User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
Will genome editing advance animal-to-human transplantation?
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
CRISPR and CRISPR-associated proteins have emerged as effective genome editing techniques that may lead to cardiac and lung models and possibly xenotransplantation, Ari A. Mennander, MD, PhD, of the Tampere (Finland) University Heart Hospital, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:492).
The concept Dr. Butler and Dr. Tector discuss involves not using antibodies to ameliorate porcine antibodies that cause rejection in humans, but rather reengineering the genetic composition of pigs to eliminate those antibodies. “According to the wildest of dreams, these genes affecting porcine glycan expression may be silenced, and the human–antiporcine humoral immunity is controlled down to the level comparable with human allograft rejection,” Dr. Mennander said.
However, such a breakthrough carries with it consequences, Dr. Mennander said. “Should one worry about the induction of zoonosis, as well as the ethical aspects of transplanting the patient a whole organ of a pig? Would even a successful xenotransplant program seriously compete with artificial hearts or allografts?” Embracing the method too early would open its advocates to ridicule, he said.
“We are to applaud the researchers for ever-lasting and exemplary enthusiasm for a futuristic new surgical solution; the future may lie as much in current clinical solutions as in innovative discoveries based on persistent scientific experiments,” Dr. Mennander said.
Dr. Mennander had no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Advances in gene editing are pushing the possibility of raising pigs for organs that may be transplanted into humans with immunosuppression regimens comparable to those now used in human-to-human transplants, coauthors James Butler, MD, and A. Joseph Tector, MD, PhD, stated in an expert opinion in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:488-92).
Developments in genome editing could bring new approaches to management of cardiopulmonary diseases, Dr. Butler and Dr. Tector noted. “Recently, cardiac-specific and lung-specific applications have been described, which will allow for the rapid creation of new models of heart and lung disease,” they said. Specifically, they noted gene targeting might eventually offer a way to treat challenging genetic problems “like the heterogeneous nature of nonsquamous cell lung cancer.”
Dr. Butler is with the department of surgery at Indiana University, Indianapolis, and Dr. Tector is with the department of surgery at the University of Alabama at Birmingham.
CRISPR technology has been used in developing multiple gene knockout pigs and neutralizing three separate porcine genes that encode human xenoantigens in a single reaction, leading to efficient methods for creating pigs with multiple genetic modifications.
According to the website of the Broad Institute of MIT and Harvard, Cambridge, Mass., where researchers perfected the system to work in eukaryotes, CRISPR works by using short RNA sequences designed by researchers to guide the system to matching sequences of DNA. When the target DNA is found, Cas9 – one of the enzymes produced by the CRISPR system – binds to the DNA and cuts it, shutting the targeted gene off.
“By facilitating high-throughput model creation, CRISPR has elucidated which modifications are necessary and which are not; despite the ability to alter many loci concurrently, recent evidence has implicated three porcine genes that are responsible for the majority of human-antiporcine humoral immunity,” Dr. Butler and Dr. Tector wrote.
Those genes are the Gal[alpha]1-3 epitope (Gal-alpha), CMAH and B4GaINT2 genes. “Each of these three genes is expressed in pigs but has been evolutionarily silenced in humans,” the coauthors added.
While CRISPR genome editing has yet to reach its full potential, researchers and clinicians should pay attention, according to Dr. Butler and Dr. Tector.
More recent modifications of CRISPR technology have shown promise in not just knocking out or turning off specific genes, but rather guiding directed replacement of genes with researcher-designed substitutes. This can enable permanent transformation of functional genes with altered behavior, according to the Broad Institute website.
Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler has no relevant financial relationships to disclose.
Key clinical point: CRISPR/Cas9 genome editing is advancing the creation of animal models for xenotransplantation into humans.
Major finding: Genome editing tools are moving xenotransplantation models quickly toward potential treatments for cardiopulmonary disease.
Data source: Expert opinion with literature review.
Disclosures: Dr. Tector disclosed he has received funding from United Therapeutics and founded Xenobridge with patents for xenotransplantation. Dr. Butler reported having no relevant financial disclosures.
