User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Analysis shows antacids do not slow IPF progression
Idiopathic pulmonary fibrosis (IPF) progressed at similar rates in patients who did and did not receive antacid therapy, based on a post hoc analysis of 52-week data from patients in three placebo-controlled trials.
“We found no association between antacid therapy and progression-free survival, mortality, or adverse events, reported Dr. Michael Krueter of the University of Heidelberg, Germany, and his colleagues (Lancet Respir Med. 2016 Mar 31;S2213-2600[16]00067-9). Further, patients who took antacids and had less than 70% forced vital capacity (FVC) had higher rates of pulmonary and nonpulmonary infections than did patients who did not receive antacid therapy.
“Long-term double-blind randomized studies are urgently needed to further investigate the potential benefit (and possible harms) of antacid therapy in patients with different stages of IPF, especially those with advanced disease,” the researchers said.
The findings challenge the conditional recommendation for antacid use in the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines on the treatment of IPF. That advice was based on previous retrospective studies that indicated antacid users had slower IPF progression, fewer acute exacerbations, and improved survival compared to patients who did not take antacids.
The new study looked at IPF patients in the placebo groups in three large, phase III trials of pirfenidone. In the placebo groups of those studies, 291 patients were receiving antacid therapy and 333 patients were not receiving antacid therapy at baseline. At 1 year, the rates of disease progression in the placebo group were not significantly different – 38% in the antacid users and 42% in those not using antacids. Disease progression was the composite endpoint of death, an absolute FVC decrease of at least 10%, and a decrease of 50 meters or more in the 6-minute walk distance (6MWD). In both groups of patients, the rates of all-cause mortality were also similar.
The risk of death from IPF at 1 year also was not significantly different; 3.9% for antacid users and 5.2% for those not using antacids.
When patients were grouped on the basis of FVC at baseline – either less than 70% FVC or at least 70% FVC or greater – little or no difference was seen in disease mortality, disease progression, changes in FVC, 6MWD, and all-cause hospital admission between patients who received antacids and those who did not receive antacids at baseline.
Prior to stratification by baseline FVC, the rates of all-cause hospital admissions, gastrointestinal adverse effects, infections, and pulmonary infections were similar for the two groups of patients. At 52 weeks, however, infections and pulmonary infections were significantly higher in patients who received antacid therapy at baseline compared with those patients who did not receive antacids at baseline (74% vs. 62%; P = .0174 and 14% vs. 6%; P = .0214, respectively).
Unlike the patients in the previous retrospective studies, few patients in the placebo arms of the clinical trials had advanced IPF and low FVC, the researchers noted. All had IPF that had been diagnosed within 48 months of entering a trial, no evidence of improvement in disease severity in the year preceding trial entry, a predicted FVC of 50% or more, and a 6MWD of 150 meters or more. Among the patients receiving antacid therapy at baseline, 88% used proton-pump inhibitors, 8% used H2 blockers and 4% used proton-pump inhibitors and H2 blockers. Of the 291 patients receiving antacid therapy, 38 stopped after baseline; of the 333 not receiving antacid therapy, 83 started receiving the therapy after baseline.
Dr. Kreuter disclosed ties with Boehringer Ingelheim and InterMune/Roche. Two coauthors disclosed ties with F. Hoffmann-La Roche, which funded the study: Derek Weycker, Ph.D., reported receiving funding from the company, and Dr. Klaus-Uwe Kirchgaessler is an employee.
Idiopathic pulmonary fibrosis (IPF) progressed at similar rates in patients who did and did not receive antacid therapy, based on a post hoc analysis of 52-week data from patients in three placebo-controlled trials.
“We found no association between antacid therapy and progression-free survival, mortality, or adverse events, reported Dr. Michael Krueter of the University of Heidelberg, Germany, and his colleagues (Lancet Respir Med. 2016 Mar 31;S2213-2600[16]00067-9). Further, patients who took antacids and had less than 70% forced vital capacity (FVC) had higher rates of pulmonary and nonpulmonary infections than did patients who did not receive antacid therapy.
“Long-term double-blind randomized studies are urgently needed to further investigate the potential benefit (and possible harms) of antacid therapy in patients with different stages of IPF, especially those with advanced disease,” the researchers said.
The findings challenge the conditional recommendation for antacid use in the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines on the treatment of IPF. That advice was based on previous retrospective studies that indicated antacid users had slower IPF progression, fewer acute exacerbations, and improved survival compared to patients who did not take antacids.
