User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Low preconception complement levels linked to adverse pregnancy outcomes in antiphospholipid syndrome
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
Abaloparatide significantly reduced fractures, increased BMD in women at high fracture risk
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).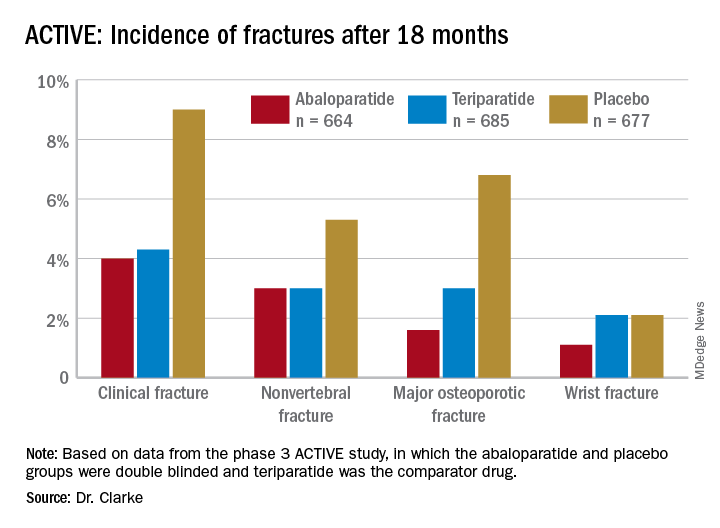
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
FROM NAMS 2021
FDA approves avacopan for rare ANCA autoimmune disease
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
U.S. regulators approved avacopan (Tavneos) for a rare immune disorder after receiving additional information to address concerns raised about the drug that were previously discussed at a public meeting in May.
ChemoCentryx, the drug’s manufacturer, today announced that the U.S. Food and Drug Administration approved the drug as an adjunctive treatment for severe active antineutrophil cytoplasmic autoantibody–associated vasculitis (also known as ANCA-associated vasculitis or ANCA vasculitis).
This systemic disease results from overactivation of the complement system, leading to inflammation and eventual destruction of small blood vessels. This can lead to organ damage and failure, with the kidney as the major target, said the company in a statement.
The avacopan approval was based in large part on the results of the ADVOCATE trial, which were highlighted in a February 2021 editorial in the New England Journal of Medicine , titled “Avacopan – Time to replace glucocorticoids?” But the FDA-approved indication for avacopan is as an adjunctive treatment of adult patients with severe active ANCA-associated vasculitis (granulomatosis with polyangiitis [GPA] and microscopic polyangiitis [MPA]) in combination with standard therapy including glucocorticoids. “Tavneos does not eliminate glucocorticoid use,” the label states.
The ADVOCATE trial was a global, randomized, double-blind, active-controlled, double-dummy phase 3 trial of 330 patients with ANCA-associated vasculitis conducted in 20 countries, ChemoCentryx said. Participants were randomly assigned to receive either rituximab or cyclophosphamide (followed by azathioprine/mycophenolate) and either avacopan or study-supplied oral prednisone.
Subjects in both treatment groups could also receive nonprotocol glucocorticoids as needed. The study met its primary endpoints of disease remission at 26 weeks and sustained remission at 52 weeks, as assessed by the Birmingham Vasculitis Activity Score (BVAS), ChemoCentryx said. Common adverse reactions among study participants included nausea, headache, hypertension, diarrhea, vomiting, rash, fatigue, upper abdominal pain, dizziness, blood creatinine increase, and paresthesia.
In the ChemoCentryx statement, Peter A. Merkel, MD, MPH, a consultant to the company and the chief of rheumatology at the University of Pennsylvania, Philadelphia, called the avacopan clearance a “first-in-a-decade approval of a medicine for ANCA-associated vasculitis.”
“Patients will now have access to a new class of medication that provides beneficial effects for the treatment of ANCA-associated vasculitis,” Dr. Merkel said.
