User login
What’s in a number? 697,633 children with COVID-19
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
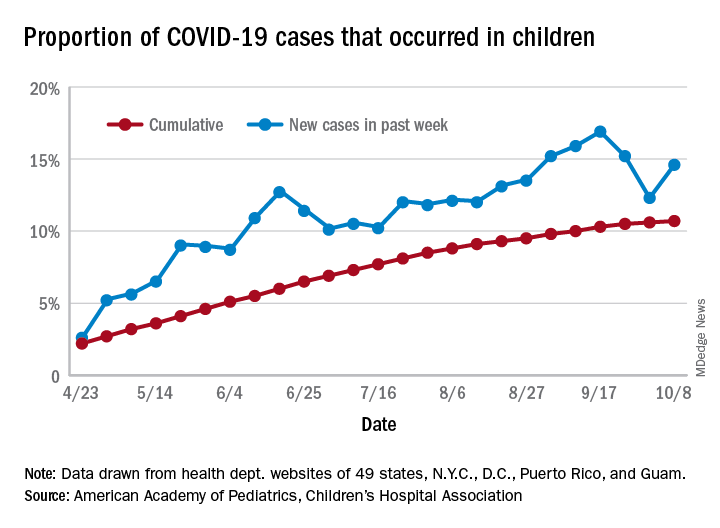
For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

For the week, 14.6% of all COVID-19 cases reported in the United States occurred in children, after 2 consecutive weeks of declines that saw the proportion drop from 16.9% to 12.3%. The cumulative rate of child cases for the entire pandemic is 10.7%, with total child cases in the United States now up to 697,633 and cases among all ages at just over 6.5 million, the AAP and the CHA said Oct. 12 in their weekly COVID-19 report.
Nationally, there were 927 cases reported per 100,000 children as of Oct. 8, with rates at the state level varying from 176 per 100,000 in Vermont to 2,221 per 100,000 in North Dakota. Two other states were over 2,000 cases per 100,000 children: Tennessee (2,155) and South Carolina (2,116), based on data from the health departments of 49 states (New York does not report age distribution), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
Severe illness continues to be rare in children, and national (25 states and New York City) hospitalization rates dropped in the last week. The proportion of hospitalizations occurring in children slipped from a pandemic high of 1.8% the previous week to 1.7% during the week of Oct. 8, and the rate of hospitalizations for children with COVID-19 was down to 1.4% from 1.6% the week before and 1.9% on Sept. 3, the AAP and the CHA said.
Mortality data from 42 states and New York City also show a decline. For the third consecutive week, children represented just 0.06% of all COVID-19 deaths in the United States, down from a high of 0.07% on Sept. 17. Only 0.02% of all cases in children have resulted in death, and that figure has been dropping since early June, when it reached 0.06%, according to the AAP/CHA report. As of Oct. 8, there have been 115 total deaths reported in children.
Flu vaccine significantly cuts pediatric hospitalizations
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
Unlike previous studies focused on vaccine effectiveness (VE) in ambulatory care office visits, Angela P. Campbell, MD, MPH, and associates have uncovered evidence of the overall benefit influenza vaccines play in reducing hospitalizations and emergency department visits in pediatric influenza patients.
“Our data provide important VE estimates against severe influenza in children,” the researchers noted in Pediatrics, adding that the findings “provide important evidence supporting the annual recommendation that all children 6 months and older should receive influenza vaccination.”
Dr. Campbell and colleagues collected ongoing surveillance data from the New Vaccine Surveillance Network (NVSN), which is a network of pediatric hospitals across seven cities, including Kansas City, Mo.; Rochester, N.Y.; Cincinnati; Pittsburgh; Nashville, Tenn.; Houston; and Seattle. The influenza season encompassed the period Nov. 7, 2018 to June 21, 2019.
A total of 2,748 hospitalized children and 2,676 children who had completed ED visits that did not lead to hospitalization were included. Once those under 6 months were excluded, 1,792 hospitalized children were included in the VE analysis; of these, 226 (13%) tested positive for influenza infection, including 211 (93%) with influenza A viruses and 15 (7%) with influenza B viruses. Fully 1,611 of the patients (90%), had verified vaccine status, while 181 (10%) had solely parental reported vaccine status. The researchers reported 88 (5%) of the patients received mechanical ventilation and 7 (<1%) died.
Most noteworthy, They further estimated a significant reduction in hospitalizations linked to A(H3N2) and A(H1N1)pdm09 viruses, even in the presence of circulating A(H3N2) viruses that differed from the A(H3N2) vaccine component.
Studies from other countries during the same time period showed that while “significant protection against influenza-associated ambulatory care visits and hospitalizations among children infected with A(H1N1)pdm09 viruses” was observed, the same could not be said for protection against A(H3N2) viruses, which varied among pediatric outpatients in the United States (24%), in England (17% outpatient; 31% inpatient), Europe (46%), and Canada (48%). They explained that such variation in vaccine protection is multifactorial, and includes virus-, host-, and environment-related factors. They also noted that regional variations in circulating viruses, host factors including age, imprinting, and previous vaccination could explain the study’s finding of vaccine protection against both A(H1N1)pdm09 and A(H3N2) viruses.
When comparing VE estimates between ED visits and hospitalizations, the researchers observed one significant difference, that “hospitalized children likely represent more medically complex patients, with 58% having underlying medical conditions and 38% reporting at lease one hospitalization in the past year, compared with 28% and 14% respectively, among ED participants.”
Strengths of the study included the prospective multisite enrollment that provided data across diverse locations and representation from pediatric hospitalizations and ED care, which were not previously strongly represented in the literature. The single-season study with small sample size was considered a limitation, as was the inability to evaluate full and partial vaccine status. Vaccine data also were limited for many of the ED patients observed.
Dr. Campbell and colleagues did caution that while they consider their test-negative design optimal for evaluating both hospitalized and ED patients, they feel their results should not be “interpreted as VE against influenza-associated ambulatory care visits or infections that are not medically attended.”
In a separate interview, Michael E. Pichichero, MD, director of the Rochester General Hospital Research Institute and a clinical professor of pediatrics at the University of Rochester (N.Y.), observed: “There are really no surprises here. A well done contemporary study confirms again the benefits of annual influenza vaccinations for children. Viral coinfections involving SARS-CoV-2 and influenza have been reported from Australia to cause heightened illnesses. That observation provides further impetus for parents to have their children receive influenza vaccinations.”
The researchers cited multiple sources of financial support for their ongoing work, including Sanofi, Quidel, Moderna, Karius, GlaxoSmithKline, Merck, AstraZeneca, and Pfizer. Funding for this study was supported by the Centers for Disease Control and Prevention. Dr. Pichichero said he had no relevant financial disclosures.
SOURCE: Campbell AP et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1368.
FROM PEDIATRICS
COVID-19 pandemic amplifies uncertainty for immigrant hospitalists
H1-B visa program needs improvement
Statistics tell the tale of immigrants in the American health care workforce in broad strokes. In an interview, though, one hospitalist shared the particulars of his professional and personal journey since arriving in the United States from India 15 years ago.
Mihir Patel, MD, MPH, FHM, came to the United States in 2005 to complete a Master’s in Public Health. Fifteen years later, he is still waiting for the green card that signifies U.S. permanent residency status. The paperwork for the application, he said, was completed in 2012. Since then, he’s been renewing his H-1B visa every three years, and he has no expectation that anything will change soon.
“If you are from India, which has a significant backlog of green cards – up to 50 years…you just wait forever,” he said. “Many people even die waiting for their green card to arrive.”
Arriving on a student visa, Dr. Patel completed his MPH in 2008 and began an internal medicine residency that same year, holding a J-1 visa for the 3 years of his US residency program.
“Post-residency, I started working in a rural hospital in an underserved area of northeast Tennessee as a hospitalist,” thus completing the 3 years of service in a rural underserved area that’s a requirement for J-1 visa holders, said Dr. Patel. “I loved this rural community hospital so much that I ended up staying there for 6 years. During my work at this rural hospital, I was able to enjoy the autonomy of managing a small ICU, doing both critical care procedures and management of intubated critical patients while working as a hospitalist,” he said. Dr. Patel served as chief of staff at the hospital for two years, and also served on the board of directors for his 400-physician medical group.
“I was a proud member of this rural community – Rogersville,” said Dr. Patel. Although he and his wife, who was completing her hospitalist residency, lived in Johnson City, Tenn., “I did not mind driving 120 miles round trip every day to go to my small-town hospital for 6 years,” he said.
Spending this time in rural Tennessee allowed Dr. Patel to finish the requirements necessary for the Physician National Interest Waiver and submit his application for permanent residency. The waiver, though, doesn’t give him priority status in the waiting list for permanent residency status.
After a stint in northern California to be closer to extended family, the pull of “beautiful northeast Tennesse and the rural community” was too strong, so Dr. Patel and his family moved back to Johnson City in 2018.
Now, Dr. Patel is a hospitalist at Ballad Health System in Johnson City. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the COVID-19 Strike Team. He co-founded and is president of the Blue Ridge Chapter of the Society of Hospital Medicine. Under another H-1B visa, Dr. Patel works part-time from home as a telehospitalist, covering six hospitals in 4 different states.
Even in ordinary circumstances, the H-1B visa comes with constraints. Although Dr. Patel’s 6-year old daughter was born in the U.S. and is a citizen, Dr. Patel and his wife have to reapply for their visas every 3 years. “If we travel outside the U.S., we have to get our visas stamped. We cannot change jobs easily due to fear of visa denial, especially with the recent political environment,” said Dr. Patel. “It feels like we are essential health care workers but non-essential immigrants.”
Having recently completed a physician executive MBA program, Dr. Patel said he’d like to start a business of his own using Lean health care principles and telemedicine to improve rural health care. “But while on an H-1B I cannot do anything outside my sponsored employment,” he said.
Ideally, health care organizations would have high flexibility in how and where staff are deployed when a surge of COVID-19 patients hits. Dr. Patel made the point that visa restrictions can make this much harder: “During this COVID crisis, this restriction can cause significant negative impact for small rural hospitals, where local physicians are quarantined and available physicians are on a visa who cannot legally work outside their primary facilities – even though they are willing to work,” he said. “One cannot even work using telemedicine in the same health system, if that is not specifically mentioned during H-1B petition filling. More than 15,000 physicians who are struck by the green card backlog are in the same situation all over U.S.,” he added.
