User login
Children and COVID: New cases up slightly, vaccinations continue to slow
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.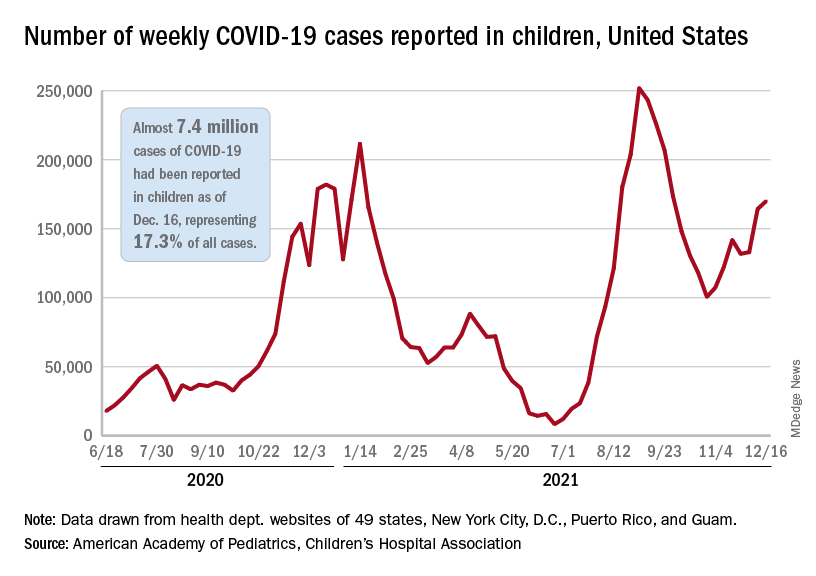
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.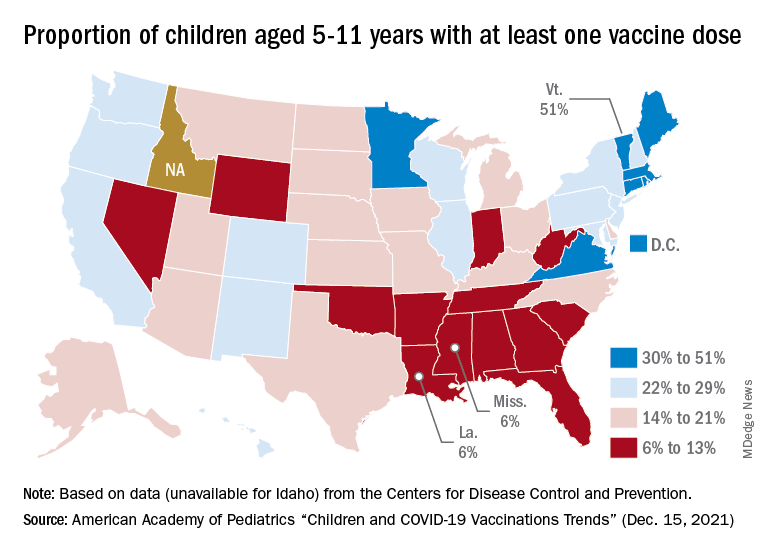
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
New COVID-19 vaccinations in children were down by almost 24% in the last week as new cases rose by just 3.5%, based on new data.
That fairly low number suggests the latest case count from the American Academy of Pediatrics and the Children’s Hospital Association has not caught up yet to the reality of the Omicron variant, which has sent new cases climbing among all ages and now represents the majority of COVID-19 infections nationwide, the Centers for Disease Control and Prevention said.
Meanwhile, in the midst of the latest surge, the United States just passed yet another sobering COVID milestone: 1,000 deaths in children aged 17 and under. The total as of Dec. 20 was 1,015, according to the CDC, with the largest share, almost 32%, occurring in children less than 5 years of age.
Regionally, the majority of that increase came in the Northeast, with a small rise in the South and decreases in the Midwest and West, the AAP and CHA said in their weekly COVID report.
At the state level, the largest percent increases in cases over the past 2 weeks were seen in Maine and New Hampshire, as well as Vermont, which has the nation’s highest vaccination rates for children aged 5-11 (51%) and 12-17 (84%), the AAP said in its vaccination trends report.