In reviewing the avacopan application, the FDA noted that the medicine is intended to treat “a rare and serious disease associated with high morbidity and increased mortality.”
“It is also a disease with high unmet need for new therapies,” the FDA staff said in a review of the ChemoCentryx application for approval of avacopan, which was posted online ahead of a meeting this past May.
Previous FDA concerns
In that review, FDA staff made public various concerns about the evidence used in seeking approval of the medicine. The FDA staff said there were “substantial uncertainties around the phase 3 study design and results, raising questions about the adequacy of this single trial to inform the benefit-risk assessment.”
Members of the FDA’s Arthritis Advisory Committee voted 10-8 on May 6 on a question of whether the risk-benefit profile of avacopan is adequate to support approval. The panel also voted 9-9 on whether the efficacy data support approval of avacopan, and 10-8 that the safety profile of avacopan is adequate to support approval.
ChemoCentryx in July said it filed an amendment to its new drug application (NDA) for avacopan. This appears to have answered regulators’ questions about the drug.
On a call with analysts Friday, ChemoCentryx officials outlined a marketing strategy for avacopan, with efforts focused on reaching influential rheumatologists and nephrologists. The company will set a U.S. wholesale acquisition cost for the drug of about $150,000-$200,000 a patient, in keeping with the range of prices often seen for orphan drugs. ChemoCentryx said it intends to offer financial support programs for the medicine.
ChemoCentryx said avacopan is also approved for the treatment of microscopic polyangiitis and granulomatosis with polyangiitis (the two main forms of ANCA-associated vasculitis) in Japan. The regulatory decision in Europe is expected by the end of this year.
A version of this article first appeared on Medscape.com.
Retraining the brain may eliminate chronic back pain
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Psychological therapy that changes an individual’s beliefs about pain not only provides lasting chronic pain relief but also alters brain regions related to pain generation, new research shows.
In the first randomized controlled test of pain-reprocessing therapy (PRT), two-thirds of patients with chronic back pain (CBP) who received 4 weeks of PRT were pain free or nearly pain free afterward – and for most patients, relief was maintained for 1 year, the researchers found.
“Primary chronic back pain can be dramatically reduced or even eliminated by psychological treatment focused on changing how threatening we perceive the pain to be,” first author Yoni Ashar, PhD, department of psychiatry, Weill Cornell Medicine, New York, said in an interview.
“ given that large reductions in pain have rarely been observed in studies that tested psychological therapies for chronic back pain.
The study was published online Sept. 29, 2021, in JAMA Psychiatry.
Rethinking pain
CBP is a leading cause of disability, and treatment is often ineffective. In about 85% of cases of primary CBP, a definitive cause of the pain can’t be identified. In these cases, fear, avoidance, and beliefs that pain indicates injury may contribute to ongoing CBP.
PRT educates patients about the role of the brain in generating chronic pain; helps them reappraise their pain as they engage in movements that they had been afraid to undertake; and helps them address emotions that may exacerbate pain.
The study included 151 adults (54% women; mean age, 41 years) who had primary CBP of low to moderate severity (mean pain intensity, 4 of 10) for an average of 10 years.
A total of 50 participants were randomly allocated to undergo PRT (one telehealth session with a physician and eight PRT sessions over 4 weeks), 51 to receive placebo (subcutaneous saline injection in the back), and 50 to continue their routine, usual ongoing care.
Large group differences in pain were observed after treatment. The mean pain score was 1.18 in the PRT group, 2.84 in the placebo group, and 3.13 in the usual-care group. Hedges’ g was –1.14 for PRT versus placebo and –1.74 for PRT versus usual care (P < .001).
Two-thirds (66%) of adults in the PRT group were pain free or nearly pain free following treatment (pain-intensity score of 0 or 1 out of 10), compared with 20% of those in the placebo group and 10% of those who received usual care.
