User login
A guide to providing wide-ranging care to newborns
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
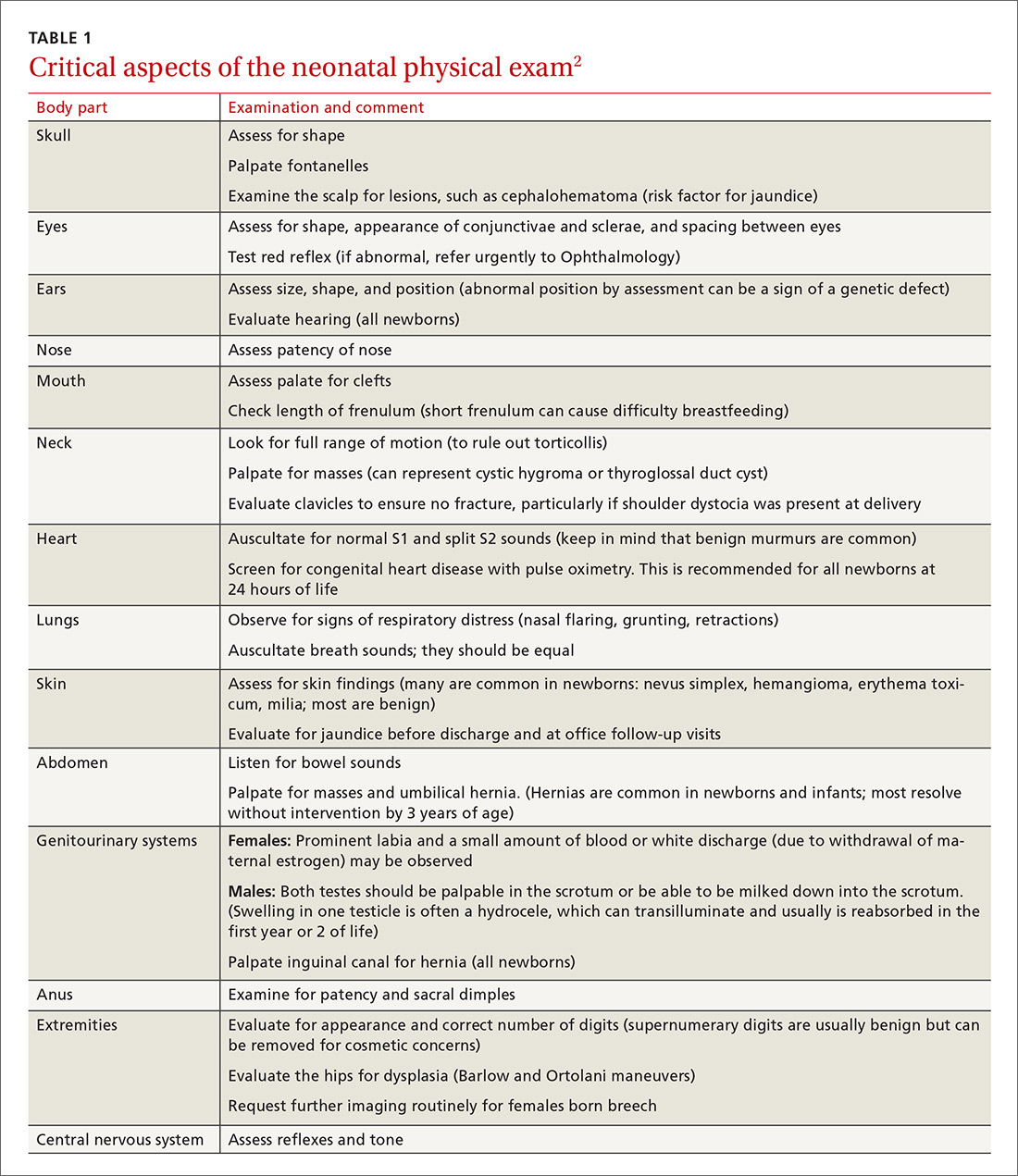
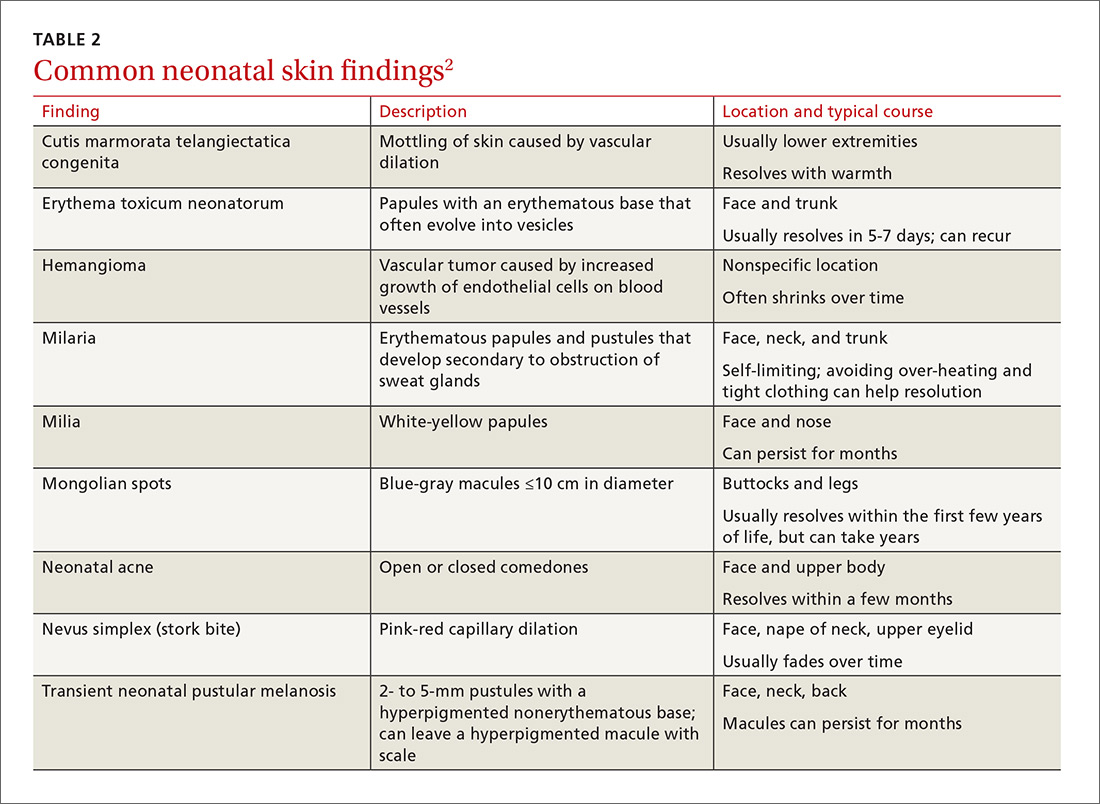
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.
Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8
Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
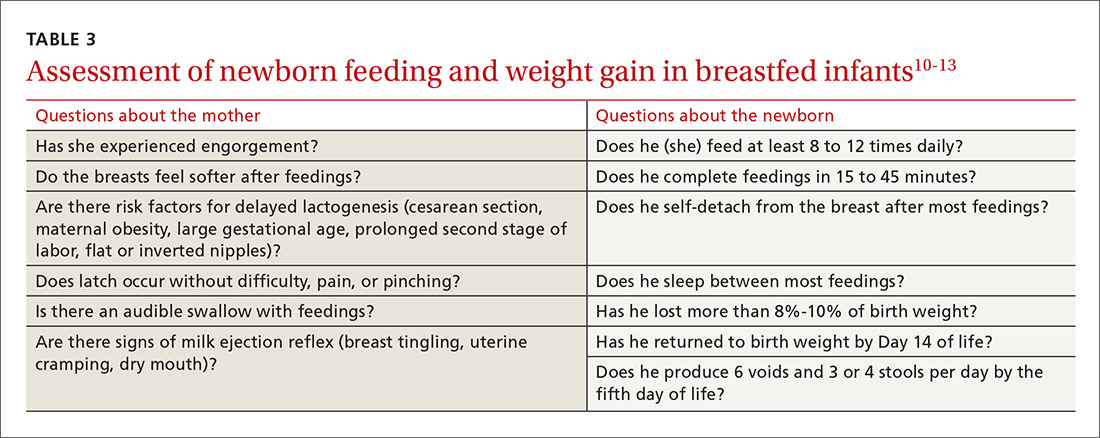
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1
Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
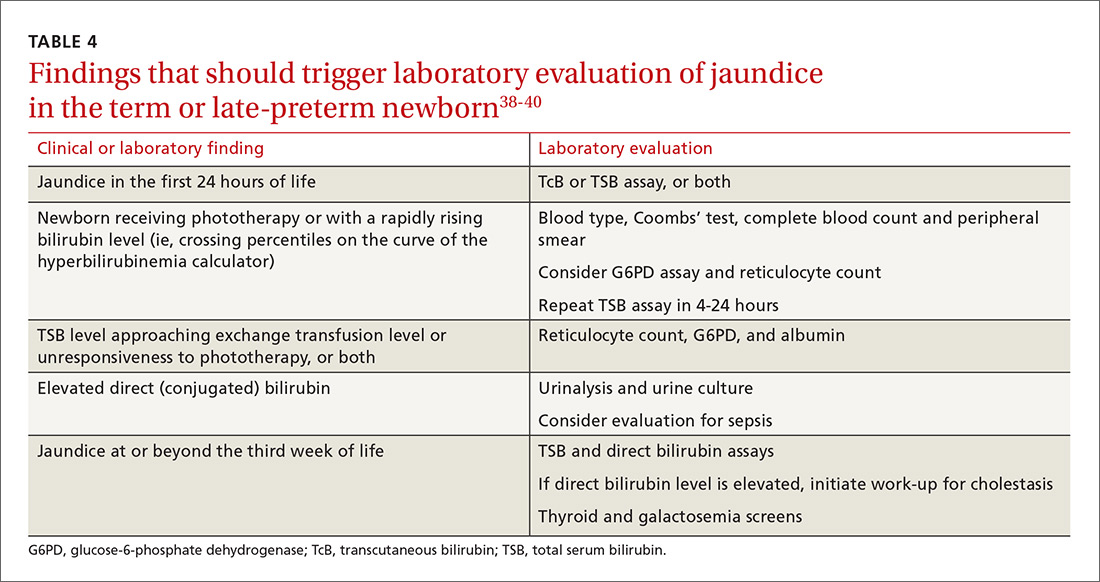
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38
Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.
