User login
Nonpharmacologic Treatment of Chronic Pain—A Critical Domains Approach
From the Department of Anesthesiology, University of Michigan, Ann Arbor, MI.
Abstract
- Objective: To provide an overview of the critical treatment domains for patients with chronic pain and describe nonpharmacologic strategies by which these domains can be addressed.
- Methods: A literature review was conducted to evaluate the evidence underlying commonly used nonpharmacologic strategies for the treatment of chronic pain, with a focus on interventions that require patient engagement.
- Results: Nonpharmacologic interventions that actively engage the patient in pain management, such as exercise, behavioral activation, sleep hygiene, and stress management, are relatively easy to implement and do not necessarily require the expertise of mental health professionals. Nonpharmacologic strategies can directly address pain and also address secondary complications, and thus serve to enhance treatment outcomes.
- Conclusion: The critical domains approach can be used to organize a comprehensive nonpharmacologic approach to treating widespread chronic pain.
According to the Institute of Medicine (IOM), chronic pain affects more Americans than coronary heart disease, diabetes, and cancer combined at an estimated cost of $635 billion per year [1]. While it has been demonstrated that we have reasonably good ability to reduce acute pain, providing pharmacologic treatment with even modest effects when addressing chronic pain remains challenging [1]. The ability to treat one form of pain successfully but not the other stems from the fact that chronic pain is not a simple extension of acute pain [2,3]; rather, the mechanisms differ and so must the treatments. The IOM report called for a cultural transformation in how pain is understood, assessed, and treated. In response, the National Pain Strategy [4] was developed. It was recommended that efficacious self-management strategies be used for individuals with chronic pain; such strategies are largely nonpharmacologic [4].
This article presents an approach to addressing chronic pain using nonpharmacologic strategies. While a number of nonpharmacologic treatments involve patients as passive recipients (eg, massage, acupuncture, balneotherapy or spa treatments), most require the patient to be engaged, eg, to exert physical energy, learn a new skill, and/or change a behavior. The approach presented here is organized around addressing critical domains, including the need to increase activity, deal with psychiatric comorbidities, address sleep problems, and tackle stress. The strategies suggested will be those that have the best evidence base and are predominantly ones that can be deployed by physicians and other health care professionals who do not necessarily have specialized training in behavioral health. A case is presented to illustrate this approach.
Case Presentation
Lisa is a 42-year-old Caucasian woman with a 2-year history of chronic low back pain presenting to a primary care clinic. She reported that the back pain began when she was working as an office manager in a busy dental clinic. The onset was sudden, occurring when she lifted a heavy box of copier paper using a “leaning and twisting motion.” The pain is described as constant (rated as 5 out of 10) and she experiences periods of more intense pain or “flares” (rated as 9 out of 10); Lisa noted that “10 is reserved for childbirth.” The flares seem to coincide with periods of stress and can result in up to 2 days of immobility, causing her to miss work at the dental office.
The pain is described as deep, aching, and throbbing but does not radiate to her legs. It is made worse by sitting still for longer than an hour and gets better if she keeps moving and gets a good night of sleep. Her sleep is generally disturbed as she has trouble falling asleep and when she does sleep, she usually wakes up feeling unrefreshed and extremely irritable. Moreover, while she knows that activity makes her pain better, Lisa can rarely find the energy or motivation to exercise.
Various evaluations by specialists have been obtained and studies conducted, including a recent MRI. All were found to be negative for a clear-cut pathology. A visit to a rheumatologist 5 years ago resulted in a diagnosis of fibromyalgia that Lisa does not accept. Upon probing, she detailed what turns out to be an almost 20-year history of chronic pain. The back pain is only the latest diagnosis in an extensive list of painful conditions including premenstrual syndrome (PMS), headache, temporomandibular joint disorder (TMJ), and fibromyalgia. There are no aspects of her history or presentation that suggest a diagnosis other than chronic musculoskeletal pain.
Lisa is a divorced mother of 2 adolescent children who are generally well-adjusted if not age-appropriately defiant. She is overweight (body mass index = 29) and admits to overeating when under stress. She says that the back pain has disrupted every aspect of her life and work is the only thing that gets adequate attention. Her salary is critical to her family’s financial stability, thus it is a priority. Lisa noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. She notes that, “I have zero joy in my life—I rarely go anywhere fun with my kids anymore, putter in my garden, and forget about going on dates. I can’t remember the last enjoyable thing I did!”
What are aspects to consider in addressing this patient’s symptoms?
Lisa’s case is likely recognizable—she presents with a long history of pain in multiple areas of her body (eg, low back pain, PMS, headache, TMJ) without clear-cut pathology. She has multiple physical and social problems and limited resources. The diagnosis of fibromyalgia is likely correct. The low back pain is probably another manifestation of a broader “centralized pain” condition [5,6]. The term centralized pain refers to the amplification of pain via changes in the central nervous system [7,8]. This does not mean that peripheral nociceptive input (ie, tissue damage or inflammation) plays no role in the pain; however, it implies that any painful stimulus is experienced with greater intensity than would be expected [5,6]. Further, psychological, behavioral, and social elements tend to be key factors in centralized pain states due in part to the exhausting challenge of living with chronic pain, as well as genetic factors that predispose to both pain and mood disturbances [9].
Due to the often complex nature of chronic pain, successful treatment usually requires addressing multiple areas of concern, including addressing behavioral, cognitive, and affective processes. It is suggested that a plan for nonpharmacologic pain management could be built around 6 domains represented by the acronym ExPRESS [10], namely Exercise, Psychological distress, Regaining function, Emotional well-being, Sleep hygiene, and Stress management. This article provides a review of the literature that focuses on systematic reviews and meta-analyses to summarize a massive literature largely supporting the use of nonpharmacologic strategies such as exercise, cognitive-behavioral therapy, mindfulness-based treatments, behavioral self-management, resilience-based interventions, and education to address the ExPRESS [10] domains using Lisa’s case as an example.
How effective is exercise for treating chronic pain and how should it be integrated into treatment?
Exercise
Over the last 5 years, a number of meta-analyses have been conducted to evaluate a robust literature regarding exercise interventions for the treatment of chronic pain [11–14]. The evidence is strong that patients with chronic pain benefit from increased physical activity and in many cases the effect size is quite substantial [14]. Meta-analytic data suggest that aerobic exercise results in significantly less pain and disability [13], improved physical fitness [14], less fatigue and better mood [14]. Exercise can be land-based or water-based [14], be conducted at a slight to moderate intensity and/or even involve only a program of walking [12]. Most established guidelines highlight the benefits of including exercise as part of the nonpharmacologic management of patients with chronic pain [15–18].
Data suggest that chronic pain patients should begin exercise training slowly starting at levels below capacity and increase duration and intensity over time until patients are exercising at low to moderate intensity (ie, 50% to 70% of age-adjusted maximum heart rate) for 20 to 30 minutes per session 2 to 3 times per week [19].
Obesity and deconditioning are common and are thought to contribute to pain sensitivity, poor sleep, and depressed mood [20]. Lisa is overweight and inactive. She injured her back and reports generally avoiding any form of exercise. Getting her moving will be imperative as an increase in physical activity could not only help her to lose weight, but could have the added benefits of decreasing her pain and stiffness, helping her sleep better and improving her mood and self-esteem. Yet, she reports not having the time or motivation.
A reasonable approach would be to not prescribe formal exercise at first but rather encourage small and immediate changes in how she already goes about her day. One concrete step would be to encourage her to stand up and stretch every 20 minutes or so while working at her computer. This is something that she cites as directly contributing to her pain. Next, an increase in physical activity such as adding a few steps every day and doing regular activities with more vigor would be a great initial step.
One of the most formidable barriers to getting patients to exercise is the perception that they must go to the gym and begin a formal program in order to achieve any benefit. As an employed single mother with two children Lisa likely lacks the time and resources for a formal exercise program. She could instead, begin a walking program that starts with reasonable goals (eg, 6000 steps per day) and builds at a slow and steady pace (eg, add 100 steps per day). Activity trackers range in price, but a simple pedometer can be found for under $10. By initiating such a walking program, the things she does already such as chores around the house all count as physical activity. She could do these with more energy and mindfulness and incrementally add activity over time.
Once a new habit of increased physical activity has been established, the strategy of branching out into new physical activities (or even more formal exercise) is usually more successful especially if they are enjoyable and feasible (ie, affordable, not too time consuming). The need to engage in more physical activity could be the impetus to encourage Lisa to do more activities with her children—walking to the park, flying a kite, and exploring the science museum are all activities that can provide physical, emotional and social benefits simultaneously.
What interventions are helpful in addressing psychiatric comorbidity?
Psychological Distress
Comorbidity with mood and anxiety disorders is often observed and complicates treatment in patients with chronic pain states [21–23]. Patients with centralized pain conditions like fibromyalgia tend to have even higher rates of psychiatric comorbidity than those with other pain conditions like arthritis alone [24–26]. While estimates vary widely, we have recently reported that 36.2% of patients evaluated in our tertiary care setting meet case criteria for depression [27]. Such psychiatric comorbidity has been shown to be associated with increased pain, worse functioning, higher costs and increased use of opioids [27–30]. Further, suicidal ideation is common in chronic pain populations, especially those with depression and anxiety, and should be carefully evaluated if suspected [31]. The presence of psychiatric comorbidity takes a toll on the individual and society. One study found that pain patients with comorbid depression utilized twice the resources that other patients without depression utilized [32]. Perhaps the most troubling element is that psychiatric comorbidity is too often not adequately addressed in medical settings [33].
Assessing for depression using a standardized measure like the PHQ-9 [34] or anxiety using the GAD-7 [35] can provide a sense of the severity of the psychiatric symptoms. More severe forms of depression and anxiety may require referral, but more mild depressive and/or anxiety symptoms may be treated by the medical personnel the patient already knows and trusts. Nonpharmacologic strategies that can be used to address depression, anxiety, and even pain in chronic pain populations include cognitive-behavioral therapy, exercise/physical activity, regulating sleep and behavioral activation (ie, getting patients engaged with valued activities, social support).
Perhaps the most effective strategy to address depression, anxiety, and pain in chronic pain populations is cognitive-behavioral therapy (CBT) [36–38]. CBT for pain consists of both cognitive and behavioral therapy interventions. Cognitive therapy proposes that modifying maladaptive thoughts will result in changes in emotions and behavior [39]. Thus, errors in thinking like catastrophizing, overgeneralizing, and minimizing positives are confronted and changed to more realistic and helpful thoughts. This results in less emotional distress and fewer self-defeating behaviors. In cognitive therapy for chronic pain, catastrophic thoughts such as “My pain is terrible and nothing I do helps” are replaced by more adaptive thoughts like “Although my pain is severe, there still are a few things I can do to make it a little better.” Several behavioral techniques are also employed such as behavioral activation (getting patients moving again), activity pacing (not overdoing it on days patients feel good and remaining active on days they feel bad), sleep hygiene (identifying then changing behaviors know to disrupt sleep), and relaxation skills (eg, breathing, imagery, progressive muscle relaxation). Meta-analyses have shown that CBT has empirical support for its effectiveness in treating patients with chronic pain [40,41].
During the visit, Lisa reported a loss of joy in her life and then began crying. Such a report should prompt a more formal exploration of the potential for depression. She would likely benefit from antidepressant medication and behavioral intervention. The physical activity prescribed above will also pertain to treating her depressive symptoms as will strategies to improve her emotional well-being, sleep and stress noted below. Perhaps the most beneficial strategy would be to refer her to CBT for pain and depressive symptoms. CBT for pain would help Lisa acquire the skills required to address many ExPRESS [10] domains including increasing physical activity, improving mood, decreasing stress, and improving sleep.
What strategy can be recommended to help patients regain function?
Regaining Function
Pain is disruptive. Patients with pain may avoid activity due to fear of re-injury or making the pain worse. Pain may keep them awake at night and lead to daytime fatigue. Pain can be so bad that a patient cannot even do simple tasks, One of the most important goals in successfully managing pain is to move away from trying to cure the pain and instead focus on regaining function—helping the patient do some of the things he/she really wants to do despite the pain. The patient may not be able to all the things he/she used to do, but new ways to do many of these activities can be found. Patients can also identify new rewarding activities to do now that things have changed.
To regain function, an evidence-based strategy comes from behavior therapy and is known as graded activation [42–46]. Here the patient is assigned one very small, manageable and incremental step towards achieving a goal. As these small goals are met, the patient feels motivated to engage in more and larger goals.
Lisa specifically mentioned giving up valued activities in light of her chronic pain. To help her re-engage a graded task assignment approach can be taken. For example, Lisa would be encouraged to first identify an activity she would like to get back to doing again. If she were to say “gardening,” then she is to next identify one small, specific, and easily achievable goal for the short term, such as “garden for 20 minutes at least once in the next week.” Help her identify the roadblocks to completing this small goal and brainstorm solutions such as “My kids have soccer and basketball practices 5 days next week so I will ask my ex-husband take them to practice at least one day next week so I can spend time in my garden.” Lisa will be told to schedule time to garden as if it were an appointment with a doctor.
Another important issue to consider is the tendency for inconsistent levels of activities across days that are predicated on how one feels that particular day. On “good days” often patients inadvertently engage in more activity than personal limitations allow and as a consequence experience several “bad days” of pain and other symptom flare up which can result in lost productivity and worse self-esteem. The goal is to have patients engage in a moderate amount of activity every day and avoid activity “binges” or days with little of no activity. Graded activation is a method of pacing that can improve physical functioning while minimizing the likelihood of pain flare-ups.
What simple strategies can be used to improve patients’ emotional well-being?
Emotional Well-Being
Psychological distress and emotional well-being occur along a continuum. Eliminating psychological distress only returns one to a state of being without distress. That is not the same as experiencing emotional well-being or happiness. People with chronic pain who also have higher levels of emotional well-being (or happiness) have decreased pain severity, fewer symptoms, better levels of functioning, and greater life satisfaction [47–49].
Recent studies provide preliminary evidence suggesting that resilience-based interventions such as keeping a gratitude journal or scheduling time to engage in pleasant activities boast equivalence or even superiority to CBT for the treatment of mood with effects that persist over time [50,51]. Two recent meta-analyses have shown that resilience-based interventions have been used to treat healthy individuals and a range of clinical conditions with a mean effect size for improving well-being ranging between 0.34 to 0.61 (ie, moderate-large effects [Cohen’s d]) [52,53]. Positive activities interventions are thought to function by increasing positive affect, which in turn, enables creativity, problem-solving, perspective-taking, and other beneficial states [54]. Such states are conducive to better mood [55,56], behavioral activation/increased physical activity [57–60], better sleep [61–63], increased social support [54,64] and physiological changes (eg, improved vagal tone, lower blood pressure, more adaptive immune responses) [57,65–69]. Recent studies have successfully adapted resilience-based interventions and shown them to be effective for individuals with pain [70–72]. Resilience-based interventions may be particularly helpful for chronic pain patients given that depression and sleep disturbances are frequent comorbidities [5,21–26,28,73,74].
Lisa stated, “I have zero joy in my life…” and later burst into tears. It is easy to surmise that her emotional well-being is quite poor. She also noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. This is a common picture for individuals with chronic pain. Valued life activities like spending quality time with loved ones, going to sporting events or doing a hobby are put aside in favor of obligatory (eg, activities of daily living) and committed (eg, work, school) activities. While this strategy might help one survive, it certainly is not conducive to thriving. To help Lisa improve her emotional well-being, there are good data supporting pleasant activity scheduling amongst other strategies. For pleasant activity scheduling Lisa would be directed to set aside time a few days a week (at least an hour) to do things that she enjoys. This time should be placed on her calendar and treated with the same level of commitment as going to work or to an appointment with her physician.
What nonpharmacologic options are available to help improve patients’ sleep?
Sleep
Lisa indicated that she has trouble falling asleep and then when she does sleep, she usually wakes up feeling unrefreshed and irritable. This is a common complaint amongst individuals with chronic pain who often report difficulty falling asleep, being awakened by pain or discomfort and awakening feeling unrefreshed and unrestored [75]. Sleep, pain and mood form a symptomatic triad such that when one aspect is affected the others are impacted. For example, when Lisa does not sleep well, her pain and mood worsen, as well. Conversely, when her pain is better, she likely sleeps better and wakes up feeling less irritable and experiences less pain.
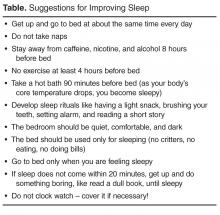
Behavioral strategies for improving sleep, if used on a regular basis, can help individuals get needed restorative sleep with the additional benefits of improving mood, pain, fatigue, and mental clarity [76]. Some of these behavioral strategies focus on maintaining regular sleep routines (go to bed at the same time every night even on weekends), engaging in sleep conducive behaviors (eg, attempting to sleep only when in feeling sleepy), and avoiding stimulating activities (eg, watching action movies, or consuming nicotine or caffeine). Studies have shown that behavioral strategies targeting sleep appear to have a direct impact on pain symptoms and on functional interference resulting from nonrestorative sleep [77,78].
What stress reduction strategies can be recommended to the patient?
Stress
Stress management has long been a target of treatment in patients with chronic pain. Progressive muscle relaxation (PRM) [79] and autogenic training have typically served as an important foundation of behavioral intervention for chronic pain [80] although there are no randomized controlled trials for PRM as a stand-alone intervention and two separate trials of autogenic training failed to find superiority for this intervention [81,82]. Despite the lack of direct evidence, clinical experience and the knowledge that both relaxation techniques are commonly part of CBT for chronic pain, their efficacy is generally accepted.
An emerging area of nonpharmacologic treatment is mindfulness-based interventions [83], which can include mindfulness-based stress reduction (MBSR) and Acceptance and Commitment Therapy [84], which can be considered a hybrid between mindfulness meditation and CBT. These interventions are still relatively new and larger, better controlled studies are needed. In MBSR, the patient is directed to focus on one thing such as a sound, a pleasant scene or their own breathing. The practitioner is encouraged to keep thoughts present oriented and analytical concerns are to be gently dismissed in favor of focusing on the sounds, scene, or breath. A recent meta-analysis evaluating 15 studies in clinical populations reported that there were small to medium effect sizes for patients with chronic pain [85]. In another new meta-analysis evaluating only studies in chronic pain the authors reported that sleep quality and pain acceptance were the 2 variables with the largest effect sizes based on the 11 studies they evaluated [83]. Similarly, a meta-analysis that included both MBSR and ACT found that 22 studies of varying quality suggest significant but small effect sizes for pain (ES = 0.37) and depression (ES = 0.32) [86]. They concluded the mindfulness-based treatments were not superior to CBT but could be a viable alternative.
For Lisa and many other chronic pain patients, the symptom flares seem to coincide with periods of stress. These flare ups are not inconsequential and have cost her days of lost productivity and potentially put her employment at risk. Moreover, she has identified stress as a trigger for over-eating which certainly contributes to her weight problems and low self-esteem. MBSR can be learned in a structured class or online--many of the principles can be taught by lay instructors.
Summary
While it is likely that health care professionals will continue to rely on pharmacological therapies in treating chronic pain, it is important to be aware that reliance on medications and procedural interventions alone is unlikely to bring adequate relief to individuals living with chronic pain [1]. Optimal pain management appears to be achieved by using a combination of both pharmacologic and nonpharmacologic approaches. Nonpharmacologic interventions that actively engage the patient in pain management such as exercise, behavioral activation, sleep hygiene and stress management are relatively easy to implement and do not necessarily require the expertise of mental health professionals. The challenge is considering pain in its biopsychosocial contexts and defining an approach that is both comprehensive and feasible. Using the ExPRESS domains to help guide care can provide a road map.
Corresponding author: Afton L. Hassett, PsyD, 24 Frank Lloyd Wright Drive, Lobby M, CPFRC, Ann Arbor, MI 48106, [email protected].
1. Institute of Medicine. Relieving pain in America a blueprint for transforming prevention, care, education, and research. In. Washington, DC: National Academy of Sciences; 2011.
2. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926.
3. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
4. National Pain Strategy. A Comprehensive Population Health-Level Strategy for Pain. 2015. Accessed at http://iprcc.nih.gov/docs/DraftHHSNationalPainStrategy.pdf.
5. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in spine pain patients presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013.
6. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain 2009;10:777-91.
7. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
8. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15.
9. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:421.
10. Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am 2009;35:393–407.
11. Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil 2015;29:1155–67.
12. O’Connor SR, Tully MA, Ryan B, et al. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:724-34 e3.
13. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil 2015;94:358–65.
14. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79.
15. Burckhardt CS, Goldenberg D, Crofford L, et al. Guideline for the management of fibromyalgia syndrome. Pain in adults and children. Glenview, IL: American Pain Society; 2005.
16. Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536–41.
17. Chou R, Huffman LH, American Pain Society, American College of Pain Medicine. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:492–504.
18. Chou R, Qaseem A, Snow V, , Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Pain Medicine, American Pain Society low back pain guidelines P. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91.
19. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther;12:R79.
20. Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood and sleep. J Pain 2010.
21. Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668-77.
22. Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 2005;113:331–9.
23. Reme SE, Tangen T, Moe T, Eriksen HR. Prevalence of psychiatric disorders in sick listed chronic low back pain patients. Eur J Pain 2011;15:1075–80.
24. Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry 2006;67:1219–25.
25. Hassett AL, Radvanski DC, Buyske S, et al. Psychiatric comorbidity and other psychological factors in patients with “chronic Lyme disease”. Am J Med 2009;122:843–50.
26. Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 1999;40:57–63.
27. Goesling J, Henry MJ, Moser SE, et al. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. J Pain 2015;16:844–51.
28. Hassett AL, Cone JD, Patella SJ, Sigal LH. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum 2000;43:2493–500.
29. Giesecke T, Williams DA, Harris RE, et al. Subgroupings of fibromyalgia patients on the basis of pressure pain thresholds and psychological factors. Arthritis Rheum 2003;48:2916–22.
30. Walen HR, Cronan PA, Bigatti SM. Factors associated with healthcare costs in women with fibromyalgia. Am J Manag Care 2001;7 Spec No:SP39-47.
31. Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr Pain Headache Rep 2014;18:436.
32. Robinson RL, Birnbaum HG, Morley MA, et al. Depression and fibromyalgia: treatment and cost when diagnosed separately or concurrently. J Rheumatol 2004;31:1621–9.
33. Fitzcharles MA. In: Canadian Rheumatology Association’s 64th Annual Meeting; 2009.
34. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
35. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7.
36. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1–13.
37. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007;26:1–9.
39. Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
39. Beck JS. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
40. Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
41. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013;9:CD009796.
42. Nielson WR, Walker C, McCain GA. Cognitive behavioral treatment of fibromyalgia syndrome: preliminary findings. J Rheumatol 1992;19:98–103.
43. Nicassio PM, Radojevic V, Weisman MH, et al. A comparison of behavioral and educational interventions for fibromyalgia. J Rheumatol 1997;24:2000–7.
44. Williams DA, Cary MA, Groner KH, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. J Rheumatol 2002;29:1280–6.
45. Lindstrom I, Ohlund C, Eek C, et al. Mobility, strength, and fitness after a graded activity program for patients with subacute low back pain. A randomized prospective clinical study with a behavioral therapy approach. Spine 1992;17:641–52.
46. Lindstrom I, Ohlund C, Eek C, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant-conditioning behavioral approach. Phys Ther 1992;72:279-90; discussion 91–3.
47. McAllister SJ, Vincent A, Hassett AL, et al. Psychological resilience, affective mechanisms and symptom burden in a tertiary-care sample of patients with fibromyalgia. Stress Health 2015;31:299–305.
48. Toussaint LL, Vincent A, McAllister SJ, et al. A comparison of fibromyalgia symptoms in patients with healthy versus depressive, low and reactive affect balance styles. Scand J Pain 2014;5:161–6.
49. Hassett AL, Simonelli LE, Radvanski DC, et al. The relationship between affect balance style and clinical outcomes in fibromyalgia. Arthritis Rheum 2008;59:833–40.
50. Zamirinejad S, Hojjat SK, Golzari M, et al. Effectiveness of resilience training versus cognitive therapy on reduction of depression in female iranian college students. Issues Ment Health Nurs 2014;35:480–8.
51. Asgharipoor N, Asgharnejad Farid A, Arshadi H, Sahebi A. A comparative study on the effectiveness of positive psychotherapy and group cognitive-behavioral therapy for the patients suffering from major depressive disorder. Iran J Psychiatry Behav Sci 2012;6:33–41.
52. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol 2009;65:467–87.
53. Bolier L, Haverman M, Westerhof GJ, et al. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health 2013;13:119.
54. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56:218–26.
55. Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol 2005;60:410–21.
56. Seligman ME, Rashid T, Parks AC. Positive psychotherapy. Am Psychol 2006;61:774–88.
57. White DK, Keysor JJ, Neogi T, et al. When it hurts, a positive attitude may help: association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care Res (Hoboken) 2012;64:1312–9.
58. Strine TW, Chapman DP, Balluz LS, et al. The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among U.S. community-dwelling adults. J Community Health 2008;33:40–50.
59. Grant N, Wardle J, Steptoe A. The relationship between life satisfaction and health behavior: a cross-cultural analysis of young adults. Int J Behav Med 2009;16:259–68.
60. Baruth M, Lee DC, Sui X, et al. Emotional outlook on life predicts increases in physical activity among initially inactive men. Health Educ Behav 2011;38:150–8.
61. Kalmbach DA, Pillai V, Roth T, Drake CL. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res 2014;23:636–45.
62. Simor P, Krietsch KN, Koteles F, McCrae CS. Day-to-day variation of subjective sleep quality and emotional states among healthy university students-a 1-week prospective study. Int J Behav Med 2015;22:625–34.
63. von Kanel R, Mausbach BT, Ancoli-Israel S, et al. Positive affect and sleep in spousal Alzheimer caregivers: a longitudinal study. Behav Sleep Med 2014;12:358–72.
64. Cohn MA, Fredrickson BL, Brown SL, et al. Happiness unpacked: positive emotions increase life satisfaction by building resilience. Emotion 2009;9:361–8.
65. Ostir GV, Berges IM, Markides KS, Ottenbacher KJ. Hypertension in older adults and the role of positive emotions. Psychosom Med 2006;68:727–33.
66. Stone AA, Cox DS, Valdimarsdottir H, et al. Evidence that secretory IgA antibody is associated with daily mood. J Pers Soc Psychol 1987;52:988–93.
67. Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A 2005;102:6508–12.
68. Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci 2013;24:1123–32.
69. Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med 2008;70:1020–7.
70. Hausmann LR, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. J Pain 2014;15:560–7.
71. Muller R, Gertz KJ, Molton IR, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin J Pain 2015.
72. Flink IK, Smeets E, Bergbom S, Peters ML. Happy despite pain: Pilot study of a positive pscyhology intervention for patients with chronic pain. Scandinavian Jounral of Pain 2015;7:71–9.
73. Hassett AL, Radvanski DC, Buyske S, et al. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum 2008;59:1742–9.
74. Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 2015;11:513–20.
75. Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med 2010;11:158–79.
76. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy Am J Psychiatry 1994;151:1172–80.
77. Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain 1996;68:363–8.
78. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165:2527–35.
79. Jacobson E. Progressive relaxation. Chicago: University of Chicago Press; 1938.
80. van Tulder MW, Koes B, Malmivaara A. Outcome of non-invasive treatment modalities on back pain: an evidence-based review. Eur Spine J 2006;15 Suppl 1:S64–81.
81. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232–8.
82. Rucco V, Feruglio C, Genco F, Mosanghini R. [Autogenic training versus Erickson’s analogical technique in treatment of fibromyalgia syndrome]. Riv Eur Sci Med Farmacol 1995;17:41–50.
83. Bawa FL, Mercer SW, Atherton RJ, et al. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. Br J Gen Pract 2015;65:e387–400.
84. Ost LG. The efficacy of acceptance and commitment therapy: an updated systematic review and meta-analysis. Behav Res Ther 2014;61:105–21.
85. Crowe M, Jordan J, Burrell B, et al. Mindfulness-based stress reduction for long-term physical conditions: a systematic review. Aust N Z J Psychiatry 2015.
86. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
From the Department of Anesthesiology, University of Michigan, Ann Arbor, MI.
Abstract
- Objective: To provide an overview of the critical treatment domains for patients with chronic pain and describe nonpharmacologic strategies by which these domains can be addressed.
- Methods: A literature review was conducted to evaluate the evidence underlying commonly used nonpharmacologic strategies for the treatment of chronic pain, with a focus on interventions that require patient engagement.
- Results: Nonpharmacologic interventions that actively engage the patient in pain management, such as exercise, behavioral activation, sleep hygiene, and stress management, are relatively easy to implement and do not necessarily require the expertise of mental health professionals. Nonpharmacologic strategies can directly address pain and also address secondary complications, and thus serve to enhance treatment outcomes.
- Conclusion: The critical domains approach can be used to organize a comprehensive nonpharmacologic approach to treating widespread chronic pain.
According to the Institute of Medicine (IOM), chronic pain affects more Americans than coronary heart disease, diabetes, and cancer combined at an estimated cost of $635 billion per year [1]. While it has been demonstrated that we have reasonably good ability to reduce acute pain, providing pharmacologic treatment with even modest effects when addressing chronic pain remains challenging [1]. The ability to treat one form of pain successfully but not the other stems from the fact that chronic pain is not a simple extension of acute pain [2,3]; rather, the mechanisms differ and so must the treatments. The IOM report called for a cultural transformation in how pain is understood, assessed, and treated. In response, the National Pain Strategy [4] was developed. It was recommended that efficacious self-management strategies be used for individuals with chronic pain; such strategies are largely nonpharmacologic [4].
This article presents an approach to addressing chronic pain using nonpharmacologic strategies. While a number of nonpharmacologic treatments involve patients as passive recipients (eg, massage, acupuncture, balneotherapy or spa treatments), most require the patient to be engaged, eg, to exert physical energy, learn a new skill, and/or change a behavior. The approach presented here is organized around addressing critical domains, including the need to increase activity, deal with psychiatric comorbidities, address sleep problems, and tackle stress. The strategies suggested will be those that have the best evidence base and are predominantly ones that can be deployed by physicians and other health care professionals who do not necessarily have specialized training in behavioral health. A case is presented to illustrate this approach.
Case Presentation
Lisa is a 42-year-old Caucasian woman with a 2-year history of chronic low back pain presenting to a primary care clinic. She reported that the back pain began when she was working as an office manager in a busy dental clinic. The onset was sudden, occurring when she lifted a heavy box of copier paper using a “leaning and twisting motion.” The pain is described as constant (rated as 5 out of 10) and she experiences periods of more intense pain or “flares” (rated as 9 out of 10); Lisa noted that “10 is reserved for childbirth.” The flares seem to coincide with periods of stress and can result in up to 2 days of immobility, causing her to miss work at the dental office.
The pain is described as deep, aching, and throbbing but does not radiate to her legs. It is made worse by sitting still for longer than an hour and gets better if she keeps moving and gets a good night of sleep. Her sleep is generally disturbed as she has trouble falling asleep and when she does sleep, she usually wakes up feeling unrefreshed and extremely irritable. Moreover, while she knows that activity makes her pain better, Lisa can rarely find the energy or motivation to exercise.
Various evaluations by specialists have been obtained and studies conducted, including a recent MRI. All were found to be negative for a clear-cut pathology. A visit to a rheumatologist 5 years ago resulted in a diagnosis of fibromyalgia that Lisa does not accept. Upon probing, she detailed what turns out to be an almost 20-year history of chronic pain. The back pain is only the latest diagnosis in an extensive list of painful conditions including premenstrual syndrome (PMS), headache, temporomandibular joint disorder (TMJ), and fibromyalgia. There are no aspects of her history or presentation that suggest a diagnosis other than chronic musculoskeletal pain.
Lisa is a divorced mother of 2 adolescent children who are generally well-adjusted if not age-appropriately defiant. She is overweight (body mass index = 29) and admits to overeating when under stress. She says that the back pain has disrupted every aspect of her life and work is the only thing that gets adequate attention. Her salary is critical to her family’s financial stability, thus it is a priority. Lisa noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. She notes that, “I have zero joy in my life—I rarely go anywhere fun with my kids anymore, putter in my garden, and forget about going on dates. I can’t remember the last enjoyable thing I did!”
What are aspects to consider in addressing this patient’s symptoms?
Lisa’s case is likely recognizable—she presents with a long history of pain in multiple areas of her body (eg, low back pain, PMS, headache, TMJ) without clear-cut pathology. She has multiple physical and social problems and limited resources. The diagnosis of fibromyalgia is likely correct. The low back pain is probably another manifestation of a broader “centralized pain” condition [5,6]. The term centralized pain refers to the amplification of pain via changes in the central nervous system [7,8]. This does not mean that peripheral nociceptive input (ie, tissue damage or inflammation) plays no role in the pain; however, it implies that any painful stimulus is experienced with greater intensity than would be expected [5,6]. Further, psychological, behavioral, and social elements tend to be key factors in centralized pain states due in part to the exhausting challenge of living with chronic pain, as well as genetic factors that predispose to both pain and mood disturbances [9].
Due to the often complex nature of chronic pain, successful treatment usually requires addressing multiple areas of concern, including addressing behavioral, cognitive, and affective processes. It is suggested that a plan for nonpharmacologic pain management could be built around 6 domains represented by the acronym ExPRESS [10], namely Exercise, Psychological distress, Regaining function, Emotional well-being, Sleep hygiene, and Stress management. This article provides a review of the literature that focuses on systematic reviews and meta-analyses to summarize a massive literature largely supporting the use of nonpharmacologic strategies such as exercise, cognitive-behavioral therapy, mindfulness-based treatments, behavioral self-management, resilience-based interventions, and education to address the ExPRESS [10] domains using Lisa’s case as an example.
How effective is exercise for treating chronic pain and how should it be integrated into treatment?
Exercise
Over the last 5 years, a number of meta-analyses have been conducted to evaluate a robust literature regarding exercise interventions for the treatment of chronic pain [11–14]. The evidence is strong that patients with chronic pain benefit from increased physical activity and in many cases the effect size is quite substantial [14]. Meta-analytic data suggest that aerobic exercise results in significantly less pain and disability [13], improved physical fitness [14], less fatigue and better mood [14]. Exercise can be land-based or water-based [14], be conducted at a slight to moderate intensity and/or even involve only a program of walking [12]. Most established guidelines highlight the benefits of including exercise as part of the nonpharmacologic management of patients with chronic pain [15–18].
Data suggest that chronic pain patients should begin exercise training slowly starting at levels below capacity and increase duration and intensity over time until patients are exercising at low to moderate intensity (ie, 50% to 70% of age-adjusted maximum heart rate) for 20 to 30 minutes per session 2 to 3 times per week [19].
Obesity and deconditioning are common and are thought to contribute to pain sensitivity, poor sleep, and depressed mood [20]. Lisa is overweight and inactive. She injured her back and reports generally avoiding any form of exercise. Getting her moving will be imperative as an increase in physical activity could not only help her to lose weight, but could have the added benefits of decreasing her pain and stiffness, helping her sleep better and improving her mood and self-esteem. Yet, she reports not having the time or motivation.
A reasonable approach would be to not prescribe formal exercise at first but rather encourage small and immediate changes in how she already goes about her day. One concrete step would be to encourage her to stand up and stretch every 20 minutes or so while working at her computer. This is something that she cites as directly contributing to her pain. Next, an increase in physical activity such as adding a few steps every day and doing regular activities with more vigor would be a great initial step.
One of the most formidable barriers to getting patients to exercise is the perception that they must go to the gym and begin a formal program in order to achieve any benefit. As an employed single mother with two children Lisa likely lacks the time and resources for a formal exercise program. She could instead, begin a walking program that starts with reasonable goals (eg, 6000 steps per day) and builds at a slow and steady pace (eg, add 100 steps per day). Activity trackers range in price, but a simple pedometer can be found for under $10. By initiating such a walking program, the things she does already such as chores around the house all count as physical activity. She could do these with more energy and mindfulness and incrementally add activity over time.
Once a new habit of increased physical activity has been established, the strategy of branching out into new physical activities (or even more formal exercise) is usually more successful especially if they are enjoyable and feasible (ie, affordable, not too time consuming). The need to engage in more physical activity could be the impetus to encourage Lisa to do more activities with her children—walking to the park, flying a kite, and exploring the science museum are all activities that can provide physical, emotional and social benefits simultaneously.
What interventions are helpful in addressing psychiatric comorbidity?
Psychological Distress
Comorbidity with mood and anxiety disorders is often observed and complicates treatment in patients with chronic pain states [21–23]. Patients with centralized pain conditions like fibromyalgia tend to have even higher rates of psychiatric comorbidity than those with other pain conditions like arthritis alone [24–26]. While estimates vary widely, we have recently reported that 36.2% of patients evaluated in our tertiary care setting meet case criteria for depression [27]. Such psychiatric comorbidity has been shown to be associated with increased pain, worse functioning, higher costs and increased use of opioids [27–30]. Further, suicidal ideation is common in chronic pain populations, especially those with depression and anxiety, and should be carefully evaluated if suspected [31]. The presence of psychiatric comorbidity takes a toll on the individual and society. One study found that pain patients with comorbid depression utilized twice the resources that other patients without depression utilized [32]. Perhaps the most troubling element is that psychiatric comorbidity is too often not adequately addressed in medical settings [33].
Assessing for depression using a standardized measure like the PHQ-9 [34] or anxiety using the GAD-7 [35] can provide a sense of the severity of the psychiatric symptoms. More severe forms of depression and anxiety may require referral, but more mild depressive and/or anxiety symptoms may be treated by the medical personnel the patient already knows and trusts. Nonpharmacologic strategies that can be used to address depression, anxiety, and even pain in chronic pain populations include cognitive-behavioral therapy, exercise/physical activity, regulating sleep and behavioral activation (ie, getting patients engaged with valued activities, social support).
Perhaps the most effective strategy to address depression, anxiety, and pain in chronic pain populations is cognitive-behavioral therapy (CBT) [36–38]. CBT for pain consists of both cognitive and behavioral therapy interventions. Cognitive therapy proposes that modifying maladaptive thoughts will result in changes in emotions and behavior [39]. Thus, errors in thinking like catastrophizing, overgeneralizing, and minimizing positives are confronted and changed to more realistic and helpful thoughts. This results in less emotional distress and fewer self-defeating behaviors. In cognitive therapy for chronic pain, catastrophic thoughts such as “My pain is terrible and nothing I do helps” are replaced by more adaptive thoughts like “Although my pain is severe, there still are a few things I can do to make it a little better.” Several behavioral techniques are also employed such as behavioral activation (getting patients moving again), activity pacing (not overdoing it on days patients feel good and remaining active on days they feel bad), sleep hygiene (identifying then changing behaviors know to disrupt sleep), and relaxation skills (eg, breathing, imagery, progressive muscle relaxation). Meta-analyses have shown that CBT has empirical support for its effectiveness in treating patients with chronic pain [40,41].
During the visit, Lisa reported a loss of joy in her life and then began crying. Such a report should prompt a more formal exploration of the potential for depression. She would likely benefit from antidepressant medication and behavioral intervention. The physical activity prescribed above will also pertain to treating her depressive symptoms as will strategies to improve her emotional well-being, sleep and stress noted below. Perhaps the most beneficial strategy would be to refer her to CBT for pain and depressive symptoms. CBT for pain would help Lisa acquire the skills required to address many ExPRESS [10] domains including increasing physical activity, improving mood, decreasing stress, and improving sleep.
What strategy can be recommended to help patients regain function?
Regaining Function
Pain is disruptive. Patients with pain may avoid activity due to fear of re-injury or making the pain worse. Pain may keep them awake at night and lead to daytime fatigue. Pain can be so bad that a patient cannot even do simple tasks, One of the most important goals in successfully managing pain is to move away from trying to cure the pain and instead focus on regaining function—helping the patient do some of the things he/she really wants to do despite the pain. The patient may not be able to all the things he/she used to do, but new ways to do many of these activities can be found. Patients can also identify new rewarding activities to do now that things have changed.
To regain function, an evidence-based strategy comes from behavior therapy and is known as graded activation [42–46]. Here the patient is assigned one very small, manageable and incremental step towards achieving a goal. As these small goals are met, the patient feels motivated to engage in more and larger goals.
Lisa specifically mentioned giving up valued activities in light of her chronic pain. To help her re-engage a graded task assignment approach can be taken. For example, Lisa would be encouraged to first identify an activity she would like to get back to doing again. If she were to say “gardening,” then she is to next identify one small, specific, and easily achievable goal for the short term, such as “garden for 20 minutes at least once in the next week.” Help her identify the roadblocks to completing this small goal and brainstorm solutions such as “My kids have soccer and basketball practices 5 days next week so I will ask my ex-husband take them to practice at least one day next week so I can spend time in my garden.” Lisa will be told to schedule time to garden as if it were an appointment with a doctor.
Another important issue to consider is the tendency for inconsistent levels of activities across days that are predicated on how one feels that particular day. On “good days” often patients inadvertently engage in more activity than personal limitations allow and as a consequence experience several “bad days” of pain and other symptom flare up which can result in lost productivity and worse self-esteem. The goal is to have patients engage in a moderate amount of activity every day and avoid activity “binges” or days with little of no activity. Graded activation is a method of pacing that can improve physical functioning while minimizing the likelihood of pain flare-ups.
What simple strategies can be used to improve patients’ emotional well-being?
Emotional Well-Being
Psychological distress and emotional well-being occur along a continuum. Eliminating psychological distress only returns one to a state of being without distress. That is not the same as experiencing emotional well-being or happiness. People with chronic pain who also have higher levels of emotional well-being (or happiness) have decreased pain severity, fewer symptoms, better levels of functioning, and greater life satisfaction [47–49].
Recent studies provide preliminary evidence suggesting that resilience-based interventions such as keeping a gratitude journal or scheduling time to engage in pleasant activities boast equivalence or even superiority to CBT for the treatment of mood with effects that persist over time [50,51]. Two recent meta-analyses have shown that resilience-based interventions have been used to treat healthy individuals and a range of clinical conditions with a mean effect size for improving well-being ranging between 0.34 to 0.61 (ie, moderate-large effects [Cohen’s d]) [52,53]. Positive activities interventions are thought to function by increasing positive affect, which in turn, enables creativity, problem-solving, perspective-taking, and other beneficial states [54]. Such states are conducive to better mood [55,56], behavioral activation/increased physical activity [57–60], better sleep [61–63], increased social support [54,64] and physiological changes (eg, improved vagal tone, lower blood pressure, more adaptive immune responses) [57,65–69]. Recent studies have successfully adapted resilience-based interventions and shown them to be effective for individuals with pain [70–72]. Resilience-based interventions may be particularly helpful for chronic pain patients given that depression and sleep disturbances are frequent comorbidities [5,21–26,28,73,74].
Lisa stated, “I have zero joy in my life…” and later burst into tears. It is easy to surmise that her emotional well-being is quite poor. She also noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. This is a common picture for individuals with chronic pain. Valued life activities like spending quality time with loved ones, going to sporting events or doing a hobby are put aside in favor of obligatory (eg, activities of daily living) and committed (eg, work, school) activities. While this strategy might help one survive, it certainly is not conducive to thriving. To help Lisa improve her emotional well-being, there are good data supporting pleasant activity scheduling amongst other strategies. For pleasant activity scheduling Lisa would be directed to set aside time a few days a week (at least an hour) to do things that she enjoys. This time should be placed on her calendar and treated with the same level of commitment as going to work or to an appointment with her physician.
What nonpharmacologic options are available to help improve patients’ sleep?
Sleep
Lisa indicated that she has trouble falling asleep and then when she does sleep, she usually wakes up feeling unrefreshed and irritable. This is a common complaint amongst individuals with chronic pain who often report difficulty falling asleep, being awakened by pain or discomfort and awakening feeling unrefreshed and unrestored [75]. Sleep, pain and mood form a symptomatic triad such that when one aspect is affected the others are impacted. For example, when Lisa does not sleep well, her pain and mood worsen, as well. Conversely, when her pain is better, she likely sleeps better and wakes up feeling less irritable and experiences less pain.
Behavioral strategies for improving sleep, if used on a regular basis, can help individuals get needed restorative sleep with the additional benefits of improving mood, pain, fatigue, and mental clarity [76]. Some of these behavioral strategies focus on maintaining regular sleep routines (go to bed at the same time every night even on weekends), engaging in sleep conducive behaviors (eg, attempting to sleep only when in feeling sleepy), and avoiding stimulating activities (eg, watching action movies, or consuming nicotine or caffeine). Studies have shown that behavioral strategies targeting sleep appear to have a direct impact on pain symptoms and on functional interference resulting from nonrestorative sleep [77,78].
What stress reduction strategies can be recommended to the patient?
Stress
Stress management has long been a target of treatment in patients with chronic pain. Progressive muscle relaxation (PRM) [79] and autogenic training have typically served as an important foundation of behavioral intervention for chronic pain [80] although there are no randomized controlled trials for PRM as a stand-alone intervention and two separate trials of autogenic training failed to find superiority for this intervention [81,82]. Despite the lack of direct evidence, clinical experience and the knowledge that both relaxation techniques are commonly part of CBT for chronic pain, their efficacy is generally accepted.
An emerging area of nonpharmacologic treatment is mindfulness-based interventions [83], which can include mindfulness-based stress reduction (MBSR) and Acceptance and Commitment Therapy [84], which can be considered a hybrid between mindfulness meditation and CBT. These interventions are still relatively new and larger, better controlled studies are needed. In MBSR, the patient is directed to focus on one thing such as a sound, a pleasant scene or their own breathing. The practitioner is encouraged to keep thoughts present oriented and analytical concerns are to be gently dismissed in favor of focusing on the sounds, scene, or breath. A recent meta-analysis evaluating 15 studies in clinical populations reported that there were small to medium effect sizes for patients with chronic pain [85]. In another new meta-analysis evaluating only studies in chronic pain the authors reported that sleep quality and pain acceptance were the 2 variables with the largest effect sizes based on the 11 studies they evaluated [83]. Similarly, a meta-analysis that included both MBSR and ACT found that 22 studies of varying quality suggest significant but small effect sizes for pain (ES = 0.37) and depression (ES = 0.32) [86]. They concluded the mindfulness-based treatments were not superior to CBT but could be a viable alternative.
For Lisa and many other chronic pain patients, the symptom flares seem to coincide with periods of stress. These flare ups are not inconsequential and have cost her days of lost productivity and potentially put her employment at risk. Moreover, she has identified stress as a trigger for over-eating which certainly contributes to her weight problems and low self-esteem. MBSR can be learned in a structured class or online--many of the principles can be taught by lay instructors.
Summary
While it is likely that health care professionals will continue to rely on pharmacological therapies in treating chronic pain, it is important to be aware that reliance on medications and procedural interventions alone is unlikely to bring adequate relief to individuals living with chronic pain [1]. Optimal pain management appears to be achieved by using a combination of both pharmacologic and nonpharmacologic approaches. Nonpharmacologic interventions that actively engage the patient in pain management such as exercise, behavioral activation, sleep hygiene and stress management are relatively easy to implement and do not necessarily require the expertise of mental health professionals. The challenge is considering pain in its biopsychosocial contexts and defining an approach that is both comprehensive and feasible. Using the ExPRESS domains to help guide care can provide a road map.
Corresponding author: Afton L. Hassett, PsyD, 24 Frank Lloyd Wright Drive, Lobby M, CPFRC, Ann Arbor, MI 48106, [email protected].
From the Department of Anesthesiology, University of Michigan, Ann Arbor, MI.
Abstract
- Objective: To provide an overview of the critical treatment domains for patients with chronic pain and describe nonpharmacologic strategies by which these domains can be addressed.
- Methods: A literature review was conducted to evaluate the evidence underlying commonly used nonpharmacologic strategies for the treatment of chronic pain, with a focus on interventions that require patient engagement.
- Results: Nonpharmacologic interventions that actively engage the patient in pain management, such as exercise, behavioral activation, sleep hygiene, and stress management, are relatively easy to implement and do not necessarily require the expertise of mental health professionals. Nonpharmacologic strategies can directly address pain and also address secondary complications, and thus serve to enhance treatment outcomes.
- Conclusion: The critical domains approach can be used to organize a comprehensive nonpharmacologic approach to treating widespread chronic pain.
According to the Institute of Medicine (IOM), chronic pain affects more Americans than coronary heart disease, diabetes, and cancer combined at an estimated cost of $635 billion per year [1]. While it has been demonstrated that we have reasonably good ability to reduce acute pain, providing pharmacologic treatment with even modest effects when addressing chronic pain remains challenging [1]. The ability to treat one form of pain successfully but not the other stems from the fact that chronic pain is not a simple extension of acute pain [2,3]; rather, the mechanisms differ and so must the treatments. The IOM report called for a cultural transformation in how pain is understood, assessed, and treated. In response, the National Pain Strategy [4] was developed. It was recommended that efficacious self-management strategies be used for individuals with chronic pain; such strategies are largely nonpharmacologic [4].
This article presents an approach to addressing chronic pain using nonpharmacologic strategies. While a number of nonpharmacologic treatments involve patients as passive recipients (eg, massage, acupuncture, balneotherapy or spa treatments), most require the patient to be engaged, eg, to exert physical energy, learn a new skill, and/or change a behavior. The approach presented here is organized around addressing critical domains, including the need to increase activity, deal with psychiatric comorbidities, address sleep problems, and tackle stress. The strategies suggested will be those that have the best evidence base and are predominantly ones that can be deployed by physicians and other health care professionals who do not necessarily have specialized training in behavioral health. A case is presented to illustrate this approach.
Case Presentation
Lisa is a 42-year-old Caucasian woman with a 2-year history of chronic low back pain presenting to a primary care clinic. She reported that the back pain began when she was working as an office manager in a busy dental clinic. The onset was sudden, occurring when she lifted a heavy box of copier paper using a “leaning and twisting motion.” The pain is described as constant (rated as 5 out of 10) and she experiences periods of more intense pain or “flares” (rated as 9 out of 10); Lisa noted that “10 is reserved for childbirth.” The flares seem to coincide with periods of stress and can result in up to 2 days of immobility, causing her to miss work at the dental office.
The pain is described as deep, aching, and throbbing but does not radiate to her legs. It is made worse by sitting still for longer than an hour and gets better if she keeps moving and gets a good night of sleep. Her sleep is generally disturbed as she has trouble falling asleep and when she does sleep, she usually wakes up feeling unrefreshed and extremely irritable. Moreover, while she knows that activity makes her pain better, Lisa can rarely find the energy or motivation to exercise.
Various evaluations by specialists have been obtained and studies conducted, including a recent MRI. All were found to be negative for a clear-cut pathology. A visit to a rheumatologist 5 years ago resulted in a diagnosis of fibromyalgia that Lisa does not accept. Upon probing, she detailed what turns out to be an almost 20-year history of chronic pain. The back pain is only the latest diagnosis in an extensive list of painful conditions including premenstrual syndrome (PMS), headache, temporomandibular joint disorder (TMJ), and fibromyalgia. There are no aspects of her history or presentation that suggest a diagnosis other than chronic musculoskeletal pain.
Lisa is a divorced mother of 2 adolescent children who are generally well-adjusted if not age-appropriately defiant. She is overweight (body mass index = 29) and admits to overeating when under stress. She says that the back pain has disrupted every aspect of her life and work is the only thing that gets adequate attention. Her salary is critical to her family’s financial stability, thus it is a priority. Lisa noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. She notes that, “I have zero joy in my life—I rarely go anywhere fun with my kids anymore, putter in my garden, and forget about going on dates. I can’t remember the last enjoyable thing I did!”
What are aspects to consider in addressing this patient’s symptoms?
Lisa’s case is likely recognizable—she presents with a long history of pain in multiple areas of her body (eg, low back pain, PMS, headache, TMJ) without clear-cut pathology. She has multiple physical and social problems and limited resources. The diagnosis of fibromyalgia is likely correct. The low back pain is probably another manifestation of a broader “centralized pain” condition [5,6]. The term centralized pain refers to the amplification of pain via changes in the central nervous system [7,8]. This does not mean that peripheral nociceptive input (ie, tissue damage or inflammation) plays no role in the pain; however, it implies that any painful stimulus is experienced with greater intensity than would be expected [5,6]. Further, psychological, behavioral, and social elements tend to be key factors in centralized pain states due in part to the exhausting challenge of living with chronic pain, as well as genetic factors that predispose to both pain and mood disturbances [9].
Due to the often complex nature of chronic pain, successful treatment usually requires addressing multiple areas of concern, including addressing behavioral, cognitive, and affective processes. It is suggested that a plan for nonpharmacologic pain management could be built around 6 domains represented by the acronym ExPRESS [10], namely Exercise, Psychological distress, Regaining function, Emotional well-being, Sleep hygiene, and Stress management. This article provides a review of the literature that focuses on systematic reviews and meta-analyses to summarize a massive literature largely supporting the use of nonpharmacologic strategies such as exercise, cognitive-behavioral therapy, mindfulness-based treatments, behavioral self-management, resilience-based interventions, and education to address the ExPRESS [10] domains using Lisa’s case as an example.
How effective is exercise for treating chronic pain and how should it be integrated into treatment?
Exercise
Over the last 5 years, a number of meta-analyses have been conducted to evaluate a robust literature regarding exercise interventions for the treatment of chronic pain [11–14]. The evidence is strong that patients with chronic pain benefit from increased physical activity and in many cases the effect size is quite substantial [14]. Meta-analytic data suggest that aerobic exercise results in significantly less pain and disability [13], improved physical fitness [14], less fatigue and better mood [14]. Exercise can be land-based or water-based [14], be conducted at a slight to moderate intensity and/or even involve only a program of walking [12]. Most established guidelines highlight the benefits of including exercise as part of the nonpharmacologic management of patients with chronic pain [15–18].
Data suggest that chronic pain patients should begin exercise training slowly starting at levels below capacity and increase duration and intensity over time until patients are exercising at low to moderate intensity (ie, 50% to 70% of age-adjusted maximum heart rate) for 20 to 30 minutes per session 2 to 3 times per week [19].