Treatment effects were maintained at 1-year follow-up. The mean pain score was 1.51 in the PRT group, 2.79 in the placebo group, and 3.00 in the usual-care group. Neither age nor sex moderated the effect of PRT on pain intensity.
Retraining the brain
The researchers said the effects of PRT on pain were mediated by lessening the belief that pain indicates tissue damage. Of note, PRT also reduced experimentally evoked back pain and spontaneous pain during functional MRI, with large effect sizes.
“The idea is that by thinking about the pain as safe rather than threatening, patients can alter the brain networks reinforcing the pain, and neutralize it,” Dr. Ashar said in a news release.
The authors noted that study participants were relatively well educated and active. The participants reported having longstanding low to moderate pain and disability at baseline.
The physician and therapists were experts in delivering PRT. Future studies should test generalizability to other patient populations, therapists, and treatment contexts.
“Our clinical experience shows that PRT is effective for other primary chronic pain conditions as well,” said Dr. Ashar, including primary knee pain and tension headache.
Restoring function
Commenting on the findings, Shaheen E. Lakhan, MD, PhD, neurologist and pain specialist in Newton, Mass., said he has long experience using psychological approaches to address pain, with good results.
“Imagine telling a person suffering from decades of chronic pain that your pain is all in your head. I’ve done that for years as a board-certified pain physician managing only the most severe and debilitating forms of pain. When used to ground brain retraining, I could ultimately restore function to people living with chronic pain,” Dr. Lakhan said.
“The statement is true – the brain ultimately processes signals from throughout the body, forms the perception of pain, and links it to emotional brain centers, among others. Pain is an important survival mechanism so that when your body is at threat of injury, you protect yourself from further damage and withdraw. The problem lies when pain outlasts its welcome and chronifies,” said Dr. Lakhan, senior vice president of research and development of Click Therapeutics in Boston.
The investigators in this study “eloquently prove” that with 4 weeks of PRT, patients can learn that chronic pain is largely a “brain-generated false alarm and that constantly affirming this truth can actually reduce or eliminate it,” Dr. Lakhan said.
“Further, the brain areas implicated with pain are calmed after going through the therapy to both resting pain and pain induced by extending the back,” he noted.
“Pain-reprocessing therapy can improve the lives of chronic [pain patients] who have low to moderate levels of pain and disability; however, much work needs to be done to make this scalable and universally available and covered by insurers as a treatment modality,” Dr. Lakhan added.
He cautioned that he has not seen therapies such as this work when there is significant depression, withdrawal, or lack of control over one’s situation such that one behaves in a helpless manner – “a terrible state of mind called learned helplessness.”
The study was funded by the National Institutes of Health, the National Center for Advancing Translational Sciences, the Radiological Society of North America, the German Research Foundation, the Psychophysiologic Disorders Association, the Foundation for the Study of the Therapeutic Encounter, and community donations. Dr. Ashar received grants from the National Institutes of Health during the conduct of the study and personal fees from UnitedHealth Group, Lin Health, Pain Reprocessing Therapy Center, and Mental Health Partners of Boulder County outside the submitted work. Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Major insurers running billions of dollars behind on payments to hospitals and doctors
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Taper, withdrawal of RA meds tested in real-life randomized trial setting
About half of patients with rheumatoid arthritis who taper or stop disease-modifying antirheumatic drugs (DMARDs) retain stable remission after 12 months, and a majority of those who do relapse regain remission when back on their original treatments, according to data from the open-label, randomized Rheumatoid Arthritis in Ongoing Remission (RETRO) study.
“Currently, 40%-50% of patients with rheumatoid arthritis reach stable remission,” as a result of factors including earlier diagnosis and a wider range of treatments, Koray Tascilar, MD, of Friedrich Alexander University Erlangen-Nuremberg (Germany) and colleagues wrote in their publication of the RETRO trial results in Lancet Rheumatology.
Previous studies have suggested that patients with RA in sustained remission may be able to taper or withdraw treatment, but data from randomized trials are limited, the researchers said.