Nationally, new COVID vaccinations in children continue to trend downward. The number of children aged 5-17 years who had received at least one dose increased by about 498,000 for the week of Dec. 13-19, down from 654,000 (–23.9%) the previous week. Children aged 5-11 years still represented the largest share (22.7%) of all vaccine initiators in the last 2 weeks, but that proportion was 42.8% just before Thanksgiving, according to data from the CDC.
On a more positive note, children aged 5-11 made up 51% of all Americans who completed the vaccine regimen during the 2 weeks ending Dec. 20. The cumulative completion count is 3.6 million in that age group, along with almost 13.4 million children aged 12-17, and the CDC data show that 6.1 million children aged 5-11 and 15.9 million children aged 12-17 have received at least one dose.
On a less positive note, however, that means almost half (47%) of 12- to 17-year-olds still are not fully vaccinated and that over a third (37%) have received no vaccine at all, according to the COVID Data Tracker.
RSV resurgence likely in wake of COVID-19
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
The impact of respiratory syncytial virus (RSV)will likely be greater in 2021 and 2022 in the United States than in previous years as a result of the ongoing COVID-19 pandemic, based on data from a simulation-modeling study involving approximately 19 million individuals.
Although RSV usually follows consistent patterns of timing and duration, the disease all but disappeared starting in March 2020 after the introduction of measures to mitigate the spread of COVID-19, Zhe Zheng, MBBS, of Yale University, New Haven, Conn., and colleagues wrote.
However, lifting of mitigation measures has resulted in emergence of RSV in various parts of the world in early 2021, and trends may be similar in the United States, but data are needed to plan for prophylaxis and hospital use, they noted.
In a study published in JAMA Network Open, the researchers developed a simulation model for epidemics of RSV based on historical data. They acquired inpatient records from New York during 2005-2014 and from California during 2003-2011. The primary clinical outcome was the estimated monthly hospitalizations for RSV.
The simulated study population was 19.45 million individuals. After evaluating several scenarios including continued low transmission associated with social distancing and other mitigation measures, the researchers focused on the likely scenario that introduction of RSV from other regions would likely spark RSV epidemics in the United States.
They determined that spring and summer 2021 would show an increase in hospitalizations for RSV. Overall, higher rates of virus introduction from other regions were associated with more intense spring and summer RSV epidemics, with the trade-off of smaller winter epidemics. In the model, the expected RSV epidemic in spring and summer 2021 in New York was small, with a peak incidence of 419 hospitalizations per 100,000 people in April; by contrast, for states with less seasonal variability, such as Florida, the model predicted a larger summer epidemic.
In the model, the mean age of hospitalization for children younger than 5 years for January 2022 was expected to be 1.17 years, compared with 0.84 years in January 2019, the researchers noted.
Across all age groups, the greatest relative increase in the incidence of RSV infection was predicted for children aged 1-4 years (ranging from 82% to 86%), as were lower respiratory infections (87%-101%) and hospitalization (99%-119%), compared with prepandemic levels.
Hospitalizations for children aged 1 year were predicted to double compared with prepandemic seasons; 707 per 100,000 children per year for 2021 and 2022 versus 355 per 100,000 children per year in a typical prepandemic season. However, the largest incidence of lower respiratory infections (30,075 per 100,000) was predicted for infants aged 3-5 months, and the largest incidence of hospitalizations (3,116 per 100,000) was predicted for infants younger than 3 months.
“Without virus importation, the risk of RSV infections across all age groups in the winter of 2021 and 2022 would be greater, as more susceptible individuals were spared from infections in the absence of summer epidemics,” the researchers noted.
The older mean hospitalization age seen in the model was similar to the reported median patient age in Australia both before the pandemic and during the reemergent RSV epidemic.
“This makes intuitive sense, since many children born in 2020 were spared from RSV infection due to the low virus activity; these children will be older when they get infected for the first time during the reemergent epidemics,” the researchers wrote. “Consequently, stakeholders should consider modifying prophylaxis guidelines to include high-risk infants less than 2 years of age for the 2021-2022 season.”
The study findings were limited by several factors including the lack of data on level of virus introduction or on the impact of lack of boosting on infants with only transplacentally acquired RSV antibodies, the researchers noted. Other limitations include the use of historical data and the lack of data on values outside those included in the model, as well as the inability to control for other factors that could influence RSV, such as vaccines or long-lasting antibodies.
However, the results suggest that the rate of imported infections is associated with RSV hospitalizations, and the model effectively captured the RSV epidemics in the United States in spring and summer 2021.
Models can guide clinical preparations
“Health care simulation modeling is a growing field, with very exciting implications,” Lenore Jarvis, MD, of George Washington University, Washington, said in an interview. The field has the potential ability to influence health care in a data-driven way, including, but not limited to, staffing and other hospital operations, as well as patient care decision-making. “In short, accurate modeling and predictions can help us to make informed health care decisions that can lead to increased quality of care, potential cost savings, and even to help save lives,” she said.
Although the details of transmission modeling were not mentioned in the study, the authors evaluated the performances of several models and scenarios. “Scenario 4, for example, was focused on in particular because it best captured the observed dynamics [for RSV] that emerged during the spring and summer of 2021,” Dr. Jarvis said.
“Pediatricians can speak to these trends firsthand. A decrease in expected RSV infections and hospitalizations in 2020, followed by an unprecedented and early increase in RSV infections and severity in 2021, and the factors that the authors account for make sense, such as reintroduction of RSV from other regions and low immunity in the population,” she said. “It also makes sense that, in these transmission modeling scenarios, the expected mean age of hospitalization because of RSV increased with a temporary (hopefully) increase in RSV hospitalizations in the 2021 season, and potentially the 2022 RSV season.”
As for additional research, Dr. Jarvis said she would like to see follow-up data on the RSV transmission modeling. “For example, with scenario 4, does this scenario continue to perform well in other time periods, such as the winter? If the modeling continues to be accurate during other periods of evaluation and reevaluation, this modeling could be very useful in helping pediatric clinics and hospitals to prepare for RSV care and hospital capacity management.”
The study was supported by grants to various researchers from the National Institute of Allergy and Infectious Diseases/National Institutes of Health, the National Center for Advancing Translational Science at the National Institutes of Health, and NIH Roadmap for Medical Research. Lead author Ms. Zheng had no financial conflicts to disclose. Her study coauthors disclosed relationships with companies including AbbVie, Merck, Pfizer, GlaxoSmithKline, MedImmune, and Janssen. Dr. Jarvis had no financial conflicts to disclose and serves on the Pediatric News editorial advisory board.
FROM JAMA NETWORK OPEN
BMJ slams ‘incompetent’ Facebook fact-checking of vaccine article
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
According to an open letter written by outgoing BMJ editor-in-chief Fiona Godlee, MD, and incoming editor-in-chief Kamran Abbasi, MD, Facebook hired a third-party contractor to evaluate the article’s findings. This resulted in “inaccurate, incompetent, and irresponsible” conclusions that “should be of concern to anyone who values and relies on sources such as the BMJ for reliable medical information.”
The article in question investigated data integrity concerns at Pfizer vaccine clinical trial sites. In September 2020, the letter states, a former employee of the research group involved in Pfizer’s main vaccine trials, Ventavia, reached out to the BMJ and “began providing ... dozens of internal company documents, photos, audio recordings, and emails.” According to the company’s website, Ventavia “played a significant part in [COVID-19 clinical trial] recruitment” and “has received recognition by Pfizer for their contribution to vaccine trials.”
It was previously reported that the whistle-blower is a former regional director who was involved in Pfizer’s vaccine trials in Texas during the fall of 2020. She alleges “the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase 3 trial.”
The images provided to the BMJ “showed needles discarded in a plastic biohazard bag instead of a sharps container box” and another displayed “vaccine packaging materials with trial participants’ identification numbers written on them left out in the open, potentially unblinding participants.”
Despite informing Ventavia, the director’s concerns went unaddressed. She then filed a complaint with the Food and Drug Administration and was subsequently fired the same day. The FDA did not investigate the director’s allegations, said Dr. Godlee and Dr. Abbasi, even though the evidence “revealed a host of poor clinical trial research practices occurring at Ventavia that could impact data integrity and patient safety.”
Article labeled as ‘hoax,’ without pointing out errors
The BMJ hired an investigative reporter to follow up on the clinical trial claims. The findings were published in an article on Nov. 2, 2021, after the article “went through ... the usual high-level legal and editorial oversight and peer review,” according to the journal.
However, by Nov. 10, the journal began receiving complaints from readers unable to share the article on social media. Others had their posts flagged with warnings, such as “missing context ... independent fact-checkers say this information could mislead people.” Administrators of various Facebook groups were notified that posts containing the article were “partly false.”
Readers were informed that Facebook contractor Lead Stories performed the article’s “fact check.” Lead Stories is “an award-winning innovative fact checking and debunking website” and “an active part of Facebook’s partnership with third-party fact checkers” – with the latter granting them “access to listings of content that has been flagged as potentially false by Facebook’s systems or its users.” The company said they “decide independently if we want to fact check it or not.”
Lead Stories stated that they “can enter our fact checks into a tool provided by Facebook and Facebook then uses our data to help slow down the spread of false information on its platform.” Although the contractor is compensated, Lead Stories claims they have “no say or influence over what we fact check or what our conclusions are.”
Both editors question the validity of the fact check performed by Lead Stories, as it failed to provide any “assertions of fact” as to what the BMJ got wrong. Moreover, the editors take issue with Lead Stories referring to the journal as a “news blog” and using the phrase “hoax-alert” in the URL when publishing the story on its site.
The BMJ has reached out to Lead Stories and Facebook, said the letter, but Lead Stories refuses to “change anything about their article or actions that have led to Facebook flagging our article.” Requests for Facebook to remove the “fact-checking” label and allow “readers to freely share the article on [Facebook’s] platform” have been unfruitful.
Dr. Godlee and Dr. Abbasi expressed concern that other “high quality information provider[s] have been affected by the incompetence of Meta’s fact checking regime.” In November, Instagram censored Cochrane, an international provider of independent systematic medical reviews. Instagram, also owned by Meta, prohibited users from tagging Cochrane because the organization “repeatedly posted ... false content about COVID-19 or vaccines.” Cochrane refuted the allegations.
While “fact checking has been a staple of good journalism for decades,” said the editors, Meta has “apparently delegated responsibility to people incompetent in carrying out this crucial task.” They urged the company to reconsider its fact-checking strategy and review the issues that contributed to the error.
This news organization reached out to Meta for comment but did not receive a response at press time.
Lead Stories has posted a reply (Lead Stories’ Response To BMJ Open Letter Objecting To A Lead Stories Fact Check) to the BMJ’s complaint on its website.
A version of this article first appeared on Medscape.com.
Axilla swelling after COVID booster puts focus on mammogram timing
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
This inflammation is caused by the enlargement of lymph nodes and can show up as an abnormal finding on mammograms and other types of chest scans, causing concern and even the need for additional imaging and follow up, wrote Constance D. Lehman, MD, PhD, and colleagues in an article published in Journal of the American College of Radiology.
Lymph node swelling is a normal immune system reaction to vaccination, and “COVID-19 vaccinations in the arm are a well-documented cause of inflammatory unilateral axillary adenopathy,” noted Dr. Lehman, in an interview. The side effect will occur on the side of the body where the patient received a vaccine, and it is not always noticeable to the woman experiencing it, she said.
“We’re finding that the patients’ bodies are responding to the booster in many ways that are similar to the initial COVID vaccines, with lymph node swelling, muscle aches and pains, headaches, and so on,” said Dr. Lehman, who is chief of breast imaging at the Massachusetts General Hospital, Boston. There have been no real differences in reactions between the Moderna and Pfizer vaccines, she added.
Because axillary lymph node swelling can obscure mammogram results, staff of at least a few imaging centers, including Penn State Breast Center in Hershey, Pa., and Providence Women’s Imaging Center in Torrance, Calif., told this news organization that they are asking women to delay mammogram imaging either 6 weeks or 4-6 weeks after getting a COVID-19 booster.
Experts’ suggestions on mammograms, boosters timing
Other experts, including Jessica Leung, MD, acknowledged that vaccine-related reactive adenopathy is seen after the booster dose and provided recommendations for the timing of getting mammograms and the booster with this in mind.
“I would recommend getting the screening mammogram first, which can be followed immediately by vaccination, even on the same day,” said Jessica Leung, MD, a professor of diagnostic radiology at the University of Texas MD Anderson Cancer Center in Houston, Tex.
“If this is not possible from the scheduling perspective, then the patient should consult her health care provider regarding whether it is okay to wait a bit after receiving the vaccine before getting her screening mammogram.”
The answer to that question will likely depend on the time interval since the prior mammogram and the patient’s personal risk factors for developing breast cancer. Dr. Leung noted. “This is all predicated on the assumption that the patient is asymptomatic. If she has any symptoms, for example a palpable breast lump, then she should seek medical attention regardless of timing of vaccination.”
The same holds true for boosters, she said.
She emphasized that careful consideration should be given before delaying the mammogram. “The medical community has a great deal more knowledge at this time than in the early days of COVID-19 vaccination, so we are often able to identify reactive adenopathy related to vaccination. If patients were to delay the mammogram, any reactive adenopathy may persist, on average, for 4-6 weeks.”
Debra Patt, MD, PhD, MBA, executive vice president at Texas Oncology, professor at the University of Texas at Austin, provided a specific example of when a patient should not delay the diagnostic imaging, which is “in the event that there is an abnormal mass in the breast that requires evaluation.”
Providers are now prepared to address these issues, she added.
Dr. Lehman’s nuanced recommendations
“It’s easy to get both a mammogram and booster, and just a matter of timing them – so that the reaction doesn’t interfere with the mammography results,” Dr. Lehman said.
But she emphasized that women should not be choosing between their mammograms or a booster. “We are now saying the same thing that we did with the initial vaccine,” said Dr. Lehman. “We don’t want patients delaying their mammograms, and we don’t want them delaying their boosters – both are critical to staying healthy.”
In her center, a model was developed to navigate vaccine-associated adenopathy. While this approach was developed for the primary vaccine series, the same applies for the booster, which is essentially a third dose of the same vaccine, explained Dr. Lehman.
When patients present for mammography, ultrasound, or MRI, the technologist will document their COVID-19 vaccination status (first or second dose or booster), the date it was given, and the location. Adding vaccination documentation to intake forms helps to support appropriate management of patients who undergo imaging after COVID-19 vaccination. Six weeks is used as the cutoff point for defining “recent” vaccination.
For patients who are getting a screening mammography or MRI, and who have no symptoms beyond unilateral axillary adenopathy on the same side of the body where they received the COVID-19 vaccination (given in the arm) within a 6-week period, the following is included in the screening mammography or screening MRI report: “In the specific setting of a patient with documented recent (within the past 6 weeks) COVID-19 vaccination in the ipsilateral arm, axillary adenopathy is a benign imaging finding. No further imaging is indicated at this time. If there is clinical concern that persists more than 6 weeks after the patient received the final vaccine dose, axillary ultrasound is recommended.”
The experts interviewed reported no conflicts of interest.
Emergency docs cite ‘dire’ situation as COVID grows, nurses scarce
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
With emergency departments straining to keep up with the latest COVID surge, the American College of Emergency Physicians
The organization said that it is “very concerned that nursing shortages in emergency departments can complicate patient access to care and add to incredible levels of stress already on physician-led care teams,” according to a press release.
ACEP President Gillian Schmitz, MD, told this news organization, “The situation is dire in many emergency departments around the country. Emergency physicians are seeing more patients with fewer resources and less staff.
“Emergency physicians in the hardest hit communities are scrambling to locate available experts, exhausting federal support, and doing all they can to adapt to the demands of the current surge – everyone is being stretched to their limit.”
The Emergency Nurses Association (ENA) agrees with ACEP’s call for a team approach to stemming the shortage.
ENA President Ron Kraus, MSN, RN, said in an interview, “The pandemic has only amplified several long-standing issues impacting emergency nurses, such as workplace violence, a healthy work environment, and concerns about staffing shortages and the pipeline of new nurses. That said, we can’t lose focus on what’s most important in these challenging moments – ensuring every patient receives the high quality of care.”
The responsibility falls on the “collaborative effort” of the emergency department with emergency nurses playing a pivotal role, he said. But the stress, fatigue, and burnout driving nurses away from their jobs “should not be viewed as added inconvenience to anyone during a pandemic, but as a long-term threat to our health care system.”
ACEP’s press release stated that with fewer nurses available in the emergency department, team members are clocking extra hours, caring for more patients, and stretched to take on additional clinical and nonclinical duties.
“I am hearing from colleagues from Washington state to Michigan to New York that this is the worst they have seen since the beginning of the pandemic,” Dr. Schmitz said. “Everyone available is filling gaps as best they can, but the current path for many frontline workers is not sustainable,” she said in the release.
Meanwhile, ACEP is also tackling violence in the emergency department and has initiatives to protect the mental health of those working on the front lines, the release states.
“Emergency physicians will continue to do everything necessary to treat patients,” Dr. Schmitz said in the release, “but it will take a collaborative effort with legislators, policymakers and health system leaders to strengthen care teams, improve access and address capacity concerns with solutions that can save lives right now and in the months ahead.”
Dr. Schmitz stated that in Washington state, ICUs are at 97% to 100% capacity and less than 30 pediatric inpatient beds are available in the western part of the state.
“In Michigan and New York, several emergency departments are overflowing, and doctors are being called in to triage people in the waiting room because all of the emergency department beds are holding admissions. There are scenarios where entire hospitals are backing up into the emergency department and waiting room and we are physically running out of space and nursing staff.”
ACEP represents its 40,000 emergency physician members.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine for younger children hits snag
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
Pfizer’s COVID-19 vaccine for children ages 2 to 5 years old fizzled in clinical trials, the company said on Friday, signaling a further delay in getting a vaccine to preschoolers just as Omicron bears down on the U.S.
In a news release, Pfizer reported that while its 3-microgram dose – which is less than one-third of the dose given to older children – generated a protective immune response in babies and toddlers ages 6 to 24 months, it didn’t generate adequate immunity in children ages 2 to 5.
The company plans to change its clinical trial to add a third dose for younger children in hopes of improving those results. It also plans to test a third dose of its 10-microgram vaccine for children ages 5 to 12.
If the trials are successful, Pfizer said it would submit data to the FDA for an emergency use authorization (EUA) in the first half of 2022.
That pushes the timeline of getting a vaccine to younger children back by several months. In November, Anthony Fauci, MD, head of the National Institute of Allergy Infectious Diseases, predicted a vaccine would be ready for preschoolers by spring.
“On one hand, parents are understandably disappointed,” said Jill Foster, MD, a pediatric infectious disease doctor at the University of Minnesota Medical School. “On the other, it shows that the system for testing vaccines is working. Children are not little adults and have complex immune systems, so it’s not just a matter of making the dose smaller and expecting that it will work,” she said, noting that data from Moderna’s KidCOVE study in preschoolers is pending.
Until there’s a vaccine, Dr. Foster says parents should protect babies and toddlers by making sure everyone around them is vaccinated, promote the use of face masks for everyone around them and for all children over age 2, and continue to avoid crowded gatherings, particularly those that are indoors.
“Hand sanitizer is important, but this virus, especially the Omicron variant, is very easily spread through the air, so keep the air clear of virus as much as possible,” she said.
While the youngest children are still waiting for an effective vaccine, there was reassuring news Dec. 16 about the safety of Pfizer’s vaccine for school-aged kids – those ages 5 through 11.
Out of more than 7 million doses given since this vaccine was authorized for emergency use in late October, most reactions to the vaccine – including arm pain, swelling, and fatigue – have been mild and gone away quickly, without the need to miss school or see a doctor, the CDC reported to a meeting of its Advisory Committee on Immunization Practices, or ACIP.
Many experts had been waiting to see if this vaccine would cause rare cases of heart inflammation called myocarditis, as a higher dose did in teens and young adults.
