User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Powered by CHEST Physician, Clinician Reviews, MDedge Family Medicine, Internal Medicine News, and The Journal of Clinical Outcomes Management.
FDA approves sublingual immunotherapy for dust mite allergies
Odactra (Merck, Sharp & Dohme) had been approved in adults aged 18-65 years, with allergic rhinitis with or without conjunctivitis. The tablets offer an alternative to subcutaneous injections, the FDA said in a statement issued March 1.
The sublingual tablets are intended to be taken daily, year-round, and the first dose must be taken under physician supervision to monitor for adverse reactions, according to the FDA. As with other sublingual immunotherapies, patients using the tablets should be simultaneously prescribed autoinjectable epinephrine.
The approval was based on results from randomized trials enrolling about 2,500 patients in Europe and the United States, according to the FDA. Patients taking the tablets saw a 16%-18% reduction in symptoms across studies, compared with placebo. Clinical benefit may be delayed by 8-14 weeks after starting the therapy, the agency said. Common adverse reactions reported in the studies included nausea, itching of the ears and mouth, and swelling of the lips and tongue.
Odactra is the fourth sublingual immunotherapy to be approved in the United States since 2014. Other approved therapies target grass and ragweed allergies.
Odactra (Merck, Sharp & Dohme) had been approved in adults aged 18-65 years, with allergic rhinitis with or without conjunctivitis. The tablets offer an alternative to subcutaneous injections, the FDA said in a statement issued March 1.
The sublingual tablets are intended to be taken daily, year-round, and the first dose must be taken under physician supervision to monitor for adverse reactions, according to the FDA. As with other sublingual immunotherapies, patients using the tablets should be simultaneously prescribed autoinjectable epinephrine.
The approval was based on results from randomized trials enrolling about 2,500 patients in Europe and the United States, according to the FDA. Patients taking the tablets saw a 16%-18% reduction in symptoms across studies, compared with placebo. Clinical benefit may be delayed by 8-14 weeks after starting the therapy, the agency said. Common adverse reactions reported in the studies included nausea, itching of the ears and mouth, and swelling of the lips and tongue.
Odactra is the fourth sublingual immunotherapy to be approved in the United States since 2014. Other approved therapies target grass and ragweed allergies.
Odactra (Merck, Sharp & Dohme) had been approved in adults aged 18-65 years, with allergic rhinitis with or without conjunctivitis. The tablets offer an alternative to subcutaneous injections, the FDA said in a statement issued March 1.
The sublingual tablets are intended to be taken daily, year-round, and the first dose must be taken under physician supervision to monitor for adverse reactions, according to the FDA. As with other sublingual immunotherapies, patients using the tablets should be simultaneously prescribed autoinjectable epinephrine.
The approval was based on results from randomized trials enrolling about 2,500 patients in Europe and the United States, according to the FDA. Patients taking the tablets saw a 16%-18% reduction in symptoms across studies, compared with placebo. Clinical benefit may be delayed by 8-14 weeks after starting the therapy, the agency said. Common adverse reactions reported in the studies included nausea, itching of the ears and mouth, and swelling of the lips and tongue.
Odactra is the fourth sublingual immunotherapy to be approved in the United States since 2014. Other approved therapies target grass and ragweed allergies.
In Oregon pertussis outbreak, unvaccinated children were affected earlier
were, according to Steve G. Robison, MPH, and Juventila Liko, MD, MPH, from the Immunization Program, Oregon Health Authority, Portland.
A total of 351 pertussis cases in children aged 2 months to 10 years were reported in Portland and the upper Willamette Valley from Jan. 1 to Nov. 1, 2012. Children who were unvaccinated accounted for 76 (22%) of the reported cases, and children who were poorly vaccinated accounted for 50 of the 275 (18%) cases in vaccinated children.
“Children who are not immunized represent a dynamic risk of spreading disease in an outbreak and have an impact that is greater than simply lessening overall community immunity levels. Diseases such as pertussis may spread across areas through the choice of parents to not immunize or to limit immunizations. Once locally present, pertussis will spread to the unimmunized and vulnerable, who in turn through the weight of exposure, may then ignite a wider outbreak in vaccinated populations,” the investigators noted.