These constraints, though, pale before the consequences of a worst-case pandemic scenario for an immigrant family, where the physician – the primary visa-holder – becomes disabled or dies. In this case, dependent family members must self-deport. “In addition, there would not be any disability or Social Security benefits for the physician or dependents, as they are not citizens or green card holders and they cannot legally stay in the US,” noted Dr. Patel. “Any hospitalist working during the COVID-19 pandemic can have this fate due to our high exposure risk.”
Reauthorizing the H1-B visa program
SHM has been advocating to improve the H1-B visa system for years, Dr. Patel said, The Fairness for High Skilled Immigrants Act passed the U.S. House of Representatives with bipartisan support, and the Society is advocating for its passage in the Senate.
The Fairness for High-Skilled Immigrants Act (S. 386) simplifies the employment-based immigration system by removing per-country caps, converting the employment-based immigration system into a “first-come, first serve” system that does not discriminate on country of origin. The act will also help alleviate the decades-long green card and permanent residency application backlogs.
Dr. Patel emphasized the importance of action by Congress to reauthorize the physician visa waiver program and expediting physician permanent residency. “This is a crisis and we are all physicians who are ready to serve, regardless of our country of origin. Please let us help this great nation by giving us freedom from visa restrictions and providing security for our families.
“During wartime, all frontline soldiers are naturalized and given citizenship by presidential mandate; this is more than war and we are not asking for citizenship – but at least give us a green card which we have already satisfied all requirements for. If not now, then when?” he asked.
H1-B visa program needs improvement
H1-B visa program needs improvement
Statistics tell the tale of immigrants in the American health care workforce in broad strokes. In an interview, though, one hospitalist shared the particulars of his professional and personal journey since arriving in the United States from India 15 years ago.
Mihir Patel, MD, MPH, FHM, came to the United States in 2005 to complete a Master’s in Public Health. Fifteen years later, he is still waiting for the green card that signifies U.S. permanent residency status. The paperwork for the application, he said, was completed in 2012. Since then, he’s been renewing his H-1B visa every three years, and he has no expectation that anything will change soon.
“If you are from India, which has a significant backlog of green cards – up to 50 years…you just wait forever,” he said. “Many people even die waiting for their green card to arrive.”
Arriving on a student visa, Dr. Patel completed his MPH in 2008 and began an internal medicine residency that same year, holding a J-1 visa for the 3 years of his US residency program.
“Post-residency, I started working in a rural hospital in an underserved area of northeast Tennessee as a hospitalist,” thus completing the 3 years of service in a rural underserved area that’s a requirement for J-1 visa holders, said Dr. Patel. “I loved this rural community hospital so much that I ended up staying there for 6 years. During my work at this rural hospital, I was able to enjoy the autonomy of managing a small ICU, doing both critical care procedures and management of intubated critical patients while working as a hospitalist,” he said. Dr. Patel served as chief of staff at the hospital for two years, and also served on the board of directors for his 400-physician medical group.
“I was a proud member of this rural community – Rogersville,” said Dr. Patel. Although he and his wife, who was completing her hospitalist residency, lived in Johnson City, Tenn., “I did not mind driving 120 miles round trip every day to go to my small-town hospital for 6 years,” he said.
Spending this time in rural Tennessee allowed Dr. Patel to finish the requirements necessary for the Physician National Interest Waiver and submit his application for permanent residency. The waiver, though, doesn’t give him priority status in the waiting list for permanent residency status.
After a stint in northern California to be closer to extended family, the pull of “beautiful northeast Tennesse and the rural community” was too strong, so Dr. Patel and his family moved back to Johnson City in 2018.
Now, Dr. Patel is a hospitalist at Ballad Health System in Johnson City. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the COVID-19 Strike Team. He co-founded and is president of the Blue Ridge Chapter of the Society of Hospital Medicine. Under another H-1B visa, Dr. Patel works part-time from home as a telehospitalist, covering six hospitals in 4 different states.
Even in ordinary circumstances, the H-1B visa comes with constraints. Although Dr. Patel’s 6-year old daughter was born in the U.S. and is a citizen, Dr. Patel and his wife have to reapply for their visas every 3 years. “If we travel outside the U.S., we have to get our visas stamped. We cannot change jobs easily due to fear of visa denial, especially with the recent political environment,” said Dr. Patel. “It feels like we are essential health care workers but non-essential immigrants.”
Having recently completed a physician executive MBA program, Dr. Patel said he’d like to start a business of his own using Lean health care principles and telemedicine to improve rural health care. “But while on an H-1B I cannot do anything outside my sponsored employment,” he said.
Ideally, health care organizations would have high flexibility in how and where staff are deployed when a surge of COVID-19 patients hits. Dr. Patel made the point that visa restrictions can make this much harder: “During this COVID crisis, this restriction can cause significant negative impact for small rural hospitals, where local physicians are quarantined and available physicians are on a visa who cannot legally work outside their primary facilities – even though they are willing to work,” he said. “One cannot even work using telemedicine in the same health system, if that is not specifically mentioned during H-1B petition filling. More than 15,000 physicians who are struck by the green card backlog are in the same situation all over U.S.,” he added.
These constraints, though, pale before the consequences of a worst-case pandemic scenario for an immigrant family, where the physician – the primary visa-holder – becomes disabled or dies. In this case, dependent family members must self-deport. “In addition, there would not be any disability or Social Security benefits for the physician or dependents, as they are not citizens or green card holders and they cannot legally stay in the US,” noted Dr. Patel. “Any hospitalist working during the COVID-19 pandemic can have this fate due to our high exposure risk.”
Reauthorizing the H1-B visa program
SHM has been advocating to improve the H1-B visa system for years, Dr. Patel said, The Fairness for High Skilled Immigrants Act passed the U.S. House of Representatives with bipartisan support, and the Society is advocating for its passage in the Senate.
The Fairness for High-Skilled Immigrants Act (S. 386) simplifies the employment-based immigration system by removing per-country caps, converting the employment-based immigration system into a “first-come, first serve” system that does not discriminate on country of origin. The act will also help alleviate the decades-long green card and permanent residency application backlogs.
Dr. Patel emphasized the importance of action by Congress to reauthorize the physician visa waiver program and expediting physician permanent residency. “This is a crisis and we are all physicians who are ready to serve, regardless of our country of origin. Please let us help this great nation by giving us freedom from visa restrictions and providing security for our families.
“During wartime, all frontline soldiers are naturalized and given citizenship by presidential mandate; this is more than war and we are not asking for citizenship – but at least give us a green card which we have already satisfied all requirements for. If not now, then when?” he asked.
Statistics tell the tale of immigrants in the American health care workforce in broad strokes. In an interview, though, one hospitalist shared the particulars of his professional and personal journey since arriving in the United States from India 15 years ago.
Mihir Patel, MD, MPH, FHM, came to the United States in 2005 to complete a Master’s in Public Health. Fifteen years later, he is still waiting for the green card that signifies U.S. permanent residency status. The paperwork for the application, he said, was completed in 2012. Since then, he’s been renewing his H-1B visa every three years, and he has no expectation that anything will change soon.
“If you are from India, which has a significant backlog of green cards – up to 50 years…you just wait forever,” he said. “Many people even die waiting for their green card to arrive.”
Arriving on a student visa, Dr. Patel completed his MPH in 2008 and began an internal medicine residency that same year, holding a J-1 visa for the 3 years of his US residency program.
“Post-residency, I started working in a rural hospital in an underserved area of northeast Tennessee as a hospitalist,” thus completing the 3 years of service in a rural underserved area that’s a requirement for J-1 visa holders, said Dr. Patel. “I loved this rural community hospital so much that I ended up staying there for 6 years. During my work at this rural hospital, I was able to enjoy the autonomy of managing a small ICU, doing both critical care procedures and management of intubated critical patients while working as a hospitalist,” he said. Dr. Patel served as chief of staff at the hospital for two years, and also served on the board of directors for his 400-physician medical group.
“I was a proud member of this rural community – Rogersville,” said Dr. Patel. Although he and his wife, who was completing her hospitalist residency, lived in Johnson City, Tenn., “I did not mind driving 120 miles round trip every day to go to my small-town hospital for 6 years,” he said.
Spending this time in rural Tennessee allowed Dr. Patel to finish the requirements necessary for the Physician National Interest Waiver and submit his application for permanent residency. The waiver, though, doesn’t give him priority status in the waiting list for permanent residency status.
After a stint in northern California to be closer to extended family, the pull of “beautiful northeast Tennesse and the rural community” was too strong, so Dr. Patel and his family moved back to Johnson City in 2018.
Now, Dr. Patel is a hospitalist at Ballad Health System in Johnson City. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the COVID-19 Strike Team. He co-founded and is president of the Blue Ridge Chapter of the Society of Hospital Medicine. Under another H-1B visa, Dr. Patel works part-time from home as a telehospitalist, covering six hospitals in 4 different states.
Even in ordinary circumstances, the H-1B visa comes with constraints. Although Dr. Patel’s 6-year old daughter was born in the U.S. and is a citizen, Dr. Patel and his wife have to reapply for their visas every 3 years. “If we travel outside the U.S., we have to get our visas stamped. We cannot change jobs easily due to fear of visa denial, especially with the recent political environment,” said Dr. Patel. “It feels like we are essential health care workers but non-essential immigrants.”
Having recently completed a physician executive MBA program, Dr. Patel said he’d like to start a business of his own using Lean health care principles and telemedicine to improve rural health care. “But while on an H-1B I cannot do anything outside my sponsored employment,” he said.
Ideally, health care organizations would have high flexibility in how and where staff are deployed when a surge of COVID-19 patients hits. Dr. Patel made the point that visa restrictions can make this much harder: “During this COVID crisis, this restriction can cause significant negative impact for small rural hospitals, where local physicians are quarantined and available physicians are on a visa who cannot legally work outside their primary facilities – even though they are willing to work,” he said. “One cannot even work using telemedicine in the same health system, if that is not specifically mentioned during H-1B petition filling. More than 15,000 physicians who are struck by the green card backlog are in the same situation all over U.S.,” he added.