The news on this front was excellent. About 6 weeks after this vaccine became available, the CDC says there have been only eight confirmed cases of myocarditis in this age group. Six more cases are under investigation.
To put this risk into context, data collected by the American Academy of Pediatrics and the Children’s Hospital Association shows that about 1% of children who test positive for COVID-19 are hospitalized for their infections, while the risk of getting a case of myocarditis after vaccination is .0002%, making it about 5,000 times more likely that a child would need to be hospitalized for COVID-19 than for myocarditis after vaccination.
John Su, MD, who is a member of the CDC’s Vaccine Safety Team, reported there had been two deaths in children after a COVID-19 vaccination. Both were girls, ages 5 and 6. Both had complicated medical histories for several medical disorders. It’s not clear their deaths were linked to the vaccine, and the causes of their deaths are still under investigation.
A version of this article first appeared on WebMD.com.
COVID cases spike as questions remain about Omicron’s threat
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
The best way to stay protected is by getting vaccinated and boosted, they said.
“For the unvaccinated, you’re looking at a winter of severe illness and death – for yourselves, families, and the hospitals who may soon overwhelm,” White House COVID-19 Response Coordinator Jeff Zients said at a news briefing Dec. 17. “We need the American people to do their part.”
The Omicron variant has been detected in at least 39 states and 75 countries, according to CDC director Rochelle Walensky, MD.
The strain is more transmissible than the already highly infectious Delta variant, and although there was early evidence that it caused more mild disease, she said that is likely because many of those infected have been vaccinated and boosted.
“Although Delta continues to circulate widely in the United States, Omicron is increasing rapidly and we expect it to become the dominant strain in the United States, as it has in other countries, in the coming weeks,” Dr. Walensky said.
The United States is averaging close to 1,300 deaths from COVID-19 each day. New cases, deaths, and hospitalizations are higher now than in the previous winter – before vaccines were so widely available. The New York Times reported on Dec. 17 that new infections in Connecticut and Maine have grown 150% in the past 2 weeks, and Ohio and Indiana are seeing hospitalization rates nearing the worst of 2020-2021’s winter surge.
Dueling reports released recently gave cause for relief and concern about Omicron.
A study from South Africa released on Dec. 14 shows lower hospitalizations during the first 3 weeks of the Omicron wave than during earlier waves from other variants. That’s the good news.
The concerning news is out of the United Kingdom, where Imperial College London reported Dec. 17 that the risk of reinfection with COVID-19 from Omicron is more than 5 times as high and that cases of Omicron-based COVID-19 are doubling every 2 days.
What’s more, the study “finds no evidence of Omicron having lower severity than Delta, judged by either the proportion of people testing positive who report symptoms, or by the proportion of cases seeking hospital care after infection. However, hospitalization data remains very limited at this time,” the researchers said.
“We have no evidence that the virus itself is more mild,” Eric Topol, MD, executive vice president of Scripps Research and editor-in-chief of Medscape, told PBS NewsHour. “Until we have that, we have to assume that people who don’t have any protection are highly vulnerable to getting very ill.”
The White House COVID-19 team continues to urge parents and guardians to get their children vaccinated, especially in anticipation of a post-holiday spike. Dr. Walensky said the CDC’s vaccine advisory board met on Dec. 16 to continue the safety discussion about COVID-19 vaccinations in children.
So far, 20 million children under 17 and 5 million under 11 have received their shots.
“Looking specifically at vaccine safety data from over 50,000 children 5-11 years old, we found no evidence of serious safety concerns,” Dr. Walensky said.
Top infectious disease expert Anthony S. Fauci, MD, highlighted the importance of getting vaccinated and boosted to avoid serious disease from Delta and Omicron.
“We’re in a situation where we are now facing a very important Delta surge and we are looking over our shoulder at an oncoming Omicron surge,” he said. “The optimum protection is fully vaccinated plus a boost.”
A version of this article first appeared on WebMD.com.
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
COVID-19 hospital data: New-onset seizures more common than breakthrough seizures
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
FROM AES 2021
Advisory on youth mental health crisis gets mixed reviews
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.