 Given the rise in antifungal resistance, the judicious use of antifungals and implementation of novel treatment strategies is essential.
While SA lacks intrinsic antifungal properties, different combinations (Whitfield ointment consisting of 3% SA and 6% benzoic acid; 2% sulfur and 2% SA) have been effective in the treatment of TV.1 It is theorized that the effectiveness of SA against TV is due to its ability to exfoliate and acidify the stratum corneum, the natural habitat of M. furfur.
SA also reduces sebum production by downregulating sebocyte lipogenesis via the sterol regulatory element-binding protein-1 pathway and suppressing the nuclear factor κB (NF-κB) pathway, a key pathway in inflammation.12 These mechanisms make SA an effective acne treatment. Additionally, M. furfur is a lipid-dependent yeast, thus the decreased lipogenesis by sebocytes may be beneficial in treating TV as well.12 A study of 25 patients with TV in India found that 88% achieved clinical and microbiological cure after 4 once-weekly treatments of a SA 30% peel.8
In a study of deployed military personnel, fungal infections affected about 11% of participants.13 Contributing factors to the development of fungal infections included excessive sweating, humid conditions, and limited access to hygiene facilities. In such settings, traditional antifungal therapies may be less effective or challenging to adhere to, making alternative treatments more desirable. SA peels could offer a practical solution in these circumstances, as they are easily applied in the clinic, require no neutralization or downtime, and do not require the patient to apply medications between visits.
In this case, the patient demonstrated significant improvement with 2 SA peels, with noted improvement in her acne. SA 30% peel was highlighted as a useful treatment option for patients with TV who struggle with topical medication adherence; furthermore, it may be particularly beneficial for patients with concomitant acne.
Conclusions
This case demonstrates the successful use of in-office SA 30% peel as a treatment for TV. The rapid improvement and resolution of lesions with minimal adverse effects suggest that SA peel may serve as a valuable alternative for patients with extensive disease in difficult-to-reach affected areas, or those who are dissatisfied with traditional therapies. Additionally, the concurrent improvement of the patient’s back acne underscores the dual therapeutic potential of this treatment. Given the ease of application, cost effectiveness, and favorable safety profile, SA 30% peel is a viable option in the management of TV, especially in cases where topical or oral antifungals are impractical. Further studies could help establish standardized protocols and assess long-term outcomes of this treatment modality.
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
- Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi:10.7573/dic.2022-9-2
- Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455-461. doi:10.2147/CCID.S84765
- Shao X, Chen Y, Zhang L, et al. Effect of 30% supramolecular salicylic acid peel on skin microbiota and inflammation in patients with moderate-to-severe acne vulgaris. Dermatol Ther. 2022;13(1):155-168. doi:10.1007/s13555-022-00844-5
- Grimes PE. The safety and efficacy of salicylic acid chemical peels in darker racial-ethnic groups. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 1999;25(1). doi:10.1046/j.1524-4725.1999.08145.x
- Kang HY, Choi Y, Cho HJ. Salicylic acid peels for the treatment of acne vulgaris in Fitzpatrick skin types IV-V: a multicenter study. Dermatol Surg. Published online 2006. doi:10.1111/j.1524-4725.2006.32146.x.
- Davis EC, Callender VD. Postinflammatory hyperpigmentation. J Clin Aesthetic Dermatol. 2010;3(7):20-31.
- Kallini JR, Riaz F, Khachemoune A. Tinea versicolor in dark-skinned individuals. Int J Dermatol. 2014;53(2):137- 141. doi:10.1111/ijd.12345
- Saoji V, Madke B. Efficacy of salicylic acid peel in dermatophytosis. Indian J Dermatol Venereol Leprol. 2021;87(5). doi:10.4103/ijdvl.IJDVL_853_18
- Arce M, Gutiérrez-Mendoza D. Pityriasis versicolor: treatment update. Curr Fungal Infect Rep 2018;12(11):195–200. https://doi.org/10.1007/s12281-018-0328-7
- Leong C, Kit JCW, Lee SM, et al. Azole resistance mechanisms in pathogenic M. furfur. Antimicrob Agents Chemother. 2021;65(5):e01975-20. doi:10.1128/AAC.01975-20
- Chang HJ, Miller HL, Watkins N, et al. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N Engl J Med. 1998;338(11):706-711. doi:10.1056/NEJM199803123381102
- Lu J, Cong T, Wen X, et al. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp Dermatol. 2019;28(7):786-794. doi:10.1111/exd.13934
- Singal A, Lipner SR. A review of skin disease in military soldiers: challenges and potential solutions. Ann Med. 2023;55(2):2267425. doi:10.1080/07853890.2023.2267425
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Successful Treatment of Tinea Versicolor With Salicylic Acid 30% Peel
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
Histology is the gold standard for cirrhosis diagnosis. However, a combination of clinical history, physical examination findings, and supportive laboratory and radiographic features is generally sufficient to make the diagnosis. Routine ultrasound and computed tomography (CT) imaging often identifies a nodular liver contour with sequelae of portal hypertension, including splenomegaly, varices, and ascites, which can suggest cirrhosis when supported by laboratory parameters and clinical features. As a result, the diagnosis is typically made clinically.1 Many patients with compensated cirrhosis go undetected. The presence of a decompensation event (ascites, spontaneous bacterial peritonitis, variceal hemorrhage, or hepatic encephalopathy) often leads to index diagnosis when patients were previously compensated. When a patient presents with suspected decompensated cirrhosis, it is important to consider other diagnoses with similar presentations and ensure that multiple disease processes are not contributing to the symptoms.
CASE PRESENTATION
A 64-year-old male with a history of intravenous (IV) methamphetamine use and prior incarceration presented with a 3-week history of progressively worsening generalized swelling. Prior to the onset of his symptoms, the patient injured his right lower extremity (RLE) in a bicycle accident, resulting in edema that progressed to bilateral lower extremity (BLE) edema and worsening fatigue, despite resolution of the initial injury. The patient gained weight though he could not quantify the amount. He experienced progressive hunger, thirst, and fatigue as well as increased sleep. Additionally, the patient experienced worsening dyspnea on exertion and orthopnea. He started using 2 pillows instead of 1 pillow at night.
The patient reported no fevers, chills, sputum production, chest pain, or paroxysmal nocturnal dyspnea. He had no known history of sexually transmitted infections, no significant history of alcohol use, and occasional tobacco and marijuana use. He had been incarcerated > 10 years before and last used IV methamphetamine 3 years before. He did not regularly take any medications.
The patient’s vital signs included a temperature of 98.2 °F; 78/min heart rate; 15/min respiratory rate; 159/109 mm Hg blood pressure; and 98% oxygen saturation on room air. He had gained 20 lbs in the past 4 months. He had pitting edema in both legs and arms, as well as periorbital swelling, but no jugular venous distention, abnormal heart sounds, or murmurs. Breath sounds were distant but clear to auscultation. His abdomen was distended with normal bowel sounds and no fluid wave; mild epigastric tenderness was present, but no intra-abdominal masses were palpated. He had spider angiomata on the upper chest but no other stigmata of cirrhosis, such as caput medusae or jaundice. Tattoos were noted.
Laboratory test results showed a platelet count of 178 x 103/μL (reference range, 140- 440 ~ 103μL).Creatinine was 0.80 mg/dL (reference range, < 1.28 mg/dL), with an estimated glomerular filtration rate (eGFR) of 99 mL/min/1.73 m2 using the Chronic Kidney Disease-Epidemiology equation (reference range, > 60 mL/min/1.73 m2), (reference range, > 60 mL/min/1.73 m2), and Cystatin C was 1.14 mg/L (reference range, < 1.15 mg/L). His electrolytes and complete blood count were within normal limits, including sodium, 134 mmol/L; potassium, 4.4 mmol/L; chloride, 108 mmol/L; and carbon dioxide, 22.5 mmol/L.
Additional test results included alkaline phosphatase, 126 U/L (reference range, < 94 U/L); alanine transaminase, 41 U/L (reference range, < 45 U/L); aspartate aminotransferase, 70 U/L (reference range, < 35 U/L); total bilirubin, 0.6 mg/dL (reference range, < 1 mg/dL); albumin, 1.8 g/dL (reference range, 3.2-4.8 g/dL); and total protein, 6.3 g/dL (reference range, 5.9-8.3 g/dL). The patient’s international normalized ratio was 0.96 (reference range, 0.8-1.1), and brain natriuretic peptide was normal at 56 pg/mL. No prior laboratory results were available for comparison.
Urine toxicology was positive for amphetamines. Urinalysis demonstrated large occult blood, with a red blood cell count of 26/ HPF (reference range, 0/HPF) and proteinuria (100 mg/dL; reference range, negative), without bacteria, nitrites, or leukocyte esterase. Urine white blood cell count was 10/ HPF (reference range, 0/HPF), and fine granular casts and hyaline casts were present.
A noncontrast CT of the abdomen and pelvis in the emergency department showed an irregular liver contour with diffuse nodularity, multiple portosystemic collaterals, moderate abdominal and pelvic ascites, small bilateral pleural effusions with associated atelectasis, and anasarca consistent with cirrhosis (Figure 1). The patient was admitted to the internal medicine service for workup and management of newly diagnosed cirrhosis.

