User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
S. aureus seen in 1% of pediatric CAP cases
SAN ANTONIO – Current guidelines on community-acquired pneumonia recommend penicillin, amoxicillin, or ampicillin as first-line treatment in children with CAP.
However, a small minority will have Staphylococcus aureus infections not treatable with these antibiotics, raising some concern about how many of these cases might be missed.
At the Pediatric Hospital Medicine 2015 meeting, Dr. Meghan E. Hofto of Children’s of Alabama at the University of Alabama, Birmingham, presented research from a study of 554 patients admitted to the hospital with community acquired pneumonia, including 78 patients with complicated pneumonia.
Seven patients in the cohort (1.3%) had S. aureus infections, Dr. Hofto and her colleagues found. Of those, six were recorded as having complicated pneumonia, characterized by pleural effusion or cavitation.
Six patients with S. aureus had been started on antibiotics in other health care settings prior to admission (amoxicillin n = 4, multiple agents n = 2). One patient positive for flu was first treated with oseltamavir only. However, all staph patients once admitted were started on vancomycin, which is effective against S. aureus, within 24 hours. Five were diagnosed by pleural fluid culture; one case was identified by clinical presentation, and another by sputum culture, Dr. Hofto said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
The S. aureus patients were younger than the cohort as a whole (median 18 months vs. 40.5 months). Length of stay was significantly longer for these patients, compared with the rest of the cohort (median 10 vs. 2 days, P less than .01), and S. aureus patients had significantly higher incidence of anemia (P less than .01), a finding that Dr. Hofto said was striking.
Within 24 hours of presentation, six out the seven staph cases had anemia, she said, while of all the 78 patients with complicated disease, 12 had anemia. Community acquired S. aureus pneumonia has been linked in other studies to severe leukopenia, Dr. Hofto noted (BMC Infect Dis. 2013;13:359) and (Paediatric Respiratory Reviews 2011 Sept;12:182-9).In an interview, Dr. Hofto said the findings supported current guidance in favor of first-line penicillin, amoxicillin, or ampicillin. “Part of what we’re looking at with guideline adherence is the barriers to treating with empiric narrow spectrum antibiotics – and obviously, one of the things people are concerned about is that are we going to miss something,” she said.
“I think we can pretty confidently say that if it’s uncomplicated CAP – if there’s no pleural effusion, no necrosis, no cavitation – you can treat with narrow spectrum, and the likelihood of it being staph is slim to none.”
If within 48 hours, patients are not responding to the first-line treatment, “you should start thinking about other causes,” Dr. Hofto said, noting that her review found all staph aureus patients were started on antibiotics effective against S. aureus – mostly vancomycin and ceftriaxone – within 24 hours of presentation.
Dr. Hofto noted as a limitation of her study, which used retrospective chart reviews of more than 3,400 children hospitalized for suspected pneumonia over a 3-year period, that additional S. aureus cases could have been missed because of a lack of proper coding or microbial confirmation. Another limitation was the single-site design and the relatively small number of S. aureus cases.
Dr. Hofto said she is conducting a more in-depth chart review ensure that no further cases of S. aureus CAP were missed in her sample.
The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
SAN ANTONIO – Current guidelines on community-acquired pneumonia recommend penicillin, amoxicillin, or ampicillin as first-line treatment in children with CAP.
However, a small minority will have Staphylococcus aureus infections not treatable with these antibiotics, raising some concern about how many of these cases might be missed.
At the Pediatric Hospital Medicine 2015 meeting, Dr. Meghan E. Hofto of Children’s of Alabama at the University of Alabama, Birmingham, presented research from a study of 554 patients admitted to the hospital with community acquired pneumonia, including 78 patients with complicated pneumonia.
Seven patients in the cohort (1.3%) had S. aureus infections, Dr. Hofto and her colleagues found. Of those, six were recorded as having complicated pneumonia, characterized by pleural effusion or cavitation.
Six patients with S. aureus had been started on antibiotics in other health care settings prior to admission (amoxicillin n = 4, multiple agents n = 2). One patient positive for flu was first treated with oseltamavir only. However, all staph patients once admitted were started on vancomycin, which is effective against S. aureus, within 24 hours. Five were diagnosed by pleural fluid culture; one case was identified by clinical presentation, and another by sputum culture, Dr. Hofto said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
The S. aureus patients were younger than the cohort as a whole (median 18 months vs. 40.5 months). Length of stay was significantly longer for these patients, compared with the rest of the cohort (median 10 vs. 2 days, P less than .01), and S. aureus patients had significantly higher incidence of anemia (P less than .01), a finding that Dr. Hofto said was striking.
Within 24 hours of presentation, six out the seven staph cases had anemia, she said, while of all the 78 patients with complicated disease, 12 had anemia. Community acquired S. aureus pneumonia has been linked in other studies to severe leukopenia, Dr. Hofto noted (BMC Infect Dis. 2013;13:359) and (Paediatric Respiratory Reviews 2011 Sept;12:182-9).In an interview, Dr. Hofto said the findings supported current guidance in favor of first-line penicillin, amoxicillin, or ampicillin. “Part of what we’re looking at with guideline adherence is the barriers to treating with empiric narrow spectrum antibiotics – and obviously, one of the things people are concerned about is that are we going to miss something,” she said.
“I think we can pretty confidently say that if it’s uncomplicated CAP – if there’s no pleural effusion, no necrosis, no cavitation – you can treat with narrow spectrum, and the likelihood of it being staph is slim to none.”
If within 48 hours, patients are not responding to the first-line treatment, “you should start thinking about other causes,” Dr. Hofto said, noting that her review found all staph aureus patients were started on antibiotics effective against S. aureus – mostly vancomycin and ceftriaxone – within 24 hours of presentation.
Dr. Hofto noted as a limitation of her study, which used retrospective chart reviews of more than 3,400 children hospitalized for suspected pneumonia over a 3-year period, that additional S. aureus cases could have been missed because of a lack of proper coding or microbial confirmation. Another limitation was the single-site design and the relatively small number of S. aureus cases.
Dr. Hofto said she is conducting a more in-depth chart review ensure that no further cases of S. aureus CAP were missed in her sample.
The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
SAN ANTONIO – Current guidelines on community-acquired pneumonia recommend penicillin, amoxicillin, or ampicillin as first-line treatment in children with CAP.
However, a small minority will have Staphylococcus aureus infections not treatable with these antibiotics, raising some concern about how many of these cases might be missed.
At the Pediatric Hospital Medicine 2015 meeting, Dr. Meghan E. Hofto of Children’s of Alabama at the University of Alabama, Birmingham, presented research from a study of 554 patients admitted to the hospital with community acquired pneumonia, including 78 patients with complicated pneumonia.
Seven patients in the cohort (1.3%) had S. aureus infections, Dr. Hofto and her colleagues found. Of those, six were recorded as having complicated pneumonia, characterized by pleural effusion or cavitation.
Six patients with S. aureus had been started on antibiotics in other health care settings prior to admission (amoxicillin n = 4, multiple agents n = 2). One patient positive for flu was first treated with oseltamavir only. However, all staph patients once admitted were started on vancomycin, which is effective against S. aureus, within 24 hours. Five were diagnosed by pleural fluid culture; one case was identified by clinical presentation, and another by sputum culture, Dr. Hofto said at the meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
The S. aureus patients were younger than the cohort as a whole (median 18 months vs. 40.5 months). Length of stay was significantly longer for these patients, compared with the rest of the cohort (median 10 vs. 2 days, P less than .01), and S. aureus patients had significantly higher incidence of anemia (P less than .01), a finding that Dr. Hofto said was striking.
Within 24 hours of presentation, six out the seven staph cases had anemia, she said, while of all the 78 patients with complicated disease, 12 had anemia. Community acquired S. aureus pneumonia has been linked in other studies to severe leukopenia, Dr. Hofto noted (BMC Infect Dis. 2013;13:359) and (Paediatric Respiratory Reviews 2011 Sept;12:182-9).In an interview, Dr. Hofto said the findings supported current guidance in favor of first-line penicillin, amoxicillin, or ampicillin. “Part of what we’re looking at with guideline adherence is the barriers to treating with empiric narrow spectrum antibiotics – and obviously, one of the things people are concerned about is that are we going to miss something,” she said.
“I think we can pretty confidently say that if it’s uncomplicated CAP – if there’s no pleural effusion, no necrosis, no cavitation – you can treat with narrow spectrum, and the likelihood of it being staph is slim to none.”
If within 48 hours, patients are not responding to the first-line treatment, “you should start thinking about other causes,” Dr. Hofto said, noting that her review found all staph aureus patients were started on antibiotics effective against S. aureus – mostly vancomycin and ceftriaxone – within 24 hours of presentation.
Dr. Hofto noted as a limitation of her study, which used retrospective chart reviews of more than 3,400 children hospitalized for suspected pneumonia over a 3-year period, that additional S. aureus cases could have been missed because of a lack of proper coding or microbial confirmation. Another limitation was the single-site design and the relatively small number of S. aureus cases.
Dr. Hofto said she is conducting a more in-depth chart review ensure that no further cases of S. aureus CAP were missed in her sample.
The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
AT PEDIATRIC HOSPITAL MEDICINE 2015
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended first-line narrow spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, 7 had S. aureus infections, 6 classed as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S. aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
Mobile health technology may encourage behavioral change
Mobile health technology such as wearable monitors, applications, and text-messaging programs may encourage behavioral change, according to a scientific statement by the American Heart Association published Aug. 13.
With cardiovascular disease, heart disease, and stroke remaining a major cause of morbidity and mortality, the AHA has identified four behaviors as part of its 2020 Strategic Impact Goals: promoting physical activity and healthful eating, and reducing weight and tobacco abuse. The AHA included blood pressure, cholesterol, and glucose as health indicators. Together these make up the AHA’s Life’s Simple 7. When taking into account these seven factors, 32% of Americans have a normal body mass index, less than 1% eat healthfully, and more than 30% have not achieved blood pressure or lipid goals.
Therefore, Lora Burke, Ph.D., of the School of Nursing at the University of Pittsburgh, and her colleagues sought to review the studies looking at mobile health interventions’ impact on the CV health indicators and targeted behaviors.
The researchers conducted an English language literature search focused on various mobile health terms and clinical diagnoses and limited to the period of 2004-2014.
Mobile health technology is beneficial for short-term weight loss when included with comprehensive lifestyle interventions. For patients who need to lose weight, physicians should consider recommending mobile health programs that are evidence based and take into account behavioral approaches, healthful eating, and physical activity. Likewise, feedback, social support, and self-monitoring capabilities should be available in the mobile health program.
More than 20% of American adults are using a form of health tracking, but there are not enough data to know if wearable tracking devices are effective at increasing physical activity. Likewise, the associated applications lack studies on their efficacy. The authors note that combining group behavioral interventions with activity trackers may result in more weight loss than with either the group intervention or tracking device alone.
Smoking cessation guidelines encourage the five As: ask, advise, assess, assist, and arrange, but clinicians are providing assessment of tobacco abuse, assistance in cessation, and arrangement for follow-up of the cessation program less often.
Several studies have shown nearly twice the abstinence rates with use of text messaging programs, but most were unsuccessful after 6 months, despite the mobile health device. Therefore, text messaging smoking cessation programs should be considered only if combined with another proven cessation intervention, the researchers noted.
In terms of the use of mobile health technology in improving glucose, blood pressure, and lipids, the data are limited.
Addressing cardiovascular disease risk factors before the development of CVD is associated with reduced CVD mortality. Given the limited face-to-face time physicians have with patients, mobile health technologies have the potential to expand the options to encourage behavioral change in patients.
“Using mHealth tools for monitoring provides the clinician data that far exceed what can be measured in the brief clinical encounter and reflect the status of physiological or behavioral measures in the person’s natural setting,” the authors concluded (Circulation. 2015 Aug. 13. doi: 10.1161/CIR.0000000000000232.)
The authors reported multiple disclosures including involvement with Scale Down, Coeus Health, Actigraph, Open mHealth, and WellDoc which are health technology companies.
Mobile health technology such as wearable monitors, applications, and text-messaging programs may encourage behavioral change, according to a scientific statement by the American Heart Association published Aug. 13.
With cardiovascular disease, heart disease, and stroke remaining a major cause of morbidity and mortality, the AHA has identified four behaviors as part of its 2020 Strategic Impact Goals: promoting physical activity and healthful eating, and reducing weight and tobacco abuse. The AHA included blood pressure, cholesterol, and glucose as health indicators. Together these make up the AHA’s Life’s Simple 7. When taking into account these seven factors, 32% of Americans have a normal body mass index, less than 1% eat healthfully, and more than 30% have not achieved blood pressure or lipid goals.
Therefore, Lora Burke, Ph.D., of the School of Nursing at the University of Pittsburgh, and her colleagues sought to review the studies looking at mobile health interventions’ impact on the CV health indicators and targeted behaviors.
The researchers conducted an English language literature search focused on various mobile health terms and clinical diagnoses and limited to the period of 2004-2014.
Mobile health technology is beneficial for short-term weight loss when included with comprehensive lifestyle interventions. For patients who need to lose weight, physicians should consider recommending mobile health programs that are evidence based and take into account behavioral approaches, healthful eating, and physical activity. Likewise, feedback, social support, and self-monitoring capabilities should be available in the mobile health program.
More than 20% of American adults are using a form of health tracking, but there are not enough data to know if wearable tracking devices are effective at increasing physical activity. Likewise, the associated applications lack studies on their efficacy. The authors note that combining group behavioral interventions with activity trackers may result in more weight loss than with either the group intervention or tracking device alone.
Smoking cessation guidelines encourage the five As: ask, advise, assess, assist, and arrange, but clinicians are providing assessment of tobacco abuse, assistance in cessation, and arrangement for follow-up of the cessation program less often.
Several studies have shown nearly twice the abstinence rates with use of text messaging programs, but most were unsuccessful after 6 months, despite the mobile health device. Therefore, text messaging smoking cessation programs should be considered only if combined with another proven cessation intervention, the researchers noted.
In terms of the use of mobile health technology in improving glucose, blood pressure, and lipids, the data are limited.
Addressing cardiovascular disease risk factors before the development of CVD is associated with reduced CVD mortality. Given the limited face-to-face time physicians have with patients, mobile health technologies have the potential to expand the options to encourage behavioral change in patients.
“Using mHealth tools for monitoring provides the clinician data that far exceed what can be measured in the brief clinical encounter and reflect the status of physiological or behavioral measures in the person’s natural setting,” the authors concluded (Circulation. 2015 Aug. 13. doi: 10.1161/CIR.0000000000000232.)
The authors reported multiple disclosures including involvement with Scale Down, Coeus Health, Actigraph, Open mHealth, and WellDoc which are health technology companies.
Mobile health technology such as wearable monitors, applications, and text-messaging programs may encourage behavioral change, according to a scientific statement by the American Heart Association published Aug. 13.
With cardiovascular disease, heart disease, and stroke remaining a major cause of morbidity and mortality, the AHA has identified four behaviors as part of its 2020 Strategic Impact Goals: promoting physical activity and healthful eating, and reducing weight and tobacco abuse. The AHA included blood pressure, cholesterol, and glucose as health indicators. Together these make up the AHA’s Life’s Simple 7. When taking into account these seven factors, 32% of Americans have a normal body mass index, less than 1% eat healthfully, and more than 30% have not achieved blood pressure or lipid goals.
Therefore, Lora Burke, Ph.D., of the School of Nursing at the University of Pittsburgh, and her colleagues sought to review the studies looking at mobile health interventions’ impact on the CV health indicators and targeted behaviors.
The researchers conducted an English language literature search focused on various mobile health terms and clinical diagnoses and limited to the period of 2004-2014.
Mobile health technology is beneficial for short-term weight loss when included with comprehensive lifestyle interventions. For patients who need to lose weight, physicians should consider recommending mobile health programs that are evidence based and take into account behavioral approaches, healthful eating, and physical activity. Likewise, feedback, social support, and self-monitoring capabilities should be available in the mobile health program.
More than 20% of American adults are using a form of health tracking, but there are not enough data to know if wearable tracking devices are effective at increasing physical activity. Likewise, the associated applications lack studies on their efficacy. The authors note that combining group behavioral interventions with activity trackers may result in more weight loss than with either the group intervention or tracking device alone.
Smoking cessation guidelines encourage the five As: ask, advise, assess, assist, and arrange, but clinicians are providing assessment of tobacco abuse, assistance in cessation, and arrangement for follow-up of the cessation program less often.
Several studies have shown nearly twice the abstinence rates with use of text messaging programs, but most were unsuccessful after 6 months, despite the mobile health device. Therefore, text messaging smoking cessation programs should be considered only if combined with another proven cessation intervention, the researchers noted.
In terms of the use of mobile health technology in improving glucose, blood pressure, and lipids, the data are limited.
Addressing cardiovascular disease risk factors before the development of CVD is associated with reduced CVD mortality. Given the limited face-to-face time physicians have with patients, mobile health technologies have the potential to expand the options to encourage behavioral change in patients.
“Using mHealth tools for monitoring provides the clinician data that far exceed what can be measured in the brief clinical encounter and reflect the status of physiological or behavioral measures in the person’s natural setting,” the authors concluded (Circulation. 2015 Aug. 13. doi: 10.1161/CIR.0000000000000232.)
The authors reported multiple disclosures including involvement with Scale Down, Coeus Health, Actigraph, Open mHealth, and WellDoc which are health technology companies.
FROM CIRCULATION
Inpatient mortality down for high-volume conditions
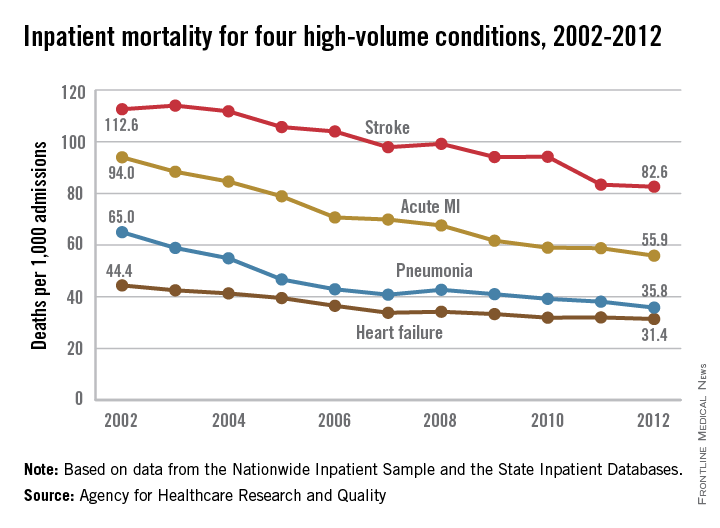
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Inpatient mortality for pneumonia, acute MI, heart failure, and stroke each fell significantly from 2002 to 2012, the Agency for Healthcare Research and Quality reported.
Over that period, mortality among adults hospitalized with pneumonia went from 65 per 1,000 admissions to 35.8 per 1,000 for a drop of 45% – largest of the four high-volume conditions. Corresponding declines for the others were 41% for acute MI, 29% for heart failure, and 27% for stroke, the AHRQ noted.

Since “death following discharge from a hospital is not reflected in these data,” the report said, measures of inpatient mortality “can reflect both improvements in health care and shifts in where end-of-life care takes place over time.”
The estimates in the report are based on data from the Nationwide Inpatient Sample (2002-2011) and State Inpatient Databases (2012).
Continuous albuterol is safe intermediate-care option in community hospitals
SAN ANTONIO – More community hospitals are offering continuous albuterol to pediatric patients as an intermediate-care service, a level of care that’s higher than usual for the pediatric ward, but not as high as in pediatric intensive care units, according to Dr. Michelle Hofmann.
A 2014 study established that continuous albuterol can be initiated safely in the nonintensive pediatric care setting, but not all hospitals have followed suit (Pediatrics 2014;134[4]:e976-82).
At the Pediatric Hospital Medicine 2015 meeting, Dr. Hofmann, a pediatric hospitalist with the University of Utah, Salt Lake City, and the Riverton (Utah) Hospital, presented findings from a study she conducted to determine whether initiating continuous albuterol outside the PICU was worthwhile, safe, and feasible in her own community hospital. She found that it was.
“When we looked at the patients we were transferring to the PICU, nothing else was happening to them – all they were getting was continuous albuterol once they got there,” Dr. Hofmann said at the conference. “And we thought, ‘we [hospitalists] are on site 24/7. How do we capitalize on our resource?”
Dr. Hofmann and her colleagues first conducted a 1-year pilot study of pediatric asthma admissions (n = 76) at Primary Children’s Hospital in Salt Lake City, which like Riverton is part of the Intermountain Healthcare network. There, children were treated with continuous albuterol in the PICU only. Dr. Hofmann and her colleagues found that most children required no additional resources beyond continuous albuterol once they were admitted to the PICU from either the floor or the emergency department.
Moreover, patients classified as severe who got started on continuous albuterol in the ED and went right to the PICU did better than those who got admitted to the floor, were given intermittent albuterol, deteriorated, and then were transferred to intensive care, Dr. Hofmann said. This indicated that best practice was not occurring, even at the children’s hospital.
Dr. Hofmann and her colleagues then developed a protocol for her community hospital in which children presenting with asthma could be started on continuous albuterol in the emergency department or on the ward. She set up a pilot study to evaluate its safety and feasibility.
Of 74 asthma admissions over the 1-year pilot period, 22% of patients (n = 16) received continuous albuterol on the floor. Most of these (75%) received all their care on the floor (mean length of stay 30 hours), while those who deteriorated were transferred to the PICU; four additional cases were transferred directly from the ED to the PICU. In only two cases transferred from the community to children’s hospital did patients require care beyond continuous albuterol.
Dr. Hofmann noted that while more community hospitals are administering continuous albuterol outside the PICU, it was important to consider the benefits or drawbacks on a case-by-case basis.
A community hospital has the advantage of “not dealing with all the different layers of physicians involved in care” in a children’s hospital, Dr. Hofmann said in an interview. “Our costs are lower, in part due to shorter length of stay, but also we have a different cost structure. It’s a small self-contained unit, and our facilities are closer to home for many families.”
However, she said, the continuous albuterol intermediate-care protocol may not suit all community hospitals. “There are significant differences in personnel, facilities, and diagnostic and treatment capabilities from hospital to hospital; there’s no set criteria that will apply at every institution for intermediate care,” Dr. Hofmann said. The feasibility of appropriate staffing and continuous monitoring capabilities and the cost-benefit of achieving these in a lower-volume program are important considerations.
Hospitals should consider “what your baseline transfer rate is, and the kinds of patients you’re already seeing. Are you really going to be able to improve it that much, to provide the extra infrastructure and work to develop this process? Will you capture that many more patients?”
Offering intermediate-care services such as continuous albuterol, which can be billed at a higher level of care, is one way to help make community hospitals’ pediatric programs more sustainable. “We tend to operate in the red,” Dr. Hofmann said, because “pediatrics is not a high revenue earner for a facility. Moving to these intermediate-level care options and figuring out what is safe and what we can keep in the community hospital is really important to us – this is just one example of ways we could do that.”
Dr. Hofmann noted as a limitation of her study the low patient volume and the fact that some asthma patients may have been transferred from the community hospital’s ED to the children’s hospital year over year, and these were only captured during the pilot study, though it may be that ED transfer rates are decreasing as well as a result of the protocol.
The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association. Dr. Hofmann reported no outside funding for her study or conflicts of interest.
SAN ANTONIO – More community hospitals are offering continuous albuterol to pediatric patients as an intermediate-care service, a level of care that’s higher than usual for the pediatric ward, but not as high as in pediatric intensive care units, according to Dr. Michelle Hofmann.
A 2014 study established that continuous albuterol can be initiated safely in the nonintensive pediatric care setting, but not all hospitals have followed suit (Pediatrics 2014;134[4]:e976-82).
At the Pediatric Hospital Medicine 2015 meeting, Dr. Hofmann, a pediatric hospitalist with the University of Utah, Salt Lake City, and the Riverton (Utah) Hospital, presented findings from a study she conducted to determine whether initiating continuous albuterol outside the PICU was worthwhile, safe, and feasible in her own community hospital. She found that it was.
“When we looked at the patients we were transferring to the PICU, nothing else was happening to them – all they were getting was continuous albuterol once they got there,” Dr. Hofmann said at the conference. “And we thought, ‘we [hospitalists] are on site 24/7. How do we capitalize on our resource?”
Dr. Hofmann and her colleagues first conducted a 1-year pilot study of pediatric asthma admissions (n = 76) at Primary Children’s Hospital in Salt Lake City, which like Riverton is part of the Intermountain Healthcare network. There, children were treated with continuous albuterol in the PICU only. Dr. Hofmann and her colleagues found that most children required no additional resources beyond continuous albuterol once they were admitted to the PICU from either the floor or the emergency department.
Moreover, patients classified as severe who got started on continuous albuterol in the ED and went right to the PICU did better than those who got admitted to the floor, were given intermittent albuterol, deteriorated, and then were transferred to intensive care, Dr. Hofmann said. This indicated that best practice was not occurring, even at the children’s hospital.
Dr. Hofmann and her colleagues then developed a protocol for her community hospital in which children presenting with asthma could be started on continuous albuterol in the emergency department or on the ward. She set up a pilot study to evaluate its safety and feasibility.
Of 74 asthma admissions over the 1-year pilot period, 22% of patients (n = 16) received continuous albuterol on the floor. Most of these (75%) received all their care on the floor (mean length of stay 30 hours), while those who deteriorated were transferred to the PICU; four additional cases were transferred directly from the ED to the PICU. In only two cases transferred from the community to children’s hospital did patients require care beyond continuous albuterol.
Dr. Hofmann noted that while more community hospitals are administering continuous albuterol outside the PICU, it was important to consider the benefits or drawbacks on a case-by-case basis.
A community hospital has the advantage of “not dealing with all the different layers of physicians involved in care” in a children’s hospital, Dr. Hofmann said in an interview. “Our costs are lower, in part due to shorter length of stay, but also we have a different cost structure. It’s a small self-contained unit, and our facilities are closer to home for many families.”
However, she said, the continuous albuterol intermediate-care protocol may not suit all community hospitals. “There are significant differences in personnel, facilities, and diagnostic and treatment capabilities from hospital to hospital; there’s no set criteria that will apply at every institution for intermediate care,” Dr. Hofmann said. The feasibility of appropriate staffing and continuous monitoring capabilities and the cost-benefit of achieving these in a lower-volume program are important considerations.
Hospitals should consider “what your baseline transfer rate is, and the kinds of patients you’re already seeing. Are you really going to be able to improve it that much, to provide the extra infrastructure and work to develop this process? Will you capture that many more patients?”
Offering intermediate-care services such as continuous albuterol, which can be billed at a higher level of care, is one way to help make community hospitals’ pediatric programs more sustainable. “We tend to operate in the red,” Dr. Hofmann said, because “pediatrics is not a high revenue earner for a facility. Moving to these intermediate-level care options and figuring out what is safe and what we can keep in the community hospital is really important to us – this is just one example of ways we could do that.”
Dr. Hofmann noted as a limitation of her study the low patient volume and the fact that some asthma patients may have been transferred from the community hospital’s ED to the children’s hospital year over year, and these were only captured during the pilot study, though it may be that ED transfer rates are decreasing as well as a result of the protocol.
The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association. Dr. Hofmann reported no outside funding for her study or conflicts of interest.
SAN ANTONIO – More community hospitals are offering continuous albuterol to pediatric patients as an intermediate-care service, a level of care that’s higher than usual for the pediatric ward, but not as high as in pediatric intensive care units, according to Dr. Michelle Hofmann.
A 2014 study established that continuous albuterol can be initiated safely in the nonintensive pediatric care setting, but not all hospitals have followed suit (Pediatrics 2014;134[4]:e976-82).
At the Pediatric Hospital Medicine 2015 meeting, Dr. Hofmann, a pediatric hospitalist with the University of Utah, Salt Lake City, and the Riverton (Utah) Hospital, presented findings from a study she conducted to determine whether initiating continuous albuterol outside the PICU was worthwhile, safe, and feasible in her own community hospital. She found that it was.
“When we looked at the patients we were transferring to the PICU, nothing else was happening to them – all they were getting was continuous albuterol once they got there,” Dr. Hofmann said at the conference. “And we thought, ‘we [hospitalists] are on site 24/7. How do we capitalize on our resource?”
Dr. Hofmann and her colleagues first conducted a 1-year pilot study of pediatric asthma admissions (n = 76) at Primary Children’s Hospital in Salt Lake City, which like Riverton is part of the Intermountain Healthcare network. There, children were treated with continuous albuterol in the PICU only. Dr. Hofmann and her colleagues found that most children required no additional resources beyond continuous albuterol once they were admitted to the PICU from either the floor or the emergency department.
Moreover, patients classified as severe who got started on continuous albuterol in the ED and went right to the PICU did better than those who got admitted to the floor, were given intermittent albuterol, deteriorated, and then were transferred to intensive care, Dr. Hofmann said. This indicated that best practice was not occurring, even at the children’s hospital.
Dr. Hofmann and her colleagues then developed a protocol for her community hospital in which children presenting with asthma could be started on continuous albuterol in the emergency department or on the ward. She set up a pilot study to evaluate its safety and feasibility.
Of 74 asthma admissions over the 1-year pilot period, 22% of patients (n = 16) received continuous albuterol on the floor. Most of these (75%) received all their care on the floor (mean length of stay 30 hours), while those who deteriorated were transferred to the PICU; four additional cases were transferred directly from the ED to the PICU. In only two cases transferred from the community to children’s hospital did patients require care beyond continuous albuterol.
Dr. Hofmann noted that while more community hospitals are administering continuous albuterol outside the PICU, it was important to consider the benefits or drawbacks on a case-by-case basis.
A community hospital has the advantage of “not dealing with all the different layers of physicians involved in care” in a children’s hospital, Dr. Hofmann said in an interview. “Our costs are lower, in part due to shorter length of stay, but also we have a different cost structure. It’s a small self-contained unit, and our facilities are closer to home for many families.”
However, she said, the continuous albuterol intermediate-care protocol may not suit all community hospitals. “There are significant differences in personnel, facilities, and diagnostic and treatment capabilities from hospital to hospital; there’s no set criteria that will apply at every institution for intermediate care,” Dr. Hofmann said. The feasibility of appropriate staffing and continuous monitoring capabilities and the cost-benefit of achieving these in a lower-volume program are important considerations.
Hospitals should consider “what your baseline transfer rate is, and the kinds of patients you’re already seeing. Are you really going to be able to improve it that much, to provide the extra infrastructure and work to develop this process? Will you capture that many more patients?”
Offering intermediate-care services such as continuous albuterol, which can be billed at a higher level of care, is one way to help make community hospitals’ pediatric programs more sustainable. “We tend to operate in the red,” Dr. Hofmann said, because “pediatrics is not a high revenue earner for a facility. Moving to these intermediate-level care options and figuring out what is safe and what we can keep in the community hospital is really important to us – this is just one example of ways we could do that.”
Dr. Hofmann noted as a limitation of her study the low patient volume and the fact that some asthma patients may have been transferred from the community hospital’s ED to the children’s hospital year over year, and these were only captured during the pilot study, though it may be that ED transfer rates are decreasing as well as a result of the protocol.
The meeting was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association. Dr. Hofmann reported no outside funding for her study or conflicts of interest.
AT PEDIATRIC HOSPITAL MEDICINE 2015
Key clinical point:With proper protocols, community hospitals can safely offer continuous albuterol in pediatric wards instead of mandating transfer to PICUs.
Major finding: Of 74 pediatric asthma admissions over a 12-month period, 22% of patients (n = 16) received continuous albuterol on the floor, with 75% receiving all their care on the floor. Most cases transferred to PICUs received no further care beyond continuous albuterol.
Data source: A single-site study at a community hospital, with comparison data also collected from a network-associated children’s hospital.
Disclosures: Dr. Hofmann had no relevant financial disclosures.
What Matters: Sleep restriction
At one time or another, insomnia afflicts nearly one-half of U.S. adults, half of whom have a clinically diagnosable disorder. This presents perpetual challenges in the face of patient populations that have been told to “ask your doctor about” sleeping medications or have received them already.
We know that the Z-drugs (zolpidem, zaleplon, and eszopiclone), some of the most widely used pharmacologics for insomnia, are benzodiazepine receptor agonists. As such, tolerance develops, and this tolerance leads to escalating doses, increased side effects, and sleepier patients.
Cognitive-behavioral therapy has been shown to be effective for insomnia, but this clinical service is not widely available. For busy clinicians trying to help these patients, we need a simple tool that can be easily explained to patients, giving them a project on which to work.
This tool is sleep restriction. The goal of sleep restriction is to consolidate fragmented sleep to increase the intrinsic sleep drive.
You might have heard your patients describe their bedroom as a “torture chamber.” Some of this torture relates to sleepless staring at the ceiling for hours on end. Sleep restriction gets them out of the chamber.
Karen Falloon, Ph.D., of the University of Auckland (New Zealand), and her colleagues conducted a randomized trial in New Zealand investigating the impact of simplified sleep restriction (SSR) for patients with primary insomnia (Br J Gen Pract. 2015 Aug;65(637):e508-15).
A total of 97 patients were randomized. All patients received sleep hygiene advice, including avoiding caffeine and developing a consistent bedtime routine. Patients in the SSR arm received a verbal and written sleep prescription establishing bedtime and wake-up times informed by a baseline 2-week daily sleep diary.
The sleep prescription was average total sleep duration plus 50% of the total time spent awake in bed. The minimum time in bed was 5 hours. If participants were sleeping less than 85% of the time in bed, the time allowed in bed was reduced to total sleep time plus 30 minutes. Sleepy patients could spend 30 more minutes in bed. All changes were made at bedtime, with wake-up time held constant.
At 6 months, the SSR group had improved perceived sleep quality and fatigue, and improved sleep efficiency as measured by actigraphy. A total of 67% of patients responded to SSR, compared with 41% of controls (number needed to treat = 4).
The efficacy of this intervention is extremely impressive. Importantly, it was delivered by a general practitioner without specialized training during two “slightly extended” visits.
Potential participants were excluded if they were on a sleeping medication, which does not imply that this would not work in a population already on Z-drugs. Consideration should to be given to possible risks when implementing sleep restriction with patients taking Z-drugs with longer half-lives (for example, eszopiclone is 6 hours, zolpidem is 3 hours, and zaleplon is 1 hour), because of higher serum concentrations upon waking.
But when these medications fail, or you have Z-drug–naive patients with insomnia, have this intervention ready.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
At one time or another, insomnia afflicts nearly one-half of U.S. adults, half of whom have a clinically diagnosable disorder. This presents perpetual challenges in the face of patient populations that have been told to “ask your doctor about” sleeping medications or have received them already.
We know that the Z-drugs (zolpidem, zaleplon, and eszopiclone), some of the most widely used pharmacologics for insomnia, are benzodiazepine receptor agonists. As such, tolerance develops, and this tolerance leads to escalating doses, increased side effects, and sleepier patients.
Cognitive-behavioral therapy has been shown to be effective for insomnia, but this clinical service is not widely available. For busy clinicians trying to help these patients, we need a simple tool that can be easily explained to patients, giving them a project on which to work.
This tool is sleep restriction. The goal of sleep restriction is to consolidate fragmented sleep to increase the intrinsic sleep drive.
You might have heard your patients describe their bedroom as a “torture chamber.” Some of this torture relates to sleepless staring at the ceiling for hours on end. Sleep restriction gets them out of the chamber.
Karen Falloon, Ph.D., of the University of Auckland (New Zealand), and her colleagues conducted a randomized trial in New Zealand investigating the impact of simplified sleep restriction (SSR) for patients with primary insomnia (Br J Gen Pract. 2015 Aug;65(637):e508-15).
A total of 97 patients were randomized. All patients received sleep hygiene advice, including avoiding caffeine and developing a consistent bedtime routine. Patients in the SSR arm received a verbal and written sleep prescription establishing bedtime and wake-up times informed by a baseline 2-week daily sleep diary.
The sleep prescription was average total sleep duration plus 50% of the total time spent awake in bed. The minimum time in bed was 5 hours. If participants were sleeping less than 85% of the time in bed, the time allowed in bed was reduced to total sleep time plus 30 minutes. Sleepy patients could spend 30 more minutes in bed. All changes were made at bedtime, with wake-up time held constant.
At 6 months, the SSR group had improved perceived sleep quality and fatigue, and improved sleep efficiency as measured by actigraphy. A total of 67% of patients responded to SSR, compared with 41% of controls (number needed to treat = 4).
The efficacy of this intervention is extremely impressive. Importantly, it was delivered by a general practitioner without specialized training during two “slightly extended” visits.
Potential participants were excluded if they were on a sleeping medication, which does not imply that this would not work in a population already on Z-drugs. Consideration should to be given to possible risks when implementing sleep restriction with patients taking Z-drugs with longer half-lives (for example, eszopiclone is 6 hours, zolpidem is 3 hours, and zaleplon is 1 hour), because of higher serum concentrations upon waking.
But when these medications fail, or you have Z-drug–naive patients with insomnia, have this intervention ready.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
At one time or another, insomnia afflicts nearly one-half of U.S. adults, half of whom have a clinically diagnosable disorder. This presents perpetual challenges in the face of patient populations that have been told to “ask your doctor about” sleeping medications or have received them already.
We know that the Z-drugs (zolpidem, zaleplon, and eszopiclone), some of the most widely used pharmacologics for insomnia, are benzodiazepine receptor agonists. As such, tolerance develops, and this tolerance leads to escalating doses, increased side effects, and sleepier patients.
Cognitive-behavioral therapy has been shown to be effective for insomnia, but this clinical service is not widely available. For busy clinicians trying to help these patients, we need a simple tool that can be easily explained to patients, giving them a project on which to work.
This tool is sleep restriction. The goal of sleep restriction is to consolidate fragmented sleep to increase the intrinsic sleep drive.
You might have heard your patients describe their bedroom as a “torture chamber.” Some of this torture relates to sleepless staring at the ceiling for hours on end. Sleep restriction gets them out of the chamber.
Karen Falloon, Ph.D., of the University of Auckland (New Zealand), and her colleagues conducted a randomized trial in New Zealand investigating the impact of simplified sleep restriction (SSR) for patients with primary insomnia (Br J Gen Pract. 2015 Aug;65(637):e508-15).
A total of 97 patients were randomized. All patients received sleep hygiene advice, including avoiding caffeine and developing a consistent bedtime routine. Patients in the SSR arm received a verbal and written sleep prescription establishing bedtime and wake-up times informed by a baseline 2-week daily sleep diary.
The sleep prescription was average total sleep duration plus 50% of the total time spent awake in bed. The minimum time in bed was 5 hours. If participants were sleeping less than 85% of the time in bed, the time allowed in bed was reduced to total sleep time plus 30 minutes. Sleepy patients could spend 30 more minutes in bed. All changes were made at bedtime, with wake-up time held constant.
At 6 months, the SSR group had improved perceived sleep quality and fatigue, and improved sleep efficiency as measured by actigraphy. A total of 67% of patients responded to SSR, compared with 41% of controls (number needed to treat = 4).
The efficacy of this intervention is extremely impressive. Importantly, it was delivered by a general practitioner without specialized training during two “slightly extended” visits.
Potential participants were excluded if they were on a sleeping medication, which does not imply that this would not work in a population already on Z-drugs. Consideration should to be given to possible risks when implementing sleep restriction with patients taking Z-drugs with longer half-lives (for example, eszopiclone is 6 hours, zolpidem is 3 hours, and zaleplon is 1 hour), because of higher serum concentrations upon waking.
But when these medications fail, or you have Z-drug–naive patients with insomnia, have this intervention ready.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition, nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article. Follow him on Twitter @jonebbert.
Proglycem found to increase pulmonary hypertension risk in infants
Infants and newborns who have been treated with Proglycem (diazoxide) for low blood pressure are at risk for pulmonary hypertension, according to a press release from the Food and Drug Administration.
As Proglycem is usually given in a hospital setting, all babies receiving it should be closely monitored by a health care professional, especially if a risk factor for pulmonary hypertension is present, such as meconium aspiration syndrome, respiratory distress syndrome, transient tachypnea of the newborn, pneumonia, sepsis, congenital diaphragmatic hernia, and congenital heart disease, the FDA said.
The FDA identified 11 cases of pulmonary hypertension since Proglycem was approved in 1973. In all cases, pulmonary hypertension was stopped or was improved by stopping Proglycem treatment.
If a parent or caregiver notices any sign of difficulty breathing such as flaring nostrils, grunting, unusual movement of their child’s chest, rapid breathing, difficulty feeding, or a bluish color of the lips or skin, he or she should contact a doctor immediately, the FDA recommended.
“FDA is continuing to investigate this safety issue and will determine whether changes are needed in the Proglycem prescribing information,” the FDA said in the press release.
Infants and newborns who have been treated with Proglycem (diazoxide) for low blood pressure are at risk for pulmonary hypertension, according to a press release from the Food and Drug Administration.
As Proglycem is usually given in a hospital setting, all babies receiving it should be closely monitored by a health care professional, especially if a risk factor for pulmonary hypertension is present, such as meconium aspiration syndrome, respiratory distress syndrome, transient tachypnea of the newborn, pneumonia, sepsis, congenital diaphragmatic hernia, and congenital heart disease, the FDA said.
The FDA identified 11 cases of pulmonary hypertension since Proglycem was approved in 1973. In all cases, pulmonary hypertension was stopped or was improved by stopping Proglycem treatment.
If a parent or caregiver notices any sign of difficulty breathing such as flaring nostrils, grunting, unusual movement of their child’s chest, rapid breathing, difficulty feeding, or a bluish color of the lips or skin, he or she should contact a doctor immediately, the FDA recommended.
“FDA is continuing to investigate this safety issue and will determine whether changes are needed in the Proglycem prescribing information,” the FDA said in the press release.
Infants and newborns who have been treated with Proglycem (diazoxide) for low blood pressure are at risk for pulmonary hypertension, according to a press release from the Food and Drug Administration.
As Proglycem is usually given in a hospital setting, all babies receiving it should be closely monitored by a health care professional, especially if a risk factor for pulmonary hypertension is present, such as meconium aspiration syndrome, respiratory distress syndrome, transient tachypnea of the newborn, pneumonia, sepsis, congenital diaphragmatic hernia, and congenital heart disease, the FDA said.
The FDA identified 11 cases of pulmonary hypertension since Proglycem was approved in 1973. In all cases, pulmonary hypertension was stopped or was improved by stopping Proglycem treatment.
If a parent or caregiver notices any sign of difficulty breathing such as flaring nostrils, grunting, unusual movement of their child’s chest, rapid breathing, difficulty feeding, or a bluish color of the lips or skin, he or she should contact a doctor immediately, the FDA recommended.
“FDA is continuing to investigate this safety issue and will determine whether changes are needed in the Proglycem prescribing information,” the FDA said in the press release.
Hypertonic saline linked to modest drop in length of stay for bronchiolitis
SAN ANTONIO – Nebulized hypertonic saline has emerged as a promising, low-risk, and low-cost treatment option for bronchiolitis, a disease for which few options exist.
Still, the evidence for hypertonic saline remains inconsistent, with many studies failing to show a clear benefit in clinical scores or length of stay compared with normal saline in children hospitalized for bronchiolitis. Supportive care remains the recommended approach for this patient group.
A 2013 Cochrane review of trials comparing normal saline 0.9% with hypertonic saline 3% suggested that hypertonic saline reduced mean hospital length of stay by 1.3 days in children with bronchiolitis as well as improved clinical severity scores. But more recent negative trials have called the Cochrane findings into question.
At the Pediatric Hospital Medicine meeting, Dr. Corinne G. Brooks of the Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., presented an updated evaluation of the Cochrane findings as well as several newer studies, which found that study heterogeneity was excessively high, though resolved by accounting for culturally expected length of stay, which varied from 2 to 7 days across studies.
Studies conducted in countries where average length of stay for bronchiolitis in the placebo arm is longer than 3 days reported a greater benefit than did those in countries like the United States, in which stays typically average fewer than 3 days. Because hospital length of stay should be correlated with severity of illness, Dr. Brooks also evaluated respiratory scores available in each study and found that higher scores also correlated with the utility of hypertonic saline.
The two Chinese studies included in the review had particularly long lengths of stay and were conducted in a city with poor air quality, and in which about 70% of patients were on systemic corticosteroids at baseline. “So there’s some question as to whether they were even measuring the same disease process,” Dr. Brooks told the conference.
Dr. Brooks and her colleagues reevaluated the Cochrane data both with and without the two outlying studies from China, and incorporated results from three additional studies not included in the Cochrane review.
Modeling cumulative results using a length of stay typical in the United States, the benefit from hypertonic saline was a reduction of 0.31 days, or 31 days/100 patients.
Dr. Brooks noted that the current data are consistent with two possibilities, that the longer a patient is treated with hypertonic saline, the better it works or that “perhaps hypertonic saline works better for sicker patients – we don’t know yet.” She acknowledged several limitations of the meta-analysis, including that patient age, additional medications, day of illness at admission, and frequency of treatment could not be correlated.
The existing data “do not support rejecting hypertonic saline for all patients or giving it to all populations,” Dr. Brooks told the conference, noting that “there are good physiologic reasons to believe hypertonic saline might have an impact based on its benefits to other respiratory populations such as cystic fibrosis – and it’s particularly appealing because of its low risk and low cost.”
However, she said, “if we’re going to use this intervention intelligently, we need to find where there is and where there isn’t a benefit.”
Dr. Brooks concluded that hypertonic saline might be worth considering for patients with an expected stay of longer than 3 days or for those with baseline clinical scores higher than average. Her study received no outside funding, and she disclosed no conflicts of interest.
SAN ANTONIO – Nebulized hypertonic saline has emerged as a promising, low-risk, and low-cost treatment option for bronchiolitis, a disease for which few options exist.
Still, the evidence for hypertonic saline remains inconsistent, with many studies failing to show a clear benefit in clinical scores or length of stay compared with normal saline in children hospitalized for bronchiolitis. Supportive care remains the recommended approach for this patient group.
A 2013 Cochrane review of trials comparing normal saline 0.9% with hypertonic saline 3% suggested that hypertonic saline reduced mean hospital length of stay by 1.3 days in children with bronchiolitis as well as improved clinical severity scores. But more recent negative trials have called the Cochrane findings into question.
At the Pediatric Hospital Medicine meeting, Dr. Corinne G. Brooks of the Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., presented an updated evaluation of the Cochrane findings as well as several newer studies, which found that study heterogeneity was excessively high, though resolved by accounting for culturally expected length of stay, which varied from 2 to 7 days across studies.
Studies conducted in countries where average length of stay for bronchiolitis in the placebo arm is longer than 3 days reported a greater benefit than did those in countries like the United States, in which stays typically average fewer than 3 days. Because hospital length of stay should be correlated with severity of illness, Dr. Brooks also evaluated respiratory scores available in each study and found that higher scores also correlated with the utility of hypertonic saline.
The two Chinese studies included in the review had particularly long lengths of stay and were conducted in a city with poor air quality, and in which about 70% of patients were on systemic corticosteroids at baseline. “So there’s some question as to whether they were even measuring the same disease process,” Dr. Brooks told the conference.
Dr. Brooks and her colleagues reevaluated the Cochrane data both with and without the two outlying studies from China, and incorporated results from three additional studies not included in the Cochrane review.
Modeling cumulative results using a length of stay typical in the United States, the benefit from hypertonic saline was a reduction of 0.31 days, or 31 days/100 patients.
Dr. Brooks noted that the current data are consistent with two possibilities, that the longer a patient is treated with hypertonic saline, the better it works or that “perhaps hypertonic saline works better for sicker patients – we don’t know yet.” She acknowledged several limitations of the meta-analysis, including that patient age, additional medications, day of illness at admission, and frequency of treatment could not be correlated.
The existing data “do not support rejecting hypertonic saline for all patients or giving it to all populations,” Dr. Brooks told the conference, noting that “there are good physiologic reasons to believe hypertonic saline might have an impact based on its benefits to other respiratory populations such as cystic fibrosis – and it’s particularly appealing because of its low risk and low cost.”
However, she said, “if we’re going to use this intervention intelligently, we need to find where there is and where there isn’t a benefit.”
Dr. Brooks concluded that hypertonic saline might be worth considering for patients with an expected stay of longer than 3 days or for those with baseline clinical scores higher than average. Her study received no outside funding, and she disclosed no conflicts of interest.
SAN ANTONIO – Nebulized hypertonic saline has emerged as a promising, low-risk, and low-cost treatment option for bronchiolitis, a disease for which few options exist.
Still, the evidence for hypertonic saline remains inconsistent, with many studies failing to show a clear benefit in clinical scores or length of stay compared with normal saline in children hospitalized for bronchiolitis. Supportive care remains the recommended approach for this patient group.
A 2013 Cochrane review of trials comparing normal saline 0.9% with hypertonic saline 3% suggested that hypertonic saline reduced mean hospital length of stay by 1.3 days in children with bronchiolitis as well as improved clinical severity scores. But more recent negative trials have called the Cochrane findings into question.
At the Pediatric Hospital Medicine meeting, Dr. Corinne G. Brooks of the Children’s Hospital at Dartmouth-Hitchcock in Lebanon, N.H., presented an updated evaluation of the Cochrane findings as well as several newer studies, which found that study heterogeneity was excessively high, though resolved by accounting for culturally expected length of stay, which varied from 2 to 7 days across studies.
Studies conducted in countries where average length of stay for bronchiolitis in the placebo arm is longer than 3 days reported a greater benefit than did those in countries like the United States, in which stays typically average fewer than 3 days. Because hospital length of stay should be correlated with severity of illness, Dr. Brooks also evaluated respiratory scores available in each study and found that higher scores also correlated with the utility of hypertonic saline.
The two Chinese studies included in the review had particularly long lengths of stay and were conducted in a city with poor air quality, and in which about 70% of patients were on systemic corticosteroids at baseline. “So there’s some question as to whether they were even measuring the same disease process,” Dr. Brooks told the conference.
Dr. Brooks and her colleagues reevaluated the Cochrane data both with and without the two outlying studies from China, and incorporated results from three additional studies not included in the Cochrane review.
Modeling cumulative results using a length of stay typical in the United States, the benefit from hypertonic saline was a reduction of 0.31 days, or 31 days/100 patients.
Dr. Brooks noted that the current data are consistent with two possibilities, that the longer a patient is treated with hypertonic saline, the better it works or that “perhaps hypertonic saline works better for sicker patients – we don’t know yet.” She acknowledged several limitations of the meta-analysis, including that patient age, additional medications, day of illness at admission, and frequency of treatment could not be correlated.
The existing data “do not support rejecting hypertonic saline for all patients or giving it to all populations,” Dr. Brooks told the conference, noting that “there are good physiologic reasons to believe hypertonic saline might have an impact based on its benefits to other respiratory populations such as cystic fibrosis – and it’s particularly appealing because of its low risk and low cost.”
However, she said, “if we’re going to use this intervention intelligently, we need to find where there is and where there isn’t a benefit.”
Dr. Brooks concluded that hypertonic saline might be worth considering for patients with an expected stay of longer than 3 days or for those with baseline clinical scores higher than average. Her study received no outside funding, and she disclosed no conflicts of interest.
AT THE PEDIATRIC HOSPITAL MEDICINE 2015 MEETING
Key clinical point: Use of nebulized hypertonic saline shortens hospital length of stay modestly in pediatric patients with bronchiolitis.
Major finding: Compared with normal saline, hypertonic saline reduced stays by 31 days per 100 patients, presuming a short length of stay of 3 days.
Data source: A meta-analysis of randomized controlled trials comparing normal saline 0.9% with hypertonic saline 3% in children with bronchiolitis; outlier results from two studies were excluded for exceptionally long lengths of stay.
Disclosures: Dr. Brooks disclosed no conflicts of interest.
Lung cancer biomarker moves into the clinic
SEATTLE – A new biomarker for bronchial epithelium that helps identify smokers with suspicious lesions who have lung cancer is now ready for clinical use. And one for nasal epithelium that could be used for screening may not be far behind.
“There is clearly a critical unmet need to develop molecular biomarkers to address some of the challenges that we now face since we have instituted CT screening for lung cancer,” Dr. Avi Spira said at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Although the National Lung Screening Trial established that annual chest CT among high-risk current and former smokers reduces their risk of death from lung cancer (N Engl J Med. 2011;365:395-409), the vast majority of those who screen positive do not have lung cancer. Also, screening only patients who meet criteria set by the trial will pick up less than half of all lung cancers in the United States.
“That leads to two critical unmet needs for molecular biomarkers in the so-called post–National Lung Screening Trial era,” said Dr. Spira, professor of medicine, pathology and laboratory medicine, and bioinformatics; chief of the division of computational biomedicine; and director of the translational bioinformatics program, Clinical and Translational Science Institute, all at Boston University.
“The first is … we desperately need molecular biomarkers that can distinguish a benign nodule found on CT versus a malignant one,” he said. “The second and arguably longer-term biomarker that we need is to distinguish which smokers would benefit from CT screening annually.”
Much of his team’s research in this area builds on the concept of field of injury. “The idea here is if you smoke, even though lung cancer tends to develop deep within the parenchyma of your lung, all of the epithelial cells that line your respiratory tract have genomic alterations that reflect the presence of that cancer,” Dr. Spira explained. Thus, profiling epithelial cells anywhere in the airway could be used for early detection and risk assessment.
He and his colleagues developed a 23-gene signature for use on bronchial epithelial cells. The biomarker was validated in the Airway Epithelium Gene Expression In the Diagnosis of Lung Cancer (AEGIS) 1 and 2 trials among 639 current and former smokers undergoing bronchoscopy for suspicious nodules seen on CT.
With 1 year of follow-up, biomarker sensitivity was 88%-89%, while specificity was 47% (N Engl J Med. 2015;373:243-251). “However the negative predictive value, which is really what drives the clinical utility of this test, is above 90%. And that’s what we believe will drive physicians to use the test – [determining] who can they avoid sending for an unnecessary [biopsy] procedure,” Dr. Spira said. Bronchoscopy alone had sensitivity of about 75%, but bronchoscopy combined with the gene signature had sensitivity of 97%.
Subgroup analyses showed the biomarker had superior sensitivity for detecting lung cancer when lesions measured no more than 3 cm or were located in the lung periphery, and when patients had early-stage disease. In addition, it performed similarly well across different types of tumors.
Of special note, among patients whose pretest probability of cancer fell in the intermediate range (10%-60%), bronchoscopy had an 83% nondiagnostic rate, but the biomarker had 88% sensitivity and a 91% negative predictive value. “That means if you have a nondiagnostic bronchoscopy in a patient who is at intermediate pretest risk for disease, a negative gene expression test would mean there is a less than 10% chance this is cancer. That’s where a physician might feel, okay, I don’t have to go on and do a biopsy, I can watch that patient serially with CT scans of the chest,” Dr. Spira said.
The biomarker test is now clinically available (Percepta, manufactured by Veracyte). “I think it’s exciting because it’s the first of what I believe are many molecular biomarkers that are going to be emerging in the clinical space for the early detection of lung cancer,” he said.
“The multimillion dollar question is why are we seeing gene expression changes in normal-appearing cells so far away from where the tumor arises? We don’t have the full answer to that yet, but based on the genes that are changing, we have developed some hypotheses,” Dr. Spira said.
Some of the down-regulated genes are involved in antioxidant and DNA repair pathways, suggesting that the smokers who ultimately get cancer have less of a protective response to smoking. And some of the up-regulated genes include ones in the PI3 kinase signaling pathway.
“I would argue that what we are seeing in the proximal airway isn’t necessarily reflecting the presence of the cancer but the susceptibility, and that’s a really important distinguishing factor because then perhaps the test could be used as a screening tool,” Dr. Spira maintained.
As not all smokers at elevated risk for lung cancer will undergo bronchoscopy, one of the investigators’ future goals is to move biomarker testing to a less invasive site. They are currently focusing on the nose, using nasal epithelium collected by brushings from the inferior turbinate.
An analysis of nasal epithelium collected at the time of bronchoscopy in the AEGIS trials has shown that a 200-gene signature performs well for distinguishing between patients with and without lung cancer, Dr. Spira reported. Furthermore, the changes in gene expression profile in the nose were similar to those seen in the bronchus.
Such a biomarker might have best clinical utility in two other settings, he proposed. The first would be in patients having nodules that are clearly not accessible by bronchoscopy, in which case the biomarker would be applied for diagnosis. The second would be in smokers being seen for routine annual exams, in which case it would be used to identify those who should have CT surveillance.
“We are hopeful that the nasal epithelium can serve as a less invasive surrogate for the bronchus and ultimately allow us to move airway profiling into the screening setting for lung cancer in the longer term,” he concluded.
Dr. Spira disclosed that he receives intellectual property rights and consulting fees from, and has an ownership interest in, Veracyte Inc.
SEATTLE – A new biomarker for bronchial epithelium that helps identify smokers with suspicious lesions who have lung cancer is now ready for clinical use. And one for nasal epithelium that could be used for screening may not be far behind.
“There is clearly a critical unmet need to develop molecular biomarkers to address some of the challenges that we now face since we have instituted CT screening for lung cancer,” Dr. Avi Spira said at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Although the National Lung Screening Trial established that annual chest CT among high-risk current and former smokers reduces their risk of death from lung cancer (N Engl J Med. 2011;365:395-409), the vast majority of those who screen positive do not have lung cancer. Also, screening only patients who meet criteria set by the trial will pick up less than half of all lung cancers in the United States.
“That leads to two critical unmet needs for molecular biomarkers in the so-called post–National Lung Screening Trial era,” said Dr. Spira, professor of medicine, pathology and laboratory medicine, and bioinformatics; chief of the division of computational biomedicine; and director of the translational bioinformatics program, Clinical and Translational Science Institute, all at Boston University.
“The first is … we desperately need molecular biomarkers that can distinguish a benign nodule found on CT versus a malignant one,” he said. “The second and arguably longer-term biomarker that we need is to distinguish which smokers would benefit from CT screening annually.”
Much of his team’s research in this area builds on the concept of field of injury. “The idea here is if you smoke, even though lung cancer tends to develop deep within the parenchyma of your lung, all of the epithelial cells that line your respiratory tract have genomic alterations that reflect the presence of that cancer,” Dr. Spira explained. Thus, profiling epithelial cells anywhere in the airway could be used for early detection and risk assessment.
He and his colleagues developed a 23-gene signature for use on bronchial epithelial cells. The biomarker was validated in the Airway Epithelium Gene Expression In the Diagnosis of Lung Cancer (AEGIS) 1 and 2 trials among 639 current and former smokers undergoing bronchoscopy for suspicious nodules seen on CT.
With 1 year of follow-up, biomarker sensitivity was 88%-89%, while specificity was 47% (N Engl J Med. 2015;373:243-251). “However the negative predictive value, which is really what drives the clinical utility of this test, is above 90%. And that’s what we believe will drive physicians to use the test – [determining] who can they avoid sending for an unnecessary [biopsy] procedure,” Dr. Spira said. Bronchoscopy alone had sensitivity of about 75%, but bronchoscopy combined with the gene signature had sensitivity of 97%.
Subgroup analyses showed the biomarker had superior sensitivity for detecting lung cancer when lesions measured no more than 3 cm or were located in the lung periphery, and when patients had early-stage disease. In addition, it performed similarly well across different types of tumors.
Of special note, among patients whose pretest probability of cancer fell in the intermediate range (10%-60%), bronchoscopy had an 83% nondiagnostic rate, but the biomarker had 88% sensitivity and a 91% negative predictive value. “That means if you have a nondiagnostic bronchoscopy in a patient who is at intermediate pretest risk for disease, a negative gene expression test would mean there is a less than 10% chance this is cancer. That’s where a physician might feel, okay, I don’t have to go on and do a biopsy, I can watch that patient serially with CT scans of the chest,” Dr. Spira said.
The biomarker test is now clinically available (Percepta, manufactured by Veracyte). “I think it’s exciting because it’s the first of what I believe are many molecular biomarkers that are going to be emerging in the clinical space for the early detection of lung cancer,” he said.
“The multimillion dollar question is why are we seeing gene expression changes in normal-appearing cells so far away from where the tumor arises? We don’t have the full answer to that yet, but based on the genes that are changing, we have developed some hypotheses,” Dr. Spira said.
Some of the down-regulated genes are involved in antioxidant and DNA repair pathways, suggesting that the smokers who ultimately get cancer have less of a protective response to smoking. And some of the up-regulated genes include ones in the PI3 kinase signaling pathway.
“I would argue that what we are seeing in the proximal airway isn’t necessarily reflecting the presence of the cancer but the susceptibility, and that’s a really important distinguishing factor because then perhaps the test could be used as a screening tool,” Dr. Spira maintained.
As not all smokers at elevated risk for lung cancer will undergo bronchoscopy, one of the investigators’ future goals is to move biomarker testing to a less invasive site. They are currently focusing on the nose, using nasal epithelium collected by brushings from the inferior turbinate.
An analysis of nasal epithelium collected at the time of bronchoscopy in the AEGIS trials has shown that a 200-gene signature performs well for distinguishing between patients with and without lung cancer, Dr. Spira reported. Furthermore, the changes in gene expression profile in the nose were similar to those seen in the bronchus.
Such a biomarker might have best clinical utility in two other settings, he proposed. The first would be in patients having nodules that are clearly not accessible by bronchoscopy, in which case the biomarker would be applied for diagnosis. The second would be in smokers being seen for routine annual exams, in which case it would be used to identify those who should have CT surveillance.
“We are hopeful that the nasal epithelium can serve as a less invasive surrogate for the bronchus and ultimately allow us to move airway profiling into the screening setting for lung cancer in the longer term,” he concluded.
Dr. Spira disclosed that he receives intellectual property rights and consulting fees from, and has an ownership interest in, Veracyte Inc.
SEATTLE – A new biomarker for bronchial epithelium that helps identify smokers with suspicious lesions who have lung cancer is now ready for clinical use. And one for nasal epithelium that could be used for screening may not be far behind.
“There is clearly a critical unmet need to develop molecular biomarkers to address some of the challenges that we now face since we have instituted CT screening for lung cancer,” Dr. Avi Spira said at a joint meeting of the Global Biomarkers Consortium and World Cutaneous Malignancies Congress.
Although the National Lung Screening Trial established that annual chest CT among high-risk current and former smokers reduces their risk of death from lung cancer (N Engl J Med. 2011;365:395-409), the vast majority of those who screen positive do not have lung cancer. Also, screening only patients who meet criteria set by the trial will pick up less than half of all lung cancers in the United States.
“That leads to two critical unmet needs for molecular biomarkers in the so-called post–National Lung Screening Trial era,” said Dr. Spira, professor of medicine, pathology and laboratory medicine, and bioinformatics; chief of the division of computational biomedicine; and director of the translational bioinformatics program, Clinical and Translational Science Institute, all at Boston University.
“The first is … we desperately need molecular biomarkers that can distinguish a benign nodule found on CT versus a malignant one,” he said. “The second and arguably longer-term biomarker that we need is to distinguish which smokers would benefit from CT screening annually.”
Much of his team’s research in this area builds on the concept of field of injury. “The idea here is if you smoke, even though lung cancer tends to develop deep within the parenchyma of your lung, all of the epithelial cells that line your respiratory tract have genomic alterations that reflect the presence of that cancer,” Dr. Spira explained. Thus, profiling epithelial cells anywhere in the airway could be used for early detection and risk assessment.
He and his colleagues developed a 23-gene signature for use on bronchial epithelial cells. The biomarker was validated in the Airway Epithelium Gene Expression In the Diagnosis of Lung Cancer (AEGIS) 1 and 2 trials among 639 current and former smokers undergoing bronchoscopy for suspicious nodules seen on CT.
With 1 year of follow-up, biomarker sensitivity was 88%-89%, while specificity was 47% (N Engl J Med. 2015;373:243-251). “However the negative predictive value, which is really what drives the clinical utility of this test, is above 90%. And that’s what we believe will drive physicians to use the test – [determining] who can they avoid sending for an unnecessary [biopsy] procedure,” Dr. Spira said. Bronchoscopy alone had sensitivity of about 75%, but bronchoscopy combined with the gene signature had sensitivity of 97%.
Subgroup analyses showed the biomarker had superior sensitivity for detecting lung cancer when lesions measured no more than 3 cm or were located in the lung periphery, and when patients had early-stage disease. In addition, it performed similarly well across different types of tumors.
Of special note, among patients whose pretest probability of cancer fell in the intermediate range (10%-60%), bronchoscopy had an 83% nondiagnostic rate, but the biomarker had 88% sensitivity and a 91% negative predictive value. “That means if you have a nondiagnostic bronchoscopy in a patient who is at intermediate pretest risk for disease, a negative gene expression test would mean there is a less than 10% chance this is cancer. That’s where a physician might feel, okay, I don’t have to go on and do a biopsy, I can watch that patient serially with CT scans of the chest,” Dr. Spira said.
The biomarker test is now clinically available (Percepta, manufactured by Veracyte). “I think it’s exciting because it’s the first of what I believe are many molecular biomarkers that are going to be emerging in the clinical space for the early detection of lung cancer,” he said.
“The multimillion dollar question is why are we seeing gene expression changes in normal-appearing cells so far away from where the tumor arises? We don’t have the full answer to that yet, but based on the genes that are changing, we have developed some hypotheses,” Dr. Spira said.
Some of the down-regulated genes are involved in antioxidant and DNA repair pathways, suggesting that the smokers who ultimately get cancer have less of a protective response to smoking. And some of the up-regulated genes include ones in the PI3 kinase signaling pathway.
“I would argue that what we are seeing in the proximal airway isn’t necessarily reflecting the presence of the cancer but the susceptibility, and that’s a really important distinguishing factor because then perhaps the test could be used as a screening tool,” Dr. Spira maintained.
As not all smokers at elevated risk for lung cancer will undergo bronchoscopy, one of the investigators’ future goals is to move biomarker testing to a less invasive site. They are currently focusing on the nose, using nasal epithelium collected by brushings from the inferior turbinate.
An analysis of nasal epithelium collected at the time of bronchoscopy in the AEGIS trials has shown that a 200-gene signature performs well for distinguishing between patients with and without lung cancer, Dr. Spira reported. Furthermore, the changes in gene expression profile in the nose were similar to those seen in the bronchus.
Such a biomarker might have best clinical utility in two other settings, he proposed. The first would be in patients having nodules that are clearly not accessible by bronchoscopy, in which case the biomarker would be applied for diagnosis. The second would be in smokers being seen for routine annual exams, in which case it would be used to identify those who should have CT surveillance.
“We are hopeful that the nasal epithelium can serve as a less invasive surrogate for the bronchus and ultimately allow us to move airway profiling into the screening setting for lung cancer in the longer term,” he concluded.
Dr. Spira disclosed that he receives intellectual property rights and consulting fees from, and has an ownership interest in, Veracyte Inc.
AT THE GLOBAL BIOMARKERS CONSORTIUM CONFERENCE
More sensitive IPD definition improves vaccine cost-effectiveness
Expanding the definition of invasive pneumococcal disease in children aged 3-42 months to include suspected cases and unspecified sepsis significantly improved the cost-effectiveness of the 10-valent pneumococcal conjugate vaccine, Dr. Arto A. Palmu and his associates reported.
In a nationwide observational follow-up study, Dr. Palmu and his associates found that the relative rate reduction for non–laboratory-confirmed invasive pneumococcal disease (IPD) and unspecified sepsis was only 34%, compared with 80% for culture-confirmed IPD. The absolute rate reduction was much higher. For culture-confirmed IPD, the absolute rate reduction was 50 cases per 100,000 person-years, and for non–laboratory-confirmed IPD and unspecified sepsis, the rate was 122 cases per 100,000 person-years, said Dr. Palmu of the National Institute for Health and Welfare in Tampere, Finland.
Mortality reduction was 51% overall. For non–culture-confirmed IPD and unspecified sepsis, the relative mortality reduction was 35%.
“Use of sensitive case definitions, such as clinically suspected IPD, in the evaluation of the total disease burden prevented by the vaccination program is important for understanding the total burden of pneumococcal disease and the cost-effectiveness of public health interventions,” the investigators concluded.
Find the full study in Pediatrics (doi:10.1542/peds.2015-0458).
Expanding the definition of invasive pneumococcal disease in children aged 3-42 months to include suspected cases and unspecified sepsis significantly improved the cost-effectiveness of the 10-valent pneumococcal conjugate vaccine, Dr. Arto A. Palmu and his associates reported.
In a nationwide observational follow-up study, Dr. Palmu and his associates found that the relative rate reduction for non–laboratory-confirmed invasive pneumococcal disease (IPD) and unspecified sepsis was only 34%, compared with 80% for culture-confirmed IPD. The absolute rate reduction was much higher. For culture-confirmed IPD, the absolute rate reduction was 50 cases per 100,000 person-years, and for non–laboratory-confirmed IPD and unspecified sepsis, the rate was 122 cases per 100,000 person-years, said Dr. Palmu of the National Institute for Health and Welfare in Tampere, Finland.
Mortality reduction was 51% overall. For non–culture-confirmed IPD and unspecified sepsis, the relative mortality reduction was 35%.
“Use of sensitive case definitions, such as clinically suspected IPD, in the evaluation of the total disease burden prevented by the vaccination program is important for understanding the total burden of pneumococcal disease and the cost-effectiveness of public health interventions,” the investigators concluded.
Find the full study in Pediatrics (doi:10.1542/peds.2015-0458).
Expanding the definition of invasive pneumococcal disease in children aged 3-42 months to include suspected cases and unspecified sepsis significantly improved the cost-effectiveness of the 10-valent pneumococcal conjugate vaccine, Dr. Arto A. Palmu and his associates reported.
In a nationwide observational follow-up study, Dr. Palmu and his associates found that the relative rate reduction for non–laboratory-confirmed invasive pneumococcal disease (IPD) and unspecified sepsis was only 34%, compared with 80% for culture-confirmed IPD. The absolute rate reduction was much higher. For culture-confirmed IPD, the absolute rate reduction was 50 cases per 100,000 person-years, and for non–laboratory-confirmed IPD and unspecified sepsis, the rate was 122 cases per 100,000 person-years, said Dr. Palmu of the National Institute for Health and Welfare in Tampere, Finland.
Mortality reduction was 51% overall. For non–culture-confirmed IPD and unspecified sepsis, the relative mortality reduction was 35%.
“Use of sensitive case definitions, such as clinically suspected IPD, in the evaluation of the total disease burden prevented by the vaccination program is important for understanding the total burden of pneumococcal disease and the cost-effectiveness of public health interventions,” the investigators concluded.
Find the full study in Pediatrics (doi:10.1542/peds.2015-0458).
Experimental Ebola vaccine up to 100% effective
A single dose of an experimental vaccine was up to 100% effective at preventing Ebola infection among those who had come into contact with recently infected individuals, according to interim results of an open-label trial conducted earlier this year in Guinea.
The study, which took place during an Ebola outbreak, included 90 clusters totaling 7,651 people who either had direct contact with someone recently diagnosed with Ebola or had contact with a direct contact. Children and pregnant or breastfeeding women were not eligible for the vaccine, and some study participants refused vaccination.
Consenting and eligible participants in 48 clusters were randomized to receive the vaccine (rVSV-ZEBOV) immediately after randomization; those in the remaining 42 clusters were vaccinated 21 days after randomization. Given that the incubation period of the virus is 10 days, the primary outcome was laboratory-confirmed Ebola infection at least 10 days after randomization to either immediate or delayed vaccination.
None of the 2,014 immediately vaccinated individuals contracted Ebola during that time, while 16 of the 1,0121 adults in the delayed vaccination group became infected, showing vaccine efficacy of 100%, Dr. Ana Maria Henao-Restrepo of the World Health Organization, Geneva, and her colleagues wrote in the Lancet.
At the cluster level, vaccine efficacy was 76% when all individuals – including children and pregnant and lactating women who were not vaccinated – were included. A total of 43 serious adverse events were reported, one of which was deemed directly related to vaccination.
“The interim results of [this trial] suggest that the efficacy of a single injection of rVSV-ZEBOV to prevent Ebola virus disease might be high, that protection can be established quickly, and that the vaccine might be effective at the population level when delivered by ring vaccination ...” the investigators wrote. The trial will be continued.
The results of this research project “will be the subject of intense scientific scrutiny and debate,” but a team under the World Health Organization’s leadership and the GAVI Alliance are ready to respectively support, and fund procurement and deployment of the vaccine, if it gets licensed, according to an editorial.
Read the full study in The Lancet (http://www.thelancet.com/pb/assets/raw/Lancet/pdfs/S0140673615611175.pdf ).
A single dose of an experimental vaccine was up to 100% effective at preventing Ebola infection among those who had come into contact with recently infected individuals, according to interim results of an open-label trial conducted earlier this year in Guinea.
The study, which took place during an Ebola outbreak, included 90 clusters totaling 7,651 people who either had direct contact with someone recently diagnosed with Ebola or had contact with a direct contact. Children and pregnant or breastfeeding women were not eligible for the vaccine, and some study participants refused vaccination.
Consenting and eligible participants in 48 clusters were randomized to receive the vaccine (rVSV-ZEBOV) immediately after randomization; those in the remaining 42 clusters were vaccinated 21 days after randomization. Given that the incubation period of the virus is 10 days, the primary outcome was laboratory-confirmed Ebola infection at least 10 days after randomization to either immediate or delayed vaccination.
None of the 2,014 immediately vaccinated individuals contracted Ebola during that time, while 16 of the 1,0121 adults in the delayed vaccination group became infected, showing vaccine efficacy of 100%, Dr. Ana Maria Henao-Restrepo of the World Health Organization, Geneva, and her colleagues wrote in the Lancet.
At the cluster level, vaccine efficacy was 76% when all individuals – including children and pregnant and lactating women who were not vaccinated – were included. A total of 43 serious adverse events were reported, one of which was deemed directly related to vaccination.
“The interim results of [this trial] suggest that the efficacy of a single injection of rVSV-ZEBOV to prevent Ebola virus disease might be high, that protection can be established quickly, and that the vaccine might be effective at the population level when delivered by ring vaccination ...” the investigators wrote. The trial will be continued.
The results of this research project “will be the subject of intense scientific scrutiny and debate,” but a team under the World Health Organization’s leadership and the GAVI Alliance are ready to respectively support, and fund procurement and deployment of the vaccine, if it gets licensed, according to an editorial.
Read the full study in The Lancet (http://www.thelancet.com/pb/assets/raw/Lancet/pdfs/S0140673615611175.pdf ).
A single dose of an experimental vaccine was up to 100% effective at preventing Ebola infection among those who had come into contact with recently infected individuals, according to interim results of an open-label trial conducted earlier this year in Guinea.
The study, which took place during an Ebola outbreak, included 90 clusters totaling 7,651 people who either had direct contact with someone recently diagnosed with Ebola or had contact with a direct contact. Children and pregnant or breastfeeding women were not eligible for the vaccine, and some study participants refused vaccination.
Consenting and eligible participants in 48 clusters were randomized to receive the vaccine (rVSV-ZEBOV) immediately after randomization; those in the remaining 42 clusters were vaccinated 21 days after randomization. Given that the incubation period of the virus is 10 days, the primary outcome was laboratory-confirmed Ebola infection at least 10 days after randomization to either immediate or delayed vaccination.
None of the 2,014 immediately vaccinated individuals contracted Ebola during that time, while 16 of the 1,0121 adults in the delayed vaccination group became infected, showing vaccine efficacy of 100%, Dr. Ana Maria Henao-Restrepo of the World Health Organization, Geneva, and her colleagues wrote in the Lancet.
At the cluster level, vaccine efficacy was 76% when all individuals – including children and pregnant and lactating women who were not vaccinated – were included. A total of 43 serious adverse events were reported, one of which was deemed directly related to vaccination.
“The interim results of [this trial] suggest that the efficacy of a single injection of rVSV-ZEBOV to prevent Ebola virus disease might be high, that protection can be established quickly, and that the vaccine might be effective at the population level when delivered by ring vaccination ...” the investigators wrote. The trial will be continued.
The results of this research project “will be the subject of intense scientific scrutiny and debate,” but a team under the World Health Organization’s leadership and the GAVI Alliance are ready to respectively support, and fund procurement and deployment of the vaccine, if it gets licensed, according to an editorial.
Read the full study in The Lancet (http://www.thelancet.com/pb/assets/raw/Lancet/pdfs/S0140673615611175.pdf ).
FROM THE LANCET