These constraints, though, pale before the consequences of a worst-case pandemic scenario for an immigrant family, where the physician – the primary visa-holder – becomes disabled or dies. In this case, dependent family members must self-deport. “In addition, there would not be any disability or Social Security benefits for the physician or dependents, as they are not citizens or green card holders and they cannot legally stay in the US,” noted Dr. Patel. “Any hospitalist working during the COVID-19 pandemic can have this fate due to our high exposure risk.”
Reauthorizing the H1-B visa program
SHM has been advocating to improve the H1-B visa system for years, Dr. Patel said, The Fairness for High Skilled Immigrants Act passed the U.S. House of Representatives with bipartisan support, and the Society is advocating for its passage in the Senate.
The Fairness for High-Skilled Immigrants Act (S. 386) simplifies the employment-based immigration system by removing per-country caps, converting the employment-based immigration system into a “first-come, first serve” system that does not discriminate on country of origin. The act will also help alleviate the decades-long green card and permanent residency application backlogs.
Dr. Patel emphasized the importance of action by Congress to reauthorize the physician visa waiver program and expediting physician permanent residency. “This is a crisis and we are all physicians who are ready to serve, regardless of our country of origin. Please let us help this great nation by giving us freedom from visa restrictions and providing security for our families.
“During wartime, all frontline soldiers are naturalized and given citizenship by presidential mandate; this is more than war and we are not asking for citizenship – but at least give us a green card which we have already satisfied all requirements for. If not now, then when?” he asked.
‘Profound human toll’ in excess deaths from COVID-19 calculated in two studies
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
However, additional deaths could be indirectly related because people avoided emergency care during the pandemic, new research shows.
Deaths linked to COVID-19 varied by state and phase of the pandemic, as reported in a study from researchers at Virginia Commonwealth University and Yale University that was published online October 12 in JAMA.
Another study published online simultaneously in JAMA took more of an international perspective. Investigators from the University of Pennsylvania and Harvard University found that in America there were more excess deaths and there was higher all-cause mortality during the pandemic than in 18 other countries.
Although the ongoing number of deaths attributable to COVID-19 continues to garner attention, there can be a lag of weeks or months in how long it takes some public health agencies to update their figures.
“For the public at large, the take-home message is twofold: that the number of deaths caused by the pandemic exceeds publicly reported COVID-19 death counts by 20% and that states that reopened or lifted restrictions early suffered a protracted surge in excess deaths that extended into the summer,” lead author of the US-focused study, Steven H. Woolf, MD, MPH, told Medscape Medical News.
The take-away for physicians is in the bigger picture – it is likely that the COVID-19 pandemic is responsible for deaths from other conditions as well. “Surges in COVID-19 were accompanied by an increase in deaths attributed to other causes, such as heart disease and Alzheimer’s disease and dementia,” said Woolf, director emeritus and senior adviser at the Center on Society and Health and professor in the Department of Family Medicine and Population Health at the Virginia Commonwealth University School of Medicine in Richmond, Virginia.
The investigators identified 225,530 excess US deaths in the 5 months from March to July. They report that 67% were directly attributable to COVID-19.
Deaths linked to COVID-19 included those in which the disease was listed as an underlying or contributing cause. US total death rates are “remarkably consistent” year after year, and the investigators calculated a 20% overall jump in mortality.
The study included data from the National Center for Health Statistics and the US Census Bureau for 48 states and the District of Columbia. Connecticut and North Carolina were excluded because of missing data.
Woolf and colleagues also found statistically higher rates of deaths from two other causes, heart disease and Alzheimer’s disease/dementia.
Altered states
New York, New Jersey, Massachusetts, Louisiana, Arizona, Mississippi, Maryland, Delaware, Rhode Island, and Michigan had the highest per capita excess death rates. Three states experienced the shortest epidemics during the study period: New York, New Jersey, and Massachusetts.
Some lessons could be learned by looking at how individual states managed large numbers of people with COVID-19. “Although we suspected that states that reopened early might have put themselves at risk of a pandemic surge, the consistency with which that occurred and the devastating numbers of deaths they suffered was a surprise,” Woolf said.
“The goal of our study is not to look in the rearview mirror and lament what happened months ago but to learn the lesson going forward: Our country will be unable to take control of this pandemic without more robust efforts to control community spread,” Woolf said. “Our study found that states that did this well, such as New York and New Jersey, experienced large surges but bent the curve and were back to baseline in less than 10 weeks.
“If we could do this as a country, countless lives could be saved.”
A global perspective
The United States experienced high mortality linked to COVID-19, as well as high all-cause mortality, compared with 18 other countries, as reported in the study by University of Pennsylvania and Harvard University researchers.
The United States ranked third, with 72 deaths per 100,000 people, among countries with moderate or high mortality. Although perhaps not surprising given the state of SARS-CoV-2 infection across the United States, a question remains as to what extent the relatively high mortality rate is linked to early outbreaks vs “poor long-term response,” the researchers note.
Alyssa Bilinski, MSc, and lead author Ezekiel J. Emanuel, MD, PhD, chair of the Department of Medical Ethics and Health Policy at the University of Pennsylvania Perelman School of Medicine in Philadelphia, calculated the difference in COVID-19 deaths among countries through Sept. 19, 2020. On this date, the United States reported a total 198,589 COVID-19 deaths.
They calculated that, if the US death rates were similar to those in Australia, the United States would have experienced 187,661 fewer COVID-19 deaths. If similar to those of Canada, there would have been 117,622 fewer deaths in the United States.
The US death rate was lower than six other countries with high COVID-19 mortality in the early spring, including Belgium, Spain, and the United Kingdom. However, after May 10, the per capita mortality rate in the United States exceeded the others.
Between May 10 and Sept. 19, the death rate in Italy was 9.1 per 100,000, vs 36.9 per 100,000.
“After the first peak in early spring, US death rates from COVID-19 and from all causes remained higher than even countries with high COVID-19 mortality,” the researchers note. “This may have been a result of several factors, including weak public health infrastructure and a decentralized, inconsistent US response to the pandemic.”
“Mortifying and motivating”
Woolf and colleagues estimate that more than 225,000 excess deaths occurred in recent months; this represents a 20% increase over expected deaths, note Harvey V. Fineberg, MD, PhD, of the Gordon and Betty Moore Foundation, in an accompanying editorial in JAMA.
“Importantly, a condition such as COVID-19 can contribute both directly and indirectly to excess mortality,” he writes.
Although the direct contribution to the mortality rates by those infected is straightforward, “the indirect contribution may relate to circumstances or choices due to the COVID-19 pandemic: for example, a patient who develops symptoms of a stroke is too concerned about COVID-19 to go to the emergency department, and a potentially reversible condition becomes fatal.”
Fineberg notes that “a general indication of the death toll from COVID-19 and the excess deaths related to the pandemic, as presented by Woolf et al, are sufficiently mortifying and motivating.”
“Profound human toll”
“The importance of the estimate by Woolf et al – which suggests that for the entirety of 2020, more than 400,000 excess deaths will occur – cannot be overstated, because it accounts for what could be declines in some causes of death, like motor vehicle crashes, but increases in others, like myocardial infarction,” write Howard Bauchner, MD, editor in chief of JAMA, and Phil B. Fontanarosa, MD, MBA, executive editor of JAMA, in another accompanying editorial.
“These deaths reflect a true measure of the human cost of the Great Pandemic of 2020,” they add.
The study from Emanuel and Bilinski was notable for calculating the excess COVID-19 and all-cause mortality to Sept. 2020, they note. “After the initial peak in early spring, US death rates from COVID-19 and from all causes remained higher than rates in countries with high COVID-19 mortality.”
“Few people will forget the Great Pandemic of 2020, where and how they lived, how it substantially changed their lives, and for many, the profound human toll it has taken,” Bauchner and Fontanarosa write.
The study by Woolf and colleagues was supported by National Center for Advancing Translational Sciences, the National Institute on Aging, and the National Institute of Allergy and Infectious Diseases. The study by Bilinski and Emanuel was partially funded by the Colton Foundation. Woolf, Emanuel, Fineberg, Bauchner, and Fontanarosa have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Fourteen-day sports hiatus recommended for children after COVID-19
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Children should not return to sports for 14 days after exposure to COVID-19, and those with moderate symptoms should undergo an electrocardiogram before returning, according to the American Academy of Pediatrics.
said Susannah Briskin, MD, a pediatric sports medicine specialist at Rainbow Babies and Children’s Hospital in Cleveland.
“There has been emerging evidence about cases of myocarditis occurring in athletes, including athletes who are asymptomatic with COVID-19,” she said in an interview.
The update aligns the AAP recommendations with those from the American College of Cardiologists, she added.
Recent imaging studies have turned up signs of myocarditis in athletes recovering from mild or asymptomatic cases of COVID-19 and have prompted calls for clearer guidelines about imaging studies and return to play.
Viral myocarditis poses a risk to athletes because it can lead to potentially fatal arrhythmias, Dr. Briskin said.
Although children benefit from participating in sports, these activities also put them at risk of contracting COVID-19 and spreading it to others, the guidance noted.
To balance the risks and benefits, the academy proposed guidelines that vary depending on the severity of the presentation.
In the first category are patients with a severe presentation (hypotension, arrhythmias, need for intubation or extracorporeal membrane oxygenation support, kidney or cardiac failure) or with multisystem inflammatory syndrome. Clinicians should treat these patients as though they have myocarditis. Patients should be restricted from engaging in sports and other exercise for 3-6 months, the guidance stated.
The primary care physician and “appropriate pediatric medical subspecialist, preferably in consultation with a pediatric cardiologist,” should clear them before they return to activities. In examining patients for return to play, clinicians should focus on cardiac symptoms, including chest pain, shortness of breath, fatigue, palpitations, or syncope, the guidance said.
In another category are patients with cardiac symptoms, those with concerning findings on examination, and those with moderate symptoms of COVID-19, including prolonged fever. These patients should undergo an ECG and possibly be referred to a pediatric cardiologist, the guidelines said. These symptoms must be absent for at least 14 days before these patients can return to sports, and the athletes should obtain clearance from their primary care physicians before they resume.
In a third category are patients who have been infected with SARS-CoV-2 or who have had close contact with someone who was infected but who have not themselves experienced symptoms. These athletes should refrain from sports for at least 14 days, the guidelines said.
Children who don’t fall into any of these categories should not be tested for the virus or antibodies to it before participation in sports, the academy said.