In the RETRO trial, researchers compared three strategies for RA patients in remission, which was defined as <2.6 on the 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR). They randomized 100 adults to continue DMARDs and glucocorticoids, 102 to taper DMARDs and glucocorticoids to half their prior doses, and 101 to reduce the doses to half for 6 months and then stop DMARDs. Patients were enrolled between May 26, 2010, and May 29, 2018, from 14 treatment centers in Germany. The final analysis included 282 patients; 92 in the continuation group, 93 in the taper group, and 96 in the stop group. The mean age of the patients was 56.5 years, and 59% were women. The mean duration of RA was 7.4 years, and the mean duration of remission was 20 months.
Overall, at 12 months, 61% of the patients remained in remission without relapse; 81.2% of the continuation group, 58.6% of the taper group, and 43.3% of the stop group. Relapses occurred in 17%, 43%, and 55% of patients in the continuation, taper, and stop groups, respectively. The median times to relapse in the three groups were 30.6 weeks, 24.3 weeks, and 26.1 weeks, respectively.
Most of the relapses occurred between weeks 24 and 36 after stopping treatment, the researchers wrote. Corresponding hazard ratios for relapse were 3.02 for the taper group and 4.34 for the stop group, compared with the continuation group. In comparison to continuing treatment, the number needed to treat for one more relapse to occur during the 12-month observation period was four for tapering and three for stopping, they noted.
The study protocol called for a return to baseline treatment for any patients who relapsed in the taper and stop groups, and most patients who relapsed regained remission after restarting their baseline treatments. Among patients who had a follow-up visit after a relapse, 10 (63%) of 16 patients in the continuation group reachieved remission before the end of the study, as did 21 (62%) of 34 in the taper group and 35 (76%) of 46 in the stop group.
The most common treatments at baseline were methotrexate (76%) and tumor necrosis factor (TNF) inhibitors (32%).
The researchers also identified several baseline characteristics associated with relapse. Overall, relapse occurred more often in biologic DMARD users than in participants treated with other drugs, more often in women than men, and more often in those with a longer disease duration, higher baseline DAS28-ESR and Health Assessment Questionnaire scores, and in those who were positive for rheumatoid factor or anti–citrullinated protein antibodies.
A total of 38 serious adverse events occurred in 29 participants during the study period, but none were deemed treatment related, and none led to study withdrawal.
The study findings were limited by several factors including the lack of masking of patients and assessors and potential underestimation of disease activity, the researchers noted. Also, the study did not include radiographic data that might have been used to confirm progression; however, such data could have produced a null result and were not feasible in the study population, they wrote.
“If RETRO had been a trial to test the superiority of 100% dose continuation, compared with tapering plus rescue treatment or stopping plus rescue treatment, we would not be able to show that continuation is superior to tapering or stopping,” the researchers noted.
The study results support “an increasingly dynamic management approach in patients with rheumatoid arthritis in stable remission,” given the changing nature of RA management, that may help reduce overtreatment in many RA patients, the researchers concluded. “Furthermore, the observation that most of the patients regained remission after reintroduction of antirheumatic treatments is helpful with regard to the benefit-risk aspect of treatment reduction.”
Real-world setting serves as starting point
The RETRO study is unique in that it tried to reflect a real-life setting by enrolling patients on baseline treatment with combinations of conventional synthetic DMARDs and biologic DMARDs seen in clinical practice rather than only patients taking biologic DMARDs – primarily TNF inhibitors – as done in previous studies of tapering and stopping DMARDs, wrote Catherine L. Hill, MD, of the University of Adelaide (Australia), in an accompanying editorial. It is also “used a simple, pragmatic one-size-fits-all treatment-tapering strategy,” she wrote.
However, she emphasized that answers are needed to questions about what relapse rates are acceptable, what duration of treatment-free time is ideal, and whether benefits to the patient outweigh risks.
