User login
Tumor Necrosis Factor α Inhibitor–Induced Lupuslike Syndrome in a Patient Prescribed Certolizumab Pegol
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.
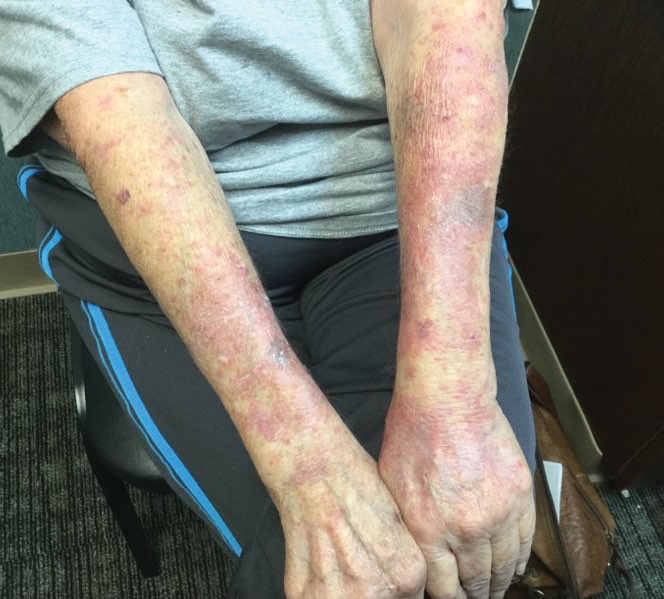
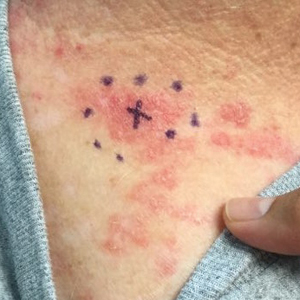
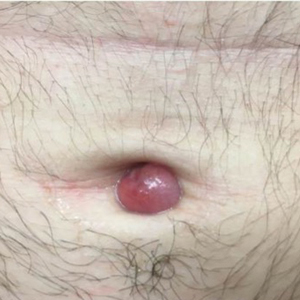
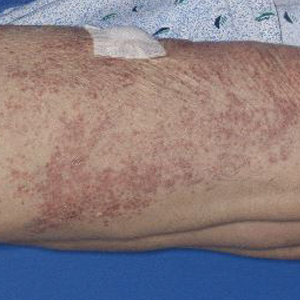
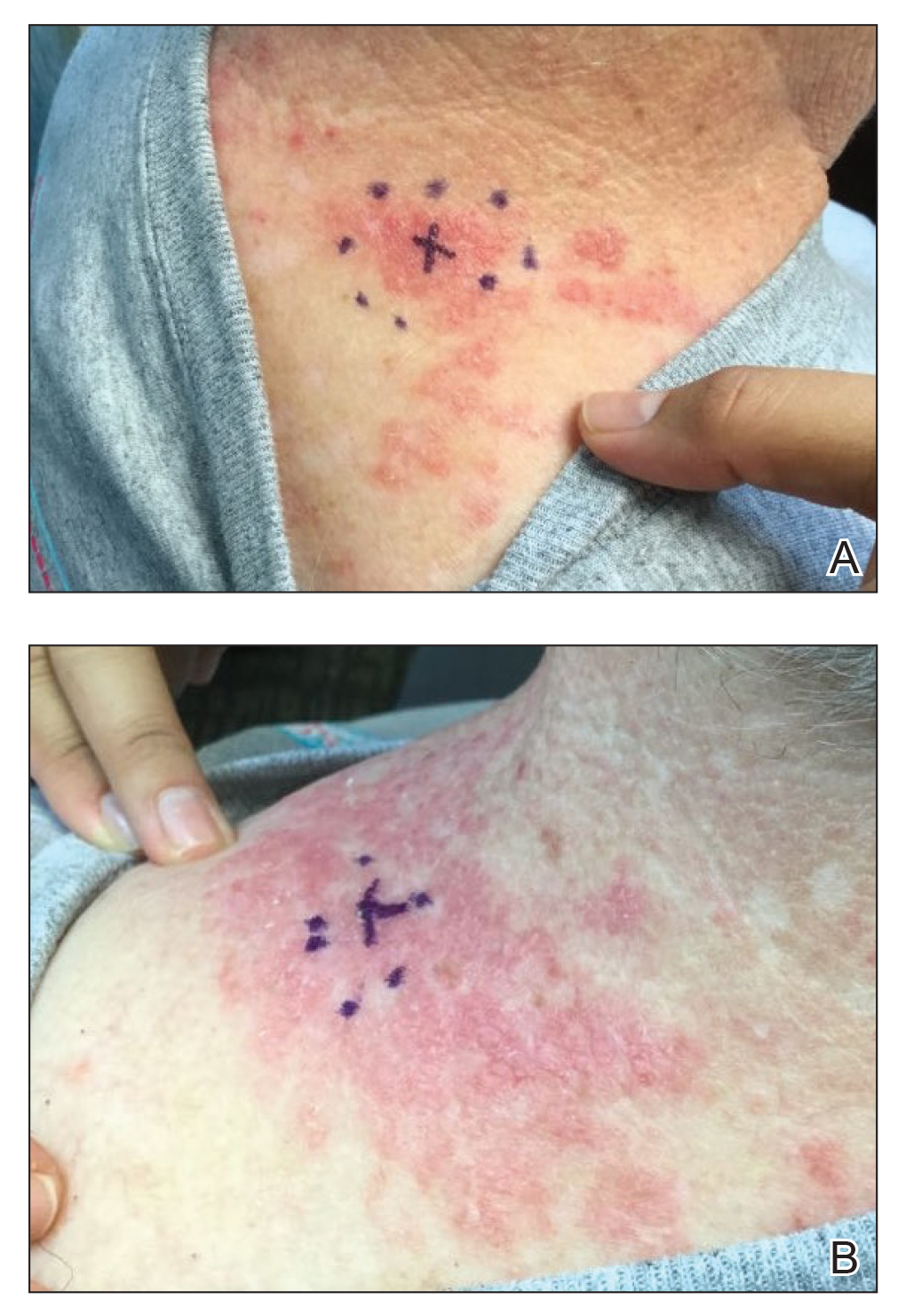
A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.
Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
To the Editor:
Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a newly described entity that refers to the onset of subacute cutaneous lupus erythematosus (SCLE) during drug therapy with TNF-α antagonists. The condition is unique because it is thought to occur via a separate pathophysiologic mechanism than all other agents implicated in the development of drug-induced lupus erythematosus (DILE). Infliximab and etanercept are the 2 most common TNF-α antagonists associated with TAILS. Although rare, adalimumab, golimumab, and certolizumab pegol have been reported to induce this state of autoimmunity. We report an uncommon presentation of TAILS in a patient taking certolizumab pegol with a brief discussion of the pathogenesis underlying TAILS.

A 71-year-old woman presented to the dermatology clinic with a rash located on the arms, face, and trunk that she reported as having been present for months. She had a medical history of rheumatoid arthritis and currently was receiving certolizumab pegol injections. Physical examination revealed erythematous patches and plaques with overlying scaling and evidence of atrophic scarring on sun-exposed areas of the body. The lesions predominantly were in a symmetrical distribution across the extensor surfaces of both outer arms as well as the posterior superior thoracic region extending anteriorly along the bilateral supraclavicular area (Figures 1 and 2). A 4-mm punch biopsy was obtained and sent for histologic analysis, along with a sample of the patient’s serum for antinuclear antibody (ANA) testing.

Hematoxylin and eosin–stained tissue sections of the right superior thoracic lesions revealed epidermal atrophy, hyperkeratosis, and vacuolar alteration of the basal layer with apoptosis, consistent with a lichenoid tissue reaction. In addition, both superficial and deep perivascular and periadnexal lymphocytic infiltrates were observed as well as increased dermal mucin. Serologic testing was performed with a comprehensive ANA panel of the patient’s serum (Table). Of note, there was a speckled ANA pattern (1:1280), with elevated anti–double-stranded DNA (anti-dsDNA) and anti–Sjögren syndrome–related antigen A (anti-SSA)(also called anti-Ro antibodies) levels. The patient’s rheumatologist was consulted; certolizumab pegol was removed from the current drug regimen and switched to a daily regimen of hydroxychloroquine and prednisone. Seven weeks after discontinuation of certolizumab pegol, the patient was symptom free and without any cutaneous involvement. Based on the histologic analysis, presence of anti-SSA (Ro) autoantibodies, and the resolution of symptoms following withdrawal of anti–TNF-α therapy, a diagnosis of TAILS was made.

