User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Drug interaction myths
A 72-year-old man with benign prostatic hypertrophy comes to clinic to discuss recent problems with erectile dysfunction. He has been treated with tamsulosin with good results for the past 3 years for his BPH. He is given a prescription for vardenafil 10 mg for his ED. The pharmacist calls and asks if you want the prescription filled despite a drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take half a tablet of vardenafil.
C. Have the patient not take vardenafil within 6 hours of taking tamsulosin.
A 22-year-old woman presents with a unilateral headache, pounding in nature, worse with exercise. She is diagnosed with migraine. She has a history of depression and is taking 40 mg of fluoxetine. She is given a prescription for sumatriptan 100 mg. The pharmacist calls you and asks if you want to make changes because of possible drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take 50 mg of sumatriptan.
C. Have her reduce her fluoxetine dose to 20 mg.
D. Do not take sumatriptan within 12 hours of taking fluoxetine.
The title of this article is drug interaction myths. These are not true myths, but in both these cases, I think the prescriptions should be filled as written, and it will be safe for the patient to take the medications despite a theoretical drug interaction.
I have received calls from the pharmacist multiple times when I have prescribed these drug combinations, and I will share with you the evidence of safety for using these medications despite potential interactions.
In 2006, the Food and Drug Administration released an alert on serotonin syndrome occurring with combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) with triptans.1 This alert was based on 29 cases that the FDA evaluated and felt justified an alert.
Dr. Randolph W. Evans did an analysis of all 29 cases to see if they met criteria for serotonin syndrome.2 He classified if the cases met two different criteria for serotonin syndrome: the Hunter criteria3 or the Sternbach criteria4.
Of the 29 case reports, 10 met the Sternbach criteria, and none of the reports met the Hunter criteria. Some of the cases included polypharmacy of other drugs that can cause serotonin syndrome. Two cases that met the Sternbach criteria were excluded because they were either not on an SSRI or had alternative compelling diagnoses.
Dr. Evans suggested the biologic implausibility of triptans causing serotonin syndrome, because serotonin syndrome is believed to be caused by activation of 5-HT1A and 5-HT2A receptors, whereas triptans act at the 5-HT1B/5-HT1D and 5-HT1F receptors.
In a prospective study of 12,339 patients with migraine who used subcutaneous sumatriptan for at least 1 year, 1,784 patients also received an SSRI.5 No episodes of serotonin syndrome were reported. David A. Sclar, Ph.D., and his colleagues estimated that in 2007-2008, 1.4 million patients were prescribed both a triptan and an SSRI or SNRI.6 That is a 36% increase from 2003-2004, despite a 50% reduction in coprescriptions from primary care physicians – suggesting neurologists were not affected by the FDA alert.7
The American Headache Society position paper on the FDA alert states, “The currently available evidence does not support limiting the use of triptans with SNRIs or SSRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome.”8
A warning will pop up on prescribing software when you prescribe a phosphodiesterase inhibitor in patients who are taking alpha-blockers. This is a common situation, because BPH and ED both become more common with age. The concern is that the combination of alpha-blocker plus phosphodiesterase inhibitor will increase the risk of hypotension.
Dr. Michel Guillaume and his colleagues studied the hemodynamic effect of doxazosin and tamsulosin in combination with tadalafil.9 A total of 45 healthy men aged 40-70 years were randomized to receive tadalafil and placebo for 28 days. Doxazosin was added after 7 days and continued for an additional 21 days. The second study included 39 men who received tadalafil and placebo for 7 days before adding tamsulosin for an additional 7 days.
There were no significant differences in change in standing systolic blood pressure with tadalafil with placebo, doxazosin, or tamsulosin.
Robert A. Kloner, M.D., Ph.D., and his colleagues reported on a randomized, double-blind, crossover trial of doxazosin 8 mg or placebo with tadalafil 20 mg and tamsulosin 0.4 mg or placebo with 10 mg or 20 mg of tadalafil.10 Tadalafil did augment the hypotensive effect of doxazosin, but it did not have any blood pressure effect on patients taking tamsulosin.
In a study of men taking both tamsulosin and vardenafil or tamsulosin and placebo for the treatment of BPH symptoms, Dr. Mauro Gacci and his colleagues found no significant difference in adverse effects in patients who received tamsulosin plus placebo, compared with men who received tamsulosin plus vardenafil.11
I think it is safe to prescribe triptans in patients who are on SSRIs and SNRIs. In patients who need both alpha-blockers and phosphodiesterase inhibitors, I think tamsulosin is the safest alpha-blocker option. It is best to not start a phosphodiesterase inhibitor at the same time as an alpha-blocker. The studies on coadministration of alpha-blockers and phosphodiesterase inhibitors have been done in either healthy volunteers, or in patients without severe systemic disease. So, the effect on blood pressure in patients taking multiple antihypertensive drugs or heart failure drugs is unknown.
References
1. U.S. Food and Drug Administration. Information for healthcare professionals: Selective serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SNRIs), 5-hydroxytryptamine receptor agonists (triptans), July 19, 2006.
2. MedGenMed. 2007 Sep 5;9(3):48.
3. QJM. 2003 Sep;96(9):635-42.
4. Am J Psychiatry. 1991 Jun;148(6):705-13.
5. Cephalalgia. 1999 Sep;19(7):668-75.
6. Headache. 2012 Feb;52(2):198-203.
7. Headache. 2012 Feb;52(2):195-7.
8. Headache. 2010 Jun;50(6):1089-99.
9. J Clin Pharmacol. 2007 Oct;47(10):1303-10.
10. J Urol. 2004 Nov;172(5 Pt 1):1935-40.
11. J Sex Med. 2012 Jun;9(6):1624-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 72-year-old man with benign prostatic hypertrophy comes to clinic to discuss recent problems with erectile dysfunction. He has been treated with tamsulosin with good results for the past 3 years for his BPH. He is given a prescription for vardenafil 10 mg for his ED. The pharmacist calls and asks if you want the prescription filled despite a drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take half a tablet of vardenafil.
C. Have the patient not take vardenafil within 6 hours of taking tamsulosin.
A 22-year-old woman presents with a unilateral headache, pounding in nature, worse with exercise. She is diagnosed with migraine. She has a history of depression and is taking 40 mg of fluoxetine. She is given a prescription for sumatriptan 100 mg. The pharmacist calls you and asks if you want to make changes because of possible drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take 50 mg of sumatriptan.