Paracentesis revealed straw-colored fluid with an ascitic fluid neutrophil count of 17/μL, a protein level of < 3 g/dL and albumin level of < 1.5 g/dL. Gram stain of the ascitic fluid showed a moderate white blood cell count with no organisms. Fluid culture showed no microbial growth.
Initial workup for cirrhosis demonstrated a positive total hepatitis A antibody. The patient had a nonreactive hepatitis B surface antigen and surface antibody, but a reactive hepatitis B core antibody; a hepatitis B DNA level was not ordered. He had a reactive hepatitis C antibody with a viral load of 4,490,000 II/mL (genotype 1a). The patient’s iron level was 120 μg/dL, with a calculated total iron-binding capacity (TIBC) of 126.2 μg/dL. His transferrin saturation (TSAT) (serum iron divided by TIBC) was 95%. The patient had nonreactive antinuclear antibody and antimitochondrial antibody tests and a positive antismooth muscle antibody test with a titer of 1:40. His α-fetoprotein (AFP) level was 505 ng/mL (reference range, < 8 ng/mL).
Follow-up MRI of the abdomen and pelvis showed cirrhotic morphology with large volume ascites and portosystemic collaterals, consistent with portal hypertension. Additionally, it showed multiple scattered peripheral sub centimeter hyperenhancing foci, most likely representing benign lesions.
The patient's spot urine protein-creatinine ratio was 3.76. To better quantify proteinuria, a 24-hour urine collection was performed and revealed 12.8 g/d of urine protein (reference range, 0-0.17 g/d). His serum triglyceride level was 175 mg/dL (reference range, 40-60 mg/dL); total cholesterol was 177 mg/ dL (reference range, ≤ 200 mg/dL); low density lipoprotein cholesterol was 98 mg/ dL (reference range, ≤ 130 mg/dL); and highdensity lipoprotein cholesterol was 43.8 mg/ dL (reference range, ≥ 40 mg/dL); C3 complement level was 71 mg/dL (reference range, 82-185 mg/dL); and C4 complement level was 22 mg/dL (reference range, 15-53 mg/ dL). His rheumatoid factor was < 14 IU/mL. Tests for rapid plasma reagin and HIV antigen- antibody were nonreactive, and the phospholipase A2 receptor antibody test was negative. The patient tested positive for QuantiFERON-TB Gold and qualitative cryoglobulin, which indicated a cryocrit of 1%.
A renal biopsy was performed, revealing diffuse podocyte foot process effacement and glomerulonephritis with low-grade C3 and immunoglobulin (Ig) G deposits, consistent with early membranoproliferative glomerulonephritis (MPGN) (Figures 2 and 3).


The patient was initially diuresed with IV furosemide without significant urine output. He was then diuresed with IV 25% albumin (total, 25 g), followed by IV furosemide 40 mg twice daily, which led to significant urine output and resolution of his anasarca. Given the patient’s hypoalbuminemic state, IV albumin was necessary to deliver furosemide to the proximal tubule. He was started on lisinopril for renal protection and discharged with spironolactone and furosemide for fluid management in the context of cirrhosis.
The patient was evaluated by the Liver Nodule Clinic, which includes specialists from hepatology, medical oncology, radiation oncology, interventional radiology, and diagnostic radiology. The team considered the patient’s medical history and characteristics of the nodules on imaging. Notable aspects of the patient’s history included hepatitis C virus (HCV) infection and an elevated AFP level, although imaging showed no lesion concerning for malignancy. Given these findings, the patient was scheduled for a liver biopsy to establish a tissue diagnosis of cirrhosis. Hepatology, nephrology, and infectious disease specialists coordinated to plan the management and treatment of latent tuberculosis (TB), chronic HCV, MPGN, compensated cirrhosis, and suspicious liver lesions.
The patient chose to handle management and treatment as an outpatient. He was discharged with furosemide and spironolactone for anasarca management, and amlodipine and lisinopril for his hypertension and MPGN. Follow-up appointments were scheduled with infectious disease for management of latent TB and HCV, nephrology for MPGN, gastroenterology for cirrhosis, and interventional radiology for liver biopsy. Unfortunately, the patient was unhoused with limited access to transportation, which prevented timely follow-up. Given these social factors, immunosuppression was not started. Additionally, he did not start on HCV therapy because the viral load was still pending at time of discharge.
DISCUSSION
The diagnosis of decompensated cirrhosis was prematurely established, resulting in a diagnostic delay, a form of diagnostic error. However, on hospital day 2, the initial hypothesis of decompensated cirrhosis as the sole driver of the patient’s presentation was reconsidered due to the disconnect between the severity of hypoalbuminemia and diffuse edema (anasarca), and the absence of laboratory evidence of hepatic decompensation (normal international normalized ratio, bilirubin, and low but normal platelet count). Although image findings supported cirrhosis, laboratory markers did not indicate hepatic decompensation. The severity of hypoalbuminemia and anasarca, along with an indeterminate Serum-Ascites Albumin Gradient, prompted the patient’s care team to consider other causes, specifically, nephrotic syndrome.
The patien’s spot protein-to-creatinine ratio was 3.76 (reference range < 0.2 mg/mg creatinine), but a 24-hour urine protein collection was 12.8 g/day (reference range < 150 mg/day). While most spot urine protein- to-creatinine ratios (UPCR) correlate with a 24-hour urine collection, discrepancies can occur, as in this case. It is important to recognize that the spot UPCR assumes that patients are excreting 1000 mg of creatinine daily in their urine, which is not always the case. In addition, changes in urine osmolality can lead to different values. The gold standard for proteinuria is a 24-hour urine collection for protein and creatinine.
The patient’s nephrotic-range proteinuria and severe hypoalbuminemia are not solely explained by cirrhosis. In addition, the patient’s lower extremity edema pointed to nephrotic syndrome. The differential diagnosis for nephrotic syndrome includes both primary and secondary forms of membranous nephropathy, minimal change disease, focal segmental glomerulosclerosis, and MPGN, a histopathological diagnosis that requires distinguishing between immune complex-mediated and complement-mediated forms. Other causes of nephrotic syndrome that do not fit in any of these buckets include amyloidosis, IgA nephropathy, and diabetes mellitus (DM). Despite DM being a common cause of nephrotic range proteinuria, it rarely leads to full nephrotic syndrome.
When considering the diagnosis, we reframed the patient’s clinical syndrome as compensated cirrhosis plus nephrotic syndrome. This approach prioritized identifying a cause that could explain both cirrhosis (from any cause) leading to IgA nephropathy or injection drug use serving as a risk factor for cirrhosis and nephrotic syndrome through HCV or AA amyloidosis, respectively. This problem representation guided us to the correct diagnosis. There are multiple renal diseases associated with HCV infection, including MPGN, membranous nephropathy, focal segmental glomerulosclerosis, and IgA nephropathy.2 MPGN and mixed cryoglobulinemia are the most common. In the past, MPGN was classified as type I, II, and III.
The patient’s urine toxicology revealed recent amphetamine use, which can also lead to acute kidney injury through rhabdomyolysis or acute interstitial nephritis (AIN).3 In the cases of rhabdomyolysis, urinalysis would show positive heme without any red blood cell on microscopic analysis, which was not present in this case. AIN commonly manifests as acute kidney injury, pyuria, and proteinuria but without a decrease in complement levels.4 While the patient’s urine sediment included white blood cell (10/high-power field), the presence of microscopic hematuria, decreased complement levels, and proteinuria in the context of HCV positivity makes MPGN more likely than AIN.
Recently, there has been greater emphasis on using immunofluorescence for kidney biopsies. MPGN is now classified into 2 main categories: MPGN with mesangial immunoglobulins and C3 deposits in the capillary walls, and MPGN with C3 deposits but without Ig.5 MPGN with Ig-complement deposits is seen in autoimmune diseases and infections and is associated with dysproteinemias.
The renal biopsy in this patient was consistent with MPGN with immunofluorescence, a common finding in patients with infection. By synthesizing these data, we concluded that the patient represented a case of chronic HCV infection that led to MPGN with cryoglobulinemia. The normal C4 and negative RF do not suggest cryoglobulinemic crisis. Compensated cirrhosis was seen on imaging, pending liver biopsy.
Treatment
The management of MPGN secondary to HCV infection relies on the treatment of the underlying infection and clearance of viral load. Direct-acting antivirals have been used successfully in the treatment of HCV-associated MPGN. When cryoglobulinemia is present, immunosuppressive therapy is recommended. These regimens commonly include rituximab and steroids.5 Rituximab is also used for nephrotic syndrome associated with MPGN, as recommended in the 2018 Kidney Disease: Improving Global Outcomes guidelines.6
When initiating rituximab therapy in a patient who tests positive for hepatitis B (HBcAb positive or HBsAb positive), it is recommended to follow the established guidelines, which include treating them with entecavir for prophylaxis to prevent reactivation or a flare of hepatitis B.7 The patient in this case needed close follow-up in the nephrology and hepatology clinic. Immunosuppressive therapy was not pursued while the patient was admitted to the hospital due to instability with housing, transportation, and difficulty in ensuring close follow-up.