The guidelines don’t vary depending on the sport. But the academy has issued separate guidance for parents and guardians to help them evaluate the risk for COVID-19 transmission by sport.
Athletes participating in “sports that have greater amount of contact time or proximity to people would be at higher risk for contracting COVID-19,” Dr. Briskin said. “But I think that’s all fairly common sense, given the recommendations for non–sport-related activity just in terms of social distancing and masking.”
The new guidance called on sports organizers to minimize contact by, for example, modifying drills and conditioning. It recommended that athletes wear masks except during vigorous exercise or when participating in water sports, as well as in other circumstances in which the mask could become a safety hazard.
They also recommended using handwashing stations or hand sanitizer, avoiding contact with shared surfaces, and avoiding small rooms and areas with poor ventilation.
Dr. Briskin disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Hospital leadership lessons in the era of COVID-19
The year 2020 has brought the COVID-19 pandemic and civil unrest and protests, which have resulted in unprecedented health care challenges to hospitals and clinics. The daunting prospect of a fall influenza season has hospital staff and administrators looking ahead to still greater challenges.
This year of crisis has put even greater emphasis on leadership in hospitals, as patients, clinicians, and staff look for direction in the face of uncertainty and stress. But hospital leaders often arrive at their positions unprepared for their roles, according to Leonard Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution at Harvard T.H. Chan School of Public Health, Boston.
“Many times what happens in medicine is that someone with the greatest technical skills or greatest clinical skills emerges to be leader of a department, or a group, or a hospital, without having really paid attention to how they can build their leadership skills,” Dr. Marcus said during the 2020 Society of Hospital Medicine Leadership Virtual Seminar, held online Sept. 16-17.
Over 2 days, Dr. Marcus discussed the complex environments faced by hospital leaders, and some of the tools and strategies that can be used to maintain calm, problem-solve, and chart a course ahead.
He emphasized that hospitals and medical systems are complex, nonlinear organizations, which could be swept up by change in the form of mergers, financial policies, patient surges due to local emergencies, or pandemics.
“Complexity has to be central to how you think about leadership. If you think you can control everything, that doesn’t work that well,” said Dr. Marcus.
Most think of leadership as hierarchical, with a boss on top and underlings below, though this is starting to change. Dr. Marcus suggested a different view. Instead of just “leading down” to those who report to them, leaders should consider “leading up” to their own bosses or oversight committees, and across to other departments or even beyond to interlinked organizations such as nursing homes.
“Being able to build that connectivity not only within your hospital, but beyond your hospital, lets you see the chain that goes through the experience of any patient. You are looking at the problem from a much wider lens. We call this meta-leadership,” Dr. Marcus said.
A key focus of meta-leadership is to create a culture where individuals are working together to help one another succeed. Leadership in hospitals is often dominated by egos, with individual leaders battling one another in a win-lose effort, and this gets in the way of incorporating different perspectives into problem-solving.
Dr. Marcus used an example from previous seminars in which he instructed participants to arm wrestle the person sitting next to them. The goal was to attain as many pins as possible in 30 seconds. About half would fight as hard as they could, and achieve a few victories. The other half worked cooperatively, letting one person win, then the other, so that they could have 30 or 40 wins each. Dr. Marcus told the story of a young nurse who was paired up with a much stronger surgeon. She let him win twice, and when he asked her why she wasn’t resisting, she took his arm and placed it in a winning position, then a losing position, and then a winning position again, and he instantly understood that the cooperative approach could be more effective. Why didn’t she just tell him? She told Dr. Marcus that she knew he wouldn’t take instruction, so she let him win and then demonstrated an alternative. “We nurses learned how to do that a long time ago,” she told Dr. Marcus.
The idea is collaborative problem-solving. “How do you orient people looking to you for leadership so that we’re in this together and we can accomplish a whole lot more in 30 seconds if we’re working together instead of always battling one another? If we’re always battling one another, we’re putting all of our effort into the contest,” said Dr. Marcus. This sort of approach is all the more important when facing the complexity experienced by hospital systems, especially during crises such as COVID-19.
A critical element of meta-leadership is emotional intelligence, which includes elements such as self-awareness, self-regulation, empathy, determining motivation of yourself and others, and the social skills to portray yourself as caring, open, and interested.
Emotional intelligence also can help recognize when you’ve entered survival mode in reaction to a crisis or incident, or something as simple as losing your car keys – what Dr. Marcus terms “going to the basement.” Responses revolve around freeze, fight, or flight. It’s helpful in the wake of a car accident, but not when trying to make managerial decisions or respond to a complex situation. It’s vital for leaders to quickly get themselves out of the basement, said Dr. Marcus, and that they help other members of the team get out as well.
He recommended protocols designed in advance, both to recognize when you’re in the basement, and to lift yourself out. Dr. Marcus uses a trigger script, telling himself “I can do this,” and then when he’s working with other people, “we can do this.” He also speaks slowly, measuring every word. Whatever you do, “it has to be a pivot you do to get yourself out of the basement,” he said. It can be helpful to predict the kinds of situations that send you “to the basement” to help recognize it when it has happened.
It’s very important not to lead, negotiate, or make important decisions while in the basement, according to Dr. Marcus. If one thinks about some of the things they’ve said to others while under duress, they are often some of the statements they regret most.
Practical leadership skills
On the second day of the Leadership Seminar, Dr. Marcus moved his focus to using leadership skills and techniques. One important technique is to incorporate multiple perspectives. He gave the example of an opaque cube with a cone inside it, with a window on the side and one on top. Viewers from the side see the cone in profile, and see it as a triangle. Viewers from the top see an aerial perspective that looks like the circular base of the cone. The two groups could argue about what’s inside the cube, but they can only identify the object if they work together.
“When dealing with complex reality, you oftentimes find there are different people with different perspectives on a problem. They may have different experiences of what the problem is, and what often happens is that people get into an adversarial fight. Looking at the problem from different perspectives actually allows a much richer and more comprehensive view,” said Dr. Marcus.
The metaphor comes from a study of the tragic events at the Twin Towers in Manhattan on Sept. 11, 2001. The New York Fire Department had a command center at the base of the building, while the police had a helicopter flying around the buildings. The helicopter could see the steel girders beginning to melt and predicted a collapse, and therefore ordered their personnel out of the buildings. But they were unable to convey that information to the firefighters, who continued to send personnel into the buildings. In all, 343 firefighters lost their lives. The police force lost 32.
To best understand a problem, a key element is the “unknown knowns.” That is, information that is available, that someone has, but is unknown to you. It takes some imagination to conceive of what “unknown knowns” might be out there, but it’s worth the effort to identify possible knowledge sources. It’s vital to seek out this information, because a common leadership mistake is to assume you know something when you really don’t.
“In many ways what you’re doing is looking for obstacles. It could be you don’t have access to the information, that it’s beyond some sort of curtain you need to overcome, or it could be people in your own department who have the information and they’re not sharing it with you,” Dr. Marcus said.
He outlined a tool called the POP-DOC loop, which is a 6-step exercise designed to analyze problems and implement solutions. Step 1 is Perceiving the situation, determining knowns and unknowns, and incorporating multiple perspectives, emotions, and politics. Step 2 is to Orient oneself: examine patterns and how they may replicate themselves as long as conditions don’t change. For example, during COVID-19, physicians have begun to learn how the virus transmits and how it affects the immune system. Step 3, based on those patterns is to make Predictions. With COVID-19, it’s predictable that people who assemble without wearing masks are vulnerable to transmission. Step 4 is to use the predictions to begin to make Decisions. Step 5 is to begin Operationalizing those decisions, and step 6 is to Communicate those decisions effectively.
Dr. Marcus emphasized that POP-DOC is not a one-time exercise. Once decisions have been made and implemented, if they aren’t having the planned effect, it’s important to incorporate the results of those actions and start right back at the beginning of the POP-DOC loop.
“The POP side of the loop is perceiving, analysis. You get out of the basement and understand the situation that surrounds you. On the DOC side, you lead down, lead up, lead across and lead beyond. You’re bringing people into the action to get things done,” Dr. Marcus said.
Another tool Dr. Marcus described, aimed at problem-solving and negotiation, is the “Walk in the Woods.” The idea is to bring two parties together to help each other succeed. The first step is Self-Interest, where both parties articulate their objectives, perspectives, and fears. The second step, Enlarged Interests, requires each party to list their points of agreement, and only then should they focus on and list their points of disagreement. During conflict, people tend to focus on their disagreements. The parties often find that they agree on more than they realize, and this can frame the disagreements as more manageable. The third step, Enlightened Interest, is a free thinking period where both parties come up with potential solutions that had not been previously considered. In step 4, Aligned Interests, the parties discuss some of those ideas that can be explored further.
The Walk in the Woods is applicable to a wide range of situations, and negotiation is central to being a leader. “Being a clinician is all about negotiating – with patients, family members, with other clinicians, with the institution,” Dr. Marcus said. “We all want the patient to have the best possible care, and in the course of those conversations if we can better understand people, have empathy, and if there are new ideas or ways we can individualize our care, let’s do it, and then at the end of the day combine our motivations so that we’re providing the best possible care.”
In the end, meta-leadership is about creating a culture where individuals strive to help each other succeed, said Dr. Marcus. “That’s the essence: involving people, making them part of the solution, and if it’s a solution they’ve created together, everyone wants to make that solution a success.”
For more information, see the book “You’re It,” coauthored by Dr. Marcus, and available on Amazon for $16.99 in hardback, or $3.99 in Kindle format.
The year 2020 has brought the COVID-19 pandemic and civil unrest and protests, which have resulted in unprecedented health care challenges to hospitals and clinics. The daunting prospect of a fall influenza season has hospital staff and administrators looking ahead to still greater challenges.
This year of crisis has put even greater emphasis on leadership in hospitals, as patients, clinicians, and staff look for direction in the face of uncertainty and stress. But hospital leaders often arrive at their positions unprepared for their roles, according to Leonard Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution at Harvard T.H. Chan School of Public Health, Boston.
“Many times what happens in medicine is that someone with the greatest technical skills or greatest clinical skills emerges to be leader of a department, or a group, or a hospital, without having really paid attention to how they can build their leadership skills,” Dr. Marcus said during the 2020 Society of Hospital Medicine Leadership Virtual Seminar, held online Sept. 16-17.
