User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Cut risedronate drug holiday to under 2 years in older patients
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
Exercise appears to improve bone structure, not density
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Opioid prescribing mapped: Alabama highest, New York lowest
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.
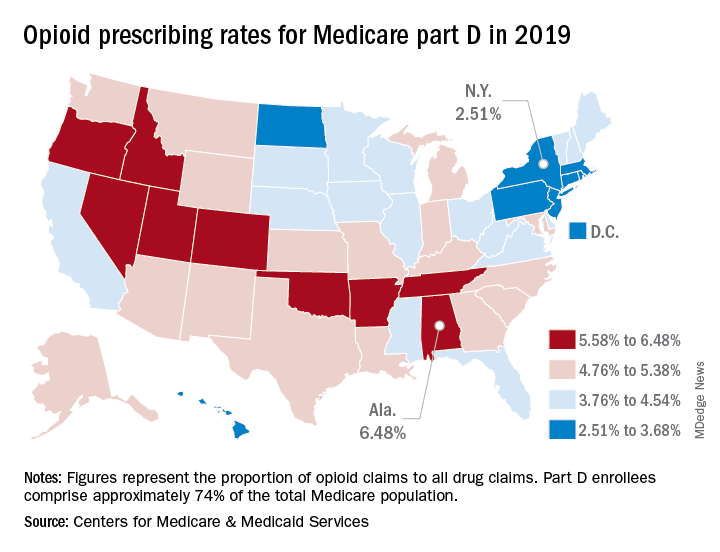
That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
Medicare beneficiaries in Alabama were more likely to get a prescription for an opioid than in any other state in 2019, based on newly released data.

That year, opioids represented 6.48% of all drug claims for part D enrollees in the state, just ahead of Utah at 6.41%. Idaho, at 6.07%, was the only other state with an opioid prescribing rate over 6%, while Oklahoma came in at an even 6.0%, according to the latest update of the Centers for Medicare & Medicaid Services’ dataset.
The lowest rate in 2019 belonged to New York, where 2.51% of drug claims, including original prescriptions and refills, involved an opioid. Rhode Island was next at 2.87%, followed by New Jersey (3.23%), Massachusetts (3.26%), and North Dakota (3.39%),
Altogether, Medicare part D processed 1.5 billion drug claims in 2019, of which 66.1 million, or 4.41%, involved opioids. Both of the opioid numbers were down from 2018, when opioids represented 4.68% (70.2 million) of the 1.5 billion total claims, and from 2014, when opioids were involved in 5.73% (81,026,831) of the 1.41 billion drug claims, the CMS data show. That works out to 5.77% fewer opioids in 2019, compared with 2014, despite the increase in total volume.
from 2014 to 2019, with Hawaii showing the smallest decline as it slipped 0.41 percentage points from 3.9% to 3.49%, according to the CMS.
In 2019, part D beneficiaries in Vermont were the most likely to receive a long-acting opioid, which accounted for 20.14% of all opioid prescriptions in the state, while Kentucky had the lowest share of prescriptions written for long-acting forms at 6.41%. The national average was 11.02%, dropping from 11.79% in 2018 and 12.75% in 2014, the CMS reported.
First-in-class TYK inhibitor shows durable effect for psoriasis
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
of follow-up, according to late-breaking data from two pivotal trials presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
From benefit reported on the two coprimary endpoints previously reported at 16 weeks, longer follow-up showed further gains out to 24 weeks and then persistent efficacy out to 52 weeks across these and multiple secondary endpoints, reported Richard Warren, MBChB, PhD, professor of dermatology and therapeutics, University of Manchester (England).
“This could be a unique oral therapy and an important treatment option for moderate to severe psoriasis,” Dr. Warren contended.
The multinational double-blind trials, called POETYK PSO-1 and PSO-2, enrolled 666 and 1,020 patients, respectively. The designs were similar. Patients with moderate to severe plaque psoriasis were randomly assigned in a 2:1:1 ratio to deucravacitinib (6 mg once daily), placebo, or apremilast (Otezla; 30 mg twice daily). At 16 weeks, those on placebo were switched to deucravacitinib.
For the coprimary endpoint of PASI 75 (75% clearance on the Psoriasis and Severity Index), the similar rate of response for deucravacitinib in the two studies (58.7%/53.6%) at week 16 was superior to the rates observed on both apremilast (35.1%/40.2%) and placebo (12.7%/9.4%).