Dr. Hill also highlighted the issue of identifying patients who are appropriate candidates for tapering or withdrawal. Stricter remission criteria may not be feasible in routine practice, and so “the development of algorithms to guide patient selection is likely to be the most practical way forward for clinicians and patients,” she wrote.
“Contemplation of treatment tapering or discontinuation in some patients with rheumatoid arthritis is remarkable and a measure of how far treatments have advanced,” Dr. Hill wrote. “However, further work to address outstanding questions on who should taper and how best to do it is still required,” she concluded.
The study received no outside funding. Lead author Dr. Tascilar disclosed lecture fees from Gilead and Union Chimique Belge; several coauthors disclosed relationships with multiple companies outside the current study. Dr. Hill had no financial conflicts to disclose.
About half of patients with rheumatoid arthritis who taper or stop disease-modifying antirheumatic drugs (DMARDs) retain stable remission after 12 months, and a majority of those who do relapse regain remission when back on their original treatments, according to data from the open-label, randomized Rheumatoid Arthritis in Ongoing Remission (RETRO) study.
“Currently, 40%-50% of patients with rheumatoid arthritis reach stable remission,” as a result of factors including earlier diagnosis and a wider range of treatments, Koray Tascilar, MD, of Friedrich Alexander University Erlangen-Nuremberg (Germany) and colleagues wrote in their publication of the RETRO trial results in Lancet Rheumatology.
Previous studies have suggested that patients with RA in sustained remission may be able to taper or withdraw treatment, but data from randomized trials are limited, the researchers said.
In the RETRO trial, researchers compared three strategies for RA patients in remission, which was defined as <2.6 on the 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR). They randomized 100 adults to continue DMARDs and glucocorticoids, 102 to taper DMARDs and glucocorticoids to half their prior doses, and 101 to reduce the doses to half for 6 months and then stop DMARDs. Patients were enrolled between May 26, 2010, and May 29, 2018, from 14 treatment centers in Germany. The final analysis included 282 patients; 92 in the continuation group, 93 in the taper group, and 96 in the stop group. The mean age of the patients was 56.5 years, and 59% were women. The mean duration of RA was 7.4 years, and the mean duration of remission was 20 months.
Overall, at 12 months, 61% of the patients remained in remission without relapse; 81.2% of the continuation group, 58.6% of the taper group, and 43.3% of the stop group. Relapses occurred in 17%, 43%, and 55% of patients in the continuation, taper, and stop groups, respectively. The median times to relapse in the three groups were 30.6 weeks, 24.3 weeks, and 26.1 weeks, respectively.
Most of the relapses occurred between weeks 24 and 36 after stopping treatment, the researchers wrote. Corresponding hazard ratios for relapse were 3.02 for the taper group and 4.34 for the stop group, compared with the continuation group. In comparison to continuing treatment, the number needed to treat for one more relapse to occur during the 12-month observation period was four for tapering and three for stopping, they noted.
The study protocol called for a return to baseline treatment for any patients who relapsed in the taper and stop groups, and most patients who relapsed regained remission after restarting their baseline treatments. Among patients who had a follow-up visit after a relapse, 10 (63%) of 16 patients in the continuation group reachieved remission before the end of the study, as did 21 (62%) of 34 in the taper group and 35 (76%) of 46 in the stop group.
The most common treatments at baseline were methotrexate (76%) and tumor necrosis factor (TNF) inhibitors (32%).
The researchers also identified several baseline characteristics associated with relapse. Overall, relapse occurred more often in biologic DMARD users than in participants treated with other drugs, more often in women than men, and more often in those with a longer disease duration, higher baseline DAS28-ESR and Health Assessment Questionnaire scores, and in those who were positive for rheumatoid factor or anti–citrullinated protein antibodies.
A total of 38 serious adverse events occurred in 29 participants during the study period, but none were deemed treatment related, and none led to study withdrawal.
