User login
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
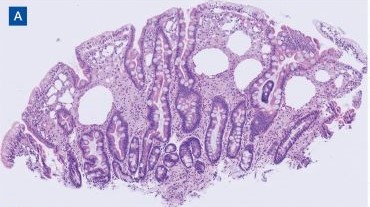
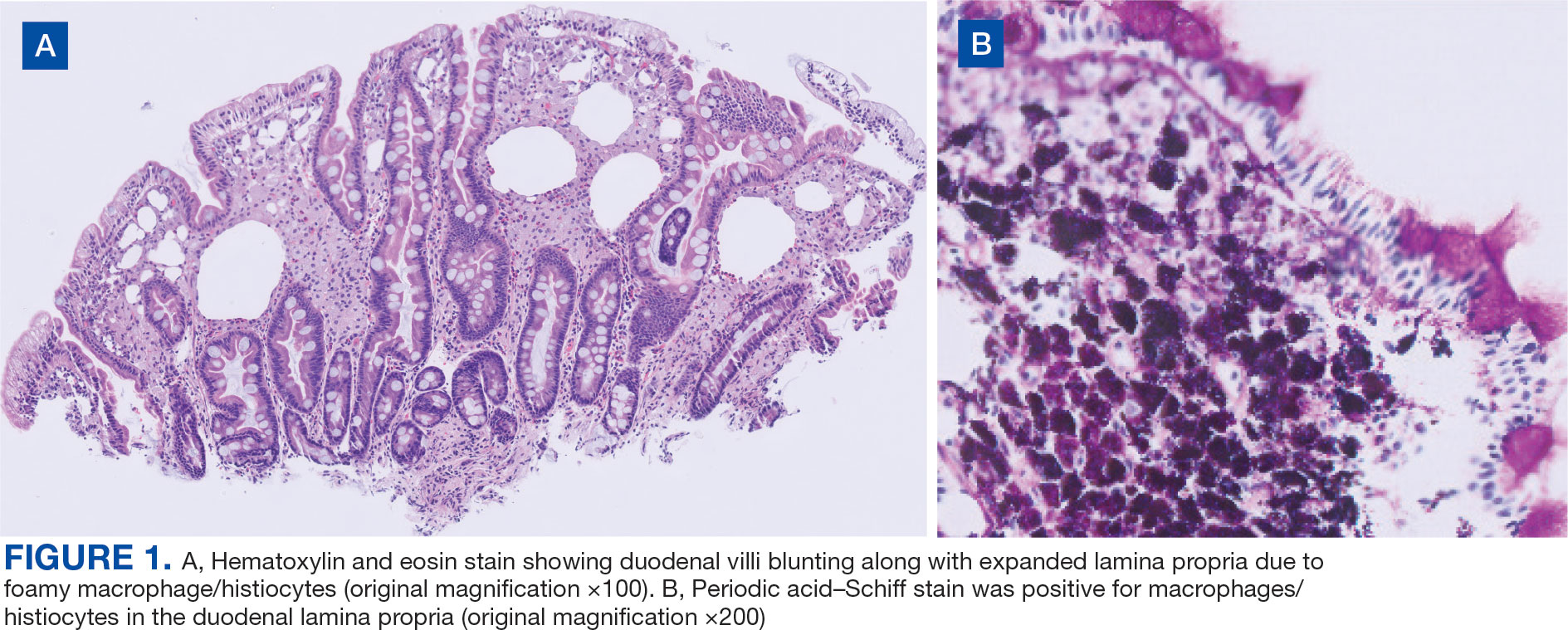
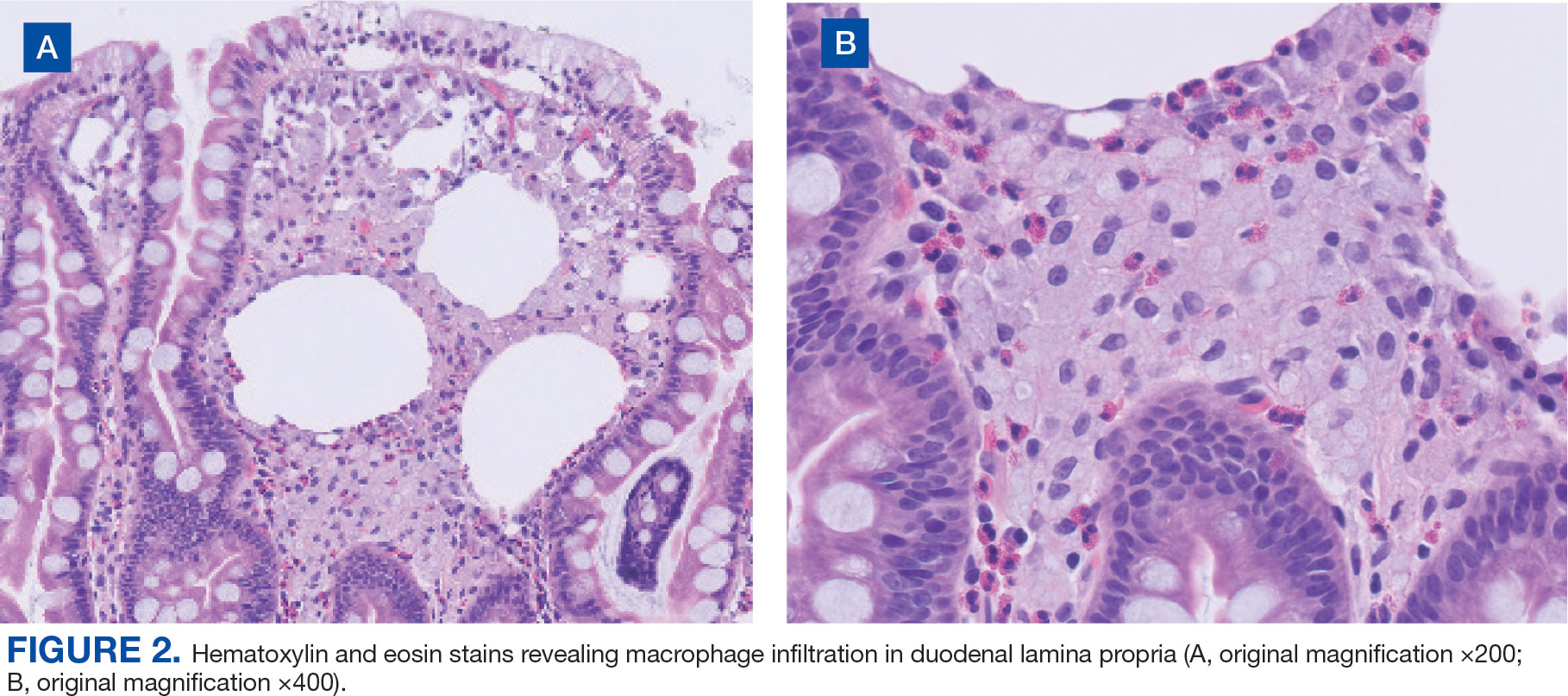
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
The Impact of a Paracentesis Clinic on Internal Medicine Resident Procedural Competency
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
Competency in paracentesis is an important procedural skill for medical practitioners caring for patients with decompensated liver cirrhosis. Paracentesis is performed to drain ascitic fluid for both diagnosis and/or therapeutic purposes.1 While this procedure can be performed without the use of ultrasound, it is preferable to use ultrasound to identify an area of fluid that is away from dangerous anatomy including bowel loops, the liver, and spleen. After prepping the area, lidocaine is administered locally. A catheter is then inserted until fluid begins flowing freely. The catheter is connected to a suction canister or collection kit, and the patient is monitored until the flow ceases. Samples can be sent for analysis to determine the etiology of ascites, identify concerns for infection, and more.
Paracentesis is a very common procedure. Barsuk and colleagues noted that between 2010 and 2012, 97,577 procedures were performed across 120 academic medical centers and 290 affiliated hospitals.2 Patients undergo paracentesis in a variety of settings including the emergency department, inpatient hospitalizations, and clinics. Some patients may require only 1 paracentesis procedure while others may require it regularly.
