User login
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
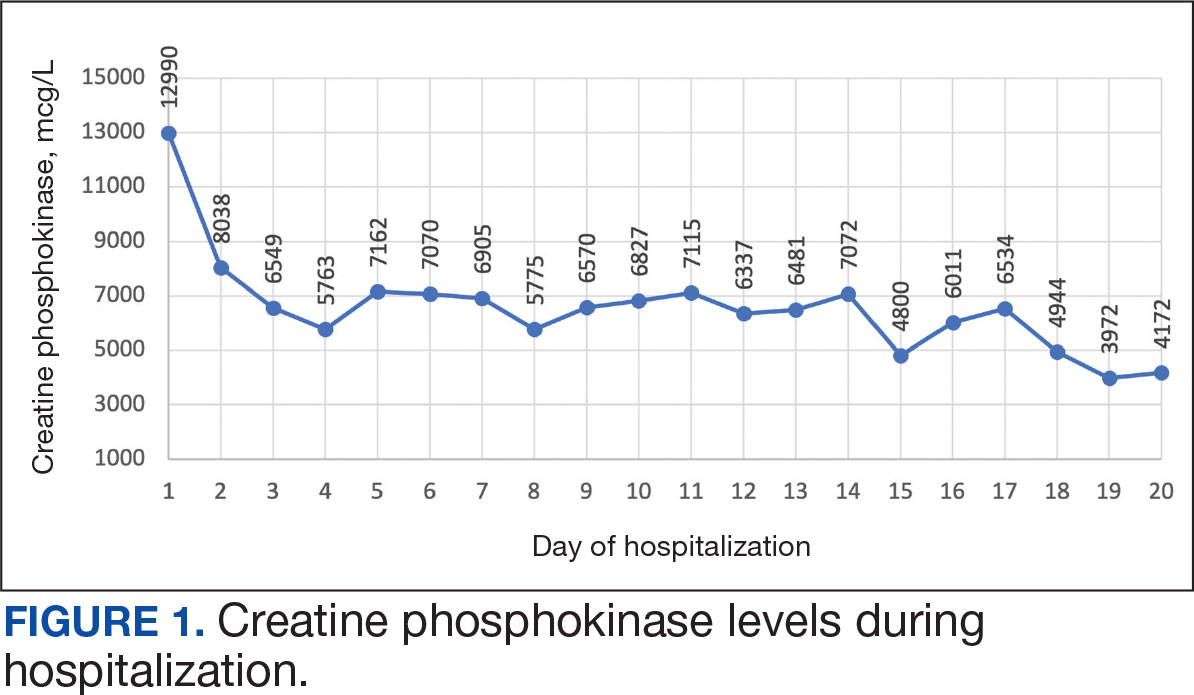
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.
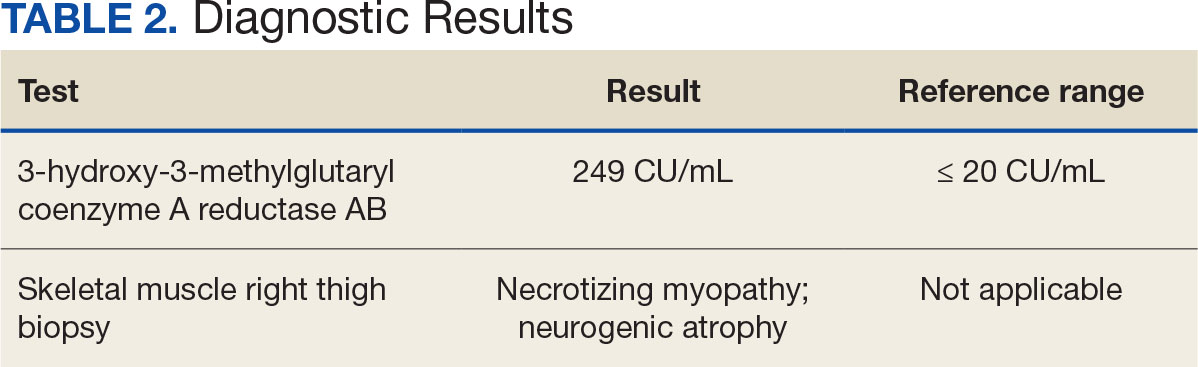
After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).
