User login
Harlequin Syndrome: Discovery of an Ancient Schwannoma
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
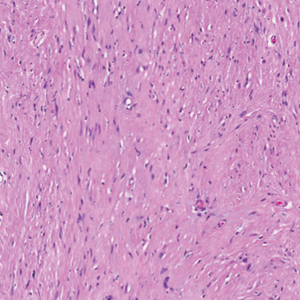
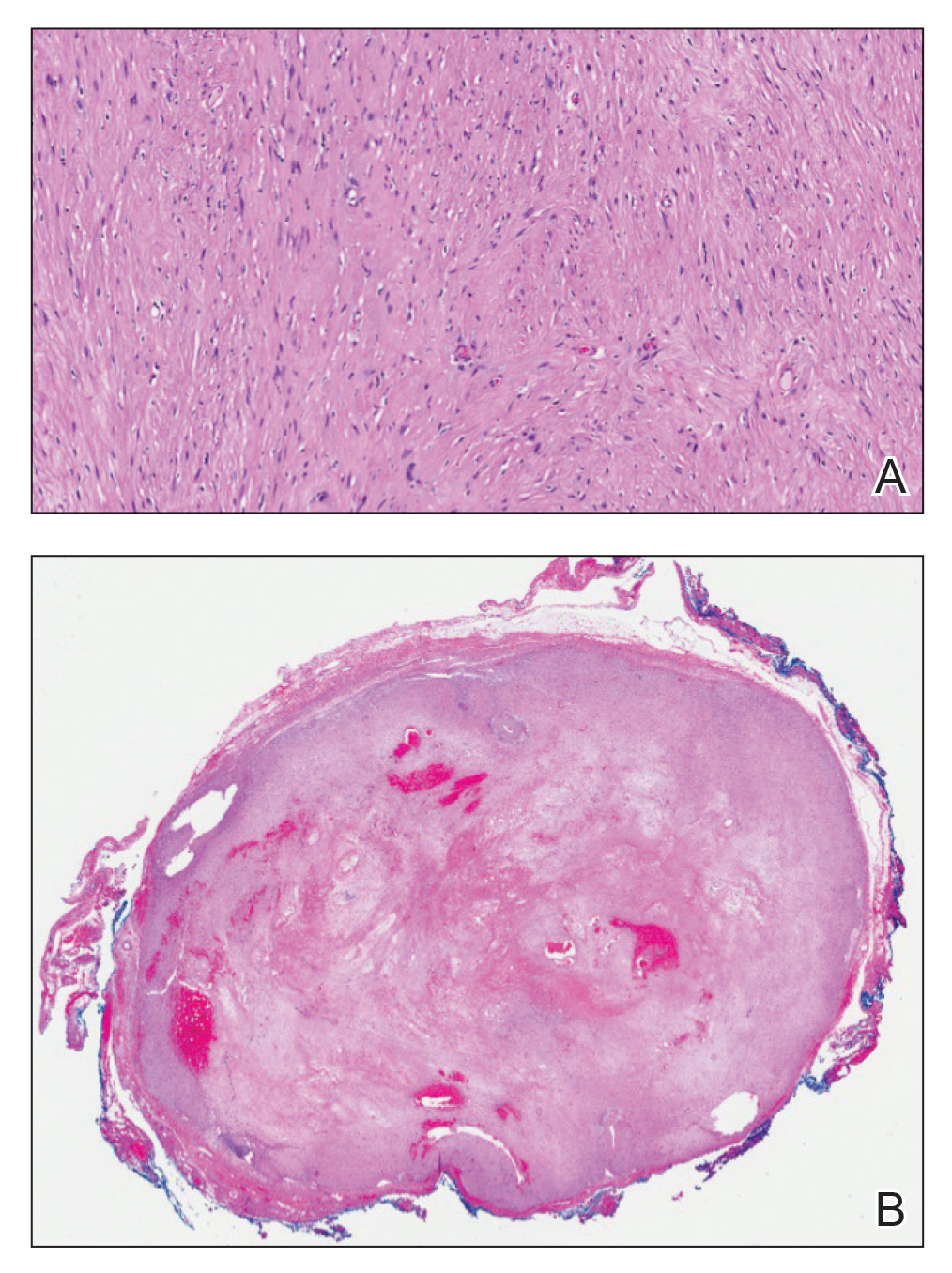
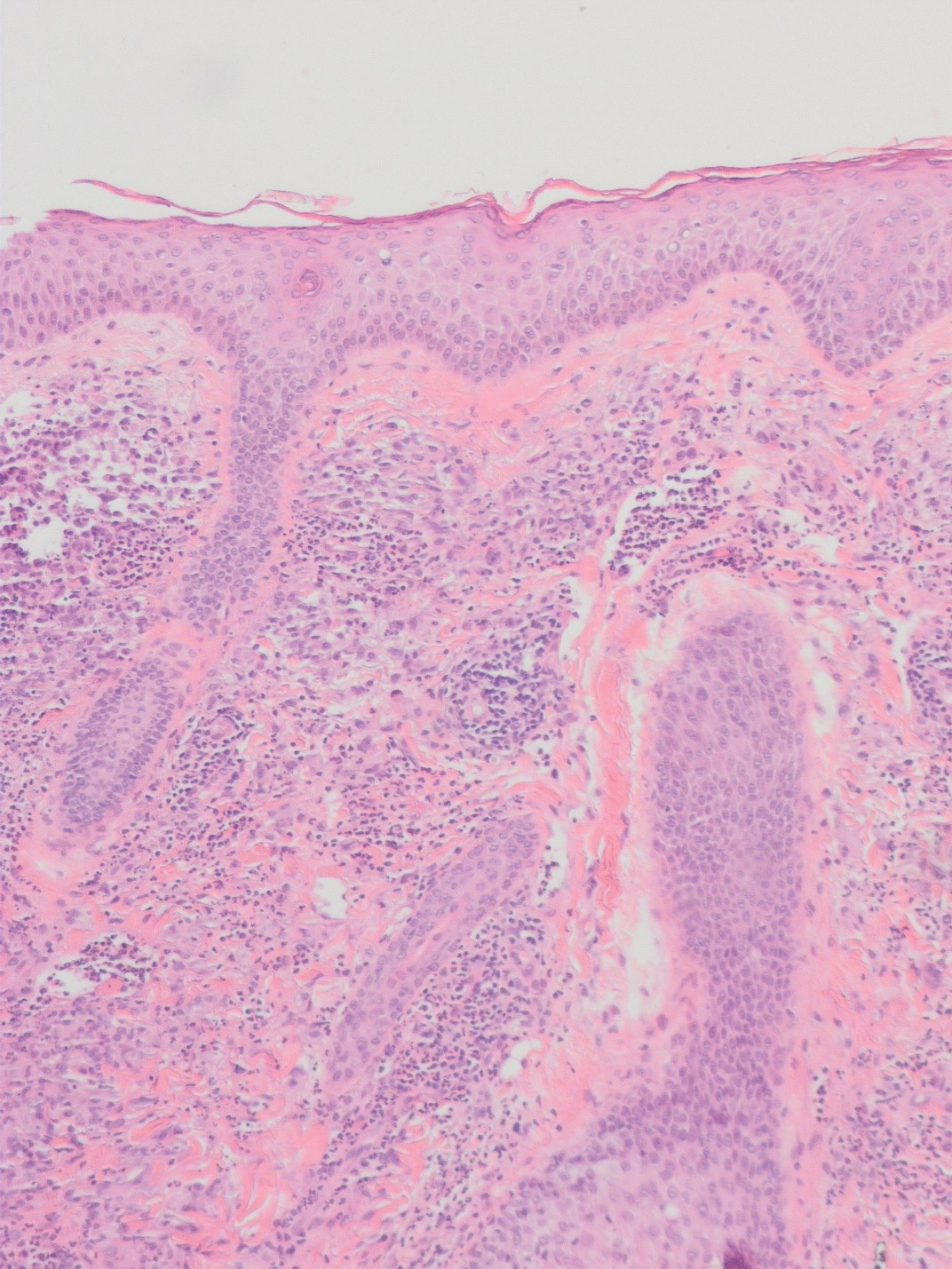
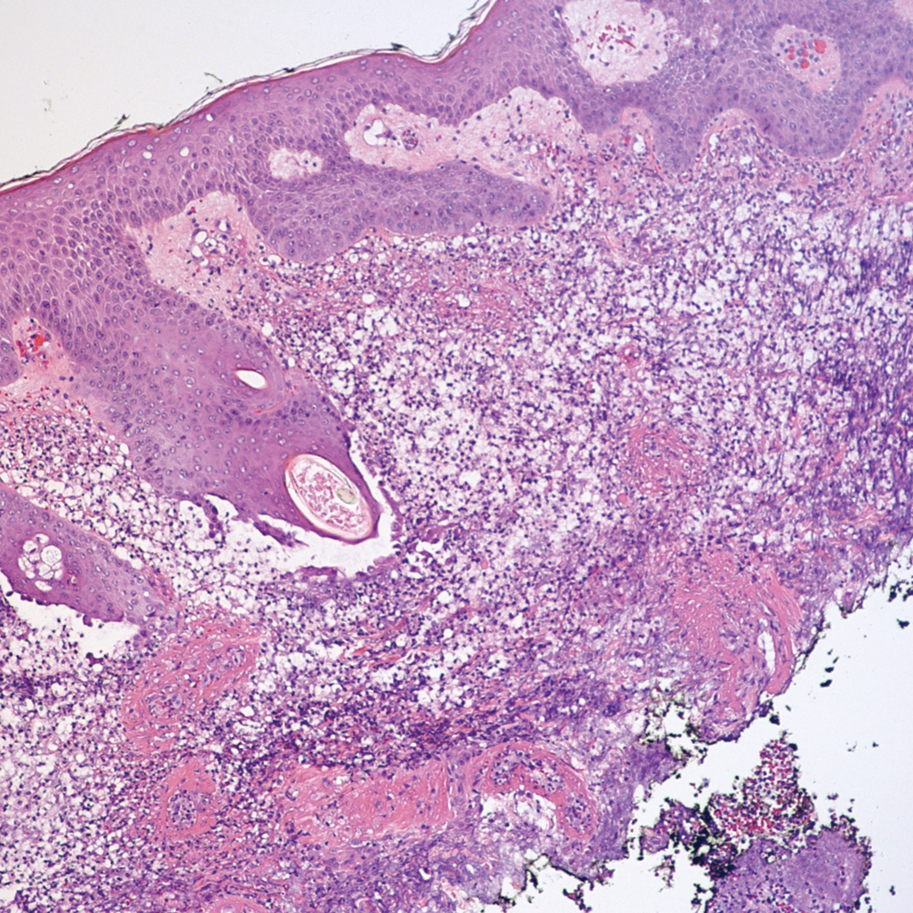
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
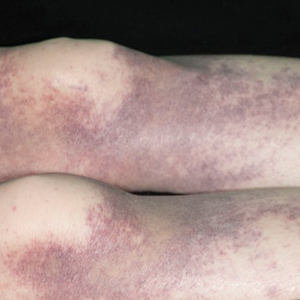
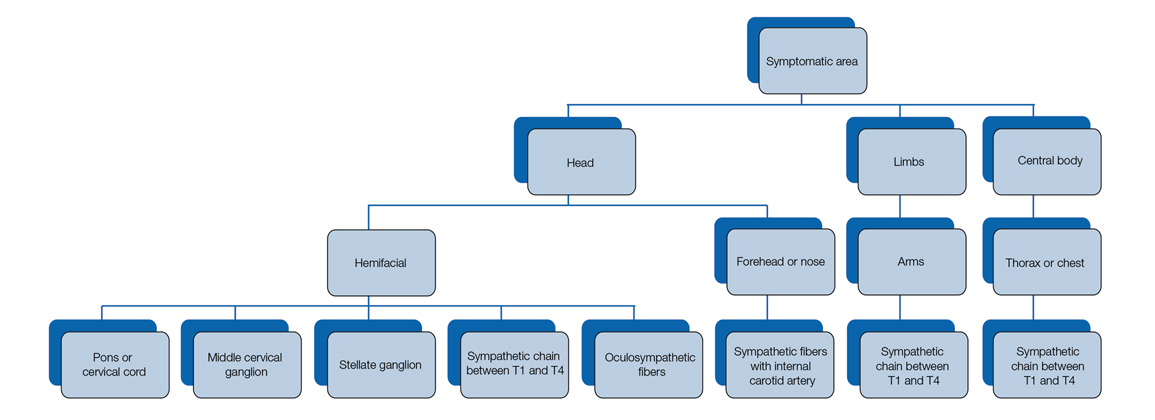
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
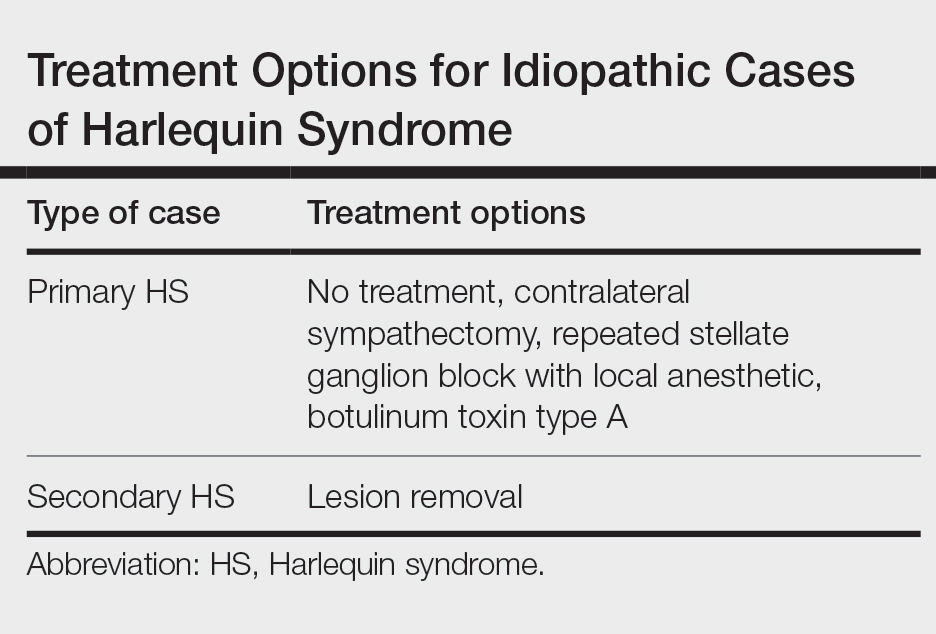
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.

Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.

Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11

- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
Practice Points
- Harlequin syndrome is a rare disorder of the sympathetic nervous system that is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.
- Secondary causes can be from schwannomas in the cervical chain ganglion.
Granuloma Faciale in Woman With Levamisole-Induced Vasculitis
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
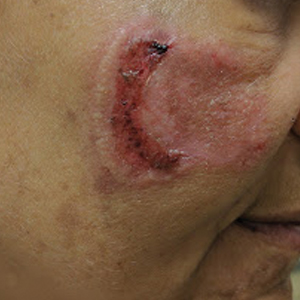
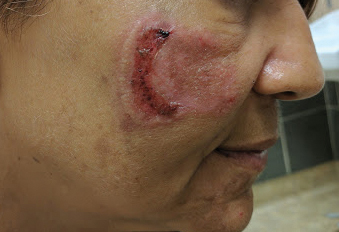
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.
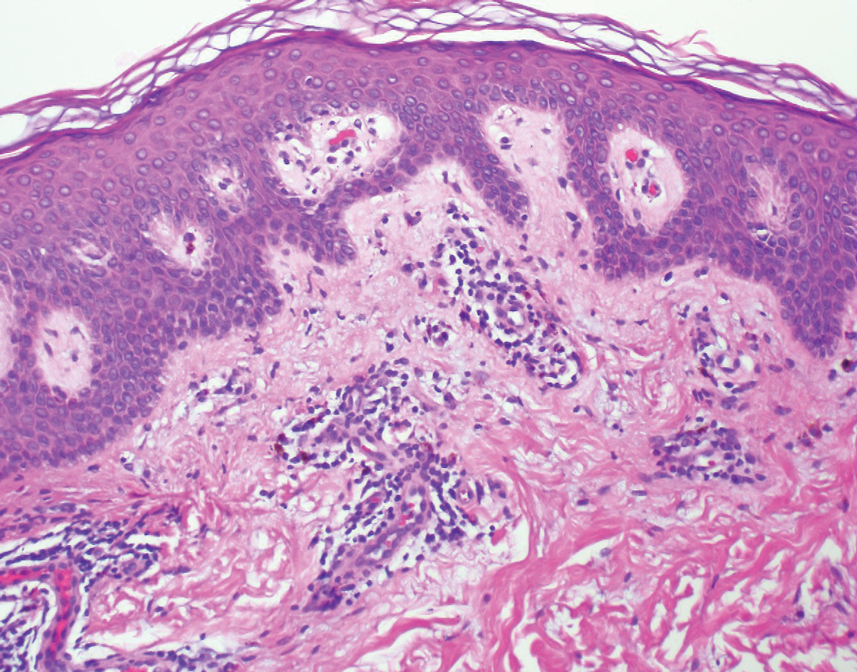
There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.
Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
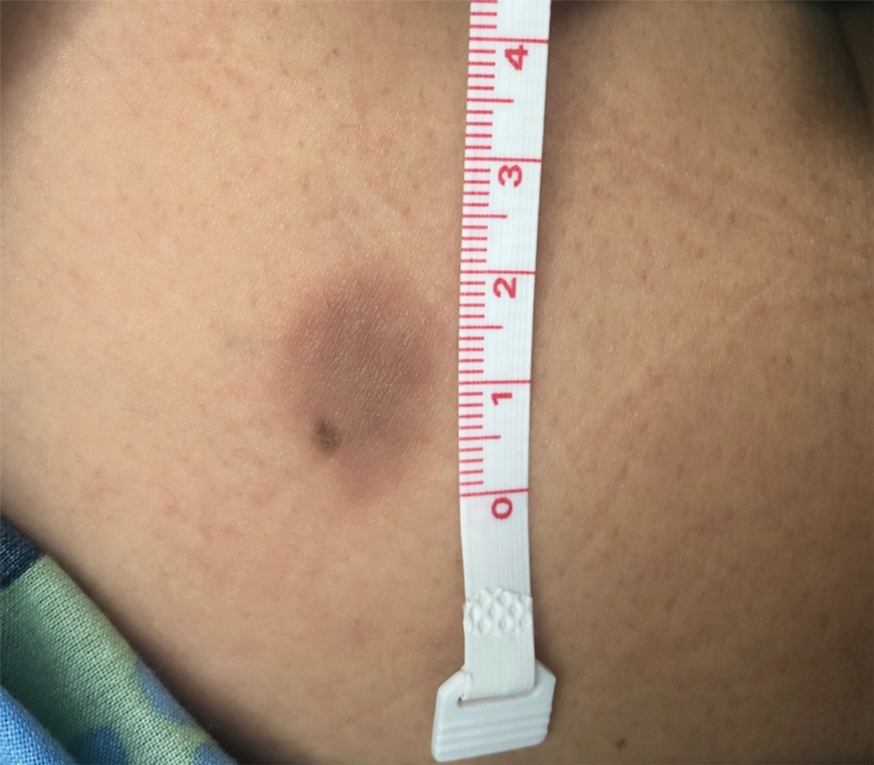
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
Practice Points
- Granuloma faciale is a benign dermal process presenting with a red-brown plaque on the face of adults that typically is not ulcerated unless physically manipulated.
- Skin biopsy often is required for correct diagnosis.
- Granuloma faciale does not resolve spontaneously and tends to be chronic.
A Fixed Drug Eruption to Medroxyprogesterone Acetate Injectable Suspension
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
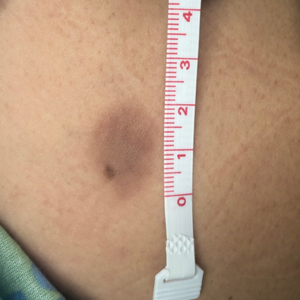
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).

An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3

Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).

An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3

Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
Practice Points
- Exogenous progesterone from the administration of the contraceptive injectable medroxyprogesterone acetate has the potential to cause a cutaneous hypersensitivity reaction in the form of a fixed drug eruption (FDE).
- Dermatologists should perform a careful and detailed review of medication history to evaluate drug eruptions.
Iododerma Following Exposure to Iodine: A Case of Explosive Acneform Eruption Overnight
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.
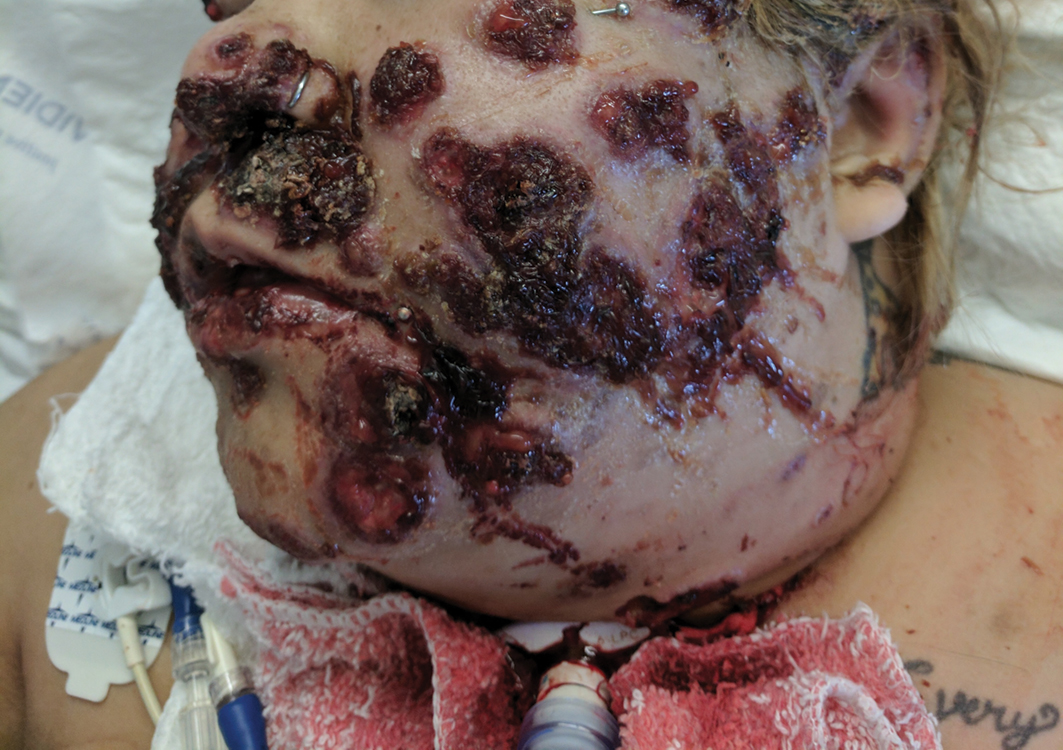
The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.
The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.