The new study looked at IPF patients in the placebo groups in three large, phase III trials of pirfenidone. In the placebo groups of those studies, 291 patients were receiving antacid therapy and 333 patients were not receiving antacid therapy at baseline. At 1 year, the rates of disease progression in the placebo group were not significantly different – 38% in the antacid users and 42% in those not using antacids. Disease progression was the composite endpoint of death, an absolute FVC decrease of at least 10%, and a decrease of 50 meters or more in the 6-minute walk distance (6MWD). In both groups of patients, the rates of all-cause mortality were also similar.
The risk of death from IPF at 1 year also was not significantly different; 3.9% for antacid users and 5.2% for those not using antacids.
When patients were grouped on the basis of FVC at baseline – either less than 70% FVC or at least 70% FVC or greater – little or no difference was seen in disease mortality, disease progression, changes in FVC, 6MWD, and all-cause hospital admission between patients who received antacids and those who did not receive antacids at baseline.
Prior to stratification by baseline FVC, the rates of all-cause hospital admissions, gastrointestinal adverse effects, infections, and pulmonary infections were similar for the two groups of patients. At 52 weeks, however, infections and pulmonary infections were significantly higher in patients who received antacid therapy at baseline compared with those patients who did not receive antacids at baseline (74% vs. 62%; P = .0174 and 14% vs. 6%; P = .0214, respectively).
Unlike the patients in the previous retrospective studies, few patients in the placebo arms of the clinical trials had advanced IPF and low FVC, the researchers noted. All had IPF that had been diagnosed within 48 months of entering a trial, no evidence of improvement in disease severity in the year preceding trial entry, a predicted FVC of 50% or more, and a 6MWD of 150 meters or more. Among the patients receiving antacid therapy at baseline, 88% used proton-pump inhibitors, 8% used H2 blockers and 4% used proton-pump inhibitors and H2 blockers. Of the 291 patients receiving antacid therapy, 38 stopped after baseline; of the 333 not receiving antacid therapy, 83 started receiving the therapy after baseline.
Dr. Kreuter disclosed ties with Boehringer Ingelheim and InterMune/Roche. Two coauthors disclosed ties with F. Hoffmann-La Roche, which funded the study: Derek Weycker, Ph.D., reported receiving funding from the company, and Dr. Klaus-Uwe Kirchgaessler is an employee.
Idiopathic pulmonary fibrosis (IPF) progressed at similar rates in patients who did and did not receive antacid therapy, based on a post hoc analysis of 52-week data from patients in three placebo-controlled trials.
“We found no association between antacid therapy and progression-free survival, mortality, or adverse events, reported Dr. Michael Krueter of the University of Heidelberg, Germany, and his colleagues (Lancet Respir Med. 2016 Mar 31;S2213-2600[16]00067-9). Further, patients who took antacids and had less than 70% forced vital capacity (FVC) had higher rates of pulmonary and nonpulmonary infections than did patients who did not receive antacid therapy.
“Long-term double-blind randomized studies are urgently needed to further investigate the potential benefit (and possible harms) of antacid therapy in patients with different stages of IPF, especially those with advanced disease,” the researchers said.
The findings challenge the conditional recommendation for antacid use in the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines on the treatment of IPF. That advice was based on previous retrospective studies that indicated antacid users had slower IPF progression, fewer acute exacerbations, and improved survival compared to patients who did not take antacids.
The new study looked at IPF patients in the placebo groups in three large, phase III trials of pirfenidone. In the placebo groups of those studies, 291 patients were receiving antacid therapy and 333 patients were not receiving antacid therapy at baseline. At 1 year, the rates of disease progression in the placebo group were not significantly different – 38% in the antacid users and 42% in those not using antacids. Disease progression was the composite endpoint of death, an absolute FVC decrease of at least 10%, and a decrease of 50 meters or more in the 6-minute walk distance (6MWD). In both groups of patients, the rates of all-cause mortality were also similar.
The risk of death from IPF at 1 year also was not significantly different; 3.9% for antacid users and 5.2% for those not using antacids.
When patients were grouped on the basis of FVC at baseline – either less than 70% FVC or at least 70% FVC or greater – little or no difference was seen in disease mortality, disease progression, changes in FVC, 6MWD, and all-cause hospital admission between patients who received antacids and those who did not receive antacids at baseline.
