User login
A guide to providing wide-ranging care to newborns
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
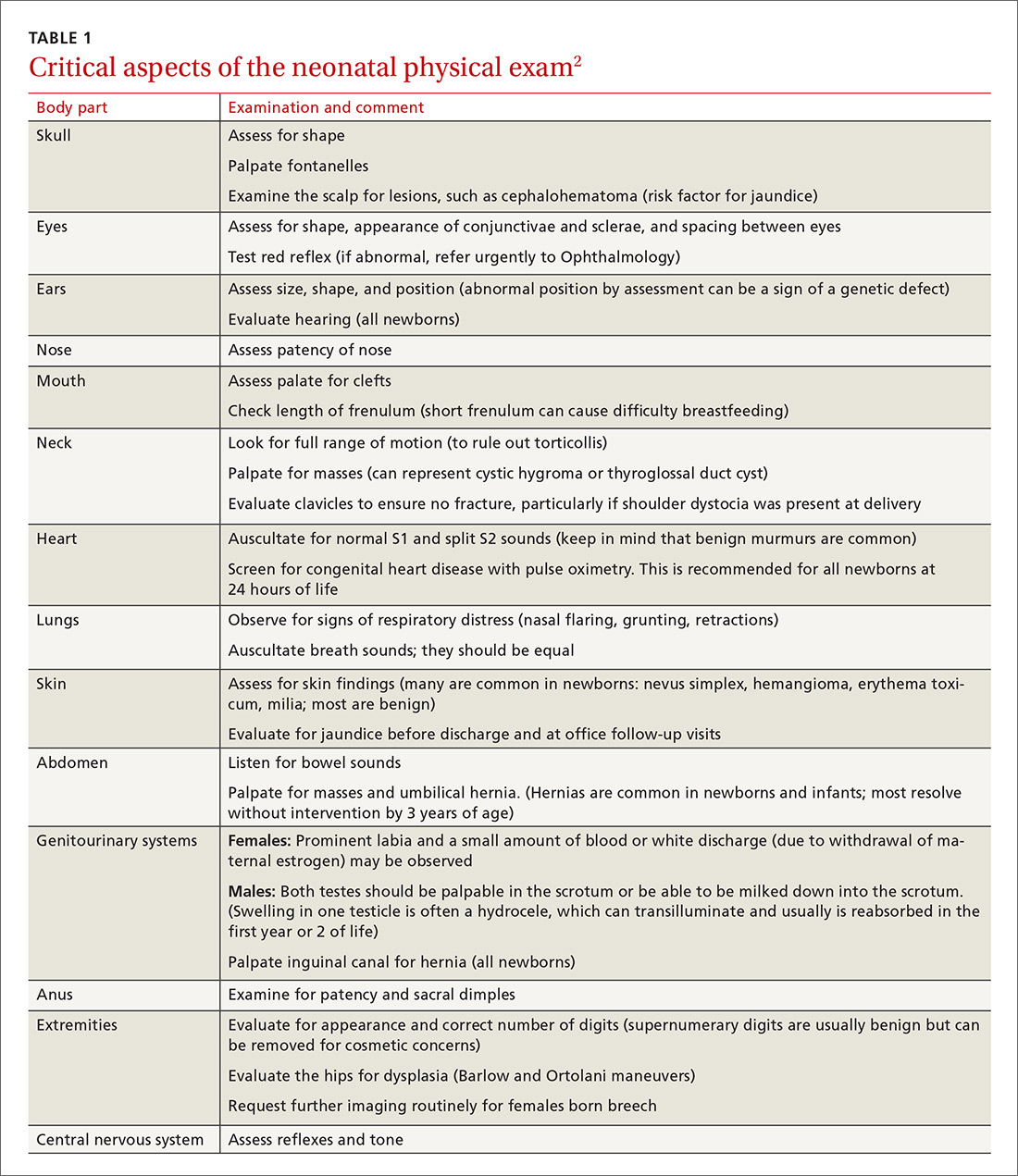
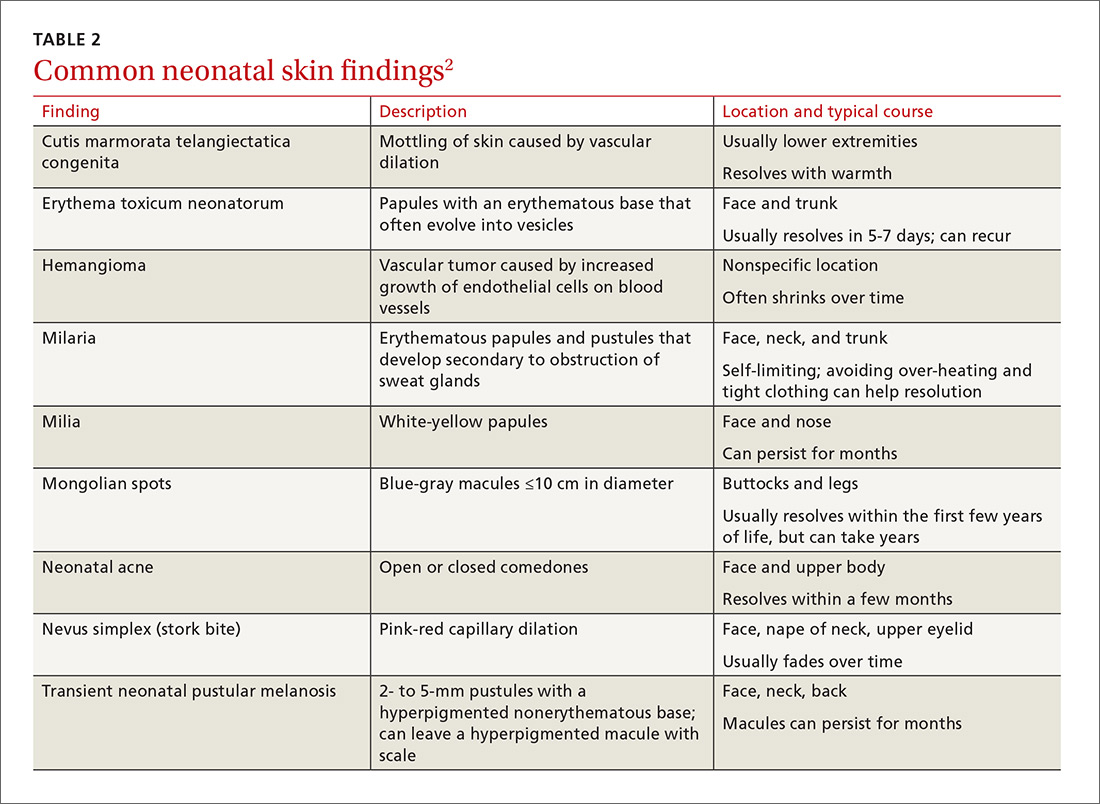
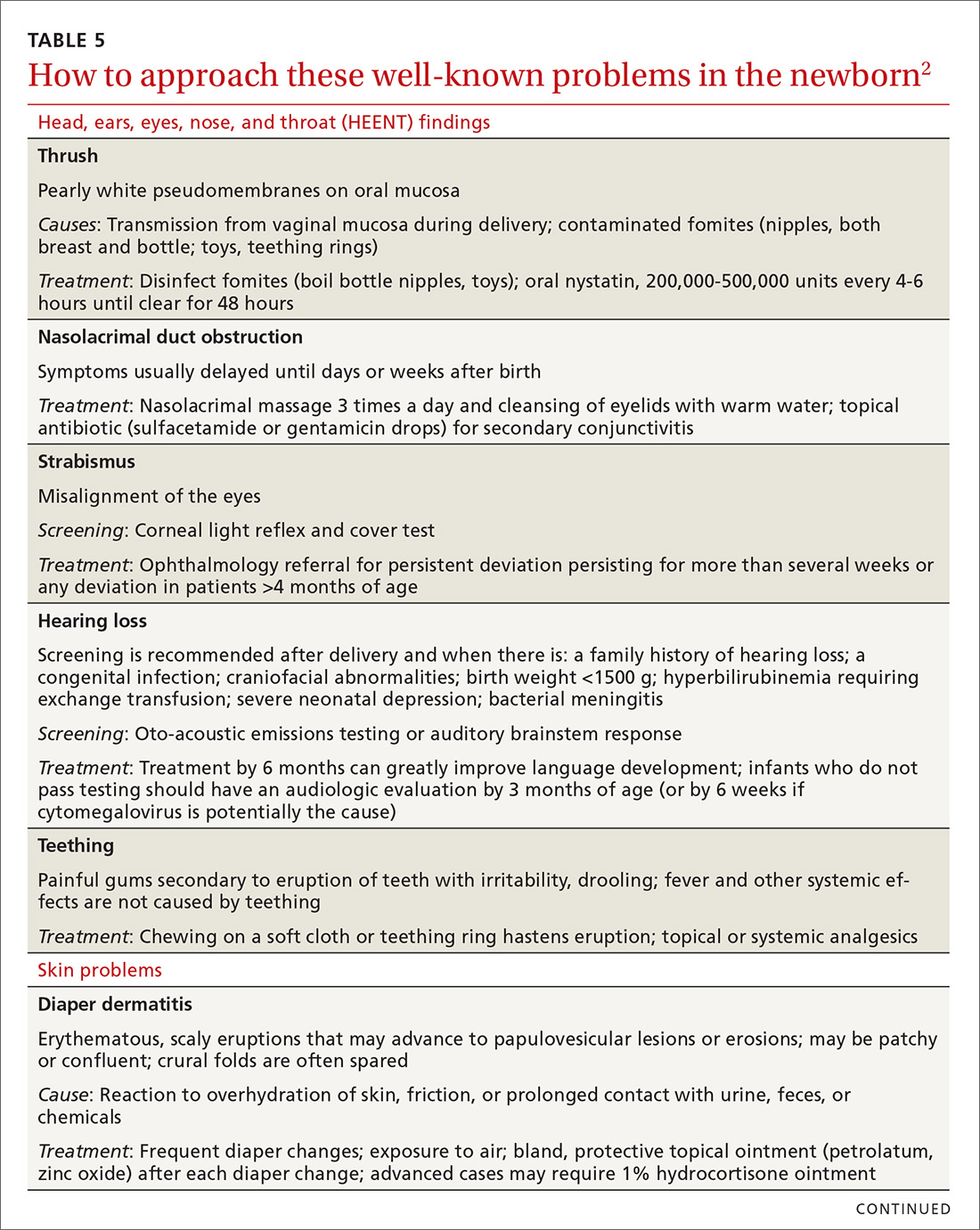
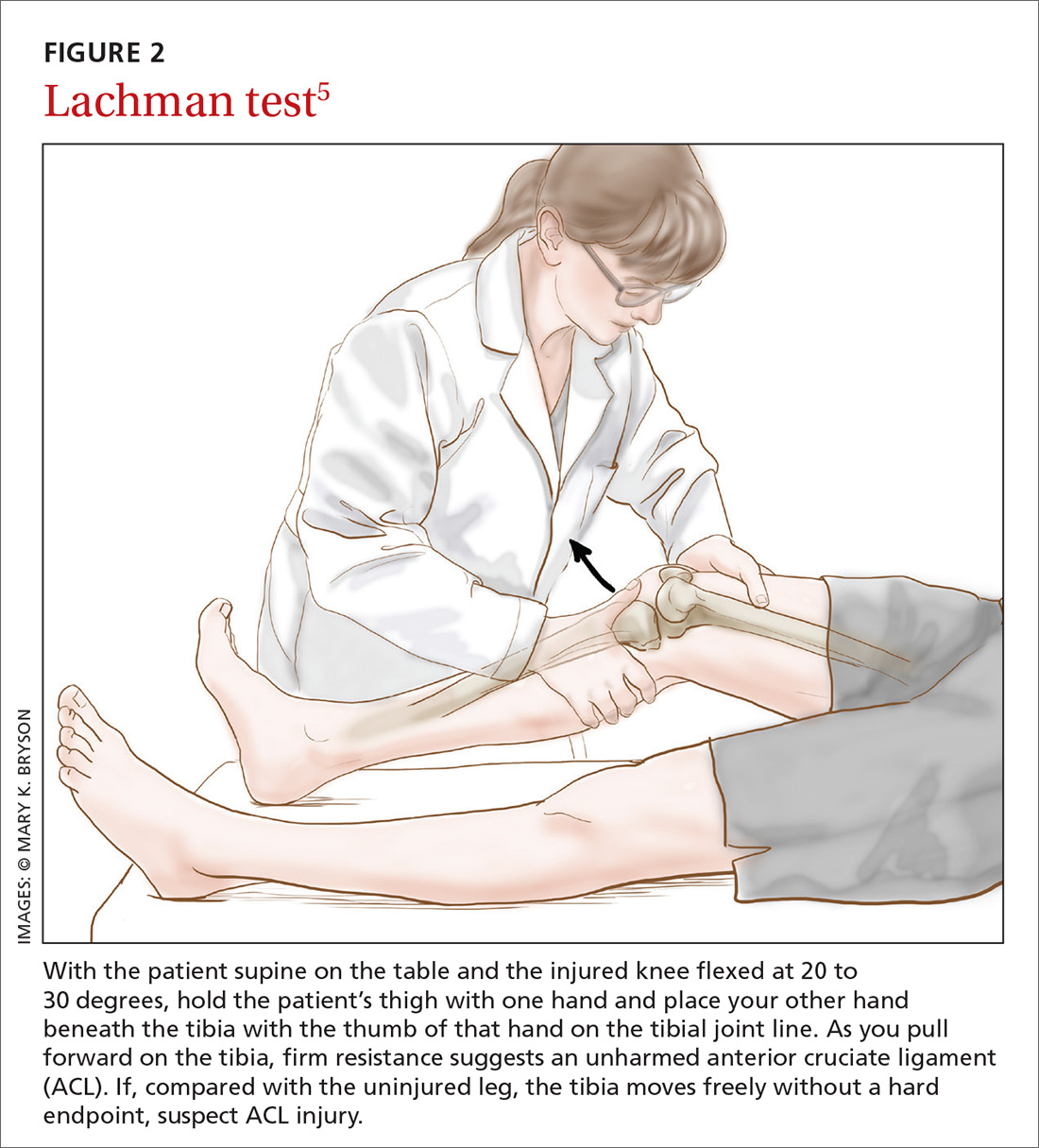
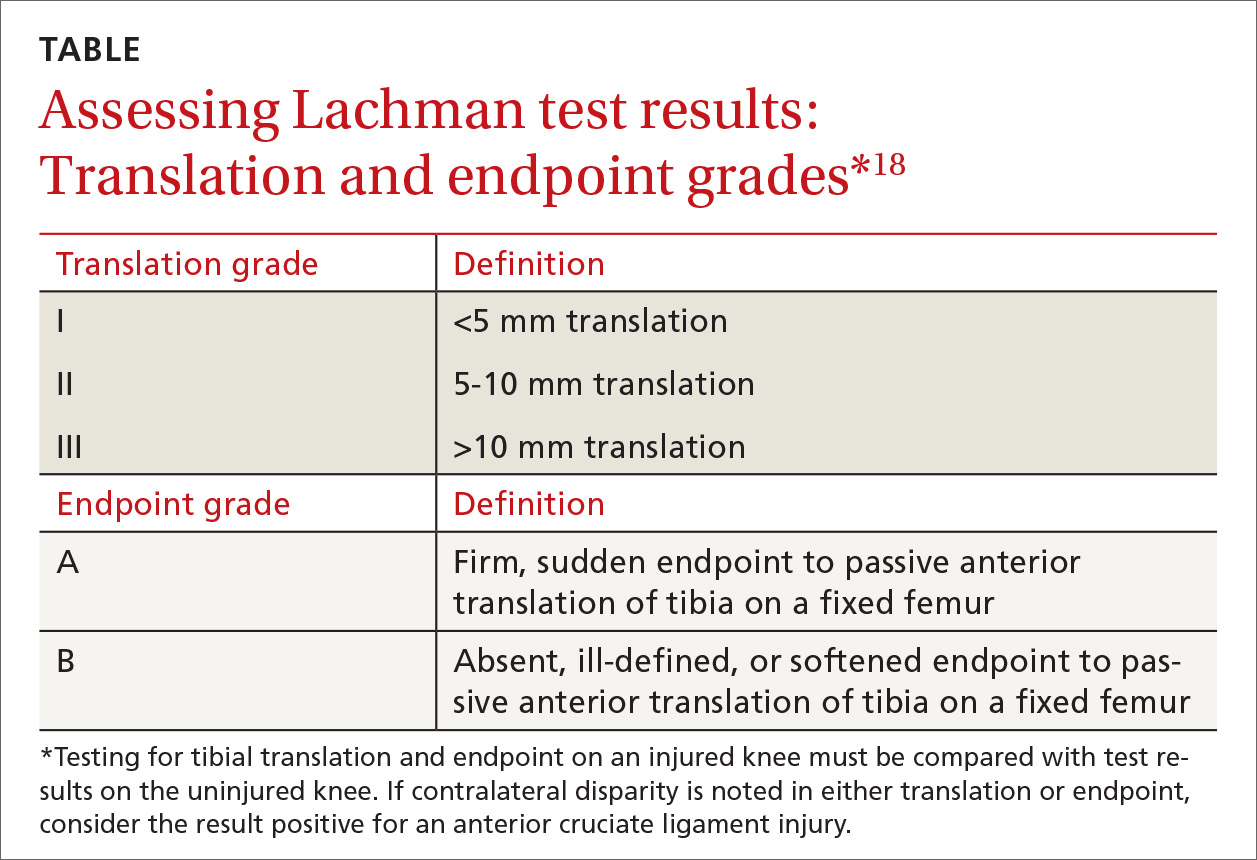
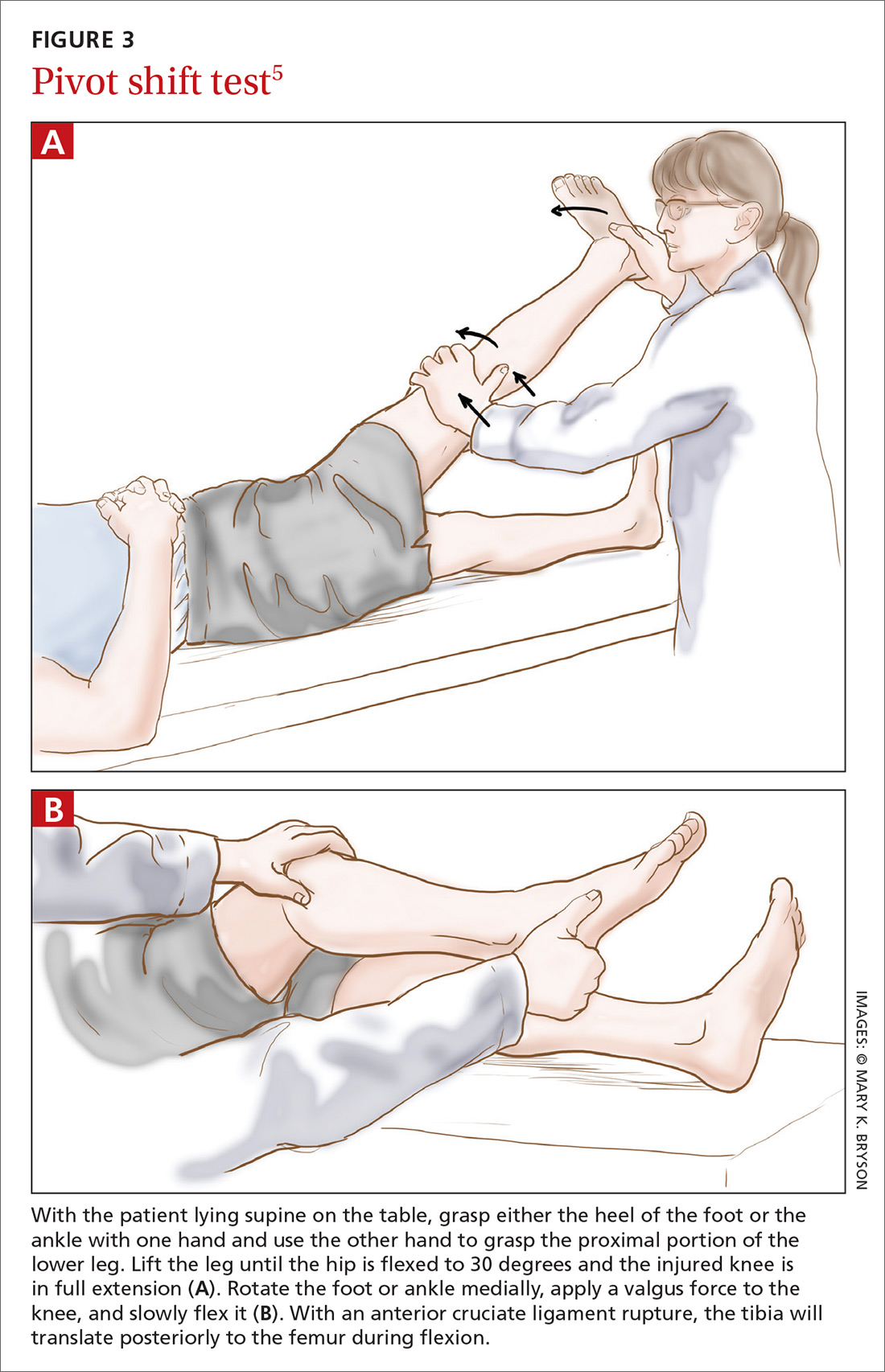
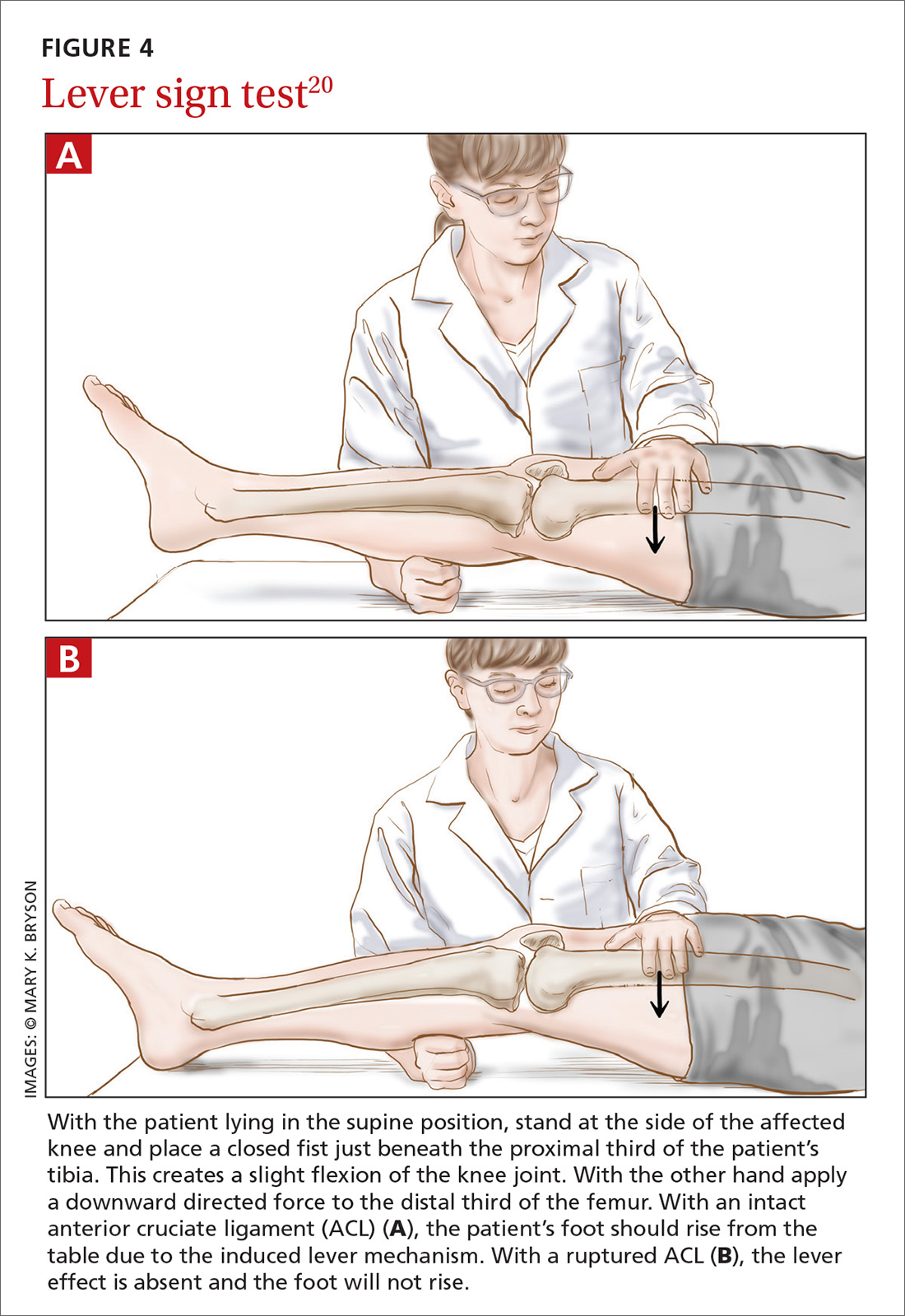
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.
Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8
Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
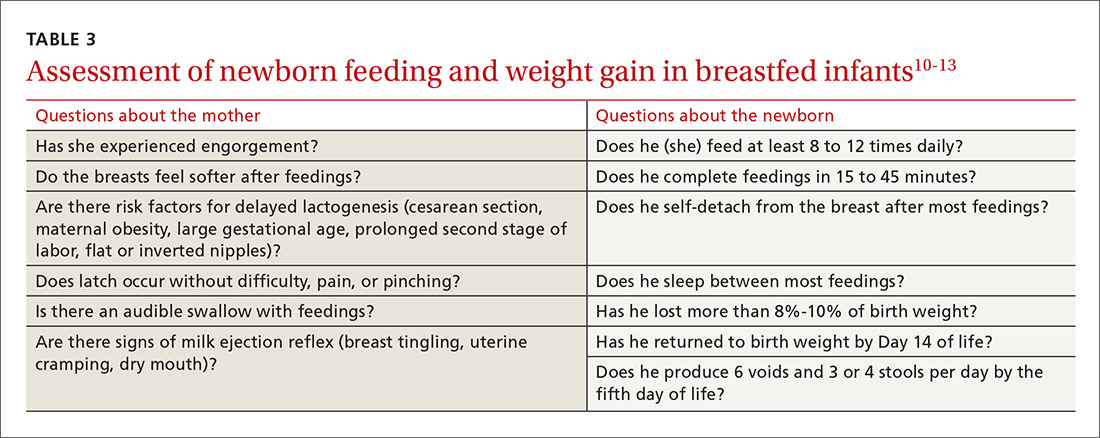
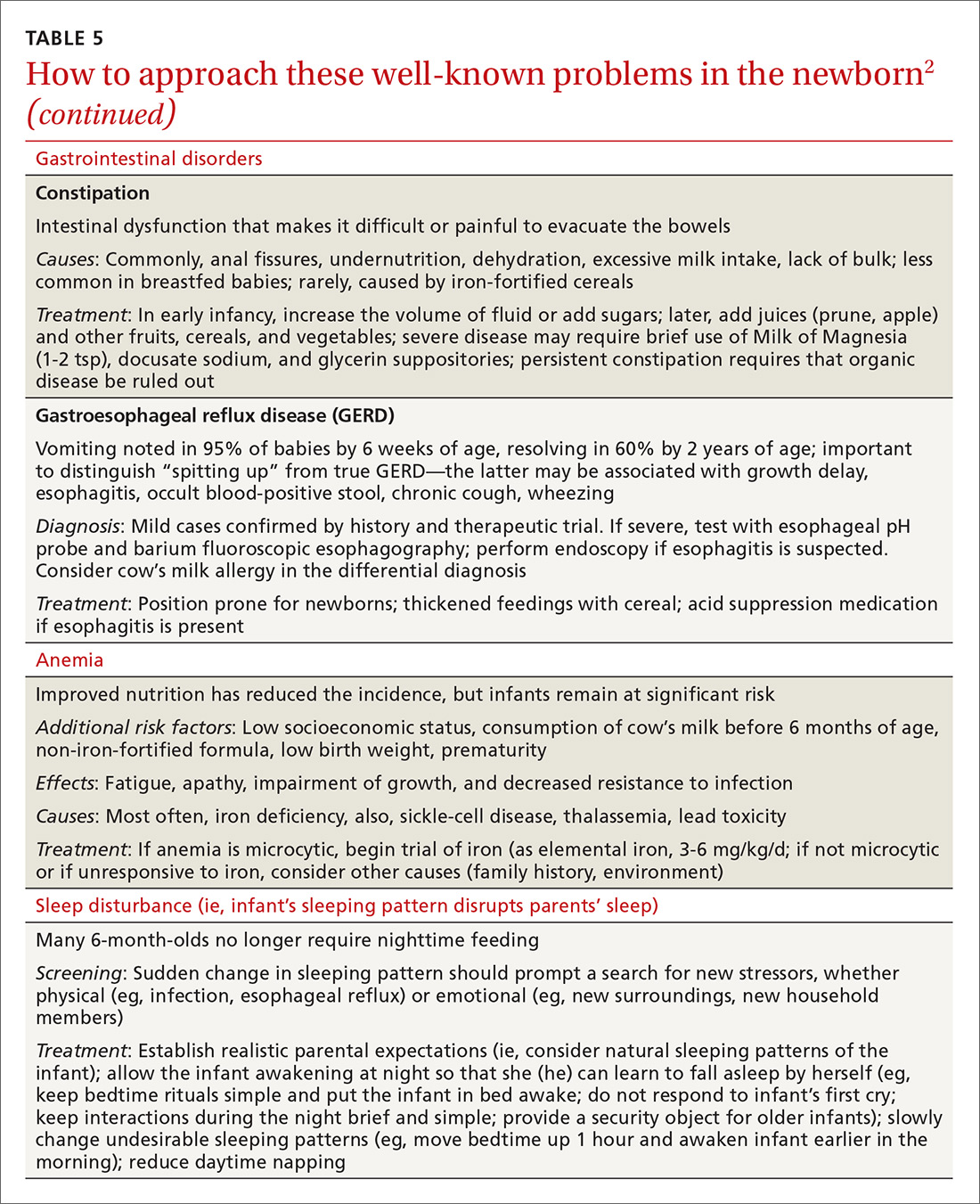
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1
Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
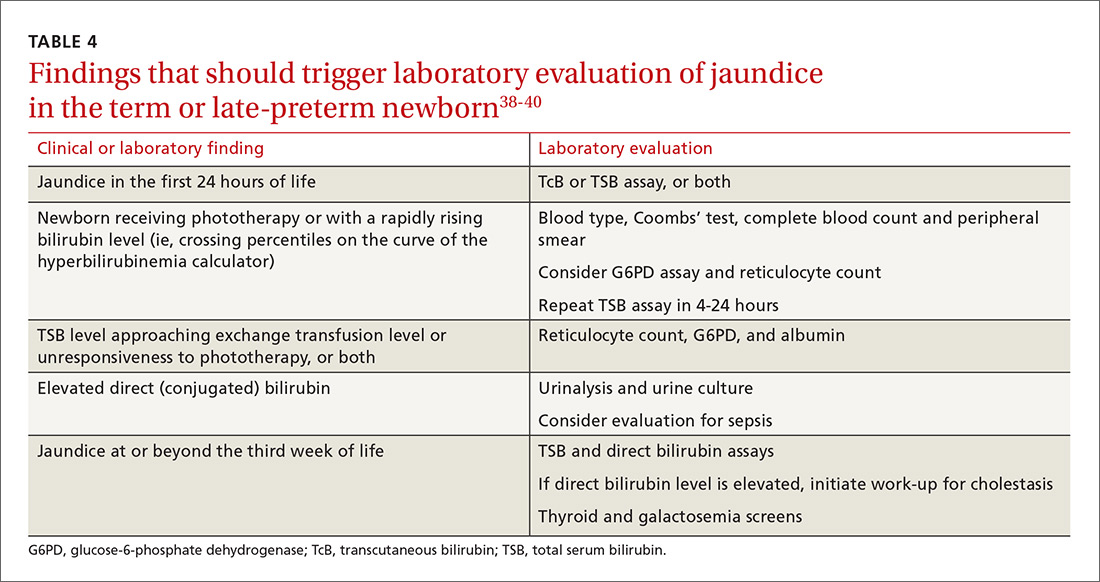
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38
Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.
To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57
Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.
CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.
Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8
Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1
Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38
Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.
To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57
Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.
CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.
Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8
Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1
Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38
Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.
To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57
Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.
CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
From The Journal of Family Practice | 2018;67(4):E4-E15.
PRACTICE RECOMMENDATIONS
› Include a full work-up and empiric antibiotics in the initial management of all febrile infants ≤28 days of age. A
› Recommend that newborns breastfeed exclusively (in the absence of contraindications) for 6 months and continue some breastfeeding until the baby is at least 12 to 24 months of age. A
› Screen all newborns for jaundice before discharge by 1) clinical assessment or 2) testing for total serum bilirubin (TSB) or transcutaneous bilirubin (TcB); measurement of TcB provides a reasonable estimate of the TSB level in healthy newborns at levels <15 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Acupuncture for pain: 7 questions answered
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.
General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.
General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
An estimated 39.4 million US adults suffer from persistent pain,1 and the National Institutes of Health indicate that pain affects more Americans than diabetes, heart disease, and cancer combined.2
As physicians, we know that conventional options to manage chronic pain leave much to be desired and that more evidence-based treatment options are sorely needed. Patients know this, too, and turn to complementary therapies for pain more than for any other diagnosis.3
Case in point: The use of acupuncture is growing. Its use in the United States tripled between 1997 and 2007.4 In addition, the research base for acupuncture is rapidly expanding. From 1991 to 2009, nearly 4000 acupuncture research studies were published, with studies on pain accounting for 41% of the acupuncture literature.4
But acupuncture is not without controversy. This is due to a lack of a universally accepted biologic mechanism, theories of use and efficacy based in an alternative medical system (traditional Chinese medicine [TCM]), and conflicting views of the evidence.
This article will help make sense of this growing body of knowledge by summarizing the latest evidence and addressing 7 common questions about acupuncture for pain conditions. Applying this information will give you the confidence to counsel patients appropriately and decide if acupuncture fits within their pain management plan.
1. What is acupuncture and how does it work?
Acupuncture, which has a 2000-year history of use, involves inserting needles at various points throughout the body to promote healing and improve function. Although acupuncture represents one piece of TCM (which is a holistic system that also includes herbal medicine, nutrition, meditation, and movement), it is often offered as an independent therapy.
Acupuncture point locations are determined either by using an underlying theoretical framework, such as TCM, or by using anatomic structures, such as muscular trigger points. Providers today often employ a hybrid approach when delivering acupuncture treatment. That is, practitioners may choose point locations based on TCM, but they may combine the practice with local treatments that are based on current knowledge of anatomy. For example, a patient presenting with low back pain may be treated utilizing traditional points located near the ankle and knee, and also by needling active trigger points in the quadratus lumborum muscle.
The mechanism of action. One of the reasons for the continuing controversy surrounding acupuncture is the lack of a clear understanding of its underlying mechanism of action. For centuries the “how” of acupuncture has been explained in poetic terms such as yin, yang, and qi. Only in the past half-century have we begun investigating the potential biologic mechanisms responsible for the physiologic effects seen with acupuncture treatment.
While research has uncovered several interesting theories, how these mechanisms interact to produce therapeutic effects is still unclear. However, looking at various components of the nervous system helps to provide some insight.
Consider the nervous system. One way to conceptualize the mechanisms of acupuncture is to consider the various levels of the nervous system and how each level is affected. In the central nervous system, needling an acupuncture point stimulates the natural endorphin system, altering the pain sensation.5 This effect is reversible with naloxone in animal models, indicating that blocking the endorphin system interferes with the analgesic benefits of acupuncture.5
Serotonergic systems are also involved centrally. Functional magnetic resonance imaging studies have shown that needling specific acupuncture points modulates areas of the brain.
In the spinal cord, the gate control theory is believed to play a role. (The gate control theory puts forth that nonpainful input closes the “gates” to painful input, which prevents pain sensations from traveling to the central nervous system.) Modulation of sensory input occurs at the level of the dorsal horn of the spinal cord during an acupuncture treatment, which can affect the physiologic pain response.6 Opioid receptors are also affected at the spinal cord level.7
Lastly, multiple chemicals released peripherally, including interleukins, substance P, and adenosine, appear to contribute to acupuncture’s analgesia.6 We know this because a local anesthetic injected around a peripheral nerve at an acupoint blocks the analgesic effect of acupuncture.8 Taken together, acupuncture treatment produces physiologic changes in the brain, spinal cord, and at the periphery, making it a truly unique therapeutic modality.
2. Is acupuncture an effective treatment for pain?
Yes, but before we look at the individual studies, it is important to mention some of the shortcomings of the research to date. First, acupuncture trials lack a standard sham control intervention. Some sham treatments involve skin penetration, while others do not. This has led to controversy regarding whether the sham interventions themselves are physiologically active, thus lessening the magnitude of effect for acupuncture. This is a point of contention in the acupuncture literature and a factor to consider when deciding if results have clinical significance.
In addition, the acupuncturist providing treatment in a trial is typically unblinded. This is also true of trials measuring other physical modalities, but it contributes to the debate surrounding the magnitude of placebo response in acupuncture studies.
Finally, many randomized trials involving acupuncture have had low methodologic quality. Fortunately, there are now several high-quality systematic reviews that have attempted to filter out the lower-quality research and provide a better representation of the evidence (TABLE9-14). A discussion of them follows.
General chronic pain. A 2012 meta-analysis15 evaluated the effectiveness of acupuncture for the treatment of chronic pain with one of 4 etiologies: nonspecific back or neck pain, chronic headache, osteoarthritis, and shoulder pain. This analysis attempted to control for the high variability of study quality in the acupuncture literature by including only studies of high methodologic character. The final analysis included 29 randomized controlled trials (N=17,922). The authors concluded that acupuncture was superior to both no acupuncture and sham (placebo) acupuncture for all pain conditions in the study. The average effect size was 0.5 standard deviations on a 10-point scale. The authors considered this to be clinically relevant, although the magnitude of benefit was modest.15
Low back pain. A 2017 systematic review by Chou et al9 evaluated 32 trials (N=5931) reviewing acupuncture for the treatment of chronic low back pain. This review found acupuncture was associated with lower pain intensity and improved function in the short term when compared with no treatment. And while acupuncture was associated with lower pain intensity when compared with a sham control, there was no difference in function between the 2 groups. Of note, 3 of the included trials compared acupuncture to standard medications used in the treatment of low back pain and found acupuncture to be superior in terms of both pain reduction and improved function.9
The authors of a 2008 systematic review that evaluated 23 trials (N=6359)10 similarly concluded that there is moderate evidence for the use of acupuncture (compared to no treatment) for the treatment of nonspecific low back pain, but did not find evidence that acupuncture was superior to sham controls.10 The 2017 American College of Physicians clinical practice guidelines support the use of acupuncture for the treatment of chronic low back pain.16
In addition to helping with chronic low back pain, acupuncture is also showing promise as a treatment for acute spinal pain. A 2013 systematic review (11 trials, N=1139) showed that acupuncture may be more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) in treating acute low back pain and may cause fewer adverse effects.17
Headache pain. Evidence favoring acupuncture in the management of headache has been fairly consistent over the past decade. An updated Cochrane review on the prevention of migraine headaches was published in 2016.11 Acupuncture was compared with no treatment in 4 trials (n=2199). The authors found moderate quality evidence that acupuncture reduces headache frequency (number needed to treat=4). Acupuncture achieved at least 50% headache reduction in 41% vs 17% in the groups that received no acupuncture. When compared with sham control groups (10 trials, n=1534), acupuncture demonstrated a small but statistically significant improvement in headache frequency. Three trials (n=744) compared acupuncture to medication prophylaxis for migraine headaches and found acupuncture had similar effectiveness with fewer adverse effects.11
Osteoarthritis (OA). Most studies have focused on OA of the knee, and, thus far, have generated conflicting results. A Cochrane review in 2010 included 4 trials (n=884) that had a wait list control and 9 trials (n=1835) that compared acupuncture to a sham control.12 When compared to a wait list control, acupuncture resulted in statistically significant and clinically relevant improvement in pain and function. However, when compared to sham treatment for OA, the review showed statistically significant improvement in pain and function for acupuncture that was unlikely to be clinically relevant.12
A more recent meta-analysis in 2016 evaluated 10 trials (N=2007) investigating acupuncture in the treatment of knee OA.13 The authors found acupuncture improved both pain and functional outcome measures when compared with either no treatment or a sham control.