The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.

The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
To the Editor:
Iododerma is a rare dermatologic condition caused by exposure to iodinated contrast media, oral iodine suspensions, or topical povidone-iodine that can manifest as eruptive acneform lesions.1-3
A 27-year-old woman in septic shock presented for worsening facial lesions that showed no improvement on broad-spectrum antibiotics, antifungals, and antivirals. She initially presented to an outside hospital with abdominal pain and underwent computed tomography (CT) with intravenous (IV) iodinated contrast; 24 hours after this imaging study, the family reported the appearance of “explosive acne overnight.” The lesions first appeared as vegetative and acneform ulcerations on the face. A second abdominal CT scan with IV contrast was performed 4 days after the initial scan, given the concern for spontaneous bacterial peritonitis. Hours after the second study, the lesions progressed to involve the buccal mucosae, tongue, mucosal airway, and distal arms and legs. She became progressively disoriented and developed an altered mentation over the course of the following week. Due to progressive facial edema, she required intubation 5 days after the second CT scan.

The patient had a medical history of end-stage renal disease secondary to crescenteric glomerulonephritis on peritoneal dialysis. Physical examination revealed numerous beefy-red, heaped-up, weepy, crusted nodules clustered on the face (Figure 1) and a few newer bullous-appearing lesions on the hands and feet. She had similar lesions involving the buccal mucosae and tongue with substantial facial edema. Infectious workup was notable for a positive skin culture growing methicillin-susceptible Staphylococcus aureus. All blood and tissue cultures as well as serologies for fungal and viral etiologies were negative. A tissue biopsy revealed necrosis with a neutrophilic infiltrate with mixed cell inflammation (Figure 2), and direct immunofluorescence was negative.

The patient initially was thought to be septic due to viral or bacterial infection. She was transferred from an outside hospital 7 days after the initial appearance of the acneform lesions, having already received IV contrast on 2 occasions within the first 48 hours of illness. Infectious disease was consulted and initiated broad-spectrum antiviral, antimicrobial, and antifungal therapy with acyclovir, linezolid, meropenem, and later micafungin without improvement. The diagnosis of iododerma ultimately was established based on the patient’s elevated urinary iodine levels with preceding iodine exposure in the context of renal failure. The preferential involvement of sebaceous areas and pathology findings were supportive of this diagnosis. Aggressive supportive measures including respiratory support, IV fluids, and dialysis were initiated. Topical iodine solutions, iodine-containing medications, and additional contrast subsequently were avoided. Despite these supportive measures, the patient died within 48 hours of admission from acute respiratory failure. Her autopsy attributed “septic complications of multifocal ulcerative cutaneous disease” as the anatomic cause of death.
Iododerma is an extremely rare neutrophilic dermatosis. The proposed mechanism of action involves a cell-mediated hypersensitivity reaction to iodine with induction of neutrophil degranulation.2 There have been documented cases with exposure to oral potassium iodide supplements, amiodarone, topical povidone-iodine, and IV iodinated contrast material.1-3 Iododerma typically presents 1 to 3 days after exposure to iodine. The most common source is IV radiocontrast. Diagnosis is based on the clinical presentation including acneform to vegetative nodular or bullous eruptions involving sebaceous areas in the context of recent iodine exposure. Elevated urinary iodine levels and histologic findings of neutrophilic infiltrate of the dermis support the diagnosis.3,4
Although there have been reported cases of iododerma in patients with normal renal function, patients with renal failure are much more susceptible due to the decreased clearance of iodine.5 The plasma half-life of radiocontrast is 23 hours in patients with end-stage renal disease vs 2 hours in patients with normal kidney function.3 Dosage adjustments for renal impairment have not been well studied, and no specific guidelines exist for the prevention of iododerma in patients with renal failure.
The first step in treating iododerma is to remove the offending iodine-containing agent. In most cases, cutaneous lesions resolve in 4 to 6 weeks after discontinuation of the source of iodine; however, there have been reported fatalities in the literature secondary to pulmonary edema in patients with iododerma.6,7 Despite the rarity and diagnostically challenging nature of iododerma, early recognition of this disease is crucial. Although our patient showed symptoms of iododerma after 1 dose of radiocontrast, she was not diagnosed at that time and received a second imaging study with contrast less than 48 hours later. These 2 consecutive exposures to iodine as well as the delayed diagnosis unfortunately resulted in rapid clinical deterioration.
The mainstay of therapy for iododerma includes avoidance of iodine-containing materials as soon as the diagnosis is suspected as well as supportive care. Patients have been successfully treated with systemic corticosteroids, with the addition of cyclosporine and hemodialysis in severe cases.3 Patients with a history of iododerma are advised to avoid iodine in their diet, in topical preparations, and in future imaging studies.8
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
- Aliagaoglu C, Turan H, Uslu E, et al. Iododerma following topical povidone-iodine application. Cutan Ocul Toxicol. 2013;32:339-340.
- Torkamani, N, Sinclair R. Iododerma in pregnancy secondary to iodinated multivitamins. Australas J Dermatol. 2015;56:235-236.
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast material. Br J Dermatol. 2014;170:1377-1379.
- Stavert R, Bunick CG, Modi B, et al. Vegetative plaques and hemorrhagic pustules. JAMA Dermatol. 2013;149:1231-1232.
- Rothman LR, Levender MM, Scharf MD, et al. Iododerma following serial computed tomography scans in a lung cancer patient. J Drugs Dermatol. 2013;12:574-576.
- Miranda-Romero A, Sánchez-Sambucety P, Gómez JE, et al. Vegetating iododerma with fatal outcome. Dermatology. 1999;198:295-297.
- Vailant L, Pengloan J, Blanchier D, et al. Iododerma and acute respiratory distress with leucocytoclastic vasculitis following the intravenous injection of contrast medium. Clin Exp Dermatol. 1990;15:232-233.
- Massé M, Flanaga V, Zhou LH. Use of topical povidone iodine resulting in an iododerma-like eruption. J Dermatol. 2008;35:744-747.
Practice Points
- Iododerma should be considered for patients who develop rapidly progressive, vegetative lesions, especially in those with renal failure. A thorough history should be obtained in these cases, focusing on medications and recent studies involving iodinated contrast.
- The most important first step in treating iododerma is to remove the iodine-containing agent to avoid continued exposure.
- Therapies for iododerma include supportive care, cyclosporine, systemic corticosteroids, and hemodialysis in severe cases.
Necrotic Ulcerations After the Use of an Over-the-counter Mole and Skin Tag Removal Product
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.
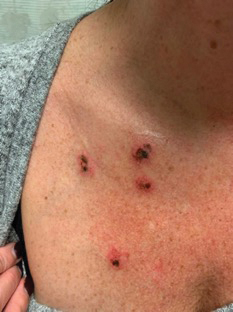
The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
To the Editor:
Several mole and skin tag removal products are available online and over the counter (OTC).1 Patients concerned with the cosmetic appearance of nevi may use these products as a do-it-yourself alternative to surgical removal. However, these products have the potential to cause harm.2 Beyond the cosmetic adverse effects of skin necrosis and scar formation, these products can mask premalignant and malignant skin lesions.2 Herein, we describe a patient with a family history of melanoma who developed facial and chest ulcerations with necrosis after applying an OTC mole and skin tag removal product.
A 45-year-old woman with fair skin presented to a clinic with multiple superficial ulcerations measuring approximately 1 cm in diameter with necrotic black bases and erythematous rims on the face, right side of the upper chest, and left earlobe after using the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set, an OTC mole and skin tag removal product. The patient reported using the product 24 hours prior for the cosmetic removal of multiple nevi. After applying the product, she observed that it “immediately melted [her] skin” and the areas where the product was applied “turned black.” She reported that the product was applied to the skin for no longer than 30 seconds, after which she developed the necrotic lesions (Figure). After removing the product, she applied an OTC ointment containing bacitracin, neomycin, and polymyxin B to the lesions.