Find the full study in the Journal of Pediatrics (doi: 10.1016/j.jpeds.2016.12.047).
were, according to Steve G. Robison, MPH, and Juventila Liko, MD, MPH, from the Immunization Program, Oregon Health Authority, Portland.
A total of 351 pertussis cases in children aged 2 months to 10 years were reported in Portland and the upper Willamette Valley from Jan. 1 to Nov. 1, 2012. Children who were unvaccinated accounted for 76 (22%) of the reported cases, and children who were poorly vaccinated accounted for 50 of the 275 (18%) cases in vaccinated children.
“Children who are not immunized represent a dynamic risk of spreading disease in an outbreak and have an impact that is greater than simply lessening overall community immunity levels. Diseases such as pertussis may spread across areas through the choice of parents to not immunize or to limit immunizations. Once locally present, pertussis will spread to the unimmunized and vulnerable, who in turn through the weight of exposure, may then ignite a wider outbreak in vaccinated populations,” the investigators noted.
Find the full study in the Journal of Pediatrics (doi: 10.1016/j.jpeds.2016.12.047).
were, according to Steve G. Robison, MPH, and Juventila Liko, MD, MPH, from the Immunization Program, Oregon Health Authority, Portland.
A total of 351 pertussis cases in children aged 2 months to 10 years were reported in Portland and the upper Willamette Valley from Jan. 1 to Nov. 1, 2012. Children who were unvaccinated accounted for 76 (22%) of the reported cases, and children who were poorly vaccinated accounted for 50 of the 275 (18%) cases in vaccinated children.
“Children who are not immunized represent a dynamic risk of spreading disease in an outbreak and have an impact that is greater than simply lessening overall community immunity levels. Diseases such as pertussis may spread across areas through the choice of parents to not immunize or to limit immunizations. Once locally present, pertussis will spread to the unimmunized and vulnerable, who in turn through the weight of exposure, may then ignite a wider outbreak in vaccinated populations,” the investigators noted.
Find the full study in the Journal of Pediatrics (doi: 10.1016/j.jpeds.2016.12.047).
FROM THE JOURNAL OF PEDIATRICS
Outpatient flu visits down slightly
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.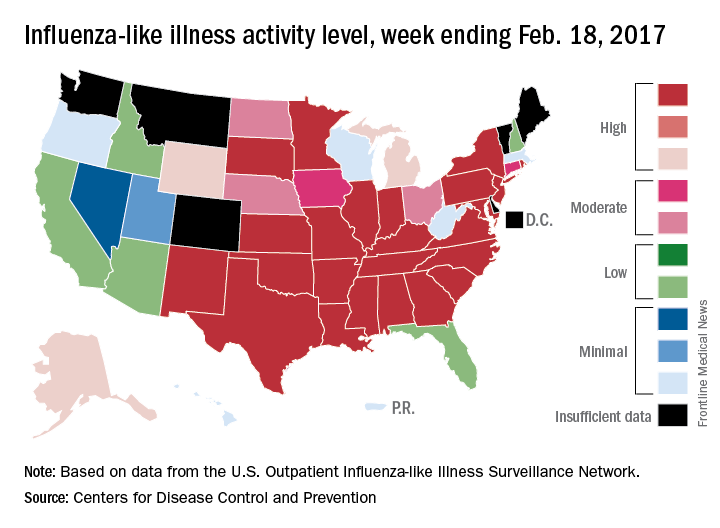
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
The overall national measure of outpatient flu activity was down for the week ending Feb. 18, and the number of states at the highest level of activity dropped from 25 to 24, according to the Centers for Disease Control and Prevention.
The national proportion of outpatient visits for influenza-like illness (ILI) decreased from 5.2% the previous week to 4.8% for the week ending Feb. 18, the CDC reported.
