User login
Bullous Pemphigoid Triggered by Liraglutide
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
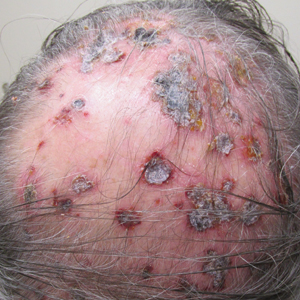
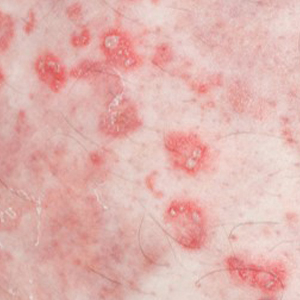
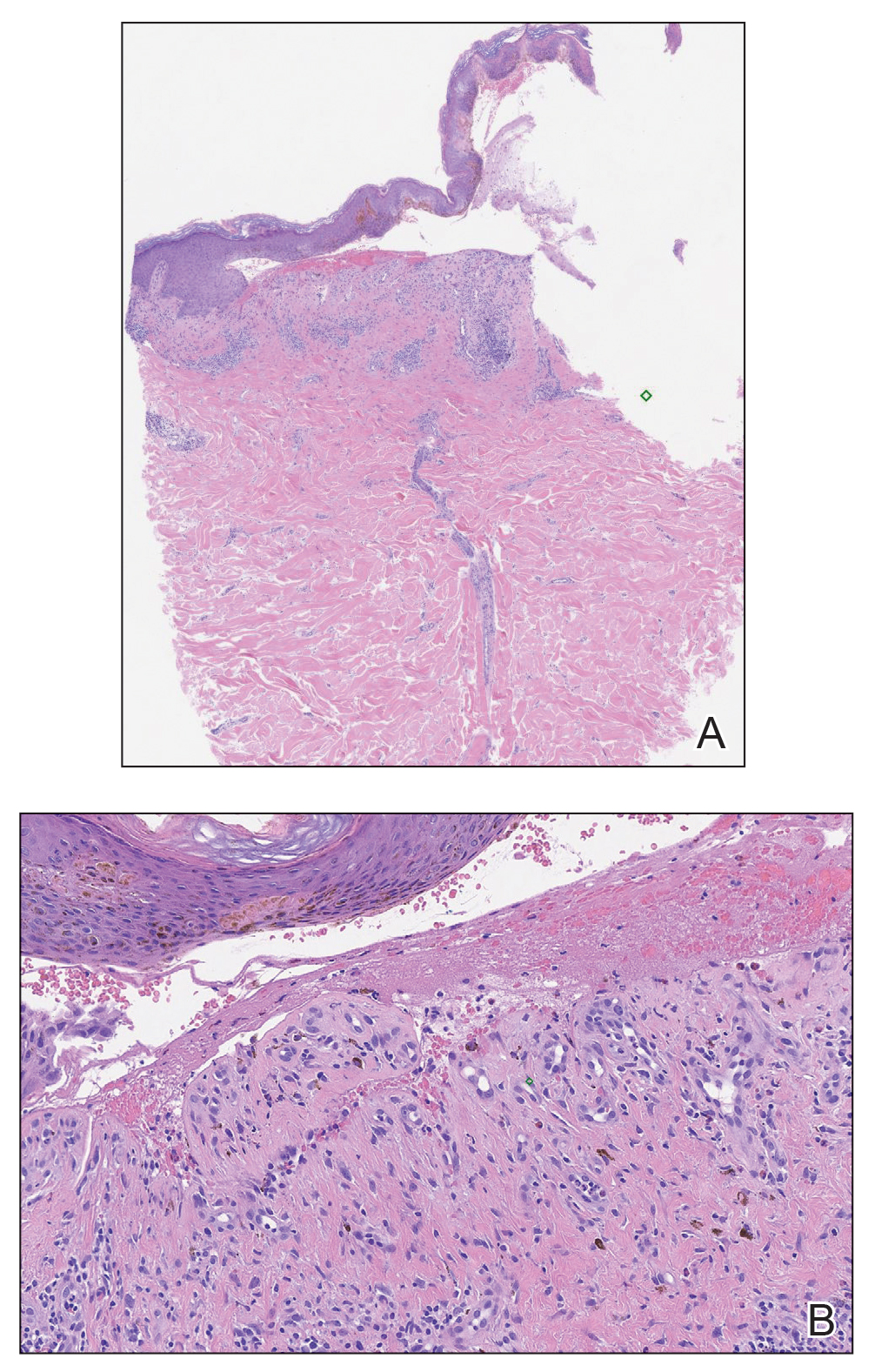
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.


Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.



Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease that typically affects the elderly, with an incidence of approximately 7 new cases per million.1 The pathogenesis of BP involves autoantibodies to BP antigens 180 and 230 at the dermoepidermal junction. Bullous pemphigoid has been associated with the use of multiple medications; vaccines; and physical damage to the skin, including trauma, radiation, and surgery.2
Several classes of medications may cause BP; one study described an association of BP with loop diuretics,3 while others found higher incidences of BP in patients taking aldosterone antagonists and neuroleptics.4 We describe a case of drug-triggered BP to liraglutide, a glucagonlike peptide 1 (GLP-1) receptor agonist.
A 75-year-old man presented to dermatology for evaluation of a vesicular eruption on the head, neck, trunk, and arms of 6 months’ duration. The eruption developed 2 weeks after starting liraglutide 1.2 mg subcutaneously daily for diabetes mellitus. The patient had a medical history of type 2 diabetes mellitus, hypertension, stroke, and prostate cancer treated with prostatectomy, and he also was taking insulin. Liraglutide was discontinued shortly after the onset of the eruption.
Physical examination revealed annular plaques on the head, neck, trunk, and arms with central hypopigmentation and hyperpigmented borders (Figure 1). Two tense bullae were evident on the left flank (Figure 2). Histopathology revealed a subepidermal blister, mixed perivascular infiltrate with numerous eosinophils, and pigment incontinence (Figure 3). Direct immunofluorescence showed linear deposition of IgG and C3 along the basement membrane zone that was localized to the roof of the blister on salt-split analysis. No microorganisms were identified on periodic acid–Schiff, Grocott-Gomori methenamine-silver, acid-fast bacilli, and Fite stains. The patient initially was treated with clobetasol ointment 0.05%, leading to marginal improvement. He declined treatment with prednisone or dapsone, and he was started on doxycycline. Seven months after stopping liraglutide and starting doxycycline, the patient had no blisters, but residual pigmentary changes remained.