Vitamin D reduces respiratory infection risk
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Key clinical point:
Major finding: For those receiving vitamin D, the odds ratio of reducing respiratory infection risk was 0.88 (P less than .001).
Data source: Systematic review and meta-analysis of data on 11,321 subjects taken from 25 different trials.
Disclosures: Funded by the National Institute for Health Research; authors reported no relevant financial disclosures.
Flu activity levels high in 23 states
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.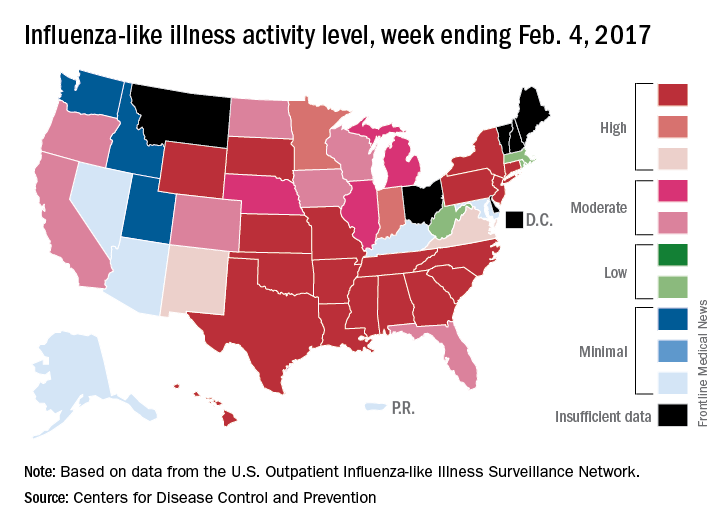
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
The number of states at the highest level of flu activity jumped from 7 to 19 during the week ending Feb. 4, 2017, according to the Centers for Disease Control and Prevention.
The 19 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity were largely concentrated in the South and lower Midwest, with another grouping mainly in the Mid-Atlantic. They were joined in the “high” range of ILI activity by four other states: Indiana and Minnesota at level 9 and New Mexico and Virginia at level 8, the CDC reported.
For the 2016-2017 season, 20 flu-related pediatric deaths have been reported: 5 were reported during the week ending Feb. 4, but 4 occurred during the week ending Jan. 28 and 1 occurred during the week ending Jan. 14, according to the CDC.
Since Oct. 1, 2016, there have been 6,804 laboratory-confirmed flu-related hospitalizations reported in the 13 states – including California, Georgia, New York, and Ohio – of the CDC’s Influenza Hospitalization Surveillance Network, for an overall hospitalization rate of 24.3 per 100,000 population. That rate, however, is “likely to be an underestimate as influenza-related hospitalizations can be missed, either because testing is not performed, or because cases may be attributed to other causes of pneumonia or other common influenza-related complications,” the CDC said.
Bronchiolitis pathway adherence tied to shorter LOS, lower costs
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
and lower health care costs, according to Mersine A. Bryan, MD, of the University of Washington, Seattle and her associates.
In a retrospective cohort study, researchers looked at 267 patients less than 24 months old diagnosed with bronchiolitis from December 2009 to July 2012. Levels of adherence were then categorized into low, middle, and high tertiles. Results show that adherence was highest for the inpatient quality indicators (mean score, 95) and lowest for the emergency department (ED) quality indicators (mean score, 79). The mean ED LOS was significantly shorter for cases with ED adherence scores in the highest versus the lowest tertile (90 vs. 140 minutes; P less than .05). There were no significant differences in mean inpatient LOS by inpatient adherence score tertiles. “However, the mean inpatient LOS was approximately 17 hours shorter for cases with combined ED/inpatient adherence scores in the highest, compared with the lowest tertile,” they said.
“Our study demonstrates that high adherence to evidence-based recommendations within a clinical pathway across the entire continuum of care, from the ED to the inpatient setting, is associated with lower costs and shorter LOS,” Dr. Bryan and associates concluded. “By improving adherence to evidence-based recommendations within a clinical pathway, we may be able to provide higher-value care by optimizing the quality of bronchiolitis care at lower costs and with shorter LOS.”
Read the full study in Pediatrics (doi: 10.1542/peds.2016-3432).
FROM PEDIATRICS
Worse outcomes with video laryngoscopy in ICU
When used in intensive care units, video laryngoscopy did not improve the chances of successful intubation on the first try, compared with direct laryngoscopy, and was associated with a significantly higher risk of severe life-threatening complications, researchers reported.
In a multicenter, randomized trial of 371 patients, first-pass intubation rates did not differ significantly whether video or direct laryngoscopy was used, at 67.7% and 70.3%, respectively, Jean Baptiste Lascarrou, MD, of District Hospital Centre, La Roche-sur-Yon, France, and his associates wrote. Meanwhile, the combined rate of death, cardiac arrest, severe cardiovascular collapse, and hypoxemia was 9.5% with video laryngoscopy and just 2.8% with direct laryngoscopy, a significant difference (JAMA. 2017 Jan 24;317[5]:483-93).
“Improved glottis visualization with video laryngoscopy did not translate into a higher success rate for first-pass intubation, because tracheal catheterization under indirect vision was more difficult, in keeping with earlier data,” the researchers concluded. “Further studies are needed to assess the comparative effectiveness of these two strategies in different clinical settings and among operators with diverse skill levels.”
Intubation in the ICU carries an inherently high risk because patients are often acutely unstable, and the intubating clinician is usually a nonexpert, the investigators noted. At the same time, the procedure must be done quickly to prevent aspiration because patients usually have not fasted. Care bundles and training on simulators have improved safety, but ICU intubations remain riskier than those done in the operating room.
Observational studies and smaller trials in ICUs seemed to support video laryngoscopy over the Macintosh laryngoscope, but raised questions about intubation time and mortality, the investigators noted. To help resolve these issues, they randomly assigned adults needing orotracheal intubation at seven ICUs in France to either video or direct Macintosh laryngoscopy, and followed them for 28 days. Patients averaged 63 years of age, and 37% were women.
For both arms, residents performed the initial intubation attempt in about 80% of cases, and successful intubation usually took 3 minutes. Video laryngoscopy did not significantly increase the combined risk of esophageal intubation, aspiration, arrhythmia, or dental injury (5.4% versus 7.7% for direct laryngoscopy). But the only death in the study occurred after video laryngoscopy, and there were four cardiac arrests after video laryngoscopy and none after direct laryngoscopy, the researchers said. Furthermore, the rate of severe hypoxemia was nearly six times higher after video laryngoscopy than with direct laryngoscopy, and the rate of hypotension was twice as high.
The researchers did not identify predictors of life-threatening complications with video laryngoscopy, but hypothesized that being able to clearly visualize the glottis might create “a false impression of safety,” especially among nonexperts. “In addition, poorer alignment of the pharyngeal axis, laryngeal axis, and mouth opening despite good glottis visualization by video laryngoscopy can lead to mechanical upper airway obstruction and faster progression to hypoxemia,” they wrote.
Centre Hospitalier Département de la Vendée sponsored the study. Dr. Lascarrou reported having no relevant conflicts of interest. Four coinvestigators disclosed ties to Fisher & Paykel, LFB, Merck Sharp & Dohme, Astellas, Basilea Pharmaceutica. Gilead, Alexion, and Cubist. The remaining coinvestigators had no disclosures.
The results of this trial illustrate the fundamental problem with video laryngoscopy: It generates excellent views of the larynx but may not facilitate tracheal intubation.
The use of video laryngoscopy can lead to the creation of blind spots, both visual and cognitive. Because the lens of the laryngoscope is located at the tip of the device, the pharynx and hypopharynx are not visualized during video laryngoscopy. Manipulating the endotracheal tube into view therefore occurs within this blind spot, and this can be difficult depending on the patient’s pharyngeal anatomy. This phenomenon has been linked to higher rates of pharyngeal soft tissue injury and longer intubation times in patients undergoing video laryngoscopy as compared with direct laryngoscopy.
The view during video laryngoscopy can also create a cognitive blind spot: laryngoscopists may fail to abort a laryngoscopy attempt in a timely manner because they have such a clear view of the larynx.