The patient had no history of nonmelanoma skin cancers or atypical nevi. She had a family history of melanoma in her mother and maternal uncle. The treatment plan was aimed primarily at reducing scar formation. We advised frequent application of petroleum-based ointments for moisture and overlying silicone scar tape to protect the area from photodamage and promote wound healing. We further advocated for sun protection and the use of a physical sunscreen on the lesions as they healed. We discussed potential laser-based scar revision options in the future.
With more than 180 reviews on Amazon and almost 70% of these reviews made within the month prior to compiling this manuscript, the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set appeared to be popular; however, the product currently is unavailable on Amazon. Testimonials and before-and-after pictures advertising the product show an all-natural, safe, and effective method as an alternative to surgical removal of skin tags and nevi. The product website claims that skin tags and moles will “fall off naturally within 7 to 10 days” and the product can be used for “almost all skin types.” Users are instructed to apply the removal product and wipe it off when the skin surrounding the mole becomes swollen. The product kit also includes a repair lotion, which claims to help heal the skin after scab formation and scar development.
The ingredients listed on the product packaging are salicylic acid 25%, Melaleuca alternifolia (tea tree) leaf oil, propylene glycol, hydroxyethylcellulose, and alcohol. Salicylic acid 25% is a superficial peeling agent that penetrates the epidermis to the dermoepidermal junction. The potential side effects are mild and include superficial desquamation and epidermolysis.3 The Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set is not regulated by the US Food and Drug Administration and may contain variable concentrations of salicylic acid and other unknown compounds. Higher concentrations of salicylic acid can penetrate the full thickness of the epidermis into the papillary dermis, which can result in postinflammatory pigmentation, superficial infection, scarring, and deeper desquamation and epidermolysis.3 The product website advertises the use of only natural ingredients and an “advanced blend of concentrated natural ingredients contributing a broad spectrum of healing properties” in the formula. Although these claims are attractive to patients seeking alternatives to surgical approaches to nevi removal, the unfounded claims and unregulated ingredients may pose a threat to unsuspecting consumers.
Other OTC and “all-natural” mole removal products previously have been reported to cause harm.2Sanguinaria canadensis, also known as bloodroot, contains an alkaloid compound (sanguinarine) that has been shown to induce mitochondrial apoptosis and activation of Bcl-2 proteins in keratinocytes.4 Some products, such as Wart & Mole Vanish cream, may claim not to contain bloodroot specifically. However, sanguinarine can be extracted from other plants and may be listed as Argemone mexicana, Chelidonium majus, or Macleaya cordata in the ingredients list.5 The use of alternative medicine products such as black or yellow salve for the removal of suspected skin cancers also is not recommended because these escharotic treatments have not been proven safe or effective, and the manufacturing process for these compounds is unregulated.6,7 Self-treatment with alternative remedies for nevi or suspected skin cancers has been associated with progression of disease and even death due to metastatic spread.2
Self-removal of moles is concerning because the nevi are masked by necrotic lesions and can no longer be assessed by dermoscopy or histopathology. Furthermore, the compounds in the Ariella Mole Corrector and Skin Tag Remover and Repair Lotion Set may have unknown effects on the transformation of premalignant cells. They also may mask an underlying process for which clinically proven and effective treatments such as cryotherapy, prescription topical agents, and surgical excision are warranted. Awareness of this product and similar products is important to educate patients on the harmful effects they may cause.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clayton R, Turner R. Cosmetic surgery: who needs surgeons when you’ve got creams? Br J Dermatol. 2007;156:1383-1384.
- McAllister JC, Petzold CR, Lio PA. Adverse effects of a mole removal cream. Pediatr Dermatol. 2009;26:628-629.
- Soleymani T, Lanoue J, Rahman Z. A practical approach to chemical peels: a review of fundamentals and step-by-step algorithmic protocol for treatment. J Clin Aesthet Dermatol. 2018;11:21-28.
- Adhami VM, Aziz MH, Mukhatar M, et al. Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes. Clin Cancer Res. 2003;9:3176-3182.
- Santos AC, Adkilen P. The alkaloids of Argemone mexicana. J Am Chem Soc. 1932;54:2923-2924.
- Osswald SS, Elston DM, Farley MF, et al. Self-treatment of a basal cell carcinoma with “black and yellow salve.” J Am Acad Dermatol. 2005;53:509-511.
- McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
Practice Point
- Self-administered mole and skin tag removal products are rising in popularity, but unregulated ingredients in over-the-counter products that are not approved by the US Food and Drug Administration may mask underlying transformation of atypical nevi.
At-Home Treatment of Pigmented Lesions With a Zinc Chloride Preparation
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
To the Editor:
Zinc chloride originally was used by Dr. Frederic Mohs as an in vivo tissue fixative during the early phases of Mohs micrographic surgery.1 Although this technique has since been replaced with fresh frozen tissue fixation, zinc chloride still is found in topical preparations that are readily available to patients. Specifically, black salve describes variably composed topical preparations that share the common ingredients zinc chloride and Sanguinaria canadensis (bloodroot).2 Patients self-treat with these unregulated compounds, but the majority do not have their lesions evaluated by a clinician prior to use and are unaware of the potential risks.3-5 Products containing zinc chloride and S canadensis that are not marketed as black salve present a new problem for the dermatology community.
A 73-year-old man presented to our dermatology clinic for the focused evaluation of scaly lesions on the face and nose. At this visit, it was recommended he undergo a total-body skin examination for skin cancer screening given his age and substantial photodamage.
Physical examination revealed more than 20 superficial, 3- to 10-mm scars predominantly over the trunk. One scar over the left mid-back had a large, 1.2-cm peripheral rim of dark brown pigment that was clinically concerning for a melanocytic neoplasm. Shave removal of this lesion was performed. Histologic examination showed melanoma in situ with a central scar. The central scar spanned the depth of the dermis, and the melanocytic component was absent in this area, raising the question if prior biopsy or treatment had been performed on this lesion. During a discussion of the results with the patient, he was questioned about prior biopsy or treatment of this lesion. He reported prior use of a topical all-natural cream containing zinc chloride and S canadensis that he purchased online, which he had used to treat this lesion as well as numerous presumed moles.
The trend of at-home mole removal products containing the traditional ingredients in black salve seems to be one of rapidly shifting product availability as well as a departure from marketing items as black salve. Many prior black salve products are no longer available.4 The product that our patient used is a topical cream marketed as a treatment for moles and skin tags.6 Despite not being marketed as black salve, it does contain zinc chloride and S canadensis. The product’s website highlights these ingredients as being a safe and effective treatment for mole removal, with claims that the product will remove the mole or skin tag without irritating the surrounding skin and can be safely used anywhere on the body without scarring.6 A Google search at the time this article was written using the term skin tag remover revealed similar products marketed as all-natural “skin tag remover and mole corrector creams.” These similar products containing zinc chloride and S canadensis were available in the United States at the time of our initial research but have since been removed and only are available outside of the United States.7
Prior reports of melanoma masked by zinc chloride and S canadensis described the use of topical agents marketed as black salve. This new wave of products marketed as all-natural creams makes continued education on the available products and their associated risks necessary for clinicians. The lack of US Food and Drug Administration oversight for these products and their frequent introduction and discontinuation in the market makes keeping updated even more challenging. Because many patients self-treat without prior evaluation by a health care provider, treatment with these products can lead to a delay in diagnosis or inaccurate staging due to scars from the chemical destruction, both of which may have occurred in our patient.5 Until these products become regulated by the US Food and Drug Administration, it is imperative that clinicians continue to educate their patients on the lack of documented benefit and clear risks of their use as well as remain up-to-date on product trends.
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
- Cohen DK. Mohs micrographic surgery: past, present, and future. Dermatol Surg. 2019;45:329-339. doi:10.1097/DSS.0000000000001701
- Eastman KL. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289. doi:10.1089/acm.2012.0377
- Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80. doi:10.5826/dpc.0403a16
- McDaniel S. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
- Clark JJ. Community perceptions about the use of black salve. J Am Acad Dermatol. 2016;74:1021-1023. doi:10.1016/j.jaad.2015.10.016
- Skinprov Cream. Skinprov. Accessed February 22, 2022. https://skinprov.net
- HaloDerm. HaloDerm Inc. Accessed February 22, 2022. https://haloderm.com/
Practice Points
- Zinc chloride preparations are readily available over the counter and unregulated.
- Patients may attempt to self-treat pigmented lesions based on claims they see online.
- When asking patients about prior treatments, it may be prudent to specifically ask about over-the-counter products and their ingredients.
Leukemia Cutis Manifesting as Nonpalpable Purpura
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.
The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).
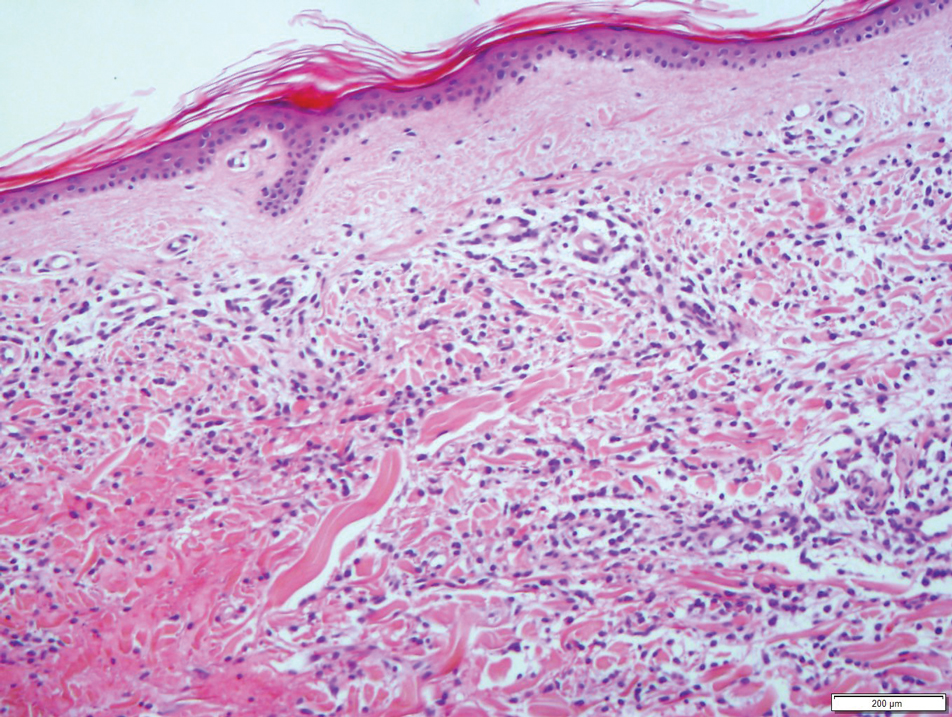
Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.
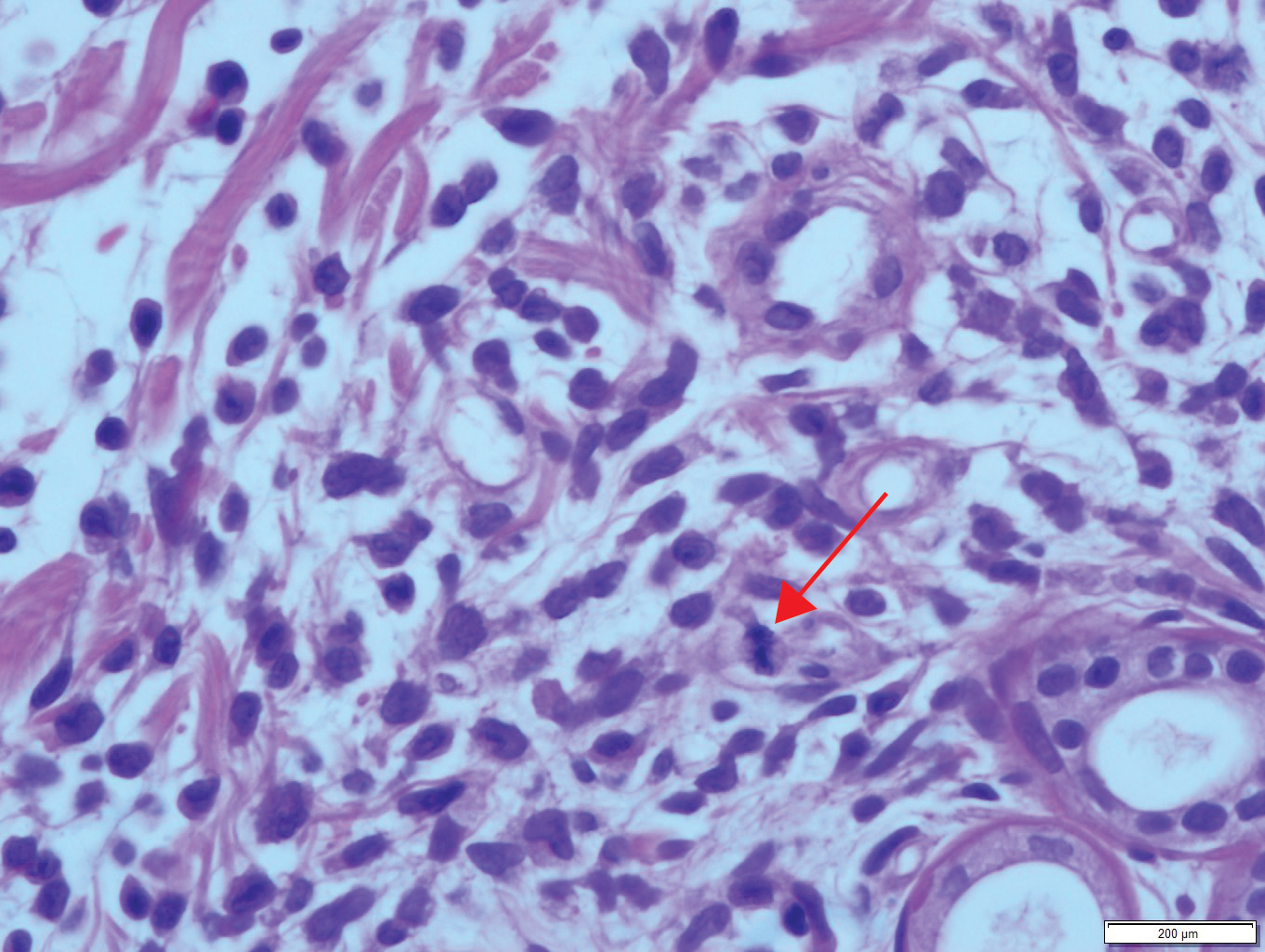
Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.
Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.

The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).

Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.

Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.

Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.

The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).

Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.

Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.

Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
Practice Points
- Leukemia cutis complicates 5% to 15% of all cases of acute myeloid leukemia (AML) in adults.
- The appearance of leukemia cutis may be highly variable. Therefore, it should be included in the differential diagnosis for any cutaneous presentation in patients with an existing diagnosis or high likelihood of AML.
- Leukemic infiltrates are associated with sites of inflammation.
Treatment of Elephantiasic Pretibial Myxedema With Rituximab Therapy
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.
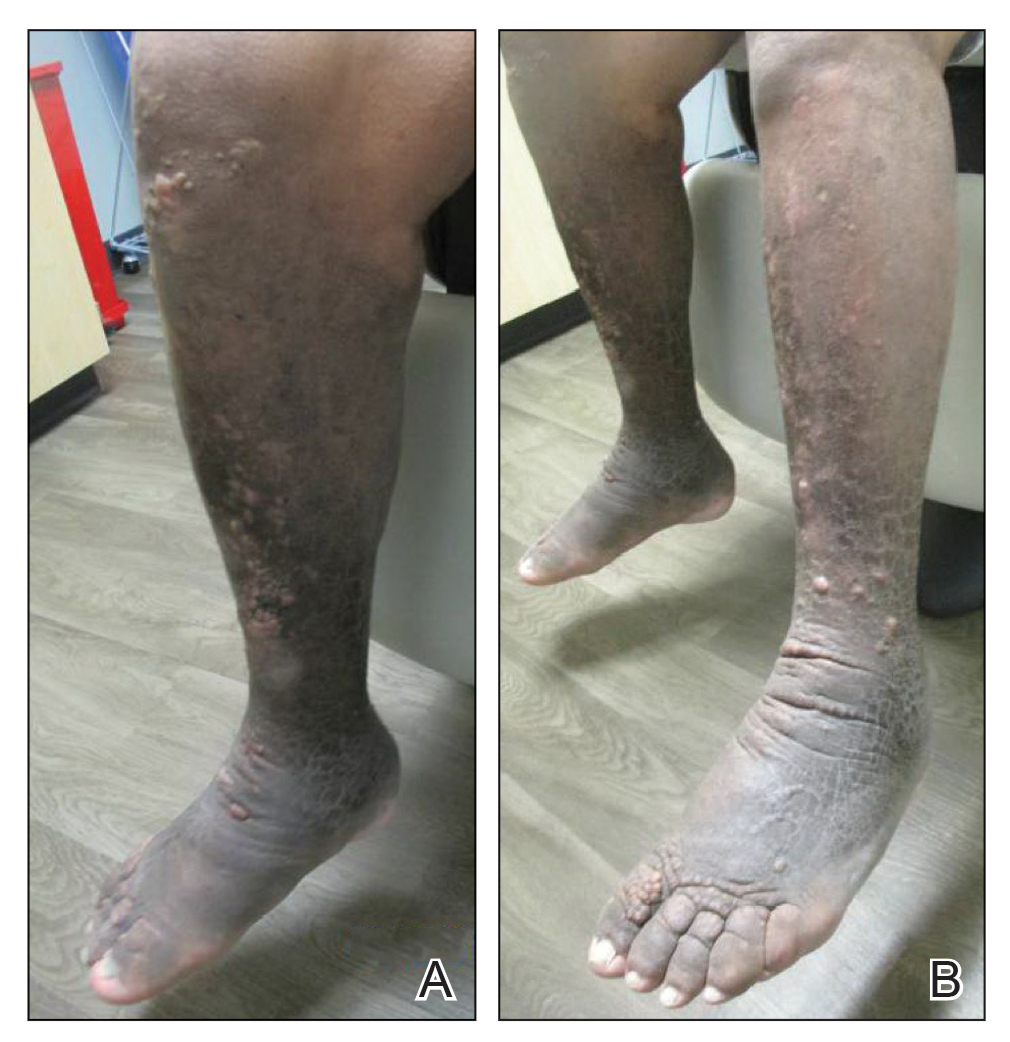
The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).
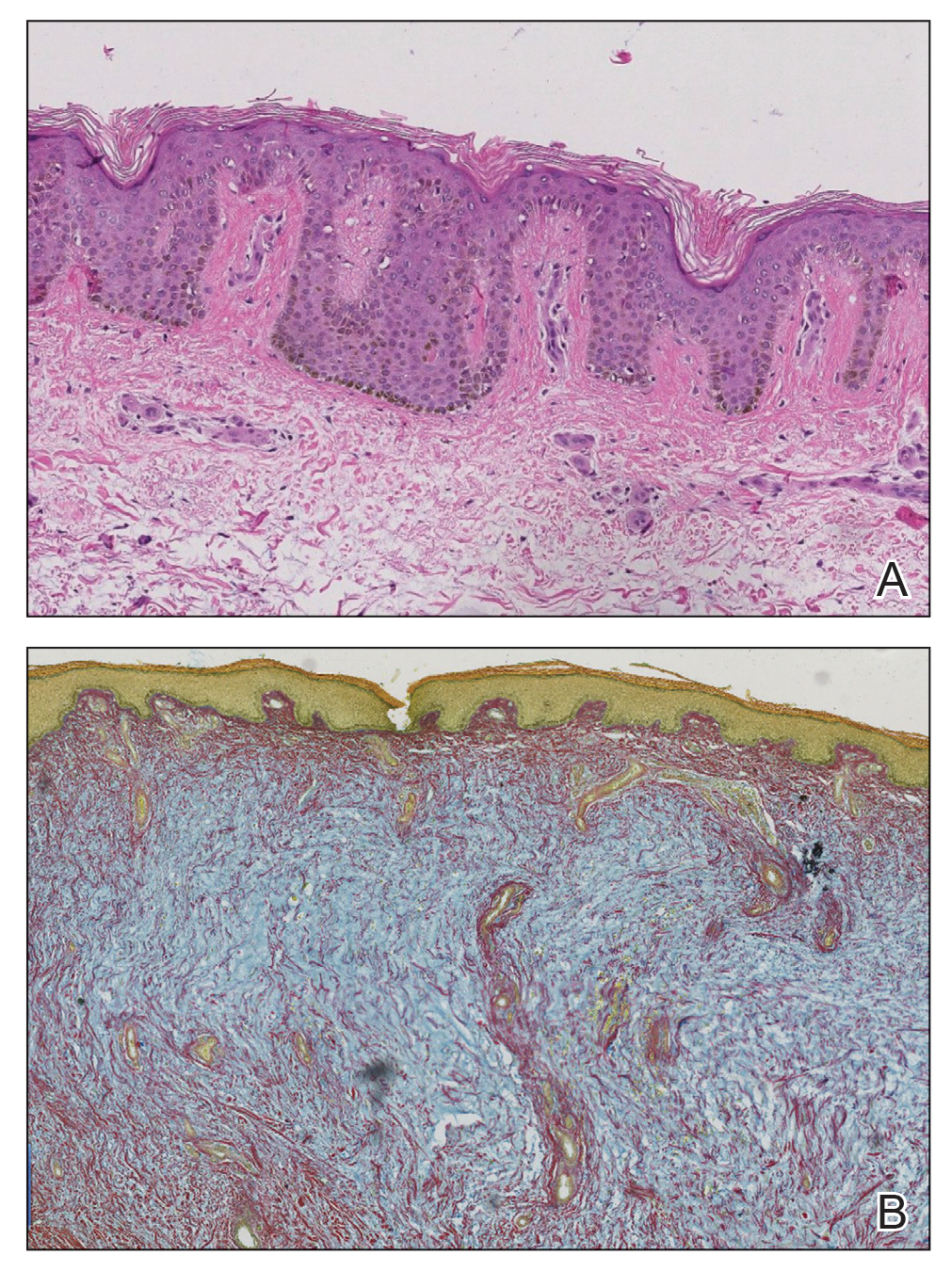
The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.
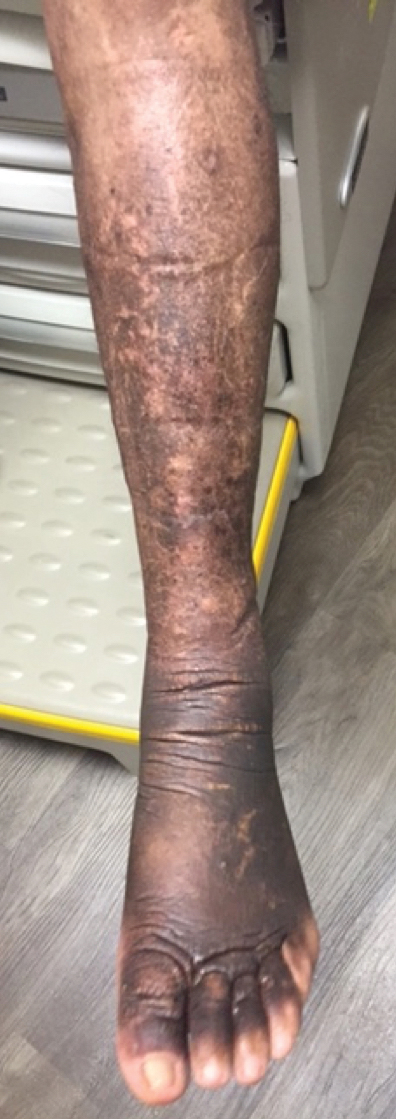
Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.

The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).

The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.

Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
To the Editor:
Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet. Pretibial myxedema occurs in approximately 0.5% to 4.3% of patients with hyperthyroidism.1 Thyroid dermopathy often is thought of as the classic nonpitting PTM with skin induration and color change. However, rarer forms of PTM, including plaque, nodular, and elephantiasic, also are important to note.2
Elephantiasic PTM is extremely rare, occurring in less than 1% of patients with PTM.2 Elephantiasic PTM is characterized by the persistent swelling of 1 or both legs; thickening of the skin overlying the dorsum of the feet, ankles, and toes; and verrucous irregular plaques that often are fleshy and flattened. The clinical differential diagnosis of elephantiasic PTM includes elephantiasis nostra verrucosa, a late-stage complication of chronic lymphedema that can be related to a variety of infectious or noninfectious obstructive processes. Few effective therapeutic modalities exist in the treatment of elephantiasic PTM. We present a case of elephantiasic PTM.
A 59-year-old man presented to dermatology with leonine facies with pronounced glabellar creases and indentations of the earlobes. He had diffuse woody induration, hyperpigmentation, and nonpitting edema of the lower extremities as well as several flesh-colored exophytic nodules scattered throughout the anterior shins and dorsal feet (Figure 1). On the left posterior calf, there was a large, 3-cm, exophytic, firm, flesh-colored nodule. Examination of the hands revealed mild hyperpigmentation of the distal digits, clubbing of the distal phalanges, and cheiroarthropathy.

The patient was diagnosed with Graves disease after experiencing the classic symptoms of hyperthyroidism, including heat intolerance, tremor, palpitations, and anxiety. He received thyroid ablation and subsequently was supplemented with levothyroxine 75 mg daily. Twelve years later, he was diagnosed with Graves ophthalmopathy with ocular proptosis requiring multiple courses of retro-orbital irradiation and surgical procedures for decompression. Approximately 1 year later, he noted increased swelling, firmness, and darkening of the pretibial surfaces. Initially, he was referred to vascular surgery and underwent bilateral saphenous vein ablation. He also was referred to a lymphedema specialist, and workup revealed an unremarkable lymphatic system. Minimal improvement was noted following the saphenous vein ablation, and he subsequently was referred to dermatology for further workup.
At the current presentation, laboratory analysis revealed a low thyrotropin level (0.03 mIU/L [reference range, 0.4–4.2 mIU/L]), and free thyroxine was within reference range. Radiography of the chest was unremarkable; however, radiography of the hand demonstrated arthrosis of the left fifth proximal interphalangeal joint. Nuclear medicine lymphoscintigraphy and lower extremity ultrasonography were unremarkable. Punch biopsies were performed of the left lateral leg and posterior calf. Hematoxylin and eosin staining demonstrated marked mucin deposition extending to the deep dermis along with deep fibroplasia and was read as consistent with PTM. Colloidal iron highlighted prominent mucin within the dermis (Figure 2).