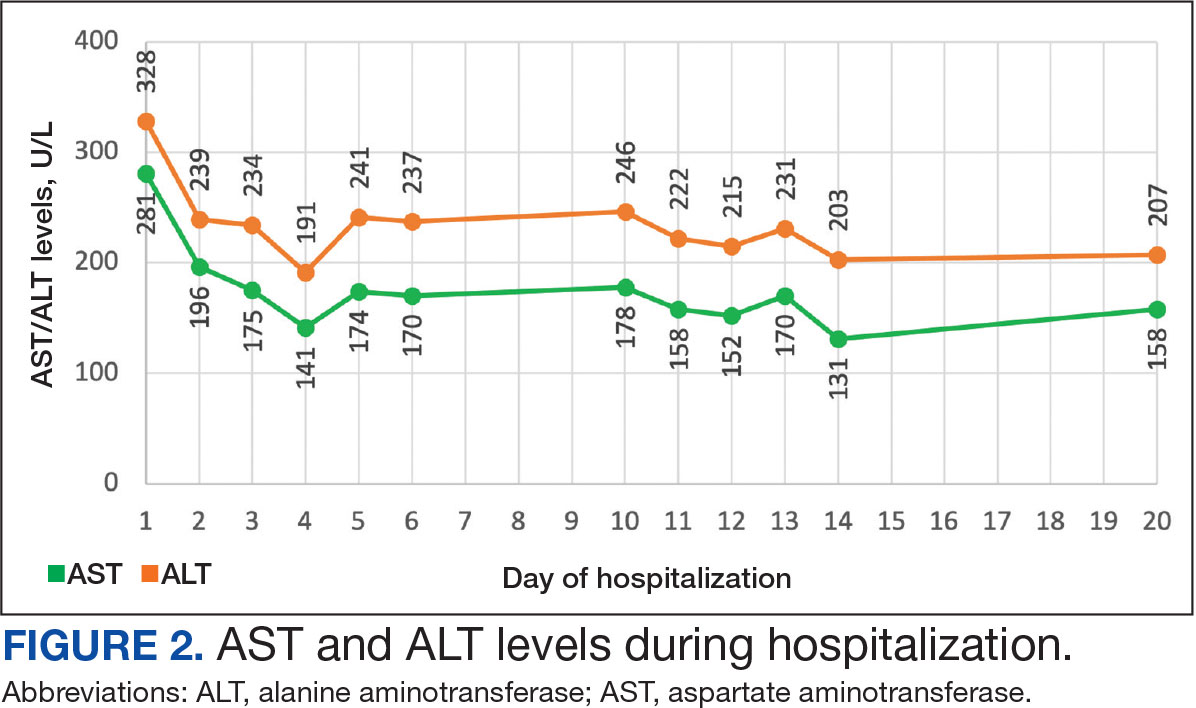
On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.