Due to the rising need for paracentesis in the Central Texas Veterans Affairs Hospital (CTVAH) in Temple, a paracentesis clinic was started in February 2018. The goal of the paracentesis clinic was multifocal—to reduce hospital admissions, improve access to regularly scheduled procedures, decrease wait times, and increase patient satisfaction.3 Through the CTVAH affiliation with the Texas A&M internal medicine residency program, the paracentesis clinic started involving and training residents on this procedure. Up to 3 residents are on weekly rotation and can perform up to 6 paracentesis procedures in a week. The purpose of this article was to evaluate resident competency in paracentesis after completion of the paracentesis clinic.
Methods
The paracentesis clinic schedules up to 3 patients on Tuesdays and Thursdays between 8
A survey was sent via email to all categorical internal medicine residents across all 3 program years at the time of data collection. Competency for paracentesis sign-off was defined as completing and logging 5 procedures supervised by a competent physician who confirmed that all portions of the procedure were performed correctly. Residents were also asked to answer questions on a scale from 1 to 10, with 1 representing no confidence and 10 representing strong confidence to practice independently (Table).
We also evaluated the number of procedures performed by internal medicine residents 3 years before the clinic was started in 2015 up to the completion of 2022. The numbers were obtained by examining procedural log data for each year for all internal medicine residents.
Results
Thirty-three residents completed the survey: 10 first-year internal medicine residents (PGY1), 12 second-year residents (PGY2), and 11 third-year residents (PGY3). The mean participation was 4.8 paracentesis sessions per person for the duration of the study. The range of paracentesis procedures performed varied based on PGY year: PGY1s performed 1 to > 10 procedures, PGY2s performed 2 to > 10 procedures, and PGY3s performed 5 to > 10 procedures. Thirty-six percent of residents completed > 10 procedures in the paracentesis clinic; 82% of PGY3s had completed > 10 procedures by December of their third year. Twenty-six residents (79%) were credentialed to perform paracentesis procedures independently after performing > 5 procedures, and 7 residents were not yet cleared for procedural independence.
In the survey, residents rated their comfort with performing paracentesis procedures independently at a mean of 7.9. The mean comfort reported by PGY1s was 7.2, PGY2s was 7.3, and PGY3s was 9.3. Residents also rated their opinion on whether or not the paracentesis clinic adequately prepared them for paracentesis procedural independence; the mean was 8.9 across all residents.
The total number of procedures performed by residents at CTVAH also increased. Starting in 2015, 3 years before the clinic was started, 38 procedures were performed by internal medicine residents, followed by 72 procedures in 2016; 76 in 2017; 58 in 2018; 94 in 2019; 88 in 2020; 136 in 2021; and 188 in 2022.
Discussion
Paracentesis is a simple but invasive procedure to relieve ascites, often relieving patients’ symptoms, preventing hospital admission, and increasing patient satisfaction.4 The CTVAH does not have the capacity to perform outpatient paracentesis effectively in its emergency or radiology departments. Furthermore, the use of the emergency or radiology departments for routine paracentesis may not be feasible due to the acuity of care being provided, as these procedures can be time consuming and can draw away critical resources and time from patients that need emergent care. The paracentesis clinic was then formed to provide veterans access to the procedural care they need, while also preparing residents to ably and confidently perform the procedure independently.
Based on our study, most residents were cleared to independently perform paracentesis procedures across all 3 years, with 79% of residents having completed the required 5 supervised procedures to independently practice. A study assessing unsupervised practice standards showed that paracentesis skill declines as soon as 3 months after training. However, retraining was shown to potentially interrupt this skill decline.5 Studies have shown that procedure-driven electives or services significantly improved paracentesis certification rates and total logged procedures, with minimal funding or scheduling changes required.6 Our clinic showed a significant increase in the number of procedures logged starting with the minimum of 38 procedures in 2015 and ending with 188 procedures logged at the end of 2022.
By allowing residents to routinely return to the paracentesis clinic across all 3 years, residents were more likely to feel comfortable independently performing the procedure, with residents reporting a mean comfort score of 7.9. The spaced repetition and ability to work with the clinic during elective time allows regular opportunities to undergo supervised training in a controlled environment and created scheduled retraining opportunities. Future studies should evaluate residents prior to each paracentesis clinic to ascertain if skill decline is occurring at a slower rate.
The inpatient effect of the clinic is also multifocal. Pham and colleagues showed that integrating paracentesis into timely training can reduce paracentesis delay and delays in care.7 By increasing the volume of procedures each resident performs and creating a sense of confidence amongst residents, the clinic increases the number of residents able and willing to perform inpatient procedures, thus reducing the number of unnecessary consultations and hospital resources. One of the reasons the paracentesis clinic was started was to allow patients to have scheduled times to remove fluid from their abdomen, thus cutting down on emergency department procedures and unnecessary admissions. Additionally, the benefits of early paracentesis procedural performance by residents and internal medicine physicians have been demonstrated in the literature. A study by Gaetano and colleagues noted that patients undergoing early paracentesis had reduced mortality of 5.5% vs 7.5% in those undergoing late paracentesis.8 This study also showed the in-hospital mortality rate was decreased with paracentesis (6.3%) vs without paracentesis (8.9%).8 By offering residents a chance to participate in the clinic, we have shown that regular opportunities to perform paracentesis may increase the number of physicians capable of independently practicing, improve procedural competency, and improve patient access to this procedure.
Limitations
Our study was not free of bias and has potential weaknesses. The survey was sent to all current residents who have participated in the paracentesis clinic, but not every resident filled out the survey (55% of all residents across 3 years completed the survey, 68.7% who had done clinic that year completed the survey). There is a possibility that those not signed off avoided doing the survey, but we are unable to confirm this. The survey also depended on resident recall of the number of paracenteses completed or looking at their procedure log. It is possible that some procedures were not documented, changing the true number. Additionally, rating comfortability with procedures is subjective, which may also create a source of potential weakness. Future projects should include a baseline survey for residents, followed by a repeat survey a year later to show changes from baseline competency.
Conclusions
A dedicated paracentesis clinic with internal medicine resident involvement may increase resident paracentesis procedural independence, the number of procedures available and performed, and procedural comfort level.
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255
1. Aponte EM, O’Rourke MC, Katta S. Paracentesis. StatPearls [internet]. September 5, 2022. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK435998
2. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. doi:10.1002/jhm.2153
3. Cheng Y-W, Sandrasegaran K, Cheng K, et al. A dedicated paracentesis clinic decreases healthcare utilization for serial paracenteses in decompensated cirrhosis. Abdominal Radiology. 2017;43(8):2190-2197. doi:10.1007/s00261-017-1406-y
4. Wang J, Khan S, Wyer P, et al. The role of ultrasound-guided therapeutic paracentesis in an outpatient transitional care program: A case series. Am J Hospice Palliat Med. 2018;35(9):1256-1260. doi:10.1177/1049909118755378
5. Sall D, Warm EJ, Kinnear B, Kelleher M, Jandarov R, O’Toole J. See one, do one, forget one: early skill decay after paracentesis training. J Gen Int Med. 2020;36(5):1346-1351. doi:10.1007/s11606-020-06242-x
6. Berger M, Divilov V, Paredes H, Kesar V, Sun E. Improving resident paracentesis certification rates by using an innovative resident driven procedure service. Am J Gastroenterol. 2018;113(suppl). doi:10.14309/00000434-201810001-00980
7. Pham C, Xu A, Suaez MG. S1250 a pilot study to improve resident paracentesis training and reduce paracentesis delay in admitted patients with cirrhosis. Am J Gastroenterol. 2022;117(10S). doi:10.14309/01.ajg.0000861640.53682.93
8. Gaetano JN, Micic D, Aronsohn A, et al. The benefit of paracentesis on hospitalized adults with cirrhosis and ascites. J Gastroenterol Hepatol. 2016;31(5):1025-1030. doi:10.1111/jgh.13255