Fibromyalgia. Systematic reviews in 2007 (5 trials, N=316)18 and 2010 (7 trials, N=385)19 showed that acupuncture did provide short-term pain relief in patients with fibromyalgia, but that the effect was not sustained at follow-up.These reviews were limited by a high risk of bias, which was noted in the studies. The authors of both reviews concluded that acupuncture could not be recommended for the treatment of fibromyalgia.
A more recent Cochrane review published in 2013 (9 trials, N=395) offered low- to moderate-level evidence of benefit for acupuncture compared with no treatment at one month follow-up.14 Of note, there was also evidence of benefit in improved sleep and global well-being, in addition to pain and stiffness measures in this review. The overall magnitude of benefit was small, but clinically significant. Acupuncture also has evidence of benefit in the treatment of conditions commonly seen in conjunction with fibromyalgia, including headaches and low back pain as described earlier.
3. What does a typical acupuncture treatment entail?
In a typical treatment, anywhere from about 5 to 20 needles are inserted into the body. Common areas of needling include the arms and legs, especially below the elbows and knees. Other frequently used areas are the scalp, ears, and structures related to the painful condition.
The needles used are very thin (typically smaller than a 30-gauge needle) and do not have a beveled tip like phlebotomy needles do. Most patients have minimal pain as the needles are inserted. During the treatment, the needles may be left alone or they may be heated or stimulated electrically. An average treatment lasts 30 to 40 minutes; many patients find the sessions relaxing.
4. Are there any adverse effects or complications of treatment?
Acupuncture is generally considered a safe therapy, with most patients experiencing no adverse effects at all. Minor adverse effects can include post-treatment fatigue, minor bruising, or vasovagal reactions from the insertion of the needles. Serious complications, such as pneumothorax, are possible, but are considered rare.20 A 2004 study estimated the incidence of severe complications to be .05 per 10,000 acupuncture treatments.21
Infections are also possible, but most reported cases were due to practitioners reusing needles.22 The standard of care in the United States is to use only sterilized, single-use needles. With this practice, infections due to acupuncture are thought to be rare.
Of note, trials that compare acupuncture to another active therapy often find that acupuncture has fewer adverse effects. This has been the case when acupuncture was compared to NSAIDs for low back pain and to topiramate for headaches.17,23
5. How does acupuncture fit into a patient’s treatment?
The simple answer is that it is often most effectively used as part of a comprehensive management plan for chronic pain.
As our understanding of the complexity of chronic pain deepens, our therapeutic armamentarium for the management of chronic pain needs to broaden. This was summed up well in a 2016 article on the multimodal management of chronic pain when the authors stated, “Many targets need more than one arrow.”24 Effective management of chronic pain involves addressing psychosocial and lifestyle factors in a patient-centered way and finding a combination of treatments that most effectively leads to improved coping and function.
It’s important to note that like medications and injections, acupuncture is a passive therapy. Although there is evidence for efficacy of improved pain with acupuncture in certain conditions, it should be combined with treatments that actively engage patients, such as exercise, behavioral treatments, development of coping skills, sleep hygiene, and educational strategies.
6. To whom do I refer patients for acupuncture treatment?
In the United States, licensed acupuncturists and physicians most commonly perform acupuncture. There are more than 50 schools that train licensed acupuncturists in the United States, and it usually takes 3 years to meet the requirements.25
SIDEBAR
Online resources
American Academy of Medical Acupuncture
www.medicalacupuncture.com
National Center for Complementary and Alternative Medicine
http://nccam.nih.gov/health/acupuncture
National Certification Commission for Acupuncture and Oriental Medicine
www.nccaom.org
Physicians are often trained through continuing medical education (CME) programs that take several months to complete. These programs often combine live lectures, distance learning, and hands-on training and are typically sponsored by a university. Most require 300 hours of CME to complete. Licensure varies by state, but in many states, having an MD or DO degree automatically allows physicians to practice acupuncture. (See “Online resources,” above for links to Web sites that can be useful in finding qualified acupuncturists in your area.)
7. Is acupuncture covered by insurance?
It depends. Insurance coverage of acupuncture is highly variable and based on region and insurance type. Medicare and Medicaid plans do not pay for acupuncture. There are some private insurance plans that do. If covered, there may be limitations regarding diagnosis, number of visits, or provider. It is best for patients to call their insurance plan directly to inquire about coverage and any limitations. If paying out of pocket, patients can expect to pay $75 to $150 per treatment session.
CORRESPONDENCE
Russell Lemmon, DO, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
1. Kennedy J, Roll JM, Schraudner T, et al. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 National Health Interview Survey. J Pain. 2014;15:979-984.
2. U.S. Department of Health and Human Services. NIH Fact Sheet. Pain management. Available at: https://www.report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=57. Accessed February 12, 2018.
3. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23.
4. Nahin RL, Barnes PM, Stussman BJ, et al. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. Natl Health Stat Report. 2009;18:1-14.
5. Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368-372.
6. Ammendolia C, Furlan AD, Imamura M, et al. Evidence-informed management of chronic low back pain with needle acupuncture. Spine J. 2008;8:160-172.
7. Zhang R, Lao L, Ren K, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482-503.
8. Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152(3 Suppl):S41-S48.
9. Chou R, Deyo R, Friedly J, et al. Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166:493-505.
10. Yuan J, Purepong N, Kerr DP, et al. Effectiveness of acupuncture for low back pain: a systematic review. Spine (Phila Pa 1976). 2008;33:E887-E900.
11. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;6:CD001218.
12. Manheimer E, Cheng K, Linde K, et al. Acupuncture for peripheral joint osteoarthritis. Cochrane Database Syst Rev. 2010;1:CD001977.
13. Lin X, Huang K, Zhu G, et al. The effects of acupuncture on chronic knee pain due to osteoarthritis: a meta-analysis. J Bone Joint Surg Am. 2016;98:1578-1585.
14. Deare JC, Zheng Z, Xue CC, et al. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013;5:CD007070.
15. Vickers AJ, Cronin AM, Maschino AC, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444-1453.
16. Qaseem A, Wilt TJ, McLean RM, et al, for the Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
17. Lee JH, Choi TY, Lee MS, et al. Acupuncture for acute low back pain: a systematic review. Clin J Pain. 2013;29:172-185.
18. Mayhew E, Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46:801-804.
19. Langhorst J, Klose P, Musial F, et al. Efficacy of acupuncture in fibromyalgia syndrome—a systematic review with a meta-analysis of controlled clinical trials. Rheumatology (Oxford). 2010;49:778-788.
20. Lao L, Hamilton GR, Fu J, et al. Is acupuncture safe? A systematic review of case reports. Altern Ther Health Med. 2003;9:72-83.
21. White A. A cumulative review of the range and incidence of significant adverse events associated with acupuncture. Acupunct Med. 2004;22:122-133.
22. Xu S, Wang L, Cooper E, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013:581203.
23. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev. 2009;1:CD001218.
24. Dale R, Stacey B. Multimodal treatment of chronic pain. Med Clin North Am. 2016;100:55-64.
25. National Certification Commission for Acupuncture and Oriental Medicine. Available at: www.nccaom.org. Accessed March 20, 2018.
From The Journal of Family Practice | 2018;67(4):224-226,228-230.
PRACTICE RECOMMENDATIONS
› Recommend acupuncture as a prophylactic treatment for migraine headaches. A
› Recommend acupuncture as a treatment option for chronic low back pain. A
› Consider using acupuncture as an adjunctive treatment in the management of fibromyalgia symptoms. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The agitated patient: Steps to take, how to stay safe
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6
The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.
Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,
Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).
Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).
Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.
CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6
The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.
Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,
Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).
Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).
Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.
CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
CASE A 40-year-old man came to our office slightly agitated. He had an acute illness that was minor in nature. However, he was not interested in answering my questions or undergoing a physical exam. The more I tried to proceed with the visit, the more agitated he became—pacing the room, muttering, avoiding eye contact. I was uncomfortable and knew that the situation could quickly escalate if it was not brought under control.
What steps would you take if this were your patient?
The scene described above occurred several years ago, but more recently, one of the institutions in my (TIM) area was affected by a shooter in the workplace. The apprehension felt by all of us who were on the periphery paled in comparison to what was experienced by those at the scene. The outcome was horrific. Communicating with those directly involved during, and immediately after, the event was heart-wrenching. The trauma that they continue to relive is unimaginable, and some are not yet able to return to work.
Situations involving agitated patients are not uncommon in health care settings, although ones that escalate to the level of a shooting are. And no matter where on the spectrum an incident involving an agitated patient falls, it can leave those involved with various levels of physical, emotional, and psychological harm. It can also leave everyone asking themselves: “How can I better prepare for such occurrences?”
This article offers some answers by providing tips and guidelines for handling agitated and/or violent patients in various settings.
[polldaddy:9948472]
Defining the problem, assessing its severity
Between 2011 and 2013, workplace assaults ranged from 23,540 and 25,630 annually, with 70% to 74% occurring in health care and social service settings.1,2
Agitation is defined as a state that may include inattention, disinhibition, emotional lability, impulsivity, motor restlessness, and aggression.3,4 Violence in a clinical setting may be seen as an extreme expression of agitation sufficient enough to cause harm to an individual or damage to an object.5,6
The causes of agitation can be grouped into categories: those due to a general medical condition, those due to a psychiatric condition, and those due to drug intoxication and/or withdrawal.7 We have chosen to add a fourth category—iatrogenic (see TABLE 13,4,7-9). They are not distinct categories, as there is sometimes overlap among areas.
Determining the level of agitation. Various scales and approaches can help determine the level of agitation in a patient (eg, the Agitated Behavior Scale [ABS; FIGURE];5 the Behavioral Activity Rating Scale [BARS]10) and the risk for violence (eg, the ABC violence risk assessment,
Scales like the ABS should be employed as soon as a patient shows signs of agitation sufficient to warrant intervention. The idea is for the family physician (FP) to be familiar enough with the tool to be able to mentally check it off, fill it out when time permits, and keep it in the patient’s chart. The first version of the form serves as a baseline so that if care is handed off to another provider, that provider can monitor whether signs and symptoms are improving or worsening.
Setting often drives the solution
Much of the evidence-based research on managing patient agitation and violence stems from inpatient psychiatric and emergency department (ED) settings. To make other health care providers aware of the experience gained in those settings, the American Association for Emergency Psychiatry created Project BETA (Best Practices in Evaluation and Treatment of Agitation). This project is designed to help promote consistency across health care settings and specialties in the way clinicians respond to agitated patients and to emphasize for all health care providers the availability of more than just pharmacologic approaches.7
De-escalating the situation. General tenets of de-escalation apply across practice settings. Among them:
- Stay calm. Avoid aggressive postures and prolonged eye contact.
- Be nonconfrontational. Acknowledge the patient’s frustration/perceptions and ask open-ended questions.
- Assess available resources such as clinical team members, family members, and silent alarms.
- Manage the situation and the patient’s underlying issues/diagnoses. This includes mobilizing other patients to avoid collateral damage and exploring solutions with the patient.
For more on de-escalation tools, see (TABLE 34,6,9,11).
Your setting matters. It’s worth noting that the settings in which clinicians practice greatly influence the resources available to de-escalate a situation and ensure the safety of the patient and others.7 The review that follows provides some issues—and tips—that are unique to different practice settings.
Ambulatory settings
Sim and colleagues9 noted that aggressive behavior in the general practice setting may stem not only from factors related to the patient’s own physical or psychological discomfort, but from patients feeling that they are being treated unfairly, whether it be because of wait times, uncomfortable waiting conditions, or something else. A number of international studies have shown high rates of abuse toward FPs.9,12 Of 831 primary care physicians surveyed in a German study, close to three-quarters indicated that within the last year, they had experienced aggression (ranging from verbal abuse and threats to physical violence and property damage) from a patient.12 This statistic increased to 91% when it included the length of their career.
Bell13 suggests that physicians be aware that transference and countertransference issues are often at play when dealing with hostile or potentially violent patients. Suggestions to prevent aggression include some practice-level approaches (eg, providing waiting room distractions, making patients aware of potential delays), as well as being aware of nonverbal cues suggesting increased agitation (eg, clenched fists, crossed arms, chin thrusts, finger pointing).9
Group practice
An FP who practices with other health care providers and clinical staff has a built-in team that can assist with de-escalation. When meeting with a patient who has a history of violence or agitation in an exam room or office, try to ensure that you can get to an exit quickly if necessary. Also, alert staff to any concerns, and have a system for at least one staff member to check in periodically during the visit.
It is also helpful to develop an evacuation plan and create a “panic room” or “safe zone” for emergencies.14,15 Such a space may be nothing more than an area or room for staff to gather. It should have access to the police or other emergency services via a land and/or cell phone line.
Solo practice
If you practice alone, institute safeguards whereby a colleague (at a different practice, building, or location) can be alerted if concerns arise. In addition, consider the following precautions: locking the door when alone after hours, screening potential patients, having a way to call for help (keep the number for the local police station and ED readily available), prohibiting potential weapons (as some states allow them to be carried), and learning some form of self-defense.15
Resources exist that offer guidelines for developing policies and procedures, checklists, and sample incident forms (eg, the International Association for Healthcare Security and Safety; iahss.org). Other organizations that can help with the development of a preparedness plan include the Occupational Safety and Health Administration (https://www.osha.gov/SLTC/workplaceviolence/evaluation.html), the Department of Homeland Security (https://www.dhs.gov/sites/default/files/publications/ISC%20Violence%20in%20%20the%20Federal%20Workplace%20Guide%20April%202013.pdf), and The Joint Commission (https://www.jointcommission.org/workplace_violence.aspx).
Long- and short-term care facilities
In long-term care settings, such as nursing homes, and shorter-term care settings, such as rehabilitation facilities, agitation may stem from causes related to a head injury or dementia or from living in an unfamiliar environment. Assessment can be accomplished using a formal scale (eg, the ABS), as well as by identifying potential underlying health-related factors that can lead to agitation, such as pain, an infection, bowel and bladder issues, seizures, wounds, endocrine anomalies, cardiac or pulmonary problems, gastrointestinal dysfunction, and metabolic abnormalities.3
Modify the environment. For this population, a primary approach involves modifying the environment to decrease the likelihood of agitation. This may involve decreasing noise or light or ensuring adequate levels of stimulation. Preventing disorientation can be addressed through verbal and visual reminders of the date, schedule, etc. If a particular situation or activity is identified as a source of agitation, attempts at modifications are called for.3
For patients with dementia, the American Psychiatric Association recommends using the lowest effective dose of an antipsychotic in conjunction with environmental and behavioral measures.16 A benzodiazepine (lorazepam, oxazepam) may be used for infrequent agitation. Trazodone or a selective serotonin reuptake inhibitor are alternatives for those without psychosis or who are intolerant to antipsychotics.16
For individuals in a rehabilitation setting, agitation can impede participation in therapy and has been associated with poorer functioning at the time of discharge.3 Agitation can also be disruptive and lead to distress for family members and caregivers, as well as for fellow patients. And because this environment has a greater likelihood of visitors unrelated to the patient being exposed to the aberrant behavior, it is especially important to have established policies and procedures for de-escalation in place.
Home care
More and more FPs and residents are conducting home visits. That’s because the Accreditation Council for Graduate Medical Education Program Requirements for Graduate Medical Education in Family Medicine now include integrating a patient’s care across settings—including the home.17 Those who do provide home care may find themselves in circumstances similar to those of domestic disputes.
The German study mentioned earlier of more than 800 primary care physicians found that while the vast majority of physicians felt safe in their offices, 66% of female doctors and 34% of male doctors did not feel safe making home visits.12
Know the neighborhood. There’s no doubt that working in the home health sector makes one vulnerable. More than 61% of home care workers report workplace violence annually.18,19 An action plan, as well as established policies and procedures, are essential when making home visits. Prior to the visit, be aware of the community and the location of the nearest police department and hospital.
Unwin and Tatum20 suggest not wearing a white coat or carrying a doctor’s bag so as not to stand out as a physician in neighborhoods where personal safety is an issue. Make sure that your cell phone is fully charged and that there is a GPS mechanism activated that allows others to locate you.21 Note the available exits in a patient’s home, and position yourself near them, if possible. Have someone call or text you at predetermined times so that the absence of a response from you will alert someone to send help.
In such situations, it is imperative to remain calm and to use the same verbal de-escalation techniques (TABLE 34,6,9,11) that would be used in any other health care setting. It is prudent to set expectations for the patient and family members prior to the home visit regarding the tools and services that will be provided in the home setting and the limitations in terms of scope of practice.
Emergency department
The ED is one of the most common settings for patient agitation and violence within the health care continuum.22 Providers must quickly determine the cause of the agitation while de-escalating the situation and ensuring that they do not miss a pertinent medical finding related to a time-sensitive issue, such as an intracerebral bleed or poisoning.7 In addition, the ED is usually heavily populated, providing an opportunity for tremendous collateral human damage should the violence escalate or weapons be deployed. The upside is that many EDs are now staffed with security personnel and, depending on the community, police officers may be on the premises or in the vicinity.22
Etiologies for agitation in the ED can range from ingestion of unknown or unidentified substances to psychiatric or medical conditions. Knowledge of etiology is necessary prior to initiation of treatment.4
As in other settings, the safety of the patient and others present is of utmost importance. Key recommendations for managing agitated patients in the ED include: 4
- Have an established plan for the management of agitated patients.
- Identify signs of agitation early, and complete an agitation rating scale.
- Attempt verbal de-escalation before using medication whenever possible.
- Employ a “show of concern” rather than “a show of force” in response to escalating agitation/violence. Doing so can strengthen the perception that interventions are coming from a place of caring.
- Use physical restraint as a last resort. When used, it should be with the intention of protecting the patient and those present, rather than as punishment.
Inpatient units
Unlike the ED, patients on units generally have a working diagnosis, and the provider has some background information with which to work, such as laboratory test results and radiology reports, facilitating more expedient and accurate situational assessment. However, the recommendations for assessment and early identification, as described for the ED, still apply.
If a provider finds him- or herself in an escalating situation, the call bells located in the rooms are of use. An alternative is to call out for help from someone in the hallway. One needs to be aware of the current policies and procedures for de-escalation, as some facilities have a specific “code” that is called for such occasions.19
Postop delirium is a common cause of agitation in the inpatient setting. Ng and colleagues11 recommend a cognitive assessment before surgery to establish a baseline in order to determine the risk for delirium after surgery. Additionally, the FP must remain aware of preexisting conditions that may surface during a hospital stay, such as dehydration or unrecognized alcohol or medication withdrawal.
