User login
Exercise-Induced Vasculitis in a Patient With Negative Ultrasound Venous Reflux Study: A Mimic of Stasis Dermatitis
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
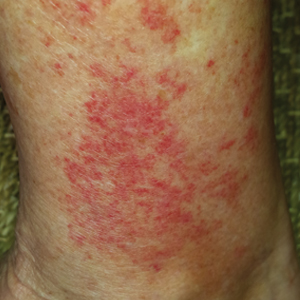
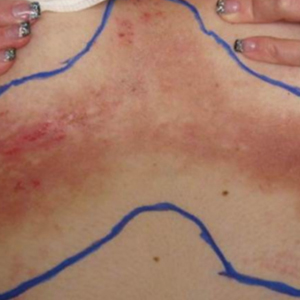
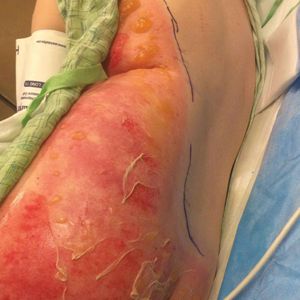
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.
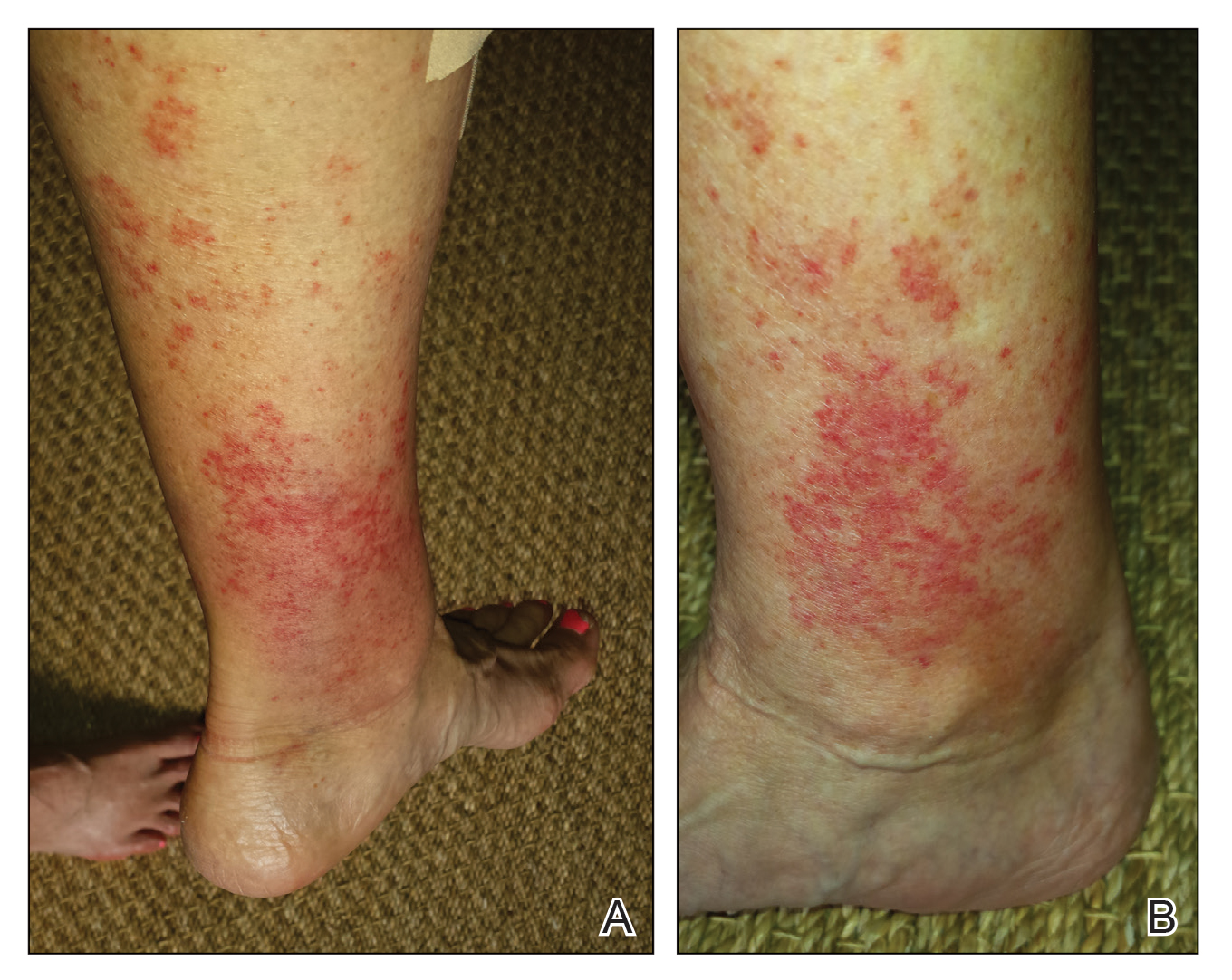
Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
To the Editor:
The transient and generic appearance of exercise-induced vasculitis (EIV) makes it a commonly misdiagnosed condition. The lesion often is only encountered through photographs brought by the patient or by taking a thorough history. The lack of findings on clinical inspection and the generic appearance of EIV may lead to misdiagnosis as stasis dermatitis due to its presentation as erythematous lesions on the medial lower legs.
A 68-year-old woman with no notable medical history was referred to our clinic for suspected stasis dermatitis. At presentation, no lesions were identified on the legs, but she brought photographs of an erythematous urticarial eruption on the medial lower legs, extending from just above the sock line to the mid-calves (Figure). The eruptions had occurred over the last 16 years, typically presenting suddenly after playing tennis or an extended period of walking and spontaneously resolving in 4 days. The lesions were painless, restricted to the calves, and were not pruritic, though the initial presentation 16 years prior included pruritic pigmented patches on the anterior thighs. Because the condition spontaneously improved within days, no treatment was attempted. An ultrasound venous reflux study ruled out venous reflux and stasis dermatitis.

Our patient stated that her 64-year-old sister had reported the same presentation over the last 8 years. Her physical activity was limited strictly to walking, and the lesions occurred after walking for many hours during the day in the heat, involving the medial aspects of the lower legs extending from the ankles to the full length of the calves. Her eruption was warm but was not painful or pruritic. It resolved spontaneously after 5 days with no therapy.
Our patient was advised to wear compression stockings as a preventative measure, but she did not adhere to these recommendations, stating it was impractical to wear compression garments while playing tennis.
Exercise-induced vasculitis most commonly is seen in the medial aspects of the lower extremities as an erythematous urticarial eruption or pigmented purpuric plaque rapidly occurring after a period of exercise.1,2 Lesions often are symmetric and can be pruritic and painful with a lack of systemic symptoms.3 These generic clinical manifestations may lead to a misdiagnosis of stasis dermatitis. One case report included initial treatment of presumptive cellulitis.4 Important clinical findings include a sparing of skin compressed by tight clothing such as socks, a lack of systemic symptoms, rapid appearance after exercise, and spontaneous resolution within a few days. No correlation with chronic venous disease has been demonstrated, as EIV can occur in patients with or without chronic venous insufficiency.5 Duplex ultrasound evaluation showed no venous reflux in our patient.
The pathophysiology of EIV remains unknown, but the concept of exercise-altered microcirculation has been proposed. Heat generated from exercise is normally dissipated by thermoregulatory mechanisms such as cutaneous vasodilation and sweat.1,6 When exercise is extended, done concomitantly in the heat, or performed in legs with preexisting edema or substantial adipose tissue that limit heat attenuation, the thermoregulatory capacity is overloaded and heat-induced muscle fiber breakdown occurs.1,7 Atrophy impairs the skeletal muscle’s ability to pump the increased venous return demanded by exercise to the heart, leading to backflow of venous return and eventual venous stasis.1 Reduction of venous return together with cutaneous vasodilation is thought to induce erythrocyte extravasation.
Histologic examination demonstrates features of leukocytoclastic vasculitis with perivascular lymphocytic and neutrophilic infiltrates.2 Erythrocyte extravasation, IgM deposits, and identification of C3 also have been reported.8,9 The spontaneous resolution of EIV has led to treatment efforts being focused on preventative measures. Several cases have reported some degree of success in preventing EIV with compression therapy, venoactive drugs, systemic steroids, and application of topical steroids before exercise.3
The clinical morphology and lower leg location of EIV leads to a common misdiagnosis of stasis dermatitis. Clinical history of a transient nature is the mainstay in the diagnosis of EIV, and ultrasound venous reflux study may be required in some cases. Preventative measures are superior to treatment and mainly include compression therapy.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
- Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
- Kelly RI, Opie J, Nixon R. Golfer’s vasculitis. Australas J Dermatol. 2005;46:11-14.
- Ramelet AA. Exercise-induced purpura. Dermatology. 2004;208:293-296.
- Cushman D, Rydberg L. A general rehabilitation inpatient with exercise-induced vasculitis. PM R. 2013;5:900-902.
- Veraart JC, Prins M, Hulsmans RF, et al. Influence of endurance exercise on the venous refilling time of the leg. Phlebology. 1994;23:120-123.
- Noakes T. Fluid replacement during marathon running. Clin J Sport Med. 2003;13:309-318.
- Armstrong RB. Muscle damage and endurance events. Sports Med. 1986;3:370-381.
- Prins M, Veraart JC, Vermeulen AH, et al. Leucocytoclastic vasculitis induced by prolonged exercise. Br J Dermatol. 1996;134:915-918.
- Sagdeo A, Gormley RH, Wanat KA, et al. Purpuric eruption on the feet of a healthy young woman. “flip-flop vasculitis” (exercise-induced vasculitis). JAMA Dermatol. 2013;149:751-756.
Practice Points
- Clinical history of a transient nature is the mainstay in the diagnosis of exercise-induced vasculitis.
- Exercise-induced vasculitis largely is documented in photographs or by history and may be misdiagnosed as stasis dermatitis due to its clinical morphology and lower leg location.
- Dermatologists should be aware of this disorder and consider performing further workup to rule out stasis dermatitis and diagnose this mimic.
- Preventative measures are superior to treatment and mainly include compression therapy.
Unilateral Nail Clubbing in a Hemiparetic Patient
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
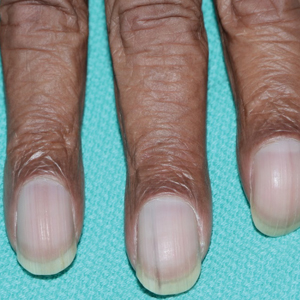
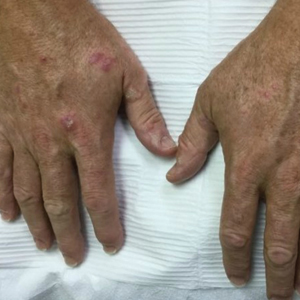
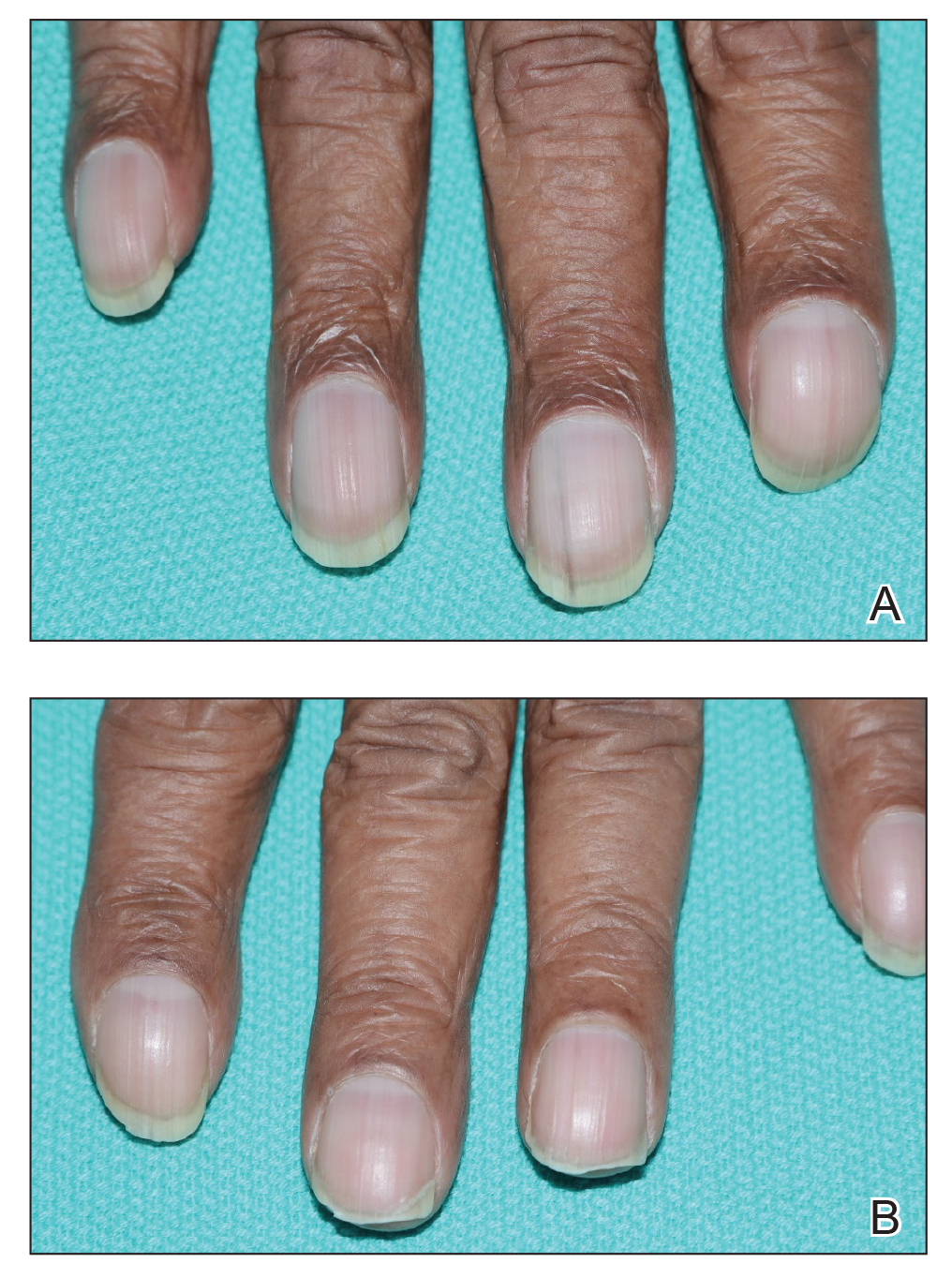
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.
Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.

Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
To the Editor:
Few cases of unilateral nail changes affecting only the hemiplegic side after a stroke have been reported. We present a case of acquired unilateral nail clubbing and longitudinal melanonychia in a hemiparetic patient.
A 79-year-old Black man with a history of smoking and stroke presented with concerns of discoloration of the fingernails. His medical history was notable for congestive heart failure; hypertension; diabetes mellitus; hypercholesterolemia; and stroke 11 years prior, which resulted in right-sided hemiparesis. Physical examination revealed longitudinal, even hyperpigmentation of several fingernails on the hands, in addition to whitening of the nail beds, sparing the tips (Terry nails). Clubbing was noted only on the fingernails of the right hand; the fingernails of the left hand exhibited normal curvature (Figure). Pulse oximetry was conducted and demonstrated the following readings: unaffected left index finger, 98%; unaffected left middle finger, 100%; affected right index finger, 95%; and affected right middle finger, 97%. The patient was diagnosed with benign longitudinal melanonychia secondary to ethnic variation, Terry nails without underlying anemia or hypoalbuminemic state, and unilateral right-sided clubbing of the fingernails in the setting of right-sided hemiparesis.

Prior reports have documented the occurrence of nail pathologies after stroke and affecting hemiplegic limbs. Unilateral digital nail clubbing following a stroke was first reported in 19751; 2 reports concluded clubbing developed in all digits affected by the stroke, and the severity of clubbing was associated with the duration of the stroke.1,2 One study noted longitudinal reddish striation, Neapolitan nails, and unilateral clubbing more commonly in hemiplegic patients.3 Longitudinal reddish striation was the most frequent condition observed in this population, always affecting the entire thumbnail of the hemiplegic limb.3 A similar report observed clubbing only on the fingernails of the hemiplegic side.4
Digital clubbing describes an exaggerated nail curvature and bulbous overgrowth of the fingertips due to an expansion of connective tissue between the nail plate and the nail bed.3,5 Clubbed fingers are found in various chronic conditions affecting the heart, lungs, and liver. Although the pathogenesis of clubbing remains unknown, many hypothesize that it is a state of proliferation in response to digital hypoxia.5 Fittingly, our patient exhibited a relative hypoperfusion of the clubbed fingers in comparison to the unaffected side.
This case provides additional support for the phenomenon of unilateral nail changes limited to hemiplegic or hemiparetic limbs. The unique presentation of longitudinal melanonychia, clubbing, and a lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
- Denham M, Hodkinson H, Wright B. Unilateral clubbing in hemiplegia. Gerontology Clin (Basel). 1975;17:7-12.
- Alveraz A, McNair D, Wildman J, et al. Unilateral clubbing of the fingernails in patients with hemiplegia. Gerontology Clin (Basel). 1975;17:1-6.
- Siragusa M, Schepis C, Cosentino F, et al. Nail pathology in patients with hemiplegia. Br J Dermatol. 2001;144:557-560.
- Gül Ü, Çakmak S, Özel S, et al. Skin disorders in patients with hemiplegia and paraplegia. J Rehabil Med. 2009;41:681-683.
- Sarkar M, Mahesh D, Madabhavi I. Digital clubbing. Lung India. 2012;29:354-362.
Practice Points
- Unilateral nail changes can be limited to hemiplegic or hemiparetic limbs.
- Lowered pulse oximetry reading only affecting the hemiparetic side demonstrates the possible connection between hypoxia and nail clubbing in this patient population.
Stump Pemphigoid Demonstrating Circulating Anti–BP180 and BP230 Antibodies
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
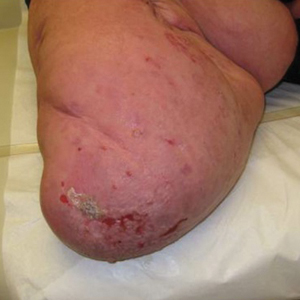
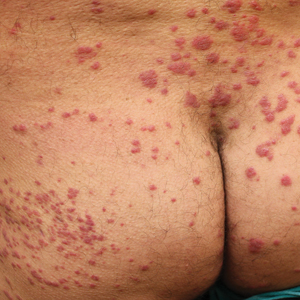
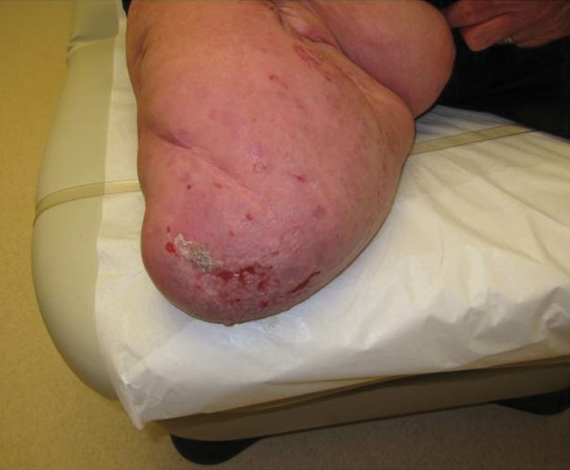
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).
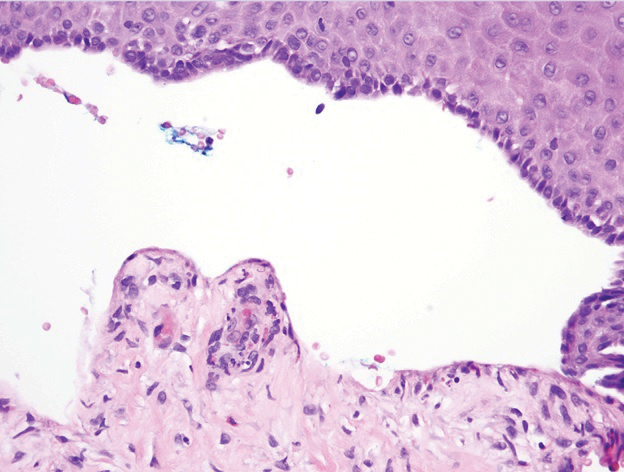
Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).


Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).


Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
Practice Points
- Bullous pemphigoid (BP) can mimic friction blisters and should be considered in amputees who present with vesicles and bullae on their amputation stump.
- Circulating anti–basement membrane antibodies BP230 and BP180 IgG may aid in diagnosis when skin biopsy results are equivocal and also may be helpful in gauging treatment response.
Scrub Typhus in Chile
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
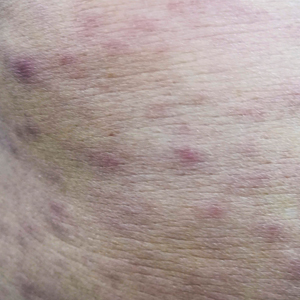
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).
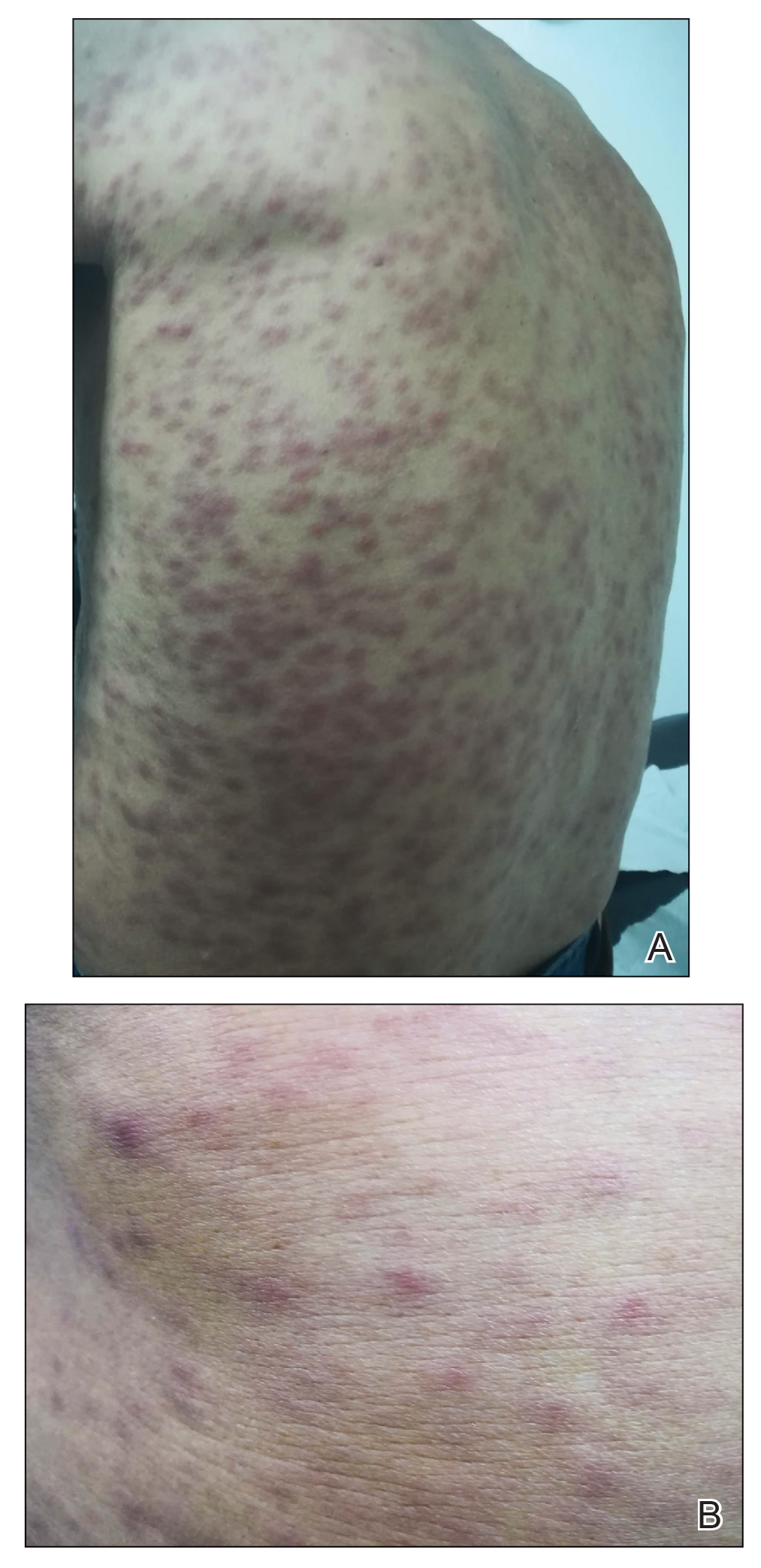
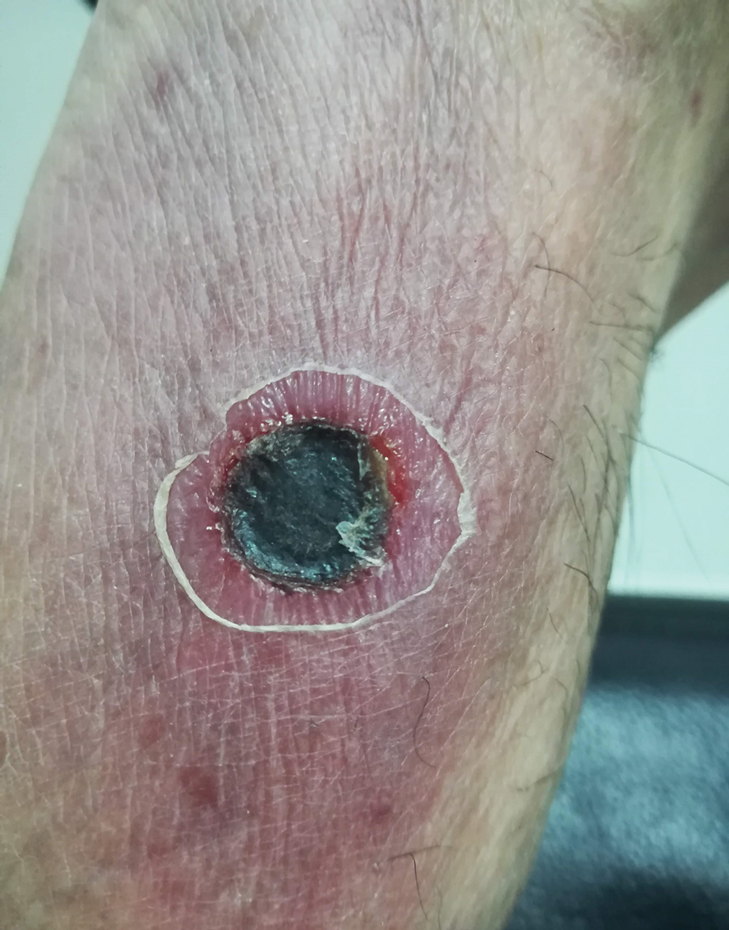
A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
Practice Points
- Scrub typhus is clinically suspected in patients who present with a febrile macular or papular rash and a characteristic necrotic eschar known as tache noire while residing in or traveling to rural areas.
- Scrub typhus can lead to serious complications. Due to its changing epidemiology, dermatologists outside the usual area of distribution should be aware in the event that new cases emerge.
Severe Refractory Hailey-Hailey Disease Treated With Electron Beam Radiotherapy and Low-Level Laser Therapy
To the Editor:
Hailey-Hailey disease (HHD), or familial benign chronic pemphigus, is a genetic disorder caused by an autosomal-dominant mutation in ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, which disrupts intracellular calcium signaling and blocks synthesis of junctional proteins required for cell-cell adhesion.1,2 As a result, patients develop acantholysis of the suprabasilar epidermis resulting in chronic flaccid blisters and erosions, particularly in intertriginous areas.3 Patients often report associated itching, pain, and burning, and they frequently present with secondary polymicrobial infections.4 Although HHD is genetic, patients may present without a family history due to variable expressivity and sporadic germline mutations.5 Therapeutic options are numerous, and patients may attempt many treatments before a benefit is observed.4 Local disease improvement was noted in a case series of 3 patients treated with electron beam radiotherapy with no disease recurrence in treated sites at 38, 33, and 9 months’ follow-up.6 Herein, we present a case of HHD refractory to numerous prior therapies that was successfully treated with electron beam radiotherapy, highlighting the potential role for palliative radiotherapy in select refractory cases of HHD.
A 35-year-old woman with a 7-year of history of HHD presented with severe recalcitrant disease including extensive erosive patches and plaques in the intertriginous areas of the bilateral axillae, groin, and inframammary folds (Figure 1). The skin eruptions first appeared at 28 years of age after her last pregnancy and continued as painful blistering that worsened with each menstrual cycle. Initially the affected desquamated skin would heal between menstrual cycles, but large areas of desquamation remained unhealed in the groin and inframammary regions throughout her full menstrual cycles by the time of referral. Family history included 4 family members with HHD: 2 aunts, an uncle, and a cousin on the paternal side of her family. Over a 7-year period, treatment with clobetasol cream, clindamycin gel, tacrolimus ointment, doxycycline, dapsone, cyclosporine, methotrexate, etanercept, isotretinoin, and prednisone (15 mg every other day) all failed. Most recently, axillary abobotulinumtoxinA injections were attempted but failed. She had been prednisone dependent for more than 1 year. Due to the ongoing refractory disease, she was referred to radiation oncology to discuss radiotherapy treatment options.
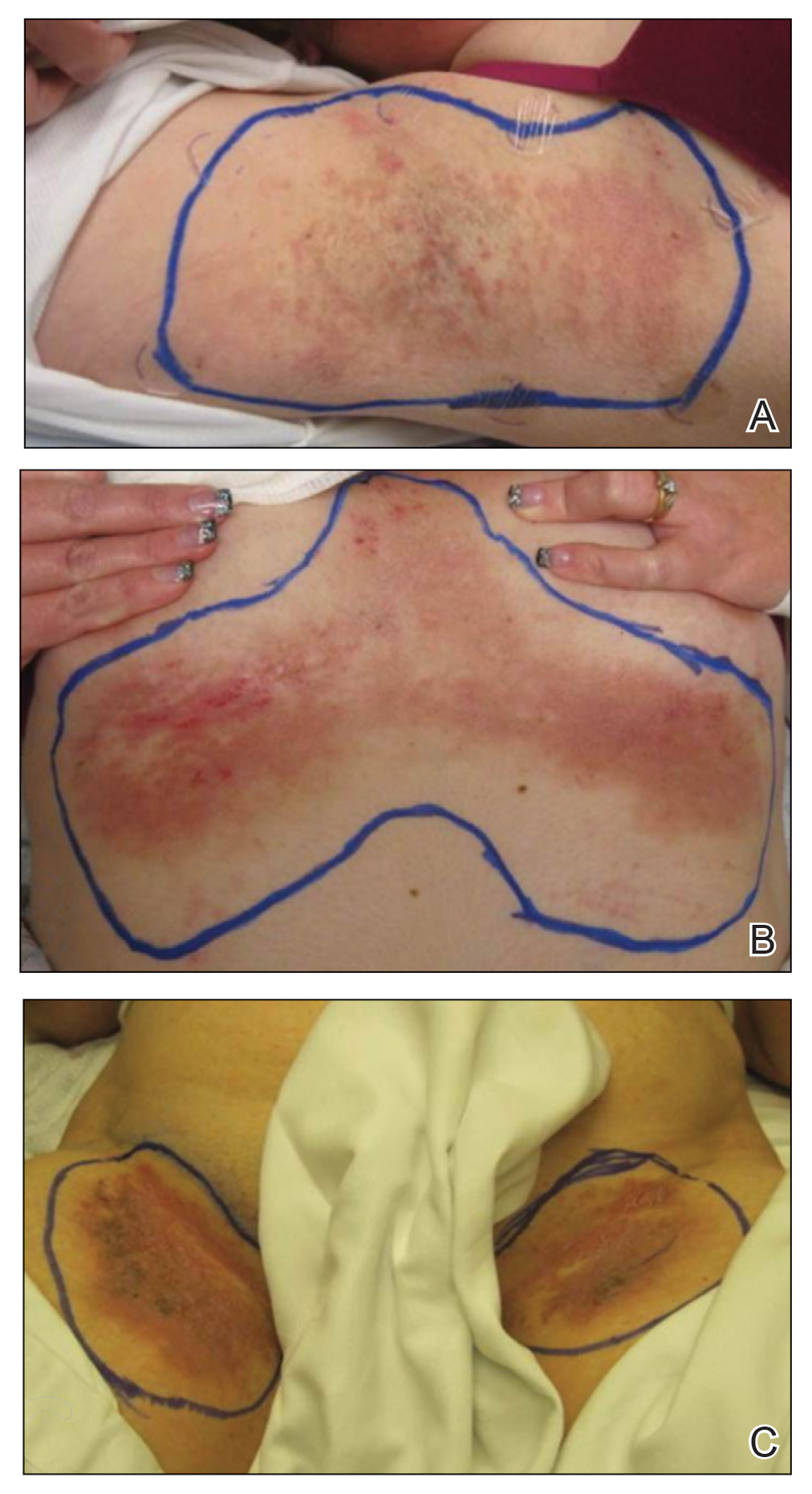
At the time of presentation to the radiation oncology clinic, she continued to have extensive involvement of the intertriginous areas as well as involvement of the right neck at sites of skin chafing from clothing. Consistent with the limited available evidence, a dose of 20 Gy in 10 fractions was first prescribed to the axillae to assess response in the event of radiosensitivity, resulting in brisk desquamation. The conventional fractionation of 2 Gy per fraction allowed for assessment of response/tolerance and the opportunity to stop the treatment in the unlikely event of a severe skin reaction. Her skin tolerated treatment well with slight dryness and mild irritation that was less severe than the typical HHD flares. Concurrently with the axillary radiotherapy, we delivered a trial of low-level laser therapy to areas of severe disease in both inguinal regions in the hope that it could be used instead of radiotherapy to avoid any associated risks of radiation. Low-level laser therapy was administered with a light-emitting diode cluster probe at 2.5 Hz for 1 minute to 2 sites in each inguinal area daily for a total of 10 treatments. Unfortunately, this therapy temporarily exacerbated exudation present in the skin before it resolved to its pretreatment state with no improvement.
One month after treatment she had total resolution of the erosive patches and plaques with mild residual hyperpigmentation in the axillae, establishing that radiation therapy was reasonably effective. To lower the total radiation dose and decrease the risk of radiation-induced malignancy, we treated the bilateral groin and the inframammary region with a treatment schedule of 8 Gy in 2 fractions at a higher dose of 4 Gy per fraction instead of 2 Gy. At 12 days posttreatment, she had a dramatic response in the groin and a mixed response in the inframammary region, with a focus that had not yet regressed. Given the dramatic response in the treated sites, the patient requested treatment to the right side of the neck. Thus, we treated this area with a similar 8 Gy in 2 fractions, and an additional 6 Gy in 2 fractions boost was added to the slow responding focus in the inframammary fold for a total of 14 Gy in 4 fractions. The patient tolerated all treatments well with mild grade 1 skin irritation that was unlike the blistering of the typical HHD flares. Two months following completion of the first course of radiotherapy to the axillae and 2 weeks from the most recent course, she tapered off her prednisone regimen (15 mg every other day) but had a relapse of disease with her subsequent menstrual cycles that presented as a blistering skin reaction in the inframammary region, which healed in response to restarting steroids. This flare was less severe than those prior to radiotherapy. At 10 months posttreatment, the patient was free of disease in the neck and axillae (Figure 2). She continued to have relapses in the inframammary region with menstrual cycles and noted new disease in the popliteal regions; however, the relapses were less severe, and her skin had improved more with radiation than any of the prior therapies.
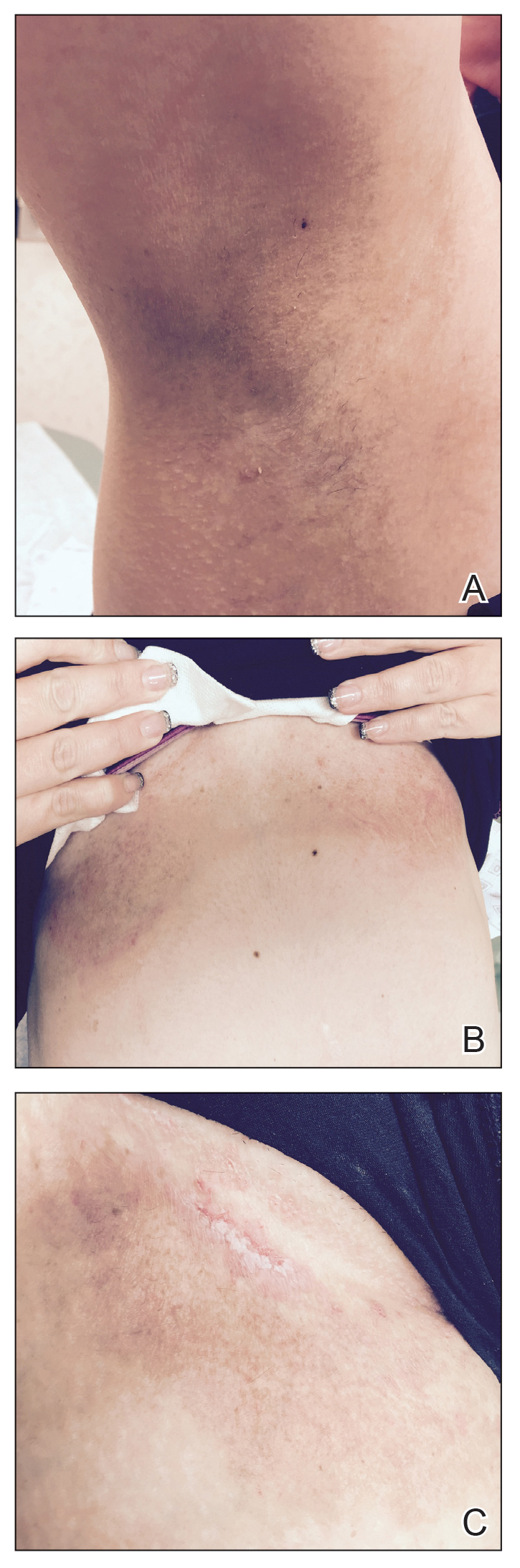
Our experience with low-level laser therapy indicated that it should be completely avoided in HHD. Our patient’s treatment with radiotherapy demonstrated excellent short-term responses in areas of pemphigus but later proved to be a mixed response with further follow-up including a mild posttreatment flare of the inframammary region that responded to steroids and new popliteal region involvement. As summarized in the Table, earlier reports of radiotherapy for the treatment of HHD have yielded varied results.6-8 The mixed response seen in our patient suggests that response to radiotherapy may be site specific, related to underlying inflammation from microbial overgrowth, or a function of the disease severity.
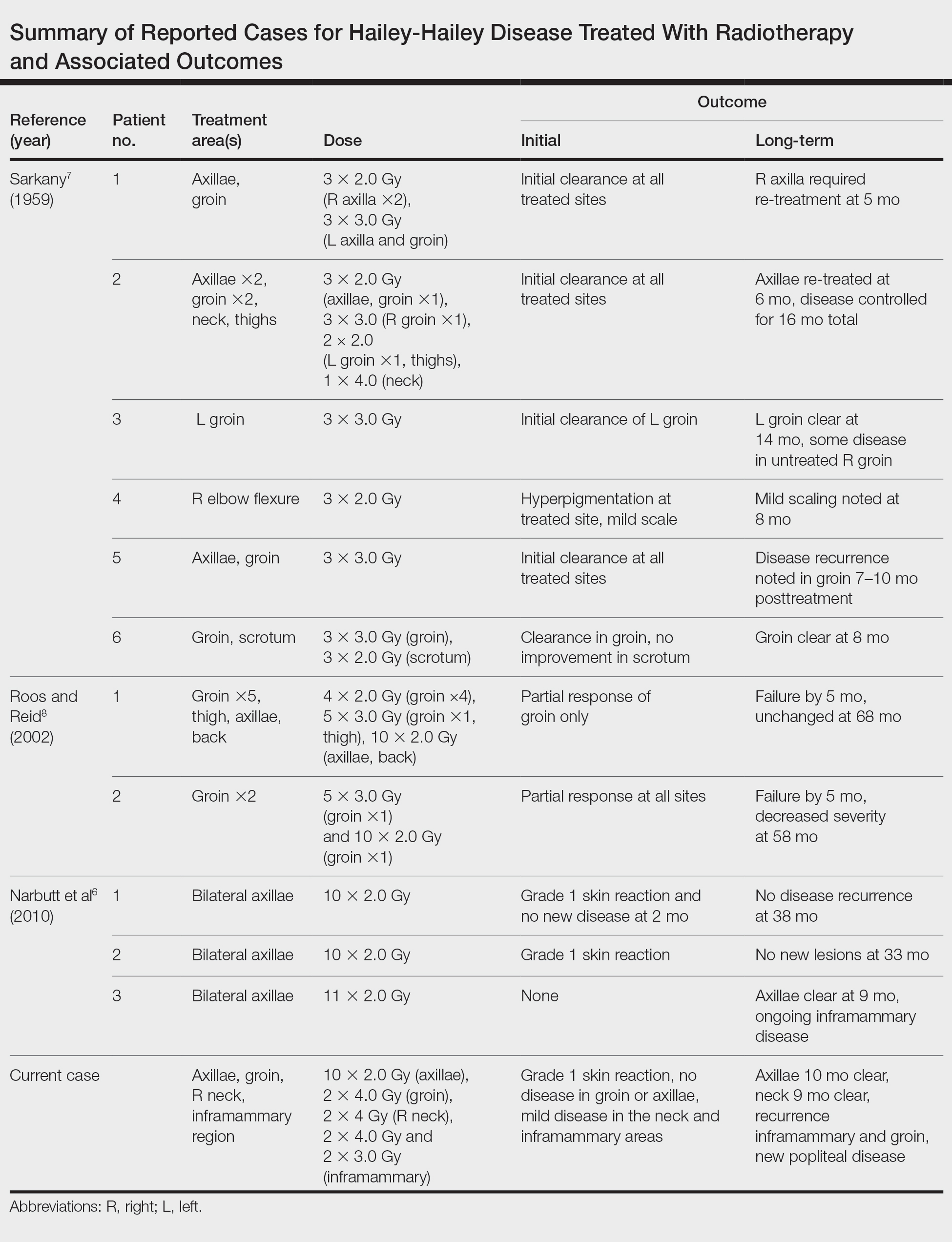
Although the number of reports on radiotherapy in HHD is small, there have been several reports of Darier disease, another acantholytic autosomal-dominant genodermatosis affecting the ATP2A2 gene, successfully treated incidentally with radiation.9,10 Total skin electron beam therapy also has been employed in the treatment of Darier disease; this patient experienced a severe flare after a relatively low treatment dose that required intensive care monitoring, possibly highlighting the potential radiosensitivity of patients with underlying genodermatoses and cautioning against radiotherapy dose escalation.11 In light of the mixed responses seen, radiation therapy should be used sparingly for severe relapsing cases that have failed a plethora of prior treatments. The risk of second cancer induction is especially of concern when using radiotherapy in a benign disease.12 We observed excellent initial responses in our patient, both with conventionally fractionated radiotherapy and hypofractionation. The risk of second malignancy induction is a linear function of radiotherapy dose.12 Thus, utilization of hypofractionated regimens such as 4 Gy times 2 fractions seems most prudent. It remains unclear if further dose de-escalation may yield a similar response, as seen in studies utilizing radiotherapy for other benign disease.13,14 Overall, given our mixed results with short follow-up, we conclude that the consideration of radiotherapy should be limited to patients with severe recalcitrant HHD.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Chiaravalloti A, Payette M. Hailey-Hailey disease and review of management. J Drugs Dermatol. 2014;13:1254-1257.
- Zhang F, Yan X, Jiang D, et al. Eight novel mutations of ATP2C1 identified in 17 Chinese families with Hailey-Hailey disease. Dermatology. 2007;215:277-283.
- Narbutt J, Chrusciel A, Rychter A, et al. Persistent improvement of previously recalcitrant Hailey-Hailey disease with electron beam radiotherapy. Acta Derm Venereol. 2010;90:179-182.
- Sarkany I. Grenz-ray treatment of familial benign chronic pemphigus. Br J Dermatol. 1959;71:247-252.
- Roos DE, Reid CM. Benign familial pemphigus: little benefit from superficial radiotherapy. Australas J Dermatol. 2002;43:305-308.
- Podgornii A, Ciammella P, Ramundo D, et al. Efficacy of the radiotherapy on Darier’s disease: an indirect evidence. Case Rep Dermatol Med. 2013;2013:907802.
- Mac Manus MP, Cavalleri G, Ball DL, et al. Exacerbation, then clearance, of mutation-proven Darier’s disease of the skin after radiotherapy for bronchial carcinoma: a case of radiation-induced epidermal differentiation? Radiat Res. 2001;156:724-730.
- Kittridge A, Wahlgren C, Fuhrer R, et al. Treatment of recalcitrant Darier’s disease with electron beam therapy. Dermatol Ther. 2010;23:302-304.
- Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381-407.
- Fröhlich D, Baaske D, Glatzel M. Radiotherapy of hidradenitis suppurativa—still valid today? [in German]. Strahlenther Onkol. 2000;176:286-289.
- Heyd R, Tselis N, Ackermann H, et al. Radiation therapy for painful heel spurs: results of a prospective randomized study. Strahlenther Onkol. 2007;183:3-9.
To the Editor:
Hailey-Hailey disease (HHD), or familial benign chronic pemphigus, is a genetic disorder caused by an autosomal-dominant mutation in ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, which disrupts intracellular calcium signaling and blocks synthesis of junctional proteins required for cell-cell adhesion.1,2 As a result, patients develop acantholysis of the suprabasilar epidermis resulting in chronic flaccid blisters and erosions, particularly in intertriginous areas.3 Patients often report associated itching, pain, and burning, and they frequently present with secondary polymicrobial infections.4 Although HHD is genetic, patients may present without a family history due to variable expressivity and sporadic germline mutations.5 Therapeutic options are numerous, and patients may attempt many treatments before a benefit is observed.4 Local disease improvement was noted in a case series of 3 patients treated with electron beam radiotherapy with no disease recurrence in treated sites at 38, 33, and 9 months’ follow-up.6 Herein, we present a case of HHD refractory to numerous prior therapies that was successfully treated with electron beam radiotherapy, highlighting the potential role for palliative radiotherapy in select refractory cases of HHD.
A 35-year-old woman with a 7-year of history of HHD presented with severe recalcitrant disease including extensive erosive patches and plaques in the intertriginous areas of the bilateral axillae, groin, and inframammary folds (Figure 1). The skin eruptions first appeared at 28 years of age after her last pregnancy and continued as painful blistering that worsened with each menstrual cycle. Initially the affected desquamated skin would heal between menstrual cycles, but large areas of desquamation remained unhealed in the groin and inframammary regions throughout her full menstrual cycles by the time of referral. Family history included 4 family members with HHD: 2 aunts, an uncle, and a cousin on the paternal side of her family. Over a 7-year period, treatment with clobetasol cream, clindamycin gel, tacrolimus ointment, doxycycline, dapsone, cyclosporine, methotrexate, etanercept, isotretinoin, and prednisone (15 mg every other day) all failed. Most recently, axillary abobotulinumtoxinA injections were attempted but failed. She had been prednisone dependent for more than 1 year. Due to the ongoing refractory disease, she was referred to radiation oncology to discuss radiotherapy treatment options.