Two types of BP have been described in response to medications: drug-induced BP and drug-triggered BP. Drug-induced BP presents as an acute, self-limited eruption that typically resolves after withdrawal of the offending agent. It tends to involve a younger population and may present with mucosal involvement and target lesions on the palms and soles. Direct immunofluorescence shows linear IgG and C3 deposition at the basement membrane zone. Patients tend to respond quickly to systemic corticosteroids and have low recurrence rates. Drug-triggered BP is a chronic form of BP that is caused by a medication and is not resolved with removal of the offending agent.5 Therefore, drug-triggered BP is more difficult to detect, especially in patients taking multiple medications.
Our patient represents a case of drug-triggered BP to liraglutide. Liraglutide is a GLP-1 receptor agonist that is US Food and Drug Administration approved for the treatment of type 2 diabetes mellitus. Glucagonlike peptide 1 is an incretin hormone that is secreted by the intestine during digestion. It binds to the GLP-1 receptor leading to an increase in glucose-dependent insulin secretion and a decrease in glucagon secretion.6 Glucagonlike peptide 1 agonists also affect the immune system; liraglutide has been shown to modestly improve psoriasis, reduce the number of dermal gamma delta T cells, and decrease IL-17 expression.7 Glucagonlike peptide 1 agonists also produce anti-inflammatory effects on multiple organs including the liver, brain, vasculature, kidney, and skin.8
Dipeptidyl peptidase 4 (DPP-4) inhibitors that function to inhibit the degradation of GLP-1 and other peptides also have been reported to cause BP. In several patients, the DPP-4 inhibitors vildagliptin and sitagliptin caused drug-induced BP that resolved with discontinuation of the medication.9 Dipeptidyl peptidase 4 is expressed in various organ systems including the skin, and inhibition of DPP-4 enhances eosinophil mobilization in the blood and recruitment to the skin in animal models.10
Although the pathogenesis of BP involves autoantibodies to BP antigens 180 and 230, these antibodies are not sufficient to cause disease, as antibasement antibodies have been detected in patients without clinically evident BP. These patients, however, may be more susceptible to developing medication-induced BP. Several hypotheses regarding the pathogenesis of medication-induced BP have been proposed, including immune dysregulation, molecular mimicry, and cross-reactivity to a prior sensitizing agent.5 Liraglutide and the DPP-4 inhibitors affect the immune system, supporting the hypothesis of immune dysregulation; however, the exact mechanism of how immune modulating medications such as GLP-1 agonists and DPP-4 inhibitors cause BP remains unclear.
The effects of liraglutide and the DPP-4 inhibitors on the immune system may play a role in the pathogenesis of drug-triggered BP and drug-induced BP, respectively. Additional studies of the immunomodulatory effects of GLP-1 agonists and DPP-4 inhibitors may help elucidate the pathogenesis of drug-triggered or drug-induced BP.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
- Serwin AB, Musialkowska E, Piascik M. Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol. 2014;53:E432-E437.
- Danescu S, Chiorean R, Macovei V, et al. Role of physical factors in the pathogenesis of bullous pemphigoid: case report series and a comprehensive review of the published work. J Dermatol. 2016;43:134-130.
- Lloyd-Lavery A, Chi CC, Wojnarowska F, et al. The associations between bullous pemphigoid and drug use: a UK case-control study. JAMA Dermatol. 2013;149:58-62.
- Bastuji-Garin S, Joly P, Picard-Dahan C, et al. Drugs associated with bullous pemphigoid. a case-control study. Arch Dermatol. 1996;132:272-276.
- Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol. 2014;28:1133-1140.
- Triplitt C, Solis-Herrera C. GLP-1 receptor agonists: practical considerations for clinical practice. Diabetes Educ. 2015;41(suppl 1):32S-46S.
- Buysschaert M, Baeck M, Preumont V, et al. Improvement of psoriasis during glucagon-like peptide-1 analogue therapy in type 2 diabetes is associated with decreasing dermal gammadelta T-cell number: a prospective case-series study. Br J Dermatol. 2014;171:155-161.
- Lee YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642.
- Skandalis K, Spirova M, Gaitanis G, et al Drug-induced bullous pemphigoid in diabetes mellitus patients receiving dipeptidyl peptidase-IV inhibitors plus metformin. J Eur Acad Dermatol Venereol. 2012;26:249-253.
- Forssmann U, Stoetzer C, Stephan M, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181:1120-1127.
Practice Points
- Liraglutide and dipeptidyl peptidase 4 inhibitors, medications used in the treatment of diabetes mellitus, may be linked to the development of bullous pemphigoid (BP).
- Further study of the mechanism of action of these medications may lead to improved understanding of the pathogenesis of BP.
Exuberant Lymphomatoid Papulosis of the Head and Upper Trunk
To the Editor:
Lymphomatoid papulosis (LyP) is a chronic, recurring, self-healing, primary cutaneous lymphoproliferative disorder. This disease affects patients of all ages but most commonly presents in the fifth decade with a slight male predominance.1 The estimated worldwide incidence is 1.2 to 1.9 cases per 1,000,000 individuals, and the 10-year survival rate is close to 100%.1 Clinically, LyP presents as a few to more than 100 red-brown papules or nodules, some with hemorrhagic crust or central necrosis, often occurring in crops and in various stages of evolution. They most commonly are distributed on the trunk and extremities; however, the face, scalp, and oral mucosa rarely may be involved. Each lesion may last on average 3 to 8 weeks, with residual hyperpigmentation or hypopigmentation of the skin or superficial varioliform scars. The clinical characteristic of spontaneous regression is crucial for distinguishing LyP from other forms of cutaneous lymphoma.2 The disease course is variable, lasting anywhere from a few months to decades. Histopathologically, LyP consists of a frequently CD30+ lymphocytic proliferation in multiple described patterns.1 We report a case of LyP in a patient who initially presented with pink edematous papules and vesicles that progressed to crusted ulcerations, nodules, and deep necrotic eschars on the scalp, neck, and upper trunk. Multiple biopsies and T-cell gene rearrangement studies were necessary to make the diagnosis.
A 73-year-old man presented with edematous crusted papules and nodules as well as scarring with serous drainage on the scalp and upper trunk of several months’ duration. He also reported pain and pruritus. He had a medical history of B-cell CD20− chronic lymphocytic leukemia (CLL) that was treated with fludarabine, cyclophosphamide, rituximab, and intravenous immunoglobulin approximately one year prior and currently was in remission; prostate cancer treated with prostatectomy; hypertension; and type 2 diabetes mellitus. His medications included metoprolol, valsartan, and glipizide.
Histopathology revealed a hypersensitivity reaction, and the clinicopathologic correlation was believed to represent an exuberant arthropod bite reaction in the setting of CLL. The eruption responded well to oral prednisone and topical corticosteroids but recurred when the medications were withdrawn. A repeat biopsy resulted in a diagnosis of atypical eosinophil-predominant Sweet syndrome. The condition resolved.
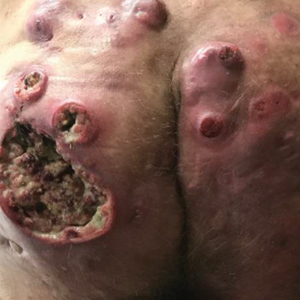
Three years later he developed multiple honey-crusted, superficial ulcers as well as serous, fluid-filled vesiculobullae on the head. A tissue culture revealed Proteus mirabilis, Staphylococcus aureus, and Enterococcus faecalis, and was negative for acid-fast bacteria and fungus. Biopsy of these lesions revealed dermal ulceration with a mixed inflammatory infiltrate and numerous eosinophils as well as a few clustered CD30+ cells; direct immunofluorescence was negative. An extensive laboratory workup including bullous pemphigoid antigens, C-reactive protein, antinuclear antibodies comprehensive profile, antineutrophil cytoplasmic antibodies, rheumatoid factor, anticyclic citrullinated peptide antibodies, serum protein electrophoresis, lactate dehydrogenase, complete blood cell count with differential, complete metabolic profile, thyroid-stimulating hormone, uric acid, C3, C4, immunoglobulin profile, angiotensin-converting enzyme level, and urinalysis was unremarkable. He improved with courses of minocycline, prednisone, and topical clobetasol, but he had periodic and progressive flares over several months with punched-out crusted ulcerations developing on the scalp (Figure 1A) and neck (Figure 1B). The oral and ocular mucosae were uninvolved, but the nasal mucosa had some involvement.
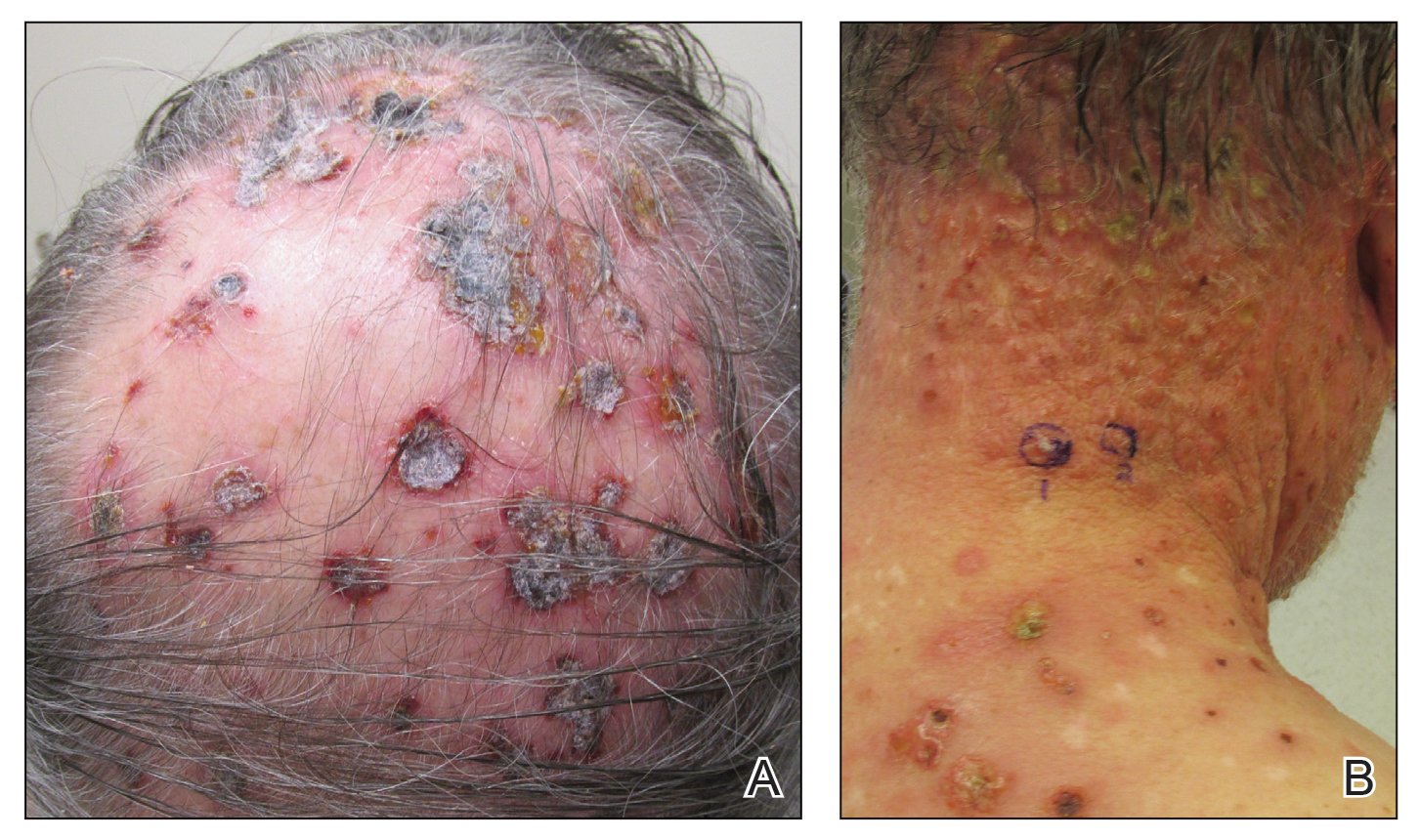
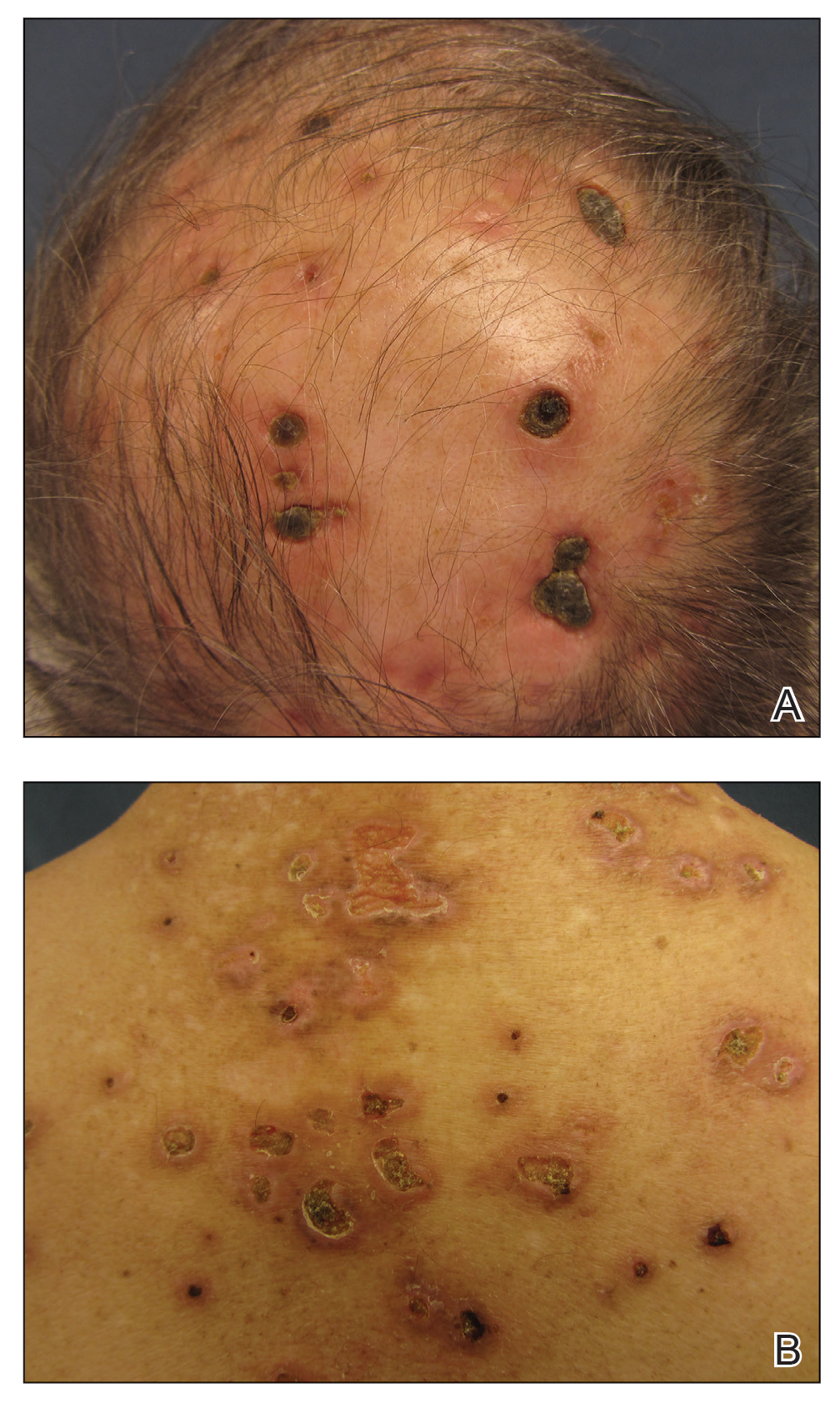
A repeat biopsy demonstrated an atypical CD30+ lymphoid infiltrate favoring LyP. T-cell clonality performed on this specimen and the prior biopsy demonstrated identical T-cell receptor β and γ clones. CD3, CD5, CD7, and CD4 immunostains highlighted the perivascular, perifollicular, and folliculotropic lymphocytic infiltrate. CD8 highlighted occasional background small T cells with only a few folliculotropic forms. A CD30 study revealed several scattered enlarged lymphocytes, and CD20 displayed a few dispersed B cells. A repeat perilesional direct immunofluorescence study was again negative. With treatment, he later formed multiple dry punched-out ulcers with dark eschars on the scalp, posterior neck, and upper back. There were multiple scars on the head, chest, and back, and no vesicles or bullae were present (Figure 2). The patient was presented at a meeting of the Philadelphia Dermatological Society and a consensus diagnosis of LyP was reached. The patient has continued to improve with oral minocycline 100 mg twice daily, topical clobetasol, and topical mupirocin.
Lymphomatoid papulosis is an indolent cutaneous lymphoma; however, it is associated with the potential development of a second hematologic malignancy, with some disagreement in the literature concerning the exact percentage.3 In some studies, lymphoma has been estimated to occur in less than 20% of cases.4,5 Wieser et al1 reported a retrospective analysis of 180 patients with LyP that revealed a secondary malignancy in 52% of patients. They also reported that the number of lesions and the symptom severity were not associated with lymphoma development.1 Similarly, Cordel et al6 reported a diagnosis of lymphoma in 41% of 106 patients. These analyses reveal that the association with lymphoma may be higher than previously thought, but referral bias may be a confounding factor in these numbers.1,5,6 Associated malignancies may occur prior to, concomitantly, or years after the diagnosis of LyP. The most frequently reported malignancies include mycosis fungoides, Hodgkin lymphoma, and primary cutaneous anaplastic large cell lymphoma.1,4
Nicolaou et al3 indicated that head involvement was more likely associated with lymphoma. Our patient had a history of CLL prior to the development of LyP, and it continues to be in remission. The incidence of CLL in patients with LyP is reported to be 0.8%.4 Our patient had an exuberant case of LyP predominantly involving the head, neck, and upper torso, which is an unusual distribution. Vesiculobullous lesions also are uncharacteristic of LyP and may have represented concomitant bullous impetigo, but bullous variants of LyP also have been reported.7 Due to the unique distribution and characteristic scarring, Brunsting-Perry cicatricial pemphigoid also was considered in the clinical differential diagnosis.
The pathogenesis of LyP associated with malignancy is not definitively known. Theories propose that progression to a malignant clonal T-cell population may come from cytogenetic events, inadequate host response, or persistent antigenic or viral stimulation.4 Studies have demonstrated overlapping T-cell receptor gene rearrangement clones in lesions in patients with both LyP and mycosis fungoides, suggesting a common origin between the diseases.8 Other theories suggest that LyP may arise from an early, reactive, polyclonal lymphoid expansion that evolves into a clonal neoplastic process.4 Interestingly, LyP is a clonal T-cell disorder, while Hodgkin lymphoma and CLL are B-cell disorders. Thus, reports of CLL occurring with LyP, as in our patient, may support the theory that LyP arises from an early stem-cell or precursor-cell defect.4
There is no cure for LyP and data regarding the potential of aggressive therapy on the prevention of secondary lymphomas is lacking. Wieser et al1 reported that treatment did not prevent the progression to lymphoma in their retrospective analysis of 180 patients. The number of lesions, frequency of outbreaks, and extent of the scarring can dictate the treatment approach for LyP. Conservative topical therapies include corticosteroids, bexarotene, and imiquimod. Mupirocin may help to prevent infection of ulcerated lesions.1,2 Low-dose methotrexate has been shown to be the most efficacious treatment in reducing the number of lesions, particularly for scarring or cosmetically sensitive areas. Oral methotrexate at a dosage of 10 mg to 25 mg weekly tapered to the lowest effective dose may suppress outbreaks of LyP lesions.1,2 Other therapies include psoralen plus UVA, UVB, interferon alfa-2a, oral bexarotene, oral acyclovir or valacyclovir, etretinate, mycophenolic acid, photodynamic therapy, oral antibiotics, excision, and radiotherapy.1,2 Systemic chemotherapy and total-skin electron beam therapy have shown efficacy in clearing the lesions; however, the disease recurs after discontinuation of therapy.2 Systemic chemotherapy is not recommended for the treatment of LyP, as risks outweigh the benefits and it does not reduce the risk for developing lymphoma.1 The prognosis generally is good, though long-term follow-up is imperative to monitor for the development of other lymphomas.
Our patient presented with LyP a few months after completing chemotherapy for his CLL. It is unknown if he developed LyP just before the time of presentation, or if he may have developed it at the same time as his CLL by a common inciting event. In the latter case, it is speculative that the LyP may have been controlled by chemotherapy for his CLL, only to become clinically apparent after discontinuation, then naturally remit for a longer period. Case reports such as ours with unusual clinical presentations, B-cell lymphoma associations, and unique timing of lymphoma onset may help to provide insight into the pathogenesis of this disease.
We highlighted an unusual case of LyP that presented clinically with crusted ulcerations as well as vesiculobullous and edematous papules that progressed into deep punched-out ulcers with eschars, nodules, and scarring on the head and upper trunk. Lymphomatoid papulosis can be difficult to diagnose histopathologically at the early stages, and multiple repeat biopsies may be necessary to confirm the diagnosis. T-cell gene rearrangement and immunohistochemistry studies are helpful along with clinical correlation to establish a diagnosis in these cases. We recommend that physicians keep LyP on the differential diagnosis for patients with similar clinical presentations and remain vigilant in monitoring for the development of secondary lymphoma.
- Wieser I, Oh C, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67.
- Duvic M. CD30+ neoplasms of the skin. Curr Hematol Malig Rep. 2011;6:245-250.
- Nicolaou V, Papadavid E, Ekonomise A, et al. Association of clinicopathological characteristics with secondary neoplastic lymphoproliferative disorders in patients with lymphomatoid papulosis. Leuk Lymphoma. 2015;56:1303-1307.
- Ahn C, Orscheln C, Huang W. Lymphomatoid papulosis as a harbinger of chronic lymphocytic leukemia. Ann Hematol. 2014;93:1923-1925.
- Kunishige J, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-5781.
- Cordelet al. Frequency and risk factors for associated lymphomas in patients with lymphomatoid papulosis. Oncologist. 2016;21:76-83.
- Sureda N, Thomas L, Bathelier E, et al. Bullous lymphomatoid papulosis. Clin Exp Dermatol. 2011;36:800-801.
- de la Garza Bravo M, Patel KP, Loghavi S, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol. 2015;46:558-569.
To the Editor:
Lymphomatoid papulosis (LyP) is a chronic, recurring, self-healing, primary cutaneous lymphoproliferative disorder. This disease affects patients of all ages but most commonly presents in the fifth decade with a slight male predominance.1 The estimated worldwide incidence is 1.2 to 1.9 cases per 1,000,000 individuals, and the 10-year survival rate is close to 100%.1 Clinically, LyP presents as a few to more than 100 red-brown papules or nodules, some with hemorrhagic crust or central necrosis, often occurring in crops and in various stages of evolution. They most commonly are distributed on the trunk and extremities; however, the face, scalp, and oral mucosa rarely may be involved. Each lesion may last on average 3 to 8 weeks, with residual hyperpigmentation or hypopigmentation of the skin or superficial varioliform scars. The clinical characteristic of spontaneous regression is crucial for distinguishing LyP from other forms of cutaneous lymphoma.2 The disease course is variable, lasting anywhere from a few months to decades. Histopathologically, LyP consists of a frequently CD30+ lymphocytic proliferation in multiple described patterns.1 We report a case of LyP in a patient who initially presented with pink edematous papules and vesicles that progressed to crusted ulcerations, nodules, and deep necrotic eschars on the scalp, neck, and upper trunk. Multiple biopsies and T-cell gene rearrangement studies were necessary to make the diagnosis.
A 73-year-old man presented with edematous crusted papules and nodules as well as scarring with serous drainage on the scalp and upper trunk of several months’ duration. He also reported pain and pruritus. He had a medical history of B-cell CD20− chronic lymphocytic leukemia (CLL) that was treated with fludarabine, cyclophosphamide, rituximab, and intravenous immunoglobulin approximately one year prior and currently was in remission; prostate cancer treated with prostatectomy; hypertension; and type 2 diabetes mellitus. His medications included metoprolol, valsartan, and glipizide.
Histopathology revealed a hypersensitivity reaction, and the clinicopathologic correlation was believed to represent an exuberant arthropod bite reaction in the setting of CLL. The eruption responded well to oral prednisone and topical corticosteroids but recurred when the medications were withdrawn. A repeat biopsy resulted in a diagnosis of atypical eosinophil-predominant Sweet syndrome. The condition resolved.
Three years later he developed multiple honey-crusted, superficial ulcers as well as serous, fluid-filled vesiculobullae on the head. A tissue culture revealed Proteus mirabilis, Staphylococcus aureus, and Enterococcus faecalis, and was negative for acid-fast bacteria and fungus. Biopsy of these lesions revealed dermal ulceration with a mixed inflammatory infiltrate and numerous eosinophils as well as a few clustered CD30+ cells; direct immunofluorescence was negative. An extensive laboratory workup including bullous pemphigoid antigens, C-reactive protein, antinuclear antibodies comprehensive profile, antineutrophil cytoplasmic antibodies, rheumatoid factor, anticyclic citrullinated peptide antibodies, serum protein electrophoresis, lactate dehydrogenase, complete blood cell count with differential, complete metabolic profile, thyroid-stimulating hormone, uric acid, C3, C4, immunoglobulin profile, angiotensin-converting enzyme level, and urinalysis was unremarkable. He improved with courses of minocycline, prednisone, and topical clobetasol, but he had periodic and progressive flares over several months with punched-out crusted ulcerations developing on the scalp (Figure 1A) and neck (Figure 1B). The oral and ocular mucosae were uninvolved, but the nasal mucosa had some involvement.