The patient’s medical history, physical examination, laboratory analysis, imaging, and biopsies were considered, and a diagnosis of elephantiasic PTM was made. Minimal improvement was noted with initial therapeutic interventions including compression therapy and application of super high–potency topical corticosteroids. After further evaluation in our multidisciplinary rheumatology-dermatology clinic, the decision was made to initiate rituximab infusions.
Two months after 1 course of rituximab consisting of two 1000-mg infusions separated by 2 weeks, the patient showed substantial clinical improvement. There was striking improvement of the pretibial surfaces with resolution of the exophytic nodules and improvement of the induration (Figure 3). In addition, there was decreased induration of the glabella and earlobes and decreased fullness of the digital pulp on the hands. The patient also reported subjective improvements in mobility.

Our patient demonstrated all 3 aspects of the Diamond triad: PTM, exophthalmos, and acropachy. Patients present with all 3 features in less than 1% of reported cases of Graves disease.3 Although all 3 features are seen together infrequently, thyroid dermopathy and acropachy often are markers of severe Graves ophthalmopathy. In a study of 114 patients with Graves ophthalmopathy, patients who also had dermopathy and acropachy were more likely to have optic neuropathy or require orbital decompression.4
After overcoming the diagnostic dilemma that the elephantiasic presentation of PTM can present, therapeutic management remains a challenge. Heyes et al5 documented the successful treatment of highly recalcitrant elephantiasic PTM with rituximab and plasmapheresis therapy. In this case, a 44-year-old woman with an 11-year history of Graves disease and elephantiasic PTM received 29 rituximab infusions and 241 plasmapheresis treatments over the course of 3.5 years. Her elephantiasic PTM clinically resolved, and she was able to resume daily activities and wear normal shoes after being nonambulatory for years.5
Rituximab is a monoclonal antibody against CD20, a protein found primarily on the surface of B-cell lymphocytes. Although rituximab initially was approved by the US Food and Drug administration for the treatment of malignant lymphoma, it has had an increasing role in the treatment of autoimmune disorders such as rheumatoid arthritis. Rituximab is postulated to target B lymphocytes and halt their progression to plasma cells. By limiting the population of long-lasting, antibody-producing plasma cells and decreasing the autoantibodies that cause many of the symptoms in Graves disease, rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.6
Although the exact mechanism is poorly understood, PTM likely is a sequela of hyperthyroidism because of the expression of thyroid-stimulating hormone receptor proteins found on normal dermal fibroblasts. Thyroid-stimulating hormone receptor autoantibodies are thought to stimulate these fibroblasts to produce glycosaminoglycans. Histopathologically, accumulation of glycosaminoglycans deposited in the reticular dermis with high concentrations of hyaluronic acid is observed in PTM.7
Treatment of elephantiasic PTM remains a therapeutic challenge. Given the rarity of the disease process and limited information on effective therapeutic modalities, rituximab should be viewed as a viable treatment option in the management of recalcitrant elephantiasic PTM.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
- Schwartz KM, Fatourechi V, Ahmed DDF, et al. Dermopathy of Graves’ disease (pretibial myxedema): long-term outcome. J Clin Endocrinol Metab. 2002;87:438-446.
- Kakati S, Doley B, Pal S, et al. Elephantiasis nostras verrucosa: a rare thyroid dermopathy in Graves’ disease. J Assoc Physicians India. 2005;53:571-572.
- Anderson CK, Miller OF 3rd. Triad of exophthalmos, pretibial myxedema, and acropachy in a patient with Graves’ disease. J Am Acad Dermatol. 2003;48:970-972.
- Fatourechi V, Bartley GB, Eghbali-Fatourechi GZ, et al. Graves’ dermopathy and acropachy are markers of severe Graves’ ophthalmopathy. Thyroid. 2003;13:1141-1144.
- Heyes C, Nolan R, Leahy M, et al. Treatment‐resistant elephantiasic thyroid dermopathy responding to rituximab and plasmapheresis. Australas J Dermatol. 2012;53:E1-E4.
- Salvi M, Vannucchi G, Campi I, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33-40.
- Heufelder AE, Dutton CM, Sarkar G, et al. Detection of TSH receptor RNA in cultured fibroblasts from patients with Graves’ ophthalmopathy and pretibial dermopathy. Thyroid. 1993;3:297-300.
Practice Points
- Pretibial myxedema (PTM) is bilateral, nonpitting, scaly thickening and induration of the skin that most commonly occurs on the anterior aspects of the legs and feet.
- Although many therapeutic modalities have been described for the management of the elephantiasis variant of PTM, few treatments have shown notable efficacy.
- Rituximab may be an effective therapy to consider in the treatment of elephantiasic PTM.
Sarcoidosis Presenting as Telangiectatic Macules
To the Editor:
Sarcoidosis is a multisystem, noncaseating, granulomatous disorder thought to occur from a combination of immunologic, genetic, and environmental factors.1 Often referred to as the “great imitator,” the cutaneous manifestations of sarcoidosis encompass many morphologies, including papules, plaques, nodules, and scars.1 We report an unusual case of sarcoidosis presenting as telangiectatic macules on the lower extremities.
A woman in her early 30s presented with a burning, pruritic, erythematous, telangiectatic eruption on the lower extremities with concurrent ankle swelling of 4 weeks’ duration. The patient denied any fevers, chills, recent infections, or new medications. Evaluation by her primary care physician during the time of the eruption included unremarkable antinuclear antibodies, thyroid stimulating hormone level, complete blood cell count, comprehensive metabolic panel, urinalysis, chest radiography, and lower-extremity Doppler ultrasonography.
Physical examination at the current presentation revealed numerous scattered, faint, erythematous, blanchable macules on the lower extremities along with mild pitting edema (Figure 1). The patient’s current medications included cetirizine, which she had been taking for years, as well as an intrauterine device. A punch biopsy from the right lower leg revealed small, well-demarcated sarcoidal granulomatous inflammation surrounding vascular structures and skin appendages (Figure 2). No foreign bodies were observed with polarized light microscopy. Microscopic findings suggestive of an infection, including caseation necrosis and suppurative inflammation, also were absent. Angiotensin-converting enzyme levels were normal. Myeloperoxidase and proteinase 3 IgG antibody levels were evaluated due to potential vascular involvement but were negative. An infectious cause of the sarcoidal granulomas was unlikely given histopathologic findings and negative tuberculosis skin testing, which the patient underwent annually for her job, so a tissue culture was not performed. The patient was prescribed triamcinolone acetonide cream 0.1% for the itching and burning at the initial visit and was continued on this treatment after the diagnosis of sarcoidosis was made. At 2-month follow-up, the patient’s eruption had nearly resolved with topical therapy.
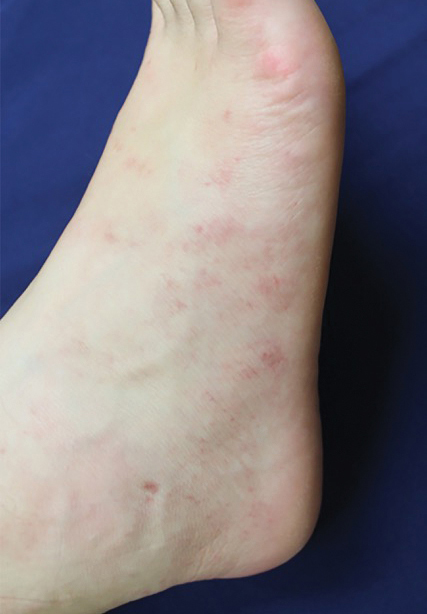
Cutaneous manifestation occurs in 20% to 35% of sarcoidosis cases and may develop in the presence or absence of systemic disease. Approximately 60% of individuals with cutaneous sarcoidosis are found to have systemic involvement; therefore, careful monitoring and diagnostic workup are important in the management of these patients.2 While most cases of cutaneous sarcoidosis are papular, it is important for clinicians to maintain a level of suspicion for sarcoidosis in any uncertain dermatologic presentation.1,2 Evidence of telangiectasias has been shown in rarer forms of sarcoidosis (eg, angiolupoid), but the lesions usually are confined to the face, ears, or neck.3 Granulomatous vasculitis has been reported in a small number of individuals with ulcerative sarcoidosis.4 In our case, no ulcerations were present, possibly indicating an early lesion or an entirely novel process. Lastly, although reticular dermal granulomas are found in drug-induced interstitial granulomatous dermatitis, these lesions often are dispersed interstitially amongst collagen bundles and are associated with necrobiosis of collagen and eosinophilic/neutrophilic infiltrates.5 The lack of these characteristic pathologic findings in our patient along with no known reported cases of cetirizine-induced granulomatous dermatitis led us to rule out reticular dermal granulomas as a diagnosis. We present our case as a reminder of the diversity of cutaneous sarcoidosis manifestations and the importance of early diagnosis of these lesions.
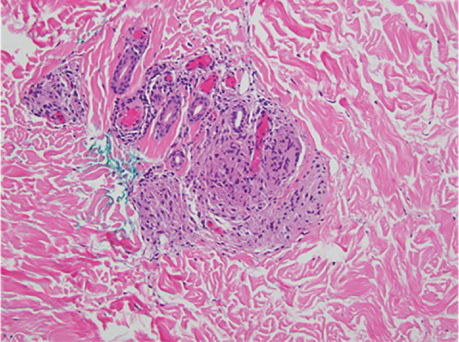
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.E1-E18.
- Yanardag H, Tetikkurt C, Bilir M, et al. Diagnosis of cutaneous sarcoidosis; clinical and the prognostic significance of skin lesions. Multidiscip Respir Med. 2013;8:26.
- Arias-Santiago S, Fernández-Pugnaire MA, Aneiros- Fernández J, et al. Recurrent telangiectasias on the cheek: angiolupoid sarcoidosis. Am J Med. 2010;123:E7-E8.
- Wei C-H, Huang Y-H, Shih Y-C, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
To the Editor:
Sarcoidosis is a multisystem, noncaseating, granulomatous disorder thought to occur from a combination of immunologic, genetic, and environmental factors.1 Often referred to as the “great imitator,” the cutaneous manifestations of sarcoidosis encompass many morphologies, including papules, plaques, nodules, and scars.1 We report an unusual case of sarcoidosis presenting as telangiectatic macules on the lower extremities.
A woman in her early 30s presented with a burning, pruritic, erythematous, telangiectatic eruption on the lower extremities with concurrent ankle swelling of 4 weeks’ duration. The patient denied any fevers, chills, recent infections, or new medications. Evaluation by her primary care physician during the time of the eruption included unremarkable antinuclear antibodies, thyroid stimulating hormone level, complete blood cell count, comprehensive metabolic panel, urinalysis, chest radiography, and lower-extremity Doppler ultrasonography.
Physical examination at the current presentation revealed numerous scattered, faint, erythematous, blanchable macules on the lower extremities along with mild pitting edema (Figure 1). The patient’s current medications included cetirizine, which she had been taking for years, as well as an intrauterine device. A punch biopsy from the right lower leg revealed small, well-demarcated sarcoidal granulomatous inflammation surrounding vascular structures and skin appendages (Figure 2). No foreign bodies were observed with polarized light microscopy. Microscopic findings suggestive of an infection, including caseation necrosis and suppurative inflammation, also were absent. Angiotensin-converting enzyme levels were normal. Myeloperoxidase and proteinase 3 IgG antibody levels were evaluated due to potential vascular involvement but were negative. An infectious cause of the sarcoidal granulomas was unlikely given histopathologic findings and negative tuberculosis skin testing, which the patient underwent annually for her job, so a tissue culture was not performed. The patient was prescribed triamcinolone acetonide cream 0.1% for the itching and burning at the initial visit and was continued on this treatment after the diagnosis of sarcoidosis was made. At 2-month follow-up, the patient’s eruption had nearly resolved with topical therapy.