The study findings were limited by several factors including the lack of masking of patients and assessors and potential underestimation of disease activity, the researchers noted. Also, the study did not include radiographic data that might have been used to confirm progression; however, such data could have produced a null result and were not feasible in the study population, they wrote.
“If RETRO had been a trial to test the superiority of 100% dose continuation, compared with tapering plus rescue treatment or stopping plus rescue treatment, we would not be able to show that continuation is superior to tapering or stopping,” the researchers noted.
The study results support “an increasingly dynamic management approach in patients with rheumatoid arthritis in stable remission,” given the changing nature of RA management, that may help reduce overtreatment in many RA patients, the researchers concluded. “Furthermore, the observation that most of the patients regained remission after reintroduction of antirheumatic treatments is helpful with regard to the benefit-risk aspect of treatment reduction.”
Real-world setting serves as starting point
The RETRO study is unique in that it tried to reflect a real-life setting by enrolling patients on baseline treatment with combinations of conventional synthetic DMARDs and biologic DMARDs seen in clinical practice rather than only patients taking biologic DMARDs – primarily TNF inhibitors – as done in previous studies of tapering and stopping DMARDs, wrote Catherine L. Hill, MD, of the University of Adelaide (Australia), in an accompanying editorial. It is also “used a simple, pragmatic one-size-fits-all treatment-tapering strategy,” she wrote.
However, she emphasized that answers are needed to questions about what relapse rates are acceptable, what duration of treatment-free time is ideal, and whether benefits to the patient outweigh risks.
Dr. Hill also highlighted the issue of identifying patients who are appropriate candidates for tapering or withdrawal. Stricter remission criteria may not be feasible in routine practice, and so “the development of algorithms to guide patient selection is likely to be the most practical way forward for clinicians and patients,” she wrote.
“Contemplation of treatment tapering or discontinuation in some patients with rheumatoid arthritis is remarkable and a measure of how far treatments have advanced,” Dr. Hill wrote. “However, further work to address outstanding questions on who should taper and how best to do it is still required,” she concluded.
The study received no outside funding. Lead author Dr. Tascilar disclosed lecture fees from Gilead and Union Chimique Belge; several coauthors disclosed relationships with multiple companies outside the current study. Dr. Hill had no financial conflicts to disclose.
About half of patients with rheumatoid arthritis who taper or stop disease-modifying antirheumatic drugs (DMARDs) retain stable remission after 12 months, and a majority of those who do relapse regain remission when back on their original treatments, according to data from the open-label, randomized Rheumatoid Arthritis in Ongoing Remission (RETRO) study.
“Currently, 40%-50% of patients with rheumatoid arthritis reach stable remission,” as a result of factors including earlier diagnosis and a wider range of treatments, Koray Tascilar, MD, of Friedrich Alexander University Erlangen-Nuremberg (Germany) and colleagues wrote in their publication of the RETRO trial results in Lancet Rheumatology.
Previous studies have suggested that patients with RA in sustained remission may be able to taper or withdraw treatment, but data from randomized trials are limited, the researchers said.
In the RETRO trial, researchers compared three strategies for RA patients in remission, which was defined as <2.6 on the 28-joint Disease Activity Score with erythrocyte sedimentation rate (DAS28-ESR). They randomized 100 adults to continue DMARDs and glucocorticoids, 102 to taper DMARDs and glucocorticoids to half their prior doses, and 101 to reduce the doses to half for 6 months and then stop DMARDs. Patients were enrolled between May 26, 2010, and May 29, 2018, from 14 treatment centers in Germany. The final analysis included 282 patients; 92 in the continuation group, 93 in the taper group, and 96 in the stop group. The mean age of the patients was 56.5 years, and 59% were women. The mean duration of RA was 7.4 years, and the mean duration of remission was 20 months.
Overall, at 12 months, 61% of the patients remained in remission without relapse; 81.2% of the continuation group, 58.6% of the taper group, and 43.3% of the stop group. Relapses occurred in 17%, 43%, and 55% of patients in the continuation, taper, and stop groups, respectively. The median times to relapse in the three groups were 30.6 weeks, 24.3 weeks, and 26.1 weeks, respectively.