CONCLUSIONS
Clinicians should maintain a broad differential even in the face of confirmatory imaging and other objective findings. In the case of anasarca, nephrotic syndrome should be considered. Key causes of nephrotic syndromes include MPGN, membranous nephropathy, minimal change disease, and focal segmental glomerulosclerosis. MPGN is a histopathological diagnosis, and it is essential to identify if it is secondary to immune complexes or only complement mediated because Ig-complement deposits are seen in autoimmune disease and infection. The management of MPGN due to HCV infection relies on antiviral therapy. In the presence of cryoglobulinemia, immunosuppressive therapy is recommended.
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
- Tapper EB, Parikh ND. Diagnosis and management of cirrhosis and its complications: a review. JAMA. 2023;329(18):1589–1602. doi:10.1001/jama.2023.5997
- Ozkok A, Yildiz A. Hepatitis C virus associated glomerulopathies. World J Gastroenterol. 2014;20(24):7544-7554. doi:10.3748/wjg.v20.i24.7544
- Foley RJ, Kapatkin K, Vrani R, Weinman EJ. Amphetamineinduced acute renal failure. South Med J. 1984;77(2):258- 260. doi:10.1097/00007611-198402000-00035
- Rossert J. Drug - induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817. doi:10.1046/j.1523-1755.2001.060002804.x
- Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31(4):341-348. doi:10.1016/j.semnephrol.2011.06.005
- Jadoul M, Berenguer MC, Doss W, et al. Executive summary of the 2018 KDIGO hepatitis C in CKD guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663-673. doi:10.1016/j.kint.2018.06.011
- Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis b virus: a review of clinical guidelines. Clin Liver Dis (Hoboken). 2020;15(4):162-167. doi:10.1002/cld.883
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Elusive Edema: A Case of Nephrotic Syndrome Mimicking Decompensated Cirrhosis
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
A 90-year-old man presented for evaluation of a large basal cell carcinoma (BCC) involving the nasal region. The lesion was a 7×4-cm pink, crusted, verrucous plaque covering the majority of the nose and extending onto the malar cheeks that originally had been biopsied 26 years prior, and repeat biopsy was performed 3 years prior. Results from both biopsies were consistent with BCC. The patient had avoided treatment for many years due to fear of losing his nose.
Given the size and location of the tumor, surgical intervention posed major challenges for both functional and cosmetic outcomes. After careful consideration and discussion of treatment options, which included Mohs micrographic surgery (MMS), wide local excision, radiation therapy, and systemic therapy, the decision was made to start the patient on vismodegib 150 mg once daily as well as L-carnitine 330 mg twice daily to help with muscle cramps. A baseline complete metabolic panel with an estimated glomerular filtration rate was unremarkable.
By the patient’s first follow-up visit after 2 months of therapy, he had experienced marked clinical improvement with notable regression of the tumor (Figure 1). He reported no adverse effects (eg, muscle cramps, dysgeusia, hair loss, nausea, vomiting, diarrhea). At subsequent follow-up visits, the patient continued to demonstrate clinical improvement. His only adverse effect was a 6-kg weight loss over the prior 6 months of initiating therapy despite no changes in taste or appetite. His dose of vismodegib was decreased to an alternative regimen of 150 mg daily for the first 2 weeks of each month with a drug holiday the rest of the month. Since that time, his weight has stabilized and he has continued with treatment.

Comment
Vismodegib was the first Hedgehog (Hh) inhibitor approved by the US Food and Drug Administration for management of selected locally advanced and metastatic BCC in adults.1,2 Genetic alterations in the Hh signaling pathway resulting in proliferation of basal cells are present in nearly all BCCs.2 In normal function, when the Hh ligand is absent at the patched (PTCH1) receptor, smoothened (SMO) is inhibited. When Hh ligand binds PTCH1, SMO is activated with downstream effects of triggering cell survival and proliferation in the nucleus via GLI. Loss of function mutations at the PTCH1 receptor or SMO-activating mutations lead to the same downstream effects, even when Hh ligand is absent.1 This allows for unregulated tumor growth.
Vismodegib is a small-molecule SMO inhibitor that blocks aberrant activation of the Hh signaling pathway, thereby slowing the growth of BCCs (Figure 2).3,4 Vismodegib and sonidegib have been used to treat patients with basal cell nevus syndrome as well as metastatic or locally advanced BCCs. At least 50% of advanced BCCs develop resistance to vismodegib, commonly via acquiring mutations in SMO.4

Basal cell carcinoma can be classified as low or high risk based on risk for recurrence. First-line treatments for low-risk BCC are surgical excision, electrodessication and curettage, and MMS.4 Second-line treatment includes radiation therapy. High-risk tumors include those involving anatomic locations of Area H near the eyelids, nose, ears, hands, feet, or genitals in addition to tumors with an aggressive histologic subtype.4,5 First-line treatments for high-risk BCC are MMS or surgical excision. Second-line treatments are radiation therapy or systemic therapy, such as vismodegib.4
Although Hh inhibitors are not a first-line treatment, our case highlights vismodegib’s effectiveness in the management of a large unresectable BCC on the nose of an elderly patient. Our patient opted out of surgical first-line options due to functional and cosmetic concerns.4 He also declined radiation treatment due to financial cost and difficulty with transportation. The patient chose to pursue systemic vismodegib therapy through shared decision-making with dermatology. Vismodegib treatment alone granted our patient a highly remarkable result.
There are limited clinical data on the effectiveness and safety profile of vismodegib in elderly patients, even though this is a high-risk population for BCC.6 In a study that categorized responses to vismodegib in 13 patients with canthal BCC, 5 experienced a complete clinical response (defined as complete regression of the tumor), and 8 achieved partial clinical response (defined as regression but not to the extent of a complete response).7 Our patient’s successful response is notable, as it reinforces vismodegib’s effectiveness as a treatment option for BCC in a sensitive facial area. In addition, our patient’s minimal adverse effect profile is evidence in support of establishing visogemib’s role as a viable treatment option in advanced BCC in the elderly.
Alternative dosing regimens of vismodegib involve the use of drug holidays.8 Utilizing a regimen of 1 week with and 3 weeks without vismodegib for 5 to 14 cycles has led to the resolution of BCC with decreased adverse effects.8 Furthermore, the MIKIE study demonstrated the efficacy of 2 dosing regimens: 12 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 12 weeks of vismodegib 150 mg and 24 weeks of vismodegib 150 mg followed by 3 cycles of 8 placebo weeks and 8 weeks of vismodegib 150 mg.9 Both regimens appeared viable to treat BCC in patients who were at risk for treatment discontinuation due to adverse effects.10
One adverse effect associated with vismodegib is muscle cramps, which are a potential cause of treatment discontinuation. The mechanism by which vismodegib causes cramps is not fully understood but is attributed to contractions from Ca2+ influx into muscle cells and a lack of adenosine triphosphate to allow muscle relaxation.11 This is due to vismodegib’s inhibition of the SMO signaling pathway and activation of the SMO–Ca2+/ AMP-related kinase axis.12 L-carnitine can be used as an adjuvant with vismodegib to address this adverse effect. L-carnitine is found in muscle cells, where its role is to produce energy by utilizing fatty acids.13 It is hypothesized that L-carnitine helps prevent cramps through production of adenosine triphosphate via fatty acid Β-oxidation that aids in stabilizing the sarcolemma and promoting muscle relaxation in skeletal muscle.13,14 Evidence suggests that making L-carnitine a common adjuvant to vismodegib can aid in preventing this adverse effect.
Vismodegib can be an effective treatment option for large nasal BCCs that are difficult to resect. Our case demonstrates both clinical efficacy and a favorable safety profile in an elderly patient. Further studies and long-term follow-up are warranted to establish the role of vismodegib in the evolving landscape of BCC management.
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
- Peris K, Fargnoli MC, Garbe C, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10-34. doi:10.1016/j.ejca.2019.06.003
- Alkeraye SS, Alhammad GA, Binkhonain FK. Vismodegib for basal cell carcinoma and beyond: what dermatologists need to know. Cutis. 2022;110:155-158. doi:10.12788/cutis.0601
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol. 2019;80:321-339. doi:10.1016/j.jaad.2018.02.083
- Wolf IH, Soyer P, McMeniman EK, et al. Actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. In: Dermatology. 5th ed. Elsevier; 2024:1888-1910. doi:10.1016/B978-0-7020-8225-2.00108-6
- National Comprehensive Cancer Network. Guidelines for patients: basal cell carcinoma. 2025. Accessed April 7, 2025. https://www.nccn.org/patients/guidelines/content/PDF/basal-cell-patient-guideline.pdf
- Ad Hoc Task Force; Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550. doi:10.1016/j .jaad.2012.06.009
- Passarelli A, Galdo G, Aieta M, et al. Vismodegib experience in elderly patients with basal cell carcinoma: case reports and review of the literature. Int J Mol Sci. 2020;21:8596. doi:10.3390/ijms21228596
- Oliphant H, Laybourne J, Chan K, et al. Vismodegib for periocular basal cell carcinoma: an international multicentre case series. Eye (Lond). 2020;34:2076-2081. doi:10.1038/s41433-020-0778-3
- Becker LR, Aakhus AE, Reich HC, et al. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153:321-322. doi:10.1001 /jamadermatol.2016.5058
- Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basalcell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. doi:10.1016 /S1470-2045(17)30072-4
- Svoboda SA, Johnson NM, Phillips MA. Systemic targeted treatments for basal cell carcinoma. Cutis. 2022;109:E25-E31. doi:10.12788/cutis.0560
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13:1540-1543. doi:10.1016/j.cgh.2014.12.005
- Teperino R, Amann S, Bayer M, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414-426. doi:10.1016/j.cell.2012.09.021
- Dinehart M, McMurray S, Dinehart SM, et al. L-carnitine reduces muscle cramps in patients taking vismodegib. SKIN J Cutan Med. 2018;2:90-95. doi:10.25251/skin.2.2.1
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
Remarkable Response to Vismodegib in a Locally Advanced Basal Cell Carcinoma on the Nose
PRACTICE POINTS
- Dermatologists should consider using vismodegib for treatment of unresectable basal cell carcinoma.
- Vismodegib dosing regimens can vary; drug holidays can be used to mitigate adverse effects while maintaining desirable treatment outcomes.
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
A 26-year-old male with Fitzpatrick skin type II presented for evaluation in the dermatology clinic after being referred by his primary care practitioner (PCP) with a complaint of spider veins. The patient reported a lifelong history of blue subcutaneous nodules that initially appeared on his face during childhood but have since involved his trunk and upper and lower extremities. The patient reported that some of the nodules were painful and increased in size with exercise. His medical history was unremarkable with no other chronic conditions or daily medication use. The patient reported no gastrointestinal (GI) symptoms, melena, or hematochezia. The patient’s mother had similar nodules but his 7 siblings did not.
Upon physical examination, numerous blue subcutaneous nodules, 2 to 8 mm in size, were scattered across his trunk, and proximal and distal extremities were present (Figure 1). The physical examination was otherwise unremarkable. Upon discussing differential diagnosis of these lesions with the patient, he was amenable to a punch biopsy for further diagnostic clarity (Figure 2).
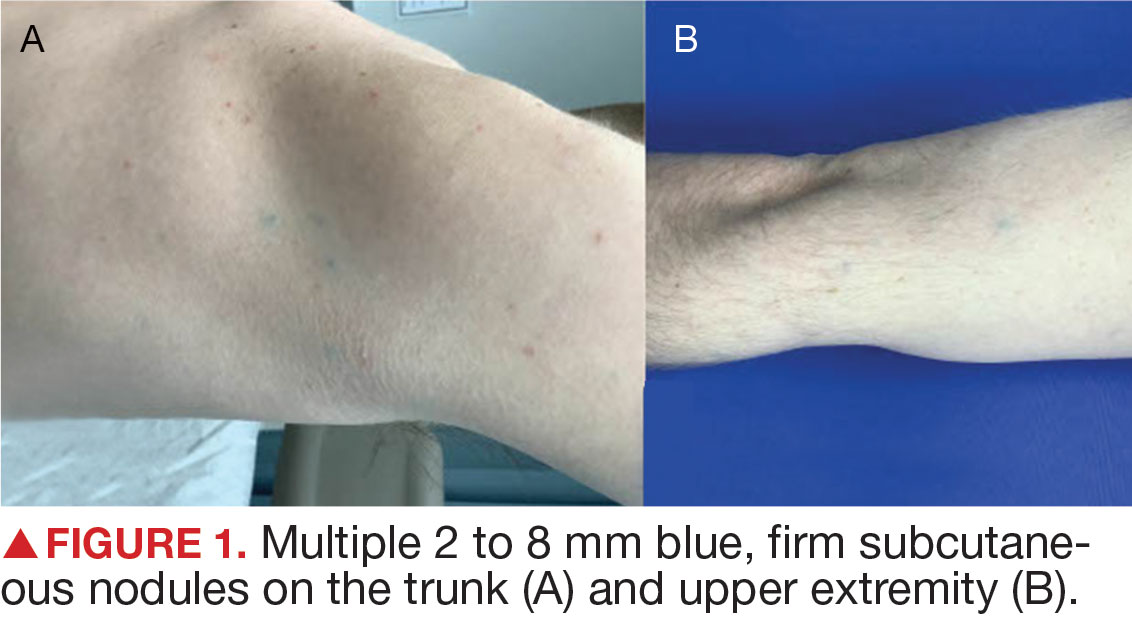
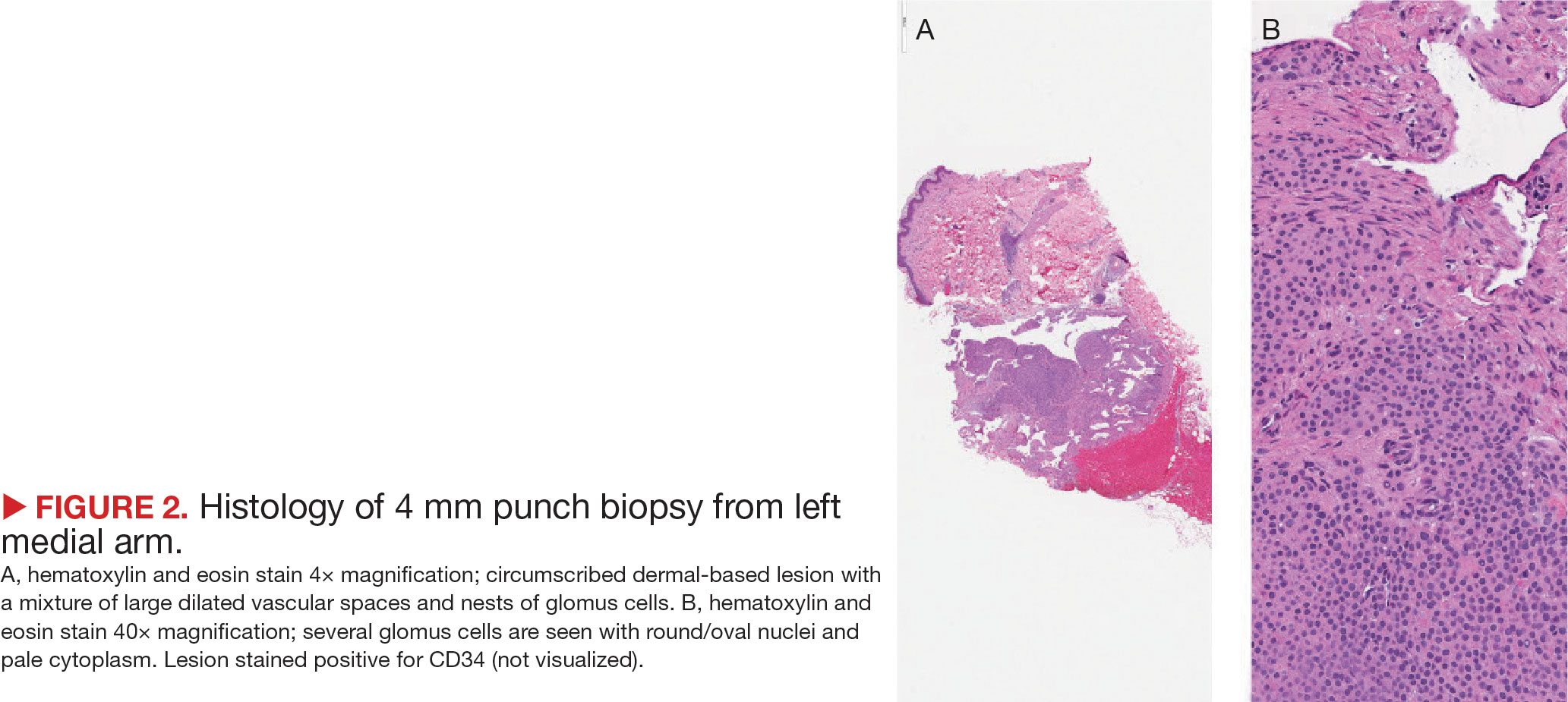
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.