Subacute cutaneous lupus erythematosus, the most common subset of DILE, typically presents with annular polycyclic or papulosquamous skin eruptions on the legs; patients often test positive for anti-SSA/Ro and/or anti–Sjögren syndrome–related antigen B (also called anti-La) antibodies. Pharmaceutical agents linked to the development of SCLE are calcium channel blockers, angiotensin-converting enzyme inhibitors, thiazide diuretics, terbinafine, the chemotherapeutic agent gemcitabine, and TNF-α antagonists.1,2 Tumor necrosis factor α antagonists are biologic agents that commonly are used in the management of systemic inflammatory diseases such as ulcerative colitis, Crohn disease, seronegative spondyloarthropathies, and rheumatoid arthritis. Among this family of therapeutics includes adalimumab (humanized monoclonal antibody), infliximab (chimeric monoclonal TNF-α antagonist), etanercept (soluble receptor fusion protein), certolizumab pegol (Fab fraction of a human IgG monoclonal antibody), and golimumab (humanized monoclonal antibody).
Tumor necrosis factor α inhibitor–induced lupuslike syndrome most commonly occurs in women in the fifth decade of life, and it is seen more often in those using infliximab or entanercept.3 Although reports do exist, TAILS rarely complicates treatment with adalimumab, golimumab, or certolizumab.4,5 Due to the lack of reports, there are no diagnostic criteria nor an acceptable theory regarding the pathogenesis. In one study in France, the estimated incidence was thought to be 0.19% for infliximab and 0.18% for etanercept.6 Tumor necrosis factor α inhibitor–induced lupuslike syndrome is unique in that it is thought to occur by a different mechanism than that of other known offending agents in the development of DILE. Molecular mimicry, direct cytotoxicity, altered T-cell gene expression, and disruption of central immune tolerance have all been hypothesized to cause drug-induced systemic lupus erythematosus, SCLE, and chronic cutaneous lupus erythematosus. Tumor necrosis factor α inhibitors, are postulated to cause the induction of SCLE via an independent route separate from not only other drugs that cause SCLE but also all forms of DILE as a whole, making it a distinctive player within the realm of agents known to cause a lupuslike syndrome. The following hypotheses may explain this occurrence:
1. Increased humoral autoimmunity: Under normal circumstances, TNF-α activation leads to upregulation in the production of cytotoxic CD8+ T lymphocytes. The upregulation of CD8+ T lymphocytes concurrently leads to a simultaneous suppression of B lymphocytes. Inhibiting the effects of TNF-α on the other hand promotes cytotoxic T-lymphocyte suppression, leading to an increased synthesis of B cells and subsequently a state of increased humoral autoimmunity.7
2. Infection: The immunosuppressive effects of TNF-α inhibitors are well known, and the propensity to develop microbial infections, such as tuberculosis, is markedly increased on the use of these agents. Infections brought on by TNF-α inhibitor usage are hypothesized to induce a widespread activation of polyclonal B lymphocytes, eventually leading to the formation of antibodies against these polyclonal B lymphocytes and subsequently SCLE.8
3. Helper T cell (TH2) response: The inhibition of TH1 CD4+ lymphocytes by TNF-α inversely leads to an increased production of TH2 CD4+ lymphocytes. This increase in the levels of circulating TH2 CD4+ lymphocytes brought on by the action of anti–TNF-α agents is thought to promote the development of SCLE.9,10
4. Apoptosis theory: Molecules of TNF-α inhibitors are capable of binding to TNF-α receptors on the cell surface. In doing so, cellular apoptosis is triggered, resulting in the release of nucleosomal autoantigens from the apoptotic cells. In susceptible individuals, autoantibodies then begin to form against the nucleosomal autoantigens, leading to an autoimmune reaction that is characterized by SCLE.11,12
Major histone compatibility (MHC) antigen testing performed by Sontheimer et al12 established the presence of the HLA class I, HLA-B8, and/or HLA-DR3 haplotypes in patients with SCLE.13,14 Furthermore, there is a well-known association between the antinuclear profile of known SCLE patients and the presence of anti-SSA (Ro) antibodies.13 Therefore, we propose that in susceptible individuals, such as those with the HLA class I, HLA-B8, or HLA-DR3 haplotypes, the initiation of a TNF-α inhibitor causes cellular apoptosis with the subsequent release of nucleosomal and cytoplasmic components (namely that of the Ro autoantigens), inducing a state of autoimmunity. An ensuing immunogenic response is then initiated in predisposed individuals for which anti-SSA (Ro) autoantibodies are produced against these previously mentioned autoantigens.
Drug-induced SCLE is most common in females (71%), with a median age of 58 years. The most common site of cutaneous manifestations is the legs.15 Although our patient was in the eighth decade of life with predominant cutaneous involvement of the upper extremity, the erythematous plaques with a symmetric, annular, polycyclic appearance in photosensitive regions raised a heightened suspicion for lupus erythematosus. Histology classically involves an interface dermatitis with vacuolar or hydropic change and lymphocytic infiltrates,16 consistent with the analysis of tissue sections from our patient. Moreover, the speckled ANA profile with positive anti-dsDNA and anti-SSA (Ro) antibodies in the absence of a negative rheumatoid factor and anticyclic citrullinated peptide antibodies strongly favored the diagnosis of SCLE over alternative diagnoses.2
The supraclavicular rash in our patient raises clinical suspicion for the shawl sign of dermatomyositis, which also is associated with musculoskeletal pain and photosensitivity. In addition, skin biopsy revealed vacuolar alteration of the basement membrane zoneand dermal mucin in both lupus erythematosus and dermatomyositis; therefore, skin biopsy is of little use in distinguishing the 2 conditions, and antibody testing must be performed. Although anti-SSA (Ro) antibodies commonly are associated with SCLE, there are reports involving positivity for the extractable nuclear antigen in cases of dermatomyositis.17 Based on our patient’s current drug regimen, including that of a known offending agent for SCLE, a presumptive diagnosis of TAILS was made. Following withdrawal of certolizumab pegol injections and subsequent resolution of the skin lesions, our patient was given a definitive diagnosis of TAILS based on clinical and pathological assessments.
The clinical diagnosis of TAILS should be made according to the triad of at least 1 serologic and 1 nonserologic American College of Rheumatology criteria, such as anti-SSA (Ro) antibodies and a photosensitive rash, respectively, as well as a relationship between the onset of symptoms and TNF-α inhibitor therapy.18 Both the definitive diagnosis and the treatment of TAILS can be made via withdrawal of the TNF-α inhibitor, which was true in our case whereby chronologically the onset of use with a TNF-α inhibitor was associated with disease onset. Furthermore, withdrawal led to complete improvement of all signs and symptoms, collectively supporting a diagnosis of TAILS. Notably, switching to a different TNF-α inhibitor has been shown to be safe and effective.19
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
- Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatological aspects. Lupus. 2009;18:935-940.
- Wiznia LE, Subtil A, Choi JN. Subacute cutaneous lupus erythematosus induced by chemotherapy: gemcitabine as a causative agent. JAMA Dermatol. 2013;149:1071-1075.
- Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol. 2011;50:619-625.
- Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. Bio Drugs. 2014;28(suppl 1):15-23.
- Mudduluru BM, Shah S, Shamah S. et al. TNF-alpha antagonist induced lupus on three different agents. Postgrad Med. 2017;129:304-306.
- De Bandt M. Anti-TNF-alpha-induced lupus. Arthritis Res Ther. 2019;21:235.
- Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to anti-tumor necrosis factor alfa agents. Semin Arthritis Rheum. 2008;37:381-387.
- Caramaschi P, Biasi D, Colombatti M. Anti-TNF alpha therapy in rheumatoid arthritis and autoimmunity. Rheumatol Int. 2006;26:209-214.
- Yung RL, Quddus J, Chrisp CE, et al. Mechanism of drug-induced lupus. I. cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154:3025-3035.
- Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest. 1996;97:2866-2871.
- Sontheimer RD, Stastny P, Gilliam JN. Human histocompatibility antigen associations in subacute cutaneous lupus erythematosus. J Clin Invest. 1981;67:312-316.
- Sontheimer RD, Maddison PJ, Reichlin M, et al. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982;97:664-671.
- Lee LA, Roberts CM, Frank MB, et al. The autoantibody response to Ro/SSA in cutaneous lupus erythematosus. Arch Dermatol. 1994;130:1262-1268.
- Deutscher SL, Harley JB, Keene JD. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci. 1988;85:9479-9483.
- DalleVedove C, Simon JC, Girolomoni G. Drug-induced lupus erythematosus with emphasis on skin manifestations and the role of anti-TNFα agents [article in German]. J Dtsch Dermatol Ges. 2012;10:889-897.
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404.
- Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8:632-637.
- De Bandt M, Sibilia J, Le Loët X, et al. Systemic lupus erythematosus induced by anti-tumour necrosis factor alpha therapy: a French national survey. Arthritis Res Ther. 2005;7:R545-R551.
- Lupu A, Tieranu C, Constantinescu CL, et al. TNFα inhibitor induced lupus-like syndrome (TAILS) in a patient with IBD. Current Health Sci J. 2014;40:285-288.
Practice Points
- Tumor necrosis factor α (TNF-α) inhibitor–induced lupuslike syndrome (TAILS) is a form of drug-induced lupus specific to patients on anti–TNF-α therapy.
- The underlying mechanism of disease development is unique compared to other types of drug-induced lupus.
- TAILS most commonly is associated with the use of infliximab and etanercept but also has been reported with adalimumab, golimumab, and certolizumab pegol.
Nodules on the Anterior Neck Following Poly-L-lactic Acid Injection
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
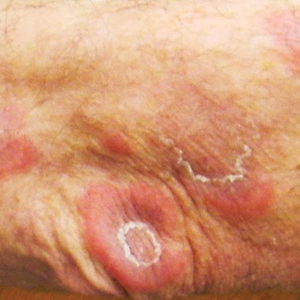
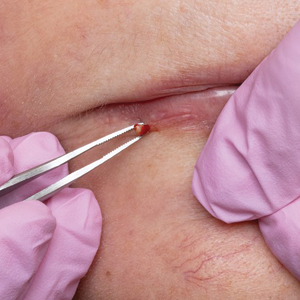
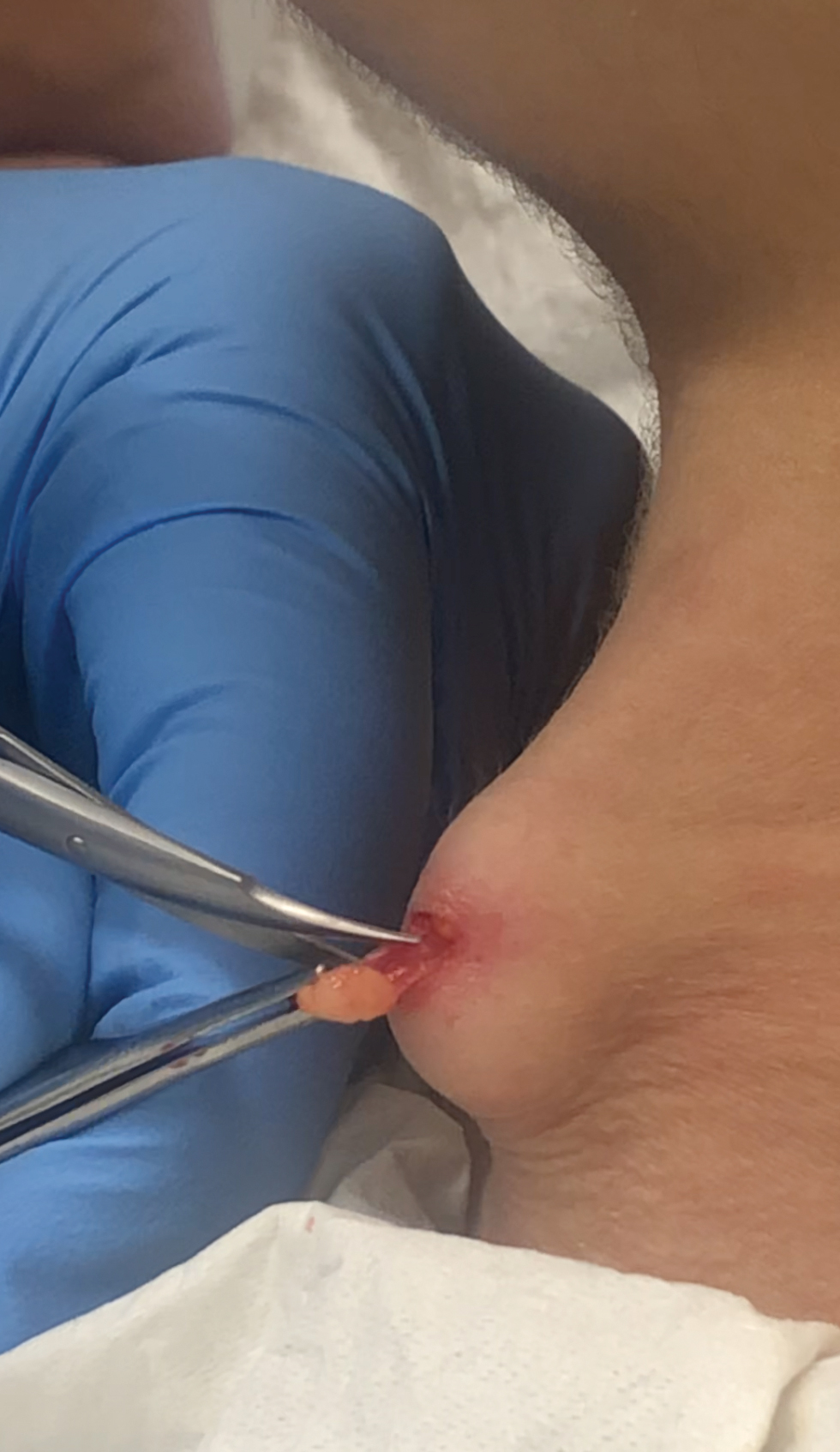
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).
Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
Poly-L-lactic acid (PLLA) is a synthetic biologic polymer that is suspended in solution and can be injected for soft-tissue augmentation. The stimulatory molecule functions to increase collagen synthesis as a by-product of its degradation.1 Poly-L-lactic acid measures 40 to 63 μm and is irregularly shaped, which inhibits product mobility and allows for precise tissue augmentation.2 Clinical trials of injectable PLLA have proven its safety with no reported cases of infection, allergies, or serious adverse reactions.3-5 The most common patient concerns generally are transient in nature, such as swelling, tenderness, pain, bruising, and bleeding. Persistent adverse events of PLLA primarily are papule and nodule formation.6 Clinical trials showed a variable incidence of papule/nodule formation between 6% and 44%.2 Nodule formation remains a major challenge to achieving optimal results from injectable PLLA. We present a case in which a hyperdiluted formulation of PLLA produced a relatively acute (3-week) onset of multiple nodule formations dispersed on the anterior neck. The nodules were resistant to less-invasive treatment modalities and were further requested to be surgically excised.
Case Report
A 38-year-old woman presented for soft-tissue augmentation of the anterior neck using PLLA to achieve correction of skin laxity and static rhytides. She had a history of successful PLLA injections in the temples, knees, chest, and buttocks over a 5-year period. Forty-eight hours prior to injection, 1 PLLA vial was hydrated with 7 cc bacteriostatic water by using a continuous rotation suspension method over the 48 hours. On the day of injection, the PLLA was further hyperdiluted with 2 cc of 2% lidocaine and an additional 7 cc of bacteriostatic water, for a total of 16 cc diluent. The product was injected using a cannula in the anterior and lateral neck. According to the patient, 3 weeks after the procedure she noticed that some nodules began to form at the cannula insertion sites, while others formed distant from those sites; a total of 10 nodules had formed on the anterior neck (Figure 1).