Obesity and deconditioning are common and are thought to contribute to pain sensitivity, poor sleep, and depressed mood [20]. Lisa is overweight and inactive. She injured her back and reports generally avoiding any form of exercise. Getting her moving will be imperative as an increase in physical activity could not only help her to lose weight, but could have the added benefits of decreasing her pain and stiffness, helping her sleep better and improving her mood and self-esteem. Yet, she reports not having the time or motivation.
A reasonable approach would be to not prescribe formal exercise at first but rather encourage small and immediate changes in how she already goes about her day. One concrete step would be to encourage her to stand up and stretch every 20 minutes or so while working at her computer. This is something that she cites as directly contributing to her pain. Next, an increase in physical activity such as adding a few steps every day and doing regular activities with more vigor would be a great initial step.
One of the most formidable barriers to getting patients to exercise is the perception that they must go to the gym and begin a formal program in order to achieve any benefit. As an employed single mother with two children Lisa likely lacks the time and resources for a formal exercise program. She could instead, begin a walking program that starts with reasonable goals (eg, 6000 steps per day) and builds at a slow and steady pace (eg, add 100 steps per day). Activity trackers range in price, but a simple pedometer can be found for under $10. By initiating such a walking program, the things she does already such as chores around the house all count as physical activity. She could do these with more energy and mindfulness and incrementally add activity over time.
Once a new habit of increased physical activity has been established, the strategy of branching out into new physical activities (or even more formal exercise) is usually more successful especially if they are enjoyable and feasible (ie, affordable, not too time consuming). The need to engage in more physical activity could be the impetus to encourage Lisa to do more activities with her children—walking to the park, flying a kite, and exploring the science museum are all activities that can provide physical, emotional and social benefits simultaneously.
What interventions are helpful in addressing psychiatric comorbidity?
Psychological Distress
Comorbidity with mood and anxiety disorders is often observed and complicates treatment in patients with chronic pain states [21–23]. Patients with centralized pain conditions like fibromyalgia tend to have even higher rates of psychiatric comorbidity than those with other pain conditions like arthritis alone [24–26]. While estimates vary widely, we have recently reported that 36.2% of patients evaluated in our tertiary care setting meet case criteria for depression [27]. Such psychiatric comorbidity has been shown to be associated with increased pain, worse functioning, higher costs and increased use of opioids [27–30]. Further, suicidal ideation is common in chronic pain populations, especially those with depression and anxiety, and should be carefully evaluated if suspected [31]. The presence of psychiatric comorbidity takes a toll on the individual and society. One study found that pain patients with comorbid depression utilized twice the resources that other patients without depression utilized [32]. Perhaps the most troubling element is that psychiatric comorbidity is too often not adequately addressed in medical settings [33].
Assessing for depression using a standardized measure like the PHQ-9 [34] or anxiety using the GAD-7 [35] can provide a sense of the severity of the psychiatric symptoms. More severe forms of depression and anxiety may require referral, but more mild depressive and/or anxiety symptoms may be treated by the medical personnel the patient already knows and trusts. Nonpharmacologic strategies that can be used to address depression, anxiety, and even pain in chronic pain populations include cognitive-behavioral therapy, exercise/physical activity, regulating sleep and behavioral activation (ie, getting patients engaged with valued activities, social support).
Perhaps the most effective strategy to address depression, anxiety, and pain in chronic pain populations is cognitive-behavioral therapy (CBT) [36–38]. CBT for pain consists of both cognitive and behavioral therapy interventions. Cognitive therapy proposes that modifying maladaptive thoughts will result in changes in emotions and behavior [39]. Thus, errors in thinking like catastrophizing, overgeneralizing, and minimizing positives are confronted and changed to more realistic and helpful thoughts. This results in less emotional distress and fewer self-defeating behaviors. In cognitive therapy for chronic pain, catastrophic thoughts such as “My pain is terrible and nothing I do helps” are replaced by more adaptive thoughts like “Although my pain is severe, there still are a few things I can do to make it a little better.” Several behavioral techniques are also employed such as behavioral activation (getting patients moving again), activity pacing (not overdoing it on days patients feel good and remaining active on days they feel bad), sleep hygiene (identifying then changing behaviors know to disrupt sleep), and relaxation skills (eg, breathing, imagery, progressive muscle relaxation). Meta-analyses have shown that CBT has empirical support for its effectiveness in treating patients with chronic pain [40,41].
During the visit, Lisa reported a loss of joy in her life and then began crying. Such a report should prompt a more formal exploration of the potential for depression. She would likely benefit from antidepressant medication and behavioral intervention. The physical activity prescribed above will also pertain to treating her depressive symptoms as will strategies to improve her emotional well-being, sleep and stress noted below. Perhaps the most beneficial strategy would be to refer her to CBT for pain and depressive symptoms. CBT for pain would help Lisa acquire the skills required to address many ExPRESS [10] domains including increasing physical activity, improving mood, decreasing stress, and improving sleep.
What strategy can be recommended to help patients regain function?
Regaining Function
Pain is disruptive. Patients with pain may avoid activity due to fear of re-injury or making the pain worse. Pain may keep them awake at night and lead to daytime fatigue. Pain can be so bad that a patient cannot even do simple tasks, One of the most important goals in successfully managing pain is to move away from trying to cure the pain and instead focus on regaining function—helping the patient do some of the things he/she really wants to do despite the pain. The patient may not be able to all the things he/she used to do, but new ways to do many of these activities can be found. Patients can also identify new rewarding activities to do now that things have changed.
To regain function, an evidence-based strategy comes from behavior therapy and is known as graded activation [42–46]. Here the patient is assigned one very small, manageable and incremental step towards achieving a goal. As these small goals are met, the patient feels motivated to engage in more and larger goals.
Lisa specifically mentioned giving up valued activities in light of her chronic pain. To help her re-engage a graded task assignment approach can be taken. For example, Lisa would be encouraged to first identify an activity she would like to get back to doing again. If she were to say “gardening,” then she is to next identify one small, specific, and easily achievable goal for the short term, such as “garden for 20 minutes at least once in the next week.” Help her identify the roadblocks to completing this small goal and brainstorm solutions such as “My kids have soccer and basketball practices 5 days next week so I will ask my ex-husband take them to practice at least one day next week so I can spend time in my garden.” Lisa will be told to schedule time to garden as if it were an appointment with a doctor.
Another important issue to consider is the tendency for inconsistent levels of activities across days that are predicated on how one feels that particular day. On “good days” often patients inadvertently engage in more activity than personal limitations allow and as a consequence experience several “bad days” of pain and other symptom flare up which can result in lost productivity and worse self-esteem. The goal is to have patients engage in a moderate amount of activity every day and avoid activity “binges” or days with little of no activity. Graded activation is a method of pacing that can improve physical functioning while minimizing the likelihood of pain flare-ups.
What simple strategies can be used to improve patients’ emotional well-being?
Emotional Well-Being
Psychological distress and emotional well-being occur along a continuum. Eliminating psychological distress only returns one to a state of being without distress. That is not the same as experiencing emotional well-being or happiness. People with chronic pain who also have higher levels of emotional well-being (or happiness) have decreased pain severity, fewer symptoms, better levels of functioning, and greater life satisfaction [47–49].
Recent studies provide preliminary evidence suggesting that resilience-based interventions such as keeping a gratitude journal or scheduling time to engage in pleasant activities boast equivalence or even superiority to CBT for the treatment of mood with effects that persist over time [50,51]. Two recent meta-analyses have shown that resilience-based interventions have been used to treat healthy individuals and a range of clinical conditions with a mean effect size for improving well-being ranging between 0.34 to 0.61 (ie, moderate-large effects [Cohen’s d]) [52,53]. Positive activities interventions are thought to function by increasing positive affect, which in turn, enables creativity, problem-solving, perspective-taking, and other beneficial states [54]. Such states are conducive to better mood [55,56], behavioral activation/increased physical activity [57–60], better sleep [61–63], increased social support [54,64] and physiological changes (eg, improved vagal tone, lower blood pressure, more adaptive immune responses) [57,65–69]. Recent studies have successfully adapted resilience-based interventions and shown them to be effective for individuals with pain [70–72]. Resilience-based interventions may be particularly helpful for chronic pain patients given that depression and sleep disturbances are frequent comorbidities [5,21–26,28,73,74].
Lisa stated, “I have zero joy in my life…” and later burst into tears. It is easy to surmise that her emotional well-being is quite poor. She also noted that she saves all of her energy for her job and has “nothing left in the tank” for her children or herself. This is a common picture for individuals with chronic pain. Valued life activities like spending quality time with loved ones, going to sporting events or doing a hobby are put aside in favor of obligatory (eg, activities of daily living) and committed (eg, work, school) activities. While this strategy might help one survive, it certainly is not conducive to thriving. To help Lisa improve her emotional well-being, there are good data supporting pleasant activity scheduling amongst other strategies. For pleasant activity scheduling Lisa would be directed to set aside time a few days a week (at least an hour) to do things that she enjoys. This time should be placed on her calendar and treated with the same level of commitment as going to work or to an appointment with her physician.
What nonpharmacologic options are available to help improve patients’ sleep?
Sleep
Lisa indicated that she has trouble falling asleep and then when she does sleep, she usually wakes up feeling unrefreshed and irritable. This is a common complaint amongst individuals with chronic pain who often report difficulty falling asleep, being awakened by pain or discomfort and awakening feeling unrefreshed and unrestored [75]. Sleep, pain and mood form a symptomatic triad such that when one aspect is affected the others are impacted. For example, when Lisa does not sleep well, her pain and mood worsen, as well. Conversely, when her pain is better, she likely sleeps better and wakes up feeling less irritable and experiences less pain.
Behavioral strategies for improving sleep, if used on a regular basis, can help individuals get needed restorative sleep with the additional benefits of improving mood, pain, fatigue, and mental clarity [76]. Some of these behavioral strategies focus on maintaining regular sleep routines (go to bed at the same time every night even on weekends), engaging in sleep conducive behaviors (eg, attempting to sleep only when in feeling sleepy), and avoiding stimulating activities (eg, watching action movies, or consuming nicotine or caffeine). Studies have shown that behavioral strategies targeting sleep appear to have a direct impact on pain symptoms and on functional interference resulting from nonrestorative sleep [77,78].
What stress reduction strategies can be recommended to the patient?
Stress
Stress management has long been a target of treatment in patients with chronic pain. Progressive muscle relaxation (PRM) [79] and autogenic training have typically served as an important foundation of behavioral intervention for chronic pain [80] although there are no randomized controlled trials for PRM as a stand-alone intervention and two separate trials of autogenic training failed to find superiority for this intervention [81,82]. Despite the lack of direct evidence, clinical experience and the knowledge that both relaxation techniques are commonly part of CBT for chronic pain, their efficacy is generally accepted.
An emerging area of nonpharmacologic treatment is mindfulness-based interventions [83], which can include mindfulness-based stress reduction (MBSR) and Acceptance and Commitment Therapy [84], which can be considered a hybrid between mindfulness meditation and CBT. These interventions are still relatively new and larger, better controlled studies are needed. In MBSR, the patient is directed to focus on one thing such as a sound, a pleasant scene or their own breathing. The practitioner is encouraged to keep thoughts present oriented and analytical concerns are to be gently dismissed in favor of focusing on the sounds, scene, or breath. A recent meta-analysis evaluating 15 studies in clinical populations reported that there were small to medium effect sizes for patients with chronic pain [85]. In another new meta-analysis evaluating only studies in chronic pain the authors reported that sleep quality and pain acceptance were the 2 variables with the largest effect sizes based on the 11 studies they evaluated [83]. Similarly, a meta-analysis that included both MBSR and ACT found that 22 studies of varying quality suggest significant but small effect sizes for pain (ES = 0.37) and depression (ES = 0.32) [86]. They concluded the mindfulness-based treatments were not superior to CBT but could be a viable alternative.
For Lisa and many other chronic pain patients, the symptom flares seem to coincide with periods of stress. These flare ups are not inconsequential and have cost her days of lost productivity and potentially put her employment at risk. Moreover, she has identified stress as a trigger for over-eating which certainly contributes to her weight problems and low self-esteem. MBSR can be learned in a structured class or online--many of the principles can be taught by lay instructors.
Summary
While it is likely that health care professionals will continue to rely on pharmacological therapies in treating chronic pain, it is important to be aware that reliance on medications and procedural interventions alone is unlikely to bring adequate relief to individuals living with chronic pain [1]. Optimal pain management appears to be achieved by using a combination of both pharmacologic and nonpharmacologic approaches. Nonpharmacologic interventions that actively engage the patient in pain management such as exercise, behavioral activation, sleep hygiene and stress management are relatively easy to implement and do not necessarily require the expertise of mental health professionals. The challenge is considering pain in its biopsychosocial contexts and defining an approach that is both comprehensive and feasible. Using the ExPRESS domains to help guide care can provide a road map.
Corresponding author: Afton L. Hassett, PsyD, 24 Frank Lloyd Wright Drive, Lobby M, CPFRC, Ann Arbor, MI 48106, [email protected].
1. Institute of Medicine. Relieving pain in America a blueprint for transforming prevention, care, education, and research. In. Washington, DC: National Academy of Sciences; 2011.
2. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926.
3. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
4. National Pain Strategy. A Comprehensive Population Health-Level Strategy for Pain. 2015. Accessed at http://iprcc.nih.gov/docs/DraftHHSNationalPainStrategy.pdf.
5. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in spine pain patients presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013.
6. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain 2009;10:777-91.
7. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
8. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15.
9. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:421.
10. Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am 2009;35:393–407.
11. Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil 2015;29:1155–67.
12. O’Connor SR, Tully MA, Ryan B, et al. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:724-34 e3.
13. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil 2015;94:358–65.
14. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79.
15. Burckhardt CS, Goldenberg D, Crofford L, et al. Guideline for the management of fibromyalgia syndrome. Pain in adults and children. Glenview, IL: American Pain Society; 2005.
16. Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536–41.
17. Chou R, Huffman LH, American Pain Society, American College of Pain Medicine. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:492–504.
18. Chou R, Qaseem A, Snow V, , Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Pain Medicine, American Pain Society low back pain guidelines P. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91.
19. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther;12:R79.
20. Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood and sleep. J Pain 2010.
21. Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668-77.
22. Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 2005;113:331–9.
23. Reme SE, Tangen T, Moe T, Eriksen HR. Prevalence of psychiatric disorders in sick listed chronic low back pain patients. Eur J Pain 2011;15:1075–80.
24. Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry 2006;67:1219–25.
25. Hassett AL, Radvanski DC, Buyske S, et al. Psychiatric comorbidity and other psychological factors in patients with “chronic Lyme disease”. Am J Med 2009;122:843–50.
26. Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 1999;40:57–63.
27. Goesling J, Henry MJ, Moser SE, et al. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. J Pain 2015;16:844–51.
28. Hassett AL, Cone JD, Patella SJ, Sigal LH. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum 2000;43:2493–500.
29. Giesecke T, Williams DA, Harris RE, et al. Subgroupings of fibromyalgia patients on the basis of pressure pain thresholds and psychological factors. Arthritis Rheum 2003;48:2916–22.
30. Walen HR, Cronan PA, Bigatti SM. Factors associated with healthcare costs in women with fibromyalgia. Am J Manag Care 2001;7 Spec No:SP39-47.
31. Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr Pain Headache Rep 2014;18:436.
32. Robinson RL, Birnbaum HG, Morley MA, et al. Depression and fibromyalgia: treatment and cost when diagnosed separately or concurrently. J Rheumatol 2004;31:1621–9.
33. Fitzcharles MA. In: Canadian Rheumatology Association’s 64th Annual Meeting; 2009.
34. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
35. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7.
36. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1–13.
37. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007;26:1–9.
39. Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
39. Beck JS. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
40. Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
41. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013;9:CD009796.
42. Nielson WR, Walker C, McCain GA. Cognitive behavioral treatment of fibromyalgia syndrome: preliminary findings. J Rheumatol 1992;19:98–103.
43. Nicassio PM, Radojevic V, Weisman MH, et al. A comparison of behavioral and educational interventions for fibromyalgia. J Rheumatol 1997;24:2000–7.
44. Williams DA, Cary MA, Groner KH, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. J Rheumatol 2002;29:1280–6.
45. Lindstrom I, Ohlund C, Eek C, et al. Mobility, strength, and fitness after a graded activity program for patients with subacute low back pain. A randomized prospective clinical study with a behavioral therapy approach. Spine 1992;17:641–52.
46. Lindstrom I, Ohlund C, Eek C, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant-conditioning behavioral approach. Phys Ther 1992;72:279-90; discussion 91–3.
47. McAllister SJ, Vincent A, Hassett AL, et al. Psychological resilience, affective mechanisms and symptom burden in a tertiary-care sample of patients with fibromyalgia. Stress Health 2015;31:299–305.
48. Toussaint LL, Vincent A, McAllister SJ, et al. A comparison of fibromyalgia symptoms in patients with healthy versus depressive, low and reactive affect balance styles. Scand J Pain 2014;5:161–6.
49. Hassett AL, Simonelli LE, Radvanski DC, et al. The relationship between affect balance style and clinical outcomes in fibromyalgia. Arthritis Rheum 2008;59:833–40.
50. Zamirinejad S, Hojjat SK, Golzari M, et al. Effectiveness of resilience training versus cognitive therapy on reduction of depression in female iranian college students. Issues Ment Health Nurs 2014;35:480–8.
51. Asgharipoor N, Asgharnejad Farid A, Arshadi H, Sahebi A. A comparative study on the effectiveness of positive psychotherapy and group cognitive-behavioral therapy for the patients suffering from major depressive disorder. Iran J Psychiatry Behav Sci 2012;6:33–41.
52. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol 2009;65:467–87.
53. Bolier L, Haverman M, Westerhof GJ, et al. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health 2013;13:119.
54. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56:218–26.
55. Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol 2005;60:410–21.
56. Seligman ME, Rashid T, Parks AC. Positive psychotherapy. Am Psychol 2006;61:774–88.
57. White DK, Keysor JJ, Neogi T, et al. When it hurts, a positive attitude may help: association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care Res (Hoboken) 2012;64:1312–9.
58. Strine TW, Chapman DP, Balluz LS, et al. The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among U.S. community-dwelling adults. J Community Health 2008;33:40–50.
59. Grant N, Wardle J, Steptoe A. The relationship between life satisfaction and health behavior: a cross-cultural analysis of young adults. Int J Behav Med 2009;16:259–68.
60. Baruth M, Lee DC, Sui X, et al. Emotional outlook on life predicts increases in physical activity among initially inactive men. Health Educ Behav 2011;38:150–8.
61. Kalmbach DA, Pillai V, Roth T, Drake CL. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res 2014;23:636–45.
62. Simor P, Krietsch KN, Koteles F, McCrae CS. Day-to-day variation of subjective sleep quality and emotional states among healthy university students-a 1-week prospective study. Int J Behav Med 2015;22:625–34.
63. von Kanel R, Mausbach BT, Ancoli-Israel S, et al. Positive affect and sleep in spousal Alzheimer caregivers: a longitudinal study. Behav Sleep Med 2014;12:358–72.
64. Cohn MA, Fredrickson BL, Brown SL, et al. Happiness unpacked: positive emotions increase life satisfaction by building resilience. Emotion 2009;9:361–8.
65. Ostir GV, Berges IM, Markides KS, Ottenbacher KJ. Hypertension in older adults and the role of positive emotions. Psychosom Med 2006;68:727–33.
66. Stone AA, Cox DS, Valdimarsdottir H, et al. Evidence that secretory IgA antibody is associated with daily mood. J Pers Soc Psychol 1987;52:988–93.
67. Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A 2005;102:6508–12.
68. Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci 2013;24:1123–32.
69. Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med 2008;70:1020–7.
70. Hausmann LR, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. J Pain 2014;15:560–7.
71. Muller R, Gertz KJ, Molton IR, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin J Pain 2015.
72. Flink IK, Smeets E, Bergbom S, Peters ML. Happy despite pain: Pilot study of a positive pscyhology intervention for patients with chronic pain. Scandinavian Jounral of Pain 2015;7:71–9.
73. Hassett AL, Radvanski DC, Buyske S, et al. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum 2008;59:1742–9.
74. Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 2015;11:513–20.
75. Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med 2010;11:158–79.
76. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy Am J Psychiatry 1994;151:1172–80.
77. Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain 1996;68:363–8.
78. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165:2527–35.
79. Jacobson E. Progressive relaxation. Chicago: University of Chicago Press; 1938.
80. van Tulder MW, Koes B, Malmivaara A. Outcome of non-invasive treatment modalities on back pain: an evidence-based review. Eur Spine J 2006;15 Suppl 1:S64–81.
81. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232–8.
82. Rucco V, Feruglio C, Genco F, Mosanghini R. [Autogenic training versus Erickson’s analogical technique in treatment of fibromyalgia syndrome]. Riv Eur Sci Med Farmacol 1995;17:41–50.
83. Bawa FL, Mercer SW, Atherton RJ, et al. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. Br J Gen Pract 2015;65:e387–400.
84. Ost LG. The efficacy of acceptance and commitment therapy: an updated systematic review and meta-analysis. Behav Res Ther 2014;61:105–21.
85. Crowe M, Jordan J, Burrell B, et al. Mindfulness-based stress reduction for long-term physical conditions: a systematic review. Aust N Z J Psychiatry 2015.
86. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
1. Institute of Medicine. Relieving pain in America a blueprint for transforming prevention, care, education, and research. In. Washington, DC: National Academy of Sciences; 2011.
2. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926.
3. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
4. National Pain Strategy. A Comprehensive Population Health-Level Strategy for Pain. 2015. Accessed at http://iprcc.nih.gov/docs/DraftHHSNationalPainStrategy.pdf.
5. Brummett CM, Goesling J, Tsodikov A, et al. Prevalence of the fibromyalgia phenotype in spine pain patients presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis Rheum 2013.
6. Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain 2009;10:777-91.
7. Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–55.
8. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15.
9. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:421.
10. Hassett AL, Gevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin North Am 2009;35:393–407.
11. Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil 2015;29:1155–67.
12. O’Connor SR, Tully MA, Ryan B, et al. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:724-34 e3.
13. Meng XG, Yue SW. Efficacy of aerobic exercise for treatment of chronic low back pain: a meta-analysis. Am J Phys Med Rehabil 2015;94:358–65.
14. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther 2010;12:R79.
15. Burckhardt CS, Goldenberg D, Crofford L, et al. Guideline for the management of fibromyalgia syndrome. Pain in adults and children. Glenview, IL: American Pain Society; 2005.
16. Carville SF, Arendt-Nielsen S, Bliddal H, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis 2008;67:536–41.
17. Chou R, Huffman LH, American Pain Society, American College of Pain Medicine. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med 2007;147:492–504.
18. Chou R, Qaseem A, Snow V, , Clinical Efficacy Assessment Subcommittee of the American College of Physicians, American College of Pain Medicine, American Pain Society low back pain guidelines P. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147:478–91.
19. Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther;12:R79.
20. Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood and sleep. J Pain 2010.
21. Gore M, Sadosky A, Stacey BR, et al. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668-77.
22. Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 2005;113:331–9.
23. Reme SE, Tangen T, Moe T, Eriksen HR. Prevalence of psychiatric disorders in sick listed chronic low back pain patients. Eur J Pain 2011;15:1075–80.
24. Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry 2006;67:1219–25.
25. Hassett AL, Radvanski DC, Buyske S, et al. Psychiatric comorbidity and other psychological factors in patients with “chronic Lyme disease”. Am J Med 2009;122:843–50.
26. Epstein SA, Kay G, Clauw D, et al. Psychiatric disorders in patients with fibromyalgia. A multicenter investigation. Psychosomatics 1999;40:57–63.
27. Goesling J, Henry MJ, Moser SE, et al. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. J Pain 2015;16:844–51.
28. Hassett AL, Cone JD, Patella SJ, Sigal LH. The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis Rheum 2000;43:2493–500.
29. Giesecke T, Williams DA, Harris RE, et al. Subgroupings of fibromyalgia patients on the basis of pressure pain thresholds and psychological factors. Arthritis Rheum 2003;48:2916–22.
30. Walen HR, Cronan PA, Bigatti SM. Factors associated with healthcare costs in women with fibromyalgia. Am J Manag Care 2001;7 Spec No:SP39-47.
31. Hassett AL, Aquino JK, Ilgen MA. The risk of suicide mortality in chronic pain patients. Curr Pain Headache Rep 2014;18:436.
32. Robinson RL, Birnbaum HG, Morley MA, et al. Depression and fibromyalgia: treatment and cost when diagnosed separately or concurrently. J Rheumatol 2004;31:1621–9.
33. Fitzcharles MA. In: Canadian Rheumatology Association’s 64th Annual Meeting; 2009.
34. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
35. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7.
36. Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1–13.
37. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007;26:1–9.
39. Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
39. Beck JS. Cognitive therapy: basics and beyond. New York: Guilford Press; 1995.
40. Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2010;37:1991–2005.
41. Bernardy K, Klose P, Busch AJ, et al. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013;9:CD009796.
42. Nielson WR, Walker C, McCain GA. Cognitive behavioral treatment of fibromyalgia syndrome: preliminary findings. J Rheumatol 1992;19:98–103.
43. Nicassio PM, Radojevic V, Weisman MH, et al. A comparison of behavioral and educational interventions for fibromyalgia. J Rheumatol 1997;24:2000–7.
44. Williams DA, Cary MA, Groner KH, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. J Rheumatol 2002;29:1280–6.
45. Lindstrom I, Ohlund C, Eek C, et al. Mobility, strength, and fitness after a graded activity program for patients with subacute low back pain. A randomized prospective clinical study with a behavioral therapy approach. Spine 1992;17:641–52.
46. Lindstrom I, Ohlund C, Eek C, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant-conditioning behavioral approach. Phys Ther 1992;72:279-90; discussion 91–3.
47. McAllister SJ, Vincent A, Hassett AL, et al. Psychological resilience, affective mechanisms and symptom burden in a tertiary-care sample of patients with fibromyalgia. Stress Health 2015;31:299–305.
48. Toussaint LL, Vincent A, McAllister SJ, et al. A comparison of fibromyalgia symptoms in patients with healthy versus depressive, low and reactive affect balance styles. Scand J Pain 2014;5:161–6.
49. Hassett AL, Simonelli LE, Radvanski DC, et al. The relationship between affect balance style and clinical outcomes in fibromyalgia. Arthritis Rheum 2008;59:833–40.
50. Zamirinejad S, Hojjat SK, Golzari M, et al. Effectiveness of resilience training versus cognitive therapy on reduction of depression in female iranian college students. Issues Ment Health Nurs 2014;35:480–8.
51. Asgharipoor N, Asgharnejad Farid A, Arshadi H, Sahebi A. A comparative study on the effectiveness of positive psychotherapy and group cognitive-behavioral therapy for the patients suffering from major depressive disorder. Iran J Psychiatry Behav Sci 2012;6:33–41.
52. Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol 2009;65:467–87.
53. Bolier L, Haverman M, Westerhof GJ, et al. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health 2013;13:119.
54. Fredrickson BL. The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. Am Psychol 2001;56:218–26.
55. Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol 2005;60:410–21.
56. Seligman ME, Rashid T, Parks AC. Positive psychotherapy. Am Psychol 2006;61:774–88.
57. White DK, Keysor JJ, Neogi T, et al. When it hurts, a positive attitude may help: association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care Res (Hoboken) 2012;64:1312–9.
58. Strine TW, Chapman DP, Balluz LS, et al. The associations between life satisfaction and health-related quality of life, chronic illness, and health behaviors among U.S. community-dwelling adults. J Community Health 2008;33:40–50.
59. Grant N, Wardle J, Steptoe A. The relationship between life satisfaction and health behavior: a cross-cultural analysis of young adults. Int J Behav Med 2009;16:259–68.
60. Baruth M, Lee DC, Sui X, et al. Emotional outlook on life predicts increases in physical activity among initially inactive men. Health Educ Behav 2011;38:150–8.
61. Kalmbach DA, Pillai V, Roth T, Drake CL. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res 2014;23:636–45.
62. Simor P, Krietsch KN, Koteles F, McCrae CS. Day-to-day variation of subjective sleep quality and emotional states among healthy university students-a 1-week prospective study. Int J Behav Med 2015;22:625–34.
63. von Kanel R, Mausbach BT, Ancoli-Israel S, et al. Positive affect and sleep in spousal Alzheimer caregivers: a longitudinal study. Behav Sleep Med 2014;12:358–72.
64. Cohn MA, Fredrickson BL, Brown SL, et al. Happiness unpacked: positive emotions increase life satisfaction by building resilience. Emotion 2009;9:361–8.
65. Ostir GV, Berges IM, Markides KS, Ottenbacher KJ. Hypertension in older adults and the role of positive emotions. Psychosom Med 2006;68:727–33.
66. Stone AA, Cox DS, Valdimarsdottir H, et al. Evidence that secretory IgA antibody is associated with daily mood. J Pers Soc Psychol 1987;52:988–93.
67. Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A 2005;102:6508–12.
68. Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci 2013;24:1123–32.
69. Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med 2008;70:1020–7.
70. Hausmann LR, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. J Pain 2014;15:560–7.
71. Muller R, Gertz KJ, Molton IR, et al. Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin J Pain 2015.
72. Flink IK, Smeets E, Bergbom S, Peters ML. Happy despite pain: Pilot study of a positive pscyhology intervention for patients with chronic pain. Scandinavian Jounral of Pain 2015;7:71–9.
73. Hassett AL, Radvanski DC, Buyske S, et al. Role of psychiatric comorbidity in chronic Lyme disease. Arthritis Rheum 2008;59:1742–9.
74. Choy EH. The role of sleep in pain and fibromyalgia. Nat Rev Rheumatol 2015;11:513–20.
75. Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med 2010;11:158–79.
76. Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy Am J Psychiatry 1994;151:1172–80.
77. Affleck G, Urrows S, Tennen H, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain 1996;68:363–8.
78. Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch Intern Med 2005;165:2527–35.
79. Jacobson E. Progressive relaxation. Chicago: University of Chicago Press; 1938.
80. van Tulder MW, Koes B, Malmivaara A. Outcome of non-invasive treatment modalities on back pain: an evidence-based review. Eur Spine J 2006;15 Suppl 1:S64–81.
81. Keel PJ, Bodoky C, Gerhard U, Muller W. Comparison of integrated group therapy and group relaxation training for fibromyalgia. Clin J Pain 1998;14:232–8.
82. Rucco V, Feruglio C, Genco F, Mosanghini R. [Autogenic training versus Erickson’s analogical technique in treatment of fibromyalgia syndrome]. Riv Eur Sci Med Farmacol 1995;17:41–50.
83. Bawa FL, Mercer SW, Atherton RJ, et al. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. Br J Gen Pract 2015;65:e387–400.
84. Ost LG. The efficacy of acceptance and commitment therapy: an updated systematic review and meta-analysis. Behav Res Ther 2014;61:105–21.
85. Crowe M, Jordan J, Burrell B, et al. Mindfulness-based stress reduction for long-term physical conditions: a systematic review. Aust N Z J Psychiatry 2015.
86. Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain 2011;152:533–42.
Delusions, hypersexuality, and a steep cognitive decline
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
CASE Inconsistent stories
Ms. P, age 56, is an Asian American woman who was brought in by police after being found standing by her car in the middle of a busy road displaying bizarre behavior. She provides an inconsistent story about why she was brought to the hospital, saying that the police did so because she wasn’t driving fast enough and because her English is weak. At another point, she says that she had stopped her car to pick up a penny from the road and the police brought her to the hospital “to experience life, to rest, to meet people.”
Upon further questioning, Ms. P reveals that she is experiencing racing thoughts, feels full of energy, has pressured speech, and does not need much sleep. She also is sexually preoccupied, talks about having extra-marital affairs, and expresses her infatuation with TV news anchors. She says she is sexually active but is unable to offer any further details, and—while giggling—asks the treatment team not to reveal this information to her husband. Ms. P also reports hearing angels singing from the sky.
Chart review reveals that Ms. P had been admitted to same hospital 5 years earlier, at which time she was given diagnoses of late-onset schizophrenia (LOS) and mild cognitive impairment. Ms. P also had 3 psychiatric inpatient admissions in the past 2 years at a different hospital, but her records are inaccessible because she refuses to allow her chart to be released.
Ms. P has not taken the psychiatric medications prescribed for her for several months; she says, “I don’t need medication. I am self-healing.” She denies using illicit substances, including marijuana, smoking, and current alcohol use, but reports occasional social drinking in the past. Her urine drug screen is negative.
The most striking revelation in Ms. P’s social history is her high premorbid functional status. She has 2 master’s degrees and had been working as a senior accountant at a major hospital system until 7 years ago. In contrast, when interviewed at the hospital, Ms. P reports that she is working at a child care center.
On mental status exam, Ms. P is half-draped in a hospital gown, casual, overly friendly, smiling, and twirling her hair. Her mood is elevated with inappropriate affect. Her thought process is bizarre and illogical. She is alert, fully oriented, and her sensorium is clear. She has persistent ambivalence and contradictory thoughts regarding suicidal ideation. Recent and remote memory are largely intact. She does not express homicidal ideation.
What could be causing Ms. P’s psychosis and functional decline?
a) major neurocognitive disorder
b) schizophrenia
c) schizoaffective disorder
d) bipolar disorder, current manic episode
HISTORY Fired from her job
According to Ms. P’s chart from her admission 5 years earlier, police brought her to the hospital because she was causing a disturbance at a restaurant. When interviewed, Ms. P reported a false story that she fought with her husband, kicked him, and spat on his face. She said that her husband then punched her in the face, she ran out of the house, and a bystander called the police. At the time, her husband was contacted and denied the incident. He said that Ms. P had gone to the store and not returned, and he did not know what happened to her.
Her husband reported a steady and progressive decline in function and behavior dating back to 8 years ago with no known prior behavioral disturbances. In the chart from 5 years ago, her husband reported that Ms. P had been a high-functioning senior executive accountant at a major hospital system 7 years before the current admission, at which time she was fired from her job. He said that, just before being fired, Ms. P had been reading the mystery novel The Da Vinci Code and believed that events in the book specifically applied to her. Ms. P would stay up all night making clothes; when she would go to work, she was caught sleeping on the job and performing poorly, including submitting reports with incorrect information. She yelled at co-workers and was unable to take direction from her supervisors.
Ms. P’s husband also reported that she believed people were trying to “look like her,” by having plastic surgery. He reported unusual behavior at home, including eating food off the countertop that had been out for hours and was not fit for consumption.
Ms. P’s husband could not be contacted during this admission because he was out of country and they were separated. Collateral information is obtained from Ms. P’s mother, who lives apart from her but in the same city and speaks no English. She confirms Ms. P’s high premorbid functioning, and reports that her daughter’s change in behavior went back as far as 10 years. She reports that Ms. P had problems controlling anger and had frequent altercations with her husband and mother, including threatening her with a knife. Self-care and hygiene then declined strikingly. She began to have odd religious beliefs (eg, she was the daughter of Jesus Christ) and insisted on dressing in peculiar ways.
No family history of psychiatric disorders, such as schizophrenia, bipolar disorder, or dementia, was reported (Table 1).
The authors’ observations
The existence of LOS as a distinct subtype of schizophrenia has been the subject of discussion and controversy as far back as Manfred Bleuler in 1943 who coined the term “late-onset schizophrenia.”1 In 2000, a consensus statement by the International Late-Onset Schizophrenia Group standardized the nomenclature, defining LOS as onset between age 40 and 60, and very-late-onset schizophrenia-like psychosis (VLOS) as onset after age 60.2 Although there is no diagnostic subcategory for LOS in DSM, DSM-5 notes that (1) women are overrepresented in late-onset cases and (2) the course generally is characterized by a predominance of psychotic symptoms with preservation of affect and social functioning.3 DSM authors comment that it is not yet clear whether LOS is the same condition as schizophrenia diagnosed earlier in life. Approximately 23% of schizophrenia cases have onset after age 40.4
Cognitive symptoms in LOS
The presence of cognitive deficits in schizophrenia is common and well-recognized. The intellectual impairment is generalized and global, and there also is specific impairment in a range of cognitive functions, such as executive functions, memory, psychomotor speed, attention, and social cognition.5 Typically these cognitive impairments are present before onset of psychotic symptoms. Although cognitive symptoms are not part of the formal diagnostic criteria, DSM-5 acknowledges their presence.3 In a systematic review on nature and course of cognitive function in LOS, Rajji and Mulsant6 report that global deficits and specific deficits in executive functions, visuospatial constructional abilities, verbal fluency, and psychomotor speech have been found consistently in studies of LOS, although the presence of deficits in memory, attention, and working memory has been less consistent.
The presence of cognitive symptoms in LOS is less well-studied and understood (Table 2). The International Consensus Statement reported that no difference in type of cognitive deficit has been found in early–onset cases (onset before age 40) compared with late-onset cases, although LOS is associated with relatively milder cognitive deficits. Additionally, premorbid educational, occupational, and psychosocial functioning are less impaired in LOS than they are in early-onset schizophrenia.2
Rajji et al7 performed a meta-analysis comparison of patients with youth-onset schizophrenia, adults with first-episode schizophrenia, and those with LOS on their cognitive profiles. They reported that patients with youth-onset schizophrenia have globally severe cognitive deficits, whereas those with LOS demonstrate minimal deficits on arithmetic, digit symbol coding, and vocabulary but larger deficits on attention, fluency, global cognition, IQ, and visuospatial construction.7
There are conflicting views in the literature with regards to the course of cognitive deficits in schizophrenia. One group of researchers believes that there is progressive deterioration in cognitive functioning over time, while another maintains that cognitive impairment in schizophrenia is largely “a static encephalopathy” with no significant progression of symptoms.8 A number of studies referenced by Rajji and Mulsant6 in their systematic review report that cognitive deficits seen in patients with LOS largely are stable on follow-up with an average duration of up to 3 years. However, 2 studies with longer follow-up report evidence of cognitive decline.9,10
Relevant findings from the literature. Brodaty et al9 followed 27 patients with LOS without dementia and 34 otherwise healthy participants at baseline, 1 year, and 5 years. They reported that 9 patients with LOS and none of the control group were found to have dementia (5 Alzheimer type, 1 vascular, and 3 dementia of unknown type) at 5-year follow-up. Some patients had no clinical signs of dementia at baseline or at 1-year follow-up, but were found to have dementia at 5-year follow-up. The authors speculated that LOS might be a prodrome of Alzheimer-type dementia.
Kørner et al10 studied 12,600 patients with LOS and 7,700 with VLOS, selected from the Danish nationwide registry; follow-up was 3 to 4.58 years. They concluded that patients with LOS and VLOS were at 2 to 3 times greater risk of developing dementia than patients with osteoarthritis or the general population. The most common diagnosis among patients with schizophrenia was unspecified dementia, with Alzheimer’s dementia (AD) being the most common diagnosis in control groups. The findings suggest that dementia in LOS and VLOS has a different basis than AD.
Zakzanis et al11 investigated which neuropsychological tests best differentiate patients with LOS and those with AD or frontotemporal dementia. They reported that Wechsler Adult Intelligence Scale-Revised (WAIS-R) Similarities subtest and the California Verbal Learning Test (both short- and long-delay free recall) can differentiate LOS from AD, and a test battery comprising the WAIS-R Vocabulary, Information, Digit Span, and Comprehension subtests, and the Hooper Visual Organization test can differentiate LOS and frontotemporal dementia.12
EVALUATION Significant impairment
CT head and MRI brain scans without contrast suggest mild generalized atrophy that is more prominent in frontal and parietal areas, but the scans are otherwise unremarkable overall. A PET scan is significant for hypoactivity in the temporal and parietal lobes but, again, the images are interpreted as unremarkable overall.
Ms. P scores 21 on the Montreal Cognitive Assessment (MoCA), indicative of significant cognitive impairment (normal score, ≥26). This is a 3-point decline on a MoCA performed during her admission 5 years earlier.
Ms. P scores 8 on the Middlesex Elderly Assessment of Mental State, the lowest score in the borderline range of cognitive function for geriatric patients. She scores 13 on the Kohlman Evaluation of Living Skills, indicating that she needs maximal supervision, structure, and support to live in the community. Particularly notable is that Ms. P failed 5 out of 6 subtests in money management—a marked decline for someone who had worked as a senior accountant.
Given Ms. P’s significant cognitive decline from premorbid functioning, verified by collateral information, and current cognitive deficits established on standardized tests, we determine that, in addition to a diagnosis of schizoaffective disorder, she might meet DSM-5 criteria for unspecified major neurocognitive disorder if her functioning does not improve with treatment.
The authors’ observations
There is scant literature on late-onset schizoaffective disorder. Webster and Grossberg13 conducted a retrospective chart review of 1,730 patients age >65 who were admitted to a geriatric psychiatry unit from 1988 to 1995. Of these patients, 166 (approximately 10%) were found to have late life-onset psychosis. The psychosis was attributed to various causes, such as dementia, depression, bipolar disorder, medical causes, delirium, medication toxicity. Two patients were diagnosed with schizophrenia and 2 were diagnosed with schizoaffective disorder (the authors did not provide additional information about the patients with schizoaffective disorder). Brenner et al14 reports a case of late-onset schizoaffective disorder in a 70-year-old female patient. Evans et al15 compared outpatients age 45 to 77 with a diagnosis of schizoaffective disorder (n = 29), schizophrenia (n = 154), or nonpsychotic mood disorder (n = 27) and concluded that late-onset schizoaffective disorder might represent a variant of LOS in clinical symptom profiles and cognitive impairment but with additional mood symptoms.16
How would you begin treating Ms. P?
a) start a mood stabilizer
b) start an atypical antipsychotic
c) obtain more history and collateral information
d) recommend outpatient treatment
The authors’ observations
Given Ms. P’s manic symptoms, thought disorder, and history of psychotic symptoms with diagnosis of LOS, we assigned her a presumptive diagnosis of schizoaffective disorder, bipolar type. From the patient report, collateral information from her mother, earlier documented collateral from her husband, and chart review, it was apparent to us that Ms. P’s psychiatric history went back only 10 years—therefore meeting temporal criteria for LOS.
Clinical assessment (Figure) and standardized tests revealed the presence of neurocognitive deficits sufficient to meet criteria for major neurocognitive disorder (Table 33). The pattern of neurocognitive deficits is consistent with an AD-like amnestic picture, although no clear-cut diagnosis was present, and the neurocognitive disorder was better classified as unspecified rather than of a particular type. It remains uncertain whether cognitive deficits of severity that meet criteria for major neurocognitive disorder are sufficiently accounted for by the diagnosis of LOS alone. Unless diagnostic criteria for schizophrenia are expanded to include cognitive deficits, a separate diagnosis of major neurocognitive disorder is warranted at present.
TREATMENT Pharmacotherapy
On the unit, Ms. P is observed by nursing staff wandering, with some pressured speech but no behavioral agitation. Her clothing had been bizarre, with multiple layers, and, at one point, she walks with her gown open and without undergarments. She also reports to the nurses that she has a lot of sexual thoughts. When the interview team enters her room, they find her masturbating.
Ms. P is started on aripiprazole, 10 mg/d, titrated to 20 mg/d, and divalproex sodium, 500 mg/d. The decision to initiate a cognitive enhancer, such as an acetylcholinesterase inhibitor or memantine, is deferred to outpatient care to allow for the possibility that her cognitive features will improve after the psychosis is treated.
By the end of first week, Ms. P’s manic features are no longer prominent but her thought process continues to be bizarre, with poor insight and judgment. She demonstrates severe ambivalence in all matters, consistently gives inconsistent accounts of the past, and makes dramatic false statements.
For example, when asked about her children, Ms. P tells us that she has 6 children—the youngest 3 months old, at home by himself and “probably dead by now.” In reality, she has only a 20-year-old son who is studying abroad. Talking about her marriage, Ms. P says she and her husband are not divorced on paper but that, because they haven’t had sex for 8 years, the law has provided them with an automatic divorce.
OUTCOME Significant improvement
Ms. P shows significant response to aripiprazole and divalproex, which are well tolerated without significant adverse effects. Her limitations in executive functioning and rational thought process lead the treatment team to consider nursing home placement under guardianship. Days before discharge, however, reexamination of her neuropsychiatric state suggests significant improvement in thought process, with improvement in cognitive features. Ms. P also becomes cooperative with treatment planning.
The treatment team has meetings with Ms. P’s mother to discuss monitoring and plans for discharge. Ms. P is discharged with follow-up arranged at community mental health services.
Bottom Line
Global as well as specific cognitive deficits are associated with late-onset schizophrenia. Studies have reported increased risk of dementia in these patients over the course of 3 to 5 years, usually unspecified or Alzheimer’s type. It is imperative to assess patients with schizophrenia, especially those age ≥40, for presence of neurocognitive disorder by means of neurocognitive testing.
Related Resources
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav. 2011;99(2):245-253.
- Radhakrishnan R, Butler R, Head L. Dementia in schizophrenia. Adv Psychiatr Treat. 2012;18(2):144-153.
Drug Brand Names
Aripiprazole • Abilify
Divalproex sodium • Depakote
Mematine • Namenda
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturer of competing products.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
1. Bleuler M. Die spätschizophrenen Krankheitsbilder. Fortschr Neurol Psychiatr. 1943;15:259-290.
2. Howard R, Rabins PV, Seeman MV, et al. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. The International Late-Onset Schizophrenia Group. Am J Psychiatry. 2000; 157(2):172-178.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull. 1988;14(1):39-55.
5. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1):4-19.
6. Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102(1-3):122-140.
7. Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2009;195(4):286-293.
8. Goldberg TE, Hyde TM, Kleinman JE, et al. Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull. 1993;19(4):797-804.
9. Brodaty H, Sachdev P, Koschera A, et al. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br J Psychiatry. 2003;183(3):213-219.
10. Kørner A, Lopez AG, Lauritzen L, et al. Late and very-late first‐contact schizophrenia and the risk of dementia—a nationwide register based study. Int J Geriatr Psychiatry. 2009;24(1):61-67.
11. Zakzanis KK, Andrikopoulos J, Young DA, et al. Neuropsychological differentiation of late-onset schizophrenia and dementia of the Alzheimer’s type. Appl Neuropsychol. 2003;10(2):105-114.
12. Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cognitive Neuropsychiatry. 2001;6(1):63-77.
13. Webster J, Grossberg GT. Late-life onset of psychotic symptoms. Am J Geriatr Psychiatry. 1998;6(3):196-202.
14. Brenner R, Campbell K, Konakondla K, et al. Late onset schizoaffective disorder. Consultant. 2014;53(6):487-488.
15. Evans JD, Heaton RK, Paulsen JS, et al. Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry. 1999;60(12):874-882.
16. Jeste DV, Blazer DG, First M. Aging-related diagnostic variations: need for diagnostic criteria appropriate for elderly psychiatric patients. Biol Psychiatry. 2005;58(4):265-271.
Treating Migraine in Teenagers
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Abstract
- Objective: To review the management of migraine in adolescent patients.
- Methods: Literature review in the context of 2 clinical cases.
- Results: Migraine is common in adolescents and can affect school and social functioning. Management options include lifestyle modifications and acute and preventative therapies. First-line medications for migraine in the adolescent population are over-the-counter medications, including ibuprofen, acetaminophen, and naproxen. Studies of efficacy of triptans in the treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. Several classes of medications are commonly used for migraine prevention, including antidepressants and antiepileptics. Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older. Biobehavioral treatments such as relaxation training, biofeedback, and cognitive behavioral therapy have been evaluated in randomized controlled trials and found to be efficacious.
- Conclusion: Approach to management of migraine in adolescents should be multifactorial with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Case Study 1
Initial Presentation
A 13-year-old left-handed boy with allergic rhinitis has been referred by his pediatrician for evaluation of headaches.
History
The headaches have been occurring since he was 7 years old. He describes a bilateral frontal and periorbital pain, which is either aching or throbbing in nature. There is associated photophobia and phonophobia, and the pain worsens with activity. When he was younger the headaches were frequently associated with emesis. He occasionally gets tenderness of his face during the headaches, mostly in the areas adjacent to his nose and above his eyes and occasionally associated rhinorrhea.
Initially the headaches were mild and occurring infrequently, but for the past 6 months they have been more severe and occurring 1 to 3 times per week, sometimes on consecutive days. They typically begin in the afternoon but at times occur soon after waking in the morning. If they do occur in the morning, they worsen after getting out of bed. He denies any type of warning symptoms indicating the headache will occur, and has no associated focal neurologic symptoms associated with the headaches. His mother tries to minimize medication intake so will typically wait to see if the headache is severe before giving him medication, typically 2 chewable children’s ibuprofen, which helps some of the time; however, his headache often does not completely abate unless he naps. He has also been taking loratadine daily due to concerns that his headaches may be secondary to chronic sinusitis.
He is a good student and enjoys school, but notes stress surrounding tests. He reports missing 4 days of school in the past 3 months as well as coming late twice. He thinks that there were 9 additional days in which he was unable to function at his full ability at school and at least 5 times he was unable to concentrate on his homework. He plays soccer 4 to 5 times per week. He says twice he had to skip soccer practice to due to headache and at least 3 other times he needed to take breaks during soccer due to headache. He is unsure how much he drinks on an average day but he drinks mostly with meals. He does not drink caffeine. He gets into bed at 10 pm and sometimes does not fall asleep until after 11 pm, often due to worry about tests. He often plays with his tablet when trying to fall asleep. He wakes up at 6 am for school. He typically eats 3 meals per day but occasionally misses breakfast if he is rushed in the morning.
Physical Examination
On examination, the patient is a well-developed, well-nourished male in no apparent distress. His weight was 50.9 kg (68th percentile), height 163.7 cm (65th percentile), BMI 19.9 (69th percentile), blood pressure 118/62 mm Hg, and heart rate 79 bpm. His general physical examination, including skin, HEENT, extremities, lung, cardiac, and abdominal examination was normal, with lungs clear to auscultation, heart with a regular rate and rhythm, and abdomen soft and tender without organomegaly.
On neurologic examination he was alert and attentive with normal mental status. Speech was fluent. Skull, spine, and meninges were normocephalic and atraumatic with a supple neck. Cranial nerves II through XII were normal with a normal fundoscopic examination including sharp disk, no papilledema, and normal fundus bilaterally. Motor examination was normal for tone and bulk with full strength throughout. Sensory examination was normal for light touch, temperature, vibration, and joint position sense. Finger-nose-finger fine finger movement and heel-knee-shin were normal. Deep tendon reflexes were symmetric and 2+ throughout with toes downgoing. Station and gait were normal including toe walking, heel walking, tandem walking, running, skipping, one-legged standing, Fog gate procedure, and there was no Romberg’s sign.
He had a normal comprehensive headache examination. There was no tenderness at typical migraine trigger points (nuchal line and mandibular process) and no tenderness at supraorbital notch. Neck was supple with normal rotation and normal bilateral trapezius muscle tightness. No bruits or palpitations were heard over carotid or jugular veins. There was a negative Muller sign with no pain on neck bending with pressure, and no signs of allergy or sinus symptoms.
What is the initial evaluation of a teen with recurrent headaches?
The overwhelming majority of children and adolescents presenting to medical attention with recurrent headaches will have a primary headache disorder, most commonly migraine [1].However, ruling out a secondary cause is typically of concern, both for providers and parents, and the decision regarding whether to image is often a daunting one. In a review of 6 large studies in which imaging was done in pediatric patients who were examined by a neurologist, only 14 patients were found to have CNS lesions requiring intervention, and all of these patients had abnormalities on physical exam [2]. An American Academy of Neurology (AAN) practice parameter published in 2002 recommended considering neuroimaging only in patients with abnormal neurologic examination, co-existing seizures, concerning associated neurologic features, or recent onset of severe headache or change in headache type [2].There are additional factors which may prompt one to consider imaging as well including atypical auras or very short (< 5 minutes) or protracted ( > 60 minutes) auras, trigeminal autonomic cephalalgia, brief headaches precipitated by cough, frequent early morning headaches or which wake the patient from sleep, headaches with primarily occipital location, headaches in a patient less than 6 years old or in a child who cannot describe the headaches well, or migrainous headaches in a child without any family history of migraine or migraine equivalents. However, recent studies continue to show that the imaging yield in patients with headaches is low and further studies are needed to elucidate which patients truly require imaging [3].
Given this, a thorough history, family history, physical exam, and detailed neurologic exam including fundoscopy are imperative. In addition to the general neurologic exam, a focused headache exam should be performed, including Mueller’s maneuver, auscultation for cranial bruits, evaluation of the temporomandibular joint, palpation of possible trigger points, and maneuvers to assess for cervical spine disease [4].
Classification of Symptoms
Once establishing the likelihood of a primary headache disorder, the International Classification of Headache Disorders, 3rd edition, beta version (ICHD IIIβ) [5]should be used to classify the headache diagnosis. Note that while this classification system was established for adults, most criteria are similar for children and adolescents, and the typical differences are noted in the comments of the ICHD IIIβ [5].Migraine is the most common type of primary headache brought to medical attention [1]. Migraine without aura is described as a recurrent headache disorder (at least 5 lifetime attacks) with attacks lasting 4 to 72 hours, typically unilateral, throbbing in nature, moderate to severe in intensity, aggravated by routine physical activity, and associated with nausea and/or photophobia and phonophobia. In young children, migraine is more frequently bilateral, the gastrointestinal symptoms often more pronounced than photophobia and phonophobia, and migraines may be shorter, lasting at least 2 hours without treatment [5].As patients reach adolescence, migraine features typically start to evolve into patterns described in adults. Differentiating migraine from other primary headaches, such as tension-type, can be challenging, especially in children in whom migraines are more likely to be shorter and bilateral. Tension-type headaches are bilateral or diffuse, pressing or tightening pain that is non-pulsatile lasting at least 30 minutes. The pain is described as mild to moderate in intensity and not aggravated by activity [5].They may be associated with photophobia or phonophobia (not both) and they may not be associated with nausea or vomiting [5].
Is any further workup indicated?
The patient has recurrent headaches which have been present for 5 years. While they have increased in frequency, there has not been any change in their quality. His headache description and history have no “red flag” features, and a thorough examination is normal. Therefore, neuroimaging and further workup would not be indicated in this case.
What is the diagnosis?