At the time of presentation to the radiation oncology clinic, she continued to have extensive involvement of the intertriginous areas as well as involvement of the right neck at sites of skin chafing from clothing. Consistent with the limited available evidence, a dose of 20 Gy in 10 fractions was first prescribed to the axillae to assess response in the event of radiosensitivity, resulting in brisk desquamation. The conventional fractionation of 2 Gy per fraction allowed for assessment of response/tolerance and the opportunity to stop the treatment in the unlikely event of a severe skin reaction. Her skin tolerated treatment well with slight dryness and mild irritation that was less severe than the typical HHD flares. Concurrently with the axillary radiotherapy, we delivered a trial of low-level laser therapy to areas of severe disease in both inguinal regions in the hope that it could be used instead of radiotherapy to avoid any associated risks of radiation. Low-level laser therapy was administered with a light-emitting diode cluster probe at 2.5 Hz for 1 minute to 2 sites in each inguinal area daily for a total of 10 treatments. Unfortunately, this therapy temporarily exacerbated exudation present in the skin before it resolved to its pretreatment state with no improvement.
One month after treatment she had total resolution of the erosive patches and plaques with mild residual hyperpigmentation in the axillae, establishing that radiation therapy was reasonably effective. To lower the total radiation dose and decrease the risk of radiation-induced malignancy, we treated the bilateral groin and the inframammary region with a treatment schedule of 8 Gy in 2 fractions at a higher dose of 4 Gy per fraction instead of 2 Gy. At 12 days posttreatment, she had a dramatic response in the groin and a mixed response in the inframammary region, with a focus that had not yet regressed. Given the dramatic response in the treated sites, the patient requested treatment to the right side of the neck. Thus, we treated this area with a similar 8 Gy in 2 fractions, and an additional 6 Gy in 2 fractions boost was added to the slow responding focus in the inframammary fold for a total of 14 Gy in 4 fractions. The patient tolerated all treatments well with mild grade 1 skin irritation that was unlike the blistering of the typical HHD flares. Two months following completion of the first course of radiotherapy to the axillae and 2 weeks from the most recent course, she tapered off her prednisone regimen (15 mg every other day) but had a relapse of disease with her subsequent menstrual cycles that presented as a blistering skin reaction in the inframammary region, which healed in response to restarting steroids. This flare was less severe than those prior to radiotherapy. At 10 months posttreatment, the patient was free of disease in the neck and axillae (Figure 2). She continued to have relapses in the inframammary region with menstrual cycles and noted new disease in the popliteal regions; however, the relapses were less severe, and her skin had improved more with radiation than any of the prior therapies.

Our experience with low-level laser therapy indicated that it should be completely avoided in HHD. Our patient’s treatment with radiotherapy demonstrated excellent short-term responses in areas of pemphigus but later proved to be a mixed response with further follow-up including a mild posttreatment flare of the inframammary region that responded to steroids and new popliteal region involvement. As summarized in the Table, earlier reports of radiotherapy for the treatment of HHD have yielded varied results.6-8 The mixed response seen in our patient suggests that response to radiotherapy may be site specific, related to underlying inflammation from microbial overgrowth, or a function of the disease severity.