A repeat biopsy demonstrated an atypical CD30+ lymphoid infiltrate favoring LyP. T-cell clonality performed on this specimen and the prior biopsy demonstrated identical T-cell receptor β and γ clones. CD3, CD5, CD7, and CD4 immunostains highlighted the perivascular, perifollicular, and folliculotropic lymphocytic infiltrate. CD8 highlighted occasional background small T cells with only a few folliculotropic forms. A CD30 study revealed several scattered enlarged lymphocytes, and CD20 displayed a few dispersed B cells. A repeat perilesional direct immunofluorescence study was again negative. With treatment, he later formed multiple dry punched-out ulcers with dark eschars on the scalp, posterior neck, and upper back. There were multiple scars on the head, chest, and back, and no vesicles or bullae were present (Figure 2). The patient was presented at a meeting of the Philadelphia Dermatological Society and a consensus diagnosis of LyP was reached. The patient has continued to improve with oral minocycline 100 mg twice daily, topical clobetasol, and topical mupirocin.

Lymphomatoid papulosis is an indolent cutaneous lymphoma; however, it is associated with the potential development of a second hematologic malignancy, with some disagreement in the literature concerning the exact percentage.3 In some studies, lymphoma has been estimated to occur in less than 20% of cases.4,5 Wieser et al1 reported a retrospective analysis of 180 patients with LyP that revealed a secondary malignancy in 52% of patients. They also reported that the number of lesions and the symptom severity were not associated with lymphoma development.1 Similarly, Cordel et al6 reported a diagnosis of lymphoma in 41% of 106 patients. These analyses reveal that the association with lymphoma may be higher than previously thought, but referral bias may be a confounding factor in these numbers.1,5,6 Associated malignancies may occur prior to, concomitantly, or years after the diagnosis of LyP. The most frequently reported malignancies include mycosis fungoides, Hodgkin lymphoma, and primary cutaneous anaplastic large cell lymphoma.1,4
Nicolaou et al3 indicated that head involvement was more likely associated with lymphoma. Our patient had a history of CLL prior to the development of LyP, and it continues to be in remission. The incidence of CLL in patients with LyP is reported to be 0.8%.4 Our patient had an exuberant case of LyP predominantly involving the head, neck, and upper torso, which is an unusual distribution. Vesiculobullous lesions also are uncharacteristic of LyP and may have represented concomitant bullous impetigo, but bullous variants of LyP also have been reported.7 Due to the unique distribution and characteristic scarring, Brunsting-Perry cicatricial pemphigoid also was considered in the clinical differential diagnosis.
The pathogenesis of LyP associated with malignancy is not definitively known. Theories propose that progression to a malignant clonal T-cell population may come from cytogenetic events, inadequate host response, or persistent antigenic or viral stimulation.4 Studies have demonstrated overlapping T-cell receptor gene rearrangement clones in lesions in patients with both LyP and mycosis fungoides, suggesting a common origin between the diseases.8 Other theories suggest that LyP may arise from an early, reactive, polyclonal lymphoid expansion that evolves into a clonal neoplastic process.4 Interestingly, LyP is a clonal T-cell disorder, while Hodgkin lymphoma and CLL are B-cell disorders. Thus, reports of CLL occurring with LyP, as in our patient, may support the theory that LyP arises from an early stem-cell or precursor-cell defect.4
There is no cure for LyP and data regarding the potential of aggressive therapy on the prevention of secondary lymphomas is lacking. Wieser et al1 reported that treatment did not prevent the progression to lymphoma in their retrospective analysis of 180 patients. The number of lesions, frequency of outbreaks, and extent of the scarring can dictate the treatment approach for LyP. Conservative topical therapies include corticosteroids, bexarotene, and imiquimod. Mupirocin may help to prevent infection of ulcerated lesions.1,2 Low-dose methotrexate has been shown to be the most efficacious treatment in reducing the number of lesions, particularly for scarring or cosmetically sensitive areas. Oral methotrexate at a dosage of 10 mg to 25 mg weekly tapered to the lowest effective dose may suppress outbreaks of LyP lesions.1,2 Other therapies include psoralen plus UVA, UVB, interferon alfa-2a, oral bexarotene, oral acyclovir or valacyclovir, etretinate, mycophenolic acid, photodynamic therapy, oral antibiotics, excision, and radiotherapy.1,2 Systemic chemotherapy and total-skin electron beam therapy have shown efficacy in clearing the lesions; however, the disease recurs after discontinuation of therapy.2 Systemic chemotherapy is not recommended for the treatment of LyP, as risks outweigh the benefits and it does not reduce the risk for developing lymphoma.1 The prognosis generally is good, though long-term follow-up is imperative to monitor for the development of other lymphomas.
Our patient presented with LyP a few months after completing chemotherapy for his CLL. It is unknown if he developed LyP just before the time of presentation, or if he may have developed it at the same time as his CLL by a common inciting event. In the latter case, it is speculative that the LyP may have been controlled by chemotherapy for his CLL, only to become clinically apparent after discontinuation, then naturally remit for a longer period. Case reports such as ours with unusual clinical presentations, B-cell lymphoma associations, and unique timing of lymphoma onset may help to provide insight into the pathogenesis of this disease.
We highlighted an unusual case of LyP that presented clinically with crusted ulcerations as well as vesiculobullous and edematous papules that progressed into deep punched-out ulcers with eschars, nodules, and scarring on the head and upper trunk. Lymphomatoid papulosis can be difficult to diagnose histopathologically at the early stages, and multiple repeat biopsies may be necessary to confirm the diagnosis. T-cell gene rearrangement and immunohistochemistry studies are helpful along with clinical correlation to establish a diagnosis in these cases. We recommend that physicians keep LyP on the differential diagnosis for patients with similar clinical presentations and remain vigilant in monitoring for the development of secondary lymphoma.
To the Editor:
Lymphomatoid papulosis (LyP) is a chronic, recurring, self-healing, primary cutaneous lymphoproliferative disorder. This disease affects patients of all ages but most commonly presents in the fifth decade with a slight male predominance.1 The estimated worldwide incidence is 1.2 to 1.9 cases per 1,000,000 individuals, and the 10-year survival rate is close to 100%.1 Clinically, LyP presents as a few to more than 100 red-brown papules or nodules, some with hemorrhagic crust or central necrosis, often occurring in crops and in various stages of evolution. They most commonly are distributed on the trunk and extremities; however, the face, scalp, and oral mucosa rarely may be involved. Each lesion may last on average 3 to 8 weeks, with residual hyperpigmentation or hypopigmentation of the skin or superficial varioliform scars. The clinical characteristic of spontaneous regression is crucial for distinguishing LyP from other forms of cutaneous lymphoma.2 The disease course is variable, lasting anywhere from a few months to decades. Histopathologically, LyP consists of a frequently CD30+ lymphocytic proliferation in multiple described patterns.1 We report a case of LyP in a patient who initially presented with pink edematous papules and vesicles that progressed to crusted ulcerations, nodules, and deep necrotic eschars on the scalp, neck, and upper trunk. Multiple biopsies and T-cell gene rearrangement studies were necessary to make the diagnosis.
A 73-year-old man presented with edematous crusted papules and nodules as well as scarring with serous drainage on the scalp and upper trunk of several months’ duration. He also reported pain and pruritus. He had a medical history of B-cell CD20− chronic lymphocytic leukemia (CLL) that was treated with fludarabine, cyclophosphamide, rituximab, and intravenous immunoglobulin approximately one year prior and currently was in remission; prostate cancer treated with prostatectomy; hypertension; and type 2 diabetes mellitus. His medications included metoprolol, valsartan, and glipizide.
Histopathology revealed a hypersensitivity reaction, and the clinicopathologic correlation was believed to represent an exuberant arthropod bite reaction in the setting of CLL. The eruption responded well to oral prednisone and topical corticosteroids but recurred when the medications were withdrawn. A repeat biopsy resulted in a diagnosis of atypical eosinophil-predominant Sweet syndrome. The condition resolved.
Three years later he developed multiple honey-crusted, superficial ulcers as well as serous, fluid-filled vesiculobullae on the head. A tissue culture revealed Proteus mirabilis, Staphylococcus aureus, and Enterococcus faecalis, and was negative for acid-fast bacteria and fungus. Biopsy of these lesions revealed dermal ulceration with a mixed inflammatory infiltrate and numerous eosinophils as well as a few clustered CD30+ cells; direct immunofluorescence was negative. An extensive laboratory workup including bullous pemphigoid antigens, C-reactive protein, antinuclear antibodies comprehensive profile, antineutrophil cytoplasmic antibodies, rheumatoid factor, anticyclic citrullinated peptide antibodies, serum protein electrophoresis, lactate dehydrogenase, complete blood cell count with differential, complete metabolic profile, thyroid-stimulating hormone, uric acid, C3, C4, immunoglobulin profile, angiotensin-converting enzyme level, and urinalysis was unremarkable. He improved with courses of minocycline, prednisone, and topical clobetasol, but he had periodic and progressive flares over several months with punched-out crusted ulcerations developing on the scalp (Figure 1A) and neck (Figure 1B). The oral and ocular mucosae were uninvolved, but the nasal mucosa had some involvement.

A repeat biopsy demonstrated an atypical CD30+ lymphoid infiltrate favoring LyP. T-cell clonality performed on this specimen and the prior biopsy demonstrated identical T-cell receptor β and γ clones. CD3, CD5, CD7, and CD4 immunostains highlighted the perivascular, perifollicular, and folliculotropic lymphocytic infiltrate. CD8 highlighted occasional background small T cells with only a few folliculotropic forms. A CD30 study revealed several scattered enlarged lymphocytes, and CD20 displayed a few dispersed B cells. A repeat perilesional direct immunofluorescence study was again negative. With treatment, he later formed multiple dry punched-out ulcers with dark eschars on the scalp, posterior neck, and upper back. There were multiple scars on the head, chest, and back, and no vesicles or bullae were present (Figure 2). The patient was presented at a meeting of the Philadelphia Dermatological Society and a consensus diagnosis of LyP was reached. The patient has continued to improve with oral minocycline 100 mg twice daily, topical clobetasol, and topical mupirocin.