The patient’s headaches are throbbing in nature, exacerbated by activity, associated with nausea, photophobia, and phonophobia, and are moderate to severe. Using the ICHD IIIβ, he meets criteria for migraine without aura. While his headaches are frequent, he is having less than 15 headache days per month, so this is episodic migraine. Note that he complains of forehead and periorbital pain and occasional rhinorrhea with his headaches, leading him to have been placed on loratadine for treatment of presumed allergic sinusitis. Children meeting criteria for migraine are very frequently misdiagnosed as having sinusitis [6]due to the overlap in location of migraine pain and proximity to the frontal and maxillary sinuses, as well as the presence of autonomic features frequently present in migraine, reportedly present in 70% of pediatric migraine patients [7].The negative headache examination, including Muller’s maneuver, points against sinus disease as well.
What is the approach to management of the adolescent with migraine?
Approach to management of migraine in adolescents should be multifactorial, with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Acute Treatment
General Approach
In counseling patients with migraine, and in particular adolescents, it should be stressed that achieving a normal level of functioning as soon as possible is the goal of therapy. Common missteps in treatment include failure to take acute medication early into the headache, incorrect dosing, incompletely treating the headache, and avoidance of participating in daily activities when headaches occur.
Adolescent patients may frequently wait to take an acute medication, typically due participation in another activity, not having medication with them, or discomfort with taking medication in front of peers or at school. Additionally, patients who have headaches beginning in early childhood, with pronounced gastrointestinal (GI) features, may be aware that their headaches resolve after vomiting, and therefore get used to not treating with medications. When these patients reach adolescence, when GI symptoms tend to become less pronounced, they will need to be educated that taking medication early is imperative. Acute medications are typically more effective when taken earlier in the course of a migraine, and the importance of pausing to take medication at the onset of the headache should be stressed to all patients and parents. With that in mind, however, care should be taken to counsel patients regarding the potential for development of medication overuse headache. When headaches are frequent, a more detailed and intricate plan may need to be devised so that adolescents and parents know which headaches to treat with medication.
Given that first-line medications for treatment of migraines are over-the-counter, underdosing occurs commonly, as dosing listed on packaging is typically age-based, not weight-based. At initial visits, young adolescent patients will frequently report that a particular medication is not effective, but this is often because they are still receiving low/less optimal dosing. Clinicians should remember to follow weights and recommend dosing changes at the initial visit and follow-up visits as well.
Treatment goals, ie, complete resolution of pain and migrainous features with ability to return to normal functioning, should be made clear to patients and families at the onset of treatment. Patients frequently fall into a pattern of continuing to treat with a medication that may lessen but not completely ablate the pain of a headache, and then sleep and avoid activity. Upon awakening, the headache may be gone, however, given incomplete initial treatment, the headache may be more likely to recur within 24 hours. At that point, the headache may be more difficult to treat and therefore cause further decrease in functioning. If this cycle perpetuates, disability can become extremely burdensome in adolescents, significantly affecting school and social functioning. Therefore, a detailed plan for initial steps in management as well as steps to take should initial therapy fail to fully break the headache should be given to every patient.
Of note, given that dehydration is now recognized as a common trigger for migraine [8,9], it is generally our recommendation to drink 16 to 32 oz (depending on weight) of a hydrating fluid together with whichever acute medication is chosen, as rehydration likely assists in breaking migraines as well [8].
Medications
Over-the-Counter Agents
The first-line medications for migraine in the pediatric and adolescent populations are over-the counter medications, including ibuprofen, acetaminophen, and naproxen sodium. Ibuprofen has been well studied in pediatrics and was found to be safe and effective in 2 studies [10,11]at doses of 7.5–10 mg/kg. Again, care should be taken to ensure timely administration of ibuprofen at onset of headache, or aura if present, with appropriate weight-based dosing. Naproxen sodium has not been studied in pediatrics for treatment of migraine. However in practice, it is often used in similar doses of 10 mg/kg, with good efficacy. Although ibuprofen and naproxen sodium are both nonsteroidal anti-inflammatory medications (NSAIDs), anecdotally many patients report successful treatment with one NSAID when another has failed. Aspirin has shown efficacy in adults for treatment of acute migraine [12].It is likely effective in the pediatric population as well, but it is generally avoided due to long-standing concerns for precipitation of Reye syndrome in children. In adolescents over 16 years old, however, it is a reasonable option if there are no contraindications.
In one study, acetaminophen was compared to ibuprofen and placebo for treatment of migraine in children and adolescents and found to be effective more frequently than placebo but not as frequently as ibuprofen [10],likely due to its only minimal anti-inflammatory effects. It is a reasonable option for children and adolescents, particularly in those who have contraindications to NSAIDs.
While these over-the-counter medications are generally safe and well tolerated, clinicians should not overlook the potential for toxicity as well as medication overuse headache, and patients should be counseled to avoid use of any of these medications for more than 2 to 3 headache days per week.
Triptans
Studies of efficacy of triptans in treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. However, some have shown efficacy over placebo, and in the past few years have received FDA approval for use in the pediatric and adolescent populations. Clinicians should remember that these medications are vasoconstrictors, so they should not be used in patients with vascular disease or in patients with migraine with brainstem aura or hemiplegic migraine. Additionally, due to the risk of serotonin syndrome, they should not be used in patients on monoamine oxidase inhibitors. It is also important to educate patients to limit use of these medications to 4 to 6 times per month to avoid precipitation of medication overuse headache.
Almotriptan was approved by the FDA in 2009 for use in patients 12 years and older, based on a large randomized controlled trial comparing doses of 6.25, 12.5, and 25 mg with placebo in patients ages 12 to 17 years old. All doses resulted in statistically significant pain relief as compared to placebo, and interestingly, the 12.5-mg dose seemed to be the most effective [13].
Rizatriptan received FDA approval in 2012 for use in patients 6 years and older. In 2 randomized controlled trials in patients 6 to 17 years old, rizatriptan (5 mg for patients < 40 kg, 10 mg for patients ≥ 40 kg) was more effective than placebo in providing pain freedom at 2 hours [14,15].One earlier trial found efficacy only on some measures (weekend treatment, decrease in nausea and functional disability) but no statistically significant difference than placebo in terms of overall efficacy in achieving pain freedom at 2 hours [16].However, this trial had a higher placebo response rate than typically seen in adult triptan trials. In a recent long-term open-label study in patients 12 to 17 years old, rizatriptan was found to be generally safe and well-tolerated with consistent efficacy of 46% to 51% pain freedom at 2 hours over time [17].
A combination pill consisting of sumatriptan and naproxen (Treximet) received FDA approval in May 2015 for patients 12 years and older. This was based on a randomized controlled parallel-group trial in patients 12 to 17 years old using the sumatriptan/naproxen combination in various dose combinations: 10/60 mg, 30/180 mg, or 85/500 [18].All doses were found to be equally effective in providing pain relief at 2 hours as compared to placebo, with a higher chance of sustained pain relief at 24 hours in the group receiving the 85/500 dose. In this trial, and in a long-term open-label safety trial [19],all doses were generally well tolerated with minimal adverse reactions.
Most recently, in June 2015, zolmitriptan nasal spray was approved for patients 12 years of age and older. In the large “Double-Diamond” study, which used a novel design to attempt to minimize placebo effect, zolmitriptan nasal spray (5 mg) was found to have a significantly higher headache response rate at 1 hour than placebo, and was significantly superior to placebo with regard to multiple secondary end-points [20].Additionally, a long-term open-label trial in patients 12 to 17 years old using oral zolmitriptan (2.5 mg or 5 mg) found it to be generally effective and well tolerated [21].
No other triptans have been approved for children or adolescents, however, most are widely used in clinical practice. There is good evidence for the efficacy and tolerability of sumatriptan nasal spray [22–26]in adolescents, and it is approved for use in adolescents with migraine in Europe. Although oral sumatriptan was the first triptan available clinically in the United States and is very widely used, there is surprisingly little published evidence for its use in the pediatric and adolescent populations. In fact, 2 randomized controlled trials in adolescents failed to show efficacy of oral sumatriptan as compared to placebo [27,28].Despite this, given its availability it is a reasonable choice for adolescent patients and is often one of the first tried. There has been 1 randomized controlled trial in adolescents for eletriptan without significant differences in efficacy at 2 hours as compared to placebo, although similar to other trials the placebo rate was high and there were some differences seen in secondary outcomes measures [29].Similarly, one randomized controlled trial of naratriptan 12.5-mg tablets failed to show efficacy as compared to placebo in pain relief [30].At present time, for frovatriptan there has only been a study looking at the pharmacokinetics and safety in adolescents, which found that it was generally well tolerated and recommended adolescent dosing similar to adult dosing [31].
In choosing a triptan, clinicians should keep in mind availability of alternate forms of administration, absence or presence of significant emesis, and the age of the patient. For patients who are unable to swallow pills or who have significant emesis associated with their migraines, nasal sprays and oral dissolving forms (melts) are good options. The nasal sprays (zolmitriptan and sumatriptan) additionally have the benefit of a quicker onset (~15 minutes) in general than the oral formulations. The downside to these nasal formulations is bad taste, which is frequently reported by patients. Patients should be counseled in proper administration of these nasal sprays (ie, avoiding inhalation, which causes the medication to enter the mouth) to minimize the bad taste and maximize absorption through the nasal mucosa. Other alternatives are the oral dissolving forms (rizatriptan and zolmitriptan). Given the FDA approval, the rizatriptan melt tablets are often the first-line triptan for children under age 12, but zolmitriptan melts are an option as well.
Preventive Treatment
General Approach
The decision to place an adolescent on a daily preventive medication should be based on a combination of headache frequency, severity, ease of breaking headaches, and overall disability as established by a disability scale (such as the PedMIDAS). Any patient with headaches occurring one or more days per week, those whose headaches are not easily treated or tend to be prolonged, and those with a PedMIDAS score of 30 or more should be considered candidate for a daily preventive. It is particularly important to consider starting a preventive early in adolescent patients given the possibility of impacting overall disease progression at a young age. While the natural history of headaches that start in the young is still being investigated, a known risk factor for transformation of episodic to chronic migraine is frequent headaches [32].It is therefore imperative to attempt to intervene early to improve quality of life in the present and also to prevent a downward cycle into chronicity, potential medication overuse, and worsening disability.
At present, there are several classes of medications that are commonly used for migraine prevention, including antidepressants, antiepileptics, antihistamines, and antihypertensives. Patients should be educated regarding the medication’s typical use, the specific way in which it is used in migraine, potential side effects, and overall expectations of efficacy. Most preventives need to be titrated up slowly to maximize tolerability, and patients need to understand that it may take time before they start to see results. In general, we recommend titrating up over the course of 4 to 12 weeks (depending on medication and dose goal), and then a substantial trial of 4 to 6 weeks on a full dose of medication before determining efficacy. If a medication shows a trend towards improvement but the patient has not met treatment goals, medication can be titrated up further at that point as tolerated. Patients and families should also understand that these medications are not intended as a “cure” for migraine but rather as a tool for improvement, which should be used in conjunction with the rest of a detailed plan. Generally, if a patient is well controlled for 4 to 6 months (ie, 3 or less headaches/month that are easily broken with medications, and a PedMIDAS < 30), attempts should be made to wean off of medication.
Medications
First-line Therapies
Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older (approved in March 2014). Three randomized double-blind placebo-controlled trials in children and adolescents have found topiramate to be superior to placebo in multiple endpoints [33–35].Doses in these trials were 2–3 mg/kg/day, 100 mg/day, and 50 or 100 mg/day, respectively. Notably, the 50 mg/day dose was not found to be superior to placebo. Multiple retrospective, open-label, and drug comparison trials have shown effectiveness and tolerability as well [36–42].We typically use a goal dose of 2 mg/kg/day, which is reached after titrating over 8 to 12 weeks, which minimizes side effects (most commonly paresthesias, memory/language dysfunction, appetite suppression, and drowsiness), but higher doses can be used if needed.
Amitriptyline has consistently shown efficacy in adult migraine trials and is therefore one of the most commonly used medications worldwide for prevention of migraine in children and adolescents. Surprisingly, there have been no published randomized controlled trials using amitriptyline in the pediatric population, although a trial comparing amitriptyline, topiramate, and placebo is currently underway (Childhood and Adolescent Migraine Prevention Study) [43].In 2 retrospective pediatric studies improvement with amitriptyline was reported in 84.2% and 89% of patients, respectively [44,45].We typically use a goal dose of 1 mg/kg/day, also with an 8- to 12-week titration. The most common side effects with amitriptyline are somnolence, dry mouth, and weight gain, but it is generally well tolerated in children and adolescents. There is also a risk of worsening depression and suicidal thoughts, so it is recommended to use caution if considering prescribing to a patient with underlying depression. It is typically administered in once daily dosing a few hours before bed to minimize morning drowsiness. There is also a concern for precipitation of arrhythmias, and while there are no guidelines recommending screening ECGs, this should be considered in patients with a family history of heart disease. Of note, many practitioners use nortriptyline in place of amitriptyline as it can be less sedating. It should be noted, however, that evidence for its efficacy is lacking. Additionally, it may carry a higher risk of arrhythmia [44].
Second-line Therapies
The second-line therapies typically considered are other antiepileptics, valproic acid, levetiracetam, and zonisamide, for which there is some evidence, although mostly in the form of open-label or retrospective studies [46–51]. Of note, despite the many promising retrospective and open-label studies for valproic acid [46–48], one randomized double-blind placebo control trial comparing various doses of extended release divalproex sodium with placebo in adolescent patients failed to show a statistically significant treatment difference between any dose and placebo [52].It is, however, frequently prescribed and anecdotally quite efficacious. Given concerns about potential for teratogenicity, and possible effects on ovarian function, as well as potential for weight gain and hair loss, it should be used with caution in adolescent females.
Antihypertensives (beta blockers and calcium channel blockers) have long been used for prevention of migraine in both the adult and pediatric population, but evidence for their use in the pediatric population is conflicting. An early double-blind crossover study of propranolol in patients 7 to 16 years old showed significant efficacy as compared with placebo [53].However, in 2 subsequent studies it failed to show efficacy as compared with placebo and self-hypnosis, respectively [54,55].Given the conflicting evidence and the potential for hypotension, depression, and exercise-induced asthma, use of propranolol has fallen out of favor by experts for use in pediatric and adolescent migraine prevention. Flunarizine, a nonselective calcium channel blocker, has demonstrated effectiveness in pediatric migraine prevention [56,57],and is actually approved in Europe for this indication, but it is not available in the United States.
Cyproheptadine is an antihistamine with antiserotonergic properties which is used frequently for migraine prevention in young children who are unable to swallow tablets, although evidence is limited to 1 retrospective study [45].However, given the propensity for weight gain with this medication, it is generally not recommended for use in adolescents.
In recent years, there has been a growing interest in nutraceuticals given their general tolerability and minimal side effects, as well as a comfort in using a more “natural” approach, particularly in pediatrics. Evidence is limited but some of the frequently used substances may be beneficial. Butterbur (petasites hybridus) has strong evidence in adults and is recommended by the AAN for migraine prevention in adults [58].There is one small pediatric RCT showing efficacy for butterbur as compared to music therapy [59],as well as one promising open-label trial [60]. Typical dosing ranges from 50–150 mg daily. However, butterbur contains pyrrolizidine alkaloids, which are hepatotoxic, and due to the concerns for inadequate monitoring of removal of these substances in the manufacturing of commercial butterbur, it has been generally avoided in the pediatric population. Coenzyme Q10 (CoQ10) is considered possibly effective for adult migraine prevention by the AAN [58].Pediatric evidence is limited to an open-label study showing improvement with supplementation of CoQ10 in deficient patients [61],and a subsequent double-blind placebo-controlled add-on study, which showed improvement in both the CoQ10 and placebo groups but faster improvement in the CoQ10 group [62].Typical pediatric dosing is 1–2 mg/kg/day. Magnesium is considered by the AAN to be a good option for migraine prevention in adults [58].One randomized controlled trial in children however had equivocal results [63],while one small prospective open-label study had positive results [64]. Magnesium supplementation may be more effective in patients who have low ionized magnesium levels, but this is difficult to measure reliably in the clinical setting. Doses of 9 mg/kg/day can be used with the most common side effects reported being gastrointestinal upset and diarrhea, generally dose dependent. Riboflavin is also considered by the AAN to be probably effective for prevention in adults [58],but again the evidence in children is limited to one positive retrospective study [65]and 2 equivocal randomized controlled trials [66,67],one of which had an unusually high placebo rate [66]. Appropriate dosing is also something of debate, as riboflavin is minimally absorbed and has a short half-life, so while studies were done using 200–400 mg daily, smaller more frequent dosing may be needed.
A potential approach to treatment with vitamins is to check for deficiencies, but currently only the study mentioned above in which CoQ10 levels were checked showed improvement with normalizing of low levels [61,62].Further research into this topic is needed to elucidate whether checking and repleting levels of specific vitamins would be beneficial in prevention of migraines in certain patients.
Healthy Habits
The importance of maintaining healthy lifestyle habits and modifying potentially detrimental ones should be stressed to patients and families, and counseling regarding these issues should be provided at every visit, as repetition is often key to patients understanding their importance. Skipping meals is a commonly reported migraine trigger [68,69].This is not an uncommon occurrence even in the most well-meaning of families; adolescents often report not feeling hungry in the morning, and in the rush to get to school will often skip breakfast. Many patients report not liking school lunches leading them to go most of the day without food. Adolescent girls may skip meals due to weight concerns. Patients need to be reminded that well-balanced meals throughout the day is imperative, and they often may need specific counseling on how to achieve this practically. Of note, unless a specific migraine trigger has been identified in a given patient, we do not generally recommend restricting any specific food lists.
Maintaining good sleep hygiene and a consistent sleep schedule is also often difficult for adolescent patients, with after-school activities, homework, and screen use (eg, television, electronic devices) often contributing to late bedtimes with then forced early morning waking for school. However, improvement in sleep hygiene has been shown to be effective in improving migraines in children and adolescents [70],and realistic plans for improvement in sleep should be discussed with patients.
Dehydration is also a common migraine trigger [8,9],so the importance of staying well hydrated should be stressed as well. Again, specific recommendations for how this can be achieved are often needed, especially given adolescents’ busy schedules. Additionally, many schools do not allow water bottles to be carried, and often a school note is needed so patients may be allowed to carry a water bottle at school and also be provided extra bathroom breaks as needed.
Also important is stressing maintenance of daily functioning throughout migraine treatment. By the time adolescents seek medical care, they may already be in a cycle of missing school due to headaches, and some may even be receiving home-schooling. The goals of staying in school and learning to function with headaches should be stressed, often with the help of coping skills, which will be discussed below, as it has been shown that functional disability generally improves before pain [71].
Behavioral Treatments
Psychological treatments are an important aspect of migraine management. Biobehavioral treatments such as relaxation training, biofeedback, and multimodal cognitive behavioral therapy (CBT) have been evaluated in randomized controlled trials and reviewed in meta-analyses in pediatric migraine populations and found to be efficacious [72–75].These treatments have been shown to reduce pain intensity and disability for children and adolescents with headaches and therapeutic gains appear to be maintained [72].
Relaxation training typically includes instruction in techniques such as diaphragmatic breathing, progressive muscle relaxation, and guided imagery. Relaxation training is most effective with children 7 years of age or older [76].Biofeedback is often used in conjunction with relaxation training to provide audio or visual feedback about normally unconscious physiological body responses associated with increased relaxation. Effective biofeedback parameters used with children and adolescents with migraine headache include electromyographic (EMG) activity and peripheral skin temperature [77].Biofeedback techniques can help children and adolescents become more aware of physical responses, better control these responses and generalize physical responses outside of therapy sessions to better cope with pain [76].CBT involves instruction in skills such as biofeedback-assisted relaxation training, activity pacing, distraction, and cognitive strategies for coping with pain. CBT was shown to be effective in a recent study comparing adolescent patients with chronic migraine receiving amitriptyline and CBT with patients receiving amitriptyline and standard headache education [78]. Patients who received CBT plus amitriptyline had greater reductions in days with headache and migraine-related disability compared with patients who received headache education plus amitriptyline [78].Hypnosis and acceptance and commitment therapy (ACT) are also psychological treatments used with children and adolescents with migraine headaches. ACT prioritizes the outcome of improved functioning above headache reduction and has broadly demonstrated efficacy for chronic pain [79]. Both treatments have shown promising benefit but there has been less evidence supporting the use of these therapies in pediatric headache than other well-established behavioral treatments.
The presence of comorbid psychiatric issues such as anxiety, depression, or ADHD can make the treatment of patients with migraine headaches more complex. A recent study found that approximately 30% of a sample of children and adolescents with chronic daily headache had a lifetime psychiatric diagnoses and having a lifetime psychiatric diagnosis was associated with poorer headache-related disability and quality of life [80].As a result, more intensive behavioral treatment for children and adolescents with a psychiatric comorbidity may be needed to focus on emotional and behavioral issues.
Pediatric psychologists can assess pain-related disability and coping difficulties and treat children and adolescents with migraine headaches. Children are often referred to a psychologist or other mental health professional if headaches are severe or impairing functioning. Unfortunately, access to this therapy is sometimes limited, but when available, should be offered to any migraine patients with significant disability requiring prevention, and specifically to patients with chronic migraine.
How should the patient be treated?
Acute Treatment
The patient reports only minimal response to ibuprofen. However, he is only taking 200 mg and does not take it until well after the headache has started. He should be instructed to take ibuprofen 600 mg (~10 mg/kg) as soon as the headache starts, along with 32 oz of a sports drink, and told that he should repeat this dose in 4 hours if his headache has not completely resolved with the first dose. As his headaches are occurring less than 3 times per week on average, he can do this with every headache. He should be given a note for school allowing him to receive the medication at school, and noting the importance of allowing him to get the medication as soon as he reports a headache. Given that some of his headaches occur on consecutive days, we suspect some may be continuations of a prior headache, so again the importance of obtaining complete pain relief from his acute medication should be stressed. If at his next follow-up visit he reports not always having complete relief with ibuprofen, we would consider trying naproxen instead, and/or adding a triptan, most likely sumatriptan 100 mg given its availability. He would need to understand that he may only use the triptan for 4 to 6 headache days/month. Depending on his response to sumatriptan, it could be used alone or in combination with his NSAID. He also may be instructed to use an NSAID alone for more mild headaches and his NSAID together with sumatriptan for more severe headaches.
Preventive Treatment
PedMIDAS score is a 25, indicating mild disability, however his migraines are frequent, occurring 1–3 times per week and clearly having a significant impact on his functioning. We would therefore recommend starting a prophylactic medication. He notes difficulty remembering to take medications as well as difficulty falling asleep, so amitriptyline would be a good choice for him. For his weight of 50.9 kg, we would start with at 12.5 mg at night and increase by 12.5 mg every 2 weeks to a dose of 50 mg (~1mg/kg/day). He and Mom should be counseled that it will likely take at least a few weeks on his goal dose before they start to see results. He should be counseled also to report to Mom immediately if he has any depressive or suicidal thoughts. Topiramate is another option, especially as it is FDA-approved for migraine prevention in children older than 12 years, although twice daily dosing may be a factor in maintaining compliance. Careful discussion with the patient and family in regards to the potential risks and benefits is important prior to starting any preventative medication.
Biobehavioral Management
The patient should be drinking 8–10 cups of non-caffeinated fluid per day and additional cups on days he plays soccer. He should be given a school note to this effect so he may carry a water bottle at school. He eats well, but the importance of not skipping breakfast should be stressed, and ideas for fitting this in should be given. The importance of a consistent bedtime routine should be stressed, with good sleep hygiene to assist with easier sleep onset. It should be made clear that he should not be using screens within an hour prior to bed. He already exercises frequently and should be commended for being active. Given his report of frequent headaches which are impairing school attendance, functioning at school, and participation in soccer activities, screening for potential co-morbid psychiatric issues and making a referral for further treatment with a pediatric pain psychologist focused on coping with chronic pain is appropriate.
Case Study 2
Initial Presentation and History
A 16-year-old right-handed girl who has been having headaches since she was 10 years old presents for evaluation. She reports unilateral mostly right-sided headaches, which are throbbing and associated with photophobia, phonophobia, osmophobia, nausea, and occasionally vomiting. She has no premonitory or aura symptoms. Her headaches typically occur once a week but she has noted that they tend to be more frequent around the time of her menses and that often these headaches do not fully respond to naproxen, which typically works to break her migraines during other times in the month. Upon further review, she believes these headaches typically start on the day or two prior to her menses, and that this happens almost every month. She often misses school due to these headaches. Her mother would like to know whether the patient should see a gynecologist to potentially be placed on birth control to control her headaches.
Physical Examination
On examination, weight was 61.5 kg (75.8 percentile), height 157.1 cm (18.4 percentile), BMI 24.91 (84 percentile), blood pressure 112/55 mm Hg, and heart rate 90 bpm. Her general physical and neurologic exam results were normal. She had tightness over the left trapezius muscles, otherwise the remainder of headache examination was unremarkable.
What is the probable diagnosis?
According to the ICHD-IIIβ, the patient meets criteria for episodic migraine without aura. Based on the history, she likely also has menstrually related migraine without aura [5].Officially, however, 3 months of prospective documentation is needed to make this diagnosis. Menstrually related migraine is included in the appendix of the ICHD-IIIβ, meaning there is ongoing debate about how it should be classified. According to the current appendix criteria, headaches should meet criteria for migraine without aura and also have documented and prospectively recorded evidence over at least 3 consecutive cycles with headaches confirmed on day 1+/– 2 (ie, 2 days prior to onset of menstruation or within the first 3 days of menstruation) in at least 2 out of 3 menstrual cycles, with migraines occurring during other times of the month as well [5]. This is distinguished from pure menstrual migraine without aura, in which migraines occur only during days 1+/–2 of menstruation but not during other times of the month [5].While we therefore cannot say that the patient meets ICHD IIIβ criteria for menstrually related migraine due to the lack of prospective documentation, her history suggests this.
Menstrually Related Migraine
An association of migraine with menses is well described in adults, occurring in up to 60% of adult female migraine patients [81].In adults, it has been observed that the migraines associated with the menstrual cycle tend to be more severe [82],associated with more nausea and vomiting [82],longer and less responsive to acute medications [83],and associated with more work-related disability [83]. Recently, analysis of data from the American Migraine Prevalence and Prevention (AMPP) Study found that women with pure menstrual migraine and menstrually associated migraine have on average higher MIDAS scores than those with non-menstrual migraine [81].
Only one study has explored menstrually related migraines in adolescents [84].It was a clinic-based study that found a similar prevalence—50% of adolescent patients who had reached menarch noted an association of migraine with their menses. Overall worse disability was not demonstrated in this study, but individual menstrual attacks were not compared with nonmenstrual attacks [84].No other studies have addressed this population, but it is clear that the pattern of menstrually related migraine exists in adolescents and it is important to recognize this pattern as these patients may require additional focused care in addition to standard migraine management.
What are treatment options?
In adults, options for more specific management of pure menstrual migraine and menstrually related migraine include intermittent prophylaxis with NSAIDs or triptans or use of hormonal contraceptives. There are no studies addressing any of these treatments in adolescents, so at this point management decisions must be based on evidence extrapolated from adult studies as well as attention to specific concerns in adolescent patients.
Generally, intermittent prophylaxis (using a medication a few days prior to and during the first few days of menses) with various medications has shown efficacy in adult studies. This approach may be more appropriate for patients with pure menstrual migraine, as there is less likelihood of precipitating medication overuse in this population. It can be considered in patients with menstrually related migraine as well if typical daily preventives have not been effective. One small open-label trial using naproxen 550 mg daily prior to and during menses (at differing schedules depending on the month) showed slightly decreased frequency and severity of headaches during that time [85].However, triptans are what are most commonly used.
Triptans
Frovatriptan is the longest-acting triptan and the one used most commonly for intermittent prophylaxis. One double-blind randomized controlled crossover study showed improvements in headache frequency, severity, and duration using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses [86].Frovatriptan 2.5 mg daily showed some efficacy as well but was not as effective as 2.5 mg BID [86].One prospective randomized placebo-controlled trial demonstrated more headache-free cycles using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses, as compared to placebo [87].
Naratriptan, also a longer-acting triptan, was studied in 3 prospective double-blind trials at doses of 1 mg BID used for 5 days total starting 2 days prior to menses, and all 3 showed a decrease in headache frequency as compared to placebo [88,89].Of note no efficacy was shown using 2.5 mg daily [88].
Less commonly used for intermittent prophylaxis are the shorter-acting triptans, but they have shown some efficacy as well. In one prospective study using oral sumatriptan 25 mg TID for 5 days each cycle starting 2 to 3 days before onset of menses, there seemed to be at least some improvement with sumatriptan in most cycles treated [90].Zolmitriptan was studied in one prospective double-blind placebo-controlled study using 2.5 mg BID or 2.5 mg TID for 7 days total with each cycle and was found to be associated with a significant reduction in headache frequency as compared with placebo [91].There has been one prospective trial using eletriptan 20 mg TID for 6 days total starting 2 days prior to menses, which also showed improvement in headache activity in 55% of patients [92].
Hormonal Contraceptives
In general for adult patients, hormonal contraceptives are not considered first-line treatment for menstrually related migraine. However, in patients who do not respond to other modes of treatment, or who plan to use hormonal contraception for contraceptive purposes, published expert opinions have recommended considering extended or continuous hormonal regimens [93,94].
Estrogen withdrawal during the luteal phase of the monthly cycle has long been speculated to be of importance in the pathophysiology of menstrually related migraine [93]and the relationship between hormonal contraceptives and migraine is complicated. Headache is a commonly reported side effect of combined hormonal contraceptives [95]; however, this effect seems to occur mostly during the hormone-free week [96]. It has been shown that headache occurs less frequently in women using an extended cycle regimen (84 or 168 days) as compared to those using a traditional monthly cyclic regimen [97,98].Studies addressing the use of combined hormonal contraceptives for women specifically with menstrually related migraine are limited. One small prospective randomized study showed improvement in menstrually related migraine in patients treated with a low dose (20 mcg ethinyl E[2]) oral hormonal contraceptive in both a 21/7 cycle and a 24/4 cycle, with more improvement in the group using a 24/4 cycle [99].Another showed improvement in menstrually related migraine in all study patients treated with a low-dose hormonal contraceptive (20 mcg ethinyl estradiol) on a 21/7 regimen, but with additional 0.9 mg conjugated equine estrogen during the placebo week [100].There has been one randomized placebo-controlled double-blind trial in patients with menstrually related migraine using an extended 168 hormonal regimen along with frovatriptan vs. placebo for 5 days during the hormone-free interval. Overall daily headache scores were decreased from pre-study cycles, and the increase in headaches during the hormone-free interval was lower in the frovatriptan group [101].A recent study showed that contraceptive-induced amenorrhea can be beneficial for decreasing migraine frequency in patients with menstrual migraine [102].There have been no studies addressing the use of hormonal contraceptives for migraine management in adolescents.
In adolescents, the decision regarding use of hormonal contraceptives is more complicated. There is less of a chance that adolescent patients, especially younger ones, would be planning to use hormones for contraception so their use may be solely for the purpose of migraine management. However, hormonal contraceptives are commonly used in adolescents for management of other menstrual-related disorders, such as menorrhagia, dysmenorrhea, and endometriosis, and extended cycle and continuous regimens have become more popular with adolescent providers in general [103].The general concern with using hormonal contraceptives in adolescents is that they have potential for longer-term use than adult patients, and the effects of using hormonal contraceptives long term are unknown. The major concerns are for potential interference with expected increase in bone mineral density in adolescents, effects on fertility, and risk for cancer and cardiovascular disease [103].Additionally, specifically in migraine patients, is the concern for increased stroke risk. The increased risk for ischemic stroke in patients with migraine, although more specifically with migraine with aura, is well known [104–106]and migraine has recently been shown to be a risk factor for ischemic stroke in adolescents as well [107]. In adults, the use of hormonal contraceptives in patients with migraine with aura is known to increase the risk of ischemic stroke [106],and this has not been studied in adolescents. Given the various unknowns in the use of hormonal contraceptives in patients with menstrually related migraines, we would not recommend this as first-line treatment. However, similarly to adults, in patients who do not respond to other methods of migraine management, or who seek to use hormonal contraceptives for contraception or for other menstrually related disorders, extended or continuous cycle hormonal contraceptives may be a reasonable option, at the lowest possible estrogen dose. However, migraine with aura should be screened for, and its presence should prompt reconsideration of combined hormonal contraceptive use.
How should this patient be managed?
The patient is having frequent and disabling migraines, so starting a preventive medication would be appropriate. She has migraines throughout the month in addition to during her menses, so a daily prophylactic would be more appropriate than intermittent prophylaxis surrounding her menstrual cycle only. At this point, our recommendation would be to start a daily preventive with either amitriptyline or topiramate. Given that naproxen is not breaking some of her migraines, she should be given a prescription for a triptan. Sumatriptan 100 mg would be an appropriate first choice, and she can be instructed to use it along with her naproxen at the onset of her menstrual migraines. She can use it for other migraines as well but she should be instructed not to use it more than 4 to 6 times per month. She should keep a diary for the next 3 months noting most importantly headache days as well as days of menstruation, so that a more definitive pattern can be confirmed and an official diagnosis based on ICHD-IIIβ criteria can be made. If her migraines do not improve with daily preventives, at that point discussion regarding potential for intermittent prophylaxis or trial of extended cycle hormonal contraception may be considered, although with caution and discussion of risks and benefits.
Corresponding author: Hope O’Brien, MD, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 2015 Cincinnati, OH 45229.
Financial disclosures: None.
1. Tepper SJ, Dahlöf CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004;44:856–64.
2. Lewis DW et al. Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2002;59:490–8.
3. Martens D, Oster I, Gottschlling S, et al. Cerebral MRI and EEG studies in the initial management of pediatric headaches. Swiss Med Wkly 2012;142:w13625.
4. Linder SL. Understanding the comprehensive pediatric headache examination. Pediatr Ann 2005;34:442–6.
5. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
6. Senbil N, Gürer YK, Uner C, et al. Sinusitis in children and adolescents with chronic or recurrent headache: a case-control study. J Headache Pain 2008;9:33–6.
7. Gelfand AA, Reider AC, Goadsby PJ. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology 2013;81:431–6.
8. Blau JN. Water deprivation: a new migraine precipitant. Headache 2005;45:757–9.
9. Martins IP, Gouveia RG. More on water and migraine. Cephalalgia 2007;27:372–4.
10. Hämäläinen ML, Hoppu K, Valkeila E, et al. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology 1997;48:103–7.
11. Lewis DW, Kellsein D, Sahl G, et al. Children’s ibuprofen suspension for the acute treatment of pediatric migraine. Headache 2002;42:780–6.
12. Lipton RB, Golstein J, Baggish JS, et al. Aspirin is efficacious for the treatment of acute migraine. Headache 2005;45:283–92.
13. Linder SL, Mathew NT, Cady RK, et al. Efficacy and tolerability of almotriptan in adolescents: a randomized, double-blind, placebo-controlled trial. Headache 2008;48:1326–36.
14. Ahonen K, Hämäläinen ML, Eerola M, et al. A randomized trial of rizatriptan in migraine attacks in children. Neurology 2006;67:1135–40.
15. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Cephalalgia 2012;32:750–65.
16. Winner P, Lewis D, Visser WH, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: a randomized, double-blind, placebo-controlled study. Headache 2002;42:49–55.
17. Hewitt DJ, Pearlman E, Hämäläinen M, et al. Long-term open-label safety study of rizatriptan acute treatment in pediatric migraineurs. Headache 2013;53:104–17.
18. Derosier FJ, Lewis D, Hershey AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics 2012;129:e1411–20.
19. McDonald SA, Hershey AD, Pearlman E, et al. Long-term evaluation of sumatriptan and naproxen sodium for the acute treatment of migraine in adolescents. Headache 2011;51:1374-87.
20. Lewis DW, Winner P, Hershey AD, et al. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics 2007;
120:390–6.
21. Linder SL, Dowson AJ. Zolmitriptan provides effective migraine relief in adolescents. Int J Clin Pract 2000;54:466–9.
22. Winner P, Rothner AD, Saper J, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106:989–97.
23. Rothner AD, Winner P, Nett R, et al. One-year tolerability and efficacy of sumatriptan nasal spray in adolescents with migraine: results of a multicenter, open-label study. Clin Ther 2000;22:1533–46.
24. Ahonen K, Hämäläinen ML, Rantala H, et al. Nasal sumatriptan is effective in treatment of migraine attacks in children: A randomized trial. Neurology 2004;62:883–7.
25. Natarajan S, Jabbour JT, Webster CJ, et al. Long-term tolerability of sumatriptan nasal spray in adolescent patients with migraine. Headache 2004;44:969–77.
26. Winner P, Rothner AD, Wooten JD, et al. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache 2006;46:212–22.
27. Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology 1997;48:1100–3.
28. Fujita M, Sato K, Nishioka H, et al. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia 2014;34:365–75.
29. Winner P, Linder SL, Lipton RB, et al. Eletriptan for the acute treatment of migraine in adolescents: results of a double-blind, placebo-controlled trial. Headache 2007;47:511–8.
30. Rothner A. Efficacy and safety of naratriptan tablets in adolescent migraine [abstract]. J Neurol Sci 1997;150:S106.
31. Elkind AH, Wade A, Ishkanian G. Pharmacokinetics of frovatriptan in adolescent migraineurs. J Clin Pharmacol 2004;44:1158–65.
32. Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep 2011;15:70–8.
33. Winner P, Pearlman EM, Linder SL, et al. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:1304–12.
34. Lakshmi CV, Singhi P, Malhi P, et al. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol 2007;22:829–35.
35. Lewis D, Winner P, Saper J, et al.Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 2009;123:924–34.
36. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of topiramate in the prevention of childhood headaches. Headache 2002;42:810–8.
37. Campistol J, Campos J, Casas C, et al. Topiramate in the prophylactic treatment of migraine in children. J Child Neurol 2005;20:251–3.
38. Unalp A, Uran N, Oztürk A. Comparison of the effectiveness of topiramate and sodium valproate in pediatric migraine. J Child Neurol 2008;23:1377–81.
39. Cruz MJ, Valencia I, Legido A, et al. Efficacy and tolerability of topiramate in pediatric migraine. Pediatr Neurol 2009;41:167–70.
40. Kim H, Byun SH, Kim JS, et al. Comparison of flunarizine and topiramate for the prophylaxis of pediatric migraines. Eur J Paediatr Neurol 2013;17:45–9.
41. Fallah R, Divanizadeh MS, Karimi M, et al. Topiramate and propranolol for prophylaxis of migraine. Indian J Pediatr 2013;80:920–4.
42. Tonekaboni SH, Ghazavi A, Fayyazi A, et al. Prophylaxis of childhood migraine: topiramate versus propranolol. Iran J Child Neurol 2013;7:9–14.
43. Hershey AD, Powers SW, Coffey CS, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double blinded, placebo controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in prevention of childhood and adolescent migraine. Headache 2013;53:799–816.
44. Hershey AD, Powers SW, Bentti AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539–49.
45. Lewis DW, Diamond S, Scott D, et al. Prophylactic treatment of pediatric migraine. Headache 2004;44:230–7.
46. Caruso JM, Brown WD, Exil G, et al. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000 Sep;40:672–6.
47. Pakalnis A, Greenberg G, Drake ME Jr, et al. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731–4.
48. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819–22.
49. Miller GS. Efficacy and safety of levetiracetem in pediatric migraine. Headache 2004;44:238–43.
50. Pakalnis A, Kring D, Meier L. Levetiracetam prophylaxis in pediatric migraine--an open label study. Headache 2007;43:427–30.
51. Pakalnis A, Kring D. Zonisamide prophylaxis in refractory pediatric headache. Headache 2006;46:804–7.
52. Apostol G, Cady RK, Laforet GA, et al. Divalproex extended-release in adolescent migraine prophylaxis: results of a randomized, double-blind, placebo-controlled study. Headache 2008;48:1012–25.
53. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol 1974;50:109–15.
54. Forsythe WI, Gillies D, Sills MA. Propanolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737–41.
55. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593–7.
56. Sorge F, DeSimone R, Marano E, et al. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled crossover study. Cephalalgia 1988;8:1–6.
57. Guidetti V, Moscato D, Ottaviano S, et al. Flunarizine and migraine in childhood: an evaluation of endocrine function. Cephalalgia 1987;7:263–6.
58. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and complementary treatments for episodic migraine treatment in adults: Report of the quality standards subcommittee of the American Academy of Neurology and American Headache Society. Neurology 2012; 78:1346–53.
59. Oelkers-Ax R, Leins A, Parzer P, et al. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain 2008;12:301–13.
60. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache 2005;45:196–203.
61. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 2007;47:73–80.
62. Slater SK, Nelson TD, Kabbouche MA, et al. A randomized, double-blind, crossover, add-on study of coenzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 2011; 31: 897–905.
63. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache 2003;43:601–10.
64. Castelli S, Meossi C, Domenici R, et al. [Magnesium in the prophylaxis of primary headache and other periodic disorders in children]. Pediatr Med Chir 1993;15:481–8. Italian.
65. Condò M, Posar A, Arbizzani A, et al. Riboflavin prophylaxis in pediatric and adolescent migraine. J Headache Pain 2009;10:361–5.
66. MacLennan SC, Wade FM, Forrest KM, et al. High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. J Child Neurol 2008;23:1300–4.
67. Bruijn J, Duivenvoorden H, Passchier J, et al. Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia 2010;30:1426–34.
68. Robbins L. Precipitating factors in migraine: a retrospective review of 494 patients. Headache 1994;34:214–6.
69. Leviton A, Slack WV, Masek B, et al. A computerized behavioral assessment for children with headaches. Headache 1984;24:182–5.
70. Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999;19 Suppl 25:57–9.
71. Lynch-Jordan AM, Sil S, Peugh J, et al. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain 2014;155:1955–61.
72. Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2014;5:CD003968.
73. Huguet A, McGrath PJ, Stinson J, et al. Efficacy of psychological treatment for headaches: an overview of systematic reviews and analysis of potential modifiers of treatment efficacy. Clin J Pain 2014;30:353–69.
74. Kropp P, Meyer B, Landgraf M, et al. Headache in children: update on biobehavioral treatments. Neuropediatrics 2013;44:20–4.
75. Kröner-Herwig B. Psychological treatments for pediatric headache. Expert Rev Neurother 2011;11:403–10.
76. Powers SW, Andrasik F. Biobehavioral treatment, disability, and psychological effects of pediatric headache. Pediatr Ann 2005;34:461–5.
77. Hermann C, Blanchard EB. Biofeedback in the treatment of headache and other childhood pain. Appl Psychophysiol Biofeedback 2002;27:143–62.
78. Powers SW, Kashikar-Zuck SM, Hershey AD, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA 2013;310:2622–30.
79. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep 2015;19:13.
80. Slater SK, O’Brien HL, Hershey AD, et al. Psychiatric comorbidity in pediatric chronic daily headache. Cephalalgia 2012;32: 1116–22.
81. Pavlovic JM, Stewart WF, Bruce CA, et al. Burden of migraine related to menses: results from the AMPP study. J Headache Pain 2015;16:24.
82. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004;63:351–3.
83. Granella F, Sances G, Allais G. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 2004;24:707–16.
84. Crawford MJ, Lehman L, Slater S, et al. Menstrual migraine in adolescents. Headache 2009;49:341–7.
85. Allais G, Bussone G, De Lorenzo C, et al. Naproxen sodium in short-term prophylaxis of pure menstrual migraine: pathophysiological and clinical considerations. Neurol Sci 2007;28(Suppl 2):S225–8.
86. Silberstein S, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology 2004;63:261–9.
87. Brandes JL, Poole A, Kallela M, et al. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia 2009;29:1133–48.
88. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache 2001;41:248–56.
89. Mannix LK, Savani N, Landy S, et al. Efficacy and tolerability of naratriptan for short-term prevention of menstrually related migraine: data from two randomized, double-blind, placebo-controlled studies. Headache 2007;47:1037–49.
90. Newman LC, Lipton RB, Lay CL, et al. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology 1998;51:307–9.
91. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs 2008;22:877–86.
92. Marcus DA, Bernstein CD, Sullivan EA, et al. Perimenstrual eletriptan prevents menstrual migraine: an open-label study. Headache 2010;50:551–6.
93. Silberstein S, Patel S. Menstrual migraine: an updated review on hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother 2014;15:2063–70.
94. MacGregor EA. Migraine management during menstruation and menopause. Continuum (Minneap Minn) 2015;21(4 Headache):990–1003.
95. Aegidius K, Zwart JA, Hagen K, et al. Oral contraceptives and increased headache prevalence: The Head-HUNT Study. Neurology 2006;66:349–53.
96. Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol 2000;95:261–6.
97. LaGuardia KD, Fisher AC, Bainbridge JD, et al. Suppression of estrogen-withdrawal headache with extended transdermal contraception. Fertil Steril 2005;83:1875–7.
98. Sulak P,Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: Impact of eliminating the standard 7-day placebo interval. Headache 2007;47:27–37.
99. De Leo V, Scolaro V, Musacchio MC, et al. Combined oral contraceptives in women with menstrual migraine without aura. Fertil Steril 2011;96:917–20.
100. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004;97:819–22.
101. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health (Larchmt) 2014;23:310–7.
102. Vetvik KG, MacGregor EA, Lundqvist C, et al. Contraceptive-induced amenorrhea leads to reduced migraine frequency in women with menstrual migraine without aura. J Headache Pain 2014;15:30.
103. Sucato GS, Gerschultz KL. Extended cycle hormonal contraception in adolescents. Curr Opin Obstet Gynecol 2005;17:461–5.
104. Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 2010;123:612–24.
105. Bigal ME, Kurth T, Santanello N, et al. Migraine and cardiovascular disease: a population-based study. Neurology 2010;74:628–35.
106. Schurks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914.
107. Gelfand AA, Fullerton HJ, Jacobson A, et al. Is migraine a risk factor for pediatric stroke? Cephalalgia 2015;35:1252–60.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Abstract
- Objective: To review the management of migraine in adolescent patients.
- Methods: Literature review in the context of 2 clinical cases.
- Results: Migraine is common in adolescents and can affect school and social functioning. Management options include lifestyle modifications and acute and preventative therapies. First-line medications for migraine in the adolescent population are over-the-counter medications, including ibuprofen, acetaminophen, and naproxen. Studies of efficacy of triptans in the treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. Several classes of medications are commonly used for migraine prevention, including antidepressants and antiepileptics. Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older. Biobehavioral treatments such as relaxation training, biofeedback, and cognitive behavioral therapy have been evaluated in randomized controlled trials and found to be efficacious.
- Conclusion: Approach to management of migraine in adolescents should be multifactorial with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Case Study 1
Initial Presentation
A 13-year-old left-handed boy with allergic rhinitis has been referred by his pediatrician for evaluation of headaches.
History
The headaches have been occurring since he was 7 years old. He describes a bilateral frontal and periorbital pain, which is either aching or throbbing in nature. There is associated photophobia and phonophobia, and the pain worsens with activity. When he was younger the headaches were frequently associated with emesis. He occasionally gets tenderness of his face during the headaches, mostly in the areas adjacent to his nose and above his eyes and occasionally associated rhinorrhea.
Initially the headaches were mild and occurring infrequently, but for the past 6 months they have been more severe and occurring 1 to 3 times per week, sometimes on consecutive days. They typically begin in the afternoon but at times occur soon after waking in the morning. If they do occur in the morning, they worsen after getting out of bed. He denies any type of warning symptoms indicating the headache will occur, and has no associated focal neurologic symptoms associated with the headaches. His mother tries to minimize medication intake so will typically wait to see if the headache is severe before giving him medication, typically 2 chewable children’s ibuprofen, which helps some of the time; however, his headache often does not completely abate unless he naps. He has also been taking loratadine daily due to concerns that his headaches may be secondary to chronic sinusitis.
He is a good student and enjoys school, but notes stress surrounding tests. He reports missing 4 days of school in the past 3 months as well as coming late twice. He thinks that there were 9 additional days in which he was unable to function at his full ability at school and at least 5 times he was unable to concentrate on his homework. He plays soccer 4 to 5 times per week. He says twice he had to skip soccer practice to due to headache and at least 3 other times he needed to take breaks during soccer due to headache. He is unsure how much he drinks on an average day but he drinks mostly with meals. He does not drink caffeine. He gets into bed at 10 pm and sometimes does not fall asleep until after 11 pm, often due to worry about tests. He often plays with his tablet when trying to fall asleep. He wakes up at 6 am for school. He typically eats 3 meals per day but occasionally misses breakfast if he is rushed in the morning.
Physical Examination
On examination, the patient is a well-developed, well-nourished male in no apparent distress. His weight was 50.9 kg (68th percentile), height 163.7 cm (65th percentile), BMI 19.9 (69th percentile), blood pressure 118/62 mm Hg, and heart rate 79 bpm. His general physical examination, including skin, HEENT, extremities, lung, cardiac, and abdominal examination was normal, with lungs clear to auscultation, heart with a regular rate and rhythm, and abdomen soft and tender without organomegaly.
On neurologic examination he was alert and attentive with normal mental status. Speech was fluent. Skull, spine, and meninges were normocephalic and atraumatic with a supple neck. Cranial nerves II through XII were normal with a normal fundoscopic examination including sharp disk, no papilledema, and normal fundus bilaterally. Motor examination was normal for tone and bulk with full strength throughout. Sensory examination was normal for light touch, temperature, vibration, and joint position sense. Finger-nose-finger fine finger movement and heel-knee-shin were normal. Deep tendon reflexes were symmetric and 2+ throughout with toes downgoing. Station and gait were normal including toe walking, heel walking, tandem walking, running, skipping, one-legged standing, Fog gate procedure, and there was no Romberg’s sign.
He had a normal comprehensive headache examination. There was no tenderness at typical migraine trigger points (nuchal line and mandibular process) and no tenderness at supraorbital notch. Neck was supple with normal rotation and normal bilateral trapezius muscle tightness. No bruits or palpitations were heard over carotid or jugular veins. There was a negative Muller sign with no pain on neck bending with pressure, and no signs of allergy or sinus symptoms.
What is the initial evaluation of a teen with recurrent headaches?
The overwhelming majority of children and adolescents presenting to medical attention with recurrent headaches will have a primary headache disorder, most commonly migraine [1].However, ruling out a secondary cause is typically of concern, both for providers and parents, and the decision regarding whether to image is often a daunting one. In a review of 6 large studies in which imaging was done in pediatric patients who were examined by a neurologist, only 14 patients were found to have CNS lesions requiring intervention, and all of these patients had abnormalities on physical exam [2]. An American Academy of Neurology (AAN) practice parameter published in 2002 recommended considering neuroimaging only in patients with abnormal neurologic examination, co-existing seizures, concerning associated neurologic features, or recent onset of severe headache or change in headache type [2].There are additional factors which may prompt one to consider imaging as well including atypical auras or very short (< 5 minutes) or protracted ( > 60 minutes) auras, trigeminal autonomic cephalalgia, brief headaches precipitated by cough, frequent early morning headaches or which wake the patient from sleep, headaches with primarily occipital location, headaches in a patient less than 6 years old or in a child who cannot describe the headaches well, or migrainous headaches in a child without any family history of migraine or migraine equivalents. However, recent studies continue to show that the imaging yield in patients with headaches is low and further studies are needed to elucidate which patients truly require imaging [3].
Given this, a thorough history, family history, physical exam, and detailed neurologic exam including fundoscopy are imperative. In addition to the general neurologic exam, a focused headache exam should be performed, including Mueller’s maneuver, auscultation for cranial bruits, evaluation of the temporomandibular joint, palpation of possible trigger points, and maneuvers to assess for cervical spine disease [4].
Classification of Symptoms
Once establishing the likelihood of a primary headache disorder, the International Classification of Headache Disorders, 3rd edition, beta version (ICHD IIIβ) [5]should be used to classify the headache diagnosis. Note that while this classification system was established for adults, most criteria are similar for children and adolescents, and the typical differences are noted in the comments of the ICHD IIIβ [5].Migraine is the most common type of primary headache brought to medical attention [1]. Migraine without aura is described as a recurrent headache disorder (at least 5 lifetime attacks) with attacks lasting 4 to 72 hours, typically unilateral, throbbing in nature, moderate to severe in intensity, aggravated by routine physical activity, and associated with nausea and/or photophobia and phonophobia. In young children, migraine is more frequently bilateral, the gastrointestinal symptoms often more pronounced than photophobia and phonophobia, and migraines may be shorter, lasting at least 2 hours without treatment [5].As patients reach adolescence, migraine features typically start to evolve into patterns described in adults. Differentiating migraine from other primary headaches, such as tension-type, can be challenging, especially in children in whom migraines are more likely to be shorter and bilateral. Tension-type headaches are bilateral or diffuse, pressing or tightening pain that is non-pulsatile lasting at least 30 minutes. The pain is described as mild to moderate in intensity and not aggravated by activity [5].They may be associated with photophobia or phonophobia (not both) and they may not be associated with nausea or vomiting [5].
Is any further workup indicated?
The patient has recurrent headaches which have been present for 5 years. While they have increased in frequency, there has not been any change in their quality. His headache description and history have no “red flag” features, and a thorough examination is normal. Therefore, neuroimaging and further workup would not be indicated in this case.
What is the diagnosis?
The patient’s headaches are throbbing in nature, exacerbated by activity, associated with nausea, photophobia, and phonophobia, and are moderate to severe. Using the ICHD IIIβ, he meets criteria for migraine without aura. While his headaches are frequent, he is having less than 15 headache days per month, so this is episodic migraine. Note that he complains of forehead and periorbital pain and occasional rhinorrhea with his headaches, leading him to have been placed on loratadine for treatment of presumed allergic sinusitis. Children meeting criteria for migraine are very frequently misdiagnosed as having sinusitis [6]due to the overlap in location of migraine pain and proximity to the frontal and maxillary sinuses, as well as the presence of autonomic features frequently present in migraine, reportedly present in 70% of pediatric migraine patients [7].The negative headache examination, including Muller’s maneuver, points against sinus disease as well.