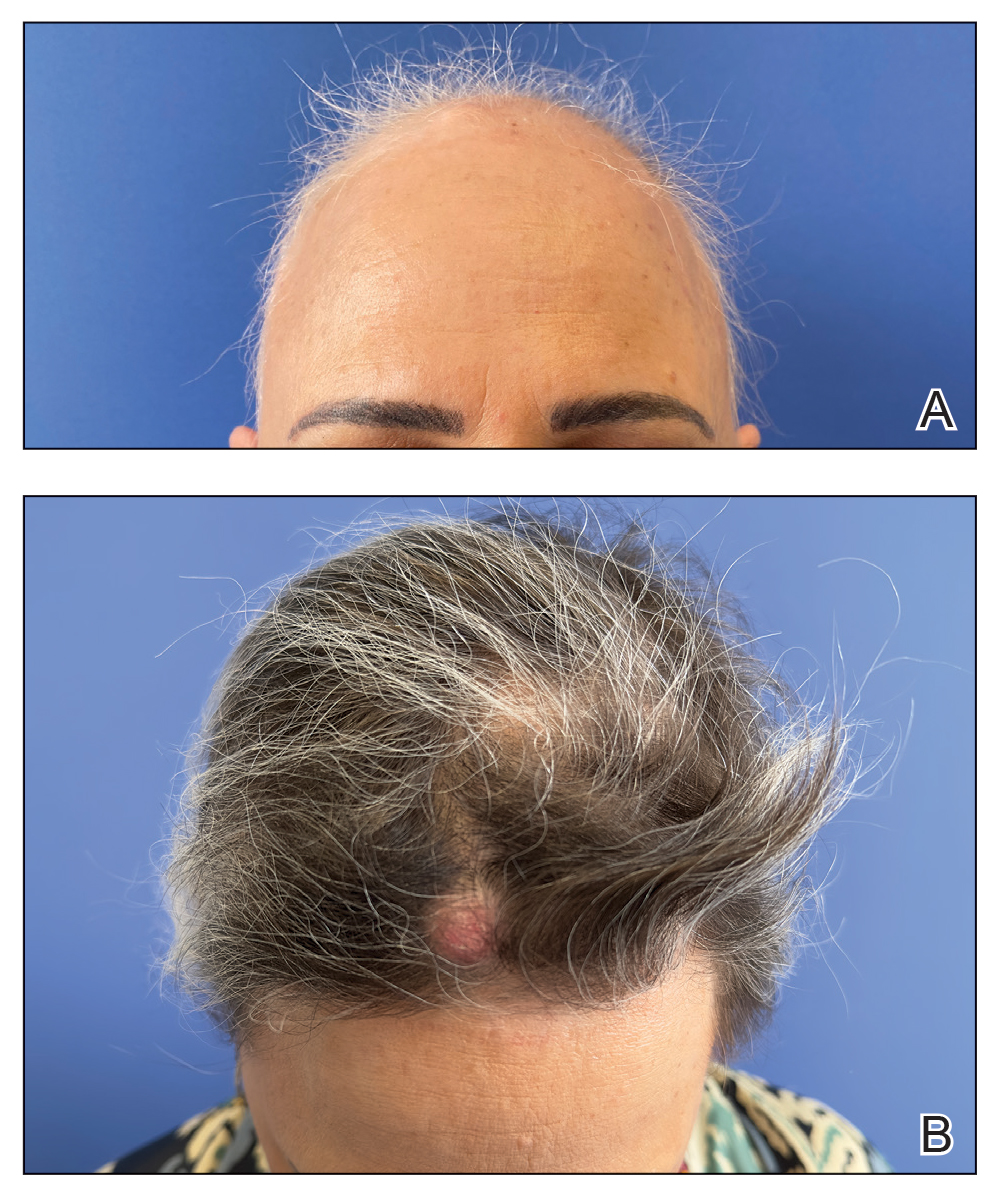
The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
To the Editor:
Alopecia areata (AA), an autoimmune disease characterized by inflammatory and nonscarring hair loss, can have a considerable impact on quality of life.1 Baricitinib is a Janus kinase inhibitor that recently was approved by the US Food and Drug Administration for treatment of severe AA in adult patients, becoming the only on-label treatment available.2 So far, the most common adverse effects reported in phase 3 trials have been acne, upper respiratory tract infections, headaches, urinary tract infections, and elevated creatine kinase levels.3
At our trichology unit in the dermatology department of a Spanish tertiary-care hospital in Seville, we have successfully used baricitinib to treat 18 patients with severe, therapy-resistant AA. Herein, we present a case of trichilemmal cyst reactivation in one of our patients following successful treatment with baricitinib.
A 53-year-old woman with a history of trichilemmal cysts presented to the dermatology department with total body hair loss of 5 years' duration that was diagnosed as AA universalis (Figure, A). The patient reported that the trichilemmal cysts had shrunk drastically 1 month after complete loss of body hair (Severity of Alopecia Tool [SALT] score, 100)(Figure, B). The largest cyst was surgically removed, and the diagnosis was histologically confirmed by a pathologist. Her mother and sister also had a history of multiple trichilemmal cysts.

The patient previously had failed treatment with oral prednisone 50 mg/d, oral cyclosporine 4 mg/kg/d, oral dexamethasone 4 mg twice weekly, and oral azathioprine 300 mg/wk. Due to the new indication of baricitinib for AA, we opted to start the patient on oral baricitinib 4 mg/d. By week 8 of treatment, she had achieved total hair regrowth (SALT score, 0). This rapid response might indicate a quick-responder phenotype, referring to a subset of patients who exhibit a fast and robust response to treatment (SALT90), generally before week 16, although more evidence is needed.
Notably, we observed the reactivation of 4 trichilemmal cysts on the scalp 6 weeks after starting baricitinib. To our knowledge, this side effect has not previously been reported. We hypothesize that reactivation of the cysts may have been due to the inhibition of the Janus kinase/signal transducer and activator of transcription pathway, which reduces the effects of cytokines and leads to reactivation of hair follicles that were inactive because of inflammation.4 As a result, the outer root sheath of the hair follicle can once again be filled with keratin, thereby reactivating the trichilemmal cysts. Based on our experience with this case, it may be relevant to consider personal and family history of trichilemmal cysts before starting treatment with baricitinib for AA and advise the patient about the possibility of this adverse effect.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83:761-770. doi:10.1007 /s40265-023-01873-w
- US Food and Drug Administration. FDA approves first systemic treatment for alopecia areata [news release]. July 13, 2022. Accessed March 17, 2025. https://www.prnewswire.com/news-releases/fda-approves-first-systemic-treatment-for-alopecia-areata-301566884.html
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056 /NEJMoa2110343
- Lensing M, Jabbari A. An overview of JAK/STAT pathways and JAK inhibition in alopecia areata. Front Immunol. 2022;13:955035. doi:10.3389/fimmu.2022.955035
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
Baricitinib-Induced Trichilemmal Cyst Reactivation in a Woman With Alopecia Areata
PRACTICE POINTS
- The rapid growth of trichilemmal cysts may serve as an indicator of a quick-responder phenotype to baricitinib in cases of alopecia areata (AA), although more evidence is needed.
- It is imperative to consider personal and family history of trichilemmal cysts prior to initiating baricitinib treatment for AA.
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.
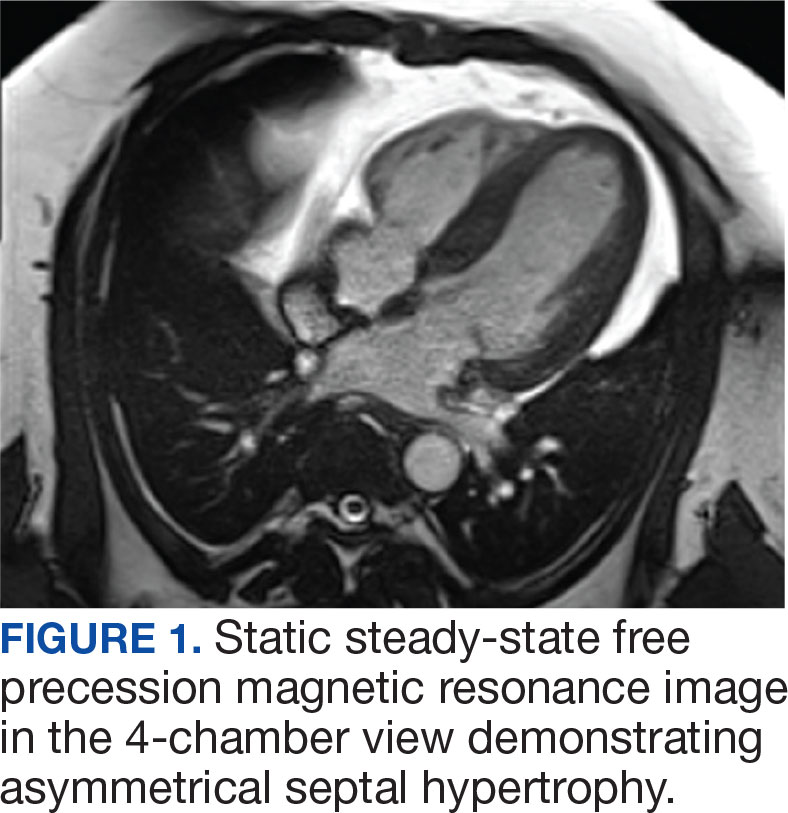
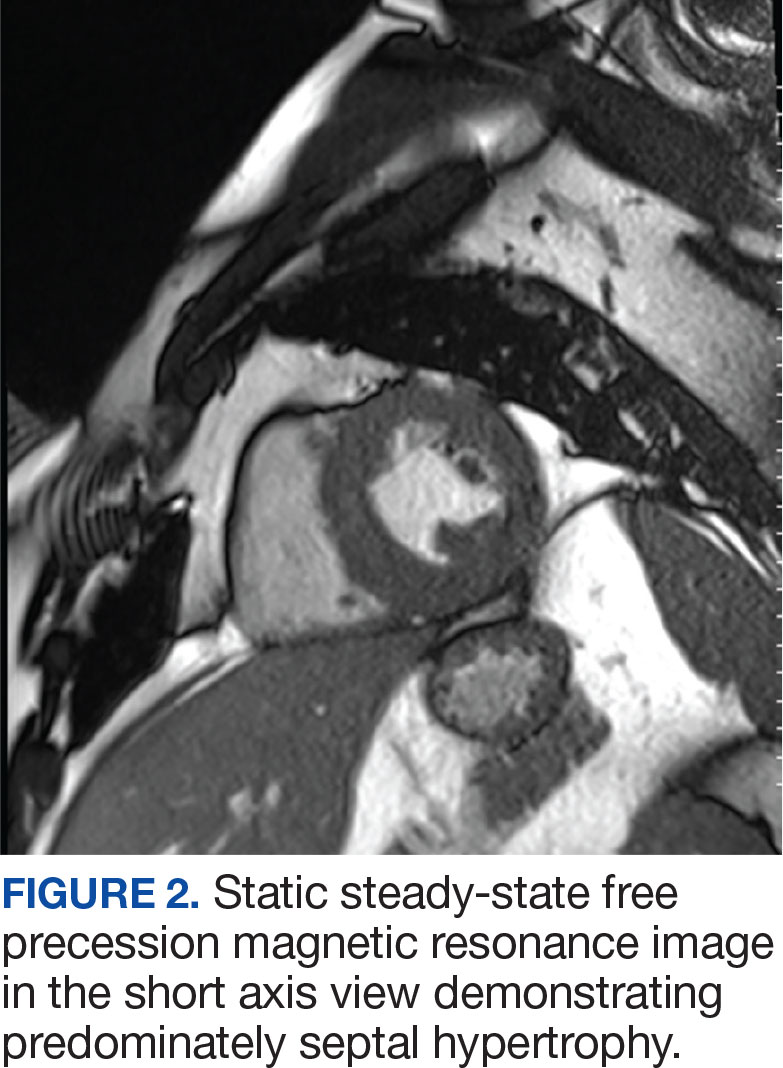
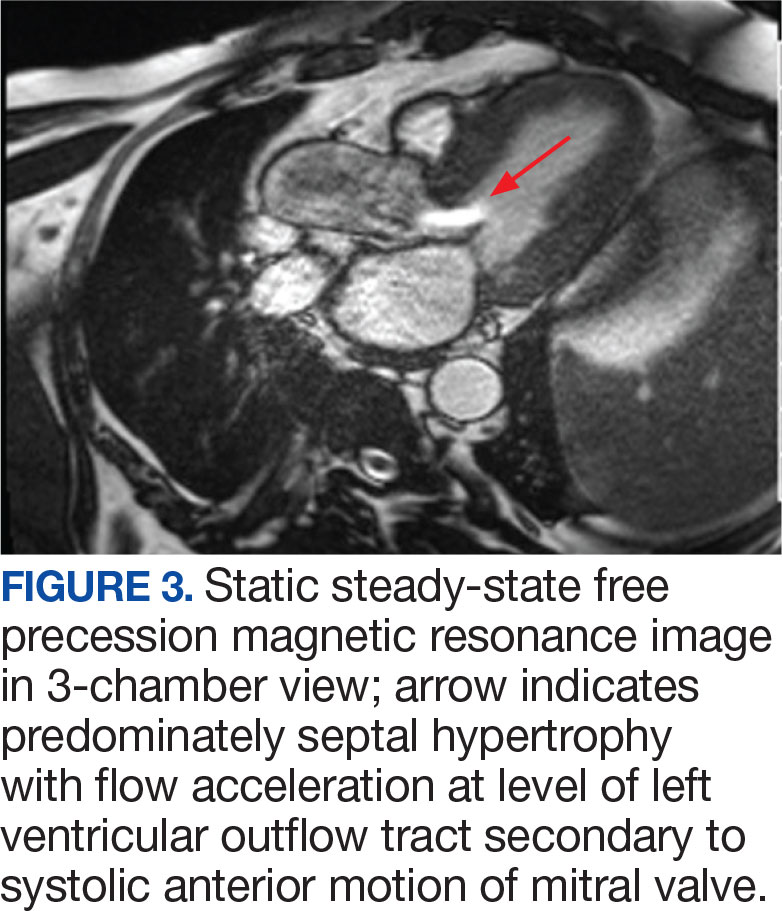
DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
Hypertrophic cardiomyopathy (HCM) is a relatively common inherited condition characterized by abnormal asymmetric left ventricular (LV) thickening. This can lead to LV outflow tract (LVOT) obstruction, which has important implications for anesthesia management. This article describes a case of previously undiagnosed HCM discovered during a preoperative physical examination prior to a routine surveillance colonoscopy.