C. Have her reduce her fluoxetine dose to 20 mg.
D. Do not take sumatriptan within 12 hours of taking fluoxetine.
The title of this article is drug interaction myths. These are not true myths, but in both these cases, I think the prescriptions should be filled as written, and it will be safe for the patient to take the medications despite a theoretical drug interaction.
I have received calls from the pharmacist multiple times when I have prescribed these drug combinations, and I will share with you the evidence of safety for using these medications despite potential interactions.
In 2006, the Food and Drug Administration released an alert on serotonin syndrome occurring with combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) with triptans.1 This alert was based on 29 cases that the FDA evaluated and felt justified an alert.
Dr. Randolph W. Evans did an analysis of all 29 cases to see if they met criteria for serotonin syndrome.2 He classified if the cases met two different criteria for serotonin syndrome: the Hunter criteria3 or the Sternbach criteria4.
Of the 29 case reports, 10 met the Sternbach criteria, and none of the reports met the Hunter criteria. Some of the cases included polypharmacy of other drugs that can cause serotonin syndrome. Two cases that met the Sternbach criteria were excluded because they were either not on an SSRI or had alternative compelling diagnoses.
Dr. Evans suggested the biologic implausibility of triptans causing serotonin syndrome, because serotonin syndrome is believed to be caused by activation of 5-HT1A and 5-HT2A receptors, whereas triptans act at the 5-HT1B/5-HT1D and 5-HT1F receptors.
In a prospective study of 12,339 patients with migraine who used subcutaneous sumatriptan for at least 1 year, 1,784 patients also received an SSRI.5 No episodes of serotonin syndrome were reported. David A. Sclar, Ph.D., and his colleagues estimated that in 2007-2008, 1.4 million patients were prescribed both a triptan and an SSRI or SNRI.6 That is a 36% increase from 2003-2004, despite a 50% reduction in coprescriptions from primary care physicians – suggesting neurologists were not affected by the FDA alert.7
The American Headache Society position paper on the FDA alert states, “The currently available evidence does not support limiting the use of triptans with SNRIs or SSRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome.”8
A warning will pop up on prescribing software when you prescribe a phosphodiesterase inhibitor in patients who are taking alpha-blockers. This is a common situation, because BPH and ED both become more common with age. The concern is that the combination of alpha-blocker plus phosphodiesterase inhibitor will increase the risk of hypotension.
Dr. Michel Guillaume and his colleagues studied the hemodynamic effect of doxazosin and tamsulosin in combination with tadalafil.9 A total of 45 healthy men aged 40-70 years were randomized to receive tadalafil and placebo for 28 days. Doxazosin was added after 7 days and continued for an additional 21 days. The second study included 39 men who received tadalafil and placebo for 7 days before adding tamsulosin for an additional 7 days.
There were no significant differences in change in standing systolic blood pressure with tadalafil with placebo, doxazosin, or tamsulosin.
Robert A. Kloner, M.D., Ph.D., and his colleagues reported on a randomized, double-blind, crossover trial of doxazosin 8 mg or placebo with tadalafil 20 mg and tamsulosin 0.4 mg or placebo with 10 mg or 20 mg of tadalafil.10 Tadalafil did augment the hypotensive effect of doxazosin, but it did not have any blood pressure effect on patients taking tamsulosin.
In a study of men taking both tamsulosin and vardenafil or tamsulosin and placebo for the treatment of BPH symptoms, Dr. Mauro Gacci and his colleagues found no significant difference in adverse effects in patients who received tamsulosin plus placebo, compared with men who received tamsulosin plus vardenafil.11
I think it is safe to prescribe triptans in patients who are on SSRIs and SNRIs. In patients who need both alpha-blockers and phosphodiesterase inhibitors, I think tamsulosin is the safest alpha-blocker option. It is best to not start a phosphodiesterase inhibitor at the same time as an alpha-blocker. The studies on coadministration of alpha-blockers and phosphodiesterase inhibitors have been done in either healthy volunteers, or in patients without severe systemic disease. So, the effect on blood pressure in patients taking multiple antihypertensive drugs or heart failure drugs is unknown.
References
1. U.S. Food and Drug Administration. Information for healthcare professionals: Selective serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SNRIs), 5-hydroxytryptamine receptor agonists (triptans), July 19, 2006.
2. MedGenMed. 2007 Sep 5;9(3):48.
3. QJM. 2003 Sep;96(9):635-42.
4. Am J Psychiatry. 1991 Jun;148(6):705-13.
5. Cephalalgia. 1999 Sep;19(7):668-75.
6. Headache. 2012 Feb;52(2):198-203.
7. Headache. 2012 Feb;52(2):195-7.
8. Headache. 2010 Jun;50(6):1089-99.
9. J Clin Pharmacol. 2007 Oct;47(10):1303-10.
10. J Urol. 2004 Nov;172(5 Pt 1):1935-40.
11. J Sex Med. 2012 Jun;9(6):1624-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 72-year-old man with benign prostatic hypertrophy comes to clinic to discuss recent problems with erectile dysfunction. He has been treated with tamsulosin with good results for the past 3 years for his BPH. He is given a prescription for vardenafil 10 mg for his ED. The pharmacist calls and asks if you want the prescription filled despite a drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take half a tablet of vardenafil.
C. Have the patient not take vardenafil within 6 hours of taking tamsulosin.
A 22-year-old woman presents with a unilateral headache, pounding in nature, worse with exercise. She is diagnosed with migraine. She has a history of depression and is taking 40 mg of fluoxetine. She is given a prescription for sumatriptan 100 mg. The pharmacist calls you and asks if you want to make changes because of possible drug interaction. What do you recommend?
A. Fill the prescription as written.
B. Have the patient take 50 mg of sumatriptan.
C. Have her reduce her fluoxetine dose to 20 mg.
D. Do not take sumatriptan within 12 hours of taking fluoxetine.
The title of this article is drug interaction myths. These are not true myths, but in both these cases, I think the prescriptions should be filled as written, and it will be safe for the patient to take the medications despite a theoretical drug interaction.
I have received calls from the pharmacist multiple times when I have prescribed these drug combinations, and I will share with you the evidence of safety for using these medications despite potential interactions.
In 2006, the Food and Drug Administration released an alert on serotonin syndrome occurring with combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) with triptans.1 This alert was based on 29 cases that the FDA evaluated and felt justified an alert.
Dr. Randolph W. Evans did an analysis of all 29 cases to see if they met criteria for serotonin syndrome.2 He classified if the cases met two different criteria for serotonin syndrome: the Hunter criteria3 or the Sternbach criteria4.