Lymphomatoid papulosis is an indolent cutaneous lymphoma; however, it is associated with the potential development of a second hematologic malignancy, with some disagreement in the literature concerning the exact percentage.3 In some studies, lymphoma has been estimated to occur in less than 20% of cases.4,5 Wieser et al1 reported a retrospective analysis of 180 patients with LyP that revealed a secondary malignancy in 52% of patients. They also reported that the number of lesions and the symptom severity were not associated with lymphoma development.1 Similarly, Cordel et al6 reported a diagnosis of lymphoma in 41% of 106 patients. These analyses reveal that the association with lymphoma may be higher than previously thought, but referral bias may be a confounding factor in these numbers.1,5,6 Associated malignancies may occur prior to, concomitantly, or years after the diagnosis of LyP. The most frequently reported malignancies include mycosis fungoides, Hodgkin lymphoma, and primary cutaneous anaplastic large cell lymphoma.1,4
Nicolaou et al3 indicated that head involvement was more likely associated with lymphoma. Our patient had a history of CLL prior to the development of LyP, and it continues to be in remission. The incidence of CLL in patients with LyP is reported to be 0.8%.4 Our patient had an exuberant case of LyP predominantly involving the head, neck, and upper torso, which is an unusual distribution. Vesiculobullous lesions also are uncharacteristic of LyP and may have represented concomitant bullous impetigo, but bullous variants of LyP also have been reported.7 Due to the unique distribution and characteristic scarring, Brunsting-Perry cicatricial pemphigoid also was considered in the clinical differential diagnosis.
The pathogenesis of LyP associated with malignancy is not definitively known. Theories propose that progression to a malignant clonal T-cell population may come from cytogenetic events, inadequate host response, or persistent antigenic or viral stimulation.4 Studies have demonstrated overlapping T-cell receptor gene rearrangement clones in lesions in patients with both LyP and mycosis fungoides, suggesting a common origin between the diseases.8 Other theories suggest that LyP may arise from an early, reactive, polyclonal lymphoid expansion that evolves into a clonal neoplastic process.4 Interestingly, LyP is a clonal T-cell disorder, while Hodgkin lymphoma and CLL are B-cell disorders. Thus, reports of CLL occurring with LyP, as in our patient, may support the theory that LyP arises from an early stem-cell or precursor-cell defect.4
There is no cure for LyP and data regarding the potential of aggressive therapy on the prevention of secondary lymphomas is lacking. Wieser et al1 reported that treatment did not prevent the progression to lymphoma in their retrospective analysis of 180 patients. The number of lesions, frequency of outbreaks, and extent of the scarring can dictate the treatment approach for LyP. Conservative topical therapies include corticosteroids, bexarotene, and imiquimod. Mupirocin may help to prevent infection of ulcerated lesions.1,2 Low-dose methotrexate has been shown to be the most efficacious treatment in reducing the number of lesions, particularly for scarring or cosmetically sensitive areas. Oral methotrexate at a dosage of 10 mg to 25 mg weekly tapered to the lowest effective dose may suppress outbreaks of LyP lesions.1,2 Other therapies include psoralen plus UVA, UVB, interferon alfa-2a, oral bexarotene, oral acyclovir or valacyclovir, etretinate, mycophenolic acid, photodynamic therapy, oral antibiotics, excision, and radiotherapy.1,2 Systemic chemotherapy and total-skin electron beam therapy have shown efficacy in clearing the lesions; however, the disease recurs after discontinuation of therapy.2 Systemic chemotherapy is not recommended for the treatment of LyP, as risks outweigh the benefits and it does not reduce the risk for developing lymphoma.1 The prognosis generally is good, though long-term follow-up is imperative to monitor for the development of other lymphomas.
Our patient presented with LyP a few months after completing chemotherapy for his CLL. It is unknown if he developed LyP just before the time of presentation, or if he may have developed it at the same time as his CLL by a common inciting event. In the latter case, it is speculative that the LyP may have been controlled by chemotherapy for his CLL, only to become clinically apparent after discontinuation, then naturally remit for a longer period. Case reports such as ours with unusual clinical presentations, B-cell lymphoma associations, and unique timing of lymphoma onset may help to provide insight into the pathogenesis of this disease.
We highlighted an unusual case of LyP that presented clinically with crusted ulcerations as well as vesiculobullous and edematous papules that progressed into deep punched-out ulcers with eschars, nodules, and scarring on the head and upper trunk. Lymphomatoid papulosis can be difficult to diagnose histopathologically at the early stages, and multiple repeat biopsies may be necessary to confirm the diagnosis. T-cell gene rearrangement and immunohistochemistry studies are helpful along with clinical correlation to establish a diagnosis in these cases. We recommend that physicians keep LyP on the differential diagnosis for patients with similar clinical presentations and remain vigilant in monitoring for the development of secondary lymphoma.
- Wieser I, Oh C, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67.
- Duvic M. CD30+ neoplasms of the skin. Curr Hematol Malig Rep. 2011;6:245-250.
- Nicolaou V, Papadavid E, Ekonomise A, et al. Association of clinicopathological characteristics with secondary neoplastic lymphoproliferative disorders in patients with lymphomatoid papulosis. Leuk Lymphoma. 2015;56:1303-1307.
- Ahn C, Orscheln C, Huang W. Lymphomatoid papulosis as a harbinger of chronic lymphocytic leukemia. Ann Hematol. 2014;93:1923-1925.
- Kunishige J, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-5781.
- Cordelet al. Frequency and risk factors for associated lymphomas in patients with lymphomatoid papulosis. Oncologist. 2016;21:76-83.
- Sureda N, Thomas L, Bathelier E, et al. Bullous lymphomatoid papulosis. Clin Exp Dermatol. 2011;36:800-801.
- de la Garza Bravo M, Patel KP, Loghavi S, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol. 2015;46:558-569.
- Wieser I, Oh C, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67.
- Duvic M. CD30+ neoplasms of the skin. Curr Hematol Malig Rep. 2011;6:245-250.
- Nicolaou V, Papadavid E, Ekonomise A, et al. Association of clinicopathological characteristics with secondary neoplastic lymphoproliferative disorders in patients with lymphomatoid papulosis. Leuk Lymphoma. 2015;56:1303-1307.
- Ahn C, Orscheln C, Huang W. Lymphomatoid papulosis as a harbinger of chronic lymphocytic leukemia. Ann Hematol. 2014;93:1923-1925.
- Kunishige J, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-5781.
- Cordelet al. Frequency and risk factors for associated lymphomas in patients with lymphomatoid papulosis. Oncologist. 2016;21:76-83.
- Sureda N, Thomas L, Bathelier E, et al. Bullous lymphomatoid papulosis. Clin Exp Dermatol. 2011;36:800-801.
- de la Garza Bravo M, Patel KP, Loghavi S, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol. 2015;46:558-569.
Practice Points
- Lymphomatoid papulosis (LyP) is a chronic, recurring, self-healing, primary cutaneous lymphoproliferative disorder characterized by red-brown papules or nodules, some with hemorrhagic crust or central necrosis, often occurring in crops and in various stages of evolution.
- Histopathologically, LyP consists of a frequently CD30Mathematical Pi LT Std+ lymphocytic proliferation in multiple described patterns.
- Lymphomatoid papulosis is an indolent cutaneous lymphoma; however, it is associated with the potential development of a second hematologic malignancy.
Home Treatment of Presumed Melanocytic Nevus With Frankincense
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
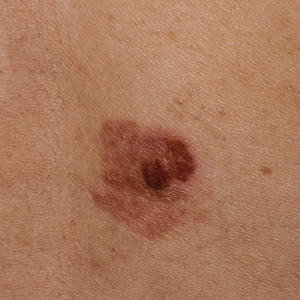
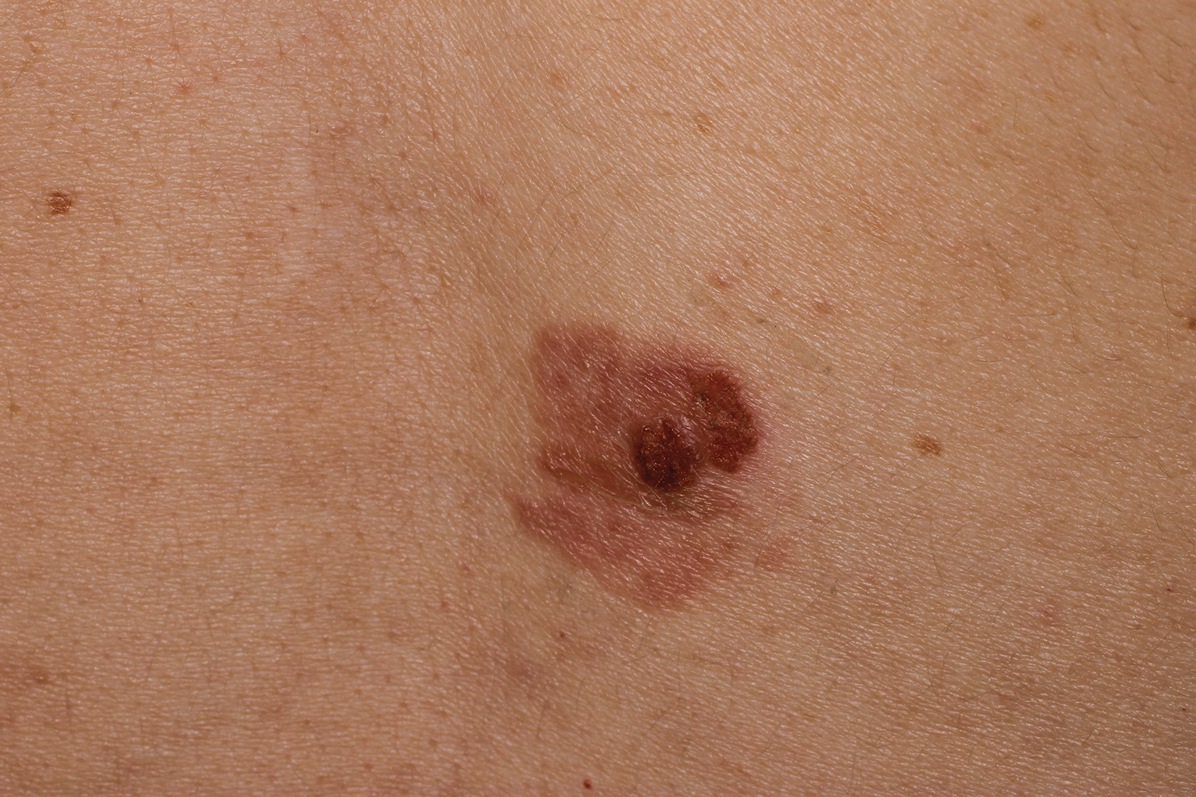
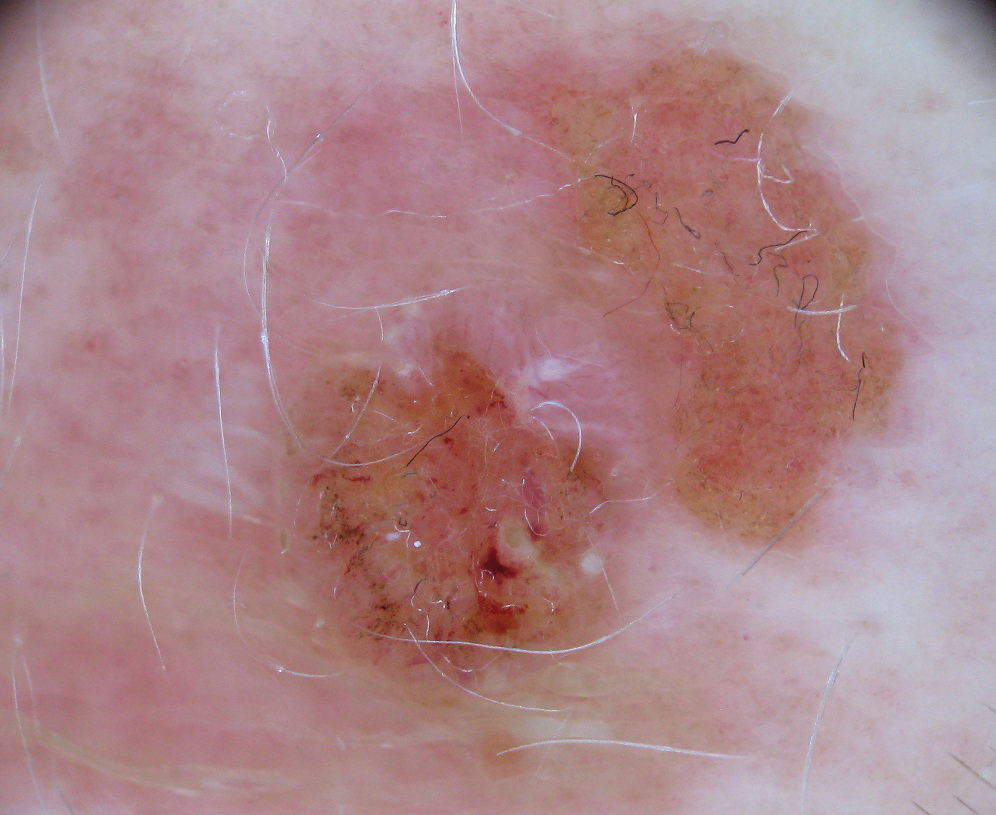
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.


Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.


Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
Practice Points
- Many patients seek natural methods of home mole removal online, including topical application of essential oils such as frankincense.
- These agents often are unregulated and can be potentially harmful when used without appropriate supervision.
- Dermatologists should be aware of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
Fatal Case of Levamisole-Induced Vasculopathy in a Cocaine User
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
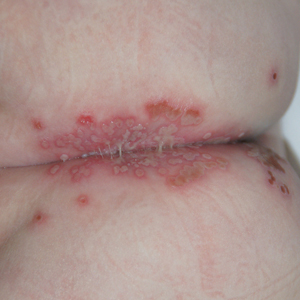
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.
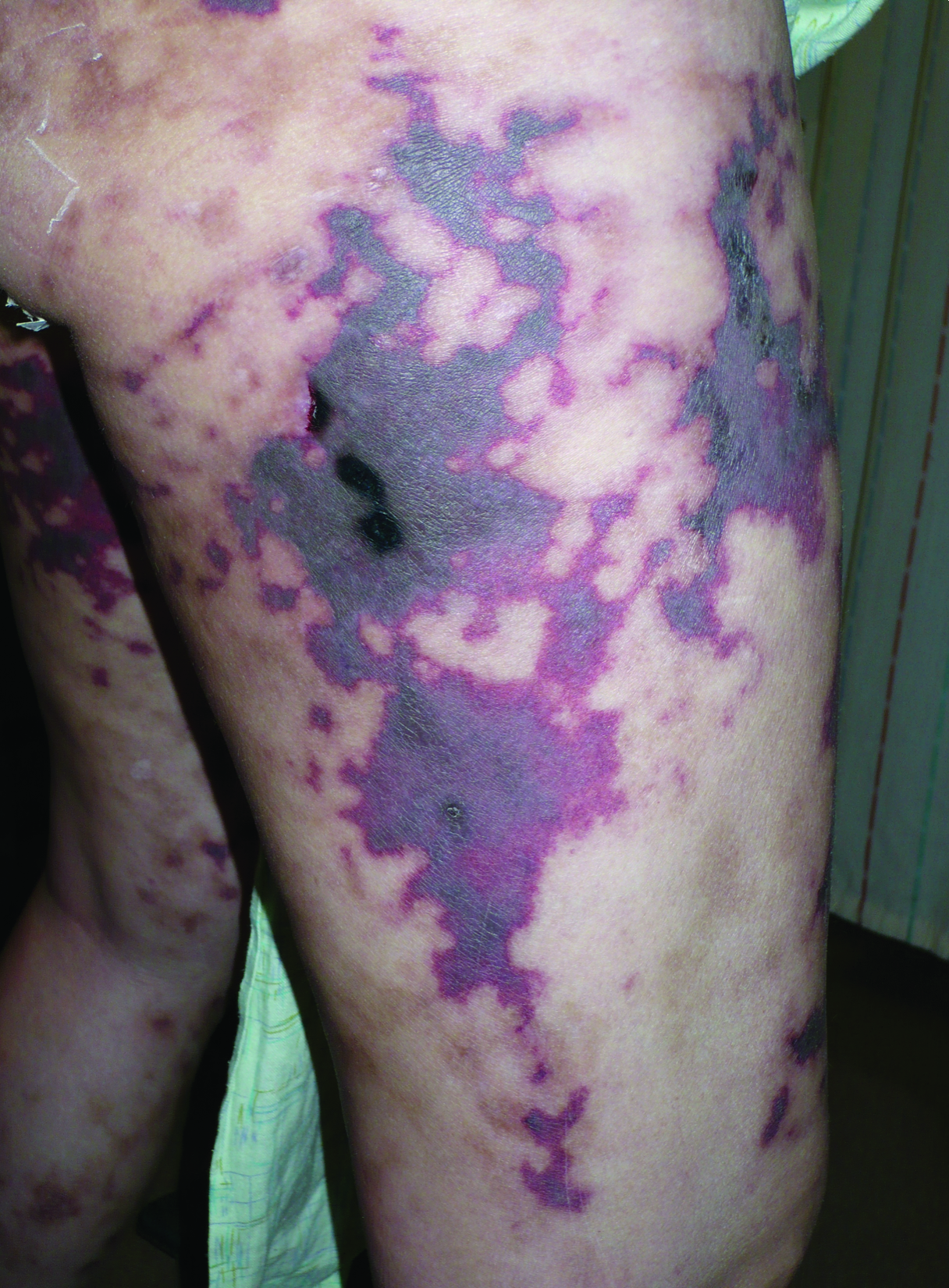
Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.

Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
Practice Points
- Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae.
- Presentation of a purpuric vasculitis, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Early recognition and discontinuation of the offending agent could be lifesaving.
Genital Primary Herpetic Infection With Concurrent Hepatitis in an Infant
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.

A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
Practice Points
- Parents with a history of herpes simplex virus (HSV) need to be educated before the baby is born to be careful about direct skin contact with the child to prevent the spread of HSV infection.
- Although systemic involvement is not typical, additional tests to rule out internal organ involvement may be required, especially in children.
Erythema Multiforme–like Dermatitis Due to Isoniazid Hypersensitivity in a Patient With Psoriasis
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
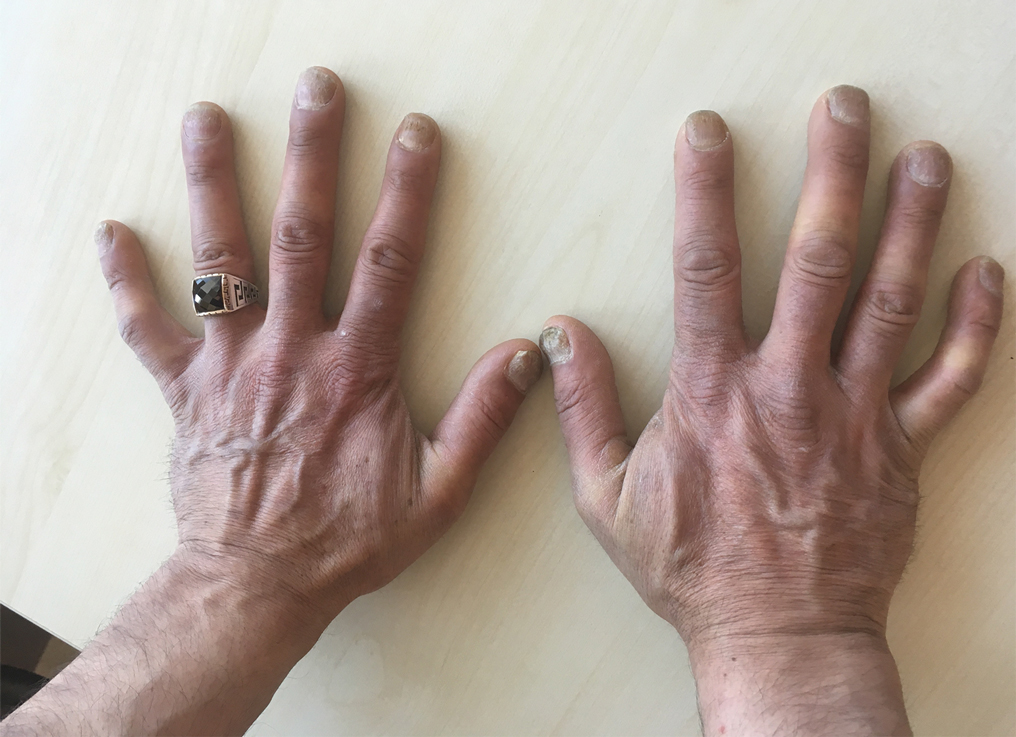
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.




This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.




This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
Practice Points
- Hypersensitivity skin reactions to antituberculosis (TB) drugs are on the rise due to the increasing use of anti–tumor necrosis factor α. Isoniazid (INH) use will be more prevalent than in the past for the treatment of latent TB.
- Even though the skin-restricted adverse events to INH are rare and minor, particular attention should be paid to patients with dermatologic diseases such as psoriasis.
Tinea Incognito Mimicking Pustular Psoriasis in a Patient With Psoriasis and Cushing Syndrome
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
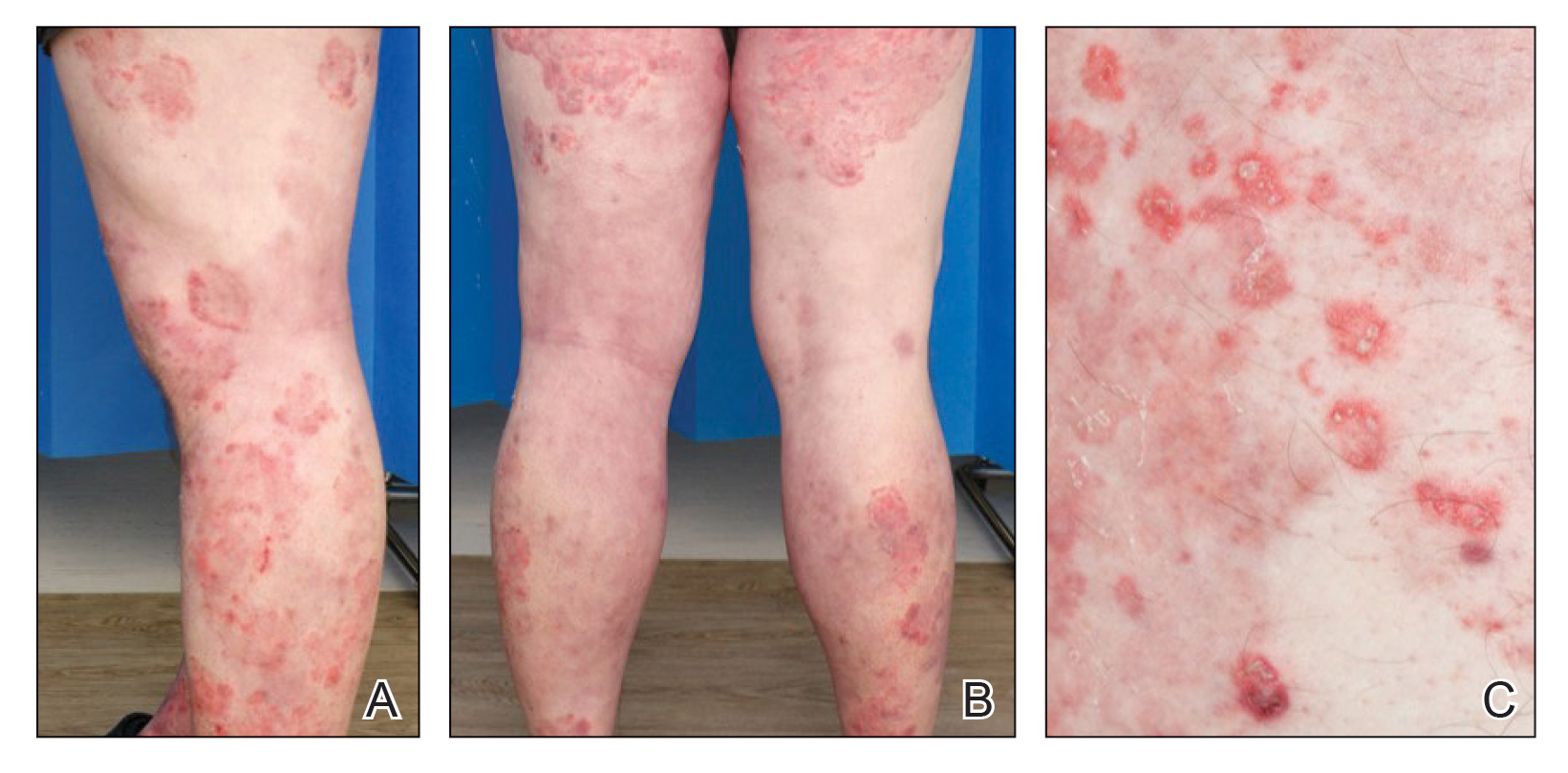
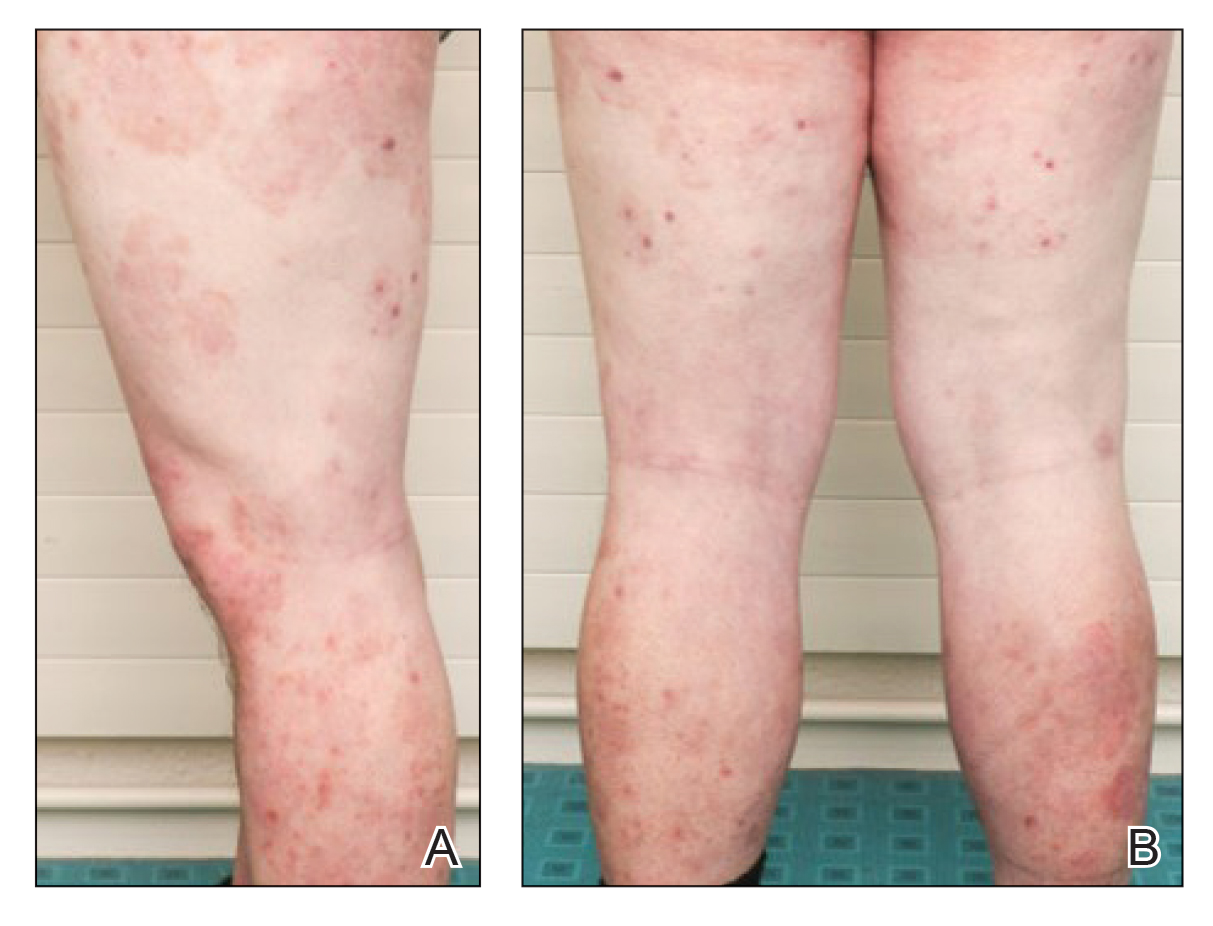
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.