CASE PRESENTATION
A 55-year-old Army veteran with a history of a sessile serrated colon adenoma presented to the preadmission testing clinic prior to planned surveillance colonoscopy under monitored anesthesia care. His medical history included untreated severe obstructive sleep apnea (53 apnea-hypopnea index score), diet-controlled hypertension, prediabetes (6.3% hemoglobin A1c), hypogonadism, and obesity (41 body mass index). Medications included semaglutide 1.7 mg injected subcutaneously weekly and testosterone 200 mg injected intramuscularly every 2 weeks, as well as lisinopril-hydrochlorothiazide 10 to 12.5 mg daily, which had recently been discontinued because his blood pressure had improved with a low-sodium diet.
A review of systems was unremarkable except for progressive weight gain. The patient had no family history of sudden cardiac death. On physical examination, the patient’s blood pressure was 119/81 mm Hg, pulse was 86 beats/min, and respiratory rate was 18 breaths/min. The patient was clinically euvolemic, with no jugular venous distention or peripheral edema, and his lungs were clear to auscultation. There was, however, a soft, nonradiating grade 2/6 systolic murmur that had not been previously documented. The murmur decreased substantially with the Valsalva maneuver, with no change in hand grip.
Laboratory studies revealed hemoglobin and renal function were within the reference range. A routine 12-lead electrocardiogram (ECG) was unremarkable. A transthoracic echocardiogram revealed moderate pulmonary hypertension (59 mm Hg right ventricular systolic pressure), asymmetric LV hypertrophy (2.1 cm septal thickness), and severe LVOT obstruction (131.8 mm Hg gradient). Severe systolic anterior motion of the mitral valve was also present. The LV ejection fraction was 60% to 65%, with normal cavity size and systolic function. These findings were consistent with severe hypertrophic obstructive cardiomyopathy (HOCM). Upon more detailed questioning, the patient reported that over the previous 5 years he had experienced gradually decreasing exercise tolerance and mild dyspnea on exertion, particularly in hot weather, which he attributed to weight gain. He also reported a presyncopal episode the previous month while working in his garage in hot weather for a prolonged period of time.
The patient’s elective colonoscopy was canceled, and he was referred to cardiology. While awaiting cardiac consultation, he was instructed to maintain good hydration and avoid any heavy physical activity beyond walking. He was told not to resume his use of lisinopril-hydrochlorothiazide. A screening 7-day Holter monitor showed no ventricular or supraventricular ectopy. After cardiology consultation, the patient was referred to a HCM specialty clinic, where a cardiac magnetic resonance imaging confirmed severe asymmetric hypertrophy with resting obstruction (Figures 1-4). Treatment options were discussed with the patient, and he underwent a trial with the Β—blocker metoprolol 50 mg daily, which he could not tolerate. Verapamil extended-release 180 mg orally once daily was then initiated; however, his dyspnea persisted. He was amenable to surgical therapy and underwent septal myectomy, with 12 g of septal myocardium removed. He did well postoperatively, with a follow-up echocardiogram showing normal LV systolic function and no LVOT gradient detectable at rest or with Valsalva maneuver. His fatigue and exertional dyspnea significantly improved. Once the patient underwent septal myectomy and was determined to have no detectable LVOT gradient, he was approved for colonoscopy which has been scheduled but not completed.




DISCUSSION
Once thought rare, HCM is now considered to be a relatively common inherited disorder, occurring in about 1 in 500 persons, with some suggesting that the actual prevalence is closer to 1 in 200 persons.1,2 Most often caused by mutations in ≥ 1 of 11 genes responsible for encoding cardiac sarcomere proteins, HCM is characterized by abnormal LV thickening without chamber enlargement in the absence of any identifiable cause, such as aortic valve stenosis or uncontrolled hypertension. The hypertrophy is often asymmetric, and in cases of asymmetric septal hypertrophy, dynamic LVOT obstruction can occur (known as HOCM). The condition is inherited in an autosomal dominant pattern with variable expression and is associated with myocardial fiber disarray, which can occur years before symptom onset.3 This myocardial disarray can lead to remodeling and an increased wall-to-lumen ratio of the coronary arteries, resulting in impaired coronary reserve.