The bacteriostatic water, lidocaine, and PLLA vial were all confirmed not to be expired. The manufacturer was contacted, and no other adverse reactions have been reported with this particular lot number of PLLA. The nodules initially were treated with injections of large boluses of bacteriostatic saline, which was ineffective. Treatment was then attempted using injections of a solution containing 1.0 mL of 5-fluorouracil (5-FU) 50 mg/mL, 0.4 mL of dexamethasone 4 mg/mL, 0.1 mL of triamcinolone 10 mg/mL, and 0.3 mL hyaluronidase. A series of 4 injections was performed in 2- to 4-week intervals. Two of the nodules resolved completely with this treatment. The remaining 8 nodules subjectively improved in size and softened to palpation but did not resolve completely. At 2 of the injection sites, treatment was complicated with steroid atrophy of the overlying skin. At the patient’s request, the remaining nodules were surgically excised (Figure 2). Histopathology revealed exogenous foreign material consistent with dermal filler (Figure 3).

Comment
Causes of Nodule Formation—Two factors that could contribute to nodule formation are inadequate dispersion of molecules and an insufficient volume of dilution. One study demonstrated that hydration for at least 24 hours is required for adequate PLLA dispersion. Furthermore, sonification for 5 minutes after a 2-hour hydration disperses molecules similarly to the 48-hour hydration.7 The PLLA in the current case was hydrated for 48 hours using a continuous rotation suspension method. Therefore, this likely did not play a role in our patient’s nodule formation. The volume of dilution has been shown to impact the incidence of nodule formation.8 At present, most injectors (60.4%) reconstitute each vial of PLLA with 9 to 10 mL of diluent.9 The PLLA in our patient was reconstituted with 16 mL; therefore, we believe that the anatomic location was the main contributor of nodule formation.

Fillers should be injected in the subcutaneous or deep dermal plane of tissue.10 The platysma is a superficial muscle that is intimately involved with the overlying skin of the anterior neck, and injections in this area could inadvertently be intramuscular. Intramuscular injections have a higher incidence of nodule formation.1 Our patient had prior PLLA injections without adverse reactions in numerous other sites, supporting the claim that the anterior neck is prone to nodule formation from PLLA injections.
Management of Noninflammatory Nodules—Initial treatment of nodules with injections of saline was ineffective. This treatment can be used in an attempt to disperse the product. Treatment was then attempted with injections of a solution containing 5-FU, dexamethasone, triamcinolone, and hyaluronidase. Combination steroid therapy may be superior to monotherapy.11 Dexamethasone may exhibit a cytoprotective effect on cells such as fibroblasts when used in combination with triamcinolone; monotherapy steroid use with triamcinolone alone induced fibroblast apoptosis at a much higher level.12 Hyaluronidase works by breaking cross-links in hyaluronic acid, a glycosaminoglycan polysaccharide prevalent in the skin and connective tissue, which increases tissue permeability and aids in delivery of the other injected fluids.13 5-Fluorouracil is an antimetabolite that may aid in treating nodules by discouraging additional fibroblast activity and fibrosis.14
The combination of 5-FU, dexamethasone, and triamcinolone has been shown to be successful in treating noninflammatory nodules in as few as 1 treatment.14 In our patient, hyaluronidase also was used in an attempt to aid delivery of the other injected fluids. If nodules do not resolve with 1 injection, it is recommended to wait at least 8 weeks before repeating the injection to prevent steroid atrophy of the overlying skin. In our patient, the intramuscular placement of the filler contributed to the nodules being resistant to this treatment. During excision, the nodules were tightly embedded in the underlying tissue, which may have prevented the solution from being delivered to the nodule (Figure 2).
Conclusion
Injectable PLLA is approved by the US Food and Drug Administration for soft-tissue augmentation of deep nasolabial folds and facial wrinkles. Off-label use of this product may cause higher incidence of nodule formation. Injectors should be cautious of injecting into the anterior neck. If nodules do form, treatment can be attempted with injections of saline. If that treatment fails, another treatment option is injection(s) of a mixture of 5-FU, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals. Finally, surgical excision is a viable treatment option, as presented in our case.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
- Bartus C, William HC, Daro-Kaftan E. A decade of experience with injectable poly-L-lactic acid: a focus on safety. Dermatol Surg. 2013;39:698-705.
- Engelhard P, Humble G, Mest D. Safety of Sculptra: a review of clinical trial data. J Cosmet Laser Ther. 2005;7:201-205.
- Mest DR, Humble G. Safety and efficacy of poly-L-lactic acid injections in persons with HIV-associated lipoatrophy: the US experience. Dermatol Surg. 2006;32:1336-1345.
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV associated facial lipoatrophy. J Am Acad Dermatol. 2005;52:233-239.
- Cattelan AM, Bauer U, Trevenzoli M, et al. Use of polylactic acid implants to correct facial lipoatrophy in human immunodeficiency virus 1-positive individuals receiving combination antiretroviral therapy. Arch Dermatol. 2006;142:329-334.
- Sculptra. Package insert. sanofi-aventis U.S. LLC; 2009.
- Li CN, Wang CC, Huang CC, et al. A novel, optimized method to accelerate the preparation of injectable poly-L-lactic acid by sonication. J Drugs Dermatol. 2018;17:894-898.
- Rossner F, Rossner M, Hartmann V, et al. Decrease of reported adverse events to injectable polylactic acid after recommending an increased dilution: 8-year results from the Injectable Filler Safety study. J Cosmet Dermatol. 2009;8:14-18.
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Sieber DA, Scheuer JF 3rd, Villanueva NL, et al. Review of 3-dimensional facial anatomy: injecting fillers and neuromodulators. Plast Reconstr Surg Glob Open. 2016;4(12 suppl Anatomy and Safety in Cosmetic Medicine: Cosmetic Bootcamp):E1166.
- Syed F, Singh S, Bayat A. Superior effect of combination vs. single steroid therapy in keloid disease: a comparative in vitro analysis of glucocorticoids. Wound Repair Regen. 2013;21:88-102.
- Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893-897.
- Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosm Investig Dermatol. 2013;6:295-316.
- Aguilera SB, Aristizabal M, Reed A. Successful treatment of calcium hydroxylapatite nodules with intralesional 5-fluorouracil, dexamethasone, and triamcinolone. J Drugs Dermatol. 2016;15:1142-1143.
Practice Points
- Injecting poly-L-lactic acid (PLLA) into the anterior neck is an off-label procedure and may cause a higher incidence of nodule formation.
- Most nodules from PLLA can be treated with injections of 5-fluorouracil, dexamethasone, triamcinolone, and hyaluronidase separated by 8-week intervals.
- Treatment-resistant nodules may require surgical excision.
Acute Alopecia Associated With Albendazole Toxicosis
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
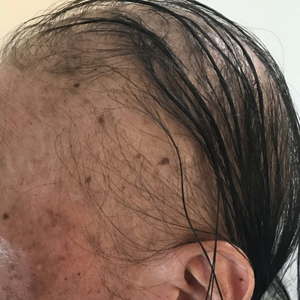
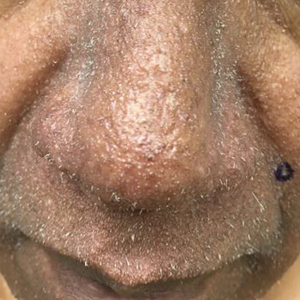
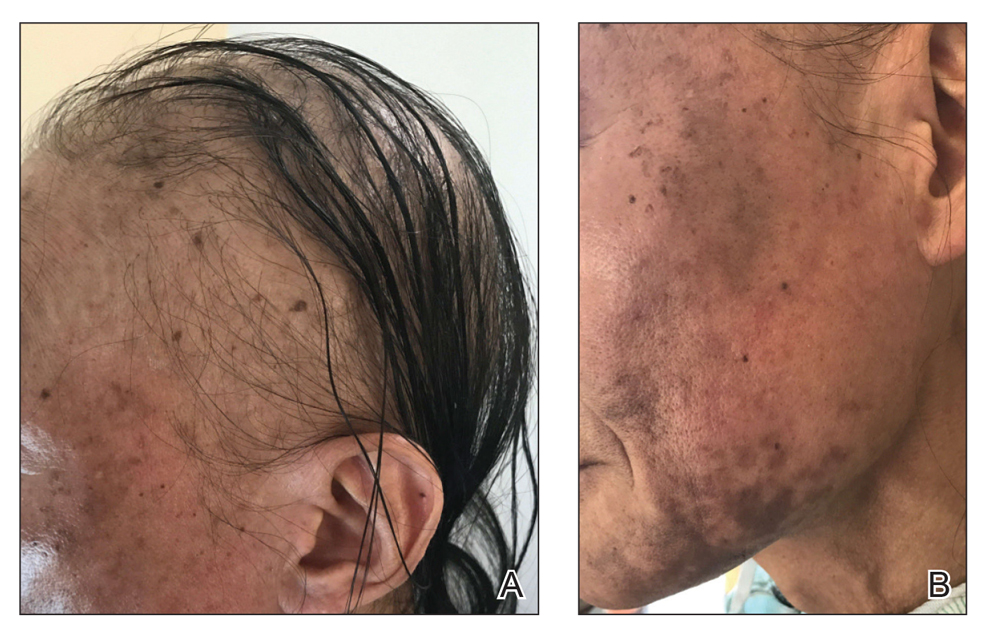
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.
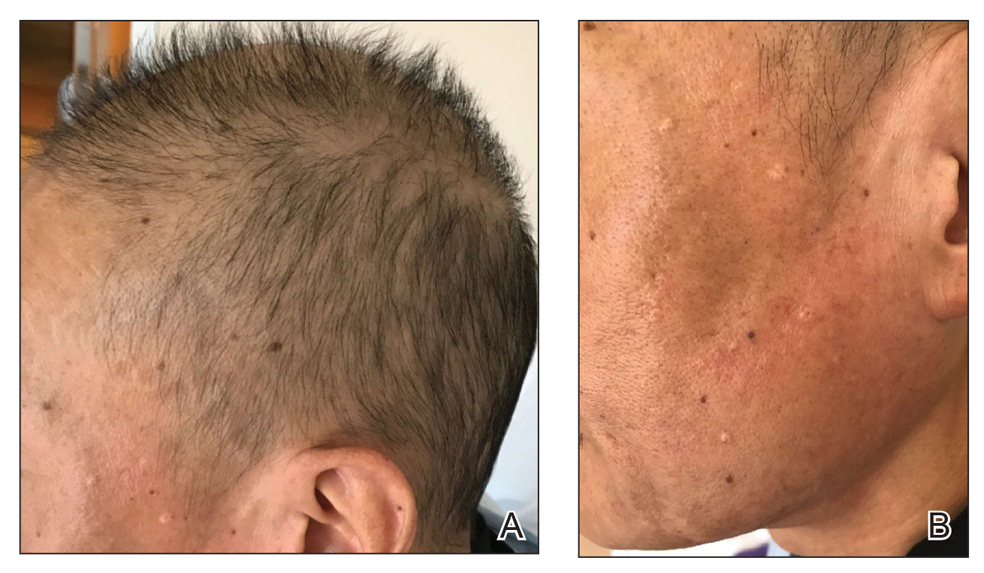
The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).
Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
To the Editor:
Albendazole is a commonly prescribed anthelmintic that typically is well tolerated. Its broadest application is in developing countries that have a high rate of endemic nematode infection.1,2 Albendazole belongs to the benzimidazole class of anthelmintic chemotherapeutic agents that function by inhibiting microtubule dynamics, resulting in cytotoxic antimitotic effects.3 Benzimidazoles (eg, albendazole, mebendazole) have a binding affinity for helminthic β-tubulin that is 25- to 400-times greater than their binding affinity for the mammalian counterpart.4 Consequently, benzimidazoles generally are afforded a very broad therapeutic index for helminthic infection.
A 53-year-old man presented to the emergency department (ED) after an episode of syncope and sudden hair loss. At presentation he had a fever (temperature, 103 °F [39.4 °C]), a heart rate of 120 bpm, and pancytopenia (white blood cell count, 0.4×103/μL [reference range, 4.0–10.0×103/μL]; hemoglobin, 7.0 g/dL [reference range, 11.2–15.7 g/dL]; platelet count, 100
The patient reported severe gastrointestinal (GI) distress and diarrhea for the last year as well as a 25-lb weight loss. He discussed his belief that his GI symptoms were due to a parasite he had acquired the year prior; however, he reported that an exhaustive outpatient GI workup had been negative. Two weeks before presentation to our ED, the patient presented to another ED with stomach upset and was given a dose of albendazole. Perceiving alleviation of his symptoms, he purchased 2 bottles of veterinary albendazole online and consumed 113,000 mg—approximately 300 times the standard dose of 400 mg.
A dermatologic examination in our ED demonstrated reticulated violaceous patches on the face and severe alopecia with preferential sparing of the occipital scalp (Figure 1). Photographs taken by the patient on his phone from a week prior to presentation showed no facial dyschromia or signs of hair loss. A punch biopsy of the chin demonstrated perivascular and perifollicular dermatitis with eosinophils, most consistent with a drug reaction.