There were 5 ILI-related pediatric deaths reported during the week, bringing the total to 34 for the season so far, but none of the 5 occurred in the current week, the CDC said. There were 89 pediatric deaths reported during the 2015-2016 season, with the peak week occurring in late March/early April (11 deaths). During the 2014-2015 season, there were 148 deaths reported, and 111 were reported in 2013-2014.
Eliminating tap water consumption may prevent M. abscessus outbreaks
Abstaining from the consumption of tap water at health care facilities can dramatically reduce the risk of Mycobacterium abscessus infections among patients and staff, according to a new study published in Clinical Infectious Diseases.
“Outbreaks of [M. abscessus] and other rapidly growing mycobacteria are common and have been associated with colonized plumbing systems in commercial buildings and health care facilities,” wrote the authors, led by Arthur W. Baker, MD, MPH, of Duke University, Durham, N.C., adding that “Infections due to M. abscessus are difficult to diagnose and typically require months of therapy using multiple antibiotics” (Clin Infect Dis. 2017 Jan 10. doi: 10.1093/cid/ciw877).
Phase 2 took place from December 2014 through June 2015; in between Phase 1 and Phase 2, tap water abstention was implemented to protect patients deemed high risk, such as those with lung transplants. Of the 71 infections that occurred during Phase 1, 39 (55%) were lung transplant patients, while 9 (13%) were in those who had a recent cardiac surgery, 5 (7%) had cancer, and 5 (7%) had hematopoietic stem cell transplants. Incidence rates decreased substantially, back to their baseline levels, and further measures were used to completely resolve the outbreak.
“Primary interventions included institution of an inpatient sterile water protocol for high-risk patients, implementation of a protocol for enhanced disinfection and sterile water use for [heater-cooler units] of [cardiopulmonary bypass] machines, and water engineering changes designed to decrease NTM [nontuberculous mycobacteria] burden in the plumbing system,” the authors explained. “Other health care facilities, particularly those with endemic NTM or newly constructed patient care facilities, should consider similar multifaceted strategies to improve water safety and decrease risk of health care–associated infection from NTM.”
The study was funded by the National Institutes of Health’s Transplant Infectious Disease Interdisciplinary Research Training Grant. Dr. Baker and his coauthors did not report any relevant financial disclosures.
Abstaining from the consumption of tap water at health care facilities can dramatically reduce the risk of Mycobacterium abscessus infections among patients and staff, according to a new study published in Clinical Infectious Diseases.
“Outbreaks of [M. abscessus] and other rapidly growing mycobacteria are common and have been associated with colonized plumbing systems in commercial buildings and health care facilities,” wrote the authors, led by Arthur W. Baker, MD, MPH, of Duke University, Durham, N.C., adding that “Infections due to M. abscessus are difficult to diagnose and typically require months of therapy using multiple antibiotics” (Clin Infect Dis. 2017 Jan 10. doi: 10.1093/cid/ciw877).
Phase 2 took place from December 2014 through June 2015; in between Phase 1 and Phase 2, tap water abstention was implemented to protect patients deemed high risk, such as those with lung transplants. Of the 71 infections that occurred during Phase 1, 39 (55%) were lung transplant patients, while 9 (13%) were in those who had a recent cardiac surgery, 5 (7%) had cancer, and 5 (7%) had hematopoietic stem cell transplants. Incidence rates decreased substantially, back to their baseline levels, and further measures were used to completely resolve the outbreak.
“Primary interventions included institution of an inpatient sterile water protocol for high-risk patients, implementation of a protocol for enhanced disinfection and sterile water use for [heater-cooler units] of [cardiopulmonary bypass] machines, and water engineering changes designed to decrease NTM [nontuberculous mycobacteria] burden in the plumbing system,” the authors explained. “Other health care facilities, particularly those with endemic NTM or newly constructed patient care facilities, should consider similar multifaceted strategies to improve water safety and decrease risk of health care–associated infection from NTM.”
The study was funded by the National Institutes of Health’s Transplant Infectious Disease Interdisciplinary Research Training Grant. Dr. Baker and his coauthors did not report any relevant financial disclosures.