Over 2 days, Dr. Marcus discussed the complex environments faced by hospital leaders, and some of the tools and strategies that can be used to maintain calm, problem-solve, and chart a course ahead.
He emphasized that hospitals and medical systems are complex, nonlinear organizations, which could be swept up by change in the form of mergers, financial policies, patient surges due to local emergencies, or pandemics.
“Complexity has to be central to how you think about leadership. If you think you can control everything, that doesn’t work that well,” said Dr. Marcus.
Most think of leadership as hierarchical, with a boss on top and underlings below, though this is starting to change. Dr. Marcus suggested a different view. Instead of just “leading down” to those who report to them, leaders should consider “leading up” to their own bosses or oversight committees, and across to other departments or even beyond to interlinked organizations such as nursing homes.
“Being able to build that connectivity not only within your hospital, but beyond your hospital, lets you see the chain that goes through the experience of any patient. You are looking at the problem from a much wider lens. We call this meta-leadership,” Dr. Marcus said.
A key focus of meta-leadership is to create a culture where individuals are working together to help one another succeed. Leadership in hospitals is often dominated by egos, with individual leaders battling one another in a win-lose effort, and this gets in the way of incorporating different perspectives into problem-solving.
Dr. Marcus used an example from previous seminars in which he instructed participants to arm wrestle the person sitting next to them. The goal was to attain as many pins as possible in 30 seconds. About half would fight as hard as they could, and achieve a few victories. The other half worked cooperatively, letting one person win, then the other, so that they could have 30 or 40 wins each. Dr. Marcus told the story of a young nurse who was paired up with a much stronger surgeon. She let him win twice, and when he asked her why she wasn’t resisting, she took his arm and placed it in a winning position, then a losing position, and then a winning position again, and he instantly understood that the cooperative approach could be more effective. Why didn’t she just tell him? She told Dr. Marcus that she knew he wouldn’t take instruction, so she let him win and then demonstrated an alternative. “We nurses learned how to do that a long time ago,” she told Dr. Marcus.
The idea is collaborative problem-solving. “How do you orient people looking to you for leadership so that we’re in this together and we can accomplish a whole lot more in 30 seconds if we’re working together instead of always battling one another? If we’re always battling one another, we’re putting all of our effort into the contest,” said Dr. Marcus. This sort of approach is all the more important when facing the complexity experienced by hospital systems, especially during crises such as COVID-19.
A critical element of meta-leadership is emotional intelligence, which includes elements such as self-awareness, self-regulation, empathy, determining motivation of yourself and others, and the social skills to portray yourself as caring, open, and interested.
Emotional intelligence also can help recognize when you’ve entered survival mode in reaction to a crisis or incident, or something as simple as losing your car keys – what Dr. Marcus terms “going to the basement.” Responses revolve around freeze, fight, or flight. It’s helpful in the wake of a car accident, but not when trying to make managerial decisions or respond to a complex situation. It’s vital for leaders to quickly get themselves out of the basement, said Dr. Marcus, and that they help other members of the team get out as well.
He recommended protocols designed in advance, both to recognize when you’re in the basement, and to lift yourself out. Dr. Marcus uses a trigger script, telling himself “I can do this,” and then when he’s working with other people, “we can do this.” He also speaks slowly, measuring every word. Whatever you do, “it has to be a pivot you do to get yourself out of the basement,” he said. It can be helpful to predict the kinds of situations that send you “to the basement” to help recognize it when it has happened.
It’s very important not to lead, negotiate, or make important decisions while in the basement, according to Dr. Marcus. If one thinks about some of the things they’ve said to others while under duress, they are often some of the statements they regret most.
Practical leadership skills
On the second day of the Leadership Seminar, Dr. Marcus moved his focus to using leadership skills and techniques. One important technique is to incorporate multiple perspectives. He gave the example of an opaque cube with a cone inside it, with a window on the side and one on top. Viewers from the side see the cone in profile, and see it as a triangle. Viewers from the top see an aerial perspective that looks like the circular base of the cone. The two groups could argue about what’s inside the cube, but they can only identify the object if they work together.
“When dealing with complex reality, you oftentimes find there are different people with different perspectives on a problem. They may have different experiences of what the problem is, and what often happens is that people get into an adversarial fight. Looking at the problem from different perspectives actually allows a much richer and more comprehensive view,” said Dr. Marcus.
The metaphor comes from a study of the tragic events at the Twin Towers in Manhattan on Sept. 11, 2001. The New York Fire Department had a command center at the base of the building, while the police had a helicopter flying around the buildings. The helicopter could see the steel girders beginning to melt and predicted a collapse, and therefore ordered their personnel out of the buildings. But they were unable to convey that information to the firefighters, who continued to send personnel into the buildings. In all, 343 firefighters lost their lives. The police force lost 32.
To best understand a problem, a key element is the “unknown knowns.” That is, information that is available, that someone has, but is unknown to you. It takes some imagination to conceive of what “unknown knowns” might be out there, but it’s worth the effort to identify possible knowledge sources. It’s vital to seek out this information, because a common leadership mistake is to assume you know something when you really don’t.
“In many ways what you’re doing is looking for obstacles. It could be you don’t have access to the information, that it’s beyond some sort of curtain you need to overcome, or it could be people in your own department who have the information and they’re not sharing it with you,” Dr. Marcus said.
He outlined a tool called the POP-DOC loop, which is a 6-step exercise designed to analyze problems and implement solutions. Step 1 is Perceiving the situation, determining knowns and unknowns, and incorporating multiple perspectives, emotions, and politics. Step 2 is to Orient oneself: examine patterns and how they may replicate themselves as long as conditions don’t change. For example, during COVID-19, physicians have begun to learn how the virus transmits and how it affects the immune system. Step 3, based on those patterns is to make Predictions. With COVID-19, it’s predictable that people who assemble without wearing masks are vulnerable to transmission. Step 4 is to use the predictions to begin to make Decisions. Step 5 is to begin Operationalizing those decisions, and step 6 is to Communicate those decisions effectively.
Dr. Marcus emphasized that POP-DOC is not a one-time exercise. Once decisions have been made and implemented, if they aren’t having the planned effect, it’s important to incorporate the results of those actions and start right back at the beginning of the POP-DOC loop.
“The POP side of the loop is perceiving, analysis. You get out of the basement and understand the situation that surrounds you. On the DOC side, you lead down, lead up, lead across and lead beyond. You’re bringing people into the action to get things done,” Dr. Marcus said.
Another tool Dr. Marcus described, aimed at problem-solving and negotiation, is the “Walk in the Woods.” The idea is to bring two parties together to help each other succeed. The first step is Self-Interest, where both parties articulate their objectives, perspectives, and fears. The second step, Enlarged Interests, requires each party to list their points of agreement, and only then should they focus on and list their points of disagreement. During conflict, people tend to focus on their disagreements. The parties often find that they agree on more than they realize, and this can frame the disagreements as more manageable. The third step, Enlightened Interest, is a free thinking period where both parties come up with potential solutions that had not been previously considered. In step 4, Aligned Interests, the parties discuss some of those ideas that can be explored further.
The Walk in the Woods is applicable to a wide range of situations, and negotiation is central to being a leader. “Being a clinician is all about negotiating – with patients, family members, with other clinicians, with the institution,” Dr. Marcus said. “We all want the patient to have the best possible care, and in the course of those conversations if we can better understand people, have empathy, and if there are new ideas or ways we can individualize our care, let’s do it, and then at the end of the day combine our motivations so that we’re providing the best possible care.”
In the end, meta-leadership is about creating a culture where individuals strive to help each other succeed, said Dr. Marcus. “That’s the essence: involving people, making them part of the solution, and if it’s a solution they’ve created together, everyone wants to make that solution a success.”
For more information, see the book “You’re It,” coauthored by Dr. Marcus, and available on Amazon for $16.99 in hardback, or $3.99 in Kindle format.
The year 2020 has brought the COVID-19 pandemic and civil unrest and protests, which have resulted in unprecedented health care challenges to hospitals and clinics. The daunting prospect of a fall influenza season has hospital staff and administrators looking ahead to still greater challenges.
This year of crisis has put even greater emphasis on leadership in hospitals, as patients, clinicians, and staff look for direction in the face of uncertainty and stress. But hospital leaders often arrive at their positions unprepared for their roles, according to Leonard Marcus, PhD, director of the Program for Health Care Negotiation and Conflict Resolution at Harvard T.H. Chan School of Public Health, Boston.
“Many times what happens in medicine is that someone with the greatest technical skills or greatest clinical skills emerges to be leader of a department, or a group, or a hospital, without having really paid attention to how they can build their leadership skills,” Dr. Marcus said during the 2020 Society of Hospital Medicine Leadership Virtual Seminar, held online Sept. 16-17.
Over 2 days, Dr. Marcus discussed the complex environments faced by hospital leaders, and some of the tools and strategies that can be used to maintain calm, problem-solve, and chart a course ahead.
He emphasized that hospitals and medical systems are complex, nonlinear organizations, which could be swept up by change in the form of mergers, financial policies, patient surges due to local emergencies, or pandemics.
“Complexity has to be central to how you think about leadership. If you think you can control everything, that doesn’t work that well,” said Dr. Marcus.
Most think of leadership as hierarchical, with a boss on top and underlings below, though this is starting to change. Dr. Marcus suggested a different view. Instead of just “leading down” to those who report to them, leaders should consider “leading up” to their own bosses or oversight committees, and across to other departments or even beyond to interlinked organizations such as nursing homes.
“Being able to build that connectivity not only within your hospital, but beyond your hospital, lets you see the chain that goes through the experience of any patient. You are looking at the problem from a much wider lens. We call this meta-leadership,” Dr. Marcus said.
A key focus of meta-leadership is to create a culture where individuals are working together to help one another succeed. Leadership in hospitals is often dominated by egos, with individual leaders battling one another in a win-lose effort, and this gets in the way of incorporating different perspectives into problem-solving.
Dr. Marcus used an example from previous seminars in which he instructed participants to arm wrestle the person sitting next to them. The goal was to attain as many pins as possible in 30 seconds. About half would fight as hard as they could, and achieve a few victories. The other half worked cooperatively, letting one person win, then the other, so that they could have 30 or 40 wins each. Dr. Marcus told the story of a young nurse who was paired up with a much stronger surgeon. She let him win twice, and when he asked her why she wasn’t resisting, she took his arm and placed it in a winning position, then a losing position, and then a winning position again, and he instantly understood that the cooperative approach could be more effective. Why didn’t she just tell him? She told Dr. Marcus that she knew he wouldn’t take instruction, so she let him win and then demonstrated an alternative. “We nurses learned how to do that a long time ago,” she told Dr. Marcus.