The patient received broad-spectrum antibiotics and supportive care. Blood count parameters normalized, and his hair began to regrow within 2 weeks after albendazole discontinuation (Figure 2).

Our patient exhibited symptoms of tachycardia, pancytopenia, and acute massive hair loss with preferential sparing of the occipital and posterior hair line; this pattern of hair loss is classic in men with chemotherapy-induced anagen effluvium.5 Conventional chemotherapeutics include taxanes and Vinca alkaloids, both of which bind mammalian β-tubulin and commonly induce anagen effluvium.
Our patient’s toxicosis syndrome was strikingly similar to common adverse effects in patients treated with conventional chemotherapeutics, including aplastic anemia with severe neutropenia and anagen effluvium.6,7 This adverse effect profile suggests that albendazole exerts an effect on mammalian β-tubulin that is similar to conventional chemotherapy when albendazole is ingested in a massive quantity.
Other reports of albendazole-induced alopecia describe an idiosyncratic, dose-dependent telogen effluvium.8-10 Conventional chemotherapy uncommonly might induce telogen effluvium when given below a threshold necessary to induce anagen effluvium. In those cases, follicular matrix keratinocytes are disrupted without complete follicular fracture and attempt to repair the damaged elongating follicle before entering the telogen phase.7 This observed phenomenon and the inherent susceptibility of matrix keratinocytes to antimicrotubule agents might explain why a therapeutic dose of albendazole has been associated with telogen effluvium in certain individuals.
Our case of albendazole-related toxicosis of this magnitude is unique. Ghias et al11 reported a case of abendazole-induced anagen effluvium. Future reports might clarify whether this toxicosis syndrome is typical or atypical in massive albendazole overdose.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
- Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948. doi:10.1001/jama.299.16.1937
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521-1532. doi:10.1016/S0140-6736(06)68653-4
- Lanusse CE, Prichard RK. Clinical pharmacokinetics and metabolism of benzimidazole anthelmintics in ruminants. Drug Metab Rev. 1993;25:235-279. doi:10.3109/03602539308993977
- Page SW. Antiparasitic drugs. In: Maddison JE, Church DB, Page SW, eds. Small Animal Clinical Pharmacology. 2nd ed. W.B. Saunders; 2008:198-260.
- Yun SJ, Kim S-J. Hair loss pattern due to chemotherapy-induced anagen effluvium: a cross-sectional observation. Dermatology. 2007;215:36-40. doi:10.1159/000102031
- de Weger VA, Beijnen JH, Schellens JHM. Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel—a review. Anticancer Drugs. 2014;25:488-494. doi:10.1097/CAD.0000000000000093
- Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:E50-E59. doi:10.1016/S1470-2045(12)70553-3
- Imamkuliev KD, Alekseev VG, Dovgalev AS, et al. A case of alopecia in a patient with hydatid disease treated with Nemozole (albendazole)[in Russian]. Med Parazitol (Mosk). 2013:48-50.
- Tas A, Köklü S, Celik H. Loss of body hair as a side effect of albendazole. Wien Klin Wochenschr. 2012;124:220. doi:10.1007/s00508-011-0112-y
- Pilar García-Muret M, Sitjas D, Tuneu L, et al. Telogen effluvium associated with albendazole therapy. Int J Dermatol. 1990;29:669-670. doi:10.1111/j.1365-4362.1990.tb02597.x
- Ghias M, Amin B, Kutner A. Albendazole-induced anagen effluvium. JAAD Case Rep. 2020;6:54-56.
PRACTICE POINTS
- Albendazole functions by inhibiting microtubule dynamics and has a remarkably greater binding affinity for helminthic β-tubulin than for its mammalian counterpart.
- An uncommon adverse effect of albendazole at therapeutic dosing is a dose-dependent telogen effluvium in susceptible persons, likely caused by the inherent susceptibility of follicular matrix keratinocytes to antimicrotubule agents.
- Massive albendazole overdose can cause anagen effluvium and myelosuppression similar to the effects of conventional chemotherapy.
Sweet Syndrome With Pulmonary Involvement Preceding the Development of Myelodysplastic Syndrome
To the Editor:
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.
With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
To the Editor:
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.

With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
To the Editor:
A 59-year-old man was referred to our clinic for a rash, fever, and night sweats following treatment for metastatic seminoma with cisplatin and etoposide. Physical examination revealed indurated erythematous papules and plaques on the trunk and upper and lower extremities, some with annular or arcuate configuration with trailing scale (Figure, A). A skin biopsy demonstrated mild papillary dermal edema with a mixed infiltrate of mononuclear cells, neutrophils, eosinophils, mast cells, lymphocytes, and karyorrhectic debris without evidence of leukocytoclastic vasculitis. The histopathologic differential diagnosis included a histiocytoid variant of Sweet syndrome (SS), and our patient’s rapid clinical response to corticosteroids supported this diagnosis.

With a relapsing and remitting course over 3 years, the rash eventually evolved into more edematous papules and plaques (Figure, B), and a repeat biopsy 3 years later was consistent with classic SS. Although the patient's condition improved with prednisone, attempts to taper prednisone invariably resulted in relapse. Multiple steroid-sparing agents were trialed over the course of 3 years including dapsone and mycophenolate mofetil, both of which resulted in hypersensitivity drug eruptions. Colchicine and methotrexate were ineffective. Thalidomide strongly was considered but ultimately was avoided due to substantial existing neuropathy associated with his prior chemotherapy for metastatic seminoma.
Four years after the initial diagnosis of SS, our patient presented with dyspnea and weight loss. Computed tomography revealed a nearly confluent miliary pattern of nodularity in the lungs. A wedge biopsy demonstrated pneumonitis with intra-alveolar fibrin and neutrophils with a notable absence of granulomatous inflammation. Fungal and acid-fast bacilli staining as well as tissue cultures were negative. He had a history of Mycobacterium kansasii pulmonary infection treated 18 months prior; however, in this instance, the histopathology, negative microbial cultures, and rapid steroid responsiveness were consistent with pulmonary involvement of SS. Over the ensuing 2 years, the patient developed worsening of his chronic anemia. He was diagnosed with myelodysplastic syndrome (MDS) by bone marrow biopsy, despite having a normal bone marrow biopsy more than 3 years prior to evaluate his anemia. At this time, thalidomide was initiated at 50 mg daily leading to notable improvement in his SS symptoms; however, he developed worsening neuropathy resulting in the discontinuation of this treatment 2 months later. An investigational combination of vosaroxin and azacytidine was used to treat his MDS, resulting in normalization of blood counts and remission from SS.
Sweet syndrome may occur in the setting of undiagnosed cancer or may signal the return of a previously treated malignancy. The first description of SS associated with solid tumors was in a patient with testicular cancer,1 which prompted continuous surveillance for recurrent seminoma in our patient, though none was found. Hematologic malignancies, as well as MDS, often are associated with SS.2 In our patient, multiple atypical features linked the development of SS to the ultimate presentation of MDS. The initial finding of a histiocytoid variant has been described in a case series of 9 patients with chronic relapsing SS who eventually developed MDS with latency of up to 7 years. The histopathology in these cases evolved over time to that of classic neutrophilic SS.3 Pulmonary involvement of SS is another interesting aspect of our case. In one analysis, 18 of 34 (53%) cases with pulmonary involvement featured hematologic pathology, including myelodysplasia and acute leukemia.4
In our patient, SS preceded the clinical manifestation of MDS by 6 years. A similar phenomenon has been described in a patient with SS that preceded myelodysplasia by 30 months and was recalcitrant to numerous steroid-sparing therapies except thalidomide, despite the persistence of myelodysplasia. Tapering thalidomide, however, resulted in recurrence of SS lesions in that patient.5 In another case, resolution of myelodysplasia from azacytidine treatment was associated with remission from SS.6
Our case represents a confluence of atypical features that seem to define myelodysplasia-associated SS, including the initial presentation with a clinically atypical histiocytoid variant, chronic relapsing and remitting course, and extracutaneous involvement of the lungs. These findings should prompt surveillance for hematologic malignancy or myelodysplasia. Serial bone marrow biopsies were required to evaluate persistent anemia before the histopathologic findings of MDS became apparent in our patient. Thalidomide was an effective treatment for the cutaneous manifestations in our patient and should be considered as a steroid-sparing agent in the treatment of recalcitrant SS. Despite the discontinuation of thalidomide therapy, effective control of our patient’s myelodysplasia with chemotherapy has kept him in remission from SS for more than 7 years of follow-up, suggesting a causal relationship between these disorders.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
- Shapiro L, Baraf CS, Richheimer LL. Sweet’s syndrome (acute febrile neutrophilic dermatosis): report of a case. Arch Dermatol. 1971;103:81-84.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Vignon-Pennamen MD, Juillard C, Rybojad M, et al. Chronic recurrent lymphocytic Sweet syndrome as a predictive marker of myelodysplasia. Arch Dermatol. 2006;142:1170-1176.
- Fernandez-Bussy S, Labarca G, Cabello F, et al. Sweet’s syndrome with pulmonary involvement: case report and literature review. Respir Med Case Rep. 2012;6:16-19.
- Browning CE, Dixon DE, Malone JC, et al. Thalidomide in the treatment of recalcitrant Sweet’s syndrome associated with myelodysplasia. J Am Acad Dermatol. 2005;53(2 suppl 1):S135-S138.
- Martinelli S, Rigolin GM, Leo G, et al. Complete remission Sweet’s syndrome after azacytidine treatment for concomitant myelodysplastic syndrome. Int J Hematol. 2014;99:663-667.
Practice Points
- Sweet syndrome is characterized by the clinical constellation of fever, a skin eruption of tender erythematous papules or plaques, and response to corticosteroids.
- Skin biopsy characteristically demonstrates marked papillary dermal edema with a dense infiltrate of mature neutrophils without leukocytoclasia.
- Sweet syndrome often is idiopathic, though it has been associated with infection, autoimmunity, medication, and malignancy.
Calcified Urachal Remnant in a Young Adult: An Unusual Case
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.
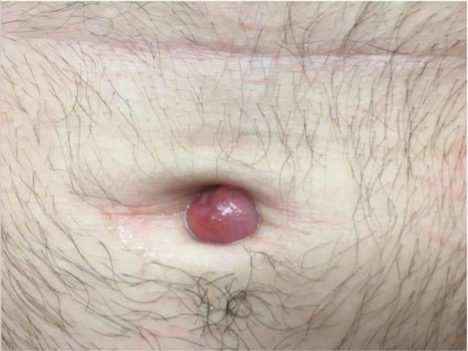
Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.
The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.
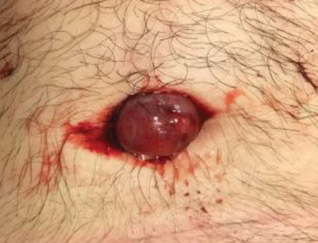
We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.

Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.

We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
To the Editor:
An otherwise healthy 26-year-old man presented to our outpatient clinic with a 15- to 20-mm, shiny, friable-appearing, red umbilical nodule with clear malodorous discharge (Figure 1). The lesion developed 2 weeks prior and gradually increased in size and discomfort. The patient reported mild associated abdominal pain. He had no fever, changes in urination or bowel movements, or prior history of umbilical growths or drainage. The abdomen was tender to palpation.

Differential diagnoses included pyogenic granuloma, umbilical hernia, epidermoid cyst or abscess, and malignancy (low suspicion). A biopsy was not performed due to concern for bleeding or communication with the bowel. A complete blood cell count, comprehensive metabolic panel, and urinalysis were unremarkable except for mild leukocytosis and elevated C-reactive protein. Ultrasonography revealed a 1.4×1.3-cm inflammatory umbilical mass with no communication with the bowel. The patient was referred to the emergency department (ED) for further evaluation. Computed tomography (CT) revealed periumbilical inflammation and an associated 1-cm calcification that appeared to be connected to a potential tract from the bladder, suggestive of a urachal remnant calcification (Figure 2). The patient was diagnosed with a persistent urachal remnant, discharged home with ciprofloxacin, and scheduled for a follow-up with urology.

The patient returned to the ED 3 days later with painful umbilical bleeding (Figure 3). While there, the patient extracted a 1-cm stone from the lesion, consistent with the calcification visualized on CT scan. Computed tomographic virtual cystoscopy showed no connection between the bladder and umbilicus. He was diagnosed with an umbilical-urachal sinus. Complete surgical excision was recommended and performed by urology without complication.

We report an unusual presentation of a symptomatic urachal remnant in an adult. During embryogenesis, the urachus connects the umbilicus to the developing bladder and normally involutes during development. Incomplete regression can cause rare pathological urachal anomalies. The clinical presentation is nonspecific and differs between children and adults, with most cases presenting during infancy or childhood.1 Pediatric urachal abnormalities often present with umbilical drainage, abdominal pain, a palpable mass, an abnormal appearance of the umbilicus, or urinary tract infections.2,3 In adults, the most common symptoms include hematuria, pain, or dysuria. Alternatively, they may be asympomatic3 or present with periumbilical dermatitis4 or abscess. Rodrigues and Gandhi5 reported another case of a symptomatic calculus formed within a urachal remnant. Calcifications in urachal remnants are rare and usually are reported as incidental radiologic findings.
Overall, visible umbilical masses occur infrequently. In addition to urachal anomalies, the differential diagnosis includes several benign and malignant pathologies. Benign causes include epidermoid cysts, foreign body granulomas, pyogenic granulomas, abscesses, hamartomas, nevi, hemangiomas, dermatofibromas, neurofibromas, lipomas, granular cell tumors, desmoid tumors, keloid scars, omphaliths, hernias, or omphalomesenteric duct remnants.6 Primary malignancies (eg, skin cancers, urachal adenocarcinoma, mesenchymal tumors) or metastasis (ie, Sister Mary Joseph nodule) also can present as umbilical nodules.
The wide range of clinical presentations of urachal anomalies combined with the rarity make diagnosis difficult. Thus, it is essential to have a high index of suspicion and awareness of how they can present. Ultrasonography and CT scan are useful tools in making the diagnosis. Urachal anomalies are prone to infection or can be associated with malignancy; therefore, timely and correct diagnosis is critical. Although surgical removal is the primary treatment for urachal anomalies, it may not be the primary treatment of the other entities included in the differential diagnosis of umbilical nodules. For example, the Sister Mary Joseph nodule can be associated with various primary malignancies, which should be treated accordingly.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
- Berman SM, Tolia BM, Laor E, et al. Urachal remnants in adults. Urology. 1988;31:17-21.
- Gleason JM, Bowlin PR, Bagli DJ, et al. A comprehensive review of pediatric urachal anomalies and predictive analysis for adult urachal adenocarcinoma. J Urol. 2015;193:632-636.
- Naiditch JA, Radhakrishnan J, Chin AC. Current diagnosis and management of urachal remnants. J Pediatr Surg. 2013;48:2148-2152.
- Cox GA, Chan I, Lloyd J, et al. Urachal sinus presenting as periumbilical dermatitis. Br J Dermatol. 2007;157:419-420.
- Rodrigues JCL, Gandhi S. Don’t get caught out! a rare case of a calcified urachal remnant mimicking a bladder calculus. J Radiol Case Rep. 2013;7:34-38.
- Ramoutar A, El Sheikh S, Aslam A. A persistent umbilical nodule. Clin Exp Dermatol. 2017;42:814-816.
Practice Points
- Visible umbilical nodules occur infrequently; the differential diagnosis is broad and consists of various benign and malignant pathologies.
- Disruption of the involution of the urachus during development can lead to various rare anomalies.
- Urachal anomalies are important to diagnose given the potential for secondary infection or malignancy.
Postirradiation Pseudosclerodermatous Panniculitis: A Rare Complication of Megavoltage External Beam Radiotherapy
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.
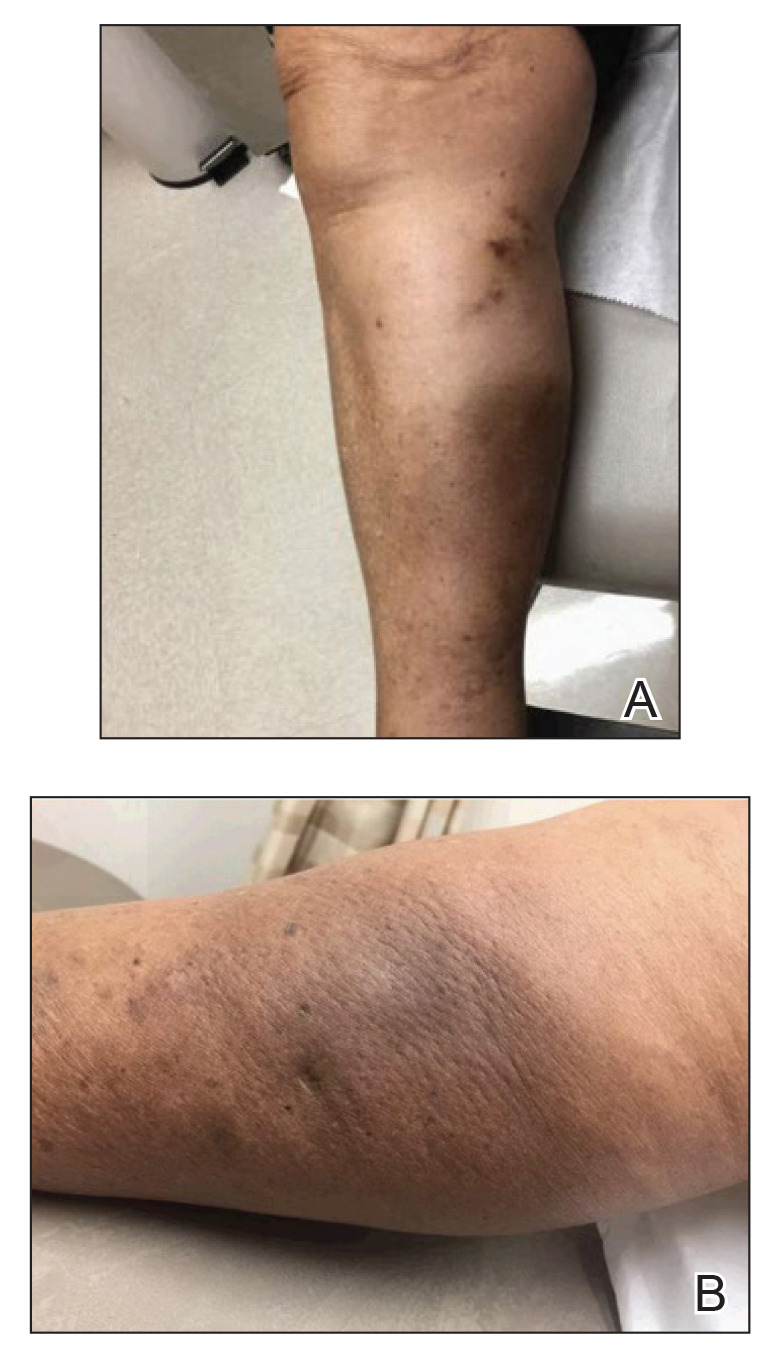
The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.
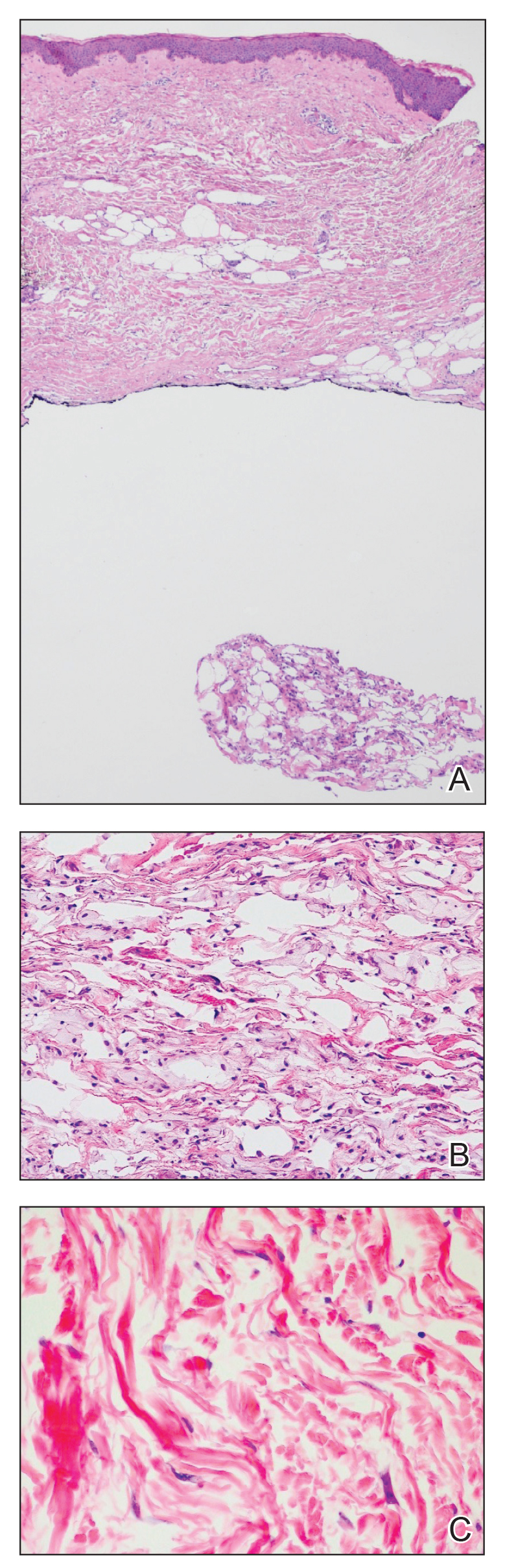
Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.