Prior to stratification by baseline FVC, the rates of all-cause hospital admissions, gastrointestinal adverse effects, infections, and pulmonary infections were similar for the two groups of patients. At 52 weeks, however, infections and pulmonary infections were significantly higher in patients who received antacid therapy at baseline compared with those patients who did not receive antacids at baseline (74% vs. 62%; P = .0174 and 14% vs. 6%; P = .0214, respectively).
Unlike the patients in the previous retrospective studies, few patients in the placebo arms of the clinical trials had advanced IPF and low FVC, the researchers noted. All had IPF that had been diagnosed within 48 months of entering a trial, no evidence of improvement in disease severity in the year preceding trial entry, a predicted FVC of 50% or more, and a 6MWD of 150 meters or more. Among the patients receiving antacid therapy at baseline, 88% used proton-pump inhibitors, 8% used H2 blockers and 4% used proton-pump inhibitors and H2 blockers. Of the 291 patients receiving antacid therapy, 38 stopped after baseline; of the 333 not receiving antacid therapy, 83 started receiving the therapy after baseline.
Dr. Kreuter disclosed ties with Boehringer Ingelheim and InterMune/Roche. Two coauthors disclosed ties with F. Hoffmann-La Roche, which funded the study: Derek Weycker, Ph.D., reported receiving funding from the company, and Dr. Klaus-Uwe Kirchgaessler is an employee.
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: Antacids don’t slow the progression of idiopathic pulmonary fibrosis.
Major finding: At 52 weeks, infections and pulmonary infections were significantly higher in patients who received antacid therapy at baseline compared with those patients who did not receive antacids at baseline (74% vs. 62%; P = .0174 and 14% vs. 6%; P = .0214, respectively).
Data source: IPF patients in the placebo groups in three large, phase III trials of pirfenidone; 291 patients were receiving antacid therapy and 333 patients were not receiving antacid therapy at baseline.
Disclosures: Dr. Kreuter disclosed ties with Boehringer Ingelheim and InterMune/Roche. Two coauthors disclosed ties with F. Hoffmann-La Roche, which funded the study: Derek Weycker, Ph.D., reported receiving funding from the company, and Dr. Klaus-Uwe Kirchgaessler is an employee.
Thousands of DNA modifications seen in infants of smoking mothers
The findings of a new, international study of 6,685 mothers and their newborns may explain why certain infant and child health problems frequently have been seen in the offspring of mothers who smoked during pregnancy, Kendall Morgan, Ph.D., wrote in the National Institutes of Health Director’s Blog.
The study, published in the American Journal of Human Genetics, “found significant differences between the epigenetic patterns of babies born to women who smoked during pregnancy and those born to nonsmokers, with many of the differences affecting genes known to play key roles in the development of the lungs, face, and nervous system,” Dr. Morgan wrote in the April 12 blog entry.
Smaller studies show there is evidence that maternal smoking can have epigenetic effects on a fetus. The new research from 13 research cohorts reaffirms and renders this finding more statistically powerful, Dr. Morgan wrote. In the infants of women who smoked every day during pregnancy, the researchers found more than 6,000 places where chemical marks on the DNA differed from those of babies born to mothers who did not smoke during pregnancy. The researchers also observed more than 4,600 epigenetic modifications in the DNA of babies of mothers who self-identified as falling under the broad category of having engaged in “any smoking during pregnancy.”
The genes thought to cause a cleft lip and/or palate were among the others to have been epigenetically modified in the fetuses of women who smoked while pregnant. The study also suggested that thousands of the epigenetic modifications seen affected children until at least age 4 years.
Read the study in the American Journal of Human Genetics (doi: 10.1016/j.ajhg.2016.02.019).
The findings of a new, international study of 6,685 mothers and their newborns may explain why certain infant and child health problems frequently have been seen in the offspring of mothers who smoked during pregnancy, Kendall Morgan, Ph.D., wrote in the National Institutes of Health Director’s Blog.
The study, published in the American Journal of Human Genetics, “found significant differences between the epigenetic patterns of babies born to women who smoked during pregnancy and those born to nonsmokers, with many of the differences affecting genes known to play key roles in the development of the lungs, face, and nervous system,” Dr. Morgan wrote in the April 12 blog entry.