Cutaneous manifestation occurs in 20% to 35% of sarcoidosis cases and may develop in the presence or absence of systemic disease. Approximately 60% of individuals with cutaneous sarcoidosis are found to have systemic involvement; therefore, careful monitoring and diagnostic workup are important in the management of these patients.2 While most cases of cutaneous sarcoidosis are papular, it is important for clinicians to maintain a level of suspicion for sarcoidosis in any uncertain dermatologic presentation.1,2 Evidence of telangiectasias has been shown in rarer forms of sarcoidosis (eg, angiolupoid), but the lesions usually are confined to the face, ears, or neck.3 Granulomatous vasculitis has been reported in a small number of individuals with ulcerative sarcoidosis.4 In our case, no ulcerations were present, possibly indicating an early lesion or an entirely novel process. Lastly, although reticular dermal granulomas are found in drug-induced interstitial granulomatous dermatitis, these lesions often are dispersed interstitially amongst collagen bundles and are associated with necrobiosis of collagen and eosinophilic/neutrophilic infiltrates.5 The lack of these characteristic pathologic findings in our patient along with no known reported cases of cetirizine-induced granulomatous dermatitis led us to rule out reticular dermal granulomas as a diagnosis. We present our case as a reminder of the diversity of cutaneous sarcoidosis manifestations and the importance of early diagnosis of these lesions.

To the Editor:
Sarcoidosis is a multisystem, noncaseating, granulomatous disorder thought to occur from a combination of immunologic, genetic, and environmental factors.1 Often referred to as the “great imitator,” the cutaneous manifestations of sarcoidosis encompass many morphologies, including papules, plaques, nodules, and scars.1 We report an unusual case of sarcoidosis presenting as telangiectatic macules on the lower extremities.
A woman in her early 30s presented with a burning, pruritic, erythematous, telangiectatic eruption on the lower extremities with concurrent ankle swelling of 4 weeks’ duration. The patient denied any fevers, chills, recent infections, or new medications. Evaluation by her primary care physician during the time of the eruption included unremarkable antinuclear antibodies, thyroid stimulating hormone level, complete blood cell count, comprehensive metabolic panel, urinalysis, chest radiography, and lower-extremity Doppler ultrasonography.
Physical examination at the current presentation revealed numerous scattered, faint, erythematous, blanchable macules on the lower extremities along with mild pitting edema (Figure 1). The patient’s current medications included cetirizine, which she had been taking for years, as well as an intrauterine device. A punch biopsy from the right lower leg revealed small, well-demarcated sarcoidal granulomatous inflammation surrounding vascular structures and skin appendages (Figure 2). No foreign bodies were observed with polarized light microscopy. Microscopic findings suggestive of an infection, including caseation necrosis and suppurative inflammation, also were absent. Angiotensin-converting enzyme levels were normal. Myeloperoxidase and proteinase 3 IgG antibody levels were evaluated due to potential vascular involvement but were negative. An infectious cause of the sarcoidal granulomas was unlikely given histopathologic findings and negative tuberculosis skin testing, which the patient underwent annually for her job, so a tissue culture was not performed. The patient was prescribed triamcinolone acetonide cream 0.1% for the itching and burning at the initial visit and was continued on this treatment after the diagnosis of sarcoidosis was made. At 2-month follow-up, the patient’s eruption had nearly resolved with topical therapy.

Cutaneous manifestation occurs in 20% to 35% of sarcoidosis cases and may develop in the presence or absence of systemic disease. Approximately 60% of individuals with cutaneous sarcoidosis are found to have systemic involvement; therefore, careful monitoring and diagnostic workup are important in the management of these patients.2 While most cases of cutaneous sarcoidosis are papular, it is important for clinicians to maintain a level of suspicion for sarcoidosis in any uncertain dermatologic presentation.1,2 Evidence of telangiectasias has been shown in rarer forms of sarcoidosis (eg, angiolupoid), but the lesions usually are confined to the face, ears, or neck.3 Granulomatous vasculitis has been reported in a small number of individuals with ulcerative sarcoidosis.4 In our case, no ulcerations were present, possibly indicating an early lesion or an entirely novel process. Lastly, although reticular dermal granulomas are found in drug-induced interstitial granulomatous dermatitis, these lesions often are dispersed interstitially amongst collagen bundles and are associated with necrobiosis of collagen and eosinophilic/neutrophilic infiltrates.5 The lack of these characteristic pathologic findings in our patient along with no known reported cases of cetirizine-induced granulomatous dermatitis led us to rule out reticular dermal granulomas as a diagnosis. We present our case as a reminder of the diversity of cutaneous sarcoidosis manifestations and the importance of early diagnosis of these lesions.

- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.E1-E18.
- Yanardag H, Tetikkurt C, Bilir M, et al. Diagnosis of cutaneous sarcoidosis; clinical and the prognostic significance of skin lesions. Multidiscip Respir Med. 2013;8:26.
- Arias-Santiago S, Fernández-Pugnaire MA, Aneiros- Fernández J, et al. Recurrent telangiectasias on the cheek: angiolupoid sarcoidosis. Am J Med. 2010;123:E7-E8.
- Wei C-H, Huang Y-H, Shih Y-C, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.E1-E18.
- Yanardag H, Tetikkurt C, Bilir M, et al. Diagnosis of cutaneous sarcoidosis; clinical and the prognostic significance of skin lesions. Multidiscip Respir Med. 2013;8:26.
- Arias-Santiago S, Fernández-Pugnaire MA, Aneiros- Fernández J, et al. Recurrent telangiectasias on the cheek: angiolupoid sarcoidosis. Am J Med. 2010;123:E7-E8.
- Wei C-H, Huang Y-H, Shih Y-C, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
Practice Points
- Cutaneous manifestations of sarcoidosis can encompass numerous morphologies. A high degree of suspicion should be maintained for any uncertain dermatologic presentation.
- Although papular eruptions are the most common cutaneous findings in sarcoidosis, this case report illustrates a less common vascular-appearing presentation.
- A systemic workup is indicated in any presentation of sarcoidosis.
Graft-vs-host Disease and Toxic Epidermal Necrolysis Following Hematopoietic Stem Cell Transplantation
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.
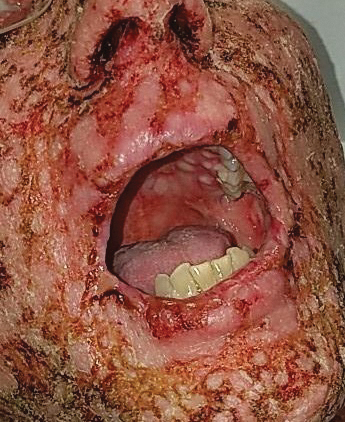
A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.
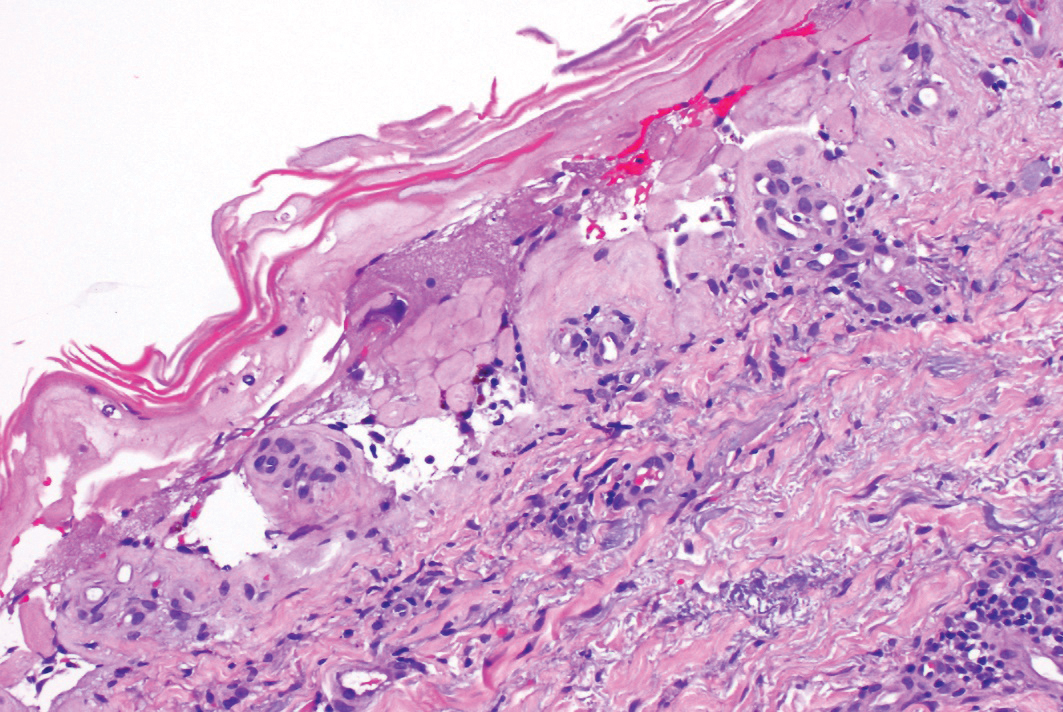
A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.
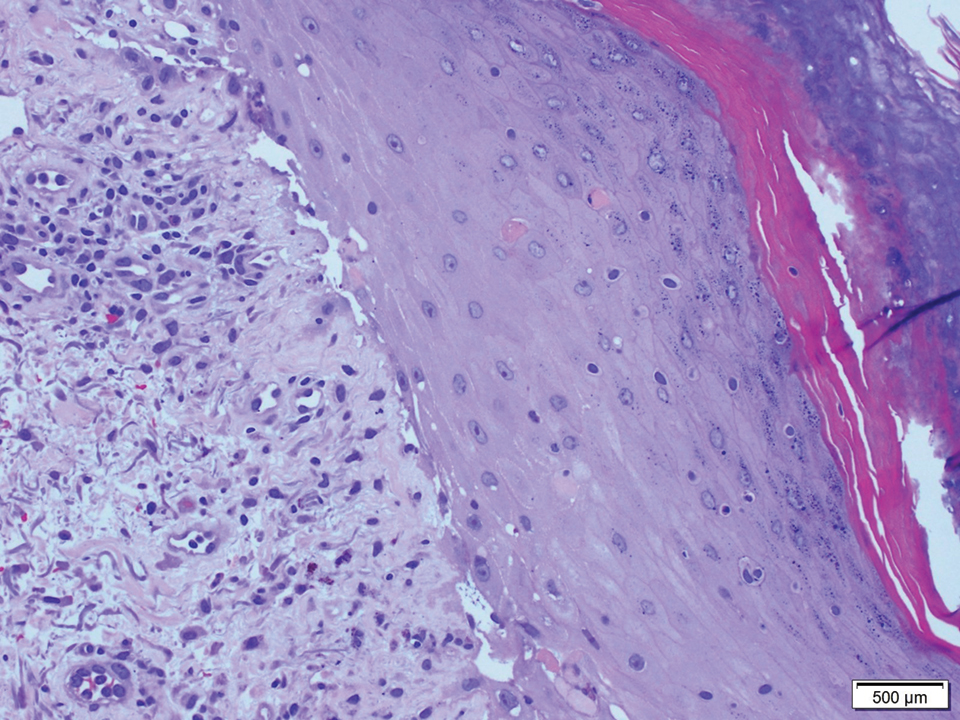
Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8
Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10
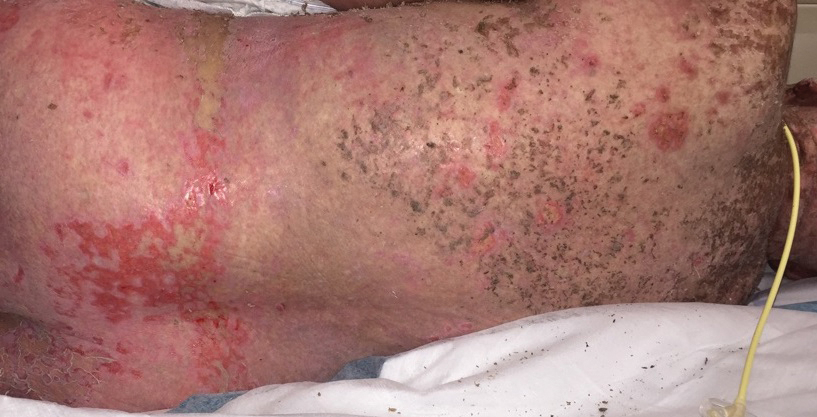
Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.