Abstaining from the consumption of tap water at health care facilities can dramatically reduce the risk of Mycobacterium abscessus infections among patients and staff, according to a new study published in Clinical Infectious Diseases.
“Outbreaks of [M. abscessus] and other rapidly growing mycobacteria are common and have been associated with colonized plumbing systems in commercial buildings and health care facilities,” wrote the authors, led by Arthur W. Baker, MD, MPH, of Duke University, Durham, N.C., adding that “Infections due to M. abscessus are difficult to diagnose and typically require months of therapy using multiple antibiotics” (Clin Infect Dis. 2017 Jan 10. doi: 10.1093/cid/ciw877).
Phase 2 took place from December 2014 through June 2015; in between Phase 1 and Phase 2, tap water abstention was implemented to protect patients deemed high risk, such as those with lung transplants. Of the 71 infections that occurred during Phase 1, 39 (55%) were lung transplant patients, while 9 (13%) were in those who had a recent cardiac surgery, 5 (7%) had cancer, and 5 (7%) had hematopoietic stem cell transplants. Incidence rates decreased substantially, back to their baseline levels, and further measures were used to completely resolve the outbreak.
“Primary interventions included institution of an inpatient sterile water protocol for high-risk patients, implementation of a protocol for enhanced disinfection and sterile water use for [heater-cooler units] of [cardiopulmonary bypass] machines, and water engineering changes designed to decrease NTM [nontuberculous mycobacteria] burden in the plumbing system,” the authors explained. “Other health care facilities, particularly those with endemic NTM or newly constructed patient care facilities, should consider similar multifaceted strategies to improve water safety and decrease risk of health care–associated infection from NTM.”
The study was funded by the National Institutes of Health’s Transplant Infectious Disease Interdisciplinary Research Training Grant. Dr. Baker and his coauthors did not report any relevant financial disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: After tap water avoidance, cases reduced from 3.0 cases per 10,000 patient-days to 0.7, the number at baseline pre-outbreak.
Data source: Prospective analysis of M. abscessus cases at a single institution during January 2013–December 2015.
Disclosures: Funded by a grant from the NIH. Authors reported no relevant disclosures.
Sarcoidosis doubles hospitalized infection risk
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
Persons with sarcoidosis were found to have double the risk of hospitalization, compared with age-matched controls in a population-based cohort study that also linked glucocorticoid use with an increased risk of hospitalization in this group.
Using data from the Rochester Epidemiology Project record-linkage system, Patompong Ungprasert, MD, an assistant professor of medicine at the Mayo Clinic in Rochester, Minn., and his colleagues identified 345 incident cases of sarcoidosis recorded between 1976 and 2013, confirmed by individual medical records (Ann Am Thorac Soc. 2017 Feb 8. doi: 10.1513/AnnalsATS.201610-750OC). Using random selection, each patient was age- and sex-matched with sarcoidosis-free controls taken from the same database. Medical records across the study were examined for community-acquired infections requiring hospitalization that occurred after the index date or the date of diagnosis.
Dr. Ungprasert and his coinvestigators found that those with sarcoidosis had double the risk of all forms of specific hospitalized infection when compared with controls – a 2.00 hazard ratio (95% confidence interval, 1.41-2.84). The results were similar when adjusted for infection risk factors: 2.13 HR (95% CI, 1.35-3.34).
The risk of hospitalized infection in the sarcoidosis arm was higher than in controls regardless of disease stage: an HR of 1.70 (95% CI, 1.12-2.58, P = .013) in those with Stage I; an HR of 2.00 (95% CI, 1.22-3.29, P = .006) among those with stage II; and an HR of 2.63 (95% CI, 1.58-4.39, P less than .001) in those with Stage III and Stage IV disease.
Biopsies taken in 251 cases resulted in 229 positive results for noncaseating granuloma, and just over half of patients had stage I disease. Stage II disease was found in 29%, Stage III in 15%, and Stage IV in 2%.