This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
Practice Points
- Tinea incognito and its altered clinical presentation can provide clinical challenges and often is diagnosed with delay.
- Immunosuppression, such as iatrogenic Cushing syndrome, is a risk factor for tinea incognito.
- Pustular tinea incognito is a differential diagnosis of pustular psoriasis that can mimic tumor necrosis factor inhibitor treatment failure in patients with psoriasis.
Squamous Cell Carcinoma in Hidradenitis Suppurativa Lesions Following Tumor Necrosis Factor α Inhibitors
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.
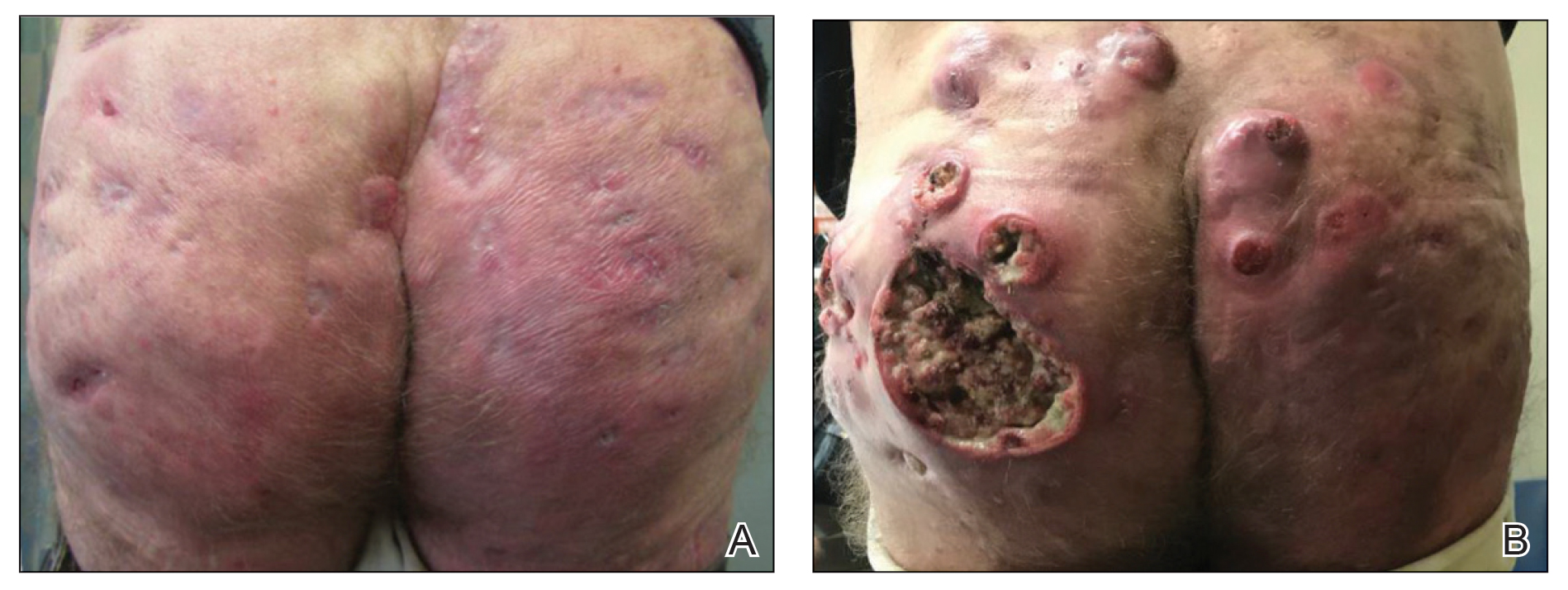
The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.
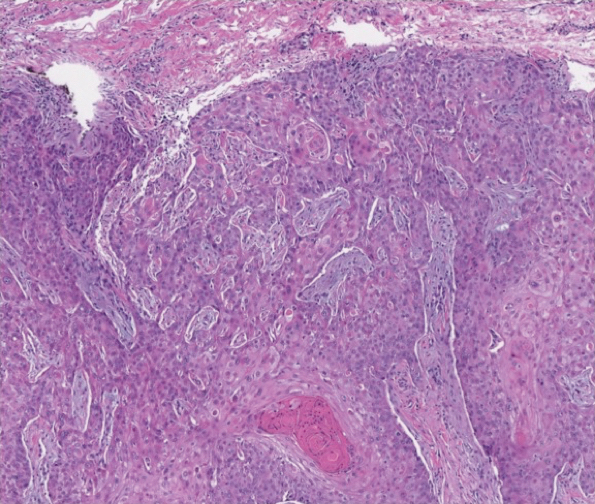
Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.
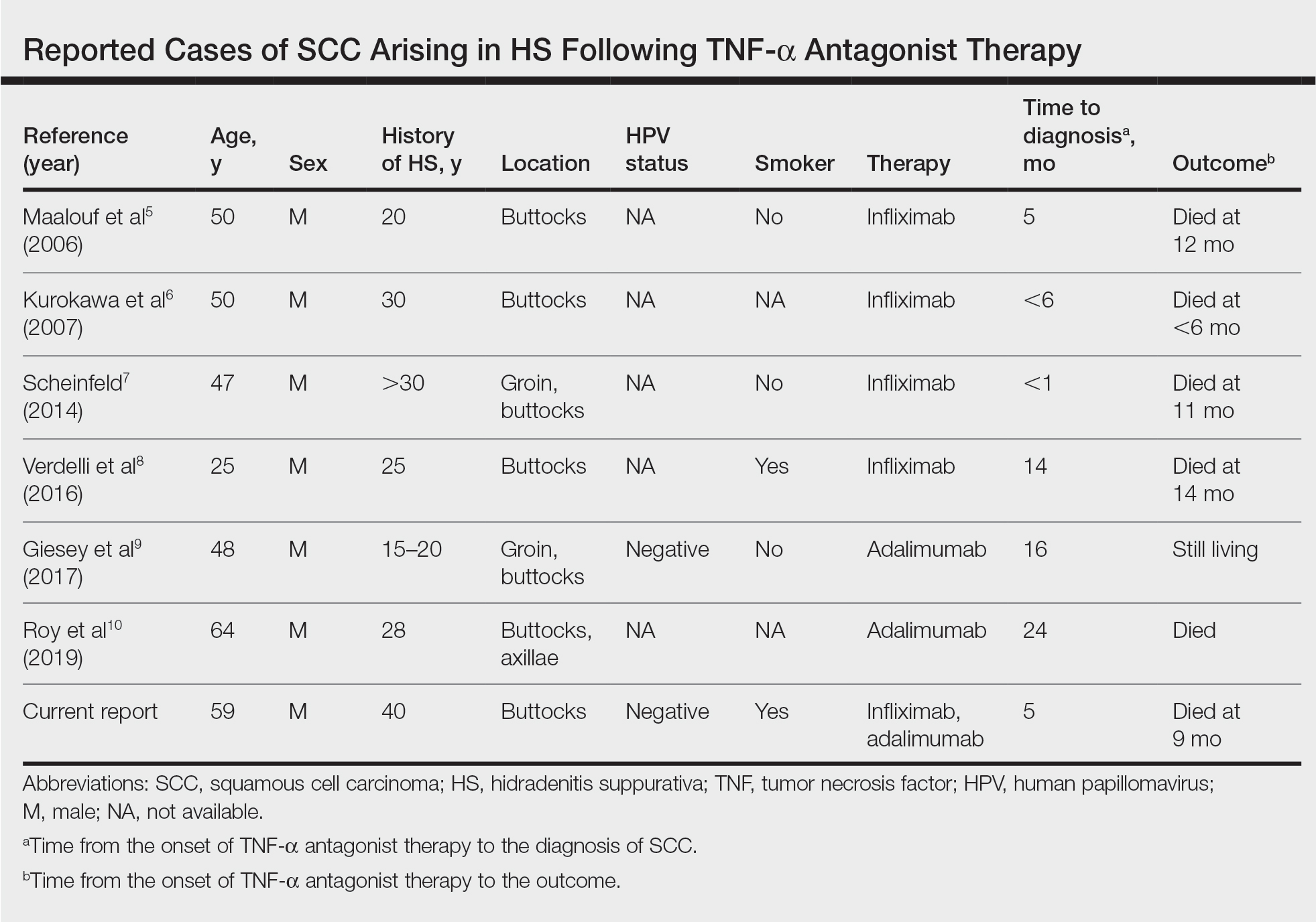
Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with high morbidity rates. Symptoms typically develop between puberty and the third decade of life, affecting twice as many females as males, with an overall disease prevalence of 1% to 4%.1 The pathogenesis is theorized to be related to an immune response to follicular occlusion and rupture in genetically susceptible individuals.
Among the complications associated with HS, the development of cutaneous squamous cell carcinoma (SCC) is 4.6-times more likely within HS lesions than in normal skin and typically is seen in the setting of long-standing disease, particularly in men with HS lesions located on the buttocks and genital region for more than 20 years.2 In 2015, the tumor necrosis factor (TNF) inhibitor adalimumab was approved by the US Food and Drug Administration for the treatment of HS. Tumor necrosis factor α inhibitors have been associated with an increased risk for skin cancer in other clinical settings.3,4 We present a case of locally advanced SCC that developed in a patient with HS who was treated with adalimumab and infliximab (both TNF-α inhibitors), ultimately leading to the patient’s death.
A 59-year-old man who smoked with a 40-year history of severe HS, who previously was lost to follow-up, presented to our dermatology clinic with lesions on the buttocks. Physical examination demonstrated confluent, indurated, boggy plaques; scattered sinus tracts with purulent drainage; scattered cystlike nodules; and tenderness to palpation consistent with Hurley stage III disease (Figure 1A). No involvement of the axillae or groin was noted. He was started on doxycycline and a prednisone taper with minimal improvement and subsequently was switched to adalimumab 3 months later. Adalimumab provided little relief and was discontinued; therapy was transitioned to infliximab 3 months later.

The patient returned to our clinic 3 months later with a severe flare and intractable pain after 4 infusions of infliximab. Physical examination showed a 7×5-cm deep malodorous ulcer with fibrinous exudate on the left buttock, several 2- to 3-cm shallow ulcers draining yellow exudate, and numerous fluctuant subcutaneous nodules on a background of scarring and sinus tracts. He was started again on doxycycline and a prednisone taper. At follow-up 2 weeks later, the largest ulcer had increased to 8 cm, and more indurated and tender subcutaneous nodules and scattered ulcerations developed (Figure 1B). Two punch biopsies of the left buttock revealed an invasive keratinizing carcinoma with no connection to the epidermis, consistent with SCC (Figure 2). Human papillomavirus (HPV) test results with probes for 37 HPV types—13 that were high risk (HPV-16, −18, −31, −33, −35, −39, −45, −51, −52, −56, −58, −59, −68)—were negative. Computerized tomography demonstrated diffuse thickening of the skin on the buttocks, inguinal adenopathy suspicious for nodal metastases, and no evidence of distant metastatic disease. Given the extent of the disease, surgical treatment was not an option, and he began receiving palliative radiotherapy. However, his health declined, and he developed aspiration pneumonia and hypotension requiring pressor support. He was transitioned to hospice care and died 3 months after presentation.

Tumor necrosis factor α antagonist treatment is being increasingly used to control HS but also may increase the risk for SCC development. We performed a search of PubMed articles indexed for MEDLINE as well as Web of Science using the terms hidradenitis suppurativa or acne inversa and one of the following—tumor necrosis factor inhibitor, infliximab, adalimumab, or etanercept—and squamous cell carcinoma or Marjolin ulcer. Seven cases of SCC arising in an HS patient treated with a TNF-α inhibitor have been reported (Table).5-10 Four cases were associated with infliximab use, 2 with adalimumab, and our case occurred after both adalimumab and infliximab treatment. All individuals were men with severe, long-standing disease of the anogenital region. In addition to smoking, HPV-16 positivity also has been reported as a risk factor for developing SCC in the setting of HS.11 In our patient, however, HPV testing did not cover all HPV strains, but several high-risk strains, including HPV-16, were negative.