Of the 29 case reports, 10 met the Sternbach criteria, and none of the reports met the Hunter criteria. Some of the cases included polypharmacy of other drugs that can cause serotonin syndrome. Two cases that met the Sternbach criteria were excluded because they were either not on an SSRI or had alternative compelling diagnoses.
Dr. Evans suggested the biologic implausibility of triptans causing serotonin syndrome, because serotonin syndrome is believed to be caused by activation of 5-HT1A and 5-HT2A receptors, whereas triptans act at the 5-HT1B/5-HT1D and 5-HT1F receptors.
In a prospective study of 12,339 patients with migraine who used subcutaneous sumatriptan for at least 1 year, 1,784 patients also received an SSRI.5 No episodes of serotonin syndrome were reported. David A. Sclar, Ph.D., and his colleagues estimated that in 2007-2008, 1.4 million patients were prescribed both a triptan and an SSRI or SNRI.6 That is a 36% increase from 2003-2004, despite a 50% reduction in coprescriptions from primary care physicians – suggesting neurologists were not affected by the FDA alert.7
The American Headache Society position paper on the FDA alert states, “The currently available evidence does not support limiting the use of triptans with SNRIs or SSRIs, or the use of triptan monotherapy, due to concerns for serotonin syndrome.”8
A warning will pop up on prescribing software when you prescribe a phosphodiesterase inhibitor in patients who are taking alpha-blockers. This is a common situation, because BPH and ED both become more common with age. The concern is that the combination of alpha-blocker plus phosphodiesterase inhibitor will increase the risk of hypotension.
Dr. Michel Guillaume and his colleagues studied the hemodynamic effect of doxazosin and tamsulosin in combination with tadalafil.9 A total of 45 healthy men aged 40-70 years were randomized to receive tadalafil and placebo for 28 days. Doxazosin was added after 7 days and continued for an additional 21 days. The second study included 39 men who received tadalafil and placebo for 7 days before adding tamsulosin for an additional 7 days.
There were no significant differences in change in standing systolic blood pressure with tadalafil with placebo, doxazosin, or tamsulosin.
Robert A. Kloner, M.D., Ph.D., and his colleagues reported on a randomized, double-blind, crossover trial of doxazosin 8 mg or placebo with tadalafil 20 mg and tamsulosin 0.4 mg or placebo with 10 mg or 20 mg of tadalafil.10 Tadalafil did augment the hypotensive effect of doxazosin, but it did not have any blood pressure effect on patients taking tamsulosin.
In a study of men taking both tamsulosin and vardenafil or tamsulosin and placebo for the treatment of BPH symptoms, Dr. Mauro Gacci and his colleagues found no significant difference in adverse effects in patients who received tamsulosin plus placebo, compared with men who received tamsulosin plus vardenafil.11
I think it is safe to prescribe triptans in patients who are on SSRIs and SNRIs. In patients who need both alpha-blockers and phosphodiesterase inhibitors, I think tamsulosin is the safest alpha-blocker option. It is best to not start a phosphodiesterase inhibitor at the same time as an alpha-blocker. The studies on coadministration of alpha-blockers and phosphodiesterase inhibitors have been done in either healthy volunteers, or in patients without severe systemic disease. So, the effect on blood pressure in patients taking multiple antihypertensive drugs or heart failure drugs is unknown.
References
1. U.S. Food and Drug Administration. Information for healthcare professionals: Selective serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SNRIs), 5-hydroxytryptamine receptor agonists (triptans), July 19, 2006.
2. MedGenMed. 2007 Sep 5;9(3):48.
3. QJM. 2003 Sep;96(9):635-42.
4. Am J Psychiatry. 1991 Jun;148(6):705-13.
5. Cephalalgia. 1999 Sep;19(7):668-75.
6. Headache. 2012 Feb;52(2):198-203.
7. Headache. 2012 Feb;52(2):195-7.
8. Headache. 2010 Jun;50(6):1089-99.
9. J Clin Pharmacol. 2007 Oct;47(10):1303-10.
10. J Urol. 2004 Nov;172(5 Pt 1):1935-40.
11. J Sex Med. 2012 Jun;9(6):1624-33.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Reslizumab especially effective in eosinophilic asthma with nasal polyps
LOS ANGELES – The interleukin-5 inhibitor reslizumab showed particularly strong efficacy in patients with severe eosinophilic asthma accompanied by chronic sinusitis and nasal polyps, as well as in patients age 65 and older, in separate analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Both presentations were post hoc, pooled analyses of two published 52-week, double-blind, pivotal phase III randomized trials of IV reslizumab at 3.0 mg/kg or placebo every 4 weeks on top of standard background therapy. The trials included a combined total of close to 1,000 patients aged 12-75 years with baseline blood eosinophil counts of at least 400 cells/mcL and inadequately controlled asthma despite being on at least moderate-dose inhaled corticosteroids. The primary endpoint – frequency of clinical asthma exacerbations – was positive in both trials, with a reduction of 54% with reslizumab compared to placebo (Lancet Respir Med. 2015 May;3[5]:355-66).
Reslizumab is a humanized monoclonal antibody of the IgG4/K isotype. On the basis of the phase III trials and other data, last December the Food and Drug Administration’s Pulmonary-Allergy Drug Advisory Committee voted 11-3 to recommend approval of the biologic in 18- to 75-year-olds with inadequately controlled eosinophilic asthma. A decision by the federal agency is expected imminently.
The two post hoc analyses were conducted to highlight the biologic’s performance in clinically important but previously understudied patient subgroups, according to investigators.
Dr. Steven F. Weinstein compared 52-week outcomes in 250 patients with chronic sinusitis, including 150 who also had nasal polyps, in juxtaposition to the total two-study population of 953 eosinophilic asthma patients. Of note, aspirin sensitivity was present in 37% of those with chronic sinusitis with nasal polyps (CSwNP) compared to 11% of total participants in the two phase III trials. The CSwNP group had higher blood eosinophil levels, too: an average of 884 cells/mcL, compared with 655/mcL in the study population as a whole.
The frequency of clinical asthma exacerbations was 3.22 episodes in 52 weeks in CSwNP patients on placebo and 0.56 in those given reslizumab, for an 83% reduction in the active treatment arm. In the overall study population, the frequency was 1.81 episodes with placebo versus 0.84 with reslizumab, for a less robust but still highly significant 54% reduction. The reduction in exacerbations among all subjects with chronic sinusitis who received reslizumab was intermediate at 70%, going from 2.81 episodes in controls to 0.83 with biologic therapy.