Depending on the degree of LVOT obstruction, patients with HCM may be classified as nonobstructive, labile, or obstructive at rest. Patients without obstruction have an outflow gradient ≤ 30 mm Hg that is not provoked with Valsalva maneuver, administration of amyl nitrite, or exercise treadmill testing.3 Patients classified as labile do not have LVOT obstruction at rest, but obstruction may be induced by provocative measures. Finally, about one-third of patients with HCM will have LVOT gradients of > 30 mm Hg at rest. These patients are at increased risk for progression to symptomatic heart failure and may be candidates for surgical myectomy or catheter-based alcohol septal ablation.4 The patient in this case had a resting LVOT gradient of 131.8 mm Hg on echocardiography. The magnitude of this gradient placed the patient at a significantly higher risk of ventricular dysrhythmias and sudden cardiac death.5
Wall thickness also has prognostic implications. 6 Although any area of the myocardium can be affected, the septum is involved in about 90% cases. In their series of 48 patients followed over 6.5 years, Spirito et al found that the risk of sudden death in patients with HCM increased as wall thickness increased. For patients with a wall thickness of < 15 mm, the risk of death was 0 per 1000 person-years; however, this increased to 18.2 per 1000 person-years for patients with a wall thickness of > 30 mm.7
While many patients with HCM are asymptomatic, others may report dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, chest pain, palpitations, presyncope/ syncope, postural lightheadedness, fatigue, or edema. Symptomatology, however, is quite variable and does not necessarily correlate with the degree of outflow obstruction. Surprisingly, some patients with significant LVOT may have minimal symptoms, such as the patient in this case, while others with a lesser degree of LVOT obstruction may be very symptomatic.3,4
Physical examination of a patient with HCM may be normal or may reveal nonspecific findings such as a fourth heart sound or a systolic murmur. In general, physical examination abnormalities are related to LVOT obstruction. Those patients without significant outflow obstruction may have a normal cardiac examination. While patients with HCM may have a variety of systolic murmurs, the 2 most common are those related to outflow tract obstruction and mitral regurgitation caused by systolic anterior motion of the mitral valve.4 The systolic murmur associated with significant LVOT obstruction has been described as a harsh, crescendo-decrescendo type that begins just after S1 and is heard best at the apex and lower left sternal border.4 It may radiate to the axilla and base but not generally into the neck. The murmur usually increases with Valsalva maneuver and decreases with handgrip or going from a standing to a sitting/ squatting position. The initial examination of the patient in this case was not suggestive of HOCM, as confirmed by 2 practitioners (a cardiologist and an internist), each with > 30 years of clinical experience. This may have been related to the patient’s hydration status at the time, with Valsalva maneuver increasing obstruction to the point of reduced flow.
About 90% of patients with HCM will have abnormalities on ECG, most commonly LV hypertrophy with a strain pattern. Other ECG findings include: (1) prominent abnormal Q waves, particularly in the inferior (II, III, and aVF) and lateral leads (I, aVL, and V4-V6), reflecting depolarization of a hypertrophied septum; (2) left axis deviation; (3) deeply inverted T waves in leads V2 through V4; and (4) P wave abnormalities indicative of left atrial (LA) or biatrial enlargement. 8 It is notable that the patient in this case had a normal ECG, given that a minority of patients with HCM have been shown to have a normal ECG.9
Echocardiography plays an important role in diagnosing HCM. Diagnostic criteria include the presence of asymmetric hypertrophy (most commonly with anterior septal involvement), systolic anterior motion of the mitral valve, a nondilated LV cavity, septal immobility, and premature closure of the aortic valve. LV thickness is measured at both the septum and free wall; values ≥ 15 mm, with a septal-to-free wall thickness ratio of ≥ 1.3, are suggestive of HCM. Asymmetric LV hypertrophy can also be seen in other segments besides the septum, such as the apex.10
HCM/HOCM is the most common cause of sudden cardiac death in young people. The condition also contributes to significant functional morbidity due to heart failure and increases the risk of atrial fibrillation and subsequent stroke. Treatments tend to focus on symptom relief and slowing disease progression and include the use of medications such as Β—blockers, nondihydropyridine calcium channel blockers, and the myosin inhibitor mavacamten.11 Select patients, such as those with severe LVOT obstruction and symptoms despite treatment with Β—blockers or nondihydropyridine calcium channel blockers, may be offered septal myectomy or catheter-based alcohol septal ablation, coupled with insertion of an implantable cardiac defibrillator to prevent sudden cardiac death in patients at high arrhythmic risk.1,12
Patients with HCM, particularly those with LVOT obstruction, pose distinct challenges to the anesthesiologist because they are highly sensitive to decreases in preload and afterload. These patients frequently experience adverse perioperative events such as myocardial ischemia, systemic hypotension, and supraventricular or ventricular arrhythmias. Acute congestive heart failure may also occur, presumably due to concomitant diastolic dysfunction. Patients with previously unrecognized HCM are of particular concern, as they may manifest unexpected and sudden hypotension with the induction of anesthesia. There may then be a paradoxical response to vasoactive drugs and anesthetic agents, which accentuate LVOT obstruction. In these circumstances, undiagnosed HCM should be considered, and intraoperative rescue transesophageal echocardiography be performed.13 Once the diagnosis is confirmed, efforts should be made to reduce myocardial contractility and sympathetic discharge (eg, with Β—blockers), increase afterload (eg, with α1 agonists), and improve preload with adequate hydration. Proper resuscitation of hypotensive patients with HCM requires a thorough understanding of disease pathology, as effective interventions may seem to be counterintuitive. Inotropic agents such as epinephrine are contraindicated in HCM because increased inotropy and chronotropy worsen LVOT obstruction. Volume status is often tenuous; while adequate preload is important, overly aggressive fluid resuscitation may promote heart failure. It is important to keep in mind that even patients without resting LVOT obstruction may develop dynamic obstruction with anesthesia induction due to sudden reductions in preload and afterload. It is also important to note that the degree of LV hypertrophy is directly correlated with arrhythmic sudden death. Those patients with LV wall thickness ≥ 30 mm are at increased risk for potentially lethal tachyarrhythmias in the operating room.14
These considerations reinforce the need for proper preoperative identification of patients with HCM. Heightened awareness is key, given the fact that HCM is relatively common and tends to be underdiagnosed in the general population. These patients are generally young, otherwise healthy, and often undergo minor operative procedures in outpatient settings. It is incumbent upon the preoperative evaluator to take a thorough medical history and perform a careful physical examination. Clues to the diagnosis include exertional dyspnea, fatigue, angina, syncope/presyncope, or a family history of sudden cardiac death or HCM. A systolic ejection murmur, particularly one that increases with standing or Valsalva maneuver, and decreases with squatting or handgrip may also raise clinical suspicion. These patients should undergo a full cardiac evaluation, including echocardiography.
CONCLUSIONS
HCM is a common condition that is important to diagnose in the preoperative clinic. Failure to do so can lead to catastrophic complications during induction of anesthesia due to the sudden reduction in preload and afterload, which may cause a significant increase in LVOT obstruction. A high index of suspicion is essential, as clinical diagnosis can be challenging. The physical examination may be deceiving and symptoms are often subtle and nonspecific. It is imperative to alert the anesthesiologist before surgery so the complex hemodynamic management of patients with HOCM can be appropriately managed.
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
- Cheng Z, Fang T, Huang J, Guo Y, Alam M, Qian H. Hypertrophic cardiomyopathy: from phenotype and pathogenesis to treatment. Front Cardiovasc Med. 2021;8:722340. doi:10.3389/fcvm.2021.722340
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249-1254. doi:10.1016/j.jacc.2015.01.019
- Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy: a review. Anesth Analg. 2015;120(3):554-569. doi:10.1213/ ANE.0000000000000538
- Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(4):372–389. doi:10.1016/j.jacc.2021.12.002
- Jorda P, Garcia-Alvarez A. Hypertrophic cardiomyopathy: sudden cardiac death risk stratification in adults. Glob Cardiol Sci Pract. 2018;3(25). doi:10.21542/gcsp.2018.25
- Wigle ED, Sasson Z, Henderson MA, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985;28(1):1-83. doi:10.1016/0033-0620(85)90024-6
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi:10.1056/ NEJM200006153422403
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy Lancet. 2017;389(10075):1253-1267. doi:10.1016/S0140-6736(16)31321-6
- Rowin EJ, Maron BJ, Appelbaum E, et al. Significance of false negative electrocardiograms in preparticipation screening of athletes for hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(7):1027-1032. doi:10.1016/j. amjcard.2012.05.035
- Losi MA, Nistri S, Galderisi M et al. Echocardiography in patients with hypertrophic cardiomyopathy: usefulness of old and new techniques in the diagnosis and pathophysiological assessment. Cardiovasc Ultrasound. 2010;8(7). doi:10.1186/1476-7120-8-7
- Tian Z, Li L, Li X, et al. Effect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. 2023;8(10):957-965. doi:10.1001/ jamacardio.2023.3030
- Fang J, Liu Y, Zhu Y, et al. First-in-human transapical beating-heart septal myectomy in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2023;82(7):575-586. doi:10.1016/j.jacc.2023.05.052
- Jain P, Patel PA, Fabbro M 2nd. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth. 2018;32(1):467-477. doi:10.1053/j.jvca.2017.04.054
- Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104(1):183-192. doi:10.1097/00000542-200601000-00025
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Importance of Recognizing Hypertrophic Cardiomyopathy in the Preoperative Clinic
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.