Medication choice should be based on the type of delirium. Hyperactive delirium (restlessness, emotional lability, hallucinations) and mixed delirium (a combination of signs of hyperactive and hypoactive dementia) both hold the potential for agitation and even violence. The approach to hyperactive delirium includes consideration of an antipsychotic medication, although the efficacy of antipsychotics is considered controversial. In the case of mixed delirium, behavioral and environmental modifications are useful (eg, reducing noise and early ambulation).11
No medications are registered with the US Food and Drug Administration for the management of delirium, and it is suggested that antipsychotics be considered only when other, less invasive, strategies have been attempted.23
Addressing caregiver stress, anxiety disorders afterward
Regardless of the setting in which FPs work, witnessing or being directly involved in a traumatic event puts one at risk for symptoms—or a full diagnosis—of posttraumatic stress disorder (PTSD), acute stress disorder, or anxiety or mood disorders.24,25 Although findings vary, studies have found that as many as 12% of ED personnel meet the criteria for PTSD26,27 and 12% to 15% report having been threatened physically.28,29 More than half of physicians in another study had witnessed a physical attack.30
Physicians and other health care personnel who have experienced a traumatic incident, or offered help to another during an incident, may attempt to cope through avoidance, cutting down on work hours, leaving the work setting in which the event took place, or leaving the profession altogether.29,31,32
There is a paucity of methodologically sound research with regard to prevention and treatment of PTSD symptoms in this population.24 According to a 2002 Cochrane review, the effectiveness of individual, single-session debriefing does not have solid research support,33 and there are concerns about potential harms due to reliving the traumatic event when sessions are led by poorly trained debriefing staff.34-36
Critical incident stress debriefing (CISD), however, holds promise in terms of facilitating a return to pretrauma functioning based on studies of first responders.34,35 This may be because CISD follows a specific protocol and that group sessions may capitalize on the social support/camaraderie within a group that has undergone a traumatic event.34,35 It is important that those providing debriefing and support be well-trained.35
Debriefing, however, is not always sufficient, and those who appear to be affected on an ongoing basis may require individual treatment for PTSD symptoms. Evidence-based treatments for PTSD, such as trauma-focused psychotherapy and/or pharmacotherapy, may be considered37 (TABLE 424,34,38).
Ongoing support in the workplace. The Cleveland Clinic has developed a “Code Lavender” to combat stress in the workplace. Like a Code Blue for medical emergencies, a Code Lavender is called when a health care worker is in need of emotional or spiritual support.38 A provider who initiates the call is met by a team of holistic nurses within 30 minutes. The team provides Reiki and massage, healthy snacks and water, and lavender arm bands to remind the individual to relax for the rest of the day. Further opportunities for spiritual support, mindfulness training, counseling, and yoga may also be made available.
CASE Sensing that the situation with my patient might escalate, I lowered my voice, relaxed my shoulders, leaned casually against the desk, and asked him to tell me how I could best help him. As he spoke, I offered him a seat (by gesturing to the chair). I did this for 2 reasons: to move him away from blocking my exit from the room, and to put him at a lower level than me so that he was entirely in my view. I didn’t interrupt him as he spoke. I just nodded or tilted my head to show I was listening. In my mind, I played out the various scenarios that could ensue.
Fortunately, I was able to get him to relax enough for an assessment, which involved a more relevant history and the exam, which he agreed to once an aide had come into the room. He did not exhibit the concerning signs of flushed skin, dilated pupils, shallow rapid respirations, or perspiration. He did have a comorbid behavioral health issue, which we were able to address. His earlier behavioral indicators of agitation were controlled with verbal and physical cues on my part. Our conversation didn't reveal an intent to harm himself or others. In this case, physical restraints were not required. Throughout the encounter the door was left open, and the patient was reminded that we were there to help.
Once he left, I made the relevant notes in the chart regarding his agitated state at the start of the visit and his final state at the end of the visit so as to assist any other providers. We (TIM, MG) also held a quick debrief after the encounter with the office staff and decided that we needed to create a policy and protocol regarding how to handle such situations in the future.
CORRESPONDENCE
Tochi Iroku-Malize, MD, MPH, MBA, Family Medicine Department, Southside Hospital, 301 East Main Street, Bay Shore, NY 11706; [email protected].
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
1. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. December 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed February 8, 2018.
2. Occupational Safety and Health Administration. Guidelines for preventing workplace violence for healthcare and social service workers. 2016. Available at: https://www.osha.gov/Publications/osha3148.pdf. Accessed February 8, 2017.
3. Mortimer DS, Berg W. Agitation in patients recovering from traumatic brain injury: nursing management. J Neurosci Nurs. 2017;49:25-30.
4. Wilson MP, Nordstrom K, Vilke GM. The agitated patient in the emergency department. Curr Emerg Hosp Med Rep. 2015;3:188-194.
5. Bogner JA, Corrigan JD, Bode RK, et al. Rating scale analysis of the Agitated Behavior Scale. J Head Trauma Rehabil. 2000;15:656-669.
6. Gaynes BN, Brown CL, Lux LJ, et al. Preventing and de-escalating aggressive behavior among adult psychiatric patients: a systematic review of the evidence. Psychiatr Serv. 2017;68:819-831.
7. Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American Association for Emergency Psychiatry Project Beta Medical Evaluation Workgroup. West J Emerg Med. 2012;13:3-10.
8. Sands N. Mental health triage: towards a model for nursing practice. J Psychiatr Ment Health Nurs. 2007;14:243-249.
9. Sim MG, Wain T, Khong E. Aggressive behaviour - prevention and management in the general practice environment. Aust Fam Physician. 2011;40:866-872.
10. Swift RH, Harrigan EP, Cappelleri JC, et al. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. J Psychiatr Res. 2002;36:87-95.
11. Ng J, Pan CX, Geube A, et al. Your postop patient is confused and agitated—next steps? J Fam Pract. 2015;64:361-366.
12. Vorderwülbecke F, Feistle M, Mehring M, et al. Aggression and violence against primary care physicians—a nationwide questionnaire survey. Dtsch Arztebl Int. 2015;112:159-165.
13. Bell HS. Curbside consultation—a potentially violent patient? Am Fam Physician. 2000;61:2237-2238.
14. Taylor H. Patient violence against clinicians: managing the risk. Innov Clin Neurosci. 2013;10:40-42.
15. Munsey C. How to stay safe in practice. APA Monitor. 2008;39:36.
16. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173:543-546.
17. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. Revised July 1, 2017. Available at: http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_family_medicine_2017-07-01.pdf . Accessed October 30, 2017.
18. Phillips JP. Workplace violence against health care workers in the United States. N Engl J Med. 2016;374:1661-1669.
19. Hanson GC, Perrin NA, Moss H, et al. Workplace violence against homecare workers and its relationship with workers health outcomes: a cross-sectional study. BMC Public Health. 2015;15:11.
20. Unwin BK, Tatum PE 3rd. House calls. Am Fam Physician. 2011;83:925-938.
21. Victor P. Safety tips for home visits from a veteran NYC social worker. National Association of Social Workers, New York. Available at: http://www.naswnyc.org/?489. Accessed June 1, 2017.
22. Kansagra SM, Rao SR, Sullivan AF, et al. A survey of workplace violence across 65 U.S. emergency departments. Acad Emerg Med. 2008;15:1268-1274.
23. Meagher D, Agar MR, Teodorczuk A. Debate article: antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry. 2017 Jul 30. doi: 10.1002/gps.4759. [Epub ahead of print].
24. Lanctot N, Guay S. The aftermath of workplace violence among healthcare workers: a systematic literature review of the consequences. Aggress Violent Behav. 2014;19:492-501.
25. Edward KL, Stephenson J, Ousey K, et al. A systematic review and meta-analysis of factors that relate to aggression perpetrated against nurses by patients/relatives or staff. J Clin Nurs. 2016;25:289-299.
26. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49-65.
27. Mills LD, Mills TJ. Symptoms of post-traumatic stress disorder among emergency medicine residents. J Emerg Med. 2005;28:1-4.
28. Laposa JM, Alden LE, Fullerton LM. Work stress and posttraumatic stress disorder in ED nurses/personnel. J Emerg Nurs. 2003;29:23-28.
29. Occupational Safety and Health Administration. Workplace violence in healthcare: understanding the challenge. 2015. Available at: https://www.osha.gov/Publications/OSHA3826.pdf. Accessed June 1, 2017.
30. Zafar W, Khan UR, Siddiqui SA, et al. Workplace violence and self-reported psychological health: coping with post-traumatic stress, mental distress, and burnout among physicians working in the emergency departments compared to other specialties in Pakistan. J Emerg Med. 2016;50:167-177.
31. de Boer J, Lok A, Van’t Verlaat E, et al. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73:316-326.
32. Shah L, Annamalai J, Aye SN, et al. Key components and strategies utilized by nurses for de-escalation of aggression in psychiatric in-patients: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2016;14:109-118.
33. Rose S, Bisson J, Churchill R, et al. Psychological debriefing for preventing post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2002;(2):CD000560.
34. Tuckey MR, Scott JE. Group critical incident stress debriefing with emergency services personnel: a randomized controlled trial. Anxiety Stress Coping. 2014;27:38-54.
35. Pack MJ. Critical incident stress management: a review of the literature with implications for social work. Int Soc Work. 2012;56: 608-627.
36. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
37. Warner CH, Warner CM, Appenzeller GN, et al. Identifying and managing posttraumatic stress disorder. Am Fam Physician. 2013;88:827-834.
38. Johnson B. Code lavender: initiating holistic rapid response at the Cleveland Clinic. Beginnings. 2014;34:10-11.
PRACTICE RECOMMENDATIONS
› Be aware of signs of agitation and use verbal de-escalation and environmental modifications whenever possible. B
› Consider group-based critical incident debriefing with a trained provider after a traumatic event. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Limited evidence guides empiric Tx of female chronic pelvic pain
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4
This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).
Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).
Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.
Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4
This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).
Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).
Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.
Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
CASE 1
Lisa G, 31 years old, gravida 0, complains of severe dysmenorrhea that began when she discontinued an oral contraceptive (OC) one year ago. Prior to stopping the OC, she had been taking an OC without interruption since she was 28, during which time she continued to have moderate symptoms of dysmenorrhea. Before taking an OC, the patient had a trial of an etonogestrel implant, which was removed because of irregular bleeding, and depot medroxyprogesterone acetate (MPA) injection, which she discontinued because of associated weight gain and fatigue.
Ms. G is not sexually active and doesn’t want to start a family at this time, but is interested in having a diagnosis. She has no other medical problems, no surgical history, and no history of sexually transmitted infection. She reports that her mother and sister had endometriosis, including pain that resolved after definitive treatment.
Ms. G reports menstrual cycles that are exquisitely painful and occur regularly (every 28 days for 4 or 5 days), with a moderate volume of bleeding that requires a regular-size tampon change every 4 to 6 hours. She reports crampy abdominal pain as 10, on a scale of one to 10; dyschezia (without hematochezia); and generalized achy abdominal pain that is continuous during menses. Pain is partially controlled by ibuprofen, 800 mg every 8 hours. Ms. G also describes gastrointestinal symptoms of bloating, constipation preceding her menstrual cycle, diarrhea during her menses, and occasionally nausea and vomiting with the severe pain.
On examination (which is not performed during menses), Ms. G appears well and is not in acute distress. Abdominal examination is benign. There is no tenderness to palpation or distension; bowel sounds are normal. Pelvic examination reveals mild tenderness upon palpation of a small and mobile uterus. Rectal examination is normal. She has no signs of hyperandrogenism (eg, male-pattern body hair, central obesity).
CASE 2
Rhonda M, 42 years old, gravida 3, para 3003, reports continuous pelvic pain for 7 years that is exacerbated by defecation, intercourse, and insertion of a tampon. She has a low level of dull baseline pain (3, on scale of one to 10) that occasionally spikes up to sharp, knifelike pain (10 on the pain scale), which, she says, brings her to tears. Ms. M describes the pain as “deep inside,” central in her pelvis, and radiating to the left and right, particularly during pain flares.
The patient’s 3 children were born by spontaneous vaginal delivery; however, she recalls that her youngest son was born via a traumatic vaginal delivery 8 years ago (he “got stuck coming out,” she reports). The only other component of Ms. M’s medical history is an anxiety disorder, for which she takes citalopram. She has a family history of cervical cancer.
Ms. M’s past diagnostic work-up for pelvic pain includes pelvic ultrasonography, endometrial biopsy, Pap smear, and diagnostic laparoscopy—all normal. She had a negative gastrointestinal work-up, including upper- and lower-tract endoscopy. Medical therapy, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), did not provide significant relief of pain.
Despite the negative work-up, Ms. M is still concerned that the pain might be related to cancer. With her family history of cervical cancer, she says that she does not want to “miss anything.”
Ms. M is thin and appears anxious. The abdomen is mildly and diffusely tender to palpation with normal bowel sounds and no distension. Pelvic examination reveals some hyperesthesia upon single-digit palpation of the pelvic floor. Placement of the speculum is difficult because of discomfort.
How would you proceed with the care of these patients?
What is chronic pelvic pain? Why is management such a challenge?
Chronic pelvic pain (CPP) is defined as chronic or intermittent cyclic or noncyclic pelvic pain lasting longer than 6 months, localized to the pelvis, diminishing a woman’s quality of life, and requiring medical intervention.1 It’s estimated that CPP affects as many as 15% of women of reproductive age in the United States each year, at a cost to the health care system of approximately $2 billion annually.2,3
CPP can result from abnormal pain responses from multiple body systems, including gynecologic conditions such as endometriosis. Notably, a nongynecologic cause is more often the major pain generator, without significant identifiable pathology (TABLE 1). Like all chronic pain disorders, CPP can also result in central sensitization of the nervous system, altering how pain is processed at the level of the pain matrix in the brain.4
This article reviews the limited evidence for treating CPP and offers recommendations for the primary care physician on providing symptomatic relief in the absence of diagnosed pathology (TABLE 25-13).
Treatment
Analgesics
NSAIDs are frequently used as first-line treatment for any kind of pain, including CPP. There is some evidence of benefit from NSAIDs, compared to placebo, in cyclic CPP secondary to dysmenorrhea and endometriosis;5,6 however, evidence of effectiveness in noncyclic CPP is absent. Because of the low cost and availability of NSAIDs, a trial is reasonable as a first-line intervention, particularly in CPP suspected to be endometriosis or of musculoskeletal origin. NSAIDs can cause adverse effects, including nausea, vomiting, headache, and drowsiness in 11% to 14% of women, although these agents are generally well-tolerated on a short-term basis.5
Opioids bind to opioid receptors in the central and peripheral nervous systems, resulting in an analgesic effect. Guidelines issued in 2016 by the Centers for Disease Control and Prevention recommend safer prescribing through careful evaluation of the risks and benefits of opioids for pain not caused by cancer and for palliation as part of end-of-life care.14
The risks of opioid use are well known in the medical community; they include tolerance, physical dependence, misuse, and death, in addition to common adverse effects such as nausea and vomiting, itching, constipation, and fatigue.14,15 Because of those risks and limited long-term benefit in nonmalignant pain disorders, opioid therapy for CPP should be avoided.14 For patients already taking an opioid, discuss a strategy for weaning and, if possible, provide home naloxone therapy in the event of accidental overdose.14
Hormonal therapy
Hormonal therapies are the most common nonsurgical treatment of noncyclic CPP, with or without a definitive diagnosis of endometriosis, in reproductive-age women with CPP.
Combined OCs, despite a lack of quality evidence, are frequently the first hormonal treatment tried in both cyclic and noncyclic CPP. A low-dosage OC may decrease cyclic pain in endometriosis, although it can increase irregular bleeding and nausea.16 As many as 53% of women with CPP reported having undergone a trial of an OC for endometriosis, despite the absence of consistent evidence showing effectiveness in CPP.17
Depot MPA, in trials, decreased pain more than placebo. It can be tried as a treatment, but its use is often limited because of adverse effects, such as weight gain and bloating.8
A trial of a levonorgestrel-releasing intrauterine device (LNG-IUD) is supported by moderate-quality evidence for women whose CPP is thought to be a symptom of endometriosis or to have another uterine origin.7
Gonadotropin-releasing hormone agonists, such as depot leuprolide and goserelin acetate implant, may be considered in a woman with a diagnosis of endometriosis whose pelvic pain is not alleviated by MPA or an LNG-IUD.9
Nonhormonal therapies
CPP shares pain mechanisms with other pain syndromes, such as neuropathic pain. Antineuropathic medications, such as gabapentin and pregabalin, may, therefore, provide benefit. These medications also produce improvement in pain disorders of the musculoskeletal system, which may contribute to their analgesic effect.18
Gabapentin and amitriptyline have been studied in CPP; both were found successful in decreasing perceived pain. Of note, patients who received gabapentin, a gamma-aminobutyric acid analogue, with or without amitriptyline, had more pain relief than those treated with amitriptyline alone.10 Adverse effects of these medications may limit their use (TABLE 319-25).
Tricyclic antidepressants are well-supported, effective treatments for chronic pain through the central increase of norepinephrine. Beginning at a low dosage to diminish adverse effects (TABLE 319-25) and increasing the dosage slowly to an effective level may increase adherence. A trial of at least 6 to 8 weeks, at a moderate dosage, is recommended before discontinuing the medication. Although amitriptyline has the most evidence for value in the management of CPP disorders,10 second-generation tricyclic antidepressants nortriptyline and desipramine have also been used for pain control, and may be better tolerated.
Duloxetine and venlafaxine—serotonin–norepinephrine reuptake inhibitors—increase serotonin in addition to norepinephrine, which is believed to result in pain control. Although a systematic review of trials of duloxetine for chronic pain showed some improvement in diabetic peripheral neuropathy, fibromyalgia, chronic low back pain, and osteoarthritis, the review excluded CPP in its analysis.26
In our opinion, a selective neurotransmitter reuptake inhibitor can be attempted to diminish the central pain sensitization of CPP. As with all drugs that increase the availability of serotonin, serotonin syndrome is a rare risk. Additionally, when stopping duloxetine, a prolonged taper may be required.
Pelvic floor dysfunction therapy
Pelvic floor dysfunction of the musculature within the bony pelvis may contribute to, or cause, CPP. The pelvic floor musculature may be hypertonic or hypotonic, and trigger points may exist. Despite the frequency of pelvic floor dysfunction, detailed examination of the pelvic floor is not routinely performed during a pelvic exam.
Because of the high prevalence of pelvic floor dysfunction in women with CPP, evaluation of the pelvic floor muscles is warranted.27 (A protocol for this evaluation is detailed in TABLE 4.) Pelvic dynamometry may indicate muscle spasm or chronic tension; palpation of the pelvic floor during the exam can also identify a pain generator.
