User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Early-wheezing Patterns Prefigure Adolescent Respiratory Outcomes and Asthma
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
FROM JAMA PEDIATRICS
Early-wheezing patterns prefigure adolescent respiratory outcomes and asthma
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
Wheezing patterns in early childhood can predict pulmonary function and the development of asthma in adolescence in a high-risk population, a study finds.
The study validates four clinically distinct early-life wheezing phenotypes identified by the landmark Tucson Children’s Respiratory Study in a high-risk population:
• Never.
• Transient early – wheezing before age 3 years but not at age 6 years.
• Late onset – wheezing at age 6 years but not before age 3 years.
• Persistent – wheezing before age 3 years and at age 6 years.
Previously, these phenotypes were shown to be associated with respiratory outcomes in adolescence but not with diagnosed asthma or in genetically predisposed children. The present study extends the associations with these early phenotypes to a high-risk adolescent population, said Meghan B. Azad, Ph.D., of the University of Manitoba, Winnipeg, and her associates.
The study findings were based on findings in 459 children previously enrolled in the Canadian Asthma Primary Prevention Study cohort; this was a prenatally randomized prevention trial in children at high genetic risk for asthma. The distribution of early-wheeze phenotypes was 51% never, 28% transient early, 9% late onset, and 13% persistent (JAMA Pediatrics. 2016 Feb 8. doi: 10.1001/jamapediatrics.2015.4127).
Across all four phenotypes, the authors found a strong gradient of decreasing lung function and increasing asthma risk by age 15 years. Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: the prevalence of asthma was 5% among never, 19% among transient early, 27% among late onset, and 42% among persistent phenotypes.
At age 15, early-wheezing phenotypes were not associated with atopic dermatitis or allergic rhinitis. Atopy before 2 years of age was associated with persistent wheeze, which was in turn associated with a 12-fold increased risk of diagnosed asthma by age 15.
“Our results are consistent with other cohorts” and the study “extends these findings through adolescence in a high-risk cohort and demonstrates that asthma-associated deficits are already present at a young age. Collectively, these data show that early wheezing patterns provide clinically meaningful information and suggest that strategies to reduce early-life wheezing and atopic sensitization could have long-term health benefits,” Dr. Azad and her colleagues concluded.
Proven strategies to prevent wheezing include avoiding dust, pets, and tobacco smoke; encouragement of breastfeeding; and delayed introduction of solid foods.
The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
FROM JAMA PEDIATRICS
Key clinical point: Persistent early-life wheezing was associated with an 12-fold increased risk of diagnosed asthma by age 15 years.
Major finding: Asthma, assessed at 15 years in 320 adolescents, was associated with early-wheeze phenotypes: prevalence of asthma was 5% among never, 19% among early transient, 27% among late onset, and 42% among persistent phenotypes.
Data source: A study of 459 children.
Disclosures: The research was supported by the Canadian Institutes of Health Research. The investigators reported no relevant financial disclosures.
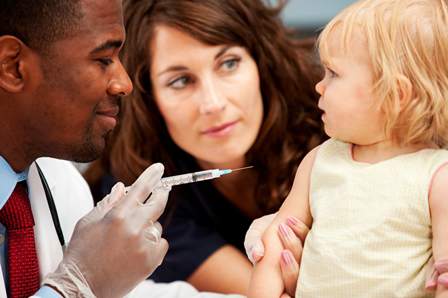
Tdap effectiveness wanes rapidly in teens
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.

|
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.

|
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
When adolescents contract whooping cough, a.k.a. pertussis, they usually don’t whoop but they cough, sometimes for 3 months. The illness is impactful for them and they are contagious to others, so the infection spreads to classmates and within families. Waning immunity after experiencing pertussis infection, after receiving the old DTwP vaccine that was discontinued in the United States years ago due to safety issues, and after receiving the newer DTaP and Tdap vaccines, has been known to occur for many years. What is new in this article is the rapidity of waning immunity in the study population.

|
Dr. Michael E. Pichichero |
First, it is important to note that the study population is from California, a state where pertussis has been circulating much more than in most other states. The exact reasons for a higher prevalence of pertussis in California are not fully understood, but a high rate of vaccine refusers may be a significant factor. Secondly, the study uses a mathematical model, so it is an estimate of waning immunity. Nevertheless, the observations alert health care providers and the community that pertussis can occur even in vaccinated persons, especially as time passes after vaccination.
The solutions are few at this time. Public health care officials are unlikely to recommend boosters more frequently than already advocated, although that is an option. Alternative formulations of Tdap to include other or additional ingredients could be a path forward, but the vaccine industry is tackling so many new diseases with vaccine development programs that a push for a better DTaP or Tdap is unlikely in the near term.
Dr. Michael E. Pichichero, specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no relevant financial disclosures.
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
The Tdap vaccine’s effectiveness against pertussis wanes so rapidly in the year after administration in teens that it provides too little protection to prevent outbreaks, according to results of a new study.
The Tdap is the booster vaccine against tetanus, diphtheria, and pertussis that adults and children ages 10 and older can receive. The corresponding acellular pertussis vaccine for children ages 6 and younger is the DTaP.
“Widespread Tdap vaccination, although associated with a transient decrease in pertussis incidence, did not prevent outbreaks among this population of teenagers who have only ever received acellular pertussis vaccines,” reported Dr. Nicola P. Klein and her associates at the Kaiser Permanente Vaccine Study Center in Oakland, Calif. “This study demonstrates that despite high rates of Tdap vaccination, the growing cohort of adolescents who have only received acellular pertussis vaccines continue to be at high risk of contracting pertussis and sustaining epidemics,” they wrote online (Pediatrics. 2016 Feb 5. [doi: 10.1542/peds.2015-3326]).
The researchers tracked 279,493 members of Kaiser Permanente Northern California who had received only DTaP vaccines, as opposed to the whole-cell DTwP formulation previously used, from age 10 onward. These included all members who were born from 1999 onward or were born from 1996-1998 and received three infant doses of DTaP, excluding children who had previously received Tdap or had pertussis. Among these, 175,094 children received the Tdap.
For the purposes of tracking pertussis cases in unvaccinated versus vaccinated adolescents, individuals were considered unvaccinated in the analysis until they received their first Tdap, after which they were vaccinated and time since vaccination was a continuous variable. Overall, 96.5% of the children were vaccinated by their 14th birthday.
Across 792,418 person-years between January 2006 and March 2015, including 418,595 vaccinated person-years for the children who received the Tdap, 1,207 cases of pertussis occurred. The vast majority of these – 85% – occurred among those ages 10-13 years. Teens aged 14-16 years comprised 15% of the cases, and only 0.5% of cases occurred among older teens. During each year of outbreaks, incidence dropped off precipitously among the age groups composed of children who would have received the whole-cell pertussis vaccine.
For example, “pertussis incidence in the 2010 outbreak sharply declined after this peak [of 10- to 11-year-olds] and stayed low at older ages, a decline that we have previously demonstrated to be associated with the receipt of whole cell instead of acellular pertussis vaccines in infancy and childhood as well as with Tdap receipt,” the authors wrote.
The researchers estimated the Tdap’s effectiveness at 69% throughout the first year after vaccination. This dropped to 57% in the second year after vaccination and then to 25% in the third year. By 4 years or later after Tdap receipt, the vaccine’s effectiveness sat at just 9%.
For each year after Tdap vaccination, children’s risk of pertussis increased 35% (hazard ratio, 1.35), and cases were mild or moderate regardless of vaccination status. Nearly all (98%) of the children with pertussis had visited the doctor within 5 days on either side of their positive polymerase chain reaction test, 86% received a diagnosis of pertussis, and 96% received a prescription for azithromycin, except for 1 for erythromycin. In addition, 4% (50) of the cases visited the emergency department. No differences in rate of ED visits or prescriptions existed between vaccinated and unvaccinated children.
“The strategy of routinely vaccinating adolescents to prevent future disease did not prevent the 2014 epidemic, arguably because the protection afforded by a dose of Tdap was too short-lived,” the authors noted. They also pointed out that Tdap waning estimates likely also include ongoing DTaP waning.“This study was unable to disentangle the waning of Tdap effectiveness from the ongoing waning of previous doses of DTaP because the years since vaccination for Tdap and the fifth DTaP dose are closely correlated,” they wrote.