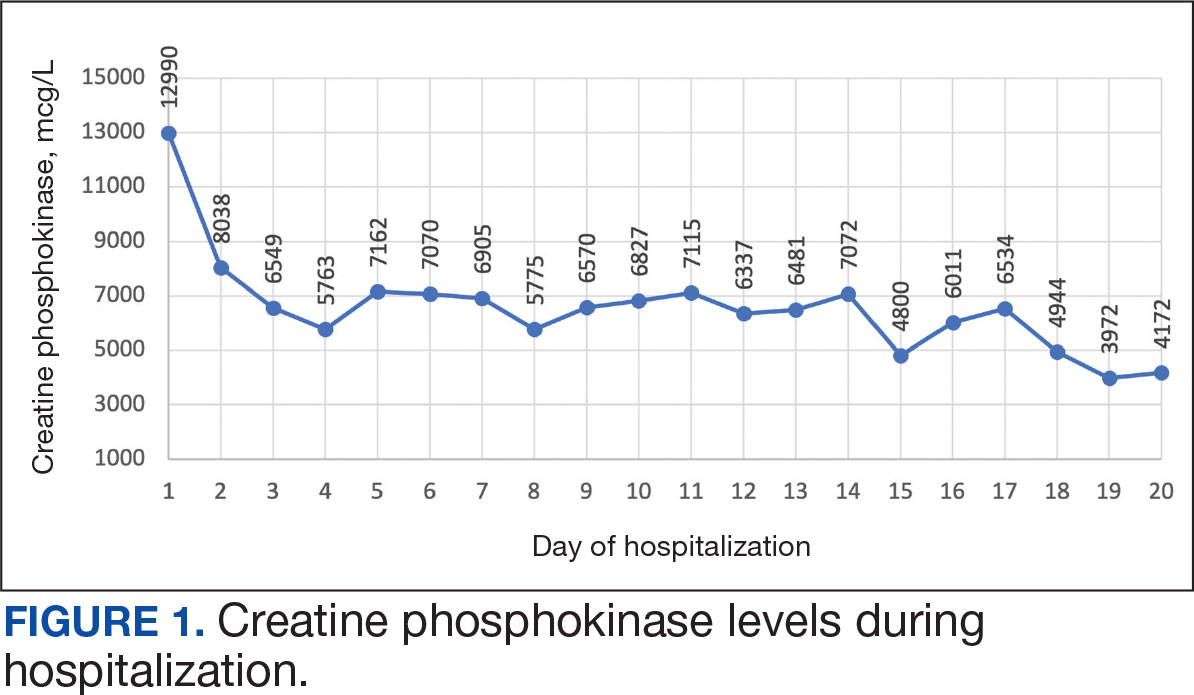
After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).
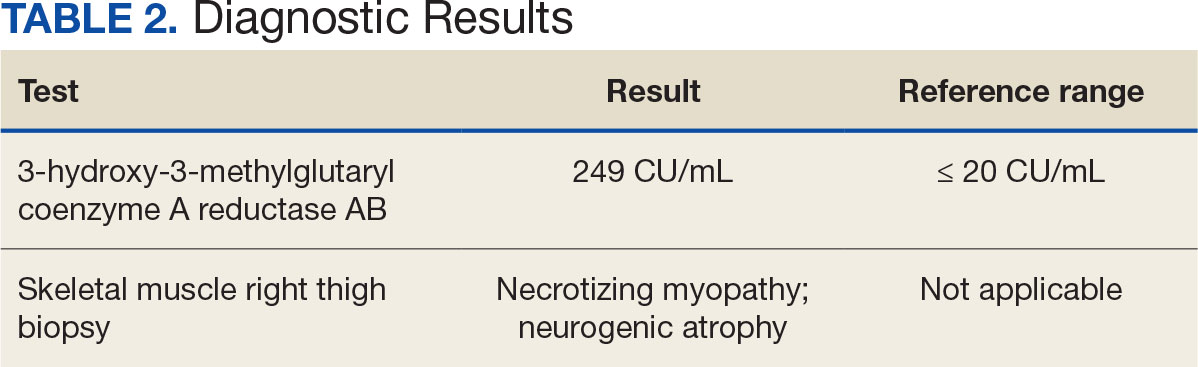
On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.
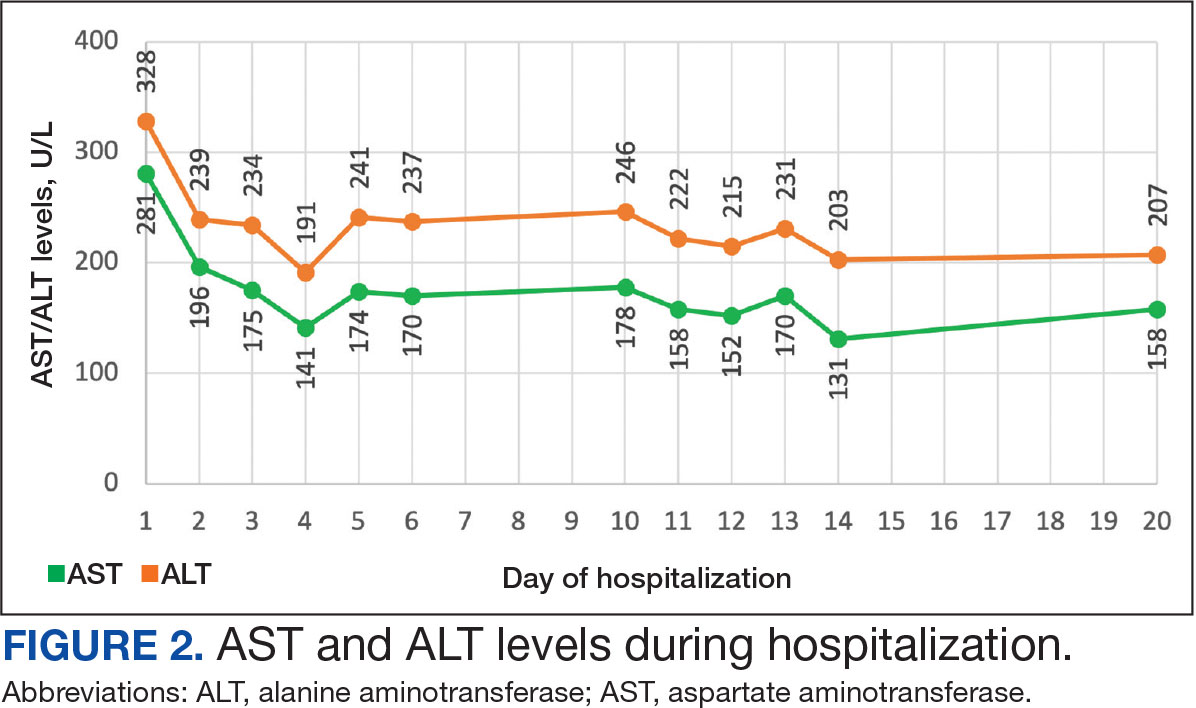
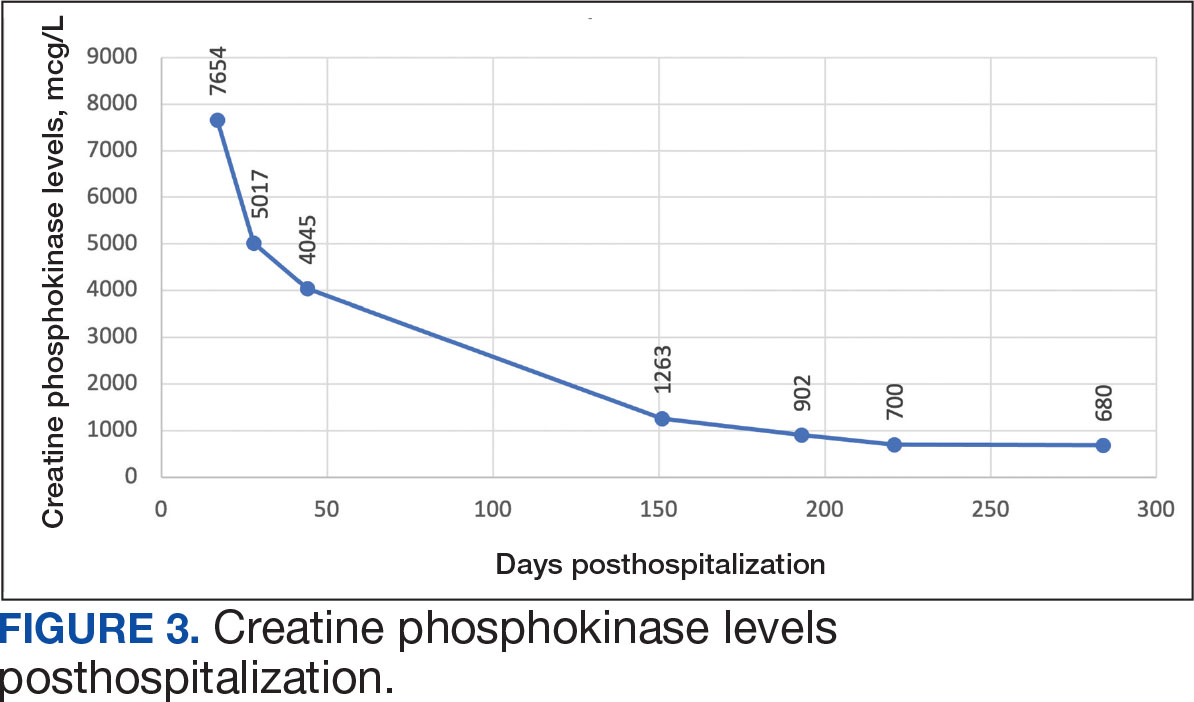
The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management