What is the approach to management of the adolescent with migraine?
Approach to management of migraine in adolescents should be multifactorial, with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Acute Treatment
General Approach
In counseling patients with migraine, and in particular adolescents, it should be stressed that achieving a normal level of functioning as soon as possible is the goal of therapy. Common missteps in treatment include failure to take acute medication early into the headache, incorrect dosing, incompletely treating the headache, and avoidance of participating in daily activities when headaches occur.
Adolescent patients may frequently wait to take an acute medication, typically due participation in another activity, not having medication with them, or discomfort with taking medication in front of peers or at school. Additionally, patients who have headaches beginning in early childhood, with pronounced gastrointestinal (GI) features, may be aware that their headaches resolve after vomiting, and therefore get used to not treating with medications. When these patients reach adolescence, when GI symptoms tend to become less pronounced, they will need to be educated that taking medication early is imperative. Acute medications are typically more effective when taken earlier in the course of a migraine, and the importance of pausing to take medication at the onset of the headache should be stressed to all patients and parents. With that in mind, however, care should be taken to counsel patients regarding the potential for development of medication overuse headache. When headaches are frequent, a more detailed and intricate plan may need to be devised so that adolescents and parents know which headaches to treat with medication.
Given that first-line medications for treatment of migraines are over-the-counter, underdosing occurs commonly, as dosing listed on packaging is typically age-based, not weight-based. At initial visits, young adolescent patients will frequently report that a particular medication is not effective, but this is often because they are still receiving low/less optimal dosing. Clinicians should remember to follow weights and recommend dosing changes at the initial visit and follow-up visits as well.
Treatment goals, ie, complete resolution of pain and migrainous features with ability to return to normal functioning, should be made clear to patients and families at the onset of treatment. Patients frequently fall into a pattern of continuing to treat with a medication that may lessen but not completely ablate the pain of a headache, and then sleep and avoid activity. Upon awakening, the headache may be gone, however, given incomplete initial treatment, the headache may be more likely to recur within 24 hours. At that point, the headache may be more difficult to treat and therefore cause further decrease in functioning. If this cycle perpetuates, disability can become extremely burdensome in adolescents, significantly affecting school and social functioning. Therefore, a detailed plan for initial steps in management as well as steps to take should initial therapy fail to fully break the headache should be given to every patient.
Of note, given that dehydration is now recognized as a common trigger for migraine [8,9], it is generally our recommendation to drink 16 to 32 oz (depending on weight) of a hydrating fluid together with whichever acute medication is chosen, as rehydration likely assists in breaking migraines as well [8].
Medications
Over-the-Counter Agents
The first-line medications for migraine in the pediatric and adolescent populations are over-the counter medications, including ibuprofen, acetaminophen, and naproxen sodium. Ibuprofen has been well studied in pediatrics and was found to be safe and effective in 2 studies [10,11]at doses of 7.5–10 mg/kg. Again, care should be taken to ensure timely administration of ibuprofen at onset of headache, or aura if present, with appropriate weight-based dosing. Naproxen sodium has not been studied in pediatrics for treatment of migraine. However in practice, it is often used in similar doses of 10 mg/kg, with good efficacy. Although ibuprofen and naproxen sodium are both nonsteroidal anti-inflammatory medications (NSAIDs), anecdotally many patients report successful treatment with one NSAID when another has failed. Aspirin has shown efficacy in adults for treatment of acute migraine [12].It is likely effective in the pediatric population as well, but it is generally avoided due to long-standing concerns for precipitation of Reye syndrome in children. In adolescents over 16 years old, however, it is a reasonable option if there are no contraindications.
In one study, acetaminophen was compared to ibuprofen and placebo for treatment of migraine in children and adolescents and found to be effective more frequently than placebo but not as frequently as ibuprofen [10],likely due to its only minimal anti-inflammatory effects. It is a reasonable option for children and adolescents, particularly in those who have contraindications to NSAIDs.
While these over-the-counter medications are generally safe and well tolerated, clinicians should not overlook the potential for toxicity as well as medication overuse headache, and patients should be counseled to avoid use of any of these medications for more than 2 to 3 headache days per week.
Triptans
Studies of efficacy of triptans in treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. However, some have shown efficacy over placebo, and in the past few years have received FDA approval for use in the pediatric and adolescent populations. Clinicians should remember that these medications are vasoconstrictors, so they should not be used in patients with vascular disease or in patients with migraine with brainstem aura or hemiplegic migraine. Additionally, due to the risk of serotonin syndrome, they should not be used in patients on monoamine oxidase inhibitors. It is also important to educate patients to limit use of these medications to 4 to 6 times per month to avoid precipitation of medication overuse headache.
Almotriptan was approved by the FDA in 2009 for use in patients 12 years and older, based on a large randomized controlled trial comparing doses of 6.25, 12.5, and 25 mg with placebo in patients ages 12 to 17 years old. All doses resulted in statistically significant pain relief as compared to placebo, and interestingly, the 12.5-mg dose seemed to be the most effective [13].
Rizatriptan received FDA approval in 2012 for use in patients 6 years and older. In 2 randomized controlled trials in patients 6 to 17 years old, rizatriptan (5 mg for patients < 40 kg, 10 mg for patients ≥ 40 kg) was more effective than placebo in providing pain freedom at 2 hours [14,15].One earlier trial found efficacy only on some measures (weekend treatment, decrease in nausea and functional disability) but no statistically significant difference than placebo in terms of overall efficacy in achieving pain freedom at 2 hours [16].However, this trial had a higher placebo response rate than typically seen in adult triptan trials. In a recent long-term open-label study in patients 12 to 17 years old, rizatriptan was found to be generally safe and well-tolerated with consistent efficacy of 46% to 51% pain freedom at 2 hours over time [17].
A combination pill consisting of sumatriptan and naproxen (Treximet) received FDA approval in May 2015 for patients 12 years and older. This was based on a randomized controlled parallel-group trial in patients 12 to 17 years old using the sumatriptan/naproxen combination in various dose combinations: 10/60 mg, 30/180 mg, or 85/500 [18].All doses were found to be equally effective in providing pain relief at 2 hours as compared to placebo, with a higher chance of sustained pain relief at 24 hours in the group receiving the 85/500 dose. In this trial, and in a long-term open-label safety trial [19],all doses were generally well tolerated with minimal adverse reactions.
Most recently, in June 2015, zolmitriptan nasal spray was approved for patients 12 years of age and older. In the large “Double-Diamond” study, which used a novel design to attempt to minimize placebo effect, zolmitriptan nasal spray (5 mg) was found to have a significantly higher headache response rate at 1 hour than placebo, and was significantly superior to placebo with regard to multiple secondary end-points [20].Additionally, a long-term open-label trial in patients 12 to 17 years old using oral zolmitriptan (2.5 mg or 5 mg) found it to be generally effective and well tolerated [21].
No other triptans have been approved for children or adolescents, however, most are widely used in clinical practice. There is good evidence for the efficacy and tolerability of sumatriptan nasal spray [22–26]in adolescents, and it is approved for use in adolescents with migraine in Europe. Although oral sumatriptan was the first triptan available clinically in the United States and is very widely used, there is surprisingly little published evidence for its use in the pediatric and adolescent populations. In fact, 2 randomized controlled trials in adolescents failed to show efficacy of oral sumatriptan as compared to placebo [27,28].Despite this, given its availability it is a reasonable choice for adolescent patients and is often one of the first tried. There has been 1 randomized controlled trial in adolescents for eletriptan without significant differences in efficacy at 2 hours as compared to placebo, although similar to other trials the placebo rate was high and there were some differences seen in secondary outcomes measures [29].Similarly, one randomized controlled trial of naratriptan 12.5-mg tablets failed to show efficacy as compared to placebo in pain relief [30].At present time, for frovatriptan there has only been a study looking at the pharmacokinetics and safety in adolescents, which found that it was generally well tolerated and recommended adolescent dosing similar to adult dosing [31].
In choosing a triptan, clinicians should keep in mind availability of alternate forms of administration, absence or presence of significant emesis, and the age of the patient. For patients who are unable to swallow pills or who have significant emesis associated with their migraines, nasal sprays and oral dissolving forms (melts) are good options. The nasal sprays (zolmitriptan and sumatriptan) additionally have the benefit of a quicker onset (~15 minutes) in general than the oral formulations. The downside to these nasal formulations is bad taste, which is frequently reported by patients. Patients should be counseled in proper administration of these nasal sprays (ie, avoiding inhalation, which causes the medication to enter the mouth) to minimize the bad taste and maximize absorption through the nasal mucosa. Other alternatives are the oral dissolving forms (rizatriptan and zolmitriptan). Given the FDA approval, the rizatriptan melt tablets are often the first-line triptan for children under age 12, but zolmitriptan melts are an option as well.
Preventive Treatment
General Approach
The decision to place an adolescent on a daily preventive medication should be based on a combination of headache frequency, severity, ease of breaking headaches, and overall disability as established by a disability scale (such as the PedMIDAS). Any patient with headaches occurring one or more days per week, those whose headaches are not easily treated or tend to be prolonged, and those with a PedMIDAS score of 30 or more should be considered candidate for a daily preventive. It is particularly important to consider starting a preventive early in adolescent patients given the possibility of impacting overall disease progression at a young age. While the natural history of headaches that start in the young is still being investigated, a known risk factor for transformation of episodic to chronic migraine is frequent headaches [32].It is therefore imperative to attempt to intervene early to improve quality of life in the present and also to prevent a downward cycle into chronicity, potential medication overuse, and worsening disability.
At present, there are several classes of medications that are commonly used for migraine prevention, including antidepressants, antiepileptics, antihistamines, and antihypertensives. Patients should be educated regarding the medication’s typical use, the specific way in which it is used in migraine, potential side effects, and overall expectations of efficacy. Most preventives need to be titrated up slowly to maximize tolerability, and patients need to understand that it may take time before they start to see results. In general, we recommend titrating up over the course of 4 to 12 weeks (depending on medication and dose goal), and then a substantial trial of 4 to 6 weeks on a full dose of medication before determining efficacy. If a medication shows a trend towards improvement but the patient has not met treatment goals, medication can be titrated up further at that point as tolerated. Patients and families should also understand that these medications are not intended as a “cure” for migraine but rather as a tool for improvement, which should be used in conjunction with the rest of a detailed plan. Generally, if a patient is well controlled for 4 to 6 months (ie, 3 or less headaches/month that are easily broken with medications, and a PedMIDAS < 30), attempts should be made to wean off of medication.
Medications
First-line Therapies
Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older (approved in March 2014). Three randomized double-blind placebo-controlled trials in children and adolescents have found topiramate to be superior to placebo in multiple endpoints [33–35].Doses in these trials were 2–3 mg/kg/day, 100 mg/day, and 50 or 100 mg/day, respectively. Notably, the 50 mg/day dose was not found to be superior to placebo. Multiple retrospective, open-label, and drug comparison trials have shown effectiveness and tolerability as well [36–42].We typically use a goal dose of 2 mg/kg/day, which is reached after titrating over 8 to 12 weeks, which minimizes side effects (most commonly paresthesias, memory/language dysfunction, appetite suppression, and drowsiness), but higher doses can be used if needed.
Amitriptyline has consistently shown efficacy in adult migraine trials and is therefore one of the most commonly used medications worldwide for prevention of migraine in children and adolescents. Surprisingly, there have been no published randomized controlled trials using amitriptyline in the pediatric population, although a trial comparing amitriptyline, topiramate, and placebo is currently underway (Childhood and Adolescent Migraine Prevention Study) [43].In 2 retrospective pediatric studies improvement with amitriptyline was reported in 84.2% and 89% of patients, respectively [44,45].We typically use a goal dose of 1 mg/kg/day, also with an 8- to 12-week titration. The most common side effects with amitriptyline are somnolence, dry mouth, and weight gain, but it is generally well tolerated in children and adolescents. There is also a risk of worsening depression and suicidal thoughts, so it is recommended to use caution if considering prescribing to a patient with underlying depression. It is typically administered in once daily dosing a few hours before bed to minimize morning drowsiness. There is also a concern for precipitation of arrhythmias, and while there are no guidelines recommending screening ECGs, this should be considered in patients with a family history of heart disease. Of note, many practitioners use nortriptyline in place of amitriptyline as it can be less sedating. It should be noted, however, that evidence for its efficacy is lacking. Additionally, it may carry a higher risk of arrhythmia [44].
Second-line Therapies
The second-line therapies typically considered are other antiepileptics, valproic acid, levetiracetam, and zonisamide, for which there is some evidence, although mostly in the form of open-label or retrospective studies [46–51]. Of note, despite the many promising retrospective and open-label studies for valproic acid [46–48], one randomized double-blind placebo control trial comparing various doses of extended release divalproex sodium with placebo in adolescent patients failed to show a statistically significant treatment difference between any dose and placebo [52].It is, however, frequently prescribed and anecdotally quite efficacious. Given concerns about potential for teratogenicity, and possible effects on ovarian function, as well as potential for weight gain and hair loss, it should be used with caution in adolescent females.
Antihypertensives (beta blockers and calcium channel blockers) have long been used for prevention of migraine in both the adult and pediatric population, but evidence for their use in the pediatric population is conflicting. An early double-blind crossover study of propranolol in patients 7 to 16 years old showed significant efficacy as compared with placebo [53].However, in 2 subsequent studies it failed to show efficacy as compared with placebo and self-hypnosis, respectively [54,55].Given the conflicting evidence and the potential for hypotension, depression, and exercise-induced asthma, use of propranolol has fallen out of favor by experts for use in pediatric and adolescent migraine prevention. Flunarizine, a nonselective calcium channel blocker, has demonstrated effectiveness in pediatric migraine prevention [56,57],and is actually approved in Europe for this indication, but it is not available in the United States.
Cyproheptadine is an antihistamine with antiserotonergic properties which is used frequently for migraine prevention in young children who are unable to swallow tablets, although evidence is limited to 1 retrospective study [45].However, given the propensity for weight gain with this medication, it is generally not recommended for use in adolescents.
In recent years, there has been a growing interest in nutraceuticals given their general tolerability and minimal side effects, as well as a comfort in using a more “natural” approach, particularly in pediatrics. Evidence is limited but some of the frequently used substances may be beneficial. Butterbur (petasites hybridus) has strong evidence in adults and is recommended by the AAN for migraine prevention in adults [58].There is one small pediatric RCT showing efficacy for butterbur as compared to music therapy [59],as well as one promising open-label trial [60]. Typical dosing ranges from 50–150 mg daily. However, butterbur contains pyrrolizidine alkaloids, which are hepatotoxic, and due to the concerns for inadequate monitoring of removal of these substances in the manufacturing of commercial butterbur, it has been generally avoided in the pediatric population. Coenzyme Q10 (CoQ10) is considered possibly effective for adult migraine prevention by the AAN [58].Pediatric evidence is limited to an open-label study showing improvement with supplementation of CoQ10 in deficient patients [61],and a subsequent double-blind placebo-controlled add-on study, which showed improvement in both the CoQ10 and placebo groups but faster improvement in the CoQ10 group [62].Typical pediatric dosing is 1–2 mg/kg/day. Magnesium is considered by the AAN to be a good option for migraine prevention in adults [58].One randomized controlled trial in children however had equivocal results [63],while one small prospective open-label study had positive results [64]. Magnesium supplementation may be more effective in patients who have low ionized magnesium levels, but this is difficult to measure reliably in the clinical setting. Doses of 9 mg/kg/day can be used with the most common side effects reported being gastrointestinal upset and diarrhea, generally dose dependent. Riboflavin is also considered by the AAN to be probably effective for prevention in adults [58],but again the evidence in children is limited to one positive retrospective study [65]and 2 equivocal randomized controlled trials [66,67],one of which had an unusually high placebo rate [66]. Appropriate dosing is also something of debate, as riboflavin is minimally absorbed and has a short half-life, so while studies were done using 200–400 mg daily, smaller more frequent dosing may be needed.
A potential approach to treatment with vitamins is to check for deficiencies, but currently only the study mentioned above in which CoQ10 levels were checked showed improvement with normalizing of low levels [61,62].Further research into this topic is needed to elucidate whether checking and repleting levels of specific vitamins would be beneficial in prevention of migraines in certain patients.
Healthy Habits
The importance of maintaining healthy lifestyle habits and modifying potentially detrimental ones should be stressed to patients and families, and counseling regarding these issues should be provided at every visit, as repetition is often key to patients understanding their importance. Skipping meals is a commonly reported migraine trigger [68,69].This is not an uncommon occurrence even in the most well-meaning of families; adolescents often report not feeling hungry in the morning, and in the rush to get to school will often skip breakfast. Many patients report not liking school lunches leading them to go most of the day without food. Adolescent girls may skip meals due to weight concerns. Patients need to be reminded that well-balanced meals throughout the day is imperative, and they often may need specific counseling on how to achieve this practically. Of note, unless a specific migraine trigger has been identified in a given patient, we do not generally recommend restricting any specific food lists.
Maintaining good sleep hygiene and a consistent sleep schedule is also often difficult for adolescent patients, with after-school activities, homework, and screen use (eg, television, electronic devices) often contributing to late bedtimes with then forced early morning waking for school. However, improvement in sleep hygiene has been shown to be effective in improving migraines in children and adolescents [70],and realistic plans for improvement in sleep should be discussed with patients.
Dehydration is also a common migraine trigger [8,9],so the importance of staying well hydrated should be stressed as well. Again, specific recommendations for how this can be achieved are often needed, especially given adolescents’ busy schedules. Additionally, many schools do not allow water bottles to be carried, and often a school note is needed so patients may be allowed to carry a water bottle at school and also be provided extra bathroom breaks as needed.
Also important is stressing maintenance of daily functioning throughout migraine treatment. By the time adolescents seek medical care, they may already be in a cycle of missing school due to headaches, and some may even be receiving home-schooling. The goals of staying in school and learning to function with headaches should be stressed, often with the help of coping skills, which will be discussed below, as it has been shown that functional disability generally improves before pain [71].
Behavioral Treatments
Psychological treatments are an important aspect of migraine management. Biobehavioral treatments such as relaxation training, biofeedback, and multimodal cognitive behavioral therapy (CBT) have been evaluated in randomized controlled trials and reviewed in meta-analyses in pediatric migraine populations and found to be efficacious [72–75].These treatments have been shown to reduce pain intensity and disability for children and adolescents with headaches and therapeutic gains appear to be maintained [72].
Relaxation training typically includes instruction in techniques such as diaphragmatic breathing, progressive muscle relaxation, and guided imagery. Relaxation training is most effective with children 7 years of age or older [76].Biofeedback is often used in conjunction with relaxation training to provide audio or visual feedback about normally unconscious physiological body responses associated with increased relaxation. Effective biofeedback parameters used with children and adolescents with migraine headache include electromyographic (EMG) activity and peripheral skin temperature [77].Biofeedback techniques can help children and adolescents become more aware of physical responses, better control these responses and generalize physical responses outside of therapy sessions to better cope with pain [76].CBT involves instruction in skills such as biofeedback-assisted relaxation training, activity pacing, distraction, and cognitive strategies for coping with pain. CBT was shown to be effective in a recent study comparing adolescent patients with chronic migraine receiving amitriptyline and CBT with patients receiving amitriptyline and standard headache education [78]. Patients who received CBT plus amitriptyline had greater reductions in days with headache and migraine-related disability compared with patients who received headache education plus amitriptyline [78].Hypnosis and acceptance and commitment therapy (ACT) are also psychological treatments used with children and adolescents with migraine headaches. ACT prioritizes the outcome of improved functioning above headache reduction and has broadly demonstrated efficacy for chronic pain [79]. Both treatments have shown promising benefit but there has been less evidence supporting the use of these therapies in pediatric headache than other well-established behavioral treatments.
The presence of comorbid psychiatric issues such as anxiety, depression, or ADHD can make the treatment of patients with migraine headaches more complex. A recent study found that approximately 30% of a sample of children and adolescents with chronic daily headache had a lifetime psychiatric diagnoses and having a lifetime psychiatric diagnosis was associated with poorer headache-related disability and quality of life [80].As a result, more intensive behavioral treatment for children and adolescents with a psychiatric comorbidity may be needed to focus on emotional and behavioral issues.
Pediatric psychologists can assess pain-related disability and coping difficulties and treat children and adolescents with migraine headaches. Children are often referred to a psychologist or other mental health professional if headaches are severe or impairing functioning. Unfortunately, access to this therapy is sometimes limited, but when available, should be offered to any migraine patients with significant disability requiring prevention, and specifically to patients with chronic migraine.
How should the patient be treated?
Acute Treatment
The patient reports only minimal response to ibuprofen. However, he is only taking 200 mg and does not take it until well after the headache has started. He should be instructed to take ibuprofen 600 mg (~10 mg/kg) as soon as the headache starts, along with 32 oz of a sports drink, and told that he should repeat this dose in 4 hours if his headache has not completely resolved with the first dose. As his headaches are occurring less than 3 times per week on average, he can do this with every headache. He should be given a note for school allowing him to receive the medication at school, and noting the importance of allowing him to get the medication as soon as he reports a headache. Given that some of his headaches occur on consecutive days, we suspect some may be continuations of a prior headache, so again the importance of obtaining complete pain relief from his acute medication should be stressed. If at his next follow-up visit he reports not always having complete relief with ibuprofen, we would consider trying naproxen instead, and/or adding a triptan, most likely sumatriptan 100 mg given its availability. He would need to understand that he may only use the triptan for 4 to 6 headache days/month. Depending on his response to sumatriptan, it could be used alone or in combination with his NSAID. He also may be instructed to use an NSAID alone for more mild headaches and his NSAID together with sumatriptan for more severe headaches.
Preventive Treatment
PedMIDAS score is a 25, indicating mild disability, however his migraines are frequent, occurring 1–3 times per week and clearly having a significant impact on his functioning. We would therefore recommend starting a prophylactic medication. He notes difficulty remembering to take medications as well as difficulty falling asleep, so amitriptyline would be a good choice for him. For his weight of 50.9 kg, we would start with at 12.5 mg at night and increase by 12.5 mg every 2 weeks to a dose of 50 mg (~1mg/kg/day). He and Mom should be counseled that it will likely take at least a few weeks on his goal dose before they start to see results. He should be counseled also to report to Mom immediately if he has any depressive or suicidal thoughts. Topiramate is another option, especially as it is FDA-approved for migraine prevention in children older than 12 years, although twice daily dosing may be a factor in maintaining compliance. Careful discussion with the patient and family in regards to the potential risks and benefits is important prior to starting any preventative medication.
Biobehavioral Management
The patient should be drinking 8–10 cups of non-caffeinated fluid per day and additional cups on days he plays soccer. He should be given a school note to this effect so he may carry a water bottle at school. He eats well, but the importance of not skipping breakfast should be stressed, and ideas for fitting this in should be given. The importance of a consistent bedtime routine should be stressed, with good sleep hygiene to assist with easier sleep onset. It should be made clear that he should not be using screens within an hour prior to bed. He already exercises frequently and should be commended for being active. Given his report of frequent headaches which are impairing school attendance, functioning at school, and participation in soccer activities, screening for potential co-morbid psychiatric issues and making a referral for further treatment with a pediatric pain psychologist focused on coping with chronic pain is appropriate.
Case Study 2
Initial Presentation and History
A 16-year-old right-handed girl who has been having headaches since she was 10 years old presents for evaluation. She reports unilateral mostly right-sided headaches, which are throbbing and associated with photophobia, phonophobia, osmophobia, nausea, and occasionally vomiting. She has no premonitory or aura symptoms. Her headaches typically occur once a week but she has noted that they tend to be more frequent around the time of her menses and that often these headaches do not fully respond to naproxen, which typically works to break her migraines during other times in the month. Upon further review, she believes these headaches typically start on the day or two prior to her menses, and that this happens almost every month. She often misses school due to these headaches. Her mother would like to know whether the patient should see a gynecologist to potentially be placed on birth control to control her headaches.
Physical Examination
On examination, weight was 61.5 kg (75.8 percentile), height 157.1 cm (18.4 percentile), BMI 24.91 (84 percentile), blood pressure 112/55 mm Hg, and heart rate 90 bpm. Her general physical and neurologic exam results were normal. She had tightness over the left trapezius muscles, otherwise the remainder of headache examination was unremarkable.
What is the probable diagnosis?
According to the ICHD-IIIβ, the patient meets criteria for episodic migraine without aura. Based on the history, she likely also has menstrually related migraine without aura [5].Officially, however, 3 months of prospective documentation is needed to make this diagnosis. Menstrually related migraine is included in the appendix of the ICHD-IIIβ, meaning there is ongoing debate about how it should be classified. According to the current appendix criteria, headaches should meet criteria for migraine without aura and also have documented and prospectively recorded evidence over at least 3 consecutive cycles with headaches confirmed on day 1+/– 2 (ie, 2 days prior to onset of menstruation or within the first 3 days of menstruation) in at least 2 out of 3 menstrual cycles, with migraines occurring during other times of the month as well [5]. This is distinguished from pure menstrual migraine without aura, in which migraines occur only during days 1+/–2 of menstruation but not during other times of the month [5].While we therefore cannot say that the patient meets ICHD IIIβ criteria for menstrually related migraine due to the lack of prospective documentation, her history suggests this.
Menstrually Related Migraine
An association of migraine with menses is well described in adults, occurring in up to 60% of adult female migraine patients [81].In adults, it has been observed that the migraines associated with the menstrual cycle tend to be more severe [82],associated with more nausea and vomiting [82],longer and less responsive to acute medications [83],and associated with more work-related disability [83]. Recently, analysis of data from the American Migraine Prevalence and Prevention (AMPP) Study found that women with pure menstrual migraine and menstrually associated migraine have on average higher MIDAS scores than those with non-menstrual migraine [81].
Only one study has explored menstrually related migraines in adolescents [84].It was a clinic-based study that found a similar prevalence—50% of adolescent patients who had reached menarch noted an association of migraine with their menses. Overall worse disability was not demonstrated in this study, but individual menstrual attacks were not compared with nonmenstrual attacks [84].No other studies have addressed this population, but it is clear that the pattern of menstrually related migraine exists in adolescents and it is important to recognize this pattern as these patients may require additional focused care in addition to standard migraine management.
What are treatment options?
In adults, options for more specific management of pure menstrual migraine and menstrually related migraine include intermittent prophylaxis with NSAIDs or triptans or use of hormonal contraceptives. There are no studies addressing any of these treatments in adolescents, so at this point management decisions must be based on evidence extrapolated from adult studies as well as attention to specific concerns in adolescent patients.
Generally, intermittent prophylaxis (using a medication a few days prior to and during the first few days of menses) with various medications has shown efficacy in adult studies. This approach may be more appropriate for patients with pure menstrual migraine, as there is less likelihood of precipitating medication overuse in this population. It can be considered in patients with menstrually related migraine as well if typical daily preventives have not been effective. One small open-label trial using naproxen 550 mg daily prior to and during menses (at differing schedules depending on the month) showed slightly decreased frequency and severity of headaches during that time [85].However, triptans are what are most commonly used.
Triptans
Frovatriptan is the longest-acting triptan and the one used most commonly for intermittent prophylaxis. One double-blind randomized controlled crossover study showed improvements in headache frequency, severity, and duration using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses [86].Frovatriptan 2.5 mg daily showed some efficacy as well but was not as effective as 2.5 mg BID [86].One prospective randomized placebo-controlled trial demonstrated more headache-free cycles using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses, as compared to placebo [87].
Naratriptan, also a longer-acting triptan, was studied in 3 prospective double-blind trials at doses of 1 mg BID used for 5 days total starting 2 days prior to menses, and all 3 showed a decrease in headache frequency as compared to placebo [88,89].Of note no efficacy was shown using 2.5 mg daily [88].
Less commonly used for intermittent prophylaxis are the shorter-acting triptans, but they have shown some efficacy as well. In one prospective study using oral sumatriptan 25 mg TID for 5 days each cycle starting 2 to 3 days before onset of menses, there seemed to be at least some improvement with sumatriptan in most cycles treated [90].Zolmitriptan was studied in one prospective double-blind placebo-controlled study using 2.5 mg BID or 2.5 mg TID for 7 days total with each cycle and was found to be associated with a significant reduction in headache frequency as compared with placebo [91].There has been one prospective trial using eletriptan 20 mg TID for 6 days total starting 2 days prior to menses, which also showed improvement in headache activity in 55% of patients [92].
Hormonal Contraceptives
In general for adult patients, hormonal contraceptives are not considered first-line treatment for menstrually related migraine. However, in patients who do not respond to other modes of treatment, or who plan to use hormonal contraception for contraceptive purposes, published expert opinions have recommended considering extended or continuous hormonal regimens [93,94].
Estrogen withdrawal during the luteal phase of the monthly cycle has long been speculated to be of importance in the pathophysiology of menstrually related migraine [93]and the relationship between hormonal contraceptives and migraine is complicated. Headache is a commonly reported side effect of combined hormonal contraceptives [95]; however, this effect seems to occur mostly during the hormone-free week [96]. It has been shown that headache occurs less frequently in women using an extended cycle regimen (84 or 168 days) as compared to those using a traditional monthly cyclic regimen [97,98].Studies addressing the use of combined hormonal contraceptives for women specifically with menstrually related migraine are limited. One small prospective randomized study showed improvement in menstrually related migraine in patients treated with a low dose (20 mcg ethinyl E[2]) oral hormonal contraceptive in both a 21/7 cycle and a 24/4 cycle, with more improvement in the group using a 24/4 cycle [99].Another showed improvement in menstrually related migraine in all study patients treated with a low-dose hormonal contraceptive (20 mcg ethinyl estradiol) on a 21/7 regimen, but with additional 0.9 mg conjugated equine estrogen during the placebo week [100].There has been one randomized placebo-controlled double-blind trial in patients with menstrually related migraine using an extended 168 hormonal regimen along with frovatriptan vs. placebo for 5 days during the hormone-free interval. Overall daily headache scores were decreased from pre-study cycles, and the increase in headaches during the hormone-free interval was lower in the frovatriptan group [101].A recent study showed that contraceptive-induced amenorrhea can be beneficial for decreasing migraine frequency in patients with menstrual migraine [102].There have been no studies addressing the use of hormonal contraceptives for migraine management in adolescents.
In adolescents, the decision regarding use of hormonal contraceptives is more complicated. There is less of a chance that adolescent patients, especially younger ones, would be planning to use hormones for contraception so their use may be solely for the purpose of migraine management. However, hormonal contraceptives are commonly used in adolescents for management of other menstrual-related disorders, such as menorrhagia, dysmenorrhea, and endometriosis, and extended cycle and continuous regimens have become more popular with adolescent providers in general [103].The general concern with using hormonal contraceptives in adolescents is that they have potential for longer-term use than adult patients, and the effects of using hormonal contraceptives long term are unknown. The major concerns are for potential interference with expected increase in bone mineral density in adolescents, effects on fertility, and risk for cancer and cardiovascular disease [103].Additionally, specifically in migraine patients, is the concern for increased stroke risk. The increased risk for ischemic stroke in patients with migraine, although more specifically with migraine with aura, is well known [104–106]and migraine has recently been shown to be a risk factor for ischemic stroke in adolescents as well [107]. In adults, the use of hormonal contraceptives in patients with migraine with aura is known to increase the risk of ischemic stroke [106],and this has not been studied in adolescents. Given the various unknowns in the use of hormonal contraceptives in patients with menstrually related migraines, we would not recommend this as first-line treatment. However, similarly to adults, in patients who do not respond to other methods of migraine management, or who seek to use hormonal contraceptives for contraception or for other menstrually related disorders, extended or continuous cycle hormonal contraceptives may be a reasonable option, at the lowest possible estrogen dose. However, migraine with aura should be screened for, and its presence should prompt reconsideration of combined hormonal contraceptive use.
How should this patient be managed?
The patient is having frequent and disabling migraines, so starting a preventive medication would be appropriate. She has migraines throughout the month in addition to during her menses, so a daily prophylactic would be more appropriate than intermittent prophylaxis surrounding her menstrual cycle only. At this point, our recommendation would be to start a daily preventive with either amitriptyline or topiramate. Given that naproxen is not breaking some of her migraines, she should be given a prescription for a triptan. Sumatriptan 100 mg would be an appropriate first choice, and she can be instructed to use it along with her naproxen at the onset of her menstrual migraines. She can use it for other migraines as well but she should be instructed not to use it more than 4 to 6 times per month. She should keep a diary for the next 3 months noting most importantly headache days as well as days of menstruation, so that a more definitive pattern can be confirmed and an official diagnosis based on ICHD-IIIβ criteria can be made. If her migraines do not improve with daily preventives, at that point discussion regarding potential for intermittent prophylaxis or trial of extended cycle hormonal contraception may be considered, although with caution and discussion of risks and benefits.
Corresponding author: Hope O’Brien, MD, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 2015 Cincinnati, OH 45229.
Financial disclosures: None.
From the Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Abstract
- Objective: To review the management of migraine in adolescent patients.
- Methods: Literature review in the context of 2 clinical cases.
- Results: Migraine is common in adolescents and can affect school and social functioning. Management options include lifestyle modifications and acute and preventative therapies. First-line medications for migraine in the adolescent population are over-the-counter medications, including ibuprofen, acetaminophen, and naproxen. Studies of efficacy of triptans in the treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. Several classes of medications are commonly used for migraine prevention, including antidepressants and antiepileptics. Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older. Biobehavioral treatments such as relaxation training, biofeedback, and cognitive behavioral therapy have been evaluated in randomized controlled trials and found to be efficacious.
- Conclusion: Approach to management of migraine in adolescents should be multifactorial with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Case Study 1
Initial Presentation
A 13-year-old left-handed boy with allergic rhinitis has been referred by his pediatrician for evaluation of headaches.
History
The headaches have been occurring since he was 7 years old. He describes a bilateral frontal and periorbital pain, which is either aching or throbbing in nature. There is associated photophobia and phonophobia, and the pain worsens with activity. When he was younger the headaches were frequently associated with emesis. He occasionally gets tenderness of his face during the headaches, mostly in the areas adjacent to his nose and above his eyes and occasionally associated rhinorrhea.
Initially the headaches were mild and occurring infrequently, but for the past 6 months they have been more severe and occurring 1 to 3 times per week, sometimes on consecutive days. They typically begin in the afternoon but at times occur soon after waking in the morning. If they do occur in the morning, they worsen after getting out of bed. He denies any type of warning symptoms indicating the headache will occur, and has no associated focal neurologic symptoms associated with the headaches. His mother tries to minimize medication intake so will typically wait to see if the headache is severe before giving him medication, typically 2 chewable children’s ibuprofen, which helps some of the time; however, his headache often does not completely abate unless he naps. He has also been taking loratadine daily due to concerns that his headaches may be secondary to chronic sinusitis.
He is a good student and enjoys school, but notes stress surrounding tests. He reports missing 4 days of school in the past 3 months as well as coming late twice. He thinks that there were 9 additional days in which he was unable to function at his full ability at school and at least 5 times he was unable to concentrate on his homework. He plays soccer 4 to 5 times per week. He says twice he had to skip soccer practice to due to headache and at least 3 other times he needed to take breaks during soccer due to headache. He is unsure how much he drinks on an average day but he drinks mostly with meals. He does not drink caffeine. He gets into bed at 10 pm and sometimes does not fall asleep until after 11 pm, often due to worry about tests. He often plays with his tablet when trying to fall asleep. He wakes up at 6 am for school. He typically eats 3 meals per day but occasionally misses breakfast if he is rushed in the morning.
Physical Examination
On examination, the patient is a well-developed, well-nourished male in no apparent distress. His weight was 50.9 kg (68th percentile), height 163.7 cm (65th percentile), BMI 19.9 (69th percentile), blood pressure 118/62 mm Hg, and heart rate 79 bpm. His general physical examination, including skin, HEENT, extremities, lung, cardiac, and abdominal examination was normal, with lungs clear to auscultation, heart with a regular rate and rhythm, and abdomen soft and tender without organomegaly.
On neurologic examination he was alert and attentive with normal mental status. Speech was fluent. Skull, spine, and meninges were normocephalic and atraumatic with a supple neck. Cranial nerves II through XII were normal with a normal fundoscopic examination including sharp disk, no papilledema, and normal fundus bilaterally. Motor examination was normal for tone and bulk with full strength throughout. Sensory examination was normal for light touch, temperature, vibration, and joint position sense. Finger-nose-finger fine finger movement and heel-knee-shin were normal. Deep tendon reflexes were symmetric and 2+ throughout with toes downgoing. Station and gait were normal including toe walking, heel walking, tandem walking, running, skipping, one-legged standing, Fog gate procedure, and there was no Romberg’s sign.
He had a normal comprehensive headache examination. There was no tenderness at typical migraine trigger points (nuchal line and mandibular process) and no tenderness at supraorbital notch. Neck was supple with normal rotation and normal bilateral trapezius muscle tightness. No bruits or palpitations were heard over carotid or jugular veins. There was a negative Muller sign with no pain on neck bending with pressure, and no signs of allergy or sinus symptoms.
What is the initial evaluation of a teen with recurrent headaches?
The overwhelming majority of children and adolescents presenting to medical attention with recurrent headaches will have a primary headache disorder, most commonly migraine [1].However, ruling out a secondary cause is typically of concern, both for providers and parents, and the decision regarding whether to image is often a daunting one. In a review of 6 large studies in which imaging was done in pediatric patients who were examined by a neurologist, only 14 patients were found to have CNS lesions requiring intervention, and all of these patients had abnormalities on physical exam [2]. An American Academy of Neurology (AAN) practice parameter published in 2002 recommended considering neuroimaging only in patients with abnormal neurologic examination, co-existing seizures, concerning associated neurologic features, or recent onset of severe headache or change in headache type [2].There are additional factors which may prompt one to consider imaging as well including atypical auras or very short (< 5 minutes) or protracted ( > 60 minutes) auras, trigeminal autonomic cephalalgia, brief headaches precipitated by cough, frequent early morning headaches or which wake the patient from sleep, headaches with primarily occipital location, headaches in a patient less than 6 years old or in a child who cannot describe the headaches well, or migrainous headaches in a child without any family history of migraine or migraine equivalents. However, recent studies continue to show that the imaging yield in patients with headaches is low and further studies are needed to elucidate which patients truly require imaging [3].
Given this, a thorough history, family history, physical exam, and detailed neurologic exam including fundoscopy are imperative. In addition to the general neurologic exam, a focused headache exam should be performed, including Mueller’s maneuver, auscultation for cranial bruits, evaluation of the temporomandibular joint, palpation of possible trigger points, and maneuvers to assess for cervical spine disease [4].
Classification of Symptoms
Once establishing the likelihood of a primary headache disorder, the International Classification of Headache Disorders, 3rd edition, beta version (ICHD IIIβ) [5]should be used to classify the headache diagnosis. Note that while this classification system was established for adults, most criteria are similar for children and adolescents, and the typical differences are noted in the comments of the ICHD IIIβ [5].Migraine is the most common type of primary headache brought to medical attention [1]. Migraine without aura is described as a recurrent headache disorder (at least 5 lifetime attacks) with attacks lasting 4 to 72 hours, typically unilateral, throbbing in nature, moderate to severe in intensity, aggravated by routine physical activity, and associated with nausea and/or photophobia and phonophobia. In young children, migraine is more frequently bilateral, the gastrointestinal symptoms often more pronounced than photophobia and phonophobia, and migraines may be shorter, lasting at least 2 hours without treatment [5].As patients reach adolescence, migraine features typically start to evolve into patterns described in adults. Differentiating migraine from other primary headaches, such as tension-type, can be challenging, especially in children in whom migraines are more likely to be shorter and bilateral. Tension-type headaches are bilateral or diffuse, pressing or tightening pain that is non-pulsatile lasting at least 30 minutes. The pain is described as mild to moderate in intensity and not aggravated by activity [5].They may be associated with photophobia or phonophobia (not both) and they may not be associated with nausea or vomiting [5].
Is any further workup indicated?
The patient has recurrent headaches which have been present for 5 years. While they have increased in frequency, there has not been any change in their quality. His headache description and history have no “red flag” features, and a thorough examination is normal. Therefore, neuroimaging and further workup would not be indicated in this case.
What is the diagnosis?
The patient’s headaches are throbbing in nature, exacerbated by activity, associated with nausea, photophobia, and phonophobia, and are moderate to severe. Using the ICHD IIIβ, he meets criteria for migraine without aura. While his headaches are frequent, he is having less than 15 headache days per month, so this is episodic migraine. Note that he complains of forehead and periorbital pain and occasional rhinorrhea with his headaches, leading him to have been placed on loratadine for treatment of presumed allergic sinusitis. Children meeting criteria for migraine are very frequently misdiagnosed as having sinusitis [6]due to the overlap in location of migraine pain and proximity to the frontal and maxillary sinuses, as well as the presence of autonomic features frequently present in migraine, reportedly present in 70% of pediatric migraine patients [7].The negative headache examination, including Muller’s maneuver, points against sinus disease as well.
What is the approach to management of the adolescent with migraine?
Approach to management of migraine in adolescents should be multifactorial, with attention to an aggressive acute treatment regimen, preventive medications when indicated, and biobehavioral management.
Acute Treatment
General Approach
In counseling patients with migraine, and in particular adolescents, it should be stressed that achieving a normal level of functioning as soon as possible is the goal of therapy. Common missteps in treatment include failure to take acute medication early into the headache, incorrect dosing, incompletely treating the headache, and avoidance of participating in daily activities when headaches occur.
Adolescent patients may frequently wait to take an acute medication, typically due participation in another activity, not having medication with them, or discomfort with taking medication in front of peers or at school. Additionally, patients who have headaches beginning in early childhood, with pronounced gastrointestinal (GI) features, may be aware that their headaches resolve after vomiting, and therefore get used to not treating with medications. When these patients reach adolescence, when GI symptoms tend to become less pronounced, they will need to be educated that taking medication early is imperative. Acute medications are typically more effective when taken earlier in the course of a migraine, and the importance of pausing to take medication at the onset of the headache should be stressed to all patients and parents. With that in mind, however, care should be taken to counsel patients regarding the potential for development of medication overuse headache. When headaches are frequent, a more detailed and intricate plan may need to be devised so that adolescents and parents know which headaches to treat with medication.
Given that first-line medications for treatment of migraines are over-the-counter, underdosing occurs commonly, as dosing listed on packaging is typically age-based, not weight-based. At initial visits, young adolescent patients will frequently report that a particular medication is not effective, but this is often because they are still receiving low/less optimal dosing. Clinicians should remember to follow weights and recommend dosing changes at the initial visit and follow-up visits as well.
Treatment goals, ie, complete resolution of pain and migrainous features with ability to return to normal functioning, should be made clear to patients and families at the onset of treatment. Patients frequently fall into a pattern of continuing to treat with a medication that may lessen but not completely ablate the pain of a headache, and then sleep and avoid activity. Upon awakening, the headache may be gone, however, given incomplete initial treatment, the headache may be more likely to recur within 24 hours. At that point, the headache may be more difficult to treat and therefore cause further decrease in functioning. If this cycle perpetuates, disability can become extremely burdensome in adolescents, significantly affecting school and social functioning. Therefore, a detailed plan for initial steps in management as well as steps to take should initial therapy fail to fully break the headache should be given to every patient.
Of note, given that dehydration is now recognized as a common trigger for migraine [8,9], it is generally our recommendation to drink 16 to 32 oz (depending on weight) of a hydrating fluid together with whichever acute medication is chosen, as rehydration likely assists in breaking migraines as well [8].
Medications
Over-the-Counter Agents
The first-line medications for migraine in the pediatric and adolescent populations are over-the counter medications, including ibuprofen, acetaminophen, and naproxen sodium. Ibuprofen has been well studied in pediatrics and was found to be safe and effective in 2 studies [10,11]at doses of 7.5–10 mg/kg. Again, care should be taken to ensure timely administration of ibuprofen at onset of headache, or aura if present, with appropriate weight-based dosing. Naproxen sodium has not been studied in pediatrics for treatment of migraine. However in practice, it is often used in similar doses of 10 mg/kg, with good efficacy. Although ibuprofen and naproxen sodium are both nonsteroidal anti-inflammatory medications (NSAIDs), anecdotally many patients report successful treatment with one NSAID when another has failed. Aspirin has shown efficacy in adults for treatment of acute migraine [12].It is likely effective in the pediatric population as well, but it is generally avoided due to long-standing concerns for precipitation of Reye syndrome in children. In adolescents over 16 years old, however, it is a reasonable option if there are no contraindications.
In one study, acetaminophen was compared to ibuprofen and placebo for treatment of migraine in children and adolescents and found to be effective more frequently than placebo but not as frequently as ibuprofen [10],likely due to its only minimal anti-inflammatory effects. It is a reasonable option for children and adolescents, particularly in those who have contraindications to NSAIDs.
While these over-the-counter medications are generally safe and well tolerated, clinicians should not overlook the potential for toxicity as well as medication overuse headache, and patients should be counseled to avoid use of any of these medications for more than 2 to 3 headache days per week.
Triptans
Studies of efficacy of triptans in treatment of pediatric migraine have been limited and results conflicting, largely due to high placebo response rates. However, some have shown efficacy over placebo, and in the past few years have received FDA approval for use in the pediatric and adolescent populations. Clinicians should remember that these medications are vasoconstrictors, so they should not be used in patients with vascular disease or in patients with migraine with brainstem aura or hemiplegic migraine. Additionally, due to the risk of serotonin syndrome, they should not be used in patients on monoamine oxidase inhibitors. It is also important to educate patients to limit use of these medications to 4 to 6 times per month to avoid precipitation of medication overuse headache.
Almotriptan was approved by the FDA in 2009 for use in patients 12 years and older, based on a large randomized controlled trial comparing doses of 6.25, 12.5, and 25 mg with placebo in patients ages 12 to 17 years old. All doses resulted in statistically significant pain relief as compared to placebo, and interestingly, the 12.5-mg dose seemed to be the most effective [13].
Rizatriptan received FDA approval in 2012 for use in patients 6 years and older. In 2 randomized controlled trials in patients 6 to 17 years old, rizatriptan (5 mg for patients < 40 kg, 10 mg for patients ≥ 40 kg) was more effective than placebo in providing pain freedom at 2 hours [14,15].One earlier trial found efficacy only on some measures (weekend treatment, decrease in nausea and functional disability) but no statistically significant difference than placebo in terms of overall efficacy in achieving pain freedom at 2 hours [16].However, this trial had a higher placebo response rate than typically seen in adult triptan trials. In a recent long-term open-label study in patients 12 to 17 years old, rizatriptan was found to be generally safe and well-tolerated with consistent efficacy of 46% to 51% pain freedom at 2 hours over time [17].
A combination pill consisting of sumatriptan and naproxen (Treximet) received FDA approval in May 2015 for patients 12 years and older. This was based on a randomized controlled parallel-group trial in patients 12 to 17 years old using the sumatriptan/naproxen combination in various dose combinations: 10/60 mg, 30/180 mg, or 85/500 [18].All doses were found to be equally effective in providing pain relief at 2 hours as compared to placebo, with a higher chance of sustained pain relief at 24 hours in the group receiving the 85/500 dose. In this trial, and in a long-term open-label safety trial [19],all doses were generally well tolerated with minimal adverse reactions.
Most recently, in June 2015, zolmitriptan nasal spray was approved for patients 12 years of age and older. In the large “Double-Diamond” study, which used a novel design to attempt to minimize placebo effect, zolmitriptan nasal spray (5 mg) was found to have a significantly higher headache response rate at 1 hour than placebo, and was significantly superior to placebo with regard to multiple secondary end-points [20].Additionally, a long-term open-label trial in patients 12 to 17 years old using oral zolmitriptan (2.5 mg or 5 mg) found it to be generally effective and well tolerated [21].
No other triptans have been approved for children or adolescents, however, most are widely used in clinical practice. There is good evidence for the efficacy and tolerability of sumatriptan nasal spray [22–26]in adolescents, and it is approved for use in adolescents with migraine in Europe. Although oral sumatriptan was the first triptan available clinically in the United States and is very widely used, there is surprisingly little published evidence for its use in the pediatric and adolescent populations. In fact, 2 randomized controlled trials in adolescents failed to show efficacy of oral sumatriptan as compared to placebo [27,28].Despite this, given its availability it is a reasonable choice for adolescent patients and is often one of the first tried. There has been 1 randomized controlled trial in adolescents for eletriptan without significant differences in efficacy at 2 hours as compared to placebo, although similar to other trials the placebo rate was high and there were some differences seen in secondary outcomes measures [29].Similarly, one randomized controlled trial of naratriptan 12.5-mg tablets failed to show efficacy as compared to placebo in pain relief [30].At present time, for frovatriptan there has only been a study looking at the pharmacokinetics and safety in adolescents, which found that it was generally well tolerated and recommended adolescent dosing similar to adult dosing [31].
In choosing a triptan, clinicians should keep in mind availability of alternate forms of administration, absence or presence of significant emesis, and the age of the patient. For patients who are unable to swallow pills or who have significant emesis associated with their migraines, nasal sprays and oral dissolving forms (melts) are good options. The nasal sprays (zolmitriptan and sumatriptan) additionally have the benefit of a quicker onset (~15 minutes) in general than the oral formulations. The downside to these nasal formulations is bad taste, which is frequently reported by patients. Patients should be counseled in proper administration of these nasal sprays (ie, avoiding inhalation, which causes the medication to enter the mouth) to minimize the bad taste and maximize absorption through the nasal mucosa. Other alternatives are the oral dissolving forms (rizatriptan and zolmitriptan). Given the FDA approval, the rizatriptan melt tablets are often the first-line triptan for children under age 12, but zolmitriptan melts are an option as well.
Preventive Treatment
General Approach
The decision to place an adolescent on a daily preventive medication should be based on a combination of headache frequency, severity, ease of breaking headaches, and overall disability as established by a disability scale (such as the PedMIDAS). Any patient with headaches occurring one or more days per week, those whose headaches are not easily treated or tend to be prolonged, and those with a PedMIDAS score of 30 or more should be considered candidate for a daily preventive. It is particularly important to consider starting a preventive early in adolescent patients given the possibility of impacting overall disease progression at a young age. While the natural history of headaches that start in the young is still being investigated, a known risk factor for transformation of episodic to chronic migraine is frequent headaches [32].It is therefore imperative to attempt to intervene early to improve quality of life in the present and also to prevent a downward cycle into chronicity, potential medication overuse, and worsening disability.
At present, there are several classes of medications that are commonly used for migraine prevention, including antidepressants, antiepileptics, antihistamines, and antihypertensives. Patients should be educated regarding the medication’s typical use, the specific way in which it is used in migraine, potential side effects, and overall expectations of efficacy. Most preventives need to be titrated up slowly to maximize tolerability, and patients need to understand that it may take time before they start to see results. In general, we recommend titrating up over the course of 4 to 12 weeks (depending on medication and dose goal), and then a substantial trial of 4 to 6 weeks on a full dose of medication before determining efficacy. If a medication shows a trend towards improvement but the patient has not met treatment goals, medication can be titrated up further at that point as tolerated. Patients and families should also understand that these medications are not intended as a “cure” for migraine but rather as a tool for improvement, which should be used in conjunction with the rest of a detailed plan. Generally, if a patient is well controlled for 4 to 6 months (ie, 3 or less headaches/month that are easily broken with medications, and a PedMIDAS < 30), attempts should be made to wean off of medication.
Medications
First-line Therapies
Currently, topiramate is the only medication approved for prevention of migraine in patients 12 years and older (approved in March 2014). Three randomized double-blind placebo-controlled trials in children and adolescents have found topiramate to be superior to placebo in multiple endpoints [33–35].Doses in these trials were 2–3 mg/kg/day, 100 mg/day, and 50 or 100 mg/day, respectively. Notably, the 50 mg/day dose was not found to be superior to placebo. Multiple retrospective, open-label, and drug comparison trials have shown effectiveness and tolerability as well [36–42].We typically use a goal dose of 2 mg/kg/day, which is reached after titrating over 8 to 12 weeks, which minimizes side effects (most commonly paresthesias, memory/language dysfunction, appetite suppression, and drowsiness), but higher doses can be used if needed.
Amitriptyline has consistently shown efficacy in adult migraine trials and is therefore one of the most commonly used medications worldwide for prevention of migraine in children and adolescents. Surprisingly, there have been no published randomized controlled trials using amitriptyline in the pediatric population, although a trial comparing amitriptyline, topiramate, and placebo is currently underway (Childhood and Adolescent Migraine Prevention Study) [43].In 2 retrospective pediatric studies improvement with amitriptyline was reported in 84.2% and 89% of patients, respectively [44,45].We typically use a goal dose of 1 mg/kg/day, also with an 8- to 12-week titration. The most common side effects with amitriptyline are somnolence, dry mouth, and weight gain, but it is generally well tolerated in children and adolescents. There is also a risk of worsening depression and suicidal thoughts, so it is recommended to use caution if considering prescribing to a patient with underlying depression. It is typically administered in once daily dosing a few hours before bed to minimize morning drowsiness. There is also a concern for precipitation of arrhythmias, and while there are no guidelines recommending screening ECGs, this should be considered in patients with a family history of heart disease. Of note, many practitioners use nortriptyline in place of amitriptyline as it can be less sedating. It should be noted, however, that evidence for its efficacy is lacking. Additionally, it may carry a higher risk of arrhythmia [44].
Second-line Therapies
The second-line therapies typically considered are other antiepileptics, valproic acid, levetiracetam, and zonisamide, for which there is some evidence, although mostly in the form of open-label or retrospective studies [46–51]. Of note, despite the many promising retrospective and open-label studies for valproic acid [46–48], one randomized double-blind placebo control trial comparing various doses of extended release divalproex sodium with placebo in adolescent patients failed to show a statistically significant treatment difference between any dose and placebo [52].It is, however, frequently prescribed and anecdotally quite efficacious. Given concerns about potential for teratogenicity, and possible effects on ovarian function, as well as potential for weight gain and hair loss, it should be used with caution in adolescent females.