Smaller studies show there is evidence that maternal smoking can have epigenetic effects on a fetus. The new research from 13 research cohorts reaffirms and renders this finding more statistically powerful, Dr. Morgan wrote. In the infants of women who smoked every day during pregnancy, the researchers found more than 6,000 places where chemical marks on the DNA differed from those of babies born to mothers who did not smoke during pregnancy. The researchers also observed more than 4,600 epigenetic modifications in the DNA of babies of mothers who self-identified as falling under the broad category of having engaged in “any smoking during pregnancy.”
The genes thought to cause a cleft lip and/or palate were among the others to have been epigenetically modified in the fetuses of women who smoked while pregnant. The study also suggested that thousands of the epigenetic modifications seen affected children until at least age 4 years.
Read the study in the American Journal of Human Genetics (doi: 10.1016/j.ajhg.2016.02.019).
The findings of a new, international study of 6,685 mothers and their newborns may explain why certain infant and child health problems frequently have been seen in the offspring of mothers who smoked during pregnancy, Kendall Morgan, Ph.D., wrote in the National Institutes of Health Director’s Blog.
The study, published in the American Journal of Human Genetics, “found significant differences between the epigenetic patterns of babies born to women who smoked during pregnancy and those born to nonsmokers, with many of the differences affecting genes known to play key roles in the development of the lungs, face, and nervous system,” Dr. Morgan wrote in the April 12 blog entry.
Smaller studies show there is evidence that maternal smoking can have epigenetic effects on a fetus. The new research from 13 research cohorts reaffirms and renders this finding more statistically powerful, Dr. Morgan wrote. In the infants of women who smoked every day during pregnancy, the researchers found more than 6,000 places where chemical marks on the DNA differed from those of babies born to mothers who did not smoke during pregnancy. The researchers also observed more than 4,600 epigenetic modifications in the DNA of babies of mothers who self-identified as falling under the broad category of having engaged in “any smoking during pregnancy.”
The genes thought to cause a cleft lip and/or palate were among the others to have been epigenetically modified in the fetuses of women who smoked while pregnant. The study also suggested that thousands of the epigenetic modifications seen affected children until at least age 4 years.
Read the study in the American Journal of Human Genetics (doi: 10.1016/j.ajhg.2016.02.019).
FROM THE AMERICAN JOURNAL OF HUMAN GENETICS
U.S. flu activity down again, except in New Jersey
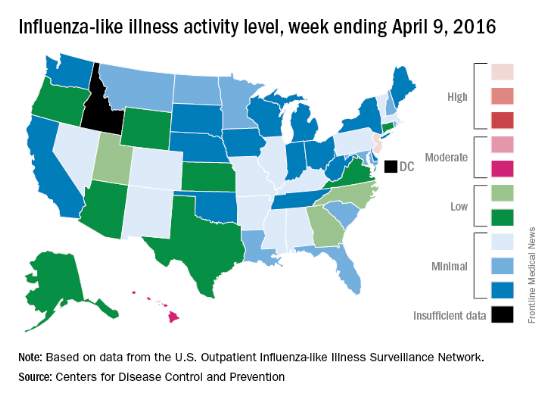
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.
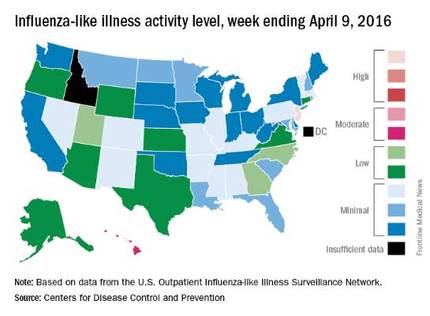
Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.

Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
Overall activity of influenza-like illness (ILI) in the United States continued to fall, but New Jersey took a turn for the worse during the week ending April 9, 2016, according to the Centers for Disease Control and Prevention.
New Jersey’s ILI activity level went from 8 the previous week to 10 on the CDC’s 1-10 scale. For the week ending April 9, it was the only U.S. state in the “high” range, with Hawaii the next highest at level 6 – the only state in the “moderate” range, the CDC reported.

Nationwide, the proportion of outpatient visits for ILI was 2.1%, which is at the national baseline of 2.1% and down from 2.5% the week before. That number has now dropped for 4 consecutive weeks since hitting a season high of 3.7% for the week ending March 12. The CDC also reported a cumulative rate of 26.6 influenza-associated hospitalizations per 100,000 population.