By week 24, the proportion of deucravacitinib patients with a PASI 75 response had reached 69.3% and 58.7% in the POETYK PSO-1 and PSO-2 trials, respectively. The proportion of patients on apremilast with PASI 75 at this time point did not increase appreciably in one study and fell modestly in the other.
By week 52, the response rates achieved with deucravacitinib at week 24 were generally unchanged and nearly double those observed on apremilast.
The pattern of relative benefit on the other coprimary endpoint, which was a score of 0 or 1, signifying clear or almost clear skin on the static Physicians Global Assessment (sPGA), followed the same pattern. At week 16, 53.6% of patients had achieved sPGA 0/1. This was significantly higher than that observed on either apremilast or placebo, and this level of response was sustained through week 52.
When patients on placebo were switched to deucravacitinib at week 16, the PASI 75 response climbed quickly. There was complete catch-up by 32 weeks. In both groups, a PASI 75 response rate of about 65% or higher was maintained for the remainder of the study.
On a prespecified analysis, prior treatment exposure was not associated with any impact on the degree of response with deucravacitinib. This included a comparison between patients exposed to no prior biologic, one prior biologic, or two or more biologics, Dr. Warren reported.
Unlike patients in POETYK PSO-1, those with a PASI 75 response at 16 weeks in the POETYK PSO-2 trial were rerandomized to remain on deucravacitinib or switch to placebo. Designed to evaluate response durability, this analysis showed a relatively gradual decline in disease control.
“The median time to a loss of response was 12 weeks,” Dr. Warren said. He was referring in this case to the PASI 75 response, but the slope of decline was similar for sPGA score 0/1. At the end of 52 weeks, 31.3% of patients who had been rerandomized to placebo still maintained a PASI 75 while 80.4% of those who stayed on deucravacitinib still had PASI 75 clearance.
In the 52-week data from these two trials, several secondary endpoints have already been examined, and Dr. Warren said more analyses are coming. So far, the pattern of response has been similar for all endpoints.
Reporting on one as an example, Dr. Warren said that sPGA 0/1 for scalp psoriasis was achieved at week 16 by 70.3% of those randomly assigned to deucravacitinib versus 17.4% of those in the placebo arm. Among those switched from placebo to deucravacitinib at 16 weeks, the scalp response had caught up to that observed in those initiated on deucravacitinib by week 28. The response was sustained out to 52 weeks in both groups.
In the long-term trials, there have been no new safety concerns, according to Dr. Warren. He described this drug as “well tolerated,” adding that no significant laboratory abnormalities have been observed on long-term treatment. Although there has been a trend for increased risk of viral infections, such as herpes zoster, relative to apremilast, cases have so far been mild.
The Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR) has been approved for psoriatic arthritis, and numerous other JAK inhibitors are now in clinical trials for plaque psoriasis. These agents vary for their relative selectivity for JAK1, 2, and 3 kinases, but deucravacitinib is the first JAK inhibitor to reach clinical trials that target TYK2, which inhibits interleukin-23 and other cytokines implicated in the pathogenesis of plaque psoriasis.
“Deucravacitinib is very distinct from the other JAK inhibitors, and I think we are seeing this in the clinical studies,” Dr. Warren said. As a result of responses in the POETYK PRO trials that rival those achieved with monoclonal antibodies, he expects this drug, if approved, to be an important option for those with moderate to severe disease who prefer oral therapies.
Mark G. Lebwohl, MD, professor of dermatology and dean for clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York, shares this opinion. In an interview, he emphasized the unique mechanism of deucravacitinib and its clinical potential.
“Unlike other less specific JAK inhibitors, deucravacitinib has a unique binding site on TYK2, the regulatory domain of the molecule. This makes deucravacitinib more targeted and therefore safer than other JAK inhibitors,” said Dr. Lebwohl.
“After cyclosporine, which has many side effects, deucravacitinib is the most effective oral therapy we have for psoriasis and one of the safest,” he added.
The POETYK PSO-1 and PSO-2 trials received funding from Bristol-Myers Squibb. Dr. Warren has financial relationships with AbbVie, Almirall, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, UCB, and Xenoport. Dr. Lebwohl has financial relationships with more than 20 pharmaceutical companies, including Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Drug cocktail significantly reduced severe COVID, death in outpatients
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
A monoclonal antibody combination of casirivimab and imdevimab (REGEN-COV) significantly reduced the risk of COVID-19–related hospitalizations and death from any cause in the phase 3 portion of an adaptive trial of outpatients.