The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.

Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
To the Editor:
Postirradiation pseudosclerodermatous panniculitis (PIPP) is a rarely reported complication of megavoltage external beam radiotherapy that was first identified in 1993 by Winkelmann et al.1 The condition presents as an erythematous or hyperpigmented indurated plaque at a site of prior radiotherapy. Lesions caused by PIPP most commonly arise several months after treatment, although they may emerge up to 17 years following exposure.2 Herein, we report a rare case of a patient with PIPP occurring on the leg who previously had been treated for Kaposi sarcoma.
An 84-year-old woman presented with a tender plaque on the right lower leg of 2 months’ duration. Her medical history was remarkable for Kaposi sarcoma, with multiple sites on the body treated with megavoltage external beam radiotherapy during the prior 4 years. The most recent treatment occurred 8 months prior to presentation, at which time she had undergone radiotherapy for lesions on the posterior lower right leg. Physical examination demonstrated a hyperpigmented and indurated plaque at the treatment site (Figure 1). Skin biopsy results showed a mildly sclerotic dermis with atypical radiation fibroblasts scattered interstitially between collagen bundles, and a lobular panniculitis with degenerated adipocytes and foamy histiocytes (Figure 2). Hyalinized dermal vessels also were present. Based on the constellation of these biopsy findings, a diagnosis of PIPP was made.

The diagnosis of PIPP is challenging and invariably requires histologic examination. Clinically, the differential diagnosis includes cutaneous metastasis of the primary neoplasm, cellulitis, lipodermatosclerosis, morphea, and chronic radiation dermatitis.

Histologically, PIPP is characterized by a lobular panniculitis without vasculitis. Typical findings include the presence of centrilobular necrotic adipocytes along with a foamy histiocytic infiltrate containing lipophagic granulomas at the periphery of the fat lobules. Septal thickening and sclerosis around fat lobules also have been described, and dermal changes associated with chronic radiation dermatitis, such as papillary dermal sclerosis, endothelial swelling, vascular hyaline arteriosclerosis, and atypical star-shaped radiation fibroblasts, may be present.2 Features of radiation-induced vasculopathy commonly are seen, although the appearance of these features varies over time. Intimal injury and mural thrombosis can develop within 5 years of radiation therapy, fibrosis of the vessel wall can occur within 10 years of radiation therapy, and atherosclerosis and periarterial fibrosis can appear within 20 years of radiation therapy.2,3 The histologic findings in our patient showed characteristic dermal findings seen in radiation dermatitis in addition to a lobular panniculitis with foamy histiocytes and mild vessel damage.
In contrast, lipodermatosclerosis is a septal and lobular panniculitis with septal fibrosis. Membranocystic fat necrosis is present, characterized by fat microcysts lined by feathery eosinophilic material. Stasis changes in the dermis and epidermis are accompanied by a mild perivascular lymphocytic infiltrate.
Patients with traumatic panniculitis, which also may enter the clinical differential diagnosis of PIPP, often demonstrate nonspecific histologic changes. Early lesions show a perivascular infiltrate of lymphocytes and macrophages. Evolving lesions show variably sized fat microcysts surrounded by histiocytes, in addition to possible calcifications and a foreign-body giant cell reaction. A fibrous capsule may develop, surrounding the fat necrosis to form a mobile encapsulated lipoma. Late lesions frequently demonstrate lipomembranous changes and calcium deposits.4
To date, nearly all cases of PIPP in the literature have been described in breast cancer patients.1,2,5,6 However, Sandoval et al7 reported a case of PIPP occurring in the leg of a patient after radiotherapy for a soft tissue sarcoma. Similar to our patient, this patient presented with a painful, dully erythematous, indurated plaque, although her symptoms arose 5 years after radiotherapy.
Megavoltage external beam radiotherapy has become a widely used modality in the treatment of various cancers. As such, PIPP may represent an underdiagnosed condition with potential cases remaining unidentified when the clinical differential diagnosis does not lead to biopsy. Effective therapies have yet to be widely reported, and our patient failed to experience notable improvement with either topical or intralesional corticosteroids. Further studies are needed in order to address this knowledge gap.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
- Winkelmann RK, Grado GL, Quimby SR, et al. Pseudosclerodermatous panniculitis after irradiation: an unusual complication of megavoltage treatment of breast carcinoma. Mayo Clin Proc. 1993;68:122-127.
- Pielasinski U, Machan S, Camacho D, et al. Postirradiation pseudosclerodermatous panniculitis: three new cases with additional histopathologic features supporting the radiotherapy etiology. Am J Dermatopathol. 2013;35:129-134.
- Butler MJ, Lane RH, Webster JH. Irradiation injury to large arteries. Br J Surg. 1980;67:341-343. Moreno A, Marcoval J, Peyri J. Traumatic panniculitis. Dermatol Clin. 2008;26:481-483.
- Shirsat HS, Walsh NM, McDonald LJ, et al. Postirradiation pseudosclerodermatous panniculitis with involvement of breast parenchyma: a dramatic example of a rare entity and a pitfall in diagnosis. J Cutan Pathol. 2016;43:444-450.
- Carrasco L, Moreno C, Pastor MA, et al. Postirradiation pseudosclerodermatous panniculitis. Am J Dermatopathol. 2001;23:283-287.
- Sandoval M, Giesen L, Cataldo K, et al. Postirradiation pseudosclerodermatous panniculitis of the leg: report of a case and review of the literature. Am J Dermatopathol. 2015;37:587-589.
Practice Points
- Postirradiation pseudosclerodermatous panniculitis presents as an erythematous or indurated plaque at a site of prior radiotherapy.
- This rare entity may be underreported and requires biopsy for accurate diagnosis.
Forceps for Milia Extraction
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.
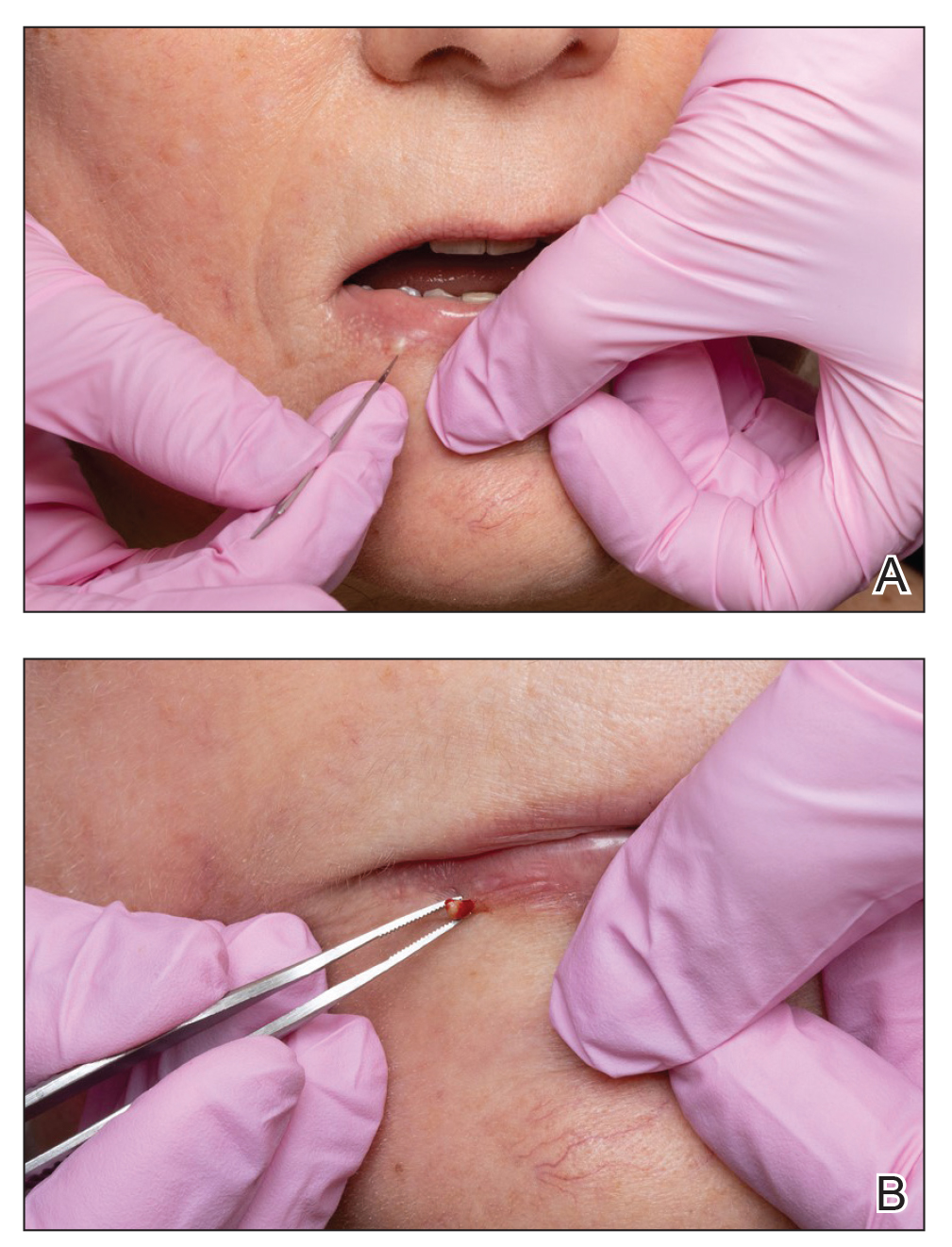
Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.

Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
To the Editor:
Several techniques can be used to destroy milia including electrocautery, electrodesiccation, and laser therapy. Manual extraction of milia uses a scalpel blade, needle, or stylet followed by the application of pressure to the lesion with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Many of these techniques fail to stabilize milia, particularly in sensitive areas such as around the eyes or mouth, which can make extraction challenging, inefficient, and painful for the patient. We report a novel technique that quickly and effectively removes milia with equipment commonly used in the practice of clinical dermatology.
A 74-year-old woman presented with an asymptomatic papule on the right lower vermilion border of several years' duration. Physical examination of the lesion revealed a 3-mm, firm, white, dome-shaped papule. Clinical features were most consistent with a benign acquired milium. The patient desired removal for cosmesis. The area was cleaned with an alcohol swab, the surface of the milium was nicked with a No. 11 blade (Figure, A), and then tips of nontoothed Adson forceps were used to gently secure and pinch the base of the papule (Figure, B). The intact cyst was quickly and effortlessly expressed through the epidermal nick. The patient tolerated the procedure well, experiencing minimal pain and bleeding.