The idea is collaborative problem-solving. “How do you orient people looking to you for leadership so that we’re in this together and we can accomplish a whole lot more in 30 seconds if we’re working together instead of always battling one another? If we’re always battling one another, we’re putting all of our effort into the contest,” said Dr. Marcus. This sort of approach is all the more important when facing the complexity experienced by hospital systems, especially during crises such as COVID-19.
A critical element of meta-leadership is emotional intelligence, which includes elements such as self-awareness, self-regulation, empathy, determining motivation of yourself and others, and the social skills to portray yourself as caring, open, and interested.
Emotional intelligence also can help recognize when you’ve entered survival mode in reaction to a crisis or incident, or something as simple as losing your car keys – what Dr. Marcus terms “going to the basement.” Responses revolve around freeze, fight, or flight. It’s helpful in the wake of a car accident, but not when trying to make managerial decisions or respond to a complex situation. It’s vital for leaders to quickly get themselves out of the basement, said Dr. Marcus, and that they help other members of the team get out as well.
He recommended protocols designed in advance, both to recognize when you’re in the basement, and to lift yourself out. Dr. Marcus uses a trigger script, telling himself “I can do this,” and then when he’s working with other people, “we can do this.” He also speaks slowly, measuring every word. Whatever you do, “it has to be a pivot you do to get yourself out of the basement,” he said. It can be helpful to predict the kinds of situations that send you “to the basement” to help recognize it when it has happened.
It’s very important not to lead, negotiate, or make important decisions while in the basement, according to Dr. Marcus. If one thinks about some of the things they’ve said to others while under duress, they are often some of the statements they regret most.
Practical leadership skills
On the second day of the Leadership Seminar, Dr. Marcus moved his focus to using leadership skills and techniques. One important technique is to incorporate multiple perspectives. He gave the example of an opaque cube with a cone inside it, with a window on the side and one on top. Viewers from the side see the cone in profile, and see it as a triangle. Viewers from the top see an aerial perspective that looks like the circular base of the cone. The two groups could argue about what’s inside the cube, but they can only identify the object if they work together.
“When dealing with complex reality, you oftentimes find there are different people with different perspectives on a problem. They may have different experiences of what the problem is, and what often happens is that people get into an adversarial fight. Looking at the problem from different perspectives actually allows a much richer and more comprehensive view,” said Dr. Marcus.
The metaphor comes from a study of the tragic events at the Twin Towers in Manhattan on Sept. 11, 2001. The New York Fire Department had a command center at the base of the building, while the police had a helicopter flying around the buildings. The helicopter could see the steel girders beginning to melt and predicted a collapse, and therefore ordered their personnel out of the buildings. But they were unable to convey that information to the firefighters, who continued to send personnel into the buildings. In all, 343 firefighters lost their lives. The police force lost 32.
To best understand a problem, a key element is the “unknown knowns.” That is, information that is available, that someone has, but is unknown to you. It takes some imagination to conceive of what “unknown knowns” might be out there, but it’s worth the effort to identify possible knowledge sources. It’s vital to seek out this information, because a common leadership mistake is to assume you know something when you really don’t.
“In many ways what you’re doing is looking for obstacles. It could be you don’t have access to the information, that it’s beyond some sort of curtain you need to overcome, or it could be people in your own department who have the information and they’re not sharing it with you,” Dr. Marcus said.
He outlined a tool called the POP-DOC loop, which is a 6-step exercise designed to analyze problems and implement solutions. Step 1 is Perceiving the situation, determining knowns and unknowns, and incorporating multiple perspectives, emotions, and politics. Step 2 is to Orient oneself: examine patterns and how they may replicate themselves as long as conditions don’t change. For example, during COVID-19, physicians have begun to learn how the virus transmits and how it affects the immune system. Step 3, based on those patterns is to make Predictions. With COVID-19, it’s predictable that people who assemble without wearing masks are vulnerable to transmission. Step 4 is to use the predictions to begin to make Decisions. Step 5 is to begin Operationalizing those decisions, and step 6 is to Communicate those decisions effectively.
Dr. Marcus emphasized that POP-DOC is not a one-time exercise. Once decisions have been made and implemented, if they aren’t having the planned effect, it’s important to incorporate the results of those actions and start right back at the beginning of the POP-DOC loop.
“The POP side of the loop is perceiving, analysis. You get out of the basement and understand the situation that surrounds you. On the DOC side, you lead down, lead up, lead across and lead beyond. You’re bringing people into the action to get things done,” Dr. Marcus said.
Another tool Dr. Marcus described, aimed at problem-solving and negotiation, is the “Walk in the Woods.” The idea is to bring two parties together to help each other succeed. The first step is Self-Interest, where both parties articulate their objectives, perspectives, and fears. The second step, Enlarged Interests, requires each party to list their points of agreement, and only then should they focus on and list their points of disagreement. During conflict, people tend to focus on their disagreements. The parties often find that they agree on more than they realize, and this can frame the disagreements as more manageable. The third step, Enlightened Interest, is a free thinking period where both parties come up with potential solutions that had not been previously considered. In step 4, Aligned Interests, the parties discuss some of those ideas that can be explored further.
The Walk in the Woods is applicable to a wide range of situations, and negotiation is central to being a leader. “Being a clinician is all about negotiating – with patients, family members, with other clinicians, with the institution,” Dr. Marcus said. “We all want the patient to have the best possible care, and in the course of those conversations if we can better understand people, have empathy, and if there are new ideas or ways we can individualize our care, let’s do it, and then at the end of the day combine our motivations so that we’re providing the best possible care.”
In the end, meta-leadership is about creating a culture where individuals strive to help each other succeed, said Dr. Marcus. “That’s the essence: involving people, making them part of the solution, and if it’s a solution they’ve created together, everyone wants to make that solution a success.”
For more information, see the book “You’re It,” coauthored by Dr. Marcus, and available on Amazon for $16.99 in hardback, or $3.99 in Kindle format.
FROM THE SHM LEADERSHIP SEMINAR
Empagliflozin cut PA pressures in heart failure patients
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
Elevated pulmonary artery diastolic pressure is “perhaps the best predictor of bad outcomes in patients with heart failure, including hospitalization and death,” and new evidence clearly showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin cuts this metric in patients by a clinically significant amount, Mikhail Kosiborod, MD, said at the virtual annual scientific meeting of the Heart Failure Society of America.
The evidence he collected from a total of 65 heart failure patients with either reduced or preserved ejection fraction is the first documentation from a randomized, controlled study to show a direct effect by a SGLT2 inhibitor on pulmonary artery (PA) pressures.
Other key findings were that the drop in PA diastolic pressure with empagliflozin treatment compared with placebo became discernible early (within the first 4 weeks on treatment), that the pressure-lowering effect steadily grew over time, and that it showed no link to the intensity of loop diuretic treatment, which held steady during 12 weeks on treatment and 13 weeks of overall monitoring.
The study’s primary endpoint was the change from baseline in PA diastolic pressure after 12 weeks on treatment. The 31 patients who completed the full 12-week course had an average drop in their PA diastolic pressure of about 1.5 mm Hg, compared with 28 patients who completed 12 weeks on placebo. Average PA diastolic pressure at baseline was about 21 mm Hg in both treatment arms, and on treatment this fell by more than 0.5 mm Hg among those who received empagliflozin and rose by close to 1 mm Hg among control patients.
“There appears to be a direct effect of empagliflozin on pulmonary artery pressure that’s not been previously demonstrated” by an SGLT2 inhibitor, Dr. Kosiborod said. “I think this is one mechanism of action” for this drug class. “If you control pulmonary artery filling pressures you can prevent hospitalizations and deaths.”
Small reductions matter
“Small pressure differences are particularly important for pulmonary hypertension,” commented Lynne W. Stevenson, MD, professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn., and the report’s designated discussant.
“In the Vanderbilt heart failure database, patients with a pulmonary artery mean pressure of 20-24 mm Hg had 30% higher mortality than patients with lower pressures,” Dr. Stevenson noted. “This has led to a new definition of pulmonary hypertension, a mean pulmonary artery pressure above at or above 20 mm Hg.”
In Dr. Kosiborod’s study, patients began with an average PA mean pressure of about 30 mm Hg, and empagliflozin treatment led to a reduction in this metric with about the same magnitude as its effect on PA diastolic pressure. Empagliflozin also produced a similar reduction in average PA systolic pressure.
A study built on ambulatory PA monitoring
The results “also provide more proof for the concept of ambulatory hemodynamic monitoring” in patients with heart failure to monitor their status, she added. The study enrolled only patients who had already received a CardioMEMS implant as part of their routine care. This device allows for frequent, noninvasive monitoring of PA pressures. Researchers collected PA pressure data from patients twice daily for the entire 13-week study.
The EMBRACE HF (Empagliflozin Impact on Hemodynamics in Patients With Heart Failure) study enrolled patients with established heart failure, a CardioMEMS implant, and New York Heart Association class II-IV symptoms at any of eight U.S. centers. Patients averaged about 65 years old, and slightly more than half had class III disease, which denotes marked limitation of physical activity.
Despite the brief treatment period, patients who received empagliflozin showed other evidence of benefit including a trend toward improved quality of life scores, reduced levels of two different forms of brain natriuretic peptide, and significant weight loss, compared with controls, that averaged 2.4 kg.
The mechanism by which empagliflozin and other drugs in its class might lower PA filling pressures is unclear, but Dr. Kosiborod stressed that the consistent level of loop diuretic use during the study seems to rule out a diuretic effect from the SGLT2 inhibitor as having a role. A pulmonary vasculature effect is “much more likely,” perhaps mediated through modified endothelial function and vasodilation, he suggested.
EMBRACE HF was funded by Boehringer Ingelheim, the company that markets empagliflozin (Jardiance) along with Eli Lilly. Dr. Kosiborod has received research support and honoraria from Boehringer Ingelheim, and he has received honoraria from several other companies. Dr. Stevenson had no disclosures.