Patients in the sarcoidosis group who had not been exposed to immunosuppressive treatment had significantly higher risk of hospitalization with an HR of 1.73 (95% CI, 1.16-2.60; P = .008) when compared with controls. The risk was even higher in study patients who had received immunosuppressive therapy: an HR of 2.41 (95% CI, 1.60-3.64; P less than .001), when compared with controls. Less than half of all sarcoidosis patients required immunosuppressive therapy at any point during follow-up: about 37% by year 30 after original diagnosis. Oral glucocorticoids were the most commonly prescribed medication, used in 113 cases.
A baseline diffusing capacity of the lung for carbon monoxide was associated with an overall increased risk of hospitalized infection, with an HR of 1.15 per decrease of 10% predicted in diffusing capacity of the lung for carbon monoxide (95% CI, 1.01-1.32). A baseline forced vital capacity was associated with an increased hospitalized pneumonia risk with an HR of 1.15 per decrease of 10% predicted in forced vital capacity (95% CI, 1.01-1.32).
Although the use of immunosuppressive agents was not significantly associated with the risk of hospitalized infection (HR, 1.43; 95% CI, 0.94-2.19), current use of oral glucocorticoids, whether alone or as adjunct to immunosuppressive therapy, significantly predicted risk of infection in patients with sarcoidosis, with an HR of 3.03 (95% CI, 1.33-6.90) for oral glucocorticoids up to 10 mg per day, and an HR of 4.48 (95% CI, 1.33-6.90) in patients taking oral glucocorticoids at more than 10 mg per day, when compared with controls.
In an interview, Dr. Ungprasert said the results were not surprising, but provided the following takeaways from this study for physicians caring for patients with sarcoidosis.
“These patients are at an increased risk of serious infection and should seek medical attention as soon as possible when they develop symptoms of infection, such as fever or chills,” he said in an interview. “Keeping current with vaccinations is also important for them.”
Dr. Ungprasert also said the study serves as a reminder to use oral glucocorticoids judiciously. “When considering their use, the physician should keep in mind that a large number of patients with sarcoidosis will have a spontaneous resolution of the disease.”
There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
[email protected]
On Twitter @whitneymcknight
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Key clinical point:
Major finding: 345 patients with sarcoidosis had a hazard ratio of 2.00 (95% CI, 1.41-2.84) for hospitalized infection, compared with controls.
Data source: Olmsted County, Minn. epidemiology records from 1976 to 2013.
Disclosures: There were no relevant disclosures. The study was funded in part by the National Institute on Aging.
Small study: Drug combo achieves negative bacterial culture in all TB patients
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
AT CROI
Key clinical point: An oral, three-drug combination led to undetectable bacteria levels.
Major finding: All of the patients in the study were culture negative at 4 months.
Data source: Open-label trial of 72 patients at two centers.
Disclosures: Dr. Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
Prednisone reduces TB-IRIS risk
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
AT CROI
Key clinical point:
Major finding: Forty-seven percent of placebo patients experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group.
Data source: Randomized, placebo-controlled trial of 240 patients.
Disclosures: Dr. Meintjes reported having no relevant financial disclosures.
Pertussis susceptibility estimates call for public health push
, according to results of research by Lana Childs and Robert A. Bednarczyk, PhD.
There were 32,971 pertussis cases reported in 2014, a 15% increase over 2013; most cases occurred in children who were too young to be fully vaccinated and in preadolescents with waning immunity from their vaccines. In the United States, vaccine coverage during childhood tends to be high overall, but DTaP coverage (84% in 2014) “remains lower than coverage for other childhood vaccinations,” they noted.
“These findings emphasize the need for public health professionals to continue efforts to increase DTaP vaccine coverage in children and Tdap coverage in pregnant women, plan for potential outbreaks, and maintain immunity levels needed to prevent the spread of pertussis.” the investigators concluded.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/INF.0000000000001537).
, according to results of research by Lana Childs and Robert A. Bednarczyk, PhD.
There were 32,971 pertussis cases reported in 2014, a 15% increase over 2013; most cases occurred in children who were too young to be fully vaccinated and in preadolescents with waning immunity from their vaccines. In the United States, vaccine coverage during childhood tends to be high overall, but DTaP coverage (84% in 2014) “remains lower than coverage for other childhood vaccinations,” they noted.