Ten flu-related pediatric deaths were reported during the week, of which only one occurred during the week. A total of 50 flu-related pediatric deaths have been reported during the 2015-2016 season, the CDC said. The overall proportion of deaths attributed to pneumonia and influenza was below the system-specific threshold in the National Center for Health Statistics Mortality Surveillance System, but above the system-specific threshold in the 122 Cities Mortality Reporting System.
Most atopic lesions colonized with Staph
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
Patients with atopic dermatitis are at an increased risk of Staphylococcus aureus colonization of both their lesional and nonlesional skin, as well as their nose, compared with healthy controls, according to a report in the British Journal of Dermatology.
Dr. J.E.E. Totté of the department of dermatology at the Erasmus MC University Medical Centre, Rotterdam, and associates conducted a systematic literature review and meta-analysis to derive pooled estimates of the prevalence and odds of colonization with S. aureus in patients with atopic dermatitis. They focused on original, human experimental, and observational studies including patients of any age with a confirmed diagnosis of atopic dermatitis (Br J Dermatol. 2016 Mar 19. doi: 10.1111/bjd.14566).
Dr. Totté and colleagues identified a total of 4,909 articles, of which 95 were found to meet the study inclusion criteria. All of the included studies were observational, with 30 comparing atopic dermatitis patients with healthy controls.
Almost three-quarters (70%) of patients had S. aureus colonization of lesional skin, while 39% had colonization of nonlesional skin, based on 81 studies including 5,231 patients and 30 studies including 1,496 patients, respectively. Nasal colonization was found in 62% of patients, based on analysis of 43 studies including 2,476 patients.
S. aureus colonization is an important factor in the pathogenesis of atopic dermatitis and should lead to evaluations of targeted antistaphylococcal therapy for the skin and nose, the investigators advised.
The authors reported that the department of dermatology of the Erasmus MC University Medical Centre Rotterdam received an unrestricted grant from Micreos Human Health. Two coauthors disclosed ties to industry sources.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Consider addressing S. aureus colonization in atopic dermatitis patients.
Major finding: Most patients (70%) were colonized with S. aureus on lesional skin, while 39% were colonized on nonlesional skin.
Data source: Literature review and meta-analysis involving 95 studies, 30 with healthy controls.
Disclosures: The study was funded by an unrestricted grant from Micreos Human Health to Erasmus MC University Medical Centre. Two coauthors disclosed ties to industry sources.
Asthma Treatment Adherence Better in Children With More Severe Symptoms
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
FROM PEDIATRIC ALLERGY AND IMMUNOLOGY
Asthma treatment adherence better in children with more severe symptoms
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
Children with better adherence to asthma treatments tended to have more severe asthma symptoms, according to Dr. Marjolein Engelkes of Erasmus University, Rotterdam (The Netherlands) and her associates.
Of the 14,303 children with asthma included in the study, short-acting beta2-agonists and inhaled corticosteroids were the most commonly prescribed treatments at 38 users/100 person-years and 31 users/100 person-years, respectively. Inhaled corticosteroid prescriptions were most common during the winter and in September, and decreased as children increased in age.
The median medication possession ratio (MPR) for inhaled corticosteroids was 56%. Children with an MPR over 87% were significantly more likely to be younger at the start of inhaled corticosteroid treatment, visit specialists more often, and to have more exacerbations than children with an MPR less than 37%.
“These findings indicate that there is room for improvement of adherence to treatment, especially in children with milder forms of asthma,” the investigators concluded.
Find the full study in Pediatric Allergy and Immunology (2016 Mar. doi: 10.1111/pai.12507).
FROM PEDIATRIC ALLERGY AND IMMUNOLOGY
ED bedside flu test accurate across flu seasons
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
FROM DIAGNOSTIC MICROBIOLOGY AND INFECTIOUS DISEASE
Key clinical point: A rapid bedside diagnostic test for influenza was accurate across four consecutive flu seasons in a pediatric ED.
Major finding: The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19.
Data source: A prospective analysis of the sensitivity and specificity of one rapid bedside diagnostic test in 764 children seen over a 4-year period.
Disclosures: Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
ED bedside flu test accurate across flu seasons
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
A rapid bedside diagnostic test for influenza showed consistent sensitivity and specificity across four consecutive flu seasons in a single pediatric ED in France, according to a report in Diagnostic Microbiology and Infectious Disease.