Clinical asthma exacerbations were defined as the use of systemic steroids by patients not already on such medication or at least a twofold increase in doses of inhaled or systemic corticosteroids for at least 3 days.
The placebo-subtracted improvement in lung function from baseline to 52 weeks in reslizumab-treated patients was 326 mL in the CSwNP group, 235 mL in all patients with chronic sinusitis, and 109 mL in the overall study population, according to Dr. Weinstein, an allergist-immunologist practicing in Huntington Beach, Calif.
A 0.5-point improvement on the validated Asthma Quality of Life Questionnaire (AQLQ) is accepted by researchers as the minimum for demonstrating clinically significant benefit. The 52-week placebo-subtracted improvement on this measure was 0.69 points in the reslizumab-treated CSwNP group, 0.47 in the total cohort of asthmatics with chronic sinusitis, and 0.27 points in the overall reslizumab-treated population.
Similarly, the average placebo-subtracted improvement on the Asthma Control Questionnaire–6 was 1.45 points in reslizumab-treated patients with CSwNP, a sixfold greater response than seen in the total study population, Dr. Weinstein noted.
The same pattern of greater-than-average efficacy on both primary and secondary study endpoints was seen with reslizumab in the 77 patients aged 65 years and older included in the two phase III trials compared with those age 18-64, according to Dr. David Bernstein, professor of medicine and environmental health at the University of Cincinnati.
Although older patients made up only a small fraction of total subjects in the two trials, it was important to examine how reslizumab performed in such patients because asthma affects an estimated 7% of Americans age 65 and up, and rates of both asthma hospitalization and mortality are higher than in younger patients, he noted.
Older and younger asthma patients in the two trials had comparable baseline characteristics. Yet in the older cohort the reduction in frequency of asthma exacerbations with reslizumab as compared to placebo was 67%, while in the younger patients it was only 53%.
Improvements in symptoms and quality of life as measured on the AQLQ, the Asthma Control Questionnaire–7, and the Asthma Symptom Utility Index were consistently larger in the reslizumab-treated older as compared to younger patients. On all three measures, only the older reslizumab-treated group successfully hurdled the bar defining minimal clinically significant improvement.
The two post hoc analyses were funded by Teva Pharmaceuticals. Both investigators serve on advisory boards for Teva and multiple other pharmaceutical companies.
LOS ANGELES – The interleukin-5 inhibitor reslizumab showed particularly strong efficacy in patients with severe eosinophilic asthma accompanied by chronic sinusitis and nasal polyps, as well as in patients age 65 and older, in separate analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Both presentations were post hoc, pooled analyses of two published 52-week, double-blind, pivotal phase III randomized trials of IV reslizumab at 3.0 mg/kg or placebo every 4 weeks on top of standard background therapy. The trials included a combined total of close to 1,000 patients aged 12-75 years with baseline blood eosinophil counts of at least 400 cells/mcL and inadequately controlled asthma despite being on at least moderate-dose inhaled corticosteroids. The primary endpoint – frequency of clinical asthma exacerbations – was positive in both trials, with a reduction of 54% with reslizumab compared to placebo (Lancet Respir Med. 2015 May;3[5]:355-66).
Reslizumab is a humanized monoclonal antibody of the IgG4/K isotype. On the basis of the phase III trials and other data, last December the Food and Drug Administration’s Pulmonary-Allergy Drug Advisory Committee voted 11-3 to recommend approval of the biologic in 18- to 75-year-olds with inadequately controlled eosinophilic asthma. A decision by the federal agency is expected imminently.
The two post hoc analyses were conducted to highlight the biologic’s performance in clinically important but previously understudied patient subgroups, according to investigators.
Dr. Steven F. Weinstein compared 52-week outcomes in 250 patients with chronic sinusitis, including 150 who also had nasal polyps, in juxtaposition to the total two-study population of 953 eosinophilic asthma patients. Of note, aspirin sensitivity was present in 37% of those with chronic sinusitis with nasal polyps (CSwNP) compared to 11% of total participants in the two phase III trials. The CSwNP group had higher blood eosinophil levels, too: an average of 884 cells/mcL, compared with 655/mcL in the study population as a whole.
The frequency of clinical asthma exacerbations was 3.22 episodes in 52 weeks in CSwNP patients on placebo and 0.56 in those given reslizumab, for an 83% reduction in the active treatment arm. In the overall study population, the frequency was 1.81 episodes with placebo versus 0.84 with reslizumab, for a less robust but still highly significant 54% reduction. The reduction in exacerbations among all subjects with chronic sinusitis who received reslizumab was intermediate at 70%, going from 2.81 episodes in controls to 0.83 with biologic therapy.
Clinical asthma exacerbations were defined as the use of systemic steroids by patients not already on such medication or at least a twofold increase in doses of inhaled or systemic corticosteroids for at least 3 days.
The placebo-subtracted improvement in lung function from baseline to 52 weeks in reslizumab-treated patients was 326 mL in the CSwNP group, 235 mL in all patients with chronic sinusitis, and 109 mL in the overall study population, according to Dr. Weinstein, an allergist-immunologist practicing in Huntington Beach, Calif.
A 0.5-point improvement on the validated Asthma Quality of Life Questionnaire (AQLQ) is accepted by researchers as the minimum for demonstrating clinically significant benefit. The 52-week placebo-subtracted improvement on this measure was 0.69 points in the reslizumab-treated CSwNP group, 0.47 in the total cohort of asthmatics with chronic sinusitis, and 0.27 points in the overall reslizumab-treated population.
Similarly, the average placebo-subtracted improvement on the Asthma Control Questionnaire–6 was 1.45 points in reslizumab-treated patients with CSwNP, a sixfold greater response than seen in the total study population, Dr. Weinstein noted.
The same pattern of greater-than-average efficacy on both primary and secondary study endpoints was seen with reslizumab in the 77 patients aged 65 years and older included in the two phase III trials compared with those age 18-64, according to Dr. David Bernstein, professor of medicine and environmental health at the University of Cincinnati.
Although older patients made up only a small fraction of total subjects in the two trials, it was important to examine how reslizumab performed in such patients because asthma affects an estimated 7% of Americans age 65 and up, and rates of both asthma hospitalization and mortality are higher than in younger patients, he noted.
Older and younger asthma patients in the two trials had comparable baseline characteristics. Yet in the older cohort the reduction in frequency of asthma exacerbations with reslizumab as compared to placebo was 67%, while in the younger patients it was only 53%.
Improvements in symptoms and quality of life as measured on the AQLQ, the Asthma Control Questionnaire–7, and the Asthma Symptom Utility Index were consistently larger in the reslizumab-treated older as compared to younger patients. On all three measures, only the older reslizumab-treated group successfully hurdled the bar defining minimal clinically significant improvement.
The two post hoc analyses were funded by Teva Pharmaceuticals. Both investigators serve on advisory boards for Teva and multiple other pharmaceutical companies.
LOS ANGELES – The interleukin-5 inhibitor reslizumab showed particularly strong efficacy in patients with severe eosinophilic asthma accompanied by chronic sinusitis and nasal polyps, as well as in patients age 65 and older, in separate analyses presented at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. Both presentations were post hoc, pooled analyses of two published 52-week, double-blind, pivotal phase III randomized trials of IV reslizumab at 3.0 mg/kg or placebo every 4 weeks on top of standard background therapy. The trials included a combined total of close to 1,000 patients aged 12-75 years with baseline blood eosinophil counts of at least 400 cells/mcL and inadequately controlled asthma despite being on at least moderate-dose inhaled corticosteroids. The primary endpoint – frequency of clinical asthma exacerbations – was positive in both trials, with a reduction of 54% with reslizumab compared to placebo (Lancet Respir Med. 2015 May;3[5]:355-66).
Reslizumab is a humanized monoclonal antibody of the IgG4/K isotype. On the basis of the phase III trials and other data, last December the Food and Drug Administration’s Pulmonary-Allergy Drug Advisory Committee voted 11-3 to recommend approval of the biologic in 18- to 75-year-olds with inadequately controlled eosinophilic asthma. A decision by the federal agency is expected imminently.
The two post hoc analyses were conducted to highlight the biologic’s performance in clinically important but previously understudied patient subgroups, according to investigators.
Dr. Steven F. Weinstein compared 52-week outcomes in 250 patients with chronic sinusitis, including 150 who also had nasal polyps, in juxtaposition to the total two-study population of 953 eosinophilic asthma patients. Of note, aspirin sensitivity was present in 37% of those with chronic sinusitis with nasal polyps (CSwNP) compared to 11% of total participants in the two phase III trials. The CSwNP group had higher blood eosinophil levels, too: an average of 884 cells/mcL, compared with 655/mcL in the study population as a whole.
The frequency of clinical asthma exacerbations was 3.22 episodes in 52 weeks in CSwNP patients on placebo and 0.56 in those given reslizumab, for an 83% reduction in the active treatment arm. In the overall study population, the frequency was 1.81 episodes with placebo versus 0.84 with reslizumab, for a less robust but still highly significant 54% reduction. The reduction in exacerbations among all subjects with chronic sinusitis who received reslizumab was intermediate at 70%, going from 2.81 episodes in controls to 0.83 with biologic therapy.
Clinical asthma exacerbations were defined as the use of systemic steroids by patients not already on such medication or at least a twofold increase in doses of inhaled or systemic corticosteroids for at least 3 days.
The placebo-subtracted improvement in lung function from baseline to 52 weeks in reslizumab-treated patients was 326 mL in the CSwNP group, 235 mL in all patients with chronic sinusitis, and 109 mL in the overall study population, according to Dr. Weinstein, an allergist-immunologist practicing in Huntington Beach, Calif.
A 0.5-point improvement on the validated Asthma Quality of Life Questionnaire (AQLQ) is accepted by researchers as the minimum for demonstrating clinically significant benefit. The 52-week placebo-subtracted improvement on this measure was 0.69 points in the reslizumab-treated CSwNP group, 0.47 in the total cohort of asthmatics with chronic sinusitis, and 0.27 points in the overall reslizumab-treated population.
Similarly, the average placebo-subtracted improvement on the Asthma Control Questionnaire–6 was 1.45 points in reslizumab-treated patients with CSwNP, a sixfold greater response than seen in the total study population, Dr. Weinstein noted.
The same pattern of greater-than-average efficacy on both primary and secondary study endpoints was seen with reslizumab in the 77 patients aged 65 years and older included in the two phase III trials compared with those age 18-64, according to Dr. David Bernstein, professor of medicine and environmental health at the University of Cincinnati.
Although older patients made up only a small fraction of total subjects in the two trials, it was important to examine how reslizumab performed in such patients because asthma affects an estimated 7% of Americans age 65 and up, and rates of both asthma hospitalization and mortality are higher than in younger patients, he noted.
Older and younger asthma patients in the two trials had comparable baseline characteristics. Yet in the older cohort the reduction in frequency of asthma exacerbations with reslizumab as compared to placebo was 67%, while in the younger patients it was only 53%.
Improvements in symptoms and quality of life as measured on the AQLQ, the Asthma Control Questionnaire–7, and the Asthma Symptom Utility Index were consistently larger in the reslizumab-treated older as compared to younger patients. On all three measures, only the older reslizumab-treated group successfully hurdled the bar defining minimal clinically significant improvement.
The two post hoc analyses were funded by Teva Pharmaceuticals. Both investigators serve on advisory boards for Teva and multiple other pharmaceutical companies.
AT 2016 AAAAI ANNUAL MEETING
Cold turkey better for smoking cessation
Quitting smoking abruptly rather than gradually leads to higher abstinence rates both at 4 weeks and 6 months, a report published online March 14 shows.
Worldwide guidelines for smoking cessation generally recommend abrupt cessation over a gradual reduction in smoking, based on data from observational studies. However a recent review of 10 randomized trials concluded that quitting “cold turkey” produces only slightly higher quit rates, said Nicola Lindson-Hawley, Ph.D., of the department of primary care health services, University of Oxford (England), and her associates.
They compared the two approaches in a noninferiority trial involving 697 adults treated at 31 primary care practices in England during a 2.5-year period. The study participants smoked at least 15 cigarettes per day and had an end-expiratory carbon monoxide concentration of at least 15 parts per million. The average age was 49 years, and the study population was evenly divided between men and women. Their mean score on the Fagerström Test for Cigarette Dependence was 6, indicating a high degree of dependence.
These participants were randomly assigned either to stop smoking abruptly on a quit date 2 weeks from baseline (355 patients) or to stop gradually, by reducing their cigarette use by half at 1 week from baseline, by half again during the second week, and completely by a quit date 2 weeks from baseline. The latter group was given a choice of three structured reduction programs to follow before the quit date, as well as nicotine patches and a choice of short-acting nicotine replacement products (gum, lozenges, nasal sprays, sublingual tablets, inhalators, or mouth sprays). The abrupt-cessation group received only the nicotine patches just before the quit day. Both groups received identical behavioral counseling, nicotine patches, and nicotine replacement products after the quit date.
The primary outcome measure, abstinence at 4 weeks, was achieved by 49% of the abrupt-cessation group, compared with only 39.2% of the gradual-cessation group (relative risk, 0.80). Thus, gradual cessation did not prove to be noninferior to abrupt cessation. The secondary outcome measure of abstinence at 6 months also was superior for the abrupt-cessation group (22%) over the gradual-cessation group (15.5%), Dr. Lindson-Hawley and her associates reported (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M14-2805).
Most of the between-group difference was attributed to the fact that fewer participants in the gradual-cessation group actually attempted to quit on their quit date (61.4% vs. 71.0%). Relapse rates were similar between the two study groups at 4 weeks (36.2% vs. 31.0%) and at 6 months (74.8% vs. 69.1%).
“These results imply that, in clinical practice, we should encourage persons to stop smoking abruptly and not gradually,” Dr. Lindson-Hawley and her associates wrote. “However, gradual cessation programs could still be worthwhile if they increase the number of persons who try to quit or take up support and medication while trying.”
The study was supported by the British Heart Foundation, Cancer Research United Kingdom, the Economic and Social Research Council, the Medical Research Council, and the National Institute for Health Research. Dr. Lindson-Hawley reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, GlaxoSmithKline, and McNeil.
The trial by Nicola Lindson-Hawley, Ph.D., is well designed and suggests that “setting a quit date and quitting abruptly increases long-term cessation rates in smokers who want to quit,” Dr. Gabriela S. Ferreira and Dr. Michael B. Steinberg wrote in an accompanying editorial. However, a gradual approach to smoking cessation still may be useful for some smokers, so that method shouldn’t be entirely abandoned just yet.
Many smokers try several times to quit abruptly but are not successful. They may not wish to set another abrupt quit date for fear of “failing” yet again. However, they may instead respond well to gradually reducing their smoking, with the eventual goal of reducing it all the way to zero.
These findings raise important questions about how clinicians should approach patients who smoke and are ready to quit, they wrote.
Dr. Ferreira and Dr. Steinberg are at the Robert Wood Johnson Medical School in New Brunswick. Dr. Ferreira reported having no relevant financial disclosures; Dr. Steinberg reported receiving personal fees from Arena Pharmaceuticals, Major League Baseball, and Pfizer outside of this work. Their remarks (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M16-0362) accompanied Dr. Lindson-Hawley’s report.
The trial by Nicola Lindson-Hawley, Ph.D., is well designed and suggests that “setting a quit date and quitting abruptly increases long-term cessation rates in smokers who want to quit,” Dr. Gabriela S. Ferreira and Dr. Michael B. Steinberg wrote in an accompanying editorial. However, a gradual approach to smoking cessation still may be useful for some smokers, so that method shouldn’t be entirely abandoned just yet.
Many smokers try several times to quit abruptly but are not successful. They may not wish to set another abrupt quit date for fear of “failing” yet again. However, they may instead respond well to gradually reducing their smoking, with the eventual goal of reducing it all the way to zero.
These findings raise important questions about how clinicians should approach patients who smoke and are ready to quit, they wrote.
Dr. Ferreira and Dr. Steinberg are at the Robert Wood Johnson Medical School in New Brunswick. Dr. Ferreira reported having no relevant financial disclosures; Dr. Steinberg reported receiving personal fees from Arena Pharmaceuticals, Major League Baseball, and Pfizer outside of this work. Their remarks (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M16-0362) accompanied Dr. Lindson-Hawley’s report.
The trial by Nicola Lindson-Hawley, Ph.D., is well designed and suggests that “setting a quit date and quitting abruptly increases long-term cessation rates in smokers who want to quit,” Dr. Gabriela S. Ferreira and Dr. Michael B. Steinberg wrote in an accompanying editorial. However, a gradual approach to smoking cessation still may be useful for some smokers, so that method shouldn’t be entirely abandoned just yet.
Many smokers try several times to quit abruptly but are not successful. They may not wish to set another abrupt quit date for fear of “failing” yet again. However, they may instead respond well to gradually reducing their smoking, with the eventual goal of reducing it all the way to zero.
These findings raise important questions about how clinicians should approach patients who smoke and are ready to quit, they wrote.
Dr. Ferreira and Dr. Steinberg are at the Robert Wood Johnson Medical School in New Brunswick. Dr. Ferreira reported having no relevant financial disclosures; Dr. Steinberg reported receiving personal fees from Arena Pharmaceuticals, Major League Baseball, and Pfizer outside of this work. Their remarks (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M16-0362) accompanied Dr. Lindson-Hawley’s report.
Quitting smoking abruptly rather than gradually leads to higher abstinence rates both at 4 weeks and 6 months, a report published online March 14 shows.
Worldwide guidelines for smoking cessation generally recommend abrupt cessation over a gradual reduction in smoking, based on data from observational studies. However a recent review of 10 randomized trials concluded that quitting “cold turkey” produces only slightly higher quit rates, said Nicola Lindson-Hawley, Ph.D., of the department of primary care health services, University of Oxford (England), and her associates.
They compared the two approaches in a noninferiority trial involving 697 adults treated at 31 primary care practices in England during a 2.5-year period. The study participants smoked at least 15 cigarettes per day and had an end-expiratory carbon monoxide concentration of at least 15 parts per million. The average age was 49 years, and the study population was evenly divided between men and women. Their mean score on the Fagerström Test for Cigarette Dependence was 6, indicating a high degree of dependence.
These participants were randomly assigned either to stop smoking abruptly on a quit date 2 weeks from baseline (355 patients) or to stop gradually, by reducing their cigarette use by half at 1 week from baseline, by half again during the second week, and completely by a quit date 2 weeks from baseline. The latter group was given a choice of three structured reduction programs to follow before the quit date, as well as nicotine patches and a choice of short-acting nicotine replacement products (gum, lozenges, nasal sprays, sublingual tablets, inhalators, or mouth sprays). The abrupt-cessation group received only the nicotine patches just before the quit day. Both groups received identical behavioral counseling, nicotine patches, and nicotine replacement products after the quit date.
The primary outcome measure, abstinence at 4 weeks, was achieved by 49% of the abrupt-cessation group, compared with only 39.2% of the gradual-cessation group (relative risk, 0.80). Thus, gradual cessation did not prove to be noninferior to abrupt cessation. The secondary outcome measure of abstinence at 6 months also was superior for the abrupt-cessation group (22%) over the gradual-cessation group (15.5%), Dr. Lindson-Hawley and her associates reported (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M14-2805).
Most of the between-group difference was attributed to the fact that fewer participants in the gradual-cessation group actually attempted to quit on their quit date (61.4% vs. 71.0%). Relapse rates were similar between the two study groups at 4 weeks (36.2% vs. 31.0%) and at 6 months (74.8% vs. 69.1%).
“These results imply that, in clinical practice, we should encourage persons to stop smoking abruptly and not gradually,” Dr. Lindson-Hawley and her associates wrote. “However, gradual cessation programs could still be worthwhile if they increase the number of persons who try to quit or take up support and medication while trying.”
The study was supported by the British Heart Foundation, Cancer Research United Kingdom, the Economic and Social Research Council, the Medical Research Council, and the National Institute for Health Research. Dr. Lindson-Hawley reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, GlaxoSmithKline, and McNeil.
Quitting smoking abruptly rather than gradually leads to higher abstinence rates both at 4 weeks and 6 months, a report published online March 14 shows.
Worldwide guidelines for smoking cessation generally recommend abrupt cessation over a gradual reduction in smoking, based on data from observational studies. However a recent review of 10 randomized trials concluded that quitting “cold turkey” produces only slightly higher quit rates, said Nicola Lindson-Hawley, Ph.D., of the department of primary care health services, University of Oxford (England), and her associates.
They compared the two approaches in a noninferiority trial involving 697 adults treated at 31 primary care practices in England during a 2.5-year period. The study participants smoked at least 15 cigarettes per day and had an end-expiratory carbon monoxide concentration of at least 15 parts per million. The average age was 49 years, and the study population was evenly divided between men and women. Their mean score on the Fagerström Test for Cigarette Dependence was 6, indicating a high degree of dependence.
These participants were randomly assigned either to stop smoking abruptly on a quit date 2 weeks from baseline (355 patients) or to stop gradually, by reducing their cigarette use by half at 1 week from baseline, by half again during the second week, and completely by a quit date 2 weeks from baseline. The latter group was given a choice of three structured reduction programs to follow before the quit date, as well as nicotine patches and a choice of short-acting nicotine replacement products (gum, lozenges, nasal sprays, sublingual tablets, inhalators, or mouth sprays). The abrupt-cessation group received only the nicotine patches just before the quit day. Both groups received identical behavioral counseling, nicotine patches, and nicotine replacement products after the quit date.
The primary outcome measure, abstinence at 4 weeks, was achieved by 49% of the abrupt-cessation group, compared with only 39.2% of the gradual-cessation group (relative risk, 0.80). Thus, gradual cessation did not prove to be noninferior to abrupt cessation. The secondary outcome measure of abstinence at 6 months also was superior for the abrupt-cessation group (22%) over the gradual-cessation group (15.5%), Dr. Lindson-Hawley and her associates reported (Ann Intern Med. 2016 Mar 15. doi: 10.7326/M14-2805).
Most of the between-group difference was attributed to the fact that fewer participants in the gradual-cessation group actually attempted to quit on their quit date (61.4% vs. 71.0%). Relapse rates were similar between the two study groups at 4 weeks (36.2% vs. 31.0%) and at 6 months (74.8% vs. 69.1%).
“These results imply that, in clinical practice, we should encourage persons to stop smoking abruptly and not gradually,” Dr. Lindson-Hawley and her associates wrote. “However, gradual cessation programs could still be worthwhile if they increase the number of persons who try to quit or take up support and medication while trying.”
The study was supported by the British Heart Foundation, Cancer Research United Kingdom, the Economic and Social Research Council, the Medical Research Council, and the National Institute for Health Research. Dr. Lindson-Hawley reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, GlaxoSmithKline, and McNeil.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: Quitting cigarette smoking abruptly rather than gradually leads to higher abstinence rates in the short and long term.
Major finding: The primary outcome measure, abstinence at 4 weeks, was achieved by 49% of the abrupt-cessation group, compared with only 39.2% of the gradual-cessation group (RR, 0.80).
Data source: A randomized, controlled noninferiority study involving 697 smokers at 31 primary care practices in England.
Disclosures: This study was supported by the British Heart Foundation, Cancer Research United Kingdom, the Economic and Social Research Council, the Medical Research Council, and the National Institute for Health Research. Dr. Lindson-Hawley reported having no relevant financial disclosures; two of her associates reported ties to Pfizer, GlaxoSmithKline, and McNeil.
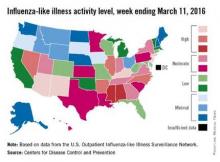
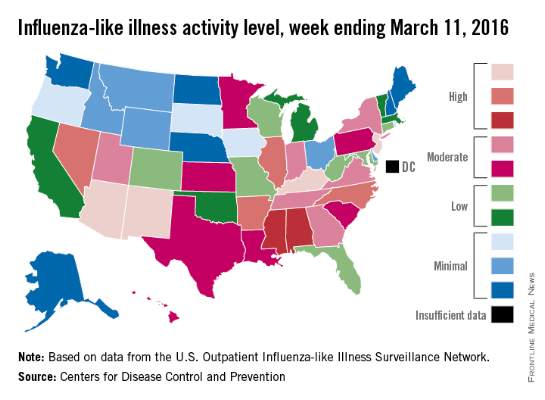
Flu activity reaches another new season high
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
Infant egg introduction can prevent sensitization at 12 months
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: If at-risk infants have negative skin prick tests (SPTs) at 4 months, tell their moms to introduce egg into their weaning diets.
Major finding: At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 children (20%) in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03).
Data source: Randomized clinical trial or 244 infants at risk for egg allergy.
Disclosures: The work was funded by the Australian government, among others. The investigators have no disclosures.
Coronary Artery Calcium Linked to Cancer, Kidney Disease, COPD
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
FROM JACC CARDIOVASCULAR IMAGING
Coronary artery calcium linked to cancer, kidney disease, COPD
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
The current report from the Multi-Ethnic Study of Atherosclerosis further expands the evidence base supporting the concept of coronary artery calcium as a marker of global health by examining its prognostic power across a diversity of noncardiovascular conditions.
Regardless of the directionality or magnitude of the connections between cardiovascular disease and non-CVD conditions, the extent to which coronary artery calcium–guided patient adherence to risk factor modification and lifestyle recommendations [affected] non-CVD conditions remains an additional link that should be explored further.
A synthesis of evidence, including the study by Handy et al., now supports the predictive ability of coronary artery calcium to estimate cardiac, cerebrovascular, and noncardiovascular conditions. We likely should come full circle in our discussion and acknowledge the far reaching implications of its predictive ability. Perhaps our index response that CAC should be fully integrated into all adult wellness and screening evaluations was on target after all!