Researchers, led by David Weinreich, MD, MBA, executive vice president of the drug cocktail’s manufacturer Regeneron, found in the randomized trial that the combination also resolved symptoms and reduced the SARS-CoV-2 viral load more quickly, compared with placebo.
Findings were published in the New England Journal of Medicine.
COVID-related hospitalization or death from any cause occurred in 18 of 1,355 patients (1.3%) in the group getting 2,400 mg infusions of the study drug, compared with 62 (4.6%) of 1,341 in the matching placebo group, indicating a relative risk reduction of 71.3%; P < .001.
Sunil Joshi, MD, president of the Duval County Medical Society Foundation and an immunologist in Jacksonville, Fla., said in an interview that these findings confirm benefits of REGEN-COV and are very good news for a patient group that includes those age 65 and older with high blood pressure, diabetes, or obesity; and for people not vaccinated, who are all at high risk of hospitalization or death if they get COVID-19.
“Vaccines are critically important,” he said, “but if you were to be infected and know that there’s a way to keep yourself out of the hospital, this is very good news.”
Researchers seek lowest doses
This trial found that the effect was similar when researchers cut the doses in half. These outcomes occurred in 7 of 736 (1%) of patients given 1,200 mg of REGEN-COV and in 24 (3.2%) of 748 in the matching placebo group (relative risk reduction, 70.4%; P = .002).
Symptoms were resolved on average 4 days earlier with each REGEN-COV dose than with placebo (10 days vs. 14 days; P < .001 for both comparisons).
Dr. Weinreich said in an interview that trials will continue to find the lowest effective doses that can stand up to all evolving variants.
“This is one of those settings where you don’t want to underdose. You’ve got one shot at this,” he said. “We’d love to do lower doses. It would be more convenient and we could treat more patients, but if it generates more clinical failures or doesn’t work with certain variants, then you’ve done a huge disservice to the world.”
Also new in this study is that researchers tested not only seronegative patients, but patients at high risk regardless of blood antibody status, he said.
“It’s the first suggestion of data that if you’re breaking through a vaccine and you’re at high risk, the use of the cocktail is something to strongly consider because treatment early is better than treatment later,” Dr. Weinreich said.
In addition to efficacy, the phase 3 trial demonstrated the cocktail had a good safety profile. Serious adverse events occurred more often in the placebo group (4%) than in the 1,200-mg group (1.1%) and the 2,400-mg group (1.3%). Infusion reactions (grade 2 or higher) occurred in less than 0.3% of patients in all groups.
William Fales, MD, state medical director for the Michigan Department of Health and Human Services, said the results confirm the promise of REGEN-COV for reducing hospitalizations and death in a peer-reviewed publication.
COVID-19 a moving target
However, Dr. Fales noted that COVID-19 is a moving target with emerging variants. The criteria for populations at high risk have also broadened since the start of the study, he said.
“A great example is pregnancy is now included as high risk, and that would have likely been a specific contraindication of patients in this clinical trial,” he said.
Dr. Fales said Michigan has been using both REGEN-COV and the Eli Lilly combination of bamlanivimab and etesevimab, which also has an emergency use authorization (EUA) from the Food and Drug Administration, with positive results.
REGEN-COV has an EUA to treat people who are at high risk of serious consequences from COVID-19, including those who are already infected (nonhospitalized) or those in certain postexposure prophylaxis settings.
“We’re seeing very low hospitalization rates and few deaths in a state that is predominately Delta,” Dr. Fales said. “So, this makes us feel that we’re doing the right thing and supports the current efforts around the country to make monoclonal antibody therapy available to high-risk patients.”
Dr. Joshi noted that trial results have been emerging from other monoclonal antibody cocktails with different COVID-19 patient groups.
However, he said in an interview, “how much more effective they would be than this is something we’d have to look at, as 71% effectiveness in keeping people out of the hospital is pretty good for any treatment.”
“These are great numbers, but vaccination itself keeps you from getting the disease in the first place and not just for a short time period. This treatment is just that – a treatment. It gets you through that episode but it doesn’t mean you won’t get sick again. You don’t develop an immune response as you do with the vaccine,” he said.