Hidradenitis suppurativa is caused by an immune response to ruptured follicles and TNF-α antagonists are useful in suppressing this response; however, immunosuppression can lead to an increased susceptibility to malignancy, especially in SCC. It is unclear whether the use of infliximab or adalimumab is causal, additive, or a confounder in the development of SCC in patients with severe HS. It is possible that these agents increase the rapidity of the development of SCC in already-susceptible patients. Although TNF-α antagonists can be an effective therapeutic option for patients with moderate to severe HS, the potential risk for contributing to skin cancer development should raise provider suspicion in high-risk patients. Given the findings in this report, it may be suitable for providers to consider a biopsy prior to initiating TNF-α therapy in men older than 20 years with moderate to severe HS of the groin or buttocks, in addition to more frequent monitoring and a lower threshold to biopsy lesions with rapid growth or ulceration.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-561; quiz 562-533.
- Lapins J, Ye W, Nyren O, et al. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137:730-734.
- Askling J, Fahrbach K, Nordstrom B, et al. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011;20:119-130.
- Mariette X, Matucci-Cerinic M, Pavelka K, et al. Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: a systematic review and meta-analysis. Ann Rheum Dis. 2011;70:1895-1904.
- Maalouf E, Faye O, Poli F, et al. Fatal epidermoid carcinoma in hidradenitis suppurativa following treatment with infliximab. Ann Dermatol Venereol. 2006;133(5 pt 1):473-474.
- Kurokawa I, Nishimura K, Yamanaka K, et al. Cytokeratin expression in squamous cell carcinoma arising from hidradenitis suppurativa (acne inversa). J Cutan Pathol. 2007;34:675-678.
- Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J. 2014;20(3).
- Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol. 2016;55:E52-E53.
- Giesey R, Delost GR, Honaker J, et al. Metastatic squamous cell carcinoma in a patient treated with adalimumab for hidradenitis suppurativa. JAAD Case Rep. 2017;3:489-491.
- Roy C, Roy S, Ghazawi F, et al. Cutaneous squamous cell carcinoma arising in hidradenitis suppurativa: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847359.
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153.
Practice Points
- Consider biopsy of representative lesions in men older than 20 years with moderate to severe disease of the groin and/or buttocks prior to initiation of tumor necrosis factor inhibitors.
- Consider more frequent clinical monitoring with a decrease in threshold to perform biopsy of any new or ulcerating lesions.
Dupilumab for the Treatment of Lichen Planus
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).
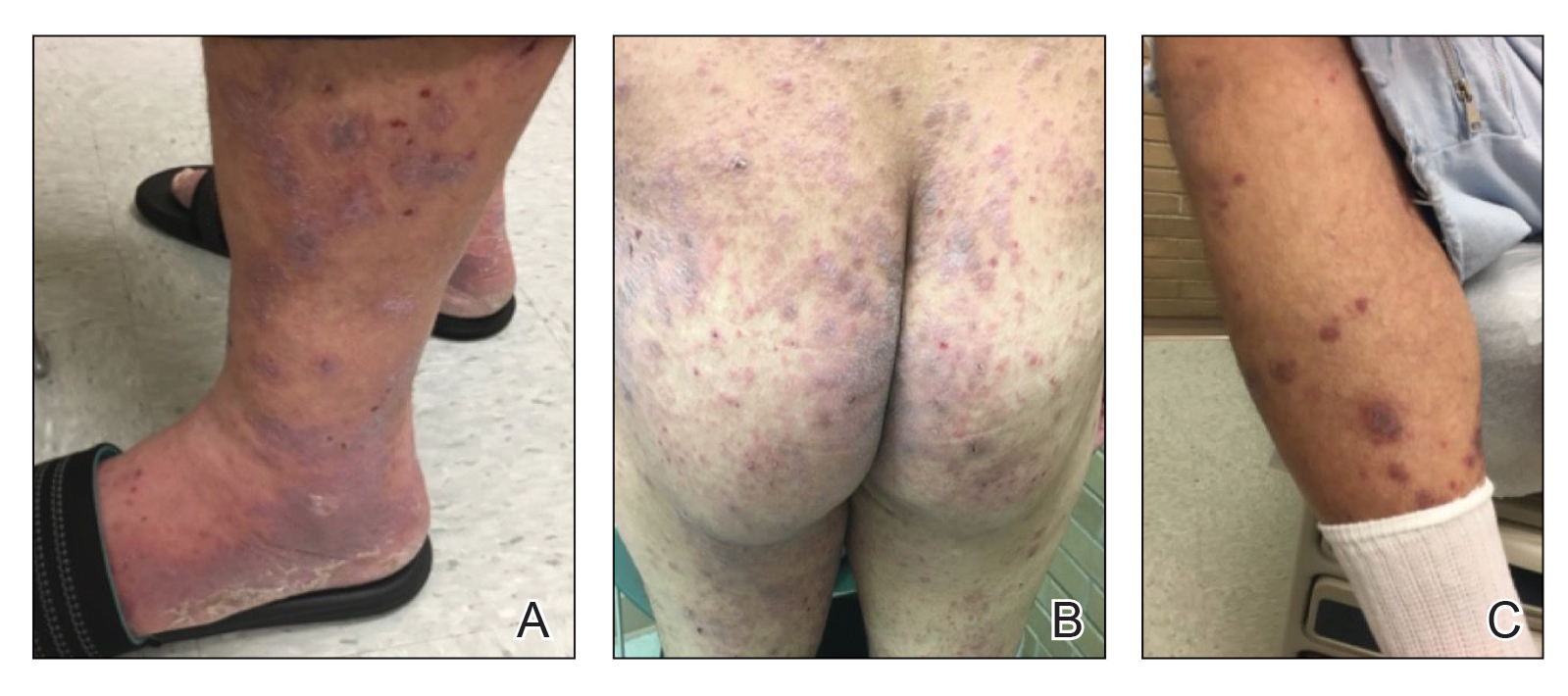
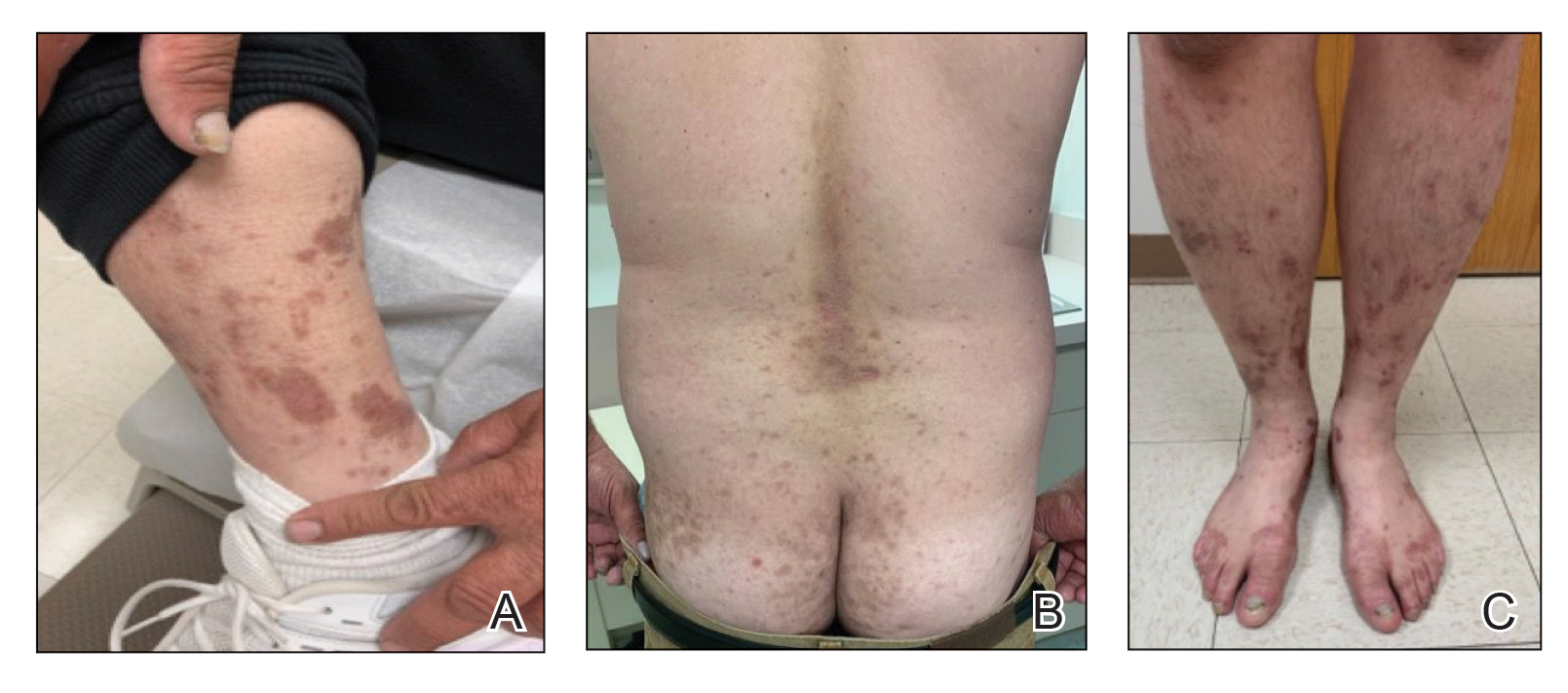
Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).


Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
To the Editor:
Lichen planus (LP) is an inflammatory mucocutaneous disorder that primarily affects adults aged 30 to 60 years.1 It can present across various regions such as the skin, scalp, oral cavity, genitalia, nails, and hair. It classically presents with pruritic, purple, polygonal papules or plaques. The proposed pathogenesis of this condition involves autoimmune destruction of epidermal basal keratinocytes.2 Management involves a stepwise approach, beginning with topical therapies such as corticosteroids and phototherapy and proceeding to systemic therapy including oral corticosteroids and retinoids. Additional medications with reported positive results include immunomodulators such as cyclosporine, tacrolimus, and mycophenolate mofetil.2-4 Dupilumab is a biologic immunomodulator and antagonist to the IL-4Rα on helper T cells (TH1). Although indicated for the treatment of moderate to severe atopic dermatitis, this medication’s immunomodulatory properties have been shown to aid various inflammatory cutaneous conditions, including prurigo nodularis.5-9 We present a case of dupilumab therapy for treatment-refractory LP.
A 52-year-old man presented with a new-onset progressive rash over the prior 6 months. He reported no history of atopic dermatitis. The patient described the rash as “severely pruritic” with a numeric rating scale itch intensity of 9/10 (0 being no itch; 10 being the worst itch imaginable). Physical examination revealed purple polygonal scaly papules on the arms, hands, legs, feet, chest, and back (Figure 1).


Three biopsies were taken, all indicative of lichenoid dermatitis consistent with LP. Rapid plasma reagin as well as HIV and hepatitis C virus serology tests were negative. Halobetasol ointment, tacrolimus ointment, and oral prednisone (28-day taper starting at 40 mg) all failed. Acitretin subsequently was initiated and failed to provide any benefit. The patient was unable to come to clinic 3 times a week for phototherapy due to his work schedule.
Due to the chronic, severe, and recalcitrant nature of his condition, as well as the lack of US Food and Drug Administration–approved treatments, the patient agreed to begin off-label treatment with dupilumab. Upon documentation, the patient’s primary diagnosis was listed as LP, clearly stating all commonly accepted treatments were attempted, except off-label therapy, and failed, and the plan was to treat him with dupilumab as if he had a severe form of atopic dermatitis. Dupilumab was approved with this documentation with a minimal co-pay, as the patient was on Medicaid. At 3-month follow-up (after 4 administrations of the medication), the patient showed remarkable improvement in appearance, and his numeric rating scale itch intensity score improved to 1/10.
Lichen planus is an immune-mediated, inflammatory condition that can affect the skin, hair, nails, and oral cavity. Although its etiology is not fully understood, research supports a primarily TH1 immunologic reaction.10 These T cells promote cytotoxic CD8 T-cell differentiation and migration, leading to subsequent destruction of epidermal basal keratinocytes. An important cytokine in this pathway—tumor necrosis factor α—stimulates a series of proinflammatory factors, including IL-1α, IL-8, and IL-6. IL-6 is of particular interest, as its elevation has been identified in the serum of patients with LP, with levels correlating to disease severity.11 This increase is thought to be multifactorial and a reliable predictor of disease activity.12,13 In addition to its proinflammatory role, IL-6 promotes the activity of IL-4, an essential cytokine in TH2 T-cell differentiation.
The TH2 pathway, enhanced by IL-6, increases the activity of downstream cytokines IL-4, IL-5, and IL-13. This pathway promotes IgE class switching and eosinophil maturation, pivotal factors in the development of atopic conditions such as allergic rhinitis, asthma, and atopic dermatitis. The role of IL-4 and TH2 cells in the pathogenesis of LP remains poorly understood.14 In prior basic laboratory studies, utilizing tissue sampling, RNA extraction, and real-time polymerase chain reaction assays, Yamauchi et al15 proposed that TH2-related chemokines played a pathogenic role in oral LP. Additional reports propose the pathogenic involvement of TH17, TH0, and TH2 T cells.16 These findings suggest that elevated IL-6 in those with LP may stimulate an increase in IL-4 and subsequent TH2 response. Dupilumab, a monoclonal antibody that targets IL-4Rα found on T cells, inhibits both IL-4 and IL-13 signaling, decreasing subsequent effector cell function.17,18 Several case reports have described dupilumab successfully treating various additional dermatoses, including prurigo nodularis, chronic pruritus, and bullous pemphigoid.5-9 Our case demonstrates an example of LP responsive to dupilumab. Our findings suggest that dupilumab interacts with the pathogenic cascade of LP, potentially implicating the role of TH2 in the pathophysiology of LP.
Treatment-refractory LP remains difficult to manage for both the patient and provider. Treatment regimens remain limited to small uncontrolled studies and case reports. Although primarily considered a TH1-mediated disease, the interplay of various alternative signaling pathways has been suggested. Our case of dupilumab-responsive LP suggests an underlying pathologic role of TH2-mediated activity. Dupilumab shows promise as an effective therapy for refractory LP, as evidenced by our patient’s remarkable response. Larger studies are warranted regarding the role of TH2-mediated inflammation and the use of dupilumab in LP.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
- Cleach LL, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;266:723-732.
- Lehman, JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Frieling U, Bonsmann G, Schwarz T, et al. Treatment of severe lichen planus with mycophenolate mofetil. J Am Acad Dermatol. 2003;49:1063-1066.
- Cribier B, Frances C, Chosidow O. Treatment of lichen planus. an evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134:1521-1530.
- Calugareanu A, Jachiet C, Lepelletier C, et al. Dramatic improvement of generalized prurigo nodularis with dupilumab. J Eur Acad Dermatol Venereol. 2019;33:E303-E304.
- Kaye A, Gordon SC, Deverapalli SC, et al. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol. 2018;154:1225-1226.
- Mollanazar NK, Qiu CC, Aldrich JL, et al. Use of dupilumab in HIV-positive patients: report of four cases. Br J Dermatol. 2019;181:1311-1312.
- Zhai LL, Savage KT, Qiu CC, et al. Chronic pruritus responding to dupilumab—a case series. Medicines (Basel). 2019;6:72.
- Mollanazar NK, Elgash M, Weaver L, et al. Reduced itch associated with dupilumab treatment in 4 patients with prurigo nodularis. JAMA Dermatol. 2019;155:121-122.
- Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. part 1. viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40-51.
- Yin M, Li G, Song H, et al. Identifying the association between interleukin-6 and lichen planus: a meta-analysis. Biomed Rep. 2017;6:571-575.
- Sun A, Chia JS, Chang YF, et al. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196-203.
- Rhodus NL, Cheng B, Bowles W, et al. Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Dis. 2006;12:112-116.
- Carrozzo M. Understanding the pathobiology of oral lichen planus. Curr Oral Health Rep. 2014;1:173-179.
- Yamauchi M, Moriyama M, Hayashida JN, et al. Myeloid dendritic cells stimulated by thymic stromal lymphopoietin promote Th2 immune responses and the pathogenesis of oral lichen planus. Plos One. 2017:12:e0173017.
- Piccinni M-P, Lombardell L, Logidice F, et al. Potential pathogenetic role of Th17, Th0, and Th2 cells in erosive and reticular oral lichen planus. Oral Dis. 2013:20:212-218.
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223-246.
- Noda S, Kruefer JG, Guttum-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135:324-336.
Practice Points
- Lichen planus (LP) is an inflammatory mucocutaneous disorder that can present across various regions of the body with pruritic, purple, polygonal papules or plaques.
- The proposed pathogenesis of LP involves autoimmune destruction of epidermal basal keratinocytes.
- The immunomodulatory properties of dupilumab have been shown to aid various inflammatory cutaneous conditions.
Isolated Perianal Erosive Lichen Planus: A Diagnostic Challenge
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.
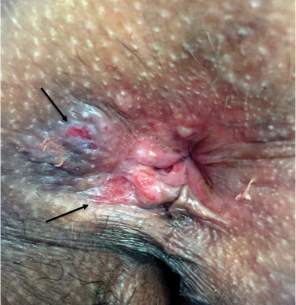
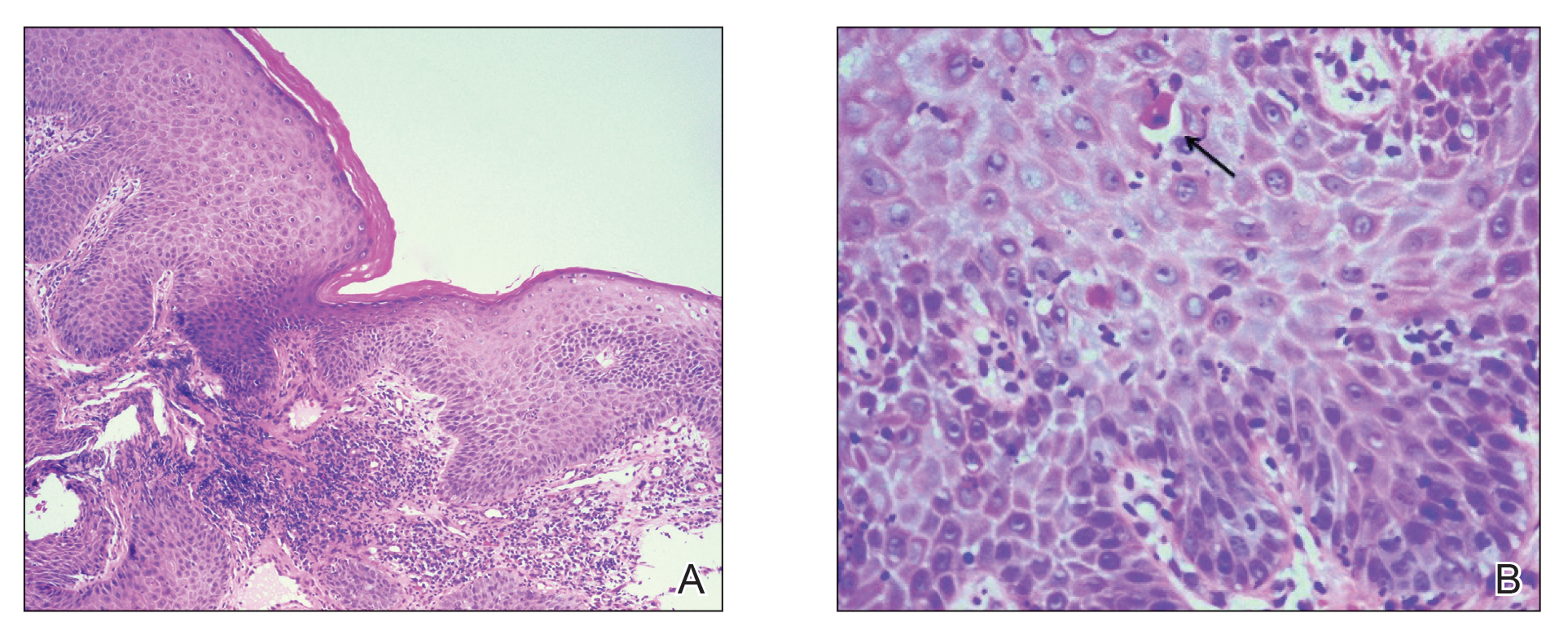
Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10
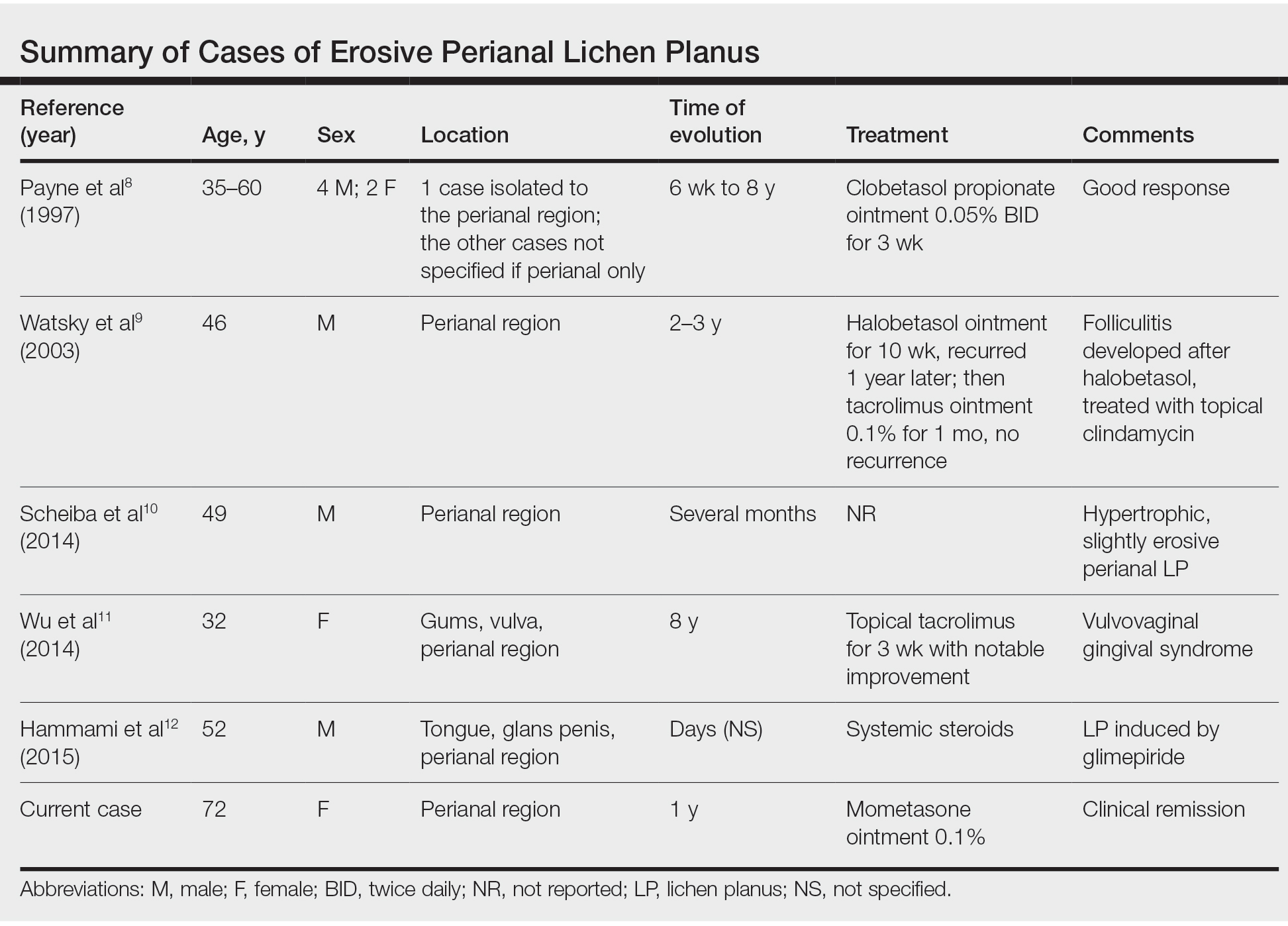
The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.


Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10

The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
To the Editor:
Erosive lichen planus (LP) often is painful, debilitating, and resistant to topical therapy making it both a diagnostic and therapeutic challenge. We report the case of an elderly woman with isolated perianal erosive LP, a rare clinical manifestation. We also review cases of erosive perianal LP reported in the literature.
A 72-year-old woman was referred to our dermatology clinic for evaluation of multiple pruritic and painful perianal lesions of 1 year’s duration. The lesions had remained stable since onset, with no other reported lesions elsewhere on body, including the mucosae. Her medical history was notable for rheumatoid arthritis, osteoporosis, hypercholesterolemia, and hypertension. She was taking methotrexate, folic acid, abatacept, alendronate, atorvastatin, and lisinopril. The patient reported she had been using abatacept for 3 years and lisinopril for 2 years. Her primary care physician initially treated the lesions as hemorrhoids but referred her to a gastroenterologist when they failed to improve. Gastroenterology evaluated the patient, and a colonoscopy was performed with unremarkable results. Thus, she was referred to dermatology for further evaluation.
Physical examination revealed 2 tender, sharply defined, angulated erosions with irregular violaceous borders involving the perianal skin (Figure 1). A biopsy of one of the lesions was taken. Histopathologic examination revealed acanthosis of the epidermis with slight compact hyperkeratosis, scattered dyskeratotic keratinocytes, and a dense bandlike lymphohistiocytic infiltrate that obliterated the dermoepidermal junction (Figure 2). A diagnosis of perianal erosive LP was made. The patient was prescribed mometasone ointment 0.1% daily with notable improvement after 2 months.


Erosive LP is an extremely rare variant of LP.1 It typically manifests as chronic painful erosions that often can progress to scarring, ulceration, and tissue destruction. Although erosive LP most commonly involves the mucosal surfaces of the genitalia and oral mucosa, it also has been reported in the palmoplantar skin, lacrimal duct, external auditory meatus, and esophagus.2-7 However, isolated perianal involvement is extremely rare. A PubMed search of articles indexed for MEDLINE using the terms erosive or ulcerative and lichen planus and perianal revealed 10 cases of perianal erosive LP, and weak data exist regarding therapy (Table).8-12 Of these cases, only 3 reported isolated perianal involvement.8-10 In most reported cases, perianal involvement manifested as extremely painful and occasionally pruritic, sharply angulated erosions and ulcers arising 0.5 to 3 cm from the anus with macerated, whitish, and violaceous borders. Most of the lesions occurred unilaterally, with only 1 case of bilateral perianal involvement.10

The differential diagnosis of perianal erosions is extensive and includes cutaneous Crohn disease, extramammary Paget disease, cutaneous malignancy, herpes simplex virus, cytomegalovirus, external hemorrhoids, lichen sclerosus, Behçet disease, lichen simplex chronicus, and drug-induced lichenoid reaction, among others. It is worth emphasizing infectious processes and cutaneous malignancies in light of our patient’s immunosuppression. Perianal cytomegalovirus has been reported in the literature in association with HIV, and it is a clinically challenging diagnosis.13 Cutaneous malignancy associated with the use of methotrexate also was considered in the differential diagnosis for our patient, given the increased risk for nonmelanoma skin cancer with the use of immunosuppresants.14
Along with a thorough patient history and physical examination, skin biopsy and clinicopathologic correlation are key to determine the exact etiology. Histologically, LP is characterized by a lichenoid interface dermatitis with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction. Other distinguishing factors include irregular acanthosis, hyperkeratosis, basal cell vacuolar degeneration, and Civatte bodies. Drug-induced LP is a possibility, but it is unclear if abatacept or lisinopril may have played a role in our patient. However, absence of eosinophils and parakeratosis suggested an idiopathic rather than drug-induced etiology. In 2016, Day et al2 published a clinicopathologic review of 60 cases of perianal lichenoid dermatoses in which only 17% of lesions were LP. Of note, 90% of perianal LP lesions were of the hypertrophic variant, and none were of the erosive variant, further supporting that our case represents a rare clinical manifestation of perianal LP.
Treatment of LP varies depending on the location and subtype of the lesions and is primarily aimed at improving symptoms. Topical corticosteroids are the standard treatment of LP; however, there is limited evidence regarding their efficacy for mucosal LP. Although randomized controlled trials assessing the efficacy of different interventions on oral erosive LP are available in the literature,15 there is a paucity of studies addressing this topic for genital or perianal LP. A review of the literature regarding perianal erosive LP suggests good response to high-potency topical steroids and calcineurin inhibitors with resolution of lesions within 3 to 4 weeks.11,15-18
Erosive LP is a painful variant that can cause erosions, ulcerations, and scarring. It rarely is seen in the perianal region alone and presents a diagnostic challenge. Treatment with high-potency topical steroid therapy seems to be effective in the few cases that have been reported as well as in our case. More comprehensive data from randomized controlled trials would be needed to evaluate their efficacy compared to other therapies.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
- Rebora A. Erosive lichen planus: what is this? Dermatology. 2002;205:226-228; discussion 227.
- Day T, Bohl TG, Scurry J. Perianal lichen dermatoses: a review of 60 cases. Australas J Dermatol. 2016;57:210-215.
- Fox LP, Lightdale CJ, Grossman ME. Lichen planus of the esophagus: what dermatologists need to know. J Am Acad Dermatol. 2011;65:175-883.
- Holmstrup P, Thorn JJ, Rindum J, et al. Malignant development of lichen planus-affected oral mucosa. J Oral Pathol. 1988;17:219-225.
- Lewi, FM, Bogliatto F. Erosive vulval lichen planus—a diagnosis not to be missed: a clinical review. Eur J Obstet Gynecol Reprod Biol. 2013;171:214-219.
- Webber NK, Setterfield JF, Lewis FM, et al. Lacrimal canalicular duct scarring in patients with lichen planus. Arch Dermatol. 2012;148:224-227.
- Martin L, Moriniere S, Machet MC, et al. Bilateral conductive deafness related to erosive lichen planus. J Laryngol Otol. 1998;112:365-366.
- Payne CM, McPartlin JF, Hawley PR. Ulcerative perianal lichen planus. Br J Dermatol. 1997;136:479.
- Watsky KL. Erosive perianal lichen planus responsive to tacrolimus. Int J Dermatol. 2003;42:217-218.
- Scheiba N, Toberer F, Lenhard BH, et al. Erythema and erosions of the perianal region in a 49-year-old man. J Dtsch Dermatol Ges. 2014;12:162-165.
- Wu Y, Qiao J, Fang H. Syndrome in question. An Bras Dermatol. 2014;89:843-844.
- Hammami S, Ksouda K, Affes H, et al. Mucosal lichenoid drug reaction associated with glimepiride: a case report. Eur Rev Med Pharmacol Sci. 2015;19:2301-2302.
- Meyerle JH, Turiansky GW. Perianal ulcer in a patient with AIDS. Arch Dermatol. 2004;140:877-882.
- Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152:164-172.
- Cheng S, Kirtschig G, Cooper S, et al. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst Rev. 2012:Cd008092.
- Gunther S. Effect of retinoic acid in lichen planus of the genitalia and perianal region. Br J Vener Dis. 1973;49:553-554.
- Vente C, Reich K, Neumann C. Erosive mucosal lichen planus: response to topical treatment with tacrolimus. Br J Dermatol. 1999;140:338-342.
- Lonsdale-Eccles AA, Velangi S. Topical pimecrolimus in the treatment of genital lichen planus: a prospective case series. Br J Dermatol. 2005;153:390-394.
Practice Points
- Erosive lichen planus (LP) is an underrecognized variant of LP presenting with painful erosions, ulcerations, and scarring.
- Although rare, perianal erosive LP should be included in the differential diagnosis of perianal erosions.
- Treatment with high-potency steroids is an effective therapeutic option resulting in notable improvement.