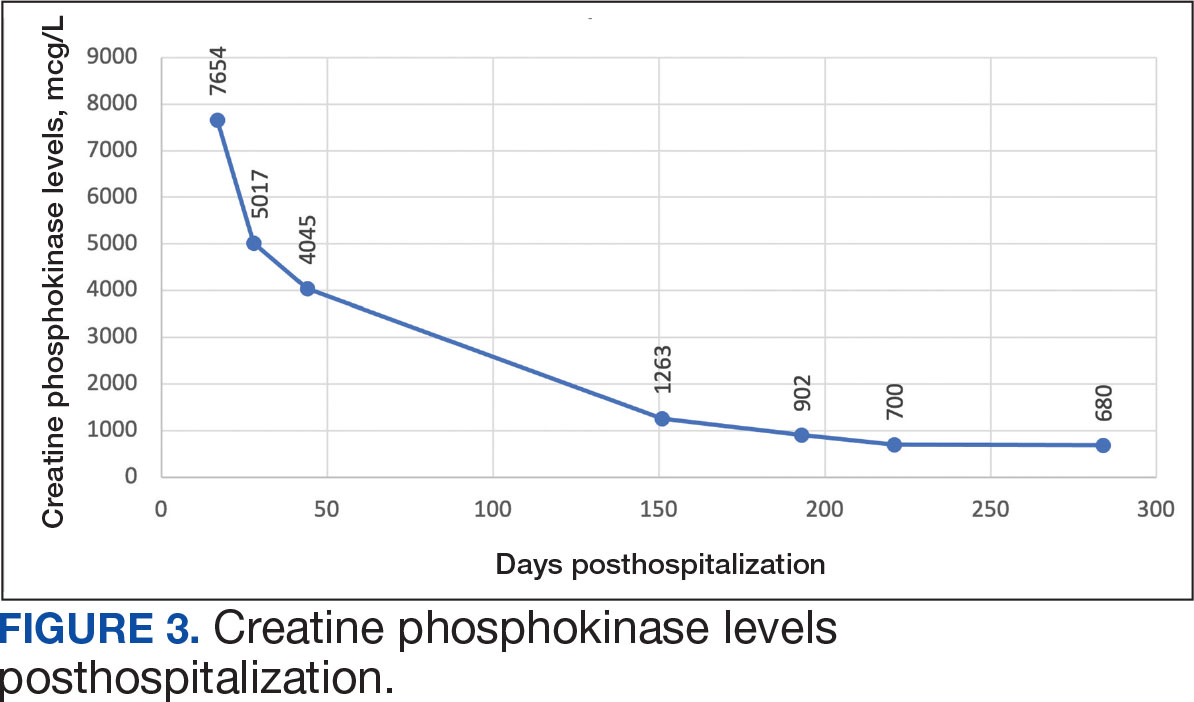
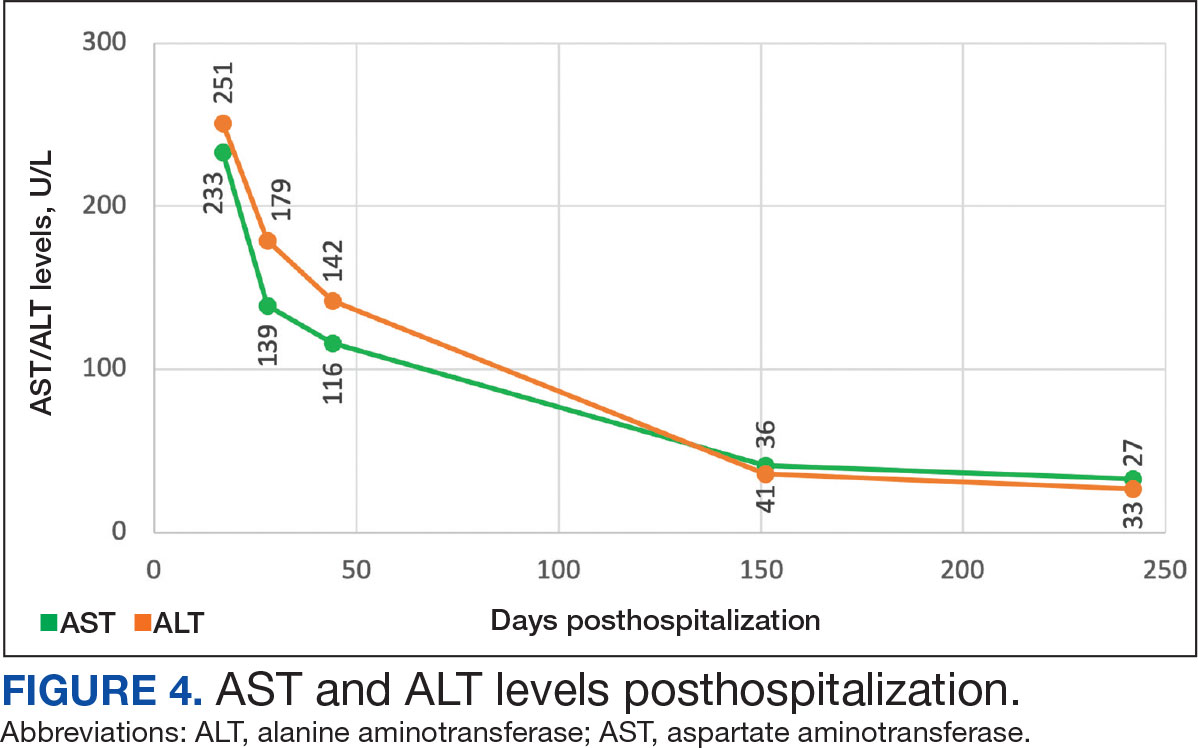
The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
Muscle-related complaints occur in 7% to 25% of patients taking statin medications.1 In most instances, these adverse effects are quickly resolved when the medication is discontinued, but in rare occurrences, the statin can trigger an autoimmune response that progresses even after stopping use. This uncommon condition is typically accompanied by symmetrical proximal muscle weakness and an elevated CPK leading to a necrotizing myopathy requiring treatment with immunosuppressive therapy. Although less common, some patients may also present with dysphagia, myalgia, weight loss, and/or skin rash.1
Statin medications have been the cornerstone of lipid-lowering therapy due to their mechanism of inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), which is the rate-limiting step within the cholesterol synthesis pathway to produce mevalonic acid. There is a proven genetic association with human leukocyte antigen (HLA)-DRB1*11:01 in adults and anti-HMGCR–associated myopathy.1 The incidence of statin-induced necrotizing autoimmune myopathy (SINAM) in relation to each specific statin agent remains unknown; however, a systematic review of case reports found higher correlations for atorvastatin and simvastatin.2
There are 2 ways to confirm a SINAM diagnosis. The first and simplest includes checking for the presence of antibodies against HMGCR. The anti-HMGCR antibody test is typically used as a definitive diagnosis because it has a high specificity for SINAM.3 The second and more invasive diagnosis method involves a muscle biopsy, which is identified as positive if the biopsy shows the presence of necrotic muscle fibers.1,3