Although the number of reports on radiotherapy in HHD is small, there have been several reports of Darier disease, another acantholytic autosomal-dominant genodermatosis affecting the ATP2A2 gene, successfully treated incidentally with radiation.9,10 Total skin electron beam therapy also has been employed in the treatment of Darier disease; this patient experienced a severe flare after a relatively low treatment dose that required intensive care monitoring, possibly highlighting the potential radiosensitivity of patients with underlying genodermatoses and cautioning against radiotherapy dose escalation.11 In light of the mixed responses seen, radiation therapy should be used sparingly for severe relapsing cases that have failed a plethora of prior treatments. The risk of second cancer induction is especially of concern when using radiotherapy in a benign disease.12 We observed excellent initial responses in our patient, both with conventionally fractionated radiotherapy and hypofractionation. The risk of second malignancy induction is a linear function of radiotherapy dose.12 Thus, utilization of hypofractionated regimens such as 4 Gy times 2 fractions seems most prudent. It remains unclear if further dose de-escalation may yield a similar response, as seen in studies utilizing radiotherapy for other benign disease.13,14 Overall, given our mixed results with short follow-up, we conclude that the consideration of radiotherapy should be limited to patients with severe recalcitrant HHD.
To the Editor:
Hailey-Hailey disease (HHD), or familial benign chronic pemphigus, is a genetic disorder caused by an autosomal-dominant mutation in ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1, which disrupts intracellular calcium signaling and blocks synthesis of junctional proteins required for cell-cell adhesion.1,2 As a result, patients develop acantholysis of the suprabasilar epidermis resulting in chronic flaccid blisters and erosions, particularly in intertriginous areas.3 Patients often report associated itching, pain, and burning, and they frequently present with secondary polymicrobial infections.4 Although HHD is genetic, patients may present without a family history due to variable expressivity and sporadic germline mutations.5 Therapeutic options are numerous, and patients may attempt many treatments before a benefit is observed.4 Local disease improvement was noted in a case series of 3 patients treated with electron beam radiotherapy with no disease recurrence in treated sites at 38, 33, and 9 months’ follow-up.6 Herein, we present a case of HHD refractory to numerous prior therapies that was successfully treated with electron beam radiotherapy, highlighting the potential role for palliative radiotherapy in select refractory cases of HHD.
A 35-year-old woman with a 7-year of history of HHD presented with severe recalcitrant disease including extensive erosive patches and plaques in the intertriginous areas of the bilateral axillae, groin, and inframammary folds (Figure 1). The skin eruptions first appeared at 28 years of age after her last pregnancy and continued as painful blistering that worsened with each menstrual cycle. Initially the affected desquamated skin would heal between menstrual cycles, but large areas of desquamation remained unhealed in the groin and inframammary regions throughout her full menstrual cycles by the time of referral. Family history included 4 family members with HHD: 2 aunts, an uncle, and a cousin on the paternal side of her family. Over a 7-year period, treatment with clobetasol cream, clindamycin gel, tacrolimus ointment, doxycycline, dapsone, cyclosporine, methotrexate, etanercept, isotretinoin, and prednisone (15 mg every other day) all failed. Most recently, axillary abobotulinumtoxinA injections were attempted but failed. She had been prednisone dependent for more than 1 year. Due to the ongoing refractory disease, she was referred to radiation oncology to discuss radiotherapy treatment options.

At the time of presentation to the radiation oncology clinic, she continued to have extensive involvement of the intertriginous areas as well as involvement of the right neck at sites of skin chafing from clothing. Consistent with the limited available evidence, a dose of 20 Gy in 10 fractions was first prescribed to the axillae to assess response in the event of radiosensitivity, resulting in brisk desquamation. The conventional fractionation of 2 Gy per fraction allowed for assessment of response/tolerance and the opportunity to stop the treatment in the unlikely event of a severe skin reaction. Her skin tolerated treatment well with slight dryness and mild irritation that was less severe than the typical HHD flares. Concurrently with the axillary radiotherapy, we delivered a trial of low-level laser therapy to areas of severe disease in both inguinal regions in the hope that it could be used instead of radiotherapy to avoid any associated risks of radiation. Low-level laser therapy was administered with a light-emitting diode cluster probe at 2.5 Hz for 1 minute to 2 sites in each inguinal area daily for a total of 10 treatments. Unfortunately, this therapy temporarily exacerbated exudation present in the skin before it resolved to its pretreatment state with no improvement.
One month after treatment she had total resolution of the erosive patches and plaques with mild residual hyperpigmentation in the axillae, establishing that radiation therapy was reasonably effective. To lower the total radiation dose and decrease the risk of radiation-induced malignancy, we treated the bilateral groin and the inframammary region with a treatment schedule of 8 Gy in 2 fractions at a higher dose of 4 Gy per fraction instead of 2 Gy. At 12 days posttreatment, she had a dramatic response in the groin and a mixed response in the inframammary region, with a focus that had not yet regressed. Given the dramatic response in the treated sites, the patient requested treatment to the right side of the neck. Thus, we treated this area with a similar 8 Gy in 2 fractions, and an additional 6 Gy in 2 fractions boost was added to the slow responding focus in the inframammary fold for a total of 14 Gy in 4 fractions. The patient tolerated all treatments well with mild grade 1 skin irritation that was unlike the blistering of the typical HHD flares. Two months following completion of the first course of radiotherapy to the axillae and 2 weeks from the most recent course, she tapered off her prednisone regimen (15 mg every other day) but had a relapse of disease with her subsequent menstrual cycles that presented as a blistering skin reaction in the inframammary region, which healed in response to restarting steroids. This flare was less severe than those prior to radiotherapy. At 10 months posttreatment, the patient was free of disease in the neck and axillae (Figure 2). She continued to have relapses in the inframammary region with menstrual cycles and noted new disease in the popliteal regions; however, the relapses were less severe, and her skin had improved more with radiation than any of the prior therapies.

Our experience with low-level laser therapy indicated that it should be completely avoided in HHD. Our patient’s treatment with radiotherapy demonstrated excellent short-term responses in areas of pemphigus but later proved to be a mixed response with further follow-up including a mild posttreatment flare of the inframammary region that responded to steroids and new popliteal region involvement. As summarized in the Table, earlier reports of radiotherapy for the treatment of HHD have yielded varied results.6-8 The mixed response seen in our patient suggests that response to radiotherapy may be site specific, related to underlying inflammation from microbial overgrowth, or a function of the disease severity.

Although the number of reports on radiotherapy in HHD is small, there have been several reports of Darier disease, another acantholytic autosomal-dominant genodermatosis affecting the ATP2A2 gene, successfully treated incidentally with radiation.9,10 Total skin electron beam therapy also has been employed in the treatment of Darier disease; this patient experienced a severe flare after a relatively low treatment dose that required intensive care monitoring, possibly highlighting the potential radiosensitivity of patients with underlying genodermatoses and cautioning against radiotherapy dose escalation.11 In light of the mixed responses seen, radiation therapy should be used sparingly for severe relapsing cases that have failed a plethora of prior treatments. The risk of second cancer induction is especially of concern when using radiotherapy in a benign disease.12 We observed excellent initial responses in our patient, both with conventionally fractionated radiotherapy and hypofractionation. The risk of second malignancy induction is a linear function of radiotherapy dose.12 Thus, utilization of hypofractionated regimens such as 4 Gy times 2 fractions seems most prudent. It remains unclear if further dose de-escalation may yield a similar response, as seen in studies utilizing radiotherapy for other benign disease.13,14 Overall, given our mixed results with short follow-up, we conclude that the consideration of radiotherapy should be limited to patients with severe recalcitrant HHD.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Chiaravalloti A, Payette M. Hailey-Hailey disease and review of management. J Drugs Dermatol. 2014;13:1254-1257.
- Zhang F, Yan X, Jiang D, et al. Eight novel mutations of ATP2C1 identified in 17 Chinese families with Hailey-Hailey disease. Dermatology. 2007;215:277-283.
- Narbutt J, Chrusciel A, Rychter A, et al. Persistent improvement of previously recalcitrant Hailey-Hailey disease with electron beam radiotherapy. Acta Derm Venereol. 2010;90:179-182.
- Sarkany I. Grenz-ray treatment of familial benign chronic pemphigus. Br J Dermatol. 1959;71:247-252.
- Roos DE, Reid CM. Benign familial pemphigus: little benefit from superficial radiotherapy. Australas J Dermatol. 2002;43:305-308.
- Podgornii A, Ciammella P, Ramundo D, et al. Efficacy of the radiotherapy on Darier’s disease: an indirect evidence. Case Rep Dermatol Med. 2013;2013:907802.
- Mac Manus MP, Cavalleri G, Ball DL, et al. Exacerbation, then clearance, of mutation-proven Darier’s disease of the skin after radiotherapy for bronchial carcinoma: a case of radiation-induced epidermal differentiation? Radiat Res. 2001;156:724-730.
- Kittridge A, Wahlgren C, Fuhrer R, et al. Treatment of recalcitrant Darier’s disease with electron beam therapy. Dermatol Ther. 2010;23:302-304.
- Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381-407.
- Fröhlich D, Baaske D, Glatzel M. Radiotherapy of hidradenitis suppurativa—still valid today? [in German]. Strahlenther Onkol. 2000;176:286-289.
- Heyd R, Tselis N, Ackermann H, et al. Radiation therapy for painful heel spurs: results of a prospective randomized study. Strahlenther Onkol. 2007;183:3-9.
- Dhitavat J, Fairclough RJ, Hovnanian A, et al. Calcium pumps and keratinocytes: lessons from Darier’s disease and Hailey-Hailey disease. Br J Dermatol. 2004;150:821-828.
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24:61-65.
- Burge SM. Hailey-Hailey disease: the clinical features, response to treatment and prognosis. Br J Dermatol. 1992;126:275-282.
- Chiaravalloti A, Payette M. Hailey-Hailey disease and review of management. J Drugs Dermatol. 2014;13:1254-1257.
- Zhang F, Yan X, Jiang D, et al. Eight novel mutations of ATP2C1 identified in 17 Chinese families with Hailey-Hailey disease. Dermatology. 2007;215:277-283.
- Narbutt J, Chrusciel A, Rychter A, et al. Persistent improvement of previously recalcitrant Hailey-Hailey disease with electron beam radiotherapy. Acta Derm Venereol. 2010;90:179-182.
- Sarkany I. Grenz-ray treatment of familial benign chronic pemphigus. Br J Dermatol. 1959;71:247-252.
- Roos DE, Reid CM. Benign familial pemphigus: little benefit from superficial radiotherapy. Australas J Dermatol. 2002;43:305-308.
- Podgornii A, Ciammella P, Ramundo D, et al. Efficacy of the radiotherapy on Darier’s disease: an indirect evidence. Case Rep Dermatol Med. 2013;2013:907802.
- Mac Manus MP, Cavalleri G, Ball DL, et al. Exacerbation, then clearance, of mutation-proven Darier’s disease of the skin after radiotherapy for bronchial carcinoma: a case of radiation-induced epidermal differentiation? Radiat Res. 2001;156:724-730.
- Kittridge A, Wahlgren C, Fuhrer R, et al. Treatment of recalcitrant Darier’s disease with electron beam therapy. Dermatol Ther. 2010;23:302-304.
- Preston DL, Shimizu Y, Pierce DA, et al. Studies of mortality of atomic bomb survivors. report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res. 2003;160:381-407.
- Fröhlich D, Baaske D, Glatzel M. Radiotherapy of hidradenitis suppurativa—still valid today? [in German]. Strahlenther Onkol. 2000;176:286-289.
- Heyd R, Tselis N, Ackermann H, et al. Radiation therapy for painful heel spurs: results of a prospective randomized study. Strahlenther Onkol. 2007;183:3-9.
Practice Points
- Hailey-Hailey disease (HHD) is a rare blistering dermatosis characterized by recurrent erythematous plaques with a predilection for the intertriginous areas.
- Electron beam radiotherapy is a potential treatment option for local control in patients with recalcitrant HHD.
Hypertrophic Lichen Planus–like Eruption Following Pembrolizumab
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
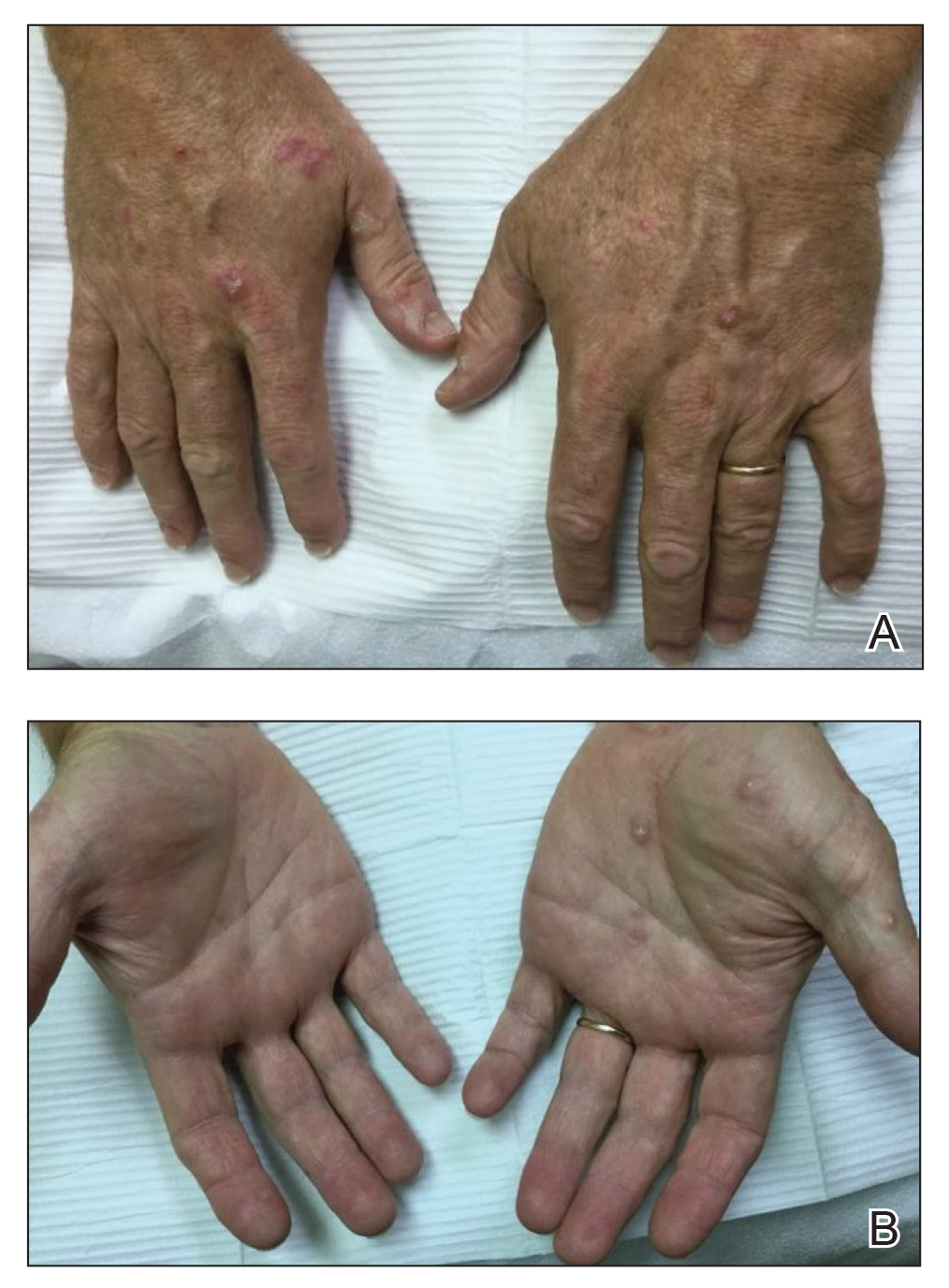
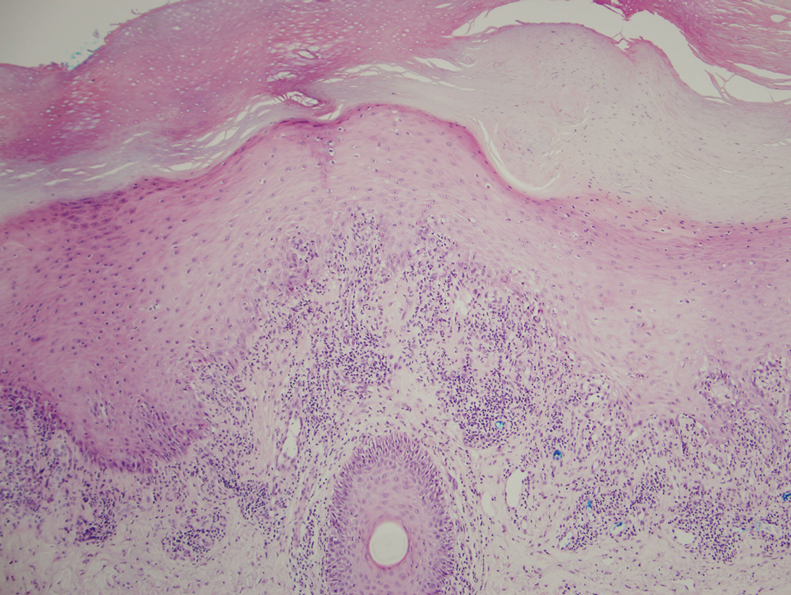
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.