Dr. Weinreich agreed: “This is not a substitute for a vaccine except for the small group who get the vaccine and their bodies can’t respond to it because they’re significantly immunocompromised.”
The results from this paper “are one piece of a large, multistudy, phase 3 program that basically spans from prophylaxis all the way to hospitalization and pretty much the gamut – all of them – have worked. All of these studies have shown dramatic improvement in whatever the definitive regulatory endpoint is,” Dr. Weinreich said.
He said discussions are ongoing for full regulatory approval in the United States and for expanding the EUA for other populations, including pre-exposure prophylaxis, “which the [United Kingdom’s] authority has already granted us but the FDA has not.”
The study is funded by Regeneron and the Department of Health & Human Services. Dr. Weinreich is a vice president of Regeneron. Dr. Joshi reported no relevant financial relationships. Dr. Fales holds stock in Eli Lilly.
A version of this article first appeared on Medscape.com.
NIAMS director reflects on her mentors, spotlights research projects underway
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
After many years at the University of California, San Francisco, Lindsey A. Criswell, MD, MPH, DSc, began a new chapter in February 2021 as the director of the National Institute of Arthritis and Musculoskeletal and Skin Disease, part of the National Institutes of Health. NIH Director Francis S. Collins, MD, PhD, selected her for the post.
“Dr. Criswell has rich experience as a clinician, researcher, and administrator,” Dr. Collins said in a prepared statement. “Her ability to oversee the research program of one of the country’s top research-intensive medical schools, and her expertise in autoimmune diseases, including rheumatoid arthritis and lupus, make her well positioned to direct NIAMS.” Dr. Criswell, a rheumatologist, was named a full professor of medicine at UCSF in 2007 and had served as vice chancellor of research at the university since 2017. She has authored more than 250 peer-reviewed scientific papers, and her efforts have contributed to the identification of more than 30 genes linked to autoimmune disorders. In her first media interview, Dr. Criswell opens up about her mentors, operational challenges posed by the COVID-19 pandemic, and highlights many NIAMS research projects underway.
Who inspired you most early in your career as a physician scientist? I have had great opportunities to work with fabulous mentors. Wallace (Wally) Epstein, MD, was my mentor when I was a rheumatology fellow and junior faculty member at UCSF. He was broadly admired for the breadth of his experience as a clinician and a researcher, and he was noteworthy at that time for his strong support for women and students of color. One of the many things I appreciated about him was his diverse range of interests outside of work, which included cello playing and woodworking.
Another mentor was Ephraim (Eph) Engleman, MD, the first academic rheumatologist in California. Eph continued to see patients beyond the age of 100. Perhaps his most important contributions were his efforts towards advocacy for funding for research and education in rheumatology. A prodigy violinist, he too had a broad range of personal interests.
What research into the genetics and epidemiology of human autoimmune disease that you have been a part of has most surprised you, in term of its ultimate clinical impact? Some of my most rewarding and impactful work has focused on the shared genetic basis of autoimmune diseases. We’ve identified dozens of genes that contribute to the risk and outcome of rheumatoid arthritis, lupus, and other autoimmune disorders. These discoveries regarding shared genes and pathways among such a diverse set of conditions have helped to inform optimal therapeutic target and treatment strategies across multiple diseases. For example, exploration of RA genes and pathways has revealed that approved agents for other conditions, such as cancer, may be appropriately repurposed for the treatment of RA. These are critical observations that have the potential to dramatically accelerate progress in developing new therapies for autoimmune diseases, such as RA.
Did you have much interaction with Stephen I. Katz, MD, PhD, your longtime predecessor who passed away unexpectedly in 2018? If so, what do you remember most about him? I regret that I had very little interaction with Steve, but I am well aware of the impact he had on NIAMS, NIH, and the research enterprise overall. He inspired so many people in a personal way, and I am energized by the legacy that he left behind.
What are your goals for the early part of your tenure as the new director of NIAMS? An important goal is getting to know the NIAMS community and expanding my knowledge of the Institute’s musculoskeletal and skin portfolios. I am also conducting outreach to Institute/Center directors and other NIH leadership to increase opportunities for input and advice. In doing this, I am identifying shared research interests, best practices, and potential partners for possible future collaborations. Another important goal is to increase NIAMS’ visibility within and beyond NIH. Ultimately, I want to contribute to the great work of the Institute and improve the lives of people with rheumatic, musculoskeletal, and skin diseases.