The anti-HMGCR antibody test can serve as a marker for disease activity because the antibodies are strongly correlated with CPK levels.1 CPK levels indicate the severity of muscle injury and is often used in addition to either of the confirmatory tests because it is faster and less expensive. Anti-HMGCR titers may remain positive while CPK returns to baseline when SINAM is dormant. In addition, clinicians may use an electromyography (EMG) test to measure the muscle response in association to nerve stimulation. 1 This test can show potential features of myopathic lesions such as positive sharp waves, spontaneous fibrillations, or myotonic repetitive potentials.
Typical treatment includes glucocorticoids as first-line agents, but SINAM can be difficult to treat due to its complicated pathophysiology processes.3 Escalation of therapy is sometimes required beyond a single agent; in these complex scenarios, methotrexate and/or intravenous (IV) immunoglobulin (IVIG) therapy are frequently added to the steroid therapy. There have been concerns with steroid use in specific patient populations due to the undesired adverse effect (AE) profile, and as a result IVIG has been used as monotherapy at a dose of 2 g/kg per month.3 Studies looking at IVIG monotherapy showed a reduction in CPK levels and improvement in strength after just 2 to 3 rounds of monthly treatment.3 Some patients receiving IVIG monotherapy even achieved baseline strength and no longer reported muscle-related symptoms, although the total treatment duration varied. A systematic review of 39 articles where glucocorticoids, IVIG, methotrexate and/or a combination were used to treat SINAM found an average time to remission of 8.6 months. Additionally, this systematic review observed more patients returned to baseline or experienced improvement in symptoms when being treated with a combination of glucocorticoid plus IVIG plus methotrexate.2 Suggested dosing recommendations are available in Table 1.

Patients diagnosed with HMGCR antibody myopathy are contraindicated for future statin therapy.1 Rechallenge of statins in this patient population has led to worsening of disease and therefore these patients should have a severe statin allergy listed in their medical documentation record.
CASE PRESENTATION
A 59-year-old male patient with a medical history including atrial fibrillation, peripheral vascular disease, type 2 diabetes mellitus (T2DM), hypertension, and peripheral neuropathy was referred by his primary care clinical pharmacist practitioner for an outpatient neurology consult. The patient reported a 4-month history of fatigue, lower extremity paresthesia, and progressive proximal muscle weakness which began in his legs, mostly noticeable when walking upstairs but quickly developed into bilateral arm weakness. The patient reported significant impact on his quality of life: he could no longer lift his arms above his head and had difficulty with daily activities such as brushing his hair or getting up from a chair. He reported multiple falls at home, and began to use a cane for assistance with ambulation. He confirmed adherence to atorvastatin over the past year. Laboratory testing on the day of the visit revealed an elevated CPK level at 9729 mcg/L (reference range for men, 30-300 mcg/L).
The patient was urged to go to the emergency department where his CPK level had increased to 12,990 mcg/L (Figure 1). The workup began to find the source of rhabdomyolysis and elevated liver enzymes differentiating autoimmune vs medication-induced myopathy. Upon admission atorvastatin was discontinued, anti-HMGCR antibody level was ordered, and IV fluids were started.

After 8 days of hospital admission with minimal improvement, Rheumatology and Neurology services were consulted in the setting of persistent CPK elevation and the potential neuropathic component of muscle weakness. Both consulting services agreed to consider muscle biopsy and EMG if the patient did not begin to show signs of improvement. The patient’s CPK levels remained elevated with minimal change in muscle weakness. The next step was a right quadricep muscle biopsy performed on Day 14 of admission. Sixteen days after admission, the anti-HMGCR antibody test (originally obtained upon admission) was positive and elevated at 249 CU/mL (reference range, < 20 CU/mL negative; reference range, ≥ 60 CU/mL strong positive), which confirmed the SINAM diagnosis (Table 2).

On Day 17 of hospitalization, the Neurology service initiated IVIG monotherapy to avoid the undesired glycemic AEs associated with glucocorticoids. The patient had a history of T2DM that was difficult to manage and his hemoglobin A1c level was the best it had ever been (6.2%) relative to a peak A1c of 11.0% 9 months prior. The patient was treated with a total IVIG dose of 2 g/kg divided into 3 daily doses while still obtaining CPK levels with daily laboratory tests to assist with trending the extent of disease severity improvement (Figures 2-4). After a 20-day hospital stay, the patient was discharged home with rehabilitation services and a scheduled outpatient EMG the following week.



The patient continued to report generalized body weakness, pain, and deconditioning upon discharge and was unable to attend the EMG neurology appointment. The patient did eventually attend a follow-up appointment about 6 weeks after hospital discharge and reported continued weakness. The Neurology service prescribed a 2-day IVIG regimen (total dose = 2 g/kg) monthly for the next 2 months. The patient returned to the neurology clinic 8 weeks later following 2 rounds of IVIG posthospitalization and reported that his muscle strength was returning, and he was able to slowly reintroduce exercise into his daily routine. During a follow-up appointment about 11 months after the initial hospitalization, the patient’s primary care clinical pharmacist provided education of effective management of cholesterol without statins, including use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as recommended by the Neurology service. At this time, the patient’s calculated low-density lipoprotein (LDL) was 110 mg/dL (reference range, 0-99 mg/dL). The patient preferred to work on a healthy diet and positive lifestyle choices before trialing any lipid lowering therapies.