Histologically, milia represent infundibular keratin-filled cysts lined with stratified squamous epithelial tissue that contains a granular cell layer. These lesions are classified as primary or secondary; the former represent spontaneous occurrence, and the latter are associated with medications, trauma, or genodermatoses.2 Multiple milia are associated with conditions such as Bazex-Dupré-Christol syndrome, Rombo syndrome, Brooke-Spiegler syndrome, oro-facial-digital syndrome type I, atrichia with papular lesions, pachyonychia congenita type 2, basal cell nevus syndrome, basaloid follicular hamartoma syndrome, and hereditary vitamin D–dependent rickets type 2.5-9 The most common subtype seen in clinical practice includes benign primary milia, which tends to favor the cheeks and eyelids.2
Although these lesions are benign, many patients seek extraction for cosmesis. Milia extraction is a common procedure performed in dermatology clinical practice. Proposed extraction techniques using destructive methods include electrocautery, electrodesiccation, and laser therapy, and manual methods include nicking the surface of the lesion with a scalpel blade, needle, or stylet and then applying tangential pressure with a curette, comedone extractor, paper clip, cotton-tipped applicator, tongue blade, or hypodermic needle.1-4 Topical retinoids have been proposed as treatment of multiple milia.10 Many of these techniques do not use equipment common to clinical practice, or they fail to stabilize milia in sensitive areas, which makes extraction challenging. We describe a case with a new manual technique that successfully extracts milia in an efficient and safe manner.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
- Parlette HL III. Management of cutaneous cysts. In: Wheeland RG, ed. Cutaneous Surgery. WB Saunders; 1994:651-652.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- George DE, Wasko CA, Hsu S. Surgical pearl: evacuation of milia with a paper clip. J Am Acad Dermatol. 2006;54:326.
- Thami GP, Kaur S, Kanwar AJ. Surgical pearl: enucleation of milia with a disposable hypodermic needle. J Am Acad Dermatol. 2002;47:602-603.
- Goeteyn M, Geerts ML, Kint A, et al. The Bazex-Dupré-Christol syndrome. Arch Dermatol. 1994;130:337-342.
- Michaëlsson G, Olsson E, Westermark P. The Rombo syndrome: a familial disorder with vermiculate atrophoderma, milia, hypotrichosis, trichoepitheliomas, basal cell carcinomas and peripheral vasodilation with cyanosis. Acta Derm Venereol. 1981;61:497-503.
- Gurrieri F, Franco B, Toriello H, et al. Oral-facial-digital syndromes: review and diagnostic guidelines. Am J Med Genet A. 2007;143A:3314-3323.
- Zlotogorski A, Panteleyev AA, Aita VM, et al. Clinical and molecular diagnostic criteria of congenital atrichia with papular lesions. J Invest Dermatol. 2001;117:1662-1665.
- Paller AS, Moore JA, Scher R. Pachyonychia congenita tarda. alate-onset form of pachyonychia congenita. Arch Dermatol. 1991;127:701-703.
- Connelly T. Eruptive milia and rapid response to topical tretinoin. Arch Dermatol. 2008;144:816-817.
Practice Points
- Milia are common benign lesions that are cosmetically undesirable to some patients.
- Although some methods of milia removal can be painful, removal with forceps is quick and effective.
Conjunctival Melanoma of the Left Lower Eyelid
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
To the Editor:
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.

A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
- Aziz HA, Gastman BR, Singh AD. Management of conjunctival melanoma: critical assessment of sentinel lymph node biopsy. Ocul Concol Pathol. 2015;1:266-273. doi:10.1159/000381719
- Shields JA, Shields CL, De Potter P. Surgical management of circumscribed conjunctival melanomas. Ophthal Plast Reconstr Surg. 1998;14:208-215.
- Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143-3152. doi:10.1158/1078-0432.CCR-13-0163
- Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664-1678. doi:10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g
- Blum ES, Yang J, Komatsubara, KM, et al. Clinical management of uveal and conjunctival melanoma. Oncology (Williston Park). 2016;30:29-32, 34-43, 48.
- Kao A, Afshar A, Bloomer M, et al. Management of primary acquired melanosis, nevus, and conjunctival melanoma. Cancer Control. 2016;23:117-125.
- Paridaens AD, McCartney AC, Minassian DC, et al. Orbital exenteration in 95 cases of primary conjunctival malignant melanoma. Br J Ophthalmol. 1994;78:520-528.
- Norregaard JC, Gerner N, Jensen OA, et al. Malignant melanoma of the conjunctiva: occurrence and survival following surgery and radiotherapy in a Danish population. Graefes Arch Clin Exp Ophthalmol. 1996;234:569-572.
- Esmaeli B, Wang X, Youssef A, et al. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108:2101-2105.
- Tuomaala S, Kivelä T. Metastatic pattern and survival in disseminated conjunctival melanoma: implications for sentinel lymph node biopsy. Ophthalmology. 2004;111:816-821.
- Demirci H, McCormick SA, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: clinical experience with histopathologic observations. Arch Ophthalmol. 2000;118:885-891.
- Shields CL. Conjunctival melanoma: risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Trans Am Ophthalmol Soc. 2000;98:471-492.
- Tuomaala S, Eskelin S, Tarkkanen A, et al. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43:3399-3408.
Practice Points
- Ophthalmologists should carefully examine palpebral and bulbar conjunctiva at each annual visit paying careful attention to pigmented nevi.
- Conjunctival abnormalities should be thoroughly documented via color photography to accurately follow for suspicious change.
Cutaneous Lupus Erythematosus–like Isotopic Response to Herpes Zoster Infection
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.
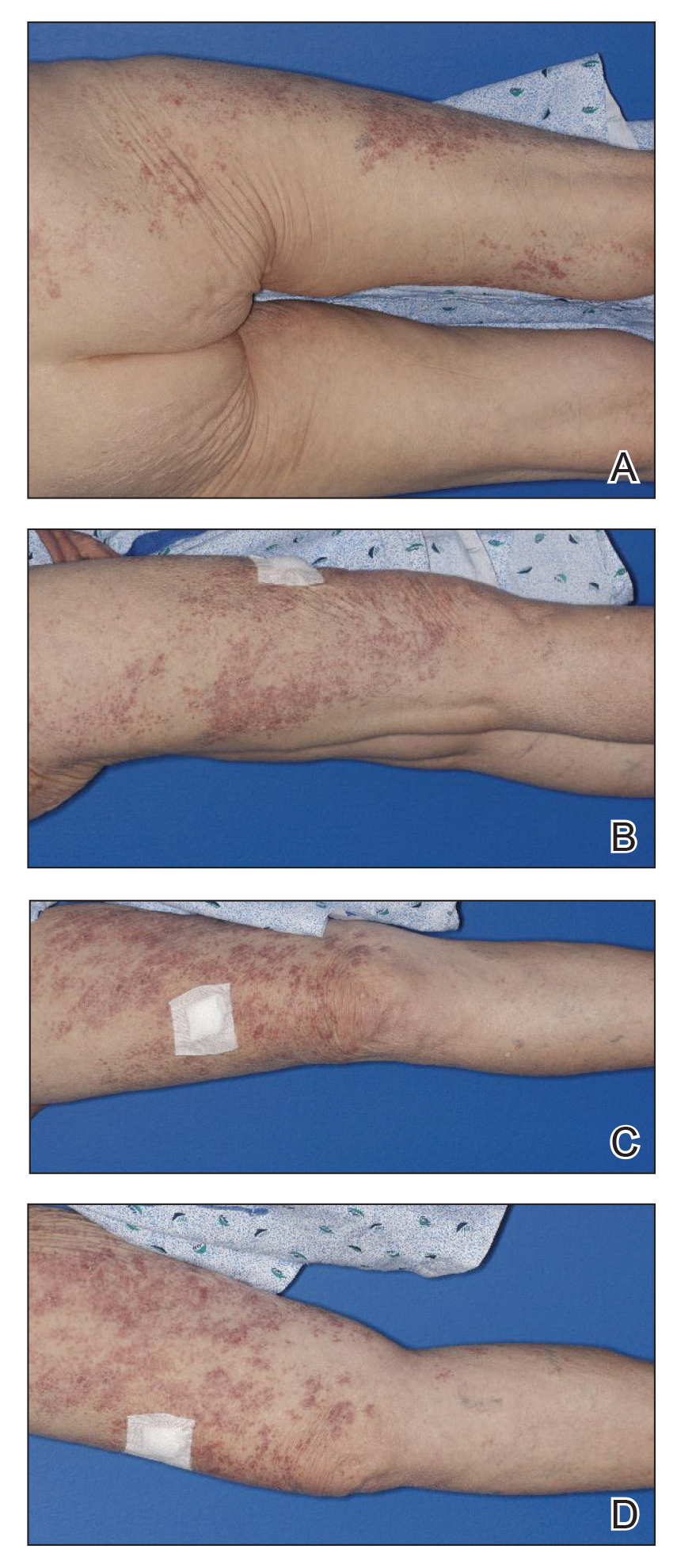
Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.
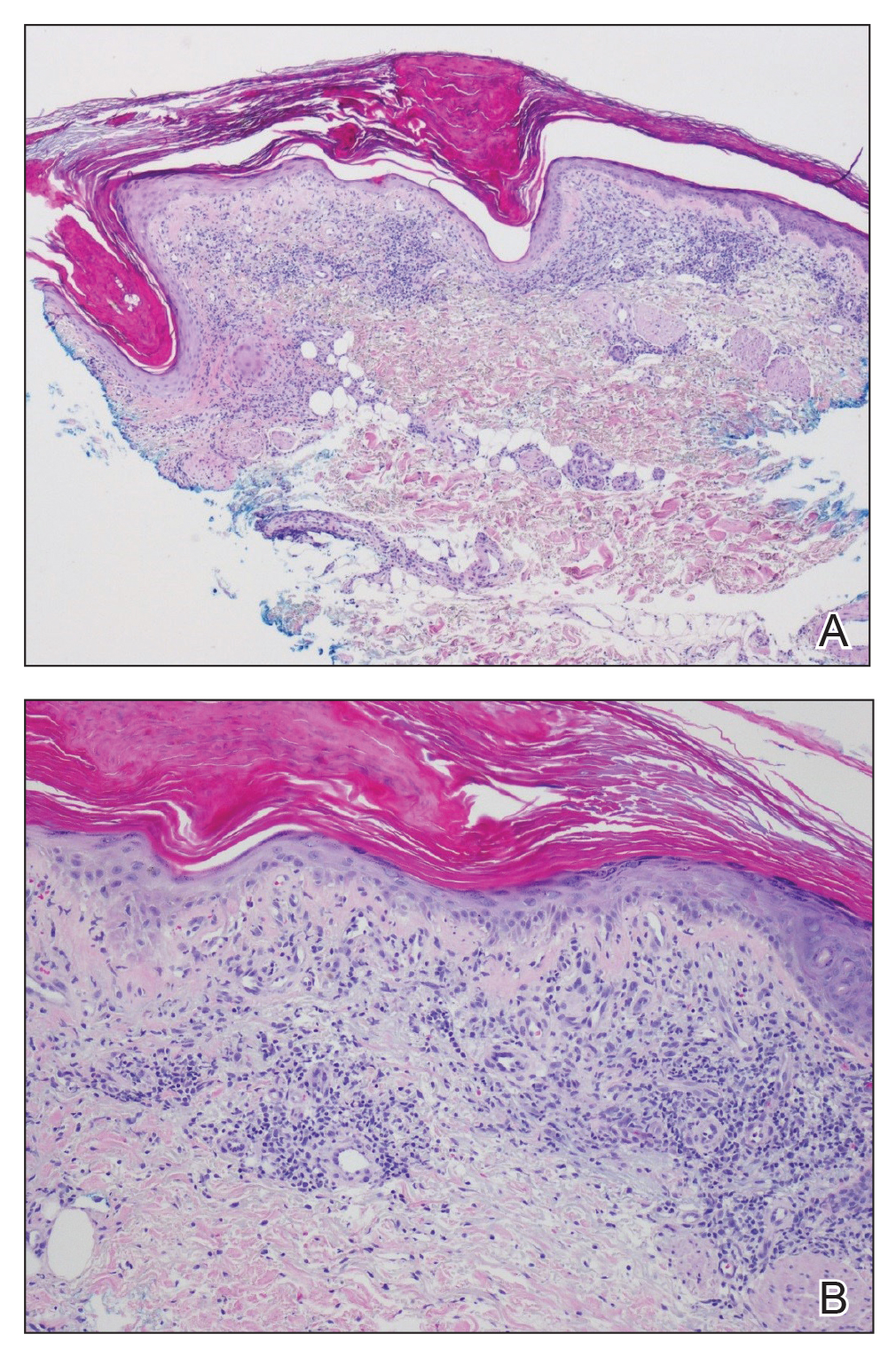
Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
To the Editor:
Wolf isotopic response describes the development of a skin disorder at the site of another healed and unrelated skin disease. Skin disorders presenting as isotopic responses have included inflammatory, malignant, granulomatous, and infectious processes. Discoid lupus erythematosus (DLE) is a rare isotopic response. We report a cutaneous lupus erythematosus–like isotopic response that presented at the site of a recent herpes zoster infection in a liver transplant recipient.
A 74-year-old immunocompromised woman was referred to the dermatology clinic for evaluation of a rash on the right leg. She was being treated with maintenance valganciclovir due to cytomegalovirus viremia, as well as tacrolimus, azathioprine, and prednisone following liver transplantation due to autoimmune hepatitis for 8 months prior to presentation. Eighteen days prior to the current presentation, she was clinically diagnosed with herpes zoster. As the grouped vesicles from the herpes zoster resolved, she developed pink scaly papules in the same distribution as the original vesicular eruption.