How would you characterize your management style? I like to lead with a flat hierarchy and work collectively to address opportunities and challenges. I value team building and tend to tap a variety of perspectives and expertise at all levels to achieve consensus, where possible.
The Accelerating Medicines Partnership (AMP) program was launched in 2014, with projects in three disease areas including the autoimmune disorders RA and lupus. What are some recent highlights from this program with respect to RA and lupus? AMP RA/SLE was dedicated to identifying promising therapeutic targets for RA and systemic lupus erythematosus. AMP-funded researchers have applied cutting-edge technologies to study cells from the synovial tissues of the joints of people with RA, and from the kidneys of people with lupus nephritis. In 2014, studying tissues in patients where the disease is active was a novel approach, since most research was conducted in mouse models or human blood samples.
The AMP RA/SLE Network developed a rich dataset that is available to the research community. Investigators are now using the data to facilitate RA and lupus research. For example, using AMP data, NIAMS-supported researchers identified potential biomarkers that could help predict an imminent RA flare. Work from another NIAMS-supported group suggests that targeting the regulatory transcription factor HIF-1, which drives inflammation and tissue damage, might be an effective approach for treating renal injury in lupus.
The data generated are accessible to the scientific community through two NIH websites: the database of Genotypes and Phenotypes (dbGaP) and the Immunology Database and Analysis Portal (IMMPORT).
Given the success of AMP RA/SLE, NIH plans to launch an “AMP 2.0” later in 2021. The AMP Autoimmune and Immune-Mediated Diseases (AMP AIM) program will provide an opportunity to leverage the accomplishments of AMP RA/SLE to new conditions, including psoriatic spectrum diseases and Sjögren’s syndrome.
What are some recent highlights from NIAMS-supported research in skin diseases? NIAMS-supported investigators continue to make significant strides in our understanding of skin biology and disease. For example, researchers recently demonstrated that imiquimod, a drug used to treat precancerous skin lesions, can help mouse ear wounds heal without scarring.
Another team addressed the safety and potential benefit of Staphylococcus hominis A9, a bacterium isolated from healthy human skin, as a topical therapy for atopic dermatitis.
Moving forward, AMP AIM will refine and extend the single-cell analysis of tissues to additional diseases, including psoriasis, setting the stage for the discovery of new therapeutic targets for the disease.
How has the COVID-19 pandemic changed the landscape of research, at least for the short term? This is a once-in-a-century pandemic that none of us were fully prepared for. We understand that it has been particularly challenging for women scientists, scientists with young children, and trainees and junior faculty who are at critically important and vulnerable stages of their careers. There isn’t a lab or clinical setting that hasn’t been negatively impacted in some way.
During the pandemic, the NIH instituted administrative flexibilities to support the grantee community, including extensions in time. In addition, the agency has issued several funding opportunities specific to COVID-19, some of which involve NIAMS participation.
What is NIAMS doing to help early/young investigators as well as female investigators and those from minority groups? Structural racism in biomedical research is a heightened concern. Earlier this year, Dr. Collins established the UNITE initiative to address structural racism and promote racial equity and inclusion at the NIH and within the larger biomedical community that we support. NIAMS is fully committed to this effort. One example is the Diversity Supplement Program, which is designed to attract and encourage eligible individuals from underrepresented populations to research careers.
Early-stage investigators are another top priority. In a tribute to the beloved former NIAMS director, NIH recently established the Stephen I. Katz Early Stage Investigator Research Grant Program. The R01 award provides support for a project unrelated to an early investigator’s area of postdoctoral study. (No preliminary data are allowed.) This award mechanism is a unique opportunity for early-stage investigators to take their research in a completely new direction.
Managing work and family life is an important concern, particularly for female investigators. Many NIH grant awards allow for reimbursement of actual, allowable costs incurred for childcare and parental leave. The NIH is exploring initiatives to promote research continuity and retention of eligible investigators facing major life events, such as pregnancy, childbirth, and adoption, at vulnerable career stages.
Who inspires you most in your work today? I am inspired by the ongoing struggles of our patients, junior investigators, and by the committed staff members on my team.
New data illustrate pandemic pivot to telehealth by patients, physicians
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.
