User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
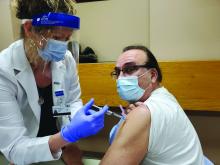
Don’t discontinue osteoporosis meds for COVID-19 vaccines, expert guidance says
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
Inpatient sodium imbalances linked to adverse COVID-19 outcomes
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Delay surgery by 7 weeks after COVID-19 diagnosis, study shows
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Nearly 20% of lupus patients have severe infection in first decade after diagnosis
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
FROM RHEUMATOLOGY
Bone loss common in kidney stone patients, yet rarely detected
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Myth busting: SARS-CoV-2 vaccine
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
MYTH: I shouldn’t get the vaccine because of potential long-term side effects
We know that 68 million people in the United States and 244 million people worldwide have already received messenger RNA (mRNA) SARS-CoV-2 vaccines (Pfizer/BioNTech and Moderna). So for the short-term side effects we already know more than we would know about most vaccines.
What about the long-term side effects? There are myths that these vaccines somehow could cause autoimmunity. This came from three publications where the possibility of mRNA vaccines to produce autoimmunity was brought up as a discussion point.1-3 There was no evidence given in these publications, it was raised only as a hypothetical possibility.
There’s no evidence that mRNA or replication-defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) produce autoimmunity. Moreover, the mRNA and replication-defective DNA, once it’s inside of the muscle cell, is gone within a few days. What’s left after ribosome processing is the spike (S) protein as an immunogen. We’ve been vaccinating with proteins for 50 years and we haven’t seen autoimmunity.
MYTH: The vaccines aren’t safe because they were developed so quickly
These vaccines were developed at “warp speed” – that doesn’t mean they were developed without all the same safety safeguards that the Food and Drug Administration requires. The reason it happened so fast is because the seriousness of the pandemic allowed us, as a community, to enroll the patients into the studies fast. In a matter of months, we had all the studies filled. In a normal circumstance, that might take 2 or 3 years. And all of the regulatory agencies – the National Institutes of Health, the FDA, the Centers for Disease Control and Prevention – were ready to take the information and put a panel of specialists together and immediately review the data. No safety steps were missed. The same process that’s always required of phase 1, of phase 2, and then at phase 3 were accomplished.
The novelty of these vaccines was that they could be made so quickly. Messenger RNA vaccines can be made in a matter of days and then manufactured in a matter of 2 months. The DNA vaccines has a similar timeline trajectory.
MYTH: There’s no point in getting the vaccines because we still have to wear masks
Right now, out of an abundance of caution, until it’s proven that we don’t have to wear masks, it’s being recommended that we do so for the safety of others. Early data suggest that this will be temporary. In time, I suspect it will be shown that, after we receive the vaccine, it will be shown that we are not contagious to others and we’ll be able to get rid of our masks.
MYTH: I already had COVID-19 so I don’t need the vaccine
Some people have already caught the SARS-CoV-2 virus that causes this infection and so they feel that they’re immune and they don’t need to get the vaccine. Time will tell if that’s the case. Right now, we don’t know for sure. Early data suggest that a single dose of vaccine in persons who have had the infection may be sufficient. Over time, what happens in the vaccine field is we measure the immunity from the vaccine, and from people who’ve gotten the infection, and we find that there’s a measurement in the blood that correlates with protection. Right now, we don’t know that correlate of protection level. So, out of an abundance of caution, it’s being recommended that, even if you had the disease, maybe you didn’t develop enough immunity, and it’s better to get the vaccine than to get the illness a second time.
MYTH: The vaccines can give me SARS-CoV-2 infection
The new vaccines for COVID-19, released under emergency use Authorization, are mRNA and DNA vaccines. They are a blueprint for the Spike (S) protein of the virus. In order to become a protein, the mRNA, once it’s inside the cell, is processed by ribosomes. The product of the ribosome processing is a protein that cannot possibly cause harm as a virus. It’s a little piece of mRNA inside of a lipid nanoparticle, which is just a casing to protect the mRNA from breaking down until it’s injected in the body. The replication defective DNA vaccines (AstraZeneca/Oxford and Johnson & Johnson) are packaged inside of virus cells (adenoviruses). The DNA vaccines involve a three-step process:
- 1. The adenovirus, containing replication-defective DNA that encodes mRNA for the Spike (S) protein, is taken up by the host cells where it must make its way to the nucleus of the muscle cell.
- 2. The DNA is injected into the host cell nucleus and in the nucleus the DNA is decoded to an mRNA.
- 3. The mRNA is released from the nucleus and transported to the cell cytoplasm where the ribosomes process the mRNA in an identical manner as mRNA vaccines.
MYTH: The COVID-19 vaccines can alter my DNA
The mRNA and replication-defective DNA vaccines never interact with your DNA. mRNA vaccines never enter the nucleus. Replication-defective DNA vaccines cannot replicate and do not interact with host DNA. The vaccines can’t change your DNA.
Here is a link to YouTube videos I made on this topic: https://youtube.com/playlist?list=PLve-0UW04UMRKHfFbXyEpLY8GCm2WyJHD.
Here is a photo of me receiving my first SARS-CoV-2 shot (Moderna) in January 2021. I received my second shot in February. I am a lot less anxious. I hope my vaccine card will be a ticket to travel in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to report.
References
1. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
2. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
3. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Joint pain in patients with hemophilia may be neuropathic
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
FROM EAHAD 2021
CDC data strengthen link between obesity and severe COVID
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Officials have previously linked being overweight or obese to a greater risk for more severe COVID-19. A report today from the U.S. Centers for Disease Control and Prevention adds numbers and some nuance to the association.
Data from nearly 150,000 U.S. adults hospitalized with COVID-19 nationwide indicate that risk for more severe disease outcomes increases along with body mass index (BMI). The risk of COVID-19–related hospitalization and death associated with obesity was particularly high among people younger than 65.
“As clinicians develop care plans for COVID-19 patients, they should consider the risk for severe outcomes in patients with higher BMIs, especially for those with severe obesity,” the researchers note. They add that their findings suggest “progressively intensive management of COVID-19 might be needed for patients with more severe obesity.”
People with COVID-19 close to the border between a healthy and overweight BMI – from 23.7 kg/m2 to 25.9 kg/m2 – had the lowest risks for adverse outcomes.
The study was published online today in Morbidity and Mortality Weekly Report.
Greater need for critical care
The risk of ICU admission was particularly associated with severe obesity. For example, those with a BMI in the 40-44.9 kg/m2 category had a 6% increased risk, which jumped to 16% higher among those with a BMI of 45 or greater.
Compared to people with a healthy BMI, the need for invasive mechanical ventilation was 12% more likely among overweight adults with a BMI of 25-29.2. The risked jumped to 108% greater among the most obese people, those with a BMI of 45 or greater, lead CDC researcher Lyudmyla Kompaniyets, PhD, and colleagues reported.
Moreover, the risks for hospitalization and death increased in a dose-response relationship with obesity.
For example, risks of being hospitalized were 7% greater for adults with a BMI between 30 and 34.9 and climbed to 33% greater for those with a BMI of 45. Risks were calculated as adjusted relative risks compared with people with a healthy BMI between 18.5 and 24.9.
Interestingly, being underweight was associated with elevated risk for COVID-19 hospitalization as well. For example, people with a BMI of less than 18.5 had a 20% greater chance of admission vs. people in the healthy BMI range. Unknown underlying medical conditions or issues related to nutrition or immune function could be contributing factors, the researchers note.
Elevated risk of dying
The risk of death in adults with obesity ranged from 8% higher in the 30-34.9 range up to 61% greater for those with a BMI of 45.
Chronic inflammation or impaired lung function from excess weight are possible reasons that higher BMI imparts greater risk, the researchers note.
The CDC researchers evaluated 148,494 adults from 238 hospitals participating in PHD-SR database. Because the study was limited to people hospitalized with COVID-19, the findings may not apply to all adults with COVID-19.
Another potential limitation is that investigators were unable to calculate BMI for all patients in the database because about 28% of participating hospitals did not report height and weight.
The study authors had no relevant financial relationships to disclose.
A version of this article first appeared on Medscape.com.
Novel lupus therapies take center stage
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
It’s been a banner year for treatment advances in systemic lupus erythematosus (SLE), with two drugs gaining approval for lupus nephritis while other promising molecules with novel mechanisms of action advanced smartly through the developmental pipeline, speakers agreed at the 2021 Rheumatology Winter Clinical Symposium.
“I think the most important thing in rheumatology in the last year is where we are now with lupus. With two drugs being approved for lupus nephritis, I think that’s really huge as we talk about treat-to-target,” said Alvin F. Wells, MD, PhD, a rheumatologist in Franklin, Wisc.
Martin Bergman, MD, concurred.
“Lupus has been blowing up in the past year. We have two new medications for lupus nephritis, we have two or three new mechanisms of action for therapy. I think that was one of the biggest things in rheumatology in the past year,” said Dr. Bergman, a rheumatologist at Drexel University in Philadelphia and in private practice in Ridley Park, Pa.
Together with Roy Fleischmann, MD, Dr. Wells spotlighted promising new molecules for the treatment of SLE, giant cell arteritis, vasculitis, rheumatoid arthritis, and osteoarthritis.
SLE
The two drugs approved in recent months specifically for lupus nephritis are voclosporin (Lupkynis) and belimumab (Benlysta), which has been approved for lupus for a decade. Voclosporin, an oral calcineurin inhibitor, is a modification of cyclosporine offering significant advantages over the older drug: It’s more potent, requires no dose titration, has a better safety profile, and is metabolized more quickly.
“A safer and easier-to-use calcineurin inhibitor is going to be huge,” Dr. Wells predicted.
Up for Food and Drug Administration review in the coming year on the basis of the positive phase 3 TULIP-1 and TULIP-2 trials is anifrolumab, a monoclonal antibody that binds to the type 1 interferon receptor subunit 1d. At 52 weeks in the pooled analysis, one or more SLE flares occurred in 33.6% of patients on anifrolumab and 42.9% of placebo-treated controls.
“This is not a blockbuster, but it’s a worthwhile addition, like belimumab,” according to Dr. Fleischmann, a rheumatologist at the University of Texas, Dallas.
Dr. Wells concurred, with a reservation: In a subgroup analysis of the TULIP trials, anifrolumab wasn’t significantly better than placebo in black patients, who tend to have more severe and tough-to-treat renal disease.
“Anifrolumab doesn’t look as effective as some other agents, and I’d be disinclined to give it to my black patients,” the rheumatologist said.
Dr. Fleischmann was far more enthusiastic about obinutuzumab (Gazyva), a humanized anti-CD20 monoclonal antibody already approved for the treatment of chronic lymphocytic leukemia and follicular lymphoma.
“It’s an anti-CD20, like rituximab. But it’s better than rituximab, it’s much more effective,” he said.
He pointed to the phase 2 NOBILITY trial, in which 125 patients with class III/IV lupus nephritis were randomized to a 1,000-mg infusion of obinutuzumab or placebo at weeks 0, 2, 24, and 26 and followed for 2 years. The complete renal response rate at 104 weeks in the obinutuzumab group was 41% and the partial renal response rate was 13%, compared to 23% and 6% in controls. The obinutuzumab group also did significantly better in terms of improvement in complement levels, double-stranded DNA, and estimated glomerular filtration rate. All this was accomplished even though the reduction in peripheral B cells dropped from 93% at week 24 to just 16% at week 104. This suggests that tissue levels of B cells in the kidney, joints, and skin may be more important than circulating B cell levels.
“This looks like a very promising agent for patients with lupus nephritis,” Dr. Wells said. “The fact that they got this long-term effect for 2 years with just four infusions is really impressive.”
Another promising drug is iberdomide, an oral modulator of the E3 ubiquitin ligase complex which decreases plasmacytoid dendritic cells and B cells while increasing T regulatory cells. In a phase 2b clinical trial in 288 patients with active SLE, all on background standard-of-care therapy, a 4-point or greater reduction in the SLE Responder Index (SRI-4) at week 24 was achieved in 54.3% of the group on iberdomide at 0.45 mg/day, a significantly better result than the 34.9% rate with placebo. This absolute 19.4% difference was even greater in the subgroup of patients with a high baseline level of the transcription factor Aiolos, where the absolute improvement over placebo was 32.9%. Similarly, the benefit of iberdomide was also enhanced in patients with a high baseline level of type 1 interferon, where the absolute difference was 26.8%. This raises the prospect that a bioassay could be developed to predict the likelihood of a favorable clinical response to the drug. Iberdomide was well tolerated, with fewer severe adverse events than in the control group.
A humanized monoclonal antibody known for now as BIIB059 demonstrated efficacy and was well tolerated in the phase 2 LILAC trial. BIIB059 binds to blood dendritic cell antigen 2 (BDCA2), a receptor specific to plasmacytoid dendritic cells, resulting in decreased production of type 1 interferon and other inflammatory cytokines. The LILAC trial included 132 SLE patients with active arthritis and skin disease who received subcutaneous injections of BIIB059 at 450 mg or placebo every 4 weeks, with an extra dose at week 2. The primary endpoint was met, with an absolute 15-joint reduction in the total number of tender or swollen joints from baseline to week 24 in the BIIB059 group, compared to an 11.6-joint reduction with placebo. In addition, the likelihood of an SRI-4 response at week 24 was 3.49-fold greater with BIIB059 than with placebo.
Dr. Wells noted that the BIIB059 group showed continued improvement from week 12 to week 24, unlike the response pattern seen with many biologics for rheumatoid arthritis, where a plateau is reached by 8-12 weeks.
Vasculitis
The positive results for the C5a receptor inhibitor avacopan for treatment of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis in the phase-3 ADVOCATE trial have been hailed by some rheumatologists as a major breakthrough, but Dr. Fleischmann isn’t so sure.
The trial randomized 331 patients to oral avacopan at 30 mg twice daily or oral prednisone, with all patients on either cyclophosphamide or rituximab. Avacopan was noninferior to prednisone in terms of remission at week 26, but superior to prednisone for sustained taper at week 52. The rate of serious adverse events was 45.1% with prednisone and 42.2% in the avacopan arm.
“This is a drug that’s going to be much, much more expensive than prednisone. There were people in our group who were ecstatic that this drug is going to come, but how much it’s going to be used, I don’t know,” Dr. Fleischmann said.
Dr. Wells said cost-benefit analyses will be needed in order to learn if avacopan’s anticipated high sticker price is offset by the cost of serious corticosteroid side effects such as avascular necrosis.
Giant cell arteritis
Mavrilimumab is a human monoclonal antibody that inhibits human granulocyte macrophage colony stimulating factor receptor alpha. It demonstrated impressive efficacy in a phase 2, double-blind, randomized, placebo-controlled trial conducted in 70 patients with biopsy-confirmed giant cell arteritis. Participants were on corticosteroids until they went into remission and were then randomized to mavrilimumab or placebo, with the steroids stopped. By week 26, 19% of patients in the mavrilimumab arm had flared, as compared to 46.4% of controls.
“This is a game changer,” Dr. Wells declared. “I struggle with these patients because I can’t get the IL-6 drugs approved for them. I need something else.”
Dr. Fleischmann has a good idea how he’ll use mavrilimumab, if it wins approval: “I think this is clearly a drug you would use in a patient you can’t get off steroids and you’re having all the steroid toxicity. I don’t know that you’d use it right away.”
Osteoarthritis
Dr. Fleischmann predicted that tanezumab, a monoclonal antibody directed against nerve growth factor, will win FDA approval in 2021 for the treatment of osteoarthritis pain in patients with an inadequate response or intolerance to standard-of-care NSAIDs and opioids. But he cautioned his colleagues not to expect too much from the biologic, which has a long and checkered developmental history.
“It works better than placebo. It does not work better than an NSAID or an opioid. So it should be reasonable in patients who cannot take an NSAID or cannot or will not take an opioid,” he said.
There are safety issues to be aware of with tanezumab, he added: clinically significant increased risks of peripheral neuropathy and joint space narrowing.
Rheumatoid arthritis
Dr. Wells thought one of the most interesting novel therapies for RA in the past year didn’t involve a pharmaceutical, but rather noninvasive auricular branch stimulation of the vagus nerve. He cited an open-label, 12-week, uncontrolled study in 27 patients with active RA who wore an ear clip for vagal nerve stimulation for 12 weeks. The mean Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP) – the primary study endpoint – improved from 6.30 at baseline to 3.76 at week 12. The number of tender joints dropped from 12.17 to 4.7, while the swollen joint count went from 7.0 to 3.44. Pain scores improved from 75.23 to 43.3. Scores on the Health Assessment Questionnaire Disability Index improved from 1.59 to 1.05. There was no significant change in CRP. All in all, a modest clinical effect achieved noninvasively.
“The thing that did it for me was the effect on MRI from baseline: decreased synovitis, osteitis, and bone erosion scores,” Dr. Wells said. “This is noninvasive, so patients who want to do medical marijuana or CBD can put an earring on their auricular nerve.”
Dr. Fleischmann scoffed. “An open-label study, 27 patients? Let me see the real study,” he quipped.
Dr. Fleischmann reported receiving clinical trial research grants from and serving as a consultant to more than a dozen pharmaceutical companies. Dr. Wells serves as a consultant to MiCare Path.
FROM RWCS 2021