Most of the relapses occurred between weeks 24 and 36 after stopping treatment, the researchers wrote. Corresponding hazard ratios for relapse were 3.02 for the taper group and 4.34 for the stop group, compared with the continuation group. In comparison to continuing treatment, the number needed to treat for one more relapse to occur during the 12-month observation period was four for tapering and three for stopping, they noted.
The study protocol called for a return to baseline treatment for any patients who relapsed in the taper and stop groups, and most patients who relapsed regained remission after restarting their baseline treatments. Among patients who had a follow-up visit after a relapse, 10 (63%) of 16 patients in the continuation group reachieved remission before the end of the study, as did 21 (62%) of 34 in the taper group and 35 (76%) of 46 in the stop group.
The most common treatments at baseline were methotrexate (76%) and tumor necrosis factor (TNF) inhibitors (32%).
The researchers also identified several baseline characteristics associated with relapse. Overall, relapse occurred more often in biologic DMARD users than in participants treated with other drugs, more often in women than men, and more often in those with a longer disease duration, higher baseline DAS28-ESR and Health Assessment Questionnaire scores, and in those who were positive for rheumatoid factor or anti–citrullinated protein antibodies.
A total of 38 serious adverse events occurred in 29 participants during the study period, but none were deemed treatment related, and none led to study withdrawal.
The study findings were limited by several factors including the lack of masking of patients and assessors and potential underestimation of disease activity, the researchers noted. Also, the study did not include radiographic data that might have been used to confirm progression; however, such data could have produced a null result and were not feasible in the study population, they wrote.
“If RETRO had been a trial to test the superiority of 100% dose continuation, compared with tapering plus rescue treatment or stopping plus rescue treatment, we would not be able to show that continuation is superior to tapering or stopping,” the researchers noted.
The study results support “an increasingly dynamic management approach in patients with rheumatoid arthritis in stable remission,” given the changing nature of RA management, that may help reduce overtreatment in many RA patients, the researchers concluded. “Furthermore, the observation that most of the patients regained remission after reintroduction of antirheumatic treatments is helpful with regard to the benefit-risk aspect of treatment reduction.”
Real-world setting serves as starting point
The RETRO study is unique in that it tried to reflect a real-life setting by enrolling patients on baseline treatment with combinations of conventional synthetic DMARDs and biologic DMARDs seen in clinical practice rather than only patients taking biologic DMARDs – primarily TNF inhibitors – as done in previous studies of tapering and stopping DMARDs, wrote Catherine L. Hill, MD, of the University of Adelaide (Australia), in an accompanying editorial. It is also “used a simple, pragmatic one-size-fits-all treatment-tapering strategy,” she wrote.
However, she emphasized that answers are needed to questions about what relapse rates are acceptable, what duration of treatment-free time is ideal, and whether benefits to the patient outweigh risks.
Dr. Hill also highlighted the issue of identifying patients who are appropriate candidates for tapering or withdrawal. Stricter remission criteria may not be feasible in routine practice, and so “the development of algorithms to guide patient selection is likely to be the most practical way forward for clinicians and patients,” she wrote.
“Contemplation of treatment tapering or discontinuation in some patients with rheumatoid arthritis is remarkable and a measure of how far treatments have advanced,” Dr. Hill wrote. “However, further work to address outstanding questions on who should taper and how best to do it is still required,” she concluded.
The study received no outside funding. Lead author Dr. Tascilar disclosed lecture fees from Gilead and Union Chimique Belge; several coauthors disclosed relationships with multiple companies outside the current study. Dr. Hill had no financial conflicts to disclose.
FROM LANCET RHEUMATOLOGY
Merck’s new COVID-19 pill: ‘Game changer’ or just one more tool?
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Web of antimicrobials doesn’t hold water
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.