A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.

A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.

Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8

Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10

Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
To the Editor:
Acute graft-vs-host disease (GVHD) remains a limitation to hematopoietic stem cell transplantation (HSCT) in 20% to 50% of patients after transplant. Furthermore, failed treatment with corticosteroids is frequent and portends a poor prognosis.1 Toxic epidermal necrolysis (TEN) is an epidermolytic skin disorder thought to represent an adverse drug reaction, though its pathogenesis remains unclear. Severe forms of acute GVHD can mimic TEN clinically and histologically. Both can present with widespread cutaneous and mucosal bullae, erosions, and desquamation. Toxic epidermal necrolysis in the context of allogeneic hematopoietic stem cell transplantation is extremely rare, with almost 100% mortality in adult patients. Features that favor acute GVHD over TEN include diarrhea, elevation in bilirubin level, and chimerism.2 However, these features might be absent, posing a therapeutic dilemma, as current treatment preferences for each of these entities differ.
Growing evidence supports the use of anti–tumor necrosis factor (TNF) α drugs for the treatment of TEN. Success has been reported with both anti–TNF-α monoclonal antibodies as well as the soluble fusion protein etanercept.3,4 The use of TNF-α inhibitors in acute GVHD remains anecdotal.

A 58-year-old man (patient 1) with a history of acute myelogenous leukemia presented with a pruritic morbilliform eruption 28 days after HSCT. There was no desquamation or mucosal involvement and the biopsy obtained was histologically suggestive of grade 2 acute GVHD. His immunosuppressive regimen included sirolimus and cyclophosphamide. He was receiving trimethoprim-sulfamethoxazole (TMP-SMX), voriconazole, and acyclovir for infectious prophylaxis. At the time of presentation, he was treated with high-dose systemic steroids (prednisone 2 mg/kg/d) for acute GVHD with partial improvement. Upon tapering of the steroids 3 weeks after initiating TMP-SMX and 1 week after initiating voriconazole, he developed painful desquamation and erosions involving 95% of the body surface area (BSA), necessitating admission to the local burn unit (Figure 1). Biopsies demonstrated full-thickness epidermal necrosis with subepidermal blistering and interface dermatitis (Figure 2). No gastrointestinal tract involvement of acute GVHD was noted. The patient was a 100% donor chimera, supporting the diagnosis of acute GVHD; however, the patient and donor carried the HLA-C*06:02 allele, which previously has been described in association with TMP-SMX–related Stevens-Johnson syndrome/TEN.5 In addition, causality assessment using the algorithm of drug causality for epidermal necrolysis indicated TMP-SMX as a probable cause and voriconazole as a possible cause. The diagnosis of TEN with a SCORe of Toxic Epidermal Necrosis (SCORTEN) of 4 in the setting of acute GVHD was favored, though grade 4 acute GVHD could not be excluded. Trimethoprim-sulfamethoxazole was discontinued, and voriconazole was changed to posaconazole. He received supportive care along with 1 dose of 25-mg subcutaneous etanercept and 3 days of intravenous immunoglobulin (IVIG). Skin re-epithelialization was complete by 3 weeks. At 4 weeks, the patient developed a new asymptomatic erythematous eruption. Biopsies demonstrated changes of acute and chronic GVHD (Figure 3) that resolved with up-titration of sirolimus. The patient remained hospitalized for 96 days and continued to follow up with his transplant team as well as ophthalmology and dermatology. He died 2 years after HSCT.

A 67-year-old woman (patient 2) with high-grade myelodysplastic syndrome presented with an erythematous morbilliform eruption on the torso on day 20 after a matched unrelated HSCT that histologically was consistent with grade 2 GVHD (Figure 4). She had been receiving sirolimus and tacrolimus for GVHD prophylaxis. Infectious prophylaxis included acyclovir, pentamidine, micafungin, and TMP-SMX. Despite high-dose systemic steroids, the rash progressed and ultimately involved 80% BSA. A positive Nikolsky sign was noted involving 21% BSA (Figure 5), in addition to oral and genital mucosal ulcers. She denied nausea, vomiting, fever, or diarrhea. Chimerism studies were negative. Trimethoprim-sulfamethoxazole was discontinued, and she was transferred to a burn unit. Biopsies showed full-thickness epidermal necrosis. A diagnosis of TEN with a SCORTEN of 4 in the setting of acute GVHD was favored; grade 4 acute GVHD could not be excluded. Steroids were discontinued. Because laboratory studies indicated IgA deficiency, IVIG was not considered as a systemic option for therapy. The patient received 1 dose of infliximab (5 mg/kg). Cyclophosphamide 1600 mg weekly was added for GVHD therapy. The wounds progressively healed, and 2 weeks into her admission she was noted to have only 3% BSA with denuded skin. The patient was transferred to the cancer treatment center for further management of the malignancy. Unfortunately, after 2 months she died due to ischemic colitis that was confirmed on autopsy.

Graft-vs-host disease and TEN are rare, life-threatening complications seen in patients with allogeneic HSCT.2 Graft-vs-host disease and TEN share clinicopathologic characteristics and effector immune mechanisms, largely the substantial role of T-cell activation and tissue destruction, which occur through mediators such as TNF-α.6-8

Given the sparse lymphocytic infiltrate, keratinocyte death in TEN is thought to result from soluble molecules, including TNF-α and TNF-related apoptosis-inducing ligand.9 Tumor necrosis factor α has been identified in blister fluid, biopsy specimens, and serum of patients with TEN. Tumor necrosis factor α increases the expression of keratinocyte-inducible nitric oxide synthase, which upregulates keratinocyte Fas ligand expression and subsequent Fas- and caspase-8–mediated keratinocyte cell death.10

Acute GVHD results from donor lymphocyte activation after infusion into damaged recipient tissues that previously have been radiated or chemoablated. Mismatches in histocompatibility complexes between donor cells and recipient tissue antigens serve as the initial trigger for immune activation. Activation of antigen-presenting cells followed by activation, proliferation, differentiation, and migration of donor T cells ultimately results in destruction of the target tissue.11 Immune mediators, such as TNF-α and lymphotoxin α (another member of the TNF superfamily), play a nonredundant role in the pathogenesis of GVHD.12
Current treatment strategies for severe acute GVHD and TEN differ. In North America, high-dose IVIG frequently is used as first-line systemic therapy, while high-dose systemic corticosteroids rarely are used.13 Studies have demonstrated successful use of anti–TNF-α drugs for the treatment of TEN.3,4 Moreover, etanercept has shown to effectively inhibit lymphotoxin α.14 Similarly, TNF inhibition in the management of steroid-refractory acute GVHD has been successful.1 These studies coupled with the underlying immune mechanisms that both diseases share encouraged initiating a trial of anti–TNF-α therapy in our patients.
Patient 1 merits further discussion because he was both a 100% donor chimera as well as a carrier of an human leukocyte antigen susceptibility candidate allele to TMP-SMX. Historical features of his presentation are consistent with either steroid-refractory GVHD or TEN superimposed on acute GVHD. His initial presentation of the more typical macular exanthem of cutaneous acute GVHD was both biopsy proven and supported by clinical improvement with steroid therapy, which was later followed by a robust blistering mucocutaneous presentation approximately 3 weeks after the administration of TMP-SMX and 1 week after initiating voriconazole that improved with IVIG and etanercept.
It is difficult to determine if TEN represents a continuum or result of the underlying drivers of acute GVHD vs a drug reaction. Although there is insufficient evidence to establish a clear-cut diagnosis of TEN, these cases illustrate the need for better diagnostic techniques to allow differentiation between TEN and grade 4 acute GVHD, and in the context of uncertainty, TNF-α inhibition poses a viable therapeutic strategy for these 2 often lethal conditions. Our cases do unequivocally indicate the benefit of this therapeutic modality, add to the current body of literature supporting the use of TNF-α inhibitors in patients such as ours without an official TEN diagnosis, and may guide future investigative efforts.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
- Couriel DR, Saliba R, de Lima M, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555-1562.
- Jeanmonod P, Hubbuch M, Grünhage F, et al. Graft-versus-host disease or toxic epidermal necrolysis: diagnostic dilemma after liver transplantation. Transpl Infect Dis. 2012;14:422-426.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Scott-Lang V, Tidman M, McKay D. Toxic epidermal necrolysis in a child successfully treated with infliximab. Pediatr Dermatol. 2014;31:532-534.
- Kingpin T, Mahasirimongkol S, Konyoung P, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. 2015;25:402-411.
- Correia O, Delgado L, Barbosa IL, et al. Increased interleukin 10, tumor necrosis factor alpha, and interleukin 6 levels in blister fluid of toxic epidermal necrolysis. J Am Acad Dermatol. 2002;47:58-62.
- French LE, Tschopp J. Fas-mediated cell death in toxic epidermal necrolysis and graft-versus-host disease: potential for therapeutic inhibition. Schweiz Med Wochenschr. 2000;130:1656-1661.
- Downey A, Jackson C, Harun N, et al. Toxic epidermal necrolysis: review of pathogenesis and management. J Am Acad Dermatol. 2012;66:995-1003.
- de Araujo E, Dessirier V, Laprée G, et al. Death ligand TRAIL, secreted by CD1a+ and CD14+ cells in blister fluids, is involved in killing keratinocytes in toxic epidermal necrolysis. Exp Dermatol. 2011;20:107-112.
- Viard-Leveugle I, Gaide O, Jankovic D, et al. TNF-α and IFN-γ are potential inducers of Fas-mediated keratinocyte apoptosis through activation of inducible nitric oxide synthase in toxic epidermal necrolysis. J Invest Dermatol. 2013;133:489-498.
- Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010;30:75-101.
- Markey KA, Burman AC, Banovic T, et al. Soluble lymphotoxin is an important effector molecule in GVHD and GVL. Blood. 2010;115:122-132.
- Dodiuk-Gad RP, Olteanu C, Jeschke MG, et al. Treatment of toxic epidermal necrolysis in North America. J Am Acad Dermatol. 2015;73:876-877.
- Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244-279.
Practice Points
- Graft-vs-host disease (GVHD) and toxic epidermal necrolysis (TEN) are rare life-threatening complications seen in patients with allogeneic hematopoietic stem cell transplantation.
- Although mild acute GVHD easily is distinguished from TEN, severe acute GVHD and TEN share overlapping features and present a diagnostic challenge.
- Therapeutic decisions and associated outcomes hinge on accurate diagnosis, as high-dose systemic corticosteroids have been associated with higher mortality rates in TEN.