To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
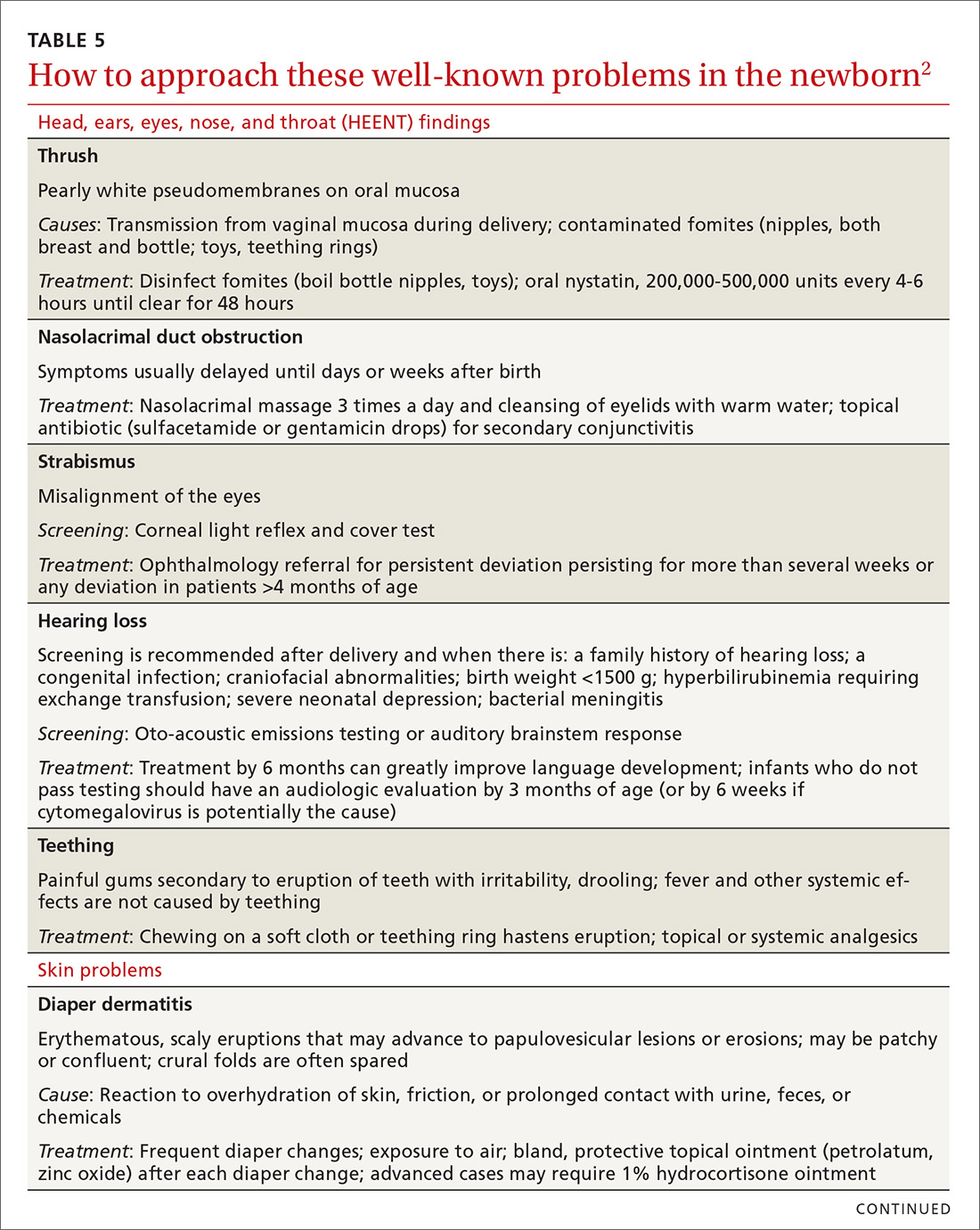
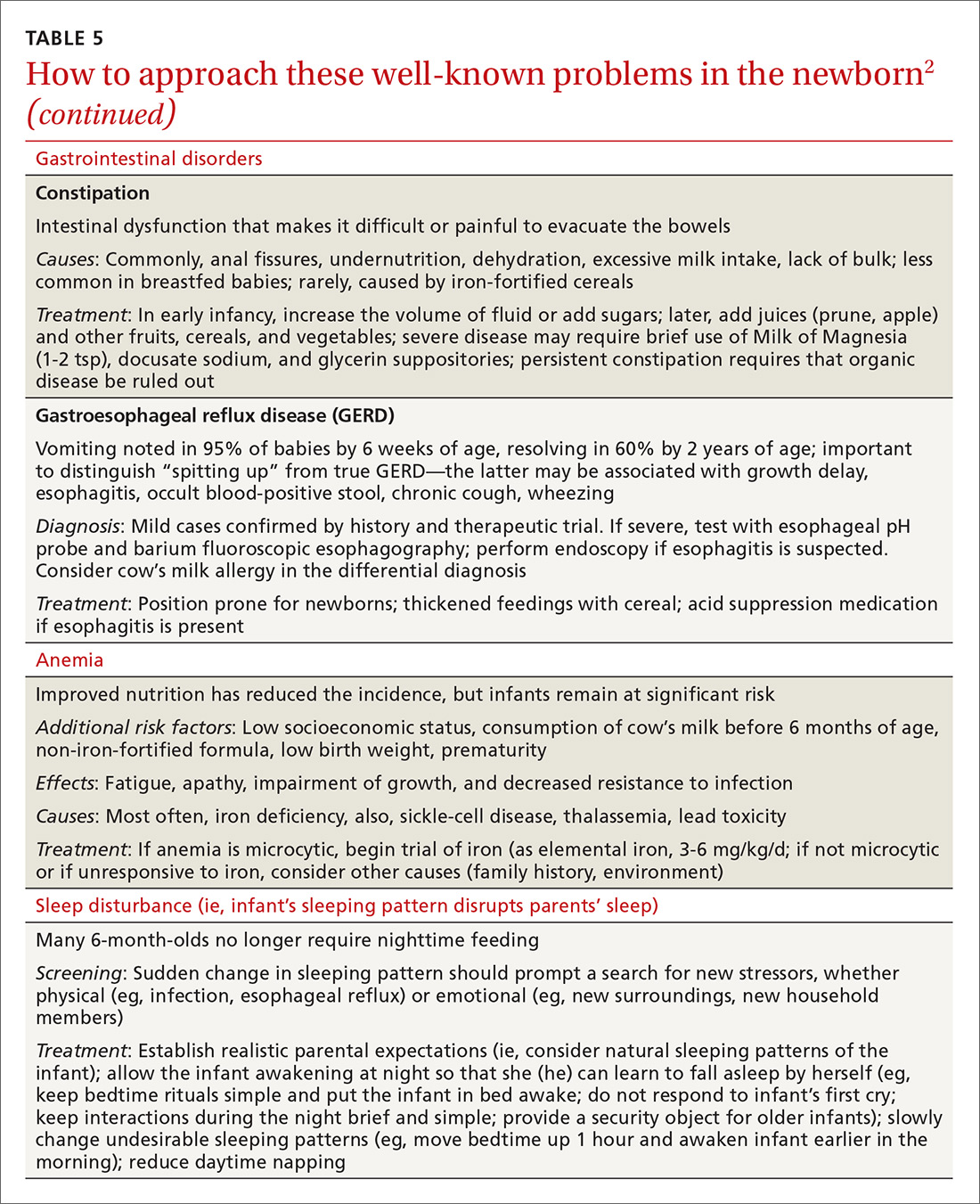
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.
CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.

Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8

Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1

Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38

Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.

To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.

CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
Caring for a newborn can be a source of joy for family physicians (FPs). In this article, we examine care provided in the first month of life, including a thorough physical examination, safe hospital discharge procedures, assessment of neonatal feeding, evaluation of jaundice and fever, and prevention of sudden infant death syndrome (SIDS). In addition, we describe how FPs can support women of childbearing age between pregnancies, with the goal of reducing the risk of adverse outcomes in future pregnancies. (See “Your role in risk assessment and interventions during the interconception period.”)
SIDEBAR
Your role in risk assessment and interventions during the interconception period
Interconception care is the care of women of childbearing age between pregnancies (from the end of a pregnancy to conception of the next). It includes medical and psychological interventions to modify their risk factors to improve future birth outcomes. In 2006, the Centers for Disease Control and Prevention Work Group and Select Panel on Preconception Care recommended risk assessment and intervention in the interconception period, especially for women who have experienced previous adverse outcomes of pregnancy.1
After the birth of a child, many women who had been receiving regular prenatal care stop seeing providers for their health care or return to a pattern of fragmented care.2-4 They often revert to behaviors, such as smoking and substance abuse, that put future pregnancies at risk.2,4,5 In addition, the maternal and family focus often shifts from caring for the woman to caring for the newborn, ignoring the health care needs of the mother.2,4,5
The IMPLICIT (Interventions to Minimize Preterm and Low birth weight Infants through Continuous Improvement Techniques) Network is a perinatal quality collaborative of family medicine residency programs and community health centers that uses continuous quality improvement processes to improve the health of women and decrease preterm birth and infant mortaility.6,7 The IMPLICIT interconception care model targets 4 risk factors that not only meet the model's requirements, but have a solid base of evidence5-8 by which to mitigate those risk factors and thus improve birth outcomes:
- tobacco use
- depression risk
- use of contraception to prolong interpregnancy interval
- use of a multivitamin with folic acid.
During newborn and well-child visits, screening for maternal health in these 4 key areas and providing point-of-care interventions can markedly improve maternal and perinatal health outcomes. Although the IMPLICIT Network continues to engage in the study of this model of addressing maternal health during newborn and infant visits, initial evidence demonstrates that these interventions exert positive effects on modifiable risk factors.6,8,9
Sidebar references
1. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care---United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. April 21, 2006. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5506a1.htm. Accessed February 1, 2018.
2. DiBari JN, Yu SM, Chao SM, et al. Use of postpartum care: predictors and barriers. J Pregnancy. 2014;2014:530769.
3. Liberto TL. Screening for depression and help-seeking in postpartum women during well-baby pediatric visits: an integrated review. J Pediatr Health Care. 2012;26:109-117.
4. Fung WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Prac. 2004;17:264-275.
5. Fang W, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264-275.
6. Rosener SE, Barr WB, Frayne DJ, et al. Interconception care for mothers during well-child visits with family physicians: an IMPLICIT Network Study. Ann Fam Med. 2016;14:350-355.
7. Bennett IM, Coco A, Anderson J, et al. Improving maternal care with a continuous quality improvement strategy: a report from the Interventions to Minimize Preterm and Low Birth Weight Infants through Continuous Improvement Techniques (IMPLICIT) Network. J Am Board Fam Med. 2009;22:380-386.
8. Conde-Agudelo A, Rosas-Bermúdez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295:1809-1823.
9. Ebbert JO, Jacobson RM. Reducing childhood tobacco smoke exposure. JAMA. 2016;315:2610-2611.
Ensuring a thorough exam, making use of a discharge checklist
Before parents leave the hospital with their newborn, it is essential that they receive written and verbal counseling on important issues in neonatal care. A discharge checklist can help make sure all topics have been covered.1 A hearing screen and pulse oximetry before discharge are required for all newborns in most states, in addition to important preventive counseling for parents. TABLE 12 and TABLE 22 summarize important newborn physical exam findings and common skin conditions. Parents should be given additional written information regarding prevention of SIDS and proper use of car seats.

Hospital physicians should assess maternal medical and psychosocial readiness for discharge. Through shared decision-making with the newborn’s parents, physicians should create a plan for outpatient follow-up. Assessment through a physician home visit can provide safe and effective care similar to what is provided at a visit to an office medical practice.3-7 A follow-up appointment should be made 2 to 5 days before discharge, preferably connecting the newborn to a medical home where comprehensive health care services are offered.1,5,6,8

Age, gestational age, risk factors for hyperbilirubinemia, and the timing and level of bilirubin testing should be considered when establishing a follow-up interval. Most newborns who are discharged before 72 hours of age should have a follow-up visit in 2 days; a newborn who has a recognized risk factor for a health problem should be seen sooner. Newborns in the “low-risk zone” (ie, no recognized risk factors) should be seen based on age at discharge or need for breastfeeding support.9
Tracking baby’s weight, ensuring proper feeding
A newborn who is discharged at 24 hours of life, or sooner, should be seen in the office within 2 days of discharge to 1) ensure that he (she) is getting proper nutrition and 2) monitor his weight1,3,5 (TABLE 310-13). All newborns should be seen again at 2 weeks of life, with additional visits more frequently if there are concerns about nutrition.1

Recording an accurate weight is critical; the newborn should be weighed completely undressed and without a diaper. Healthy newborns can safely lose up to 10% of birth weight within the first week of life; they should be back to their birth weight by approximately 2 weeks of life.10,11 A healthy newborn loses approximately 0.5 to 1 oz a day;11 greater than 10% loss of birth weight should trigger a thorough medical work-up and feeding assessment.
Breastfeeding. For breastfeeding mothers, physicians should recommend on-demand feeding or a feeding at least every 2 or 3 hours. Adequate intake in breastfed infants can be intimidating for new parents to monitor, but they can use a written chart or any of several available smartphone applications to document length and timing of feeds and frequency of urination and bowel movements. By the fifth day of life, a newborn should be having at least 6 voids and 3 or 4 stools a day.10-12
In addition, physicians can counsel parents on what to look for—in the mother and the newborn—to confirm that breastfeeding is successful, with adequate nutritional intake (TABLE 310-13). Physicians should recommend against providing a pacifier to breastfeeding infants during the first several weeks of life—or until breastfeeding is well established (usually at 3 or 4 weeks of age). The World Health Organization (WHO) recommends against providing bottles, pacifiers, and artificial nipples to breastfeeding newborns.14 Liquids other than colostrum or breast milk should not be given unless there is a documented medical need, such as inadequate weight gain or feeding difficulty.15 If the newborn experiences early latch difficulties, supplementation with expressed breast milk is preferable to supplementation with formula. Assistance from a trained lactation consultant is a key element in the support of the breastfeeding dyad.11,12,16
Breastfeeding optimizes development of the newborn’s immune system, thus bolstering disease prevention; it also assists with maternal postpartum weight loss and psychological well-being. Exclusively or primarily formula-fed newborns are at increased risk of gastrointestinal, ear, and respiratory infections throughout infancy and childhood; type 1 diabetes mellitus; asthma; childhood and adult obesity; and leukemia.17,18 Mothers who feed their newborn primarily formula increase their own risk of obesity, type 2 diabetes mellitus, ovarian and breast cancer, and depression.17-22
Infant feeding is a personal and family choice but, in the absence of medical contraindications—such as maternal human immunodeficiency virus infection and galactosemia—exclusive breastfeeding should be recommended.17,18 FPs are well suited to support the mother–infant breastfeeding dyad in the neonatal period, based on expert recommendations. Specifically, the American Academy of Family Physicians (AAFP) and American Academy of Pediatrics (AAP) recommend that all infants be exclusively breastfed for the first 6 months of life and continue some breastfeeding through the first year or longer.17,18 WHO recommends breastfeeding until 24 months of age—longer if mother and infant want to, unless breastfeeding is contraindicated.14,17,18
Physicians should provide up-to-date information to parents regarding the risks and benefits of feeding choices. Support for breastfeeding mothers postnatally has been shown to be helpful in lengthening the time of exclusive breastfeeding.12 Certain medications pass through breast milk, and updated guides to medication cautions can be found at the National Institutes of Health’s LACTMED Web site (https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm).13 In many cases, when a maternal medication is incompatible with breastfeeding, the family physician can consider substituting another appropriate medication that is compatible.
Physician recommendation and support improves the rate of breastfeeding, but many mother–infant dyads require additional support to maintain breastfeeding for the recommended duration; such support can take the form of a certified lactation consultant or counselor, doula, or peer counselor.23-25 Although structured breastfeeding education in the antenatal period has been demonstrated to be effective in improving breastfeeding initiation and duration, recent research shows that support groups and assistance from the professionals previously mentioned also improve the breastfeeding rate.26-28
The AAFP recommends that FPs’ offices adopt specific, evidence-based practices that can have an impact on breastfeeding initiation and duration. Such practices include phone and in-person breastfeeding support from nursing staff and removing any formula advertisements from the office.17
Formula feeding. When parents choose formula feeding, most infants tolerate cow’s milk-based formula.29 For healthy term infants, differences between brands of formula are generally insignificant. Soy-protein formulas are of value only if lactose intolerance is strongly suspected, such as after prolonged episodes of loose stools. Even then, intolerance is usually transient and cow’s milk-based formula can be tried again in 2 to 4 weeks.
Physicians should recommend 20 kcal/oz of iron-fortified formula for infants who are fed formula—except in special circumstances, such as premature newborns, who may require a more calorie-dense formula. Parents should pay special attention to the manufacturer’s instructions for mixing formula with water because overdilution can cause hyponatremia. Typical volume for newborns should be at least 15 to 30 mL/feed for the first few days; newborns should not go more than 4 hours between feedings. Within the first week, newborns will start taking 60 to 90 mL/feed and increase that gradually to approximately 120 mL/feed by the end of the first month of life. On average, infants need a little more than 100 kcal/kg of body weight a day; for a 3.5-kg infant, that is at least 500 mL of formula over the course of a day.17,22
Because formula does not contain fluoride, physicians should recommend that parents mix formula that is provided as a powder with fluoridated water. Low-iron formula offers no advantage; feeding with it will cause iron-deficiency anemia in most infants.
When tongue-tie interferes with feeding
Tongue-tie—or ankyloglossia, an atypically short or thick lingual frenulum—is present in 3% to 16% of all births. The condition can make breastfeeding difficult; result in poor neonatal weight gain; and cause sore nipples in 25% to 44% of cases.30 Once tongue-tie is noted, the physician should talk to the mother about the history of feeding success, including whether her nipples are sore and whether the newborn is having difficulty feeding (ie, transferring milk). The Hazelbaker Assessment Tool for Lingual Frenulum Function and the simpler Bristol Tongue Assessment Tool can be used to assess the severity of tongue-tie.30-35
When tongue-tie interferes with feeding, a physician who is not trained in treatment can refer the mother and infant to a specialist in the community. Frenotomy has been used for many years as a treatment for tongue-tie; improvement in nipple pain and the mother-reported breastfeeding score have been reported postoperatively in several studies.30-33
Ensure proper vitamin D intake through supplementation
Newborns should consume 400 IU/d of supplemental vitamin D to prevent deficiency and its clinical manifestation, rickets, or other associated abnormalities of calcium metabolism. Deficiency of vitamin D has also been linked to a number of other conditions, including developmental delay and, possibly, type 1 diabetes mellitus in childhood and cardiovascular disease later in life.36
In the first months of life, few infants who are solely formula-fed will consume a full liter daily; for them, supplementation of vitamin D for at least one month should be prescribed.35 For breastfed infants, high-dosage maternal vitamin D supplementation may be effective, precluding infant oral vitamin D supplementation36; however, neither the AAFP nor the AAP has issued guidance promoting maternal supplementation in lieu of direct oral infant supplementation.37
Jaundice prevention—and recognition
An elevated bilirubin level is seen in most newborns in the first days of life because of increased production and decreased clearance of bilirubin—a condition known as physiologic jaundice. Conditions that aggravate physiologic hyperbilirubinemia include inborn errors of metabolism, ABO blood-group incompatibility, hemoglobin variants, and inflammatory states such as sepsis. It is important to distinguish physiologic jaundice from exaggerated physiologic and pathologic forms of hyperbilirubinemia; the latter is a medical emergency. Before we get to that, a word about prevention.
Prevention. Because poor caloric intake and dehydration are associated with hyperbilirubinemia, physicians should advise breastfeeding mothers to feed their newborn at least 8 to 12 times daily during the first week of life. However, routine supplementation of liquids other than breast milk should be discouraged in newborns who are not dehydrated.38
All pregnant women should be tested for ABO and Rh (D) blood types and undergo serum screening for isoimmune antibodies. Randomized trials have demonstrated that the incidence of significant hyperbilirubinemia can be reduced if, for Rh-negative mothers and those who did not undergo prenatal blood-group testing, infant cord blood is tested for 1) ABO and Rh (D) types and 2) direct antibody (Coombs’ test).38,39
Screening and assessment. It is recommended that all newborns be screened for jaundice before discharge by 1) assessment of clinical risk factors or 2) testing of transcutaneous bilirubin (TcB) or total serum bilirubin (TSB). Furthermore, because evidence shows that treating clinical jaundice can improve outcomes and rehospitalization, TSB should be measured in every newborn who has clinical jaundice in the first 24 hours of life. Measurement of TcB or TSB should also be performed on all infants in whom there appears to be clinical jaundice that is excessive for age.38,39
During routine clinical care, TcB measurement provides a reasonable estimate of the TSB level in healthy newborns at levels less than 15 mg/dL,40 although TcB testing might not be available in the outpatient office. An AAP management algorithm can help determine when a newborn should be seen for outpatient follow-up based on risk of hyperbilirubinemia; higher-risk newborns should be reevaluated in 24 hours.9 Outpatient visual assessment of jaundice for cephalocaudal progression—in a well-lit room, with a fully undressed newborn—correlates well with TSB test results. However, visual assessment should not be used alone to screen for hyperbilirubinemia; recent studies have demonstrated that such assessment lacks clinical reliability.40
Laboratory assessment. All bilirubin levels should be interpreted based on the newborn’s age in hours. The need for phototherapy should be based on the zone (low, low-intermediate, high-intermediate, or high, as categorized in the AAP nomogram38 in which the TSB level falls. TABLE 438-40 provides recommendations for laboratory studies based on risk factors. Standard curves for risk stratification have been developed by the AAP.37,38

Treatment. Decisions to initiate treatment should be based on the AAP algorithm.38 When initiating phototherapy, precautions include ensuring adequate fluid intake, patching eyes, and monitoring temperature. Phototherapy can generally be stopped when the TSB level falls by 5 mg/dL or below 14 mg/dL. Home phototherapy, using a fiberoptic blanket, for uncomplicated jaundice (in carefully selected newborns with reliable parents) allows continued breastfeeding and bonding with the family, and can significantly decrease the rate of rehospitalization for infants older than 34 weeks.41
Breastfeeding is often associated with a higher bilirubin level than is seen in infants fed formula exclusively; increasing the frequency of feeding usually reduces the bilirubin level. So-called breast-milk jaundice is a delayed, but common, form of jaundice that is usually diagnosed in the second week of life and peaks by the end of the second week, resolving gradually over one to 4 months. If evaluation reveals no pathologic source, breastfeeding can generally be continued. Temporary discontinuation of breastfeeding to consider a diagnosis of breast-milk jaundice or other reasons for an elevated bilirubin level increases the risk of breastfeeding failure and is usually unnecessary.12,37,39
Fever—a full work-up, thorough history are key
Concern about serious bacterial illness (SBI) makes the evaluation of fever critical for those who care for newborns. Many studies have attempted to identify which newborns might be able to be cared for safely as outpatients to prevent unnecessary testing and antibiotics.5,42 Regrettably, SBI in infants remains difficult to predict, and protocols that have been developed may miss as many as 1 of every 10 newborns who has SBI.43 Initial management of all infants 28 days old or younger with fever must therefore include a full work-up, including lumbar puncture and empiric antibiotics.44
Evaluation. When an infant younger than 28 days has a fever, the physician should first verify that the temperature was taken rectally and how it was documented. In an infant who has a history of prematurity, it is crucial to correct for chronological age when deciding on proper evaluation.
Additional important findings in the history include a significant change in behavior, associated symptoms, and exposure to sick contacts. The maternal and birth history, including prolonged rupture of membranes, colonization with group B Streptococcus, administration of antibiotics at delivery, and genital herpes simplex virus (HSV) infection may suggest a cause for fever.45
The evaluation of fever might include the white blood cell (WBC) count, blood culture, measurement of markers of inflammation, urine studies, lumbar puncture, stool culture, and chest radiograph. Traditionally, the WBC count has been utilized as a standard marker for sepsis, although it has a low sensitivity and specificity for SBI, especially in newborns.46 Blood cultures should be obtained routinely in the newborn with fever, and before antibiotics are administered in older infants.