Telehealth use, although much higher than before the COVID-19 pandemic, accounted for less than 20% of weekly outpatient visits 6 months into the pandemic, according to a new report from the American Medical Association. Ten percent of weekly visits were conducted via videoconferencing, and 8.1% of visits were conducted using the telephone.
Those figures may overstate the true level of telehealth use in fall 2020. A study by the Commonwealth Fund, Harvard University, Boston, and Phreesia found that in December of that year, only 8% of outpatient visits involved the use of telemedicine – and that was up from 6% in October. In contrast to the AMA results, which came from its 2020 benchmark survey of physicians, the Commonwealth Fund study used data from practice management systems and an online patient registration platform, as well as electronic health record data.
A more recent survey of hospital executives found that as of September 2021, hospital telehealth visits had leveled off at 10% to 20% of appointments. Similarly, a McKinsey survey in July showed that telehealth encounters made up 13% to 17% of evaluation and management visits across all specialties.
Big jump during pandemic
The AMA report offers a wealth of data on how physicians use telehealth and the differences between specialties in this area.
The report found that 70.3% of physicians worked in practices that used videoconferencing to provide patient visits in September 2020, compared to 14.3% of physicians in September 2018. Sixty-seven percent of physicians worked in practices that used telephone visits (the comparable figure for 2018 was unavailable).
Overall, 79% of physicians worked in a practice that used telehealth, compared to 25% in 2018.
Not every doctor in practices that utilized telehealth conducted virtual visits. In contrast to the 70.3% of doctors who were in practices that had video visits, only 59.1% of the respondents had personally conducted a videoconferencing visit in the previous week. The average numbers of weekly video and telephone visits per physician were 9.9 and 7.6, respectively, including those who did none.
There were big differences in virtual visit use among specialties as well. Eighty-five percent of psychiatrists were in practices that provided online appointments, according to the AMA survey, and three-quarters of primary care physicians said their practices offered telehealth appointments. Pediatricians were much less likely than family practice/general practice physicians (FPs/GPs) or general internists to do so.
The practices of many medical specialists were also highly likely to provide telehealth. Over 75% of practices in cardiology, endocrinology/diabetes, gastroenterology, nephrology, and neurology offered telehealth visits. About 88% of hematologists/oncologists offered video visits. Far fewer surgeons reported that their practice used virtual visits; the exceptions were urologists and dermatologists, 87% of whose practices used telehealth.
How telehealth was used
Across all specialties, 58% of physicians said clinicians in their practices used it to diagnose or treat patients; 59.2%, to manage patients with chronic disease; 50.4%, to provide acute care; and 34.3%, to provide preventive care.
Seventy-two percent of FP/GP and pediatric practices used telehealth to diagnose or treat patients. Just 64.9% of internists said their practices did so, and only 61.9% of them said their practices provided acute care via telehealth, versus 70% of FPs/GPs and pediatricians.
Among medical specialties, endocrinologists/diabetes physicians were those most likely to report the practice-level use of telehealth to diagnose or treat patients (71.9%), manage patients with chronic disease (92.1%), and provide preventive care (52.6%).
Significantly, 33% of medical specialists said their practices used remote patient monitoring. This finding was driven by high rates of use among cardiology practices (63.3%) and endocrinology practices (41.6%). Overall, the practice-level use of remote patient monitoring rose from 10.4% of practices in 2018 to 19.9% in 2020.
Virtual consults with peers
Some practices used telehealth to enable physicians to consult with colleagues. Twelve percent of respondents said their practices used telehealth to seek a second opinion from a health care professional in 2020, compared to 6.9% in 2018. Formal consultations via telehealth were also increasingly common: 17.2% of doctors said their practices did this in 2020, compared to 11.3% in 2018.
Also of note, 22.4% of physicians said their practices used telehealth for after-hours care or night calls in 2020, versus 9.9% in 2018.
The AMA report credited telehealth and expanded coverage and payment rules for enabling physician practices to keep their revenue streams positive and their practices open. However, the Commonwealth Fund study found “a substantial cumulative reduction in visits across all specialties over the course of the pandemic in 2020.” These ranged from a drop of 27% in pediatric visits to a decline of 8% in rheumatology visits during the period from March to December 2020.
A version of this article first appeared on Medscape.com.