Most of the Tdap vaccines administered were Adacel (Sanofi Pasteur), but Boostrix (GlaxoSmithKline) was used as well. Waning occurred in both brands, and although not directly compared, no major differences in waning seemed to exist.
“We expect future pertussis epidemics to be larger as the cohort that has only received acellular pertussis vaccines ages,” the authors concluded. “The results in this study raise serious questions regarding the benefits of routinely administering a single dose of Tdap to every adolescent aged 11 or 12 years.”
The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech, and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
FROM PEDIATRICS
Key clinical point: Tdap protection against pertussis in adolescents wanes rapidly after the first year.
Major finding: Tdap effectiveness against pertussis dropped from 68.6% effective in the first year after vaccination to 8.9% by 4 years later; each year after Tdap increased the risk of pertussis in vaccinated teens by 35% (hazard ratio per year: 1.35).
Data source: The findings are based on analysis of 1,207 cases of pertussis among 279,493 California teens who were unvaccinated or who received the Tdap booster after recommended childhood doses of DTaP.
Disclosures: The research was funded by Kaiser Permanente. Dr. Klein has received research funding from GlaxoSmithKline for a separate pertussis vaccine effectiveness study, and Dr. Klein and one coauthor also have received research funding from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Novartis, Protein Science, Nuron Biotech and MedImmune. The remaining coauthors said they had no relevant financial disclosures. GlaxoSmithKline and Sanofi Pasteur manufacture the pertussis vaccines purchased by Kaiser Permanente for this study.
Majority of children aged 6-23 months are not vaccinated for flu
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
FROM PEDIATRICS
Try behavioral therapies first, then melatonin for pediatric insomnia
NEW YORK – In children with insomnia, melatonin is the appropriate first-line drug therapy, but pharmacologic treatments come after behavioral interventions, according to an evidence-based summary presented at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Medication should rarely be our first choice. We should always be trying to combine it with behavioral therapies, because they work just as well and last longer,” reported Dr. Jess P. Shatkin, a professor in the department of child and adolescent psychiatry, New York University.
The number of randomized trials for sleep medications in children is limited, and there is no pharmacotherapy approved by the Food and Drug Administration for this indication, Dr. Shatkin said. Clinicians often extrapolate from adult studies, but Dr. Shatkin said these data are not necessarily transferable. He noted, for example, that a study of zolpidem in children, which is approved for adults, was negative.
The antihistamine diphenhydramine also has been studied in children, and results were mixed. In one of two double-blind, placebo-controlled pediatric studies, parents reported improvement in getting children to sleep. In the other, conducted in children aged 6 months to 15 months, no significant advantage was found for this agent over placebo.
“[Diphenhydramine] Benadryl may make your kids sleep, it may make your patients sleep, it may make you sleep, which is fine, but the data do not convince us that [diphenhydramine] Benadryl is a great treatment for sleep in children,” Dr. Shatkin reported.
Rather, the best data are with melatonin, an endogenous hormone produced on a circadian rhythm correlating with the end-of-day phenomenon known as dim-light melatonin onset (DLMO). In one study conducted in otherwise healthy children aged 6 to 12 years with chronic sleep-onset insomnia, the administration of exogenous melatonin decreased sleep-onset latency by 35 minutes as measured with actigraphy, according to Dr. Shatkin. Similar benefit has been observed in studies conducted in children with attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorders (ASD).
“Melatonin is efficacious in typically developing children and those with neurodevelopmental disorders. It imposes minimal effects on sleep architecture, it is associated with a low risk of side effects, the cost is low, and it is widely available,” Dr. Shatkin said. However, he advised against using this drug in children younger than 6 months old.
“The timing of the dosing is critical and should be based on DLMO,” Dr. Shatkin said. Specifically, he recommended a starting dose of 0.2 to 0.5 mg administered 2-3 hours before sleep time. The dose can be increased as needed by 0.2-0.5 mg per week to a maximum of 3.0 mg. Dr. Shatkin reported that there is “little evidence” to support extended-release formulations, but he did warn that over-the-counter preparations may vary in quality.
As an alternative, clonidine was listed as a second choice for treating insomnia in children. Although this therapy is not supported by controlled data, several open-label studies and chart reviews suggest benefit, and this therapy is less likely than diphenhydramine to produce next-day drowsiness.
Yet, he reiterated that the best evidence-based treatment of sleep problems in children is cognitive-behavioral therapy. He called the techniques – such as regular bedtimes, avoidance of stimuli, and creating a relaxing bedtime ritual – as easy to learn and teach to parents. Obvious problems in the sleep routine, such as irregular bedtimes, typically can be identified with a sleep history. There are numerous strategies to wean children from requiring the presence of a parent to fall asleep, such as “graduated extinction,” which involves incrementally distancing the parent from the child’s bedside.
Empathetic to the frequency of sleep disturbances in children, Dr. Shatkin cited data suggesting that 50% of preschool children, 30% of school-age children, and 40% of adolescents report sleep problems. A survey of child psychiatrists found that most acknowledged prescribing a sleep medication within the past month for pediatric insomnia, but Dr. Shatkin emphasized that behavioral therapies often may produce a longer-lasting result.
For treating pediatric sleep disturbances, “our educational and behavioral efforts really should trump our medications,” Dr. Shatkin said. “We should be using any medication sparingly.”
Dr. Shatkin reported financial relationships with Assurex Health, Edgemont Pharmaceuticals, Eli Lilly, Forest Laboratories, and Lundbeck.
NEW YORK – In children with insomnia, melatonin is the appropriate first-line drug therapy, but pharmacologic treatments come after behavioral interventions, according to an evidence-based summary presented at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Medication should rarely be our first choice. We should always be trying to combine it with behavioral therapies, because they work just as well and last longer,” reported Dr. Jess P. Shatkin, a professor in the department of child and adolescent psychiatry, New York University.
The number of randomized trials for sleep medications in children is limited, and there is no pharmacotherapy approved by the Food and Drug Administration for this indication, Dr. Shatkin said. Clinicians often extrapolate from adult studies, but Dr. Shatkin said these data are not necessarily transferable. He noted, for example, that a study of zolpidem in children, which is approved for adults, was negative.
The antihistamine diphenhydramine also has been studied in children, and results were mixed. In one of two double-blind, placebo-controlled pediatric studies, parents reported improvement in getting children to sleep. In the other, conducted in children aged 6 months to 15 months, no significant advantage was found for this agent over placebo.
“[Diphenhydramine] Benadryl may make your kids sleep, it may make your patients sleep, it may make you sleep, which is fine, but the data do not convince us that [diphenhydramine] Benadryl is a great treatment for sleep in children,” Dr. Shatkin reported.
Rather, the best data are with melatonin, an endogenous hormone produced on a circadian rhythm correlating with the end-of-day phenomenon known as dim-light melatonin onset (DLMO). In one study conducted in otherwise healthy children aged 6 to 12 years with chronic sleep-onset insomnia, the administration of exogenous melatonin decreased sleep-onset latency by 35 minutes as measured with actigraphy, according to Dr. Shatkin. Similar benefit has been observed in studies conducted in children with attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorders (ASD).
“Melatonin is efficacious in typically developing children and those with neurodevelopmental disorders. It imposes minimal effects on sleep architecture, it is associated with a low risk of side effects, the cost is low, and it is widely available,” Dr. Shatkin said. However, he advised against using this drug in children younger than 6 months old.
“The timing of the dosing is critical and should be based on DLMO,” Dr. Shatkin said. Specifically, he recommended a starting dose of 0.2 to 0.5 mg administered 2-3 hours before sleep time. The dose can be increased as needed by 0.2-0.5 mg per week to a maximum of 3.0 mg. Dr. Shatkin reported that there is “little evidence” to support extended-release formulations, but he did warn that over-the-counter preparations may vary in quality.
As an alternative, clonidine was listed as a second choice for treating insomnia in children. Although this therapy is not supported by controlled data, several open-label studies and chart reviews suggest benefit, and this therapy is less likely than diphenhydramine to produce next-day drowsiness.
Yet, he reiterated that the best evidence-based treatment of sleep problems in children is cognitive-behavioral therapy. He called the techniques – such as regular bedtimes, avoidance of stimuli, and creating a relaxing bedtime ritual – as easy to learn and teach to parents. Obvious problems in the sleep routine, such as irregular bedtimes, typically can be identified with a sleep history. There are numerous strategies to wean children from requiring the presence of a parent to fall asleep, such as “graduated extinction,” which involves incrementally distancing the parent from the child’s bedside.
Empathetic to the frequency of sleep disturbances in children, Dr. Shatkin cited data suggesting that 50% of preschool children, 30% of school-age children, and 40% of adolescents report sleep problems. A survey of child psychiatrists found that most acknowledged prescribing a sleep medication within the past month for pediatric insomnia, but Dr. Shatkin emphasized that behavioral therapies often may produce a longer-lasting result.
For treating pediatric sleep disturbances, “our educational and behavioral efforts really should trump our medications,” Dr. Shatkin said. “We should be using any medication sparingly.”
Dr. Shatkin reported financial relationships with Assurex Health, Edgemont Pharmaceuticals, Eli Lilly, Forest Laboratories, and Lundbeck.
NEW YORK – In children with insomnia, melatonin is the appropriate first-line drug therapy, but pharmacologic treatments come after behavioral interventions, according to an evidence-based summary presented at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
“Medication should rarely be our first choice. We should always be trying to combine it with behavioral therapies, because they work just as well and last longer,” reported Dr. Jess P. Shatkin, a professor in the department of child and adolescent psychiatry, New York University.
The number of randomized trials for sleep medications in children is limited, and there is no pharmacotherapy approved by the Food and Drug Administration for this indication, Dr. Shatkin said. Clinicians often extrapolate from adult studies, but Dr. Shatkin said these data are not necessarily transferable. He noted, for example, that a study of zolpidem in children, which is approved for adults, was negative.
The antihistamine diphenhydramine also has been studied in children, and results were mixed. In one of two double-blind, placebo-controlled pediatric studies, parents reported improvement in getting children to sleep. In the other, conducted in children aged 6 months to 15 months, no significant advantage was found for this agent over placebo.
“[Diphenhydramine] Benadryl may make your kids sleep, it may make your patients sleep, it may make you sleep, which is fine, but the data do not convince us that [diphenhydramine] Benadryl is a great treatment for sleep in children,” Dr. Shatkin reported.
Rather, the best data are with melatonin, an endogenous hormone produced on a circadian rhythm correlating with the end-of-day phenomenon known as dim-light melatonin onset (DLMO). In one study conducted in otherwise healthy children aged 6 to 12 years with chronic sleep-onset insomnia, the administration of exogenous melatonin decreased sleep-onset latency by 35 minutes as measured with actigraphy, according to Dr. Shatkin. Similar benefit has been observed in studies conducted in children with attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorders (ASD).
“Melatonin is efficacious in typically developing children and those with neurodevelopmental disorders. It imposes minimal effects on sleep architecture, it is associated with a low risk of side effects, the cost is low, and it is widely available,” Dr. Shatkin said. However, he advised against using this drug in children younger than 6 months old.
“The timing of the dosing is critical and should be based on DLMO,” Dr. Shatkin said. Specifically, he recommended a starting dose of 0.2 to 0.5 mg administered 2-3 hours before sleep time. The dose can be increased as needed by 0.2-0.5 mg per week to a maximum of 3.0 mg. Dr. Shatkin reported that there is “little evidence” to support extended-release formulations, but he did warn that over-the-counter preparations may vary in quality.
As an alternative, clonidine was listed as a second choice for treating insomnia in children. Although this therapy is not supported by controlled data, several open-label studies and chart reviews suggest benefit, and this therapy is less likely than diphenhydramine to produce next-day drowsiness.
Yet, he reiterated that the best evidence-based treatment of sleep problems in children is cognitive-behavioral therapy. He called the techniques – such as regular bedtimes, avoidance of stimuli, and creating a relaxing bedtime ritual – as easy to learn and teach to parents. Obvious problems in the sleep routine, such as irregular bedtimes, typically can be identified with a sleep history. There are numerous strategies to wean children from requiring the presence of a parent to fall asleep, such as “graduated extinction,” which involves incrementally distancing the parent from the child’s bedside.
Empathetic to the frequency of sleep disturbances in children, Dr. Shatkin cited data suggesting that 50% of preschool children, 30% of school-age children, and 40% of adolescents report sleep problems. A survey of child psychiatrists found that most acknowledged prescribing a sleep medication within the past month for pediatric insomnia, but Dr. Shatkin emphasized that behavioral therapies often may produce a longer-lasting result.
For treating pediatric sleep disturbances, “our educational and behavioral efforts really should trump our medications,” Dr. Shatkin said. “We should be using any medication sparingly.”
Dr. Shatkin reported financial relationships with Assurex Health, Edgemont Pharmaceuticals, Eli Lilly, Forest Laboratories, and Lundbeck.
EXPERT ANALYSIS FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
5% prevalence of expiratory central airway collapse in COPDGene study
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Expiratory central airway collapse is associated with a worse respiratory-related quality of life among current and former smokers.
Major finding: Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years).
Data source: A cross-sectional cohort study comparing inspiratory and expiratory CT scans for 8,820 adults participating in a COPD study.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Acetazolamide doesn’t reduce duration of mechanical ventilation
Acetazolamide failed to reduce the duration of invasive mechanical ventilation in chronic obstructive pulmonary disease (COPD) patients who had pure or mixed metabolic alkalosis, according to results from a clinical trial published online Feb. 2 in JAMA.
Acetazolamide is a carbonic anhydrase inhibitor used as a respiratory stimulant in patients who have COPD and metabolic alkalosis. Recent studies suggested that high doses of the drug (1,000 mg/day or more) might shorten the duration of mechanical ventilation in critically ill COPD patients who require invasive mechanical ventilation, by markedly lowering serum bicarbonate and raising minute ventilation, said Dr. Christophe Faisy of the medical intensive care unit, European Georges Pompidou Hospital, Paris, and his associates.
To test this hypothesis, Dr. Faisy and his associates in the DIABOLO trial assessed 380 adults with COPD who were being treated at 15 French ICUs. One patient was receiving ventilation through a tracheotomy tube and the rest through endotracheal intubation. These study participants were randomly assigned in a double-blind fashion to receive either 500 mg acetazolamide twice daily or 1,000 mg acetazolamide twice daily if they were concomitantly receiving loop diuretics (187 patients), or a matching placebo (193 patients), administered as a slow intravenous injection.
Patients in the active-treatment group achieved larger reductions in serum bicarbonate and had fewer days with metabolic alkalosis. Nevertheless, the primary efficacy outcome – the duration of invasive ventilation – did not differ significantly between the two study groups. The median duration of ventilation was 136.5 hours with acetazolamide and 163.0 hours with placebo, which is clinically substantial but did not reach statistical significance, the investigators said (JAMA. 2016 Feb 2. doi: 10.1001/jama.2016.0019).
Acetazolamide didn’t exert a respiratory stimulant effect as measured by changes in respiratory rate, tidal volume, or minute ventilation. And there were no significant differences between the two study groups in secondary outcomes such as time to weaning off ventilation, rate of successful weaning, number of spontaneous breathing trials, use of tracheotomy or noninvasive ventilation after extubation, unplanned extubations, episodes of ventilator-associated pneumonia, laboratory values, length of ICU stay, or in-ICU mortality.
In addition, rates of adjunctive treatment using loop diuretics, glucocorticoids, beta-agonists, or catecholamines were the same between the two study groups, and left ventricular ejection fraction at weaning from ventilation also was the same. The rate of serious adverse events also was comparable.
“Taken together, these findings indicate that the inhibition of the renal carbonic anhydrase enzyme and the resulting serum bicarbonate reduction did not trigger a sufficient respiratory-stimulating effect to affect outcomes of critically ill patients with COPD,” Dr. Faisy and his associates wrote.
However, they noted that in both study groups the median duration of invasive mechanical ventilation was shorter than had been anticipated when the trial was designed, which likely decreased the statistical power of the primary endpoint. “It is possible that the study was underpowered to establish statistical significance,” the researchers said.
It is also possible that higher doses of acetazolamide may have exerted a greater effect on respiratory parameters. However, higher doses also may have increased the workload of the respiratory muscles and induced respiratory muscle fatigue, they added.
Acetazolamide failed to reduce the duration of invasive mechanical ventilation in chronic obstructive pulmonary disease (COPD) patients who had pure or mixed metabolic alkalosis, according to results from a clinical trial published online Feb. 2 in JAMA.
Acetazolamide is a carbonic anhydrase inhibitor used as a respiratory stimulant in patients who have COPD and metabolic alkalosis. Recent studies suggested that high doses of the drug (1,000 mg/day or more) might shorten the duration of mechanical ventilation in critically ill COPD patients who require invasive mechanical ventilation, by markedly lowering serum bicarbonate and raising minute ventilation, said Dr. Christophe Faisy of the medical intensive care unit, European Georges Pompidou Hospital, Paris, and his associates.
To test this hypothesis, Dr. Faisy and his associates in the DIABOLO trial assessed 380 adults with COPD who were being treated at 15 French ICUs. One patient was receiving ventilation through a tracheotomy tube and the rest through endotracheal intubation. These study participants were randomly assigned in a double-blind fashion to receive either 500 mg acetazolamide twice daily or 1,000 mg acetazolamide twice daily if they were concomitantly receiving loop diuretics (187 patients), or a matching placebo (193 patients), administered as a slow intravenous injection.
Patients in the active-treatment group achieved larger reductions in serum bicarbonate and had fewer days with metabolic alkalosis. Nevertheless, the primary efficacy outcome – the duration of invasive ventilation – did not differ significantly between the two study groups. The median duration of ventilation was 136.5 hours with acetazolamide and 163.0 hours with placebo, which is clinically substantial but did not reach statistical significance, the investigators said (JAMA. 2016 Feb 2. doi: 10.1001/jama.2016.0019).
Acetazolamide didn’t exert a respiratory stimulant effect as measured by changes in respiratory rate, tidal volume, or minute ventilation. And there were no significant differences between the two study groups in secondary outcomes such as time to weaning off ventilation, rate of successful weaning, number of spontaneous breathing trials, use of tracheotomy or noninvasive ventilation after extubation, unplanned extubations, episodes of ventilator-associated pneumonia, laboratory values, length of ICU stay, or in-ICU mortality.
In addition, rates of adjunctive treatment using loop diuretics, glucocorticoids, beta-agonists, or catecholamines were the same between the two study groups, and left ventricular ejection fraction at weaning from ventilation also was the same. The rate of serious adverse events also was comparable.
“Taken together, these findings indicate that the inhibition of the renal carbonic anhydrase enzyme and the resulting serum bicarbonate reduction did not trigger a sufficient respiratory-stimulating effect to affect outcomes of critically ill patients with COPD,” Dr. Faisy and his associates wrote.
However, they noted that in both study groups the median duration of invasive mechanical ventilation was shorter than had been anticipated when the trial was designed, which likely decreased the statistical power of the primary endpoint. “It is possible that the study was underpowered to establish statistical significance,” the researchers said.
It is also possible that higher doses of acetazolamide may have exerted a greater effect on respiratory parameters. However, higher doses also may have increased the workload of the respiratory muscles and induced respiratory muscle fatigue, they added.
Acetazolamide failed to reduce the duration of invasive mechanical ventilation in chronic obstructive pulmonary disease (COPD) patients who had pure or mixed metabolic alkalosis, according to results from a clinical trial published online Feb. 2 in JAMA.
Acetazolamide is a carbonic anhydrase inhibitor used as a respiratory stimulant in patients who have COPD and metabolic alkalosis. Recent studies suggested that high doses of the drug (1,000 mg/day or more) might shorten the duration of mechanical ventilation in critically ill COPD patients who require invasive mechanical ventilation, by markedly lowering serum bicarbonate and raising minute ventilation, said Dr. Christophe Faisy of the medical intensive care unit, European Georges Pompidou Hospital, Paris, and his associates.
To test this hypothesis, Dr. Faisy and his associates in the DIABOLO trial assessed 380 adults with COPD who were being treated at 15 French ICUs. One patient was receiving ventilation through a tracheotomy tube and the rest through endotracheal intubation. These study participants were randomly assigned in a double-blind fashion to receive either 500 mg acetazolamide twice daily or 1,000 mg acetazolamide twice daily if they were concomitantly receiving loop diuretics (187 patients), or a matching placebo (193 patients), administered as a slow intravenous injection.
Patients in the active-treatment group achieved larger reductions in serum bicarbonate and had fewer days with metabolic alkalosis. Nevertheless, the primary efficacy outcome – the duration of invasive ventilation – did not differ significantly between the two study groups. The median duration of ventilation was 136.5 hours with acetazolamide and 163.0 hours with placebo, which is clinically substantial but did not reach statistical significance, the investigators said (JAMA. 2016 Feb 2. doi: 10.1001/jama.2016.0019).
Acetazolamide didn’t exert a respiratory stimulant effect as measured by changes in respiratory rate, tidal volume, or minute ventilation. And there were no significant differences between the two study groups in secondary outcomes such as time to weaning off ventilation, rate of successful weaning, number of spontaneous breathing trials, use of tracheotomy or noninvasive ventilation after extubation, unplanned extubations, episodes of ventilator-associated pneumonia, laboratory values, length of ICU stay, or in-ICU mortality.
In addition, rates of adjunctive treatment using loop diuretics, glucocorticoids, beta-agonists, or catecholamines were the same between the two study groups, and left ventricular ejection fraction at weaning from ventilation also was the same. The rate of serious adverse events also was comparable.
“Taken together, these findings indicate that the inhibition of the renal carbonic anhydrase enzyme and the resulting serum bicarbonate reduction did not trigger a sufficient respiratory-stimulating effect to affect outcomes of critically ill patients with COPD,” Dr. Faisy and his associates wrote.
However, they noted that in both study groups the median duration of invasive mechanical ventilation was shorter than had been anticipated when the trial was designed, which likely decreased the statistical power of the primary endpoint. “It is possible that the study was underpowered to establish statistical significance,” the researchers said.
It is also possible that higher doses of acetazolamide may have exerted a greater effect on respiratory parameters. However, higher doses also may have increased the workload of the respiratory muscles and induced respiratory muscle fatigue, they added.
FROM JAMA
Key clinical point: Acetazolamide failed to reduce the duration of invasive mechanical ventilation in COPD patients with metabolic alkalosis.
Major finding: The median duration of ventilation was 136.5 hours with acetazolamide and 163.0 hours with placebo, a nonsignificant difference.
Data source: A multicenter randomized double-blind trial involving 380 adults followed for 28 days after initiating invasive ventilation.
Disclosures: This study was supported by the French Ministry of Health and Sanofi-Aventis. Dr. Faisy reported having no relevant financial disclosures; his associates reported numerous ties to industry sources.
Adult immunization schedule undergoes minor changes for 2016
The federal Advisory Committee on Immunization Practices’ 2016 adult immunization schedule differs from the previous schedule in small but significant ways – chief among them that the nine-valent human papillomavirus vaccine (9vHPV) has been added.
“This vaccine can be used for routine vaccination against HPV as one of three HPV vaccines (bivalent HPV vaccine [2vHPV], quadrivalent HPV vaccine [4vHPV], and 9vHPV) recommended for females and one of two HPV vaccines (4vHPV and 9vHPV) recommended for males,” according to the new schedule, which is recommended and released by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (Ann Intern Med. 2016 Feb 2. doi: 10.7326/M15-3005).
Another noteworthy change is in the amount of time recommended between receiving the 13-valent pneumococcal conjugate vaccine (PCV13) and the subsequent 23-valent pneumococcal polysaccharide vaccine (PPSV23) in adults aged 65 years and older who are deemed “immunocompetent.” That interval has been increased to “at least 1 year”; previously, it was 6-12 months.
In addition, adults aged 19 years or older should receive the PPSV23 vaccine at least 8 weeks after PCV13 only if they have “anatomical or functional asplenia, cerebrospinal fluid leak, or cochlear implant,” or if they are deemed immunocompromised.
The schedule for meningococcal serogroup B (MenB) has also been changed slightly. It is now recommended for all individuals aged 10 years or older who are considered to be “at increased risk for [MenB],” which ACIP outlines as persons with “anatomical or functional asplenia or persistent complement component deficiencies, microbiologists who are routinely exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.”
The recommendations note that the MenB series of vaccines can be given to any adolescents and young adults between the ages of 16 and 23 years to provide protection against the disease on a short-term basis, with a recommended age range of 16-18 years as optimal for vaccination.
Other changes are more minor, consisting of alterations to the vaccine schedule’s figures and accompanying text.
The row pertaining to “Meningococcal” vaccinations has been changed to “Meningococcal 4-valent conjugate (MenACWY) or polysaccharide (MPSV4),” in order to clarify that “there are two types of serogroup A, C, W, and Y meningococcal vaccines available for adults.” Furthermore, MenB has been given its own row on the immunization schedule.
The text regarding hepatitis A has been amended from “2 doses” to “2 to 3 doses depending on vaccine” because of differences between the hepatitis A and B vaccine series. The other changes are to texts detailing alternate dosing schedules for common vaccines, such as “Measles, mumps, and rubella,” to show that those recommendations can be changed “depending on indication.”
The 2016 adult immunization schedule was reviewed and approved by the American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American College of Nurse-Midwives.
The federal Advisory Committee on Immunization Practices’ 2016 adult immunization schedule differs from the previous schedule in small but significant ways – chief among them that the nine-valent human papillomavirus vaccine (9vHPV) has been added.
“This vaccine can be used for routine vaccination against HPV as one of three HPV vaccines (bivalent HPV vaccine [2vHPV], quadrivalent HPV vaccine [4vHPV], and 9vHPV) recommended for females and one of two HPV vaccines (4vHPV and 9vHPV) recommended for males,” according to the new schedule, which is recommended and released by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (Ann Intern Med. 2016 Feb 2. doi: 10.7326/M15-3005).
Another noteworthy change is in the amount of time recommended between receiving the 13-valent pneumococcal conjugate vaccine (PCV13) and the subsequent 23-valent pneumococcal polysaccharide vaccine (PPSV23) in adults aged 65 years and older who are deemed “immunocompetent.” That interval has been increased to “at least 1 year”; previously, it was 6-12 months.
In addition, adults aged 19 years or older should receive the PPSV23 vaccine at least 8 weeks after PCV13 only if they have “anatomical or functional asplenia, cerebrospinal fluid leak, or cochlear implant,” or if they are deemed immunocompromised.
The schedule for meningococcal serogroup B (MenB) has also been changed slightly. It is now recommended for all individuals aged 10 years or older who are considered to be “at increased risk for [MenB],” which ACIP outlines as persons with “anatomical or functional asplenia or persistent complement component deficiencies, microbiologists who are routinely exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.”
The recommendations note that the MenB series of vaccines can be given to any adolescents and young adults between the ages of 16 and 23 years to provide protection against the disease on a short-term basis, with a recommended age range of 16-18 years as optimal for vaccination.
Other changes are more minor, consisting of alterations to the vaccine schedule’s figures and accompanying text.
The row pertaining to “Meningococcal” vaccinations has been changed to “Meningococcal 4-valent conjugate (MenACWY) or polysaccharide (MPSV4),” in order to clarify that “there are two types of serogroup A, C, W, and Y meningococcal vaccines available for adults.” Furthermore, MenB has been given its own row on the immunization schedule.
The text regarding hepatitis A has been amended from “2 doses” to “2 to 3 doses depending on vaccine” because of differences between the hepatitis A and B vaccine series. The other changes are to texts detailing alternate dosing schedules for common vaccines, such as “Measles, mumps, and rubella,” to show that those recommendations can be changed “depending on indication.”
The 2016 adult immunization schedule was reviewed and approved by the American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American College of Nurse-Midwives.
The federal Advisory Committee on Immunization Practices’ 2016 adult immunization schedule differs from the previous schedule in small but significant ways – chief among them that the nine-valent human papillomavirus vaccine (9vHPV) has been added.
“This vaccine can be used for routine vaccination against HPV as one of three HPV vaccines (bivalent HPV vaccine [2vHPV], quadrivalent HPV vaccine [4vHPV], and 9vHPV) recommended for females and one of two HPV vaccines (4vHPV and 9vHPV) recommended for males,” according to the new schedule, which is recommended and released by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (Ann Intern Med. 2016 Feb 2. doi: 10.7326/M15-3005).
Another noteworthy change is in the amount of time recommended between receiving the 13-valent pneumococcal conjugate vaccine (PCV13) and the subsequent 23-valent pneumococcal polysaccharide vaccine (PPSV23) in adults aged 65 years and older who are deemed “immunocompetent.” That interval has been increased to “at least 1 year”; previously, it was 6-12 months.
In addition, adults aged 19 years or older should receive the PPSV23 vaccine at least 8 weeks after PCV13 only if they have “anatomical or functional asplenia, cerebrospinal fluid leak, or cochlear implant,” or if they are deemed immunocompromised.
The schedule for meningococcal serogroup B (MenB) has also been changed slightly. It is now recommended for all individuals aged 10 years or older who are considered to be “at increased risk for [MenB],” which ACIP outlines as persons with “anatomical or functional asplenia or persistent complement component deficiencies, microbiologists who are routinely exposed to isolates of Neisseria meningitidis, and persons identified at increased risk because of a serogroup B meningococcal disease outbreak.”
The recommendations note that the MenB series of vaccines can be given to any adolescents and young adults between the ages of 16 and 23 years to provide protection against the disease on a short-term basis, with a recommended age range of 16-18 years as optimal for vaccination.
Other changes are more minor, consisting of alterations to the vaccine schedule’s figures and accompanying text.
The row pertaining to “Meningococcal” vaccinations has been changed to “Meningococcal 4-valent conjugate (MenACWY) or polysaccharide (MPSV4),” in order to clarify that “there are two types of serogroup A, C, W, and Y meningococcal vaccines available for adults.” Furthermore, MenB has been given its own row on the immunization schedule.
The text regarding hepatitis A has been amended from “2 doses” to “2 to 3 doses depending on vaccine” because of differences between the hepatitis A and B vaccine series. The other changes are to texts detailing alternate dosing schedules for common vaccines, such as “Measles, mumps, and rubella,” to show that those recommendations can be changed “depending on indication.”
The 2016 adult immunization schedule was reviewed and approved by the American College of Physicians, the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American College of Nurse-Midwives.
FROM ANNALS OF INTERNAL MEDICINE
Ebola research update: January 2016
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Individuals from areas of poverty were associated with high rates of transmission and spread of Ebola to other regions, according to a study in PLOS Neglected Tropical Diseases. A research team at the Yale School of Public Health analyzed the heterogeneity of Ebola incidence and transmission factors among 300 communities, categorized by socioeconomic status, within Montserrado County, Liberia, and found that infected individuals from communities of middle and low socioeconomic status were associated with 1.5 and 3.5 times as many secondary cases as those from communities of high socioeconomic status. This suggests Ebola could most effectively be prevented or contained if disease interventions were targeted to areas of extreme poverty and funding were dedicated to development projects that meet basic needs.
A recent study published online in the Journal of Virology shows a promising mechanism for attacking the Ebola virus. A team at the Texas Biomedical Research Institute used ultradeep sequencing to reveal that the spontaneous mutation frequency for Ebola virus was high and similar to that of other RNA viruses, although the Ebola virus had very limited ability to tolerate spontaneous changes in the genome. Testing the drug ribavirin in mouse and monkey models of postexposure therapy, researchers delayed the time to subject death and increased survival.
Dr. Tom Koch, a medical geographer at the University of British Columbia, Vancouver, reflects in a review essay in the International Journal of Epidemiology on what could have been done differently in the efforts to prevent the Ebola epidemic in 2014. He said the limits of patient location and travel mapping are reasons why it was difficult to contain Ebola from spreading. Maps and census data were almost nonexistent for the region in Guinea where the outbreak occurred, and aggressive quarantine programs were not quickly enacted.
A clinical team at an Ebola treatment unit in Sierra Leone has published guidelines for the treatment of children with Ebola virus disease. The aggressive approach they recommend calls for giving children fluids intravenously; treating other possible infections; feeding them highly fortified food; and greatly increasing the amount of bedside care they receive.
A rapid screening assay has been used for the first time in a standard open laboratory to identify and test promising anti-Ebola drugs. Research on new Ebola therapies has been limited by an inability to compare antiviral effectiveness, since cell model systems, treatment regimens and results are so varied that it is difficult to compare effectiveness amongst the compounds.
Researchers reported in Emerging Infectious Diseases that polymerase chain reaction–based testing for Plasmodium spp. parasitemia can be implemented easily and safely in laboratories performing Ebola virus disease diagnostics to assist with case-patient management during EVD outbreaks in malaria-endemic areas.
A researcher team at Vanderbilt University Medical Center, Nashville, Tenn., and the University of Texas Medical Branch in Galveston reported in the journal Cell that they have isolated human monoclonal antibodies from Ebola survivors that can neutralize multiple species of the virus. Investigators said the results provide a “roadmap to develop a single antibody-based treatment.”
In a letter to the editor of Emerging Infectious Diseases, clinicians who worked during the Ebola virus disease outbreak in West Africa recommend that Ebola virus–positive women stop breastfeeding immediately and that breastfeeding not be resumed until two negative reverse transcription polymerase chain reaction tests of the breast milk have been confirmed.
A report in MMWR describes the design and implementation of the Ebola active monitoring program established by the New York City Department of Health and Mental Hygiene (DOHMH) during its first 6 months (October 2014-April 2015) of operation.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Individuals from areas of poverty were associated with high rates of transmission and spread of Ebola to other regions, according to a study in PLOS Neglected Tropical Diseases. A research team at the Yale School of Public Health analyzed the heterogeneity of Ebola incidence and transmission factors among 300 communities, categorized by socioeconomic status, within Montserrado County, Liberia, and found that infected individuals from communities of middle and low socioeconomic status were associated with 1.5 and 3.5 times as many secondary cases as those from communities of high socioeconomic status. This suggests Ebola could most effectively be prevented or contained if disease interventions were targeted to areas of extreme poverty and funding were dedicated to development projects that meet basic needs.
A recent study published online in the Journal of Virology shows a promising mechanism for attacking the Ebola virus. A team at the Texas Biomedical Research Institute used ultradeep sequencing to reveal that the spontaneous mutation frequency for Ebola virus was high and similar to that of other RNA viruses, although the Ebola virus had very limited ability to tolerate spontaneous changes in the genome. Testing the drug ribavirin in mouse and monkey models of postexposure therapy, researchers delayed the time to subject death and increased survival.
Dr. Tom Koch, a medical geographer at the University of British Columbia, Vancouver, reflects in a review essay in the International Journal of Epidemiology on what could have been done differently in the efforts to prevent the Ebola epidemic in 2014. He said the limits of patient location and travel mapping are reasons why it was difficult to contain Ebola from spreading. Maps and census data were almost nonexistent for the region in Guinea where the outbreak occurred, and aggressive quarantine programs were not quickly enacted.
A clinical team at an Ebola treatment unit in Sierra Leone has published guidelines for the treatment of children with Ebola virus disease. The aggressive approach they recommend calls for giving children fluids intravenously; treating other possible infections; feeding them highly fortified food; and greatly increasing the amount of bedside care they receive.
A rapid screening assay has been used for the first time in a standard open laboratory to identify and test promising anti-Ebola drugs. Research on new Ebola therapies has been limited by an inability to compare antiviral effectiveness, since cell model systems, treatment regimens and results are so varied that it is difficult to compare effectiveness amongst the compounds.
Researchers reported in Emerging Infectious Diseases that polymerase chain reaction–based testing for Plasmodium spp. parasitemia can be implemented easily and safely in laboratories performing Ebola virus disease diagnostics to assist with case-patient management during EVD outbreaks in malaria-endemic areas.
A researcher team at Vanderbilt University Medical Center, Nashville, Tenn., and the University of Texas Medical Branch in Galveston reported in the journal Cell that they have isolated human monoclonal antibodies from Ebola survivors that can neutralize multiple species of the virus. Investigators said the results provide a “roadmap to develop a single antibody-based treatment.”
In a letter to the editor of Emerging Infectious Diseases, clinicians who worked during the Ebola virus disease outbreak in West Africa recommend that Ebola virus–positive women stop breastfeeding immediately and that breastfeeding not be resumed until two negative reverse transcription polymerase chain reaction tests of the breast milk have been confirmed.
A report in MMWR describes the design and implementation of the Ebola active monitoring program established by the New York City Department of Health and Mental Hygiene (DOHMH) during its first 6 months (October 2014-April 2015) of operation.
On Twitter @richpizzi
The struggle to defeat Ebola virus disease continues globally, although it may not always make the headlines. To catch up on what you may have missed, here are some notable news items and journal articles published over the past few weeks that are worth a second look.
Individuals from areas of poverty were associated with high rates of transmission and spread of Ebola to other regions, according to a study in PLOS Neglected Tropical Diseases. A research team at the Yale School of Public Health analyzed the heterogeneity of Ebola incidence and transmission factors among 300 communities, categorized by socioeconomic status, within Montserrado County, Liberia, and found that infected individuals from communities of middle and low socioeconomic status were associated with 1.5 and 3.5 times as many secondary cases as those from communities of high socioeconomic status. This suggests Ebola could most effectively be prevented or contained if disease interventions were targeted to areas of extreme poverty and funding were dedicated to development projects that meet basic needs.
A recent study published online in the Journal of Virology shows a promising mechanism for attacking the Ebola virus. A team at the Texas Biomedical Research Institute used ultradeep sequencing to reveal that the spontaneous mutation frequency for Ebola virus was high and similar to that of other RNA viruses, although the Ebola virus had very limited ability to tolerate spontaneous changes in the genome. Testing the drug ribavirin in mouse and monkey models of postexposure therapy, researchers delayed the time to subject death and increased survival.
Dr. Tom Koch, a medical geographer at the University of British Columbia, Vancouver, reflects in a review essay in the International Journal of Epidemiology on what could have been done differently in the efforts to prevent the Ebola epidemic in 2014. He said the limits of patient location and travel mapping are reasons why it was difficult to contain Ebola from spreading. Maps and census data were almost nonexistent for the region in Guinea where the outbreak occurred, and aggressive quarantine programs were not quickly enacted.
A clinical team at an Ebola treatment unit in Sierra Leone has published guidelines for the treatment of children with Ebola virus disease. The aggressive approach they recommend calls for giving children fluids intravenously; treating other possible infections; feeding them highly fortified food; and greatly increasing the amount of bedside care they receive.
A rapid screening assay has been used for the first time in a standard open laboratory to identify and test promising anti-Ebola drugs. Research on new Ebola therapies has been limited by an inability to compare antiviral effectiveness, since cell model systems, treatment regimens and results are so varied that it is difficult to compare effectiveness amongst the compounds.
Researchers reported in Emerging Infectious Diseases that polymerase chain reaction–based testing for Plasmodium spp. parasitemia can be implemented easily and safely in laboratories performing Ebola virus disease diagnostics to assist with case-patient management during EVD outbreaks in malaria-endemic areas.
A researcher team at Vanderbilt University Medical Center, Nashville, Tenn., and the University of Texas Medical Branch in Galveston reported in the journal Cell that they have isolated human monoclonal antibodies from Ebola survivors that can neutralize multiple species of the virus. Investigators said the results provide a “roadmap to develop a single antibody-based treatment.”
In a letter to the editor of Emerging Infectious Diseases, clinicians who worked during the Ebola virus disease outbreak in West Africa recommend that Ebola virus–positive women stop breastfeeding immediately and that breastfeeding not be resumed until two negative reverse transcription polymerase chain reaction tests of the breast milk have been confirmed.
A report in MMWR describes the design and implementation of the Ebola active monitoring program established by the New York City Department of Health and Mental Hygiene (DOHMH) during its first 6 months (October 2014-April 2015) of operation.
On Twitter @richpizzi
Smoking Cessation What Should You Recommend?
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.
IN THIS ARTICLE
- The 2008 USPHS guideline: 10 key recommendations
- USPHS smoking cessation guideline: An evidence summary
- Medications for smoking cessation: Dosing, advantages, and adverse effects
In its 2014 report, The Health Consequences of Smoking—50 Years of Progress,1 the US Surgeon General concluded that, while significant improvements have been made since the publication of its landmark 1964 report, cigarette smoking remains a major public health problem. It is the leading cause of preventable death, increasing the risk for such common causes of mortality as cardiovascular disease, pulmonary disease, and malignancy. Cigarette smoking is responsible for an estimated 443,000 deaths annually.2
Overall, 42 million US adults and about 3 million middle and high school students smoke, despite the availability of an array of pharmacologic interventions to help them quit.1 Half of those who continue to smoke will die from a tobacco-related cause. Stopping before the age of 50 cuts the risk in half, and quitting before age 30 almost completely negates it.3
The most recent comprehensive smoking cessation guideline, sponsored by the US Public Health Service, was published in 2008.4 The US Preventive Services Task Force (USPSTF) recommendation that “clinicians ask all adults about tobacco use and provide tobacco cessation interventions” for those who smoke was issued one year later.5 Since then, multiple studies have assessed the merits of the various medications, forms of nicotine replacement therapy (NRT), and counseling aimed at helping smokers maintain abstinence from tobacco.
This article reviews the guideline and provides family practice providers with an evidence-based update.
Continue for treating tobacco use and dependence >>
The guideline: Treating tobacco use and dependence
Prescribing a firstline medication (bupropion SR, varenicline, nicotine gum, nicotine inhaler, nicotine lozenge, nicotine nasal spray, or nicotine patch) for every patient who seeks to quit smoking is a key component of the 2008 guideline (see Table 1).4 The only exceptions: patients for whom such agents are medically contraindicated and groups for which there is insufficient evidence of effectiveness, such as pregnant women and adolescents.
The use of any of these medications as a single agent nearly doubles the likelihood of success compared with placebo, with an average cessation rate of 25% (see Table 2).4 Combination therapy (pairing a nicotine patch and an additional agent) was found to be even more effective, with some combinations attaining success rates as high as 65%.4
Second-line therapies, including clonidine and nortriptyline, were also cited as effective, with an average cessation rate of 24%.4 Although the meta-analyses that these averages were based on did not include head-to-head comparisons, subsequent studies that also showed efficacy did include such comparisons.
Continue for counseling is an essential component >>
Counseling is an essential component
In one of the meta-analyses on which the guideline was based, the combination of counseling and medication proved to be more effective than either intervention alone. Individual, group, and telephone counseling were all effective (odds ratios [ORs], 1.7, 1.3, and 1.2, respectively), provided they included practical help that emphasized problem solving and skills training, as well as social support. The benefits of a team-based approach were evident from the finding that counseling provided by more than one type of clinician had higher effect sizes (OR, 2.5 when two different clinical disciplines were involved and 2.4 for three or more disciplines).4
The guideline also found state-sponsored quit lines, accessible at no charge via 800-QUIT-NOW, are an effective option. (For more information about this and other resources, see Table W1.) For patients who aren’t ready to stop smoking, the guideline recommends motivational interviewing4—a direct, patient-centered technique used to explore and work through ambivalence. Further information about this method is available at www.motivational interviewing.org.
In counseling patients who are considering a quit attempt, it is important to present all options. A smoking history is needed, too, because factors such as the number of cigarettes smoked per day, how long a patient is typically awake before smoking the first cigarette of the day, and level of dependence are important factors in determining medication and dosage. Consider the advantages and disadvantages of the various medications (see Table 3) or methods used for prior quit attempts and reasons for relapse, if appropriate, as well as patient preference.4,6,7
Continue for evidence update >>
Evidence update: What’s best?
Since 2009, many clinical trials have examined the best way to help smokers quit. Here’s a closer look at the latest evidence.
NRT boosts long-term cessation
A 2012 Cochrane review examined 150 trials and found that every type of NRT—gum, lozenge, patch, inhaler, and nasal spray—was associated with long-term cessation (relative risk [RR], 1.60).8 This effect was essentially unchanged regardless of the duration, setting, or intensity of supportive therapy offered, and no single type of NRT was more effective than any other. However, combining a short-acting form, such as a lozenge, with a long-acting patch was found to be more effective than either one alone (RR, 1.34).
Starting the NRT before the patient quit did not improve cessation rates over traditional start times (RR, 1.18). Neither was there an added benefit to using NRT beyond the recommended 24-week prescription period,9 although doing so was found to be safe. Another 2012 Cochrane review looked specifically at the use of pharmacologic smoking cessation interventions during pregnancy and concluded that there was still not sufficient data to document efficacy for this patient population.10
Adherence. In deciding on which type of NRT to prescribe, it is important to consider not only patient preference and previous efforts but also the latest evidence. A study comparing various NRT formulations found patient adherence to be highest with the patch, followed by nicotine gum, which had a higher compliance rate than either the nicotine inhaler or nasal spray.11
Varenicline is still a firstline agent
Since the 2008 guideline recommended this partial nicotinic receptor agonist/antagonist as a firstline pharmacologic agent, additional meta-analyses have confirmed its long-term efficacy in smokers who are ready to quit.12,13 A 2012 Cochrane review found varenicline to increase long-term cessation compared with placebo (RR, 2.27).13 It also showed varenicline to be more effective than bupropion SR (RR, 1.52), but about as effective as NRT (RR, 1.13).
Newer data suggest that varenicline may also be effective for those who are willing to cut down on smoking but not yet ready to give up cigarettes completely. Used for 24 weeks by those who were initially resistant to quitting, researchers found varenicline nearly tripled the cessation rate at 52 weeks compared with placebo (RR, 2.7).14
Latest evidence on safety. Additional concerns about the safety of varenicline have been raised, however, since the 2008 guideline was published. In 2009, the FDA required that black box warnings be added to the labels of both varenicline and bupropion SR based on postmarketing safety reports showing risk for neuropsychiatric symptoms, including suicidality.15 In 2011, a large case-control study by the FDA Adverse Event Reporting System also showed an increased risk for suicidality in patients taking these drugs.16
Follow-up studies, however, including a large prospective cohort study and a large meta-analysis, failed to show an increased association with neuropsychiatric adverse effects.17,18 A smaller randomized controlled trial (RCT) showed that in smokers diagnosed with schizophrenia and bipolar disorder, maintenance therapy with varenicline was effective in preventing smoking relapse for up to 52 weeks. Abstinence rates were 60% for those in the varenicline group versus 19% for those in the placebo group (OR, 6.2). Although no increased risk for adverse psychiatric events was found in this study, it was not powered to detect them.19 Also of note: A meta-analysis of 14 RCTs showed an increased rate of cardiovascular events associated with varenicline.20 There are concerns about methodologic flaws in this meta-analysis, however, and two subsequent meta-analyses failed to find a cardiovascular risk.21,22
The higher quality studies that have been published since the original concerns about varenicline’s safety are reassuring, but it is still essential to carefully weigh the risks and benefits of varenicline. Review cardiac and psychiatric history and conduct a suicidality assessment before prescribing it as a smoking cessation aid, and provide close follow-up.
Continue for a closer look at antidepressants >>
A closer look at antidepressants
Bupropion SR, an atypical antidepressant, was also listed as a firstline treatment in the 2008 guideline. A 2014 Cochrane review of 90 studies confirmed the evidence for this recommendation.6 Monotherapy with this agent was found to significantly increase rates of long-term cessation (RR, 1.62). No increased risk for serious adverse events was identified compared with placebo. As already noted, associations with neuropsychiatric symptoms were found, but this risk must be considered with any antidepressant.
Bupropion’s efficacy was not significantly different from that of NRT, but moderate evidence suggests that it is less effective than varenicline (RR, 0.68). Other classes of antidepressants, including selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and monoamine oxidase inhibitors, were found to be ineffective for smoking cessation.6
Nortriptyline, a tricyclic antidepressant, was not significantly different from bupropion SR (RR, 1.30) in efficacy for smoking cessation, but it lacks FDA approval for this purpose and is not considered a firstline agent.6
Second-line agents
Clonidine is an alpha-2 adrenergic receptor agonist that was originally used to treat hypertension but found to be effective for smoking cessation in a meta-analysis performed for the 2008 guideline.4 Like nortriptyline, however, clonidine is not FDA-approved for this purpose and is not considered a firstline agent.5 A 2013 Cochrane meta-analysis further showed that clonidine is effective for smoking cessation versus placebo (RR, 1.63)7 but suggested that its significant dose-related adverse effects, including postural hypotension and sedation, could limit its usefulness.
Combination therapies are highly effective
Evidence for various combinations of smoking cessation pharmacotherapy continues to mount.23-26 Perhaps the most compelling evidence comes from a comparative effectiveness trial that randomized 1,346 patients in 12 primary care clinics to nicotine patches, nicotine lozenges, bupropion SR, a combination of patch plus lozenge, and bupropion SR plus lozenge. The six-month abstinence rate was 30% for the bupropion SR plus lozenge combination, the most effective option. The combination was superior to either patch or bupropion SR monotherapy (ORs, 0.56 and 0.54, respectively).23 Based on data from the 2008 guideline, similar combinations (eg, nicotine patch plus nicotine gum or bupropion SR plus the patch) are likely to be equally effective. The 2008 guideline also supports a nicotine patch and nicotine inhaler combination.
Another study found varenicline combined with the patch to be highly effective, with a 65% abstinence rate at 24 weeks compared with 47% for varenicline alone (number needed to treat [NNT], 6).24
In heavy smokers—defined as those who smoke 20 or more cigarettes daily—a varenicline and bupropion SR combination was more effective than varenicline alone (NNT, 9), but the combination can lead to increased anxiety and depression.25 A smaller study found triple therapy using nicotine patch plus inhaler plus bupropion SR to be more effective than the nicotine patch alone (35% abstinence vs 19% abstinence at 26 weeks; NNT, 6).26 Consider using these combinations in patients who have high nicotine dependency levels or who have been unable to quit using a single firstline agent.
Continue to the role e-cigarettes play >>
What role do e-cigarettes play?
The use of electronic cigarettes or “vapes”—battery-operated devices that deliver nicotine to the user through vapor—has increased significantly since their US introduction in 2007. A recent study found that “ever use” of e-cigarettes increased from 1.8% in 2010 to 13% in 2013; current use increased from 0.3% to 6.8% in the same time frame.27 Vaping, as inhaling on an e-cigarette is sometimes known, causes a sensor to detect airflow and initiate the heating element to vaporize the liquid solution within the cartridge, which contains propylene glycol, flavoring, and nicotine.
There is limited evidence of the efficacy of e-cigarettes for smoking cessation, but there is support for their role in reducing the quantity of conventional cigarettes smoked. A 2014 Cochrane review of two RCTs evaluating e-cigarette efficacy for smoking cessation or reduction found evidence of increased abstinence at six months in users of e-cigarettes containing nicotine, compared with placebo e-cigarettes (9% vs 4%; RR, 2.29). Additionally, e-cigarette use was associated with a more than 50% decrease in cigarette smoking versus placebo (36% vs 27%; RR,1.31) or patch (61% vs 44%; RR, 1.41).28
A survey published after the review also showed a correlation between cigarette reduction (but not cessation) after one year of e-cigarette use.29 A subsequent RCT conducted in a controlled laboratory setting found that e-cigarettes were highly effective in reducing cessation-related cravings.30 And at eight-month follow-up, 44% of those using e-cigarettes were found to have at least a 50% reduction in the use of conventional cigarettes—and complete cessation in some cases.
Concerns about health effects
E-cigarettes have generally been thought to be safer than conventional cigarettes, given that they mainly deliver nicotine and propylene glycol instead of the more toxic chemicals—eg, benzene, carbon monoxide, and formaldehyde—released by conventional cigarettes.31 Carcinogens have also been found in e-cigarettes, but at significantly lower levels.31 However, a systematic review found wide variation in the toxin content of e-cigarettes.32 In addition, recent reports have detailed incidents in which e-cigarette devices were alleged to have exploded, causing severe bodily harm.33
Adverse effects of e-cigarettes include minor irritation of the throat, mouth, and lungs. Among cigarette-naive patients, lightheadedness, throat irritation, dizziness, and cough were most commonly reported. No serious adverse events have been reported, but the lack of long-term safety data is a source of concern.32
Additionally, minimal regulatory oversight of the e-cigarette industry exists. Currently, the FDA only has authority to regulate e-cigarettes that are marketed for therapeutic purposes, although the agency is seeking to extend its oversight to all e-cigarettes.
The bottom line: More data on safety and regulatory oversight are needed before recommendations on the use of e-cigarettes as a smoking cessation tool can be made.
Continue for looking ahead >>
Looking ahead
Several novel pharmacotherapies have been evaluated for smoking cessation in recent years. Among them is a nicotine vaccine that several drug companies have been pursuing. In theory, such a vaccine would create an immunologic reaction to nicotine in a smoker, thereby preventing the substance from reaching the brain and providing rewarding stimuli. A 2008 Cochrane review of four trials assessing the efficacy of nicotine vaccines for tobacco cessation found that none showed efficacy.34
Naltrexone, an opioid antagonist, has shown efficacy in helping those with opioid or alcohol dependence achieve abstinence from these substances, raising the possibility that it might aid in smoking cessation as well. A 2013 Cochrane review of eight trials found that this was not the case: Compared with placebo, naltrexone was not beneficial when used alone (RR, 1.00) or as an adjunct to NRT compared with NRT alone (RR, 0.95).35
Cytisine, an extract from plants in the Faboideae family, has been used in Eastern Europe for decades for smoking cessation. It appears to work as a nicotine receptor partial agonist similar to varenicline. The extract does not have FDA approval, but the National Institutes of Health’s Center for Complementary and Integrative Health is sponsoring early-stage safety trials that could lead to its approval in the US.36
A 2012 Cochrane review identified two recent RCTs evaluating cytisine and found it to be effective in increasing smoking cessation rates, compared with placebo (RR, 3.98).13
The authors thank Matt Orr, PhD, and Kathryn E. Bornemann for their help with this manuscript.
References
1. National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.ncbi.nlm.nih.gov/pubmed/24455788. Accessed January 21, 2016.
2. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226-1228.
3. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519.
4. US Public Health Service. A clinical practice guideline for treating tobacco use and dependence: 2008 update. Am J Prev Med. 2008;35:158-176.
5. US Preventive Services Task Force. Tobacco use in adults and pregnant women: counseling and interventions. April 2009. www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed January 21, 2016.
6. Hughes JR, Stead LF, Hartmann-Boyce J, et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2014;(1):CD000031.
7. Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329.
8. Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD000146.
9. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175: 504-511.
10. Coleman T, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev. 2012;(9):CD010078.
11. Hajek P, West R, Foulds J, et al. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Arch Intern Med. 1999;159:2033-2038.
12. Eisenberg MJ, Filion KB, Yavin D, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135-144.
13. Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;(4):CD006103.
14. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313:687-694.
15. FDA. Reports of suicidality associated with use of varenicline (marketed as CHANTIX) and bupropion (marketed as ZYBAN and GENERICS). FDA Drug Safety News. 2009.
16. Moore TJ, Furberg CD, Glenmullen J, et al. Suicidal behavior and depression in smoking cessation treatments. PLoS One. 2011;6:e27016.
17. Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704.
18. Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109.
19. Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311:145-154.
20. Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359-1366.
21. Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856.
22. Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
23. Smith SS, McCarthy DE, Japuntich SJ, et al. Comparative effectiveness of five smoking cessation pharmacotherapies in primary care clinics. Arch Intern Med. 2009;169:2148-2155.
24. Koegelenberg CFN, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation. JAMA. 2014;312:155-161.
25. Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311:155-163.
26. Steinberg MB, Greenhaus S, Schmelzer AC, et al. Triple-combination pharmacotherapy for medically ill smokers: a randomized trial. Ann Intern Med. 2009;150:447-454.
27. McMillen RC, Gottlieb MA, Shaefer RMW, et al. Trends in electronic cigarette use among US adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195-1202.
28. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev. 2014;(12):CD010216.
29. Brose LS, Hitchman SC, Brown J, et al. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction. 2015;110:1160-1168.
30. Adriaens K, Van Gucht D, Declerck P, et al. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11:11220-11248.
31. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133-139.
32. Pisinger C, Døssing M. A systematic review of health effects of electronic cigarettes. Prev Med (Baltim). 2014;69C:248-260.
33. Bowerman M. Fla man hospitalized after e-cigarette explodes in face. USA Today Network. October 29, 2015. www.usatoday.com/story/news/nation-now/2015/10/29/fla-man-hospitalized-e-cigarette-explodes-face/74791722/. Accessed January 21, 2016.
34. Hatsukami D, Cahill K, Stead LF. Nicotine vaccines for smoking cessation. Cochrane Database Syst Rev. 2008;(2):CD007072.
35. David SP, Lancaster T, Stead LF, et al. Opioid antagonists for smoking cessation. Cochrane Database Syst Rev. 2013;(6):CD003086.
36. Frankel T. Pill that quashes tobacco urge found in plain sight. Washington Post. May 15, 2015. www.washingtonpost.com/business/economy/pill-promises-a-safercheaper-way-than-chantix-to-quit-smoking/2015/05/15/8ce5590c-f830-11e4-9030-b4732caefe81_story.html. Accessed January 21, 2016.