Although it might be difficult to distinguish pelvic floor myofascial pain as the primary or secondary cause of pain, pelvic floor physical therapy may clarify the role of the pelvic floor response (depending on the patient’s clinical exam and history). A low-quality retrospective case study on pelvic floor physical therapy reported significant improvement in pain that was proportional to the number of sessions completed.11 Trigger-point injections and injections of botulinum toxin A have been used with reported improvement in the pelvic floor pain profile, and there is evidence to support the benefit of such injections in pelvic muscle dysfunction.12
Psychotherapy
Cognitive behavioral therapy (CBT) is well established as an option to manage a patient’s response to pain, including teaching coping skills for a chronic pain disorder and pain flares. Evidence supports using CBT or mindfulness techniques over usual care in reducing the intensity of pain in chronic low back pain,28 and may be helpful in CPP. Patients with CPP who received 10 treatments of Mensendieck somatocognitive therapy (a mind–body therapy technique popular in Europe) over 90 days, compared with standard treatment alone, demonstrated improvement in pain, motor function, and psychological distress that persisted 9 months after treatment.13
Lifestyle changes, complementary and alternative therapies
Although medical and nonpharmacotherapeutic treatments are often important in the
Diet modifications may relieve pain in some women with CPP. Although a systematic review in 2011 highlighted the lack of data available for the efficacy of dietary therapies for treating CPP, the authors did present data that a diet rich in antioxidants might alleviate pain sysmptoms.29 Also, a gluten-free diet might reduce the symptoms of pain related to endometriosis and, thus, improve physical functioning, among other health domains.30
Exercise can be an important factor in the management of CPP, as with other chronic pain syndromes. In functional pain syndromes, the addition or maintenance of an exercise program has been shown to decrease the amount of pain medications required, improve depressive symptoms, increase energy, and decrease stress. Exercise also improves sleep quality and one’s ability to cope with pain.31
Yoga provides a good balance of aerobic and muscle-building activity and, in the authors’ experience, is tolerated by most women with CPP.
Acupuncture has limited evidence in the treatment of pelvic pain in women. Of the available studies, most are limited to pain related to endometriosis.32
Sleep hygiene may be an important consideration in managing CPP. Sleep disturbances are reported in more than 80% of women with CPP,33 including excessive time in bed and frequent napping, resulting in daytime fatigue and feeling generally unrested. A recent meta-analysis reported mild-to-moderate immediate improvement in patients’ pain after nonpharmacotherapeutic sleep interventions.34 The National Sleep Foundation has produced a patient guide to assist in sleep hygiene.35
Devising a management strategy despite sparse evidence
Because the cause of noncyclic CPP may be multifactorial, and because the literature on the etiology of CPP is limited (and, when there is research, it is inconclusive or of poor quality36), there are few evidence-based recommendations for treating CPP. Given the paucity of quality evidence, physicians should treat patients empirically, based on their experience and their familiarity with the range of medical and nonpharmacotherapeutic options used to manage other chronic pain syndromes.
CASE 1
Ms. G’s cyclic pelvic pain was present only during menses. The dyschezia, severe pain that began only after she discontinued a combined OC, aching pain, and severe menstrual cramps are, taken together, suggestive of endometriosis, despite a normal physical exam.
Medical and surgical options were reviewed with Ms. G. She elected to undergo diagnostic laparoscopy. Several extrauterine foci of endometrial tissue were noted and excised; an LNG-IUD was inserted. Her pain improved significantly after surgery.
CASE 2
Ms. M was found to have significant pain on single-digit examination of the pelvic floor muscles, indicating likely pelvic floor muscle dysfunction. Pelvic dynamometry revealed significant tightness and spasm in the pelvic floor muscles—specifically, the levator ani complex.
Ms. M was started on gabapentin to reduce baseline pain and was referred for pelvic floor physical therapy. She felt reassured that her risk of cancer was low, considering her negative work-up, and that cancer was not the cause of her pain. Her symptoms improved greatly with a regimen of medical and physical therapy, although she continues to experience pain flares.
CORRESPONDENCE
Wendy S. Biggs, MD, Central Michigan University College of Medicine, 1632 Stone St., Saginaw, MI 48602; [email protected].
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
1. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 51. Chronic pelvic pain. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2004;103:589-605.
2. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: prevalence, health related quality of life, and economic correlates. Obstet Gynecol. 1996;87:321-327.
3. Ahangari A. Prevalence of chronic pelvic pain among women: an updated review. Pain Phys. 2014;17:e141-e147.
4. Rodriguez MA, Afari N, Buchwald DS; National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain. Evidence of overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
5. Allen C, Hopewell S, Prentice A, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. 2009;(2):CD004753.
6. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. 2015;(7):CD001751.
7. Brown J, Farquhar C. Endometriosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(3):CD009590.
8. Cheong YC, Smotra G, Williams AC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;(3):CD008797.
9. Ling FW. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Pelvic Pain Study Group. Obstet Gynecol. 1999;93:51-58.
10. Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochenschr. 2005;117:761-768.
11. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
12. Abbott JA, Jarvis SK, Lyons SC, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108:915-923.
13. Haugstad GK, Haugstad TS, Kirste UM, et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am J Obstet Gynecol. 2008;199:615.e1-e8.
14. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315:1624-1645.
15. Darnall BD, Stacey BR, Chou R. Medical and psychological risks and consequences of long-term opioid therapy in women. Pain Med. 2012;13:1181-1211.
16. Harada T, Momoeda M, Taketani Y, et al. Low-dose contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008:90:1583-1588.
17. De Graaff AA, D’Hooghe TM, Dunselman GA, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human Reprod. 2013;28:2677-2685.
18. Haviv Y, Rettman A, Aframian D, et al. Myofascial pain: an open study on the pharmacotherapeutic response to stepped treatment with tricyclic antidepressants and gabapentin. J Oral Facial Pain Headache. 2015;29:144-151.
19. Drugs.com. Amitriptyline dosing information. Available at: https://www.drugs.com/amitriptyline.html. Accessed January 4, 2018.
20. Drugs.com. Nortriptyline dosage. Available at: https://www.drugs.com/dosage/nortriptyline.html. Accessed January 4, 2018.
21. Drugs.com. Desipramine (oral route). Available at: https://www.drugs.com/cons/desipramine.html. Accessed January 4, 2018.
22. Drugs.com. Duloxetine capsules. Available at: https://www.drugs.com/pro/duloxetine-capsules.html. Accessed January 4, 2018.
23. Drugs.com. Venlafaxine. Available at: https://www.drugs.com/pro/venlafaxine.html. Accessed January 4, 2018.
24. Drugs.com. Gabapentin. Available at: https://www.drugs.com/pro/gabapentin.html. Accessed January 4, 2018.
25. Drugs.com. Pregabalin. Available at: https://www.drugs.com/monograph/pregabalin.html. Accessed January 4, 2018.
26. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;(1):CD007115.
27. Howard FM. Chronic pelvic pain. Obstet Gynecol. 2003;101:594-611.
28. Cherkin DC, Sheman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240-1249.
29. Sesti F, Capozzolo T, Pietropolli A, et al. Dietary therapy: a new strategy for management of chronic pelvic pain. Nutr Res Rev. 2011;24:31-38.
30. Marziali M, Venza M, Lazzaro A, et al. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. 2012;67:499-504.
31. Henningsen P, Zipfel S, Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946-955.
32. Zhu X, Hamilton KD, McNicol ED. Acupuncture for pain in endometriosis. Cochrane Database Syst Rev. 2011;(9):CD007864.
33. Cosar E, Çakır Güngör A, Gencer M, et.al. Sleep disturbance among women with chronic pelvic pain. Int J Gynaecol Obstet. 2014;126:232-234.
34. Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751-1764.
35. National Sleep Foundation. Healthy sleep tips. Available at: http://sleepfoundation.org/sleep-tools-tips/healthy-sleep-tips. Accessed December 26, 2017.
36. Yunker A, Sathe NA, Reynolds WS, et al. Systematic review of therapies for noncyclic chronic pelvic pain in women. Obstet Gynecol Survey. 2012;67:417-425.
PRACTICE RECOMMENDATIONS
› Consider the levonorgestrel-releasing intrauterine device for relief of chronic pelvic pain (CPP) from endometriosis; it’s been found to be more effective than expectant management. B
› Prescribe a trial of depot medroxyprogesterone acetate, which was more effective than placebo for CPP for as long as 9 months. B
› Use gabapentin—with or without amitriptyline—to provide greater relief of CPP than amitriptyline alone. B
› Recommend pelvic physical therapy for CPP; the pelvic pain score can be reduced in proportion to the number of sessions. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
ACL injury: How do the physical examination tests compare?
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.
The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.
The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18
A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.
The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.
The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.
The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.
The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18
A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.
The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.
The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
CASE An athletic 25-year-old woman presents to her family physician complaining of a painful and swollen knee. She says that she injured the knee the day before during a judo match. The injury occurred when her upper body suddenly changed direction while her foot remained planted and her knee rotated medially. A cruciate ligament injury immediately comes to mind, but other potential diagnoses include meniscal injury, collateral ligament injury, and patellar instability. The first step in determining an accurate diagnosis is to evaluate the stability of the knee by physical examination—often a difficult task immediately following an injury.
How would you proceed?
Rupture of the anterior cruciate ligament (ACL), partial or complete, is a common injury, especially in athletes who hurt their knee in a pivoting movement.1 The number of patients who present with ACL injury is estimated at 252,000 per year.2 Cruciate ligament injury may lead to complaints of instability with subsequent inability to engage in sports activities. Cruciate ligament injury is also associated with premature development of osteoarthritis later in life.3 Operative treatment seems to be superior to conservative treatment in improving both subjective and objective measures of knee instability and in helping athletes return to their former level of activity.4
Because early detection is key to achieving the best clinical outcome, it is essential that the most accurate physical examination tests are performed during the acute phase. Primary care physicians, emergency room doctors, physical therapists, and athletic trainers are the ones who most often see these patients immediately following the injury, and they often have only the physical examination with which to assess ACL injury. Their task is to identify the patient with potential ACL injury and to refer the patient swiftly.
Three physical examination tests are most commonly used to evaluate cruciate ligament injury. The best known and most frequently used technique is the anterior drawer test. The other 2 tests, the Lachman test and the pivot shift test, are more difficult to perform and are used less often, especially by physicians untrained in their use. In addition, there is a relatively new diagnostic test: the lever sign test. The aim of our article is to provide a short, clinically relevant overview of the literature and to assess the diagnostic value of physical examination for the primary care physician.
Anterior drawer test
How it’s done. In this test, the patient lies supine on the examination table with hips flexed to 45 degrees and knees flexed to 90 degrees (FIGURE 1).5 The examiner sits on the table with a leg resting on the patient's foot, grasps the tibia of the injured leg just below the knee, and draws the tibia forward. If the tibia, compared with the tibia of the uninjured leg, moves farther anteriorly, or if the endpoint feels softened or is absent, the result is positive for an ACL injury.
The literature. Nine systematic reviews conclude that the anterior drawer test is inferior to the Lachman test,6-14 which we’ll describe in a moment. This is due, in part, to the anterior drawer test’s unacceptably low sensitivity and specificity in the clinical setting—especially during the acute phase.10 The most recent meta-analysis on the anterior drawer test reports a sensitivity of 38% and a specificity of 81%.9 In other words, out of 100 ruptured ligaments, only 38 will test positive with the anterior drawer test.
The literature offers possible explanations for findings on the test’s validity. First, rupture of the ACL is often accompanied by swelling of the knee caused by hemarthrosis and reactive synovitis that can prevent the patient from flexing the knee to 90 degrees. Second, the joint pain may induce a protective muscle action, also called guarding of the hamstrings, that creates a vector opposing the passive anterior translation.15
Apart from the matter of a test’s validity, it's also important to consider the test’s inter- and intra-rater reliability.16 Compared with the Lachman test, the anterior drawer test is inferior in reliability.7
Lachman test
How it’s done. The Lachman test is performed with the patient supine on the table and the injured knee flexed at 20 to 30 degrees (FIGURE 2).5 The examiner holds the patient’s thigh with one hand and places the other hand beneath the tibia with the thumb of that hand on the tibial joint line. As the tibia is pulled forward, firm resistance suggests an uninjured ACL. Free movement without a hard endpoint, compared with the uninjured knee, indicates ACL injury.
The literature. The Lachman test is the most accurate of the 3 diagnostic physical procedures. The most recent meta-analysis reports a sensitivity of 68% for partial ruptures and 96% for complete ACL ruptures.6 According to a recently published overview of systematic reviews, the Lachman test has high diagnostic value in confirming or ruling out an ACL injury.17
Two factors are important when assessing results of the Lachman test. The quantity of anterior translation of the tibia relative to the femur is as important as the quality of the endpoint of the anterior translation. Quantity of translation must always be compared with the unaffected knee. Quality of the endpoint in passive anterior translation should be assessed as “firm” or “sudden,” indicating an intact ACL, or as “absent, ill-defined, or softened,” indicating ACL pathology (TABLE).18
A drawback of the Lachman test is that it is challenging to perform correctly.19 The patient’s ability to relax the upper leg musculature is critically important. It is also essential to stabilize the distal femur, which can be problematic if the examiner has small hands relative to the size of the patient's leg musculature.10 These difficulties might be resolved by conducting the Lachman test with the patient in the prone position, known as the Prone Lachman.19 However, good evidence is not yet available to support this proposed solution. One systematic review, though, reports that the Prone Lachman test has the highest inter-rater reliability of all commonly used physical examination tests.7
The Lachman test is known as the test with highest validity on physical examination. When the outcome of a correctly performed Lachman test is negative, a rupture of the ACL is very unlikely.
Pivot shift test
How it’s done.
The literature. The pivot shift test is technically more challenging to perform than the other 2 tests and is, therefore, less practical in the primary care setting. However, when this test is done correctly, a positive result is highly specific for ACL injury.9,10 Reported sensitivity values are contradictory. The most recent meta-analysis reports a sensitivity of 85%.6 Two other studies cite much lower values: 24% and 28%.9,10 These data suggest that the pivot shift test, when carried out correctly, can be of use in confirming a possible ACL rupture. However, the test should not be used alone in ruling out a possible ACL injury.
New diagnostic test: Lever sign test
How it’s done. The lever sign test (FIGURE 4),20 introduced in the mid-2010s, is also performed with the patient lying in the supine position. The examiner stands at the side of the affected knee of the patient, places a closed fist just beneath the proximal third of the patient’s tibia, creating a slight flexion of the knee joint. With the other hand, the examiner applies a downward directed force to the distal third of the femur. With an intact ACL, the patient’s foot should rise from the table due to the induced lever mechanism. With a ruptured ACL, the lever effect is absent and the foot will not rise.
The literature. In the prospective clinical study that introduced the lever sign test, the sensitivity rate was reported at 100%—higher than that seen with the other commonly used tests.20 Another study has reported that the lever sign test was easily adopted in clinical practice and showed higher sensitivity than the Lachman test (94% vs 80% in pre-anesthesia assessment).21 However, a more recent study has shown a sensitivity of 77% for the lever sign.22 The lever sign test is relatively easy to perform and requires less examiner strength than does the Lachman test. These factors enhance applicability of the lever sign test in the primary care office and in other settings such as physical therapy centers and emergency departments.
Applying this information in primary care
Given the importance of physical examination in diagnosing ACL injury, how can the current evidence best be applied in primary care practice? Based on its good test properties and feasibility, the Lachman test is preferred in primary care. The anterior drawer test can be used, but its low accuracy must be considered in making an assessment. The pivot shift test, given its difficulty of execution, should not be used by physicians unacquainted with it.
If future research supports early reports of the lever sign test’s accuracy, it could be very helpful in family practice. Going forward, research should aim at developing a constructive strategy for applying these physical examination tests in both primary care and specialty settings.
CORRESPONDENCE
Christiaan H. Koster, Department of Trauma Surgery, VU University Medical Centre, P.O. Box 7057, 1081 HV Amsterdam, The Netherlands; [email protected].
ACKNOWLEDGEMENTS
We thank Frits Oosterveld, PhD, for critically reviewing the manuscript and Ralph de Vries for his assistance in the literature search.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
1. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-50.
2. American Academy of Orthopedic Surgeons. Management of anterior cruciate ligament injuries. Evidence-based clinical practice guideline. 2014. Available at: http://www.aaos.org/research/guidelines/ACLGuidelineFINAL.pdf. Accessed January 26, 2018.
3. Simon D, Mascarenhas R, Saltzman BM, et al. The relationship between anterior cruciate ligament injury and osteoarthritis of the knee. Adv Orthop. 2015;2015:928301. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4410751/. Accessed January 26, 2018.
4. Hinterwimmer S, Engelschalk M, Sauerland S, et al. [Operative or conservative treatment of anterior cruciate ligament rupture: a systematic review of the literature.] Unfallchirurg. 2003;106:374-379.
5. Brown JR, Trojian TH. Anterior and posterior cruciate ligament injuries. Prim Care. 2004;31:925-956.
6. Leblanc MC, Kowalczuk M, Andruszkiewicz N, et al. Diagnostic accuracy of physical examination for anterior knee instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2015;10:2805-2813.
7. Lange T, Freiberg A, Dröge P, et al. The reliability of physical examination tests for the diagnosis of anterior cruciate ligament rupture – a systematic review. Man Ther. 2015;20:402-411.
8. Swain MS, Henschke N, Kamper SJ, et al. Accuracy of clinical tests in the diagnosis of anterior cruciate ligament injury: a systematic review. Chiropr Man Therap. 2014;22:25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4152763/. Accessed January 26, 2018.
9. van Eck CF, van den Bekerom MP, Fu FH, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc. 2013;21:1895-1903.
10. Benjaminse A, Gokeler A, van der Schans CP. Clinical diagnosis of an anterior cruciate ligament rupture: a meta-analysis. J Orthop Sports Phys Ther. 2006;36:267-288.
11. Jackson J, O’Malley PG, Kroenke K. Evaluation of acute knee pain in primary care. Ann Intern Med. 2003;139:575-588.
12. Malanga GA, Andrus S, Nadler SF, et al. Physical examination of the knee: a review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592-603.
13. Scholten RJ, Opstelten W, van der Plas CG, et al. Accuracy of physical diagnostic tests for assessing ruptures of the anterior cruciate ligament: a meta-analysis. J Fam. Pract. 2003;52:689-694.
14. Solomon DH, Simel DL, Bates DW, et al. The rational clinical examination. Does this patient have a torn meniscus or ligament of the knee? Value of the physical examination. JAMA. 2001;286:1610-1620.
15. Gurtler RA, Stine R, Torg JS. Lachman test evaluated. Quantification of a clinical observation. Clin Orthop Relat Res. 1987;216:141-150.
16. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217-238.
17. Décary S, Ouellet P, Vendittoli PA, et al. Diagnostic validity of physical examination tests for common knee disorders: an overview of systematic reviews and meta-analysis. Phys Ther Sport. 2017;23:143-155.
18. Mulligan EP, McGuffie DQ, Coyner K, et al. The reliability and diagnostic accuracy of assessing the translation endpoint during the Lachman test. Int J Sports Phys Ther. 2015;10:52-61.
19. Floyd RT, Peery DS, Andrews JR. Advantages of the prone Lachman versus the traditional Lachman. Orthopedics. 2008;31:671-675.
20. Lelli A, Di Turi RP, Spenciner DB, et al. The "Lever Sign": a new clinical test for the diagnosis of anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2016;24:2794-2797.
21. Deveci A, Cankaya D, Yilmaz S, et al. The arthroscopical and radiological corelation of lever sign test for the diagnosis of anterior cruciate ligament rupture. Springerplus. 2015;4:830. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4695483/. Accessed January 26, 2018.
22. Jarbo KA, Hartigan DE, Scott KL, et al. Accuracy of the Lever Sign Test in the diagnosis of anterior cruciate ligament injuries. Orthop J Sports Med. 2017;5(10):2325967117729809. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5639970/. Accessed January 26, 2018.
PRACTICE RECOMMENDATIONS
› Consider using the Lachman test, known to have higher validity than other anterior cruciate ligament (ACL) physical examination tests. When the outcome of a correctly performed test is negative, a rupture of the ACL is unlikely. A
› Use the pivot shift test to confirm a possible ACL rupture only if good execution is assured. Do not use the pivot shift test alone to rule out a possible ACL injury. A
› Familiarize yourself with the lever sign test, which is easy to perform but has yielded varying reports on sensitivity and specificity for ACL rupture. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Point-of-care ultrasound: Coming soon to primary care?
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).
Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).


Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
Point-of-care ultrasound (POCUS) has been gaining greater traction in recent years as a way to quickly (and cost-effectively) assess for conditions including systolic dysfunction, pleural effusion, abdominal aortic aneurysms (AAAs), and deep vein thrombosis (DVT). It involves limited and specific ultrasound protocols performed at the bedside by the health care provider who is trying to answer a specific question and, thus, help guide treatment of the patient.
POCUS was first widely used by emergency physicians starting in the early 1990s with the widespread adoption of the Focused Assessment with Sonography in Trauma (FAST) scan.1,2 Since that time, POCUS has expanded beyond trauma applications and into family medicine.
One study assessed physicians’ perceptions of POCUS after its integration into a military family medicine clinic. The study showed that physicians perceived POCUS to be relatively easy to use, not overly time consuming, and of high value to the practice.3 In fact, the literature tells us that POCUS can help decrease the cost of health care and improve outcomes,4-7 while requiring a relatively brief training period.
If residencies are any indication, POCUS may be headed your way
Ultrasound units are becoming smaller and more affordable, and medical schools are increasingly incorporating ultrasound curricula into medical student training.8 As of 2016, only 6% of practicing FPs reported using non-obstetric POCUS in their practices.9 Similarly, a survey from 2015 reported that only 2% of family medicine residency programs had established POCUS curricula.10 However, 50% of respondents in the 2015 survey reported early-stage development or interest in developing a POCUS curriculum.
Since then a validated family medicine residency curriculum has been published,11 and the American Academy of Family Physicians (AAFP) recently released a POCUS Curriculum Guideline for residencies (https://www.aafp.org/dam/AAFP/documents/medical_education_residency/program_directors/Reprint290D_POCUS.pdf).
[polldaddy:9928416]
The potential applications of POCUS in family medicine are numerous and have been reviewed in several recent publications.12,13 In this article, we will review the evidence for the use of POCUS in 4 areas: the cardiovascular exam (FIGURES 1 and 2), the lung exam (FIGURES 3-6), the screening exam for AAAs (FIGURE 7), and the evaluation for DVT (FIGURES 8 and 9). (Obstetric and musculoskeletal applications have been sufficiently covered elsewhere.14-17) For all of these applications, POCUS is safe, accurate, and beneficial and can be performed with a relatively small amount of training by non-radiology specialists, including FPs (TABLEs 1 and 2).


Just 2 hours of cardio POCUS training enhanced Dx accuracy
The American Society of Echocardiography (ASE) issued an expert consensus statement for focused cardiac ultrasound in 2013.18 The guideline supports non-cardiologists utilizing POCUS to assess for pericardial effusion and right and left ventricular enlargement, as well as to review global cardiac systolic function and intravascular volume status. Cardiovascular POCUS protocols are relatively easy to learn; even small amounts of training and practice can yield competency.
For example, a 2013 study showed that after 2 hours of training with a pocket ultrasound device, medical students and junior physicians inexperienced with POCUS were able to improve their diagnostic accuracy for heart failure from 50% to 75%.19 In another study, internal medicine residents with limited cardiac ultrasound training (ie, 20 practice exams) were able to detect decreased left ventricular ejection fraction using a handheld ultrasound device with 94% sensitivity and specificity in patients admitted to the hospital with acute decompensated heart failure.20 Similarly, after only 8 hours of training, a group of Norwegian general practitioners were able to obtain measurements of systolic function with a pocket ultrasound device that were not statistically different from a cardiologist’s measurements.21
In another study, rural FPs attended a 4-day course and then performed focused cardiac ultrasounds on primary care patients with a clinical indication for an echocardiogram.22 The scans were uploaded to a Web-based program for remote interpretation by a cardiologist. There was high concordance between the FPs’ interpretations of the focused cardiac ultrasounds and the cardiologist’s interpretations. Only 32% of the patients in the study group required a formal follow-up echocardiogram.
Kimura et al published a POCUS protocol for the rapid assessment of patients with heart failure, called the Cardiopulmonary Limited Ultrasound Exam (CLUE).23 The CLUE protocol utilizes 4 views to assess left ventricular systolic and diastolic function along with signs of pulmonary edema or systemic volume overload (TABLE 323). The presence of pulmonary edema or a plethoric inferior vena cava (IVC) was highly prognostic of in-hospital mortality. The CLUE protocol has been successfully used by novices including internal medicine residents after brief training (ie, up to 60 supervised scans) and can be performed in less than 5 minutes.24,25
Inpatient use. In addition to its use as an outpatient diagnostic tool, POCUS may be able to help guide therapy in patients admitted to the hospital with heart failure. Increasing collapse of the IVC directly correlates with the amount of fluid volume removed during hemodialysis.26 Goonewardena et al showed that IVC collapsibility was an independent predictor of 30-day hospital readmission even when demographics, signs and symptoms, and volume of diuresis were otherwise equal.27 However, whether the use of IVC collapsibility to guide management improves outcomes in heart failure remains to be validated in a prospective trial.
More sensitive, specific than x-rays for pulmonary diagnoses
The chest x-ray has traditionally been the imaging modality of choice to evaluate primary care pulmonary complaints. However, POCUS can be more sensitive and specific than a chest x-ray for evaluating several pulmonary diagnoses including pleural effusion, pneumonia, and pulmonary edema.
Pleural effusion can be difficult to detect with a physical exam alone. A systematic review showed that the physical exam is not sensitive for effusions <300 mL and can have even lower utility in obese patients.28 While an upright lateral chest x-ray can accurately detect effusions as small as 50 mL, portable x-rays have sensitivities of only 53% to 71% for small- or moderate-sized effusions.29,30 Ultrasound, however, has a sensitivity of 97% for small effusions.31
A 2016 meta-analysis showed that POCUS had a pooled sensitivity and specificity of 94% and 98%, respectively, for pleural effusions, while chest x-ray had a pooled sensitivity and specificity of 51% and 91%, respectively, when compared with computed tomography (CT) and expert sonography.32 POCUS evaluation for pleural effusion is technically simple, and at least one study showed that even novice users can achieve high diagnostic accuracy after only 3 hours of training.33
Pneumonia is the eighth leading cause of death in the United States and the single leading cause of infectious disease death in children worldwide.34-36 Pneumonia is a difficult diagnosis to make based on a history and physical examination alone, and the Infectious Diseases Society of America recommends diagnostic imaging to make the diagnosis.37
The adult and pediatric literature clearly demonstrate that lung ultrasound is accurate at diagnosing pneumonia. In a 2015 meta-analysis of the pediatric literature, lung ultrasound had a sensitivity of 96% and a specificity of 93% and positive and negative likelihood ratios of 15.3 and 0.06, respectively.38 In adults, a 2016 meta-analysis of lung ultrasound showed a pooled sensitivity and specificity of 90% and 88%, respectively, with positive and negative likelihood ratios of 6.6 and 0.08, respectively.39
In 2015, a prospective study compared the accuracy of lung ultrasound and chest x-ray using CT as the gold standard.40 Lung ultrasound had a significantly better sensitivity of 82% compared to a sensitivity of 64% for chest x-ray. Specificities were comparable at 94% for ultrasound and 90% for chest x-ray.40
At least one study found novice sonographers to be accurate with lung POCUS for the diagnosis of pneumonia after only two 90-minute training sessions.41 Moreover, ultrasound has a more favorable safety profile, greater portability, and lower cost compared with chest x-ray and CT.
Pulmonary edema. Lung ultrasound can identify interstitial pulmonary edema via artifacts called B lines, which are produced by the reverberation of sound waves from the pleura due to the widening of the fluid-filled interlobular septa. These are distinctly different from the A-line pattern of repeating horizontal lines that is seen with normal lungs, making lung ultrasound more accurate than chest x-ray for identification of pulmonary edema.42,43 When final diagnosis via blinded chart review is used as the reference standard, bilateral B lines on a lung ultrasound image have a sensitivity of 86% to 100% and a specificity of 92% to 98% for the diagnosis of pulmonary edema compared to chest x-ray’s sensitivity of 56.9% and specificity of 89.2%.44 There is also a linear correlation between the number of B lines present and the extent of pulmonary edema.42,45,46 The number of B lines decreases in real time as volume is removed in dialysis patients.47
POCUS evaluation for B lines can be learned very quickly. Exams of novices who have performed only 5 prior exams correlate highly with those of experts who have performed more than 100 exams.48
Simple, efficient screening method for abdominal aortic aneurysm
AAAs are present in up to 7% of men over the age of 50.49 The mortality rate of a ruptured AAA is as high as 80% to 95%.50 There is, however, a long prodromal period when interventions can make a significant difference, which is why accurate screening is so important.
AAA screening with ultrasound has been shown to decrease mortality.51 The current recommendation of the US Preventive Services Task Force (USPSTF) is a one-time AAA screening for all men ages 65 to 75 years who have ever smoked (Grade B).52 Despite the recommendations of the USPSTF, screening rates are low. One study found that only 9% of eligible patients in primary care practices received appropriate screening.51
Ultrasound performed by specialists is known to be an excellent screening test for AAA with a sensitivity of 98.9% and a specificity of 99.9%.53 POCUS use by emergency medicine physicians for the evaluation of symptomatic AAA is well established in the literature. A meta-analysis including 7 studies and 655 patients showed a pooled sensitivity of 99% and a specificity of 98%.54 Multiple studies also support primary care physicians performing POCUS AAA screening in the clinic setting.
For example, a 2012 prospective, observational study performed in Canada compared office-based ultrasound screening exams performed by a rural FP to scans performed in the hospital on the same patients.55 The physician completed 50 training examinations. The average discrepancy in aorta diameters between the 2 was only 2 mm, which is clinically insignificant, and the office-based scans had a sensitivity and specificity of 100%.
Similarly, a second FP study performed in Barcelona, showed that an FP who performed POCUS AAA screening had 100% concordance with a radiologist.56 Additionally, POCUS screening for AAA was not time consuming; it was performed in under 4 minutes per patient.55,57
Ruling out DVT
DVT is a relatively rare occurrence in the ambulatory setting. However, patients who present with a painful, swollen lower extremity are much more common, and DVT must be considered and ruled out in these situations.
Although isolated distal DVTs that occur in the calf veins are usually self-limited and have a very low risk of embolization, they can progress to proximal DVTs of the thigh veins up to 20% of time.58,59 Similarly, thrombophlebitis of the superficial lower extremity veins rarely embolizes, but can progress to a proximal DVT, especially if large segments are involved or if the segments are within 5 cm of the junction to the deep venous system.59 The risk of missing a proximal leg DVT is high because embolization occurs up to 60% of the time if the DVT is left untreated.60
The current standard for diagnosis of DVT is the lower extremity Doppler ultrasound examination, but obtaining same-day Doppler evaluations can be difficult in the ambulatory setting. In these instances, the American College of Chest Physicians (ACCP) recommends that even low-risk patients receive anticoagulation pending the evaluation if it cannot be obtained in the first 24 hours.59 This approach not only increases the cost of care, but also exposes patients—many of whom will not be diagnosed with thrombosis in the end—to the risks of anticoagulation.
D-dimer blood tests have drawbacks, too. While a negative high-sensitivity D-dimer blood test in a patient with a low pre-test probability of DVT can effectively rule out a DVT, laboratory testing is not always immediately available in the ambulatory setting either.61 Additionally, false-positive rates are high, and positive D-dimer exams still require evaluation by Doppler ultrasound.
Given these limitations, performing an ultrasound at the bedside or in the exam room can allow for more timely and cost-effective care. In fact, research shows that a limited ultrasound, called the 2-region compression exam, which follows along the course of the common femoral vein and popliteal vein only, ignoring the femoral and calf veins, is highly accurate in assessing for proximal leg DVTs. As such, it has been adopted for POCUS use by emergency medicine physicians.62
Multiple studies show that physicians with minimal training can perform the 2-region compression exam with a high degree of accuracy when full-leg Doppler ultrasound was used as the gold standard.63,64 In these studies, hands-on training times ranged from only 10 minutes to 5 hours, and the exam could be performed in less than 4 minutes. A systematic review of 6 studies comparing emergency physician-performed ultrasound with radiology-performed ultrasound calculated an overall sensitivity of 0.95 (95% CI, 0.87-0.99) and specificity of 0.96 (95% CI, 0.87-0.99) for those performed by emergency physicians.65
The main concern with the 2-region compression exam is that it can miss a distal leg DVT. As stated earlier, distal DVTs are relatively benign and tend to resolve without treatment; however, up to 20% can progress to become a dangerous proximal leg DVT.58 Researchers have validated several methods by prospective trials to address this limitation.
Specifically, researchers have demonstrated that patients with a low pre-test probability of DVT per the Wells scoring system could have DVT effectively ruled out with a single 2-region compression ultrasound without further evaluation.66 In another study, researchers evaluated all patients (regardless of pretest probability) with a 2-point compression exam and found that those with negative exams could be followed with a second exam in 7 to 10 days without initiating anticoagulation. If the second one was negative, no further evaluation was needed.67,68
And finally, researchers demonstrated that a negative 2-point compression ultrasound in combination with a concurrent negative D-dimer test was effective at ruling out DVT, regardless of pre-test probability.69,70
A preferred approach
Given this data and the fact that in the ambulatory setting it is often easier and faster to perform a 2-region compression examination than to obtain a D-dimer laboratory test or a formal full-leg Doppler ultrasound, what follows is our preferred approach to a patient with suspected DVT in the outpatient setting (FIGURE 10).
We first assess pre-test probability using the Wells scoring system. We then perform the 2-region compression ultrasound. If the patient has low pre-test risk according to the Wells score, we rule out DVT. If the patient has moderate or high risk with a negative 2-region compression ultrasound, the patient gets a D-dimer test. If the D-dimer test is negative, we rule out DVT. If the D-dimer test is positive, we schedule the patient for a repeat 2-region compression ultrasound in 7 to 10 days. If at any time the 2-region compression evaluation is positive, we treat the patient for DVT.
CORRESPONDENCE
Paul Bornemann, MD, Palmetto Health Family Medicine Residency, Department of Family and Preventive Medicine, University of South Carolina School of Medicine, 3209 Colonial Drive, Columbia, SC 29203; [email protected].
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
1. Hahn RG, Davies TC, Rodney WM. Diagnostic ultrasound in general practice. Fam Pract. 1988;5:129-135.
2. Deutchman ME, Hahn RG, Rodney WMM. Diagnostic ultrasound imaging by physicians of first contact: extending the family medicine experience into emergency medicine. Ann Emerg Med. 1993;22:594-596.
3. Bornemann P, Bornemann G. Military family physicians’ perceptions of a pocket point-of-care ultrasound device in clinical practice. Mil Med. 2014;179:1474-1477.
4. Smith-Bindman R, Aubin C, Bailitz J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110.
5. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal imaging: medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5:182-188.
6. Gordon CE, Feller-Kopman D, Balk EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332-339.
7. Calvert N, Hind D, McWilliams RG, et al. The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation. Health Technol Assess. 2003;7:1-84.
8. Hoppmann RA, Rao VV, Bell F, et al. The evolution of an integrated ultrasound curriculum (iUSC) for medical students: 9-year experience. Crit Ultrasound J. 2015;7:18.
9. Clinical procedures performed by physicians at their practice. American Academy of Family Physicians Member Census, December 31, 2016. Available at: http://www.aafp.org/about/the-aafp/family-medicine-facts/table-12(rev).html. Accessed June 26, 2017.
10. Hall JW, Holman H, Bornemann P, et al. Point of care ultrasound in family medicine residency programs: a CERA study. Fam Med. 2015;47:706-711.
11. Bornemann P. Assessment of a novel point-of-care ultrasound curriculum’s effect on competency measures in family medicine graduate medical education. J Ultrasound Med. 2017;36:1205-1211.
12. Steinmetz P, Oleskevich S. The benefits of doing ultrasound exams in your office. J Fam Pract. 2016;65:517-523.
13. Flick D. Bedside ultrasound education in family medicine. J Ultrasound Med. 2016;35:1369-1371.
14. Dresang LT, Rodney WM, Rodney KM. Prenatal ultrasound: a tale of two cities. J Natl Med Assoc. 2006;98:167-171.
15. Dresang LT, Rodney WM, Dees J. Teaching prenatal ultrasound to family medicine residents. Fam Med. 2004;36:98-107.
16. Rodney WM, Deutchman ME, Hartman KJ, et al. Obstetric ultrasound by family physicians. J Fam Pract. 1992;34:186-194.
17. Broadhurst NA, Simmons N. Musculoskeletal ultrasound - used to best advantage. Aust Fam Physician. 2007;36:430-432.
18. Spencer KT, Kimura BJ, Korcarz CE, et al. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:567-581.
19. Panoulas VF, Daigeler AL, Malaweera AS, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323-330.
20. Razi R, Estrada JR, Doll J, et al. Bedside hand-carried ultrasound by internal medicine residents versus traditional clinical assessment for the identification of systolic dysfunction in patients admitted with decompensated heart failure. J Am Soc Echocardiogr. 2011;24:1319-1324.
21. Mjølstad OC, Snare SR, Folkvord L, et al. Assessment of left ventricular function by GPs using pocket-sized ultrasound. Fam Pract. 2012;29:534-540.
22. Evangelista A, Galuppo V, Méndez J, et al. Hand-held cardiac ultrasound screening performed by family doctors with remote expert support interpretation. Heart. 2016;102:376-382.
23. Kimura BJ, Yogo N, O’Connell CW, et al. Cardiopulmonary limited ultrasound examination for “quick-look” bedside application. Am J Cardiol. 2011;108:586-590.
24. Kimura BJ, Amundson SA, Phan JN, et al. Observations during development of an internal medicine residency training program in cardiovascular limited ultrasound examination. J Hosp Med. 2012;7:537-542.
25. Kimura BJ, Shaw DJ, Amundson SA, et al. Cardiac limited ultrasound examination techniques to augment the bedside cardiac physical examination. J Ultrasound Med. 2015;34:1683-1690.
26. Brennan JM, Ronan A, Goonewardena S, et al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol. 2006;1:749-753.
27. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of hand-carried ultrasound assessment of the inferior vena cava and N-terminal pro-brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure. JACC Cardiovasc Imaging. 2008;1:595-601.
28. Wong CL, Holroyd-Leduc J, Straus SE. Does this patient have a pleural effusion? JAMA. 2009;301:309-317.
29. Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol. 1996;3:103-109.
30. Kitazono MT, Lau CT, Parada AN, et al. Differentiation of pleural effusions from parenchymal opacities: accuracy of bedside chest radiography. Am J Roentgenol. 2010;194:407-412.
31. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12:12-16.
32. Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran, Iran). 2016;4:1-10.
33. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40:1475-1480.
34. World Health Organization. Pneumonia. Fact Sheet No. 331. Available at: http://www.who.int/mediacentre/factsheets/fs331/en/. Accessed June 26, 2017.
35. Gereige RS, Laufer PM. Pneumonia. Pediatr Rev. 2013;34:438-456.
36. National Center for Health Statistics. Leading causes of death. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessed July 2, 2017.
37. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2:S27-S72.
38. Pereda MA, Chavez MA, Hooper-Miele CC, et al. Lung ultrasound for the diagnosis of pneumonia in children: a meta-analysis. Pediatrics. 2015;135:714-722.
39. Xia Y, Ying Y, Wang S, et al. Effectiveness of lung ultrasonography for diagnosis of pneumonia in adults: a systematic review and meta-analysis. J Thorac Dis. 2016;8:2822-2831.
40. Nazerian P, Volpicelli G, Vanni S, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33:620-625.
41. Filopei J, Siedenburg H, Rattner P, et al. Impact of pocket ultrasound use by internal medicine housestaff in the diagnosis of dyspnea. J Hosp Med. 2014;9:594-597.
42. Lichtenstein D, Mezière G. A lung ultrasound sign allowing bedside distinction between pulmonary edema and COPD: the comet-tail artifact. Intensive Care Med. 1998;24:1331-1334.
43. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014;12:25.
44. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23:223-242.
45. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689-696.
46. Picano E, Frassi F, Agricola E, et al. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356-363.
47. Noble VE, Murray AF, Capp R, et al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis: time course for resolution. Chest. 2009;135:1433-1439.
48. Gullett J, Donnelly JP, Sinert R, et al. Interobserver agreement in the evaluation of B-lines using bedside ultrasound. J Crit Care. 2015;30:1395-1399.
49. Guirguis-Blake JM, Beil TL, Sun X, et al. Primary Care Screening for Abdominal Aortic Aneurysm: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Evidence Syntheses No. 109. Rockville, MD; 2014.
50. Metcalfe D, Holt PJE, Thompson MM. The management of abdominal aortic aneurysms. BMJ. 2011;342:d1384.
51. Thompson SG, Ashton HA, Gao L, et al. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Brit J Surg. 2012;99:1649-1656.
52. LeFevre ML. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:281-290.
53. Lindholt JS, Vammen S, Juul S, et al. The validity of ultrasonographic scanning as screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 1999;17:472-475.
54. Rubano E, Mehta N, Caputo W, et al. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;20:128-138.
55. Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58:e172-e178.
56. Sisó-Almirall A, Gilabert Solé R, Bru Saumell C, et al. Feasibility of hand-held-ultrasonography in the screening of abdominal aortic aneurysms and abdominal aortic atherosclerosis. Med Clin (Barc). 2013;141:417-422.
57. Sisó-Almirall A, Kostov B, Navarro González M, et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS One. 2017;12:e0176877.
58. Philbrick JT, Becker DM. Calf deep venous thrombosis: a wolf in sheep’s clothing? Arch Intern Med. 1988;148:2131-2138.
59. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e351S-418S.
60. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
61. Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227-1235.
62. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep-vein thrombosis by real-time B-mode ultrasonography. N Engl J Med. 1989;320:342-345.
63. Crisp JG, Lovato LM, Jang TB. Compression ultrasonography of the lower extremity with portable vascular ultrasonography can accurately detect deep venous thrombosis in the emergency department. Ann Emerg Med. 2010;56:601-610.
64. Blaivas M, Lambert MJ, Harwood RA, et al. Lower-extremity doppler for deep venous thrombosis—can emergency physicians be accurate and fast? Acad Emerg Med. 2000;7:120-126.
65. Burnside PR, Brown MD, Kline JA. Systematic review of emergency physician-performed ultrasonography for lower-extremity deep vein thrombosis. Acad Emerg Med. 2008;15:493-498.
66. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795-1798.
67. Birdwell BG, Raskob GE, Whitsett TL, et al. The clinical validity of normal compression ultrasonography in outpatients suspected of having deep venous thrombosis. Ann Intern Med. 1998;128:1-7.
68. Cogo A, Lensing AW, Koopman MM, et al. Compression ultrasonography for diagnostic management of patients with clinically suspected deep vein thrombosis: prospective cohort study. BMJ. 1998;316:17-20.
69. Tick LW, Ton E, Van Voorthuizen T, et al. Practical diagnostic management of patients with clinically suspected deep vein thrombosis by clinical probability test, compression ultrasonography, and D-dimer test. Am J Med. 2002;113:630-635.
70. Stevens
Inpatient antibiotic resistance: Everyone’s problem
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
CASE
A 68-year-old woman is admitted to the hospital from home with acute onset, unrelenting, upper abdominal pain radiating to the back and nausea/vomiting. Her medical history includes bile duct obstruction secondary to gall stones, which was managed in another facility 6 days earlier with endoscopic retrograde cholangiopancreatography and stenting. The patient has type 2 diabetes (managed with metformin and glargine insulin), hypertension (managed with lisinopril and hydrochlorothiazide), and cholesterolemia (managed with atorvastatin).
On admission, the patient's white blood cell count is 14.7 x 103 cells/mm3, heart rate is 100 bpm, blood pressure is 90/68 mm Hg, and temperature is 101.5° F. Serum amylase and lipase are 3 and 2 times the upper limit of normal, respectively. A working diagnosis of acute pancreatitis with sepsis is made. Blood cultures are drawn. A computed tomography scan confirms acute pancreatitis. She receives one dose of meropenem, is started on intravenous fluids and morphine, and is transferred to the intensive care unit (ICU) for further management.
Her ICU course is complicated by worsening sepsis despite aggressive fluid resuscitation, nutrition, and broad-spectrum antibiotics. On post-admission Day 2, blood culture results reveal Escherichia coli that is resistant to gentamicin, amoxicillin/clavulanate, ceftriaxone, piperacillin/tazobactam, imipenem, trimethoprim/sulfamethoxazole, ciprofloxacin, and tetracycline. Additional susceptibility testing is ordered.
The Centers for Disease Control and Prevention (CDC) conservatively estimates that antibiotic-resistant bacteria are responsible for 2 billion infections annually, resulting in approximately 23,000 deaths and $20 billion in excess health care expenditures annually.1 Infections caused by antibiotic-resistant bacteria typically require longer hospitalizations, more expensive drug therapies, and additional follow-up visits.1 They also result in greater morbidity and mortality compared with similar infections involving non-resistant bacteria.1 To compound the problem, antibiotic development has steadily declined over the last 3 decades, with few novel antimicrobials developed in recent years.2 The most recently approved antibiotics with new mechanisms of action were linezolid in 2000 and daptomycin in 2003, preceded by the carbapenems 15 years earlier. (See “New antimicrobials in the pipeline.”)
New antimicrobials in the pipeline
The Generating Antibiotic Incentives Now (GAIN) Act was signed into law in 2012, creating a new designation—qualified infectious diseases products (QIDPs)—for antibiotics in development for serious or life-threatening infections (https://www.congress.gov/112/plaws/publ144/PLAW-112publ144.pdf). QIDPs are granted expedited FDA approval and an additional 5 years of patent exclusivity in order to encourage new antimicrobial development.
Five antibiotics have been approved with the QIDP designation: tedizolid, dalbavancin, oritavancin, ceftolozane/tazobactam, and ceftazidime/avibactam, and 20 more agents are in development including a new fluoroquinolone, delafloxacin, for acute bacterial skin and skin structure infections including those caused by methicillin-resistant Staphylococcus aureus (MRSA), and a new tetracycline, eravacycline, for complicated intra-abdominal infections and complicated UTIs. Eravacycline has in vitro activity against penicillin-resistant Streptococcus pneumoniae, MRSA, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Enterobacteriaceae, and multidrug-resistant A. baumannii. Both drugs will be available in intravenous and oral formulations.
Greater efforts aimed at using antimicrobials sparingly and appropriately, as well as developing new antimicrobials with activity against multidrug-resistant pathogens, are ultimately needed to address the threat of antimicrobial resistance. This article describes the evidence-based management of inpatient infections caused by resistant bacteria and the role family physicians (FPs) can play in reducing further development of resistance through antimicrobial stewardship practices.
Health care-associated methicillin-resistant Staphylococcus aureus
S. aureus is a common culprit of hospital-acquired infections, including central line-associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated pneumonia, and nosocomial skin and soft tissue infections. In fact, nearly half of all isolates from these infections are reported to be methicillin-resistant S. aureus (MRSA).3
Patients at greatest risk for MRSA infections include those who have been recently hospitalized, those receiving recent antibiotic therapy or surgery, long-term care residents, intravenous drug abusers, immunocompromised patients, hemodialysis patients, military personnel, and athletes who play contact sports.4,5 Patients with these infections often require the use of an anti-MRSA agent (eg, vancomycin, linezolid) in empiric antibiotic regimens.6,7 The focus of this discussion is on MRSA in hospital and long-term care settings; a discussion of community-acquired MRSA is addressed elsewhere. (See “Antibiotic stewardship: The FP’s role,” J Fam Pract. 2016;65:876-885.8)
Efforts are working, but problems remain. MRSA accounts for almost 60% of S. aureus isolates in ICUs.9 Thankfully, rates of health care-associated MRSA are now either static or declining nationwide, as a result of major initiatives targeted toward preventing health care-associated infection in recent years.10
Methicillin resistance in S. aureus results from expression of PBP2a, an altered penicillin-binding protein with reduced binding affinity for beta-lactam antibiotics. As a result, MRSA isolates are resistant to most beta-lactams.9 Resistance to macrolides, azithromycin, aminoglycosides, fluoroquinolones, and clindamycin is also common in health care-associated MRSA.9
The first case of true vancomycin-resistant S. aureus (VRSA) in the United States was reported in 2002.11 Fortunately, both VRSA and vancomycin-intermediate S. aureus (VISA) have remained rare throughout the United States and abroad.9,11 Heterogeneous VISA (hVISA), which is characterized by a few resistant subpopulations within a fully susceptible population of S. aureus, is more common than VRSA or VISA. Unfortunately, hVISA is difficult to detect using commercially available susceptibility tests. This can result in treatment failure with vancomycin, even though the MRSA isolate may appear fully susceptible and the patient has received clinically appropriate doses of the drug.12
Treatment. Vancomycin is the mainstay of therapy for many systemic health care-associated MRSA infections. Alternative therapies (daptomycin or linezolid) should be considered for isolates with a vancomycin minimum inhibitory concentration (MIC) >2 mcg/mL or in the setting of a poor clinical response.4 Combination therapy may be warranted in the setting of treatment failure. Because comparative efficacy data for alternative therapies is lacking, agent selection should be tailored to the site of infection and patient-specific factors such as allergies, drug interactions, and the risk for adverse events (TABLE 113-17).
Ceftaroline, the only beta-lactam with activity against MRSA, is approved by the US Food and Drug Administration (FDA) for use with acute bacterial skin and skin structure infections (ABSSIs) and community-acquired bacterial pneumonia.18 Tedizolid, a new oxazolidinone similar to linezolid, as well as oritavancin and dalbavancin—2 long-acting glycopeptides—were also recently approved for use with ABSSIs.13,14,19
Oritavancin and dalbavancin both have dosing regimens that may allow for earlier hospital discharge or treatment in an outpatient setting.13,14 Telavancin, quinupristin/dalfopristin, and tigecycline are typically reserved for salvage therapy due to adverse event profiles and/or limited efficacy data.15
Vancomycin-resistant enterococci (VRE)
Enterococci are typically considered normal gastrointestinal tract flora. However, antibiotic exposure can alter gut flora allowing for VRE colonization, which in some instances, can progress to the development of a health care-associated infection.15 Therefore, it is important to distinguish whether a patient is colonized or infected with VRE because treatment of colonization is unnecessary and may lead to resistance and other adverse effects.15
Enterococci may be the culprit in nosocomially-acquired intra-abdominal infections, bacteremia, endocarditis, urinary tract infections (UTIs), and skin and skin structure infections, and can exhibit resistance to ampicillin, aminoglycosides, and vancomycin.15 VRE is predominantly a health care-associated pathogen and may account for up to 77% of all health care-associated Enterococcus faecium infections and 9% of Enterococcus faecalis infections.1
Treatment. Antibiotic selection for VRE infections depends upon the site of infection, patient comorbidities, the potential for drug interactions, and treatment duration. Current treatment options include linezolid, daptomycin, quinupristin/dalfopristin (for E. faecium only), tigecycline, and ampicillin if the organism is susceptible (TABLE 113-17).15 For cystitis caused by VRE (not urinary colonization), fosfomycin and nitrofurantoin are additional options.16
Resistant Enterobacteriaceae
Resistant Enterobacteriaceae such as Escherichia coli and Klebsiella pneumoniae have emerged as a result of increased broad-spectrum antibiotic utilization and have been implicated in health care-associated UTIs, intra-abdominal infections, bacteremia, and even pneumonia.1 Patients with prolonged hospital stays and invasive medical devices, such as urinary and vascular catheters, endotracheal tubes, and endoscopy scopes, have the highest risk for infection with these organisms.20
The genotypic profiles of resistance among the Enterobacteriaceae are diverse and complex, resulting in different levels of activity for the various beta-lactam agents (TABLE 221-24).25 Furthermore, extended-spectrum beta-lactamase (ESBL)-producers and carbapenem-resistant Enterobacteriaceae (CRE) are often resistant to other classes of antibiotics, too, including aminoglycosides and fluoroquinolones.20,25 The increasing diversity among beta-lactamase enzymes has made the selection of appropriate antibiotic therapy challenging, since the ability to identify specific beta-lactamase genes is not yet available in the clinical setting.
ESBLs emerged shortly after the widespread use of cephalosporins in practice and are resistant to a variety of beta-lactams (TABLE 221-24). Carbapenems are considered the mainstay of therapy for ESBL-producing Enterobacteriaceae.20,26 An alternative for urinary and biliary tract infections can be piperacillin-tazobactam,21,26 but the combination may be subject to the inoculum effect, in which MIC and risk for treatment failure increase in infections with a high bacterial burden (colony-forming units/mL) such as pneumonias (TABLE 320,22,,23,25,27-42).22
Cefepime may retain activity against some ESBL-producing isolates, but it is also susceptible to the inoculum effect and should only be used for non–life-threatening infections and at higher doses.23 Fosfomycin has activity against ESBL-producing bacteria, but is only approved for oral use in UTIs in the United States.20,27 Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) were approved in 2014 and 2015, respectively, by the FDA for the management of complicated urinary tract and intra-abdominal infections caused by susceptible ESBL-producing Enterobacteriaceae. In order to preserve the antimicrobial efficacy of these 2 newer agents, however, they are typically reserved for definitive therapy when in vitro susceptibility is demonstrated and there are no other viable options.
AmpC beta-lactamases are resistant to similar agents as the ESBLs, in addition to cefoxitin and the beta-lactam/beta-lactamase inhibitor combinations containing clavulanic acid, sulbactam, and in some cases, tazobactam. Resistance can be induced and emerges in certain pathogens while patients are on therapy.28 Fluoroquinolones and aminoglycosides have a low risk of developing resistance while patients are on therapy, but are more likely to cause adverse effects and toxicity compared with the beta-lactams.28 Carbapenems have the lowest risk of emerging resistance and are the empiric treatment of choice for known AmpC-producing Enterobacteriaceae in serious infections.20,28 Cefepime may also be an option in less severe infections, such as UTIs or those in which adequate source control has been achieved.28,29
Carbapenem-resistant Enterobacteriaceae (CRE) have become a serious threat as a result of increased carbapenem use. While carbapenem resistance is less common in the United States than worldwide, rates have increased nearly 4-fold (1.2% to 4.2%) in the last decade, with some regions of the country experiencing substantially higher rates.24 The most commonly reported CRE genotypes identified in the United States include the serine carbapenemase (K. pneumoniae carbapenemase, or KPC), and the metallo-beta-lactamases (Verona integrin-encoded metallo-beta-lactamase, or VIM, and the New Dehli metallo-beta-lactamase, or NDM), with each class conferring slightly different resistance patterns (TABLE 221-24).20,30
Few treatment options exist for Enterobacteriaceae producing a serine carbapenemase, and, unfortunately, evidence to support these therapies is extremely limited. Some CRE isolates retain susceptibility to the polymyxins, the aminoglycosides, and tigecycline.30 Even fewer options exist for treating Enterobacteriaceae producing metallo-beta-lactamases, which are typically only susceptible to the polymyxins and tigecycline.43-45
Several studies have demonstrated lower mortality rates when combination therapy is utilized for CRE bloodstream infections.31,32 Furthermore, the combination of colistin, tigecycline, and meropenem was found to have a significant mortality advantage.32 Double carbapenem therapy has been effective in several cases of invasive KPC-producing K. pneumoniae infections.33,34 However, it is important to note that current clinical evidence comes from small, single-center, retrospective studies, and additional research is needed to determine optimal combinations and dosing strategies for these infections.
Lastly, ceftazidime/avibactam (Avycaz) was recently approved for the treatment of complicated urinary tract and intra-abdominal infections, and has activity against KPC-producing Enterobacteriaceae, but not those producing metallo-beta-lactamases, like VIM or NDM. In the absence of strong evidence to support one therapy over another, it may be reasonable to select at least 2 active agents when treating serious CRE infections. Agent selection should be based on the site of the infection, susceptibility data, and patient-specific factors (TABLE 320,22,,23,25,27-42). The CDC also recommends contact precautions for patients who are colonized or infected with CRE.35
Multi-drug resistant Pseudomonas aeruginosa
Pseudomonas aeruginosa is a gram-negative rod that can be isolated from nosocomial infections such as UTIs, bacteremias, pneumonias, skin and skin structure infections, and burn infections.20 Pseudomonal infections are associated with high morbidity and mortality and can cause recurrent infections in patients with cystic fibrosis.20 Multidrug-resistant P. aeruginosa (MDR-P) infections account for approximately 13% of all health care-associated pseudomonal infections nationally.1 Both fluoroquinolone and aminoglycoside resistance has emerged, and multiple types of beta-lactamases (ESBL, AmpC, carbapenemases) have resulted in organisms that are resistant to nearly all anti-pseudomonal beta-lactams.20
Treatment. For patients at risk for MDR-P, some clinical practice guidelines have recommended using an empiric therapy regimen that contains antimicrobial agents from 2 different classes with activity against P. aeruginosa to increase the likelihood of susceptibility to at least one agent.6 De-escalation can occur once culture and susceptibility results are available.6 Dose optimization based on pharmacodynamic principles is critical for ensuring clinical efficacy and minimizing resistance.36 The use of high-dose, prolonged-infusion beta-lactams (piperacillin/tazobactam, cefepime, ceftazidime, and carbapenems) is becoming common practice at institutions with higher rates of resistance.36-38
A resurgence of polymyxin (colistin) use for MDR-P isolates has occurred, and may be warranted empirically in select patients, based on local resistance patterns and patient history. Newer pharmacokinetic data are available, resulting in improved dosing strategies that may enhance efficacy while alleviating some of the nephrotoxicity concerns associated with colistin therapy.39
Ceftolozane/tazobactam (Zerbaxa) and ceftazidime/avibactam (Avycaz) are options for complicated urinary tract and intra-abdominal infections caused by susceptible P. aeruginosa isolates. Given the lack of comparative efficacy data available for the management of MDR-P infections, agent selection should be based on site of infection, susceptibility data, and patient-specific factors.
Multi-drug resistant Acinetobacter baumannii
A. baumannii is a lactose-fermenting, gram-negative rod sometimes implicated in nosocomial pneumonias, line-related bloodstream infections, UTIs, and surgical site infections.20 Resistance has been documented for nearly all classes of antibiotics, including carbapenems.1,20 Over half of all health care-associated A. baumannii isolates in the United States are multidrug resistant.1
Treatment. Therapy options for A. baumannii infections are often limited to polymyxins, tigecycline, carbapenems (except ertapenem), aminoglycosides, and high-dose ampicillin/sulbactam, depending on in vitro susceptibilities.40,41 When using ampicillin/sulbactam for A. baumannii infections, sulbactam is the active ingredient. Doses of 2 to 4 g/d of sulbactam have demonstrated efficacy in non-critically ill patients, while critically ill patients may require higher doses (up to 12 g/d).40 Colistin is considered the mainstay of therapy for carbapenem-resistant A. baumannii. It should be used in combination with either a carbapenem, rifampin, an aminoglycoside, or tigecycline.42
Drug therapies for nosocomial-resistant gram-negative infections, along with clinical pearls for use, are summarized in TABLE 3.20,22,23,25,27-42 Because efficacy data are limited for treating infections caused by these pathogens, appropriate antimicrobial selection is frequently guided by location of infection, susceptibility patterns, and patient-specific factors such as allergies and the risk for adverse effects.
Antimicrobial stewardship
Antibiotic misuse has been a significant driver of antibiotic resistance.46 Efforts to improve and measure the appropriate use of antibiotics have historically focused on acute care settings. Broad interventions to reduce antibiotic use include prospective audit with intervention and feedback, formulary restriction and preauthorization, and antibiotic time-outs.47,48
Pharmacy-driven interventions include intravenous-to-oral conversions, dose adjustments for organ dysfunction, pharmacokinetic or pharmacodynamic interventions to optimize treatment for organisms with reduced susceptibility, therapeutic duplication alerts, and automatic-stop orders.47,48
Diagnosis-specific interventions include order sets for common infections and the use of rapid diagnostic assays (TABLE 449,50). Rapid diagnostic testing is increasingly being considered an essential component of stewardship programs because it permits significantly shortened time to organism identification and susceptibility testing and allows for improved antibiotic utilization and patient outcomes when coupled with other effective stewardship strategies.49
Key players in acute care antibiotic stewardship programs (ASPs) often include physicians, pharmacists, infectious disease specialists, epidemiologists, microbiologists, nurses, and experts in quality improvement and information technology.
The core elements. The CDC has defined the core elements of successful inpatient ASPs.46 These include:
- commitment from hospital leadership
- a physician leader who is responsible for overall program outcomes
- a pharmacist leader who co-leads the program and is accountable for enterprise-wide improvements in antibiotic use
- implementation of at least one systemic intervention (broad, pharmacy-driven, or infection/syndrome-specific)
- monitoring of prescribing and resistance patterns
- reporting antibiotic use and resistance patterns to all involved in the medication use process
- Education directed at the health care team about optimal antibiotic use.
Above all, success with antibiotic stewardship is dependent on identified leadership and an enterprise-wide multidisciplinary approach.
The FP’s role in hospital ASPs can take a number of forms. FPs who practice inpatient medicine should work with all members of their department and be supportive of efforts to improve antibiotic use. Prescribers should help develop and implement hospital-specific treatment recommendations, as well as be responsive to measurements and audits aimed at determining the quantity and quality of antibiotic use. Hospital-specific updates on antibiotic prescribing and antibiotic resistance should be shared widely through formal and informal settings. FPs should know if patients with resistant organisms are hospitalized at institutions where they practice, and should remain abreast of infection rates and resistance patterns.
When admitting a patient, the FP should ask if the patient has received medical care elsewhere, including in another country. When caring for patients known to be currently or previously colonized or infected with resistant organisms, the FP should follow the appropriate precautions and insist that all members of the health care team follow suit.
CASE
A diagnosis of carbapenem-resistant E.coli sepsis is eventually made. Additional susceptibility test results reported later the same day revealed sensitivity to tigecycline and colistin, with intermediate sensitivity to doripenem. An infectious disease expert recommended contact precautions and combination treatment with tigecycline and doripenem for at least 7 days. The addition of a polymyxin was also considered; however, the patient’s renal function was not favorable enough to support a course of that agent. Longer duration of therapy may be required if adequate source control is not achieved.
After a complicated ICU stay, including the need for surgical wound drainage, the patient responded satisfactorily and was transferred to a medical step-down unit for continued recovery and eventual discharge.
CORRESPONDENCE
Dora E. Wiskirchen, PharmD, BCPS, Department of Pharmacy, St. Francis Hospital and Medical Center, 114 Woodland St., Hartford, CT 06105; Email: [email protected].
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed January 9, 2018.
2. Boucher HW, Talbot GH, Benjamin DK Jr, et al. 10 × ‘20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685-1694.
3. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312:1438-1446.
4. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18-e55.
5. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520-532.
6. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61-e111.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173
8. Wiskirchen DE, Summa M, Perrin A, et al. Antibiotic stewardship: The FP’s role. J Fam Pract. 2016;65:876-885.
9. Stryjewski ME, Corey GR. Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 2014;58 Suppl 1:S10-S19.
10. Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970-1978.
11. Askari E, Tabatabai SM, Arianpoor A, et al. VanA-positive vancomycin-resistant Staphylococcus aureus: systematic search and review of reported cases. Infect Dis Clin Pract. 2013;21:91-93.
12. van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405-410.
13. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206334s000lbl.pdf. Accessed January 10, 2018.
14. Dalvance [package insert]. Parsippany, NJ: Allergan; 2016. Available at: https://www.allergan.com/assets/pdf/dalvance_pi. Accessed January 10, 2018.
15. Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-1243.
16. Heintz BH, Halilovic J, Christensen CL. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy. 2010;30:1136-1149.
17. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-278.
18. Teflaro [package insert]. Parsippany, NJ: Allergan; 2016. Available at: http://www.allergan.com/assets/pdf/teflaro_pi. Accessed January 10, 2018.
19. Sivextro [package insert]. Whitehouse Station, NJ: Merck & Co; 2015. Available at: https://www.merck.com/product/usa/pi_circulars/s/sivextro/sivextro_pi.pdf. Accessed January 10, 2018.
20. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multi-drug resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011;86:250-259.
21. Rodríguez-Baño J, Navarro MD, Retamar P, et al. β-lactam/β-lactamase inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli; a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167-174.
22. Peterson LR. Antibiotic policy and prescribing strategies for therapy of extended-spectrum beta-lactamase-producing Enterobacteriaceae: the role of piperacillin-tazobactam. Clin Microbiol Infect. 2008;14 Suppl 1:181-184.
23. Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and β-lactam/β-lactamase inhibitors in the treatment of infections caused by extended-spectrum-β-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871-880.
24. Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1-14.
25. Toussaint KA, Gallagher JC. β-lactam/β-lactamase inhibitor combinations: from then to now. Ann Pharmacother. 2015;49:86-98.
26. Curello J, MacDougall C. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum β-lactamases. J Pediatr Pharmacol Ther. 2014;19:156-164.
27. Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant Gram-negative bacterial infections. Pharmacotherapy. 2014;34:845-857.
28. MacDougall C. Beyond susceptible and resistant, part I: treatment of infections due to Gram-negative organisms with inducible β-lactamases. J Pediatr Pharmacol Ther. 2011;16:23-30.
29. Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae. Clin Infect Dis. 2013;57:781-788.
30. Morrill HJ, Pogue JM, Kaye KS, et al. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2:1-15.
31. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment of bacteremia due to KPC-producing Klebsiella pneumonia: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113.
32. Tumbarello M, Viale P, Viscoli C, et al. Predictors of morality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumonia: importance of combination therapy. Clin Infect Dis. 2012;55:943-950.
33. Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388-2390.
34. Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900-2901.
35. Centers for Disease Control and Prevention. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Available at: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf. Accessed January 9, 2018.
36. Crandon JL, Nicolau DP. Pharmacodynamic approaches to optimizing beta-lactam therapy. Crit Car Clin. 2011;27:77-93.
37. Zavascki AP, Carvalhaes CG, Picão RC, et al. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8:71-93.
38. Crandon JL, Ariano RE, Zelenitsky SA, et al. Optimization of meropenem dosage in the critically ill population based on renal function. Intensive Care Med. 2011;37:632-638.
39. Ortwine JK, Kaye KS, Li J, et al. Colistin: understanding and applying recent pharmacokinetic advances. Pharmacotherapy. 2015;35:11-16.
40. Adnan S, Paterson DL, Lipman J, et al. Ampicillin/sulbactam: its potential use in treating infections in critically ill patients. Int J Antimicrob Agents. 2013:42:384-389.
41. Munoz-Price LS, Weinstein RA, et al. Acinetobacter infection. N Engl J Med. 2008;358:1271-1281.
42. Pogue JM, Mann T, Barber KE, et al. Carbapenem-resistant Acinetobacter baumannii: epidemiology, surveillance and management. Expert Rev of Anti Infect Ther. 2013;11:383-393.
43. Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597-602.
44. Moellering RC Jr. NDM-1—a cause for worldwide concern. N Engl J Med. 2010;363:2377-2379.
45. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis. 2013;19:870-878.
46. Centers for Disease Control and Prevention. 2014. The core elements of hospital antibiotic stewardship programs. Available at: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf. Accessed January 9, 2018.
47. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159-177.
48. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antimicrobial stewardship program: guidelines by the Infectious Diseases Society of American and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016:62:e51-e77.
49. Bauer KA, Perez KK, Forrest GN, et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis. 2014;59 Suppl 3:S134-S145.
50. Wong Y. An introduction to antimicrobial rapid diagnostic testing. Pharmacy One Source 2015. Available at: http://blog.pharmacyonesource.com/an-introduction-to-antimicrobial-rapid-diagnostic-testing. Accessed July 20, 2015.
51. Pakyz AL, MacDougall C, Oinonen M, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254-2260.
52. Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007;44:664-670.
53. Toth NR, Chambers RM, Davis SL. Implementation of a care bundle for antimicrobial stewardship. Am J Health Syst Pharm. 2010;67:746-749.
From The Journal of Family Practice | 2018;67(2):E1-E11.
PRACTICE RECOMMENDATIONS
› Consider alternatives to vancomycin for health care-associated methicillin-resistant Staphylococcus aureus isolates with a vancomycin minimum inhibitory concentration >2 mcg/mL or in the setting of poor clinical response. A
› Identify colonization vs infection with vancomycin-resistant enterococci (VRE) in the gastrointestinal tract following antibiotic exposure to minimize inappropriate antibiotic prescribing for VRE. C
› Use carbapenems as first-line treatment for severe infections caused by Enterobacteriaceae-producing extended-spectrum beta-lactamases. C
› Treat invasive carbapenem-resistant Enterobacteriaceae infections with combination therapy; site of infection, susceptibility patterns, and patient-specific factors should guide antibiotic selection. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Osteoporosis: A quick update
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10
Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10

Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
Researchers estimate that approximately 10.2 million Americans have osteoporosis, and an additional 43 million have low bone density.1 Equally stark are the ramifications of these numbers. About one out of every 2 Caucasian women will experience an osteoporosis-related fracture at some point in their lifetime, as will approximately one in 5 men.2 Although African American women tend to have a higher bone mineral density (BMD) than white women throughout their lives, those who have osteoporosis have the same elevated risk for fractures as Caucasians.
Osteoporotic fractures are associated with increased risk of disability, mortality, and nursing home placement. Given the aging population, researchers expect annual direct costs from osteoporosis to reach $25.3 billion by 2025.3
Family physicians (FPs) can have a meaningful impact on the extent to which this condition affects the population. To that end, we’ve put together a brief summary of the screening recommendations to keep in mind and a comparison of the different agents used to treat and prevent osteoporosis. The reference tables throughout will put these details at your fingertips.
Screening recommendations vary, Dx doesn’t require BMD testing
Guidelines for screening for osteoporosis vary considerably by professional organization. For example, the US Preventive Services Task Force (USPSTF) recommends screening all women ≥65 years, and younger women whose fracture risk is the same, or greater than, that of a 65-year-old white woman who has no additional risk factors (TABLE 14).5 In addition, the USPSTF concludes that the current evidence is insufficient to recommend routine screening for osteoporosis in men.5
The National Osteoporosis Foundation (NOF) recommends that BMD testing be performed in all women ≥65 years and in men ≥70 years.6 In terms of frequency, NOF recommends BMD testing one to 2 years after initiating therapy to reduce fracture risk and every 2 years thereafter. The American College of Obstetricians and Gynecologists recommends BMD screening for women no more than every 2 years starting at age 65 years.7 It also recommends selective screening in women younger than 65 years of age if they are postmenopausal and have other risk factors for osteoporosis.7
The most recent guideline regarding osteoporosis was published in May 2017 by the American College of Physicians (ACP) and endorsed by the American Academy of Family Physicians.8 But the guideline focuses on treatment rather than screening.
Although guidelines vary by society, most experts agree with BMD assessment in all women ≥65 years and postmenopausal women <65 years if one or more of the risk factors identified in TABLE 14 are present.
Diagnosis. Osteoporosis can be diagnosed using dual energy x-ray absorptiometry (DXA) and T-score (TABLE 26),9 but BMD testing is not always necessary to establish the diagnosis. For example, osteoporosis can be diagnosed clinically in both men and women who have sustained a hip fracture (with or without BMD testing). Osteoporosis may also be diagnosed in patients with osteopenia (determined by DXA and T-score) who have had a vertebral, proximal humeral, or pelvic fracture. Generally speaking, a detailed history and physical together with BMD assessment, vertebral imaging to diagnose vertebral fractures, and, when appropriate, the World Health Organization’s 10-year estimated fracture probability, are all utilized to establish patients’ fracture risk.6,10

Treatment: Which agents and for how long?
Once a patient is diagnosed with osteoporosis, answering the following questions will help with selection of the best therapy for the patient:
- Where on the body is BMD the lowest (vertebral, nonvertebral, or hip) and, consequently, at highest risk for a fracture?
- Does the patient have any conditions that would interfere with therapy (difficulty swallowing, esophageal/gastrointestinal irritation)? This is important, as certain agents are associated with severe esophagitis.
- Does the patient have any issues that would prevent adherence? Adherence may improve with therapy that is administered less frequently (weekly, monthly, once every 3 months, or annually).
TABLE 36,11-14 lists the prescription medications used to treat and prevent osteoporosis, their effect on the risk of vertebral, hip, and nonvertebral fractures, and contraindications/major adverse effects. First-line therapies are recommended based on clinical trials comparing the medication to placebo and evaluating their effectiveness in lowering the risk of vertebral, hip, and nonvertebral fractures.15 Given the absence of studies comparing these drugs to one another, TABLE 36,11-14 should not be used to make direct comparisons.
A new monoclonal antibody, romosozumab, has shown statistically significant decreases in the risk of new vertebral and nonvertebral fractures compared to alendronate after 12 months of use.16 However, there was a statistically significant higher number of patients who had a cardiac ischemic event or revascularization while taking romosozumab compared with those taking alendronate in the one-year double-blind period of the study.16 As of press time, the US Food and Drug Administration has not approved romosozumab.
Duration of treatment should be individualized based on specific patient factors, the pharmacologic agent, and, of course, adverse effects. However, no pharmacologic agent should be used indefinitely.6 In its clinical practice guidelines, the ACP recommends that patients be treated for 5 years with an appropriate pharmacologic therapy.8 The American Society for Bone and Mineral Research (ASBMR) Task Force recommends a review of therapy after 3 years with an intravenous bisphosphonate (BP; strength of recommendation [SOR]=C).17
A review of 2 recent long-term trials analyzing the effects of BPs offers some additional guidance regarding duration of therapy in Caucasian postmenopausal women.18 In one study, women who received 10 years of therapy with alendronate reported fewer vertebral fractures than those who were switched to placebo after 5 years of treatment.19
In the second trial, which studied zoledronic acid, there were fewer morphometric vertebral fractures for those participants given annual injections for 6 years vs 3 years.20 This trial found a significant transient increase in serum creatinine >0.5 mg/dL in the zoledronic acid treatment group.
These findings have prompted some experts in the field of osteoporosis to call for physicians to consider longer therapy with a BP (10 years with oral therapy or 6 years with intravenous therapy) in high-risk postmenopausal women (older women, those with a low hip T-score or high fracture risk score, those with a previous major osteoporotic fracture, and those who experienced fracture while on therapy) (SOR=B).18
Two rare adverse effects to keep in mind
The incidence of atypical femoral fracture, although rare (2-100 per 100,000 women), increases with duration of BP use. As a result, a drug holiday of 2 to 3 years should be considered for women with a low risk for fracture after 3 to 5 years of BP therapy (SOR=C).18
Osteonecrosis of the jaw (ONJ), also known as antiresorptive-associated osteonecrosis of the jaw, is a rare adverse effect of BPs that is associated with higher drug potency, higher cumulative dose, and parenteral route of administration, as well as other risk factors.17,21 The American Association of Maxillofacial Surgeons (AAOMS) states that the risk of developing ONJ increases with use of oral BPs for more than 4 years;22 however, the Task Force of the ASBMR states that the evidence to support this is of poor quality.18 No recommendations on duration of therapy based on risk for ONJ have been made; however, AAOMS recommends discontinuation of oral BPs for a period of 2 months prior to, and 3 months following (or until osseous healing has occurred), elective invasive dental surgery for patients who have been taking an oral BP ≥4 years (SOR=C).22
If a long-term drug holiday is selected, patients should be reassessed in 2 years. Shorter duration of follow-up is warranted for patients taking denosumab, teriparatide, or raloxifene, since bone loss will resume once therapy is discontinued.18
Because the benefits of BPs (in terms of reducing the risk of vertebral fracture) are significantly greater than the risks of an atypical fracture or ONJ, therapy should be started in appropriate patients, but duration of therapy should be monitored closely.
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CORRESPONDENCE
Lovedhi Aggarwal, MD, 95-390 Kuahelani Avenue, Mililani, HI 96789; [email protected].
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
1. Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520-2526.
2. Office of the Surgeon General (US). Bone health and osteoporosis: a report of the Surgeon General. Rockville (MD); 2004.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17 Suppl 6:S164-S169.
4. Kanis JA, McCloskey EV, Johansson H, et al, on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23-57.
5. Screening for Osteoporosis: U.S. Preventive Services Task Force Final Recommendation Statement. Ann Intern Med. 2011;154:356-364.
6. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis (National Osteoporosis Foundation). Osteoporos Int. 2014;25:2359-2381.
7. Committee on Practice Bulletins-Gynecology, The American College of Obstetricians and Gynecologists. ACOG Practice Bulletin N. 129. Osteoporosis. Obstet Gynecol. 2012;120:718-734.
8. Qaseem A, Forciea MA, McLean RM, et al. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:818-839.
9. Jeremiah MP, Unwin BK, Greenawald MH, et al. Diagnosis and management of osteoporosis. Am Fam Physician. 2015;92:261-268.
10. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395-2411.
11. Lexicomp Online. Clinical Drug Information. Available at: https://online.lexi.com/lco/action/home. Accessed June 30, 2016.
12. Crandall CJ, Newberry SJ, Diamant A, et al. Treatment to Prevent Fractures in Men and Women with Low Bone Density and Osteoporosis: Update of a 2007 Report. Comparative Effectiveness Review No. 53. Rockville, MD: Agency for Healthcare Research and Quality; March 2012. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/osteoporosis-bone-fracture_research.pdf. Accessed January 10, 2018.
13. O’Connell MB, Borchert JS. Chapter 73. Osteoporosis and other metabolic bone diseases. In: DiPiro JT, Talbert RL, Yee GC, eds. Pharmacotherapy: a pathophysiologic approach. 9th ed. McGraw-Hill Education; 2014.
14. Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med. 2014;161:711-723.
15. Watts NB, Bilezikian JP, Camacho PM, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16(Supp 3):1-37. Available at: https://www.aace.com/files/osteo-guidelines-2010.pdf. Accessed June 17, 2016.
16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427.
17. Adler RA, El-Hajj Fuleihan G, Bauer DC, et al. Managing osteoporosis in patients on long-term bisphosphonate treatment: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2016;31:16-35.
18. Adler RA. Duration of anti-resorptive therapy for osteoporosis. Endocrine. 2015;51:222-224.
19. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
20. Black DM, Reid IR, Boonen S, et al. The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res. 2012;27:243-254.
21. Denosumab (Xgeva, Prolia); intravenous bisphosphonates: osteonecrosis of the jaw—further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk. Accessed June 30, 2016.
22. Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72:1938-1956.
From The Journal of Family Practice | 2018;67(2):59-62,64-65.
PRACTICE RECOMMENDATIONS
› Use bisphosphonates (except ibandronate) and denosumab as first-line pharmacologic treatment for osteoporosis. A
› Treat patients for 5 years with oral bisphosphonates and 3 years with intravenous bisphosphonates before reviewing therapy, unless there are complications. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series