During flu seasons, it is difficult to distinguish young children who have the flu from those who have serious bacterial infections because clinical symptoms alone cannot differentiate the two conditions and fever may be the only symptom during the onset of a bacterial infection. Rapid influenza diagnostic tests purport to help ED clinicians estimate the probability of influenza at the bedside, which in turn can reduce the need for further diagnostic testing, length of ED stay, inappropriate use of antibiotics, and the costs of care, said Dr. E. Avril of the pediatric ED, University Hospital in Nantes, France, and associates.
To assess the diagnostic value of one rapid influenza diagnostic test used in this setting every winter, the investigators studied 764 patients younger than age 5 years who were admitted to the ED during four consecutive flu seasons with fever of no known origin. The prevalence of influenza varied widely during the study period, from a low of 30% to a high of 62%.
The rapid diagnostic test performed comparably well across the four flu seasons, with only a modest decrease in sensitivity and specificity during the 2010 H1N1 flu pandemic. The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19. These results are similar to those of two previous small-scale studies that found sensitivities of 69%-85% and specificities of 83%-98%, Dr. Avril and associates said (Diag Microbiol Infect Dis. 2016 doi:10.1016/j.diagmicrobio.2016.03.015).
These findings “support the rational use of rapid influenza diagnostic tests in clinical practice for young children presenting with fever without a source during flu season,” the investigators said.
Dr. Avril and associates added that they assessed only one rapid diagnostic test for influenza (QuickVue) – the only one available in their ED because of cost – but that there are 22 such tests commercially available. Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
FROM DIAGNOSTIC MICROBIOLOGY AND INFECTIOUS DISEASE
Key clinical point: A rapid bedside diagnostic test for influenza was accurate across four consecutive flu seasons in a pediatric ED.
Major finding: The bedside test had an overall sensitivity of 0.82, a specificity of 0.98, a positive likelihood ratio of 37.8, and a negative likelihood ratio of 0.19.
Data source: A prospective analysis of the sensitivity and specificity of one rapid bedside diagnostic test in 764 children seen over a 4-year period.
Disclosures: Nantes University Hospital supported the study. Dr. Avril and associates reported having no relevant disclosures.
Chronic illness associated with lower developmental readiness for school entry
Chronic illnesses such as otitis media, respiratory disease, and epilepsy increase the likelihood that children will be developmentally at risk for difficulties at school entry, a study showed.
The authors’ findings indicated “that chronically poor health in early childhood is a risk for school readiness, over and above the disadvantage conferred by socioeconomic factors,” Megan F. Bell of the University of Western Australia in Perth and her associates reported online (Pediatrics. 2016 April 13. doi: 10.1542/peds.2015-2475). “This increased risk was particularly evident for social and emotional capacities.”
The researchers analyzed developmental school readiness among 22,890 children (average age, 5 years), within the context of having chronic illness. The children were born in Western Australia in 2003-2004, underwent an early development assessment for school readiness in 2009, and did not have special needs, cerebral palsy, or a diagnosed developmental disorder. The assessments’ five domains included physical health and well-being, social competence, emotional maturity, language and cognitive skills, and communication skills and general knowledge. Children were designated “developmentally vulnerable” if they scored in the bottom 10% of a domain and “at risk” if they scored in the bottom 10%-25%. The top 75% were classified as “on track.”
Among 2,879 children with chronic illness, involving 13% of the overall sample, 93% of these children had one chronic illness diagnosis, and 7% had at least two. The most common illnesses were chronic otitis media (71%), chronic respiratory disease (27%), and epilepsy (3%).
Using health and demographic data for 19,227 mothers and 19,030 fathers of the children, the researchers accounted for sociodemographic characteristics of each child, their community, and their parents, including parental chronic illness. After these adjustments, children with a chronic illness were approximately 20%-35% more likely to fall within the vulnerable or at-risk categories in all five developmental domains, compared with their healthier peers. Children with multiple diagnoses, however, were not significantly more likely to be less developmentally ready for school in any of the five domains than their peers with one diagnosis, suggesting that “just one chronic illness is enough to increase a child’s risk for lower school readiness,” the authors wrote.
A 15%-35% increase in the risk of developmental vulnerability was seen in all domains for 1,859 children with a single diagnosis of otitis media and a 24%-45% increase was seen for 618 children with a single diagnosis of respiratory disease. However, there was no significant increase in risk for the 63 children with a diagnosis of epilepsy; all probability values were greater than .05.