At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.


At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
Practice Points
- With an increased use of immunotherapy medications such as pembrolizumab for various cancers, it is important that dermatologists are aware of the wide range of adverse cutaneous reactions that can occur, including lichenoid reactions.
- Hypertrophic lichen planus should be considered in the differential diagnosis of patients with cutaneous lesions in addition to nail findings developing after starting programmed cell death protein 1 inhibitor therapy.
Testosterone Pellet–Induced Generalized Drug Eruption
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
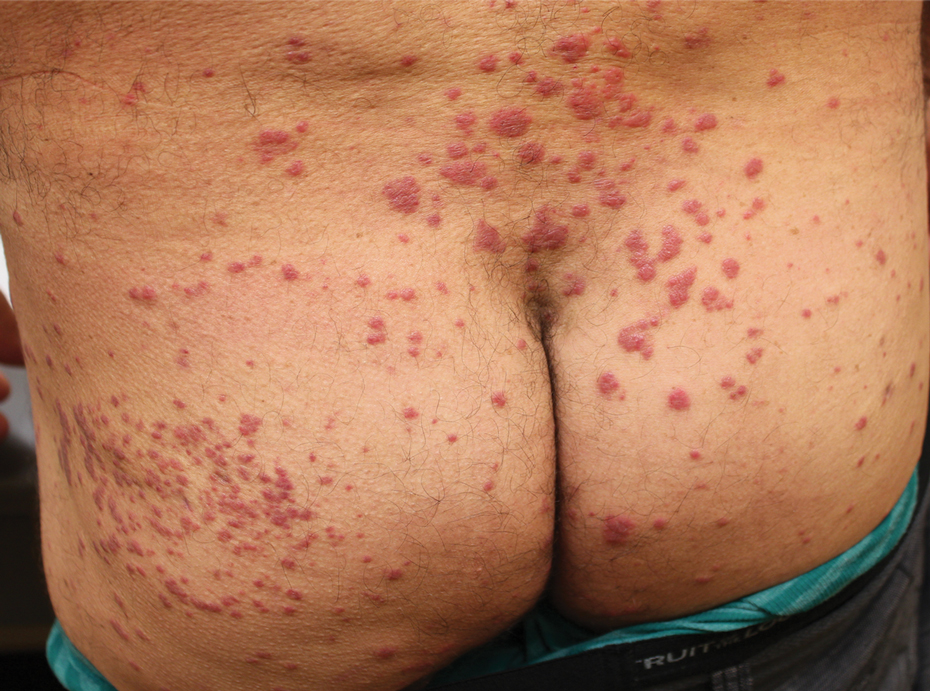
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
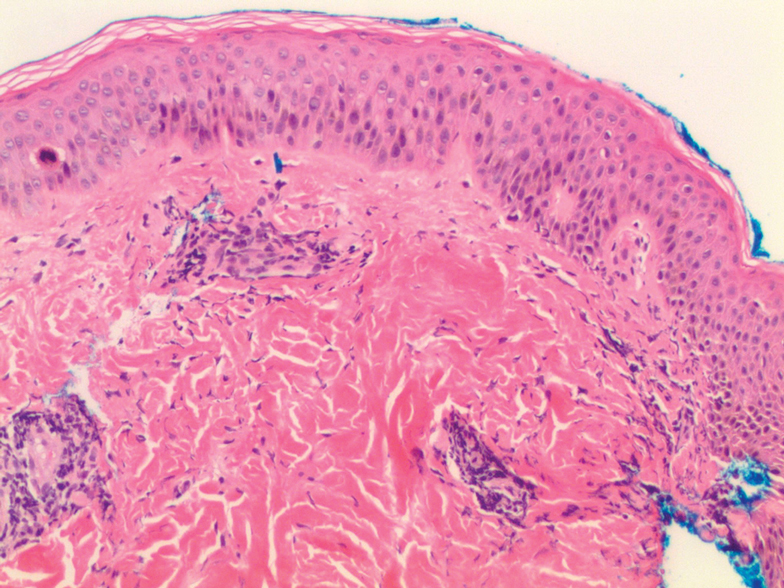
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.

Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).

Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.

Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
Practice Points
- Dermatologists should be aware that testosterone pellet implantation can cause dermatitis overlying the implantation site, which can generalize and differ in morphologic presentation.
- For patients presenting with a suspected case of testosterone pellet–induced dermatitis, a high-dose oral corticosteroid can be deployed as an effective therapy.
High-Grade Ovarian Serous Carcinoma Presenting as Androgenetic Alopecia
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).
Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.
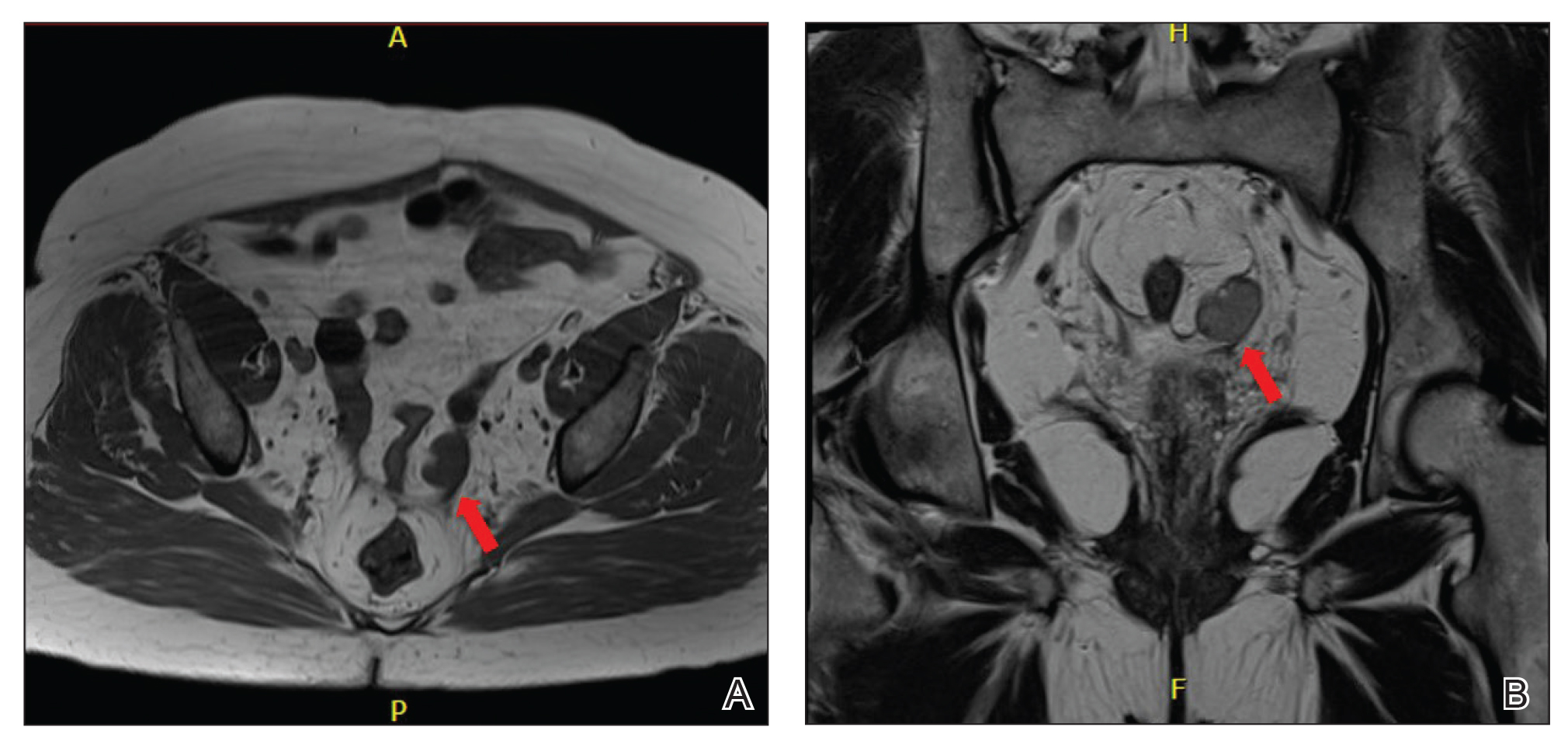
Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).

Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.

Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
To the Editor:
Female pattern hair loss is common, and the literature suggests that up to 56% of women experience hair thinning in their lifetime, with increased prevalence in older women.1 Pathophysiology is incompletely understood and involves the nonscarring progressive miniaturization of hair follicles, causing decreased production of terminal hairs relative to more delicate vellus hairs. Because vellus hairs have a shorter anagen growth phase than terminal hairs, hair loss is expedited. Androgen excess, when present, hastens the process by inducing early transition of hair follicles from the anagen phase to the senescent telogen phase. Serum testosterone levels are within reference range in most female patients with hair loss, suggesting the presence of additional contributing factors.2
Given the high prevalence of female pattern hair loss and the harm of overlooking androgen excess and an androgen-secreting neoplasm, dermatologists must recognize indications for further evaluation. Additional signs of hyperandrogenism, such as menstrual irregularities, acne, hirsutism, anabolic appearance, voice deepening, and clitoromegaly, are reasons for concern.3 Elevated serum androgen levels also should raise suspicion of malignancy. Historically, a total testosterone level above 200 ng/dL or a dehydroepiandrosterone sulfate (DHEA-S) level greater than 700 µg/dL prompted evaluation for a tumor.4 More recent studies show that tumor-induced increases in serum androgen levels are highly variable, challenging the utility of these cutoffs.5
A 70-year-old woman presented with hair loss over the last 12 years with accentuated thinning on the frontal and vertex scalp. The patient’s primary care physician previously made a diagnosis of androgenetic alopecia and recommended topical minoxidil. Although the patient had a history of excess facial and body hair since young adulthood, she noted a progressive increase in the density of chest and back hair, prominent coarsening of the texture of the facial and body hair, and new facial acne in the last 3 years. Prior to these changes, the density and texture of the scalp and body hair had been stable for many years.
Although other postmenopausal females in the patient’s family displayed patterned hair loss, they did not possess coarse and dense hair on the face and trunk. Her family history was notable for ovarian cancer in her mother (in her 70s) and breast cancer in her maternal grandmother (in her 80s).
A review of systems was notable only for decreased energy. Physical examination revealed a well-appearing older woman with coarse terminal hair growth on the cheeks, submental chin, neck, chest, back, and forearms. Scalp examination indicated diffusely decreased hair density, most marked over the vertex, crown, and frontal scalp, without scale, erythema, or loss of follicular ostia (Figure 1).

Laboratory evaluation revealed elevated levels of total testosterone (106 ng/dL [reference range, <40 ng/dL]) and free testosterone (32.9 pg/mL [reference range, 1.8–10.4 pg/mL]) but a DHEA-S level within reference range, suggesting an ovarian source of androgen excess. The CA-125 level was elevated (89 U/mL [reference range, <39 U/mL]).
Pelvic ultrasonography was suspicious for an ovarian pathology. Follow-up pelvic magnetic resonance imaging (MRI) demonstrated a 2.5-cm mass abutting the left ovary (Figure 2). The patient was given a diagnosis of stage IIIA high-grade ovarian serous carcinoma with lymph node involvement. Other notable findings from the workup included a BRCA2 mutation and concurrent renal cell carcinoma. After bilateral salpingo-oophorectomy, partial nephrectomy, and chemotherapy with carboplatin and paclitaxel, the testosterone level returned to within reference range and remained stable for the next 2 years of follow-up.

Female pattern hair loss is common in postmenopausal women and is a frequent concern in patients presenting to dermatology. Although most cases of androgenetic alopecia are isolated or secondary to benign conditions, such as polycystic ovary syndrome or nonclassic congenital adrenal hyperplasia, a small minority(<1% of women presenting with signs of hyperandrogenism) have an androgen-secreting tumor.6
Rapid onset or worsening of clinical hyperandrogenism, as seen in our patient, should raise concern for pathology; serum total testosterone and DHEA-S levels should be evaluated. Abnormally elevated serum androgens are associated with malignancy; however, there is variability in the recommended cutoff levels to prompt suspicion for an androgen-producing tumor and further workup in postmenopausal women.7 In the case of testosterone elevation, classic teaching designates a testosterone level greater than 200 ng/dL as the appropriate threshold for concern, but this level is now debated. In a series of women with hyperandrogenism referred to a center for suspicion of an androgen-secreting tumor, those with a tumor had, on average, a significantly higher (260 ng/dL) testosterone level than women who had other causes (90 ng/dL)(P<.05).6 The authors of that study proposed a cutoff of 1.4 ng/mL because women in their series who had a tumor were 8.4 times more likely to have a testosterone level of 1.4 ng/mL or higher than women without a tumor. However, this cutoff was only 92% sensitive and 70% specific.6 The degree of androgen elevation is highly variable in both tumorous and benign pathologies with notable overlap, challenging the notion of a clear cutoff.
Imaging is indicated for a patient presenting with both clinical and biochemical hyperandrogenism. Patients with an isolated testosterone level elevation can be evaluated with transvaginal ultrasonography; however, detection and characterization of malignancies is highly dependent on the skill of the examiner.8,9 The higher sensitivity and specificity of pelvic MRI reduces the likelihood of missing a malignancy and unnecessary surgery. Tumors too small to be visualized by MRI rarely are malignant.10
Sex cord-stromal cell tumors, despite representing fewer than 10% of ovarian tumors, are responsible for the majority of androgen-secreting malignancies. Our patient presented with clinical hyperandrogenism with an elevated testosterone level in the setting of a serous ovarian carcinoma, which is an epithelial neoplasm. Epithelial tumors are the most common type of ovarian tumor and typically are nonfunctional, though they have been reported to cause hyperandrogenism through indirect mechanisms. It is thought that both benign and malignant epithelial tumors can induce stromal hyperplasia or luteinization, leading to an increase in androgen levels.6
Due to the high prevalence of androgenetic alopecia and hirsutism in aging women, identification of androgen-secreting neoplasms by clinical presentation is challenging. A wide range of serum testosterone levels is possible at presentation, which complicates diagnosis. This case highlights the importance of correlating clinical and biochemical hyperandrogenism in raising suspicion of malignancy in older women presenting with hair loss.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
- Carmina E, Azziz R, Bergfeld W, et al. Female pattern hair loss and androgen excess: a report from the multidisciplinary androgen excess and PCOS committee. J Clin Endocrinol Metab. 2019;104:2875-2891.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:e9860.
- Rothman MS, Wierman ME. How should postmenopausal androgen excess be evaluated? Clin Endocrinol (Oxf). 2011;75:160-164.
- Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968-973.
- Kaltsas GA, Isidori AM, Kola BP, et al. The value of the low-dose dexamethasone suppression test in the differential diagnosis of hyperandrogenism in women. J Clin Endocrinol Metab. 2003;88:2634-2643.
- Sarfati J, Bachelot A, Coussieu C, et al; Study Group Hyperandrogenism in Postmenopausal Women. Impact of clinical, hormonal, radiological, immunohistochemical studies on the diagnosis of postmenopausal hyperandrogenism. Eur J Endocrinol. 2011;165:779-788.
- Glintborg D, Altinok ML, Petersen KR, et al. Total testosterone levels are often more than three times elevated in patients with androgen-secreting tumours. BMJ Case Rep. 2015;2015:bcr2014204797.
- Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adnexal lesion characterization. AJR Am J Roentgenol. 2010;194:311-321.
- Rauh-Hain JA, Krivak TC, Del Carmen MG, et al. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15-21.
- Horta M, Cunha TM. Sex cord-stromal tumors of the ovary: a comprehensive review and update for radiologists. Diagn Interv Radiol. 2015;21:277-286.
Practice Points
- Laboratory assessment for possible androgen excess should be performed in patients with female pattern hair loss and include baseline serum total testosterone and dehydroepiandrosterone sulfate.
- Rapid onset or worsening of clinical hyperandrogenism should raise suspicion of malignancy.
- Transvaginal ultrasonography and possible pelvic magnetic resonance imaging are indicated for patients with clinical hyperandrogenism and an isolated testosterone level elevation.
Symmetric Drug-Related Intertriginous and Flexural Exanthema
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.
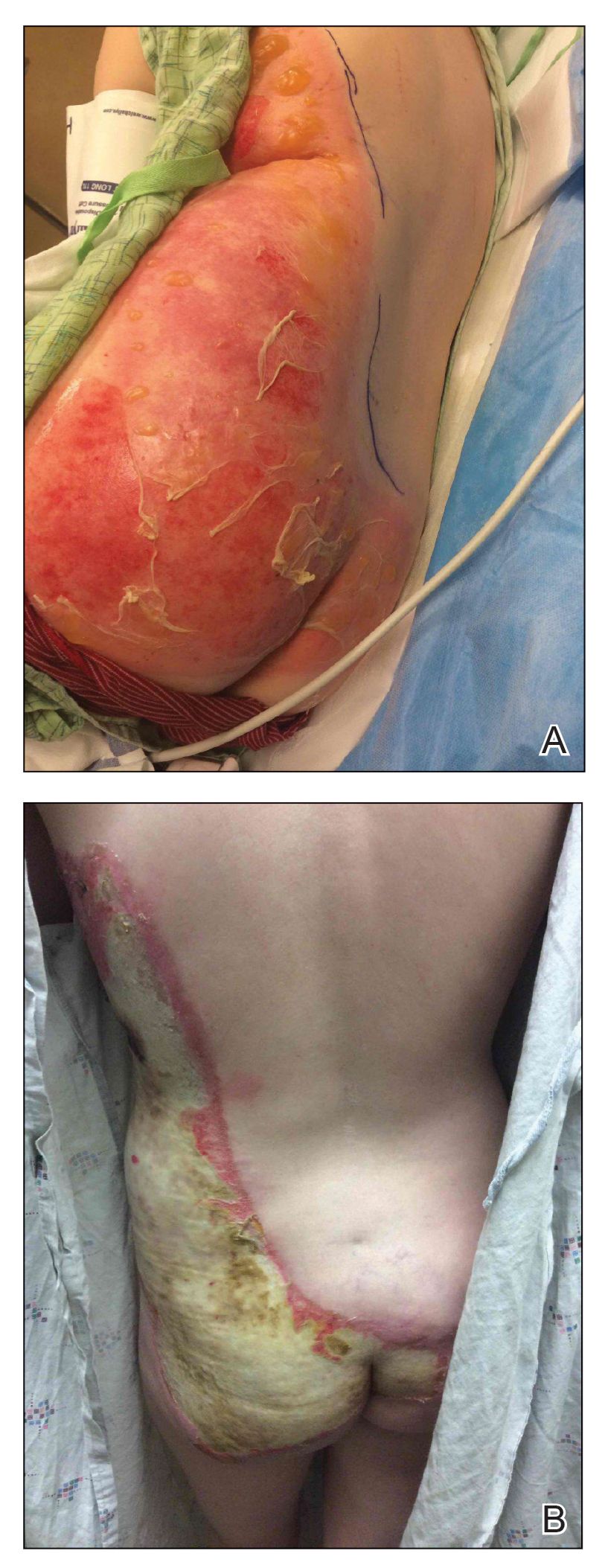
Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4
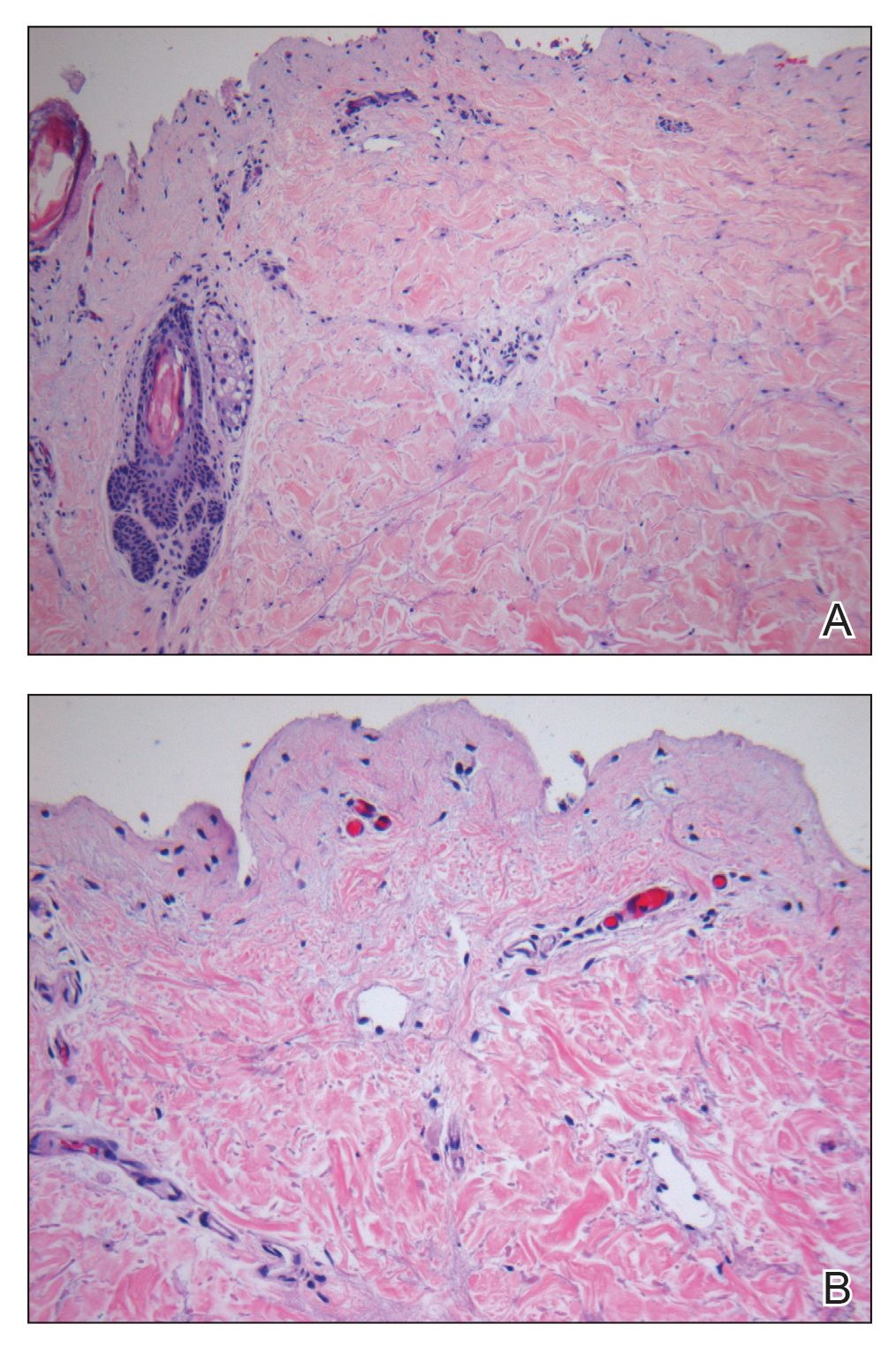
Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.

Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4

Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
To the Editor:
Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) is a curious disorder that has undergone many clinical transformations since first being described by Andersen et al1 in 1984 using the term baboon syndrome. Initially described as a mercury hypersensitivity reaction resulting in an eruption resembling the red-bottomed baboon, this exanthema has expanded in definition with inciting agents, clinical features, and diagnostic criteria. Its prognosis, however, has remained stable and favorable throughout the decades. The condition is almost universally benign and self-limited.1-3 As new cases are reported in the literature and the paradigm of SDRIFE continues to shift, its prognosis also may warrant reconsideration and respect as a potentially destructive reaction.
A 39-year-old woman who was otherwise healthy presented to the emergency department after developing a rapidly evolving and blistering rash on the left flank. Hours later, the rash had progressed to a sharply demarcated, confluent, erythematous plaque with central ulceration and large flaccid bullae peripherally, encompassing 18% of total body surface area and extending from the gluteal cleft to the tip of the scapula along the left flank (Figure 1) with no vaginal or mucosal involvement. The patient recently had completed a 10-day course of amoxicillin–clavulanic acid 2 days prior for a cat bite on the right dorsal wrist. Additional history confirmed the absence of prodromal fever, fatigue, or chills. Inciting trauma, including chemical and thermal burns, was denied. Potential underlying psychosocial cofounders were explored and were unrevealing.

Laboratory test results, including complete blood cell count and metabolic panel as well as vital signs were unremarkable, except for slight leukocytosis at 14,000/µL (reference range 4500–11,000/µL). A punch biopsy was taken from the patient’s left upper back at the time of admission, which revealed a sparse, superficial, perivascular infiltrate of lymphocytes and rare neutrophils with largely absent epidermis and an occasional focal necrosis of adnexal epithelium (Figure 2). Immunofluorescence was negative for specific deposition of IgG, IgA, IgM, C3, or fibrinogen. Wound culture also returned negative, and the Naranjo adverse drug reaction probability scale score was calculated to be 4 out of 12, indicating possible adverse drug reaction.4

Given the extent and distribution of the rash as well as the full-thickness dermal involvement, the patient was transferred to the burn unit for subsequent care. At 8-month follow-up, she experienced severe, symptomatic, hypertrophic scarring and was awaiting intralesional triamcinolone acetonide injections. The patient subsequently was lost to follow up.
The clinical picture of SDRIFE has remained obscure over the last 30 years, likely owing to its rarity and unclear pathogenesis. Diagnostic criteria for SDRIFE were first proposed by Häusermann et al2 in 2004 and contained 5 elements: (1) occurrence after (re)exposure to systemic drugs, (2) sharply demarcated erythema of the gluteal region or V-shaped erythema of the inguinal area, (3) involvement of at least 1 other intertriginous location, (4) symmetry of affected areas, and (5) absence of systemic symptoms and signs. Based on these clinical criteria, our patients fulfilled 3 of 5 elements, with deductions for symmetry of affected areas and involvement of other intertriginous locations. Histopathologic findings in SDRIFE predominantly are nonspecific with superficial perivascular mononuclear infiltrates; however, prior reports have confirmed the potential for vacuolar changes and hydropic degeneration in the basal cell layer with subepidermal bullae formation.5,6 Similarly, although the presence of bullae are somewhat atypical in SDRIFE, it has been described.3 Taken together, we speculate that these findings may support a diagnosis of SDRIFE with atypical presentation, though an alternative diagnosis of bullous fixed drug eruption (FDE) cannot be ruled out.
Historically, SDRIFE has been associated with a benign course. The condition typically arises within a few hours to days following administration of the offending agent, most commonly amoxicillin or another β-lactam antibiotic.1 Most cases spontaneously resolve via desquamation within 1 to 2 weeks. We present an unusual case of amoxicillin-induced full-thickness epidermal necrosis resulting in symptomatic sequelae, which exhibits findings of SDRIFE, bullous FDE, or Stevens-Johnson syndrome/toxic epidermal necrolysis, suggesting the possibility for a common pathway underlying the pathogenesis of these conditions.
The diagnostic uncertainty that commonly accompanies these various toxic drug reactions may in part relate to their underlying immunopathogenesis. Although the exact mechanism by which SDRIFE results in its characteristic skin lesions has not been fully elucidated, prior work through patch testing, lymphocyte transformation assays, and immunohistochemical staining of biopsies suggests a type IV delayed hypersensitivity (DTH) reaction.7-10 Specifically, SDRIFE appears to share features of both DTH type IVa—involving CD4+ helper T cells (TH1), monocytes, and IFN-γ signaling—and DTH type IVc—involving cytotoxic CD4 and CD8 cells, granzyme B action, and FasL signaling.11,12 A similar inflammatory milieu has been implicated in numerous toxic drug eruptions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and FDE.11,13 This mechanistic overlap may explain the overlap seen clinically among such conditions.
In the undifferentiated patient, categorization of the clinical syndrome proves helpful in prognostication and therapeutic approach. The complexities and commonalities intrinsic to these syndromes, however, may simultaneously preclude certain cases from neatly following the predefined rules. These atypical presentations, while diagnostically challenging, can in turn offer a unique opportunity to reexamine the current state of disease understanding to better allow for appropriate classification.
Despite its rarity, SDRIFE should be considered in the differential of undiagnosed drug eruptions, particularly as new clinical presentations emerge. Careful documentation and timely declaration of future cases will prove invaluable for diagnostic and therapeutic advancements should this once-benign condition develop a more destructive potential.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr TH, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011;11:313-318.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Elmariah SB, Cheung W, Wang N, et al. Systemic drug-related intertriginous and flexural exanthema (SDRIFE). Dermatol Online J. 2009;15:3.
- Hembold P, Hegemann B, Dickert C, et al. Symptomatic psychotropic and nonpigmenting fixed drug eruption due to cimetidine (so-called baboon syndrome). Dermatology. 1998;197:402-403.
- Barbaud A, Trechot P, Granel F, et al. A baboon syndrome induced by intravenous human immunoglobulins: a report of a case and immunological analysis. Dermatology. 1999;199:258-260.
- Miyahara A, Kawashima H, Okubo Y, et al. A new proposal for a clinical-oriented subclassification of baboon syndrome and review of baboon syndrome. Asian Pac J Allergy Immunol. 2011;29:150-160.
- Goossens C, Sass U, Song M. Baboon syndrome. Dermatology. 1997;194:421-422.
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:123-129.
- Ozkaya E. Current understanding of baboon syndrome. Expert Rev Dermatol. 2009;4:163-175.
- Ozakaya E. Fixed drug eruption: state of the art. J Dtsch Dermatol Ges. 2008;6:181-188.
Practice Points
- Symmetric drug-related intertriginous and flexural exanthema (SDRIFE) appears in the absence of systemic signs and symptoms such as fever, which may help differentiate it from infectious causes.
- β-Lactam antibiotics, particularly amoxicillin, are common offenders in the pathogenesis of SDRIFE, but new drug relationships frequently are being described.
- Symmetric drug-related intertriginous and flexural exanthema commonly follows a benign course but warrants respect, as it may have devastating potential.
Multiple Glomangiomas in a Patient With a History of Metastatic Melanoma
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
To the Editor:
A 32-year-old man presented to the dermatology clinic with multiple asymptomatic blue lesions on the arms and upper torso of 15 years’ duration. His medical history was notable for a recent diagnosis of malignant melanoma following excision of a mole on the upper back 4 months prior. He reported that the mole had been present since childhood, but his sister noticed that it increased in size and changed in color over the course of a year. Physical examination showed multiple blue subcutaneous nodules on the bilateral arms and lower back. The nodules were soft and nontender, and some had telangiectasia on the overlying skin.
Given the atypical distribution of nodules and the patient’s recent history of melanoma, there was concern for cutaneous metastases. A punch biopsy of one of the nodules on the right upper arm was performed. Microscopic examination of the biopsy specimen revealed a proliferation of multiple cavernous vessels surrounded by several rows of monotonous round cells with moderate eosinophilic cytoplasm and monomorphic nuclei, which was consistent with a diagnosis of glomangioma (Figure 1). Immunohistochemical analysis showed diffuse positive staining for smooth muscle actin (Figure 2); CD34 immunostain was positive in endothelial cells and negative in tumor cells (Figure 3).



Two weeks after the first punch biopsy, the patient returned for follow-up. He noted a new soft, painless, nontender mass in the left axillary region. Positron emission tomography–computed tomography and a lymphoscintigram were performed to assess for lymphadenopathy, but they were not contributory. Subsequently, the patient underwent bilateral axillary sentinel lymph node dissection, which revealed the presence of metastatic melanoma in one lymph node in the left axilla. No metastatic disease was identified in the right axillary sentinel lymph nodes. A second skin biopsy was performed on another blue nodule to confirm the diagnosis and to exclude the possibility of sampling error. The histopathologic examination again revealed glomangioma, which established the diagnosis of multiple glomangiomas.
Glomus tumors arise from modified smooth muscle cells located in glomus bodies. The glomus body is a component of the dermis involved in regulation of body temperature that is composed of an afferent arteriole and an efferent venule. The arterial end of this apparatus, known as the Sucquet-Hoyer canal, is surrounded by glomus cells that have a contractile capability similar to smooth muscle cells. Glomus tumors usually present as painful masses on the fingers with a typical subungual location and almost always are solitary.1 Glomangiomas, sometimes known as glomuvenous malformations, tend to be larger and usually are painless. They mostly are found on the trunk and extremities and can appear in groups.2,3 Histopathologically, glomus tumors are circumscribed lesions that show a predominance of glomus cells surrounding inconspicuous blood vessels. Glomangiomas are less well-circumscribed and show a more vascular architecture with prominent dilated vessels and a smaller number of glomus cells.4
We present a case of a patient with multiple glomangiomas. There are few reports of multiple glomangiomas in the literature. This case is particularly interesting in that our patient had a history of malignant melanoma, and there was a concern for skin metastases. Despite the patient’s personal history of blue lesions that predated the diagnosis of melanoma for many years, we could not exclude the possibility of cutaneous metastases without performing biopsies.
Tumors of glomus cell origin usually are benign. It has been suggested to replace the term glomangioma with glomuvenous malformations to emphasize the hamartomatous nature of these lesions.5 Glomuvenous malformations, or glomangiomas, can occur sporadically or can be inherited as a familial disorder. Inheritable glomangioma has been linked to the chromosome 1p21-22 locus and mutations in the glomulin gene, GLMN, with variable penetrance.6 Our patient did not report a family history of such lesions.
Glomangiomas typically are solitary but rarely can present as multiple lesions in fewer than 10% of cases.7 Multiple glomangiomas are classified into 3 subtypes: localized, disseminated, and congenital plaque type. Localized multiple glomangiomas present as blue nodules confined to 1 anatomic location such as the hand or arm. Disseminated glomangiomas are more widely distributed and involve more than 1 anatomic location.8 Plaque-type glomangiomas consist of numerous confluent lesions occurring either as solitary or multiple plaques.2 Clinically, glomangiomas manifest as painless to mildly painful cutaneous nodules. Compared to venous malformations, glomangiomas are less compressible under external pressure.
Histopathologically, glomangiomas appear as nonencapsulated tumors with large, irregular, prominent vessels lined by glomus cells. Glomus cells may be so sparse that the distinction from venous malformations and hemangiomas becomes difficult. Immunohistochemistry can play an important role in diagnosis. As modified smooth muscle cells, glomus cells stain positive with a-smooth muscle actin, while CD34 highlights the vascular endothelium.1The clinical differential diagnosis of multiple blue or violaceous subcutaneous nodules includes blue rubber bleb nevus syndrome, Maffucci syndrome, glomus tumor, pyogenic granuloma, hemangioma, spiradenoma, angiolipoma, leiomyoma, or hemangiopericytoma.9-12
Different treatment modalities are available for solitary glomangiomas, including surgical excision, sclerotherapy, and laser application. Treatment of multiple glomangiomas may not be feasible, and excision of isolated symptomatic lesions may be the only option; however, it is crucial to reach the correct diagnosis in these patients to avoid improper treatments and interventions.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
- Patterson JW. Weedon’s Skin Pathology. 4th ed. Edinburgh, Scotland: Churchill Livingstone Elsevier; 2016.
- Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006;142:892-896.
- Boon L, Mulliken JB, Enjolras O, et al. Glomuvenous malformation (glomangioma) and venous malformation distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140:971-976.
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132:1448-1452.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70:866-874.
- Brouillard P, Ghassibé M, Penington A, et al. Four common glomulin mutations cause two thirds of glomuvenous malformations (“familial glomangiomas”): evidence for a founder effect. J Med Genet. 2005;42:E13.
- Goodman TF, Abele DC. Multiple glomus tumors. a clinical and electron microscopic study. Arch Dermatol. 1971;103:11-23.
- Miyamoto H, Wada H. Localized multiple glomangiomas on the foot. J Dermatol. 2009;36:604-607.
- Borovaya A, Kunte C, Flaig MJ, et al. Disseminated cutaneousglomangiomas in an adolescent boy. Acta Derm Venereol. 2012;92:324-325.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16:11.
- Ertem D, Acar Y, Kotiloglu E, et al. Blue rubber bleb nevus syndrome. Pediatrics. 2001;107:418-420.
- Faik A, Allali F, El Hassani S, et al. Maffucci’s syndrome: a case report. Clin Rheumatol. 2006;25:88-91.
Practice Points
- The diagnosis of glomus tumor and glomangioma is easily suspected when the lesions are in the digital or subungual region.
- Multiple glomangiomas are rare and can clinically pose a diagnostic challenge to dermatologists.
- In patients with a recent history of malignancy, multiple glomangiomas may mimic cutaneous metastases. Therefore, multiple biopsies and histologic examination may be necessary.