Brian O’Gara, MD, and Daniel Talmor, MD, of Harvard University, Boston, and Samuel Brown, MD, MS, of the University of Utah School, Murray, Utah, made these comments in an accompanying editorial (JAMA. 2017 Feb 7; doi: 10.1001/jama.2016.21036) . None of the authors had relevant financial disclosures.
The results of this trial illustrate the fundamental problem with video laryngoscopy: It generates excellent views of the larynx but may not facilitate tracheal intubation.
The use of video laryngoscopy can lead to the creation of blind spots, both visual and cognitive. Because the lens of the laryngoscope is located at the tip of the device, the pharynx and hypopharynx are not visualized during video laryngoscopy. Manipulating the endotracheal tube into view therefore occurs within this blind spot, and this can be difficult depending on the patient’s pharyngeal anatomy. This phenomenon has been linked to higher rates of pharyngeal soft tissue injury and longer intubation times in patients undergoing video laryngoscopy as compared with direct laryngoscopy.
The view during video laryngoscopy can also create a cognitive blind spot: laryngoscopists may fail to abort a laryngoscopy attempt in a timely manner because they have such a clear view of the larynx.
Brian O’Gara, MD, and Daniel Talmor, MD, of Harvard University, Boston, and Samuel Brown, MD, MS, of the University of Utah School, Murray, Utah, made these comments in an accompanying editorial (JAMA. 2017 Feb 7; doi: 10.1001/jama.2016.21036) . None of the authors had relevant financial disclosures.
The results of this trial illustrate the fundamental problem with video laryngoscopy: It generates excellent views of the larynx but may not facilitate tracheal intubation.
The use of video laryngoscopy can lead to the creation of blind spots, both visual and cognitive. Because the lens of the laryngoscope is located at the tip of the device, the pharynx and hypopharynx are not visualized during video laryngoscopy. Manipulating the endotracheal tube into view therefore occurs within this blind spot, and this can be difficult depending on the patient’s pharyngeal anatomy. This phenomenon has been linked to higher rates of pharyngeal soft tissue injury and longer intubation times in patients undergoing video laryngoscopy as compared with direct laryngoscopy.
The view during video laryngoscopy can also create a cognitive blind spot: laryngoscopists may fail to abort a laryngoscopy attempt in a timely manner because they have such a clear view of the larynx.
Brian O’Gara, MD, and Daniel Talmor, MD, of Harvard University, Boston, and Samuel Brown, MD, MS, of the University of Utah School, Murray, Utah, made these comments in an accompanying editorial (JAMA. 2017 Feb 7; doi: 10.1001/jama.2016.21036) . None of the authors had relevant financial disclosures.
When used in intensive care units, video laryngoscopy did not improve the chances of successful intubation on the first try, compared with direct laryngoscopy, and was associated with a significantly higher risk of severe life-threatening complications, researchers reported.
In a multicenter, randomized trial of 371 patients, first-pass intubation rates did not differ significantly whether video or direct laryngoscopy was used, at 67.7% and 70.3%, respectively, Jean Baptiste Lascarrou, MD, of District Hospital Centre, La Roche-sur-Yon, France, and his associates wrote. Meanwhile, the combined rate of death, cardiac arrest, severe cardiovascular collapse, and hypoxemia was 9.5% with video laryngoscopy and just 2.8% with direct laryngoscopy, a significant difference (JAMA. 2017 Jan 24;317[5]:483-93).
“Improved glottis visualization with video laryngoscopy did not translate into a higher success rate for first-pass intubation, because tracheal catheterization under indirect vision was more difficult, in keeping with earlier data,” the researchers concluded. “Further studies are needed to assess the comparative effectiveness of these two strategies in different clinical settings and among operators with diverse skill levels.”
Intubation in the ICU carries an inherently high risk because patients are often acutely unstable, and the intubating clinician is usually a nonexpert, the investigators noted. At the same time, the procedure must be done quickly to prevent aspiration because patients usually have not fasted. Care bundles and training on simulators have improved safety, but ICU intubations remain riskier than those done in the operating room.
Observational studies and smaller trials in ICUs seemed to support video laryngoscopy over the Macintosh laryngoscope, but raised questions about intubation time and mortality, the investigators noted. To help resolve these issues, they randomly assigned adults needing orotracheal intubation at seven ICUs in France to either video or direct Macintosh laryngoscopy, and followed them for 28 days. Patients averaged 63 years of age, and 37% were women.
For both arms, residents performed the initial intubation attempt in about 80% of cases, and successful intubation usually took 3 minutes. Video laryngoscopy did not significantly increase the combined risk of esophageal intubation, aspiration, arrhythmia, or dental injury (5.4% versus 7.7% for direct laryngoscopy). But the only death in the study occurred after video laryngoscopy, and there were four cardiac arrests after video laryngoscopy and none after direct laryngoscopy, the researchers said. Furthermore, the rate of severe hypoxemia was nearly six times higher after video laryngoscopy than with direct laryngoscopy, and the rate of hypotension was twice as high.
The researchers did not identify predictors of life-threatening complications with video laryngoscopy, but hypothesized that being able to clearly visualize the glottis might create “a false impression of safety,” especially among nonexperts. “In addition, poorer alignment of the pharyngeal axis, laryngeal axis, and mouth opening despite good glottis visualization by video laryngoscopy can lead to mechanical upper airway obstruction and faster progression to hypoxemia,” they wrote.
Centre Hospitalier Département de la Vendée sponsored the study. Dr. Lascarrou reported having no relevant conflicts of interest. Four coinvestigators disclosed ties to Fisher & Paykel, LFB, Merck Sharp & Dohme, Astellas, Basilea Pharmaceutica. Gilead, Alexion, and Cubist. The remaining coinvestigators had no disclosures.
When used in intensive care units, video laryngoscopy did not improve the chances of successful intubation on the first try, compared with direct laryngoscopy, and was associated with a significantly higher risk of severe life-threatening complications, researchers reported.
In a multicenter, randomized trial of 371 patients, first-pass intubation rates did not differ significantly whether video or direct laryngoscopy was used, at 67.7% and 70.3%, respectively, Jean Baptiste Lascarrou, MD, of District Hospital Centre, La Roche-sur-Yon, France, and his associates wrote. Meanwhile, the combined rate of death, cardiac arrest, severe cardiovascular collapse, and hypoxemia was 9.5% with video laryngoscopy and just 2.8% with direct laryngoscopy, a significant difference (JAMA. 2017 Jan 24;317[5]:483-93).
“Improved glottis visualization with video laryngoscopy did not translate into a higher success rate for first-pass intubation, because tracheal catheterization under indirect vision was more difficult, in keeping with earlier data,” the researchers concluded. “Further studies are needed to assess the comparative effectiveness of these two strategies in different clinical settings and among operators with diverse skill levels.”
Intubation in the ICU carries an inherently high risk because patients are often acutely unstable, and the intubating clinician is usually a nonexpert, the investigators noted. At the same time, the procedure must be done quickly to prevent aspiration because patients usually have not fasted. Care bundles and training on simulators have improved safety, but ICU intubations remain riskier than those done in the operating room.
Observational studies and smaller trials in ICUs seemed to support video laryngoscopy over the Macintosh laryngoscope, but raised questions about intubation time and mortality, the investigators noted. To help resolve these issues, they randomly assigned adults needing orotracheal intubation at seven ICUs in France to either video or direct Macintosh laryngoscopy, and followed them for 28 days. Patients averaged 63 years of age, and 37% were women.
For both arms, residents performed the initial intubation attempt in about 80% of cases, and successful intubation usually took 3 minutes. Video laryngoscopy did not significantly increase the combined risk of esophageal intubation, aspiration, arrhythmia, or dental injury (5.4% versus 7.7% for direct laryngoscopy). But the only death in the study occurred after video laryngoscopy, and there were four cardiac arrests after video laryngoscopy and none after direct laryngoscopy, the researchers said. Furthermore, the rate of severe hypoxemia was nearly six times higher after video laryngoscopy than with direct laryngoscopy, and the rate of hypotension was twice as high.
The researchers did not identify predictors of life-threatening complications with video laryngoscopy, but hypothesized that being able to clearly visualize the glottis might create “a false impression of safety,” especially among nonexperts. “In addition, poorer alignment of the pharyngeal axis, laryngeal axis, and mouth opening despite good glottis visualization by video laryngoscopy can lead to mechanical upper airway obstruction and faster progression to hypoxemia,” they wrote.
Centre Hospitalier Département de la Vendée sponsored the study. Dr. Lascarrou reported having no relevant conflicts of interest. Four coinvestigators disclosed ties to Fisher & Paykel, LFB, Merck Sharp & Dohme, Astellas, Basilea Pharmaceutica. Gilead, Alexion, and Cubist. The remaining coinvestigators had no disclosures.
FROM JAMA
Key clinical point: Among patients in intensive care units, video laryngoscopy did not improve the chances of successful intubation on the first try, when compared with standard Macintosh laryngoscopy, and was associated with a significantly higher risk of severe life-threatening events.
Major finding: Rates of first-pass intubation were 67.7% for video laryngoscopy and 70.3% for direct laryngoscopy (P = .6). The combined rate of death, cardiac arrest, severe cardiovascular collapse, and hypoxemia was 9.5% with video laryngoscopy and 2.8% with direct laryngoscopy (P = .01).
Data source: A multicenter randomized trial of 371 ICU patients.
Disclosures: Centre Hospitalier Département de la Vendée sponsored the study. Dr. Lascarrou reported having no relevant conflicts of interest. Four coinvestigators disclosed ties to Fisher & Paykel, LFB, Merck Sharp & Dohme, Astellas, Basilea Pharmaceutica. Gilead, Alexion, and Cubist. The remaining coinvestigators had no disclosures.
Rituximab is dramatically effective in IgG4-related disease
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
IgG4-related disease can strike any organ system
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Review offers reassurance on prenatal Tdap vaccination safety
Combined tetanus, diphtheria, and pertussis (Tdap) vaccination during the second or third trimester of pregnancy does not appear to be associated with clinically significant harm to the fetus or neonate, according to findings from a systematic review of the literature.
However, the findings are limited by a dearth of randomized, placebo-controlled trials.
Point estimates for all anomalies after Tdap vaccination ranged from 1.20 to 1.60, Mark McMillan of the University of Adelaide, North Adelaide, Australia and his colleagues reported (Obstet Gynecol. 2017;129:560-73).
“Statistical imprecision for combined ‘all anomalies’ outcomes meant that upper 95% [confidence intervals] were 2.0 or above,” the researchers wrote. “Statistical imprecision was even greater in the individual congenital anomaly outcomes and little confidence can be placed in these estimates.”
Additionally, one of three studies assessing chorioamnionitis showed a small but significant increase in risk (relative risk, 1.19) after vaccination.
Among the studies examining medically attended adverse events, no association was seen between vaccination and such events or reactions, including neurologic events, gestational diabetes, preeclampsia or eclampsia, and cardiac events. Maternal effects included fever in 1%-3% of subjects, and headache, malaise, and myalgia, which were more common.
“Overall, despite the limitations described, the review offers reassurance for antenatal Tdap or Tdap-IPV [diphtheria, tetanus, pertussis, and polio] vaccination administered during the second or third trimester of pregnancy,” the researchers wrote. “These findings need to be interpreted in the context of the evidence of effectiveness of antenatal vaccination programs at preventing serious morbidity and mortality from pertussis in young infants.”
Mr. McMillan received travel support from GlaxoSmithKline, and institutional research grants from GSK and Sanofi Pasteur. Other researchers also reported receiving research funding and/or travel support from these companies.
Combined tetanus, diphtheria, and pertussis (Tdap) vaccination during the second or third trimester of pregnancy does not appear to be associated with clinically significant harm to the fetus or neonate, according to findings from a systematic review of the literature.
However, the findings are limited by a dearth of randomized, placebo-controlled trials.
Point estimates for all anomalies after Tdap vaccination ranged from 1.20 to 1.60, Mark McMillan of the University of Adelaide, North Adelaide, Australia and his colleagues reported (Obstet Gynecol. 2017;129:560-73).
“Statistical imprecision for combined ‘all anomalies’ outcomes meant that upper 95% [confidence intervals] were 2.0 or above,” the researchers wrote. “Statistical imprecision was even greater in the individual congenital anomaly outcomes and little confidence can be placed in these estimates.”
Additionally, one of three studies assessing chorioamnionitis showed a small but significant increase in risk (relative risk, 1.19) after vaccination.
Among the studies examining medically attended adverse events, no association was seen between vaccination and such events or reactions, including neurologic events, gestational diabetes, preeclampsia or eclampsia, and cardiac events. Maternal effects included fever in 1%-3% of subjects, and headache, malaise, and myalgia, which were more common.
“Overall, despite the limitations described, the review offers reassurance for antenatal Tdap or Tdap-IPV [diphtheria, tetanus, pertussis, and polio] vaccination administered during the second or third trimester of pregnancy,” the researchers wrote. “These findings need to be interpreted in the context of the evidence of effectiveness of antenatal vaccination programs at preventing serious morbidity and mortality from pertussis in young infants.”
Mr. McMillan received travel support from GlaxoSmithKline, and institutional research grants from GSK and Sanofi Pasteur. Other researchers also reported receiving research funding and/or travel support from these companies.
Combined tetanus, diphtheria, and pertussis (Tdap) vaccination during the second or third trimester of pregnancy does not appear to be associated with clinically significant harm to the fetus or neonate, according to findings from a systematic review of the literature.
However, the findings are limited by a dearth of randomized, placebo-controlled trials.
Point estimates for all anomalies after Tdap vaccination ranged from 1.20 to 1.60, Mark McMillan of the University of Adelaide, North Adelaide, Australia and his colleagues reported (Obstet Gynecol. 2017;129:560-73).
“Statistical imprecision for combined ‘all anomalies’ outcomes meant that upper 95% [confidence intervals] were 2.0 or above,” the researchers wrote. “Statistical imprecision was even greater in the individual congenital anomaly outcomes and little confidence can be placed in these estimates.”
Additionally, one of three studies assessing chorioamnionitis showed a small but significant increase in risk (relative risk, 1.19) after vaccination.
Among the studies examining medically attended adverse events, no association was seen between vaccination and such events or reactions, including neurologic events, gestational diabetes, preeclampsia or eclampsia, and cardiac events. Maternal effects included fever in 1%-3% of subjects, and headache, malaise, and myalgia, which were more common.
“Overall, despite the limitations described, the review offers reassurance for antenatal Tdap or Tdap-IPV [diphtheria, tetanus, pertussis, and polio] vaccination administered during the second or third trimester of pregnancy,” the researchers wrote. “These findings need to be interpreted in the context of the evidence of effectiveness of antenatal vaccination programs at preventing serious morbidity and mortality from pertussis in young infants.”
Mr. McMillan received travel support from GlaxoSmithKline, and institutional research grants from GSK and Sanofi Pasteur. Other researchers also reported receiving research funding and/or travel support from these companies.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Point estimates for all anomalies after Tdap vaccination ranged from 1.20 to 1.60.
Data source: A systematic review of 21 studies.
Disclosures: Mr. McMillan received travel support from GlaxoSmithKline, and institutional research grants from GSK and Sanofi Pasteur. Other researchers also reported receiving research funding and/or travel support from these companies.
ACIP releases updated guidance for adult vaccinations
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
FROM ANNALS OF INTERNAL MEDICINE
High levels of flu activity reported in 15 states
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.
Fifteen states experienced high levels of flu activity for the week ending Jan. 28 as the 2016-2017 season moved past last season’s peak, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 3.9% for the week ending Jan. 28, 2017, the CDC reported. That level is higher than the national baseline level of 2.2% and higher than the peak of 3.6% for the 2015-2016 season.
The week also brought reports of seven flu-related pediatric deaths, although five actually occurred during previous weeks. There have now been 15 flu-related pediatric deaths during the 2016-2017 season, the CDC reported.