“School-based programs targeted at enhancing social and emotional abilities have been shown to lead to improvements in behavioral, social, and academic outcomes,” the authors wrote. “This may therefore be an important focus of intervention for chronically ill children.
“Our findings suggest there is a need to broaden the scope of health conditions eligible for additional support at school entry. For instance, the most prevalent diagnosis was chronic otitis media, a common childhood condition that is associated with delayed language development, reading and spelling difficulties, and auditory processing deficits. Although recurrent ear infections may not be associated with significant limitations in daily activities, our study demonstrated that children with this condition are at increased risk of poor school readiness, even without having a more severe comorbid condition,” Ms. Bell and her associates wrote.
The research was funded by the Australian Research Council Linkage Project. The authors reported no relevant financial disclosures.
Chronic illnesses such as otitis media, respiratory disease, and epilepsy increase the likelihood that children will be developmentally at risk for difficulties at school entry, a study showed.
The authors’ findings indicated “that chronically poor health in early childhood is a risk for school readiness, over and above the disadvantage conferred by socioeconomic factors,” Megan F. Bell of the University of Western Australia in Perth and her associates reported online (Pediatrics. 2016 April 13. doi: 10.1542/peds.2015-2475). “This increased risk was particularly evident for social and emotional capacities.”
The researchers analyzed developmental school readiness among 22,890 children (average age, 5 years), within the context of having chronic illness. The children were born in Western Australia in 2003-2004, underwent an early development assessment for school readiness in 2009, and did not have special needs, cerebral palsy, or a diagnosed developmental disorder. The assessments’ five domains included physical health and well-being, social competence, emotional maturity, language and cognitive skills, and communication skills and general knowledge. Children were designated “developmentally vulnerable” if they scored in the bottom 10% of a domain and “at risk” if they scored in the bottom 10%-25%. The top 75% were classified as “on track.”
Among 2,879 children with chronic illness, involving 13% of the overall sample, 93% of these children had one chronic illness diagnosis, and 7% had at least two. The most common illnesses were chronic otitis media (71%), chronic respiratory disease (27%), and epilepsy (3%).
Using health and demographic data for 19,227 mothers and 19,030 fathers of the children, the researchers accounted for sociodemographic characteristics of each child, their community, and their parents, including parental chronic illness. After these adjustments, children with a chronic illness were approximately 20%-35% more likely to fall within the vulnerable or at-risk categories in all five developmental domains, compared with their healthier peers. Children with multiple diagnoses, however, were not significantly more likely to be less developmentally ready for school in any of the five domains than their peers with one diagnosis, suggesting that “just one chronic illness is enough to increase a child’s risk for lower school readiness,” the authors wrote.
A 15%-35% increase in the risk of developmental vulnerability was seen in all domains for 1,859 children with a single diagnosis of otitis media and a 24%-45% increase was seen for 618 children with a single diagnosis of respiratory disease. However, there was no significant increase in risk for the 63 children with a diagnosis of epilepsy; all probability values were greater than .05.
“School-based programs targeted at enhancing social and emotional abilities have been shown to lead to improvements in behavioral, social, and academic outcomes,” the authors wrote. “This may therefore be an important focus of intervention for chronically ill children.
“Our findings suggest there is a need to broaden the scope of health conditions eligible for additional support at school entry. For instance, the most prevalent diagnosis was chronic otitis media, a common childhood condition that is associated with delayed language development, reading and spelling difficulties, and auditory processing deficits. Although recurrent ear infections may not be associated with significant limitations in daily activities, our study demonstrated that children with this condition are at increased risk of poor school readiness, even without having a more severe comorbid condition,” Ms. Bell and her associates wrote.
The research was funded by the Australian Research Council Linkage Project. The authors reported no relevant financial disclosures.
Chronic illnesses such as otitis media, respiratory disease, and epilepsy increase the likelihood that children will be developmentally at risk for difficulties at school entry, a study showed.
The authors’ findings indicated “that chronically poor health in early childhood is a risk for school readiness, over and above the disadvantage conferred by socioeconomic factors,” Megan F. Bell of the University of Western Australia in Perth and her associates reported online (Pediatrics. 2016 April 13. doi: 10.1542/peds.2015-2475). “This increased risk was particularly evident for social and emotional capacities.”