Procalcitonin (PCT; a calcitonin precursor) and the inflammatory marker C-reactive protein (CRP) have been shown, in several large studies, to have relatively high sensitivity and specificity for SBI; measurement of these constituents may enhance detection of serious illness.46-49 In a large study of 2047 febrile infants older than 30 months, the PCT level was determined to be more accurate than the CRP level, the WBC count, and the absolute neutrophil count in predicting SBI.48,49 PCT shows the most promise for preventing a full fever work-up and empiric antibiotics. It has not yet been widely translated into practice, however, because of a lack of clear guidance on how to combine PCT levels with other laboratory markers and clinical decision-making.48-50
Urinalysis (UA) should be obtained for all newborns who present with fever. Traditionally, it was recommended that urine should be cultured for all newborns with fever; however, more recent data show that the initial urinalysis is much more sensitive than once thought. In a study, UA was positive (defined as pyuria or a positive leukocyte esterase test, or both) in all but 1 of 203 infants who had bacteremic UTI (sensitivity, 99.5%).51
Stool culture is necessary in newborns only when they present with blood or mucus in diarrhea. Lumbar puncture should be performed in all febrile newborns and all newborns for whom empiric antibiotics have been prescribed.43,44 A chest radiograph may be useful in diagnosis when a newborn has any other sign of pulmonary disease: respiratory rate >50/min, retractions, wheezing, grunting, stridor, nasal flaring, cough, and positive findings on lung examination.43,44
Treatment. Management for all newborns who have a rectal temperature ≥38° C includes admission to the hospital and empiric antibiotics; guidance is based primarily on expert consensus. Common pathogens for SBI include group B Strep, Escherichia coli, Enterococcus spp., and Listeria monocytogenes.43,44 Empiric antibiotics, including ampicillin (to cover L monocytogenes) and cefotaxime or gentamicin should be started immediately after sending for blood, urine, and cerebrospinal fluid (CSF) cultures.43-45
All infants who are ill-appearing or have vesicles, seizures, or a maternal history of genital HSV infection should also be started on empiric acyclovir. Vesicles should be cultured and CSF should be sent for HSV DNA polymerase chain reaction before acyclovir is administered.43-45
Sudden infant death syndrome: Steps to take to minimize risk
SIDS is defined as the sudden death of a child younger than 1 year that remains unexplained after a thorough case investigation and comprehensive review of the clinical history. The risk of SIDS in the United States is less than 1 for every 1000 live births; incidence peaks between 2 and 4 months of age.52 In the United States, SIDS and other sleep-related infant deaths, such as strangulation in bed or accidental suffocation, account for more than 4000 deaths a year.53 The incidence of SIDS declined markedly after the “Back to Sleep” campaign was launched in 2003, but has leveled off since 2005.53-55
Numerous risk factors for SIDS have been identified, including maternal factors (young maternal age, maternal smoking during pregnancy, late or no prenatal care) and infant and environmental factors (prematurity, low birth weight, male gender, prone sleeping position, sleeping on a soft surface or with bedding accessories, bed-sharing (ie, sleeping in the parents’ bed), and overheating. In many cases, the risk factors are modifiable; sleeping in the prone position is the most meaningful modifiable risk factor.

To minimize the risk for SIDS, parents should be educated on the risk factors—prenatally as well as at each infant well visit. Home monitors have not been proven to reduce the incidence of SIDS and are not recommended for that purpose.54-57 Although evidence is strongest for supine positioning as a preventive intervention for SIDS, other evidence-based recommendations include use of a firm sleep surface; breastfeeding; use of a pacifier; room-sharing with parents without bed-sharing; routine immunization; avoidance of overheating; avoiding falling asleep with the infant on a chair or couch; and avoiding exposure to tobacco smoke, alcohol, and drugs of abuse.55,56 A recent systematic review showed that large-scale community interventions and education campaigns can play a significant role in parental and community adoption of safe sleep recommendations; however, families and communities rarely exhibit complete adherence to safe sleep practices.57

Other concerns in the first month of life and immediately beyond
In TABLE 5,2 we list additional common newborn problems not reviewed in the text of this article and summarize evidence-based treatment strategies.

CORRESPONDENCE
Scott Hartman, MD, Associate Professor, Department of Family Medicine, University of Rochester Medical Center, 777 South Clinton Avenue, Rochester, NY 14620; [email protected].
Acknowledgement
We thank Nancy Phillips for her assistance in the preparation of this article.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
1. Langan RC. Discharge procedures for healthy newborns. Am Fam Physician. 2006;73:849-852.
2. Hartman S, Taylor A. Problems of the newborn and infant. In: Paulman PM, Taylor RB, Paulman AA, et al, eds. Family Medicine: Principles and Practice. 7th ed. Cham, Switzerland: Springer Cham; 2016:217-239.
3. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619-1627.
4. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
5. Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125-131.
6. Escobar GJ, Braveman PA, Ackerson L, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics. 2001;108:719-727.
7. Meara E, Kotagal UR, Atherton HD, et al. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113:1619–1627.
8. Benitz WE; Committee on Fetus and Newborn, American Academy of Pediatrics. Hospital stay for healthy term newborn infants. Pediatrics. 2015;135:948-953.
9. Maisels MJ, Vinod VK, Bhutani D, et al. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198.
10. Crossland DS, Richmond S, Hudson M, et al. Weight change in the term baby in the first 2 weeks of life. Acta Paediatrica. 2008;97:425-429.
11. Noel-Weiss J, Courant G, Woodend AK. Physiological weight loss in the breastfed neonate: a systematic review. Open Med. 2008;2:e99-e110.
12. Holmes AV, McLeod AY, Bunik M. ABM Clinical Protocol #5: Peripartum breastfeeding management for the healthy mother and infant at term. Breastfeed Med. 2013;8:469-473.
13. National Library of Medicine. Drugs and Lactation Database (LactMed). Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed February 1, 2018.
14. World Health Organization. Guideline: Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. Available at: http://www.who.int/nutrition/publications/guidelines/breastfeeding-facilities-maternity-newborn/en/. Accessed March 23, 2018.
15. Chantry CJ, Dewey KG, Peerson JM, et al. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164:1339-1345.
16. Patel S, Patel S. The effectiveness of lactation consultants and lactation counselors on breastfeeding outcomes. J Hum Lact. 2015;32:530-541.
17. Position Paper: Breastfeeding, family physicians supporting. American Academy of Family Physicians Breastfeeding Advisory Committee. Available at: www.aafp.org/about/policies/all/breastfeeding-support.html. 2017. Accessed February 1, 2018.
18. Eidelman AI, Schanler RJ; Section on Breastfeeding. Policy Statement: Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827-e841.
19. Ip S, Chung M, Raman G, et al. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17-S30.
20. Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974-982.
21. Luan NN, Wu QJ, Gong TT, et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J Clin Nutr. 2013;98:1020-1031.
22. Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep). 2007;(153):1-186.
23. Hartman S, Barnett J, Bonuck KA. Implementing international board-certified lactation consultants intervention into routine care: barriers and recommendations. Clinical Lactation. 2012;3:131-137.
24. Hodnett ED, Gates S, Hofmeyr GJ, et al. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
25. Lassi ZS, Das JK, Salam RA, et al. Evidence from community-level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health. 2014;11(Suppl 2):S2.
26. Chapman DJ, Pérez-Escamilla R. Breastfeeding among minority women: moving from risk factors to interventions. Adv Nutr. 2012;3:95-104.
27. Rosen-Carole C, Hartman S; Academy of Breastfeeding Medicine. ABM Clinical Protocol #19: Breastfeeding promotion in the prenatal setting, revision 2015. Breastfeed Med. 2015;10:451-457.
28. Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. Effects of CenteringPregnancy group prenatal care on breastfeeding outcomes. J Midwifery Womens Health. 2013;58:389-395.
29. Singhal A, Kennedy K, Lanigan J, et al. Dietary nucleotides and early growth in formula-fed infants: a randomized controlled trial. Pediatrics. 2010;126:e946-e953.
30. Demirci JR, Bogen DL, Holland C, et al. Characteristics of breastfeeding discussions at the initial prenatal visit. Obstet Gynecol. 2013;122:1263-1270.
31. Ingram J, Johnson D, Copeland M, et al. The development of a tongue assessment tool to assist with tongue tie identification. Arch Dis Child Fetal Neonatal Ed. 2015;100:F344-F348.
32. Power RF, Murphy JF. Tongue tie and frenotomy in infants with breastfeeding difficulties: achieving a balance. Arch Dis Child. 2015;100:489-494.
33. Buryk M, Bloom D, Shope T. Efficacy of neonatal release of ankyloglossia: a randomized trial. Pediatrics. 2011;128:280-288.
34. Francis DO, Krishnaswami S, McPheeters M. Treatment of ankyloglossia and breastfeeding outcomes: a systematic review. Pediatrics. 2015;135:e1458-e1466.
35. Amir LH, James JP, Donath SM. Reliability of the Hazelbaker Assessment Tool for Lingual Frenulum Function. Int Breastfeed J. 2006;1:3.
36. Misra M, Pacaud D, Petryk A, et al; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
37. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634.
38. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114;297-316 [erratum: Pediatrics. 2004;114:1138].
39. Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114:e130-e153.
40. Taylor JA, Burgos AE, Flaherman V, et al. Discrepancies between transcutaneous and serum bilirubin measurements. Pediatrics. 2015:135:224-231.
41. Newman TB. Data suggest visual assessment of jaundice in newborns is helpful. J Pediatr. 2009;154:466; author reply 466-467.
42. Roberts KB. Young, febrile infants: a 30-year odyssey ends where it started. JAMA. 2004;291:1261-1262.
43. Bhatti M, Chu A, Hageman JR, et al. Future directions in the evaluation and management of neonatal sepsis. NeoReviews. 2012;13:e103-e110.
44. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530-545.
45. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:pii: e20162013.
46. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Disease J. 2014;33:342-344.
47. Bressan S, Gomez B, Mintegi S, et al. Diagnostic performance of the lab-score in predicting severe and invasive bacterial infections in well-appearing young febrile infants. Pediatr Infect Dis J. 2012;31:1239-1244.
48. Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62-69.
49. Kuppermann N, Mahajan P. Role of serum procalcitonin in identifying young febrile infants with invasive bacterial infections: one step closer to the Holy Grail? JAMA Pediatr. 2016;170:17-18.
50. England JT, Del Vecchio MT, Aronoff SC. Use of serum procalcitonin in evaluation of febrile infants: a meta-analysis of 2317 patients. J Emerg Med. 2014;47:682-688.
51. Schroeder AR, Chang PW, Shen MW, et al. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135:965-971.
52. Salm Ward TC, Balfour GM. Infant safe sleep interventions, 1990-2015: a review. J Community Health. 2016;41:180-196.
53. Goldstein RD, Trachtenberg FL, Sens MA, et al. Overall postneonatal mortality and rates of SIDS. Pediatrics. 2016;137:e20152298.
54. Task Force on Sudden Infant Death Syndrome, Moon RY. SIDS and other sleep-related infant deaths: expansion of recommendations for a safe infant sleeping environment. Pediatrics. 2011;128:e1341-1367.
55. Smith LA, Geller NL, Kellams AL, et al. Infant sleep location and breastfeeding practices in the United States: 2011-2014. Acad Pediatr. 2016;16:540-549.
56. Task Force on Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: updated 2016 recommendations for a safe infant sleeping environment. Pediatrics. 2016;138;e20162938.
57. Corriveau SK, Drake, EE. Kellams AL, et al. Evaluation of an office protocol to increase exclusivity of breastfeeding. Pediatrics. 2013;131:942-950.
From The Journal of Family Practice | 2018;67(4):E4-E15.
PRACTICE RECOMMENDATIONS
› Include a full work-up and empiric antibiotics in the initial management of all febrile infants ≤28 days of age. A
› Recommend that newborns breastfeed exclusively (in the absence of contraindications) for 6 months and continue some breastfeeding until the baby is at least 12 to 24 months of age. A
› Screen all newborns for jaundice before discharge by 1) clinical assessment or 2) testing for total serum bilirubin (TSB) or transcutaneous bilirubin (TcB); measurement of TcB provides a reasonable estimate of the TSB level in healthy newborns at levels <15 mg/dL. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