Physical examination revealed numerous erythematous, 2- to 3-mm, scaly papules that coalesced into small plaques with serous crusts; they originated above the supragluteal cleft and extended rightward in the L3 and L4 dermatomes to the right knee (Figure 1). A 3-mm punch biopsy specimen was obtained from the right anterior thigh. Histologic analysis revealed interface lymphocytic inflammation with squamatization of basal keratinocytes, basement membrane thickening, and follicular plugging by keratin (Figure 2). There was a moderately intense perivascular and periadnexal inflammatory infiltrate of mature lymphocytes with rare eosinophils within the papillary and superficial reticular dermis. There was no evidence of a viral cytopathic effect, and an immunohistochemical stain for varicella-zoster virus protein was negative. The histologic findings were suggestive of cutaneous involvement by DLE. A diagnosis of a cutaneous lupus erythematosus–like Wolf isotopic response was made, and the patient’s rash resolved with the use of triamcinolone cream 0.1% applied twice daily for 2 weeks. At 6-week follow-up, there were postinflammatory pigmentation changes at the sites of the prior rash and persistent postherpetic neuralgia. Recent antinuclear antibody screening was negative, coupled with the patient’s lack of systemic symptoms and quick resolution of rash, indicating that additional testing for systemic lupus was not warranted.

Wolf isotopic response describes the occurrence of a new skin disorder at the site of a previously healed and unrelated skin disorder. The second disease may appear within days to years after the primary disease subsides and is clearly differentiated from the isomorphic response of the Koebner phenomenon, which describes an established skin disorder appearing at a previously uninvolved anatomic site following trauma.1 As in our case, the initial cutaneous eruption resulting in a subsequent Wolf isotopic response frequently is herpes zoster and less commonly is herpes simplex virus.2 The most common reported isotopic response is a granulomatous reaction.2 Rare reports of leukemic infiltration, lymphoma, lichen planus, morphea, reactive perforating collagenosis, psoriasis, discoid lupus, lichen simplex chronicus, contact dermatitis, xanthomatous changes, malignant tumors, cutaneous graft-vs-host disease, pityriasis rosea, erythema annulare centrifugum, and other infectious-based isotopic responses exist.2-6
Our patient presented with Wolf isotopic response that histologically mimicked DLE. A PubMed search of articles indexed for MEDLINE using the terms isotopic response and lupus revealed only 3 cases of cutaneous lupus erythematosus presenting as an isotopic response in the English-language literature. One of those cases occurred in a patient with preexisting systemic lupus erythematosus, making a diagnosis of Koebner isomorphic phenomenon more appropriate than an isotopic response at the site of prior herpes zoster infection.7 The remaining 2 cases were clinically defined DLE lesions occurring at sites of prior infection—cutaneous leishmaniasis and herpes zoster—in patients without a prior history of cutaneous or systemic lupus erythematosus.8,9 The latter case of DLE-like isotopic response occurring after herpes zoster infection was further complicated by local injections at the zoster site for herpes-related local pain. Injection sites are reported as a distinct nidus for Wolf isotopic response.9
The pathogenesis of Wolf isotopic response is unclear. Possible explanations include local interactions between persistent viral particles at prior herpes infection sites, vascular injury, neural injury, and an altered immune response.1,5,6,10 The destruction of sensory nerve fibers by herpesviruses cause the release of neuropeptides that then modulate the local immune system and angiogenic responses.5,6 Our patient’s immunocompromised state may have further propagated a local altered immune cell infiltrate at the site of the isotopic response. Despite its unclear etiology, Wolf isotopic response should be considered in the differential diagnosis for any patient who presents with a dermatomal eruption at the site of a prior cutaneous infection, particularly after infection with herpes zoster. Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses with an excellent prognosis.11
We present a case of a cutaneous lupus erythematosus–like isotopic response that occurred at the site of a recent herpes zoster eruption in an immunocompromised patient without prior history of systemic or cutaneous lupus erythematosus. Clinical recognition of Wolf isotopic response is important for accurate histopathologic diagnosis and management. Continued investigation into the underlying pathogenesis should be performed to fully understand and better treat this process.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
- Sharma RC, Sharma NL, Mahajan V, et al. Wolf’s isotopic response: herpes simplex appearing on scrofuloderma scar. Int J Dermatol. 2003;42:664-666.
- Wolf R, Wolf D, Ruocco E, et al. Wolf’s isotopic response. Clin Dermatol. 2011;29:237-240.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Wolf D. “Wolf’s isotopic response”: the originators speak their mind and set the record straight. Clin Dermatol. 2017;35:416-418.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Lee NY, Daniel AS, Dasher DA, et al. Cutaneous lupus after herpes zoster: isomorphic, isotopic, or both? Pediatr Dermatol. 2013;30:110-113.
- Bardazzi F, Giacomini F, Savoia F, et al. Discoid chronic lupus erythematosus at the site of a previously healed cutaneous leishmaniasis: an example of isotopic response. Dermatol Ther. 2010;23:44-46.
- Parimalam K, Kumar D, Thomas J. Discoid lupus erythematosis occurring as an isotopic response. Indian Dermatol Online J. 2015;6:50-51.
- Wolf R, Lotti T, Ruocco V. Isomorphic versus isotopic response: data and hypotheses. J Eur Acad Dermatol Venereol. 2003;17:123-125.
- James W, Elston D, Treat J, et al. Viral diseases. In: James W, Elston D, Treat J, et al, eds. Andrew’s Diseases of the Skin. 13th ed. Elsevier; 2020:362-420.
Practice Points
- Wolf isotopic response describes the occurrence of a new skin condition at the site of a previously healed and unrelated skin disorder; a granulomatous reaction is a commonly reported isotopic response.
- Treatment with topical or intralesional corticosteroids usually suffices for inflammatory-based isotopic responses.
Facial Follicular Spicules: A Rare Cutaneous Presentation of Trichodysplasia Spinulosa
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
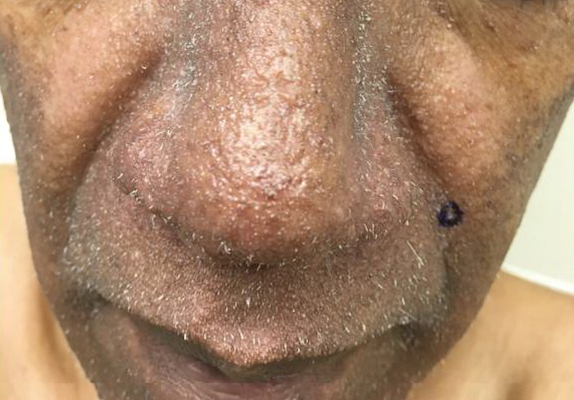
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
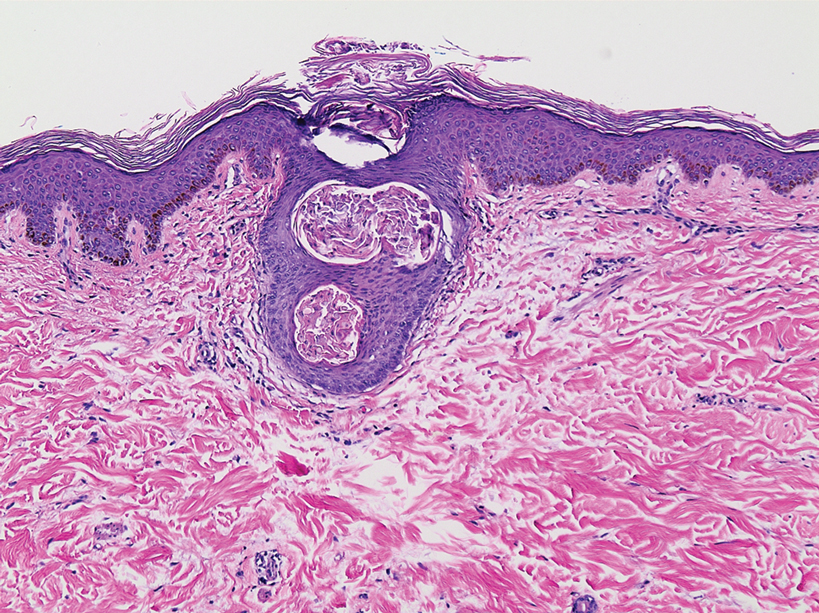
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
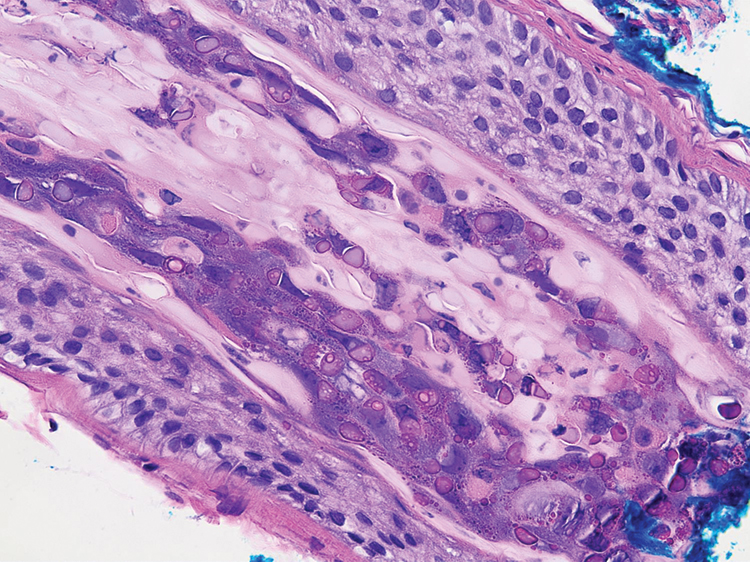
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.

A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.

Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.

Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5

Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.

Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
Practice Points
- Trichodysplasia spinulosa (TS) is a rare skin disease caused by primary TS-associated polyomavirus (TSPyV) infecting follicular keratinocytes in immunocompromised patients.
- Trichodysplasia spinulosa typically presents with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes.
- The viral protein can be verified through immunohistochemistry TSPyV major capsid protein VP1 staining or can be visualized on transmission electron microscopy.
- Follicular spicules of multiple myeloma should be ruled out before initiating treatment with cidofovir gel 1% for TS.