The researchers analyzed developmental school readiness among 22,890 children (average age, 5 years), within the context of having chronic illness. The children were born in Western Australia in 2003-2004, underwent an early development assessment for school readiness in 2009, and did not have special needs, cerebral palsy, or a diagnosed developmental disorder. The assessments’ five domains included physical health and well-being, social competence, emotional maturity, language and cognitive skills, and communication skills and general knowledge. Children were designated “developmentally vulnerable” if they scored in the bottom 10% of a domain and “at risk” if they scored in the bottom 10%-25%. The top 75% were classified as “on track.”
Among 2,879 children with chronic illness, involving 13% of the overall sample, 93% of these children had one chronic illness diagnosis, and 7% had at least two. The most common illnesses were chronic otitis media (71%), chronic respiratory disease (27%), and epilepsy (3%).
Using health and demographic data for 19,227 mothers and 19,030 fathers of the children, the researchers accounted for sociodemographic characteristics of each child, their community, and their parents, including parental chronic illness. After these adjustments, children with a chronic illness were approximately 20%-35% more likely to fall within the vulnerable or at-risk categories in all five developmental domains, compared with their healthier peers. Children with multiple diagnoses, however, were not significantly more likely to be less developmentally ready for school in any of the five domains than their peers with one diagnosis, suggesting that “just one chronic illness is enough to increase a child’s risk for lower school readiness,” the authors wrote.
A 15%-35% increase in the risk of developmental vulnerability was seen in all domains for 1,859 children with a single diagnosis of otitis media and a 24%-45% increase was seen for 618 children with a single diagnosis of respiratory disease. However, there was no significant increase in risk for the 63 children with a diagnosis of epilepsy; all probability values were greater than .05.
“School-based programs targeted at enhancing social and emotional abilities have been shown to lead to improvements in behavioral, social, and academic outcomes,” the authors wrote. “This may therefore be an important focus of intervention for chronically ill children.
“Our findings suggest there is a need to broaden the scope of health conditions eligible for additional support at school entry. For instance, the most prevalent diagnosis was chronic otitis media, a common childhood condition that is associated with delayed language development, reading and spelling difficulties, and auditory processing deficits. Although recurrent ear infections may not be associated with significant limitations in daily activities, our study demonstrated that children with this condition are at increased risk of poor school readiness, even without having a more severe comorbid condition,” Ms. Bell and her associates wrote.
The research was funded by the Australian Research Council Linkage Project. The authors reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: Chronic illness, including otitis media, is associated with lower developmental readiness for school entry among children.
Major finding: Preschool children with chronic conditions are 20%-35% more likely to be developmentally at risk.
Data source: The findings are based on a cross-sectional cohort analysis of chronic illnesses and developmental assessments for school readiness among 22,890 Western Australian children, with an average age of 5 years.
Disclosures: The research was funded by the Australian Research Council Linkage Project. The authors reported no relevant financial disclosures.
Asthma, Eczema in Children Unrelated to Allergic Sensitization
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
Atopy was not related to development of eczema or asthma in children under age 13 years, according to Ann-Marie Malby Schoos, Ph.D., and her associates at the University of Copenhagen.
Allergic sensitization increased with age in the 399 children tested, rising from 12% at 6 months to 54% at 13 years. The incidence of asthma was highest at age 4 years at 16%, but decreased afterward, falling to 12% at 13 years. The incidence of eczema peaked at 39% in children aged 1.5 years old, but decreased steadily to only 12% in 13-year-olds.
Asthma and allergic sensitization were related only in late childhood, with an odds ratio of 4.49 in 13-year-olds. This pattern was seen throughout allergic sensitization subgroups. There were strong associations between eczema and allergic sensitization at 6 months (OR, 6.02), 1.5 years (OR, 2.06), and 6 years (OR, 2.77), but no association at 13 years. The proportion of children with allergic sensitization who did not have asthma or eczema also increased with age.
“The tradition of using atopy as a particular endotype of asthma and eczema seems unfounded because it depends on the method of testing for sensitization, type of allergens, and age of the patient. This questions the relevance of the terms atopic asthma and atopic eczema as true endotypes,” the investigators concluded.
Find the full study in the Journal of Allergy and Clinical Immunology (doi: 10.1016/j.jaci.2015.10.004).
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY