User login
Epidermodysplasia Verruciformis and the Risk for Malignancy
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
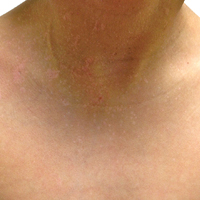
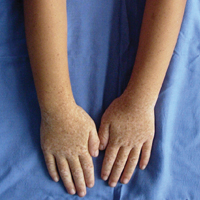
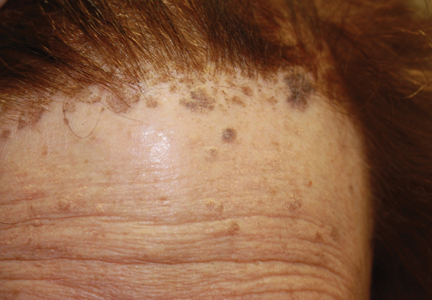
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.
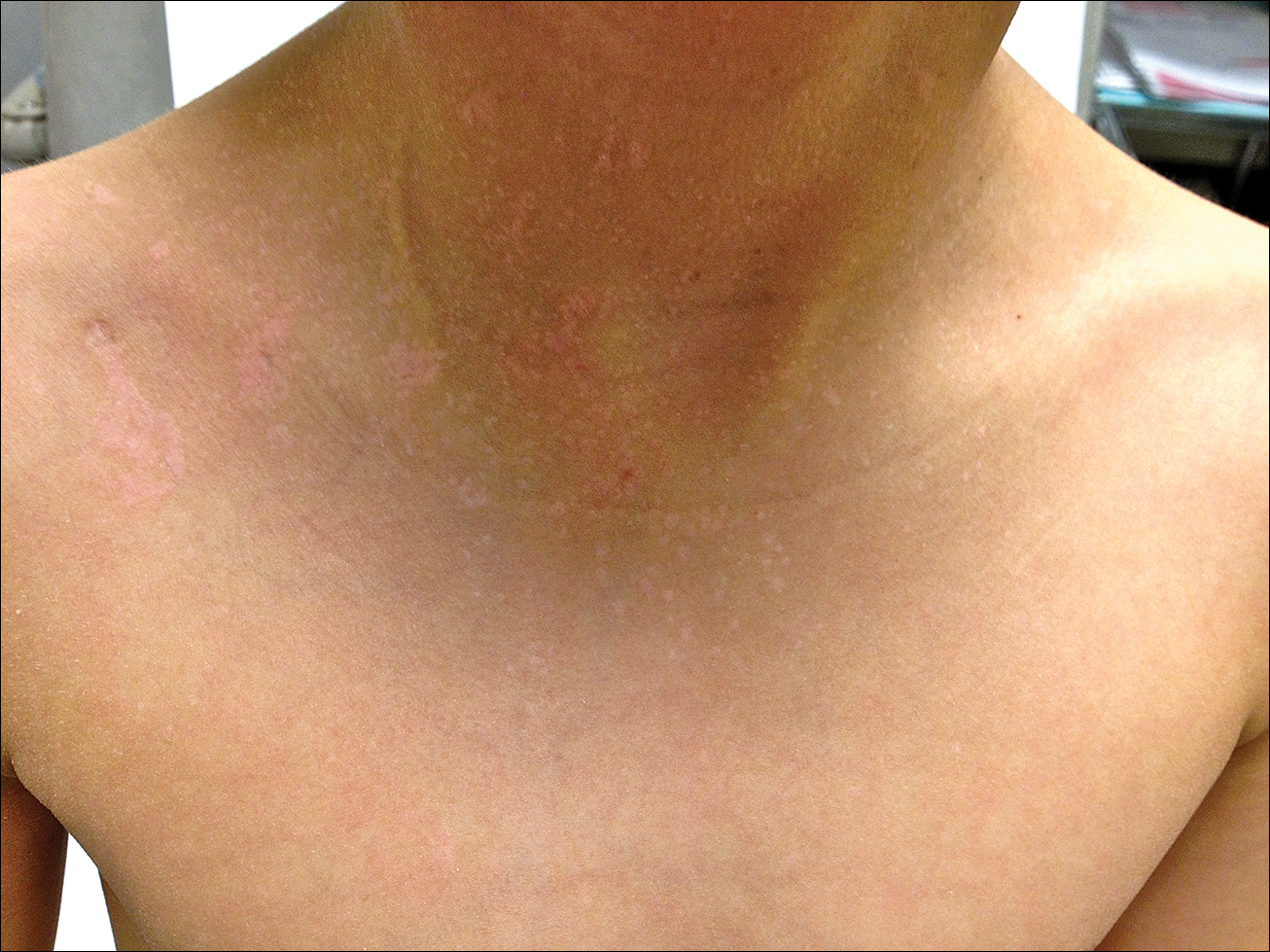
The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
To the Editor:
Epidermodysplasia verruciformis (EV) is a rare autosomal-recessive genodermatosis characterized by widespread infection with specific strains of human papillomavirus (HPV). Patients with EV have a unique susceptibility to acquire HPV due to defects in cellular immunity to the presenting antigens.1 These defects may be related to mutations of the EVER genes or due to acquisition of an immunosuppressive condition.2,3 Infections with HPV-3 and HPV-10 do not lead to the development of malignancies. However, infection with HPV-5, HPV-8, and HPV-14 can lead to the development of nonmelanoma skin cancers, usually squamous cell carcinomas (SCCs), in approximately 60% of patients.3,4 This viral condition lasts throughout the patient’s lifetime and presents as tinea versicolor–like macules and patches. These lesions may be confused with seborrheic keratosis or verruca plana.5 Lesions typically are hypopigmented but occasionally may be hyperpigmented or erythematous. They often are found on the trunk, but lesions on the face, arms, palms, legs, and soles have been reported.5 Mucous membranes are always spared. Epidermodysplasia verruciformis often presents in childhood, except in cases related to acquired immunosuppression. The condition has no sex or racial predilection and no geographical preference.5
A 7-year-old boy (Fitzpatrick skin type V) presented with an asymptomatic rash on the trunk (Figure 1), dorsal aspect of the hands, and forehead. The lesions first appeared 5 years prior on the upper back and upper chest and had recently spread to the forehead and frontal aspect of the scalp. The patient had a history of myelomeningocele, which was corrected at birth with surgical placement of a ventriculoperitoneal shunt. The patient was otherwise healthy and met all appropriate developmental milestones for his age group. Family history revealed consanguinity of the patient’s paternal grandparents who were first cousins. The patient’s mother denied any other family member having similar rashes or lesions.

The patient had been treated for pityriasis versicolor on and off for 2 years by another dermatologist. His mother reported faithfully applying ketoconazole cream twice daily for several months with no improvement. She also reported using topical steroids, which did not provide any benefit. The patient and mother denied any associated pruritus, bleeding, burning, or physical discomfort.
Skin examination revealed diffuse, flat, polymorphous, hypopigmented and salmon-colored hyperkeratotic macules and patches with mild scaling on the upper region of the anterior aspect of the chest and upper back (Figure 2A). Additionally, the patient had an extensive number of lesions on the forehead and frontal aspect of the scalp (Figure 2B).

A shave biopsy demonstrated a thick basket weave stratum corneum, koilocytes, and large pale keratinocytes with characteristic blue cytoplasm. These findings were characteristic for EV.
At the patient’s 3-month follow-up visit, he again denied any symptoms associated with the lesions and reported that the appearance was diminishing in severity. On examination there was no evidence of SCC. The mother was advised to discontinue all topical treatments for the patient and return to the office every 3 to 6 months for regular skin surveillance. The mother was further advised to protect the patient from UV radiation with sunscreen and sun-protective clothing.
Epidermodysplasia verruciformis was first reported by Lewandowsky and Lutz6 in 1922. This rare condition often presents in childhood and is characterized by a persistent HPV infection and an autosomal-recessive inheritance pattern. Reports in the literature frequently involve kindreds. Often, patients with EV have a family history of first-degree or second-degree consanguinity.7
The clinical presentation of EV often resembles a pityriasis versicolor–like eruption. However, pityriasis versicolor is less commonly seen in childhood and is more prevalent in patients aged 21 to 30 years, likely due to increased sebum production and changing hormone levels. Furthermore, it is unusual to see pityriasis versicolor affect the face and scalp.8 Lesions of EV vary from hypopigmented and pinkish red macules to confluent patches and hyperkeratotic verrucalike lesions.3 Clinical characteristics also may include dyschromic patches; lesions that resemble flat warts on the trunk, face, and distal arms; and/or lesions that appear similar to seborrheic keratoses on the dorsal aspect of the hands.9,10
Mutations of the EVER gene downregulate a cell’s ability to adequately attack the HPV antigens.11 Although some patients with EV are found to have mutations of the EVER1 and EVER2 genes, a notable portion of patients with EV lack these mutations. Three other causes of EV include acquisition of immunosuppressive conditions including lymphoma, solid organ transplant, and human immunodeficiency virus. If one suspects autosomal-recessive inheritance of EV, genetic testing such as polymerase chain reaction DNA fragment analysis can be performed to determine if there are mutations on the EVER1 or EVER2 genes.12
The inability of patients with EV to mount an immune response to multiple types of HPV increases the risk for developing cutaneous malignancies.7 Additionally, it is known that UV radiation diminishes skin cell immunity, and the combination of EV and UV radiation further increases the risk for developing SCCs.11 The development of nonmelanoma skin cancers usually occurs on sun-exposed skin 20 to 30 years after the onset of lesions, with the highest occurrence of SCCs presenting in the fourth decade of life.1
Protection from UV light exposure is critical to reduce the risk for malignancy. Treatment options for EV lesions have included topical imiquimod 5%, 5-fluorouracil, oral isotretinoin, and intralesional interferon alfa, but patients are often refractory to these interventions. Curettage, surgical excision, electrosurgery, and laser ablation can be effective for individual lesions but carry a greater risk for scarring.1 Photodynamic therapy with aminolevulinic acid and blue light represents a promising option that deserves further study.
Epidermodysplasia verruciformis should be considered as a differential diagnosis in all patients presenting with disseminated lesions resembling pityriasis versicolor that are unresponsive to treatment. A biopsy will help to establish the diagnosis. Patients should minimize sun exposure and report any skin lesions that are changing in appearance.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
- Hoffner MV, Camacho FM. Surgical treatment of epidermodysplasia verruciformis. Dermatol Surg. 2010;36:363-367.
- McDermott D, Gammon B, Snijders P. Autosomal dominant epidermodysplasia verruciformis lacking a known EVER1 or EVER2 mutation. Pediatr Dermatol. 2009;26:306-310.
- Patel T, Morrison K, Rady P, et al. Epidermodysplasia verruciformis and susceptibility to HPV. Dis Markers. 2010;29:199-206.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:308-311.
- Oliveira W, Netu C, Rady P, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
- Lewandowsky F, Lutz W. Ein Fall einer bisher nicht beschriebenen Hauterkrankung (epidermodysplasia verruciformis). Arch Dermatol Syphilol. 1922;141:193-203.
- Prystowsky S, Herndon J, Freeman R, et al. Epidermodysplasia verruciformis. Am J Dis Child. 1976;130:437-440.
- Kyriakis KP, Terzoudi S, Palamaras I, et al. Pityriasis versicolor prevalence by age and gender. Mycoses. 2006;49:517-518.
- Nuovo G, Ishag M. The histologic spectrum of epidermodysplasia verruciformis. Am J Surg Pathol. 2000;24:1400-1406.
- Jacobelli S, Laude H, Carlotti A, et al. Epidermodysplasia verruciformis in human immunodeficiency virus-infected patients: a marker of human papillomavirus-related disorders not affected by antiretroviral therapy. Arch Dermatol. 2011;147:590-596.
- Rogers HD, MacGregor JL, Nord KM, et al. Acquired epidermodysplasia verruciformis. J Am Acad Dermatol. 2009;60:316-320.
- Gober MD, Rady PL, He Q, et al. Novel homozygous frameshift mutation of EVER1 gene in an epidermodysplasia verruciformis patient. J Invest Dermatol. 2007;127:817-820.
Practice Points
- Epidermodysplasia verruciformis (EV) is a rare genodermatosis that usually presents in early childhood and presents as verrucous papules and plaques most commonly on the skin of the head, neck, and upper extremities. It often is misdiagnosed at pityriasis versicolor.
- Mutations of the EVER1 and EVER2 genes have been identified as a source for developing EV.
- Epidermodysplasia verruciformis produces wartlike lesions in individuals who have a unique susceptibility to acquiring the human papillomavirus and early onset of nonmelanoma skin cancers, most commonly squamous cell carcinomas related to viral oncogenesis.
- Avoidance and protection from UV exposure is a critical component of treatment plans for patients with EV.
Diagnosis of a Rapidly Growing Preauricular Nodule: Chondroid Syringoma or Pleomorphic Adenoma?
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
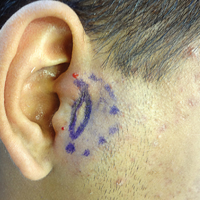
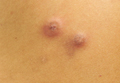
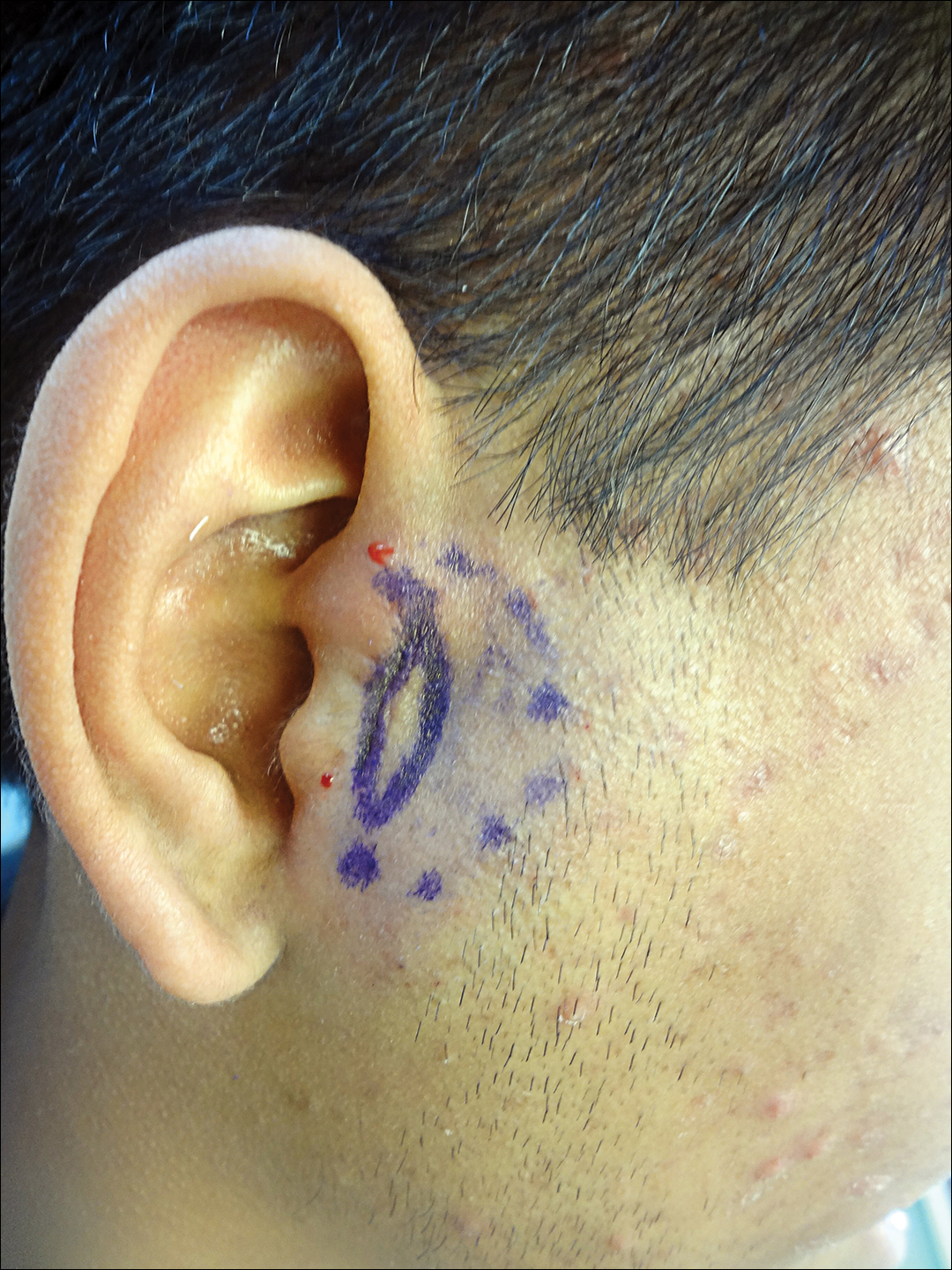
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.
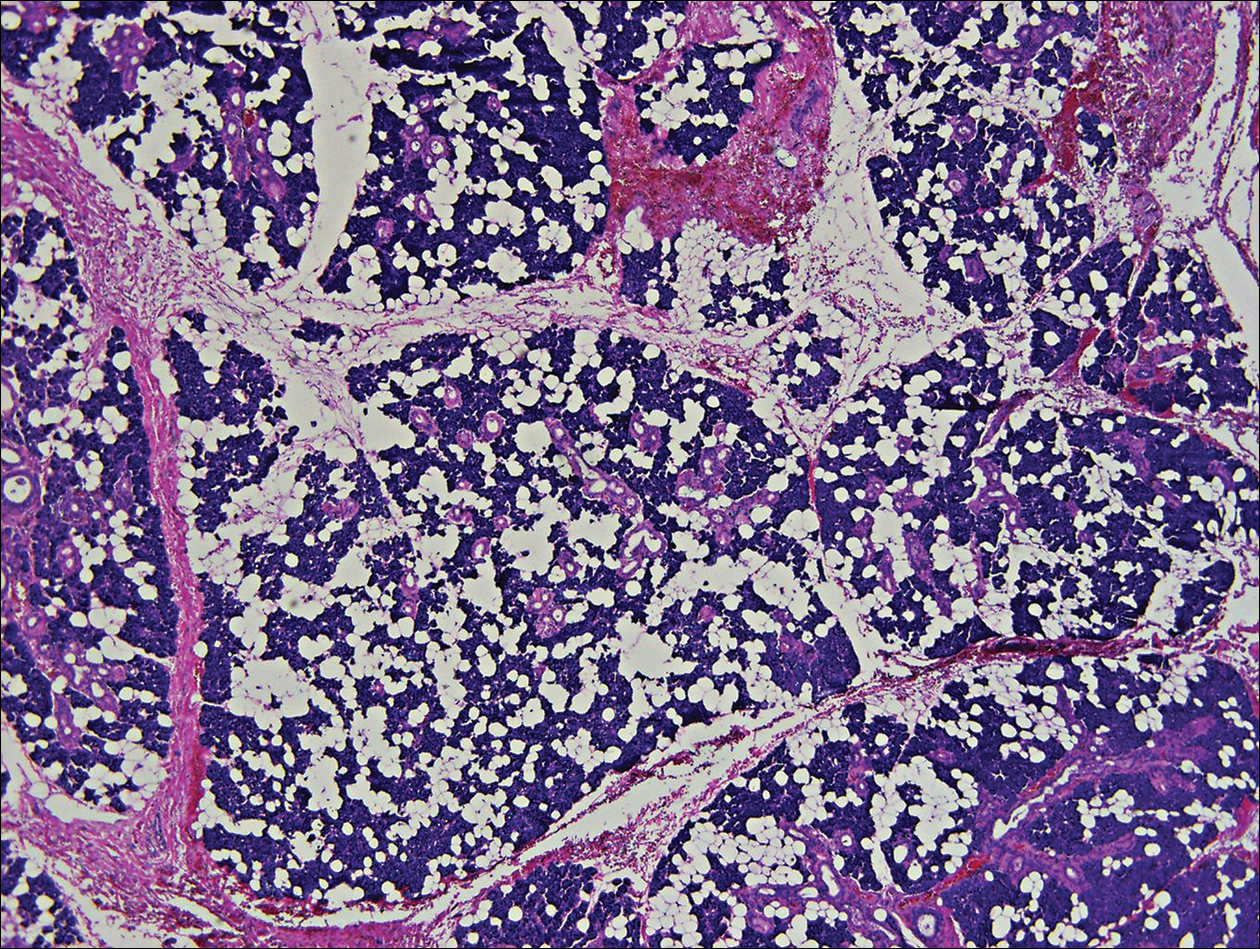
Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.
Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.

Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.

Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
To the Editor:
Chondroid syringoma is a rare benign mixed tumor that originates from the sweat glands, typically presenting with both epithelial and mesenchymal components.1 It differs from pleomorphic adenoma, which arises from salivary glands. The surgical approach for complete excision is different for the 2 tumors; therefore, definitive diagnosis is important. For chondroid syringoma, total excision is recommended,2 while for pleomorphic adenoma, extracapsular dissection or superficial parotidectomy is commonly indicated. We report a case of a preauricular nodule at presentation and highlight the importance of differentiating a chondroid syringoma from a pleomorphic adenoma. This case is unique because of the anatomic location of the nodule, making diagnosis difficult because the tumor was abutting the parotid gland and a biopsy included normal salivary gland cells.
A 19-year-old man with a history of moderate acne on the shoulders, back, and face presented with a rapidly growing, painless nodule on right preauricular region of 6 months’ duration. The nodule was originally thought to be acne related and monitored, as the patient was asymptomatic. On examination the patient was found to have a firm, fixed, nontender, subcutaneous nodule overlying the right temporomandibular joint just anterior to the right tragus (Figure 1). Laboratory results were unremarkable. Computed tomography showed a subcutaneous nonaggressive-appearing soft-tissue mass measuring 16×17×12 mm just anterior and inferior to the external auditory canal cartilaginous segment with no bony abnormalities. The patient was initially treated with incomplete excision of the area for a presumed sebaceous cyst; 2 months later, an abnormal biopsy prompted a complete excisional biopsy.

Histologically, the initial incomplete excision biopsy revealed myxoid and chondroid tissue with glandular elements and adjacent lymph node with strong positivity for S-100 protein and moderate positivity for glial fibrillary acid protein, consistent with chondroid syringoma (Figure 2). Histological findings on second excision biopsy performed 2 months later showed tumor cells surrounded by normal salivary gland cells; therefore, it was difficult to define the origin of this tumor. Subsequent magnetic resonance imaging showed no evidence of the tumor and normal parotid gland borders.

Chondroid syringoma is a rare nonmelanoma skin tumor of the head and neck, mostly benign in nature but with malignant potential. Predominantly, it presents in males as an asymptomatic, slow-growing, nontender nodule.2 Malignant chondroid syringomas are more rare, typically appear on the trunk and legs of females, and present as rapidly growing hard nodules. They can arise de novo or from a preexisting chondroid syringoma and can metastasize.3,4
Clinically and histologically, chondroid syringoma resembles a pleomorphic adenoma. Its diagnosis is dependent on the clinical location to exclude origin in a salivary gland.5 Folliculosebaceous and myoepithelial differentiation within the tumor has been reported.6 Immunocytochemistry is the same in both types and is used to identify 2 prominent components—epithelial and mesenchymal—found in both chondroid syringoma and pleomorphic adenoma. Immunocytochemistry differentiates the epithelial component, which expresses cytokeratin, epithelial membrane antigen, and carcinoembryonic antigen. In contrast, the mesenchymal component expresses S-100, vimentin, and neuron‐specific enolase, and less often glial fibrillary acidic protein, smooth muscle actin, calponin, or p63.5,7,8 Identification of both layers is a distinctive trait of both tumors, rendering it apart from other conditions in the differential diagnosis.5
Typical treatment options include excision, electrodesiccation, dermabrasion, and argon or CO2 laser. Total excision is recommended if there is a benign tumor and complete excision is a cure.2 One case of recurrent benign chondroid syringoma was treated by Mohs micrographic surgery on the eyebrow9; however, Mohs surgery was not recommended in our case due to concerns of spread if malignant as well as an unknown tumor depth, as these tumors have a tendency for deep infiltration.
Due to its anatomical location and presentation as an anterior preauricular mass, it was difficult to differentiate between chondroid syringoma from sweat gland origin and pleomorphic adenoma from the salivary gland. As seen in our case, it is important for physicians to be aware of the differential diagnosis for mixed tumors because it can have a notable effect on the type of surgical therapy and follow-up management.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
- Hirsch P, Helwig EB. Chondroid syringoma. Arch Dermatol. 1961;84:835-847.
- Turhan-Haktanir N, Sahin O, Bukulmez A, et al. Chondroid syringoma in a child. Pediatr Dermatol. 2007;24:505-507.
- Mathiasen RA, Rasgon BM, Rumore G. Malignant chondroid syringoma of the face: a first reported case. Otolaryngol Head Neck Surg. 2005;133:305-307.
- Barnett MD, Wallack MK, Zuretti A, et al. Recurrent malignant chondroid syringoma of the foot: a case report and review of the literature. Am J Clin Oncol. 2000;23:227-232.
- Dubb M, Michelow P. Cytologic features of chondroid syringoma in fine needle aspiration biopsies a report of 3 cases. Acta Cytol. 2010;54:183-186.
- Rauso R, Santagata M, Tartaro G, et al. Chondroid syringoma: rare tumor of orofacial region. Minerva Stomatol. 2009;58:383-388.
- Metzler G, Schaumburg-Lever G, Hornstein O, et al. Malignant chondroid syringoma: immunohistopathology. Am J Dermatopathol. 1996;18:83-89.
- Argenyi ZB, Balogh K, Goeken JA. Immunohistochemical characterization of chondroid syringomas. Am J Clin Pathol. 1988;90:662-669.
- Walls AC, Deng A, Geist DE. Mohs micrographic surgery for recurrent chondroid syringoma of the eyebrow. Dermatol Surg. 2012;38:800-802.
Practice Points
- Clinically and histologically, pleomorphic adenomas and chondroid syringoma both have identical presentations. Differentiation can be determined by knowing where the mixed tumor originated.
- Both lesions warrant different surgical management techniques. Pleomorphic adenoma requires extracapsular dissection or superficial parotidectomy, while complete excision is recommended for chondroid syringoma.
Mucous Membrane Pemphigoid Involving the Trachea and Bronchi: An Extremely Rare and Life-Threatening Presentation
To the Editor:
Mucous membrane pemphigoid (MMP) is an autoimmune blistering disorder that causes subepithelial damage and scarring of mucosal surfaces with or without skin involvement.1 The clinical presentation is highly variable. The oropharynx is the most common site of initial presentation, followed by ocular, nasopharyngeal, anogenital, skin, laryngeal, and esophageal involvement.2 Patients often present to a variety of specialists depending on initial symptoms, and due to the diverse clinical manifestations, MMP often is misdiagnosed. Our patient presented an even greater challenge because the disease progressed to tracheal and bronchial involvement.
A 37-year-old man presented to his primary care physician with a chief concern of a sore throat and oral ulcers. The patient was treated with a course of antibiotics followed by a nystatin oral solution. He continued to develop ulcerative lesions on the soft palate, posterior pharynx, and nasal mucosae. He sought treatment from 2 otolaryngologists (ENTs) and a gastroenterologist, and continued to be treated with multiple oral antibiotics, fluconazole, and topical nystatin. Despite treatment, the patient developed pansinusitis and laryngitis and presented to the ENT department at our institution with severe hoarseness and dyspnea on exertion. Examination by the ENT department revealed ulcerative lesions of the nares with stenosis and ulcers along the soft palate. Videolaryngostroboscopy showed remarkable supraglottic edema with thick endolaryngeal mucus. The patient worked as a funeral director and had notable formaldehyde exposure. He also hunted wild game and performed taxidermy regularly.
The patient was admitted and treated with intravenous dexamethasone for a compromised airway. Subsequently, he was taken to the operating room and had biopsies performed of the posterior pharynx. Given his exposure history, the infectious disease department was consulted and he was evaluated for multiple viral, bacterial, and fungal suspects including leishmania and tularemia. Age-appropriate screening, physical examination, and review of systems were negative for an underlying neoplasm. Histopathologic examination revealed a subepithelial vesicular mucositis with a mixed infiltrate of lymphocytes and histiocytes. Direct immunofluorescence microscopy demonstrated strong linear fluorescence along the epithelial-subepithelial junction with IgG and C3. Based on these findings, the diagnosis of MMP was made.
Further testing for bullous pemphigoid antigen 1 (BP230) and bullous pemphigoid antigen 2 (BP180) were negative. On one occasion the patient tested positive for anti-BP230 IgG, but it was at a level judged to be insignificant (7.5 [reference range, <9]). The patient also was negative for autoantibodies against desmoglein 1 and 3. Indirect immunofluorescence using rat bladder epithelium was not performed.
The patient was started on methotrexate and oral prednisone by the rheumatology department, but after 1 week, he presented in respiratory distress and was taken for an emergency tracheostomy. The patient eventually was referred to the dermatology department where methotrexate was discontinued and the patient was started on titrating doses of prednisone and mycophenolate mofetil. Eight weeks later, the patient became completely aphonic and was taken by ENT for dilation of the supraglottic, glottic, and subglottic stenosis with mucosal triamcinolone injections. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily was initiated in addition to mycophenolate mofetil 3 g and prednisone 80 mg, but again the patient developed near-complete tracheal stenosis just proximal to the tracheostomy entry site. At 16 weeks, balloon dilation was repeated with dexamethasone injections and topical mitomycin C. Subsequently, the patient regained some use of his voice. Although the next several laryngoscopes showed improvement in the patient’s epiglottis and glottis, the trachea continued to require debridement and dilation.
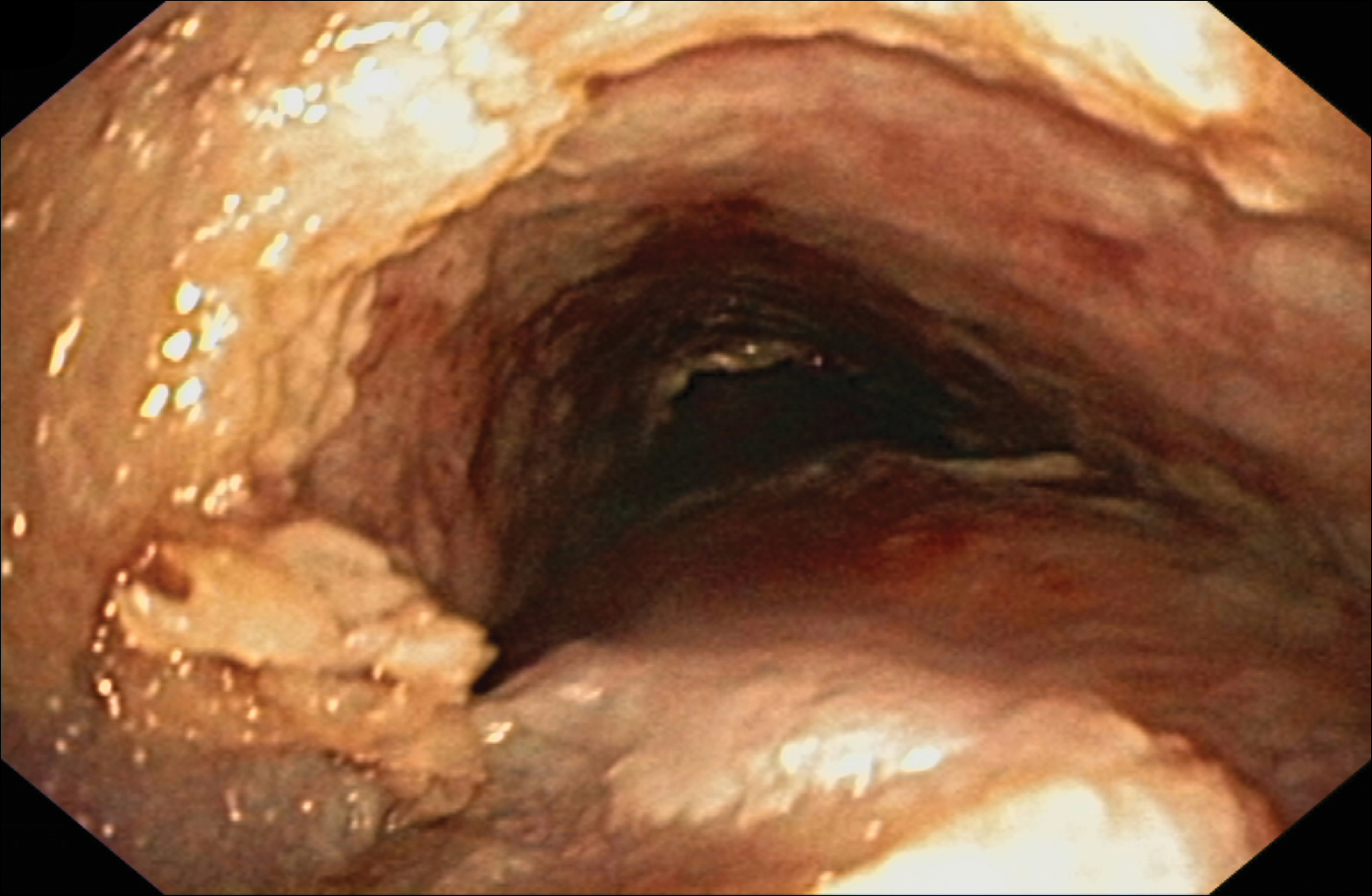
Despite maximal medical therapy and surgical interventions, the patient had little improvement in his voice and large clots of blood obstructed his tracheostomy daily. He was unable to sleep in his preferred position on the stomach (prone) due to dyspnea but had less distress sleeping on his back (supine). The patient was referred to the pulmonology department for an endotracheobronchoscopy to further evaluate the airway. It was discovered that the mucosa of the trachea from the level of the tracheostomy to the carina was friable with active erosions and thick bloody secretions (Figure 1). Lesions extended as far as the scope was able to visualize to the left upper lobe takeoff and the right mainstem bronchus (Figure 2). Biopsies of the carinal mucosa showed 3+/3+ linear fluorescence with IgG along the dermoepidermal junction. Salt-split studies were performed, but because the specimen was fragmented, it was not possible to assess if the fluorescence was present at the floor or at the roof of the split.

Given the severity of disease and failure to respond to other aggressive immunosuppressive therapies as well as having been with a tracheostomy for 22 months, the patient was started on 2 doses of intravenous rituximab 1 g 2 weeks apart along with trimethoprim-sulfamethoxazole (3 times weekly) for pneumocystis pneumonia prophylaxis. No complications were observed during infusions. After 2 rituximab infusions, he was weaned off of prednisone and a repeat bronchoscopy showed no airway ulcers beyond the distal trachea or endobronchial obstruction. However, the subglottic space and area above the tracheostomy showed remarkable stenosis with a cobblestone pattern and granulation tissue with continued narrowing of the subglottic area. The ENT performed further dilation and after 34 months, the tracheostomy was removed and a T-tube was placed. The patient required cleaning out of the T-tube approximately every 3 months, and after 2 years the original T-tube was replaced with a new one. At the time of this report, the ENT recommended removing the T-tube, but the patient was reluctant to do so; therefore, a second T-tube replacement is planned. He continues to do well without relapse and has been off all medical therapy for nearly 4 years.
Mucous membrane pemphigoid is an acquired autoimmune subepithelial blistering disease that predominantly affects mucous membranes with or without skin involvement. This condition has been referred to as cicatricial pemphigoid, oral pemphigoid, and ocular cicatricial pemphigoid, among other names. It is characterized by linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone. According to the international consensus on MMP, the target antigens identified in the epithelial basement membrane zone include bullous pemphigoid antigen 1 (BP230), bullous pemphigoid antigen 2 (BP180), laminin 5 (α3, β3, γ2 chains), laminin 6 (α3 chain), type VII collagen, and integrin β4 subunit.3 Not all patients with MMP will have circulating autoantibodies to the above components, and although our patient did have detectable anti-BP230 IgG, it was not considered clinically significant. Furthermore, the type of autoantibody does not impact decisions regarding therapy selection.3
Although rare, MMP is well-known to dermatologists and ophthalmologists who manage a large majority of MMP patients depending on which mucosa is involved. Mucous membrane pemphigoid is extremely rare in the lower respiratory tract, and when these lesions are discovered, it often is in the face of life-threatening respiratory distress. Mucous membrane pemphigoid is a challenging disease to treat, even more so when the primary specialty physician is unable to visualize the affected areas. Our patient’s disease was limited primarily to the pharynx, larynx, trachea, and bronchi with few oral lesions. According to a PubMed search of articles indexed for MEDLINE using the terms mucous membrane pemphigus, cicatricial pemphigoid, trachea, bronchus, and fatal, 8 reports (7 case reports and 1 prospective study) of MMP involving the lower respiratory tract have been published.4-11 Of the case reports, each patient also presented with involvement of the eyes or skin.4,5,7-11 Four of these cases were fatal secondary to cardiopulmonary arrest.5,7,9,10 In the prospective study, 110 consecutive patients with clinical, histologic, and immunologic criteria of MMP were examined with a flexible nasopharyngolaryngoscope.6 Thirty-eight patients had nose or throat symptoms but only 10 had laryngeal involvement and 5 had acute dyspnea. The nasal valves, choanae, pharynx, and/or larynx were severely scarred in 7 patients, which was fatal in 3.6
Medical treatment should be based on the following factors of the patient’s disease: site, severity, and rapidity of progression.3 High-risk patients can be defined as those who have lesions at any of the following sites: ocular, genital, nasopharyngeal, esophageal, and laryngeal mucosae. As our patient had involvement at several high-risk sites, in particular sites only visualized by various scoping procedures, a team of physicians including dermatologists, ENT physicians, pulmonologists, and oncologists was necessary to facilitate his care. Scarring is the hallmark of MMP and prevention of scarring is the most important aspect of treatment of MMP. Surgical repair of the previously involved mucosa is difficult, as the tissue is prone to re-scarring and difficult to heal. Over the last several years, there has been increasing evidence for the use of rituximab in autoimmune bullous skin diseases including pemphigus vulgaris, epidermolysis bullosa acquisita, and MMP.12-14 After 2 infusions of rituximab, our patient had clearance of his disease and currently is doing well with a T-tube.
Acknowledgments
We thank Kim Yancey, MD (Dallas, Texas), for providing access to the patient’s diagnostic laboratory immunology and reviewing biopsy specimens; Luis Angel, MD (San Antonio, Texas), for providing bronchoscopy photographs; and C. Blake Simpson, MD (San Antonio, Texas), for co-managing this challenging case.
- James WD, Berger TG, Elston D. Chronic blistering diseases. In: James WD, Berger TG, Elston D. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Sanders Elsevier; 2010:448-467.
- Neff AG, Turner M, Mutasim DF. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag. 2008;4:617-626.
- Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370-379.
- Kato K, Moriyama Y, Saito H, et al. A case of mucous membrane pemphigoid involving the trachea and bronchus with autoantibodies to β3 subunit of laminin-332. Acta Derm Venereol. 2014;94:237-238.
- Gamm DM, Harris A, Mehran RJ, et al. Mucous membrane pemphigoid with fatal bronchial involvement in a seventeen-year-old girl. Cornea. 2006;25:474-478.
- Alexandre M, Brette MD, Pascal F, et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore). 2006;85:239-252.
- de Carvalho CR, Amato MB, Da Silva LM, et al. Obstructive respiratory failure in cicatricial pemphigoid. Thorax. 1989;44:601-602.
- Müller LC, Salzer GM. Stenosis of left mainstem bronchus in a case of cicatricial pemphigoid. Eur J Cardiothorac Surg. 1988;2:284-286.
- Camisa C, Allen CM. Death from CP in a young woman with oral, laryngeal, and bronchial involvement. Cutis. 1987;40:426-429.
- Derbes VJ, Pitot HC, Chernosky ME. Fatal cicatricial mucous membrane pemphigoid of the trachea. Dermatol Trop Ecol Geogr. 1962;1:114-117.
- Wieme N, Lambert J, Moerman M, et al. Epidermolysis bullosa acquisita with combined features of bullous pemphigoid and cicatricial pemphigoid. Dermatology. 1999;198:310-313.
- Taylor J, McMillan R, Shephard M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid [published online March 11, 2015]. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:161.e20-171.e20.
- Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid [published online July 9, 2013]. Ocul Surf. 2013;11:259-266.
- Maley A, Warren M, Haberman I, et al. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid (MMP) [published online February 28, 2016]. J Am Acad Dermatol. 2016;74:835-840.
To the Editor:
Mucous membrane pemphigoid (MMP) is an autoimmune blistering disorder that causes subepithelial damage and scarring of mucosal surfaces with or without skin involvement.1 The clinical presentation is highly variable. The oropharynx is the most common site of initial presentation, followed by ocular, nasopharyngeal, anogenital, skin, laryngeal, and esophageal involvement.2 Patients often present to a variety of specialists depending on initial symptoms, and due to the diverse clinical manifestations, MMP often is misdiagnosed. Our patient presented an even greater challenge because the disease progressed to tracheal and bronchial involvement.
A 37-year-old man presented to his primary care physician with a chief concern of a sore throat and oral ulcers. The patient was treated with a course of antibiotics followed by a nystatin oral solution. He continued to develop ulcerative lesions on the soft palate, posterior pharynx, and nasal mucosae. He sought treatment from 2 otolaryngologists (ENTs) and a gastroenterologist, and continued to be treated with multiple oral antibiotics, fluconazole, and topical nystatin. Despite treatment, the patient developed pansinusitis and laryngitis and presented to the ENT department at our institution with severe hoarseness and dyspnea on exertion. Examination by the ENT department revealed ulcerative lesions of the nares with stenosis and ulcers along the soft palate. Videolaryngostroboscopy showed remarkable supraglottic edema with thick endolaryngeal mucus. The patient worked as a funeral director and had notable formaldehyde exposure. He also hunted wild game and performed taxidermy regularly.
The patient was admitted and treated with intravenous dexamethasone for a compromised airway. Subsequently, he was taken to the operating room and had biopsies performed of the posterior pharynx. Given his exposure history, the infectious disease department was consulted and he was evaluated for multiple viral, bacterial, and fungal suspects including leishmania and tularemia. Age-appropriate screening, physical examination, and review of systems were negative for an underlying neoplasm. Histopathologic examination revealed a subepithelial vesicular mucositis with a mixed infiltrate of lymphocytes and histiocytes. Direct immunofluorescence microscopy demonstrated strong linear fluorescence along the epithelial-subepithelial junction with IgG and C3. Based on these findings, the diagnosis of MMP was made.
Further testing for bullous pemphigoid antigen 1 (BP230) and bullous pemphigoid antigen 2 (BP180) were negative. On one occasion the patient tested positive for anti-BP230 IgG, but it was at a level judged to be insignificant (7.5 [reference range, <9]). The patient also was negative for autoantibodies against desmoglein 1 and 3. Indirect immunofluorescence using rat bladder epithelium was not performed.
The patient was started on methotrexate and oral prednisone by the rheumatology department, but after 1 week, he presented in respiratory distress and was taken for an emergency tracheostomy. The patient eventually was referred to the dermatology department where methotrexate was discontinued and the patient was started on titrating doses of prednisone and mycophenolate mofetil. Eight weeks later, the patient became completely aphonic and was taken by ENT for dilation of the supraglottic, glottic, and subglottic stenosis with mucosal triamcinolone injections. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily was initiated in addition to mycophenolate mofetil 3 g and prednisone 80 mg, but again the patient developed near-complete tracheal stenosis just proximal to the tracheostomy entry site. At 16 weeks, balloon dilation was repeated with dexamethasone injections and topical mitomycin C. Subsequently, the patient regained some use of his voice. Although the next several laryngoscopes showed improvement in the patient’s epiglottis and glottis, the trachea continued to require debridement and dilation.
Despite maximal medical therapy and surgical interventions, the patient had little improvement in his voice and large clots of blood obstructed his tracheostomy daily. He was unable to sleep in his preferred position on the stomach (prone) due to dyspnea but had less distress sleeping on his back (supine). The patient was referred to the pulmonology department for an endotracheobronchoscopy to further evaluate the airway. It was discovered that the mucosa of the trachea from the level of the tracheostomy to the carina was friable with active erosions and thick bloody secretions (Figure 1). Lesions extended as far as the scope was able to visualize to the left upper lobe takeoff and the right mainstem bronchus (Figure 2). Biopsies of the carinal mucosa showed 3+/3+ linear fluorescence with IgG along the dermoepidermal junction. Salt-split studies were performed, but because the specimen was fragmented, it was not possible to assess if the fluorescence was present at the floor or at the roof of the split.


Given the severity of disease and failure to respond to other aggressive immunosuppressive therapies as well as having been with a tracheostomy for 22 months, the patient was started on 2 doses of intravenous rituximab 1 g 2 weeks apart along with trimethoprim-sulfamethoxazole (3 times weekly) for pneumocystis pneumonia prophylaxis. No complications were observed during infusions. After 2 rituximab infusions, he was weaned off of prednisone and a repeat bronchoscopy showed no airway ulcers beyond the distal trachea or endobronchial obstruction. However, the subglottic space and area above the tracheostomy showed remarkable stenosis with a cobblestone pattern and granulation tissue with continued narrowing of the subglottic area. The ENT performed further dilation and after 34 months, the tracheostomy was removed and a T-tube was placed. The patient required cleaning out of the T-tube approximately every 3 months, and after 2 years the original T-tube was replaced with a new one. At the time of this report, the ENT recommended removing the T-tube, but the patient was reluctant to do so; therefore, a second T-tube replacement is planned. He continues to do well without relapse and has been off all medical therapy for nearly 4 years.
Mucous membrane pemphigoid is an acquired autoimmune subepithelial blistering disease that predominantly affects mucous membranes with or without skin involvement. This condition has been referred to as cicatricial pemphigoid, oral pemphigoid, and ocular cicatricial pemphigoid, among other names. It is characterized by linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone. According to the international consensus on MMP, the target antigens identified in the epithelial basement membrane zone include bullous pemphigoid antigen 1 (BP230), bullous pemphigoid antigen 2 (BP180), laminin 5 (α3, β3, γ2 chains), laminin 6 (α3 chain), type VII collagen, and integrin β4 subunit.3 Not all patients with MMP will have circulating autoantibodies to the above components, and although our patient did have detectable anti-BP230 IgG, it was not considered clinically significant. Furthermore, the type of autoantibody does not impact decisions regarding therapy selection.3
Although rare, MMP is well-known to dermatologists and ophthalmologists who manage a large majority of MMP patients depending on which mucosa is involved. Mucous membrane pemphigoid is extremely rare in the lower respiratory tract, and when these lesions are discovered, it often is in the face of life-threatening respiratory distress. Mucous membrane pemphigoid is a challenging disease to treat, even more so when the primary specialty physician is unable to visualize the affected areas. Our patient’s disease was limited primarily to the pharynx, larynx, trachea, and bronchi with few oral lesions. According to a PubMed search of articles indexed for MEDLINE using the terms mucous membrane pemphigus, cicatricial pemphigoid, trachea, bronchus, and fatal, 8 reports (7 case reports and 1 prospective study) of MMP involving the lower respiratory tract have been published.4-11 Of the case reports, each patient also presented with involvement of the eyes or skin.4,5,7-11 Four of these cases were fatal secondary to cardiopulmonary arrest.5,7,9,10 In the prospective study, 110 consecutive patients with clinical, histologic, and immunologic criteria of MMP were examined with a flexible nasopharyngolaryngoscope.6 Thirty-eight patients had nose or throat symptoms but only 10 had laryngeal involvement and 5 had acute dyspnea. The nasal valves, choanae, pharynx, and/or larynx were severely scarred in 7 patients, which was fatal in 3.6
Medical treatment should be based on the following factors of the patient’s disease: site, severity, and rapidity of progression.3 High-risk patients can be defined as those who have lesions at any of the following sites: ocular, genital, nasopharyngeal, esophageal, and laryngeal mucosae. As our patient had involvement at several high-risk sites, in particular sites only visualized by various scoping procedures, a team of physicians including dermatologists, ENT physicians, pulmonologists, and oncologists was necessary to facilitate his care. Scarring is the hallmark of MMP and prevention of scarring is the most important aspect of treatment of MMP. Surgical repair of the previously involved mucosa is difficult, as the tissue is prone to re-scarring and difficult to heal. Over the last several years, there has been increasing evidence for the use of rituximab in autoimmune bullous skin diseases including pemphigus vulgaris, epidermolysis bullosa acquisita, and MMP.12-14 After 2 infusions of rituximab, our patient had clearance of his disease and currently is doing well with a T-tube.
Acknowledgments
We thank Kim Yancey, MD (Dallas, Texas), for providing access to the patient’s diagnostic laboratory immunology and reviewing biopsy specimens; Luis Angel, MD (San Antonio, Texas), for providing bronchoscopy photographs; and C. Blake Simpson, MD (San Antonio, Texas), for co-managing this challenging case.
To the Editor:
Mucous membrane pemphigoid (MMP) is an autoimmune blistering disorder that causes subepithelial damage and scarring of mucosal surfaces with or without skin involvement.1 The clinical presentation is highly variable. The oropharynx is the most common site of initial presentation, followed by ocular, nasopharyngeal, anogenital, skin, laryngeal, and esophageal involvement.2 Patients often present to a variety of specialists depending on initial symptoms, and due to the diverse clinical manifestations, MMP often is misdiagnosed. Our patient presented an even greater challenge because the disease progressed to tracheal and bronchial involvement.
A 37-year-old man presented to his primary care physician with a chief concern of a sore throat and oral ulcers. The patient was treated with a course of antibiotics followed by a nystatin oral solution. He continued to develop ulcerative lesions on the soft palate, posterior pharynx, and nasal mucosae. He sought treatment from 2 otolaryngologists (ENTs) and a gastroenterologist, and continued to be treated with multiple oral antibiotics, fluconazole, and topical nystatin. Despite treatment, the patient developed pansinusitis and laryngitis and presented to the ENT department at our institution with severe hoarseness and dyspnea on exertion. Examination by the ENT department revealed ulcerative lesions of the nares with stenosis and ulcers along the soft palate. Videolaryngostroboscopy showed remarkable supraglottic edema with thick endolaryngeal mucus. The patient worked as a funeral director and had notable formaldehyde exposure. He also hunted wild game and performed taxidermy regularly.
The patient was admitted and treated with intravenous dexamethasone for a compromised airway. Subsequently, he was taken to the operating room and had biopsies performed of the posterior pharynx. Given his exposure history, the infectious disease department was consulted and he was evaluated for multiple viral, bacterial, and fungal suspects including leishmania and tularemia. Age-appropriate screening, physical examination, and review of systems were negative for an underlying neoplasm. Histopathologic examination revealed a subepithelial vesicular mucositis with a mixed infiltrate of lymphocytes and histiocytes. Direct immunofluorescence microscopy demonstrated strong linear fluorescence along the epithelial-subepithelial junction with IgG and C3. Based on these findings, the diagnosis of MMP was made.
Further testing for bullous pemphigoid antigen 1 (BP230) and bullous pemphigoid antigen 2 (BP180) were negative. On one occasion the patient tested positive for anti-BP230 IgG, but it was at a level judged to be insignificant (7.5 [reference range, <9]). The patient also was negative for autoantibodies against desmoglein 1 and 3. Indirect immunofluorescence using rat bladder epithelium was not performed.
The patient was started on methotrexate and oral prednisone by the rheumatology department, but after 1 week, he presented in respiratory distress and was taken for an emergency tracheostomy. The patient eventually was referred to the dermatology department where methotrexate was discontinued and the patient was started on titrating doses of prednisone and mycophenolate mofetil. Eight weeks later, the patient became completely aphonic and was taken by ENT for dilation of the supraglottic, glottic, and subglottic stenosis with mucosal triamcinolone injections. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily was initiated in addition to mycophenolate mofetil 3 g and prednisone 80 mg, but again the patient developed near-complete tracheal stenosis just proximal to the tracheostomy entry site. At 16 weeks, balloon dilation was repeated with dexamethasone injections and topical mitomycin C. Subsequently, the patient regained some use of his voice. Although the next several laryngoscopes showed improvement in the patient’s epiglottis and glottis, the trachea continued to require debridement and dilation.
Despite maximal medical therapy and surgical interventions, the patient had little improvement in his voice and large clots of blood obstructed his tracheostomy daily. He was unable to sleep in his preferred position on the stomach (prone) due to dyspnea but had less distress sleeping on his back (supine). The patient was referred to the pulmonology department for an endotracheobronchoscopy to further evaluate the airway. It was discovered that the mucosa of the trachea from the level of the tracheostomy to the carina was friable with active erosions and thick bloody secretions (Figure 1). Lesions extended as far as the scope was able to visualize to the left upper lobe takeoff and the right mainstem bronchus (Figure 2). Biopsies of the carinal mucosa showed 3+/3+ linear fluorescence with IgG along the dermoepidermal junction. Salt-split studies were performed, but because the specimen was fragmented, it was not possible to assess if the fluorescence was present at the floor or at the roof of the split.


Given the severity of disease and failure to respond to other aggressive immunosuppressive therapies as well as having been with a tracheostomy for 22 months, the patient was started on 2 doses of intravenous rituximab 1 g 2 weeks apart along with trimethoprim-sulfamethoxazole (3 times weekly) for pneumocystis pneumonia prophylaxis. No complications were observed during infusions. After 2 rituximab infusions, he was weaned off of prednisone and a repeat bronchoscopy showed no airway ulcers beyond the distal trachea or endobronchial obstruction. However, the subglottic space and area above the tracheostomy showed remarkable stenosis with a cobblestone pattern and granulation tissue with continued narrowing of the subglottic area. The ENT performed further dilation and after 34 months, the tracheostomy was removed and a T-tube was placed. The patient required cleaning out of the T-tube approximately every 3 months, and after 2 years the original T-tube was replaced with a new one. At the time of this report, the ENT recommended removing the T-tube, but the patient was reluctant to do so; therefore, a second T-tube replacement is planned. He continues to do well without relapse and has been off all medical therapy for nearly 4 years.
Mucous membrane pemphigoid is an acquired autoimmune subepithelial blistering disease that predominantly affects mucous membranes with or without skin involvement. This condition has been referred to as cicatricial pemphigoid, oral pemphigoid, and ocular cicatricial pemphigoid, among other names. It is characterized by linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone. According to the international consensus on MMP, the target antigens identified in the epithelial basement membrane zone include bullous pemphigoid antigen 1 (BP230), bullous pemphigoid antigen 2 (BP180), laminin 5 (α3, β3, γ2 chains), laminin 6 (α3 chain), type VII collagen, and integrin β4 subunit.3 Not all patients with MMP will have circulating autoantibodies to the above components, and although our patient did have detectable anti-BP230 IgG, it was not considered clinically significant. Furthermore, the type of autoantibody does not impact decisions regarding therapy selection.3
Although rare, MMP is well-known to dermatologists and ophthalmologists who manage a large majority of MMP patients depending on which mucosa is involved. Mucous membrane pemphigoid is extremely rare in the lower respiratory tract, and when these lesions are discovered, it often is in the face of life-threatening respiratory distress. Mucous membrane pemphigoid is a challenging disease to treat, even more so when the primary specialty physician is unable to visualize the affected areas. Our patient’s disease was limited primarily to the pharynx, larynx, trachea, and bronchi with few oral lesions. According to a PubMed search of articles indexed for MEDLINE using the terms mucous membrane pemphigus, cicatricial pemphigoid, trachea, bronchus, and fatal, 8 reports (7 case reports and 1 prospective study) of MMP involving the lower respiratory tract have been published.4-11 Of the case reports, each patient also presented with involvement of the eyes or skin.4,5,7-11 Four of these cases were fatal secondary to cardiopulmonary arrest.5,7,9,10 In the prospective study, 110 consecutive patients with clinical, histologic, and immunologic criteria of MMP were examined with a flexible nasopharyngolaryngoscope.6 Thirty-eight patients had nose or throat symptoms but only 10 had laryngeal involvement and 5 had acute dyspnea. The nasal valves, choanae, pharynx, and/or larynx were severely scarred in 7 patients, which was fatal in 3.6
Medical treatment should be based on the following factors of the patient’s disease: site, severity, and rapidity of progression.3 High-risk patients can be defined as those who have lesions at any of the following sites: ocular, genital, nasopharyngeal, esophageal, and laryngeal mucosae. As our patient had involvement at several high-risk sites, in particular sites only visualized by various scoping procedures, a team of physicians including dermatologists, ENT physicians, pulmonologists, and oncologists was necessary to facilitate his care. Scarring is the hallmark of MMP and prevention of scarring is the most important aspect of treatment of MMP. Surgical repair of the previously involved mucosa is difficult, as the tissue is prone to re-scarring and difficult to heal. Over the last several years, there has been increasing evidence for the use of rituximab in autoimmune bullous skin diseases including pemphigus vulgaris, epidermolysis bullosa acquisita, and MMP.12-14 After 2 infusions of rituximab, our patient had clearance of his disease and currently is doing well with a T-tube.
Acknowledgments
We thank Kim Yancey, MD (Dallas, Texas), for providing access to the patient’s diagnostic laboratory immunology and reviewing biopsy specimens; Luis Angel, MD (San Antonio, Texas), for providing bronchoscopy photographs; and C. Blake Simpson, MD (San Antonio, Texas), for co-managing this challenging case.
- James WD, Berger TG, Elston D. Chronic blistering diseases. In: James WD, Berger TG, Elston D. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Sanders Elsevier; 2010:448-467.
- Neff AG, Turner M, Mutasim DF. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag. 2008;4:617-626.
- Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370-379.
- Kato K, Moriyama Y, Saito H, et al. A case of mucous membrane pemphigoid involving the trachea and bronchus with autoantibodies to β3 subunit of laminin-332. Acta Derm Venereol. 2014;94:237-238.
- Gamm DM, Harris A, Mehran RJ, et al. Mucous membrane pemphigoid with fatal bronchial involvement in a seventeen-year-old girl. Cornea. 2006;25:474-478.
- Alexandre M, Brette MD, Pascal F, et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore). 2006;85:239-252.
- de Carvalho CR, Amato MB, Da Silva LM, et al. Obstructive respiratory failure in cicatricial pemphigoid. Thorax. 1989;44:601-602.
- Müller LC, Salzer GM. Stenosis of left mainstem bronchus in a case of cicatricial pemphigoid. Eur J Cardiothorac Surg. 1988;2:284-286.
- Camisa C, Allen CM. Death from CP in a young woman with oral, laryngeal, and bronchial involvement. Cutis. 1987;40:426-429.
- Derbes VJ, Pitot HC, Chernosky ME. Fatal cicatricial mucous membrane pemphigoid of the trachea. Dermatol Trop Ecol Geogr. 1962;1:114-117.
- Wieme N, Lambert J, Moerman M, et al. Epidermolysis bullosa acquisita with combined features of bullous pemphigoid and cicatricial pemphigoid. Dermatology. 1999;198:310-313.
- Taylor J, McMillan R, Shephard M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid [published online March 11, 2015]. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:161.e20-171.e20.
- Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid [published online July 9, 2013]. Ocul Surf. 2013;11:259-266.
- Maley A, Warren M, Haberman I, et al. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid (MMP) [published online February 28, 2016]. J Am Acad Dermatol. 2016;74:835-840.
- James WD, Berger TG, Elston D. Chronic blistering diseases. In: James WD, Berger TG, Elston D. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, PA: Sanders Elsevier; 2010:448-467.
- Neff AG, Turner M, Mutasim DF. Treatment strategies in mucous membrane pemphigoid. Ther Clin Risk Manag. 2008;4:617-626.
- Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138:370-379.
- Kato K, Moriyama Y, Saito H, et al. A case of mucous membrane pemphigoid involving the trachea and bronchus with autoantibodies to β3 subunit of laminin-332. Acta Derm Venereol. 2014;94:237-238.
- Gamm DM, Harris A, Mehran RJ, et al. Mucous membrane pemphigoid with fatal bronchial involvement in a seventeen-year-old girl. Cornea. 2006;25:474-478.
- Alexandre M, Brette MD, Pascal F, et al. A prospective study of upper aerodigestive tract manifestations of mucous membrane pemphigoid. Medicine (Baltimore). 2006;85:239-252.
- de Carvalho CR, Amato MB, Da Silva LM, et al. Obstructive respiratory failure in cicatricial pemphigoid. Thorax. 1989;44:601-602.
- Müller LC, Salzer GM. Stenosis of left mainstem bronchus in a case of cicatricial pemphigoid. Eur J Cardiothorac Surg. 1988;2:284-286.
- Camisa C, Allen CM. Death from CP in a young woman with oral, laryngeal, and bronchial involvement. Cutis. 1987;40:426-429.
- Derbes VJ, Pitot HC, Chernosky ME. Fatal cicatricial mucous membrane pemphigoid of the trachea. Dermatol Trop Ecol Geogr. 1962;1:114-117.
- Wieme N, Lambert J, Moerman M, et al. Epidermolysis bullosa acquisita with combined features of bullous pemphigoid and cicatricial pemphigoid. Dermatology. 1999;198:310-313.
- Taylor J, McMillan R, Shephard M, et al. World Workshop on Oral Medicine VI: a systematic review of the treatment of mucous membrane pemphigoid [published online March 11, 2015]. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:161.e20-171.e20.
- Sobolewska B, Deuter C, Zierhut M. Current medical treatment of ocular mucous membrane pemphigoid [published online July 9, 2013]. Ocul Surf. 2013;11:259-266.
- Maley A, Warren M, Haberman I, et al. Rituximab combined with conventional therapy versus conventional therapy alone for the treatment of mucous membrane pemphigoid (MMP) [published online February 28, 2016]. J Am Acad Dermatol. 2016;74:835-840.
Practice Points
- Mucous membrane pemphigoid (MMP) can present with diverse clinical manifestations, making the diagnosis challenging for many clinicians, including experienced dermatologists.
- If not treated early and aggressively, MMP can lead to scarring and is a potentially life-threatening disease.
Novel De Novo Heterozygous Frameshift Mutation of the ADAR1 Gene in Heavy Dyschromatosis Symmetrica Hereditaria
To the Editor:
Dyschromatosis symmetrica hereditaria (DSH)(Online Mendelian Inheritance in Man 127400), also called reticulate acropigmentation of Dohi, is a pigmentary genodermatosis characterized by a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Linkage analysis has revealed that the DSH gene locus resides on chromosome 1q11-q21,1 and the adenosine deaminase RNA specific gene, ADAR1 (also called DSRAD), in this region has been identified as being responsible for the development of DSH.2 We report a sporadic case of severe DSH with the ADAR1 gene detected in a mutation analysis.
A 6-year-old girl presented with a mixture of hyperpigmented and hypopigmented macules on the dorsal aspects of the hands and feet and the curved side of the wrists, heels, and knees, as well as scattered frecklelike and depigmented spots on the face, ears, neck, arms, and upper back (Figure 1). Her parents noted that hyperpigmented and hypopigmented macules on the dorsal aspects of the hands developed at 5 months of age. Exacerbation after exposure to sunlight resulted in the eruption becoming remarkable in summer and fainter in winter. The skin lesions gradually became more progressive. Physical examination revealed that the patient generally was healthy.
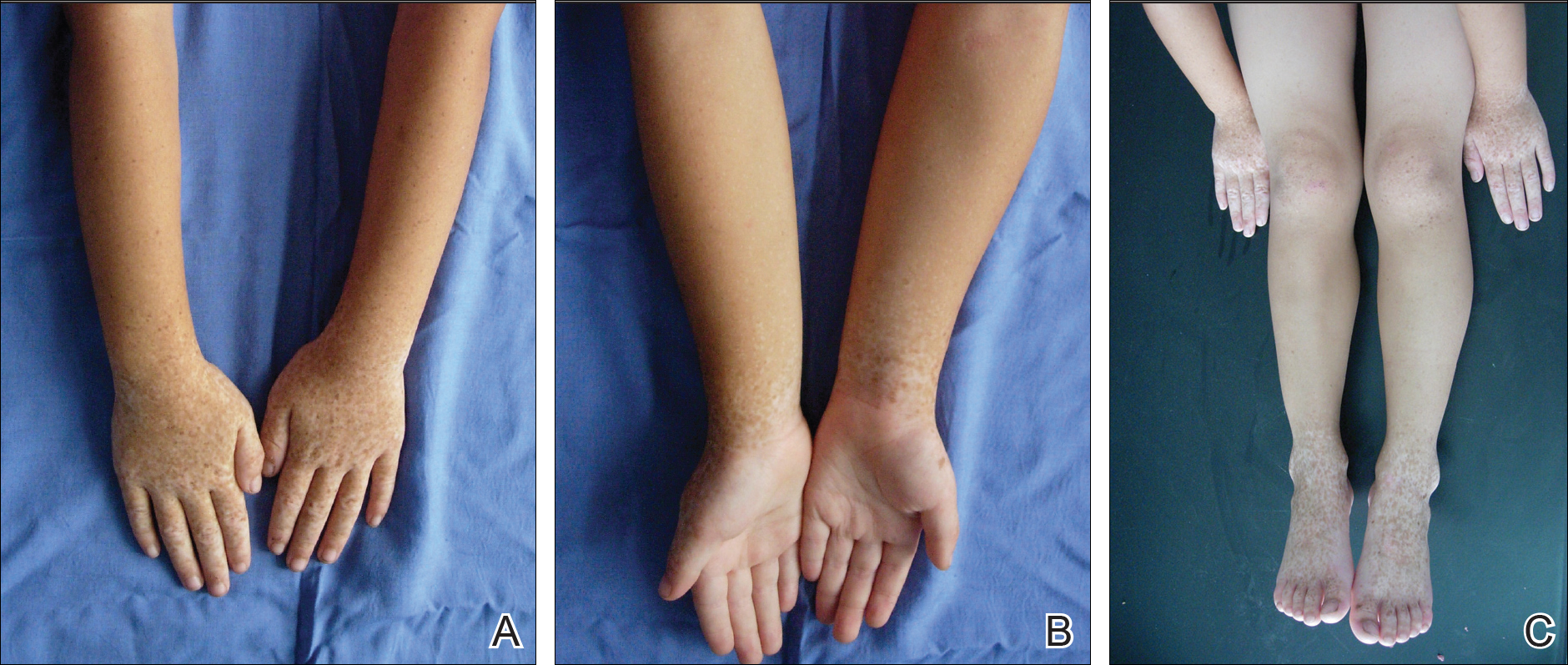
After obtaining informed consent, we performed a mutation analysis of the ADAR1 gene in our patient and her parents. We used a kit to extract genomic DNA from peripheral blood, which was then used to amplify the exons of the ADAR1 gene with intronic flanking sequences by polymerase chain reaction with the primer.3 After amplification, polymerase chain reaction products were purified. We sequenced the ADAR1 gene. Sequence comparisons and analysis found that the patient (proband) carried a heterozygous insertional mutation c.2253insG in exon 6 of the ADAR1 gene. This mutation was not detected in the proband’s healthy parents and 100 normal individuals (Figure 2).
Dyschromatosis symmetrica hereditaria is acquired by autosomal-dominant inheritance and is mainly reported in Asians, especially in Japan and China. Oyama et al4 reviewed 185 cases of DSH in Japan and found the onset of this disease usually was during infancy or childhood; 73% of patients developed the skin lesions before 6 years of age. Suzuki et al5 reported 10 unrelated Japanese patients and found the onset of disease ranged from 1 year of age to childhood. Zhang et al1,6 investigated 78 Chinese patients with DSH including 8 multigenerational families and 2 sporadic patients and found the age of disease onset ranged from 6 months to 15 years of age. The age of onset in our patient (5 months) was younger than these prior reports.
Patients with DSH have a characteristic appearance including a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Few patients have similar lesions on the knees and elbows. Many patients have frecklelike macules on the face and arms.1-6 One patient has been described with scattered depigmented spots on the face and chest.1 Our patient had a characteristic appearance as well as some special manifestations including skin lesions on the curved side of the wrist, ears, neck, and upper back.
The human ADAR1 gene spans 30 kilobase and contains 15 exons. It encodes RNA-specific adenosine deaminase composed of 1226 amino acid residues. This enzyme is important for various functions such as site-specific RNA editing and nuclear translation. This enzyme has 2 Z-alpha domains, 3 double-stranded RNA–binding domains, and the putative deaminase domain corresponding to exon 2, exons 2 to 7, and exons 9 to 14 of ADAR1, respectively.6
Mutation analysis of the ADAR1 gene in this case showed heterozygous insertion mutation c.2253insG in exon 6 of the ADAR1 gene, which changed the reading frame, and 475 amino acid residues in C-terminus are replaced by 90 amino acid residues (TSSRAQVRLPSKSWGSLVPSRLRTQQEA RQAGSSRCGSPCLDWGEREGRTHGFHRG NPSDRGQSQKNYAPPLKVPRSTAKT DTPSHWQHLP). This mutation was not detected in the proband’s healthy parents and the 100 control individuals, which indicated that it was a de novo mutation and the pathogenic mutation of DSH rather than a common polymorphism.
In conclusion, we report a novel mutation of the ADAR1 gene with a heavy clinical phenotype in DSH. This study expands the spectrum of clinical manifestations and demonstrates the ADAR1 mutation in DSH.
Acknowledgments
We are most grateful to the patient and her family for taking part in our study.
- Zhang XJ, Gao M, Li M, et al. Identification of a locus for dyschromatosis symmetrica hereditaria at chromosome 1q11-1q21. J Invest Dermatol. 2003;120:776-780.
- Miyamura Y, Suzuki T, Kono M, et al. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria [published online August 11, 2003]. Am J Hum Genet. 2003;73:693-699.
- Li M, Li C, Hua H, et al. Identification of two novel mutations in Chinese patients with dyschromatosis symmetrica hereditaria [published online October 8, 2005]. Arch Dermatol Res. 2005;297:196-200.
- Oyama M, Shimizu H, Ohata Y, et al. Dyschromatosis symmetrica hereditaria (reticulate acropigmentation of Dohi): report of a Japanese family with the condition and a literature review of 185 cases. Br J Dermatol. 1999;140:491-496.
- Suzuki N, Suzuki T, Inagaki K, et al. Ten novel mutations of the ADAR1 gene in Japanese patients with dyschromatosis symmetrica hereditaria [published online August 17, 2006]. J Invest Dermatol. 2007;127:309-311.
- Zhang XJ, He PP, Li M, et al. Seven novel mutations of the ADAR gene in Chinese families and sporadic patients with dyschromatosis symmetrica hereditaria (DSH). Hum Mutat. 2004;23:629-630.
To the Editor:
Dyschromatosis symmetrica hereditaria (DSH)(Online Mendelian Inheritance in Man 127400), also called reticulate acropigmentation of Dohi, is a pigmentary genodermatosis characterized by a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Linkage analysis has revealed that the DSH gene locus resides on chromosome 1q11-q21,1 and the adenosine deaminase RNA specific gene, ADAR1 (also called DSRAD), in this region has been identified as being responsible for the development of DSH.2 We report a sporadic case of severe DSH with the ADAR1 gene detected in a mutation analysis.
A 6-year-old girl presented with a mixture of hyperpigmented and hypopigmented macules on the dorsal aspects of the hands and feet and the curved side of the wrists, heels, and knees, as well as scattered frecklelike and depigmented spots on the face, ears, neck, arms, and upper back (Figure 1). Her parents noted that hyperpigmented and hypopigmented macules on the dorsal aspects of the hands developed at 5 months of age. Exacerbation after exposure to sunlight resulted in the eruption becoming remarkable in summer and fainter in winter. The skin lesions gradually became more progressive. Physical examination revealed that the patient generally was healthy.

After obtaining informed consent, we performed a mutation analysis of the ADAR1 gene in our patient and her parents. We used a kit to extract genomic DNA from peripheral blood, which was then used to amplify the exons of the ADAR1 gene with intronic flanking sequences by polymerase chain reaction with the primer.3 After amplification, polymerase chain reaction products were purified. We sequenced the ADAR1 gene. Sequence comparisons and analysis found that the patient (proband) carried a heterozygous insertional mutation c.2253insG in exon 6 of the ADAR1 gene. This mutation was not detected in the proband’s healthy parents and 100 normal individuals (Figure 2).

Dyschromatosis symmetrica hereditaria is acquired by autosomal-dominant inheritance and is mainly reported in Asians, especially in Japan and China. Oyama et al4 reviewed 185 cases of DSH in Japan and found the onset of this disease usually was during infancy or childhood; 73% of patients developed the skin lesions before 6 years of age. Suzuki et al5 reported 10 unrelated Japanese patients and found the onset of disease ranged from 1 year of age to childhood. Zhang et al1,6 investigated 78 Chinese patients with DSH including 8 multigenerational families and 2 sporadic patients and found the age of disease onset ranged from 6 months to 15 years of age. The age of onset in our patient (5 months) was younger than these prior reports.
Patients with DSH have a characteristic appearance including a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Few patients have similar lesions on the knees and elbows. Many patients have frecklelike macules on the face and arms.1-6 One patient has been described with scattered depigmented spots on the face and chest.1 Our patient had a characteristic appearance as well as some special manifestations including skin lesions on the curved side of the wrist, ears, neck, and upper back.
The human ADAR1 gene spans 30 kilobase and contains 15 exons. It encodes RNA-specific adenosine deaminase composed of 1226 amino acid residues. This enzyme is important for various functions such as site-specific RNA editing and nuclear translation. This enzyme has 2 Z-alpha domains, 3 double-stranded RNA–binding domains, and the putative deaminase domain corresponding to exon 2, exons 2 to 7, and exons 9 to 14 of ADAR1, respectively.6
Mutation analysis of the ADAR1 gene in this case showed heterozygous insertion mutation c.2253insG in exon 6 of the ADAR1 gene, which changed the reading frame, and 475 amino acid residues in C-terminus are replaced by 90 amino acid residues (TSSRAQVRLPSKSWGSLVPSRLRTQQEA RQAGSSRCGSPCLDWGEREGRTHGFHRG NPSDRGQSQKNYAPPLKVPRSTAKT DTPSHWQHLP). This mutation was not detected in the proband’s healthy parents and the 100 control individuals, which indicated that it was a de novo mutation and the pathogenic mutation of DSH rather than a common polymorphism.
In conclusion, we report a novel mutation of the ADAR1 gene with a heavy clinical phenotype in DSH. This study expands the spectrum of clinical manifestations and demonstrates the ADAR1 mutation in DSH.
Acknowledgments
We are most grateful to the patient and her family for taking part in our study.
To the Editor:
Dyschromatosis symmetrica hereditaria (DSH)(Online Mendelian Inheritance in Man 127400), also called reticulate acropigmentation of Dohi, is a pigmentary genodermatosis characterized by a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Linkage analysis has revealed that the DSH gene locus resides on chromosome 1q11-q21,1 and the adenosine deaminase RNA specific gene, ADAR1 (also called DSRAD), in this region has been identified as being responsible for the development of DSH.2 We report a sporadic case of severe DSH with the ADAR1 gene detected in a mutation analysis.
A 6-year-old girl presented with a mixture of hyperpigmented and hypopigmented macules on the dorsal aspects of the hands and feet and the curved side of the wrists, heels, and knees, as well as scattered frecklelike and depigmented spots on the face, ears, neck, arms, and upper back (Figure 1). Her parents noted that hyperpigmented and hypopigmented macules on the dorsal aspects of the hands developed at 5 months of age. Exacerbation after exposure to sunlight resulted in the eruption becoming remarkable in summer and fainter in winter. The skin lesions gradually became more progressive. Physical examination revealed that the patient generally was healthy.

After obtaining informed consent, we performed a mutation analysis of the ADAR1 gene in our patient and her parents. We used a kit to extract genomic DNA from peripheral blood, which was then used to amplify the exons of the ADAR1 gene with intronic flanking sequences by polymerase chain reaction with the primer.3 After amplification, polymerase chain reaction products were purified. We sequenced the ADAR1 gene. Sequence comparisons and analysis found that the patient (proband) carried a heterozygous insertional mutation c.2253insG in exon 6 of the ADAR1 gene. This mutation was not detected in the proband’s healthy parents and 100 normal individuals (Figure 2).

Dyschromatosis symmetrica hereditaria is acquired by autosomal-dominant inheritance and is mainly reported in Asians, especially in Japan and China. Oyama et al4 reviewed 185 cases of DSH in Japan and found the onset of this disease usually was during infancy or childhood; 73% of patients developed the skin lesions before 6 years of age. Suzuki et al5 reported 10 unrelated Japanese patients and found the onset of disease ranged from 1 year of age to childhood. Zhang et al1,6 investigated 78 Chinese patients with DSH including 8 multigenerational families and 2 sporadic patients and found the age of disease onset ranged from 6 months to 15 years of age. The age of onset in our patient (5 months) was younger than these prior reports.
Patients with DSH have a characteristic appearance including a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet. Few patients have similar lesions on the knees and elbows. Many patients have frecklelike macules on the face and arms.1-6 One patient has been described with scattered depigmented spots on the face and chest.1 Our patient had a characteristic appearance as well as some special manifestations including skin lesions on the curved side of the wrist, ears, neck, and upper back.
The human ADAR1 gene spans 30 kilobase and contains 15 exons. It encodes RNA-specific adenosine deaminase composed of 1226 amino acid residues. This enzyme is important for various functions such as site-specific RNA editing and nuclear translation. This enzyme has 2 Z-alpha domains, 3 double-stranded RNA–binding domains, and the putative deaminase domain corresponding to exon 2, exons 2 to 7, and exons 9 to 14 of ADAR1, respectively.6
Mutation analysis of the ADAR1 gene in this case showed heterozygous insertion mutation c.2253insG in exon 6 of the ADAR1 gene, which changed the reading frame, and 475 amino acid residues in C-terminus are replaced by 90 amino acid residues (TSSRAQVRLPSKSWGSLVPSRLRTQQEA RQAGSSRCGSPCLDWGEREGRTHGFHRG NPSDRGQSQKNYAPPLKVPRSTAKT DTPSHWQHLP). This mutation was not detected in the proband’s healthy parents and the 100 control individuals, which indicated that it was a de novo mutation and the pathogenic mutation of DSH rather than a common polymorphism.
In conclusion, we report a novel mutation of the ADAR1 gene with a heavy clinical phenotype in DSH. This study expands the spectrum of clinical manifestations and demonstrates the ADAR1 mutation in DSH.
Acknowledgments
We are most grateful to the patient and her family for taking part in our study.
- Zhang XJ, Gao M, Li M, et al. Identification of a locus for dyschromatosis symmetrica hereditaria at chromosome 1q11-1q21. J Invest Dermatol. 2003;120:776-780.
- Miyamura Y, Suzuki T, Kono M, et al. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria [published online August 11, 2003]. Am J Hum Genet. 2003;73:693-699.
- Li M, Li C, Hua H, et al. Identification of two novel mutations in Chinese patients with dyschromatosis symmetrica hereditaria [published online October 8, 2005]. Arch Dermatol Res. 2005;297:196-200.
- Oyama M, Shimizu H, Ohata Y, et al. Dyschromatosis symmetrica hereditaria (reticulate acropigmentation of Dohi): report of a Japanese family with the condition and a literature review of 185 cases. Br J Dermatol. 1999;140:491-496.
- Suzuki N, Suzuki T, Inagaki K, et al. Ten novel mutations of the ADAR1 gene in Japanese patients with dyschromatosis symmetrica hereditaria [published online August 17, 2006]. J Invest Dermatol. 2007;127:309-311.
- Zhang XJ, He PP, Li M, et al. Seven novel mutations of the ADAR gene in Chinese families and sporadic patients with dyschromatosis symmetrica hereditaria (DSH). Hum Mutat. 2004;23:629-630.
- Zhang XJ, Gao M, Li M, et al. Identification of a locus for dyschromatosis symmetrica hereditaria at chromosome 1q11-1q21. J Invest Dermatol. 2003;120:776-780.
- Miyamura Y, Suzuki T, Kono M, et al. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria [published online August 11, 2003]. Am J Hum Genet. 2003;73:693-699.
- Li M, Li C, Hua H, et al. Identification of two novel mutations in Chinese patients with dyschromatosis symmetrica hereditaria [published online October 8, 2005]. Arch Dermatol Res. 2005;297:196-200.
- Oyama M, Shimizu H, Ohata Y, et al. Dyschromatosis symmetrica hereditaria (reticulate acropigmentation of Dohi): report of a Japanese family with the condition and a literature review of 185 cases. Br J Dermatol. 1999;140:491-496.
- Suzuki N, Suzuki T, Inagaki K, et al. Ten novel mutations of the ADAR1 gene in Japanese patients with dyschromatosis symmetrica hereditaria [published online August 17, 2006]. J Invest Dermatol. 2007;127:309-311.
- Zhang XJ, He PP, Li M, et al. Seven novel mutations of the ADAR gene in Chinese families and sporadic patients with dyschromatosis symmetrica hereditaria (DSH). Hum Mutat. 2004;23:629-630.
Practice Points
- The adenosine deaminase RNA specific gene, ADAR1, has been identified as being responsible for the development of dyschromatosis symmetrica hereditaria (DSH).
- The characteristic appearance of DSH is a mixture of hyperpigmented and hypopigmented macules of various sizes on the dorsal aspects of the hands and feet.
Desmoplastic Hairless Hypopigmented Nevus
To the Editor:
We report 2 cases of desmoplastic hairless hypopigmented nevi (DHHN), which are giant congenital melanocytic nevi (GCMN) that show sclerosis with progressive loss of pigment and hair. These changes in GCMN could be considered signs of regression.
A 6-year-old boy presented in the dermatology department with an asymptomatic skin lesion on the right buttock since birth. The parents claimed that the lesion was darkly pigmented at birth and gradually increased in size, with progressive reduction in color in the last 2 years. Physical examination revealed a 10×6-cm, well-defined, raised plaque on the upper medial side of the right buttock (Figure 1). The plaque was firm with a shiny smooth surface and was devoid of hair. The surface was flesh colored with scattered pigmented spots. A punch biopsy of the lesion showed increased melanin content in the basal cell layer. The upper dermis showed small nests of epithelioid nevus cells, most of them containing melanin pigment (Figure 2). In the lower two-thirds of the dermis, nevus cells were both epithelioid and spindle shaped and were arranged in between thick sclerotic collagen bundles with an increased number of fibroblasts. There was a marked reduction in the number of hair follicles. Immunohistochemical staining results were S-100 positive and CD34 negative.
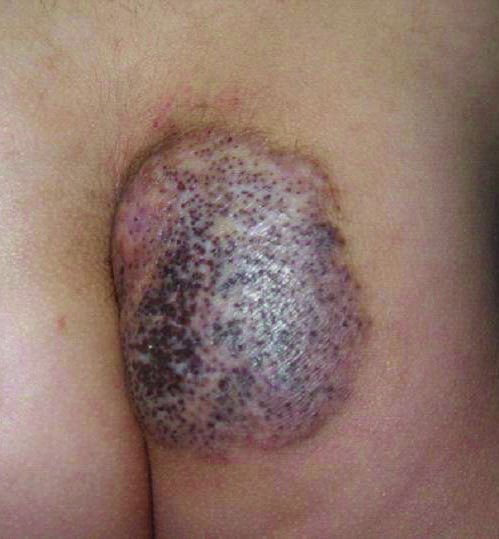
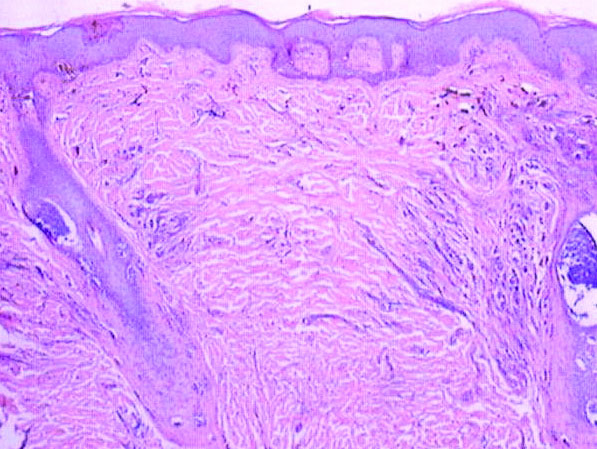
A 5-year-old boy presented in the dermatology department with a large hairy GCMN covering most of the trunk since birth. In the last 1.5 years the parents noted gradual fading of color, decreased hair density, and increased induration of the nevus. Physical examination revealed a large plaque covering the anterior aspect of the trunk (Figure 3) and the back extending down to the buttocks. The lesion formed large skin folds that were more pronounced on the back. The nevus was darkly pigmented with large areas of lighter color that were indurated, devoid of hair, and showed small spots of dark pigmentation. A punch biopsy from the lesion showed small nests of nevus cells in the upper part of the reticular dermis. In the lower part of the dermis, nevus cells were arranged in single units in between thick collagen bundles.
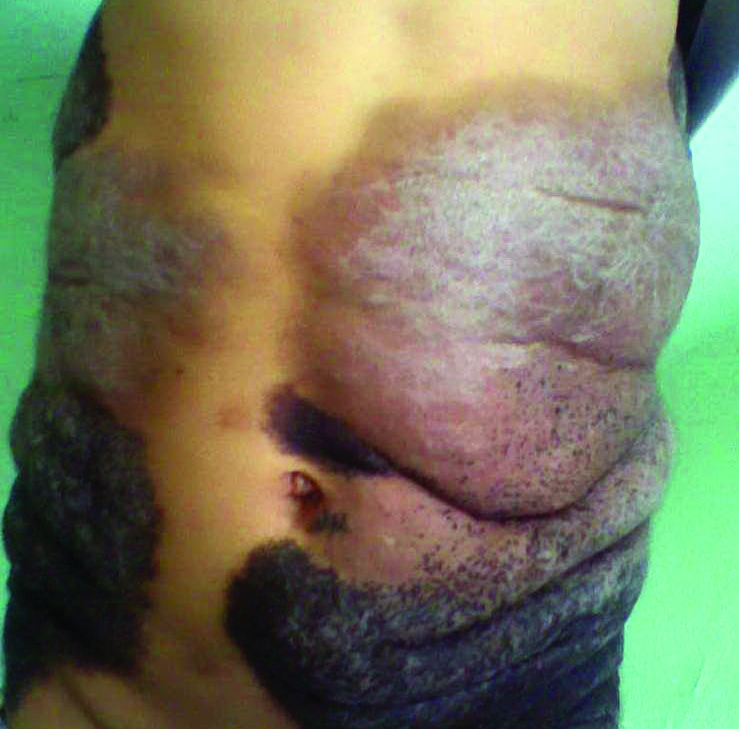
In 2003, Ruiz-Maldonado et al1 described 4 cases of GCMN that showed progressive loss of pigmentation, sclerosis, and hair loss. They proposed the term desmoplastic hairless hypopigmented nevus for their cases and considered it as a variant of GCMN.1 Prior to these reported cases, 2 similar cases were described. The first was a report by Hogan et al2 in 1988 of a 7-month-old girl with a GCMN involving the occipital area and the upper back that became indurated and ulcerated with progressive involution that led to complete disappearance of the nevus. The second was a report by Pattee et al3 in 2001 of a newborn with a GCMN located on the trunk with progressive sclerodermiform reaction. After surgical excision of the nevus, the sclerotic margin disappeared.3
Following the report by Ruiz-Maldonaldo et al,1 5 more cases of DHHN were described.4-8 All cases of DHHN share the same clinical and histopathological features. The clinical features include a GCMN present since birth with progressive sclerosis over time and loss of both pigmentation and hair. Histologically, DHHN shows the typical changes of a congenital melanocytic nevus with decreased numbers of nevus cells, thick sclerotic collagen bundles of the reticular dermis, increased number of fibroblasts, and decreased number of hair follicles. The progressive reduction in the number of nevus cells in melanocytic nevi is considered a sign of regression. Spontaneous regression was rarely described in GCMN, and all the reported cases of regression were associated with desmoplasia.4 Desmoplasia is thought to be induced by either melanocytes that function as adaptive fibroblasts or by fibroblasts themselves, as fibroblasts can show multifunctional differentiation capabilities.9 The direct correlation between the increased induration of DHHN and pigment depletion supports the former hypothesis. The absence of inflammatory cells within the sections of DHHN lesions is against the possibility of an immune-mediated reaction as a cause for the clinical and histological changes seen in this rare form of GCMN. The progressive hair loss in DHHN may be explained by the progressive fibrotic changes in the reticular dermis that affect the blood supply to follicles, leading to atrophy or even absence of the follicles. The progressive reduction in the number of nevus cells in DHHN reduces the potential for malignant transformation and hence following a watchful waiting strategy is a reasonable way to manage these nevi.
We present 2 patients with DHHN, which is a rare form of GCMN that shows signs of regression. The cause of these changes is still unclear.
- Ruiz-Maldonado R, Orozco-Covarrubias L, Ridaura-Sanz C, et al. Desmoplastic hairless hypopigmented naevus: a variant of giant congenital melanocytic naevus. Br J Dermatol. 2003;148:1253-1257.
- Hogan DJ, Murphy F, Bremner RM. Spontaneous resolution of a giant congenital melanocytic nevus. Pediatr Dermatol. 1988;5:170-172.
- Pattee SF, Hansen RC, Bangert JL, et al. Giant congenital nevus with progressive sclerodermoid reaction in a newborn. Pediatr Dermatol. 2001;18:321-324.
- Boente MC, Asial RA. Desmoplastic hairless hypopigmented nevus (DHHN). a distinct variant of giant melanocytic nevus. Eur J Dermatol. 2005;15:451-453.
- Bushby SA, Rajan NJ, Shehade SA. Spontaneous resolution of a giant melanocytic naevus involving a desmoplastic process. Br J Dermatol. 2005;153(suppl 1):13-19.
- Martin JM, Jorda E, Monteagudo C, et al. Desmoplastic giant congenital nevus with progressive depigmentation. J Am Acad Dermatol. 2007;56(suppl 2):S10-S14.
- Hermandez-Martin A, Torrelo A, Echevarria C, et al. Ulcerated sclerotic giant congenital melanocytic naevus: case report and review of the literature. Clin Exp Dermatol. 2007;32:529-532.
- Werner B, Carvalho VO, Nacif SB, et al. Desmoplastic hypopigmented hairless nevus: a variant with progressive depigmentation, induration and overgrowth [published online May 16, 2011]. Pediatr Dermatol. 2012;29:336-340.
- Fearns C, Dowdle EB. The desmoplastic response: induction of collagen synthesis by melanoma cells in vitro. Int J Cancer. 1992;50:621-627.
To the Editor:
We report 2 cases of desmoplastic hairless hypopigmented nevi (DHHN), which are giant congenital melanocytic nevi (GCMN) that show sclerosis with progressive loss of pigment and hair. These changes in GCMN could be considered signs of regression.
A 6-year-old boy presented in the dermatology department with an asymptomatic skin lesion on the right buttock since birth. The parents claimed that the lesion was darkly pigmented at birth and gradually increased in size, with progressive reduction in color in the last 2 years. Physical examination revealed a 10×6-cm, well-defined, raised plaque on the upper medial side of the right buttock (Figure 1). The plaque was firm with a shiny smooth surface and was devoid of hair. The surface was flesh colored with scattered pigmented spots. A punch biopsy of the lesion showed increased melanin content in the basal cell layer. The upper dermis showed small nests of epithelioid nevus cells, most of them containing melanin pigment (Figure 2). In the lower two-thirds of the dermis, nevus cells were both epithelioid and spindle shaped and were arranged in between thick sclerotic collagen bundles with an increased number of fibroblasts. There was a marked reduction in the number of hair follicles. Immunohistochemical staining results were S-100 positive and CD34 negative.


A 5-year-old boy presented in the dermatology department with a large hairy GCMN covering most of the trunk since birth. In the last 1.5 years the parents noted gradual fading of color, decreased hair density, and increased induration of the nevus. Physical examination revealed a large plaque covering the anterior aspect of the trunk (Figure 3) and the back extending down to the buttocks. The lesion formed large skin folds that were more pronounced on the back. The nevus was darkly pigmented with large areas of lighter color that were indurated, devoid of hair, and showed small spots of dark pigmentation. A punch biopsy from the lesion showed small nests of nevus cells in the upper part of the reticular dermis. In the lower part of the dermis, nevus cells were arranged in single units in between thick collagen bundles.

In 2003, Ruiz-Maldonado et al1 described 4 cases of GCMN that showed progressive loss of pigmentation, sclerosis, and hair loss. They proposed the term desmoplastic hairless hypopigmented nevus for their cases and considered it as a variant of GCMN.1 Prior to these reported cases, 2 similar cases were described. The first was a report by Hogan et al2 in 1988 of a 7-month-old girl with a GCMN involving the occipital area and the upper back that became indurated and ulcerated with progressive involution that led to complete disappearance of the nevus. The second was a report by Pattee et al3 in 2001 of a newborn with a GCMN located on the trunk with progressive sclerodermiform reaction. After surgical excision of the nevus, the sclerotic margin disappeared.3
Following the report by Ruiz-Maldonaldo et al,1 5 more cases of DHHN were described.4-8 All cases of DHHN share the same clinical and histopathological features. The clinical features include a GCMN present since birth with progressive sclerosis over time and loss of both pigmentation and hair. Histologically, DHHN shows the typical changes of a congenital melanocytic nevus with decreased numbers of nevus cells, thick sclerotic collagen bundles of the reticular dermis, increased number of fibroblasts, and decreased number of hair follicles. The progressive reduction in the number of nevus cells in melanocytic nevi is considered a sign of regression. Spontaneous regression was rarely described in GCMN, and all the reported cases of regression were associated with desmoplasia.4 Desmoplasia is thought to be induced by either melanocytes that function as adaptive fibroblasts or by fibroblasts themselves, as fibroblasts can show multifunctional differentiation capabilities.9 The direct correlation between the increased induration of DHHN and pigment depletion supports the former hypothesis. The absence of inflammatory cells within the sections of DHHN lesions is against the possibility of an immune-mediated reaction as a cause for the clinical and histological changes seen in this rare form of GCMN. The progressive hair loss in DHHN may be explained by the progressive fibrotic changes in the reticular dermis that affect the blood supply to follicles, leading to atrophy or even absence of the follicles. The progressive reduction in the number of nevus cells in DHHN reduces the potential for malignant transformation and hence following a watchful waiting strategy is a reasonable way to manage these nevi.
We present 2 patients with DHHN, which is a rare form of GCMN that shows signs of regression. The cause of these changes is still unclear.
To the Editor:
We report 2 cases of desmoplastic hairless hypopigmented nevi (DHHN), which are giant congenital melanocytic nevi (GCMN) that show sclerosis with progressive loss of pigment and hair. These changes in GCMN could be considered signs of regression.
A 6-year-old boy presented in the dermatology department with an asymptomatic skin lesion on the right buttock since birth. The parents claimed that the lesion was darkly pigmented at birth and gradually increased in size, with progressive reduction in color in the last 2 years. Physical examination revealed a 10×6-cm, well-defined, raised plaque on the upper medial side of the right buttock (Figure 1). The plaque was firm with a shiny smooth surface and was devoid of hair. The surface was flesh colored with scattered pigmented spots. A punch biopsy of the lesion showed increased melanin content in the basal cell layer. The upper dermis showed small nests of epithelioid nevus cells, most of them containing melanin pigment (Figure 2). In the lower two-thirds of the dermis, nevus cells were both epithelioid and spindle shaped and were arranged in between thick sclerotic collagen bundles with an increased number of fibroblasts. There was a marked reduction in the number of hair follicles. Immunohistochemical staining results were S-100 positive and CD34 negative.


A 5-year-old boy presented in the dermatology department with a large hairy GCMN covering most of the trunk since birth. In the last 1.5 years the parents noted gradual fading of color, decreased hair density, and increased induration of the nevus. Physical examination revealed a large plaque covering the anterior aspect of the trunk (Figure 3) and the back extending down to the buttocks. The lesion formed large skin folds that were more pronounced on the back. The nevus was darkly pigmented with large areas of lighter color that were indurated, devoid of hair, and showed small spots of dark pigmentation. A punch biopsy from the lesion showed small nests of nevus cells in the upper part of the reticular dermis. In the lower part of the dermis, nevus cells were arranged in single units in between thick collagen bundles.

In 2003, Ruiz-Maldonado et al1 described 4 cases of GCMN that showed progressive loss of pigmentation, sclerosis, and hair loss. They proposed the term desmoplastic hairless hypopigmented nevus for their cases and considered it as a variant of GCMN.1 Prior to these reported cases, 2 similar cases were described. The first was a report by Hogan et al2 in 1988 of a 7-month-old girl with a GCMN involving the occipital area and the upper back that became indurated and ulcerated with progressive involution that led to complete disappearance of the nevus. The second was a report by Pattee et al3 in 2001 of a newborn with a GCMN located on the trunk with progressive sclerodermiform reaction. After surgical excision of the nevus, the sclerotic margin disappeared.3
Following the report by Ruiz-Maldonaldo et al,1 5 more cases of DHHN were described.4-8 All cases of DHHN share the same clinical and histopathological features. The clinical features include a GCMN present since birth with progressive sclerosis over time and loss of both pigmentation and hair. Histologically, DHHN shows the typical changes of a congenital melanocytic nevus with decreased numbers of nevus cells, thick sclerotic collagen bundles of the reticular dermis, increased number of fibroblasts, and decreased number of hair follicles. The progressive reduction in the number of nevus cells in melanocytic nevi is considered a sign of regression. Spontaneous regression was rarely described in GCMN, and all the reported cases of regression were associated with desmoplasia.4 Desmoplasia is thought to be induced by either melanocytes that function as adaptive fibroblasts or by fibroblasts themselves, as fibroblasts can show multifunctional differentiation capabilities.9 The direct correlation between the increased induration of DHHN and pigment depletion supports the former hypothesis. The absence of inflammatory cells within the sections of DHHN lesions is against the possibility of an immune-mediated reaction as a cause for the clinical and histological changes seen in this rare form of GCMN. The progressive hair loss in DHHN may be explained by the progressive fibrotic changes in the reticular dermis that affect the blood supply to follicles, leading to atrophy or even absence of the follicles. The progressive reduction in the number of nevus cells in DHHN reduces the potential for malignant transformation and hence following a watchful waiting strategy is a reasonable way to manage these nevi.
We present 2 patients with DHHN, which is a rare form of GCMN that shows signs of regression. The cause of these changes is still unclear.
- Ruiz-Maldonado R, Orozco-Covarrubias L, Ridaura-Sanz C, et al. Desmoplastic hairless hypopigmented naevus: a variant of giant congenital melanocytic naevus. Br J Dermatol. 2003;148:1253-1257.
- Hogan DJ, Murphy F, Bremner RM. Spontaneous resolution of a giant congenital melanocytic nevus. Pediatr Dermatol. 1988;5:170-172.
- Pattee SF, Hansen RC, Bangert JL, et al. Giant congenital nevus with progressive sclerodermoid reaction in a newborn. Pediatr Dermatol. 2001;18:321-324.
- Boente MC, Asial RA. Desmoplastic hairless hypopigmented nevus (DHHN). a distinct variant of giant melanocytic nevus. Eur J Dermatol. 2005;15:451-453.
- Bushby SA, Rajan NJ, Shehade SA. Spontaneous resolution of a giant melanocytic naevus involving a desmoplastic process. Br J Dermatol. 2005;153(suppl 1):13-19.
- Martin JM, Jorda E, Monteagudo C, et al. Desmoplastic giant congenital nevus with progressive depigmentation. J Am Acad Dermatol. 2007;56(suppl 2):S10-S14.
- Hermandez-Martin A, Torrelo A, Echevarria C, et al. Ulcerated sclerotic giant congenital melanocytic naevus: case report and review of the literature. Clin Exp Dermatol. 2007;32:529-532.
- Werner B, Carvalho VO, Nacif SB, et al. Desmoplastic hypopigmented hairless nevus: a variant with progressive depigmentation, induration and overgrowth [published online May 16, 2011]. Pediatr Dermatol. 2012;29:336-340.
- Fearns C, Dowdle EB. The desmoplastic response: induction of collagen synthesis by melanoma cells in vitro. Int J Cancer. 1992;50:621-627.
- Ruiz-Maldonado R, Orozco-Covarrubias L, Ridaura-Sanz C, et al. Desmoplastic hairless hypopigmented naevus: a variant of giant congenital melanocytic naevus. Br J Dermatol. 2003;148:1253-1257.
- Hogan DJ, Murphy F, Bremner RM. Spontaneous resolution of a giant congenital melanocytic nevus. Pediatr Dermatol. 1988;5:170-172.
- Pattee SF, Hansen RC, Bangert JL, et al. Giant congenital nevus with progressive sclerodermoid reaction in a newborn. Pediatr Dermatol. 2001;18:321-324.
- Boente MC, Asial RA. Desmoplastic hairless hypopigmented nevus (DHHN). a distinct variant of giant melanocytic nevus. Eur J Dermatol. 2005;15:451-453.
- Bushby SA, Rajan NJ, Shehade SA. Spontaneous resolution of a giant melanocytic naevus involving a desmoplastic process. Br J Dermatol. 2005;153(suppl 1):13-19.
- Martin JM, Jorda E, Monteagudo C, et al. Desmoplastic giant congenital nevus with progressive depigmentation. J Am Acad Dermatol. 2007;56(suppl 2):S10-S14.
- Hermandez-Martin A, Torrelo A, Echevarria C, et al. Ulcerated sclerotic giant congenital melanocytic naevus: case report and review of the literature. Clin Exp Dermatol. 2007;32:529-532.
- Werner B, Carvalho VO, Nacif SB, et al. Desmoplastic hypopigmented hairless nevus: a variant with progressive depigmentation, induration and overgrowth [published online May 16, 2011]. Pediatr Dermatol. 2012;29:336-340.
- Fearns C, Dowdle EB. The desmoplastic response: induction of collagen synthesis by melanoma cells in vitro. Int J Cancer. 1992;50:621-627.
Cutaneous Artifactual Disease Represented as Recurrent Toxic Epidermal Necrolysis
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
To the Editor:
Lyell1 coined the term cutaneous artifactual disease to describe the spectrum of factitious disorders associated with skin presentations. Interestingly, Lyell was the first to name toxic epidermal necrolysis (TEN).2,3 We present a rare case of factitial TEN, a dangerous and life-threatening manifestation of factitial disease.
A 49-year-old homeless man with a history of Stevens-Johnson syndrome (SJS)/TEN from trimethoprim-sulfamethoxazole (TMP-SMX) was admitted on 4 separate occasions over an 18-month period for recurrent exposure to the medication producing SJS/TEN. Originally, this patient was given TMP-SMX for a skin infection and 10 days later presented with 15% body surface area (BSA) involvement of SJS/TEN. He was successfully treated with intravenous immunoglobulin (IVIg) in the burn intensive care unit (BICU) and discharged. Several months later, the patient was given TMP-SMX for a leg infection by a different clinic. He was admitted to the BICU with 40% BSA, treated with IVIg, and survived. Eight months later, the patient was again admitted to the BICU with 30% BSA and treated with IVIg; however, this admission required intubation due to complications secondary to volume resuscitation. He was evaluated by psychiatry and confessed to purposely seeking TMP-SMX, stating that he “liked the food and care in the hospital.” He was diagnosed with factitial disorder and given a referral for further treatment at an outpatient facility. Two months later, the patient was again admitted to the BICU after taking a single dose of TMP-SMX obtained from a “friend.” He had 10% BSA with conjunctival involvement and was again successfully treated with IVIg. He was discharged with the state psychiatric system for further treatment and evaluation.
Factitial disease in dermatology is difficult to diagnose. Its incidence is unknown, as only case reports exist in the literature. In factitial disease, patients “perform self-mutilating and clinically relevant damage to themselves without the direct intention of suicide.”4 Harth et al4 described 3 subcategories of factitious disorders: dermatitis artefacta syndrome, dermatitis para-artefacta syndrome, or malingering. Dermatitis artefacta syndrome is “a dissociated self-injury or behavior where the patient unconsciously simulated disease with intention to be cared for as a patient.”4 Dermatitis para-artefacta syndrome was described as a disorder of impulse control in which a patient will produce or manipulate a specific dermatosis presentation. The patient usually admits to doing it in a semiconscious state. Dermatitis artefacta and dermatitis para-artefacta differ from malingering in that malingering patients knowingly fake symptoms for external gain, which can be monetary or the avoidance of responsibility.4 More familiar examples to dermatologists of factitial disease include factitial panniculitis,1 direct applications of caustic agents to the skin, and excoriations from instruments or fingernails.4,5
This case illustrates the difficult and potentially dangerous nature of factitial disorders, specifically dermatitis para-artefacta syndrome. Our patient was intensely preoccupied with the outcome of being a patient in a hospital. Our patient sought out a medication from multiple providers to produce a deadly and severe life-threatening reaction. If his main intentions were solely to obtain a bed and 3 square meals a day, then malingering would have represented his factitial disease. However, his main intent was to be seen as a patient, and then doted on and cared for by medical professionals in a hospital setting. From this assessment, the patient’s behavior would fall under the factitial disorder of dermatitis para-artefacta syndrome.
Factitious disorders pose immense challenges for diagnosis and treatment. It is prudent for physicians to learn to recognize patterns of history and examination that do not coincide. The first step in treatment is the recognition and early involvement of psychiatry to aid in curbing this behavior. Remission of factitial disorders can be induced with proper diagnosis and treatment. Patients with the highest chance of remission are those with treatment centered on behavioral therapy in conjunction with psychotropic medications.1
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
- Lyell A. Cutaneous artifactual disease. J Am Acad Dermatol. 1979;1:391-402.
- Palmieri TL, Greenhalgh, DG, Saffle JR, et al. A multicenter review of toxic epidermal necrolysis treated in U.S. burn centers at the end of the twentieth century. J Burn Care Rehab. 2002;23:87-96.
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol. 1956;68:355-361.
- Harth W, Gieler U, Kusnir D, et al. Primarily psychogenic dermatoses. In: Clinical Management in Psychodermatology. 1st ed. Leipzog, Germany: Springer-Verlag Berlin Heidelberg; 2009:11-19.
- Sanmartin O, Requena C, Requena L. Factitial panniculitis. Dermatol Clin. 2008;26:519-527.
Practice Points
- It is important to consider an underlying psychiatric disorder (eg, factitial disorders) in dermatologic patients, even when an exogenous cause can be identified.
- On occasion, dermatologic disease is best treated and prevented with routine psychiatric care and psychotropic therapy.
Multiple Eruptive Dermatofibromas in a Patient With Sarcoidosis
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.
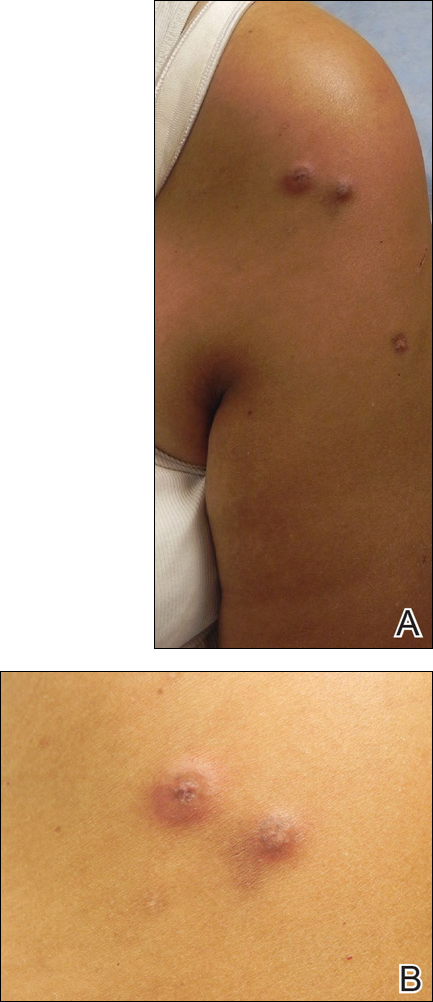
A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).
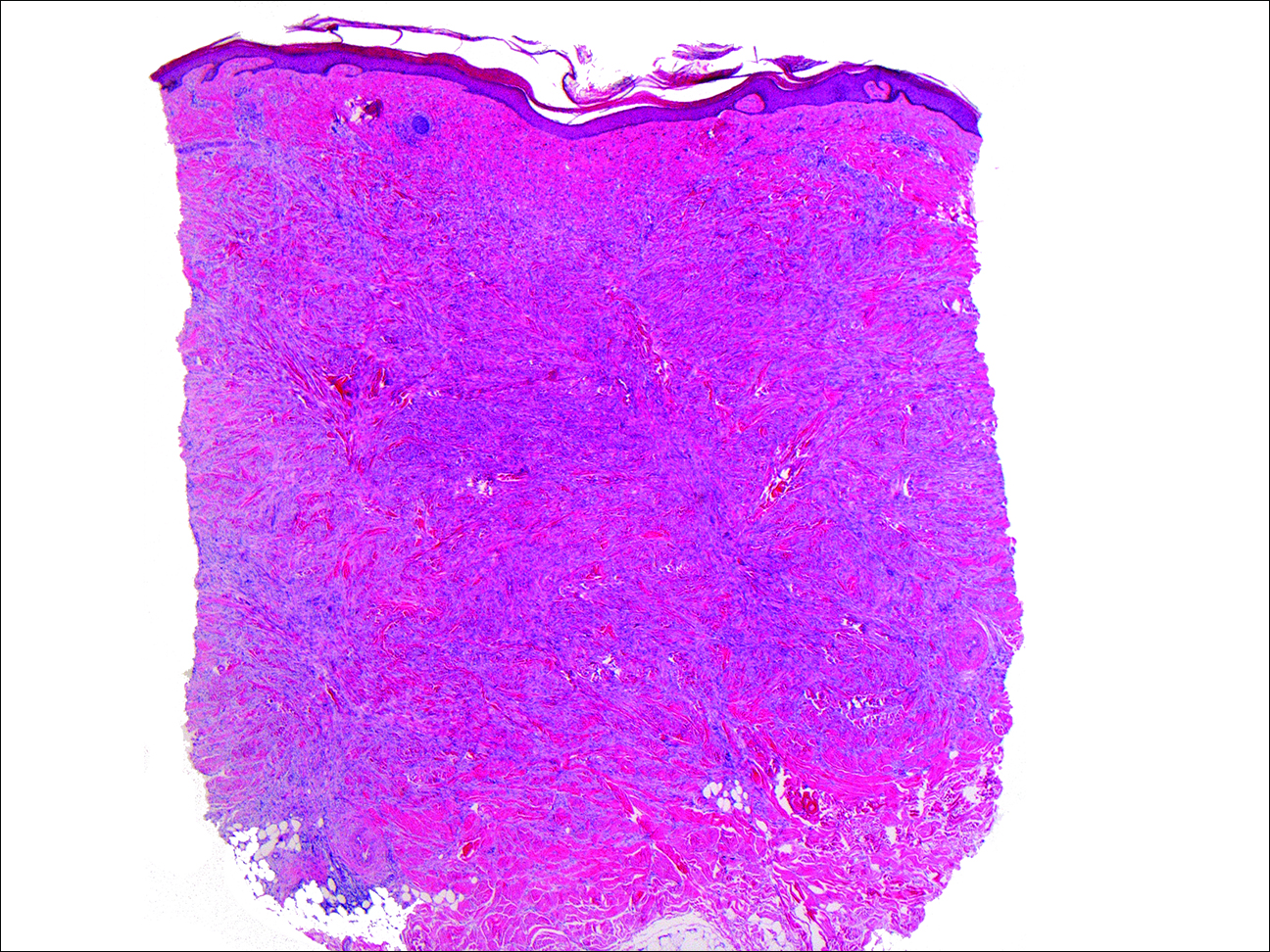
Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.

A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).

Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
To the Editor:
Dermatofibromas, the most common fibrohistiocytic tumors of the skin, are typically solitary lesions. Clustering of and multiple dermatofibromas (multiple eruptive dermatofibromas [MEDFs]) are relatively less common. The association between MEDF and systemic immunoaltered disease states such as systemic lupus erythematosus (SLE) or human immunodeficiency virus infection has been described and led to speculation that MEDF might be a result of an abnormal immune response. We report a patient with sarcoidosis who developed multiple large dermatofibromas, some clustered, on the neck, left shoulder, and back.

A 61-year-old woman with a history of mild pulmonary sarcoidosis confirmed by transbronchial biopsies presented to our clinic with a 2-year history of hyperpigmented papules on the trunk and extremities with subjective enlargement and increased erythema of a papule on the left shoulder over the last 6 months. She had associated pain and pruritus in the area. She was not on any systemic medications for sarcoidosis at the time. Physical examination revealed 2 large, firm, hyperpigmented nodules on the left shoulder, one with overlying erythema and mild scale (Figure 1). There also were multiple scattered hyperpigmented papules on the back, chest, and right arm that dimpled when compressed. A biopsy was obtained because of clinical concern for cutaneous sarcoidosis. Histopathologic evaluation of the largest nodule demonstrated epidermal hyperplasia with effacement of the rete ridges and a proliferation of spindle cells that wrapped around collagen fibers in the dermis, consistent with a dermatofibroma (Figure 2).

Dermatofibromas are common fibrohistiocytic neoplasms in the skin that typically present as a solitary lesion. A clustering of dermatofibromas, MEDFs, is relatively less common, representing only 0.3% of all dermatofibromas.1,2 Histopathologically, similar to solitary dermatofibromas,3 MEDF has classically been defined as more than 15 lesions, though another definition includes the appearance of several dermatofibromas over a relatively short period of time.2
Multiple eruptive dermatofibromas have been described in association with several underlying diseases. A strong association between MEDFs and immune dysregulation appears to exist, with 69% of reported cases of MEDF associated with an underlying disease, 83% of which were related to dysregulated immunity. Systemic lupus erythematosus was the most common underlying disease associated with MEDF, representing 25% of published cases.3 Multiple eruptive dermatofibromas also have been linked to other autoimmune disorders such as myasthenia gravis,4,5 Hashimoto thyroiditis,4 diabetes mellitus,6 Sjögren syndrome,7,8 dermatomyositis,9 and Graves-Basedow disease.10
Multiple eruptive dermatofibromas also have been linked to immunosuppression, including human immunodeficiency virus11; malignancy12,13; and immunosuppressive or immunomodulatory drugs such as corticosteroids,14 cyclophosphamide,5 methotrexate,9 efalizumab,15 and interferon alfa.12 The degree of immunosuppression, however, does not seem to correlate to the number of MEDFs.3 In addition, MEDFs have been reported in pregnancy and a variety of other systemic disorders including atopic dermatitis,1,16,17 hypertriglyceridemia,8 and pulmonary hypertension.18 We report a case of MEDF in a patient with sarcoidosis who was not treated with immunosuppressive medication. A report of sarcoidosis and MEDF was previously published, but the patient had been treated for many years with prednisone.19 Most reports of SLE-associated MEDF occurred in the setting of steroid use.
Although the etiology of dermatofibromas is unclear, the link between MEDFs and altered immunity has led to speculation that dermatofibromas could be a manifestation of defective autoimmune inflammatory regulation. This hypothesis has been supported by the observation that the lesions are often associated with cells that express class II MHC molecules and also bear morphologic similarity to dermal antigen-presenting cells.20 Reports of familial cases of MEDF suggest that there could be a genetic predisposition.1
The association of MEDFs and underlying immune disorders is important for clinicians to know for appropriate evaluation of potential systemic associations, including sarcoidosis. In addition, biopsy should be considered to confirm the diagnosis with large or atypical lesions to exclude other potential diagnoses. Given the protean nature of sarcoidosis, skin biopsy often is indicated to identify whether cutaneous findings are granulomatous sarcoid-related manifestations. The association of MEDFs with sarcoidosis requires further evaluation but might provide keys to understanding the pathophysiology of these lesions.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Niiyama S, Katsuoka K, Happle R, et al. Multiple eruptive dermatofibromas: a review of the literature. Acta Derm Venereol. 2002;82:241-244.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Kimura Y, Kaneko T, Akasaka E, et al. Multiple eruptive dermatofibromas associated with Hashimoto’s thyroiditis and myasthenia gravis. Eur J Dermatol. 2010;20:538-539.
- Bargman HB, Fefferman I. Multiple eruptive dermatofibromas in a patient with myasthenia gravis treated with prednisone and cyclophosphamide. J Am Acad Dermatol. 1986;14:351-352.
- Gelfarb M, Hyman AB. Multiple noduli cutanei. an unusual case of multiple noduli cutanei in a patient with hydronephrosis. Arch Dermatol. 1962;85:89-94.
- Yamamoto T, Katayama I, Nishioka K. Mast cell numbers in multiple dermatofibromas. Dermatology. 1995;190:9-13.
- Tsunemi Y, Tada Y, Saeki H, et al. Multiple dermatofibromas in a patient with systemic lupus erythematosus and Sjögren’s syndrome. Clin Exp Dermatol. 2004;29:483-485.
- Huang PY, Chu CY, Hsiao CH. Multiple eruptive dermatofibromas in a patient with dermatomyositis taking prednisolone and methotrexate. J Am Acad Dermatol. 2007;57(suppl 5):S81-S84.
- Lopez N, Fernandez A, Bosch RJ, et al. Multiple eruptive dermatofibromas in a patient with Graves-Basedow disease. J Eur Acad Dermatol Venereol. 2008;22:402-403.
- Gualandri L, Betti R, Cerri A, et al. Eruptive dermatofibromas and immunosuppression. Eur J Dermatol. 1999;9:45-47.
- Alexandrescu DT, Wiernik PH. Multiple eruptive dermatofibromas occurring in a patient with chronic myelogenous leukemia. Arch Dermatol. 2005;141:397-398.
- Chang SE, Choi JH, Sung KJ, et al. Multiple eruptive dermatofibromas occurring in a patient with acute myeloid leukaemia. Br J Dermatol. 2000;142:1062-1063.
- Cohen PR. Multiple dermatofibromas in patients with autoimmune disorders receiving immunosuppressive therapy. Int J Dermatol. 1991;30:266-270.
- Santos-Juanes J, Coto-Segura P, Mallo S, et al. Multiple eruptive dermatofibromas in a patient receiving efalizumab. Dermatology. 2008;216:363.
- Stainforth J, Goodfield MJ. Multiple dermatofibromata developing during pregnancy. Clin Exp Dermatol. 1994;19:59-60.
- Ashworth J, Archard L, Woodrow D, et al. Multiple eruptive histiocytoma cutis in an atopic. Clin Exp Dermatol. 1990;15:454-456.
- Lee HW, Lee DK, Oh SH, et al. Multiple eruptive dermatofibromas in a patient with primary pulmonary hypertension. Br J Dermatol. 2005;153:845-847.
- Veraldi S, Drudi E, Gianotti R. Multiple, eruptive dermatofibromas. Eur J Dermatol. 1996;6:523-524.
- Nestle FO, Nickeloff BJ, Burg G. Dermatofibroma: an abortive immunoreactive process mediated by dermal dendritic cells? Dermatology. 1995;190:265-268.
Practice Points
- Sarcoidosis can present with multiple cutaneous morphologies and dermatologists should have a low threshold to perform skin biopsy to confirm sarcoidal granulomatous inflammation.
- Dermatofibromas can occur in greater numbers in patients with immune dysregulation such as human immunodeficiency virus and systemic lupus erythematosus.
Eruptive Seborrheic Keratoses Secondary to Telaprevir-Related Dermatitis
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
To the Editor:
Telaprevir is a hepatitis C virus (HCV) protease inhibitor used with ribavirin and interferon for the treatment of increased viral load clearance in specific HCV genotypes. We report a case of eruptive seborrheic keratoses (SKs) secondary to telaprevir-related dermatitis.
A 65-year-old woman with a history of depression, basal cell carcinoma, and HCV presented 5 months after initiation of antiviral treatment with interferon, ribavirin, and telaprevir. Shortly after initiation of therapy, the patient developed a diffuse itch with a “pricking” sensation. The patient reported that approximately 2 months after starting treatment she developed an erythematous scaling rash that covered 75% of the body, which led to the discontinuation of telaprevir after 10 weeks of therapy; interferon and ribavirin were continued for a total of 6 months. In concert with the eczematous eruption, the patient noticed many new hyperpigmented lesions with enlargement of the few preexisting SKs. She presented to our clinic 6 weeks after the discontinuation of telaprevir for evaluation of these lesions.
On examination, several brown, hyperpigmented, stuck-on papules and plaques were noted diffusely on the body, most prominently along the frontal hairline (Figure 1). A biopsy of the right side of the forehead showed a reticulated epidermis, horn pseudocysts, and increased basilar pigment diagnostic of an SK (Figure 2).

[[{"attributes":{},"fields":{}}]][[{"attributes":{},"fields":{}}]]
Telaprevir is an HCV protease inhibitor that is given in combination with interferon and ribavirin for increased clearance of genotype 1 HCV infection. Cutaneous reactions to telaprevir are seen in 41% to 61% of treated patients and include Stevens-Johnson syndrome, drug reaction with eosinophilia and systemic symptoms, sarcoidosis, pityriasis rubra pilaris–like drug eruption, and most commonly telaprevir-related dermatitis.1-3 Telaprevir-related dermatitis accounts for up to 95% of cutaneous reactions and presents at a median of 15 days (interquartile range, 4–41 days) after initiation of therapy. Nearly 25% of cases occur in the first 4 days and 46% of cases occur within 4 weeks. It presents as an erythematous eczematous dermatitis commonly associated with pruritus in contrast to the common morbilliform drug eruption. Secondary xerosis, excoriation, and lichenification can be appreciated. With appropriate treatment, resolution occurs in a median of 44 days.1 Treatment of the dermatitis can allow completion of the recommended 12-week course of telaprevir and involves oral antihistamines and topical corticosteroids. Severe cases may require oral corticosteroids and discontinuation of telaprevir. If the cutaneous eruption does not resolve, discontinuation of ribavirin also may be required, as it can cause a similar cutaneous eruption.4
Eruptive SKs may be appreciated in 2 clinical circumstances: associated with an internal malignancy (Leser-Trélat sign), or secondary to an erythrodermic eruption. Flugman et al5 reported 2 cases of eruptive SKs in association with erythroderma. Their first patient developed erythroderma after initiating UVB therapy for psoriasis. The second patient developed an erythrodermic drug hypersensitivity reaction after switching to generic forms of quinidine gluconate and ranitidine. The SKs spontaneously resolved within 6 months and 10 weeks of the resolution of erythroderma, respectively.5 Most of our patient’s eruptive SKs resolved within a few months of their presentation, consistent with the time frame reported in the literature.
Telaprevir-related dermatitis presumably served as the inciting factor for the development of SKs in our patient, as the lesions improved after discontinuation of telaprevir despite continued therapy with ribavirin. As noted by Flugman et al,5 SKs may be seen in erythroderma due to diverse etiologies such as psoriasis, pityriasis rubra pilaris, or allergic contact dermatitis. We hypothesize that the eruption immunologically releases cytokines and/or growth factors that stimulate the production of the SKs. Fibroblast growth factor receptor 3 mutations have been associated with SKs.6 An erythrodermic milieu may incite such mutations in genetically predisposed patients.
We present a case of eruptive SKs related to telaprevir therapy. Our report expands the clinical scenarios in which the clinician can observe eruptive SKs. Although further research is necessary to ascertain the pathogenesis of these lesions, patients may be reassured that most lesions will spontaneously resolve.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
- Roujeau J, Mockenhaupt M, Tahan S, et al. Telaprevir-related dermatitis. JAMA Dermatol. 2013;149:152-158.
- Stalling S, Vu J, English J. Telaprevir-induced pityriasis rubra pilaris-like drug eruption. Arch Dermatol. 2012;148:1215-1217.
- Hinds B, Sonnier G, Waldman M. Cutaneous sarcoidosis triggered by immunotherapy for chronic hepatitis C: a case report. J Am Acad Dermatol. 2013;68:AB47.
- Lawitz E. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol. 2011;7:469-471.
- Flugman SL, McClain SA, Clark RA. Transient eruptive seborrheic keratoses associated with erythrodermic psoriasis and erythrodermic drug eruption: report of two cases. J Am Acad Dermatol. 2001;45(6 suppl):S212-S214.
- Hafner C, Hartman A, van Oers JM, et al. FGFR3 mutations in seborrheic keratoses are already present in flat lesions and associated with age and localization. Mod Pathol. 2007;20:895-903.
Practice Points
- Cutaneous reactions presenting as eczematous dermatitis are common (41%–61%) during telaprevir treatment.
- Telaprevir-related dermatitis can lead to eruptive seborrheic keratoses that may spontaneously resolve.
Enhanced Radiation Dermatitis Associated With Concurrent Palliative Radiation and Vemurafenib Therapy
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.
The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.
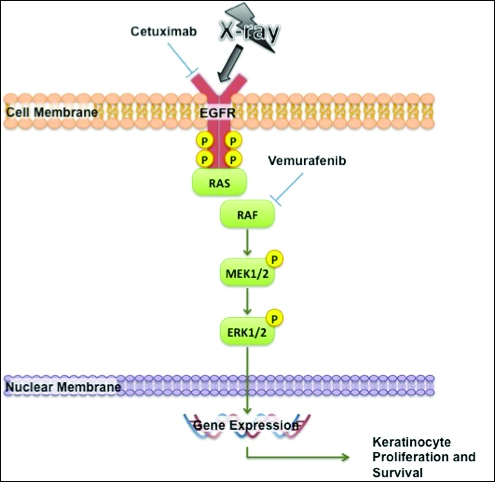
We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
To the Editor:
Vemurafenib is a selective BRAF inhibitor that was approved by the US Food and Drug Administration (FDA) in August 2011 for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an approved test. Both malignant and nonmalignant cutaneous findings have been well documented in association with vemurafenib, including squamous cell carcinoma, keratoacanthomas, UVA photosensitivity, keratosis pilaris–like eruptions, seborrheic dermatitis, follicular plugging, follicular hyperkeratosis, and eruptive melanocytic nevi.1 As more patients with metastatic melanoma are treated with vemurafenib, the use of concomitant palliative or adjuvant radiation therapy with vemurafenib will inevitably occur in greater frequency. Therefore, it is critical to understand the potential cutaneous side effects of this combination.
A predisposition to enhanced radiation dermatitis has been well described with concurrent use of targeted chemotherapies such as the epidermal growth factor receptor inhibitor cetuximab with radiotherapy.2 We report a case of radiation dermatitis occurring shortly after initiating radiation therapy in a patient on vemurafenib.
A 53-year-old man with initial stage IIIB melanoma, Breslow depth 2.2 mm with histologic ulceration, and a mitotic index of 2/mm2 on the right buttock underwent wide local excision and sentinel lymph node biopsy followed by complete lymph node dissection with a total of 2 of 10 positive lymph nodes. The patient subsequently underwent 1 year of adjuvant high-dose interferon therapy. Four years after his initial presentation he developed metastases to the lungs, pelvis, and both femurs. He was started on oral vemurafenib 960 mg twice daily. Due to painful bony metastases in the pelvis, the patient also was started on concurrent palliative radiation therapy to both femurs, L5 vertebra, and the sacrum 1 day after initiation of vemurafenib. Three days after initiation of radiation therapy at a cumulative radiation dose of 0.75 Gy, the patient developed severe, painful, well-demarcated, erythematous plaques in the anterior and posterior pelvic distribution overlying the radiation field (Figure 1) that subsequently evolved to eroded desquamative plaques with copious transudate. The patient also developed hyperkeratotic papules on the chest and thighs consistent with the keratosis pilaris–like eruptions associated with vemurafenib therapy.1 Five months later the patient developed worsening neurologic symptoms, and magnetic resonance imaging of the brain revealed multiple brain metastases. Given his disease progression, vemurafenib was discontinued. Ten days later, the patient underwent palliative whole-brain radiation therapy. He received a total dose of 3.25 Gy to the whole brain without any cutaneous sequelae.

The pathophysiology of radiation dermatitis is caused by a dose-dependent loss of basal and endothelial cells following irradiation.3 If surviving basal cells are able to repopulate the basal monolayer, normal skin barrier function is preserved. Dose tolerance is exceeded when cell loss without replacement occurs, resulting in necrosis and clinical evidence of radiation dermatitis, which is characterized by painful erythema or hyperpigmentation followed by desquamation and skin necrosis. In general, occurrence and severity of radiation dermatitis when radiation therapy is used alone in the absence of concurrent chemotherapy is dose dependent, with cutaneous evidence of radiation dermatitis occurring at doses ranging from as low as 2 Gy but most commonly 5 to 10 Gy.4 A report of radiation recall dermatitis in 2 patients who received vemurafenib after completing a full course of radiotherapy5 supports the hypothesis that vemurafenib is a radiosensitizing medication. Enhanced radiation dermatitis was reported in a single case of a patient on vemurafenib who developed radiation dermatitis after completing 3.25 Gy of radiation to the lumbar spine. Although this case likely depicted enhanced radiation dermatitis secondary to concurrent vemurafenib use, it was inconclusive whether vemurafenib contributed to the cutaneous effect, as the patient developed a cutaneous skin reaction 1 week after receiving a cumulative radiation dose of 3.25 Gy, a level at which radiation alone has been shown to cause skin toxicity.6 In our patient, cutaneous manifestations were noted 3 days after initiation of radiation treatment, at which point he had received a total radiation dose of 0.75 Gy, which is well below the threshold commonly recognized to cause radiation-induced skin toxicities. In addition, rechallenge in this patient with higher-dose radiotherapy while off of vemurafenib treatment led to no skin toxicity, despite the common side effects of whole-brain radiation therapy including radiation dermatitis and alopecia.7
The exact mechanism of increased radiosensitivity caused by targeted chemotherapies such as cetuximab and vemurafenib is unclear. One possible explanation is that the drug interferes with the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in controlling cell survival and regeneration following radiation exposure.8 Disruption of this signaling pathway through targeted therapies leads to impaired keratinocyte cell survival and recovery, and thus may enhance susceptibility to radiation-induced skin injury (Figure 2). In vivo studies have demonstrated that the epidermal growth factor receptor is activated following UV irradiation in human keratinocytes, leading to activation of the downstream MAPK signal transduction pathway required for cellular proliferation mediated via the RAF family of proteins.9,10 Further supporting the importance of this pathway in keratinocyte survival and recovery are findings that somatic deletion of BRAF in fibroblasts results in decreased growth factor–induced MAPK activation and enhanced apoptosis,8 whereas activated BRAF has been shown to exert protective effects against oxidative stress as well as tumorigenesis.11 The observation that mutant BRAF melanoma cells demonstrated increased radiosensitivity following BRAF inhibition with vemurafenib12 is consistent with our hypothesis that increased radiosensitivity occurs when signal transduction mediated by MAPK pathway is blocked, thereby inhibiting cell survival. As a result, radiation dermatitis is likely to occur more frequently and at a lower dose when signaling pathways upstream in the MAPK pathway required for keratinocyte regeneration, such as epidermal growth factor receptor and BRAF, are inhibited by targeted therapies. This hypothesis supports the observation that patients on medications that inhibit these signaling pathways, such as cetuximab and vemurafenib, develop enhanced sensitivity to both UV radiation and radiation therapy.

We report a case of enhanced radiation dermatitis occurring at a total dose of 0.75 Gy of radiotherapy, well below the threshold commonly recognized to cause radiation-induced skin toxicities. Our observation suggests that vemurafenib likely acts as a radiosensitizing agent that notably decreases the threshold for radiotherapy-related skin toxicities. Furthermore, the radiosensitizing effect of vemurafenib appears to be transient, as our patient showed no evidence of any skin reaction to subsequent radiation treatment soon after vemurafenib was discontinued. As more patients with metastatic melanoma are treated with vemurafenib, the combination of palliative or adjuvant radiation therapy with vemurafenib will likely be used more frequently. Caution should be exercised in patients on vemurafenib who receive concurrent radiotherapy, even at low radiation doses.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Studer G, Brown M, Dalgueiro E, et al. Grade 3/4 dermatitis in head and neck cancer patients treated with concurrent cetuximab and IMRT. Int J Radiat Oncol Biol Phys. 2011;81:110-117.
- Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31:1171-1185.
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341.
- Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149:855-857.
- Churilla TM, Chowdhry VK, Pan D, et al. Radiation-induced dermatitis with vemurafenib therapy. Pract Radiat Oncol. 2013;3:e195-e198.
- Anker CJ, Grossmann KF, Atkins MB, et al. Avoiding severe toxicity from combined BRAF inhibitor and radiation treatment: consensus guidelines from the Eastern Cooperative Oncology Group (ECOG). Int J Radiat Oncol Biol Phys. 2016;95:632-646.
- Dent P, Yacoub A, Fisher PB, et al. MAPK pathways in radiation responses. Oncogene. 2003;22:5885-5896.
- Cao C, Lus S, Jiang Q, et al. EGFR activation confers protections against UV-induced apoptosis in cultured mouse skin dendritic cells. Cell Signal. 2008;20:1830-1838.
- Xu Y, Shao Y, Zhou J, et al. Ultraviolet irradiation-induces epidermal growth factor receptor (EGFR) nuclear translocation in human keratinocytes. J Cell Biochem. 2009;107:873-880.
- Valerie K, Yacoub A, Hagan M, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789-801.
- Sambade M, Peters E, Thomas N, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98:394-399.
Practice Points
- Given the increased frequency of palliative and adjuvant radiation therapy in patients with metastatic melanoma, it is critical to understand the potential cutaneous side effects of vemurafenib when used in conjunction with radiotherapy.
- Clinicians should be aware of the increased risk for severe radiation dermatitis in patients on vemurafenib who are receiving concurrent palliative radiation therapy.
Fingernail Photo-onycholysis After Aminolevulinic Acid–Photodynamic Therapy Under Blue Light for Treatment of Actinic Keratoses on the Face
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5
Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5

Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
To the Editor:
Topical photodynamic therapy (PDT) is one of several effective treatments of actinic keratoses (AKs). Photodynamic therapy involves selection of a lesion field, application of a photosensitizer drug, incubation for an explicit period of time, and illumination of the area from a light source corresponding to the absorption spectrum of the chosen drug.1 A photosensitizer drug used in PDT to target AK is aminolevulinic acid (ALA). Aminolevulinic acid converts disease tissue to photoactivatable porphyrins, especially protoporphyrin IX, which has its largest absorption peak (410 nm) in the blue spectrum, with smaller absorption peaks at 505, 540, 580, and 630 nm. Photodynamic therapy treatments historically have been carried out under red light (peak emissions, 630 nm) to improve tissue penetration, which is superior in efficacy when treating Bowen disease and basal cell carcinoma.1,2 Broadband blue light (peak emission, 417 nm) now is routinely used and has been proven effective in combination with ALA for the treatment of AK.3 It was approved by the US Food and Drug Administration for AKs in 1999.4
Photo-onycholysis is a photosensitivity reaction defined as separation of the nail plate from the nail bed. There are 4 different types of photo-onycholysis characterized by appearance and by the number of digits affected: Type I is denoted by the involvement of several fingers, with half-moon–shaped separations of the nail plate. Type II affects a single finger and corresponds to a brown, defined, circular notch opening distally. Type III, which involves several fingers, is defined as round yellow stains in the central portion of the nail that turn red after 5 to 10 days. Type IV has been associated with bullae under the nails.5 There have been cases of photo-onycholysis arising after exposure to UV light following ingestion of certain prescription drugs or spontaneously,6 and a single case following PDT to the hands with red light.5 We report a case of fingernail photo-onycholysis resulting from ALA-PDT for the treatment of perioral AK.
A 65-year-old woman was treated for AKs on the perioral region of the face with PDT. Aminolevulinic acid hydrochloride 20% was applied to the lips and allowed to incubate for 60 minutes. Her face was illuminated with 10 J/cm² of blue light (417 nm) for 16 minutes and 40 seconds. Sunscreen (sun protection factor 40) was applied to the area immediately after treatment, and the patient was thoroughly counseled to avoid sunlight for the next 48 hours and to use sun protection. Within 72 hours following treatment, the patient reported all 10 fingernails noticeably separated from the nail bed with minimal pain, corresponding to type I photo-onycholysis (Figure). The patient’s only medications were vitamin D (1000 mg once daily) and calcium supplements (1500 mg twice daily). Although the patient exercised strict UV light avoidance for the face, her hands were not protected when she went gardening directly after the treatment. At 5 weeks, the patient returned for her second ALA-PDT treatment of perioral AK and a fungal culture was taken of the left third fingernail, which returned negative results. Poly-ureaurethane nail lacquer 16% was prescribed and was used once daily to protect and strengthen the fingernails. The patient returned for follow-up in clinic after 13 weeks and photo-onycholysis was resolving. Photo-onycholysis is categorized as a phototoxic reaction often associated with drug intake, more specifically with the use of tetracyclines, psoralens, and fluoroquinolones; less commonly with oral contraceptives; or spontaneously.6 It usually is recognized as a crescent-shaped distal separation of the nail surrounded by pigment. The action spectrum is believed to include UVA and UVB, though the exact mechanisms have not been confirmed.5

Our case provides evidence for risks involving the development of photo-onycholysis following PDT. We have no reason to believe there was systemic absorption of ALA, as there were no visible vesicles on the arms or hands after the treatment. Negative fungal culture results excluded onychomycosis. It is our hypothesis that the patient touched her face with her fingernails during the 60-minute incubation time prior to ALA-PDT treatment under blue light, inadvertently collecting ALA under the fingernails. Once she exposed her hands to sunlight while gardening after treatment, the nails likely reacted with the ALA in response to the UV radiation, thus triggering photo-onycholysis.
This case represents a report of fingernail photo-onycholysis from ALA-PDT under blue light as well as a report following treatment of AK not located on the hands with PDT. Although the photo-onycholysis did resolve within a few months of treatment, our case demonstrates the importance of counseling patients more specifically about isolating the ALA treatment zone from nontreated areas on the body during incubation. Improper UV light protection following ALA-PDT is known to produce phototoxic reactions and our case supports this outcome.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Hauschild A. Photodynamic therapy for actinic keratoses: procedure matters? Br J Dermatol. 2012;166:3-5.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24:16-25.
- Babilas P, Schreml S, Landthaler M, et al. Photodynamic therapy in dermatology: state-of-the-art. Photodermatol Photoimmunol Photomed. 2010;26:118-132.
- Hanneken S, Wessendorf U, Neumann NJ. Photodynamic onycholysis: first report of photo-onycholysis after photodynamic therapy. Clin Exp Dermatol. 2008;33:659-660.
- Baran R, Juhlin L. Photoonycholysis. Photodermatol Photoimmunol Photomed. 2002;18:202-207.
Practice Points
- Photodynamic therapy with aminolevulinic acid (ALA) is an effective treatment of actinic keratoses but can produce unexpected side effects in locations distant from initial therapy sites.
- It is important to counsel patients prior to initiating photodynamic therapy with ALA about isolating the ALA treatment zone from nontreated areas on the body during incubation.