Although CAC has not been without its critics and is not supported as a reimbursable procedure, its expansive evidence warrants a more thoughtful discussion within the CVD community that this powerful procedure provides valuable information to guide health care decision making.
Dr. Mosaab Awad, Dr. Parham Eshtehardi, and Leslee J. Shaw, Ph.D., of Emory University Clinical Cardiovascular Research Institute, Emory University, Atlanta, made these comments in an editorial (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.021). They had no disclosures.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
Patients whose coronary artery calcium scores exceeded 400 were significantly more likely to develop cancer, chronic obstructive pulmonary disease, chronic kidney disease, and hip fractures, compared with adults with undetectable CAC, in an analysis of the Multi-Ethnic Study of Atherosclerosis reported March 9 in JACC Cardiovascular Imaging.
The study is the first to examine the relationship between CAC and significant noncardiovascular diseases, said Dr. Catherine Handy of the Johns Hopkins Ciccarone Center for the Prevention of Heart Disease, Baltimore. Patients with CAC scores of zero represent a unique group of “healthy agers,” she and her associates said. Conversely, 20% of initial non-CVD events occurred in the 10% of patients with CAC scores over 400, and 70% of events occurred in patients with scores greater than zero, they reported.
While CAC is an established indicator of vascular aging, CVD risk, and all-cause mortality, its relationship with non-CVD is unclear. To elucidate the issue, the researchers analyzed data from the prospective, observational Multi-Ethnic Study of Atherosclerosis, which included 6,814 adults aged 45-84 years from six U.S. cities. Patients had no CVD and were not receiving cancer treatment.
Over a median follow-up period of 10.2 years, and after controlling for demographic factors and predictors of CVD, patients with CAC scores exceeding 400 were significantly more likely to develop cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71) and hip fracture (HR, 4.29), compared with patients without detectable CAC. Patients with CAC scores of zero were at significantly lower risk of these diagnoses, compared with patients with scores greater than zero (JACC Cardiovasc Imaging. 2016 Mar 9. doi: 10.1016/j.jcmg.2015.09.02).
Doubling of CAC was a modest but significant predictor of cancer, chronic kidney disease, pneumonia, and chronic obstructive pulmonary disease in the subgroup of adults aged 65 years and older. However, CAC was not associated with dementia or deep vein thrombosis or pulmonary embolism.
Sparse diagnoses of hip fractures and DVT/PE meant that the study might be underpowered to clearly link CAC with risk of these events, said the researchers. There also might not have been enough follow-up time to uncover risk in participants with the lowest CAC scores, they said. “At this time, our data are not powered for stratifying results based on gender or race,” they added.
The National Institutes of Health funded the study. The researchers had no conflicts of interest.
FROM JACC CARDIOVASCULAR IMAGING
Key clinical point: High coronary artery calcium scores were significantly associated with noncardiovascular disease events in a prospective study.
Major finding: Patients with CAC scores exceeding 400 were at significantly greater risk of cancer (hazard ratio, 1.53), chronic kidney disease (HR, 1.70), pneumonia (HR, 1.97), chronic obstructive pulmonary disease (HR, 2.71), and hip fracture (HR, 4.29), compared with patients without detectable CAC.
Data source: A prospective, observational cohort study of 6,814 adults aged 45-84 years.
Disclosures: The National Institutes of Health funded the study. The researchers had no conflicts of interest.
AAAAI: Albuterol Dry Powder Inhaler Offers Simplified Approach for Young Kids
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
AT 2016 AAAAI ANNUAL MEETING
AAAAI: Albuterol Dry Powder Inhaler Offers Simplified Approach for Young Kids
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
AT 2016 AAAAI ANNUAL MEETING
AAAAI: Albuterol dry powder inhaler offers simplified approach for young kids
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
LOS ANGELES – Young asthmatic children on bronchodilator therapy may soon gain access to a novel albuterol multidose dry powder inhaler that’s already proved popular with teen and adult patients with reversible obstructive airway disease because of its ease of use.
A phase III randomized, double-blind multicenter trial of the albuterol multidose dry powder inhaler (MDPI) versus placebo in 184 asthmatic children aged 4-11 years not on systemic corticosteroids met its primary and secondary lung function endpoints, with safety and tolerability similar to placebo, Dr. Tushar P. Shah reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
The albuterol MDPI is already marketed by Teva Pharmaceuticals as the ProAir RespiClick in patients aged 12 and older. The purpose of this phase III clinical trial was to obtain an expanded indication in 4- to 11-year-olds. The company has submitted its request to the Food and Drug Administration and anticipates smooth sailing based upon the new data, according to Dr. Shah, senior vice president for global respiratory research and development at Teva in Frazer, Pa.
The albuterol MDPI fills an unmet need for a simplified approach to rescue medication, the allergist said in an interview.
“This is a breath-actuated inhaler. Many patients – especially kids – have a hard time coordinating a conventional multidose inhaler actuation with inhalation. They have trouble getting the timing right, so the drug doesn’t get to the distal lung. That’s why this albuterol MDPI has been very well received in adults. For kids, I think it’s going to be even better because this is a very simple and intuitive device. All they do is open the cap, inhale, [and] close the cap,” he explained.
The young study participants used the albuterol MDPI at two inhalations four times daily, with a total daily albuterol dose of 720 mcg.
The primary study endpoint was the short-term improvement in lung function seen during testing performed after the very first study dose and again after the final dose of medication 3 weeks later. This was expressed as the area under the baseline-adjusted percent-predicted forced expiratory volume in 1 second effect-time curve from predose to 6 hours post dose. On both occasions, a sharp jump in opening of the airways was demonstrated within 5 minutes of dosing, with the effect remaining significantly better than with placebo for more than 2 hours.
Moreover, the maximum change from baseline in peak expiratory flow rate seen within 2 hours after dosing was a 26% increase with the albuterol MDPI, a significantly better result than the 14% increase with placebo.
No adverse events attributable to the study drug were seen.
The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: The albuterol multidose dry powder inhaler that’s now indicated for asthma patients aged 12 years and older may become available to those aged 4-11 years.
Major finding: Lung function measurements improved sharply within 5 minutes after dosing with this bronchodilator used for acute symptom relief, with the effect lasting for longer than 2 hours.
Data source: A phase III, double-blind, multicenter, placebo-controlled randomized trial involving 184 asthmatic children aged 4-11 years.
Disclosures: The study was sponsored by Teva Pharmaceuticals. The presenter is a senior company employee.