FROM HFSA 2020
Remdesivir effective, well-tolerated in final trial report
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
Drug beats placebo across multiple endpoints in COVID-19 patients
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
The socioeconomic revolving door of 30-day heart failure readmissions
Patients receiving even top-notch hospital care for heart failure (HF) are, once discharged to home, at higher short-term risk of another HF hospitalization if home is in a socioeconomically deprived neighborhood. That helps explain why Blacks in the United States have a much higher 30-day HF readmission risk than Whites, a disparity that only worsens with the level of neighborhood deprivation, a new analysis suggests.
Some systemic and entrenched socioeconomic inequities that health care providers have little sway over, and which disproportionately affect Black individuals, are independent and robust predictors of worsened HF outcomes, Alanna A. Morris, MD, MSc, Emory University, Atlanta, said during her presentation at the virtual annual scientific meeting of the Heart Failure Society of America.
In a retrospective cohort study, Blacks had a 45% higher risk of 30-day readmission than Whites (P < .001) independent of cardiovascular risk factors, clinical history, comorbidities, type and location of hospital, and type of third-party payer coverage. The analysis included more than 30,000 patients with at least one HF hospitalization at centers in a major metropolitan health system.
The racial disparity widened with worsening socioeconomic deprivation of patients’ residential neighborhoods, that is, with rising quartiles of neighborhood scores on the Social Deprivation Index (SDI).
The SDI, based on U.S. census data, incorporates seven socioeconomic criteria, including household income, education level, employment, and prevalence of rented housing and households that are without a car, single parent, or overcrowded.
There was a 4–percentage point gap in adjusted 30-day readmission rate between Blacks and Whites in the lowest quartile that widened to more than 8 points by the third quartile; the disparity in both the second and fourth quartiles was the same, at about 5.5 percentage points.
A remaining question, Dr. Morris said in an interview, is why the outcomes disparity between Blacks and Whites peaks in the third SDI quartile but drops a bit in the fourth quartile representing the most severe neighborhood deprivation.
“Our hypothesis is that when you look at patients who are the poorest, who live in the most deprived neighborhoods, race may be less of a factor,” she said. Socioeconomic deprivation may have similar consequences for everyone “regardless of race, ethnicity, gender, or other demographic characteristics if you live in a neighborhood that’s highly deprived.”
Based on the current study, “it does appear that increased heart failure incident rates are related to living in deprived neighborhoods, and it raises important clinical and public health concerns that must be addressed,” Keith C. Ferdinand, MD, Tulane University, New Orleans, said as invited discussant after the presentation from Dr. Morris.
“These findings could serve as an aid to policy makers, going forward, in terms of allocating resources for primary health care,” he said. “And it’s important looking at these data and other [data] that we target heart failure patients who reside in deprived neighborhoods before, during, and [after] hospitalization.”
Dr. Morris agreed that policy makers are in a better position to attack the racial disparity in HF readmission rates identified in the study. “This is not a problem that can be fixed within the health care system.”
If the reported interpretation is correct, it could add a twist to the public health care debate in the United States, observed session moderator Mandeep R. Mehra, MD, Brigham and Woman’s Hospital in Boston.
That debate, he noted, has often focused on insurability, access to coverage, and the merits or shortcomings of a single-payer system. Yet the study suggests outcomes disparities stemming from neighborhood deprivation will not be corrected by improved access to health insurance, a conclusion he finds “startling,” Dr. Mehra said in an interview.
Some proposed explanations for the disparities by race blame unequal access to health care and or variable health insurance coverage, Dr. Morris observed in an interview. But “that may not fully explain the increased risk that we see.”
Black patients followed at Emory University’s advanced HF clinic still have a higher risk of rehospitalization than Whites. “These are patients who have insurance, who are followed by advanced heart failure providers, who are on equal amounts of guideline-recommended medical therapy – and you still see about a 50% higher risk of rehospitalization,” Dr. Morris said, citing data that isn’t part of the current analysis.
“We can say that these patients are certainly able to access care, because they are able to access our emergency room and be taken care of within the hospital setting,” he said. The study controlled for whether health coverage was by private insurance, Medicare, or Medicaid.
Instead, the current analysis points to socioeconomic and environmental factors as a major source of the disparity in 30-day readmissions, Dr. Morris said.
“When patients are discharged from our healthcare systems, they still go back into environments where they don’t have the same resources as patients who live in higher-SDI neighborhoods,” she explained.
For example, “we tell them to eat low-sodium [foods], exercise, eat fresh fruits and vegetables, take their medicines, but the reality is that certain neighborhoods within the United States – and this is much more true for Blacks – make it very difficult to follow those self-care recommendations.”
The analysis included 16,147 Black patients and 14,483 White patients hospitalized with HF within the Emory Healthcare system at least once from 2010-2018, Dr. Morris reported. Compared with Whites, Blacks were younger (63.5 vs 69.1 years) and less likely to be 65 or older (48.9% vs. 66.5%); more likely to be women (53.5% vs. 42.2%), more likely to reside in deprived census tracts and to have diabetes, hypertension, or chronic kidney disease; and had higher comorbidity scores.
In all, 20.6% of Black and 13.5% of White patients were readmitted for HF within 30 days of discharge, for an unadjusted risk ratio of 1.52 (95% CI, 1.44-1.61).
The RR hardly budged, 1.45 (95% CI, 1.37-1.54, P < .001), after adjustment for age, sex, type of insurance, type of HF, vital signs and laboratory values, medical history (diabetes, hypertension, atrial fibrillation, coronary disease, chronic kidney disease, and chronic pulmonary disease), Charlson Comorbidity Index, discharging medical specialty, and hospital location.
The excess in 30-day HF readmissions for Black, compared with White patients climbed from the first to the third neighborhood SDI quartile, the disparity peaking at 8.2 absolute percentage points.
A major criticism of the Hospital Readmissions Reduction Program component of the Affordable Care Act, Dr. Morris said in a Q&A discussion after her presentation, is that it can hold hospitals “responsible for structural inequalities that exist beyond the health care system,” including neighborhood deprivation.
“But public policy makers have to realize that there are certain patients we take care of who don’t have the resources to carry out the therapeutic lifestyle changes that will allow them to live healthy.”
The HRRP’s 30-day HF readmission metric that steers reimbursement “is penalizing health care systems across the United States” with its premise that hospital performance can be measured by 30-day HF readmission rates, Dr. Morris said in an interview.
“The reality is that some of these patients are going to a postdischarge environment that is inherently high risk, and that many of them are going to come back to us within 30 days,” she said. “We would like to make sure that we don’t put excess penalties on health care systems that take care of disproportionate numbers of African Americans in neighborhoods that have fewer resources.”
Dr. Morris and Dr. Ferdinand have disclosed no relevant financial relationships. Dr. Mehra discloses consulting or serving on an advisory board for Abbott, Medtronic, Janssen, Leviticus, NupulseCV, FineHeart, Portola, Bayer, the Baim Institute for Clinical Research, and Mesoblast.
A version of this article originally appeared on Medscape.com.
Patients receiving even top-notch hospital care for heart failure (HF) are, once discharged to home, at higher short-term risk of another HF hospitalization if home is in a socioeconomically deprived neighborhood. That helps explain why Blacks in the United States have a much higher 30-day HF readmission risk than Whites, a disparity that only worsens with the level of neighborhood deprivation, a new analysis suggests.
Some systemic and entrenched socioeconomic inequities that health care providers have little sway over, and which disproportionately affect Black individuals, are independent and robust predictors of worsened HF outcomes, Alanna A. Morris, MD, MSc, Emory University, Atlanta, said during her presentation at the virtual annual scientific meeting of the Heart Failure Society of America.
In a retrospective cohort study, Blacks had a 45% higher risk of 30-day readmission than Whites (P < .001) independent of cardiovascular risk factors, clinical history, comorbidities, type and location of hospital, and type of third-party payer coverage. The analysis included more than 30,000 patients with at least one HF hospitalization at centers in a major metropolitan health system.
The racial disparity widened with worsening socioeconomic deprivation of patients’ residential neighborhoods, that is, with rising quartiles of neighborhood scores on the Social Deprivation Index (SDI).
The SDI, based on U.S. census data, incorporates seven socioeconomic criteria, including household income, education level, employment, and prevalence of rented housing and households that are without a car, single parent, or overcrowded.
There was a 4–percentage point gap in adjusted 30-day readmission rate between Blacks and Whites in the lowest quartile that widened to more than 8 points by the third quartile; the disparity in both the second and fourth quartiles was the same, at about 5.5 percentage points.
A remaining question, Dr. Morris said in an interview, is why the outcomes disparity between Blacks and Whites peaks in the third SDI quartile but drops a bit in the fourth quartile representing the most severe neighborhood deprivation.
“Our hypothesis is that when you look at patients who are the poorest, who live in the most deprived neighborhoods, race may be less of a factor,” she said. Socioeconomic deprivation may have similar consequences for everyone “regardless of race, ethnicity, gender, or other demographic characteristics if you live in a neighborhood that’s highly deprived.”
Based on the current study, “it does appear that increased heart failure incident rates are related to living in deprived neighborhoods, and it raises important clinical and public health concerns that must be addressed,” Keith C. Ferdinand, MD, Tulane University, New Orleans, said as invited discussant after the presentation from Dr. Morris.
“These findings could serve as an aid to policy makers, going forward, in terms of allocating resources for primary health care,” he said. “And it’s important looking at these data and other [data] that we target heart failure patients who reside in deprived neighborhoods before, during, and [after] hospitalization.”
Dr. Morris agreed that policy makers are in a better position to attack the racial disparity in HF readmission rates identified in the study. “This is not a problem that can be fixed within the health care system.”
If the reported interpretation is correct, it could add a twist to the public health care debate in the United States, observed session moderator Mandeep R. Mehra, MD, Brigham and Woman’s Hospital in Boston.
That debate, he noted, has often focused on insurability, access to coverage, and the merits or shortcomings of a single-payer system. Yet the study suggests outcomes disparities stemming from neighborhood deprivation will not be corrected by improved access to health insurance, a conclusion he finds “startling,” Dr. Mehra said in an interview.
Some proposed explanations for the disparities by race blame unequal access to health care and or variable health insurance coverage, Dr. Morris observed in an interview. But “that may not fully explain the increased risk that we see.”