“These findings emphasize the need for public health professionals to continue efforts to increase DTaP vaccine coverage in children and Tdap coverage in pregnant women, plan for potential outbreaks, and maintain immunity levels needed to prevent the spread of pertussis.” the investigators concluded.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/INF.0000000000001537).
, according to results of research by Lana Childs and Robert A. Bednarczyk, PhD.
There were 32,971 pertussis cases reported in 2014, a 15% increase over 2013; most cases occurred in children who were too young to be fully vaccinated and in preadolescents with waning immunity from their vaccines. In the United States, vaccine coverage during childhood tends to be high overall, but DTaP coverage (84% in 2014) “remains lower than coverage for other childhood vaccinations,” they noted.
“These findings emphasize the need for public health professionals to continue efforts to increase DTaP vaccine coverage in children and Tdap coverage in pregnant women, plan for potential outbreaks, and maintain immunity levels needed to prevent the spread of pertussis.” the investigators concluded.
Read more at (Ped Inf Dis J. 2017. doi: 10.1097/INF.0000000000001537).
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
Twenty-five states at highest flu activity level
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.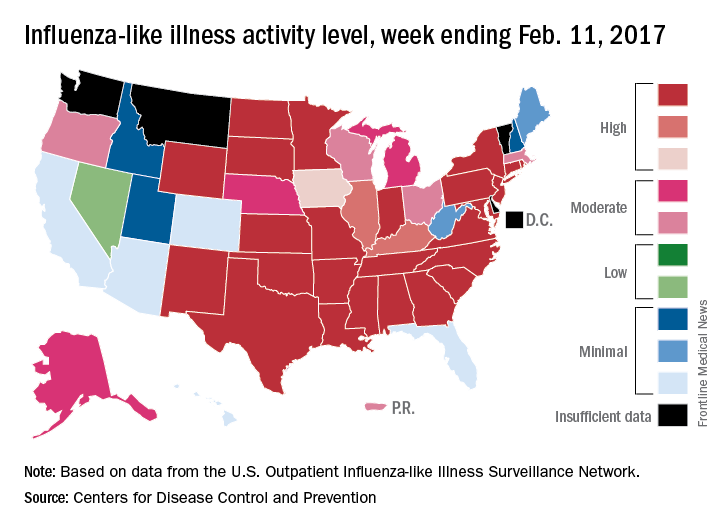
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Flu activity in the United States continued to increase as half of the states reached the highest level of influenza-like illness (ILI) activity in the week ending Feb. 11, according to the Centers for Disease Control and Prevention.
For the week, the 25 states at level 10 on the CDC’s 1-10 scale of ILI activity were joined in the high range by Illinois and Kentucky at level 9 and Iowa at level 8, the CDC reported. The previous week, there were 23 states in the high range.
Of the nine flu-related pediatric deaths reported to the CDC during the latest week, eight occurred in earlier weeks. For the 2016-2017 season so far, 29 flu-related pediatric deaths have been reported, the CDC said.
Can a nomogram foretell invasive pulmonary adenocarcinoma?
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The nomogram Dr. Jin and coauthors present can be a valuable tool for determining the extent of resection of subsolid pulmonary nodules and to distinguish invasive from preinvasive disease where preoperative needle biopsy and intraopertiave frozen section typically cannot, Bryan Burt, MD, of Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:460-1).
“However,” Dr. Burt added, “as the accuracy of frozen section for this disease improves, as it has in select centers, the clinical utility of such a nomogram will diminish.”
Use of the nomogram relies on experienced chest radiologists to aid in scoring variables and a validation methodology that a retrospective trial cannot meet, Dr. Burt said. “Of note, this nomogram was constructed from a dataset composed of only surgically resected lesions, and it will be imperative to validate these methods among a larger cohort of individuals with subsolid pulmonary nodules treated both surgically and nonsurgically, ideally in a prospective trial,” Dr. Burt concluded.
Dr. Burt had no relevant financial disclosures.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
The diagnosis of solitary peripheral subsolid nodule carries with it an undefined risk of invasive pulmonary carcinoma, but clinicians have not had a tool that can help guide their planning for surgery. However, researchers in China have developed a nomogram that they said may aid clinicians to predict the risk of invasive pulmonary adenocarcinoma in these patients.
“Validation by the use of bootstrap resampling revealed optimal discrimination and calibration, indicating that the nomogram may have clinical utility,” said Chenghua Jin, MD, and Jinlin Cao, MD, of Zhejiang University, Hangzhou, China, and coauthors. They reported their findings in the February issue of the Journal of Thoracic and Cardiovascular Surgery (2017;153:42-9).
The nomogram accounts for the following factors: computed tomography attenuation; nodule size; spiculation; signs of vascular convergence; pleural tags; and solid proportion. “The nomogram showed a robust discrimination with an area under the receiver operating characteristic curve of 0.894,” Dr. Jin and coauthors reported. An area under the curve of 1 is equivalent to 100%, so the area under the curve this study reported shows close to 90% accuracy.
The study involved a retrospective analysis of 273 consecutive patients who had resection of a solitary peripheral subsolid nodule at Zhejiang University School of Medicine from January 2013 to December 2014. Subsolid pulmonary nodules include pure ground-glass nodules and part-solid nodules that feature both solid and ground-glass components. “The optimal management of patients with a subsolid nodule is of growing clinical concern, because the most common diagnosis for resected subsolid nodules is lung adenocarcinoma,” Dr. Jin and colleagues indicated.
Of the study population, 58% were diagnosed with invasive pulmonary adenocarcinoma. Other diagnoses within the group were benign (13%), atypical adenomatous hyperplasia (1%), adenocarcinoma in situ (6.5%) and minimally invasive adenocarcinoma (21%).
Results of the multivariable analyses showed that invasive pulmonary adenocarcinoma correlated with the following characteristics: lesion size; spiculation; vascular convergence; and pleural tag. Factors that were not significant included age, family history of lung cancer, CT attenuation, and solid proportion. However, the researchers did include CT attenuation, along with solid proportion, in the final regression analysis based on their contributions to the statistical analysis.
For the model, CT attenuation of –500 to –200 Hounsfield units carried an odds ratio of 1.690 (P = .228) while CT attenuation greater than –200 HU had an OR of 1.791 (P = .645). Positive spiculation had an OR of 3.312 (no P value given) and negative vascular convergence an OR of 0.300 (no P value given).
While a number of prediction models have been devised and validated to evaluate the likelihood of malignancy in pulmonary nodules, they have not given subsolid nodules “specific or detailed consideration,” Dr. Jin and and coauthors said. “To our knowledge, this study was the first to construct a quantitative nomogram to predict the probability of invasive pulmonary adenocarcinoma in patients with subsolid nodules,” the researchers wrote.
One limitation of the study is its selection bias toward patients with a greater probability of having a malignancy. Also, validation of the nomogram requires external analysis with additional databases from other countries and with more diverse ethnic groups. Another shortcoming is the retrospective nature of the study and a small number of patients who had positron emission tomography. “Further data collection, wider geographic recruitment, and incorporation of positron emission tomography results and some molecular factors could improve this model for future use,” Dr. Jin and coauthors concluded.
Dr. Jin and Dr. Cao had no relevant financial disclosures. The study received funding from the Zhejiang Province Science and Technology Plan.
EXPERT ANALYSIS FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Investigators developed a nomogram that may help predict the risk of invasive pulmonary adenocarcinoma for patients with a solitary peripheral subsolid nodule.
Major finding: This nomogram may help clinicians individualize each patient’s prognosis for invasive pulmonary adenocarcinoma and develop treatment plans accordingly.
Data source: Retrospective analysis of 273 consecutive patients who had surgery to remove a solitary peripheral subsolid nodule at a single center.
Disclosure: The investigators received support from the Zhejiang Province Science and Technology Plan. Dr. Jin and Dr. Cao reported having no relevant financial disclosures.