Antihypertensives (beta blockers and calcium channel blockers) have long been used for prevention of migraine in both the adult and pediatric population, but evidence for their use in the pediatric population is conflicting. An early double-blind crossover study of propranolol in patients 7 to 16 years old showed significant efficacy as compared with placebo [53].However, in 2 subsequent studies it failed to show efficacy as compared with placebo and self-hypnosis, respectively [54,55].Given the conflicting evidence and the potential for hypotension, depression, and exercise-induced asthma, use of propranolol has fallen out of favor by experts for use in pediatric and adolescent migraine prevention. Flunarizine, a nonselective calcium channel blocker, has demonstrated effectiveness in pediatric migraine prevention [56,57],and is actually approved in Europe for this indication, but it is not available in the United States.
Cyproheptadine is an antihistamine with antiserotonergic properties which is used frequently for migraine prevention in young children who are unable to swallow tablets, although evidence is limited to 1 retrospective study [45].However, given the propensity for weight gain with this medication, it is generally not recommended for use in adolescents.
In recent years, there has been a growing interest in nutraceuticals given their general tolerability and minimal side effects, as well as a comfort in using a more “natural” approach, particularly in pediatrics. Evidence is limited but some of the frequently used substances may be beneficial. Butterbur (petasites hybridus) has strong evidence in adults and is recommended by the AAN for migraine prevention in adults [58].There is one small pediatric RCT showing efficacy for butterbur as compared to music therapy [59],as well as one promising open-label trial [60]. Typical dosing ranges from 50–150 mg daily. However, butterbur contains pyrrolizidine alkaloids, which are hepatotoxic, and due to the concerns for inadequate monitoring of removal of these substances in the manufacturing of commercial butterbur, it has been generally avoided in the pediatric population. Coenzyme Q10 (CoQ10) is considered possibly effective for adult migraine prevention by the AAN [58].Pediatric evidence is limited to an open-label study showing improvement with supplementation of CoQ10 in deficient patients [61],and a subsequent double-blind placebo-controlled add-on study, which showed improvement in both the CoQ10 and placebo groups but faster improvement in the CoQ10 group [62].Typical pediatric dosing is 1–2 mg/kg/day. Magnesium is considered by the AAN to be a good option for migraine prevention in adults [58].One randomized controlled trial in children however had equivocal results [63],while one small prospective open-label study had positive results [64]. Magnesium supplementation may be more effective in patients who have low ionized magnesium levels, but this is difficult to measure reliably in the clinical setting. Doses of 9 mg/kg/day can be used with the most common side effects reported being gastrointestinal upset and diarrhea, generally dose dependent. Riboflavin is also considered by the AAN to be probably effective for prevention in adults [58],but again the evidence in children is limited to one positive retrospective study [65]and 2 equivocal randomized controlled trials [66,67],one of which had an unusually high placebo rate [66]. Appropriate dosing is also something of debate, as riboflavin is minimally absorbed and has a short half-life, so while studies were done using 200–400 mg daily, smaller more frequent dosing may be needed.
A potential approach to treatment with vitamins is to check for deficiencies, but currently only the study mentioned above in which CoQ10 levels were checked showed improvement with normalizing of low levels [61,62].Further research into this topic is needed to elucidate whether checking and repleting levels of specific vitamins would be beneficial in prevention of migraines in certain patients.
Healthy Habits
The importance of maintaining healthy lifestyle habits and modifying potentially detrimental ones should be stressed to patients and families, and counseling regarding these issues should be provided at every visit, as repetition is often key to patients understanding their importance. Skipping meals is a commonly reported migraine trigger [68,69].This is not an uncommon occurrence even in the most well-meaning of families; adolescents often report not feeling hungry in the morning, and in the rush to get to school will often skip breakfast. Many patients report not liking school lunches leading them to go most of the day without food. Adolescent girls may skip meals due to weight concerns. Patients need to be reminded that well-balanced meals throughout the day is imperative, and they often may need specific counseling on how to achieve this practically. Of note, unless a specific migraine trigger has been identified in a given patient, we do not generally recommend restricting any specific food lists.
Maintaining good sleep hygiene and a consistent sleep schedule is also often difficult for adolescent patients, with after-school activities, homework, and screen use (eg, television, electronic devices) often contributing to late bedtimes with then forced early morning waking for school. However, improvement in sleep hygiene has been shown to be effective in improving migraines in children and adolescents [70],and realistic plans for improvement in sleep should be discussed with patients.
Dehydration is also a common migraine trigger [8,9],so the importance of staying well hydrated should be stressed as well. Again, specific recommendations for how this can be achieved are often needed, especially given adolescents’ busy schedules. Additionally, many schools do not allow water bottles to be carried, and often a school note is needed so patients may be allowed to carry a water bottle at school and also be provided extra bathroom breaks as needed.
Also important is stressing maintenance of daily functioning throughout migraine treatment. By the time adolescents seek medical care, they may already be in a cycle of missing school due to headaches, and some may even be receiving home-schooling. The goals of staying in school and learning to function with headaches should be stressed, often with the help of coping skills, which will be discussed below, as it has been shown that functional disability generally improves before pain [71].
Behavioral Treatments
Psychological treatments are an important aspect of migraine management. Biobehavioral treatments such as relaxation training, biofeedback, and multimodal cognitive behavioral therapy (CBT) have been evaluated in randomized controlled trials and reviewed in meta-analyses in pediatric migraine populations and found to be efficacious [72–75].These treatments have been shown to reduce pain intensity and disability for children and adolescents with headaches and therapeutic gains appear to be maintained [72].
Relaxation training typically includes instruction in techniques such as diaphragmatic breathing, progressive muscle relaxation, and guided imagery. Relaxation training is most effective with children 7 years of age or older [76].Biofeedback is often used in conjunction with relaxation training to provide audio or visual feedback about normally unconscious physiological body responses associated with increased relaxation. Effective biofeedback parameters used with children and adolescents with migraine headache include electromyographic (EMG) activity and peripheral skin temperature [77].Biofeedback techniques can help children and adolescents become more aware of physical responses, better control these responses and generalize physical responses outside of therapy sessions to better cope with pain [76].CBT involves instruction in skills such as biofeedback-assisted relaxation training, activity pacing, distraction, and cognitive strategies for coping with pain. CBT was shown to be effective in a recent study comparing adolescent patients with chronic migraine receiving amitriptyline and CBT with patients receiving amitriptyline and standard headache education [78]. Patients who received CBT plus amitriptyline had greater reductions in days with headache and migraine-related disability compared with patients who received headache education plus amitriptyline [78].Hypnosis and acceptance and commitment therapy (ACT) are also psychological treatments used with children and adolescents with migraine headaches. ACT prioritizes the outcome of improved functioning above headache reduction and has broadly demonstrated efficacy for chronic pain [79]. Both treatments have shown promising benefit but there has been less evidence supporting the use of these therapies in pediatric headache than other well-established behavioral treatments.
The presence of comorbid psychiatric issues such as anxiety, depression, or ADHD can make the treatment of patients with migraine headaches more complex. A recent study found that approximately 30% of a sample of children and adolescents with chronic daily headache had a lifetime psychiatric diagnoses and having a lifetime psychiatric diagnosis was associated with poorer headache-related disability and quality of life [80].As a result, more intensive behavioral treatment for children and adolescents with a psychiatric comorbidity may be needed to focus on emotional and behavioral issues.
Pediatric psychologists can assess pain-related disability and coping difficulties and treat children and adolescents with migraine headaches. Children are often referred to a psychologist or other mental health professional if headaches are severe or impairing functioning. Unfortunately, access to this therapy is sometimes limited, but when available, should be offered to any migraine patients with significant disability requiring prevention, and specifically to patients with chronic migraine.
How should the patient be treated?
Acute Treatment
The patient reports only minimal response to ibuprofen. However, he is only taking 200 mg and does not take it until well after the headache has started. He should be instructed to take ibuprofen 600 mg (~10 mg/kg) as soon as the headache starts, along with 32 oz of a sports drink, and told that he should repeat this dose in 4 hours if his headache has not completely resolved with the first dose. As his headaches are occurring less than 3 times per week on average, he can do this with every headache. He should be given a note for school allowing him to receive the medication at school, and noting the importance of allowing him to get the medication as soon as he reports a headache. Given that some of his headaches occur on consecutive days, we suspect some may be continuations of a prior headache, so again the importance of obtaining complete pain relief from his acute medication should be stressed. If at his next follow-up visit he reports not always having complete relief with ibuprofen, we would consider trying naproxen instead, and/or adding a triptan, most likely sumatriptan 100 mg given its availability. He would need to understand that he may only use the triptan for 4 to 6 headache days/month. Depending on his response to sumatriptan, it could be used alone or in combination with his NSAID. He also may be instructed to use an NSAID alone for more mild headaches and his NSAID together with sumatriptan for more severe headaches.
Preventive Treatment
PedMIDAS score is a 25, indicating mild disability, however his migraines are frequent, occurring 1–3 times per week and clearly having a significant impact on his functioning. We would therefore recommend starting a prophylactic medication. He notes difficulty remembering to take medications as well as difficulty falling asleep, so amitriptyline would be a good choice for him. For his weight of 50.9 kg, we would start with at 12.5 mg at night and increase by 12.5 mg every 2 weeks to a dose of 50 mg (~1mg/kg/day). He and Mom should be counseled that it will likely take at least a few weeks on his goal dose before they start to see results. He should be counseled also to report to Mom immediately if he has any depressive or suicidal thoughts. Topiramate is another option, especially as it is FDA-approved for migraine prevention in children older than 12 years, although twice daily dosing may be a factor in maintaining compliance. Careful discussion with the patient and family in regards to the potential risks and benefits is important prior to starting any preventative medication.
Biobehavioral Management
The patient should be drinking 8–10 cups of non-caffeinated fluid per day and additional cups on days he plays soccer. He should be given a school note to this effect so he may carry a water bottle at school. He eats well, but the importance of not skipping breakfast should be stressed, and ideas for fitting this in should be given. The importance of a consistent bedtime routine should be stressed, with good sleep hygiene to assist with easier sleep onset. It should be made clear that he should not be using screens within an hour prior to bed. He already exercises frequently and should be commended for being active. Given his report of frequent headaches which are impairing school attendance, functioning at school, and participation in soccer activities, screening for potential co-morbid psychiatric issues and making a referral for further treatment with a pediatric pain psychologist focused on coping with chronic pain is appropriate.
Case Study 2
Initial Presentation and History
A 16-year-old right-handed girl who has been having headaches since she was 10 years old presents for evaluation. She reports unilateral mostly right-sided headaches, which are throbbing and associated with photophobia, phonophobia, osmophobia, nausea, and occasionally vomiting. She has no premonitory or aura symptoms. Her headaches typically occur once a week but she has noted that they tend to be more frequent around the time of her menses and that often these headaches do not fully respond to naproxen, which typically works to break her migraines during other times in the month. Upon further review, she believes these headaches typically start on the day or two prior to her menses, and that this happens almost every month. She often misses school due to these headaches. Her mother would like to know whether the patient should see a gynecologist to potentially be placed on birth control to control her headaches.
Physical Examination
On examination, weight was 61.5 kg (75.8 percentile), height 157.1 cm (18.4 percentile), BMI 24.91 (84 percentile), blood pressure 112/55 mm Hg, and heart rate 90 bpm. Her general physical and neurologic exam results were normal. She had tightness over the left trapezius muscles, otherwise the remainder of headache examination was unremarkable.
What is the probable diagnosis?
According to the ICHD-IIIβ, the patient meets criteria for episodic migraine without aura. Based on the history, she likely also has menstrually related migraine without aura [5].Officially, however, 3 months of prospective documentation is needed to make this diagnosis. Menstrually related migraine is included in the appendix of the ICHD-IIIβ, meaning there is ongoing debate about how it should be classified. According to the current appendix criteria, headaches should meet criteria for migraine without aura and also have documented and prospectively recorded evidence over at least 3 consecutive cycles with headaches confirmed on day 1+/– 2 (ie, 2 days prior to onset of menstruation or within the first 3 days of menstruation) in at least 2 out of 3 menstrual cycles, with migraines occurring during other times of the month as well [5]. This is distinguished from pure menstrual migraine without aura, in which migraines occur only during days 1+/–2 of menstruation but not during other times of the month [5].While we therefore cannot say that the patient meets ICHD IIIβ criteria for menstrually related migraine due to the lack of prospective documentation, her history suggests this.
Menstrually Related Migraine
An association of migraine with menses is well described in adults, occurring in up to 60% of adult female migraine patients [81].In adults, it has been observed that the migraines associated with the menstrual cycle tend to be more severe [82],associated with more nausea and vomiting [82],longer and less responsive to acute medications [83],and associated with more work-related disability [83]. Recently, analysis of data from the American Migraine Prevalence and Prevention (AMPP) Study found that women with pure menstrual migraine and menstrually associated migraine have on average higher MIDAS scores than those with non-menstrual migraine [81].
Only one study has explored menstrually related migraines in adolescents [84].It was a clinic-based study that found a similar prevalence—50% of adolescent patients who had reached menarch noted an association of migraine with their menses. Overall worse disability was not demonstrated in this study, but individual menstrual attacks were not compared with nonmenstrual attacks [84].No other studies have addressed this population, but it is clear that the pattern of menstrually related migraine exists in adolescents and it is important to recognize this pattern as these patients may require additional focused care in addition to standard migraine management.
What are treatment options?
In adults, options for more specific management of pure menstrual migraine and menstrually related migraine include intermittent prophylaxis with NSAIDs or triptans or use of hormonal contraceptives. There are no studies addressing any of these treatments in adolescents, so at this point management decisions must be based on evidence extrapolated from adult studies as well as attention to specific concerns in adolescent patients.
Generally, intermittent prophylaxis (using a medication a few days prior to and during the first few days of menses) with various medications has shown efficacy in adult studies. This approach may be more appropriate for patients with pure menstrual migraine, as there is less likelihood of precipitating medication overuse in this population. It can be considered in patients with menstrually related migraine as well if typical daily preventives have not been effective. One small open-label trial using naproxen 550 mg daily prior to and during menses (at differing schedules depending on the month) showed slightly decreased frequency and severity of headaches during that time [85].However, triptans are what are most commonly used.
Triptans
Frovatriptan is the longest-acting triptan and the one used most commonly for intermittent prophylaxis. One double-blind randomized controlled crossover study showed improvements in headache frequency, severity, and duration using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses [86].Frovatriptan 2.5 mg daily showed some efficacy as well but was not as effective as 2.5 mg BID [86].One prospective randomized placebo-controlled trial demonstrated more headache-free cycles using frovatriptan 2.5 mg BID for 6 days total starting 2 days prior to menses, as compared to placebo [87].
Naratriptan, also a longer-acting triptan, was studied in 3 prospective double-blind trials at doses of 1 mg BID used for 5 days total starting 2 days prior to menses, and all 3 showed a decrease in headache frequency as compared to placebo [88,89].Of note no efficacy was shown using 2.5 mg daily [88].
Less commonly used for intermittent prophylaxis are the shorter-acting triptans, but they have shown some efficacy as well. In one prospective study using oral sumatriptan 25 mg TID for 5 days each cycle starting 2 to 3 days before onset of menses, there seemed to be at least some improvement with sumatriptan in most cycles treated [90].Zolmitriptan was studied in one prospective double-blind placebo-controlled study using 2.5 mg BID or 2.5 mg TID for 7 days total with each cycle and was found to be associated with a significant reduction in headache frequency as compared with placebo [91].There has been one prospective trial using eletriptan 20 mg TID for 6 days total starting 2 days prior to menses, which also showed improvement in headache activity in 55% of patients [92].
Hormonal Contraceptives
In general for adult patients, hormonal contraceptives are not considered first-line treatment for menstrually related migraine. However, in patients who do not respond to other modes of treatment, or who plan to use hormonal contraception for contraceptive purposes, published expert opinions have recommended considering extended or continuous hormonal regimens [93,94].
Estrogen withdrawal during the luteal phase of the monthly cycle has long been speculated to be of importance in the pathophysiology of menstrually related migraine [93]and the relationship between hormonal contraceptives and migraine is complicated. Headache is a commonly reported side effect of combined hormonal contraceptives [95]; however, this effect seems to occur mostly during the hormone-free week [96]. It has been shown that headache occurs less frequently in women using an extended cycle regimen (84 or 168 days) as compared to those using a traditional monthly cyclic regimen [97,98].Studies addressing the use of combined hormonal contraceptives for women specifically with menstrually related migraine are limited. One small prospective randomized study showed improvement in menstrually related migraine in patients treated with a low dose (20 mcg ethinyl E[2]) oral hormonal contraceptive in both a 21/7 cycle and a 24/4 cycle, with more improvement in the group using a 24/4 cycle [99].Another showed improvement in menstrually related migraine in all study patients treated with a low-dose hormonal contraceptive (20 mcg ethinyl estradiol) on a 21/7 regimen, but with additional 0.9 mg conjugated equine estrogen during the placebo week [100].There has been one randomized placebo-controlled double-blind trial in patients with menstrually related migraine using an extended 168 hormonal regimen along with frovatriptan vs. placebo for 5 days during the hormone-free interval. Overall daily headache scores were decreased from pre-study cycles, and the increase in headaches during the hormone-free interval was lower in the frovatriptan group [101].A recent study showed that contraceptive-induced amenorrhea can be beneficial for decreasing migraine frequency in patients with menstrual migraine [102].There have been no studies addressing the use of hormonal contraceptives for migraine management in adolescents.
In adolescents, the decision regarding use of hormonal contraceptives is more complicated. There is less of a chance that adolescent patients, especially younger ones, would be planning to use hormones for contraception so their use may be solely for the purpose of migraine management. However, hormonal contraceptives are commonly used in adolescents for management of other menstrual-related disorders, such as menorrhagia, dysmenorrhea, and endometriosis, and extended cycle and continuous regimens have become more popular with adolescent providers in general [103].The general concern with using hormonal contraceptives in adolescents is that they have potential for longer-term use than adult patients, and the effects of using hormonal contraceptives long term are unknown. The major concerns are for potential interference with expected increase in bone mineral density in adolescents, effects on fertility, and risk for cancer and cardiovascular disease [103].Additionally, specifically in migraine patients, is the concern for increased stroke risk. The increased risk for ischemic stroke in patients with migraine, although more specifically with migraine with aura, is well known [104–106]and migraine has recently been shown to be a risk factor for ischemic stroke in adolescents as well [107]. In adults, the use of hormonal contraceptives in patients with migraine with aura is known to increase the risk of ischemic stroke [106],and this has not been studied in adolescents. Given the various unknowns in the use of hormonal contraceptives in patients with menstrually related migraines, we would not recommend this as first-line treatment. However, similarly to adults, in patients who do not respond to other methods of migraine management, or who seek to use hormonal contraceptives for contraception or for other menstrually related disorders, extended or continuous cycle hormonal contraceptives may be a reasonable option, at the lowest possible estrogen dose. However, migraine with aura should be screened for, and its presence should prompt reconsideration of combined hormonal contraceptive use.
How should this patient be managed?
The patient is having frequent and disabling migraines, so starting a preventive medication would be appropriate. She has migraines throughout the month in addition to during her menses, so a daily prophylactic would be more appropriate than intermittent prophylaxis surrounding her menstrual cycle only. At this point, our recommendation would be to start a daily preventive with either amitriptyline or topiramate. Given that naproxen is not breaking some of her migraines, she should be given a prescription for a triptan. Sumatriptan 100 mg would be an appropriate first choice, and she can be instructed to use it along with her naproxen at the onset of her menstrual migraines. She can use it for other migraines as well but she should be instructed not to use it more than 4 to 6 times per month. She should keep a diary for the next 3 months noting most importantly headache days as well as days of menstruation, so that a more definitive pattern can be confirmed and an official diagnosis based on ICHD-IIIβ criteria can be made. If her migraines do not improve with daily preventives, at that point discussion regarding potential for intermittent prophylaxis or trial of extended cycle hormonal contraception may be considered, although with caution and discussion of risks and benefits.
Corresponding author: Hope O’Brien, MD, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave., MLC 2015 Cincinnati, OH 45229.
Financial disclosures: None.
1. Tepper SJ, Dahlöf CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004;44:856–64.
2. Lewis DW et al. Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2002;59:490–8.
3. Martens D, Oster I, Gottschlling S, et al. Cerebral MRI and EEG studies in the initial management of pediatric headaches. Swiss Med Wkly 2012;142:w13625.
4. Linder SL. Understanding the comprehensive pediatric headache examination. Pediatr Ann 2005;34:442–6.
5. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
6. Senbil N, Gürer YK, Uner C, et al. Sinusitis in children and adolescents with chronic or recurrent headache: a case-control study. J Headache Pain 2008;9:33–6.
7. Gelfand AA, Reider AC, Goadsby PJ. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology 2013;81:431–6.
8. Blau JN. Water deprivation: a new migraine precipitant. Headache 2005;45:757–9.
9. Martins IP, Gouveia RG. More on water and migraine. Cephalalgia 2007;27:372–4.
10. Hämäläinen ML, Hoppu K, Valkeila E, et al. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology 1997;48:103–7.
11. Lewis DW, Kellsein D, Sahl G, et al. Children’s ibuprofen suspension for the acute treatment of pediatric migraine. Headache 2002;42:780–6.
12. Lipton RB, Golstein J, Baggish JS, et al. Aspirin is efficacious for the treatment of acute migraine. Headache 2005;45:283–92.
13. Linder SL, Mathew NT, Cady RK, et al. Efficacy and tolerability of almotriptan in adolescents: a randomized, double-blind, placebo-controlled trial. Headache 2008;48:1326–36.
14. Ahonen K, Hämäläinen ML, Eerola M, et al. A randomized trial of rizatriptan in migraine attacks in children. Neurology 2006;67:1135–40.
15. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Cephalalgia 2012;32:750–65.
16. Winner P, Lewis D, Visser WH, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: a randomized, double-blind, placebo-controlled study. Headache 2002;42:49–55.
17. Hewitt DJ, Pearlman E, Hämäläinen M, et al. Long-term open-label safety study of rizatriptan acute treatment in pediatric migraineurs. Headache 2013;53:104–17.
18. Derosier FJ, Lewis D, Hershey AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics 2012;129:e1411–20.
19. McDonald SA, Hershey AD, Pearlman E, et al. Long-term evaluation of sumatriptan and naproxen sodium for the acute treatment of migraine in adolescents. Headache 2011;51:1374-87.
20. Lewis DW, Winner P, Hershey AD, et al. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics 2007;
120:390–6.
21. Linder SL, Dowson AJ. Zolmitriptan provides effective migraine relief in adolescents. Int J Clin Pract 2000;54:466–9.
22. Winner P, Rothner AD, Saper J, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106:989–97.
23. Rothner AD, Winner P, Nett R, et al. One-year tolerability and efficacy of sumatriptan nasal spray in adolescents with migraine: results of a multicenter, open-label study. Clin Ther 2000;22:1533–46.
24. Ahonen K, Hämäläinen ML, Rantala H, et al. Nasal sumatriptan is effective in treatment of migraine attacks in children: A randomized trial. Neurology 2004;62:883–7.
25. Natarajan S, Jabbour JT, Webster CJ, et al. Long-term tolerability of sumatriptan nasal spray in adolescent patients with migraine. Headache 2004;44:969–77.
26. Winner P, Rothner AD, Wooten JD, et al. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache 2006;46:212–22.
27. Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology 1997;48:1100–3.
28. Fujita M, Sato K, Nishioka H, et al. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia 2014;34:365–75.
29. Winner P, Linder SL, Lipton RB, et al. Eletriptan for the acute treatment of migraine in adolescents: results of a double-blind, placebo-controlled trial. Headache 2007;47:511–8.
30. Rothner A. Efficacy and safety of naratriptan tablets in adolescent migraine [abstract]. J Neurol Sci 1997;150:S106.
31. Elkind AH, Wade A, Ishkanian G. Pharmacokinetics of frovatriptan in adolescent migraineurs. J Clin Pharmacol 2004;44:1158–65.
32. Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep 2011;15:70–8.
33. Winner P, Pearlman EM, Linder SL, et al. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:1304–12.
34. Lakshmi CV, Singhi P, Malhi P, et al. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol 2007;22:829–35.
35. Lewis D, Winner P, Saper J, et al.Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 2009;123:924–34.
36. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of topiramate in the prevention of childhood headaches. Headache 2002;42:810–8.
37. Campistol J, Campos J, Casas C, et al. Topiramate in the prophylactic treatment of migraine in children. J Child Neurol 2005;20:251–3.
38. Unalp A, Uran N, Oztürk A. Comparison of the effectiveness of topiramate and sodium valproate in pediatric migraine. J Child Neurol 2008;23:1377–81.
39. Cruz MJ, Valencia I, Legido A, et al. Efficacy and tolerability of topiramate in pediatric migraine. Pediatr Neurol 2009;41:167–70.
40. Kim H, Byun SH, Kim JS, et al. Comparison of flunarizine and topiramate for the prophylaxis of pediatric migraines. Eur J Paediatr Neurol 2013;17:45–9.
41. Fallah R, Divanizadeh MS, Karimi M, et al. Topiramate and propranolol for prophylaxis of migraine. Indian J Pediatr 2013;80:920–4.
42. Tonekaboni SH, Ghazavi A, Fayyazi A, et al. Prophylaxis of childhood migraine: topiramate versus propranolol. Iran J Child Neurol 2013;7:9–14.
43. Hershey AD, Powers SW, Coffey CS, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double blinded, placebo controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in prevention of childhood and adolescent migraine. Headache 2013;53:799–816.
44. Hershey AD, Powers SW, Bentti AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539–49.
45. Lewis DW, Diamond S, Scott D, et al. Prophylactic treatment of pediatric migraine. Headache 2004;44:230–7.
46. Caruso JM, Brown WD, Exil G, et al. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000 Sep;40:672–6.
47. Pakalnis A, Greenberg G, Drake ME Jr, et al. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731–4.
48. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819–22.
49. Miller GS. Efficacy and safety of levetiracetem in pediatric migraine. Headache 2004;44:238–43.
50. Pakalnis A, Kring D, Meier L. Levetiracetam prophylaxis in pediatric migraine--an open label study. Headache 2007;43:427–30.
51. Pakalnis A, Kring D. Zonisamide prophylaxis in refractory pediatric headache. Headache 2006;46:804–7.
52. Apostol G, Cady RK, Laforet GA, et al. Divalproex extended-release in adolescent migraine prophylaxis: results of a randomized, double-blind, placebo-controlled study. Headache 2008;48:1012–25.
53. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol 1974;50:109–15.
54. Forsythe WI, Gillies D, Sills MA. Propanolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737–41.
55. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593–7.
56. Sorge F, DeSimone R, Marano E, et al. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled crossover study. Cephalalgia 1988;8:1–6.
57. Guidetti V, Moscato D, Ottaviano S, et al. Flunarizine and migraine in childhood: an evaluation of endocrine function. Cephalalgia 1987;7:263–6.
58. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and complementary treatments for episodic migraine treatment in adults: Report of the quality standards subcommittee of the American Academy of Neurology and American Headache Society. Neurology 2012; 78:1346–53.
59. Oelkers-Ax R, Leins A, Parzer P, et al. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain 2008;12:301–13.
60. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache 2005;45:196–203.
61. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 2007;47:73–80.
62. Slater SK, Nelson TD, Kabbouche MA, et al. A randomized, double-blind, crossover, add-on study of coenzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 2011; 31: 897–905.
63. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache 2003;43:601–10.
64. Castelli S, Meossi C, Domenici R, et al. [Magnesium in the prophylaxis of primary headache and other periodic disorders in children]. Pediatr Med Chir 1993;15:481–8. Italian.
65. Condò M, Posar A, Arbizzani A, et al. Riboflavin prophylaxis in pediatric and adolescent migraine. J Headache Pain 2009;10:361–5.
66. MacLennan SC, Wade FM, Forrest KM, et al. High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. J Child Neurol 2008;23:1300–4.
67. Bruijn J, Duivenvoorden H, Passchier J, et al. Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia 2010;30:1426–34.
68. Robbins L. Precipitating factors in migraine: a retrospective review of 494 patients. Headache 1994;34:214–6.
69. Leviton A, Slack WV, Masek B, et al. A computerized behavioral assessment for children with headaches. Headache 1984;24:182–5.
70. Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999;19 Suppl 25:57–9.
71. Lynch-Jordan AM, Sil S, Peugh J, et al. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain 2014;155:1955–61.
72. Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2014;5:CD003968.
73. Huguet A, McGrath PJ, Stinson J, et al. Efficacy of psychological treatment for headaches: an overview of systematic reviews and analysis of potential modifiers of treatment efficacy. Clin J Pain 2014;30:353–69.
74. Kropp P, Meyer B, Landgraf M, et al. Headache in children: update on biobehavioral treatments. Neuropediatrics 2013;44:20–4.
75. Kröner-Herwig B. Psychological treatments for pediatric headache. Expert Rev Neurother 2011;11:403–10.
76. Powers SW, Andrasik F. Biobehavioral treatment, disability, and psychological effects of pediatric headache. Pediatr Ann 2005;34:461–5.
77. Hermann C, Blanchard EB. Biofeedback in the treatment of headache and other childhood pain. Appl Psychophysiol Biofeedback 2002;27:143–62.
78. Powers SW, Kashikar-Zuck SM, Hershey AD, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA 2013;310:2622–30.
79. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep 2015;19:13.
80. Slater SK, O’Brien HL, Hershey AD, et al. Psychiatric comorbidity in pediatric chronic daily headache. Cephalalgia 2012;32: 1116–22.
81. Pavlovic JM, Stewart WF, Bruce CA, et al. Burden of migraine related to menses: results from the AMPP study. J Headache Pain 2015;16:24.
82. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004;63:351–3.
83. Granella F, Sances G, Allais G. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 2004;24:707–16.
84. Crawford MJ, Lehman L, Slater S, et al. Menstrual migraine in adolescents. Headache 2009;49:341–7.
85. Allais G, Bussone G, De Lorenzo C, et al. Naproxen sodium in short-term prophylaxis of pure menstrual migraine: pathophysiological and clinical considerations. Neurol Sci 2007;28(Suppl 2):S225–8.
86. Silberstein S, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology 2004;63:261–9.
87. Brandes JL, Poole A, Kallela M, et al. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia 2009;29:1133–48.
88. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache 2001;41:248–56.
89. Mannix LK, Savani N, Landy S, et al. Efficacy and tolerability of naratriptan for short-term prevention of menstrually related migraine: data from two randomized, double-blind, placebo-controlled studies. Headache 2007;47:1037–49.
90. Newman LC, Lipton RB, Lay CL, et al. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology 1998;51:307–9.
91. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs 2008;22:877–86.
92. Marcus DA, Bernstein CD, Sullivan EA, et al. Perimenstrual eletriptan prevents menstrual migraine: an open-label study. Headache 2010;50:551–6.
93. Silberstein S, Patel S. Menstrual migraine: an updated review on hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother 2014;15:2063–70.
94. MacGregor EA. Migraine management during menstruation and menopause. Continuum (Minneap Minn) 2015;21(4 Headache):990–1003.
95. Aegidius K, Zwart JA, Hagen K, et al. Oral contraceptives and increased headache prevalence: The Head-HUNT Study. Neurology 2006;66:349–53.
96. Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol 2000;95:261–6.
97. LaGuardia KD, Fisher AC, Bainbridge JD, et al. Suppression of estrogen-withdrawal headache with extended transdermal contraception. Fertil Steril 2005;83:1875–7.
98. Sulak P,Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: Impact of eliminating the standard 7-day placebo interval. Headache 2007;47:27–37.
99. De Leo V, Scolaro V, Musacchio MC, et al. Combined oral contraceptives in women with menstrual migraine without aura. Fertil Steril 2011;96:917–20.
100. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004;97:819–22.
101. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health (Larchmt) 2014;23:310–7.
102. Vetvik KG, MacGregor EA, Lundqvist C, et al. Contraceptive-induced amenorrhea leads to reduced migraine frequency in women with menstrual migraine without aura. J Headache Pain 2014;15:30.
103. Sucato GS, Gerschultz KL. Extended cycle hormonal contraception in adolescents. Curr Opin Obstet Gynecol 2005;17:461–5.
104. Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 2010;123:612–24.
105. Bigal ME, Kurth T, Santanello N, et al. Migraine and cardiovascular disease: a population-based study. Neurology 2010;74:628–35.
106. Schurks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914.
107. Gelfand AA, Fullerton HJ, Jacobson A, et al. Is migraine a risk factor for pediatric stroke? Cephalalgia 2015;35:1252–60.
1. Tepper SJ, Dahlöf CG, Dowson A, et al. Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the Landmark Study. Headache 2004;44:856–64.
2. Lewis DW et al. Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2002;59:490–8.
3. Martens D, Oster I, Gottschlling S, et al. Cerebral MRI and EEG studies in the initial management of pediatric headaches. Swiss Med Wkly 2012;142:w13625.
4. Linder SL. Understanding the comprehensive pediatric headache examination. Pediatr Ann 2005;34:442–6.
5. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808.
6. Senbil N, Gürer YK, Uner C, et al. Sinusitis in children and adolescents with chronic or recurrent headache: a case-control study. J Headache Pain 2008;9:33–6.
7. Gelfand AA, Reider AC, Goadsby PJ. Cranial autonomic symptoms in pediatric migraine are the rule, not the exception. Neurology 2013;81:431–6.
8. Blau JN. Water deprivation: a new migraine precipitant. Headache 2005;45:757–9.
9. Martins IP, Gouveia RG. More on water and migraine. Cephalalgia 2007;27:372–4.
10. Hämäläinen ML, Hoppu K, Valkeila E, et al. Ibuprofen or acetaminophen for the acute treatment of migraine in children: a double-blind, randomized, placebo-controlled, crossover study. Neurology 1997;48:103–7.
11. Lewis DW, Kellsein D, Sahl G, et al. Children’s ibuprofen suspension for the acute treatment of pediatric migraine. Headache 2002;42:780–6.
12. Lipton RB, Golstein J, Baggish JS, et al. Aspirin is efficacious for the treatment of acute migraine. Headache 2005;45:283–92.
13. Linder SL, Mathew NT, Cady RK, et al. Efficacy and tolerability of almotriptan in adolescents: a randomized, double-blind, placebo-controlled trial. Headache 2008;48:1326–36.
14. Ahonen K, Hämäläinen ML, Eerola M, et al. A randomized trial of rizatriptan in migraine attacks in children. Neurology 2006;67:1135–40.
15. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized, double-blind, placebo-controlled trial using a novel adaptive enrichment design. Cephalalgia 2012;32:750–65.
16. Winner P, Lewis D, Visser WH, et al. Rizatriptan 5 mg for the acute treatment of migraine in adolescents: a randomized, double-blind, placebo-controlled study. Headache 2002;42:49–55.
17. Hewitt DJ, Pearlman E, Hämäläinen M, et al. Long-term open-label safety study of rizatriptan acute treatment in pediatric migraineurs. Headache 2013;53:104–17.
18. Derosier FJ, Lewis D, Hershey AD, et al. Randomized trial of sumatriptan and naproxen sodium combination in adolescent migraine. Pediatrics 2012;129:e1411–20.
19. McDonald SA, Hershey AD, Pearlman E, et al. Long-term evaluation of sumatriptan and naproxen sodium for the acute treatment of migraine in adolescents. Headache 2011;51:1374-87.
20. Lewis DW, Winner P, Hershey AD, et al. Efficacy of zolmitriptan nasal spray in adolescent migraine. Pediatrics 2007;
120:390–6.
21. Linder SL, Dowson AJ. Zolmitriptan provides effective migraine relief in adolescents. Int J Clin Pract 2000;54:466–9.
22. Winner P, Rothner AD, Saper J, et al. A randomized, double-blind, placebo-controlled study of sumatriptan nasal spray in the treatment of acute migraine in adolescents. Pediatrics 2000;106:989–97.
23. Rothner AD, Winner P, Nett R, et al. One-year tolerability and efficacy of sumatriptan nasal spray in adolescents with migraine: results of a multicenter, open-label study. Clin Ther 2000;22:1533–46.
24. Ahonen K, Hämäläinen ML, Rantala H, et al. Nasal sumatriptan is effective in treatment of migraine attacks in children: A randomized trial. Neurology 2004;62:883–7.
25. Natarajan S, Jabbour JT, Webster CJ, et al. Long-term tolerability of sumatriptan nasal spray in adolescent patients with migraine. Headache 2004;44:969–77.
26. Winner P, Rothner AD, Wooten JD, et al. Sumatriptan nasal spray in adolescent migraineurs: a randomized, double-blind, placebo-controlled, acute study. Headache 2006;46:212–22.
27. Hämäläinen ML, Hoppu K, Santavuori P. Sumatriptan for migraine attacks in children: a randomized placebo-controlled study. Do children with migraine respond to oral sumatriptan differently from adults? Neurology 1997;48:1100–3.
28. Fujita M, Sato K, Nishioka H, et al. Oral sumatriptan for migraine in children and adolescents: a randomized, multicenter, placebo-controlled, parallel group study. Cephalalgia 2014;34:365–75.
29. Winner P, Linder SL, Lipton RB, et al. Eletriptan for the acute treatment of migraine in adolescents: results of a double-blind, placebo-controlled trial. Headache 2007;47:511–8.
30. Rothner A. Efficacy and safety of naratriptan tablets in adolescent migraine [abstract]. J Neurol Sci 1997;150:S106.
31. Elkind AH, Wade A, Ishkanian G. Pharmacokinetics of frovatriptan in adolescent migraineurs. J Clin Pharmacol 2004;44:1158–65.
32. Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep 2011;15:70–8.
33. Winner P, Pearlman EM, Linder SL, et al. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache 2005;45:1304–12.
34. Lakshmi CV, Singhi P, Malhi P, et al. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol 2007;22:829–35.
35. Lewis D, Winner P, Saper J, et al.Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 2009;123:924–34.
36. Hershey AD, Powers SW, Vockell AL, et al. Effectiveness of topiramate in the prevention of childhood headaches. Headache 2002;42:810–8.
37. Campistol J, Campos J, Casas C, et al. Topiramate in the prophylactic treatment of migraine in children. J Child Neurol 2005;20:251–3.
38. Unalp A, Uran N, Oztürk A. Comparison of the effectiveness of topiramate and sodium valproate in pediatric migraine. J Child Neurol 2008;23:1377–81.
39. Cruz MJ, Valencia I, Legido A, et al. Efficacy and tolerability of topiramate in pediatric migraine. Pediatr Neurol 2009;41:167–70.
40. Kim H, Byun SH, Kim JS, et al. Comparison of flunarizine and topiramate for the prophylaxis of pediatric migraines. Eur J Paediatr Neurol 2013;17:45–9.
41. Fallah R, Divanizadeh MS, Karimi M, et al. Topiramate and propranolol for prophylaxis of migraine. Indian J Pediatr 2013;80:920–4.
42. Tonekaboni SH, Ghazavi A, Fayyazi A, et al. Prophylaxis of childhood migraine: topiramate versus propranolol. Iran J Child Neurol 2013;7:9–14.
43. Hershey AD, Powers SW, Coffey CS, et al. Childhood and Adolescent Migraine Prevention (CHAMP) study: a double blinded, placebo controlled, comparative effectiveness study of amitriptyline, topiramate, and placebo in prevention of childhood and adolescent migraine. Headache 2013;53:799–816.
44. Hershey AD, Powers SW, Bentti AL, et al. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache 2000;40:539–49.
45. Lewis DW, Diamond S, Scott D, et al. Prophylactic treatment of pediatric migraine. Headache 2004;44:230–7.
46. Caruso JM, Brown WD, Exil G, et al. The efficacy of divalproex sodium in the prophylactic treatment of children with migraine. Headache 2000 Sep;40:672–6.
47. Pakalnis A, Greenberg G, Drake ME Jr, et al. Pediatric migraine prophylaxis with divalproex. J Child Neurol 2001;16:731–4.
48. Serdaroglu G, Erhan E, Tekgul H, et al. Sodium valproate prophylaxis in childhood migraine. Headache 2002;42:819–22.
49. Miller GS. Efficacy and safety of levetiracetem in pediatric migraine. Headache 2004;44:238–43.
50. Pakalnis A, Kring D, Meier L. Levetiracetam prophylaxis in pediatric migraine--an open label study. Headache 2007;43:427–30.
51. Pakalnis A, Kring D. Zonisamide prophylaxis in refractory pediatric headache. Headache 2006;46:804–7.
52. Apostol G, Cady RK, Laforet GA, et al. Divalproex extended-release in adolescent migraine prophylaxis: results of a randomized, double-blind, placebo-controlled study. Headache 2008;48:1012–25.
53. Ludvigsson J. Propranolol used in prophylaxis of migraine in children. Acta Neurol 1974;50:109–15.
54. Forsythe WI, Gillies D, Sills MA. Propanolol (‘Inderal’) in the treatment of childhood migraine. Dev Med Child Neurol 1984;26:737–41.
55. Olness K, MacDonald JT, Uden DL. Comparison of self-hypnosis and propranolol in the treatment of juvenile classic migraine. Pediatrics 1987;79:593–7.
56. Sorge F, DeSimone R, Marano E, et al. Flunarizine in prophylaxis of childhood migraine. A double-blind, placebo-controlled crossover study. Cephalalgia 1988;8:1–6.
57. Guidetti V, Moscato D, Ottaviano S, et al. Flunarizine and migraine in childhood: an evaluation of endocrine function. Cephalalgia 1987;7:263–6.
58. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and complementary treatments for episodic migraine treatment in adults: Report of the quality standards subcommittee of the American Academy of Neurology and American Headache Society. Neurology 2012; 78:1346–53.
59. Oelkers-Ax R, Leins A, Parzer P, et al. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain 2008;12:301–13.
60. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache 2005;45:196–203.
61. Hershey AD, Powers SW, Vockell AL, et al. Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache 2007;47:73–80.
62. Slater SK, Nelson TD, Kabbouche MA, et al. A randomized, double-blind, crossover, add-on study of coenzyme Q10 in the prevention of pediatric and adolescent migraine. Cephalalgia 2011; 31: 897–905.
63. Wang F, Van Den Eeden SK, Ackerson LM, et al. Oral magnesium oxide prophylaxis of frequent migrainous headache in children: a randomized, double-blind, placebo-controlled trial. Headache 2003;43:601–10.
64. Castelli S, Meossi C, Domenici R, et al. [Magnesium in the prophylaxis of primary headache and other periodic disorders in children]. Pediatr Med Chir 1993;15:481–8. Italian.
65. Condò M, Posar A, Arbizzani A, et al. Riboflavin prophylaxis in pediatric and adolescent migraine. J Headache Pain 2009;10:361–5.
66. MacLennan SC, Wade FM, Forrest KM, et al. High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. J Child Neurol 2008;23:1300–4.
67. Bruijn J, Duivenvoorden H, Passchier J, et al. Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia 2010;30:1426–34.
68. Robbins L. Precipitating factors in migraine: a retrospective review of 494 patients. Headache 1994;34:214–6.
69. Leviton A, Slack WV, Masek B, et al. A computerized behavioral assessment for children with headaches. Headache 1984;24:182–5.
70. Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalalgia 1999;19 Suppl 25:57–9.
71. Lynch-Jordan AM, Sil S, Peugh J, et al. Differential changes in functional disability and pain intensity over the course of psychological treatment for children with chronic pain. Pain 2014;155:1955–61.
72. Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2014;5:CD003968.
73. Huguet A, McGrath PJ, Stinson J, et al. Efficacy of psychological treatment for headaches: an overview of systematic reviews and analysis of potential modifiers of treatment efficacy. Clin J Pain 2014;30:353–69.
74. Kropp P, Meyer B, Landgraf M, et al. Headache in children: update on biobehavioral treatments. Neuropediatrics 2013;44:20–4.
75. Kröner-Herwig B. Psychological treatments for pediatric headache. Expert Rev Neurother 2011;11:403–10.
76. Powers SW, Andrasik F. Biobehavioral treatment, disability, and psychological effects of pediatric headache. Pediatr Ann 2005;34:461–5.
77. Hermann C, Blanchard EB. Biofeedback in the treatment of headache and other childhood pain. Appl Psychophysiol Biofeedback 2002;27:143–62.
78. Powers SW, Kashikar-Zuck SM, Hershey AD, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA 2013;310:2622–30.
79. Smitherman TA, Wells RE, Ford SG. Emerging behavioral treatments for migraine. Curr Pain Headache Rep 2015;19:13.
80. Slater SK, O’Brien HL, Hershey AD, et al. Psychiatric comorbidity in pediatric chronic daily headache. Cephalalgia 2012;32: 1116–22.
81. Pavlovic JM, Stewart WF, Bruce CA, et al. Burden of migraine related to menses: results from the AMPP study. J Headache Pain 2015;16:24.
82. MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004;63:351–3.
83. Granella F, Sances G, Allais G. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 2004;24:707–16.
84. Crawford MJ, Lehman L, Slater S, et al. Menstrual migraine in adolescents. Headache 2009;49:341–7.
85. Allais G, Bussone G, De Lorenzo C, et al. Naproxen sodium in short-term prophylaxis of pure menstrual migraine: pathophysiological and clinical considerations. Neurol Sci 2007;28(Suppl 2):S225–8.
86. Silberstein S, Elkind AH, Schreiber C, et al. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology 2004;63:261–9.
87. Brandes JL, Poole A, Kallela M, et al. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia 2009;29:1133–48.
88. Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache 2001;41:248–56.
89. Mannix LK, Savani N, Landy S, et al. Efficacy and tolerability of naratriptan for short-term prevention of menstrually related migraine: data from two randomized, double-blind, placebo-controlled studies. Headache 2007;47:1037–49.
90. Newman LC, Lipton RB, Lay CL, et al. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology 1998;51:307–9.
91. Tuchman MM, Hee A, Emeribe U, et al. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs 2008;22:877–86.
92. Marcus DA, Bernstein CD, Sullivan EA, et al. Perimenstrual eletriptan prevents menstrual migraine: an open-label study. Headache 2010;50:551–6.
93. Silberstein S, Patel S. Menstrual migraine: an updated review on hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother 2014;15:2063–70.
94. MacGregor EA. Migraine management during menstruation and menopause. Continuum (Minneap Minn) 2015;21(4 Headache):990–1003.
95. Aegidius K, Zwart JA, Hagen K, et al. Oral contraceptives and increased headache prevalence: The Head-HUNT Study. Neurology 2006;66:349–53.
96. Sulak PJ, Scow RD, Preece C, et al. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol 2000;95:261–6.
97. LaGuardia KD, Fisher AC, Bainbridge JD, et al. Suppression of estrogen-withdrawal headache with extended transdermal contraception. Fertil Steril 2005;83:1875–7.
98. Sulak P,Willis S, Kuehl T, Coffee A, Clark J. Headaches and oral contraceptives: Impact of eliminating the standard 7-day placebo interval. Headache 2007;47:27–37.
99. De Leo V, Scolaro V, Musacchio MC, et al. Combined oral contraceptives in women with menstrual migraine without aura. Fertil Steril 2011;96:917–20.
100. Calhoun AH. A novel specific prophylaxis for menstrual-associated migraine. South Med J 2004;97:819–22.
101. Coffee AL, Sulak PJ, Hill AJ, et al. Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health (Larchmt) 2014;23:310–7.
102. Vetvik KG, MacGregor EA, Lundqvist C, et al. Contraceptive-induced amenorrhea leads to reduced migraine frequency in women with menstrual migraine without aura. J Headache Pain 2014;15:30.
103. Sucato GS, Gerschultz KL. Extended cycle hormonal contraception in adolescents. Curr Opin Obstet Gynecol 2005;17:461–5.
104. Spector JT, Kahn SR, Jones MR, et al. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med 2010;123:612–24.
105. Bigal ME, Kurth T, Santanello N, et al. Migraine and cardiovascular disease: a population-based study. Neurology 2010;74:628–35.
106. Schurks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914.
107. Gelfand AA, Fullerton HJ, Jacobson A, et al. Is migraine a risk factor for pediatric stroke? Cephalalgia 2015;35:1252–60.
Concomitant Sensitization to Inhaled Budesonide and Oral Nystatin Presenting as Allergic Contact Stomatitis and Systemic Allergic Contact Dermatitis
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8
In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
Practice Points
- When lesions develop in the oral cavity during treatment with inhaled corticosteroids, delayed contact allergy should be considered in the differential diagnosis along with fungal infection.
- Although it generally is not considered to be allergenic due to its poor intestinal absorption, oral nystatin may induce systemic allergic disorders.
- All drugs involved in a presumed allergic reaction must be evaluated since concomitant sensitization to multiple drugs could be present. Patch and challenge testing should be conducted to diagnose allergic contact dermatitis and assess drug cross-reactivity.
Manic and nonadherent, with a diagnosis of breast cancer
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Diagnosis, mood changes
Ms. A, age 58, is a white female with a history of chronic bipolar I disorder who is being evaluated as a new patient in an academic psychiatric clinic. Recently, she was diagnosed with ER+, PR+, and HER2+ ductal carcinoma. She does not take her prescribed mood stabilizers.
After her cancer diagnosis, Ms. A experiences new-onset agitation, including irritable mood, suicidal thoughts, tearfulness, decreased need for sleep, fast speech, excessive spending, and anorexia. She reports that she hears the voice of God telling her that she could cure her breast cancer through prayer and herbal remedies. Her treatment team, comprising her primary care provider and surgical oncologist, consider several medication adjustments, but are unsure of their effects on Ms. A’s mental health, progression of cancer, and cancer treatment.
What is the most likely cause of Ms. A’s psychiatric symptoms?
a) anxiety from having a diagnosis of cancer
b) stress reaction
c) panic attack
d) manic or mixed phase of bipolar I disorder
The authors’ observations
Treating breast cancer with concurrent severe mental illness is complex and challenging for the patient, family, and health care providers. Mental health and oncology clinicians must collaborate when treating these patients because of overlapping pathophysiology and medication interactions. A comprehensive evaluation is required to tease apart whether a patient is simply demoralized by her new diagnosis, or if a more serious mood disorder is present.
Worldwide, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women.1 The mean age of women diagnosed with breast cancer is 61 years; 61% of these women are alive 15 years after diagnosis, representing the largest group of female cancer survivors.
The incidence of breast cancer is reported to be higher in women with bipolar disorder compared with the general population.2-4 This positive correlation might be associated with a high rate of smoking, poor health-related behaviors, and, possibly, medication side effects. A genome-wide association study found significant associations between bipolar disorder and the breast cancer-related genes BRCA2 and PALB2.5
Antipsychotics and prolactin
Antipsychotics play an important role in managing bipolar disorder; several, however, are known to raise the serum prolactin level 10- to 20-fold. A high prolactin level could be associated with progression of breast cancer. All antipsychotics have label warnings regarding their use in women with breast cancer.
The prolactin receptor is overexpressed in >95% of breast cancer cells, regardless of estrogen-receptor status. The role of prolactin in development of new breast cancer is open to debate. The effect of a high prolactin level in women with diagnosed breast cancer is unknown, although available preclinical data suggest that high levels should be avoided. Psychiatric clinicians should consider checking the serum prolactin level or switching to a treatment strategy that avoids iatrogenic prolactin elevation. This risk must be carefully weighed against the mood-stabilizing properties of antipsychotics.6
TREATMENT Consider comorbidities
Ms. A receives supportive psychotherapy in addition to quetiapine, 400 mg/d, and valproic acid, 1,500 mg/d. This regimen helps her successfully complete the initial phase of breast cancer treatment, which consists of a single mastectomy, adjuvant chemotherapy (doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab). She is now on endocrine therapy with tamoxifen.
Ms. A, calm, much improved mood symptoms, and euthymic, has questions regarding her mental health, cancer prognosis, and potential medication side effects with continued cancer treatment.
Which drug used to treat breast cancer might relieve Ms. A’s manic symptoms?
a) cyclophosphamide
b) tamoxifen
c) trastuzumab
d) pamidronate
The authors’ observations
Recent evidence suggests that tamoxifen reduces symptoms of bipolar mania more rapidly than many standard medications for bipolar disorder. Tamoxifen is the only available centrally active protein kinase C (PKC) inhibitor,7 although lithium and valproic acid also might inhibit PKC activity. PKC regulates presynaptic and postsynaptic neurotransmission, neuronal excitability, and neurotransmitter release. PKC is thought to be overactive during mania, possibly because of an increase in membrane-bound PKC and PKC translocation from the cytosol to membrane.7,8
Preliminary clinical trials suggest that tamoxifen significantly reduces manic symptoms in patients with bipolar disorder within 5 days of initiation.7 These findings have been confirmed in animal studies and in 1 single-blind and 4 double-blind placebo-controlled clinical studies over the past 15 years.9
Tamoxifen is a selective estrogen-receptor modulator used to prevent recurrence in receptor-positive breast cancer. Cytochrome P450 (CYP) 2D6 is the principal enzyme that converts tamoxifen to its active metabolite, endoxifen. Inhibition of tamoxifen conversion to endoxifen by CYP2D6 inhibitors could decrease the efficacy of tamoxifen therapy and might increase the risk of breast cancer recurrence. Although antidepressants generally are not recommended as a first-line agent for bipolar disorder, several selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are potent, moderate, or mild inhibitors of CYP2D610 (Table 1). Approximately 7% of women have nonfunctional CYP2D6 alleles and have a lower endoxifen level.11
Treating breast cancer
The mainstays of breast cancer treatment are surgery, radiation therapy, chemotherapy, hormone therapy, and targeted monoclonal antibody therapy. The protocol of choice depends on the stage of cancer, estrogen receptor status, expression of human epidermal growth factor receptor 2 (HER-2), treatment history, and the patient’s menopausal status. Overexpression of HER-2 oncoprotein, found in 25% to 30% of breast cancers, has been shown to promote cell transformation. HER-2 overexpression is associated with aggressive tumor phenotypes, lymph node involvement, and resistance to chemotherapy and endocrine therapy. Therefore, the HER-2 oncoprotein is a key target for treatment. Often, several therapies are combined to prevent recurrence of disease.
Breast cancer treatment often can cause demoralization, menopausal symptoms, sleep disturbance, impaired sexual function, infertility, and disturbed body image. It also can trigger psychiatric symptoms in patients with, or without, a history of mental illness.
Trastuzumab is a recombinant humanized monoclonal antibody against HER-2, and is approved for treating HER-2 positive breast cancer. However, approximately 50% of patients with HER-2 overexpression do not respond to trastuzumab alone or combined with chemotherapy, and nearly all patients develop resistance to trastuzumab, leading to recurrence.12 This medication is still used in practice, and research regarding antiepileptic drugs working in synergy with this monoclonal antibody is underway.
OUTCOME Stability achieved
Quetiapine and valproic acid are first-line choices for Ms. A because (1) she would be on long-term tamoxifen to maintain cancer remission maintenance and (2) she is in a manic phase of bipolar disorder. Tamoxifen also could improve her manic symptoms. This medication regimen might enhance the action of cancer treatments and also could reduce adverse effects of cancer treatment, such as insomnia associated with tamoxifen.
After the team educates Ms. A about how her psychiatric medications could benefit her cancer treatment, she becomes more motivated to stay on her regimen. Ms. A does well on these medications and after 18 months has not experienced exacerbation of psychiatric symptoms or recurrence of cancer.
The authors’ observations
There are 3 major classes of mood stabilizers for treating bipolar disorder: lithium, antiepileptic drugs, and atypical antipsychotics.13 In a setting of cancer, mood stabilizers are prescribed for managing mania or drug-induced agitation or anxiety associated with steroid use, brain metastases, and other medical conditions. They also can be used to treat neuropathic pain and hot flashes and seizure prophylaxis.13
Valproic acid
Valproic acid can help treat mood lability, impulsivity, and disinhibition, whether these symptoms are due to primary psychiatric illness or secondary to cancer metastasis. It is a first-line agent for manic and mixed bipolar states, and can be titrated quickly to achieve optimal benefit. Valproic acid also has been described as a histone deacetylase (HDAC) inhibitor, known to attenuate apoptotic activity, making it of interest as a treatment for cancer.14 HDAC inhibitors have been shown to:
- induce differentiation and cell cycle arrest
- activate the extrinsic or intrinsic pathways of apoptosis
- inhibit invasion, migration, and angiogenesis in different cancer cell lines.15
In regard to breast cancer, valproic acid inhibits growth of cell lines independent of estrogen receptors, increases the action of such breast cancer treatments as tamoxifen, raloxifene, fulvestrant, and letrozole, and induces solid tumor regression.14 Valproic acid also reduces cancer cell viability and could act as a powerful antiproliferative agent in estrogen-sensitive breast cancer cells.16
Valproic acid reduces cell growth-inducing apoptosis and cell cycle arrest in ERα-positive breast cancer cells, although it has no significant apoptotic effect in ERα-negative cells.16 However, evidence does support the ability of valproic acid to restore an estrogen-sensitive phenotype in ERα-negative breast cancer cells, allowing successful treatment with the anti-estrogen tamoxifen in vitro.10
Antipsychotics
Antipsychotics act as dopamine D2 receptor antagonists within the hypothalamic-pituitary-adrenal axis, thus increasing the serum prolactin level. Among atypicals, risperidone and its active metabolite, paliperidone, produce the greatest increase in the prolactin level, whereas quetiapine, clozapine, and aripiprazole minimally elevate the prolactin level.
Hyperprolactinemia correlates with rapid breast cancer progression and inferior prognosis, regardless of breast cancer receptor typing. Therefore, prolactin-sparing antipsychotics are preferred when treating a patient with comorbid bipolar disorder and breast cancer. Checking the serum prolactin level might help guide treatment. The literature is mixed regarding antipsychotic use and new mammary tumorigenesis; current research does not support antipsychotic choice based on future risk of breast cancer.6
Other adverse effects from antipsychotic use for bipolar disorder could have an impact on patients with breast cancer. Several of these medications could ameliorate side effects of advanced cancer and chemotherapy. Quetiapine, for example, might improve tamoxifen-induced insomnia in women with breast cancer because of its high affinity for serotonergic receptors, thus enhancing central serotonergic neurotransmitters and decreasing excitatory glutamatergic transmission.17
In any type of advanced cancer, nausea and vomiting are common, independent of chemotherapy and medication regimens. Metabolic derangement, vestibular dysfunction, CNS disorders, and visceral metastasis all contribute to hyperemesis. Olanzapine has been shown to significantly reduce refractory nausea and can cause weight gain and improved appetite, which benefits cachectic patients.18
Last, clozapine is one of the more effective antipsychotic medications, but also carries a risk of neutropenia. In patients with neutropenia secondary to chemotherapy, clozapine could increase the risk of infection in an immunocompromised patient.19 Granulocyte colony stimulating factor might be useful as a rescue medication for treatment-emergent neutropenia.19
Treatment considerations
Cancer patients might be unable or unwilling to seek services for mental health during their cancer treatment, and many who have a diagnosis of psychiatric illness might stop following up with psychiatric care when cancer treatment takes priority. It is critical for clinicians to be aware of the current literature regarding the impact of mood-stabilizing medication on cancer treatment. Monitoring for drug interactions is essential, and electronic drug interaction tools, such as Lexicomp, may be useful for this purpose.13 Because of special vulnerabilities in this population, cautious and judicious prescribing practices are advised.
The risk-benefit profile for medications for bipolar disorder must be considered before they are initiated or changes are made to the regimen (Table 2). Changing an effective mood stabilizer to gain benefits in breast cancer prognosis is not recommended in most cases, because benefits have been shown to be only significant in preclinical research; currently, there are no clinical guidelines. However, medication adjustments should be made with these theoretical benefits in mind, as long as the treatment of bipolar disorder remains effective.
Regardless of what treatment regimen the health team decides on, several underlying issues that affect patient care must be considered in this population. Successfully treating breast cancer in a woman with severe mental illness only can be accomplished when her mental illness is under control. Once she is psychiatrically stable, it is important for her to have a basic understanding of how cancer can affect the body and know the reasons behind treatment.
It is imperative that physicians provide their patients with a general understanding of their comorbid disorders, and find ways to help patients remain adherent with treatment of both diseases. Many patients feel demoralized by a cancer diagnosis and adherence to a medication regimen might be a difficult task among those with bipolar disorder who also are socially isolated, lack education, or have poor recall of treatment recommendations.20
Bottom Line
Managing comorbid bipolar disorder and breast cancer might seem daunting,
but treatments for the 2 diseases can work in synergy. You have an opportunity to
educate patients and colleagues in treating bipolar disorder and comorbid breast
cancer. Optimizing care using known psychopharmacologic data can not only lead
to better outcomes, but might additionally offer some hope and reason to remain
treatment-adherent for patients suffering from this complex comorbidity.
Related Resources
• Agarwala P, Riba MB. Tailoring depression treatment for women with breast cancer. Current Psychiatry. 2010;9(11): 39-40,45-46,48-49.
• Cunningham R, Sarfati D, Stanley J, et al. Cancer survival in the context of mental illness: a national cohort study. Gen Hosp Psychiatry. 2015;37(6):501-506.
Drug Brand Names
Amiodarone • Cordarone
Aripiprazole • Abilify
Asenapine • Saphris
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Cyclophosphamide • Cytoxan, Neosar
Doxorubicin • Doxil, Adriamycin
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fulvestrant • Faslodex
Iloperidone • Fanapt
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paclitaxel • Onxol
Paliperidone • Invega
Pamidronate • Aredia
Paroxetine • Paxil
Quetiapine • Seroquel
Raloxifene • Evista
Risperidone • Risperdal
Sertraline • Zoloft
Tamoxifen • Nolvadex
Thioridazine • Mellaril
Trastuzumab • Herceptin
Valproic acid • Depakene
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
2. American Cancer Society. Cancer facts and figures 2014. Atlanta, GA: American Cancer Society; 2014.
3. BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108(1-2):43-48.
4. Hung YP, Liu CJ, Tsai CF, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology. 2013;22(10):2227-2234.
5. Tesli M, Athanasiu L, Mattingsdal M, et al. Association analysis of PALB2 and BRCA2 in bipolar disorder and schizophrenia in a scandinavian case–control sample. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1276-1282.
6. Rahman T, Clevenger CV, Kaklamani V, et al. Antipsychotic treatment in breast cancer patients. Am J Psychiatry. 2014;171(6):616-621.
7. Armani F, Andersen ML, Galduróz JC. Tamoxifen use for the management of mania: a review of current preclinical evidence. Psychopharmacology (Berl). 2014;231(4):639-649.
8. Zarate CA Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561-570.
9. Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23(7):569-582.
10. Fortunati N, Bertino S, Costantino L, et al. Valproic acid restores ER alpha and antiestrogen sensitivity to ER alpha-negative breast cancer cells. Mol Cell Endocrinol. 2010;314(1):17-22.
11. Thekdi SM, Trinidad A, Roth A. Psychopharmacology in cancer. Curr Psychiatry Rep. 2014;17(1):529.
12. Meng Q, Chen X, Sun L, et al. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Mol Cell Biochem. 2011;348(1-2):165-171.
13. Altamura AC, Lietti L, Dobrea C, et al. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11(1):85-99.
14. Chateauvieux S, Morceau F, Dicato M, et al. Molecular and therapeutic potential and toxicity of valproic acid [published online July 29, 2010]. J Biomed Biotechnol. doi: 10.1155/2010/479364.
15. Jafary H, Ahmadian S, Soleimani M. The enhanced apoptosis and antiproliferative response to combined treatment with valproate and nicotinamide in MCF-7 breast cancer cells. Tumour Biol. 2013;35(3):2701-2710.
16. Fortunati N, Bertino S, Costantino L, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259(2):156-164.
17. Pasquini M, Speca A, Biondi M. Quetiapine for tamoxifen-induced insomnia in women with breast cancer. Psychosomatics. 2009;50(2):159-161.
18. Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage. 2003;25(6):578-582.
19. Sankaranarayanan A, Mulchandani M, Tirupati S. Clozapine, cancer chemotherapy and neutropenia - dilemmas in management. Psychiatr Danub. 2013;25(4):419-422.
20. Cole M, Padmanabhan A. Breast cancer treatment of women with schizophrenia and bipolar disorder from Philadelphia, PA: lessons learned and suggestions for improvement. J Cancer Educ. 2012;27(4):774-779.
Agitated and hallucinating, with a throbbing headache
CASE Psychotic, and nonadherent
Mr. K, a 42-year-old Fijian man, is brought to the emergency department by his older brother for evaluation of behavioral agitation. Mr. K is belligerent and threatens to kill his family members. Three years earlier, he was given a diagnosis of schizophrenia and treated at an inpatient psychiatric unit.
At that time, Mr. K was stabilized on risperidone, 4 mg/d. However, he did not follow-up with treatment after discharge and has not taken any psychotropic medications since that time.
His brother reports that Mr. K has been slowly deteriorating, talking to himself, staying up at night, and getting into arguments with his family over his delusional beliefs. Although Mr. K once worked as a security guard, he has not worked in 8 years. He has been living with his family, who are no longer willing to accept him into their home because they fear he might harm them.
In the emergency department, Mr. K reports that he has a throbbing headache. Blood pressure is 177/101 mm Hg; heart rate is 103 beats per minute; respiratory rate is 18 breaths per minute; weight is 185 lb; and body mass index (BMI) is 31.8. Physical examination is unremarkable.
Laboratory values show that sodium is 131 mEq/L; potassium, 3.7 mEq/L; bicarbonate, 26 mEq/L; glucose, 420 mg/dL; hemoglobin A1c,12.7; and urine glucose, 3+. Mr. K denies being told he has diabetes.
What are Mr. K’s risk factors for diabetes?
a) schizophrenia
b) physical inactivity
c) obesity
d) Fijian ethnicity
e) all of the above
The authors’ observations
The prevalence of type 2 diabetes mellitus (T2DM) in persons with schizophrenia or a schizoaffective disorder is twice that of the general population.1-4 Multiple variables contribute to the increased prevalence of diabetes in this population, including genetic predisposition, environmental and cultural factors related to diet and physical activity, a high rate of smoking,5,6 iatrogenic causes (metabolic dysregulation and weight gain from antipsychotic treatment), and socioeconomic factors (poverty, lack of access to health care). In addition, symptoms of psychosis such as thought disorder, delusions, hallucinations, and cognitive decline in persons with chronic schizophrenia and schizoaffective disorder can make basic health maintenance difficult.
In Mr. K’s case, premorbid risk of diabetes was elevated because of his Fijian ethnicity.7 With a BMI of 31.8, obesity further increased that risk.5,6,8 In addition, his untreated chronic mental illness, lack of access to health care, low socioeconomic status, long-standing smoking habit, and previous exposure to antipsychotics also increased his risk of T2DM.
The interaction between diabetes and psychosis contributes to a vicious cycle that makes both conditions worse if either, or both, are untreated. In general, medical comorbidities are associated with depression and neurocognitive impairment in persons with schizophrenia.9 Specifically, diabetes is associated with lower global cognitive functioning among persons with schizophrenia.10 Poor cognitive functioning can, in turn, decrease the patient’s ability to manage his medical illness. Also, persons with schizophrenia are less likely to be treated for diabetes, dyslipidemia, and hypertension, as in Mr. K’s case.11
How would you treat Mr. K’s newly diagnosed diabetes?
a) refer him to a primary care physician
b) start an oral agent
c) start sliding-scale insulin
d) start long-acting insulin
e) recommend a carbohydrate-controlled diet=
TREATMENT Stabilization
Mr. K is admitted to the medical unit for treatment of hyperglycemia. The team starts him on amlodipine, 5 mg/d, for hypertension; aripiprazole, 10 mg/d, for psychosis; and sliding-scale insulin (lispro) and 20 units of insulin (glargine) nightly for diabetes. Mr. K’s blood glucose level is well regulated on this regimen; after being medically cleared, he is transferred to the inpatient psychiatric unit.
EVALUATION Denies symptoms
Mr. K appears older than his stated age, is poorly groomed, and is dressed in a hospital gown. He is isolated and appears to be internally preoccupied. He repeatedly denies hearing auditory hallucinations, but often is overheard responding to internal stimuli and mumbling indecipherably in a low tone. His speech is decreased and his affect is flat and guarded. He states that he is not “mental” but that he came to the hospital for “tooth pain.” Every day he asks when he can return home and he asks the staff to call his family to take him home. When informed that his family is not able to care for him, Mr. K states that he would live in a house he owns in Fiji, which his family members state is untrue.
How would you treat Mr. K’s psychosis?
a) continue aripiprazole
b) switch to risperidone, an agent to which he previously responded
c) switch to olanzapine because he has not been sleeping well
d) switch to haloperidol because of diabetes
The authors’ observations
Pharmacotherapy for patients with comorbid schizophrenia and diabetes requires consideration of clinical and psychosocial factors unique to this population.
Antipsychotic choice
Selection of an antipsychotic agent to address psychosis requires considering its efficacy, side-effect profile, and adherence rates, as well as its potential effects on metabolic regulation and weight (Table 1). Typical antipsychotics are less likely than atypical antipsychotics to cause metabolic dysregulation.12 When treatment with atypical antipsychotics cannot be avoided—such as when side effects or an allergic reaction develop—consider selecting a relatively weight-neutral drug with a lower potential for metabolic dysregulation, such as aripiprazole. However, many times, using an agent with significant effects on metabolic regulation cannot be avoided, making treating and monitoring the resulting metabolic effects even more significant.
Glycemic control
Initiating an agent for glycemic control in persons with newly diagnosed diabetes requires weighing many factors, including mode of delivery (oral or subcutaneous), level of glycemic control required, comorbid medical illness (such as renal impairment and heart failure), cost, and potential long-term side effects of the medication (Table 2).13 Oral agents have a slower effect on lowering the blood glucose level, and each agent carries contraindications, but generally they are more affordable. Many present a low risk of hypoglycemia but sulfonylureas are an exception. In addition, metformin could lead to weight loss over the long term, which further lowers the risk of diabetes.
If, however, tight and immediate glycemic control is needed, subcutaneous insulin injections might be required, although this method is more costly, carries an increased risk of hypoglycemic episodes acutely, and leads to weight gain in the long run because of induced hunger and increased appetite.
Psychosocial factors
In addition to the clinical variables above, treating diabetes in patients with comorbid schizophrenia requires considering other psychosocial factors that might not be readily apparent (Table 3). For example, patients with schizophrenia might have decreased motivation, self-efficacy, and capacity for self-care when it comes to health maintenance and medication adherence.14 Chronically mentally ill persons might have reduced cognitive functioning that could affect their ability to maintain complicated medication regimens, such as administering insulin and monitoring blood glucose.
In addition, easy access to hypodermic needles and large amounts of insulin could become a safety concern in patients with ongoing hallucinations, delusions, and thought disorder, despite antipsychotic treatment. For example, a patient with schizophrenia and diabetes who has been maintained on insulin might begin hearing voices that tell her to inject her eyeballs with insulin. Similarly, although the risk of hypoglycemic episodes in patients treated with insulin is well known, patients with schizophrenia have a higher risk of acute complications of diabetes than other patients with diabetes.15Psychosocial factors, such as placement, support system, and follow-up care need to be considered. In some instances, the need to administer daily subcutaneous insulin could be a barrier to placement if the facility does not have the staff or expertise to monitor blood glucose and administer insulin.
If the patient is returning home, then the patient or a caretaker will need to be trained to monitor blood glucose and administer insulin. This might be difficult for persons with chronic mental illness who have lost the support and care of their family. Also, consider the issue of storing insulin, which requires refrigeration. Because of the potential complications involved in using insulin in patients with schizophrenia, practitioners should consider managing non-insulin dependent diabetes with an oral hypoglycemic agent, when possible, along with lifestyle modifications.
OUTCOME Weight loss, discharge
Mr. K is transitioned from aripiprazole to higher-potency oral risperidone, titrated to 6 mg/d. At this dosage, he continues to maintain a delusion about owning a house in Fiji, but was seen responding to internal stimuli less often. The treatment team places him on a diabetic and low-sodium diet of 1,800 kcal/d. His fasting blood glucose levels range in the 70s to 110s mg/dL during his first week of hospitalization.
The treatment team starts Mr. K on oral metformin, titrated to 1,000 mg twice daily, while tapering him off insulin lispro and glargine over the course of hospitalization. The transition from insulin to metformin also is considered because Mr. K’s daily insulin requirement is rather low (<0.5 units/kg).
Mr. K’s course is prolonged because his treatment team initiates the process of conservatorship and placement in the community. Approximately 6 months after his admission, Mr. K is discharged to an unlocked facility with support from an intensive outpatient mental health program. At 6 months follow-up with his outpatient provider, Mr. K’s hemoglobin A1c is 7.0 and he weighs 155 lb with a BMI of 26.5.
The authors’ observations
Despite Mr. K’s initial elevated hemoglobin A1c of 12.7 and weight of 185 lb, over approximately 6 months he experiences a 5.7-unit drop in hemoglobin A1c and weight loss of 30 lb with dietary management and metformin—without the need for other agents. Other options for weight-neutral treatment of T2DM include exenatide, which also is available as a once weekly injectable formulation, canagliflozin, and the gliptins (sitagliptin, saxagliptin, and linagliptin) (Table 4).
Mr. K’s improved control of his diabetes occurred despite initiation of an atypical antipsychotic, which would have been expected to cause additional weight gain and make his diabetes worse secondary to metabolic effects.12 Treatment with metformin in particular has been associated with weight loss in patients with16 and without17 comorbid schizophrenia, including those with antipsychotic-induced weight gain.18-20
Bottom Line
Persons with schizophrenia are at higher risk of type 2 diabetes mellitus for many reasons, including metabolic effects of antipsychotics, reduced cognitive functioning, poor self-care, and limited access to health care. Typical antipsychotics are less likely to cause metabolic dysregulation but, if an atypical is needed, one that is relatively weight-neutral should be selected. When choosing an agent for glycemic control, consider a patient’s or a caretaker’s ability to administer medications, monitor blood glucose, and follow dietary recommendations.
Related Resources
• Cohn T. The link between schizophrenia and diabetes. Current Psychiatry. 2012;11(10):28-34,46.
• Annamalai A, Tek C. An overview of diabetes management in schizophrenia patients: office based strategies for primary care practitioners and endocrinologists [published online March 23, 2015]. Int J Endocrinol. 2015;2015:969182. doi: 10.1155/2015/969182.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Asenapine • Saphris
Canagliflozin • Invokana
Exenatide • Bydureon, Byetta
Glargine • Lantus
Linagliptin • Tradjenta
Lispro • Humalog
Lurasidone • Latuda
Metformin • Glucophage
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Saxagliptin • Onglyza
Sitagliptin • Januvia
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
2. Heald A. Physical health in schizophrenia: a challenge for antipsychotic therapy. Eur Psychiatry. 2010;25(suppl 2):S6-S11.
3. El-Mallakh P. Doing my best: poverty and self-care among individuals with schizophrenia and diabetes mellitus. Arch Psychiatr Nurs. 2007;21(1):49-60; discussion 61-63.
4. Rouillon F, Sorbara F. Schizophrenia and diabetes: epidemiological data. Eur Psychiatry. 2005;20(suppl 4):S345-S348.
5. Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654-2664.
6. Dome P, Lazary J, Kalapos MP, et al. Smoking, nicotine and neuropsychiatric disorders. Neurosci Biobehav Rev. 2010;34(3):295-342.
7. National Institute of Diabetes and Digestive and Kidney Diseases. For people of African, Mediterranean, or Southeast Asian heritage: important information about diabetes blood tests. http://www.diabetes.niddk.nih.gov/dm/ pubs/asianamerican/index.htm. Published October 2011. Accessed November 18, 2015.
8. Shaten BJ, Smith GD, Kuller LH, et al. Risk factors for the development of type 2 diabetes among men enrolled in the usual care group of the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(10):1331-1339.
9. Chwastiak LA, Rosenheck RA, McEvoy JP, et al. Interrelationships of psychiatric symptom severity, medical comorbidity, and functioning in schizophrenia. Psychiatr Serv. 2006;57(8):1102-1109.
10. Takayanagi Y, Cascella NG, Sawa A, et al. Diabetes is associated with lower global cognitive function in schizophrenia. Schizophr Res. 2012;142(1-3):183-187.
11. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
12. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
13. Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006; 355(18):1903-1911.
14. Chen SR, Chien YP, Kang CM, et al. Comparing self-efficacy and self-care behaviours between outpatients with comorbid schizophrenia and type 2 diabetes and outpatients with only type 2 diabetes. J Psychiatr Ment Health Nurs. 2014;21(5):414-422.
15. Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care. 2011;34(2):398-402.
16. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731-737.
17. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
18. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-430. doi: 10.4088/JCP.12m08186.
19. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
20. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6): 1385-1403.
CASE Psychotic, and nonadherent
Mr. K, a 42-year-old Fijian man, is brought to the emergency department by his older brother for evaluation of behavioral agitation. Mr. K is belligerent and threatens to kill his family members. Three years earlier, he was given a diagnosis of schizophrenia and treated at an inpatient psychiatric unit.
At that time, Mr. K was stabilized on risperidone, 4 mg/d. However, he did not follow-up with treatment after discharge and has not taken any psychotropic medications since that time.
His brother reports that Mr. K has been slowly deteriorating, talking to himself, staying up at night, and getting into arguments with his family over his delusional beliefs. Although Mr. K once worked as a security guard, he has not worked in 8 years. He has been living with his family, who are no longer willing to accept him into their home because they fear he might harm them.
In the emergency department, Mr. K reports that he has a throbbing headache. Blood pressure is 177/101 mm Hg; heart rate is 103 beats per minute; respiratory rate is 18 breaths per minute; weight is 185 lb; and body mass index (BMI) is 31.8. Physical examination is unremarkable.
Laboratory values show that sodium is 131 mEq/L; potassium, 3.7 mEq/L; bicarbonate, 26 mEq/L; glucose, 420 mg/dL; hemoglobin A1c,12.7; and urine glucose, 3+. Mr. K denies being told he has diabetes.
What are Mr. K’s risk factors for diabetes?
a) schizophrenia
b) physical inactivity
c) obesity
d) Fijian ethnicity
e) all of the above
The authors’ observations
The prevalence of type 2 diabetes mellitus (T2DM) in persons with schizophrenia or a schizoaffective disorder is twice that of the general population.1-4 Multiple variables contribute to the increased prevalence of diabetes in this population, including genetic predisposition, environmental and cultural factors related to diet and physical activity, a high rate of smoking,5,6 iatrogenic causes (metabolic dysregulation and weight gain from antipsychotic treatment), and socioeconomic factors (poverty, lack of access to health care). In addition, symptoms of psychosis such as thought disorder, delusions, hallucinations, and cognitive decline in persons with chronic schizophrenia and schizoaffective disorder can make basic health maintenance difficult.
In Mr. K’s case, premorbid risk of diabetes was elevated because of his Fijian ethnicity.7 With a BMI of 31.8, obesity further increased that risk.5,6,8 In addition, his untreated chronic mental illness, lack of access to health care, low socioeconomic status, long-standing smoking habit, and previous exposure to antipsychotics also increased his risk of T2DM.
The interaction between diabetes and psychosis contributes to a vicious cycle that makes both conditions worse if either, or both, are untreated. In general, medical comorbidities are associated with depression and neurocognitive impairment in persons with schizophrenia.9 Specifically, diabetes is associated with lower global cognitive functioning among persons with schizophrenia.10 Poor cognitive functioning can, in turn, decrease the patient’s ability to manage his medical illness. Also, persons with schizophrenia are less likely to be treated for diabetes, dyslipidemia, and hypertension, as in Mr. K’s case.11
How would you treat Mr. K’s newly diagnosed diabetes?
a) refer him to a primary care physician
b) start an oral agent
c) start sliding-scale insulin
d) start long-acting insulin
e) recommend a carbohydrate-controlled diet=
TREATMENT Stabilization
Mr. K is admitted to the medical unit for treatment of hyperglycemia. The team starts him on amlodipine, 5 mg/d, for hypertension; aripiprazole, 10 mg/d, for psychosis; and sliding-scale insulin (lispro) and 20 units of insulin (glargine) nightly for diabetes. Mr. K’s blood glucose level is well regulated on this regimen; after being medically cleared, he is transferred to the inpatient psychiatric unit.
EVALUATION Denies symptoms
Mr. K appears older than his stated age, is poorly groomed, and is dressed in a hospital gown. He is isolated and appears to be internally preoccupied. He repeatedly denies hearing auditory hallucinations, but often is overheard responding to internal stimuli and mumbling indecipherably in a low tone. His speech is decreased and his affect is flat and guarded. He states that he is not “mental” but that he came to the hospital for “tooth pain.” Every day he asks when he can return home and he asks the staff to call his family to take him home. When informed that his family is not able to care for him, Mr. K states that he would live in a house he owns in Fiji, which his family members state is untrue.
How would you treat Mr. K’s psychosis?
a) continue aripiprazole
b) switch to risperidone, an agent to which he previously responded
c) switch to olanzapine because he has not been sleeping well
d) switch to haloperidol because of diabetes
The authors’ observations
Pharmacotherapy for patients with comorbid schizophrenia and diabetes requires consideration of clinical and psychosocial factors unique to this population.
Antipsychotic choice
Selection of an antipsychotic agent to address psychosis requires considering its efficacy, side-effect profile, and adherence rates, as well as its potential effects on metabolic regulation and weight (Table 1). Typical antipsychotics are less likely than atypical antipsychotics to cause metabolic dysregulation.12 When treatment with atypical antipsychotics cannot be avoided—such as when side effects or an allergic reaction develop—consider selecting a relatively weight-neutral drug with a lower potential for metabolic dysregulation, such as aripiprazole. However, many times, using an agent with significant effects on metabolic regulation cannot be avoided, making treating and monitoring the resulting metabolic effects even more significant.
Glycemic control
Initiating an agent for glycemic control in persons with newly diagnosed diabetes requires weighing many factors, including mode of delivery (oral or subcutaneous), level of glycemic control required, comorbid medical illness (such as renal impairment and heart failure), cost, and potential long-term side effects of the medication (Table 2).13 Oral agents have a slower effect on lowering the blood glucose level, and each agent carries contraindications, but generally they are more affordable. Many present a low risk of hypoglycemia but sulfonylureas are an exception. In addition, metformin could lead to weight loss over the long term, which further lowers the risk of diabetes.
If, however, tight and immediate glycemic control is needed, subcutaneous insulin injections might be required, although this method is more costly, carries an increased risk of hypoglycemic episodes acutely, and leads to weight gain in the long run because of induced hunger and increased appetite.
Psychosocial factors
In addition to the clinical variables above, treating diabetes in patients with comorbid schizophrenia requires considering other psychosocial factors that might not be readily apparent (Table 3). For example, patients with schizophrenia might have decreased motivation, self-efficacy, and capacity for self-care when it comes to health maintenance and medication adherence.14 Chronically mentally ill persons might have reduced cognitive functioning that could affect their ability to maintain complicated medication regimens, such as administering insulin and monitoring blood glucose.
In addition, easy access to hypodermic needles and large amounts of insulin could become a safety concern in patients with ongoing hallucinations, delusions, and thought disorder, despite antipsychotic treatment. For example, a patient with schizophrenia and diabetes who has been maintained on insulin might begin hearing voices that tell her to inject her eyeballs with insulin. Similarly, although the risk of hypoglycemic episodes in patients treated with insulin is well known, patients with schizophrenia have a higher risk of acute complications of diabetes than other patients with diabetes.15Psychosocial factors, such as placement, support system, and follow-up care need to be considered. In some instances, the need to administer daily subcutaneous insulin could be a barrier to placement if the facility does not have the staff or expertise to monitor blood glucose and administer insulin.
If the patient is returning home, then the patient or a caretaker will need to be trained to monitor blood glucose and administer insulin. This might be difficult for persons with chronic mental illness who have lost the support and care of their family. Also, consider the issue of storing insulin, which requires refrigeration. Because of the potential complications involved in using insulin in patients with schizophrenia, practitioners should consider managing non-insulin dependent diabetes with an oral hypoglycemic agent, when possible, along with lifestyle modifications.
OUTCOME Weight loss, discharge
Mr. K is transitioned from aripiprazole to higher-potency oral risperidone, titrated to 6 mg/d. At this dosage, he continues to maintain a delusion about owning a house in Fiji, but was seen responding to internal stimuli less often. The treatment team places him on a diabetic and low-sodium diet of 1,800 kcal/d. His fasting blood glucose levels range in the 70s to 110s mg/dL during his first week of hospitalization.
The treatment team starts Mr. K on oral metformin, titrated to 1,000 mg twice daily, while tapering him off insulin lispro and glargine over the course of hospitalization. The transition from insulin to metformin also is considered because Mr. K’s daily insulin requirement is rather low (<0.5 units/kg).
Mr. K’s course is prolonged because his treatment team initiates the process of conservatorship and placement in the community. Approximately 6 months after his admission, Mr. K is discharged to an unlocked facility with support from an intensive outpatient mental health program. At 6 months follow-up with his outpatient provider, Mr. K’s hemoglobin A1c is 7.0 and he weighs 155 lb with a BMI of 26.5.
The authors’ observations
Despite Mr. K’s initial elevated hemoglobin A1c of 12.7 and weight of 185 lb, over approximately 6 months he experiences a 5.7-unit drop in hemoglobin A1c and weight loss of 30 lb with dietary management and metformin—without the need for other agents. Other options for weight-neutral treatment of T2DM include exenatide, which also is available as a once weekly injectable formulation, canagliflozin, and the gliptins (sitagliptin, saxagliptin, and linagliptin) (Table 4).
Mr. K’s improved control of his diabetes occurred despite initiation of an atypical antipsychotic, which would have been expected to cause additional weight gain and make his diabetes worse secondary to metabolic effects.12 Treatment with metformin in particular has been associated with weight loss in patients with16 and without17 comorbid schizophrenia, including those with antipsychotic-induced weight gain.18-20
Bottom Line
Persons with schizophrenia are at higher risk of type 2 diabetes mellitus for many reasons, including metabolic effects of antipsychotics, reduced cognitive functioning, poor self-care, and limited access to health care. Typical antipsychotics are less likely to cause metabolic dysregulation but, if an atypical is needed, one that is relatively weight-neutral should be selected. When choosing an agent for glycemic control, consider a patient’s or a caretaker’s ability to administer medications, monitor blood glucose, and follow dietary recommendations.
Related Resources
• Cohn T. The link between schizophrenia and diabetes. Current Psychiatry. 2012;11(10):28-34,46.
• Annamalai A, Tek C. An overview of diabetes management in schizophrenia patients: office based strategies for primary care practitioners and endocrinologists [published online March 23, 2015]. Int J Endocrinol. 2015;2015:969182. doi: 10.1155/2015/969182.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Asenapine • Saphris
Canagliflozin • Invokana
Exenatide • Bydureon, Byetta
Glargine • Lantus
Linagliptin • Tradjenta
Lispro • Humalog
Lurasidone • Latuda
Metformin • Glucophage
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Saxagliptin • Onglyza
Sitagliptin • Januvia
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Psychotic, and nonadherent
Mr. K, a 42-year-old Fijian man, is brought to the emergency department by his older brother for evaluation of behavioral agitation. Mr. K is belligerent and threatens to kill his family members. Three years earlier, he was given a diagnosis of schizophrenia and treated at an inpatient psychiatric unit.
At that time, Mr. K was stabilized on risperidone, 4 mg/d. However, he did not follow-up with treatment after discharge and has not taken any psychotropic medications since that time.
His brother reports that Mr. K has been slowly deteriorating, talking to himself, staying up at night, and getting into arguments with his family over his delusional beliefs. Although Mr. K once worked as a security guard, he has not worked in 8 years. He has been living with his family, who are no longer willing to accept him into their home because they fear he might harm them.
In the emergency department, Mr. K reports that he has a throbbing headache. Blood pressure is 177/101 mm Hg; heart rate is 103 beats per minute; respiratory rate is 18 breaths per minute; weight is 185 lb; and body mass index (BMI) is 31.8. Physical examination is unremarkable.
Laboratory values show that sodium is 131 mEq/L; potassium, 3.7 mEq/L; bicarbonate, 26 mEq/L; glucose, 420 mg/dL; hemoglobin A1c,12.7; and urine glucose, 3+. Mr. K denies being told he has diabetes.
What are Mr. K’s risk factors for diabetes?
a) schizophrenia
b) physical inactivity
c) obesity
d) Fijian ethnicity
e) all of the above
The authors’ observations
The prevalence of type 2 diabetes mellitus (T2DM) in persons with schizophrenia or a schizoaffective disorder is twice that of the general population.1-4 Multiple variables contribute to the increased prevalence of diabetes in this population, including genetic predisposition, environmental and cultural factors related to diet and physical activity, a high rate of smoking,5,6 iatrogenic causes (metabolic dysregulation and weight gain from antipsychotic treatment), and socioeconomic factors (poverty, lack of access to health care). In addition, symptoms of psychosis such as thought disorder, delusions, hallucinations, and cognitive decline in persons with chronic schizophrenia and schizoaffective disorder can make basic health maintenance difficult.
In Mr. K’s case, premorbid risk of diabetes was elevated because of his Fijian ethnicity.7 With a BMI of 31.8, obesity further increased that risk.5,6,8 In addition, his untreated chronic mental illness, lack of access to health care, low socioeconomic status, long-standing smoking habit, and previous exposure to antipsychotics also increased his risk of T2DM.
The interaction between diabetes and psychosis contributes to a vicious cycle that makes both conditions worse if either, or both, are untreated. In general, medical comorbidities are associated with depression and neurocognitive impairment in persons with schizophrenia.9 Specifically, diabetes is associated with lower global cognitive functioning among persons with schizophrenia.10 Poor cognitive functioning can, in turn, decrease the patient’s ability to manage his medical illness. Also, persons with schizophrenia are less likely to be treated for diabetes, dyslipidemia, and hypertension, as in Mr. K’s case.11
How would you treat Mr. K’s newly diagnosed diabetes?
a) refer him to a primary care physician
b) start an oral agent
c) start sliding-scale insulin
d) start long-acting insulin
e) recommend a carbohydrate-controlled diet=
TREATMENT Stabilization
Mr. K is admitted to the medical unit for treatment of hyperglycemia. The team starts him on amlodipine, 5 mg/d, for hypertension; aripiprazole, 10 mg/d, for psychosis; and sliding-scale insulin (lispro) and 20 units of insulin (glargine) nightly for diabetes. Mr. K’s blood glucose level is well regulated on this regimen; after being medically cleared, he is transferred to the inpatient psychiatric unit.
EVALUATION Denies symptoms
Mr. K appears older than his stated age, is poorly groomed, and is dressed in a hospital gown. He is isolated and appears to be internally preoccupied. He repeatedly denies hearing auditory hallucinations, but often is overheard responding to internal stimuli and mumbling indecipherably in a low tone. His speech is decreased and his affect is flat and guarded. He states that he is not “mental” but that he came to the hospital for “tooth pain.” Every day he asks when he can return home and he asks the staff to call his family to take him home. When informed that his family is not able to care for him, Mr. K states that he would live in a house he owns in Fiji, which his family members state is untrue.
How would you treat Mr. K’s psychosis?
a) continue aripiprazole
b) switch to risperidone, an agent to which he previously responded
c) switch to olanzapine because he has not been sleeping well
d) switch to haloperidol because of diabetes
The authors’ observations
Pharmacotherapy for patients with comorbid schizophrenia and diabetes requires consideration of clinical and psychosocial factors unique to this population.
Antipsychotic choice
Selection of an antipsychotic agent to address psychosis requires considering its efficacy, side-effect profile, and adherence rates, as well as its potential effects on metabolic regulation and weight (Table 1). Typical antipsychotics are less likely than atypical antipsychotics to cause metabolic dysregulation.12 When treatment with atypical antipsychotics cannot be avoided—such as when side effects or an allergic reaction develop—consider selecting a relatively weight-neutral drug with a lower potential for metabolic dysregulation, such as aripiprazole. However, many times, using an agent with significant effects on metabolic regulation cannot be avoided, making treating and monitoring the resulting metabolic effects even more significant.
Glycemic control
Initiating an agent for glycemic control in persons with newly diagnosed diabetes requires weighing many factors, including mode of delivery (oral or subcutaneous), level of glycemic control required, comorbid medical illness (such as renal impairment and heart failure), cost, and potential long-term side effects of the medication (Table 2).13 Oral agents have a slower effect on lowering the blood glucose level, and each agent carries contraindications, but generally they are more affordable. Many present a low risk of hypoglycemia but sulfonylureas are an exception. In addition, metformin could lead to weight loss over the long term, which further lowers the risk of diabetes.
If, however, tight and immediate glycemic control is needed, subcutaneous insulin injections might be required, although this method is more costly, carries an increased risk of hypoglycemic episodes acutely, and leads to weight gain in the long run because of induced hunger and increased appetite.
Psychosocial factors
In addition to the clinical variables above, treating diabetes in patients with comorbid schizophrenia requires considering other psychosocial factors that might not be readily apparent (Table 3). For example, patients with schizophrenia might have decreased motivation, self-efficacy, and capacity for self-care when it comes to health maintenance and medication adherence.14 Chronically mentally ill persons might have reduced cognitive functioning that could affect their ability to maintain complicated medication regimens, such as administering insulin and monitoring blood glucose.
In addition, easy access to hypodermic needles and large amounts of insulin could become a safety concern in patients with ongoing hallucinations, delusions, and thought disorder, despite antipsychotic treatment. For example, a patient with schizophrenia and diabetes who has been maintained on insulin might begin hearing voices that tell her to inject her eyeballs with insulin. Similarly, although the risk of hypoglycemic episodes in patients treated with insulin is well known, patients with schizophrenia have a higher risk of acute complications of diabetes than other patients with diabetes.15Psychosocial factors, such as placement, support system, and follow-up care need to be considered. In some instances, the need to administer daily subcutaneous insulin could be a barrier to placement if the facility does not have the staff or expertise to monitor blood glucose and administer insulin.
If the patient is returning home, then the patient or a caretaker will need to be trained to monitor blood glucose and administer insulin. This might be difficult for persons with chronic mental illness who have lost the support and care of their family. Also, consider the issue of storing insulin, which requires refrigeration. Because of the potential complications involved in using insulin in patients with schizophrenia, practitioners should consider managing non-insulin dependent diabetes with an oral hypoglycemic agent, when possible, along with lifestyle modifications.
OUTCOME Weight loss, discharge
Mr. K is transitioned from aripiprazole to higher-potency oral risperidone, titrated to 6 mg/d. At this dosage, he continues to maintain a delusion about owning a house in Fiji, but was seen responding to internal stimuli less often. The treatment team places him on a diabetic and low-sodium diet of 1,800 kcal/d. His fasting blood glucose levels range in the 70s to 110s mg/dL during his first week of hospitalization.
The treatment team starts Mr. K on oral metformin, titrated to 1,000 mg twice daily, while tapering him off insulin lispro and glargine over the course of hospitalization. The transition from insulin to metformin also is considered because Mr. K’s daily insulin requirement is rather low (<0.5 units/kg).
Mr. K’s course is prolonged because his treatment team initiates the process of conservatorship and placement in the community. Approximately 6 months after his admission, Mr. K is discharged to an unlocked facility with support from an intensive outpatient mental health program. At 6 months follow-up with his outpatient provider, Mr. K’s hemoglobin A1c is 7.0 and he weighs 155 lb with a BMI of 26.5.
The authors’ observations
Despite Mr. K’s initial elevated hemoglobin A1c of 12.7 and weight of 185 lb, over approximately 6 months he experiences a 5.7-unit drop in hemoglobin A1c and weight loss of 30 lb with dietary management and metformin—without the need for other agents. Other options for weight-neutral treatment of T2DM include exenatide, which also is available as a once weekly injectable formulation, canagliflozin, and the gliptins (sitagliptin, saxagliptin, and linagliptin) (Table 4).
Mr. K’s improved control of his diabetes occurred despite initiation of an atypical antipsychotic, which would have been expected to cause additional weight gain and make his diabetes worse secondary to metabolic effects.12 Treatment with metformin in particular has been associated with weight loss in patients with16 and without17 comorbid schizophrenia, including those with antipsychotic-induced weight gain.18-20
Bottom Line
Persons with schizophrenia are at higher risk of type 2 diabetes mellitus for many reasons, including metabolic effects of antipsychotics, reduced cognitive functioning, poor self-care, and limited access to health care. Typical antipsychotics are less likely to cause metabolic dysregulation but, if an atypical is needed, one that is relatively weight-neutral should be selected. When choosing an agent for glycemic control, consider a patient’s or a caretaker’s ability to administer medications, monitor blood glucose, and follow dietary recommendations.
Related Resources
• Cohn T. The link between schizophrenia and diabetes. Current Psychiatry. 2012;11(10):28-34,46.
• Annamalai A, Tek C. An overview of diabetes management in schizophrenia patients: office based strategies for primary care practitioners and endocrinologists [published online March 23, 2015]. Int J Endocrinol. 2015;2015:969182. doi: 10.1155/2015/969182.
Drug Brand Names
Amlodipine • Norvasc
Aripiprazole • Abilify
Asenapine • Saphris
Canagliflozin • Invokana
Exenatide • Bydureon, Byetta
Glargine • Lantus
Linagliptin • Tradjenta
Lispro • Humalog
Lurasidone • Latuda
Metformin • Glucophage
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Saxagliptin • Onglyza
Sitagliptin • Januvia
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
2. Heald A. Physical health in schizophrenia: a challenge for antipsychotic therapy. Eur Psychiatry. 2010;25(suppl 2):S6-S11.
3. El-Mallakh P. Doing my best: poverty and self-care among individuals with schizophrenia and diabetes mellitus. Arch Psychiatr Nurs. 2007;21(1):49-60; discussion 61-63.
4. Rouillon F, Sorbara F. Schizophrenia and diabetes: epidemiological data. Eur Psychiatry. 2005;20(suppl 4):S345-S348.
5. Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654-2664.
6. Dome P, Lazary J, Kalapos MP, et al. Smoking, nicotine and neuropsychiatric disorders. Neurosci Biobehav Rev. 2010;34(3):295-342.
7. National Institute of Diabetes and Digestive and Kidney Diseases. For people of African, Mediterranean, or Southeast Asian heritage: important information about diabetes blood tests. http://www.diabetes.niddk.nih.gov/dm/ pubs/asianamerican/index.htm. Published October 2011. Accessed November 18, 2015.
8. Shaten BJ, Smith GD, Kuller LH, et al. Risk factors for the development of type 2 diabetes among men enrolled in the usual care group of the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(10):1331-1339.
9. Chwastiak LA, Rosenheck RA, McEvoy JP, et al. Interrelationships of psychiatric symptom severity, medical comorbidity, and functioning in schizophrenia. Psychiatr Serv. 2006;57(8):1102-1109.
10. Takayanagi Y, Cascella NG, Sawa A, et al. Diabetes is associated with lower global cognitive function in schizophrenia. Schizophr Res. 2012;142(1-3):183-187.
11. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
12. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
13. Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006; 355(18):1903-1911.
14. Chen SR, Chien YP, Kang CM, et al. Comparing self-efficacy and self-care behaviours between outpatients with comorbid schizophrenia and type 2 diabetes and outpatients with only type 2 diabetes. J Psychiatr Ment Health Nurs. 2014;21(5):414-422.
15. Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care. 2011;34(2):398-402.
16. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731-737.
17. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
18. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-430. doi: 10.4088/JCP.12m08186.
19. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
20. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6): 1385-1403.
1. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
2. Heald A. Physical health in schizophrenia: a challenge for antipsychotic therapy. Eur Psychiatry. 2010;25(suppl 2):S6-S11.
3. El-Mallakh P. Doing my best: poverty and self-care among individuals with schizophrenia and diabetes mellitus. Arch Psychiatr Nurs. 2007;21(1):49-60; discussion 61-63.
4. Rouillon F, Sorbara F. Schizophrenia and diabetes: epidemiological data. Eur Psychiatry. 2005;20(suppl 4):S345-S348.
5. Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654-2664.
6. Dome P, Lazary J, Kalapos MP, et al. Smoking, nicotine and neuropsychiatric disorders. Neurosci Biobehav Rev. 2010;34(3):295-342.
7. National Institute of Diabetes and Digestive and Kidney Diseases. For people of African, Mediterranean, or Southeast Asian heritage: important information about diabetes blood tests. http://www.diabetes.niddk.nih.gov/dm/ pubs/asianamerican/index.htm. Published October 2011. Accessed November 18, 2015.
8. Shaten BJ, Smith GD, Kuller LH, et al. Risk factors for the development of type 2 diabetes among men enrolled in the usual care group of the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(10):1331-1339.
9. Chwastiak LA, Rosenheck RA, McEvoy JP, et al. Interrelationships of psychiatric symptom severity, medical comorbidity, and functioning in schizophrenia. Psychiatr Serv. 2006;57(8):1102-1109.
10. Takayanagi Y, Cascella NG, Sawa A, et al. Diabetes is associated with lower global cognitive function in schizophrenia. Schizophr Res. 2012;142(1-3):183-187.
11. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
12. Meyer JM, Davis VG, Goff DC, et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101(1-3):273-286.
13. Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006; 355(18):1903-1911.
14. Chen SR, Chien YP, Kang CM, et al. Comparing self-efficacy and self-care behaviours between outpatients with comorbid schizophrenia and type 2 diabetes and outpatients with only type 2 diabetes. J Psychiatr Ment Health Nurs. 2014;21(5):414-422.
15. Becker T, Hux J. Risk of acute complications of diabetes among people with schizophrenia in Ontario, Canada. Diabetes Care. 2011;34(2):398-402.
16. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731-737.
17. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
18. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-430. doi: 10.4088/JCP.12m08186.
19. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
20. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6): 1385-1403.
A girl refuses to eat solid food because she is afraid of choking
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Refusing solid food
Ms. B, age 11, is admitted to a pediatric medical inpatient unit for unintentional weight loss of 14 lb (15% total body weight) over the past month. She reports having 2 traumatic episodes last month: choking on a piece of cheese and having a swab specimen taken for a rapid strep test, which required several people to restrain her (the test was positive). Since then, she has refused to ingest solids, despite hunger and a desire to eat.
Ms. B reports diffuse abdominal pain merely “at the sight of food” and a fear of swallowing solids. She denies difficulty or pain upon swallowing, nausea, vomiting, or any change in bowel habits.
Her mother reports that, on the rare occasion that Ms. B has attempted to eat solid food, she spent as long as an hour cutting it into small pieces before bringing it to her mouth—after which she put the food down without eating. Her mother also witnessed Ms. B holding food in her mouth for “a very long time,” then spitting it out.
Ms. B says she is distressed about the weight loss and recognizes that her fear of solid food is excessive.
What would your diagnosis of Ms. B’s problem be?
a) anorexia nervosa
b) avoidant/restrictive food intake disorder (ARFID)
c) specific phobia (swallowing solids or choking)
d) generalized anxiety disorder (GAD)
The authors’ observations
DSM-5 describes a new eating disorder called ARFID, which replaces the DSM-IV-TR diagnosis of feeding disorder of infancy or early childhood</keyword>. DSM-5 diagnostic criteria define ARFID as:
An eating or feeding disturbance (eg, avoidance based on the sensory characteristics of food…) as manifested by persistent failure to meet appropriate nutritional and/or energy needs associated with at least one of the following: 1. Significant weight loss (or failure to achieve expected weight gain or faltering growth in children). 2. Significant nutritional deficiency. 3. Dependence on enteral feeding or oral nutritional supplements. 4. Marked interference with psychosocial functioning.1
DSM-5 also specifies that the disorder cannot be caused by lack of available food or traditional cultural practices; cannot coexist with anorexia or bulimia nervosa; and is not attributable to a concurrent medical or psychiatric disorder.
Because it is a newly defined diagnosis, the epidemiology of ARFID is unclear. Patients with ARFID have a wide variety of eating symptoms that do not meet diagnostic criteria for anorexia or bulimia nervosa. One study found that, among a cohort of mostly adolescent patients who presented for evaluation of an eating disorder, 14% met diagnostic criteria for ARFID.2 Another retrospective case-control study found a similar prevalence among patients age 8 to 18 (13.8% of 712 patients).3 Because of the variety of maladaptive feeding behaviors seen in ARFID, there is little evidence that pharmacotherapy is effective.4
HISTORY Premature birth
Ms. B’s medical history states that she is twin A of a premature birth at 26 weeks (birth weight, 1,060 g), with a 90-day neonatal intensive care unit hospitalization, during which she required supplemental oxygen and nasogastric tube feeding. She has mild cerebral palsy, and had motor delay of walking at 2.5 years old. Currently, she has no motor difficulties.
Ms. B does not have a psychiatric history and does not take medications. Her mother has a history of major depressive disorder that is well controlled with an unspecified selective serotonin reuptake inhibitor. Ms. B’s maternal uncle has poorly controlled schizophrenia.
During Ms. B’s 6-day hospitalization, her mental status exams are unremarkable. She is shy but cooperative and open. Her mood ranges from “sad” and “nervous” on admission to “fine” with mood-congruent affect. She denies suicidal or homicidal thoughts and hallucinations, and demonstrates good insight and judgment. All laboratory values are within normal limits except for mild hypophosphatemia (3.7 mg/dL) and mild hyperalbuminemia (4.9 g/dL) on admission, which may have been related to her nutritional status.
DIAGNOSIS Not solely psychiatric
The psychiatric differential diagnosis includes:
- ARFID
- specific phobia of swallowing solids or choking (pseudodysphagia)
- GAD
- unspecified feeding disorder.
Ms. B meets diagnostic criteria for ARFID, particularly that of profound acute weight loss due to restrictive eating behaviors. Her presentation also is similar to that of a specific phobia, namely profound anxiety upon even the thought of solid food (phobic stimulus) and recognition that her fear is excessive. However, she fails to meet diagnostic criteria for phobia in children, which require duration of at least 6 months. GAD also is less likely because she has had symptoms for 1 month (also requires 6-month duration) and her anxiety is limited to feeding behaviors.
The treatment team starts exposure therapy, encouraging Ms. B to begin taking small bites of textured foods, such as oatmeal.
On hospital Day 5, barium esophagogram reveals extrinsic compression of the esophagus. To identify the precise cause of compression, chest magnetic resonance angiogram reveals that Ms. B has a right-sided aortic arch with an aberrant left subclavian artery at T4 that, with the ligamentum arteriosum and left pulmonary artery, form a vascular ring impinging around the esophagus.
The authors’ observations
Dysphagia lusoria, coined in 1789 from the root for “natural abnormality,”5 is caused by an aberrant right subclavian artery, which persists because of abnormal involution of the right fourth aortic arch during embryogenesis6 (Figure 1). The condition is diagnosed incidentally in most affected adults, who are asymptomatic throughout life and do not require operative management.6
Symptoms of dysphagia lusoria can include dysphagia and recurrent aspiration if significant esophageal impingement is present.7 It is thought that patients tend to show more symptoms as they age because of sclerosis, aneurysm, or atherosclerosis of the impinging vessel.7 Cough and dyspnea caused by impingement of the trachea also have been reported, and might be more common in children because of tracheal flexibility.5
This case illustrates the complex interrelationship of physical and psychiatric conditions. After the treatment team discovered a physical cause of Ms. B’s symptoms, the initial psychiatric diagnosis became problematic, but remained critically important for long-term treatment of her comorbid eating phobia.
TREATMENT Therapy, surgery
The treatment team is faced with the question of whether dysphagia lusoria fully accounts for Ms. B’s status. The anatomic anomaly might explain the initial choking incident if the food particle was lodged at the site of impingement, but does not account for development of a severe acute eating disorder and subsequent malnutrition.
Because of the presence of dysphagia lusoria, the team concludes that Ms. B does not meet diagnostic criteria for ARFID. She is given a diagnosis of unspecified eating disorder.
Ms. B is discharged and referred to a pediatric cardiothoracic surgeon for operative consult of symptomatic dysphagia lusoria. By discharge, she is successfully eating yogurt with fruit chunks, which she had rejected earlier. She also expresses optimism about eating cake at her birthday party, scheduled for 2 weeks after discharge.
The treatment team strongly recommends outpatient psychiatric follow-up to manage Ms. B’s unspecified eating disorder with cognitive-behavioral therapy and exposure and response prevention, which helped to mildly decrease her anxiety during the hospital stay.
Ms. B’s surgeon deems the case severe enough to warrant surgery. Surgery for dysphagia lusoria can be indicated to prevent progression of symptoms into adulthood8; options include division of the ligamentum arteriosum to loosen the vascular ring and re-implantation of the aberrant subclavian artery.9,10 (On the other hand, dietary modification might be therapeutic in patients whose symptoms are mild.5)
The surgeon elects to divide the ligamentum arteriosum to relieve the pressure of the vascular ring on the esophagus. Intraoperatively, he discovers that Ms. B has a mildly patent ductus arteriosus (PDA) (Figure 2), which is more common in premature infants than in full-term births. The PDA is clipped on both sides and divided. This immediately causes the vascular ring created by the aberrant left subclavian artery, right-sided aorta, and PDA to spring open, releasing pressure on the esophagus.
The aberrant left subclavian artery emerges from an abnormal bulging of aorta, known as Kommerell’s diverticulum. After the vascular ring is released, the diverticulum is not observed to impinge on the esophagus; however, as a preventive measure, the surgeon sutures it to the anterior spinous ligament. This will prevent the diverticulum from enlarging and impinging on the esophagus as Ms. B grows to adulthood.
The surgery is completed without complications. Ms. B tolerates the procedure well.
Ten days after surgery, Ms. B is recovering well. She and her mother report satisfaction with the procedure to release the vascular ring. She discontinues her pain medication after 2 days and slowly begins reintroducing solid foods. Her fear of dysphagia and choking rapidly diminish.
Bottom Line
The diagnosis and management of eating disorders, including avoidant/restrictive food intake disorder and choking phobia, can be challenging. Furthermore, if an anatomical or organic anomaly is found, it is important to question how a patient’s eating disorder can be managed best through interdisciplinary collaboration between medical and behavioral specialties.
Related Resources
• Bryant-Waugh R. Feeding and eating disorders in children. Curr Opin Psychiatry. 2013;26(6):537-542.
• Norris ML, Robinson A, Obeid N, et al. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47(5):495-499.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
1. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
2. Ornstein RM, Rosen DS, Mammel KA, et al. Distribution of eating disorders in children and adolescents using the proposed DSM-5 criteria for feeding and eating disorders. J Adolesc Health. 2013;53(2):303-305.
3. Fisher MM, Rosen DS, Ornstein RM, et al. Characteristics of avoidant/restrictive food intake disorder in children and adolescents: a “new disorder” in DSM-5. J Adolesc Health. 2014;55(1):49-52.
4. Kelly NR, Shank LM, Bakalar JL, et al. Pediatric feeding and eating disorders: current state of diagnosis and treatment. Curr Psychiatry Rep. 2014;16(5):446.
5. Janssen M, Baggen MG, Veen HF, et al. Dysphagia lusoria: clinical aspects, manometric findings, diagnosis, and therapy. Am J Gastroenterol. 2000;95(6):1411-1416.
6. Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: anatomical curiosity or clinical entity. Int J Surg. 2009;7(2):106-109.
7. Calleja F, Eguaras M, Montero J, et al. Aberrant right subclavian artery associated with common carotid trunk. A rare cause of vascular ring. Eur J Cardiothorac Surg. 1990;4(10):568-570.
8. Jalaie H, Grommes J, Sailer A, et al. Treatment of symptomatic aberrant subclavian arteries. Eur J Vasc Endovasc Surg. 2014;48(5):521-526.
9. Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg. 1946;124:532-534.
10. Morrris CD, Kanter KR, Miller JI Jr. Late-onset dysphagia lusoria. Ann Thorac Surg. 2001;71(2):710-712.
Differentiation of Latex Allergy From Irritant Contact Dermatitis
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Practice Points
- The term latex allergy often is used as a general diagnosis to describe 3 types of reactions to natural rubber latex, including irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity reaction), and true latex allergy (type I hypersensitivity reaction).
- The latex skin prick test is considered the gold standard for diagnosis of true latex allergy, but this method is not available in the United States. In vitro assay for latex-specific immunoglobulin E antibodies is the best alternative.
Evaluation and Management of Pancreatic Cystic Lesions
From the Department of Medicine, Stanford University School of Medicine, Stanford, CA.
Abstract
- Objective: To review the diagnosis and management of pancreatic cystic lesions.
- Methods: Narrative review of the literature.
- Results: Pancreatic cystic lesions are clinically relevant as some are precursor lesions to pancreatic adenocarcinoma. Mucinous cystic neoplasms and intraductal papillary mucinous neoplasms are 2 commonly encountered pre-cancerous pancreatic cysts. Many cysts are identified incidentally due to frequent use of high-resolution CT and MRI imaging technology. Proposed diagnostic and management algorithms exist to guide clinical practice but are limited by a lack of evidence and discordance among various guidelines. New cyst fluid biomarkers are under development to diagnose cyst types and risk of cancer.
- Conclusion: Pancreatic cysts are increasingly encountered in clinical practice and represent a growing problem. Diagnostic and management algorithms are available to assist practice but are limited by the available evidence. A multidisciplinary approach is recommended.
In the United States there were an estimated 46,420 new cases of pancreatic cancer in 2014 [1]. Of all major cancers, pancreatic cancer had the lowest 5-year survival rate at 6% [1]. Of the 3 known precursor lesions to pancreas adenocarcinoma, 2 are pancreatic cysts [2]. Correctly identifying those with cancer, those with cancer potential (premalignant), and those that are benign (harboring no malignant potential) can be difficult.
Case 1
A 57-year-old male had a 1.5-cm pancreatic cyst located in the head that was found on computed tomography (CT) imaging for suspected renal colic. He had no history or complaints suspicious for pancreatic disease. A CT pancreas protocol scan was obtained, which demonstrated a simple appearing cyst with no mural nodules. The pancreatic and biliary ducts were normal. His laboratory evaluations including liver function testing and lipase were normal.
• What is the approach to incidentally discovered pancreatic cysts?
While many pancreatic cysts are first discovered by cross sectional imaging (CT or MRI), the diagnostic accuracy of defining cyst type and the presence of malignancy is imperfect. The area under the curve (AUC) for differentiating malignant from benign pancreatic cysts ranges from 0.64 to 0.82 for CT and 0.73 to 0.91 for MRI, and no difference between the 2 were observed [12,13]. Several guidelines are currently available to offer guidance on management [6,14,15,16,17]. Much of the current evidence includes retrospective case series with no randomized control trials. The guidelines, therefore, mostly represent consensus-based expert extrapolation of available data.
The American Gastroenterological Association (AGA) recently produced guidelines in 2015 [14]. In comparison to the international consensus guidelines, there are a few key differences, which have now become a point of vigorous debate and disagreement among pancreatologists and confusion among general gastroenterologists and surgeons in the community. Where the international consensus guidelines have stricter criteria to define the appropriateness of surgery, the AGA guidelines are more liberal. AGA defined cysts appropriate for surgery as having 2 out of 3 of the following features: (1) cyst size ≥ 3 cm, (2) presence of a solid component in the cyst, and (3) dilation of the main pancreatic duct. Those having 1 out of 3 criteria were defined as needing further investigation with EUS [14]. These criteria are more relaxed and will likely lead to more surgical resections.
Another difference involves surveillance recommendations. The international consensus guidelines do not define a period when surveillance can be safely stopped. The AGA guidelines define 5 years as the period where if there is no significant change in the cyst from surveillance MRIs performed every 1 to 2 years, then surveillance can be stopped [14]. As the natural history of these cysts remain substantially uncertain, with evidence that malignant transformation occurs after 5 years, this particular recommendation by the AGA remains highly controversial [18,19]. Other differences between these 2 guidelines are summarized in Table 2 [6,14].
Until the surveillance recommendations by the AGA are validated with further studies, we generally follow the 2012 international consensus guidelines. We generally prefer MRI for initial and surveillance evaluations of pancreatic cysts. Besides the lack of radiation exposure, some studies show MRI to have better inter-reader variability [20], better resolution to show cyst communication with the main pancreatic duct [21], and better characterization of peripheral pancreatic cysts [22]. At our center, when a solid mass is suspected, a CT pancreas protocol is preferred in lieu of an MRI by our surgical team.
Case 1 Continued
Despite the reassuring CEA and cytology results, a high concern for a malignant cystic lesion remained based on cyst size, main pancreatic duct dilation, and atrophy noted in the distal pancreas. The patient underwent surgical resection including subtotal pancreatectomy, splenectomy, subtotal gastrectomy, and superior mesenteric and portal vein resection with reconstruction. Pathology revealed the cyst to be a benign pseudocyst.
This case reflects some of the critical challenges in current management of pancreatic cysts. By history, this patient had no suspicion for pancreatitis, making a pseudocyst less likely in a differential diagnosis. When the patient presented 7 years later, again with no reported history of pancreatitis, there was clinical concern for a branch duct IPMN. Although the cyst fluid CEA and cytology were reassuring, the patient met surgical criteria by the 2006 international consensus criteria and the more recent AGA guidelines. Interestingly, the narrowed 2012 international consensus guidelines for surgical resection would have recommended observation. This case highlights the need for better diagnostic tests.
• What is the epidemiology of pancreatic fluid collections and how do they present?
Pancreatic fluid collections are not true cysts as they lack an epithelial cell lining. They often occur in the context of either acute or chronic pancreatitis, and are considered benign or nonmucinous cysts [7,8]. Duct disruption occurs causing pancreatic fluid accumulation, initially defined as an acute peri-pancreatic fluid collection, or an acute necrotic collection if necrosis is present. Over about 4 weeks a more defined cyst wall forms and the cyst is now classified as either a pseudocyst or walled-off pancreatic necrosis [23]. In one review, the median age at presentation was 49 with a male:female ratio of 2:1. Only 52% of fluid collections were discovered following an acute attack of pancreatitis [24]. The risk factors for pancreatic fluid collections are similar to the risk factors for pancreatitis, with the most common being alcohol use and gallstones [24]. Potential symptoms include abdominal pain, weight loss, gastrointestinal bleeding from pseudoaneurysms, obstructive symptoms, sepsis from super infection, and obstructive jaundice [8,24,25,26,27].
• How are pancreatic fluid collections diagnosed and managed?
Clinical suspicion for pancreatic fluid collections should increase if a cyst is diagnosed in the context of acute or chronic pancreatitis [28]. However, other types of cysts can cause ductal obstruction and pancreatitis, so further investigation may be needed, including review of prior imaging if available. The presence of internal debris, the presence of imaging findings of acute or chronic pancreatitis, and fluid extension beyond the pancreas and taking the shape of the retroperitoneum are often characteristics found in pancreatic fluid collections [29,28,30]. If needed, FNA with assay of amylase may be helpful. An amylase value of 5680 IU/L or greater was 84% sensitive and 64% specific (AUC 0.69) for pseudocysts in one study [31].
Management of pancreatic fluid collections is largely based on surgical series. Drainage procedures for pancreatic fluid collections are often undertaken for intractable symptoms or concerns of infection [8,24,25,26]. Asymptomatic pseudocysts can be safely followed. Specific techniques used for pancreatic fluid collection management vary by institutional expertise. Endoscopic drainage can be done by transpapillary stenting if main duct communication is present, or transgastric/transduodenal stenting if the cyst wall is mature and accessible by these approaches [32]. If necrosis is present this can be debrided endoscopically [33]. Laparoscopic surgical options are preferred to open approaches, and can be performed in 1 procedure where endoscopic approaches may require multiple procedures. The most common approach is to drain the cyst by creating a cyst-gastrostomy, or when not feasible a cyst-duodenostomy or cyst-jejunostomy [26,34]. Percutaneous drainage is less commonly performed and used for unstable patients as it can lead to cutaneous fistulas [34]. The best technique for intervention should be decided in the context of a multidisciplinary team. The option for conservative management should be considered as well because about 60% of those managed conservatively will have resolution in 1 year [35].
Case 2
• What is the role of endoscopic ultrasound with FNA in the diagnosis of pancreatic cysts?
While more invasive than CT or MRI, EUS provides detailed imaging to characterize relevant clinical features and allows fine needle aspiration of the cyst fluid and tissue of intra-cystic masses (Table 2) [6,14]. While MRI imaging resolution is continuing to improve [22,36,37], EUS is generally considered superior [38] for diagnosing high-risk lesions. A limitation of EUS, however, is significant inter-observer variability when compared with MRI [37,39,40].
EUS enables FNA of the cyst, which offers unique oppor-tunities for diagnosis. Cyst fluid cytology unfortunately has limited diagnostic yield, with a recent meta-analysis showing sensitivity 63%, specificity 88%, and AUC of 0.89 in differentiating mucinous from nonmucinous cysts [41]. The low sensitivity is likely because cyst fluid is paucicellular. Techniques that include targeting the cyst wall are under investigation and may improve the diagnostic yield of cytological analysis [42]. Tumor markers such as CEA have been widely used in the cyst fluid, with a value > 192 ng/mL having 63% sensitivity and 88% specificity (AUC of 0.79) for mucinous pancreatic cysts [43]. Other cyst fluid markers have been or are being developed including mutated KRAS DNA [44], mutated GNAS DNA [45,46], micro RNA [47,48], glucose [49], proteomic analysis [50], and multiple other molecules [51]. At this time, many of these markers are under investigation.
Case 2 Continued
An endoscopic ultrasound was performed and showed normal main pancreatic and common bile ducts. No intra-cystic mural nodules were observed. FNA was performed. Cytology showed “paucicellular fluid” and the cyst fluid CEA was 319 ng/mL. Having met the original consensus criteria for surgical resection [17] based on size, the presence of mural nodules, and due to suspicion for a mucinous cyst based on the CEA level, the patient underwent a Whipple procedure. The final pathology was a branch-duct IPMN with moderate dysplasia.
• What interventions exist for treating pancreatic cysts?
Surgery is the mainstay of treatment for pancreatic cysts. The most common surgical procedure for worrisome cysts in the head of the pancreas is a pancreatoduodenectomy (Whipple procedure). For cysts in the distal pancreas, a laparoscopic distal pancreatectomy can be performed [52,53]. Middle pancreatectomy, total pancreatectomy, and enucleation are less commonly performed and remain under investigation. The most common complications after surgery are surgical site and nonsurgical site infection, bleeding, pancreatic fistula, and delayed gastric emptying [52,53]. Overall complication rate for pancreatic cyst surgery is 27% to 39%, and perioperative mortality is 0.5% to 4% at high-volume centers [52,53].
An area of active investigation involves EUS-directed chemical cyst ablation. Prior studies using ethanol intra-cystic injection alone showed cyst resolution in 33% of patients [54]. A combination of ethanol and paclitaxel showed cyst resolution in 62% of patients [55]. Though these techniques offer a less invasive alternative to surgery, the complete eradication of dysplastic cystic epithelium remains uncertain and long-term efficacy is unclear. Thus, these techniques should only be considered in the context of a clinical trial or perhaps in patients who are not surgical candidates [56].
Case 3
• What is the epidemiology of IPMNs and how do they present?
IPMNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas involving either the main or branch ducts that have the potential to develop into pancreatic adenocarcinoma [57]. The mean age at presentation for both branch duct IPMNs (BD-IPMNs) and main duct IPMNs (MD-IPMNs) is around 65 years [58,59]. In the United States, the male to female prevalence ratio is equal, though there is some geographic variation among different countries [58]. Risk factors for IPMN formation include diabetes, chronic pancreatitis, and a family history of pancreatic adenocarcinoma [60]. Presentation is often asymptomatic but may present with pancreatitis, abdominal pain, weight loss, jaundice, and pancreatic exocrine insufficiency [61]. They tend to occur in the pancreatic head [29]. IPMNs involve either the main pancreatic duct or branch duct or both [62], but this is not always visible by imaging [21]. MRI with MRCP is considered superior to CT in characterizing these lesions, specifically in identifying a connection with the pancreatic ducts [21].
• How are IPMNs diagnosed and managed?
MD-IPMNs harbor a higher risk of malignancy than BD-IPMNs. In one series, 64% of MD-IPMN resected specimens contained cancer [63]. Because of the high cancer risk, all guidelines recommend surgical resection for appropriate patients [6,14,15,16,17]. BD-IPMNs have a lower risk of cancer at diagnosis, present in 19.5% of resected specimens in one study [63]. As a surgical series, this may overstate the true prevalence, which is supported by another study. A cohort of 103 suspected BD-IPMNs patients were observed and those with high-risk features were resected. The overall rate of cancer at 5 years was 2.6%, and only 1 of 103 patients developed non-resectable disease [64]. For these reasons, suspected BD-IPMNs can often be safely monitored if they do not harbor any high risk stigmata as defined by the international consensus criteria (Table 2)[6]. Otherwise, suspected BD-IPMNs are managed in a similar manner to other pancreatic cysts (Table 2) [6,14].
Prognosis after resection is more favorable for IPMNs than for pancreatic adenocarcinoma, possibly due to earlier stage of detection. The 5-year survival for BD-IPMN is 90% after resection, and 47% for MD-IPMN after resection [62]. Survival rates for IPMNs with invasive adenocarcinoma are lower with a combined overall survival 24% to 42% at 5 years. Survival rates are similar to the survival rate for non-cystic pancreatic adenocarcinoma when controlling for size, invasiveness, and lymph node metastasis [65,66].
Guidelines for surveillance after resection have even fewer applicable studies. The 5-year postoperative recurrence rate is 0 to 20% for IPMNs [6]. The revised international consensus guidelines recommends surveillance 6 months after resection with CT or MRI for all IPMNs, but with no recommendation given on how long to continue surveillance [6]. For patients with invasive disease, the same follow up is recommended as for standard invasive adenocarcinoma [6]. The AGA recommends yearly MRI only for only patients with high-grade dysplasia or invasive disease, with consideration for lifelong surveillance [14].
Case 4
• What is the epidemiology of MCNs and how do they present?
MCNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas histologically defined by the presence of ovarian stroma [67]. They have the potential to develop into pancreatic adenocarcinoma. Unlike IPMNs, MCNs occur almost exclusively in women, and patients are generally younger. In one series, 99.7% of MCNs occurred in females, with a mean age of 47 [67]. Presenting symptoms, as with other cysts, are often vague. These include abdominal pain, fatigue, weight loss, pancreatitis, and a palpable mass. Only 25% of patients are asymptomatic [68].
• How are MCNs diagnosed and managed?
Approximately 95% of MCNs are located in the body or tail of the pancreas [67]. These lesions do not communicate with the pancreatic ducts unlike IPMNs, though they may still cause ductal obstruction and dilation [29]. They are often one large unilocular cyst with a thick cyst wall, but in 20% of cases they can have multiple septations [29]. Peripheral eggshell calcification on CT is present in roughly 25% of cases, which is sometimes helpful in differentiating these lesions from serous cystic neoplasms, which often have central calcification [69].
When diagnosed, MCNs are surgically removed [6,14]. A recent surgical series found that the rate of high-grade dysplasia in resected specimens was 5.5%, and the risk of invasive disease was 4.4% [70]. This data suggests that a more conservative approach of observation rather than immediate resection may be reasonable for some patients [70]. The prognosis is very good after MCN resection, with a 5-year survival of 97% to 100% for all comers [68,70]. However, invasive MCNs have a lower 5-year survival rate ranging from 15% to 75% [70,71]. Per the AGA guidelines, patients with invasive disease or dysplasia should undergo yearly surveillance with MRI [14]. This recommendation is based on a potential field defect described with IPMNs. However, the international consensus guidelines only recommends surveillance if invasive disease is present [6,68,71,72].
Case 5
A 59-year-old male presents for evaluation of sudden onset abdominal pain and an 8-pound weight loss over the past few months. Seven years ago a pancreatic cyst was diagnosed and has since been observed by serial imaging. His lipase was 400 U/L (normal < 82) with normal liver function tests. A CT scan of the abdomen and pelvis showed peri-pancreatic stranding consistent with pancreatitis and a large complex cyst in the head of the pancreas.
• What is the epidemiology of SCNs and how do they present?
SCNs are benign non–mucin-producing cystic lesions that are characterized by a glycogen-rich epithelium on histology [73]. Of patients with SCNs, 74% are female, with a median age 58 [10]. When diagnosed, most patients are usually asymptomatic (61%), but 27% present with abdominal pain. Other symptoms include jaundice, pancreatitis, nausea, and presence of a palpable abdominal mass. SCNs are more common in patients with von Hippel-Lindau syndrome [74].
• How are SCNs diagnosed and managed?
These cysts have fairly even distribution when discovered in the pancreas [53]. About 74% of lesions have smaller micro-cystic components [75]. About 20% of lesions have a characteristic honeycomb appearance, which is highly suggestive of an SCN [76]. About 30% of patients have a characteristic central stellate scar on CT which is also highly suggestive of an SCN [76]. Unlike mucinous neoplasms, peripheral calcification is usually not seen [69].
Malignancy associated with these cysts is very rare, with the largest cohort study reporting a rate of 0.1% [10]. The diagnosis can commonly be made by its unique imaging appearance [10]. Diagnostic biomarkers that may identify such cysts with more certainty are under active investigation [77]. Resection is reasonable and often performed for SCNs when they cause debilitating symptoms including refractory abdominal pain or pancreatitis. When resected and confirmed by pathology, no surveillance is required [14,78].
Conclusion
Pancreatic cysts are common incidental findings in clinical practice today. Many cause anxiety due to their association with pancreas cancer, but most are indolent and safe to observe. Even those cysts with malignant potential grow slowly and immediate surgery is often unnecessary. Several guidelines have been developed, and while there are similarities between them, there are enough critical differences unfortunately to cause some confusion among practitioners today. Further robust research is needed to help address and reconcile these differences. In the meantime, a multidisciplinary approach is highly recommended at dedicated centers of excellence for pancreatic diseases.
Corresponding author: Walter G. Park, MD, MS, 300 Pasteur Drive, MC: 5187, Stanford, CA 94305, [email protected].
Funding/support: Dr. Park is funded by an American College of Gastroenterology Junior Faculty Development Award and is a subcontinent for the National Cancer Institute's Early Detection Research Network.
Financial disclosures: None.
Author contributions: conception and design, TZ, WGP; analysis and interpretation of data, WGP; drafting of article, TZ, WGP; critical revision of the article, TZ, WGP.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71.
2. Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am 2007;36:831–49.
3. Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008;191:802–7.
4. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105:2079–84.
5. Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol 20(1 Suppl):S71–93.
6. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97.
7. Galanis C, Zamani A, Cameron J, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg 2007;11:820–6.
8. Vitas G, Sarr M. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 1992;111:123–30.
9. Jani N, Bani Hani M, Schulick RD, et al. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc 2011;2011:478913.
10. Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2015 Jun 4.
11. Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc 2015;48:31–8.
12. Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. Am J Roentgenol 2007;189:648–56.
13. Lee H-J, Kim M-J, Choi J-Y, et al. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol 2011;66:315–21.
14. Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–48.
15. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2010;7:754–73.
16. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49.
17. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2006;6(1-2).
18. Ahn D-W, Lee SH, Kim J, et al. Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 2012;6:493–500.
19. Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2012;12:198–202.
20. Grieser C, Heine G, Stelter L, et al. Morphological analysis and differentiation of benign cystic neoplasms of the pancreas using computed tomography and magnetic resonance imaging. Fortschr Röntgenstr 2013;185:219–27.
21. Waters J, Schmidt CM, Pinchot J, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg 2008;12:101–9.
22. Duconseil P, Turrini O, Ewald J, et al. “Peripheric” pancreatic cysts: performance of CT scan, MRI and endoscopy according to final pathological examination. HPB 2015;17:485–9.
23. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11.
24. Sanfey H, Aguilar M, Jones R. Pseudocysts of the pancreas, a review of 97 cases. Am Surg 1994;60:661–8.
25. Cheruvu CVN, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl 2003;85:313–6.
26. Usatoff V, Brancatisano R, Williamson RCN. Operative treatment of pseudocysts in patients with chronic pancreatitis. Br J Surg 2000;87:1494–9.
27. Marshall G, Howell DA, Hansen BL, et al. Multidisciplinary approach to pseudoaneurysms complicating pancreatic pseudocysts: Impact of pretreatment diagnosis. Arch Surg 1996;131:278–83.
28. Sahani DV, Kadavigere R, Saokar A, et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. RadioGraphics 2005;25:1471–84.
29. Tirkes T, Aisen A, Cramer H, et al. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging 2014;39:1088–101.
30. Macari M, Finn ME, Bennett GL, et al. Differentiating Pancreatic cystic neoplasms from pancreatic pseudocysts at MR Imaging: value of perceived internal debris. Radiology 2009;251:77–84.
31. Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011;40:42–5.
32. Braden B, Dietrich CF. Endoscopic ultrasonography-guided endoscopic treatment of pancreatic pseudocysts and walled-off necrosis: New technical developments. World J Gastroenterol WJG 2014;20:16191–6.
33. van Brunschot S, Fockens P, Bakker O, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc 2014;28:1425–38.
34. Pan G, Wan MH, Xie K-L, et al. Classification and management of pancreatic pseudocysts. Medicine (Baltimore). 2015;94:e960.
35. Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990;170:411–7.
36. Chebib I, Yaeger K, Mino-Kenudson M, Pitman MB. The role of cytopathology and cyst fluid analysis in the preoperative diagnosis and management of pancreatic cysts >3 cm. Cancer Cytopathol 2014;122:804–9.
37. Kim JH, Eun HW, Park H-J, et al. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol 2012;81:2927–35.
38. Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? the incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42:717–21.
39. de Jong K, Verlaan T, Dijkgraaf MG, et al. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy 2011;43:579–84.
40. Harinck F, Konings ICAW, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2015 May 18.
41. Thosani N, Thosani S, Qiao W, et al. Role of EUS-FNA based cytology in diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756–66.
42. Hong S-KS, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc 2012;75:775–82.
43. Thornton G, McPhail M, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48–57.
44. Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009;May;69:1095–102.
45. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66.
46. Springer S, Wang Y, Molin MD, et al. A Combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015 Aug 4.
47. Ryu JK, Matthaei H, dal Molin M, et al. Elevated microRNA miR-21 Levels in Pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology 2011;11:343–50.
48. Matthaei H, Wylie D, Lloyd MB, et al. miRNA Biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res 2012;18 :4713–24.
49. Zikos T, Pham K, Bowen R, et al. Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts. Am J Gastroenterol 2015;110:909–14.
50. Corcos O, Couvelard A, Dargère D, et al. Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2012;41:169–74.
51. Thiruvengadam N, Park W. Systematic review of pancreatic cyst fluid biomarkers: the path forward. Clin Transl Gastroenterol 2015;11:e88.
52. Plichta JK, Brosius JA, Pappas SG, et al. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surg 2015;2015:791704.
53. Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4–12.
54. DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc 2009;70:710–23.
55. Oh H-C, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011;140:172–9.
56. Gavini H, Lee JH. Endoscopic ultrasound-guided endotherapy. J Clin Gastroenterol 2015;49:185–93.
57. Grutzmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Ooncologist 2010;15:1294–309.
58. Ingkakul T, Warshaw AL, Fernández-Del Castillo C. Epidemiology of intraductal papillary mucinous neoplasms of the pancreas: sex differences between 3 geographic regions. Pancreas 2011;40:779–80.
59. Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218–26.
60. Capurso G, Boccia S, Salvia R, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol 2013n;108:1003–9.
61. Lubezky N, Ben-Haim M, Nakache R, et al. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg 2010;34:126–32.
62. Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg Chic Ill 1960. 1999;134:1131–6.
63. Serikawa M, Sasaki T, Fujimoto Y, et al. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol 2006;40:856–62.
64. Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 2010;42:1077–84.
65. Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 201 Mar;251:470–6.
66. Worni M, Akushevich I, Gloor B, et al. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol 2012;19:1316–23.
67. Goh BKP, Tan Y-M, Chung Y-FA, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg 2006;30:2236–45.
68. Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008;247:571–9.
69. Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000;175:99–103.
70. Park JW, Jang J-Y, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatology 2014;14:131–6.
71. Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: Part II. Mucinous cystic neoplasms. Surg Oncol 2011;20:e93–101.
72. Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg 2000;231:205–12.
73. Reid MD, Choi H, Balci S, et al. Serous cystic neoplasms of the pancreas: Clinicopathologic and molecular characteristics. Pancreat Neoplasms 2014;31:475–83.
74. Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg Off J Soc Surg Aliment Tract 2012;16:1422–8.
75. Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41):380–7.
76. Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg Off J Soc Surg Aliment Tract 2003;7:417–28.
77. Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg 2014;218:608–17.
78. Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013;45:703–11.
From the Department of Medicine, Stanford University School of Medicine, Stanford, CA.
Abstract
- Objective: To review the diagnosis and management of pancreatic cystic lesions.
- Methods: Narrative review of the literature.
- Results: Pancreatic cystic lesions are clinically relevant as some are precursor lesions to pancreatic adenocarcinoma. Mucinous cystic neoplasms and intraductal papillary mucinous neoplasms are 2 commonly encountered pre-cancerous pancreatic cysts. Many cysts are identified incidentally due to frequent use of high-resolution CT and MRI imaging technology. Proposed diagnostic and management algorithms exist to guide clinical practice but are limited by a lack of evidence and discordance among various guidelines. New cyst fluid biomarkers are under development to diagnose cyst types and risk of cancer.
- Conclusion: Pancreatic cysts are increasingly encountered in clinical practice and represent a growing problem. Diagnostic and management algorithms are available to assist practice but are limited by the available evidence. A multidisciplinary approach is recommended.
In the United States there were an estimated 46,420 new cases of pancreatic cancer in 2014 [1]. Of all major cancers, pancreatic cancer had the lowest 5-year survival rate at 6% [1]. Of the 3 known precursor lesions to pancreas adenocarcinoma, 2 are pancreatic cysts [2]. Correctly identifying those with cancer, those with cancer potential (premalignant), and those that are benign (harboring no malignant potential) can be difficult.
Case 1
A 57-year-old male had a 1.5-cm pancreatic cyst located in the head that was found on computed tomography (CT) imaging for suspected renal colic. He had no history or complaints suspicious for pancreatic disease. A CT pancreas protocol scan was obtained, which demonstrated a simple appearing cyst with no mural nodules. The pancreatic and biliary ducts were normal. His laboratory evaluations including liver function testing and lipase were normal.
• What is the approach to incidentally discovered pancreatic cysts?
While many pancreatic cysts are first discovered by cross sectional imaging (CT or MRI), the diagnostic accuracy of defining cyst type and the presence of malignancy is imperfect. The area under the curve (AUC) for differentiating malignant from benign pancreatic cysts ranges from 0.64 to 0.82 for CT and 0.73 to 0.91 for MRI, and no difference between the 2 were observed [12,13]. Several guidelines are currently available to offer guidance on management [6,14,15,16,17]. Much of the current evidence includes retrospective case series with no randomized control trials. The guidelines, therefore, mostly represent consensus-based expert extrapolation of available data.
The American Gastroenterological Association (AGA) recently produced guidelines in 2015 [14]. In comparison to the international consensus guidelines, there are a few key differences, which have now become a point of vigorous debate and disagreement among pancreatologists and confusion among general gastroenterologists and surgeons in the community. Where the international consensus guidelines have stricter criteria to define the appropriateness of surgery, the AGA guidelines are more liberal. AGA defined cysts appropriate for surgery as having 2 out of 3 of the following features: (1) cyst size ≥ 3 cm, (2) presence of a solid component in the cyst, and (3) dilation of the main pancreatic duct. Those having 1 out of 3 criteria were defined as needing further investigation with EUS [14]. These criteria are more relaxed and will likely lead to more surgical resections.
Another difference involves surveillance recommendations. The international consensus guidelines do not define a period when surveillance can be safely stopped. The AGA guidelines define 5 years as the period where if there is no significant change in the cyst from surveillance MRIs performed every 1 to 2 years, then surveillance can be stopped [14]. As the natural history of these cysts remain substantially uncertain, with evidence that malignant transformation occurs after 5 years, this particular recommendation by the AGA remains highly controversial [18,19]. Other differences between these 2 guidelines are summarized in Table 2 [6,14].
Until the surveillance recommendations by the AGA are validated with further studies, we generally follow the 2012 international consensus guidelines. We generally prefer MRI for initial and surveillance evaluations of pancreatic cysts. Besides the lack of radiation exposure, some studies show MRI to have better inter-reader variability [20], better resolution to show cyst communication with the main pancreatic duct [21], and better characterization of peripheral pancreatic cysts [22]. At our center, when a solid mass is suspected, a CT pancreas protocol is preferred in lieu of an MRI by our surgical team.
Case 1 Continued
Despite the reassuring CEA and cytology results, a high concern for a malignant cystic lesion remained based on cyst size, main pancreatic duct dilation, and atrophy noted in the distal pancreas. The patient underwent surgical resection including subtotal pancreatectomy, splenectomy, subtotal gastrectomy, and superior mesenteric and portal vein resection with reconstruction. Pathology revealed the cyst to be a benign pseudocyst.
This case reflects some of the critical challenges in current management of pancreatic cysts. By history, this patient had no suspicion for pancreatitis, making a pseudocyst less likely in a differential diagnosis. When the patient presented 7 years later, again with no reported history of pancreatitis, there was clinical concern for a branch duct IPMN. Although the cyst fluid CEA and cytology were reassuring, the patient met surgical criteria by the 2006 international consensus criteria and the more recent AGA guidelines. Interestingly, the narrowed 2012 international consensus guidelines for surgical resection would have recommended observation. This case highlights the need for better diagnostic tests.
• What is the epidemiology of pancreatic fluid collections and how do they present?
Pancreatic fluid collections are not true cysts as they lack an epithelial cell lining. They often occur in the context of either acute or chronic pancreatitis, and are considered benign or nonmucinous cysts [7,8]. Duct disruption occurs causing pancreatic fluid accumulation, initially defined as an acute peri-pancreatic fluid collection, or an acute necrotic collection if necrosis is present. Over about 4 weeks a more defined cyst wall forms and the cyst is now classified as either a pseudocyst or walled-off pancreatic necrosis [23]. In one review, the median age at presentation was 49 with a male:female ratio of 2:1. Only 52% of fluid collections were discovered following an acute attack of pancreatitis [24]. The risk factors for pancreatic fluid collections are similar to the risk factors for pancreatitis, with the most common being alcohol use and gallstones [24]. Potential symptoms include abdominal pain, weight loss, gastrointestinal bleeding from pseudoaneurysms, obstructive symptoms, sepsis from super infection, and obstructive jaundice [8,24,25,26,27].
• How are pancreatic fluid collections diagnosed and managed?
Clinical suspicion for pancreatic fluid collections should increase if a cyst is diagnosed in the context of acute or chronic pancreatitis [28]. However, other types of cysts can cause ductal obstruction and pancreatitis, so further investigation may be needed, including review of prior imaging if available. The presence of internal debris, the presence of imaging findings of acute or chronic pancreatitis, and fluid extension beyond the pancreas and taking the shape of the retroperitoneum are often characteristics found in pancreatic fluid collections [29,28,30]. If needed, FNA with assay of amylase may be helpful. An amylase value of 5680 IU/L or greater was 84% sensitive and 64% specific (AUC 0.69) for pseudocysts in one study [31].
Management of pancreatic fluid collections is largely based on surgical series. Drainage procedures for pancreatic fluid collections are often undertaken for intractable symptoms or concerns of infection [8,24,25,26]. Asymptomatic pseudocysts can be safely followed. Specific techniques used for pancreatic fluid collection management vary by institutional expertise. Endoscopic drainage can be done by transpapillary stenting if main duct communication is present, or transgastric/transduodenal stenting if the cyst wall is mature and accessible by these approaches [32]. If necrosis is present this can be debrided endoscopically [33]. Laparoscopic surgical options are preferred to open approaches, and can be performed in 1 procedure where endoscopic approaches may require multiple procedures. The most common approach is to drain the cyst by creating a cyst-gastrostomy, or when not feasible a cyst-duodenostomy or cyst-jejunostomy [26,34]. Percutaneous drainage is less commonly performed and used for unstable patients as it can lead to cutaneous fistulas [34]. The best technique for intervention should be decided in the context of a multidisciplinary team. The option for conservative management should be considered as well because about 60% of those managed conservatively will have resolution in 1 year [35].
Case 2
• What is the role of endoscopic ultrasound with FNA in the diagnosis of pancreatic cysts?
While more invasive than CT or MRI, EUS provides detailed imaging to characterize relevant clinical features and allows fine needle aspiration of the cyst fluid and tissue of intra-cystic masses (Table 2) [6,14]. While MRI imaging resolution is continuing to improve [22,36,37], EUS is generally considered superior [38] for diagnosing high-risk lesions. A limitation of EUS, however, is significant inter-observer variability when compared with MRI [37,39,40].
EUS enables FNA of the cyst, which offers unique oppor-tunities for diagnosis. Cyst fluid cytology unfortunately has limited diagnostic yield, with a recent meta-analysis showing sensitivity 63%, specificity 88%, and AUC of 0.89 in differentiating mucinous from nonmucinous cysts [41]. The low sensitivity is likely because cyst fluid is paucicellular. Techniques that include targeting the cyst wall are under investigation and may improve the diagnostic yield of cytological analysis [42]. Tumor markers such as CEA have been widely used in the cyst fluid, with a value > 192 ng/mL having 63% sensitivity and 88% specificity (AUC of 0.79) for mucinous pancreatic cysts [43]. Other cyst fluid markers have been or are being developed including mutated KRAS DNA [44], mutated GNAS DNA [45,46], micro RNA [47,48], glucose [49], proteomic analysis [50], and multiple other molecules [51]. At this time, many of these markers are under investigation.
Case 2 Continued
An endoscopic ultrasound was performed and showed normal main pancreatic and common bile ducts. No intra-cystic mural nodules were observed. FNA was performed. Cytology showed “paucicellular fluid” and the cyst fluid CEA was 319 ng/mL. Having met the original consensus criteria for surgical resection [17] based on size, the presence of mural nodules, and due to suspicion for a mucinous cyst based on the CEA level, the patient underwent a Whipple procedure. The final pathology was a branch-duct IPMN with moderate dysplasia.
• What interventions exist for treating pancreatic cysts?
Surgery is the mainstay of treatment for pancreatic cysts. The most common surgical procedure for worrisome cysts in the head of the pancreas is a pancreatoduodenectomy (Whipple procedure). For cysts in the distal pancreas, a laparoscopic distal pancreatectomy can be performed [52,53]. Middle pancreatectomy, total pancreatectomy, and enucleation are less commonly performed and remain under investigation. The most common complications after surgery are surgical site and nonsurgical site infection, bleeding, pancreatic fistula, and delayed gastric emptying [52,53]. Overall complication rate for pancreatic cyst surgery is 27% to 39%, and perioperative mortality is 0.5% to 4% at high-volume centers [52,53].
An area of active investigation involves EUS-directed chemical cyst ablation. Prior studies using ethanol intra-cystic injection alone showed cyst resolution in 33% of patients [54]. A combination of ethanol and paclitaxel showed cyst resolution in 62% of patients [55]. Though these techniques offer a less invasive alternative to surgery, the complete eradication of dysplastic cystic epithelium remains uncertain and long-term efficacy is unclear. Thus, these techniques should only be considered in the context of a clinical trial or perhaps in patients who are not surgical candidates [56].
Case 3
• What is the epidemiology of IPMNs and how do they present?
IPMNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas involving either the main or branch ducts that have the potential to develop into pancreatic adenocarcinoma [57]. The mean age at presentation for both branch duct IPMNs (BD-IPMNs) and main duct IPMNs (MD-IPMNs) is around 65 years [58,59]. In the United States, the male to female prevalence ratio is equal, though there is some geographic variation among different countries [58]. Risk factors for IPMN formation include diabetes, chronic pancreatitis, and a family history of pancreatic adenocarcinoma [60]. Presentation is often asymptomatic but may present with pancreatitis, abdominal pain, weight loss, jaundice, and pancreatic exocrine insufficiency [61]. They tend to occur in the pancreatic head [29]. IPMNs involve either the main pancreatic duct or branch duct or both [62], but this is not always visible by imaging [21]. MRI with MRCP is considered superior to CT in characterizing these lesions, specifically in identifying a connection with the pancreatic ducts [21].
• How are IPMNs diagnosed and managed?
MD-IPMNs harbor a higher risk of malignancy than BD-IPMNs. In one series, 64% of MD-IPMN resected specimens contained cancer [63]. Because of the high cancer risk, all guidelines recommend surgical resection for appropriate patients [6,14,15,16,17]. BD-IPMNs have a lower risk of cancer at diagnosis, present in 19.5% of resected specimens in one study [63]. As a surgical series, this may overstate the true prevalence, which is supported by another study. A cohort of 103 suspected BD-IPMNs patients were observed and those with high-risk features were resected. The overall rate of cancer at 5 years was 2.6%, and only 1 of 103 patients developed non-resectable disease [64]. For these reasons, suspected BD-IPMNs can often be safely monitored if they do not harbor any high risk stigmata as defined by the international consensus criteria (Table 2)[6]. Otherwise, suspected BD-IPMNs are managed in a similar manner to other pancreatic cysts (Table 2) [6,14].
Prognosis after resection is more favorable for IPMNs than for pancreatic adenocarcinoma, possibly due to earlier stage of detection. The 5-year survival for BD-IPMN is 90% after resection, and 47% for MD-IPMN after resection [62]. Survival rates for IPMNs with invasive adenocarcinoma are lower with a combined overall survival 24% to 42% at 5 years. Survival rates are similar to the survival rate for non-cystic pancreatic adenocarcinoma when controlling for size, invasiveness, and lymph node metastasis [65,66].
Guidelines for surveillance after resection have even fewer applicable studies. The 5-year postoperative recurrence rate is 0 to 20% for IPMNs [6]. The revised international consensus guidelines recommends surveillance 6 months after resection with CT or MRI for all IPMNs, but with no recommendation given on how long to continue surveillance [6]. For patients with invasive disease, the same follow up is recommended as for standard invasive adenocarcinoma [6]. The AGA recommends yearly MRI only for only patients with high-grade dysplasia or invasive disease, with consideration for lifelong surveillance [14].
Case 4
• What is the epidemiology of MCNs and how do they present?
MCNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas histologically defined by the presence of ovarian stroma [67]. They have the potential to develop into pancreatic adenocarcinoma. Unlike IPMNs, MCNs occur almost exclusively in women, and patients are generally younger. In one series, 99.7% of MCNs occurred in females, with a mean age of 47 [67]. Presenting symptoms, as with other cysts, are often vague. These include abdominal pain, fatigue, weight loss, pancreatitis, and a palpable mass. Only 25% of patients are asymptomatic [68].
• How are MCNs diagnosed and managed?
Approximately 95% of MCNs are located in the body or tail of the pancreas [67]. These lesions do not communicate with the pancreatic ducts unlike IPMNs, though they may still cause ductal obstruction and dilation [29]. They are often one large unilocular cyst with a thick cyst wall, but in 20% of cases they can have multiple septations [29]. Peripheral eggshell calcification on CT is present in roughly 25% of cases, which is sometimes helpful in differentiating these lesions from serous cystic neoplasms, which often have central calcification [69].
When diagnosed, MCNs are surgically removed [6,14]. A recent surgical series found that the rate of high-grade dysplasia in resected specimens was 5.5%, and the risk of invasive disease was 4.4% [70]. This data suggests that a more conservative approach of observation rather than immediate resection may be reasonable for some patients [70]. The prognosis is very good after MCN resection, with a 5-year survival of 97% to 100% for all comers [68,70]. However, invasive MCNs have a lower 5-year survival rate ranging from 15% to 75% [70,71]. Per the AGA guidelines, patients with invasive disease or dysplasia should undergo yearly surveillance with MRI [14]. This recommendation is based on a potential field defect described with IPMNs. However, the international consensus guidelines only recommends surveillance if invasive disease is present [6,68,71,72].
Case 5
A 59-year-old male presents for evaluation of sudden onset abdominal pain and an 8-pound weight loss over the past few months. Seven years ago a pancreatic cyst was diagnosed and has since been observed by serial imaging. His lipase was 400 U/L (normal < 82) with normal liver function tests. A CT scan of the abdomen and pelvis showed peri-pancreatic stranding consistent with pancreatitis and a large complex cyst in the head of the pancreas.
• What is the epidemiology of SCNs and how do they present?
SCNs are benign non–mucin-producing cystic lesions that are characterized by a glycogen-rich epithelium on histology [73]. Of patients with SCNs, 74% are female, with a median age 58 [10]. When diagnosed, most patients are usually asymptomatic (61%), but 27% present with abdominal pain. Other symptoms include jaundice, pancreatitis, nausea, and presence of a palpable abdominal mass. SCNs are more common in patients with von Hippel-Lindau syndrome [74].
• How are SCNs diagnosed and managed?
These cysts have fairly even distribution when discovered in the pancreas [53]. About 74% of lesions have smaller micro-cystic components [75]. About 20% of lesions have a characteristic honeycomb appearance, which is highly suggestive of an SCN [76]. About 30% of patients have a characteristic central stellate scar on CT which is also highly suggestive of an SCN [76]. Unlike mucinous neoplasms, peripheral calcification is usually not seen [69].
Malignancy associated with these cysts is very rare, with the largest cohort study reporting a rate of 0.1% [10]. The diagnosis can commonly be made by its unique imaging appearance [10]. Diagnostic biomarkers that may identify such cysts with more certainty are under active investigation [77]. Resection is reasonable and often performed for SCNs when they cause debilitating symptoms including refractory abdominal pain or pancreatitis. When resected and confirmed by pathology, no surveillance is required [14,78].
Conclusion
Pancreatic cysts are common incidental findings in clinical practice today. Many cause anxiety due to their association with pancreas cancer, but most are indolent and safe to observe. Even those cysts with malignant potential grow slowly and immediate surgery is often unnecessary. Several guidelines have been developed, and while there are similarities between them, there are enough critical differences unfortunately to cause some confusion among practitioners today. Further robust research is needed to help address and reconcile these differences. In the meantime, a multidisciplinary approach is highly recommended at dedicated centers of excellence for pancreatic diseases.
Corresponding author: Walter G. Park, MD, MS, 300 Pasteur Drive, MC: 5187, Stanford, CA 94305, [email protected].
Funding/support: Dr. Park is funded by an American College of Gastroenterology Junior Faculty Development Award and is a subcontinent for the National Cancer Institute's Early Detection Research Network.
Financial disclosures: None.
Author contributions: conception and design, TZ, WGP; analysis and interpretation of data, WGP; drafting of article, TZ, WGP; critical revision of the article, TZ, WGP.
From the Department of Medicine, Stanford University School of Medicine, Stanford, CA.
Abstract
- Objective: To review the diagnosis and management of pancreatic cystic lesions.
- Methods: Narrative review of the literature.
- Results: Pancreatic cystic lesions are clinically relevant as some are precursor lesions to pancreatic adenocarcinoma. Mucinous cystic neoplasms and intraductal papillary mucinous neoplasms are 2 commonly encountered pre-cancerous pancreatic cysts. Many cysts are identified incidentally due to frequent use of high-resolution CT and MRI imaging technology. Proposed diagnostic and management algorithms exist to guide clinical practice but are limited by a lack of evidence and discordance among various guidelines. New cyst fluid biomarkers are under development to diagnose cyst types and risk of cancer.
- Conclusion: Pancreatic cysts are increasingly encountered in clinical practice and represent a growing problem. Diagnostic and management algorithms are available to assist practice but are limited by the available evidence. A multidisciplinary approach is recommended.
In the United States there were an estimated 46,420 new cases of pancreatic cancer in 2014 [1]. Of all major cancers, pancreatic cancer had the lowest 5-year survival rate at 6% [1]. Of the 3 known precursor lesions to pancreas adenocarcinoma, 2 are pancreatic cysts [2]. Correctly identifying those with cancer, those with cancer potential (premalignant), and those that are benign (harboring no malignant potential) can be difficult.
Case 1
A 57-year-old male had a 1.5-cm pancreatic cyst located in the head that was found on computed tomography (CT) imaging for suspected renal colic. He had no history or complaints suspicious for pancreatic disease. A CT pancreas protocol scan was obtained, which demonstrated a simple appearing cyst with no mural nodules. The pancreatic and biliary ducts were normal. His laboratory evaluations including liver function testing and lipase were normal.
• What is the approach to incidentally discovered pancreatic cysts?
While many pancreatic cysts are first discovered by cross sectional imaging (CT or MRI), the diagnostic accuracy of defining cyst type and the presence of malignancy is imperfect. The area under the curve (AUC) for differentiating malignant from benign pancreatic cysts ranges from 0.64 to 0.82 for CT and 0.73 to 0.91 for MRI, and no difference between the 2 were observed [12,13]. Several guidelines are currently available to offer guidance on management [6,14,15,16,17]. Much of the current evidence includes retrospective case series with no randomized control trials. The guidelines, therefore, mostly represent consensus-based expert extrapolation of available data.
The American Gastroenterological Association (AGA) recently produced guidelines in 2015 [14]. In comparison to the international consensus guidelines, there are a few key differences, which have now become a point of vigorous debate and disagreement among pancreatologists and confusion among general gastroenterologists and surgeons in the community. Where the international consensus guidelines have stricter criteria to define the appropriateness of surgery, the AGA guidelines are more liberal. AGA defined cysts appropriate for surgery as having 2 out of 3 of the following features: (1) cyst size ≥ 3 cm, (2) presence of a solid component in the cyst, and (3) dilation of the main pancreatic duct. Those having 1 out of 3 criteria were defined as needing further investigation with EUS [14]. These criteria are more relaxed and will likely lead to more surgical resections.
Another difference involves surveillance recommendations. The international consensus guidelines do not define a period when surveillance can be safely stopped. The AGA guidelines define 5 years as the period where if there is no significant change in the cyst from surveillance MRIs performed every 1 to 2 years, then surveillance can be stopped [14]. As the natural history of these cysts remain substantially uncertain, with evidence that malignant transformation occurs after 5 years, this particular recommendation by the AGA remains highly controversial [18,19]. Other differences between these 2 guidelines are summarized in Table 2 [6,14].
Until the surveillance recommendations by the AGA are validated with further studies, we generally follow the 2012 international consensus guidelines. We generally prefer MRI for initial and surveillance evaluations of pancreatic cysts. Besides the lack of radiation exposure, some studies show MRI to have better inter-reader variability [20], better resolution to show cyst communication with the main pancreatic duct [21], and better characterization of peripheral pancreatic cysts [22]. At our center, when a solid mass is suspected, a CT pancreas protocol is preferred in lieu of an MRI by our surgical team.
Case 1 Continued
Despite the reassuring CEA and cytology results, a high concern for a malignant cystic lesion remained based on cyst size, main pancreatic duct dilation, and atrophy noted in the distal pancreas. The patient underwent surgical resection including subtotal pancreatectomy, splenectomy, subtotal gastrectomy, and superior mesenteric and portal vein resection with reconstruction. Pathology revealed the cyst to be a benign pseudocyst.
This case reflects some of the critical challenges in current management of pancreatic cysts. By history, this patient had no suspicion for pancreatitis, making a pseudocyst less likely in a differential diagnosis. When the patient presented 7 years later, again with no reported history of pancreatitis, there was clinical concern for a branch duct IPMN. Although the cyst fluid CEA and cytology were reassuring, the patient met surgical criteria by the 2006 international consensus criteria and the more recent AGA guidelines. Interestingly, the narrowed 2012 international consensus guidelines for surgical resection would have recommended observation. This case highlights the need for better diagnostic tests.
• What is the epidemiology of pancreatic fluid collections and how do they present?
Pancreatic fluid collections are not true cysts as they lack an epithelial cell lining. They often occur in the context of either acute or chronic pancreatitis, and are considered benign or nonmucinous cysts [7,8]. Duct disruption occurs causing pancreatic fluid accumulation, initially defined as an acute peri-pancreatic fluid collection, or an acute necrotic collection if necrosis is present. Over about 4 weeks a more defined cyst wall forms and the cyst is now classified as either a pseudocyst or walled-off pancreatic necrosis [23]. In one review, the median age at presentation was 49 with a male:female ratio of 2:1. Only 52% of fluid collections were discovered following an acute attack of pancreatitis [24]. The risk factors for pancreatic fluid collections are similar to the risk factors for pancreatitis, with the most common being alcohol use and gallstones [24]. Potential symptoms include abdominal pain, weight loss, gastrointestinal bleeding from pseudoaneurysms, obstructive symptoms, sepsis from super infection, and obstructive jaundice [8,24,25,26,27].
• How are pancreatic fluid collections diagnosed and managed?
Clinical suspicion for pancreatic fluid collections should increase if a cyst is diagnosed in the context of acute or chronic pancreatitis [28]. However, other types of cysts can cause ductal obstruction and pancreatitis, so further investigation may be needed, including review of prior imaging if available. The presence of internal debris, the presence of imaging findings of acute or chronic pancreatitis, and fluid extension beyond the pancreas and taking the shape of the retroperitoneum are often characteristics found in pancreatic fluid collections [29,28,30]. If needed, FNA with assay of amylase may be helpful. An amylase value of 5680 IU/L or greater was 84% sensitive and 64% specific (AUC 0.69) for pseudocysts in one study [31].
Management of pancreatic fluid collections is largely based on surgical series. Drainage procedures for pancreatic fluid collections are often undertaken for intractable symptoms or concerns of infection [8,24,25,26]. Asymptomatic pseudocysts can be safely followed. Specific techniques used for pancreatic fluid collection management vary by institutional expertise. Endoscopic drainage can be done by transpapillary stenting if main duct communication is present, or transgastric/transduodenal stenting if the cyst wall is mature and accessible by these approaches [32]. If necrosis is present this can be debrided endoscopically [33]. Laparoscopic surgical options are preferred to open approaches, and can be performed in 1 procedure where endoscopic approaches may require multiple procedures. The most common approach is to drain the cyst by creating a cyst-gastrostomy, or when not feasible a cyst-duodenostomy or cyst-jejunostomy [26,34]. Percutaneous drainage is less commonly performed and used for unstable patients as it can lead to cutaneous fistulas [34]. The best technique for intervention should be decided in the context of a multidisciplinary team. The option for conservative management should be considered as well because about 60% of those managed conservatively will have resolution in 1 year [35].
Case 2
• What is the role of endoscopic ultrasound with FNA in the diagnosis of pancreatic cysts?
While more invasive than CT or MRI, EUS provides detailed imaging to characterize relevant clinical features and allows fine needle aspiration of the cyst fluid and tissue of intra-cystic masses (Table 2) [6,14]. While MRI imaging resolution is continuing to improve [22,36,37], EUS is generally considered superior [38] for diagnosing high-risk lesions. A limitation of EUS, however, is significant inter-observer variability when compared with MRI [37,39,40].
EUS enables FNA of the cyst, which offers unique oppor-tunities for diagnosis. Cyst fluid cytology unfortunately has limited diagnostic yield, with a recent meta-analysis showing sensitivity 63%, specificity 88%, and AUC of 0.89 in differentiating mucinous from nonmucinous cysts [41]. The low sensitivity is likely because cyst fluid is paucicellular. Techniques that include targeting the cyst wall are under investigation and may improve the diagnostic yield of cytological analysis [42]. Tumor markers such as CEA have been widely used in the cyst fluid, with a value > 192 ng/mL having 63% sensitivity and 88% specificity (AUC of 0.79) for mucinous pancreatic cysts [43]. Other cyst fluid markers have been or are being developed including mutated KRAS DNA [44], mutated GNAS DNA [45,46], micro RNA [47,48], glucose [49], proteomic analysis [50], and multiple other molecules [51]. At this time, many of these markers are under investigation.
Case 2 Continued
An endoscopic ultrasound was performed and showed normal main pancreatic and common bile ducts. No intra-cystic mural nodules were observed. FNA was performed. Cytology showed “paucicellular fluid” and the cyst fluid CEA was 319 ng/mL. Having met the original consensus criteria for surgical resection [17] based on size, the presence of mural nodules, and due to suspicion for a mucinous cyst based on the CEA level, the patient underwent a Whipple procedure. The final pathology was a branch-duct IPMN with moderate dysplasia.
• What interventions exist for treating pancreatic cysts?
Surgery is the mainstay of treatment for pancreatic cysts. The most common surgical procedure for worrisome cysts in the head of the pancreas is a pancreatoduodenectomy (Whipple procedure). For cysts in the distal pancreas, a laparoscopic distal pancreatectomy can be performed [52,53]. Middle pancreatectomy, total pancreatectomy, and enucleation are less commonly performed and remain under investigation. The most common complications after surgery are surgical site and nonsurgical site infection, bleeding, pancreatic fistula, and delayed gastric emptying [52,53]. Overall complication rate for pancreatic cyst surgery is 27% to 39%, and perioperative mortality is 0.5% to 4% at high-volume centers [52,53].
An area of active investigation involves EUS-directed chemical cyst ablation. Prior studies using ethanol intra-cystic injection alone showed cyst resolution in 33% of patients [54]. A combination of ethanol and paclitaxel showed cyst resolution in 62% of patients [55]. Though these techniques offer a less invasive alternative to surgery, the complete eradication of dysplastic cystic epithelium remains uncertain and long-term efficacy is unclear. Thus, these techniques should only be considered in the context of a clinical trial or perhaps in patients who are not surgical candidates [56].
Case 3
• What is the epidemiology of IPMNs and how do they present?
IPMNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas involving either the main or branch ducts that have the potential to develop into pancreatic adenocarcinoma [57]. The mean age at presentation for both branch duct IPMNs (BD-IPMNs) and main duct IPMNs (MD-IPMNs) is around 65 years [58,59]. In the United States, the male to female prevalence ratio is equal, though there is some geographic variation among different countries [58]. Risk factors for IPMN formation include diabetes, chronic pancreatitis, and a family history of pancreatic adenocarcinoma [60]. Presentation is often asymptomatic but may present with pancreatitis, abdominal pain, weight loss, jaundice, and pancreatic exocrine insufficiency [61]. They tend to occur in the pancreatic head [29]. IPMNs involve either the main pancreatic duct or branch duct or both [62], but this is not always visible by imaging [21]. MRI with MRCP is considered superior to CT in characterizing these lesions, specifically in identifying a connection with the pancreatic ducts [21].
• How are IPMNs diagnosed and managed?
MD-IPMNs harbor a higher risk of malignancy than BD-IPMNs. In one series, 64% of MD-IPMN resected specimens contained cancer [63]. Because of the high cancer risk, all guidelines recommend surgical resection for appropriate patients [6,14,15,16,17]. BD-IPMNs have a lower risk of cancer at diagnosis, present in 19.5% of resected specimens in one study [63]. As a surgical series, this may overstate the true prevalence, which is supported by another study. A cohort of 103 suspected BD-IPMNs patients were observed and those with high-risk features were resected. The overall rate of cancer at 5 years was 2.6%, and only 1 of 103 patients developed non-resectable disease [64]. For these reasons, suspected BD-IPMNs can often be safely monitored if they do not harbor any high risk stigmata as defined by the international consensus criteria (Table 2)[6]. Otherwise, suspected BD-IPMNs are managed in a similar manner to other pancreatic cysts (Table 2) [6,14].
Prognosis after resection is more favorable for IPMNs than for pancreatic adenocarcinoma, possibly due to earlier stage of detection. The 5-year survival for BD-IPMN is 90% after resection, and 47% for MD-IPMN after resection [62]. Survival rates for IPMNs with invasive adenocarcinoma are lower with a combined overall survival 24% to 42% at 5 years. Survival rates are similar to the survival rate for non-cystic pancreatic adenocarcinoma when controlling for size, invasiveness, and lymph node metastasis [65,66].
Guidelines for surveillance after resection have even fewer applicable studies. The 5-year postoperative recurrence rate is 0 to 20% for IPMNs [6]. The revised international consensus guidelines recommends surveillance 6 months after resection with CT or MRI for all IPMNs, but with no recommendation given on how long to continue surveillance [6]. For patients with invasive disease, the same follow up is recommended as for standard invasive adenocarcinoma [6]. The AGA recommends yearly MRI only for only patients with high-grade dysplasia or invasive disease, with consideration for lifelong surveillance [14].
Case 4
• What is the epidemiology of MCNs and how do they present?
MCNs are mucin-producing lesions (mucinous cysts) of the exocrine pancreas histologically defined by the presence of ovarian stroma [67]. They have the potential to develop into pancreatic adenocarcinoma. Unlike IPMNs, MCNs occur almost exclusively in women, and patients are generally younger. In one series, 99.7% of MCNs occurred in females, with a mean age of 47 [67]. Presenting symptoms, as with other cysts, are often vague. These include abdominal pain, fatigue, weight loss, pancreatitis, and a palpable mass. Only 25% of patients are asymptomatic [68].
• How are MCNs diagnosed and managed?
Approximately 95% of MCNs are located in the body or tail of the pancreas [67]. These lesions do not communicate with the pancreatic ducts unlike IPMNs, though they may still cause ductal obstruction and dilation [29]. They are often one large unilocular cyst with a thick cyst wall, but in 20% of cases they can have multiple septations [29]. Peripheral eggshell calcification on CT is present in roughly 25% of cases, which is sometimes helpful in differentiating these lesions from serous cystic neoplasms, which often have central calcification [69].
When diagnosed, MCNs are surgically removed [6,14]. A recent surgical series found that the rate of high-grade dysplasia in resected specimens was 5.5%, and the risk of invasive disease was 4.4% [70]. This data suggests that a more conservative approach of observation rather than immediate resection may be reasonable for some patients [70]. The prognosis is very good after MCN resection, with a 5-year survival of 97% to 100% for all comers [68,70]. However, invasive MCNs have a lower 5-year survival rate ranging from 15% to 75% [70,71]. Per the AGA guidelines, patients with invasive disease or dysplasia should undergo yearly surveillance with MRI [14]. This recommendation is based on a potential field defect described with IPMNs. However, the international consensus guidelines only recommends surveillance if invasive disease is present [6,68,71,72].
Case 5
A 59-year-old male presents for evaluation of sudden onset abdominal pain and an 8-pound weight loss over the past few months. Seven years ago a pancreatic cyst was diagnosed and has since been observed by serial imaging. His lipase was 400 U/L (normal < 82) with normal liver function tests. A CT scan of the abdomen and pelvis showed peri-pancreatic stranding consistent with pancreatitis and a large complex cyst in the head of the pancreas.
• What is the epidemiology of SCNs and how do they present?
SCNs are benign non–mucin-producing cystic lesions that are characterized by a glycogen-rich epithelium on histology [73]. Of patients with SCNs, 74% are female, with a median age 58 [10]. When diagnosed, most patients are usually asymptomatic (61%), but 27% present with abdominal pain. Other symptoms include jaundice, pancreatitis, nausea, and presence of a palpable abdominal mass. SCNs are more common in patients with von Hippel-Lindau syndrome [74].
• How are SCNs diagnosed and managed?
These cysts have fairly even distribution when discovered in the pancreas [53]. About 74% of lesions have smaller micro-cystic components [75]. About 20% of lesions have a characteristic honeycomb appearance, which is highly suggestive of an SCN [76]. About 30% of patients have a characteristic central stellate scar on CT which is also highly suggestive of an SCN [76]. Unlike mucinous neoplasms, peripheral calcification is usually not seen [69].
Malignancy associated with these cysts is very rare, with the largest cohort study reporting a rate of 0.1% [10]. The diagnosis can commonly be made by its unique imaging appearance [10]. Diagnostic biomarkers that may identify such cysts with more certainty are under active investigation [77]. Resection is reasonable and often performed for SCNs when they cause debilitating symptoms including refractory abdominal pain or pancreatitis. When resected and confirmed by pathology, no surveillance is required [14,78].
Conclusion
Pancreatic cysts are common incidental findings in clinical practice today. Many cause anxiety due to their association with pancreas cancer, but most are indolent and safe to observe. Even those cysts with malignant potential grow slowly and immediate surgery is often unnecessary. Several guidelines have been developed, and while there are similarities between them, there are enough critical differences unfortunately to cause some confusion among practitioners today. Further robust research is needed to help address and reconcile these differences. In the meantime, a multidisciplinary approach is highly recommended at dedicated centers of excellence for pancreatic diseases.
Corresponding author: Walter G. Park, MD, MS, 300 Pasteur Drive, MC: 5187, Stanford, CA 94305, [email protected].
Funding/support: Dr. Park is funded by an American College of Gastroenterology Junior Faculty Development Award and is a subcontinent for the National Cancer Institute's Early Detection Research Network.
Financial disclosures: None.
Author contributions: conception and design, TZ, WGP; analysis and interpretation of data, WGP; drafting of article, TZ, WGP; critical revision of the article, TZ, WGP.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71.
2. Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am 2007;36:831–49.
3. Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008;191:802–7.
4. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105:2079–84.
5. Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol 20(1 Suppl):S71–93.
6. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97.
7. Galanis C, Zamani A, Cameron J, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg 2007;11:820–6.
8. Vitas G, Sarr M. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 1992;111:123–30.
9. Jani N, Bani Hani M, Schulick RD, et al. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc 2011;2011:478913.
10. Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2015 Jun 4.
11. Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc 2015;48:31–8.
12. Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. Am J Roentgenol 2007;189:648–56.
13. Lee H-J, Kim M-J, Choi J-Y, et al. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol 2011;66:315–21.
14. Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–48.
15. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2010;7:754–73.
16. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49.
17. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2006;6(1-2).
18. Ahn D-W, Lee SH, Kim J, et al. Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 2012;6:493–500.
19. Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2012;12:198–202.
20. Grieser C, Heine G, Stelter L, et al. Morphological analysis and differentiation of benign cystic neoplasms of the pancreas using computed tomography and magnetic resonance imaging. Fortschr Röntgenstr 2013;185:219–27.
21. Waters J, Schmidt CM, Pinchot J, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg 2008;12:101–9.
22. Duconseil P, Turrini O, Ewald J, et al. “Peripheric” pancreatic cysts: performance of CT scan, MRI and endoscopy according to final pathological examination. HPB 2015;17:485–9.
23. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11.
24. Sanfey H, Aguilar M, Jones R. Pseudocysts of the pancreas, a review of 97 cases. Am Surg 1994;60:661–8.
25. Cheruvu CVN, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl 2003;85:313–6.
26. Usatoff V, Brancatisano R, Williamson RCN. Operative treatment of pseudocysts in patients with chronic pancreatitis. Br J Surg 2000;87:1494–9.
27. Marshall G, Howell DA, Hansen BL, et al. Multidisciplinary approach to pseudoaneurysms complicating pancreatic pseudocysts: Impact of pretreatment diagnosis. Arch Surg 1996;131:278–83.
28. Sahani DV, Kadavigere R, Saokar A, et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. RadioGraphics 2005;25:1471–84.
29. Tirkes T, Aisen A, Cramer H, et al. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging 2014;39:1088–101.
30. Macari M, Finn ME, Bennett GL, et al. Differentiating Pancreatic cystic neoplasms from pancreatic pseudocysts at MR Imaging: value of perceived internal debris. Radiology 2009;251:77–84.
31. Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011;40:42–5.
32. Braden B, Dietrich CF. Endoscopic ultrasonography-guided endoscopic treatment of pancreatic pseudocysts and walled-off necrosis: New technical developments. World J Gastroenterol WJG 2014;20:16191–6.
33. van Brunschot S, Fockens P, Bakker O, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc 2014;28:1425–38.
34. Pan G, Wan MH, Xie K-L, et al. Classification and management of pancreatic pseudocysts. Medicine (Baltimore). 2015;94:e960.
35. Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990;170:411–7.
36. Chebib I, Yaeger K, Mino-Kenudson M, Pitman MB. The role of cytopathology and cyst fluid analysis in the preoperative diagnosis and management of pancreatic cysts >3 cm. Cancer Cytopathol 2014;122:804–9.
37. Kim JH, Eun HW, Park H-J, et al. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol 2012;81:2927–35.
38. Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? the incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42:717–21.
39. de Jong K, Verlaan T, Dijkgraaf MG, et al. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy 2011;43:579–84.
40. Harinck F, Konings ICAW, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2015 May 18.
41. Thosani N, Thosani S, Qiao W, et al. Role of EUS-FNA based cytology in diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756–66.
42. Hong S-KS, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc 2012;75:775–82.
43. Thornton G, McPhail M, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48–57.
44. Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009;May;69:1095–102.
45. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66.
46. Springer S, Wang Y, Molin MD, et al. A Combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015 Aug 4.
47. Ryu JK, Matthaei H, dal Molin M, et al. Elevated microRNA miR-21 Levels in Pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology 2011;11:343–50.
48. Matthaei H, Wylie D, Lloyd MB, et al. miRNA Biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res 2012;18 :4713–24.
49. Zikos T, Pham K, Bowen R, et al. Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts. Am J Gastroenterol 2015;110:909–14.
50. Corcos O, Couvelard A, Dargère D, et al. Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2012;41:169–74.
51. Thiruvengadam N, Park W. Systematic review of pancreatic cyst fluid biomarkers: the path forward. Clin Transl Gastroenterol 2015;11:e88.
52. Plichta JK, Brosius JA, Pappas SG, et al. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surg 2015;2015:791704.
53. Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4–12.
54. DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc 2009;70:710–23.
55. Oh H-C, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011;140:172–9.
56. Gavini H, Lee JH. Endoscopic ultrasound-guided endotherapy. J Clin Gastroenterol 2015;49:185–93.
57. Grutzmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Ooncologist 2010;15:1294–309.
58. Ingkakul T, Warshaw AL, Fernández-Del Castillo C. Epidemiology of intraductal papillary mucinous neoplasms of the pancreas: sex differences between 3 geographic regions. Pancreas 2011;40:779–80.
59. Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218–26.
60. Capurso G, Boccia S, Salvia R, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol 2013n;108:1003–9.
61. Lubezky N, Ben-Haim M, Nakache R, et al. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg 2010;34:126–32.
62. Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg Chic Ill 1960. 1999;134:1131–6.
63. Serikawa M, Sasaki T, Fujimoto Y, et al. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol 2006;40:856–62.
64. Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 2010;42:1077–84.
65. Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 201 Mar;251:470–6.
66. Worni M, Akushevich I, Gloor B, et al. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol 2012;19:1316–23.
67. Goh BKP, Tan Y-M, Chung Y-FA, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg 2006;30:2236–45.
68. Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008;247:571–9.
69. Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000;175:99–103.
70. Park JW, Jang J-Y, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatology 2014;14:131–6.
71. Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: Part II. Mucinous cystic neoplasms. Surg Oncol 2011;20:e93–101.
72. Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg 2000;231:205–12.
73. Reid MD, Choi H, Balci S, et al. Serous cystic neoplasms of the pancreas: Clinicopathologic and molecular characteristics. Pancreat Neoplasms 2014;31:475–83.
74. Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg Off J Soc Surg Aliment Tract 2012;16:1422–8.
75. Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41):380–7.
76. Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg Off J Soc Surg Aliment Tract 2003;7:417–28.
77. Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg 2014;218:608–17.
78. Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013;45:703–11.
1. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71.
2. Hruban RH, Maitra A, Kern SE, Goggins M. Precursors to pancreatic cancer. Gastroenterol Clin North Am 2007;36:831–49.
3. Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008;191:802–7.
4. Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105:2079–84.
5. Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol 20(1 Suppl):S71–93.
6. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97.
7. Galanis C, Zamani A, Cameron J, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg 2007;11:820–6.
8. Vitas G, Sarr M. Selected management of pancreatic pseudocysts: operative versus expectant management. Surgery 1992;111:123–30.
9. Jani N, Bani Hani M, Schulick RD, et al. Diagnosis and management of cystic lesions of the pancreas. Diagn Ther Endosc 2011;2011:478913.
10. Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2015 Jun 4.
11. Kim YS, Cho JH. Rare nonneoplastic cysts of pancreas. Clin Endosc 2015;48:31–8.
12. Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. Am J Roentgenol 2007;189:648–56.
13. Lee H-J, Kim M-J, Choi J-Y, et al. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin Radiol 2011;66:315–21.
14. Scheiman JM, Hwang JH, Moayyedi P. American Gastroenterological Association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:824–48.
15. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2010;7:754–73.
16. Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol 2007;102:2339–49.
17. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2006;6(1-2).
18. Ahn D-W, Lee SH, Kim J, et al. Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 2012;6:493–500.
19. Khannoussi W, Vullierme MP, Rebours V, et al. The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 2012;12:198–202.
20. Grieser C, Heine G, Stelter L, et al. Morphological analysis and differentiation of benign cystic neoplasms of the pancreas using computed tomography and magnetic resonance imaging. Fortschr Röntgenstr 2013;185:219–27.
21. Waters J, Schmidt CM, Pinchot J, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg 2008;12:101–9.
22. Duconseil P, Turrini O, Ewald J, et al. “Peripheric” pancreatic cysts: performance of CT scan, MRI and endoscopy according to final pathological examination. HPB 2015;17:485–9.
23. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11.
24. Sanfey H, Aguilar M, Jones R. Pseudocysts of the pancreas, a review of 97 cases. Am Surg 1994;60:661–8.
25. Cheruvu CVN, Clarke MG, Prentice M, Eyre-Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Ann R Coll Surg Engl 2003;85:313–6.
26. Usatoff V, Brancatisano R, Williamson RCN. Operative treatment of pseudocysts in patients with chronic pancreatitis. Br J Surg 2000;87:1494–9.
27. Marshall G, Howell DA, Hansen BL, et al. Multidisciplinary approach to pseudoaneurysms complicating pancreatic pseudocysts: Impact of pretreatment diagnosis. Arch Surg 1996;131:278–83.
28. Sahani DV, Kadavigere R, Saokar A, et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. RadioGraphics 2005;25:1471–84.
29. Tirkes T, Aisen A, Cramer H, et al. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging 2014;39:1088–101.
30. Macari M, Finn ME, Bennett GL, et al. Differentiating Pancreatic cystic neoplasms from pancreatic pseudocysts at MR Imaging: value of perceived internal debris. Radiology 2009;251:77–84.
31. Park WG, Mascarenhas R, Palaez-Luna M, et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 2011;40:42–5.
32. Braden B, Dietrich CF. Endoscopic ultrasonography-guided endoscopic treatment of pancreatic pseudocysts and walled-off necrosis: New technical developments. World J Gastroenterol WJG 2014;20:16191–6.
33. van Brunschot S, Fockens P, Bakker O, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surg Endosc 2014;28:1425–38.
34. Pan G, Wan MH, Xie K-L, et al. Classification and management of pancreatic pseudocysts. Medicine (Baltimore). 2015;94:e960.
35. Yeo CJ, Bastidas JA, Lynch-Nyhan A, et al. The natural history of pancreatic pseudocysts documented by computed tomography. Surg Gynecol Obstet 1990;170:411–7.
36. Chebib I, Yaeger K, Mino-Kenudson M, Pitman MB. The role of cytopathology and cyst fluid analysis in the preoperative diagnosis and management of pancreatic cysts >3 cm. Cancer Cytopathol 2014;122:804–9.
37. Kim JH, Eun HW, Park H-J, et al. Diagnostic performance of MRI and EUS in the differentiation of benign from malignant pancreatic cyst and cyst communication with the main duct. Eur J Radiol 2012;81:2927–35.
38. Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts? the incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms. Pancreas 2013;42:717–21.
39. de Jong K, Verlaan T, Dijkgraaf MG, et al. Interobserver agreement for endosonography in the diagnosis of pancreatic cysts. Endoscopy 2011;43:579–84.
40. Harinck F, Konings ICAW, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2015 May 18.
41. Thosani N, Thosani S, Qiao W, et al. Role of EUS-FNA based cytology in diagnosis of mucinous pancreatic cystic lesions: a systematic review and meta-analysis. Dig Dis Sci 2010;55:2756–66.
42. Hong S-KS, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc 2012;75:775–82.
43. Thornton G, McPhail M, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology 2013;13:48–57.
44. Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009;May;69:1095–102.
45. Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66.
46. Springer S, Wang Y, Molin MD, et al. A Combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015 Aug 4.
47. Ryu JK, Matthaei H, dal Molin M, et al. Elevated microRNA miR-21 Levels in Pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology 2011;11:343–50.
48. Matthaei H, Wylie D, Lloyd MB, et al. miRNA Biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res 2012;18 :4713–24.
49. Zikos T, Pham K, Bowen R, et al. Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts. Am J Gastroenterol 2015;110:909–14.
50. Corcos O, Couvelard A, Dargère D, et al. Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2012;41:169–74.
51. Thiruvengadam N, Park W. Systematic review of pancreatic cyst fluid biomarkers: the path forward. Clin Transl Gastroenterol 2015;11:e88.
52. Plichta JK, Brosius JA, Pappas SG, et al. The changing spectrum of surgically treated cystic neoplasms of the pancreas. HPB Surg 2015;2015:791704.
53. Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: A 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4–12.
54. DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc 2009;70:710–23.
55. Oh H-C, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011;140:172–9.
56. Gavini H, Lee JH. Endoscopic ultrasound-guided endotherapy. J Clin Gastroenterol 2015;49:185–93.
57. Grutzmann R, Niedergethmann M, Pilarsky C, et al. Intraductal papillary mucinous tumors of the pancreas: biology, diagnosis, and treatment. Ooncologist 2010;15:1294–309.
58. Ingkakul T, Warshaw AL, Fernández-Del Castillo C. Epidemiology of intraductal papillary mucinous neoplasms of the pancreas: sex differences between 3 geographic regions. Pancreas 2011;40:779–80.
59. Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218–26.
60. Capurso G, Boccia S, Salvia R, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol 2013n;108:1003–9.
61. Lubezky N, Ben-Haim M, Nakache R, et al. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg 2010;34:126–32.
62. Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg Chic Ill 1960. 1999;134:1131–6.
63. Serikawa M, Sasaki T, Fujimoto Y, et al. Management of intraductal papillary-mucinous neoplasm of the pancreas: treatment strategy based on morphologic classification. J Clin Gastroenterol 2006;40:856–62.
64. Sawai Y, Yamao K, Bhatia V, et al. Development of pancreatic cancers during long-term follow-up of side-branch intraductal papillary mucinous neoplasms. Endoscopy 2010;42:1077–84.
65. Poultsides GA, Reddy S, Cameron JL, et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann Surg 201 Mar;251:470–6.
66. Worni M, Akushevich I, Gloor B, et al. Adjuvant radiotherapy in the treatment of invasive intraductal papillary mucinous neoplasm of the pancreas: an analysis of the surveillance, epidemiology, and end results registry. Ann Surg Oncol 2012;19:1316–23.
67. Goh BKP, Tan Y-M, Chung Y-FA, et al. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg 2006;30:2236–45.
68. Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 2008;247:571–9.
69. Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000;175:99–103.
70. Park JW, Jang J-Y, Kang MJ, et al. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatology 2014;14:131–6.
71. Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited: Part II. Mucinous cystic neoplasms. Surg Oncol 2011;20:e93–101.
72. Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg 2000;231:205–12.
73. Reid MD, Choi H, Balci S, et al. Serous cystic neoplasms of the pancreas: Clinicopathologic and molecular characteristics. Pancreat Neoplasms 2014;31:475–83.
74. Charlesworth M, Verbeke CS, Falk GA, et al. Pancreatic lesions in von Hippel-Lindau disease? A systematic review and meta-synthesis of the literature. J Gastrointest Surg Off J Soc Surg Aliment Tract 2012;16:1422–8.
75. Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012;41):380–7.
76. Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg Off J Soc Surg Aliment Tract 2003;7:417–28.
77. Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg 2014;218:608–17.
78. Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013;45:703–11.
Malignant catatonia and aphasia follow multiple-drug overdose
CASE Improvement, then decline
Ms. M, age 37, is brought to the hospital after her husband found her at home, after an unknown duration of impaired consciousness. Her husband reports that Ms. M had normal cognitive functioning before this event, with no difficulty completing activities of daily living. Ms. M’s medical and psychiatric histories are notable for type 2 diabetes mellitus, unspecified bipolar disorder, and opioid, cocaine, and alcohol use disorders. Her medications include paroxetine, 40 mg/d, and gabapentin, 1,200 mg/d.
First admission. Poor inspiratory effort and oxygen saturation of 70% leads to emergent intubation. Serum laboratory studies reveal a white blood cell (WBC) count at 10,900/μL and creatinine phosphokinase level of 25,000 U/L. Urine drug screen is positive for tetrahydrocannabinol, cocaine, and opioids.
Ms. M is admitted to the ICU for management of rhabdomyolysis and multi-organ system failure, including acute hypoxic kidney injury.
By hospital Day 7, the tube is extubated with no recorded physical neurologic deficits. Mental status exam is normal, except for impaired memory of events surrounding the admission. Ms. M is discharged home with a recommendation for outpatient follow-up.
2 Weeks later. Ms. M is brought to the emergency department after a progressive decrease in social interaction, limited oral intake, decline in activities of daily living, and urinary incontinence. Results from laboratory studies are within normal limits; brain MRI is negative; EEG shows generalized moderate slowing.
During psychiatric evaluation, Ms. M is mute and staring continuously. Examination reveals oppositional paratonia (gegenhalten), catalepsy, prominent negativism, and waxy flexibility, all suggestive of catatonia. IV lorazepam is initiated at 1 mg every 8 hours, titrated to 2 mg, 3 times a day.
Ms. M is transferred to a psychiatric hospital for further treatment of catatonia.
Second admission. Evaluation with the Bush-Francis Catatonia Rating Scale supported a diagnosis of catatonia, with the presence of >3 features from the 14-item screen and a score of 16 on the 23-item rating scale.1 After titrating lorazepam to 9 mg/d with minimal therapeutic impact, the psychiatry team consults the electroconvulsive therapy (ECT) service, who deems Ms. M to be an appropriate candidate and petitions for court-ordered ECT.
On hospital Day 8, Ms. M has a fever of 104°F, tachycardia at 180 beats per minute, increased rigidity, and a WBC count of 17,800/μL. She is transferred to the ICU, with a presumptive diagnosis of malignant catatonia.
The medical evaluation, including general laboratory studies, EEG, and spinal fluid analysis, is unremarkable. Because of vital sign instability, 2 ECT treatments are completed in the general hospital before Ms. M resumes psychiatric inpatient care.
By the tenth ECT treatment, Ms. M is no longer febrile and experiences no further autonomic instability or psychomotor features of catatonia. Despite these improvements, she is noted to have persistent word-finding difficulty.
Which test would you order as the next step in your work up?
a) EEG
b) lumbar puncture
c) MRI
d) CT
The authors’ observations
In approximately 25% of cases, catatonia is caused by a general medical condition2; as such, a comprehensive medical workup is vital for assessment and management of catatonic patients. In Ms. M’s case, we considered several medical causes, including nutritional deficiency, infection, a toxin, renal or hepatic impairment, hypothyroidism, seizure, and stroke. Evaluation included measurement of thyroid-stimulating hormone, vitamin B12, and folic acid levels; urinalysis and urine drug screen; chest radiography; lumbar puncture; neuroimaging; and EEG (Table 1).
Several conditions in the differential diagnosis were noteworthy. Ms. M’s severe and sudden neurologic decline, along with a positive urine drug screen for substances of abuse, raised concern about overdose leading to toxic encephalopathy or hypoxic brain injury. Ms. M’s oxygen saturation when she was found was moderately hypoxic at 70%, which is not a level associated with hypoxic brain damage.
We also considered posterior reversible encephalopathy syndrome (PRES), which presents variably with nausea, visual impairment, disturbance in consciousness, seizures, and focal neurologic signs.3 Although 67% to 80% of patients with PRES also have acute hypertension, blood pressure elevation is not necessary for the diagnosis.4 Similar to toxic leukoencephalopathy, PRES is diagnosed by brain MRI, with classic signs of posterior white-matter edema.
Case reports also describe an uncommon demyelinating syndrome, delayed post-hypoxic leukoencephalopathy (DPHL), which develops several weeks or months after a cerebral anoxic insult.5 In Ms. M’s case, brain MRI performed during her second medical hospitalization, 7 days after the initial neuropsychiatric decline, was unremarkable. Using this result to rule out DPHL would have been premature because pathognomonic abnormalities can appear as long as 40 days after the anoxic insult. Given our differential diagnosis, we ordered a repeat MRI.
Etiology and pathophysiology
First described in 1979, DPHL is rare, posing diagnostic challenges for clinical providers.6 Although the exact incidence of DPHL is unknown, the precipitating event typically involves cerebral anoxia, which can occur through carbon monoxide (CO) poisoning, strangulation, cardiac arrest, respiratory failure, and overdose from sedatives and narcotics (Table 2).7 DPHL was first observed in a small percentage (2.75%) of patients suffering from CO poisoning.8,9 Progression of the disease generally includes a period of unconsciousness, then a lucid interval that can last 2 to 40 days, followed by the abrupt onset of neuropsychiatric symptoms.10 The specific pathophysiologic mechanism is unknown, but has been hypothesized to involve inferior compensatory response to decreased oxygenation in the white matter.
Diagnosis and clinical features
DPHL can be divided into 2 clinical variations: parkinsonism and akinetic mutism. The former consists of conventional parkinsonian features along with agitation, apathy, hallucinations, dystonic posturing, and odd behaviors. The latter variant presents with apathy, minimal response to pain, functional bowel and bladder incontinence, mutism, and, at times, inappropriate laughter or tearfulness.5 Both variants share similar features with hypokinetic forms of catatonia.
DPHL is a diagnosis of exclusion. A careful history is critical to establish the possibility of a recent anoxic event. MRI findings, including hyperintensities in the cerebral white matter on T2-based sequencing, are suggestive of the disease. A choline peak on magnetic resonance spectroscopy also might be present in patients with DPHL, although it is not specific to the diagnosis.
Early reports of DPHL suggested an associated deficiency of arylsulfatase A, an enzyme required in the modulation of myelin; however, more recent case reports are conflicting.11 Familial mutations in the gene for arylsulfatase A also result in metachromatic leukodystrophy, and adult onset can present with psychiatric symptoms, including delusions and hallucinations.12
Treatment and prognosis
The treatment of DPHL consists primarily of supportive care and rehabilitation with physical, occupational, and speech therapy.11 With these measures, most patients improve after 3 to 6 months; however, a large percentage sustain some long-term cognitive deficit, the most prevalent symptom being frontal executive dysfunction.5
OUTCOME Supportive care
A second MRI shows diffuse hyperintensities in the white matter that spare the cerebellum and brainstem (Figure). This finding is pathognomonic for DPHL.
ECT is discontinued because there is no evidence to support ECT-associated improvement in DPHL. Moreover, ECT might worsen the clinical course through increased stress and metabolic demand on the brain.13
Because the primary treatment of DPHL is early rehabilitation, we consider that Ms. M would benefit most from increased supportive care and therapy. She is discharged to a brain injury rehabilitation facility, where metoprolol is prescribed for mild tachycardia, along with thiamine and vitamins B12 and D. Physical, occupational, and speech therapy are continued.
Approximately 3 weeks after admission to the rehabilitation program, Ms. M is discharged home. Although she improves in overall activities of daily living, she continues to experience moderate communication deficits and occasional external distractibility.
Bottom Line
Although delayed post-hypoxic leukoencephalopathy is considered rare, consider it in the differential diagnosis when a patient has a recent history of an anoxic event followed by the abrupt onset of neuropsychiatric symptoms. Keep in mind that the condition can be missed if an MRI is obtained too early, and the clinical signs can mimic hypokinetic catatonia.
Related Resources
• Meyer MA. Delayed post-hypoxic leukoencephalopathy: case report with a review of disease pathophysiology. Neurol Int. 2013;5(3):e13. doi: 10.4081/ni.2013.e13.
• Aljarallah S, Al-Hussain F. Acute fatal posthypoxic leukoencephalopathy following benzodiazepine overdose: a case report and review of the literature. BMC Neurol. 2015;15:69.
Drug Brand Names
Gabapentin • Neurontin
Lorazepam • Ativan
Metoprolol • Lopressor
Paroxetine • Paxil
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of com
1. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
2. Azzam PN, Gopalan P. Prototypes of catatonia: diagnostic and therapeutic challenges in the general hospital. Psychosomatics. 2013;54(1):88-93.
3. Tormoehlen LM. Toxic leukoencephalopathies. Neurol Clin. 2011;29(3):591-605
4. Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. In: Vincent JL, ed. Annual update in intensive care and emergency medicine. Heidelberg, Germany: Springer Berlin Heidelberg; 2011:631-653.
5. Schprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26(1):65-72.
6. Wallace IR, Dynan C, Esmonde T. One confused patient, many confused physicians: a case of delayed post-hypoxic leucoencephalopathy. QJM. 2010;103(3):193-194.
7. Lou M, Jing CH, Selim MH, et al. Delayed substantia nigra damage and leukoencephalopathy after hypoxic-ischemic injury. J Neurol Sci. 2009;277(1-2):147-149.
8. Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40(7):433-435.
9. Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27(8):1763-1765.
10. Shprecher DR, Flanigan KM, Smith AG, et al. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20(4):473-477.
11. Lee BH, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42(6):530-533.
12. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49(4):401-406.
13. Quinn DK, Abbott CC. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics. 2014;55(6):525-535.
CASE Improvement, then decline
Ms. M, age 37, is brought to the hospital after her husband found her at home, after an unknown duration of impaired consciousness. Her husband reports that Ms. M had normal cognitive functioning before this event, with no difficulty completing activities of daily living. Ms. M’s medical and psychiatric histories are notable for type 2 diabetes mellitus, unspecified bipolar disorder, and opioid, cocaine, and alcohol use disorders. Her medications include paroxetine, 40 mg/d, and gabapentin, 1,200 mg/d.
First admission. Poor inspiratory effort and oxygen saturation of 70% leads to emergent intubation. Serum laboratory studies reveal a white blood cell (WBC) count at 10,900/μL and creatinine phosphokinase level of 25,000 U/L. Urine drug screen is positive for tetrahydrocannabinol, cocaine, and opioids.
Ms. M is admitted to the ICU for management of rhabdomyolysis and multi-organ system failure, including acute hypoxic kidney injury.
By hospital Day 7, the tube is extubated with no recorded physical neurologic deficits. Mental status exam is normal, except for impaired memory of events surrounding the admission. Ms. M is discharged home with a recommendation for outpatient follow-up.
2 Weeks later. Ms. M is brought to the emergency department after a progressive decrease in social interaction, limited oral intake, decline in activities of daily living, and urinary incontinence. Results from laboratory studies are within normal limits; brain MRI is negative; EEG shows generalized moderate slowing.
During psychiatric evaluation, Ms. M is mute and staring continuously. Examination reveals oppositional paratonia (gegenhalten), catalepsy, prominent negativism, and waxy flexibility, all suggestive of catatonia. IV lorazepam is initiated at 1 mg every 8 hours, titrated to 2 mg, 3 times a day.
Ms. M is transferred to a psychiatric hospital for further treatment of catatonia.
Second admission. Evaluation with the Bush-Francis Catatonia Rating Scale supported a diagnosis of catatonia, with the presence of >3 features from the 14-item screen and a score of 16 on the 23-item rating scale.1 After titrating lorazepam to 9 mg/d with minimal therapeutic impact, the psychiatry team consults the electroconvulsive therapy (ECT) service, who deems Ms. M to be an appropriate candidate and petitions for court-ordered ECT.
On hospital Day 8, Ms. M has a fever of 104°F, tachycardia at 180 beats per minute, increased rigidity, and a WBC count of 17,800/μL. She is transferred to the ICU, with a presumptive diagnosis of malignant catatonia.
The medical evaluation, including general laboratory studies, EEG, and spinal fluid analysis, is unremarkable. Because of vital sign instability, 2 ECT treatments are completed in the general hospital before Ms. M resumes psychiatric inpatient care.
By the tenth ECT treatment, Ms. M is no longer febrile and experiences no further autonomic instability or psychomotor features of catatonia. Despite these improvements, she is noted to have persistent word-finding difficulty.
Which test would you order as the next step in your work up?
a) EEG
b) lumbar puncture
c) MRI
d) CT
The authors’ observations
In approximately 25% of cases, catatonia is caused by a general medical condition2; as such, a comprehensive medical workup is vital for assessment and management of catatonic patients. In Ms. M’s case, we considered several medical causes, including nutritional deficiency, infection, a toxin, renal or hepatic impairment, hypothyroidism, seizure, and stroke. Evaluation included measurement of thyroid-stimulating hormone, vitamin B12, and folic acid levels; urinalysis and urine drug screen; chest radiography; lumbar puncture; neuroimaging; and EEG (Table 1).
Several conditions in the differential diagnosis were noteworthy. Ms. M’s severe and sudden neurologic decline, along with a positive urine drug screen for substances of abuse, raised concern about overdose leading to toxic encephalopathy or hypoxic brain injury. Ms. M’s oxygen saturation when she was found was moderately hypoxic at 70%, which is not a level associated with hypoxic brain damage.
We also considered posterior reversible encephalopathy syndrome (PRES), which presents variably with nausea, visual impairment, disturbance in consciousness, seizures, and focal neurologic signs.3 Although 67% to 80% of patients with PRES also have acute hypertension, blood pressure elevation is not necessary for the diagnosis.4 Similar to toxic leukoencephalopathy, PRES is diagnosed by brain MRI, with classic signs of posterior white-matter edema.
Case reports also describe an uncommon demyelinating syndrome, delayed post-hypoxic leukoencephalopathy (DPHL), which develops several weeks or months after a cerebral anoxic insult.5 In Ms. M’s case, brain MRI performed during her second medical hospitalization, 7 days after the initial neuropsychiatric decline, was unremarkable. Using this result to rule out DPHL would have been premature because pathognomonic abnormalities can appear as long as 40 days after the anoxic insult. Given our differential diagnosis, we ordered a repeat MRI.
Etiology and pathophysiology
First described in 1979, DPHL is rare, posing diagnostic challenges for clinical providers.6 Although the exact incidence of DPHL is unknown, the precipitating event typically involves cerebral anoxia, which can occur through carbon monoxide (CO) poisoning, strangulation, cardiac arrest, respiratory failure, and overdose from sedatives and narcotics (Table 2).7 DPHL was first observed in a small percentage (2.75%) of patients suffering from CO poisoning.8,9 Progression of the disease generally includes a period of unconsciousness, then a lucid interval that can last 2 to 40 days, followed by the abrupt onset of neuropsychiatric symptoms.10 The specific pathophysiologic mechanism is unknown, but has been hypothesized to involve inferior compensatory response to decreased oxygenation in the white matter.
Diagnosis and clinical features
DPHL can be divided into 2 clinical variations: parkinsonism and akinetic mutism. The former consists of conventional parkinsonian features along with agitation, apathy, hallucinations, dystonic posturing, and odd behaviors. The latter variant presents with apathy, minimal response to pain, functional bowel and bladder incontinence, mutism, and, at times, inappropriate laughter or tearfulness.5 Both variants share similar features with hypokinetic forms of catatonia.
DPHL is a diagnosis of exclusion. A careful history is critical to establish the possibility of a recent anoxic event. MRI findings, including hyperintensities in the cerebral white matter on T2-based sequencing, are suggestive of the disease. A choline peak on magnetic resonance spectroscopy also might be present in patients with DPHL, although it is not specific to the diagnosis.
Early reports of DPHL suggested an associated deficiency of arylsulfatase A, an enzyme required in the modulation of myelin; however, more recent case reports are conflicting.11 Familial mutations in the gene for arylsulfatase A also result in metachromatic leukodystrophy, and adult onset can present with psychiatric symptoms, including delusions and hallucinations.12
Treatment and prognosis
The treatment of DPHL consists primarily of supportive care and rehabilitation with physical, occupational, and speech therapy.11 With these measures, most patients improve after 3 to 6 months; however, a large percentage sustain some long-term cognitive deficit, the most prevalent symptom being frontal executive dysfunction.5
OUTCOME Supportive care
A second MRI shows diffuse hyperintensities in the white matter that spare the cerebellum and brainstem (Figure). This finding is pathognomonic for DPHL.
ECT is discontinued because there is no evidence to support ECT-associated improvement in DPHL. Moreover, ECT might worsen the clinical course through increased stress and metabolic demand on the brain.13
Because the primary treatment of DPHL is early rehabilitation, we consider that Ms. M would benefit most from increased supportive care and therapy. She is discharged to a brain injury rehabilitation facility, where metoprolol is prescribed for mild tachycardia, along with thiamine and vitamins B12 and D. Physical, occupational, and speech therapy are continued.
Approximately 3 weeks after admission to the rehabilitation program, Ms. M is discharged home. Although she improves in overall activities of daily living, she continues to experience moderate communication deficits and occasional external distractibility.
Bottom Line
Although delayed post-hypoxic leukoencephalopathy is considered rare, consider it in the differential diagnosis when a patient has a recent history of an anoxic event followed by the abrupt onset of neuropsychiatric symptoms. Keep in mind that the condition can be missed if an MRI is obtained too early, and the clinical signs can mimic hypokinetic catatonia.
Related Resources
• Meyer MA. Delayed post-hypoxic leukoencephalopathy: case report with a review of disease pathophysiology. Neurol Int. 2013;5(3):e13. doi: 10.4081/ni.2013.e13.
• Aljarallah S, Al-Hussain F. Acute fatal posthypoxic leukoencephalopathy following benzodiazepine overdose: a case report and review of the literature. BMC Neurol. 2015;15:69.
Drug Brand Names
Gabapentin • Neurontin
Lorazepam • Ativan
Metoprolol • Lopressor
Paroxetine • Paxil
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of com
CASE Improvement, then decline
Ms. M, age 37, is brought to the hospital after her husband found her at home, after an unknown duration of impaired consciousness. Her husband reports that Ms. M had normal cognitive functioning before this event, with no difficulty completing activities of daily living. Ms. M’s medical and psychiatric histories are notable for type 2 diabetes mellitus, unspecified bipolar disorder, and opioid, cocaine, and alcohol use disorders. Her medications include paroxetine, 40 mg/d, and gabapentin, 1,200 mg/d.
First admission. Poor inspiratory effort and oxygen saturation of 70% leads to emergent intubation. Serum laboratory studies reveal a white blood cell (WBC) count at 10,900/μL and creatinine phosphokinase level of 25,000 U/L. Urine drug screen is positive for tetrahydrocannabinol, cocaine, and opioids.
Ms. M is admitted to the ICU for management of rhabdomyolysis and multi-organ system failure, including acute hypoxic kidney injury.
By hospital Day 7, the tube is extubated with no recorded physical neurologic deficits. Mental status exam is normal, except for impaired memory of events surrounding the admission. Ms. M is discharged home with a recommendation for outpatient follow-up.
2 Weeks later. Ms. M is brought to the emergency department after a progressive decrease in social interaction, limited oral intake, decline in activities of daily living, and urinary incontinence. Results from laboratory studies are within normal limits; brain MRI is negative; EEG shows generalized moderate slowing.
During psychiatric evaluation, Ms. M is mute and staring continuously. Examination reveals oppositional paratonia (gegenhalten), catalepsy, prominent negativism, and waxy flexibility, all suggestive of catatonia. IV lorazepam is initiated at 1 mg every 8 hours, titrated to 2 mg, 3 times a day.
Ms. M is transferred to a psychiatric hospital for further treatment of catatonia.
Second admission. Evaluation with the Bush-Francis Catatonia Rating Scale supported a diagnosis of catatonia, with the presence of >3 features from the 14-item screen and a score of 16 on the 23-item rating scale.1 After titrating lorazepam to 9 mg/d with minimal therapeutic impact, the psychiatry team consults the electroconvulsive therapy (ECT) service, who deems Ms. M to be an appropriate candidate and petitions for court-ordered ECT.
On hospital Day 8, Ms. M has a fever of 104°F, tachycardia at 180 beats per minute, increased rigidity, and a WBC count of 17,800/μL. She is transferred to the ICU, with a presumptive diagnosis of malignant catatonia.
The medical evaluation, including general laboratory studies, EEG, and spinal fluid analysis, is unremarkable. Because of vital sign instability, 2 ECT treatments are completed in the general hospital before Ms. M resumes psychiatric inpatient care.
By the tenth ECT treatment, Ms. M is no longer febrile and experiences no further autonomic instability or psychomotor features of catatonia. Despite these improvements, she is noted to have persistent word-finding difficulty.
Which test would you order as the next step in your work up?
a) EEG
b) lumbar puncture
c) MRI
d) CT
The authors’ observations
In approximately 25% of cases, catatonia is caused by a general medical condition2; as such, a comprehensive medical workup is vital for assessment and management of catatonic patients. In Ms. M’s case, we considered several medical causes, including nutritional deficiency, infection, a toxin, renal or hepatic impairment, hypothyroidism, seizure, and stroke. Evaluation included measurement of thyroid-stimulating hormone, vitamin B12, and folic acid levels; urinalysis and urine drug screen; chest radiography; lumbar puncture; neuroimaging; and EEG (Table 1).
Several conditions in the differential diagnosis were noteworthy. Ms. M’s severe and sudden neurologic decline, along with a positive urine drug screen for substances of abuse, raised concern about overdose leading to toxic encephalopathy or hypoxic brain injury. Ms. M’s oxygen saturation when she was found was moderately hypoxic at 70%, which is not a level associated with hypoxic brain damage.
We also considered posterior reversible encephalopathy syndrome (PRES), which presents variably with nausea, visual impairment, disturbance in consciousness, seizures, and focal neurologic signs.3 Although 67% to 80% of patients with PRES also have acute hypertension, blood pressure elevation is not necessary for the diagnosis.4 Similar to toxic leukoencephalopathy, PRES is diagnosed by brain MRI, with classic signs of posterior white-matter edema.
Case reports also describe an uncommon demyelinating syndrome, delayed post-hypoxic leukoencephalopathy (DPHL), which develops several weeks or months after a cerebral anoxic insult.5 In Ms. M’s case, brain MRI performed during her second medical hospitalization, 7 days after the initial neuropsychiatric decline, was unremarkable. Using this result to rule out DPHL would have been premature because pathognomonic abnormalities can appear as long as 40 days after the anoxic insult. Given our differential diagnosis, we ordered a repeat MRI.
Etiology and pathophysiology
First described in 1979, DPHL is rare, posing diagnostic challenges for clinical providers.6 Although the exact incidence of DPHL is unknown, the precipitating event typically involves cerebral anoxia, which can occur through carbon monoxide (CO) poisoning, strangulation, cardiac arrest, respiratory failure, and overdose from sedatives and narcotics (Table 2).7 DPHL was first observed in a small percentage (2.75%) of patients suffering from CO poisoning.8,9 Progression of the disease generally includes a period of unconsciousness, then a lucid interval that can last 2 to 40 days, followed by the abrupt onset of neuropsychiatric symptoms.10 The specific pathophysiologic mechanism is unknown, but has been hypothesized to involve inferior compensatory response to decreased oxygenation in the white matter.
Diagnosis and clinical features
DPHL can be divided into 2 clinical variations: parkinsonism and akinetic mutism. The former consists of conventional parkinsonian features along with agitation, apathy, hallucinations, dystonic posturing, and odd behaviors. The latter variant presents with apathy, minimal response to pain, functional bowel and bladder incontinence, mutism, and, at times, inappropriate laughter or tearfulness.5 Both variants share similar features with hypokinetic forms of catatonia.
DPHL is a diagnosis of exclusion. A careful history is critical to establish the possibility of a recent anoxic event. MRI findings, including hyperintensities in the cerebral white matter on T2-based sequencing, are suggestive of the disease. A choline peak on magnetic resonance spectroscopy also might be present in patients with DPHL, although it is not specific to the diagnosis.
Early reports of DPHL suggested an associated deficiency of arylsulfatase A, an enzyme required in the modulation of myelin; however, more recent case reports are conflicting.11 Familial mutations in the gene for arylsulfatase A also result in metachromatic leukodystrophy, and adult onset can present with psychiatric symptoms, including delusions and hallucinations.12
Treatment and prognosis
The treatment of DPHL consists primarily of supportive care and rehabilitation with physical, occupational, and speech therapy.11 With these measures, most patients improve after 3 to 6 months; however, a large percentage sustain some long-term cognitive deficit, the most prevalent symptom being frontal executive dysfunction.5
OUTCOME Supportive care
A second MRI shows diffuse hyperintensities in the white matter that spare the cerebellum and brainstem (Figure). This finding is pathognomonic for DPHL.
ECT is discontinued because there is no evidence to support ECT-associated improvement in DPHL. Moreover, ECT might worsen the clinical course through increased stress and metabolic demand on the brain.13
Because the primary treatment of DPHL is early rehabilitation, we consider that Ms. M would benefit most from increased supportive care and therapy. She is discharged to a brain injury rehabilitation facility, where metoprolol is prescribed for mild tachycardia, along with thiamine and vitamins B12 and D. Physical, occupational, and speech therapy are continued.
Approximately 3 weeks after admission to the rehabilitation program, Ms. M is discharged home. Although she improves in overall activities of daily living, she continues to experience moderate communication deficits and occasional external distractibility.
Bottom Line
Although delayed post-hypoxic leukoencephalopathy is considered rare, consider it in the differential diagnosis when a patient has a recent history of an anoxic event followed by the abrupt onset of neuropsychiatric symptoms. Keep in mind that the condition can be missed if an MRI is obtained too early, and the clinical signs can mimic hypokinetic catatonia.
Related Resources
• Meyer MA. Delayed post-hypoxic leukoencephalopathy: case report with a review of disease pathophysiology. Neurol Int. 2013;5(3):e13. doi: 10.4081/ni.2013.e13.
• Aljarallah S, Al-Hussain F. Acute fatal posthypoxic leukoencephalopathy following benzodiazepine overdose: a case report and review of the literature. BMC Neurol. 2015;15:69.
Drug Brand Names
Gabapentin • Neurontin
Lorazepam • Ativan
Metoprolol • Lopressor
Paroxetine • Paxil
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of com
1. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
2. Azzam PN, Gopalan P. Prototypes of catatonia: diagnostic and therapeutic challenges in the general hospital. Psychosomatics. 2013;54(1):88-93.
3. Tormoehlen LM. Toxic leukoencephalopathies. Neurol Clin. 2011;29(3):591-605
4. Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. In: Vincent JL, ed. Annual update in intensive care and emergency medicine. Heidelberg, Germany: Springer Berlin Heidelberg; 2011:631-653.
5. Schprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26(1):65-72.
6. Wallace IR, Dynan C, Esmonde T. One confused patient, many confused physicians: a case of delayed post-hypoxic leucoencephalopathy. QJM. 2010;103(3):193-194.
7. Lou M, Jing CH, Selim MH, et al. Delayed substantia nigra damage and leukoencephalopathy after hypoxic-ischemic injury. J Neurol Sci. 2009;277(1-2):147-149.
8. Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40(7):433-435.
9. Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27(8):1763-1765.
10. Shprecher DR, Flanigan KM, Smith AG, et al. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20(4):473-477.
11. Lee BH, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42(6):530-533.
12. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49(4):401-406.
13. Quinn DK, Abbott CC. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics. 2014;55(6):525-535.
1. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
2. Azzam PN, Gopalan P. Prototypes of catatonia: diagnostic and therapeutic challenges in the general hospital. Psychosomatics. 2013;54(1):88-93.
3. Tormoehlen LM. Toxic leukoencephalopathies. Neurol Clin. 2011;29(3):591-605
4. Legriel S, Pico F, Azoulay E. Understanding posterior reversible encephalopathy syndrome. In: Vincent JL, ed. Annual update in intensive care and emergency medicine. Heidelberg, Germany: Springer Berlin Heidelberg; 2011:631-653.
5. Schprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26(1):65-72.
6. Wallace IR, Dynan C, Esmonde T. One confused patient, many confused physicians: a case of delayed post-hypoxic leucoencephalopathy. QJM. 2010;103(3):193-194.
7. Lou M, Jing CH, Selim MH, et al. Delayed substantia nigra damage and leukoencephalopathy after hypoxic-ischemic injury. J Neurol Sci. 2009;277(1-2):147-149.
8. Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40(7):433-435.
9. Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27(8):1763-1765.
10. Shprecher DR, Flanigan KM, Smith AG, et al. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20(4):473-477.
11. Lee BH, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42(6):530-533.
12. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49(4):401-406.
13. Quinn DK, Abbott CC. Catatonia after cerebral hypoxia: do the usual treatments apply? Psychosomatics. 2014;55(6):525-535.