The patient appeared to tolerate this treatment regimen following 7 rounds of IVIG. He noted fatigue for about 24 hours after his infusion sessions but otherwise reported no additional AEs. He has continued to attend weekly physical therapy sessions and is able to walk without the assistance of a cane. He can now walk a mile before he begins to feel fatigued or experience bilateral lower leg pain. The pain appears neuropathic in nature, as the patient reports ongoing “pins and needles” sensation in his legs and feet. The patient has noticed a major improvement in his overall function, strength, and exercise tolerance since starting IVIG treatments and although he is not yet back to his baseline, he is motivated to continue his recovery. Neurology is considering ongoing treatment with IVIG monthly infusions given his continued clinical improvement.
DISCUSSION
There is limited evidence on the use of IVIG monotherapy for SINAM, although it may be a viable option for patients deemed poor candidates for glucocorticoid or methotrexate therapy. This particularly applies to patients with DM for which there may be concerns for managing blood glucose levels with steroid use. The Johns Hopkins Myositis Center evaluated 3 patients with SINAM who declined glucocorticoid therapy and had documented DM and weakness in the proximal arms and legs. Following 2 to 3 monthly rounds of IVIG 2 g/kg monotherapy, these patients had reduced CPK levels and had improvement in both arm and hip-flexion strength. Two patients reported no muscle-related symptoms after completing IVIG monotherapy treatment for 9 and 19 months.3
The optimal treatment duration for IVIG monotherapy for SINAM is still uncertain given the limited available data. The patient in this case report showed clinically significant muscle-related improvement following 7 monthly rounds of 2 g/kg IVIG treatments. The mechanism of action for IVIG in this setting is still unknown, although the medication may allow muscle regeneration to surpass muscle destruction, thus leading to resolution of the muscle-related symptoms.3
There are numerous concerns with IVIG use to consider prior to initiating treatment, including expense, AEs, patient response, and comorbidities. IVIG is considerably more expensive than glucocorticoid and methotrexate alternatives. Systemic reactions have been shown to occur in 5% to 15% of patients receiving IVIG infusion.4 The majority of these infusion reactions occur early during infusion or within a few hours after administration is complete.5 Early AEs to monitor for include injection site reactions, flu-like symptoms, dermatologic reactions, anaphylaxis, transfusion-related acute lung injury, and transfusion-associated circulatory overload. Additional AEs may be delayed, including thromboembolic events, acute kidney injury, aseptic meningitis, hemolysis, neutropenia, and blood-borne infection.6 IVIG has a boxed warning for thrombosis, renal dysfunction, and acute renal failure risk.7 There are multiple strategies documented to reduce the risk of IVIG reactions including slowing the infusion rate, ensuring adequate hydration, and/or giving analgesics, antihistamines, or steroids prior to infusion.6 The patient in this case had monthly IVIG infusions without the need of any pretreatment medications and only reported fatigue for about 24 hours following the infusion.
An essential question is how to provide safe cholesterol management for patients with SINAM. Some evidence has suggested that other lipid-lowering medications that avoid the mevalonate pathway, such as fenofibrate or ezetimibe, may be used cautiously initially at lower doses.1 Due to the severity of SINAM, it is crucial to closely monitor and ensure tolerability as new lipid-lowering agents are introduced. More evidence suggests that PCSK9 inhibitors are a safer option.8 PCSK9 inhibitors avoid the mevalonate pathway and block PCSK9 from binding to LDL receptors, allowing LDL to be removed from circulation.
Tiniakou et al followed 8 individuals for a mean 1.5 years who had anti-HMGCR immune-mediated myopathy at high cardiovascular risk. Muscle strength, CPK levels, and serum anti-HMGCR antibody titers were assessed at baseline and again after initiation of PCSK9 inhibitor. None of the patients experienced a decline in their muscle strength. CPK, anti-HMGCR antibody levels, and LDL trended down in all participants and 2 patients were able to reduce their immunosuppression treatment while still achieving clinical improvement. Tiniakou et al suggest that PCSK9 inhibitors are a safe and effective option to lower cholesterol in patients with SINAM.8
Alirocumab is the preferred PCSK9 inhibitor for patients at the US Department of Veterans Affairs (VA). The VA Pharmacy Benefits Management (PBM) Service guidance recommends alirocumab for patients with a history of atherosclerotic cardiovascular disease (ASCVD) or severe hypercholesterolemia.9 PBM guidance suggests alirocumab use for patients with a contraindication, intolerance, or insufficient LDL reduction with a maximally tolerated dose of statin and ezetimibe with a desire to reduce ASCVD risk by lowering LDL. Per the PBM Criteria for Use guidance, patients should follow the stepwise approach and trial ezetimibe prior to being considered for PCSK9 inhibitor therapy. Given the patient’s contraindication to future statin use and severity of myopathy, in this case the Neurology Service felt that the safest option to reach goal LDL reduction would be a PCSK9 inhibitor. Consideration can be made for alirocumab use when considering an alternative lipid lowering therapy.
CONCLUSIONS
This report demonstrates a case of SINAM caused by atorvastatin therapy. Patients presenting with proximal muscle weakness and elevated CPK even after statin discontinuation should be considered for a full workup to determine whether SINAM may be involved. This uncommon form of myopathy can be diagnosed based on the detection of anti-HMGCR antibodies and/or presence of necrosis on muscle biopsy. A combination of glucocorticoid, methotrexate, and IVIG is recommended for a patient’s best chance of muscle symptom improvement. IVIG monotherapy should be considered for patients with glycemic control concerns.
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
- Tiniakou E. Statin-associated autoimmune myopathy: current perspectives. Ther Clin Risk Manag. 2020;16:483-492. doi:10.2147/TCRM.S197941
- Somagutta MKR, Shama N, Pormento MKL, et al. Statin-induced necrotizing autoimmune myopathy: a systematic review. Reumatologia. 2022;60(1):63-69. doi:10.5114/reum.2022.114108
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680-1682. doi:10.1056/NEJMc1506163
- Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171-178. doi:10.1016/j.tmrv.2013.05.004
- Ameratunga R, Sinclair J, Kolbe J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin Exp Immunol. 2004;136(1):111-113. doi:10.1111/j.1365-2249.2004.02412.x
- Abbas A, Rajabally YA. Complications of immunoglobulin therapy and implications for treatment of inflammatory neuropathy: a review. Curr Drug Saf. 2019;14(1):3-13. doi:10.2174/1574886313666181017121139
- Privigen. Prescribing information. CSL Behring LLC; 2022. Accessed March 17, 2025. https://labeling.cslbehring.com/PI/US/Privigen/EN/Privigen-Prescribing-Information.pdf
- Tiniakou E, Rivera E, Mammen AL, Christopher-Stine L. Use of proprotein convertase subtilisin/Kexin Type 9 inhibitors in statin-associated immune-mediated necrotizing myopathy: a case series. Arthritis Rheumatol. 2019;71(10):1723-1726. doi:10.1002/art.40919
- US Department of Veterans Affairs, Pharmacy Benefits Management (PBM) Services. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9 Inhibitor) (Alirocumabpreferred, Evolocumab-non-preferred) Criteria for Use. June 2024. Accessed March 25, 2025. https://www.va.gov/formularyadvisor/DOC/128
- Jayatilaka S, Desai K, Rijal S, Zimmerman D. Statin-induced autoimmune necrotizing myopathy. J Prim Care Community Health. 2021;12:21501327211028714. doi:10.1177/21501327211028714
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management
Statin-Induced Necrotizing Autoimmune Myopathy in a Patient With Complex Diabetes Management