Black patients followed at Emory University’s advanced HF clinic still have a higher risk of rehospitalization than Whites. “These are patients who have insurance, who are followed by advanced heart failure providers, who are on equal amounts of guideline-recommended medical therapy – and you still see about a 50% higher risk of rehospitalization,” Dr. Morris said, citing data that isn’t part of the current analysis.
“We can say that these patients are certainly able to access care, because they are able to access our emergency room and be taken care of within the hospital setting,” he said. The study controlled for whether health coverage was by private insurance, Medicare, or Medicaid.
Instead, the current analysis points to socioeconomic and environmental factors as a major source of the disparity in 30-day readmissions, Dr. Morris said.
“When patients are discharged from our healthcare systems, they still go back into environments where they don’t have the same resources as patients who live in higher-SDI neighborhoods,” she explained.
For example, “we tell them to eat low-sodium [foods], exercise, eat fresh fruits and vegetables, take their medicines, but the reality is that certain neighborhoods within the United States – and this is much more true for Blacks – make it very difficult to follow those self-care recommendations.”
The analysis included 16,147 Black patients and 14,483 White patients hospitalized with HF within the Emory Healthcare system at least once from 2010-2018, Dr. Morris reported. Compared with Whites, Blacks were younger (63.5 vs 69.1 years) and less likely to be 65 or older (48.9% vs. 66.5%); more likely to be women (53.5% vs. 42.2%), more likely to reside in deprived census tracts and to have diabetes, hypertension, or chronic kidney disease; and had higher comorbidity scores.
In all, 20.6% of Black and 13.5% of White patients were readmitted for HF within 30 days of discharge, for an unadjusted risk ratio of 1.52 (95% CI, 1.44-1.61).
The RR hardly budged, 1.45 (95% CI, 1.37-1.54, P < .001), after adjustment for age, sex, type of insurance, type of HF, vital signs and laboratory values, medical history (diabetes, hypertension, atrial fibrillation, coronary disease, chronic kidney disease, and chronic pulmonary disease), Charlson Comorbidity Index, discharging medical specialty, and hospital location.
The excess in 30-day HF readmissions for Black, compared with White patients climbed from the first to the third neighborhood SDI quartile, the disparity peaking at 8.2 absolute percentage points.
A major criticism of the Hospital Readmissions Reduction Program component of the Affordable Care Act, Dr. Morris said in a Q&A discussion after her presentation, is that it can hold hospitals “responsible for structural inequalities that exist beyond the health care system,” including neighborhood deprivation.
“But public policy makers have to realize that there are certain patients we take care of who don’t have the resources to carry out the therapeutic lifestyle changes that will allow them to live healthy.”
The HRRP’s 30-day HF readmission metric that steers reimbursement “is penalizing health care systems across the United States” with its premise that hospital performance can be measured by 30-day HF readmission rates, Dr. Morris said in an interview.
“The reality is that some of these patients are going to a postdischarge environment that is inherently high risk, and that many of them are going to come back to us within 30 days,” she said. “We would like to make sure that we don’t put excess penalties on health care systems that take care of disproportionate numbers of African Americans in neighborhoods that have fewer resources.”
Dr. Morris and Dr. Ferdinand have disclosed no relevant financial relationships. Dr. Mehra discloses consulting or serving on an advisory board for Abbott, Medtronic, Janssen, Leviticus, NupulseCV, FineHeart, Portola, Bayer, the Baim Institute for Clinical Research, and Mesoblast.
A version of this article originally appeared on Medscape.com.
Patients receiving even top-notch hospital care for heart failure (HF) are, once discharged to home, at higher short-term risk of another HF hospitalization if home is in a socioeconomically deprived neighborhood. That helps explain why Blacks in the United States have a much higher 30-day HF readmission risk than Whites, a disparity that only worsens with the level of neighborhood deprivation, a new analysis suggests.
Some systemic and entrenched socioeconomic inequities that health care providers have little sway over, and which disproportionately affect Black individuals, are independent and robust predictors of worsened HF outcomes, Alanna A. Morris, MD, MSc, Emory University, Atlanta, said during her presentation at the virtual annual scientific meeting of the Heart Failure Society of America.
In a retrospective cohort study, Blacks had a 45% higher risk of 30-day readmission than Whites (P < .001) independent of cardiovascular risk factors, clinical history, comorbidities, type and location of hospital, and type of third-party payer coverage. The analysis included more than 30,000 patients with at least one HF hospitalization at centers in a major metropolitan health system.
The racial disparity widened with worsening socioeconomic deprivation of patients’ residential neighborhoods, that is, with rising quartiles of neighborhood scores on the Social Deprivation Index (SDI).
The SDI, based on U.S. census data, incorporates seven socioeconomic criteria, including household income, education level, employment, and prevalence of rented housing and households that are without a car, single parent, or overcrowded.
There was a 4–percentage point gap in adjusted 30-day readmission rate between Blacks and Whites in the lowest quartile that widened to more than 8 points by the third quartile; the disparity in both the second and fourth quartiles was the same, at about 5.5 percentage points.
A remaining question, Dr. Morris said in an interview, is why the outcomes disparity between Blacks and Whites peaks in the third SDI quartile but drops a bit in the fourth quartile representing the most severe neighborhood deprivation.
“Our hypothesis is that when you look at patients who are the poorest, who live in the most deprived neighborhoods, race may be less of a factor,” she said. Socioeconomic deprivation may have similar consequences for everyone “regardless of race, ethnicity, gender, or other demographic characteristics if you live in a neighborhood that’s highly deprived.”
Based on the current study, “it does appear that increased heart failure incident rates are related to living in deprived neighborhoods, and it raises important clinical and public health concerns that must be addressed,” Keith C. Ferdinand, MD, Tulane University, New Orleans, said as invited discussant after the presentation from Dr. Morris.
“These findings could serve as an aid to policy makers, going forward, in terms of allocating resources for primary health care,” he said. “And it’s important looking at these data and other [data] that we target heart failure patients who reside in deprived neighborhoods before, during, and [after] hospitalization.”
Dr. Morris agreed that policy makers are in a better position to attack the racial disparity in HF readmission rates identified in the study. “This is not a problem that can be fixed within the health care system.”
If the reported interpretation is correct, it could add a twist to the public health care debate in the United States, observed session moderator Mandeep R. Mehra, MD, Brigham and Woman’s Hospital in Boston.
That debate, he noted, has often focused on insurability, access to coverage, and the merits or shortcomings of a single-payer system. Yet the study suggests outcomes disparities stemming from neighborhood deprivation will not be corrected by improved access to health insurance, a conclusion he finds “startling,” Dr. Mehra said in an interview.
Some proposed explanations for the disparities by race blame unequal access to health care and or variable health insurance coverage, Dr. Morris observed in an interview. But “that may not fully explain the increased risk that we see.”
Black patients followed at Emory University’s advanced HF clinic still have a higher risk of rehospitalization than Whites. “These are patients who have insurance, who are followed by advanced heart failure providers, who are on equal amounts of guideline-recommended medical therapy – and you still see about a 50% higher risk of rehospitalization,” Dr. Morris said, citing data that isn’t part of the current analysis.
“We can say that these patients are certainly able to access care, because they are able to access our emergency room and be taken care of within the hospital setting,” he said. The study controlled for whether health coverage was by private insurance, Medicare, or Medicaid.
Instead, the current analysis points to socioeconomic and environmental factors as a major source of the disparity in 30-day readmissions, Dr. Morris said.
“When patients are discharged from our healthcare systems, they still go back into environments where they don’t have the same resources as patients who live in higher-SDI neighborhoods,” she explained.
For example, “we tell them to eat low-sodium [foods], exercise, eat fresh fruits and vegetables, take their medicines, but the reality is that certain neighborhoods within the United States – and this is much more true for Blacks – make it very difficult to follow those self-care recommendations.”
The analysis included 16,147 Black patients and 14,483 White patients hospitalized with HF within the Emory Healthcare system at least once from 2010-2018, Dr. Morris reported. Compared with Whites, Blacks were younger (63.5 vs 69.1 years) and less likely to be 65 or older (48.9% vs. 66.5%); more likely to be women (53.5% vs. 42.2%), more likely to reside in deprived census tracts and to have diabetes, hypertension, or chronic kidney disease; and had higher comorbidity scores.
In all, 20.6% of Black and 13.5% of White patients were readmitted for HF within 30 days of discharge, for an unadjusted risk ratio of 1.52 (95% CI, 1.44-1.61).
The RR hardly budged, 1.45 (95% CI, 1.37-1.54, P < .001), after adjustment for age, sex, type of insurance, type of HF, vital signs and laboratory values, medical history (diabetes, hypertension, atrial fibrillation, coronary disease, chronic kidney disease, and chronic pulmonary disease), Charlson Comorbidity Index, discharging medical specialty, and hospital location.
The excess in 30-day HF readmissions for Black, compared with White patients climbed from the first to the third neighborhood SDI quartile, the disparity peaking at 8.2 absolute percentage points.
A major criticism of the Hospital Readmissions Reduction Program component of the Affordable Care Act, Dr. Morris said in a Q&A discussion after her presentation, is that it can hold hospitals “responsible for structural inequalities that exist beyond the health care system,” including neighborhood deprivation.
“But public policy makers have to realize that there are certain patients we take care of who don’t have the resources to carry out the therapeutic lifestyle changes that will allow them to live healthy.”
The HRRP’s 30-day HF readmission metric that steers reimbursement “is penalizing health care systems across the United States” with its premise that hospital performance can be measured by 30-day HF readmission rates, Dr. Morris said in an interview.
“The reality is that some of these patients are going to a postdischarge environment that is inherently high risk, and that many of them are going to come back to us within 30 days,” she said. “We would like to make sure that we don’t put excess penalties on health care systems that take care of disproportionate numbers of African Americans in neighborhoods that have fewer resources.”
Dr. Morris and Dr. Ferdinand have disclosed no relevant financial relationships. Dr. Mehra discloses consulting or serving on an advisory board for Abbott, Medtronic, Janssen, Leviticus, NupulseCV, FineHeart, Portola, Bayer, the Baim Institute for Clinical Research, and Mesoblast.
A version of this article originally appeared on Medscape.com.
More data on impact of corticosteroids on COVID-19 mortality in patients with COPD
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
FROM LANCET RESPIRATORY MEDICINE